 Reconstructing Dunkleosteus terrelli (Placodermi: Arthrodira): A new look for an iconic Devonian predator
Reconstructing Dunkleosteus terrelli (Placodermi: Arthrodira): A new look for an iconic Devonian predator
Article number: 27.3.a45
https://doi.org/10.26879/1343
Copyright Society for Vertebrate Paleontology, September 2024
Author biography
Plain-language and multi-lingual abstracts
PDF version
Appendices
Submission: 22 September 2023. Acceptance: 4 August 2024.
ABSTRACT
Dunkleosteus is a widely-recognized prehistoric organism, yet its life appearance, paleobiology, and even basic morphology remain poorly understood. A new reconstruction of D. terrelli is presented here based on examination of complete, three-dimensionally mounted dermal skeletons and a review of available paleontological evidence. Despite the post-thoracic body of D. terrelli being poorly known, its morphology and body shape can be constrained based on preserved elements and conserved anatomical patterns seen both within arthrodires and across fishes more broadly. Trunk armor proportions, estimated body length, and the locations of the fin bases suggest D. terrelli had a relatively stout, deep trunk. Its trunk armor is apomorphically deep among arthrodires, resulting in a body shape reminiscent of other pelagic vertebrates (lamnids, thunnins, ichthyosaurs). The anterior trunk is stiffened due to the interlocking ventral shield plates and fused spine restricting lateral motion, and its anatomy suggests extremely large lateral trunk muscles and a well-developed horizontal septum, compatible with thunniform swimming. Body depth is positively allometric in D. terrelli, resembling other arthrodires. Eubrachythoracid arthrodires likely had incomplete lateral lines. The pectoral fin base of Dunkleosteus is located at an extreme anterior position on the body, and the pelvic girdle is unusually small. Arthrodires appear more disparate in body shape than previously assumed, and many taxa may have been well-adapted to active nektonic life, though their rigid dermal armor and generally stocky bodies imply swimming kinematics unlike most living fishes. Many questions about their biology and biomechanics remain unanswered, representing ideal targets for future research.
Russell K. Engelman, Department of Biology, Case Western Reserve University, 10900 Euclid Ave., Cleveland, Ohio 44106, U.S.A., neovenatoridae@gmail.com
Keywords: paleoart; reconstruction; fish; arthrodire; placoderm; thunniform
Final citation: Engelman, Russell K. 2024. Reconstructing Dunkleosteus terrelli (Placodermi: Arthrodira): A new look for an iconic Devonian predator. Palaeontologia Electronica, 27(3):a45.
https://doi.org/10.26879/1343
palaeo-electronica.org/content/2024/5307-dunkleosteus-reconstruction
Copyright: September 2024 Society for Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0.
INTRODUCTION
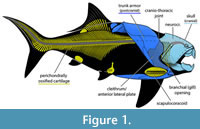 The Late Devonian (Famennian) arthrodire placoderm Dunkleosteus terrelli is a flagship taxon for Paleozoic vertebrate paleontology. Best known from the Cleveland Shale Member of the Ohio Shale (hereafter the Cleveland Shale) of Ohio, USA, this fish has fascinated people for over 150 years due to its blade-like gnathal plates and extensive dermal armor (Newberry, 1873). However, in spite of this, Dunkleosteus terrelli remains poorly understood as an organism. This is primarily because despite its heavily ossified skull and trunk armor, the endoskeleton of this taxon is mostly composed of cartilage (with a thin, external layer of perichondral bone; Johanson et al., 2019; van Mesdag et al., 2020), resulting in the dermal armor being the only elements usually preserved in the fossil record (Figure 1). Some specimens occasionally preserve endoskeletal elements, including the pectoral fin (Carr et al., 2010), synarcual (Johanson et al., 2013), anteriormost vertebrae (Johanson et al., 2019), and pelvic girdle (present study), but the post-pelvic body remains unknown. This greatly limits available morphological information for this taxon (Heintz, 1932; Carr et al., 2010; Ferrón et al., 2017a; Engelman, 2023a, 2023b), and has resulted in a large number of uncertainties and misconceptions regarding its morphology, size, and paleobiology.
The Late Devonian (Famennian) arthrodire placoderm Dunkleosteus terrelli is a flagship taxon for Paleozoic vertebrate paleontology. Best known from the Cleveland Shale Member of the Ohio Shale (hereafter the Cleveland Shale) of Ohio, USA, this fish has fascinated people for over 150 years due to its blade-like gnathal plates and extensive dermal armor (Newberry, 1873). However, in spite of this, Dunkleosteus terrelli remains poorly understood as an organism. This is primarily because despite its heavily ossified skull and trunk armor, the endoskeleton of this taxon is mostly composed of cartilage (with a thin, external layer of perichondral bone; Johanson et al., 2019; van Mesdag et al., 2020), resulting in the dermal armor being the only elements usually preserved in the fossil record (Figure 1). Some specimens occasionally preserve endoskeletal elements, including the pectoral fin (Carr et al., 2010), synarcual (Johanson et al., 2013), anteriormost vertebrae (Johanson et al., 2019), and pelvic girdle (present study), but the post-pelvic body remains unknown. This greatly limits available morphological information for this taxon (Heintz, 1932; Carr et al., 2010; Ferrón et al., 2017a; Engelman, 2023a, 2023b), and has resulted in a large number of uncertainties and misconceptions regarding its morphology, size, and paleobiology.
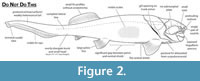 Reconstructions of Dunkleosteus vary wildly in their anatomy, often differing dramatically in fin position/morphology, head shape, location of the gill opening, distribution of integument and soft tissues, and body length/shape (Figure 2). This variability is driven by artistic license, believed to be afforded by the assumption that “nothing is known of Dunkleosteus beyond the ‘head’” (often mistakenly including the trunk armor as part of the head), leaving the rest of the animal’s anatomy up for debate. In many cases this results in depictions of Dunkleosteus whose anatomy directly contradicts features observable from fossils (Figure 2), such as inaccurately treating the trunk armor as an operculum and/or part of the head, restoring Dunkleosteus with a pointed shark-like snout, or dislocating the pectoral fin from its known position within the pectoral fenestra (Carr et al., 2010). Reconstructions of Dunkleosteus are often claimed to be modeled after other, more complete arthrodires (especially Coccosteus cuspidatus; see Heintz, 1932; and discussion in Ferrón et al., 2017a). However, there has been no formal attempt to determine how the comparative anatomy of these forms map onto taxa like Dunkleosteus.
Reconstructions of Dunkleosteus vary wildly in their anatomy, often differing dramatically in fin position/morphology, head shape, location of the gill opening, distribution of integument and soft tissues, and body length/shape (Figure 2). This variability is driven by artistic license, believed to be afforded by the assumption that “nothing is known of Dunkleosteus beyond the ‘head’” (often mistakenly including the trunk armor as part of the head), leaving the rest of the animal’s anatomy up for debate. In many cases this results in depictions of Dunkleosteus whose anatomy directly contradicts features observable from fossils (Figure 2), such as inaccurately treating the trunk armor as an operculum and/or part of the head, restoring Dunkleosteus with a pointed shark-like snout, or dislocating the pectoral fin from its known position within the pectoral fenestra (Carr et al., 2010). Reconstructions of Dunkleosteus are often claimed to be modeled after other, more complete arthrodires (especially Coccosteus cuspidatus; see Heintz, 1932; and discussion in Ferrón et al., 2017a). However, there has been no formal attempt to determine how the comparative anatomy of these forms map onto taxa like Dunkleosteus.
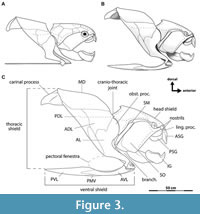 Heintz (1931b, 1932) published a formal reconstruction of the armor of Dunkleosteus terrelli, which has not been superseded by later authors and has served as the basis for most subsequent reconstructions. However, when compared to well-preserved, three-dimensionally mounted specimens of D. terrelli in the Cleveland Museum of Natural History (CMNH), this reconstruction significantly differs in its proportions and is missing several plates (Figure 3). Heintz (1932: p. 115–116, 123) was aware of the CMNH specimens but explicitly did not consider them in his study to avoid scooping his colleague Jesse Hyde, who at that time was working on a series of monographs describing the CMNH Cleveland Shale collection. Instead, Heintz’s study focused on Dunkleosteus material from the American Museum of Natural History (AMNH), which are mostly isolated plates and/or from juveniles (Hussakof, 1905: p. 27; 1906; Dean, 1909a; Heintz, 1932: p. 116; Engelman, pers. obs.) limiting the amount of data Heintz had on the proportions of adult individuals to inform his reconstruction. Hyde never completed his intended monographs on the Cleveland Shale fauna (Morris, 1937: 168–169), and the morphology of the near-complete Dunkleosteus specimens at the CMNH remains almost undescribed. Certain features of these specimens have been briefly mentioned in later studies (Dunkle and Bungart, 1942, 1946; Heintz, 1968; Anderson and Westneat, 2007), though primarily in relation to other topics of interest. Other studies on Dunkleosteus were agnostic to this taxon’s comparative anatomy and/or body shape (Anderson and Westneat, 2007, 2009; Carr, 2010; Snively et al., 2010; Ferrón et al., 2017a) or focus on specimens preserving endochondral elements (Carr et al., 2010; Johanson et al., 2013; Johanson et al., 2019). Heintz’s (1932) monograph remains one of the most important papers ever published on Dunkleosteus, but additional material collected over the last 90 years has substantially expanded our understanding of this taxon’s anatomy.
Heintz (1931b, 1932) published a formal reconstruction of the armor of Dunkleosteus terrelli, which has not been superseded by later authors and has served as the basis for most subsequent reconstructions. However, when compared to well-preserved, three-dimensionally mounted specimens of D. terrelli in the Cleveland Museum of Natural History (CMNH), this reconstruction significantly differs in its proportions and is missing several plates (Figure 3). Heintz (1932: p. 115–116, 123) was aware of the CMNH specimens but explicitly did not consider them in his study to avoid scooping his colleague Jesse Hyde, who at that time was working on a series of monographs describing the CMNH Cleveland Shale collection. Instead, Heintz’s study focused on Dunkleosteus material from the American Museum of Natural History (AMNH), which are mostly isolated plates and/or from juveniles (Hussakof, 1905: p. 27; 1906; Dean, 1909a; Heintz, 1932: p. 116; Engelman, pers. obs.) limiting the amount of data Heintz had on the proportions of adult individuals to inform his reconstruction. Hyde never completed his intended monographs on the Cleveland Shale fauna (Morris, 1937: 168–169), and the morphology of the near-complete Dunkleosteus specimens at the CMNH remains almost undescribed. Certain features of these specimens have been briefly mentioned in later studies (Dunkle and Bungart, 1942, 1946; Heintz, 1968; Anderson and Westneat, 2007), though primarily in relation to other topics of interest. Other studies on Dunkleosteus were agnostic to this taxon’s comparative anatomy and/or body shape (Anderson and Westneat, 2007, 2009; Carr, 2010; Snively et al., 2010; Ferrón et al., 2017a) or focus on specimens preserving endochondral elements (Carr et al., 2010; Johanson et al., 2013; Johanson et al., 2019). Heintz’s (1932) monograph remains one of the most important papers ever published on Dunkleosteus, but additional material collected over the last 90 years has substantially expanded our understanding of this taxon’s anatomy.
Engelman (2023a) presented a life reconstruction of Dunkleosteus terrelli in association with their research on this taxon’s body size (Appendix 1). That study did not go into detail on the anatomical basis for their reconstruction, instead deferring to a future manuscript devoted to that topic: the present contribution. This goal can be further broken down into three main objectives:
1) present additional evidence for the shorter, stockier body plan of Dunkleosteus terrelli proposed by Engelman (2023b), drawn from comparative anatomy and examination of original Dunkleosteus material rather than allometric size estimates.
2) provide a detailed overview of available evidence for the life appearance of Dunkleosteus, in the hope of providing a guide to paleoartists to help standardize depictions of this species.
3) highlight new research directions raised by this research and other recent studies suggesting many eubrachythoracid arthrodires had well-developed nektonic habits (Carr, 2010; Carr et al., 2010; Ferrón et al., 2017a; Jobbins et al., 2022; Engelman, 2023b; Jobbins et al., 2024), which have important implications for the swimming kinematics and general paleobiology of these animals.
MATERIALS AND METHODS
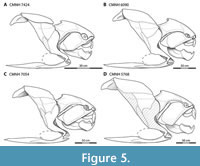
 The present reconstruction of Dunkleosteus (Figure 4) is largely based on specimens from the Cleveland Museum of Natural History (CMNH). These include five near-complete, three-dimensionally mounted heads and thoracic armors (CMNH 6194, CMNH 7424, CMNH 6090, CMNH 7054, and CMNH 5768, from smallest to largest), representing associated plates from single individuals (Figure 5). These specimens span an ontogenetic series ranging from juveniles (CMNH 6194 and CMNH 7424), to subadults or young adults (CMNH 6090 and CMNH 7054), to large, likely mature adults (CMNH 5768). CMNH 5768 is among the upper fifth percentile of the extensive CMNH hypodigm (600+ specimens) in terms of overall size, though a few slightly larger individuals up to 20-25% larger in linear dimensions (CMNH 5936, CMNH 7568, CMNH 9951) do exist (Engelman, 2023b). The sample size of D. terrelli is large enough to suggest these individuals are probably close to the maximal size achieved by D. terrelli in the Cleveland Shale (Mallon and Hone, 2024), though the average large individual is closer in size to CMNH 5768 than these “supergiants”. These factors suggest CMNH 5768 should be representative of the size and proportions of an adult D. terrelli. Anatomical observations were made either from the original material or 3D digital models uploaded by the Cleveland Museum of Natural History to Morphosource (https://www.morphosource.org/teams/000373825) and the University of Michigan Museum of Paleontology UMORF (rf.ummp.lsa.umich.edu/wp/specimen-data/?Model_ID=1336). Observations were made prior to the remounting of CMNH 5768, CMNH 6090, and CMNH 7424 in late 2022/2023.
The present reconstruction of Dunkleosteus (Figure 4) is largely based on specimens from the Cleveland Museum of Natural History (CMNH). These include five near-complete, three-dimensionally mounted heads and thoracic armors (CMNH 6194, CMNH 7424, CMNH 6090, CMNH 7054, and CMNH 5768, from smallest to largest), representing associated plates from single individuals (Figure 5). These specimens span an ontogenetic series ranging from juveniles (CMNH 6194 and CMNH 7424), to subadults or young adults (CMNH 6090 and CMNH 7054), to large, likely mature adults (CMNH 5768). CMNH 5768 is among the upper fifth percentile of the extensive CMNH hypodigm (600+ specimens) in terms of overall size, though a few slightly larger individuals up to 20-25% larger in linear dimensions (CMNH 5936, CMNH 7568, CMNH 9951) do exist (Engelman, 2023b). The sample size of D. terrelli is large enough to suggest these individuals are probably close to the maximal size achieved by D. terrelli in the Cleveland Shale (Mallon and Hone, 2024), though the average large individual is closer in size to CMNH 5768 than these “supergiants”. These factors suggest CMNH 5768 should be representative of the size and proportions of an adult D. terrelli. Anatomical observations were made either from the original material or 3D digital models uploaded by the Cleveland Museum of Natural History to Morphosource (https://www.morphosource.org/teams/000373825) and the University of Michigan Museum of Paleontology UMORF (rf.ummp.lsa.umich.edu/wp/specimen-data/?Model_ID=1336). Observations were made prior to the remounting of CMNH 5768, CMNH 6090, and CMNH 7424 in late 2022/2023.
 The CMNH mounts were created in the 1920–30s by retrodeforming complete but crushed and disarticulated individuals (Chapman et al., 2006; J. Tait, pers. comm. September 2022). The proportions of these mounts are consistent between specimens and resemble undistorted, three-dimensionally preserved Dunkleosteus material from Morocco (e.g., GPIT/PD/9; Rücklin and Clément, 2017: fig. 5), suggesting they accurately reflect the proportions and anatomy of this species with minimal taphonomic/reconstructive distortion. The numerous sutures, articulations, and contacts between elements of arthrodire armors greatly limit possible distortion in reconstructions, as incorrect retrodeformation would prevent proper articulation between plates (see discussion in Heintz, 1932: p. 153–158). Specifically, the paired cranio-thoracic joints between the head shield and anterior dorsolateral plates must be horizontally oriented or else their hinge-like motion is impossible (ibid). The spacing and orientation of these articulations determines the width and cross-sectional shape of the trunk armor, meaning if the head shield and anterior dorsolateral plates are preserved the general shape of the trunk armor can be reconstructed with a reasonable degree of accuracy (Heintz, 1931b, 1932; Young, 2005).
The CMNH mounts were created in the 1920–30s by retrodeforming complete but crushed and disarticulated individuals (Chapman et al., 2006; J. Tait, pers. comm. September 2022). The proportions of these mounts are consistent between specimens and resemble undistorted, three-dimensionally preserved Dunkleosteus material from Morocco (e.g., GPIT/PD/9; Rücklin and Clément, 2017: fig. 5), suggesting they accurately reflect the proportions and anatomy of this species with minimal taphonomic/reconstructive distortion. The numerous sutures, articulations, and contacts between elements of arthrodire armors greatly limit possible distortion in reconstructions, as incorrect retrodeformation would prevent proper articulation between plates (see discussion in Heintz, 1932: p. 153–158). Specifically, the paired cranio-thoracic joints between the head shield and anterior dorsolateral plates must be horizontally oriented or else their hinge-like motion is impossible (ibid). The spacing and orientation of these articulations determines the width and cross-sectional shape of the trunk armor, meaning if the head shield and anterior dorsolateral plates are preserved the general shape of the trunk armor can be reconstructed with a reasonable degree of accuracy (Heintz, 1931b, 1932; Young, 2005).
The plates of the CMNH mounts generally articulate properly, especially in the trunk armor. Some minor distortion can be identified when one specimen’s morphology differs from the remaining three, but this has little effect on the proportions or overall shape of the animal. Relevant exceptions are noted where appropriate (see “Ventral Shield” and “Potential Errors in Armor Reconstruction of CMNH 5768”). For the ventral shield, which was never physically retrodeformed in the CMNH specimens (see “Ventral Shield”), two attempts were made to retrodeform the ventral shield based on either visual approximation or using a curve modifier in Blender 3.5. This results in a ventral shield similar to CMNH Dunkleosteus material that preserves uncrushed plates and the three-dimensionally preserved dunkleosteoid Eastmanosteus calliaspis (Dennis-Bryan, 1987).
Additional observations were made on other less complete or unmounted Dunkleosteus specimens and isolated plates housed at the CMNH, as well as Dunkleosteus specimens at the American Museum of Natural History (AMNH), Cincinnati Museum Center (CMC), and the Natural History Museum of London (NHMUK) (Appendix 2). Observations of non -Dunkleosteus arthrodires were drawn from the previously published literature or specimens housed at the aforementioned institutions as well as the Field Museum of Natural History (FMNH), Musée d’Histoire naturelle de Miguasha (MNHM), National Museum of Scotland (NMS), or Royal Ontario Museum (ROM) (Appendix 2). Data for extant fishes were collected from the previously published literature or from examination of skeletonized or fluid-preserved specimens in the collections of the CMNH, Florida State Biodiversity Collection (FSBC), or Ohio State University Museum of Biodiversity (OSUM) (Appendix 2). Several additional morphometric comparative analyses on pre-pectoral length, pectoral fin base size, pre-pelvic length, snout-vent length, and caudal peduncle height were conducted using these specimens and the previously published literature. These analyses and details on their methodology were conducted in R 4.3.1 (R Core Team, 2020) and can be found in Appendix 3. Raw measurement data and references can be found in Appendix 4 and Appendix 5, respectively.
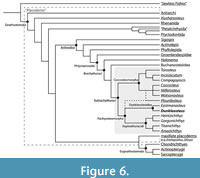 Dunkleosteus is phylogenetically bracketed by arthrodires known from body outlines or extensive post-thoracic remains (Figure 6). This means features consistently present across Arthrodira also likely occur in Dunkleosteus unless directly contradicted by fossils (Bryant and Russell, 1992; Witmer, 1995). Anatomical features in extinct organisms can be inferred using one of three criteria: 1) direct fossil evidence, 2) phylogenetic bracketing, and 3) indirect evidence like form-function relationships and paleoenvironment (Bryant and Russell 1992). These lines of evidence form a decreasing gradient of certainty, with the latter always deferring to the former if available. When direct evidence and/or phylogenetic bracketing result in multiple equally likely interpretations, indirect evidence such as functional patterns and paleoenvironment can be used to identify the most likely alternative. A list of the major features considered and the evidence used to support them can be found in Table 1.
Dunkleosteus is phylogenetically bracketed by arthrodires known from body outlines or extensive post-thoracic remains (Figure 6). This means features consistently present across Arthrodira also likely occur in Dunkleosteus unless directly contradicted by fossils (Bryant and Russell, 1992; Witmer, 1995). Anatomical features in extinct organisms can be inferred using one of three criteria: 1) direct fossil evidence, 2) phylogenetic bracketing, and 3) indirect evidence like form-function relationships and paleoenvironment (Bryant and Russell 1992). These lines of evidence form a decreasing gradient of certainty, with the latter always deferring to the former if available. When direct evidence and/or phylogenetic bracketing result in multiple equally likely interpretations, indirect evidence such as functional patterns and paleoenvironment can be used to identify the most likely alternative. A list of the major features considered and the evidence used to support them can be found in Table 1.
Anatomical terminology for arthrodire plates follows Heintz (1932), Miles and Westoll (1968), Denison (1978), and Miles and Dennis (1979). Plate abbreviations in Figure 3C and elsewhere follow Miles and Dennis (1979). Homologies between placoderm plates and elements in other gnathostomes follow Zhu et al. (2013) and Zhu et al. (2016a). “Trunk armor” is used here to refer to the combined thoracic and ventral shields (Figure 3C), though in most non-eubrachythoracid arthrodires the thoracic and ventral shields do not form separate structures (Miles, 1969). Broader anatomical terminology for fishes follows standard ichthyological definitions (Compagno, 1984; Hubbs et al., 2004). Fineness ratio (f) is calculated as precaudal length/body depth, as in many prior studies (e.g., Aleev, 1969). Note that some studies prefer to use an alternative method calculating fineness ratio relative to mean body diameter (Walker et al., 2013), but the simpler method is used here to more readily enable comparisons between taxa. There is little difference if using these alternate methods except the f values for adult Dunkleosteus are brought slightly closer to values seen in other arthrodires (f ~ 3.0–3.5), due to the former having trunks that are slightly deeper than wide (see below).
Fishes in this study are described in terms of one of four life habit categories: benthic, demersal, neritic, and pelagic (Table 2). These represent broad, widely-recognized divisions within the spectrum of fish locomotor behavior and life habits, and are easily recognized based on functional morphology and body shape. However, because the boundaries between certain functional groups or their terminology are sometimes inconsistent, they are defined here to make their use clear to the reader. Particular attention is drawn to “pelagic” versus “neritic” fishes. Both are nektonic but differ significantly in functional anatomy and body shape. These differences, such as adaptations for prolonged, rapid swimming, are thought to be driven by biomechanical and adaptational challenges to living in the open ocean (Allen and Cross, 2006; Helfman et al., 2009).
Habitus categories for extinct taxa follow the previously published literature. Conclusions in those studies were largely based on functional morphology and paleoenvironment (Dean, 1909b; Harris, 1951; Miles and Westoll, 1968; Trewin, 1986; Compagno, 1990; Carr, 1995, 2010; Jobbins et al., 2022). Previous studies have considered Dunkleosteus a nektonic, pelagic cruiser (Carr, 2010; Ferrón et al., 2017a) based on paleoenvironmental data. The Cleveland Shale is a black shale with a distinct absence of bioturbation or benthos, suggesting a highly stratified water column inhabited by nektonic organisms living over an anoxic bottom (see below, and Carr, 2010), implying Dunkleosteus was a strongly nektonic and likely pelagic animal. Paleoenvironmental data were not used to reconstruct body form unless otherwise specified, and when discussing the overall paleobiology of this taxon features lacking direct positive or negative constraints from fossil data were not considered to avoid circular reasoning.
Reconstruction of Coccosteus cuspidatus
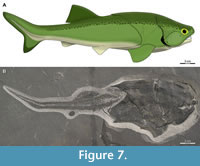 The reconstruction of Coccosteus cuspidatus used here (Figure 7) was modeled after Miles and Westoll (1968) with modifications. Complete specimens of C. cuspidatus (e.g., ROM VP 52664, Figure 7B) show key differences from the classic Miles and Westoll (1968) reconstruction. These include a shorter abdomen and caudal fin relative to the head and trunk armor (Appendix 6), and a more anterior position of the pelvic girdle (see also Trinajstic et al., 2015, who made a similar observation regarding the latter). Many specimens examined by Miles and Westoll (1968) only preserved part of the post-thoracic body, which may account for this discrepancy, though at least some of their specimens show a more anterior pelvic girdle similar to the material examined here (Miles and Westoll, 1968: pl. 5–9). The armor of that reconstruction is also slightly distorted (sheared and flattened) compared to more complete specimens, which may be due to being reconstructed from crushed specimens prior to the description of three-dimensionally preserved armor in the Gogo arthrodires that could be used as a guide. The reconstruction in Miles and Westoll (1968) was modified here to include data from these newer, better-preserved specimens (particularly ROM VP 52664).
The reconstruction of Coccosteus cuspidatus used here (Figure 7) was modeled after Miles and Westoll (1968) with modifications. Complete specimens of C. cuspidatus (e.g., ROM VP 52664, Figure 7B) show key differences from the classic Miles and Westoll (1968) reconstruction. These include a shorter abdomen and caudal fin relative to the head and trunk armor (Appendix 6), and a more anterior position of the pelvic girdle (see also Trinajstic et al., 2015, who made a similar observation regarding the latter). Many specimens examined by Miles and Westoll (1968) only preserved part of the post-thoracic body, which may account for this discrepancy, though at least some of their specimens show a more anterior pelvic girdle similar to the material examined here (Miles and Westoll, 1968: pl. 5–9). The armor of that reconstruction is also slightly distorted (sheared and flattened) compared to more complete specimens, which may be due to being reconstructed from crushed specimens prior to the description of three-dimensionally preserved armor in the Gogo arthrodires that could be used as a guide. The reconstruction in Miles and Westoll (1968) was modified here to include data from these newer, better-preserved specimens (particularly ROM VP 52664).
The shape of the dorsal fin follows Greenfield (2020) but with a lower aspect ratio. Greenfield (2020) modeled his fin shape after the carpet sharks Chiloscyllium and Orectolobus, but these taxa have two small dorsal fins whereas Coccosteus has a single dorsal fin with a long base, resulting in an abnormally tall dorsal fin if the shape in these chondrichthyan taxa is uncritically applied to Coccosteus. The shape shown here more closely resembles the dorsal fin traces figured in Trewin (1986). The sharper heterocercal angle of the caudal fin and more prominent hypochordal (ventral) caudal lobe are based on ROM VP 52664 (Figure 7B), as well as other specimens of Coccosteus (see “Caudal Fin”).
Paleoenvironmental Context of the Cleveland Shale
The paleoenvironment of the Cleveland Shale provides important, independent lines of evidence constraining Dunkleosteus' likely body shape. Carr (2010) previously argued Dunkleosteus was a nektonic, pelagic cruiser based on the paleoenvironment of the Cleveland Shale; this section largely builds on those arguments and the reader is highly encouraged to read Carr (2010) for further details on that study.
The Cleveland Shale is a fine-grained black shale interpreted as representing an open water, offshore environment (Lewis and Schwietering, 1971; Hlavin, 1976; Hansen, 1996; Carr, 2010; Baird et al., 2023 and references therein; but see Dunkel et al., 2022 for an alternate opinion) with a vertically stratified water column separated into an oxygenated surface layer and a dysxoic to anoxic bottom (Rimmer et al., 2010; Martinez et al., 2019). The anoxic zone in some areas spanned nearly 80 km in width based on the geographic extent of the Cleveland Shale perpendicular to the paleo-coastline in Appalachia (Prosser, 1913; Lewis and Schwietering, 1971; Daeschler and Cressler, 2011; Baird et al., 2023). This extent is probably an underestimate, as the lateral margins of the black shale appear to have been removed by erosion (Baird et al., 2023). This differs from some other dysaerobic Paleozoic fossil sites, where the anoxic zone is geographically restricted and immediately distal to otherwise oxygenated waters (Zorn et al., 2005; Gaines, 2014) allowing benthic and demersal organisms to be transported into the area and preserved.
The anoxic benthic zone of the Cleveland Shale is thought to have been uninhabitable to benthic or demersal organisms. The soft, fine-grained muds that made up the seafloor would have further excluded benthic life, especially large, benthic fishes (Wignall, 1993; Carr, 2010). These factors are thought to be responsible for the high-quality preservation of vertebrate remains in this unit, with organisms sinking into the anoxic zone from the oxygenated surface waters (Hansen, 1996; Carr and Jackson, 2008; Carr, 2010; Braun et al., 2014). Benthic and demersal organisms are nearly absent except for rare, hypoxia-tolerant lingulid brachiopods (Hlavin, 1976; Dunkel et al., 2022; Baird et al., 2023). Most preserved invertebrate taxa are nektonic species, including thylacocephalans (Williams, 1990; Saja and Hannibal, 2018) and ammonoids (House et al., 1986). Bioturbation is near-absent (Dunkel et al., 2022), and vertebrate fossils lack encrusting epibionts (Carr, 2010; Engelman, pers. obs.). Remains of fishes in the Cleveland Shale are often articulated or at least associated (Dunkle and Bungart, 1945; Dunkle, 1947; Carr, 2010; Carr et al., 2010; Braun et al., 2014) with many chondrichthyans even showing extensive soft-tissue preservation (Dean, 1894, 1902, 1909b; Harris, 1951; Braun et al., 2014; Tomita, 2015), suggesting an absence of benthic scavengers. These factors further suggest the absence of benthic fauna in the Cleveland Shale reflects a real phenomenon and is not caused by reducing conditions destroying invertebrate remains.
The Cleveland Shale fish fauna is also indicative of a pelagic environment. Over 65 species of fishes are known from this unit (Carr and Jackson, 2008; Carr, 2018), mostly pachyosteomorph arthrodires (28 species) and chondrichthyans (32 species) along with 4 species of actinopterygians and one sarcopterygian (Carr and Jackson, 2008). All taxa with preserved fin outlines or body fossils show strongly nektonic body plans, often with pelagic specializations such as caudal keels (Dean, 1909b; Harris, 1951; Dunkle, 1964; Dunkle and Schaeffer, 1973; Compagno, 1990; Carr et al., 2010). Cleveland Shale arthrodires generally show features suggestive of nektonic habits such as reduced armor, large pectoral fenestra, and large orbits (Miles, 1969; Carr, 1995). Otherwise common benthic or demersal Devonian fish groups are rare or absent, including antiarchs, ptyctodonts, and non-eubrachythoracid arthrodires. Sarcopterygians, another group often interpreted as benthic or demersal and associated with coastal or freshwater habitats (Andrews and Westoll, 1970; Campbell and Barwick, 1988; Long, 1991; Ahlberg, 1992; Johanson and Ahlberg, 1998; but see Frey et al.. 2018), are only represented by a single specimen of the dipnoan Ctenodus wagneri (Newberry, 1889; Carr and Jackson, 2008; see Kemp, 1996, regarding taxonomy). Some specimens of the onychodontiform Onychodus ortoni have been described from the underlying Huron Shale Member, leading Babcock (2024) to suggest the Huron and Cleveland Shale may have been more oxygenated and inhabited by more benthos than previously thought, given onychodonts have traditionally been interpreted as benthic or demersal (Andrews et al., 2005). However, undescribed pelagic onychodonts from black shales in Morocco (Frey et al., 2018) indicate that late Devonian onychodonts had expanded into pelagic niches, and thus their presence in the Huron Shale does not necessarily indicate oxygenated conditions. Coccosteomorph arthrodires (generally regarded as more demersal than pachysteomorphs; Miles, 1969; Carr, 1995) are present but rare, represented by taxa such as “Coccosteus” cuyahogae (Hlavin, 1976; Carr and Jackson, 2008). However, Hlavin (1976) notes similarities between “C.” cuyahogae and the possible pachyosteomorph “Heintzichthys” mixeri (Hussakof and Bryant, 1918), making it unclear if coccosteomorphs are present in the Cleveland Shale at all. The fauna of the Cleveland Shale suggest this taphocoenosis represents an epipelagic, open ocean ecosystem effectively captured in isolation, with the local environment excluding influences from more coastal habitats. Similar paleoenvironmental conclusions have been reached for other Devonian black shales (e.g., the Maïder Basin of Morocco; Frey et al., 2018) based on the abundance of pelagic organisms and rare-to-absent benthos.
Dunkleosteus terrelli is the most common vertebrate fossil in the Cleveland Shale, representing 20% of all vertebrate specimens and 32% of all vertebrate material identifiable to genus in the CMNH collections (n = 680; A. McGee and C. Colleary, pers. comm. June 2022). This is undoubtedly influenced by collections bias as large arthrodire plates are easier to recognize in the field (Dunkle and Bungart, 1945; Carr and Jackson, 2008; A. McGee, pers. comm. 2022). However, the sheer number of specimens collected still suggests D. terrelli was relatively common within the Cleveland Shale paleoenvironment. Dunkleosteus fossils do not vary in abundance across the anoxic zone (Carr, 2010), with many specimens being found close to the center of the laterally extensive black shale deposits (e.g., at Black River, Rocky River). This would require a significant amount of transportation either in vivo or postmortem if the organisms originally inhabited coastal regions, and if this was the case Dunkleosteus fossils would be expected to be most abundant closer to the paleo-coastline (which is not the case; Carr, 2010). The presence of Dunkleosteus specimens with associated plates and a lack of abrasion further suggests they were not washed into the basin after death (Carr, 2010). Dunkleosteus fossils from the Cleveland Shale span a wide range of sizes and ontogenetic stages, suggesting these animals spent most of their lifespan in the local area rather than being a demersal/benthic taxon that only migrated into/through oceanic habitats to breed, like some freshwater eels (Anguillidae). This all agrees with broader conclusions about Cleveland Shale taphonomy, which suggests fossils in this unit represent local animals preserved with limited post-mortem transportation (Carr, 2010; Braun et al., 2014).
The above evidence indicates Dunkleosteus terrelli was a common, autochthonous inhabitant of the Cleveland Shale area, not a benthic or demersal taxon that occasionally wandered into the area or had its remains transported there postmortem (see Carr, 2010 for a more extensive discussion). Given the extensive, inhospitable anoxic seafloor, Dunkleosteus could not have survived in this area unless it was nektonic (as argued by Carr, 2010), limiting this taxon to body shapes seen in highly nektonic, neritic to pelagic taxa. The abundant, widespread distribution of Dunkleosteus within the anoxic zones (whose oxygenated upper layers can be thought of as epipelagic zones in isolation) strongly implies pelagic habits. Despite this, most of the conclusions presented here about Dunkleosteus' pelagic habits and body shape are drawn from data independent from the Cleveland Shale paleoenvironment unless otherwise specified. The broader point is that evidence from both comparative anatomy and paleoenvironment favor the presented body shape of Dunkleosteus.
Institutional abbreviations. AA.MEM.DS, Université Cadi Ayyad, Marrakech, Morocco; AMNH, American Museum of Natural History, New York, New York, USA; CMC, Cincinnati Museum Center, Cincinnati, Ohio, USA; CMNH, Cleveland Museum of Natural History, Cleveland, Ohio, USA; FMNH, Field Museum of Natural History, Chicago, USA; FSBC, Florida State Biodiversity Collection, St. Petersburg, Florida, USA; GPIT, Institut für Geowissenschaften, Eberhard Karls Universität Tübingen, Tübingen, Germany; LDUCZ, Grant Museum of Zoology, University College, London, U.K.; MCZ, Museum of Comparative Zoology, Harvard, Massachusetts, USA; MHNM, Musée d’Histoire naturelle de Miguasha, Nouvelle, Quebec, Canada; MNHN, Musée National d’Histoire Naturelle, Paris, France; MZL; Musée Cantonal de Zoologie, Lausanne, Switzerland; NMS, National Museum of Scotland, Edinburgh, UK; NHMUK, Natural History Museum, London, UK.; NMP, National Museum of Victoria, Melbourne, Australia; ROM, Royal Ontario Museum, Toronto, Ontario Canada; USNM, Smithsonian National Museum of Natural History, Washington, D.C., USA; WAM, Western Australian Museum, Perth, Western Australia, Australia.
LIFE APPEARANCE OF DUNKLEOSTEUS TERRELLI
Length and Body Shape
Length. Body size estimates of Dunkleosteus have been poorly constrained until recently due to the rarity of post-thoracic material. Historical length estimates have ranged from 4.5–6 m (15–20 ft) at the low extreme (Newberry, 1889; Carr, 2010; Long, 2010) to 8–10 m (26–33 ft) at the upper (Romer, 1966; Sallan and Galimberti, 2015; Ferrón et al., 2017a). These studies, with the exception of Hussakof (1905) and Ferrón et al. (2017a), do not report their data or methods, and thus their length estimates are not reproducible. Some estimates are implied to be calculated by scaling from Coccosteus cuspidatus (compare with Titanichthys in Dean, 1909c), but which elements were scaled to produce estimates for Dunkleosteus are not specified and attempts to replicate these methods generally produce much shorter lengths (Engelman, 2023b: p. 39 and tab. 3). More recent studies sampling both arthrodires known from whole body fossils and a broad sample of 971 living fish species strongly suggest traditional lengths for Dunkleosteus are overestimates (Engelman, 2023a, 2023b). The reader is referred to those studies for further details, but their findings will be briefly summarized for context.
