 Unveiling the cheilostome bryozoan fauna of Daidokutsu submarine cave (Okinawa, Japan) over the last 7,000 years
Unveiling the cheilostome bryozoan fauna of Daidokutsu submarine cave (Okinawa, Japan) over the last 7,000 years
Article number: 28.1.a7
https://doi.org/10.26879/1433
Copyright Palaeontological Association, February 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 30 July 2024. Acceptance: 11 January 2025.
ABSTRACT
The study of marine bryozoans in Japan dates to the late 1800s, with research primarily focusing on Pleistocene and Recent faunas in shallow-water shelf habitats. This paper aims to address gaps in the study of Holocene bryozoan assemblages from cave habitats in Japan by examining the cheilostome bryozoan fauna of a sediment core (Core 19) spanning the last 7,000 years extracted from Daidokutsu cave, a submarine cave on Ie Island, Okinawa. The study describes and illustrates using scanning electron microscopy, 63 cheilostome bryozoan species, comprising 17 anascan-grade and 46 ascophoran-grade cheilostomes, and introduces three new genera and 30 new species, a significant proportion consistent with previous studies on other Japanese bryozoan faunas. Additionally, we transfer the family Crepidacanthidae from Mamilloporoidea to Adeonoidea. This research establishes a critical taxonomic foundation for future ecological studies aimed at understanding the responses of the bryozoan community in this cave habitat to environmental and climate changes through the Holocene. The inclusion of bryozoan data expands the multi-taxon approach that was previously focused on other groups of organisms (ostracods, molluscs, foraminifera), thus providing a more comprehensive understanding of the historical biodiversity and ecological dynamics of the region.
Emanuela Di Martino. Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università di Catania, Corso Italia 57, 95129, Catania, Italy. (Corresponding author) emanuela.dimartino@unict.it; ORCID: 0000-0002-3892-4036
Antonietta Rosso. Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Università di Catania, Corso Italia 57, 95129, Catania, Italy. rosso@unict.it; ORCID: 0000-0001-5565-9513
Paul D. Taylor. Natural History Museum, Cromwell Road, London, SW7 5BD, UK. p.taylor@nhm.ac.uk; ORCID: 0000-0002-3127-080X
Ruby W.T. Chiu School of Biological Sciences, and Swire Institute of Marine Science, The University of Hong Kong, Kadoorie Biological Sciences Building, Hong Kong, China. rubychiu@ymail.com; ORCID: 0000-0001-6663-9526
Kazuhiko Fujita. Department of Physics and Earth Sciences, Faculty of Science and Tropical Biosphere Research Center, University of the Ryukyus, Okinawa, 903-0213, Japan. fujitaka@sci.u-ryukyu.ac.jp; ORCID: 0000-0002-9833-007X
Akihisa Kitamura. Institute of Geosciences, Shizuoka University, Shizuoka, 422-8529, Japan. seakita@ipc.shizuoka.ac.jp; ORCID: 0000-0002-9928-3746
Moriaki Yasuhara. School of Biological Sciences, Area of Ecology and Biodiversity, Swire Institute of Marine Science, Institute for Climate and Carbon Neutrality, Musketeers Foundation Institute of Data Science, and State Key Laboratory of Marine Pollution, The University of Hong Kong, Kadoorie Biological Sciences Building, Hong Kong, China. yasuhara@hku.hk; ORCID: 0000-0003-0990-1764
Keywords: Taxonomy; new species; new genera; Holocene; Cheilostomatida
Final citation: Di Martino, Emanuela, Rosso, Antonietta, Taylor, Paul D., Chiu, Ruby W.T., Fujita, Kazuhiko, Kitamura, Akihisa, and Yasuhara, Moriaki. 2025. Unveiling the cheilostome bryozoan fauna of Daidokutsu submarine cave (Okinawa, Japan) over the last 7,000 years. Palaeontologia Electronica, 28(1):a7.
https://doi.org/10.26879/1433
palaeo-electronica.org/content/2025/5441-holocene-bryozoans-from-japan
Copyright: February 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
https://zoobank.org/6E7554EF-C09B-4860-AC2A-FA1A6FD53B03
INTRODUCTION
In his review of bryozoan diversity in Japanese waters, Hirose (2017) noted that the study of marine bryozoans in Japan dates to the H.M.S. Challenger expedition of 1873-1876 (Busk, 1884), while Ortmann (1890) produced the first major report on these benthic animals. As of 2008, over 1,000 bryozoan species were estimated to inhabit Japanese waters (Scholz et al., 2008). The most studied regions from north to south, include Hokkaido and the Kurile Islands, which host 185 species of cheilostome bryozoans (e.g., Mawatari, 1956; Mawatari and Mawatari, 1981; Grischenko et al., 2007); Tohoku (NE Japan), with 51 species on the Pacific side (e.g., Okada, 1928, 1929; Okada and Mawatari, 1937), and 27 species on the Sea of Japan side (e.g., Hayami, 1973); Tokyo Bay and Sagami Bay/Sea, with 179 species (e.g., Ortmann, 1890; Yanagi and Okada, 1918; Okada, 1934; Okada and Mawatari, 1935, 1936); the Noto Peninsula (Sea of Japan, middle Honshu) with 92 species (e.g., Mawatari, 1962; Sakakura, 1935); the Kii Peninsula (Pacific Coast, middle Honshu) with 144 species (e.g., Mawatari, 1952); the Ogasawara Islands, with 94 species (e.g., Silén, 1941); the Korea (Tsushima) Strait, between the East China Sea and the Sea of Japan, with 40 species (e.g., Okada, 1923); the Seto Inland Sea, with 14 species (e.g., Okada, 1934; Okada and Mawatari, 1938); Shikoku (Pacific coast), with four species (e.g., Ortmann, 1890; Okada and Mawatari, 1938); Kyushu, between the East China Sea and the southern part, with 63 species (e.g., Silén, 1941, 1947); and Okinawa, including Nansei Islands, with 26 species (e.g., Mawatari, 1987), and Sesoko Island, with 52 species (Dick and Grischenko, 2017). The most thoroughly investigated habitats are nearshore shelf regions, with fewer studies conducted in both intertidal sandy bottoms and deep waters (Hirose, 2017).
In their review of Pleistocene bryozoans from Japan, Dick et al. (2008) reported 334 cheilostomes from four main regions: the Boso Peninsula, on the Pacific side of central Honshu with c. 145 species (e.g., Sakakura, 1935, 1938; Arakawa, 1995); SW Hokkaido and N Honshu near the Tsugaru Strait, with c. 129 species (e.g., Kataoka, 1957; Hayami, 1971, 1973, 1975); the Noto Peninsula, with c. 26 species (Hayami, 1975); and Niigata, in central Honshu, Sea of Japan, and Kikai-jima Island in the Nansei Archipelago south of Kyushu, with c. 133 species (e.g., Kataoka, 1961). The palaeoenvironments for these occurrences are shallow-water shelf habitats.
Many habitats and specific time intervals remain unexplored, including submarine caves of Holocene age (the last c. 11,700 years). This paper aims to address this gap by describing and illustrating the taxonomy of 63 species of cheilostome bryozoans, found in a sediment core spanning the last 7,000 years, extracted from a dark, oligotrophic submarine cave on the east coast of Ie Island, Okinawa, Japan. We introduce three new genera and 30 new species. Descriptions and illustrations of the cyclostome species from the same samples will be presented in a separate work currently in preparation.
This study establishes the taxonomic foundation necessary for an ecological investigation into the ecosystem-scale response of the cave fauna to environmental and climate changes through most of the Holocene. Previous research in this cave has focused on ostracods, larger benthic foraminifera, and bivalves (Kase and Hayami, 1992; Hayami and Kase, 1993, 1996; Tabuki and Hanai, 1994, 1999; Kitamura et al., 2007a, b; Yamamoto et al., 2009, 2010; Omori et al., 2010; Chiu et al., 2016, 2017). The inclusion of bryozoan data will extend this multitaxon approach to a new group, for which substantial information on cave assemblages exists from other regions of the world (e.g., Harmelin, 1986, 1997, 2000, 2003; Rosso et al., 2013a, b, 2018a, 2019, 2021a).
MATERIAL AND METHODS
Material used for this study was sorted from 62 samples (Table 1), each corresponding to 1 cm section of a sediment core (Core 19) obtained from a depth of 29 m inside the Daidokutsu submarine cave (26.72ºN, 127.83ºE; see Yamamoto et al., 2009; Chiu et al., 2017, figure 1A, B) on the east coast of Ie Island, Okinawa, Japan (Kitamura et al., 2007). The sampling site of Core 19 was located approximately 32.5 m from the entrance of the cave (see Chiu et al., 2017, figure 1C, for more details on the sampling site). The core, which was 5 cm in diameter and 233 cm long, covers approximately the last 7,000 years based on radiocarbon dating (Yamamoto et al., 2009; Kitamura et al., 2013). The samples were wet-sieved using a 63 µm sieve, dried, and then dry-sieved using 150 µm, 250 µm, 500 µm, and 1 mm sieves. Bryozoans were picked from the > 500 µm size fractions.
Scanning electron microscopy was conducted on selected, uncoated specimens using low-vacuum scanning electron microscopes (SEMs) in back-scattered mode, a LEO VP-1455 and a JEOL IT500 at the Natural History Museum, London, UK (NHMUK), a Tescan Vega 2 LMU at the Department of Biological, Geological and Environmental Sciences of the University of Catania (Italy), and a Hitachi TM4000plus Tabletop at the Natural History Museum, University of Oslo, Norway.
Morphometric measurements were taken from SEM images using the image processing program ImageJ (available at https://imagej.nih.gov/). Each measurement is given in the text as the mean value plus or minus standard deviation, observed range, number of specimens used and total number of measurements made (the latter two values enclosed in parentheses).
Abbreviations for the measurements are: AvCyL, avicularium cystid length; AvCyW, avicularium cystid width; AvL, avicularium length; AvW, avicularium width; AvOpL, avicularium opesia length; AvOpW, avicularium opesia width; AZL, A-type zooid length; AZW, A-type zooid width; AZOpL, A-type zooid opesia length; AZOpW, A-type zooid opesia width; BZL, B-type zooid length; BZW, B-type zooid width; BZOpL, B-type zooid opesia length; BZOpW, B-type zooid opesia width; CryL, cryptocyst length; CryW, cryptocyst width; FoD, foramina diameter; FoN, foramina number; GymL, proximal gymnocyst length; KzL, kenozooid length; KzW, kenozooid width; LatGymW, lateral gymnocyst width; LyW, lyrula width; OpL, opesia length; OpW, opesia width; OpesL, opesiule length; OpesW, opesiule width; OvL, ooecium length; OvW, ooecium width; PeL, peristome length; ScuL, scutum length; ScuW, scutum width; SinL, orifice sinus length; SinW, orifice sinus width; VibrL, vibraculum length; VibrW, vibraculum width; ZL, zooid length; ZW, zooid width.
The synonymy lists provided are not exhaustive. They include the original paper in which the species was first described, and in cases where the species names are currently accepted under a different combination, any subsequent work first adopting the new nomenclature. They also encompass works that specifically mention the presence of the species in Japan, along with those providing SEM micrographs.
Three of us (EDM, AR and PDT) were responsible for the systematic part of this paper and are to be considered the authors for the new taxa described.
All specimens, including type and figured specimens, are deposited in the collections of the Palaeontological Museum (PMC) of the Department of Biological, Geological, and Environmental Sciences of the University of Catania (Italy), under the catalogue numbers reported for each species.
SYSTEMATIC PALAEONTOLOGY
Phylum BRYOZOA Ehrenberg, 1831
Class GYMNOLAEMATA Allman, 1856
Order CHEILOSTOMATIDA Busk, 1852
Superfamily THALAMOPORELLOIDEA Levinsen, 1902
Family THALAMOPORELLIDAE Levinsen, 1902
Genus THALAMOPORELLA Hincks, 1887
Thalamoporella sp. 1
Figure 1
Figured material. PMC EDM-Collection J.H.B.119a, sample 19206 (Figure 1A-B) and sample 19084 (Figure 1C-E); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
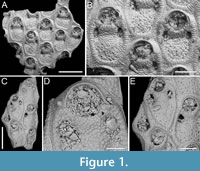 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, outlined by a thin rim of smooth gymnocyst, rectangular to hexagonal, elongate (mean ZL/ZW 1.49). Cryptocyst extensive, consisting of a raised, wide (27-64 µm), rope-like rim along proximal and lateral zooidal margins, convex, sloping towards the centre of zooid frontal surface between opesia and opesiules, slightly depressed and flat below opesiules, coarsely granular (granule diameter 14-29 µm), with a few, sparse pseudopores concentrated mainly in the proximal portion (pseudopore diameter 5-10 µm); pierced by two symmetrical, elliptical opesiules placed in the distal third of the zooid, just below the proximal margin of opesia. Gymnocyst reduced, visible distally and laterally to the opesia, usually more extensive laterally, reaching width of 43-76 µm; in a single zooid a preserved adoral tubercle of c. 33 µm in diameter. Opesia eye-shaped, occupying the distal third of zooidal length (mean OpL/ZL 0.30). A single heterozooid observed, lozenge-shaped, shorter than autozooid, symmetrical; cryptocyst coarsely granular, imperforate, lacking condyles or pivotal bar; distal margin triangular, opesia oval; sibling zooids not torqued towards heterozooid. Ovicells not seen.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, outlined by a thin rim of smooth gymnocyst, rectangular to hexagonal, elongate (mean ZL/ZW 1.49). Cryptocyst extensive, consisting of a raised, wide (27-64 µm), rope-like rim along proximal and lateral zooidal margins, convex, sloping towards the centre of zooid frontal surface between opesia and opesiules, slightly depressed and flat below opesiules, coarsely granular (granule diameter 14-29 µm), with a few, sparse pseudopores concentrated mainly in the proximal portion (pseudopore diameter 5-10 µm); pierced by two symmetrical, elliptical opesiules placed in the distal third of the zooid, just below the proximal margin of opesia. Gymnocyst reduced, visible distally and laterally to the opesia, usually more extensive laterally, reaching width of 43-76 µm; in a single zooid a preserved adoral tubercle of c. 33 µm in diameter. Opesia eye-shaped, occupying the distal third of zooidal length (mean OpL/ZL 0.30). A single heterozooid observed, lozenge-shaped, shorter than autozooid, symmetrical; cryptocyst coarsely granular, imperforate, lacking condyles or pivotal bar; distal margin triangular, opesia oval; sibling zooids not torqued towards heterozooid. Ovicells not seen.
Measurements (µm). ZL 569±43, 514-653 (2, 12); ZW 381±48, 272-449 (2, 12); OpL 172±12, 149-187 (2, 12); OpW 203±16, 166-224 (2, 12); OpesL 75±12, 56-92 (2, 12); OpesW 54±5, 47-62 (2, 12); AvL 453 (1, 1); AvW 262 (1, 1); AvOpL 207 (1, 1); AvOpW 147 (1, 1).
Remarks. Typically, vicarious avicularia in Thalamoporella have a prominently elevated rostrum with smooth lateral ‘wings’, and robust condyles or a complete crossbar. These characteristics, however, are absent in the single heterozooid observed, raising the possibility that it might be a kenozooid. Kenozooids, which are polymorphs that often form to fill geometric gaps within the colony, are common in species of Thalamoporella. Examples include the middle Miocene T. polygonalis Di Martino, Taylor and Portell, 2017 from Florida, and the Recent T. lanceolata Soule, Soule and Chaney, 1999 from Fiji and Samoa. The Recent Thalamoporella stapifera Levinsen, 1909 from Indonesia bears the closest resemblance in overall appearance and autozooid size range to the Okinawan species. However, the absence of more detailed features, such as the clear presence of vicarious avicularia, prevents further comparison and identification at the species level.
Family STEGINOPORELLIDAE Hincks, 1884
Genus STEGINOPORELLA Smitt, 1873
Steginoporella sp. 1
Figure 2
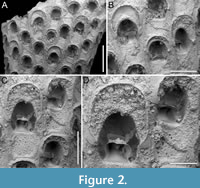 Figured material. PMC EDM-Collection J.H.B.120a, sample 19210; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.120a, sample 19210; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect, forming cylindrical branches of up to at least 15 longitudinal series of autozooids; maximum diameter of the available branch 3.95 mm. Zooids dimorphic (A- and B-type) with B-zooids on average 1.4 times larger than A-zooids and with a more extensive distal oral shelf (distal shelf length 84±15 µm [53-108 µm] in A-zooids vs 270±16 µm [240-285 µm] in B-zooids); A-zooids more common than B-zooids (AZ:BZ 3:1). All zooids elongate rectangular (mean AZL/AZW 1.66 and mean BZL/BZW 1.97) with arcuate distal and proximal borders, and finely granular cryptocyst occupying about half length of frontal surface, depressed, flat, pseudoporous (pseudopore diameter 9-14 µm). Opesia semielliptical, longer than wide (mean OpL/OpW 1.25 in both zooid types), delimited proximally by median process (c. 130 µm long by 245 µm wide in A-zooid, 230-275 µm long by 230-270 µm wide in B-zooids) with deep cavity; polypide tube placed centrally and vertically with its distal margin slightly above the level of median process; polypide tube diameter 185 µm in A-zooids and 210 µm in B-zooids. Gymnocyst smooth, reduced distally, forming lateral opercular condyles.
Measurements (µm). AZL 846±72, 706-953 (1, 20); AZW 508±37, 447-573 (1, 20); AZOpL 455±24, 410-504 (1, 14); AZOpW 365±25, 311-394 (1, 14); BZL 1185±52, 1121-1261 (1, 7); BZW 602±52, 519-675 (1, 7); BZOpL 500±20, 478-530 (1, 6); BZOpW 400±38, 352-437 (1, 6).
Remarks. The widespread Steginoporella magnilabris (Busk, 1854) stands as the sole species within the genus reported from Japan (e.g., Ortmann, 1890; Silén, 1941). It is notable for forming bryozoan thickets in Sagami Bay, spanning depths from 7 to 126 m (Hirose et al., 2013). Among the Steginoporella species characterized by dimorphic zooids and a median process with a deep cavity, S. magnilabris bears the closest resemblance to this specimen from Daidokutsu cave. However, while S. magnilabris is known for its tendency to form large, encrusting uni- to multilaminar sheets (Tilbrook, 2006) and erect rigid foliaceous colonies (Hirose et al., 2013), it does not typically form erect vincularian colonies with cylindrical branches, as observed in this instance. Steginoporella magnilabris also differs in the general appearance of the cryptocyst, which is more coarsely ornamented, and the shape and surface texture of the median process, which projects less outwards and is more granular than in Steginoporella sp. 1 (see Bock, 2024 https://bryozoa.net/cheilostomata/steginoporellidae/steginoporella_magnilabris.html).
