 Geographic, taxonomic and temporal interrogation of bradoriid diversity and carapace disparity
Geographic, taxonomic and temporal interrogation of bradoriid diversity and carapace disparity
Article number: 27.3.a56
https://doi.org/10.26879/1424
Copyright Palaeontological Association, December 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendix 1
Submission: 19 June 2024. Acceptance: 8 November 2024.
ABSTRACT
Bradoriids are an abundant order of extinct marine arthropods that ranged from the early Cambrian to the mid-Ordovician, with a global distribution. The bivalved carapace of bradoriids varies in size, shape and ornamentation. This study presents the first quantitative analysis of bradoriid morphological disparity, quantifying the outline shape of carapaces with respect to a range of variables. Elliptical Fourier analysis (EFA) was used to quantify the carapace outline shape of 139 specimens, representing 128 species, from material figured in the literature. The mean bradoriids carapace was postplete. Families broadly overlapped in the morphospace, with Beyrichonidae the most disparate. Similarly, bradoriids from different continents mostly overlap in the morphospace, with Avalonian bradoriids occupying the most extreme positions. Bradoriid disparity changed through time, peaking at Stage 4. However, the reductions in disparity following extinction events represents a ‘thinning’ rather than culling of particular morphologies, showing that extinctions were not selective for particular bradoriids carapace shapes. Centroid position does show a shift in the morphospace over time, representing a change in mean valve shape from postplete to amplete, however, this is likely due to retention of extreme forms and lower diversity in younger samples. Pelagic bradoriids display similar carapace outlines to benthic ones. Our results are likely impacted by the overrepresentation of bradoriids from early Cambrian deposits and uneven geographic sampling. Studying the morphological variation of bradoriid carapace shape can provide context for understanding patterns in their distribution, species longevity, and their demise in the late Cambrian.
Alicia Cox. Homerton College, University of Cambridge, Hills Road, Cambridge, CB2 8PH, United Kingdom; and Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ, United Kingdom. Current address: University of Birmingham, Birmingham, B15 2TT, United Kingdom. aliciamcox24@gmail.com. Stephen Pates. Homerton College, University of Cambridge, Hills Road, Cambridge, CB2 8PH, United Kingdom; Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ, United Kingdom; and Centre for Ecology and Conservation, University of Exeter, Penryn, Cornwall, TR10 9FE, United Kingdom. s.pates@exeter.ac.uk.
Keywords: Bradoriida; Cambrian; Ordovician; morphospace; Elliptical Fourier Analysis
Final citation: Cox, Alicia and Pates, Stephen. 2024. Geographic, taxonomic, and temporal interrogation of bradoriid diversity and carapace disparity. Palaeontologia Electronica, 27(3):a56.
https://doi.org/10.26879/1424
palaeo-electronica.org/content/2024/5385-bradoriid-carapace-disparity
Copyright: December 2024 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Arthropods are the most diverse and successful group of animals alive today, with over one million extant species and are found in almost every ecosystem across the world, where they have important roles as predators, prey, organic decomposers, pollinators, and parasites, and make up the majority of animal biomass (Giribet and Edgecombe, 2012; Bar-On et al., 2018). Indeed, arthropods have been diverse, successful, and ecologically important since their origin over 500 mya during the Cambrian period (Daley et al., 2018). The oldest arthropod trace fossils are Terreneuvian in age (between 541–521 mya), while a diverse array of stem-group and crown-group body fossils appear abruptly at the base of Stage 3 c. 521 mya (Jensen, 2003; Budd and Telford, 2009; Daley et al., 2018). This diversification was traditionally seen as part of the Cambrian explosion, resulting from interlinked genetic, ecological and environmental processes (Erwin et al., 2011; Smith and Harper, 2013). However, more recently the Cambrian explosion has instead been suggested to form part of an Ediacaran Cambrian Transition and/or early Palaeozoic radiation (e.g., Mángano et al., 2014; Zhuravlev and Wood, 2018; Wood et al., 2019; Servais et al., 2023) rather than representing a discrete evolutionary event.
The extinct order of Bradoriida first appear in the fossil record around 521 mya (Series 2 Stage 3), just before the first trilobites (Hou et al., 2001), and thus represent the oldest known total-group arthropod body fossils. Bradoriids were small (c. 2–20 mm) marine bivalved arthropods (Williams et al., 2007).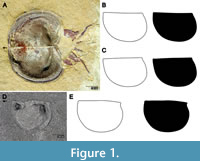 The majority of bradoriids are suggested to have had a benthic lifestyle, due to their prominence in shelf marine deposits, and this group was an important part of the microbenthos in oxygenated shelf environments during Cambrian times (e.g., Williams et al., 2007, 2011). Indeed, the bradoriids Liangshanella and Kunmingella (Figure 1A) have been reported as among the most or the most abundant taxa within the Burgess Shale (and nearby deposits) and Chengjiang Lagerstätten respectively (Caron and Jackson, 2008; Zhao et al., 2010; Nanglu et al., 2020). Due to their large numbers, bradoriids played an important role in early Cambrian ecosystems, feeding on detritus, recycling organic material on the seafloor, and providing a food source for other organisms (Williams et al., 2007). A few species were likely pelagic and active in the water column, as inferred from a combination of environmental, morphological and palaeogeographical data (Williams et al., 2015).
The majority of bradoriids are suggested to have had a benthic lifestyle, due to their prominence in shelf marine deposits, and this group was an important part of the microbenthos in oxygenated shelf environments during Cambrian times (e.g., Williams et al., 2007, 2011). Indeed, the bradoriids Liangshanella and Kunmingella (Figure 1A) have been reported as among the most or the most abundant taxa within the Burgess Shale (and nearby deposits) and Chengjiang Lagerstätten respectively (Caron and Jackson, 2008; Zhao et al., 2010; Nanglu et al., 2020). Due to their large numbers, bradoriids played an important role in early Cambrian ecosystems, feeding on detritus, recycling organic material on the seafloor, and providing a food source for other organisms (Williams et al., 2007). A few species were likely pelagic and active in the water column, as inferred from a combination of environmental, morphological and palaeogeographical data (Williams et al., 2015).
Exceptional fossils preserving the soft anatomy of bradoriids from Konservat Lagerstätten such as the Chengjiang and Burgess Shale (e.g., Figure 1A) have also provided details of their anatomy, including appendage morphologies (e.g., Hou et al., 1996, 2010). More recent studies utilising micro-CT scanning techniques have revealed further appendage details demonstrating extensive specialisations and likely convergence with a range of other arthropod groups (Zhai et al., 2019). Details of the soft part anatomy of bradoriids has also been crucial for resolving their phylogenetic position within arthropods. Originally considered ostracods (e.g., Sylvester-Bradley, 1961; Siveter 2008) on account of similarities in their small size and bivalved carapaces, bradoriids have most recently been recovered as stem-group arthropods (Zhai, et al., 2019), having been previously recovered as sister to total-group mandibulates (Legg et al., 2013) or as stem-group crustaceans (Hou et al., 2010). Within bradoriids, nine (possibly 10) families are recognised. These are Bradoriidae, Beyrichonidae, Cambriidae, Comptalutidae, Duibianellidae, Hipponicharionidae, Kunmingellidae, Monasteriidae, Svealuitdae, and possibly Zhexiellidae (Jones and Kruse, 2009). Seven of these are considered Bradoriida sensu stricto, with numerous uncertain forms, Duibianellidae and Monasteriidae perhaps not falling within monophyletic Bradoriida (Williams et al., 2007) or bradoriids perhaps not being monophyletic (Zhai et al., 2019).
