 A new genus of ptyctodont (Placodermi) from the Late Devonian of Baltic area
A new genus of ptyctodont (Placodermi) from the Late Devonian of Baltic area
Article number: 22.2.23
https://doi.org/10.26879/890
Copyright Society for Vertebrate Paleontology, May 2019
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 May 2018. Acceptance: 18 March 2019
ABSTRACT
The ptyctodont fish first referred to as Chelyophorus pskovensis Obruchev, 1947 from the Early Frasnian (Late Devonian) of Velikaya River, Pskov Region, Russia, is redescribed following the collection of additional materials from the contemporaneous Meeksi Mill outcrop, Estonia; Piskovichi and Snetnaya Gora outcrops, Russia and is here referred to Meeksiella gen. nov. With the exception of an articulated skull roof, the fossils occur as three dimensionally preserved isolated plates, and this has allowed accurate reconstruction of the dermal skeleton. A phylogenetic analysis resolves Meeksiella pskovensis gen. et sp. nov. within a previously recovered cluster of taxa which includes Ctenurella from Europe and Austroptyctodus from Western Australia, demonstrating global distribution of this clade during the Late Devonian.
Kate Trinajstic. School of Molecular and Life Sciences, Curtin University, Bently, Western Australia 6845, Australia. K.Trinajstic@curtin.edu.au
John Long. College of Science and Engineering, Flinders University, Sturt Rd, Bedford Park South Australia 5042, Australia; Research School of Earth Sciences, The Australian National University, Canberra, 0200, Australia. john.long@flinders.edu.au
Alexander O. Ivanov. Institute of Earth Sciences, St. Petersburg State University, St. Petersburg, 199178, Russia; Kazan Federal University, Kazan, Russia. IvanovA-Paleo@yandex.ru
Elga Mark-Kurik. Department of Geology, Tallinn University of Technology, Tallinn, 19086 Estonia (posthumous)
Keywords: new genus; Placoderm; Ptyctodont; phylogeny; Frasnian; Estonia; Pskov Region
Final citation: Trinajstic, Kate, Long, John A., Ivanov, Alexander O., and Mark-Kurik, Elga. 2019. A new genus of ptyctodont (Placodermi) from the Late Devonian of Baltic area. Palaeontologia Electronica 22.2.23A 1-19. https://doi.org/10.26879/890
palaeo-electronica.org/content/2019/2490-a-new-baltic-ptyctodont
Copyright: May 2019 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0/creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/A0E8E9EA-5A21-4F1F-9E5D-BAD240B4981F
INTRODUCTION
Ptyctodont remains are common in Middle to Late Devonian sediments globally, however, they occur mostly as isolated tooth plates, with body fossils being rare. Complete, articulated specimens showing three-dimensional preservation are even rarer, with three taxa known from the early Frasnian Gogo Formation in Western Australia (Austroptyctodus and Campbellodus (Miles and Young, 1977; Long, 1988, 1997) and Materpiscis (Long et al., 2008; Long and Trinajstic, 2010; Trinajstic et al., 2012), and a single taxon, Chelyophorus (Agassiz, 1844) from the Famennian of the Oryol Region, Russia. Although preserved two dimensionally, detailed morphology of several taxa has been described for Ctenurella (Ørvig, 1960), Rhynchodus (Newberry, 1873), Ptyctodopsis (Denison, 1985) and Rhamphodopsis (Watson, 1938). Taxa identified from isolated plates, preserved in 3D, include Denisonodus (Johnson and Elliott, 1996) from North America, Kimbryanodus (Trinajstic and Long, 2009) from Western Australia and Desmoporella (Ørvig, 1971) from Germany. Despite the low number of specimens, the excellent preservation found in these rare examples means that the general morphology of the pytctodonts is well known; however, until recently there remained some controversy in the orientation of parts of the braincase and branchial skeleton (Young, 1986; Goujet, 1984; Long, 1997; Carr et al., 2009).
Additional, more complete specimens of Campbellodus (Long, 1988) and Austroptyctodus (Long, 1997) led to new reconstructions of the head shield and braincase of ptyctodonts (Long, 1997). This revision determined that the dermal plates of the skull roof did not abut in all taxa, and the orbital ossification was repositioned in a horizontal position (Long, 1997), rather than the vertical orientation proposed by Miles and Young (1977). A new taxon, Materpiscis, and another new specimen of Austroptyctodus both with the perichondral ossification of the skull preserved in situ resulted in a new reconstruction of the endoskeleton of the jaw (Trinajstic et al., 2012) and confirmed the presence of a separate opercular cartilage and hyomandibular bone (Young, 1986; Long, 1997 contra Goujet, 1984; Carr et al., 2009). In addition, these two specimens provided new morphological information of the braincase showing separated ethmoid, orbital and occipital ossifications (Trinajstic et al., 2012), the occipital fused along the midline and confirmed the vertical orientation of the orbital ossification (Miles and Young, 1977) contra Long (1997). Re-examination of the postcranial skeleton in Campbellodus, Ctenurella and Rhamphodopisis revealed the presence of dermal pelvic plates in addition to the perichondral elements of the pelvic girdle (Trinajsic et al., 2015). Following these discoveries, it was determined that the female basal plates and male claspers were separate from the pelvic girdle and fins, a condition common to all placoderms in which intromittent organs are known (Trinajstic et al., 2015; Long et al., 2015).
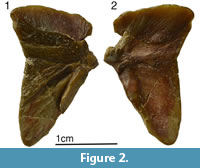
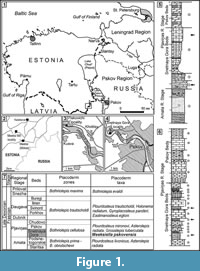 Here we described a new genus of ptyctodont from the Frasnian (Upper Devonian) sediments of the Baltic area, which is also referred to as the Main Devonian Field (Figure 1.1-1.4). The holotype (Figure 2.1-2.2), a single anterior dorsolateral (ADL) plate, was originally referred to the genus Chelyophorus (Obruchev, 1947). The author did not provide a detailed description of the new species only indicating that the ADL plate is longer and lower in comparison with the ADL plate of Chelyophorus verneuili Agassiz, 1844. Gross (1942) illustrated an upper tooth plate from the Kārļu Muiža (Karlsruhe) outcrop on Amata River, Latvia, which he named as Rhynchodus sp., but this specimen is now known to belong to the new ptyctodont genus described herein. Obrucheva (1983) suggested that Ctenurella, or a closely related taxon, is present in the Snetnaya Gora Beds of Pskov Region and Estonia and attributed the specimen to the genus Ctenurella. Previously the only detailed description was of the shoulder girdle and associated endoskeleton (Mark-Kurik et al., 1991) but the species was never formally described.
Here we described a new genus of ptyctodont from the Frasnian (Upper Devonian) sediments of the Baltic area, which is also referred to as the Main Devonian Field (Figure 1.1-1.4). The holotype (Figure 2.1-2.2), a single anterior dorsolateral (ADL) plate, was originally referred to the genus Chelyophorus (Obruchev, 1947). The author did not provide a detailed description of the new species only indicating that the ADL plate is longer and lower in comparison with the ADL plate of Chelyophorus verneuili Agassiz, 1844. Gross (1942) illustrated an upper tooth plate from the Kārļu Muiža (Karlsruhe) outcrop on Amata River, Latvia, which he named as Rhynchodus sp., but this specimen is now known to belong to the new ptyctodont genus described herein. Obrucheva (1983) suggested that Ctenurella, or a closely related taxon, is present in the Snetnaya Gora Beds of Pskov Region and Estonia and attributed the specimen to the genus Ctenurella. Previously the only detailed description was of the shoulder girdle and associated endoskeleton (Mark-Kurik et al., 1991) but the species was never formally described.
The above specimens, in addition to new material from the head and trunk shield and perichondral ossifications of the braincase, all preserved in three dimensions, indicate that these remains belong to a new genus Meeksiella pskovensis gen. et comb. nov. This work includes a description of the new taxon and places this new genus within a phylogenetic analysis of the Ptyctodontida.