Steginoporella sp. 2
Figure 3
Figured material. PMC EDM-Collection J.H.B.121a., sample 19210; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
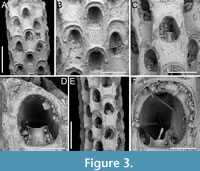 Description. Colony erect, forming cylindrical branches of up to at least eight longitudinal series of autozooids; maximum diameter of the available branch 1.63 mm. Zooids dimorphic (A- and B-type) with B-zooids on average 1.3 times longer than A-zooids, with a more extensive distal oral shelf (distal shelf length 93±11 µm [68-115 µm] in A-zooids vs 241±16 µm [219-268 µm] in B-zooids); A-zooids more common than B-zooids (AZ:BZ 3:1). All zooids elongate rectangular (mean AZL/AZW 1.69 and mean BZL/BZW 2.02) with arcuate proximal border; distal border arcuate in A-zooids, straight in B-zooids; cryptocyst finely granular occupying about half length of frontal surface, depressed, flat, pseudoporous (pseudopore diameter 8-12 µm). Opesia semielliptical, longer than wide (mean OpL/OpW 1.28 in A-zooids, OpL/OpW 1.36 in B-zooids), delimited proximally by a median process (c. 142-145 µm long by 176-196 µm wide in A-zooids, 168-175 µm long by 179-190 µm wide in B-zooids) with moderately deep cavity; polypide tube placed centrally and vertically not visible in frontal view, its distal margin slightly below the level of median process; polypide tube diameter c. 130 µm in A-zooids and c. 160 µm in B-zooids. Gymnocyst smooth, reduced distally, forming lateral opercular condyles.
Description. Colony erect, forming cylindrical branches of up to at least eight longitudinal series of autozooids; maximum diameter of the available branch 1.63 mm. Zooids dimorphic (A- and B-type) with B-zooids on average 1.3 times longer than A-zooids, with a more extensive distal oral shelf (distal shelf length 93±11 µm [68-115 µm] in A-zooids vs 241±16 µm [219-268 µm] in B-zooids); A-zooids more common than B-zooids (AZ:BZ 3:1). All zooids elongate rectangular (mean AZL/AZW 1.69 and mean BZL/BZW 2.02) with arcuate proximal border; distal border arcuate in A-zooids, straight in B-zooids; cryptocyst finely granular occupying about half length of frontal surface, depressed, flat, pseudoporous (pseudopore diameter 8-12 µm). Opesia semielliptical, longer than wide (mean OpL/OpW 1.28 in A-zooids, OpL/OpW 1.36 in B-zooids), delimited proximally by a median process (c. 142-145 µm long by 176-196 µm wide in A-zooids, 168-175 µm long by 179-190 µm wide in B-zooids) with moderately deep cavity; polypide tube placed centrally and vertically not visible in frontal view, its distal margin slightly below the level of median process; polypide tube diameter c. 130 µm in A-zooids and c. 160 µm in B-zooids. Gymnocyst smooth, reduced distally, forming lateral opercular condyles.
Measurements (µm). AZL 801±69, 674-873 (1, 18); AZW 474±33, 412-570 (1, 18); AZOpL 397±43, 353-478 (1, 6); AZOpW 311±28, 263-348 (1, 6); BZL 1052±37, 1002-1120 (1, 7); BZW 521±44, 462-594 (1, 7); BZOpL 440±9, 426-448 (1, 5); BZOpW 323±33, 282-370 (1, 5).
Remarks. Based on the available evidence, this species seems to differ from its Daidokutsu congener in having narrower branches made of fewer longitudinal series, and in the squared distal end of the B-zooids. Among the Steginoporella species characterized by dimorphic zooids, a median process with a moderately deep cavity, and straight distal margins of B-zooids, S. haddoni Harmer, 1900 is the most similar in the general zooidal appearance, as depicted in both Harmer (1900, figure 11) and Pouyet and David (1979, figure 2). Nevertheless, S. haddoni differs in having encrusting colonies and B-zooids twice the size of the A-zooids.
Superfamily CALLOPOROIDEA Norman, 1903
Family CALLOPORIDAE Norman, 1903
Genus COPIDOZOUM Harmer, 1926
Copidozoum rhoae Yang, Seo and Gordon, 2018
Figure 4
v. 2018 Copidozoum rhoae Yang, Seo and Gordon, p. 496, figs. 2-5.
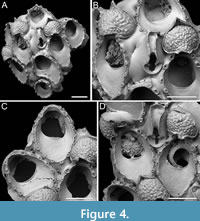 Figured material. PMC EDM-Collection J.H.B.122a, sample 19033 (Figure 4A-B), sample 19017 (Figure 4C), and sample 19021 (Figure 4D); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.122a, sample 19033 (Figure 4A-B), sample 19017 (Figure 4C), and sample 19021 (Figure 4D); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, delineated by deep interzooidal furrows, variably shaped, ranging from oval to elliptical, or irregularly lozenge- or pear-shaped, longer than wide (mean ZL/ZW 1.56). Gymnocyst smooth and convex, most extensive proximally, tapering laterally and distally, accommodating 12-17 circumopesial spines (with an average of 15±2), constantly 12 in zooid distal to ovicellate zooids; spine bases 20-27 µm in diameter (with an average of 23±2 µm), with the second distalmost pair having a larger diameter of 27-41 µm (with an average of 33±4 µm). Cryptocyst forming a raised beaded rim, with rim granules measuring 7-9 µm in diameter, sloping inwards to create an extensive, depressed, flat shelf, transitioning from coarse granular proximally to progressively smooth towards the proximal margin of the opesia. Opesia ovoidal to bell-shaped, slightly longer than wide, occupying slightly less than half of the zooidal surface (mean OpL/ZL 0.41). Avicularia subvicarious, large, slightly over half the size of an autozooid, resting on a convex cystid of smooth gymnocyst, scimitar-shaped, with a rounded rostrum measuring 206-240 µm in length (with an average of 223±17 µm); rostrum tip slightly curved on one side, distally directed, opesia elongate and figure eight-shaped, palate extensive and smooth, condyles robust. Ovicells hyperstomial, globular; endooecium coarsely granular (granule diameter 8-21 µm), ectooecium mostly uncalcified except for a narrow peripheral band, 16-18 µm wide, of smooth calcification forming an acute distal spike, 40-56 µm high, and proximal raised convolutions. Mid-distal, elliptical pore-chamber windows observed, c. 30 µm long by 60 µm wide.
Measurements (µm). ZL 472±41, 416-513 (3, 7); ZW 303±16, 277-327 (3, 7); CryL 181±36, 96-223 (3, 12); GymL 77±21, 49-118 (3, 10); OpL 194±21, 169-230 (3, 7); OpW 170±16, 151-197 (3, 7); AvCyL 324±12, 313-337 (2, 3); AvCyW 212±25, 193-240 (2, 3); AvL 282±18, 266-301 (2, 3); AvW 129±4, 127-134 (2, 3); AvOpL 118±7, 110-124 (2, 3); AvOpL 42±8, 33-47 (2, 3); OvL 201±20, 182-234 (3, 8); OvW 235±28, 186-273 (3, 8).
Remarks. Copidozoum rhoae stands out as one of the commonest components of the bryozoan assemblage in the Daidokutsu cave core. Specimens have larger autozooids and avicularia compared to the Recent material described from South Korea by Yang et al. (2018). Our morphological measurements closely align with those given in the original description of Copidozoum kikaijimense Kataoka, 1961, a Pleistocene Japanese species found in the Ryukyu Limestone of Kagoshima Prefecture, Japan. This species also bears a striking resemblance to C. rhoae, except for the ovicell, which is described as smooth, the presence of septula as interzooidal communications, and the smaller size of the avicularia. Unfortunately, the poor quality of the image in Kataoka (1961) prevents a more certain comparison between Copidozoum kikaijimense and specimens of C. rhoae from both Japan and South Korea.
Genus AMMATOPHOROIDES gen. nov. Di Martino, Rosso, and Taylor
zoobank.org/49F124DF-2C76-4699-B923-D0DF6BE8AE89
Type species. Ammatophoroides angeloi gen. et sp. nov. Di Martino, Rosso, and Taylor.
Etymology. Greek suffix ‘-oides’ meaning -like, referring to its similarity to species of the genus Ammatophora Norman, 1903.
Diagnosis. Ammatophora -like calloporid with blunt autozooidal boundaries, smooth gymnocyst filling the space between autozooids, flat to slightly convex especially around the lateral and distal zooidal boundaries, sometimes nodular but never forming thick tubercles as in Ammatophora; interzooidal communications via uniporous septula. An oval to ovoidal beaded rim outlining the cryptocystal portion of the zooid; cryptocyst granular, forming a flat depressed shelf proximally, occupying one-third to half of the frontal surface, narrowing and sloping gradually laterally, tapering distally, completely disappearing in ovicellate zooids. Opesia rounded trapezoidal to bell-shaped; muscle scars visible through the opesia. Ovicells subimmersed; ooecium cap-like, smooth, imperforate. Spines and avicularia absent.
Remarks. Among calloporid genera, Ammatophora is the most similar to the new genus. Both genera share a granular cryptocyst forming a distinct shelf proximal to the opesia, and both lack spines, avicularia, and pore chambers. The main differences are the absence of large interzooidal nodular kenozooids in Ammatophoroides gen. nov., which are typical of Ammatophor a (Winston and Vieira, 2013), and the ovicell, which is subimmersed in the new genus and hyperstomial in Ammatophora (Hayward and Ryland, 1998).
Ammatophoroides angeloi gen. et sp. nov. Di Martino, Rosso, and Taylor
Figure 5
zoobank.org/6597F524-5F13-4A81-A2EB-D25E8C08BB01
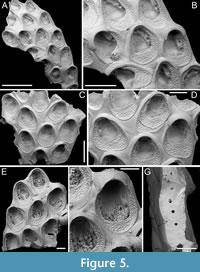 Type material. Holotype PMC. B44. 29.7.2024a, sample 19027 (Figure 5A-B); paratype PMC. B44. 29.7.2024b1, sample 19016 (Figure 5C-D); paratype PMC. B44. 29.7.2024b2, sample 19002 (Figure 5E-F); paratype PMC. B44. 29.7.2024b3, sample 19018 (Figure 5G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Type material. Holotype PMC. B44. 29.7.2024a, sample 19027 (Figure 5A-B); paratype PMC. B44. 29.7.2024b1, sample 19016 (Figure 5C-D); paratype PMC. B44. 29.7.2024b2, sample 19002 (Figure 5E-F); paratype PMC. B44. 29.7.2024b3, sample 19018 (Figure 5G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Etymology. Named after a close friend of the first author, Angelo Cavallaro, geologist and natural science teacher with a deep passion for rocks and fossils.
Diagnosis. As for Ammatophoroides gen. nov.
Description. Colony encrusting, multiserial, unilaminar; zooidal communications through numerous uniporous septula along lateral, vertical walls, with pore diameters ranging from 9 to 26 µm. Gymnocyst smooth, generally flat and more extensive proximally, slightly convex and narrower laterally, sometimes nodular but never developing into nodular kenozooids, sloping towards the oval to ovoidal cryptocystal rim outlining autozooids, longer than wide (mean ZL/ZW 1.38). Cryptocyst forming a raised, beaded rim, 8-19 µm wide, developing into an extensive proximal shelf occupying one-third to half of the frontal wall, slightly depressed, narrower laterally, gradually sloping towards the opesia, tapering distally and completely disappearing in ovicellate zooids, finely and evenly granular with granules arranged in radial rows becoming almost horizontal and parallel along the lateral margins. Opesia rounded trapezoidal to bell-shaped, occupying two-thirds to half of the frontal surface (mean OpL/ZL 0.51); elliptical muscle scars visible through the opesia in its distal part. Ovicell subimmersed; ooecium cap-like, smooth, imperforate, with lateral and median sutures sometimes visible, and proximolateral corners projecting into the opesia, forming a slight constriction. Spines and avicularia absent. Signs of repair visible in the cryptocyst of some zooids.
Measurements (µm). ZL 690±75, 570-903 (4, 20); ZW 501±64, 360-649 (4, 20); OpL 352±25, 305-391 (4, 20); OpW 274±19, 233-311 (4, 20); CryL 185±30, 121-237 (4, 20); GymL 119±50, 46-243 (4, 20); OvL 111±11, 98-121 (3, 4); OvW 383±24, 354-410 (3, 4).
Remarks. This species is one of the commonest in samples from Daidokutsu cave.
Family ANTROPORIDAE Vigneaux, 1949
Genus ANTROPORA Norman, 1903
Antropora typica (Canu and Bassler, 1928)
Figure 6
v. 1928 Dacryonella typica Canu and Bassler, p. 87, pl. 5, figs. 4-8; pl. 32, figs. 11, 12.
v. 1967 Antropora typica (Canu and Bassler); Rucker, p. 817, fig. 12b.
v. 1986 Antropora typica (Canu and Bassler); Winston, p. 5, figs. 1, 2.
v. 1998 Antropora typica (Canu and Bassler); Tilbrook, p. 37, figs. 1F, 3A.
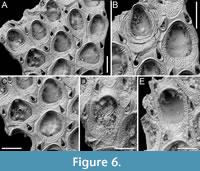 Figured material. PMC EDM-Collection J.H.B.123a, sample 19072 (Figure 6A-C) and sample 19230 (Figure 6D-E); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.123a, sample 19072 (Figure 6A-C) and sample 19230 (Figure 6D-E); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by fine furrows, oval, quincuncially arranged, longer than wide (mean ZL/ZW 1.21). Gymnocyst smooth, generally narrow, visible entirely around perimeter of zooid, more extensive proximally, tapering laterally and distally; cryptocyst outlined by a raised, beaded rim, slightly depressed, flat, and granular, broader proximally, narrowing laterally before disappearing distally. Opesia oval to pear-shaped, occupying two-thirds of total autozooid length (mean OpL/ZL 0.69). Avicularia interzooidal, small, teardrop-shaped, typically paired, located at zooidal distolateral corners, with raised, sharply triangular rostrum, directed distolaterally and inwardly, two faint condyles and a pear-shaped opesia. Ovicell endozooidal, small, partly immersed beneath distal autozooid; ooecium cap-like, imperforate, smooth. Vicarious avicularia not observed.
Measurements (µm). ZL 352±28, 309-408 (1, 13); ZW 291±37, 230-348 (1, 13); OpL 244±15, 222-273 (1, 9); OpW 197±28, 161-232 (1, 9); CryL 85±21, 45-123 (2, 14); GymL 37±17, 16-71 (2, 12); AvL 121±9, 104-136 (2, 20); AvW 75±7, 64-90 (2, 20); OvL 63±7, 58-68 (1, 2); OvW 172±1, 171-173 (1, 2).
Remarks. This species, originally described from northern Cuba and later identified in Brazil, Malaysia and Mauritius, has also been recorded off the coast of Japan, including locations such as Okinose near Tokyo and Shikoku (Tilbrook, 1998). As observed by Tilbrook (1998), the size of the autozooids in this species varies both within colonies and across different geographical regions. In the holotype, autozooids average 0.46 × 0.29 mm, whereas specimens from Okinawa display shorter autozooids, averaging 0.35 mm in length but which are of the same width as the holotype.
Family CHAPERIIDAE Jullien, 1888
Genus CHAPERIA Jullien, 1881
Chaperia robusta sp. nov. Di Martino, Rosso and Taylor
Figure 7
zoobank.org/CA4BE9D3-DDBD-4684-A936-3F098A300D92
Type material. Holotype PMC. B45. 29.7.2024a, sample 19081 (Figure 7A-D); paratype PMC. B45. 29.7.2024b, sample 19059 (Figure 7E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Etymology. Latin, meaning robust, referring to the stout appearance of the autozooids.
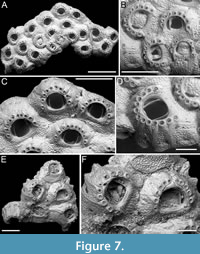 Diagnosis. Chaperia with stout autozooids and thick, prominently exposed lateral walls; cryptocyst coarsely granular, extensive, forming both frontal and lateral walls; gymnocyst reduced to a smooth calcification encasing spines and creating vertical ridges separating groups of spines; opesia eye-shaped, occupying less than half of the frontal surface, with robust occlusor laminae; 9-13 distolateral oral spines; tubercular closure plates developing in ancestrula and periancestrular zooids.
Diagnosis. Chaperia with stout autozooids and thick, prominently exposed lateral walls; cryptocyst coarsely granular, extensive, forming both frontal and lateral walls; gymnocyst reduced to a smooth calcification encasing spines and creating vertical ridges separating groups of spines; opesia eye-shaped, occupying less than half of the frontal surface, with robust occlusor laminae; 9-13 distolateral oral spines; tubercular closure plates developing in ancestrula and periancestrular zooids.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep grooves, irregularly arranged, rhomboidal, squat, as long as wide (mean ZL/ZW 1.01), with thick, prominently exposed, lateral walls (180-300 µm thick) and small, circular pore-chamber windows at their base. Frontal surface mostly occupied by a well-developed cryptocyst, coarsely granular proximally and finer towards the proximal margin of the opesia, flat to slightly convex; lateral walls made of tubercular cryptocyst; gymnocyst reduced to a smooth calcification encasing the oral spines and forming vertical ridges between spine clusters. Opesia eye-shaped, occupying less than half of zooidal surface (mean OpL/ZL 0.44), with well-developed occlusor laminae positioned around one-sixth of the width of the proximal margin of the opesia on each side; spine bases arranged in an arc along distolateral margin of opesia, with proximalmost pair aligned with or slightly below the proximal margin of opesia; 9-10 spines in the first budded zooids, 9-13 in subsequent ones, each spine bases with two concentric rings and external diameter 40-56 µm. Ancestrula resembling later autozooids but more slender and smaller, 522 µm long by 394 µm wide, opesia 187 µm long by 210 µm wide, with nine spines, budding two zooids, one distally and the other distolaterally; first budded autozooids slightly smaller than subsequent ones, 420-430 µm long by 515-560 µm wide, with opesiae 216-233 µm long by 265-290 µm wide. Closure plates with tubercular texture closing the opesia of both ancestrula and the first two generations of autozooids, leaving uncovered areas lateral to the occlusor laminae. Ovicells and avicularia not observed.
Measurements (µm). ZL 571±91, 439-701 (2, 13); ZW 567±65, 471-688 (2, 13); OpL 254±22, 225-287 (2, 13); OpW 302±17, 268-321 (2, 13).
Remarks. The stout appearance of the autozooids with thick and granular, exposed lateral walls, the large number of oral spines, and the unique features of the cryptocyst and gymnocyst distinguish this species from all known Chaperia species. Among Chaperia species recorded from Japan, C. acanthina (Lamouroux, 1824), a species that is widespread and otherwise restricted to the southern hemisphere with the doubtful exception of Japan (Harmer, 1926; Silén, 1941), differs in having fewer spines (e.g., 7-10 in Harmer, 1926; 8-10 in Hirose, 2010; 4-5 in Boonzaaier-Davids et al., 2023), and in the oval shape of the opesia, which also occupies a larger proportion of the frontal surface of the zooids. The unnamed new species of Chaperia from Sagami Bay (Japan) in Hirose (2010) not only has fewer spines (6-7) but also differs in the general appearance of the zooids having an extensive, oval opesia. Among species with numerous spines, the Indo-Pacific C. judex (Kirkpatrick, 1888) differs in having 15-20 spines (Gordon, 1984; Hayward, 1988), while C. multispinosa Gordon, 1984, from the Kermadec Ridge and SE Australia, differs in having the spines arranged in two semicircular rows (Gordon, 1984).
Family FOVEOLARIIDAE Gordon and Winston, 2005 in Winston, 2005
Genus FOVEOLARIA Busk, 1884
Foveolaria retiformis Harmer, 1926
Figure 8
v. 1926 Foveolaria retiformis Harmer, p. 247, pl. 14, figs. 21-23.
v. 2010 Foveolaria retiformis Harmer; Hirose, p. 37, pl. 62.
