 A feeding organ the basihyal and thyrohyal tells which size of prey do true baleen whales (Cetacea, Chaeomysticeti) eat
A feeding organ the basihyal and thyrohyal tells which size of prey do true baleen whales (Cetacea, Chaeomysticeti) eat
Article number: 27.1.a8
https://doi.org/10.26879/1311
Copyright Society of Vertebrate Paleontology, January 2024
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 June 2023. Acceptance: 10 January 2024.
ABSTRACT
Modern baleen whales are filter feeders. The Chaeomysticeti is a group of true baleen whales including extinct and all extant baleen whales. The early feeding strategy of the Chaeomysticeti has been discussed but not enough. Considering evolution of feeding strategy of baleen whales, fossils of the Chaeomysticeti are important key to describe how filter feeding in whales began and diversified.
To answer such questions, this study examined the relationships of the basihyal-thyrohyal shape and feeding strategy among extinct and extant baleen whales, and hypothesized evolution of the prey types.
As the result of analysis, small prey feeders such as balaenids, Caperea marginata, and Eschrichtius robustus share the basihyal-thyrohyal with small articular processes, and a wide and shallow notch between the articular processes. On the other hand, large prey feeders eating fish primarily show very long articular processes and anteriorly oriented lateral portions of the basihyal-thyrohyal, which is a unique condition owned by Balaenoptera edeni among baleen whales. A member of the most basal chaeomysticete: Yamatocetus canaliculatus was plotted close to the cluster of the small prey feeders.
This result suggests that the early Chaeomysticeti fed on small prey using the baleen plates for filtering. In the Miocene, the Cetotheriidae and Balaenopteridae started having both large and small prey. Then, a few members of Balaenopteridae such as Balaenoptera musculus and B. edeni were specialized in prey types. In short, prey type of the Chaeomysticeti started from small-sized prey such as small invertebrates then diversified through evolution.
Yoshihiro Tanaka, Osaka Museum of Natural History, Nagai Park 1-23, Higashi-Sumiyoshi-ku, Osaka, 546-0034, Japan. tanaka@omnh.jp
Keywords: feeding strategy; prey types; hyoid; Mammalia; rorquals; evolution
Final citation: Tanaka, Yoshihiro. 2024. A feeding organ the basihyal and thyrohyal tells which size of prey do true baleen whales (Cetacea, Chaeomysticeti) eat. Palaeontologia Electronica, 27(1):a8.
https://doi.org/10.26879/1311
palaeo-electronica.org/content/2024/5120-whale-hyoid
Copyright: January 2024 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Modern baleen whales (the Mysticeti) are filter feeders, who feed on much smaller prey to develop and maintain huge bodies. The Chaeomysticeti is a group of toothless and true baleen whales including extinct and all extant baleen whales. Considering evolution of feeding strategy of baleen whales, fossils of the Chaeomysticeti are important key to describe how filter feeding whales began and diversified.
Chaeomysticetes with the baleen plates appeared in the Late Oligocene about 30 to 27 m.y.a. (Boessenecker and Fordyce, 2017). Prior to them, there were toothed mysticetes from the latest Eocene (about 36 m.y.a.) (Lambert et al., 2017). For example, an early toothed mysticete Mystacodon having large teeth with worn crowns is thought as a raptorial feeder (De Muizon et al., 2019).
Feeding strategies such as prey capture tactics and prey types of extant baleen whales are varied. Prey capture tactics of extant baleen whales are known as skim, lunge, and benthic suction feedings (Werth, 2000). Skim feeding is employed by the Balaenidae (right and bowhead whales) and Caperea marginata (pygmy right whale) and is “generating continuous negative pressure” within the mouth cavity with a steady forward propulsion (Brodie and Vikingsson, 2009). Lunge feeding is employed by most of balaenopterids (rorquals and humpback whales) and is “intermittent engulfment and subsequent filtration” (Croll et al., 2018). Benthic suction feeding is employed by Eschrichtius robustus (gray whale) and involves the filtering of invertebrates from the sea bottom (Ray and Schevill, 1974). Some baleen whales such as Balaenoptera borealis (sei whale) and E. robustus employ combinations of such feeding tactics (Nemoto, 1970; Jefferson et al., 2008; Werth, 2000), which can be called multiple prey capture tactics (Tanaka, 2022).
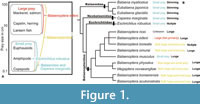 Baleen whales feed on different sizes of prey (Figure 1). Such prey can be divided into small size prey (about 0.1 to 5 cm) and large size prey (about 10 cm or larger). The Balaenidae and Caperea marginata feed on small size prey of copepods and other invertebrates with skim feeding (Nemoto, 1970; Jefferson et al., 2008). Most members of the Balaenopteridae feed on a wide range of prey such as copepods to large fish with lunge feeding (Nemoto, 1970; Jefferson et al., 2008). Only a few balaenopterids are different from the others. Balaenoptera edeni (Bryde’s whales) primarily feeds schooling fish such as pilchard, anchovy, sardine, mackerel, and herring (Jefferson et al., 2008). Balaenoptera musculus (blue whales) feeds krill with lunge feeding (Jefferson et al., 2008). Balaenoptera borealis uses multiple prey capture tactics, which includes not only lunge but also skim feedings. B. borealis eats copepods and small prey with skim feeding, and krill, sardines and anchovies with lunge feeding (Jefferson et al., 2008; Brodie and Vikingsson, 2009). Eschrichtius robustus also uses multiple prey capture tactics including benthic suction, skim and lunge feedings, and feed on mysids, amphipods, tube worm, and so on (Scammon, 1874; Nemoto, 1970; Werth, 2000; Jefferson et al., 2008). Such variety of feeding strategies might be related to niche partitioning, diversification, and evolution (Woodward et al., 2006; Werth et al., 2018).
