 Designing scientifically-grounded paleoart for augmented reality at La Brea Tar Pits
Designing scientifically-grounded paleoart for augmented reality at La Brea Tar Pits
Article number: 25.1.a9
https://doi.org/10.26879/1191
Copyright Society for Vertebrate Paleontology, March 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 1 October 2021. Acceptance: 10 January 2022
ABSTRACT
Paleoart is an important medium that communicates scientific understanding about prehistoric life to both the public and researchers. However, despite its broad influence, the scientific and aesthetic decisions that go into paleoart are rarely described in formal academic literature or subjected to peer review. This is unfortunate, as paleoart can easily create and perpetuate misconceptions that are carried through generations of iterative popular media. As an example of what we hope will become a standard article type in paleontological journals, we describe the process and latest scientific research used to develop 13 new paleoart reconstructions of Ice Age animals found in the La Brea Tar Pits, including the saber-toothed cat, dire wolf, and teratorn. We adopted a stylized low polygon aesthetic for these three-dimensional (3D), animated virtual models both to support learning objectives and to optimize performance for smartphone based augmented reality (AR) experiences. We encourage all researchers to follow the example of this article by publishing paleoart descriptions for any major new work that, at a minimum, reference the aesthetic and scientific reasoning behind general posture and proportions, gross appearance of soft tissues, coloration, and behavior.
Matt Davis. Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007, USA. mdavis@nhm.org
Benjamin D. Nye. University of Southern California, Institute for Creative Technologies, 12015 Waterfront Drive, Playa Vista, CA 90094, USA. nye@ict.usc.edu
Gale M. Sinatra. Rossier School of Education, University of Southern California, 3470 Trousdale Parkway, Los Angeles, CA 90089, gsinatra@usc.edu
William Swartout. University of Southern California, Institute for Creative Technologies, 12015 Waterfront Drive, Playa Vista, CA 90094, USA. swartout@ict.usc.edu
Molly Sjӧberg. Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007, USA. msjorber@nhm.org
Molly Porter. Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007, USA. mollyporter@nhm.org
David Nelson. University of Southern California, Institute for Creative Technologies, 12015 Waterfront Drive, Playa Vista, CA 90094, USA. dnelson@ict.usc.edu
Alana A.U. Kennedy. University of Southern California Rossier School of Education, 3470 Trousdale Parkway, Los Angeles, CA 90089-4036, USA. alana.kennedy@usc.edu
Imogen Herrick. University of Southern California Rossier School of Education, 3470 Trousdale Parkway, Los Angeles, CA 90089-4036, USA. iherrick@usc.edu
Danaan DeNeve Weeks. La Brea Tar Pits, 5801 Wilshire Blvd., Los Angeles, CA 90036, USA. ddeneveweeks@tarpits.org
Emily Lindsey. La Brea Tar Pits, 5801 Wilshire Blvd., Los Angeles, CA 90036, USA. elindsey@tarpits.org
Institute of the Environment and Sustainability, University of California Los Angeles, 619 Charles E. Young Drive East, Los Angeles, CA 90095, USA and Department of Earth Sciences, University of Southern California, 3651 Trousdale Pkwy, Los Angeles, CA 90089, USA
Keywords: paleoart; augmented reality; Pleistocene; La Brea Tar Pits; museums; 3D
Final citation: Davis, Matt, Nye, Benjamin D., Sinatra, Gale M., Swartout, William, Sjӧberg, Molly, Porter, Molly, Nelson, David, Kennedy, Alana A.U., Herrick, Imogen, DeNeve Weeks, Danaan, and Lindsey, Emily. 2022. Designing scientifically-grounded paleoart for augmented reality at La Brea Tar Pits. Palaeontologia Electronica, 25(1):a9. https://doi.org/10.26879/1191
palaeo-electronica.org/content/2022/3524-la-brea-tar-pits-paleoart
Copyright: March 2022 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Rancho La Brea (commonly called La Brea Tar Pits) is a famous lagerstatte in California, USA preserving plant and animal remains from the last 60 ka of the Los Angeles Basin. The bubbling asphalt seeps, still active today, are renowned for their preservation of entire Quaternary ecosystems in great detail from pollen and insects to whole trees and mammoths (Stock and Harris, 1992). Still under excavation, the Tar Pits are the only active paleontological dig site located within a major metropolis and provide a unique opportunity to show the public the entire process of paleontology from excavation and preparation to research and display. A popular tourist destination that is featured in many movies, tv shows, and comics, La Brea Tar Pits looms large in the public’s perceptions (and misperceptions) of prehistoric life.
 Paleoart, any original artistic manifestation attempting to reconstruct prehistoric life according to current scientific evidence (paraphrasing Ansón et al., 2015), has always been integral to how scientists and educators communicate the paleontological discoveries of the Tar Pits. Decades before the first museum was built on the site, sculptures of saber-toothed cats, American lions, short-faced bears, and giant ground sloths dotted Hancock Park, where the tar pits are located, to show visitors what the area might have looked like during the Ice Age (Scott, 1985). A tableau sculpted by Howard Ball in 1968 of a female Columbian mammoth sinking into the asphalt while her worried mate and offspring look on (Figure 1) is one of the most iconic pieces of public artwork in Los Angeles (Scott, 1985; Nakano, 2021).
Paleoart, any original artistic manifestation attempting to reconstruct prehistoric life according to current scientific evidence (paraphrasing Ansón et al., 2015), has always been integral to how scientists and educators communicate the paleontological discoveries of the Tar Pits. Decades before the first museum was built on the site, sculptures of saber-toothed cats, American lions, short-faced bears, and giant ground sloths dotted Hancock Park, where the tar pits are located, to show visitors what the area might have looked like during the Ice Age (Scott, 1985). A tableau sculpted by Howard Ball in 1968 of a female Columbian mammoth sinking into the asphalt while her worried mate and offspring look on (Figure 1) is one of the most iconic pieces of public artwork in Los Angeles (Scott, 1985; Nakano, 2021).
Paleoart, like the sculptures and paintings found at the Tar Pits, is not only valuable for public education and paleontology themed merchandising. Developing more realistic artistic reconstructions has required scientists to push beyond typical questions and data, leading to important research into the biology and behavior of many extinct organisms from Ediacaran vendobionts to early hominids (Grazhdankin and Seilacher, 2002; Antón et al., 2009; Gurche, 2013). Increasingly, researchers can use sophisticated methods to infer the external appearance of extinct organisms, something that was previously relegated to guesswork. Limb proportion analysis can provide statistically likely countershading patterns (Kamilar and Bradley, 2011), electron microscopes can reveal pigmentation and structural coloration in feathered dinosaurs (Vinther, 2020), and extinct fluorescent phenotypes can be synthesized in the lab from ancestral state reconstructions of genotypes (Randall et al., 2016). Such techniques have only increased the responsibility for scientists and artists to produce reconstructions that accurately reflect our current knowledge and portray biologically realistic extinct organisms. Paleoart (or perhaps bad paleoart) is very effective at promoting and perpetuating misconceptions (Ross et al., 2018). This influence is not limited to the layperson. The inaccurate visual trope of wooly mammoths frozen in blocks of crystalline ice rather than permafrost influenced scientific discourse on catastrophism from the writings of Charles Lyell to top scientific journals well into the 1960s (Glass et al., 2016).
 The paleoart created for La Brea Tar Pits spans a wide range of scientific accuracy and artistic value: Harlan’s ground sloth is reconstructed with a worryingly inconsistent number of toes, one mural features flamingoes gracefully wading into asphalt pools despite the fact that they are not known from Ice Age or present-day California, a newer mural botches perspective to present western camels that are only half their true size. Even paleoart classics like Charles Knight’s archetypal 1925 La Brea mural (Figure 2A), considered highly accurate at the time of its creation, can perpetuate misconceptions. Mark Hallett’s 1988 mural teeming with small life (Figure 2B) was commissioned in part to counteract the belief, common among visitors who had seen Knight’s mural or its many derivatives, that the Tar Pits only captured megafauna engaged in life or death struggles with dire wolves and saber-toothed cats. The sculpture of a Columbian mammoth sinking into the bubbling Lake Pit outside the Tar Pits Museum (Figure 1), while iconic, reinforces the misconception that animals sunk into deep asphalt pools like quicksand (Kennedy et al., 2021); most asphalt seeps were probably only a few centimeters deep and trapped animals more like sticky fly paper (Akersten et al., 1983). The Lake Pit itself isn’t even a natural seep, but the remains of a nineteenth century asphalt mining operation.
The paleoart created for La Brea Tar Pits spans a wide range of scientific accuracy and artistic value: Harlan’s ground sloth is reconstructed with a worryingly inconsistent number of toes, one mural features flamingoes gracefully wading into asphalt pools despite the fact that they are not known from Ice Age or present-day California, a newer mural botches perspective to present western camels that are only half their true size. Even paleoart classics like Charles Knight’s archetypal 1925 La Brea mural (Figure 2A), considered highly accurate at the time of its creation, can perpetuate misconceptions. Mark Hallett’s 1988 mural teeming with small life (Figure 2B) was commissioned in part to counteract the belief, common among visitors who had seen Knight’s mural or its many derivatives, that the Tar Pits only captured megafauna engaged in life or death struggles with dire wolves and saber-toothed cats. The sculpture of a Columbian mammoth sinking into the bubbling Lake Pit outside the Tar Pits Museum (Figure 1), while iconic, reinforces the misconception that animals sunk into deep asphalt pools like quicksand (Kennedy et al., 2021); most asphalt seeps were probably only a few centimeters deep and trapped animals more like sticky fly paper (Akersten et al., 1983). The Lake Pit itself isn’t even a natural seep, but the remains of a nineteenth century asphalt mining operation.
It is the power of paleoart to communicate scientific concepts and its frequent inability to portray even basic anatomy like digit numbers correctly that makes paleoart advocates lament what they see as a pervasive laissez-faire approach from museums and scientists towards paleoart accuracy (Witton et al., 2014). The scientific and artistic decisions involved in any piece of paleoart are rarely public, and if they are published, it is usually in popular press monographs (Gurche, 2013; Campbell, R.M. et al., 2021) or online blogs (Witton, 2019), rather than peer reviewed scientific literature (Antón et al., 1998). Prominent paleoartists have recently called for professionalizing the field by subjecting work to more rigorous scientific and artistic debate (Witton, 2017a). We agree that given paleoart’s contributions to understanding past life and its vast reach and lengthy staying power with the public, it should be treated with the same rigor as any research paper (Campbell, R.M. et al., 2021). Here, we present a large new collection of Ice Age paleoart so that our aesthetic and scientific influences can be properly cited and the decisions we made can be recorded, debated, and referenced for future work. Given that low poly virtual models are likely an uncommon paleoart medium to many readers, we also summarize our production process and how these models can be used in a variety of education and outreach applications.
METHODS
Project Origin
Although our low poly models have now been used for a variety of purposes, the original impetus for their creation was the Tar AR project, a National Science Foundation Advancing Informal Science Learning (NSF AISL 1811014; 1810984) research grant to study how differences in visual immersion and interactivity affect learning and engagement in augmented reality (AR) experiences (Herrick et al., 2021; Kennedy et al., 2021). Tar AR was primarily designed to help museum visitors learn core concepts (e.g., ecosystem relationships, timelines) and correct misconceptions (e.g., animals sinking into the tar rather than getting stuck to it) (Kennedy et al., 2021). This work also concerned testing museum AR experiences on inexpensive, commonly-available AR hardware such as smartphones, as opposed to specialized or high performance head mounted displays. Tar AR is still ongoing and various aspects of the theory, experimental design, and initial results have already been discussed elsewhere (Davis, 2019; Davis, 2021; Herrick et al., 2021; Kennedy et al., 2021). Below, we describe some features of the experimental design as they directly influenced the style and mode of paleoart used for our models.
 Two experiences were developed for Tar AR. The first is a tabletop AR experience focusing on paleohabitat reconstruction using fossils. The second is more akin to an “Ice Age safari” where users view virtual, life-size animals and make observations about an entrapment event. To test the effects of immersion and interactivity, research participants were randomly assigned to one of five different yet content equivalent conditions: control, high interactivity/high immersion, high interactivity/low immersion, low interactivity/high immersion, and low interactivity/low immersion. For the control conditions, we used the content and imagery of the AR experiences to make standard printed labels typical for museums. High and low interactivity AR conditions varied on how much the user was required to manipulate features in the virtual environment. Low immersion AR conditions used handheld smartphones (Figure 3A) whereas in high immersion AR conditions, the same smartphones were placed in simple, inexpensive headsets to provide passthrough AR optics (Figure 3B). To create binocular vision for headsets, visuals are split into left and right images on the smartphone screen, greatly reducing the number of pixels (i.e., resolution) available (Figure 3C) while simultaneously taxing the device’s processor twice as hard as it now needs to render two separate images at once (Trivedi, 2019).
Two experiences were developed for Tar AR. The first is a tabletop AR experience focusing on paleohabitat reconstruction using fossils. The second is more akin to an “Ice Age safari” where users view virtual, life-size animals and make observations about an entrapment event. To test the effects of immersion and interactivity, research participants were randomly assigned to one of five different yet content equivalent conditions: control, high interactivity/high immersion, high interactivity/low immersion, low interactivity/high immersion, and low interactivity/low immersion. For the control conditions, we used the content and imagery of the AR experiences to make standard printed labels typical for museums. High and low interactivity AR conditions varied on how much the user was required to manipulate features in the virtual environment. Low immersion AR conditions used handheld smartphones (Figure 3A) whereas in high immersion AR conditions, the same smartphones were placed in simple, inexpensive headsets to provide passthrough AR optics (Figure 3B). To create binocular vision for headsets, visuals are split into left and right images on the smartphone screen, greatly reducing the number of pixels (i.e., resolution) available (Figure 3C) while simultaneously taxing the device’s processor twice as hard as it now needs to render two separate images at once (Trivedi, 2019).
As a result of our experimental setup, the virtual models needed to be optimized for a number of criteria:
1) Ecosystem representation: Models needed to be high performance, in that a large number could be presented as interacting simultaneously (e.g., herds of bison, packs of wolves) through real-time rendering on smartphones.
2) Salient features: Models needed to be clearly visible against real-life backgrounds and easily discernible from each other (e.g., dire wolves could be distinguished from coyotes and mammoths could be distinguished from mastodons even for someone unfamiliar with these taxa).
3) Counter misconceptions: Models needed to correct common misconceptions held by the public and avoid introducing additional misconceptions (i.e., ambiguity should be preferred over realism in the case of uncertain reconstructions).
For example, essential learning objectives were not about what color dire wolves were but rather why predators like dire wolves are so overrepresented in fossils found in the tar pits. These objectives required optimizing the animals for presenting realistic behavior and interactions (i.e., perceptual realism), often at a distance or on a very small section of a smartphone screen (Trivedi, 2019).
Technical and Aesthetic Considerations
Low polygon or “low poly” is a highly stylized visual aesthetic where three dimensional forms are recreated using a simplified mesh of polygons, usually triangles (Trivedi, 2019). Low poly is a relative term with no absolute cutoff but our reconstructions, which average only 1,455 triangles (tris) per animal, clearly fit the designation, especially when compared with prerendered Hollywood movies with animals made of millions of triangles. Originally a necessity to simplify virtual shapes so they could be rendered in real time while playing video games, the style is now often used both in 2D and 3D art as a deliberate aesthetic choice (Trivedi, 2019). Although console based video game systems have become much more powerful and no longer require low poly graphics, rendering high fidelity and complex models on mobile hardware is still computationally expensive and leads to longer rendering times (Trivedi, 2019). AR and virtual reality (VR) applications require high frame rates to provide smooth motion of objects and an increased sense of presence (Trivedi, 2019). Using low polygon models decreases rendering times and lowers model file sizes, making low poly a particularly useful style for both AR and VR (collectively known as XR) applications (Trivedi, 2019).