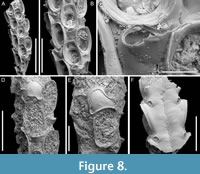 Figured material. PMC EDM-Collection J.H.B.124a, sample 19072 (Figure 8A-C), sample 19232 (Figure 8D-E), and sample 19227 (Figure 8F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.124a, sample 19072 (Figure 8A-C), sample 19232 (Figure 8D-E), and sample 19227 (Figure 8F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect with bi- tri- or quadriserial, unilaminar branches, flat to slightly convex, straight or slightly curved, c. 500 µm wide. Autozooids distinct, separated by fine furrows as well as the raised rim of the cryptocyst surrounding the opesia, rectangular to club-shaped, elongate (mean ZL/ZW 2.23), arranged alternately in longitudinal rows. Gymnocyst smooth, extensive proximally, thin laterally and distally; cryptocyst wrinkled, moderately wide, uniformly encircling the opesia, raised marginally and steeply sloping into the opesia. Opesia oval, occupying most of the frontal surface (mean OpL/ZL 0.67); muscle scars visible through the opesia at distolateral corners; spines absent. Avicularia adventitious, lodged on proximal gymnocyst, touching the proximal cryptocystal rim, pear-shaped, transversely oriented; rostrum raised, narrow, truncated, channelled, curved and serrated, directed laterally upwardly, resting on the distal cryptocystal rim of the neighbouring autozooid; two faint condyles and oval opesia. Ovicells hyperstomial, globular; ooecium smooth having a slightly raised and bent proximal margin; endooecium mostly exposed except for a distal band of calcified ectooecium, 26-40 µm wide. Dorsal side flat or slightly concave, smooth, with central median line and divergent wrinkles; a pair of circular rootlet pores consistently present at the level of proximal and distal ends of zooids external to the branch.
Measurements (µm). ZL 559±40, 515-627 (2, 9); ZW 251±9, 236-261 (2, 9); CryL 53±13, 36-74 (2, 8); GymL 165±15, 146-184 (2, 8); OpL 372±24, 342-415 (2, 12); OpW 230±16, 207-257 (2, 12); AvL 177±13, 156-200 (2, 8); AvW 93±6, 86-106 (2, 8); OvL 254±14, 244-264 (1, 2); OvW 288±11, 280-296 (1, 2).
Remarks. The available fragments do not provide sufficient evidence to confirm the reticulate colony shape characteristic of this species. However, all other observed characters align with the original description of the species by Harmer (1926) from Sulu Archipelago in the Philippines.
Superfamily BUGULOIDEA Gray, 1848
Family CANDIDAE d’Orbigny, 1851
Genus AMASTIGIA Busk, 1852
Amastigia okinawaensis sp. nov. Di Martino, Rosso and Taylor
Figure 9
zoobank.org/7E61AF79-B0B6-4ECC-B934-D7FB0FC62AC5
Type material. Holotype PMC. B46. 29.7.2024a, sample 19035 (Figure 9A-B, E-F); paratype PMC. B46. 29.7.2024b, sample 19065 (Figure 9C-D); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Etymology. Referring to the type locality, the Japanese islands of Okinawa where Daidokutsu cave is located.
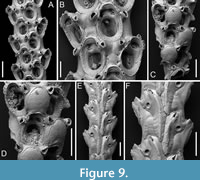 Diagnosis. Amastigia with biserial branches; club-shaped autozooids arranged in longitudinal alternating series; extensive, smooth gymnocyst proximally; narrow granular cryptocyst outlining the opesia proximally and laterally; opesia oval with 1-2 inner and 2-3 outer distolateral spines; 0-2 teardrop-shaped avicularia on raised cystids on the gymnocyst, adjacent to opesia, directed proximolaterally inwards but distolaterally outwards in autozooids distal to an ovicell; ovicell globular, ooecium smooth, ectooecium limited to a peripheral band framing the endooecium laterally and distally; vibracula developed longitudinally for the entire length of the zooid. Frontal scuta and gigantic frontal avicularia apparently absent.
Diagnosis. Amastigia with biserial branches; club-shaped autozooids arranged in longitudinal alternating series; extensive, smooth gymnocyst proximally; narrow granular cryptocyst outlining the opesia proximally and laterally; opesia oval with 1-2 inner and 2-3 outer distolateral spines; 0-2 teardrop-shaped avicularia on raised cystids on the gymnocyst, adjacent to opesia, directed proximolaterally inwards but distolaterally outwards in autozooids distal to an ovicell; ovicell globular, ooecium smooth, ectooecium limited to a peripheral band framing the endooecium laterally and distally; vibracula developed longitudinally for the entire length of the zooid. Frontal scuta and gigantic frontal avicularia apparently absent.
Description. Colony erect with relatively narrow (400-475 µm), biserial branches, presumably non-articulated, bifurcations not observed. Autozooids distinct, separated by thin grooves, club-shaped, elongate (mean ZL/ZW 2.12), alternately arranged in two longitudinal series. Gymnocyst smooth, forming an extensive, flat shelf proximally, occupying one-quarter to a half of the frontal surface, accommodating the avicularia; cryptocyst coarsely granular to nodular, narrow, more extensive proximally, tapering laterally, disappearing distally, gradually sloping towards the opesia proximally, steeply sloping laterally; cryptocystal granules 7-10 µm in diameter, arranged in concentric series, more prominent at the periphery. Opesia immersed, oval to pear-shaped, occupying half or more of the frontal surface, with communication pores and muscle scars visible through it; 1-2 inner and 2-3 outer distolateral spines present, indenting the opesial rim, usually with one having a slightly larger diameter than the others, spine diameter at the base 14-22 µm; frontal scuta absent. Avicularia either absent, single or more commonly paired, placed at the distal margin of the gymnocyst adjacent to the proximal margin of the opesia on raised, columnar cystids, small, teardrop-shaped with acutely triangular rostrum, commonly directed proximolaterally inwards but oriented distolaterally outwards when associated with an autozooid placed distally of an ovicell; crossbar lacking, two blunt condyles present; gigantic frontal avicularia apparently absent. Ovicell hyperstomial, globular, occupying the entire length of the proximal gymnocyst of the distal zooid, often indenting or covering the proximal margin of the opesia; ooecium smooth, imperforate; ectooecium limited to a peripheral band, 22-34 µm wide, framing the endooecium laterally and distally; proximal margin of the endooecium slightly curved upwards. Dorsal side occupied by two alternating series of Q-shaped (on the left portion of the branch) or P-shaped (on the right portion of the branch) vibracula, extending longitudinally for the entire length of the associated zooid, each with a large (42-66 µm in diameter), circular, radicular pore placed distolaterally.
Measurements (µm). ZL 371±17, 354-408 (2, 9); ZW 175±14, 159-200 (2, 9); OpL 235±10, 221-252 (2, 10); OpW 157±10, 146-174 (2, 9); CryL 41±7, 32-54 (2, 17); GymL 135±26, 113-196 (2, 17); AvL 65±9, 55-82 (2, 16); AvW 44±6, 39-60 (2, 16); OvL 195±1, 194-195 (1, 2); OvW 190±8, 184-196 (1, 2); VibrL 413±30, 368-441 (1, 6); VibrW 183±6, 176-193 (1, 6).
Remarks. Three species of Amastigia have been previously reported from the North Pacific: A. rudis (Busk, 1852), A. biseriata Osburn, 1950, and A. tricervicornis Gluhak, Lewis and Popijak, 2007. Vieira et al. (2010) listed A. rudis among Pacific species without scuta, which aligns with the SEM image in Bock (2024) (https://bryozoa.net/cheilostomata/candidae/amastigia_rudis.html). However, in Busk’s (1852, p. 38, pl. 46) original description and illustration of the species, the author depicts autozooids with scuta, as does Osburn (1950, p. 127, pl. 16, figures 3-5). The new species differs by being biserial and lacking the giant frontal avicularia. Additionally, the vibraculum on the back develops longitudinally rather than transversely, following the entire length of the zooid. The absence of scuta seems to be genuine and not the result of preservational issues, as there are no internal attachment structures adjacent to the opesia that would indicate the presence of a scutum. The report of A. rudis throughout the Pacific — from Australia (Busk, 1852) to Japan (Ortmann, 1890) and on the eastern side, including California, Mexico, and Costa Rica (Osburn, 1950) — suggests it might be a complex of species. Amastigia biseriata from California is similar in having autozooids arranged in two alternating longitudinal series but differs by having small lateral avicularia and a single spine on each side (Osburn, 1950, pl. 15, figures 1-3). Amastigia tricervicornis from Taiwan differs by having a single frontal avicularium placed horizontally with a curved upward rostrum (Gluhak et al., 2007, figure 4).
Genus CANDA Lamouroux, 1816
Canda foliifera Harmer, 1926
Figure 10
v. 1926 Canda foliifera Harmer, p. 386, pl. 26, figs. 21-23.
v. 2007 Canda foliifera Harmer; Gluhak, Lewis and Popijac, p. 403, fig. 7A-D.
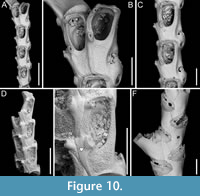 Figured material. PMC EDM-Collection J.H.B.125a, sample 19071 (Figure 10A-C, F) and sample 19200 (Figure 10D-E); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.125a, sample 19071 (Figure 10A-C, F) and sample 19200 (Figure 10D-E); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect with relatively narrow (460-470 µm), rectilinear, biserial, keeled branches, triangular in cross-section, dichotomously branched; articulating nodes not observed. Autozooids distinct, separated by shallow grooves, rounded rectangular, elongate (mean ZL/ZW 1.84), slightly asymmetrical, bent towards the external side of the branch, starting at zooidal mid-length, arranged alternately in two longitudinal series; three narrower autozooids present at bifurcations (187-231 µm wide). Frontal surface with minimal gymnocyst slightly wider in the proximal half of autozooids and around spines and the attachment base of the putative scutum; cryptocystal area outlined by a raised, undulating rim framing the proximal cryptocystal shelf and the opesia, forming a slightly prominent distal edge, and a mostly straight to slightly convex proximal edge, sometimes rounded, particularly in autozooids at bifurcations. Cryptocyst slightly depressed, occupying slightly less than half of the frontal surface (mean CryL/ZL 0.38), finely and densely granular, with granules around the opesia projecting into it, giving a spinose appearance, extensive proximally, very narrow laterally and distolaterally, absent distally. Opesia elongate, ovoidal with straight distal edge, occupying more than half of the frontal surface (mean OpL/ZL 0.59); a single spine present on the inner distolateral corner, 24-35 µm in diameter at the base, 0-1 spine on the outer distolateral corner, 13-24 µm in diameter at the base; an elliptical basal structure at the proximolateral edge, 50-90 µm long, interpreted as the base of a detached scutum; spines and putative base of scutum indenting the outlining rim. Avicularia absent. Ovicells not observed. Dorsal side occupied by vibracula with a long, curved, deep setal groove oriented distolaterally; a shallow sinuous median furrow corresponding to zooidal boundaries; a large, circular radicular pore, measuring 50-70 µm in diameter, placed proximally on each vibraculum on the outer side of the branch.
Measurements (µm). ZL 471±33, 419-530 (3, 15); ZW 255±14, 229-274 (3, 10); OpL 276±39, 205-333 (3, 13); OpW 147±8, 134-158 (3, 10); CryL 178±19, 159-233 (3, 15); VibrL 261±18, 246-310 (2, 10); VibrW 177±12, 153-194 (2, 10).
Remarks. Canda foliifera is an extant species reported from various locations in the Indo-Pacific. Initially identified as Canda retiformis Pourtales, 1867 by Thornely (1905, 1912) in Zanzibar and the Seychelles and by Waters (1913) in Sri Lanka, these records were later synonymized by Harmer (1926) with his new species found at several Indonesian stations during the Siboga Expedition. More recently, it has been found in samples from Taiwan (Gluhak et al., 2007). The main features of C. foliifera are the extensive cryptocyst with a straight proximal edge, the lack of frontal avicularia, and a large, asymmetrical scutum (Gluhak et al., 2007). In our subfossil specimens, the scutum appears to be detached, but its presence can be inferred from the robust base observed on the proximolateral inner corner of the opesial rim (Figure 10E, arrowed). This structure closely resembles that illustrated by Gluhak et al. (2007, p. 403, figure 7C) in their Taiwanese material.
Canda scutata Harmer, 1926
Figure 11
v. 1926 Canda pecten var. scutata Harmer, p. 389, pl. 26, fig. 24.
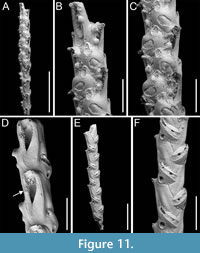 Figured material. PMC EDM-Collection J.H.B.126a, samples 19200 (Figure 11A-C), sample 19029 (Figure 11D), and sample 19227 (Figure 11E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.126a, samples 19200 (Figure 11A-C), sample 19029 (Figure 11D), and sample 19227 (Figure 11E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect with narrow (c. 265 µm), rectilinear, biserial, keeled branches; articulating nodes and bifurcations not observed. Autozooids distinct, separated by shallow grooves, rounded rectangular, elongate (mean ZL/ZW 2.13), arranged alternately in two longitudinal, series. Gymnocyst minimal, smooth, convex. Cryptocystal area outlined by a shallow furrow; cryptocyst slightly depressed, occupying slightly less than half of the frontal surface (mean CryL/ZL 0.36), smooth to nodular, granular around the opesia with granules projecting into it, giving a serrated appearance, extensive proximally, narrow laterally nearest branch midline, wider and wedge-shaped on the outer side, absent distally. Opesia asymmetrical, almost triangular (i.e., comma-shaped), tapering proximally, occupying half of the frontal surface (mean OpL/ZL 0.50); a single spine on each distolateral corner, 10-20 µm in diameter at the base, visible also in ovicellate zooids; a spine-base-like structure laterally at about opesia mid-length on the inner side of the branch, with a larger diameter (30-55 µm) than spine bases, appearing to be the base of a detached scutum; spines and putative base of scutum indenting the outline of the cryptocystal area. Vestigial avicularia associated with ovicells, distally placed, rounded triangular directed distolaterally, alternating on either side. Ovicells globular, relatively depressed, obliquely placed, directed inwards from the opesia, with a pointed distal margin due to the presence of the avicularium; the series of convergent and alternating ovicells from the two zooidal rows forming the central elevated part of the branch. Ooecium smooth; ectooecium with an irregularly elliptical central fenestra, exposing the endooecium. Dorsal side occupied by vibracula with a long, curved, deep setal groove oriented distolaterally; a shallow sinuous median furrow corresponding to zooidal boundaries; a large, circular radicular pore measuring 40-50 µm in diameter, placed proximally on each vibraculum on the outer side.
Measurements (µm). ZL 351±22, 307-395 (4, 20); ZW 165±22, 121-202 (4, 20); OpL 174±12, 160-194 (2, 7); OpW 60±6, 51-65 (2, 7); CryL (proximal) 128±13, 110-154 (4, 20); VibrL 179±14, 163-198 (2, 9); VibrW 115±11, 94-134 (2, 9).
Remarks. Harmer (1926) described this species as a variety of Canda pecten Thornely, 1907, distinguishing it by the presence of minute scuta in the new variety (see also Tilbrook, 2006). The specimens from Daidokutsu cave show the main diagnostic characters of the species, including asymmetrical opesiae and obliquely placed ovicells with associated vestigial avicularia and broad ectooecial fenestra. Branch bifurcations were not observed in the examined samples, which prevented the observation of another key diagnostic feature, i.e., the presence of large frontal avicularia, either single or paired, which are developed on each branch above the bifurcation. Our specimens also lack a scutum, but the attachment base is clearly recognizable (Figure 11D, arrowed), leading to their identification as C. scutata rather than C. pecten. While C. pecten has been already recorded in Japan (Tilbrook, 2006), C. scutata had not been previously reported in this region. It has been documented in New Guinea and the Loyalty Islands (Harmer, 1957), the Kermadec Ridge (Gordon, 1984), and the Nansha Islands (Liu, 1991). The specimens illustrated by Gordon (1984, pl. 13D-E) differ slightly from the specimens found in the Daidokutsu cave. The differences include more prominent ovicells with apparently different length-to-width proportions, varying development of the frontal fenestra, the possible occurrence of a second spine at one distal corner, and narrower vibracula.
Canda lunata sp. nov. Di Martino, Rosso and Taylor
Figure 12
zoobank.org/56822F86-59BA-45FC-B8D1-791A06758EA7
Type material. Holotype PMC. B47. 29.7.2024a, sample 19029 (Figure 12A-E); paratype PMC. B47. 29.7.2024b, sample 19227 (Figure 12F-G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
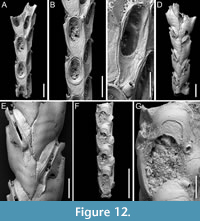 Etymology. Latin, meaning crescent-shaped, referring to the shape of the ectooecial fenestra.
Etymology. Latin, meaning crescent-shaped, referring to the shape of the ectooecial fenestra.
Diagnosis. Canda with biserial branches becoming triserial at bifurcations, triangular in cross-section; autozoids club-shaped, arranged in alternating series; gymnocyst absent; proximal cryptocyst extensive smooth and pitted; opesia transversely elliptical with two distolateral spines, one on each side; scutum lacking; small drop-shaped avicularia of two types: adventitious distolateral and frontal interzooidal, both on a columnar cystid; ovicells covering the proximal margin of the opesia of the distal zooid, ooecium smooth with crescent-shaped ectooecial fenestra; vibracula on the dorsal side with an obliquely placed setal groove only slightly curved.
Description. Colony erect with narrow (245-395 µm) branches; branches rectilinear, biserial transitioning to triserial at bifurcations, triangular in cross-section; articulating nodes not observed. Autozooids distinct, separated by shallow grooves and a slightly raised rim, club-shaped, elongate (mean ZL/ZW 1.93), arranged alternately in two longitudinal, series. Gymnocyst absent; cryptocyst smooth, pitted, slightly depressed, extensive proximally, occupying two-fifths of the frontal surface (mean CryL/ZL 0.36), tapering laterally, absent distally. Opesia elliptical, nearly parallel-sided, occupying two-thirds of the frontal surface (mean OpL/ZL 0.65); a single spine on each distolateral corner, 10-18 µm in diameter at the base, concealed in ovicellate zooids; scutum lacking; two oval communication pores and muscle scars visible through the opesia. Avicularia small, drop-shaped, occurring in two types: adventitious, placed distolaterally adjacent to the opesia externally, atop a conical cystid, slightly sloping outwards and directed laterally; frontal interzooidal, positioned on a columnar cystid directed proximo- or distolaterally; cystids 60-90 µm in length; crossbar lacking. Ovicells globular, placed on the cryptocyst of the distal zooid, partially covering its proximal margin and more of the inner corner; ooecium smooth with an ectooecium featuring a transversely placed, crescent-shaped fenestra, 55-75 µm long by 100-120 µm wide, exposing the endooecium. Dorsal side occupied by vibracula with a long, obliquely placed, only slightly curved, deep setal groove; a shallow sinuous median furrow corresponding to zooidal boundaries; on each vibraculum a large, circular radicular pore measuring 17-23 µm in diameter, located proximally on the outer side.