Head-trunk proportions appear to be strongly constrained in fishes (Engelman, 2023a, 2023b). In particular, the anteroposterior length of the head excluding the snout (i.e., “orbit-opercular length” or OOL) strongly correlates with overall length (r2 = 0.947; Engelman, 2023b),predicting total length within ±17.6% of the actual value across fishes. OOL scales isometrically with body length within arthrodires and across fishes more generally, with arthrodires and eugnathostomes following the same allometric regression line. This relationship is consistent across fishes spanning a wide range of body sizes, from the ~1 cm Paedocypris and Priocharax to 5–9 m Rhincodon, Cetorhinus, and Megachasma, making extrapolation error unlikely. OOL predicts total length within ±12.5% of actual length in complete arthrodires. 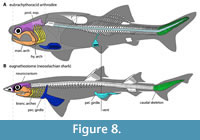 The reliability of this metric is further supported by the conserved anteroposterior location of several key anatomical landmarks, such as the positions of the glossopharyngeal and vagus nerves (both associated with the gill chamber), across arthrodires and other gnathostomes (Carr et al., 2009: p. 797–798). This indicates the underlying anatomy of the head such as the location of the brain, head-trunk boundary, and gill chamber is consistent across these groups, despite superficial differences in external morphology (Figure 8; see also “Branchial Opening”, below).
The reliability of this metric is further supported by the conserved anteroposterior location of several key anatomical landmarks, such as the positions of the glossopharyngeal and vagus nerves (both associated with the gill chamber), across arthrodires and other gnathostomes (Carr et al., 2009: p. 797–798). This indicates the underlying anatomy of the head such as the location of the brain, head-trunk boundary, and gill chamber is consistent across these groups, despite superficial differences in external morphology (Figure 8; see also “Branchial Opening”, below).
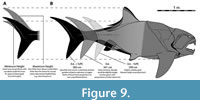 OOL produces lengths of about 3.0–3.4 m for subadults to typical adults of Dunkleosteus terrelli, with the largest individuals estimated to measure approximately 4.1 m long (Engelman, 2023b). Margins of error in these methods mean lengths as high as 3.8/4.5 m for average/maximum adult individuals of D. terrelli (respectively) remain possible, but lengths greater than +8–10% (30–40 cm) of the estimated value are unlikely (Figure 9, see also Engelman, 2023b). Such lengths would require D. terrelli to exhibit proportions significantly outside the range of head-trunk proportions seen in non-anguilliform fishes, including other arthrodires (Engelman, 2023a). Historical length estimates for D. terrelli require a head only 8% of total length versus 18–30% like in most non-anguilliform fishes (Engelman, 2023a). By contrast, OOL results in D. terrelli having a relative head size much closer to other fishes, though still below average (~18% total length).
OOL produces lengths of about 3.0–3.4 m for subadults to typical adults of Dunkleosteus terrelli, with the largest individuals estimated to measure approximately 4.1 m long (Engelman, 2023b). Margins of error in these methods mean lengths as high as 3.8/4.5 m for average/maximum adult individuals of D. terrelli (respectively) remain possible, but lengths greater than +8–10% (30–40 cm) of the estimated value are unlikely (Figure 9, see also Engelman, 2023b). Such lengths would require D. terrelli to exhibit proportions significantly outside the range of head-trunk proportions seen in non-anguilliform fishes, including other arthrodires (Engelman, 2023a). Historical length estimates for D. terrelli require a head only 8% of total length versus 18–30% like in most non-anguilliform fishes (Engelman, 2023a). By contrast, OOL results in D. terrelli having a relative head size much closer to other fishes, though still below average (~18% total length).
Fork length (length to the notch in the posterior margin of the caudal fin) and precaudal (= standard) length correlate closely with total length (r2 ~ 0.96–0.99; Engelman, 2023b), and therefore can be estimated if approximate total length is known. While anteroposterior lengths of the body must be determined indirectly, body depth and width can be measured from the trunk armor of mounted specimens. With values for the dimensions of the three major body axes, a well-constrained reconstruction of the body shape of D. terrelli can be created (Figure 4).
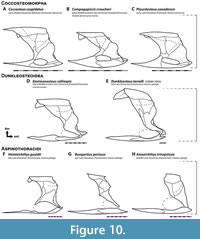 Body Shape. This reconstruction of Dunkleosteus has a relatively deep trunk, ~25–28% total length in subadult to adult individuals (fineness ratio or f = 2.66–3.18). This appears to be an apomorphy of Dunkleosteus terrelli, supported by several lines of evidence. Arthrodires generally have stockier bodies than eugnathostomes (Engelman, 2023b), but the trunk of Dunkleosteus is unusually deep even among arthrodires. This is evident when scaling the trunk armor of various eubrachythoracid arthrodires to the same anteroposterior length; under these conditions the trunk armor of Dunkleosteus stands out as considerably deeper than most other taxa (Figure 10). Deep trunk armors are consistently present in all large (inferred subadult to adult) specimens of D. terrelli, suggesting these proportions cannot be attributed to an incorrect reconstruction of the trunk armor. These proportions are not seen in trunk armors of other relatively large, pelagically-interpreted arthrodires, like Amazichthys, Bungartius, and Heintzichthys (Figure 10F‒H, see also Appendix 7). The arthrodire most closely resembling Dunkleosteus in terms of its trunk armor proportions is the dunkleosteoid Eastmanosteus calliaspis (Figure 10D), which has a more robust trunk than other Gogo forms yet lacks the highly elongate ventral shield of many aspinothoracidans. This suggests the deep trunk of Dunkleosteus may represent an extreme endpoint of a broader trend within dunkleosteoids.
Body Shape. This reconstruction of Dunkleosteus has a relatively deep trunk, ~25–28% total length in subadult to adult individuals (fineness ratio or f = 2.66–3.18). This appears to be an apomorphy of Dunkleosteus terrelli, supported by several lines of evidence. Arthrodires generally have stockier bodies than eugnathostomes (Engelman, 2023b), but the trunk of Dunkleosteus is unusually deep even among arthrodires. This is evident when scaling the trunk armor of various eubrachythoracid arthrodires to the same anteroposterior length; under these conditions the trunk armor of Dunkleosteus stands out as considerably deeper than most other taxa (Figure 10). Deep trunk armors are consistently present in all large (inferred subadult to adult) specimens of D. terrelli, suggesting these proportions cannot be attributed to an incorrect reconstruction of the trunk armor. These proportions are not seen in trunk armors of other relatively large, pelagically-interpreted arthrodires, like Amazichthys, Bungartius, and Heintzichthys (Figure 10F‒H, see also Appendix 7). The arthrodire most closely resembling Dunkleosteus in terms of its trunk armor proportions is the dunkleosteoid Eastmanosteus calliaspis (Figure 10D), which has a more robust trunk than other Gogo forms yet lacks the highly elongate ventral shield of many aspinothoracidans. This suggests the deep trunk of Dunkleosteus may represent an extreme endpoint of a broader trend within dunkleosteoids.
The high depth/length ratio of the trunk armor in Dunkleosteus appears driven by an expansion in dorsoventral height relative to other arthrodires, rather than an anteroposterior shortening. When the dermal skeleton of Dunkleosteus is scaled to the same head length as complete arthrodires like Coccosteus (and to a lesser degree Amazichthys), the ventral shield ends at a similar anteroposterior position relative to total length (Engelman, 2023b: fig. 11), indicating the trunk armor is not greatly reduced in anteroposterior length compared to other arthrodires. Similarly, a Dunkleosteus -like dermal armor can be produced from a generalized eubrachythoracid by simply expanding the body dorsoventrally (Appendix 8). Aside from features differentiating coccosteomorphs from pachyosteomorphs (presence of a posterior spine on the median dorsal plate, reduced nuchal gap), the overall shape of the armor and the anteroposterior location of major landmarks like triple junctions between plates are similar. Dunkleosteus also has a relatively deep head among eubrachythoracid arthrodires, whether scaled by the total height of the skull or the height of the cranium to the ventral margin of the cheek unit (Appendix 7). Because relative cranial and trunk heights in fishes are closely correlated (Ward and Mehta, 2010; Engelman, 2023b), this further supports the idea that Dunkleosteus had a deep trunk.
Available evidence does not support the idea that the thoracic and ventral shield of Dunkleosteus were both anteroposteriorly shortened. This would require the thoracic shield, ventral shield, and head to all be shortened relative to total length to near identical degrees without any difference in their proportions relative to one another, which is highly unlikely. Similarly, a longer, narrower body for Dunkleosteus requires distorting or ignoring anatomical proportions and relationships otherwise consistent among arthrodires, such as the association between the pelvic girdle and end of the ventral shield (see below).
The trunk armor of Dunkleosteus remains unusually deep even when scaled against other anatomical metrics, such as head length or the length of the posterior ventrolateral plate of the ventral shield (Appendix 7). Indeed, dimensions of the ventral shield provide a useful independent test for the body shape as reconstructed here. Ventral shield length is consistently around 30–33% total length in brachythoracid arthrodires known from complete remains (Appendix 6). These include Holonema westolli (30.4%), Coccosteus cuspidatus (30–32%), Incisoscutum ritchei (~34%), and Amazichthys trinajsticae (29.6–31.6%). Extensive data for the coccosteids Dickosteus and Watsonosteus are unavailable, but examination of available specimens (NHMUK PV OR 49663, NMS 1987.7.118, NMS G.1995.4.2) suggest proportions similar to Coccosteus. Unpublished material of Dickosteus and Watsonosteus have ventral shields that are 32.4% and 32.8% total length and a head + trunk armor that represents 49.5% and 45.2% total length, respectively (R. Jones and M. Newman, pers. comm. June 2024).
Specimens of Millerosteus (FMNH PF 1090, NMS 1859.33.986, NMS 1965.37.1) seem to show a proportionally longer ventral shield than other arthrodires (~40% total length), but these specimens may not be complete. Assuming the preserved length of these specimens represents total length requires Millerosteus to have an incredibly short post-thoracic body (~1/3 total length), with almost no space for a functional caudal fin. The post-thoracic axial skeleton of these specimens is not well defined, and it is possible much of the caudal skeleton may be missing or hidden under matrix. The specimen NHMUK PV P16795 suggests this is the case. This specimen’s ventral shield is ~41% of its preserved length, but the specimen is clearly not complete as preserved, being broken off approximately at the level of the caudal peduncle (Trinajstic et al., 2015: fig. 15A). The ventral shield length relative to precaudal length in this specimen is similar to other arthrodires (Appendix 6).
The ventral shield is proportionally shorter relative to total length (25% TL) in the more basal (non-brachythoracid) arthrodire Cowralepis (Ritchie, 2005), but this appears to be driven by the extremely long caudal fin of this taxon (Appendix 6). Other landmarks like the relative locations of the caudal peduncle, pelvis, and ventral shield appear consistent with other arthrodires. It is possible the unusual proportions of Cowralepis relative to other arthrodires are driven by ecology, some benthic sharks like nurse (Ginglymostoma cirratum) and zebra (Stegostoma tigrinum) sharks are known to have unusually long caudal fins relative to precaudal length. Ritchie (2005) also notes the armored proportion of Cowralepis is positively allometric across ontogeny, which appears primarily driven by a shortening of the caudal fin. If scaled to precaudal length, the relative length of the ventral shield of Cowralepis (38.0–41.5%) is similar to other arthrodires (Holonema, 40.4%; Coccosteus, 38.4–41.4%; Millerosteus, 41.3%; Incisoscutum, 42.3%; Amazichthys, 37.4–38.1%; Appendix 6).
Overall, ventral shield length seems to correspond relatively closely with total length in eubrachythoracid arthrodires, and appears to scale close to isometry. This conserved proportion might be expected, given the ventral shield may function as an protective cover for the visceral cavity (Trinajstic et al., 2022b). OOL-based length estimates for Dunkleosteus (particularly for CMNH 5768, 6090, and 7054) also result in a ventral shield length ~30–32% total length (Figure 4, Appendix 7), resembling other arthrodires. Length estimates as low as 3.8 m (the upper end of the margins of error in Engelman, 2023b) would result in an unusually short ventral shield among arthrodires. Even if Dunkleosteus was assumed to have a shorter ventral shield with proportions similar to Cowralepis it would not be possible to replicate total lengths of 6–10 m suggested by prior studies. Similarly, even if for the sake of argument the ventral shield is assumed to show slight negative allometry relative to total length (~27% total length), this would still only result in lengths of 3.8 m for CMNH 5768 and 4.76 m for CMNH 5936 (assuming this specimen is 25% larger than CMNH 5768 in linear dimensions).
The posterior ventrolateral plate of the ventral shield provides another potentially useful size proxy, as this plate has been noted to scale near-isometrically in arthrodires (Trinajstic, 1995; J. Long, pers. comm. February 2022). This may be because this plate spans the bases of the pectoral and pelvic girdles, and thus its size is highly correlated with trunk length and by extension body size. Posterior ventrolateral plate length in Dunkleosteus is comparable to other arthrodires when scaled by OOL-estimated body length (Appendix 7). These methods, along with scaling based on other dimensions of individual plates (see Engelman, 2023b), suggests OOL-based body length estimates — and the deeper body fineness ratio resulting as a consequence of this — approximate the actual values for Dunkleosteus.
This deeper body plan remains consistent even under greater lengths for Dunkleosteus terrelli. A length of 3.8 m for CMNH 5768, at the upper end of the possible lengths in Engelman (2023b), only results in an f of ~3 (Figure 9, Appendix 3: fig. 4.2). This results in a head around 16% total length, close to the most extreme head-trunk proportions in non-anguilliform fishes (e.g., Acanthocybium, Coryphaena, Scomberomorus), making greater lengths for Dunkleosteus unlikely. Indeed, a length of 3.8 m results in an unusually short ventral shield and anterior position of the paired fins and cloaca (see below and Figure 9), suggesting it may be an overly generous estimate.
The low fineness ratios calculated for Dunkleosteus terrelli are not unusual when compared to well-preserved eubrachythoracid arthrodires. Coccosteus cuspidatus (ROM VP 52664, Figure 7) has an f of ~3.6 and no greater than 3.8. Incisoscutum has an f of ~3.8–3.9 (Trinajstic et al., 2013: fig. 1). Amazichthys has a reconstructed f of ~3.7–3.9 (Jobbins et al., 2022: figs. 9–10; Engelman, 2023b: fig. 11C) but this value could be as high as 4 depending on how the trunk armor is reconstructed, fitting with the comparatively elongate trunk of this taxon. However, the fineness ratio of Dunkleosteus is still comparatively lower than these taxa due to its proportionally deeper trunk. All of these values for arthrodires are much lower than the theoretical optimum f of 4.6 (Alexander, 1967) and are distinctly below-average among fishes (Appendix 3: fig. 4.1), though fusiform fishes with fineness ratios similar to Dunkleosteus do exist (e.g., Bramidae, Hyperoglyphe spp., Serranidae, some Thunnus spp.). These values are also significantly lower than fineness ratios for elasmobranchs (x̄ = 6.42, n = 162, range = 3.46–14.76; Appendix 3: table 4.1), further supporting the conclusions of Engelman (2023b) that eubrachythoracid arthrodires and sharks are not comparable in body shape.
Trunk armor and torso shape in arthrodires appear to be correlated. This is evident in the few arthrodire taxa known from body outlines (Miles and Westoll, 1968; Jobbins et al., 2022), and is supported by arthrodire soft tissue anatomy (Trinajstic et al., 2013; Trinajstic et al., 2022b). Previously, these features had been treated as unrelated, with remarkably little discussion over how the trunk armor might reflect body shape. This is one reason why the trunk armor in many earlier reconstructions of Dunkleosteus appears poorly integrated into the overall body (Figure 2). This correlation has significant implications for future research, as it provides new avenues for studying the trunk morphology of arthrodires in taxa where postcranial elements other than the trunk armor are unknown (i.e., most of them).
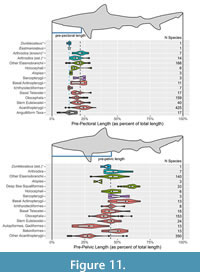 A short, deep body for Dunkleosteus is further supported by other anatomical landmarks such as the probable location of the pelvic girdle and cloaca. The pelvic girdle of arthrodires (when known) is invariably located at or near the posterior end of the ventral shield, and this also appears to be true for Dunkleosteus (see “Pelvic Fins” below). In most non-acanthopterygian fishes (including complete arthrodires) prepelvic length is ~45–50% total length (with most taxa having a value between 35–55% total length; Figure 11B; Appendix 3: section 4.5), and the pelvic fins are just posterior to the center of mass (Lauder and Drucker, 2004; Standen, 2008). These relationships are relatively conserved among non-acanthopterygian fishes (Appendix 3: section 4.5), even in groups with wildly disparate body shapes such as the elongate-bodied Chirocentridae (prepelvic length = 42% total length) and Ichthyodectiformes (prepelvic length = 45% total length) or the discoid Lampridae (prepelvic length = 41% total length) and Serrasalmidae (prepelvic length = 39% total length). This consistency may be driven by developmental and/or biomechanical constraints, but pelvic fin function in non-acanthopterygians remains poorly understood (Harris, 1938; Lauder and Drucker, 2004; Standen, 2008). By contrast, an anterior position of the pelvic fins (< 35% total length) is a derived state restricted to several neoteleost lineages (Acanthopterygii and some Aulopiformes and Paracanthopterygii; Yamanoue et al., 2010; see also Appendix 3). In these taxa anterior migration of the pelvic fin is correlated with a more dorsal position of the pectoral fins aligned with the center of mass, a greater emphasis on pectoral fin oscillation in propulsion and steering, and the combined use of the pectoral and pelvic fins in braking (Harris, 1953; Lauder and Drucker, 2004; Yamanoue et al., 2010), seemingly driven by developmental changes relative to the ancestral gnathostome condition (Tanaka, 2011). Dunkleosteus and most arthrodires do not exhibit these features, making a more anterior position of the pelvic girdle unlikely.
A short, deep body for Dunkleosteus is further supported by other anatomical landmarks such as the probable location of the pelvic girdle and cloaca. The pelvic girdle of arthrodires (when known) is invariably located at or near the posterior end of the ventral shield, and this also appears to be true for Dunkleosteus (see “Pelvic Fins” below). In most non-acanthopterygian fishes (including complete arthrodires) prepelvic length is ~45–50% total length (with most taxa having a value between 35–55% total length; Figure 11B; Appendix 3: section 4.5), and the pelvic fins are just posterior to the center of mass (Lauder and Drucker, 2004; Standen, 2008). These relationships are relatively conserved among non-acanthopterygian fishes (Appendix 3: section 4.5), even in groups with wildly disparate body shapes such as the elongate-bodied Chirocentridae (prepelvic length = 42% total length) and Ichthyodectiformes (prepelvic length = 45% total length) or the discoid Lampridae (prepelvic length = 41% total length) and Serrasalmidae (prepelvic length = 39% total length). This consistency may be driven by developmental and/or biomechanical constraints, but pelvic fin function in non-acanthopterygians remains poorly understood (Harris, 1938; Lauder and Drucker, 2004; Standen, 2008). By contrast, an anterior position of the pelvic fins (< 35% total length) is a derived state restricted to several neoteleost lineages (Acanthopterygii and some Aulopiformes and Paracanthopterygii; Yamanoue et al., 2010; see also Appendix 3). In these taxa anterior migration of the pelvic fin is correlated with a more dorsal position of the pectoral fins aligned with the center of mass, a greater emphasis on pectoral fin oscillation in propulsion and steering, and the combined use of the pectoral and pelvic fins in braking (Harris, 1953; Lauder and Drucker, 2004; Yamanoue et al., 2010), seemingly driven by developmental changes relative to the ancestral gnathostome condition (Tanaka, 2011). Dunkleosteus and most arthrodires do not exhibit these features, making a more anterior position of the pelvic girdle unlikely.
Similarly, the posterior end of the visceral cavity and cloaca in arthrodires appear to be located just posterior to the ventral shield, based on arthrodires with preserved organs from the Gogo Formation (Trinajstic et al., 2022b) and a specimen of Heintzichthys gouldii with gut contents partially expelled from the body cavity postmortem (AMNH FF 2826; Dean, 1896; Engelman, 2023b: p. 34–35). The cloaca is also interpreted to end behind the ventral shield in Sigaspis (Goujet, 1973) and Kujdanowiaspis (Stensiö, 1963; Dupret, 2010) based on scalation patterns and ventral shield shape. This strongly suggests the ventral shield represents an external protective cover for the visceral cavity, with ventral shield length approximating visceral cavity length. Goujet (1984: p. 205–206) questioned if this applied to eubrachythoracid arthrodires based on the relationship between the ventral shield and pelvic girdle in Rhachiosteus (Miles, 1966) and the position of the pelvis in Coccosteus as reconstructed by Miles and Westoll (1968). These are resolved respectively in “Pelvic Fins” below and “Reconstruction of Coccosteus cuspidatus” above, eliminating this potential discrepancy and suggesting the end of the ventral shield does indeed correlate with vent position in eubrachythoracids.
The proportion of snout-vent length to total length is remarkably conserved across gnathostomes, consistently representing ~50% of total length (Appendix 3: section 4.3). Chondrichthyans and osteichthyans do not significantly differ in vent position relative to total length (Appendix 3: section 4.3.2). The position of the pelvic and anal fins relative to the cloaca differs between these groups, but appears to represent a rearrangement of fin bases around a conserved vent position (Appendix 3: fig. 4.4). In chondrichthyans the cloaca is associated with the pelvic fin and claspers, but in extant osteichthyans is closer to the anal fin origin. The presence of claspers near the pelvic fins indicate arthrodires were similar to chondrichthyans in the position of the cloaca relative to the fins (Miles and Westoll, 1968; Goujet, 1973: p. 84; Ahlberg et al., 2009; Long et al., 2009; Trinajstic et al., 2015; Trinajstic et al., 2022b; see also Figure 7B here), given the claspers must be near the vent to function. The claspers of arthrodires appear to be non-homologous with those of chondrichthyans (Trinajstic et al., 2015), but are still close to the pelvis in a manner analogous to these taxa (Ahlberg et al., 2009; Trinajstic et al., 2015). In Coccosteus cuspidatus and Incisoscutum ritchei the cloaca is estimated to be around 50% total length based on clasper position (Appendix 3), similar to extant eugnathostomes. The present reconstruction of Dunkleosteus results in a similar position of the cloaca.
These comparative patterns support the body shape for Dunkleosteus proposed here. Breaking any of these proportional relationships, as would be required by a longer trunk, results in a shape strongly deviating from the basic arthrodire body plan, and there is currently no anatomical evidence D. terrelli was an outlier in these relationships.
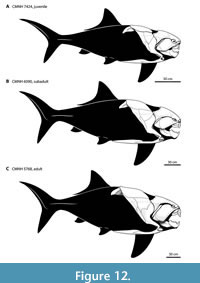 Body Shape Ontogeny. The trunk armor of Dunkleosteus became deeper throughout ontogeny (Figure 5). Small Dunkleosteus specimens (CMNH 6194 and CMNH 7424) have shallow trunk armors (20% of estimated body length, 1.12 times head length, f ~ 3.5 in CMNH 6194 and 3.85 in CMNH 7424), intermediate-sized specimens (CMNH 6090 and 7054) have deeper bodies (25% of estimated length, 1.35–1.45 times head length, f = 2.92–3.18), and CMNH 5768 has a trunk armor height that is at least 28% estimated total length and 1.55 times head length (f = 2.66), using the shallowest possible interpretation of the body armor (see below). Greater possible estimates of thoracic depth in CMNH 5768 potentially result in trunk heights up to 1.85 times head length. Other specimens of D. terrelli follow this pattern. CMC VP8294 and CMNH 8982, although not three-dimensionally mounted, have narrower trunk armors similar to CMNH 69144 and CMNH 7424 (approximate f for CMC VP8294 using methods similar to other specimens ~3.6–3.8). USNM V 21314 and the mounted specimen described by Stetson (1930) represent larger, older individuals and show deeper trunk armors more similar to CMNH 5768, CMNH 6090, and CMNH 7054. The ventral shield also appears to become proportionally broader throughout ontogeny. This suggests Dunkleosteus showed positive allometry in body fineness, with adults having deeper bodies and potentially a more thunniform shape than juveniles (Figure 12). Many fishes show slight positive allometry in relative body depth and girth (Jones et al., 1999; Froese, 2006; Engelman, pers. obs.), though in extant fishes these changes are usually driven by soft tissues and not reflected in their bony anatomy.
Body Shape Ontogeny. The trunk armor of Dunkleosteus became deeper throughout ontogeny (Figure 5). Small Dunkleosteus specimens (CMNH 6194 and CMNH 7424) have shallow trunk armors (20% of estimated body length, 1.12 times head length, f ~ 3.5 in CMNH 6194 and 3.85 in CMNH 7424), intermediate-sized specimens (CMNH 6090 and 7054) have deeper bodies (25% of estimated length, 1.35–1.45 times head length, f = 2.92–3.18), and CMNH 5768 has a trunk armor height that is at least 28% estimated total length and 1.55 times head length (f = 2.66), using the shallowest possible interpretation of the body armor (see below). Greater possible estimates of thoracic depth in CMNH 5768 potentially result in trunk heights up to 1.85 times head length. Other specimens of D. terrelli follow this pattern. CMC VP8294 and CMNH 8982, although not three-dimensionally mounted, have narrower trunk armors similar to CMNH 69144 and CMNH 7424 (approximate f for CMC VP8294 using methods similar to other specimens ~3.6–3.8). USNM V 21314 and the mounted specimen described by Stetson (1930) represent larger, older individuals and show deeper trunk armors more similar to CMNH 5768, CMNH 6090, and CMNH 7054. The ventral shield also appears to become proportionally broader throughout ontogeny. This suggests Dunkleosteus showed positive allometry in body fineness, with adults having deeper bodies and potentially a more thunniform shape than juveniles (Figure 12). Many fishes show slight positive allometry in relative body depth and girth (Jones et al., 1999; Froese, 2006; Engelman, pers. obs.), though in extant fishes these changes are usually driven by soft tissues and not reflected in their bony anatomy.
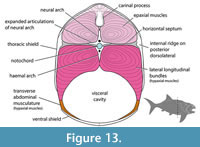 The cross-sectional shape of the trunk armor also changes across ontogeny in D. terrelli. The trunk armor of CMNH 6194 and CMNH 7424 is nearly circular cross-section with a depth/width ratio close to 1, similar to coccosteomorphs. CMNH 6090 and CMNH 7054 have armors that are ovate and slightly deeper than wide, with depth/width ratios of 1.21–1.29 (Figure 13; Engelman, 2023a: fig. 1A). Other specimens of Dunkleosteus (e.g., CMC VP8294, CMNH 5768, CMNH 8982, USNM V 21314) seem to follow this correlation between cross-sectional shape and size. CMNH 5768 as mounted has a wider, near-circular cross-section, but this appears to be inaccurate and due to many of the lateral trunk shield plates being reconstructed (see “Potential Errors in Armor Reconstruction in CMNH 5768, below); the spacing between the plates of the ventral shield suggests a narrower trunk similar to CMNH 6090 or CMNH 7054. This narrower cross-section means the frontal area of Dunkleosteus is less than traditionally assumed (i.e., f calculated from mean body diameter is 3.3–3.5 for CMNH 6090 and CMNH 7054 versus 2.92–3.18 if calculating based on maximum body height), reducing issues with drag that would arise if considering body shape using traditional mounts of CMNH 5768. Notably, subadult/adult Dunkleosteus only have a mediolaterally narrow body relative to the subcircular cross-sections of coccosteomorph arthrodires and chondrichthyans (Appendix 9). The trunk in these specimens is still significantly wider than in most teleosts, which have trunk height/width ratios close to 2. Adult/subadult Dunkleosteus have a cross-sectional shape more similar to extant non-teleost actinopterygians and tuna-like scombrids (Thunnini and Sardini), as well as other pachyosteomorph arthrodires (Appendix 9). Pachyosteomorphs in general seem to differ from coccosteomorphs in showing a mediolaterally narrower trunk (see also Gross, 1932). Overall, these patterns support the idea that ontogenetic changes in body shape in D. terrelli are driven by a dorsoventrally deepening of the body.
The cross-sectional shape of the trunk armor also changes across ontogeny in D. terrelli. The trunk armor of CMNH 6194 and CMNH 7424 is nearly circular cross-section with a depth/width ratio close to 1, similar to coccosteomorphs. CMNH 6090 and CMNH 7054 have armors that are ovate and slightly deeper than wide, with depth/width ratios of 1.21–1.29 (Figure 13; Engelman, 2023a: fig. 1A). Other specimens of Dunkleosteus (e.g., CMC VP8294, CMNH 5768, CMNH 8982, USNM V 21314) seem to follow this correlation between cross-sectional shape and size. CMNH 5768 as mounted has a wider, near-circular cross-section, but this appears to be inaccurate and due to many of the lateral trunk shield plates being reconstructed (see “Potential Errors in Armor Reconstruction in CMNH 5768, below); the spacing between the plates of the ventral shield suggests a narrower trunk similar to CMNH 6090 or CMNH 7054. This narrower cross-section means the frontal area of Dunkleosteus is less than traditionally assumed (i.e., f calculated from mean body diameter is 3.3–3.5 for CMNH 6090 and CMNH 7054 versus 2.92–3.18 if calculating based on maximum body height), reducing issues with drag that would arise if considering body shape using traditional mounts of CMNH 5768. Notably, subadult/adult Dunkleosteus only have a mediolaterally narrow body relative to the subcircular cross-sections of coccosteomorph arthrodires and chondrichthyans (Appendix 9). The trunk in these specimens is still significantly wider than in most teleosts, which have trunk height/width ratios close to 2. Adult/subadult Dunkleosteus have a cross-sectional shape more similar to extant non-teleost actinopterygians and tuna-like scombrids (Thunnini and Sardini), as well as other pachyosteomorph arthrodires (Appendix 9). Pachyosteomorphs in general seem to differ from coccosteomorphs in showing a mediolaterally narrower trunk (see also Gross, 1932). Overall, these patterns support the idea that ontogenetic changes in body shape in D. terrelli are driven by a dorsoventrally deepening of the body.
Similar ontogenetic patterns occur in other placoderms. In Gogo coccosteomorphs like Compagopiscis, the body armor becomes distinctly deeper and wider with age (Trinajstic and McNamara, 1999; Trinajstic and Hazelton, 2007). This results in juvenile specimens having relatively slender and narrow trunk armors compared to adults (Gardiner and Miles, 1994; Long, 1994), much like Dunkleosteus. Dunkleosteus also resembles Compagopiscis in the head shield becoming increasingly broader across ontogeny. The head shield in the smallest individuals of D. terrelli (CMNH 6194, CMNH 7424) is longer than wide (length/width =1.25), head shield length/width ratios in intermediate-sized specimens (CMNH 6090, CMNH 7054) are close to 1:1, and head shields in the largest specimens (CMNH 5768) are wider than long (length/width = 0.77) (Appendix 10). This suggests similar ontogenetic and allometric patterns are operating across both taxa, with the broadening of the head shield in Dunkleosteus reflecting broadening of the body.
Similarly, in E. calliaspis, the largest individuals have the proportionally deepest trunk armors, regardless of whether trunk height is scaled by the length of the head, infragnathal, nuchal, or anterior ventrolateral plates (see Dennis-Bryan, 1987: tab. 2). This pattern also occurs in some non-arthrodire placoderms like the antiarch Bothriolepis, where juveniles are relatively slender-bodied and adults are much wider and squatter (Stensiö, 1948; Downs et al., 2011: fig 9, but see Werdelin and Long, 1986), though Hemmings (1978) reported the antiarch Pterichthyodes became more elongate with age. Positive allometry of trunk height and/or width throughout ontogeny may characterize all arthrodires, if not all placoderms.
These patterns do not represent negative allometry of the head and trunk armor relative to a conserved body shape. That requires an unlikely scenario where the head and trunk armor show near-identical, negative allometries with no change in the relative proportions of plates. The anteroposterior location of major anatomical landmarks and triple junctions between plates remain conserved across ontogeny in Dunkleosteus, the armor simply deepens dorsoventrally. Negative allometry of the trunk armor would also require significant negative allometry of elements otherwise known to scale isometrically in arthrodires, like the posterior ventrolateral plates (Trinajstic, 1995). It would also require the end of the ventral shield — and thus pelvic girdle — be positioned increasingly anterior throughout ontogeny, which is not seen in other arthrodires or basal eugnathostomes like sharks (Garrick, 1982, 1985; Engelman, 2023b). A far more parsimonious explanation is the body armor of Dunkleosteus simply became deeper with age, especially given similar patterns are present in other placoderms.
Potential Errors in Armor Reconstruction of CMNH 5768. The reconstruction in this study is primarily based on CMNH 5768, the only mounted specimen of a large, adult Dunkleosteus terrelli at the CMNH. This mount is missing several lateral trunk plates, including the posterior dorsolateral, posterior lateral, and anterior lateral plate (Figure 5D), which are replaced by sculpted elements. This, along with the mount being created by retrodeformation of a crushed specimen, means further examination is necessary to determine if its armor proportions are reliable.
The absence of anterior lateral and spinal plates in CMNH 5768 is a significant concern. These plates connect the thoracic and ventral shields, and thus are important in defining the height of the trunk. The suture between the anterior lateral and anterior dorsolateral plates as originally mounted is oriented at a shallow, anteriorly inclined angle (Appendix 1A). This is unlike CMNH 6090, CMNH 7054, and CMNH 7424 where this suture is much more oblique (Figure 5A–C). Examination of CMNH 5768 finds the preserved anterior dorsolateral plate has a sharply angled contact for the anterior lateral plate, similar to other specimens, but this suture is obscured by the reconstructed anterior lateral plate. Restoring the anterior lateral plate based on the morphology seen in other specimens results in a less posterodorsally protruding median dorsal and anterior dorsolateral plates, reducing the trunk height of CMNH 5768 by up to 20 cm. This interpretation is followed in the present reconstruction compared to that in Engelman (2023b) (Appendix 1). One caveat is some large, isolated anterior lateral plates of Dunkleosteus (AMNH FF 5, CMNH 7054) seem to seem to show a posterior wing at their contact with the anterior dorsolateral plate, which could result in a shallower angle of this suture (Hussakof, 1906: fig. 19), but this is not entirely clear.
Outside of the orientation of the suture between the anterior lateral and anterior dorsolateral plates, other dimensions of the reconstructed anterior lateral plate, and thus the deep trunk of CMNH 5768, appear accurate. CMNH 5768 preserves the skull and gnathal plates, median dorsal plate, anterior dorsolateral plate and a nearly complete ventral shield. The morphology of the anterior lateral plate is constrained by its interactions with several of these elements. The obstantic process of the anterior lateral plate protrudes anteriorly to fit within the subobstantic fossa of the head shield, the V-shaped postbranchial embayment of the anterior lateral plate is occupied by the postsuborbital and submarginal plates, the anteroventral wing of the anterior lateral and interolateral plates form a trough which limit the position of the mandible, and the ventral edge of the anterior lateral plate borders the pectoral fenestra dorsally (Figure 3). These elements strongly constrain the height and shape of the anterior lateral plate. Additionally, the preserved cranio-thoracic joint between the head shield and anterior dorsolateral plates, the sutures between the anterior dorsolateral and median dorsal plates, and the width of the ventral shield greatly constrains the shape of the armor (see Materials and Methods). This suggests while the improperly reconstructed suture between the anterior lateral and anterior dorsolateral plates exaggerate trunk height, the overall shape of the trunk is fairly well constrained and remains deep after this is accounted for. CMNH 7054, a slightly smaller (~3 m; Engelman, 2023b) individual of D. terrelli with a complete trunk armor shows a similar (if slightly shallower) trunk armor to CMNH 5768, further suggesting the deep trunk of the latter is accurate.
Regardless of how the anterior lateral is reconstructed, the thoracic armor of CMNH 5768 is clearly deep. Scaling the anterior lateral plate of other specimens of Dunkleosteus (e.g., CMNH 6090 or CMNH 7054) to the size of CMNH 5768 suggests the overall height of the plate is probably reliable, even if the orientation of the suture with the anterior dorsolateral plate is questionable. Even assuming a modified anterior lateral plate similar to these smaller individuals, maximum thoracic depth in CMNH 5768 cannot be reduced below 28–30% estimated total length (95–100 cm). Reducing trunk armor depth further requires an oblique, anterodorsal-posteroventral contact between the median dorsal plate and anterior/posterior dorsolateral plates (instead of near-horizontal like other arthrodires, including other specimens of Dunkleosteus) or crushing the pectoral fenestra to the point the scapulocoracoid cannot fit within it. CMNH 5768 clearly has a deeper thoracic armor than the smaller CMNH 6090 or CMNH 7054 (in which body depth is ~25% total length) regardless of how the armor is reconstructed. This agrees with the positive allometry in body depth seen in the remaining specimens of D. terrelli.
Ventral Shield. The ventral shield of Dunkleosteus is reconstructed as curved, following other arthrodires. Historically Dunkleosteus was depicted with a flat ventral shield (Branson, 1908; Dean, 1909a; Heintz, 1932: p. 71; see also Figure 3A of this study), because of its assumed benthic habits (D. Chapman, pers. comm. 2014; see discussion in Carr, 2010). However, three-dimensionally Gogo Formation specimens show the ventral shield of most eubrachythoracids was anteroposteriorly and mediolaterally curved, making the trunk armor ovate in cross-section (e.g., Miles and Dennis, 1979; Gardiner and Miles, 1994). Some demersal eubrachythoracids (Coccosteus, Millerosteus) do show slightly flattened ventral shields (Miles and Westoll, 1968), but not to the degree required by old reconstructions of Dunkleosteus.
