 Towards sustainable treatments to preserve fossils from weathering, as part of the garden redevelopment project at the Natural History Museum
Towards sustainable treatments to preserve fossils from weathering, as part of the garden redevelopment project at the Natural History Museum
Article number: 27.1.a6
https://doi.org/10.26879/1274
Copyright Paleontological Society, January 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 17 February 2023. Acceptance: 15 December 2023.
ABSTRACT
The redevelopment of the gardens at the Natural History Museum (London, UK) required the preparation and stabilisation of several large fossil specimens to enable them to withstand exposure to typical weathering in an urban setting within the UK. Several specimens were considered to be at risk from damage: limestone dinosaur trackways, limestone invertebrate fossil blocks and some silicified sections of fossil tree. This project explores several options using literature searches, scanning electron microscopy and tape tests to identify sustainable but effective methods of preservation. The most appropriate treatment chosen for the limestones is CaLoSiL® E25 with TiO2 and for the silicious fossils SILRES(®) BS OH 100.
Lu Allington-Jones, Principal Conservator, The Natural History Museum, Cromwell Road, London SW7 5BD, UK. l.allington-jones@nhm.ac.uk
Kieran Miles, Fossil Preparator, The Natural History Museum, Cromwell Road, London SW7 5BD, UK. k.miles@nhm.ac.uk
Innes Clatworthy, Electron Microscopist, The Natural History Museum, Cromwell Road, London SW7 5BD, UK. i.Clatworthy@nhm.ac.uk
Keywords: nanolime; fossil; conservation; weathering; protection; consolidation
Final citation: Allington-Jones, Lu, Miles, Kieran, and Clatworthy, Innes. 2024. Towards sustainable treatments to preserve fossils from weathering, as part of the garden redevelopment project at the Natural History Museum. Palaeontologia Electronica, 27(1):a6.
https://doi.org/10.26879/1274
palaeo-electronica.org/content/2024/5104-sustainable-preservation
Copyright: January 2024 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
 Several limestone-based specimens plus sections of silicified wood have been selected for the garden refurbishment project. The largest block measures 180 cm in length and 1,500 kg in weight. Some of the smaller specimens are shown in Figure 1 and Figure 2. Specimens include an ammonite and oyster block (shelly limestone with detrital quartz from brackish to freshwater lagoonal deposition) from the Purbeck Group, Durlston Formation, Stair Hole Member, Cretaceous period in age (Berriasian Stage) ~ 145-140 million years old. The individual ammonites are from the Portland Stone Formation, Tisbury Member, Jurassic period (Tithonian Stage) ~ 150-145 million years old and are internal moulds in fine grained sandy limestone (quartzose glauconitic biocalcarenite). The fossil wood blocks are silicified conifer from the Purbeck Group, Lulworth Formation, Jurassic period (Tithonian Stage) ~ 150-145 million years old, they show some minor delamination in outer layers. Some of the theropod footprints are impressions in lagoonal shelly low-porosity limestone from Purbeck Quarry, Swanage, Dorset and are ~163-145 million years old and others are from Purbeck Group, Durlston Formation, Stair Hole Member, Cretaceous period (Berriasian Stage) ~ 145-140 million years old. The ornithopod footprints are also from the Purbeck Group (Durlston Formation, Stair Hole Member, Cretaceous period (Berriasian Stage) ~ 145-140 million years old) but the matrix is more crumbly and yellow in colour, due to a higher sand content in the biomicrite.
Several limestone-based specimens plus sections of silicified wood have been selected for the garden refurbishment project. The largest block measures 180 cm in length and 1,500 kg in weight. Some of the smaller specimens are shown in Figure 1 and Figure 2. Specimens include an ammonite and oyster block (shelly limestone with detrital quartz from brackish to freshwater lagoonal deposition) from the Purbeck Group, Durlston Formation, Stair Hole Member, Cretaceous period in age (Berriasian Stage) ~ 145-140 million years old. The individual ammonites are from the Portland Stone Formation, Tisbury Member, Jurassic period (Tithonian Stage) ~ 150-145 million years old and are internal moulds in fine grained sandy limestone (quartzose glauconitic biocalcarenite). The fossil wood blocks are silicified conifer from the Purbeck Group, Lulworth Formation, Jurassic period (Tithonian Stage) ~ 150-145 million years old, they show some minor delamination in outer layers. Some of the theropod footprints are impressions in lagoonal shelly low-porosity limestone from Purbeck Quarry, Swanage, Dorset and are ~163-145 million years old and others are from Purbeck Group, Durlston Formation, Stair Hole Member, Cretaceous period (Berriasian Stage) ~ 145-140 million years old. The ornithopod footprints are also from the Purbeck Group (Durlston Formation, Stair Hole Member, Cretaceous period (Berriasian Stage) ~ 145-140 million years old) but the matrix is more crumbly and yellow in colour, due to a higher sand content in the biomicrite.