Measurements (µm). ZL 359±9, 353-369 (1, 3); ZW 186±6, 182-193 (1, 3); OpL 234±28, 209-264 (1, 3); OpW 123±29, 104-156 (1, 3); CryL (proximal) 131±12, 113-150 (2, 8); AvL (adventitious) 36±2, 34-39 (2, 5); AvW (adventitious) 18±4, 14-24 (2, 5); AvL (interzooidal) 33±2, 31-36 (2, 6); AvW (interzooidal) 23±4, 19-28 (2, 6); OvL 154±11, 139-164 (1, 4); OvW 185±7, 177-192 (1, 4); VibrL 179±14, 163-198 (2, 9); VibrW 115±11, 94-134 (2, 9).
Remarks. The absence of a scutum in this subfossil species is not due to preservational issues as there are no basal structures from which it might have detached. Among the Recent and fossil Indo-Pacific species of Canda lacking a scutum, C. giorgioi Di Martino and Taylor, 2014, and C. federicae Di Martino and Taylor, 2014, from the Miocene of East Kalimantan (Indonesia), differ in their lack of avicularia and a more curved setal groove of the vibracula. However, C. federicae is the most similar in the overall appearance of the autozooids. Canda pecten differs in having a triangular asymmetrical opesia, while C. clypeata (Haswell, 1880) has large frontal avicularia with a curved rostrum directed towards the centre of the branch (Tilbrook, 2006).
In the original description of the Indonesian species reported by Di Martino and Taylor (2014, pp. 57, 58), the authors did not designate a holotype specimen. Following the re-examination of this material for this work, we designate specimen NHMUK PI BZ 6882 as the lectotype (NHMUK PI BZ 6883 as the paralectotype) for C. giorgioi, and specimen NHMUK PI BZ 6884 as the lectotype (NHMUK PI BZ 6885 as the paralectotype) for C. federicae.
Genus POMOCELLARIA Vieira, Spencer Jones, Winston, Migotto and Marques, 2014
Pomocellaria spatulata sp. nov. Di Martino, Rosso and Taylor
Figure 13
zoobank.org/F77AB514-8076-4EE1-A7B7-5DB21424592D
Type material. Holotype PMC. B48. 29.7.2024a, sample 19032; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
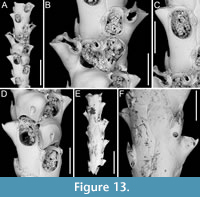 Etymology. Latin, meaning spatula-like (i.e., broad and flat), referring to the shape of the dimorphic lateral avicularium.
Etymology. Latin, meaning spatula-like (i.e., broad and flat), referring to the shape of the dimorphic lateral avicularium.
Diagnosis. Pomocellaria with rounded rectangular autozooids, smooth extensive proximal gymnocyst, negligible smooth cryptocyst bordering the proximal margin of opesia; elliptical to pear-shaped opesia with one inner and two outer distolateral spines, also visible in ovicellate zooids; scutum attachment base located on the inner side at approximately half opesia length; frontal avicularium single or absent, small, drop-shaped, directed distolaterally; lateral avicularium dimorphic, either medium-sized triangular with a hooked rostrum or giant and spatulate with duckbill-shaped rostrum; ovicells with an upside-down, drop-shaped medioproximal fenestra.
Description. Colony erect with narrow (330-390 µm) branches; branches rectilinear, biserial, flat, elliptical in cross-section; articulating nodes or bifurcations not observed. Autozooids distinct, separated by shallow grooves, rounded rectangular, elongate (mean ZL/ZW 2.18), arranged alternately in two longitudinal series. Proximal gymnocyst smooth, extensive; cryptocyst smooth, negligible, bordering the proximal margin of the opesia. Opesia elliptical to pear-shaped, occupying approximately half of the frontal surface (mean OpL/ZL 0.49); a single spine on the inner and two on the outer distolateral corners, 13-22 µm in diameter at the base; spines also visible in ovicellate zooids. Scutum detached, originating laterally from the inner side, attached at about half the length of the opesia, with an attachment base measuring 50-60 µm in maximum diameter. Adventitious avicularia occurring both frontally and laterally. Frontal avicularium often absent; a single damaged example observed, small, drop-shaped with an acutely triangular rostrum directed distolaterally outwards, associated with an ovicell and displaced towards the branch midline, projecting into the inner distolateral corner of the opesia of the adjacent autozooid. Lateral avicularium consistently present in two forms: type 1, a medium-sized triangular type with an upward hooked rostrum tip directed laterally, atop a conical cystid 115-140 µm long; type 2, an enlarged spatulate type with duckbill-shaped rostrum with an undulate margin, facing obliquely and sloping towards the front of the branch while raised towards the back, with a narrow, 8-shaped opening, positioned atop a fan-shaped cystid 190-205 µm long; crossbar seemingly absent in all avicularia types. Ovicells globular, occupying the entire proximal gymnocyst of the distal zooid; ooecium smooth with an almost completely calcified ectooecium, featuring a small, elliptical fenestra with a upside-down drop-shaped, external outline, 70-80 µm long by 35-60 µm wide, located medioproximally, exposing the endooecium. Dorsal side occupied by sac-shaped vibracula with a long, obliquely placed, straight, deep setal groove, and a sinuous median furrow; each vibraculum with a large, circular radicular pore measuring 35-45 µm in diameter, located proximally on the outer side of the branch.
Measurements (µm). ZL 414±14, 397-428 (1, 7); ZW 189±17, 170-226 (1, 7); OpL 201±15, 180-218 (1, 8); OpW 127±6, 119-135 (1, 8); GymL 199±7, 193-208 (1, 4); CryL 15±5, 9-21 (1, 8); AvL (lateral type 1) 86±15, 67-108 (1, 5); AvL (lateral type 2) 150±38, 123-177 (1, 2); AvW (lateral type 2, proximally) 76±1, 75-77 (1, 2); AvW (lateral type 2, spatulate rostrum) 118±10, 111-125 (1, 2); AvL (frontal) 69 (1, 1); AvW (frontal) 33 (1, 1); OvL 204±1, 203-205 (1, 2); OvW 203±33, 180-227 (1, 2); VibrL 184±13, 67-97 (1, 4); VibrW 86±13, 67-97 (1, 4).
Remarks. The genus Pomocellaria was introduced by Vieira et al. (2014) for Scrupocellaria -like species with an opesia occupying half the length of the zooid, a reduced cryptocyst, dimorphic lateral avicularia, a single frontal fenestra in the ooecium, and vibracula with a straight setal groove. They assigned five species from the NE Pacific to their new genus. This new species extends the known range of Pomocellaria to the NW Pacific. The spatulate shape of the giant dimorphic lateral avicularium distinguishes this species from other known Pomocellaria species.
Among Scrupocellaria species of uncertain classification, i.e., not yet reassigned to a genus following Vieira et al. (2014), there is one species recorded from Japan, Scrupocellaria muricata (Lamouroux, 1816). According to Tilbrook and Vieira (2012), who examined specimens from Japan housed at the Museum national d’Histoire naturelle (MNHN) in Paris, and identified by Lamouroux himself as Crisia muricata, this species can be assigned to a complex of species belonging to the genus Tricellaria, characterised by the absence of basal vibracula.
Genus SCRUPOCABEREA Vieira, Spencer Jones, Winston, Migotto and Marques, 2014
Scrupocaberea contraria sp. nov. Di Martino, Rosso and Taylor
Figure 14
zoobank.org/4C3BE02F-CE91-4E99-A8A7-587E790D87F3
Type material. Holotype PMC. B49. 29.7.2024a, sample 19032 (Figure 14C-E); paratype PMC. B49. 29.7.2024b1, sample 19065 (Figure 14A-B, F-G); paratype PMC. B49. 29.7.2024b2, sample 19023 (not figured); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
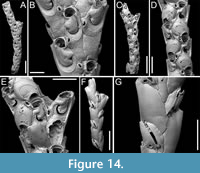 Etymology. Latin, meaning opposite, referring to the change in direction (from proximolateral to distolateral) of the frontal avicularia when the proximal zooid is ovicellate.
Etymology. Latin, meaning opposite, referring to the change in direction (from proximolateral to distolateral) of the frontal avicularia when the proximal zooid is ovicellate.
Diagnosis. Scrupocaberea with rounded rectangular or club-shaped autozooids; smooth extensive proximal gymnocyst, short smooth cryptocyst bordering the proximolateral margins of opesia; bean-shaped opesia with two faint distolateral spines, one on each side, typically hidden in ovicellate zooids; lung-shaped scutum nearly completely covering the proximal portion of opesia, with robust attachment base; frontal avicularium small, drop-shaped, placed at zooidal mid-length on the inner side, adjacent to the proximolateral margin of the opesia, directed proximolaterally when distal to a non-ovicellate zooid, shifting to distolateral when distal to an ovicellate zooid; frontal giant avicularium rare, placed atop a raised cystid; lateral avicularium also small and drop-shaped; ovicells with large semicircular ectooecial fenestra with concave proximal margin; dorsal side with tulip-shaped vibracula with oblique, slightly curved setal groove.
Description. Colony erect with narrow biserial branches (245-295 µm wide) transitioning to triserial at bifurcations (460-480 µm wide); branches rectilinear to slightly curved, flat, subelliptical in cross-section; articulating nodes not observed. Autozooids distinct, separated by shallow grooves, rounded rectangular to club-shaped, elongate (mean ZL/ZW 2.61), arranged alternately in two longitudinal series. Proximal gymnocyst smooth, extensive; cryptocyst smooth, slightly depressed, relatively wide proximally, tapering laterally, absent distally. Opesia bean-shaped, occupying slightly less than half of the frontal surface (mean OpL/ZL 0.42) with two faint distolateral spines, one on each corner, 8-16 µm in diameter at the base, typically concealed in ovicellate zooids. Scutum originating laterally from the inner side, attached at about half the opesia length, covering nearly the entire proximal portion and leaving a semicircular to rounded rectangular orifice distally, lung-shaped, with a robust attachment structure measuring 52-95 µm in width. Adventitious avicularia small, drop-shaped, occurring in two types: a single (sometimes absent) frontal avicularium, at the distal termination of the gymnocyst leaning on the lateral zooidal corner, on the inner side of the branch, and adjacent to the opesia, possibly modifying the outline of the cryptocystal rim, positioned on a lozenge-shaped slightly raised cystid, with an acutely triangular rostrum directed proximolaterally, changing orientation to distolateral when distal to an ovicellate zooid; giant frontal avicularium rarely present, located atop a smooth, raised cystid constricted at the base (44 µm wide) and enlarged distally (116 µm wide); constant lateral avicularium positioned on a small conical cystid, with an acutely triangular rostrum directed laterally and sloping downward; all avicularia with small condyles. Ovicells globular, occupying the entire proximal gymnocyst of the distal zooid, modifying its cryptocystal rim; ooecium smooth with a partially calcified ectooecium, featuring a central large semicircular fenestra with a concave proximal margin, 68-100 µm long by 84-120 µm wide, exposing the endooecium. Dorsal side occupied by tulip-shaped vibracula with a long, obliquely placed, slightly curved, deep setal groove, and a shallow undulose median furrow corresponding to zooidal boundaries; each vibraculum with a medium-sized, circular to transversely elliptical radicular pore measuring 18-22 µm in maximum diameter, located proximally on the outer side.
Measurements (µm). ZL 413±13, 395-438 (1, 10); ZW 158±22, 124-195 (1, 10); GymL 165±12, 153-195 (1, 12); CryL 50±9, 41-72 (2, 17); OpL 175±12, 153-198 (2, 17); OpW 80±9, 65-97 (2, 17); AvL (frontal standard) 48±7, 36-57 (2, 16); AvW (frontal standard) 31±5, 24-39 (2, 16); AvL (frontal enlarged) 216 (1, 1); AvW (frontal enlarged) 116 (1, 1); AvL (lateral) 34±2, 31-36 (2, 5); AvW (lateral) 20±4, 14-24 (2, 5); ScuL 126±16, 106-156 (2, 14); ScuW 65±10, 48-80 (2, 16); OvL 177±7, 166-185 (1, 8); OvW 166±13, 139-186 (1, 8); VibrL 243±26, 198-284 (2, 10); VibrW 127±18, 92-142 (2, 10).
Remarks. Two Scrupocaberea species were found in Daidokutsu cave: S. contraria sp. nov. and S. maderensis (Busk, 1860) (see description below and Figure 15). These species mainly differ in the texture of the cryptocyst, which is smooth in S. contraria sp. nov. and granular in S. maderensis, the shape of the ectooecial fenestra, which is large, semicircular and crescent-shaped in S. contraria sp. nov. but small and circular to elliptical in S. maderensis, the number and position of the opesial spines, with two faint distolateral spines, one on each corner, in S. contraria sp. nov., and two spines on the inner and three on the outer distolateral corners in S. maderensis. Additionally, the dorsal side differs in the shape, size and location of the vibracula with respect to the zooid to which they are associated. The general appearance of the branch is also very different, being serrated in S. maderensis and more linear in S. contraria sp. nov.
The ectooecial fenestra of S. contraria sp. nov. resembles that of Canda lunata sp. nov., and both species exhibit a change in avicularia orientation when distal to an ovicellate zooid.
Compared to other known Scrupocaberea species, S. contraria sp. nov. has distinct features: S. dongolensis (Waters, 1909) from Sri Lanka shares a similar scutum and zooidal shape, and frontal avicularia in similar position, but has a greater number of more robust outer distolateral spines, larger lateral avicularia, and a significantly reduced ectooecial fenestra (Vieira et al., 2014). Similarly, S. ornithorhynchus (Thomson, 1858) from Australia also has a very reduced fenestra, large lateral avicularia, and a denticulated margin of the opesia in ovicellate zooids (Vieira et al., 2014). Scrupocaberea gilbertensis (Maplestone, 1909) from the southwest Pacific differs by having a granular cryptocyst (Vieira et al., 2014).
Scrupocaberea maderensis (Busk, 1860)
Figure 15
v. 1860 Scrupocellaria maderensis Busk, p. 280.
v. 2014 Scrupocaberea maderensis (Busk); Vieira, Spencer Jones, Winston, Migotto and Marques, p. 18, fig. 15A-C.
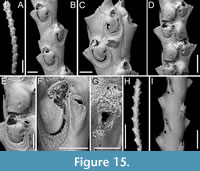 Figured material. PMC EDM-Collection J.H.B.127a, sample 19019 (Figure 15A-C, H-I), sample 19016 (Figure 15D-E), and sample 19147 (Figure 15F-G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.127a, sample 19019 (Figure 15A-C, H-I), sample 19016 (Figure 15D-E), and sample 19147 (Figure 15F-G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect with relatively narrow (270-350 µm) branches; branches rectilinear to slightly curved, biserial transitioning to triserial at bifurcations, flat; articulating nodes not observed. Autozooids distinct, separated by shallow grooves, rounded rectangular to club-shaped, elongate (mean ZL/ZW 2.68), arranged in two longitudinal, alternating series. Proximal gymnocyst smooth, extensive; cryptocyst nodular to finely granular (granules 7-11 µm in diameter), slightly depressed, relatively wide proximally, tapering laterally, absent distally. Opesia pear-shaped, occupying slightly less than half of the frontal surface (mean OpL/ZL 0.41) with beaded proximolateral margins; two spines on the inner and three on the outer distolateral corner, 7-18 µm in diameter at the base, the proximalmost pair larger; spines visible also in ovicellate zooids. Scutum very robust, originating laterally from the inner side, attached at about two-thirds of opesia length, covering the proximal portion entirely and leaving a semicircular orifice distally, kidney-shaped, truncated at the top, with a robust attachment structure measuring 55-77 µm in diameter. Adventitious avicularia, small, drop-shaped, occurring as two types: a single (often absent) frontal avicularium at the distal termination of the gymnocyst adjacent to the opesia, leaning towards the median axis of the branch, positioned atop a raised cystid c. 90 µm long, with acutely triangular rostrum directed distolaterally on either side; a constant lateral avicularium positioned on a conical cystid 100-120 µm long, with acutely triangular hooked rostrum directed laterally; both types with complete crossbar. Ovicells globular, occupying the entire proximal gymnocyst of the distal zooid; ooecium smooth with an almost completely calcified ectooecium apart from a small, circular to transversely elliptical fenestra, 25-30 µm long by 25-40 µm wide, located medioproximally, exposing the endooecium. Opesia of ovicellate zooids with denticulated distal margin. Dorsal side occupied by triangular vibracula with a long, slightly curved, deep setal groove; a shallow zigzag median furrow corresponding to zooidal boundaries; each vibraculum with a large, circular radicular pore measuring 25-35 µm in diameter, located proximally on the outer side.
Measurements (µm). ZL 439±12, 411-452 (1, 13); ZW 164±9, 146-178 (1, 13); GymL 221±19, 197-270 (1, 14); CryL 50±6, 38-60 (2, 19); OpL 183±11, 157-198 (1, 16); OpW 100±10, 80-113 (1, 16); AvL (frontal) 50±2, 48-53 (2, 6); AvW (frontal) 27±1, 26-29 (2, 6); AvL (lateral) 50±2, 48-53 (1, 16); ScuL 120±9, 109-140 (2, 17); ScuW 76±6, 64-84 (2, 17); OvL 189±2, 187-190 (1, 3); OvW 143±13, 128-152 (1, 3); VibrL 164±11, 147-181 (1, 7); VibrW 96±6, 84-103 (1, 7).
Remarks. Scrupocaberea maderensis, originally described from the Azores, has since been recorded worldwide, including Japan (Tilbrook, 2006). It has been recognized as a complex of species by Vieira et al. (2014), who distinguished two additional species from specimens previously identified as S. maderensis by Harmer (1926). This suggests that further records might reveal additional species upon re-analysis. However, when comparing our specimens with the syntype figured in Vieira et al. (2014, figure 15A-C), no significant differences were observed in morphological traits or size, indicating that the North-Pacific specimens are similar to those from the Atlantic. Despite this, the geographical distance raises doubts about their conspecificity. Vieira et al. (2014) described the cryptocyst of S. maderensis as smooth with beading around the opesial margin. However, in the images of the syntype and also in our specimens, the cryptocyst appears nodular to finely granular. Differences between S. maderensis and the congener in the same samples, S. contraria sp. nov., are discussed in the Remarks section above.
Superfamily MICROPOROIDEA Gray, 1848
Family PORICELLARIIDAE Harmer, 1926
Genus PORICELLARIA d’Orbigny, 1854
Poricellaria ratoniensis (Waters, 1887)
Figure 16
v. 1887 Micropora ratoniensis Waters, p. 185, pl. 4, fig. 5.
v. 1926 Poricellaria ratoniensis (Waters); Harmer, p. 134, pl. 17, fig. 14, pl. 23, figs. 3-8.
v. 1984 Poricellaria ratoniensis (Waters); Ryland, p. 70, fig. 39.21.
v. 2018 Poricellaria ratoniensis (Waters); Cook, Bock, Hayward and Gordon, p. 137, fig. 3.81.
Figured material. PMC EDM-Collection J.H.B.128a, sample 19085 (Figure 16A-B, D) and sample 19048 (Figure 16C); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
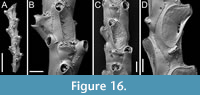 Description. Colony erect, flexible, articulated; branches narrow, 230-280 µm in width, quadriserial. Autozooids distinct, separated by thin furrows, asymmetrical, oval, elongate (mean ZL/ZW 2.24), arranged alternately in longitudinal rows. Gymnocyst well developed both proximally and laterally, smooth, convex. Mural rim thin, slightly elevated. Cryptocyst forming an extensive flat, slightly and asymmetrically depressed shelf mediodistally, rising proximal to orifice, nodular and smooth; a single slit-like opesiule, 77-88 µm long by 14-19 µm wide, positioned on the same side towards which the orifice inclines at the midpoint of the cryptocyst. Orifice semicircular, slightly wider than long, facing laterally, protruding from branch axis. Adventitious avicularia placed on the proximal gymnocyst, very close to the mural rim, salient; rostrum acutely triangular, narrow, long, channelled, directed proximolaterally almost transversely to branch axis, opposite to orifice; crossbar complete. Rhizoidal pore adjacent to avicularian rostrum tip, 5-9 µm in diameter, placed at the centre of a circular depression 29-37 µm in diameter. Ovicells not observed.