Baleen whales feed on different sizes of prey (Figure 1). Such prey can be divided into small size prey (about 0.1 to 5 cm) and large size prey (about 10 cm or larger). The Balaenidae and Caperea marginata feed on small size prey of copepods and other invertebrates with skim feeding (Nemoto, 1970; Jefferson et al., 2008). Most members of the Balaenopteridae feed on a wide range of prey such as copepods to large fish with lunge feeding (Nemoto, 1970; Jefferson et al., 2008). Only a few balaenopterids are different from the others. Balaenoptera edeni (Bryde’s whales) primarily feeds schooling fish such as pilchard, anchovy, sardine, mackerel, and herring (Jefferson et al., 2008). Balaenoptera musculus (blue whales) feeds krill with lunge feeding (Jefferson et al., 2008). Balaenoptera borealis uses multiple prey capture tactics, which includes not only lunge but also skim feedings. B. borealis eats copepods and small prey with skim feeding, and krill, sardines and anchovies with lunge feeding (Jefferson et al., 2008; Brodie and Vikingsson, 2009). Eschrichtius robustus also uses multiple prey capture tactics including benthic suction, skim and lunge feedings, and feed on mysids, amphipods, tube worm, and so on (Scammon, 1874; Nemoto, 1970; Werth, 2000; Jefferson et al., 2008). Such variety of feeding strategies might be related to niche partitioning, diversification, and evolution (Woodward et al., 2006; Werth et al., 2018).
For understanding true baleen whale evolution, early members of the Chaeomysticeti are keys. Early feeding strategy of the Chaeomysticeti has been discussed recently, but not enough. An early work briefly mentioned that fossil mysticetes were structurally similar to balaenopterids and Eschrichtius robustus, in a review about the baleen whale feeding mechanism (Pivorunas, 1979). A later work more clearly mentioned that none of archaic baleen whales have arched or robust rostra like the Balaenidae and E. robustus, thus lunge feeding was earned by archaic baleen whales (Fordyce and de Muizon, 2001). After the studies, some studies discussed about early Chaeomysticeti feeding strategy as benthic feeding based on osteosclerotic bones (Beatty and Dooley, 2009), skim feeding based on lack of gulp feeders’ features (Boessenecker and Fordyce, 2015), lunge feeding based on mandible morphology (Tsai and Fordyce 2018). One of the latest study analyzed rostrum morphology variation among the Chaeomysticeti and stated that the feeding strategy of early chaeomysticetes was not skim and benthic feedings, but the answer is still unsolved (Tanaka, 2022).
There are difficulties for restoring feeding strategies of extinct animals. Even among living whales, we cannot observe what is happening in their mouth. Thus, creating hypotheses and running experimental works are two ways to improve our understanding on the black box (Werth and Potvin, 2016; Goldbogen et al., 2017). There is another way in paleobiology. Comparing the shape among living species and tracing the shape change of fossil species helps in understanding their evolution.
An analysis of relationships between the rostrum shape and prey capture tactics of the Chaeomysticeti recognized convergent evolution (Tanaka, 2022). The Balaenidae and Caperea marginata shifted from unknown feedings (some sort of primitive ones) to skim feeding independently through evolution. Tanaka (2022) could not find certain variation in rostral morphology among lunge feeders of the Balaenopteridae, which has various prey types as above. Because lunge feeding can be observed only among modern balaenopterids, and it was not possible to recognize convergent evolution. However, analysis of different anatomical elements might reveal different factors of feeding strategy adaptation to understand baleen whale evolution.
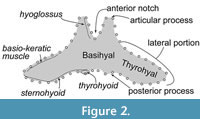 A single fused element of the basihyal-thyrohyal (Figure 2) forms a part of the hyoid. The hyoid has an important role for feeding. It provides numerous attachments to muscles to the larynx, sternum, and tongue (Werth, 2007), which work for moving the tongue and swallowing (Gray et al., 1887). An early study of Balaenoptera borealis introduced five muscles from the basihyal and thyrohyal (Schulte, 1916), and the knowledge of the bones and muscles’ relationships and their function on baleen whales has been developed as shown in Table 1 and Figure 2 show (Reidenberg and Laitman, 1994; Kienle et al., 2015; Werth and Ito, 2017).
A single fused element of the basihyal-thyrohyal (Figure 2) forms a part of the hyoid. The hyoid has an important role for feeding. It provides numerous attachments to muscles to the larynx, sternum, and tongue (Werth, 2007), which work for moving the tongue and swallowing (Gray et al., 1887). An early study of Balaenoptera borealis introduced five muscles from the basihyal and thyrohyal (Schulte, 1916), and the knowledge of the bones and muscles’ relationships and their function on baleen whales has been developed as shown in Table 1 and Figure 2 show (Reidenberg and Laitman, 1994; Kienle et al., 2015; Werth and Ito, 2017).
The basihyal and thyrohyal have been compared among extant baleen whales (Omura, 1964; Werth, 2007), and analyzed extant whales, dolphins, and porpoises (Johnston and Berta, 2011). But the element has not been analyzed with extinct members of the Chaeomysticeti yet. A member of early Chaeomysticeti, Yamatocetus canaliculatus is a key taxon showing an early condition of the basihyal and thyrohyal among the true baleen whale group. This study examines the basihyal and thyrohyal using geometric morphometric analysis, recognizes shared features of the element, then finds morphological traits with feeding strategies to describe evolutionary history of feeding strategy among the Chaeomysticeti.