Besides the lower computational load, the low poly aesthetic has some other benefits. As it is commonly used for popular AR applications like Pokemon Go, many people are already used to interacting with characters of this aesthetic in an augmented space on their smartphones. Even if more complex models could be rendered quickly enough for our applications, chasing realism is a difficult race to win. The cutting-edge computer graphics of Ice Age nature shows 10 years ago now look outdated. By deliberately using a highly stylized aesthetic that acknowledges its own artificiality, we create something outside of the normal realism spectrum. Our models should look as good in 10 years as they do now. More complex models also require more complex paleoart that may have to overcommit to detailed physical features for which we do not yet have sufficient fossil evidence. A low poly style can be thought of as a conservative form of 3D paleoart where organisms are just detailed enough to be recognizable without additional details that would require more speculation or inference. In other words, the models are more accurate than high resolution 3D models but purposely more imprecise where precision is not possible.
It is surprising that even a small number of polygons can approximate the general shape of most large mammals reasonably well, but the stylized nature of the low poly aesthetic limits certain aspects of realism. Coloration is usually applied in blocks where each individual polygon is a specific color. This works reasonably well for the tan and white countershading commonly found in mammals but fails to capture more complex patterns or gradients and coloration that do not map along body contours. We’ve “cheated” by introducing some slight gradients and subtle fur or spot patterns to suggest that animals either had longer hair or patterned pelage. Complex motions like running are also very difficult to replicate accurately in low poly models because the limited polygonal surfaces don’t bend and flex in the same way muscles and flesh would. Many low poly games apply simple smoothers that blend together the blocky, polygonal shapes of models into more natural looking features; for example, the hard angels of a hexagon would be smoothed out to form a circle. Although this might make our models look more natural or realistic, we weren’t pleased with initial trials and decided to eschew smoothers to accentuate our stylized, angular low poly aesthetic.
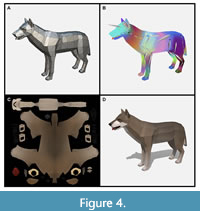 To help us build our models, we contracted a development firm specializing in low poly assets for video games called Polyperfect. This made more sense than retraining in-house artists in specialized aesthetics and software. In addition to being paid an appropriate sum, Polyperfect was allowed to sell the models we made through their own store. Encouraging video game developers (and even other museums) to use our scientifically accurate models made more educational sense than locking up the intellectual property. Models were developed and paid for in batches during a rapid production schedule. We delivered Polyperfect a packet of information including detailed descriptions of how we wanted the organisms to look, scaled pictures of mounted skeletons, details of complex features like feet, reference images from other paleoart, and videos of behavior found in related species. An artist first produced a generically colored mesh of the organism, which went through several rounds of scientific review before the shape of the organism was approved (Figure 4A). Once the mesh was locked, the development could proceed on two parallel tracks: animation and skinning.
To help us build our models, we contracted a development firm specializing in low poly assets for video games called Polyperfect. This made more sense than retraining in-house artists in specialized aesthetics and software. In addition to being paid an appropriate sum, Polyperfect was allowed to sell the models we made through their own store. Encouraging video game developers (and even other museums) to use our scientifically accurate models made more educational sense than locking up the intellectual property. Models were developed and paid for in batches during a rapid production schedule. We delivered Polyperfect a packet of information including detailed descriptions of how we wanted the organisms to look, scaled pictures of mounted skeletons, details of complex features like feet, reference images from other paleoart, and videos of behavior found in related species. An artist first produced a generically colored mesh of the organism, which went through several rounds of scientific review before the shape of the organism was approved (Figure 4A). Once the mesh was locked, the development could proceed on two parallel tracks: animation and skinning.
On one track, an animator rigged the model by placing generic bones inside to approximate the animal’s skeleton and joints. Next, the animator painted weights onto the model, a difficult process that determines how the shape of the model (i.e., its muscles and skin) will stretch and bend around its internal skeleton (Figure 4B). Rigging models is a time-consuming process, and any changes to the model’s mesh (i.e., its shape) usually necessitate redoing the rig and repainting weights. We worked hard with the team at Polyperfect to review and approve models following a strict workflow to avoid unnecessary replication of steps and wasted effort. After rigging, the animator followed frame-by-frame reference videos of extant animals (where available) to accurately reconstruct behavior.
Each model had three basic behaviors or animations that were designed with our AR applications in mind: movement, idle, and selection. Movement was typically the animal walking at a slow pace although birds also had flying animations and our fish had a swimming animation. Programmatically, idle is the animation the animal displays during its neutral state. We generally showed herbivores grazing and carnivores sniffing the air, looking around, or grooming. Although many of the herbivores we animated were likely mixed feeders or browsers, we typically showed them chewing low to the ground like grazers. This was done purely for logistical simplicity. Unless we add a shrub or tree nearby, an animal feeding at head height looks like it is just chewing the air during its idle animation. The ground, however, will always be at ground level and provides the animal with a more realistic feeding motion regardless of which plants we place around it in the virtual environment. Selection is the animation animals display when they are clicked on by the user. This was usually a roar or some other vocalization associated with clear movement.
Although the behaviors we show in our animations are realistic, the animals probably move too much, especially during their idle animations. Real animals exhibit an economy of movement; a large predator might sit relatively motionless for long periods of the day. However, if a user opens an AR app on their phone and all the animals are static, they are likely to interpret the lack of motion as the app crashing rather than the animals displaying realistic behavior. For this reason, we decided to prioritize perceptual realism over biological realism and made sure the animals had some clearly visible motion, even when they were sitting relatively still. Like the meshes, all animations went through several rounds of review before approval. Due to logistical constraints, we decided to wait until the models were completely finalized before syncing sounds to the behaviors. Sound production is still in process and is being carried out by skilled in-house audio technicians at the Tar Pits Museum. Except where called out below in individual species descriptions, sound effects are speculative and generally were taken from recordings of extinct species’ closest living relatives.
While the animations were underway, an artist worked on skinning a model by digitally painting on the fur and skin tones. This “texture” is drawn as a 2D image (Figure 4C) that is wrapped around the mesh using a process called UV mapping (Figure 4D). The UV mapping pattern is analogous to a map projection and determines how the 3D surface can be represented as a flat 2D image. All our UV maps were done by hand to ensure that seams didn’t show by placing them in convenient locations like the border between sharply countershaded sides and underbellies. These textures represent the base colors of the animals: the grey of mammoth skin, the black of raccoon tail stripes, etc. Real time computer graphics also often use specular maps that designate how shiny or metallic surfaces should be. We created these for all the animals but rarely used these in applications as the texture maps created sufficient realism without additional computational load. However, specular maps would be more important to approximate the appearance of insects, birds, and fish where reflective structural coloration is common.
Once the textures and animations were scientifically approved, an art director reviewed them for aesthetic qualities and consistency with the look and feel of the applications we were developing. Models were also reviewed by our programmers to ensure that files were generated correctly and they displayed no bugs in our development environment. Once final approval was given, Polyperfect sent us the models optimized to work in the Unity game engine, a popular video game production software. Models are formatted as.fbx files, which stores the 3D meshes, UV mapping, and animations. Textures are formatted as.png files and stored separately.
Biological Considerations
For our initial AR experiences, we created low poly models of 22 species of plants and animals excavated from the Tar Pits. Several of these species, like rainbow trout (Oncorhynchus mykiss) and clustered tarweed (Hemizonia fasciculata) still live in the Los Angeles Basin today. While these extant, non-megafauna species serve important learning goals in our AR experiences, their reconstruction does not warrant lengthy description here as their appearance can be readily observed in the wild. However, some extant species like coyotes (Canis latrans) have measurably changed their morphology since the Pleistocene (Meachen et al., 2014). We chose to use modern measurements and limb proportions for these species rather than fossil measurements as they were planned for use in experiences highlighting Los Angeles’ current flora and fauna and thus modern proportions are desirable. However, we don’t think using these models in an Ice Age scene is problematic as the intraspecific morphological differences between time periods, only apparent to the specialist with calipers, would not be noticed by the average user, especially given the models’ blocky, low poly aesthetic.
The dimensions of models for the extinct species (when viewed “life size” in AR) are mostly derived from mounted adult skeletons displayed in the Tar Pits Museum rather than average sizes given in the literature. As these models were made for use at the Tar Pits, we think they should reflect the morphotypes found at the Tar Pits. Associated skeletal material is very rare at the Tar Pits due to pre-burial scavenging, disarticulation from struggles of entrapped animals, and churning of asphalt by subsurface gasses (Woodard and Marcus, 1973; Friscia et al., 2008) so the mounted fossil skeletons are composites of many individuals except in rare cases mentioned below (Scott, 1988). We don’t know the exact process that previous exhibitions staff used to select fossil bones for mounting (Shaw, 1988) but it is likely that they chose elements from very common size ranges rather than trying to build a skeleton made from the largest and most robust bones, which would be much rarer assuming no taphonomic size bias within species. We checked this assumption by comparing femur dimensions of mounted skeletons for seven species that could be accurately measured while on display to a random sample of isolated femurs within the collection. The femurs on most mounted skeletons were within one standard deviation of the average size for isolated femurs in the collection, suggesting that the mounted skeletons on display at the Tar Pits Museum and the models we based off of them likely represent modal sizes for fauna living in the area, or at least those that became entrapped. The femurs on two mounted skeletons were much larger than isolated femurs though. Although they are composites, the mounted Harlan's ground sloth (Paramylodon harlani) and the mounted American lion (Panthera atrox) likely represent large individuals. This shouldn’t have a huge effect on our models though. The American lion was one model where we didn’t rely on the composite, mounted skeleton as we were able to use an associated skeleton from the collection (LACMP23-555) instead. The difference between our large Harlan’s ground sloth and an average sloth, a 3.5 cm difference on femurs ~52 cm long, is visually trivial at the coarse scale of our low poly models.
Several paleoartists have critiqued the field’s culture of copying where visual tropes from coloration patterns, to poses, and even whole scenes are reproduced over and over, perpetuating inaccuracies and reducing creative compositions and artistic ideas (Witton et al., 2014). This has led to the “All Yesterdays Movement” (Conway et al., 2012) where paleoartists deliberately depict underused compositions and seemingly bizarre anatomies, as long as they are biologically defensible, in an effort to broaden our conceptions of what extinct species possibly looked like. While we think the critiques of paleoart homogeneity by the All Yesterdays Movement are completely valid, we intentionally tried to perpetuate visual tropes in the reconstructions for this project. Once installed, museum displays are hard to change. Several sculptures at the Tar Pits have been on continual display for over 80 years and older paleoart is frequently adapted and modified for new programing, exhibits, and marketing. Any new paleoart in a museum has to coexist with a range of other reconstructions of different vintage, style, medium, and accuracy in addition to labels of various antiquity using outdated common and scientific names. For any reconstruction, we try to follow older paleoart in the Museum unless there is strong scientific evidence that an organism’s appearance should be changed. For example, melanism is a relatively common polymorphism in many felid species (Allen et al., 2011). It is probable that some saber-toothed cat individuals were melanistic, and illustrating this morph in paleoart could lead to fruitful discussion regarding the genetics and adaptive benefits of dark coat coloration. But painting a black saber-toothed cat on a mural in our Museum would likely just confuse visitors and lead them to believe that the animal they see is a different species than the felid they see during the Ice Age Encounters puppet show, the 3D movie, or their visit to the gift shop, etc. Far better to copy the coat coloration of existing saber-toothed cat assets already used in the Museum as long as they are biologically realistic and scientifically supported.
We would also argue that late Pleistocene mammals, like those found at La Brea, have greater scientific constraints on how they can be reconstructed compared to many other prehistoric fauna. This does not forgive all tropes or copying but similar looking reconstructions may partially reflect greater certainty about what animals looked like. Ground sloths aside, many Ice Age mammals have very close living relatives whose behavior and soft tissue can serve as reasonable models. For several species, we have DNA, muscle tissue, and hair preserved (McDonald, H.G., 2003; Boeskorov et al., 2021). Some species were even observed by humans and illustrated by early artists (Antón et al., 2009). Lastly, mammals are more morphologically constrained in their external appearance and coloration than many other taxa (Caro, 2013). While it is reasonable to reconstruct a saber-toothed cat as dappled, tawny, or even melanistic, we would never add on dorsal frills, metallic green stripes, or fleshy cranial crests, all perfectly plausible features for a range of dinosaur species where no soft tissue is preserved.
SPECIES ACCOUNTS
Below, we provide the 3D, animated model for each extinct animal we reconstructed along with a description of the aesthetic and scientific considerations that went into the model. These descriptions are split into two sections: appearance and behavior. Appearance covers the general external morphology of the animal and its coloration. Behavior includes specific actions like locomotion as well as sounds and sociality. These descriptions vary considerably in length. Where life appearance is generally agreed on, we usually followed established and scientifically supported reconstructions described elsewhere. Descriptions are necessarily longer for species with more controversial life reconstructions.
Teratorn
Accipitriformes; Teratornithidae;
Teratornis merriami
(Figure 5)
 Appearance. The avifauna of Rancho La Brea is understudied compared to the mammalian megafauna and deserves a detailed, modern revision. Probably the best known of Rancho La Brea’s birds though is the teratorn (Teratornis merriami), a large condor-like bird with a wingspan of 3-4 m and a mass of 13.7 kg (Campbell, K.E., Jr. and Tonni, 1983). With Teratornithidae perhaps sister to Cathartidae, the teratorn closely resembled New World vultures in postcranial appearance (Mayr, 2009). “Consequently, all reconstructions of teratorns have pictured them as slightly larger versions of condors, usually sitting in a tree waiting for a trapped animal to die, or feeding in groups on large carcasses as vultures are wont to do.” (Campbell, K.E., Jr. and Tonni, 1981, p. 265). The large number of teratorn skeletons found in the tar pits supported this inference that they fell into the asphalt while greedily devouring the carcasses of large mammals that had already become stuck (Howard, 1930).
Appearance. The avifauna of Rancho La Brea is understudied compared to the mammalian megafauna and deserves a detailed, modern revision. Probably the best known of Rancho La Brea’s birds though is the teratorn (Teratornis merriami), a large condor-like bird with a wingspan of 3-4 m and a mass of 13.7 kg (Campbell, K.E., Jr. and Tonni, 1983). With Teratornithidae perhaps sister to Cathartidae, the teratorn closely resembled New World vultures in postcranial appearance (Mayr, 2009). “Consequently, all reconstructions of teratorns have pictured them as slightly larger versions of condors, usually sitting in a tree waiting for a trapped animal to die, or feeding in groups on large carcasses as vultures are wont to do.” (Campbell, K.E., Jr. and Tonni, 1981, p. 265). The large number of teratorn skeletons found in the tar pits supported this inference that they fell into the asphalt while greedily devouring the carcasses of large mammals that had already become stuck (Howard, 1930).
This vulture-like reconstruction is featured in Knight’s iconic mural (Figure 2A) and several other pieces of paleoart at the Museum. It prevailed until two papers by Campbell, K.E., Jr. and Tonni (1981; 1983) argued that teratorns were morphologically incapable of vulture-like scavenging and were instead active terrestrial predators of small mammals. This ecology is displayed in Mark Hallett’s 1988 reconstruction at middle right of Figure 2B. Rather than the naked-headed condor with brown/black plumage, Campbell, K.E., Jr. and Tonni promoted a reconstruction of teratorns analogous to secretarybirds with greyish wings and white countershading. The coloring was speculative but Campbell, K.E., Jr. reasonably believed that countershading and a white head would help camouflage a stalking bird from the view of small prey looking upward towards their demise (Campbell, K.E., Jr., personal commun., 2019). This coloring didn’t appear in Hallett’s mural (Figure 2B) but was adopted for several more recent pieces of paleoart at the Museum including a life size teratorn puppet.