 Deterioration of stone (and fossils) left open to weathering is caused by micro-organisms, salt migration, acid rain, sulphur dioxide, nitrous oxides and other peroxides in pollution (Artesani et al., 2020). Limestone (formed of calcium carbonate) is prone to physical and chemical deterioration due to pollution, salt migration, acid rain and bio-colonisation. The main mechanism of deterioration caused by pollution involves sulphur dioxide, which reacts to form gypsum. Gypsum is more soluble and more porous than calcium carbonate. The increased porosity of the gypsum crust allows particulate matter to be trapped and a blackened layer is formed. This whole process is accelerated by the presence of nitrous oxides and other peroxides in polluted air (Artesani et al., 2020).
Deterioration of stone (and fossils) left open to weathering is caused by micro-organisms, salt migration, acid rain, sulphur dioxide, nitrous oxides and other peroxides in pollution (Artesani et al., 2020). Limestone (formed of calcium carbonate) is prone to physical and chemical deterioration due to pollution, salt migration, acid rain and bio-colonisation. The main mechanism of deterioration caused by pollution involves sulphur dioxide, which reacts to form gypsum. Gypsum is more soluble and more porous than calcium carbonate. The increased porosity of the gypsum crust allows particulate matter to be trapped and a blackened layer is formed. This whole process is accelerated by the presence of nitrous oxides and other peroxides in polluted air (Artesani et al., 2020).
The ideal treatment for the specimens will re-establish grain to grain cohesion but without creating an impermeable layer or preventing retreatment. It should also be available in small quantities and environmentally sustainable.
Polymer coatings, traditionally used to protect exterior stone, will yellow with age due to photoxidation. UV radiation, water, frost action and salts also negatively affect synthetic organic polymers reducing their reversibility, colour and integrity (Bernardi et al., 2012). This makes them less soluble and more difficult to remove without causing damage to the substrate. Traditionally used epoxy resins have very poor ageing characteristics and are irreversible (Tesser et al., 2018). Reversibility is important in case a treatment is found to cause damage or has been applied in error. Although polymethyl methacrylates have performed well under some laboratory conditions (Kallaf et al., 2011), Paraloid B67, Paraloid B72 and silicone-based product Dri-Film 104 performed poorly as coatings under UV exposure (Favaro et al., 2006). Traditional polymers can also crack, allowing water onto the stone below, but also preventing it from evaporating again, causing further issues. In very porous limestone acrylic and epoxy resins form a hard layer reaching to a depth of a few mm (Ferreira Pinto and Delgado Rodrigues, 2008). Locking-in water with impermeable coatings can be disastrous, leading to delamination and fissuring of the outer layer (Varas-Muriel et al., 2015).
To replace the use of polymer coatings, there have been four major advancements in exterior limestone protection within the last decade: fluorinated polymer nanocomposites, bacterial films, nanolime and diammonium hydrogen phosphate. All these treatments are far more sustainable and ecologically friendlier than the use of traditional polymer coatings. All the treatments discussed below are effectively irreversible and would not be considered for normal museum specimens, but they are considered suitable for specimens acquired specifically for display in the gardens, which would otherwise suffer significant damage if untreated and which will not be required for research.
Nano-composites
Silane and siloxane polymers, such as Rhodorsil H224 (Antonelli and Tesser, 2018), or fluorinated elastomers, such as Akeoguard CO (Ruffolo et al., 2010) and Fluoline HY (Pelosi et al., 2020), can be used as a base for nano-composites containing nitrogen and zinc oxides or zinc and tin oxides. The extreme hydrophobicity of the nano-composites is supposed to prevent water damage and also have biocidal properties. The photo-catalytic nature of the metal nano-particles will decompose pollutants that settle on the surface, effectively creating a self-cleaning layer (Ruffolo et al., 2010). High concentrations of nano-particles within the composites will, however, cause a colour change on application. Organosilicone compounds have low surface tension, good elasticity and resistance to thermal stress and good chemical stability resulting from the high strength of the silicone-oxygen bond (Tesser et al., 2014). Rhodorsil consolidant RC90 is recommended by Antonelli and Tesser (2018), it provides waterproofing properties and is very durable with respect to UV and thermo oxidation (Tesser et al., 2014). But like Akeogard and H224, RC90 is a water repellant, not a consolidant, which is undesirable for this project. Studies show that the lower levels of buildings suffer more deterioration than upper levels due to the water uptake from the ground (Fassina et al. 2016), the specimens within this project will all be positioned on bare ground, so preventing moisture egress from the upper surfaces would lead to severe issues. Another problem with using products recommended for building conservation, is managing to source smaller amounts for only a few blocks, and it is difficult to purchase any of these named products in small quantities. Calcium and magnesium alkoxide treatments are currently being developed for treatment but are not yet commercially available (Bernardi, personal commun., 2021). TEOS treatment requires white spirit, which is damaging for the environment and TEOS, ethyl silicates and other alkoxysilanes need the presence of quartz to work well (Hansen et al., 2003; Maravelaki-Kalaitzaki et al., 2008; Ferreira Pinto and Delgado Rodrigues, 2008) and are therefore unsuitable for treating limestones. None of these options are suitable for treatment in this project because of their extreme hydrophobicity and creation of an impermeable outer layer. Bacterial carbonatogenesis is a more appealing option.