The CMNH Dunkleosteus mounts have a flat ventral shield, but this is not natural. Cleveland Shale arthrodire specimens are typically flattened during fossilization and lose most of their original curvature (Heintz, 1932: p. 153–159). When the CMNH mounts were created in the 1920s-1930s, the preparators attempted to restore this curvature by physically breaking and resetting the plates into the proper shape (D. Chapman, pers. comm., 2014). At that time, the plates of the ventral shield were not retrodeformed, because of the aforementioned assumption Dunkleosteus was benthic (ibid). However, later specimens of Dunkleosteus (e.g., CMNH 9951) preserve some of the original curvature and show the ventral shield was slightly curved, resulting in an ovate cross-section like the Gogo arthrodires (D. Chapman, pers. comm., 2014; R. Carr, pers. comm., 2023). Nevertheless, the ventral shields of the CMNH specimens were not remounted following this discovery because by that time retrodeformation was considered too destructive to the original material (D. Chapman, pers. comm., 2014). Retained curvature in less distorted ventral plates of Dunkleosteus suggests greatest body height was located at the center of the posterior median ventral plate in the center of the ventral shield, like other arthrodires. The curvature of these plates suggests posterior to this point the trunk was already starting to taper towards the caudal peduncle, supporting the shorter trunk for Dunkleosteus presented here.
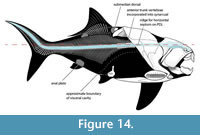 Spinal Posture. The spinal cord of Dunkleosteus appears to have been located at a relatively dorsoventrally high position on the body based on several factors. First, in arthrodires the foramen magnum is located at the same dorsoventral level as the paired cranio-thoracic joints (Heintz, 1932; Stensiö, 1963; Miles and Westoll, 1968). The occiput must be at this position to allow motion across the hinge-like cranio-thoracic joints (Heintz, 1932: p. 196). Because the cranio-thoracic joint of Dunkleosteus is relatively high on the body, the spinal cord exits the skull at a high position (Figure 14).
Spinal Posture. The spinal cord of Dunkleosteus appears to have been located at a relatively dorsoventrally high position on the body based on several factors. First, in arthrodires the foramen magnum is located at the same dorsoventral level as the paired cranio-thoracic joints (Heintz, 1932; Stensiö, 1963; Miles and Westoll, 1968). The occiput must be at this position to allow motion across the hinge-like cranio-thoracic joints (Heintz, 1932: p. 196). Because the cranio-thoracic joint of Dunkleosteus is relatively high on the body, the spinal cord exits the skull at a high position (Figure 14).
Secondly, spinal cord position is strongly constrained by the size of the carinal process (Figure 3). This is a large, bony process of unclear function (Heintz, 1932; Miles, 1969; Engelman et al., in press) that projects ventrally from the internal border of the median dorsal plate. In Dunkleosteus and some other large arthrodires (Gorgonichthys, Titanichthys; Dunkle and Bungart, 1940; Boyle and Ryan, 2017) the carinal process is particularly large and projects posteriorly beyond the edge of the median dorsal plate (Figure 14). The spinal cord must pass beneath the carinal process, strongly limiting the possible narrowness of the trunk. The carinal process of Dunkleosteus is of appropriate size for the spinal cord to travel beneath this process without significant deflection (Figure 5), like other arthrodires. In Coccosteus the carinal process directly articulates with the axial skeleton via a notch connecting to the neural process of the fifth neural arch (Miles and Westoll, 1968: p. 447), meaning spinal cord position is directly correlated with carinal process morphology. However, not all arthrodires exhibit this relationship; this notch is absent in Dunkleosteus, and other taxa (mostly aspinothoracidans) have a nearly vestigial carinal process that would preclude such a relationship (Dunkle, 1947; Carr, 1991, 1996; Jobbins et al., 2022).
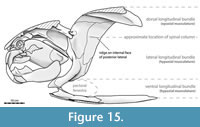 Third, the trunk armor of Dunkleosteus has a horizontal ridge on the internal face of the posterior dorsolateral plate (Figure 15; see also Heintz, 1932: pp. 165–166), readily visible in CMNH 6090, CMNH 7054, and CMNH 7424. This ridge is located at the same height as the main trunk canal of the lateral line and the cranio-thoracic joint. The location of this ridge and its anteroposterior orientation suggests it represents an attachment site for the horizontal septum, and thus the approximate position of the spinal cord (Figure 13, Figure 14, Figure 15). Analogous attachments for axial connective tissues occurs in other fishes with dermal plates, such as seahorses (Syngnathidae) (Neutens et al., 2014). This ridge is absent in the Gogo arthrodires (K. Trinajstic, pers. comm., April 2023), but Gogo specimens with preserved myomeres show the spine and horizontal septum are located at the same height as the cranio-thoracic joint and dorsal branch of the main lateral line canal (Trinajstic et al., 2013). Miles and Westoll (1968: p. 449) report preserved traces of the horizontal septum in Coccosteus suggesting it is located at the same level as the spine, as in all other fishes. These features support the identification of the ridge on the posterior dorsolateral plate of Dunkleosteus as an osteological correlate of the horizontal septum.
Third, the trunk armor of Dunkleosteus has a horizontal ridge on the internal face of the posterior dorsolateral plate (Figure 15; see also Heintz, 1932: pp. 165–166), readily visible in CMNH 6090, CMNH 7054, and CMNH 7424. This ridge is located at the same height as the main trunk canal of the lateral line and the cranio-thoracic joint. The location of this ridge and its anteroposterior orientation suggests it represents an attachment site for the horizontal septum, and thus the approximate position of the spinal cord (Figure 13, Figure 14, Figure 15). Analogous attachments for axial connective tissues occurs in other fishes with dermal plates, such as seahorses (Syngnathidae) (Neutens et al., 2014). This ridge is absent in the Gogo arthrodires (K. Trinajstic, pers. comm., April 2023), but Gogo specimens with preserved myomeres show the spine and horizontal septum are located at the same height as the cranio-thoracic joint and dorsal branch of the main lateral line canal (Trinajstic et al., 2013). Miles and Westoll (1968: p. 449) report preserved traces of the horizontal septum in Coccosteus suggesting it is located at the same level as the spine, as in all other fishes. These features support the identification of the ridge on the posterior dorsolateral plate of Dunkleosteus as an osteological correlate of the horizontal septum.
Finally, the anterior spinal column of Dunkleosteus is highly rigid and extensively fused, with at least 12 additional vertebrae partially incorporated into the synarcual (Johanson et al., 2019), which itself is composed of ~8–14 undifferentiated vertebrae (Johanson et al., 2013). This extensive fusion effectively extends the synarcual almost to the end of the thoracic shield (Johanson et al., 2019: fig. 1.2). By contrast, the synarcual of Compagopiscis is composed of only five vertebrae and ends well beneath the median dorsal plate (Johanson et al., 2019). The vertebral column of Dunkleosteus also shows laterally-expanded, anteroposteriorly broad articular surfaces (Johanson et al., 2019: figs. 2–3). This would greatly restrict anterior trunk flexion as the trunk could not bend too far laterally or else the articular surfaces would overlap, similar to how large, flattened processes parallel to the axis of rotation and well-developed zygapophyses restrict axial motion in other vertebrates (Long, 1987; Hebrank et al., 1990; Galis et al., 2014). Thus, the spinal column of Dunkleosteus is extremely stiff between the occiput and end of the thoracic armor (at least), restricting flexion in life (Z. Johanson, pers. comm., 2023).
These features suggest the spinal cord of Dunkleosteus was positioned relatively high on the body, about 66% total body height in smaller individuals (CMNH 7424) and 75% greatest height in larger specimens (CMNH 6090 and CMNH 7054). The high position of the spinal cord and proportionally deep trunk imply relatively large hypaxial and epaxial muscles (Figure 13, Figure 15), particularly the lateral longitudinal bundles. In sharks (De Iuliis and Pulerà, 2011) and smaller arthrodires (Trinajstic et al., 2013: figs. 1C and S2A) the lateral longitudinal bundles extend approximately between the horizontal septum and the dorsal border of the pectoral fin base. In these taxa the lateral longitudinal bundles span about ~1/3 of total trunk height, whereas in larger individuals of D. terrelli (CMNH 6090 and 7054) this region represents approximately 1/2 total trunk height (Figure 13, Figure 15). The regions for these muscles in Dunkleosteus compared to sharks or arthrodires are thus large both relative to organismal size (whether it is scaled by estimated total length, head length, or, among arthrodires, armor length) or in terms of their proportional trunk height.
In nearly all fishes the spine is near-straight posterior to the foramen magnum (Figure 8; see also figures in Gregory and Conrad, 1937; Romeo and Mansueti, 1962; Cousteau et al., 2008; Kamminga et al., 2017; Andrade et al., 2019; Stone and Shimada, 2019; Bonani Mateussi et al., 2020; Kim et al., 2021). A slight dorsal curvature sometimes exists over the ribcage, but typically the foramen magnum and caudal peduncle are nearly dorsoventrally level. The only exceptions are highly compressiform/discoid acanthopterygians and coelacanths (Lund and Lund, 1984), where the spine is strongly arched with the caudal peduncle lower than the occiput. Other compressiform/discoid fishes, like serrasalmids and the “palaeoniscoid” Dorypterus, lack this feature (Westoll, 1941; Andrade et al., 2019; Bonani Mateussi et al., 2020; bony scales make this difficult to evaluate in other “palaeoniscoids”). Given a near-straight spine is the plesiomorphic and more widespread condition, it would also be expected in arthrodires. The caudal peduncle of Dunkleosteus cannot be positioned significantly lower than depicted in Figure 4 without significantly arching the spinal cord, unlike nearly all non-compressiform fishes.
Some arthrodire reconstructions show a sharp ventral flexure of the spinal cord posterior to the occiput and within the trunk armor (Miles, 1966; Miles and Westoll, 1968; Johanson et al., 2013). This appears to derive from the reconstruction of Coccosteus in Miles and Westoll (1968: p. 48), which is often considered as representative of generalized eubrachythoracid anatomy. The trunk armor of this reconstruction is dorsoventrally compressed and geometrically sheared compared to complete specimens of Coccosteus cuspidatus, requiring the spinal cord to bend ventrally around the carinal process to exit the trunk armor. If this reconstruction is retrodeformed following complete specimens of C. cuspidatus, the spinal cord can travel posterior to the foramen magnum in a relatively straight line without unnecessary bending (Figure 8A). Similarly in three-dimensionally preserved eubrachythoracids from the Gogo Formation, the cranio-thoracic joint (and thus the foramen magnum) is generally at or below the ventral border of the carinal process (Dennis and Miles, 1981; Dennis-Bryan, 1987; Gardiner and Miles, 1990, 1994), and the spinal cord does not need to bend around it. Any resting flexure of the spinal column in arthrodires is unlikely.
Scales and Lateral Line
Scales. No visible scales were added to the present reconstruction, as most eubrachythoracid arthrodires were probably scaleless (Stensiö, 1963; Carr, 1995; Janvier, 2003; K. Trinajstic, pers. comm., January 2023). Scales have been reported for some non-eubrachythoracid arthrodires, like Actinolepis, Holonema, and Sigaspis (Goujet, 1973; Mark-Kurik, 1985; Burrow and Turner, 1999; Trinajstic, 1999), but in eubrachythoracids scales are only known in the early-diverging coccosteomorphs Coccosteus and Eldenosteus (Miles and Westoll, 1968; Johnson and Elliott, 1995; Burrow and Turner, 1999). The scales of Coccosteus are extremely small (0.1–0.2 mm in diameter; Miles and Westoll, 1968), whereas those of Eldenosteus are much larger (~1 cm in diameter), heavily ornamented, and lack areas of overlap despite pertaining to a smaller animal (Johnson and Elliott, 1995). This morphological disparity makes it unclear if one or both actually represent scales. Scales are invariably absent in eubrachythoracid specimens from Gogo (K. Trinajstic, pers. comm., January 2023), despite being preserved in actinopterygians (Choo, 2012), sarcopterygians (Andrews et al., 2005), chondrichthyans (Long et al., 2015), and non-eubrachythoracids (Holonema; Trinajstic, 1999) at this site. Similarly, scales are not preserved in association with well-preserved eubrachythoracids at other localities like the Cleveland Shale. This is unusual given fossil fishes are frequently associated with isolated or partially articulated patches of scales. The consistent absence of scales in eubrachythoracid fossils (unlike most fossil fishes) suggests they were probably secondarily lost within this group.
Possible Dunkleosteus skin fragments do exist. Jesse Hyde (Hyde in Heintz, 1938: p. 38) reported possible “Dinichthys” (= Dunkleosteus) scales in passing, described as “much larger than those in Coccosteus, but reminiscent of the latter in shape”. However, figures, specimen numbers, and evidence referring this material to Dunkleosteus were not provided. The specimen CMNH 8735 preserves what appear to be pyritized skin fragments in close association with a Dunkleosteus paranuchal, pelvic girdles, and partially articulated Orodus tooth files. These fragments are covered in fine, unornamented tubercles, the largest being only ~0.5 mm in diameter, with an overall leathery texture resembling the skin of catfishes and rays. However, no clear placoid or rhomboid scales are present. The texture of this material is similar to that of purported Coccosteus scales (Miles and Westoll, 1968: pl. 10E), agreeing with Hyde’s description and suggesting it represents Dunkleosteus skin. However, it cannot be the material reported by Hyde as it was collected in the 1960s. It also suggests this material does not pertain to Orodus, which has prominent rhomboid scales (Zangerl, 1981: p. 92). This material (and the “scales” of Coccosteus) significantly differ from the scales of other arthrodires, which are generally rhomboid structures with obvious tubercular ornamentation (Johnson and Elliott, 1995; Burrow and Turner, 1999; Trinajstic, 1999). This raises the question as to whether the “scales” reported for Coccosteus are actually scales or leathery skin, though the possibility exists that they represent microdenticles as seen in some rays. Future research is needed to confirm whether this material is genuinely skin, along with a wider search for soft tissue preservation in other Cleveland Shale arthrodire remains.
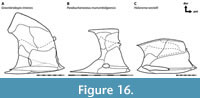 Lateral Line. Dunkleosteus terrelli, as well as most eubrachythoracid arthrodires, appear to have had incomplete lateral lines that did not extend the entire length of its body. Some previous studies have implied eubrachythoracid arthrodires had incomplete lateral lines (i.e., Northcutt, 1997), but did not discuss it in detail. In Dunkleosteus, the main trunk canal stops before the end of the trunk shield in a slight upturn in the center of the posterior dorsolateral plate (Figure 5; see also Heintz, 1932). This would seemingly preclude a lateral line extending the entire length of the body. Only a single main trunk canal is present, whereas in some other arthrodires like Holonema, buchanosteoids, and many coccosteomorphs there are multiple branches of the main trunk canal (Figure 10A–C, Figure 16B–C; see also Miles and Westoll, 1968; Miles and White, 1971; Gardiner and Miles, 1994; Long et al., 2014).
Lateral Line. Dunkleosteus terrelli, as well as most eubrachythoracid arthrodires, appear to have had incomplete lateral lines that did not extend the entire length of its body. Some previous studies have implied eubrachythoracid arthrodires had incomplete lateral lines (i.e., Northcutt, 1997), but did not discuss it in detail. In Dunkleosteus, the main trunk canal stops before the end of the trunk shield in a slight upturn in the center of the posterior dorsolateral plate (Figure 5; see also Heintz, 1932). This would seemingly preclude a lateral line extending the entire length of the body. Only a single main trunk canal is present, whereas in some other arthrodires like Holonema, buchanosteoids, and many coccosteomorphs there are multiple branches of the main trunk canal (Figure 10A–C, Figure 16B–C; see also Miles and Westoll, 1968; Miles and White, 1971; Gardiner and Miles, 1994; Long et al., 2014).
A main trunk canal terminating before the end of the thoracic shield occurs in most eubrachythoracids, though additional interspecific variation exists within this pattern. In coccosteoid coccosteomorphs (e.g., Coccosteus, Millerosteus, Plourdosteus; Figure 10A, C) the main trunk canal is more extensive than in Dunkleosteus but still terminates before the posterior border of the thoracic armor (Miles and Westoll, 1968; Desmond, 1974; Vézina, 1988). Instead, this canal turns dorsally on the posterior dorsolateral plate and continues onto the median dorsal plate. In some specimens and/or taxa, the left and right side of the main trunk canal are actually in midline contact (Miles and Westoll, 1968; Desmond, 1974; Vézina, 1988). By contrast, incisoscutoid coccosteomorphs (e.g., Compagopiscis; Figure 10B) have a main trunk canal almost entirely restricted to the anterior dorsolateral plate (except in Torosteus and Incisoscutum; Dennis and Miles, 1981; Gardiner and Miles, 1990), though remnants of the trunk canal are sometimes present on the median dorsal plate. In camuropiscids the main trunk canal terminates on the anterior lateral plate, and thus is considerably reduced compared to other coccosteomorphs (Dennis and Miles, 1979a, 1979b; Long, 1988). Coccosteomorphs often show dorsal and ventral divisions of the main trunk canal, but neither extends beyond the posterior margin of the armor. Based on its shape the trunk canal in Dunkleosteus is likely homologous with the dorsal division in coccosteomorphs, with the ventral division having been lost.
Other dunkleosteoids, including Eastmanosteus calliaspis, Kiangyousteus yohii, and dunkleosteoid material from Iran that may pertain to Golshanichthys resemble Dunkleosteus terrelli in the main trunk canal ending in a slight upturn on the posterior dorsolateral plate (Figure 10D; see also Lelievre et al., 1981; Dennis-Bryan, 1987; Zhu and Zhu, 2013). Kiangyousteus and some specimens of E. calliaspis also preserve remnants of the main trunk canal on the median dorsal plate (Lelievre et al., 1981; Dennis-Bryan, 1987; Zhu and Zhu, 2013), unlike Dunkleosteus. In aspinothoracidans (Figure 10F, H) the trunk canal usually terminates on the anterior dorsolateral plate (Carr, 1991, 1994, 1996; Rücklin, 2011; Jobbins et al., 2022) though in some individuals of Amazichthys it extends slightly onto the posterior dorsolateral plate (Jobbins et al., 2022). These patterns (along with comparisons in non-eubrachythoracids, see below) suggest an overall reduction in the trunk canal over arthrodire evolutionary history, reaching its greatest extreme in aspinothoracidans and some camuropiscid coccosteomorphs.
Unlike eubrachythoracids, most non-eubrachythoracid arthrodires have a main trunk canal that continues beyond the posterior edge of the trunk armor (Figure 16). This is present in groenlandaspids (Ritchie, 1975; Daeschler et al., 2003; Gess and Trinajstic, 2017), buchanosteoids (Long et al., 2014), Bryantolepis (Denison, 1962), and Holonema (Miles and White, 1971; Trinajstic, 1999), among others. When scales are known for these taxa, they show the main trunk canal was continuous between the trunk armor and body squamation (Goujet, 1973; Burrow and Turner, 1999; Trinajstic, 1999; and in undescribed groenlandaspids, J. Long, pers. comm. March 2023). Similar patterns occur in non-arthrodiran “placoderms” like rhenanidans, antiarchs, Entelognathus, and seemingly Xiushanosteus (Gross, 1963; Béchard et al., 2014; Wang and Zhu, 2022; Zhu et al., 2022; Cui et al., 2023). This further suggests the lateral line of eubrachythoracids ended on the thoracic armor and did not span the entire body. Non-eubrachythoracid arthrodires lack the upturned trunk canal seen in many eubrachythoracids, but instead often show a distinct “zigzag” of the trunk canal on the posterior dorsolateral plate that then continues beyond the armor (Figure 16). Non-arthrodiran placoderms lack either the upturned trunk canal or the “zigzag”. This suggests these features may be phylogenetically relevant characters, with the initial flexure of the “zigzag” of non-eubrachythoracid arthrodires being modified into the upturned posterior canal seen in eubrachythoracids.
Based on this evidence, most eubrachythoracid arthrodires appear to have had incomplete lateral lines. Incomplete lateral lines are fairly common among fishes, independently evolving in many groups (Webb, 1989b). In some extant fishes, the lateral line may continue beyond the end of the trunk canal with canal neuromasts being replaced by superficial neuromasts (Webb, 2014). However, this is unlikely for eubrachythoracid arthrodires. In eubrachythoracids the entire trunk canal deviates from the horizontal axis of the body to terminate on the dorsum of the trunk armor, rather than maintaining a consistent path and merely being replaced posteriorly by superficial neuromasts. The trunk canal may be further reduced, as in camuropiscids and pachyosteomorphs, but this appears to be the ancestral pattern for this group. The extensive dermal ornamentation of the trunk armor suggests additional trunk canals or superficial neuromasts are unlikely to have been present but overlooked, because such structures would conspicuously disrupt dermal ornamentation. A good example of this are the “pit-line complexes” of Ørvig (1971), which are externally visible and may represent osteological correlates of superficial neuromasts. However, Ørvig (1971) only discusses these structures as present on the head shield; he does not report them as present on the trunk armor extending the main trunk canal and the author has been unable to identify similar structures at the end of the trunk canal in Dunkleosteus.
A disjunct main trunk canal is possible but highly unlikely. Disjunct lateral lines are rare in fishes and most restricted to compressiform percomorphs (e.g., Cichlidae, Plesiopidae, Pomacentridae; Webb, 1989a). In these taxa the disruption of the trunk canal is almost universally located over the anal fin (Webb, 1989a, 1990b, 1990a), but eubrachythoracids would require a disruption over the anterior trunk. Discontinuous lateral lines in percomorphs are the result of a unique, bidirectional mechanism of trunk canal formation, unlike the anterior-to-posterior sequence of most fishes (Webb, 1990b). A disjunct lateral line canal in eubrachythoracid arthrodires requires invoking unique anatomical patterns not seen in other fishes.
The apparent absence of a post-thoracic lateral line in eubrachythoracid arthrodires may relate to their relatively deep, wide bodies. A wide and deep body might be expected to create a larger wake, and thus less pressure differential over the trailing side (Ristroph et al., 2015). This could reduce post-thoracic lateral line effectiveness and select for its loss, but this requires further biomechanical testing. The size, morphology, and distribution of lateral line canals in fishes is thought to significantly correlate with ecology and developmental biology, but the significance of a given morphology is unclear (Webb, 2014). The absence of a post-thoracic lateral line in Dunkleosteus does not preclude this taxon from being an active, pelagic animal. Several extant active pelagic fishes, including herrings and anchovies (Clupeiformes) and adult swordfish (Xiphias gladius) show an even more extreme condition than most eubrachythoracids in the trunk canal being entirely absent (Nakamura, 1985; Webb, 2014).
Head and Mouthparts
The cranial osteology of Dunkleosteus terrelli has been extensively discussed by other researchers (e.g., Heintz, 1932; Dunkle and Bungart, 1942, 1946; Engelman et al., in press; and references therein), and so will not be discussed in detail here. Jaw musculature of D. terrelli, which is expected to have some influence on life appearance, has been previously discussed by Heintz (1932), Dunkle and Bungart (1946), Anderson and Westneat (2007, 2009), and Engelman et al. (in press). The present study primarily focuses on aspects of the cranium unrelated to jaw function. Whether the armor plates were visible in living representative of Dunkleosteus and the presence or absence of “lips” is an extensive enough question to deserve separate treatment, and is not considered here in the interest of space. However, it can be noted this reconstruction shows the dermal armor as still externally visible and while the oral region has more soft tissue than often depicted the gnathal plates are still partially exposed (Figure 4).
One plate that affects life appearance is the submarginal plate (Figure 3C). This is an elongate, dermal ossification associated with the hyomandibula located along the posterodorsal margin of the cheek unit (Gardiner and Miles, 1990; Carr et al., 2009; Hu et al., 2017). Heintz (1932) was unaware of the submarginal plate in Dunkleosteus, and reconstructed the suborbital plate and head shield in direct contact along the former’s posterolateral margin (Figure 3A). Most depictions of Dunkleosteus have followed Heintz (1932) and therefore often lack the submarginal plate. The submarginal of Dunkleosteus is unusually elongate compared to other arthrodires (L/W ratio > 7.8, versus < 5.5 in most arthrodires, see Engelman et al., in press), almost rod-shaped rather than rectangular (Figure 4, Figure 7), and appears to have lacked sutural contacts with either the head shield or the remaining cheek plates (Engelman et al., in press).
Mouthparts. The mouth of Dunkleosteus became larger and more protruding (i.e., terminal) throughout ontogeny (Figure 5). Several studies have documented positive mouth allometry in Dunkleosteus based on changes in the shape of the suborbital plate (Heintz, 1932), general mouth dimensions (Engelman, 2023a), and an overall elongation of the infragnathal across ontogeny (Boyle et al., 2016; Engelman, 2023b). Arthrodires generally exhibit positive allometry of the mouth (Trinajstic, 1995; Trinajstic and Hazelton, 2007), which may be related to the infragnathal growing by apposition of new bone at the posterior and ventral edges of the oral region (Ørvig, 1980; Rücklin et al., 2012; Lebedev et al., 2023). Dunkleosteus has a particularly long cheek unit and jaw relative to other arthrodires. This can be demonstrated quantitatively by scaling cheek/jaw length against other parts of the body (Engelman, 2023a), has been noted by previous authors (Denison, 1978) and resembles patterns in other eubrachythoracids where more macropredatory species have proportionally longer cheeks and jaws (Miles, 1969: p. 151; Dennis-Bryan, 1987: p. 21).
The linguiform process of the suborbital plate, which indirectly supports with the posterior supragnathals via the autopalatine cartilage (Heintz, 1932; Long, 1995; Engelman et al., in press), also shows positive allometry, resulting in increasingly prognathic gnathals throughout ontogeny (Engelman et al., in press). The youngest individuals of D. terrelli (e.g., CMNH 6194, CMNH 7424) have small linguiform processes (Figure 5A) and supragnathals positioned close to the external margins of the suborbital plate like many Gogo arthrodires (Dennis and Miles, 1981; Gardiner and Miles, 1994; Long, 1995). This process becomes proportionally larger in subadult/adult individuals of D. terrelli due to the hypertrophy of the likely m. preorbitalis (Engelman et al., in press), resulting in the mouthparts projecting beyond the external margins of the suborbital and the anterior border of the supragnathals reaching or extending beyond the anterior end of the head shield in larger individuals (Figure 5B–D). The anterior supragnathals cannot be positioned more posteriorly, or else their large lateral ridge conflicts for space with the linguiform process, supporting the ontogenetic prognathism seen in the CMNH material. Increasing prognathism throughout ontogeny occurs in Incisoscutum ritchei (Long, 1994: fig. 4), whether it occurs in other Gogo arthrodires is unclear. The well-developed linguiform process results in Dunkleosteus having a terminal mouth versus the subterminal and slightly superiorly oriented mouth of Coccosteus and most of the Gogo arthrodires.
Branchial Opening. The musculature of the ventral head and branchial region was restored after Heintz (1932), Dunkle and Bungart (1946), Long (1995), and Johanson (2003). The opening for the gill chamber in Dunkleosteus and other arthrodires is located in the cleft between the head and thoracic armor (Figure 1 and Figure 3). The location of this feature can be identified by the presence of denticles (postbranchial lamina) on the interolateral plates representing the posterior face of the gill chamber (Heintz, 1932: p. 199–202; Miles and Dennis, 1979; Johanson and Smith, 2003; Carr et al., 2009). Anterodorsal to this, the cucullaris fossa of the head shield in some arthrodires (Cowralepis) is partially denticulated, suggesting this structure represents (at least in part) the dorsal border of the parabranchial cavity (Carr et al., 2009). Suggestions that the parabranchial chambers of arthrodires extended posterior to the cranio-thoracic joint (Stensiö, 1963) are not supported by anatomical features (Johanson and Smith, 2003; Carr et al., 2009). Many reconstructions of Dunkleosteus incorrectly reconstruct the location of the branchial opening due to mistaking the trunk armor as part of the head (an operculum). This results in the gill opening either being covered with soft tissue (Adams, 1919) or depicted as posterior to the trunk armor (Figure 2; e.g., Garrod, 2021).
In chondrichthyans and osteichthyans the gills are usually posterior to the cranium, but in arthrodires the gills are internal to the cheek and jaws (Figure 8) (Heintz, 1932; Carr et al., 2009). Glossopharyngeal and vagus nerve position are conserved across gnathostomes (Carr et al., 2009) and arthrodires and eugnathostomes show similar head-trunk proportions (Stensiö, 1963: p. 13; Engelman, 2023a, 2023b). This suggests differences in cranial morphology between arthrodires and eugnathostomes are not due to changes in branchial chamber location, but a posterior expansion of the cranium and cheek plate relative to a conserved head-trunk boundary (Figure 8; see also Carr et al., 2009; Young, 2010: fig. 1A). Like most arthrodires (Miles, 1969), the cheek unit of Dunkleosteus (specifically the postsuborbital plate) fits into the anterior notch of the anterior lateral, forming a pseudo-operculum externally covering the gill slits in life. This would leave only a single, large cleft between the head and thoracic armor. The individual gill slits of arthrodires would not be visible unless the head was moved from a neutral position, such as when the mouth was opened.
Eyes and Sclerotic Rings. Dunkleosteus terrelli is frequently depicted with small, beady eyes, but orbits in complete specimens are relatively large (8.1 cm wide in CMNH 5768). Preliminary research on eye size in arthrodires (Engelman, in prep) suggests orbit size in Dunkleosteus is unremarkably average among fishes, comparable to a tuna (Thunnus spp.) of the same body mass. Specimens of Dunkleosteus with associated sclerotic rings (CMNH 6090, CMNH 6194, CMNH 7424) show they occupied the entire orbit. The small eyes in previous reconstructions of Dunkleosteus are primarily because the historic mount of CMNH 5768 (and by extension its many replicas) has sclerotic rings significantly smaller than its orbital diameter (~5.8 cm). These are actually plaster replicas, likely from a smaller individual. Part of the original sclerotic rings of CMNH 5768 are preserved on the ventral surface of the head shield. These fragments are much larger than the sclerotic rings of the mount and indicate the complete sclerotic ring would have filled the entire orbit, like other specimens of D. terrelli.
 Whether the sclerotic ring of Dunkleosteus terrelli was visible in life is unclear. Witton (2018) suggested sclerotic rings in extinct vertebrates would have been covered by soft tissues in life, based on the condition in living birds, reptiles, and fishes. However, unlike living vertebrates, the sclerotic rings of most arthrodires have considerable dermal ornamentation (Dennis and Miles, 1979a, 1980, 1981; Dennis-Bryan, 1987), potentially suggesting a different arrangement. Even some aspinothoracidans with otherwise unornamented dermal armor like Gymnotrachelus hydei and Steneosteus angustopectus retain prominent sclerotic ornamentation (Figure 17A–B). Sclerotic ornamentation is usually restricted to a band around the aperture in eubrachythoracids, but the entire ring may be ornamented in non-eubrachythoracids (Heintz, 1933; Goujet, 1984: fig. 19; Burrow et al., 2005). Dermal ornamentation is typically restricted to surfaces exposed or near-exposed to the external environment in arthrodires, like the external surface of the dermal armor and the postbranchial lamina of the gill chamber (Johanson and Smith, 2005), suggesting the sclerotic ring had little to no external soft tissue. Dunkleosteus and some other arthrodires (Heintzichthys, Gorgonichthys) lack sclerotic tuberculation, but this may be due to the general loss of armor tuberculation in these taxa rather than a more internally positioned sclerotic ring. The ornamented sclerotic rings of arthrodires resemble the denticle-covered eyeballs of whale sharks (Rhincodon typus), particularly in their distribution on the orbit’s surface (Tomita et al., 2020: fig. 2). In R. typus these denticles are suggested to protect the eye from mechanical damage (Tomita et al., 2020), and the ornamented sclerotic rings of arthrodires may have functioned similarly.
Whether the sclerotic ring of Dunkleosteus terrelli was visible in life is unclear. Witton (2018) suggested sclerotic rings in extinct vertebrates would have been covered by soft tissues in life, based on the condition in living birds, reptiles, and fishes. However, unlike living vertebrates, the sclerotic rings of most arthrodires have considerable dermal ornamentation (Dennis and Miles, 1979a, 1980, 1981; Dennis-Bryan, 1987), potentially suggesting a different arrangement. Even some aspinothoracidans with otherwise unornamented dermal armor like Gymnotrachelus hydei and Steneosteus angustopectus retain prominent sclerotic ornamentation (Figure 17A–B). Sclerotic ornamentation is usually restricted to a band around the aperture in eubrachythoracids, but the entire ring may be ornamented in non-eubrachythoracids (Heintz, 1933; Goujet, 1984: fig. 19; Burrow et al., 2005). Dermal ornamentation is typically restricted to surfaces exposed or near-exposed to the external environment in arthrodires, like the external surface of the dermal armor and the postbranchial lamina of the gill chamber (Johanson and Smith, 2005), suggesting the sclerotic ring had little to no external soft tissue. Dunkleosteus and some other arthrodires (Heintzichthys, Gorgonichthys) lack sclerotic tuberculation, but this may be due to the general loss of armor tuberculation in these taxa rather than a more internally positioned sclerotic ring. The ornamented sclerotic rings of arthrodires resemble the denticle-covered eyeballs of whale sharks (Rhincodon typus), particularly in their distribution on the orbit’s surface (Tomita et al., 2020: fig. 2). In R. typus these denticles are suggested to protect the eye from mechanical damage (Tomita et al., 2020), and the ornamented sclerotic rings of arthrodires may have functioned similarly.
The sclerotic rings, like most elements of Dunkleosteus terrelli, are typically flattened when preserved. In rare specimens with uncrushed sclerotic rings, like CMNH 5074, CMNH 6090, and CMNH 7424, the rings are cup-shaped and bulge externally (Figure 17C). Other taxa like Heintzichthys (CMNH 5291) and Phlyctaenius (Heintz, 1933) show a similar condition. How this affected the eye’s life appearance is unclear; it could suggest the eyes were telescoped and bulged outwards, or possibly the rings were positioned more internally and only their outer edges were visible. Heintz (1933) suggested Phlyctaenius acadicus had telescoped (= bulging) eyes based on this feature, but whether this can be applied to eubrachythoracids is unclear.
Nostrils. Dunkleosteus had two pairs of external nostrils, like most jawed fishes (Zhu and Ahlberg, 2004). One pair was located between the ventrolateral face of the rostral plate of the head shield and the anteromedial face of the postnasal plate. The nostril opening is formed by a well-developed anteromedial notch in the postnasal plate, which in other arthrodires has a highly denticulated rim (Miles and Westoll, 1968). The ventral edge of this notch forms an interfenestral process separating the two pairs of nostrils (Miles and Westoll, 1968; Desmond, 1974; Long, 1995). The second pair of nostrils are identifiable by a broad notch on the medial face of the ethmoid ossification (Engelman et al., in press), and are medial and ventral to the first pair. The number and position of nasal openings in Dunkleosteus resembles other arthrodires like Mcnamaraspis (Long, 1995) and Latocamurus (Long, 1988).
The snout of Dunkleosteus is rounded and blunt. The head shield, suborbital plate, postnasal plate, and ethmoid cartilages interlock to form a nearly continuous covering over the anterior face of the head (Figure 3; see also Engelman et al., in press). Both pairs of external nostrils open within this bony covering. The gnathals extend anteriorly to the level of the nostrils or slightly beyond, resulting in no space for a protruding rostrum like in neoselachians. Most reconstructions of Dunkleosteus show a rounded snout, but some show a slightly pointed, shark-like rostrum extending anterior to the mouth (e.g., Stensiö, 1963, as well as some other, more recent depictions), which is not supported by fossils. Stensiö (1963) restored arthrodires with large, protruding annular cartilages, but later studies show most arthrodires were snub-nosed and the annular cartilages simply fit on the end of the blunt snout without creating a pointed profile (Miles and Westoll, 1968; Miles and White, 1971; Long, 1995).
Fins
Dunkleosteus terrelli was reconstructed here with one dorsal fin, one pair of pectoral fins, one pair of pelvic fins, and one anal fin. Fin number (with the possible exception of the anal fin) is highly conserved in arthrodires, despite body outlines being known for several distantly related taxa of varying inferred life habits (e.g., Amazichthys, Coccosteus, Incisoscutum). In particular, these taxa consistently show a single dorsal fin, suggesting arthrodires lacked second dorsal fins. Fin profiles in this reconstruction were expanded from their bases with ceratotrichia, given the presence of ceratotrichia in the fins of D. terrelli and other arthrodires (Carr et al., 2010; Greenfield, 2020; Jobbins et al., 2022). Reconstructions of Dunkleosteus often show visible fin rays, an actinopterygian feature not present in placoderms. There is no evidence for fin spines in arthrodires, though in non-eubrachythoracids and some coccosteomorphs the median dorsal, spinal, or posterior ventrolateral plate may be modified to fulfill a similar role (Westoll, 1947; Miles and Westoll, 1968; Miles, 1969; Gess and Trinajstic, 2017). These modifications are not seen in Dunkleosteus.
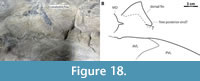 The fins of Dunkleosteus were reconstructed with somewhat pointed tips, similar to a shark. This agrees with the pectoral fin outline reported for CMNH 8982 by Carr et al. (2010), which has a somewhat pointed shape (though missing the extreme tip). Soft tissue fin outlines are virtually unknown for other arthrodires. Coccosteus specimens are reported to occasionally preserve soft tissue traces (Trewin, 1986: p. 34), but complete fin outlines have not been described. The aspinothoracidan Amazichthys trinajsticae has distinctly rounded fin tips (Jobbins et al., 2022). While it is tempting to speculate that rounded fin tips characterize arthrodires more broadly, another aspinothoracidan specimen (CMC VP8545, Figure 18) preserves the carbonaceous outline of a dorsal (?) fin with a pointed tip, indicating fin tip shape was variable in these animals.
