 Fossil calibrations for molecular analyses and divergence time estimation for true crabs (Decapoda: Brachyura)
Fossil calibrations for molecular analyses and divergence time estimation for true crabs (Decapoda: Brachyura)
Article number: 27.2.a38
https://doi.org/10.26879/1332
Copyright Palaeontological Association, August 2024
Proceedings of the 8th Symposium on Fossil Decapod Crustaceans
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 19 August 2023. Acceptance: 17 July 2024.
ABSTRACT
True crabs, or Brachyura, comprise over 7,600 known species and are among the most ecologically dominant, economically significant, and popularly recognized groups of extant crustaceans. There are over 3,000 fossil brachyuran species known from mid and upper Jurassic, Cretaceous, and Cenozoic deposits across the globe, many of them preserved in exquisite detail, but their origins and early evolution remain unresolved. This uncertainty hinders the identification of the stratigraphically earliest occurrence of major brachyuran groups in the fossil record, obscuring our understanding of their phylogenetic relationships and thus the ability to estimate divergence times to answer large-scale macroevolutionary questions. We present 36 vetted fossil node calibration points for molecular phylogenetic analysis of crabs (one Anomura and 35 Brachyura) and reassess the earliest occurrences of several key clades based on recent fossil discoveries or re-examination of previous studies. For each calibrated node, we provide minimum and tip maximum ages for the stratigraphically oldest fossil that can be reliably assigned to the group. Disentangling the anatomical disparity of fossil forms and their phylogenetic relationships is crucial to recognizing the earliest branching members among brachyuran groups. This represents a critical first step in understanding the evolution of carcinization and decarcinization, the appearance of key adaptations, and the transition from sea to land and freshwater in brachyurans. The identification and critical examination of reliable fossils for deep time calibrations, both as tips and nodes, is pivotal to ensure not only precise but more accurate divergence time estimations when reconstructing the crab tree of life.
Javier Luque. Department of Zoology, Museum of Zoology, University of Cambridge, Downing Street, Cambridge CB2 3EJ, UK; Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA; Institute of Environment, Department of Biological Sciences, Florida International University-Biscayne Bay Campus, North Miami, FL 33181, USA; and Smithsonian Tropical Research Institute, Balboa–Ancón 0843–03092, Panamá, Panamá. Corresponding author. jl2351@cam.ac.uk.
Heather D. Bracken-Grissom. Institute of Environment, Department of Biological Sciences, Florida International University-Biscayne Bay Campus, North Miami, FL 33181, USA and Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave NW, Washington, DC 20560, USA. hbracken@fiu.edu
Javier Ortega-Hernández. Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA. jortegahernandez@fas.harvard.edu
Joanna M. Wolfe. Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA. jowolfe@g.harvard.edu
Keywords: crown group; evolution; marine; non-marine; stem group; phylogeny
Final citation: Luque, Javier, Bracken-Grissom, Heather D., Ortega-Hernández, Javier, and Wolfe, Joanna M. 2024. Fossil calibrations for molecular analyses and divergence time estimation for true crabs (Decapoda: Brachyura). Palaeontologia Electronica, 27(2):a38.
https://doi.org/10.26879/1332
palaeo-electronica.org/content/2024/5285-fossil-calibrations-true-crabs
Copyright: August 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
The origins and evolution of true crabs, or Brachyura, have sparked the fascination of scientists and the public alike in the last decades, thanks to their astonishing anatomical diversity (disparity) and multiple convergent instances of gain/loss of the crab-like body plan (i.e., carcinization and decarcinization) (Scholtz, 2014; Luque et al., 2019a; Wolfe et al., 2021). With over 7,600 extant species and more than 3,000 fossil species known (e.g., Ng et al., 2008; Schweitzer et al., 2010; Luque et al., 2019a; Poore and Ahyong, 2023, and references therein), brachyurans are one of the most speciose groups of crustaceans. The monophyly of Brachyura is widely accepted, based on morphological and molecular grounds, and they are considered reciprocal sister groups with Anomura (collectively known as false crabs and allies), both forming a clade referred to as Meiura (Scholtz and Richter, 1995; Schram and Dixon, 2004; Hegna et al., 2020).
The systematics and classification of brachyurans have changed considerably in the last few decades, especially concerning family-level ranks and above. New lines of evidence have become available in the form of comprehensive phylogenetic studies, new fossil discoveries, and the critical re-interpretations of previous ones using novel technologies. Molecular and morphological phylogenetics are key to better understanding the evolutionary relationships through common ancestry among and within brachyurans, allowing us to quantitatively examine previous views and hypotheses largely based on traditional alpha taxonomy. This is particularly important when considering problematic groups such as the so-called podotremes and the heterotreme eubrachyurans, now widely recognized as paraphyletic groups, as well as the relationships among primary freshwater groups (e.g., Tan et al., 2018; Wang et al., 2018; Ma et al., 2019; Luque et al., 2019a; Tang et al., 2020; Wang et al., 2020; Luque et al., 2021; Tsang et al., 2022; Zhang et al., 2022; Poore and Ahyong, 2023; Wolfe et al., 2023).
While molecular and morphological phylogenies provide different types of evidence for understanding evolutionary relationships among organisms, fossils offer critical anatomical, spatial, and temporal information inaccessible from extant species alone (Luque et al., 2019a). Thus, accurate calibrations in a phylogenetic framework require reliable fossils, both in terms of their systematic affinities and their chronostratigraphic occurrences, since the use of equivocal (e.g., superficially similar but unrelated) or poorly constrained temporal and stratigraphic fossils may lead to precise yet inaccurate dating (either as tips or nodes), and divergence time estimates, and therefore may confound interpretations of macroevolutionary patterns and processes over time.
The fossil record of brachyuran crabs consists mainly of marine taxa, which tend to be the most abundant and are often well preserved. Conversely, fossils of non-marine crabs (i.e., terrestrial, semi-terrestrial, and freshwater) are remarkably scarce and often fragmentary, largely due to the relatively dynamic and high-energy environments they inhabit, the geochemistry of the substrates, and scavenging of their corpses or even re-working and consumption of their own exuviae (e.g., Locatelli, 2013; Luque, 2017; Luque et al., 2018). Such biases limit the number of remains that can fossilize, often restricted to the most resilient biomineralized body parts (e.g., claw dactyli and propodi remains), with whole organisms and soft to lightly-biomineralized tissues only preserved under exceptional conditions (Luque et al., 2019a; Luque et al., 2021). In turn, these biases constrain the number of fossils suitable as reliable calibration points in molecular estimations of time divergence, and consequently the available fossil data that can be used to examine the evolution of terrestrialization and the transition from marine to non-marine habitats (Tsang et al., 2014; Luque et al., 2021; Watson-Zink, 2021; Tsang et al., 2022; Wolfe et al., 2023). Understanding the early origins of marine and non-marine brachyurans by means of their fossil record requires not only new paleontological discoveries, but also a critical re-examination of previous records. This holds true for marine crab fossils as well.
General Considerations When Selecting Fossils and Their Age Ranges for Molecular Calibrations and Divergence Time Estimation
Molecular biologists face limitations when selecting fossils for internal node calibrations related to evaluating specific details of such fossil occurrences, like their chronostratigraphic and lithostratigraphic context, and the reliability of their systematic placement, especially with fragmentary material (Gandolfo et al., 2008; Parham et al., 2012; Wolfe et al., 2016). Among anomurans and brachyurans, and specifically within Brachyura, anatomical convergence of their body and body parts is pervasive (Scholtz, 2014; Luque et al., 2019a), and there is a trove of extinct and extant groups that superficially may resemble each other, particularly in their dorsal carapaces. This may lead to inappropriate selection of fossils that are not in fact related and, as a result, introduce large errors and inaccuracies into the estimation of divergence dates when they are used to calibrate particular nodes and tips.
Fossils collected in situ are often assigned a tentative relative age based on the chronostratigraphic age of the geological formation that contains them. However, geological formations can span tens to hundreds of meters in thickness and hundreds of thousands to millions of years in age, whereas the age of a unitary fossil sample represents a single point in time, making it difficult to constrain its age with respect to the age of the entire lithostratigraphic unit. For example, if a formation is known to be ‘Eocene’ in age, but its base and top have not been better constrained via absolute (radiometric isotope) or relative (e.g., biostratigraphy, stratigraphic position) dating, it means that it could, hypothetically, range chronostratigraphically anywhere from 56.0 to 33.9 Ma. Even if the relative age of such formation is further constrained, for example to ‘early Eocene (Ypresian)’, the age bracket would range from 56.0 to 47.8 Ma, in which case the fossil would be assigned these as its tip maximum and minimum ages (e.g., several Italian fossil occurrences, see below). This can be circumvented when other components of the fossil assemblages shed light on the biostratigraphic ranges of other micro and/or macrofossils (e.g., the porcellanid crab in calibration 1 here), when reliable radioisotopic data are associated to the same strata as the fossils (e.g., the pseudothelphusid freshwater crab in calibration 16 herein), or if magnetostratigraphic information is available (e.g., the parthenopid crab in calibration 23 herein) (Table 1).
An issue that is often oversimplified, and sometimes hard to circumvent, is the extrapolation of the age range of a formation in a specific locality to other geographically separated localities where the same formation occurs. While, in principle, the horizontality and superposition of beds in a normal sequence would allow for regional correlations, extrapolating ages is not always straightforward. This is particularly important for fossils near the lower and upper boundaries of a lithostratigraphic unit, principally due to the irregular geometry of the basins, unconformities and other erosional surfaces, eustasy, differential subsidence, diachronism, lateral facial changes, and pinching of the strata, to name some examples. Thus, assigning the age of a formation in one locality to a fossil found in the same formation in a different locality would be better informed by gaining some level of understanding of the local and regional geological and stratigraphic context.
Lastly, unless the ages near the top and/or the bottom of a sequence are known, an absolute age from a sample coming from a bed within the stratigraphic interval can inform about the overall age range of the formation, but does not represent the age of the formation per se, and therefore the age of the fossils present in the formation, unless the fossils come from or near the dated bed itself (as in the case in calibration 16 herein) (Table 1). Nonetheless, an approximate age bracket for a fossil occurring between two dated layers is as the best possible estimate, given the available data, in which case the minimum and tip maximum ages of the fossil will be informed and constrained by such absolute ages (e.g., as in the pinnotherid crab in calibration 10 herein) (Table 1).
MATERIALS AND METHODS
Materials
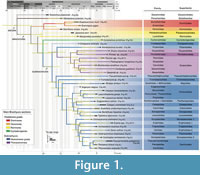 Here we present a set of 36 phylogenetically vetted fossil calibration points for molecular analyses and divergence time estimations of true crabs (one anomuran outgroup, 35 brachyuran ingroups) (based on the crown group topologies inferred by Wolfe et al., 2023) (Figure 1), and provide information on the type of material investigated, its repository and catalogue number, phylogenetic justification, minimum and tip maximum ages, age justification, and a discussion, following best practices (Parham et al., 2012) (Table 1).
Here we present a set of 36 phylogenetically vetted fossil calibration points for molecular analyses and divergence time estimations of true crabs (one anomuran outgroup, 35 brachyuran ingroups) (based on the crown group topologies inferred by Wolfe et al., 2023) (Figure 1), and provide information on the type of material investigated, its repository and catalogue number, phylogenetic justification, minimum and tip maximum ages, age justification, and a discussion, following best practices (Parham et al., 2012) (Table 1).
Methods
For crown Meiura (Anomura + Brachyura) (Table 1, calibrations 1-7), we provide a soft maximum age of 191.8 Ma based on the estimated maximum age for the brachyuran fossil Eocarcinus praecursor Withers, 1932, from the Lower Jurassic (Pliensbachian) of England (Figure 2A; Table 1). We did not consider Platykotta akaina Chablais, Feldmann, and Schweitzer, 2011, from the Late Triassic (Norian-Rhaetian) of United Arab Emirates, as a suitable calibration for Meiura due to its overall poor preservation and incompleteness (Hegna et al., 2020), which precludes a systematic affiliation with Meiura beyond doubt, whereas E. praecursor is represented by more specimens and fits better within the overall meiuran and brachyuran anatomical range (e.g., De Grave et al., 2009; Haug and Haug, 2014; Scholtz, 2014; Hegna et al., 2020).
For crown Eubrachyura (Table 1, calibrations 8-19 and 21-36), we provide a soft maximum age of 100.9 Ma based on the estimated maximum age for the crown eubrachyuran fossil Cretapsara athanata Luque, in Luque et al., 2021, from the mid-Cretaceous (uppermost Albian-lowermost Cenomanian) of Burma (Figure 3A; Table 1). For crown Portunoidea (calibration 20), we provide a soft maximum age of 97.0 Ma based on the estimated maximum age for the stem group portunoid fossil Eogeryon elegius Ossó, 2021, from the mid-Cretaceous (Cenomanian) of Spain (Ossó, 2016, 2021; Luque et al., 2021) (Figure 4D; Table 1).
For each vetted calibration, we reassess the earliest occurrences of several key groups considering recent fossil findings and re-examination of previous ones. In some cases, the selection of a specific fossil calibration point from the literature does not necessarily represent the oldest record that has been putatively assigned to a given family, but the oldest reliable occurrence that can be referred to the clade with optimal confidence in its morphology. Minimum and tip maximum ages were either obtained from the ages reported in the original publications, or from stratigraphically constrained absolute or relative ages from the same localities as the fossil material or localities nearby, whenever possible. Numerical ages for geological time intervals follow the International Chronostratigraphic Chart by the International Commission on Stratigraphy (Cohen et al., 2013; updated 2023).
Institutional Repositories and Abbreviations
AR: New Zealand Geological Survey, Lower Hutt, New Zealand.
BEG: Bureau of Economic Geology, University of Texas at Austin, USA.
GHUNLPam: Geology Collections at National University of La Pampa, Santa Rosa, La Pampa, Argentina.
HNHM: Hungarian Natural History Museum, Department of Earth Sciences and Palaeontology.
I.G.: Museo Civico “G. Zannato” di Montecchio Maggiore, Vicenza, northern Italy.
IGM: Museo Geológico José Royo y Gómez, Colombian Geological Survey.
IGVR: Museo di Storia Naturale di Verona, Italy.
IHNFG: Museo de Paleontología “Eliseo Palacios Aguilera” (Secretaría de Medio Ambiente e Historia Natural), Calzada de los Hombres Ilustres s/n, Tuxtla Gutiérrez, Chiapas, Mexico.
LYAM: Longyin Amber Museum, Xishan District, Kunming, Yunnan Province, China.
MCV: Museo Civico "D. Dal Lago" di Valdagno (MCV), Vicenza, northern Italy.
MCZ: Museo di Archeologia e Scienze Naturali “G. Zannato” di Montecchio Maggiore, Vicenza, northern Italy.
MFM: Mizunami Fossil Museum (Yamanouchi, Mizunami, Japan).
MGB: Museu de Geologia de Barcelona, Spain.
MGSB: Museo Geológico del Seminario de Barcelona (MGSB), Catalonia, Spain.
MHN-UABCS: Museo de Historia Natural, Universidad Autónoma de Baja California Sur, La Paz, Baja California Sur, México (MHN-UABCS).
MNHN: Muséum national d’Histoire naturelle, Paris, France.
MPZ: Palaeontological collection of the Museo de Ciencias Naturales de la Universidad de Zaragoza, Spain.
MUSM - CTA: Museo de Historia Natural de la Universidad Nacional Mayor San Marcos de Lima (MUSM), Peru; Contamana (CTA).
NHM / In: Natural History Museum (NHM) London, Palaeontological Department, London, England (including specimens from the formerly British Museum (Natural History), under acronym BM In).
NHMW: Geological-palaeontological collections of the Natural History Museum at Vienna, Austria (Naturhistorisches Museum Wien, NHMW).
NNM: NCB - Naturalis, Leiden, The Netherlands.
PAL-ULPGC: Palaeontological collections of the Universidad de Las Palmas de Gran Canarias, Spain.
UF: Invertebrate Paleontology collections, Florida Museum of Natural History, USA.
USNM: Smithsonian Institution, National Museum of Natura History (NMHN), USA.
VR: Museo di Storia naturale di Verona, Italy.
FOSSIL CALIBRATIONS
Anomura: Galatheoidea: Porcellanidae (crown)
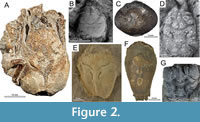 Fossil specimen. Vibrissalana jurassica Robins and Klompmaker, 2019. Naturhistorisches Museum Wien, Vienna, Austria. Holotype NHMW 2007z0149/0405, a somewhat complete dorsal carapace, with a broken left side (Figure 2B).
Fossil specimen. Vibrissalana jurassica Robins and Klompmaker, 2019. Naturhistorisches Museum Wien, Vienna, Austria. Holotype NHMW 2007z0149/0405, a somewhat complete dorsal carapace, with a broken left side (Figure 2B).
Phylogenetic justification. The overall carapace shape of the holotype and sole specimen of V. jurassica fits well within the general diagnosis of Galatheoidea. The ovate, wide, and flattened dorsal carapace, plus the limited dorsal ornamentation and groove pattern, match previous diagnoses for Porcellanidae (Ahyong et al., 2010; Schweitzer and Feldmann, 2012; Robins and Klompmaker, 2019).
Minimum age. 145.0 Ma.
Tip maximum age. 152.0 Ma.
Soft maximum age. 191.8 Ma.
Age justification. This fossil was collected from the Ernstbrunn Limestone, exposed in the Ernstbrunn Quarries, Ernstbrunn, Austria. Although the age of the Ernstbrunn Limestone, based on microfossil and ammonite biostratigraphy, has been considered to range from the middle Tithonian (Upper Jurassic) to the Berriasian (Lower Cretaceous) (e.g., Moshammer and Schlagintweit, 1999; Zeiss, 2001; Schneider et al., 2013), the age of the rocks bearing meiurans has been consistently treated as middle upper Tithonian (Richterella richteri Zone to Micracanthoceras microcanthum Zone, Simplisphinctes Subzone) (e.g., Zeiss, 2001; Feldmann and Schweitzer, 2009; Schweitzer and Feldmann, 2010a; Robins et al., 2013; Fraaije et al., 2019; Robins and Klompmaker, 2019), for which a tip maximum age and a minimum age for Vibrissalana jurassica can be bracketed between ~152.0 and 145.0 Ma.
As described in Wolfe et al. (2019), a soft maximum age is obtained by phylogenetic bracketing. The oldest crown Brachyura is debatable; Eocarcinus praecursor Withers, 1932, (Figure 2A) and Eoprosopon klugi Förster, 1986, have both been proposed, but both lack some crown group characters. Nevertheless, stem lineage positions of these taxa (Scholtz, 2020) allow a calibration of crown group Meiura with a soft maximum from the base of the Pliensbachian, at 191.8 Ma.
Discussion. Jurellana tithonia Schweitzer and Feldmann, 2010a, also from the Ernstbrunn Limestone, was previously considered the oldest porcellanid, although a recent revision of the taxon has suggested an affinity with homolodromioid brachyurans (Robins and Klompmaker, 2019). As such, Vibrissalana jurassica stands as the putatively oldest crown porcellanid (~152-145 Mya) and represents, to date, a better calibration point for the crown group Porcellanidae.
Brachyura: Dromiacea: Dromioidea: Dynomenidae (crown)
Fossil specimen. Graptocarcinus muiri Stenzel, 1944. Bureau of Economic Geology, University of Texas at Austin. Holotype BEG00021288.000, a large and complete dorsal carapace (Figure 2C).
Phylogenetic justification. The overall ovate carapace outline, being wider than long, with discrete but low-relief dorsal grooves, fits the diagnosis of Dynomenidae (Schweitzer et al., 2012; Van Bakel et al., 2012a).
Minimum age. 100.5 Ma.
Tip maximum age. 113.0 Ma.
Soft maximum age. 191.8 Ma.
Age justification. This fossil was collected in the Taniniúl limestone, upper Lower Cretaceous (Albian), from Choy Cave in Sierra del Abra between Las Palmas and Taninil, at kilometre 550 on the railroad between Tampico and San Luis Potosí, State of San Luis Potosí, Mexico (Stenzel, 1944). Soft maximum age as for calibration 1.
Discussion. Six species of the genus Graptocarcinus Roemer, 1887, ranging from the Lower Cretaceous (Albian) to the middle Eocene (lower Lutetian) (Schweitzer et al., 2012; Beschin et al., 2016a), are currently included within the subfamily Graptocarcininae Van Bakel, Guinot, Corral and Artal, 2012a. Two isolated dactyli from the Barremian “Coulés boueuses”, Serre de Bleyton, France, have been assigned to Graptocarcinus (Hyžný and Kroh, 2015). Due the fragmentary nature of the samples, we considered for calibration the earliest occurrence of fossils with more and better diagnostic features that would warrant inclusion within crown Dynomenidae.
Several taxonomic studies have highlighted the issues of synonymy among some species within the genus Graptocarcinus, for instance, Graptocarcinus muiri, from the Albian of San Luis Potosí, Mexico, and Graptocarcinus texanus Roemer, 1887, from the Albian and Cenomanian of the USA and Europe, with some of them concluding that G. miuri is a junior synonym of G. texanus (e.g., Klompmaker, 2013; Kocova-Veselská et al., 2014, and references therein), whereas other studies have maintained them as separate, valid species (e.g., Schweitzer et al., 2012; Van Bakel et al., 2012a; Beschin et al., 2016a, and references therein). Since the earliest confirmed occurrences of well-preserved fossils of both species are Albian in age, choosing one species over the other would impact minimally the time calibration bracket for the genus (113-100 Mya).
Brachyura: Dromiacea: Dromioidea: Dromiidae (crown)
Fossil specimen. Dromiopsis gigas Forir, 1887. Mineral collections of the Université de Liège, Belgium. Holotype 4936, a large, fragmented dorsal carapace in volume, right side.
Phylogenetic justification. Dromiopsis is an extinct genus with over a dozen species known to date (Schweitzer et al., 2010). The overall circular carapace shape nearly as wide as long, the fronto-orbital configuration, and the well-developed set of dorsal grooves delimiting the dorsal carapace regions, guarantee placement within the crown Dromiidae (Schweitzer et al., 2012).
Minimum age. 93.9 Ma.
Tip maximum age. 100.5 Ma.
Soft maximum age. 191.8 Ma.
Age justification. The type material of Dromiopsis gigas comes from the Tourtias (Formation?), Tournai, Belgium, which has been dated as Cenomanian based on ammonite biozonation (Kennedy et al., 2011).
Discussion. Costadromia hajzeri Feldmann and Schweitzer, 2019, from the Campanian (Upper Cretaceous, ~83-72 Mya) Wenonah Formation in New Jersey, USA, has been recently proposed as the earliest known sponge crab, and included within Dromiidae sensu lato based mostly on details of the frontal margin (Feldmann and Schweitzer, 2019). While C. hajzeri does seem to fit well within the total group Dromiidae, the presence of Dromiopsis gigas in mid-Cretaceous (Cenomanian, ~100-93 Mya) rocks of Belgium, and its conspicuous similarity to other genera within crown Dromiidae, indicate that the type material of D. gigas provides a better current calibration point for crown Dromiidae. Soft maximum age as for calibration 1.
Brachyura: Homoloida: Homoloidea: Homolidae (stem)
Fossil specimen. Doerflesia ornata Feldmann and Schweitzer, 2009. Naturhistorisches Museum Wien (NHMW). Holotype 2007z0149/0015, a small dorsal carapace nearly complete (Figure 2D).
Phylogenetic justification. The genus Doerflesia has several of the diagnostic features of the family Homolidae, and it shows some resemblance to extant genera (Feldmann and Schweitzer, 2009; Feldmann et al., 2012). While a homolid affinity is most likely, its position within the crown group cannot be confirmed beyond doubt with the available fossil material (a dorsal carapace of the holotype and sole specimen), therefore, we have chosen to treat it as a stem group member of Homolidae.
Minimum age. 145.0 Ma.
Tip maximum age. 152.0 Ma.
Soft maximum age. 191.8 Ma.
Age justification. This fossil was collected from the Ernstbrunn Limestone, exposed in the Ernstbrunn Quarries, near the village of Dörfles, Austria. Soft maximum age as for calibration 1.
Brachyura: Raninoida: Palaeocorystoidea: Palaeocorystidae (stem)
Fossil specimen. Joeranina kerri (Luque, Feldmann, Schweitzer, Jaramillo, and Cameron, 2012) (as Notopocorystes). Colombian Geological Survey (formerly INGEOMINAS), Museo Geológico José Royo y Gómez, Bogotá DC, Colombia. Holotype IGM p881128, a nearly complete dorsal carapace (Luque et al., 2012, pp. 411-413, figure 4A, B.; Luque et al., 2017, p. 20, figure 8B) (Figure 2E).
Phylogenetic justification. Recent morphological phylogenetic analyses have shown consistently that crabs of the extinct superfamily Palaeocorystoidea occupy an intermediate place between the crab-like Necrocarcinoidea and the frog-like Raninoidea (Karasawa et al., 2014; Luque, 2015b; Schweitzer et al., 2016b; Luque et al., 2019a). As such, Palaeocorystoidea is recovered as part of the stem group of Raninoidea, the latter of which includes all of the extant genera of frog crabs.
Minimum age. 113.0 Ma.
Tip maximum age. 118.0 Ma.
Soft maximum age. 191.8 Ma.
Age justification. The holotype of Joeranina kerri was discovered in situ by one of us (J.L.) in grey shales of the upper portion of the Lower Cretaceous Paja Formation, exposed along the road between the towns of San Gil and Curití, in the Department of Santander, Colombia. The occurrence of the gastropod Turritella (Haustator) columbiana Jaworski, 1938, and the ammonite Acanthohoplites eleganteante Etayo-Serna, 1979, stratigraphically below the horizon yielding the holotype of J. kerri, indicate an upper Aptian age in Colombia (see in Luque et al., 2012; Luque, 2014). Soft maximum age as for calibration 1.
Discussion. Frog crabs and allies, together constituting the Section Raninoida (Ahyong et al., 2007), are among the most anatomically disparate brachyuran groups (Schweitzer et al., 2012; Van Bakel et al., 2012b; Karasawa et al., 2014; Hartzell et al., 2022). Recent phylogenetic studies have shown that early-branching forms, referred to as the crab-like raninoidans (e.g., Orithopsidae, Paranecrocarcinidae, Necrocarcinidae, Cenomanocarcinidae) are sister groups to a clade formed by mostly decarcinized, frog-like raninoidan families such as the extinct Palaeocorystidae and the extant Raninidae and allied relatives (Luque, 2015b; Schweitzer et al., 2016b; Luque et al., 2019a). As such, the most recent common ancestor of Palaeocorystidae + Raninidae must be as old or older than the oldest fossils known within Palaeocorystidae, which corresponds to Joeranina kerri from the upper Aptian (Lower Cretaceous, 118-113 Mya) of Colombia, South America, as indicated above.
Brachyura: Raninoida: Raninoidea: Lyreididae (stem)
Fossil specimen. Marylyreidus punctatus (Rathbun, 1935, as Notopocorystes). University of Texas. Holotype BEG-21207 (=BEG00021207.000), a nearly complete dorsal carapace in volume (not illustrated herein). A non-type specimen, USNM 559038, is illustrated in Figure 2F.
Phylogenetic justification. The type material of Marylyreidus punctatus, together with additional specimens known from different localities and preserving dorsal, ventral, cuticular, and pleonal details, strongly show a crown Raninoidea affinity, with a sternal configuration suggesting a close proximity to lyreidids (Van Bakel et al., 2012b; Karasawa et al., 2014; Franţescu et al., 2016; Schweitzer et al., 2018). This is consistent with the phylogenetic position of Marylyreidus, recovered in a parsimony analysis as sister to Bournelyreidus van Bakel, Guinot, Artal, Fraaije, and Jagt, 2012b, both forming an early diverging stem group sister to the remainder of the lyreidid-like frog crabs (Karasawa et al., 2014).
Minimum age. 100.5 Ma.
Tip maximum age. 104.0 Ma.
Soft maximum age. 191.8 Ma.
Age justification. The type specimen of Marylyreidus punctatus was discovered in rocks of the Lower Cretaceous (Albian), indicated by Rathbun (1935) as belonging to the ‘Denton Clay, lower Comanche Series, Washita Formation’, cropping out in Grayson County, two miles north of Denison, Texas, USA (Rathbun, 1935). Currently, the Washita ‘Formation’ in Texas is considered at the Group level, and constituted at its thickest part by seven formations, in ascending order: Kiamichi, Duck Creek, Fort Worth, Denton, Weno, Paw Paw, and Main Street formations (Scott et al., 2002; Scott et al., 2016). The entire Washita Group from base to top is thought to have been deposited between 104 and 94.4 Ma, with its lower boundary in the lowermost upper Albian, and with the upper Albian-lower Cenomanian boundary (100.5 Ma) represented in the uppermost Main Street Limestone (Scott et al., 2000; Scott et al., 2002). The Denton Formation has been dated as upper Albian, based on the occurrence of ammonites from the Mortoniceras (Subschloenbachia) rostratum Zone (Kennedy et al., 2005). Additional specimens assigned to M. punctatus have been recovered from several localities and stratigraphic intervals in Texas, including the slightly younger but also upper Albian Paw Paw Formation (Franţescu et al., 2016). As such, relaxing the lowermost and uppermost potential occurrences of M. punctatus within the Washita Group, its tip maximum and minimum ages can be bracketed within 104.0-100.5 Ma. Soft maximum age as for calibration 1.