Bacterial Carbonatogenesis
Calcite and vaterite deposition, using naturally occurring local bacteria, can be used to consolidate and protect limestones. The non-pathogenic naturally abundant soil bacteria deposit calcium carbonates onto the limestone, reducing porosity, and creating a biopatina, effectively preventing colonisation by other organisms (Artesani et al., 2020). Upon drying, the bacteria dies, so uncontrolled growth spreading out from the treated surface is not a risk (Rodriguez-Navarro et al., 2015). This treatment creates bacterial cement which binds loose grains and lines pores (without plugging), as shown in SEM investigations (Rodriguez-Navarro et al., 2015).
This process can be achieved by spraying the surface with a nutritional solution. This alkaline culture medium encourages the deposition of calcium carbonate and prevents growth of acid-producing organisms during treatment (Jroundi et al., 2010). The use of calcium acetate as the calcium source allows the acetic/acetate pair to act as a buffer against an eventual pH drop (Jroundi et al., 2010). A sterile nutrient solution is sufficient, using endemic microbiota in the stone, there is no need to add cultured bacteria (Rodriguez-Navarro et al., 2015). Chromatic changes over four years have been judged acceptable and were even lower than in untreated rocks, and the population of fungal spores was found to be reduced in the long-term (Rodriguez-Navarro et al., 2015). Depending on rock type, treatment was proved effective for 2.5-4 years (Rodriguez-Navarro et al., 2015).
This treatment is claimed to provide equivalent strengthening to applications of ethyl silicate (Rodriguez-Navarro et al., 2015). Bacterial carbonatogenesis has worked in the field but these tests were undertaken in a warmer climate: Rodriguez-Navarro et al. (2015) undertook treatment at a constant 20°C using warm air circulation at night. For this project it was felt that tests were required in the UK climate to see if halting the functioning of bacteria metabolism at night, and indeed most of the day, is an issue.
Nanolime
This treatment requires spraying dry surfaces with a low dilution of alcohol-based calcium hydroxide e.g. dilute CaLoSil®E25 (25g/L) in ethanol or isopropanol to 5g/L, followed by a second undiluted treatment 24 hr later (IBZ-Schalzchémie, 2020). Precipitation of calcium hydroxide occurs as the alcohol evaporates, which reacts with atmospheric carbon dioxide to create calcium carbonate and leads to consolidation (IBZ-Schalzchémie, 2020). In addition, the alcohol, which is used in the dilute solution, is also anti-microbial (IBZ-Schalzchémie, 2020) working as an initial surface decontaminant, although not as a long-term preventive. CaLoSil®E25 has been found to be a good consolidant (Daehne and Herm, 2013), increasing resistance to salt crystallization, frost and thermal shock and long-term resistance to dissolution. Nanolime treatments have also been found to increase the compressive strength of archaeological limestone by 37% and the drilling resistance by 75% (Ahmad Al-Omary et al., 2018). Bernardi (2015), however, found it ineffective in scotch tape testing on the stone within their project. Individual tests are therefore always necessary to determine if this treatment is appropriate on different stone types.
CaLoSil®E25 has been found to compare favourably with metal alkoxide treatments (Natali et al., 2015) but to gain even more benefit, titanium oxide can be added to the nanolime at 7.4% wt/vol (Nuño et al., 2015). TiO2 is insoluble in water so requires sonication in an ultrasonic bath or using a probe in order to obtain a stable colloidal suspension. Titanium oxide has catalytic, photo-catalytic and biocidal properties, making it a self-cleaning agent (Ortega-Morales et al., 2018). It has been found to be more effective in preventing algae and lichen colonisation than conventional biocides in both laboratory and on-site studies (Ortega-Morales et al., 2018). The metal alkoxides also work on the principle of depositing calcium or magnesium carbonates (Natali et al., 2015) and are more eco-friendly than polymer coatings. Water and titanium oxide has even been found to work as a biocide and photocatalyst on its own in the lab but not in the field on porous, rough surfaces (Quagliarini et al., 2018). For the purposes of this project, TiO2 could be added to a third application of CaLoSil®E25, after a slight reduction in porosity has already been created, since nanolime does decrease capilliarity but not completely (Taglieri et al., 2017). Ahmad Al-Omary et al. (2018) found a reduction in porosity of 4.6% in archaeological stone. This will hopefully give a certain level of protection to the limestones but will not trap salts or completely prevent water egress. TiO2 causes a slight increase in water repellence, but not as much as siloxane polymers (Giancristofaro et al., 2014).
