 Healed antler fracture from a giant deer (Megaloceros giganteus) from the Pleistocene in Poland
Healed antler fracture from a giant deer (Megaloceros giganteus) from the Pleistocene in Poland
Article number: 17.2.23A
https://doi.org/10.26879/448
Copyright Palaeontological Association, May 2014
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 5 December 2013. Acceptance: 27 March 2014
{flike id=732}
ABSTRACT
We evaluated the skull of an ancient giant deer with a deformity of one antler. The skull was found in the 1930s in the San River near Barycz, in southeastern Poland. Its dating (39,800±1000 yr BP) corresponds to MIS-3, when the giant deer was widespread in Europe. Our diagnostics for the antler included gross morphology, radiography, computed tomography, and histopathology. We noted signs of fracture healing of the affected antler, including disordered arrangement of lamellae, absence of osteons, and numerous Volkmann's canals remaining after blood vessel loss. The antler deformity appears to be of traumatic origin, with a healing component. No similar evaluation process has been described previously for this species.
Kamilla Pawłowska. Institute of Geology, Adam Mickiewicz University, Maków Polnych 16, Poznań 61-606, Poland, koka@amu.edu.pl
Krzysztof Stefaniak. Division of Palaeozoology, Department of Evolutionary Biology and Ecology, Faculty of Biological Sciences, University of Wrocław, Sienkiewicza 21, Wrocław 50-335, Poland, stefanik@biol.uni.wroc.pl
Dariusz Nowakowski. Department of Anthropology, Wroclaw University of Environmental and Life Sciences, Kożuchowska 6/7, Wrocław 51-631, Poland, darekn@hot.pl
Keywords: giant deer; Megaloceros giganteus; paleopathology; Pleistocene; Poland
Final citation: Pawłowska, Kamilla, Stefaniak, Krzysztof, and Nowakowski, Dariusz. 2014. Healed antler fracture from a giant deer (Megaloceros giganteus) from the Pleistocene in Poland. Palaeontologia Electronica Vol. 17, Issue 1;23A; 9p. https://doi.org/10.26879/448
palaeo-electronica.org/content/2014/732-antler-fracture-in-giant-deer
INTRODUCTION
The giant deer (Megaloceros giganteus Blumenbach 1799)[AUTHOR this reference is needed for reference list] was widespread in Pleistocene Eurasia (Stuart et al., 2004; Van der Made, 2006; Pushkina, 2007; Van der Made and Tong, 2008; Shpansky, 2011; Vislobokova, 2011, 2012). Giant deer antlers are robust, palmate, and extend laterally (Van der Made and Tong, 2008; Stuart et al., 2004), often spanning 3.5 m or more and weighing up to 45 kg (Lister, 1994). The roles of such large antlers have been discussed (Barnosky, 1985; Stuart et al., 2004; Benton and Harper, 2009). Antlers in cervids, including giant deer, are used for display and for combat between males seeking to establish territories (Barnosky, 1985; Benton and Harper, 2009), and are also employed when males compete for dominance and access to females in the rut (e.g., Lincoln, 1992).
The seminal study of the antlers of giant deer, specifically concerning their positive allometry, was performed by Gould (1974). Based on the measurements of 79 skulls and antlers, he showed that giant deer have about the predicted antler size for their body size, in contrast with other cervids, such as moose and fallow deer (which have, respectively, smaller and larger antlers than expected). Stuart et al. (2004) indicate that it is likely that the huge antlers would have excluded giant deer males from even moderately dense woodland, at least for part of the year.
Morphological (Breda, 2005), paleoecological (Van der Made and Tong, 2008; Chritz et al., 2009), paleobiogeographical (Stuart et al., 2004), evolutionary (Vislobokova, 2012), and phylogenetic (Lister et al., 2005; Hughes et al., 2006; Van der Made and Tong, 2008) studies, among others, of giant deer have been published. However, morphological reports have not included the histological detail of normal and pathological antler tissue. Antlers are very tough structures that are able to absorb repeated severe impacts with low likelihood of antler fracture (Currey, 2002).
Here, we report evaluation of a misshapen right antler from a giant deer. This rare finding offers a unique opportunity for detailed study of the healing of fractured antlers of ancient animals, using morphological observations, radiography, computed tomography, and histology.
