 An early Cambrian pelago-benthic acorn worm and the origin of the hemichordate larva
An early Cambrian pelago-benthic acorn worm and the origin of the hemichordate larva
Article number: 27.1.a17
https://doi.org/10.26879/1356
Copyright Paleontological Society, March 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 9 November 2023. Acceptance: 13 February 2024.
ABSTRACT
Enteropneusts (acorn worms) are hemichordates, the sister group to echinoderms. Together they form the clade Ambulacraria, which is closely related to chordates. All three groups appear in the lower Cambrian, but their interrelationships remain problematic, which impedes the understanding of early deuterostome evolution. Enteropneusts are also extremely rare in the fossil record, only a few species are known from Lagerstätten-type deposits. Here, we describe the earliest known enteropneust, Cambrobranchus pelagobenthos gen. et sp. nov. based on soft-bodied specimens, including tornaria larvae and juveniles, from the lower Cambrian (Epoch 2, Stage 3) Haiyan Lagerstätte, Chengjiang biota, of China. The enteropneust larvae and post-metamorphic juveniles are the first reported in the fossil record and provide direct evidence for a pelago-benthic lifestyle in a Cambrian deuterostome animal, bolstering the hypothesis that an indirect development is primitive to the enteropneusts and maybe the hemichordates or whole of Ambulacraria.
Xianfeng Yang. Yunnan Key Laboratory for Palaeobiology, Institute of Palaeontology, Yunnan University, 650500 Kunming, Yunnan, China and MEC International Joint Laboratory for Palaeobiology and Palaeoenvironment, Yunnan University, 650500 Kunming, Yunnan, China. (Corresponding author) 279751300@qq.com
Julien Kimmig. Abteilung Geowissenschaften, Staatliches Museum für Naturkunde Karlsruhe, Karlsruhe, 76133, Germany and The Harold Hamm School of Geology & Geological Engineering, University of North Dakota, Grand Forks, North Dakota 58202, USA. (Corresponding author) julien.kimmig@smnk.de
Christopher B. Cameron. Université de Montréal, Département de sciences biologiques, C.P. 6128, Succ. Centre-ville, Montréal, QC, H3C 3J7, Canada. c.cameron@umontreal.ca
Karma Nanglu. Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, Massachusetts 02138, USA. knanglu@fas.harvard.edu
Sara R. Kimmig. Karlsruhe Institute of Technology (KIT), Institute of Geography and Geoecology, Reinhard-Baumeister-Platz 1, 76131 Karlsruhe, Germany. sara.kimmig@kit.edu
Danielle de Carle. Department of Ecology and Evolutionary Biology, University of Toronto, 25 Willcocks Street, Toronto, ON, M5S 2B4, Canada. danielle.decarle@mail.utoronto.ca
Caixia Zhang. Yunnan Key Laboratory for Palaeobiology, Yunnan University, North Cuihu Road 2, 650091, Kunming, China and MEC International Joint Laboratory for Palaeobiology and Palaeoenvironment, Yunnan University, 650091, Kunming, China. fyz@mail.ynu.edu.cn
Mengxiao Yu. Yunnan Key Laboratory for Palaeobiology, Yunnan University, North Cuihu Road 2, 650091, Kunming, China and MEC International Joint Laboratory for Palaeobiology and Palaeoenvironment, Yunnan University, 650091, Kunming, China. ymx720@126.com
Shanchi Peng. State Key Laboratory of Palaeobiology and Stratigraphy, Chinese Academy of Sciences, Nanjing, 210008, China. scpeng@nigpas.ac.cn
Keywords: new genus; new species; lower Cambrian; Chengjiang; Enteropneusta; Ambulacraria
Final citation: Yang, Xianfeng, Kimmig, Julien, Cameron, Christopher B., Nanglu, Karma, Kimmig, Sara R., de Carle, Danielle, Zhang, Caixia, Yu, Mengxiao, and Peng, Shanchi. 2024. An early Cambrian pelago-benthic acorn worm and the origin of the hemichordate larva. Palaeontologia Electronica, 27(1):a17.
https://doi.org/10.26879/1356
palaeo-electronica.org/content/2024/5163-origin-of-the-hemichordate-larva
Copyright: March 2024 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/E8EB1F93-2F23-4FF3-A5E1-306D0B978EB6
INTRODUCTION
A complex, biphasic life history including a benthic, reproductive adult and a pelagic, feeding larva is phylogenetically widespread among extant marine taxa (Thorson, 1950; Mileikovsky, 1973; Levin, 2006; Cowen and Sponaugle, 2009; Dyachuk and Odintsova, 2013). Larvae contribute to the survivorship of species by diversifying both larval and adult life forms and thereby the habitat and resources available. The larval stage is usually morphologically distinct from the adult and the similarities in larva between some phyla are generally attributable to either shared ancestry or to convergence due to small size, mechanical, and developmental constraints (Haug, 2020). For these reasons, larvae provide an overlooked role in unlocking metazoan ecology, evolution, and diversification. The origin of larvae is widely debated and at the root of our understanding of animal evolution. The debate entails whether Cambrian animals were holobenthic and the planktotrophic larva added later, or pelago-benthic with a distinct larval stage (Signor and Vermeij, 1994; Peterson et al., 2005; Wolfe, 2017).
We describe the previously unknown lower Cambrian acorn worm, Cambrobranchus pelagobenthos, based on 39 specimens from the Haiyan Lagerstätte of China. Juveniles, adults, and tornaria larva fossils are preserved, providing direct evidence for a pelago-benthic life history early in hemichordate evolution (Figure 1, Figure 2, Figure 3, Appendix 1, Appendix 2). This exceptional finding from one of the premiere fossil localities in the early Cambrian, the Haiyan Lagerstätte of the Chengjiang biota (Yang et al., 2021), shows that animals were exploiting benthic and pelagic Cambrian habitats at different life stages.
MATERIALS AND METHODS
Terminology
We follow the morphological terminology used in previous studies of fossil and extant enteropneusts (Urata and Yamaguchi, 2004; Caron et al., 2013; Urata et al., 2014; Nanglu et al., 2016, 2020a; Gonzalez et al., 2017, 2018).
Studied Material
The specimens reported here were collected as part of a broader study of the Haiyan Lagerstätte (Yang et al., 2021), a 40 cm thick interval of alternating event and background mudstone bed sets, near the bottom of the Yu’anshan Member, Chiungchussu Formation (Cambrian, Epoch 2, Stage 3), on the eastern shore of Dianchi Lake, near Kunming, ~24 km northwest of Chengjiang County, China. The enteropneusts were recovered from the lowermost 5 cm of the Lagerstätte interval. To minimize bias (Whitaker and Kimmig, 2020), sampling efforts collected all visible, (principally) complete fossils.
Close-ups and microphotographs were taken with a Leica DFC 500 digital camera mounted on a Leica M205-C stereoscope. Macrophotographs of the fossils were taken with a Canon EOS 5D digital SLR camera equipped with a Canon 50 mm macro lens and cross-polarized lights.
Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy Analyses
The backscattered electron (BSE) imaging and energy-dispersive X-ray spectroscopy (EDX) of uncoated specimens was conducted with a FEI Quanta 650 FEG field emission scanning electron microscope (SEM) at the Yunnan Key Laboratory for Palaeobiology, Institute of Palaeontology, Yunnan University, Kunming, China (YKLP). All imaging analyses were conducted with the following operating conditions: 14 mm ± 1 mm working distance (minor differences to allow for variation in sample thickness or topography) for basic imaging and EDX, 20 keV beam accelerating voltage, 10 nA beam current, 20 Pa chamber pressure (low vacuum), 50 µm aperture for imaging, and 40 µm aperture for EDX analysis.
X-Ray Tomographic Microscopy
One specimen (YKLP14443) was scanned using an Xradia MicroXCT-520 Versa system (Carl Zeiss XRM) at YKLP. The scan was conducted with the source operating at 70 kV, 6 W, pixel size 6.95 μm over 206º sample rotation (-103º to 103º), with no filter, for 204 minutes. The exposure time of each projection was 4 seconds. Geometric and optical magnification settings were chosen to collect projections with XY pixel dimensions of 6.95 μm. During the scan,1572 radiographs were registered and saved as TIFF stacks for 3D reconstruction with Drishti 2.4 software.