Diammonium Hydrogen Phosphate
A solution of diammonium hydrogen phosphate (DAP) and calcium hydroxide nanoparticles in ethanol and water can also be applied to limestone (Nazel et al., 2021). Hydroxyapatite (HAP) can be formed from the reaction between Ca2+ ions deriving either from partial dissolution of the stone and PO 4 3- ions coming from the aqueous solution of DAP. Treatment with DAP solution, however, has four drawbacks: (1) not only HAP, but also other metastable calcium phosphate phases are formed; (2) small unreacted phosphate fractions remain in the stone (Nazel et al., 2021); (3) it is not ideal for conservation ethics that this process dissolves part of the specimen; (4) over-strengthening of an outer layer could lead to damage because it has different properties to the bulk matrix and could cause detachment (Sassoni et al., 2016).
Shekofteh et al. (2019) compared nanolime and DAP treatments: spectrophotometry revealed a yellowish colour in the samples treated with DAP and during FE-SEM observations, some diffused microcracks were detected on the surface. When subjected to accelerated aging tests, both nanolime and DAP treatments delayed the formation of microcracks during freeze-thaw tests, and both showed excellent stability during salt crystallization cycles (Shekofteh et al., 2019). This makes nanolime more compatible with darker coloured limestone and previous research found that lime has a low propensity for biological growth, supporting its use as a consolidating product for the stone used in open-air archeological sites that are prone to microbial growth (Shekofteh et al., 2019). Both DAP treatment (Sassoni et al., 2014) and nanolime (Ahmad Al-Omary et al., 2018) improve mechanical strength of limestone but DAP treatment is vulnerable to the presence of salts (possible in the specimens chosen for the garden project), forming soluble calcium phosphates instead of HAP (Praticὸ et al., 2019). In other cases, however, DAP has been found to be more effective than nanolime in resisting salt weathering (Shekofteh et al., 2019). Treatment conditions, solvent, stone composition, porosity and pore distribution may affect individual results.
The two treatments selected for trial on the limestone specimens were therefore bacterial carbonatogenesis and nanolime with TiO2.
Treatment for Silicified Trees
Chemical affinity between consolidant and substrate is necessary to ensure that conservation treatments are successful (Praticὸ et al., 2019). Treatments which are suitable for limestones are therefore not suitable for silicates due to their differing chemistry. There is more universal consensus on the best treatments for outdoor silicates than for limestone: ethyl silicates are widely recommended (Ferron and Matero, 2011; Moropoulou et al., 2003; Wheeler, 2005; Young et al., 2007) and especially for fossil wood (Lόpez-Polín, personal commun., 2022). Of these, Wacker SILRES BS OH100 has been chosen because it is not a water repellent (Mustoe et al., 2022) (so will not create a locked layer) and is commercially available in small quantities.
METHODS
Samples
Samples (100 mm x 50 mm x 20 mm) were taken from the matrix of the different limestone-based specimens selected for the garden refurbishment project: Ammonite and oyster block, Stair Hole Member; Ammonites, Portland Stone Formation; Ornithopod footprints, Stair Hole Member; Theropod footprints, Stair Hole Member. Two samples of each rock type were used for each experiment.
Bacterial Carbonatogenesis Treatment
Samples were sprayed with alkaline nutritional solution M-3P twice daily for five days to enable biomineralization by local calcium-depositing bacteria (1% wt/vol Bacto Casitone, 1% wt/vol calcium acetate hydrate Ca(CH3 COO)2 ⋅4H2O, 0.2% wt/vol potassium carbonate-1.5-hydrate K2 CO3 ⋅1/2H2 O in a 10 mM phosphate buffer, pH 8) at a volume of 1-1.5 ml/cm2 in distilled water (Jroundi et al., 2010; Rodriguez-Navarro et al., 2015). Samples were kept covered from the sun with foil (not touching the specimen) during treatment and for three further days to prevent rapid drying.
For this project more pH buffer was required than in the recommended recipe, because when first mixed the solution was at 6.5-7 pH (presumably because the deionised water was at 5.5 pH). Different samples were left outside in the gardens within the chalk downland area (an established ecosystem with imported soil) and the grassed area with standard London soil, other samples were positioned inside the laboratory, some with chalk downland soil and others with local London soil (in case soil bacteria played a contributing role). The maximum daytime temperature for the outdoor samples was 18°C. These were also plagued by slugs, foxes and flies, attracted by the nutrient solution. After treatment the samples were left for a further one week in the laboratory then gently rinsed with local rainwater (pH 5.5) before air-drying.
Nanolime Treatments
Samples were consolidated in a laboratory environment with three surface saturation treatments of CaLoSil®E25 spray: (1) at 5x dilution (recommended by IBZ-Schalzchémie (2020)); (2) at standard dilution; and (3) standard dilution with titanium oxide additive 7.4% wt/vol (which was added to the solution just before application and sonicated for 20 seconds); 24 hrs were left between spray treatments. Samples were then briefly rinsed in pH 5.5 local rainwater. This treatment was repeated on different samples but with no TiO2 and with only 0.5% wt/vol TiO2.