MATERIALS AND METHODS
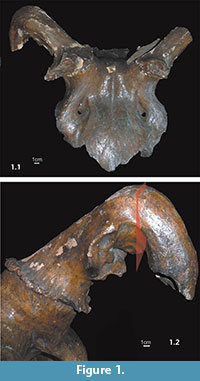 The skull of this giant deer was found in the 1930s in the San River near Barycz (49o49'N, 22o03'E) (Niezabitowski, 1935) (Figure 1.1). Presently, it is curated in the collections of the Institute of Geology, Adam Mickiewicz University, Poznań, Poland (IG-Br.12F1).
The skull of this giant deer was found in the 1930s in the San River near Barycz (49o49'N, 22o03'E) (Niezabitowski, 1935) (Figure 1.1). Presently, it is curated in the collections of the Institute of Geology, Adam Mickiewicz University, Poznań, Poland (IG-Br.12F1).
The skull was described initially by Niezabitowski (1935) who gave a detailed description of its morphology, antler deformity, and measurements. However, his investigations were limited by the research methods available at the time. For example, the observed antler deformity was described without the use of any method such as radiography. For the same reason, a revision of the measurements of the skull using modern methods was made by one of us (KP) and has been described by Croitor et al. (2014), with the conclusion that the skull morphometry corresponds to that of larger specimens from Ireland according to Lister (1994).
The Niezabitowski (1935) study also lacked a taphonomic analysis. Two hypotheses were given by the author: (i) the flexion of the antler is most likely the result of being hit by the paw of a predator, and (ii) the deer was killed by a predator attack that has left marks in the form of cavities within the occipital part of the skull. Both hypotheses are discussed here in the light of the results of paleopathological and taphonomic research.
The phylogenetic position of the giant deer, based on mtDNA sequences (Lister et al., 2005; Hughes et al., 2006), is closest to extant Dama species. Since the giant deer is extinct, we based our comparative evaluation on the antler development of extant cervids, especially Dama dama.
Craniofacial (norma frontalis) digital radiographs were made, with focus-object distance 1 m, 85 kV and 30 mAs (using a GE Silhouette VR X-ray device, USA/China). Computed tomography (CT) was performed in frontal and lateral (Figure 1.2) planes, scanning the object every 6 mm, 130 kV, 30 mAs (using a Siemens Tomaton Emotion 6 X-ray device). Radiographs and CT were performed at the Department of General and Clinical Anatomy, Pomeranian Medical University, Szczecin, Poland.
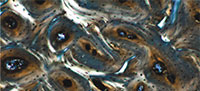
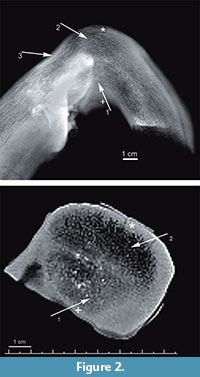 Ground sections of antler were obtained for examination by light microscopy, in white and polarized light (Nikon Eclipse, 80i with lens Nikon Plan Fluor 10x/0.30, Japan, at the Department of Anthropology, Wroclaw University of Environmental and Life Sciences). Based on radiographic images, three antler foci were selected for histological analysis: (a) a radiographically normal site (Figure 2.1, arrow 2); (b) a site of morphologically obvious deformation (Figure 2.1, arrow 1); (c) a morphologically obvious bony outgrowth. Histological analysis concerned both the compact and cancellous antler bone tissues. We present histological images of compact antler bone tissue from two selected locations within the foci (Figure 2.1-2, asterisk and cross). Samples for histological examination were cut with a diamond saw (Dremel multitool, model 4000 with diamond cutting wheel, model SC545, shank diameter 3.2 mm, working diameter 38.0 mm, Dremel Europe, The Netherland) in the plane shown in Figure 1.2. Sections were made using the method of Schultz (2003), with the modifications of de Boer et al. (2013). Unstained slides (a cross-section of the antler, 20–30 μm in thickness) were examined histologically using light microscopy. Photos were taken with a camera (Canon Eos 50D, 4752×3168 dpi) mounted on the microscope.
