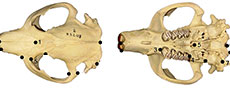
 Comparison of Miocene to early Pleistocene-aged Castor californicus (Rodentia: Castoridae) to extant beavers and implications for the evolution of Castor in North America
Comparison of Miocene to early Pleistocene-aged Castor californicus (Rodentia: Castoridae) to extant beavers and implications for the evolution of Castor in North America
Article number: 26.3.a35
https://doi.org/10.26879/1284
Copyright Society of Vertebrate Paleontology, September 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 24 March 2023. Acceptance: 18 August 2023.
ABSTRACT
The beaver, genus Castor, is represented in North America today by Castor canadensis and in Eurasia by C. fiber. Historically, the fossil Miocene to early Pleistocene-aged North American beaver C. californicus has been considered a distinct species from C. canadensis due to its larger size. In this study, we test the hypothesis that the morphology of Miocene to early Pleistocene-aged fossils of C. californicus differs from that of the extant C. canadensis. Specimens of fossil and extant Castor were compared using 2D geometric morphometrics of skull and dentary material and linear measurements of postcranial material to analyze morphological differences between species and determine whether C. californicus fits within the range of intraspecific variation seen in C. canadensis. Results show that C. canadensis is highly variable in both skull and postcranial morphology, and C. californicus falls largely within the range of variation seen within the extant species. The morphological similarities between the two species suggest that they can be treated as ecological analogs and may represent change in a single species through time, although a rigorous evaluation of whether they are conspecific will require more data.
Kelly E. Lubbers. The Mammoth Site, Hot Springs, South Dakota 57747, USA and Department of Geosciences, East Tennessee State University, Johnson City, Tennessee 37614, USA. kellyl@mammothsite.org
Joshua X. Samuels. Department of Geosciences, East Tennessee State University, Johnson City, Tennessee 37614, USA and Don Sundquist Center of Excellence in Paleontology, East Tennessee State University, Johnson City, Tennessee, 37614, USA. samuelsjx@etsu.edu
Keywords: morphology; geometric morphometrics; chronospecies; semi-aquatic; Castoridae
Final citation: Lubbers, Kelly E. and Samuels, Joshua X. 2023. Comparison of Miocene to early Pleistocene-aged Castor californicus (Rodentia: Castoridae) to extant beavers and implications for the evolution of Castor in North America. Palaeontologia Electronica, 26(3):a35.
https://doi.org/10.26879/1284
palaeo-electronica.org/content/2023/3943-fossil-beaver-morphology
Copyright: September 2023 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Beavers (family Castoridae) first appeared in North America during the late Eocene and from there dispersed into Eurasia (Korth, 1994; Flynn and Jacobs, 2008). The fossil record of beavers includes approximately 30 genera, with diverse lineages adapted to fossorial, terrestrial, and semiaquatic lifestyles (Martin and Bennett, 1977; Martin, 1989; Korth, 1994; Rybczynski, 2007; Samuels and Van Valkenburgh, 2008; Samuels and Van Valkenburgh, 2009; Calede, 2022). The semiaquatic lineage of beavers likely arose in the late Oligocene (Korth and Samuels, 2015; Calede, 2022), and members of both Castorinae and Castoroidinae diversified greatly in the Miocene (Rybczynski, 2007; Rybczynski et al., 2010). The genus Castor likely appeared in the late Miocene, as represented by Castor neglectus from Germany (Hugueney, 1999; Flynn and Jacobs, 2008). Little is known about the dispersals of Castor between North America and Eurasia, though it is likely those migrations were facilitated by the Bering land bridge throughout the Cenozoic (Rybczynski, 2007; Flynn and Jacobs, 2008; Samuels and Zancanella, 2011).
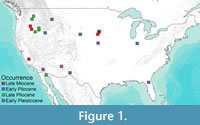 Castor has been present in North America since the late Miocene, with the earliest records of Castor californicus dating to around 7 m.y.a. (Samuels and Zancanella, 2011). Castor californicus was first discovered in the Kettleman Hills in California (Kellogg, 1911). Kellogg (1911) designated it as a separate species from C. canadensis based on features of the upper third molar. Other specimens of C. californicus, including skull, postcranial, and dental material, have been discovered and described in Miocene, Pliocene, and early Pleistocene-aged localities across the United States (Figure 1) (Zakrzewski, 1969; Shotwell, 1970; Kurtén and Anderson, 1980; Samuels and Zancanella, 2011). A second species of fossil beaver in North America, Castor accessor, was initially described by Hay (1927) and designated as a separate species based on differences in tooth striae lengths compared to C. californicus and C. canadensis (Hay, 1927; Kurtén and Anderson, 1980). However, due to similarities in size and temporal distribution, C. accessor is generally considered a junior synonym of C. californicus (Stirton, 1935; Flynn and Jacobs, 2008).
Castor has been present in North America since the late Miocene, with the earliest records of Castor californicus dating to around 7 m.y.a. (Samuels and Zancanella, 2011). Castor californicus was first discovered in the Kettleman Hills in California (Kellogg, 1911). Kellogg (1911) designated it as a separate species from C. canadensis based on features of the upper third molar. Other specimens of C. californicus, including skull, postcranial, and dental material, have been discovered and described in Miocene, Pliocene, and early Pleistocene-aged localities across the United States (Figure 1) (Zakrzewski, 1969; Shotwell, 1970; Kurtén and Anderson, 1980; Samuels and Zancanella, 2011). A second species of fossil beaver in North America, Castor accessor, was initially described by Hay (1927) and designated as a separate species based on differences in tooth striae lengths compared to C. californicus and C. canadensis (Hay, 1927; Kurtén and Anderson, 1980). However, due to similarities in size and temporal distribution, C. accessor is generally considered a junior synonym of C. californicus (Stirton, 1935; Flynn and Jacobs, 2008).
Castor canadensis is one of two extant members of the genus Castor, the other being C. fiber. Although the two species are similar in morphology, they are genetically and chromosomally different (C. canadensis 2n=40, C. fiber 2n=48) (Jenkins and Buscher, 1979; Rosell et al., 2005; Brazier et al., 2020). Castor canadensis has a distribution extending throughout North America, whereas the distribution of C. fiber extends through Eurasia (Jenkins and Buscher, 1979; Halley et al., 2020).
Since Stirton (1935), Castor specimens from the Miocene to early Pleistocene of North America have been referred to as Castor californicus (due to shift of the Plio-Pleistocene boundary from 1.8 Ma to 2.5 Ma (Gibbard and Head, 2010), many C. californicus specimens originally considered Pliocene are now considered early Pleistocene), whereas those from the late Pleistocene to recent are referred to the living species Castor canadensis (Kurtén and Anderson, 1980; Flynn and Jacobs, 2008). Although no rigorous analysis has yet been undertaken of this pattern, multiple studies have noted a decrease in size within Castor from the Miocene through the Pleistocene of North America (Stirton, 1935; Shotwell, 1970), but otherwise there has been little other morphological change in the taxon (Martin, 1989; Samuels and Zancanella, 2011). This raises the question of whether C. canadensis and C. californicus are distinct species or represent anagenetic change in a single species over time. The primary purpose of this study is to evaluate whether the Miocene to early Pleistocene-aged C. californicus and the late Pleistocene-aged and extant C. canadensis are distinct in skull and postcranial morphology. This will improve understanding of the evolution of Castor in North America over time and help resolve whether the two species can be treated as conspecific and/or ecological analogs. Based on the age of studied specimens (Pliocene and early Pleistocene), we expect C. californicus to either show 1) intermediate morphological features between C. fiber and C. canadensis, possibly reflecting retention of ancestral Castor morphology or evolutionary divergence of Eurasian beavers and North American beavers that increases over time, 2) distinct morphological features that differentiate C. californicus from both C. canadensis and C. fiber, reflecting its status as a distinct species, or 3) strong morphological similarity to C. canadensis, reflecting their shared lineage and potential status as chronomorphs of a single synonymous species.