Description. Colony erect, flexible, articulated; branches narrow, 230-280 µm in width, quadriserial. Autozooids distinct, separated by thin furrows, asymmetrical, oval, elongate (mean ZL/ZW 2.24), arranged alternately in longitudinal rows. Gymnocyst well developed both proximally and laterally, smooth, convex. Mural rim thin, slightly elevated. Cryptocyst forming an extensive flat, slightly and asymmetrically depressed shelf mediodistally, rising proximal to orifice, nodular and smooth; a single slit-like opesiule, 77-88 µm long by 14-19 µm wide, positioned on the same side towards which the orifice inclines at the midpoint of the cryptocyst. Orifice semicircular, slightly wider than long, facing laterally, protruding from branch axis. Adventitious avicularia placed on the proximal gymnocyst, very close to the mural rim, salient; rostrum acutely triangular, narrow, long, channelled, directed proximolaterally almost transversely to branch axis, opposite to orifice; crossbar complete. Rhizoidal pore adjacent to avicularian rostrum tip, 5-9 µm in diameter, placed at the centre of a circular depression 29-37 µm in diameter. Ovicells not observed.
Measurements (µm). ZL 424±33, 380-472 (3, 13); ZW 189±16, 163-220 (3, 13); CryL 261±41, 199-318 (3, 13); CryW 143±24, 108-176 (3, 13); GymL 146±20, 121-173 (3, 7); LatGymW 55±15, 36-77 (3, 7); OpL 57±4, 49-62 (3, 13); OpW 66±4, 61-73 (3, 13); AvL 105±20, 79-136 (3, 10); AvW 47±12, 36-67 (3, 10).
Remarks. Our specimens correspond to Poricellaria ratoniensis, a species extensively found across the Indo-Pacific. Previous records of P. ratoniensis have been documented in Indonesia (Harmer, 1926), Australia (Waters, 1887; Ryland, 1984; Cook et al., 2018), New Guinea and Zanzibar (Cook et al., 2018), and the South China Sea (Gordon, 2016). Additionally, this species has also been recorded in the Miocene of southeastern Australia (Bock, 2024). To the best of our knowledge, this is the first record of this species in Japan.
Superfamily CELLARIOIDEA Fleming, 1828
Family CELLARIIDAE Fleming, 1828
Genus CELLARIA Ellis and Solander, 1786
Cellaria levigata sp. nov. Di Martino, Rosso and Taylor
Figure 17
zoobank.org/9477DA69-5BF0-472C-8E74-33D2F39AA820
Type material. Holotype PMC. B50. 29.7.2024a, sample 19200 (Figure 17A-C); paratype PMC. B50. 29.7.2024b1, sample 19227 (Figure 17D-F); paratype PMC. B50. 29.7.2024b2, sample 19230 (Figure 17G-J); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
 Etymology. Latin, meaning smooth, referring to the smooth appearance of the cryptocyst.
Etymology. Latin, meaning smooth, referring to the smooth appearance of the cryptocyst.
Diagnosis. Cellaria with hexagonal to rhomboidal zooids arranged in whorls of 3-4; cryptocyst smooth, with thick raised ridges flanking the orifice; orifice transversely crescent-shaped with two rounded triangular denticles; vicarious avicularia elongate pear-shaped with condyles associated with fertile autozooids; fertile autozooids clustered in whorls locally inflating internode diameter; ovicell endotoichal, appearing as a fissure with lateral indentations, protected by the raised cryptocystal distal margin of the maternal zooid.
Description. Colony erect, jointed, flexible; internodes cylindrical, narrow (230-350 µm wide), tapering slightly near the node (190-200 µm), rectilinear to slightly curved, becoming inflated near clusters of fertile zooids. Autozooids distinct, separated by thin furrows between raised margins, hexagonal with parallel lateral sides and acute to rounded triangular distal and proximal margins, longer than wide (mean ZL/ZW 1.55), arranged in whorls of 3-4. Cryptocyst smooth, slightly depressed compared to the margins, but slightly convex, with the frontal area marked by two lateral raised ridges flanking the opesia, sometimes closing proximally in a V-shape or remaining open like two brackets, 12-22 µm thick. Opesia outlined by a thin (c. 10 µm) raised rim, transversely crescent-shaped with a markedly convex proximal margin, placed at the distal third of zooidal length; two rounded triangular denticles, 13-14 µm long by 16 µm wide at the base, present on the proximal margin, positioned at a certain distance from the proximal corners. Vicarious avicularia rare, pear-shaped (215 µm long by 100 µm wide), placed on larger triangular cystid, with a raised, rounded acicular rostrum, directed slightly distolaterally; two small pointed condyles directed downwards. Fertile zooids clustered in whorls causing inflation of internode width, rhomboidal with a straight to slightly convex distal margin, wider and squatter (mean ZL/ZW 1.10) and with a slightly broader opesia than autozooids. Ovicells endotoichal, appearing as a fissure 70-90 µm long by 12-24 µm wide, with two small U-shaped indentations (Figure 17D, arrowed), c. 20 µm deep, at the corners, covered by a visor-like rectangular portion of the distal rim of the maternal autozooid.
Measurements (µm). ZL 334±37, 280-396 (4, 14); ZW 216±39, 153-278 (4, 14); OpL 54±6, 46-69 (4, 16); OpW 92±10, 78-109 (4, 16); ZL (fertile) 318±45, 276-363 (3, 4); ZW (fertile) 287±65, 232-372 (3, 4); OpL (fertile) 57±9, 48-67 (3, 4); OpW (fertile) 111±19, 93-128 (3, 4); AvL (cystid) 309 (1, 1); AvW (cystid) 189 (1, 1).
Remarks. Cellaria levigata sp. nov. is distinct from all Recent Japanese, North-Pacific and Indo-Pacific species of Cellaria. Cellaria anceps Harmer, 1926 differs by having internodes with six zooids in a whorl, a granular cryptocyst, and the absence of frontal ridges; Cellaria boninensis Silén, 1938 has a granular cryptocyst, more centrally placed opesia, and an avicularium with a slightly curved and asymmetrical rostrum (Di Martino, 2023); Cellaria granulata Canu and Bassler, 1929 is similar to the new species in having inflated internodes where there are fertile zooids, and fertile zooids with a convex transverse opening, but differs by having an ovicell fissure covered by a cryptocystal lamella raising from the distal zooid, and a granular cryptocyst; Cellaria japonica Canu and Bassler, 1929 shares a smooth cryptocyst with the new species but differs by having internodes larger in diameter, more than four zooids in a whorl, maternal zooids with an orbicular pore, non-inflated fertile internodes, and more centrally placed opesia; Cellaria mandibulata Hincks, 1882, also has a smooth cryptocyst but is distinguished by its avicularia, which are shorter and twice as wide as the autozooids; Cellaria punctata (Busk, 1852) has a granular cryptocyst, more centrally placed opesia, and an avicularium with triangular squat rostrum (Tilbrook et al., 2001); Cellaria tenuirostris (Busk, 1852), in addition to a granular cryptocyst, also has a narrower and more pointed avicularian rostrum (Gordon, 1984; Achilleos et al., 2020; Bock, 2024).
Superfamily CRIBRILINOIDEA Hincks, 1879
Family CRIBRILINIDAE Hincks, 1879
Genus CRIBRILARIA Canu and Bassler, 1929
Cribrilaria harmeri Ristedt, 1985
Figure 18
v. 1985 Cribrilaria harmeri Ristedt, p. 26, figs. 1, 6-9.
v. 1988 Cribrilaria harmeri Ristedt; Hayward, p. 290, pl. 3c.
v. 2006 Puellina harmeri (Ristedt); Dick, Tilbrook and Mawatari, p. 2213, fig. 6A, C, E, G.
v. 2017 Puellina harmeri (Ristedt); Dick and Grischenko, p. 170, fig. 9.
v. 2018 Puellina harmeri (Ristedt); Yang, Seo and Gordon, p. 229, figs. 36-38.
 Figured material. PMC EDM-Collection J.H.B.129a, sample 19069 (Figure 18A-B), sample 19019 (Figure 18C-E), sample 19072 (Figure 18F), and sample 19113 (Figure 18G-I); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.129a, sample 19069 (Figure 18A-B), sample 19019 (Figure 18C-E), sample 19072 (Figure 18F), and sample 19113 (Figure 18G-I); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, oval to rounded polygonal, longer than wide (mean ZL/ZW 1.31). Frontal shield flat to slightly convex, consisting of 14-18 (mean 16) costae, including suboral pair, maximum basal width 23-48 µm, narrowing towards the centre, meeting along a straight or curved midline, with opposing tips either touching or alternating; pelmatidium inconspicuous, 2-5 µm in diameter. Costae connected by intercostal bridges creating 5-10 slit-like to reniform intercostal lacunae, 8-16 µm in length. Suboral pair of coastal number forming a small umbo in the midline, either leaving a conspicuous, rounded to heart-shaped suboral pore, measuring 10-24 µm in diameter, or a tiny suture, possibly related to ontogenesis. Gymnocyst smooth, exposed along zooidal margins, typically wider proximally. Orifice semicircular, almost as long as wide, with straight proximal margin; seven, evenly spaced oral spine bases, 10-20 µm in diameter, with the proximalmost pair placed slightly above the proximal oral margin; four spines in ovicellate zooids (three observed in one instance). Avicularia both interzooidal and adventitious. Interzooidal avicularia emerging from distolateral pore-chamber windows, resting on the substrate atop a small, bulbous cystid of smooth gymnocyst; rostrum smooth, long, slender, needle-like, resting on the frontal costate shield of distal zooid. Adventitious avicularia small, placed on the latero-oral gymnocyst without touching the substrate, usually paired, associated with ovicells; rostrum short, acutely triangular, directed distally or distolaterally outwards. Both types of avicularia with small mandibular pivots and finely denticulate rostrum. Ovicells of types A (produced by a distal autozooid; Figure 18D) and B (produced by a distal kenozooid; Figure 18C, F) as defined by Bishop and Househam (1987), and type 1 of Ostrovsky (2013); ooecium smooth with central ridge, reflecting the continuation of the costa from the distal zooid or kenozooid into the ooecium, and a small central umbo; distal portion of kenozooid almost always visible. Kenozooids elliptical to rounded polygonal, with costate shield of 13-16 radial costae. Pore-chamber windows seen along lateral walls of zooids at colony periphery, rounded to elliptical, 20-37 µm long by 6-17 µm wide.
Measurements (µm). ZL 416±41, 322-487 (4, 20); ZW 318±46, 212-417 (4, 20); GymL 71±27, 33-142 (4, 20); OL 76±5, 65-83 (4, 20); OW 75±4, 65-81 (4, 20); AvL (adventitious) 106±18, 83-142 (3, 10); AvW (adventitious) 46±4, 42-53 (3, 10); AvL (interzooidal) 206±10, 197-217 (2, 3); AvW (interzooidal) 50±2, 49-53 (2, 3); OvL 147±20, 119-183 (3, 10); OvW 184±24, 139-215 (3, 10); KzL 305±53, 215-344 (3, 5); KzW 247±42, 198-291 (3, 5).
Remarks. Cribrilaria harmeri has been recorded widely from localities including the Philippines (Ristedt, 1985), Mauritius (Hayward, 1988), Hawaii (Dick et al., 2006), Jeju Island in South Korea (Yang et al., 2018), and Okinawa (Dick and Grischenko, 2017). This species shows considerable intraspecific and intracolonial variability, particularly in the denticulation of the avicularian rostra, which can range from smooth to finely denticulate or markedly serrated. Variability is also observed in the formation of the ovicells. Ooecia can be derived from either the distal autozooid or kenozooid. The development of the kenozooid associated with the ooecium can also vary. Some are well developed and visible in frontal view, with as many as ten costae, while others are less developed, with the frontal part not visible. An additional variation observed in the present specimens is in the number of intercostal lacunae, which are more numerous in the Japanese material than in previous descriptions.
This species stands out by having both interzooidal and adventitious avicularia. This characteristic complicates the task of assigning it to a specific genus, particularly because one of the primary distinctions between Cribrilaria and Glabrilaria is the type of avicularia they possess (Rosso et al., 2018b). Canu and Bassler (1929) first established Cribrilaria as a genus. However, Bishop and Househam (1987) later reclassified it as a subgenus of Puellina, while introducing a second subgenus, Glabrilaria, for species with pedunculate avicularia. Rosso et al. (2018b) subsequently elevated all three taxa — Cribrilaria, Glabrilaria, and Puellina — to genus level. This taxonomic shift awaits validation through molecular sequence analysis. The presence of a species like C. harmeri, which features both types of avicularia, questions the legitimacy of the distinction between Cribrilaria and Glabrilaria. However, we prefer to keep the species in Cribrilaria because if future molecular studies suggest merging the two genera, Cribrilaria would have priority over Glabrilaria as it was established earlier.
Genus GLABRILARIA Bishop and Househam, 1987
Glabrilaria biavicularia (Kataoka, 1961)
Figure 19
v. 1961 Cribrilaria biavicularia Kataoka, p. 242, pl. 27, fig. 5.
F igured material. PMC EDM-Collection J.H.B.130a, sample 19025; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
igured material. PMC EDM-Collection J.H.B.130a, sample 19025; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, oval to rhomboidal or rarely polygonal, longer than wide (mean ZL/ZW 1.37). Frontal shield flat, consisting of 12-15 (mean 14) narrow costae including suboral pair, maximum basal width 20-37 µm, tapering towards the centre, converging along an irregular midline, adorned with a prominent, pointed pelmatidium at the base. Costae connected by intercostal bridges creating 5-7 slit-like to reniform intercostal lacunae, 8-18 µm in length. Suboral pair of costae extremely reduced, originating within the length of orifice proximal margin not laterally relative to the orifice, extending proximally (almost longitudinally to the zooid elongation) and creating a distinctive suboral area with a large, rounded to upside-down triangular suboral pore, measuring 23-32 × 27-34 µm. Gymnocyst exposed along zooidal margins, typically wider proximally (proximal axial length 50-104 µm). Orifice semicircular with straight proximal margin; five, regularly spaced oral spine bases, 10-15 µm in diameter, the proximalmost pair placed slightly above the proximal oral margin. Avicularia teardrop-shaped, mostly paired and of different sizes, occasionally single, emerging from basal pore chambers lateral to the orifice, positioned either at the same level as the proximal margin of the orifice or slightly above aligned with the proximalmost pair of spines; rostrum slender and needle-like, oriented distomedially around distal edge of orifice, resting beside oral spines; small mandibular pivots present. A single kenozooid observed, polygonal, with reduced costate shield of six radial costae, 116 × 114 µm. Pore-chamber windows visible along lateral walls of zooids at colony periphery, rounded to elliptical, 9-26 µm long by 5-10 µm wide. Ovicells not observed.
Measurements (µm). ZL 348±41, 298-415 (1, 11); ZW 254±11, 239-269 (1, 11); OL 60±2, 58-63 (1, 10); OW 65±3, 60-70 (1, 10); AvL 98±16, 77-142 (1, 16); AvW 41±4, 36-49 (1, 16); KzL 189 (1, 1); KzW 192 (1, 1).
Remarks. The characteristics observed, including the number of oral spines, costae, and avicularia, as well as the position and orientation of the avicularia, and size measurements fall within the documented variability range for this species as described by Kataoka (1961). In the single fragment recovered, the number of spines consistently remains at five, while Kataoka (1961) noted a range of 4-6 spines in his Pleistocene specimens. Additionally, there is also a slight discrepancy in the variability of coastal number: 12-15 in the Holocene fragment compared to 11-14 in the Pleistocene specimens. It is possible that the vestigial suboral costae were not accounted for by Kataoka (1961). Furthermore, upon examination of the holotype image, albeit with caution due to its poor quality, there appears to be a potential difference in the development of the gymnocyst, which seems less extensive than in our material. Kataoka (1961) did not mention the presence of pointed pelmatidia, which may be due to preservational issues, as observed also in some zooids within our fragment.
Glabrilaria ossuosa sp. nov. Di Martino, Rosso and Taylor
Figure 20
zoobank.org/F5D22272-058D-48AE-B629-E69B4164CB60
Type material. Holotype PMC. B51. 29.7.2024a, sample 19071 (Figure 20A-D); paratype PMC. B51. 29.7.2024b, sample 19053 (Figure 20E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
 Etymology. Latin, meaning bony, referring to the narrow, sharp, angular appearance of the costae.
Etymology. Latin, meaning bony, referring to the narrow, sharp, angular appearance of the costae.
Diagnosis. Glabrilaria with 14-17 narrow, angular, ridged costae, 3-8 intercostal lacunae, single, minute, flat pelmatidium at the outer end of each costa, large rounded-to-kidney-shaped oral lacuna without a mucro; seven oral spine bases in autozooids, four retained in ovicellate zooids; single or paired adventitious, semi-erect latero-oral avicularia with a needle-like rostrum, directed distolaterally medially, either transversely positioned on the costate frontal shield of the distal zooid or covering one side of the ovicell; ooecium globular, smooth, with central suture beginning proximally and closing around mid-length.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, oval to rhomboidal, occasionally polygonal, longer than wide (mean ZL/ZW 1.33). Frontal shield flat to slightly convex, consisting of 14-17 (mean 15) costae including suboral pair; costae narrow, angular, ridged, with a maximum basal width of 17-29 µm, tapering and converging towards the centre, along an undulate midline; each costa with a minute, flat, circular pelmatidium, measuring 2-4 µm in diameter, at its outer end. Costae connected by intercostal bridges creating 3-8 elliptical intercostal lacunae, 11-17 µm in length. Suboral pair of costae greatly reduced, forming a large, rounded to kidney-shaped suboral lacuna, measuring 8-16 × 12-27 µm; in some instances the suboral costae not fusing, creating a trifoliate lacuna, while in other cases bifurcating and meeting at two separate points, resulting in two lacunae of different sizes, with the distalmost larger than the proximalmost; suboral costae flat, not forming a suboral mucro. Gymnocyst exposed along zooidal margins, typically wider proximally, 28-81 µm in length. Orifice transversely D-shaped with straight proximal margin; seven, regularly spaced oral spine bases, 10-14 µm in diameter, with the proximalmost pair placed slightly above the proximal oral margin. Avicularia teardrop-shaped, mostly paired, occasionally single, semi-erect, emerging from basal pore chambers lateral to the orifice, positioned at the same level as the suboral or rarely the second distalmost pair of costae; rostrum slender and needle-like, oriented distolaterally and medially, either resting on the costate frontal shield of the distal zooid or occupying one side of the ovicell; small mandibular pivots present. Ovicell hyperstomial, globular, either kenozooidal with the kenozooid not visible in frontal view, or formed by the distal autozooid; ooecium smooth, with median suture beginning proximally and closing at about halfway along its length. Kenozooids present, typically polygonal, often rounded triangular, with reduced costate shield of 10 radial costae and 3-5 intercostal lacunae. Pore-chamber windows visible along lateral walls of zooids at colony periphery, rounded to elliptical, 6-75 µm long by 5-50 µm wide.
