 A Pliocene goodeid fish of the Paleolake Amajac, Sanctórum, Hidalgo, Mexico
A Pliocene goodeid fish of the Paleolake Amajac, Sanctórum, Hidalgo, Mexico
Article number: 26.2.a30
https://doi.org/10.26879/1259
Copyright Paleontological Society, August 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 16 December 2022. Acceptance: 24 July 2023.
ABSTRACT
The splitfin fossil species Paleocharacodon guzmanae gen. and sp. nov. is erected based on the osteological study of 14 fossil male and female specimens recovered in the Pliocene deposits of the Paleolake Amajac, in Sanctórum, Hidalgo, Mexico. This new cyprinodontiform fish exhibits the diagnostic features of the family Goodeidae and subfamily Goodeinae; like all the goodeids, its premaxilla has a straight distal end, and its premaxillary ascending process is small; and, like the goodeines, this new species was viviparous, its first anal fin ray is rudimentary, and the males show an andropodium. Although P. guzmanae displays numerous primitive features, it is not possible to place it in any of the goodeine tribes, which currently are vaguely defined by osteological features. This new species seems to be closely related to Characodon; both share a peculiar osteological character; the articular facet for the quadrate is a donut-like structure, in which the retroarticular forms the central region, and a couple of semicircular anguloarticular processes form the surrounding part. This species differs from other goodeids mainly in two features; it has a posttemporal bone with small anteroventral processes, and the openings of its supraorbital canal show the formula1-2a, 2b-3a, 3b-4a, 4b-5a, and 5b-7. The discovery of this extinct goodeid species in the great Pánuco-Salado Basin on the eastern slope of Mexican territory represents an unexpected historical element.
Carmen Caballero-Viñas. Departamento de Paleontología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito de la Investigación S/N, Ciudad Universitaria, Coyoacán, Ciudad de México, 04510, Mexico. c-caballero@live.com.mx
Jesús Alvarado-Ortega. Departamento de Paleontología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito de la Investigación S/N, Ciudad Universitaria, Coyoacán, Ciudad de México, 04510, México. alvarado@geología.unam.mx
Kleyton Magno Cantalice Severiano. Departamento de Paleontología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito de la Investigación S/N, Ciudad Universitaria, Coyoacán, Ciudad de México, 04510, México. kleytonmc@geologia.unam.mx
Keywords: new genus; new species; goodeids; Pliocene; fossil; Mexico
Final citation: Caballero-Viñas, Carmen, Alvarado-Ortega, Jesús, and Magno Cantalice Severiano, Kleyton. 2023. A Pliocene goodeid fish of the Paleolake Amajac, Sanctórum, Hidalgo, Mexico. Palaeontologia Electronica, 26(2):a30.
https://doi.org/10.26879/1259
palaeo-electronica.org/content/2023/3919-pliocene-goodeid-from-mexico
Copyright: August 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/DD338EE2-B59D-4A46-877D-8675A5543BA8
INTRODUCTION
Jordan and Gilbert (1883) erected the family Goodeidae to include the “splitfins.” This group of small freshwater cyprinodontiforms fishes is endemic to the United States of America (USA) and Mexico (Doadrio and Domínguez, 2004). This family comprises two geographically disjunct subfamilies (Foster and Piller, 2018). The subfamily Empetrichthyinae consists of four living species gathered into two genera, which are oviparous and opportunistic omnivores fishes that inhabit springs and pools restricted to the south-western Great Basin of the USA (Williams and Williams, 1982). In contrast, the subfamily Goodeinae consists of about 18 genera and 44 species of viviparous fishes that have different eating habits and inhabit lakes, creeks, marshes, canals, and large rivers of the Mesa Central of Mexico and adjacent regions (Webb et al., 2004; Domínguez-Domínguez et al., 2010). The empetrichthyines, once included in the Cyprinodontidae family and now considered part of Goodeidae (Parenti, 1981), are oviparous organisms with no pelvic fins. In contrast, goodeines are viviparous, have internal fertilization, exhibit embryonic feeding by matrotrophy through trophotaeniae, and the males develop an elaborate courtship (Deacon and Williams, 1984; Doadrio and Domínguez, 2004; Domínguez-Domínguez et al., 2010). In recent studies, Goodeidae and Profundulidae are closely related (Costa, 1998; Webb et al., 2004; Nelson et al., 2016; Piller et al., 2022).
Although the origin of Goodeidae dates to the early Miocene (Foster and Piller, 2018, fig. 2), the fossil record of these fishes is relatively scarce and usually involves skeletal remains, often so fragmentary and isolated that their taxonomic identity is hardly determinable. In Mexico, fossil remains of living species have been recovered in the Neogene lacustrine and fluvial deposits scattered within the Trans-Mexican Volcanic Belt (TMVB), like those of the middle Pliocene and Plio-Pleistocene age near the Chapala and Zacoalco lakes, in the Jocotepec and Zacoalco municipalities, Jalisco, and those of Pleistocene age discovered in the Tlapacoya Municipality, Estado de Mexico (Smith, 1980; Guzmán and Polaco, 2009; Guzmán, 2015).
Up to now, only two fossil species of goodeid have been described based on complete and relatively well-preserved specimens. Empetrichthys erdisi Uyeno and Miller, 1962, is an empetrichthyine recovered from the Pliocene deposits of Santa Clara River Valley in the Piru Mountains, Ventura County, southwestern California, USA. Tapatia occidentalis Álvarez and Arriola-Longoria, 1972, is a goodeine from the Miocene lacustrine deposits at the Santa Rosa Ravin, Amatitán Municipality, Jalisco (Miller and Smith, 1986; Guzmán et al., 1998). At the beginning of this century, Becerra Martínez et al. (2002) reported complete goodeid specimens from the Pliocene strata of the Amajac Paleolake belonging to the Atotonilco El Grande Formation that outcrop in Sanctórum village within the Atotonilco El Grande Municipality, Hidalgo, central Mexico. Later, Becerra Martínez (2003) suggested the presence of two species among these fossils, an indeterminate Goodeidae and a possible member of the genus Goodea Jordan, 1880.
Despite its importance in understanding the history and composition of the North American continental fish communities, studying fossil freshwater fishes from Mexico still needs to be completed. Therefore, the authors of this work launched a project to recover and study these fossils, an effort that now has some achievements (Alvarado-Ortega and Carranza-Castañeda, 2002a-c; Alvarado-Ortega et al., 2006; Espinosa-Arrubarrena et al., 2009; Mendoza-Reynosa and Alvarado-Ortega, 2015; Mendoza-Reynosa et al., 2013). Today, we are studying the Pliocene ichthyofauna of Sanctórum; hence, the present manuscript aims to determine the taxonomical identity of that fish previously referred to as an indeterminate Goodeidae species by Becerra Martínez (2003).
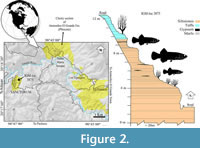
 The fossil goodeids studied in this work are from the lacustrine deposits of volcanic ashes and clays that outcrop within the Sanctórum village, located between the coordinates 20°18ʹ16ʺN, 98°45ʹ51ʺW (Figure 1), about 34 km northeast of the city of Pachuca (Arellano-Gil et al., 2005). The fossiliferous sequence of Sanctórum was discovered in the 1980s by Dr. Oscar Carranza Castañeda and Mr. Gerardo Álvarez Reyes, paleontologists from the Instituto de Geology (Igl) of the National Autonomous University of Mexico (UNAM). In 2008, the second of the authors of the present work began a systematic collection in a small outcrop on the outskirts of this town, called "Sanctórum-JAO site" (Figure 2), registered as a locality IGM-loc in the Colección Nacional de Paleontología at Igl-UNAM. These strata belong to the Atotonilco El Grande Formation, a lithostratigraphic unit described by Segerstrom (1961) as a sequence of clastic rocks and limestone lenses, covered or locally interspersed by basalt currents, which together are 500 to 600 m thick and were deposited into the basins of the Metztitlán and Amajac rivers.
The fossil goodeids studied in this work are from the lacustrine deposits of volcanic ashes and clays that outcrop within the Sanctórum village, located between the coordinates 20°18ʹ16ʺN, 98°45ʹ51ʺW (Figure 1), about 34 km northeast of the city of Pachuca (Arellano-Gil et al., 2005). The fossiliferous sequence of Sanctórum was discovered in the 1980s by Dr. Oscar Carranza Castañeda and Mr. Gerardo Álvarez Reyes, paleontologists from the Instituto de Geology (Igl) of the National Autonomous University of Mexico (UNAM). In 2008, the second of the authors of the present work began a systematic collection in a small outcrop on the outskirts of this town, called "Sanctórum-JAO site" (Figure 2), registered as a locality IGM-loc in the Colección Nacional de Paleontología at Igl-UNAM. These strata belong to the Atotonilco El Grande Formation, a lithostratigraphic unit described by Segerstrom (1961) as a sequence of clastic rocks and limestone lenses, covered or locally interspersed by basalt currents, which together are 500 to 600 m thick and were deposited into the basins of the Metztitlán and Amajac rivers.
 The oldest lithostratigraphic units in the Atotonilco El Grande region are the El Doctor Formation Albian-Cenomanian limestones and the Mendez Formation Campanian-Maastrichtian marls. Such Cretaceous strata are discordantly covered by sediments of the Pachuca Group, which consists of the basal Eocene-Oligocene Amajac conglomerates that are gradually replaced by the Miocene-Pliocene strata of sandstones and silt-sandy intercalated by limonite, loamy shale, and loams. Above these lies the Pliocene-Pleistocene lacustrine and volcano-sedimentary sequence of the Atotonilco El Grande Formation. Finally, the youngest local sediments are Quaternary alluviums (Beltrán-Romero and Luna-Gómez, 1994; Kowallis et al., 1998; Salvador-Flores, 2001). The Pliocene section of the Atotonilco El Grande Formation consists of fine-grained lacustrine sediments deposited under high altitude, shallow, and low energy conditions, which allow the fossil preservation of plants, invertebrates, coprolites, and vertebrates (Arellano-Gil et al., 2005). The fossil assemblage of Sanctórum includes impressions of terrestrial and aquatic plants, charophytes, silicified wood fragments, insects, ostracods, gastropods, coprolites, frogs, salamanders, as well as a rodent jaw, a snake, and numerous fishes (Beltrán-Romero and Luna-Gómez, 1994; Velasco-de León et al., 2000; Reyes-Torres et al., 2002; Velasco-de León and Aguilar-Arellano, 2002; Zaragoza-Caballero and Velasco-de León, 2003; Palma-Ramírez et al., 2012; Avendaño Pazos, 2020; among others).
The oldest lithostratigraphic units in the Atotonilco El Grande region are the El Doctor Formation Albian-Cenomanian limestones and the Mendez Formation Campanian-Maastrichtian marls. Such Cretaceous strata are discordantly covered by sediments of the Pachuca Group, which consists of the basal Eocene-Oligocene Amajac conglomerates that are gradually replaced by the Miocene-Pliocene strata of sandstones and silt-sandy intercalated by limonite, loamy shale, and loams. Above these lies the Pliocene-Pleistocene lacustrine and volcano-sedimentary sequence of the Atotonilco El Grande Formation. Finally, the youngest local sediments are Quaternary alluviums (Beltrán-Romero and Luna-Gómez, 1994; Kowallis et al., 1998; Salvador-Flores, 2001). The Pliocene section of the Atotonilco El Grande Formation consists of fine-grained lacustrine sediments deposited under high altitude, shallow, and low energy conditions, which allow the fossil preservation of plants, invertebrates, coprolites, and vertebrates (Arellano-Gil et al., 2005). The fossil assemblage of Sanctórum includes impressions of terrestrial and aquatic plants, charophytes, silicified wood fragments, insects, ostracods, gastropods, coprolites, frogs, salamanders, as well as a rodent jaw, a snake, and numerous fishes (Beltrán-Romero and Luna-Gómez, 1994; Velasco-de León et al., 2000; Reyes-Torres et al., 2002; Velasco-de León and Aguilar-Arellano, 2002; Zaragoza-Caballero and Velasco-de León, 2003; Palma-Ramírez et al., 2012; Avendaño Pazos, 2020; among others).
MATERIAL AND METHODS
Institutional abbreviations. The acronyms of the institutions involved with this work include CMR, Colección de Materiales Recientes (a subcollection into the Colección Nacional de Paleontología). CPUM, Colección de Peces, Universidad Michoacana de San Nicolás de Hidalgo, Michoacán. ENCB, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Ciudad de México.IGM, Colección Nacional de Paleontología, Instituto de Geología (previously Instituto Geológico de México) of the Universidad Nacional Autónoma de México, Ciudad de México. LaNaBio, Laboratorio Nacional de Biodiversidad, Instituto de Biología, UNAM. LUMIR, Laboratorio Universitario de Microtomografía de Rayos X, Centro de Geociencias of UNAM, Campus Juriquilla, Querétaro.
Preparation methods. All the fish specimens described here belong to the Colección Nacional de Paleontología. These are housed in the Museo María del Carmen Perrilliat M. belonging to the Igl-UNAM. These fossils are mechanically prepared. Under a binocular stereoscopic microscope, fine needles helped to remove the small patches of sediments and uncover the fossil osseous structures. When necessary, a solution of plexigum and methacrylate acetate, applied with fine brushes, glued hardened the fossil bones. The specimens were, directly and indirectly, observed under a stereoscopic microscope and a collection of high-quality photographs obtained in a Low Vacuum Scanning Electron Microscope (SEM). We got digital photographs with a single-lens reflex Camara (SLR) under different illumination conditions, including the natural, white, and long-wave UV (254 nm) lights, as well as with a Confocal Laser Scanning Microscope (CLSM). We obtained virtual micro-computed tomographies (μCT) of recent and fossil specimens. We generated a total of 1200 images with a voxels size of 21.86 μm of each treated fish under a protocol of 110kV, 72 μA, and 500 ms (the electronic files obtained so far will be available for further research because they will be delivered and will be part of the electronic files housed in the Colección Nacional de Paleontología). As noted below, different individuals of living species were cleared and stained according to the technique of Dingerkus and Uhler (1977). The disarticulated dry skeletons of recent goodeids included in this study were obtained by alkaline digestion when complete specimens were immersed in a saturated solution of KOH for 3 hrs and subsequently washed in clean water for 24 hrs. Bones were separated from the soft tissue debris using dissection needles under the microscope and left to dry in the shade at room temperature.
