 Phylogeny of iguanodontian dinosaurs and the evolution of quadrupedality
Phylogeny of iguanodontian dinosaurs and the evolution of quadrupedality
Article number: 25.3.a30
https://doi.org/10.26879/702
Copyright Society for Vertebrate Paleontology, November 2022
Author biography
Plain-language and multi-lingual abstracts
PDF version
http://morphobank.org/permalink/?P4581
Submission: 18 July 2016. Acceptance: 25 October 2022.
ABSTRACT
Iguanodontians are a large and biogeographically widespread group of dinosaurs, known from every modern continent, with a temporal range from the Late Jurassic through the Late Cretaceous. While the nested hadrosauroids have been studied extensively, the phylogeny of non-hadrosauroid iguanodontians remains less clear. This study presents a character matrix with 323 characters, and both parsimony and time-calibrated Bayesian analyses. While these result in different topologies, they both recover a Thescelosauridae outside of Iguanodontia. Within Iguanodontia, they both recover Muttaburrasaurus and Tenontosaurus as sister taxa to Rhabdodontidae, with a larger group of Gondwanan Rhabdodontoidea (nomen cladi novum) in the Bayesian analysis. A small Dryosauridae forms the sister group to Ankylopollexia, which has Uteodon and Camptosaurus as the most basally branching taxa. Within Styracosterna two distinct clades are recovered: Iguanodontidae, and a group of taxa with robust forelimbs. The holotype of Mantellisaurus is sister to “Dollodon”, supporting the hypothesis that these taxa are synonymous.
The “hatchet-shaped” sternal thought to be a synapomorphy of Styracosterna occurs in two taxa recovered outside that group: Macrogryphosaurus and the unnamed taxon from the Kirkwood Formation of South Africa. Characters associated with quadrupedality are mapped on the phylogeny, indicating a transition from bipedality to quadrupedality occurred in a stepwise manner at the base of Ankylopollexia. Based on synapomorphies of the groups, the major innovations in Ankylopollexia were postcranial, while those of hadrosauroids were centered on dentition and the dentaries. There is a clear faunal succession from the Late Jurassic to Early Cretaceous non-hadrosauroid ankylopollexians to the Late Cretaceous hadrosauroids.
Karen Poole. Department of Basic Sciences, New York Institute of Technology College of Osteopathic Medicine at Arkansas State University, Wilson Hall, 2405 Aggie Rd., Jonesboro, Arkansas 72401, USA. kpoole@nyit.edu
Keywords: Iguanodontia; Ornithopoda; Ornithischia; phylogeny; parsimony; Bayesian
Final citation: Poole, Karen E. 2022. Phylogeny of iguanodontian dinosaurs and the evolution of quadrupedality. Palaeontologia Electronica, 25(3):a30. https://doi.org/10.26879/702
palaeo-electronica.org/content/2022/3707-iguanodontian-phylogeny
Copyright: November 2022 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
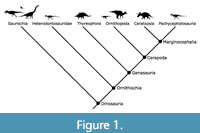 Iguanodontians are a clade of herbivorous dinosaurs within the diverse Ornithischia; more specifically, they are derived ornithopods (Figure 1). The first genus named in the group was Iguanodon Mantell 1825, which long served as a wastebasket taxon; as such, the genus and the clades to which it belongs have a complex taxonomic history. Currently, the generally accepted phylogeny has Iguanodontia nested within Ornithopoda and Hadrosauroidea nested within Iguanodontia. This phylogeny creates paraphyletic groupings of non-hadrosaurid iguanodontians (hereafter iguanodontians) and non-iguanodontian ornithopods. There are several major changes in morphology between the more basal taxa and hadrosaurids that make this group ideal for studying character evolution. The premaxillary teeth are lost while maxillary and dentary teeth become more numerous, higher crowned, and closely packed into dental batteries. Additionally, there is a general trend toward increased size in iguanodontians, which shift from bipedality to quadrupedality. The most notable morphological change associated with this is seen in the carpal elements of iguanodontians, which become massive and fused together, while those of hadrosauroids are diminutive or lost entirely.
Iguanodontians are a clade of herbivorous dinosaurs within the diverse Ornithischia; more specifically, they are derived ornithopods (Figure 1). The first genus named in the group was Iguanodon Mantell 1825, which long served as a wastebasket taxon; as such, the genus and the clades to which it belongs have a complex taxonomic history. Currently, the generally accepted phylogeny has Iguanodontia nested within Ornithopoda and Hadrosauroidea nested within Iguanodontia. This phylogeny creates paraphyletic groupings of non-hadrosaurid iguanodontians (hereafter iguanodontians) and non-iguanodontian ornithopods. There are several major changes in morphology between the more basal taxa and hadrosaurids that make this group ideal for studying character evolution. The premaxillary teeth are lost while maxillary and dentary teeth become more numerous, higher crowned, and closely packed into dental batteries. Additionally, there is a general trend toward increased size in iguanodontians, which shift from bipedality to quadrupedality. The most notable morphological change associated with this is seen in the carpal elements of iguanodontians, which become massive and fused together, while those of hadrosauroids are diminutive or lost entirely.
Iguanodontians are known from all modern continents and have a temporal span of roughly 100 million years from the Late Jurassic through the end of the Cretaceous Period. During this time, the supercontinent of Pangaea was rifting into smaller landmasses; by the end of the Cretaceous, these were recognizable as the modern continents.
This study reviews the taxonomic history of Iguanodontia and uses parsimony and time-calibrated Bayesian methods to produce phylogenetic hypotheses and map the geographic distribution of taxa.
Taxonomic History
Early work. The term Iguanodontia is often attributed to Dollo (1888), but Norman (2015) observed that Dollo never used this term. In fact, he offered new definitions of Iguanodontidae and Camptonotidae.
The name Iguanodontia was first used by Baur in 1891 and by his definition included the families Iguanodontidae, Hypsilophodontidae, Hadrosauridae, Scelidosauridae, Stegosauridae, Agathaumidae, and possibly Ornithomimidae. This grouping (excluding the saurischian clade Ornithomimidae) is equivalent to Ornithischia (Seeley, 1887), though Baur gave no explanation for why the latter name would not suffice. Following Seeley, he did not consider Dinosauria to be a natural group and intended the name Iguanodontia to be an order or suborder, at an equivalent level to Crocodylia. The term Iguanodontia appeared in the literature through the early 20th century (e.g., Lull, 1908), though Williston (1905) drew a distinction between iguanodontians and other “predentate” dinosaurs such as Stegosaurus and Scelidosaurus, and Osborn (1906) distinguished between iguanodontians and ceratopsians. Already, the term had a more restricted meaning than Baur’s original definition. In 1911, Lull described three groups belonging to the Ornithopoda: Iguanodontia, Ceratopsia, and Stegosauria. He equated the name Ornithopoda with Predentata (Marsh, 1896). Lull (1911) defined iguanodontians as those ornithopods that were unarmored and without horns.
The family-level name Iguanodontidae was first used by Gervais (1853), though no definition of the group was given. Huxley (1870) described Iguanodontidae as one of three groups within Dinosauria, the others being Scelidosauridae and Megalosauridae. He assigned to this family Cetiosaurus, Iguanodon, Hypsilophodon, Hadrosaurus, and possibly Stenopelix. This grouping is more equivalent to later conceptions of Ornithopoda, with the obvious exception of the sauropod Cetiosaurus.
Through the middle of the twentieth century, the term Iguanodontia fell out of use, while Iguanodontidae remained a commonly used family name. The more ambiguous ‘iguanodonts’ was also commonly used (e.g., Gilmore, 1909; Osborn, 1912). Romer’s (1956) classification included Iguanodontidae as a family within suborder Ornithopoda including the genera Iguanodon, Camptosaurus, and Rhabdodon (though excluding Dryosaurus and Dysalotosaurus, which he placed in Hypsilophodontidae).
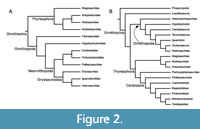 The Cladistic Era. With the advent of cladistics, nomenclature changed rapidly. In 1984, Norman and Sereno each presented a phylogeny of Ornithischia at the Third Symposium on Mesozoic Terrestrial Ecosystems (Figure 2). Norman’s phylogeny used only families as operational taxonomic units (OTUs), and found Iguanodontidae as sister to Hadrosauridae, with Dryosauridae just outside this node. He recovered Hypsilophodontidae outside of a clade containing Dryosauridae, Iguanodontidae, Hadrosauridae, and Ceratopsia. While this hypothesis was not supported for many years, it is interesting to note that some recent analyses have found at least some taxa considered to be “basal ornithopods” outside of Cerapoda (Butler et al., 2008; Boyd, 2012, 2015; Spencer, 2013). Sereno (1984) used a mix of family and genus level OTUs, and found Hadrosauridae to be nested within ‘iguanodonts’, thus making the traditional grouping of Iguanodontidae paraphyletic. Sereno (1986) renewed the term Iguanodontia for this larger clade, while also naming the less inclusive clades Dryomorpha, Ankylopollexia, and Styracosterna (see Table 1). He diagnosed Iguanodontia based on the following characters: “the absence of premaxillary teeth, the presence of leaf-shaped denticles in the cheek teeth, and the loss of one phalanx from manus digit III”. In his phylogeny, Iguanodontia included Tenontosaurus, Dryosaurus, Camptosaurus, Probactrosaurus, Iguanodon, and Ouranosaurus, as well as Hadrosauridae. His analysis recovered Hypsilophodontidae as a monophyletic group, sister to Iguanodontia, which together comprise Euornithopoda. The most basal ornithopods in the analysis are the Heterodontosauridae. The subsequent phylogenetic analyses of Forster (1990), Weishampel and Heinrich (1992), and Sereno (1998) found similar results.
The Cladistic Era. With the advent of cladistics, nomenclature changed rapidly. In 1984, Norman and Sereno each presented a phylogeny of Ornithischia at the Third Symposium on Mesozoic Terrestrial Ecosystems (Figure 2). Norman’s phylogeny used only families as operational taxonomic units (OTUs), and found Iguanodontidae as sister to Hadrosauridae, with Dryosauridae just outside this node. He recovered Hypsilophodontidae outside of a clade containing Dryosauridae, Iguanodontidae, Hadrosauridae, and Ceratopsia. While this hypothesis was not supported for many years, it is interesting to note that some recent analyses have found at least some taxa considered to be “basal ornithopods” outside of Cerapoda (Butler et al., 2008; Boyd, 2012, 2015; Spencer, 2013). Sereno (1984) used a mix of family and genus level OTUs, and found Hadrosauridae to be nested within ‘iguanodonts’, thus making the traditional grouping of Iguanodontidae paraphyletic. Sereno (1986) renewed the term Iguanodontia for this larger clade, while also naming the less inclusive clades Dryomorpha, Ankylopollexia, and Styracosterna (see Table 1). He diagnosed Iguanodontia based on the following characters: “the absence of premaxillary teeth, the presence of leaf-shaped denticles in the cheek teeth, and the loss of one phalanx from manus digit III”. In his phylogeny, Iguanodontia included Tenontosaurus, Dryosaurus, Camptosaurus, Probactrosaurus, Iguanodon, and Ouranosaurus, as well as Hadrosauridae. His analysis recovered Hypsilophodontidae as a monophyletic group, sister to Iguanodontia, which together comprise Euornithopoda. The most basal ornithopods in the analysis are the Heterodontosauridae. The subsequent phylogenetic analyses of Forster (1990), Weishampel and Heinrich (1992), and Sereno (1998) found similar results.
Sereno (1998) defined Iguanodontia based on phylogenetic relationships rather than a suite of characters as all euornithopods closer to Parasaurolophus than to Hypsilophodon. He defined Ornithopoda as the least inclusive clade containing Heterodontosaurus and Parasaurolophus, but excluding Pachycephalosaurus, Triceratops, and Ankylosaurus. He defined the clade Euornithopoda as further excluding Heterodontosaurus (Sereno, 1999, 2005).
Subsequent studies at the genus level failed to recover Hypsilophodontidae as a monophyletic group (Scheetz, 1999; Winkler et al., 1997; Weishampel et al., 2003). Treating these genera as separate OTUs recovered largely pectinate topologies with ‘hypsilophodonts’ as a paraphyletic group with respect to Iguanodontia. Due to inconsistent relationships among hypsilophodontids, Sereno (2005) emended the definition of Iguanodontia to the most inclusive clade containing Parasaurolophus walkeri but not Hypsilophodon foxii or Thescelosaurus neglectus.
In a paper describing the new genus of Patagonian dinosaur Gasparinisaura, Coria and Salgado (1996) named the clade Euiguanodontia, and defined it as Gasparinisaura and Dryomorpha, excluding Tenontosaurus and hypsilophodontids. However, subsequent analyses (e.g., Weishampel et al., 2003; Butler et al., 2008; this study) find Gasparinisaura arising from a more inclusive node than Tenontosaurus. In this topology, Euiguanodontia is nonsensical, and the name has not been adopted.
In the second edition of The Dinosauria, phylogenies of basal ornithopods and basal iguanodontians were analyzed separately, thus little can be concluded about higher-level relationships (Norman, 2004; Norman et al., 2004). However, phylogenetic definitions were given for these groups: Ornithopoda was defined as a stem-based taxon composed of all cerapodans closer to Edmontosaurus than to Triceratops—this included Heterodontosaurus (Norman et al., 2004). Iguanodontia was defined as all euornithopods closer to Edmontosaurus than to Thescelosaurus (Norman, 2004).
The explosion of iguanodontian taxa. The number of iguanodontian genera increased quickly beginning in the 1990s, as new fossils were described for the first time (Rich and Rich, 1989; Coria and Salgado, 1996; Head, 1998; Kirkland, 1998; Taquet and Russell, 1999; DiCroce and Carpenter, 2001; Wang and Xu, 2001; Coria and Calvo, 2002; Kobayashi and Azuma, 2003; You et al., 2003; Novas et al., 2004; You et al., 2005; Gilpin et al., 2006; Calvo et al., 2007; Sues and Averianov, 2009; Dalla Vecchia 2010; McDonald et al., 2010b; McDonald et al., 2010c; Wu et al., 2010; You et al., 2011; Godefroit et al., 2012; McDonald et al., 2012; Coria et al., 2013), and previously known specimens were reanalyzed (Weishampel et al., 2003; Paul, 2007; Paul, 2008; Carpenter and Ishida, 2010; Norman 2010; McDonald et al., 2010a; McDonald, 2011; Paul, 2012). There remains disagreement about some of these taxon assignments; this study predominantly follows the taxonomy laid out by Norman (2013).
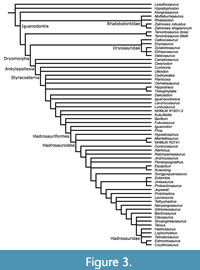 McDonald et al. (2010c) and McDonald (2012a) made the first attempts at analyzing the phylogenetic relationships of the plethora of new taxa, including as many as 66 ornithopod genera in the analyses (Figure 3). However, these studies had a small number of characters (135) relative to the number of taxa, and the consensus tree was resolved poorly, with a largely pectinate topology.