Discussion. Wolfe et al., 2023 recovered a monophyletic Lyreididae nested within a paraphyletic ‘Raninidae’. As such, despite Marylyreidus being part of the stem group to Lyreididae (or Lyreininae, if confirmed to be a subfamily within Raninidae), it belongs to the crown Raninoidea. Besides M. punctatus, only a few other raninoideans are currently known from upper Albian rocks worldwide, both initially assigned to the genus Hemioon Bell, 1863, traditionally considered a Lyreididae. These are ‘H. cunningtoni’ Bell, 1863, the type species of the genus and known from the upper Albian of England, and ‘Hemioon yanini’ Ilyin and Alekseev, 1998, known from the upper Albian of Crimea. However, both records are problematic. Firstly, ‘Hemioon’ has been a problematic taxon due to the few specimens known and their poor preservation, resulting in some authors considering it a valid taxon, whereas others have synonymized it with Raninella A. Milne-Edwards, 1862b (see discussions in Bishop and Williams, 2000; Waugh et al., 2009; Van Bakel et al., 2012b; Karasawa et al., 2014; Schweitzer et al., 2018 and references therein). Secondly, Raninella ‘cunningtoni’ might be a juvenile of Raninella elongata Milne-Edwards, 1862a (see Bishop and Williams, 2000; Van Bakel et al., 2012b; Karasawa et al., 2014 and references therein). Thirdly, Raninella is currently considered a member of the subfamily Ranininae within the Raninidae, in which case this family would also have an upper Albian record. Unfortunately, besides the conflicting views on their systematic affinities, the exact age of ‘H. cunningtoni’ needs further revision (B. van Bakel, pers. comm. to J.L., February 2023).
Regarding ‘Hemioon yanini’, its age is better constrained than the species above discussed, being referable to the upper Albian based on its occurrence in the Mortoniceras infiatum Zone (Ilyin and Alekseev, 1998; Ilyin, 2005), but its systematic placement is equally convoluted. Van Bakel et al. (2012b) included this species within Raninella, whereas Karasawa et al. (2014) included it within the genus Macroacaena Tucker, 1998, under the lyreidid subfamily Macroacaeninae Karasawa, Schweitzer, Feldmann, and Luque, 2014 (Schweitzer et al., 2018). Regardless the generic and specific affinities of ‘H. cunningtoni’ and ‘H. yanini’, the presence of disparate raninidoid forms in the early Albian of USA, UK, and Crimea, indicate that the most recent common ancestor of Raninodiea and all its descendants must have originated before late Albian.
Brachyura: Cyclodorippoida: Cyclodorippoidea: Cymonomidae (crown)
Fossil specimen. Cymonomus primitivus Müller and Collins, 1991. Természettudományi Múzeum, Föld-és oslénytar, VIII Muzeum krt. 14-16, H-1088 Budapest, Hungary. Holotype HNHM M.91-135, a partial dorsal carapace (Müller and Collins, 1991, p. 61, 63-64, pl. 3, figure 6) (Figure 2G).
Phylogenetic justification. The Hungarian specimen can be referred to crown Cyclodorippoidea and is assignable to Cymonomidae based on diagnostic characteristics of the subquadrate dorsal carapace, the narrow fronto-orbital margin, and the configuration of the dorsal grooves and regions, which fit well within the diagnosis of crown Cymonomidae (Müller and Collins, 1991; Tavares, 1993; Ahyong, 2019).
Minimum age. 33.9 Ma.
Tip maximum age. 37.71 Ma.
Soft maximum age. 191.8 Ma.
Age justification. Cymonomus primitivus was collected from 4-5 m thick coral-bearing limestones of Facies 4 of the Upper Eocene (Priabonian) Szépvölgy Limestone Formation, cropping out at the Ruprecht quarry, Budapest, Hungary, with the coral fauna indicating a Priabonian age (Müller and Collins, 1991). Soft maximum age as for calibration 1.
Discussion. Besides Cymonomus primitivus, other presumed cymonomids are known from the Eocene, e.g., Spathanomus felicianensis De Angeli, 2016, and Caporiondolus bericus De Angeli, 2016, from the Priabonian of the Orgiano quarry, Monti Berici, Italy, and Eonomus californianus Nyborg, Garassino, and Slak, 2017, from the early to middle Eocene Llajas Formation, Simi Valley, California, USA. We selected C. primitivus as our vetted calibration point for crown Cymonomidae (~37-33 Ma), since Cymonomus is an extant genus, whereas Spathanomus, Caporiondolus, and Eonomus are all extinct genera, and their family-level affinities are considered by some authors as uncertain (e.g., Schweitzer et al., 2017).
Brachyura: Eubrachyura: Potamoidea: Potamidae (crown)
 Fossil specimen. Alontecarcinus buratoi De Angeli and Caporiondo, 2019. Museo di Storia Naturale di Verona. Holotype IGVR 19.38, a complete dorsal carapace (Figure 3B).
Fossil specimen. Alontecarcinus buratoi De Angeli and Caporiondo, 2019. Museo di Storia Naturale di Verona. Holotype IGVR 19.38, a complete dorsal carapace (Figure 3B).
Phylogenetic justification. The type material of Alontecarcinus buratoi shows remarkable similarities with the dorsal carapaces of extant potamid crabs, including their oval outline and wide carapace, the well-developed cervical and gastro-cardiac grooves, a front that is entire and directed downwards, and the convex lateral margins that are smooth and bear a single epibranchial tooth (De Angeli and Caporiondo, 2019).
Minimum age. 37.71 Ma.
Tip maximum age. 41.2 Ma.
Soft maximum age. 100.9 Ma.
Age justification. This fossil was collected from middle Eocene limestones of the Alonte quarry in Berici Mounts, Vicenza, Northern Italy (De Angeli and Caporiondo, 2019). The stratigraphic position of the Alonte quarry Limestones, overlying rocks of middle Eocene age, and underlying rocks of Priabonian age, together with associations of gastropods and bivalves (e.g., Ampullina, Cerithium, Campanile, Natica, Corbis, Glycimeris), echinoids (e.g., Leiopedina, Sismondia, Echinolampas, Schizaster, Cidaris), and calcareous nannofossils, indicate a Bartonian age for the Alonte quarry Limestones (41.2-37.71 Ma) (Beccaro, 2003; De Angeli and Alberti, 2016; De Angeli and Caporiondo, 2019).
As this node is within Eubrachyura, a soft maximum age is based on Cretapsara athanata Luque in Luque et al., 2021, which is the oldest crown group Eubrachyura (Figure 3A). The position of C. athanata relative to modern eubrachyuran families is not clear, but its membership within the crown group, based on morphological phylogenetic analysis (Luque et al., 2021), indicates that the common ancestor of typical eubrachyuran forms must be older than the earliest Late Cretaceous. The fossil was found in Burmese amber, for which the exact age estimates can vary, depending on the source. The limited radioisotopic U-Pb information available for Kachin burmite indicates an age of 98.8 ± 0.6 Ma (Shi et al., 2012), which would suggest a bracketed tip maximum age and minimum age for C. athanata between 99.4 Ma and 98.2 Ma. Radiometric estimates pertain primarily to the sediments and not necessarily the amber itself (see discussion in Luque et al., 2021). Therefore, we calibrate a soft maximum close to the Albian-Cenomanian boundary 100.5 Ma ± 0.4 Myr = 100.9 Ma.
Brachyura: Eubrachyura: Ocypodoidea: Ocypodidae (crown)
Fossil specimens. Afruca miocenica (Artal, 2008, as Uca). Museo Geológico del Seminario de Barcelona (MGSB), Catalonia, Spain. Holotype MGSB 68653, a complete and well-preserved dorsal carapace (Figure 3C), and paratypes 68654a to 68654e, including well-preserved dorsal and ventral males and females with pleon, maxillipeds, pereopods, and the male major cheliped.
Phylogenetic justification. The type material of Afruca miocenica can be confidently assigned to the genus Afruca Crane, 1975, based on the diagnostic enlarged and flattened dactyli and pollices of the male major cheliped, and it is closest to the extant Afruca tangeri (Eydoux, 1835), which is the only recognized species of fiddler crabs living today in the Iberic Peninsula and north Atlantic Africa and is sister to the tropical American clade Uca Leach, 1814 (Crane, 1975; Rosenberg, 2001; de Gibert et al., 2013; Luque et al., 2018). While A. miocenica might indeed represent its own species, its overall similarities with A. tangeri have invited the question of whether they are synonymous, with the former representing ontogenetic variations like those seen in younger individuals of the latter (de Gibert et al., 2013). In either scenario, the proximity between A. miocenica and A. tangeri is remarkable, and the phylogenetic assignment of the fossils from Catalonia to the extant genus Afruca–and thus to the crown Ocypodidae–are confirmed.
Minimum age. 13.82 Ma.
Tip maximum age. 15.97 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The fossil material of Afruca miocenica was collected in situ in terrigenous yellow claystones from a Middle Miocene (Langhian, 15.97-13.82 Ma) locality of the Rubí (Vallés), Province of Barcelona, Catalonia, NE Iberian Peninsula (Artal, 2008). These deposits are part of the Vallès-Penedès Basin, and represent marine to coastal mangrove facies deposited during the maximum transgression of the Langhian in the area (Batllori and García, 1997; de Gibert and Robles, 2005; Artal, 2008; Garassino et al., 2009), overlying continental Burdigalian facies (Casanovas-Vilar et al., 2011), and underlying continental Serravallian facies (Batllori and García, 1997; de Gibert and Robles, 2005; Casanovas-Vilar et al., 2016). Soft maximum age as for calibration 8.
Discussion. The fossil record of fiddler crabs is sparse and fragmentary, with only half a dozen species recognized from fossilized carapaces (e.g., Artal, 2008; Domínguez Alonso, 2008; de Gibert et al., 2013; Luque et al., 2017; Luque et al., 2018; Lima et al., 2020). Besides Afruca miocenina, the only other Miocene fiddler crab known is Uca maracoani (Latreille, 1802) from Brazil (Brito, 1972; Luque et al., 2017; Luque et al., 2018; Lima et al., 2020). The fossil material of U. maracoani was collected in rocks of the Pirabas Formation, in the village of Baunilha Grande, Baia de Quatipuru, Pará State, Brazil (Brito, 1972), and subsequent material from the area has been reported recently (Lima et al., 2020). However, the exact age and stratigraphic provenance of these specimens is unclear. Overall, palynological, lithological, petrographic, and geochemical data indicate that the Pirabas Formation as a whole is constituted by two distinct depositional groups: the oldest and lowermost one, assigned to the uppermost lower Miocene, deposited in a shallow marine environment, and the youngest and uppermost one, assigned to the upper middle Miocene to upper Miocene, deposited in a coastal environment near mangroves (e.g., Aguilera et al., 2022; Gomes et al., 2023). In localities such as Capanema, where the lowermost depositional unit outcrops, absolute 87Sr/86 Sr dating obtained from pectinid bivalve shells have yielded ages of 17.3-16.0 Ma, suggesting a latest early Miocene (upper Burdigalian) age (Martinez et al., 2017; Gomes et al., 2023). In localities such as Praia do Atalaia and Praia do Maçarico, where the uppermost depositional unit outcrops, palynomorphs of the T16 biozone (Jaramillo et al., 2011) indicate an age of 12.7 to 7.1 Ma, suggesting a latest middle Miocene to late Miocene age (mid Serravalian to lowermost lower Messinian) (Gomes et al., 2023). The fiddler crab-bearing facies of the Pirabas Formation at the ‘Furo de Baunilha Grande’ locality correspond to the latter, younger unit, with a most probable age of uppermost middle Miocene to late Miocene, roughly corresponding to the Serravallian-Tortonian (13.82-7.246 Ma) (Gomes et al., 2023). Moreover, these fiddler crab-bearing nodules are not in situ, but found loose on the Baunilla stream floor (Lima et al., 2020; Orangel Aguilera, pers. comm. to J.L., February 2023), and although previous works have presumably recovered palynological samples associated to these nodules that would suggest a uppermost lower Miocene rock age (e.g., Antonioli et al., 2015), more recent and comprehensive biostratigraphic and paleonvironmental studies across localities have confirmed the latest middle Miocene to late Miocene age of the Baunilha Grande rocks (Gomes et al., 2023). As such, we opt to use the apparently older and in situ occurrence of Afruca miocenica (Langhian, 15.97-13.82 Ma) as our calibration point for Ocypodidae.
Aside from the two fiddler crab species above mentioned, the only other Ocypodidae with Miocene fossils known to date is Ocypode vericoncava Casadío et al., 2005, a single damaged dorsal carapace from the upper Miocene (Tortonian? 11.63-7.246 Ma) of Argentina. Despite the poor preservation of the holotype and sole specimen, the overall shape and fronto-orbital configuration match those of Ocypode. Thus, this occurrence from Argentina, together with those of Uca from Brazil and Afruca in Catalonia, indicate that Ocypodidae was already widespread by the mid-Miocene, and that the most recent common ancestor of Ocypodidae and its divergence from other thoracotremes must have occurred in the pre-Neogene, most likely in the Paleogene or even the Cretaceous (Wolfe et al., 2023).
Brachyura: Eubrachyura: Pinnotheroidea: Pinnotheridae (crown)
Fossil specimen. Pinnixa sp. Invertebrate Paleontology collections, Florida Museum of Natural History. Specimen UF 115397, a complete dorsal carapace (Luque et al., 2017, figure 12Q) (Figure 3D).
Phylogenetic justification. Based on the overall carapace shape and dorsal patterns, this fossil specimen can be assigned with confidence to the genus Pinnixa. Recent molecular studies, however, have shown that Pinnixa species may not form a monophyletic genus, but instead may be distributed among three Pinnotheridae subfamilies (e.g., Palacios Theil et al., 2016). Despite this, they all can be assigned to the crown-group Pinnotheridae, justifying the inclusion of the fossil Pinnixa from the lower Miocene of Panama in the crown group.
Minimum age. 18.7 Ma.
Tip maximum age. 21.68 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The specimen was collected in situ by one of us (J.L.) in marine grey mudstones from the middle part of the upper member of the Culebra Formation in the Panama Canal Zone, Lirio section, Culebra Cut (formerly known as Gaillard Cut). The marine rocks of the Culebra Formation are lower Miocene (uppermost Aquitanian-lower Burdigalian), and not Oligocene as assumed by Rathbun, 1918 [1919] and repeated as such by subsequent authors (see notes in Luque et al., 2017). At its base, the marine Culebra Formation overlies in sharp unconformity the terrestrial upper Oligocene-lower Miocene rocks of the Las Cascadas Formation, and its top, it underlies conformably the coarser terrestrial rocks of the upper lower to middle Miocene Cucaracha Formation (Montes et al., 2012; Buchs et al., 2019; LeBlanc, 2021). Kirby et al. (2008) reported Sr isotope dating ages for different parts of the formation, including four ages from Acropora corals and pectinids in the middle member of the Culebra Formation (Emperador Limestone), ranging between 21.24±0.44 and 20.62±0.58 Ma, and two ages from a pectinid and an ostreid (19.83±0.39 and 19.12±0.42 Ma, respectively), from the lowermost portion of the Cucaracha Formation, above the top of the upper member of the Culebra Formation (Kirby et al., 2008, figure 6, table 2). Montes et al. (2012) reported a U/Pb age of 19.3 ±0.4 Ma from magmatic zircons in an ash tuff from the lower half of the upper member of the Culebra Formation, and MacFadden et al. (2014) reported U/Pb and Ar/Ar ages of 18.81±0.3 and 18.96±0.9 Ma, respectively, from detrital zircons in an ash tuff from the overlying Cucaracha Formation. As such, based on the currently available absolute ages available, the age of the crab-bearing beds in the middle part of the upper member of the Culebra Formation–and thus that of Pinnixa sp.–can be bracketed with confidence between 21.68 and 18.7 Ma, most likely around 20-19 Ma (see Farris et al., 2017), in the lowermost Burdigalian, near the Aquitanian-Burdigalian boundary. Soft maximum age as for calibration 8.
Discussion. The fossil record of pinnotherids is among the most controversial among eubrachyurans, in part because of their overall small sizes, their largely fragmentary fossil record, and the overall carapace outline similarities of some fossils to some species of other eubrachyurans (e.g., some hexapodids and aphanodactylids). This obscures the systematic affinities of several fossils, and thus casts doubt of their usefulness for node calibrations of crown Pinnotheridae.
The extinct genus Viapinnixa Schweitzer and Feldmann, 2001, comprises four species that range in age from early Paleocene to early Eocene (e.g., Philippi, 1887; Vega et al., 2001; Vega et al., 2007; Vega et al., 2008; Armstrong et al., 2009), which are remarkably similar to species within Hexapodidae. As such, the genus cannot be assigned with certainty to the pinnotheroids (Luque et al., 2017), and even less so to the crown group Pinnotheridae. Other fossil currently placed in the family Pinnotherinae include the spectacularly preserved extinct genus Pharkidodes Feldmann, Schweitzer, Casadío and Griffin, Feldmann et al., 2011b, from the middle Miocene of Tierra del Fuego, Argentina, which unfortunately cannot be placed within the crown group with certainty.
Three fossil species have been dubiously assigned to Pinnotheres, i.e., P. ? araucana Philippi, 1887, from the ‘Tertiary’ of Chile, P. ? elatus Milne-Edwards, 1873, from the upper Miocene of France, and P. ? promaucanus Philippi, 1887, from the Miocene of Chile (Schweitzer et al., 2010). Over half a dozen species have been previously assigned to Pinnixa. ‘Pinnixa’ eocenica Rathbun, 1926, from the Eocene of Washington, USA, is a junior homonym of Pinnixa eocenica Woods, 1922, and it is currently recognized as a hexapodid: Palaeopinnixa rathbunae Schweitzer, Feldmann, Tucker, and Berglund, 2000.
Pinnixa navidadensis Feldmann, Schweitzer, and Encinas, 2005, from the middle Miocene of Chile (Feldmann et al., 2010; Jagt et al., 2015); and Pinnixa sp., from the middle Pleistocene of Florida (Agnew, 2001; Portell and Agnew, 2004), can be assigned to Pinnixa, but they are younger than Pinnixa sp. from the lower Miocene Culebra Formation in Panama. Pinnixa aequipunctata Morris and Collins, 1991, and P. omega Morris and Collins, 1991, come from the Pliocene Upper Miri Formation, Brunei. Pinnixa microgranulosa Collins, Lee, and Noad 2003, from the Miocene Sandakan Formation, Sarawak, may also belong to the hexapodid genus Palaeopinnixa.
As such, given the reliable systematic placement and the constrained stratigraphic age of Pinnixa sp. from the lower Miocene Culebra Formation in the Panama Canal Zone (Luque et al., 2017), this is to date the most reliable calibration datum for the crown group Pinnotheridae, while the most recent common ancestor of all extant pinnotherids must have originated in the pre-Neogene, most likely the Paleogene or Late Cretaceous (see Wolfe et al., 2023).
Brachyura: Eubrachyura: Grapsoidea: Percnidae (crown)
Fossil specimen. Percnon paleogenicus De Angeli, 2023. Museo Civico “D. Dal Lago” di Valdagno (MCV), Vicenza, northern Italy. Holotype MCV.23/738-22.341, a complete dorsal carapace, part and counterpart (Figure 3F).
Phylogenetic justification. Seven extant species in the genus Percnon constitute the monophyletic family Percnidae Stevcic, 2005, which is nested within polyphyletic Grapsoidea (e.g., Schubart and Cuesta, 2010; Wolfe et al., 2023). The fossil species P. paleogenicus has a carapace shape and an orbitofrontal configuration that are typical of Percnidae close to Percnon, and thus is a reliable calibration point for the family.
Minimum age. 33.9 Ma.
Tip maximum age. 37.71 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The holotype of P. paleogenicus was collected from calcarenites and calcareous algae levels of the Collina di San Feliciano (Orgiano, Vicenza), which has been dated as upper Eocene (Priabonian, ~37.71-33.9 Mya) based on nannofossils (Beccaro, 2003, in De Angeli and Garassino, 2021a, b) (Figure 1).
Discussion. Until recently, the only and thus oldest fossil assignable to Percnidae was Percnon santurbanensis Ceccon and De Angeli, 2019, represented by a well-preserved dorsal carapace collected from the lower Oligocene (Rupelian, ~33.90-27.82 Ma) of the Berici eastern Lessini mountains in Montecchio Maggiore, Vicenza, northern Italy (Ceccon and De Angeli, 2019) (Figure 3E). We used this fossil recently in Wolfe et al., 2023) as the calibration point for Percnidae, since P. paleogenicus was published after the completion of the phylogenetic study and became available to us at a later stage. What is clear is that there is more than one fossil occurrence referrable to Percnidae and Percnon per se, and that the crown group Percnidae must have a pre-Priabonian origin, probably into the Paleocene or latest Cretaceous.
Brachyura: Eubrachyura: Grapsoidea: Grapsidae (crown)
Fossil specimen. Pachygrapsus hungaricus Müller, 1974. Hungarian Natural History Museum, department of Earth Sciences and Palaeontology. Holotype HNHM 2004.163.1, a nearly complete dorsal carapace (Figure 3G).
Phylogenetic justification. Morphological and molecular phylomitogenomic studies indicate that Pachygrapsus is phylogenetically well nested within crown Grapsidae, close to Grapsus (e.g., Karasawa and Kato, 2001; Chen et al., 2019; Lü et al., 2022; Tsang et al., 2022; Zhang et al., 2022; Wolfe et al., 2023).
Minimum age. 12.6 Ma.
Tip maximum age. 13.82 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Pachygrapsus hungaricus was collected from a patch reef with Porites of the Leitha Limestone Formation, exposed at Téteny-plateau, western part of the Budapest area, Hungary, dated as middle Miocene (late Badenian, Decapod Zone 4 sensu Müller, 1984) (Hyžný, 2016; Hyžný and Dulai, 2021). Several tens of additional specimens referred to P. hungaricus have also been reported from coeval rocks in several localities in the Budapest area (Gyakorló út, Örs vezér tere, and Rákos), as well as in its vicinity (i.e., Biatorbágy and Diósd) in Hungary, and from coeval strata in Austria and Poland (Müller, 1984, 1996; Hyžný, 2016; Hyžný and Dulai, 2021). Other conspecific occurrences are known from slightly older strata as well (Decapod Zone 3 sensu Müller, 1984). Thus, P. hungaricus has been reliably documented from the entire span of the upper Badenian (13.82-12.6 Ma) (Kováč et al., 2018; M. Hyžný, pers. comm. to J.L., February 2023) (Figure 1, Figure 3G).
The age of the lower and upper boundaries of the Paratethyan Badenian stage/age have been a matter of ongoing discussion. While some authors have considered that the age of the Badenian lower boundary is positioned at the top of chron C5Cn.2n (16.303 Ma), roughly equivalent to the uppermost part of the global Lower Miocene Burdigalian stage/age (e.g., Piller et al., 2007; Hohenegger et al., 2014; Wagreich et al., 2014), others have considered the age of the Badenian lower boundary to be closer to the base of the global Langhian stage/age (15.97 Ma) (Krijgsman and Piller, 2012; Reichenbacher et al., 2013; Gozhyk et al., 2015; Kováč et al., 2018). Similarly, for several authors, the age of the Badenian upper boundary is positioned at the top of polarity chron C5Ar.2n (12.829), roughly equivalent to the middle part of the global Middle Miocene Serravallian stage/age boundary (e.g., Hohenegger et al., 2014; Wagreich et al., 2014), whereas for others, the Badenian/Sarmatian boundary is slightly younger (12.7-12.6 Ma) (e.g., Piller et al., 2007; Krijgsman and Piller, 2012; Kováč et al., 2018, see also Hyžný and Dulai, 2021 and references therein). This reflects the issues of interpreting minimum and tip maximum ages for taxa based on the literature alone, since the lithostratigraphic boundary ages in different localities might vary in relation with eustatic sea-level changes and local subsidence, and rarely a crab fossil occurrence is tied to absolute dating.
Since the goal of our calibration is not to resolve the exact stratigraphic age of a given occurrence but to provide a confidence range that brackets the minimum and tip maximum ages, we opt to consider the minimum age of P. hungaricus as 12.6 Ma, and the tip maximum age as 13.82 Ma, which roughly delimit the beginning and the end of the late Badenian (Kováč et al., 2018). Soft maximum age as for calibration 8.
Discussion. Fossils of grapsid crabs are rare, in part due to the relatively dynamic and high-energy environments they inhabit (Luque et al., 2018). Among grapsids, the genera Goniopsis De Haan, 1833-1850, and Leptograpsus Milne Edwards, 1853(monotypic) have no confirmed fossil record. The genera Geograpsus and Grapsus have fossils known from the Holocene of Hawaii, i.e., Geograpsus severnsi Paulay and Starmer, 2011, and the Holocene of the Pacific in Panama, i.e., G. aff. G. grapsus (Luque et al., 2018). The extant genus Metopograpsus has two extinct species currently assigned to it, e.g., M. badenis Müller, 2006, from the same middle Miocene (Badenian) locality in Hungary as Pachygrapsus hungaricus, and M. traxleri Müller, 1998, from the lower Miocene (Karpatian) of Austria (Hyžný, 2016; Hyžný and Dulai, 2021). While any of the fossil metopograpsid species could serve as potential good candidates as vetted calibration points for crown Grapsidae, the fragmentary holotypes missing the fronto-orbital margins make their systematic placement less confident than those fossils referred to P. hungaricus.
The extinct genus Miograpsus Fleming, 1981, and its sole species M. papaka Fleming, 1981, is represented by a well-preserved female holotype and a ventrally exposed paratype from a “silty sandstone pebble, not in place” (ex-situ) (Fleming, 1981, p. 105), presumably from the lower Hurupi Formation, lower part of Tongaporutuan Stage (upper Miocene) of New Zealand. Although the genus Miograpsus has been placed within Grapsidae by several authors (e.g., Feldmann, 1993; Schweitzer et al., 2010), it seems to have closer affinities to genera within ‘Varuninae’ (now considered its own family, Varunidae) (e.g., Fleming, 1981; Karasawa and Kato, 2001; Webber et al., 2010). Since Miograpsus is 1) an extinct genus, 2) slightly younger than the fossil record of crown genera such as Pachygrapsus and Metopograpsus, and especially 3) may have putative varunid affinities, we refrain from using this taxon as a calibration point for crown Grapsidae. ‘Nautilograpsoides’ prior Smirnov, 1929, a fossil species from the lower Miocene of the Caucasus, is currently envisioned as a juvenile form of the portunid genus Liocarcinus (Hyžný et al., 2022, and references therein).
Pachygrapsus, on the other hand, has a couple of fossil occurrences that can be assigned to this genus. Fragmentary cheliped remains of Pachygrapsus sp. are known from the upper Pleistocene of Jamaica (Morris, 1993; Luque et al., 2017), and P. hungaricus is known from several tens of specimens from the middle Miocene (Badenian) of Europe (see above). Since Pachygrapsus is an extant genus with a good Miocene fossil record, as represented by the type series and numerous additional specimens of P. hungaricus, we selected the latter as our calibration point for crown Grapsidae. The presence of Pachygrapus and Metopograpsus in the Miocene indicates that the most recent common ancestor of crown Grapsidae and all its descendants must have a pre-Miocene origin, and likely rooted into the early Paleogene or Late Cretaceous.
Brachyura: Eubrachyura: Grapsoidea: Varunidae (crown)
Fossil specimen. Brachynotus corallinus Beschin, Busulini, De Angeli and Tessier, 2007. Museo di Archeologia e Scienze Naturali “G. Zannato” di Montecchio Maggiore, Vicenza, northern Italy. Holotype MCZ 1794, a nearly complete dorsal carapace (Figure 3H).
Phylogenetic justification. The overall carapace morphology indicates that the holotype of Brachynotus corallinus can be referred to crown Varunidae, which is distantly related to Grapsidae, the eponym of the polyphyletic superfamily Grapsoidea (Chen et al., 2019; Lü et al., 2022; Tsang et al., 2022; Wolfe et al., 2023)
Minimum age. 47.8 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. This coral-associated fossil was collected from lower Eocene (Ypresian) marine calcareous rocks, exposed at the Contrada Gecchelina of Monte di Malo in Vicenza, northern Italy. The faunal association includes foraminifera, corals, calcareous algae, and molluscs. Foraminifera like Nummulites cf. partschi, N. tauricus, and N. nitidus, indicate a middle-upper Ypresian age (Beschin et al., 2007), and are stratigraphically correlated with the nearby ‘Rossi’ quarry (cava ‘Rossi’) (Beschin et al., 1998).
Discussion. Other Paleogene fossils of crown Varuninae include Brachynotus oligocenicus De Angeli, Garassino and Ceccon, 2010b, a nicely preserved dorsal carapace from the lower Oligocene of Vicenza, Italy (De Angeli et al., 2010b), confirming the presence of the genus and the family early in the Paleogene.
Brachyura: Eubrachyura: Grapsoidea: Gecarcinidae (crown)
Fossil specimen. Cardisoma guanhumi Latreille, in Latreille, Le Peletier, Serville and Guérin, 1828. NCB - Naturalis, Leiden, the Netherlands, specimen NNM RGM 544 482, a large and mostly complete right chela preserving the dactylus and pollex. Specimen illustrated in Figure 3I corresponds to a dactylus of C. guanhumi from the Pleistocene of Bermuda (Luque, 2017).
Phylogenetic justification. Cardisoma guanhumi is an extant gecarcinid species closely related to Gecarcinus, the type genus of the family Gecarcinidae. The phylogenetic position of Gecarcinidae is contentious with respect to other families within Grapsoidea. Recent nuclear and mitochondrial phylogenetic studies have recovered a non-monophyletic Grapsoidea, with gecarcinids closer to taxa within the families Sesarmidae and even Varunidae than to any taxon within Grapsidae (e.g., Chen et al., 2019; Liu et al., 2021; Lü et al., 2022; Tsang et al., 2022; Wolfe et al., 2023).
Minimum age. 0.0117 Ma.
Tip maximum age. 0.129 Ma.
Soft maximum age. 100.9 Ma.
Age justification. This fossil was collected in the Port Morant Formation, parish of St. Thomas, east Port Morant Harbour, southeast Jamaica (Collins and Donovan, 1997, 2010). The Port Morant Formation has been dated as middle-late Pleistocene (132 ± 7 kyr to 125 ± 9 kyr, Sangamonian Stage) based on electron spin resonance dating on samples of the coral species Solenastrea bournoni and Siderastrea radians (Mitchell et al., 2000; Mitchell et al., 2001; James-Williamson and Mitchell, 2012). Given the age uncertainty of the fossil specimens from the Port Morant Formation with respect to the coral samples dated, we bracketed the soft maximum and minimum ages for the fossil occurrences of C. guanhumi between the base and the top of the Late Pleistocene (0.129-0.0117 Ma), although we expect the family as a whole to be much older than Pleistocene, most likely originating in the Paleogene or possibly late Cretaceous (see in Wolfe et al., 2023). Soft maximum age as for calibration 8.