MATERIALS AND METHODS
Cranial Analyses
A total of 67 specimens were used in the analysis of cranial material (Appendix 1). Fifty-nine specimens of Castor canadensis, and four of C. fiber accounted for the modern sample. For the fossil specimens, three Pliocene and early Pleistocene-aged specimens of C. californicus (HAFO 2243, UF 225200, USNM 26154), and one Pleistocene C. fiber (FMNH 1537) were used. Castor fiber had limited modern sampling in this analysis due to lack of available specimens in collections visited. While specimens of fossil C. californicus are common in North America, limited material was used in this analysis due to fragmentary nature of available fossil specimens.
Forty dentaries of both fossil and modern species were also used for this study (Appendix 2). Modern specimens included 35 Castor canadensis, which came from all over North America, and two C. fiber. Fossil specimens included two specimens of C. californicus (UF 225200, USNM 26154) and one C. accessor (UOMNCH 16338), which has been considered synonymous with C. californicus, with specimens ranging from Pliocene to early Pleistocene in age.
To minimize the effects of allometry, only adult specimens (approximately 5 years old or greater) were used in the analysis. Adults were selected based on the fusion of the suture between the basioccipital and basisphenoid following Roberson and Shadle (1954). Most dentaries included in the analysis were associated with craniums, however, four isolated dentaries were included from previous identification of specimens being classified as adult. Fossil specimens were selected based on assumptions of age groupings sharing similar characteristics to those of extant beavers; however, an extensive evaluation of age classifications in fossil Castor californicus compared to extant C. canadensis are outside the scope of this study.
Cranial material was photographed in dorsal, lateral, and ventral views, whereas dentaries were photographed in both lateral and medial views. In dorsal and ventral view specimens were photographed with the palate parallel to the photographic plane, and in lateral view the midline of the palate was aligned perpendicular to the photographic plane. The dentary was photographed with the occlusal surface of the cheek teeth aligned perpendicular to the photographic plane. Scale bars were used with all photos to calibrate known scale when setting landmarks.  Specimens were photographed using either a Nikon D810 DSLR camera with an AF-S Micro Nikkor 60 mm lens at a resolution of 7360 x 4912 pixels or a Mercury CyberPix E5205 digital camera at a resolution of 2560 x 1920 pixels. Photographs were taken at a distance using a camera stand to minimize the effects of parallax. Regardless of the camera used, imaged specimens were over 1000 pixels wide, and the linearity of photographs was tested at all magnification levels used. Thin-plate spline (TPS) files were created from JPEG files for all image views using the program tpsUtil32 (v.1.61), in preparation for landmark digitization (Rohlf, 2015). The program tpsDig2 (v. 2.31) was used for digital placement of landmarks on specimen images (Rohlf, 2021). Landmark placement for cranial material (Figure 2; Table 1) followed those previously used in Samuels and Van Valkenburgh (2009) and Rybczynski et al. (2010), and dentary landmarks (Figure 2; Table 2) followed those previously used in Monteiro et al. (2005). To minimize the potential effects of asymmetry, only one side was used for the dorsal and ventral cranial views.
Specimens were photographed using either a Nikon D810 DSLR camera with an AF-S Micro Nikkor 60 mm lens at a resolution of 7360 x 4912 pixels or a Mercury CyberPix E5205 digital camera at a resolution of 2560 x 1920 pixels. Photographs were taken at a distance using a camera stand to minimize the effects of parallax. Regardless of the camera used, imaged specimens were over 1000 pixels wide, and the linearity of photographs was tested at all magnification levels used. Thin-plate spline (TPS) files were created from JPEG files for all image views using the program tpsUtil32 (v.1.61), in preparation for landmark digitization (Rohlf, 2015). The program tpsDig2 (v. 2.31) was used for digital placement of landmarks on specimen images (Rohlf, 2021). Landmark placement for cranial material (Figure 2; Table 1) followed those previously used in Samuels and Van Valkenburgh (2009) and Rybczynski et al. (2010), and dentary landmarks (Figure 2; Table 2) followed those previously used in Monteiro et al. (2005). To minimize the potential effects of asymmetry, only one side was used for the dorsal and ventral cranial views.
Data Analysis
Landmark data were first analyzed via Relative Warp Analysis (RWA) performed in the tpsRelW program (v 1.70) (Rohlf, 2015). The RWA used generalized least square Procrustes analysis to scale, rotate, and align coordinate sets for each view of the skull and dentary. Two stepwise canonical variates analyses (CVA) were run using partial warp scores and uniform components from all RWA, the first CVA used both Castor canadensis and C. fiber as a priori categories with Castor californicus treated as unknown for the classification phase of the analysis, and the second CVA categorized all three species individually into a priori categories. The classification stage of the CVA was used to determine the accuracy for individual specimens to be assigned into species by the models. Classification occurred in two steps: group classifications and cross-validation, where specimens were excluded from the model and reevaluated using the remaining specimens in the analysis. CVA were run in IBM SPSS Statistics 26.0 and shapes associated with CV scores visualized with tpsRegr (Rohlf, 2011).
The hierarchical cluster analysis was run to examine the phenetic similarity between specimens based on their morphology, without the need for a priori grouping of species. The cluster analysis was run in IBM SPSS Statistics 26.0 using an unweighted paired group means analysis method (UPGMA) and average algorithm with partial warp scores and uniform component scores as variables.
Postcranial Analyses
The analysis of postcranial material used a total of 59 individuals (Appendix 3). Modern specimens included 30 Castor canadensis and two C. fiber. Fossils included 26 Pliocene-aged specimens identified as C. californicus and one Pleistocene-aged Castor sp. 64 postcranial characteristics were recorded where possible, measured features (following Samuels and Van Valkenburgh, 2008) included total lengths of bones, midshaft diameters, prominent articular dimensions, and muscle attachments (Table 3). Measurements were collected in millimeters (to 0.01 mm) using Mitutoyo digital calipers.
Measurement data were input to IBM SPSS Statistics 26.0 where descriptive statistics were run for each species. The analysis computed mean, standard deviation, and minimum and maximum values for the measurements of each species. From those data, coefficients of variation were also calculated for each measurement across species. Coefficients of variation compare the range of variation seen within groups (Sokal and Braumann, 1980; Cope and Lacy, 1995; Emery-Wetherell and Davis, 2018), in this case species were compared for various postcranial measurements to determine if the level of variation in Castor calfornicus fits within the level of intraspecific variation for C. canadensis. Only species measurements with more than three specimens were used to calculate coefficients of variation, as fewer than three would represent insufficient sampling. Coefficients of variation were corrected for small sample size bias using the equation previously used in Sokal and Braumann (1980). In addition to the descriptive statistics, an analysis of variance (ANOVA) was run for each of the measurements allowing assessment of differences in mean values between species groups.
Institutional Abbreviations
ETMNHV, East Tennessee State University Museum of Natural History, Zoology Collection (Johnson City, TN, USA); FMNH, Field Museum of Natural History (Chicago, IL, USA); HAFO, Hagerman Fossil Beds National Monument (Hagerman, Idaho, USA); IMNH, Idaho Museum of Natural History (Pocatello, Idaho, USA); LACM, Natural History Museum of Los Angeles County (Los Angeles, California, USA); MVZ, University of California Berkley Museum of Vertebrate Zoology (Berkley, California, USA); UCLA, University of California Los Angeles, Donald R. Dickey Collection (Los Angeles, California, USA); UF, Florida Museum of Natural History (Gainesville, Florida, USA); UOMNCH, University of Oregon Museum of Natural and Cultural History (Eugene, Oregon, USA); USNM, Smithsonian Institution National Museum of Natural History (Washington D.C., USA); UWBM, University of Washington Burke Museum (Seattle, Washington, USA).