Bradoriids are not only known from Konservat Lagerstätten. They have been recovered from different sediment types, preserved as body fossils of isolated carapace valves, phosphatised, as Small Carbonaceous Fossils (SCFs) and as Small Shelly Fossils (SSFs) (e.g., Zhang, 2007; Liu et al., 2008; Slater et al., 2018; Wallet et al., 2021; Skovsted et al., 2021). These data reveal that bradoriids had a global distribution, with some cosmopolitan species. Bradoriids are known from all major landmasses except for South America and sub-Saharan Africa (Williams et al., 2007; Collette et al., 2011). As with many other groups which first appear during the Cambrian, bradoriids rapidly diversified following their origination during the ‘Cambrian Arthropod Radiation Event’ in Stage 3 approximately 521 mya (Williams et al., 2011). However, various extinction events drastically reduced their diversity towards the end of the Cambrian, and only a small number of species persisted until their final extinction in the Ordovician (Williams et al., 2007).
However, despite their diversity and ecological importance, aspects of the evolution of bradoriids remain unexplored. In particular, the extent to which the morphological disparity of the group is impacted by diversity, geography, taxonomy, and how it changed through time remains unquantified. This study presents the first broad morphometric analysis of bradoriids caparace shape, applying Elliptical Fourier Analysis (EFA) to quantify differences in the outline shape of complete bradoriid carapaces. These outline shapes were then visualised and separated into groups to explore trends in diversity and disparity. These analyses quantified bradoriids diversity and carapace outline disparity in relation to their taxonomy, palaeogeography, and through time, further investigating whether a pelagic mode of life is associated with novel carapace morphologies, to determine the role of these variables in the morphology of this important Cambrian group.
MATERIAL AND METHODS
Bradoriid Carapace Outline Terminology
Bradoriid carapace valves are often described as ‘amplete’, ‘preplete’, or ‘postplete’ in outline (e.g., McMenamin, 2020). These refer to the position of maximum valve height (maximum perpendicular distance from the hinge connecting two carapace valves to the margin of a valve) relative to the anterior and posterior of the carapace.
Amplete: Maximum height of the carapace valve is near the midpoint between anterior and posterior.
Preplete: Maximum height of the carapace valve is near the anterior.
Postplete: Maximum height of the carapace valve is near the posterior.
Data Collection
A list of bradoriid species was created using the appendix of Williams et al. (2007). Species described after this time were found by performing a literature search (July 2022) of electronic databases using keywords such as “bradoriid”, “bradoriids”, “Bradoriida”, “new”, and “species” and filtering the year of publication to after 2006. The list of genera in McMenamin (2020) was used subsequently to find individual species, which may have been missed, by searching the literature for each genus name. A database was created which included taxonomic information and geological occurrence for each of the species. Biozones and regional stages were correlated to their respective global stages using the biozone correlation chart from Geyer (2019). The total list of species was used to calculate species diversity for each analysis, while photographs of complete carapaces were used for the outline creation and analysis (below). Literature sources for these outlines are provided in the Appendix.
Outline Creation
Photographs of specimen valves were collected from the online literature and stored digitally. Images of both single valves and open carapaces were used (Figure 1), with the latter being split into respective left and right valves. At least one carapace image was collected for each species where possible. Valves were manually traced using the Bezier curve function in Inkscape, and then filled in to create a black silhouette on a white background. Outlines were not created for specimens described as damaged, or where significant damage could be seen in the photograph. For specimens which were only slightly incomplete, for example with a small divot to the margin, the outlines were reconstructed to remove the damage (Figure 1D). As both left and right valves were digitized, outlines were mirrored so they were in the same orientation. Outlines were exported from Inkscape as.png files at a resolution of 300dpi, converted into.jpg format, and imported into R using the ‘import_jpg’ function from the ‘Momocs’ package (Bonhomme et al., 2014). R code and outlines are provided in the OSF project that accompanies this article (Cox and Pates, 2024).
Data Analysis
All data analysis was conducted in R Studio version 4.1.3 (RStudioTeam, 2021). A total of 139 outlines were sampled at 64 points per outline, scaled, and centred. The R package ‘Momocs’ (Bonhomme et al., 2014) was used for elliptical Fourier analysis (EFA) and subsequent data visualisation. The function ‘calibrate_harmonicpower_efourier’ was used to calculate the number of harmonics required to capture 99.9% of the variation in the sample. EFA was performed using the ‘efourier’ function. Sum of variance (SOV), Sum of ranges (SOR), average neighbour distance, and average displacement values were used to measure morphospace occupation and disparity and were calculated using the R package ‘dispRity’ (Guillerme, 2018). Sum of ranges and sum of variances measure the size of morphospace occupation, and therefore the morphological disparity, average neighbour distance measures the density, and average displacement measures the position from the centre of the morphospace (0,0) (Guillerme et al., 2020).
Similar quantitative analyses have been performed on ostracods to study morphological disparity relationships of carapace shape with ecology, ontogeny, longevity, and other metrics (summarised in Baltanás and Danielopol, 2011).
Bradoriid diversity and disparity were quantified and visualised (section below) in relation to their taxonomy, palaeogeography, and through time. To investigate the role of taxonomy on bradoriid disparity, carapace outlines were grouped by family, into the 10 proposed families, with an eleventh group for uncertain forms. These families include both bradoriids sensu stricto and those which may not fall within monophyletic bradoriids (e.g., Williams et al., 2007; Zhai et al., 2019). To explore the role of palaeogeography on bradoriids carapace disparity, a ‘continent analysis’ was performed. This analysis separated bradoriids into groups relating to which palaeocontinent they have been collected from. Nine palaeocontinents were used in the analysis: Australia, Avalonia, Baltica, Gondwana, Kazakhstania, Laurentia, North China, Siberia, and South China. The third analysis considered how bradoriids carapace disparity changed through time. For this analysis, bradoriid carapaces were separated into groups depending on the geological stage(s) from which they are known. Some taxa are known from multiple stages and so were included in the analysis once for each stage. All Cambrian stages from Stage 3 were included, as was the Early Ordovician. A final analysis was conducted to address whether genera for which a pelagic mode of life has been considered suitable had distinct carapace outlines to benthic genera. For this, the area of morphospace occupation for two genera, Anabarochilina and Liangshanella, were compared to all other bradoriids.
PCA Visualisation
The morphospace and principal components were visualised using the ‘plot.PCA’ function from the ‘Momocs’ package (Bonhomme et al., 2014). PCA plots showing individual convex hulls were visualised using the ‘ggplot2’ package (Wickham, 2016). For analyses considering changing disparity through time, some bradoriids are known from multiple Cambrian stages. These were included in the analysis multiple times, slightly altering the morphospace and the values on the PC axes. For the ‘continent analysis’, the PC2 scores were inverted to those of the family and time analysis and have been flipped to aid comparison.
RESULTS
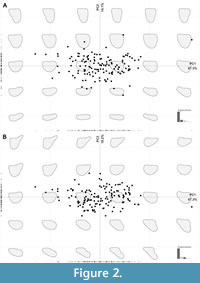 For analyses of taxonomy and geography, the first three principal components describe 91.6% of the variation in outline shape, with PC1 representing 67.3%, PC2 14.1%, and PC3 10.2%. This variation is mainly focused on the length and width ratios of the valve, and the anterior-posterior asymmetry (Figure 2). The PC1 axis describes changes in the shape of the valve, with positive scores associated with a slightly more postplete valve and negative scores corresponding to a slightly more preplete valve shape. The PC2 axis describes changes in the ‘roundness’ of the valve, with positive values reflecting shorter and wider valves which are close to triangular, and negative values indicating longer and thinner valves that are more oval shaped. PC3 describes variation in the asymmetry of the valve, with positive scores linked to valves which are wider at the anterior end (preplete), and negative values reflecting those which are wider at the posterior end (postplete).
For analyses of taxonomy and geography, the first three principal components describe 91.6% of the variation in outline shape, with PC1 representing 67.3%, PC2 14.1%, and PC3 10.2%. This variation is mainly focused on the length and width ratios of the valve, and the anterior-posterior asymmetry (Figure 2). The PC1 axis describes changes in the shape of the valve, with positive scores associated with a slightly more postplete valve and negative scores corresponding to a slightly more preplete valve shape. The PC2 axis describes changes in the ‘roundness’ of the valve, with positive values reflecting shorter and wider valves which are close to triangular, and negative values indicating longer and thinner valves that are more oval shaped. PC3 describes variation in the asymmetry of the valve, with positive scores linked to valves which are wider at the anterior end (preplete), and negative values reflecting those which are wider at the posterior end (postplete).