Discussion. The fossil record of gecarcinid land crabs is sparse and fragmentary, and largely represented by isolated claw remains (e.g., Luque, 2017) (Figure 3I). To date, all the known fossils assignable to Cardisoma are Quaternary in age, i.e., late Pleistocene (Collins and Donovan, 1997; Luque, 2017) or late Holocene (Luque, 2017; Luque et al., 2017; Luque et al., 2018), making the record from the upper Pleistocene of Jamaica the oldest fossil material referable to Gecarcinidae with confidence.
Brachyura: Eubrachyura: Grapsoidea: Sesarmidae (crown)
Fossil specimens. Sesarmidae gen. et sp. indet., in Serrano-Sánchez et al. (2016). Museo de Paleontología “Eliseo Palacios Aguilera” (Secretaría de Medio Ambiente e Historia Natural), Calzada de los Hombres Ilustres s/n, Tuxtla Gutiérrez, Chiapas, Mexico, specimens with acronym IHNFG (Instituto de Historia Natural, Fósil Geográfico) IHNFG-4969, IHNFG-4970, IHNFG-4991, IHNFG-4992, and IHNFG-5555. A handful of specimens are preserved in amber, mostly represented by complete dorsal carapaces and ventral thoracic sterna with limbs attached (Serrano-Sánchez et al., 2016, figures 3, 4.1, 4.3-47; Luque et al., 2017, figure 13L) (Figure 3J).
Phylogenetic justification. Despite the unclear generic and specific affinities of the fossil grapsoids in amber from the Miocene of Chiapas, Mexico, the fossils can be assigned with confidence to crown Sesarmidae based on the overall morphology of the dorsal carapace, the construction of the orbitofrontal margin, I shape and size of the chelipeds and pereopods, and the stratigraphic and palaeoecological setting (Serrano-Sánchez et al., 2016; Luque et al., 2017).
Minimum age. 23.0 Ma.
Tip maximum age. 23.03 Ma.
Soft maximum age. 100.9 Ma.
Age justification. These fossils were collected in rocks of the upper La Quinta Formation (Finca Carmitto Member) in Chiapas, México, dated as early Miocene (Aquitanian, 22.8 Ma, in Serrano-Sánchez et al., 2015) based on the biostratigraphy of corals, molluscs, microfossils, and Strontium isotopes (Vega et al., 2009; Serrano-Sánchez et al., 2015). Bernot et al. (2022) highlighted that the absence of radiometric ages for La Quinta Formation precludes a more precise constrain of the possible age of the crab-bearing amber samples, and propose some tentative minimum ages based on previously reported benthic foraminiferal data from the overlying Mazantic Shale (Solórzano Kraemer, 2007), while indicating a 87Sr/86Sr radiometric date of 23.0 Ma, roughly at the Oligocene-Miocene boundary, reported in Vega et al. (2009) (see Bernot et al. (2022) supplementary materials and reference therein). As such, given the uncertainty on the age of the crab-yielding amber deposits, we constrain the minimum age to the date reported by Serrano-Sánchez et al. (2015), and the tip maximum age to the base of the Aquitanian and thus the Miocene (23.03 Ma). Soft maximum age as for calibration 8.
Discussion. Sesarmid crabs trapped in amber are known from the lower Miocene (Aquitanian) of Simojovel, Chiapas, Mexico (Grimaldi, 1996; Boucot and Poinar Jr., 2010; Serrano-Sánchez et al., 2016; Luque et al., 2017). ‘Sesarma’ paraensis Beurlen, 1958, presumably a fossil sesarmid from the Miocene Pirabas Formation of Pará, Brazil, cannot be confirmed to be a sesarmid as the specimen is not illustrated in the original paper by Beurlen (1958), but only a schematic line drawing reconstruction (Beurlen, 1958, pl. IV, figure 4). As such, as it stands today, this record cannot be reliably confirmed as a sesarmid. The only other sesarmid fossils known are cheliped remains from the upper Pleistocene of Jamaica previously described as a new species, Sesarma primigenium Collins, Mitchell and Donovan, 2009, but now considered a junior synonym of the extant species Sesarma cookei Hartnoll, 1971, to which the fossilized cheliped remains above mentioned belong (see discussion in Luque et al., 2017, p. 68, note 3).
Brachyura: Eubrachyura: Pseudothelphusoidea: Pseudothelphusidae (crown)
 Fossil specimen. Pseudothelphusidae gen. et sp. indet. (in Luque et al., 2019b). Invertebrate Paleontology collections, Florida Museum of Natural History. Specimen UF 354202, a complete tri-dimensinally preserved crab, dorsally and ventrally, with chelipeds, eyes, and proximal podomeres of pereopods (Figure 4A).
Fossil specimen. Pseudothelphusidae gen. et sp. indet. (in Luque et al., 2019b). Invertebrate Paleontology collections, Florida Museum of Natural History. Specimen UF 354202, a complete tri-dimensinally preserved crab, dorsally and ventrally, with chelipeds, eyes, and proximal podomeres of pereopods (Figure 4A).
Phylogenetic justification. The detailed preservation of diagnostic features of the orbitofrontal margin in the fossil specimen, the overall form of its dorsal carapace and the groove patterns, its lack of dorsal and lateral ornamentation, together with its stratigraphic and paleoenviromental context, e.g., continental leaf-rich level of the freshwater Pedro Miguel Formation, allow its placement with certainty within the freshwater group Pseudothelphusidae, and thus as part of the crown Pseudothelphusoidea (Luque et al., 2019b).
Minimum age. 17.93Ma.
Tip maximum age. 18.18 Ma.
Soft maximum age. 100.9 Ma.
Age justification. This fossil pseudothelphusid specimen comes in situ from a leaf-rich level of tuffaceous mudstones of the Pedro Miguel Formation, Panama Canal, Panama. U/Pb absolute ages from detrital zircons collected in situ in two tuffaceous conglomerates that bracket a leaf-rich horizon reported by Londoño et al. (2018) from which the fossil pseudothelphusid comes, yielded an estimated depositional age of 18.01±0.17 Ma. Previous Ar/Ar dating from the Pedro Miguel Formation in other parts of the Canal by Wegner et al. (2011) has suggested an age of 18.4 ± 1.07 Ma, and while details of the exact provenance of such samples within the formation are unclear (Montes et al., 2012; Farris et al., 2017), a corrected datum by MacFadden et al. (2014) suggests an age of 18.90±0.59 Ma.
Since the tuffaceous mudstone layer yielding the fossil leaves of Londoño et al. (2018) and the in situ pseudothelphusid are bracketed by the same lower and upper tuffaceous conglomerates, both dated as 18.01±0.17 Ma, we assign the fossil a tip maximum age of 18.18 Ma, and a minimum age of 17.93 Ma, which are closer to the middle Burdigalian (late Early Miocene). As this fossil is clearly part of the crown Pseudothelphusidae (Luque et al., 2019b), the most recent common ancestor of all pseudothelphusids must have originated in the pre-Burdigalian, most likely the Paleogene. Soft maximum age as for calibration 8.
Discussion. There are only two extant superfamilies of freshwater crabs in the Neotropics: Trichodactylidae and Pseudothelphusidae. While the fossil record of Trichodactylidae is represented by numerous cheliped fragments (see below), the fossil record of Pseudothelphusidae is otherwise unknown. The new fossil represents not only the first and thus the oldest known fossil of the family, but one of the most complete fossil freshwater crabs known to date (Luque et al., 2019b).
Brachyura: Eubrachyura: Trichodactyloidea: Trichodactylidae (crown)
Fossil specimen. Trichodactylidae genus and species indet. Museo de Historia Natural de la Universidad Nacional Mayor San Marcos de Lima (MUSM), Peru. Three claw fragments: CTA 47 (one specimen) (Figure 4B), and CTA 66 (two specimens), representing a small pollex (CTA 47), and a very small dactylus and a small pollex (CTA 66) (Klaus et al., 2017, figure 3D-E).
Phylogenetic justification. The preservation of diagnostic features on the fossilized dactyli and propodi and the teeth and interteeth on their cutting edges, together with their stratigraphic and continental limnic paleoenviromental contexts, allow their placement within the family Trichodactylidae and not Pseudothelphusoidea, which together, despite not being closely related, are the only freshwater crab clades in the Americas (Klaus et al., 2017; Luque et al., 2017).
Minimum age. 40.94 Ma.
Tip maximum age. 43.44 Ma.
Soft maximum age. 100.9 Ma.
Age justification. These fossils were collected in the lower member of the Pozo Formation, middle and upper middle Eocene (lower Barrancan, >41.6-40.94 Ma, 43.44 ± 2.5 Ma), Contamana area, Loreto, Peru. The ages are based on mammalian biochronology and 40Ar/39Ar radiometric age (Antoine et al., 2016; Klaus et al., 2017). The South American land mammal age (SALMA) known as Barrancan is largely equivalent to the uppermost global Lutetian and most of the Bartonian. Soft maximum age as for calibration 8.
Brachyura: Eubrachyura: Epialtidae + Mithracidae (crown)
Fossil specimen. ‘Micippa’ antiqua Beschin, De Angeli, and Checchi, 2001. Museo Civico “G. Zannato” di Montecchio Maggiore, Vicenza, northern Italy. Holotype I.G. 286477, a complete dorsal carapace (Figure 4C).
Phylogenetic justification. Micippa Leach, 1817, is an extant genus that has been recovered by several authors as not clustering together with other mithracids (see Windsor and Felder, 2014 and references therein). Windsor and Felder (2014) included Micippa and Stenocionops Desmarest, 1823, within the family Mithracidae sensu lato, and Klompmaker et al. (2015) and Schweitzer et al., 2020 also included them within this family in their revision of the fossil record of Mithracidae (see discussion below). Alternatively, Poore and Ahyong (2023) include Micippa within Epialtidae. As such, we consider ‘Micippa’ antiqua as a calibration point for Epialtidae + Mithracidae (crown) (see in Wolfe et al., 2023). The fossil record of pre-Cenozoic majoid crabs is one of the most problematic for brachyurans due to the anatomical disparity seen across taxa and its often-fragmentary nature, and it needs future thorough revisions.
Minimum age. 27.82 Ma.
Tip maximum age. 33.9 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The holotype of ‘Micippa’ antiqua was collected associated to shallow-water corals-bearing limestones from the Castelgomberto Formation (“Formazione di Castelgomberto”) in Vicenza, northern Italy (Beschin et al., 2001). Large foraminifera such as Nummulites fichteli, N. vascus, Operculina complanata, Praerhapydionina delicata, Spirolina cylindracea, Peneroplis glynnjonesi, and Asterigerina rotula haeringensis in the Castelgomberto Formation are indicative of the lower Oligocene (Rupelian, 33.9-27.82 Ma) biozones SB21-22A (Ungaro, 1978; Cahuzac and Poignant, 1997; Nebelsick et al., 2013). Soft maximum age as for calibration 8.
Discussion. Among the extant mithracid genera with a known fossil record, Micippa has the oldest putatively confirmed occurrences, as represented by ‘M.’ antiqua from the lower Oligocene of Italy. Dorsal carapaces of two other micippid species, i.e., M. annamariae Gatt and De Angeli, 2010, and M. hungarica (Lőrenthey in Lőrenthey and Beurlen, 1929), are known from the middle Miocene of Malta and the middle-upper Miocene of Poland, Hungary, and Austria, respectively. This, together with some Miocene and several Pliocene and Pleistocene fossils referable to Mithracidae s.s. from the Caribbean (Klompmaker et al., 2015; Luque et al., 2017), indicate that mithracids were already present and widespread during the late Paleogene-early Neogene.
‘Stenocionops’ suwanneeana Rathbun, 1935, from the upper Eocene of Florida, USA, is a propodus that cannot be placed with confidence within any extant mithracid genus. “Stenocionops” primus Rathbun, 1935, presumably from the Upper Cretaceous (Santonian?) of Arkansas, USA, is a fragmented cheliped manus of unclear affinities. Antarctomithrax thomsoni Feldmann, 1994, from the Eocene of Antarctica, is an extinct monotypic genus described based on a partially preserved dorsal carapace that cannot be ascribed to Mithracidae with confidence.
Brachyura: Eubrachyura: Portunoidea: Eogeryonidae (stem)
Fossil specimen. Eogeryon elegius Ossó, 2021. Museu de Geologia de Barcelona, holotype MGB 69151, a complete dorsal and ventral body in volume with associated cheliped (Ossó, 2016, 2021) (Figure 4D).
Phylogenetic justification. In a recent morphological phylogenetic study incorporating fossil and extant brachyurans, Eogeryonidae has been recovered as stem Portunoidea, sister to a clade formed by the extant families Carcinidae, Geryonidae, and Portunidae, and all of them together forming the total group Portunoidea (Luque et al., 2021).
Minimum age. 93.9 Ma.
Tip maximum age. 97.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Eogeryon elegius comes from sandy limestones of the Villa de Vés Formation, exposed near the village of Condemios de Arriba, Northern Guadalajara Province, Spain. The presence of the ammonite Vascoceras gamai Choffat, 1898, in the Villa de Vés Formation represents the upper part of the upper Cenomanian (lowermost Upper Cretaceous, 95-93 Ma), and it is found below the Spathites (Jeanrogericeras) subconciliatus Zone, which marks the end of the Cenomanian (Ossó, 2016). Soft maximum age as for calibration 8.
Discussion. The anatomical similarities of Eogeryon to portunoids such as Geryon suggest a potential proximity of Eogeryonidae to Geryonidae (Ossó, 2016, 2021). While this remains open to debate, the only morphological phylogenetic work that has tested the position of E. elegius has recovered it as a stem group portunoid, sister to the crown group Portunoidea (Luque et al., 2021). It is conceivable that the earliest relatives of the most recent common ancestor for crown Portunoidea were less portunoid-looking than crown members, and thus, the large claw of E. elegius, which is atypical for portunoids (but compare it to geryonids and scyllids), does not preclude it from being a stem portunoid. Therefore, at present, a stem-portunoid is the best systematic placement for E. elegius, given the fossil and phylogenetic information available. Several small dorsal carapaces of the eubrachyuran Romualdocarcinus salesi Prado and Luque in Prado et al., 2018, from the upper Lower Cretaceous (Aptian-Albian, ~115-110 Ma) of Brazil, share some diagnostic features with Eogeryon elegius that suggest a plausible eogeryonid affinity, but due to their incompleteness, especially regarding the lack of ventral and cheliped material associated, a more precise phylogenetic placement of R. salesi is not possible at this time (Prado et al., 2018). Eogeryon, Romualdocarcinus, and other modern-looking crown eubrachyuran fossils, also share several general features with the remarkably well-preserved Cretapsara athanata Luque in Luque, Xing et al., 2021, from the lowermost Cenomanian (~99 Ma) of Burma (Myanmar), but Cretapsara differs from them in having a bilobate rostrum and lacking orbital fissures (Luque et al., 2021).
Brachyura: Eubrachyura: Portunoidea: Geryonidae (crown)
Fossil specimen. Chaceon helmstedtense (Bachmayer and Mundlos, 1968) (as Coeloma ?). Geological-palaeontological collections of the Natural History Museum Vienna, Austria. Holotype NHMW 1968-0773/0002, a complete dorsal carapace in volume with chelipeds and legs preserved (Figure 4E).
Phylogenetic justification. The fossil species Chaceon helmstedtense can be assigned to the extant genus Chaceon Manning and Holthuis, 1989, and thus the family Geryonidae, based on diagnostic features of the dorsal carapace outline, the orbitofrontal and anterolateral margins, the thoracic sternum, and the chelipeds. Molecular and morphological phylogenetic studies have recovered Chaceon and Geryonidae as nested within crown Portunoidea (e.g., Karasawa et al., 2008; Spiridonov et al., 2014; Evans, 2018; Luque et al., 2021; Wolfe et al., 2023).
Minimum age. 27.82 Ma.
Tip maximum age. 33.9 Ma.
Soft maximum age. 97.0 Ma.
Age justification. The type locality of Chaceon helmstedtense is a marl/clay horizon (Mergel/Ton-Horizont, crab zone K I) in Helmstedt near Braunschweig, Lower Saxony, Northwestern Germany, considered early Oligocene in age (Rupelian, 33.9-27.82 Ma). Associated molluscan fauna in the marl/clay beds includes “Pecten corneus, Isocardia multicostata, Cardita latesulcata, Ostrea ventilabrum, and Ostrea queteleti” (Bachmayer and Mundlos, 1968). As crown group members of Portunoidea cannot be older than their common ancestor shared with the stem group fossil Eogeryon (Ossó, 2016), a soft maximum age is taken from the maximum uncertainty on the age of the Villa de Vés Formation, at 97.0 Ma.
Discussion. Besides Chaceon helmstedtense, another occurrence of Chaceon in the Paleogene is C. heimertingensis (Bachmayer and Wagner, 1957), from the uppermost Oligocene (Chattian) of Austria. Chaceon peruvianus (d’Orbigny, 1842, as Portunus) is an iconic Miocene fossil crab from southern South America–mainly Argentina, but also Chile, Peru, and Uruguay (e.g., Casadío et al., 2005; Luque et al., 2017; Perea et al., 2020, and references therein). This species has been reported from the Estancia 25 de Mayo Formation (previously known as the Centinela Formation) in Santa Cruz Province, Argentina, whose lower portion has been dated as late Oligocene-early Miocene (Chattian-Aquitanian, 27.28-20.44 Ma) based on 87Sr/86Sr radioisotopic dating ages between 25.5-21.5 Ma from a valve of the oyster Crassostrea ? hatcheri (Ortmann), collected below a tuff bed near the Oligocene-Miocene (Chattian-Aquitanian) boundary (Casadío et al., 2000; Casadio et al., 2001; Guerstein et al., 2004), and the palynomorphs Operculodinium israelianum, Spiniferites ramosus, Lingulodinium machaerophorum, Reticulatosphaera actinocoronata, Nematosphaeropsis cf. N. lativittata, Baumannipolis variapertatus, Mutisiapollis viteauensis, Nothofagidites spp., and Podocarpidites spp. (Guerstein et al., 2004). As such, the most recent common ancestor of Geryonidae and all its descendants must have originated in the pre-Oligocene.
Brachyura: Eubrachyura: Portunoidea: Portunidae: Thalamitinae (crown)
Fossil specimen. Lessinithalamita gioiae De Angeli and Ceccon, 2015. Museo Civico di Valdagno, Italy. Holotype MCV14/15, a fairly complete dorsal carapace (Figure 4F).
Phylogenetic justification. The extinct, monotypic genus Lessinithalamita De Angeli and Ceccon, 2015, shows remarkable affinities with the extant genera Thalamita Latreille, 1829, and Thranita Evans, 2018, justifying its inclusion within the subfamily Thalaminitae, which is nested within the crown group Portunidae (Evans, 2018).
Minimum age. 47.8 Ma.
Tip maximum age. 53.3 Ma.
Soft maximum age. 97 Ma.
Age justification. The specimen studied comes from compact calcarenites rich in coralline algae (e.g., Lithothamnium bolcensis) and calcareous nannofossils rocks cropping out near Monte Magrè, Vicenza, Italy, and dated approximately as lower Eocene (middle and upper Ypresian, ~53.3-47.8 Ma) (Beccaro, 2003, in De Angeli and Ceccon, 2012). Soft maximum age as for calibration 20.
Discussion. Besides Lessinithalamita gioiae, there are two other species of Eocene thalamitine crabs belonging to the extinct genus Eocharybdis Beschin, Busulini, De Angeli and Tessier, 2002, i.e., E. rugosa Beschin, Busulini, Tessier and Zorzin, 2016b, from the lower Eocene (Ypresian) of Monte di Malo, Verona, NE Italy, and E. cristata Beschin, Busulini, De Angeli and Tessier, 2002, from the middle Eocene (Lutetian) Cava “Main” di Arzignano, Vicenza, Italy. While both of these species are suitable candidates for calibration points for the subfamily Thalamitinae, the completeness of the type material of L. gioiae and its similarities to Thalamita and Thranita, support a more reliable affiliation to the crown Thalamitinae.
Brachyura: Eubrachyura: Portunoidea: Polybiidae (crown)
Fossil specimen. Liocarcinus heintzi Schweitzer and Feldmann, 2010b. Muséum national d’Histoire naturelle, Paris. Holotype MNHN R03778, a well-preserved dorsal carapace (Figure 4G).
Phylogenetic justification. Liocarcinus Stimpson, 1858, is an extant portunoid genus nested within crown Polybiidae (Evans, 2018). The overall sub-hexagonal carapace of L. heintzi, slightly wider than long, with four anterolateral spines, the concave posterolateral margins, a convex posterior margin, the orbits directed forward, and marked dorsal regions, seem to conform with the forms seen among species of Liocarcinus (Schweitzer et al., 2021d).
Minimum age. 27.82 Ma.
Tip maximum age. 33.9 Ma.
Soft maximum age. 97 Ma.
Age justification. The studied specimen comes from the carbonatic Calcaire à Astéries Formation, cropping out near Monségur, Gironde, Western Aquitaine, France (Schweitzer and Feldmann, 2010b). The Calcaire à Astéries Formation has been dated as early Oligocene (Rupelian, 33.9-27.82 Ma), based on the recognition of the foraminifera biozones SBZ 21 and 22A, containing Nummulites fichteli, N. intermedius N. vascus, Spiroclypeus, Operculina, Halkyardia spp., Neorotalia spp., Peneroplis, and Arenagula and the ostreid Crassostrea longirostris (Cahuzac and Londeix, 2012; Sztrákos and Steurbaut, 2017). Soft maximum age as for calibration 20.
Discussion. Besides Liocarcinus heintzi, there are several other occurrences of polybiid and polybiid-like genera and species in the Paleogene. Archaeogeryon corsolini (Casadío, De Angeli, Feldmann, Garassino, Hetler, Parras and Schweitzer, 2004, as Proterocarcinus), and A. lophos (Feldmann, Casadío, Chirino-Gálvez, and Aguirre-Urreta, 1995), are known from the middle Oligocene and Paleocene (Danian, 66.0-61.6 Ma) of Argentina, respectively (Feldmann et al., 1995; Casadío et al., 2004; Casadío et al., 2005; Feldmann et al., 2011b). The latter species, represented by the holotype and sole specimen, is moderately preserved and shows some dorsal and sternal features that suggest affinity with portunids, but the fronto-orbital margin and part of the anterolateral margins are eroded, precluding a detailed comparison with polybiids (e.g., García-Raso et al., 2024). Moreover, the exact phylogenetic position of Archaeogeryon Colosi, 1924, among portunoids, as well as that of the also extinct genus Gecchelicarcinus Beschin, Busulini, De Angeli, and Tessier, 2007, known from two species from the Ypresian of Italy, is unclear. Both taxa could represent extinct polybiid genera, but they cannot be assigned to the crown group with certainty.
The presumably polybiid monotypic extinct genera Boschettia Busulini, Tessier, Beschin, and De Angeli, 2003, from the lower and middle Eocene (Ypresian to Lutetian, 56.0-41.2 Ma) of Italy, and Falsiportunites Collins and Jakobsen, 2003, from the middle Eocene (?Lutetian, 47.8-41.2 Ma) of Denmark, show a combination of anatomical features on their fronto-orbital margins, anterolateral margins, and dorsal carapaces that differ from those seen among polybiids (see García-Raso et al., 2024 for comparisons) and thus cannot be accommodated satisfactorily among the crown group either.
Among the extant genera with known fossil records, Liocarcinus oligocenicus (Paucă, 1929, as Portunus), and L. atropatanus (Aslanova and Dschafarova, 1975), the latter now synonymized with L. oligocenicus (Hyžný et al., 2022) are known from the lower and upper Oligocene (Rupelian-Chattian, 33.9-23.3 Ma) of Romania and Azerbaijan, respectively (Schweitzer et al., 2009; Beschin et al., 2016a; Hyžný, 2016), as well as from Czech Republic, Ukraine, Hungary, Poland, and Russia (Hyžný et al., 2022), which confirm the presence of the genus during Oligocene times. Liocarcinus priscus Beschin, De Angeli, Checci, and Zarantonello, 2016a, from the lower Eocene (lower Lutetian, 47.8-41.2 Ma), may represent the oldest occurrence of the genus and thus a more approximate soft maximum age of calibration for the polybiid node, but due to its remarkable differences with the type species of the genus Liocarcinus, i.e., L. holsatus (Fabricius, 1798), to which L. heintzi is anatomically closer, we favor the younger fossil for a calibration point. Regardless of which of these fossils is considered the oldest reliable record of the family, the clear occurrence of several Paleogene genera and species attributable to Polybiidae indicate that the most recent common ancestor of the family and all its descendants originated most likely during the early Paleogene (Wolfe et al., 2023), or even the Late Cretaceous.
Brachyura: Eubrachyura: Parthenopoidea: Parthenopidae+Dairoididae (crown)
Fossil specimen. Aragolambrus collinsi Ferratges, Zamora, and Aurell, 2019. Palaeontological collection of the Museo de Ciencias Naturales de la Universidad de Zaragoza, Spain. Holotype MPZ-2019/211, a nearly complete dorsal carapace with chelipeds associated (Figure 4H).
Phylogenetic justification. In a recent molecular phylogenetic study, Wolfe et al. (2023) recovered Dairoides kusei (Sakai, 1938) as sister to a monophyletic Parthenopidae. Ferratges et al. (2023), based on a morphological phylogenetic study of Parthenopoidea, recovered the extinct monotypic genus Aragolambrus Ferratges, Zamora, and Aurell, 2019, near the also extinct genus Phrynolambrus Bittner, 1893, and the extant genus Dairoides Stebbing, 1920. All three genera form a well-supported monophyletic subfamily Dairoidinae sister to Daldorfiinae, nested within a paraphyletic Parthenopidae (Ferratges et al., 2023). Future comprehensive molecular phylogenetic studies with larger taxon coverage across multiple parthenopoid genera will shed light on the internal relationships among these groups.
Minimum age. 53.02 Ma.
Tip maximum age. 57.1 Ma.
Soft maximum age. 100.9 Ma.
Age justification. Aragolambrus collinsi comes from the coral-algal Reef Limestones interval (middle member) of the Serraduy Formation, in the Barranco de Ramals outcrop near Puebla de Roda and Serraduy, Huesca province, Spain (Ferratges et al., 2019). The Reef Limestones interval develops over the underlying Alveolina Limestones interval and the overlying marls of the Riguala Member (Serra-Kiel et al., 1994; Pujalte et al., 2009). Serra-Kiel et al. (1994) assigned an age of lower Ilerdian to the Alveolina Limestones interval, which is located within the magnetostratigraphic Chron C24r (57.101-53.9 Ma, Francescone et al., 2019; Ogg, 2020), and contains the macroforaminifera Alveolina cucumiformis, A. ellipsoidalis, and Nummulites fraasi, from the NP9, BB1, and P6a Biozones. For the marls of the Riguala Member, the same authors assigned an age of middle Ilerdian, roughly located between the top of the magnetostratigraphic Chron C24r and Chron C24n.1r (~53.9-53.02 Ma, Francescone et al., 2019; Ogg, 2020), and contains the macroforaminifera Alveolina moussoulensis, A. corbarica, Nummulites robustifrons, and N. exilis, from the BB1-BB2, NP9-NP10, and P6a-P6b Biozones (Serra-Kiel et al., 1994). Due to the lateral facies’ discontinuity of the Reef Limestones interval, which is bounded at the bottom by the Alveolina Limestones and at the top by the Riguala Member, we bracket the tip maximun and minimum ages of A. collinsi between 57.1-53.02 Ma, with a possible age of the fossil itself closer to 53.5 Ma (F. Ferratges, pers. comm. to J. Luque, Feb. 2023). Soft maximum age as for calibration 8.
Discussion. Aragolambrus and Phrynolambrus are two of the oldest fossils of crown Parthenopidae known, sharing with Dairoides the presence of two to three anterolateral spines, an ornamented epistomia with one or more rows of tubercles, and the pterygostome with grooves, which are synapomorphies that unite them among parthenopoids under the subfamily Dairoidinae (Ferratges et al., 2023).
The extinct genera Eogarthambrus De Angeli, Garassino, and Alberti, 2010a, and Mesolambrus Müller and Collins, 1991, known from the Eocene (middle Ypresian to Priabonian, ~52.0-33.9 Ma) of Italy and Hungary, were recovered by Ferratges et al. (2023) outside the crown Parthenopoidea. The monotypic extinct genus Braggilambrus De Angeli and Caporiondo, 2016, from the Ypresian of Italy, seems to fit within the general parthenopoidean body form, but due to its incompleteness and therefore lack of key diagnostic characters, its affinities at the family and subfamily levels are still unclear.
The presence of several crown parthenopid forms across the early to late Eocene indicate that the most recent common ancestor of Parthenopoidea and all its descendants must have Paleocene or even Late Cretaceous origin.
Brachyura: Eubrachyura: Calappoidea: Calappidae (crown)
Fossil specimen. Calappa zinsmeisteri Feldmann and Wilson, 1988. Smithsonian Institution, National Museum of Natura History (NMHN), USA. Holotype USNM 404877, claws only (Figure 4I, J).
Phylogenetic justification. Calappa Weber, 1795, is the type genus of the family Calappidae De Haan, 1833, and thus phylogenetically nested within the crown Calappoidea (Lu et al., 2020).
Minimum age. 33.9 Ma.
Tip maximum age. 41.2 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Calappa zinsmeisteri comes from the uppermost La Meseta Formation on Seymour Island, Antarctica, Locality 14, of Feldmann and Wilson (1988). This locality corresponds to the Telms VI-VII of Sadler (1988), which have been dated as middle to late Eocene (Bartonian-Priabonian, ~42.0-34.0 Ma) based on 87Sr/86Sr radiometric dating ages from Cucullaea shells from Telm VII between 46.13 and 34.69 Ma (Dutton et al., 2002), and the occurrences of the neogastropods Prosipho lawsi, P. lamesetaensis, Austroficopsis meridionalis, Microfulgur byrdi, Fusinus ?suraknisos, Eupleura suroabdita, ?Adelomelon suropsilos, Zemacies finlayi, and Aforia canalomos (Crame et al., 2014). Soft maximum age as for calibration 8.