McDonald et al. (2010c) and McDonald (2012a) made the first attempts at analyzing the phylogenetic relationships of the plethora of new taxa, including as many as 66 ornithopod genera in the analyses (Figure 3). However, these studies had a small number of characters (135) relative to the number of taxa, and the consensus tree was resolved poorly, with a largely pectinate topology.
Norman (2015) restricted his analysis to well-preserved taxa (27) and used just over a hundred characters (105). As in McDonald’s analyses, Norman included Hypsilophodon as the only representative basal ornithopod, although he also included the basal ornithischian Lesothosaurus, 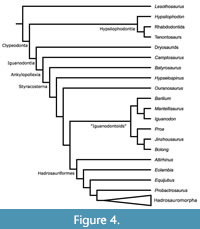 on which the tree was rooted (Figure 4). His analysis recovered a monophyletic group of ‘iguanodontoids’, including, in addition to Iguanodon, the recently discovered Proa, Jinzhousaurus, and Bolong, as well as genera created recently for species formerly assigned to Iguanodon: Barilium dawsoni and Mantellisaurus atherfieldensis. He assigned the name Clypeodonta to “Hypsilophodon foxii, Edmontosaurus regalis, their most recent common ancestor, and all of its descendants.” However, Hypsilophodon was the only basal ornithopod present in this analysis, so it is unclear how Clypeodonta relates to Ornithopoda sensu Norman et al. (2004).
on which the tree was rooted (Figure 4). His analysis recovered a monophyletic group of ‘iguanodontoids’, including, in addition to Iguanodon, the recently discovered Proa, Jinzhousaurus, and Bolong, as well as genera created recently for species formerly assigned to Iguanodon: Barilium dawsoni and Mantellisaurus atherfieldensis. He assigned the name Clypeodonta to “Hypsilophodon foxii, Edmontosaurus regalis, their most recent common ancestor, and all of its descendants.” However, Hypsilophodon was the only basal ornithopod present in this analysis, so it is unclear how Clypeodonta relates to Ornithopoda sensu Norman et al. (2004).
The work of Butler et al. (2008) examined the phylogeny of Ornithischia. It did not include many iguanodontians; however, it informed the selection of the more basal taxa included in the current analysis. Butler et al. did not offer a definition of Iguanodontia, but defined ornithopods as “All genasaurians more closely related to Parasaurolophus walkeri Parks, 1922, than to Triceratops horridus Marsh, 1889”, closely following the definition of Norman et al. (2004) rather than the definition given by Sereno (1998). Most importantly, this analysis found Heterodontosauridae as a basally branching member of Ornithischia, making Sereno’s definition of Ornithopoda (1998) nearly equivalent to Ornithischia (though it is node-based, rather than stem-based). They also recovered several genera previously considered to be basally branching ornithopods (e.g., Norman et al., 2004) outside of Cerapoda, as basally branching neornithischians. These include Agilisaurus, Hexinlusaurus, and Othnielia.
Although it does not have precedence, the stem-based definition of Ornithopoda used by Butler et al. (2008) has already been used widely (Buchholz, 2002; Wagner, 2004; Norman et al., 2004). As there is a need for a term to describe the sister group of Marginocephalia within Cerapoda, and Ornithopoda is already recognized as such, this usage is adopted here, despite the lack of priority for the definition.
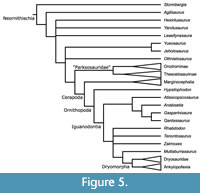 Finally, Boyd (2012, 2015) recovered many putative basally branching ornithopods outside of Cerapoda (Figure 5), leaving Hypsilophodon as the sole non-iguanodontian ornithopod. As there are no marginocephalians in the current analysis, it is impossible to say whether certain taxa are basally branching ornithopods or basally branching neornithischians. Therefore, the more inclusive term “basal neornithischian” is preferred here–a future analysis including marginocephalians will help to clarify this ambiguity.
Finally, Boyd (2012, 2015) recovered many putative basally branching ornithopods outside of Cerapoda (Figure 5), leaving Hypsilophodon as the sole non-iguanodontian ornithopod. As there are no marginocephalians in the current analysis, it is impossible to say whether certain taxa are basally branching ornithopods or basally branching neornithischians. Therefore, the more inclusive term “basal neornithischian” is preferred here–a future analysis including marginocephalians will help to clarify this ambiguity.
By using a larger character matrix than those of previous studies, and including many basal neornithischians as outgroups, this analysis seeks to test which taxa belong within Iguanodontia, and to define this and any subclades both phylogenetically and via character-based diagnoses.
Biogeography
As basal neornithischians were diversifying in the Early Jurassic, Pangaea was still contiguous, with the Tethys Ocean lying between Asia and Eastern Gondwana (Blakey, 2008). Gondwana and Laurasia were united via northern Africa. Rifting between Africa and North America began in the Triassic, but the formation of oceanic crust (and therefore continental separation) did not occur until the Early Jurassic, approximately 190-180 Ma (Bartolini and Larson, 2001; Veevers, 2004). Through the Cretaceous, the Laurasian continents maintained intermittent contact, although epeiric seas divided North America in two large landmasses, and Europe into a series of smaller landmasses (Esmerode et al., 2007; Miall et al., 2008; Scotese, 2014). Meanwhile, rifting continued in Gondwana, producing the southern continents we are familiar with today. Given these physical constraints, one would expect that endemism within neornithischians would increase from the Triassic through the Cretaceous, and any late appearing clade would have more restricted ranges.
Biogeography of iguanodontians has been analyzed quantitatively by several researchers (Milner and Norman, 1984; Upchurch et al. 2002; Ezcurra and Angolín, 2012; Boyd, 2015). Upchurch et al. (2002) used tree reconciliation analysis (TRA) to examine biogeographical patterns across Dinosauria. This approach was important in reconstructing overall patterns of vicariance through the Mesozoic. Ezcurra and Agnolin (2012) also used TRA across a wide range of taxa and found frequent links between European and Gondwanan taxa. Boyd (2015) used both parsimony and likelihood models to infer ancestral areas of nodes within Ornithischia, finding diversification of Neornithischia within Africa in the Early Jurassic, dispersing to Asia by the Late Jurassic. The ancestral area reconstruction for Iguanodontia is resolved more poorly, with different models finding this in Europe or in Asia, and differing dispersal within the group. This study employs model-based approaches such as Dispersal-Extinction-Cladogenesis (DEC) (Ree and Smith, 2008; Matzke 2012, 2013b) for ancestral area reconstruction (AAR).
Institutional Abbreviations
AM, Albany Museum, Grahamstown, South Africa; AMNH, American Museum of Natural History, New York City, New York, USA; IMM, Inner Mongolia Museum, Hohhot, China; MC, Museum Crúzy, Crúzy, France; MNHN, Museum National d’Histoire Naturelle, Paris, France; MPT, Museo Provincial de Teruel, Spain; MUCPv, Museo de Geologia y Paleontologia de la Universidad Nacional del Comahue, Neuquén, Argentina; NHMUK, Natural History Museum, London, United Kingdom; NMV, National Museum of Victoria, Melbourne, Australia; NRRU, Nakhon Ratchasima Rajabhat University, Thailand; PRC, Palaeontological Collection, Palaeontological Research and Education Centre, Mahasarakham University, Thailand; RBINS (formerly IRSNB), Royal Belgian Institute of Natural Sciences, Brussels, Belgium; SDSM, Museum of Geology, South Dakota School of Mines and Technology, Rapid City, South Dakota, USA; USNM, National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA; YPM, Yale Peabody Museum, New Haven, Connecticut, USA.
METHODS
Character Selection
A matrix of 323 characters was compiled, with 171 (53%) from the postcranial skeleton. The character list is given in Appendix 1, and the character matrix in Appendix 2. The matrix was constructed in Mesquite (version 2.72; Maddison and Maddison, 2009) and combines characters drawn from Norman (2002), Weishampel et al. (2003), Butler et al. (2008), Boyd et al. (2009), Prieto-Márquez and Salinas (2010), and McDonald (2012a).
After the characters and character states from all these studies were combined, duplicate characters were eliminated, and those that were uninformative to the analysis were deleted. Characters were reworded, where necessary, to follow a standardized format (Sereno, 2007). Characters that were inapplicable to some taxa were revised to use reductive coding (Strong and Lipscomb, 1999). This approach produced 184 characters, with 104 from the cranial and dental regions. Then 139 new characters were added (48 cranial, 91 postcranial), noted as “new” in Appendix 1. The measured values for states of all ratio-based characters were graphed, and states were determined based on gaps present in those values.
Taxon Selection
All putative genera of iguanodontians were considered in the analysis, including many non-iguanodontian neornithischians and a few basally branching ornithischians as outgroups. Of the 73 selected OTUs, specimens of 45 were examined directly (62%), while the others were coded from literature descriptions (Appendix 3). Taxa with large amounts of missing data were not excluded a priori (Kearney and Clark, 2003; Wiens and Moen, 2008; Wiens and Morrill, 2011). Some named taxa were excluded from the analysis a priori, either because they do not possess apomorphies or a unique suite of character states, or because they are junior synonyms of other taxa. These taxa and the rationale for their exclusion is provided in Appendix 4.
Naming conventions follow the rules and recommendations of the International Code of Phylogenetic Nomenclature, version 4c (Cantino and deQueiroz, 2010).
Phylogenetic Analysis
Both parsimony and time-calibrated Bayesian analyses were performed. Despite the potential for conflict between parsimony and model-based methods (Felsenstein, 1978; Farris, 1983; Swofford et al., 2001; Wright and Hillis, 2014), comparison of the resulting trees shows which areas of the topology are robust and supported by both methods, addressing concerns that Bayesian analyses with missing data will recover nodes with erroneously high support values (Simmons, 2012, 2014; Xu and Pol, 2013) by allowing comparison between results of the parsimony and Bayesian analyses. Matching nodes found under both methods indicate which areas of the tree are well supported by the data, while differences in topologies or relative support show which areas remain less certain.
The introduction of relaxed-clock Bayesian methods that create time-calibrated phylogenies is useful particularly within paleontology (Drummond et al., 2006; Pyron, 2011; Ronquist et al., 2012). While developed with combined data sets of extant and extinct taxa using molecular and morphological data, these methods have also been used for analysis of entirely extinct groups known only from morphological data (Gorscak and O’Connor, 2016).
Ultimately, both parsimony and Bayesian methods are useful, though the results must be interpreted differently. Parsimony provides the simplest representation of the data, with no added parameters. Clades in these analyses tend to be supported by a higher number of characters, but those characters are more labile (Lee and Worthy, 2012). It does not take temporal data into account and allows long ghost lineages.
Bayesian analyses allow us to model evolutionary processes more explicitly and to account for time as a factor in the phylogeny. This tends to disfavor long ghost lineages. It is also important to note that the Bayesian tree presented here is not a consensus, but a Maximum Clade Credibility tree: of the trees sampled, it is the single tree with the highest overall posterior probability.
Parsimony analysis. A parsimony analysis was conducted in TNT 1.1 (Goloboff et al., 2008). A New Technology Search was conducted using a driven search set to find the best score 500 times using default settings of sectorial searches, ratchet, drift, and tree fusing. Pruned consensus (reduced consensus of Wilkinson [2003]) was used to determine which taxa acted as wild cards for removal post hoc from the consensus tree, resulting in removal of six taxa: Oryctodromeus, Atlascopscosaurus, Planicoxa, Cumnoria, Cedrorestes, and NHMUK R28860 (“Kukufeldia”). After pruning, a strict consensus tree was calculated. Jackknife supports were calculated using 10,000 replicates in a traditional search. Initially, the default value of 0.33 was used for the chance of dropping a character from the matrix. As this resulted in overall low values, the analysis was redone using a 0.1 probability of dropping characters. This results in overall higher values but allows for a better assessment of the relative support of the nodes present in the tree. Bremer supports were calculated by performing a traditional search with 5,000 RAS and tree bisection reconnection (TBR), while keeping trees suboptimal up to seven steps. The trees were rooted on the basal ornithischian Eocursor (Butler et al., 2007).
To assess the impact of adding many new postcranial characters to the matrix, an additional parsimony analysis was run in which the 80 new postcranial characters added to this matrix were excluded.
Tip-dated Bayesian analysis. A time-calibrated analysis was carried out in BEAST 2.0 (Bouckaert et al., 2014) with an xml file created in BEASTmasteR (Matzke, 2014). Ages of tips were determined based on temporal data given in descriptions, or from studies dating relevant beds (Appendix 4). The entire possible age range, including error, was used in this analysis (O’Reilly et al., 2014). For taxa constrained to a particular stage or substage, numerical dates were assigned according to Gradstein et al. (2012). However, note that error estimates are not given for substage boundaries. Uniform priors were assigned to age ranges. Two tips known from well-dated localities were assigned fixed ages: Tenontosaurus dossi at 113 ma (Jacobs et al., 1991) and Protohadros at 95 ma (Kennedy and Cobban, 1990). The root of the tree was constrained with a lognormal prior with both mean and standard deviation equal to 1. This creates a 95% probability of the root occurring between 215 and 228 ma, while allowing the possibility of older dates.
A relaxed clock model was implemented in which branch lengths were uncorrelated to adjacent branches (Drummond et al., 2006; Rannala and Yang, 2007; Lepage, et al., 2007), and drawn from a lognormal distribution with mean=0.001 and standard deviation=1. Characters used an Mk ordered or Mk unordered model, as appropriate (Lewis, 2001).
A Birth-Death Skyline (BDSKY) model with serial sampling was used as a tree model (Stadler et al., 2013). This is an extension of a birth-death model that allows those rates to change across time bins. Bins were assigned by geologic stages: Late Triassic, Early, Middle, and Late Jurassic, and Early and Late Cretaceous. Broad priors were assigned to speciation rate (λ), extinction rate (μ), and sampling rate (ψ), with each allowed to vary between 0 and 10. The sampling rate is the rate at which a lineage is sampled and allows for taxa of different ages to be included in the analysis; this should not be confused with the sampling probability (ρ), which represents the probability of sampling a lineage within a particular time bin. Sampling probability was fixed at 0.1, as assigning broad priors to all tree model variables provides too little constraint to the model, which often fails to converge (Drummond and Bouckaert, 2015). Matzke (2014) suggested fixing ρ=1, but this would indicate perfect sampling. Given the incomplete nature of the fossil record, sampling probability is more likely (to an order of magnitude estimate) in the range of 1% to 10%. Analyses were conducted with ρ=0.1 and ρ=0.01 to determine the effect of different values of sampling probability. While, as expected, it altered the posterior distributions of ʎ, µ, and ψ, there was no effect on tree topology.
Both analyses were run for 50 million generations, saving trees every 10,000 generations, producing a total sample of 5,001 trees. Convergence was assessed based on Estimated Sample Size (ESS) of all posterior values: ESS>200 was considered the minimum threshold for convergence. In addition, the trace plots for variables were assessed visually to ensure that stationarity and sufficient mixing occurred. TreeAnnotator was used to find the Maximum Clade Credibility (MCC) tree, with a burn-in of 20%. Synapomorphies were identified on the MCC tree by importing the tree to the character matrix in Mesquite and mapping the characters.