Phylogenetic Analysis and Ancestral State Estimation
We performed phylogenetic analyses using a modified version of the morphological character matrix published in Nanglu et al. (2020a). The data matrix consists of 29 taxa and 113 characters (Appendix 3). The Bayesian phylogenetic analysis was performed using MrBayes 3.2.6 with the following search parameters: 10 million generations, sampling every 1000 generations, 25% burn-in, using an mkv model with a gamma distribution. Additionally, we performed a parsimony analysis, the strict consensus tree for which is presented in the Appendix 1. The Appendix 3 and Appendix 4 includes a detailed list of characters used in both analyses based on Cameron (2005) and Nanglu et al. (2020a). To test the hypothesis that a bi-phasic lifestyle is ancestral for both Hemichordata and Ambulacraria, we used MrBayes to empirically estimate ancestral states for nodes of interest, as detailed by Huelsenbeck and Bollback (2001). This approach allowed us to account for uncertainty in tree topology and all other parameters. For this, and Bayesian phylogenetic analysis, Tracer 1.7.1 (Rambaut et al., 2018) was used to confirm that runs had converged (i.e., effective sample size for all parameters > 200; average standard deviation of split frequencies < 0.01; estimates for all parameter values were ~ normally distributed). Results are detailed in the Appendix 1.
Repositories and Institutional Abbreviations
Types, figures, and other specimens examined in this study are deposited in the following institution: Yunnan Key Laboratory for Palaeobiology, Institute of Palaeontology, Yunnan University, Kunming, China (YKLP).
SYSTEMATIC PALEONTOLOGY
Phylum HEMICHORDATA Bateson, 1885 (emend. Fowler, 1892)
Class ENTEROPNEUSTA Gegenbaur, 1870
Genus CAMBROBRANCHUS gen. nov.
zoobank.org/7176AE24-7C5C-407A-BB93-E1B7FFC637E7
Type species. Cambrobranchus pelagobenthos gen. et sp. nov.
Cambrobranchus pelagobenthos gen. et sp. nov.
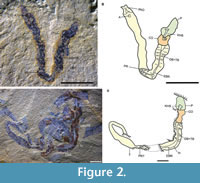
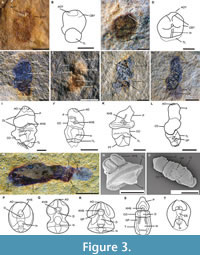
Figure 1A-F, Figure 2A-D, Figure 3A-M, Figure 4A-P, Figure 5
zoobank.org/A0088DD9-1F6F-4666-BFF6-5430FF782D87
 Type material. Holotype, YKLP14443 (Figure 1A-C, Appendix 1) from the Yu’anshan Member, Chiungchussu Formation, Cambrian, Epoch 2, Stage 3, Wutingaspis - Eoredlichia Biozone, on the eastern shore of Dianchi Lake, near Kunming, ~24 km northwest of Chengjiang County, China (Repository: YKLP). Paratypes, YKLP14529-14537, 14543, 14544 (Figure 1D-F, Figure 3A-H, Appendix 1).
Type material. Holotype, YKLP14443 (Figure 1A-C, Appendix 1) from the Yu’anshan Member, Chiungchussu Formation, Cambrian, Epoch 2, Stage 3, Wutingaspis - Eoredlichia Biozone, on the eastern shore of Dianchi Lake, near Kunming, ~24 km northwest of Chengjiang County, China (Repository: YKLP). Paratypes, YKLP14529-14537, 14543, 14544 (Figure 1D-F, Figure 3A-H, Appendix 1).
Other specimens. YKLP14538-14540, 14542, 14545-14568 (Figure 3M, Appendix 1).
Diagnosis. An enteropneust possessing a tripartite vermiform body consisting of proboscis, collar, and trunk. Proboscis approximately two times longer than wide; collar approximately two times longer than wide. A series of gill and tongue bars (GB+TB) accounting for 1/3-1/2 of the length of the trunk. The trunk is usually U-shaped or sinuous. Gut is straight with a post-anal organ structure. Indirect development with a tornaria larva.
 Etymology. Genus name Cambro, for the period (Cambrian) in which the fossils are found; branchus for gills. Species name pelagobenthos (Latin), for the lifestyle.
Etymology. Genus name Cambro, for the period (Cambrian) in which the fossils are found; branchus for gills. Species name pelagobenthos (Latin), for the lifestyle.
Type locality and horizon. Bottom 5 cm of the Haiyan Lagerstätte (Yang et al., 2021), Yu’anshan Member, Chiungchussu Formation, Cambrian, Epoch 2, Stage 3, Wutingaspis - Eoredlichia Biozone, on the eastern shore of Dianchi Lake, near Kunming, ~24 km northwest of Chengjiang County, China.
Description. Specimens are entirely soft-bodied and typically preserved as two-dimensional compressions. Body is tripartite, divided into proboscis, collar, and trunk. Specimens range from 500 μm to 33 mm in total length. The outer surface of the trunk preserves GB+TB, along with gill pores. There is an ovoid post-anal organ. The extension of GB+TB extends to one third or half of the trunk (Figure 1D-F, Appendix 1). The post-anal organ of a few specimens is adhered to unidentified biological fragments (Appendix 1). In some cases, individuals are found in clusters on a single slab (Appendix 1), co-occurring with tornaria larvae (Figure 3A, C) and numerous juveniles (Figure 3E-H, M).
 The proboscis is well-preserved in almost all specimens, excluding tornaria, and is usually contracted, the exception being the YKLP14443 and YKLP14534 (Figure 1A, Appendix 1) where it is extended. The proboscis is conical to cylindrical in shape (Figure 1A-F, Figure 2A-D, Figure 3E-H, M, Appendix 1). There is an axial zone within the proboscis coelom (Figure 2A, Figure 3M, Appendix 1) and at its anterior, an irregular elliptical region with a slight bulge, usually darker or more reflective than its surroundings (Figure 3M, Appendix 1). The length of this major axis varies from 0.1 mm to 0.4 mm. The structure is herein interpreted as kidney-heart-stomochord complex (KHS) (Figure 1A-F, Figure 2A-D, Figure 3E-G, M, Appendix 1), as it is in the same area as the KHS in extant enteropneusts (Balser and Ruppert, 1990) and fossil species from the Cambrian Burgess Shale (Caron et al., 2013; Nanglu et al., 2016, 2020a).
The proboscis is well-preserved in almost all specimens, excluding tornaria, and is usually contracted, the exception being the YKLP14443 and YKLP14534 (Figure 1A, Appendix 1) where it is extended. The proboscis is conical to cylindrical in shape (Figure 1A-F, Figure 2A-D, Figure 3E-H, M, Appendix 1). There is an axial zone within the proboscis coelom (Figure 2A, Figure 3M, Appendix 1) and at its anterior, an irregular elliptical region with a slight bulge, usually darker or more reflective than its surroundings (Figure 3M, Appendix 1). The length of this major axis varies from 0.1 mm to 0.4 mm. The structure is herein interpreted as kidney-heart-stomochord complex (KHS) (Figure 1A-F, Figure 2A-D, Figure 3E-G, M, Appendix 1), as it is in the same area as the KHS in extant enteropneusts (Balser and Ruppert, 1990) and fossil species from the Cambrian Burgess Shale (Caron et al., 2013; Nanglu et al., 2016, 2020a).
A 360-degree collar annulation with a raised outline attaches to the anterior of the trunk. The holotype collar is twice as long as wide (Figure 1A-C), and the length to width ratio of the proboscis, collar, and trunk are 2.5, 1.25, and 9, respectively (Appendix 1). Collar ridges are discernable at the anterior and posterior margins (Appendix 1), and many transversally corrugated bands are preserved. There are at least six rows of corrugated bands in specimen YKLP14529. The bands can also be found in some of the juvenile specimens (Figure 3E-H, M).