Discussion. Among the extant calappid genera, only Calappa and Mursia Leach, in Desmarest, 1823, have recognized Eocene fossils, to our knowledge. Four species of Calappa (including C. zinsmeisteri) are known from the middle and upper Eocene of USA, Antarctica, and presumably Venezuela, and are constituted largely of cheliped (propodi) remains with diagnostic anatomical details that can be assigned with certainty to crown Calappidae, and most likely to Calappa (Ross et al., 1964; Feldmann and Wilson, 1988; Luque et al., 2017). One species of Mursia, i.e., Mursia aspina Schweitzer and Feldmann, 2000b, from the upper Eocene of Washington, USA, is represented by a handful of dorsal carapaces that strongly resemble Mursia, although they lack some of the diagnostic features of the genus such as developed lateral spines. While we consider the cheliped remains of Calappa zinsmeisteri as our calibration point given their putative affinities to the type genus of the family, M. aspina, which is represented by dorsal carapace material of similar age as C. zinsmeisteri, could also be a good candidate to calibrate the node for crown Calappidae.
Among the extinct calappid genera, Calappilia A. Milne-Edwards in de Bouillé, 1873, bears conspicuous anatomical similitudes with Calappa, and has over 20 species known, of which more than 15 are Eocene in age (e.g., Williams and Child, 1988; Schweitzer et al., 2010; Busulini et al., 2014; Rumsey et al., 2016; Luque et al., 2017; Feldmann et al., 2019, and references therein). There are also several extinct genera known exclusively from the Eocene, such as the monotypic genera Paracorallomursia Beschin, Busulini, Tessier and Zorzin, 2016b, and Pseudocorallomursia Beschin, Busulini, Tessier and Zorzin, 2016b, from the Ypresian of Italy, Carinocalappa Beschin, Busulini, and Tessier in Beschin et al., 2018, from the Priabonian of Italy, and Tavernolesia Artal and Onetti, 2017, from the Lutetian of Spain. Corallomursia De Angeli and Ceccon, 2014, comprises two species, both from the Ypresian of Italy (Beschin et al., 2015). All these extinct genera seem to fit well within the total group Calappidae, but their precise affinities with crown calappids remain unclear.
Calappid crabs were already diverse, speciose, and widespread by the Eocene, indicating that the most recent common ancestor of Calappidae and all its descendants must have originated in the earliest Paleogene (Paleocene) or the Cretaceous.
Brachyura: Eubrachyura: Cancroidea: Cancridae (crown)
Fossil specimen. Anatolikos undecimspinosus Schweitzer, Feldmann, González-Barba, and Ćosović, 2006. Museo de Historia Natural, Universidad Autónoma de Baja California Sur, La Paz, Baja California Sur, México (MHN-UABCS). Holotype MHN-UABCS/Ba12-9, a partially preserved dorsal left carapace (Figure 4K).
Phylogenetic justification. The extant genus Anatolikos Schweitzer and Feldmann, 2000a, can be placed with confidence within the family Cancridae (Schweitzer et al., 2006; Schram and Ng, 2012; Wolfe et al., 2023). The diagnostic shape and arrangement of the eleven anterolateral spines seen in the extant genus Anatolikos and thus in A. undecimspinosus, together with the presence of a nearly straight rostrum with “five coalesced spines separated by fissures” (Schweitzer and Feldmann, 2000a) are unique traits not shared with other genera within the family (Schweitzer et al., 2006; Poore and Ahyong, 2023).
Minimum age. 41.2 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Anatolikos undecimspinosus comes from middle Eocene rocks of the Bateque Formation, cropping out at locality Waypoint 29 of Schweitzer et al. (2006), northwest of La Paz, near the village of San Ignacio, Baja California Sur, Mexico. The age of the Bateque Formation has been constrained between the early Eocene and the late middle Eocene (56.0-41.2 Ma) based on foraminiferal, nannoplankton, and diatom assemblages, more than 150 macroinvertebrates, and ostracods (Mina-Uhink, 1957; Sorensen, 1982; McLean et al., 1987; Carreño and Cronin, 1993; Morales-Ortega et al., 2016). Soft maximum age as for calibration 8.
Discussion. Among the cancrids with known fossil record, only the Cancrinae genera Anatolikos and presumably Cancer Linnaeus, 1758, have Eocene occurrences. However, Cancer gabbi Rathbun, 1926, from the Eocene of California, is represented by claw fragments only that cannot be confirmed to belong to the group. Cancer meticuriensis Thurmann, 1853, from the Oligocene and presumably from the lower Eocene of France, is too incomplete, with only the sternum and the underside of the anterior pereopods known, which A. Milne-Edwards (1865) considered to most likely belong to a portunid. At least six species (C. aldenardensis, C. archiaci, C. flandricus, C. pratti, C. rotnacensis, and C. villabersiani) from the Ypresian-Lutetian of Europe, are all nomina nuda, many of which have never been described or figured, and therefore their generic affinities are dubious (Van Straelen, 1920; Nations, 1975). Cancer santosi (Rathbun, 1937, as Lobocarcinus ?) from the upper Eocene of Panama, is a poorly preserved right dorsal carapace.
Three extinct monotypic genera with potential Carcininae affinities also have early to middle Eocene occurrences, i.e., Santeecarcinus Blow and Manning, 1996; Sarahcarcinus Blow and Manning, 1996, both from USA, and Notocarcinus Schweitzer and Feldmann, 2000a, from Argentina. However, given that these three genera are extinct only, we consider them suboptimal candidates for the calibration of the Cancridae node, given the presence of fossil taxa assigned to extant genera, e.g., Anatolikos undecimspinosus, in rocks of similar ages.
A few other extinct cancroid genera currently placed within the subfamily Lobocarcininae Beurlen, 1930 (e.g., De Grave et al., 2009; Schweitzer et al., 2010), have also their oldest or only records in the Eocene, e.g., Nicoliscarcinus Beschin, Busulini, Tessier and Zorzin, 2016b, and Ramacarcinus De Angeli and Ceccon, 2017, from Italy. In addition, two out of the three species of the extinct genus Ceronnectes De Angeli and Beschin, 1998, and several species of Lobocarcinus Reuss, 1857, also occur in the middle Eocene (e.g., Anderson and Feldmann, 1995; De Angeli and Beschin, 1998; Feldmann et al., 1998; Schweitzer et al., 2010; Beschin et al., 2016b).
Brachyura: Eubrachyura: Dorippoidea (crown)
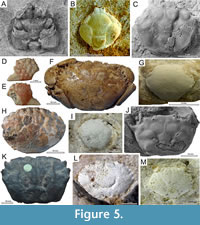 Fossil specimen. Bartethusa hepatica Quayle and Collins, 1981. Palaeontological Department, Lon’on's Natural History Museum (formerly British Museum [Natural History]), England, holotype In.61704, a well-preserved dorsal carapace (Figure 5A).
Fossil specimen. Bartethusa hepatica Quayle and Collins, 1981. Palaeontological Department, Lon’on's Natural History Museum (formerly British Museum [Natural History]), England, holotype In.61704, a well-preserved dorsal carapace (Figure 5A).
Phylogenetic justification. Bartethusa hepatica has an overall carapace outline, fronto-orbital construction, and dorsal carapace region configuration that matches the range of forms seen across crown dorippoids. While Bartethusa hepatica could be allied to either Ethusidae Guinot, 1977 (e.g., Quayle and Collins, 1981; Van Bakel et al., 2021) or Dorippidae MacLeay, 1838a (e.g., Luque, 2015a; Schweitzer et al., 2021b; Guinot, 2023), in either case it will still be nested within the crown Dorippoidea MacLeay, 1838a.
Minimum age. 37.71 Ma.
Tip maximum age. 41.2 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Bartethusa hepatica was collected in rocks of the Horizon A3, Barton Beds, Christchurch Bay, Isle of Wight, England (Quayle and Collins, 1981), which have been dated as middle Eocene (Bartonian, 41.2-37.71 Ma) based on larger and smaller foraminifera, calcareous nannofossils, palynomorphs, and paleomagnetism (see Cotton et al., 2021, and references therein). Tsang et al. (2014) also used B. hepatica as their calibration point, but they assigned it an age of early Eocene (Ypresian, 56.0-47.8 Ma), which seems to be an overestimation of the stratigraphic age as above indicated. Soft maximum age as for calibration 8.
Discussion. Dorippoid crabs–extant and fossil–are among the most puzzling of the eubrachyurans in terms of their phylogenetic affinities, given their unique combination of plesiomorphic and apomorphic characters and their relatively low representation in previous molecular phylogenies compared to most other crabs. Based on morphology alone, dorippoids have been typically regarded as some of the oldest crown eubrachyurans, and thus one of the earliest splitting branches in the eubrachyuran tree, with their earliest known representatives, e.g., Telamonocarcinidae Larghi, 2004, and potentially Tepexicarcinidae Luque, 2015a, already present in the Early Cretaceous (Luque, 2015a; Luque et al., 2021; Van Bakel et al., 2021). Although their overall anatomy is strongly reminiscent of the array of body forms across modern dorippoids in terms of their dorsal carapaces, thoracic sternum, and shape and size of their pereopods (P2-P5), they cannot be assigned to any of the two crown families recognized today, i.e., Dorippidae and Ethusidae. Some of these other extinct families, however, may represent convergent morphology, as dorippoids consistently nest well within heterotremes in molecular phylogenies (Tsang et al., 2014; Wolfe et al., 2023).
Among the extinct post-Cretaceous dorippoid forms, Goniochele Bell, 1858, presumably belonging to its own family Goniochelidae Schweitzer and Feldmann, 2011, has a confirmed fossil record restricted to two Eocene species: G. angulata Bell, 1858, from the Ypresian of England, and G. madseni Collins and Jakobsen, 2003, from the Ypresian-Lutetian of Denmark. However, the few similarities between Goniochelidae and some members of Dorippoidea s.s. might be convergent and not reflecting shared ancestry (Guinot, 2023). Rathbun (1918 [1919]), based on an isolated dactylus from the Early Miocene (not Oligocene, as Rathbun suggested) Culebra Formation in the Panama Canal, erected a third species of Goniochele, G. armata Rathbun, 1918 [1919], that currently cannot be ascribed to this genus with certainty (see Luque et al., 2017). Another extinct taxon, Archaeocypoda veronensis Secrétan, 1975, known from the lower Eocene (upper Ypresian) of Italy (Pasini et al., 2019), has been presumably assigned to Dorippidae based on the sub-oval carapace outline, the broad front and continuous supraorbital margin, the smooth anterolateral and posterolateral margins, the inflated protogastric and branchial regions, the smooth antero- and posterolateral margins (Casadío et al., 2005; Pasini et al., 2019; Schweitzer et al., 2021b). While these features match some of the diagnostic characters of dorippids, the possession of at least three pairs of well-developed pereopods (i.e., P2-P4) dramatically differs from the apomorphic dorippoid possession of two large pereopods (P2-P3) and two reduced, subchelate, and sub-dorsally carried pereopods (P4-P5), which is a feature seen across crown dorippoids and even stem dorippoids from the Cretaceous (e.g., Larghi, 2004; Casadío et al., 2005; Luque, 2015a; Guinot et al., 2019; Guinot, 2023). As such, while A. veronensis might (or might not) be related to crown Dorippoidea, its systematic affinities remain unclear. Another taxon, the presumed genus Titanodorippe Blow and Manning, 1996, from the middle Eocene of USA, is a single cheliped propodus that cannot be assigned with confidence to Dorippidae or even Dorippoidea.
Among the extant dorippoid genera, only Ethusa Roux, 1830 [in Roux, 1828-1830], has its oldest record in the uppermost Eocene (Priabonian, 37.71-33.9 Ma) (Müller and Collins, 1991), while Dorippe Weber, 1795, and Medorippe Manning and Holthuis, 1991, are known from the lower-middle Miocene (Burdigalian, 20.44-15.97 Ma) (Studer, 1892; Karasawa, 2000). Given the proximity in age between the occurrences of Bartethusa and Ethusa from the middle and late Eocene, respectively, the most recent common ancestor of crown Dorippoidea and all of its descendants must have originated during the early Paleogene or even the Cretaceous, while the presence of several potential stem dorippoids in the Early and Late Cretaceous worldwide support a late Mesozoic origin for the superfamily.
Brachyura: Eubrachyura: Leucosioidea: Leucosiidae (crown)
Fossil specimen. Typilobus alponensis Beschin, De Angeli, and Zorzin, 2009. Museo di Storia Naturale di Verona. Holotype IGVR78539, a complete dorsal carapace in volume (Figure 5B).
Phylogenetic justification. The overall oval and domed carapace about as long as wide, with convex anterolateral and posterolateral margins, a narrow posterior margin, the small and round orbits, and the presence of a projected bilobed front/rostrum that is mesially depressed, are typical features of Leucosiidae, an extant family nested within Leucosioidea (Beschin et al., 2009).
Minimum age. 47.8 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The holotype and sole specimen of Typilobus alponensis comes from lower Eocene (Ypresian, 56.0-47.8 Ma) rocks cropping out in the valley of Alpone, near Monte Serea, San Giovanni Ilarione, Verona, Italy (Beschin et al., 2009). Soft maximum age as for calibration 8.
Discussion. Typilobus Stoliczka, 1871, is an extinct genus with presumably over a dozen species ranging in age from early Eocene (Ypresian) to Miocene and widely distributed across Europe, the UK, North Africa, Asia, and the Indo-Pacific (Karasawa, 1998; Beschin et al., 2009; Artal and Hyžný, 2016; Karasawa et al., 2019). However, several of these species are considerably different from the type species of the genus, T. granulosus Stoliczka, 1871, from the Early Miocene of Pakistan, casting doubts on the monophyletic nature of the genus as currently envisioned. Regardless of the generic affinities of Typilobus alponensis, this species fits well within the overall body plan seen across crown Leucosiidae (see above); hence, this is our species selected for calibration of the node. Other Eocene species assigned to Typilobus that might be related to T. alponensis and thus belong to the crown Leucosiidae are T. belli Quayle and Collins, 1981, from the Bartonian of the UK; T. prevostianus (Desmarest, 1822, as Leucosia), from the Lutetian of the Paris Basin; T. semseyanus Lőrenthey, 1898, from the Lutetian-Priabonian of Hungary and Italy (Lőrenthey and Beurlen, 1929; Beschin et al., 1998); and T. trispinosus Lőrenthey, 1907, from the Lutetian-Priabonian of Egypt (Beschin et al., 1998; Feldmann et al., 2011a; Artal and Hyžný, 2016).
The type material of the monotypic genus Zannatoius Beschin, Busulini and Tessier in Beschin, Busulini, Fornaciari, Papazzoni, and Tessier, 2018, from the upper Eocene of Italy, is somewhat damaged and missing the fronto-orbital margin, but overall it conforms with the leucosiid body plan. Among the extant leucosiid genera with known fossil record, Ebalia Leach, 1817, has its earliest putative occurrence in the upper Eocene (Priabonian, 37.71-33.9 Ma) of Germany (Förster and Mundlos, 1982; Karasawa et al., 2019). As such, the presence of crown Leucosiidae in the early and late Eocene suggests that the family must have originated at least during the early Paleogene.
Brachyura: Eubrachyura: Goneplacoidea: Euryplacidae (crown)
Fossil specimens. Chirinocarcinus wichmanni (Feldmann, Casadío, Chirino-Galvez, and Aguirre-Urreta, 1995) (as ?Glyphithyreus). Geology Collections at National University of La Pampa, Santa Rosa, La Pampa, Argentina. Holotype GHUNLPam 7015, a nearly complete dorsal carapace (Figure 5C).
Phylogenetic justification. Chirinocarcinus wichmanni has been included within Euryplacidae based on the relatively broad fronto-orbital margin and large orbits, the well-defined supraorbital angle, and short anterolateral margins (Feldmann et al., 1995; Karasawa and Kato, 2003; Castro and Ng, 2010).
Minimum age. 61.6 Ma.
Tip maximum age. 66.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Chirinocarcinus wichmanni was collected from lowermost Paleocene (lower Danian) rocks of the Roca Formation at the type section, located to the north of General Roca, Rio Negro, Neuquén Province, Argentina (Feldmann et al., 1995). The Roca Formation has been dated as latest Cretaceous (Maastrichtian) to early Paleocene (Danian), based on foraminifera, calcareous nannoplankton, and palynomorphs (del Rio et al., 2011 and references therein). The crab-bearing intervals of the Roca Formation are comprised within the calcareous nannofossil zones NP3 and NP4, with Chiasmolithus danicus, Neochiastozygus modestus, Hornibrookina edwardsii, H. teuriensis, and Toweius africanus from NP3, indicative of the lower Danian (del Rio et al., 2011). Soft maximum age as for calibration 8.
Discussion. Goneplacoidea, as traditionally envisioned, is among the most anatomically disparate and problematic brachyuran groups in terms of the clarity about their internal phylogenetic affinities, and thus their likely polyphyletic relationships with other eubrachyuran groups (Ng et al., 2008; Castro et al., 2010). The extant family Euryplacidae, however, seems to be monophyletic (Castro and Ng, 2010) and nested within the crown Goneplacoidea (Karasawa and Kato, 2003).
Among the euryplacid genera, the monotypic Chirinocarcinus Karasawa and Schweitzer, 2004, is the only taxon known from the Paleocene. Five other monotypic genera currently assigned to Euryplacidae are known from the lower Eocene (Ypresian) of Italy and the middle Eocene (Lutetian) of Spain (Karasawa and Kato, 2003; Beschin and De Angeli, 2011; Beschin et al., 2016b), as is the genus Corallicarcinus Müller and Collins, 1991, from Eocene fossils from Hungary and Italy (Müller and Collins, 1991; Karasawa and Kato, 2003; Beschin et al., 2018). Similarly, the genus Orbitoplax Tucker and Feldmann, 1990, presumably also referable to Euryplacidae, is known from four Eocene species occurring in Mexico and USA (e.g., Rathbun, 1926; Tucker and Feldmann, 1990; Schweitzer, 2000; Vega et al., 2001).
Among the extant euryplacid genera, Eucrate De Haan, 1835, has its oldest putative record in the Oligocene, as represented by a fairly complete ventral sternum with chelipeds of E. martini Rathbun, 1926, from USA, and E. puliensis Hu and Tao, 1996, from Taiwan. ?Euryplax culebrensis Rathbun, 1918 [1919], from the Panama Canal Zone, is of early Miocene age and not Oligocene, just like the other fossil decapods from the Culebra Formation in the Panama Canal, as initially assumed by Rathbun and repeated by several authors (Luque et al., 2017, and comments therein).
The number of extinct and extant euryplacids known from the Paleogene, and the proximity of C. wichmanni to the Cretaceous/Paleogene boundary, indicates that Euryplacidae must have a pre-Cenozoic origin, most likely in the Cretaceous.
Brachyura: Eubrachyura: Eriphioidea: Eriphiidae (crown)
Fossil specimens. Eriphia verrucosa (Forskal, 1775), in Betancort et al., 2014). Palaeontological collections of the Universidad de Las Palmas de Gran Canarias, Spain. Specimen number PAL-ULPGC 1460. Single left cheliped fragment with palm and pollex (Figure 5 D, E).
Phylogenetic justification. The type species of Eriphia Latreille, 1817, is E. verrucosa (Forskal, 1775) [ E. spinifrons (Herbst, 1782)], by subsequent designation (Koh and Ng, 2008; Ng et al., 2008). Although Eriphioidea as previously envisioned is shown to be polyphyletic (Wolfe et al., 2023), Eriphia is the type genus of the family Eriphiidae and thus the superfamily Eriphioidea. As such, it is reliably placed within the crown group.
Minimum age. 4.1 Ma.
Tip maximum age. 9.3 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The specimen of E. verrucosa reported by Betancort et al. (2014) comes from rocks of the Papagayo cliff, Lanzarote, Canary Islands, Spain, dated as late Miocene (Messinian?) to early Pliocene based on the K/Ar radiometric dating, ranging between 9.3 and 4.1 Ma (Betancort et al., 2014, and references therein). Soft maximum age as for calibration 8.
Discussion. The family Eriphiidae, as currently recognized, includes only the extant genera Eriphia Latreille, 1817, and Eriphides Rathbun, 1897 (Ng et al., 2008; De Grave et al., 2009). While Eriphides is monotypic, Eriphia is represented by eight extant and two fossil species (Koh and Ng, 2008; Schweitzer et al., 2010). The extinct Eriphia cocchii Ristori, 1886, is known from well-preserved specimens in volume as well as from isolated dactyli from the lower Pliocene (Zanclean-Piacentian, ~5,33-3.6 Ma) of Italy (Ristori, 1886; De Angeli et al., 2009) (Figure 5F). Based solely on an isolated propodus, Collins and Donovan (1997), reported a presumably new species, Eriphia xaymacaensis Collins and Donovan, 1997, from the upper Pleistocene of Jamaica (Luque et al., 2017). The latter species, however, might be a junior synonym of Eriphia gonagra Fabricius, 1781, an extant species. Along these lines, among the extant species with putative fossils, Varola (1981) reported isolated dactyli of the extant species E. verrucosa from the mid-Pliocene of Italy, and Luque et al., 2018), reported a cheliped of E. squamata Stimpson, 1859, from the Quaternary of Panama.
As we show here, the fossil record of Eriphiidae is meager and restricted to the uppermost Neogene and Quaternary, explaining why we opted for the calibration above discussed. As such, the most recent common ancestor of extant eriphiids and all its descendants must have originated at least in the early Neogene or, most likely, in the Paleogene.
Brachyura: Eubrachyura: Trapezioidea: Trapeziidae (crown)
Fossil specimen. Archaeotetra lessinea De Angeli and Ceccon, 2013. Museo Civico “D. Dal Lago” of Valdagno (Vicenza) (MCV). Holotype MCV 12/05-I.G.360311, a complete dorsal carapace (Figure 5G).
Phylogenetic justification. Phylogenetically, Trapeziidae Miers, 1886, seems to be a monophyletic family nested within Trapezioidea s.l. (Castro et al., 2004; Lai et al., 2009; Wolfe et al., 2023), but the monophyly of Trapezioidea as a whole, containing Tetraliidae Castro, Ng, and Ahyong, 2004, has been questioned (see Wolfe et al., 2023). Despite this, the distinctive carapace outline of Archaeotetra Schweitzer, 2005, and thus of Archaeotetra lessinea, with a wide and slightly bilobed front, the fronto-orbital margin being about 80-90% as wide as the carapace maximum width, the orbits placed anterolaterally, the smooth dorsal carapace, and the seemingly spineless, short, and nearly parallel anterolateral margins, conforms with the body form typical of genera and species in the family Trapeziidae (Schweitzer, 2005; Karasawa and Schweitzer, 2006; De Angeli and Ceccon, 2013; Poore and Ahyong, 2023).
Minimum age. 47.8 Ma.
Tip maximum age. 53.3 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The material studied comes from micritic limestones with abundant nullipore coralline algae and fragments of invertebrates, cropping out in the vicinity of Monte Magré, eastern margin of Lessini Mounts, Italy. Age as for calibration 21. Soft maximum age as for calibration 8.
Discussion. Among the few trapeziid crab fossils known, at least four species besides Archaeotetra lessinea have been recovered from Eocene deposits. Archaeotetra inornata Schweitzer, 2005, is known from the lower to middle Eocene of Mexico; Paratetralia convexa Beschin, Busulini, De Angeli, and Tessier, 2007, from the lower Eocene of Italy; and Eomaldivia pannonica and E. trispinosa Müller and Collins, 1991, from the upper Eocene of Hungary. This indicates that the most recent common ancestor of trapeziid crabs and all its descendants must have a pre-Eocene origin (Wolfe et al., 2023).
Brachyura: Eubrachyura: Eriphioidea: Oziidae (crown)
Fossil specimen. Ozius collinsi Karasawa, 1992. Mizunami Fossil Museum (Yamanouchi, Mizunami, Japan). Holotype MFM39001, a complete dorsal carapace (Figure 5H).
Phylogenetic justification. The extant genus Ozius Milne Edwards, 1834 [in H. Milne Edwards, 1834-1840] is the type genus of the family Oziidae. The overall anatomy of Ozius collinsi fits well the diagnosis of the genus, and thus can be considered within the crown Oziidae s.s.
Minimum age. 13.82 Ma.
Tip maximum age. 15.97 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Ozius collinsi comes from mudstones of the Yoshino Formation, Katsuta Group, Locality KTT13, in Niida, Tsuyama City, Okayama Prefecture, Japan (Karasawa, 1992). The Yoshino Formation is considered as uppermost lower Miocene to middle Miocene (Burdigalian to Langhian), with some estimated ages of 17.9± 2.1 Ma and 18.5-16 Ma based on the fission track method and palynomorphs (Mori and Yamanoi, 2003; Suzuki et al., 2003; Ando et al., 2016), and on mollusc assemblages and planktonic foraminifera biostratigraphic ages, zones N8 to N10 (Blow, 1969; Yoshimoto, 1979; Karasawa, 1992 and Taguchi, 2002), which correspond to the global Burdigalian. Other authors have restricted the age of the oldest fossil Oziidae from Japan to the Langhian (Schweitzer et al., 2021a). Soft maximum age as for calibration 8.
Discussion. In some recent mitogenomic studies, oziid taxa such as Epixathus frontalis have been recovered outside the traditional Eriphioidea, highlighting the polyphyletic nature of the superfamily as traditionally envisioned (Tan et al., 2018; Lü et al., 2022), and further corroborated in Wolfe et al., 2023. Moreover, previous groupings of genera within the family Oziidae have been shown to be non-monophyletic (Lai et al., 2014), further adding to the phylogenetic issues highlighted above. This also adds to the problem that several extinct forms that might superficially resemble oziids might not necessarily belong to Oziidae s.s., which, together with their incompleteness, preclude a confident systematic placement. As such, due to the reliable placement of O. collinsi within the genus Ozius, and due to its early-middle Miocene age, we consider it as the best calibration point for the family currently available.
Brachyura: Eubrachyura: Pilumnoidea: Pilumnidae (crown)
Fossil specimen. Glabropilumnus trispinosus Beschin, Busulini, and Tessier, in Beschin et al., 2016b. Museo di Storia naturale di Verona. Holotype VR 94508, a complete dorsal carapace in volume (Figure 5I).
Phylogenetic justification. The overall carapace shape of G. trispinosus, with smooth dorsal surface and lacking dorsal ridges, and the anterolateral margin with three spines, being the posterior spine the smallest of all, match well the diagnosis of Glabropilumnus (Poore and Ahyong, 2023). Glabropilumnus Balss, 1932, is an extant genus represented by six extant species (Ng et al., 2008), and the only genus in the family Pilumnidae and the subfamily Pilumninae Samouelle, 1819, with a fossil record extending into the Eocene (see below), hence our taxon selection for calibration.
Minimum age. 47.8 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. Glabropilumnus trispinosus is known from its type locality around Rama di Bolca (Verona), NE Italy, in calcareous rocks of the ‘Calcari Nummulitici’ that have been ascribed to the lower Eocene (Ypresian) based on the presence of Nummulites partschi, Alveolina cremae, A. croatica, A. decastroi, A. cf. dainellii, and A. distefanoi (Beschin et al., 2016b, and references therein). Soft maximum age as for calibration 8.
Discussion. Pilumnid genera and species show a considerable anatomical disparity. Although previous molecular and mitogenomic phylogenetic studies have suggested that Pilumnidae, in the broad sense, is monophyletic (Lai et al., 2014; Duan et al., 2022), the monophyly of Pilumnidae as currently envisioned is questionable (Wolfe et al., 2023). Despite this, Glabropilumnus can be referred to the subfamily Pilumninae sensu stricto, and thus to the crown Pilumnidae (Poore and Ahyong, 2023).
There are several extinct genera of Eocene crabs from Europe and USA that have been tentatively assigned to Pilumnidae and, specifically, the subfamily Pilumninae. However, the only extant pilumnid genus with Eocene representatives known is Glabropilumnus Balss, 1932. Besides G. trispinosus, G. bizzarinii Beschin, Busulini, and Tessier, in Beschin et al., 2019, is known from the upper Eocene (Priabonian) of Italy, together with G. cf. granulatus De Angeli, Garassino and Ceccon, 2010b, which was previously known solely from the lower Oligocene of Italy (Beschin et al., 2019). As such, the most recent common ancestor of Pilumnidae and all of its descendants must be pre-Eocene.
Brachyura: Eubrachyura: Goneplacoidea: Goneplacidae (crown)
Fossil specimens. Carcinoplax temikoensis Feldmann and Maxwell, 1990. New Zealand Geological Survey, Lower Hutt, New Zealand. Holotype AR 1943, a complete dorsal carapace and a cheliped (Figure 5J).
Phylogenetic justification. Carcinoplax temikoensis can be assigned to Carcinoplax Milne Edwards, 1852, based on the transversely rectangular carapace, the forward directed, narrow orbits, and the presence of three spines on the anterolateral margin, including the one forming the outer orbital angle (Feldmann and Maxwell, 1990; Ng and Castro, 2020). Carcinoplax is nested within Goneplacidae (Karasawa and Kato, 2003).
Minimum age. 34.6 Ma.
Tip maximum age. 39.1 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Carcinoplax temikoensis was collected from the lower part (unit 2) of the Island Sandstone at Te Miko, locality K30/D7A, exposed along the coast between Perpendicular Point and Punakaiki, north of Greymouth, Westland, New Zealand, ascribed to the upper Eocene (Kaiatan to Runangan) based on foraminiferal assemblages (Feldmann and Maxwell, 1990). The Kaiatan (39.1-36.7 Ma) and Runangan (36.7-34.6 Ma) stages in New Zealand roughly correspond to the global uppermost Bartonian (41.2-37.71 Ma) and Priabonian (37.71-33.9 Ma) ages (Raine et al., 2015). Given the unclear exact age of the layers bearing the type material of C. temikoensis, for the time being, we bracket its tip maximum and minimum ages between the base of the Kaiatan and the top of the Runangan (i.e., 39.1-34.6 Ma). Soft maximum age as for calibration 8.