The active terrestrial predator reconstruction focused on analysis of the teratorn’s skull (Campbell, K.E., Jr. and Tonni, 1981) and hind limbs (Campbell, K.E., Jr. and Tonni, 1983). Campbell, K.E., Jr. and Tonni (1983) agreed with previous research that the postcranial skeleton of teratorns closely resembled that of the California condor (Gymnogyps californianus) except for the teratorn’s larger overall size and proportionally much larger sternum (Fisher, 1945). The body mass of the teratorn was ~33% larger than that of the California condor but its pectoral musculature would have been about ~200% greater (Chatterjee et al., 2007). Campbell, K.E., Jr. and Tonni (1983) disputed Fisher’s (1945) analysis that this likely gave teratorns pelican-like flapping abilities but agreed the teratorn could have soared much like a California condor and likely faced similar constraints getting aloft.
Early researchers noted the interesting mix of vulturine and aquiline features of the teratorn’s skull (Stock, 1930). Quantitative (Hertel, 1995) and qualitative analyses (Campbell, K.E., Jr. and Tonni, 1981) showed that it had many features in common with piscivorous birds like albatrosses or cormorants that use their hooked bills to actively catch prey. However, stable isotopes show that teratorns fed exclusively from the terrestrial realm on a mix of grazers and browsers (Fox-Dobbs et al., 2006). Based on the high kinesis of the skull and several features of the mandible and maxilla, Campbell, K.E., Jr. and Tonni (1981) concluded that teratorns could not be scavengers. They also hypothesized that a predentary bone was present, a feature unknown in extant birds. Regardless of what they ate, it is clear that teratorns’ skulls don’t fit neatly into any extant morphology.
Campbell, K.E., Jr. and Tonni (1983) disputed Fisher’s (1945) conclusion that teratorns would have been slow and awkward on the ground by noting that the angle of the pelvis more closely matches walking birds like storks. However, they acknowledged that their legs were still short and probably incapable of running or grasping prey. They imagined teratorns actively stalking through a savannah habitat, surprising small prey, hooking it with their beaks, and eating it whole.
Without disputing any of the individual findings of Campbell, K.E., Jr. and Tonni’s (1981; 1983) detailed analyses, we find their reconstruction of the teratorn as an active terrestrial predator problematic. Imagine the ecology of a bird that flies like a condor yet hunts small mammals and herps on the ground. The teratorn wakes up from its presumably elevated roost. It soars to high altitudes on thermals, covering large areas looking for prey. Once it spots a mouse, it swoops down to a nearby area as it cannot catch the prey immediately with its feet like a raptor. It folds up its 4 m wings, then quietly stalks up on the mouse, catching it, and swallowing it whole. It cannot chase prey into the shrubs common in the chaparral landscape around the tar pits because it would risk entangling its large wings and getting eaten by one of the many mammalian predators in the area (Campbell, K.E., Jr. and Tonni, 1983). Thus, it likely takes back to the air after only a few hunting attempts. What other bird lives like this? Terrestrial avian predators like caracaras or secretarybirds have clear cursorial adaptations in their hind limbs. The teratorn had shorter legs than the Andean condor (Vultur gryphus), hardly a species considered fleet footed on the ground (Campbell, K.E., Jr. and Tonni, 1983). Condor-like soaring makes more sense for spatially rare, high reward resources like predator kills, not small mammals. Metabolic scaling analyses of the much larger teratornithid Argentavis magnificens also showed that its presumed life history traits make much more sense if the bird was a soaring scavenger rather than an active predator (Palmqvist and Vizcaíno, 2003). The skull of the teratorn was different than condors but Campbell, K.E., Jr. and Tonni’s (1983, p. 390) assertion that “They were functionally incapable of feeding by tearing pieces of flesh from carcasses as vultures do.” seems overstated. Many birds like eagles and corvids that do not have vulture-like beaks feed regularly on carrion. Lastly, reconstructing the teratorn as something other than a specialist of large mammal carcasses begs the question of why this species went extinct at the end of the Pleistocene at the same time as the megafauna (Campbell, K.E., Jr. and Tonni, 1983).
The taphonomic evidence for teratorns feeding on large mammal carcasses (their high abundance in the predator trap tar pits) is admittedly muddled, though. Nine of the ten most common birds found in Rancho La Brea’s early excavations are (or likely were) carnivorous (Howard, 1930). But of predatory birds found in the asphalt, only one half of individuals are from species that are (or likely were) obligate carrion feeders (Howard, 1930). The second most common species with 517 individuals, the extinct California turkey (Parapavo californicus), was likely omnivorous like extant turkeys and may have become stuck in asphalt in such high numbers due to its large mass, gregariousness, and foraging habit of scratching away ground litter, thus exposing the underlying sticky goo (Howard, 1930; Bocheński and Campbell, K.E., Jr., 2006). Bocheński and Campbell, K.E., Jr. (2006) hypothesized that the most common bird, the golden eagle (Aquila chyrsaetos), with 880 individuals (Howard, 1930) may have become stuck predating the second most common bird, the aforementioned California turkey. High speed aerial attack dives were likely hard to recover from when the prey was glued in place. Golden eagles are also common opportunistic scavengers, though and this likely explains some of their overabundance (Wilmers et al., 2003). The bird found at La Brea whose foraging most closely resembles Campbell, K.E., Jr. and Tonni’s (1983) terrestrial hunting teratorn is the caracara (either the extant Caracara cheriway or a similar extinct form), at 251 individuals, the third most common bird preserved (Howard, 1930). What this means for the habits of the teratorn (tenth most common with 108 individuals) is unclear (Howard, 1930). But the fact that the remains of two terrestrial opportunistic generalists (with varying degrees of carnivory) are more abundant than Rancho La Brea’s vultures (Coragyps occidentalis, Neophrontops americanus), condors (Gymnogyps californianus), and bald eagles (Haliaeetus leucocephalus), all obligate or common carrion feeders, shows that Knight’s iconic image of scavengers fluttering down to feast on the carcasses of megafauna entrapped in asphalt (Figure 2A) was apparently not the only way, or even the most common way, for birds to enter the fossil record at Rancho La Brea (Howard, 1930; Wilmers et al., 2003). More research needs to be done comparing the fossil bird assemblage at Rancho La Brea to actualistic predator kills like has been carried out with mammals (Carbone et al., 2009). And the life habits of the iconic teratorn are due for reanalysis using all the modern techniques now at our disposal.
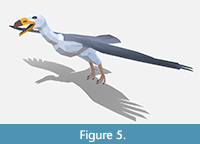
Reconstructing the general body contours and bone placement of teratorns, we followed two mounted skeletons from our Museum posed in both perching and soaring positions (Scott, 1988) and a reconstruction by Scott Hartman (2013) showing a teratorn walking. Given the controversial reconstructions of teratorns, we decided to hedge our bets on its external appearance. We followed the white and grey coloration of several recent reconstructions in the Museum but deliberately left the neck narrower than would be expected with full plumage (Figure 5). This will allow us to draw a new texture to wrap around the mesh that reconstructs the teratorn with a more condor-like naked head should we choose to do so in the future.
Behavior. We created both walking and soaring behaviors closely following the movements of California condors. We will likely display teratorns both eating carrion and actively walking around the tar pits to reflect their uncertain status as scavengers.
Dwarf Pronghorn
Artiodactyla; Antilocapridae;
Capromeryx minor
(Figure 6)
 Appearance. The extinct dwarf pronghorn is understudied. This is especially regrettable given that the dwarf pronghorn doesn’t seem to fit the pattern of the end-Pleistocene megafaunal extinction. Based on its reconstructed small size (~10.5 kg), mixed diet, and open forest habitat, the dwarf pronghorn should have easily survived the many larger grazing ungulates that went extinct in North America (Bravo-Cuevas et al., 2013; Pérez-Crespo et al., 2016; Davis, 2017). In a reversal of the pattern seen with most other Rancholabrean mammalian families (ursids, felids, canids, bovids), the dwarf pronghorn became extinct while the larger species of the group (Antilocapra americana) survived. More detailed analysis of this seemingly anomalous extinction could add more context to the ongoing debate surrounding the causes of the end-Pleistocene extinction pulse (Davis, 2017).
Appearance. The extinct dwarf pronghorn is understudied. This is especially regrettable given that the dwarf pronghorn doesn’t seem to fit the pattern of the end-Pleistocene megafaunal extinction. Based on its reconstructed small size (~10.5 kg), mixed diet, and open forest habitat, the dwarf pronghorn should have easily survived the many larger grazing ungulates that went extinct in North America (Bravo-Cuevas et al., 2013; Pérez-Crespo et al., 2016; Davis, 2017). In a reversal of the pattern seen with most other Rancholabrean mammalian families (ursids, felids, canids, bovids), the dwarf pronghorn became extinct while the larger species of the group (Antilocapra americana) survived. More detailed analysis of this seemingly anomalous extinction could add more context to the ongoing debate surrounding the causes of the end-Pleistocene extinction pulse (Davis, 2017).
We followed a reconstruction of a dwarf pronghorn originally sculpted by William Otto for the old Hancock Hall at NHMLAC sometime between 1923 and 1946 (Scott, 1985). It is unclear whether Capromeryx exhibited sexual dimorphism in horncore structure like A. americana does but all C. minor specimens with prominent horncores are typically regarded as males (White and Morgan, 2011). Thus, this reconstruction with horns probably represents a male. The coloring of the sculpture, a simplified version of the countershading of A. americana, is speculative but reasonable given it is a common pattern for many small ungulates; dappling would also make sense (White and Morgan, 2011). Although this reconstruction is very old, it is still on display at La Brea Tar Pits and the small amount of research on dwarf pronghorns since its creation has not generated any findings rendering it obsolete. As mentioned above, unless strong scientific evidence compelled us otherwise, we aimed to reuse older reconstructions to provide a consistent visual language for species at La Brea Tar Pits.
Behavior. Little is known about the dwarf pronghorn’s sociality or behavior but given its small size, rareness in the fossil record, and likely habitat, it has been inferred to have a lifestyle similar to extant small forest dwelling ungulates like duiker or brocket deer (White and Morgan, 2011; Davis, 2017). We portrayed the dwarf pronghorn as solitary and furtive, freezing at the sound of predators.
Ancient Bison
Artiodactyla; Bovidae;
Bison antiquus
(Figure 7)
 Appearance. Morphological and genetic data suggest the modern plains bison (Bison bison) evolved directly from the ancient bison around 10,000 14C BP so the two species were likely very similar in appearance and behavior except for the earlier species being larger (McDonald, J.N. and Lammers, 2002; Shapiro et al., 2004; Wilson et al., 2008). We followed McDonald (1981) who undertook a detailed reconstruction of several North American fossil bison species. Considering life history and appearance patterns common in extant ungulates, he suggested that the ancient bison would have had a much-reduced bonnet and pantaloons (shaggy hair covering the head and front legs) compared to extant plains bison (Figure 7).
Appearance. Morphological and genetic data suggest the modern plains bison (Bison bison) evolved directly from the ancient bison around 10,000 14C BP so the two species were likely very similar in appearance and behavior except for the earlier species being larger (McDonald, J.N. and Lammers, 2002; Shapiro et al., 2004; Wilson et al., 2008). We followed McDonald (1981) who undertook a detailed reconstruction of several North American fossil bison species. Considering life history and appearance patterns common in extant ungulates, he suggested that the ancient bison would have had a much-reduced bonnet and pantaloons (shaggy hair covering the head and front legs) compared to extant plains bison (Figure 7).
Behavior. The large number of ancient bison bones found in the Tar Pits, as well as Paleoindian kill sites, suggest the species lived in large herds numbering in the hundreds of individuals (Stock, 1930; Ben Wheat et al., 1972). Although we never displayed herds this size due to computational constraints, we did show the ancient bison in small groups of conspecifics in our AR experiences.
Western Camel
Artiodactyla; Camelidae;
Camelops hesternus
(Figure 8)
 Appearance. Genetic data (Heintzman et al., 2015) shows that Camelops is sister to Camelus (extant Bactrian and dromedary camels). Given this close relationship and the similarity, both in size and general appearance, between the skeletons of western camels and dromedaries (Camelus dromedarius), our reconstruction (Figure 8) closely resembles the latter species. Webb (1965) noted several differences that separated western camels from dromedaries: they had proportionally longer and deeper heads with longer muzzles that flexed downward more steeply, more muscular upper lips, proportionally more elongate limbs, and feet that may have been more llama-like. Given the limitations of our low poly aesthetic, the more gracile nature of the western camel is likely the only difference readily apparent in our model. Confirmation of a hump awaits more soft tissue preservation but neural spine anatomy led Webb (1965) to believe that the western camel likely had a single hump spanning most of the rib cage, though potentially placed farther forward than the hump in dromedaries. In several older pieces of paleoart at the Tar Pits, western camels are illustrated with what can best be described as a “humplett”, an ambiguous mass of intermediate size somewhere between large, true hump and dorsal clump of long, dark hair. We followed this purposefully ambiguous reconstruction although our “humplett” is likely too far back on the body as it follows placement in dromedaries.
Appearance. Genetic data (Heintzman et al., 2015) shows that Camelops is sister to Camelus (extant Bactrian and dromedary camels). Given this close relationship and the similarity, both in size and general appearance, between the skeletons of western camels and dromedaries (Camelus dromedarius), our reconstruction (Figure 8) closely resembles the latter species. Webb (1965) noted several differences that separated western camels from dromedaries: they had proportionally longer and deeper heads with longer muzzles that flexed downward more steeply, more muscular upper lips, proportionally more elongate limbs, and feet that may have been more llama-like. Given the limitations of our low poly aesthetic, the more gracile nature of the western camel is likely the only difference readily apparent in our model. Confirmation of a hump awaits more soft tissue preservation but neural spine anatomy led Webb (1965) to believe that the western camel likely had a single hump spanning most of the rib cage, though potentially placed farther forward than the hump in dromedaries. In several older pieces of paleoart at the Tar Pits, western camels are illustrated with what can best be described as a “humplett”, an ambiguous mass of intermediate size somewhere between large, true hump and dorsal clump of long, dark hair. We followed this purposefully ambiguous reconstruction although our “humplett” is likely too far back on the body as it follows placement in dromedaries.
Behavior. We used footage of modern dromedary and Bactrian camels for locomotion references, and assumed that western camels also exhibited the unusual stride pattern where both limbs on one side move forward at a time.
Dire Wolf
Carnivora; Canidae;
Aenocyon dirus
(Figure 9)
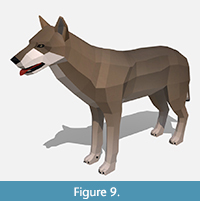 Appearance. Our reconstruction of the extinct dire wolf was developed before recent genetic analyses shattered the long held belief that dire wolves and extant grey wolves (Canis lupus) were closely related (Perri et al., 2021). Perri et al. (2021) found that dire wolves likely belonged to an isolated New World lineage that split from living canids about 5.7 million years ago and should thus be placed in the monotypic genus Aenocyon. It is unclear how this evidence should alter the traditional reconstruction of dire wolves as slightly larger and stockier grey wolves; even this new study arguing for reclassification confirmed that dire and grey wolves were morphologically very similar in their skeletal anatomy. In publicity for their study (Grimm, 2021), but not their research paper itself, the authors of the genetic study (Perri et al., 2021) suggested that because dire wolves lived in warmer latitudes of North America, they may have had characteristics of animals in these areas like rounded ears, bushy tails, and red fur. Perri said they may have resembled, “a giant, reddish coyote” (Grimm, 2021). Accordingly, a new reconstruction of dire wolves with reddish orange coats by paleoartist Maurico Antón accompanied press releases for the new study (Grimm, 2021). In a tweet (2021), Antón explained that for the coat color he, “mixed features from dholes (which I found to be even more variable than I assumed), Simien wolves, dingoes and even maned wolves for good measure!” While dire wolves could have certainly been reddish, we don’t find anything in Perri (2021) that specifically suggests this coloration is more probable than any other. The wide latitudinal range of dire wolves has long been known, and they were common near ice sheets in mammoth steppe habitats and the high-altitude Bolivian altiplano, as well as much warmer climes like Pleistocene Southern California, Venezuela, and coastal Peru (Dundas, 1999). The reddish coats do work well to counteract perceptions that dire wolves resembled large versions of grey wolves though.