RESULTS
Cranial Analyses
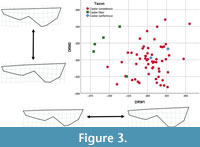 Relative warp analysis. Relative warp analyses were run for each view of the cranium and dentary. The relative warp scores for the dorsal view produced seven significant warps (Eigenvalue >1), explaining 81.5% of the observed shape variation. Dorsal relative warps 1 and 2 showed separation of Castor fiber from overlapping C. canadensis and C. californicus (Figure 3). Dorsal relative warp 1 (DRW1) explained 27.64% of the variation, with C. fiber clustered with negative scores whereas C. californicus clustered with positive scores. Castor canadensis clustered near zero, spanning both positive and negative values. Positive DRW1 scores are associated with shorter nasals, wider occiput, and posteriorly positioned orbit. Negative DRW1 scores are associated with elongated nasals, narrow occiput, and anteriorly positioned orbit. DRW2 explained 17.03% of the variation. Both C. fiber and C. californicus had positive scores (except for one C. fiber), whereas C. canadensis displayed a wide range of both positive and negative scores. Positive DRW2 scores are associated with shorter rostrum, narrow posterior cranium, and wider zygomatic arches. Negative DRW2 scores are associated with elongated rostrum, wider occiput, and narrow zygomatic arches.
Relative warp analysis. Relative warp analyses were run for each view of the cranium and dentary. The relative warp scores for the dorsal view produced seven significant warps (Eigenvalue >1), explaining 81.5% of the observed shape variation. Dorsal relative warps 1 and 2 showed separation of Castor fiber from overlapping C. canadensis and C. californicus (Figure 3). Dorsal relative warp 1 (DRW1) explained 27.64% of the variation, with C. fiber clustered with negative scores whereas C. californicus clustered with positive scores. Castor canadensis clustered near zero, spanning both positive and negative values. Positive DRW1 scores are associated with shorter nasals, wider occiput, and posteriorly positioned orbit. Negative DRW1 scores are associated with elongated nasals, narrow occiput, and anteriorly positioned orbit. DRW2 explained 17.03% of the variation. Both C. fiber and C. californicus had positive scores (except for one C. fiber), whereas C. canadensis displayed a wide range of both positive and negative scores. Positive DRW2 scores are associated with shorter rostrum, narrow posterior cranium, and wider zygomatic arches. Negative DRW2 scores are associated with elongated rostrum, wider occiput, and narrow zygomatic arches.
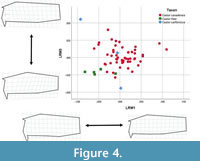 Relative warp scores for the lateral view produced eight significant warps, explaining 92.73% of the observed shape variation. Relative warps 1 and 3 showed (good?) separation of Castor fiber and C. canadensis (Figure 4). Lateral relative warp 1 (LRW1) explained 45.97% of the variation, with C. fiber having negative scores, C. californicus near zero, and C. canadensis displaying both positive and negative scores. Negative LRW1 scores are associated with elongated nasals and dorsoventrally shorter occiput. Positive LRW1 scores are associated with shorter nasals and dorsoventrally deeper occiput. LRW3 explained 16.57% of the variation, with C. fiber having exclusively negative scores whereas C. canadensis and C. californicus showed widespread distribution across positive and negative scores. Negative LRW3 scores are associated with the anteroventral extension of the nasals and dorsoventrally shallower occiput. Positive LRW3 scores are associated with shorter nasals and dorsoventrally deeper occiput.
Relative warp scores for the lateral view produced eight significant warps, explaining 92.73% of the observed shape variation. Relative warps 1 and 3 showed (good?) separation of Castor fiber and C. canadensis (Figure 4). Lateral relative warp 1 (LRW1) explained 45.97% of the variation, with C. fiber having negative scores, C. californicus near zero, and C. canadensis displaying both positive and negative scores. Negative LRW1 scores are associated with elongated nasals and dorsoventrally shorter occiput. Positive LRW1 scores are associated with shorter nasals and dorsoventrally deeper occiput. LRW3 explained 16.57% of the variation, with C. fiber having exclusively negative scores whereas C. canadensis and C. californicus showed widespread distribution across positive and negative scores. Negative LRW3 scores are associated with the anteroventral extension of the nasals and dorsoventrally shallower occiput. Positive LRW3 scores are associated with shorter nasals and dorsoventrally deeper occiput.
The relative warp scores for the ventral view produced six significant warps, explaining 83.52% of the observed shape variation (Table 4). None of the six relative warps showed clear separation of species in morphospace and are therefore not described further here.
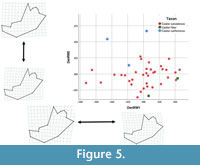 The relative warp scores for the dentary produced eleven significant warps, explaining 92.87% of the observed shape variation. Relative warps 1 and 2 showed clear separation of groups within morphospace (Figure 5). Dentary relative warp 1 (DenRW1) explained 27.71% of the variation, with Castor fiber clustering in positive scores, C. californicus in negative and low positive scores, and C. canadensis spanning both positive and negative scores. Negative DenRW1 scores are associated with a posterior placement of the anterior margin of the pterygoid insertion as well as larger coronoid and angular processes. Positive DenRW1 scores are associated with the anterior placement of the anterior margin of the pterygoid insertion as well as smaller coronoid and angular processes. DenRW2 explained 14.62% of the variation, with C. fiber displaying negative scores, C. californicus positive scores, and C. canadensis both negative and positive scores clustering around zero. Positive DenRW2 scores are associated with a widened area between the mandibular condyle and angular process, a posterior widening of the ascending ramus (from the coronoid process to the angular process), and an elevated anteroventral border of the incisor alveolus. Negative DenRW2 scores are associated with a narrowed area between the mandibular condyle and angular process, a posterior constriction of the ascending ramus (between the coronoid process to the angular process), and a ventrally depressed anteroventral border of the incisor alveolus.
The relative warp scores for the dentary produced eleven significant warps, explaining 92.87% of the observed shape variation. Relative warps 1 and 2 showed clear separation of groups within morphospace (Figure 5). Dentary relative warp 1 (DenRW1) explained 27.71% of the variation, with Castor fiber clustering in positive scores, C. californicus in negative and low positive scores, and C. canadensis spanning both positive and negative scores. Negative DenRW1 scores are associated with a posterior placement of the anterior margin of the pterygoid insertion as well as larger coronoid and angular processes. Positive DenRW1 scores are associated with the anterior placement of the anterior margin of the pterygoid insertion as well as smaller coronoid and angular processes. DenRW2 explained 14.62% of the variation, with C. fiber displaying negative scores, C. californicus positive scores, and C. canadensis both negative and positive scores clustering around zero. Positive DenRW2 scores are associated with a widened area between the mandibular condyle and angular process, a posterior widening of the ascending ramus (from the coronoid process to the angular process), and an elevated anteroventral border of the incisor alveolus. Negative DenRW2 scores are associated with a narrowed area between the mandibular condyle and angular process, a posterior constriction of the ascending ramus (between the coronoid process to the angular process), and a ventrally depressed anteroventral border of the incisor alveolus.
Overall, Castor fiber and C. canadensis showed minimal overlap within the relative warp analyses. Castor californicus plotted either within or near the range of C. canadensis and consistently fell outside of the plotted range for C. fiber in morphospace.