Discussion. Currently, there are 25 species referred to Carcinoplax (Ng and Castro, 2020). As previously discussed for Euryplacidae (see above), the superfamily Goneplacoidea is an anatomically disparate and problematic brachyuran groups in terms of the clarity about its internal phylogenetic affinities (Ng et al., 2008; Castro et al., 2010). Despite this, Carcinoplax fits well within Goneplacidae and thus belongs to the crown Goneplacoidea.
There are over a dozen species of Carcinoplax known from fossils, from which only C. temikoensis occur in the Eocene. Extinct, monotypic genera known from the Eocene and presumably with goneplacid affinities include Amydrocarcinus Schweitzer, Feldmann, González-Barba, and Vega, 2002, from the Bartonian of Mexico, and Gonioplacoides Quayle and Collins, 2012, from the upper Eocene of UK. The presence of extinct and extant goneplacid genera in the Eocene indicate that the most recent common ancestor of Goneplacidae and all its descendants must be at least early Eocene in age, but, as previously seen with most other families already discussed, its origins might lie in the Paleocene (Wolfe et al., 2023).
Brachyura: Eubrachyura: Xanthoidea: Pseudorhombilidae (crown)
Fossil specimens. Pseudorhombila patagonica Glaessner, 1933. Collections of the British Museum. Holotype In. 28031, a complete dorsal carapace (Figure 5K).
Phylogenetic justification. The genus Pseudorhombila H. Milne Edwards, 1834 [in 1834-1840], has been recovered nested within a monophyletic Xanthoidea, but its familial placement has been included within Pseudorhombilidae Alcock, 1900 (e.g., Thoma et al., 2014), within the subfamily Pseudorhombilinae under the family Panopeidae Ortmann, 1893, or besides other subfamilies such as Panopeinae, Melybiinae, and an unnamed subfamily (e.g., Mendoza et al., 2022). In our recent phylogenetic study (Wolfe et al., 2023), Pseudorhombila was recovered in a clade formed by several genera typically ascribed to Pseudorhombilidae, which in turn was recovered as sister to a clade formed by several genera ascribed to Panopeidae. As such, here we consider Pseudorhombilidae as a discrete family.
Minimum age. 5.333 Ma.
Tip maximum age. 23.03 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type specimen of Pseudorhombila patagonica was collected from the so-called Patagonian Beds, in Santa Cruz Province, Argentina, which was considered Miocene in age (Glaessner, 1933, 1969). Unfortunately, it comes from an old collection, and its precise lithostratigraphic and chronostratigraphic context is unclear, hence the broad minimum and soft maximum age bracket. Soft maximum age as for calibration 8.
Discussion. Pseudorhombilidae Alcock, 1900, is a small family of xanthoid crabs with eight extant genera and over a dozen species known to date (Ng et al., 2008; De Grave et al., 2009; Schweitzer et al., 2021c). In our study (Wolfe et al., 2023), species within Pseudorhombilidae form a monophyleic group sister to Panopeidae. To date, the only known fossil referable to the family is P. patagonica, a member of the extant genus Pseudorhombila, therefore it is our selection of fossil for calibration.
Brachyura: Eubrachyura: Xanthoidea: Panopeidae (crown)
Fossil specimens. Panopeus incisus Beschin, Busulini, De Angeli, and Tessier, 2007. Museo di Archeologia e Scienze Naturali “G. Zannato” di Montecchio Maggiore, Vicenza, northern Italy. Holotype MCZ 2009, a nearly complete dorsal carapace (Figure 5L).
Phylogenetic justification. Panopeus incisus was ascribed to Panopeus H. Milne Edwards, 1834 [in 1834-1840], based on the wide, subhexagonal carapace, the broad and mesially notched front, the sub-ovate orbits bearing two orbital fissures, and the well-developed anterolateral margin with four spines excluding the outer orbital spine (Schweitzer, 2000; Beschin et al., 2007). Panopeus is an extant genus, the type genus of the family Panopeidae Ortmann, 1893, and together with other panopeid species forms a clade nested within Xanthoidea (Thoma et al., 2014; Jennings et al., 2021; Mendoza et al., 2022; Wolfe et al., 2023).
Minimum age. 47.8 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. The type material of Panopeus incisus comes from lower Eocene rocks cropping out at the Contrada Gecchelina of the Monte di Malo in Vicenza, northern Italy (Beschin et al., 2007, see also Beschin et al., 2016b). Age as for calibration 13. Soft maximum age as for calibration 8.
Discussion. Panopeidae is a speciose group of xanthoid crabs with an early fossil record extending back into the Paleogene period. Among the extant panopeid genera, only Panopeus, Lophopanopeus Rathbun, 1898, and Metopocarcinus Stimpson, 1860, have putative occurrences in the Eocene (Beschin et al., 2016a; Pasini et al., 2019), with Panopeus being represented by multiple species (e.g., Schweitzer, 2000; Vega et al., 2008; Gatt and De Angeli, 2010; Beschin et al., 2013; Beschin et al., 2016b; Beschin et al., 2018, and references therein). While there are numerous extinct presumed panopeid genera with Eocene representatives, only Pakicarcinus Schweitzer, Feldmann and Gingerich, 2004, and Glyphithyreus Reuss, 1859, have pre-Eocene (upper? Paleocene) occurrences (Collins and Morris, 1978; Charbonnier et al., 2013; Schweitzer et al., 2016a).
The presence of numerous species of extinct and extant panopeid genera in the Eocene and apparently the late Paleocene, suggest that the origins of the family are rooted into the early Paleogene.
Brachyura: Eubrachyura: Xanthoidea: Xanthidae (crown)
Fossil specimens. Phlyctenodes edwardsi Beschin, Busulini, Tessier, and Zorzin, 2016b. Museo di Storia naturale di Verona. Holotype VR 94284, a complete dorsal carapace (Figure 5M).
Phylogenetic justification. The genus Phlyctenodes Milne-Edwards, 1862b is ascribed to Xanthidae, specifically the subfamily Actaeinae Alcock, 1898, based on the ovate and wide carapace with poorly defined dorsal regions, the large and rimmed sub-circular orbits lacking orbital fissures, the anterolateral margins moderately convex and bearing several small tubercles or spines, the smooth posterolateral margins nearly straight to weakly concave, the overall fronto-orbital margin configuration, a front that is wide and bearing four or more tubercles, and the overall outline of the frontal and anterolateral margins forming a wide arch (Busulini et al., 2006; Beschin et al., 2016b; Schweitzer et al., 2021c).The monophyletic status of the family Xanthidae MacLeay, 1838b, has been corroborated by several molecular and total evidence phylogenetic studies (e.g., Lai et al., 2011; Tsang et al., 2014; Mendoza et al., 2022; Wolfe et al., 2023).
Minimum age. 47.8 Ma.
Tip maximum age. 56.0 Ma.
Soft maximum age. 100.9 Ma.
Age justification. Phlyctenodes edwardsi is known from its type locality around Rama di Bolca (Verona), NE Italy, in calcareous rocks of the ‘Calcari Nummulitici’ that have been ascribed to the lower Eocene (Ypresian) (Beschin et al., 2016b). Age as for calibration 32. Soft maximum age as for calibration 8.
Discussion. Phlyctenodes is an extinct genus with most of its species occurring in the Eocene of Europe (Busulini et al., 2006). We chose P. edwardsi as our calibration point given its Ypresian (56.0-47.8 Ma) age, which is similar to the age assigned to P. multituberculatus Beschin, Busulini, De Angeli, and Tessier, 2007, and P. nicolisi Bittner, 1884, from the Ypresian of Italy. Phlyctenodes tuberculosus Milne-Edwards, 1862b, from the middle-upper Eocene (41.2-47.8 Ma) of France and Italy, was used by Tsang et al. (2014), Wolfe et al. (2019), and Mendoza et al. (2022) as their calibration points. Beschin et al. (2016b) reported this species also from the Ypresian of Italy, in the Bolca region from were P. edwardsi originated. As such, given the numerous species of Phlyctenodes now known from the Ypresian, any of those species would represent equally parsimonious choices of calibration points, and all would indicate that the most recent common ancestor of xanthid crabs and all its descendants must have originated in the pre-Eocene.
CONCLUSIONS
In this work, we highlight some general considerations when selecting fossils and their age ranges for molecular calibrations and divergence time estimations, irrespective of the taxon. While molecular and morphological phylogenies provide different types of evidence for understanding evolutionary relationships among organisms, fossils offer critical anatomical, spatial, and temporal information inaccessible from extant species alone.
As a case study, we present 36 vetted fossil node calibration points for molecular phylogenetic analysis of crabs and reassess the earliest occurrences of several key clades based on recent fossil discoveries, re-examination of previous studies, their systematic affinities and, whenever possible, their stratigraphically constrained absolute or relative ages. For each calibrated node, we provide minimum and tip maximum ages for the stratigraphically oldest fossil that can be reliably assigned to the group. In some cases, the selection of a specific fossil calibration point does not represent the oldest record that has been putatively assigned to a given family, but the oldest occurrence that can be referred to the clade with optimal confidence in its morphology.
For crown Meiura (Anomura + Brachyura), we provide a soft maximum age of 191.8 Ma based on the estimated maximum age for the brachyuran fossil Eocarcinus praecursor from the Lower Jurassic. The slightly older Platykotta akaina, from the Upper Triassic, was not considered as a suitable calibration for Meiura due to its overall poor preservation and incompleteness, which precludes a systematic affiliation with Anomura or even Meiura beyond doubt. For crown Eubrachyura, we provide a soft maximum age of 100.9 Ma based on the estimated maximum age for Cretapsara athanata from the mid-Cretaceous. Its phylogenetic position, and its derived, modern-looking anatomy, place it well nested within crown Eubrachyura, compared to other coeval forms.
Molecular biologists working on phylogenetics and using fossils for calibrations and divergence time estimations would greatly benefit from working closer with palaeobiologists and geobiologists, which may inform better whether some of the purported older records of a given group are reliable for calibrations, as well as their lithostratigraphic context and chronostratigraphically constrained absolute or relative ages to increase accuracy.
The identification and critical examination of key fossil occurrences is crucial to recognize the lower age limits of Brachyura, ensuring not only precise but accurate calibrations. Reconstructed divergence times for the crab tree of life will help disentangle the anatomical disparity of crabs, and key events such as the origins of carcinization, decarcinization, and the transition from sea to land and freshwater.
ACKNOWLEDGEMENTS
We dedicate this work to the memory of Rodney M. Feldmann (1939-2024), a colleague, friend, and mentor of the lead author, whose contribution to brachyuran palaeobiology and systematics transformed the field of palaeocarcinology. We thank M. Hyžný, À. Ossó, B. van Bakel, F.A. Ferratges, C. Schweitzer, and O. Aguilera, for facilitating us bibliographic references and for valuable discussion about the systematic affinities and potential ages of some of taxa here investigated. Special thanks to P.O. Antoine (Université de Montpellier, France), P. Artal (Museo Geológico del Seminario de Barcelona, Spain), B. van Bakel (Oertijdmuseum De Groene Poort, Boxten, the Netherlands), J.F. Betancort (Universidad de Las Palmas de Gran Canaria, Spain), L. Boucher and A. Molienux (UT Austin, USA), A. Busulini (Museo di Storia Naturale, Venezia, Italy), A. De Angeli (Museo Civico “G. Zannato”, Montecchio Maggiore, Vicenza, Italy), A. Dulai (Hungarian Natural History Museum, Hungary), R. Feldmann and C. Schweitzer (Kent State University, USA), F.A. Ferratges (Universidad de Zaragoza, Spain), R. Howard (NHM London, UK), M. Hyžný (Comenius University, Slovakia), H. Karasawa (Mizunami Fossil Museum, Japan), A. Klompmaker (Alabama), À. Ossó (Tarragona, Catalonia), and F. Vega (UNAM, Mexico), for their kind help providing several of the images of the fossil specimens here illustrated. We thank the National Science Foundation (NSF), DEB grants #1856667 and #1856679 (USA) for supporting this work, and C. Schweitzer and M. Hyžný for their thoughtful and constructive comments. This is contribution #1717 from the Coastlines and Oceans Division of the Institute of Environment at Florida International University.
AUTHOR CONTRIBUTION
J.L. conceived and designed the manuscript, wrote the main draft, made the figures and tables. J.M.W. helped conceptually on the project. J.L., H.D.B-G., J.O-H., J.M.W. edited the manuscript and confirmed the content of the paper.
REFERENCES
Agnew, J. 2001. Taxonomy, taphonomy, and paleoecology of the Plio-Pleistocene shell beds at 101 Ranch Pit, Okeechobbe County, Florida. Masters of Science thesis, Gainesville, Florida, USA.
Aguilera, O., Martins, M.V.A., Linhares, A.P., Kütter, V.T., and Coletti, G. 2022. Palaeoenvironment of the Miocene Pirabas Formation mixed carbonate-siliciclastic deposits, Northern Brazil: Insights from skeletal assemblages. Marine and petroleum geology, 145:105855.
https://doi.org/10.1016/j.marpetgeo.2022.105855
Ahyong, S.T. 2019. The cymonomid crabs of New Zealand and Australia (Crustacea: Brachyura: Cyclodoripoida). Records of the Australian Museum, 71:33-69.
https://doi.org/10.3853/j.2201-4349.71.2019.1682
Ahyong, S.T., Lai, J.C.Y., Sharkey, D., Colgan, D.J., and Ng, P.K.L. 2007. Phylogenetics of the brachyuran crabs (Crustacea: Decapoda): The status of Podotremata based on small subunit nuclear ribosomal RNA. Molecular Phylogenetics and Evolution, 45:576-586.
https://doi.org/10.1016/j.ympev.2007.03.022
Ahyong, S.T., Baba, K., Macpherson, E., and Poore, G.C. 2010. A new classification of the Galatheoidea (Crustacea: Decapoda: Anomura). Zootaxa, 2676:57-68.
https://doi.org/10.11646/zootaxa.2676.1.4
Alcock, A. 1898. Materials for a carcinological fauna of India. No. 3. The Brachyura Cyclometopa. Part I. The family Xanthidae. Journal of the Asiatic Society of Bengal, 67:67-233.
Alcock, A. 1900. Materials for a carcinological fauna of India, No. 6. The Brachyura Catometopa, or Grapsoidea. Journal of the Asiatic Society of Bengal, 69:279-456.
https://doi.org/10.5962/bhl.title.15344
Anderson, J.L. and Feldmann, R.M. 1995. Lobocarcinus lumacopius (Decapoda: Cancridae), a new species of cancrid crab from the Eocene of Fayum, Egypt. Journal of Paleontology, 69:922-932.
https://doi.org/10.1017/s0022336000035575
Ando, Y., Kishimoto, S., and Kawano, S. 2016. Two new species of Thalassina (Decapoda, Thalassinidae) from the Miocene of Japan. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen:107-117.
https://doi.org/10.1127/njgpa/2016/0568
Antoine, P.-O., Abello, M.A., Adnet, S., Sierra, A.J.A., Baby, P., Billet, G., Boivin, M., Calderón, Y., Candela, A., and Chabain, J. 2016. A 60-million-year Cenozoic history of western Amazonian ecosystems in Contamana, eastern Peru. Gondwana Research, 31:30-59.
https://doi.org/10.1016/j.gr.2015.11.001
Antonioli, L., de Araújo Távora, V., and Dino, R. 2015. Palynology of carcinolites and limestones from the Baunilha Grande Ecofacies of the Pirabas Formation (Miocene of Pará state, northeastern Brazil). Journal of South American Earth Sciences, 62:134-147.
https://doi.org/10.1016/j.jsames.2015.05.005
Armstrong, A., Nyborg, T., Bishop, G.A., Ossó-Morales, À., and Vega, F.J. 2009. Decapod crustaceans from the Paleocene of Central Texas, USA. Revsta Mexicana de Ciencias Geológicas, 26:745-763.
https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1026-87742009000300015
Artal, P. 2008. Uca miocenica (Crustacea, Decapoda), nueva especie del Mioceno de la Prov. de Barcelona (Cataluña, España). Scripta Musei Geologici Seminarii Barcinonensis (Palaeontologica), 6:3-18.
Artal, P. and Hyžný, M. 2016. An appraisal of Typilobus Stoliczka, 1871 (Crustacea, Brachyura, Leucosioidea), with description of a new family and genus. Zootaxa, 4117:387-398.
https://doi.org/10.11646/zootaxa.4117.3.6
Artal, P. and Onetti, A. 2017. Tavernolesia, new genus (Crustacea, Decapoda, Brachyura), from the Eocene of the Iberian Peninsula. Batalleria, 24:6-12.
https://www.mgsb.es/pdfs%20Batalleria%2024/Tavernolesia.pdf
Aslanova, S. and Dschafarova, J. 1975. Some finds of Decapoda from the Tertiary deposits of Azerbaijan. Akademia nauk Azerbaidzhanskoi Soviet Socialist Republic Doklady, 31:41-46.
Bachmayer, F. and Wagner, R. 1957. Geryon heimertingensis n. sp., eine Krabbbe aus dem Chattian der Erdölbohrung Heimertingen (bayerische Vorlandmolasse). Paläontologische Zeitschrift, 31:99-102.
https://doi.org/10.1007/bf02988967
Bachmayer, F. and Mundlos, R. 1968. Die tertiären Krebse von Helmstedt bei Braunschweig, Deutschland. Annalen des Naturhistorischen Museums in Wien, 72:649-692.
https://www.jstor.org/stable/41781809
Balss, H. 1932. Über einige systematisch interessante Xanthidae (Crustacea, Decapoda, Brachyura) der Harmsschen Reisen nach dem Sundaarchipel. Zeitschrift für Wissenschaftliche Zoologie, 142:510-519.
Batllori, J. and García, J.J. 1997. Malacofauna d'un manglar del Miocè de Bellaterra (depressió del Vallès-Penedès, Barcelona). Butlletí de la Institució Catalana d'Història Natural:15-21.
https://raco.cat/index.php/ButlletiICHN/article/view/14669/303398
Beccaro, L. 2003. Revisioni stratigrafiche nel Paleocene del Veneto occidentale. Dottorato di Ricerca in Scienze della Terra, Ciclo XVI. Unpublished Ph.D. Thesis, Università degli Studi di Padova.
Bell, T. 1858. A monograph of the fossil malacostracous Crustacea of Great Britain. Part I. Crustacea of the London Clay. Monograph of the Palaeontographical Society, 10:i-viii, 1-44, 11 pls.
https://doi.org/10.1080/02693445.1858.12027913
Bell, T. 1863. A monograph of the Fossil Malacostracous Crustacea of Great Britain. Part II. Crustacea of the Gault and Greensand. Monograph of the Palaeontographical Society, 14:vii + 40 pp., pls. 41-11.
https://doi.org/10.1080/02693445.1863.12027935
Bernot, J.P., Owen, C.L., Wolfe, J.M., Meland, K., Olesen, J., and Crandall, K.A. 2022. Major revisions in pancrustacean phylogeny and evidence of sensitivity to taxon sampling. Molecular Biology and Evolution, 40:msad175.
https://doi.org/10.1093/molbev/msad175
Beschin, C., Busulini, A., De Angeli, A., Tessier, G., and Ungaro, S. 1998. Crostacei eocenici di “Cava Rossi” presso Monte di Malo (Vicenz - Italia settentrionale). Studi Trentini di Scienze Naturali - Acta Geologica, 73:7-34.
Beschin, C., De Angeli, A., and Checchi, A. 2001. Crostacei decapodi associati a coralli della “Formazione di Castelgomberto”(Oligocene)(Vicenza–Italia settentrionale). Studi e Ricerche, Associazione Amici del Museo, Museo Civico ‘‘G. Zannato’’, Montecchio Maggiore (Vicenza), 2001:13-30.
Beschin, C., Busulini, A., De Angeli, A., and Tessier, G. 2002. Aggiornamento ai crostacei eocenici di cava “Main” di Arzignano (Vicenza-Italia settentrionale) (Crustacea, Decapoda). Studi e Ricerche, Associazione Amici del Museo–Museo Civico “G. Zannato”(Montecchio Maggiore), 2002:7-28.
Beschin, C., Busulini, A., De Angeli, A., and Tessier, G. 2007. I decapodi dell'Eocene inferiore di Contrada Gecchelina (Vicenza, Italia settentrionale) (Anomura e Brachyura). Museo di archeologia e scienze naturali G. Zannato:1-84.
Beschin, C., De Angeli, A., and Zorzin, R. 2009. Crostacei fossili del Veneto: una inedita fauna eocenica dei Lessini orientali (Monte Serea di San Giovanni Ilarione, Verona), con descrizione di tre nuove specie. Bollettino del Museo civico di Storia naturale di Verona, 33:59-83.
Beschin, C. and De Angeli, A. 2011. Baldoplax bonizzatoi gen. nov., sp. nov., (Crustacea, Decapoda, Euryplacidae) dell'Eocene di Ferrara di Monte Baldo (Verona, Italia settentrionale). Studi e Ricerche - Associazione Amici del Museo- Museo Civico "G. Zannato", 18:5-10.
Beschin, C., Busulini, A., and Tessier, g. 2013. Crostacei medio-eocenici della "Pietra di Nanto" (Monti Berici, Vicenza- Italia settentrionale). Lavori-Società Veneziana di Scienze Naturali, 38:111-146.
Beschin, C., Busulini, A., and Tessier, G. 2015. Nuova segnalazione di crostacei associati a coralli nell'Eocene inferiore dei Lessini orientali (Vestenanova-Verona). Lavori Società veneziana di Scienze naturali, 40:47-109.
Beschin, C., De Angeli, A., Checchi, A., and Zarantonello, G. 2016a. Crostacei Decapodi del "tufo a Lophoranina" (Luteziano inferiore) della Valle del Chiampo (Vicenza, Italia nordorientale). Museo di Archeologia e Scienze Naturali "G. Zannato", Montecchio Maggiore (Vicenza).
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2016b. I crostacei associati a coralli nell'Eocene inferiore dell'area di Bolca: Verona e Vicenza, Italia nordorientale, Memorie del Museo civico di storia naturale di Verona.
Beschin, C., Busulini, A., Fornaciari, E., Papazzoni, C.A., and Tessier, G. 2018. La fauna di crostacei associati a coralli dell'Eocen Superiore di Campolongo di Val Liona (Monti Berici, Vicenza, Italia Nordorientale). Bolletino del Museo di Storia Naturale di Venezia, 69:129-215.
Beschin, C., Busulini, A., Tessier, G., and Zorzin, R. 2019. La fauna di crostacei dell'Eocene superiore di Parona di Verona (Italia nordorientale): nuovi ritrovamenti. Bollettino del Museo di Storia Naturale di Venezia, 70:71-142.
Betancort, J.F., Lomoschitz Mora-Figueroa, A., and Meco, J. 2014. Mio-Pliocene crustaceans from the Canary Islands, Spain. Rivista Italiana di paleontologia e Stratigrafia, 120:337-349. https://doi.org/10.13130/2039-4942/6076
Beurlen, K. 1958. Contribuição à paleontologia do estado do Para: Crustáceos Decápodos da Formação Pirabas. Boletim do Museu Paraense Emilio Goeldi, (nova série) (Geologia), 5:1-48.
Bishop, G.A. and Williams, A.B. 2000. Fossil crabs from tepee buttes, submarine seeps of the Late Cretaceous Pierre Shale, South Dakota and Colorado, USA. Journal of Crustacean Biology, 20:286-300.
https://doi.org/10.1163/1937240x-90000031
Bittner, A. 1893. Decapoden des pannonischen Tertiärs. Sitzungsberichte der kaiserlichen Akademie der Wissenschaften in Wien, 102:10-37.
Blow, W.C. and Manning, R.B. 1996. Preliminary descriptions of 25 new decapod crustaceans from the middle Eocene of the Carolinas, U.S.A. Tulane Studies in Geology and Paleontology, 29:1-26. https://journals.tulane.edu/tsgp/article/view/611
Blow, W.H. 1969. Late Middle Eocene to Recent planktonic foraminiferal biostratigraphy. In Proceedings of the first international conference on planktonic microfossils (Geneva, 1967) 1969, Volume 1, Leiden, EJ Brill, p. 199-422.
https://doi.org/10.1163/9789004616455_018
Boucot, A.J. and Poinar Jr, G.O. 2010. Fossil Behavior Compendium. CRC Press, Boca Raton.
https://doi.org/10.1201/9781439810590
Brito, I.M. 1972. Contribuição ao Conhecimento dos Crustáceos Decápodos da Formação Pirabas. II – O Gênero Uca (Brachyura-Ocypodidae). Anais da Academia Brasileira de Ciências, 44:95-98.
Buchs, D.M., Irving, D., Coombs, H., Miranda, R., Wang, J., Coronado, M., Arrocha, R., Lacerda, M., Goff, C., and Almengor, E. 2019. Volcanic contribution to emergence of Central Panama in the Early Miocene. Scientific reports, 9:1-16.
https://doi.org/10.1038/s41598-018-37790-2
Busulini, A., Tessier, G., Beschin, C., and De Angeli, A. 2003. Boschettia giampietroi, nuovo genere e specie di Portunidae (Crustacea, Decapoda) dell’Eocene medio della Valle del Chiampo (Vicenza, Italia settentrionale). Studi e Ricerche-Associazione Amici del Museo-Museo civico “G. Zannato”-Montecchio Maggiore (Vicenza):13-18.
Busulini, A., Tessier, G., and Beschin, C. 2006. The genus Phlyctenodes Milne Edwards, 1862 (Crustacea: Decapoda: Xanthidae) in the Eocene of Europe. Revista mexicana de ciencias geológicas, 23:350-360.
https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1026-87742006000300011
Busulini, A., Beschin, C., and Tessier, G. 2014. A re-evaluation of extinct European crabs referred to the genus Calappilia A. Milne Edwards in Bouillé, 1873 (Brachyura, Calappidae). Scripta Geologica, 147:193-219.
Cahuzac, B. and Poignant, A. 1997. Essai de biozonation de l’Oligo-Miocène dans les bassins européens à l’aide des grands foraminifères néritiques. Bulletin de la Société géologique de France, 168:155-169.
Cahuzac, B. and Londeix, L. 2012. Biostratigraphie appliquée à l’étage Aquitanien [Contribution à la connaissance de l'étage Aquitanien, n° 8] Bulletin de la Société Linnéenne de Bordeaux:213-226.
Carreño, A.L. and Cronin, T.M. 1993. Middle Eocene Ostracoda from Baja California Sur, Mexico. Journal of Micropalaeontology, 12:141-153.
https://doi.org/10.1144/jm.12.2.141
Casadío, S., Guerstein, G., Marenssi, S., Santillana, S., Feldmann, R., Parras, A., and Montalvo, C. 2000. Evidencias para una edad oligocena de la Formación Centinela, suroeste de Santa Cruz, Argentina. Ameghiniana, 37:71R.
Casadio, S., Parras, A., Marenssi, S., and Griffin, M. 2001. Edades 87Sr/86Sr de Crassostrea ?hatcheri Ortmann (Bivalvia, Ostreoidea) en el “Patagoniano” de Santa Cruz, Argentina, Reunión de Comunicaciones de la Asociación Paleontológica Argentina. 29-30 de noviembre, 2001, Volume 38. Ameghiniana, Suplemento.
Casadío, S., De Angeli, A., Feldmann, R.M., Garassino, A., Hetler, J.L., Parras, A., and Schweitzer, C.E. 2004. New decapod crustaceans (Thalassinidea, Galatheoidea, Brachyura) from the middle Oligocene of Patagonia, Argentina. Annals of Carnegie Museum, 73:25-47.
https://doi.org/10.5962/p.215152
Casadío, S., Feldmann, R.M., Parras, A., and Schweitzer, C.E. 2005. Miocene fossil Decapoda (Crustacea: Brachyura) from Patagonia, Argentina, and their paleoecological setting. Annals of Carnegie Museum, 74:151-188.
https://doi.org/10.2992/0097-4463(2005)74[151:mfdcbf]2.0.co;2
Casanovas-Vilar, I., DeMiguel, D., Galindo, J., Robles, J.M., Garcés, M., and Cabrera, L. 2011. The continental Burdigalian (Early Miocene) of the Vallès-Penedès Basin (Catalonia, Spain), p. 93-100. In Pérez-García, A., Gascó, F., Gasulla, J.M., and Escaso, F. (eds.), Viajando a mundos pretéritos: Ayuntamiento de Morella, Morella.
Casanovas-Vilar, I., Madern, A., Alba, D.M., Cabrera, L., García-Paredes, I., Van den Hoek Ostende, L.W., DeMiguel, D., Robles, J.M., Furió, M., and van Dam, J. 2016. The Miocene mammal record of the Vallès-Penedès Basin (Catalonia). Comptes Rendus Palevol, 15:791-812.
https://doi.org/10.1016/j.crpv.2015.07.004
Castro, P., Ng, P.K., and Ahyong, S.T. 2004. Phylogeny and systematics of the Trapeziidae Miers, 1886 (Crustacea: Brachyura), with the description of a new family. Zootaxa, 643:1-70.
https://doi.org/10.11646/zootaxa.643.1.1
Castro, P., Guinot, D., and Ng, P.K.L. 2010. A new family for Sotoplax robertsi Guinot, 1984, with a diagnosis and key to the Goneplacoidea MacLeay, 1838 (Crustacea: Decapoda: Brachyura). Zootaxa, 2356:36-56.
https://doi.org/10.11646/zootaxa.2356.1.2
Castro, P. and Ng, P.K.L. 2010. Revision of the family Euryplacidae Stimpson, 1871 (Crustacea: Decapoda: Brachyura: Goneplacoidea). Zootaxa, 2375:1-130.
https://doi.org/10.11646/zootaxa.2375.1.1
Ceccon, L. and De Angeli, A. 2019. Un nuovo costaceo Percnidae dell’Oligocene di Sant’Urbano, Vicenza, Italia settentrionale. Bolletino del Museo Civico di Storia Naturale di Verona, 43:17-22.