Scanning Electron Microscopy
Samples were placed on SEM stubs and coated with gold-palladium (80/20) with a Cressington 208 HR sputter coater. Scanning electron microscopy was used to observe deposition of new cement at up to x10000 magnification using a Zeiss UltraPlus FEGSEM.
Tape Test
Tape peels can be used to evaluate the cohesivity of a stone surface and are commonly used in building conservation. The sticky surface of the tape removes any loose material from the surface, the tape is then weighed and can be used to compare the effectiveness of different consolidants. Tape peel tests following the method of Drdácký et al. (2012) were undertaken on control samples and samples treated with CaLoSiL® E25 or the bacterial carbonatogenesis technique. The tape strips were weighed before and after application to the surface (90 seconds application, 90° removal angle at a constant 10 mm/second removal rate). Tests were repeated 10 times in same location. There are several other methods of assessing cohesion, but these were not available during this study.
Fossil Preparation
Fossil preparation was requested for the large block containing oysters and ammonites and the large ammonites (Figure 1) from Chicksgrove Quarry, Wiltshire. As display specimens the aim was to enhance their aesthetic appeal by removing excess matrix, enhancing relief and increasing the visibility of the basic morphology. The request included the caveat that the preparation work be as discrete as possible, keeping tool marks as unobtrusive as possible.
For the large ammonites, bulk matrix removal was carried out by mallet and chisel. Chisel marks were obscured where possible using a combination of a HW 70 Eurofossils air pen (or air scribe) and an Accuflo by Comco Inc air abrasive with sodium bicarbonate. It became apparent that where rock was removed, the sharp contrast between the bright white of the fresh broken surfaces and the weathered outer surfaces was too obvious. To try to artificially replicate the look of the weathered surface, very dilute (1%) acetic acid was applied to the prepared surfaces of one of the specimens by pipette, with successful results.
Due to the weight (1,500 kg) of the large block containing ammonites and oysters, the decision was made to move this piece to the garden and carry out the preparation work in situ to avoid moving the block multiple times. A portable compressor was taken outside, and the preparation carried out by air pen.
Repairs
CaLoSil(®) E25 is not recommended as an adhesive for exterior stone (Daehne and Herm, 2013) but polyvinyl butryal has proved effective (Young et al., 2007) and has the advantage of compatibility with nanolime if dissolved in ethanol. To maintain this compatibility, glass microballoons can be used as a filler in silicious specimens and marble dust for limestones. A selection of adhesives mixed with marble dust filler were applied to limestone samples and left outside for a 12-month period: Butvar B98, Paraloid B72, Paraloid B48N and Paraloid B44.
RESULTS AND DISCUSSION
Scanning Electron Microscopy
 The only sample to display new crystal growth resulting from the nutrient treatment was the ornithopod trackway sample, which had been treated within the laboratory in association with chalk downland soil. The crystals formed were smaller and more platey in habit than those observed by Rodriguez-Navarro et al. (2003, 2015). This result may be due to the different bioreceptivity of the different limestones (Ortega-Morales et al., 2018) and because the exterior temperatures were too low for effective colonisation over the short period.
The only sample to display new crystal growth resulting from the nutrient treatment was the ornithopod trackway sample, which had been treated within the laboratory in association with chalk downland soil. The crystals formed were smaller and more platey in habit than those observed by Rodriguez-Navarro et al. (2003, 2015). This result may be due to the different bioreceptivity of the different limestones (Ortega-Morales et al., 2018) and because the exterior temperatures were too low for effective colonisation over the short period.
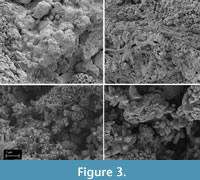
Following treatment with CaLoSiL® E25 the ornithopod trackway sample at x32 and x500 magnification showed cracks in valleys where the liquid pooled and dried. Observations of higher points at x500 and x10000 magnification revealed new crystal growth (Figure 3). The theropod trackway, Purbeck and ammonite and oyster block samples also showed new formation of calcium carbonate, again in different crystal habits. Liu et al. (2021) also found that the calcite precipitates as different crystal morphologies.
Tape Test
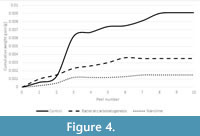 The results of the tape peel tests are shown in Figure 4. These were only undertaken on the ornithopod trackway samples since this was the only limestone type that showed new crystal growth following bacterial carbonatogenesis treatment. Both treatments created an increase in surface cohesion, but the nanolime was more effective than the nutrient solution.
The results of the tape peel tests are shown in Figure 4. These were only undertaken on the ornithopod trackway samples since this was the only limestone type that showed new crystal growth following bacterial carbonatogenesis treatment. Both treatments created an increase in surface cohesion, but the nanolime was more effective than the nutrient solution.