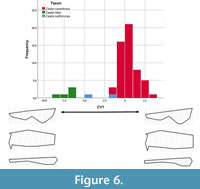 Stepwise canonical variate analyses. The first cranial stepwise model, with Castor californicus categorized as unknown, included nine of the 21 partial warps and showed good separation of groups (Wilk’s lambda = 0.173, F (1,56) = 24.360, p < 0.001). The analysis yielded one canonical variate which accounted for 100% of the variance in the dataset (Table 5). Canonical variate 1 (CV1) showed separation of C. fiber with high negative scores and C. canadensis with both positive and negative scores distributed around 0, whereas C. californicus had lower negative scores (Figure 6). Positive CV1 scores are associated with shorter nasals, posteromedial position of the orbit, and wider occiput.
Stepwise canonical variate analyses. The first cranial stepwise model, with Castor californicus categorized as unknown, included nine of the 21 partial warps and showed good separation of groups (Wilk’s lambda = 0.173, F (1,56) = 24.360, p < 0.001). The analysis yielded one canonical variate which accounted for 100% of the variance in the dataset (Table 5). Canonical variate 1 (CV1) showed separation of C. fiber with high negative scores and C. canadensis with both positive and negative scores distributed around 0, whereas C. californicus had lower negative scores (Figure 6). Positive CV1 scores are associated with shorter nasals, posteromedial position of the orbit, and wider occiput.
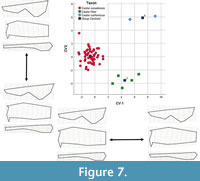 The second cranial stepwise model, with all specimens categorized a priori, included 11 of the 21 partial warps and showed significant separation of groups (Wilk’s lambda 0.056, F(1,56) = 13.198, p < 0.001). This analysis yielded two canonical variates, which accounted for 100% of the variance in the dataset (Table 6). CV1 accounted for 63.5% of the variance and showed good separation of taxa (Figure 7); Castor fiber and C. californicus both had positive CV1 scores, whereas C. canadensis clustered around 0, with both positive and negative scores. Negative CV1 scores are associated with shorter nasals, wider occiput, and wider premaxillae. CV2 accounted for 36.5% of the variance and additionally showed good separation of species; Castor fiber had negative scores, C. californicus had high positive scores, and C. canadensis had scores close to 0, with some in both positive and negative scores. Positive CV2 scores associated are with shorter nasals and wider occiput.
The second cranial stepwise model, with all specimens categorized a priori, included 11 of the 21 partial warps and showed significant separation of groups (Wilk’s lambda 0.056, F(1,56) = 13.198, p < 0.001). This analysis yielded two canonical variates, which accounted for 100% of the variance in the dataset (Table 6). CV1 accounted for 63.5% of the variance and showed good separation of taxa (Figure 7); Castor fiber and C. californicus both had positive CV1 scores, whereas C. canadensis clustered around 0, with both positive and negative scores. Negative CV1 scores are associated with shorter nasals, wider occiput, and wider premaxillae. CV2 accounted for 36.5% of the variance and additionally showed good separation of species; Castor fiber had negative scores, C. californicus had high positive scores, and C. canadensis had scores close to 0, with some in both positive and negative scores. Positive CV2 scores associated are with shorter nasals and wider occiput.
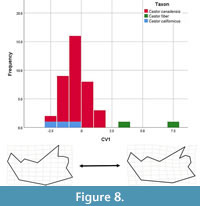 The dentary stepwise model, which initially had Castor californicus categorized as unknowns, included six of the 11 partial warps and showed some separation of groups (Wilk’s lambda = 0.351, F (1, 40) = 9.246, p < 0.00). The analysis yielded one canonical variate, which accounted for 100% of the variance in the dataset (Table 7). CV1 showed good separation of groups (Figure 8); C. fiber had high positive scores, C. californicus overlapped with C. canadensis, but only as negative and near 0 scores, and C. canadensis centered just negative of zero and contained positive and negative scores. Negative CV1 scores are associated with anterior positioned anterior margin of angular process, more obtuse curvature between coronoid and condylar processes, and anteriorly oriented coronoid process.
The dentary stepwise model, which initially had Castor californicus categorized as unknowns, included six of the 11 partial warps and showed some separation of groups (Wilk’s lambda = 0.351, F (1, 40) = 9.246, p < 0.00). The analysis yielded one canonical variate, which accounted for 100% of the variance in the dataset (Table 7). CV1 showed good separation of groups (Figure 8); C. fiber had high positive scores, C. californicus overlapped with C. canadensis, but only as negative and near 0 scores, and C. canadensis centered just negative of zero and contained positive and negative scores. Negative CV1 scores are associated with anterior positioned anterior margin of angular process, more obtuse curvature between coronoid and condylar processes, and anteriorly oriented coronoid process.
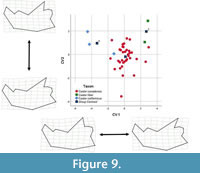 The second dentary stepwise model, with all species categorized a priori, included three of the 11 partial warps and showed significant separation of groups (Wilk’s lambda = 0.329, F(2,40) = 8.658, p < 0.00). This analysis yielded two canonical variates, which accounted for 100% of the variance in the dataset (Table 8). CV1 accounted for 80.5% of the variance and showed separation of Castor californicus with negative scores from C. fiber with positive scores, and C. canadensis with scores centered around 0, with some spread into positive and negative scores (Figure 9). Positive CV1 scores are associated with posterior positioning of coronoid process, shorter condylar process, dorsal positioning of angular process, and anteriorly extended ascending ramus. CV2 accounted for 19.5% of the variance and showed some separation of groups, C. californicus had positive scores and near 0 values, C. fiber had highly positive values, and C. canadensis clustered near 0 in both positive and negative scores. Positive CV2 scores associated with posterior positioning of the ascending ramus, ventral expansion of the angular process, and anteriorly oriented coronoid process.
The second dentary stepwise model, with all species categorized a priori, included three of the 11 partial warps and showed significant separation of groups (Wilk’s lambda = 0.329, F(2,40) = 8.658, p < 0.00). This analysis yielded two canonical variates, which accounted for 100% of the variance in the dataset (Table 8). CV1 accounted for 80.5% of the variance and showed separation of Castor californicus with negative scores from C. fiber with positive scores, and C. canadensis with scores centered around 0, with some spread into positive and negative scores (Figure 9). Positive CV1 scores are associated with posterior positioning of coronoid process, shorter condylar process, dorsal positioning of angular process, and anteriorly extended ascending ramus. CV2 accounted for 19.5% of the variance and showed some separation of groups, C. californicus had positive scores and near 0 values, C. fiber had highly positive values, and C. canadensis clustered near 0 in both positive and negative scores. Positive CV2 scores associated with posterior positioning of the ascending ramus, ventral expansion of the angular process, and anteriorly oriented coronoid process.
The first cranial CVA classification resulted in 100% correct classification of individuals with 98.3% correct classification when cross-validated (Table 9). One specimen of Castor canadensis (MVZ 183809) was misclassified as C. fiber in cross-validation. Castor californicus, which was assigned as unknown for this cranial CVA, included specimens classified as both C. fiber and C. californicus. Those classifications had low conditional probabilities, which suggests low likelihood of those fossil specimens belonging to those species (Table 10).
The second cranial CVA classification, in which all species were assigned a priori, resulted in 100% correct classification of individuals with 98.3% correct classification when cross validated (Table 11). One specimen of Castor californicus (UF 225200) was classified as C. canadensis in cross-validation. The classification of that specimen had a low conditional probability, which suggests low likelihood of belonging to C. canadensis (Table 12).
Dentary CVA classification, where Castor californicus was assigned as unknown, resulted in 100% correct classification of individuals with 97.3% correct classification when cross-validation (Table 13). Castor californicus specimens were classified as C. canadensis and one specimen of C. fiber (USNM 174938) was misclassified as C. canadensis in cross-validation. Conditional probabilities for C. californicus indicate those specimens fall within the range of variation for C. canadensis (Table 14).