Comparative materials. CPUM donated recent females and males of 34 Mexican goodeid species are used here for comparative purposes. These specimens include Ameca splendens Miller and Fitzsimons, 1971: Complete specimens cleared and stained, CMR 1247, a male of 41.75 mm SL; CMR 1248, a female of 43.3 mm of SL. Characodon audax Smith and Miller, 1986: Complete specimens cleared and stained, CMR 1249, a male of 36.88 mm of SL; CMR 1250, a female of 37.97 mm of SL. Characodon lateralis Günter, 1866: Complete specimens cleared and stained, CMR 1251, a male of 35.83 mm of SL; CMR 1252, a female of 38.38 mm of SL. Goodea atripinnis Jordan, 1880: Disarticulated dry skeleton including CMR 1253, a male of 58 mm of SL; CMR 1254, a female of 64 mm of SL; complete specimens cleared and stained, CMR 1255, a male of 35.97 mm of SL; CMR 1256, a female of 65.74 mm of SL. Hubbsina turneri De Buen, 1940: CMR 1257, a female of 46.70 mm of SL; CMR 1258, a pregnant female of 35.08 mm of SL. Tapatia occidentalis Álvarez and Arriola-Longoria, 1972: P 3661, holotype, a complete specimen of 15.93 mm of SL (deposited in the collection of the Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional); IGM 7966, a complete specimen.
Anatomical nomenclature and abbreviations. The osteological nomenclature, abbreviations, and measurements considered in this manuscript and its figures and tables follow that of previous similar studies (e.g., Costa, 1998; Webb, 1998; Kobelkowsky, 2005).
SYSTEMATIC PALEONTOLOGY
Order Cyprinodontiformes Berg, 1940.
Family Goodeidae sensu Parenti, 1981.
Subfamily Goodeinae Jordan and Gilbert, 1883.
Tribe indeterminate
Genus Paleocharacodon gen. nov.
zoobank.org/AB23EFF9-A960-46BE-9BE6-53BB556A50C0
Species included. Paleocharacodon guzmanae sp. nov., described below.
Etymology. The genus name derives from “paleo” or “ancient” in Greek, plus “Characodon” in reference to the genus named by Günther (1886).
Diagnosis. As in the type and unique species of the genus, see below.
Paleocharacodon guzmanae sp. nov.
(Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14; Table 1-Table 2)
zoobank.org/8F4FF55A-56B2-44DC-8CFF-A69A04A1C151
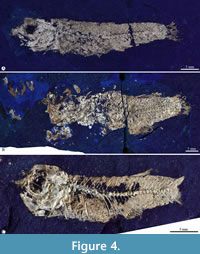
 Holotype. IGM 13117, a complete female specimen of 37.29 mm of standard length, preserved in part and counterpart (Figure 3, Table 1).
Holotype. IGM 13117, a complete female specimen of 37.29 mm of standard length, preserved in part and counterpart (Figure 3, Table 1).
Paratypes. IGM 13118, IGM 13119, IGM 13120 (Figure 4A), and IGM 13121 (Figure 4B) are almost complete and articulated males. IGM 13122 and IGM 13123 are incomplete and significantly disarticulated males. IGM 13124 and IGM 13125 (Figure 4C) are almost entirely well-preserved females. IGM 13126, IGM 13127, IGM 13128, IGM 13129, and IGM 13130 are incomplete specimens of unknown sex (Table 1).
Type locality and age horizon. Sanctórum-JAO site (recorded as IGM-loc 3875 in the Colección Nacional de Paleontología, Igl-UNAM), Atotonilco El Grande Municipality, Hidalgo, Mexico. Pliocene lacustrine deposits of the Paleolake Amajac belonging to the Atotonilco el Grande Formation (Figure 1 and Figure 2).
Etymology. The species epithet honors our colleague, Dr. Ana Fabiola Guzmán Camacho, who passed away in 2022, for her contributions to Mexican archaeozoology and paleoichthyology. Together the genus and species names mean “the Guzmán’s ancient Characodon fish.”
Diagnosis. Goodeinae fish with a unique combination of characters and a maximum total length is near to 60 mm, the body is elongated; slightly humped behind the head; fins rounded; pelvic fin rising at the beginning of the posterior half of the body at 57.3% of SL in males and 64.8% of SL in females, and in both cases its rear edge does not reach the anterior end of the dorsal fin base; unpaired fins opposed to each other, located far back of the body, and behind the 69% of SL; total vertebrae 35 in males including 15 abdominals plus 20 caudals while there are 31-32 in females including 14 abdominals and 17-18 caudals; anal fin rays typically 16; in males the most anterior anal rays is rudimentary and this and the following five forms the andropodium; dorsal fin rays 15 in males and 12 in females; pectoral rays 12 in males and 13-14 in females; pelvic rays 6 in both sexes; ribs 15 in males and 12-13 in females; the lateral edges of the anterior end of the parasphenoid are parallel and have no lateral wings; jaws shows a dental battery composed of a labial row of long and robust teeth distally bicuspid and acute with bases co-ossified with the bone plus smaller conic and unicuspid teeth are located lingually; the palatoquadrate arch is anteriorly inclined; the articular facet for the quadrate is donut-like with the central part formed by the retroarticular, and the surrounding region is formed by a couple of curved processes of the anguloarticular; the palatine head has an anterolateral projection; the ventroposterior end of coracoid is convex and has the posterior edge vertically tilted and notched. This species is unique in having the posttemporal with a small anteroventral process, and the openings of the supraorbital canal show the formula 1-2a, 2b-3a, 3b-4a, 4b-5a, 5b-7.
Description
Body shape and general proportions. Table 1 shows the body measurements and proportions of Paleocharacodon guzmanae gen. and sp. nov. The fossil fishes from Sanctórum show different modes of conservation. Occasionally, their bones are totally or partially disarticulated and dispersed. Although it is common to find them relatively complete, well-articulated, and resting, showing one of the body flanks, in general, the bones of the anterior part of the head, the jaws, the nape, and the abdominal region are a little dislocated causing some imprecision in the body measurements. The present description is based on the holotype and added data observed in the paratypes. The larger specimens are the female IGM 13117 (its total and standard lengths are 48.27 and 37.29 mm) and IGM 13130, a sex-undefined and incomplete individual that may be near 100 mm of SL (not included in Table 1).
 This elongated fish has a triangular head, almost as high as long, in which the snout is terminal. The maximum body height (MBH) is allometrically variable in the predorsal region of the trunk, in which the back becomes a kind of hump in the larger specimens (Table 1; Figure 3, Figure 4). Compared with females, males' bodies and heads are shorter and lower (the SL range is 21.88-40.32 mm vs. 25.38-37.29 mm). Regarding the SL, the MBH is 16.65-22.79% vs. 28.94-35.66%; the head length is 26.66-28.01% vs. 28.77-36.12%; and the head height is 21.67-27.23% vs. 27.39-30.08%). All fins have round terminal margins. The pectoral fin extends in the first third of the abdominal region and rises between the vertebral column and the ventral edge of the body. The pelvic fin is short, located in the posterior half of the body (57.3% of SL in males and 64.8% of SL in females), and its distal ends extension does not reach the cloaca. The unpaired fins are short (concerning the SL, the dorsal fin length is 69.3% in males and 71.87% in females while the anal fin length is 70.56% in males and 73.26% in females), opposite to each other, placed on the last third of the body, and represent about 10 to 15% of SL. The caudal peduncle is robust. The caudal fin is entirely round, its base is as high as the caudal peduncle, and its shorter rays are about half its longest.
This elongated fish has a triangular head, almost as high as long, in which the snout is terminal. The maximum body height (MBH) is allometrically variable in the predorsal region of the trunk, in which the back becomes a kind of hump in the larger specimens (Table 1; Figure 3, Figure 4). Compared with females, males' bodies and heads are shorter and lower (the SL range is 21.88-40.32 mm vs. 25.38-37.29 mm). Regarding the SL, the MBH is 16.65-22.79% vs. 28.94-35.66%; the head length is 26.66-28.01% vs. 28.77-36.12%; and the head height is 21.67-27.23% vs. 27.39-30.08%). All fins have round terminal margins. The pectoral fin extends in the first third of the abdominal region and rises between the vertebral column and the ventral edge of the body. The pelvic fin is short, located in the posterior half of the body (57.3% of SL in males and 64.8% of SL in females), and its distal ends extension does not reach the cloaca. The unpaired fins are short (concerning the SL, the dorsal fin length is 69.3% in males and 71.87% in females while the anal fin length is 70.56% in males and 73.26% in females), opposite to each other, placed on the last third of the body, and represent about 10 to 15% of SL. The caudal peduncle is robust. The caudal fin is entirely round, its base is as high as the caudal peduncle, and its shorter rays are about half its longest.
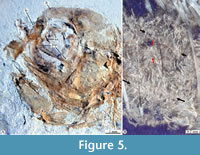 Skull. In most specimens studied here, the skull bones are so crushed or fragmented and isolated that it is difficult to recognize them accurately. Despite this situation, these exhibit the following details (Figure 5, Figure 6, Figure 7).
Skull. In most specimens studied here, the skull bones are so crushed or fragmented and isolated that it is difficult to recognize them accurately. Despite this situation, these exhibit the following details (Figure 5, Figure 6, Figure 7).
In the dorsal view, the skull is trapezoidal with no medial fontanels, in which the interfrontal suture is a somewhat sinuous interfrontal suture, and the frontals and supraoccipital bones are in contact separating the parietals. 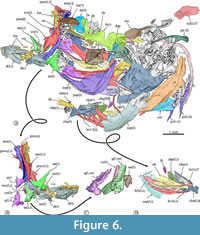 Anteriorly, each nasal is a flat oval bone with a large pore near its anterior that may correspond to pore 1 of the supraorbital sensory canal (Figure 6, Figure 8A). Each lateral ethmoid bone is a robust, complex C-shaped bone attached to the anterior ventral region of the respective frontal and extends up to the anterior end of the orbital part of the parasphenoid (Figure 6, Figure 7, Figure 8A).
Anteriorly, each nasal is a flat oval bone with a large pore near its anterior that may correspond to pore 1 of the supraorbital sensory canal (Figure 6, Figure 8A). Each lateral ethmoid bone is a robust, complex C-shaped bone attached to the anterior ventral region of the respective frontal and extends up to the anterior end of the orbital part of the parasphenoid (Figure 6, Figure 7, Figure 8A).
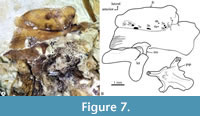 Each frontal is laminar dorsally expanded and axe-shaped, which forms 70% of the skull length, and markedly broad in its anterior two-thirds. The surface of each frontal shows three sections or low domes joined by inconspicuous ventral ridges, in which the bone thickens (Figure 7). The medial section of the frontal is the largest, dorsally smooth, and ventrally has a series of anterior parallel longitudinal ridges in which the respective lateral ethmoid bone attaches. The lateral section of this bone is roughly triangular and displays a series of 10 large pores alongside its borders with the medial and posterior sections, which corresponds to the pores 2b, 3a, 3b, 4a, 4b, 5a, 5b, 6, and 7 of the supraorbital sensory canal (Figure 6, Figure 7). Since pores 1 and 2 of this sensory canal open in the nasal bone, the formula of these openings is 1-2a, 2b-3a, 3b-4a, 4b-5a, and 5b-7. Remarkably, the 2b of these sensory pores is complex; this opens into a broad depression in which at least two other associated pores open (Figure 7). The posterior section of the frontal is the smallest, has a triangular shape, and is behind the lateral section. Near its posteromedial limit, the lateral section has a pair of conspicuous, laminar, and parallel ridges forming a bony canal projected lateromedially, which opens dorsally, encloses pore 6, and ends right where pore 7 opens. In the most posterior part of the frontal, where this bone bends ventrally and exposes more laterally, there is a wide shallow depression that seems to be the dorsal end of the dilator fossa (Figure 6).
Each frontal is laminar dorsally expanded and axe-shaped, which forms 70% of the skull length, and markedly broad in its anterior two-thirds. The surface of each frontal shows three sections or low domes joined by inconspicuous ventral ridges, in which the bone thickens (Figure 7). The medial section of the frontal is the largest, dorsally smooth, and ventrally has a series of anterior parallel longitudinal ridges in which the respective lateral ethmoid bone attaches. The lateral section of this bone is roughly triangular and displays a series of 10 large pores alongside its borders with the medial and posterior sections, which corresponds to the pores 2b, 3a, 3b, 4a, 4b, 5a, 5b, 6, and 7 of the supraorbital sensory canal (Figure 6, Figure 7). Since pores 1 and 2 of this sensory canal open in the nasal bone, the formula of these openings is 1-2a, 2b-3a, 3b-4a, 4b-5a, and 5b-7. Remarkably, the 2b of these sensory pores is complex; this opens into a broad depression in which at least two other associated pores open (Figure 7). The posterior section of the frontal is the smallest, has a triangular shape, and is behind the lateral section. Near its posteromedial limit, the lateral section has a pair of conspicuous, laminar, and parallel ridges forming a bony canal projected lateromedially, which opens dorsally, encloses pore 6, and ends right where pore 7 opens. In the most posterior part of the frontal, where this bone bends ventrally and exposes more laterally, there is a wide shallow depression that seems to be the dorsal end of the dilator fossa (Figure 6).