MATERIALS AND METHODS
The anatomical terms follow Reidenberg and Laitman (1994) and Werth (2007) for the basihyal and thyrohyal and Schulte (1916) for myology. In Schulte (1916), the label 8 in figure 7 did not have a name of muscle, and the label 7 genioglossus did not match for the explanation in the text, which said that the muscle runs from the mandible to the tongue. Here, label 7 genioglossus is not followed, and the label number 8 is stated as the hyoglossus following later anatomical studies (Werth, 2007; Kienle et al., 2015).
 In total 85 Basihyal-thyrohyal data were collected from photos and illustrations having the right views in previous studies (Figure 3 and Table 2, see also Appendix 1). They include 14 extant species of 81 specimens, representing all extant baleen whale species except Eubalaena australis. Bryde’s whales are treated as Balaenoptera edeni (Table 2), following the latest list of species and subspecies of Society for Marine Mammalogy (Committee on Taxonomy, 2022).
In total 85 Basihyal-thyrohyal data were collected from photos and illustrations having the right views in previous studies (Figure 3 and Table 2, see also Appendix 1). They include 14 extant species of 81 specimens, representing all extant baleen whale species except Eubalaena australis. Bryde’s whales are treated as Balaenoptera edeni (Table 2), following the latest list of species and subspecies of Society for Marine Mammalogy (Committee on Taxonomy, 2022).
Fossil toothless baleen whales (members of the Chaeomysticeti) were selected through the preservation of their basihyal-thyrohyal. Some specimens were reconstructed using a preserved right or left side of the specimens. An early Chaeomysticeti Yamatocetus canaliculatus has the basihyal and thyrohyal with broken lateral ends, which is identified as a minor damage based on preserved rounded outline.
The materials were classified by feeding strategies (Heithaus and Dill, 2018). Feeding strategies includes foraging tactics (skim, lunge, or multiple prey capture tactics) (Nemoto, 1970; Croll et al., 2018; Tanaka, 2022), and prey type (feed on small, large, or both small and large prey) (Jefferson et al., 2008; Cerchio and Yamada, 2018). The feeding strategies of Balaenoptera ricei, which is a recently established extant species and extinct true baleen whales were assigned as unknown (not observed) (Rosel et al., 2021).
Institutional Abbreviations
AMNH, American Museum of Natural History, New York, USA. KMNH, Kitakyushu Museum of Natural History, Fukuoka, Japan. MNHN, Muséum national d’Histoire naturelle, Paris, France. MPST, Museo Paleontologico di Salsomaggiore Terme, Italy. NFL, Numata Fossil Museum, Hokkaido, Japan. NMNS, National Museum of Nature and Science, Tsukuba, Japan. NMV, Museum Victoria, Australia. OMNH, Osaka Museum of Natural History, Osaka, Japan. USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA.
Data Collection
Landmark acquisition was managed using the TPS program package such as tpsUtil v1.78 and tpsDig v2.31 (Rohlf, 2015). Semi-landmarks (Figure 2) were measured on each specimen. Lines on the outline of the basihyal-thyrohyal were taken as semi-landmarks from/to the most posterior position of the anterior margin of the basihyal-thyrohyal at the center, on pictures in dorsoventral view. They were divided into 50 semi-landmarks at equal distances. Non-shape information (size and rotation) was removed from the landmark configurations using the New Procrustes Fit implemented in MorphoJ 1.07a (Klingenberg, 2011).
Morphometric Analysis
Geometric morphometric analysis was used to access the shape variation of basihyal-thyrohyal morphology and test the hypothesis that specific basihyal-thyrohyal morphologies facilitate specific feeding strategies. Analyzes were run with MorphoJ 1.07a (Klingenberg, 2011) and PAST 4.13 (Hammer et al., 2001).
To reduce the dimensionality of the data and display the major axes of variation for extant and extinct true baleen whales, principal component analysis (PCA) was used in MorphoJ (Figure 4 and Figure 5) and was tested on PC1 and 2 by Multivariate Analysis of Variance (MANOVA) in PAST.
Hypotheses were fixed as below. If specific basihyal-thyrohyal morphologies facilitate specific feeding strategies, then clusters of phylogenetically separated linages with the same feeding strategies will be closely associated. For example, on prey capture tactics, some phylogenetically separated groups having small prey such as balaenids, Caperea marginata, Eschrichtius robustus, and Balaenoptera musculus will be grouped together in the morphospace. Only one primary fish feeder Balaenoptera edeni will be separated from other balaenopterids. For example, on prey type, Balaenoptera borealis has not only lunge but also skim feedings (multiple prey capture tactics) and will appear far from other lunge feeder balaenopterids in the morphospace, but will be close to Eschrichtius robustus, which also has a multiple prey capture tactics. In addition, examined specimens will appear as clusters of prey types.
To examine which groups united by feeding strategy (prey capture tactics and prey types) differ from each other, pairwise Hotelling’s tests was used in PAST (Table 3).
Cladograms
To consider the evolution of feeding strategies among the Chaeomysticeti, strategies of both extant and extinct baleen whales are adapted to cladograms. The cladograms represent two phylogenetic hypotheses on the phylogenetic position of included three named extinct taxa analyzed in this study. There are numerous phylogenetic hypotheses, which can be recognized as two types of Pelocetus calvertensis positions. Type A locates P. calvertensis basal to the Balaenopteridae (Lambert et al., 2017; Buono et al., 2017; De Muizon et al., 2019; Marx et al., 2019; de Lavigerie et al., 2020; Kimura and Hasegawa, 2021). Type B locates P. calvertensis basal to the Balaenopteridae+Cetotheriidae clade (Gol’din, 2018). These cladograms were chosen from numerous phylogenetic hypotheses with confluency of molecular phylogenetic analysis (Nikaido et al., 2006; Sasaki et al., 2006; McGowen et al., 2009; Steeman et al., 2009; Rosel et al., 2021).