Appearance. Our reconstruction of the extinct dire wolf was developed before recent genetic analyses shattered the long held belief that dire wolves and extant grey wolves (Canis lupus) were closely related (Perri et al., 2021). Perri et al. (2021) found that dire wolves likely belonged to an isolated New World lineage that split from living canids about 5.7 million years ago and should thus be placed in the monotypic genus Aenocyon. It is unclear how this evidence should alter the traditional reconstruction of dire wolves as slightly larger and stockier grey wolves; even this new study arguing for reclassification confirmed that dire and grey wolves were morphologically very similar in their skeletal anatomy. In publicity for their study (Grimm, 2021), but not their research paper itself, the authors of the genetic study (Perri et al., 2021) suggested that because dire wolves lived in warmer latitudes of North America, they may have had characteristics of animals in these areas like rounded ears, bushy tails, and red fur. Perri said they may have resembled, “a giant, reddish coyote” (Grimm, 2021). Accordingly, a new reconstruction of dire wolves with reddish orange coats by paleoartist Maurico Antón accompanied press releases for the new study (Grimm, 2021). In a tweet (2021), Antón explained that for the coat color he, “mixed features from dholes (which I found to be even more variable than I assumed), Simien wolves, dingoes and even maned wolves for good measure!” While dire wolves could have certainly been reddish, we don’t find anything in Perri (2021) that specifically suggests this coloration is more probable than any other. The wide latitudinal range of dire wolves has long been known, and they were common near ice sheets in mammoth steppe habitats and the high-altitude Bolivian altiplano, as well as much warmer climes like Pleistocene Southern California, Venezuela, and coastal Peru (Dundas, 1999). The reddish coats do work well to counteract perceptions that dire wolves resembled large versions of grey wolves though.
For his latest reddish orange dire wolf reconstruction (Grimm, 2021), Antón used an older musculoskeletal study he had originally developed for a book on canid evolution (Wang and Tedford, 2008) saying that, “dire wolf anatomy remains the same” (Antón, 2021). This is the same reconstruction we used for our model (Figure 9), albeit with its original more wolf/coyote like coat. We think that reddish coats and more traditional wolf/coyote coats are both reasonable for dire wolves. Until we find new paleontological or genetic evidence, the coloration of dire wolves remains speculative.
Behavior. For animations, we followed the locomotion and behavior of extant grey wolves. The large number of dire wolf skeletons preserved in the Tar Pits suggests social, pack-like behavior (Carbone et al., 2009) so we showed them in small groups when possible. As with bison, the group sizes we displayed virtually were smaller than they likely would have been in reality due to space constraints in the animated scenes. For vocalizations, we used a grey wolf pitched lower based on recent morphological analyses of preserved dire wolf hyoids that hypothesized they could have sounded like lower frequency grey wolves (Flores et al., 2020).
American Lion
Carnivora; Felidae;
Panthera atrox
(Figure 10, Figure 11)
 Appearance. For the muscle placement of our American lion (Panthera atrox) reconstruction, we followed (Cuff et al., 2017) who used a nearly complete skeleton found at the Tar Pits (LACMP23-555) to digitally flesh out the species with realistic virtual muscles. General body shape followed a reconstruction by Mauricio Antón (2013a) and the appearance of extant African lions (Panthera leo).
Appearance. For the muscle placement of our American lion (Panthera atrox) reconstruction, we followed (Cuff et al., 2017) who used a nearly complete skeleton found at the Tar Pits (LACMP23-555) to digitally flesh out the species with realistic virtual muscles. General body shape followed a reconstruction by Mauricio Antón (2013a) and the appearance of extant African lions (Panthera leo).
While it is clear that American lions were closely related to African lions (Barnett et al., 2009) and followed their general body shape albeit at a much larger size, the pelage of American lions remains controversial (Yamaguchi et al., 2004). Recent descriptions (published after we developed our models) of several well-preserved frozen cubs of European cave lions (Panthera spelaea), the sister taxon to P. atrox (Tseng et al., 2014), show that at least young of that species closely resembled extant African lions (Boeskorov et al., 2021). The European cave lions differed in several key respects though including lighter, greyer coats; thicker fur undercoats; and dark fur along the dorsal midline. Even though two of the European cave lions were thought to be 1-2 months old, they also lacked the dark, circular markings found on the coats of extant African lion cubs (Boeskorov et al., 2021). The fur around the European cave lion cubs’ faces seem to be consistent with cave paintings of P. spelaea that frequently show a contrasting “drip line” descending from the eye to the cheek as well as a dark patch between the eye and ear (Boeskorov et al., 2021).
 In contrast to the preserved soft tissues and cave art representations of the European cave lion, the American lion was known only from skeletal remains until Chimento and Agnolin (2017) reanalyzed the morphology of several South American fossils once attributed to a giant, extinct jaguar, “Panthera onca mesembrina”, and reassigned them to Panthera atrox. Based on skin associated with fossils of “P. o. mesembrina” and cave art, they reconstructed P. atrox as a large lion with a jaguar-like appearance including black spots over a rufous coat and yellowish striped forelimbs (Chimento and Agnolin, 2017). American lions and jaguars (Panthera onca) have often been mistaken for each other; in fact, all extant Panthera species possess highly similar skeletal morphology and are often difficult to distinguish by cranial characteristics alone (Christiansen and Harris, 2009). Metcalf et al. (2016) sampled 17 fossils of “P. o. mesembrina” from across South America for ancient DNA and found they all clustered into a genetically distinct clade sister to modern jaguars (P. onca), not modern African lions (P. leo). Twelve of these samples came from Cueva del Milodon in Ultima Esperanza, Chile: the same location of the skeletal and skin material examined in Chimento and Agnolin (2017). Crucially, a distal fragment of a right humerus (MLP 94-VIII-10-15) that Chimento and Agnolin (2017) assigned to P. atrox based on morphology had DNA that matched P. o. mesembrina instead (Metcalf et al., 2016). The isolated fragment of skin (MLP 94-VIII-10-71) pictured in Chimento and Agnolin (2017, figure 8) purported to be P. atrox looks more like the countershading on a mountain lion (Puma concolor) than the spots of a jaguar. Unfortunately, the rufous patch of skin associated with a “P. o. mesembrina” skull was not pictured. Additionally, the cave art evidence for a jaguar-like American lion seems overstated. Chimento and Agnolin (2017) claim that one spotted figure from El Ceibo, Santa Cruz province, Argentina (Cardich, 1987, figures 16 and 17) likely represents an American lion because it is reddish and larger than other animals portrayed at the same site. Could it not represent a giant, extinct subspecies of jaguar that there now seems to be ample morphological and genetic evidence for? Or a modern jaguar that the artist painted larger to reflect its importance (Chimento and Agnolin, 2017)? The various patches of skin Chimento and Agnolin (2017) attributed to P. atrox need to be genetically tested to ascertain whether they actually represent the coloration of American lions and an expansion of this species’ known range into South America.
In contrast to the preserved soft tissues and cave art representations of the European cave lion, the American lion was known only from skeletal remains until Chimento and Agnolin (2017) reanalyzed the morphology of several South American fossils once attributed to a giant, extinct jaguar, “Panthera onca mesembrina”, and reassigned them to Panthera atrox. Based on skin associated with fossils of “P. o. mesembrina” and cave art, they reconstructed P. atrox as a large lion with a jaguar-like appearance including black spots over a rufous coat and yellowish striped forelimbs (Chimento and Agnolin, 2017). American lions and jaguars (Panthera onca) have often been mistaken for each other; in fact, all extant Panthera species possess highly similar skeletal morphology and are often difficult to distinguish by cranial characteristics alone (Christiansen and Harris, 2009). Metcalf et al. (2016) sampled 17 fossils of “P. o. mesembrina” from across South America for ancient DNA and found they all clustered into a genetically distinct clade sister to modern jaguars (P. onca), not modern African lions (P. leo). Twelve of these samples came from Cueva del Milodon in Ultima Esperanza, Chile: the same location of the skeletal and skin material examined in Chimento and Agnolin (2017). Crucially, a distal fragment of a right humerus (MLP 94-VIII-10-15) that Chimento and Agnolin (2017) assigned to P. atrox based on morphology had DNA that matched P. o. mesembrina instead (Metcalf et al., 2016). The isolated fragment of skin (MLP 94-VIII-10-71) pictured in Chimento and Agnolin (2017, figure 8) purported to be P. atrox looks more like the countershading on a mountain lion (Puma concolor) than the spots of a jaguar. Unfortunately, the rufous patch of skin associated with a “P. o. mesembrina” skull was not pictured. Additionally, the cave art evidence for a jaguar-like American lion seems overstated. Chimento and Agnolin (2017) claim that one spotted figure from El Ceibo, Santa Cruz province, Argentina (Cardich, 1987, figures 16 and 17) likely represents an American lion because it is reddish and larger than other animals portrayed at the same site. Could it not represent a giant, extinct subspecies of jaguar that there now seems to be ample morphological and genetic evidence for? Or a modern jaguar that the artist painted larger to reflect its importance (Chimento and Agnolin, 2017)? The various patches of skin Chimento and Agnolin (2017) attributed to P. atrox need to be genetically tested to ascertain whether they actually represent the coloration of American lions and an expansion of this species’ known range into South America.
Whether American lions had manes is also controversial. Summarizing various lines of evidence including prehistoric art clearly depicting both sexes, Yamaguchi et al. (2004) concluded that manes are a secondary sexual character that evolved with extant African lions and likely did not occur in extinct European cave lions (P. spelaea). Given that American lions probably evolved from a subpopulation of Beringean P. spelaea, it is likely that they lacked manes as well (Barnett et al., 2009). Guthrie (1990) pointed to cave art in Les Combarelles, France that he interpreted as a male lion with discrete dorsal and ventral manes that were not as contrastingly colored as modern African lion manes. However, this same painting has also been interpreted as a bison, not a lion (Yamaguchi et al., 2004).
To represent the uncertain appearance of American lions, we made two, somewhat chimeric models (Figure 10, Figure 11). Overall, they follow extant African lions but have slightly redder coats and subtle spots patterns on their flanks. One model represents a lioness or generic maneless lion (Figure 10). The second follows Guthrie’s (1990) reconstruction of a male lion with distinct dorsal and ventral manes (Figure 11). So far, we have only used the lioness/generic maneless version in our AR experiences to skirt the question of whether male American lions possessed manes. If we were to make these models again, we would probably make the coats lighter and greyer to reflect new evidence from the frozen European cave lion cubs (Boeskorov et al., 2021) as well as move the jaguar-like spots lower on the body and legs or eliminate them all together. The dark drip lines and patches found on cave lions’ faces as well as the dorsal stripe should also be added but given the blocky coloration of our low poly style, incorporating fine features like this might be difficult.
Behavior. Given their close relationship and morphology, we used modern African lions as a locomotion reference for American lions. Whether the two species shared the same social structure remains controversial. Modern African lions are the only extant felids whose females live in groups. Cave art showing multiple lions together suggests that European cave lions lived in some kind of group (Yamaguchi et al., 2004). However, American lion remains are incredibly rare at the Tar Pits compared to the overabundant remains of presumably social dire wolves, coyotes, and saber-toothed cats (Smilodon fatalis) (Carbone et al., 2009). The model of tar pits as predator traps (Stock and Harris, 1992) for social species is consistent with observations in modern ecosystems, where social predators like spotted hyenas (Crocuta crocuta), and lions are the most common animals that come to investigate playbacks of prey distress calls in African parks (Carbone et al., 2009). Was the American lion solitary or merely rare in the region around the Pleistocene tar pits? Other solitary carnivores like mountain lions and bears (Ursus arctos, U. americanus, and Arctodus simus) are relatively rare in Tar Pits deposits (Carbone et al., 2009). We think showing the American lion alone or in pairs and triplets is reasonable given their phylogenetic affinities and avoids making any strong statements about whether they lived in large packs.
Saber-toothed Cat
Carnivora; Felidae;
Smilodon fatalis
(Figure 12)
 Appearance. Our saber-toothed cat model followed thoroughly-researched reconstructions by Antón and others (Antón et al., 1998; Antón, 2013b). The dappled coat of our model (Figure 12) is a speculative but commonly used pattern for saber-toothed cat reconstructions, consistent with the common interpretation of Smilodon fatalis as an ambush predator that lived in mixed habitats (Antón, 2013b). In an attempt to produce a more robust estimate of what saber-toothed cats’ pelts might look like, we combined the models of Meloro et al. (Meloro et al., 2013), which predicted habitat type from skeletal morphology of big cats, with the models of Allen et al. (Allen et al., 2011), which used habitat type (among other variables) to predict coat pattern in extant felids. However, we were unable to find any strong signal linking skeletal morphology directly to quantitative features of coat pattern. This remains a promising line of research for future paleoartistic reconstructions.
Appearance. Our saber-toothed cat model followed thoroughly-researched reconstructions by Antón and others (Antón et al., 1998; Antón, 2013b). The dappled coat of our model (Figure 12) is a speculative but commonly used pattern for saber-toothed cat reconstructions, consistent with the common interpretation of Smilodon fatalis as an ambush predator that lived in mixed habitats (Antón, 2013b). In an attempt to produce a more robust estimate of what saber-toothed cats’ pelts might look like, we combined the models of Meloro et al. (Meloro et al., 2013), which predicted habitat type from skeletal morphology of big cats, with the models of Allen et al. (Allen et al., 2011), which used habitat type (among other variables) to predict coat pattern in extant felids. However, we were unable to find any strong signal linking skeletal morphology directly to quantitative features of coat pattern. This remains a promising line of research for future paleoartistic reconstructions.
Behavior. For simple animations of walking and attacking prey, we used the similarly-sized extant African lion as an analogue. However, sociality in saber-toothed cats, like that of American lions, remains controversial with various lines of evidence from brain size to healed fracture rates put forth to support conflicting conclusions on their sociality (McCall et al., 2003). The preponderance of the evidence, including the overabundance of S. fatalis fossils at Rancho La Brea (where it represents the second most common megafauna species after dire wolves) as well as the prevalence of healed injuries (Shaw and Ware, 2018) matches expectations for a group-living animal lured into a predator trap (Carbone et al., 2009). We chose to display saber-toothed cats in small groups of two or three individuals as a gregarious social structure seems supported by the taphonomic evidence and matches multiple earlier works of sculpture and paintings found at the La Brea Tar Pits.
Short-faced Bear
Carnivora; Ursidae;
Arctodus simus
(Figure 13)
 Appearance. Our model of the short-faced bear follows the reconstruction done by Oscar San-Isidro for Figueirido et al. (2010). The coloration (Figure 13) is based on the closest extant relative of Arctodus simus, the spectacled bear (Tremarctos ornatus). Pelage can vary markedly among closely-related groups, especially bears; despite the name “black” bear, Ursus americanus famously has multiple distinctive color morphs besides black including white, cinnamon, and brown (Caro, 2013). T. ornatus differs from A. simus in numerous life history characteristics including size, carnivory, and arboreality (Vela-Vargas et al., 2021). However, T. ornatus inhabits a very wide variety of habitats today from cloud forest through scrub desert (Vela-Vargas et al., 2021) and black is a common color for most extant bears (Caro, 2013), so there is no reason to think this coloration could not have been successful for A. simus in the Pleistocene savanna ecosystem of the Los Angeles Basin.
Appearance. Our model of the short-faced bear follows the reconstruction done by Oscar San-Isidro for Figueirido et al. (2010). The coloration (Figure 13) is based on the closest extant relative of Arctodus simus, the spectacled bear (Tremarctos ornatus). Pelage can vary markedly among closely-related groups, especially bears; despite the name “black” bear, Ursus americanus famously has multiple distinctive color morphs besides black including white, cinnamon, and brown (Caro, 2013). T. ornatus differs from A. simus in numerous life history characteristics including size, carnivory, and arboreality (Vela-Vargas et al., 2021). However, T. ornatus inhabits a very wide variety of habitats today from cloud forest through scrub desert (Vela-Vargas et al., 2021) and black is a common color for most extant bears (Caro, 2013), so there is no reason to think this coloration could not have been successful for A. simus in the Pleistocene savanna ecosystem of the Los Angeles Basin.
