 Linking burrow morphology to the behaviors of predatory soil arthropods: Applications to continental ichnofossils
Linking burrow morphology to the behaviors of predatory soil arthropods: Applications to continental ichnofossils
Article number: 26.2.a22
https://doi.org/10.26879/1257
Copyright Paleontological Society, June 2023
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 December 2022. Acceptance: 8 June 2023.
ABSTRACT
Predatory arthropods are known from terrestrial environments since the Silurian. Many of these animals have developed morphological and behavioral adaptations for living within soil environments. Ichnofossils are common in Paleozoic paleosols, yet most are of uncertain origin and may record a hidden diversity of predatory arthropods. These ichnofossils are especially important given the relatively poor preservation potential of soil invertebrates in the environments they inhabit. To better understand the morphology and uses of predatory soil arthropod burrows, laboratory experiments were conducted with centipedes, scorpions, whip scorpions, and spiders. Specimens were placed in sediment-filled terrariums for 1 to 36 weeks. The animals were observed continuously using digital recordings to monitor their behaviors and use of their burrows. Open burrows were cast and described qualitatively and quantitatively. The animals burrowed using various techniques including intrusion, compression, excavation, and backfilling. Some burrows were occupied for short intervals (2-5 days) before being abandoned, whereas others were permanently occupied. Burrows ranged from simple vertical shafts to complex, branching networks that served as temporary to permanent dwellings, and most were used as sites for ambush predation or as prey traps. The different predatory arthropods produced unique burrows that could be linked to specific behaviors. Distinct burrow features were linked to predatory activities including vertical shafts, multiple surface openings, branching tunnel networks, and expanded chambers. These data can be applied to continental ichnofossils to improve our understanding of the evolution of terrestrial predatory arthropods, their distribution through time, and interpretations of the paleoecology of ancient soil ecosystems.
Daniel I. Hembree. Department of Earth and Planetary Sciences, University of Tennessee Knoxville, Knoxville, Tennessee 37996, USA, dhemre2@utk.edu
Keywords: trace fossil; arthropod; terrestrial; paleosol; paleoecology
Final citation: Hembree, Daniel I. 2023. Linking burrow morphology to the behaviors of predatory soil arthropods: Applications to continental ichnofossils. Palaeontologia Electronica, 26(2):a22.
https://doi.org/10.26879/1257
palaeo-electronica.org/content/2023/3869-burrows-of-arthropod-predators
Copyright: June 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Predatory arthropods are known to have been present in terrestrial environments since the Silurian (Dunlop, 2010). Many of these animals have acquired behavioral adaptations suited for soil environments, including fossorial habits, and are integral parts of soil ecosystems (Lavelle and Spain, 2005; Potopov et al., 2022). These animals engage in a variety of activities and behaviors in the substrate and produce a wide array of biogenic sedimentary structures, or ichnofossils, in the process (e.g., Hasiotis, 2007; Hembree, 2016; Genise, 2017). Ichnofossils are common even in Paleozoic paleosols (Retallack, 2001), yet most are of uncertain origin and may record a hidden diversity of predatory arthropods. Understanding the origin of these ichnofossils is especially important given the relatively poor preservation potential of soil invertebrates in the environments they inhabit (Martin, 1999). In addition, continental ichnofossils have a wide range of uses in paleontological, sedimentological, and sequence stratigraphic studies (Hasiotis, 2007; MacEachern et al., 2012a, 2012b; Mángano et al., 2012).
Modern soils contain a diverse and abundant biota, including members of all terrestrial phyla, forming elaborate food webs containing several trophic levels (Lavelle and Spain, 2005; Potapov et al., 2022). These animals contribute significantly to pedogenesis, including the mixing, mounding, and aeration of soils (Schaetzl and Anderson, 2005). Key to soil food webs, like most, are large carnivores. In soil food webs, arthropods such as centipedes, scorpions, and spiders are the major predators (Lavelle and Spain, 2005; Potapov et al., 2022). These animals occupy soil environments on every continent except Antarctica, in arid to tropical climates from the equator to high latitudes (Schaetzl and Anderson, 2005). Soil arthropod carnivores can be semi-fossorial to fossorial, occupying the substrate temporarily to permanently, feeding on a wide variety of prey, from nematodes, annelids, gastropods, arthropods, and even vertebrates (Lavelle and Spain, 2005).
Soil arthropod carnivores have a long, if sparse, fossil record, owing to their lack of mineralized hard parts and the generally destructive nature of their habitats (Dunlop, 2010). Body fossils of scorpions are known from the Silurian (Ludfordian) and Devonian (Dunlop and Selden, 2013; Gess, 2013), centipedes from the Devonian (Shear and Bonamo, 1988), spiders from the Carboniferous (Stephanian) (Selden, 1996), and whip scorpions from the Pennsylvanian (Moscovian) (Tetlie and Dunlop, 2008). What is generally lacking, however, is a clear fossil record of the bioturbation produced by these types of soil animals which, given their geologic range, should be abundant in paleosols from the Paleozoic to the recent.
This paucity of ichnofossil data is most likely an issue of recognition rather than a problem of preservation. To better understand the morphology and behavioral significance of predatory soil arthropod burrows in the fossil record, observations of extant animals in the laboratory and field are essential. The study of modern trace-making organisms allows for the interpretation of ichnofossils, including the behaviors involved in their production, the environments that influenced their production, and the organisms that produced them (Bromley, 1996). These actualistic studies provide the data that makes ichnofossils invaluable to paleoecological and paleoenvironmental reconstructions. While many investigations of the traces produced by modern soil animals have been published (e.g. Jackson, 2000; Tschinkel, 2003; Gobetz, 2005; Hembree and Hasiotis, 2006, 2007; Lawfield and Pickerill, 2006; M’rabet et al., 2007; Rodríguez-Tovar, 2007; Smith and Hasiotis, 2008; Counts and Hasiotis, 2009; Hembree, 2009; Halfen and Hasiotis, 2010; Hembree et al., 2012; Kinlaw and Grasmueck, 2012; Melchor et al., 2012; Platt et al., 2012; Genise et al., 2013; Hembree, 2013, 2014; Mikuś and Uchman, 2013; Bowen and Hembree, 2014; Catena and Hembree, 2014; Doody et al., 2014; Dzenowski and Hembree, 2014; Sarzetti et al., 2014; Cantil et al., 2014, 2015; Doody et al., 2015; Hils and Hembree, 2015; Monaenkova et al., 2015; Adams et al., 2016; Belmontes et al., 2018; Uchman et al., 2018; Hembree, 2019; Nascimento et al., 2021; Thacker and Hembree, 2021), direct connections between predatory behavior and burrow morphology are not well documented (e.g., Lehane and Ekdale, 2013).
In this study the connections between the behaviors of soil arthropod carnivores and specific morphological traits of their burrows were analyzed for the purpose of identifying similar types of behaviors and trace makers in the fossil record based on ichnofossil morphology. These data can be applied to continental ichnofossils to improve understanding of the evolution of terrestrial predatory arthropods, their distribution through time, and interpretations of the paleoecology of ancient soil ecosystems.
MATERIALS AND METHODS
 This study involved the compilation of the results of neoichnological experiments with 15 species of burrowing predatory arthropods, including three species of centipedes (Scolopendra viridis, Scolopendra polymorpha, Hemiscolopendra marginata), five species of scorpions (Pandinus imperator, Heterometrus spinifer, Hadrurus arizonensis, Smeringurus mesaensis, Uroctonus mordax), one species of whip scorpion (Mastigoproctus giganteus), and six species of spiders (Hogna lenta, Gorgyrella inermis, Myrmekiaphilia sp., Aphonopelma chalcodes, Hysterocrates gigas, Pelinobius muticus) (Figure 1, Table 1) (Hembree et al., 2012; Hembree, 2013, 2014; Hils and Hembree, 2015; Hembree, 2016, 2017, 2019). In these experiments the studied animals were placed in sediment-filled 38-, 75-, 114-, 212-, and 246-liter terrariums depending on the animal size (Figure 2). The enclosures were prepared with known environmental parameters including soil consistency, composition, and moisture, modeled after the natural conditions of each species (Table 1). See the referenced papers for details on the experimental design used for each species.