GEOLOGICAL SETTING AND AGE OF THE FAUNA
The north-western region of the East European Platform is also called the Main Devonian Field, due to the large exposure of Devonian deposits. It includes regions of the northern part of Belarus, Lithuania, Latvia, Estonia and the north-west part of Russia (Pskov, Leningrad, Novgorod and Vologda regions). This area occupied an equatorial palaeolatitude and was deposited along the northern part of a shallow-water, epi-cratonic basin in the Devonian time. The Frasnian of the Main Devonian Field is subdivided into the Amata, Pļaviņas, Dubnik, Daugava, Snezha and Prilovat regional stages (Figure 1.7). The position of the Givetian/Frasnian boundary on the East European Platform has not been determined precisely. However, the Amata Regional Stage probably belongs to the Frasnian (Ivanov et al., 2005). The Pļaviņas Regional Stage has been subdivided into the Snetnaya Gora, Pskov and Chudovo beds (or substages) (Figure 1.7). The Amata Regional Stage and Snetnaya Gora Beds have been dated as the Early Frasnian based on the presence of Polygnathus lanei (Ivanov et al., 2005; Mark-Kurik and Poldvere, 2012), which is equivalent to the M. falsiovalis - early Pa. transitans zones of the Standard conodont zonations (Ziegler and Sandberg, 1990).
The Snetnaya Gora Beds that yielded the described ptyctodont remains are represented by light marl, dolomitic limestone and dolomite with clay-rich intercalations. The deposits in the stratotype area are up to 10 m in thickness. The fossils reported from the Snetnaya Gora Beds include abundant and diverse vertebrates, rare brachiopods, conodonts, ostracods, gastropods, algae remains and diverse trace fossils (Sorokin, 1978; Ivanov et al., 2005).
The deposits of Snetnaya Gora Beds or their analogous extends from Lithuania, central and eastern Latvia, southern Estonia, Pskov and Leningrad regions to Lake Onega in Vologda Region, Russia. The so called ‘Snetnaya Gora’ vertebrate assemblage extends into the overlying Pskov Beds. The Bothriolepis cellulosa placoderm Zone belongs to the Snetnaya Gora - Pskov interval (Figure 1.7). This assemblage was first described by Gross (1933, 1941,1942) and has yielded over 20 species of psammosteid agnathans, placoderm, acanthodian, actinopterygian and sarcopterygian fishes (Ivanov, 1990). Most of the vertebrate taxa such as: Psammosteus maeandrinus Agassiz 1844; Plourdosteus mironovi Obruchev 1933; B. cellulosa (Pander in Keyserling 1846), Grossilepis tuberculata Gross 1941; Haplacanthus perseensis Gross 1942; Latvius grenwingki Gross 1933; Eusthenopteron saevesoederberghi Jarvik 1944; Rhinodipterus secans Gross 1956 and Moythomasia perforata Gross 1942, occur in that interval. However, Meeksiella pskovensis Obruchev 1947; Strunius rolandi Gross 1956 and Griphognathus minutidens Gross 1956; were recorded only in the Snetnaya Gora Beds (Figure 1.7).
The remains of the new ptyctodont genus have been collected from the Piskovichi, Snetnaya Gora and Slavyanskie Klyuchi outcrops in Pskov Region, Russia; Meeksi Mill outcrop, Meeksi Creek, Võru County, Estonia; Īļaku iezis and Kārļu muiža, Amata River, Latvia (Figure 1.1-1.4). The holotype and most of the described specimens come from the Piskovichi and Snetnaya Gora outcrops. The Piskovichi outcrop (Figure 1.5) is located on the right bank of the Velikaya River, near the village of Piskovichi, 8 km to the north-west from Pskov city (Figure 1.3). The deposits of the upper part of the Amata Regional Stage and Snetnaya Gora Beds (Figure 1.5) are exposed in a cliff, and the beds are approximately 15 m high and 150 m long. The Snetnaya Gora Beds are represented by dolomite, clay-rich dolomite, dolomitic marl and carbonate clay (Ivanov et al., 2005). The numerous remains of the new ptyctodont have been found in two levels of the outcrop; in the greenish and violet grey dolomite and, in the light grey dolomitic marl (Figure 1.5). The Snetnaya Gora outcrop (Figure 1.6) is situated on the right bank of Velikaya River (Figure 1.4), in a north-western suburb of Pskov city, and comprises a cliff more than 15 m high and 200 m long. The Snetnaya Gora and Pskov beds are exposed there. The deposits of the Snetnaya Gora Beds alternate between dolomites, dolomitic marls, marls with the intercalations of clays, siltstones and dolomitic limestones. The remains of the new ptyctodont occur in the grey dolomitic marl in three horizons of the outcrop (Figure 1.6). Three plates have also been found in the clay-rich limestones from the Slavyanskie Klyuchi outcrop, near the town of Izborsk in Pskov Region (Ivanov et al., 2005).
Some plates of the new ptyctodont genus have been collected in the dolomitic limestones of Meeksi Mill outcrop, Meeksi Creek (Figure 1.2), as well as of Orava and Loosi outcrops, south-eastern Estonia. Rare remains of that taxon are recorded in the marl of Koknese Member (analogous of Snetnaya Gora Beds) from two outcrops on Amata River, Īļaku iezis and Kārļu muiža, in Latvia. The specimens which, were collected from the latter outcrop were mentioned by Gross (1942).
MATERIALS AND METHODS
Material from resistant limestone or dolomite beds of the Snetnaya Gora Beds were either extracted through disaggregating the rock in 10% acetic or 5% formic acids; other specimens were mechanically prepared.
The internal structure of skull roof was reconstructed using Bruker-microCT SkyScan 1172 (the Center for Geo-Environmental Research and Modeling “GEOMODEL”, Research Park of St. Petersburg State University). The specimen (GIT196-14) was scanned by applying different parameters according to the sample type at a voltage of 40 - 100 kV and a current of 100 - 250 μA, with a copper and aluminium filter for a half rotation of 180° at the highest camera resolution. The images of virtual cross-sections were generated from the 3D reconstructions using software DataViewer, CTAn and CTVox.
The described specimens are housed in the: Borissiak Palaeontological Institute of the Russian Academy of Sciences, Moscow; Department of Geology at Tallinn University of Technology; Palaeontological Museum of St. Petersburg State University and the Swedish Museum of Natural History, Stockholm.
Abbreviations
Anatomical. aorb, area for orbital articulation; ar.cn, articular condyle; arta, area for articular condyle; Ce, central plate; kllc, main lateral line canal; oaMD, overlap area of median dorsal plate; M, marginal plate; Nu, nuchal plate; oa ADL, overlap area anterodorsolateral plate; oa AL, overlap area anterior lateral plate; oa PNu, overlap area for the paranuchal plate; oa PRO, overlap area fro the preorbital plate; om, orbital margin; P, pineal plate; p. gr, posterior groove on the median dorsal plate; PNu, paranuchal plate; pp, posterior pitline; ppl. posterior pitline canal on the central plate; ppr, posterior process of skull roof; pr, ventral process; PrO, preorbital plate; PtO, postorbital plate; ri, ridge; soc, supraorbital sensory canal; sp.n, spiracular notch; vpr, ventral process.
Institutions. GIT, Department of Geology at Tallinn University of Technology, Tallinn, Estonia; MNHN ARD, Muséum national d’Histoire naturelle, Paris, France; MNHN URP, Agassiz collection, Muséum national d’Histoire naturelle, Paris, France; NHM P., Natural History Museum, London, United Kingdom; PIN, Borissiak Palaeontological Institute of the Russian Academy of Sciences, Moscow, Russia; PM SPU, Palaeontological Museum of St. Petersburg State University, St Petersburg, Russia; SUI, Museum of Natural History Geological Repository, University of Iowa, Iowa City, USA; SMNH, Swedish Museum of Natural History, Stockholm, Sweden; WAM, Western Australian Museum, Perth, Australia.