Of all our models, A. simus is probably the one we are least satisfied with from a realism perspective. In our efforts to accurately match our fossil proportions, we fell prey to the common paleoart pitfall of “shrink wrapping” soft tissues around skeletal anatomy. This led to a rather gangly looking bear. Given another chance, we would probably bulk out the model a bit to account for the large fat reserves thought to be ancestral to Tremarctinae (Fowler et al., 2021) and long hair that the short-faced bear likely had. Although preserved skin is unknown for the short-faced bear, it seems reasonable that it would have had a full coat even though its large size may have pushed it toward gigantothermy. Their closest living relatives, spectacled bears, have long fur and even large bears like Mexican grizzlies (Ursus arctos horribilis) that lived in hot, dry habits had long, shaggy fur (Merriam, 1914).
Behavior. We used the polar bear (Ursus maritimus) as a reference for walking animations as it has similar leg proportions to the short-faced bear and is the largest extant bear (Figueirido et al., 2010). We avoided the long running controversy over short-faced bears’ cursorial habits by only showing them in a meandering gait (Figueirido et al., 2010).
Western Horse
Perissodactyla; Equidae;
Equus occidentalis
(Figure 14)
 Appearance. Extinct North American Equus is perhaps the taxon where our understanding of external appearance lags the most behind available evidence and methods. The modern horse husbandry industry has developed a detailed understanding of the genetics underlying different coat coloration and patterns (Pruvost et al., 2011). Combined with a prevalent fossil record (Barrón-Ortiz et al., 2017), many sequenced ancient genomes (Orlando, 2020), well-preserved frozen carcasses (Boeskorov et al., 2014), and (at least in Europe) numerous cave paintings (Pruvost et al., 2011), we have the potential to accurately reconstruct the appearance of several fossil equid species. However, application of these techniques in North America is hindered by the century old debate of American fossil horse taxonomy and difficulty matching up genetically identified clades with traditional morphospecies (Barrón-Ortiz et al., 2017). While out of the scope of this paper, synthesizing evidence from frozen remains and matching up existing DNA samples of extinct American horses with known coat patterning genes offers a promising direction for future research on North American fossil equids.
Appearance. Extinct North American Equus is perhaps the taxon where our understanding of external appearance lags the most behind available evidence and methods. The modern horse husbandry industry has developed a detailed understanding of the genetics underlying different coat coloration and patterns (Pruvost et al., 2011). Combined with a prevalent fossil record (Barrón-Ortiz et al., 2017), many sequenced ancient genomes (Orlando, 2020), well-preserved frozen carcasses (Boeskorov et al., 2014), and (at least in Europe) numerous cave paintings (Pruvost et al., 2011), we have the potential to accurately reconstruct the appearance of several fossil equid species. However, application of these techniques in North America is hindered by the century old debate of American fossil horse taxonomy and difficulty matching up genetically identified clades with traditional morphospecies (Barrón-Ortiz et al., 2017). While out of the scope of this paper, synthesizing evidence from frozen remains and matching up existing DNA samples of extinct American horses with known coat patterning genes offers a promising direction for future research on North American fossil equids.
For our model (Figure 14) of the western horse (Equus occidentalis), we followed the general appearance of Przewalski’s horse (Equus ferus przewalskii). Although now thought to actually represent the feral descendants of a very early domestic horse, their dun coats are considered a common ancestral wildtype for horses (Orlando, 2019).
Behavior. We used Przewalski’s horse as an animation reference. We displayed the western horse in groups as all living horses are herd animals, and E. occidentalis is the second most common herbivore found at Rancho La Brea (Stock and Harris, 1992; Bennett and Hoffman, 1999).
Shasta Ground Sloth
Pilosa; Megatheriidae;
Nothrotheriops shastensis
(Figure 15)
 Appearance. Reconstructions of extinct ground sloths are likely more tenuous than those of extinct carnivorans and ungulates because ground sloths lack close living relatives (Davis et al., 2018) or even functional analogues among modern fauna (Lopes et al., 2016; Davis, 2017). However, due to well preserved remains of the Shasta ground sloth (Nothrotheriops shastensis) (Lull, 1929), we likely know more about its appearance than any other extinct sloth (Kurtén and Anderson, 1980). Our model (Figure 15) follows an archetypal plasticine sculptural reconstruction by Lull (1929) based on a desiccated Shasta ground sloth (YPM 13198) found preserved in guano inside a fumarole in Aden Crater, New Mexico. There is little doubt his reconstruction accurately captures the general posture of the living sloth as the bones of YPM 13198 were held in articulation by preserved ligaments and tendons (Lull, 1929). Additionally, five patches of skin including one with 45 mm long pale-yellow hair were also found on the specimen. For cranial anatomy, we relied on the detailed muscular reconstructions of Naples (1987) although we gave the Shasta ground sloth face coloration common to three-toed sloths (Bradypus spp.) for purely artistic reasons.
Appearance. Reconstructions of extinct ground sloths are likely more tenuous than those of extinct carnivorans and ungulates because ground sloths lack close living relatives (Davis et al., 2018) or even functional analogues among modern fauna (Lopes et al., 2016; Davis, 2017). However, due to well preserved remains of the Shasta ground sloth (Nothrotheriops shastensis) (Lull, 1929), we likely know more about its appearance than any other extinct sloth (Kurtén and Anderson, 1980). Our model (Figure 15) follows an archetypal plasticine sculptural reconstruction by Lull (1929) based on a desiccated Shasta ground sloth (YPM 13198) found preserved in guano inside a fumarole in Aden Crater, New Mexico. There is little doubt his reconstruction accurately captures the general posture of the living sloth as the bones of YPM 13198 were held in articulation by preserved ligaments and tendons (Lull, 1929). Additionally, five patches of skin including one with 45 mm long pale-yellow hair were also found on the specimen. For cranial anatomy, we relied on the detailed muscular reconstructions of Naples (1987) although we gave the Shasta ground sloth face coloration common to three-toed sloths (Bradypus spp.) for purely artistic reasons.
One shortcoming of both the Shasta and Harlan’s ground sloth (Paramylodon harlani, discussed below) models (Figure 15, Figure 16) is the blockiness of the appendages. Given the constraints of our low poly style and how the models needed to be rigged and animated, these models necessarily exhibit a somewhat clumsy rendition of the proportions of the various digits and claws. Although much research has gone into the skeletal structure of ground sloth appendages (McDonald, H.G., 2012), it is not entirely clear what the external appearance of most ground sloth pedes and manus would have looked like. Digits on the same pes or manus can range from vestigial nubs to long claws with keratinous sheathes. Detailed comparative analyses and reexamination of specimens like YPM 13198 could lead to more accurate external reconstructions of these important features. We acknowledge helpful expert guidance from H. Gregory McDonald on the appendages of both ground sloth species discussed here.
Behavior. The general movements of our animations follow those of the Shasta ground sloth done for Ice Age Giants (2013), a large budget BBC documentary featuring La Brea Tar Pits.
Harlan’s Ground Sloth
Pilosa; Mylodontidae;
Paramylodon harlani
(Figure 16)
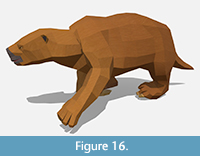 Appearance. For the head on our Harlan’s ground sloth model (Figure 16), we followed a detailed cranial reconstruction of Glossotherium robustum (Bargo et al., 2006) since Glossotherium and Paramylodon are morphologically very similar (McAfee, 2009), to the point of often being confused for one another (Naples, 1989) (in fact, La Brea Tar Pits P. harlani material was originally catalogued as Glossotherium before the genera were split). Although the Shasta ground sloth clearly had fur (Lull, 1929), we are unaware of any similarly preserved soft tissue for Harlan’s ground sloth. Some authors have reasonably questioned the traditional paleoartistic reconstructions of ground sloths with shaggy coats, especially for truly gigantic taxa like Megatherium, which could have easily generated enough body heat to keep warm without fur given their extreme size (Fariña, 2002). However, patches of skin with embedded osteoderms and 3-5 cm reddish-blond hair are known from Mylodon, a mylodontid ground sloth of similar size and morphology to Paramylodon (Nitiu et al., 2016) (which also has osteoderms) so it seems reasonable that medium-sized sloths like Paramylodon would have had hair. The reddish color of hair on our Harlan’s ground sloth model (Figure 16) is consistent with Mylodon pelage and matches several previous works of paleoart at La Brea Tar Pits, visually separating it from the smaller, blond Shasta ground sloth.
Appearance. For the head on our Harlan’s ground sloth model (Figure 16), we followed a detailed cranial reconstruction of Glossotherium robustum (Bargo et al., 2006) since Glossotherium and Paramylodon are morphologically very similar (McAfee, 2009), to the point of often being confused for one another (Naples, 1989) (in fact, La Brea Tar Pits P. harlani material was originally catalogued as Glossotherium before the genera were split). Although the Shasta ground sloth clearly had fur (Lull, 1929), we are unaware of any similarly preserved soft tissue for Harlan’s ground sloth. Some authors have reasonably questioned the traditional paleoartistic reconstructions of ground sloths with shaggy coats, especially for truly gigantic taxa like Megatherium, which could have easily generated enough body heat to keep warm without fur given their extreme size (Fariña, 2002). However, patches of skin with embedded osteoderms and 3-5 cm reddish-blond hair are known from Mylodon, a mylodontid ground sloth of similar size and morphology to Paramylodon (Nitiu et al., 2016) (which also has osteoderms) so it seems reasonable that medium-sized sloths like Paramylodon would have had hair. The reddish color of hair on our Harlan’s ground sloth model (Figure 16) is consistent with Mylodon pelage and matches several previous works of paleoart at La Brea Tar Pits, visually separating it from the smaller, blond Shasta ground sloth.
Behavior. Walking animations followed the biomechanical reconstructions of McDonald (2007), which were based on tracks attributed to Paramylodon harlani found at Nevada State Prison near Carson City, Nevada.
Columbian Mammoth
Proboscidea; Elephantidae;
Mammuthus columbi
(Figure 17)
 Appearance. Our virtual Columbian mammoth model (Figure 17) followed a life-sized sculpture of Mammuthus columbi recently installed in the Tar Pits Museum. The detailed, life-sized reconstruction of the Columbian mammoth was originally produced for the Field Museum’s travelling exhibition Mammoths and Mastodons: Titans of the Ice Age by Blue Rhino Studios, a well-known museum exhibition fabricator. Little is known about the soft tissue of Columbian mammoths and purported finds of preserved hair (De Pastino, 2015) have not been formally described yet. Given their large size, they likely had no trouble conserving heat in relatively warm environments like Pleistocene Southern California (or Mexico where they are also found) and are typically reconstructed with minimal hair in paleoart both at the Tar Pits (Figure 1, Figure 2) and elsewhere.
Appearance. Our virtual Columbian mammoth model (Figure 17) followed a life-sized sculpture of Mammuthus columbi recently installed in the Tar Pits Museum. The detailed, life-sized reconstruction of the Columbian mammoth was originally produced for the Field Museum’s travelling exhibition Mammoths and Mastodons: Titans of the Ice Age by Blue Rhino Studios, a well-known museum exhibition fabricator. Little is known about the soft tissue of Columbian mammoths and purported finds of preserved hair (De Pastino, 2015) have not been formally described yet. Given their large size, they likely had no trouble conserving heat in relatively warm environments like Pleistocene Southern California (or Mexico where they are also found) and are typically reconstructed with minimal hair in paleoart both at the Tar Pits (Figure 1, Figure 2) and elsewhere.
Behavior. We used modern African elephants (Loxodonta africana), as animation references. Based on the behavior of modern elephants and numerous monodominant localities (i.e., “crowd” finds) of Columbian mammoth fossils, Mammuthus columbi probably lived in herds of around 20 individuals (Haynes, 1991; Haynes and Klimowicz, 2003; Hoppe, 2004). Although it would take up too much room to show a full herd of mammoths, we showed several mammoths at a time when we could in our virtual experiences.
American Mastodon
Proboscidea; Mammutidae;
Mammut americanum
(Figure 18)
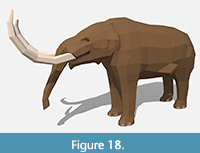 Behavior. Our model of an American mastodon (Figure 18) follows typical reconstructions (e.g., Figure 2A) of Mammut americanum as a stocky elephant-like animal with shaggy brown fur (Witton, 2020). Although this general appearance is well supported by numerous skeletal finds, there is actually little direct evidence that mastodons were covered in hair despite over a century of paleoart that almost exclusively reconstructs them with dense fur (Witton, 2020). There has been only one find of preserved mastodon fur: a skull from Wisconsin with two small patches of hairy skin (Hallin and Gabriel, 1981; Hallin, 1983; 1989). Unfortunately, these patches are described only briefly and have never been figured in the scientific literature beyond an SEM picture of one hair (Hallin, 1989). The patches consisted of hollow guard hairs with a furry undercoat similar to that of aquatic mammals (Hallin, 1983). Hallin and Gabriel (1981, p. 199) concluded that, “This occurrence of mastodon hair supports the accuracy of illustrations which depict mastodons as having been hairy.”, although they interestingly thought the hair pointed to a semiaquatic lifestyle for mastodons instead of cold weather tolerance, the typical reason given for mastodons’ (hypothetical) fur coat (Hallin, 1983).
Behavior. Our model of an American mastodon (Figure 18) follows typical reconstructions (e.g., Figure 2A) of Mammut americanum as a stocky elephant-like animal with shaggy brown fur (Witton, 2020). Although this general appearance is well supported by numerous skeletal finds, there is actually little direct evidence that mastodons were covered in hair despite over a century of paleoart that almost exclusively reconstructs them with dense fur (Witton, 2020). There has been only one find of preserved mastodon fur: a skull from Wisconsin with two small patches of hairy skin (Hallin and Gabriel, 1981; Hallin, 1983; 1989). Unfortunately, these patches are described only briefly and have never been figured in the scientific literature beyond an SEM picture of one hair (Hallin, 1989). The patches consisted of hollow guard hairs with a furry undercoat similar to that of aquatic mammals (Hallin, 1983). Hallin and Gabriel (1981, p. 199) concluded that, “This occurrence of mastodon hair supports the accuracy of illustrations which depict mastodons as having been hairy.”, although they interestingly thought the hair pointed to a semiaquatic lifestyle for mastodons instead of cold weather tolerance, the typical reason given for mastodons’ (hypothetical) fur coat (Hallin, 1983).
 Are these two patches of hair enough to conclude that mastodons were entirely covered in thick fur? While mastodons, like modern elephants, certainly had some hair, it is unclear if they needed thick coats to keep warm at the northern, interglacial limits of their range in the Arctic and Subarctic (Zazula et al., 2014; Larramendi, 2016). These large-bodied animals certainly would not have needed thick fur in the subtropical climate of Florida where they were also common (Zazula et al., 2014). If the Columbian mammoth was not thought to have extensive fur, it is also unclear why the similarly sized, yet even more compactly proportioned, mastodon needed a thick fur coat. As they cooccurred in Pleistocene Southern California, reconstructions at the Tar Pits often show mammoths and mastodons in the same scene, the former nearly naked and the later thickly furred (e.g., Figure 2B). Surely mastodons’ mixed or closed habitat preferences (Haynes and Klimowicz, 2003) were not enough to account for this thermal disparity (Figure 2B). Interestingly, there is one piece of paleoart at La Brea Tar Pits that displays a mastodon without thick fur (Figure 19). However, this 1968 life-sized sculpture by Howard Ball is located at the west end of the Lake Pit, an area currently under construction and partially obscured by vegetation with low visitor traffic. It is likely one of the least viewed and reproduced pieces of paleoart onsite.