Dentary CVA classification in which all species were assigned a priori resulted in 95% correct classification of individuals with 92.5% correct classification when cross-validated (Table 15). In this analysis, some specimens were misclassified between groups. One specimen of Castor canadensis (LACM 93330) was classified as C. fiber in both the original classification and cross validation. One specimen of C. fiber (USNM 174938) was misclassified as C. canadensis in cross validation and one specimen of C. californicus (USNM 26154) was classified as C. canadensis. Conditional probabilities indicate those specimens fall within the range of variation for the assigned species (Table 16).
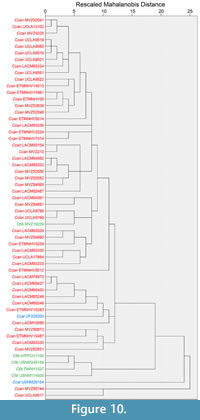 Cluster analysis. The cluster analysis of cranial data resulted in some separation between species (Figure 10). All Castor fiber specimens grouped together, except for one specimen (MVZ 19229), which clustered with C. canadensis. This specimen consistently clustered separately from the other C. fiber specimens, as shown in the relative warp graphs produced from the RWA. This separation could be attributed to MVZ 19229 having distinct morphological differences compared to the other C. fiber specimens included in the analysis, including an elongated rostrum, narrow zygomatic arches, and widened posterior cranium.
Cluster analysis. The cluster analysis of cranial data resulted in some separation between species (Figure 10). All Castor fiber specimens grouped together, except for one specimen (MVZ 19229), which clustered with C. canadensis. This specimen consistently clustered separately from the other C. fiber specimens, as shown in the relative warp graphs produced from the RWA. This separation could be attributed to MVZ 19229 having distinct morphological differences compared to the other C. fiber specimens included in the analysis, including an elongated rostrum, narrow zygomatic arches, and widened posterior cranium.
 Nearly all North American Castor specimens grouped together, with C. californicus nested within the C. canadensis cluster (Figure 10). Castor fiber specimens clustered together, except for MVZ 19229, which grouped apart from other specimens of the species in other analyses. An outgroup, formed by three specimens, formed outside of the C. canadensis and C. fiber clusters. Those specimens included two C. canadensis (MVZ 80744 and UCLA 9517) and one C. californicus (USNM 26154). Uniform components and partial warp scores of those three outlier specimens showed no clear indication of similarities or extreme variation in scores which might separate those specimens from the other C. canadensis group.
Nearly all North American Castor specimens grouped together, with C. californicus nested within the C. canadensis cluster (Figure 10). Castor fiber specimens clustered together, except for MVZ 19229, which grouped apart from other specimens of the species in other analyses. An outgroup, formed by three specimens, formed outside of the C. canadensis and C. fiber clusters. Those specimens included two C. canadensis (MVZ 80744 and UCLA 9517) and one C. californicus (USNM 26154). Uniform components and partial warp scores of those three outlier specimens showed no clear indication of similarities or extreme variation in scores which might separate those specimens from the other C. canadensis group.
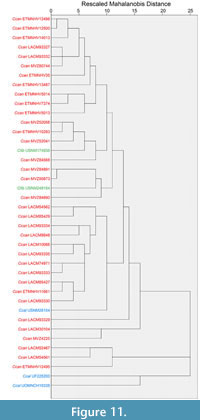
The cluster analysis of dentary data resulted in two prominent groupings (Figure 11). All Castor canadensis grouped together, with C. fiber specimens (USNM 174938 and USNM 248154) grouped within C. canadensis specimens. An early Pleistocene aged C. californicus (and a specimen previously referred to C. accessor) clustered together, forming the outgroup from the extant species.
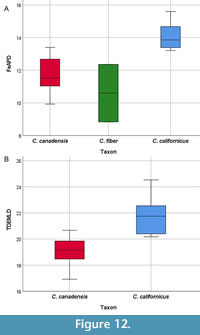 Postcranial Analysis
Postcranial Analysis
Descriptive statistics and ANOVA. Castor californicus had significantly larger mean values than C. canadensis for most variables in this analysis (Table 17). Maximum and minimum range values show C. californicus has substantial overlap in size when compared to C. canadensis. Comparisons to C. fiber were limited due to inadequate sampling.
Postcranial elements with little overlap in range values include FeAPD and TDEMLD (Figure 12). Castor californicus had a broad femur anteroposterior diameter (FeAPD) compared to C. canadensis. Castor californicus also has a wider mediolateral diameter on the distal end of the tibia (TDEMLD) than C. canadensis.
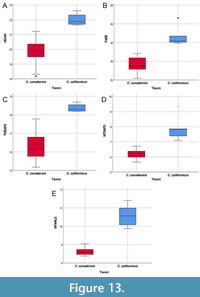 Castor californicus and C. canadensis do not overlap in several measurements (Figure 13). Castor californicus has a wider humeral distal articular width (HDAW), greater anteroposterior diameter of the third metatarsal (MT3APD), and greater mediolateral diameter of the fourth metatarsal (MT4MLD) than C. canadensis. Castor californicus also has an anteroposteriorly broader distal end of the tibia (TDEAPD) and greater femoral epicondylar breadth (FeEB) than C. canadensis.
Castor californicus and C. canadensis do not overlap in several measurements (Figure 13). Castor californicus has a wider humeral distal articular width (HDAW), greater anteroposterior diameter of the third metatarsal (MT3APD), and greater mediolateral diameter of the fourth metatarsal (MT4MLD) than C. canadensis. Castor californicus also has an anteroposteriorly broader distal end of the tibia (TDEAPD) and greater femoral epicondylar breadth (FeEB) than C. canadensis.
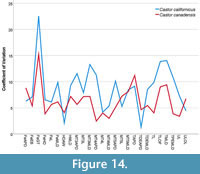
Coefficients of variation. Coefficients of variation were calculated for measurements with more than three samples per species, which were sample size corrected included with descriptive statistics and ANOVA results (Table 17). Species overall showed high levels of variation within postcranial elements (Figure 14).
DISCUSSION AND CONCLUSIONS
 The earliest occurrence of the genus Castor in North America is from the late Miocene, as represented by Castor californicus (Samuels and Zancanella, 2011). Although previously described as a different species from the extant North American beaver C. canadensis, morphological similarities between Miocene to early Pleistocene-aged fossil and extant North American beavers warrant a re-evaluation of the fossil taxa to determine if C. californicus is distinct from C. canadensis. Here we present a morphological evaluation between the Miocene to early Pleistocene-aged C. californicus and the late Pleistocene-aged and extant C. canadensis.
The earliest occurrence of the genus Castor in North America is from the late Miocene, as represented by Castor californicus (Samuels and Zancanella, 2011). Although previously described as a different species from the extant North American beaver C. canadensis, morphological similarities between Miocene to early Pleistocene-aged fossil and extant North American beavers warrant a re-evaluation of the fossil taxa to determine if C. californicus is distinct from C. canadensis. Here we present a morphological evaluation between the Miocene to early Pleistocene-aged C. californicus and the late Pleistocene-aged and extant C. canadensis.
Morphological Similarities
Castor canadensis shows high levels of variation in skull morphology. The geometric morphometric analysis all resulted in widespread distribution of the species within morphospace. It has been noted in previous literature that C. canadensis is highly variable (Stefen, 2009), as at one time it was separated into subspecies based on phenotypic characteristics and regional distribution across North America (Rhoads, 1898; Jenkins and Buscher, 1979; Long, 2000). Specimens of C. canadensis used in this study here were collected from across North America (Appendix 1 and Appendix 2); therefore, the resulting variation seen within the species is a good representation of the variation seen across the continent in the recent past and present. Some of the variation seen in C. canadensis may also be partially attributed to ontogenetic changes in skull shape within older adults (greater than 5 years old) after reaching sexual maturity (Segura et al., 2023), as has been previously noted with the sagittal crest of extant beavers (Hinze, 1950). Castor fiber may also have similarly high morphological variability in the skull, suggested by the wide separation of MVZ 19229 from other specimens across analyses, but limited sampling here precludes rigorous evaluation of that possibility. Prior work has also documented high intraspecific variability in the dentition of both C. canadensis and C. fiber (Stefen, 2009), with much of the variation attributable to ontogenetic changes.