This study involved the compilation of the results of neoichnological experiments with 15 species of burrowing predatory arthropods, including three species of centipedes (Scolopendra viridis, Scolopendra polymorpha, Hemiscolopendra marginata), five species of scorpions (Pandinus imperator, Heterometrus spinifer, Hadrurus arizonensis, Smeringurus mesaensis, Uroctonus mordax), one species of whip scorpion (Mastigoproctus giganteus), and six species of spiders (Hogna lenta, Gorgyrella inermis, Myrmekiaphilia sp., Aphonopelma chalcodes, Hysterocrates gigas, Pelinobius muticus) (Figure 1, Table 1) (Hembree et al., 2012; Hembree, 2013, 2014; Hils and Hembree, 2015; Hembree, 2016, 2017, 2019). In these experiments the studied animals were placed in sediment-filled 38-, 75-, 114-, 212-, and 246-liter terrariums depending on the animal size (Figure 2). The enclosures were prepared with known environmental parameters including soil consistency, composition, and moisture, modeled after the natural conditions of each species (Table 1). See the referenced papers for details on the experimental design used for each species.
 Once placed in their enclosure, the burrowing techniques, activities, and behaviors of the animals were observed for periods of 1-36 weeks. The observations included daily written records and illustrations, photographs of the sides and surface of the enclosures, and digital recordings of the animals when active. In addition to documenting activities of the animals, modifications to and use of specific portions of their burrows were recorded.
Once placed in their enclosure, the burrowing techniques, activities, and behaviors of the animals were observed for periods of 1-36 weeks. The observations included daily written records and illustrations, photographs of the sides and surface of the enclosures, and digital recordings of the animals when active. In addition to documenting activities of the animals, modifications to and use of specific portions of their burrows were recorded.
Once the experiments were concluded, the animals were removed from the enclosures, and their burrows were cast using a quick-drying plaster to document their three-dimensional morphology. The architectural and surficial morphology of the burrows of each species were then described qualitatively and quantitatively using the same methodology. Qualitative architectural morphology included the general burrow form, orientation, cross-sectional shape, type and amount of branching, and interconnectedness between different burrow elements (e.g., Bertling et al., 2006). 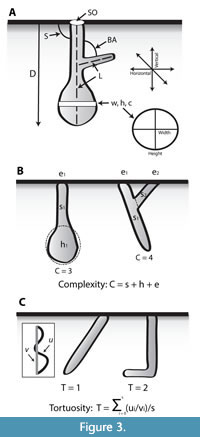 Surficial morphology included features on burrow surfaces such as scratches, bumps, and linings that record evidence of burrow construction, maintenance, and locomotion styles. Quantitatively the burrow casts were described with nine properties: 1) number of surface openings; 2) maximum depth; 3) total length; 4) width; 5) height; 6) width to height ratio; 7) circumference; 8) burrow slope with respect to the surface; and 9) branching angles if present (Figure 3A). Burrow complexity and tortuosity, two semi-quantitative, scale independent properties, were also determined (e.g., Meadows, 1991; Hembree and Hasiotis, 2006). Complexity is the sum of the number of different tunnel segments, surface openings, and chambers in a burrow system (Figure 3B). Tortuosity is a measure of the deviation of the morphology of the shafts and tunnels from a straight line (Figure 3C).
Surficial morphology included features on burrow surfaces such as scratches, bumps, and linings that record evidence of burrow construction, maintenance, and locomotion styles. Quantitatively the burrow casts were described with nine properties: 1) number of surface openings; 2) maximum depth; 3) total length; 4) width; 5) height; 6) width to height ratio; 7) circumference; 8) burrow slope with respect to the surface; and 9) branching angles if present (Figure 3A). Burrow complexity and tortuosity, two semi-quantitative, scale independent properties, were also determined (e.g., Meadows, 1991; Hembree and Hasiotis, 2006). Complexity is the sum of the number of different tunnel segments, surface openings, and chambers in a burrow system (Figure 3B). Tortuosity is a measure of the deviation of the morphology of the shafts and tunnels from a straight line (Figure 3C).
The observed techniques used by the different species to construct and maintain their burrows were compared to find similarities and differences and consistent associated burrow architectural elements. Digital recordings were analyzed to determine the activities and behaviors associated with burrows as a whole and individual burrow elements, including the amount of time spent in different portions of the burrow. The qualitative and quantitative properties of the burrow casts of each species and group of arthropods were then compared to find distinctive features common to each type of arthropod predator and arthropod predators in general.
RESULTS
Burrowing Techniques
The studied arthropod carnivores began constructing burrows within minutes (centipedes) to several days (tarantulas and scorpions) after the experiments began, but typically within 24 h. In most experiments, some burrows were started, but then abandoned soon after construction began, often within 12-24 h. The animals were observed burrowing using four basic techniques: intrusion, compression, excavation, backfilling, and lining (e.g., Bromley, 1996). Excavation was the most common technique employed by all the studied species.
 Intrusion involved the animal forcing its way into the substrate, typically along zones of weakness or in loose substrates (Figure 4A-B; Appendix 1). Intrusion was used by all three centipede species and three of the spider species (Hogna lenta, Myrmekiaphilia sp., Pelinobius muticus). While simple, this technique was highly effective and was one of the fastest means of entering the substrate. Intrusion was used in conjunction with other burrowing techniques, including compression and excavation, once the animal was below the surface. Intrusion alone did not result in well-defined burrows as the sediment collapsed behind the animal as it moved.
Intrusion involved the animal forcing its way into the substrate, typically along zones of weakness or in loose substrates (Figure 4A-B; Appendix 1). Intrusion was used by all three centipede species and three of the spider species (Hogna lenta, Myrmekiaphilia sp., Pelinobius muticus). While simple, this technique was highly effective and was one of the fastest means of entering the substrate. Intrusion was used in conjunction with other burrowing techniques, including compression and excavation, once the animal was below the surface. Intrusion alone did not result in well-defined burrows as the sediment collapsed behind the animal as it moved.
Compression involved the compacting of sediment along the sides of the burrow, reducing the porosity of the surrounding sediment to produce an open space (Figure 4C-D; Appendix 2). This process was most effective in loose substrates with low sand content. Compression was used by all three centipede species and three spider species (Hogna lenta, Gorgyrella inermis, Myrmekiaphilia sp.). Sediment was compacted with the arthropod’s appendages, body, or a combination of the two. This method was generally initiated after entry into the substrate by intrusion, or after burrow construction had already been initiated by excavation. Burrows produced by compression tended to be cylindrical, circular to ovoid in cross section, and had smooth interior surfaces.
Burrowing by excavation occurred in more cohesive, dense substrates and generally involved the removal of sediment from the subsurface by kicking or throwing it from near the burrow opening or carrying it out of the developing burrow (Figure 4E-G; Appendix 3). Excavation was used by all the studied animals except for Hogna lenta. The arthropods used a variety of appendages to excavate including their walking legs, pedipalps, and maxilla. Excavated sediment was deposited at the surface directly by the burrow entrance or carried up to several body lengths away from the entrance. Burrows produced by excavation had a variety of forms from vertical shafts to complex mazeworks. Most burrows constructed by excavation were of irregular width and height and had rough interior surfaces.
Backfilling involved the deposition of excavated sediment within the burrow, filling most or all of the tunnel or shaft behind the animal (Figure 4H; Appendix 4). Two of the studied centipedes (Scolopendra polymorpha, Hemiscolopendra marginata) and the whip scorpion (Mastigoproctus giganteus) often engaged in backfilling of their burrows, packing previously excavated tunnels with sediment dug out from new tunnels. This process resulted in open burrow architectures that shifted over time, from complex to simple. Whip scorpions also used backfilling to isolate themselves from the surface for several weeks.
Burrows could be modified by the addition of a lining, typically to provide additional structural support or as a substrate for more effective chimney climbing. This process was most evident in the spider species, all of which would coat the burrow walls with thin to thick layers of silk (Figure 4I-J; Appendix 5). These silk linings were also used for prey detection as they tended to extend up to the surface as either mats or strings (Figure 4K). The addition of a lining resulting in burrow casts with smooth sides as well as surficial features such as scratch marks.
Burrow Associated Activities
The burrows produced by the arthropod predators in these experiments served various functions. Many of the activities and behaviors were observed in association with the specific burrow elements and burrow architectures.