Are these two patches of hair enough to conclude that mastodons were entirely covered in thick fur? While mastodons, like modern elephants, certainly had some hair, it is unclear if they needed thick coats to keep warm at the northern, interglacial limits of their range in the Arctic and Subarctic (Zazula et al., 2014; Larramendi, 2016). These large-bodied animals certainly would not have needed thick fur in the subtropical climate of Florida where they were also common (Zazula et al., 2014). If the Columbian mammoth was not thought to have extensive fur, it is also unclear why the similarly sized, yet even more compactly proportioned, mastodon needed a thick fur coat. As they cooccurred in Pleistocene Southern California, reconstructions at the Tar Pits often show mammoths and mastodons in the same scene, the former nearly naked and the later thickly furred (e.g., Figure 2B). Surely mastodons’ mixed or closed habitat preferences (Haynes and Klimowicz, 2003) were not enough to account for this thermal disparity (Figure 2B). Interestingly, there is one piece of paleoart at La Brea Tar Pits that displays a mastodon without thick fur (Figure 19). However, this 1968 life-sized sculpture by Howard Ball is located at the west end of the Lake Pit, an area currently under construction and partially obscured by vegetation with low visitor traffic. It is likely one of the least viewed and reproduced pieces of paleoart onsite.
If the evidence isn’t conclusive for mastodons’ fur, why did we reconstruct them with it? Because brown fur matches most of the previous reconstructions at the Tar Pits Museum, and it visually separates mastodons from mammoths. While the stockier skeleton, straighter tusks, and conical teeth of mastodons are readily visible to the professional paleontologist, the average museum patron often treats mammoths and mastodons as interchangeable (Hallin, 1989). For example, across the street from La Brea Tar Pits, the Los Angeles Metro displays a large banner proudly announcing the discovery of “Hayden”, a juvenile mammoth, while constructing the Wilshire/Fairfax subway station. The reconstruction of Hayden the mammoth they use is a tracing of a mastodon reconstruction previously found on the Museum’s website. A recent PBS documentary filmed at La Brea Tar Pits even showed a stock picture of a woolly mammoth for their mastodon reconstruction. The low poly aesthetic of our mastodon (Figure 18) is ambiguous enough that we feel comfortable publishing it. If we do find more evidence that mastodons had fur, then the model will be accurate. However, if detailed studies of American mastodons’ thermoregulatory capabilities reveal that naked skin is more likely, the model could still reasonably function as a naked mastodon, albeit one with light brown skin, an artistic choice that makes it easily distinguishable from our Columbian mammoth model (Figure 17).
We note that Dooley et al. (Dooley et al., 2019) recently proposed assigning all specimens of Mammut found at La Brea Tar Pits to a newly erected species, Mammut pacificus. If this designation holds, it is unlikely to change our reconstruction. Of the morphological differences proposed between M. americanum and M. pacificus, only the lack of mandibular tusks in M. pacificus would be discernable in a model like ours (Dooley et al., 2019). As our model lacks mandibular tusks, it should function well as either M. pacificus or M. americanum, given that not all M. americanum individuals possessed mandibular tusks.
Behavior. We used modern Asian elephants (Elephas maximus) for animation references given their similar size to mastodons. Group size in mastodons is controversial. The perceived overabundance of solitary American mastodon finds compared to the large number of Columbian mammoth “crowd” finds may not represent actual behavioral differences between the two species but rather taphonomic and publication biases based on mastodons’ mixed and closed habitat preferences (Haynes and Klimowicz, 2003). We conservatively only show solitary mastodons as even if they lived in herds, one would still expect to see solitary mastodons wandering around just like with extant forest dwelling elephants.
DISCUSSION
Our virtual models have received strong positive reviews from visitors and led to learning gains in both AR and traditional museum label treatments that used them (Herrick et al., 2021). Test participants frequently recalled modelled animals by name and described in detail ecosystem attributes they were associated with, showing their high salience (Herrick et al., 2021). The simple models also performed well on consumer grade smartphones. Running on a typical Android device, seven animals could be rendered in AR at the same time with ~50,000 total triangles rendered per scene. With a backend render time of ~7 ms per frame, our AR experiences can consistently achieve a smartphone’s maximum framerate of 60 frames per second while only taxing the device at about 44% efficiency, helping reduce heat generated by the processor. These results indicate that a low poly design can be an effective way to balance visitor engagement, performance considerations, and scientific grounding.
 Although they were created specifically to serve experimental applications for Tar AR, our NSF AISL grant investigating differences in AR immersiveness and interactivity, the forethought we put into designing them and their standard format (.fbx) has made them easy to adapt to other uses. The low poly models were simple enough that we were able to port them into a Web XR framework for a prototype AR viewfinder called Perceptoscope (NSF SBIR 1820238). Even a few animated models walking around La Brea Tar Pits in AR were enough to interest visitors and create an engaging AR experience (Davis, 2020). The models were also easy to import into popular AR platforms like Snapchat. Through a partnership with Snap Inc., members of a Tar Pits teen educational initiative were able to program their own interactive AR experiences with saber-toothed cats, dire wolves, and Shasta ground sloths (Figure 20) in lieu of typical in-person activities at the Museum during the COVID-19 pandemic. We are also using the models to block out space while mocking up a new Tar Pits themed Mobile Museum and investigating turning the animals into 3D printed tokens for more tactile, “hands on” applications. These extended use cases demonstrate how virtual paleoart can be adopted into many different experiences and modalities both at a physical museum and through virtual outreach.
Although they were created specifically to serve experimental applications for Tar AR, our NSF AISL grant investigating differences in AR immersiveness and interactivity, the forethought we put into designing them and their standard format (.fbx) has made them easy to adapt to other uses. The low poly models were simple enough that we were able to port them into a Web XR framework for a prototype AR viewfinder called Perceptoscope (NSF SBIR 1820238). Even a few animated models walking around La Brea Tar Pits in AR were enough to interest visitors and create an engaging AR experience (Davis, 2020). The models were also easy to import into popular AR platforms like Snapchat. Through a partnership with Snap Inc., members of a Tar Pits teen educational initiative were able to program their own interactive AR experiences with saber-toothed cats, dire wolves, and Shasta ground sloths (Figure 20) in lieu of typical in-person activities at the Museum during the COVID-19 pandemic. We are also using the models to block out space while mocking up a new Tar Pits themed Mobile Museum and investigating turning the animals into 3D printed tokens for more tactile, “hands on” applications. These extended use cases demonstrate how virtual paleoart can be adopted into many different experiences and modalities both at a physical museum and through virtual outreach.
The main downside of low poly models, or any 3D virtual paleoart, is that the format still requires specialized software to generate and view. Most museum staff have familiarity working with flat assets but developing the skills, workflows, and shared vocabularies necessary to manipulate animated 3D models and export them for various uses can be challenging. Despite some advances like 3D PDFs, virtual fossils or reconstructions are also still difficult to incorporate into formal peer reviewed literature, which has been based on 2D images for centuries (Lautenschlager and Rücklin, 2014). However, the growing adoption of virtual paleontology has created strong momentum in the field for simple and inexpensive pipelines that can turn 3D models of fossils generated for research into engaging outreach and education tools (Rahman et al., 2012; Ziegler et al., 2020). Now with just a little training, anyone can produce realistic 3D photogrammetry models of fossils using only a smartphone and free software, a process that once required expensive, specialized equipment (Cunningham, 2021). Given software like Sketchfab that allows models to be viewed by anyone with a web browser (Cunningham, 2021), we believe 3D virtual paleoart could soon become standard in the repertoires of many museums.
CONCLUSION
Paleoart is highly influential, both to the public (Ross et al., 2018) and professionals (Witton, 2020), and can live on long after its initial intended use. However, museums and scientists rarely treat paleoart with the same academic rigor and transparency as other paleontological research (Witton et al., 2014; Witton, 2017a; Campbell, R.M. et al., 2021). Analogous to growing calls for reproducibility through data and code sharing requirements, we suggest that researchers and museums publish sufficiently detailed paleoart descriptions whenever releasing new paleoart (Campbell, R.M. et al., 2021). These could either be included within the supporting material of broader manuscripts or published on their own as a novel type of article.
This work documents the biological, practical, and artistic considerations that went into creating a menagerie of 3D, animated Ice Age animal models. We believe this approach has merit and warrants formalization of methods. We challenge other researchers to also subject their paleoartistic reconstructions to rigorous peer review, making the reasoning behind these decisions publicly available for scientists, educators, and artists in the future. By having different institutions document their paleoart, best-practices may be identified that enable the creation of effective guidelines and standards for describing paleoart. Moreover, close documentation can highlight promising avenues of research, such as in this work, where we noted genetic markers for known coat patterns that have not been matched to available genetic sequences of fossil North American horses.
Researchers
While no responsible researcher would publish a graph without explaining the data and methods that went into creating the figure, paleoart frequently accompanies paleontological research with little to no explanation of the data or decisions that went into creating it (Campbell, R.M. et al., 2021). Often this is because paleoart is not part of the scientific manuscript itself but rather is created for media use after acceptance of the manuscript. The paleoart can portray hypotheses to the public that were not raised in the formal study or subjected to peer review. In one previously noted example, a press release suggested that dire wolves had reddish coats (Grimm, 2021) although the peer reviewed publication it was advertising did not (Perri et al., 2021).
Why not move this paleoart to a figure in the study itself (Bertozzo et al., 2020), as authors increasingly do? Journal editors pressed for space may not deem paleoart serious enough to include with anatomical photographs and graphs. However, articles are most commonly read in electronic form where paleoart can be readily included. Moreover, good paleoart contains just as much research as any other figure and will often prove more influential. In particular, paleoart accompanying any new fossil species description should especially be included in the original manuscript with an appropriate description of the various scientific and aesthetic decisions that went into making it. Including the paleoart directly into scientific papers and subjecting it to peer review should also encourage scientists to work more closely with artists on their reconstructions (Witton, 2017b; 2017a). Researchers should not distance themselves from the work as mere “artist’s interpretations” to the press or public, but should own and publish their reconstructions as they do any other conclusions of their written study. Even minimal paleoart like ubiquitous black silhouette bone maps should include some statement describing their origin (Mateus and Tschopp, 2017) as life appearance reconstructions are built upon skeletal anatomy reconstructions, which often lack their own scientific and artistic justifications (Campbell, R.M. et al., 2021).
Researchers may understandably lament that they rarely have enough money to pay for open access publication, let alone professional paleoartists through multiple rounds of peer review (Witton, 2017a). We have no ready solution to this other than that funding agencies and scientists alike need to understand that the communication of research needs to be budgeted for and funded just as much as its production. Paleoart that accompanies research carries an authoritative weight (Witton, 2017a) and often generates numerous derivative reconstructions (if not outright copies) in other scientific and popular media (Naish, 2017). Universities looking to expand their academic influence and prestige would do well to have their media offices fund original, scientifically supported paleoart. Given the pervasive culture of copying in paleoart (Witton et al., 2014), a few thousand dollar investment could generate substantial positive return, including influencing the appearance of a species for decades to come as well as improving the scientific accuracy of the species as it is portrayed to the public in books, movies, and video games.
Museums
Museums are ranked as one of the most trustworthy sources for information (Dilenschneider, 2017), which means they bear an even greater responsibility than researchers to produce, and explain, scientifically grounded paleoart used in their exhibitions and programs (Campbell, R.M. et al., 2021). With the exception perhaps of major Hollywood franchises like Jurassic Park, museums and exhibitions likely produce the most lasting and culturally influential pieces of paleoart such as The Crystal Palace’s prehistoric menagerie by sculptor Benjamin Waterhouse Hawkins or The Yale Peabody Museum’s The Age of Reptiles mural by Rudolph Zallinger. Blockbuster travelling exhibitions like Extreme Mammals or T. rex the Ultimate Predator often create major new works of independent paleoart that will be seen by millions of visitors during their runs. The American Museum of Natural History’s T. rex the Ultimate Predator claimed to feature the “most scientifically accurate model of T. rex ever” (Snyder, 2019, pg. 1). An exhibition press release billed their life-sized model as, “...the definitive representation of this prehistoric predator” (emphasis in the original) (Snyder, 2019, p. 1). Surely the research that went into this captivating reconstruction is worthy of publication but we are unaware of any scientific article describing the features that make it definitive or the evidence behind this paleoart.
This is not to claim that museums rarely explain their paleoart. Paleoart, and the process of creating it, is often highlighted in web articles, programs, or even physical exhibitions. The curators of T. rex the Ultimate Predator did, in fact, describe their model in popular press interviews (Quain, 2019), just not in easily citable formal academic literature with the benefits of references and clear anatomical illustrations. While the focus of this paper is on academic descriptions of new paleoart, we encourage museums to expand on their existing paleoart descriptions for the general public as a way to bring art into science and better explain the iterative process of science. The Creation Museum near Cincinnati has already weaponized public misunderstanding of the paleoartistic process in one of their exhibits to suggest that using the same underlying fossil evidence, artists can plausibly reconstruct the hominin Australopithecus afarensis as anything from a stereotypical light skinned cave man to a quadrupedal orangutan (Henderson, 2013; Campbell, R.M. et al., 2021). It would serve museums well to counteract these arguments by being transparent with the public about the scientific and artistic choices going into their work (Campbell, R.M. et al., 2021). This applies especially to paleoart reconstructing extinct hominins, which has a well known history of promulgating racist ideas beneath the veneer of objective scientific authority (Campbell, R.M. et al., 2021).
We believe that any new paleoart of sufficient importance produced by a museum should be accompanied by a description of scientific and aesthetic choices in a peer reviewed journal, as we have done here. This may seem like an unnecessary responsibility added to academics’ already busy schedules but curators should welcome the opportunity to write paleoart descriptions. They turn exhibition consultation and curation, a time-consuming activity that is sometimes treated as a burdensome service requirement, into valuable, citable research publications. Given the production logistics of museum exhibitions, one shortcoming of submitting paleoart for peer review is that any corrections suggested by reviewers will unlikely be incorporated into the new exhibition in the form of updated paleoart. However, these suggestions still have merit. If incorporated into the article text, they could serve as valuable critiques and research avenues for generating more accurate paleoart in the future.
Sufficiently Detailed Paleoart Descriptions
Others have provided minimum guidelines for creating scientifically credible paleoart (Witton, 2017a) but what should the requirements of a supporting paleoart description be? We suggest the following items at a minimum:
• Identification of any individual specimens used for the reconstruction.
• Citation of any previous artwork, reconstructions, or research that were influential in the making of the new reconstruction.
• Descriptions of general posture and proportions, gross appearance of soft tissue, coloration, and behavior explaining whether the appearance of these features was drawn from direct fossil evidence, reasonable inference (e.g., phylogenetic bracketing), or aesthetic choice.
The more detailed these descriptions become, the more valuable they are to the advancement of paleoart and our understanding of fossil organisms. Just because some feature is widely accepted in paleoartistic reconstructions, does not mean it is undeserved of description and justification. Even if they are used in a new reconstruction, calling out tropes that lack strong evidence, such as thick mastodon hair (Witton, 2020), helps inoculate against paleontological “just so” stories and generate new research ideas and visuals. As including code and raw data aimed at reproducibility can lead to better quantitative research, we also encourage the inclusion of intermediate work generated on the way to the final paleoart composition. Color and deep tissue studies, while not intended as final work, can show the steps and inferences used to flesh out an extinct animal. Orthogonal views can let researchers and artists evaluate detailed anatomy and body proportions without the complication of foreshortened perspectives or atmospheric lighting used to generate dynamism in a final composition. These requirements and suggestions for explanatory material should not be used to limit the artistic style or value of paleoart. We think our own Ice Age models presented here are scientifically well supported while also being highly stylized.
We hope our example here of a new type of research article focused on the scientific and aesthetic justifications for paleoart is adopted by paleontological journals. Citable, peer reviewed descriptions could do much to help advance both the field of paleoart and our understanding of prehistoric life.