The fins of Dunkleosteus were reconstructed with somewhat pointed tips, similar to a shark. This agrees with the pectoral fin outline reported for CMNH 8982 by Carr et al. (2010), which has a somewhat pointed shape (though missing the extreme tip). Soft tissue fin outlines are virtually unknown for other arthrodires. Coccosteus specimens are reported to occasionally preserve soft tissue traces (Trewin, 1986: p. 34), but complete fin outlines have not been described. The aspinothoracidan Amazichthys trinajsticae has distinctly rounded fin tips (Jobbins et al., 2022). While it is tempting to speculate that rounded fin tips characterize arthrodires more broadly, another aspinothoracidan specimen (CMC VP8545, Figure 18) preserves the carbonaceous outline of a dorsal (?) fin with a pointed tip, indicating fin tip shape was variable in these animals.
Dorsal Fin. Dorsal fin elements are unknown for Dunkleosteus, but the position of this fin can be constrained by other body elements. In most nektonic vertebrates, such as sharks, cetaceans, and ichthyosaurs, the dorsal fin is located roughly over the center of mass to aid as a stabilizer (Aleev, 1969; Fish, 2002; Lingham-Soliar, 2005), though in some like tarpon (Megalopidae) and Ichthyodectiformes the dorsal fin is more posterior. Additionally, in many tachynektonic acanthopterygians like scombrids and sphyraenids the anterior spiny dorsal is either small and/or folds back during steady swimming and the soft dorsal (which functionally analogous to the dorsal fin in other taxa) is located posterior to the center of mass (Lauder and Drucker, 2004; Fish and Lauder, 2017). A dorsal fin located over the center of mass might be expected for Dunkleosteus, given this taxon has been interpreted as a nektonic organism (Carr, 2010; Ferrón et al., 2017a). However, the location of the dorsal fin origin is limited anteriorly by the large carinal process and associated submedian dorsal plate; if the fin is located too far anterior its basals conflict for space with these other midline dorsal structures (Figure 14, compare with Figure 7, Figure 8). The extent of the carinal process and submedian dorsal plate prevent the dorsal fin of Dunkleosteus from being located over the deepest part of the body, unlike many large marine vertebrates.
A correlation between dorsal fin position and the morphology of the carinal process/submedian dorsal plate is supported by the condition in other arthrodires. Dorsal fin elements are known in several coccosteomorphs (e.g., Miles and Westoll, 1968; Desmond, 1974; Dennis and Miles, 1981) and the aspinothoracidans Amazichthys (Jobbins et al., 2022), Heintzichthys (Dean, 1896), and possibly an undescribed selenosteid (CMC VP8545, Figure 18). In all of these taxa the dorsal fin is posterior to the carinal process. In the latter three taxa, generally considered as pelagic animals, the dorsal fin origin is anterior to the pelvic girdle. In Amazichthys and CMC VP8545 the dorsal fin origin is located at the carinal process, while in Heintzichthys it is further posterior, closer to the pelvis and end of the ventral shield. By contrast, in coccosteomorphs like Coccosteus (Figure 7, see also Miles and Westoll, 1968), Dickosteus (NHMUK PV OR 49663), Watsonosteus (NMS G.1995.4.2), Plourdosteus (MHNM 02-901), and Incisoscutum (Dennis and Miles, 1981: fig. 21), the dorsal fin origin is posterior to the pelvis and the center of mass. Dorsal fin elements are unknown in the dunkleosteoid Eastmanosteus but the extent of the thoracic shield and carinal process would prevent it from being located over the center of mass (Figure 10D), like coccosteomorphs and Dunkleosteus. The dorsal fin in Figure 5 was reconstructed as far anterior as possible before it conflicted for space with the carinal process and submedian dorsal plate. This results in a dorsal fin position more similar to Heintzichthys and Amazichthys while still obeying the constraints of the thoracic shield.
 Complete submedian dorsal plates are unknown for Dunkleosteus, but one specimen (CMC VP8294) preserves a fragment of this plate in situ (Figure 19). The preserved fragment shows an articulation with the fossa on the posterior face of the carinal process like other arthrodires (Miles and Westoll, 1968), but no other details can be determined. The posteroventrally protruding shape of the carinal process in Dunkleosteus means the articulation between this structure and the submedian dorsal plate is oriented anterodorsally, but without a complete submedian dorsal plate this significance of this morphology is unclear.
Complete submedian dorsal plates are unknown for Dunkleosteus, but one specimen (CMC VP8294) preserves a fragment of this plate in situ (Figure 19). The preserved fragment shows an articulation with the fossa on the posterior face of the carinal process like other arthrodires (Miles and Westoll, 1968), but no other details can be determined. The posteroventrally protruding shape of the carinal process in Dunkleosteus means the articulation between this structure and the submedian dorsal plate is oriented anterodorsally, but without a complete submedian dorsal plate this significance of this morphology is unclear.
The function of the submedian dorsal plate in arthrodires and its associated soft tissues have not been extensively investigated. It was clearly not a basal plate for the dorsal fin, as the submedian dorsal plate and dorsal fin base are not associated in most arthrodires (Miles and Westoll, 1968: pl. 5–9; Dennis and Miles, 1981: fig. 21). Stensiö (1963) considered the submedian dorsal plate to support a second, anterior dorsal fin composed only of ceratotrichia, but later analyses found this proposed fin was actually an artifact of preparation (Miles and Westoll, 1968: p. 450). Additionally, in early eubrachythoracids like Millerosteus and Coccosteus, the carinal process is near the center of the median dorsal plate, resulting in the submedian dorsal plate being internal to this plate and not exposed to the organism’s dorsum (Miles and Westoll, 1968; Desmond, 1974: fig. 4). The median dorsal plate in these taxa also has a long, posteriorly projecting spine, further separating the carinal process and submedian dorsal plate from any external midline structure (ibid). These features would make it difficult for tissues to extend from the carinal process and submedian dorsal plate to external structures on the dorsal midline of the body.
The extent of the thoracic armor/size of the carinal process may constrain dorsal fin position in arthrodires. Many later nektonic arthrodires have an anteroposteriorly short thoracic shield (Miles, 1969; Carr, 1995), which could allow the dorsal fin to be more anteriorly positioned over the center of mass. Species with a shortened thoracic shield do not also shorten the ventral shield (Carr, 1995), indicating this trend is driven by selective pressures acting on the dorsum versus a general reduction of the trunk armor allowing more of the trunk to participate in lateral undulation. Many aspinothoracidans, particularly selenosteids, take this trend even further by greatly reducing the carinal process and possibly losing the submedian dorsal plate (Dunkle, 1947; Carr, 1991, 1996; Jobbins et al., 2022), and some even show specialized features allowing for a more anterior position of the dorsal fin. Amazichthys has an autapomorphic posterior embayment of the median dorsal plate, allowing the dorsal fin to extend anterior to the posterior margin of the thoracic armor (Figure 10H; Jobbins et al., 2022). Similarly, Bungartius has a “notch” in the posterior end of the median dorsal plate (Dunkle, 1947: fig. 4). The carinal process of Bungartius is almost vestigial and lacks a clear facet for the submedian dorsal plate. This suggests the notch is not related to the usual structures at the posterior end of the trunk armor in arthrodires, but based on its position could represent an articulation between the first basal of the dorsal fin and the trunk armor.
In contrast to these taxa, massive, posteriorly-projecting carinal processes seem to have independently evolved in several large-bodied arthrodires, including Dunkleosteus, Gorgonichthys, Titanichthys, and the Heterostiidae (Dunkle and Bungart, 1940; Boyle and Ryan, 2017; Schultze and Cumbaa, 2017). This would prevent the dorsal fin from being located over the deepest part of the body/center of mass, despite these taxa (except Heterostiidae) being considered pelagic. This suggests other factors are selecting for a large carinal process and overriding selective pressure for a more anteriorly positioned dorsal fin, potentially creating an evolutionary trade-off. However, testing this hypothesis depends on identifying the function of the carinal process and submedian dorsal plate. Previous authors have suggested the carinal process served as the origin for the m. levator capitis major, a major jaw opening muscle/cranial elevator (Heintz, 1932), potentially implying a trade-off between cranial elevation ability and dorsal fin position, but later authors doubted this and preserved muscle attachment sites show the m. levator capitis major originates well anterior to the carinal process (Miles, 1969; Trinajstic et al., 2013). The function of the carinal process and submedian dorsal plate in arthrodires remain unclear.
The dorsal fin outline of Dunkleosteus is unknown. The few pachyosteomorphs with known dorsal fins (all aspinothoracidans) show highly diverse shapes. Heintzichthys has a small dorsal fin base (comparable in relative size to a tarpon, Megalops spp.), and the fin outline is not preserved (Dean, 1896). Amazichthys, on the other hand, shows a long dorsal fin base (Jobbins et al., 2022), longer than that of even demersal coccosteomorphs, and the fin is tall and rounded similar to a mahi mahi (Coryphaena spp.). CMC 8545 preserves a small, triangular dorsal (?) fin, similar to a nektonic shark but smaller and not as tall (Figure 18). Such diversity in dorsal fin shape might be expected for pelagic arthrodires given the fin shape diversity seen in living fishes. For the present study Dunkleosteus was reconstructed assuming a relatively conservative fin size base (similar to a coccosteomorph) but with this fin’s ceratotrichia forming a triangular shape, given similar dorsal fin shapes occur in many groups of marine vertebrates.
Pectoral Fin. The position of the pectoral fin in Dunkleosteus is well constrained by the pectoral fenestra between the anterior lateral, spinal, and anterior ventrolateral plates. Several specimens even preserve the scapulocoracoid and fin basals in situ (CMC VP8294, CMNH 8982; see Carr et al., 2010). The pectoral fin of Dunkleosteus is located at an extreme anterior position on the body. This is facilitated by the unusual shape of the anterior lateral plate, which is narrow, strongly L-shaped, and has an anteriorly projecting ventral wing, allowing the scapulocoracoid to be more anteriorly positioned than in other arthrodires (Figure 10). Approximately half of the pectoral fenestra underlies the head, with its anterior border reaching the level of the suture between the suborbital and postsuborbital plates of the cheek unit. This results in the pectoral fin base being largely ventral to the gill chamber (Figure 5). No other arthrodire shows such an anteriorly positioned pectoral fin (Figure 10). In most taxa, the pectoral fin origin is at or near the level of the cranio-thoracic joint and significantly posterior to the gill chamber. The only arthrodire that approaches the condition in Dunkleosteus is Eastmanosteus calliaspis, in which the anterior 1/4th of the pectoral fenestra underlies the gill chamber (Figure 10D). Some selenosteids may have shown an analogous condition (Stensiö, 1959, 1963).
The prepectoral length of Dunkleosteus terrelli is proportionally shorter than most extant fishes (Figure 11A; Appendix 3: fig. 4.8), though still within the range of variation in other species. Notably, this is calculated relative to the body length estimates of Engelman (2023b). Greater body lengths would make the pectoral fin position even more extreme; values as low as 4.2 m for typical adult D. terrelli (e.g., CMNH 5768) produce a significantly more anterior pectoral fin than almost any non-anguilliform fish, living or extinct (Appendix 3: fig. 4.9). It is possible this is related to the stocky body plan of D. terrelli. A more anteriorly located pectoral fin relative to the center of mass creates a longer lever arm that allows it to apply more torque and function as a more effective rudder (Aleev, 1969; Fish, 2002; Fish and Lauder, 2017). Thus, fishes with comparatively heavyset bodies might require more anterior pectoral fins for effective steering. However, the pectoral fins cannot be located too far anterior on the body or else risk destabilizing the animal, as the long lever arm means even slight changes in fin position can create large torques (Fish and Lauder, 2017). Traditional longer-bodied depictions of D. terrelli would result in the pectoral fin being so far anterior it potentially makes the animal highly unstable, especially compared to the reconstruction depicted here. These factors further support a comparatively shorter, robust body plan in D. terrelli.
The fins of arthrodires have often been compared to sharks in terms of gross morphology (presence of basals and ceratotrichia) and function (Stensiö, 1959; Carr, 1995; Carr et al., 2010; Jobbins et al., 2022). In spite of this, Dunkleosteus differs from modern sharks in having a proportionally larger pectoral fenestra (and by extension the pectoral fin/fin base) relative to body length. In most arthrodires the scapulocoracoid fills the entire pectoral fenestra/foramen (Carr et al., 2010), and therefore is a good proxy for pectoral fin base size. In D. terrelli the pectoral fenestra (measured from the anterior ventrolateral plate) is about 8.5–10% total length, whereas in most extant large-bodied sharks it is about 5–7% total length (Appendix 3). In CMC VP8294 the articulation facet on the scapulocoracoid is approximately 83% the total length of this element, though soft tissues may have expanded the fin base (Stensiö, 1959: p. 22–23). Even if the pectoral fin base of Dunkleosteus did not occupy the entire pectoral fenestra, the fin was still relatively large and at the least comparable in size to modern sharks.
When compared to other arthrodires, the pectoral fin base of Dunkleosteus is larger than coccosteomorphs (3.9–8.5% total length) but is closer in size to the pachyosteomorphs Amazichthys, Brachyosteus, Eastmanosteus, Enseosteus, and Heintzichthys (8.5–11.9% total length) (Appendix 3: fig. 4.15). Based on fin basal count, this appears due to an increase in pectoral fin base size, rather than a shorter head and body armor relative to a conserved pectoral fin size. Pachyosteomorph arthrodires consistently show 1.3–2 times the number of fin basals of coccosteomorphs (7–9 basals versus 12+; Carr et al., 2010: table 2). Dunkleosteus actually has one of the highest basal counts among eubrachythoracids, with a minimum of 14–16 basals identified in two specimens (CMC VP8294 and CMNH 8982; Carr et al., 2010). This is actually greater than Heintzichthys, which has been noted to have an extremely large pectoral fin base (Carr, 1991, 1995) but a maximum basal count of 13 (Carr et al., 2010). This agrees with the observations of previous authors (Stensiö, 1959; Miles, 1969; Carr, 1995), who noted an expansion in pectoral fin base size in pachyosteomorphs associated with the pectoral fin opening on the trunk armor changing from a small, enclosed pectoral foramen in to a wide pectoral fenestra. A broader examination of pectoral fin base size evolution in arthrodires would require greater sampling of aspinothoracidans, and is beyond the scope of this study.
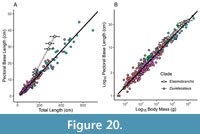 The relatively larger pectoral fin base of Dunkleosteus terrelli appears driven by differences in body shape between this taxon and modern sharks. Dunkleosteus has a larger pectoral fin base than that of sharks if scaling by body length (Figure 20A), but if scaling by body mass D. terrelli has a pectoral fin base expected for an animal of its size (Figure 20B). This suggests the seemingly larger pectoral fin base of Dunkleosteus is a consequence of its stockier body. Specifically, because the pectoral fin plays a role in steering (Aleev, 1969) a more robust body might require a proportionally larger pectoral fin, and thus pectoral fin size might be expected to scale with body mass rather than length. A much longer body for D. terrelli would potentially make the pectoral fin unusually small relative to length or mass, supporting the smaller sizes for D. terrelli presented here and elsewhere (Engelman, 2023b).
The relatively larger pectoral fin base of Dunkleosteus terrelli appears driven by differences in body shape between this taxon and modern sharks. Dunkleosteus has a larger pectoral fin base than that of sharks if scaling by body length (Figure 20A), but if scaling by body mass D. terrelli has a pectoral fin base expected for an animal of its size (Figure 20B). This suggests the seemingly larger pectoral fin base of Dunkleosteus is a consequence of its stockier body. Specifically, because the pectoral fin plays a role in steering (Aleev, 1969) a more robust body might require a proportionally larger pectoral fin, and thus pectoral fin size might be expected to scale with body mass rather than length. A much longer body for D. terrelli would potentially make the pectoral fin unusually small relative to length or mass, supporting the smaller sizes for D. terrelli presented here and elsewhere (Engelman, 2023b).
The pectoral fin of Dunkleosteus terrelli shows clear positive allometry. The pectoral fenestra is about 8% total length and 45% skull length in smaller specimens (CMNH 7424, CMNH 8982) and 10% total length and 55% skull length in larger ones (CMNH 6090, CMNH 5768) (Appendix 10). CMNH 7054 is an exception, but this may be due to taphonomic elongation of this specimen’s skull. This allometry is reflected in the ventral shield, with the pectoral fenestra representing about 27% total ventral shield length in CMNH 7424 but 33% in CMNH 5768. The scapulocoracoid (and thus pectoral fin base) fills the entire pectoral fenestra, meaning larger individuals of D. terrelli had proportionally larger pectoral fins (Figure 20A). This is unlike sharks, where pectoral fin size remains constant across ontogeny (e.g., Garrick, 1982; Garrick, 1985). This may be because sharks generally exhibit body shape isometry across ontogeny, unlike arthrodires. The increasing robustness of the body across ontogeny may explain the proportionately larger pectoral fins of adult Dunkleosteus.
Carr et al. (2010) describe a specimen of Dunkleosteus terrelli (CMNH 8982) with a partial pectoral fin outline and basals articulated with the scapulocoracoid. This fin has a relatively elongate, pointed shape with an aspect ratio of ~1.3 (Carr et al., 2010: p. 116). Its shape resembles modern pelagic chondrichthyans like lamnids and alopiids, but is somewhat broader at its base. Carr et al. (2010: p. 120) estimate the pectoral fin span of CMNH 8982 is roughly twice its thorax width. Assuming a conserved aspect ratio, the positive allometry of the pectoral fin base suggests this proportion was maintained across ontogeny in Dunkleosteus, despite positive allometry in trunk height. Carr et al. (2010: p. 120) describe a specimen of Dunkleosteus marsasi (MNHN-MCD 162) in which the basals are oriented at a nearly 90° angle to the anteroposterior axis, which suggests in life the fin was oriented almost lateral to the body with a near-straight leading edge, resembling many lamnids. This is supported by CMNH 8982, whose fin outline appears to have a straight anterior edge (Carr et al., 2010). Near-perpendicular basals are not universal among arthrodires; some taxa like Heintzichthys have basals oriented at a 130° angle to the body (Carr et al., 2010: p. 120), suggesting a posterolaterally oriented pectoral fin in vivo as seen in life photos of modern carcharhinids (Engelman, pers. obs.; P. Sternes pers. comm., December 2023).
The presence of a free posterior edge to the pectoral fin is unclear, but likely. Early chondrichthyans were originally reconstructed with fins protruding directly from the body and lacking a free end (Dean, 1909b), but this is contradicted by specimens of Cladoselache (Tomita, 2015) and the ctenacanths Ctenacanthus and Dracopristis (Hodnett et al., 2021). The free posterior end of the fin is typically located close to the body, and thus can be easily obscured by the trunk when specimens are preserved as carbonized body outlines. The unnamed selenosteid CMC VP8545 possibly preserves a free posterior edge on its putative dorsal fin (Figure 18). These features suggest free posterior ends of fins may be more widely distributed in fishes, potentially including arthrodires.
Whether Dunkleosteus had plesodic or aplesodic pectoral fins is unclear. A few specimens of Dunkleosteus preserve radial elements in addition to basals, but preservation makes it unclear how far distally they extended (Carr et al., 2010). The large, distal articulations on the basals suggest the radials were well-developed and the fin was robust, especially compared to arthrodires like Heintzichthys (Carr et al., 2010), but this does not determine how much of the fin was supported by cartilage. Distal radials have not been described for any other arthrodire, including Heintzichthys and Amazichthys (Carr et al., 2010; Jobbins et al., 2022). The question of plesodic or aplesodic fins in Dunkleosteus is important because virtually all living aplesodic sharks are relatively slow swimmers (the most active being some squalids) and are almost entirely non-pelagic (Hoffmann et al., 2020). This is because plesodic fins are stiffer and function better as hydrofoils, whereas aplesodic fins allow the distal fin greater maneuverability and freedom of motion, and thus generally occur in taxa living in closed environments (Wilga and Lauder, 2004; Hoffmann et al., 2020). Plesodic fins are a labile feature, independently evolving in most clades of tachynektonic chondrichthyans, including Carcharhiniformes, Lamniformes (Hoffmann et al., 2020), Symmoriformes (Tomita, 2015), and Ctenacanthiformes (Hodnett et al., 2021), suggesting they are not out of the question for Dunkleosteus. The pectoral fin outline of CMNH 8982 more closely resembles the pointed fin of plesodic sharks than the more rounded pectoral fin typical of aplesodic ones, potentially hinting at a more plesodic fin, but a precise answer is not available at this time.
Pelvic Fins. Several undescribed pelvic girdles of Dunkleosteus terrelli exist in the CMNH collections, some of which have associated basals. A detailed description of the anatomy of these elements is currently under preparation. Therefore, only features directly related to the life appearance of D. terrelli, specifically the relative size of the pelvic fins and their position on the body, are considered here.
Previous authors have remarked that the pelvic girdle of arthrodires is typically immediately posterior to the end of the ventral shield (Goujet, 1984; Young, 2010; Trinajstic et al., 2015; Wilson, 2019; Engelman, 2023b). A more detailed survey of this condition in arthrodires with the pelvic girdle in situ (Table 3) confirms this observation and shows it to be present in a wide variety of taxa. In most of these taxa the pelvic girdle is almost in contact with the posterior end of the posterior ventrolateral plates. Specimens of the aspinothoracidans Heintzichthys and Amazichthys show a slight (3–5 cm) gap between the posterior ventrolateral plate and pelvic girdle, but the two are still associated. The ventral shield and/or pelvic girdles of these specimens appears displaced from life position, suggesting the gap may be a taphonomic artifact, especially as the ventral shield is often pulled anteriorly postmortem in arthrodires (Storrs et al., 2008; Trinajstic et al., 2022b). Even if a similar gap is applied to Dunkleosteus it would be less than 20 cm long (< 5–6% total length) for adult individuals. Pelvic girdles are known for the dunkleosteoid Eastmanosteus calliaspis, but in situ position was not explicitly described (Dennis-Bryan, 1987). However, figures in Trinajstic et al. (2007: fig. 1I–J) seem to imply they were located in a similar position to other arthrodires if accounting for the “exploded” dermal skeleton. Some of these reported pelves may represent misidentified claspers (K. Trinajstic, pers. comm., May 2022; S. van Mesdag, pers. comm., February 2024). However, this should not affect interpretations of pelvic girdle position, as the pelvic girdles of arthrodires are invariably between the ventral shield and claspers (Ahlberg et al., 2009; Trinajstic et al., 2015), constraining the possible position of the pelvic girdle to this region. The only arthrodire to exhibit a sizable gap between the ventral shield and pelvic girdle is Rhachiosteus pterygiatus, but this appears due to the holotype and only known specimen lacking all ventral shield plates except for the anterior ventrolateral plate. Miles (1966, p. 380) speculated these plates were secondarily lost in Rhachiosteus, but the holotype may be immature (Denison, 1978), and it is possible they were unossified.
Most non-arthrodire placoderms retaining a complete set of ventral shield plates also show a close association between the ventral shield and pelvic girdle, including antiarchs (Zhu et al., 2012), Xiushanosteus (Zhu et al., 2022), and the maxillate placoderms Bianchengichthys (Li et al., 2021), Qilinyu (Zhu et al., 2016a), and Entelognathus (Cui et al., 2023). Rhenanidans, stensioellids, and ptyctodonts have large gaps between the pelvis and ventral shield (Miles, 1967b; Denison, 1978), but like Rhachiosteus this is correlated with an absence or loss of ventral shield plates (especially the posterior ventrolateral plate) rather than an anteroposterior shortening of a complete ventral shield or an obvious change in pelvis position. When placoderms reduce the extent of their trunk armor, they consistently do so via the loss of ventral shield plates rather than shortening the ventral shield as a whole, contra traditional depictions of Dunkleosteus (Figure 2). Placoderms retaining a complete set of ventral shield plates invariably have pelvises closely associated with the end of the ventral shield.
The pelvic girdle of Dunkleosteus terrelli appears to have been located at the end of the ventral shield, as in other arthrodires. CMC VP8294 preserves what appear to be pelvic fin basals near the posterior end of the ventral shield. (Figure 19). These basals are unlikely to be from the pectoral or dorsal fins as they are much smaller than the preserved pectoral fin basals and are too far anterior for anal fin basals. Their position and size agree with pelvic fin position in other arthrodires and the size of the pelvic girdle in Dunkleosteus (see below). The girdle itself is not visible, but may be beneath unprepared matrix near the partially exposed basals or on the missing corner of the concretion. CMC VP8294 suggests the pelvic fins of Dunkleosteus, like other arthrodires, were located at the posterior end of the trunk armor, lacking even a small gap as potentially seen in Amazichthys. A significant gap between the ventral shield and the pelvic fins, common in depictions of D. terrelli (Figure 2), is unlikely.
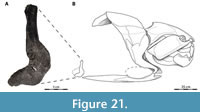 At least three specimens of Dunkleosteus terrelli, CMNH 6090, CMNH 7054 (Figure 21) and CMNH 7568, are known to preserve pelvic girdles associated with partial or complete dermal armor. The position of these girdles in-situ is not recorded (A. McGee, pers. comm., June 2023), but collectors in the Cleveland Shale historically only excavated in the immediate area of the body armor due to difficulties in collecting from steep cliff faces and riverbanks (Hyde, 1926; D. Chapman and A. McGee, pers. comm., March 2023). These excavations often stopped once the trunk armor was exposed assuming the rest of the body was not preserved, but later fieldwork has found many elements of the post-thoracic skeleton often were present further back into the cliff face and were simply overlooked due to being small and difficult to recognize (Carr and Jackson, 2008; G. Kampouris, pers. comms., March 2023). The three specimens mentioned above were collected in the 1930–40s, suggesting the pelvic girdles were likely found closely associated with the body armor as in other arthrodires. If the pelvis of Dunkleosteus was not associated with the ventral shield in life, it would have been preserved in isolation from the dermal skeleton and therefore be unlikely to have been collected.
At least three specimens of Dunkleosteus terrelli, CMNH 6090, CMNH 7054 (Figure 21) and CMNH 7568, are known to preserve pelvic girdles associated with partial or complete dermal armor. The position of these girdles in-situ is not recorded (A. McGee, pers. comm., June 2023), but collectors in the Cleveland Shale historically only excavated in the immediate area of the body armor due to difficulties in collecting from steep cliff faces and riverbanks (Hyde, 1926; D. Chapman and A. McGee, pers. comm., March 2023). These excavations often stopped once the trunk armor was exposed assuming the rest of the body was not preserved, but later fieldwork has found many elements of the post-thoracic skeleton often were present further back into the cliff face and were simply overlooked due to being small and difficult to recognize (Carr and Jackson, 2008; G. Kampouris, pers. comms., March 2023). The three specimens mentioned above were collected in the 1930–40s, suggesting the pelvic girdles were likely found closely associated with the body armor as in other arthrodires. If the pelvis of Dunkleosteus was not associated with the ventral shield in life, it would have been preserved in isolation from the dermal skeleton and therefore be unlikely to have been collected.
The pelvic girdle of Dunkleosteus is invariably small. The largest are only 20 cm in height (Figure 21), and many are significantly smaller. The available pelvic girdles cannot all pertain to juveniles, as they are frequently associated with large individuals. The pelvic girdle of CMNH 7054 is 18 cm tall (6.0% estimated total length), and the pelvic girdle of CMNH 7568 is 20 cm tall (5.7% estimated total length), measurements are not available for CMNH 6090. Another specimen, CMNH 9951, likely represents one of the largest Dunkleosteus individuals known (comparable to CMNH 5936, estimated length ~4 m; Engelman, 2023b) based on the size of its posterior ventrolateral plates (71 and 78 cm, left and right, respectively in length). This specimen has a pelvic girdle only 19 cm in height, roughly 4.2% estimated length.
The pelvic girdle of Dunkleosteus terrelli is smaller and less robust than in other arthrodires, suggesting a smaller pelvic fin. The dunkleosteoid Eastmanosteus calliapsis has a pelvic girdle with a height 9.3–10.6% of total length (Appendix 3: section 5.2),roughly 1.7 times the relative size of this element in D. terrelli. E. calliaspis is interpreted as neritic (Trinajstic et al., 2022a), suggesting the smaller pelvises of Dunkleosteus may be due to pelagic habits. This resembles the pelagic aspinothoracidans Amazichthys and Heintzichthys, which also have relatively small, gracile pelvises among arthrodires (Appendix 3: section 5.2; Dean, 1896; Carr, 1991; Jobbins et al., 2022). It also resembles pelagic sharks (e.g., many lamniforms), which have small pelvic fins relative to non-pelagic taxa. How to reconstruct the size and shape of the pelvic fins in Dunkleosteus given the small pelvis remains unclear. Sharks have elongate basipterygia which allow even small pelvic girdles to support relatively large pelvic fins (e.g., see figures in Stone and Shimada, 2019), but elongated basipterygia are unknown in arthrodires. Instead the pelvic fin basals articulated directly with the girdle (Miles and Westoll, 1968: fig. 49; Trinajstic et al., 2015), potentially resulting in a much smaller pelvic fin base. Fin basals are preserved in association with Dunkleosteus pelvises (Williams, 1990: fig. 243), suggesting the pelvic fins and girdle were not vestigial in this taxon. Nevertheless, how they functioned given their small size is unclear. For the present reconstruction (Figure 4) pelvic fin shape was reconstructed using lamnids as an extant analogue, with the caveat their size may be conservatively large.
Claspers. Claspers were likely present in Dunkleosteus, given this taxon is bracketed by other clasper-bearing arthrodires (Figure 6), including the coccosteomorphs Millerosteus, Coccosteus, Compagopiscis, and Incisoscutum, the holonematid Holonema, and the phyllolepids Austrophyllolepis and Cowralepis (Trinajstic et al., 2015), but claspers have yet to be described for any pachyosteomorph. Further study is needed to determine if claspers were present in pachyosteomorphs or if their absence reflects secondary loss.
Anal Fin. Of the five fin loci in this reconstruction (dorsal, pectoral, pelvic, anal, and caudal), the presence and morphology of the anal fin is the most difficult to constrain. Several arthrodires such as Coccosteus (see Miles and Westoll, 1968), Watsonosteus (NMS G.1995.4.2), Incisoscutum (NHMUK PVP 50934 [visible under x-ray, Z. Johanson and J. Long, pers. comm., February 2022]; WAM 03.3.28), and Plourdosteus (MHNM 02-901) preserve an anal plate where the anal fin is in other fishes (Figure 7B, Figure 8). Anal plates have not been identified in any pachyosteomorph, including Dunkleosteus, Heintzichthys (Carr, 1991), or Amazichthys (M. Jobbins, pers. comm., July 2022).
Whether the anal plate actually supported a fin has been controversial. Basals or fin outlines of the anal fin are unknown in eubrachythoracid arthrodires. This includes Amazichthys, though it is possible the anal fin is simply not preserved in known specimens (M. Jobbins, pers. comm., July 2022). R. S. Miles suggested the anal fin was absent in eubrachythoracids (see Miles, 1966; Miles and Westoll, 1968; Moy-Thomas and Miles, 1971), given the anal plate (= postanal plate of those studies) had a thin distal edge apparently lacking articulations for radials. Miles and Westoll (1968: p. 450) suggested the plate served as an attachment site for unidentified muscles or represents a vestigial fin base in eubrachythoracid arthrodires. Most placoderm workers seem to have tacitly disagreed with Miles’ assessment and consider an anal fin present in eubrachythoracid arthrodires (Trinajstic et al., 2013; K. Trinajstic, pers. comm., July 2022), but evidence for this interpretation or a formal rebuttal to Miles’ hypothesis have not been presented in the literature. An anal fin is present in the groenlandaspid Africanaspis (Gess and Trinajstic, 2017). This implies an anal fin was absent in eubrachythoracid arthrodires it was a secondary loss. Unfortunately, specimens of Africanaspis do not preserve endoskeletal elements, obscuring its anal fin morphology. Ultimately the functional morphology of the anal plate in eubrachythoracid arthrodires and whether it correlates with an anal fin requires further study. Dunkleosteus is conservatively reconstructed with an anal fin here, given it is the general consensus among arthrodire workers.
Caudal Fin. The caudal fin is inherently the most speculative aspect of any arthrodire reconstruction. Caudal fin skeletons have only been reported for Holonema, Plourdosteus, and the coccosteids from the Orcadian Basin (Miles and Westoll, 1968; Vézina, 1988; Trinajstic, 1999; they are currently unknown for any Gogo eubrachythoracid, see Trinajstic et al., 2022a), and soft tissue outlines are only known for Africanaspis and Amazichthys (Gess and Trinajstic, 2017; Jobbins et al., 2022). Although significant advances have been made in the study of arthrodire caudal fin shape (Ferrón et al., 2017a; Jobbins et al., 2022), caudal material has not been described for Dunkleosteus terrelli. Thus, whereas all other anatomical regions in this manuscript are at least partly based on data from Dunkleosteus fossils, caudal fin morphology must be inferred based on paleobiology and biomechanical or functional comparisons with other fishes, which fortunately seem to be a much stronger influence on caudal fin shape than phylogenetic history (Nursall, 1958; Aleev, 1969; Ferrón et al., 2017a).
The shape of the caudal fin here generally follows Ferrón et al. (2017a), with modifications. Although Dunkleosteus terrelli is often reconstructed with an anguilliform or macruriform caudal fin in older works (Figure 2; e.g., Heintz, 1932), Ferrón et al. (2017a) demonstrated the caudal fin of this taxon was most likely lunate. This conclusion was further supported by the discovery of a lunate fin in the pachyosteomorph arthrodire Amazichthys (Jobbins et al., 2022). Although the author agrees with the broader conclusions of Ferrón et al. (2017a), methodological issues suggest the shape predicted in that study may need adjustment. Ferrón et al. (2017a) predicted caudal fin shape in Dunkleosteus via an allometric regression analysis derived from geometric morphometric data in extant sharks, but the only predictive information considered from Dunkleosteus were estimated length (based on upper jaw perimeter) and inferred pelagic habits (Ferrón et al., 2017a: p. 5). No geometric landmark data from Dunkleosteus fossils were used in this prediction. Thus, the caudal fin shape predicted by this study is not necessarily the expected caudal fin shape for Dunkleosteus, but the expected caudal fin shape of a pelagic shark with a similar-sized mouth to Dunkleosteus. If Dunkleosteus substantially differed from sharks in body shape (such as having a deeper trunk), the predicted caudal fin shape would need adjustment. This may be one reason why the caudal fin in the reconstruction presented by Ferrón et al. (2017a: fig. 4B) appears so gracile and has a small span relative to the animal’s body.
Trunk height and caudal fin span are closely correlated in marine vertebrates (Aleev, 1969: p. 120–129; Motani et al., 1996; Jobbins et al., 2022) especially those that use body and caudal fin (BCF) propulsion, with the caudal fin spans of most nektonic fishes (e.g., Carcharhiniformes, Carangiformes, Istiophoriformes, Lamniformes, Scombridae) being equal to or greater than trunk height. Exceptions to this pattern are taxa (often discoid acanthopterygians) that are either partially or entirely median and paired fin (MPF) swimmers (Aleev, 1969). This correlation may be due to wake, with the caudal fin span needing to be close to trunk height for the fin tips project to beyond the drag vortices created by the trunk and allow the fin to function as a rudder (Aleev, 1969: p. 120–121). The deep trunk of Dunkleosteus would completely rule out a slightly heterocercal or anguilliform caudal fin for this taxon, typical of reconstructions prior to Ferrón et al. (2017a). Because the thrust generated by a paddle (caudal fin) is proportional to its surface area and given the stocky body of arthrodires, the caudal fin of Dunkleosteus may also require a deeper and broader than predicted in Ferrón et al. (2017a) to provide sufficient thrust. These conclusions are supported by the caudal fin shape of Amazichthys trinajsticae, which although superficially resembling a pelagic shark is also relatively broader and taller (Jobbins et al., 2022: p. 16–17).
The caudal fin of Dunkleosteus may also need to be significantly more homocercal and have a larger ventral lobe than Ferrón et al. (2017a) predicted, given the depth of the trunk and dorsoventrally high position of the spinal cord. The caudal fin shape proposed by Ferrón et al. (2017a) results in a ventral lobe that does not reach the ventral edge of the trunk, even if caudal fin span is greatly increased. This is unlike most fishes using BCF propulsion and highly forked fins, and would potentially make the ventral lobe unable to escape the wake produced by a deeper torso (Aleev, 1969: p. 132–134). This is also why the reconstruction in Engelman (2023b), which uncritically used the degree of heterocercality proposed by Ferrón et al. (2017a), has a caudal fin that appears lopsided (Appendix 1). The only way to retain the caudal fin shape proposed by Ferrón et al. (2017a) and allow the ventral lobe to reach the ventral level of the trunk is to expand the fin until the height of the dorsal lobe alone is almost as tall as the entire trunk. Given the deep trunk of Dunkleosteus, this seems unlikely. The only way to fix this biomechanical issue is to restore the caudal fin of Dunkleosteus with a larger ventral lobe (though still possibly within the range of variation in ventral lobe size proposed by Ferrón et al., 2017a: fig. 4A). The presence of a larger ventral lobe is supported by living fishes with caudal peduncles higher than the center of gravity (e.g., Pelecus cultratus, see Aleev, 1969: fig. 85). These fishes usually compensate for such an arrangement via a larger ventral lobe, which results in the center of area of the caudal fin remaining at the dorsoventral level of the center of mass (Aleev, 1969: p. 132–134).