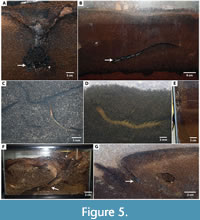 Most commonly the burrows served as permanent dwellings, used for occupation, concealment, and protection (Figure 5A-B). These burrows were constructed quickly, usually over 1-3 days after the animal was placed in the enclosure. Dwelling burrows were occupied for periods of weeks to months during which time the animals rarely moved to the surface and were engaged in continual maintenance, modification, and expansion of the burrow. Dwelling burrows were kept open with one to several surface openings, consisted of simple shafts or ramps, U-shaped burrows, or mazeworks of varying depths from a few centimeters to 10s of centimeters, and, with scorpions, whip scorpions, and spiders, typically had one or more chambers. Surface openings were sealed with excavated sediment (backfilled) in association with molting. With spiders, this sediment was typically bound with silk.
Most commonly the burrows served as permanent dwellings, used for occupation, concealment, and protection (Figure 5A-B). These burrows were constructed quickly, usually over 1-3 days after the animal was placed in the enclosure. Dwelling burrows were occupied for periods of weeks to months during which time the animals rarely moved to the surface and were engaged in continual maintenance, modification, and expansion of the burrow. Dwelling burrows were kept open with one to several surface openings, consisted of simple shafts or ramps, U-shaped burrows, or mazeworks of varying depths from a few centimeters to 10s of centimeters, and, with scorpions, whip scorpions, and spiders, typically had one or more chambers. Surface openings were sealed with excavated sediment (backfilled) in association with molting. With spiders, this sediment was typically bound with silk.
Movement, or locomotion, through shafts and tunnels could be observed where the burrows intersected the side of the enclosure (Figure 5C; Appendix 6). Locomotion could be limited to a range of 10s of centimeters in simple burrows to 100s of centimeters in a mazework. Two main styles of locomotion were observed in burrows, pacing, and chimney climbing. Pacing involved normal movement along a horizontal or subhorizontal plane, typically along the ‘floor’ of the burrow. This locomotion style was observed in all the studied arthropods. Chimney climbing was vertical movement, where the appendages were in contact with the burrow along all sides. This locomotion style was observed in spiders, centipedes, and whip scorpions. While observed, locomotion was limited during the experiments and associated with burrow construction and maintenance and active hunting. The animals were stationary for most of the time they were observed.
While some hunting was observed on the sediment surface, the burrows served as ambush sites for the arthropod carnivores, so they did not need to move to the surface. Burrows used in this manner generally had openings that were partially or fully concealed, such as by a constructed trap door or an object on surface. The prey was captured at the surface as the carnivore waited inside the burrow, just below the surface opening (Figure 5D-E; Appendix 7). Spiders and centipedes most often used their burrows in ambush predation. These burrows were characterized by vertical shafts near the surface, but could be more complex (U-shaped, Y-shaped, or branching) below the surface. Most of the studied spiders that engaged in ambush predation used constructed “trapdoors” or simple sediment plugs to conceal the surface opening of their burrow (Figure 4J-K). The centipedes did not conceal their burrow openings.
Burrows also served as prey traps, where the prey animals entered the burrow and were captured below the surface (Figure 5F-G; Appendix 8). In some cases, the predator waited just inside the burrow, 5-10 cm from the opening, for prey to enter and capture was directly observed. In others, the predator could not be seen, but the prey animals entered the burrow opening and did not emerge. Burrows used in this manner generally possessed multiple surface openings and below the surface were multiple interconnected shafts and tunnels. This was a typical burrow form produced by scorpions from both arid and tropical regions as well as centipedes and whip scorpions. However, burrows that served as prey traps also included simple ramps and helical burrows with a single opening produced by tarantulas and scorpions. Key features of burrows used as prey traps were unconcealed openings and entrance tunnels with a shallow slope.
Burrow Morphology
The burrows produced by the studied centipedes, scorpions, whip scorpions, and spiders possessed distinct features related to their level of evolutionary similarity. For example, it was possible to differentiate the burrows of centipedes, scorpions, whip scorpions, and spiders and even the burrows of the different species of each of these groups. However, there were also features that were shared by the burrows of all the arthropod carnivores.
 Burrows produced by all the studied animals were kept connected to the surface for a significant portion of the time of occupation, most likely to provide access to prey based on observations (Figure 6A-C). The openings may have been concealed or covered (Figure 4K), but the connection to the surface was still maintained. The number of openings varied based on the type of animal or even by species. Spiders typically only had one surface opening except for Pelinobius muticus and Hogna lenta, which rarely had two openings. The studied scorpions were more variable in the number of surface openings; burrows of the tropical to temperate scorpions Pandinus imperator and Heterometrus spinifer, and Uroctonus mordax had only one opening whereas those of the arid scorpions Hadrurus arizonensis and Smeringurus mesaensis often had multiple openings. Burrows of Mastigoproctus giganteus had one to several openings depending on the time of occupation. Centipede burrows were characterized by multiple surface openings.
Burrows produced by all the studied animals were kept connected to the surface for a significant portion of the time of occupation, most likely to provide access to prey based on observations (Figure 6A-C). The openings may have been concealed or covered (Figure 4K), but the connection to the surface was still maintained. The number of openings varied based on the type of animal or even by species. Spiders typically only had one surface opening except for Pelinobius muticus and Hogna lenta, which rarely had two openings. The studied scorpions were more variable in the number of surface openings; burrows of the tropical to temperate scorpions Pandinus imperator and Heterometrus spinifer, and Uroctonus mordax had only one opening whereas those of the arid scorpions Hadrurus arizonensis and Smeringurus mesaensis often had multiple openings. Burrows of Mastigoproctus giganteus had one to several openings depending on the time of occupation. Centipede burrows were characterized by multiple surface openings.
There were multiple architectures of burrows produced by the soil arthropod predators depending on the type of animal and the style of prey capture (ambush vs. prey trap). Largely vertically oriented burrows were quite common among many of the studied animals, particularly the spiders, whip scorpions, and centipedes. Spider burrows were the most vertical, consisting of a single or primary shaft with a circular cross section (Figure 6D). Most spider burrows were unbranched, but multiple horizontal branches were present in burrows of P. muticus, and H. lenta produced rare Y-shaped burrows (Figure 6E). Spider burrows generally had an enlarged chamber at or below the surface; the level of enlargement of the chamber varied considerably (Figure 6F). An exception to this trend in spider burrow morphology were the burrows of Aphonopelma chalcodes, which consisted of subhorizontal ramps and helical tunnels (Figure 6G). This difference in architecture coincided with a difference in locomotion style within the burrows; A. chalcodes were not observed using chimney climbing but walked solely on the floor of their tunnels.
Whip scorpion burrows had significant vertical components, especially at the entrance. These burrows had pronounced J-, U-, or Y-shapes which transitioned into a horizontal element in the subsurface (Figure 6H-J). The cross-sectional form of the shafts and tunnels was irregular but tended to be more elliptical, with a greater width than height. Enlarged chambers occurred at burrow terminations. As the time of occupation increased, additional burrow elements were added to produce complex mazeworks composed of multiple J-, U-, and Y-shaped burrows connected by short horizontal tunnels (Figure 6K). In all cases, however, the vertical elements were the dominant component.
This same trend was observed in the centipede burrows, which were largely J- to U-shaped, becoming interconnected over time (Figure 6L-M). Connection to the surface was vertical, with horizontal elements occurring in the subsurface. Centipede burrows also tended to be elliptical in cross section, but no defined chambers were observed.
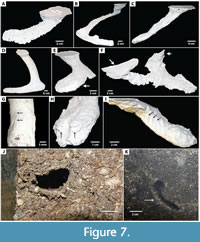 Scorpion burrows were largely subhorizontal in orientation, consisting of simple, low-sloping ramps or helical tunnels (Figure 7A-D). These burrows had a pronounced elliptical cross section and most possessed laterally expanded chambers at the ends of tunnels (Figure 7E). Hadrurus arizonensis did produce a more complex set of burrow forms, including U-shaped burrows and mazeworks composed of multiple, interconnected ramps and U-shaped burrows (Figure 7F).
Scorpion burrows were largely subhorizontal in orientation, consisting of simple, low-sloping ramps or helical tunnels (Figure 7A-D). These burrows had a pronounced elliptical cross section and most possessed laterally expanded chambers at the ends of tunnels (Figure 7E). Hadrurus arizonensis did produce a more complex set of burrow forms, including U-shaped burrows and mazeworks composed of multiple, interconnected ramps and U-shaped burrows (Figure 7F).
