 Adding to the diversity of Katlasidae (Hemiptera: Fulgoromorpha: Fulgoridoidea) – a new genus and species from mid-Cretaceous Kachin amber of northern Myanmar
Adding to the diversity of Katlasidae (Hemiptera: Fulgoromorpha: Fulgoridoidea) – a new genus and species from mid-Cretaceous Kachin amber of northern Myanmar
Article number: 28.3.a46
https://doi.org/10.26879/1575
Copyright Paleontological Society, October 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 28 April 2025. Acceptance: 14 September 2025.
ABSTRACT
A new genus and species - Lucidusmacula lihanjieae gen. et sp. nov. is described from mid-Cretaceous amber of northern Myanmar. It is the third genus and species of the planthopper family Katlasidae, and clearly differs from the two other known genera of Katlasidae by the combination of morphological characters of the frons, pronotum, tegminal venation and its structure. This taxon adds new morphological information for Cretaceous fulgoridioid planthoppers.
Lining Wang. School of GeoSciences, Yangtze University, Wuhan, Hubei, 430100, China. State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, 210008, China. wanglining.stu@yangtzeu.edu.cn
Jacek Szwedo. Laboratory of Evolutionary Entomology and Museum of Amber Inclusions, Department of Invertebrate Zoology and Parasitology, Faculty of Biology, University of Gdansk, PL80-308 Gdańsk, Poland. jacek.szwedo@ug.edu.pl
De Zhuo. Beijing Xiachong Amber Museum, Beijing 100023, China. zhuode113@163.com
Chuantao Xiao. School of GeoSciences, Yangtze University, Wuhan, Hubei, 430100, China. (Corresponding author) ctxiao@yangtzeu.edu.cn
Cihang Luo. State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, 210008, China. (Corresponding author) chluo@nigpas.ac.cn
Keywords: Myanmar; planthopper; Mesozoic; taxonomy; new genus; new species
https://zoobank.org/60291E6F-98B3-4FE2-B21F-2E8B4B72D747
Final citation: Wang, Lining, Szwedo, Jacek, Zhuo, De, Xiao, Chuantao, Luo, Cihang. 2025. Adding to the diversity of Katlasidae (Hemiptera: Fulgoromorpha: Fulgoridoidea) – a new genus and species from mid-Cretaceous Kachin amber of northern Myanmar. Palaeontologia Electronica, 28(3):a46.
https://doi.org/10.26879/1543
palaeo-electronica.org/content/2025/5669-a-new-genus-of-planthopper-from-kachin-amber
Copyright: October 2025 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Hemiptera Linnaeus, 1758 is the order with the highest species number among the hemimetabolous insects, and includes over 330 known extant and extinct families (Szwedo, 2018; Boderau et al., 2025). As an important group of Hemiptera, the planthoppers (suborder Fulgoromorpha Evans, 1946) contain three extinct superfamilies (Coleoscytoidea Martynov, 1935, Surijokocixioidea Shcherbakov, 2000 and Fulgoridioidea Handlirsch, 1939), three extant superfamilies (Delphacoidea Leach, 1815, Fulgoroidea Latreille, 1807 and Meenoploidea Fieber, 1872), and several other families with no precise superfamily attribution (e.g., Jubisentidae Zhang, Ren et Yao, 2019, Mimarachnidae Shcherbakov, 2007a, Neazoniidae Szwedo, 2007 and Perforissidae Shcherbakov, 2007b) (Bourgoin and Szwedo 2022, 2023; Deng et al., 2025).
Kachin amber is currently the Cretaceous amber with the highest diversity of bioinclusions in the world (Ross, 2025), containing various fossils such as vertebrates, invertebrates, plants. Insects are the most abundant group in Kachin amber. Among them, twelve planthopper families have been discovered: Achilidae Stål, 1866 (Brysz et al., 2023), Cixiidae Spinola, 1839 (Luo et al., 2021; Wang et al., 2022), Derbidae Spinola, 1839 (Emeljanov and Shcherbakov, 2020), Dorytocidae Emeljanov & Shcherbakov, 2018 (Emeljanov and Shcherbakov, 2018; Song et al., 2021), Fulgoridiidae Handlirsch, 1939 (Poinar et al., 2022), Inoderbidae Emeljanov & Shcherbakov, 2021 (Shcherbakov and Emeljanov, 2021; Luo et al., 2022), Jubisentidae Zhang, Ren et Yao, 2019 (Zhang et al., 2019; Shcherbakov, 2020), Katlasidae Luo, Jiang & Szwedo, 2020 (Luo et al., 2020b; Zhang et al., 2023), Mimarachnidae Shcherbakov, 2007a (e.g., Shcherbakov, 2017), Nogodinidae Melichar, 1898 (Luo et al., 2023), Perforissidae Shcherbakov, 2007b (Shcherbakov, 2007b; Zhang et al., 2017; Luo et al., 2020a; but see reservation in Bucher et al., 2024 and Ross, 2025), and Yetkhatidae Song, Szwedo & Bourgoin, 2019 (Song et al., 2019). The unique preservation of these planthoppers in Kachin amber allows for a detailed morphological examination, shedding light on the evolutionary processes that shaped the diversity of planthoppers during this period (Bourgoin and Szwedo 2022, 2023; Deng et al., 2025; Boderau et al., 2025).
Katlasidae Luo, Jiang et Szwedo, 2020 is a planthopper family established by Luo et al. (2020b), representing a unique group within Fulgoridioidea (Zhang et al., 2023). Katlasidae contains two genera and species, i.e., Katlasus xiai Luo, Jiang et Szwedo, 2020 and Dumpyawmus hpungwanus Zhang, Luo et Szwedo, 2023, both from Kachin amber (Luo et al., 2020b; Zhang et al., 2023).
Herein we describe the third genus and species of this family, Lucidusmacula lihanjieae gen. et sp. nov., from mid-Cretaceous Kachin amber in Myanmar, adding new data on the taxonomic diversity and morphological disparity of both the family and planthoppers as a whole.
MATERIALS AND METHODS
The studied specimen comes from a Cretaceous amber mine, near Danai (Tanai) Town (26°210′33.41″ N, 96°43′11.88″ E; palaeolatitude 5.0 ± 4.7°S) in the Hukawng Valley of Myanmar, see figure 1 in Jiang et al. (2019) (Thu and Zaw, 2017; Westerweel et al., 2019). Radiometric U-Pb zircon dating of the volcaniclastic matrix of the amber constrained a refined age of 98.79 ± 0.62 Ma (earliest Cenomanian) (Shi et al., 2012), which is also supported by the ammonite trapped in the amber (Yu et al., 2019).