Influence of Taxonomy on Bradoriid Carapace Disparity
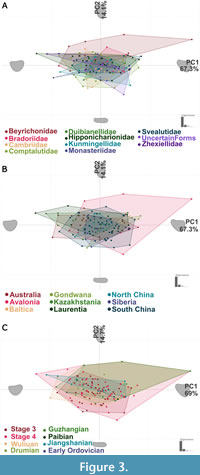 There is a large amount of overlap between bradoriid families, which is concentrated close to the origin of the morphospace (Figure 3A, Figure 4). The exception to this is the positive PC1, positive PC2 region, which corresponds to a triangular carapace space, and is exclusively occupied by Beyrichonidae. Beyrichonidae covers the largest morphospace area (SOR), with specimens spanning the majority of the PC1 axis in the positive PC2 region. Bradoriidae, Duibianellidae,and Svealutidae are mostly found between values of -0.25 to 0.25 for PC1, and between -0.1 to 0.1 for PC2. Hipponicharionidae also occupies this area, except for a specimen with an extreme PC1 value of 0.5. Variation in Cambriidae is mainly across the PC2 axis, which represents changes in the length to width ratio, with PC2 values ranging from -0.2 to 0.15. Comptalutidae occupies the area between -0.25 to 0.25 for PC1, and -0.2 to 0.1 for PC2. Kunmingellidae occupies the area between -0.25 to 0.25 for PC1, and -0.15 to 0.1 for PC2.
There is a large amount of overlap between bradoriid families, which is concentrated close to the origin of the morphospace (Figure 3A, Figure 4). The exception to this is the positive PC1, positive PC2 region, which corresponds to a triangular carapace space, and is exclusively occupied by Beyrichonidae. Beyrichonidae covers the largest morphospace area (SOR), with specimens spanning the majority of the PC1 axis in the positive PC2 region. Bradoriidae, Duibianellidae,and Svealutidae are mostly found between values of -0.25 to 0.25 for PC1, and between -0.1 to 0.1 for PC2. Hipponicharionidae also occupies this area, except for a specimen with an extreme PC1 value of 0.5. Variation in Cambriidae is mainly across the PC2 axis, which represents changes in the length to width ratio, with PC2 values ranging from -0.2 to 0.15. Comptalutidae occupies the area between -0.25 to 0.25 for PC1, and -0.2 to 0.1 for PC2. Kunmingellidae occupies the area between -0.25 to 0.25 for PC1, and -0.15 to 0.1 for PC2. 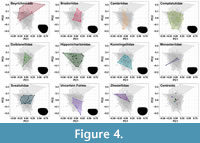 Monasteriidae is concentrated around the origin, except for a single specimen occupying an extreme in the negative PC1, negative PC2 region, which is likely responsible for the high disparity (SOR) found in this family (Table 1). Zhexiellidae occupies mainly the negative PC1, positive PC2 area of the morphospace with PC1 values between -0.5 and 0.25, and PC2 values between 0 to 0.1. The average carapace shape for most families is postplete, while Hipponicharionidae and Zhexiellidae are amplete, and Monasteriidae is preplete. The centroids are positioned in the centre of the morphospace, except for Beyrichonidae and Zhexiellidae whose centroids have more positive PC2 values and the highest displacement values (Table 1). Comptaluidae, Hipponicharionidae, and Svealutidae have the largest sample sizes, however, Beyrichonidae, has the highest SOR value (Table 1) and therefore shows the highest morphological disparity (followed by Hipponicharionidae and Comptalutidae). Beyrichondiae also has the highest average neighbour distance, reflecting the high disparity of the family.
Monasteriidae is concentrated around the origin, except for a single specimen occupying an extreme in the negative PC1, negative PC2 region, which is likely responsible for the high disparity (SOR) found in this family (Table 1). Zhexiellidae occupies mainly the negative PC1, positive PC2 area of the morphospace with PC1 values between -0.5 and 0.25, and PC2 values between 0 to 0.1. The average carapace shape for most families is postplete, while Hipponicharionidae and Zhexiellidae are amplete, and Monasteriidae is preplete. The centroids are positioned in the centre of the morphospace, except for Beyrichonidae and Zhexiellidae whose centroids have more positive PC2 values and the highest displacement values (Table 1). Comptaluidae, Hipponicharionidae, and Svealutidae have the largest sample sizes, however, Beyrichonidae, has the highest SOR value (Table 1) and therefore shows the highest morphological disparity (followed by Hipponicharionidae and Comptalutidae). Beyrichondiae also has the highest average neighbour distance, reflecting the high disparity of the family.
Influence of Geography on Bradoriid Carapace Disparity
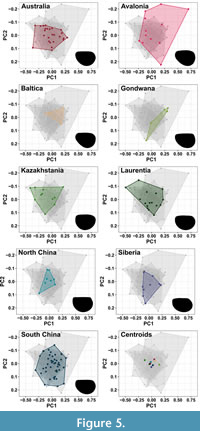 There is again a large amount of overlap in the centre of the morphospace when specimens are grouped by continents (Figure 3B, Figure 5). Avalonia covers the largest area (SOR) and exclusively occupies a large amount of the positive PC1 section. Avalonia shows the greatest variation in the length to width ratio, with specimens ranging across the entire PC2 axis, while PC1 values range between -0.25 to 0.75. Baltica, North China, and Siberia mostly occupy the area of the morphospace with values between -0.25 to 0.25 for PC1, and between -0.1 to 0.1 for PC2. Australia and Laurentia also mainly occupy this space, with the addition of specimens with more negative PC1 values. Gondwana has specimens in both the negative PC1 positive PC2 and positive PC1 negative PC2 quadrants, showing variation in both the shape and width of the valve. Kazakhstania occupies the negative PC1 region of the morphospace, with PC2 values between -0.1 to 0.1. South China occupies the region between -0.25 to 0.25 for PC1, and between -0.15 to 0.15 for PC2. The average carapace shape for most geographic regions is postplete, however, for Kazahkstania and Laurentia it is amplete. Centroids for each geographical region are clustered around the origin, except for Gondwana and Kazakhstania, whose centroids have the highest displacement values (Table 2). The centroid for Gondwana falls in the positive PC1 positive PC2 region, while that of Kazakhstania falls in the negative PC1 negative PC2 region. Continents with the largest sample sizes (South China, Australia, and Laurentia) have higher morphological disparity as expected (Table 2). South China also has the lowest average nearest neighbour distance, reflecting the high density of carapaces for this continent. Morphological disparity is greatest for Avalonia, followed by Laurentia. The high disparity and average neighbour distance of Avalonia is likely due to specimens which occupy extreme positions in the morphospace.
There is again a large amount of overlap in the centre of the morphospace when specimens are grouped by continents (Figure 3B, Figure 5). Avalonia covers the largest area (SOR) and exclusively occupies a large amount of the positive PC1 section. Avalonia shows the greatest variation in the length to width ratio, with specimens ranging across the entire PC2 axis, while PC1 values range between -0.25 to 0.75. Baltica, North China, and Siberia mostly occupy the area of the morphospace with values between -0.25 to 0.25 for PC1, and between -0.1 to 0.1 for PC2. Australia and Laurentia also mainly occupy this space, with the addition of specimens with more negative PC1 values. Gondwana has specimens in both the negative PC1 positive PC2 and positive PC1 negative PC2 quadrants, showing variation in both the shape and width of the valve. Kazakhstania occupies the negative PC1 region of the morphospace, with PC2 values between -0.1 to 0.1. South China occupies the region between -0.25 to 0.25 for PC1, and between -0.15 to 0.15 for PC2. The average carapace shape for most geographic regions is postplete, however, for Kazahkstania and Laurentia it is amplete. Centroids for each geographical region are clustered around the origin, except for Gondwana and Kazakhstania, whose centroids have the highest displacement values (Table 2). The centroid for Gondwana falls in the positive PC1 positive PC2 region, while that of Kazakhstania falls in the negative PC1 negative PC2 region. Continents with the largest sample sizes (South China, Australia, and Laurentia) have higher morphological disparity as expected (Table 2). South China also has the lowest average nearest neighbour distance, reflecting the high density of carapaces for this continent. Morphological disparity is greatest for Avalonia, followed by Laurentia. The high disparity and average neighbour distance of Avalonia is likely due to specimens which occupy extreme positions in the morphospace.