Ground sections of antler were obtained for examination by light microscopy, in white and polarized light (Nikon Eclipse, 80i with lens Nikon Plan Fluor 10x/0.30, Japan, at the Department of Anthropology, Wroclaw University of Environmental and Life Sciences). Based on radiographic images, three antler foci were selected for histological analysis: (a) a radiographically normal site (Figure 2.1, arrow 2); (b) a site of morphologically obvious deformation (Figure 2.1, arrow 1); (c) a morphologically obvious bony outgrowth. Histological analysis concerned both the compact and cancellous antler bone tissues. We present histological images of compact antler bone tissue from two selected locations within the foci (Figure 2.1-2, asterisk and cross). Samples for histological examination were cut with a diamond saw (Dremel multitool, model 4000 with diamond cutting wheel, model SC545, shank diameter 3.2 mm, working diameter 38.0 mm, Dremel Europe, The Netherland) in the plane shown in Figure 1.2. Sections were made using the method of Schultz (2003), with the modifications of de Boer et al. (2013). Unstained slides (a cross-section of the antler, 20–30 μm in thickness) were examined histologically using light microscopy. Photos were taken with a camera (Canon Eos 50D, 4752×3168 dpi) mounted on the microscope.
RESULTS AND DISCUSSION
The giant deer appeared about 0.4 Myr ago (Lister, 1994) and survived till around 6900 radiocarbon yr BP (about 7,700 yr ago) in western Siberia (Stuart et al., 2004). In Poland, giant deer remains have been found mainly in the south (Kowalski, 1958; Stefaniak et al., 2009), and more recently in the central part of the country (Pawłowska, unpublished data).
Niezabitowski (1935) mentioned that the skull was found in Barycz, near the banks of the river San, but gave no details of dating. The obtained radiocarbon date for the healthy antler fragment (uncalibrated date of 39,800 ± 1000 yr BP, laboratory #Poz-52083, Poznan Radiocarbon Laboratory, Poznań, Poland) corresponds to MIS-3, when the giant deer was very common in the higher and middle latitudes of Europe.
Cervids are the only animals possessing antlers, which are entirely bony structures (after velvet shedding). They should thus not be confused with horns, which are found only in bovids and are composed of a scabbard-like keratinous sheath covering a permanent bony horn core (Davis et al., 2011). The specimen from Barycz came from an adult individual with incomplete fusion of cranial sutures.
The presence of antlers indicates that the individual was male. Antlers develop only in the male (Geist, 1998), with the exception of reindeer (Rangifer tarandus), in which both sexes may have antlers, and pathological cases in cervids such as hermaphroditism (Jaczewski, 1992). Footnote 1 The fully developed burr in the skull from Barycz indicates fully grown antlers that are present only in the autumn and winter, based on knowledge of antler cycles in the fallow deer, Dama dama (Barnosky, 1985). Thus, the individual probably died prior to annual shedding of the antlers (Lister, 1994).
The specimen is incomplete, with preserved fragments including damaged occipital bone, fragments of right and left temporal, parietal, lacrimal, and sphenoidal bones, and frontal bone with basal parts of antlers (Figure 1.1). The preserved left antler fragment is 110 mm long, and consists of burr, incomplete brow tine, and a portion of the main beam. The right antler features a downward bend in its main beam of 110o at a distance of about 150 mm from the burr (Figure 1.1). A bony outgrowth of 66.4 mm × 38.8 mm (anterioposterior × mediolateral) and of maximum depth 13.8 mm, is concave and visible just above the brow tine, in the posterior part of the bend (Figure 1.2). Inside this structure, gravel is preserved, supporting our conclusion (below) about the specimen's movement within the sandy-gravel fraction. No part of the antler is preserved distal to about 140 mm from the bend.
Radiography of the antler cross-section shows an area (1 x 2 cm) of higher-density tissue in the more ventral part of the antler, at the bend, reaching to half of its diameter (Figure 2.1, arrow 1). Above this feature, the structure of the cancellous core of antler is normal (Figure 2.1, arrow 2). A narrow area (3 cm long and 0.5 cm thick) on the opposite margin of the antler shows a focus of increased tissue density (Figure 2.1, arrow 3). CT scan of the antler cross-section at its bend reveals two foci of different tissue density within a cancellous core; the more ventral focus has greater density (Figure 2.2, arrow 1), while the more dorsal focus suggests normal antler structure (Figure 2.2, arrow 2).