Qualitative Comparison
To see differences and unique features among the Balaenopteridae, detailed morphological features in the basihyal-thyrohyal such as notches and projections were compared (Table 4).
To know conditions among early mysticetes, Fucaia buelli phylogenetically placed basal to the Chaeomysticeti (Marx et al., 2015) is included in qualitative comparison as an outgroup. This specimen has isolated basihyal and thyrohyal. Thus, it does not show original orientation of the lateral portion. Limitedly preserved holotype bone of Pelocetus calvertensis is also included in qualitative comparison.
RESULTS
Principal Component Analysis
The first two PCs combined explain 83.3% of the variation (PC1=54.1%, PC2=29.2%, PC3=5.9%, PC4=3.1%, PC5= 2.6%, PC6 = 0.8%), and the results of MANOVA in the shape of feeding strategies were significant (P<0.001) (Appendix 2).
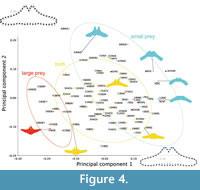 Principal component 1 represents the orientation of the lateral portion and the curvature of the posterior border of the basihyal-thyrohyal. To the right end (the positive side) of PC 1, the posterior border of the basihyal-thyrohyal is strongly anteriorly excavated and the lateral portion of the basihyal-thyrohyal oriented posterolaterally. By contrast, to the left (the negative side), the posterior border of the basihyal-thyrohyal is posteriorly expanded and the lateral portion of the basihyal-thyrohyal oriented anterolaterally (Figure 4).
Principal component 1 represents the orientation of the lateral portion and the curvature of the posterior border of the basihyal-thyrohyal. To the right end (the positive side) of PC 1, the posterior border of the basihyal-thyrohyal is strongly anteriorly excavated and the lateral portion of the basihyal-thyrohyal oriented posterolaterally. By contrast, to the left (the negative side), the posterior border of the basihyal-thyrohyal is posteriorly expanded and the lateral portion of the basihyal-thyrohyal oriented anterolaterally (Figure 4).
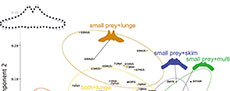
A primary fish feeder Balaenoptera edeni has negative PC1 scores associated with an anterolaterally oriented lateral portion of the basihyal-thyrohyal. Some of small prey feeders such as the Balaenidae, Caperea marginata, and Eschrichtius robustus have positive PC1 scores associated with posterolaterally oriented lateral portions of the basihyal-thyrohyal. However, another small prey feeder Balaenoptera musculus has near zero PC1 scores like other balaenopterids feed on both small and large prey plotted between the two groups of large prey feeders and small prey feeders (Figure 4).
Principal component 2 is characterized by relative position of the articular process against the lateral portion. Positive PC2 scores were related to an anteriorly elongated articular process against the whole part of the basihyal-thyrohyal with an angle at the lateral portion, that can be seen Balaenoptera musculus, which has very high PC2 scores. Balaenoptera edeni and B. borealis show relatively low PC2 scores. However, this does not mean that Balaenoptera edeni and B. borealis has short articular process (see Qualitative morphological comparison).
As a result, the Balaenidae, Caperea marginata, and Eschrichtius robustus share the basihyal-thyrohyal morphology by eating small-size prey. However, Balaenoptera musculus is plotted far from the three linages (the Balaenidae, Caperea marginata, and Eschrichtius robustus).
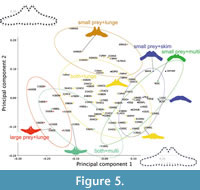 Prey capture tactics do not affect strongly in PCA (Figure 5). Lunge feeders are not bound closely and separated into three groups by prey types (Figure 5). Thus, prey types effect morphology of the basihyal-thyrohyal strongly.
Prey capture tactics do not affect strongly in PCA (Figure 5). Lunge feeders are not bound closely and separated into three groups by prey types (Figure 5). Thus, prey types effect morphology of the basihyal-thyrohyal strongly.
This is also supported by the result of pairwise Hotelling’s tests. P-values for pairwise Hotelling’s tests are all significant (p <0.05) in groups categorized by prey types, but are limitedly in groups categorized by feeding tactics (Table 3). Multiple prey capture tactics are discriminated from lunge feeding significantly, but from skim feeding not significantly.
Most fossil taxa are closely associated with the clusters of the lunge feeders having both small and large prey (most of balaenopterids), and small prey feeders with skimming (the Balaenidae and Caperea marginata) or multiple prey capture tactics (Eschrichtius robustus), but far from the clusters of the lunge feeders having fish primary (Balaenoptera edeni) and small invertebrates (Balaenoptera musculus) in the morphospace (Figure 5).
The early Chaeomysticeti Yamatocetus canaliculatus shows that high positive PC1 score among the analyzed specimens, which makes Yamatocetus canaliculatus closest to the clusters of the small prey feeders (the Balaenidae, Caperea marginata, and Eschrichtius robustus).
Qualitative Morphological Comparison
Balaenoptera edeni shows a unique combination of features such as very long articular processes, deep notch between the articular processes, large and wide processes at the posterior margin, and anterolaterally oriented lateral portions (Table 4). On the other hand, most of baleen whales have a short articular process, wide notch between the articular process, and laterally oriented lateral portions.
Among the Chaeomysticeti including the Balaenopteridae, only Balaenoptera musculus shows anteroposteriorly expanded lateral portions. This expansion forms an angle at the middle of the lateral portion of the basihyal-thyrohyal.
The all extant non-balaenopterids (the Balaenidae, Caperea marginata, and Eschrichtius robustus) show small articular and posterior processes, shallow notch, and posteriorly oriented lateral portions.