The dorsal branchial-pharynx region of the trunk is comprised of thick and thin gill bars alternating, herein interpreted as the GB+TB. Few of the gill slits have been completely exposed due to the taphonomy and possible ecology of the animals, but in specimen YKLP14545 (Appendix 1), a laterally compressed adult, there are about five sets of GB+TB for nearly every 500 μm (Appendix 1). The GB+TB are paired and symmetrically arranged on either side of an epibranchial ridge (EBR) (Figure 1D-F, Appendix 1). The gill slits are defined by gill bars and connect to the outside gill pores by atrial cavities. At least two to four gill pores can be identified in our collection (Figure 3H, L, Appendix 1). The parabranchial ridges (PR) merge posteriorly (Figure 1D-F, Appendix 1). A single pair and serial pairs of GB+TB are well-preserved in YKLP14568 and YKLP14545, respectively (Appendix 1). Some relatively well-preserved GB+TB can be discerned in YKLP14562-14563, 14565-14567 (Appendix 1). The post pharynx region is a simple tube and best preserved in YKLP14565-14566 (Figure 1D-F, Appendix 1).
Digestive tract: The digestive system of C. pelagobenthos consists of a mouth, an enlarged pharyngeal area, an oesophageal organ (OO), a posterior intestine, and an anus. The mouth is ventrally located near the most anterior part of the collar (Figure 1A, Figure 3M, Appendix 1). The pharyngeal region extends from one third to almost half the length of the trunk (Figure 1D-F, Figure 2A-B, Appendix 1) and preserves an end-pharyngeal pouch (PH) at the anterior end (Appendix 1). A post-pharyngeal ovoid structure is visible in several specimens (Appendix 1) that is here interpreted as the OO, like the one present in other Cambrian and extant enteropneusts (Caron et al., 2013; Nanglu et al., 2016, 2020a). The OO is followed by the intestine, which terminates in the anus. The anus is rarely preserved but appears to be non-terminal, followed by a post-anal organ (PAO) of roughly ovoid shape. The length of the post-anal organ ranges from 57 μm in juvenile specimens to 454 μm in the largest adult specimen. It is present in several specimens (Figure 2A-B, Figure 3M, Appendix 1) and is sometimes preserved with three-dimensional relief (Figure 2A-B, Appendix 1).
 Ontogeny and larval development: Two potential larval specimens have been identified (Figure 3A-D, Figure 4), based on their morphology and compared with modern tornaria. There are other potential larvae present, but the preservation quality of these specimens prevents assignment. The specimens co-occur with juvenile, sub-adult, and adult specimens (Appendix 1), which is common among extant long-lived planktotrophic larva, including tornaria, which routinely settle in the adult sedimentary habitats (Flint and Kalke, 1986; Nielsen and Hay-Schmidt, 2007). The first (Figure 3A-B) is oval shaped, 1.5 mm long, showing the posterior ciliated telotroch swimming band. An outline of the larval body appears above the telotroch, potentially showing a ciliated feeding band and dark apical organ (Figure 3A). The second (Figure 3C-D) is 1.6 mm in length, in anterior view, with a telotroch and feed bands, gut, and a retracted apical organ comparable to extant analogues that characterize the tornaria of modern acorn worms, including Schizocardium (Figure 3N), Glandiceps, and ptychoderid species (Urata and Yamaguchi, 2004; Urata et al., 2014; Gonzalez et al., 2017, 2018).
Ontogeny and larval development: Two potential larval specimens have been identified (Figure 3A-D, Figure 4), based on their morphology and compared with modern tornaria. There are other potential larvae present, but the preservation quality of these specimens prevents assignment. The specimens co-occur with juvenile, sub-adult, and adult specimens (Appendix 1), which is common among extant long-lived planktotrophic larva, including tornaria, which routinely settle in the adult sedimentary habitats (Flint and Kalke, 1986; Nielsen and Hay-Schmidt, 2007). The first (Figure 3A-B) is oval shaped, 1.5 mm long, showing the posterior ciliated telotroch swimming band. An outline of the larval body appears above the telotroch, potentially showing a ciliated feeding band and dark apical organ (Figure 3A). The second (Figure 3C-D) is 1.6 mm in length, in anterior view, with a telotroch and feed bands, gut, and a retracted apical organ comparable to extant analogues that characterize the tornaria of modern acorn worms, including Schizocardium (Figure 3N), Glandiceps, and ptychoderid species (Urata and Yamaguchi, 2004; Urata et al., 2014; Gonzalez et al., 2017, 2018).
Four additional fossils are interpreted as recently metamorphosed early juvenile developmental stages (cf. Urata and Yamaguchi, 2004) (Figure 3E-H). The specimens show differentiation into a tripartite body with an elongated conical proboscis, collar, and trunk. The trunk is comparatively short with respect to that of adult specimens. YKLP14549 (Figure 3H) represents an early juvenile specimen with at least two pairs of preserved oval-shaped gill pores (GP1 and GP2). Its length is 1.8 mm, and the position of the gill pores is like that of modern acorn worms (Urata and Yamaguchi, 2004; Urata et al., 2014).
 Another seven specimens represent juveniles (Figure 3M, Appendix 1). They are fully developed and morphologically comparable to extant juvenile specimens. The PAO is comparable to extant harrimaniid acorn worms (Figure 3M), a probable homologue to the pterobranch stalk. The trunk of the juveniles has a length of ~1.6 mm in YKLP14551 (Figure 3M). Those of juvenile worms to adults range from 2.2 mm to 33 mm long (Figure 1A-F, Appendix 1). A reconstruction of the different metamorphic stages can be seen in Figure 5.
Another seven specimens represent juveniles (Figure 3M, Appendix 1). They are fully developed and morphologically comparable to extant juvenile specimens. The PAO is comparable to extant harrimaniid acorn worms (Figure 3M), a probable homologue to the pterobranch stalk. The trunk of the juveniles has a length of ~1.6 mm in YKLP14551 (Figure 3M). Those of juvenile worms to adults range from 2.2 mm to 33 mm long (Figure 1A-F, Appendix 1). A reconstruction of the different metamorphic stages can be seen in Figure 5.
Remarks. Maletz (2019) reported the presence of pterobranchs from the Fortunian (Stage 1; Epoch 1). This suggests that pterobranchs and enteropneusts were already differentiated at that point, and that Cambrobranchus pelagobenthos may not represent the most basal enteropneust.
DISCUSSION
Preservation
All specimens of Cambrobranchus pelagobenthos were collected from the lowermost 5 cm of the Haiyan Lagerstätte. They are preserved in finely laminated mudstones, featuring the typical alternations of event and background layers known from the Chengjiang biota (Zhao et al., 2009). All fossils are found in the event layers, which are represented by thin, yellowish-grey layers interbedded in greyish mudstones. The event beds are suggested to have formed by accumulation of suspended mud from periodical storms, tsunamis, or turbidites (Zhao et al., 2009). Temporary high particle suspension aiding preservation in the Chengjiang biota has also recently been suggested (Saleh et al., 2022). As the enteropneust larvae appear to have been planktonic, similar to their modern equivalents (Gonzalez et al., 2018), they must have been killed off in the water column shortly after they hatched or once they settled in the adult sedimentary habitats, possibly by anoxia or a toxic gas release, which supports a ‘boom-and-bust’ environment (Yang et al., 2021). Additionally, modern enteropneust larvae are very delicate (Nielsen and Hay-Schmidt, 2007), supporting the idea that the specimens were quickly buried by suspended mud.
The fine external details that were preserved through initial pyrite replacement, later weathered to iron oxides, and the intact preservation of internal organs and structures suggest that the C. pelagobenthos specimens were rapidly buried, but not in a high energy event, as they would have either decayed relatively quickly on the oxygenated ocean floor or within the uppermost oxic- to sub-oxic layers of sediment, given their infaunal nature (Butler et al., 2015; Sansom, 2016). This can be seen in other deposits where specimens were exposed (Kimmig and Pratt, 2016; Leibach et al., 2021). Additionally, evidence of scavenging would likely be observed, unless the whole animal was consumed.