Comparatively larger ventral lobes (relative to sharks) may characterize eubrachythoracid arthrodires more broadly (see also Figure 7). Amazichthys is also characterized by a proportionally deeper ventral lobe than extant sharks, resulting in its caudal fin shape plotting slightly outside extant shark morphospace (Jobbins et al., 2022: p. 15–16). The strongly heterocercal caudal fin and small ventral lobe of sharks and acipenserids (sturgeons) may be related to the cambered, ventrally flattened trunk (used to generate lift; Aleev, 1969), which means the ventral lobe does not have to be as tall to reach the ventral margin. Eubrachythoracid arthrodires generally lack cambered trunks, given most have a ventrally curved trunk armor (Figure 10; see also Dennis and Miles, 1979a; Dennis-Bryan, 1987; Gardiner and Miles, 1990, 1994). Other features such as the insertions of the m. coracomandibularis and m. coracohyoideus being dorsally elevated relative to their origins on the interolateral plate of the trunk armor when the mouth was closed (see previous citation, as well as Johanson, 2003; Sanchez et al., 2013) also imply their head was ventrally curved, not flattened — contra the condition in sharks and acipenserids. Arthrodires also generally show a deeper trunk than sharks and acipenserids (Engelman, 2023b), which would suggest a broader caudal fin span. Some early-diverging eubrachythoracids like Millerosteus and Coccosteus are a partial exception due to their flatter ventral shield (Miles and Westoll, 1968; Desmond, 1974; Engelman, 2023a), but this may be driven by their inferred demersal habits instead of lift (Miles and Westoll, 1968; Trewin, 1986). A relationship between body cambering and caudal fin shape is further supported by polyodontids and most Paleozoic actinopterygians, which have non-cambered bodies and caudal fins that are internally heterocercal but externally homocercal or only slightly heterocercal (Grande and Bemis, 1991; Schultze et al., 2021). It is also worth noting that pelagic sharks like lamnids also tend to have caudal fins that are near-homocercal externally (Thomson and Simanek, 1977; Sternes and Shimada, 2020). In this respect paleoenvironment and the preserved anatomy of Dunkleosteus converge in their suggested caudal fin shape.
Other evidence suggests caudal fins with well-developed heterocercal angles and/or caudal forks may have been more widely distributed in Arthrodira than previously thought. Arthrodires are frequently reconstructed with weakly heterocercal or even macruriform caudal fins based on Coccosteus cuspidatus (Heintz, 1931a, 1938; Stensiö, 1963; Miles and Westoll, 1968). However, the caudal morphology of well-preserved specimens of C. cuspidatus differs from “classic” reconstructions in several aspects. This is best demonstrated by ROM VP 52664, which shows a spectacularly preserved caudal skeleton (Figure 7). The caudal fin of this specimen is noticeably shorter than previous reconstructions (21.6% total length versus 32% total length) and has a sharper, more pronounced heterocercal angle (24.5° versus 18°). Other specimens of C. cuspidatus (NMS 1897.55.6, NMS 1900.12.12, NMS 1901.106.1) show similar proportions (Appendix 6). The caudal skeleton of Coccosteus resembles a carcharhinid shark in exhibiting a well-developed caudal bend. By contrast, other shark groups often compared with Coccosteus, such as Squaliformes, Heterodontiformes, or Orectolobiformes, lack a sharp bend in their caudal skeleton, and the heterocercality of the fin is formed by soft tissue (Compagno, 1984; Little and Bemis, 2004; De Iuliis and Pulerà, 2011; Motani and Shimada, 2023). This indicates a more strongly heterocercal fin in Coccosteus than previously thought.
ROM VP 52264 shows eight enlarged haemal spines at the base of the caudal bend, potentially suggesting a larger ventral (hypochordal) lobe of the caudal fin in Coccosteus cuspidatus than previously assumed (Figure 7). Heintz (1938) and Miles and Westoll (1968: p. 449) also noticed these structures in their specimens and used them to suggest Coccosteus had a hypocercal lobe, though they restored it as extremely small. No reasoning was given, but this was likely based on the historical assumption that arthrodires lacked distal radials/ceratotrichia and the smooth gradient in the height of the haemal spines (see discussion in Heintz, 1931a, 1938; Carr et al., 2010; Greenfield, 2020). Distal fin cartilages and ceratotrichia have been since identified in several arthrodire taxa (Trewin, 1986; Carr et al., 2010; Jobbins et al., 2022), suggesting the caudal fin profiles of arthrodires were probably larger than previously thought. Prominent hypochordal lobes and forked caudal fins are not reflected in the gross morphology of the endoskeleton in many extant sharks (Little and Bemis, 2004; Kim et al., 2013; Moreira et al., 2019), suggesting a Coccosteus -like caudal skeleton does not necessarily imply an anguilliform or macruriform caudal fin. Indeed, ROM VP 52264 shows a sharp difference in height between the enlarged haemal spines at the base of the caudal bend and those further posterior, which could be interpreted as evidence of a caudal fork (Figure 7), but this transition is less clear in other specimens of Coccosteus.
This evidence suggests caudal fins with pronounced heterocercal angles and caudal forks might be more widely distributed in arthrodires than once thought. Further examination of haemal arches in heterocercal fishes might identify features useful for determining the presence or extent of a hypochordal lobe. Examining the caudal skeleton morphology of Coccosteus in more detail is beyond the scope of this study, but current evidence suggests inferences of macruriform, anguilliform, or weakly heterocercal caudal fins in eubrachythoracid arthrodires (i.e., other coccosteomorphs) need more rigorous evaluation. In the case of Dunkleosteus, the anteroposteriorly short, sharply angled caudal skeleton of Coccosteus further argues against an elongate “eel-like” caudal fin, commonly seen in depictions of D. terrelli prior to Ferrón et al. (2017a).
Arthrodires seem to have proportionally deep caudal peduncles among fishes. Demersal arthrodires like Coccosteus, Plourdosteus, Watsonosteus, and Incisoscutum seem to have much deeper caudal peduncles (~40% greatest body depth) than sharks with analogous life habits (e.g., Triaenodon). Amazichthys also has a deep peduncle, roughly 40% greatest body depth (Jobbins et al., 2022), despite this fish being interpreted as pelagic. This condition is unusual, as most extant pelagic fishes have caudal peduncles < 25% maximum body height, though it resembles megalopids, rachycentrids, and sphyraenids (Appendix 3: fig. 4.16). The deep peduncle of Amazichthys cannot be attributed to taphonomic deformation. Assuming an originally narrow lamnid, scombrid, or istiophoriform-like peduncle would require the peduncle to nearly double in height due to taphonomic distortion, which is highly unlikely, especially as a deep peduncle is present in multiple specimens of Amazichthys (Jobbins et al., 2022).
The deep peduncle of arthrodires may result from their generally stocky body plan (Engelman, 2023b). Due to their relatively massive bodies for their length, the deeper and sturdier peduncle of arthrodires may provide more force through the caudal fin and thus more thrust for a shorter, more massive body. However, the possibility that the deep peduncle of Amazichthys is reflective of a megalopid, rachycentrid, or sphyraenid-like mode of life cannot be ruled out. For Dunkleosteus, because of its likely pelagic habits (see “Paleoenvironmental Context of the Cleveland Shale”), the author reconstructed the peduncle as deeper than that of extant thunniform taxa but narrower than other arthrodires. Preliminary data suggest peduncle height is influenced by both phylogeny and anatomy (Appendix 3: section 4.8.2), confounding a straightforward estimation of peduncle height in fossil fishes. Attempting to estimate the peduncle height of CMNH 5768 without assuming pelagic habits produces a possible range of 7–30 cm, roughly 2–9% estimated total length (Appendix 3: section 4.8.2), which is relatively narrow compared to the animal’s deep trunk. However, the author recommends this estimate be considered with significant skepticism given the overall imprecision in this model.
The caudal fin of Dunkleosteus was reconstructed without a terminal caudal lobe (Figure 2). Free terminal caudal lobes are present in all modern (neoselachian) sharks, which represent the majority of extant heterocercal fishes. However, they are absent in all other heterocercal fishes, including the arthrodire Amazichthys (Jobbins et al., 2022), non-neoselachian chondrichthyans like Symmoriformes (Dean, 1909b), Eugeneodontiformes (Zangerl, 1981), Ctenacanthiformes (Hodnett et al., 2021), and Hybodontiformes (Maisey, 1989), and heterocercal actinopterygians like “palaeoniscoids” (Schultze et al., 2021), and acipenseriforms (Grande and Bemis, 1991). A free terminal caudal lobe appears to be a neoselachian apomorphy, and thus unlikely to be present in Dunkleosteus.
Lateral keels on the caudal peduncle are unknown in Dunkleosteus but highly likely, given these structures have evolved independently multiple times in pelagic fishes. These include Chondrichthyes (Cladoselache, eugeneodonts, lamnids; Dean, 1894; Garrick, 1967; Zangerl, 1981), Actinopterygii (scombrids and xiphiids; LaMonte, 1955; Walters, 1962), and the aspinothoracidan arthrodire Amazichthys (Jobbins et al., 2022). This suggests lateral caudal keels are a particularly plastic trait in fish evolution, agreeing with previous observations that caudal fin morphology in fishes is more influenced by function than phylogenetic history (Nursall, 1958; Ferrón et al., 2017a). If Dunkleosteus was pelagic as favored here, these structures were likely present.
DISCUSSION
Paleobiology and Swimming Kinematics of Dunkleosteus
Body Shape in Dunkleosteus. Multiple lines of evidence (trunk armor shape, head proportions, OOL, entering angle, position of the fin bases, etc.; see present study and Engelman, 2023b) suggest Dunkleosteus had a relatively stocky body plan with a deep trunk. Deep trunks and/or stocky bodies occur in a number of fish ecomorphs, but few of these are associated with open water habitats like those interpreted for the Cleveland Shale. For example, groupers (Serranidae), giant sea bass (Stereolepis), and coelacanths (Latimeria) are stocky-bodied but are also generally demersal taxa that remain close to the substrate and rarely range into open water (Bullock and Smith, 1991; Musick et al., 1991; Allen and Andrews, 2012). Given the Cleveland Shale paleoenvironment has been interpreted as uninhabitable to non-nektonic organisms due to its anoxic bottom (see “Paleoenvironmental Context of the Cleveland Shale”), this constrains Dunkleosteus to the relative narrow range of body shapes seen in pelagic fishes and neritic fishes that sometimes venture into open water habitats like tiger sharks (Galeocerdo cuvier) and barracudas (Sphyraenidae) (Allen and Cross, 2006; Compagno, 2008; Helfman et al., 2009: fig. 18.6). Among open water fishes, most taxa with relatively deep trunks and stocky bodies are thunniform species, such as thunnins and lamnid sharks (Helfman et al., 2009: fig. 18.6). The only other pelagic fishes with deep-bodies are taxa like opah (Lampridae) and molas (Molidae), which Dunkleosteus is unlikely to have resembled as these taxa are highly specialized MPF swimmers. Within the limited range of body forms seen in open water fishes, a thunniform body shape seems most likely for Dunkleosteus.
Several other features of Dunkleosteus are potentially suggestive of thunniform locomotion. The anterior vertebral column is highly stiffened by partial fusion and extensive lateral articular facets (Johanson et al., 2019). The trunk would have been further stiffened anteriorly by the laterally-inflexible dermal armor, and while dermal armor is present in all arthrodires the ventral shield of Dunkleosteus is particularly inflexible laterally due to extensive interlocking processes between the anterior and posterior ventrolateral plates (see below). These patterns broadly resemble other thunniform vertebrates, which often show adaptations enhancing the rigidity of the anterior vertebral column and trunk (Fierstine and Walters, 1968; Motani et al., 1996; Fish, 2002), though the exact mechanisms used to achieve this differ between groups. Thunnins use elongate zygapophyses and restriction of the notochord (Fierstine and Walters, 1968; Baxter et al., 2022), thunnosaurian ichthyosaurs use large, discoidal centra (Motani et al., 1996), cetaceans use highly compressed cervical and anterior thoracic centra (Fish, 2002), and Dunkleosteus exhibits partial vertebral fusion and laterally expanded articular processes. Additional characters proposed to be useful in identifying extinct thunniform swimmers are restricted to the caudal and posterior axial skeleton (Motani and Shimada, 2023), regions currently unknown for Dunkleosteus, and thus cannot be evaluated.
The trunk armor of Dunkleosteus suggests the presence of well-developed axial musculature, with the area spanned by the lateral longitudinal bundles being particularly large compared to other arthrodires (Figure 13 and Figure 15). In most fishes the lateral longitudinal bundles are the main muscles involved in trunk undulation, suggesting Dunkleosteus may have been capable of powerful lateral flexion of the posterior trunk and tail. Much of this musculature would have been located anterior to the end of the ventral shield (Figure 4 and Figure 15), somewhat resembling thunnins and lamnids, which show an anteriorization of muscle mass (especially red muscle) compared to non-thunniform taxa (Graham et al., 1983; Carey et al., 1985). However, it also means much of the musculature in Dunkleosteus would be in an area incapable of lateral undulation due to the inflexible trunk armor and partially fused spinal column. Extant thunniform fishes solve similar biomechanical issues of locomoting with a stiff body via large, well-developed lateral tendons and other connective tissues, which transmit forces generated by muscles in the otherwise stiff anterior trunk to the posterior trunk and caudal peduncle (Donley et al., 2004; Shadwick and Gemballa, 2005; Gemballa et al., 2006). The prominent internal ridge on the posterior lateral in Dunkleosteus terrelli, currently unique to this taxon, suggests an unusually well-developed horizontal septum compared to other arthrodires, with strong stabilizing connections to the trunk armor. In thunnins the horizontal septum is one of the main tissues involved in transmitting force from the anterior axial musculature to the caudal peduncle (Westneat et al., 1993), though in lamnids an analogous function is instead performed by the hypaxial lateral tendons (Donley et al., 2004). This suggests Dunkleosteus may have resembled some extant thunniform fishes in having a well-developed horizontal septum that could be used to transmit force from the anterior to the posterior trunk.
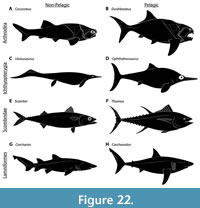 Other lines of anatomical evidence support pelagic and tachynektonic habits in Dunkleosteus. These do not directly support a thunniform body plan, but do suggest the deep trunk of Dunkleosteus cannot be attributed to this taxon having a non-pelagic lifestyle. The small pelvic girdle of D. terrelli resembles many other pelagic, tachynektonic vertebrate clades (Figure 22) which have convergently reduced or outright lost the pelvic girdle (e.g., some arthrodires like Amazichthys, Lamniformes, Symmoriformes, Eugeneodontida, Thunnosauria, Cetacea; Zangerl, 1981; Compagno, 1990; Gutarra et al., 2019; Gutarra et al., 2022; Jobbins et al., 2022), likely because large, paired fins contribute significantly to drag (Gutarra et al., 2019; Gutarra et al., 2022). The smooth, unornamented dermal armor of Dunkleosteus might also be indicative of pelagic habits, as other arthrodires lacking tuberculated armor (e.g., selenosteids) are interpreted as tachynektonic species. Notably, virtually all Cleveland Shale arthrodires have unornamented armor regardless of their phylogenetic position, with a few exceptions that are rare in the fauna (Glyptaspis; Boylan and Murphy, 1978). Cleveland Shale arthrodires appear to represent at least two independent losses of armor tuberculation (once in Dunkleosteus terrelli and at least once in aspinothoracidans; Dennis-Bryan, 1987; Carr and Hlavin, 2010; Zhu et al., 2016b), further suggesting this is driven by paleobiology rather than phylogeny. This contrasts with other arthrodire faunas (e.g., Gogo, Wee Jasper, the various Orcadian Basin faunas) in which most if not all species have tuberculated armor (e.g., Miles and Westoll, 1968; Desmond, 1974; Dennis and Miles, 1979b; Miles and Dennis, 1979; Dennis-Bryan, 1987; Young, 2004, 2009). This resembles what is seen in extant fishes, where open water taxa such as tunas (Thunnini), barracudas (Sphyraenidae), and tarpon (Megalopidae) have smooth or unornamented dermal skeletons, whereas benthic/demersal fishes or those associated with cluttered habitats such as gars (Lepisosteidae), sturgeons (Acipenseridae), pacu (Serrasalmidae), many catfishes (Ariidae, Loricariidae, Callichthyidae, Doradidae, Pimelodidae) show ornamented bone texture (Engelman, pers. obs.). However, a more in-depth survey of this phenomenon is beyond the scope of this study.
Other lines of anatomical evidence support pelagic and tachynektonic habits in Dunkleosteus. These do not directly support a thunniform body plan, but do suggest the deep trunk of Dunkleosteus cannot be attributed to this taxon having a non-pelagic lifestyle. The small pelvic girdle of D. terrelli resembles many other pelagic, tachynektonic vertebrate clades (Figure 22) which have convergently reduced or outright lost the pelvic girdle (e.g., some arthrodires like Amazichthys, Lamniformes, Symmoriformes, Eugeneodontida, Thunnosauria, Cetacea; Zangerl, 1981; Compagno, 1990; Gutarra et al., 2019; Gutarra et al., 2022; Jobbins et al., 2022), likely because large, paired fins contribute significantly to drag (Gutarra et al., 2019; Gutarra et al., 2022). The smooth, unornamented dermal armor of Dunkleosteus might also be indicative of pelagic habits, as other arthrodires lacking tuberculated armor (e.g., selenosteids) are interpreted as tachynektonic species. Notably, virtually all Cleveland Shale arthrodires have unornamented armor regardless of their phylogenetic position, with a few exceptions that are rare in the fauna (Glyptaspis; Boylan and Murphy, 1978). Cleveland Shale arthrodires appear to represent at least two independent losses of armor tuberculation (once in Dunkleosteus terrelli and at least once in aspinothoracidans; Dennis-Bryan, 1987; Carr and Hlavin, 2010; Zhu et al., 2016b), further suggesting this is driven by paleobiology rather than phylogeny. This contrasts with other arthrodire faunas (e.g., Gogo, Wee Jasper, the various Orcadian Basin faunas) in which most if not all species have tuberculated armor (e.g., Miles and Westoll, 1968; Desmond, 1974; Dennis and Miles, 1979b; Miles and Dennis, 1979; Dennis-Bryan, 1987; Young, 2004, 2009). This resembles what is seen in extant fishes, where open water taxa such as tunas (Thunnini), barracudas (Sphyraenidae), and tarpon (Megalopidae) have smooth or unornamented dermal skeletons, whereas benthic/demersal fishes or those associated with cluttered habitats such as gars (Lepisosteidae), sturgeons (Acipenseridae), pacu (Serrasalmidae), many catfishes (Ariidae, Loricariidae, Callichthyidae, Doradidae, Pimelodidae) show ornamented bone texture (Engelman, pers. obs.). However, a more in-depth survey of this phenomenon is beyond the scope of this study.
Biogeography further supports pelagic life habits for Dunkleosteus. Fossils of Dunkleosteus have been found in Laurentia, Africa (Morocco), and Baltica (Carr, 2009; Szrek and Wilk, 2018; Lebedev et al., 2023). The material from Baltica has been assigned to a distinct species (D. tuderensis; Lebedev et al., 2023), but D. terrelli from Laurentia and D. marsaisi from Africa may be synonymous (Rücklin, 2002). Some of these landmasses were potentially separated by ocean basins during the Late Devonian (Golonka, 2020), suggesting Dunkleosteus was capable of crossing the open ocean. Large arthrodires like Dunkleosteus, Gorgonichthys, and Titanichthys generally show wide biogeographical distributions (Carr, 2009; Boyle and Ryan, 2017; Coatham et al., 2020; Szrek et al., 2021), agreeing with suggestions of ocean-going habits.
Body Shape Evolution in Pelagic Vertebrates. The stocky body shape interpreted for Dunkleosteus relative to other arthrodires is not unusual among pelagic vertebrates. Body plans with deeper, stockier trunks and lower fineness ratios compared to demersal or neritic outgroups have convergently evolved in a number of thunniform pelagic vertebrate clades (Figure 22), including lamniform sharks, two lineages of scombrids (thunnins and Gasterochisma) (Block and Finnerty, 1994; Bernal et al., 2001; Donley et al., 2004; Shadwick, 2005), eosauropterygians (Wintrich et al., 2017), ichthyosaurs (Buchholtz, 2001; Motani, 2004; Gutarra et al., 2019), and cetaceans (Gingerich, 1998; Bebej and Smith, 2018). Arthrodires simply appear to be part of this broader trend, with Dunkleosteus potentially being one of the geologically oldest examples of a thunniform body plan. Dunkleosteus appears slightly stockier than the other pelagic taxa in Figure 22, but this may be because arthrodires are generally stockier and more robust than other fishes regardless of life habits (Engelman, 2023b: p. 33–38). Thus, the shape of Dunkleosteus may reflect selection for a shorter, deeper body applied on top of an ancestrally stocky body plan.
 Despite thunniform body plans convergently evolving in a number of pelagic vertebrates, the underlying reason for stockier body shapes and lower fineness ratios in these taxa remains poorly understood. A short, deep body shape has been suggested to reduce surface area-to-volume ratio and therefore drag (Alexander, 1967; Vogel, 2008), but biomechanical modeling has been equivocal on this idea (Gutarra et al., 2019). One possibility is this phenomenon may be driven by endothermy (Block and Finnerty, 1994). Most pelagic vertebrates with stocky bodies and high fineness ratios, including thunnins, Gasterochisma, xiphiids, lamnids, cetaceans, ichthyosaurs, and lamprids, are almost exclusively endothermic (Block and Finnerty, 1994; Bernard et al., 2010; Wegner et al., 2015), whereas other pelagic vertebrates such as non-thunnin scombrids (e.g., Acanthocybium, Scomberomorus), istiophorids, coryphaenids, other nektonic sharks like carcharhinids, etc., are predominantly ectothermic and often show elongate body plans (Figure 23). The link between body shape and thermophysiology is controversial in fishes (Sternes et al., 2024), but this is primarily due to regional endothermy being identified in non-thunniform taxa, there are few if any “false positives” of ectothermic pelagic fishes with stocky bodies. This pattern may be due to a squatter, fatter tuna-like body plan being better at retaining heat due to the square-cube law, and therefore more optimal for endothermic organisms (Block and Finnerty, 1994). Size may also play an important role in determining which of these morphotypes is functionally optimal. Most tuna-like taxa are relatively large, whereas most ectothermic pelagic vertebrates are smaller (compare the 3.5 m Dunkleosteus versus 1 m Amazichthys). Because drag also scales following the square-cube law (proportional to surface area), larger animals may be less penalized by drag produced by the higher fineness ratios of thunniform body plans (Gutarra et al., 2019; Gutarra et al., 2022). Ferrón et al. (2017b) suggested larger body size may also promote endothermy (and potentially by extension a thunniform body shape), because it allows large pelagic animals to range farther to find food to support their large size. All of these factors may work together to promote thunniform shapes in larger pelagic organisms.
Despite thunniform body plans convergently evolving in a number of pelagic vertebrates, the underlying reason for stockier body shapes and lower fineness ratios in these taxa remains poorly understood. A short, deep body shape has been suggested to reduce surface area-to-volume ratio and therefore drag (Alexander, 1967; Vogel, 2008), but biomechanical modeling has been equivocal on this idea (Gutarra et al., 2019). One possibility is this phenomenon may be driven by endothermy (Block and Finnerty, 1994). Most pelagic vertebrates with stocky bodies and high fineness ratios, including thunnins, Gasterochisma, xiphiids, lamnids, cetaceans, ichthyosaurs, and lamprids, are almost exclusively endothermic (Block and Finnerty, 1994; Bernard et al., 2010; Wegner et al., 2015), whereas other pelagic vertebrates such as non-thunnin scombrids (e.g., Acanthocybium, Scomberomorus), istiophorids, coryphaenids, other nektonic sharks like carcharhinids, etc., are predominantly ectothermic and often show elongate body plans (Figure 23). The link between body shape and thermophysiology is controversial in fishes (Sternes et al., 2024), but this is primarily due to regional endothermy being identified in non-thunniform taxa, there are few if any “false positives” of ectothermic pelagic fishes with stocky bodies. This pattern may be due to a squatter, fatter tuna-like body plan being better at retaining heat due to the square-cube law, and therefore more optimal for endothermic organisms (Block and Finnerty, 1994). Size may also play an important role in determining which of these morphotypes is functionally optimal. Most tuna-like taxa are relatively large, whereas most ectothermic pelagic vertebrates are smaller (compare the 3.5 m Dunkleosteus versus 1 m Amazichthys). Because drag also scales following the square-cube law (proportional to surface area), larger animals may be less penalized by drag produced by the higher fineness ratios of thunniform body plans (Gutarra et al., 2019; Gutarra et al., 2022). Ferrón et al. (2017b) suggested larger body size may also promote endothermy (and potentially by extension a thunniform body shape), because it allows large pelagic animals to range farther to find food to support their large size. All of these factors may work together to promote thunniform shapes in larger pelagic organisms.
Given the evidence suggesting a stocky, likely thunniform body shape in Dunkleosteus, this taxon could potentially be the oldest known endotherm. This would not be the first time endothermy has been proposed for Late Devonian arthrodires, Ferrón et al. (2017b) predicted Dunkleosteus would eventually be identified as endothermic given most marine predators of similar size (~1000–10000 kg) and activity level are regional or whole-body endotherms. The question of whether Dunkleosteus exhibited regional or whole-body endothermy is a potentially fruitful area for future research, but without more direct evidence (e.g., histology; see Cubo et al., 2020) must be treated as speculative for now.
Turning Performance in Dunkleosteus. Despite its short, deep body plan, extensive dermal armor, and evidence for a stiffened trunk, Dunkleosteus may have shown comparatively greater maneuverability than extant thunniform taxa. Short, deep body plans (i.e., low fineness ratios) are often associated with high acceleration and turning ability in fishes (Alexander, 1967; Howe et al., 2021). A short, deep body results in more of the body being closer to the center of mass, minimizing the moment of inertia and allowing tighter pivoting on the animal’s long axis (Alexander, 1967; Webb, 2005). Similarly, a shorter body minimizes turning drag because parts of the body more distal to the center of mass move faster when turning, creating more drag (Alexander, 1967: p. 45). These selective pressures would potentially be more relevant for stiffer-bodied organisms (like arthrodires), for which the ability to bank into a turn (as more flexible-bodied fishes do; Gray, 1933; Weihs, 1972) was limited. These biomechanical challenges would be exaggerated at larger body sizes, because drag ∝ surface area ∝ body length2, whereas moment of inertia ∝ distal body mass ∝ body length3. This would potentially result in selection for increasingly stocky shapes (higher length-weight relationships) at large body sizes in arthrodires to minimize these effects. The stocky body shape and allometric patterns in trunk armor morphology described here for Dunkleosteus could be interpreted as supporting this idea.
The large size of the pectoral fin in Dunkleosteus terrelli and its comparatively anterior location on the body would have further enhanced turning ability. Pectoral fins become more effective rudders the further anterior they are to the center of mass (Aleev, 1969; Nakaya, 1995; Fish, 2002), as this creates a longer lever arm and thus more torque. Theoretically, turning ability is optimized if the rudder is at the anterior tip of the body, yet in most fishes the anterior extent of the pectoral fin is constrained by the length of the snout/head (novelly solved in Sphyrnidae via the cephalofoil; Nakaya, 1995). Dunkleosteus, with its short snout, deep head, and anteriorly positioned pectoral fenestra, had a considerably more anterior pectoral fin than most extant nektonic fishes (Table 4). Along with the large size of the fin base, this would have potentially made this taxon effective at turning. These specializations may have served to compensate for the bulky and relatively stiff trunk, but the extreme degree of specialization seen in Dunkleosteus suggests these features may not have been mutually exclusive. The size of the pectoral fins by themselves can also enhance maneuverability. The proportionally larger pectoral fins of humpback whales (Megaptera novaeangeliae) make them substantially more maneuverable than other baleen whales, which otherwise tend to swim in a relatively rectilinear and stiff-bodied fashion due to sheer size (Fish and Battle, 1995; Fish, 1999). It is possible that the large pectoral fenestrae seen in many seemingly nektonic pachyosteomorph arthrodires (Stensiö, 1959; Miles, 1969; Carr, 1995), including Dunkleosteus, reflect the presence of large, mobile pectoral fins which served to compensate for their stiffened anterior trunks. Ironically, while modern thunniform swimmers like lamnids and thunnins are typically characterized by stiff bodies and poor turning performance (Blake et al., 1995; Blake, 2004; Syme and Shadwick, 2011; Downs et al., 2023), the anatomy of Dunkleosteus suggests it may have been comparatively better at turning performance within the adaptive landscape of thunniform body plans.
The short, deep body of Dunkleosteus also suggests it was likely capable of faster initial bursts of acceleration than extant sharks. Deeper bodies potentially result in more muscle mass relative to length (Webb, 1978), as well as longer myomeres relative to body length (Jayne and Lauder, 1994, 1996) and therefore an increase in the force each myomere can generate. The more posterior position of the dorsal fin relative to the center of gravity in Dunkleosteus is also more biomechanically similar to “fast-start” predatory fishes capable of high initial acceleration (e.g., esocids and sphyraenids; Aleev, 1969; Weihs and Webb, 1983; Drucker and Lauder, 2001; Maia and Wilga, 2013). This is thought to be because the more posterior dorsal fin can stabilize flow over the caudal fin and be recruited to expand the lateral surface area of the posterior body oscillated during lateral undulation (ibid). However, the position of the dorsal fin in Dunkleosteus may be due to pre-existing constraints of the arthrodire body plan (i.e., the development of the carinal process) rather than any selective pressure for high acceleration.
Any morphological specialization creates tradeoffs. While the available evidence suggests Dunkleosteus most likely had a thunniform shape, the comparatively deeper body and larger frontal profile (as well as the much blunter snout) would have created more drag than lamnid sharks or tuna. Thus, when compared to extant thunniform fishes Dunkleosteus may have performed better at maneuverability and burst acceleration at the expense of endurance and sustained swimming speed (i.e., a sprinter versus long distance pursuit predator). Arthrodires, like sharks and dolphins, also lack the bony tails that allow tachynektonic actinopterygians like scombrids and istophoriforms to ignore cavitation damage (Iosilevskii and Weihs, 2008), further limiting practical maximum swimming speed. However, the reduced performance of Dunkleosteus in sustained swimming compared to lamnids or tunas should not be taken as an indication that this animal was a less effective organism. Rather, these three groups of fishes all emphasized slightly different adaptational strategies (speed versus maneuverability) within the broader ecomorphospace of a convergent, thunniform body shape.
Obviously, understanding the swimming behavior and hydrodynamic performance of a taxon depends on the shape and structure of the entire organism, rather than evaluating its features in isolation. Dunkleosteus shows an unusual mix of features, some of which have differing implications for performance. Dunkleosteus has a highly stiffened anterior trunk, well-developed attachments for a horizontal septum, and (compared to its relatives) a much deeper trunk with expanded areas for lateral trunk musculature, which are suggestive of thunniform locomotion and a general resistance to resistance to roll and pitch. Other features like small pelvic fins and unornamented armor suggest tachynektonic habits of some form. However, Dunkleosteus differs from extant thunniform taxa in its larger frontal profile, extremely anterior and ventral pectoral fins, and relatively posterior dorsal fin position. These features would be expected to promote maneuverability and burst performance at the cost of increasing drag and instability to yaw (though the pectoral fins may have also aided in stabilization). Some of the features that might be expected to negatively impact performance in these areas seem to be retained arthrodire plesiomorphies and thus the result of phylogenetic signal and/or evolutionary constraints rather than function. Other features, such as caudal fin morphology, cannot be directly evaluated and await the discovery of more complete material. Nevertheless, these features converge to paint a picture of a taxon that is generally thunniform in shape, but that seems to occupy a slightly different ecomorphological niche than living thunniform species. More complex, holistic evaluation of Dunkleosteus ’ swimming performance would require whole-body modelling of hydrodynamic function, and awaits future analysis.
Swimming Biomechanics of Arthrodires
Arthrodire Swimming Behavior. The recognition that arthrodires, especially Dunkleosteus, exhibited stockier, more robust body plans than most extant fishes (Engelman, 2023b, this study) raises questions about these animals’ swimming kinematics. Swimming kinematics in arthrodires have rarely been discussed, given how few species are known from post-thoracic material (see Miles, 1967a; Miles and Westoll, 1968; Carr, 1995; Carr et al., 2010; Ferrón et al., 2017a; Jobbins et al., 2022 for exceptions). Arthrodires have historically been considered anguilliform swimmers (Stensiö, 1963; Miles, 1969; Carr, 1995) but Carr (1995) noted most arthrodires had to be at least subcarangiform to carangiform swimmers, as the rigid bony armor covering the anterior 1/3 to 1/2 of the animal prevented undulation of the anterior trunk. The proportion of the trunk capable of lateral flexion does not appear to increase across arthrodire evolution (Carr, 1995: p. 107; see also Appendix 6), even though the trunk muscles become less restricted laterally. Even later pachyosteomorphs with reduced thoracic shields (Miles, 1969) still have a ventral shield extending to the level of the pelvis and vent (Carr, 1995; Jobbins et al., 2022; Trinajstic et al., 2022b; Engelman, 2023b), largely limiting lateral undulation to the post-pelvic region. The anterior trunk of arthrodires does not appear capable of moving independently of the trunk armor, given the extensive transverse abdominal musculature spanning the base of the myomeres and the internal surface of the posterior ventrolateral plates (Trinajstic et al., 2013).
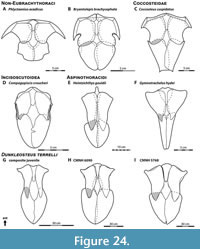 There is little evidence the ventral shield was flexible enough to allow anterior trunk undulations. Indeed, most incisoscutoid and pachyosteomorph arthrodires have an interlocking-W-shaped suture extending across the transverse midline of the ventral shield, formed by large triangular, posteriorly-projecting processes of the anterior ventrolateral plate that fit into V-shaped facets on the posterior ventrolateral plate (Figure 24D–F; see also Dunkle and Bungart, 1945: fig. 3C; Dunkle, 1947: fig. 5C; Dennis-Bryan, 1987: fig. 21A; Long, 1988: fig. 9; Long, 1995: fig. 13; among others). The shape of this suture would make the ventral shield more resistant to lateral flexion than in more basal arthrodires where this feature is absent (Figure 24A–C), such as coccosteids (Miles and Westoll, 1968; Desmond, 1974), Plourdosteus (Vézina, 1988), Holonema (Miles and White, 1971), buchanosteoids (Long et al., 2014), and non-brachythoracids (Heintz, 1933; Denison, 1962; Goujet, 1973, 1984; Mark-Kurik, 1985). In Dunkleosteus terrelli the posteriorly projecting wedge of the anterior ventrolateral plate is particularly large (Figure 24G–I), and partial fusion of the anterior vertebral column (Johanson et al., 2019) would have further restricted anterior trunk flexibility. Thus, the trunk of Dunkleosteus was extremely rigid, even by arthrodire standards.
There is little evidence the ventral shield was flexible enough to allow anterior trunk undulations. Indeed, most incisoscutoid and pachyosteomorph arthrodires have an interlocking-W-shaped suture extending across the transverse midline of the ventral shield, formed by large triangular, posteriorly-projecting processes of the anterior ventrolateral plate that fit into V-shaped facets on the posterior ventrolateral plate (Figure 24D–F; see also Dunkle and Bungart, 1945: fig. 3C; Dunkle, 1947: fig. 5C; Dennis-Bryan, 1987: fig. 21A; Long, 1988: fig. 9; Long, 1995: fig. 13; among others). The shape of this suture would make the ventral shield more resistant to lateral flexion than in more basal arthrodires where this feature is absent (Figure 24A–C), such as coccosteids (Miles and Westoll, 1968; Desmond, 1974), Plourdosteus (Vézina, 1988), Holonema (Miles and White, 1971), buchanosteoids (Long et al., 2014), and non-brachythoracids (Heintz, 1933; Denison, 1962; Goujet, 1973, 1984; Mark-Kurik, 1985). In Dunkleosteus terrelli the posteriorly projecting wedge of the anterior ventrolateral plate is particularly large (Figure 24G–I), and partial fusion of the anterior vertebral column (Johanson et al., 2019) would have further restricted anterior trunk flexibility. Thus, the trunk of Dunkleosteus was extremely rigid, even by arthrodire standards.
The laterally inflexible ventral shield may be one reason for the comparatively wide bodies of arthrodires. The body plans of most extant fishes are laterally narrow in profile, as this allows greater lateral flexibility (Aleev, 1969). Sharks and osteichthyans both show low body widths relative to anteroposterior length, despite being otherwise dissimilar in body shape (Engelman, 2023b: supplementary figure 7.7). Arthrodires instead show relative body widths comparable to stockier fishes like scombrids, siluriforms, and Latimeria, which is evident even in arthrodires known from complete remains (e.g., Coccosteus; Miles and Westoll, 1968). Laterally narrow bodies may enhance trunk flexibility and turning ability, but if the body is laterally inflexible due to pre-existing constraints (i.e., the dermal armor) there will be no selective pressure to minimize body width. Indeed, relaxing constraints for a narrower body may allow for larger trunk muscles to help generate more thrust and aid in flexing the posterior trunk. The absence of a mediolaterally narrow body shape in some nektonic fishes, like certain catfishes (e.g., Ariidae, Pimelodidae) and coelacanths, warrants further functional morphological research.