Castor californicus consistently plots within the observed range of variation of C. canadensis across both relative warp and canonical variate analyses of the cranium (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9). Within the classification stage of both the cranial and dentary CVA, C. californicus primarily categorized with C. canadensis (Table 10, Table 11, Table 12, Table 13, Table 14, Table 15, Table 16). Castor californicus clustered within C. canadensis in the cranial hierarchical cluster analysis (Figure 10), indicating strong, shared morphological similarities between the two species.
This suggests cranial morphological features are more similar in Castor canadensis and C. californicus than either is with C. fiber. Across relative warp analyses, C. fiber consistently plots separate from C. canadensis and C. californicus, highlighting the distinct morphological differences between extant beaver species (Troszyński, 1975; Flynn and Jacobs, 2008; Danilov et al., 2011; Kauhala and Timonen, 2016). Previous studies on the mitochondrial DNA of C. canadensis and C. fiber show that the two species last shared a common ancestor as early as 7.5 m.y.a. (Horn et al., 2011). This timing corresponds with the oldest known record of C. californicus in North America from the Rattlesnake Formation in Oregon (Samuels and Zancanella, 2011).
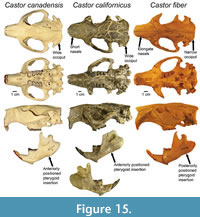 Cranial morphological similarities between Castor canadensis and C. californicus broadly include shorter nasals, wider occiput, and posteriorly positioned orbits when compared to C. fiber (Figure 15). Dentaries of C. canadensis and C. californicus both display anterior placement of the anterior mar gin of the pterygoid insertion and greater spread of the posterior processes (coronoid, condylar, angular) (Figure 15). These findings suggest both North American species have higher mechanical advantage and potentially larger pterygoid muscles than C. fiber, and a broader nuchal region which represents the insertion area of the head-stabilizing neck muscles.
Cranial morphological similarities between Castor canadensis and C. californicus broadly include shorter nasals, wider occiput, and posteriorly positioned orbits when compared to C. fiber (Figure 15). Dentaries of C. canadensis and C. californicus both display anterior placement of the anterior mar gin of the pterygoid insertion and greater spread of the posterior processes (coronoid, condylar, angular) (Figure 15). These findings suggest both North American species have higher mechanical advantage and potentially larger pterygoid muscles than C. fiber, and a broader nuchal region which represents the insertion area of the head-stabilizing neck muscles.
Studied specimens of Castor californicus fit largely within the wide range of morphological variation seen in C. canadensis postcrania (Table 17). The postcranial analysis of C. fiber is limited due to inadequate sampling, precluding detailed comparisons to either North American species. The postcranial elements, which were measured, did document differences from those of C. canadensis, though not enough data were collected to confidently evaluate morphological differences between these species.
Castor canadensis and C. californicus show high levels of variation in postcranial morphology, as evidenced by coefficients of variation, which were also highly variable for both species even with sample size corrections. Previous studies of C. californicus described it as closely resembling the extant C. canadensis but larger in size (Stirton, 1935; Shotwell, 1970). High intraspecific variability in both taxa and substantial overlap in measurements suggests that differentiating these species based on size is not reliable. Other studies have found size is not generally a reliable metric for identifying and distinguishing species (Koch, 1986; Stefen, 2010; Emery-Wetherell and Davis, 2018; Martin et al., 2018). Given the degree of morphological variation observed in extant C. canadensis (data presented here and for dentition by Stefen, 2009), size alone should not be used to distinguish C. californicus from C. canadensis. Although certain postcranial features showed differences in range between C. californicus and C. canadensis, most C. californicus elements measured in the study fit within the observed range of variation for C. canadensis.
Morphological Distinctions
Castor canadensis and C. californicus show some morphological distinctions within the dentary and postcrania. Most specimens of C. californicus fell outside of the range of C. canadensis, in the relative warp analysis of the dentary (Figure 5). The hierarchical cluster analysis of the dentary showed greater separation between species, with most specimens of C. californicus forming the outgroup from C. canadensis and C. fiber (Figure 11), reflecting morphological differences from the extant species.
Semi-aquatic rodents exhibit a wide range of osteological specializations for their lifestyles (Howell, 1930; Gingerich, 2003; Samuels and Van Valkenburgh, 2008; Calede, 2022). Postcranial characteristics include shortening of the femur, robust limb elements, enlarged muscle attachment sites for the hind limb, and elongated hindfoot to aid in movement through the water (Samuels and Van Valkenburgh, 2008). These characteristics hold true for Castor canadensis and C. californicus (Table 17, Appendix 3, Figure 12 and Figure 13).
The femur anteroposterior diameter (FeAPD) in Castor canadensis is low, exhibiting an extreme flattening of the femur while C. californicus exhibits a more robust anteroposterior diameter than C. canadensis. The femoral epicondylar breadth (FeEB) is wider in C. californicus than in C. canadensis. A wider FeEB would allow for greater muscle attachments to help with swimming (Samuels and Van Valkenburgh, 2008) and would be expected in an animal of larger body mass. The anteroposterior and mediolateral diameters at the distal end of the tibia (TDEAPD and TDEMLD) are slightly wider in C. californicus than in C. canadensis, suggesting that C. californicus had more robust articular distal ends on the hindlimbs than C. canadensis. In the pes, the anteroposterior diameter of the third metatarsal (MT3APD) and mediolateral diameter of the fourth metatarsal (MT4MLD) are both more robust in C. californicus than C. canadensis. Increasing the size of the pes can aid in enlarging the surface area of the hindfoot for amplified propulsion through the water (Samuels and Van Valkenburgh, 2008), which would also be expected at larger body mass. The articular width at the distal end of the humerus (HDAW) is wider in C. californicus than C. canadensis, suggesting that C. californicus had more robust articular distal ends on the forelimbs than C. canadensis, which would accommodate larger size and may have allowed a wider range of motions used in both swimming and digging.
Ecological Role and Taxonomic Validity of Castor californicus
Overall, analyses employed here have documented strong morphological similarity between the late Miocene to early Pleistocene-aged beaver Castor californicus and the extant North American beaver C. canadensis. Both taxa show high degrees of variability in size, and substantial overlap in both skull (cranium and dentary) and postcranial size and shape. Subtle, but notable morphological differences in the skull of C. californicus include a wider occiput and posteriorly positioned orbit (Figure 15). Dentary morphology in C. californicus displays distinctly wider separation between condylar and angular processes and anteroventrally depressed incisor alveolus (Figure 15). Postcranial morphology of C. californicus is distinguished from extant species of beaver by less dorsoventrally flattened the femur, greater robustness of hindlimb elements, and greater metatarsal widths. These differences may be attributable to body size and allometry, but do not likely represent substantial differences in function of either the cranial or postcranial skeleton.