Some of the less well-preserved internal organs, likely underwent rapid decay, as has been observed for non-cuticular interior organs in decay experiments (Sansom, 2016). These include the KHS, which is preserved as a darker mass between the base of the proboscis and the collar, which corresponds to its position in all modern hemichordates (Cameron et al., 2010) and has been inferred in Cambrian hemichordates (Caron et al., 2013; Nanglu et al., 2015, 2016).
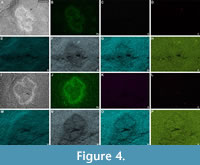
Pyrite or its iron oxide- and/or oxyhydroxide-weathering are the main form of preservation of the fossils (Figure 4, Appendix 1), including the internal organs, with rare patches of organic carbon, similar to fossils from other Chengjiang deposits (Gabbott et al., 2004; Saleh et al., 2020; Yang et al., 2021). While this form of preservation can initially be thought of as inept for the preservation of internal and fine external structures, it has shown to preserve remarkable details in the Chengjiang biota (Liu et al., 2014, 2016; Hou et al., 2017). While oxidized pyrite preservation is observationally evident by a rusty red-orange coloration of the fossils, this mode of preservation is confirmed by the presence of iron-bearing framboidal textures indicating weathering-induced pseudomorphs after the original pyrite (Appendix 1). Iron-bearing mineral concentrations usually follow the margins of the specimens and have also replaced the internal organs (Appendix 1).
While the idea of preserving planktotrophic larvae adjacent to adults of the same species might be counterintuitive, long lived extant planktotrophic larva, including tornaria, routinely settle in the adult sedimentary habitats (Flint and Kalke, 1986; Shanks et al., 2003; Nielsen and Hay-Schmidt, 2007; Shanks, 2009; Freckelton et al., 2022). This has also been observed in the fossil record, i.e., the Ordovician of Siberia, where Isotelus -like protaspides (planktonic larvae) have been found in the same beds as their interpreted adult stages (Lerosey-Aubril and Laibl, 2021) and the Ordovician of Canada where Isotelus protaspides are found together with benthic juveniles (meraspides) and adults (holaspides) (Chatterton, 1980). The mechanism, inferred for most marine planktotrophic larvae, is that settlement and metamorphosis are induced by local chemical cues. The best studied examples include biofilms and adult pheromones (Zimmer-Faust and Tamburri, 1994; Browne and Zimmer, 2001; Dobretsov and Rittschof, 2020).
When considering the abundance of larval and juvenile fossils found in the Haiyan Lagerstätte, we also must consider its interpretation as a ‘boom-and-bust deposit’ (Yang et al., 2021), which increases the likelihood of any planktotrophic larvae to be preserved adjacent to adult specimens as we are dealing with rapidly deposited event beds, with each layer representing a single burial event. The layers preserve relatively short periods of time compared to time-averaged deposits hosting many other fossils, suggesting that planktotrophic larvae would be trapped during such events. This also indicates that deposition in the Haiyan Lagerstätte was likely not ecologically selective (Saleh et al., 2021), at least in certain event beds, as endobenthic, nektobenthic/epibenthic, and nektonic/planktonic taxa are preserved (Chen, 2004; Yang et al., 2021).
The Differences between the Chengjiang and the Burgess Shale Specimens
There are 114 species of extant enteropneusts in four crown group families: Harrimaniidae, Spengelidae, Torquaratoridae, and Ptychoderidae (Jabr et al., 2018) and seven fossil species (Cameron, 2018). The previously earliest fossil specimens are from the Burgess Shale of Canada and include Spartobranchus tenuis Walcott 1911 and Oesia disjuncta Walcott 1911. These two fossil body plans are harrimaniid-like in that they lack gill bar synapticula, genital wings, and hepatic sacs (Caron et al., 2013; Nanglu et al., 2016), but unlike any extant forms in that they are tubicolous and possess posterior grasping appendages. The remaining fossil species have been put into crown group families by Cameron (2018) and include Mazoglossus ramsdelli Bardack 1997 and Saccoglossus testa Cameron 2016 from the Carboniferous Mazon Creek of Illinois, USA, Megaderaion sinemuriense Arduini et al., 1981 and Ptychodera callovianum Alessandrello et al., 2004 from the Lower and Middle Jurassic of northern Italy and southern France, respectively, and Mesobalanoglossus buergeri Bechly and Frickhinger in Frickhinger 1999 from the Upper Jurassic Solnhofen Limestones of southern Germany.
Cambrobranchus pelagobenthos gen. et sp. nov. is the first hemichordate exhibiting enteropneust traits from the early Cambrian Chengjiang biota and predates all other known enteropneust fossils. Its body plan is like the Burgess Shale S. tenuis and O. disjuncta in that it has a tripartite body composed of a proboscis, collar, and trunk. The three species preserve gill bars, tongue bars, a post-anal organ (or bulb), and some evidence of a KHS (Caron et al., 2013; Nanglu et al., 2016). Also noted are external gill pores (GP) and a well-preserved collar with circumferential collar bands (Figure 1), features that are lacking from the Burgess Shale specimens. The histology of circumferential collar bands is well known: The anterior lip can be pliable and exploratory or stiff and immotile, and the subsequent regions characterized by dense cilia, abundant mucous cells, or dark staining secretory mulberry cells (Benito and Pardos, 1997). The functional biology of this complex collar is largely unknown, but it is ancient. For a detailed comparison of C. pelagobenthos with the Burgess Shale acorn worms see Appendix 2.
The new taxon differs ecologically from the Burgess Shale enteropneust species, which were tubicolous. Spartobranchus tenius was probably a facultative filter feeder and deposit feeder, whereas Oesia disjuncta was a suspension feeder (Caron et al., 2013; Nanglu et al., 2016). Cambrobranchus pelagobenthos lacks a tube and is therefore interpreted here as an epibenthic deposit feeder because several specimens have well-preserved fragments of shells and cuticle in the mouth, pharynx, oesophageal organ, and intestine (Appendix 1). While this does not preclude the possibility that C. pelagobenthos facultatively filter fed like some modern harrimaniids (Cameron, 2002), the gut contents suggest that it was primarily a deposit feeder. Like the priapulid worm Ottoia, which has an excellent record of intestinal residues (Vannier, 2012), the low nutritional value of hyolithids and brachiopods was likely offset by the intake of tissues from carcasses. We cannot rule out that it is also suspension fed, akin to some modern acorn worms. Evidence of gut contents in C. pelagobenthos demonstrates that deposit feeding evolved early in the enteropneust line. When considered together with Spartobranchus, Oesia, and the stem-group hemichordate Gyaltsenglossus, there is increasing evidence that hemichordates had come to occupy several marine niches by the early to middle Cambrian. Extant acorn worms that are epibenthic include the deep-sea rock dwelling Saxipendium coronatum Woodwick and Sensenbaugh 1985, the Alaskan kelp holdfast dwelling Harrimania maculosa Ritter 1900, and most members of the deep-sea family Torquaratoridae. The remainder are burrowers (Jensen et al., 1992; Holland et al., 2012). We cannot rule out burrowing in C. pelagobenthos either because trace fossils are rarely preserved in the Haiyan Lagerstätte due to the soft nature of the substrate.
Phylogeny
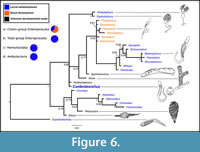 The results of our Bayesian phylogenetic analysis (Figure 6) resolve Cambrobranchus as a total-group hemichordate with high support, but its exact relationship with the extant Classes is uncertain. More specifically, it is placed in a polytomy with pterobranchs, total-group Enteropneusta (including the fossils Oesia and Spartobranchus), and the tentaculate Gyaltsenglossus. To further investigate the most supported positioning for Cambrobranchus, we considered the number of trees in the posterior distribution (n = 20,001) in which Hemichordata and Enteropneusta were monophyletic, as both are unambiguously supported by all recent molecular phylogenies (Cannon et al., 2014; Kapli et al., 2021). This yielded 14,096 total trees, 51% of which supported Cambrobranchus as a stem-group enteropneust, 43.44% of which supported Cambrobranchus as a stem-group hemichordate, and 5.56% of which supported Cambrobranchus as a stem-pterobranch. The posterior distribution of trees is available in Appendix 4.