Most of examined extinct species show the same conditions of the features to those of other extant groups (the Balaenidae, Caperea marginata, and Eschrichtius robustus). An aetiocetid Fucaia buelli and an early member of the Chaeomysticeti Yamatocetus canaliculatus show primitive conditions of the basihyal-thyrohyal. They have very short and not anteriorly well-projected articular processes. Yamatocetus canaliculatus shows most posteriorly oriented lateral portion. A Middle to Late Miocene cetotheriid Piscobalaena nana and a Late Miocene chaeomisticeti Pelocetus calvertensis have short articular processes, which are similar to these of extant balaenopterids. An Early Pliocene balaenopterid “Megaptera” hubachi has laterally oriented lateral portion.
Prey size and the size of the basihyal-thyrohyal are varied. The size of the basihyal-thyrohyal of small prey feeders are from a small size of the Balaenidae (about 70 to 90 cm wide) to large size of Balaenoptera musculus (about 120 to 130 cm wide). The size of the basihyal-thyrohyal of both large and small prey feeders are from small size of Balaenoptera acutorostrata (about 30 to 40 cm wide) to large size of Balaenoptera borealis (about 60 to 80 cm wide).
DISCUSSION
Morphological Traits by Prey Types
Some morphological features are related to prey types more strongly instead of prey capture tactics based on PCA and pairwise Hotelling’s tests (Figure 5 and Table 3). The clusters of small prey feeders with different prey capture tactics such as balaenids, Caperea marginata, and Eschrichtius robustus are plotted very closely (Figure 4). Such small prey feeders share the basihyal-thyrohyal with a small articular process, and a wide and shallow notch between the articular processes.
A lunge + small prey feeder Balaenoptera musculus is differentiated from other small prey feeders (balaenids, Caperea marginata, and Eschrichtius robustus) by having the basihyal-thyrohyal with a large articular process, and a narrow and deep notch between the articular processes. In addition, B. musculus uniquely shows an anteroposteriorly expanded lateral portion of the basihyal-thyrohyal. This fact implies that B. musculus are specialized and adapted to consume small prey in different ways.
Most of balaenopterids’ feeding of both small and large prey are located at the center in the morphospace (Figure 4 and Figure 5). Such balaenopterids have moderate-sized articular process and narrow notch between the processes.
On the other hand, the primarily fish feeders (Balaenoptera edeni) are distant from the center of the confidence ellipse of balaenopterids. The primarily fish feeder Balaenoptera edeni having a very long articular process and anterolaterally oriented lateral portion. These features are very rare among the Chaeomysticeti.
Some Balaenoptera borealis is similar to B. edeni in having slender articular process. However, the articular process of B. borealis is shorter than that of B. edeni. In B. borealis, orientation of lateral portion is varied, but some of them have anterolaterally oriented ones. B. borealis is known as a generalist feeding wider range of prey compared to other extant baleen whales (Horwood, 2018). B. borealis might have more fish compared to other balaenopterids excluding B. edeni.
Morphology of the Basihyal-Thyrohyal and Function of the Tongue
Since the hyoid bone provides muscles for moving the tongue and swallowing, differences of the bone shape may reflect difference of the tongues themselves such as hardness, function of the tongue, and swalloing manner.
How whales use their tongues? The members of the Balaenidae and Caperea marginata are skim feeders, and they use their sturdy tongues for cleaning baleen with trapped prey and swallowing (Werth and Crompton, 2023). Eschrichtius robustus retract their muscular tongue to expand the oral cavity and generate negative pressure to take prey from the mud sea floor (Werth, 2001). The members of the Balaenopteridae are thought to use their flabby tongues for transporting accumulated prey posteriorly in their mouth (Werth and Ito, 2017).
The hardness of the tongue possibly relates to the basihyal-thyrohyal morphology. The tongues of Eschrichtius robustus and the Balaenidae are muscular, but the ones of the Balaenopteridae are soft. Whales with muscular tongues share the basihyal-thyrohyal morphology with a small articular process, and a wide and shallow notch between the articular processes. The tongue of Balaenoptera edeni was described with other balaenopterids such as B. borealis, B. acutorostrata, and Megaptera novaeangliae as a poorly muscled tongue (Nemoto, 1970; Lambertsen, 1983).
It might be possible that among the Balaenopteridae detailed comparison of the tongue in not only hardness, but also dimensions and shape. B. edeni can be differentiated from other members. The relationships between basihyal-thyrohyal morphology and tongue are still unclear. At least, B. edeni has some sort of different tongue usage from other balaenopterids.
Having a long articular process and anteriorly oriented lateral portion are unique features for B. edeni, which can be thought as a features of fish eaters.
Having a long articular process possibly allows to provide the insertions of the hyoglossus muscle longer than these of other balaenopterids. The hyoglossus is a paired muscle on the left and right sides of the tongue (Kienle et al., 2015). The hyoglossus is originated from the articular process of the basihyal-thyrohyal (Carte and Macalister, 1868; Schulte, 1916). Having long articular process associated to having an anteroposteriorly long origin for the hyoglossus.
The lateral portion provides muscle attachments such as the sternohyoid muscle running to the sternum and the geniohyoid muscle running to the mandibular symphysis (Werth and Ito, 2017). The sternohyoid muscle retracts and depresses the tongue root (Werth and Ito, 2017). As mentioned above, the members of the Balaenopteridae use their flabby tongues for transporting accumulated prey posteriorly in their mouth.
Anteriorly oriented lateral portion makes the anteroposterior distance between the lateral portion and anterior border of the anterior process shorter in B. edeni than these of other balaenopterids. These differencese of B. edeni from other balaenopterids might be related to swallowing large size prey such as fish.