The amber piece was collected in 2015, before November 2017 when the Myanmar military closed the Kachin amber mine. The fossil reported in this study was not involved in armed conflict and ethnic strife in Myanmar or linked with such events in any conceivable way. The amber piece with the holotype LYU-BL2003 described here is now permanently deposited in the Institute of Geology and Paleontology at Linyi University in Linyi, China, in full compliance with the International Code of Zoological Nomenclature (ICZN, 1999), Statement of the International Palaeoentomological Society (Szwedo et al., 2020), and the policies proposed by Haug et al. (2020).
Observations were performed using a Zeiss Stemi 508 microscope. The photographs were taken with a Zeiss Stereo Discovery V16 microscope system in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China; and measurements were taken using CorelDraw 2023 graphic software. Photomicrographic composites of about 20 individual focal planes were digitally stacked as obtained using the software Helicon Focus 6.7.1 for a better illustration of 3D structures. Photographs were adjusted using Adobe Lightroom Classic and line drawings were prepared using CorelDraw 2023 graphic software.
Morphological terms are used after the proposals of Anufriev and Emeljanov (1988), Bartlett et al. (2014) and Brożek et al. (2024). The nomenclature of the fore wing (tegmen) follows the interpretation proposed by Bourgoin et al. (2015) and Stroiński (2020), for hind wings after Anufriev and Emeljanov (1988): CA, costal margin (costa anterior); Pc+CP, precosta + costa posterior; ScP+R, subcosta posterior + radius; RA, radius anterior; RP, radius posterior; MP, media posterior; CuA, cubitus anterior; CuP, cubitus posterior; Pcu, postcubitus; A1, first anal vein; A2, second anal vein. The morphological terminology used in this study mostly follows Luo et al. (2020b).
SYSTEMATIC PALAEONTOLOGY
Order HEMIPTERA Linnaeus, 1758
Suborder FULGOROMORPHA Evans, 1946
Superfamily FULGORIDIOIDEA Handlirsch, 1939
Family KATLASIDAE Luo, Jiang et Szwedo, 2020 in Luo et al., 2020b
Genus LUCIDUSMACULA
Wang, Szwedo et Luo, gen. nov.
(Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6)
zoobank.org/C27B00F8-5B3C-4B7F-9F6A-A360DE40E098
Type species. Lucidusmacula lihanjieae Wang, Szwedo et Luo, sp. nov.; by present designation and monotypy.
 Etymology. The generic name is derived from the combination of two words in Latin, “lucidus”, meaning “brighter” and “macula”, meaning “spot” and refers to the distinct brighter area present between ScP and RA on tegmen. Gender: feminine.
Etymology. The generic name is derived from the combination of two words in Latin, “lucidus”, meaning “brighter” and “macula”, meaning “spot” and refers to the distinct brighter area present between ScP and RA on tegmen. Gender: feminine.
Diagnosis. vertex with trigons visible in dorsal view (not visible in Katlasus); frons with distinct median carina, extending almost to postclypeus, postclypeus with median carina and two lateral carinate (same as Katlasus, not visible in Dumpyawnus); rostrum clearly exceeding metacoxae (same as Dumpyawnus, just reaching metacoxae in Katlasus). Antennal pedicel barrel-shaped (same as Katlasus, pedicel cone-shaped in Dumpyawnus). Pronotum without distinct median carina (median carina present in Katlasus). Tegmen with a distinct brighter area between ScP and RA, terminals of ScP+RA all single (same as Katlasus, forked with two terminals in Dumpyawnus), ScP single, RA with about five terminals (ten terminals in Dumpyawnus, nine terminals in Katlasus); RP with at least eight terminals (only two terminals in Katlasus and Dumpyawnus); MP with about nine terminals (Dumpyawnus with only five terminals and the Katlasus with 14 terminals); MP3 forked; CuA2a single; CuA2b with about four terminals. Hind wing with ScP+RA forked with three terminals (same as Katlasus, four terminals in Dumpyawnus), RP forked with about four terminals (five terminals in Katlasus, three terminals in Dumpyawnus); MP1+2 forked with three terminals (two terminals in Katlasus and Dumpyawnus), MP3+4 single (two terminals in Katlasus and Dumpyawnus); CuA forked with three terminals (same as Katlasus, five terminals in Dumpyawnus). Metatibia with three ectodermal lateral spines (as in Dumpyawnus; two visible spines in Katlasus).
Age and occurrence. Mid-Cretaceous (lowermost Cenomanian); amber from Kachin State, northern Myanmar.
Lucidusmacula lihanjieae
Wang, Szwedo et Luo, sp. nov.
(Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6)
zoobank.org/B0078C47-8C5D-4631-B127-6DE165AF1516
 Etymology. The specific name is dedicated to Hanjie Li, the daughter of Mr. De Zhuo’s friend.
Etymology. The specific name is dedicated to Hanjie Li, the daughter of Mr. De Zhuo’s friend.
Material. Holotype. Kachin amber, cabochon, 23 × 20 × 9 mm. No syninclusions. Specimen No. LYU-BL2003, deposited in the Institute of Geology and Paleontology at Linyi University in Linyi, China.
Locality and horizon. Kachin amber, from deposits near Tanai Village in the Hukawng Valley of northern Myanmar, lowermost Cenomanian.
Diagnosis. As for the genus.
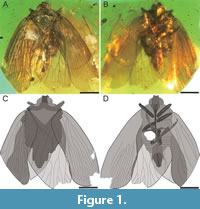
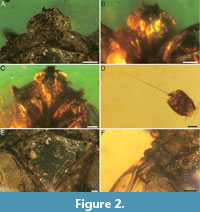
Description. Adult, male (Figure 1). Total length ca. 10.79 mm, body 8.15 mm long. Head with compound eyes 1.71 mm wide (½ as wide as pronotum). 0.88 mm long in midline. Compound eyes large, bulging laterally, anterior margin distinctly exceeding half of head length; right compound eye 0.67 mm long, 0.57 mm wide, left compound eye 0.68 mm long, 0.56 mm wide. Vertex double trapezoid, 0.56 mm long in mid line, 0.59 mm wide, lateral margins converging anteriad, carinate, anterior margin slightly concave (Figure 2A), posterior margin concave, slightly elevated. Frons with a distinct median carina, almost extending to postclypeus, lateral carinae converging anteriad (Figure 2B, C). Frontoclypeal suture slightly concave, distinct. Postclypeus somewhat shield-like, with distinct median carina extending to anteclypeus, and two lateral carinate; anteclypeus narrow, with median carina; rostrum long (in total 2.68 mm), clearly exceeding metacoxae. Antennal pedicel barrel-shaped, with few distinct sensory plaque organs, 0.29 mm long, 0.18 mm wide; flagellum whip-like, 0.83 mm long (Figure 2D).