Bradoriid Carapace Disparity in Time
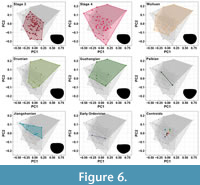 For this analysis, which included a slightly larger sample than the previous ones due to some carapaces being included in multiple time slices, PC1, PC2, and PC3 represented 69%, 14.7%, and 8.3% of the shape variation, but the overall positions of individual taxa are very comparable to the other two analyses (Figure 3). Categorisation of specimens by geological stage again shows large amounts of overlap in the centre of the morphospace (Figure 3C, Figure 6). The area correlating with positive PC1 and positive PC2 values is occupied by the consecutive time stages of Stage 4, Wuliuan, Drumian and Guzhangian. Stage 3, with the highest sample size, occupies the region between -0.25 to 0.25 for PC1 and between -0.15 to 0.15 for PC2. This region is also covered by most specimens from Stage 4, Wuliuan, Drumian and Guzhangian. The Jiangshanian mainly occupies the negative PC1 space, with PC2 values ranging from -0.05 to 0.1. Except for the Paibian and Early Ordovician groups (which have low sample sizes), centroid position appears to shift through time from a negative PC1 and negative PC2 position towards a positive PC1 and positive PC2 position, showing a change in morphology of the mean bradoriids carapace from postplete to amplete. There is a shift in centroid position going from Stage 3 to Stage 4, and from the Wuliuan to Drumian (Table 3). Stage 3, which has the highest sample size and diversity, has a lower morphological disparity (SOR) than Stage 4, Wuliuan, Drumian and Guzhangian, and has the lowest average nearest neighbour distance reflecting the high density of the group and lack of carapaces in the extreme parts of the space.
For this analysis, which included a slightly larger sample than the previous ones due to some carapaces being included in multiple time slices, PC1, PC2, and PC3 represented 69%, 14.7%, and 8.3% of the shape variation, but the overall positions of individual taxa are very comparable to the other two analyses (Figure 3). Categorisation of specimens by geological stage again shows large amounts of overlap in the centre of the morphospace (Figure 3C, Figure 6). The area correlating with positive PC1 and positive PC2 values is occupied by the consecutive time stages of Stage 4, Wuliuan, Drumian and Guzhangian. Stage 3, with the highest sample size, occupies the region between -0.25 to 0.25 for PC1 and between -0.15 to 0.15 for PC2. This region is also covered by most specimens from Stage 4, Wuliuan, Drumian and Guzhangian. The Jiangshanian mainly occupies the negative PC1 space, with PC2 values ranging from -0.05 to 0.1. Except for the Paibian and Early Ordovician groups (which have low sample sizes), centroid position appears to shift through time from a negative PC1 and negative PC2 position towards a positive PC1 and positive PC2 position, showing a change in morphology of the mean bradoriids carapace from postplete to amplete. There is a shift in centroid position going from Stage 3 to Stage 4, and from the Wuliuan to Drumian (Table 3). Stage 3, which has the highest sample size and diversity, has a lower morphological disparity (SOR) than Stage 4, Wuliuan, Drumian and Guzhangian, and has the lowest average nearest neighbour distance reflecting the high density of the group and lack of carapaces in the extreme parts of the space.
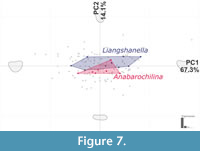 Influence of Pelagic Life Mode on Bradoriids Carapace Shape
Influence of Pelagic Life Mode on Bradoriids Carapace Shape
The pelagic genera Anabarochilina and Liangshanella occupy positions between -0.25 and 0.32 in PC1 and -0.1 and 0.1 on PC2.These genera fall in the most densely occupied part of the morphospace and are not distinct from the carapace shapes of benthic taxa (Figure 7).
DISCUSSION
Bradoriid valve shapes include ‘postplete’, ‘amplete’, and ‘preplete’, with previous work suggesting that the majority of bradoriids were postplete (e.g., McMenamin, 2020). The morphospace shows bradoriids with all three types of shape, with the highest density close to the origin of the plot, representing a carapace shape that is postplete.
Influence of taxonomy on bradoriid carapace disparity
The EFA results show that all families, including Zhexiellidae, overlap the origin in the morphospace (Figure 3A). However, while this broad overlap in the central part of the morphospace limits the utility of carapace shape alone in distinguishing bradoriids taxa even to the family level, there are differences in the extreme areas of morphospace occupied by different families- though the centroid positions indicate only very limited differences in this case. Thus, while some differences can be observed, valve shape when quantified through EFA is limited in terms of taxonomic use at the family level when all other features of the carapace are not taken into account. Bradoriid taxa are differentiated from one another by broad scale differences in valve shape (amplete, postplete, preplete), as well as hinge and sulcus morphology. These differences are captured by analyses of carapace outlines. Further diagnostic characters of bradoriids, such as the shape and size of lobes, nodes, or the presence of ornamentation and spines are not captured by outline analyses, and thus our measure of disparity only captures some of the total morphological variation within the group. Just as for other groups (e.g., trilobites; Holmes, 2023), disparity measures of different aspects of morphology will capture different patterns. This finding draws similarities with a previous analysis quantifying carapace outlines for the ostracod superfamily Cypridoidea, which found a large overlap in morphospace between families, and that morphological disparity is not linked to diversity of taxonomic groups (Sánchez-González et al., 2004).
These other morphological features are also important for determining the life mode of bradoriids, for example whether they were pelagic or not. Two genera of the Svealutidae family, Anabarochilina and Liangshanella, are suggested to have been pelagic based on lithofacies distribution and anatomy suggesting an active swimming lifestyle (Williams et al., 2007, 2015; Collette et al., 2011). Indeed the pelagic life mode of these Svealutids may be the reason they were able to disperse and escape extinction when anoxic water spread onto the marine shelf (Williams et al., 2011), and this may have contributed to Svealutidae being one of the longer ranging bradoriid families, from Cambrian Stage 3 until the end of the Cambrian. While carapace shape was likely important for streamlining in larger bivalved arthropods in Cambrian oceans (e.g., Vannier and Chen, 2000; Vannier et al., 2009; Pates et al., 2021), this would have been more limited at the lower Reynolds numbers of the much smaller bradoriids. Appendage morphology and swimming power from muscles are more important for extant micro-arthropods in the pelagic realm. Extant copepods and ostracods only swim rapidly (and at high Reynolds numbers) during escape reactions that last around one second (e.g., Strickler, 1975), using setiose appendages as paddles to provide thrust (e.g., Hunt et al., 2007) during normal swimming. Characters that facilitated a pelagic mode of life in Anabarochilina such as adductor muscles supporting well-developed swimming appendages (Williams et al., 2015) may have impacted on the carapace outlines, or correlated with carapace outline shape, as they had to be accommodated beneath bradoriid carapaces. Indeed, carapace outline in some ostracods does vary with where they live. An analysis of 30 ostracod carapaces found that populations from shallow and deep environments occupied different areas of morphospace, albeit with a lower variation between habitats than the asymmetry between the valves (Baltanás and Danielopol, 2011). However, comparison of the carapace shape of the pelagic svealutids with benthic bradoriids shows no clear difference in the morphology of the outline shapes, and thus our results show no evidence that carapace shape correlates with life mode. Thus at this point details of the muscles and/or swimming appendages, alongside well known distribution patterns and presence in multiple different sediment types are required to support a pelagic mode of life in microscopic arthropods (Williams et al., 2015; see also Siveter et al., 1991, 2022; Perrier et al., 2011), and a pelagic mode cannot be inferred from carapace shape alone. This will limit the number of bradoriid taxa from which a pelagic mode can be inferred, as not all are preserved with equal fidelity, nor are global and facies distribution patterns equally well known. Indeed data from South America and sub-Saharan Africa are lacking (Williams et al., 2007), and only a few specimens have been reported from India (Collette et al., 2011). These latter specimens were crucial for inferring a pelagic mode of life in Anabarochilinia, demonstrating the possible limitations of life mode inference based off current data.