Biogeographic Analysis
Ancestral areas were calculated for the consensus time-calibrated tree using the R package BioGeoBEARS (Matzke, 2013a), using DEC, DIVAlike, and BAYAREA models, without the J parameter allowing founder events. Given the wide geographic range of the taxa and the sparseness of the fossil record, including founder events in the model does not seem theoretically sound. While it does create a model better fitted to the data, this is more likely due to fossil lineages appearing suddenly in the record of an area due to changes in sedimentology regimes or access to outcrops.
Models were compared using the Akaike Information Criterion (AIC) to determine which model best fit the tree. A time stratified approach was used, in which switching areas was given equal weight through the Kimmeridgian, after which switching areas between Laurasian and Gondwanan areas became less likely by a factor of 10.
RESULTS
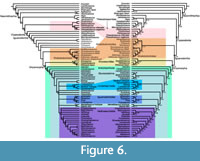 The topology recovered in the parsimony (Figure 6, Figure 7) and Bayesian analyses (Figure 6, Figure 8) is similar within Dryosauridae and Ankylopollexia. However, there are substantial differences in the basal section of the tree. In the parsimony tree, this region is pectinate with long ghost lineages, though thescelosaurids and rhabdodontoids form small clades (Figure 7). In the Bayesian topology, most of the non-ankylopollexian iguanodontians are included within a large Hypsilophodontidae (which includes Thescelosauridae) and rhabdodontoids (which includes many more genera than in the parsimony topology).
The topology recovered in the parsimony (Figure 6, Figure 7) and Bayesian analyses (Figure 6, Figure 8) is similar within Dryosauridae and Ankylopollexia. However, there are substantial differences in the basal section of the tree. In the parsimony tree, this region is pectinate with long ghost lineages, though thescelosaurids and rhabdodontoids form small clades (Figure 7). In the Bayesian topology, most of the non-ankylopollexian iguanodontians are included within a large Hypsilophodontidae (which includes Thescelosauridae) and rhabdodontoids (which includes many more genera than in the parsimony topology). 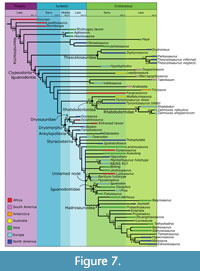 These topological differences occur because of differences in the models: the birth-death model implemented in the Bayesian analysis favors a bifurcating topology without multiple long branches, whereas parsimony analyses tend towards a pectinate topology when there is not a strong phylogenetic signal. While the recovered topologies of each method will be discussed below, further work is needed to resolve the relationships in this area of the tree.
These topological differences occur because of differences in the models: the birth-death model implemented in the Bayesian analysis favors a bifurcating topology without multiple long branches, whereas parsimony analyses tend towards a pectinate topology when there is not a strong phylogenetic signal. While the recovered topologies of each method will be discussed below, further work is needed to resolve the relationships in this area of the tree.
 There are several shared features of the two topologies: (1) Tenontosaurus and Muttaburrasaurus are sister taxa to Rhabdodontidae, forming a more inclusive Rhabdodontoidea, (2) Uteodon is the most basal ankylopollexian, (3) the small clade Iguanodontidae includes Iguanodon, Equijubus, Proa, and Fukuisaurus, and (4) there is clear stratigraphic separation between non-hadrosauroid ankylopollexians and hadrosauroids. Unambiguous synapomorphies for both analyses listed in Appendix 5.
There are several shared features of the two topologies: (1) Tenontosaurus and Muttaburrasaurus are sister taxa to Rhabdodontidae, forming a more inclusive Rhabdodontoidea, (2) Uteodon is the most basal ankylopollexian, (3) the small clade Iguanodontidae includes Iguanodon, Equijubus, Proa, and Fukuisaurus, and (4) there is clear stratigraphic separation between non-hadrosauroid ankylopollexians and hadrosauroids. Unambiguous synapomorphies for both analyses listed in Appendix 5.
Parsimony Analysis
Initial parsimony analysis yielded 2843 MPTs with a tree length of 1394 steps. After running “pruned trees” in TNT, six ingroup taxa were removed from the strict consensus: Oryctodromeus, Atlascopscosaurus, Planicoxa, Cumnoria, Cedrorestes, and NHMUK R28860 (“Kukufeldia”). Rerunning the analysis without these taxa yielded 114 MPTs; the strict consensus of these is shown in Figure 6.
The strict consensus of 114 MPTs is shown along with the Bayesian tree in Figure 6. A time scaled tree showing known temporal ranges of taxa is shown in Figure 7. Jackknife values at most nodes are low, but Bremer supports show some areas of the tree to be well supported. The phylogenetic definitions used here are summarized in Table 2.
The most notable features of this topology are the inclusion of Tenontosaurus and Muttaburrasaurus in a clade with Rhabdodontidae. Additionally, there is a clade of styracosternans that includes Barilium, Hypselospinus, and Lurdusaurus. This group is characterized by robust forearm elements, presence of a radial tuberosity for insertion of m. biceps brachii and enlarged pollex unguals (at least 25% of radial length). There is also a small clade that includes Iguanodon and is here referred to as Iguanodontidae, though this is a smaller, more closely related group of taxa than the historical conception of iguanodontids. While there is some overlap with the ‘iguanodontoids’ of Norman (2015), several taxa from that clade are recovered in other groups in this topology.
Also of note is that the holotype of Mantellisaurus atherfieldensis (NHMUK R5764) forms a clade with the holotype of “D. bampingi” (RBINS R57). This supports the contention that Dollodon bampingi is a junior subjective synonym of M. atherfieldensis, as discussed by McDonald (2012b) and Norman (2013, 2015). This Mantellisaurus clade is the sister to hadrosauroids.
It is also worth noting that based on previous definitions, Ornithopoda cannot be delineated easily on this phylogeny, as there are no marginocephalians included. Following the topology of Butler et al. (2008), Ornithopoda here would lie at the node basal to Thescelosauridae and Clypeodonta, but this is contradicted by the phylogeny of Boyd (2012), in which Thescelosauridae lies outside of Cerapoda (Ornithopoda + Marginocephalia). Further analysis with a wider sampling of basal ornithischians, and especially marginocephalians, is necessary to determine whether Thescelosauridae is included in Ornithopoda.
Bayesian Analysis
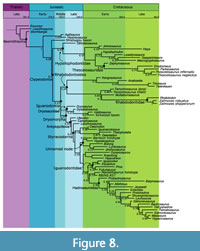
The MCC tree with posterior probabilities is shown along with the parsimony tree in Figure 6, and the time-calibrated tree is Figure 8. The value of ESS was well over 200 for most variables except the birth, death, and sampling rates for time bins 5 and 6 (Early Jurassic and Late Triassic). Given that only three taxa are present across both these bins, this is unsurprising. As determining birth and death rates near the root of the tree is not the goal of this study, and there are no differences in topology with the parsimony tree or Bayesian analyses without a skyline component, the smaller ESS for these parameters is not a cause for concern. The ESS and posterior distributions for key variables are given in Table 3.
Notable features of the topology of the Bayesian tree are a clade of hypsilophodontids that form the sister group to Iguanodontia. This hypsilophodontid clade includes thescelosaurids. Within Iguanodontia, there are two clades: Dryomorpha and Rhabdodontoidea, which includes Rhabdodontidae, Tenontosaurus, Muttaburrasaurus, Talenkauen, Trinisaura, Anabisetia, and Kangnasaurus. Within Dryomorpha, the topology of the Bayesian tree is similar to that of the parsimony tree, with Uteodon and Camptosaurus as the only non-styracosternan ankylopollexians, a small Iguanodontidae, and a clade of taxa with robust forelimbs. Mantellisaurus groups with RBINS R57, and these are sister to Hadrosauroidea. Within Hadrosauroidea, the topology is consistent with the parsimony analysis (though polytomies in the parsimony tree are resolved into bifurcating branches in the Bayesian results) with one exception. Tethyshadros is recovered branching from a node basal to Bactrosaurus and Gilmoreosaurus in the parsimony tree, but at a less inclusive node in the Bayesian tree. The genera found in Ankylopollexia, Styracosterna, Iguanodontidae, and Hadrosauroidea are all identical in the parsimony and Bayesian topologies. This overall consistency indicates that the topology is supported by the data, regardless of the model.
SYSTEMATIC PALEONOTOLOGY
Thescelosauridae Sternberg, 1937
Phylogenetic definition. All neornithischians more closely related to Thescelosaurus neglectus Gilmore, 1913 than to Hypsilophodon foxii Huxley, 1869, Dryosaurus altus (Marsh, 1878), or Parasaurolophus walkeri Parks, 1922.
Unambiguous synapomorphies. In the parsimony analysis (Figure 7), thescelosaurids are characterized by three unambiguous synapomorphies: dentary tooth row straight in dorsal view (98.0), 10-12 ridges on the maxillary teeth (136.3), and acromion process of scapula does not extend beyond the edge of the coracoid (196.0).
In the Bayesian analysis (Figure 8), a more extensive Thescelosauridae is characterized by two unambiguous synapomorphies: 10-12 ridges on the maxillary teeth (136.3) and 9-11 ridges on the dentary teeth (137.3).
Topology. Thescelosauridae is recovered in the parsimony analysis (Figure 6, Figure 7) containing Thescelosaurus, Parksosaurus, Orodromeus, and Zephyrosaurus, though the group has poor support (Jackknife value=7). Unlike in Boyd (2012), Haya is recovered at a node basal to thescelosaurids, rather than within the Thescelosaurinae. Macrogryphosaurus and Talenkauen, which Boyd also found within Thescelosaurinae, are recovered within iguanodontia, based on the presence of opisthocoelus cervical vertebrae, and a postacetabular process that is less than 30% of total ilium length.
In the Bayesian analysis (Figure 6, Figure 8), Thescelosauridae is nested within a larger Hypsilophodontidae. Though it only has moderate support (PP=0.59), the internal nodes have higher posterior probabilities. The genera included in this group match those recovered in the parsimony analysis; the one difference in topology is that Orodromeus and Zephyrosaurus form successive outgroups to Thescelosaurus + Parksosaurus instead of forming a distinct Orodrominae (as found by Boyd 2012). Though Haya and Macrogryphosaurus are recovered as closely related hypsilophodontids, they are not found within Thescelosauridae as proposed in Boyd (2012).
CLYPEODONTA Norman, 2015
Phylogenetic definition. Hypsilophodon foxii, Edmontosaurus regalis, their most recent common ancestor, and all of its descendants (Norman, 2015).
Unambiguous synapomorphies. For the topology recovered by parsimony, Clypeodonta has nine unambiguous synapomorphies: presence of a quadrate buttress or "hamular process" (63.1), quadrate with a lateral condyle that is larger than the medial condyle (69.2), mandibular articulation that is horizontal to dorsomedially inclined in caudal view (70.0/1), maxillary and dentary teeth with crowns that taper toward the root (127.1, 128.1), with the base of the crown defined by an everted lip which makes the crown slightly inset from the root (146.1, 147.1), presence of a primary ridge on labial side of maxillary teeth (139.1), and elongate centra of postaxial cervical vertebrae, with craniocaudal length more than twice the dorsoventral height (159.1).
Within the Bayesian topology, Clypeodonta is characterized primarily by features of the teeth and jaws: presence of a diastema in the maxilla (16.1), equal lengths in the oral margin of the premaxilla and predentary (84.1), a coronoid process that extends more than one crown height dorsal to the tooth row (101.1), surangular with a small fenestra positioned dorsally on or near the dentary joint (111.1), surangular foramen rostral to the lateral lip of the glenoid (114.1), cheek teeth with asymmetrically distributed enamel (134.1), ridges running the full length of the crown on the labial side of maxillary teeth and the lingual side of dentary teeth (135.1), and a femoral head separated from the greater trochanter by a distinct constriction (292.1).
Topology. In the parsimony tree (Figure 6, Figure 7), Clypeodonta is the sister clade to Thescelosauridae. This node has a jackknife value of 15, but a relatively high Bremer support of 4. Hypsilophodon is recovered as the only non-iguanodontian clypeodontan.
In the Bayesian topology (Figure 6, Figure 8), Hypsilophodon is recovered within a large Hypsilophodontidae. Consequently, Clypeodonta is a more inclusive clade than in the parsimony tree, including Thescelosauridae, a clade with Haya, Jeholosaurus, and Othnielosaurus, and Leaellynasaura, Gasparinisaura, and Macrogryphosaurus. It excludes only a few basal neornithischians such as Hexinlusaurus and Agilisaurus. It is moderately supported, with a posterior probability (PP) of 0.60.
IGUANODONTIA Sereno, 1986
Phylogenetic definition. The most inclusive clade containing Parasaurolophus walkeri Parks, 1922 but not Hypsilophodon foxii Huxley, 1869, or Thescelosaurus neglectus Gilmore, 1913 (Sereno, 2005).
Unambiguous synapomorphies. In the parsimony analysis, Iguanodontia is characterized by a maxilla with a broad and triangular dorsal process of the maxilla (21.1), a quadrate extending ventrally such that the quadratojugal is well removed from the mandibular condyle (60.1), a single wear facet on each cheek tooth (131.1), opisthocoelus post-axial cervical vertebrae (157.1), a distinct indentation on the scapula superior to the glenoid, termed here the supraglenoid fossa (199.1), and a manual digit III with three or fewer phalanges (236.1).
Two other synapomorphies recovered for this clade are elongate prezygopophyses on the distal caudal vertebrae (185.1), and chevrons that are strongly and asymmetrically expanded distally (188.1). The former of these is found only in Gasparinisaura and Leaellynasaura, and the latter in these genera plus Parksosaurus and Macrogryphosaurus. While they are present at the base of the clade, these characters are not widespread, and therefore not useful in diagnosing the clade.
There is only one overlapping character here with the diagnosis of Sereno (1986); the reduction of phalanges in digit III. The presence of “leaf-shaped” or mamillated denticles is more restricted within Styracosterna, and while most iguanodontians lack premaxillary teeth, both Talenkauen and Tenontosaurus dossi have one premaxillary tooth. Iguanodontia is recovered with jackknife support of 19 and Bremer support of 4.
Within the Bayesian topology, Iguanodontia (PP=0.43) lacks the basal pectinate region found in the parsimony analysis and is instead composed of the sister groups of rhabdodontoids and Dryomorpha. Gasparinisaura, Leaellynasaura, and Macrogryphosaurus are excluded from Iguanodontia, and are recovered instead with the hypsilophodontids. This rearrangement of taxa leads to different synapomorphies for Iguanodontia between the parsimony and Bayesian analyses. Synapomorphies for the Bayesian topology include: premaxilla flaring laterally to form a floor of the narial fossa (3.1), small antorbital fenestra (31.1), predentary with denticulate oral margin (87.1), ventral process of predentary deeply bifurcated (89.1), cheek teeth with crowns tapering toward the root (127.1, 128.1), cheek teeth that are closely packed without spaces between roots (126.1), cheek teeth with one wear facet on each tooth (131.1), cheek teeth lacking a basal ridge (“cingulum”) (148.1), caudal vertebrae with distal facets for chevrons much larger than proximal facets (183.0), humerus with an elongate deltopectoral crest (>43% humeral length) (214.1), manual digit III with three or fewer phalanges (236.1), first phalanx of manual digits II-IV more than twice the length of the second phalanx (239.1), ischium with an untwisted shaft (283.1), ischium with an expanded distal end (288.1), femur with a cranial intercondylar sulcus (300.1), and a caudal intercondylar sulcus partially roofed by the medial condyle (302.1).