Pronotum saddle-shaped, 0.75 mm long in mid line, 3.27 mm wide, with two incomplete, diverging posteriad lateral carinae, delimiting disc. Postocular carinae present, widely diverging behind compound eyes; pronotal disc elevated; lateral sections of pronotum descending laterad; anterior margin of disc slightly convex, anterolateral margins widely diverging posteriad, anterolateral angles acute, posterolateral angles obtusely angulate, posterior margin widely concave (Figure 2A). Mesonotum transversely lozenge-shaped, convex, slightly transversely wrinkled on its surface, mesoscutellum not distinctly, delimited by sculpture, with rounded apex; mesonotum 1.87 mm long in mid line and 2.85 mm wide, without distinct carinae (Figure 2E).
Tegula relatively large (0.51 mm long, 0.39 mm wide from the dorsal view), strongly arched (Figure 2F).
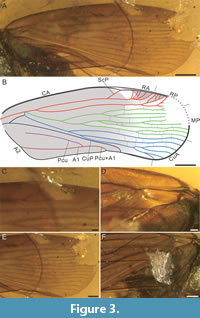 Tegmen (Figure 3) macropterous, membranous, translucent, brown at basal ¾ of its length (corium) and becoming brighter at distal ¼ of its length, on membrane (Figure 3A, B); a distinct brighter area present in stigmal area, between ScP and RA; Figure 3C), tegmen broadest at slightly apicad of ¾ of its length, about 2.6 times as long as wide, tegmen 8.55 mm long, 3.24 mm wide (Figure 3A, B). Costal margin slightly arcuate at base, then slightly arcuate; anteroapical angle widely rounded, posteroapical angle rounded, apical margin mildly rounded, tornus arcuate, claval margin slightly concave, postclaval margin concave. Margin of tegmen surrounded by ambient vein from basal part of costal margin to end of Pcu+A1, with densely transverse veins between ambient vein and margin from slightly before distal end of costal area to claval apex. Basal cell elongated, about five times as long as wide (Figure 3D), basal cell 0.93 mm long, 0.18 mm wide. Stems ScP+R and MP leaving basal cell from same point (terminal of basal cell). Stems ScP+RA and RP with a short common stalk, then forked; stem ScP+RA arcuate, then forked at slightly basad ⅗ of tegminal length; branch ScP single, branch RA with five terminals reaching margin basad of anteroapical angle; branch RP slightly sinuate, then forked at slightly basad of ¾ of tegminal length, reaching margin with at least eight terminals at about anteroapical angle. Stem MP leaving basal cell with a short stalk, about 1.5 times as long as basal cell, reaching margin with at least nine terminals in total; stem MP slightly concave, then forked at slightly basad of ⅓ of tegminal length; branch MP1+2 arcuate, then forked at slightly apicad of ½ of tegminal length; MP1 sinuate, then forked at slightly apicad of ⅘ of tegminal length, reaching margin with two terminals at least; MP2 reaching margin with at least two terminals; branch MP3+4 slightly concave, then forked at slightly apicad of 2/5 of tegminal length; MP3 arcuate, then forked at half of tegminal length; MP3a slightly arcuate at base, then slightly concave, then forked, reaching margin with at least two terminals; MP3b sinuate, single; MP4 slightly sinuate, then forked at of 0.7 of tegminal length, reaching margin with two terminals. Stem CuA with six terminals in total; stem CuA slightly concave at base, then almost straight, then forked at ⅓ of tegminal length; branch CuA1 sinuate, single; branch CuA2 straight, then forked at slightly apicad of ⅓ of tegminal length; CuA2a straight, then arcuate, single; CuA2b slightly sinuate, then forked at of 0.7 of tegminal length, reaching margin with four terminals. Stem CuP slightly arcuate, then almost straight, then arcuate distally, reaching margin at about 3/5 of tegminal length (Figure 3E). Claval veins Pcu and A1 fused at slightly apicad 2/5 of tegminal length, common portion Pcu+A1 distinctly shorter than free portion of Pcu; Pcu almost straight, then slightly arcuate distally; A1 concave; common portion of Pcu+A1 arcuate distally, reaching claval margin (vein A2) at slightly apicad of ½ of tegminal length (Figure 3F). Transverse veinlets (cross-veins) are mostly distributed in apical half of tegmen and not arranged in regular lines. Costal area absent; cell C1 longest, about 5.3 times as long as basal cell, delimited posteriorly by a transverse veinlet ir, 4.95 mm long, 0.33 mm wide; cell C3 shortest, about 2.4 times as long as basal cell, 2.26 mm long, 0.33 mm wide; cell C5 shorter than cell C1, about 4.75 times as long as basal cell, delimited posteriorly by a transverse veinlet icu, 4.42 mm long, 0.27 mm wide.
Tegmen (Figure 3) macropterous, membranous, translucent, brown at basal ¾ of its length (corium) and becoming brighter at distal ¼ of its length, on membrane (Figure 3A, B); a distinct brighter area present in stigmal area, between ScP and RA; Figure 3C), tegmen broadest at slightly apicad of ¾ of its length, about 2.6 times as long as wide, tegmen 8.55 mm long, 3.24 mm wide (Figure 3A, B). Costal margin slightly arcuate at base, then slightly arcuate; anteroapical angle widely rounded, posteroapical angle rounded, apical margin mildly rounded, tornus arcuate, claval margin slightly concave, postclaval margin concave. Margin of tegmen surrounded by ambient vein from basal part of costal margin to end of Pcu+A1, with densely transverse veins between ambient vein and margin from slightly before distal end of costal area to claval apex. Basal cell elongated, about five times as long as wide (Figure 3D), basal cell 0.93 mm long, 0.18 mm wide. Stems ScP+R and MP leaving basal cell from same point (terminal of basal cell). Stems ScP+RA and RP with a short common stalk, then forked; stem ScP+RA arcuate, then forked at slightly basad ⅗ of tegminal length; branch ScP single, branch RA with five terminals reaching margin basad of anteroapical angle; branch RP slightly sinuate, then forked at slightly basad of ¾ of tegminal length, reaching margin with at least eight terminals at about anteroapical angle. Stem MP leaving basal cell with a short stalk, about 1.5 times as long as basal cell, reaching margin with at least nine terminals in total; stem MP slightly concave, then forked at slightly basad of ⅓ of tegminal length; branch MP1+2 arcuate, then forked at slightly apicad of ½ of tegminal length; MP1 sinuate, then forked at slightly apicad of ⅘ of tegminal length, reaching margin with two terminals at least; MP2 reaching margin with at least two terminals; branch MP3+4 slightly concave, then forked at slightly apicad of 2/5 of tegminal length; MP3 arcuate, then forked at half of tegminal length; MP3a slightly arcuate at base, then slightly concave, then forked, reaching margin with at least two terminals; MP3b sinuate, single; MP4 slightly sinuate, then forked at of 0.7 of tegminal length, reaching margin with two terminals. Stem CuA with six terminals in total; stem CuA slightly concave at base, then almost straight, then forked at ⅓ of tegminal length; branch CuA1 sinuate, single; branch CuA2 straight, then forked at slightly apicad of ⅓ of tegminal length; CuA2a straight, then arcuate, single; CuA2b slightly sinuate, then forked at of 0.7 of tegminal length, reaching margin with four terminals. Stem CuP slightly arcuate, then almost straight, then arcuate distally, reaching margin at about 3/5 of tegminal length (Figure 3E). Claval veins Pcu and A1 fused at slightly apicad 2/5 of tegminal length, common portion Pcu+A1 distinctly shorter than free portion of Pcu; Pcu almost straight, then slightly arcuate distally; A1 concave; common portion of Pcu+A1 arcuate distally, reaching claval margin (vein A2) at slightly apicad of ½ of tegminal length (Figure 3F). Transverse veinlets (cross-veins) are mostly distributed in apical half of tegmen and not arranged in regular lines. Costal area absent; cell C1 longest, about 5.3 times as long as basal cell, delimited posteriorly by a transverse veinlet ir, 4.95 mm long, 0.33 mm wide; cell C3 shortest, about 2.4 times as long as basal cell, 2.26 mm long, 0.33 mm wide; cell C5 shorter than cell C1, about 4.75 times as long as basal cell, delimited posteriorly by a transverse veinlet icu, 4.42 mm long, 0.27 mm wide.