Influence of geography on bradoriid carapace disparity
Strong endemism has been reported from Cambrian Stage 3 and Stage 4 bradoriids (e.g., Siveter et al., 1997; Hou et al., 2002; Zhang, 2007; Topper et al., 2011), while cluster analyses have been used to provide support for faunal links and associations (e.g., Williams et al., 2007; Topper et al., 2011). These studies have found support for a faunal association between Australia and South China, and of Avalonia, Baltica, West Gondwana, Laurentia and Siberia (Williams et al., 2007; Topper et al., 2011). When carapace morphologies are separated by continent (Figure 5), there is broad overlap towards the centre of the morphospace. However, Avalonian bradoriids occupy extremes in both positive PC1, positive PC2 and positive PC1, negative PC2 quadrants of the space, while South China bradoriids are found in a more constrained part of the morphospace (Figure 5). Bradoriids from the second cluster (Baltica, West Gondwana, Laurentia and Siberia) are generally limited to PC1 values between -0.25 and 0.25, and PC2 values between -0.1 and 0.1 (Figure 5), but cannot be easily separated from bradoriids not found within the faunal associations, such as those from Kazakhstania or North China. The broad overlap between continent morphologies may reflect the global distribution of many bradoriids, a result of the wide-range dispersal capabilities of genera such as Kunmingella, Beyrichona and Hipponicharion (Williams et al., 2007), alongside the limited differences in carapace morphologies when considered at the family level (Figure 4).
This analysis of differences in the morphological variation of bradoriids across continents is further limited by the uneven knowledge of bradoriids geographically. Although bradoriids have been described from almost all modern continents, there are certainly gaps in our knowledge. Williams et al., (2007) noted that the geological areas which are least represented in the bradoriid fossil record are India, South America, the Middle East and sub-Saharan Africa. While bradoriids have now been found in some of these locations, the Middle East (Elicki, 2012) and India (Collette et al., 2011), large gaps remain and these areas have received far less study than for example Europe, North America and South China. The prevalence of bradoriids from well-studied geographic locations will also have influenced the analyses of diversity and disparity through time (section below), with South China deposits most common in Stage 3, Australia in Stage 4, and Laurentia in Stage 5 and the Drumian. However, given the broad overlap in morphospace across continents, these geographic sampling biases will be less impactful than if broad differences in carapace shape were observed between different continents, allowing more confidence to be placed in the temporal patterns than otherwise would be the case.
Diversity and Disparity through Time
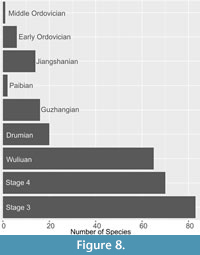 Importantly, the area of morphospace occupied does not correlate with overall diversity, when separated by Cambrian stages (Table 1, Table 3). The highest diversity of bradoriids is recovered for Stage 3 (Figure 8), supporting previous genus level diversity curves (Williams et al., 2011; McMenamin, 2020), whereas the highest area of the morphospace, as indicated by the sum of ranges, is in Stage 4, and the highest dispersal within the morphospace, as indicated by the sum of variances and average neighbour distances, falls in the Guzhangian (Table 3; the larger average neighbour distance for the Paibian is an artefact of the very low sample size). The increase in overall morphospace occupation in Stage 4 is associated with increases in the most extreme positions on both PC1 and PC2 occupied by bradoriids. This is most striking for the Avalonian beyrichonid Beyrichona papilio, which with their triangular carapace outlines occupy the most extreme positions in the positive PC1, positive PC2 quadrant of the morphospace. These originate in Stage 4 and persist until the Guzhangian (Williams et al., 2007). Indeed, the changing centroid position through time (Figure 6) is most likely a result of the interaction of the presence of these extreme morphological forms and a reducing diversity within the space, rather than reflecting a change in the overall average shape of bradoriid carapaces. Similarly, the increase in displacement from previous time period (Table 3) metric is likely being significantly impacted by the presence of these extreme forms in the morphospace and reduced overall bradoriid diversity.
Importantly, the area of morphospace occupied does not correlate with overall diversity, when separated by Cambrian stages (Table 1, Table 3). The highest diversity of bradoriids is recovered for Stage 3 (Figure 8), supporting previous genus level diversity curves (Williams et al., 2011; McMenamin, 2020), whereas the highest area of the morphospace, as indicated by the sum of ranges, is in Stage 4, and the highest dispersal within the morphospace, as indicated by the sum of variances and average neighbour distances, falls in the Guzhangian (Table 3; the larger average neighbour distance for the Paibian is an artefact of the very low sample size). The increase in overall morphospace occupation in Stage 4 is associated with increases in the most extreme positions on both PC1 and PC2 occupied by bradoriids. This is most striking for the Avalonian beyrichonid Beyrichona papilio, which with their triangular carapace outlines occupy the most extreme positions in the positive PC1, positive PC2 quadrant of the morphospace. These originate in Stage 4 and persist until the Guzhangian (Williams et al., 2007). Indeed, the changing centroid position through time (Figure 6) is most likely a result of the interaction of the presence of these extreme morphological forms and a reducing diversity within the space, rather than reflecting a change in the overall average shape of bradoriid carapaces. Similarly, the increase in displacement from previous time period (Table 3) metric is likely being significantly impacted by the presence of these extreme forms in the morphospace and reduced overall bradoriid diversity.
This pattern of disparity increases from Stage 3 to Stage 4, and corresponding decrease in diversity, differs from the ‘early burst’ model for disparity within groups that is often observed in disparity studies (e.g., Hughes et al., 2013). Under the ‘early burst’ model, maximal disparity is established early on, prior to a peak in taxonomic diversity. For bradoriids, it seems that diversity peaked ahead of disparity, with the most extreme forms appearing in Stage 4. This time is associated with expansion of bradoriids into high and low latitudes, and new environments including shallow shelf, deep shelf, and marginal marine settings (e.g., Siveter and Williams, 1995; Siveter et al., 1995 Williams et al., 2007; Loughlin and Hillier, 2011), raising the possibility of ecological release permitting expansion of bradoriids into these extreme areas of morphospace. Notably, the ecological release of bradoriids into the pelagic realm did not lead to a further expansion of the morphospace area occupied (Figure 7).
Despite significant reductions in bradoriid diversity during Cambrian extinction events (Figure 8; Williams et al., 2011), a broad area of the morphospace remains occupied until the end of the Guzhangian (Figure 8). For example, there is only a slight decrease in disparity (SOR) between the Wuliuan and Drumian (Table 3), compared to the substantial loss of diversity (from >60 to 20 species; Figure 8). Instead, extinctions appear to have ‘thinned’ the density of bradoriids within morphospace, leading to increased average neighbour distances and sum of variances (Table 3). This reduced density indicates that these extinctions were not selective for a particular morphology of bradoriids. When compared to other benthic invertebrates, similar patterns can be observed. For example, hyoliths peaked in diversity in Stage 4, before declining in numbers, with a corresponding thinning of the morphospace occupation but no clear shift in the space (Liu et al., 2024). Changes in absolute size of a range of benthic invertebrates have been recorded from across the early to middle Cambrian (e.g., Zhuravlev and Wood, 2020). A future analysis incorporating size into the study of bradoriids morphological changes might be informative as to whether oxygen peaks facilitated growth to broader size ranges than other times during this interval, and size data would complement the morphological data presented here.