Each parietal is a small and smooth rectangular bone that joins the rear of the frontal and the lateral edge of the supraoccipital (Figure 6). The supraoccipital bone is an oblong, laminar, and dorsally convex bone with a large posterior crest that is bifid and expanded posteriorly (Figure 6). The basioccipital bone is triangular, anteriorly tapered, as high as its vertebral articulation, and hardly expanded laterally. In the dorsal view, the sphenotic bone is rectangular with a stout lateral process. The epiotic is a small oblong bone located in the posterior region of the skull that bears a straight posterior thick process (Figure 6).
 The parasphenoid is a complex unpaired bone that is expanded laterally in its otic region, whereas its orbital part forms a rod-like structure, evenly straight. The anterior end of parasphenoid becomes dorsoventrally flat and bifid. The middle orbital section of this bone changes allometrically; in small individuals, its transversal section is “+” shaped because it has low wings expanded dorsal, ventral, and laterally whereas, in large specimens, the cross-section becomes T-shaped because the dorsal wing disappears (Figure 6, Figure 7, Figure 8A). This bone displays a triangular basipterygoid process projected dorsally and laterally at the union of its orbital and otic sections.
The parasphenoid is a complex unpaired bone that is expanded laterally in its otic region, whereas its orbital part forms a rod-like structure, evenly straight. The anterior end of parasphenoid becomes dorsoventrally flat and bifid. The middle orbital section of this bone changes allometrically; in small individuals, its transversal section is “+” shaped because it has low wings expanded dorsal, ventral, and laterally whereas, in large specimens, the cross-section becomes T-shaped because the dorsal wing disappears (Figure 6, Figure 7, Figure 8A). This bone displays a triangular basipterygoid process projected dorsally and laterally at the union of its orbital and otic sections.
Otoliths. Although some specimens studied show remains of the three pairs of otoliths, only the sagittal otolith is well-preserved (Figure 8A, Figure 9, Table 2). In life, these inner ear stones are solid crystalline structures of calcium carbonate; however, the otoliths of our fossils have a grainy appearance, probably due to the partial dissolution of their calcium carbonate.
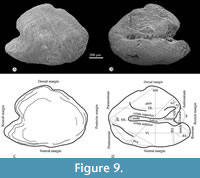 The sagittal otolith is a thick lenticular structure crudely resembling an arrowhead pointing backward, in which the ventral section is a little longer than the dorsal one (Figure 9). Its lateral surface is smooth, has a hardly protruding middle region, and peripherical concentric growth lines. On the contrary, its medial surface is irregular; it has two longitudinal grooves, one corresponding to an expanded deep concave dorsal area, and the other is a slender medial sulcus, dorsally and ventrally bordered by the cristae superior and inferior, respectively.
The sagittal otolith is a thick lenticular structure crudely resembling an arrowhead pointing backward, in which the ventral section is a little longer than the dorsal one (Figure 9). Its lateral surface is smooth, has a hardly protruding middle region, and peripherical concentric growth lines. On the contrary, its medial surface is irregular; it has two longitudinal grooves, one corresponding to an expanded deep concave dorsal area, and the other is a slender medial sulcus, dorsally and ventrally bordered by the cristae superior and inferior, respectively.
The contour of the sagittal otolith is slightly sinuous all around except for a conspicuous notch in the middle of the dorsal margin (Figure 9). In the rostral margin, the rostrum and antirostrum are prominent convex projections deeply separated by a very open exisura that rises from a sharp notch. In the posterior margin, the middle part is a noticeable convex process projected beyond the postrostrum and pararostrum, which are inconspicuous convex projections.
Table 2 summarizes the measurements and proportion observed in the left sagittal otolith obtained in specimen IGM 13119. This inner ear stone has 1237.1 μm of total length. Its ratio total length/maximum height is 1.36 (the maximum height is 73.3% of its total length). Its ratio ventral length/maximum heigh is 1.01 (the ventral length is 74.3% of its total length). Its relative dorsal length (DL/VL) is 98.5. Its relative medial length (ML/VL) is 1.08. Its relative antirostrum height (AH/MH) and length (AL/VL) are 0.385 and 0.099, respectively. Its relative rostrum height (RH/MH) and length (RL/VL) are 0.401 and 0.263, respectively. The exisura opens about 90°, and the posterior and postventral angles are 60 and 128°, respectively.
Circumorbital series. The circumorbital series is highly reduced and only consists of the lacrimal and dermosphenotic bones. The lacrimal bone is somewhat rectangular, about twice higher as long (Figure 5); unfortunately, none of the specimens studied here has a lacrimal that clearly shows the trajectory or the number of pores of the preorbital sensory canal. The dermosphenotic is smooth and thickened tubular bone, ventrodorsally projected, which ends with a blunt tip and borders the dorsoposterior region of the orbit. The dermosphenotic is hollow, carries the postorbital branch of the supraorbital sensory canal, and has at least four pores of that sensory canal (Figure 6).
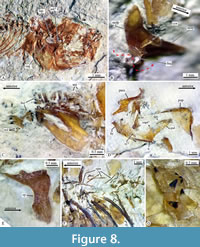
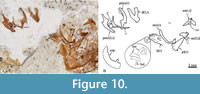 Upper jaw. Each limb of this jaw consists of the premaxilla and the maxilla. In lateral view, the premaxilla is a sinuous bone divided into two parts. The anterior half of this bone corresponds to its toothed section, a stout bar, homogenously high, and ventrally and medially curved, in which the proximal end becomes thick, dorsally expanded, and forms a straight inter-premaxillary articular surface and a short triangular ascending process. In contrast, the untoothed section of the premaxilla is a rhomboidal-like expanded structure that includes a rectangular and flat ventral process, a short dorsal spine posteriorly projected, and a straight posterior process (Figure 6, Figure 8D, Figure 10).
Upper jaw. Each limb of this jaw consists of the premaxilla and the maxilla. In lateral view, the premaxilla is a sinuous bone divided into two parts. The anterior half of this bone corresponds to its toothed section, a stout bar, homogenously high, and ventrally and medially curved, in which the proximal end becomes thick, dorsally expanded, and forms a straight inter-premaxillary articular surface and a short triangular ascending process. In contrast, the untoothed section of the premaxilla is a rhomboidal-like expanded structure that includes a rectangular and flat ventral process, a short dorsal spine posteriorly projected, and a straight posterior process (Figure 6, Figure 8D, Figure 10).
The maxilla is a long, curved, untoothed bone (Figure 6A-B, Figure 7, Figure 10). In this bone, the anterior third is cylindrical, medially bent, and ends in an expanded rounded structure with two globular dorsal processes and an elongated triangular dorsal process with an acute tip. The posterior half of this bone becomes straight, flat, and broader.
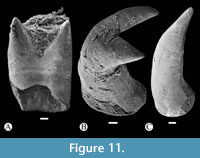 In toothed elements of jaws are the premaxilla in the upper jaw and dentary in the lower jaw; these bones show two kinds of teeth, bicuspid or bifid sharp teeth and unicuspid acute teeth (Figure 6, Figure 8, Figure 10). The toothed surface of the premaxilla is broad and shows a labial or principal tooth row that consists of eight or nine bicuspid teeth that are long, thick, and have a stake-like base co-ossified with the bone. In these bifid teeth, the base is ovoid, expanded, and laterally compressed; the middle part is progressively thinner; and the terminal end becomes labio-lingually flat, broader, and divided into two symmetrical acute triangular tips with lateral slightly curved edges (Figure 11). Additionally, numerous secondary teeth are in a lingual position; these are conic, unicuspid, smaller, and shorter. Although many teeth of the principal row have the tips broken, their bases are with the dentary and premaxillary (Figure 8). Secondary teeth appear to be just tightly attached to the surface of the bone.
In toothed elements of jaws are the premaxilla in the upper jaw and dentary in the lower jaw; these bones show two kinds of teeth, bicuspid or bifid sharp teeth and unicuspid acute teeth (Figure 6, Figure 8, Figure 10). The toothed surface of the premaxilla is broad and shows a labial or principal tooth row that consists of eight or nine bicuspid teeth that are long, thick, and have a stake-like base co-ossified with the bone. In these bifid teeth, the base is ovoid, expanded, and laterally compressed; the middle part is progressively thinner; and the terminal end becomes labio-lingually flat, broader, and divided into two symmetrical acute triangular tips with lateral slightly curved edges (Figure 11). Additionally, numerous secondary teeth are in a lingual position; these are conic, unicuspid, smaller, and shorter. Although many teeth of the principal row have the tips broken, their bases are with the dentary and premaxillary (Figure 8). Secondary teeth appear to be just tightly attached to the surface of the bone.
Lower jaw. Each limb of the lower jaw consists of four bones, the dentary, anguloarticular, retroarticular, and coronomeckelian. The dentary is a trapezoidal bone in which the coronoid process is absent, and the posterior end is expanded and deeply bifid. In this bone, the anterior and ventral edges bend medially, the ventral edge is notched, and the symphysis is straight and high (Figure 6A, Figure 8B-D, Figure 10). Here, the toothed border is straight and extended about the anterior two-thirds of this bone. The mandibular sensory canal runs alongside the ventral edge of the dentary and opens through at least four large pores; among these, the second and third pores are the most extensive and form long and wide grooves.
The anguloarticular is a small boomerang-like structure that shows limbs slightly differently shaped, forming an interior angle somewhat obtuse. This bone has a small articular process protruding in the base of its posterior edge and has a trapezoidal ventral process near its rear that sutures with the anterior border of the retroarticular (Figure 6A-C, Figure 8B, Figure 10). Here, the anterior and dorsal processes form an interior angle of about 110-120°; the anterior process has straight edges, shows an acute tip, and is about 1.3 longer, whereas the dorsal process shows somewhat convex edges and a rounded tip. This bone does not carry the mandibular sensory canal. At the base of the retroarticular articular process, the anguloarticular and retroarticular forms part of the articular facet for the quadrate. This facet is a shallow and ovoidal depression with a donut-like configuration, in which the retroarticular's dorsal surface forms the central region of such facet, while the anguloarticular has two curved or C-shaped processes forming the surrounding part of this articular surface. The tips of these surrounding processes extend almost to join at the back of the retroarticular (Figure 6A-C, Figure 8B, Figure 10).
The retroarticular is a pyramidal and stout bone (Figure 6). This bone forms the ventroposterior end of the lower jaw because it is attached laterally between the articular and ventral processes of the anguloarticular bone. In most of the specimens studied here, the retroarticular and anguloarticular are disarticulated, suggesting that these bones showed cartilaginous sutures in life. The coronomeckelian is a small, flat, untoothed, and comma-shaped bone medially attached to the anterior process near the interior angle of the anguloarticular (Figure 6, Figure 8B, Figure 10).
The toothed surface of the dentary shows teeth similarly shaped and ordered as those described in the premaxilla (Figure 6A-C, Figure 8B, Figure 10, Figure 11). This bone also has two kinds of teeth; six or seven long bicuspid teeth, thick and stake-like based along the labial or principal row, and an indeterminate number of smaller unicuspid teeth forming a lingual or secondary tooth patch (Figure 8C, Figure 10, Figure 11).
Suspensorium. IGM 13123 shows the bones of the suspensorium series complete and articulated (Figure 8D). This comprises the palatine, quadrate, hyomandibula, symplectic, and endopterygoid. These bones are ordered, forming an U-shaped structure. The metapterygoid and ectopterygoid bones are absent. Here, the palatoquadrate arch, formed on the anterior edge and between the heads of the quadrate and palatine, is straight and strongly tilted vertically. The palatine is a triangular laminar bone, about two times higher than long, smooth, with an imbricated suture for the quadrate and endopterygoid. The palatine articular head is narrow and has a small dorsal process protruding anterolaterally (Figure 6A, Figure 8D).
The quadrate bone is a triangular bone. Its posterior edge is somewhat concave, whereas its ventral and anterior edges are relatively straight, form an obtuse angle close to 115°, and join the base of the mandibular articular head. This head is robust, slightly protuberant forward, and bicondylar, in which the medial condyle is larger than the lateral one. The incisura for the symplectic is broad and deep and separates the laminar body of this bone from its posterior process, which is markedly thicker and has a sharp tip.
The symplectic is a long triangular bone, anteriorly acute, posteriorly expanded, and dorsoposteriorly projected (Figure 6A). It is almost two times longer than the quadrate and joins at the base of the hyomandibular. The endopterygoid [also identified as mesopterygoid by Kobelkowsky (2005, fig. 2) and ectopterygoid by Smith (1980, fig. 15)] is a weak laminar and oblong bone, ventrally expanded, and dorsally tapered. The posteroventral part of the endopterygoid occupies the quadrate's posterior notch, and its anterodorsal end is attached to the base of the rear edge of the palatine. The hyomandibular is an axe-shaped bone with a short handle, an expanded rectangular body, and two long articular facets (Figure 8D). The opercular process barely projects near the middle of the posterior edge of the hyomandibula.
Opercular bones. This bone series includes the opercle, preopercle, subopercle, and interopercle (Figure 6). The opercle is a triangular bone laminar, slightly longer than high, and superficially convex; in which the dorsal edge is slightly concave; the anterior border is straight and thick; and the posterodorsal end becomes narrow, somewhat elongated, and rounded. Anterodorsally, the opercle exhibits a short rectangular articular process (= preopercular arm) that protrudes anterodorsally and forms an obtuse angle of about 130-140° with the anterior edge of the bone. On the contrary, in the medial surface of this bone, near its anterior border and at the base of its articular facet (=opercular facet), is a shallow and semispherical concavity that occupies the center of a superficial and oblong structure that protrudes medially.