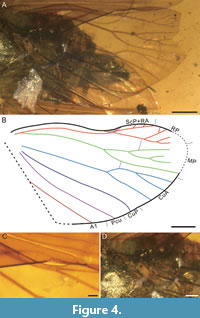 Hind wing (Figure 4) membranous, transparent, subtriangular, shorter than tegmen, about 1.75 times as long as wide, 7.88 mm long, 4.52 mm wide (Figure 4A, B). Costal margin arcuate, then concave; anteroapical angle widely rounded, posteroapical angle rounded, tornus curved, wing-coupling lobe (WCL) present (Figure 4C). Margin of hind wing surrounded by ambient vein at least from anteroapical angle to distal end of postclaval margin, with densely transverse wrinkles between ambient and margin from anteroapical angle to near distal end of postclaval margin. Stem ScP+R subparallel to margin, then forked at about 3/5 of length of hindwing; stem ScP+RA arcuate, then forked slightly before 0.7 of length of hindwing, reaching margin with three terminals; branch RP almost straight, then forked at about ¾ of length of hindwing, reaching margin with four terminals at least. Stem MP slightly arcuate, then forked apicad of ½ of length of hindwing; MP1+2 slightly arcuate, then forked slightly before 0.7 of length of hindwing; MP1 single(?); MP2 concave, then forked at slightly basad of 4/5 of length of hindwing, reaching margin with at least two terminals; MP3+4 concave, single. Stem CuA slightly arcuate at base, then almost straight, forked at basad of 3/5 of length of hindwing; CuA1 strongly arcuate at base, then forked, with two terminals; CuA2 single. Stem CuP almost straight, then arcuate distally, single. Stem Pcu concave, then almost straight at distally, single. Stem A1 concave, then almost straight at distally, single. Transverse veinlets sparse, with one r-m and one m-cu.
Hind wing (Figure 4) membranous, transparent, subtriangular, shorter than tegmen, about 1.75 times as long as wide, 7.88 mm long, 4.52 mm wide (Figure 4A, B). Costal margin arcuate, then concave; anteroapical angle widely rounded, posteroapical angle rounded, tornus curved, wing-coupling lobe (WCL) present (Figure 4C). Margin of hind wing surrounded by ambient vein at least from anteroapical angle to distal end of postclaval margin, with densely transverse wrinkles between ambient and margin from anteroapical angle to near distal end of postclaval margin. Stem ScP+R subparallel to margin, then forked at about 3/5 of length of hindwing; stem ScP+RA arcuate, then forked slightly before 0.7 of length of hindwing, reaching margin with three terminals; branch RP almost straight, then forked at about ¾ of length of hindwing, reaching margin with four terminals at least. Stem MP slightly arcuate, then forked apicad of ½ of length of hindwing; MP1+2 slightly arcuate, then forked slightly before 0.7 of length of hindwing; MP1 single(?); MP2 concave, then forked at slightly basad of 4/5 of length of hindwing, reaching margin with at least two terminals; MP3+4 concave, single. Stem CuA slightly arcuate at base, then almost straight, forked at basad of 3/5 of length of hindwing; CuA1 strongly arcuate at base, then forked, with two terminals; CuA2 single. Stem CuP almost straight, then arcuate distally, single. Stem Pcu concave, then almost straight at distally, single. Stem A1 concave, then almost straight at distally, single. Transverse veinlets sparse, with one r-m and one m-cu.