The results of our Bayesian phylogenetic analysis (Figure 6) resolve Cambrobranchus as a total-group hemichordate with high support, but its exact relationship with the extant Classes is uncertain. More specifically, it is placed in a polytomy with pterobranchs, total-group Enteropneusta (including the fossils Oesia and Spartobranchus), and the tentaculate Gyaltsenglossus. To further investigate the most supported positioning for Cambrobranchus, we considered the number of trees in the posterior distribution (n = 20,001) in which Hemichordata and Enteropneusta were monophyletic, as both are unambiguously supported by all recent molecular phylogenies (Cannon et al., 2014; Kapli et al., 2021). This yielded 14,096 total trees, 51% of which supported Cambrobranchus as a stem-group enteropneust, 43.44% of which supported Cambrobranchus as a stem-group hemichordate, and 5.56% of which supported Cambrobranchus as a stem-pterobranch. The posterior distribution of trees is available in Appendix 4.
The most important result of these analyses is that Cambrobranchus provides direct fossil evidence of indirect development as present among middle Cambrian hemichordates and, when considered with the distribution of taxa with biphasic lifestyles in our analysis, supports metamorphosis as an ancestral character to both Hemichordata and Ambulacraria (Figure 6). Among the included taxa, the harrimaniid enteropneusts are the only direct developers, suggesting that this is a derived trait within this clade of acorn worms and not the plesiomorphic condition. It is also likely that other Cambrian hemichordates were indirect developers with planktonic larvae as well given their largely sessile habits (Caron et al., 2013; Nanglu et al., 2016, 2020a; Maletz, 2019). However, these juvenile forms have yet to be discovered, further highlighting the significance of the discovery of Cambrobranchus.
As we discuss in greater detail below, the origin of a pelago-benthic life history is debated, and similarities between echinoderm and hemichordate larvae have historically been used to recognize the relatedness of these two disparate phyla (Nezlin, 2000; Röttinger and Lowe, 2012). Moreover, the direct vs. indirect development of hemichordates has factored heavily into theories about the ecomorphology of the last common ancestor of the deuterostome (Romer, 1967). The discovery of Cambrobranchus, and its larvae, also re-affirms recent discoveries that enteropneusts, which until 2013 had a fossil record dating only to the Carboniferous (Caron et al., 2013), were a significant part of Cambrian ecosystems filling a wide array of marine niches. An ancestral capacity for larval dispersal may also explain the wide biogeographic distribution of Cambrian tube building hemichordate fossils from Laurentia, Canada (Kimmig and Pratt, 2015; Nanglu et al., 2016, 2020b; Nanglu and Caron, 2021) and Utah (Conway Morris and Robison 1988; Kimmig et al., 2019; Foster et al., 2022), South China, Yunnan (Hu et al., 2018), and Siberia (Nielsen, 1998).
Evidence for an Early Pelago-Benthic Life Cycle
The origin of larvae is widely debated and at the root of our understanding of animal evolution. The debate encompasses whether early animals existed initially as holobenthic organisms, adding pelagic larvae later (late Cambrian-Early Ordovician) due to predation pressure (Signor and Vermeij, 1994; Peterson et al., 2005) or the availability of planktonic food (Nützel et al., 2006; Kroeck et al., 2022; Laibl et al., 2023), or if they had a pelagic larva from the beginning (i.e., a pelago-benthic life history) (Peterson et al., 2005). More controversially, it has been suggested that early animals were holopelagic larvae-like organisms (Zhang and Pratt, 1993). Here, we provide the first direct fossil evidence for a Cambrian deuterostome larva. Cambrian acorn worm adults, juveniles, and larvae were found on the same rock slab, supporting the hypothesis that early animals had a pelago-benthic life cycle (Figure 3, Figure 5). The only other record of Cambrian soft-bodied larvae are the Orsten-type arthropod faunas from Sweden and China (Müller and Walossek, 1986; Andres, 1989; Waloszek and Dunlop, 2002; Steiner et al., 2004). These findings have significant macroevolutionary implications because the larva is often the dispersal phase of animal life cycles that permits the establishment of new populations in spatially separated habitats, which in turn maintains genetic diversity. Multiple distant populations mean that a species develops resistance to extinction following a disaster of a local population. A further macro-evolutionary consequence is the longevity of a species (Hansen, 1978, 1980). Populations that overlap genetically due to a widely dispersed larva are expected to have low speciation rates compared to equivalent populations that lack larvae (Jablonski, 1986). In this context it has also been shown that metamorphosis was likely ancestral for crown arthropods and not direct development, and a planktonic ecology may have evolved in the Ediacaran, or earlier (Wolfe, 2017). The same study also shows that, under a variety of phylogenetic hypotheses, metamorphosis is supported as the most likely ancestral state for ecdysozoans and euarthropods, suggesting it was a more common trait than the fossil record shows. Additionally, larval stages might not have preserved well in the fossil record, or not have been collected because of a variety of collections biases (Whitaker and Kimmig, 2020).
The discovery of C. pelagobenthos tornaria is the first fossil evidence of a hemichordate larva (Figure 3A-D) and suggests that the modern enteropneust larva (Figure 3J-O) evolved early in the history of the clade (Urata and Yamaguchi, 2004; Urata et al., 2014; Gonzalez et al., 2017). When fossils, including these new larval forms, and the predominant developmental modes among echinoderms are considered, we can reject the hypothesis that direct development, like that seen in the familiar Saccoglossus, is plesiomorphic to this Hemichordata (Sly et al., 2002, 2003; Raff and Byrne, 2006; Raff, 2008; Gonzalez et al., 2017). This finding is further supported by ancestral state estimation, which shows that a bi-phasic lifestyle is ancestral for Hemichordata and for Ambulacraria more broadly (Figure 6). Surprisingly, metamorphosis and juvenile morphology reflect those of extant acorn worms (Figure 3J-O). Metamorphic and early juvenile specimens are predominantly proboscis, with a distinct collar, and trunk with a post-anal organ. This mode of development appears to have been highly conserved since at least 525 million years ago (Jablonski and Lutz, 1983; Strathmann, 1985, 1990). The best evidence that direct developing acorn worms, like Saccoglossus, have evolved from an indirect developing ancestor is that early developmental stages have a ciliated telotroch band: This vestigial structure is a larval feature.
CONCLUSIONS
Here, we have described a new acorn worm, Cambrobranchus pelagobenthos gen. et sp. nov., from the early Cambrian Haiyan Lagerstätte, Chengjiang biota, of China. In addition to adult specimens, we also report juvenile specimens and evidence for the first tornaria larvae in the fossil record. The most important result of this study is that Cambrobranchus provides direct fossil evidence of indirect development as present among middle Cambrian hemichordates and, when considered with the distribution of taxa with biphasic lifestyles in our analysis, supports metamorphosis as an ancestral character to both Hemichordata and Ambulacraria.
ACKNOWLEDGEMENTS
This paper is a contribution to IGCP668, Equatorial Gondwanan History and Early Palaeozoic Evolutionary Dynamics. X.Y. was supported by the National Natural Science Foundation of China (grant nos. 42162001, 41562001, 41062001) and the State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (grant no. 103113). S.P. was supported by the State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (grant no. 20191101). We would like to thank the reviewers and the Handling Editor (Z. Barkaszi) for their time and comments.
REFERENCES
Alessandrello, A., Bracchi, G., and Riou, B. 2004. Polychaete, sipunculan and enteropneust worms from the Lower Callovian (Middle Jurassic) of La Voulte-sur-Rhône (Ardèche, France). Memoire della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano (Fascicolo I), 32:1–16.