Chablais, J., Feldmann, R.M., and Schweitzer, C.E. 2011. A new Triassic decapod, Platykotta akaina, from the Arabian shelf of the northern United Arab Emirates: earliest occurrence of the Anomura. Paläontologische Zeitschrift, 85:93-102.
https://doi.org/10.1007/s12542-010-0080-y
Charbonnier, S., Garassino, A., Pasini, G., Métais, G., Merle, D., Bartolini, A., Brohi, I.A., Solangi, S.H., Lashari, R.A., Welcomme, J.-L., and Marivaux, L. 2013. Early Paleogene decapod crustaceans from the Sulaiman and Kirthar Ranges, Pakistan. Annales de Paléontologie, 99:101-117.
https://doi.org/10.1016/j.annpal.2012.12.003
Chen, J., Xing, Y., Yao, W., Xu, X., Zhang, C., Zhang, Z., and Liu, Q. 2019. Phylomitogenomics reconfirm the phylogenetic position of the genus Metaplax inferred from the two grapsid crabs (Decapoda: Brachyura: Grapsoidea). PLoS ONE, 14:e0210763.
https://doi.org/10.1371/journal.pone.0210763
Choffat, P.L. 1898. Recueil d´études paléontologiques sur la Faune Crétacique du Portugal, II p. 45, Section des Travaux Géologiques du Portugal, Lisbonne.
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.-X. 2013; updated 2023. The ICS International Chronostratigraphic Chart. Episodes, 36:199-204.
http://www.stratigraphy.org/ICSchart/ChronostratChart2023-04.pdf
Collins, J.S.H. and Morris, S.F. 1978. New Lower Tertiary crabs from Pakistan. Palaeontology, 21:957-981.
Collins, J.S.H. and Donovan, S.K. 1997. Some new crab records (Crustacea: Decapoda) from the late Pleistocene Port Morant Formation of southeast Jamaica. Bulletin of the Mizunami Fossil Museum, 24:73-77.
Collins, J.S.H. and Jakobsen, S.L. 2003. New crabs (Crustacea, Decapoda) from the Eocene (Ypresian/Lutetian) Lillebælt Clay Formation of Jutland, Denmark. Bulletin of the Mizunami Fossil Museum, 30:63-96.
Collins, J.S.H., Lee, C., and Noad, J. 2003. Miocene and Pleistocene crabs (Crustacea, Decapoda) from Sabah and Sarawak. Journal of Systematic Palaeontology, 1:187-226.
https://doi.org/10.1017/s1477201903001068
Collins, J.S.H., Mitchell, S.F., and Donovan, S.K. 2009. A new species of land crab, Sesarma Say, 1817 (Decapoda, Brachyura), from the Pleistocene of Jamaica. Scripta Geologica, 138:11-21.
Collins, J.S.H. and Donovan, S.K. 2010. Pleistocene decapod crustaceans of eastern Jamaica. Caribbean Journal of Science, 46:133-142.
https://doi.org/10.18475/cjos.v46i2.a1
Colosi, G. 1924. Una specie fossile de Gerionide (Decapodi brachiuri). Bolettino della Societá dei Naturalisti in Napoli, Series 2, 15 (for 1923):248-255.
Cotton, L., Rivero-Cuesta, L., Franceschetti, G., Monechi, S., Iakovleva, A., Alegret, L., Dinares-Turell, J., Hooker, J., King, C., and Fluegeman, R. 2021. Reassessing the Bartonian unit stratotype at Alum Bay (Isle of Wight, UK): an integrated approach. Newsletters on Stratigraphy, 54:17-42.
https://doi.org/10.1127/nos/2020/0563
Crame, J.A., Beu, A.G., Ineson, J.R., Francis, J.E., Whittle, R.J., and Bowman, V.C. 2014. The early origin of the Antarctic marine fauna and its evolutionary implications. PLoS ONE, 9:e114743. https://doi.org/10.1371/journal.pone.0114743
Crane, J. 1975. Fiddler Crabs of the World (Ocypodidae: genus Uca). Princeton University Press.
De Angeli, A. 2016. Nuovi crostacei Cymonomidae (Decapoda: Brachyura) dell’Eocene dei Monti Berici (Vicenza, Italia settentrionale). Studi Trentini di Scienze Naturali, 95:25-32.
De Angeli, A. and Beschin, C. 1998. Ceronnectes, nuovo genere di brachyuro dell’Eocene di Ungheria e italia. Lavori Società Veneziana di Scienze Naturali, 23:87-91.
De Angeli, A., Garassino, A., and Pasini, G. 2009. New reports of anomurans and brachyurans from the Cenozoic of Tuscany (Italy). Atti della Società italiana di scienze naturali e del museo civico di storia naturale di Milano, 150:163-196.
De Angeli, A., Garassino, A., and Alberti, R. 2010a. Eogarthambrus guinotae n. gen. and n. sp. (Decapoda, Brachyura, Parthenopidae) from the Eocene of Vicenza, Italy, p. 107-116, Studies on Brachyura: a homage to Danièle Guinot: Brill.
https://doi.org/10.1163/ej.9789004170865.i-366.71
De Angeli, A., Garassino, A., and Ceccon, L. 2010b. New report of the coral-associated decapods from the “Formazione di Castelgomberto” (early Oligocene) (Vicenza, NE Italy). Atti della Società italiana di Scienze naturali e del Museo civico di Storia naturale in Milano, 151:145-177.
De Angeli, A. and Ceccon, L. 2012. Eouroptychus montemagrensis n. gen., n. sp. (Crustacea, Decapoda, Anomura, Chirostylidae) dell'Eocene inferiore (Ypresiano) di Monte Magrè (Vicenza, Italia settentrionale). Lavori Società veneziana di Scienze naturali, 37:19-24.
De Angeli, A. and Ceccon, L. 2013. Tetraliidae and Trapeziidae (Crustacea, Decapoda, Brachyura) from the Early Eocene of Monte Magrè (Vicenza, NE Italy). Natural History Sciences, 154:25-40.
https://doi.org/10.4081/nhs.2013.25
De Angeli, A. and Ceccon, L. 2014. Nuovi Brachyura (Decapoda) dell'Eocene inferiore di Monte Magrè (Vicenza, Italia settentrionale). Lavori Società veneziana di Scienze naturali, 39:77-92.
De Angeli, A. and Ceccon, L. 2015. Nuovi crostacei Brachyura dell'Eocene di Monte Magrè (Vicenza, Italia settentrionale). Lavori Società veneziana di Scienze naturali, 40:119-138.
De Angeli, A. and Alberti, R. 2016. Tethyscarpilius bericus n. gen., n. sp.(Decapoda, Brachyura, Carpiliidae) dell’Eocene superiore dei Monti Berici (Vicenza, Italia settentrionale). Lavori Società Veneziana di Scienze Naturali, 41:121-128.
De Angeli, A. and Caporiondo, F. 2016. Un nuovo Parthenopidae (Crustacea, Decapoda, Brachyura) dell'Eocene inferiore dei Monti Lessini orientali (Verona− Italia settentrionale). Lavori Società veneziana di Scienze naturali, 41:137-144.
De Angeli, A. and Ceccon, L. 2017. Contributo ai crostacei decapodi dell’Eocene inferiore dei Monti Lessini orientali (Italia nordorientale). Natura Vicentina, 20:5-38.
De Angeli, A. and Caporiondo, F. 2019. Alontecarcinus buratoi n. gen., n. sp. (Decapoda, Brachyura, Potamonidae) un nuovo crostaceo d’acqua dolce dell’Eocene (Bartoniano) di Alonte (Monti Berici, Vicenza, Italia settentrionale). Bollettino del Museo Civico di Storia Naturale di Verona, 43:5-16.
De Angeli, A. and Garassino, A. 2021a. New report of fossil crabs (Decapoda, Brachyura) from the late Eocene of San Feliciano Hill (Orgiano, Monti Berici, Vicenza, NE Italy). Boletín de la Sociedad Geológica Mexicana, 73:A120221.
https://doi.org/10.18268/bsgm2021v73n3a120221
De Angeli, A. and Garassino, A. 2021b. Report of ethusid crabs (Brachyura, Ethusidae) from the late Eocene of San Francisco hill (Monti Berici, Vicenza, NW Italy). Neues Jahrbuch fuer Geologie und Palaeontologie. Abhandlungen, 301:17-23.
https://doi.org/10.1127/njgpa/2021/0995
De Angeli, A. 2023. Nuovi crostacei decapodi dell’Eocene superiore dei Monti Berici (Vicenza, Italia nordorientale). Lavori - Società Veneziana di Scienze Naturali, 48:169 -186.
de Bouillé, R. Paléontologie de Biarritz. In Proceedings Compte Rendu Travaux Congrès Scientifique de France, 39e session, Pau1873, p. 11, pl. 14.
de Gibert, J.M. and Robles, J.M. 2005. Firmground ichnofacies recording high-frequency marine flooding events (Langhian transgression, Vallès-Penedès Basin, Spain). Geologica Acta, 3:295-305.
http://www.publicacions.ub.es/doi/documents/1397.pdf
de Gibert, J.M., Muñiz, F., Belaústegui, Z., and Hyžný, M. 2013. Fossil and modern fiddler crabs (Uca tangeri: Ocypodidae) and their burrows from SW Spain: ichnologic and biogeographic implications. Journal of Crustacean Biology, 33:537-551.
https://doi.org/10.1163/1937240x-00002151
De Grave, S., Pentcheff, N.D., Ahyong, S.T., Chan, T.Y., Crandall, K.A., Dworschak, P.C., Felder, D.L., Feldmann, R.M., Fransen, C.H.J.M., Goulding, L.Y.D., Lemaitre, R., Low, M.E.Y., Martin, J.W., Ng, P.K.L., Schweitzer, C.E., Tan, S.H., Tshudy, D., and Wetzer, R. 2009. A classification of recent and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology Supplement, 21:1-109.
https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/10/app/uploads/2017/06/s21rbz1-109.pdf
De Haan, W. 1833-1850. Crustacea, p. i-xvii, i-xxxi, ix-xvi, 1-243, pls. A-J, L-Q, 241-255, table 242. In Siebold, P.F.v. (ed.), Fauna Japonica sive Descriptio Animalium, Quae in Itinere per Japoniam, Jussu et Auspiciis Superiorum, qui Summum in India Batava Imperium Tenent, Suscepto, Annis 1823-1830 Collegit, Notis, Observationibus et Adumbrationibus Illustravit: Lugduni-Batavorum, Leiden.
https://doi.org/10.5962/bhl.title.124951
del Rio, C.J., Concheyro, A., and Martınez, S.A. 2011. The Maastrichtian-Danian at General Roca (Patagonia, Argentina): a reappraisal of the chronostratigraphy and biostratigraphy of a type locality. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 259:129-156.
https://doi.org/10.1127/0077-7749/2011/0103
Desmarest, A.G. 1822. Les crustacés proprement dits, p. 67-154. In Brongniart, A. and Desmarest, A.G. (eds.), Histoire naturelle des crustacés fossiles: F.-G. Levrault, Paris.
https://doi.org/10.5962/bhl.title.9102
Desmarest, A.G. 1823. Malacostracés, Malacostraca. (Crust.), p. 138-425. In Cuvier, F. (ed.), Dictionnaire des Sciences Naturelles, dans lequel on trait Méthodiquement des Différens étres de la Nature, considérés soit en eux-mêmes, d’après l’état actuel de nos connoissances, soit relativement a l’utilité qu’en peuvent retirer la Médecine, l’Agriculture, le Commerce et les Arts. Suivi d’une biographie des plus Célèbres Naturalistes. Ouvrage destiné aux médecins, aux agriculteurs, aux commerçans, aux artistes, aux manufacturiers, et à tous ceux qui ont intérêt à connoître les productions de la nature, leurs caractères génériques et spécifiques, leur lieu natal, leurs propiétés et leurs usages, Volume 28: F.G. Levrault et Le Normant, Strasbourg et Paris.
Domínguez Alonso, P. 2008. Nuevo cangrejo violinista (género Uca, Ocypodidae) en el Plio-Pleistoceno del litoral pacífico de Honduras. Ameghiniana, 45:663-676.
Duan, X., Dong, X., Li, J., Lü, J., Guo, B., Xu, K., and Ye, Y. 2022. The complete mitochondrial genome of Pilumnopeus makianus (Brachyura: Pilumnidae), novel gene rearrangements, and phylogenetic relationships of Brachyura. Genes, 13:1943.
https://doi.org/10.3390/genes13111943
Dutton, A.L., Lohmann, K.C., and Zinsmeister, W.J. 2002. Stable isotope and minor element proxies for Eocene climate of Seymour Island, Antarctica. Paleoceanography, 17:6-1-6-13.
https://doi.org/10.1029/2000pa000593
Etayo-Serna, F. 1979. Zonation of the Cretaceous of central Colombia by ammonites. Ministerio de Minas y Energia, Instituto Nacional de Investigaciones Geológico-Mineras, Bogotá, Colombia.
https://doi.org/10.32685/10.143.1979.729
Evans, N. 2018. Molecular phylogenetics of swimming crabs (Portunoidea Rafinesque, 1815) supports a revised family-level classification and suggests a single derived origin of symbiotic taxa. PeerJ, 6:e4260.
https://doi.org/10.7717/peerj.4260
Eydoux, F. 1835. Nouvelle espèce de Gélasime. Magasin de Zoologie, 5:29-32.
Fabricius, J. 1781. Species insectorum exhibentes eorum dffirentias specificas, sryonrya auctorum, loca natalia, rnetarnorphosis adjectis obseruationibus, descriptionibus. Ernest Bohnii, Hamburg and Cologne.
https://doi.org/10.5962/bhl.title.36509
Fabricius, J. 1798. Supplementatum Entomologiae systematicae. Proft et Storch, Hafniae.
https://doi.org/10.5962/bhl.title.65803
Farris, D.W., Cardona, A., Montes, C., Foster, D., and Jaramillo, C. 2017. Magmatic evolution of Panama Canal volcanic rocks: A record of arc processes and tectonic change. PLoS ONE, 12:e0176010.
https://doi.org/10.1371/journal.pone.0176010
Feldmann, R.M. 1993. Additions to the fossil decapod crustacean fauna of New Zealand. New Zealand Journal of Geology and Geophysics, 36:201-211.
https://doi.org/10.1080/00288306.1993.9514568
Feldmann, R.M. 1994. Antarctomithrax thomsoni, a new genus and species of crab (Brachyura; Majidae) from the La Meseta Formation (Eocene) of Seymour Island, Antarctica. Journal of Paleontology, 68:174-176.
https://doi.org/10.1017/s0022336000025725
Feldmann, R.M. and Wilson, M.T. 1988. Eocene decapod crustaceans from Antarctica. Geological Society of America Memoir, 169:465-488.
Feldmann, R.M. and Maxwell, P.A. 1990. Late Eocene decapod Crustacea from North Westland, South Island, New Zealand. Journal of Paleontology, 64:779-797.
https://doi.org/10.1017/s0022336000018989
Feldmann, R.M., Casadío, S., Chirino-Galvez, L., and Aguirre-Urreta, M. 1995. Fossil decapod crustaceans from the Jagüel and Roca formations (Maastrichtian-Danian) of the Neuquén Basin, Argentina. Journal of Paleontology, 69:1-22.
https://doi.org/10.1017/s0022336000061060
Feldmann, R.M., Bice, K.L., Schweitzer-Hopkins, C.E., Salva, E.W., and Pickford, K. 1998. Decapod crustaceans from the Eocene Castle Hayne Formation, North Carolina: Paleoceanographic implications. Memoir (The Paleontological Society), 48:1-28.
https://doi.org/10.1017/s0022336000059916
Feldmann, R.M., Schweitzer, C.E., and Encinas, A. 2005. New decapods from the Navidad Formation (Miocene) of Chile. Journal of Crustacean Biology, 25:427-449.
https://doi.org/10.1651/c-2547
Feldmann, R.M. and Schweitzer, C.E. 2009. Revision of Jurassic Homoloidea De Haan, 1839, from the Ernstbrunn and Štramberk limestones, Austria and the Czech Republic. Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie:183-205.
https://www.jstor.org/stable/41701784
Feldmann, R.M., Schweitzer, C.E., and Encinas, A. 2010. Neogene decapod Crustacea from Southern Chile. Annals of Carnegie Museum 78:337-366.
https://doi.org/10.2992/007.078.0404
Feldmann, R.M., Schweitzer, C.E., Bennett, O., Franţescu, O.D., Resar, N., and Trudeau, A. 2011a. New Eocene Brachyura (Crustacea: Decapoda) from Egypt. Neues Jahrbuch fur Geologie und Palaontologie-Abhandlungen, 262:1-31.
https://doi.org/10.1127/0077-7749/2011/0202
Feldmann, R.M., Schweitzer, C.E., Casadío, S., and Griffin, M. 2011b. New Miocene Decapoda (Thalassinidea; Brachyura) from Tierra del Fuego, Argentina: Paleobiogeographic implications. Annals of the Carnegie Museum, 79:91-123.
https://doi.org/10.2992/007.079.0202
Feldmann, R.M., Schweitzer, C.E., and Karasawa, H. 2012. Part R, Revised, Volume 1, Chapter 8M: Systematic Descriptions: Infraorder Brachyura, Section Homoloida. Treatise Online, 52:1-12.
https://doi.org/10.17161/to.v0i0.4337
Feldmann, R.M. and Schweitzer, C.E. 2019. Earliest known sponge crab (Bacrhyura: Dromiidae) from the Upper Cretaceous Wenonah Formation, New Jersey, USA. Bulletin of the Mizunami Fossil Museum, 45:1-6.
Feldmann, R.M., Schweitzer, C.E., and Phillips, G. 2019. Paleogene Decapoda (Caridea, Anomura, Axiidea, Brachyura) from Alabama and Mississippi, USA. Journal of Crustacean Biology, 39:279-302.
https://doi.org/10.1093/jcbiol/ruz002
Ferratges, F.A., Zamora, S., and Aurell, M. 2019. A new genus and species of Parthenopidae MacLeay, 1838 (Decapoda: Brachyura) from the lower Eocene of Spain. Journal of Crustacean Biology, 39:303-311.
https://doi.org/10.1093/jcbiol/ruz014
Ferratges, F.A., Luque, J., Dominguez, J.L., Ossó, À., Aurell, M., and Zamora, S. 2023. The oldest dairoidid crab (Decapoda: Brachyura: Parthenopoidea) from the Eocene of Spain. Papers in Palaeontology, 9:e1494.
https://doi.org/10.1002/spp2.1494
Fleming, C.A. 1981. A new grapsid crab from the upper Miocene of New Zealand. Journal of the Royal Society of New Zealand, 11:103-108.
https://doi.org/10.1080/03036758.1981.10419444
Forir, H. 1887. Contributions à l’étude du système crétacé de la Belgique. II. Études complémentaires sur les crustacés. Annales de la Société géologique de Belgique, 14:155-175.
Forskal, P. 1775. Descriptiones Animalium, Avium, Amphibiorum, Piscium, Insectorum, Vermium, quae in itinere orientali observavit Petrus Forskal. Post mortem auct. ed. C. Niebuhr: ex officina Mölleri, Hafniae (= Copenhagen).
Förster, R. 1986. Der erste Nachweis eines brachyuren Krebses aus dem Lias (Oberes Pliensbach) Mitteleuropas. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Historische Geologie, 26:25-31.
Förster, R. and Mundlos, R. 1982. Krebse aus dem Alttertiär von Helmstedt und Handorf (Niedersachsen). Palaeontographica (A), 179:148-184.
Fraaije, R.H., Robins, C., van Bakel, B.W., Jagt, J.W., and Bachmayer, F. 2019. Paguroid anomurans from the Tithonian Ernstbrunn Limestone, Austria-the most diverse extinct paguroid assemblage on record. Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie, 121:257-290.
Francescone, F., Lauretano, V., Bouligand, C., Moretti, M., Sabatino, N., Schrader, C., Catanzariti, R., Hilgen, F., Lanci, L., and Turtù, A. 2019. A 9 million-year-long astrochronological record of the early-middle Eocene corroborated by seafloor spreading rates. Bulletin, 131:499-520.
https://doi.org/10.1130/b32050.1
Franţescu, O., Feldmann, R.M., and Schweitzer, C.E. 2016. Cretaceous fossil Raninoida De Haan, 1839 (Crustacea, Decapoda, Brachyura) from northeast Texas. Journal of Paleontology, 90:1118-1132.
https://doi.org/10.1017/jpa.2016.106
Gandolfo, M.A., Nixon, K.C., and Crepet, W.L. 2008. Selection of Fossils for Calibration of Molecular Dating Models. Annals of the Missouri Botanical Garden, 95:34-42.
https://doi.org/10.3417/2007064
Garassino, A., Artal, P., and Pasini, G. 2009. Upogebia miocenica n. sp.(Crustacea, Thalassinidea, Upogebiidae) from the Miocene of Catalonia (Spain). Atti della Società italiana di Scienze naturali e del Museo civico di Storia naturale di Milano, 150:61-68.
García-Raso, E., d’Udekem d’Acoz, C., Moukrim, A., Schubart, C.D., and Cuesta, J.A. 2024. A new cryptic species of Polybiidae (Crustacea: Decapoda: Portunoidea) from the East Atlantic, with considerations on the genus Polybius. European journal of taxonomy, 930:277-313.
https://doi.org/10.5852/ejt.2024.930.2501
Gatt, M. and De Angeli, A. 2010. A new coral‐associated decapod assemblage from the Upper Miocene (Messinian) Upper Coralline Limestone of Malta (Central Mediterranean). Palaeontology, 53:1315-1348.
https://doi.org/10.1111/j.1475-4983.2010.01008.x
Glaessner, M.F. 1933. I.–New tertiary crabs in the collection of the British Museum. Journal of Natural History Series 10, 12:1-28.
https://doi.org/10.1080/00222933308673747
Glaessner, M.F. 1969. Decapoda, p. R399-R651. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology. Part R. Arthropoda, Volume 4(2): Geological Society of America and University of Kansas Press, Lawrence.
Gomes, B.T., Aguilera, O., da Silva-Caminha, S.A.F., D’Apolito, C., Cárdenas, D., Hocking, E.P., and Lemes, K.K.B. 2023. Biostratigraphy and Paleoenvironments of the Pirabas Formation (Neogene, Pará State-Brazil). Marine Micropaleontology:102218.
https://doi.org/10.1016/j.marmicro.2023.102218
Gozhyk, P., Semenenko, V., Andreeva-Grigorovich, A., and Maslun, N. 2015. The correlation of the Neogene of Central and Eastern Paratethys segments of Ukraine with the International Stratigraphic Chart based on planktonic microfossils. Geologica Carpathica, 66:235-244.
https://doi.org/10.1515/geoca-2015-0022
Grimaldi, D.A. 1996. Amber: Window to the Past, American Museum of Natural History, New York.
Guerstein, G., Guler, M., and Casadío, S. 2004. Palynostratigraphy and palaeoenvironments across the Oligocene-Miocene boundary within the Centinela Formation, southwestern Argentina. Geological Society, London, Special Publications, 230:325-343.
https://doi.org/10.1144/gsl.sp.2004.230.01.17
Guinot, D. 1977. Propositions pour une nouvelle classification des Crustacés Décapodes Brachyoures. Comptes rendus hebdomadaires des séances de l’Académie des sciences, 285:1049-1052.
Guinot, D. 2023. A new subfamily classification of the highly diversified Dorippidae H. Milne Edwards, 1837 (Crustacea, Decapoda, Brachyura, Dorippoidea), using morphological, molecular and palaeotonlogical data, with special emphasis on its unique female reproductive system. Zoosystema, 45:225-372.
https://doi.org/10.5252/zoosystema2023v45a9
Guinot, D., Charbot-Chanona, G., and Vega, F.J. 2019. Archaeochiapasidae n. fam., a new early Cenomanian brachyuran family from Chiapas, Mexico, new hypothesis on Lecythocaridae Schweitzer & Feldmann, 2009, and phylogenetic implications (Crustacea, Decapoda, Brachyura, Eubrachyura). Geodiversitas, 41:285-322.
https://doi.org/10.5252/geodiversitas2019v41a7
Hartnoll, R.G. 1971. Sesarma cookei n. sp., a grapsid crab from Jamaica (Decapoda: Brachyura). Crustaceana, 20:257-262.
https://doi.org/10.1163/156854071x00049
Hartzell, S.M., Schweitzer, C.E., and Feldmann, R.M. 2022. Extinction and survival of raninoid crabs (Decapoda: Brachyura: Raninoida) from the Early Cretaceous to the present. Journal of Crustacean Biology, 42:ruac053.
https://doi.org/10.1093/jcbiol/ruac053
Haug, J.T. and Haug, C. 2014. Eoprosopon klugi (Brachyura) - the oldest unequivocal and most “primitive” crab reconsidered. Palaeodiversity, 7:149-158.
https://www.palaeodiversity.org/pdf/07/08Palaeodiversity_7-14_Haug_3.pdf
Hegna, T.A., Luque, J., and Wolfe, J.M. 2020. The fossil record of the Pancrustacea, p. 21-52. In Poore, G. and Thiel, M. (eds.), The Natural History of the Crustacea: Evolution and Biogeography of the Crustacea, Volume 8, Volume 8: Oxford University Press.
https://doi.org/10.1093/oso/9780190637842.003.0002
Herbst, J.F.W. 1782-1804. Versuch einer Naturgeschichte der Krabben und Krebse, nebst einer systematischen Beschreibung ihrer verschiedenen Arten. Strals und GA Lange.
https://doi.org/10.5962/bhl.title.62813
Hohenegger, J., Coric, S., and Wagreich, M. 2014. Timing of the middle miocene Badenian stage of the central Paratethys. Geologica Carpathica, 65:55-66.
https://doi.org/10.2478/geoca-2014-0004
Hyžný, M. 2016. Diversity and distribution patterns of the Oligocene and Miocene decapod crustaceans (Crustacea: Malacostraca) of the Western and Central Paratethys. Geologica Carpathica, 67:471-494.
https://doi.org/10.1515/geoca-2016-0030
Hyžný, M. and Kroh, A. 2015. Barremian decapod crustaceans from Serre de Bleyton (Drôme, SE France). Annalen des Naturhistorischen Museums in Wien. Serie A, Fur Mineralogie und Petrographie, Geologie und Palaontologie, Anthropologie und Prahistorie, 117:121-152.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4471114/
Hyžný, M. and Dulai, A. 2021. Badenian decapods of Hungary. GeoLitera Publishing House, Institute of Geosciences, University of Szeged, Hungary.
Hyžný, M., Kovalchuk, O., Świdnicka, E., Berezovsky, A., Dumitriu, S., Grădianu, I., Stefaniak, K., and Barkaszi, Z. 2022. Revisiting brachyuran crabs (Malacostraca: Decapoda) from Oligocene and Miocene fish beds of Europe. Geologica Carpathica, 73:579-597.
https://doi.org/10.31577/geolcarp.73.6.3
Ilyin, I.V. and Alekseev, A.S. 1998. Novye vidy krabov (Decapoda, Brachyura) iz nizhnego mela ugo-zapadnogo Kryma. Paleontologicheskii Zhurnal, 1998/6:46-49.
Ilyin, I.V. 2005. Cretaceous and Paleogene decapod crustaceans of the western part of Northern Eurasia (in Russian). Moscow State University Press, Moscow.
Jagt, J.W.M., Van Bakel, B.W.M., Guinot, D., Fraaije, R.H., and Artal, P. 2015. Fossil Brachyura, p. 847-920. In Castro, P., Davie, P.J.F., Guinot, D., Schram, F.R., and Vaupel Klein, J.C.v. (eds.), Decapoda: Brachyura (Part 2). Treatise on Zoology – Anatomy, Taxonomy, Biology. The Crustacea, Vol. 9C-, Volume 9C-II: Brill, Leiden & Boston.
James-Williamson, S.A. and Mitchell, S.F. 2012. Revised lithostratigraphy of the Coastal Group of south-eastern St. Thomas, Jamaica. Caribbean Journal of Earth Science, 44:9-17.
https://caribjes.com/CJESpdf/CJES44-03-JamesMitchellCoastalGp.pdf
Jaramillo, C.A., Rueda, M., and Torres, V. 2011. A palynological zonation for the Cenozoic of the Llanos and Llanos Foothills of Colombia. Palynology, 35:46-84.
https://doi.org/10.1080/01916122.2010.515069
Jaworski, E. 1938. Gasterópodos del Cretácico inferior de Colombia. Estudios geológicos y Paleontológicos sobre la Cordillera Oriental de Colombia, Parte 3:109-112.
Jennings, L.A., Blakeslee, A., McCoy, K.A., Behringer, D.C., and Bojko, J. 2021. Systematic assessment of the Panopeidae and broader Eubrachyura (Decapoda: Brachyura) using mitochondrial genomics. Arthropod Systematics and Phylogeny, 79:569-585.
https://doi.org/10.3897/asp.79.e70234
Karasawa, H. 1992. The crab Ozius collinsi sp. nov. (Xanthoidea: Decapoda: Crustacea) from the Miocene Katsuta Group, southwest Japan. Tertiary Research, 14:19-24.
Karasawa, H. 1998. Typilobus kishimotoi, a new leucosiid crab (Crustacea: Decapoda: Brachyura) from the Miocene Katsuta Group, Japan. Proceedings of the Biological Society of Washington, 111:97-101.
Karasawa, H. 2000. Medorippe tanabei, a new species of Miocene dorippid crab (Crustacea: Decapoda: Brachyura) from the Katsuta Group, West Honshu, Japan. Proceedings of the Biological Society of Washington, 113:810-814.
Karasawa, H. and Kato, H. 2001. The systematic status of the genus Miosesarma Karasawa, 1989 with a phylogenetic analysis within the family Grapsidae and a review of fossil records (Crustacea: Decapoda: Brachyura). Paleontological Research, 5:259-275.