ACKNOWLEDGMENTS
This work was funded by NSF AISL collaborative grant "Re-Living Paleontology: Studying How Augmented Reality Immersion and Interaction Impact Engagement and Communicating Science to the Public" (1811014; 1810984). We thank two anonymous reviewers for their insightful comments and help improving this article.
REFERENCES
Akersten, W.A., Shaw, C.A., Page, G.C., and Jefferson, G.T. 1983. Rancho La Brea: Status and future. Paleobiology, 9:211-217. https://doi.org/10.1017/S0094837300007648
Allen, W.L., Cuthill, I.C., Scott-Samuel, N.E., and Baddeley, R. 2011. Why the leopard got its spots: relating pattern development to ecology in felids. Proceedings of the Royal Society B, 278:1373-1380. https://doi.org/10.1098/rspb.2010.1734
Ansón, M., Hernández Fernández, M., and Saura Ramos, P.A. 2015. Paleoart: term and conditions (a survey among paleontologists). Current Trends in Paleontology and Evolution, Conference proceedings of the XIII Encuentro en Jóvenes Investigadores en Paleontología (XIII EJIP), Cercedilla, Spain, p. 28-34.
Antón, M. 2013a. A clash of titans: lion vs. scimitar-toothed cat. Chasing Sabretooths blog. 16 May 2013. Accessed 13 August 2021. https://chasingsabretooths.wordpress.com/2013/05/16/a-clash-of-titans-lion-vs-scimitar-tooth-cat/
Antón, M. 2013b. Sabertooth. Indiana University Press, Bloomington.
Antón, M. 2021. (Post on Twitter.com, Jan 13, 2021). Accessed 13 August 2021. https://twitter.com/MAntonPaleoart/status/1349481462119211008
Antón, M., García-Perea, R., and Turner, A. 1998. Reconstructed facial appearance of the sabretoothed felid Smilodon. Zoological Journal of the Linnean Society, 124:369-386.
Antón, M., Salesa, M.J., Turner, A., Galobart, A., and Pastor, J.F. 2009. Soft tissue reconstruction of Homotherium latidens (Mammalia, Carnivora, Felidae). Implications for the possibility of representations in Palaeolithic art. Geobios, 42:541-551. https://doi.org/10.1016/j.geobios.2009.02.003
Bargo, M.S., Toledo, N., and Vizcaíno, S.F. 2006. Muzzle of South American Pleistocene ground sloths (Xenarthra, Tardigrada). Journal of Morphology, 267:248-263.
Barnett, R., Shapiro, B., Barnes, I., Ho, S.Y., Burger, J., Yamaguchi, N., Higham, T.F., Wheeler, H., Rosendahl, W., Sher, A.V., Sotnikova, M., Kuznetsova, T., Baryshnikov, G.F., Martin, L.D., Harington, C.R., Burns, J.A., and Cooper, A. 2009. Phylogeography of lions (Panthera leo ssp.) reveals three distinct taxa and a late Pleistocene reduction in genetic diversity. Molecular Ecology, 18:1668-1677.
Barrón-Ortiz, C.I., Rodrigues, A.T., Theodor, J.M., Kooyman, B.P., Yang, D.Y., and Speller, C.F. 2017. Cheek tooth morphology and ancient mitochondrial DNA of late Pleistocene horses from the western interior of North America: Implications for the taxonomy of North American Late Pleistocene Equus. PLoS ONE, 12:e0183045. https://doi.org/10.1371/journal.pone.0183045
Ben Wheat, J., Malde, H.E., and Leopold, E.B. 1972. The Olsen-Chubbuck site: a paleo-indian bison kill. Memoirs of the Society for American Archaeology, 1-180.
Bennett, D. and Hoffman, R.S. 1999. Equus caballus. Mammalian Species, 628:1-14.
Bertozzo, F., Manucci, F., Dempsey, M., Tanke, D.H., Evans, D.C., Ruffell, A., and Murphy, E. 2020. Description and etiology of paleopathological lesions in the type specimen of Parasaurolophus walkeri (Dinosauria: Hadrosauridae), with proposed reconstructions of the nuchal ligament. Journal of Anatomy, 238:1055-1069. https://doi.org/10.1111/joa.13363
Bocheński, Z.M. and Campbell, K.E., Jr. 2006. The extinct California turkey, Meleagris californica, from Rancho La Brea. Contributions in Science Natural History Museum of Los Angeles County, 509:1-92.
Boeskorov, G.G., Potapova, O.R., Mashchenko, E.N., Protopopov, A.V., Kuznetsova, T.V., Agenbroad, L., and Tikhonov, A.N. 2014. Preliminary analyses of the frozen mummies of mammoth (Mammuthus primigenius), bison (Bison priscus) and horse (Equus sp.) from the Yana‐Indigirka Lowland, Yakutia, Russia. Integrative Zoology, 9:471-480. https://doi.org/10.1111/1749-4877.12079
Boeskorov, G.G., Plotnikov, V.V., Protopopov, A.V., Baryshnikov, G.F., Fosse, P., Dalén, L., Stanton, D.W.G., Pavlov, I.S., Suzuki, N., and Tikhonov, A.N. 2021. The preliminary analysis of cave lion cubs Panthera spelaea (Goldfuss, 1810) from the permafrost of Siberia. Quaternary, 4:1-16. https://doi.org/10.3390/quat4030024
Bravo-Cuevas, V.M., Jimenez-Hidalgo, E., Cabral-Perdomo, M.A., and Priego-Vargas, J. 2013. Taxonomy and notes on the paleobiology of the late Pleistocene (Rancholabrean) antilocaprids (Mammalia, Artiodactyla, Antilocapridae) from the state of Hidalgo, central Mexico. Revista Mexicana De Ciencias Geologicas, 30:601-613.
Campbell, K.E., Jr. and Tonni, E.P. 1981. Preliminary observations on the paleobiology and evolution of teratorns (Aves: Teratornithidae). Journal of Vertebrate Paleontology, 1:265-272. https://doi.org/10.1080/02724634.1981.10011901
Campbell, K.E., Jr. and Tonni, E.P. 1983. Size and locomotion in teratorns (Aves: Teratornithidae). The Auk, 100:390-403.
Campbell, R.M., Vinas, G., Henneberg, M., and Diogo, R. 2021. Visual depictions of our evolutionary past: a broad case study concerning the need for quantitative methods of soft tissue reconstruction and art-science collaborations. Frontiers in Ecology and Evolution, 9:639048. https://doi.org/10.3389/fevo.2021.639048
Carbone, C., Maddox, T., Funston, P.J., Mills, M.G.L., Grether, G.F., and Van Valkenburgh, B. 2009. Parallels between playbacks and Pleistocene tar seeps suggest sociality in an extinct sabretooth cat, Smilodon. Biology Letters, 5:81-85. https://doi.org/10.1098/rsbl.2008.0526
Cardich, A. 1987. Arqueología de Los Toldos y El Ceibo (provincia de Santa Cruz, Argentina). Estudios Atacameños, 8:95-113. https://doi.org/10.22199/S07181043.1987.0008.00008
Caro, T. 2013. The colours of extant mammals. Seminars in Cell & Developmental Biology, 24:542-552. https://doi.org/10.1016/j.semcdb.2013.03.016
Chatterjee, S., Templin, R.J., and Campbell, K.E., Jr. 2007. The aerodynamics of Argentavis, the world’s largest flying bird from the Miocene of Argentina. Proceedings of the National Academy of Sciences, 104:12398-12403.
Chimento, N.R. and Agnolin, F.L. 2017. The fossil American lion (Panthera atrox) in South America: Palaeobiogeographical implications. Comptes Rendus Palevol, 1-16. https://doi.org/10.1016/j.crpv.2017.06.009
Christiansen, P. and Harris, J.M. 2009. Craniomandibular morphology and phylogenetic affinities of Panthera atrox: Implications for the evolution and paleobiology of the lion lineage. Journal of Vertebrate Paleontology, 29:934-945. https://doi.org/10.1671/039.029.0314
Conway, J., Kosemen, C.M., and Naish, D. 2012. All Yesterdays. lulu.com.
Cuff, A.R., Goswami, A., and Hutchinson, J.R. 2017. Reconstruction of the musculoskeletal system in an extinct lion. Palaeontologia Electronica, 20.2.23A:1-20. https://doi.org/10.26879/688
Cunningham, J.A. 2021. The use of photogrammetric fossil models in palaeontology education. Evolution: Education and Outreach, 14:1-7. https://doi.org/10.1186/s12052-020-00140-w
Davis, M. 2017. What North America's skeleton crew of megafauna tells us about community disassembly. Proceedings of the Royal Society B, 284:20162116. https://doi.org/10.1098/rspb.2016.2116
Davis, M. 2019. Virtual reality, augmented reality, and real reality: thinking holistically about the spectrum of immersive technologies in museums. PaleoBios, 36(Supplement 1):118. https://doi.org/10.5070/P9361044177
Davis, M. 2020. Towards frictionless augmented reality. American Alliance of Museums blog. Accessed 24 August 2021. https://www.aam-us.org/2020/06/15/towards-frictionless-augmented-reality/
Davis, M. 2021. Tar AR: bringing the past to life in place-based augmented reality science learning. Presented at the MuseWeb. https://mw21.museweb.net/proposal/tar-ar-bringing-the-past-to-life-in-place-based-augmented-reality-science-learning/
Davis, M., Faurby, S., and Svenning, J.C. 2018. Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proceedings of the National Academy of Sciences, 115:11262-11267. https://doi.org/10.1073/pnas.1804906115
De Pastino, B. 2015. First Columbian mammoth with hair discovered on California farm. Western Digs Blog. Accessed 8 August 8 2021. http://westerndigs.org/first-columbian-mammoth-with-hair-discovered-on-california-farm/
Dilenschneider, C. 2017. People trust museums more than newspapers. Here is why that matters right now (DATA). Colleen Dilen Blog. 26 April 2017. Accessed 18 September 2021. https://www.colleendilen.com/2017/04/26/people-trust-museums-more-than-newspapers-here-is-why-that-matters-right-now-data/
Dooley, A.C., Scott, E., Green, J., Springer, K.B., Dooley, B.S., and Smith, G.J. 2019. Mammut pacificus sp. nov., a newly recognized species of mastodon from the Pleistocene of western North America. PeerJ, 7:e6614. https://doi.org/10.7717/peerj.6614
Dundas, R.G. 1999. Quaternary records of the dire wolf, Canis dirus, in North and South America. Boreas, 28:375-385.
Fariña, R.A. 2002. Megatherium, el pelado: sobre la apariencia de los grandes perezosos (Mammalia; Xenarthra) cuaternarios. Ameghiniana, 39:241-244.
Figueirido, B., Perez-Claros, J.A., Torregrosa, V., Martin-Serra, A., and Palmqvist, P. 2010. Demythologizing Arctodus simus, the “short-faced” long-legged and predaceous bear that never was. Journal of Vertebrate Paleontology, 30:262-275. https://doi.org/10.1080/02724630903416027
Fisher, H.I. 1945. Locomotion in the fossil vulture Teratornis. American Midland Naturalist, 33:725-742. https://doi.org/10.2307/2421186
Flores, D., Eldridge, E.I., Elminowski, E.E., Dickinson, E., and Hartstone-Rose, A. 2020. The howl of Rancho La Brea: Comparative anatomy of modern and fossil canid hyoid bones. Journal of Morphology, 281:646-652. https://doi.org/10.1002/jmor.21130
Fowler, N.L., Spady, T.J., Wang, G., Leopold, B.D., and Belant, J.L. 2021. Denning, metabolic suppression, and the realisation of ecological opportunities in Ursidae. Mammal Review, 51:465-481. https://doi.org/10.1111/mam.12246
Fox-Dobbs, K., Stidham, T.A., Bowen, G.J., Emslie, S.D., and Koch, P.L. 2006. Dietary controls on extinction versus survival among avian megafauna in the late Pleistocene. Geology, 34:685-4. https://doi.org/10.1130/G22571.1
Friscia, A.R., Van Valkenburgh, B., Spencer, L., and Harris, J.M. 2008. Chronology and spatial distribution of large mammal bones in Pit 91, Rancho La Brea. Palaios, 23:35-42. https://doi.org/10.2110/palo.2005.p05-143r
Gelt, J., Nakano, C., and Vankin, D. 2021. What is L.A.’s most beloved landmark? We put it to a vote. Los Angeles Times. 8 September 2021. Accessed 15 September 2021. https://www.latimes.com/entertainment-arts/story/2021-09-08/los-angeles-landmarks-architecture
Glass, J.R., Davis, M., Walsh, T.J., Sargis, E.J., and Caccone, A. 2016. Was frozen mammoth or giant ground sloth served for dinner at The Explorers Club? PLoS ONE, 11:e0146825. https://doi.org/10.1371/journal.pone.0146825
Grazhdankin, D. and Seilacher, A. 2002. Underground Vendobionta from Namibia. Palaeontology, 45:57-78. https://doi.org/10.1111/1475-4983.00227
Grimm, D. 2021. The legendary dire wolf may not have been a wolf at all. Science News. 13 January 2021. Accessed 13 August 2021. https://doi.org/10.1126/science.abg5607
Gurche, J. 2013. Shaping Humanity. Yale University Press, New Haven.
Guthrie, R.D. 1990. Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe. The University of Chicago Press, Chicago.
Hallin, K.F. 1989. Wisconsin's Ice Age tuskers: Ice Age elephants and mastodonts. Wisconsin Academy Review, 35:6-10.
Hallin, K.F. 1983. Hair of the American mastodon indicates an adaptation to a semiaquatic habitat. American Zoologist, 23:949.
Hallin, K.F. and Gabriel, D. 1981. The first specimen of mastodon hair. Geological Society of America 34th Annual Meeting of the Rocky Mountain Section, Abstracts with Program, 13(4):199.
Hartman, S. 2013. Teratornis merriami. Skeletal Drawing. Accessed 30 June 2020. https://www.skeletaldrawing.com/theropods/teratornis
Haynes, G. 1991. Mammoths, Mastodonts, and Elephants: Biology, Behavior, and the Fossil Record. Cambridge University Press, New York.
Haynes, G. and Klimowicz, J. 2003. Mammoth (Mammuthus spp.) and American mastodont (Mammut americanum) bonesites: what do the differences mean? Deinsea, 9:185-204.
Heintzman, P.D., Zazula, G.D., Cahill, J.A., Reyes, A.V., MacPhee, R.D.E., and Shapiro, B. 2015. Genomic data from extinct North American Camelops revise camel evolutionary history. Molecular Biology and Evolution, 32:2433-2440. https://doi.org/10.1093/molbev/msv128
Henderson, D. 2013. Bringing Lucy to Life. Answers in Genesis. 1 January 2013. Accessed 23 September 2021. https://answersingenesis.org/human-evolution/lucy/bringing-lucy-to-life/
Herrick, I., Sinatra, G.M., Kennedy, A.A.U., Nye, B.D., Swartout, W., and Lindsey, E. 2021. Tar AR: Connecting the past with the present in informal science learning. Presented at the AERA Annual Meeting 2021. https://par.nsf.gov/servlets/purl/10282905
Hertel, F. 1995. Ecomorphological indicators of feeding behavior in recent and fossil raptors. The Auk, 112:890-903.
Hoppe, K.A. 2004. Late Pleistocene mammoth herd structure, migration patterns, and Clovis hunting strategies inferred from isotopic analyses of multiple death assemblages. Paleobiology, 30:129-145.
Howard, H. 1930. A census of the Pleistocene birds of Rancho La Brea from the collections of the Los Angeles Museum. The Condor, 32:81-88.
Ice Age Giants. 2013. Ice Age Giants. BBC. https://www.bbc.co.uk/programmes/p018c9fm
Kamilar, J.M. and Bradley, B.J. 2011. Countershading is related to positional behavior in primates. Journal of Zoology, 283:227-233.