Measurements (µm). ZL 321±34, 265-396 (3, 20); ZW 241±37, 192-310 (3, 20); GymL 51±13, 28-81 (3, 20); OL 51±3, 48-57 (3, 18); OW 66±4, 61-72 (3, 18); AvL 100±28, 59-162 (3, 20); AvW 35±3, 31-41 (3, 20); OvL 103±16, 76-128 (2, 16); OvW 148±13, 113-163 (2, 16); KzL 181±52, 145-218 (1, 2); KzW 167±86, 107-228 (1, 2).
Remarks. Glabrilaria ossuosa sp. nov. shares several characteristics with the two other cribrilinid species identified in the Okinawa core samples. It resembles G. biavicularia in the appearance of its costate frontal shield, which consists of narrow, angular costae, and in the presence of adventitious latero-oral avicularia. However, G. ossuosa sp. nov. differs from G. biavicularia in the number of oral spines, consistently seven in the former species, consistently five in the latter species, in the shape of the pelmatidium, flat in G. ossuosa sp. nov. and tubercular in G. biavicularia, and in the shape of the orifice, which is more elongate in G. biavicularia (mean OL/OW 0.92 in G. biavicularia vs 0.78 in G. ossuosa sp. nov.). In addition to these differences, the suboral area of G. biavicularia is also very distinctive.
Glabrilaria ossuosa sp. nov. also shares the consistent number of oral spines with Cribrilaria harmeri, both having seven. They differ in the ooecium type, that if kenozooidal in C. harmeri generally has a visible kenozooid, which is not apparent in G. ossuosa sp. nov. In addition, G. ossuosa sp. nov. lacks interzooidal avicularia and the adventitious avicularia are differently directed.
The new species differs from all other Glabrilaria with seven spine bases. Glabrilaria africana (Hayward and Cook, 1983), from South Africa, has a tubercular pelmatidium, an oooecium with an umbo, and a suboral area containing up to five large pores along with several smaller ones. Glabrilaria antoniettae Ramalho and Moraes, 2021, found in the West Atlantic off the coast of Brazil, has a distinct suboral mucro and avicularia with pronounced serrations of rostra. Glabrilaria corbula Bishop and Househam, 1987, described from the English Channel, is distinguished by its ooecium, which has radial ridges and lacks a median suture, and avicularium with a curved rostral tip forming a beak-like projection. Glabrilaria hirsuta Rosso in Rosso et al., 2018b, also from the West Atlantic, has a spiny costate frontal shield. Glabrilaria septemspinosa (d’Hondt, 1986) comb. nov. from New Caledonia, has fewer costae compared to G. ossuosa sp. nov., as well as differently directed avicularia.
Superfamily CATENICELLOIDEA Busk, 1852
Family CATENICELLIDAE Busk, 1852
Genus AURICELLINA gen. nov. Di Martino, Rosso and Taylor
zoobank.org/9D103D7B-EB39-4509-BB3D-61D990BDABB7
Type species. Auricellina biserialis gen. et sp. nov. Di Martino, Rosso and Taylor.
Etymology. Latin, prefix meaning ear, referring to the position of the paired pore areas flanking the orifice, and suffix meaning small cell, commonly used in bryozoan genus names.
Diagnosis. Colony erect, jointed, branching. Internodes uni- to biserial, uni- to multizooidal. Autozooids claviform, narrowing proximally. Frontal shield largely gymnocystal, smooth, with no costae or foramina. Orifice monomorphic, transversely D-shaped with a shallowly concave proximal margin, centrally interrupted by a short suture. Two symmetrical, large, slightly depressed, drop-shaped, cryptocystal pore areas (vittae) flanking the orifice and extending proximally beyond it, with sparse, minute, circular pores; additional smaller pore areas possibly present on the sides or the back. Ovicell subimmersed, elongate, occupying the caudal and one-third of proximal portion of the distal zooid; ooecium frontally flat, sometimes developing a median longitudinal carena, and with an ectooecial slit-like fissure on its proximal third, placed medially, extending horizontally, slightly arched, sometimes closed centrally and separated into two openings of different sizes and shapes obliquely oriented; ooecial opening quadrangular, likely not closed by zooidal operculum. Avicularia not observed, seemingly lacking.
Remarks. Gordon (1993) described a new species of the catenicellid genus Talivittaticella Gordon and d’Hondt, 1985, namely T. nuda Gordon, 1993, from Mindoro Strait, Philippines, at depths of 92-97 m. He noted that although no other genus could accommodate this species, it was very unusual among species of Talivittaticella in lacking costae and foramina. However, the small suture on the proximal margin of the orifice suggested a relationship with species having a costate shield. The absence of an ovicell in Gordon’s specimens prevented further taxonomic action at that time.
Our study of the present material, though limited to a single internode, allowed for the observation of the ooecium. As in other Catenicellidae (e.g., Pterocella Levinsen, 1909), the fertile zooid develops as part of a complex that includes the ovicell and the distal zooid (Ostrovsky, 2013). The distinctive feature of the ooecium is a slit-like ectooecial fenestra, which can calcify over to form two smaller windows. This unique characteristic distinguishes this genus not only from Talivittaticella but also from other genera of Catenicellidae. In other Catenicellidae, the ectooecium is completely or partially uncalcified, leaving numerous small windows or a few (one or two) large fenestrae, through which the endooecium is visible. The endooecium can vary from smooth, to reticulate, finely porous, spinous or a combination of these patterns.
Differences between this new genus and other genera in the family extend beyond the ooecium. Here, we briefly highlight the most salient distinctions.
Calpidium Busk, 1852 and Claviporella MacGillivray, 1887, have a keyhole-shaped orifice and costate frontal shields. Costate frontal shields are characteristic of Costaticella Maplestone, 1899, Pterocella and the fossil genus Costatimorpha Zágoršek, 2003, as well as Digenopora Maplestone, 1899. Additionally, Calpidium has a frontal shield with multiple gymnocystal regions separated by ridges, and has terminal ovicells and dimorphic fertile zooids. This latter feature is also found in Cornuticellina Stach, 1935 and Strophipora MacGillivray, 1895. Claviporella and Strophipora also have frontal shield with a median suture and an uncalcified central opening.
Catenicella Busk, 1852, Cornuticella Canu and Bassler, 1927, Scalicella Harmer, 1957 and Terminocella Harmer, 1957 have vittae along the lateral margins, rather than on the side of the orifice. In Catenicella, avicularia are frequent, while in Cornuticella, the distal compartments of autozooids develop into long spinose processes. Cribricellina Canu and Bassler, 1927 and Paracribricellina Wass and Yoo, 1976 have densely and evenly porous gymnocysts and also fertile zooid with enlarged dimorphic orifice. In Cribricellina, the ooecium has numerous radially arranged pores, while Paracribricellina has a deep, wide ectooecial fenestra exposing the endooecium, which can be finely porous, smooth, or papillate. Orthoscuticella Wass and Yoo, 1975 has several windows on the frontal shield and dimorphic fertile zooids, in addition to an orifice with a small central notch. Scuticella Levinsen, 1909 has large windows in the gymnocyst unequal in size, and dimorphic fertile zooids with an ectooecium calcified only proximally and a largely exposed endooecium, which is spinous and porous with pores organized in a V-pattern. Strongylopora Maplestone, 1899 and Vasignyella Gordon, 1989a have numerous windows in the frontal gymnocyst, mainly confined to the lateral margins in Strongylopora, but extending centrally in one species of Vasignyella. The distal corners can develop to different extents into spinose processes, but they always bear avicularia.
Despite T. nuda lacking ovicells, the consistent presence of certain features (e.g., pore areas adjacent to orifice and their shape) and absence of others (e.g., costae and foramina) warrant the introduction of a new genus to encompass this species and the new species described here. Therefore, we propose the new combination Auricellina nuda (Gordon, 1993) comb. nov.
The differentiation between genera with uni-, bi-, and trizooidal segments versus those with segments comprising more than three autozooids (i.e., multizooidal) has traditionally been a key feature for distinguishing genera in the subfamilies Catenicellinae Stach, 1935 and Ditaxiporinae Stach, 1935 (Gordon and Braga, 1994; Vieira et al., 2007). However, this new genus includes one species with uni- to bizooidal internodes and another species with multizooidal segments. This variability is also seen in Vasygniella, which can have both uniserial chains of unizooidal internodes and biserial multizooidal internodes, with ovicellate zooids always occurring in multizooidal internodes (Vieira et al., 2007). This suggests that if infertile internodes of the newly described species A. biserialis gen. et sp. nov. (see below) were found, they could be uni- or bizooidal, while fertile segments of A. nuda comb. nov. might be multizooidal.
The new genus differs also from other genera within the subfamily Ditaxiporinae. Bryosartor Gordon and Braga, 1994 has a costate frontal shield, a largely uncalcified ectooecium, granular endooecium, and avicularia. Caberoides Canu, 1908 has a dorsal side with vibracular-like chambers. Ditaxipora MacGillivray, 1895 and Ditaxiporina Stach, 1935 have keyhole-shaped orifices and distolateral avicularia. Ditaxipora additionally has a frontal shield largely occupied by vittae, while Ditaxiporina has numerous gymnocystal windows. Plagiopora MacGillivray, 1895 also has an elongate orifice, typically paired distolateral avicularia, and vittae on the frontal side that are continuous with those on the dorsal side.
Auricellina biserialis sp. nov. Di Martino, Rosso and Taylor
Figure 21
zoobank.org/CB9F4D60-17BC-4888-9A2A-53E590D84641
Type material. Holotype PMC. B52. 29.7.2024a, sample 19132; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Etymology. Latin, meaning two rows, referring to the arrangement of autozooids in two alternating series within the internode.
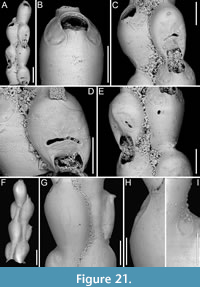 Diagnosis. Catenicellid with multizooidal, biserial internodes. Autozooids claviform, arranged in alternating series. Frontal shield smooth, with symmetrical pore areas (vittae) adjacent to the orifice extending proximally beyond it, and minute pores scattered along the margin. Orifice transversely D-shaped with shallowly concave proximal margin interrupted medially by a short suture. Ovicell elongate, flat frontally, with a horizontal median slit-like fissure or two uncalcified areas on each lateral half; sometimes with a longitudinal carena. Avicularia seemingly absent. Dorsal side smooth with a small drop-shaped or narrowly elliptical pore area, with a few pores, placed at the distolateral corner of autozooids or distal margin of the ooecium.
Diagnosis. Catenicellid with multizooidal, biserial internodes. Autozooids claviform, arranged in alternating series. Frontal shield smooth, with symmetrical pore areas (vittae) adjacent to the orifice extending proximally beyond it, and minute pores scattered along the margin. Orifice transversely D-shaped with shallowly concave proximal margin interrupted medially by a short suture. Ovicell elongate, flat frontally, with a horizontal median slit-like fissure or two uncalcified areas on each lateral half; sometimes with a longitudinal carena. Avicularia seemingly absent. Dorsal side smooth with a small drop-shaped or narrowly elliptical pore area, with a few pores, placed at the distolateral corner of autozooids or distal margin of the ooecium.
Description. Colony erect, likely jointed and branching; branching pattern unknown. Internodes multizooidal and biserial. Autozooid distinct, separated by deep grooves, claviform, tapering proximally forming a tubular cauda 180-220 µm long and c. 65 µm wide, much longer than wide (mean ZL/ZW 3 including cauda), arranged in alternating series. Frontal shield convex, smooth, largely gymnocystal except for two symmetrical, cryptocystal pore areas (vittae) adjacent to the orifice, extending proximally beyond it, slightly depressed in relation to their rim and the surrounding gymnocyst, 80-105 µm long by 40-75 µm wide, with minute pores scattered along the margin; pores circular, 1-2 µm in diameter; costae and foramina absent. Orifice monomorphic, transversely D-shaped, with proximal margin starting with straight corners sloping towards the centre forming a shallowly concave median proximal part, interrupted medially by a short, vertical suture, 8-12 µm long. Ovicell subimmersed, elongate (mean OvL/OvW 1.55), occupying the cauda and one-third of the proximal portion of the distal zooid; ooecium flat frontally, with an ectooecial slit-like fenestra (45-100 µm long by 5-10 µm wide) on its proximal third, placed medially, extending horizontally, slightly arched, sometimes separated into two openings of different size and shape, obliquely oriented; sometimes developing a median longitudinal carena; ooecial opening quadrangular, c. 45 µm long by 65-80 µm wide, likely not closed by zooidal operculum. Dorsal side smooth, entirely gymnocystal except for a drop-shaped or narrowly elliptical (c. 70 µm long) cryptocystal pore area smaller than those on the frontal (40-45 µm long by 20-25 µm wide); cryptocystal pore area placed at the dorsal distolateral corner of autozooids or if ovicellate at the dorsal distolateral margin of ooecium, with 1-2 circular pores, 3 µm in diameter; a pronounced suture between the back of the ooecium and the margin of the cauda visible proximally, distally the dorsal side of the ooecium seemingly fusing with the surface of the cauda. Avicularia seemingly absent.
Measurements (µm). ZL 531±28, 507-561 (1, 3); ZW 186±7, 179-194 (1, 3); OL 62±7, 57-67 (1, 2); OW 79.5±1, 79-80 (1, 2); OvL 255±10, 248-262 (1, 2); OvW 163.5±11, 156-171 (1, 2).
Remarks. This species differs from the only other species assigned to the genus in the arrangement of internodes. In Auricellina nuda (Gordon, 1993) comb. nov., internodes consist of 1-2 autozooids, while in A. biserialis gen. et sp. nov. there are at least five, but probably more, autozooids per internode. Another difference is in the presence of pore areas on the sides of the cauda in A. nuda comb. nov., absent in A. biserialis sp. nov., and in the number of pores in the vittae, which are more abundant in A. nuda comb. nov. than in A. biserialis sp. nov.
Superfamily ARACHNOPUSIOIDEA Jullien, 1888
Family EXECHONELLIDAE Harmer, 1957
Genus EXECHONELLA Canu and Bassler in Duvergier, 1924
Exechonella coronida sp. nov. Di Martino, Rosso and Taylor
Figure 22
zoobank.org/A0297EDF-09F0-4B12-A0C4-AD5C572DCF8B
Type material. Holotype PMC. B53. 29.7.2024a, sample 19010 (Figure 22A-C); paratype PMC. B53. 29.7.2024b1, sample 19007 (Figure 22D); paratype PMC. B53. 29.7.2024b2, sample 19035 (not figured); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
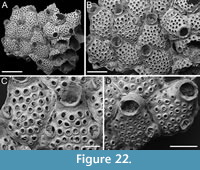 Etymology. Greek ‘koronída’, meaning coronis, i.e., a curved punctuation mark similar to a small apostrophe or a curved line used in ancient Greek to show a crasis (the contraction of two vowels into one), referring to the coronis-like shape of its avicularia.
Etymology. Greek ‘koronída’, meaning coronis, i.e., a curved punctuation mark similar to a small apostrophe or a curved line used in ancient Greek to show a crasis (the contraction of two vowels into one), referring to the coronis-like shape of its avicularia.
Diagnosis. Exechonella of the magna -complex with numerous, medium-sized, rounded, and spaced foramina with narrow rims, single or paired curved avicularia with rounded triangular rostrum proximally directed having a complete crossbar, and a few small marginal pores between avicularia and zooidal margins.
Description. Colony encrusting, multiserial, uni- to multilaminar due to self-overgrowth. Autozooids distinct, separated by deep grooves and thin raised sutures, lozenge-shaped, longer than wide (mean ZL/ZW 1.21). Frontal shield slightly convex, nodular, evenly and densely perforated by 20-42 medium-sized, regularly spaced, rounded foramina with a slightly raised, narrow rim; no frontal projections. Primary orifice concealed by a collar-like peristome; condyles not seen. Peristome pustulose, imperforate; secondary orifice semicircular to elliptical to eye-shaped. Avicularia adventitious, sporadic; if present, single or paired, placed approximately at zooidal mid-length, laterally, adjacent to zooidal lateral corners deriving from a marginal areolar pore and associated with a foramen. Foramina associated with avicularia having modified shape compared to foramina elsewhere on frontal shield, appearing more elongate elliptical or resembling a fissure; a raised rim outlines both the avicularium and the associated foramen, with the total diameter outlined by this rim, occupied by the avicularium for two-thirds, and by the foramen itself for one-third. Avicularia curved, rostrum rounded triangular, proximally directed, external lateral edge raised; crossbar complete; 3-5 small (14-28 µm in diameter), circular marginal areolar pores present between the external lateral edge of the avicularium and the margin of the zooid. Clusters of up to five circular marginal areolar pores often developing in the same position, even in the absence of an avicularium.
Measurements (µm). ZL 913±115, 687-1144 (3, 20); ZW 752±80, 614-940 (3, 20); OL (secondary) 185±15, 169-198 (1, 3); OW (secondary) 235±15, 222-251 (1, 3); PeL 117±35, 61-161 (3, 8); FoN 30±5, 20-42 (3, 20); FoD 45±9, 32-59 (3, 20); AvL 151±18, 118-173 (3, 20); AvW 93±14, 78-122 (3, 20).
Remarks. This species belongs to the Exechonella magna -complex, which is characterized by lozenge-shaped zooids, low collar-like peristomes, numerous large foramina with free space in between, and proximally directed triangular avicularia equipped with a complete crossbar (Cook and Bock, 2004; Cáceres-Chamizo et al., 2017). Although efforts have been made to disentangle other Exechonella species-complexes such as the E. ampullacea -complex, E. antillea -complex, E. brasiliensis -complex and E. verrucosa -complex (see Cáceres-Chamizo et al., 2017), the E. magna -complex remains unresolved. Exechonella coronida sp. nov. is easily distinguished from all other specimens currently identified as E. magna (McGillivray, 1895) because of the curved shape of its avicularia. In specimens of E. magna from the Miocene of Muddy Creek (Australia), avicularia are either lozenge-shaped (Cook and Bock, 2004, figure 2A) or drop-shaped (Cook and Bock, 2004, figure 3A), consistently with an acutely triangular rostrum. Recent specimens attributed to E. magna from the Solomon Islands also have drop-shaped avicularia (Tilbrook, 2006, pl. 18E), while avicularia are described as triangular for the Philippines’ specimens of Canu and Bassler (1929).
The general shape and distribution of foramina in E. coronida sp. nov. are reminiscent of species in the E. brasiliensis -complex. However, avicularia in this group lack a crossbar and have a central nipple-like structure (Cáceres-Chamizo et al., 2017).
Exechonella juliae sp. nov. Di Martino, Rosso and Taylor
Figure 23
zoobank.org/9C66D176-4D5D-4DDD-92E7-308A1E367398
Type material. Holotype PMC. B54. 29.7.2024a, sample 19014 (Figure 23D-E); paratype PMC. B54. 29.7.2024b1, sample 19059 (Figure 23A-B); paratype PMC. B54. 29.7.2024b2, sample 19015 (Figure 23C); paratype PMC. B54. 29.7.2024b3, sample 19042 (Figure 23F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
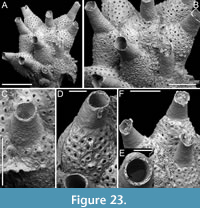 Etymology. Named in honour of Dr Julia Cáceres-Chamizo, recognizing her significant contribution to the study of Exechonella and her efforts to disentangle its species complexes.