Radiography and CT reveal the foci with abnormal tissue density in the more ventral focus. A further interpretation of this structure was based on histological studies (see below). The more dorsal focus is normal tissue; the boundary between the two areas resulting from the fracture is distinct. This means the two foci are next to each other, within one section, and there is no transitory zone. The remaining parts of the antler appear to be normal.
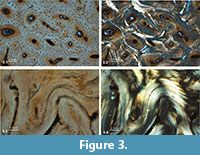 Histological evaluation of antler structure, development, and pathology has been focused on extant cervids, including sika deer (Cervus nippon) (Gao et al., 2010) and fallow deer (Dama dama) (Kierdorf et al., 2004). Similar information on antler histology of extinct cervids is scanty (Paral et al., 2007). Our histological examination of the focus having a normal radiological shadow (Figure 2.1, asterisk) reveals the expected features of orderly arrangement of osteons of similar diameter, and well-developed Haversian systems (compact bone tissue, Figure 3.1-2). The osteons are clearly primary osteons that filled in vascular spaces within a trabecular framework, corresponding to that described for the compact antler tissue of the red deer (Cervus elaphus) (Gomez et al., 2013).
Histological evaluation of antler structure, development, and pathology has been focused on extant cervids, including sika deer (Cervus nippon) (Gao et al., 2010) and fallow deer (Dama dama) (Kierdorf et al., 2004). Similar information on antler histology of extinct cervids is scanty (Paral et al., 2007). Our histological examination of the focus having a normal radiological shadow (Figure 2.1, asterisk) reveals the expected features of orderly arrangement of osteons of similar diameter, and well-developed Haversian systems (compact bone tissue, Figure 3.1-2). The osteons are clearly primary osteons that filled in vascular spaces within a trabecular framework, corresponding to that described for the compact antler tissue of the red deer (Cervus elaphus) (Gomez et al., 2013).
The pathological focus, with higher radiodensity (Figure 2.1, cross), reveals disorganized primary bone, prior to osteoclast-mediated remodeling. No osteons are visible, lamellar bone is disorganized, and numerous blood vessels are seen (Figure 3.3-4). This also is true for the examined tissue from the bony outgrowth. These observations are consistent with a fracture healing process and callus formation (Grupe and Peters, 2006).
A previous histological study of hard antlers in extant cervids revealed living bone with regions presenting living osteocytes, active osteoblasts, osteoid seams, and even early stages of trabecular microcallus formation, thus indicating continuous bone remodeling (Rolf and Enderle, 1999). In a more recent study, however, there is clear evidence that, following velvet shedding, no further mineralization occurs within the antler bone and the antler dies due to ischemic necrosis (Gomez et al., 2013). Hence, in the case of the specimen from Barycz, the observed remodeling process is related to the healing of a fractured velvet antler.
Many factors can cause or affect the expression of antler abnormalities, including genetics, age, nutrition, injury, hormones, and disease (Heffelfinger, 2006). In this study, results indicate that the reason for the antler malformation was mechanical damage on the underside of the antler, causing tissue discontinuity that encompassed half of the antler thickness. As a result of impact, the antler was first displaced upward, and then the fractured part shifted downward under gravity and became fixed in this position, due to the formation of a fracture callus. The fracture of the velvet antler did not lead to the loss of the distal fragment, because it was apparently held by the skin and periosteum.
There are known cases of fractured antler that has lost the distal fractured fragment—for example, a red deer specimen from Croatia, in which the fracture resulted from the impaired mechanical stability of the antler caused by an inflammation resulting from an injury to the velvet antler (Kierdorf et al., 2013). By contrast, fractured antlers without damaged velvet are usually associated with the formation of the antler so-called Blasengeweih, not observed here, as a result of hemorrhage or abscess, later overgrown by tissue and ossified (Bubenik, 1966; Jaczewski, 1992).