Although comparisons with thunniform fishes work well for inferred pelagic arthrodires like Dunkleosteus, they are more difficult to apply to non-pelagic species like Coccosteus or Incisoscutum. This is part of a broader problem in arthrodire paleobiology in that few suitable extant models exist for the swimming kinematics of these animals. Most biomechanical studies on fishes have been conducted on flexible-bodied species (Gray, 1933; Lindsey, 1978; Webb, 1984; Schrank et al., 1999; Gemballa and Roder, 2004; Di Santo et al., 2021) or those with entirely rigid trunks (Blake, 1977; Walker, 2000; Hove et al., 2001; Parson et al., 2011), and these taxa generally show limited positional variation in axial flexibility. Eubrachythoracid arthrodires do not conform to either of these categories due to their pronounced anteroposterior variability in trunk stiffness. The anterior trunk of eubrachythoracids may have been laterally stiffened (particularly at the cranio-thoracic joint), but their lateral embayed trunk armor and reduced scalation means the posterior trunk was substantially more flexible than extant fishes with extensive trunk armor like boxfishes. The closest identifiable extant analogues for the condition seen in eubrachythoracids, including an intermediate whole-body stiffness between rigid-bodied swimmers and “typical” fishes along with substantial anteroposterior variation in trunk stiffness, are, ironically, tuna (Fish, 1999; Luo et al., 2020; Downs et al., 2023: fig. 6). Body flexibility has been recognized as an important axis of functional variation in aquatic vertebrates, but how to recognize, quantify, and interpret this variation remains poorly understood (Jimenez et al., 2023).
Additionally, most extant fishes with stiff trunks are exclusively MPF swimmers (Miles, 1967a; Hove et al., 2001; Blake, 2004; Parson et al., 2011) or combine BCF swimming with MPF oscillation (Gordon et al., 2020). By contrast, eubrachythoracid arthrodires generally show features consistent with BCF swimming. Complete eubrachythoracids show well-developed tails with large caudal fins (e.g., Miles and Westoll, 1968; Jobbins et al., 2022), and their pectoral fin anatomy and position are similar to sharks, suggesting a passive role in locomotion (Stensiö, 1959; Carr, 1995; Carr et al., 2010; but see also below). Eubrachythoracids lack features correlated with MPF swimming, like obliquely oriented pectoral fin bases located along the dorsoventral midline of the body as in labriform swimmers (Drucker et al., 2005) or robust, near-symmetrical dorsal and anal fins like ballistiform swimmers (Dornburg et al., 2011). Arthrodires in general show little evidence of adaptations for MPF swimming, with the exception of the basal clade Williamsaspididae (e.g., Elvaspis) which have been suggested to exhibit a similar mode of life to modern boxfishes (Ostracioidea) (Young, 2009). These factors make it difficult to interpret the functional anatomy of arthrodires, especially in non-pelagic forms.
Anterior “Tipping”. The unusual body plan of arthrodires would have created several biomechanical challenges not typically seen in living fishes. Compared to living fishes, arthrodires are distinctly “front-heavy”, with their dermal armor asymmetrically distributed around the anterior half of the body. This results in the center of mass potentially being significantly anterior to the center of buoyancy. If not controlled for, this would produce significant anterior tipping (torque), potentially causing the animal to face-plant into the substrate when attempting to locomote. Similar issues occur in some living fishes (e.g., Cichlasoma; Ting and Yang, 2008), causing these taxa to adopt a tilted-downward posture when swimming. In most fishes the distance between the center of mass and center of buoyancy is generally less than 1–3% total length, and rarely > 5% total length (Aleev, 1969: p. 31), likely for this reason. Even though the armor of eubrachythoracid arthrodires appears to have weighed less than traditionally assumed (~7% total armor-free mass; Engelman, 2023b), this problem would remain at any value of armor weight or body length simply because the armor is unevenly distributed across the body (Gordon et al., 2020; Trinajstic et al., 2022b).
There are several possible ways arthrodires could compensate for this problem. First, this effect can be lessened if the body is anteroposteriorly short. This places the center of mass closer to the center of buoyancy, reducing the lever arm and thus torque. This could explain why arthrodires tend to exhibit short and stocky bodies compared to many other fishes. A short body plan does not entirely fix the problem, but does alleviate it. Second, an anteriorly located organ of positive buoyancy would negate the negative anterior buoyancy of the trunk armor, bringing the center of buoyancy and mass closer together and solving this problem. Extant fishes with extensive trunk armor (Ostracioidea) compensate for negative buoyancy of their armor via swim bladders (Hove et al., 2001; Gordon et al., 2020), which do not appear to have been present in arthrodires (Trinajstic et al., 2022b). However, it is possible the significantly more anterior position of the arthrodire liver compared to sharks (Trinajstic et al., 2022b), roughly in the center of volume of the thoracic armor, is related to compensation for the armor weight. Further study is needed.
Third, arthrodires could have employed combined BCF + pectoral fin swimming and flapped their pectoral fins to provide compensatory anterior lift, similar to what has been proposed for mosasaurs (Formoso et al., 2019) and what occurs in extant “front-heavy” fishes (Ting and Yang, 2008). Carr (1995: p. 107) suggested some aspinothoracidans (e.g., Heintzichthys) could have used combined BCF + pectoral fin swimming given their large pectoral fin bases, but noted that the distally tapered basals in these forms contradicted this idea and suggested they used their fins for steering and passive lift like sharks. The basals of Dunkleosteus are more distally robust than other arthrodires (Carr et al., 2010), and it is tempting to speculate this could be related to the pectoral fin actively providing more anterior lift to a bulky body. However, this seems unlikely; the ventrolateral position and horizontal orientation of the pectoral fenestra/scapulocoracoid in Dunkleosteus resemble sharks, not labriform swimmers (Blake, 2004). The basals of Dunkleosteus are better interpreted as supporting a robust (plesodic?) pectoral fin that did not provide active lift.
Head Oscillation. Lateral head and trunk movements are an important component of locomotion in most extant fishes. Trunk undulation during BCF swimming produces lateral motion (yaw) of the head as a side effect (Lindsey, 1978; Webb, 1984; Rowe et al., 1993), which is compensated for by contralateral movement of the head (Webb and Weihs, 2015; Di Santo et al., 2021). Lateral flexion of the head and trunk is also critical in initiating and controlling turns (Gray, 1933; Aleev, 1969; Weihs, 1972). However, in distinct contrast to most modern fishes, arthrodires were incapable of lateral flexion of the head and anterior trunk. The occiput and laterally paired cranio-thoracic joints are aligned along a single mediolateral axis, with their ginglymoid shape only allowing dorsoventral cranial elevation (Heintz, 1932: p. 196, figs. 35, 68, 83; Miles and Westoll, 1968: fig. 24B; Miles and Dennis, 1979: fig. 9). This creates a highly stable system with three points of articulation incapable of lateral motion. Experimental manipulations of three-dimensionally preserved Gogo arthrodires find lateral motion was impossible across the cranio-thoracic joint (K. Trinajstic, pers. comm., June 2023). The trunk armor would further limit flexibility of the anterior body. Although some arthrodires show evidence of small (mm-sized) gaps between trunk armor plates (Young, 2005: p. 217) likely filled by cartilage in vivo, these are not large enough to allow significant flexion of the anterior trunk.
This means, unlike most fishes, arthrodires could not laterally flex their head, and thus had no innate ability to compensate for head yaw produced by BCF locomotion. This would potentially impose severe biomechanical penalties, as without some compensatory mechanism for head yaw, lateral head motion would constantly disorient a swimming arthrodire. Similarly, arthrodires would be entirely reliant on using their medial and paired fins as rudders to initiate and control turns, given head and anterior trunk flexion were impossible. The significance of this issue is supported by aracanid boxfishes, which resemble arthrodires in having an anteriorly stiffened and laterally inflexible trunk; these fishes are slow, highly unstable swimmers showing high rates of head and trunk yaw during locomotion (Gordon et al., 2020). This issue has little effect on fitness in aracanids, which are demersal fishes that feed on benthic invertebrates and live in sheltered environments like seagrass beds and rocky reefs (Matsuura, 2008), but aracaniform swimming is impractical for large, nektonic organisms that consume other vertebrates and cannot rely on hiding in seagrass beds or reef crevices to escape predators. These issues cannot be explained away by a perceived “primitiveness” or “inefficiency” of the arthrodire body plan. If this were not compensated for in vivo, the organism would not be viable.
In theory, this would support a more thunniform shape for many arthrodires. Thunniform fishes are traditionally thought to show the least amount of head oscillation by restricting lateral undulation to the tail, whereas anguilliform fishes are thought to show the highest (Lindsey, 1978; Webb, 1984; Di Santo et al., 2021). However, more recent studies show all methods of BCF swimming involve some amount of head oscillation. Thunniform fishes swim so frantically they produce a significant amount of head yaw despite their stiffened body, whereas anguilliform and carangiform fishes are able to compensate for yaw more than previously thought (Di Santo et al., 2021). Extant fishes that do have immobile heads and stiff torsos (e.g., Chimaeriformes, Aulostomoidea, Ostraciidae; Miles, 1967a) are almost exclusively MPF swimmers that do not employ lateral trunk undulation, unlike eubrachythoracid arthrodires.
Head oscillation has been previously acknowledged as a biomechanical issue in arthrodires (Westoll, 1947: p. 384–385; White, 1952; Miles, 1967a: p. 62), though often only in passing and without proposing a clear biomechanical solution. The closest any author came was White (1952: p. 290), who agreed it was an issue but considered it irrelevant to the early, non-eubrachythoracid arthrodires he was studying because these taxa were “bottom-haunting and poor swimmers”. How later eubrachythoracid arthrodires would have been affected was not discussed. In general, most studies have deferred discussing this issue because it was traditionally assumed arthrodires were poor swimmers. Many arthrodires are now recognized to show specializations for active, nektonic life, yet still maintained cranio-thoracic joints and anteriorly stiff trunks (Jobbins et al., 2022), indicating this issue can no longer be ignored. Unfortunately, the present author is also unable to propose a useful solution, only highlight that more research is needed on this topic.
Selective Pressures of the Arthrodire Body Plan. The body plan of arthrodires imposed novel constraints but may have also created evolutionary opportunities not present in other fishes. The rigid trunk armor could be co-opted to produce additional attachment sites for thoracic musculature. This may have encouraged a squatter body plan by emphasizing hypertrophy and specialization of the anterior trunk musculature over general axial elongation. Arthrodire specimens with preserved muscle tissue from the Gogo Formation exhibit extensive transverse abdominal musculature, otherwise unknown in non-tetrapods (Trinajstic et al., 2013). These unique pre-pelvic muscles attach to the trunk armor (the posterior ventrolateral plate), suggesting the axial musculature in arthrodires exhibit atypical anteroposterior regionalization among fishes. The well-developed connection between the horizontal septum and the trunk armor on the posterior dorsolateral in Dunkleosteus also supports this, if the septum is interpreted as transmitting force from the axial musculature to the peduncle as in thunnins.
The carinal process of the median dorsal and associated submedian dorsal plate may also imply axial musculature regionalization. While these structures were likely embedded in the epaxial musculature, their function is unclear. Heintz (1931b, 1932) proposed the carinal process served as the origin of the m. levator capitis major, but Miles (1969) disputed this interpretation. Arthrodires with preserved muscle fibers show this muscle originates on the flattened internal face of the median dorsal anterior to the carinal process (Trinajstic et al., 2013). The function of the submedian dorsal plate is equally unclear. Miles and Westoll (1968) proposed it may have served as an anchoring point for powerful epaxial muscles, given the dorsoventrally oriented articulation between the plate and the carinal process. They suggested this eased strain on the trunk armor during lateral undulation by allowing for flexion between the two plates.
Evolution of Nektonic Habits within Arthrodira
In his initial description of Dunkleosteus terrelli, Newberry (1875) wrote:
“We are compelled, however, to regard the complete and impenetrable armor, and the massive and formidable jaws of the great Placoderms, as heavy and rude first models, rather than the light, elegant, and efficient machines which are the perfected results of a long process of improvement. The heavy armor worn by the knights of old has long since been laid aside, for the mail-clad warriors of the middle ages would be clumsy and powerless antagonists to our light-armed troops, carrying repeating rifles and revolvers, and moving with the celerity and precision of modern tactics. So in the progress of ichthyic life, increased intelligence, rapidity of movement and address, have proved in the struggle of life more than a match for the impenetrable but cumbrous defenses of the sluggish and over-loaded Placoderms.” Newberry (1875: p. 17).
For nearly 150 years, this idea that arthrodires were “slow, sluggish swimmers eventually outcompeted by faster, more efficient bony fishes and sharks” has been the dogma in treatments of this group (Hussakof, 1906; Moy-Thomas and Miles, 1971; Barron and Ettensohn, 1981; Benton, 2005; Rhodes, 2016; Abel and Grubbs, 2020), with most authors historically regarding nearly all eubrachythoracid arthrodires, including Dunkleosteus, as benthic animals (Stensiö, 1963; Miles, 1969; Denison, 1978; see discussion in Carr, 2010). Even today, there remains a general sentiment that even if some arthrodires were pelagic animals, they must have been poorly adapted to nektonic life compared to modern marine vertebrates (pers. comms. of others between 2016 and 2023 to R. Engelman). This assumption is driven by several factors, including 1) a perceived inferiority and primitiveness of the arthrodire body plan due to their geologic age and lack of living representatives, 2) an idea that Devonian oceans were lower energy and structured differently from modern ones, or 3) a belief that arthrodires existed for such a short geologic time that evolution could not produce a well-adapted nektonic body plan (again, compare with Newberry, 1875).
However, evidence has been gradually building suggesting eubrachythoracid arthrodires (and Dunkleosteus in particular) were more active, faster-moving, and more nektonic animals than previously thought. Arthrodires, particularly eubrachythoracids, have long been noted to show increasing nektonic specializations over evolutionary time (Stensiö, 1959, 1963; Miles, 1969; Carr, 1995), though most studies (except Carr, 1995) still regarded them as near-exclusively benthic. These specializations include the loss of a dorsoventrally flattened body plan, expansion of the nuchal gap, reduction of the lateral trunk armor (particularly the posterior lateral and posterior dorsolateral plates), separation of the thoracic and ventral shields via loss of the contact between posterior ventrolateral and posterior lateral plates, enlargement of the pectoral fin base, reduction of the spinal plate (with its eventual loss in aspinothoracidans), anteroposterior reduction of the dorsal thoracic shield (primarily in aspinothoracidans), enlargement of the orbits, and loss of body squamation (Stensiö, 1959, 1963; Miles, 1969; Carr, 1995). Pachyosteomorphs have also been argued to show reduced bone density in the vertebral column compared to coccosteomorphs (van Mesdag et al., 2020), similar to how some secondarily aquatic tetrapod groups show a secondary reversal of pachyostosis with increasing nektonic habits (Bianucci et al., 2023). However, the rarity of complete arthrodire fossils makes it difficult to determine how specialized these animals were for a nektonic lifestyle compared to other aquatic vertebrates. More recently, several studies over the last 15 years have presented evidence suggesting eubrachythoracid arthrodires were more ecologically diverse than previously thought and many species (including Dunkleosteus) were likely active, nektonic animals, with several potentially being pelagic (Carr, 1995, 2010; Carr et al., 2010; Ferrón et al., 2017a; Jobbins et al., 2022; Trinajstic et al., 2022a; Trinajstic et al., 2022b: supplementary information; Engelman, 2023b; Jobbins et al., 2024). The present study and prior work by the present author (Engelman, 2023b) adds further support to these conclusions by identifying features directly observable in fossils of Dunkleosteus (deep torso and likely body shape, small pelvic girdle) suggesting active, pelagic habits in this taxon.
Arthrodires are often regarded as early evolutionary experiments that did not exist long enough to evolve a well-adapted, nektonic body plan, but this does not agree with the long evolutionary history of this group. Arthrodires are known from the entirety of the Devonian period, approximately 59.7 Ma (Carr, 1995; Johnson et al., 2000; Dupret and Zhu, 2008; Becker et al., 2020), though they likely have a ghost lineage extending into the Silurian (Zhu et al., 2013; Zhu et al., 2022). Thus, the evolutionary history of Arthrodira spans roughly the same length of time as the entire Cenozoic era (~66 Ma). Within the same time frame, whales (Cetacea) transitioned from terrestrial mammals with even fewer initial adaptions for nektonic life than early arthrodires to highly specialized nektonic forms, and in fact did so within 8–12 Ma of becoming aquatic (Thewissen et al., 2007; Thewissen, 2014; McGowen et al., 2020). Similar patterns are seen in ichthyosaurs (McGowan and Motani, 2003; Jiang et al., 2016; Gutarra et al., 2019) and mosasaurs (Lindgren et al., 2007; Madzia and Cau, 2020), which achieve their most extreme degrees of aquatic specialization in less than 20–30 Ma of returning to the water. finds Rapid specialization for nektonic life may be typical for marine vertebrates; most clades of marine mammals seemingly reaching asymptotic degrees of specialization for nektonic life within 10–12 Ma of entering this adaptive zone (Uhen, 2022). Although eubrachythoracid arthrodires are not tetrapods, marine tetrapods represent a useful analogue for understanding their evolutionary history, as both evolved nektonic habits from non-nektonic ancestors and may show analogous functional patterns (e.g., the development and secondary loss of bone mass increase as mentioned above). The primary difference is eubrachythoracid arthrodires evolved from primarily benthic, detritivorous animals (Miles, 1969; Young, 2010) rather than descending from a terrestrial, non-aquatic ancestor. Length of niche occupation does not guarantee specialization, but based on the rapid specialization seen in other groups of vertebrates and features hinting at nektonic specializations within Arthrodira, the Devonian lasted long enough for Late Devonian arthrodires to develop specialized nektonic body plans. However, the timing and pattern of this transition needs further investigation (see Miles, 1969; Carr, 1995; Zhu et al., 2016b for prior studies discussing this topic).
The argument that Devonian marine ecosystems significantly differed in trophic structure or activity levels from modern ones overlooks important changes in marine ecosystem structure across this period. Some studies suggest Early and Middle Devonian marine ecosystems had lower activity levels than modern ones (Bambach, 1999; Dahl et al., 2010), but these studies also consider Late Devonian (Frasnian-Famennian) ecosystems to have activity levels closer to younger (Carboniferous and onward) Phanerozoic faunas, particularly in vertebrate guilds. These changes are reflected in several evolutionary trends across the Devonian, including an expansion in vertebrate size and appearance of vertebrate megafauna (Dahl et al., 2010; Choo et al., 2014; Sallan and Galimberti, 2015; Engelman, 2023b), radiations of ammonoids and nektonic gnathostomes (Klug et al., 2010), independent evolution of tachypelagic body plans in Late Devonian chondrichthyans and arthrodires (Compagno, 1990; Jobbins et al., 2022), increasing complexity of aquatic food webs (Chevrinais et al., 2017), increasing morphological specialization among gnathostomes (Coatham et al., 2020; Klug et al., 2023; Jobbins et al., 2024), and the appearance of ecological niches still present in modern ecosystems like vertebrate megaplanktivores (Coatham et al., 2020). Overall, Late Devonian nektonic guilds appear more similar to younger Phanerozoic faunas than Early-Middle Devonian ones in their overall structure and degree of specialization.
The ecological success of arthrodires within the nektonic adaptive zone also cannot be explained by incumbency and a lack of competition. Arthrodires were not the first nektonic, predatory vertebrates. Eubrachythoracids seemingly diversified in the Eifelian or Givetian (~395–380 Ma; Denison, 1978; Becker et al., 2020), with older members of this group (non-eubrachythoracids) being predominantly benthic and/or detritivorous (Miles, 1969; Lebedev et al., 2009). Nektonic, predatory vertebrates were already long established by this time, with sarcopterygians and acanthodians occupying these niches since the Late Silurian (~420 Ma; Yu, 1998; Lebedev et al., 2009; Choo et al., 2014; Blais, 2017). While it could be argued armored fishes and eugnathostomes formed “parallel food webs”, with eugnathostomes being too fast for armored fishes to catch but armored fishes being too heavily armored for eugnathostomes to eat, this is at odds with extensive evidence of Eifelian–Givetian arthrodires (and other armored fishes) and eugnathostomes preying on one another (Miles and Westoll, 1968; Trewin, 1986; Long, 1991; Newman et al., 2021; Choo et al., 2023). Arthrodires were capable enough nektonic predators to carve out an ecological niche in an adaptive zone already occupied by more typical looking fishes, rather than simply occupying it due to incumbency.
Similarly, Famennian pelagic pachyosteomorph arthrodires coexisted with highly specialized, pelagic chondrichthyans (such as Ctenacanthiformes [e.g., Ctenacanthus], Symmoriformes [e.g., Cladoselache], Phoebodontiformes [e.g., Diademodus]; Dean, 1909b; Harris, 1951; Compagno, 1990; Carr and Jackson, 2008; Frey et al., 2019; Klug et al., 2023) and osteichthyans (e.g., Tegeolepis; Dunkle and Schaeffer, 1973) for nearly 13 Ma. Again, the Cleveland Shale shows extensive evidence that arthrodires and eugnathostomes ecologically interacted with each other as both predator and prey (Hlavin, 1990; Williams, 1990), meaning arthrodires showed high enough fitness in pelagic environments to coexist with these tachynektonic taxa without being driven to extinction. Carr (1995) suggested possible active or passive competitive exclusion between arthrodires and eugnathostomes during the Famennian, but a long (13 Ma) period of coexistence argues against competitive exclusion (Scott and Anderson, 2021). Opportunistic replacement may better explain this pattern, especially given the catastrophic Kellwasser and Hangenberg Events immediately before and after the Famennian which dramatically reduced Late Devonian arthrodire diversity (Racki, 2005; Sallan and Coates, 2010; Kaiser et al., 2016; Xue et al., 2024). This possibility was also considered by Carr (1995), though considered a less attractive explanation. The ecological role of arthrodires within Devonian fish faunas requires further study.
CONCLUSIONS
Despite the posterior body of Dunkleosteus being poorly known, a surprising amount of its anatomy is constrained by known elements of the skeleton. The length of the body, shape of the trunk, and location of the dorsal and pelvic fins is well constrained by the comparative anatomy of arthrodires. Eubrachythoracid arthrodires have a relatively conserved bauplan allowing body length and shape to be inferred with some confidence even when post-thoracic remains are unknown. Broader adaptational, biomechanical, and proportional patterns across fishes help further refine these constraints. Building on the results of previous works (Engelman, 2023a, 2023b), traditionally proposed lengths of 5–10 m for Dunkleosteus terrelli cannot be justified based on available anatomical evidence. There is currently no evidence for any arthrodire reaching sizes greater than ~4.5 m, though it is possible very large individuals of Titanichthys may have slightly exceeded this.
The stocky, deep body shape for Dunkleosteus proposed by Engelman (2023b) is not unusual in context. Many pelagic vertebrates show stocky, deep (thunniform) body plans compared to their non-pelagic relatives. Dunkleosteus resembles other placoderms in showing positive allometry in body depth, body width, and mouth size. This is partially responsible for the deep trunk of adults. Other features of the anatomy of Dunkleosteus, such as the deep trunk armor relative to other arthrodires, stiffened anterior spine, size and position of the pectoral fenestra, and extremely small pelvis, are suggestive of a highly pelagic thunniform body plan.
Dunkleosteus appears to have been an active, fast-moving animal, as suggested by features such as the large zones for lateral trunk musculature. Compared to other thunniform taxa Dunkleosteus seems to have been better adapted to short, fast bursts of acceleration and high maneuverability, but would probably have performed less well in sustained speed and endurance as a tradeoff. This further supports the idea that arthrodires were much more active animals than previously thought, and that vertebrates comparable in specialization to modern pelagic taxa had already arisen by the end of the Devonian.
The stocky body plan of arthrodires compared to living fishes may be driven by biomechanical challenges created by their unique anatomy. A short body keeps the center of mass closer to the center of buoyancy, which would reduce anterior torque created by the unevenly distributed dermal armor. It also reduces drag and moment of inertia when turning, which would be important given the limited lateral flexibility of arthrodires. Modern fishes are usually mediolaterally narrow to promote lateral flexibility and turning ability, but these functional patterns are not applicable to arthrodires as their body armor already limits lateral flexibility of the trunk.
Overall, arthrodires challenge our general understanding of fish swimming biomechanics and functional anatomy. Most fishes have bodies with low width-to-length ratios. Arthrodires do not, though their proportions are similar to some living fishes like thunnins and catfishes. Most fishes with stiffened anterior trunks and laterally immobile heads are MPF swimmers. Arthrodires have these features but otherwise resemble BCF swimmers. Most fishes can laterally flex their head to initiate turns or compensate for head yaw. Arthrodires could not. Most extant fishes without swim bladders (i.e., sharks) have cambered torsos with flattened bellies to provide lift. Eubrachythoracid arthrodires did not. Most fishes show a largely uniform axial musculature, whereas arthrodires have a body plan suggesting anteroposterior specialization and regionalization. Most fishes have long, pointed snouts, to improve hydrodynamic efficiency. Most arthrodires had blunt, short snouts. These features are evident in any arthrodire known from complete body outlines or relatively complete dermal skeletons (e.g., those in Miles and Westoll, 1968; Jobbins et al., 2022).
Despite this, arthrodires appear to have been highly competent swimmers, given some show streamlined body plans with highly lunate caudal fins similar to modern pelagic cruisers (Jobbins et al., 2022) despite retaining all of the constraints listed above. Given arthrodires are neither sharks nor bony fishes and appear to have evolved nektonic habits independently from other eugnathostomes (Miles, 1969; Lebedev et al., 2009), it is unsurprising they would evolve distinct solutions to biomechanical problems. Arthrodires challenge our understanding of fish biomechanics because they represent a functional strategy with few living analogues. This suggests arthrodire swimming kinematics will be a productive area for future research.
ACKNOWLEDGMENTS
I thank C. Colleary, A. McGee, and H. Majewski for access to fossil specimens in the CMNH collections and assistance in collecting data on mounted specimens of D. terrelli, as well as R. Muehlheim (CMNH) for access to modern ichthyological specimens in their care. I thank A. Gishlick and J. Maisey (AMNH), G. Storrs and C. Schwalbach (CMC), B. Simpson, L. Grande, and C. McGarrity (FMNH), E. Price (FSBC), J. Kerr (MHNM), E. Benard (NHMUK), S. Walsh (NMS), and M. Daly and M. Kibbey (OSU) for access to specimens in their care; M. Benard, R. Carr, B. Carroll, B. Choo, D. Croft, V. Di Santo, N. Gardner, Y. Haridy, R. Hawley, P. Jambura, Z. Johanson, R. Jones, O. Lebedev, J. Long, M. Mehle, A. McGee, M. Mihalistis, M. Newman, P. Princehouse, R. Shell, S. Simpson, P. Sternes, A. Teresi, K. Trinajstic, R. Ward, and S. van Mesdag for discussions on arthrodire and/or fish anatomy; D. Chapman, J. Hannibal, G. Kampouris, A. McGee, E. Scott, and J. Tait for discussion on the scientific history of Dunkleosteus; D. Evans and N. Woods (ROM) for permission to use their photo of ROM VP 52664; and F. Mollen for permission to use their image of Carcharias; and two anonymous reviewers whose comments helped improve this manuscript. Digital models of arthrodires consulted in this study were created by NSF DBI 2230809 to C. Colleary and the CMNH.
REFERENCES
Abel, D.C. and Grubbs, R.D. 2020. Shark Biology and Conservation: Essentials for Educators, Students, and Enthusiasts. John Hopkins University Press, Baltimore.
Adams, L.A. 1919. A memoir on the jaw muscles in recent and fossil vertebrates. Annals of the New York Academy of Sciences, 28:120–127.
https://doi.org/10.1111/j.1749-6632.1918.tb55350.x
Ahlberg, P. 1992. The palaeoecology and evolutionary history of the porolepiform sarcopterygians, p. 71–90. In Mark-Kurik, E. (ed.), Fossil Fishes as Living Animals. Academy of Sciences of Estonia, Tallinn.
Ahlberg, P., Trinajstic, K., Johanson, Z., and Long, J. 2009. Pelvic claspers confirm chondrichthyan-like internal fertilization in arthrodires. Nature, 460:888–889.
https://doi.org/10.1038/nature08176
Aleev, Y.G. 1969. Function and gross morphology in fish. Israel Program for Scientific Translation, Jerusalem.
Alexander, R.M. 1967. Functional Design in Fishes. Hutchinson University Library, London.
Allen, L.G. and Andrews, A.H. 2012. Bomb radiocarbon dating and estimated longevity of Giant Sea Bass (Stereolepis gigas). Bulletin of the Southern California Academy of Sciences, 111:1–14.
https://doi.org/10.3160/0038-3872-111.1.1
Allen, L.G., and Cross, J.N. 2006. Surface Waters, p. 320–341. In Allen, L.G., Pondella, D.J., and Horn, M.H. (eds.), The Ecology of Marine Fishes: California and Adjacent Waters. University of California Press, Berkeley.
Anderson, P.S.L. and Westneat, M.W. 2007. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biology Letters, 3:77–80.
https://doi.org/10.1098/rsbl.2006.0569
Anderson, P.S.L. and Westneat, M.W. 2009. A biomechanical model of feeding kinematics for Dunkleosteus terrelli (Arthrodira, Placodermi). Paleobiology, 35:251–269.
https://doi.org/10.1666/08011.1
Andrade, M.C., López-Fernández, H., and Liverpool, E.A. 2019. New Myloplus from Essequibo River basin, Guyana, with discussion on the taxonomic status of Myleus pacu (Characiformes: Serrasalmidae). Neotropical Ichthyology, 17:e190026.
https://doi.org/10.1590/1982-0224-20190026
Andrews, M., Long, J., Ahlberg, P., Barwick, R., and Campbell, K. 2005. The structure of the sarcopterygian Onychodus jandemarrai n. sp. from Gogo, Western Australia: with a functional interpretation of the skeleton. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 96:197–307.
https://doi.org/10.1017/S0263593300001309
Andrews, S.M. and Westoll, T.S. 1970. The Postcranial Skeleton of Eusthenopteron foordi Whiteaves. Transactions of the Royal Society of Edinburgh, 68:207–329.
https://doi.org/10.1017/s008045680001471x
Babcock, L.E. 2024. Some vertebrate types (Chondrichthyes, Actinopterygii, Sarcopterygii, and Tetrapoda) from two Paleozoic Lagerstätten of Ohio, U.S.A. Journal of Vertebrate Paleontology, 43:e2308621.
https://doi.org/10.1080/02724634.2024.2308621
Baird, G.C., Harper, J.A., Over, D.J., Hannibal, J.T., McKenzie, S.C., and Tesmer, I.H. 2023. Late Famennian Conneaut Group to basal Mississippian Stratigraphic succession and geochronology, New York/Pennsylvania borderland and Lake Erie region, p. 113–209. In Ver Straeten, C.A., Over, D.J. and Woodrow, D. (eds.), Devonian of New York, Volume 3: Frasnian to Famennian (Upper Devonian) stages and the Devonian terrestrial system in New York.
https://doi.org/10.32857/bap.2023.407.04
Bambach, R.K. 1999. Energetics in the global marinefauna: A connection between terrestrial diversification and change in the marine biosphere. Geobios, 32:131–144.
https://doi.org/10.1016/S0016-6995(99)80025-4
Barron, L.S. and Ettensohn, F.R. 1981. Paleoecology of the Devonian-Mississippian black-shale sequence in eastern Kentucky with an atlas of some common fossils. U.S. Department of Energy, Morgantown Energy Technology Center, Morgantown.
Baxter, D., Cohen, K.E., Donatelli, C.M., and Tytell, E.D. 2022. Internal vertebral morphology of bony fishes matches the mechanical demands of different environments. Ecology and Evolution, 12:e9499.
https://doi.org/10.1002/ece3.9499
Bebej, R.M. and Smith, K.M. 2018. Lumbar mobility in archaeocetes (Mammalia: Cetacea) and the evolution of aquatic locomotion in the earliest whales. Zoological Journal of the Linnean Society, 182:695–721.
https://doi.org/10.1093/zoolinnean/zlx058
Béchard, I., Arsenault, F., Cloutier, R., and Kerr, J. 2014. The Devonian placoderm fish Bothriolepis canadensis revisited with three-dimensional digital imagery. Palaeontologia Electronica, 17:1–19.
https://doi.org/10.26879/417
Becker, R.T., Marshall, J.E.A., Da Silva, A.C., Agterberg, F.P., Gradstein, F.M., and Ogg, J.G. 2020. The Devonian Period, p. 733–810. In Gradstein, F.M., Ogg, J.G., Schmitz, M.D. and Ogg, G.M. (eds.), Geologic Time Scale 2020. Elsevier.
https://doi.org/10.1016/B978-0-12-824360-2.00022-X
Benton, M.J. 2005. Vertebrate Palaeontology, Third Edition edition. Wiley-Blackwell, Oxford.
Bernal, D., Dickson, K.A., Shadwick, R.E., and Graham, J.B. 2001. Analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 29:695–726.
https://doi.org/10.1016/S1095-6433(01)00333-6
Bernard, A., Lécuyer, C., Vincent, P., Amiot, R., Bardet, N., Buffetaut, E., Cuny, G., Fourel, F., Martineau, F., Mazin, J.-M., and Prieur, A. 2010. Regulation of Body Temperature by Some Mesozoic Marine Reptiles. Science, 328:1379–1382.
https://doi.org/10.1126/science.1187443
Bianucci, G., Lambert, O., Urbina, M., Merella, M., Collareta, A., Bennion, R., Salas-Gismondi, R., Benites-Palomino, A., Post, K., de Muizon, C., Bosio, G., Di Celma, C., Malinverno, E., Pierantoni, P.P., Villa, I.M., and Amson, E. 2023. A heavyweight early whale pushes the boundaries of vertebrate morphology. Nature, 620:824–829.
https://doi.org/10.1038/s41586-023-06381-1
Blais, S.A. 2017. Precise occlusion and trophic niche differentiation indicate specialized feeding in Early Devonian jawed vertebrates. FACETS, 2:513–530.
https://doi.org/10.1139/facets-2016-0030
Blake, R.W. 1977. On ostraciiform locomotion. Journal of the Marine Biological Association of the United Kingdom, 57:1047–1055.
https://doi.org/10.1017/S0025315400026114
Blake, R.W. 2004. Fish functional design and swimming performance. Journal of Fish Biology, 65:1193–1222.
https://doi.org/10.1111/j.0022-1112.2004.00568.x
Blake, R.W., Chatters, L.M., and Domenici, P. 1995. Turning radius of yellowfin tuna (Thunnus albacares) in unsteady swimming manoeuvres. Journal of Fish Biology, 46:536–538.
https://doi.org/10.1111/j.1095-8649.1995.tb05994.x
Block, B.A. and Finnerty, J.R. 1994. Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures. Environmental Biology of Fishes, 40:283–302.
https://doi.org/10.1007/BF00002518
Bonani Mateussi, N.T., Melo, B.F., and Oliveira, C. 2020. Molecular delimitation and taxonomic revision of the wimple piranha Catoprion (Characiformes: Serrasalmidae) with the description of a new species. Journal of Fish Biology, 97:668–685.
https://doi.org/10.1111/jfb.14417
Boylan, J.C. and Murphy, P.A. 1978. The ventral armor and feeding biomechanics of Glyptaspis verrucosa Newberry, a placoderm from the Fammenian Cleveland Shale. American Museum Novitates, 2655:1–12.
Boyle, J.T., Ryan, M., Snively, E., and Hlavin, W.J. 2016. Jaw ontogeny of the Late Devonian " T. rex " with implications for feeding strategies and life history of the arthrodire Dunkleosteus terrelli. Journal of Vertebrate Paleontology, SVP Program and Abstracts Book 2016, 103.
Boyle, J. and Ryan, M.J. 2017. New information on Titanichthys (Placodermi, Arthrodira) from the Cleveland Shale (Upper Devonian) of Ohio, USA. Journal of Paleontology, 91:318–336.
https://doi.org/10.1017/jpa.2016.136
Branson, E.B. 1908. Notes on Dinichthys terrelli Newberry, with a restoration. The Ohio Naturalist, 8:363–369.
Braun, M.A., Ryan, M.J., Saja, D.B., Riedel, J.A., and Chapman, D. 2014. Shark taphonomy in the Cleveland Shale of northeastern Ohio, USA: a story of disarticulation. Geological Society of America Abstracts with Programs, 46:330.
Bryant, H.N. and Russell, A.P. 1992. The role of phylogenetic analysis in the inference of unpreserved attributes of extinct taxa. Philosophical Transactions: Biological Sciences, 337:405–418.
Buchholtz, E.A. 2001. Swimming styles in Jurassic ichthyosaurs. Journal of Vertebrate Paleontology, 21:61–73.
https://doi.org/10.1671/0272-4634(2001)021[0061:SSIJI]2.0.CO;2
Bullock, L.H. and Smith, G.B. 1991. Seabasses (Pisces: Serranidae). Memoirs of the Hourglass Cruises, 8:1–206.
Burrow, C.J., Jones, A.S., and Young, G.C. 2005. X-ray microtomography of 410 million-year-old optic capsules from placoderm fishes. Micron, 36:551–557.
https://doi.org/10.1016/j.micron.2005.05.005
Burrow, C.J. and Turner, S. 1999. A review of placoderm scales, and their significance in placoderm phylogeny. Journal of Vertebrate Paleontology, 19:204–219.
https://doi.org/10.1080/02724634.1999.10011135
Campbell, K.S.W., and Barwick, R.E. 1988. Geological and palaeontological information and phylogenetic hypotheses. Geological Magazine, 125:207–227.
https://doi.org/10.1017/S0016756800010165
Carey, F.G., Casey, J.G., Pratt, H.L., Jr., Urquhart, D., and McCosker, J.E. 1985. Temperature, heat production, and heat exchange in lamnid sharks. Memoirs of the Southern California Academy of Sciences, 9:92–109.
Carr, R.K. 1991. Reanalysis of Heintzichthys gouldii (Newberry), an aspinothoracid arthrodire (Placodermi) from the Famennian of northern Ohio, with a review of brachythoracid systematics. Zoological Journal of the Linnean Society, 103:349–390.
https://doi.org/10.1111/j.1096-3642.1991.tb00909.x
Carr, R.K. 1994. A redescription of Gymnotrachelus (Placodermi: Arthrodira) from the Cleveland Shale (Famennian) of northern Ohio, U.S.A. Kirtlandia, 48:3–21.