Andres, D. 1989. Phosphatisierte Fossilien aus dem unteren Ordoviz von Südschweden. Berliner geowissenschaftliche Abhandlungen (A), 106:9–19.
Arduini, P., Pinna, G., and Terruzzi, G. 1981. Megaderaion sinemuriense n.g. n.sp., a new fossil enteropneust of the Sinemurian of Osteno in Lombardy. Atti della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, 122:104–108.
https://www.biodiversitylibrary.org/part/325194
Balser, E.J. and Ruppert, E.E. 1990. Structure, ultrastructure, and function of the preoral heart-kidney in Saccoglossus kowalevskii (Hemichordata, Enteropneusta) including new data on the stomochord. Acta Zoologica, 71:235–249.
https://doi.org/10.1111/j.1463-6395.1990.tb01082.x
Bardack, D. 1997. Wormlike animals: Enteropeusta, p. 89–92. In Shabica, C.W. and Hay, A.A. (eds.), Richardson’s Guide to the fossil fauna of Mazon Creek. Northeastern Illinois University, Chicago.
Bateson, W. 1885. The later stages in the development of Balanoglossus kowalevskii. Quarterly Journal of Microscopical Science, 25:81–122.
https://doi.org/10.1242/jcs.s2-25.s1.81
Bechly, G. and Frickhinger, K.A. 1999. Acorn worms, p. 76–79. In Frickhinger, K.A. (ed.), The fossils of Solnhofen 2: New specimens, new details, new results. Goldschneck-Verlag, Korb.
Benito, J. and Pardos, F. 1997. Hemichordata, p. 15–101. In Harrison, F.W. and Ruppert, E.E. (eds.), Microscopic anatomy of invertebrates. Vol. 15. Wiley-Liss, New York.
Browne, K.A. and Zimmer, R.K. 2001. Controlled field release of a waterborne chemical signal stimulates planktonic larvae to settle. The Biological Bulletin (Woods Hole), 200:87–91.
https://doi.org/10.2307/1543088
Butler, A.D., Cunningham, J.A., Budd, G.E., and Donoghue, P.C.J. 2015. Experimental taphonomy of Artemia reveals the role of endogenous microbes in mediating decay and fossilization. Proceedings of the Royal Society B, 282:20150476.
https://doi.org/10.1098/rspb.2015.0476
Cameron, C.B. 2002. The anatomy, life habits, and later development of a new species of enteropneust, Harrimania planktophilus (Hemichordata: Harrimaniidae) from Barkley Sound. The Biological Bulletin, 202:182–191.
https://doi.org/10.2307/1543654
Cameron, C.B. 2005. A phylogeny of the hemichordates based on morphological characters. Canadian Journal of Zoology, 83:196–215.
https://doi.org/10.1139/z04-190
Cameron, C.B. 2016. Saccoglossus testa from the Mazon Creek fauna (Pennsylvanian of Illinois) and the evolution of acorn worms (Enteropneusta: Hemichordata). Palaeontology, 59:329–336.
https://doi.org/10.1111/pala.12235
Cameron, C.B. 2018. Class Enteropneusta: Introduction, Morphology, Life Habits, Systematic Descriptions, and Future Research. Treatise Online, 109:1–22.
https://doi.org/10.17161/to.v0i0.7889
Cameron, C.B., Deland, C., and Bullock, T.H. 2010. A revision of the genus Saccoglossus (Hemichordata: Enteropneusta: Harrimaniidae) with taxonomic descriptions of five new species from the Eastern Pacific. Zootaxa, 2483:1–22.
https://doi.org/10.11646/zootaxa.2483.1.1
Cannon, J.T., Kocot, K.M., Waits, D.S., Weese, D.A., Swalla, B.J., Santos, S.R., and Halanych, K.M. 2014. Phylogenomic resolution of the hemichordate and echinoderm clade. Current Biology, 24:2827–2832.
https://doi.org/10.1016/j.cub.2014.10.016
Caron, J.-B., Conway Morris, S., and Cameron, C.B. 2013. Tubicolous enteropneusts from the Cambrian period. Nature, 495:503–506.
https://doi.org/10.1038/nature12017
Chatterton, B.D.E. 1980. Ontogenetic studies of Middle Ordovician Trilobites from the Esbataottine Formation, Mackenzie Mountains, Canada. Palaeontographica Abteilung A, 171:1–74.
Chen, J.-Y. 2004. The Dawn of Animal World. Jiangsu Science and Technology Publishing House, Nanjing. [In Chinese]
Conway Morris, S. and Robison, R.A. 1988. More soft-bodied animals and algae from the middle Cambrian of Utah and British Columbia. University of Kansas Paleontological Contributions, 122:1–48.
https://kuscholarworks.ku.edu/handle/1808/3691
Cowen, R.K. and Sponaugle, S. 2009. Larval dispersal and marine population connectivity. Annual Review of Marine Science, 1:443–466.
https://doi.org/10.1146/annurev.marine.010908.163757
Dobretsov, S. and Rittschof, D. 2020. Love at first taste: Induction of larval settlement by marine microbes. International Journal of Molecular Science, 21:731.
https://doi.org/10.3390/ijms21030731
Dyachuk, V. and Odintsova, N. 2013. Larval myogenesis in Echinodermata: conserved features and morphological diversity between class-specific larval forms of Echinoidae, Asteroidea, and Holothuroidea. Evolution & Development, 15:5–17.
https://doi.org/10.1111/ede.12010
Flint, R.W. and Kalke, R.D. 1986. Biological enhancement of estuarine benthic community structure. Marine Ecology-Progress Series, 31:23–33.
https://doi.org/10.3354/meps031023
Foster, J.R., Sroka, S.D., Howells, T.F., Cothren, H.R., Dehler, C.M., and Hagadorn, J.W. 2022. New Cambrian vermiform organisms from Burgess Shale-type deposits of the western United States. Bulletin of Geosciences 97:269–288.
https://doi.org/10.3140/bull.geosci.1858
Fowler, G. 1892. Note on the structure of Rhabdopleura. Proceedings of the Royal Society of London, 52:7–9.
https://doi.org/10.1098/rspl.1892.0061
Freckelton, M.L., Nedved, B.T., Cai, Y.-S., and Hadfield, M.G. 2022. Bacterial lipopolysaccharide induces settlement and metamorphosis in a marine larva. Proceedings of the National Academy of Sciences of the United States of America, 119:e2200798119.
https://doi.org/10.1101/851519
Gabbott, S.E., Hou, X.-G., Norry, M.J., and Siveter, D.J. 2004. Preservation of Early Cambrian animals of the Chengjiang biota. Geology, 32:901–904.
https://doi.org/10.1130/g20640.1
Gegenbaur, C. 1870. Grundzüge der vergleichenden Anatomie, Zweite, umgearbeitete Auflage. Wilhelm Engelmann, Leipzig.
https://doi.org/10.11588/diglit.15089#0004
Gonzalez, P., Uhlinger, K.R., and Lowe, C.J. 2017. The adult body plan of indirect developing hemichordates develops by adding a Hox-patterned trunk to an anterior larval territory. Current Biology, 27:87–95.
https://doi.org/10.1016/j.cub.2016.10.047
Gonzalez, P., Jiang, J.Z., and Lowe, C.J. 2018. The development and metamorphosis of the indirect developing acorn worm Schizocardium californicum (Enteropneusta: Spengelidae). Frontiers in Zoology, 15:26.
https://doi.org/10.1186/s12983-018-0270-0
Hansen, T.A. 1978. Larval dispersal and species longevity in lower tertiary gastropods. Science, 199:885–887.
https://doi.org/10.1126/science.199.4331.885
Hansen, T.A. 1980. Influence of larval dispersal and geographic distribution on species longevity in neogastropods. Paleobiology, 6:193–207.
https://doi.org/10.1017/S0094837300006758
Haug, J.T. 2020. Why the term “larva” is ambiguous, or what makes a larva? Acta Zoologica, 101:167–188.