The hypothesis of Niezabitowski that the flexion of the antler is most likely the result of impact from the paw of a predator cannot be verified, because claw marks are unlikely to remain on the velvet antler. This finding is rare, taking into consideration the Pleistocene age of the skull cap. However, the abnormality of the antler in cervids is not uncommon (Goss, 1983), and examples of similar antler malformation have been given by Bubenik (1966), Jaczewski (1992), and others.
The visible phases of osseous substance formation and callus reabsorption that we observed could take place only when the animal was alive. These observations suggest a healing process of approximately 2-3 months, since antler tissue regeneration progresses more rapidly than other bone tissue regeneration (Kierdorf et al., 2004; Li, 2012). Consequently, the death of the animal appears to have occurred during antler healing, or may have occurred following velvet shedding from the healed antler, but prior to antler shedding.
Mineralogical analysis of giant deer antler, and especially the orientation of hydroxyapatite crystals, suggests that bulls used their antlers not only for display, but also for dominance fighting during the rut (Moen et al., 1999). Thus, the fracture probably had an effect on the bull's hierarchical position, negatively impacting its reproductive position in the herd. Further, competitive disadvantage could have resulted in deterioration of the animal's physical condition, secondary to foraging consequences of reduced social position.
The application of taphonomical analysis provides the possibility of reconstructing the environmental conditions under which the skeleton or bone complex was preserved, as well as the depositional history of the bones (post-consumption remnants, flood remains, and accumulation of bones by predators) (Pawłowska, 2010). In the case of the skull cap from Barycz, the described damage and deficiencies are, in most cases, predepositional, thus allowing them to be interpreted in relation to the depositional context. Both the burr and the brow tine of the left and right antler have rounded and shiny edges. Also, in some places, the surface is shiny and exhibits small rounded indentations. All of these features are characteristic of the movement of the bone in water with a gravel or sand sediment (Pawłowska et al., 2014; with further references), which is also corroborated by the gravel present inside the boney outgrowth. In conclusion, the specimen should be considered as being in a secondary, rather than a primary, context.
Furthermore, the taphonomic analysis showed no traces of predator-caused modifications (such as gnawing or scratches), in contrast to earlier suggestions (Niezabitowski, 1935) that a predator attack was the direct cause of death of the animal on the basis of marks left in the form of two triangular cavities within the occipital part of the skull. These cavities are indeed predepositional, but their rounded edges again suggest damage during water movement, and are thus associated with the depositional history of the specimen. In spite of this, the possibility that the deer was killed by a predator, but that the attack left no traces on the recovered element, should be taken into account.
ACKNOWLEDGEMENTS
The research was funded as the project of the Polish Ministry of Science and Higher Education (NN 307050139; K. Pawłowska was the grant manager). We are thankful to the anonymous reviewers for their very helpful comments on the manuscript. We also thank Prof. B. Pokryszko (Museum of Natural History, Wrocław University) for translating previous version of this paper into English.
REFERENCES
Barnosky, A.D. 1985. Taphonomy and herd structure of the extinct Irish elk (Megaloceros giganteus). Science, 228:340-344.
Benton, M.J. and Harper, D.A.T. 2009. Introduction to Paleobiology and the Fossil Record. Wiley-Blackwell.
Blumenbach, J. H. 1799. Handbuch der Naturgeschichte (6th ed.), Gottingen, Germany:
J. H. Dietrich.
Breda, M. 2005. The morphological distinction between the postcranial skeleton of Cervalces/Alces and Megaloceros giganteus and comparison between the two Alceini genera from the Upper Pliocene–Holocene of Western Europe. Geobios, 38:151-170.
Bubenik, A.B. 1966. Das Geweih. Entwicklung, Aufbau und Ausformung der Geweihe und Gehörne und ihre Bedeutung für das Wild und für die Jagd. Verlag Paul Parey, Hamburg und Berlin, 214 pp.
Chritz, K.L., Dyke, G.J., Zazzo, A., Lister, A.M., Monaghan, N.T., and Sigwart, J.D. 2009. Palaeobiology of an extinct Ice Age mammal: Stable isotope and cementum analysis of giant deer teeth. Palaeogeography, Palaeoclimatology, Palaeoecology, 282:133-144.