Karasawa, H. and Kato, H. 2003. The family Goneplacidae MacLeay, 1838 (Crustacea: Decapoda: Brachyura): Systematics, phylogeny, and fossil records. Paleontological Research, 7:129-151.
https://doi.org/10.2517/prpsj.7.129
Karasawa, H. and Schweitzer, C.E. 2004. Revision of the genus Glyphithyreus Reuss, 1859 (Crustacea, Decapoda, Brachyura, Xanthoidea) and recognition of a new genus. Paleontological Research, 8:143-154.
https://doi.org/10.2517/prpsj.8.143
Karasawa, H. and Schweitzer, C.E. 2006. A new classification of the Xanthoidea sensu lato (Crustacea: Decapoda: Brachyura) based on phylogenetic analysis and traditional systematics and evaluation of all fossil Xanthoidea sensu lato. Contributions to Zoology, 75:23-73.
https://doi.org/10.1163/18759866-0750102002
Karasawa, H., Schweitzer, C.E., and Feldmann, R.M. 2008. Revision of the Portunoidea Rafinesque, 1815 (Decapoda: Brachyura) with emphasis on the fossil genera and families. Journal of Crustacean Biology, 28:82-127.
https://doi.org/10.1651/07-2882r.1
Karasawa, H., Schweitzer, C.E., Feldmann, R.M., and Luque, J. 2014. Phylogeny and classification of the Raninoida (Decapoda: Brachyura). Journal of Crustacean Biology, 34:216-272.
https://doi.org/10.1163/1937240X-00002216
Karasawa, H., Schweitzer, C.E., and Feldmann, R.M. 2019. Part R, Revised, Volume 1, Chapter 8T3: Systematic Descriptions: Superfamily Leucosioidea. Treatise Online, 115:1-22.
https://doi.org/10.17161/to.v0i0.9756
Kennedy, W., Cobban, W., Hancock, J., and Gale, A. 2005. Upper Albian and Lower Cenomanian ammonites from the Main Street Limestone, Grayson Marl and Del Rio Clay in northeast Texas. Cretaceous Research, 26:349-428.
https://doi.org/10.1016/j.cretres.2004.11.018
Kennedy, W.J., Amédro, F., Robaszynski, F., and Jagt, J.W. 2011. Ammonite faunas from condensed Cenomanian-Turonian sections (‘Tourtias’) in southern Belgium and northern France. Netherlands Journal of Geosciences, 90:209-238.
https://doi.org/10.1017/s0016774600001128
Kirby, M.X., Jones, D.S., and MacFadden, B.J. 2008. Lower Miocene srtratigraphy along the Panama Canal and its bearing on the Central American Peninsula. PLoS ONE, e2791:1-14.
https://doi.org/10.1371/journal.pone.0002791
Klaus, S., Magalhães, C., Salas-Gismondi, R., Gross, M., and Antoine, P.-O. 2017. Paleogene and Neogene brachyurans of the Amazon Basin: A revised first appearance date for primary freshwater crabs (Crustacea, Brachyura, Trichodactylidae). Crustaceana:953-967.
https://doi.org/10.1163/15685403-00003629
Klompmaker, A.A. 2013. Extreme diversity of decapod crustaceans from the mid-Cretaceous (late Albian) of Spain: Implications for Cretaceous decapod paleoecology. Cretaceous Research, 41:150-185.
https://doi.org/10.1016/j.cretres.2012.12.003
Klompmaker, A.A., Portell, R.W., Klier, A.T., Prueter, V., and Tucker, A.L. 2015. Spider crabs of the Western Atlantic with special reference to fossil and some modern Mithracidae. PeerJ, 3:e1301.
https://doi.org/10.7717/peerj.1301
Kocova-Veselská, M., Koci, T., and Kubajko, M. 2014. Dynomenid crabs (Decapoda, Brachyura) and stalked barnacles (Cirripedia, Scalpelliformes) from upper Cenomanian-lower Turonian nearshore, shallow-water strata in the Bohemian Cretaceous Basin, Czech Republic. Scripta Geologica, 147:49-81.
https://repository.naturalis.nl/pub/523864/SG147_049-081.pdf
Koh, S. and Ng, P.K. 2008. A revision of the shore crabs of the genus Eriphia (Crustacea: Brachyura: Eriphiidae). Raffles Bulletin of Zoology, 56:327-355.
Kováč, M., Halásová, E., Hudáčková, N., Holcová, K., Hyžný, M., Jamrich, M., and Ruman, A. 2018. Towards better correlation of the Central Paratethys regional time scale with the standard geological time scale of the Miocene Epoch. Geologica Carpathica, 69:283-300.
https://doi.org/10.1515/geoca-2018-0017
Krijgsman, W. and Piller, W.E. 2012. Central and Eastern Paratethys, p. 935-937. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D., and Ogg, G. (eds.), The Geologic Time Scale 2012: Elsevier, Amsterdam.
Lai, J.C.Y., Ahyong, S.T., Jeng, M.-S., and Ng, P.K. 2009. Are coral-dwelling crabs monophyletic? A phylogeny of the Trapezioidea (Crustacea: Decapoda: Brachyura). Invertebrate Systematics, 23:402-408.
https://doi.org/10.1071/is09012
Lai, J.C.Y., Mendoza, J.C.E., Guinot, D., Clark, P.F., and Ng, P.K.L. 2011. Xanthidae MacLeay, 1838 (Decapoda: Brachyura: Xanthoidea) systematics: A multi-gene approach with support from adult and zoeal morphology. Zoologischer Anzeiger - A Journal of Comparative Zoology, 250:407-448.
https://doi.org/10.1016/j.jcz.2011.07.002
Lai, J.C.Y., Thoma, B.P., Clark, P.F., Felder, D.L., and Ng, P.K. 2014. Phylogeny of eriphioid crabs (Brachyura, Eriphioidea) inferred from molecular and morphological studies. Zoologica Scripta, 43:52-64.
https://doi.org/10.1111/zsc.12030
Larghi, C. 2004. Brachyuran decapod Crustacea from the Upper Cretaceous of Lebanon. Journal of Paleontology, 78:528-541.
https://doi.org/10.1666/0022-3360(2004)078<0528:bdcftu>2.0.co;2
Latreille, P.A. 1802. Histoire Naturelle, générale et particulière des Crustacés et des Insectes: Ouvrage faisant suite aux Oeuvres de Leclerc de Buffon, et Partie du Cours complet d'Histoire Naturelle rédigé par C. S. Sonnini, Membre de plusieurs Sociétés Savantes. F. Dufart, Paris. 10.5962/bhl.title.15764
Latreille, P.A. 1817. Les crustacés, les arachnides et les insectes, p. xxix + 653 pp. In Cuvier, G., (ed., Le règne animal distribué d’après son organization, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée, Volume Edition 1, 3, Paris.
Latreille, P.A. 1825-1828. Encyclopédie méthodique, Histoire naturelle: Entomologie, ou histoire naturelle des crustacés, des arachnides et des insectes. Chez Mme veuve Agasse, Imprimerie-Libraire, Paris, v. (1, 2).
Latreille, P.A. 1829. Crustacés, arachnides et partie des insectes. (2nd Edition), p. 1-584. In Cuvier, G. (ed.), Le règne animal distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Nouvelle édition, revue et augmentée., Volume 4: Chez Déterville, Libraire; et Chez Crochard, Libraire, Paris.
Leach, W.E. 1813-1815. Crustaceology, p. 383-384 [1813], 1385-1437, 1765-1766 [1814], Plate 1221 [1815]. In Webster, D. (ed.), The Edinburgh Encyclopædia, Volume 7: Edinburgh, Balfour.
Leach, W.E. 1817. Monograph on the genera and species of the malacostracous family Leucosidea, p. 17-26. In Leach, W.E. (ed.), The Zoological Miscellany; being descriptions of new, or interesting animals, Volume 3: E. Nodder and Son, Covent Garden and London.
LeBlanc, J. 2021. Stratigraphic Lexicon: The Onshore Cenozoic Sedimentary Formations of The Republic of Panama. Biosis: Biological Systems, 2:1-173.
https://doi.org/10.37819/biosis.002.01.0095
Lima, D., Tavares, M., Lopes, R.T., de Araújo, O.M.O., and Aguilera, O. 2020. Uca maracoani (Crustacea, Decapoda, Ocypodidae) from a Miocene paleomangrove in Brazil: A case of evolutionary stasis among tropical American fiddler crabs. Journal of South American Earth Sciences, 99:102517.
https://doi.org/10.1016/j.jsames.2020.102517
Linnaeus, C.v. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Laurentii Salvii, Holmiae.
https://doi.org/10.5962/bhl.title.157601
Liu, Q.-N., Tang, Y.-Y., Yang, T.-T., Li, Y.-T., and Yu, X.-M. 2021. Phylogenetic relationships of Grapsoidea and insights into the higher phylogeny of Brachyuran. Genomics, 113:429-439.
https://doi.org/10.1016/j.ygeno.2020.08.033
Locatelli, E.R. 2013. Preservation potential of gecarcinid land crabs (Decapoda, Brachyura, Gecarcinidae) from San Salvador. Palaios, 28:867-874.
https://doi.org/10.2110/palo.2013.083
Londoño, L., Royer, D.L., Jaramillo, C., Escobar, J., Foster, D.A., Cárdenas‐Rozo, A.L., and Wood, A. 2018. Early Miocene CO 2 estimates from a Neotropical fossil leaf assemblage exceed 400 ppm. American Journal of Botany, 105:1929-1937.
https://doi.org/10.1002/ajb2.1187
Lőrenthey, E. 1898. Beiträge zur Decapodenfauna des Ungarischen Tertiärs. Termész Füzetek, Budapest, 21:1-133.
Lőrenthey, E. 1907. Palaeontologiai tanulmányok a harmadkorú rákok köréből. V. Adatok Egyptom eocenkori rákfaunájához. Mathematikai és Természettudományi Közlemények, 29:195-242.
Lőrenthey, E. and Beurlen, K. 1929. Die fossilen Decapoden der Länder der Ungarischen Krone. Geologica Hungarica (Palaeontologica), 3:1-421, 412 tables, 416 pls.
Lü, J., Xia, L., Liu, X., Ma, Y., Li, J., Ye, Y., and Guo, B. 2022. The mitochondrial genome of Grapsus albolineatus (Decapoda: Brachyura: Grapsidae) and phylogenetic associations in Brachyura. Scientific Reports, 12:1-12.
https://doi.org/10.1038/s41598-022-06080-3
Lu, X., Gong, L., Zhang, Y., Chen, J., Liu, L., Jiang, L., Lü, Z., Liu, B., Tong, G., and Wei, X. 2020. The complete mitochondrial genome of Calappa bilineata: the first representative from the family Calappidae and its phylogenetic position within Brachyura. Genomics, 112(3), pp.2516-2523. Genomics, 112:2516-2523.
https://doi.org/10.1016/j.ygeno.2020.02.003
Luque, J. 2014. A new genus and species of raninoidian crab (Decapoda, Brachyura), from the Lower Cretaceous of Colombia, South America. Scripta Geologica, 147:27-34.
https://repository.naturalis.nl/pub/523862/SG147_027-034.pdf
Luque, J. 2015a. The oldest higher true crabs (Crustacea: Decapoda: Brachyura): insights from the Early Cretaceous of the Americas. Palaeontology, 58:251-263.
https://doi.org/10.1111/pala.12135
Luque, J. 2015b. A puzzling frog crab (Crustacea: Decapoda: Brachyura) from the Early Cretaceous Santana Group of Brazil: Frog first or crab first? Journal of Systematic Palaeontology, 13:153-166.
https://doi.org/10.1080/14772019.2013.871586
Luque, J. 2017. Fossil hermit and land crabs (Anomura and Brachyura) from the Quaternary of Antigua and Bermuda. Journal of Crustacean Biology, 37:151-156.
https://doi.org/10.1093/jcbiol/rux007
Luque, J., Feldmann, R.M., Schweitzer, C.E., Jaramillo, C., and Cameron, C.B. 2012. The oldest frog crabs (Decapoda: Brachyura: Raninoida) from the Aptian of northern South America. Journal of Crustacean Biology, 32:405-420.
https://doi.org/10.1163/193724012X626539
Luque, J., Schweitzer, C.E., Santana, W., Portell, R.W., Vega, F.J., and Klompmaker, A.A. 2017. Checklist of fossil decapod crustaceans from tropical America, Part I: Anomura and Brachyura. Nauplius 25:e2017025.
https://doi.org/10.1590/2358-2936e2017025
Luque, J., Christy, J.H., Hendy, A.J.W., Rosenberg, M.S., Portell, R.W., Kerr, K.A., and Palmer, A.R. 2018. Quaternary intertidal and supratidal crabs (Decapoda, Brachyura) from tropical America and the systematic affinities of fossil fiddler crabs. Journal of Systematic Palaeontology, 16:1037-1055.
https://doi.org/10.1080/14772019.2017.1362599
Luque, J., Feldmann, R.M., Vernygora, O., Schweitzer, C.E., Cameron, C.B., Kerr, K.A., Vega, F.J., Duque, A., Strange, M., Palmer, A.R., and Jaramillo, C. 2019a. Exceptional preservation of mid-Cretaceous marine arthropods and the evolution of novel forms via heterochrony. Science Advances, 5:eaav3875.
https://doi.org/10.1126/sciadv.aav3875
Luque, J., Allison, W.T., Bracken-Grissom, H.D., Jenkins, K.M., Palmer, A.R., Porter, M.L., and Wolfe, J.M. 2019b. Evolution of crab eye structures and the utility of ommatidia morphology in resolving phylogeny. bioRxiv preprint:786087.
https://doi.org/10.1101/786087
Luque, J., Xing, L., Briggs, D.E.G., Clark, E.G., Duque, A., Hui, J., Mai, H., and McKellar, R.C. 2021. Crab in amber reveals an early colonization of non-marine environments during the Cretaceous. Sciences Advances, 7:eabj5689.
https://doi.org/10.1126/sciadv.abj5689
Ma, K.Y., Qin, J., Lin, C.-W., Chan, T.-Y., Ng, P.K.L., Chu, K.H., and Tsang, L.M. 2019. Phylogenomic analyses of brachyuran crabs support early divergence of primary freshwater crabs. Molecular Phylogenetics and Evolution, 135:62-66.
https://doi.org/10.1016/j.ympev.2019.02.001
MacFadden, B.J., Bloch, J.I., Evans, H., Foster, D.A., Morgan, G., Rincón, A., and Wood, A.R. 2014. Temporal Calibration and Biochronology of the Centenario Fauna, Early Miocene of Panama. The Journal of Geology, 122:113-135.
https://doi.org/10.1086/675244
MacLeay, W.S. 1838a. On the brachyurous decapod Crustacea brought from the Cape by Dr. Smith, p. 53-71. In Smith, A. (ed.), Illustrations of the Annulosa of South Africa; consisting chiefly of figures and descriptions of the objects of natural history collected during an expedition into the interior of South Africa, in the years 1834, 1835, and 1836; fitted out by “The Cape of Good Hope Association for Exploring Central Africa...”: Smith, Elder and Company, London.
MacLeay, W.S. 1838b. On the brachyurous decapod Crustacea brought from the Cape by Dr. Smith, p. 53-71, pp. 52 plates. In Smith, A. (ed.), Illustrations of the Annulosa of South Africa; being a portion of the objects of natural history chiefly collected during an expedition into the interior of South Africa, under the directin of Dr. Andrew Smith, in the years 1834, 1835. and 1836; fitted out by “The Cape of Good Hope Association for Exploring Central Africa”. Smith, Elder, and Co., London.
Manning, R.B. and Holthuis, L.B. 1989. Two new genera and nine new species of geryonid crabs (Crustacea, Decapoda, Geryonidae). Proceedings of the Biological Society of Washington, 102:50-77.
Manning, R.B. and Holthuis, L.B. 1991. West African Brachyuran Crabs (Crustacea: Decapoda). Smithsonian Contributions to Zoology, 396:1-379.
https://doi.org/10.5479/si.00810282.306
Martinez, S., Ramos, M.I.F., McArthur, J.M., Del Río, C.J., and Thirlwall, M.F. 2017. Late Burdigalian (Miocene) age for pectinids (Mollusca-Bivalvia) from the Pirabas Formation (northern Brazil) derived from Sr-isotope (87 Sr/86 Sr) data. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 284:57-64.
https://doi.org/10.1127/njgpa/2017/0650
McLean, H., Hausback, B., and Knapp, J. 1987. The geology of west-central Baja California Sur, Mexico: US Geological Survey.
https://doi.org/10.3133/b1579
Mendoza, J.C., Chan, K.O., Lai, J.C., Thoma, B.P., Clark, P.F., Guinot, D., Felder, D.L., and Ng, P.K. 2022. A comprehensive molecular phylogeny of the brachyuran crab superfamily Xanthoidea provides novel insights into its systematics and evolutionary history. Molecular Phylogenetics and Evolution, 177:107627.
https://doi.org/10.1016/j.ympev.2022.107627
Miers, E.J. 1886. Report on the Brachyura collected by H.M.S. Challenger during the years 1873-1876 p. 1-362. In Murray, J. (ed.), Report of the Scientific Results of the Voyage of H.M.S. Challenger during the years 1873-1876, (Zoology), Under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N.: Neil and Company, Edinburgh.
Milne Edwards, H. 1834-1840. Histoire naturelle des Crustacés, comprenant l’anatomie, la physiologie et la classification de ces animaux. Librairie Encyclopédique de Roret, Roret. Paris, v. 2.
https://doi.org/10.5962/bhl.title.6234
Milne Edwards, H. 1853. Observations sur les affinités zoologiques et la classification naturelle des Crustacés. In Proceedings Annales des Sciences Naturelles. Zoologie. Série 3, 18:109-166, pls 3, 4. (second part in Annales des Sciences naturelles, Zoologie, Série 3, 20:163-228, pls 6-11 [1853])1852.
Milne Edwards, H. 1853. Mémoire sur la famille des Ocypodides. Suite (1). Deuxième Tribu Principale. Annales des Sciences Naturelles, 3e série, 20:163-228.
Milne-Edwards, A. 1862a. Sur l’existence de Crustacés de la famille des Raniniens pendant la période crétacée. Comptes Rendus de l’Academie des Sciences de Paris, 55:492-494.
Milne-Edwards, A. 1862b. Monographie des Crustacés fossiles de la famille des Cancériens. Annales des Sciences Naturelles, Zoologie, série 4, 18:31-85.
Milne-Edwards, A. 1865. Monographie des Crustacés Fossiles de la Famille des Cancériens. Annales des Sciences Naturelles, 5e série, 3:297-351.
Milne-Edwards, A. 1873. Crustacés fossiles nouveaux des terrains tertiaires de la Gironde. Actes de la Société Linnéenne de Bordeaux, 29:64-66.
Mina-Uhink, F. 1957. Bosquejo geoldgico del territorio de la Baja California. Boletin de la Asociacidn Mexicana de Geólogos Petroleros, Mexico, D.F., 9:139-269.
Mitchell, S.F., Pickerill, R.K., Blackwell, B.A.B., and Skinner, A.R. 2000. The age of the Port Morant Formation, south-eastern Jamaica. Caribbean Journal of Earth Science, 34:1-4.
https://caribjes.com/CJESpdf/CJES%2034-1-SFM.pdf
Mitchell, S.F., Pickerill, R.K., and Stemann, T.A. 2001. The Port Morant Formation (Upper Pleistocene, Jamaica): high resolution sedimentology and paleoenvironmental analysis of a mixed carbonate clastic lagoonal succession. Sedimentary Geology, 144:291-306.
https://doi.org/10.1016/s0037-0738(01)00101-4
Montes, C., Cardona, A., McFadden, R., Morón, S., Silva, C., Restrepo-Moreno, S., Ramírez, D., Hoyos, N., Wilson, J., and Farris, D. 2012. Evidence for middle Eocene and younger land emergence in central Panama: Implications for Isthmus closure. Geological Society of America Bulletin, 124:780-799.
https://doi.org/10.1130/b30528.1
Morales-Ortega, P., González-Barba, G., Nava-Sánchez, E.H., and Vera-Dimas, D.R. 2016. New Eocene bivalves from Bateque Formation, Baja California Sur, Mexico. Paleontología Mexicana, 5:1-19.
http://www.ojs-igl.unam.mx/index.php/Paleontologia/article/view/197/127
Mori, M. and Yamanoi, T. 2003. Pollen assemblages and palaeovegetation changes of the Bihoku and Katsuta Groups in Hiroshima Prefecture and Okayama Prefecture, southwest Japan. Japanese Journal of Palynology, 49:9-20.
Morris, S.F. and Collins, J.S.H. 1991. Neogene crabs from Brunei, Sabah and Sarawak. Bulletin of the British Museum (Natural History), (Geology), 47:1-33.
https://biostor.org/reference/101333
Morris, S.F. 1993. The fossil arthropods of Jamaica, p. 115-124. In Wright, R.M. and Robinson, E. (eds.), Biostratigraphy of Jamaica. Geological Society of America Memoirs, Volume 182.
https://doi.org/10.1130/mem182-p115
Moshammer, B. and Schlagintweit, F. 1999. The Ernstbrunn Limestone (Lower Austria): new data on biostratigraphy and applied geology. Abhandlungen der Geologischen Bundesanstalt, 56:553-566.
Müller, P. 1974. Decapoda (Crustacea) fauna a budapesti miocénből (1). [Les faunes de crustacés décapodes des calcaires miocènes de Budapest (1)]. Foldtani Kozlony, 104:119-132 [in Hungarian with French summary].
Müller, P. 1984. Decapod Crustacea of the Badenian. Institutum Geologicicum Hungaricum, Geologica Hungarica, Series Palaeontologica, 42:3-317.
Müller, P. 1996. Middle Miocene decapod Crustacea from southern Poland. Prace Muzeum Ziemi, 43:3-14.
Müller, P. 1998. Decapode Crustacea aus dem Karpat des Korneuburger Beckens (Unter-Miozän, Niederösterreich). Beiträge zur Paläontologie, 23:273-281.
Müller, P. 2006. New decapods from the Miocene of Hungary–with remarks about their environment. Földtani Közlöny, 136:37-50.
Müller, P. and Collins, J.S.H. 1991. Late Eocene coral-associated decapods (Crustacea) from Hungary. Contributions to Tertiary and Quaternary Geology, 28:47-92.
Nations, J.D. 1975. The genus Cancer (Crustacea: Brachyura): systematics, biogeography and fossil record. Science Bulletin of the National History Museum of Los Angeles County, 23:1-104.
Nebelsick, J.H., Bassi, D., and Lempp, J. 2013. Tracking paleoenvironmental changes in coralline algal-dominated carbonates of the Lower Oligocene Calcareniti di Castelgomberto formation (Monti Berici, Italy). Facies, 59:133-148.
https://doi.org/10.1007/s10347-012-0349-6
Ng, P.K. and Castro, P. 2020. A revision of Carcinoplax abyssicola (Miers, 1885) and seven related species of Carcinoplax H. Milne Edwards, 1852, with the description of two new species and an updated key to the genus (Crustacea, Decapoda, Brachyura, Goneplacidae). Zoosystema, 42:239-284.
https://doi.org/10.5252/zoosystema2020v42a17
Ng, P.K.L., Guinot, D., and Davie, P.J.F. 2008. Systema Brachyurorum: Part I. An Annotated Checklist of Extant Brachyuran Crabs of the World. The Raffles Bulletin of Zoology, 17:1-286.
https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/10/app/uploads/2017/04/s17rbz.pdf
Nyborg, T., Garassino, A., and Slak, G. 2017. Eonomus californianus n. gen., n. sp.(Crustacea, Brachyura, Cymon-omidae) from the Eocene Llajas Formation, California (USA). Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 286:189-193.
https://doi.org/10.1127/njgpa/2017/0694
Ogg, J.G. 2020. Geomagnetic polarity time scale, p. 159-192, Geologic time scale 2020: Elsevier.
https://doi.org/10.1016/b978-0-12-824360-2.00005-x
Orbigny, A.D.d. 1842. Voyage dans l’Amérique méridional, 1826-1833. Géologie et Paléontologie, Paris.
https://doi.org/10.5962/bhl.title.77334
Ortmann, A.E. 1893. Die Decapoden-Krebse des Strassburger Museums, mit besonderer Berücksichtigung der von Herrn Dr. Döderlein bei Japan und bei den Liu-Kiu-Inseln gesammelten und zur Zeit im Strassburger Museum aufbewahrten Formen. VII. Theil. Abtheilung: Brachyura (Brachyura genuina Boas) II. Unterabtheilung: Cancroidea, 2. Section: Cancrinea, 1. Gruppe: Cyclometopa. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Thiere, 7:411-495.
https://doi.org/10.5962/bhl.part.24064
Ossó, À. 2016. Eogeryon elegius n. gen. and n. sp. (Decapoda: Eubrachyura: Portunoidea), one of the oldest modern crabs from late Cenomanian of the Iberian Peninsula. Boletín de la Sociedad Geológica Mexicana, 68:231-246.
https://doi.org/10.18268/bsgm2016v68n2a5
Ossó, À. 2021. Un cranc nou, Eogeryon elegius gen. nov., sp. nov. del Cenomanià tardà de la península Ibèrica. Nemus. Revista de l'Ateneu de Natura:144-158.
Palacios Theil, E., Cuesta, J.A., and Felder, D.L. 2016. Molecular evidence for non-monophyly of the pinnotheroid crabs (Crustacea: Brachyura: Pinnotheroidea), warranting taxonomic reappraisal. Invertebrate Systematics, 30:1-27.
https://doi.org/10.1071/IS15023
Parham, J.F., Donoghue, P.C., Bell, C.J., Calway, T.D., Head, J.J., Holroyd, P.A., Inoue, J.G., Irmis, R.B., Joyce, W.G., and Ksepka, D.T. 2012. Best practices for justifying fossil calibrations. Systematic Biology, 61:346-359.
https://doi.org/10.1093/sysbio/syr107
Pasini, G., Garassino, A., De Angeli, A., Hyžný, M., Giusberti, L., and Zorzin, R. 2019. Eocene decapod faunas from the Konservat-Lagerstätten laminites of “Pesciara”(Bolca, Verona) and Monte Postale (Altissimo, Vicenza) in northeast Italy: a review and update. Neues Jahrbuch für Geologie und Paläontologie‐Abhandlungen, 293:233-270.
https://doi.org/10.1127/njgpa/2019/0840
Paucă, M. 1929. Zwei neue fossile Decapoden aus Oligozanen Clupea crenata Schiefern von Suslăneşti-Muscel und über die Bildung der Menilitschiefern. Bulletin de la Section Scientifique de l'Académie Roumaine, 12:40-44.
Paulay, G. and Starmer, J. 2011. Evolution, insular restriction, and extinction of oceanic land crabs, exemplified by the loss of an endemic Geograpsus in the Hawaiian Islands. PLoS ONE, 6:e19916.
https://doi.org/10.1371/journal.pone.0019916
Perea, D., Verde, M., Toriño, P., Montenegro, F., Ubilla, M., and Manzuetti, A. 2020. A Complex Association of Invertebrates, Vertebrates and Trace Fossils in the Marine Camacho Formation (Late Miocene of Uruguay): Biostratigraphy and Paleoenvironments. Ameghiniana, 57:266-277.
https://doi.org/10.5710/amgh.29.02.2020.3327
Philippi, R.A. 1887. Die tertiären und quartären Versteinerunge Chiles, Brockhaus, Leipzig.
https://doi.org/10.5962/bhl.title.15048
Piller, W.E., Harzhauser, M., and Mandic, O. 2007. Miocene Central Paratethys stratigraphy-current status and future directions. Stratigraphy, 4:151-168.
https://doi.org/10.29041/strat.04.2.09
Poore, G.C. and Ahyong, S.T. 2023. Marine Decapod Crustacea: A Guide to Families and Genera of the World. CSIRO PUBLISHING.
https://doi.org/10.1071/9781486311798
Portell, R.W. and Agnew, J. 2004. Pliocene and Pleistocene decapod crustaceans. Florida Fossil Invertebrates, 4:1-29.
Prado, L.A.C., Luque, J., Barreto, A.M.F., and Palmer, A.R. 2018. New brachyuran crabs from the Aptian-Albian Romualdo Formation, Santana Group of Brazil: Evidence for a Tethyan connection to the Araripe Basin. Acta Palaeontologica Polonica, 63:737-750.
https://doi.org/10.4202/app.00480.2018
Pujalte, V., Schmitz, B., Baceta, J.I., Orue-Echebarría, X., Bernaola, G., Dinarès-Turell, J., Payros, A., Apellaniz, E., and Caballero, F. 2009. Correlation of the Thanetian-Ilerdian turnover of larger foraminifera and the Paleocene-Eocene thermal maximum: confirming evidence from the Campo area (Pyrenees, Spain). Geologica Acta:161-175.
https://doi.org/10.11137/2006_1_500-501
Quayle, W.J. and Collins, J.S.H. 1981. New Eocene crabs from the Hampshire Basin. Palaeontology, 24:733-758.
https://www.biodiversitylibrary.org/page/49722137#page/309/mode/1up
Quayle, W.J. and Collins, J.S.H. 2012. A review of the decapod crustaceans from the Tertiary of the Isle of Wight, Hampshire, UK, with description of three new species. Bulletin of the Mizunami Fossil Museum, 38:33-51.
Raine, J.I., Beu, A.G., Boyes, A.F., Campbell, H.J., Cooper, R.A., Crampton, J.S., Crundwell, M.P., Hollis, C.J., and Morgans, H.E.G. 2015. A revised calibration of the New Zealand geological timescale: NZGT2015/1. GNSScience Report 2012/39.
https://doi.org/10.1190/ice2015-2211449
Rathbun, M.J. 1897. A revision of the nomenclature of the Brachyura. Proceedings of the Biological Society of Washington, 11:153-167.
https://www.biodiversitylibrary.org/page/3942279
Rathbun, M.J. 1898. The Brachyura of the biological expedition to the Florida Keys and the Bahamas in 1893. Bulletin from the Laboratories of Natural History, State University of Iowa, 4:250-294.
https://www.biodiversitylibrary.org/page/15405204
Rathbun, M.J. 1918 [1919]. Decapod crustaceans from the Panama region, p. 123-184. In Vaughan, T.W. (ed.), Contributions to the geology and paleontology of the Canal Zone, Panama and geologically related areas in Central America and the West Indies. Bulletin of the United States National Museum, Volume 103.
https://doi.org/10.5962/bhl.title.31557
Rathbun, M.J. 1926. The fossil stalk-eyed Crustacea of the Pacific slope of North America. US Government Printing Office, Bulletin of the United States National Museum.
https://doi.org/10.5479/si.03629236.138.i
Rathbun, M.J. 1935. Fossil Crustacea of the Atlantic and Gulf Coastal Plain. Geological Society of America, Special Paper:i-viii, 1-160.
https://doi.org/10.1130/spe2
Rathbun, M.J. 1937. Cretaceous and Tertiary crabs from Panama and Colombia. Journal of Paleontology, 11:26-28.