Carr, R.K. 1995. Placoderm diversity and evolution. Bulletin du Muséum National d'Histoire Naturelle, 4:85–125.
Carr, R.K. 1996. Stenosteus angustopectus sp. nov. from the Cleveland Shale (Fammenian) of northern Ohio with a review of selenosteid (Placodermi) systematics. Kirtlandia, 49:19–43.
Carr, R.K. 2009. A big fish story: New links between the Appalachian Basin and Morocco in the Late Devonian. Cincinatti Museum Scientific Contributions, 3:204.
Carr, R.K. 2010. Paleoecology of Dunkleosteus terrelli. Kirtlandia, 57:36–45.
Carr, R.K. 2018. A new aspinothoracid arthrodire from the Late Devonian of Ohio, U.S.A. Acta Geologica Polonica, 68:363–379.
https://doi.org/10.1515/agp-2018-0021
Carr, R.K. and Hlavin, W.J. 2010. Two new species of Dunkleosteus Lehman, 1956, from the Ohio Shale Formation (USA, Famennian) and the Kettle Point Formation (Canada, Upper Devonian), and a cladistic analysis of the Eubrachythoraci (Placodermi, Arthrodira). Zoological Journal of the Linnean Society, 159:195–222.
https://doi.org/10.1111/j.1096-3642.2009.00578.x
Carr, R.K. and Jackson, G.L. 2008. The vertebrate fauna of the Cleveland Member (Famennian) of the Ohio Shale, p. 1–17. In Hannibal, J.T. (ed.), Guide to the Geology and Paleontology of the Cleveland Member of the Ohio Shale.
Carr, R.K., Johanson, Z., and Ritchie, A. 2009. The phyllolepid placoderm Cowralepis mclachlani: Insights into the evolution of feeding mechanisms in jawed vertebrates. Journal of Morphology, 270:775–804.
https://doi.org/10.1002/jmor.10719
Carr, R.K., Lelièvre, H., and Jackson, G.L. 2010. The ancestral morphotype for the gnathostome pectoral fin revisited and the placoderm condition, p. 107–122. In Elliott, D.K., Maisey, J.G., Yu, X., and Miao, D. (eds.), Morphology, Phylogeny and Paleobiogeography of Fossil Fishes. Verlag Dr. Friedrich Pfeil, München.
Chapman, D.A., Jackson, G.L., Dunn, D.W., and Ryan, M.J. 2006. The casting program of the Cleveland Museum of Natural History. Geological Society of America Abstracts with Programs, 38:76.
Chevrinais, M., Jacquet, C., and Cloutier, R. 2017. Early establishment of vertebrate trophic interactions: Food web structure in Middle to Late Devonian fish assemblages with exceptional fossilization. Bulletin of Geosciences, 92:491–510.
https://doi.org/10.3140/bull.geosci.1651
Choo, B. 2012. Revision of the actinopterygian genus Mimipiscis (= Mimia) from the Upper Devonian Gogo Formation of Western Australia and the interrelationships of the early Actinopterygii. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 102:77–104.
https://doi.org/10.1017/S1755691011011029
Choo, B., Holland, T., Clement, A.M., King, B., Challands, T., Young, G., and Long, J.A. 2023. A new stem-tetrapod fish from the Middle–Late Devonian of central Australia. Journal of Vertebrate Paleontology, 43:e2285000.
https://doi.org/10.1080/02724634.2023.2285000
Choo, B., Zhu, M., Zhao, W., Jia, L., and Zhu, Y. 2014. The largest Silurian vertebrate and its palaeoecological implications. Scientific Reports, 4:5242.
https://doi.org/10.1038/srep05242
Coatham, S.J., Vinther, J., Rayfield, E.J., and Klug, C. 2020. Was the Devonian placoderm Titanichthys a suspension feeder? Royal Society Open Science, 7:200272.
https://doi.org/10.1098/rsos.200272
Compagno, L.J.V. 1984. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1. Hexanchiformes to Lamniformes. Food and Agriculture Organization of the United Nations, Rome.
Compagno, L.J.V. 1990. Alternative life-history styles of cartilaginous fishes in time and space. Environmental Biology of Fishes, 28:33–75.
https://doi.org/10.1007/BF00751027
Compagno, L.J.V. 1999. Endoskeleton, p. 69–92. In Hamlett, W.C. (ed.), Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes. John Hopkins University Press, Baltimore.
Compagno, L.J.V. 2008. Pelagic elasmobranch diversity, p. 14–23. In Camhi, M.D., Pikitch, E. K. and Babcock, E.A. (eds.), Sharks of the Open Ocean: Biology, Fisheries & Conservation. Blackwell Publishing, Oxford.
Cousteau, J.-M., Pauly, D., and Parenti, L.R. 2008. Ichthyo: The Architecture of Fish. X-rays from the Smithsonian Institution. Chronicle Books, San Francisco.
Cubo, J., Sena, M.V.A., Aubier, P., Houee, G., Claisse, P., Faure-Brac, M.G., Allain, R., Andrade, R.C.L.P., Sayão, J.M., and Oliveira, G.R. 2020. Were Notosuchia (Pseudosuchia: Crocodylomorpha) warm-blooded? A palaeohistological analysis suggests ectothermy. Biological Journal of the Linnean Society, 131:154–162.
https://doi.org/10.1093/biolinnean/blaa081
Cui, X., Friedman, M., Yu, Y., Zhu, Y.-a., and Zhu, M. 2023. Bony-fish-like scales in a Silurian maxillate placoderm. Nature Communications, 14:7622.
https://doi.org/10.1038/s41467-023-43557-9
Daeschler, E.B. and Cressler, W.L., III 2011. Late Devonian paleontology and paleoenvironments at Red Hill and other fossil sites in the Catskill Formation of north-central Pennsylvania. Geological Society of America Field Guide, 20:1–16.
https://doi.org/10.1130/2011.0020(01)
Daeschler, E.B., Frumes, A.C., and Mullison, C.F. 2003. Groenlandaspidid placoderm fishes from the Late Devonian of North America. Records of the Australian Museum, 55:45–60.
https://doi.org/10.3853/j.0067-1975.55.2003.1374
Dahl, T.W., Hammarlund, E.U., Anbar, A.D., Bond, D.P.G., Gill, B.C., Gordon, G.W., Knoll, A.H., Nielsen, A.T., Schovsbo, N.H., and Canfield, D.E. 2010. Devonian rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proceedings of the National Academy of Sciences, 107:17911–17915.
https://doi.org/10.1073/pnas.1011287107
De Iuliis, G. and Pulerà, D. 2011. The dissection of vertebrates: a laboratory manual, Will Second edition. Academic Press, New York.
De Maddalena, A., Glaizot, O., and Olivier, G. 2003. On the Great White Shark, Carcharodon carcharias (Linnaeus, 1758), preserved in the Museum of Zoology in Lausanne. Marine Life, 13:53–59.
Dean, B. 1894. Contributions to the morphology of Cladoselache. Journal of Morphology, 9:87–115.
https://doi.org/10.1002/jmor.1050090103
Dean, B. 1896. On the vertebral column, fins, and ventral armoring of Dinichthys. Proceedings of the New York Academy of Sciences, 15:157–188.
Dean, B. 1902. The preservation of muscle-fibres in sharks of the Cleveland Shale. American Scientist, 30:273–278.
Dean, B. 1909a. A mounted specimen of Dinichthys terrelli. Memoirs of the American Museum of Natural History, 9:268–269.
Dean, B. 1909b. The Cladoselachian Sharks. Memoirs of the American Museum of Natural History, 9:211–248.
Dean, B. 1909c. Notes on a newly mounted Titanichthys. Memoirs of the American Museum of Natural History, 9:270–271.
Denison, R.H. 1962. A reconstruction of the shield of the arthrodire Bryantolepis brachycephalus (Bryant). Fieldiana Geology, 14:99–104.
https://doi.org/10.5962/bhl.title.5196
Denison, R.H. 1978. Placodermi, p. 1–128. In Schultze, H.-P. (ed.), Handbook of Palaeoichthyology. Gustav Fischer Verlag, Stuttgart.
Dennis-Bryan, K. 1987. A new species of eastmanosteid arthrodire (Pisces: Placodermi) from Gogo, Western Australia. Zoological Journal of the Linnean Society, 90:1–64.
https://doi.org/10.1111/j.1096-3642.1987.tb01347.x
Dennis, K. and Miles, R.S. 1979a. A second eubrachythoracid arthrodire from Gogo, Western Australia. Zoological Journal of the Linnean Society, 67:1–29.
https://doi.org/10.1111/j.1096-3642.1979.tb01102.x
Dennis, K. and Miles, R.S. 1979b. Eubrachythoracid arthrodires with tubular rostral plates from Gogo, Western Australia. Zoological Journal of the Linnean Society, 67:297–328.
https://doi.org/10.1111/j.1096-3642.1979.tb01118.x
Dennis, K. and Miles, R.S. 1980. New durophagous arthrodires from Gogo, Western Australia. Zoological Journal of the Linnean Society, 69:43–85.
https://doi.org/10.1111/j.1096-3642.1980.tb01932.x
Dennis, K. and Miles, R.S. 1981. A pachyosteomorph arthrodire from Gogo, Western Australia. Zoological Journal of the Linnean Society, 73:213–258.
https://doi.org/10.1111/j.1096-3642.1981.tb01594.x
Desmond, A.J. 1974. On the coccosteid arthrodire Millerosteus minor. Zoological Journal of the Linnean Society, 54:277–298.
https://doi.org/10.1111/j.1096-3642.1974.tb00804.x
Di Santo, V., Goerig, E., Wainwright Dylan, K., Akanyeti, O., Liao James, C., Castro-Santos, T., and Lauder George, V. 2021. Convergence of undulatory swimming kinematics across a diversity of fishes. Proceedings of the National Academy of Sciences, 118:e2113206118.
https://doi.org/10.1073/pnas.2113206118
Donley, J.M., Sepulveda, C.A., Konstantinidis, P., Gemballa, S., and Shadwick, R.E. 2004. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature, 429:61–65.
https://doi.org/10.1038/nature02435
Dornburg, A., Sidlauskas, B., Santini, F., Sorenson, L., Near, T.J., and Alfaro, M.E. 2011. The influence of an innovative locomotor strategy on the phenotypic diversification of triggerfish (family: Balistidae). Evolution, 65:1912–1926.
https://doi.org/10.1111/j.1558-5646.2011.01275.x
Downs, A.M., Kolpas, A., Block, B.A., and Fish, F.E. 2023. Multiple behaviors for turning performance of Pacific bluefin tuna (Thunnus orientalis). Journal of Experimental Biology, 226:jeb244144.
https://doi.org/10.1242/jeb.244144
Downs, J.P., Criswell, K.E., and Daeschler, E.B. 2011. Mass Mortality of Juvenile Antiarchs (Bothriolepis sp.) from the Catskill Formation (Upper Devonian, Famennian Stage), Tioga County, Pennsylvania. Proceedings of the Academy of Natural Sciences of Philadelphia, 161:191–203. https://doi.org/10.1635/053.161.0111
Drucker, E.G. and Lauder, G.V. 2001. Locomotor function of the dorsal fin in teleost fishes: experimental analysis of wake forces in sunfish. Journal of Experimental Biology, 204:2943–2958.
https://doi.org/10.1242/jeb.204.17.2943
Drucker, E.G., Walker, J.A., and Westneat, M.W. 2005. Mechanics of Pectoral Fin Swimming in Fishes, p. 369–423. Fish Physiology. Academic Press.
https://doi.org/10.1016/S1546-5098(05)23010-8
Dunkel, C.A., Vázquez-Ortega, A., and Evans, J.E. 2022. Black shale–gray shale transitions in a Late Devonian shale succession, Central Appalachian Basin (Northern Ohio): Sedimentary and geochemical evidence for terrestrial organic matter input driving anoxia events. Palaeogeography, Palaeoclimatology, Palaeoecology, 608:111271.
https://doi.org/10.1016/j.palaeo.2022.111271
Dunkle, D.H. 1947. A new genus and species of arthrodiran fish from the upper Devonian Cleveland Shale. Kirtlandia, 8:103–117.
Dunkle, D.H. 1964. Preliminary description of a palaeoniscoid fish from the Upper Devonian of Ohio. Scientific Publications of the Cleveland Museum of Natural History, 3:1–16.
Dunkle, D.H. and Bungart, P.A. 1940. On one of the least known of the Cleveland Shale Arthrodira. Kirtlandia, 8:29–47.
Dunkle, D.H. and Bungart, P.A. 1942. The infero-gnathal plates of Titanichthys. Kirtlandia, 8:49–59.
Dunkle, D.H. and Bungart, P.A. 1945. A new arthrodiran fish from the upper Devonian Ohio Shales. Kirtlandia, 8:85–95.
Dunkle, D.H. and Bungart, P.A. 1946. The antero-supragnathal of Gorgonichthys. American Museum Novitates, 1316:1–10.
Dunkle, D.H. and Schaeffer, B. 1973. Tegeolepis clarki (Newberry), a palaeonisciform from the upper Devonian Ohio Shale. Palaeontographica Abteilung A, 143:151–158.
Dupret, V. 2010. Revision of the genus Kujdanowiaspis Stensiö, 1942 (Placodermi, Arthrodira, “Actinolepida”) from the Lower Devonian of Podolia (Ukraine). Geodiversitas, 32:5–63.
https://doi.org/10.5252/g2010n1a1
Dupret, V., Goujet, D., and Mark-Kurik, E. 2007. A new genus of placoderm (Arthrodira: ‘Actinolepida’) from the Lower Devonian of Podolia (Ukraine). Journal of Vertebrate Paleontology, 27:266–284.
https://doi.org/10.1671/0272-4634(2007)27[266:ANGOPA]2.0.CO;2
Dupret, V. and Zhu, M. 2008. The earliest phyllolepid (Placodermi, Arthrodira) from the Late Lochkovian (Early Devonian) of Yunnan (South China). Geological Magazine, 145:257–278.
https://doi.org/10.1017/S0016756807004207
Engelman, R.K. 2023a. Giant, swimming mouths: Oral dimensions of extant sharks do not accurately predict body size in Dunkleosteus terrelli (Placodermi: Arthrodira). PeerJ, 11:1–34.
https://doi.org/10.7717/peerj.15131
Engelman, R.K. 2023b. A Devonian fish tale: A new method of body length estimation suggests much smaller sizes for Dunkleosteus terrelli (Placodermi: Arthrodira). Diversity, 15:318.
https://doi.org/10.3390/d15030318
Engelman, R.K., Carr, R., Trinajstic, K., Lebedev, O.A., and Johanson, Z. In Press. Functional anatomy, jaw mechanisms and feeding behavior of Dunkleosteus terrelli (Placodermi, Arthrodira).
Ferrón, H.G., Martinez-Perez, C., and Botella, H. 2017a. Ecomorphological inferences in early vertebrates: reconstructing Dunkleosteus terrelli (Arthrodira, Placodermi) caudal fin from palaeoecological data. PeerJ, 5:e4081.
https://doi.org/10.7717/peerj.4081
Ferrón, H.G., Martínez-Pérez, C., and Botella, H. 2017b. The evolution of gigantism in active marine predators. Historical Biology, 30:712–716.
https://doi.org/10.1080/08912963.2017.1319829
Fierstine, H.L. and Walters, V. 1968. Studies in locomotion and anatomy of scombrid fishes. Memoirs of the Southern California Academy of Sciences, 6:1–31.
Fish, F.E. 1999. Performance constraints on the maneuverability of flexible and rigid biological systems, p. 394–406. Proceedings of the Eleventh International Symposium on Unmanned Untethered Submersible Technology. Autonomous Undersea Systems Institute, Durham.
Fish, F.E. 2002. Balancing requirements for stability and maneuverability in cetaceans. Integrative and Comparative Biology, 42:85–93.
https://doi.org/10.1093/icb/42.1.85
Fish, F.E. and Battle, J.M. 1995. Hydrodynamic design of the humpback whale flipper. Journal of Morphology, 225:51–60.
https://doi.org/10.1002/jmor.1052250105
Fish, F.E. and Lauder, G.V. 2017. Control surfaces of aquatic vertebrates: active and passive design and function. Journal of Experimental Biology, 220:4351–4363.
https://doi.org/10.1242/jeb.149617
Formoso, K., Habib, M., and Bottjer, D. 2019. Reassessment of the mosasaur pectoral girdle and its role in locomotion. Geological Society of America Abstracts with Programs, 51:
https://doi.org/10.1130/abs/2019AM-333823
Frey, L., Rücklin, M., Korn, D., and Klug, C. 2018. Late Devonian and Early Carboniferous alpha diversity, ecospace occupation, vertebrate assemblages and bio-events of southeastern Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology, 496:1–17.
https://doi.org/10.1016/j.palaeo.2017.12.028
Frey, L., Coates, M., Ginter, M., Hairapetian, V., Rucklin, M., Jerjen, I., and Klug, C. 2019. The early elasmobranch Phoebodus: phylogenetic relationships, ecomorphology and a new time-scale for shark evolution. Proceedings of the Royal Society B: Biological Sciences, 286:20191336.
https://doi.org/10.1098/rspb.2019.1336
Froese, R. 2006. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. Journal of Applied Ichthyology, 22:241–253.
https://doi.org/10.1111/j.1439-0426.2006.00805.x
Gaines, R.R. 2014. Burgess Shale-type preservation and its distribution in space and time. The Paleontological Society Papers, 20:123–146.
https://doi.org/10.1017/S1089332600002837
Galis, F., Carrier, D.R., van Alphen, J., van der Mije, S.D., Van Dooren, T.J., Metz, J.A., and ten Broek, C.M. 2014. Fast running restricts evolutionary change of the vertebral column in mammals. Proceedings of the National Academy of Sciences, 111:11401–11406.
https://doi.org/10.1073/pnas.1401392111
Gardiner, B.G. and Miles, R.S. 1990. A new genus of eubrachythoracid arthrodire from Gogo, Western Australia. Zoological Journal of the Linnean Society, 99:159–204.
https://doi.org/10.1111/j.1096-3642.1990.tb00566.x
Gardiner, B.G. and Miles, R.S. 1994. Eubrachythoracid arthrodires from Gogo, Western Australia. Zoological Journal of the Linnean Society, 112:443–477.
https://doi.org/10.1111/j.1096-3642.1994.tb00331.x
Garrick, J.A.F. 1967. Revision of sharks of genus Isurus with description of a new species (Galeoidea, Lamnidae). Proceedings of the United Stated National Museum, 118:663–690.
Garrick, J.A.F. 1982. Sharks of the genus Carcharhinus. NOAA Technical Report NMFS Circular, 445:1–194.
Garrick, J.A.F. 1985. Additions to a revision of the shark genus Carcharhinus: Synonymy of Aprionodon and Hypoprion, and description of a new species of Carcharhinus (Carcharhinidae). NOAA Technical Report NMFS, 34:1–25.
Garrod, B. 2021. Dunkleosteus. Extinct - The Story of Life on Earth. Zephyr Press, Brookline.
Gemballa, S., Konstantinidis, P., Donley, J.M., Sepulveda, C., and Shadwick, R.E. 2006. Evolution of high-performance swimming in sharks: Transformations of the musculotendinous system from subcarangiform to thunniform swimmers. Journal of Morphology, 267:477–493.
https://doi.org/10.1002/jmor.10412
Gemballa, S. and Roder, K. 2004. From head to tail: the myoseptal system in basal actinopterygians. Journal of Morphology, 259:155–71.
https://doi.org/10.1002/jmor.10175
Gess, R.W. and Trinajstic, K.M. 2017. New morphological information on, and species of placoderm fish Africanaspis (Arthrodira, Placodermi) from the Late Devonian of South Africa. PLoS ONE, 12:e0173169.
https://doi.org/10.1371/journal.pone.0173169
Gingerich, P.D. 1998. Paleobiological Perspectives on Mesonychia, Archaeoceti, and the Origin of Whales, p. 423–449. In Thewissen, J.G.M. (ed.), The Emergence of Whales: Evolutionary Patterns in the Origin of Cetacea. Springer US, Boston, MA.
https://doi.org/10.1007/978-1-4899-0159-0_15
Golonka, J. 2020. Late Devonian paleogeography in the framework of global plate tectonics. Global and Planetary Change, 186:103129.
https://doi.org/10.1016/j.gloplacha.2020.103129
Goode, G.B. 1884. The Fisheries And Fishery Industries Of The United States: Natural History of Useful Aquatic Animals. Government Printing Office, Washington D.C.
Gordon, M.S., Lauritzen, D.V., Wiktorowicz-Conroy, A.M., and Rutledge, K.M. 2020. Aracaniform Swimming: A Proposed New Category of Swimming Mode in Bony Fishes (Teleostei: Tetraodontiformes: Aracanidae). Physiological and Biochemical Zoology, 93:235–242.
https://doi.org/10.1086/708163
Goujet, D. 1973. Sigaspis, un nouvel arthrodire du Dévonien inférieur du Spitzberg. Palaeontographica Abteilung A, 143:73–88.
Goujet, D. 1984. Les poissons placodermes du Spitsberg. CNRS, Paris.
Graham, J.B., Koehrn, F.J., and Dickson, K.A. 1983. Distribution and relative proportions of red muscle in scombrid fishes: consequences of body size and relationships to locomotion and endothermy. Canadian Journal of Zoology, 61:2087–2096.
https://doi.org/10.1139/z83-274
Grande, L. and Bemis, W.E. 1991. Osteology and phylogenetic relationships of fossil and recent paddlefishes (Polyodontidae) with comments on the interrelationships of Acipenseriformes. Journal of Vertebrate Paleontology, 11:1–121.
https://doi.org/10.1080/02724634.1991.10011424
Gray, J. 1933. Directional control of fish movement. Proceedings of the Royal Society B: Biological Sciences, 113:115–125.
https://doi.org/10.1098/rspb.1933.0035
Greenfield, T. 2020. Ceratotrichia and dorsal fin shape in arthrodires. Incertae Sedis. Accessed April 9, 2022.
https://incertaesedisblog.wordpress.com/2020/11/03/ceratotrichia-and-dorsal-fin-shape-in-arthrodires/
Gregory, W.K. and Conrad, G.M. 1937. The comparative osteology of the swordfish (Xiphias) and the sailfish (Istiophorus). American Museum Novitates, 952:1–25.
Gross, W. 1932. Die Arthrodira Wildungens. Geologische und Paläeontologische Abhandlungen, 19:1–61.
Gross, W. 1963. Gemuendina stuertzi Traquair. Neuuntersuchung. Notizblatt des Hessischen Landesamtes für Bodenforschung, 91:36–73.
Gutarra, S., Moon, B.C., Rahman, I.A., Palmer, C., Lautenschlager, S., Brimacombe, A.J., and Benton, M.J. 2019. Effects of body plan evolution on the hydrodynamic drag and energy requirements of swimming in ichthyosaurs. Proceedings of the Royal Society B: Biological Sciences, 286:20182786.
https://doi.org/10.1098/rspb.2018.2786
Gutarra, S., Stubbs, T.L., Moon, B.C., Palmer, C., and Benton, M.J. 2022. Large size in aquatic tetrapods compensates for high drag caused by extreme body proportions. Communications Biology, 5:380.
https://doi.org/10.1038/s42003-022-03322-y
Hansen, M.C. 1996. Phylum Chordata-vertebrate fossils, p. 288–369. In Feldmann, R.M. and Hackathorn, M. (eds. Fossils of Ohio. Ohio Division of Geological Survey, Columbus.
Harris, J.E. 1938. The Role of the Fins in the Equilibrium of the Swimming Fish: II. The Role of the Pelvic Fins. Journal of Experimental Biology, 15:32–47.
https://doi.org/10.1242/jeb.15.1.32
Harris, J.E. 1951. Diademodus hydei, a new fossil shark from the Cleveland Shale. Proceedings of the Zoological Society of London, 120:683–697.
https://doi.org/10.1111/j.1096-3642.1951.tb00672.x
Harris, J.E. 1953. Fin patterns and mode of life in fishes, p. 17–28. In Marshall, S.M. and Orr, P. (eds.), Essays in Marine Biology. Oliver and Boyd, Edinburgh.
Hebrank, J.H., Hebrank, M.R., Long, J.H., Jr., Block, B.A., and Wainwright, S.A. 1990. Backbone mechanics of the blue marlin Makaira nigricans (Pisces, Istiophoridae). Journal of Experimental Biology, 148:449–459.
https://doi.org/10.1242/jeb.148.1.449
Heintz, A. 1931a. Revision of the structure of Coccosteus decipiens Ag. Norsk Geologisk Tiddskrift, 12:291–313.
Heintz, A. 1931b. A new reconstruction of Dinichthys. American Museum Novitates, 457:1–5.
Heintz, A. 1932. The structure of Dinichthys: a contribution to our knowledge of the Arthrodira, p. 115–224. In Gudger, E.W. (ed.), The Bashford Dean Memorial Volume. American Museum of Natural History, New York.
Heintz, A. 1933. Some remarks about the structure of Phlyctaenaspis acadica Whiteaves. Norsk Geologisk Tiddskrift, 14:127–144.
Heintz, A. 1938. Notes on Arthrodira. Norsk geologisk Tidsskrift, 18:1–27.
Heintz, A. 1968. The spinal plate in Homostius and Dunkleosteus, p. 145–151. In Ørvig, Tor (ed.), Current Problems of Lower Vertebrate Phylogeny. Almqvist and Wiksell, Stockholm.
Helfman, G.S., Collete, B.B., Facey, D.E., and Bowen, B.W. 2009. The Diversity of Fishes: Biology, Evolution, and Ecology, Second edition. Wiley-Blackwell, West Sussex.
Hemmings, S.K. 1978. The Old Red Sandstone Antiarchs of Scotland: Pterichthyodes and Microbrachius. Monographs of the Palaeontographical Society, 131:1–62.
https://doi.org/10.1080/25761900.2022.12131737
Hlavin, W.J. 1976. Biostratigraphy of the late Devonian black shales on the cratonal margin of the Appalachian geosyncline. PhD thesis. Boston University Graduate School, Ann Arbor. 211 pp.
Hlavin, W.J. 1990. Arthrodire-ctenacanth shark, p. 192–195. In Boucot, A.J. (ed.), Evolutionary Paleobiology of Behavior and Coevolution. Elsevier, New York.
Hodnett, J.-P.M., Grogan, E.M., Lund, R., Lucas, S.G., Suazo, T., Elliott, D.K., and Pruitt, J. 2021. Ctenacanthiform sharks from the late Pennsylvanian (Missourian) Tinajas Member of the Atrasado Formation, central New Mexico. New Mexico Museum of Natural History and Science Bulletin, 84:391–424.
Hoffmann, S.L., Buser, T.J., and Porter, M.E. 2020. Comparative morphology of shark pectoral fins. Journal of Morphology, 281:1501–1516.
https://doi.org/10.1002/jmor.21269
House, M.R., Gordon, M., and Hlavin, W.J. 1986. Late Devonian ammonoids from Ohio and adjacent states. Journal of Paleontology, 60:126–144.
https://doi.org/10.1017/S0022336000021582
Hove, J.R., O’Bryan, L.M., Gordon, M.S., Webb, P.W., and Weihs, D. 2001. Boxfishes (Teleostei: Ostraciidae) as a model system for fishes swimming with many fins: kinematics. Journal of Experimental Biology, 204:1459–1471.
https://doi.org/10.1242/jeb.204.8.1459
Howe, S., Bryant, K., Duff, A., and Astley, H. 2021. Testing the effects of body depth on fish maneuverability via robophysical models. Bioinspiration and Biomimetics, 17: 016002.
https://doi.org/10.1088/1748-3190/ac33c1
Hu, Y., Lu, J., and Young, G.C. 2017. New findings in a 400 million-year-old Devonian placoderm shed light on jaw structure and function in basal gnathostomes. Scientific Reports, 7:7813.
https://doi.org/10.1038/s41598-017-07674-y
Hubbs, C., Lagler, K., and Smith, G. 2004. Fishes of the Great Lakes Region, Revised Edition, Revised edition. University of Michigan Press, Ann Arbor.
https://doi.org/10.3998/mpub.17658
Hussakof, L. 1905. Notes on the Devonian “placoderm” Dinichthys intermedius Newb. Bulletin of the American Museum of Natural History, 21:27–36.
Hussakof, L. 1906. Studies on the Arthrodira. Memoirs of the American Museum of Natural History, 9:103–155.
Hussakof, L. and Bryant, W.L. 1918. Catalog of the fossil fishes in the Museum of the Buffalo Society of Natural Sciences. Bulletin of the Buffalo Society of Natural Sciences, 12:1–198.
Hussakof, L. and Kepler, W. 1905. On the structure of two imperfectly known dinichthyids. Bulletin of the American Museum of Natural History, 21:409–414.
Hyde, J.E. 1926. Collecting fossil fishes from the Cleveland Shale. Natural History, 26:497–504.
Iosilevskii, G. and Weihs, D. 2008. Speed limits on swimming of fishes and cetaceans. Journal of the Royal Society Interface, 5:329–38.
https://doi.org/10.1098/rsif.2007.1073
Janvier, P. 2003. Early Vertebrates. Oxford, Clarendon Press.
Jayne, B.C. and Lauder, G.V. 1994. Comparative morphology of the myomeres and axial skeleton in four genera of centrarchid fishes. Journal of Morphology, 220:185–205.
https://doi.org/10.1002/jmor.1052200207
Jayne, B.C. and Lauder, G.V. 1996. New data on axial locomotion in fishes: how speed affects diversity of kinematics and motor patterns. American Zoologist, 36:642–655.
https://doi.org/10.1093/icb/36.6.642
Jiang, D.-Y., Motani, R., Huang, J.-D., Tintori, A., Hu, Y.-C., Rieppel, O., Fraser, N.C., Ji, C., Kelley, N.P., Fu, W.-L., and Zhang, R. 2016. A large aberrant stem ichthyosauriform indicating early rise and demise of ichthyosauromorphs in the wake of the end-Permian extinction. Scientific Reports, 6:26232.
https://doi.org/10.1038/srep26232
Jimenez, Y.E., Lucas, K.N., Long, J.H., Jr., and Tytell, E.D. 2023. Flexibility is a hidden axis of biomechanical diversity in fishes. Journal of Experimental Biology, 226:jeb245308.
https://doi.org/10.1242/jeb.245308
Jobbins, M., Rücklin, M., Ferrón, H.G., and Klug, C. 2022. A new selenosteid placoderm from the Late Devonian of the eastern Anti-Atlas (Morocco) with preserved body outline and its ecomorphology. Frontiers in Ecology and Evolution, 10:969158.
https://doi.org/10.3389/fevo.2022.969158
Jobbins, M., Rücklin, M., Sánchez Villagra, M.R., Lelièvre, H., Grogan, E., Szrek, P., and Klug, C. 2024. Extreme lower jaw elongation in an alien-looking placoderm from Morocco. Royal Society Open Science, 11:231747.
https://doi.org/10.1098/rsos.231747
Johanson, Z. 2003. Placoderm branchial and hypobranchial muscles and origins in jawed vertebrates. Journal of Vertebrate Paleontology, 23:735–749.
https://doi.org/10.1671/2
Johanson, Z. and Ahlberg, P.E. 1998. A complete primitive rhizodont from Australia. Nature, 394:569–573.
https://doi.org/10.1038/29058
Johanson, Z. and Smith, M.M. 2003. Placoderm fishes, pharyngeal denticles, and the vertebrate dentition. Journal of Morphology, 257:289–307.
https://doi.org/10.1002/jmor.10124
Johanson, Z. and Smith, M.M. 2005. Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms. Biological Reviews, 80:303–345.
https://doi.org/10.1017/S1464793104006682
Johanson, Z., Trinajstic, K., Carr, R., and Ritchie, A. 2013. Evolution and development of the synarcual in early vertebrates. Zoomorphology, 132:95–110.
https://doi.org/10.1007/s00435-012-0169-9
Johanson, Z., Trinajstic, K., Cumbaa, S., and Ryan, M.J. 2019. Fusion in the vertebral column of the pachyosteomorph arthrodire Dunkleosteus terrelli (‘Placodermi’). Palaeontologia Electronica, 22.2.20A:1–13.
https://doi.org/10.26879/872
Johnson, H., Elliott, D.K., and Wittke, J.H. 2000. A new actinolepid arthrodire (Class Placodermi) from the Lower Devonian Sevy Dolomite, East-Central Nevada. Zoological Journal of the Linnean Society, 129:241–266.
https://doi.org/10.1006/zjls.1999.0206
Johnson, H.G. and Elliott, D.K. 1995. A Redescription of Eldenosteus arizonensis (Placodermi: Arthrodira) from the Upper Devonian Martin Formation of Northern Arizona. Journal of Vertebrate Paleontology, 15:221–234.
https://doi.org/10.1080/02724634.1995.10011226
Jones, R.E., Petrell, R.J., and Pauly, D. 1999. Using modified length–weight relationships to assess the condition of fish. Aquacultural Engineering, 20:261–276.
https://doi.org/10.1016/S0144-8609(99)00020-5
Kaiser, S.I., Aretz, M., and Becker, R.T. 2016. The global Hangenberg Crisis (Devonian–Carboniferous transition): review of a first-order mass extinction. Geological Society, London, Special Publications, 423:387.
https://doi.org/10.1144/SP423.9
Kamminga, P., De Bruin, P.W., Geleijns, J., and Brazeau, M.D. 2017. X-ray computed tomography library of shark anatomy and lower jaw surface models. Scientific Data, 4:170047.
https://doi.org/10.1038/sdata.2017.47
Kemp, A. 1996. Sagenodus (Proceratodus) carlinvillensis (Romer and Smith 1934), (Osteichthyes: Dipnoi), Short Ridge Anomaly and Classification of Dipnoans. Journal of Vertebrate Paleontology, 16:16–19.
https://doi.org/10.1080/02724634.1996.10011279
Kim, S.H., Shimada, K., and Rigsby, C.K. 2013. Anatomy and evolution of heterocercal tail in lamniform sharks. The Anatomical Record, 296:433–42.
https://doi.org/10.1002/ar.22647
Kim, S.W., Yuen, A.H.L., Poon, C.T.C., Hwang, J.O., Lee, C.J., Oh, M.-K., Kim, K.T., Kim, H.J., Giri, S.S., Kim, S.G., Kwon, J., Lee, S.B., Choi, M.C., and Park, S.C. 2021. Cross-sectional anatomy, computed tomography, and magnetic resonance imaging of the banded houndshark (Triakis scyllium). Scientific Reports, 11:1165.
https://doi.org/10.1038/s41598-020-80823-y
Klug, C., Coates, M., Frey, L., Greif, M., Jobbins, M., Pohle, A., Lagnaoui, A., Haouz, W.B., and Ginter, M. 2023. Broad snouted cladoselachian with sensory specialization at the base of modern chondrichthyans. Swiss Journal of Palaeontology, 142:2.
https://doi.org/10.1186/s13358-023-00266-6
Klug, C., Kröger, B., Kiessling, W., Mullins, G.L., Servais, T., Frýda, J., Korn, D., and Turner, S. 2010. The Devonian nekton revolution. Lethaia, 43:465–477.
https://doi.org/10.1111/j.1502-3931.2009.00206.x
LaMonte, F. 1955. A review and revision of the marlins, genus Makaira. Bulletin of the American Museum of Natural History, 107:319–358.
Lauder, G.V. and Drucker, E.G. 2004. Morphology and experimental hydrodynamics of fish fin control surfaces. IEEE Journal of Oceanic Engineering, 29:556–571.
https://doi.org/10.1109/JOE.2004.833219
Lebedev, O.A., Engelman, R.K., Skutschas, P.P., Johanson, Z., Smith, M.M., Kolchanov, V.V., Trinajstic, K., and Linkevich, V.V. 2023. Structure, Growth and Histology of Gnathal Elements in Dunkleosteus (Arthrodira, Placodermi), with a Description of a New Species from the Famennian (Upper Devonian) of the Tver Region (North-Western Russia). Diversity, 15:648.
https://doi.org/10.3390/d15050648
Lebedev, O.A., Mark-Kurik, E., Karatajūtė-Talimaa, V.N., Lukševičs, E., and Ivanov, A. 2009. Bite marks as evidence of predation in early vertebrates. Acta Zoologica, 90:344–356.
https://doi.org/10.1111/j.1463-6395.2008.00344.x
Lelievre, H., Janvier, P., and Goujet, D. 1981. Les vertébrés dévoniens de l'Iran central: IV: Arthrodires et ptyctodontes. Geobios, 14:677–709.
https://doi.org/10.1016/S0016-6995(81)80148-9
Lewis, T.L. and Schwietering, J.F. 1971. Distribution of the Cleveland Black Shale in Ohio. GSA Bulletin, 82:3477–3482.
https://doi.org/10.1130/0016-7606(1971)82[3477:DOTCBS]2.0.CO;2
Li, Q., Zhu, Y.A., Lu, J., Chen, Y., Wang, J., Peng, L., Wei, G., and Zhu, M. 2021. A new Silurian fish close to the common ancestor of modern gnathostomes. Current Biology, 31:3613–3620 e2.
https://doi.org/10.1016/j.cub.2021.05.053
Lindgren, J., Jagt, J.W.M., and Caldwell, M.W. 2007. A fishy mosasaur: the axial skeleton of Plotosaurus (Reptilia, Squamata) reassessed. Lethaia, 40:153–160.
https://doi.org/10.1111/j.1502-3931.2007.00009.x
Lindsey, C.C. 1978. Form, Function, and Locomotory Habits in Fish, p. 1–100. In Hoar, W.S. and Randall, D.J. (eds.), Fish Physiology. Academic Press.
https://doi.org/10.1016/S1546-5098(08)60163-6
Lingham-Soliar, T. 2005. Dorsal fin in the white shark, Carcharodon carcharias: A dynamic stabilizer for fast swimming. Journal of Morphology, 263:1–11.
https://doi.org/10.1002/jmor.10207
Little, C.D. and Bemis, W.E. 2004. Observations on the skeleton of the heterocercal tail of sharks (Chondrichthyes: Elasmobranchii, p. 563–573. In Arratia, G., Wilson, M.V.H., and Cloutier, R. (eds.), Recent advances in the origin and early radiation of vertebrates. Verlag Dr. Friedrich Pfeil, München.