Croitor, R., Stefaniak, K., Pawłowska, K., Ridush, B., Wojtal, P., and Stach, M., 2014. Giant deer Megaloceros giganteus Blumenbach, 1799 (Cervidae, Mammalia) from Palaeolithic of Eastern Europe. Quaternary International, 326-327:91-104.
Currey, J.D. 2002. Bones: Structure and Mechanics. Princeton University Press.
Davis, E.B., Brakora, K.A., and Lee, A.H. 2011. Evolution of ruminant headgear: a review. Proceedings of the Royal Society B (2011), 278:2857-2865.
de Boer, H.H., Aarents, M.J., and Maat, G.J.R. 2013. Manual for the preparation and staining of embedded natural dry bone tissue sections for microscopy. International Journal of Osteoarchaeology, 23:83-93.
Duetsch, J. and Peterson, R. 2012. Using pelvis morphology to identify sex in moose skeletal remains. Alces: A Journal Devoted to the Biology and Management of Moose, 48:1-6.
Edwards, J.K., Marchinton, R.L., and Smith, G.F. 1982. Pelvic girdle criteria for sex determination of white-tailed deer. The Journal of Wildlife Management, 46(2):544-547.
Gao, X., Yang, F., Zhao, H., Wang, W., and Li, Ch. 2010. Antler transformation is advanced by inversion of antlerogenic periosteum implants in Sika Deer (Cervus nippon). Anatomical Record, 293:1787-1796.
Geist, V. 1998. Deer of the World: Their Evolution, Behavior and Ecology. Stackpole Books, Mechanicsburg.
Gomez, S., Garcia, A.J., Luna, S., Kierdorf, U., Kierdorf, H., Gallego, L., and Landete-Castillejos, T. 2013. Labeling studies on cortical bone formation in the antlers of red deer (Cervus elaphus). Bone, 52:506-515.
Goss, R.J. 1983. Deer Antlers: Regeneration, Function and Evolution. Academic Press, London.
Gould, S.J. 1974. The origin and function of 'bizarre' structures: antler size and skull size in the 'Irish Elk,' Megaloceros giganteus. Evolution, 28:191-220.
Grupe, G. and Peters, J. 2006. Histomorphological perspectives of human and animal bone, and soft tissue, p. 15-103. In Grupe, G. and Peters, J. (eds.), Microscopic Examinations of Bioarchaeological Remains. Keeping a Close Eye on Ancient Tissues. Verlag Marie Leidorf GmbH, Leidorf.
Heffelfinger, J. 2006. Deer of the Southwest: a Complete Guide to the Natural History, Biology, and Management of Southwestern Mule Deer and White-tailed Deer. TAMU Press.
Hughes, S., Hayden, T., Douady, C.J., Tougard, C., Germonpré, M., Stuart, A., Lbova, L., Carden, R.F., Hänni, C., and Say, L. 2006. Molecular phylogeny of the extinct giant deer, Megaloceros giganteus. Molecular Phylogenetics and Evolution, 40:285-291.
Jaczewski, Z. 1992. Deer Antlers. Państwowe Wydawnictwo Rolnicze i Leśne, Warszawa (In Polish).
Kierdorf, U., Kierdorf, H., and Konjević, D. 2013. Pathological fracture of a red deer antler secondary to purulent inflammation-a case report. Veterinarski arhiv, 83(3):347-356.
Kierdorf, U., Kierdorf, H., Schultz, M., and Rolf, H.J. 2004. Histological structure of antlers in castrated male Fallow Deer (Dama dama). Anatomical Record Part A, 281A:1352-1362.
Kowalski, K. 1958. A Catalogue of the Pleistocene Mammals of Poland. PWN, Warszawa–Wrocław (In Polish).
Li, Ch. 2012. Deer antler regeneration: A stem cell-based epimorphic process. Birth Defects Research Part C, 96:51-62.
Lincoln, G.A. 1992. Biology of antlers. Journal of Zoology, 226:517-528.
Lister, A.M. 1994. The evolution of the giant deer, Megaloceros giganteus (Blumenbach). Zoological Journal of the Linnean Society, 112:65-100.