Bioglyphs, or markings produced by the trace maker such are scratch marks from appendages or mouth parts, were present along the burrow surfaces of many of the arthropods and were produced during construction, maintenance, and movement through the burrow. Finer scale scratch marks associated with continual chimney climbing were produced by the trapdoor spiders (Figure 7G). Scorpions, whip scorpions, and tarantulas produced larger scale features associated with sediment excavation with appendages (Figure 7H-I). Lining or constructed walls were not common among the studied animals. Heavy silk linings were present in some of the spider burrows, some of which incorporated surrounding sediment (Figure 7J). Thin, discontinuous, compressional linings were present in some centipede tunnels when observed in cross sections (Figure 7K).
DISCUSSION
Associations of Burrow Morphology, Soil Arthropods, and Predation Activity
Experimental studies of extant burrowing animals are vital to the recognition of the traces they produce in the fossil record (Hembree, 2016). These studies provide the defining features, or ichnotaxobases, that can be used to properly interpret the behavioral significance of the trace and potential trace makers (Bromley, 1996; Bertling et al., 2006; Hembree, 2016; Bertling et al., 2022). Critical ichnotaxobases include internal structure (or fill), bioglyphs, cross sectional shape, orientation with respect to the sediment surface, and architecture (Bromley, 1996; Bertling et al., 2006). The experiments described in this study revealed several key ichnotaxobases produced by predatory soil arthropods that can be useful in identifying their burrows in the fossil record.
As a result of the maintained connection of the burrows to the surface, the burrows would be largely passively filled when preserved (e.g., Bromley, 1996). The plaster poured into the burrows from the surface openings was able to fill most of the burrows, even when they were composed of multiple elements. This suggests that the burrows have a good preservation potential in the event of the rapid emplacement of fluidized sediment on the landscape (e.g., Bromley, 1996; Savrda, 2007). This feature of the studied burrows, occurring in all four types of soil arthropods, was related to their use as a permanent dwelling and the need of the arthropod trace makers to access prey at the surface (e.g., Williams, 1987). This same feature has been observed in a variety of predatory arthropod burrows, in particular among spiders and scorpions (e.g., Shorthouse and Marple, 1980; Polis et al., 1986; Adams et al., 2016; Belmontes et al., 2018; Uchman et al., 2018; Nascimento et al., 2021). Some portions of the burrows could show evidence of active filling as the result of burrow maintenance activities, such as when old portions of burrow complexes were filled in with the material excavated from new areas as observed in centipedes and whip scorpions. This active filling is massive in nature and generally matches the composition of the surrounding sediment. Primary differences between backfill and the surrounding matrix may include a different texture and higher porosity due to the excavation process (e.g., Bromley, 1996).
The presence of bioglyphs on tunnel and shaft surfaces was common among the studied burrows. These features have a high degree of preservation potential and are important ichnotaxobases (Bertling et al., 2006). They are also critical to the interpretation of the behavioral significance of fossil burrows and their potential trace makers (Bertling et al., 2006; Gobetz, 2005; Knaust, 2012). The bioglyphs observed in the burrow casts from this study, including fine scratch marks and large protrusions, were produced during burrow construction and occupation, either as a result of sediment excavation or locomotion through the burrow. Similar bioglyphs have been observed in burrows of other wolf spiders (Belmontes et al., 2018) and trapdoor spiders (Nascimento et al., 2021). Both activities are expected of burrows that are kept open, regularly maintained or modified, and occupied for extended periods of time as a dwelling structure (Bromley, 1996; Hasiotis, 2007). The most typical feeding style of macrofauna associated with these burrow characteristics in terrestrial environments is carnivory (e.g., Lavelle and Spain, 2005; Potopov et al., 2022).
The cross-sectional shape of the burrows ranged from circular to elliptical and was generally consistent throughout individual burrows. The cross section of the tunnels and shafts was largely related to the body morphology of the trace-making arthropod. Arthropods with a body morphology with a high width-to-height ratio, such as scorpions, whip scorpions, and centipedes, produced burrows with elliptical cross sections. Those arthropods with a body width-to-height ratio close to 1, such as spiders, produced burrows with a more circular cross section. The cross-sectional relationships between body form and burrow were consistent even with differences in burrow orientation (vertical or subhorizontal). The same cross-sectional shapes have been observed in the burrows of other scorpions (Williams, 1966; Koch, 1978; Polis et al., 1986; Adams et al., 2016) and spiders (Miller and Miller, 1984; Belmontes et al., 2018; Uchman et al., 2018; Nascimento et al., 2021) studied in the field. In the experimental studies, the body-burrow cross section relationship was best expressed in burrow elements deeper within the substrate as opposed to elements that connected to the surface, as those became distorted in shape as a result of burrow maintenance and predation activities.
The recognition of the diversity of burrow orientations and architectures produced by predatory soil arthropods is key to identifying their burrows in the fossil record. Burrows of the four different types of predatory arthropods had their own defining architectural elements. It is, in many cases, possible to identify the type of trace maker (e.g., spider, scorpion, whip scorpion, centipede) based on the burrow morphology (e.g., Hembree, 2016). Even burrows produced by different types of the same group of arthropods, such as trapdoor spiders and tarantulas, can be distinguished (Hembree, 2017). However, while there was no one burrow morphology produced by all four types of the studied arthropods, they did have common elements related to similarities in their life activities and behaviors.
The two end member burrow architectures that were produced could be separated into those that were primarily vertical and those that were primarily subhorizontal in orientation with respect to the sediment surface. Among the vertical burrows, simple shafts were common among spiders, but U- to Y-shaped burrows were distinctive vertical architectures frequently produced by a variety of the studied animals, including centipedes, whip scorpions, scorpions, and spiders. The same architectures have been observed in wolf spiders (Miller and Miller, 1984; Belmontes et al., 2018; Uchman et al., 2018), trapdoor spiders (Nascimento et al., 2021), and scorpions (Harington, 1978). These architectures had a strong connection with ambush predation activities in which the predatory arthropod was able to position itself just below the surface opening. The vertical nature of the connecting burrow element allowed the animal to remain concealed from prey animals at the surface in addition to, in some cases, the presence of a sediment cover or constructed trapdoor (Bond and Coyle, 1995; Hils and Hembree, 2015; Hembree, 2017). The vertical nature of the burrow also appeared to enhance the ambush nature of prey capture, increasing both the speed of attack and the degree of surprise in the prey animal (Bond and Coyle, 1995; Hils and Hembree, 2015).
Subhorizontal burrows, largely produced by tarantulas and scorpions, ranged from straight ramps to helical ramps. These burrows tended to have low slopes, from 10-35°, and elliptical cross sections. Helical ramps, while seemingly complex, simply consisted of repeating series of connected straight ramps that were turned in a new direction at different intervals. The same architectures have been observed in a number of different types of scorpions (Koch, 1978; Shorthouse and Marples, 1980; Polis et al., 1986; Adams et al., 2016). These architectures were largely used as prey trap structures, as observed in these experiments and in the field (Williams, 1987; Hembree et al., 2012; Hembree, 2014, 2017). In general, it was observed in these experiments that prey animals most often entered burrows with a lower slope at the entrance (subhorizontal burrows rather than vertical). It was also more difficult for the predatory arthropods to conceal themselves close enough to the surface for these burrows to be effectively used in ambush predation. More commonly, these animals waited deeper inside the burrow for prey to enter (Williams, 1987; Hembree et al., 2012; Hembree, 2014, 2017). Surface animals will typically enter the substrate through existing openings for concealment and protection, making this an effective strategy for acquiring prey (Lavelle and Spain, 2005).
Paired with their maintained surface openings, which would result in passive infilling and bioglyphs produced by excavation and long-term occupation, these major architectural end members are good indicators of predatory feeding strategies in soil environments. This combination of ichnotaxobases would be essential for interpretation of this activity in ichnofossils.