Fossil Species
Morphology of the basihyal-thyrohyal of fossil species suggests that prey type of the Chaeomysticeti started from small-sized prey such as small invertbrates then diversified through evolution.
The early Chaeomysticeti: a member of the Eomysticetidae Yamatocetus canaliculatus is closest to the clusters of small prey feeders (balaenids, Caperea marginata, and Eschrichtius robustus), but far from balaenopterids in the morphospace (Figure 5). An aetiocetid Fucaia buelli is included in qualitative comparison as outgroup, which shares some conditions with the early Chaeomysticeti: Y. canaliculatus has very small and not anteriorly well-projected anterior process and wide notch (Table 4). These conditions are supposed as primitive condition at least among the Chaeomysticeti based on comparison with outgroup.
In this study, morphology of the basihyal and thyrohyal represents prey types. Y. canaliculatus can be hypothesized as a small prey feeder. Previously, Y. canaliculatus was identified as “not a skim and multiple prey capture feeder” as seen on modern baleen whales based on rostrum morphology and was closer to the cluster of lunge feeders (Tanaka, 2022). Of note, Y. canaliculatus has very small and not anteriorly well- projected articular processes and more posteriorly oriented lateral portions, which are not the same to all compared baleen whales (Table 4). The Eomysticetidae: an early member of the Chaeomysticeti likely had unknown feeding strategies different from all extant baleen whales.
"Megaptera" hubachi and NFL 2634 are plotted in the three overlapped clusters of small prey, and both small and large prey feeders (Figure 5). Their prey types are still unknown, but at least, they were not large prey feeders like Balaenoptera edeni (Table 5).
A cetotheriid Piscobalaena nana possibly had both small and large prey. An amazingly preserved fossil stomach of a cetotheriid from the Pisco Formation, Peru contained Sardinops scales and bones (Collareta et al., 2015). 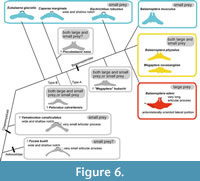 Unusual among the Malacostraca, Krill have no unequivocal fossil records (Jarman, 2001), because difficulty of preservation. Thus, the fossil cetotheriid including P. nana might have not only fish but also krill.
Unusual among the Malacostraca, Krill have no unequivocal fossil records (Jarman, 2001), because difficulty of preservation. Thus, the fossil cetotheriid including P. nana might have not only fish but also krill.
Here, prey type diversification among the Chaeomysticeti is hypothesized (Figure 6). Early members of the Chaeomysticeti started to have small size invertebrates, then some linages such as the Cetotheriidae and Balaenopteridae adapted to have fish in the Miocene. Among the Balaenopteridae, at least one linage of B. musculus being back to a small invertebrate feeder but in different way to other linages (balaenids, Caperea marginata, and Eschrichtius robustus). Another linage in the Balaenopteridae B. edeni adapted to feeding fish primarily.
CONCLUSION
This study examined the relationships of the basihyal-thyrohyal shape and feeding strategy among extinct and extant baleen whales, and hypothesized evolution of the prey types. As the result of analysis, small prey feeders such as balaenids, Caperea marginata, and Eschrichtius robustus share the basihyal-thyrohyal with small articular processes, and a wide and shallow notch between the articular processes. On the other hand, large prey feeders eating fish primarily show very long articular processes and anteriorly oriented lateral portions of the basihyal-thyrohyal, which is a unique condition owned by Balaenoptera edeni among baleen whales. A member of the most basal family Eomysticetidae: Yamatocetus canaliculatus was plotted close to the cluster of the small prey feeders. This result suggests that the early Chaeomysticeti fed small prey using the baleen plates for filtering. In the Miocene, the Cetotheriidae and Balaenopteridae started having both large and small prey. Then, a few members of Balaenopteridae such as Balaenoptera musculus and B. edeni were specialized in prey types. In short, morphology of the basihyal-thyrohyal of fossil species suggests that prey type of the Chaeomysticeti started from small-sized prey such as small invertebrates then diversified through evolution. This study revealed that the early member of the Chaeomysticeti represents a combination of prey type and prey capture tactics, which is not the same with combinations of any extant baleen whales. We still do not know how primitive feeding strategies worked among the Chaeomysticeti. More morphological comparison possibly allows us to provide better idea to consider combination of prey capture tactics, prey types and so on.
ACKNOWLEDGMENTS
I thank H. Taruno for his constructive comments on this project, Y. Tajima and T.K. Yamada (NMNS) for examination of analyzed specimens, T.K. Yamada for information of Balaenoptera omurai. I also thank two anonymous reviewers giving constructive comments.
REFERENCES
Beatty, B. and Dooley, A. 2009. Injuries in a mysticete skeleton from the Miocene of Virginia, with a discussion of buoyancy and the primitive feeding mode in the Chaeomysticeti. Jeffersoniana, 20:1–28.
Boessenecker, R.W. and Fordyce, R.E. 2015. Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: a new genus and species of Eomysticetidae (Mammalia: Cetacea) from the Oligocene of New Zealand. PeerJ, 3:e1129.
https://doi.org/10.7717/peerj.1129
Boessenecker, R.W. and Fordyce, R.E. 2017. A new eomysticetid from the Oligocene Kokoamu Greensand of New Zealand and a review of the Eomysticetidae (Mammalia, Cetacea). Journal of Systematic Palaeontology, 15:429–469.
https://doi.org/10.1080/14772019.2016.1191045
Brodie, P. and Vikingsson, G. 2009. On the feeding mechanisms of the sei whale (Balaenoptera borealis). Journal of Northwest Atlantic Fishery Science, 42:49–54.
https://doi.org/10.2960/J.v42.m646
Buono, M.R., Fernández, M.S., Cozzuol, M.A., Cuitiño, J.I., and Fitzgerald, E.M.G. 2017. December. The early Miocene balaenid Morenocetus parvus from Patagonia (Argentina) and the evolution of right whales. PeerJ, 5:e4148.
https://doi.org/10.7717/peerj.4148
Carte, A. and Macalister, A. 1868. On the anatomy of Balænoptera rostrata. Philosophical Transactions of the Royal Society of London, 158:201–261.