Lister, A.M., Edwards, C.J., Nock, D.A.W., Bunce, M., van Pijlen, I.A., Bradley, D.G., Thomas, M.G., and Barnes, I. 2005. The phylogenetic position of the giant deer Megaloceros giganteus. Nature, 438:850-853.
Moen, R.A., Pastor, J., and Cohen, Y. 1999. Antler growth and extinction of Irish elk. Evolutionary Ecology Research, 1:235-249.
Niezabitowski, E.L. 1935. Skull of Giant deer (Cervus euryceros Aldr.) with abnormal antlers of Barycz on the San river. Prace Komisji Mat.-Przyr. PTPN. Seria B 7, 3:25-29 (In Polish).
Paral, V., Witter, K., and Tonar, Z. 2007. Microscopic examination of ground sections – a simple method for distinguishing between bone and antler? International Journal of Osteoarchaeology, 17:627-634.
Pawłowska, K. 2010. The usefulness of a taphonomic approach for studies of Pleistocene mammals. Geologos, 16:183-189.
Pawłowska, K., Greenfield, H., and Czubla, P., 2014. ‘Steppe’ mammoth (Mammuthus trogontherii) remains in their geological and cultural context from Bełchatów (Poland): A consideration of human exploitation in the Middle Pleistocene, Quaternary International, 326-327:448-468.
Pushkina, D. 2007. The Pleistocene easternmost distribution in Eurasia of the species associated with the Eemian Palaeoloxodon antiquus assemblage. Mammal Review, 37: 224-245.
Rolf, H.J. and Enderle, A. 1999. A hard Fallow Deer antler: A living bone till antler casting? Anatomical Record, 255:69-77.
Schultz, M. 2003. Light microscopic analysis in skeletal paleopathology, p. 73-108. In Ortner, D.J. (ed.), Identification of Pathological Conditions in Human Skeletal Remains. Academic Press, San Diego.
Shpansky, A.B. 2011. Gigantskiye oleni Megaloceros giganteus (Blum.) (Mammalia, Artiodactyla) neopleystotsena Yugo-Vostoka Zapadno-Sibirskoy Ravniny. Biulleten'Moskovskogo obshchestva ispytatelei prirody. Otdel geologicheskii, 86:18-30.
Stefaniak, K., Socha, P., Nadachowski, A., and Tomek, T. 2009. Palaeontological studies in the Częstochowa Upland, p. 85-144. In Stefaniak, K., Socha, P., and Tyc, A. (eds.), Karst of the Częstochowa Upland and the Eastern Sudetes: Palaeoenvironments and Protection. Studies of the Faculty of Earth Sciences 56. University of Silesia, Sosnowiec-Wrocław.
Stuart, A.J., Kosintsev, P.A., Higham, T.F.G., and Lister, A.M. 2004. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature, 431:684-689.
Taber, R.D. 1956. Characteristics of the pelvic girdle in relation to sex in black-tailed and white-tailed deer. California Fish and Game, 42:15-21.
Van der Made, J. 2006. The evolution and biogeography of the Pleistocene giant deer. Megaloceros giganteus (Cervidae, Mammalia), Courier Forschungs-Institut Senckenberg, 256:117-129.
Van der Made, J. and Tong, H. 2008. Phylogeny of the giant deer with palmate brow tines Megaloceros from west and Sinomegaceros from east Eurasia. Quaternary International, 179:135-162.
Vislobokova, I.A. 2011. Historical development and geographical distribution of Giant Deer (Cervidae, Megacerini). Paleontological Journal, 45:674-688.
Vislobokova, I.A. 2012. Giant deer: Origin, evolution, role in the biosphere. Paleontological Journal, 46:643-775.
Footnote 1 (It is worth noting, that, apart from antlers, sex in cervids may be morphologically determined on the basis of the pelvis (in the elk or moose Alces alces, by the angle created by the ischiatic arch (Duetsch and Peterson, 2012); in Odocoileus virginianus, the white-tailed deer, and Odocoileus hemionus columbianus, the black-tailed deer, of two or more years of age, by the presence or absence of the pelvic suspensory tuberosity (Taber, 1956); in white-tailed deer of 1.5 years old, by the position of the iliopectineal eminences (Edwards et al., 1982)).)