Topology. In the parsimony analysis (Figure 6, Figure 7), the basally branching portion of Iguanodontia forms a pectinate topology outside of Dryomorpha, which includes Gasparinisaura, Leaellynasaura, Macrogryphosaurus, Talenkauen, Valdosaurus, Anabisetia, Trinisaura, and Kangnasaurus. It is supported by a jackknife value of 19 and a Bremmer support of 4.
In the Bayesian analysis (Figure 6, Figure 8), Iguanodontia bifurcates into rhabdodontoids and dryomorphans. Iguanodontia is supported by a posterior probability of 0.43.
RHABDODONTOIDEA new clade name
Phylogenetic definition. A stem-based taxon including all taxa more closely related to Zalmoxes robustus and Rhabdodon priscus than to Dryosaurus altus.
Unambiguous synapomorphies. The smaller clade found in the parsimony analysis is characterized by four unambiguous synapormorphies: a sub-rectangular orbit (34.1), sinuous ventral edge of the jugal (56.1), caudodorsally extending postacetabular process of the ilium (265.1), and a femur that is straight in lateral view (290.0).
The larger Bayesian clade has two unambiguous synapomorphies: maxilla with a broad triangular dorsal process (21.1), and a straight maxillary toothrow in ventral view (26.2).
Topology. The parsimony analysis (Figure 6, Figure 7) recovers a clade containing rhabdodontids, Tenontosaurus, and Muttaburrasaurus; this is supported by a jackknife value of 23, and Bremer support of 4.
The Bayesian analysis (Figure 6, Figure 8) also finds Tenontosaurus and Muttaburrasaurus as the sister taxa to Rhabdodontidae, but this is included within a larger clade with many Gondwanan taxa (Kangnasaurus, Anabisetia, Trinisaura, and Talenkauen). The basal node of the clade is poorly supported (PP=0.24), though the node supporting Rhabdodontidae, Muttaburrasaurus, and Tenontosaurus has stronger support (PP=0.85).
RHABDODONTIDAE
Weishampel, Jianu, Csiki, and Norman, 2003
Phylogenetic definition. A node-based taxon consisting of the most recent common ancestor of Zalmoxes robustus and Rhabdodon priscus and all the descendants of this common ancestor (Weishampel et al., 2003).
Unambiguous synapomorphies. Rhabdodontidae is characterized by the presence of apicobasally extending ridges on the cutting surfaces of unworn cheek teeth (lingual surface of maxillary teeth, labial surface of dentary teeth) (138.1), acromion process of scapula does not extend beyond the edge of the coracoid (196.0), medial condyle of the humerus transversely wider than the lateral (216.2), proximal flange present on the ulna (219.1), preacetabular process of the ilium twisted around its long axis (249.1), pubic peduncle of the ischium dorsoventrally compressed (280.1), and distal condyles of the femur expanded both cranially and caudally (304.1), metatarsal V absent (320.1).
Topology. This small clade contains two genera, Zalmoxes and Rhabdodon, both known from the Late Cretaceous of Europe. This clade is recovered in the parsimony analysis with a jackknife value of 81 and a Bremer support of 4, and in the Bayesian analysis with a posterior probability of 1.
DRYOMORPHA Sereno, 1986
Phylogenetic definition. The most inclusive clade containing Dryosaurus altus (Marsh, 1878) and Parasaurolophus walkeri Parks, 1922 (Sereno, 2005).
Unambiguous synapomorphies. In both parsimony and Bayesian analyses, Dryomorpha is characterized by a premaxilla with a posterolateral process that contacts the lacrimal (6.1), a maxilla with a rostrolateral process (18.1), a lacrimal that fits into a slot in the dorsal process of the maxilla (27.1), a quadratojugal that lacks a dorsal process (57.1), a coronoid process with subequal craniocaudal widths of dentary and surangular (109.1), cheek teeth with secondary ridges that are thin and formed only from enamel (145.1), a radius that is triangular in distal view (224.1), and an ischium with the obturator process within the proximal 25% of the ischium (287.1).
In the parsimony analysis, Dryomorpha is further supported by a maxillary tooth row that is medially bowed in ventral view (26.0), a jugal that is excluded from the antorbital fenestra by lacrimal-maxilla contact (47.0), a quadratojugal that lacks a foramen through the center of the element (58.0), and absent ossified hypaxial tendons on the caudal vertebrae (322.0).
In the Bayesian analysis, Dryomorpha is also supported by the presence of a quadrate buttress or "hamular process" (63.1), presence of a quadrate (or paraquadratic) foramen or notch on the boundary between the quadrate and quadratojugal (65.1), and presence of a short midline process of the predentary dorsal to the dentary symphysis (90.1)
Topology. In both parsimony and Bayesian analyses (Figure 6), Dryomorpha contains two sister clades: Dryosauridae and Ankylopollexia. Support for this node is moderate (jackknife=19, Bremmer support=4, PP=0.27).
DRYOSAURIDAE Milner and Norman, 1984
Phylogenetic definition. The most inclusive clade containing Dryosaurus altus (Marsh, 1878) but not Parasaurolophus walkeri Parks, 1922 (Sereno, 2005).
Unambiguous synapomorphies. Dryosauridae is characterized by nine unambiguous synapomorphies: premaxilla with a medial dorsal (nasal) process that does not contact the nasal (9.1), palpebrals that traverse the entire width of orbit (42.1), a dentary with dorsal and ventral margins (under the tooth row) that converge anteriorly (97.0), maxillary teeth with primary ridges that are centered mesio-distally (143.0), 15 or fewer dorsal vertebrae (161.0), posterior dorsal vertebrae with transverse processes longer than the dorsoventral height of the centrum (167.1), scapula with a weakly developed acromion process (195.0), prepubic process with a horizontal ridge on medial side (273.1) and a pubis with an obturator foramen completely enclosed by bone (275.0)
Topology. In addition to Dryosaurus and Dysalotosaurus, which have been found previously to form a clade (McDonald et al., 2010b), this analysis finds the unnamed Kirkwood taxon from South Africa within the group, as sister to Dysalotosaurus. The clade is well supported, with a jackknife value of 60, and Bremer support of 4. In the Bayesian topology, Dryosauridae also contains Valdosaurus as sister to the Kirkwood taxon (PP=0.27).
ANKYLOPOLLEXIA Sereno, 1986
Phylogenetic definition. The least inclusive clade containing Camptosaurus dispar (Marsh, 1879), Uteodon aphanoecetes (Carpenter and Wilson 2008), and Parasaurolophus walkeri Parks, 1922 (emended from Sereno, 1986).
Unambiguous synapomorphies. Ankylopollexia is characterized by nine unambiguous synapomorphies: deltoid ridge of the scapula close to parallel to the long axis of the scapula (198.0), humerus with a well-developed deltopectoral crest (212.0), ulna with a flange on the proximal end that wraps around the lateral edge of the radius (219.1) some fusion of the carpals (227.1), manual digit I oriented at least 45 degrees from the antebrachial axis (232.1), metacarpal I short and block-like (233.1), ungual of manual digit I subconical (241.1), brevis fossa of ilium not well defined by a lateral lip (259.0), ossified epaxial and hypaxial tendons arranged in a double-layered lattice (323.1).
Topology. This is a well-supported clade, with a Jackknife value of 35 and Bremer support of 6 in the parsimony analysis (Figure 7), and a posterior probability of 0.91 in the Bayesian tree (Figure 8). In both topologies, the most basally branching taxon is Uteodon, and Camptosaurus is recovered as the sister to Styracosterna. These two genera are the only non-styracosternan ankylopollexians.
STYRACOSTERNA Sereno, 1986
Phylogenetic definition. The most inclusive clade containing Parasaurolophus walkeri Parks 1922 but not Camptosaurus dispar (Marsh, 1879) or Uteodon aphanoecetes (Carpenter and Wilson 2008).
Unambiguous synapomorphies. Styracosterna is characterized by five unambiguous synapomorphies: presence of denticulation on the oral margin of the premaxilla (5.1), 18-28 maxillary tooth positions (123.2), dentary teeth with a maximum of two to four ridges extending from the base to the tip of the crown on lingual side of teeth (137.1), maxillary tooth crowns mesiodistally narrower than dentary crowns (149.1), mid to posterior dorsal vertebrae with length much shorter than height (164.1).
Topology. Styracosterna has strong support in both topologies (JV=35, BS=4, PP=0.91), but within this group, relationships are resolved poorly. The parsimony tree (Figure 7) has several polytomies and the Bayesian tree (Figure 8) recovers several small clades within Styracosterna, but most have low support.
UNNAMED NODE
Comments. No formal definition is given for this clade of styracosternans with robust forelimbs. Variations on it are recovered in both parsimony and Bayesian analyses, but the lack of strong support and variability in included taxa makes this grouping tentative.
Unambiguous synapomorphies. This group is characterized by seven unambiguous synapomorphies: neural spines of caudal vertebrae bowed (180.1), robust ulna with length less than nine times diameter at mid-shaft (217.0), olecranon process of the ulna greater than 17% of ulna length (218.2), robust radius with minimal radial width equal to or greater than 12% radial length (221.1), presence of tubercle near proximal end of radius for insertion of M. biceps (223.1) manual ungual I greater than 30% the length of the radius (242.1), and obturator foramen of the pubis circular (276.1).
Topology. The parsimony analysis (Figure 6, Figure 7) recovers a small clade of styracosternans that includes Hypselospinus, the holotype of Barilium, and Lurdusaurus. In the majority rule consensus, Bolong and Jinzhousaurus are also included in this clade. This larger clade including Bolong and Jinzhousaurus is also recovered in the Bayesian analysis (Figure 6, Figure 8), supported with a posterior probability of 0.14. The clade can be diagnosed by several synapomorphies, and some genera share character states that are found nowhere else in the analysis: Hypselospinus and Lurdusaurus each have olecranon processes that compose more than 17% of the ulna length, and a radial tubercle for the insertion of M. biceps.
IGUANODONTIDAE Gervais, 1853
Phylogenetic definition. A stem-based taxon including all taxa more closely related to Iguanodon bernissartensis than to Edmontosaurus regalis.
Unambiguous synapomorphies. This clade is characterized by three unambiguous synapomorphies: palpebrals that traverse the entire width of orbit (42.1), postorbital with a bifurcated squamosal process (45.1) small fenestra near the suture of the dentary and surangular lies within the surangular (112.1). (Note that this fenestra is not present in Iguanodon or Equijubus.)
Topology. This is another small clade of styracosternans that includes Iguanodon, along with Equijubus, Proa, and Fukuisaurus. As such, the name Iguanodontidae is appropriate, although the taxa included within it are far more closely related to each other than those within the historical conception of Iguanodontidae (e.g., Huxley (1870) included Hypsilophodon and hadrosaurs within Iguanodontidae). The support for the group is not high, with a jackknife value=12 and a Bremer support of 2, but the same topology is recovered in the Bayesian analysis (with a posterior probability of 0.46).
MANTELLISAURUS + “DOLLODON”
Unambiguous synapomorphies. This grouping is supported by seven unambiguous synapomorphies: presence of a fossa or foramen just dorsal to the oral margin along the premaxilla-maxilla boundary (15.1), diastema of the maxilla one to two tooth widths long (17.0), strongly downturned rostral end of dentary ramus (95.1), subvertical coronoid process of dentary (105.1), mid to posterior dorsal vertebral centra with craniocaudal length subequal to or longer than dorsoventral height (164.0), humerus less than 62% of femoral length (211.0), metacarpal III long and slender with length more than 5.5 times minimum transverse width (230.1).
Topology. The holotype specimen of Mantellisaurus is recovered as the sister taxon to IRSNB 1551, referred to Dollodon by Paul (2008). The clade is supported with jackknife of 55 and a Bremer support of 3. This clade is also recovered in the Bayesian analysis (PP=0.71).
HADROSAUROIDEA Sereno, 1986
Phylogenetic definition. The most inclusive taxon containing Parasaurolophus walkeri Parks, 1922 but not Iguanodon bernissartensis Boulenger, 1881 (Sereno, 2005).
Unambiguous synapomorphies. Hadrosauroids are characterized largely by features related to the “dental battery”, which become even more exaggerated in the hadrosaurids. The clade has 18 unambiguous synapomorphies: antorbital fenestra absent (29.1), left and right squamosals separated by only a narrow band of the parietal (73.1), long diastema of the dentary, the width of three or more teeth (93.1), dentary, rostral extent of Meckel's groove meets the dentary symphysis: absent, ends more caudally (94.1), caudal extent of dentary tooth row is in line with or caudal to the apex of the coronoid process (103.2), coronoid process of the dentary oriented near vertically (105.1), coronoid process of the dentary with a rostrocaudally expanded apex (107.1), maxillary teeth with a primary ridge only (136.0), dentary tooth row with one functional tooth rostrally and caudally, and up to two teeth at and approaching the middle of the dental battery (150.1), maximum of two replacement dentary teeth (151.1), dentary without discrete alveoli, but parallel grooves lining a continuous dental battery (152.1), most proximal chevron placed at distal end of third caudal vertebra or more distally (186.2), coracoid, length of the scapular articulation less than 1.25 times the length of the lateral margin of the glenoid (207.1), radius length greater than 70% of humeral length (220.1), metacarpal III long and slender, length greater than 5.5 times the transverse width at midshaft (230.1), preacetabular process of the ilium is parallel-sided or slightly tapering at its distal end (251.0), base of the preacetabular process of the ilium is not transversely thickened ventrally (252.0), ischium shaft straight in lateral view (281.0), distal condyles of femur with rounded articular surfaces (305.1).
Topology. Support in the parsimony tree is low (jackknife=7, Bremer support=2), but there is strong support in the Bayesian tree (posterior probability=0.96). It includes Probactrosaurus, Batyrosaurus, Altirhinus, Jeyawati, Eolambia, Protohadros, Shuangmiaosaurus, Levnesovia, Gilmoreosaurus, Bactrosaurus, Tethyshadros, Telmatosaurus, Edmontosaurus, Maiasaura, and Hypacrosaurus. This topology agrees with the results of other recent studies (e.g., Gates and Scheetz, 2014; Prieto-Márquez, 2012).
Ancestral Area Reconstruction
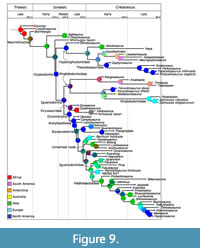 Among the models used (DEC, DIVALIKE and BAYAREA), the DIVALIKE model had the lowest AICc score, and AAR from this model is shown in Figure 9. Few clades in this analysis form distinct geographic clusters. Ankylopollexians are found primarily in Laurasia, with the exception of Ouranosaurus and Lurdusaurus in northern Africa. Based on the maximum likelihoods found in the AAR, ankylopollexians are likely to have originated in North America, and hadrosauroids are likely to have evolved in Asia.