Cerchio, S. and Yamada, T.K. 2018. Omura’s whale: Balaenoptera omurai, p. 656–659. In Würsig, B., Thewissen, J.G.M., and Kovacs, K.M. (eds.), Encyclopedia of Marine Mammals (Third Edition). Academic Press, London.
Collareta, A., Landini, W., Lambert, O., Post, K., Tinelli, C., Di Celma, C., Panetta, D., Tripodi, M., Salvadori, P.A., and Caramella, D. 2015. Piscivory in a Miocene Cetotheriidae of Peru: first record of fossilized stomach content for an extinct baleen-bearing whale. The Science of Nature, 102:1–12.
https://doi.org/10.1007/s00114-015-1319-y
Committee on Taxonomy. 2022. Retrieved February 27, 2023, from
https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies/
Croll, D.A., Tershy, B.R., Newton, K.M., de Vos, A., Hazen, E., and Goldbogen, J.A. 2018. Filter feeding, p.363–368. In Würsig, B., Thewissen, J.G.M., and Kovacs, K.M. (eds.), Encyclopedia of Marine Mammals (Third Edition). Academic Press, London.
De Muizon, C., Bianucci, G., Martinez-Caceres, M., and Lambert, O. 2019. Mystacodon selenensis, the earliest known toothed mysticete (Cetacea, Mammalia) from the late Eocene of Peru: anatomy, phylogeny, and feeding adaptations. Geodiversitas, 41:401–499.
https://doi.org/10.5252/geodiversitas2019v41a11
Fordyce, R.E. and de Muizon, C. 2001. Evolutionary history of whales: a review, p. 169–234. In Mazin, J.-M. and de Buffrenil, V. (eds.), Secondary Adaptation of Tetrapods to Life in Water. Pfeil, München.
Gaskin, D.E. 1982. The ecology of whales and dolphins. Heinemann, London.
Goldbogen, J., Cade, D., Calambokidis, J., Friedlaender, A., Potvin, J., Segre, P., and Werth, A. 2017. How baleen whales feed: the biomechanics of engulfment and filtration. Annual Review of Marine Science, 9:367–386.
https://doi.org/10.1146/annurev-marine-122414-033905
Gol’din, P. 2018. New Paratethyan dwarf baleen whales mark the origin of cetotheres. PeerJ, 6:e5800.
https://doi.org/10.7717/peerj.5800
Gray, H., Holden, L., Keen, W.W., and Pick, T.P. 1887. Anatomy, Descriptive and Surgical 50th Ed. Lea Brothers & Co., Philadelphia.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontogia Electronica 4:4.
https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Heithaus, M.R. and Dill, L.M. 2018. Feeding strategies and tactics, p. 354–363. In Würsig, B., Thewissen, J.G.M., and Kovacs, K.M. (eds.), Encyclopedia of Marine Mammals (Third Edition). Academic Press, London.
Horwood, J. 2018. Sei whale: Balaenoptera borealis, p. 845–847. In Würsig, B., Thewissen, J.G.M., and Kovacs, K.M. (eds.), Encyclopedia of Marine Mammals (Third Edition). Academic Press, London.
Jarman, S.N. 2001. The evolutionary history of krill inferred from nuclear large subunit rDNA sequence analysis. Biological Journal of the Linnean Society, 73:199–212.
https://doi.org/10.1006/bijl.2001.0538
Jefferson, T.A., Webber, M.A., and Pitman, R.L. 2008. Marine Mammals of the World: A Comprehensive Guide to their Identification. Academic Press, Oxford, UK.
Johnston, C. and Berta, A. 2011. Comparative anatomy and evolutionary history of suction feeding in cetaceans. Marine Mammal Science, 27:493–513.
https://doi.org/10.1111/j.1748-7692.2010.00420.x
Kienle, S.S., Ekdale, E.G., Reidenberg, J.S., and Deméré, T.A. 2015. Tongue and hyoid musculature and functional morphology of a neonate gray whale (Cetacea, Mysticeti, Eschrichtius robustus). The Anatomical Record, 298:660–674.
https://doi.org/10.1002/ar.23107
Kimura, T. and Hasegawa, Y. 2021. Second report on the new material of Joumocetus shimizui from the Miocene Haraichi Formation, Annaka Group, Gunma, Japan. Bulletin of Gunma Museum of Natural History, 25:59–64.
Klingenberg, C.P. 2011. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11:353–357.
https://doi.org/10.1111/j.1755-0998.2010.02924.x
Lambert, O., Martínez-Cáceres, M., Bianucci, G., Di Celma, C., Salas-Gismondi, R., Steurbaut, E., Urbina, M., and de Muizon, C. 2017. Earliest mysticete from the Late Eocene of Peru sheds new light on the origin of baleen whales. Current Biology, 27:1535–1541.
https://doi.org/10.1016/j.cub.2017.04.026
Lambertsen, R.H. 1983. Internal mechanism of rorqual feeding. Journal of Mammalogy, 64:76–88.
https://doi.org/10.2307/1380752
de Lavigerie, G.D., Bosselaers, M., Goolaerts, S., Park, T., Lambert, O., and Marx, F.G. 2020. New Pliocene right whale from Belgium informs balaenid phylogeny and function. Journal of Systematic Palaeontology, 18:1141–1166.