Comparative Material
Austroptyctodus gardineri Miles and Young, 1977; (revised Long 1997), holotype WAM 70.4.253 additional material WAM 86.9.662; NHM P50906, NHM P50908, NHM P50910, NHM P57654, NHM P57655; Campbellodus decipiens Miles and Young, 1977, holotype WAM 86.9.672, additional material WAM 70.4.252, WAM 95.6.112, NHM P50907; Chelyophorus verneuili Agassiz 1844, URP.2, URP.4-6, URP.10-14, NHM P7483; Ctenurella gladbachensis Ørvig 1960 ARD232, ARD230a and b, NHM P48255-6, NHM P48238-9, NHM P48249, NHM P53605, NHM P53574; Kimbryanodus williamburyensis Trinajstic and Long, 2009, holotype WAM 99.8.1, additional material WAM 99.8.1 - WAM 99.8.20; Materpiscis attenboroughi Long et al., 2008 (revised Trinajstic et al., 2012), holotype WAM 07.12.1; Ptyctodopsis menzeli Denison, 1985, holotype SUI 33860; Rhamphodopsis threiplandi Watson, 1938, NHMP48664,NHMP52979,NHMP52954; Rhynchodus tetrodon Newberry, 1873, NHM P48771-2.
SYSTEMATIC PALAEONTOLOGY
Subclass PLACODERMI Mc’Coy, 1848
Order PTYCTODONTIDA Gross, 1932
Family PTYCTODONTIDAE Woodward, 1891
Genus Meeksiella gen. nov.
(Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8)
zoobank.org/7B5A1FCE-805D-4C2A-8289-35B6F75D8ED0
1947 Chelyophorus Obruchev, p. 201, Pl. LIII, fig. 4.
1981 Ctenurella Obrucheva, p. 163, fig. 2 D.
 Type species. Chelyophorus pskovensis Obruchev, 1947.
Type species. Chelyophorus pskovensis Obruchev, 1947.
Etymology. The generic name refers to the locality Meeksi Mill in Estonian (near the town of Miikse) where several specimens were found (Figure 1.2).
Distribution. Lower Frasnian, Main Devonian Field, East European Platform.
Diagnosis. same for species by monotypy.
Meeksiella pskovensis (Obruchev 1947) comb. nov.
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7
1942 Rhynchodus sp.sp., Gross, S. 425, Abb. 14 A.
1947 Chelyophorus pskovensis sp. nov., Obruchev, p. 201, Pl. LIII, fig. 4.
1981 Ctenurella pskovensis (Obruchev), Obrucheva, p. 163, fig. 2 D.
1991 Ctenurella pskovensis (Obruchev), Mark-Kurik, Ivanov, Obrucheva, p. 160-163, Pl. I, figs. 4, 5.
1992 Ctenurella pskovensis (Obruchev), Ivanov, p. 133, Pl. II, figs. 4, 7.
2005 Ctenurella pskovensis (Obruchev), Ivanov et al., p. 36, 37, figs. 22, 25 A-C.
Holotype. A single anterior dorsolateral plate (Figure 2), PIN 54/247 (collected by D.V. Obruchev in 1929).
Type locality and horizon. Piskovichi site (Figure 1.3), Velikaya River, Pskov, Russia; Snetnya Gora Beds, Pļaviņas Regional Stage, Early Frasnian.
Referred material. A partial specimen (GIT 196-14) comprising and articulated skull roof with associated orbital ossification, marginal (GIT 196-31) and post marginal plates. Numerous isolated plates: preorbital (GIT 196-16, GIT 196-17, GIT 196-18, GIT 196-19, GIT 196-33, GIT 196-34); postorbital (GIT 196-35, GIT 196-36, GIT 196-37, GIT 196-38); central (GIT 196-23, GIT 196-24, GIT 196-39, GIT 196-40); nuchal (GIT 196-15); paranuchal (GIT 196-31, GIT 196-32, GIT 196-41); marginal (GIT 196-20, GIT 196-42, GIT 196-43, PM SPU 3-15); lower tooth (GIT 196-26, GIT 196-27, GIT 196-44); upper tooth (GIT 196-25, GIT 196-45, GIT 196-46, SMNH P.4546) quadrate (GIT 196-56), median dorsal (GIT 196-3, GIT 196-4, GIT 196-11, GIT 196-12, GIT 196-13, GIT 196-22, GIT 196-29, GIT 196-30); anterior dorsolateral (GIT 196-7, GIT 196-8, GIT 196-47, GIT 196-53, PIN 54/248, PIN 54/249), anterior lateral (GIT 196-1, GIT 196-2, GIT 196-9, GIT 196-10, GIT 196-28), anterior median ventral (GIT 196-5,), anterior ventrolateral (GIT 196-6), dermal clasper J-element (GIT 196-50).
Locality and horizon. Most specimens are from the Piskovichi and Snetnaya Gora outcrops (Figure 1.5 - 1.6), Pskov Region, Russia (Figure 1.3 - 1.4), some specimens from Meeksi Mill outcrop, Vastseliina, Võru County, Estonia (Figure 1.2), and few specimens from Īļaku iezis and Kārļu muiža, Amata River, Latvia. Snetnaya Gora Beds (or Koknese Member), Pļaviņas Regional Stage, Lower Frasnian, Late Devonian.
Revised diagnosis. Shallow convex skull roof longer than wide, broadest across the postorbital-paranuchal junction. Skull roof pattern shows medial contact between the preorbital plates, with a deep median U-shaped embayment for the pineal plate. Posteriorly tapering nuchal plate, anterior point separates paired central plates, straight posterior margin forms back of the headshield in dorsal view however central plate underlap areas connect beneath the posterior margin of the nuchal plate; paranuchal plate is approximately the same size as the postorbital plate and two-thirds the length of the large preorbital plate. Central plate with a well-developed posterior process. Paranuchal and postorbital plates with strong dorsolateral inflexion forming the dorsal portion of a deep cheek. Large marginal plate with slender lateral postorbital division, small median ventral process and dorsally irregular attachment margin to postorbital plate. Upper toothplate longer than deep, short anterior dorsal process and lacking an anterior ridge; lower toothplate narrows anteriorly and lacking a ventral process. Trunk shield with short, broad and relatively flat triangular median dorsal plate with a low posterior dorsal crest, anterior boss; anterior dorsolateral plate with a distinct dorsolateral inflexion and v-shaped insertion for the anterior lateral plate.
Description and Comparisons
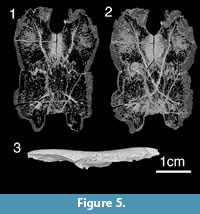
 Head shield. The head shield is rectangular in shape being longer than broad (Figure 3.1-3.2, Figure 4, Figure 5). Anteriorly the skull roof is slightly convex and posteriorly is strongly convex across the rostrocaudal axis (Figure 3.1, Figure 4.1-4.2). The skull roof bones all abut (Figure 6.1), as in Kimbryanodus (Figure 6.2) Ptyctodopsis (Figure 6.3) and Ctenurella (Figure 6.4). Anteromesially there is a narrow embayment for the pineal plate (Figure 3.1). Earlier photographs indicate the presence of the pineal plate in the articulated skull roof (GIT 196-14) but the plate now seems to have been misplaced. It was broadest posteriorly with an irregular anterior margin forming the mesial portion of the anterior skull roof margin (Figure 4.1) as in Ctenurella (Figure 6.4) and Rhamphodopsis. Specimen GIT 196-14 (Figure 3.1-3.2, Figure 4.1-4.2) shows fusion of part of the left margin of the nuchal to the left central plate, although isolated examples of both the nuchal (e.g., GIT 196-15) (Figure 3.7) and the left central plates (e.g., GIT 196-23) (Figure 3.11) in addition to scans of GIT 196-14 (Figure 5.1-5.2) in which the margins of the nuchal are visible, indicate that the fusion is pathological. The nuchal plate forms the mesial posterior margin of the skull roof (Figure 3.1, Figure 4, Figure 5) as in Kimbryanodus (Figure 6.2), Rhychodus (Figure 6.5), Campbellodus (Figure 6.6), Materpiscis (Figure 6.7) and Austroptyctodus (Figure 6.8). However, unlike these taxa, the underlap margins of the central plates abut under the nuchal plate (Figure 3.2, Figure 5.2). On the skull roof the ornament is concentrated on the outer margins of the plates and comprises elongated tubercles, joined to form radiating ridges, interspersed with a series of pits and grooves (Figure 3.1).