Among the models used (DEC, DIVALIKE and BAYAREA), the DIVALIKE model had the lowest AICc score, and AAR from this model is shown in Figure 9. Few clades in this analysis form distinct geographic clusters. Ankylopollexians are found primarily in Laurasia, with the exception of Ouranosaurus and Lurdusaurus in northern Africa. Based on the maximum likelihoods found in the AAR, ankylopollexians are likely to have originated in North America, and hadrosauroids are likely to have evolved in Asia.
While the large rhabdodontoid group recovered in the Bayesian analysis is not well supported, if this clade existed it was geographically widespread, and the ancestral area is not clear. A small clade within the rhabdodontoids is restricted to Gondwana; this includes the genera Kangnasaurus from South Africa, Anabisetia and Talenkauen from Argentina, and Trinisaura from Antarctica. The estimated mean age of the node at which this clade branches from the rest of the rhabdodontoids is 145 ma, with a 95% confidence interval ranging from a minimum age of 126 ma to a maximum of 168 ma. The large range here is likely due to the poorly constrained age of Kangnasaurus. While this range overlaps with the opening of the North Atlantic Ocean and the separation of Laurasia from Gondwana (Blakey, 2008), the large range of potential node ages makes it difficult to determine with any certainty whether this clade diverged due to vicariance.
The more basal Hypsilophodontidae are geographically wide-ranging, although they are best represented in the Late Cretaceous by Thescelosauridae from North America. The basal position of Othnielosaurus also helps to increase the likelihood of the group originating in North America. The age of the nodes Clypeodonta and Iguanodontia indicate that late Early through Middle Jurassic aged deposits should be productive for finding fossils that elucidate the relationships at the base of Clypeodonta, and across basal Ornithischia.
Effect of Postcranial Characters
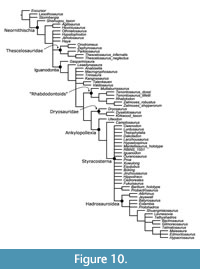 The parsimony analysis that excluded the 80 new postcranial characters produced 3,086 MPTs, with a strict consensus that is considerably less resolved than the primary analysis discussed here (Figure 10). Styracosterna is recovered as a large polytomy including 18 terminals and two clades. Both Iguanodontidae and the unnamed styracosternan clade collapse in this analysis. Thescelosauridae, rhabdodontoids, and Dryosauridae are recovered in this tree, and Camptosaurus and Uteodon are recovered as basal ankylopollexians outside of Styracosterna, which is a slight improvement over the strict consensus found by McDonald (2012a), in which Ankylopollexia formed a large polytomy. The poor resolution of this tree indicates the improvement in resolution achieved with a concerted effort to include more postcranial characters.
The parsimony analysis that excluded the 80 new postcranial characters produced 3,086 MPTs, with a strict consensus that is considerably less resolved than the primary analysis discussed here (Figure 10). Styracosterna is recovered as a large polytomy including 18 terminals and two clades. Both Iguanodontidae and the unnamed styracosternan clade collapse in this analysis. Thescelosauridae, rhabdodontoids, and Dryosauridae are recovered in this tree, and Camptosaurus and Uteodon are recovered as basal ankylopollexians outside of Styracosterna, which is a slight improvement over the strict consensus found by McDonald (2012a), in which Ankylopollexia formed a large polytomy. The poor resolution of this tree indicates the improvement in resolution achieved with a concerted effort to include more postcranial characters.
DISCUSSION
Tree Topology and Nomenclature
In overall structure, this analysis agrees largely with previous work (Sereno, 1998; McDonald et al., 2010b; McDonald et al., 2012; Norman, 2015) in finding a series of nested clades within Iguanodontia: Dryomorpha, Ankylopollexia, Styracosterna, and Hadrosauroidea. The sister group to Ankylopollexia is Dryosauridae; together these form the node-based Dryomorpha, and the sister group to this is the rhabdodontoids.
The topology found here agrees with that of Boyd (2012, 2015) in recovering a Thescelosauridae (=”Parksosauridae”) outside of Iguanodontia, although there are some differences in the taxa included. Haya is recovered at a node basal to thescelosaurids, rather than within the Thescelosaurinae. Macrogryphosaurus and Talenkauen, which Boyd also found within Thescelosaurinae, are recovered within Iguanodontia. Given that Thescelosauridae falls within Clypeodonta in the Bayesian topology, it is possible that the latter term may be equivalent or at least similar in taxonomic composition to Ornithopoda. To test this further, several marginocephalians and relevant characters would need to be added to the analysis.
The Clypeodonta recovered in both the parsimony and Bayesian trees differs from that of Norman (2015), largely due to the inclusion of more non-iguanodontian neornithischians. Some of the characters cited by Norman to diagnose Clypeodonta (e.g., the presence of ridges on the labial surface of maxillary teeth) are more widespread, also occurring in Thescelosauridae.
As in the work of McDonald (2012a) and Dieudonné et al. (2016), and congruent with observations of Molnar (1996), this analysis recovers Muttaburrasaurus in a clade with Rhabdodontidae. Dieudonné et al. (2016) erected a node-based clade, Rhabdodontomorpha, for this group. In the parsimony analysis, rhabdodontomorphans also includes Tenontosaurus, while the Bayesian analysis finds Tenontosaurus as the sister to Rhabdodontomorpha. It also recovers a larger group of rhabdodontoids including more Gondwanan taxa. The taxa included in Rhabdodontoidea are likely to shift as new analyses are done, but the stem-based definition given here will create some stability. This grouping is congruent with biogeographic analyses linking European and Gondwanan taxa (Ezcurra and Agnolín, 2012). Rhabdodontoids are united primarily by dental features: teeth that are relatively wide mesiodistally, with a high number of secondary ridges that are thick, composed of both dentine and enamel (rather than being formed only of small crenulations in the enamel). While it is likely that these taxa form a clade, it is also possible that these character states are parallelisms, due to similar diets or chewing mechanisms.
When Ankylopollexia was named, Uteodon aphanoecetes had not yet been recognized and the specimens which now represent the taxon were referred to Camptosaurus dispar, which Sereno (1986) used to define Ankylopollexia. Considering these taxonomic changes, and Sereno’s original description of ankylopollexians as those iguanodontians that displayed fused carpals, it seems prudent to emend Sereno’s definition to include both U. aphanoecetes and C. dispar as reference taxa for Ankylopollexia. It should be noted that all nine of the unambiguous synapomorphies of this group are postcranial, with four associated with carpal fusion and modifications in the first manual digit. In addition to these, the presence of a flange on the ulna serves as an indicator of quadrupedality (Maidment and Barrett, 2014, and see below). Thus, the fundamental shift between non-ankylopollexian iguanodontians and Ankylopollexia lies in the forelimbs, and in a shift to functional quadrupedality.
As with Ankylopollexia, the definition of Styracosterna is emended to exclude both C. dispar and U. aphanoecetes. It should also be noted that the eponymous feature of this group, the “hatchet-shaped” sternal, with a caudolateral process projecting from the main plate of the sternal, is more widespread than previously recognized, and found in at least two genera outside of Styracosterna: the basal iguanodontian Macrogryphosaurus, and the unnamed dryosaurid from the Kirkwood Formation.
This analysis agrees partly with that of Norman (2015) in finding a clade within Styracosterna including Iguanodon and Proa, but differs in placing Batyrosaurus, Barilium, Bolong, Jinzhousaurus, and Mantellisaurus outside this clade. Aside from Batyrosaurus which is recovered as a basal hadrosauroid, these taxa are all found in the large polytomy of styracosternans, just one node outside of iguanodontids. In that sense, this topology is not drastically different from that of Norman (2015), but the larger number of taxa included here may have caused the strict consensus to collapse.
The holotype of Mantellisaurus and RBINS R57 (=“Dollodon”) being recovered as sister taxa within their own small clade lends support to the taxonomic conclusion of Norman (2013, 2015) and McDonald (2012b) that Dollodon should be considered a subjective junior synonym of Mantellisaurus.
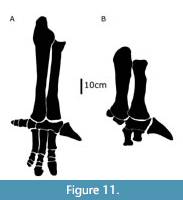 There are two non-hadrosauroid clades within Styracosterna, iguanodontoids and an unnamed clade with more robust forelimb elements and a larger ungual on the first digit (Figure 11). The lack of clear temporal succession or geographic separation between these two groups (Figure 9) indicates this was not due to faunal turnover or vicariance and could represent niche partitioning. As many of the synapomorphies are associated with heavier, more muscular forearms and a larger pollex ungual, it is also possible that these characters are linked functionally and represent parallelisms within Styracosterna.
There are two non-hadrosauroid clades within Styracosterna, iguanodontoids and an unnamed clade with more robust forelimb elements and a larger ungual on the first digit (Figure 11). The lack of clear temporal succession or geographic separation between these two groups (Figure 9) indicates this was not due to faunal turnover or vicariance and could represent niche partitioning. As many of the synapomorphies are associated with heavier, more muscular forearms and a larger pollex ungual, it is also possible that these characters are linked functionally and represent parallelisms within Styracosterna.
Morphological Patterns
One of the most surprising outcomes of this analysis in terms of character distribution is the homoplasy found in “hatchet shaped” sternals (those with a caudolateral process). This feature was thought to be diagnostic of Styracosterna, even giving the name to the group (Sereno, 1986). However, it seems to have arisen at least three times within Iguanodontia: in Macrogryphosaurus, in the Kirkwood taxon (Dryosauridae), and in Styracosterna (Figure 12). The position of Macrogryphosaurus is quite different between the parsimony and Bayesian analyses, but in both cases it is well outside of Styracosterna. A similar morphology also occurs in Pachycephalosauridae (Maryańska and Osmólska, 1974; Perle et al., 1982).
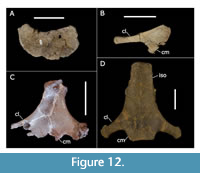 The rugose caudal end of the process indicates it served as an attachment point for costal cartilage. This relationship appears to be present in non-styracosternan neornithischians such as Hypsilophodon (Galton, 1974; Butler and Galton, 2008) and Thescelosaurus (NCSM 15728), which both show the sternal articulated with ossified segments of costal cartilage. While this may explain the function of the caudolateral process of the sternal, it fails to indicate any functional reason for the difference in morphology. Previous studies of comparative myology (e.g., Dilkes, 2000) have not discussed the sternal in detail. While it would have served as the origin of M. pectoralis, it remains unclear what changes to the myology might occur with the presence of a caudolateral process of the sternal.
The rugose caudal end of the process indicates it served as an attachment point for costal cartilage. This relationship appears to be present in non-styracosternan neornithischians such as Hypsilophodon (Galton, 1974; Butler and Galton, 2008) and Thescelosaurus (NCSM 15728), which both show the sternal articulated with ossified segments of costal cartilage. While this may explain the function of the caudolateral process of the sternal, it fails to indicate any functional reason for the difference in morphology. Previous studies of comparative myology (e.g., Dilkes, 2000) have not discussed the sternal in detail. While it would have served as the origin of M. pectoralis, it remains unclear what changes to the myology might occur with the presence of a caudolateral process of the sternal.
There are some clear trends among dental characters that are helpful in distinguishing ankylopollexians, rhabdodontoids, and thescelosaurids.
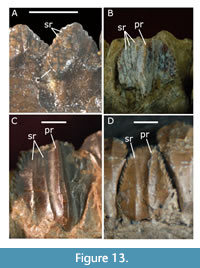 Thescelosauridae retain the most plesiomorphic teeth, with five to six premaxillary teeth, cheek teeth with a distinct angle between crown and root, spaces between roots, and a basal ridge (=cingulum) (Figure 13A). Rhabdodontoidea, Dryosauridae, and Ankylopollexia all have a reduction or complete loss of premaxillary teeth, cheek teeth that are closely packed with crowns that taper toward the root, and no basal ridge. While rhabdodontoids trend toward fewer but wider teeth with many ridges (Figure 13B), the teeth of ankylopollexians become more numerous and narrower (especially in the maxilla), with fewer ridges. The morphology of the dental ridges also differs: rhabdodontoids display thick, regularly spaced secondary ridges (Figure 13B), while ankylopollexians have thin, often irregular secondary ridges that merge or split with each other (Figure 13D). In this respect, Tenontosaurus is similar to ankylopollexians (Figure 13C). Within hadrosauroids, the secondary ridges are lost as the tooth crowns become even narrower and more numerous.
Thescelosauridae retain the most plesiomorphic teeth, with five to six premaxillary teeth, cheek teeth with a distinct angle between crown and root, spaces between roots, and a basal ridge (=cingulum) (Figure 13A). Rhabdodontoidea, Dryosauridae, and Ankylopollexia all have a reduction or complete loss of premaxillary teeth, cheek teeth that are closely packed with crowns that taper toward the root, and no basal ridge. While rhabdodontoids trend toward fewer but wider teeth with many ridges (Figure 13B), the teeth of ankylopollexians become more numerous and narrower (especially in the maxilla), with fewer ridges. The morphology of the dental ridges also differs: rhabdodontoids display thick, regularly spaced secondary ridges (Figure 13B), while ankylopollexians have thin, often irregular secondary ridges that merge or split with each other (Figure 13D). In this respect, Tenontosaurus is similar to ankylopollexians (Figure 13C). Within hadrosauroids, the secondary ridges are lost as the tooth crowns become even narrower and more numerous.
Locomotor Changes
Maidment and Barrett (2014) surveyed ornithischians to determine osteological correlates for quadrupedality using taxa that were either clearly bipedal or clearly quadrupedal. They found five characters that consistently correlated with quadrupedality, four of which are examined here (their “transversely broadened ilium” does not clearly correlate to any of the characters in this analysis). Changes in these character states are mapped onto the Bayesian phylogeny (Figure 14).
 The four characters that Maidment and Barrett found to be correlates of quadrupedality that are present in this analysis are:
The four characters that Maidment and Barrett found to be correlates of quadrupedality that are present in this analysis are:
219.1 Ulna, flange on proximal end that wraps around the lateral edge of the radius: present.
243.1 Manus unguals II and III, shape: dorsoventrally compressed, with a rounded tip.
289.1 Femur, length relative to tibia: longer than the tibia.
296.1 Femur, fourth trochanter, shape: prominent ridge (not pendant).
These characters change in a stepwise manner near the base of Ankylopollexia, indicating that quadrupedality evolved near the base of this group. The accumulation of traits associated with quadrupedality across several nodes indicates that the evolution of quadrupedality occurred in a stepwise manner, such that the basal ankylopollexians Uteodon and Camptosaurus were likely facultative quadrupeds, while styracosternans were more likely obligate quadrupeds. Other characters distinguish ankylopollexians from dryosaurids and may indicate anatomical changes associated with quadrupedality. One is the muscle scar for M. caudofemoralis longus, which is a large oblong depression on the medial side of the fourth trochanter in ankylopollexians, but a small depression on the shaft of the femur in dryosaurids (Character 299). Another is the more complex arrangement of the ossified tendons (Character 323). The synchondrosis in the carpals and metacarpals may also be adaptive for quadrupedality, but this hypothesis is confounded by the co-occurring enlargement of the pollex ungual. Two of these correlates of quadrupedality also occur within rhabdodontoids. While not as clear as the shift at the base of Ankylopollexia, there may have been some degree of quadrupedality in this group.