Etymology. Named in honour of Dr Julia Cáceres-Chamizo, recognizing her significant contribution to the study of Exechonella and her efforts to disentangle its species complexes.
Diagnosis. Exechonella of the ampullacea -complex with numerous, medium-sized, rounded foramina with narrow rims, limited number of flat frontal projections, and tubular peristome with flared and jagged rim.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep grooves, bottle-shaped, longer than wide (mean ZL/ZW 1.34). Frontal shield convex, pustulose, evenly and densely perforated by 32-44 medium-sized, rounded foramina with a slightly raised, narrow rim; one to three foramina per zooid bearing a flat, blunt or pointed, frontal projection reaching up to 50-90 µm in length; shape of foramina modified to reniform or semicircular when bearing the frontal projection. Primary orifice oval, concealed by a long, tubular, distally tapering peristome; condyles not seen. Peristome pustulose externally, with pustules 20-35 µm in diameter, longitudinally striated on its internal surface with a distance between striations of 25-38 µm, imperforate, facing obliquely frontally, forming an angle of 15-70º in respect to the colony surface; peristome margin slightly flared and jagged; secondary orifice circular to elliptical. Avicularia absent. Kenozooids not observed.
Measurements (µm). ZL 813±68, 707-943 (5, 20); ZW 606±69, 519-791 (5, 20); OL (secondary) 220±30, 172-257 (3, 10); OW (secondary) 227±33, 155-262 (3, 10); PeL 493±89, 301-652 (5, 20); FoN 38±6, 32-44 (2, 5); FoD 41±5, 31-51 (5, 20).
Remarks. This species belongs to the Exechonella ampullacea -complex, which is characterized by bottle-like zooids, long tubular peristomes, numerous mid-sized foramina and the absence of avicularia (Cáceres-Chamizo et al., 2017). The Japanese species closely resembles E. safagaensis Cáceres-Chamizo, Sanner, Tilbrook and Ostrovsky, 2017 from the Red Sea, and E. variperforata Cáceres-Chamizo, Sanner, Tilbrook and Ostrovsky, 2017 from the Great Barrier Reef, sharing some traits with each. It shares the length of autozooids and the number of foramina with E. safagaensis but differs in having smaller foramina with narrower rims, fewer and flatter frontal projections, longer peristomes, and wider autozooids. With E. variperforata, it shares the flat shape of the frontal projections and the narrower rims of the foramina but differs in having more numerous, smaller foramina, fewer frontal projections, and longer, narrower peristomes.
Exechonella verrucosa (Canu and Bassler, 1927)
Figure 24
v. 1927 Coleopora verrucosa Canu and Bassler, p. 6, 42, pl. 1, fig. 7.
v. 1929 Coleopora verrucosa Canu and Bassler; Canu and Bassler, p. 267-268, pl. 20, fig. 4, pl. 26, fig. 9.
v. 2017 Exechonella verrucosa (Canu and Bassler); Cáceres-Chamizo, Sanner, Tilbrook and Ostrovsky, p. 56, fig. 23.
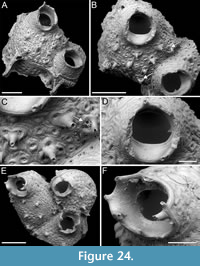 Figured material. PMC EDM-Collection J.H.B.131a, sample 19074 (Figure 24A-D) and sample 19125 (Figure 24E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.131a, sample 19074 (Figure 24A-D) and sample 19125 (Figure 24E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting. Autozooids distinct, separated by deep grooves, oval to rhomboidal, slightly longer than wide (mean ZL/ZW 1.13). Frontal shield slightly convex, wrinkled, tessellated, evenly and densely punctured by over 40 minute, circular foramina, c. 15 µm in diameter; foramina centrally located within a larger funnel-shaped gymnocystal rim, measuring between 30-40 µm in diameter; often, rims of 2-3 foramina fusing together, forming distinctive reniform to trifoliate clusters adorned with tubular, hollow, spike-like frontal projections, 75-125 µm long, 25-40 µm in diameter at the tip; projections randomly distributed across the frontal shield, with about 6-8 per zooid in the specimens examined; numerous circular marginal pores, roughly 15 µm in diameter, distributed around zooidal periphery. Primary orifice subcircular, slightly wider than long, surrounded by a short, collar-like peristome; stout, triangular condyles, 35-40 µm long by 40-45 µm wide, medially directed, placed at two-thirds of orifice length, separating the orifice into equally wide anter and poster, with anter occupying two-thirds and poster one-third of orifice length. Peristome externally pustulose, internally smooth to slightly wrinkled, forming a sloping shelf proximally, developing spiny processes around its rim; eight such processes counted in the single zooid in which they were all preserved, with the lateral one showing a bifurcated structure. Avicularia present on the rims of lateral or proximal foramina producing tubular projections, characterized by a distinctive nipple-like structure with a central pore.
Measurements (µm). ZL 1159±96, 1059-1251 (2, 3); ZW 1028±59, 975-1092 (2, 3); OL 275±3, 273-278 (2, 3); OW 322±26, 298-349 (2, 3); PeL 221±55, 182-260 (2, 2).
Remarks. To date, Exechonella verrucosa is unequivocally known only from the Philippines. Records from other geographical areas have been reassigned to new species in an effort to clarify the E. verrucosa species-complex (Cáceres-Chamizo et al., 2017). Due to the limited availability of material, consisting of only a few fragments with one or two zooids each, it is challenging to observe morphological variation. However, the key characteristics of our specimens fit the updated description of E. verrucosa, which appears to be the closest match among those described within this genus, also in terms of size, number and distribution of foramina. Notable differences we have observed compared to the Recent type specimens include the absence of well-defined longitudinal striations on the inner surface of the peristome; instead, only wrinkles concentric to the proximal orifice margin have been observed. Additionally, adventitious kenozooids associated with the avicularia are also absent in our specimens.
Superfamily ADEONOIDEA Busk, 1884
Family CREPIDACANTHIDAE Levinsen, 1909
Genus CREPIDACANTHA Levinsen, 1909
Crepidacantha longiseta Canu and Bassler, 1928
Figure 25
v. 1928 Crepidacantha longiseta Canu and Bassler, p. 135, pl. 21, figs. 3, 4.
v. 2001 Crepidacantha longiseta Canu and Bassler; Tilbrook, Hayward and Gordon, p. 92, fig. 16B.
v. 2006 Crepidacantha longiseta Canu and Bassler; Dick, Tilbrook and Mawatari, p. 2239, fig. 13g, h.
v. 2016 Crepidacantha longiseta Canu and Bassler; Dick and Grischenko, p. 231, fig. 32.
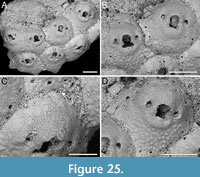 Figured material. PMC EDM-Collection J.H.B.143a, sample 19119; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.143a, sample 19119; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, outlined by deep furrows, nearly as long as wide (mean ZL/ZW 1.03), rounded hexagonal to pentagonal. Frontal shield flat to slightly convex proximally, becoming more convex around the orifice, coarsely tubercular, imperforate except for a row of circular to elliptical marginal areolae (max diameter 8-20 µm) along the zooid perimeter, often obscured by neighbouring zooids; peripheral spines likely present, a single base, measuring 10 µm in diameter, observed. Orifice keyhole-shaped, with horseshoe-shaped anter, two pointed, downward directed condyles, and an almost straight to faintly convex proximal margin. Paired avicularia laterally to orifice, with rostrum tips at level with orifice condyles, directed proximally. Ovicells not observed.
Measurements (µm). ZL 417±45, 363-496 (1, 6); ZW 406±42, 334-456 (1, 6); OL 90±5, 85-95 (1, 4); OW 83±7, 77-91 (1, 4); AvL 48±3, 45-50 (1, 4); AvW 32±3, 29-35 (1, 4).
Remarks. Two species of Crepidacantha with latero-oral avicularia directed proximally have been previously recorded from Japan (i.e., C. crinispina Levinsen, 1909 and C. longiseta Canu and Bassler, 1928) (e.g., Hirose, 2010; Dick and Grischenko, 2017). The two species mainly differ in the curvature of the proximal margin of the orifice, which is flatter in C. longiseta compared to C. crinispina (see Tilbrook, 2006).
We hereby reclassify the family Crepidacanthidae, moving it from the superfamily Mamilloporoidea Canu and Bassler, 1927 to the superfamily Adeonoidea. This decision is based on the molecular phylogenetic analysis conducted by Orr et al. (2022), which demonstrated that Crepidacantha is well nested within Adeonoidea. Shared characters, such as numerous basal pore-chambers, support this reallocation. The previous classification under Mamilloporoidea was primarily due to the superficial similarity of the pseudoporous ovicells.
Superfamily LEPRALIELLOIDEA Vigneaux, 1949
Family ROMANCHEINIDAE Jullien, 1888
Genus ESCHAROIDES Milne-Edwards, 1836
Escharoides cavernicolus sp. nov. Di Martino, Rosso and Taylor
Figure 26
zoobank.org/F3A4DDF7-89DB-4A74-BA7A-939006E21AFE
Type material. Holotype PMC. B55. 29.7.2024a, sample 19132 (Figure 26A-D); paratype PMC. B55. 29.7.2024b1, sample 19105 (Figure 26E-F); paratype PMC. B55. 29.7.2024b2, sample 19125 (Figure 26G); paratype PMC. B55. 29.7.2024b3, sample 19094 (Figure 26H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
 Etymology. Latin, meaning cave-dweller, referring to its commonness in the samples from Daidokutsu cave.
Etymology. Latin, meaning cave-dweller, referring to its commonness in the samples from Daidokutsu cave.
Diagnosis. Escharoides with 1-2 series of marginal areolar pores surrounding zooids avicularium cystids; 2-4 oral spines in autozooids, two if ovicellate; proximal peristome with extended median denticle; single or paired parallel-sided avicularia, with serrated rostrum, of variable size and position, either placed laterally to orifice juxtaposed with its margin and distally directed, or placed below orifice on the side of zooids, directed proximolaterally; ovicell globular with marginal pores and central umbo; dorsal side with conical pillars; ancestrula tatiform.
Description. Colony encrusting, multiserial, unilaminar, with dorsal side featuring pillars developing medially in correspondence with most zooids. Autozooids distinct, separated by thin furrows, oval to rounded hexagonal, longer than wide (mean ZL/ZW 1.42). Frontal shield smooth, slightly nodular, imperforate except for one or two rows of marginal pores, circular, elliptical or drop-shaped margins, consistently and orderly arranged between ridges along zooidal margins (35-85 µm in maximum diameter), and around avicularian cystids (15-35 µm in maximum diameter). Primary orifice situated deep inside the peristome, rounded quadrangular, approximately as long as wide (158 µm in the single zooid measured); peristome well-developed proximally and laterally, bearing a rectangular median denticle measuring 30-40 µm in length by 30-60 µm in width at the base, extending approximately 120 µm inside the peristome; 2-4 oral spine bases, 35-50 µm in diameter, two visible in ovicellate zooids. Avicularia adventitious, present in two sizes and positions, either small and placed laterally to orifice, juxtaposed with its lateral margin, directed distally, or large and placed on zooidal lateral sides, directed proximolaterally. Four avicularian configurations observed: i) single small avicularium on one side of zooid; ii) a small avicularium juxtaposed with lateral margin of orifice and a large one on the zooidal side; iii) two small avicularia each juxtaposed with orifice side; iv) two large avicularia on each zooidal side. Rostrum raised, serrated, parallel-sided or slightly spatulate; opesia rounded triangular; crossbar complete. Ovicell hyperstomial, globular; ooecium smooth, nodular, with peripheral pores, 50-92 µm in diameter, small umbo centrally, and proximal margin raised and bent. Ancestrula tatiform, elliptical, 300 µm long by 270 µm wide (excluding gymnocyst), with extensive, wrinkled gymnocyst (c. 180 µm long) proximally, and narrow, smooth cryptocyst steeply sloping into the circular opesia, about 200 µm in diameter; 11 spines on the gymnocyst, indenting the cryptocyst, with circular or elliptical bases, 30-60 µm in maximum diameter. Three zooids budded directly from the ancestrula, one distally and two distolaterally, slightly smaller than later autozooids (610-720 µm long by 330-420 µm wide), with a single avicularium, slightly smaller than those found on later autozooids, placed laterally.
Measurements (µm). ZL 968±64, 873-1056 (1, 6); ZW 681±67, 591-743 (1, 6); AvL (small type) 230±34, 159-278 (2, 13); AvW (small type) 86±11, 65-98 (2, 13); AvL (large type) 430±75, 296-544 (2, 11); AvW (large type) 136±21, 91-167 (2, 11); OvL 360±30, 317-385 (1, 4); OvW 454±47, 385-494 (1, 4).
Remarks. Escharoides cavernicolus sp. nov. is one of the most common and abundant species found in the Daidokutsu cave samples. The configuration of its avicularia is highly distinctive. Other Recent and fossil Escharoides species described from Japan include: the Pleistocene to Recent E. adeonelloides (Ortmann, 1890), which exhibits erect bilaminar colonies with oral spines present only in early ontogeny (Mawatari and Kii, 1952; Hirose, 2010); E. hataii Hayami, 1975, a Pleistocene species with or without a single avicularium and 1-3 spines, lacking a peristomial denticle (Hayami, 1975); E. ramulosum Okada and Mawatari, 1937, characterized by erect colonies with cylindrical branches; and E. sauroglossa Levinsen, 1909, which has paired avicularia, with one typically being very spatulate, consistently placed laterally to the orifice, directed distolaterally (Mawatari and Kii, 1952; Hirose, 2010).
We have used the masculine ending “-us” in naming our new species, in accordance with Article 30 of the International Code of Zoological Nomenclature (ICZN, 1999), which states that genera ending with the suffix “-oides” are always masculine unless otherwise specified by the author. In Milne-Edwards (1836), there is no clear definition of the genus or its gender, therefore the ICZN should be followed. Currently, in Escharoides, there are various combinations, indicating that different authors have treated this genus as masculine, feminine (likely due to its inferred derivation from Eschara, which contains the majority of species), or neuter. This situation should be revised according to the code, which clearly states how the gender of genus-group names should be determined. The same issue likely affects many other bryozoan genera.
Genus HIPPOPLEURIFERA Canu and Bassler, 1925
Hippopleurifera hirosei sp. nov. Di Martino, Rosso and Taylor
Figure 27
zoobank.org/DFB2D636-AA1B-4926-9CE4-5C42561DEBD2
Type material. Holotype PMC. B56. 29.7.2024a, sample 19143 (Figure 27A-D); paratype PMC. B56. 29.7.2024b1, sample 19227 (Figure 27E); paratype PMC. B56. 29.7.2024b2, sample 19135 (Figure 27F); paratype PMC. B56. 29.7.2024b3, sample 19217 (Figure 27G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
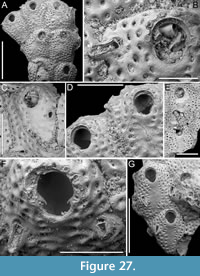 Etymology. Named in honour of Dr Masato Hirose, recognizing his significant contribution to the study of Japanese bryozoans.
Etymology. Named in honour of Dr Masato Hirose, recognizing his significant contribution to the study of Japanese bryozoans.
Diagnosis. Hippopleurifera with nodular frontal shield marked by ridges, with 3-4 rows of marginal areolar pores and narrow, elliptical, imperforate central region; orifice keyhole-shaped with downwardly hooked condyles and 6-9 robust, distolateral oral spine bases; adventitious avicularia large and spoon-shaped or small and pear-shaped, placed either laterally to orifice or frontally, single or paired, directed proximally or proximolaterally externally; ooecium seemingly with pitted fenestrae.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep furrows, slightly longer than wide (mean ZL/ZW 1.21), rectangular to irregularly polygonal. Frontal shield flat to slightly convex, nodular, marked by faint radial ridges, with 3-4 rows of circular to elliptical marginal areolar pores, 20-45 µm in maximum diameter, and a narrow, elliptical, imperforate central region, 360-515 µm long by 150-240 µm wide. Orifice keyhole-shaped with arched anter separated from a shallow, bowl-shaped poster by two downwardly hooked condyles; 6-9 oral spine bases, 28-45 µm in diameter, arranged in an arch surrounding the anter with the proximalmost pair above or at level with condyles. Avicularia adventitious, single or paired, of two sizes and shapes: type 1, large and spoon-shaped placed laterally to orifice level with the proximalmost spines or laterally on the frontal shield at approximately zooidal mid-length or distal-third; or type 2, small and pear-shaped exclusively observed on the frontal shield; both types directed proximally or proximolaterally externally with complete crossbar. Broken ooecia observed, appearing globular, one measuring approximately 440 µm in length by 570 µm in width, with pitted fenestrae.
Measurements (µm). ZL 868±77, 729-972 (3, 10); ZW 717±102, 583-839 (3, 10); OL 248±28, 188-287 (3, 9); OW 211±17, 176-231 (3, 9); AvL (type 1) 193±41, 148-294 (4, 10); AvW (type 1) 75±22, 40-123 (4, 10); AvL (type 2) 105±8, 99-111 (2, 2); AvW (type 2) 66±7, 61-71 (2, 2).
Remarks. The definitions of the genera Hippopleurifera and Hippomenella Canu and Bassler, 1917 suffers from ambiguity. Canu and Bassler’s diagnosis of Hippomenella, particularly with reference to the ovicell, included fossil specimens that could potentially be attributed to Hippopleurifera (see Berning, 2013). Our new species is ascribed to Hippopleurifera based on features from both the ovicell and frontal shield, following Berning (2013): the frontal shield is nearly entirely perforated by areolar pores, indicating an extremely reduced umbonuloid part, and the structure of the ooecium, albeit poorly preserved, which seems to possess fenestrae. As noted in an earlier study of the Cenozoic bryozoan fauna of East Kalimantan, certain species exhibit a combination of characters from both genera, showcasing a bifenestrate ooecium typical of Hippopleurifera and an almost imperforate frontal shield characteristic of Hippomenella, thereby constituting an argument for the synonymization of these two genera (Di Martino and Taylor, 2015).
This new species differs from Pacific species assigned to both Hippopleurifera and Hippomenella. Hippopleurifera lateralis (MacGillivray, 1891) has 10 spines and proximomedially directed avicularia. Hippopleurifera? philippinensis Canu and Bassler, 1929 has six spines and avicularia directed distally. Hippopleurifera porosa (Canu and Bassler, 1929) has small, triangular avicularia and only two rows of marginal areolae. Hippopleurifera repugnans (Canu and Bassler, 1929) has 10-12 oral spines and large avicularia placed distally to the orifice and directed distolaterally. Hippomenella avicularis (Livingstone, 1926) has large spatulate avicularia directed distolaterally (see Gordon and d’Hondt, 1997). Hippomenella chepigae Gontar, 1993 has four spines, and paired avicularia located laterally to the orifice. Although described as directed distally, the drawing depicts them as directed proximally (Gontar, 1993, p. 44, figure 6). Hippomenella coronula (Ortmann, 1890) differs in the shape, size and direction of the avicularia, with those placed laterally to the orifice directed distolaterally. Hippomenella rudicula Tilbrook, 2006 has 14 spines. Hippomenella sp. nov. described in the doctoral dissertation of Hirose (2010), and yet to be formalized, has a distinctly elongate orifice and is devoid of spines. The Pleistocene species Hippomenella konnoi Kataoka, 1961 has 11 spines and a distolaterally directed avicularium placed laterally to the orifice.