More complex burrows were produced by many of the studied animals, including at least one species per arthropod group (Table 2). These burrows are defined as mazeworks as their components spread throughout a three-dimensional space in the subsurface (Bromley, 1996). Production of similarly complex burrows has been observed in other scorpions (Shivashankar, 1994). The complex burrows were largely composed of repeating simple elements, such as shafts, ramps, or U-shaped burrows, that were constructed individually and connected over time (Figure 6I, K-M). These simple elements were the same architectural end members described above. While the way they were constructed varied, the complex burrows possessed all the key features (fill, bioglyphs, cross section, orientation) of the individual elements, which were useful in interpreting behavioral significance and trace maker identity. For example, complex burrows were observed in animals that dominantly used their burrows as either sites of ambush predation or as prey traps. Those burrow complexes that were used for ambush predation tended to have vertical elements connected to the surface (Figure 6K, M) whereas those used as prey traps had subhorizontal connections (Figure 7F). As a result, the recognition of a complex burrow or mazework in the fossil record may provide evidence of the activity of a predatory arthropod, but analysis of the individual elements would be critical to the interpretation.
Finally, many of the burrows produced by predatory soil arthropods were large in scale (Figure 6G, Figure 7B, E), especially those of spiders and scorpions. This included tunnel and shaft diameter as well as total length and depth of the burrows. These burrow dimensions were not necessarily correlated to animal body size, especially when the burrows were occupied by multiple individuals in the case of scorpions (Hembree, 2014). Burrowing by excavation tended to result in burrows with larger dimensions than the trace maker’s body. This connection corresponded to the nature of sediment extraction techniques, which tended to be irregular in nature, and the need for many of the animals to turn their bodies in the shafts and tunnels to move to the surface while carrying loads of sediment (Hembree, 2013, 2017, 2019). This observation should provide caution when assessing potential trace makers based on burrow size; large burrows in paleosols do not necessarily require a vertebrate trace maker (e.g., Hembree, 2016).
Application to and Comparisons with Ichnofossils
The interpretation of the behavioral significance of ichnofossils requires the known association of trace morphology with animal activities in the modern (e.g., Bromley, 1996). Experimental studies of burrowing animals in the laboratory and observations of them in the field make these associations possible and provide sets of useful ichnotaxobases that can be applied to ichnofossils interpretation. Ichnofossils interpreted as record of predatory feeding activities have generally consisted of bite marks or drill holes in hard substrates (Oichnus), coprolites containing animal material, or close associations of trails or trackways with other ichnofossils (i.e., Cruziana/Rusophycus and Planolites) (Buatois and Mángano, 2011). These types of ichnofossils show direct interaction with prey material (bites, holes, coprolites) or assumed interactions due to the proximity of two different traces. The former is more definitive and based on comparisons with modern feeding traces (e.g., Nebelsick and Kowaleski, 1999; Kelley and Hansen, 2003). The latter, however, is far less certain as co-occurring or even overlapping ichnofossils may have been produced at different times (Bromley, 1996). The recognition of specific burrow morphologies produced by terrestrial predatory animals, especially those where the burrow is used in the acquisition of prey, can provide another means of interpreting predation activity in the fossil record.
The key characteristics of predatory soil arthropods burrows revealed by this study were primarily tied to how the burrow was used to acquire prey, as a site for ambush or as a prey trap. Burrows used as sites for ambush predation tend to be vertical in orientation (single shaft, U-, or Y-shaped), at least near the surface. Branching horizontal tunnels may be present, but they do not constitute the majority of the total burrow. The shafts and tunnels have cross sections that are generally circular and have a maintained surface connection. This connection, however, may be covered by a maintained trapdoor or sediment plug that may inhibit passive infilling. Chambers are typically present and occur at the burrow termination and at the surface opening. Burrows used as prey traps tend to be subhorizontal in overall orientation, consisting of a single straight to helical tunnel or interconnected networks of tunnels with multiple surface openings. Branches are common as are chambers at the ends of tunnels. Regardless of specific predatory activity, tunnels and shafts could be either lined or unlined depending on the trace maker, related to specific burrowing techniques. However, all burrows would be kept open and primarily preserved by passive infilling.
Occurrences of various continental ichnogenera have been interpreted as the burrows of different predatory arthropods, particularly those of spiders (Dunlop and Braddy, 2011). Simple burrows that have been associated with predatory arthropod trace makers include Skolithos, Cylindricum, and Macanopsis. Morrissey et al. (2012) suggested arachnids may have produced Skolithos associated with Late Silurian to Early Devonian paleosols from the Lower Old Red Sandstone of the Anglo-Welsh Basin. Melchor et al. (2006) and Sciscio et al. (2021) also listed spiders as possible tracemakers of Skolithos from Early Mesozoic fluvial successions of Argentina and Zimbabwe, respectively. The described burrows do match the general morphology of simple vertical spider burrows observed in the experiments, in particular those of trapdoor spiders. Smith et al. (2008) included spiders as a possible trace maker of Cylindricum. These burrows are similar in morphology and scale to the simple, inclined burrows produced by wolf spiders and smaller tarantulas in the experiments. Macanopsis has also been associated with spiders when present in paleosols (Bown and Kraus, 1983; Hasiotis, 2002; Catena et al., 2016) owing to the enlarged terminal chamber. Trapdoor spiders in particular produced burrows similar to Macanopsis. It should be noted, however, that in all of these examples, additional possible trace makers were mentioned including beetles, bees, wasps, and many other insects. Mikuś and Uchman (2013), for example, clearly demonstrated the morphological similarity between beetle burrows and Macanopsis. In the case of burrows with simple morphologies, clear associations with specific behaviors or trace makers are difficult to discern. Additional details that may support an arthropod predator as a trace maker may include the wide spacing of individual burrows, smooth surfaces of the burrow interior resulting from a silk lining, or bioglyphs such as scratch marks indicating repeated movement through the burrow.
Fossil burrows associated with scorpions are less common but have been described from the Jurassic eolian dunes of North America (Loope, 2008, Engelmann et al., 2014; Good and Ekdale, 2014) and Permian floodplains of Europe (Dunlop et al., 2016). The burrows described from dune deposits have a number of forms and are often irregular in appearance, but in general tend to have low angle slopes, elliptical to circular cross sections, passive to active fill, and sharp contacts with the surrounding matrix without a defined lining or wall or bioglyphs (Loope, 2008, Engelmann et al., 2014; Good and Ekdale, 2014). While alternative trace makers for these burrows were described (i.e., reptiles or mammals) they do possess some of the ichnotaxobases associated with modern scorpion burrows. The burrow described by Dunlop et al. (2016) is unique in that the terminal chamber contains the complete body fossil of a scorpion. The burrow itself is not well defined due to the nature of the specimen’s collection and preparation, but it did appear to include a widened terminal chamber with a connected tunnel. None of the burrows associated with scorpions were attributed to specific ichnotaxa.
In the experiments, centipedes, scorpions, spiders, and whip scorpions produced burrows generally similar to both Arenicolites and Psilonichnus, having forms that were U-shaped to Y-shaped. These are burrow forms that are generally not associated with predators in continental settings, and so this direct association is important for interpretations of ichnofossils assemblages in paleosols. The complex burrows of centipedes, consisting of series of multiple, interconnected U-shaped burrows, most closely resemble Treptichnus, although they are significantly larger and with more pronounced U-shaped segments. Continental examples of Treptichnus have been interpreted as burrows of insect larvae, but these have morphologies that are distinct from those produced by centipedes (Getty et al., 2016). The more complex mazeworks observed in the experiments, such as those produced by the scorpions and whip scorpions, would likely require the establishment of new ichnotaxa if found in the fossil record. Given their prevalence of production by these animals, high burrow complexity could be used as an indication of predation-related activities.
CONCLUSIONS
Improving the interpretation of the behavioral significance of continental ichnofossils as well as their potential trace makers required detailed knowledge of the burrowing techniques, behaviors, and burrows of modern animals. Soil arthropods represent a significant proportion of the trace-making community with continental ecosystems, and likely have been since the Middle Paleozoic, and are, therefore, critical to understand within an ichnologic context. The experimental study of soil arthropods, such as those described here, is an important step in fully realizing the diversity of burrow forms produced by different taxonomic groups, as well as how and why these burrows are produced. While recognition of specific trace makers is an important goal, proper interpretation of the behavioral significance is critically important and typically more attainable (Bromley, 1996). This is especially true when investigating ichnofossils with simple architectures; while one simple burrow form may have been produced by a variety of animals, the behavioral significance in the specific environmental setting is more restricted.