 Proleg (Figure 5A): profemur margins carinate, covered with few short setae, left profemur 2.23 mm long, 0.35 mm wide, right profemur 2.24 mm long, 0.36 mm wide; protibia narrow and long, margins carinate, dorsally incised, left protibia 1.12 mm long, 0.14 mm wide, right protibia 1.35 mm long, 0.13 mm wide; tarsomere not visible. Mesoleg (Figure 5B, C): mesofemur margins carinate, covered with few short setae, left mesofemur 1.82 mm long, 0.28 mm wide, right mesofemur 1.09 mm long, 0.41 mm wide; mesotibia distinctly narrower and longer than mesofemur, margins carinate, covered with few setae, dorsally incised, apical with few longer setae, left mesotibia 1.31 mm long, 0.14 mm wide, right mesotibia 2.34 mm long, 0.16 mm wide; mesotarsomere distinctly narrower than mesotibia, basimesotarsomere shortest, dorsally incised, left basimesotarsomere 0.10 mm long, 0.21 mm wide, right basimesotarsomere 0.07 mm long, 0.20 mm wide; midmesotarsomere longer than basimesotarsomere, left midmesotarsomere 0.30 mm long, 0.16 mm wide, right midmesotarsomere 0.26 mm long, 0.13 mm wide; apical mesotarsomere longest, left apical mesotarsomere 0.51 mm long, 0.08 mm wide, right apical mesotarsomere 0.42 mm long, 0.08 mm wide; claws invisible. Metaleg (Figure 5D-I): metafemur narrow and long, margins carinate, left metafemur 1.34 mm long, 0.37 mm wide, right metafemur 1.58 mm long, 0.27 mm wide; metatibia narrow and long, margins carinate, widened apical, apical part dorsally incised, with three lateral spines (Figure 5E), with nine apical teeth (Figure 5F), left metatibia 2.59 mm long, 0.31 mm wide, right metatibia 2.63 mm long, 0.37 mm wide; metatarsomere distinctly narrower than metatibia, basimetatarsomere longest, distinctly widened apicad, covered with numerous setae,
Proleg (Figure 5A): profemur margins carinate, covered with few short setae, left profemur 2.23 mm long, 0.35 mm wide, right profemur 2.24 mm long, 0.36 mm wide; protibia narrow and long, margins carinate, dorsally incised, left protibia 1.12 mm long, 0.14 mm wide, right protibia 1.35 mm long, 0.13 mm wide; tarsomere not visible. Mesoleg (Figure 5B, C): mesofemur margins carinate, covered with few short setae, left mesofemur 1.82 mm long, 0.28 mm wide, right mesofemur 1.09 mm long, 0.41 mm wide; mesotibia distinctly narrower and longer than mesofemur, margins carinate, covered with few setae, dorsally incised, apical with few longer setae, left mesotibia 1.31 mm long, 0.14 mm wide, right mesotibia 2.34 mm long, 0.16 mm wide; mesotarsomere distinctly narrower than mesotibia, basimesotarsomere shortest, dorsally incised, left basimesotarsomere 0.10 mm long, 0.21 mm wide, right basimesotarsomere 0.07 mm long, 0.20 mm wide; midmesotarsomere longer than basimesotarsomere, left midmesotarsomere 0.30 mm long, 0.16 mm wide, right midmesotarsomere 0.26 mm long, 0.13 mm wide; apical mesotarsomere longest, left apical mesotarsomere 0.51 mm long, 0.08 mm wide, right apical mesotarsomere 0.42 mm long, 0.08 mm wide; claws invisible. Metaleg (Figure 5D-I): metafemur narrow and long, margins carinate, left metafemur 1.34 mm long, 0.37 mm wide, right metafemur 1.58 mm long, 0.27 mm wide; metatibia narrow and long, margins carinate, widened apical, apical part dorsally incised, with three lateral spines (Figure 5E), with nine apical teeth (Figure 5F), left metatibia 2.59 mm long, 0.31 mm wide, right metatibia 2.63 mm long, 0.37 mm wide; metatarsomere distinctly narrower than metatibia, basimetatarsomere longest, distinctly widened apicad, covered with numerous setae,  dorsally deeply incised, with at least eleven apical teeth and numerous subapical platellae (robust setae-like structure; see also figure 6D of Brysz et al., 2024), except lateral teeth (Figure 5G), left basimetatarsomere 0.87 mm long, 0.17 mm wide, right basimetatarsomere 0.83 mm long, 0.16 mm wide; midmetatarsomere distinctly widened apicad, covered with numerous setae, dorsally deeply incised, with at least nine apical teeth and subapical platellae, except the external teeth (Figure 5H), left midmetatarsomere 0.39 mm long, 0.14 mm wide, right midmetatarsomere 0.41 mm long, 0.15 mm wide; apical metatarsomere shortest, becoming narrower apicad, left apical metatarsomere 0.54 mm long, 0.10 mm wide, right apical metatarsomere 0.53 mm long, 0.08 mm wide; claws small, without distinct arolium (Figure 5I).
dorsally deeply incised, with at least eleven apical teeth and numerous subapical platellae (robust setae-like structure; see also figure 6D of Brysz et al., 2024), except lateral teeth (Figure 5G), left basimetatarsomere 0.87 mm long, 0.17 mm wide, right basimetatarsomere 0.83 mm long, 0.16 mm wide; midmetatarsomere distinctly widened apicad, covered with numerous setae, dorsally deeply incised, with at least nine apical teeth and subapical platellae, except the external teeth (Figure 5H), left midmetatarsomere 0.39 mm long, 0.14 mm wide, right midmetatarsomere 0.41 mm long, 0.15 mm wide; apical metatarsomere shortest, becoming narrower apicad, left apical metatarsomere 0.54 mm long, 0.10 mm wide, right apical metatarsomere 0.53 mm long, 0.08 mm wide; claws small, without distinct arolium (Figure 5I).
Abdomen 5.04 mm long with male terminalia. Male terminalia (Figure 6A, B) tubular, 2.2 mm long, 1.3 mm wide; visible median carina on ventral side of genital plate, genital style narrow toward apex and rounded apically.
DISCUSSION
Lucidusmacula gen. nov. can be assigned to Hemiptera according to its piercing-sucking mouthparts and can be attributed to Fulgoromorpha due to the structure of the head capsule with carinae, antennae positioned below the compound eyes, “Y-shape” veins on the clavus (Pcu and A1), the tegmen with wrinkled peripheral membrane and the presence of the metatibio-tarsal pecten (Bourgoin and Szwedo, 2022, 2023). Lucidusmacula gen. nov. can be placed in Katlasidae Luo, Jiang et Szwedo, 2020 mainly based on its tegminal structure: absence of the costal area, closed clavus, elongated tornus, widened membrane, increased branching complexity in ScP+RA, MP, and CuA2 veins; unforked CuA1; absence of nodal line, the first fork of CuA2 occurs basal to the claval apex, as well as the hind wing venation ScP+RA, RP, MP, and CuA veins has three to five terminals (Luo et al., 2020b; Zhang et al., 2023).
However, Lucidusmacula gen. nov. clearly differs from the other two genera of Katlasidae by a set of characters. The head with trigons is visible in Lucidusmacula gen. nov. in the dorsal view, which is not the case in Katlasus. The rostrum of Lucidusmacula gen. nov. clearly exceeds the metacoxae, a condition shared with Dumpyawnus, which is longer than the rostrum of Katlasus (just reaching the metacoxae). The pronotum of Lucidusmacula gen. nov. lacks a distinct median carina, which is also different from the pattern observed in Katlasus. Numerous shared and differing characters are present on the tegmen, for example, the presence of a distinct brighter area at stigmal area (between ScP and RA) is a striking feature of Lucidusmacula gen. nov.; such structure is absent in Katlasus and Dumpyawnus. The single terminal ScP of Lucidusmacula gen. nov. is shared with Katlasus, while it is forked with two terminals in Dumpyawnus. Lucidusmacula gen. nov. also clearly separates from the other two genera in having RA forked with five terminals, whereas Dumpyawnus exhibits a branching pattern characterized by over ten terminals, and Katlasus has nine terminals. Striking differences are also observed in RP, which bears at least eight terminals in Lucidusmacula gen. nov., compared to only two in both Katlasus and Dumpyawnus. Regarding the number of MP terminals, Lucidusmacula gen. nov. occupies an intermediate position with nine terminals. This contrasts with Dumpyawnus, which possesses five terminals, and Katlasus, which exhibits 14 terminals. Significantly, Lucidusmacula gen. nov. shares the characters of a single CuA2a with Dumpyawnus, whereas Katlasus has two terminals. Furthermore, CuA2b in Lucidusmacula gen. nov. has four terminals, differing from Katlasus (five terminals) and Dumpyawnus (six terminals). The hind wings also provide diagnostically significant characters. For example, ScP+RA bearing three terminals is identical to the condition in Katlasus but contrasts with Dumpyawnus where it presents four terminals. RP is forked, resulting in four terminals in Lucidusmacula gen. nov. Conversely, Katlasus exhibits a higher count of five RP terminals, while Dumpyawnus displays a reduced count of only three terminals. CuA forked with three branches, represents a shared character state between Lucidusmacula gen. nov. and Katlasus. In contrast, CuA in Dumpyawnus bears five terminals.