Reichenbacher, B., Krijgsman, W., Lataster, Y., Pipperr, M., Van Baak, C.G., Chang, L., Kälin, D., Jost, J., Doppler, G., and Jung, D. 2013. A new magnetostratigraphic framework for the lower Miocene (Burdigalian/Ottnangian, Karpatian) in the North Alpine Foreland Basin. Swiss Journal of Geosciences, 106:309-334.
https://doi.org/10.1007/s00015-013-0142-8
Reuss, A.E. 1857. Zur Kenntnis fossiler Krabben. Sitzungsberichte der Akademie der Wissenschaften, 27:161-166.
Reuss, A.E. 1859. Zur Kenntnis fossiler Krabben. Denkschriften der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe, 17:1-90.
https://doi.org/10.5962/bhl.title.10650
Ristori, G. 1886. I crostacei brachiuri e anomuri del pliocene italiano. Bollettino della Società Geologica Italiana, 5:93-128.
Robins, C.M., Feldmann, R.M., and Schweitzer, C.E. 2013. Nine new genera and 24 new species of the Munidopsidae (Decapoda: Anomura: Galatheoidea) from the Jurassic Ernstbrunn Limestone of Austria, and notes on fossil munidopsid classification. Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie:167-251.
https://www.jstor.org/stable/43923069
Robins, C.M. and Klompmaker, A.A. 2019. Extreme diversity and parasitism of Late Jurassic squat lobsters (Decapoda: Galatheoidea) and the oldest records of porcellanids and galatheids. Zoological Journal of the Linnean Society, 187:1131-1154.
https://doi.org/10.1093/zoolinnean/zlz067
Roemer, F.A. 1887. Graptocarcinus texanus, ein Brachyure aus der oberen Kreide von Texas. Leonhardt und Bronn’s Neues Jahrbuch für Mineralogie, Geologie, und Paläontologie, 1:173-176.
Rosenberg, M.S. 2001. The systematics and taxonomy of fiddler crabs: a phylogeny of the genus Uca. Journal of Crustacean Biology, 21:839-869.
https://doi.org/10.1163/20021975-99990176
Ross, A., Lewis, J.E., and Scolaro, R.J. 1964. New Eocene decapods from Florida. Quarterly Journal of the Florida Academy of Sciences, 27:187-196.
http://www.jstor.org/stable/24315065
Roux, P. 1828-1830. Crustacés de la Mediterranée et de son littoral. Décrits et Lithographiés par Polydore Roux, Conservateur du Cabinet d’histoire naturelle de la Ville de Marseille.. Levrault, Paris.
https://doi.org/10.5962/bhl.title.8729
Rumsey, B.T., Klompmaker, A.A., and Portell, R.W. 2016. Paleobiogeography of the fossil box crab Calappilia (Brachyura: Calappidae) with a new species from the Eocene-Oligocene of Florida, USA. Journal of Crustacean Biology, 36:329-337.
https://doi.org/10.1163/1937240x-00002422
Sadler, P.M. 1988. Geometry and stratification of uppermost Cretaceous and Paleogene units on Seymour Island, Northern Antarctic Peninsula, p. 303-320. In Feldmann, R. M., and Woodburne, M., eds.), Geology and Paleontology of Seymour Island, Antarctic Peninsula. Memoirs of the Geological Society of America, Volume 169: Geological Society of America, California.
https://doi.org/10.1130/mem169-p303
Sakai, T. 1938. Studies on the crabs of Japan, III. Brachygnatha, Oxyrhyncha. Yokendo Co, Tokyo, 3:193-364.
Samouelle, G. 1819. The entomologist’s useful compendium; or an introduction to the knowledge of British insects, comprising the best means of obtaining and preserving them, and a description of the apparatus generally used; together with the genera of Linné, and the modern method of arranging the classes Crustacea, Myriapoda, Spiders, Mites and Insects, from their affinities and structure, according to the views of Dr. Leach, London.
https://doi.org/10.5962/bhl.title.120094
Schneider, S., Harzhauser, M., Kroh, A., Lukeneder, A., and Zuschin, M. 2013. Ernstbrunn Limestone and Klentnice beds (Kimmeridgian Berriasian; Waschberg-dánice Unit; NE Austria and SE Czech Republic): state of the art and bibliography. Bulletin of Geosciences, 88.
https://doi.org/10.3140/bull.geosci.1360
Scholtz, G. and Richter, S. 1995. Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca). Zoological Journal of the Linnaean Society, 113:289-328.
https://doi.org/10.1111/j.1096-3642.1995.tb00936.x
Scholtz, G. 2014. Evolution of crabs – history and deconstruction of a prime example of convergence. Contributions to Zoology, 83:87-105.
https://doi.org/10.1163/18759866-08302001
Scholtz, G. 2020. Eocarcinus praecursor Withers, 1932 (Malacostraca, Decapoda, Meiura) is a stem group brachyuran. Arthropod Structure & Development, 59:100991.
https://doi.org/10.1016/j.asd.2020.100991
Schram, F.R. and Dixon, C.J. 2004. Decapod phylogeny: addition of fossil evidence to a robust morphological cladistic data set. Bulletin of the Mizunami Fossil Museum, 31:1-19.
Schram, F.R. and Ng, P.K.L. 2012. What is Cancer ? Journal of Crustacean Biology, 32:665-672.
https://doi.org/10.1163/193724012x640650
Schubart, C.D. and Cuesta, J.A. 2010. Phylogenetic relationships of the Plagusiidae Dana, 1851 (Brachyura), with description of a new genus and recognition of Percnidae Stevcic, 2005, as an independent family. Crustaceana Monographs, 11:279-299.
https://doi.org/10.1163/ej.9789004170865.i-366.171
Schweitzer, C.E. 2000. Tertiary Xanthoidea (Crustacea: Decapoda: Brachyura) from the west coast of North America. Journal of Crustacean Biology, 20:715-742.
https://doi.org/10.1163/20021975-99990095
Schweitzer, C.E. 2005. The Trapeziidae and Domeciidae (Decapoda: Brachyura: Xanthoidea) in the fossil record and a new Eocene genus from Baja California Sur, Mexico. Journal of Crustacean Biology, 25:625-636.
https://doi.org/10.1651/c-2575.1
Schweitzer, C.E. and Feldmann, R.M. 2000a. Re-evaluation of the Cancridae Latreille, 1803 (Decapoda: Brachyura) including three new genera and three new species. Contributions to Zoology, 69:223-250.
https://doi.org/10.1163/18759866-06904002
Schweitzer, C.E. and Feldmann, R.M. 2000b. New species of calappid crabs from western North America and reconsideration of the Calappidae sensu lato. Journal of Paleontology, 74:230-246.
https://doi.org/10.1017/s0022336000031450
Schweitzer, C.E., Feldmann, R.M., Tucker, A.B., and Berglund, R.E. 2000. Eocene decapod crustaceans from Pulali Point, Washington. Annals of the Carnegie Museum, 69:23-67.
https://doi.org/10.5962/p.215187
Schweitzer, C.E. and Feldmann, R.M. 2001. Differentiation of the fossil Hexapodidae Miers, 1886 (Decapoda: Brachyura) from similar forms. Journal of Paleontology, 75:330-345.
https://doi.org/10.1017/s0022336000018138
Schweitzer, C.E., Feldmann, R.M., González-Barba, G., and Vega, F.J. 2002. New crabs from the Eocene and Oligocene of Baja California Sur, Mexico and an assessment of the evolutionary and paleobiogeographic Implications of Mexican fossil decapods. Paleontological Society Memoir, 76:1-43.
https://doi.org/10.1666/0022-3360(2002)76[1:ncftea]2.0.co;2
Schweitzer, C.E., Feldmann, R.M., and Gingerich, P.D. 2004. New Decapoda (Crustacea) from the middle and late Eocene of Pakistan and a revision of Lobonotus A. Milne Edwards, 1864. University of Michigan, Contributions from the Museum of Paleontology, 31:89-118.
Schweitzer, C.E., Feldmann, R.M., González-Barba, G., and Cosovic, V. 2006. New Decapoda (Anomura, Brachyura) from the Eocene Bateque and Tepetate Formations, Baja California Sur, Mexico. Bulletin of the Mizunami Fossil Museum, 33:21-45.
Schweitzer, C.E., Feldmann, R.M., and Lazar, I. 2009. Fossil Crustacea (excluding Cirripedia and Ostracoda) in the University of Bucharest Collections, Romania, including two new species. Bulletin of the Mizunami Fossil Museum, 35:1-14.
Schweitzer, C.E., Feldmann, R.M., Garassino, A., Karasawa, H., and Schweigert, G. 2010. Systematic List of Fossil Decapod Crustacean Species. Crustaceana Monographs, 10:222.
https://doi.org/10.1163/ej.9789004178915.i-222
Schweitzer, C.E. and Feldmann, R.M. 2010a. Earliest known Porcellanidae (Decapoda: Anomura: Galatheoidea) (Jurassic: Tithonian). Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 258:243-248.
https://doi.org/10.1127/0077-7749/2010/0096
Schweitzer, C.E. and Feldmann, R.M. 2010b. New fossil decapod crustaceans from the Remy Collection, Muséum national d'Histoire naturelle, Paris. Geodiversitas, 32:399-415.
https://doi.org/10.5252/g2010n3a3
Schweitzer, C.E. and Feldmann, R.M. 2011. New fossil Brachyura (Decapoda: Homoloidea, Dorippoidea, Carpilioidea) from the United Kingdom. Bulletin of the Mizunami Fossil Museum, 37:1-11.
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2012. Part R, Revised, Volume 1, Chapter 8M: Systematic Descriptions: Section Dromiacea. Treatise Online, 51:1-43, 24 figs.
https://doi.org/10.17161/to.v0i0.4336
Schweitzer, C.E. and Feldmann, R.M. 2012. Revision of Decapoda deposited in The Muséum national d’Histoire naturelle, Paris. Bulletin of the Mizunami Fossil Museum, 38:15-27.
Schweitzer, C.E., Odumodu, C.F.R., and Feldmann, R.M. 2016a. New Eocene crabs from Nigeria (Decapoda: Brachyura: Heterotremata). Annals of Carnegie Museum, 84:59-73.
https://doi.org/10.2992/007.084.0107
Schweitzer, C.E., Karasawa, H., Luque, J., and Feldmann, R.M. 2016b. Phylogeny and classification of Necrocarcinoidea Förster, 1968 (Brachyura: Raninoida) with description of two new genera. Journal of Crustacean Biology, 36:338-372.
https://doi.org/10.1163/1937240x-00002432
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2017. Part R, Revised, Volume 1, Chapter 8R: Systematic Descriptions: Section Cyclodorippoida. Treatise Online, 97:1-6, 4 figs.
https://doi.org/10.17161/to.v0i0.6659
Schweitzer, C.E., Feldmann, R.M., Karasawa, H., and Luque, J. 2018. Part R, Revised, Volume 1, Chapter 8S: Systematic Descriptions: Section Raninoida. Treatise Online, 113:1-42, 20 figs.
https://doi.org/10.17161/to.v0i0.8246
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2020. Part R, Revised, Volume 1, Chapter 8T11: Systematic Descriptions: Superfamily Majoidea, p. 1-31, Treatise Online, Volume 136: KU Paleontological Institute, The University of Kansas, Lawrence, Kansas, USA.
https://doi.org/10.17161/to.vi.14519
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2021a. Part R, Revised, Volume 1, Chapter 8T10: Systematic descriptions: Superfamily Eriphioidea, p. 1-8, Treatise Online, Volume 132.
https://doi.org/10.17161/to.vi.13577
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2021b. Part R, Revised, Volume 1, Chapter 8T17: Systematic descriptions: Superfamily Dorippoidea, p. 1-8, Treatise Online, Volume 159.
https://doi.org/10.17161/to.vi.16310
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2021c. Part R, Revised, Volume 1, Chapter 8T16: Systematic descriptions: Superfamilies Trapezioidea and Xanthoidea, p. 1-42, Treatise Online, Volume 153.
https://doi.org/10.17161/to.vi.15848
Schweitzer, C.E., Feldmann, R.M., and Karasawa, H. 2021d. Part R, Revised, Volume 1, Chapter 8T15: Systematic descriptions: Superfamily Portunoidea, p. 1-40, Treatise Online, Volume 151.
https://doi.org/10.17161/to.vi.15392
Scott, R.W., Schlager, W., Fouke, B., and Nederbragt, S.A. 2000. Are mid-Cretaceous eustatic events recorded in Middle East carbonate platforms?, p. 73-84. In Alsharhan, A. S., and Scott, R. W., eds.), Middle East models of Jurassic/Cretaceous Carbonate Systems: SEPM Special Publication, Volume 69.
https://doi.org/10.2110/pec.00.69.0077
Scott, R.W., Benson, D.G., Morin, R.W., Shaffer, B.L., and Oboh-Ikuenobe, F.E. 2002. Integrated Albian-Lower Cenomanian chronostratigraphy standard, Trinity River section, Texas. US Gulf Coast Cretaceous stratigraphy and paleoecology: Gulf Coast Section, Society of Economic Paleontologists and Mineralogists, Bob F. Perkins Memorial Volume, 23:277-334.
Scott, R.W., Campbel, W., Hojnacki, R., Wang, Y., and Lai, X. 2016. Albian rudist biostratigraphy (Bivalvia), Comanche shelf to shelf margin, Texas. Carnets de Géologie (Notebooks on Geology), 16:513-541.
https://doi.org/10.4267/2042/61701
Secrétan, S. 1975. Les Crustacés du Monte Bolca. Studi e Ricerche sui Giacimenti Terziari di Bolca II, Miscellanea Paleontologica. Museo Civico di Storia Naturale - Studi e Ricerche sui giacimenti terziari di Bolca, Verona, 2:315-388, pls. I-XXXVII.
Serra-Kiel, J., Canudo, J., Dinares, J., Molina, E., Ortiz, N., Pascual, J., Samsó, J., and Tosquella, J. 1994. Cronoestratigrafía de los sedimentos marinos del Terciario inferior de la Cuenca de Graus-Tremp (Zona Central Surpirenaica). Revista de la Sociedad Geológica de España, 7:273-297.
Serrano-Sánchez, M., Hegna, T.A., Schaaf, P., Pérez, L., Centeno-García, E., and Vega, F.J. 2015. The aquatic and semiaquatic biota in Miocene amber from the Campo La Granja mine (Chiapas, Mexico): Paleoenvironmental implications. Journal of South American Earth Sciences, 62:243-256.
https://doi.org/10.1016/j.jsames.2015.06.007
Serrano-Sánchez, M.d.L., Guerao, G., Centeno-García, E., and Vega, F.J. 2016. Crabs (Brachyura: Grapsoidea: Sesarmidae) as inclusions in Lower Miocene amber from Chiapas, Mexico. Boletín de la Sociedad Geológica Mexicana, 68:37-43.
https://doi.org/10.18268/bsgm2016v68n1a6
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37:155-163.
https://doi.org/10.1016/j.cretres.2012.03.014
Solórzano Kraemer, M. 2007. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from Mexican amber. Palaeontographica Abteilung A:1-133.
https://doi.org/10.1127/pala/282/2007/1
Sorensen, P.A. 1982. Sedimentology and sedimentary petrology of a Paleoce Basin near Laguna San Ignacio, Baja California Sur, Mexico. Master of Arts, University of California, Santa Barbara, California, United States.
Spiridonov, V.A., Neretina, T.V., and Schepetov, D. 2014. Morphological characterization and molecular phylogeny of Portunoidea Rafinesque, 1815 (Crustacea Brachyura): implications for understanding evolution of swimming capacity and revision of the family-level classification. Zoologischer Anzeiger - A Journal of Comparative Zoology, 253:404-429.
https://doi.org/10.1016/j.jcz.2014.03.003
Stebbing, T.R.R. 1920. The Malacostraca of Durban Bay. Durban Museum Novitates, 2:263-278.
https://journals.co.za/doi/abs/10.10520/AJA0012723X_1599
Stenzel, H.B. 1944. A new Cretaceous crab, Graptocarcinus muiri, from Mexico. Journal of Paleontology, 18:550-552.
https://www.jstor.org/stable/1299080
Stevcic, Z. 2005. The reclassification of brachyuran crabs (Crustacea: Decapoda: Brachyura). Natura Croatica, 14:1-159.
Stimpson, W. 1858. Prodromus descriptionis animalium evertebratorum, quae in Expeditione ad Oceanum Pacificum Septentrionalem, a Republica Federata missa, Cadwaladaro Ringgold et Johanne Rodgers ducibus, observavit et descripsit. Pars VII. Crustacea Anomura. Proceedings of the Academy of Natural Sciences of Philadelphia, 10:225-252 [Pages 263-290 on separate].
https://doi.org/10.5962/bhl.title.51447
Stimpson, W. 1859. Notes on North American Crustacea in the Museum of the Smithsonian Institution No. 1. Annals of the Lyceum of Natural History, New York, 7:49-93.
Stimpson, W. 1860. Notes on North American Crustacea in the Museum of the Smithsonian Institution No. 2. Annals of the Lyceum of Natural History, New York, 7:176-246.
Stoliczka, F. 1871. Observations on fossil crabs from Tertiary deposits in Sind and Kutch. Memoirs of the Geological Survey of India, Palaeontologica Indica, (7) (14), 1:1-16.
Studer, T. 1892. Ueber zwei fossile dekapode Krebse aus den Molasseablagerungen des Belpberges. Abhandlungen der Schweizerischen Paläontologischen Gesellschaft, 19:1-8.
Suzuki, S., Danhara, T., and Tanaka, H. 2003. Fission-track dating of Tertiary tuff samples from the Kibi Plateau Area, Okayama prefecture, Southwest Japan. Journal of Geography, 112:35-49.
https://doi.org/10.5026/jgeography.112.35
Sztrákos, K. and Steurbaut, E. 2017. Révision lithostratigraphique et biostratigraphique de l'Oligocène d'Aquitaine occidentale (France). Geodiversitas, 39:741-781.
https://doi.org/10.5252/g2017n4a6
Taguchi, E. 2002. Stratigraphy, molluscan fauna and paleoenvironment of the Miocene Katsuta Group in Okayama Prefecture, Southwest Japan. Bulletin of the Mizunami Fossil Museum, 29:95-133.
Tan, M.H., Gan, H.M., Lee, Y.P., Linton, S., Grandjean, F., Bartholomei-Santos, M.L., Miller, A.D., and Austin, C.M. 2018. ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Molecular Phylogenetics and Evolution, 127:320-331.
https://doi.org/10.1016/j.ympev.2018.05.015
Tang, Y.-Y., Tang, B.-P., Xin, Z.-Z., Li, Y.-T., Zha, X.-H., Zhang, D.-Z., Sun, Y., Liu, Q.-N., and Ma, Y.-F. 2020. Characterization of the complete mitochondrial genome of Helice latimera and its phylogenetic implications in Brachyura. Genomics, 112:5180-5187.
https://doi.org/10.1016/j.ygeno.2020.08.013
Tavares, M. 1993. Crustacea Decapoda: les Cyclodorippidae et Cymonomidae de l'Indo-Ouest-Pacifique à l'exclusion du genre Cymonomus, p. 253-313. In Crosnier, A. (ed.), Résultats des Campagnes MUSORSTOM, Volume 10. Mémoires du Muséum national d’Histoire naturelle, Volume 156.
Thoma, B.P., Guinot, D., and Felder, D.L. 2014. Evolutionary relationships among American mud crabs (Crustacea: Decapoda: Brachyura: Xanthoidea) inferred from nuclear and mitochondrial markers, with comments on adult morphology. Zoological Journal of the Linnean Society, 170:86-109.
https://doi.org/10.1111/zoj.12093
Thurmann, J. 1853. Lettres écrites du Jura à la Société d'histoire naturelle de Berne: Lettre IX, Mitteilungen der Naturforschenden Gesellschaft Bern.
Tsang, C.T., Schubart, C.D., Chu, K.H., Ng, P.K., and Tsang, L.M. 2022. Molecular phylogeny of Thoracotremata crabs (Decapoda, Brachyura): Toward adopting monophyletic superfamilies, invasion history into terrestrial habitats and multiple origins of symbiosis. Molecular Phylogenetics and Evolution, 177:107596.
https://doi.org/10.1016/j.ympev.2022.107596
Tsang, L.M., Schubart, C.D., Ahyong, S.T., Lai, J.C.Y., Au, E.Y.C., Chan, T.-Y., Ng, P.K.L., and Chu, K.H. 2014. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Molecular Biology and Evolution, 31:1173-1187.
https://doi.org/10.1093/molbev/msu068
Tucker, A.B. 1998. Systematics of the Raninidae (Crustacea: Decapoda: Brachyura), with accounts of three new genera and two new species. Proceedings of the Biological Society of Washington, 111:320-371.
Tucker, A.B. and Feldmann, R.M. 1990. Fossil decapod crustaceans from the lower Tertiary of the Prince William Sound region, Gulf of Alaska. Journal of Paleontology, 64:409-427.
https://doi.org/10.1017/s0022336000018643
Ungaro, S. 1978. L’Oligocene dei Colli Berici. Rivista, Italiana di Paleontologiia e Stratigrafia, 84:199-278.
Van Bakel, B.W., Guinot, D., Corral, J.C., and Artal, P. 2012a. Graptocarcininae n. subfam., an extinct subfamily of Dynomenidae Ortmann, 1892 (Crustacea, Brachyura, Podotremata). Zootaxa, 3534:40-52.
https://doi.org/10.11646/zootaxa.3534.1.3
Van Bakel, B.W.M., Guinot, D., Artal, P., Fraaije, R.H.B., and Jagt, J.W.M. 2012b. A revision of the Palaeocorystoidea and the phylogeny of raninoidian crabs (Crustacea, Decapoda, Brachyura, Podotremata). Zootaxa, 3215:1-216.
https://doi.org/10.11646/zootaxa.3215.1.1
Van Bakel, B.W., Mychko, E.V., Spiridonov, A., Jagt, J.W., and Fraaije, R.H. 2021. New Cretaceous crabs (Crustacea, Brachyura) from Moscow Oblast and Dagestan (Russia): patterns in phylogeny and morphospace of the oldest eubrachyurans (Dorippoidea). Cretaceous Research, 119:104675.
https://doi.org/10.1016/j.cretres.2020.104675
Van Straelen, V. 1920. Catalogue des crustacés décapodes des terrains Tertiaires de la Belgique. Annales de la Société royale zoologique de Belgique, 51:111-131.
Varola, A. 1981. Crostacei Decapodi neogenici della penisola salentina (Italia). Thalassia salentina, 11:3-51.
Vega, F.J., Cosma, T., Coutiño, M.A., Feldmann, R.M., Nyborg, T., Schweitzer, C.E., and Waugh, D.A. 2001. New middle Eocene decapods (Crustacea) from Chiapas, Mexico. Journal of Paleontology, 75:929-946.
https://doi.org/10.1017/s002233600003986x
Vega, F.J., Nyborg, T., Fraaye, R.H., and Espinosa, B. 2007. Paleocene decapod Crustacea from the Rancho Nuevo Formation (Parras Basin-Difunta Group), Northeastern México. Journal of Paleontology, 81:1432-1441.
https://doi.org/10.1666/06-018r.1
Vega, F.J., Nyborg, T., Coutiño, M.A., and Hernández-Monzón, O. 2008. Review and additions to the Eocene decapod Crustacea from Chiapas, Mexico. Bulletin of the Mizunami Fossil Museum, 34:51-71.
Vega, F.J., Nyborg, T., Coutiño, M.A., Solé, J., and Hernández-Monzón, O. 2009. Neogene Crustacea from Southeastern Mexico. Bulletin of the Mizunami Fossil Museum, 35:51-69.
Wagreich, M., Hohenegger, J., and Ćorić, S. 2014. Base and new definition of the Lower Badenian and the age of the Badenian stratotype (Middle Miocene, Central Paratethys). In Proceedings STRATI 2013: First International Congress on Stratigraphy At the Cutting Edge of Stratigraphy 2014, Springer, p. 615-618.
https://doi.org/10.1007/978-3-319-04364-7_118
Wang, Q., Tang, D., Guo, H., Wang, J., Xu, X., and Wang, Z. 2020. Comparative mitochondrial genomic analysis of Macrophthalmus pacificus and insights into the phylogeny of the Ocypodoidea & Grapsoidea. Genomics, 112:82-91.
https://doi.org/10.1016/j.ygeno.2019.12.012
Wang, Z., Wang, Z., Shi, X., Wu, Q., Tao, Y., Guo, H., Ji, C., and Bai, Y. 2018. Complete mitochondrial genome of Parasesarma affine (Brachyura: Sesarmidae): Gene rearrangements in Sesarmidae and phylogenetic analysis of the Brachyura. International journal of biological macromolecules, 118:31-40.
https://doi.org/10.1016/j.ijbiomac.2018.06.056
Watson-Zink, V.M. 2021. Making the grade: physiological adaptations to terrestrial environments in decapod crabs. Arthropod Structure & Development, 64:101089.
https://doi.org/10.1016/j.asd.2021.101089
Waugh, D.A., Feldmann, R.M., and Schweitzer, C.E. 2009. Systematic evaluation of raninid cuticle microstructure. Bulletin of the Mizunami Fossil Museum, 35:15-41.
Webber, W., Fenwick, G., Bradford-Grieve, J., Eagar, S., Buckeridge, J., Poore, G., Dawson, E., Watling, L., Jones, J., Wells, J., Bruce, N., Ahyong, S., Larsen, K., Chapman, M., Olesen, J., Ho, J., Green, J., Shiel, R., Rocha, C., Lorz, A., Bird, G., and Charleston, W. 2010. Phylum Arthropoda Subphylum Crustacea: shrimps, crabs, lobsters, barnacles, slaters, and kin, p. 98-232. In Gordon, D.P. (ed.), New Zealand Inventory of Biodiversity: Volume Two: Kingdom Animalia - Chaetognatha, Ecdysozoa, Ichnofossils,: Canterbury University Press, New Zealand.
https://hdl.handle.net/2440/65423
Weber, F. 1795. Nomenclator Entomologicus Secundum Entomologiam Systematicum ill: Fabricii Adjectis Speciebus Recens Detectis et Varietatibus. C. E. Bohn. Chilonii and Hamburgi, Chilonii et Hamburgi.
https://doi.org/10.5962/bhl.title.12297
Wegner, W., Wörner, G., Harmon, R.S., and Jicha, B.R. 2011. Magmatic history and evolution of the Central American Land Bridge in Panama since Cretaceous times. Geological Society of America Bulletin, 123:703-724.
https://doi.org/10.1130/b30109.1
Williams, A.B. and Child, C.A. 1988. Comparison of some genera and species of box crabs (Brachyura: Calappidae), southwestern North Atlantic, with description of a new genus and species. Fishery Bulletin, Washington, 87:105-121.
https://research.nhm.org/pdfs/27673/27673.pdf
Windsor, A.M. and Felder, D.L. 2014. Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda: Brachyura: Majoidea). Invertebrate Systematics, 28:145-173.
https://doi.org/10.1071/is13011
Withers, T.H. 1932. XXXIII.–A Liassic crab, and the origin of the Brachyura. Annals and Magazine of Natural History, 9:313-323.
https://doi.org/10.1080/00222933208673499
Wolfe, J.M., Daley, A.C., Legg, D.A., and Edgecombe, G.D. 2016. Fossil calibrations for the arthropod Tree of Life. Earth-Science Reviews, 160:43-110.
https://doi.org/10.1016/j.earscirev.2016.06.008
Wolfe, J.M., Breinholt, J.W., Crandall, K.A., Lemmon, A.R., Moriarty Lemmon, E., Timm, L.E., Siddall, M.E., and Bracken-Grissom, H.D. 2019. A phylogenomic framework, evolutionary timeline and genomic resources for comparative studies of decapod crustaceans. Proceedings of the Royal Society B, 286:20190079.
https://doi.org/10.1098/rspb.2019.0079
Wolfe, J.M., Luque, J., and Bracken-Grissom, H.D. 2021. How to Become a Crab: Phenotypic Constraints on a Recurring Body Plan. Bioessays, 43:1-14.
http://doi.org/10.1002/bies.202100020
Wolfe, J.M., Ballou, L., Luque, J., Watson-Zink, V.M., Ahyong, S.T., Barido-Sottani, J., Chan, T.-Y., Chu, K.H., Crandall, K.A., Daniels, S.R., Felder, D.L., Mancke, H., Martin, J.W., Ng, P.K.L., Ortega-Hernández, J., Palacios Theil, E., Pentcheff, N.D., Robles, R., Thoma, B.P., Tsang, L.M., Wetzer, R., Windsor, A.M., and Bracken-Grissom, H.D. 2024. Convergent adaptation of true crabs (Decapoda: Brachyura) to a gradient of terrestrial environments. Systematic Biology, 73(2):247–262.
https://doi.org/10.1093/sysbio/syad066
Woods, H. 1922. Crustacea from the Eocene deposits of Peru, p. 114-118. In Bosworth, T.O. (ed.), Geology of the Tertiary and Quaternary Periods in the northwest part of Peru: Macmillan and Co., London.
Yoshimoto, Y. 1979. 38. Tsuyama basin, Okayama Prefecture, p. 113-114. In Tsuchi, R. (ed.), Fundamental data on Japanese Neogene Bio- and Chronostratigraphy: IGCO-114 National Working Group of Japan, Shizuoka (in Japanese).
Zeiss, A. 2001. Die Ammonitenfauna der Tithonklippen von Ernstbrunn, Niederösterreich. Neue Denkschriften des Naturhistorischen Museums in Wien, Neue Serie, 6:1-117.
Zhang, Y., Gong, L., Lu, X., Miao, Z., Jiang, L., Liu, B., Liu, L., Li, P., Zhang, X., and Lü, Z. 2022. Comparative mitochondrial genome analysis of Varunidae and its phylogenetic implications. Acta Oceanologica Sinica, 41:119-131.
https://doi.org/10.1007/s13131-021-1927-7