The centipede, scorpion, whip scorpion, and spider species investigated in this study engaged in predation activities closely tied to their burrows, including ambush predation and prey trapping. The burrow morphologies were related to both the body form of the trace maker as well as the functional use of the burrow. Each group of animals studied produced burrows that were generally distinct, yet they possessed similar components, or ichnotaxobases, due to these similar uses. Vertical elements were typically used in ambush predation, whereas horizontal burrows with multiple openings were typically used as prey traps. In addition to serving as sites for prey acquisition, the burrows were also permanent dwellings and were regularly maintained and modified during periods of occupation. The burrows produced were similar in morphology to Skolithos, Palaeophycus, Macanopsis, Arenicolites, Psilonichnus, and Treptichnus. There were also more complex burrows that did not fit within any described ichnogenera.
The recognition of predatory soil arthropod burrows in the fossil record has the potential to increase our understanding of the evolution of predatory arthropods as well as their changing geographic and environmental distribution through time. Ichnofossils of these types of animals are especially important due to the poor preservation potential of soil arthropods in the environments they occupy. Ultimately if we can use continental ichnofossils to recognize all the components of the soil fauna, we will be able to greatly improve interpretations of ancient soil ecosystems including those components of terrestrial food webs that are not preserved as body fossils.
ACKNOWLEDGEMENTS
I would like to thank many students, both undergraduate and graduate, who have helped with this research over the years in the Continental Ichnology Research Laboratory (CIRL) including B. Atkinson, S. Barot, A. Durkee, S. Hampton, M. Hils, M. Ingle, L. Johnson, L. Johnson, M. Reichle, E. Swaninger, R. Tenwalde, H. Thacker, and R. Wawrzynski, as well as funding from the National Science Foundation (EAR-0844256), American Chemical Society Petroleum Research Fund (49387-UNI8), and the Ohio University Office of Research and Sponsored Programs. I would also like to thank two anonymous reviewers and the Handling Editor, L. Laibl, for their valuable comments and assistance.
REFERENCES
Adams, A.M., Marais, E., Turner, J.S., Prendini, L., and Pinshow, B. 2016. Similar burrow architecture of three arid-zone scorpion species implies similar ecological function. Naturwissenschaften, 103(7-8):56. https://doi.org/10.1007/s00114-016-1374-z
Belmontes, F.M., Melchor, R.N., and Piacentini, L.N. 2018. Wolf spider burrows from a modern saline sandflat in central Argentina: morphology, taphonomy and clues for recognition of fossil examples. PeerJ, 6:e5054. https://doi.org/10.7717/peerj.5054
Bertling, M., Braddy, S.J., Bromley, R.G., Demathiey, G.R., Genise, J., Mikuláš, R., Nielsen, J.K., Rindsberg, A.K., Schlirf, M., and Uchman, A. 2006. Names for trace fossils: a uniform approach. Lethaia, 39:265-286. https://doi.org/10.1080/00241160600787890
Bertling, M., Buatois, L.A., Knaust, D., Laing, B., Mángano, M.G., Meyer, N., Mikuláš, R., Minter, N.J., Neumann, C., Rindsberg, A.K., Uchman, A., amd Wisshak, M. 2022. Names for trace fossils 2.0: theory and practice in ichnotaxonomy. Lethaia, 55(3):1-19.
https://doi.org/10.18261/let.55.3.3
Bond, J.E. and Coyle, F.A. 1995. Observation on the natural history of an Ummidia trapdoor spider from Costa Rica (Aranae, Ctenizidae). Journal of Arachnology, 23(3):157-164.
Bowen, J. and Hembree, D. 2014. Neoichnology of two spirobolid millipedes: improving the understanding of the burrows of soil detritivores. Palaeontologia Electronica, 17.1.18A:1–48. https://doi.org/10.26879/395
Bown, T.M. and Kraus, M.J. 1983. Ichnofossils of the alluvial Willwood Formation (Lower Eocene), Bighorn Basin, Northwest Wyoming, USA. Palaeogeography, Palaeoclimatology, Palaeoecology, 43:95-128. https://doi.org/10.1016/0031-0182(83)90050-0
Bromley, R.G. 1996. Trace Fossils: Biology, Taphonomy and Applications. Chapman and Hall, London, United Kingdom.
Buatois, L. and Mángano, G. 2011. Ichnology: Organism-Substrate Interactions in Space and Time. Cambridge University Press, Cambridge, United Kingdom.
Cantil, L.F., Sánchez, M.V., and Genise, J.F. 2014. The nests and brood balls of Canthon (Canthon) virens aff. paraguayanus Balthasar (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 63:384-386.
Cantil, L.F., Sánchez, M.V., Sarzetti, L., Molina, A., and Genise, J.F. 2015. Nests and brood balls of Coprophanaeus (Coprophanaeus) cyanescens (Olsoufieff, 1924) (Coleoptera: Scarabaeidae: Scarabaeinae). The Coleopterists Bulletin, 69:153-158.
Catena, A.M. and Hembree, D.I. 2014. Biogenic structures of burrowing skinks: neoichnology of Mabuya multifasciata (Squamata: Scincidae), p. 343-369. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Topics in Geobiology, 41. Springer Publishing, Dordrecht.
Catena, A.M., Hembree, D.I., Saylor, B.Z., Anaya, F., and Croft, D.A. 2016. Paleoenvironmental analysis of the Neotropical fossil mammal site of Cerdas, Bolivia (middle Miocene) based on ichnofossils and paleopedology. Palaeogeography, Palaeoclimatology, Palaeoecology, 459:423-439. https://doi.org/10.1016/j.palaeo.2016.07.028
Counts, R.R. and Hasiotis, S.T. 2009. Neoichnological experiments with masked chafer beetles (Coleoptera: Scarabaeidae): implications for backfilled continental trace fossils. PALAIOS, 24: 74-91. https://doi.org/10.2110/palo.2008.p08-026r
Doody, J.S., James, H., Colyvas, K., McHenry, C.R., and Clulow, S. 2015. Deep nesting in a lizard, déjà vu devil’s corkscrew: first helical reptile burrow and deepest vertebrate nest. Biological Journal of the Linnean Society, 116:13-26. https://doi.org/10.1670/13-006
Doody, J.S., James, H., Ellis, R., Gibson, N., Raven, M., Mahony, S., Hamilton, D.G., Rhind, D., Clulow, S., and McHenry, C.R. 2014. Cryptic and complex nesting in the yellow-spotted monitor, Varanus panoptes. Journal of Herpetology, 48:363-370. https://doi.org/10.1670/13-006
Dunlop, J.A. 2010. Geologic history and phylogeny of Chelicerata. Arthropod Structure & Development, 39:124-142. https://doi.org/10.1016/j.asd.2010.01.003
Dunlop, J.A. and Braddy, S.J. 2011. Cteniza bavincourti and the nomenclature of arachnid-related trace fossils. The Journal of Arachnology, 39(2):250-257. https://doi.org/10.1636/CHa10-70.1
Dunlop, J.A. and Selden, P.A. 2013. Scorpion fragments from the Silurian of Powys, Wales. Arachnology, 16(1):27-32. https://doi.org/10.13156/arac.2013.16.1.27
Dunlop, J.A., Legg, D.A., Selden, P.A., Fet, V., Schneider, J.W., and Rößler, R. 2016. Permian scorpions from the Petrified Forest of Chemnitz, Germany. BMC Evolutionary Biology, 16:72. https://doi.org/10.1186/s12862-016-0634-z
Dzenowski, N.D. and Hembree, D.I., 2014. The neoichnology of two terrestrial salamanders: quantifying amphibian burrows using modern analogs, p. 305-341. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Topics in Geobiology 41. Springer Publishing, Dordrecht.
Engelmann, G.F., Chure, D.J., and Good, T.R. 2014. Large burrows in the dunes of the Nugget Sandstone, Early Jurassic?, of NE Utah. New Mexico Museum of Natural History Bulletin, 62:197-203.
Genise, J.F. 2017. Ichnoentomology: Insect Traces in Soils and Paleosols. Topics in Geobiology 37. Springer Publishing, Dordrecht.