Further comparisons between beaver species are necessary for a rigorous evaluation of the taxonomic validity of Castor californicus. Detailed examination of dental dimensions, occlusal patterns, and how teeth change ontogenetically would supplement the findings of this study. Additionally, including more C. californicus, modern C. fiber, and Pleistocene-aged C. canadensis and C. fiber specimens will help provide a better representative sample for future analyses. When combined, that would allow more confidence in assessing the validity of species. Our findings are consistent with Castor californicus and C. canadensis representing chronospecies, with subtle differences in morphology because of anagenetic changes in beavers over the last 7 million years. That time interval includes dramatic changes in climate, floras, and faunas in North America and the rest of the world (e.g., Webb, 1977; Graham, 1999; Jacobs et al., 1999; Janis et al., 2002; Retallack, 2007; Edwards et al., 2010; Stromberg, 2011; Samuels and Hopkins, 2017; Westerhold et al., 2020), and thus some evolutionary changes within persisting lineages should not be surprising. Other well-studied rodents of North America have been interpreted similarly (anagenetic changes in a lineage, sometimes with recognizable chronospecies or subspecies). For example, muskrats (Ondatra zibethicus) are common in the fossil record and what were formerly considered distinct species are now treated as chrono-subspecies in a lineage that evolved greatly over the Pliocene and Pleistocene (Martin et al., 1996; Martin, 2019).
Regardless of taxonomic assignments of specimens, the strong morphological similarity of these two beaver species indicates they can be considered ecological analogs, having similar dietary and locomotor ecology. This is also what had been noted in prior ecomorphological studies of rodents, which inferred the ecology of Castor californicus to have been a semi-aquatic herbivore specialized for feeding on fibrous plant matter (Samuels and Van Valkenburgh, 2008; Samuels, 2009). Consequently, we can confidently infer the impacts of beavers on ecosystems in North America (i.e., tree-felling, dam building, watershed altering) to have been occurring since the late Miocene, at least 7 m.y.a. (Rybczynski, 2008; Samuels and Zancanella, 2011). Ongoing studies of beaver evolution and distribution will improve understanding of how both beavers and the ecosystems they inhabit have changed through time.
ACKNOWLEDGEMENTS
We would like to thank Dr. B. Schubert and Dr. A. Joyner at East Tennessee State University for their feedback and assistance throughout the project. We would also like to thank Dr. C. Stefen, Dr. J. Mead, and the other anonymous reviewers for their constructive feedback and revisions which significantly helped improve the manuscript. This project was supported by the Department of Geosciences at East Tennessee State University.
REFERENCES
Brazier, R.E., Puttock, A., Graham, H.A., Auster, R.E., Davies, K.H., and Brown, C.M.L. 2020. Beaver: Nature’s ecosystem engineers. WIREs Water, 8:1.
https://doi.org/10.1002/wat2.1494
Calede, J.J. 2022. The oldest semi-aquatic beaver in the world and a new hypothesis for the evolution of locomotion in Castoridae. Royal Society Open Science, 9(8):220926.
https://doi.org/10.1098/rsos.220926
Cope, D.A. and Lacy, M.G. 1995. Comparative application of the coefficient of variation and range-based statistics for assessing the taxonomic composition of fossil samples. Journal of Human Evolution, 29:549-579.
https://doi.org/10.1006/jhev.1995.1075
Danilov, P.I., Kanshiev, V.Y., and Fedorov, F.V. 2011. Differences of the morphology of the North American and Eurasian beavers in Karelia, pp. 50-54. In Sjöberg, G. and Ball, J.P. (eds.), Restoring the Eurasian beaver: 50 years of experience. Pensoft Publishers, Sofia, Bulgaria.
Edwards, E.J., Osborne, C.P., Strömberg, C.A.E., Smith, S.A., and Consortium, C.G. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science, 328:587-591.
https://doi.org/10.1126/science.1177216
Emery-Wetherell, M.M. and Davis, E.B. 2018. Dental measurements do not diagnose modern artiodactyl species: Implications for the systematics of Merycoidodontoidea. Palaeontologia Electronica, 21.2.23A:1-28.
https://doi.org/10.26879/748
Flynn, L.J. and Jacobs, L.L. 2008. Castoroidea, pp. 391-405. In Janis, C.M., Gunnell, G.F., and Uhen, M.D. (eds.), Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals. Cambridge University Press, Cambridge, UK.
Gibbard, P.L. and Head, M.J. 2010. The newly-ratified definition of the Quaternary system/period and redefinition of the Pleistocene series/epoch, and comparison of proposals advanced prior to formal ratification. Episodes Journal of International Geosciences, 33(3):152-158.
https://doi.org/10.18814/epiiugs/2010/v33i3/002
Gingerich, P.D. 2003. Land-to-sea transition in early whales: evolution of Eocene Archaeoceti (Cetacea) in relation to skeletal proportions and locomotion of living semiaquatic mammals. Paleobiology, 29:429-454.
https://doi.org/10.1666/0094-8373(2003)029<0429:LTIEWE>2.0.CO;2
Graham, A. 1999. Late Cretaceous and Cenozoic History of North American Vegetation (North of Mexico). Oxford University Press, New York.
https://doi.org/10.1093/oso/9780195113426.001.0001
Halley, D.J., Saveljev, A.P., and Rosell, F. 2020. Population and distribution of beavers Castor fiber and Castor canadensis in Eurasia. Mammal Review, 51:1-24.
https://doi.org/10.1111/mam.12216
Hay, O.P. 1927. The Pleistocene mammals of the Western Region of North America and its Vertebrated Animals. Carnegie Institute of Washington, Washington D.C.
Hinze, G. 1950. Der Biber: Körperbau und Lebensweise. Verbreitung und Geschichte. Akademie Verlag, Berlin, Germany.
Horn, S., Durka, W., Wolf, R., Ermala, A., Stubbe, A., Stubbe, M., and Hofreiter, M. 2011. Mitochondrial genomes reveal slow rates of molecular evolution and the timing of speciation in beavers (Castor), one of the largest rodent species. PLoS ONE, 6(1):e14622.
https://doi.org/10.1371/journal.pone.0014622
Howell, A.B. 1930. Aquatic mammals; their adaptations to life in the water. Dover Publications, New York.
https://doi.org/10.5962/bhl.title.6582
Hugueney, M. 1999. Family Castoridae, pp. 1-516. In Rössner, G.E. and Heissig, K. (eds.). The Miocene land mammals of Europe. Verlag Friedrich Pfeil, München, Germany.
Jacobs, B.F., Kingston, J.D., and Jacobs, L.L. 1999. The origins of grass-dominated ecosystems. Annals of the Missouri Botanical Garden, 86(2):590-643.
https://doi.org/10.2307/2666186
Janis, C.M., Damuth, J., and Theodor, J.M. 2002. The origins and evolution of the North American grassland biome: The story from the hoofed mammals. Palaeogeography, Palaeoclimatology, Palaeoecology, 177:183-198.
https://doi.org/10.1016/S0031-0182(01)00359-5
Jenkins, S.H. and Busher, P.E. 1979. Castor canadensis. Mammalian Species, 120:1-8.
https://doi.org/10.2307/3503787
Kauhala, K. and Timonen, P. 2016. Mitä majavien kallot kertovat? Suomen Riista, 62:7-18. (In Finnish, with English summary)
Kellogg, L. 1911. A fossil beaver from the Kettleman Hills, California. University of California Press, 6:401-402.
Koch, P.L. 1986. Clinal geographic variation in mammals: implications for the study of chronoclines. Paleobiology, 12(3):269-281.
https://doi.org/10.1017/S0094837300013774
Korth, W.W. 1994. The Tertiary record of rodents in North America. Springer, New York.
https://doi.org/10.1007/978-1-4899-1444-6
Korth, W.W. and Samuels, J.X. 2015. New rodent material from the John Day Formation (Arikareean, middle Oligocene to early Miocene) of Oregon. Annals of Carnegie Museum, 83(1):19-84.
https://doi.org/10.2992/007.083.0102
Kurtén, B. and Anderson, E. 1980. Pleistocene mammals of North America. Columbia University Press, New York.
Long, K. 2000. Beaver: a wildlife handbook. Johnson Books, Boulder, Colorado.