Future Work
The relationships within Styracosterna remain difficult to resolve, and some of this may be due to poor taxonomy. A specimen-level phylogeny could determine which specimens form natural groupings and can be considered part of the same species. This may alleviate problems arising from incorrect a priori assumptions about relationships between specimens and help to resolve the phylogeny.
More fossils are needed to determine the origins of Iguanodontia (both within and outside of the clade). These will be found, most likely, in Middle to Late Jurassic and Early Cretaceous strata. North America has a high likelihood as the ancestral area for these groups, though the data are skewed due to the high number of fossils already recovered there. Given the uncertainty of the topology at the base of Iguanodontia, and the geographic arrangement of the continents at the time, these basally branching taxa are likely to be geographically widespread.
The long ghost lineages in the basal portion of the tree in both parsimony and Bayesian analyses indicate that sampling for these generally smaller taxa has been poorer than that for ankylopollexians. Only in scrutinizing the fossil record for these more diminutive species will we be able to understand the evolution of Iguanodontia.
ACKNOWLEDGMENTS
I am grateful to C. Forster for many comments on early drafts of this work, and discussions of morphological characters. Helpful comments were also provided by J. Clark, M. Carrano, and D. Weishampel. Thanks also to A. Pyron for advice on Bayesian analysis, and to N. Matzke for answering questions related to BeastMaster and BioGeoBEARS. For enlightening conversations about ornithischian morphology, thanks to P. Barrett and A. Nabavizadeh. Thanks as well to three anonymous reviewers whose comments greatly improved this manuscript.
I am thankful to the many people who provided access to collections: R. Allain, D. Brinkman, K. Carpenter, M. Carrano, I. Cerda, S. Chapman, J.-P. Chenet, R. Coria, B. de Klerk, A. Folie, P. Halvik, S. Kaal, J. Kirkland, C. Levitt, C. Mehling, D. Pagnac, D. Pickering, J. Porfiri, T. Rich, J. Scanella, R. Scheetz, T. Schossleitner, D. Schwarz-Wings, J. Sertich, K. Spring, T. Tortosa, D. Weishampel, and Xu Xing.
Finally, my thanks to everyone for their patience with this paper, which I have kept in the works far too long.
REFERENCES
Barrett, P.M. and Han, F.L. 2009. Cranial anatomy of Jeholosaurus shangyuanensis (Dinosauria: Ornithischia) from the Early Cretaceous of China. Zootaxa, 2072:31-55. https://doi.org/10.11646/zootaxa.2072.1.2
Barrett, P.M., Butler, R.J., and Knoll, F. 2005. Small-bodied ornithischian dinosaurs from the Middle Jurassic of Sichuan, China. Journal of Vertebrate Paleontology, 25:823-834. https://doi.org/10.1671/0272-4634(2005)025[0823:sodftm]2.0.co;2
Barrett, P.M., Butler, R.J., Xiao-Lin, W., and Xing, X. 2009. Cranial anatomy of the iguanodontoid ornithopod Jinzhousaurus yangi from the Lower Cretaceous Yixian Formation of China. Acta Palaeontologica Polonica, 54:35-48. https://doi.org/10.4202/app.2009.0105
Barrett, P.M., Butler, R.J., Twitchett, R.J., and Hutt, S. 2011. New material of Valdosaurus canaliculatus (Ornithischia: Ornithopoda) from the Lower Cretaceous of southern England. Studies on Fossil Tetrapods, 86:131-163. https://doi.org/10.24199/j.mmv.2016.74.04
Bartolini, A. and Larson, R.L. 2001. Pacific microplate and the Pangea supercontinent in the Early to Middle Jurassic. Geology, 29:735-738. https://doi.org/10.1130/0091-7613(2001)029%3C0735:pmatps%3E2.0.co;2
Baur, G. 1891. Remarks on the reptiles generally called Dinosauria. The American Naturalist, 25:434-454. https://doi.org/10.1086/275329
Blakey, R.C. 2008. Gondwana paleogeography from assembly to breakup–A 500 my odyssey. Geological Society of America Special Papers, 441:1-28. https://doi.org/10.1130/2008.2441(01)
Bouckaert, R., Heled, J., Kühnert, D., Vaughan, T., Wu, C-H., Xie, D., Suchard, MA., Rambaut, A., and Drummond, A.J. 2014. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10:e1003537.
https://doi.org/10.1371/journal.pcbi.1003537
Boulenger G.A. 1881. Sur l’arc pelvien chez les dinosauriens de Bernissart. Bulletin de l’Académie royal de Belgique, 1:3-11.
Boyd, C.A. 2012. Taxonomic revision of latest Cretaceous North American basal neornithischian taxa and a phylogenetic analysis of basal ornithischian relationships. Ph.D. dissertation, The University of Texas at Austin, Austin, Texas.
Boyd, C.A. 2015. The systematic relationships and biogeographic history of ornithischian dinosaurs. PeerJ 3:e1523. https://doi.org/10.7717/peerj.1523
Boyd, C.A., Brown, C.M., Scheetz, R.D., and Clarke, J.A. 2009. Taxonomic revision of the basal neornithischian taxa Thescelosaurus and Bugenasaura. Journal of Vertebrate Paleontology, 29:758-770. https://doi.org/10.1671/039.029.0328
Brill, K. and Carpenter, K. 2007. A description of a new ornithopod from the Lytle Member of the Purgatoire Formation (Lower Cretaceous) and a reassessment of the skull of Camptosaurus, p. 49-67. In Carpenter, K. (ed.), Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs. Indiana University Press, Bloomington. https://doi.org/10.2307/j.ctt1zxz1md.8
Brown, B. 1908. The Ankylosauridae, a new family of armored dinosaurs from the Upper Cretaceous. Bulletin of the AMNH, 24(12):187-201.
Buchholz, P.W. 2002. Phylogeny and biogeography of basal Ornithischia, p. 18-34. In Brown, D.E. (ed.), The Mesozoic in Wyoming. Tate Geological Museum, Casper.
Butler, R.J. 2005. The ‘fabrosaurid’ ornithischian dinosaurs of the upper Elliot Formation (Lower Jurassic) of South Africa and Lesotho. Zoological Journal of the Linnaean Society, 145:175-218. https://doi.org/10.1111/j.1096-3642.2005.00182.x
Butler, R.J. and Galton, P.M. 2008. The ‘dermal armour’ of the ornithopod dinosaur Hypsilophodon from the Wealden (Early Cretaceous: Barremian) of the Isle of Wight: a reappraisal. Cretaceous Research, 29:636-642. https://doi.org/10.1016/j.cretres.2008.02.002
Butler, R.J., Smith, R.M., and Norman, D.B. 2007. A primitive ornithischian dinosaur from the Late Triassic of South Africa, and the early evolution and diversification of Ornithischia. Proceedings of the Royal Society of London B: Biological Sciences, 274:2041-2046. https://doi.org/10.1098/rspb.2007.0367
Butler, R.J., Upchurch, P., and Norman, D.B. 2008. The phylogeny of the ornithischian dinosaurs. Journal of Systematic Palaeontology, 6:1-40. https://doi.org/10.1017/s1477201907002271
Calvo, J.O., Porfiri, J.D., and Novas, F.E. 2007. Discovery of a new ornithopod dinosaur from the Portezuelo formation (Upper Cretaceous), Neuquen, Patagonia, Argentina. Arquivos do Museu Nacional, 65:471-483.
Cambiaso, A.V. 2007. Los ornitópodos e iguanodontes basales (Dinosauria, Ornithischia) del Cretácico de Argentina y Antártida. Ph.D. dissertation, Universidad de Buenos Aires, Buenos Aires.
Campione, N.E. and Evans, D.C. 2011. Cranial growth and variation in Edmontosaurus (Dinosauria: Hadrosauridae): implications for latest Cretaceous megaherbivore diversity in North America. PLOS ONE, 6:e25186. https://doi.org/10.1371/journal.pone.0025186s
Cantino, P.D. and de Queiroz, K. 2010. PhyloCode: a phylogenetic code of biological nomenclature, version 4c. https://doi.org/10.1201/9780429446320
Carpenter, K. and Ishida, Y. 2010. Early and “Middle” Cretaceous iguanodonts in time and space. Journal of Iberian Geology, 36:145-164. https://doi.org/10.5209/rev_jige.2010.v36.n2.3
Carpenter, K. and Wilson, Y. 2008. A new species of Camptosaurus (Ornithopoda: Dinosauria) from the Morrison Formation (Upper Jurassic) of Dinosaur National Monument, Utah, and a biomechanical analysis of its forelimb. Annals of Carnegie Museum, 76:227-263. https://doi.org/10.2992/0097-4463(2008)76[227:ansoco]2.0.co;2
Chanthasit, P. 2010. The ornithopod dinosaur Rhabdodon from the Late Cretaceous of France: anatomy, systematics and paleobiology. Ph.D. dissertation, Université Claude Bernard-Lyon I, Lyon.
Cooper, M.R. 1985. A revision of the ornithischian dinosaur Kangnasaurus coetzeei Haughton, with a classification of the Ornithischia. Annals of the South African Museum, 95:281-317.
Coria, R.A. and Calvo, J.O. 2002. A new iguanodontian ornithopod from Neuquen Basin, Patagonia, Argentina. Journal of Vertebrate Paleontology, 22:503-509. https://doi.org/10.1671/0272-4634(2002)022[0503:aniofn]2.0.co;2
Coria, R.A. and Salgado, L. 1996. A basal iguanodontian (Ornithischia: Ornithopoda) from the Late Cretaceous of South America. Journal of Vertebrate Paleontology, 16:445-457. https://doi.org/10.1080/02724634.1996.10011333
Coria, R.A., Moly, J.J., Reguero, M., Santillana, S., Marenssi, S. 2013. A new ornithopod (Dinosauria; Ornithischia) from Antarctica. Cretaceous Research, 41:186-193. https://doi.org/10.1016/j.cretres.2012.12.004
Crompton, A.W. and Charig, A.J. 1962. A new ornithischian from the Upper Triassic of South Africa. Nature, 196:1074-1077. https://doi.org/10.1038/1961074a0
Dalla Vecchia, F.M. 2009. Tethyshadros insularis, a new hadrosauroid dinosaur (Ornithischia) from the Upper Cretaceous of Italy. Journal of Vertebrate Paleontology, 29:1100-1116. https://doi.org/10.1671/039.029.0428
DiCroce, T. and Carpenter, K. 2001. New ornithopod from the Cedar Mountain Formation (Lower Cretaceous) of eastern Utah. p. 183-196. In Tanke, D.H. and Carpenter, K. (eds.), Mesozoic Vertebrate Life. Indiana University Press, Bloomington.
Dieudonné, P.E., Tortosa, T., Fernández-Baldor, F.T., Canudo, J.I., and Díaz-Martínez, I. 2016. An unexpected early rhabdodontid from Europe (Lower Cretaceous of Salas de los Infantes, Burgos Province, Spain) and a re-examination of basal iguanodontian relationships. PLOS ONE, 11:e0156251. https://doi.org/10.1371/journal.pone.0156251
Dilkes, D.W. 2000. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Cretaceous (Campanian) of Montana. Transactions of the Royal Society of Edinburgh: Earth Sciences, 90:87-125. https://doi.org/10.1017/s0263593300007185
Dollo L. 1888. Iguanodontidae et Camptonotidae. Comptes Rendus hebdomadaires des séances de l’Académie des Sciences Paris, CVI(106):775-777.
Drummond A.J. and Bouckaert R.R. 2015. Bayesian evolutionary analysis with BEAST. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9781139095112
Drummond, A.J., Ho, S.Y.W., Phillips, M.J., and Rambaut, A. 2006. Relaxed phylogenetics and dating with confidence. PLOS Biology, 4:e88. https://doi.org/10.1017/cbo9781139095112
Esmerode, E.V., Lykke-Andersen, H., and Surlyk, F. 2007. Ridge and valley systems in the Upper Cretaceous chalk of the Danish Basin: contourites in an epeiric sea. Geological Society, London, Special Publications, 276:265-282.
https://doi.org/10.1144/gsl.sp.2007.276.01.13
Ezcurra, M.D. and Angolín, F.L. 2012. A new global palaeobiogeographical model for the late Mesozoic and early Tertiary. Systematic Biology, 61:553-566. https://doi.org/10.1093/sysbio/syr115
Farris, J.S. 1983. The logical basis of phylogenetic analysis, p. 1-47. In Platnick, N.I. and Funk, V.A. (eds.), Advances in Cladistics, Volume 2, Proceedings of the Second Meeting of the Willi Hennig Society. Columbia University Press, New York.
Felsenstein, J. 1978. Cases in which parsimony or compatibility methods will be positively misleading. Systematic Biology, 27:401-410. https://doi.org/10.1093/sysbio/27.4.401
Forster, C.A. 1990. The postcranial skeleton of the ornithopod dinosaur Tenontosaurus tilletti. Journal of Vertebrate Paleontology, 10:273-294. https://doi.org/10.1080/02724634.1990.10011815
Galton, P.M. 1974. The ornithischian dinosaur Hypsilophodon from the Wealden of the Isle of Wight. Bulletin of the British Museum (Natural History), 25(1):1-152. https://doi.org/10.5962/p.313819
Galton, P.M. 1981. Dryosaurus, a hypsilophodontid dinosaur from the Upper Jurassic of North America and Africa postcranial skeleton. Paläontologische Zeitschrift, 55:271-312. https://doi.org/10.1007/bf02988144
Galton, P.M. 1983. The cranial anatomy of Dryosaurus, a hypsilophodontid dinosaur from the Upper Jurassic of North America and East Africa, with a review of hypsilophodontids from the Upper Jurassic of North America. Geologica et Palaeontologica, 17:207-243. https://doi.org/10.1007/bf02988144
Galton, P.M. 2009. Notes on Neocomian (Lower Cretaceous) ornithopod dinosaurs from England- Hypsilophodon, Valdosaurus, “Camptosaurus”, “Iguanodon” -and referred specimens from Romania and elsewhere. Revue de Paléobiologie, 28:211-273.
Galton, P.M. and Taquet, P. 1982. Valdosaurus, a hypsilophodontid dinosaur from the Lower Cretaceous of Europe and Africa. Geobios, 15:147-159.
Gasca, J., Moreno-Azanza, M., Ruiz-Omeñaca, J., and Canudo, J. 2015. New material and phylogenetic position of the basal iguanodont dinosaur Delapparentia turolensis from the Barremian (Early Cretaceous) of Spain. Journal of Iberian Geology, 41:57-70. https://doi.org/10.5209/rev_jige.2015.v41.n1.48655
Gates, T.A. and Scheetz, R. 2014. A new saurolophine hadrosaurid (Dinosauria: Ornithopoda) from the Campanian of Utah, North America. Journal of Systematic Palaeontology, 13:711-725. https://doi.org/10.1080/14772019.2014.950614
Gervais, P. 1853. Observations relatives aux reptiles fossils de France. Comptes-rendus de l’Academie des Sciences de Paris, 36:374-377.
Gilmore, C.W. 1909. Osteology of the Jurassic reptile Camptosaurus: with a revision of the species of the genus, and description of two new species. Proceedings of the United States National Museum, 36:197-332. https://doi.org/10.5479/si.00963801.36-1666.197
Gilmore, C.W. 1913. A new dinosaur from the Lance Formation of Wyoming. Smithsonian Miscellaneous Collections, 61(5):1-5.