Colour Change with TiO2
Colour change with 7.4% TiO2 was higher than accepted in cultural heritage surface treatments, a phenomenon also observed by Frazoni et al. (2014). In addition, excess titania on the surface decreases contact angle of water droplets, greatly increasing the absorption rate of the stone, leading to increased frost damage and chemical attack (Frazoni et al., 2014). Ahmad Al-Omary et al. (2018) found that nanolime worked very well by itself, significantly improving mechanical properties and resistance to salt crystallisation damage but not reducing porosity. Within this study nanolime was found to create new crystal formation on all the limestone substrates and increase surface cohesion. A 0.5% level of TiO2 at the 3rd application of CaLoSiL® E25 is considered a viable option for the garden specimens and tests found no discernible colour change by eye.
Adhesives
After 12 months all adhesive joins were found to be intact, so all were considered of sufficient strength and durability. Butvar B98 was selected for use in repairs of the limestone trackways due to its compatibility with CaLoSiL® E25 and because it would not interfere with future treatments.
CONCLUSIONS
Bacterial carbonatogenesis has proved a very successful sustainable option for treatment of exterior stone by other researchers but was unsuccessful in this case, partly due to the colder climate and partly because different substrates have differing bioreceptivity. This implies that bacterial carbonatogensis should always be tested before wider application. The test samples had to be carefully wrapped between treatments since they attracted so much wildlife, and this could be problematic on a larger scale.
The chosen treatment for the limestone specimens is CaLoSiL® E25 with 0.5% TiO2 and for the silicates SILRES BS OH100. Minor repairs were made by adhesion with Butvar B98 with marble dust filler in limestones and glass microballoons for silicious material.
Nanolime treatments only penetrate about 5 mm depth depending on porosity (Ahmad Al-Omary et al., 2018) so these specimens will require annual checks and may require retreatment. If these treatments prove insufficient and deterioration does occur over time, less sustainable options may be considered such as treatment with with a nano-composite mixture (Akeoguard CO (Poly(vinylidene fluoride-co-hexafluoropropylene)) 5% in acetone and 0.5% ZnTiO3 nanoparticles (Ruffolo et al., 2010) or Rhodorsil H224 (alkyl poly-siloxane) 6% v/v in white spirit (Pelosi et al., 2020) with 0.5% ZnTiO3 nanoparticles.
ACKNOWLEDGEMENTS
Many thanks for the valuable advice and contributions of the reviewers and editorial team, and to P. Kenrick, Principal Researcher at the Natural History Museum in London UK.
REFERENCES
Ahmad Al-Omary, R., Al-Naddaf, M., and Al Sekhaneh, W. 2018. Laboratory evaluation of nanolime consolidation of limestone structures in the Roman site of Jerash, Jordan. Mediterranean Archaeology and Archaeometry, 18:35–43.
https://doi.org/10.5281/zenodo.1323865
Antonelli, F. and Tesser, E. 2018. Evaluation of silicone-based products used in the past as today for the consolidation of Venetian monumental stone surfaces. Mediterranean Archaeology and Archaeometry, 18:159–170. https://doi.org/10.5281/zenodo.1285902
Artesani, A., Di Turo, F., Zucchelli, M., and Traviglia, A. 2020. Recent advances in protective coatings for cultural heritage - an overview. Coatings, 10:217.
https://doi.org/10.3390/coatings10030217
Bernardi, A. 2015. Final report summary – NANOMATCH (Nano-systems for the conservation of immoveable and moveable polymaterial cultural heritage in a changing environment). Accessed 30 December 2021.
https://cordis.europa.eu/project/id/283182/reporting
Bernardi, A., Favaro, M., Nijland, T., García, O., Detalle, V., Wittstadt, K., Romero Sanchez, M.D., Pockelé, L., Kunday, B., Verhey, B., Brinkmann, U., de Micheli, G., Labouré, M., Möller, B., and Olteanu, I. D. 2012. Nanomatch: a European project to develop consolidants through the synthesis of new inorganic nanomaterials for the conservation of built heritage. International Journal of Heritage in the Digital Era, 1 (supplement 1):307–312.
https://doi.org/10.1260/2047-4970.1.0.307
Daehne, A. and Herm, C. 2013. Calcium hydroxide nanosols for the consolidation of porous building materials – results from EU-STONECORE. Heritage Science, 1:11.
https://doi.org/10.1186/2050-7445-1-11
Drdácký, M., Lesák, J., Rescic, S., Slízkova, Z., Tiano, P., and Valach, J. 2012. Standardization of peeling tests for assessing the cohesion and consolidation characteristics of historic stone surfaces. Materials and Structures, 45:505–520.
https://doi.org/10.1617/s11527-011-9778-x
Fassina, V., Benchiarin, S., and Molin, G. 2016. Laboratory and in situ evaluation of restoration treatments in two important monuments in Padua: “Loggia Cornaro” and “Stele of Minerva”. 13th International Congress on the Deterioration and Conservation of Stone: Case Studies. The International Institute for Conservation of Historic and Artistic Works, London, 1111–1118.