Martin, J.M., Mead, J.I., and Barboza, P.S. 2018. Bison body size and climate change. Ecology and Evolution, 8:4564-4574.
https://doi.org/10.1002/ece3.4019
Martin, L.D. 1989. Plio-Pleistocene rodents in North America. In series: Black, C.C. and Dawson, M.R. (eds.), Papers on fossil rodents in honor of Albert Elmer Wood, Science Series 33. Natural History Museum of Los Angeles County, Los Angeles, California.
Martin, L.D. and Bennett, D. 1977. The burrows of the Miocene beaver Palaeocastor, Western Nebraska, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology, 22:173-193.
https://doi.org/10.1016/0031-0182(77)90027-X
Martin, R.A. 2019. Body mass and correlated ecological variables in the North American muskrat lineage: evolutionary rates and the tradeoff of large size and speciation potential. Historical Biology, 31(5):631-643.
https://doi.org/10.1080/08912963.2017.1384474
Martin, R.A., Stewart, K., and Seymour, K. 1996. Dental evolution and size change in the North American muskrat: classification and tempo of a presumed phyletic sequence, pp. 431-457. Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals: Tributes to the Career of CS (Rufus) Churcher. University of Toronto Press, Toronto, Ontario.
Monteiro, L.R., Bonato, V., and dos Reis, S.F. 2005. Evolutionary integration and morphological diversification in complex morphological structures: mandible shape divergence in spiny rats (Rodentia, Echimyidae). Evolution Development, 7:429-439.
https://doi.org/10.1111/j.1525-142X.2005.05047.x
Retallack, G.J. 2007. Cenozoic paleoclimate on land in North America. The Journal of Geology, 115:271-294. https://doi.org/10.1086/512753
Rhoads, S.N. 1898. Contributions to a revision of the North American beavers, otters and fishers. Transactions of the American Philosophical Society, 19:417-439.
https://doi.org/10.2307/1005498
Robertson, R.A. and Shadle, A.R. 1954. Osteologic criteria of age in beavers. Journal of Mammalogy, 35:197-203.
https://doi.org/10.2307/1376033
Rosell, F., Bozer, O., Collen, P., and Parker, H. 2005. Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mammal Review, 35:248-276.
https://doi.org/10.1111/j.1365-2907.2005.00067.x
Rohlf, F.J. 2011. tpsRegr, shape regression, version 1.40. State University of New York at Stony Brook, Department of Ecology and Evolution.
Rohlf, F.J. 2015a. The tps series of software. Hystrix, the Italian Journal of Mammalogy, 26:1-4.
https://doi.org/10.4404/hystrix-26.1-11264
Rohlf, F.J. 2015b. tpsRelw: relative warps analysis. State University of New York at Stony Brook, Department of Ecology and Evolution.
Rohlf, F.J. 2021. tpsDig2, version 2.31. State University of New York at Stony Brook, Department of Ecology and Evolution.
Rybczynski, N. 2007. Castorid phylogenetics: Implications for the evolution of swimming and tree-exploitation in beavers. Journal of Mammalian Evolution, 14:1-35.
https://doi.org/10.1007/s10914-006-9017-3
Rybczynski, N. 2008. Woodcutting behavior in beavers (Castoridae, Rodentia): estimating ecological performance in modern and fossil taxon. Paleobiology, 34:389-402.
https://doi.org/10.1666/06085.1
Rybczynski, N., Ross, E.M., Samuels, J.X., and Korth, W.W. 2010. Re-evaluation of Sinocastor (Rodentia: Castoridae) with implications on the origin of modern beavers. PLoS ONE, 5:e13990.
https://doi.org/10.1371/journal.pone.0013990
Samuels, J.X. 2009. Cranial morphology and dietary habits of rodents. Zoological Journal of the Linnaean Society, 156: 864-888.
https://doi.org/10.1111/j.1096-3642.2009.00502.x
Samuels, J.X. and Hopkins, S.S.B. 2017. The impacts of Cenozoic climate and habitat changes on small mammal diversity of North America. Global and Planetary Change, 149:36-52.
https://doi.org/10.1016/j.gloplacha.2016.12.014
Samuels, J.X. and Van Valkenburgh, B. 2008. Skeletal indicators of locomotor adaptations in living and extinct rodents. Journal of Morphology, 269:1387-1411.
https://doi.org/10.1002/jmor.10662
Samuels, J.X. and Van Valkenburgh, B. 2009. Craniodental adaptations for digging in extinct burrowing beavers. Journal of Vertebrate Paleontology, 29:254-268.
https://doi.org/10.1080/02724634.2009.10010376
Samuels, J.X. and Zancanella, J. 2011. An early Hemphillian occurrence of Castor (Castoridae) from the Rattlesnake Formation of Oregon. Journal of Paleontology, 85:930-935.
https://doi.org/10.1666/11-016.1
Segura, V., Flores, D., and Deferrari, G. 2023. Comparison of skull growth in two ecosystem modifiers: beavers Castor canadensis (Rodentia: Castoridae) and muskrats Ondatra zibethicus (Rodentia: Cricetidae). Zoologischer Anzeiger, 304:61-72.
https://doi.org/10.1016/j.jcz.2023.03.004
Shotwell, J.A. 1970. Pliocene mammals of Southeast Oregon and adjacent Idaho. Bulletin of the Museum of Natural History, University of Oregon, Eugene, Oregon, 17:1-103.
Sokal, R.R. and Braumann, C.A. 1980. Significance tests for coefficients of variation and variability profiles. Systematic Zoology, 29:50-66.
https://doi.org/10.1093/sysbio/29.1.50
Stefen, C. 2009. Intraspecific variability of beaver teeth (Castoridae: Rodentia). Zoological Journal of the Linnean Society, 155(4):926-936.
https://doi.org/10.1111/j.1096-3642.2008.00467.x
Stefen, C. 2010. Morphometric considerations of the teeth of the palaeocastorine beavers Capacikala, Palaeocastor and “Capatanka”. Palaeontologia Electronica, 13(1):1-34.
https://palaeo-electronica.org/2010_1/191/index.html
Stirton, R.A. 1935. A review of the Tertiary beavers. University of California Press, Berkeley, California.
Strömberg, C.A.E. 2011. Evolution of grasses and grassland ecosystems. Annual Review of Earth and Planetary Sciences, 39:517-544.
https://doi.org/10.1146/annurev-earth-040809-152402
The NOW Community 2023. New and old worlds database of fossil mammals (NOW). Licensed under CC BY 4.0. Retrieved 05 July 2023 from https://nowdatabase.org/now/database/
http://doi.org/10.5281/zenodo.4268068
Troszyński, W. 1975. The main differences in the structure of the skull of both the Canadian beaver (Castor canadensis Kuhl) and the European beaver (Castor fiber Linnaeus). Przegląd Zoologiczny, 19:481-486.
Webb, S.D. 1977. A history of savanna vertebrates in the New World. Part I. North America. Annual Review of Ecology and Systematics, 8:355-380.
https://doi.org/10.1146/annurev.es.08.110177.002035
Westerhold, T., Marwan, N., Drury, A.J., Liebrand, D., Agnini, C., Anagnostou, E., Barnet, J.S.K., Bohaty, S.M., De Vleeschouwer, D., Florindo, F., Frederichs, T., Hodell, D.A., Holbourn, A.F., Kroon, D., Lauretano, V., Littler, K., Lourens, L.J., Lyle, M., Pälike, H., Röhl, U., Tian, J., Wilkens, R.H., Wilson, P.A., and Zachos, J.C. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science, 369(6509):1383-1387.
http://doi.org/10.1126/science.aba6853
Zakrzewski, R.J. 1969. The rodents from the Hagerman local fauna, Upper Pliocene of Idaho. Museum of Paleontology, University of Michigan, Ann Arbor, Michigan.