Gilmore, C.W. 1931. A new species of troödont dinosaur from the Lance Formation of Wyoming. Proceedings of the United States National Museum, 79(2875):1-6.
Gilpin, D., DiCroce T., and Carpenter, K. 2007. A possible new basal hadrosaur from the Lower Cretaceous Cedar Mountain Formation of eastern Utah, p. 79-89. In Carpenter K. (ed.), Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs. Indiana University Press, Bloomington. https://doi.org/10.2307/j.ctt1zxz1md.10
Godefroit, P., Dong, Z.M., Bultynck, P., Li, H., and Feng, L. 1998. New Bactrosaurus (Dinosauria: Hadrosauroidea) material from Iren Dabasu (Inner Mongolia, PR China). Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terra, 68 (Supplement):3-70.
Godefroit, P., Li, H., and Shang, C.Y. 2005. A new primitive hadrosauroid dinosaur from the Early Cretaceous of Inner Mongolia (PR China). Comptes Rendus Palevol, 4:697-705.
Godefroit, P., Escuillié, F., Bolotsky, Y.L., and Lauters, P. 2012. A new basal hadrosauroid dinosaur from the Upper Cretaceous of Kazakhstan, p. 335-358. In Godefroit, P. (ed.), Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems. Indiana University Press, Bloomington.
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics, 24:774-786. https://doi.org/10.1111/j.1096-0031.2008.00217.x
Gorscak, E. and O ‘Connor, P.M. 2016. Time-calibrated models support congruency between Cretaceous continental rifting and titanosaurian evolutionary history. Biology Letters, 12:20151047. https://doi.org/10.1098/rsbl.2015.1047
Gradstein, F.M., Ogg, J., Schmitz, M.A., and Ogg, G. 2012. A geologic time scale. Elsevier Publishing Company, Amsterdam.
Han, F.L., Barrett, P.M., Butler, R.J., and Xu, X. 2012. Postcranial anatomy of Jeholosaurus shangyuanensis (Dinosauria, Ornithischia) from the Lower Cretaceous Yixian Formation of China. Journal of Vertebrate Paleontology, 32:1370-1395.
https://doi.org/10.1080/02724634.2012.694385
Head, J.J. 1998. A new species of basal hadrosaurid (Dinosauria, Ornithischia) from the Cenomanian of Texas. Journal of Vertebrate Paleontology, 18:718-738. https://doi.org/10.1080/02724634.1998.10011101
Hooley RW. 1925. On the skeleton of Iguanodon atherfieldensis sp. nov., from the Wealden shales of Atherfield (Isle of Wight). Quarterly Journal of the Geological Society of London, 81:1-61.
Horner, J.R. 1983. Cranial osteology and morphology of the type specimen of Maiasaura peeblesorum (Ornithischia: Hadrosauridae), with a discussion of its phylogenetic position. Journal of Vertebrate Paleontology, 3:29-38.
https://doi.org/10.1080/02724634.1983.10011954
Horner, J.R. and Currie, P.J. 1994. Embryonic and neonatal morphology and ontogeny of a new species of Hypacrosaurus (Ornithischia, Lambeosauridae) from Montana and Alberta, p. 312-336. In Carpenter, K., Hirsch, K.F., and Horner, J.R. (eds.), Dinosaur Eggs and Babies. Cambridge University Press, Cambridge.
Horner, J.R., Weishampel, D.B., and Forster, C.A. 2004. Hadrosauridae, p. 438-463. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.), The Dinosauria: Second Edition. University of California Press, Berkeley.
Hübner, T.R. and Rauhut, O.W.M. 2010. A juvenile skull of Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia), and implications for cranial ontogeny, phylogeny, and taxonomy in ornithopod dinosaurs. Zoological Journal of the Linnean Society, 160:366-396. https://doi.org/10.1111/j.1096-3642.2010.00620.x
Huxley, T.H. 1869. On Hypsilophodon, a new genus of Dinosauria. Abstracts of the Proceedings of the Geological Society (of London), 204:3-4.
Huxley, T.H. 1870. On the classification of the Dinosauria, with observations on the Dinosauria of the Trias. Quarterly Journal of the Geological Society, 26:32-51. https://doi.org/10.1144/gsl.jgs.1870.026.01-02.09
Jacobs, L.L., Winkler, D.A., and P.A. Murry, P.A. 1991. On the age and correlation of Trinity mammals, Early Cretaceous of Texas, USA. Newsletters on Stratigraphy, 24:35-43. https://doi.org/10.1127/nos/24/1991/35
Janensch, W. 1955. Der Ornithopode Dysalotosaurus der Tendaguruschichten. Palaeontographica-Supplementbände, 7:105-176.
Kearney, M. and Clark, J.M. 2003. Problems due to missing data in phylogenetic analyses including fossils: a critical review. Journal of Vertebrate Paleontology, 23:263-274. https://doi.org/10.1671/0272-4634(2003)023[0263:pdtmdi]2.0.co;2
Kennedy, W.J. and Cobban, W.A. 1990. Cenomanian ammonite faunas form the Woodbine Formation and lower part of the Eagle-Ford Group, Texas. Paleontology, 33:75-154.
Kirkland, J.I. 1998. A new hadrosaurid from the upper Cedar Mountain Formation (Albian-Cenomanian: Cretaceous) of eastern Utah - the oldest known hadrosaurid (lambeosaurine?). In: Lucas SG, Kirkland JI, Estep JW, (eds). Lower and Middle Cretaceous Terrestrial Ecosystems. New Mexico Museum of Natural History and Science Bulletin 14:283-295.
Kobayashi, Y. and Azuma, Y. 2003. A new iguanodontian (Dinosauria: Ornithopoda) from the Lower Cretaceous Kitadani Formation of Fukui Prefecture, Japan. Journal of Vertebrate Paleontology, 23:166-175. https://doi.org/10.1671/0272-4634(2003)23[166:anidof]2.0.co;2
Lambe, L.M. 1920. The hadrosaur Edmontosaurus from the Upper Cretaceous of Alberta. Geological Survey of Canada, Memoir 120:1-79. https://doi.org/10.4095/101655
Lee, M.S. and Worthy, T.H. 2012. Likelihood reinstates Archaeopteryx as a primitive bird. Biology Letters, 8:299-303. https://doi.org/10.1098/rsbl.2011.0884
Leidy, J. 1858. Hadrosaurus foulkii, a new saurian from the Cretaceous of New Jersey, related to Iguanodon. Proceedings of the Academy of Natural Sciences of Philadelphia, 10:213-218.
Lepage, T., Bryant, D., Philippe, H., and Lartillot, N. 2007. A general comparison of relaxed molecular clock models. Molecular Biology and Evolution, 24:2669-2680. https://doi.org/10.1093/molbev/msm193
Lewis, P.O. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50:913-925. https://doi.org/10.1080/106351501753462876
Lull, R.S. 1908. The cranial musculature and the origin of the frill in the ceratopsian dinosaurs. American Journal of Science, 25:387-399. https://doi.org/10.2475/ajs.s4-25.149.387
Lull, R.S. 1911. Cretaceous Dinosaurs. Ten years’ progress in vertebrate paleontology. Bulletin of the Geological Society of America, 28:208-212.
Lydekker R. 1889. Notes on new and other dinosaur remains. Geological Magazine, VI:352-356.
Maddison, W.P. and Maddison, D.R. 2009. Mesquite: a modular system for evolutionary analysis. Version 2.72. http://mesquiteproject.org
Maidment, S.C. and Barrett, P.M. 2012. Osteological correlates for quadrupedality in ornithischian dinosaurs. Acta Palaeontologica Polonica, 59:53-70. https://doi.org/10.4202/app.2012.0065
Main, D.J. 2013. Appalachian delta plain paleoecology of the Cretaceous Woodbine Formation at the Arlington Archosaur Site north Texas. Ph.D. dissertation, The University of Texas at Austin, Austin, Texas.
Makovicky, P.J., Kilbourne, B.M., Sadleir, R.W., and Norell, M.A. 2011. A new basal ornithopod (Dinosauria, Ornithischia) from the Late Cretaceous of Mongolia. Journal of Vertebrate Paleontology, 31:626-640. https://doi.org/10.1080/02724634.2011.557114
Mantell, G.A. 1825. Notice on the Iguanodon, a newly discovered fossil reptile, from the sandstone of Tilgate forest, in Sussex. Philosophical Transactions of the Royal Society, 115:179–186. https://doi.org/10.1098/rstl.1825.0010
Marsh, O.C. 1877. A new order of extinct Reptilia (Stegosauria) from the Jurassic of the Rocky Mountains. American Journal of Science, 3(84):513-514.
Marsh, O.C. 1878. Principal characters of American Jurassic dinosaurs. American Journal of Science, s3-16(95):411-416.
Marsh, O.C. 1879. Notice of new Jurassic reptiles. American Journal of Science, 3(108):501-505.
Marsh, O.C. 1889. Notice of gigantic horned Dinosauria from the Cretaceous. American Journal of Science, 3(224):173-176.
Marsh, O.C. 1896. The Dinosaurs of North America. USGS Survey, 16:133-415. https://doi.org/10.5962/bhl.title.60562
Maryańska, T. and Osmólska, H. 1974. Pachycephalosauria, a new suborder of ornithischian dinosaurs. Palaeontologia Polonica, 30:45-102.
Matheron P. 1869. Notes sur les reptiles fossiles des dépôts fluvio-lacustres crétacés du bassin à lignite de Fuveau. Bulletin de la Société Géologiques de France (ser. 2), 26:781-795.
Matzke, N.J. 2012. Founder-event speciation in BioGeoBEARS package dramatically improves likelihoods and alters parameter inference in Dispersal-Extinction-Cladogenesis (DEC) analyses. Frontiers of Biogeography, 4(suppl. 1):210.
Matzke, N.J. 2013a. BioGeoBEARS: BioGeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. http://CRAN.Rproject.org/package=BioGeoBEARS
Matzke, N.J. 2013b. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Frontiers of Biogeography, 5:242-248. https://doi.org/10.21425/f55419694
Matzke, N.J. 2014. BEASTmasteR: Automated conversion of NEXUS data to BEAST2 XML format, for fossil tip-dating and other uses. Online at PhyloWiki. http://phylo.wikidot.com/beastmaster
McDonald, A.T. 2011. The taxonomy of species assigned to Camptosaurus (Dinosauria: Ornithopoda). Zootaxa, 2783:52-68. https://doi.org/10.11646/zootaxa.2783.1.4
McDonald, A.T. 2012a. Phylogeny of basal iguanodonts (Dinosauria: Ornithischia): An update. PLoS ONE 7(5):e36745. https://doi.org/10.1371/journal.pone.0036745
McDonald, A.T. 2012b. The status of Dollodon and other basal iguanodonts (Dinosauria: Ornithischia) from the Lower Cretaceous of Europe. Cretaceous Research, 33:1-6. https://doi.org/10.1016/j.cretres.2011.03.002
McDonald, A.T., Barrett, P.M., and Chapman, S.D. 2010a. A new basal iguanodont (Dinosauria: Ornithischia) from the Wealden (Lower Cretaceous) of England. Zootaxa, 2569:1-43. https://doi.org/10.11646/zootaxa.2569.1.1
McDonald, A.T., Kirkland, J.I., DeBlieux, D.D., Madsen, S.K., and Cavin, J. 2010b. New basal iguanodonts from the Cedar Mountain Formation of Utah and the evolution of thumb-spiked dinosaurs. PLoS ONE, 5:e14075. https://doi.org/10.1371/journal.pone.0014075
McDonald, A.T., Wolfe, D.G., and Kirkland, J.I. 2010c. A new basal hadrosauroid (Dinosauria: Ornithopoda) from the Turonian of New Mexico. Journal of Vertebrate Paleontology, 30:799-812. https://doi.org/10.1080/02724631003763516
McDonald, A.T., Espilez, E., Mampel, L., Kirkland, J.I., and Alcala, L. 2012. An unusual new basal iguanodont (Dinosauria: Ornithopoda) from the Lower Cretaceous of Teruel, Spain. Zootaxa, 3595:61-76. https://doi.org/10.11646/zootaxa.3595.1.3
Miall, A.D., Catuneanu, O., Vakarelov, B.K., and Post, R. 2008. The western interior basin. Sedimentary Basins of the World, 5:329-362. https://doi.org/10.1016/s1874-5997(08)00009-9
Milner, A.R. and Norman, D.B. 1984. The biogeography of advanced ornithopod dinosaurs (Archosauria: Ornithischia)―a cladistic-vicariance model, p. 145-150. In: Reif, W.-E. and Westphal, F. (eds.), Third Symposium on Mesozoic Terrestrial Ecosystems. Attempto Verlag, Tübingen.
Molnar, R.E. 1996. Observations on the Australian ornithopod dinosaur, Muttaburrasaurus. Memoirs-Queensland Museum, 39:639-652.
Nopcsa, F. 1902. Dinosaurierreste aus Siebenbürgen II. (Schädelreste von Mochlodon). Mit einem Anhange: zur Phylogenie der Ornithopodiden. Denkschriften der königlichen Akademie der Wissenschaften, Mathematisch‐Naturwissenschaftlichen Klasse, 72:149-175.
Norman, D.B. 1980. On the ornithischian dinosaur Iguanodon bernissartensis from the Lower Cretaceous of Bernissart (Belgium). Institut Royal des Sciences Naturelles de Belgique Memoire, 178:1-103.
Norman, D.B. 1984. A systematic reappraisal of the reptile order Ornithischia, p. 157-162. In: Reif, W.-E. and Westphal, F. (eds.), Third Symposium on Mesozoic Terrestrial Ecosystems. Attempto Verlag, Tübingen.
Norman, D.B. 1986. On the anatomy of Iguanodon atherfieldensis (Ornithischia: Ornithopoda). Bulletin-Institut Royal des Sciences Naturelles de Belgique. Sciences de la Terre, 56:281-372.
Norman, D.B. 2002. On Asian ornithopods (Dinosauria: Ornithischia). 4. Probactrosaurus Rozhdestvensky, 1966. Zoological Journal of the Linnean Society, 136:113-144. https://doi.org/10.1046/j.1096-3642.2002.00027.x
Norman, D.B. 2004. Basal Iguanodontia, p. 413-437. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.), The Dinosauria (2nd ed.). University of California Press, Berkeley. https://doi.org/10.1525/california/9780520242098.003.0022
Norman, D.B. 2010. A taxonomy of iguanodontians (Dinosauria: Ornithopoda) from the lower Wealden Group (Cretaceous: Valanginian) of southern England. Zootaxa, 2489:47-66. https://doi.org/10.11646/zootaxa.2489.1.3
Norman, D.B. 2013. On the taxonomy and diversity of Wealden iguanodontian dinosaurs (Ornithischia: Ornithopoda). Revue de Paléobiologie, 32:385-404.