Head shield. The head shield is rectangular in shape being longer than broad (Figure 3.1-3.2, Figure 4, Figure 5). Anteriorly the skull roof is slightly convex and posteriorly is strongly convex across the rostrocaudal axis (Figure 3.1, Figure 4.1-4.2). The skull roof bones all abut (Figure 6.1), as in Kimbryanodus (Figure 6.2) Ptyctodopsis (Figure 6.3) and Ctenurella (Figure 6.4). Anteromesially there is a narrow embayment for the pineal plate (Figure 3.1). Earlier photographs indicate the presence of the pineal plate in the articulated skull roof (GIT 196-14) but the plate now seems to have been misplaced. It was broadest posteriorly with an irregular anterior margin forming the mesial portion of the anterior skull roof margin (Figure 4.1) as in Ctenurella (Figure 6.4) and Rhamphodopsis. Specimen GIT 196-14 (Figure 3.1-3.2, Figure 4.1-4.2) shows fusion of part of the left margin of the nuchal to the left central plate, although isolated examples of both the nuchal (e.g., GIT 196-15) (Figure 3.7) and the left central plates (e.g., GIT 196-23) (Figure 3.11) in addition to scans of GIT 196-14 (Figure 5.1-5.2) in which the margins of the nuchal are visible, indicate that the fusion is pathological. The nuchal plate forms the mesial posterior margin of the skull roof (Figure 3.1, Figure 4, Figure 5) as in Kimbryanodus (Figure 6.2), Rhychodus (Figure 6.5), Campbellodus (Figure 6.6), Materpiscis (Figure 6.7) and Austroptyctodus (Figure 6.8). However, unlike these taxa, the underlap margins of the central plates abut under the nuchal plate (Figure 3.2, Figure 5.2). On the skull roof the ornament is concentrated on the outer margins of the plates and comprises elongated tubercles, joined to form radiating ridges, interspersed with a series of pits and grooves (Figure 3.1).
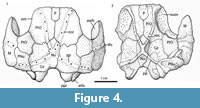 The preorbital plates (Figure 3.1-3.4, Figure 4, Figure 5.2) are sub rectangular in shape, and there is an interdigitated contact with the anterior margin of the central plate similar to that in Kimbryanodus (WAM 96.4.8) (Figure 6.2) and Ctenuralla (MNHN ARD 232) (Figure 6.4). Posterior to the opening for the pineal plate, the mesial margins of the left and right preorbital plates abut (Figure 3.1, Figure 4.1, Figure 5.1-5.2). The anterior part of the preorbital plate is relatively flat above the orbits (Figure 5.3) and like Materpiscis (WAM 07.12.1; fig. 6; Trinajstic et al., 2012) does not curve around the lateral margin of the skull roof. In dorsal view the path of the supraorbital sensory canal (soc) can be recognised as a series of a few large pores running diagonally across some plates (e.g., GIT 196-17, GIT 196-18; Figure 3.1, 3.3, Figure 4.1) although the number of pore canals varies and in GIT 196-14 they are completely overgrown (Figure 3.4). In visceral view the canal is preserved as bony tubes in all preorbital plates, as in most other ptyctodontids (Figure 3.2, Figure 4.2, Figure 5.1-5.2). The path of the sensory line canals is best determined by scans (Figure 5.2). The visceral surface of the preorbital plate is dominated by the supraorbital vault (Figure 3.2, Figure 4.2, Figure 5.3), which extends from the anterolateral corner of the plate posterolaterally and terminates at the posterolateral margin of the plate. The prominent preorbital and supraorbital ridge delineates the supraorbital vault (Figure 3.2, Figure 4.2, Figure 5.3) and is more prominent than those known for other ptyctodonts.
The preorbital plates (Figure 3.1-3.4, Figure 4, Figure 5.2) are sub rectangular in shape, and there is an interdigitated contact with the anterior margin of the central plate similar to that in Kimbryanodus (WAM 96.4.8) (Figure 6.2) and Ctenuralla (MNHN ARD 232) (Figure 6.4). Posterior to the opening for the pineal plate, the mesial margins of the left and right preorbital plates abut (Figure 3.1, Figure 4.1, Figure 5.1-5.2). The anterior part of the preorbital plate is relatively flat above the orbits (Figure 5.3) and like Materpiscis (WAM 07.12.1; fig. 6; Trinajstic et al., 2012) does not curve around the lateral margin of the skull roof. In dorsal view the path of the supraorbital sensory canal (soc) can be recognised as a series of a few large pores running diagonally across some plates (e.g., GIT 196-17, GIT 196-18; Figure 3.1, 3.3, Figure 4.1) although the number of pore canals varies and in GIT 196-14 they are completely overgrown (Figure 3.4). In visceral view the canal is preserved as bony tubes in all preorbital plates, as in most other ptyctodontids (Figure 3.2, Figure 4.2, Figure 5.1-5.2). The path of the sensory line canals is best determined by scans (Figure 5.2). The visceral surface of the preorbital plate is dominated by the supraorbital vault (Figure 3.2, Figure 4.2, Figure 5.3), which extends from the anterolateral corner of the plate posterolaterally and terminates at the posterolateral margin of the plate. The prominent preorbital and supraorbital ridge delineates the supraorbital vault (Figure 3.2, Figure 4.2, Figure 5.3) and is more prominent than those known for other ptyctodonts.
 The postorbital plate (PtO; Figure 4.1) is oval- to diamond-shaped and is approximately the same size as the paranuchal plate as in Materpicsis (Figure 6.7) and Austroptyctodus (Figure 6.8). There is a marked dorsolateral inflexion, and the plate is broken along the inflection point (Figure 3.5-3.6). The greater part of the plate forms the cheek, and there is a notch in the posterolateral margin, which forms a small postorbital fenestra between the paranuchal plate (Figure 3.5). This differs from the high narrow fenestral opening seen in Materpiscis, Kimbryanodus, Campbellodus and Austroptyctodus. In visceral and lateral views, a prominent ridge delineates the posterior portion of the supraorbital vault (Figure 3.2, Figure 5.3).
The postorbital plate (PtO; Figure 4.1) is oval- to diamond-shaped and is approximately the same size as the paranuchal plate as in Materpicsis (Figure 6.7) and Austroptyctodus (Figure 6.8). There is a marked dorsolateral inflexion, and the plate is broken along the inflection point (Figure 3.5-3.6). The greater part of the plate forms the cheek, and there is a notch in the posterolateral margin, which forms a small postorbital fenestra between the paranuchal plate (Figure 3.5). This differs from the high narrow fenestral opening seen in Materpiscis, Kimbryanodus, Campbellodus and Austroptyctodus. In visceral and lateral views, a prominent ridge delineates the posterior portion of the supraorbital vault (Figure 3.2, Figure 5.3).
The nuchal plate (Nu, Figure 3.1, 3.7, Figure 4, Figure 5.2) broadens slightly anteriorly and terminates in an anterior point, which extends between left and right preorbital plates. The posterior margin of the nuchal plates forms the mesial posterior margin of the headshield (Figure 3.1, Figure 4.1, Figure 5.1-5.2) as in Austroptyctodus (Figure 6.8), Campbellodus (Figure 6.6), Kimbryanodus (Figure 6.2), and Rhynchodus (Figure 6.5). The sensory lines are not clearly visible in dorsal view, however, scans clearly show their path (Figure 5.1-5.2), and in visceral view a prominent X-shaped raised tube of bone is present (Figure 3.2). Unlike Austroptyctodus, Campbellodus and Denisonodus, where the bony tubes end at the posterior corners of the Nu plate, in Meeksiella, Kimbryanodus and Materpiscis the bony tubes extend onto the central plates from just below the mid-section of the nuchal plate (Figure 3.2, Figure 5.1-5.2).
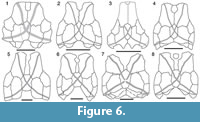 The central plates (Figure 3.1-2, 3.11, Figure 4.1, Figure 5) are shorter and broader than the preorbital plates, unlike in Ctenurella (Figure 6.4) and Austoptyctodus (Figure 6.8) in which the preorbital and central plates are approximately the same size. The anterior plate margin is irregular (Figure 3.1, Figure 4.1, Figure 5.1-5.2) and interdigitates with the posterior margin of the preorbital plate. There is a posterior process that contacts the paranuchal plate (Figure 3.1, Figure 4.1, Figure 5.1). The margin of the central plate, which is in contact with the paranuchal plate, is strongly convex in dorsal view where the skull roof bends down (Figure 3.5).