https://doi.org/10.1111/azo.12283
Holland, N.D., Kuhnz, L.A., and Osborn, K.J. 2012. Morphology of a new deep-sea acorn worm (class Enteropneusta, phylum Hemichordata): A part-time demersal drifter with externalized ovaries. Journal of Morphology, 273:661–671.
https://doi.org/10.1002/jmor.20013
Hou, X.-G., Siveter, D.J., Siveter, D.J., Aldridge, R.J., Cong, P.-Y., Gabbott, S.E., Ma, X.-Y., Purnell, M.A., and Williams, M. 2017. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life (second edition). Wiley, New York.
https://doi.org/10.1002/9781118896372
Hu, S., Erdtmann, B.-D., Steiner, M., Zhang, Y., Zhao, F., Zhang, Z., and Han, J. 2018. Malongitubus: a possible pterobranch hemichordate from the early Cambrian of South China. Journal of Paleontology, 92:26–32.
https://doi.org/10.1017/jpa.2017.134
Huelsenbeck, J.P. and Bollback, J.P. 2001. Empirical and hierarchical Bayesian estimation of ancestral states. Systematic Biology, 50:351–366.
https://doi.org/10.1080/106351501300317978
Jablonski, D. 1986. Larval ecology and macroevolution in marine invertebrates. Bulletin of Marine Science, 39:565–587.
https://doi.org/10.1111/j.1469-185x.1983.tb00380.x
Jablonski, D. and Lutz, R.A. 1983. Larval ecology of marine benthic invertebrates: paleobiological implications. Biological Reviews, 58:21–89.
Jabr, N., Archambault, P., and Cameron, C.B. 2018. Biogeography and adaptations of torquaratorid acorn worms (Hemichordata: Enteropneusta) including two new species from the Canadian Arctic. Canadian Journal of Zoology, 96:1221–1229.
https://doi.org/10.1139/cjz-2017-0214
Jensen, P., Emrich, R., and Weber, K. 1992. Brominated metabolites and reduced numbers of meiofauna organisms in the burrow wall lining of the deep-sea enteropneust Stereobalanus canadensis. Deep Sea Research Part A. Oceanographic Research Papers, 39:1247–1253.
https://doi.org/10.1016/0198-0149(92)90067-4
Kapli, P., Natsidis, P., Leite, D.J., Fursman, M., Jeffrie, N., Rahman, I.A., Philippe, H., Copley, R.R., and Telford, M.J. 2021. Lack of support for Deuterostomia prompts reinterpretation of the first Bilateria. Science Advances, 7:eabe2741.
https://doi.org/10.1101/2020.07.01.182915
Kimmig, J. and Pratt, B.R. 2015. Soft-bodied biota from the middle Cambrian (Drumian) Rockslide Formation, Mackenzie Mountains, northwestern Canada. Journal of Paleontology, 89:51–71.
https://doi.org/10.1017/jpa. 2014.5
Kimmig, J. and Pratt, B.R. 2016. Taphonomy of the middle Cambrian (Drumian) Ravens Throat River Lagerstätte, Rockslide Formation, Mackenzie Mountains, Northwest Territories, Canada. Lethaia, 49:150–169.
https://doi.org/10.1111/let.12135
Kimmig, J., Strotz, L.C., Kimmig, S.R., Egenhoff, S.O., and Lieberman, B.S. 2019. The Spence Shale Lagerstätte: An important window into Cambrian biodiversity. Journal of the Geological Society, 176:609–619.
https://doi.org/10.1144/jgs2018-195
Kroeck, D.M., Mullins, G., Zacaï, A., Monnet, C., and Servais, T. 2022. A review of Paleozoic phytoplankton biodiversity: Driver for major evolutionary events? Earth-Science Reviews, 232:104113.
https://doi.org/10.1016/j.earscirev.2022.104113
Laibl, L., Saleh, F., and Pérez-Peris, F. 2023. Drifting with trilobites: the invasion of early post-embryonic trilobite stages to the pelagic realm. Palaeogeography, Palaeoclimatology, Palaeoecology, 613:111403.
https://doi.org/10.1016/j.palaeo.2023.111403
Leibach, W.W., Lerosey-Aubril, R., Whitaker, A.F., Schiffbauer, J.D., and Kimmig, J. 2021. First palaeoscolecid from the Cambrian (Drumian, Miaolingian) Marjum Formation of western Utah, USA. Acta Palaeontologica Polonica, 66:663–678.
https://doi.org/10.4202/app.00875.2021
Lerosey-Aubril, R. and Laibl, L. 2021. Protaspid larvae are unique to trilobites. Arthropod Structure and Development, 63:101059.
https://doi.org/10.1016/j.asd.2021.101059
Levin, L.A. 2006. Recent progress in understanding larval dispersal: new directions and digressions. Integrative and Comparative Biology, 46:282–297.
https://doi.org/10.1093/icb/icj024
Liu, Y., Haug, J.T., Haug, C., Briggs, D.E.G., and Hou, X. 2014. A 520 million year-old chelicerate larva. Nature Communications, 5:4440.
https://doi.org/10.1073/pnas.1522899113
Liu, Y., Melzer, R.R., Haug, J.T., Haug, C., Briggs, D.E.G., Hornig, M.K., He, Y.Y., and Hou, X.G. 2016. Three-dimensionally preserved minute larva of a great-appendage arthropod from the early Cambrian Chengjiang biota. Proceedings of the National Academy of Sciences of the United States of America, 113:5542–5546.
Maletz, J. 2019. Tracing the evolutionary origins of the Hemichordata (Enteropneusta and Pterobranchia). Palaeoworld, 28:58–72.
https://doi.org/10.1016/j.palwor.2018.07.002
Mileikovsky, S. 1973. Speed of active movement of pelagic larvae of marine bottom invertebrates and their ability to regulate their vertical position. Marine Biology, 23:11–17.
https://doi.org/10.1007/bf00394107
Müller, K.J. and Walossek, D. 1986. Arthropod larvae from the Upper Cambrian of Sweden. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 77:157–179.
https://doi.org/10.1017/s0263593300010804
Nanglu, K. and Caron, J.-B. 2021. Symbiosis in the Cambrian: enteropneust tubes from the Burgess Shale co-inhabited by commensal polychaetes. Proceedings of the Royal Society B, 288:20210061.
https://doi.org/10.1098/rspb.2021.0061
Nanglu, K., Caron, J.-B., and Cameron, C.B. 2015. Using experimental decay of modern forms to reconstruct the early evolution and morphology of fossil enteropneusts. Paleobiology, 41:460–478.
https://doi.org/10.1017/pab.2015.11
Nanglu, K., Caron, J.-B., Conway Morris, S., and Cameron, C.B. 2016. Cambrian suspension-feeding tubicolous hemichordates. BMC Biology, 14:56.
https://doi.org/10.1186/s12915-016-0271-4
Nanglu, K., Caron, J.-B., and Cameron, C.B. 2020a. Cambrian tentaculate worms and the origin of the hemichordate body plan. Current Biology, 30:4238–4244.
https://doi.org/10.1016/j.cub.2020.07.078
Nanglu, K., Caron, J.B., and Gaines, R.R. 2020b. The Burgess Shale paleocommunity with new insights from Marble Canyon, British Columbia. Paleobiology, 46:58–81.
https://doi.org/10.1017/pab.2019.42
Nezlin, L.P. 2000. Tornaria of hemichordates and other dipleurula-type larvae: a comparison. Journal of Zoological Systematics and Evolutionary Research, 38:149–156.
https://doi.org/10.1046/j.1439-0469.2000.383144.x
Nielsen, C. 1998. Origin and evolution of animal life cycles. Biological Reviews, 73:125–155.
https://doi.org/10.1111/j.1469-185x.1997.tb00027.x
Nielsen, C. and Hay‐Schmidt, A. 2007. Development of the enteropneust Ptychodera flava: ciliary bands and nervous system. Journal of Morphology, 268:551–570.
https://doi.org/10.1046/j.1525-142x.2001.01051.x
Nützel, A., Lehnert, O., and Frýda, J. 2006. Origin of planktotrophy–evidence from early molluscs. Evolution & Development, 8:325–330.