The presence of a new representative of Katlasidae in Kachin amber adds new morphological information for Cretaceous fulgoridioid planthoppers. The oldest Fulgoridioidea - belonging to the family Szeiinidae Zhang, Jiang, Szwedo et Zhang, 2021 - is known from the Late Triassic of Huanglong County, Shaanxi Province, China (Zhang et al., 2021). The Jurassic was dominated by numerous Fulgoridiidae Handlirsch, 1939 (Szwedo et al., 2004; Bucher et al., 2024), with the latest known representative described from Kachin amber - Stonymetopus megus Poinar, Brown et Bourgoin, 2022 (Poinar et al., 2022). Inclusions of the Fulgoridioidea in Kachin amber represent high taxonomic diversity and morphological disparity, offering a number of extinct families of this lineage: Dorytocidae Emeljanov et Shcherbakov, 2018 (Emeljanov and Shcherbakov 2018; Song et al., 2021), Inoderbidae Shcherbakov et Emeljanov, 2021, with two subfamilies (Shcherbakov and Emeljanov 2021; Luo et al., 2022; Zhang et al., 2024), and Katlasidae Luo, Jiang et Szwedo, 2020 (Luo et al., 2020; Zhang et al., 2023). In addition, several taxa described from Kachin amber that were originally placed in other families have been moved to this lineage (Bucher et al., 2024). Morphologically, this new fossil of Katlasidae matches the set of diagnostic characters proposed by Bourgoin and Szwedo (2022) for Fulgoridioidea, viz. a long stem ScP+RA, CuA posterior branch CuA2 forking again at least once well before the nodal line, anterior branches at these nodes leaving anteriorly, posterior branches almost aligned with previous ones. Its tegminal structure clearly places Lucidusmacula gen. nov. in Katlasidae, adding new data on morphological disparity of this family. Katlasidae presents another interesting feature - presence of lateral spines on the metatibia - the structure has recently been discussed in Cixiidae (Brożek et al., 2024), but in Katlasidae, this structure developed as ectodermal expansions, similarly as in Fulgoroidea. Also, the presence of subapical platellae appears to be another important feature of Katlasidae, as these were shown as structures of importance for classification and evolutionary reconstructions; however, this structure requires more attention and further investigations (e.g., Song et al., 2019; Brysz et al., 2023; Brożek et al., 2024; Deng et al., 2025).
CONCLUSION
The third genus of the planthopper family Katlasidae is described from mid-Cretaceous Kachin amber. The discovery of this new genus significantly advances our understanding of phylogenetic diversity and structural disparity within Katlasidae and Fulgoridioidea, giving a new insight to their evolution. However, more specimens are needed to reveal the precise systematic position of Katlasidae within Fulgoridioidea.
ACKNOWLEDGEMENTS
We are grateful to the two anonymous reviewers for their thoughtful and constructive comments. We also thank the editors, Dr. Sergio Álvarez-Parra and Prof. Dr. Carolin Haug, for their valuable feedback and support. This research has been supported by the National Natural Science Foundation of China (42293280) and Jiangsu Innovation Support Plan for International Science and Technology Cooperation Programme (BZ2023068). JS is grateful to the Chinese Academy of Sciences for support under the President’s International Fellowship Initiative (PIFI No. 2021VCA0009). This paper is a contribution to the IUGS “Deep-time Digital Earth” Big Science Program, Geobiodiversity Database.
REFERENCES
Anufriev, G.A. and Emeljanov, A.F. 1988. Volume II: Homoptera and Heteroptera, p. 496. In Lehr, P.A. (eds.), Keys to the Insects of the Far East of the USSR in Six Volumes. Nauka Publishing, St. Petersburg, Russia.
Bartlett, C.R., O’Brien, L.B., Wilson, S.W. 2014. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Memoirs of the American Entomological Society, 50(1): 1-287.
https://doi.org/10.3157/061.149.0104
Boderau, M., Nel, A., Jouault, C. 2025. Diversification and extinction of Hemiptera in deep time. Communication Biology, 8: 352.
https://doi.org/10.1038/s42003-025-07773-x
Bourgoin, T., Szwedo, J. 2022. Toward a new classification of planthoppers Hemiptera Fulgoromorpha: 1. What do Fulgoridiidae really cover? Annales Zoologici, 72(4): 951-962.
https://doi.org/10.3161/00034541anz2022.72.4.011
Bourgoin, T., Szwedo, J. 2023. Toward a new classification of planthoppers Hemiptera Fulgoromorpha: 2. Higher taxa, their names and their composition. Zootaxa, 5297(4): 562-568.
https://doi.org/10.11646/zootaxa.5297.4.5
Bourgoin, T., Wang, R.R., Asche, M., Hoch, H., Soulier-Perkins, A., Stroinski, A., Yap, S., Szwedo, J. 2015. From micropterism to hyperpterism: recognition strategy and standardized homology-driven terminology of the forewing venation patterns in planthoppers (Hemiptera: Fulgoromorpha). Zoomorphology, 134(1): 63-77.
https://doi.org/10.1007/s00435-014-0243-6
Brożek, J., Stroiński, A., Romaniak, A., Bourgoin, T. 2024. Disparity of metatibial and metatarsal cuticular and sensory structures in Cixiidae (Hemiptera: Fulgoromorpha) with a metatibiotarsal diagnosis for the tribes. Zoological Letters, 10(16): 1-31.
https://doi.org/10.1186/s40851-024-00239-8
Brysz, A.M., Mueller, P., Szwedo, J. 2023. First fossil representative of the tribe Amphignomini (Hemiptera: Fulgoromorpha: Achilidae) from mid-Cretaceous Kachin amber and its significance. European Journal of Entomology, 120: 42-49.