The central plates (Figure 3.1-2, 3.11, Figure 4.1, Figure 5) are shorter and broader than the preorbital plates, unlike in Ctenurella (Figure 6.4) and Austoptyctodus (Figure 6.8) in which the preorbital and central plates are approximately the same size. The anterior plate margin is irregular (Figure 3.1, Figure 4.1, Figure 5.1-5.2) and interdigitates with the posterior margin of the preorbital plate. There is a posterior process that contacts the paranuchal plate (Figure 3.1, Figure 4.1, Figure 5.1). The margin of the central plate, which is in contact with the paranuchal plate, is strongly convex in dorsal view where the skull roof bends down (Figure 3.5).
The paranuchal plate (PNu, Figure 3.1, 3.5, 3.9, Figure 4.1) has a strong dorso-ventral inflexion, the majority of the plate forming the posterior margin of the cheek (Figure 3.5). The ventral margin of the plate has a deep embayment in which the marginal plate inserts (Figure 3.6). There is a prominent spatulate glenoid fossa surrounded by a bony ring (Figure 3.6).
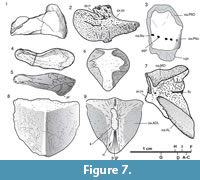 The overall shape of the marginal plate (M, Figure 3.5-3.6, 3.8, Figure 4.1-4.2, Figure 7.2) most closely resembles those of Kimbryanodus, Ctenurella and Materpiscis with its deeply convex posterior margin; however, rather than having a square ended process on the ventral margin, the process is rounded (Figure 3.5-3.6, Figure 7.2). The orbital division of the marginal plate (or.m, Figure 7.2) is shorter than the postorbital division as in Ctenurella and Materpiscis. Like Austroptyctodus, Kimbyranodus and Materpiscis there is a well-developed, thick, inwardly projecting mesial lamina on the smooth visceral surface (Figure 3.6, 3.8) a strong ridge of bone delineates that postorbital region (Figure 3.6, 3.8).
The overall shape of the marginal plate (M, Figure 3.5-3.6, 3.8, Figure 4.1-4.2, Figure 7.2) most closely resembles those of Kimbryanodus, Ctenurella and Materpiscis with its deeply convex posterior margin; however, rather than having a square ended process on the ventral margin, the process is rounded (Figure 3.5-3.6, Figure 7.2). The orbital division of the marginal plate (or.m, Figure 7.2) is shorter than the postorbital division as in Ctenurella and Materpiscis. Like Austroptyctodus, Kimbyranodus and Materpiscis there is a well-developed, thick, inwardly projecting mesial lamina on the smooth visceral surface (Figure 3.6, 3.8) a strong ridge of bone delineates that postorbital region (Figure 3.6, 3.8).
Dentition. The lower toothplate (Ifg, Figure 3.14-15, Figure 7.4-7.5) is deep with a curved ventral margin and a narrow sectorial, occlusal surface. A developed process forms the attachment to the quadrate (Figure 7.5, pr). The upper toothplate has a low anterior dorsal process (Figure 3.12, Figure 7.1) that articulates with the autopalatine, and in lateral view there is a prominent anterior ridge (Figure 3.12) and, posterior to the ridge, the toothplate expands laterally, similar to the condition in Kimbryanodus. The quadrate is well ossified and square shaped in cross section as in Campbellodus (Miles and Young, 1977). However, the anterior is only slightly expanded and there is no anterior flange. The exterior ventral margin is bulbous and articulates with the dorsal surface of the articular.
 Thoracic shield. The median dorsal plate is triangular in shape (MD; Figure 7.8-7.9, Figure 8.5-8.7), is strongly arched with a low median crest (mcr; Figure 7.9, Figure 8.7) and similar in height to that of Kimbryanodus. The anterior margin is concave, although there is a small median point, which is raised to form a small anterior crest (Figure 8.5). There are no overlap areas to suggest the presence of a median dorsal spine. There is a deep groove on the posterior underside of the plate where the basal plate articulates (Figure 7.9, Figure 8.7).
Thoracic shield. The median dorsal plate is triangular in shape (MD; Figure 7.8-7.9, Figure 8.5-8.7), is strongly arched with a low median crest (mcr; Figure 7.9, Figure 8.7) and similar in height to that of Kimbryanodus. The anterior margin is concave, although there is a small median point, which is raised to form a small anterior crest (Figure 8.5). There are no overlap areas to suggest the presence of a median dorsal spine. There is a deep groove on the posterior underside of the plate where the basal plate articulates (Figure 7.9, Figure 8.7).
The anterior dorsolateral plate (ADL; Figure 2, Figure 7.7, Figure 8.4, 8.8-8.9) has a distinct, dorso-lateral angular inflexion, comparable to Chelyophorus, Kimbryanodus and Rhynchodus. The dorsal margin is broad being approximately twice as long as the ventral margin and in this respect is similar to Austroptyctodus, Ctenurella and Kimbryanodus, whereas in Materpiscis and Rhamphodopsis the dorsal and ventral margins are of similar dimensions whereas in Campbellodus the ventral margin is broader. A narrow ventral process of ornamented bone meets the anterior lateral plate, defining a V-shaped overlap area for that plate (Figure 2.1, Figure 7.7, Figure 8.4) which is similar to that described for Austroptyctodus, Chelyophorus, Ctenurella, Kimbryanodus and Materpiscis. The key-shaped glenoid process is well developed (ar.cn, Figure 8.4). There are two pore openings on the dermal surface (Figure 8.4), and these open into a sensory line canal, enclosed by bone on the visceral surface (Figure 2.2, Figure 8.9).
The dorsal margin of the anterior lateral plate (AL, Figure 8.1-8.3) is not well preserved although the overlap margin on the anterior dorsolateral plate indicates that it formed an acute apex at the contact with the anterior dorsolateral plate (Figure 2.1, Figure 8.4). The mesial lamina has four rows of fan-shaped dermal tubercles (Figure 8.1); however, the row number of tubercles varies in species where more than one specimen is known (e.g., Austropytctodus, Ctenurella). The reticulate ornament on the external surface is sparse, although the plate does appear to be worn (Figure 8.1).
The anterior medioventral plate is rhomboid in shape, with an anterior extension of the ornamented part, with shallow overlap areas for the interolateral plates (Figure 7.6).
Reproductive structures. The anterior element of a single, male, hook-shaped “clasper” is preserved in internal view (Figure 8.10). There are four acutely pointed serrations on the posterior end of the laterally curving clasper similar to Austroptyctodus (Miles and Young, 1977). The clasper is anteriorly expanded with a V-shaped concavity, delineated by high lateral walls that converge at the base of the concavity to form a single median ridge that extends to the distal margin.
PTYCTODONT PHYLOGENY
Taxon Selection
The ptyctodont taxa, used for the phylogenetic analysis, are known from articulated specimens, with the exception of Denisonodus plutonensis (Johnson and Elliott, 1996) and Kimbryanodus williamburyensis (Trinajstic and Long, 2009), which are known from associated plates. Basal outgroup taxa used were the acanthothoracids Romundina stellina Ørvig, 1975 and Brindabellaspis stensioi Young 1980 the petalichthyids Lunaspis broilii Gross, 1937 and Eurycaraspis incilis Liu 1991to assist in character polarization.
Tree Computing Method
A data matrix of 32 morphological characters coded as in Table 1, unordered and unweighted for 15 genera, was run through PAUP (Swofford, 2017 trial version for MAC) using the stepwise heuristic search algorithm and ACCTRAN optimisation. One thousand replications with random taxon addition sequences were used. A branch and bound search was also undertaken. Identical results were obtained.