The preopercle shows the typical inverted L-shape with the horizontal and vertical limbs similarly shaped, forming an angle of about 90° plus a well-developed medial shelf, uniformly expanded, and a rounded anterior edge (Figure 6A, Figure 8D). Alongside the ventral and posterior edge, this bone displays a deep groove roofed by a thin laminar shelf, in which four or five pores of the preopercular sensory canal open, three of them in the horizontal limb and the others in the vertical one (these pores are visible under the microscope with the specimen wet with alcohol).
The subopercle is a sickle-shaped laminar bone with an anterior ascending process that is small, thorn-like, and subterminal (Figure 6, Figure 10). This bone and the posteroventral edge of the opercle are equally long. In the subopercle, the anterior border is high and convex, the dorsal and ventral edges are harmoniously curved, and the posterior end is acute. The interopercle is a long triangular bone, slightly curved dorsally, rounded posteriorly, and sharp anteriorly (Figure 6). The preopercle covers a large part of the interopercle; however, its anterior tip is so long that it almost reaches the articular head of the quadrate.
Hyoid arch and branchial apparatus. IGM 13127 shows the bones of the hyoid arch and branchial apparatus (Figure 6A, D). The ventral hypohyal is a solid pyramidal structure anteriorly pointed. Behind this, the anterior ceratohyal is an unpierced rectangular bone, lateromedially flattened, slightly constrained in its anterior half, and expanded in its anterior and posterior ends. The posterior ceratohyal is a solid triangular bone, probably equally longer than high. The anterior ceratohyal is anteriorly and posteriorly tightly attached to the respective ventral hypohyal and posterior ceratohyal. The urohyal is a long bone with a slight anterior articular head plus two triangular longitudinal wings posteriorly projected; the horizontal one forms the ventral edge of the bone, and the vertical one projects dorsally.
Although sometimes poorly preserved, in this fish, there are always five pairs of branchiostegal rays (Figure 6A, D). The shape of these laminar, long, and curved bones varies in anteroposterior order, from thread-like to saber-like structures. Among these bones, the anterior four are proximally joining the anterior ceratohyal, while the posterior one does this with the posterior ceratohyal.
Numerous elements of the branchial apparatus are obscured or poorly preserved in the fossils studied here. In IGM 00024 (Figure 6A, D), the basihyal is a long bone, trapezoidal, somewhat slender and expanded anteriorly, and has a small posterior articular head. The same specimen also shows a minute thorn-like interhyal bone; however, this does not preserve the basibranchial bones that once separated the basihyal and interhyal bones.
Other bones of the branchial apparatus, such as the hypobranchials, ceratobranchials, epibranchials, pharyngobranchials, and gill rakers, seem present in different specimens studied herein; however, these are so cracked and incomplete that their identification and description are difficult. These include at least a lower and an upper pair of expanded gracile-toothed pharyngobranchial plates and associated posterior branchial bones. In these plates, the teeth are conic, very robust, and irregularly sized, with the tip curved backward and implanted in shallow sockets (Figure 8G). Although these teeth are usually unattached and dispersed between the medial surfaces of the opercular bones, those placed in the middle are so high that they equal or exceed the thickness of such plates.
Axial skeleton. The numbers of centra and ribs vary slightly in males and females (Table 1). In males, the vertebral column consists of 34-35 total centra, including 15 abdominals and 20 caudals. On the contrary, females have 31-32 total centra, 13-14 abdominals centra, and 17-89 caudals. The rib pairs are 15 in males and 12-13 in females. All the centra are autogenous, but the last three fuse into the caudal complex (Figure 14).
All centra are hollow, deep amphycelic, and hourglass-shaped; these are constrained in the middle, equally expanded anterior and posterior, and show circular intervertebral surfaces. Although it is difficult to determine if most anterior abdominal vertebrae have unfused neural arches, all the other centra fuse with the respective hemal and neural arches. At least four abdominal centra (probably the 2 to 5) show neural crests expanded, rectangular, and laminar. The ribs are long, thin, and slightly curved, enclosing almost the entire abdominal cavity; these join the dorsal surface of the paraphyses (= transverse processes in Kobelkowsky, 2005, figure 4), which are straight, long spiny-like structures projected laterally from the dorsal part of the centra, that show a lateromedial groove in its dorsal surface. At least, the anterior half of the ribs are associated with thin epineural bones, which are about two centra long and lie horizontally (Figure 8F).
Pectoral fin and girdle. The pectoral fin is rounded and has 12 rays in males and 13-14 in females (Table 1). These rays are distally branched and segmented, uniformly thin, and extend, covering the anterior third of the abdominal region. At least four rectangular radials support this fin.
The posttemporal is a long triradial bone (Figure 6A). This bone is oblong, thin, short, and posteroventrally projected. In contrast, its anteroventral process is small and triangular, while its anterodorsal limb is a bar-like structure, uniformly wide, curved dorsoventrally, slightly flattened, and about four times longer than the anteroventral process. The supracleithrum is a flat bone, short and drop-like, that rests above the dorsoposterior end of the cleithrum. Since the highly compressed preservation of the specimens studied here, only the lateral surface of the cleithrum is identifiable; this thin bone is crescent- shaped and bears a laminar posterior extension with a slightly curved edge. Two postcleithra are present; the first is an ovoid plate in the rear corner of the cleithrum, and the other is a sinuous rod-like structure projected ventrally. The posterior wing of the cleithrum covers a large part of the scapula; however, its rear surface is exposed, showing its articulation with the pelvic radials. Otherwise, only the lateral surface of the coracoid appears as an elongated laminar and triangular structure that is posteriorly expanded and has a ventroposterior end convex, with a posterior margin vertically tilted and anteriorly limited by a narrow and deep notch (Figure 6A).
 Pelvic fin and girdle. The pelvic fin is entirely opposed to the predorsal region of the trunk. It rises in the posterior half of the body, at 57.3 SL in males and 64.8% of SL in females. The posterior edge of the fin does not reach the origin of the dorsal fin. This small and rounded fin consists of six pelvic rays that are distally segmented, branched, and have bifid articular heads (Table 1, Figure 12). There are no pelvic radials.
Pelvic fin and girdle. The pelvic fin is entirely opposed to the predorsal region of the trunk. It rises in the posterior half of the body, at 57.3 SL in males and 64.8% of SL in females. The posterior edge of the fin does not reach the origin of the dorsal fin. This small and rounded fin consists of six pelvic rays that are distally segmented, branched, and have bifid articular heads (Table 1, Figure 12). There are no pelvic radials.
The pelvic bone is triangular, about two times longer than wide, laterally and medially almost straight, and anteriorly acute. The dorsal surface of this bone bears a middle thickening bar or keel. The posterior edge of the pelvic bone is concave and shows small facets to join the pelvic rays. In small specimens, this bone shows a shallow posterior notch and three processes relatively small, including an inconspicuous lateral process, a small and rounded medial process, and a stout triangular ischial process (Figure 12A). On the contrary, the pelvic bone of larger fishes has a posterior notch conspicuous, a lateral process wider and rectangular, and a long thick medial process that is somewhat sinuous (Figure 12B).
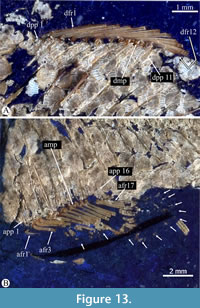 Dorsal fin. The dorsal fin is rounded, long, and about equally high than long (Figure 13A). The number of rays from this fin is sexually variable; males have 15 dorsal rays, while females show only 12. The dorsal rays are uniformly thin and distally branched and segmented. Here, the first three and last rays are comparatively smaller than the others.
Dorsal fin. The dorsal fin is rounded, long, and about equally high than long (Figure 13A). The number of rays from this fin is sexually variable; males have 15 dorsal rays, while females show only 12. The dorsal rays are uniformly thin and distally branched and segmented. Here, the first three and last rays are comparatively smaller than the others.
A series of long and straight dorsal proximal pterygiophores support the dorsal fin rays in a numerical relation 1:1. The series has no distal pterygiophores but includes small square-like medial dorsal pterygiophores in at least three anterior quarters of the dorsal fin. Along the series, the size of the proximal dorsal pterygiophores is almost the same and are so long that they penetrate the interneural spaces present immediately below.
Anal fin. The anal fin is high, long, and rounded; in general, this is a little smaller and located slightly behind the dorsal fin (Figure 13B, Table 1). This fin comprises around 16 rays (between 15 and 18) in both sexes; the first ray is rudimentary. In males, the subsequent six rays form the andropodium; these rays are segmented, unbranched, shorter than the posteriors, and crowded together. Due to the difference in size, the andropodial rays are separated from the subsequent by a small notch. Posterior anal rays are distally segmented, branched, and at least 20 % longer than the last andropoidal rays. The rear three anal rays decrease in length progressively.
A series of proximal pterygiophores internally support the anal fin. These rod-like bones are straight, and so elongated that they penetrate the interhemal spaces. In extreme cases, these proximal pterygiophores cover three-quarters of the distance between the vertebral column and the base of the fin (Figure 13B). The numerical relation between anal rays and proximal pterygiophores is 1:1, except for the first pterygiophore that joins the first two anal rays. There are no distal pterygiophores. The anterior medial pterygiophores are absent or probably fused with the articular head of the anterior seven proximal pterygiophores, which are close-set, mainly the first five; therefore, these proximal pterygiophores are the only ones supporting the andropodial rays. Beyond, a robust rectangular medial pterygiophore is present between the posterior proximal pterygiophores.
 Caudal fin. The caudal skeleton involves the neural and hemal spines of the preural 1-3 plus those bones associated with the ural centra (Figure 14). These spines become thicker, broader, and slightly less tilted than those of previous centra. The parhypural and the hemal spines of preural centra 2 and 3 are so broad that they are in contact. The parhypural has a wide proximal tip, probably fused with preural 1. A single wide epural fills the space between the most posterior neural spine and the ural plate. As in other cyprinodontiforms, the end of the vertebral column, the caudal complex involves the fusion of the preural 1, urals 1, and an undetermined number of hypurals (see Thieme et al., 2021). The hypurals form a fan structure with a long conspicuous interhypural foramen longitudinally bordered by two shallow ridges. Near its base, this caudal complex shows three small processes; the urostyle is a small and rectangular anterordorsal process that is projected upward and seems to embrace the anterior tip of the epural; in the base of the hypural plate, there is a small spine-shaped process and a keel-shaped process laterally expanded in dorsal and ventral position, respectively.
Caudal fin. The caudal skeleton involves the neural and hemal spines of the preural 1-3 plus those bones associated with the ural centra (Figure 14). These spines become thicker, broader, and slightly less tilted than those of previous centra. The parhypural and the hemal spines of preural centra 2 and 3 are so broad that they are in contact. The parhypural has a wide proximal tip, probably fused with preural 1. A single wide epural fills the space between the most posterior neural spine and the ural plate. As in other cyprinodontiforms, the end of the vertebral column, the caudal complex involves the fusion of the preural 1, urals 1, and an undetermined number of hypurals (see Thieme et al., 2021). The hypurals form a fan structure with a long conspicuous interhypural foramen longitudinally bordered by two shallow ridges. Near its base, this caudal complex shows three small processes; the urostyle is a small and rectangular anterordorsal process that is projected upward and seems to embrace the anterior tip of the epural; in the base of the hypural plate, there is a small spine-shaped process and a keel-shaped process laterally expanded in dorsal and ventral position, respectively.
The caudal fin is rounded and posteriorly expanded. Its dorsal and ventral lobes form a harmonious convex profile, while the ventral and dorsal edges of the fin are straight. This fin is relatively short, and its length is about 1.2 times the postanal length of the trunk. Regarding those caudal rays anteriorly associated with the caudal complex, the caudal formula is vi+ I+9—8+I+v. In both lobes, the procurrent rays become longer and thicker in anterior to posterior order.
Scales. The scales cover the whole body, head, jaws, and caudal skeleton 8 (Figure 3, Figure 4, Figure Figure 5, Figure 8F, Figure 13, Figure 14). These are cycloid scales, rounded to slightly ovoid, in which the center half is smooth. The surrounding scale surface has 4-9 concentric circuli that are sinuous, unbranched, and evenly separated. Additionally, most scales covering the anterior third of the trunk have 3-12 straight, horizontal, and parallel radii. In small specimens, only a few scales of the middle part of the trunk show radii, while in larger fishes, the radii are present in more scales. Regardless of the size of the specimens, the scales on the head and jaw have no radii. On the trunk, scales are evenly sized; however, those on the occiput and the head become smaller. Middle longitudinal scale lines on the skull extend symmetrically on each side up to the frontal-mesethmoid contact. Nine to 10 scale rows probably cover the height of the trunk, and about 32 rows extend above the vertebral column between the head and the anterior tips of the caudal fin rays.
Coloration pattern. UV light allows us to recognize some details of the color pattern of Paleocharacodon guzmanae gen. and sp. nov., which are invisible under white light. IGM 13117 shows 14 wide dark color spots covering the posterior surface of the abdominal cavity, four behind the pectoral girdle and over the first vertebrae, and at least another five or six (barely observable in the specimen) forming a longitudinal row above the vertebral column, between the pectoral girdle to the middle part of the anal fin. Additionally, a large dark color spot is present along the basal half of the anal fin (Figure 3).
Such spots of color are not present in all the fishes of Sanctórum, probably because the multiple taphonomic factors that controlled and allowed their conservation did not affect all individuals uniformly. Otherwise, the rare preservation of these color spots could respond to the fact that, when collected, many of these fish open in half and therefore do not show their exterior surface details.