A lack of specificity of bradoriid extinctions based on carapace shape is perhaps to be expected, given the results of the family-level analysis and lack of a link between bradoriids carapace outline and a pelagic mode of life. As there is broad overlap in the central region of the morphospace, the loss of entire families, such as the Kunmingellidae during late Stage 3 (e.g., Williams et al., 2011) did not remove an area of the morphospace, but rather ‘thinned’ it. Similarly, the origination of new bradoriids groups did not lead to occupation of vastly new areas of carapace morphospace. Further, broad dispersal of some groups and a pelagic mode of life of some taxa, which has been suggested to have contributed to the longevity of some svealutids (e.g., Williams et al., 2011, 2015), is not associated with a different part of the morphospace. Thus, selection for animals able to escape deep water anoxia would not be reflected by a shift in the occupation area of the morphospace, as these taxa have carapaces occupying positions close to the origin of the morphospace (Figure 7).
CONCLUSIONS
Bradoriid carapace outlines are quantified using EFA, and morphospace occupation quantified with respect to family, geography, and geological stage. These results are limited by the current sampling biases present in the bradoriid fossil record. There is broad overlap in morphospace occupation across all these metrics, with the highest disparity represented by Beyrichonidae, Avalonia, and Cambrian Stage 4, respectively. Bradoriids do not follow the ‘early burst’ model for morphological disparity. There is no clear difference in the outline shape of pelagic bradoriids from benthic ones, and extinctions are linked to ‘thinning’ of bradoriids morphospace rather than big reductions, indicating that they were not selective for particular carapace morphologies.
ACKNOWLEDGEMENTS
We thank the Handling Editor, N. Nasser, and two anonymous reviewers for their constructive comments on the manuscript. We thank D.J. Siveter, Y. Xiaoli, and X. Ma for kindly providing photographs of bradoriids used in Figure 1. SP acknowledges funding from a Herchel Smith Postdoctoral Fellowship (University of Cambridge) and Independent Research Fellowship NE/X017745/1 (NERC).
REFERENCES
Álvarez, M.E.D., Gozalo, R., Cederström, P., and Ahlberg, P. 2008. Bradoriid arthropods from the lower-middle Cambrian of Scania, Sweden. Acta Palaeontologica Polonica, 53:647–656.
https://doi.org/10.4202/app.2008.0409
Andersson, H. 2014. Bradoriids from the middle Cambrian 'thin' Stephen Formation at Odaray Mountain, Canadian Rocky Mountains. Självständigt arbete Nr 93, Uppsala Universitet.
Baltanás, Á. and Danielopol, D.L. 2011. Geometric morphometrics and its use in ostracod research: a short guide. Joannea Geologie und Palaontologie, 11:235–272.
Bar-On, Y.M., Phillips, R., and Milo, R. 2018. The biomass distribution on Earth. Proceedings of the National Academy of Sciences, 115:6506–6511.
https://doi.org/10.1073/pnas.1711842115
Betts, M.J., Topper, T.P., Valentine, J.L., Skovsted, C.B., Paterson, J.R., and Brock, G.A. 2014. A new early Cambrian bradoriid (Arthropoda) assemblage from the northern Flinders Ranges, South Australia. Gondwana Research, 25:420–437.
https://doi.org/10.1016/j.gr.2013.05.007
Betts, M.J., Paterson, J.R., Jago, J.B., Jacquet, S.M., Skovsted, C.B., Topper, T.P., and Brock, G.A. 2016. A new lower Cambrian shelly fossil biostratigraphy for South Australia. Gondwana Research, 36:176–208.
https://doi.org/10.1016/j.gr.2016.05.005
Betts, M.J., Paterson, J.R., Jago, J.B., Jacquet, S.M., Skovsted, C.B., Topper, T.P., and Brock, G.A. 2017. Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone. Gondwana Research, 46:240–279.
https://doi.org/10.1016/j.gr.2017.02.007
Bonhomme, V., Picq, S., Gaucherel, C., and Claude, J. 2014. Momocs: Outline Analysis Using R. Journal of Statistical Software, 56:1–24.
https://doi.org/10.18637/jss.v056.i13
Budd, G.E. and Telford, M.J. 2009. The origin and evolution of arthropods. Nature, 7231:812–817.
https://doi.org/10.1038/nature07890
Caron, J.B. and Jackson, D.A. 2008. Paleoecology of the greater phyllopod bed community, Burgess Shale. Palaeogeography, Palaeoclimatology, Palaeoecology, 258:222–256.
https://doi.org/10.1016/j.palaeo.2007.05.023
Collette, J.H., Hughes, N.C., and Peng, S. 2011. The first report of a Himalayan bradoriid arthropod and the paleogeographic significance of this form. Journal of Paleontology, 85:76–82.
https://doi.org/10.1666/10-063.1
Cox, A. and Pates, S. Supplementary information for Geographic, taxonomic and temporal interrogation of bradoriid diversity and carapace disparity. Open Science Framework:osf.io/sy295.
https://doi.org/10.17605/OSF.IO/SY295
Daley, A.C., Antcliffe, J.B., Drage, H.B., and Pates, S. 2018. Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences, 115:5323–5331.
https://doi.org/10.1073/pnas.1719962115
Elicki, O. 2012. First skeletal microfauna from the Cambrian Series 3 of the Jordan Rift Valley (Middle East). Memoirs of the Association of Australasian Palaeontologists, 42:153–173.
Erwin, D.H., Laflamme, M., Tweedt, S.M., Sperling, E.A., Pisani, D., and Peterson, K.J. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science, 334:1091–1097.
https://doi.org/10.1126/science.1206375
Geyer, G. 2019. A comprehensive Cambrian correlation chart. Episodes Journal of International Geoscience, 42:321–332.
https://doi.org/10.18814/epiiugs/2019/019026
Giribet, G. and Edgecombe, G.D. 2012. Reevaluating the arthropod tree of life. Annual review of entomology, 57:167–186.
https://doi.org/10.1146/annurev-ento-120710-100659
Guillerme, T. 2018. dispRity: A modular R package for measuring disparity. Methods in Ecology and Evolution, 9:1755–1763.
https://doi.org/10.1111/2041-210x.13022
Guillerme, T., Puttick, M.N., Marcy, A.E., and Weisbecker, V. 2020. Shifting spaces: Which disparity or dissimilarity measurement best summarize occupancy in multidimensional spaces? Ecology and Evolution, 10:7261–7275.
https://doi.org/10.1002/ece3.6452
Hinz-Schallreuter, I. and Jones, P. J. 1994. Gladioscutum lauriei n. gen. n. sp.(Archaeocopida) from the Middle Cambrian of the Georgina basin, central Australia. Paläontologische Zeitschrift, 68:361–375.
https://doi.org/10.1007/bf02991349
Hinz-Schallreuter, I., Gozalo, R., and Liñán, E. 2007. New bradorid arthropods from the Lower Cambrian of Spain. Micropaleontology, 53: 497–510.
https://doi.org/10.2113/gsmicropal.53.6.497
Holmes, J.D. 2023. Contrasting patterns of disparity suggest differing constraints on the evolution of trilobite cephalic structures during the Cambrian ‘explosion’. Palaeontology, e12647:1–9.
https://doi.org/10.1111/pala.12647
Hou, S., Cui, Z., Wang, X., and Zhang, Y. 1989. The geological succession and geographical distribution of Cambrian Bradoriida from China. Journal of Southeast Asian Earth Sciences, 3:197–118.
https://doi.org/10.1016/0743-9547(89)90014-7
Hou, X., Siveter, D.J., Williams, M., Walossek, D., and Bergström, J. 1996. Appendages of the arthropod Kunmingella from the early Cambrian of China: its bearing on the systematic position of the Bradoriida and the fossil record of the Ostracoda. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 351:1131–1145.
https://doi.org/10.1098/rstb.1996.0098
Hou, X., Siveter, D.J., Williams, M., and Feng, X. 2001. A monograph of the Bradoriid arthropods from the Lower Cambrian of SW China. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 92:347–409.