Character Analysis
Characters 1-26 are taken from Trinajstic and Long (2009)
Character 7, Dermal bone forms a sheath to cartilaginous claspers (males): 0, absent; 1, present (Johnson and Elliott, 1996; Trinajstic and Long, 2009) has been removed for this analysis. The presence of claspers, previously considered to be unique to pyctodonts within the placoderms (Watson, 1938; Miles and Young, 1977; Johnson and Elliott, 1996; Trinajstic and Long, 2009), are now recognised as a synapomorphy of the Placodermi (Long et al., 2015, King et al., 2016).
The recognition of new taxa, Materpiscis (Long et al., 2008; Trinajstic et al., 2012) and Meeksiella gen et sp nov. in addition to new specimens of Austroptyctodus (Trinajstic et al., 2012) showing additional morphology has enabled the character set to be expanded.
Characters
1. Lateral line canals form an X pattern on visceral surface of the nuchal plate: 0, absent; 1, present.
2. Sensory-line canals expressed on the dermal surface of bones as pores: 0, absent; 1, present. In Chelyophorus pore holes are visible within external grooves.
3. Median dorsal plate: 0, multiple; 1, one plate (modified from character 8 of Johnson and Elliott, 1996).
4. Scales: 0, present; 1, absent
5. Only the posterior supragnathals are present: 0, absent; 1, present.
6. Gnathal plates are flat plates with ornament or cusps: 0, present; 1, absent, laterally compressed as a crushing plate.
7. Central plates meet posterior to the nuchal plate: 0, absent (do not meet); 1, present (meet) (character 10 of Johnson and Elliott, 1996).
8. Preorbital plates meet mesially: 0, present; 1, absent (character 1 of Johnson and Elliott, 1996).
9. Pineal plate is part of anterior margin of skull roof: 0, absent, rostral plate forms the anterior margin; 1, present, pineal plate forms anterior margin of skull; 2, absent, preorbital plates form the anterior margin of the skull.
10. Upper tooth plates have an anterior dorsal process (state 2 is here defined as when the dorsal process is more than twice the height of the median height of the tooth plate): 0, absent; 1, present short and thick; 2, present high (modified from character 13 of Johnson and Elliott, 1996).
11. Tritorial lower tooth plates: 0, present; 1, absent.
12. Median dorsal plate with high spine: 0 present; 1, absent (character 6 of Johnson and Elliott, 1996).
13. Spinal plate: 0, present, large; 1, present, small; 2, absent (modified character 5 of Johnson and Elliott, 1996).
14. Contact between anterior lateral and anterior dorsolateral plates a simple inverted V-shape: 0 present; 1, absent and has an M-shape.
15. Elongated anterior median ventral plate: 0, present; 1, absent.
16. Ginglymoid neck joint: 0, present; 1, absent (character 9 of Forey and Gardiner, 1986).
17. Median dorsal plate has a straight or concave anterior margin: 0, present; 1, absent, has a median point.
18. Preorbital plates longer than central plates: 0, present; 1, absent.
19. Anterior margin of the nuchal plate is square: 0; is pointed, 1.
20. The point at which the postorbital, central and paranuchal plates meet is anterior to the posterior margin of the nuchal plate: 0; posterior to or level with the posterior margin the nuchal plate, 1.
21. Relative size of the postorbital division of the marginal plate: 0, larger than the orbital division; 1, smaller than the orbital division (marginal plate crescent-shaped).
22. Nuchal plate shape: 0, elongate being more than twice as long as wide; 1, short, relatively broad.
23. Nuchal plate with a median groove: 0, absent; 1, present.
24. Postorbital plate is irregular in outline: 0, postorbital plate is triangular in outline; 1, this character in unique to P. menzeli.
25. Anterior border of the interolateral plate: 0, scalloped; 1, straight.
26. Ventral process present on the marginal plate: 0, absent 1, present.
27. Ventral process on the lower toothplate: 0, absent; 1, present.
28. Dermal pelvic girdle: 0, absent; 1, present.
29. Median dorsal crest: 0, absent; 1, present.
30. Postorbital fenestra: 0, absent; 1, present.
31. Size of the postorbital plate relative to the paranuchal plate: parnuchal plate smaller, 0; paranuchal and postorbital plates equal, 1; postorbital plate larger than the paranuchal plate, 2,
32. Sensory lines of the nuchal plate: extend to the posterior margin, 0; do not extend, 1
Phylogenetic Reconstruction
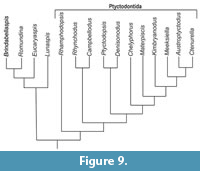 The parsimony analysis produced a single tree (Figure 9) with a CI = 0.52, RI = 0.68, and a tree length of 68 steps. Rhamphodopsis is resolved as the most basal of the ptyctodonts in agreement with Trinajstic and Long (2009). Contra Trinajstic and Long (2009), but in agreement with Johnson and Elliot (1996) Campbellodus and Rhynchodus form a separate clade from the two North American genera Ptyctodopsis (Figure 6.3) and Denisonodus (Figure 9). Rhynchodus (Figure 6.5) and Campbellodus (Figure 6.6) are united by the presence of preorbital plates with no mesial contact (character 9; 0⇒1, character 1 of Johnson and Elliott, 1996), a character that is independently acquired in Materpiscis (Figure 6.7) and Austroptyctodus (Figure 6.8). The North American taxa are united (Figure 9) by three characters: the centrals participating in the posterior border of the skull roof (character 8, ®01); a median point on the anterior margin of the median dorsal plate, which projects in front of the anterior dorsolateral plates (character 18; 0⇒1) and the presence of crushing, lower tooth plates (character 12; 1⇒0). A crushing type dentition also occurs in Campbellodus and Materpiscis and is most likely convergent, reflecting similar feeding strategies rather than relationship (Trinajstic and Long, 2009).
The parsimony analysis produced a single tree (Figure 9) with a CI = 0.52, RI = 0.68, and a tree length of 68 steps. Rhamphodopsis is resolved as the most basal of the ptyctodonts in agreement with Trinajstic and Long (2009). Contra Trinajstic and Long (2009), but in agreement with Johnson and Elliot (1996) Campbellodus and Rhynchodus form a separate clade from the two North American genera Ptyctodopsis (Figure 6.3) and Denisonodus (Figure 9). Rhynchodus (Figure 6.5) and Campbellodus (Figure 6.6) are united by the presence of preorbital plates with no mesial contact (character 9; 0⇒1, character 1 of Johnson and Elliott, 1996), a character that is independently acquired in Materpiscis (Figure 6.7) and Austroptyctodus (Figure 6.8). The North American taxa are united (Figure 9) by three characters: the centrals participating in the posterior border of the skull roof (character 8, ®01); a median point on the anterior margin of the median dorsal plate, which projects in front of the anterior dorsolateral plates (character 18; 0⇒1) and the presence of crushing, lower tooth plates (character 12; 1⇒0). A crushing type dentition also occurs in Campbellodus and Materpiscis and is most likely convergent, reflecting similar feeding strategies rather than relationship (Trinajstic and Long, 2009).
The remaining taxa (Figure 9), with the exception of Kimbryanodus, which does have a spinal plate, comprises the aspinothoracid ptyctodontids all of which possess an anterior dorsal lateral plate with a simple inverted V-shape articulation to the anterior lateral plate (character 15) possess large orbital division on the marginal plate (character 21; 1⇒0) although Meeksiella maintains the larger postorbital divisions (character 21; 1⇒0) and has a straight overlap margin between the interolateral and anterior dorsolateral plates (character 26; 0⇒1). They all lack a dorsal spine on the median dorsal plate (character 13; 0⇒1). Austropytctodus, Ctenurella, Kimbryanodus Materpiscis and Meeksiella nov. are further united by a short broad anterior median ventral plate (character 16; 0⇒1) and a postorbital fenestra (character 30; 0⇒1). Austroptyctodus, Ctenurella, Kimbryanodus and Meeksiella nov. all have a ventral process on the marginal plate (Character 26; 0⇒1) and a ventral process on the lower tooth plate (character 27; 0⇒1).