https://doi.org/10.1080/14772019.2020.1746422
Marx, F.G., Post, K., Bosselaers, M., and Munsterman, D.K. 2019. February. A large Late Miocene cetotheriid (Cetacea, Mysticeti) from the Netherlands clarifies the status of Tranatocetidae. PeerJ, 7:e6426.
https://doi.org/10.7717/peerj.6426
Marx, F.G., Tsai, C.-H., and Fordyce, R.E. 2015. A new Early Oligocene toothed ‘baleen’ whale (Mysticeti: Aetiocetidae) from western North America: one of the oldest and the smallest. Royal Society Open Science, 2:150476.
https://doi.org/10.1098/rsos.150476
McGowen, M.R., Spaulding, M., and Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution, 53:891–906.
https://doi.org/10.1016/j.ympev.2009.08.018
Nemoto, T. 1970. Feeding pattern of baleen whales in the ocean, p. 241–252. In Steele, J.H. (ed.), Marine Food Chains. University of California Press, Berkeley, CA.
Nikaido, M., Hamilton, H., Makino, H., Sasaki, T., Takahashi, K., Goto, M., Kanda, N., Pastene, L.A., and Okada, N. 2006. Baleen whale phylogeny and a past extensive radiation event revealed by SINE insertion analysis. Molecular Biology and Evolution, 23:866–873.
https://doi.org/10.1093/molbev/msj071
Omura, H. 1964. A systematic study of the hyoid bones in the baleen whales. Scientific Reports of the Whales Research Institute, 18:149–170.
Pivorunas, A. 1979. The feeding mechanisms of baleen whales. American Scientist, 67:432–440.
Ray, G.C. and Schevill, W.E. 1974. Feeding of a captive gray whale. Marine Fisheries Review, 36:31–38.
Reidenberg, J.S. and Laitman, J.T. 1994. Anatomy of the hyoid apparatus in Odontoceti (toothed whales): Specializations of their skeleton and musculature compared with those of terrestrial mammals. The Anatomical Record, 240:598–624. https://doi.org/10.1002/ar.1092400417
Rohlf, F.J. 2015. The tps series of software. Hystrix, 26:9–12.
https://doi.org/10.4404/hystrix-26.1-11264
Rosel, P.E., Wilcox, L.A., Yamada, T.K., and Mullin, K.D. 2021. A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Marine Mammal Science, 37:577–610.
https://doi.org/10.1111/mms.12776
Sasaki, T., Nikaido, M., Wada, S., Yamada, T.K., Cao, Y., Hasegawa, M., and Okada, N. 2006. Balaenoptera omurai is a newly discovered baleen whale that represents an ancient evolutionary lineage. Molecular Phylogenetics and Evolution, 41:40–52.
https://doi.org/10.1016/j.ympev.2006.03.032
Scammon, C.M. 1874. The marine mammals of the north-western coast of North America: described and illustrated; together with an account of the American whale-fishery. JH Carmany, San Francisco.
Schulte, W. 1916. Anatomy of a foetus of Balaenopterus borealis. Memoirs of the American Museum of Natural History, 1:389–502.
Steeman, M.E., Hebsgaard, M.B., Fordyce, R.E., Ho, S.Y.W., Rabosky, D.L., Nielsen, R., Rahbek, C., Glenner, H., Sørensen, M.V., and Willerslev, E. 2009. Radiation of extant cetaceans driven by restructuring of the oceans. Systematic Biology, 58:573–585.
https://doi.org/10.1093/sysbio/syp060
Tanaka, Y. 2022. Rostrum morphology and feeding strategy of the baleen whale indicates that right whales and pygmy right whales became skimmers independently. Royal Society Open Science, 9:221353.
https://doi.org/10.1098/rsos.221353
Tsai, C.-H. and Fordyce, R.E. 2018. A new archaic baleen whale Toipahautea waitaki (early Late Oligocene, New Zealand) and the origins of crown Mysticeti. Royal Society Open Science, 5:172453.
https://doi.org/10.1098/rsos.172453
Werth, A. 2001. How do mysticetes remove prey trapped in baleen? Bulletin of the Museum of Comparative Zoology, 156:189–203.
Werth, A.J. 2000. Feeding in marine mammals, p. 475–514. In Schwenk, K. (ed.), Feeding: form, function and evolution in tetrapod vertebrates. Academic Press, San Diego.
Werth, A.J. 2007. Adaptations of the cetacean hyolingual apparatus for aquatic feeding and thermoregulation. The Anatomical Record, 290:546–568.
https://doi.org/10.1002/ar.20538
Werth, A.J. and Crompton, A. 2023. Cetacean tongue mobility and function: A comparative review. Journal of Anatomy, 243:343–373.
https://doi.org/10.1111/joa.13876
Werth, A.J. and Ito, H. 2017. Sling, scoop, and squirter: anatomical features facilitating prey transport, processing, and swallowing in rorqual whales (Mammalia: Balaenopteridae). The Anatomical Record, 300:2070–2086.
https://doi.org/10.1002/ar.23606
Werth, A.J. and Potvin, J. 2016. Baleen hydrodynamics and morphology of cross-flow filtration in balaenid whale suspension feeding. PLoS ONE, 11:e0150106.
https://doi.org/10.1371/journal.pone.0150106
Werth, A.J., Potvin, J., Shadwick, R.E., Jensen, M.M., Cade, D.E., and Goldbogen, J.A. 2018. Filtration area scaling and evolution in mysticetes: trophic niche partitioning and the curious cases of sei and pygmy right whales. Biological Journal of the Linnean Society, 125:264–279.
https://doi.org/10.1093/biolinnean/bly121
Woodward, B.L., Winn, J.P., and Fish, F.E. 2006. Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. Journal of Morphology, 267:1284–1294.
https://doi.org/10.1002/jmor.10474