https://doi.org/10.1111/j.1525-142x.2006.00105.x
Peterson, K.J., McPeek, M.A., and Evans, D.A. 2005. Tempo and mode of early animal evolution: inferences from rocks, Hox, and molecular clocks. Paleobiology, 31:36–55.
https://doi.org/10.1666/0094-8373(2005)031[0036:tamoea]2.0.co;2
Raff, R.A. 2008. Origins of the other metazoan body plans: the evolution of larval forms. Philosophical Transactions of the Royal Society B, 363:1473–1479.
https://doi.org/10.1038/sj.hdy.6800866
Raff, R.A. and Byrne, M. 2006. The active evolutionary lives of echinoderm larvae. Heredity, 97:244–252.
https://doi.org/10.1038/sj.hdy.6800866
Rambaut, A., Drummond, A.J., Xie, D., Baele, G., and Suchard, M.A. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67:901–904.
https://doi.org/10.1093/sysbio/syy032
Ritter, W.E. 1900. Papers from the Harriman Alaska Expedition. II. Harrimania maculosa, a new genus and species of Enteropneusta from Alaska, with special regard to the character of its notochord. Proceedings of the Washington Academy of Sciences, 2:111–132.
https://www.jstor.org/stable/24525859
Romer, A. 1967. Major steps in vertebrate evolution. Science, 158:1629–1637.
https://doi.org/10.1126/science.158.3809.1629
Röttinger, E. and Lowe, C.J. 2012. Evolutionary crossroads in developmental biology: hemichordates. Development, 139:2463–2475.
https://doi.org/10.1242/dev.066712
Saleh, F., Antcliffe, J.B., Lefebvre, B., Pittet, B., Laibl, L., Peris, F.P., Lustri, L., Gueriau, P., and Daley, A.C. 2020. Taphonomic bias in exceptionally preserved biotas. Earth and Planetary Science Letters, 529:115873.
https://doi.org/10.1016/j.epsl.2019.115873
Saleh, F., Bath-Enright, O.G., Daley, A.C., Lefebvre, B., Pittet, B., Vite, A., Ma, X., Mángano, M.G., Buatois, L.A., and Antcliffe, J.B. 2021. A novel tool to untangle the ecology and fossil preservation knot in exceptionally preserved biotas. Earth and Planetary Science Letters, 569:117061.
https://doi.org/10.1016/j.epsl.2021.117061
Saleh, F. Qi, C., Buatois, L.A., Mángano, M.G., Paz, M., Vaucher, R., Zheng, Q., Hou, X.-G., Gabbott, S.E., and Ma, X.-Y. 2022. The Chengjiang Biota inhabited a deltaic environment. Nature Communications, 13:1569.
https://doi.org/10.1038/s41467-022-29246-z
Sansom, R.S. 2016. Preservation and phylogeny of Cambrian ecdysozoans tested by experimental decay of Priapulus. Scientific Reports, 6:32817.
https://doi.org/10.1038/srep32817
Shanks, A.L. 2009. Pelagic larval duration and dispersal distance revisited. The Biological Bulletin, 216:373–385.
https://doi.org/10.1086/bblv216n3p373
Shanks, A.L., Grantham, B.A., and Carr, M.H. 2003. Propagule dispersal distance and the size and spacing of marine reserves. Ecological Applications, 13:159–169.
https://doi.org/10.1890/1051-0761(2003)013[0159:pddats]2.0.co;2
Signor, P.W. and Vermeij, G.J. 1994. The plankton and the benthos: origins and early history of an evolving relationship. Paleobiology, 20:297–319.
https://doi.org/10.1017/s0094837300012793
Sly, B.J., Hazel, J.C., Popodi, E.M., and Raff, R.A. 2002. Patterns of gene expression in the developing adult sea urchin central nervous system reveal multiple domains and deep-seated neural pentamery. Evolution & Development, 4:189–204.
https://doi.org/10.1046/j.1525-142x.2002.02002.x
Sly, B.J., Snoke, M.S., and Raff, R.A. 2003. Who came first-larvae or adults? Origins of bilaterian metazoan larvae. The International Journal of Developmental Biology, 47:623–632.
https://ijdb.ehu.eus/article/14756338
Steiner, M., Zhu, M., Li, G., Qian, Y., and Erdtmann, B.D. 2004. New Early Cambrian bilaterian embryos and larvae from China. Geology, 32:833–836.
https://doi.org/10.1130/g20567.1
Strathmann, R.R. 1985. Feeding and nonfeeding larval development and life-history evolution in marine invertebrates. Annual Review of Ecology, Evolution, and Systematics, 16:339–361.
https://doi.org/10.1146/annurev.es.16.110185.002011
Strathmann, R.R. 1990. Why life histories evolve differently in the sea. American Zoologist, 30:197–207.
https://doi.org/10.1093/icb/30.1.197
Thorson, G. 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews, 25:1–45.
https://doi.org/10.1111/j.1469-185x.1950.tb00585.x
Urata, M. and Yamaguchi, M. 2004. The Development of the Enteropneust Hemichordate Balanoglossus misakiensis Kuwano. Zoological Science, 21:533–540.
https://doi.org/10.2108/zsj.21.533
Urata, M., Iwasaki, S., Ohtsuka, S., and Yamaguchi, M. 2014. Development of the swimming acorn worm Glandiceps hacksi: similarity to holothuroids. Evolution & Development, 16:149–154.
https://doi.org/10.1111/ede.12075
Vannier, J. 2012. Gut contents as direct indicators for trophic relationships in the Cambrian marine ecosystem. PLoS ONE, 7:52200.
https://doi.org/10.1371/journal.pone.0052200
Walcott, C.D. 1911. Cambrian Geology and Paleontology II: No.5-Middle Cambrian annelids. Smithsonian Miscellaneous Collections, 57:109–145.
https://repository.si.edu/handle/10088/34820
Waloszek, D. and Dunlop, J.A. 2002. A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian ‘Orsten’ of Sweden, and the phylogenetic position of pycnogonids. Palaeontology, 45:421–446.
https://doi.org/10.1111/1475-4983.00244
Whitaker, A.F. and Kimmig, J. 2020. Anthropologically introduced biases in natural history collections, with a case study on the invertebrate paleontology collections from the middle Cambrian Spence Shale Lagerstätte. Palaeontologia Electronica, 23:a58.
https://doi.org/10.26879/1106
Wolfe, J.M. 2017. Metamorphosis is ancestral for crown euarthropods and evolved in the Cambrian or earlier. Integrative and Comparative Biology, 57:499–509.
https://doi.org/10.1093/icb/icx039
Woodwick, K.H. and Sensenbaugh, T. 1985. Saxipendium coronatum, new genus, new species (Hemichordata: Enteropneusta): the unusual spaghetti worms of the Galápagos Rift hydrothermal vents. Proceedings of the Biological Society of Washington, 98:351–365.
https://www.biodiversitylibrary.org/part/46566
Yang, X., Kimmig, J., Zhai, D., Liu, Y., Kimmig, S.R., and Peng, S. 2021. A juvenile-rich palaeocommunity of the lower Cambrian Chengjiang biota sheds light on palaeo-boom or palaeo-bust environments. Nature Ecology & Evolution, 5:1082–1090.
https://doi.org/10.1038/s41559-021- 01490-4
Zhang, X.-G. and Pratt, B.R. 1993. Early Cambrian ostracode larvae with a univalved carapace. Science, 262:93–94.
https://doi.org/10.1126/science.262.5130.93
Zhao, F.-C., Caron, J.-B., Hu, S.-X., and Zhu, M.-Y. 2009. Quantitative analysis of taphofacies and paleocommunities in the Early Cambrian Chengjiang Lagerstätten. Palaios, 24:826–839.
https://doi.org/10.2110/palo.2009.p09-004r
Zimmer-Faust, R.K. and Tamburri, M.N. 1994. Chemical identity and ecological implications of a waterborne, larval settlement cue. Limnology and Oceanography, 39:1075–1087.
https://doi.org/10.4319/lo.1994.39.5.1075