Favaro, M., Mendichi, R., Ossola, F., Russo, U., Simon, S., Tomasin, P., and Vigato, P. A. 2006. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part 1: photo-oxidative weathering. Polymer Degradation and Stability, 91:3083–3096.
https://doi.org/10.1016/j.polymdegradstab.2006.08.012
Ferreira Pinto, A.P. and Delgado Rodrigues, J. 2008. Stone consolidation: the role of treatment procedures. Journal of Cultural Heritage, 9:38–53.
https://doi.org/10.1016/j.culher.2007.06.004
Ferron, A. and Matero, F. G. 2011. A comparative study of ethyl-silicate-based consolidants on earthen finishes. Journal of the American Institute of Conservation, 50:49–72.
https://doi.org/10.1179/019713611804488964
Franzoni, E., Fregni, A., Gabrielli, R., Graziani, G., and Sassoni, E. 2014. Compatibility of photocatalytic TiO2 based finishing for renders in architectural restoration: a preliminary study. Building and Environment, 80:125–135.
https://doi.org/10.1016/j.buildenv.2014.05.027
Giancristofaro, C., D’Amato, R., Caneve, L., Pilloni, L., Rinaldi, A., and Persia, F. 2014. Performance of nanocomposites for preservation of artistic stones. AIP Conference Proceedings, 1603:86.
https://doi.org/10.1063/1.4883046
Hansen, E., Doehne, E., Fidler, J., Larson, J., Martin, B., Matteini, M., Rodriguez-Navarro, C., Pardo, E.S., de Tagle, A., Teutonico, J.M., and Weiss, N. 2003. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Studies in Conservation, 48 (supplement 1):13–25.
https://doi.org/10.1179/sic.2003.48.Supplement-1.13
IBZ-Schalzchémie. 2020. CaLoSil® conservation of stone, plaster and mortar. Accessed 30 December 2021.
http://www.hirst-conservation.com/site/wp-content/uploads/2020/04/CaLoSiL-application-recommendations_IBZ.pdf
Jroundi, F., Fernández-Vivas, A., Rodriguez-Navarro, C., Bedmar, E.J., and González-Muñoz, M.T. 2010. Bioconservation of deteriorated monumental calcarenite stone and identification of bacteria with carbonatogenic activity. Microbial Ecology, 60:39–54.
https://doi.org/10.1007/s00248-010-9665-y
Kallaf, M.K., El-Midany, A.A., and El-Mofty, S.E. 2011. Influence of acrylic coatings on the interfacial, physical and mechanical properties of stone-based monuments. Progress in Organic Coatings, 72:592–598. https://doi.org/10.1016/j.porgcoat.2011.06.021
Liu, X., Zarfel, G., van der Weijden, R., Loiskandl, W., Bitschnau, B., Dinkla, I., Fuchs, E. C., and Paulitsch-Fuchs, A.H. 2021. Density-dependent microbial calcium carbonate precipitation by drinking water bacteria via amino acid metabolism and biosorption. Water Research, 202:117444.
https://doi.org/10.1016/j.watres.2021.117444
Maravelaki-Kalaitzaki, P., Kallithrakas-Kontos, N., Agioutantis, Z., Maurigiannakis, S., and Korakaki, D. 2008. A comparative study of porous limestones treated with silicon-based strengthening agents. Progress in Organic Coatings, 62:49–60.
https://doi.org/10.1016/j.porgcoat.2007.09.020
Moropoulou, A., Haralampopoulos, G., Tsiourva Th., Auger F., and Birginie J.M. 2003 Artificial weathering and non-destructive tests for the performance evaluation of consolidation materials applied on porous stones. Materials and Structures, 36:201–217.
https://doi.org/10.1007/BF02479613
Mustoe, G.E., Aranyanark, C., and Boonchai, N. 2022. A new look at Cenozoic fossil wood from Thailand. Geosciences, 12:291.
https://doi.org/10.3390/geosciences12080291
Natali, I., Tomasin, P., Becherini, F., Bernardi, A., Ciantelli, C., Favaro, M., Favoni, O., Forrat Pérez, V.J., Olteanu, I.D., Romero Sanchez, M.D., Vivarelli, A., and Bonazza, A. 2015. Innovative consolidating products for stone materials: field exposure tests as a valid approach for assessing durability. Heritage Science, 3:6.
https://doi.org/10.1186/s40494-015-0036-3
Nazel, T., Abd El-Latif, M.L., Abuel-ela, R., and Abo El-Magd, M. 2021. Experimental comparative study on the performance of nanolime and phosphate-based treatment as consolidants for monumental limestone. The Journal of Scientific and Engineering Research, 8(3):40–49.
Nuño, M., Pesce, G.L., Bowen, C.R., and Ball, R.J. 2015. Environmental performance of nano-structured Ca(OH)2/TiO2 photocatalytic coatings for buildings. Building and Environment, 92:734–742.
https://doi.org/10.1016/j.buildenv.2015.05.028
Ortega-Morales, B.O., Reyes-Estebanez, M.M., Gaylarde, C.C., Camacho-Chab, J.C., Sanmartin, P., Chan-Bacab, M.J., Granados-Echegoyen, C.A., and Pereañez-Sacarias, J.E. 2018. Antimicrobial properties of nanomaterials use to control microbial colonisation of stone substrata, p. 277–298. In Hosseini, M. and Karapanagiotis, I. (eds.), Advanced Materials for the Conservation of Stone. Springer International Publishing AG, Cham.