DISCUSSION
The first phylogenetic analysis of ptyctodonts, was undertaken by Johnson and Elliott (1996), using seven in-group taxa and 15 character states. However, since this initial analysis, two additional taxa (Kimbryanodus and Materpiscis) have been described, all showing three-dimensional preservation (Trinajstic and Long, 2009; Trinajstic et al., 2012), in addition to a new specimen of Austroptyctodus (Trinajstic et al., 2012) has been prepared, which shows the complete toothplates and ventral margin of the marginal plate for the first time. These new discoveries, in addition to Meeksiella gen. nov. described herein, have allowed the recognition of additional character states such as the presence of a ventral process on the lower toothplates, and marginal plate common to Austroptyctodus, Ctenurella, Kimbryanodus and Meeksiella nov. and the position of the central line canals on the visceral surface the nuchal plate indicating that further phylogenetic analysis was required. The new genus most closely resembles Kimbryanodus williamburyensis in having a similar arrangement of the skull roof plates (Figure 6.1-6.2), a large postorbital plate and a ventral process on the lower toothplate and marginal plate, the latter characters also shared with Austroptyctodus and Ctenurella. It differs from these taxa in that the pineal opening is U-shaped, in visceral view the central plates connect under the nuchal plate, and the shape of the median dorsal plate is triangular (Figure 3.2).
Long (1997) reconstructed the headshields of Austropyctodus, Campbellodus and Rhynchodus determining from the three-dimensional preservation of the Australian material that not all the plates of the headshield overlapped. The North American taxa and Rhamphodopsis were not included in Long’s (1997) redescription. Articulated headshields of Rhamphodopsis (Miles, 1967) are known, showing that the preorbital and pineal plates all overlap, in addition the nuchal plate is anteriorly pointed. However, in Austroptyctodus, Campbellodus, and Rhynchodus the anterior margin of the nuchal plates is square and the preorbital plates are separated along the midline of the skull roof (Figure 6.5-6.8). Denisonodus also possesses a square anterior margin on the nuchal plate, however, the skull roof of Denisonodus was reconstructed (Johnson and Elliot, 1996) with the preorbital plates having mesial contact. The square shape of the anterior margin of the nuchal plate in Denisonodus suggests that the preorbital plates in this taxon may not have medial contact, and a review of this material is required.
The aspinothoracids ptyctodonts are here united as a clade, primarily through the loss of basal characters. A large and projecting spinal plate such as occurs in Denisonodus, Ptyctodopsis and Rhamphodopsis is considered to represent the basal condition as does the presence of scales on the pelvic fins and, in the case of Campbellodus the body, and a high median dorsal spine. These characters are also present in the outgroup.
It must be noted that many more ptyctodont toothplates have been recovered than dermal body plates (Miles and Young, 1977; Carr, 1995), indicating that there was a greater diversity of ptyctodonts than represented, in this analysis. In particular no complete body fossils from lower Devonian strata are known, although isolated headshield plates, one articulated trunkshield (Tollodus) and toothplates occurr as early as the Eifelian (Denison, 1978). The lack of early Devonian body fossils may therefore affect the polarity of characters.
CONCLUSION
Meeksiella pskovensis represents a complete Early Frasnian (Late Devonian) ptyctodont from the Main Devonian Fields (Baltic area). The increase in the number of ptyctodont taxa known from complete body armour has enabled an increase in the number of morphological characters considered in a phylogenetic analysis. The hypothesis of relationships resolved Meeksiella pskovensis as more derived than the Famennian Russian taxon Chelyophorus although the genus Chelyophorus is in need of revision as it is probable that two ptyctodont taxa have been assigned to this genus. The lack of other Famennian taxa and older Devonian taxa may have resulted in the polarity of characters not being fully realised.
ACKNOWLEDGMENTS
The authors are grateful to U. Toom (Department of Geology at Tallinn University of Technology) for kindly providing some of the material for this study; G. Baranov (Department of Geology at Tallinn University of Technology) for photography of Estonian specimens; O. Lebedev (Borissiak Palaeontological Institute of the Russian Academy of Sciences, Moscow) for information on the collection of D. Obruchev; S. Bagirov (Borissiak Palaeontological Institute of the Russian Academy of Sciences, Moscow) for photography of the holotype, and S. Nilov (Institute of the Earth Sciences, St. Petersburg State University, St. Petersburg) for the microtomographic images. The work was performed partly in accordance to the Russian Government Program of Competitive Growth of Kazan Federal University. The scientific research completed by AI was performed at the Center for X-ray Diffraction Studies, Research Park of St. Petersburg State University. Research funding for KT and JL was through an Australian Research Council Discovery Project Grant (DP140104161) with additional funding to KT through a Curtin Research Fellowship, Curtin University. We thank the reviewers for helpful comments.
REFERENCES
Agassiz, L. 1844. Monographie des Poissons Fossils du Vieux Grès Rouge ou Système Dévonien (Old Red Sandstone) des îles Britanniques et de Russie.Soleure, Chez Jent et Gassmann, Neuchâtel, Switzerland.
Carr, R.K. 1995. Placoderm diversity and evolution. Bulletin du Muséum National d’Histoire Naturelle, serie C Paris, 14:3-13.
Carr, R.K., Johanson, Z., and Ritchie, A. 2009. The phyllolepid placoderm Cowralepis mclachlani: insights into the evolution of feeding mechanisms in jawed vertebrates. Journal of Morphology, 270:775-804. https://doi.org/10.1002/jmor.10719
Denison, R.H. 1985. A new ptyctodontid placoderm, Ptyctodopsis, from the Middle Devonian of Iowa. Journal of Paleontology, 59:511-522. https://www.jstor.org/stable/1304971
Forey, P.L. and Gardiner, B.G. 1986. Observations on Ctenurella (Ptyctodontida) and the classification of placoderm fishes. Zoological Journal of the Linnean Society, 86:43-74. https://doi.org/10.1111/j.1096-3642.1986.tb01807.x
Goujet, D. 1984. Placoderm interrelationships: a new interpretation, with a short review of placoderm classification. Proceedings of the Linnean Society of New South Wales, 107:211-241.
Gross, W. 1932. Die Arthrodira Wildungens. Geologische und paläontologische Abhandlungen, Neue Folge, 19:1-61.
Gross, W. 1933. Die Fische des Baltischen Devons. Palaeontographica, 79:1-74. https://www.schweizerbart.de/papers/pala/list/A079#issue1-2
Gross, W. 1937. Die Wirbeltiere des rheinischen Devons. Abhandlungen Preuss Geologische Landesanstält, 176:5-83.
Gross, W. 1941. Die Bothriolepis -Arten der Cellulosa-Mergel Lettlands. Kunglia Svenska Vetenskaps-akademiens Handlingar, 19 (5):1-79.
Gross, W. 1942. Die Fischfaunen des baltischen Devons und ihre biostratigraphische Bedeutung. Korresponedz-blatt des Naturforscher-Vereins zu Riga, 64:373-436.
Gross, W. 1956. Über Crossopterygier und Dipnoer aus dem baltischen Oberdevon im Zusammenhangeiniger vergleichenden Utersucgung des Porenkanalslystem paläozoischer Agnathan und Fische. Kunglia Svenska Vetenskaps-akademiens Handlingar, 5 (6):1-40.
Ivanov, A.O. 1990. Snetnaya Gora vertebrate assemblage from the Main Devonian Field and its biostratigraphical significance. Vestnik Leningradskogo Universiteta, Series 7, Geology and Stratigraphy, 1:94-98. (In Russian)
Ivanov, A.O. 1992. Occurrences of ptyctodontids (Placodermi) in the Upper Devonian of the East European Platforms. Problems of Palaeontology, 10:133-137. (In Russian)
Ivanov, A., Zhuravlev, A., Stinkulis, G., Evdokimova, I., Dronov, A., Sokiran, E., Shishlov, S., Broushkin, A., and Myshkina, N. 2005. Devonian Sections of North-West of East European Platform. Guidebook of the Post-Conference Field Trip. Saint Petersburg. https://pureportal.spbu.ru/ru/publications/devonian-sections-of-north-west-of-east-european-platform-guidebo
Jarvik, E.1944. On the exoskeletal shoulder girdle on teleostomian fishes, with special reference to Eusthenopteron foordi Whiteaves. Kungliga Svenska Vetenskapsakademiens Handlingar, 21:1-32.