https://doi.org/10.1017/s0263593300000286
Hou, X., Williams, M., Siveter, D.J., Siveter, D.J., Aldridge, R.J., and Sansom, R.S. 2010. Soft-part anatomy of the Early Cambrian bivalved arthropods Kunyangella and Kunmingella: significance for the phylogenetic relationships of Bradoriida. Proceedings of the Royal Society B: Biological Sciences, 277:1835–1841.
https://doi.org/10.1098/rspb.2009.2194
Hughes, M., Gerber, S., and Wills, M.A. 2013. Clades reach highest morphological disparity early in their evolution. Proceedings of the National Academy of Sciences USA, 111:13875–13879.
https://doi.org/10.1073/pnas.1302642110
Hunt, G., Park, L.E., and Labarbera, M. 2007. A novel crustacean swimming stroke: coordinated four-paddled locomotion in the cypridoidean ostracode Cypridopsis vidua (Muller). The Biological Bulletin, 212:67–73.
https://doi.org/10.2307/25066581
Jensen, S., 2003. The Proterozoic and earliest Cambrian trace fossil record; patterns, problems and perspectives. Integrative and Comparative Biology, 43:219–228.
https://doi.org/10.1093/icb/43.1.219
Jones, P.J. and McKenzie, K.G. 1980. Queensland Middle Cambrian Bradoriida (Crustacea): new taxa, palaeobiogeography and biological affinities. Alcheringa, 4:203–225.
https://doi.org/10.1080/03115518008618932
Jones, P.J. and Laurie, J.R. 2006. Bradoriida and Phosphatocopida (Arthropoda) from the Arthur Creek Formation (Middle Cambrian), Georgina Basin, central Australia. Memoirs of the Association of Australasian Palaeontologists, 32:205.
Jones, P.J. and Kruse, P.D. 2009. New Middle Cambrian bradoriids (Arthropoda) from the Georgina Basin, central Australia. Memoirs of the Association of Australasian Palaeontologists, 37:55–86.
Legg, D.A., Sutton, M.D., and Edgecombe, G.D. 2013. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nature Communications, 4:2485.
https://doi.org/10.1038/ncomms3485
Liu, F., Topper, T.P., Strotz, L.C., Liang, Y., Hu, Y., Skovsted, C.B., and Zhang, Z. 2024. Morphological disparity and evolutionary patterns of Cambrian hyoliths. Papers in Palaeontology, e1554.
https://doi.org/10.1002/spp2.1554
Liu, Y., Zhang, X.L., Liu, W., and Zhang, Q. 2008. New bradoriids from the lower Cambrian Yanwangbian formation of southern Shaanxi Province, Central China. Palaeoworld, 17:102–107.
https://doi.org/10.1016/j.palwor.2008.02.003
Loughlin, J.D. and Hillier, R.D. 2011. Early Cambrian Teichichnus- dominated ichnofabrics and palaeoenvironmental analysis of the Caerfai Group, Southwest Wales, UK. Palaeogeography, Palaeoclimatology, Palaeoecology, 297:239–251.
https://doi.org/10.1016/j.palaeo.2010.07.030
Mángano, M.G. and Buatois, L.A. 2014. Decoupling of body-plan diversification and ecological structuring during the Ediacaran-Cambrian transition: evolutionary and geobiological feedback. Proceedings of the Royal Society B: Biological Sciences, 281:20140038.
https://doi.org/10.1098/rspb.2014.0038
McMenamin, M.A. 2020. Bradoriids (Arthropoda) and the Cambrian diversification. Geosciences, 10:119.
https://doi.org/10.3390/geosciences10040119
Melnikova, L.M. 1998. Revision of some Cambrian bradoriids (Crustacea) from the Siberian platform. Paleontologicheskii Zhurnal, 4:36–40.
Melnikova, L.M. 2003. Cambrian Bradoriida (Arthropoda) of the Malyi Karatau (Kyr-Shabakty section). Paleontological Journal, 37:394–399.
Melnikova, L.M., Siveter, D.J., and Williams, M. 1997. Cambrian Bradoriida and Phosphatocopida (Arthropoda) of the former Soviet Union. Journal of Micropalaeontology, 16:179–191.
https://doi.org/10.1144/jm.16.2.179
Nanglu, K., Caron, J.B., and Gaines, R.R. 2020. The Burgess Shale paleocommunity with new insights from Marble Canyon, British Columbia. Paleobiology, 46:58–81.
https://doi.org/10.1017/pab.2019.42
Pates, S., Daley, A.C., Legg, D.A., and Rahman, I.A. 2021. Vertically migrating Isoxys and the early Cambrian biological pump. Proceedings of the Royal Society B, 288:20210464.
https://doi.org/10.1098/rspb.2021.0464
Peel, J.S. 2017. Systematics and biogeography of some early Cambrian (Series 2) bradoriids (Arthropoda) from Laurentia (Greenland). Canadian Journal of Earth Sciences, 54:961-972.
https://doi.org/10.1139/cjes-2017-0101
Peel, J.S. and Streng, M. 2015. A new middle Cambrian bradoriid arthropod from Greenland and western Canada. Journal of Paleontology, 89:96–102.
https://doi.org/10.1017/jpa.2014.8
Peng, J., Feng, H., Fu, X., Zhao, Y., and Yao, L. 2010. New bradoriid arthropods from the early Cambrian Balang Formation of eastern Guizhou, South China. Acta Geologica Sinica (English Edition), 84:56–68.
https://doi.org/10.1111/j.1755-6724.2010.00170.x
Perrier, V., Vannier, J., and Siveter, D.J. 2011. Silurian bolbozoids and cypridinids (Myodocopa) from Europe: pioneer pelagic ostracods. Palaeontology, 54:1361–1391.
https://doi.org/10.1111/j.1475-4983.2011.01096.x
Rode, A.L., Lieberman, B.S., and Rowell, A.J. 2003. A new early Cambrian bradoriid (Arthropoda) from East Antarctica. Journal of Paleontology, 77:691–697.
https://doi.org/10.1666/0022-3360(2003)077<0691:anecba>2.0.co;2
RStudioTeam, 2021. RStudio: Integrated Development Environment for R. Boston (MA): RStudio, PBC.
Sánchez-González, J.R., Baltanás, Á., and Danielopol, D.L. 2004. Patterns of morphospace occupation in recent Cypridoidea (Crustacea, Ostracoda). Revista Española de Micropaleontología, 36:13–27.
Servais, T., Cascales-Miñana, B., Harper, D.A., Lefebvre, B., Munnecke, A., Wang, W., and Zhang, Y. 2023. No (Cambrian) explosion and no (Ordovician) event: A single long-term radiation in the early Palaeozoic. Palaeogeography, Palaeoclimatology, Palaeoecology, 623:111592.
https://doi.org/10.1016/j.palaeo.2023.111592
Shu, D. and Chen, L. 1994. Cambrian palaeobiogeography of Bradoriida. Journal of Southeast Asian Earth Sciences, 9:289–299.
https://doi.org/10.1016/0743-9547(94)90036-1
Siveter, D.J. 2008. Ostracods in the Palaeozoic? Senckenbergiana lethaea, 88:1–9.
https://doi.org/10.1007/bf03043973
Siveter, D.J. and Williams, M. 1995. An early Cambrian assignment for the Caerfai Group of South Wales. Journal of the Geological Society, London, 152:221–224.
https://doi.org/10.1144/gsjgs.152.2.0221
Siveter, D.J. and Williams, M. 1997. Cambrian bradoriid and phosphatocopid arthropods of North America. Special Papers in Palaeontology, 57:1–69.
https://doi.org/10.1017/s0016756899461771
Siveter, D.J., Vannier, J.M., and Palmer, D. 1991. Silurian myodocopes: pioneer pelagic ostracods and the chronology of an ecological shift. Journal of Micropalaeontolgy, 10:151–173.
https://doi.org/10.1144/jm.10.2.151
Siveter, D.J., Williams, M., Peel, J.S., and Siveter, D.J. 1995. Bradoriida (Arthropoda) from the early Cambrian of North Greenland. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 86:113–121.
https://doi.org/10.1017/s0263593300006374
Siveter, D.J., Perrier, V., and Williams, M. 2022. Silurian myodocopes display adaptations for a nektobenthic lifestyle: The paleobiological evidence. Marine Micropaleontology, 174:101906.
https://doi.org/10.1016/j.marmicro.2020.101906
Skovsted, C.B. 2005. A carapace of the bradoriid arthropod Mongolitubulus from the Early Cambrian of Greenland. GFF, 127:217–220.
https://doi.org/10.1080/11035890501273217
Skovsted, C.B., Brock, G.A., and Paterson, J.R. 2006. Bivalved arthropods from the Lower Cambrian Mernmerna Formation, Arrowie Basin, South Australia and their implications for identification of Cambrian 'small shelly fossils'. Association of Australasian Palaeontologists memoirs, 32:7–41.