Reproduction. Skeletal remains attributable to unborn small fishes preserved in the abdominal cavity of some of the females, as IGM 13117 and IGM 13125 (Figure 5B), evidence the viviparity of Paleocharacodon guzmanae gen. and sp. nov. Although it is challenging to identify complete individuals in these remains, here, we rule out the possibility that these remains represent stomach contents because these do not show any signs of decay by digestion.
DISCUSSION
 For almost 150 years, at the same time as the knowledge of the taxonomic diversity of fishes now included in the family Goodeidae increased, different authors discussed their possible interrelationships under both pre and post-Hennigan considerations (Figure 15). Unfortunately, in most of these efforts, the evidence considered is non-osteological, with unverifiable attributes in fossil specimens. These first studies only considered general phenotypic body traits and soft structures, particularly those linked to their reproductive strategies (Hubbs and Turner, 1939; Gosline, 1949; Fitzsimons, 1981). Subsequently, the phylogenetic studies carried out in the last 25 years involved molecular data of alloenzymes and different genetic materials, show somewhat contradictory results (Grudzien et al., 1992; Webb, 1998; Doadrio and Domínguez, 2004; Domínguez-Domínguez, 2010; Parker, 2017; Parker et al., 2019). Today, determining the taxonomic identity and the phylogenetic relationships of fossil goodeids, such as Paleocharacodon guzmanae gen. and sp. nov. and Tapatia occidentalis, are complex tasks because the osteological features of these extinct fishes have been unnoticed in such studies. Therefore, the present authors have begun an osteological descriptive and comparative analysis of members of the family Goodeidae, which now gathers about 21 genera and 50 species (Table 3), an effort that will still take us several years. Despite this restrictive scenario, those osteological features of fossils and extant goodeids previously discussed by different authors (e.g., Miller and Fitzsimons, 1971; Smith, 1980; Parenti, 1981; Webb, 1998), together with the results of our observations on recent specimens, are used here to recognize the taxonomical peculiarity and affinities of P. guzmanae.
For almost 150 years, at the same time as the knowledge of the taxonomic diversity of fishes now included in the family Goodeidae increased, different authors discussed their possible interrelationships under both pre and post-Hennigan considerations (Figure 15). Unfortunately, in most of these efforts, the evidence considered is non-osteological, with unverifiable attributes in fossil specimens. These first studies only considered general phenotypic body traits and soft structures, particularly those linked to their reproductive strategies (Hubbs and Turner, 1939; Gosline, 1949; Fitzsimons, 1981). Subsequently, the phylogenetic studies carried out in the last 25 years involved molecular data of alloenzymes and different genetic materials, show somewhat contradictory results (Grudzien et al., 1992; Webb, 1998; Doadrio and Domínguez, 2004; Domínguez-Domínguez, 2010; Parker, 2017; Parker et al., 2019). Today, determining the taxonomic identity and the phylogenetic relationships of fossil goodeids, such as Paleocharacodon guzmanae gen. and sp. nov. and Tapatia occidentalis, are complex tasks because the osteological features of these extinct fishes have been unnoticed in such studies. Therefore, the present authors have begun an osteological descriptive and comparative analysis of members of the family Goodeidae, which now gathers about 21 genera and 50 species (Table 3), an effort that will still take us several years. Despite this restrictive scenario, those osteological features of fossils and extant goodeids previously discussed by different authors (e.g., Miller and Fitzsimons, 1971; Smith, 1980; Parenti, 1981; Webb, 1998), together with the results of our observations on recent specimens, are used here to recognize the taxonomical peculiarity and affinities of P. guzmanae.
Webb (1998) performed a phylogenetic study of Goodeidae, combining molecular and morphological data. He identified five Goodeinae tribes, including Characodontini (Characodon), Goodiini (Goodea and Ataenobius), Ilyodontini (Ilyodon, Allodontichthys, and Xenotaenia), Girardichthyini (Girardinichthys, Allotoca, and Hubbsina), and Chapalichthyini (Chapalichthys, Allophorus, Ameca, Xenotoca, Xenoophorus, and Xanotaenia) (Figure 15); additionally, in his hypotheses, Skiffia and Zoogoneticus are like jumping-joker cards because their relationships are problematic. Currently, such tribe names are used even though, in the most recent hypotheses (Figure 15), the groups obtained are not always those of Webb’s. In this manuscript, we consider these tribe names.
Paleocharacodon guzmanae, a New Goodeidae
Smith (1980) divided Goodeidae into two large groups. His Characodon-group shares two features, the anguloarticular bone (=articular-angular) has a dorsal process projected almost vertically, forming a nearly right angle with the anterior process, and the articular head of the quadrate is comparatively short and hardly protrudes from the anterior edge of the palatopterygoid complex. On the contrary, in his Goodea-group, the anguloarticular has a dorsal process strongly projected backward beyond the articular process and forming an obtuse angle with the anterior process; additionally, here, the articular head of the quadrate is comparatively longer and projects in advance of the anterior edge of the palatopterygoid complex (Smith, 1980, figures 16, 17). Although Smith (1980) considered Ameca, Hubbsina, and Tapatia occidentalis as goodeids of uncertain position, the subsequent reviews of these taxa reveal that their anguloarticulars have a vertical dorsal process (Guzmán 2010, figure 4b; Webb, 1998), suggesting that these may form part of his Characodon-group.
In this context, it is impossible to include Paleocharacodon guzmanae gen. and sp. nov. in any of the goodeid groups proposed by Smith (1980). This Mexican fossil fish shows the condition of the anguloarticular described in the Characodon-group, as well as the quadrate having an articular head comparatively long and protruding forward as in the Goodea-group (Figure 6, Figure 8D). Additionally, Tapatia occidentalis and P. guzmanae differ in the shape of the anguloarticular and jaw teeth. In T. occidentalis, the length of the anguloarticular anterior process is at least 2.5 times the height of its dorsal process. The teeth in the frontal rows of jaws show a bicuspid or bifid shape, end in two square lobes loosely separated, and are distally truncated or straight (Guzmán, 2010, figures 4b, 5 c”). On the contrary, in P. guzmanae, the proportions of the anguloarticular are less contrasting. That length is just about 1.3 times such height, and the teeth of principal rows have bicuspid and sharp ends (Figure 6, Figure 10, Figure 11).
Also, Paleocharacodon guzmanae differs from Hubbsina and Ameca in the size and position of the dorsal fin. In P. guzmanae, the dorsal fin is short, has 12-15 rays, and is entirely opposed to the anal fin (Table 1, Figure 13). Although the dorsal fin in Ameca is short and has only 13-14 rays, in Hubssina, this fin is peculiarly long and consists of 29-37 rays; in both cases, the dorsal fin is placed more to the front and opposes the anal fin only in part (De Buen, 1940; Miller and Fitzsimons, 1971; Domínguez-Domínguez et al., 2005, p. 540, 552). As a result, P. guzmanae is considered a new genus and species.
Unfortunately, after the efforts of Parenti (1981) and Webb (1998), who included some osteological features to define the naturalness of the family Goodeidae and recognize its interrelations under the cladistic scope (Figure 15), this type of data ceased to be considered in phylogenetic assays. Therefore, under these conditions, it is necessary to analyze, recognize and assess the possible osteological differences between these fishes, a task already in progress that will take a few more years to be helpful. In this paper, we explore the affinities of Paleocharacodon guzmanae gen. and sp. nov. within Goodeidae, analyzing the distribution of its osteological features along the branches of those hypotheses proposed by these authors. Table 4 summarizes the comparative analysis of P. guzmanae and other extant and extinct goodeid taxa.
In the restructuring of Goodeidae suggested by Parenti (1981, p. 515-516) to include the subfamilies Goodeinae and Empetrichtyinae, she noted that these fishes share four diagnostic features (1-4 below and in Figure 15). Later, Webb (1998) discovered other distinctive features of the family (5-7 in this paragraph and Figure 15); however, among these features, only two are synapomorphies (3 and 5). In this context, Paleocharacodon guzmanae gen. and sp. nov. is an unquestionable member of Goodeidae because it shows six of these diagnostic features (Figure 15):
1) In the anal fin, the most anterior 2 to 7 medial pterygiophores (= middle radials) are absent as autogenous bones because these are fused with the respective proximal pterygiophores (= proximal radials of Parenti, 1981, figure 83, node A) (Figure 13).
2) The dorsal process of the maxilla is strongly reduced (Parenti, 1981, figure 83, node A) (Figure 6, Figure 8D).
3) The distal arm of the premaxilla is straight (Smith, 1980; Parenti, 1981, figures 40C, 83 node A) (Figure 6A-B; Figure 8D); according to Webb (1998, ch. 649), this is a synapomorphy.
4) The anguloarticular (=articular in Parenti, 1981, p. 406, figures 33B, C, D, and node A in figure 83) is significantly reduced, and its medial extension does not carry the mandibular sensory canal.
5) In lateral view, the lacrimal bone is somewhat rectangular and at least twice as higher as long; according to Webb (1998, figures III.22B, ch. 658) is a synapomorphy (Figure 5).
6) The ascending process of the premaxilla is small or reduced (Parenti, 1981, figure 39C; Webb, 1998, ch. 650) (Figure 6). In goodeids, in dorsal view, the pterotic (=autopterotic) bones show a narrow autopterotic fossa
7) in Figure 15), in which the otic sensory canal runs (Uyeno and Miller, 1962; Parenti, 1981; Webb, 1998, figures III.12B, ch. 641); unfortunately, any of the specimens of P. guzmanae herein studied show that fossa.
Paleocharacodon guzmanae, a New Goodeinae
Paleocharacodon guzmanae gen. and sp. nov. is also an undeniable member of Goodeinae because it was a viviparous species and shows both the homoplasy and synapomorphy that support this subfamily (8 and 9 in Figure 15, Table 4). Following the numbering of the anterior paragraph, these features include:
8) The anterior anal fin ray is rudimentary (Parenti, 1981, figure 83, node B) (Figure 13).
9) The andropodium or pseudophallus is present and formed by the unbranched anterior anal-fin rays that crowd together (Figure 13), separated by a notch from the rest of the fin, and reduced between 50-80% concerning the subsequent posterior rays; Webb (1998, p. 75, ch. 684 in part) discovered this remarkable osteological synapomorphy. Additionally, Webb (1998, p. 61 and 92, ch. 637) suggested that the appearance of a shallow neurocranium (in which the height is equal to or less than half of its width) also supports the Goodeinae monophyly; however, this condition may represent a plesiomorphy because it is present in Empetrichthyinae and other Cyprinodontiformes, too.
Eight osteological features support the naturalness of Empetrichthyinae. These include four synapomorphies (11-18 in Figure 15, Table 4), previously noticed by Parenti (1981) and Webb (1998). Paleocharacodon guzmanae gen. and sp. nov. differ from Empetrichthyinae because it does not have any of its diagnostic features. Following the numbering of the anterior paragraphs, these features include:
10) The lack of the pelvic fin and girdle (Parenti,1981, node C in figure 83; Webb, 1998, ch. 673). On the contrary, the pelvic fin and girdle are well-developed in P. guzmanae (Figure 12).
11) The epibranchial 1 is nearly Y-shaped, notched at its base, and has a lateral process (Parenti, 1981, node C in figure 83 and figure 47B; Webb, 1998, ch. 668). Unfortunately, the epibranchials are unknown in P. guzmanae, but among goodeines this bone is rod-shaped.
12) The surface of neurocranial, suspensorium, and hypural bones are rugose and irregular due to pits and ridges (Webb, 1998, ch. 643). On the contrary, these bones are superficially smooth in P. guzmanae (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8D, Figure 14).
13) The anterior margin of the vomer is abruptly concave medially (Uyeno and Miller, 1962; Webb, 1998, figure III.9C, ch. 629). Sadly, the vomer is unknown in P. guzmanae.
14 and 15). The frontal has a supraorbital canal partially ossified and does not show the break 4a-4b (Gosline, 1949; Fitzsimons, 1981; Webb, 1998, chs. 632 and 634) (Figure 15). On the contrary, as in other goodeines, in P. guzmanae, the frontal bone entirely encloses the supraorbital canal that shows the break 4a-4b (Figure 7).
16) The dorsal and ventral pharyngobranchials are relatively robust; their bases are deep, ossified, and higher than their teeth (Webb, 1998, ch. 667). Although in P. guzmanae, the toothed pharyngobranchial plates are not well-exposed, in this species, such plates are rather gracile and bear large teeth of varying size, usually rest unattached and scattered; here, the most prominent pharyngobranchial teeth are higher than the pharyngobranchial plates.
17) Third postcleithrum with a posteriorly directed laminar plate at its mid-length (Webb, 1998, ch. 672). P. guzmanae has only two postcleithra.
Palaocharacodon guzmanae and Other Goodeines
Paleocharacodon guzmanae gen. and sp. nov., Goodiini, Characodon, Xenotaenia, and some species of Allotoca share a primitive feature; the dorsal fin is far in the back of the body (the predorsal length is equal or greater than 67% of SL). Hence, P. guzmanae differs from Ilyodontini, Girardichthyini (less some species of Allotoca), Chapalichthyini (including Zoogoneticus and less Xenotaenia), and Skiffia that have the dorsal fin located not so far back (the predorsal length is equal or less than 67% of SL)
18) (in Figure 15, Table 4) (Miller, 1948; Webb, 1998, p. 77, ch. 689).
According to Webb (1998, p. 61, ch. 637), all goodeines except Goodiini, Ilyodontini, and Alloophorus show a neurocranium primitively low; in the posterior view, its height/width ratio is 0.45 to 0.52. On the contrary, in other goodeines, the neurocranium shows a higher derived condition, and the range of such proportion is 0.58 to 0.66
19) (in Figure 15, Table 4). This character had a regressive change to the shallow state in Ilyodontini and Alloophorus (19* in Figure 15). Unfortunately, the neurocranial proportions of Paleocharacodon guzmanae gen. and sp. nov. and Tapatia occidentalis are unknown.