Norman, D.B. 2015. On the history, osteology, and systematic position of the Wealden (Hastings group) dinosaur Hypselospinus fittoni (Iguanodontia: Styracosterna). Zoological Journal of the Linnean Society, 173:92-189. https://doi.org/10.1111/zoj.12193
Norman, D.B., Sues, H.D., Witmer, L.M., and Coria, R.A. 2004. Basal Ornithopoda, p. 393-412. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.), The Dinosauria (2nd ed.). University of California Press, Berkeley.
https://doi.org/10.1525/california/9780520242098.003.0021
Novas, F.E., Cambiaso A.V., and Ambrosio A. 2004. A new basal iguanodontian (Dinosauria, Ornithischia) from the Upper Cretaceous of Patagonia. Ameghiniana, 41:75-82.
O'Reilly, J., Donoghue, P., Dos Reis, M., and Yang, Z. 2014. Evaluating the performance of node versus tip based fossil calibration of the molecular clock. Journal of Vertebrate Paleontology, 34(supplement):199.
Osborn, H.F. 1906. Tyrannosaurus, Upper Cretaceous carnivorous dinosaur: (second communication). Bulletin of the American Museum of Natural History, 22:281-296.
Osborn, H.F. 1912. Integument of the iguanodont dinosaur Trachodon. Memoirs of the American Museum of Natural History, New Series, 1:33-54.
Parks, W.A. 1922. Parasaurolophus walkeri: a new genus and species of crested trachodont dinosaur. University of Toronto Studies, Geological Series, 13:1-32.
Parks, W.A. 1926. Thescelosaurus warreni, a new species of orthopodous dinosaur from the Edmonton Formation of Alberta. University of Toronto Studies, Geological Series, 21:1-42.
Paul, G.S. 2007. Turning the old into the new: a separate genus for the gracile iguanodont from the Wealden of England, p. 69-77. In Carpenter, K. (ed.), Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs. Indiana University Press, Bloomington.
https://doi.org/10.2307/j.ctt1zxz1md.9
Paul, G.S. 2008. A revised taxonomy of the iguanodont dinosaur genera and species. Cretaceous Research, 29(2):192-216. https://doi.org/10.1016/j.cretres.2007.04.009
Paul, G. 2012. Notes on the rising diversity of iguanodont taxa, and iguanodonts named after Darwin, Huxley, and evolutionary science. Actas V Jornadas Internacionales sobre Paleontología de Dinosaurios y su Entorno, Salas de los Infantes, Burgos, 121-131.
Perle, A., Maryańska, T., and Osmólska, H. 1982. Goyocephale lattimorei gen. et sp. n., a new flat-headed pachycephalosaur (Ornithischia, Dinosauria) from the Upper Cretaceous of Mongolia. Acta Palaeontologica Polonica, 27(1-4):115-127.
Prieto-Márquez, A. 2012. The skull and appendicular skeleton of Gryposaurus latidens, a saurolophine hadrosaurid (Dinosauria: Ornithopoda) from the early Campanian (Cretaceous) of Montana, USA. Canadian Journal of Earth Sciences, 49:510-532.
https://doi.org/10.1139/e11-069
Prieto-Márquez, A. and Norell, M.A. 2010. Anatomy and relationships of Gilmoreosaurus mongoliensis (Dinosauria: Hadrosauroidea) from the Late Cretaceous of Central Asia. American Museum Novitates, 3694:1-49. https://doi.org/10.1206/3694.2
Prieto-Márquez, A. and Salinas, G.C. 2010. A re-evaluation of Secernosaurus koerneri and Kritosaurus australis (Dinosauria, Hadrosauridae) from the Late Cretaceous of Argentina. Journal of Vertebrate Paleontology, 30:813-837.
https://doi.org/10.1080/02724631003763508
Pyron, R.A. 2011. Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Systematic Biology, 60:1-16. https://doi.org/10.1093/sysbio/syr047
Rannala, B. and Yang, Z. 2007. Inferring speciation times under an episodic molecular clock. Systematic Biology, 56:453-466. https://doi.org/10.1080/10635150701420643
Ree, R.H. and Smith, S.A. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology, 57:4-14. https://doi.org/10.1080/10635150701883881
Rich, T.H. and Rich, P.V. 1988. A juvenile dinosaur brain from Australia. National Geographic Research, 4:148.
Rich, T.H. and Rich, P.V. 1989. Polar dinosaurs and biotas of the Early Cretaceous of southeastern Australia. National Geographic Research, 5:15-53.
Romer, A.S. 1956. Osteology of the Reptiles. University of Chicago Press, Chicago, Illinois.
Ronquist, F., Klopfstein, S., Vilhelmsen, L., Schulmeister, S., Murray, D.L., and Rasnitsyn, A.P. 2012. A total-evidence approach to dating with fossils, applied to the early radiation of the Hymenoptera. Systematic Biology, 61:973-999. https://doi.org/10.1093/sysbio/sys058
Ruiz-Omeñaca, J.I. 2011. Delapparentia turolensis nov. gen et sp., un nuevo dinosaurio iguanodontoideo (Ornithischia: Ornithopoda) en el Cretácico Inferior de Galve. Estudios geológicos, 67(1):83-110.
Scheetz, R.D. 1999. Osteology of Orodromeus makelai and the phylogeny of basal ornithopod dinosaurs. Ph.D. dissertation, Montana State University, Bozeman.
Scotese, C.R. 2014. Atlas of Earth History, Volume 1, Paleogeography. PALEOMAP Project, Arlington, Texas.
Seeley, H.G. 1887. Classification of the Dinosauria. Geological Magazine (Decade III), 5:45-46. https://doi.org/10.1017/s0016756800156006
Sereno, P.C. 1984. The phylogeny of the Ornithischia: a reappraisal, p. 219-226. In: Reif, W.-E. and Westphal, F. (eds.), Third Symposium on Mesozoic Terrestrial Ecosystems. Attempto Verlag, Tübingen.
Sereno, P.C. 1986. Phylogeny of the bird-hipped dinosaurs (Order Ornithischia). National Geographic Research, 2:234-256.
Sereno, P.C. 1991. Lesothosaurus, “fabrosaurids,” and the early evolution of Ornithischia. Journal of Vertebrate Paleontology, 11:168-197. https://doi.org/10.1080/02724634.1991.10011386
Sereno, P.C. 1998. A rationale for phylogenetic definitions, with application to the higher level taxonomy of Dinosauria. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 210:41-83. https://doi.org/10.1127/njgpa/210/1998/41
Sereno, P.C. 1999. The evolution of dinosaurs. Science, 284:2137-2147. https://doi.org/10.1126/science.284.5423.2137
Sereno, P. C. 2005. Stem Archosauria–TaxonSearch. http://www.taxonsearch.org/Archive/stem-archosauria-1.0.php
Sereno, P.C. 2007. Logical basis for morphological characters in phylogenetics. Cladistics, 23:1-23. https://doi.org/10.1111/j.1096-0031.2007.00161.x
Shibata, M., Jintasakul, P., and Azuma, Y. 2011. A new iguanodontian dinosaur from the Lower Cretaceous Khok Kruat Formation, Nakhon Ratchasima in northeastern Thailand. Acta Geologica Sinica‐English Edition, 85:969-976.
Simmons, M.P. 2012. Misleading results of likelihood‐based phylogenetic analyses in the presence of missing data. Cladistics, 28:208-222. https://doi.org/10.1111/j.1096-0031.2011.00375.x
Simmons, M.P. 2014. A confounding effect of missing data on character conflict in maximum likelihood and Bayesian MCMC phylogenetic analyses. Molecular Phylogenetics and Evolution, 80:267-280. https://doi.org/10.1016/j.ympev.2014.08.021
Spencer, M.R. 2013. Phylogenetic and Biogeographic Assessment of Ornithischian Diversity Throughout the Mesozoic: A Species-Level Analysis from Origin to Extinction. Ph.D. dissertation, University of Iowa, Iowa City.
Stadler, T., Kühnert, D., Bonhoeffer, S., and Drummond, A.J. 2013. Birth-death skyline plot reveals temporal changes of epidemic spread in HIV and Hepatitis C virus (HCV). Proceedings of the National Academy of Sciences, 110:228-233.
https://doi.org/10.1073/pnas.1207965110
Sternberg, C.M. 1937. A classification of Thescelosaurus, with a description of a new species. Proceedings of the Geological Society of America, 1936:375.
Strong, E.E. and Lipscomb, D. 1999. Character coding and inapplicable data. Cladistics, 15:363-371. https://doi.org/10.1111/j.1096-0031.1999.tb00272.x
Sues, H.D. 1980. Anatomy and relationships of a new hypsilophodontid dinosaur from the Lower Cretaceous of North America. Palaeontographica, Abteilung A: Paläozoologie - Stratigraphie, 169:51-72.
Sues, H.D. and Averianov, A. 2009. A new basal hadrosauroid dinosaur from the Late Cretaceous of Uzbekistan and the early radiation of duck-billed dinosaurs. Proceedings of the Royal Society B: Biological Sciences, 276:2549-2555.
https://doi.org/10.1098/rspb.2009.0229
Swofford, D.L., Waddell, P.J., Huelsenbeck, J.P., Foster, P.G., Lewis, P.O., and Rogers, J.S. 2001. Bias in phylogenetic estimation and its relevance to the choice between parsimony and likelihood methods. Systematic Biology, 50:525-539.
https://doi.org/10.1080/106351501750435086
Taquet, P. and Russell, D.A. 1999. A massively constructed iguanodont from Gadoufaoua, Lower Cretaceous of Niger. Annales de Paléontologie, 85:85-96. https://doi.org/10.1016/s0753-3969(99)80009-3
Upchurch, P., Hunn, C.A., and Norman, D. B. 2002. An analysis of dinosaurian biogeography: evidence for the existence of vicariance and dispersal patterns caused by geological events. Proceedings of the Royal Society B, 269:613-621. https://doi.org/10.1098/rspb.2001.1921
Varricchio, D.J., Martin, A.J., and Katsura, Y. 2007. First trace and body fossil evidence of a burrowing, denning dinosaur. Proceedings of the Royal Society of London B: Biological Sciences, 274:1361-1368. https://doi.org/10.1098/rspb.2006.0443
Veevers, J.J. 2004. Gondwanaland from 650-500 Ma assembly through 320 Ma merger in Pangea to 185-100 Ma breakup: Supercontinental tectonics via stratigraphy and radiometric dating. Earth-Science Reviews, 68(1):1-132. https://doi.org/10.1016/j.earscirev.2004.05.002
Wagner, J.R. 2004. The phylogenetic nomenclature of ornithischian dinosaurs (Vertebrata: Reptilia) and a consideration of the difficulties in converting the names of extinct clades, p. 28. First International Phylogenetic Nomenclature Meeting, Paris.
Wang, X. and Xu, X. 2001. A new iguanodontid (Jinzhousaurus yangi gen. et sp. nov.) from the Yixian Formation of western Liaoning, China. Chinese Science Bulletin, 46:1669-1672. https://doi.org/10.1007/bf02900633
Wang, X., Pan, R., Butler, R.J., and Barrett, P.M. 2010. The postcranial skeleton of the iguanodontian ornithopod Jinzhousaurus yangi from the Lower Cretaceous Yixian Formation of western Liaoning, China. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 101:135-159. https://doi.org/10.1017/s1755691010009266
Weishampel, D.B. and Heinrich, R.E. 1992. Systematics of Hypsilophodontidae and basal Iguanodontia (Dinosauria: Ornithopoda). Historical Biology, 6:159-184. https://doi.org/10.1080/10292389209380426
Weishampel, D.B. and Horner, J.R. 1986. The hadrosaurid dinosaurs from the Iren Dabasu Fauna (People's Republic of China, Late Cretaceous). Journal of Vertebrate Paleontology, 6:38-45. https://doi.org/10.1080/02724634.1986.10011597
Weishampel, D.B., Norman, D.B., and Grigorescu, D. 1993. Telmatosaurus transsylvanicus from the Late Cretaceous of Romania: the most basal hadrosaurid dinosaur. Palaeontology, 36:361-385.
Weishampel, D.B., Jianu, C.-M., Csiki, Z., and Norman, D.B. 2003. Osteology and phylogeny of Zalmoxes (n.g.), an unusual euornithopod dinosaur from the Latest Cretaceous of Romania. Journal of Systematic Palaeontology, 1(2):65-123.
https://doi.org/10.1017/s1477201903001032
Wiens, J.J. and Moen, D.S. 2008. Missing data and the accuracy of Bayesian phylogenetics. Journal of Systematics and Evolution, 46:307-314.
Wiens, J.J. and Morrill, M.C. 2011. Missing data in phylogenetic analysis: reconciling results from simulations and empirical data. Systematic Biology, 60:719-731. https://doi.org/10.1093/sysbio/syr025
Wilkinson, M. 2003. Missing entries and multiple trees: instability, relationships, and support in parsimony analysis. Journal of Vertebrate Paleontology, 23:311-323. https://doi.org/10.1671/0272-4634(2003)023[0311:meamti]2.0.co;2
Williston, S.W. 1905. The Hallopus, Baptanodon, and Atlantosaurus Beds of Marsh. The Journal of Geology, 13:338-350. https://doi.org/10.1086/621233
Winkler, D.A., Murry, P.A., and Jacobs, L.L. 1997. A new species of Tenontosaurus (Dinosauria: Ornithopoda) from the Early Cretaceous of Texas. Journal of Vertebrate Paleontology, 17:330-348. https://doi.org/10.1080/02724634.1997.10010978
Wright, A.M. and Hillis, D.M. 2014. Bayesian analysis using a simple likelihood model outperforms parsimony for estimation of phylogeny from discrete morphological data. PLoS ONE 9: e109210. https://doi.org/10.1371/journal.pone.0109210
Wu, W.H. and Godefroit, P. 2012. Anatomy and relationships of Bolong yixianensis, an Early Cretaceous iguanodontid dinosaur from western Liaoning, China, p. 293-333 In Godefroit, P. (ed.), Bernissart dinosaurs and Early Cretaceous terrestrial ecosystems. Indiana University Press, Bloomington.
Wu, W.H., Godefroit, P., and Hu, D.Y. 2010. Bolong yixianensis gen. et sp. nov.: a new iguanodontoid dinosaur from the Yixian Formation of western Liaoning, China. Geology and Resources, 19:127-133.
Xu, X. and Pol, D. 2014. Archaeopteryx, paravian phylogenetic analyses, and the use of probability-based methods for palaeontological datasets. Journal of Systematic Palaeontology, 12:323-334. https://doi.org/10.1080/14772019.2013.764357
You, H., Ji, Q., Li, J., and Li, Y. 2003. A new hadrosauroid dinosaur from the mid‐Cretaceous of Liaoning, China. Acta Geologica Sinica (English Edition), 77:148-154. https://doi.org/10.1111/j.1755-6724.2003.tb00557.x
You, H., Ji, Q., and Li, D. 2005. Lanzhousaurus magnidens gen. et sp. nov. from Gansu Province, China: the largest-toothed herbivorous dinosaur in the world. Geological Bulletin of China, 24:785-794.
You, H., Li, D., and Liu, W. 2011. A new hadrosauriform dinosaur from the Early Cretaceous of Gansu Province, China. Acta Geologica Sinica (English Edition), 85(1):51-57. https://doi.org/10.1111/j.1755-6724.2011.00377.x