Johnson, H-M. and Elliott, D. 1996. A new ptyctodont (Placodermi) from the Upper Devonian Martin Formation of northern Arizona, and an analysis of ptyctodont phylogeny. Journal of Paleontology, 70 (6):997-1003. https://doi.org/10.1017/S0022336000038695
King, B., Qiao, T., Lee, M.S., Zhu, M., and Long, J.A. 2016. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Systematic Biology, p.syw107. https://doi.org/10.1093/sysbio/syw107
Liu, Y.H. 1991. On a new petalichthyid, Eurycaraspis incilis gen. et sp. nov., from the middle Devonian of Zhanyi, Yunnan, p. 139-177. In Chang, M.M., Liu, Y.H., and Zhang, G.R. (eds.), Early Vertebrates and Related Problems of Evolutionary Biology. Science Press. Beijing.
Long, J.A. 1988. Campbellodus sp. (Placodermi: Ptyctodontida) from the Upper Devonian Napier range, Canning Basin. Records of the Western Australian Museum, 14:141-144.
Long, J.A. 1997. Ptyctodontid fishes (Vertebrata, Placodermi) from the Late Devonian Gogo Formation, Western Australia, with a revision of the European genus Ctenurella Ørvig, 1960. Geodiversitas, 19 (3):515-555.
Long, J.A. and Trinajstic, K. 2010. The Late Devonian Gogo Formation lägerstatte of Western Australia: exceptional early vertebrate preservation and diversity. Annual Review of Earth and Planetary Sciences, 38:255-279. https://doi.org/10.1146/annurev-earth-040809-152416
Long, J.A., Trinajstic, K., Young, C., and Senden, T. 2008. Live birth in the Devonian. Nature, 453:650 --653. https://doi.org/10.1038/nature06966
Long, J.A., Mark-Kurik, E., Johanson, Z., Lee, M.S., Young, G.C., Min, Z., Ahlberg, P.E., Newman, M., Jones, R., den Blaauwen, J., and Choo, B. and Trinajstic, K. 2015. Copulation in antiarch placoderms and the origin of gnathostome internal fertilization. Nature, 517(7533):196-199. https://doi.org/10.1038/nature13825
Mark-Kurik, E. 1977. The structure of the shoulder girdle in early ptyctodonts, p. 61-70. In Menner, V. V. (ed.), Ocherki po filogenii i sistematike iskopaemykh ryb i beschelyustnykh [= Essays on Phylogeny and Systematics of Fossil Agnathans and Fishes]. Nauka. Moscow. (In Russian)
Mark-Kurik, E., Ivanov, A., and Obrucheva, O. 1991. The endoskeleton of shoulder girdle in ptyctodonts (Placodermi). Proceedings of the Estonian Academy of Sciences, Geology, 40 (4):160-164.
Mark-Kurik, E. and Poldvere, A. 2012. Devonian stratigraphy in Estonia: current state and problems. Estonian Journal of Earth Sciences, 61(1):33-47. https://doi.org/10.3176/earth.2012.1.03
McCoy, F. 1848. On some new fossil fish of the Carboniferous period. Annals and Magazine of Natural History, 2:1-10.
Miles, R.S. 1967. Observations on the ptyctodont fish, Rhamphodopsis Watson. Zoological Journal of the Linnean Society, 47:99-120. https://doi.org/10.1111/j.1096-3642.1967.tb01398.x
Miles, R.S. and Young, G.C. 1977. Placoderm interrelationships reconsidered in the light of new ptyctodontids from Gogo, Western Australia. Linnean Society of London, Symposium Series, 4:123-198.
Newberry, J.S. 1873. Descriptions of fossil fishes. Geological Survey Report 1, pt 2: (Paleontology):243-355.
Obruchev, D.V. 1947. Chordata, p. 191-206. In Nalivkin, D.V. (ed.), Atlas iskopaemykh faun SSSR [= Atlas of Fossil Faunas of USSR], III: Devonian. Gosgeolizdat. (In Russian)
Obrucheva, O.P. 1966. New data on the coccosteids (armoured fishes) from the Devonian of the Baltics, p. 151-190. In Grigelis, A.A. (ed.)., Paleontologiya i stratigrafiya Pribaltiki i Belorussii [Palaeontology and Stratigraphy of Baltics and Belorussia], I (VI). Mintis. Vilnius. (In Russian)
Obrucheva, O.P. 1981. Placoderm Devonian fishes, ptyctodontids, p. 155-167. In Menner, V.V. and Druschits, V.V. (eds.), Zakonomernosti istoricheskogo razvitiya iskopaemykh organizmov [= Patterns of Historical Development in Fossil Organisms]. MSU. Moscow. (In Russian)
Obrucheva, O.P. 1983. Genus Chelyophorus from deposits of the Main Devonian Field, p. 28-36. In Novitskaya, L.I. (ed.), Problemy sovremennoy paleoikhthiologii [= Problems of Modern Palaeoichthyology]. Nauka. Moscow. (In Russian)
Ørvig, T. 1960. New finds of acanthodians, arthrodires, crossopterygians, ganoids and dipnoans in the upper Middle Devonian Calcareous Flags (Oberer Plattenkalk) of the bergisch-Paffrath Trough (Part 1). Paläontologische Zietschrift, 34:295-335. https://doi.org/10.1007/BF02986872
Ørvig, T. 1971. Comments on the lateral line system of some brachythoracid and ptyctodontid arthrodires. Zoologica Scripta, 1:5-35. https://doi.org/10.1111/j.1463-6409.1971.tb00710.x
Ørvig, T. 1975. Description, with special reference to the dermal skeleton, of a new radontinid arthrodire from the Gedinnian of Arctic Canada, p. 41-71. In Lehman, J.P. (ed.), P roblèmes Actuels de p\Paléontologie: Evolution des Vertébrés, Colloques internationaux du Centre National de la Recherche scientifique, 218. Paris.
Pander, C. In Keyserling, K. 1846. Wissen-schaftliche Beobachtungen auf einer Reise in das Petschora-Land. Buchdruckerei der Kaiserlische Akademia der Wissenschaften, Saint-Petersburg.
Sorokin, V.S. 1978. Etapy razvitiya severo-zapada Russkoy platformy vo franskom veke [= Development Stages of the North-Western Part of Russian Platform in the Frasnian Stage]. Zinātne. Rīga. (In Russian)
Swofford, D.L. 2001. PAUP* 4.0 b: phylogenetic analysis of parsimony (and other methods). Sinauer and Associates, Sunderland, Massachusetts.
Trinajstic, K. and Long, J.A., 2009. A new genus and species of Ptyctodont (Placodermi) from the Late Devonian Gneudna Formation, Western Australia, and an analysis of Ptyctodont phylogeny. Geological Magazine, 146 (5):743-760. https://doi.org/10.1017/S001675680900644X
Trinajstic, K., Long, J.A., Johanson, Z., Young, G., and Senden, T., 2012. New morphological information on the ptyctodontid fishes (Placodermi, Ptyctodontida) from Western Australia. Journal of Vertebrate Paleontology, 32 (4):757-780 https://doi.org/10.1080/02724634.2012.661379
Trinajstic, K., Boisvert, C., Long, J., Maksimenko, A., and Johanson, Z., 2015. Pelvic and reproductive structures in placoderms (stem gnathostomes). Biological Reviews, 90 (2):467-501. https://doi.org/10.1111/brv.12118
Watson, D.M.S. 1938. On Rhamphodopsis, a ptyctodont from the Middle Old Red sandstone of Scotland. Transactions of the Royal Society of Edinburgh, 59:397410. https://doi.org/10.1017/S0080456800009157
Woodward, A.S. 1891. Catalogue of the fossil fishes in the British Museum (Natural History). Volume 2. 567 pp. London: Trustees of the British Museum (Natural History).
Young, G.C. 1980. A new Early Devonian placoderm from New South Wales, Australia, with a discussion of placoderm phylogeny. Palaeontographica, 167A:10-76.
Young, G.C. 1986. The relationships of placoderm fishes. Zoological Journal of the Linnean Society, 88:1-57. https://doi.org/10.1111/j.1096-3642.1986.tb00876.x
Ziegler, W. and Sandberg, C.A. 1990. The Late Devonian standard conodont zonation. Courier Forshungsinstitut Senckenberg, 121:1-115.