Into Goodeidae, only Goodiini and Empetrichthyinae possess rostral cartilages (Webb, 1998, p. 63, ch. 644). Other goodeids have no rostral cartilage
(20 in Figure 15, Table 4). The presence or absence of this cartilage is unknown in Paleocharacodon guzmanae gen. and sp. nov. and Tapatia occidentalis.
According to Webb (1998), Goodea and Ataenobius form the tribe Goodiini. In one of his hypotheses, this tribe is the sister group of other goodeines, while in the other, this constitutes a natural group with Skiffia. Two unambiguous characters, a synapomorphy, and a homoplasy, support this tribe in Webb's hypotheses. The first is the presence of lateral wings in the anterior end of the parasphenoid
21) (in Figure 15 and Table 4; Webb, 1998, p. 61, ch. 638). The homoplasy is the presence of a pelvic fin relatively short because its posterior edge does not reach the dorsal fin origin
22) (in Figure 15 and Table 4; Webb, 1998, p. 72, ch. 675). A short pelvic fin is also present in Characodon and Allotoca (see Domínguez- Domínguez et al., 2005, p. 545, 547). In other goodeids, the parasphenoid has no wings because its anterior lateral edges are parallel, and the pelvic fin is long and goes beyond the dorsal fin origin. Although the pelvic fin of Paleocharacodon guzmanae gen. and sp. nov. is short, it is recognized as non-Goodiini fish because its parasphenoid is not winged.
In Webb’s (1998) hypotheses, the long and well-developed posterior process in the epioccipital bone represents a derived condition of Goodiini and Skiffia
23) (in Figure 15 and Table 4; Webb, 1998, p. 62, ch. 640). These taxa, Ilyodontiini (except Allodontichthys polylepis, A. tamazulae, and A, zonistius), Ameca, and Xenoophorus have 20 to 54 gill rackers on the first branchial arch
24) (in Figure 15 and Table 4); Webb, 1998, p. 70, ch. 665). In other goodeids, the epioccipital process is tiny, and the first branchial arch has only 9 to 19 gill rackers. Unfortunately, Paleocharacodon guzmanae gen. and sp. nov. and Tapatia occidentalis do not expose either of these features.
25 and 26) (in Figure 15 and Table 4; Webb, 1998, chs. 645-647, respectively). The crowns of primary jaw teeth are distally flattened, bicuspid, and straight or broadly blunted, and its bases are loosely attached to the jaws by cartilage in Goodiini, Skiffia, Ilyodon, and Xenoophorus also shows (e.g. Hubbs and Turner, 1939). On the contrary, other goodeids and Paleocharacodon guzmanae gen. and sp. nov. (Figures 8, 10, 11) have primary jaw teeth bicuspid, acute, which bases are co-ossified with the bone of jaws. According to Webb (1998), Allodontichthys shows tricuspid or shouldered teeth and large specimens of Skiffia multipunctata also have strong tooth attachments.
Additionally, Goodiini, Skiffia, and Ilyodon share a derived condition of the anguloarticular bone; its dorsal process projects backward, forming an angle of about 180°
27) (in Figure 15; Smith, 1980, figure 17B; Guzmán, 2010, figure 9; Webb, 1998, p. 66, ch. 654). On the contrary, Paleocharacodon guzmanae gen. and sp. nov. and other goodeids show the alternative primitive condition of this bone; its dorsal process is projected dorsally and forms an angle of about 90° with its anterior process (Figure 6, Figure 8B, Figure 10).
Among goodeids the ventroposterior coracoid region is variable (Webb, 1998, p. 71-72, ch. 671). This region shows a straight angle in Empetrichthyinae, most Goodeiinae, and probably in Tapatia occidentalis; on the contrary, in Skiffia, this is broadly convex
28) (in Figure 15 and Table 4), and in Ilyodon varies between straight and convex. Only in Ameca, Xenotoca variata, and now Paleocharacodon guzmanae gen. and sp. nov, this region is convex, has a posterior margin vertically tilted, and is anteriorly limited by a narrow and deep notch (Figure 6; Webb, 1998, figure III.27). In this case, the condition shared by Ameca, X. variata, and P. guzmanae seem an evolutive convergence.
Furthermore, Webb (1998, p. 67-68, ch. 657) noted that in Goodiini and Skiffia, the palatoquadrate arch (between the articular heads of the palatine and quadrate bones is about vertically inclined
29) (in Figure 15 and Table 4). On the contrary, this arch is bent forward in other goodeids, including Paleocharacodon guzmanae gen. and sp. nov. (Figure 8D).
Likewise, Goodiini, Skiffia, Ilyodon, and Ameca share a homoplasic and derived feature, the retroarticular bone forms the lingual part of the articular facet for the quadrate
30) (in Figure 15 and Table 4; Webb, 1998, p. 67, ch. 655). On the contrary, this bone does not form that facet in other goodeids. In Paleocharacodon guzmanae gen. and sp. nov., that facet has a donut-like configuration in which the retroarticular forms its central region and the anguloarticular has two curved processes forming its surrounding part (Figure 6, Figure 8B). Although this donut-like configuration of the articular facet for the quadrate (added in this work as
character 31 (in Figure 15 and Table 4) was observed only in specimens of Characodon, additional observations are required to clarify its possible occurrence in other goodeids.
Based on Webb’s hypotheses, among goodeids, there is a primitive anterolateral projection in the palatine head of Empetrichthyinae, Goodiini, Ilyiodontini, and most Chapalichthyini (except for Alloophorus, Zoogoneticus, and Skiffia francesae). A convergent derived state of these characters, the lack of such projection in the palatine occurs in Characodontini, Girardinichthyini, Alloophorus, Zoogoneticus, and S. francesae
32) (in Figure 15 and Table 4, Webb, 1998, p. 67, ch. 656). Herein, Paleocharacodon guzmanae gen. and sp. nov. and is excluded from Chacarondontini and Girardinichthyini because it shows the primitive anterolateral projection of the palatine.
Herein, we identify that Paleocharacodon guzmanae gen. sp. nov. and Characodon spp. Differ from each other in at least three different features. In the last, the openings of the supraorbital canal show a formula—1-2a, 2b-7— as in some Allodontichthys species (Fitzsimons, 1981, table 1); its posttemporal is unforked because the anteroventral process is absent as in Empetrichthyinae, Girardichthyini, and Zoogoneticus (33 in Figure 15 and Table 4; Webb, 1998, p. 71, ch. 669); and the parasphenoid bone shows outstanding dorsal wings extended in its orbital part. On the contrary, in the fossil species, the openings of the supraorbital canal show a formula never recorded before among goodeids—1-2a, 2b-3a, 3b-4a, 4b-5a, 5b-7—(Figure 7); its parasphenoid bone shows tiny ventral and lateral wings in its orbital part, (Figure 6); and its posttemporal bone shows a small anteroventral process that may be an intermediate condition observed in other goodeids (Figure 6).
Unfortunately, in his hypotheses, Webb (1998), most no-Goodiini, and no-Characodontini groups do not have osteological supporting features. Therefore, it is not easy to compare the anatomy of such groups with those features observed here in Paleocharacodon guzmanae gen. sp. nov. In the present context, after mapping the osteological features of P. guzmanae into Webb’s hypotheses, it is possible to point out that this fossil is part of the family Goodeidae and the subfamily Goodeinae (it shows the characters 1-6, 8-9 in Figure 15). In addition, the mixture of characters of this fossil goodeid does not allow us to recognize it as part of any of the tribes of Goodeinae. Besides, this fossil goodeid does not share features with the tribes and shows different characters to those that support such tribes or groups of tribes (numbers in italics in Figure 15). On the other hand, this fossil and the tribe Characodontini shared more features (numbers in bold in Figure 15), but it shows significative differences with its single member, Characodon. Therefore, the species described here was included in its genus, Paleocharacodon, to highlight its similarity with Characodon, including a feature observed for the first time, the donut-like articular facet for the quadrate.
Phylogenetic and Biogeographic Implications
Despite numerous recently published hypotheses (Doadrio and Domínguez, 2004; Webb et al., 2004; Domínguez-Domínguez et al., 2010; Foster and Piller, 2018), the phylogenetic and biogeographic relationships of Goodeidae are still unclear (Figure 15). This situation probably results from the lack of suitable fossil evidence that allows us to recognize the history of the morphological changes of Goodeidae, as well as the geological-environmental conditions in which they occurred. The discovery of Paleocharacodon guzmanae gen. and sp. nov. not only complements our knowledge about the diversity of the family, but it also reveals itself as an element to consider in future biogeography studies.
The accurate osteological description of living goodeids is a pending task that avoids the comparison of its extinct and recent species. Despite this, the analysis of Paleocharacodon guzmanae gen. and sp. nov. and some data published of other goodeids reveals that this new fish does not belong to any goodeine tribes. The putative primitive features of P. guzmanae suggest that it may represent a primitive goodeinae. Such observation is congruent since P. guzmanae is a Pliocene species of at least 4 Mya. In any case, assessing the relationships of fossil goodeids, such as P. guzmanae, Goodea-like from Sanctórum, and Tapatia occidentalis requires a comprehensive phylogenetic study involving osteological data of recent and fossil goodeids.
 In the biogeographic hypotheses of Goodeidae so far proposed, Goodeinae arose in the northwestern region of Mexico and had different episodes of diversification in the central part of this country. Such diversification seems more successful in rivers and lakes of the western and southern of the country, which today concentrate most of the known species. Later, some of its genera experienced exchanges between these regions (Gesundheit and Macías, 2005; Domínguez-Domínguez et al., 2006, 2010; Pérez‐Rodríguez et al., 2015, Foster and Piller, 2018; among others). The finding of Paleocharacodon guzmanae gen. and sp. nov. and his contemporary Goodea-like in Sanctórum is significant. The Pliocene strata of the extinct Paleolake Amajac are within the domains of the Pánuco-Salado Basin on the eastern side of Mexico, where there are only six recent species (Table 3, Figure 16). The contrasting diversity of goodeids between the western-southern and eastern regions of Mexico seems linked to the geological-environmental history of the country. On the one hand, during the Neogene, the development of the TMVB strongly and continuously affected its western and southern regions, while the eastern area occupied by the Pánuco-Salado basin was relatively more stable. For now, the uncertain relationships of P. guzmanae within the goodeids hinder the recognition of whether this supports or rejects the biogeographic hypotheses already proposed; however, our study suggests that this extinct species of the eastern slope of Mexico is not directly related to any species from the Pánuco-Salado Basin.
In the biogeographic hypotheses of Goodeidae so far proposed, Goodeinae arose in the northwestern region of Mexico and had different episodes of diversification in the central part of this country. Such diversification seems more successful in rivers and lakes of the western and southern of the country, which today concentrate most of the known species. Later, some of its genera experienced exchanges between these regions (Gesundheit and Macías, 2005; Domínguez-Domínguez et al., 2006, 2010; Pérez‐Rodríguez et al., 2015, Foster and Piller, 2018; among others). The finding of Paleocharacodon guzmanae gen. and sp. nov. and his contemporary Goodea-like in Sanctórum is significant. The Pliocene strata of the extinct Paleolake Amajac are within the domains of the Pánuco-Salado Basin on the eastern side of Mexico, where there are only six recent species (Table 3, Figure 16). The contrasting diversity of goodeids between the western-southern and eastern regions of Mexico seems linked to the geological-environmental history of the country. On the one hand, during the Neogene, the development of the TMVB strongly and continuously affected its western and southern regions, while the eastern area occupied by the Pánuco-Salado basin was relatively more stable. For now, the uncertain relationships of P. guzmanae within the goodeids hinder the recognition of whether this supports or rejects the biogeographic hypotheses already proposed; however, our study suggests that this extinct species of the eastern slope of Mexico is not directly related to any species from the Pánuco-Salado Basin.
Additionally, divergence dates in the Goodeidae evolution are available in the most recent phylogenetic studies (Figure 15); Foster and Piller, 2018; however, the discovery of Paleocharacodon guzmanae gen and sp. nov. suggests that a different goodeinae lineage without living representatives must have existed. According to these data, the family Goodeidae and the subfamilies Goodeinae and Empetrichthyinae rose during the early Miocene, before 15 Mya; in the later 5 My and during the middle Miocene, between 15 and 10 Mya, the goodeines tribes separated; and finally, since then the diversification of these fishes led to the establishment of all genera and species hitherto known. As demonstrated in the previous section, the available evidence from Tapatia occidentalis and P. guzmanae indicates that these Miocene and Pliocene fossil species are part of Goodeinae. However, their inclusion at the tribe level is impossible and may represent unknown members of other lineages.
The situations described in the two previous paragraphs suggest that the biogeographic history of goodeids is more complex than those described so far. Such a process may include at least two alternative early scenarios: A) Early goodeids were widely distributed in Mexico and B) The eastern slope of Mexico received an ancient group of goodeids from the northwest region, subsequently displaced by more recent species.
The geographic distributions of Goodeidae and Profundulidae are disjoint, adjacent, and separated by the Sierra Madre del Sur. The first family inhabits the north, between the southwestern USA and the Mesa Central of Mexico, plus its neighboring regions. At the same time, Profundulidae is found further south, from the Pacific slope of the Sierra Madre del Sur and the Mixtec Basin to Guatemala, El Salvador, and Honduras (Morcillo Alonso, 2004, figure 1). Such distributional patterns respond to the development of TMVB, which began 10-20 million years ago. In this scenario, fossils of Paleocharacodon guzmanae gen. and sp. nov. are geographically nearer to the current distribution of Profundulidae and temporally closer to the origin of TMVB; however, its importance in the effort to understand the separation of these two families is minimal because Profundulidae has no fossil record.