Pelosi, C., Lanteri, L., Agresti, G., Rubino, G., Persia, F., Bonifazi, G., Serranti, S., and Capobianco, G. 2020. Experimental tests for evaluating the stability of a new nano-silica based protective for sperone stone in comparison to traditional products. Nanoinnovation, AIP Conference Proceedings, 2257:020012.
https://doi.org/10.1063/5.0023721
Praticὸ, Y., Caruso, F., Delgado Rodrigues, J., Girardet, F., Sassoni, E., Scherer, G.W., Vergès-Belmin, V., Weiss, N.R., Wheeler, G., and Flatt, R. J. 2019. Stone consolidation: a critical discussion of theoretical insights and field practice. RILEM Technical Letters, 4:145–153.
https://doi.org/10.21809/rilemtechlett.2019.101
Quagliarini, E., Graziani, L., Diso, D., Licciulli, A., and D’Orazio, M. 2018. Is nano-TiO2 alone an effective strategy for the maintenance of stones in cultural heritage? Journal of Cultural Heritage, 30:81–91. https://doi.org/10.1016/j.culher.2017.09.016
Rodriguez-Navarro, C., Jroundi, F., and Gonzalez-Muñoz, M.T. 2015. Stone consolidation by bacterial carbonatogenesis: evaluation of in situ applications. Restoration of Buildings and Monuments, 21:9–20.
https://doi.org/10.1515/rbm-2015-0002
Rodriguez-Navarro, C., Rodriguez-Gallego, M., Chekroun, K.B., and Gonzalez-Munoz, M.T. 2003. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Applied and Environmental Microbiology, 69:2182–2193.
https://doi.org/10.1128/aem.69.4.2182-2193.2003
Ruffolo, S.A., la Russa, M.F., Malagodi, M., Rossi, C.O., Palermo, A.M., and Crisci, G.M. 2010. ZnO and ZnTiO3 nanopowders for antimicrobial stone coating. Applied Physics A: Materials Science and Processing, 100:829–834.
https://doi.org/10.1007/s00339-010-5658-4
Sassoni, E., Franzoni, G., Graziani, G., and Sagripanti, F. 2014. Limestone resistance to sodium sulfate degradation after consolidation by hydroxyapatite and TEOS. 3rd International Conference on Salt Weathering of Buildings and Stone Sculptures, 14-16 October 2014, 335–343.
Sassoni, E., Graziani, G., and Franzoni, E. 2016. An innovative phosphate-based consolidant for limestone. Part 1: effectiveness and compatibility in comparison with ethyl silicate. Construction and Building Materials, 102(P1):918–930.
https://doi.org/10.1016/j.conbuildmat.2015.04.026
Shekofteh, A., Molina, E., Rueda-Quero, L., Arizzi, A., and Cultrone, G. 2019. The efficiency of nanolime and dibasic ammonium phosphate in the consolidation of beige limestone from the Pasargadae World Heritage Site. Archaeological and Anthropological Sciences, 11:5065–5080.
https://doi.org/10.1007/s12520-019-00863-y
Taglieri, G., Daniele, V., Rosatelli, G., Sfarra, S., Mascolo, M.C., and Mondelli, C. 2017. Eco-compatible protective treatments on an Italian historic mortar (XIV century). Journal of Cultural Heritage, 25:135–141.
https://doi.org/10.1016/j.culher.2016.12.008
Tesser, E., Antonelli, F., Sperni, L., Ganzerla, R., and Maravelaki, N. 2014. Study of the stability of siloxane stone strengthening agents. Polymer Degradation and Stability, 110:232–240.
https://doi.org/10.1016/j.polymdegradstab.2014.08.022
Tesser, E., Lazzarini, L., and Bracci, S. 2018. Investigation on the chemical structure and ageing transformations of the cycloaliphatic epoxy resin EP2101 used as a stone consolidant. Journal of Cultural Heritage, 31:72–82.
https://doi.org/10.1016/j.culher.2017.11.002
Varas-Muriel, M.J., Pérez-Monserrat, E.M., Vázquez-Calvo, C., and Fort, R. 2015. Effect of conservation treatments on heritage stone: characterisation of decay processes in a case study. Construction and Building Materials, 95:611–622.
https://doi.org/10.1016/j.conbuildmat.2015.07.087
Wheeler, G. 2005. Alkoxysilanes and the Consolidation of Stone. Los Angeles, Getty Publications.
Young, J.L., Meyer, H.W., and Mustoe, G. 2007. Conservation of an Eocene petrified forest at Florissant Fossil Beds National Monument: investigation of strategies and techniques for stabilizing in situ fossil stumps. The Geological Society of America, Special Paper, 435:141–157.