Genise, J.F., Cantil, L.F., Dinghi, P.A., Sánchez, M.V., and Sarzetti, L. 2013. The aestivation chamber of the giant earthworm Glossoscolex bergi (Glossoscolecidae) in the subtropical rainforest of Misiones (Argentina). Ichnos, 20:116-119. https://doi.org/10.1080/10420940.2013.812964
Gess, R.W. 2013. The earliest record of terrestrial animals in Gondwana: a scorpion from the Famennian (Late Devonian) Witpoort Formation of South Africa. African Invertebrates, 54(2):373-379. https://doi.org/10.5733/afin.054.0206
Getty, P.R., McCarthy, T.D., Hsieh, S., and Bush, A.M. 2016. A new reconstruction of continental Treptichnus based on exceptionally preserved material from the Jurassic of Massachusetts. Journal of Paleontology, 90(2):269-278. https://doi.org/10.1017/jpa.2016.20
Gobetz, K.E. 2005. Claw impressions in the walls of modern mole (Scalopus aquaticus) tunnels as a means to identify fossil burrows and interpret digging movements. Ichnos, 12:227-231. https://doi.org/10.1080/10420940591009006
Good, T.R. and Ekdale, A.A. 2014. Paleoecology and taphonomy of trace fossils in the eolian Upper Triassic/Lower Jurassic Nugget Sandstone, northeastern Utah. PALAIOS, 29:401-413. https://doi.org/10.2110/palo.2014.013
Halfen, A.F. and Hasiotis, S.T. 2010. Neoichnological study of the traces and burrowing behaviors of the western harvester ant Pognomyrmex occidentalis (Insecta: Hymenoptera: Formicidea): paleopedogenic and paleoecologic implications. PALAIOS, 25:703-720.
https://doi.org/10.2110/palo.2010.p10-005r
Harington, A. 1978. Burrowing biology of the scorpion Cheloctonus jonesii (Arachnida: Scorpionida: Scorpionidae). Journal of Arachnology, 5:243-249.
Hasiotis, S.T. 2002. Continental Trace Fossils. SEPM Short Course Notes, 51. Society for Sedimentary Geology, Tulsa, Oklahoma, USA.
Hasiotis, S.T. 2007. Continental ichnology: fundamental processes and controls on trace fossil distribution. The Continental Realm, p. 268-284. In Miller, W. (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier Science.
Hembree, D.I. 2009. Neoichnology of burrowing millipedes: linking modern burrow morphology, organism behavior, and sediment properties to interpret continental ichnofossils. PALAIOS, 24:425-439. https://doi.org/10.2110/palo.2008.p08-098r
Hembree, D.I. 2013. Neoichnology of the whip scorpion Mastigoproctus giganteus: complex burrows of predatory terrestrial arthropods. PALAIOS, 28:141-162. https://doi.org/10.2110/palo.2012.p12-079r
Hembree, D.I. 2014. Large, complex burrows of terrestrial invertebrates: neoichnology of Pandinus imperator, p. 229-263. In Hembree, D.I., Platt, B.F., and Smith, J.J. (eds.), Experimental Approaches to Understanding Fossil Organisms: Lessons from the Living. Topics in Geobiology 41. Springer Publishing, Dordrecht.
Hembree, D.I. 2016. Using experimental neoichnology and quantitative analyses to improve the interpretation of continental trace fossils. Ichnos, 23:262-297. https://doi.org/10.1080/10420940.2016.1202684
Hembree, D.I. 2017. Neoichnology of tarantulas (Araneae: Theraphosidae): criteria for recognizing spider burrows in the fossil record: Palaeontologia Electronica, 20.3.45A:1-30. https://doi.org/10.26879/780
Hembree, D.I. 2019. Burrows and ichnofabric produced by centipedes: modern and ancient examples. PALAIOS, 34:468-489. https://doi.org/10.2110/palo.2019.059
Hembree, D.I. and Hasiotis, S.T. 2006. The identification and interpretation of reptile ichnofossils in paleosols through modern studies. Journal of Sedimentary Research, 76:575-588. https://doi.org/10.2110/JSR.2006.049
Hembree, D.I. and Hasiotis, S.T. 2007. Biogenic structures produced by sand-swimming snakes: a modern analog for interpreting continental ichnofossils. Journal of Sedimentary Research, 77:389-397. https://doi.org/10.2110/jsr.2007.034
Hembree, D.I., Johnson, L.M., and Tenwalde, R.W. 2012. Neoichnology of the desert scorpion Hadrurus arizonensis: burrows to biogenic cross lamination. Palaeontologia Electronica, 15.1.10A:1-14. https://doi.org/10.26879/292
Hils, J.M. and Hembree, D.I. 2015. Neoichnology of the burrowing spiders Gorgyrella inermis (Mygalomorphae: Idiopidae) and Hogna lenta (Araneomorphae: Lycosidae). Palaeontologica Electronica, 18.1.7A:1-62. https://doi.org/10.26879/500
Jackson, T.P. 2000. Adaptation to living in an open arid environment: lessons from the burrow structure of the two southern African whistling rats, Parotomys brantsii and P. littledalei. Journal of Arid Environments, 46:345-355. https://doi.org/10.1006/jare.2000.0683
Kelley, P. and Hansen, T.A. 2003. The fossil record of drilling predation on bivalves and gastropods, p. 113-139. In Kelley, P.H., Kowalewski, M., and Hansen, T.A. (eds.), Predator-Prey Interactions in the Fossil Record. Topics in Geobiology, vol 20. Springer, Boston.
https://doi.org/10.1007/978-1-4615-0161-9_6
Kinlaw, A. and Grasmueck, M. 2012. Evidence for and geomorphologic consequences of a reptilian ecosystem engineer: the burrowing cascade initiated by the gopher tortoise. Geomorphology, 157-158:108-121. https://doi.org/10.1016/j.geomorph.2011.06.030
Knaust, D. 2012. Trace-fossil systematics, p. 79-102. In Knaust, D. and Bromley, R.G. (eds.), Trace Fossils as Indicators of Sedimentary Environments. Elsevier, Amsterdam, the Netherlands.
Koch, L.E. 1978. A comparative study of the structure, function, and adaptation to different habitats of burrows in the scorpion genus Urodacus (Scorpionida, Scorpionidae). Records of the Western Australian Museum, 6:119-146.
Lavelle, P. and Spain, A.V. 2005. Soil Ecology. Springer Publishing, Dordrecht, the Netherlands.
Lawfield, A.M.W. and Pickerell, R.K. 2006. A novel contemporary fluvial ichnocoenose: Unionid bivalves and the Scoyenia-Mermia ichnofacies transition. PALAIOS, 21:391-396. https://doi.org/10.2110/palo.2006.P06-08
Lehane, J.R. and Ekdale, A.A. 2013. Pitfalls, traps, and webs in ichnology: traces and trace fossils of an understudied behavioral strategy. Palaeogeography, Palaeoclimatology, Palaeoecology, 375:59-69. https://doi.org/10.1016/j.palaeo.2013.02.014
Loope, D.B. 2008. Life beneath the surfaces of active Jurassic dunes: burrows from the Entrada Sandstone of south-central Utah. PALAIOS, 23:411-419. https://doi.org/10.2110/palo.2006.p06-133r
MacEachern, J.A., Bann, K.L., Gingras, M.K., Zonneveld, J.P., Dashtgard, S.E., and Pemberton, S.G. 2012a. The ichnofacies paradigm, p. 329-378. In Knaust, D. and Bromley, R.G. (eds.), Trace Fossils as Indicators of Sedimentary Environments. Elsevier, Amsterdam, the Netherlands.
MacEachern, J.A., Dashtgard, S.E., Knaust, D., Catuneanu, O., Bann, K.L., and Pemberton, S.G., 2012b. Sequence stratigraphy, p. 157-194. In Knaust, D. and Bromley, R.G. (eds.), Trace Fossils as Indicators of Sedimentary Environments. Elsevier, Amsterdam, the Netherlands.
Mángano, M.G., Buatois, L.A., and MacNaughton, R.B. 2012. Ichnostratigraphy, p. 195-212. In Knaust, D. and Bromley, R.G. (eds.), Trace Fossils as Indicators of Sedimentary Environments. Elsevier, Amsterdam, the Netherlands.
Martin, R.E. 1999. Taphonomy: A Process Approach. Cambridge University Press, Cambridge, United Kingdom.
Meadows, P.S. 1991. The environmental impact of bur- rows and burrowing animals - conclusions and a model, p. 327-388. In Meadows, P.S. and Meadows, A. (eds.), The Environmental Impact of Burrowing Animals and Animal Burrows. Clarendon Press, Oxford, United Kingdom.