Skovsted, C.B., Topper, T.P., McLoughlin, S., Johansson, O., Liu, F., and Vajda, V. 2021. First discovery of Small Shelly Fossils and new occurrences of brachiopods and trilobites from the early Cambrian (Stage 4) of the Swedish Caledonides, Lapland. GFF, 143:134–150.
https://doi.org/10.1080/11035897.2021.1895303
Slater, B.J., Willman, S., Budd, G.E., and Peel, J.S. 2018. Widespread preservation of small carbonaceous fossils (SCFs) in the early Cambrian of North Greenland. Geology, 46:107–110.
https://doi.org/10.1130/g39788.1
Smith, M.P. and Harper, D.A. 2013. Causes of the Cambrian explosion. Science, 341:1355–1356.
https://doi.org/10.1126/science.1239450
Smith, M.P., Brock, G.A., Paterson, J.R. and Topper, T.P. 2014. New bradoriid arthropods from the Giles Creek Dolostone (Cambrian Series 3, Stage 5; Templetonian), Amadeus basin, Central Australia. Memoirs of the Association of Australasian Palaeontologists, 45:233–248.
https://search.informit.org/doi/10.3316/informit.343769503348397
Streng, M. and Geyer, G. 2019. Middle Cambrian Bradoriida (Arthropoda) from the Franconian Forest, Germany, with a review of the bradoriids described from West Gondwana and a revision of material from Baltica. PalZ, 93:567–591.
https://doi.org/10.1007/s12542-019-00448-z
Strickler, J.R. 1975. Swimming of planktonic Cyclops species (Copepoda, Crustacea): pattern, movements and their control, p. 599–613. In Wu, T.Y.T., Brokaw, C.J., and Brennen, C. (eds.), Swimming and Flying in Nature: Volume 2., Springer US, Boston.
https://doi.org/10.1007/978-1-4757-1326-8_9
Sun, H., Zhao, F., Steiner, M., Li, G., Na, L., Pan, B., Yin, Z., Zeng, H., Van Iten, H., and Zhu, M. 2020. Skeletal faunas of the lower Cambrian Yu'anshan formation, eastern Yunnan, China: metazoan diversity and community structure during the Cambrian age 3. Palaeogeography, Palaeoclimatology, Palaeoecology, 542:109580.
https://doi.org/10.1016/j.palaeo.2019.109580
Sylvester-Bradley, P.C. 1961. Archaeocopida, p. Q100–Q103. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology: Part Q, Arthropoda 3. Geological Society of America and University of Kansas Press, Boulder.
https://doi.org/10.17161/dt.v0i0.5618
Topper, T.P., Skovsted, C.B., Brock, G.A., and Paterson, J.R. 2007. New bradoriids from the lower Cambrian Mernmerna Formation, South Australia: systematics, biostratigraphy and biogeography. Memoirs of the Association of Australasian Palaeontologists, 33:67–100.
Topper, T.P., Skovsted, C.B., Brock, G.A., and Paterson, J.R. 2011. The oldest bivalved arthropods from the early Cambrian of East Gondwana: Systematics, biostratigraphy and biogeography. Gondwana Research, 19:310–326.
https://doi.org/10.1016/j.gr.2010.05.012
Vannier, J. and Chen, J.Y. 2000. The Early Cambrian colonization of pelagic niches exemplified by Isoxys (Arthropoda). Lethaia, 4:295–311.
https://doi.org/10.1080/002411600750053862
Vannier, J., Williams, M., Alvaro, J.J., Vizcaïno, D., Monceret, S., and Monceret, E. 2005. New Early Cambrian bivalved arthropods from southern France. Geological Magazine, 142:751–763.
https://doi.org/10.1017/s0016756805001093
Vannier, J., García-Bellido, D.C., Hu, S.X., and Chen, A.L. 2009. Arthropod visual predators in the early pelagic ecosystem: evidence from the Burgess Shale and Chengjiang biotas. Proceedings of the Royal Society B: Biological Sciences, 276:2567–2574.
https://doi.org/10.1098/rspb.2009.0361
Wallet, E., Slater, B.J., Willman, S., and Peel, J.S. 2021. Small carbonaceous fossils (SCF s) from North Greenland: New light on metazoan diversity in early Cambrian shelf environments. Papers in Palaeontology, 7:1403–1433.
https://doi.org/10.1002/spp2.1347
Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York.
https://doi.org/10.1007/978-0-387-98141-3
Williams, M., Siveter, D.J., Popov, L.E., and Vannier, J.M. 2007. Biogeography and affinities of the bradoriid arthropods: cosmopolitan microbenthos of the Cambrian seas. Palaeogeography, Palaeoclimatology, Palaeoecology, 248:202–232.
https://doi.org/10.1016/j.palaeo.2006.12.004
Williams, M., Vannier, J., Corbari, L., and Massabuau, J.C. 2011. Oxygen as a driver of early arthropod micro-benthos evolution. PLoS ONE, 6:e28183.
https://doi.org/10.1371/journal.pone.0028183
Williams, M., Vandenbroucke, T.R., Perrier, V., Siveter, D.J., and Servais, T. 2015. A link in the chain of the Cambrian zooplankton: bradoriid arthropods invade the water column. Geological Magazine, 152: 923–934.
https://doi.org/10.1017/s0016756815000059
Wood, R., Liu, A.G., Bowyer, F., Wilby, P.R., Dunn, F.S., Kenchington, C.G., Hoyal Cuthill, J.F., Mitchell, E.G., and Penny, A. 2019. Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nature Ecology and Evolution, 3:528–538.
https://doi.org/10.1038/s41559-019-0821-6
Wrona, R. 2009. Early Cambrian bradoriide and phosphatocopide arthropods from King George Island, West Antarctica: biogeographic implications. Polish Polar Research, 30:4.
Zhai, D., Williams, M., Siveter, D.J., Harvey, T.H.P., Sansom, R.S., Gabbott, S.E., Siveter, D.J., Ma, X., Zhou, R., Liu, Y., and Hou, X. 2019. Variation in appendages in early Cambrian bradoriids reveals a wide range of body plans in stem-euarthropods. Communications biology, 2:329.
https://doi.org/10.1038/s42003-019-0573-5
Zhang, X.G. 2007. Phosphatized Bradoriids (Arthropoda) from the Cambrian of China. Palaeontographica Abteilung A, 281:93–173.
https://doi.org/10.1127/pala/281/2007/93
Zhao, F., Zhu, M., and Hu, S. 2010. Community structure and composition of the Cambrian Chengjiang biota. Science China Earth Sciences, 53:1784–1799.
https://doi.org/10.1007/s11430-010-4087-8
Zhuravlev, A.Y. and Wood, R.A. 2018. The two phases of the Cambrian Explosion. Scientific Reports, 8:16656.
https://doi.org/10.1038/s41598-018-34962-y
Zhuravlev, A.Y. and Wood, R.A. 2020. Dynamic and synchronous changes in metazoan body size during the Cambrian Explosion. Scientific Reports, 10:6784.
https://doi.org/10.1038/s41598-020-63774-2