CONCLUSIONS
The review of the Pliocene fish from the Paleolake Amajac deposits in Sanctórum, Hidalgo, Mexico, reveals that these represent two species of the family Goodeidae and subfamily Goodeinae. Here, one is described and named Paleocharacodon gu zmanae gen. and sp. nov.; the other, still poorly known, resembles Goodea (Becerra Martínez, 2003). The principal jaw teeth of P. guzmanae, with bicuspid and symmetrical acute-ends, noticeably differ from those of other Mexican goodeid fossils, Tapatia occidentalis and the Goodea-like of Sanctórum, in which bicuspid teeth have wide spatula-ends. A unique combination of non-exclusive and exclusive features shared with other goodeids supports the singularity of P. guzmanae. These features include the unpaired fins opposed and located far back of the body, behind the 69% of SL; the rudimentary anterior anal ray; the andropodium of males; the strong jaw teeth with distal acute and bicuspid ends; and the donut-like articular facet for the quadrate. The exclusive features of the species include the small anteroventral process of posttemporal and the formula 1-2a, 2b-3a, 3b-4a, 4b-5a, 5b-7 of the supraorbital canal openings.
As its name implies, Paleocharacodon gen. nov. shares some osteological similarities with Characodon; however, the mapping of the osteological features of this fish into the different branches of the phylogenetic hypotheses published by Webb (1998) allows us to recognize that this does not belong to any tribe of the subfamily Goodeinae. Developing a phylogenetic study of Goodeidae based on osteological data, which enables the inclusion of fossil species such as P. guzmanae or Tapatia occidentalis, still requires the accurate characterization of most of the living species of this family. Meanwhile, the inclusion of P. guzmanae in any tribe is unfounded.
The discovery of Paleocharacodon guzmanae gen. nov. in a restricted area within the Great Pánuco-Salado Basin is significant because it will provide new data for future phylogenetic and biogeographic investigations. On the one hand, this new species shares some osteological characteristics (mainly primitive) previously observed in other specimens; however, at the same time, it shows osteological novelties that may represent the key to improving our understanding of the evolution of goodeids. The recovery of this and other fossils in different Miocene-Pleistocene Mexican sites is a tempting invitation to review the skeletal anatomy of the extant and extinct goodeids and seek new evidence of their phylogenetic biogeographic interrelationships. On the other hand, the finding of P. guzmanae and Goodea-like of Sanctórum forces us to reconsider the phylogenetic and biogeographic hypotheses already published. These Mexican fossils encourage us to visualize new possible scenarios where the distribution of extinct goodeids may indicate that the origin or, at least some episodes of the early diversification of this clade, have occurred on the eastern slope of central Mexico, closer to the south of the country, where the sister group of Goodeidae, the family Profundulidae inhabits.
ACKNOWLEDGMENTS
We are deeply indebted to Mrs. E. Liberto Álvarez and her family, inhabitants of Sanctórum and owners of the land of the Sanctórum paleontological site. The UNAM entirely funded this work through the postdoctoral scholarship of DGAPA assigned to CCV and the subventions DGAPA-PAPIIT IN115223 and IN209017 assigned to JAO and KMC. We thank Mr. J.M. Contreras for his work in the obtention of part of the photographs illustrated in this work, Biol. S. Guzmán Gómez of LaNaBio-UNAM, for her technical assistance in photography through the multifocal microscope; Dr. A.M.Reyes Salas and Chem. B.S. Angeles García of the LANGEM-UNAM and Igl-UNAM for their help using and obtention photographs in the SEM; and M. in Sc. D. Arteaga Martínez of LUMIR, Centro de Geociencias-UNAM Juriquilla, for the microtomography images included in this study. We thank G. Arratia and H.P. Shultz from the University of Kansas; Mr. G. Alvarez Reyes and C. Nuñez Alfaro, technicians of the Laboratorio de Paleontología de Vertebrados at the IGl-UNAM; as well as volunteers, undergraduate, and graduate students of both the Facultad de Ciencias and the Posgrado en Ciencias Biológicas of the UNAM, including A. Guadarrama Pérez, B. Carranza Becerra, D.P. Heredia Jiménez, E. Sánchez Fernández, E. Mendoza-Roynosa, J.J. Avedaño Pazos, M. del Pilar Melgarejo Damián. O. Hilario Bautista, P. Moctezuma Ducloud, S. Pacheco Ordaz, and T. Mejía Farfán for their invaluable help during the fieldwork in Sanctórum. Finally, we greatly appreciate the annotations of two anonymous reviewers whose reviews and comments improved this manuscript.
REFERENCES
Aguilar-Arellano, F.J. and Velasco-de León, M.P. 2002. El clima durante el Plioceno en la región de Santa María Amajac, Hidalgo, México. Boletín de la Sociedad Botánica de México, 71:71-81.
Alvarado-Ortega, J. and Carranza-Castañeda, O. 2002a. Los peces fósiles de la región de Tula, Hidalgo, p. 25. In VIII Congreso Nacional de Paleontología. Guadalajara, Jalisco.
Alvarado-Ortega, J. and Castañeda-Carranza, O. 2002b. Evidencias fósiles de la Presencia de un Lago durante el Plioceno en las cercanías de Tula, Hidalgo, p. 304-305. In III Reunión Nacional de Ciencias de la Tierra (IIIRNCT), Puerto Vallarta, Jalisco.
Alvarado-Ortega, J. and Castañeda-Carranza, O. 2002c. The fossil fishes from Pliocene localities near Tula de Allende, Hidalgo, México. Journal of Vertebrate Paleontology, 22 (Supplement to No. 3):32A.
Alvarado-Ortega, J., Carranza Castañeda O., and Álvarez Reyes, G. 2006. A new fossil species of Ictiobus (Teleostei: Catostomidae) from Pliocene lacustrine sediments near Tula de Allende, Hidalgo, México. Journal of Paleontology, 80:993-1008.
https://doi.org/10.1666/0022-3360(2006)80[993:ANFSOI]2.0.CO;2
Álvarez, J. 1959. Contribución al conocimiento del género Neoophorus (Pisc., Goodeidae). Ciencia, México, 19:13-22.
Álvarez, J. 1963. Ictiologia Michoacána. III. Los peces de San Juanico y de Tocumbo. Anales de la Escuela Nacional de Ciencias Biológicas, 12:111-138.
Álvarez, J. and Arriola-Longoria, J. 1972. Primer goodeido fósil procedente del Plioceno jalisciense (Pisces: Teleostomi). Boletín de la Sociedad de Ciencias Naturales de Jalisco, 6:6-15.
Arellano-Gil, J., Velasco-de Léon, P., Silva-Pineda, A., Salvador-Flores, R., and Beltrán-Romero, F. 2005. Origen y características geológicas del paleo-Lago de Amajac, Hidalgo. Revista Mexicana de Ciencias Geológicas, 22:199-211.
Avendaño Pazos, J.J. 2020, Determinación taxonómica de una serpiente fósil del plioceno de Sanctórum, Hidalgo, México. Unpublished Bachelor Thesis, Universidad Nacional Autónoma de México, Ciudad de México, México.
Bean, T. 1887. Descriptions of five new species of fishes sent by Prof. A. Dugès from the Province of Guanajuato, Mexico. Proceeding of the United States National Museum, 10:370-375.
Bean, T. 1892. Notes on fishes collected in Mexico by Prof. Alfredo Dugès, with descriptions of new species. Proceeding of the United States National Museum, 15:283-287.
Bean, T. 1898. Notes on a collection of fishes from Mexico, with description of a new species of Platypoecilus. Proceeding of the United States National Museum, 21:539-542.
Becerra Martínez, C.A. 2003. Estudio anatómico de las aletas impares de los goodeidos fósiles procedentes de Sanctórum (Formación Atotonilco El Grande), Hidalgo. Unpublished Bachelor Thesis, Universidad Nacional Autónoma de México, Distrito Federal, México.
Becerra Martínez, C.A., Guzmán, A.F., and Velasco de León, M.P. 2002. First fossil record of Goodeidae from Hidalgo State, p. 20-21. In Grier, H. and Uribe, M.C. (eds.), Proceedings of the II International Symposium of Live-bearing Fishes. Querétaro, Mexico.
Beltrán-Romero, F. and Luna-Gómez, P. 1994. Estudio geológico de la región de Santa María Amajac, Municipio de Atotonilco el Grande, Estado de Hidalgo. Unpublished Bachelor Thesis, Universidad Nacional Autónoma de México, Distrito Federal, México.
Berg, L.S. 1940. Classification of fishes, both Recent and fossil. Trudy Zoologicheskogo Instituta Akademii Nauk SSSR, 5:85-517.
Bustamante, M. 1837. Cyprinus viviparus (Vulgo) Mexclapique. El Mosaico Mexicano, 2:116.
Costa, W.J.E.M. 1998. Phylogeny and classification of the Cyprinodontiformes (Euteleostei: Atherinomorpha): A reappraisal, p. 519-536. In Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M., and Lucena, C.A.S. (eds.), Phylogeny and Classification of Neotropical Fishes. Edipucrs, Porto Alegre.
De Buen, F. 1940. Lista de los Peces de Agua Dulce de México. En Preparación de su Catálogo. Departamento de la Marina Nacional. Departamento de Pesca e Industrias Marítimas. Estación Limnológica de Pátzcuaro, Pátzcuaro, Michoacán, México.
De Buen, F. 1942. Los peces de agua dulce de la familia Goodeidae. Boletín Biológico. Universidad de Puebla, 2(3):111-148.
Deacon, J.E. and Williams, J.E. 1984. Annotated list of the fishes of Nevada. Proceedings of the Biological Society of Washington, 97(1):103-118.
Dingerkus, G. and Uhler, L.D. 1977. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technology, 52(4):229-232.
https://doi.org/10.3109/10520297709116780
Doadrio, I. and Domínguez, O. 2004. Phylogenetic relationships within the fish family Goodeidae based on cytochrome b sequence data. Molecular Phylogenetics and Evolution, 31(2):416-430.
https://doi.org/10.1016/j.ympev.2003.08.022
Domínguez-Domínguez, O., Bernal-Zuñiga, D.M., and Piller, K.R. 2016. Two new species of the genus Xenotoca Hubbs and Turner, 1939 (Teleostei, Goodeidae) from central-western Mexico. Zootaxa, 4189(1):81-98.
https://doi.org/10.11646/zootaxa.4189.1.3
Domínguez‐Domínguez, O., Doadrio, I., and Pérez‐Ponce de León, G. 2006. Historical biogeography of some river basins in central Mexico evidenced by their goodeine freshwater fishes: a preliminary hypothesis using secondary Brooks parsimony analysis. Journal of Biogeography, 33(8):1437-1447.
https://doi.org/10.1111/j.1365-2699.2006.01526.x
Domínguez-Domínguez, O., Mercado-Silva, N., Lyons, J., Grier, and Harry, J. 2005. The viviparous goodeid species, p. 525-569. In Uribe, M.C. and Grier, H.J. (eds.), Viviparous Fishes. New Life Publications, Mexico.
Domínguez-Domínguez, O., Pedraza-Lara, C., Gurrola-Sánchez, N., Pérez-Rodríguez, R., Israde-Alcántara, I., Garduño-Monroy, V.H., Doadrio, I., Pérez-Ponce de León, G., and Brooks, D.R. 2010. Historical biogeography of the Goodeinae (Cyprinodontiforms), p. 33-74. In Uribe, M.C. and Grier, H.J. (eds.), Viviparous Fishes II. New Life Publications, Mexico.
Domínguez-Domínguez, O., Pérez-Rodríguez, R., and Doadrio, I. 2008. Morphological and genetic comparative analyses of populations of Zoogoneticus quitzeoensis (Cyprinodontiformes: Goodeidae) from Central Mexico, with description of a new species. Revista Mexicana de Biodiversidad, 79:373-383.
Espinosa-Arrubarrena L., Alvarado-Ortega, J., Castañeda Posadas, C., and Núñez Utrilla, J.P. 2009. El registro mexicano del pez fósil Ictiobus, incluyendo un ejemplar casi completo, p. 21. In XI Congreso Nacional de Paleontología, Juriquilla, Queretaro. México.
Fitzsimons, J.M. 1972. A revision of two genera of goodeid fishes (Cyprinodontiformes, Osteichthyes) from the Mexican Plateau. Copeia, 1972(4):728-756.
Fitzsimons, J.M. 1981. Sensory head pores and canals in goodeid fishes. Occasional Papers of the of Natural Science, Louisiana State University, 60(1):1-10.
https://doi.org/10.31390/opmns.060
Foster, K.L. and Piller, K.R. 2018. Disentangling the drivers of diversification in an imperiled group of freshwater fishes (Cyprinodontiformes: Goodeidae). BMC Evolutionary Biology, 18:116.
https://doi.org/10.1186/s12862-018-1220-3
Gesundheit, P. and Macías, C. 2005. Biogeografía cladística de la familia Goodeidae (Cyprinodontiformes), p. 319-338. In Llorente-Bousquets, J. and Morrone, J.J. (eds.), Regionalización biogeográfica en Iberoamérica y tópicos afines. Facultad de Ciencias, Universidad Nacional Autónoma de México.
Gilbert, C.H. 1893. Report on the fishes of the Death Valley expedition collected in southern California and Nevada in 1891, with descriptions of new species. North American Fauna, 7 (2):229-384.
Gosline, W.A. 1949. The sensory canals of the head in some cyprinodont fishes, with reference to the genus Fundulus. Occasional Papers of the Museum of Zoology, University of Michigan, 519:1-17.