https://doi.org/10.14411/eje.2023.006
Brysz, A. M., Stroiński, A., Szwedo, J. 2024. A new tribe of scaphocephalic Achilidae from South Africa (Hemiptera: Fulgoromorpha). European Journal of Taxonomy, 958: 151-176.
https://doi.org/10.5852/ejt.2024.958.2667
Bucher, M., Gignoux, G., Szwedo, J., Bourgoin, T. 2024. Time-traveling through fossil planthopper tegmina in the Paleozoic and Mesozoic eras (Insecta: Hemiptera: Fulgoromorpha). Palaeoentomology, 7(1): 1-67.
https://doi.org/10.11646/palaeoentomology.7.1.1
Deng J.C., Stroiński A., Szwedo J., Ghanavi H.R., Yapar E., Franco D.C., Prus-Frankowska M., Michalik A., Wahlberg N., Łukasik P. 2025. Phylogenomics resolves the relationship and the evolutionary history of planthoppers (Insecta: Hemiptera: Fulgoromorpha). Systematic Entomology, 50 (3), 495–518.
https://doi.org/10.1111/syen.12666
Emeljanov, A.F., Shcherbakov, D.E. 2018. The longest-nosed Mesozoic Fulgoroidea (Homoptera): A new family from mid-Cretaceous Burmese amber. Far Eastern Entomologist, 354: 1-14.
https://doi.org/10.25221/fee.354.1
Emeljanov, A.F., Shcherbakov, D.E. 2020. The first Mesozoic Derbidae (Homoptera: Fulgoroidea) from Cretaceous Burmese amber. Russian Entomological Journal, 29(3): 237-246.
https://doi.org/10.15298/rusentj.29.3.02
Evans, J.W. 1946. A natural classification of leaf-hoppers (Jassoidea, Hemiptera). Part 1. External morphology and systematic position. Transactions of the Royal Entomological Society of London, 96: 47-60.
https://doi.org/10.1111/j.1365-2311.1946.tb00442.x
Fieber, F.X. 1872. Katalog der europäischen Cicadinen, nach Originalien mit Benützung der neuesten Literatur.
Handlirsch, A. 1939. Neue Untersuchungen über die fossilen Insekten, Teil 2. Annalen des Naturhistorischen Museums in Wien, 49: 1-240.
Haug, J.T., Azar, D., Ross, A.J., Szwedo, J., Wang, B., Arillo, A., Baranov, V., Bechteler, J., Beutel, R.G., Blagoderov, V., Delclòs, X., Feldberg, K., Feldmann, R., Foth, C., Fraaije, R.H.B., Gehler, A., Harms, D., Hedenäs, L., Hyžný, M., Jagt, J.W.M., Jagt-Yazykova, E.A., Jarzembowski, E.A., Kerp, H., Khine, P.K., Kirejtshuk, A.G., Klug, C., Kopylov, D.S., Kotthoff, U., Kriwet, J., McKellar, R.C., Nel, A., Nützel, A., Peñalver, E., Perrichot, V., Pint, A., Ragazzi, E., Regalado, L., Reich, M., Rikkinen, J., Schmidt, A.R., Schneider, H., Schram, F.R., Schweigert, G., Selden, P.A., Solórzano-Kraemer, M.M., Stilwell, J.D., van-Bakel, B.W.M., Vega, F.J., Wang, Y., Xing, L.D., Haug, C. 2020 Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated April 21, 2020. regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber. Paläontologische Zeitschrift, 94: 431-437.
https://doi.org/10.1007/s12542-020-00522-x
ICZN. 1999. International code of zoological nomenclature. Fourth Edition. The International Trust for Zoological Nomenclature, London.
Latreille, P.A. 1807. Genera crustaceorum et insectorum secundum ordinem naturalem in familias disposita, iconibus exemplisque plurimis explicata. apud Amand Koenig, Parisiis et Argentorati.
Leach, W.E. 1815. Entomology. The Edinburgh Encyclopedia, 9: 57-172.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis, 10th Edition ed. Laurentii Salvii, Stockholm.
Luo, C., Gnezdilov, V.M., Zhuo, D., Song, Z. 2023. First mid-Cretaceous nogodinid planthopper (Hemiptera: Fulgoromorpha: Fulgoroidea) from Kachin amber with an extant relative from the Neotropics. Cretaceous Research, 150: 105591.
https://doi.org/10.1016/j.cretres.2023.105591
Luo, C., Jiang, T., Szwedo, J., Wang, B., Xiao, C. 2020a. A new genus and species of Perforissidae (Hemiptera: Fulgoromorpha) from mid-Cretaceous Kachin amber. Cretaceous Research, 114: 104518.
https://doi.org/10.1016/j.cretres.2020.104518
Luo, C., Jiang, T., Szwedo, J., Wang, B., Xiao, C. 2020b. A new planthopper family Katlasidae fam. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea) from mid-Cretaceous Kachin amber. Cretaceous Research, 115: 104532.
https://doi.org/10.1016/j.cretres.2020.104532
Luo, C., Song, Z., Liu, X., Jiang, T., Jarzembowski, E.A., Szwedo, J. 2022. Ingensalinae subfam. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea: Inoderbidae), a new planthopper subfamily from mid-Cretaceous Kachin amber from Myanmar. Fossil Record, 24(2): 455-465.
https://doi.org/10.5194/fr-24-455-2022
Luo, Y., Bourgoin, T., Szwedo, J., Feng, JN. 2021. Acrotiarini trib. nov., in the Cixiidae (Insecta, Hemiptera, Fulgoromorpha) from mid-Cretaceous amber of northern Myanmar, with new insights in the classification of the family. Cretaceous Research, 128: 104959.
https://doi.org/10.1016/j.cretres.2021.104959
Martynov, A.V. 1935. Permian fossil insects from Arkhangelsk district. Part.5. Homoptera. Trudy Paleozoologischeskogo Instituta Akademii Nauk SSSR, 4: 1-35.
Poinar, G., Brown, A.E., Bourgoin, T. 2022. Stonymetopus megus gen. et sp. nov. (Hemiptera: Fulgoromorpha), the first Fulgoridiidae genus from mid-Cretaceous Burmese amber. Palaeodiversity, 15(1): 83-90.
https://doi.org/10.18476/pale.v15.a6
Ross, A.J. 2025. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2024. Palaeoentomology, 8(1): 12-28.
https://doi.org/10.11646/palaeoentomology.8.1.4
Spinola, M. 1839. Essai sur les Fulgorelles. sous tribu de la tribu des cicadaires, ordre des Rhyngotes. Annales de la Société entomologique de France, 8: 133-454.