Melchor, R.N., Bedatou, E. de Valais, S., and Genise, J.F. 2006. Lithofacies distribution of invertebrate and vertebrate trace fossil assemblages in an Early Mesozoic ephemeral fluvio-lacustrine system from Argentina: implications for the Scoyenia ichnofacies. Palaeogeography, Palaeoclimatology, Palaeoecology, 239(3-4):253-285. https://doi.org/10.1016/j.palaeo.2006.01.011
Melchor, R.N., Genise, J.F., Umazano, A.M., and Superina, M. 2012. Pink fairy armadillo meniscate burrows and ichnofabrics from Miocene and Holocene interdune deposits of Argentina: palaeoenvironmental and palaeoecological significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 350-352:149-170. https://doi.org/10.1016/j.palaeo.2012.06.026
Mikuś, P. and Uchman, A. 2013. Beetle burrows with a terminal chamber: A contribution to the knowledge of the trace fossil Macanopsis in continental sediments. PALAIOS, 28:403-413. https://doi.org/10.2110/palo.2012.p12-129r
Miller, G.L. and Miller, P.R. 1984. Correlations of burrow characteristics and body size in burrowing wolf spiders (Aranae: Lycosidae). The Florida Entomologist, 67(2):314-317.
Monaenkova, D., Gravish, N., Rodriguez, G., Kutner, R., Goodisman, M.A.D., and Goldman, D.I. 2015. Behavioral and mechanical determinants of collective subsurface nest excavation. The Journal of Experimental Biology, 218:1295-1305. https://doi.org/10.1242/jeb.113795
Morrissey, L.B., Hillier, R.D., and Marriott, S.B. 2012. Late Silurian and Early Devonian terrestrialisation: ichnological insights from the Lower Old Red Sandstone of the Anglo-Welsh Basin, U.K. Palaeogeography, Palaeoclimatology, Palaeoecology, 337-338:194-215.
https://doi.org/10.1016/j.palaeo.2012.04.018
M’rabet, S.M., Henaut, Y., Sepulveda, A., Rojo, R., Calme, S., and Geissen, V. 2007. Soil preference and burrow structure of an endangered tarantula, Brachypelma vagans (Mygalomorphae: Theraphosidae). Journal of Natural History, 41:1025-1033.
https://doi.org/10.1080/00222930701384547
Nascimento, D.L., Netto, R.G., and Indicatti, R.P. 2021. Neoichnology of mygalomorph spiders: improving the recognition of spider burrows in the fossil record. Journal of South American Earth Science, 108:103178. https://doi.org/10.1016/j.jsames.2021.103178
Nebelsick, J.H. and Kowaleski, M. 1999. Drilling predation on recent clypeasteroid echinoids from the Red Sea. PALAIOS, 14:127-144.
https://doi.org/10.2307/3515369
Platt, B.F., Hasiotis, S.T., and Hirmas, D.R. 2012. Empirical determination of physical controls on megafaunal footprint formation through neoichnological experiments with elephants. PALAIOS, 27:725-737. https://doi.org/10.2110/palo.2012.p12-006r
Polis, G.A., Myers, C., and Quinlan, M. 1986. Burrowing biology and spatial distribution of desert scorpions. Journal of Arid Environments, 10:137-146.
Potopov, A.M., Beaulier, F., Birkhofer, K., Bluhm, S.L., Degtyarev, M.I., Devetter, M., Goncharov, A.A., Gongalsky, K.B., Klarner, B., Korobushkin, D.I., Liebke, D.F., Maraun, M., McDonnell, R.J., Pollierer, M.M., Schaefer, I., Shrubovych, J., Semenyuk, I.I., Sendra, A., Tuma, J., Tůmová, M., Vassilieva, A.B., Chen, T.W., Geisen, S., Schmidt, O., Tiunov, A.V., and Scheu, S. 2022. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biological Reviews, 97:1057-1117. https://doi.org/10.1111/brv.12832
Retallack, G.J., 2001. Soils of the Past, second edition. Blackwell Science, Oxford, United Kingdom.
Rodríguez-Tovar, F.J. 2007. Substrate firmness controlling nesting behavior of Bembix oculata (Hymenoptera, Bembicinae), p. 353-359. In Bromley, R.G., Buatois, L.A., Mángano, G., Genise, J.F., and Melchor, R.N. (eds.), Sediment-Organism Interactions: A Multifaceted Ichnology. Society for Sedimentary Geology, Tulsa.
Sarzetti, L.C., Genise, J.F., and Sanchez, M.V. 2014. Nest architecture of Oxaea austere (Andrenidae, Oxaeinae) and its significance for the interpretation of Uruguayan fossil bee cells. Journal of Hymenoptera Research, 39:59-70. https://doi.org/10.3897/JHR.39.8201
Savrda, C.E. 2007. Taphonomy of trace fossils, p. 92–109. In Miller, W. III (ed.), Trace Fossils: Concepts, Problems, Prospects. Elsevier, Amsterdam, the Netherlands.
Schaetzl, R. and Anderson, S. 2005. Soils: Genesis and Geomorphology. Cambridge University Press, Cambridge, United Kingdom.
Scisco, L., Broderick, T.J., Barrett, P.M., Munyikwa, D., Zondo, M., and Choiniere, J.N. 2021. Invertebrate and plant trace fossils from the terrestrial Late Triassic of Zimbabwe. PALAIOS, 36:129-140. http://doi.org/10.2110/palo.2020.071
Selden, P.A. 1996. First fossil mesothele spider, from the Carboniferous of France. Revue Suisse de Zoologie, 2:585-596.
Shear, W.A. and Bonamo, P.M. 1988. Devonobiomorpha, a new order of centipeds (Chilopoda) from the Middle Devonian of Gilboa, New York State, USA, and the phylogeny of centiped orders. American Museum Novitates, 2927:1-30.
Shivashankar, T. 1994. Advanced subsocial behaviour in the scorpion Heterometrus fulvipes Brunner (Arachnida). Journal of Biosciences, 19:81-90.
Shorthouse, D.J. and Marples, T.G. 1980. Observations on the burrow and associated behavior of the arid-zone scorpion Urodacus yaschenkoi (Birula). Australian Journal of Zoology, 28:581-590. https://doi.org/10.1071/ZO9800581
Smith, J.J. and Hasiotis, S.T. 2008. Traces and burrowing behaviors of the cicada nymph Cicadetta calliope: neoichnology and paleoecological significance of extant soil-dwelling insects. PALAIOS, 23:503-513. http://doi.org/10.2110/palo.2007.p07-063r
Smith, J.J., Hasiotis, S.T., Woody, D.T., and Kraus, M.J. 2008. Paleoclimatic implications of crayfish-mediated prismatic structures in paleosols of the Paleogene Willwood Formation, Bighorn Basin, Wyoming, U.S.A. Journal of Sedimentary Research, 78:323-334.
https://doi.org/10.2110/jsr.2008.040
Tetlie, O.E. and Dunlop, J.A. 2008. Geralinura carbonaria (Arachnia; Uropygi) from Mazon Creek, Illinois, USA, and the origin of subchelate pedipalps in whip scorpions. Journal of Paleontology, 82:299-312. https://doi.org/10.1666/06-138.1
Thacker, H.A. and Hembree, D.I. 2021. Neoichnological study of two species of burrowing darkling beetles (Coleoptera: Tenebrionidae) from larval to adult stages. Ichnos, 28(4):290-308. https://doi.org/10.1080/10420940.2021.1941001
Tschinkel, W.R. 2003. Subterranean ant nests: trace fossil past and future. Palaeogeography, Palaeoclimatology, Palaeoecology, 192:321-333.
https://doi.org/10.1016/j.palaeo.2011.05.046
Uchman, A., Vrenozi, B., and Muceku, B. 2018. Spider burrows in ichnological context: a review of literature data and burrows of the wolf spider Trochosa hispanica Simon, 1870 from Albania. Rendiconti Lincei. Scienze Fisiche e Naturali, 29:67-79.
https://doi.org/10.1007/s12210-017-0662-7
Williams, S.C. 1966. Burrowing activities of the scorpion Anuroctonus phaeodactylus (Wood) (Scorpionida: Vaejovidae). Proceedings of the California Academy of Sciences, 34:419-428.
Williams, S.C. 1987. Scorpion bionomics. Annual Review of Entomology, 32:275-295.