Long, J. 1988. A new camuropiscid arthrodire (Pisces: Placodermi) from Gogo, Western Australia. Zoological Journal of the Linnean Society, 94:233–258.
https://doi.org/10.1111/j.1096-3642.1988.tb01194.x
Long, J. 1994. A second incisoscutid arthrodire (Pisces, Placodermi) from the Late Devonian Gogo Formation, Western Australia). Alcheringa, 18:59–69.
https://doi.org/10.1080/03115518.1994.9638763
Long, J.A. 1987. A new dinichthyid fish (Placodermi: Arthrodira) from the Upper Devonian of Western Australia, with a discussion of dinichthyid interrelationships. Records of the Western Australian Museum, 13:515–540.
Long, J.A. 1991. Arthrodire predation by Onychodus (Pisces: Crossopterygii) from the late Devonian Gogo Formation, Western Australia. Records of the Western Australian Museum, 15:479–481.
Long, J.A. 1995. A new plourdosteid arthrodire from the Upper Devonian Gogo Formation of Western Australia. Palaeontology, 38:39–62.
Long, J.A. 2010. The Rise of Fishes: 500 Million Years of Evolution, Second edition. Johns Hopkins University Press, Baltimore.
Long, J.A., Anderson, M.E., Gess, R., and Hiller, N. 1997. New placoderm fishes from the Late Devonian of South Africa. Journal of Vertebrate Paleontology, 17:253–268.
https://doi.org/10.1080/02724634.1997.10010973
Long, J.A., Trinajstic, K., and Johanson, Z. 2009. Devonian arthrodire embryos and the origin of internal fertilization in vertebrates. Nature, 457:1124–1127.
https://doi.org/10.1038/nature07732
Long, J.A., Mark-Kurik, E., and Young, G.C. 2014. Taxonomic revision of buchanosteoid placoderms (Arthrodira) from the Early Devonian of south-eastern Australia and Arctic Russia. Australian Journal of Zoology, 62:26–43.
https://doi.org/10.1071/ZO13081
Long, J.A., Burrow, C.J., Ginter, M., Maisey, J.G., Trinajstic, K.M., Coates, M.I., Young, G.C., and Senden, T.J. 2015. First shark from the Late Devonian (Frasnian) Gogo Formation, Western Australia sheds new light on the development of tessellated calcified cartilage. PLoS ONE, 10:e0126066.
https://doi.org/10.1371/journal.pone.0126066
Lund, R. and Lund, W. 1984. New genera and species of coelacanths from the Bear Gulch Limestone (Lower Carboniferous) of Montana (U.S.A.). Geobios, 17:237–244.
https://doi.org/10.1016/S0016-6995(84)80145-X
Luo, Y., Xiao, Q., Shi, G., Pan, G., and Chen, D. 2020. The effect of variable stiffness of tuna-like fish body and fin on swimming performance. Bioinspiration and Biomimetics, 16:016003.
https://doi.org/10.1088/1748-3190/abb3b6
Madzia, D. and Cau, A. 2020. Estimating the evolutionary rates in mosasauroids and plesiosaurs: discussion of niche occupation in Late Cretaceous seas. PeerJ, 8:e8941.
https://doi.org/10.7717/peerj.8941
Maia, A. and Wilga, C.A. 2013. Function of dorsal fins in bamboo shark during steady swimming. Zoology, 116:224–231.
https://doi.org/10.1016/j.zool.2013.05.001
Maisey, J.G. 1989. Hamiltonichthys mapesi, g. & sp. nov. (Chondrichthyes, Elasmobranchii), from the Upper Pennsylvanian of Kansas. American Museum Novitates, 2931:1–42.
Mallon, J.C., and Hone, D.W.E. 2024. Estimation of maximum body size in fossil species: A case study using Tyrannosaurus rex. Ecology and Evolution, 14:e11658.
https://doi.org/10.1002/ece3.11658
Mark-Kurik, E. 1985. Actinolepis spinosa n. sp. (Arthrodira) from the Early Devonian of Latvia. Journal of Vertebrate Paleontology, 5:287–292.
https://doi.org/10.1080/02724634.1985.10011866
Martinez, A.M., Boyer, D.L., Droser, M.L., Barrie, C., and Love, G.D. 2019. A stable and productive marine microbial community was sustained through the end-Devonian Hangenberg Crisis within the Cleveland Shale of the Appalachian Basin, United States. Geobiology, 17:27–42.
https://doi.org/10.1111/gbi.12314
Matsuura, K. 2008. Family Ostraciidae. Boxfishes, Trunkfishes, p. 842–856. In Gomon, M., Bray, D., and Kuiter, R. (eds.), Fishes of Australia's Southern Coast. New Holland Publishers, Wahroonga.
McGowan, C. and Motani, R. 2003. Ichthyopterygia, p. 1–178. In Sues, H.-D. (ed.), Handbuch der Palaoherpetologie. Ger.:Verlag Dr. Friedrich Pfeil, München.
McGowen, M.R., Tsagkogeorga, G., Álvarez-Carretero, S., dos Reis, M., Struebig, M., Deaville, R., Jepson, P.D., Jarman, S., Polanowski, A., Morin, P.A., and Rossiter, S.J. 2020. Phylogenomic Resolution of the Cetacean Tree of Life Using Target Sequence Capture. Systematic Biology, 69:479–501.
https://doi.org/10.1093/sysbio/syz068
Miles, R.S. 1966. The Placoderm Fish Rhachiosteus pterygiatus Gross and its Relationships. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 66:377–392.
https://doi.org/10.1017/S0080456800023693
Miles, R.S. 1967a. The cervical joint and some aspects of the origin of the Placodermi. Colloques Internationaux du Centre National de la Recherche Scientifique, 163:49–71.
Miles, R.S. 1967b. Observations on the ptyctodont fish, Rhamphodopsis Watson. Zoological Journal of the Linnean Society, 47:99–120.
https://doi.org/10.1111/j.1096-3642.1967.tb01398.x
Miles, R.S. 1969. Features of placoderm diversification and the evolution of the arthrodire feeding mechanism. Transactions of the Royal Society of Edinburgh, 68:123–170.
https://doi.org/10.1017/S0080456800014629
Miles, R.S. and Dennis, K. 1979. A primitive eubrachythoracid arthrodire from Gogo, Western Australia. Zoological Journal of the Linnean Society, 66:31–62.
https://doi.org/10.1111/j.1096-3642.1979.tb01900.x
Miles, R.S. and Westoll, T.S. 1968. The Placoderm Fish Coccosteus cuspidatu s Miller ex Agassiz from the Middle Old Red Sandstone of Scotland. Part I. Descriptive Morphology. Transactions of the Royal Society of Edinburgh, 67:373–476.
https://doi.org/10.1017/S0080456800024078
Miles, R.S. and White, E.I. 1971. The Holonematidae (placoderm fishes), a review based on new specimens of Holonema from the Upper Devonian of Western Australia. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 263:101–234.
https://doi.org/10.1098/rstb.1971.0111
Mollen, F.H. 2019. Making Louis Agassiz's wish come true: combining forces and a new protocol for collecting comparative skeletal material of sharks, skates and rays, as a comment and an addition to 'The need of providing tooth morphology in descriptions of extant elasmobranch species' by Guinot et al. (2018). Zootaxa, 4571:295–300.
https://doi.org/10.11646/zootaxa.4571.2.13
Moreira, R.A., Gomes, U.L., and de Carvalho, M.R. 2019. Systematic implications of the caudal fin skeletal anatomy in ground sharks, order Carcharhiniformes (Chondrichthyes: Elasmobranchii). Zoological Journal of the Linnean Society, 185:193–211.
https://doi.org/10.1093/zoolinnean/zly038
Morris, F.K. 1937. Memorial of Jesse Earl Hyde, p. 163–174. Proceedings of the Geological Society of America for 1936.
Motani, R. 2004. Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints. Annual Review of Earth and Planetary Sciences, 33:395–420.
https://doi.org/10.1146/annurev.earth.33.092203.122707
Motani, R., You, H., and McGowan, C. 1996. Eel-like swimming in the earliest ichthyosaurs. Nature, 382:347–348.
https://doi.org/10.1038/382347a0
Motani, R. and Shimada, K. 2023. Skeletal convergence in thunniform sharks, ichthyosaurs, whales, and tunas, and its possible ecological links through the marine ecosystem evolution. Scientific Reports, 13:16664.
https://doi.org/10.1038/s41598-023-41812-z
Moy-Thomas, J.A. and Miles, R.S. 1971. Subclass Placodermi, p. 161–205. In Moy-Thomas, J. A. and Miles, R.S. (eds.), Palaeozoic Fishes. Springer US, Boston, MA.
https://doi.org/10.1007/978-1-4684-6465-8_8
Musick, J.A., Bruton, M.N., and Balon, E.K. 1991. The biology of Latimeria chalumnae and the evolution of coelacanths. Springer Science, Dordrecht.
Nakamura, I. 1985. Billfishes of the world. An annotated and illustrated catalogue of marlins, sailfishes, spearfishes and swordfishes known to date. Food and Agriculture Organization of the United Nations, Rome.
Nakaya, K. 1995. Hydrodynamic function of the head in the hammerhead sharks (Elasmobranchii: Sphyrnidae). Copeia, 1995:330–336.
https://doi.org/10.2307/1446895
Neutens, C., Adriaens, D., Christiaens, J., De Kegel, B., Dierick, M., Boistel, R., and Van Hoorebeke, L. 2014. Grasping convergent evolution in syngnathids: a unique tale of tails. Journal of Anatomy, 224:710–723.
https://doi.org/10.1111/joa.12181
Newberry, J.S. 1873. Fossil Fishes, p. 245–355. Report of the Geological Survey of Ohio. Volume II. Geology and Paleontology. Nevins and Myers, State Printers, Columbus.
Newberry, J.S. 1875. Descriptions of fossil fishes, p. 1–64. Report of the Geological Survey of Ohio. Volume II. Geology and Paleontology. Nevins and Myers, State Printers, Columbus.
Newberry, J.S. 1889. Paleozoic Fishes of North America. Monographs of the U.S. Geological Survey, 16:1–228.
https://doi.org/10.5962/bhl.title.14705
Newman, M.J., den Blaauwen, J., Burrow, C., and Jones, R. 2021. Earliest vertebrate embryos in the fossil record (Middle Devonian, Givetian). Palaeontology, 64:21–30.
https://doi.org/10.1111/pala.12511
Northcutt, R.G. 1997. Evolution of gnathostome lateral line ontogenies. Brain, Behavior and Evolution, 50:25–37.
https://doi.org/10.1159/000113319
Nursall, J.R. 1958. The caudal fin as a hydrofoil. Evolution, 12:116–120.
https://doi.org/10.2307/2405913
Ørvig, T. 1971. Comments on the lateral line system of some brachythoracid and ptyctodontid arthrodires. Zoologica Scripta, 1:5–35.
https://doi.org/10.1111/j.1463-6409.1971.tb00710.x
Ørvig, T. 1980. Histological studies of ostracoderms, placoderms and fossil elasmobranchs. 3. Structure and growth of the gnathalia of certain arthrodires. Zoologica Scripta, 9:141–159.
https://doi.org/10.1111/j.1463-6409.1980.tb00660.x
Parson, J.M., Fish, F.E., and Nicastro, A.J. 2011. Turning performance of batoids: Limitations of a rigid body. Journal of Experimental Marine Biology and Ecology, 402:12–18.
https://doi.org/10.1016/j.jembe.2011.03.010
Prosser, C.S. 1913. The Huron and Cleveland Shales of Northern Ohio. The Journal of Geology, 21:323–362.
Qiao, T., King, B., Long, J.A., Ahlberg, P.E., and Zhu, M. 2016. Early gnathostome phylogeny revisited: Multiple method consensus. PLoS ONE, 11:e0163157.
https://doi.org/10.1371/journal.pone.0163157
R Core Team 2020. R: A Language and Environment for Statistical Computing, Ver. 4.0.3. R Foundation for Statistical Computing, Vienna, Austria.
Racki, G. 2005. Toward understanding Late Devonian global events: few answers, many questions, p. 5–36. In Over, D.J., Morrow, J.R. and Wignall, P.B. (eds.), Developments in Palaeontology and Stratigraphy. Elsevier.
https://doi.org/10.1016/S0920-5446(05)80002-0
Rhodes, F.H.T. 2016. Origins: The Search for Our Prehistoric Past. Cornell University Press, Ithaca.
Rimmer, S.M., Rowe, H.D., Hawkins, S.J., and Francis, H. 2010. Geochemistry of the Cleveland Member of the Ohio Shale, Appalachian Basin: Indicators of depositional environment during sediment accumulation. Kirtlandia, 57:3–12.
Ristroph, L., Liao, J.C., and Zhang, J. 2015. Lateral line layout correlates with the differential hydrodynamic pressure on swimming fish. Physical Review Letters, 114:018102.
https://doi.org/10.1103/PhysRevLett.114.018102
Ritchie, A. 1975. Groenlandaspis in Antarctica, Australia and Europe. Nature, 254:569–573.
https://doi.org/10.1038/254569a0
Ritchie, A. 2005. Cowralepis, a new genus of phyllolepid fish (Pisces, Placodermi) from the Late Middle Devonian of New South Wales, Australia. Proceedings of the Linnean Society of New South Wales, 126:215–259.
Rivas, L.R. 1955. A comparison between giant bluefin tuna (Thunnus thynnus) from the Straits of Florida and the Gulf of Maine, with reference to migration and population identity. Proceedings of the Gulf and Caribbean Fisheries Institute Seventh Annual Session, 133–149.
Romeo, J. and Mansueti, A.J. 1962. Little Tuna, Euthynnus alletteratus, in Northern Chesapeake Bay, Maryland, with an Illustration of Its Skeleton. Chesapeake Science, 3:257–263.
https://doi.org/10.2307/1350633
Romer, A.S. 1966. Vertebrate Paleontology, Third edition. University of Chicago Press, Chicago.
Rowe, D.M., Denton, E.J., and Batty, R.S. 1993. Head turning in herring and some other fish. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 341:141–148.
https://doi.org/10.1098/rstb.1993.0098
Rücklin, M. 2002. New finds of placoderms from the Late Devonian of Morocco. 7th European Workshop on Vertebrate Palaeontology, Sibiu, Romania, p. 31
Rücklin, M. 2011. First selenosteid placoderms from the eastern Anti-Atlas of Morocco; osteology, phylogeny and palaeogeographical implications. Palaeontology, 54:25–62.
https://doi.org/10.1111/j.1475-4983.2010.01026.x
Rücklin, M. and Clément, G. 2017. A review of the placoderms and sarcopterygians from the Devonian of Morocco, p. 81–101. In Zouhri, S. (ed.), Vertebrate Paleontology of Morocco: The state of knowledge. La Société Géologique de France, Paris.
Rücklin, M., Donoghue, P.C.J., Johanson, Z., Trinajstic, K., Marone, F., and Stampanoni, M. 2012. Development of teeth and jaws in the earliest jawed vertebrates. Nature, 491:748–751.
https://doi.org/10.1038/nature11555
Russell, F.S. 1934. Tunny investigations made in the North Sea on Col. E. T. Peel’s Yacht, “St. George,” Summer, 1933. Part I. Biometric data. Journal of the Marine Biological Association of the United Kingdom, 19:503–522.
https://doi.org/10.1017/S0025315400046592
Saja, D.B. and Hannibal, J.T. 2018. Reinterpretation of putative “arthrodire egg cases” from the Famennian Cleveland Member as extremely large thylacocephalan arthropods. Geological Society of America Abstracts with Programs, 53:
https://doi.org/10.1130/abs/2021NC-362794
Sallan, L. and Galimberti, A.K. 2015. Body-size reduction in vertebrates following the end-Devonian mass extinction. Science, 350:812–815.
https://doi.org/10.1126/science.aac7373
Sallan, L.C. and Coates, M.I. 2010. End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proceedings of the National Academy of Sciences, 107:10131–10135.
https://doi.org/10.1073/pnas.0914000107
Sanchez, S., Dupret, V., Tafforeau, P., Trinajstic, K.M., Ryll, B., Gouttenoire, P.-J., Wretman, L., Zylberberg, L., Peyrin, F., and Ahlberg, P.E. 2013. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. PLoS ONE, 8:e56992.
https://doi.org/10.1371/journal.pone.0056992
Schrank, A.J., Webb, P.W., and Mayberry, S. 1999. How do body and paired-fin positions affect the ability of three teleost fishes to maneuver around bends? Canadian Journal of Zoology, 77:203–210.
https://doi.org/10.1139/z98-209
Schultze, H.-P. and Cumbaa, S.L. 2017. A new Early Devonian (Emsian) arthrodire from the Northwest Territories, Canada, and its significance for paleogeographic reconstruction. Canadian Journal of Earth Sciences, 54:461–476.
https://doi.org/10.1139/cjes-2017-0013
Schultze, H.-P., Mickle, K.E., Poplin, C., Hilton, E.J., and Grande, L. 2021. Actinopterygii I. Palaeoniscimorpha, Stem Neopterygii, Chondrostei. Verlag Dr. Friedrich Pfeil, Munich.
Scott, B. and Anderson, P. 2021. Identifying competitive exclusion in the vertebrate fossil record: lessons from early vertebrate morphometrics. 9th Annual Meeting Canadian Society of Vertebrate Paleontology, Online, p. 33–34
Shadwick, R.E. 2005. How tunas and lamnid sharks swim: An evolutionary convergence. American Scientist, 93:524–531.
https://doi.org/10.1511/2005.6.524
Shadwick, R.E. and Gemballa, S. 2005. Structure, kinematics, and muscle dynamics In undulatory swimming, p. 241–280. In Shadwick, R.E. and Lauder, G.V. (eds.), Fish Physiology. Academic Press.
https://doi.org/10.1016/S1546-5098(05)23007-8
Snively, E., Anderson, P.S.L., and Ryan, M.J. 2010. Functional and ontogenetic implications of bite stress in arthrodire placoderms. Kirtlandia, 57:53–60.
Standen, E.M. 2008. Pelvic fin locomotor function in fishes: three-dimensional kinematics in rainbow trout (Oncorhynchus mykiss). Journal of Experimental Biology, 211:2931–2942.
https://doi.org/10.1242/jeb.018572
Stensiö, E.A. 1948. On the Placodermi of the Upper Devonian of East Greenland. 11. Antiarcha: subfamily Bothriolepinae. Palaeozoologica Groenlandica, 2:1–622.
Stensiö, E.A. 1959. On the pectoral fin and shoulder girdle of the arthrodires. Kungligar Svenska Vetenskapakadamiens Handlingar, 8:1–229.
Stensiö, E.A. 1963. Anatomical studies on the arthrodiran head. Pt. 1. Preface, geological and geographical distribution, the organisation of the arthrodires, the anatomy of the head in the Dolichothoraci, Coccosteomorphi and Pachyosteomorphi. Kungligar Svenska Vetenskapakadamiens Handlingar, 9:1–419.
Sternes, P.C. and Shimada, K. 2020. Body forms in sharks (Chondrichthyes: Elasmobranchii) and their functional, ecological, and evolutionary implications. Zoology, 140:125799.
https://doi.org/10.1016/j.zool.2020.125799
Sternes, P.C., Jambura, P.L., Türtscher, J., Kriwet, J., Siversson, M., Feichtinger, I., Naylor, G.J.P., Maisey, J.G., Tomita, T., Moyer, J.K., Higham, T.E., da Silva, J.P.C.B., Bornatowski, H., Long, D.J., Perez, V.J., Collareta, A., Underwood, C., Ward, D.J., Vullo, R., González-Barba, G., Maisch, H.M. IV, Griffiths, M.L., Becker, M.A., Wood, J.J., and Shimada, K. 2024. White shark comparison reveals a slender body for the extinct megatooth shark, Otodus megalodon (Lamniformes: Otodontidae). Palaeontologia Electronica, 27:a2.
https://doi.org/10.26879/1345
Stetson, H. 1930. Notes on the structure of Dinichthys and Macropetalichthys. Bulletin of the Museum of Comparative Zoology, 71:19–39.
Stone, N.R. and Shimada, K. 2019. Skeletal anatomy of the bigeye sand tiger shark, Odontaspis noronhai (Lamniformes: Odontaspididae), and Its implications for lamniform phylogeny, taxonomy, and conservation biology. Copeia, 107:632–652.
https://doi.org/10.1643/CG-18-160
Storrs, G., Kampouris, G., and Carr, R.K. 2008. New insights into the anatomy and function of Dunkleosteus terrelli (Newberry), a giant arthrodire from the Famennian Cleveland Shale of Ohio. Journal of Vertebrate Paleontology, Program and Abstracts, 2008, 148A.
Syme, D.A. and Shadwick, R.E. 2011. Red muscle function in stiff-bodied swimmers: there and almost back again. Philosophical Transactions of the Royal Society B: Biological Sciences, 366:1507–1515.
https://doi.org/10.1098/rstb.2010.0322
Szrek, P., Kozłowski, W., and Wilk, O. 2021. A large predatory arthrodire (Vertebrata, Placodermi) from the Famennian (Upper Devonian) of the Holy Cross Mountains, Poland. Journal of Vertebrate Paleontology, 41:e1930019.
https://doi.org/10.1080/02724634.2021.1930019
Szrek, P. and Wilk, O. 2018. A large Late Devonian arthrodire (Vertebrata, Placodermi) from Poland. Estonian Journal of Earth Sciences, 67:33–42.
https://doi.org/10.3176/earth.2018.02
Tanaka, M. 2011. Revealing the mechanisms of the rostral shift of pelvic fins among teleost fishes. Evolution & Development, 13:382–390.
https://doi.org/10.1111/j.1525-142X.2011.00493.x
Thewissen, J.G.M. 2014. The walking whales: from land to water in eight million years. University of California Press, Oakland.
Thewissen, J.G.M., Cooper, L.N., Clementz, M.T., Bajpai, S., and Tiwari, B.N. 2007. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature, 450:1190–1194.
https://doi.org/10.1038/nature06343
Thomson, K.S. and Simanek, D.E. 1977. Body form and locomotion in sharks. American Zoologist, 17:343–354.
https://doi.org/10.1093/icb/17.2.343
Ting, S.C. and Yang, J.T. 2008. Pitching stabilization via caudal fin-wave propagation in a forward-sinking parrot cichlid (Cichlasoma citrinellum × Cichlasoma synspilum). Journal of Experimental Biology, 211:3147–3159.
https://doi.org/10.1242/jeb.020263
Tomita, T. 2015. Pectoral fin of the Paleozoic shark, Cladoselache: new reconstruction based on a near-complete specimen. Journal of Vertebrate Paleontology, 35:e973029.
https://doi.org/10.1080/02724634.2015.973029
Tomita, T., Murakumo, K., Komoto, S., Dove, A., Kino, M., Miyamoto, K., and Toda, M. 2020. Armored eyes of the whale shark. PLoS ONE, 15:e0235342.
https://doi.org/10.1371/journal.pone.0235342
Trewin, N.H. 1986. Palaeoecology and sedimentology of the Achanarras fish bed of the Middle Old Red Sandstone, Scotland. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 77:21–46.
https://doi.org/10.1017/S0263593300010737
Trinajstic, K. 1995. The role of heterochrony in the evolution of eubrachythoracid arthrodires with special reference to Compagopiscis croucheri and Incisoscutum ritchei from the Late Devonian Gogo Formation, Western Australia. Geobios, 28:125–128.
https://doi.org/10.1016/S0016-6995(95)80099-9
Trinajstic, K. 1999. New anatomical information on Holonema (Placodermi) based on material from the Frasnian Gogo Formation and the Givetian-Frasnian Gneudna Formation, Western Australia. Geodiversitas, 21:69–84.
Trinajstic, K. and Hazelton, M. 2007. Ontogeny, phenotypic variation and phylogenetic implications of arthrodires from the Gogo Formation, Western Australia. Journal of Vertebrate Paleontology, 27:571–583.
https://doi.org/10.1671/0272-4634(2007)27[571:OPVAPI]2.0.CO;2
Trinajstic, K. and McNamara, K.J. 1999. Heterochrony in the Late Devonian arthrodiran fishes Compagopiscis and Incisoscutum. Records of the Western Australian Museum, 57:77–91.
Trinajstic, K., Marshall, C., Long, J., and Bifield, K. 2007. Exceptional preservation of nerve and muscle tissues in Late Devonian placoderm fish and their evolutionary implications. Biology Letters, 3:197–200.
https://doi.org/10.1098/rsbl.2006.0604
Trinajstic, K., Sanchez, S., Dupret, V., Tafforeau, P., Long, J., Young, G., Senden, T., Boisvert, C., Power, N., and Ahlberg, P.E. 2013. Fossil musculature of the most primitive jawed vertebrates. Science, 341:160–4.
https://doi.org/10.1126/science.1237275
Trinajstic, K., Boisvert, C., Long, J., Maksimenko, A., and Johanson, Z. 2015. Pelvic and reproductive structures in placoderms (stem gnathostomes). Biological Reviews, 90:467–501.
https://doi.org/10.1111/brv.12118
Trinajstic, K., Briggs, D.E.G., and Long, J.A. 2022a. The Gogo Formation Lagerstätte: a view of Australia's first great barrier reef. Journal of the Geological Society, 179:jgs2021–105.
https://doi.org/10.1144/jgs2021-105
Trinajstic, K., Long, J.A., Sanchez, S., Boisvert, C.A., Snitting, D., Tafforeau, P., Dupret, V., Clement, A.M., Currie, P.D., Roelofs, B., Bevitt, J.J., Lee, M.S.Y., and Ahlberg, P.E. 2022b. Exceptional preservation of organs in Devonian placoderms from the Gogo lagerstätte. Science, 377:1311–1314.
https://doi.org/10.1126/science.abf3289
Uhen, M.D. 2022. Assessing commitment to the life aquatic in mammals. Journal of Vertebrate Paleontology, SVP Program and Abstracts Book, 2022, 332–333.
van Mesdag, S.N.K., den Blaauwen, J., Dean, M.N., and Johanson, Z. 2020. Hyperossification in the vertebral column of Devonian placoderm fishes (Arthrodira). Journal of Vertebrate Paleontology, 40:e1766477.
https://doi.org/10.1080/02724634.2020.1766477
Vézina, D. 1988. Plourdosteus canadensis (Woodward 1892), un Arthrodire du Frasnien inférieur du Canada: contribution à l'étude morphologique et phylogénétique des Plourdosteidae (Vertebrata, Placodermi) du Dévonien moyen et supérieur. PhD thesis. l'Université de Paris VII, Paris. 341 pp.
Vogel, S. 2008. Modes and scaling in aquatic locomotion. Integrative and Comparative Biology, 48:702–712.
https://doi.org/10.1093/icb/icn014
Walker, J.A. 2000. Does a rigid body limit maneuverability? Journal of Experimental Biology, 203:3391–3396.
https://doi.org/10.1242/jeb.203.22.3391
Walker, J.A., Alfaro, M.E., Noble, M.M., and Fulton, C.J. 2013. Body fineness ratio as a predictor of maximum prolonged-swimming speed in coral reef fishes. PLoS ONE, 8:e75422.
https://doi.org/10.1371/journal.pone.0075422
Walters, V. 1962. Body form and swimming performance in the scombroid fishes. American Zoologist, 2:143–149.
https://doi.org/10.1093/icb/2.2.143
Wang, Y. and Zhu, M. 2022. Squamation and scale morphology at the root of jawed vertebrates. eLife, 11:e76661.
https://doi.org/10.7554/eLife.76661
Ward, A.B. and Mehta, R.S. 2010. Axial elongation in fishes: Using morphological approaches to elucidate developmental mechanisms in studying body shape. Integrative and Comparative Biology, 50:1106–1119.
https://doi.org/10.1093/icb/icq029
Webb, J.F. 1989a. Gross morphology and evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behavior and Evolution, 33:44–53.
https://doi.org/10.1159/000316061
Webb, J.F. 1989b. Developmental constraints and evolution of the lateral line system in teleost fishes, p. 79–97. In Coombs, S., Görner, P., and Münz, H. (eds.), The Mechanosensory Lateral Line. Springer New York, New York, NY.
Webb, J.F. 1990a. Ontogeny and phylogeny of the trunk lateral line system in cichlid fishes. Journal of Zoology, 221:405–418.
https://doi.org/10.1111/j.1469-7998.1990.tb04010.x
Webb, J.F. 1990b. Comparative morphology and evolution of the lateral line system in the Labridae (Perciformes: Labroidei). Copeia, 1990:137–146.
https://doi.org/10.2307/1445830
Webb, J.F. 2014. Morphological diversity, development, and evolution of the mechanosensory lateral line system, p. 17–72. In Coombs, S., Bleckmann, H., Fay, R.R., and Popper, A. N. (eds.), The Lateral Line System. Springer New York, New York, NY.
https://doi.org/10.1007/2506_2013_12
Webb, P.W. 1978. Fast-start performance and body form In seven species of teleost fish. Journal of Experimental Biology, 74:211–226.
https://doi.org/10.1242/jeb.74.1.211
Webb, P.W. 1984. Form and function in fish swimming. Scientific American, 251:72–83.
https://doi.org/10.1038/scientificamerican0784-72
Webb, P.W. 2005. Stability and Maneuverability, p. 281–332. Fish Physiology. Academic Press.
https://doi.org/10.1016/S1546-5098(05)23008-X
Webb, P.W. and Weihs, D. 2015. Stability versus maneuvering: Challenges for stability during swimming by fishes. Integrative and Comparative Biology, 55:753–764.
https://doi.org/10.1093/icb/icv053
Wegner, N.C., Snodgrass, O.E., Dewar, H., and Hyde, J.R. 2015. Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science, 348:786–789.
https://doi.org/10.1126/science.aaa8902
Weihs, D. 1972. A hydrodynamical analysis of fish turning manoeuvres. Proceedings of the Royal Society of London. Series B. Biological Sciences, 182:59–72.
https://doi.org/10.1098/rspb.1972.0066
Weihs, D. and Webb, P.W. 1983. Optimization of locomotion, p. 339–371. In Webb, P.W. and Weihs, D. (eds.), Fish Biomechanics. Prager, New York.
Werdelin, L. and Long, J.A. 1986. Allometry in the placoderm Bothriolepis canadensis and its significance to antiarch evolution. Lethaia, 19:161–169.
https://doi.org/10.1111/j.1502-3931.1986.tb00727.x
Westneat, M.W., Hoese, W., Pell, C.A., and Wainwright, S.A. 1993. The horizontal septum: Mechanisms of force transfer in locomotion of scombrid fishes (Scombridae, Perciformes). Journal of Morphology, 217:183–204.
https://doi.org/10.1002/jmor.1052170207
Westoll, T.S. 1941. The Permian fishes Dorypterus and Lekanichthys. Proceedings of the Zoological Society of London, B111:39–58.
https://doi.org/10.1111/j.1469-7998.1941.tb00042.x
Westoll, T.S. 1947. The paired fins of placoderms. Earth and Environmental Science Transactions of The Royal Society of Edinburgh, 61:381–398.
https://doi.org/10.1017/S0080456800004804
White, E.I. 1952. Australian arthrodires. Bulletin of the British Museum (Natural History), Geology, London, 1:249–304.
Wignall, P.B. 1993. Distinguishing between oxygen and substrate control in fossil benthic assemblages. Journal of the Geological Society, 150:193–196.
https://doi.org/10.1144/gsjgs.150.1.0193
Wilga, C.D. and Lauder, G.V. 2004. Biomechanics of locomotion in sharks, rays, and chimeras, p. 139–164. In Carrier, J.C., Musick, J.A., and Heithaus, M.R. (eds.), Biology of Sharks and Their Relatives. CRC Press, Boca Raton.
Williams, M.E. 1990. Feeding behavior in Cleveland Shale fishes, p. 273–287. In Boucot, A.J. (ed.), Evolutionary Biology of Behavior and Coevolution. Elsevier, Amsterdam.
Wilson, H. 2019. Dunkleosteus terrelli size. Accessed April 9, 2022.
https://www.deviantart.com/harry-the-fox/art/Dunkleosteus-terrelli-size-808872651
Wintrich, T., Hayashi, S., Houssaye, A., Nakajima, Y., and Sander, P.M. 2017. A Triassic plesiosaurian skeleton and bone histology inform on evolution of a unique body plan. Science Advances, 3:e1701144.
https://doi.org/10.1126/sciadv.1701144
Witmer, L.M. 1995. The Extant Phylogenetic Bracket and the importance of reconstructing soft tissues in fossils, p. 19–33. In Thomason, J.J. (ed.), Functional Morphology in Vertebrate Paleontology. Cambridge University Press, New York.
Witton, M.P. 2018. Palaeoartist’s Handbook: Recreating Prehistoric Animals in Art. The Crowood Press, Ramsbury.
Xue, Q.-Y., Yu, Y.-L., Pan, Z.-H., Zhu, Y.-A., and Zhu, M. 2024. Decline in phylogenetic diversity of Arthrodira (stem-group Gnathostomata) correlates with major Devonian bioevents. Vertebrata PalAsiatica, 62:1–12.
https://doi.org/10.19615/j.cnki.2096-9899.231124
Yamanoue, Y., Setiamarga, D.H., and Matsuura, K. 2010. Pelvic fins in teleosts: structure, function and evolution. Journal of Fish Biology, 77:1173–208.
https://doi.org/10.1111/j.1095-8649.2010.02674.x
Young, G.C. 2004. Large brachythoracid arthrodires (placoderm fishes) from the early Devonian of Wee Jasper, New South Wales, Australia, with a discussion of basal brachythoracid characters. Journal of Vertebrate Paleontology, 24:1–17.
https://doi.org/10.1671/1942-1
Young, G.C. 2005. A New Middle Devonian Arthrodire (Placoderm Fish) from the Broken River Area, Queensland. Records of the Australian Museum, 57:211–220.
https://doi.org/10.3853/j.0067-1975.57.2005.1443
Young, G.C. 2009. New arthrodires (Family Williamsaspididae) from Wee Jasper, New South Wales (Early Devonian), with comments on placoderm morphology and palaeoecology. Acta Zoologica, 90:69–82.
https://doi.org/10.1111/j.1463-6395.2008.00366.x
Young, G.C. 2010. Placoderms (Armored Fish): Dominant Vertebrates of the Devonian Period. Annual Review of Earth and Planetary Sciences, 38:523–550.
https://doi.org/10.1146/annurev-earth-040809-152507
Yu, X. 1998. A new porolepiform-like fish, Psarolepis romeri, gen. et sp. nov. (Sarcopterygii, Osteichthyes) from the Lower Devonian of Yunnan, China. Journal of Vertebrate Paleontology, 18:261–274.
https://doi.org/10.1080/02724634.1998.10011055
Zangerl, R. 1981. Chondrichthyes I. Paleozoic Elasmobranchii. Gustav Fisher, New York.
Zhu, M. and Ahlberg, P.E. 2004. The origin of the internal nostril of tetrapods. Nature, 432:94–97. https://doi.org/10.1038/nature02843
Zhu, M., Yu, X., Choo, B., Wang, J., and Jia, L. 2012. An antiarch placoderm shows that pelvic girdles arose at the root of jawed vertebrates. Biology Letters, 8:453–456.
https://doi.org/10.1098/rsbl.2011.1033
Zhu, M., Yu, X., Ahlberg, P.E., Choo, B., Lu, J., Qiao, T., Qu, Q., Zhao, W., Jia, L., Blom, H., and Zhu, Y.a. 2013. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature, 502:188–193.
https://doi.org/10.1038/nature12617
Zhu, M., Ahlberg, P.E., Pan, Z., Zhu, Y., Qiao, T., Zhao, W., Jia, L., and Lu, J. 2016a. A Silurian maxillate placoderm illuminates jaw evolution. Science, 354:334–336.
https://doi.org/10.1126/science.aah3764
Zhu, Y.-A. and Zhu, M. 2013. A redescription of Kiangyousteus yohii (Arthrodira: Eubrachythoraci) from the Middle Devonian of China, with remarks on the systematics of the Eubrachythoraci. Zoological Journal of the Linnean Society, 169:798–819.
https://doi.org/10.1111/zoj.12089
Zhu, Y.-A., Zhu, M., and Wang, J.-Q. 2016b. Redescription of Yinostius major (Arthrodira: Heterostiidae) from the Lower Devonian of China, and the interrelationships of Brachythoraci. Zoological Journal of the Linnean Society, 176:806–834.
https://doi.org/10.1111/zoj.12356
Zhu, Y.-A., Li, Q., Lu, J., Chen, Y., Wang, J., Gai, Z., Zhao, W., Wei, G., Yu, Y., Ahlberg, P.E., and Zhu, M. 2022. The oldest complete jawed vertebrates from the early Silurian of China. Nature, 609:954–958.
https://doi.org/10.1038/s41586-022-05136-8
Zorn, M.E., Caldwell, M.W., and Wilson, M.V.H. 2005. Lithological analysis of the Lower Devonian vertebrate-bearing beds at the MOTH locality, N.W.T., Canada: insights to taphonomy and depositional setting. Canadian Journal of Earth Sciences, 42:763–775.
https://doi.org/10.1139/E05-015