Stål, C. 1866. Hemiptera Homoptera Latr. Hemiptera Africana, 4: 1-276.
Shcherbakov, D.E. 2000. Permian faunas of Homoptera (Hemiptera) in relation to phytogeography and the Permo-Triassic crisis. Paleontologicheskii Zhurnal, 34: S251-S267.
Shcherbakov, D.E. 2007a. Mesozoic spider mimics-Cretaceous Mimarachnidae fam. n. (Homoptera: Fulgoroidea). Russian Entomological Journal, 16: 259-264.
Shcherbakov, D.E. 2007b. An extraordinary new family of Cretaceous planthoppers (Homoptera: Fulgoroidea). Russian Entomological Journal, 16(2): 139-154.
Shcherbakov, D.E. 2017. First record of the Cretaceous family Mimarachnidae (Homoptera: Fulgoroidea) in amber. Russian Entomological Journal, 26(4): 389-392.
Shcherbakov, D.E. 2020. The earliest fully brachypterous Auchenorrhynchan from Cretaceous Burmese amber (Homoptera: Fulgoroidea: Jubisentidae). Russian Entomological Journal, 29(1): 6-11.
https://doi.org/10.15298/rusentj.29.1.02
Shcherbakov, D.E., Emeljanov, A.F. 2021. Paradoxical derbid-like planthopper (Homoptera: Fulgoroidea) from Cretaceous Burmese amber. Russian Entomological Journal, 30(2): 135-139.
https://doi.org/10.15298/rusentj.30.2.03
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research, 37: 155-163.
https://doi.org/10.1016/j.cretres.2012.03.014
Song, Z.S., Xu, G.H., Liang, A.P., Szwedo, J., Bourgoin, T. 2019. Still greater disparity in basal planthopper lineage: A new planthopper family Yetkhatidae (Hemiptera, Fulgoromorpha, Fulgoroidea) from mid-Cretaceous Myanmar amber. Cretaceous Research, 101: 47-60.
https://doi.org/10.1016/j.cretres.2019.03.023
Song, Z.S., Zhang, C.L., Xi, H.Y., Szwedo, J., Bourgoin, T. 2021. First record of adult Dorytocidae Dorytocus jiaxiaoae Song, Szwedo & Bourgoin sp. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea) from mid-Cretaceous Kachin amber. Cretaceous Research, 125: 104863.
https://doi.org/10.1016/j.cretres.2021.104863
Szwedo, J. 2007. Nymphs of a new family Neazoniidae fam. n.(Hemiptera: Fulgoromorpha: Fulgoroidea) from the Lower Cretaceous Lebanese amber. African Invertebrates, 48(1): 127-143.
Szwedo, J. 2018. The unity, diversity and conformity of bugs (Hemiptera) through time. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 107(2-3): 109-128.
https://doi.org/10.1017/s175569101700038x
Szwedo, J., Bourgoin, T., Lefebvre, F. 2004 Fossil Planthoppers (Hemiptera: Fulgoromorpha) of the World. An annotated catalogue with Notes on Hemiptera Classification. Studio 1, Warszawa, pp. 1-199+8 Pls.
Szwedo, J., Wang, B., Soszyńska-Maj, A., Azar, A., Ross, A.J., 2020. International Palaeoentomological Society Statement. Palaeoentomology, 3(3): 221-222.
Thu, K., Zaw, K. 2017. Gem deposits of Myanmar. Geological Society, London Memoirs, 48: 497-529.
https://doi.org/10.1144/m48.23
Wang, M., Liang, F., Bourgoin, T. 2022. A new cixiid fossil genus of the tribe Acrotiarini from Mid-Cretaceous Burmese amber (Insecta, Hemiptera, Fulgoromorpha). Insects, 13(1):102.
https://doi.org/10.3390/insects13010102
Westerweel, J., Roperch, P., Licht, A., Dupont-Nivet, G., Win, Z., Poblete, F., Aung, D.W. 2019. Burma Terrane part of the Trans-Tethyan arc during collision with India according to palaeomagnetic data. Nature Geoscience, 12(10): 863-868.
https://doi.org/10.1038/s41561-019-0443-2
Yu, T., Kelly, R., Mu, L., Ross, A., Kennedy, J., Broly, P., Xia, F.Y., Zhang, H.C., Wang, B., Dilcher, D. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences of the United States of America, 116(23): 11345-11350.
https://doi.org/10.1073/pnas.1821292116
Zhang, F., Bourgoin, T., Song, Z., Chen, R., Xiao, C., Luo, C. 2024. Imbricatala novitas gen. et sp. nov., a new planthopper of family Inoderbidae (Hemiptera: Fulgoromorpha: Fulgoridioidea) from mid-Cretaceous Kachin amber of northern Myanmar. Palaeontographica Abteilung A, 328(1-6): 123-134.
https://doi.org/10.1127/pala/2024/0151
Zhang, Q., Jiang, T., Szwedo, J., Zhang, H. 2021. A new family of Triassic planthoppers (Hemiptera: Fulgoromorpha: Fulgoroidea) from the Shaanxi Province of China. Alcheringa: An Australasian Journal of Palaeontology, 45(1): 86-90.
https://doi.org/10.1080/03115518.2021.1919206
Zhang, X., Ren, D., Yao, Y. 2017. A new species of Foveopsis Shcherbakov (Hemiptera: Fulgoromorpha: Fulgoroidea: Perforissidae) from mid-Cretaceous Burmese amber. Cretaceous Research, 79: 35-42.
https://doi.org/10.1016/j.cretres.2017.07.002
Zhang, X., Ren, D., Yao, Y. 2019. A new family Jubisentidae fam. nov. (Hemiptera: Fulgoromorpha: Fulgoroidea) from the mid-Cretaceous Burmese amber. Cretaceous Research, 94: 1-7.
https://doi.org/10.1016/j.cretres.2018.10.012
Zhang, X., Yao, Y., Ren, D., Pang, H. 2021. A new species of Cretodorus (Hemiptera: Fulgoromorpha: Fulgoroidea: Mimarachnidae) from Upper Cretaceous amber of northern Myanmar. Cretaceous Research, 128: 104988.
https://doi.org/10.1016/j.cretres.2021.104988
Zhang, X., Luo, C., Song, Z., Szwedo, J. 2023. Dumpyawnus hpungwanus gen. et sp. nov., the second genus and species of Katlasidae (Hemiptera: Fulgoromorpha: Fulgoridoidea) from mid-Cretaceous Kachin amber, northern Myanmar. Cretaceous Research, 150: 105585.
https://doi.org/10.1016/j.cretres.2023.105585