Grudzien, T.A., White, M.M., and Turner, B.J. 1992. Biochemical systematics of the viviparous fish family Goodeidae. Journal of Fish Biology, 40(5):801-814.
https://doi.org/10.1111/j.1095-8649.1992.tb02626.x
Günther, A.1866. Catalogue of the Physostomi, containing the families Salmonidae, Percopsidae, Galaxidae, Mormyridae, Gymnarchidae, Esocidae, Umbridae, Scombresocidae, Cyprinodontidae, in the collection of the British Museum. Catalogue of the fishes in the British Museum, 6:1-368.
Guzmán, A.F. 2010. Microscopical analysis of the fossil goodeid Tapatia occidentalis, p. 75-86. In Uribe, M.C. and Grier, H.J. (eds.), Viviparous fishes II. New Life Publications, Mexico.
Guzmán, A.F. 2015. El registro fósil de los peces mexicanos de agua dulce. Revista Mexicana de Biodiversidad, 86(3): 661-673.
https://doi.org/10.1016/j.rmb.2015.05.003
Guzmán, A.F. and Polaco, O.J. 2009. Peces fósiles mexicanos de agua dulce, p. 316-340. In Ortega Reyes, J., Sedeño Díaz, J.E., and López López, E., (compilators), Setenta y cinco años de la Escuela Nacional de Ciencias Biológicas. Instituto Politécnico Nacional, México.
Guzmán, A.F., Stinnesbeck, W., Robles-Camacho, J., and Polaco, O.J. 1998. El paleolago de Amatitán Jalisco: estratigrafía, sedimentología y paleontología de la localidad tipo de Tapatia occidentalis (Osteichthyes: Goodeidae). Revista de la Sociedad Mexicana de Paleontología, 8(2):127-134.
Hubbs, C.L. 1924. Studies of the fishes of the Order Cyprinodontes. V. Notes on species of Goodea and Skiffia. Occasional Papers of the Museum of Zoology University of Michigan, 148:1-8.
Hubbs, C.L. 1932. Studies of the fishes of the order Cyprinodontes, XII: A new genus related to Empetrichthys. Occasional Papers of the Museum of Zoology, University of Michigan, 252:1-5.
Hubbs, C.L. and Turner, C.L. 1939. Studies of the fishes of the order Cyprinodontes. XVI. A revision of the Goodeidae. Miscellaneous Publications, Museum of Zoology, University of Michigan, 42:1-80.
Jordan, D.S. 1880. Notes on a collection of fishes obtained in the streams of Guanajuato and in Chapala Lake, Mexico, by Prof. A. Dugès. Proceedings of the United States National Museum, 2(94):298-301.
https://doi.org/10.5479/si.00963801.94.298
Jordan, D.S. and Evermann, B.W. 1898. The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America north of the Isthmus of Panama. Part III. Bulletin of the United States National Museum, 47:2183-3136.
https://doi.org/10.5962/bhl.title.46755
Jordan, D.S. and Gilbert, C.H. 1882. Catalogue of fishes collected by Mr. John Xantus at Cape San Lucas, which are now in the U.S. National Museum, with descriptions of eight new species. Proceedings of the United States National Museum, 5:353-371.
https://doi.org/10.5479/si.00963801.5-290.353
Jordan, D.S. and Gilbert, C.H. 1883. Synopsis of the fishes of North America. Bulletin of the United States National Museum, 16:1-1018.
https://doi.org/10.5479/si.03629236.16.i
Jordan, D.S. and Snyder, J.O. 1899. Notes on a collection of fishes from the rivers of Mexico, with description of twenty new species. Bulletin of the United States Fisheries Commission, 19:115-147.
Kingston, D.I. 1978. Skiffia francesae, a new species of goodeid fish from western Mexico. Copeia, 1978(3):503-508.
https://doi.org/10.2307/1443618
Kobelkowsky, A. 2005. General anatomy and sexual dimorphism of Goodea atripinnis (Teleostei: Goodeidae), p. 483-498. In Uribe, M.C. and Grier, H.J. (eds.), Viviparous fishes. New Life Publications, Mexico.
Kowallis, B.J., Carl, C.S., Carranza-Castañeda, O., Millar, W.E., and Tingey, D.G. 1998. Fission-track and single-crystal 40Ar/39Ar laser-fusion ages from volcanic ash layers in fossil-bearing Pliocene sediments in Central México. Revista Mexicana de Ciencias Geológicas,15(2):157-160.
Meek, S.E. 1902. A contribution to the ichthyology of Mexico. Field Columbian Museum Publication, Zoological Series, 3(6):63-128.
Meek, S.E. 1904. The fresh-water fishes of Mexico north of the Isthmus of Tehuantepec. Field Columbian Museum Publication, 5(93):136-144.
Mendoza-Reynosa, E. and Alvarado-Ortega, J. 2015. La importancia del goodeido fósil de Sanctorúm, Hidalgo en la Historia evolutiva de la familia Goodeidae. Paleontología Mexicana, 1:46.
Mendoza-Reynosa, E., Alvarado-Ortega, J., and Domínguez-Domínguez, O. 2013. Los goodeidos fósiles (Cyprinidintiformes: Goodeidae) de Sanctórum, Hidalgo vs. Goodea atripinnis, p. 65. In VIII Congreso Latinoamericano de Paleontología & XIII Congreso Nacional de Paleontología. Guanajuato, México.
Meyer, M.K. and Förster, W. 1983. Eine neue Ilyodon-Art aus Guerrero, Mexiko (Osteichthys, Goodeidae). Zoologische Abhandlungen (Dresden), 38(16):257-263.
Meyer, M.K., Radda, A.C., and Domínguez, O.D. 2001. Notes on the genera Neoophorus Hubbs & Turner, 1937 and Allotoca Hubbs & Turner, 1937, with a description of a new species of Allotoca from Laguna de Zacapu, Michoacán, Mexico (Teleostei, Cyprinodontiformes: Goodeidae). Annalen des Naturhistorischen Museums in Wien, Serie B für Botanik und Zoologie, 2001:453-460.
Miller, R.R. 1948. The cyprinodont fishes of the Death Valley System of eastern California and southwestern Nevada. Miscellaneous Publications, Museum of Zoology, University of Michigan, 68:1-5.
Miller, R.R. and Fitzsimons, J.M. 1971. Ameca splendens, a new genus and species of goodeid fish from western Mexico, with remarks on the classification of the Goodeidae. Copeia, 1971(1):1-13.
Miller, R.R. and Uyeno, T. 1980. Allodontichthys hubbsi, a new species of goodeid fish from southwestern Mexico. Occasional Papers of the Museum of Zoology, University of Michigan, 692:1-13.
Miller, R.R. and Smith, M.L. 1986. Origin and geography of the fishes of Central Mexico, p. 487-517. In Hocutt, C.H. and Wiley, E.O. (eds.), The Zoogeography of North American freshwater fishes. John Wiley and Sons, New York, USA.
Miranda, R., Galicia, D., Monks, S., and Pulido-Flores, G., 2010. First record of Goodea atripinnis (Cyprinodontiformes: Goodeidae) in the state of Hidalgo (Mexico) and some considerations about its taxonomic position. Hidrobiológica, 20:185-190.
Morcillo Alonso, F. 2004. El género Profundulus Hubbs, 1924 (Actinopterygii: Profundulidae): sistemática, filogenia y biogeografía. Tesis Doctoral. Facultad de Ciencias, Universidad Autónoma de Madrid, España.
Nelson, J.S., Grande, T.C., and Wilson, M.V.H. 2016. Fishes of the World. John Wiley & Sons, Hoboken, New Jersey, USA.
Palma-Ramírez, A., Morer, E.L.P., and Santamaría, J.J.V. 2012. Datos paleomagnéticos preliminares del paleolago Plio-Pleistoceno de Santa María Amajac (Hidalgo, Mexico). Geotemas, 13:1216-1219.
Parenti, L.R. 1981. A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). Bulletin of the American Museum of Natural History, 168(4):335-557.
Parker, E. 2017. Molecular Phylogenetics and Biogeography of the Subfamily Goodeinae (Cyprinodontiformes: Goodeidae). Unpublished Master Thesis, Southeastern Louisiana University at Hammond, Louisiana, USA.
Parker, E., Dornburg, A., Domínguez-Domínguez, O., and Piller, K.R. 2019. Assessing phylogenetic information to reveal uncertainty in historical data: an example using Goodeinae (Teleostei: Cyprinodontiformes: Goodeidae). Molecular Phylogenetics and Evolution, 134:282-290.
https://doi.org/10.1016/j.ympev.2019.01.025
Paulo-Maya, J. and Trujillo-Jiménez, P. 2000. Nueva especie de Ilyodon (Cyprinodontiformes: Goodeidae) de la cuenca del río Balsas, México. Revista de Biología Tropical, 48:465-472.
Pellegrin, J. 1901. Poissons recueillis par M. L. Diguet dans l'État de Jalisco (Mexique). Bulletin du Muséum National d'Histoire Naturelle, 7:204-207.
Pérez‐Rodríguez, R., Domínguez‐Domínguez, O., Doadrio, I., Cuevas‐García, E., and Pérez‐Ponce de León, G. 2015. Comparative historical biogeography of three groups of Nearctic freshwater fishes across central Mexico. Journal of Fish Biology, 86(3):993-1015.
https://doi.org/10.1111/jfb.12611
Piller, K.R., Parker, E., Lemmon, A.R., and Lemmon, E.M. 2022. Investigating the utility of Anchored Hybrid Enrichment data to investigate the relationships among the Killifishes (Actinopterygii: Cyprinodontiformes), a globally distributed group of fishes. Molecular Phylogenetics and Evolution, 173:107482.
https://doi.org/10.1016/j.ympev.2022.107482
Rauchenberger, M. 1988. A new species of Allodontichthys (Cyprinodontiformes: Goodeidae), with comparative morphometrics for the genus. Copeia, 1988(2):433-441.
Reyes-Torres, A., Vázquez-Rodríguez, S.D., Carreño, A.L., and Velasco-de León, M.P. 2002. Ostrácodos lacustres del Plioceno-Pleistoceno inferior de la Formación Atotonilco El Grande, Hidalgo, México, p. 128. In VIII Congreso Nacional de Paleontología, Guadalajara, Jalisco.
Rutter, C.L. 1896. Notes on freshwater fishes of the Pacific slope of North America. Proceedings of the California Academy of Sciences, 6:245-267.
Salvador-Flores R. 2001. Origen sedimentológico y estratigrafía del Paleolago de Amajac, Hidalgo. Unpublished Bachelor Thesis, Universidad Nacional Autónoma de México. Distrito Federal, México.
Segerstrom, K. 1961. Geología del suroeste del estado de Hidalgo y del noreste del estado de México. Boletín de la Asociación Mexicana de Geológos Petroleros, 13:147-168.
Smith, M.L. 1980. The evolutionary and ecological history of the fish fauna of the Rio Lerma basin, Mexico. Unpublished PhD Thesis, University of Michigan at Ann Arbor, Michigan, USA.
Smith, M.L. and Miller, R.R. 1980. Allotoca maculata, a new species of goodeid fish from western Mexico, with comments on Allotoca dugesi. Copeia, 1980(3):408-417.
Smith, M.L. and Miller, R.R. 1986. Mexican goodeid fishes of the genus Characodon, with a description of a new species. American Museum Novitates, 2851:1-14.
Smith, M.L. and Miller, R.R. 1987. Allotoca goslinei, a new species of goodeid fish from Jalisco, Mexico. Copeia, 1987(3):610-616.
Thieme, P., Warth, P., and Moritz, T. 2021. Development of the caudal-fin skeleton reveals multiple convergent fusions within Atherinomorpha. Frontiers in Zoology, 18:20.
https://doi.org/10.1186/s12983-021-00408-x
Turner, C.L. 1946. A contribution to the taxonomy and zoogeography of the goodeid fishes. Occasional Papers of the Museum of Zoology, University of Michigan, 495:1-13.
Uyeno, T. and Miller, R.R. 1962. Relationships of Empetrichthys erdisi, a Pliocene cyprinodontid fish from California, with remarks on the Fundulinae and Cyprinodontinae. Copeia, 1962(3):20-532.
Velasco-de León, M.P. and Aguilar-Arellano, F.J. 2002. La fisonomía foliar y el paleoclima de Santa María Amajac, Hidalgo, p. 83. In VIII Congreso Latinoamericano de Botánica & II Congreso Colombiano de Botánica, Cartagena de Indias, Colombia.
Velasco-de León, M.P. and Arellano-Gil, J., Silva-Pineda A. 2000, La secuencia lacustre y su biota de la Formación Atotonilco El Grande de Santa María Amajac, en el Estado de Hidalgo. GEOS, 20(3):302.
Webb, S.A. 1998. A phylogenetic analysis of the Goodeidae (Teleostei: Cyprinodontiformes). Unpublished PhD Thesis, University of Michigan at Ann Arbor, Michigan, USA.
Webb, S.A. and Miller, R.R. 1998. Zoogoneticus tequila, a new goodeid fish (Cyprinodontiformes) from the Ameca drainage of Mexico, and a rediagnosis of the genus. Occasional Papers of the Museum of Zoology, University of Michigan, 725:1-23.
Webb, S.A., Graves, J.A., Macias-Garcia, C., Magurran, A.E., Foighil, D.Ó., and Ritchie, M.G., 2004. Molecular phylogeny of the live-bearing Goodeidae (Cyprinodontiformes). Molecular Phylogenetics and Evolution, 30(3):527-544.
https://doi.org/10.1016/S1055-7903(03)00257-4
Williams, C.D. and Williams, J.E. 1982. Summer food habits of fishes from two springs in east-Central Nevada. Southwest Naturalists, 27:437-445.
https://doi.org/10.2307/3670718
Zaragoza-Caballero, S. and Velasco-de León, P. 2003. Una especie nueva de Epicauta (Coleoptera: Meloidae) del Plioceno del estado de Hidalgo, México. Revista Mexicana de Ciencias Geológicas, 20(2):154-159.