Kennedy, A.A.U., Thacker, I., Nye, B.D., Sinatra, G.M., Swartout, W., and Lindsey, E. 2021. Promoting interest, positive emotions, and knowledge using augmented reality in a museum setting. International Journal of Science Education, Part B, 1-17. https://doi.org/10.1080/21548455.2021.1946619
Kurtén, B. and Anderson, E. 1980. Pleistocene mammals of North America. Columbia University Press.
Larramendi, A. 2016. Shoulder height, body mass, and shape of proboscideans. Acta Palaeontologica Polonica, 61:537-574. https://doi.org/10.4202/app.00136.2014
Lautenschlager, S. and Rücklin, M. 2014. Beyond the print--virtual paleontology in science publishing, outreach, and education. Journal of Paleontology, 88:727-734. https://doi.org/10.1666/13-085
Lopes, R.P., Frank, H.T., Buchmann, F.S. de C., and Caron, F. 2016. Megaichnus igen. nov.: giant paleoburrows attributed to extinct Cenozoic mammals from South America. Ichnos, 24:133-145. https://doi.org/10.1080/10420940.2016.1223654
Lull, R.S. 1929. A Remarkable Ground Sloth. Memoirs of the Peabody Museum of Natural History, Yale University, 3:1-39.
Mateus, S. and Tschopp, E. 2017. Scientific illustration and reconstruction of a skull of the diplodocid sauropod dinosaur Galeamopus. Journal of Paleontological Techniques, 17:1-11. https://www.jpaleontologicaltechniques.org/pasta3/JPT%20N17/Bulletin.html
Mayr, G. 2009. Paleogene Fossil Birds. Springer, Berlin.
McAfee, R.K. 2009. Reassessment of the cranial characters of Glossotherium and Paramylodon (Mammalia: Xenarthra: Mylodontidae). Zoological Journal of the Linnean Society, 155:885-903. https://doi.org/10.1111/j.1096-3642.2008.00468.x
McCall, S., Naples, V., and Martin, L. 2003. Assessing behavior in extinct animals: was Smilodon social? Brain, Behavior and Evolution, 61:159-164. https://doi.org/10.1159/000069752
McDonald, H.G. 2003. Sloth remains from North American caves and associated karst features, p. 1-16. In Schubert, B.W., Mead, J.I., Graham, R.W. (eds.), Ice Age Cave Faunas of North America. Indiana University Press, Bloomington.
McDonald, H.G. 2007. Biomechanical inferences of locomotion in ground sloths: integrating morphological and track data. New Mexico Museum of Natural History and Science Bulletin, 42:201-208.
McDonald, H.G. 2012. Evolution of the pedolateral foot in ground sloths: patterns of change in the astragalus. Journal of Mammalian Evolution, 19:209-215.
McDonald, J.N. 1981. North American Bison. University of California Press, Berkeley.
McDonald, J.N. and Lammers, G.E. 2002. Bison antiquus from Kenora, Ontario, and notes on the evolution of North American Holocene Bison. Smithsonian Contributions to Paleobiology, 93:83-97.
Meachen, J.A., Janowicz, A.C., Avery, J.E., and Sadleir, R.W. 2014. Ecological changes in coyotes (Canis latrans) in response to the ice age megafaunal extinctions. PLoS ONE, 9:e116041. https://doi.org/10.1371/journal.pone.0116041
Meloro, C., Elton, S., Louys, J., Bishop, L.C., and Ditchfield, P. 2013. Cats in the forest: predicting habitat adaptations from humerus morphometry in extant and fossil Felidae (Carnivora). Paleobiology, 39:323-344.
Merriam, C.H. 1914. Descriptions of thirty apparently new grizzly and brown bears from North America. Proceedings of the Biological Society of Washington, 27:173-196.
Metcalf, J.L., Turney, C., Barnett, R., Martin, F., Bray, S.C., Vilstrup, J.T., Orlando, L., Salas-Gismondi, R., Loponte, D., Medina, M., De Nigris, M., Civalero, T., Fernández, P.M., Gasco, A., Duran, V., Seymour, K.L., Otaola, C., Gil, A., Paunero, R., Prevosti, F.J., Bradshaw, C.J.A., Wheeler, J.C., Borrero, L., Austin, J.J., and Cooper, A. 2016. Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the Last Deglaciation. Science Advances, 2:e1501682. https://doi.org/10.1126/sciadv.1501682
Naish, D. 2017. Palaeoart memes and the unspoken status quo in palaeontological popularization. Tetrapod Zoology.10 February 2017. Accessed 22 September 2021. https://blogs.scientificamerican.com/tetrapod-zoology/palaeoart-memes-and-the-unspoken-status-quo-in-palaeontological-popularization/
Naples, V.L. 1987. Reconstruction of cranial morphology and analysis of function in the Pleistocene ground sloth Nothrotheriops shastense (Mammalia, Megatheriidae). Natural History Museum of Los Angeles County, Contributions in Science, 389:1-21.
Naples, V.L. 1989. The feeding mechanism in the Pleistocene ground sloth, Glossotherium. Natural History Museum of Los Angeles County, Contributions in Science, 415:1-23.
Nitiu, D.S., Mallo, A., Saparrat, M., and Santa Cruz, M.G. 2016. Survey of the state of conservation of the Mylodon listai (Xenarthra-Mylodontidae) skin fragment from the Pleistocene of Argentina kept at the Museum of La Plata (Argentina). Ge-Conservación, 10:44-53.
Orlando, L. 2019. An ancient DNA perspective on horse evolution, p. 325-351. In Lindqvist, C. and Rajora, O.P. (eds.), Paleo-Genomics. Springer, Cham. https://doi.org/10.1007/13836_2018_23
Orlando, L. 2020. The Evolutionary and Historical Foundation of the Modern Horse: Lessons from Ancient Genomics. Annual Review of Genetics, 54:563-581. https://doi.org/10.1146/annurev-genet-021920-011805
Palmqvist, P. and Vizcaíno, S.F. 2003. Ecological and reproductive constraints of body size in the gigantic Argentavis magnificens (Aves, Theratornithidae) from the Miocene of Argentina. Ameghiniana, 40:379-385.
Pérez-Crespo, V.A., Barrón-Ortiz, C.R., Arroyo-Cabrales, J., Morales-Puente, P., Cienfuegos-Alvarado, E., and Otero, F.J. 2016. Preliminary data on the diet and habitat preferences of Capromeryx mexicana (Mammalia: Antilocapridae) from the late Pleistocene of Cedral, San Luis Potosí, Mexico. The Southwestern Naturalist, 61:152-155.
Perri, A.R., Mitchell, K.J., Mouton, A., lvarez-Carretero, S.A.X., Hulme-Beaman, A., Haile, J., Jamieson, A., Meachen, J., Lin, A.T., Schubert, B.W., Ameen, C., Antipina, E.E., Bover, P., Brace, S., Carmagnini, A., e, C.C.X., Castruita, J.A.S., Chatters, J.C., Dobney, K., Reis, M., Evin, A., Gaubert, P., Gopalakrishnan, S., Gower, G., Heiniger, H., Helgen, K.M., Kapp, J., Kosintsev, P.A., Linderholm, A., Ozga, A.T., Presslee, S., Salis, A.T., Saremi, N.F., Shew, C., Skerry, K., Taranenko, D.E., Thompson, M., Sablin, M.V., Kuzmin, Y.V., Collins, M.J., Sinding, M.-H.S., Gilbert, M.T.P., Stone, A.C., Shapiro, B., Valkenburgh, B., Wayne, R.K., Larson, G., Cooper, A., and Frantz, L.A.F. 2021. Dire wolves were the last of an ancient New World canid lineage. Nature, 1-9. https://doi.org/10.1038/s41586-020-03082-x
Pruvost, M., Bellone, R., Benecke, N., Sandoval-Castellanos, E., Cieslak, M., Kuznetsova, T., Morales-Muñiz, A., O’Connor, T., Reissmann, M., Hofreiter, M., and Ludwig, A. 2011. Genotypes of predomestic horses match phenotypes painted in Paleolithic works of cave art. Proceedings of the National Academy of Sciences of the United States of America, 108:18626-18630. https://doi.org/10.1073/pnas.1108982108
Quain, J.R. 2019. What did T. rex look like? A new exhibit has the “ultimate predator” in feathers. Gizmodo. 3 September 2019. Acessed 22 September 2021. https://gizmodo.com/what-did-t-rex-look-like-a-new-exhibit-has-the-ultima-1833164920
Rahman, I.A., Adcock, K., and Garwood, R.J. 2012. Virtual fossils: a new resource for science communication in paleontology. Evolution: Education and Outreach, 5:635-641. https://doi.org/10.1007/s12052-012-0458-2
Randall, R.N., Radford, C.E., Roof, K.A., Natarajan, D.K., and Gaucher, E.A. 2016. An experimental phylogeny to benchmark ancestral sequence reconstruction. Nature Communications, 7:12847. https://doi.org/10.1038/ncomms12847
Ross, R.M., Duggan-Haas, D., and Allmon, W.D. 2018. The posture of Tyrannosaurus rex: why do student views lag behind the science? Journal of Geoscience Education, 61:145-160. https://doi.org/10.5408/11-259.1
Scott, E. 1985. They live again: sixty years of sculpture in Hancock Park. Terra: the quarterly magazine of the Natural History Museum of Los Angeles County, Natural History Museum of Los Angeles County, 24:23-29.
Scott, E. 1988. Treasures of the tar pits: Mount up! The skeletons of Rancho La Brea on display. Terra: the quarterly magazine of the Natural History Museum of Los Angeles County, Natural History Museum of Los Angeles County, 26:11-13.
Shapiro, B., Drummond, A.J., Rambaut, A., Wilson, M.C., Matheus, P.E., Sher, A.V., Pybus, O.G., Gilbert, M.T.P., Barnes, I., Binladen, J., Willerslev, E., Hansen, A.J., Baryshnikov, G.F., Burns, J.A., Davydov, S., Driver, J.C., Froese, D.G., Harington, C.R., Keddie, G., Kosintsev, P., Kunz, M.L., Martin, L.D., Stephenson, R.O., Storer, J., Tedford, R., Zimov, S., and Cooper, A. 2004. Rise and fall of the Beringian steppe bison. Science, 306:1561-1565. https://doi.org/10.1126/science.1101074
Shaw, C.A. 1988. Body by Fischer and Bessom. Terra: the quarterly magazine of the Natural History Museum of Los Angeles County, 26:13-16.
Shaw, C.A. and Ware, C.S. 2018. Smilodon paleopathology: a summary of research at Rancho La Brea, p. 196-206. In Werdelin, L., McDonald, H.G., Shaw, C.A. (eds.), Smilodon. Baltimore.
Snyder, K. 2019. T. rex: The Ultimate Predator opens at the American Museum of Natural History. AMNH press release. https://www.amnh.org/content/download/242378/4173605/file/T-Rex-Ultimate-Predator-Opens-AMNH-Press-Release.pdf
Stock, C. 1930. Rancho La Brea: a record of Pleistocene life in California. Los Angeles Museum of History, Science and Art Science Series, 1:1-84.
Stock, C. and Harris, J.M. 1992. Rancho La Brea, 7 ed. Natural History Museum of Los Angeles County, Los Angeles.
Trivedi, V. 2019. Virtual and Augmented Reality. How to Speak Tech. Berkeley, 157-173. https://doi.org/10.1007/978-1-4842-4324-4_17
Tseng, Z.J., Wang, X., Slater, G.J., Takeuchi, G.T., Li, Q., Liu, J., and Xie, G. 2014. Himalayan fossils of the oldest known pantherine establish ancient origin of big cats. Proceedings of the Royal Society B, 281:20132686. https://doi.org/10.1098/rspb.2013.2686
Vela-Vargas, I.M., Jorgenson, J.P., González-Maya, J.F., and Koprowski, J.L. 2021. Tremarctos ornatus (Carnivora: Ursidae). Mammalian Species, 53:78-94. https://doi.org/10.1093/mspecies/seab008
Vinther, J. 2020. Reconstructing vertebrate paleocolor. Annual Review of Earth and Planetary Sciences, 48:345-375. https://doi.org/10.1146/annurev-earth-073019-045641
Wang, X. and Tedford, R.H. 2008. Dogs: Their fossil relatives and evolutionary history. Columbia University Press, New York.
Webb, S.D. 1965. The osteology of Camelops. Bulletin of the Los Angeles County Museum, Science, 1:1-65.
White, R. and Morgan, G.S. 2011. Capromeryx (Artiodactyla: Antilocapridae) from the Rancholabrean Tramperos Creek fauna, Union County, New Mexico, with a review of the occurrence and paleobiology of Capromeryx in the Rancholabrean of New Mexico. New Mexico Museum of Natural History and Science Bulletin, 53:641-651.
Wilmers, C.C., Stahler, D.R., Crabtree, R.L., Smith, D.W., and Getz, W.M. 2003. Resource dispersion and consumer dominance: scavenging at wolf- and hunter-killed carcasses in Greater Yellowstone, USA. Ecology Letters, 6:996-1003. https://doi.org/10.1046/j.1461-0248.2003.00522.x
Wilson, M.C., Hills, L.V., and Shapiro, B. 2008. Late Pleistocene northward-dispersing Bison antiquus from the Bighill Creek Formation, Gallelli Gravel Pit, Alberta, Canada, and the fate of Bison occidentalis. Canadian Journal of Earth Sciences, 45:827-859. https://doi.org/10.1139/E08-027
Witton, M.P. 2020. The “palaeontological folklore” of mastodon hair. Mark Witton Blog. 16 August 2020. Accessed 23 August 2021. https://markwitton-com.blogspot.com/2020/08/the-palaeontological-folklore-of.html
Witton, M.P. 2017a. Scientists: please pay more attention to palaeoart. Mark Witton Blog. 17 February 2017 Accessed 10 June 2020a. http://markwitton-com.blogspot.com/2017/02/scientists-please-pay-more-attention-to.html
Witton, M.P. 2017b. Scientist-palaeoartist collaborations - what palaeontologists can, and probably should, critique when reviewing palaeoart, Mark Witton Blog. 31 March 2017. Accessed 22 September 2021b. http://markwitton-com.blogspot.com/2017/03/scientist-palaeoartist-collaborations.html
Witton, M.P. 2019. We need to talk about teratorns. Mark Witton Blog. 30 August 2019. Accessed 24 August 2021. https://markwitton-com.blogspot.com/2019/08/we-need-to-talk-about-teratorns.html
Witton, M.P., Naish, D., and Conway, J. 2014. State of the Palaeoart. Palaeontologia Electronica, 17:5E. https://doi.org/10.26879/145
Woodard, G.D. and Marcus, L.F. 1973. Rancho La Brea fossil deposits: a re-evaluation from stratigraphic and geological evidence. Journal of Paleontology, 47:54-69.
Yamaguchi, N., Cooper, A., Werdelin, L., and Macdonald, D.W. 2004. Evolution of the mane and group‐living in the lion (Panthera leo): a review. Journal of Zoology, 263:329-342. https://doi.org/10.1017/S0952836904005242
Zazula, G.D., MacPhee, R.D.E., Metcalfe, J.Z., Reyes, A.V., Brock, F., Druckenmiller, P.S., Groves, P., Harington, C.R., Hodgins, G.W.L., Kunz, M.L., Longstaffe, F.J., Mann, D.H., McDonald, H.G., Nalawade-Chavan, S., and Southon, J.R. 2014. American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proceedings of the National Academy of Sciences of the United States of America, 111:18460-18465. https://doi.org/10.1073/pnas.1416072111
Ziegler, M.J., Perez, V.J., Pirlo, J., Narducci, R.E., Moran, S.M., Selba, M.C., Hastings, A.K., Vargas-Vergara, C., Antonenko, P.D., and MacFadden, B.J. 2020. Applications of 3D paleontological data at the Florida Museum of Natural History. Frontiers in Earth Science, 8:600696. https://doi.org/10.3389/feart.2020.600696