Superfamily SMITTINOIDEA Levinsen, 1909
Family SMITTINIDAE Levinsen, 1909
Genus HEMISMITTOIDEA Soule and Soule, 1973
Hemismittoidea dicki sp. nov. Di Martino, Rosso and Taylor
Figure 28
zoobank.org/B2A515A0-3D97-4FE2-A469-8BF29B2126B1
Type material. Holotype PMC. B57. 29.7.2024a, sample 19061 (Figure 28A-C); paratype PMC. B57. 29.7.2024b, sample 19210 (Figure 28D); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
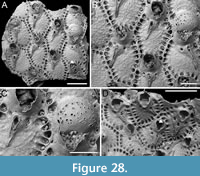 Etymology. Named in honour of Prof. Matthew Dick, recognizing his significant contribution to the study of Japanese bryozoans.
Etymology. Named in honour of Prof. Matthew Dick, recognizing his significant contribution to the study of Japanese bryozoans.
Diagnosis. Hemismittoidea with rounded hexagonal to lozenge-shaped autozooids, each showing a single row of 18-25 closely spaced, marginal areolae within funnel-like depressions separated by distinct ridges; orifice with anvil-shaped lyrula occupying more than half its width; presence of oral spines uncertain; peristome with lateral flaps and sharp constrictions; avicularium positioned asymmetrically between two peristomial pores, needle-like, with acutely triangular, serrated rostrum pointing proximolaterally; ooecium striated and pseudoporous, except for a proximal imperforate portion, with a narrow distal band of secondary calcification.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, slightly longer than wide (mean ZL/ZW 1.23), rounded hexagonal to lozenge-shaped, quincuncially or irregularly arranged. Frontal shield flat or slightly convex, coarsely granular, imperforate except for a single row of 18-25 (with an average of 20) circular to elliptical marginal areolar pores, 19-43 µm in maximum diameter, closely spaced and located within funnel-like depressions separated by distinct ridges; an additional pair of pores of different sizes positioned on each side of distal margin of avicularium, the smallest measuring 12-14 µm in diameter, the largest 22-30 µm in diameter, in a few instances two pores on one side and only one on the other. Primary orifice elliptical, wider than long, with anvil-shaped lyrula occupying more than half of its width (LyW/OW 0.56), and acutely triangular condyles medially directed; short peristome surrounding and hiding primary orifice, more developed laterally, forming two flaps; secondary orifice keyhole-shaped, 160-185 µm long by 150-180 µm wide, with acutely triangular proximal constrictions defining a U-shaped pseudosinus; presence of oral spines uncertain; single, putative spine base rarely observed. Avicularium adventitious, single, needle-like, suboral, asymmetrically positioned, 30-50 µm from peristomial pseudosinus; rostrum raised, elongate, acutely triangular, with roughly serrated edges, directed proximolaterally on either side, with the tip sometimes resting on the marginal areolae; crossbar complete, thicker laterally, thin and fragile centrally, without ligula, creating a small, circular opening distally and a larger upside-down triangular to trapezoidal opening proximally. Ovicell hyperstomial, globular, formed by the distal zooid, not closed by zooidal operculum; ooecium smooth, radially striated, pierced by 40 circular to elliptical pseudopores arranged radially, maximum diameter of 5-30 µm, the proximal rectangular portion flat and imperforate, with a narrow, 30-35 µm wide, peripheral band of secondary calcification spreading from adjacent zooids.
Measurements (µm). ZL 626±78, 512-721 (2, 9); ZW 507±80, 427-678 (2, 9); OL 130 (1, 1); OW 165 (1, 1); AvL 235±52, 143-300 (2, 14); AvW 55±7, 40-66 (2, 14); OvL 256 (1, 1); OvW 310 (1, 1).
Remarks. This new species is attributed to Hemismittoidea because of its asymmetrically developed submedial avicularium, which appears to bud from a distolateral areola and a median peristomial pore. This feature is a key differentiator between Hemismittoidea and Smittoidea Osburn, 1952 (Soule and Soule, 1973; Gordon, 1984).
Hemismittoidea dicki sp. nov. stands out from other known species of Hemismittoidea because of the lack of obvious oral spines. In two instances, a small, circular pore (12 µm in diameter) has been observed on the distal margin of the peristome (see arrows in Figure 28A, D) that could potentially be the base of an ephemeral peristomial spine. Although the original diagnosis of the genus specifies, 2-6 oral spines as a typical character, we believe that the absence of oral spines is a less critical feature when compared to the distinctive submedial avicularium.
The only other Hemismittoidea species from the Northwest Pacific, H. taiwanensis Gluhak, Lewis and Popijak, 2007, described from the southeastern coast of Taiwan, differs in having a convex frontal shield, less conspicuous marginal areolae, and rounded peristomial constrictions, in addition to 4-6 robust oral spines (Gluhak et al., 2007).
Genus SMITTOIDEA Osburn, 1952
Smittoidea ligata sp. nov. Di Martino, Rosso and Taylor
Figure 29
zoobank.org/76B02F1B-D891-4C69-948B-CB3793409E93
Type material. Holotype PMC. B58. 29.7.2024a, sample 19052 (Figure 29A-D); paratype PMC. B58. 29.7.2024b1, sample 19185 (Figure 29E-F); paratype PMC. B58. 29.7.2024b2, sample 19210 (Figure 29G-H); paratype PMC. B58. 29.7.2024b3, sample 19044 (Figure 29I-K); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
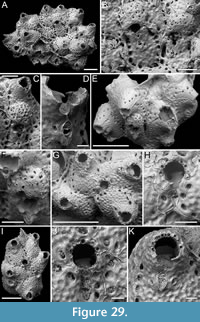 Etymology. Latin, meaning linked, referring to the raised rim linking the proximal peristomial pseudosinus to the avicularium.
Etymology. Latin, meaning linked, referring to the raised rim linking the proximal peristomial pseudosinus to the avicularium.
Diagnosis. Smittoidea with autozooids separated by thin, raised sutures; nodular frontal shield with single row of marginal areolae, and additional pores around the avicularium; orifice with anvil-shaped lyrula occupying more than half of its width and acutely triangular condyles; peristome curved frontally with small marginal pores on its backside, forming a U-shaped pseudosinus proximally; 7-8 oral spine bases, 0-2 visible in ovicellate zooids; suboral avicularium teardrop-shaped, proximally directed, located a short distance from pseudosinus, connected to pseudosinus by a perpendicular suture; crossbar with blunt ligula; ooecium nodular and pseudoporous.
Description. Colony encrusting, multiserial, uni- to multilaminar. Autozooids distinct, separated by thin raised sutures of smooth calcification, elongate (mean ZL/ZW 1.53), oval to irregularly polygonal, irregularly arranged. Frontal shield flat to slightly convex, nodular, imperforate except for a single row of 10-18 (average 16) circular to elliptical marginal areolar pores, 14-45 µm in maximum diameter, placed in deep, funnel-like depressions; additional pores, 23-33 µm in diameter, placed around the avicularium, one on each side and one proximally. Primary orifice subcircular, almost as long as wide, with an anvil-shaped lyrula occupying more than half of its width (LyW/OW 0.66), with slightly downwardly curved tips; acutely triangular condyles directed medially or slightly downward, aligned with the lyrula distal margin; orifice surrounded by a short, tubular peristome, forming a U-shaped pseudosinus proximally; 7-8 (most commonly 8) oral spine bases, 14-26 µm in diameter, arranged in an arch, the proximalmost pair aligned with or slightly above the distal margin of the lyrula; 0-2 spine bases visible in ovicellate zooids, encased between the rim outlining the frontal shield and the rim of the projecting proximolateral margins of the ovicell; autozooids at colony edge with peristome curved frontally, its backside with small marginal pores. Avicularium adventitious, single, suboral, median, teardrop-shaped, positioned 15-50 µm from pseudosinus, connected to pseudosinus by a perpendicular raised suture of calcification; rostrum acutely triangular, directed proximally; crossbar complete with a small ligula outlining two elliptical openings, the smaller one distally. Ovicell hyperstomial, globular, formed by the distal zooid, not closed by zooidal operculum; ooecium smooth, striated (nodular if covered by secondary calcification), pseudoporous, with approximately 15 circular pores, 12-26 µm in diameter. Pseudoancestrula partially overgrown, resembling later autozooids but smaller, measuring approximately 248-366 µm in length by 212-224 µm in width, with 7-8 oral spines and an avicularium 69-89 µm long by 39-49 µm wide, surrounded by 4-5 autozooids slightly smaller than later ones, measuring 465-610 µm in length by 280-360 µm in width.
Measurements (µm). ZL 573±45, 527-625 (1, 5); ZW 374±66, 276-457 (1, 5); OL 114 (1, 1); OW 116 (1, 1); AvL 94±5, 87-100 (1, 10); AvW 49±5, 41-56 (1, 10); OvL 239±13, 225-250 (1, 3); OvW 323±35, 284-352 (1, 3).
Remarks. Smittoidea ligata sp. nov. differs from all Northern Pacific species described to date, including those reported from Japan. Considering the Japanese species: the Pleistocene Smittoidea reticulata ascoporoides (Sakakura, 1935) differs in having significantly longer avicularia, measuring approximately 0.17 mm, and a pseudosinus that becomes a rounded pore separated from the proximal peristome (see also Kataoka, 1961); Smittoidea reticulata okadai Sakakura, 1938, also from the Pleistocene, features similarly long and pointed avicularia; the Pliocene Smittoidea matobai Hayami, 1975 is distinct in that it lacks spines; and S. notoensis Hayami, 1975 is characterized by four distal spines that are consistently concealed in ovicellate zooids. Among the Smittoidea species documented from Sagami Bay by Hirose (2010): S. levis (Kirkpatrick, 1890) has the avicularium positioned adjacent to the proximal margin of the peristome, its ligula is better developed, and the orifice has a denticulate anter; S. pacifica (Soule and Soule, 1973) differs in having an avicularium centrally located on the frontal shield, and a lyrula spanning nearly the entire length of the proximal margin. Hirose (2010) also identified two potential new species: Smittoidea n. sp. 1 stands out because of its narrower and deeper pseudosinus, absence of spines, and a distinct pattern of secondary calcification extending from the distal peristome to cover the proximal part of the ooecium, which is remarkably flat and embedded; Smittoidea n. sp. 2 is characterized by the lack of spines, a denticulate anter, and a larger avicularium that is differently shaped and positioned more proximally compared to the new Holocene species. In addition, the North Pacific species Smittoidea marginata (Canu and Bassler, 1929), from the Philippines, has a long, narrow avicularium, which is more centrally located on the frontal shield.
Smittoidea notoensis Hayami, 1975
Figure 30
v. 1975 Smittoidea notoensis Hayami, p. 114, pl. 16, fig. 8.
Figured material. PMC EDM-Collection J.H.B.132a, sample 19072; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
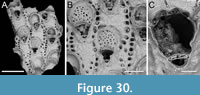 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, elongate (mean ZL/ZW 1.68), rectangular to irregularly polygonal, seemingly arranged in alternating longitudinal rows. Frontal shield flat, slightly convex around the orifice, nodular, imperforate except for a single row of 13-15 (most commonly 15) circular to elliptical marginal areolar pores, 18-65 µm in maximum diameter, placed in deep, rounded quadrangular, funnelling depressions; an additional smaller pore, 12-18 µm in diameter, placed centrally below the avicularium. Primary orifice subcircular, almost as long as wide, with a rectangular lyrula occupying approximately one-third of its width; orifice surrounded by a short peristome developed proximally and laterally, forming two lateral flaps hiding condyles; secondary orifice keyhole-shaped, 100-110 µm long by 130-145 µm wide, with rounded triangular proximal constrictions; four distal oral spine bases, 20-28 µm in diameter, hidden in ovicellate zooids. Avicularium adventitious, single, suboral, median, embedded in proximal peristome with its distal margin adjacent to the base of lyrula, plectrum-shaped; rostrum rounded triangular, directed proximally; crossbar complete with a ligula outlining a slit-like opening distally and a transversely C-shaped opening proximally. Ovicell hyperstomial, globular, formed by the distal zooid, not closed by zooidal operculum; ooecium smooth, striated, densely and evenly pierced by 30-35 circular to elliptical pseudopores, with a maximum diameter of 9-27 µm, semilunar proximal margin imperforate, sloping inwards; secondary calcification spreading from adjacent zooids forming a narrow (25-50 µm wide distally, 35-55 µm laterally), nodular, peripheral perimeter.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, elongate (mean ZL/ZW 1.68), rectangular to irregularly polygonal, seemingly arranged in alternating longitudinal rows. Frontal shield flat, slightly convex around the orifice, nodular, imperforate except for a single row of 13-15 (most commonly 15) circular to elliptical marginal areolar pores, 18-65 µm in maximum diameter, placed in deep, rounded quadrangular, funnelling depressions; an additional smaller pore, 12-18 µm in diameter, placed centrally below the avicularium. Primary orifice subcircular, almost as long as wide, with a rectangular lyrula occupying approximately one-third of its width; orifice surrounded by a short peristome developed proximally and laterally, forming two lateral flaps hiding condyles; secondary orifice keyhole-shaped, 100-110 µm long by 130-145 µm wide, with rounded triangular proximal constrictions; four distal oral spine bases, 20-28 µm in diameter, hidden in ovicellate zooids. Avicularium adventitious, single, suboral, median, embedded in proximal peristome with its distal margin adjacent to the base of lyrula, plectrum-shaped; rostrum rounded triangular, directed proximally; crossbar complete with a ligula outlining a slit-like opening distally and a transversely C-shaped opening proximally. Ovicell hyperstomial, globular, formed by the distal zooid, not closed by zooidal operculum; ooecium smooth, striated, densely and evenly pierced by 30-35 circular to elliptical pseudopores, with a maximum diameter of 9-27 µm, semilunar proximal margin imperforate, sloping inwards; secondary calcification spreading from adjacent zooids forming a narrow (25-50 µm wide distally, 35-55 µm laterally), nodular, peripheral perimeter.
Measurements (µm). ZL 613±37, 563-665 (1, 5); ZW 365±13, 351-386 (1, 5); OL 135 (1, 1); OW 137 (1, 1); AvL 52±5, 45-58 (1, 6); AvW 70±2, 68-73 (1, 6); OvL 243±2, 242-245 (1, 3); OvW 292±6, 286-296 (1, 3).
Remarks. Smittoidea notoensis was previously known in Japan only as a fossil. The holotype was found in the Pleistocene Hiradoko Formation of Suzu City, while additional specimens have been discovered in the Pliocene Hamada Formation of Chikagawa and the Shibikawa Formation of Anden (Hayami, 1975). Our Holocene specimen matches the morphological description provided by Hayami (1975) and falls within the specified size range for ovicells, but has larger autozooids compared to those of the holotype (length 0.44-0.42 mm, width 0.24-0.32 mm).
Genus PARASMITTINA Osburn, 1952
Parasmittina cf. delicatula (Busk, 1884)
Figure 31
cf. 1884 Mucronella delicatula Busk, p. 156, pl. 18, fig. 2.
cf. 1973 Parasmittina delicatula (Busk); Soule and Soule, p. 401, fig. 6D-F.
Figured material. PMC EDM-Collection J.H.B.133a, sample 19210; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
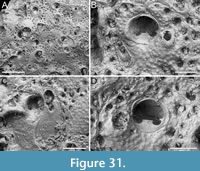 Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct, separated by shallow grooves, slightly longer than wide (mean ZL/ZW 1.13), oval to irregularly polygonal, chaotically arranged due to frontal budding. Frontal shield flat to slightly convex, coarsely granular, imperforate except for a single row of typically more than 20 circular to elliptical marginal areolar pores, 15-45 µm in maximum diameter; additional smaller pores, about 10 µm in diameter, flanking the avicularium. Primary orifice orbicular, with rectangular lyrula occupying approximately one-third of its width (mean LyW/OW 0.32), and robust triangular condyles, medially directed, 10-12 µm long by 11-13 µm wide; orifice encircled by a rim of smooth calcification, sometimes extending laterally as open flaps; oral spine bases numbering one or absent, distal, medial, 14-20 µm in diameter. Avicularia adventitious, located lateral to orifice, usually single, sometimes paired, proximally directed, of three types: type 1, small-sized rounded triangular; type 2, medium-sized elliptical to slightly spatulate; type 3, large, almost as long as an autozooid, broadly spatulate; complete crossbar not observed. When observed paired, one of the avicularia is small and the other medium-sized. Ovicells not seen.
Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct, separated by shallow grooves, slightly longer than wide (mean ZL/ZW 1.13), oval to irregularly polygonal, chaotically arranged due to frontal budding. Frontal shield flat to slightly convex, coarsely granular, imperforate except for a single row of typically more than 20 circular to elliptical marginal areolar pores, 15-45 µm in maximum diameter; additional smaller pores, about 10 µm in diameter, flanking the avicularium. Primary orifice orbicular, with rectangular lyrula occupying approximately one-third of its width (mean LyW/OW 0.32), and robust triangular condyles, medially directed, 10-12 µm long by 11-13 µm wide; orifice encircled by a rim of smooth calcification, sometimes extending laterally as open flaps; oral spine bases numbering one or absent, distal, medial, 14-20 µm in diameter. Avicularia adventitious, located lateral to orifice, usually single, sometimes paired, proximally directed, of three types: type 1, small-sized rounded triangular; type 2, medium-sized elliptical to slightly spatulate; type 3, large, almost as long as an autozooid, broadly spatulate; complete crossbar not observed. When observed paired, one of the avicularia is small and the other medium-sized. Ovicells not seen.
Measurements (µm). ZL 437±31, 409-483 (1, 6); ZW 386±38, 325-435 (1, 6); OL 132±6, 124-142 (1, 9); OW 134±9, 122-150 (1, 9); AvL (type 1) 77±10, 66-87 (1, 5); AvW (type 1) 65±10, 48-71 (1, 5); AvL (type 2) 124±6, 119-132 (1, 4); AvW (type 2) 63±6, 58-72 (1, 4); AvL (type 3) 371 (1, 1); AvW (type 3) 191 (1, 1).
Remarks. Parasmittina delicatula was originally described from Hawaii and has since been reported widely across the Pacific, including Japan. Hayward and Parker (1994), who designated a lectotype, noted that this species is highly variable. Despite this variability, there are two traits that seem to remain constant: 1) the orbicular shape of the primary orifice, which is also equipped with a lyrula having a straight distal edge and gently concave sides, along with thin, pointed condyles slightly curving downward, and distal border bearing a single median spine, absent in some autozooids; 2) the enlarged spatulate avicularia that can be as long as an autozooid and have a slender complete crossbar.
In our specimen, the condyles are more robust and are not downwardly curved but are instead directed medially. Avicularian crossbars were not observed, likely due to their delicate structure, and no ovicells were found, hindering further comparisons. Compared to the description and illustration of P. delicatula by Hirose (2010), our specimen differs again in having more robust condyles not downwardly curved. In addition, in the Recent specimen from Sagami Bay, the medium-sized avicularia may also point distally, while the small triangular avicularia were not observed.
Parasmittina ligulata sp. nov. Di Martino, Rosso and Taylor
Figure 32
zoobank.org/FA9A9F18-2303-495A-A27B-85F929110C58
Type material. Holotype PMC. B59. 29.7.2024a, sample 19178; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
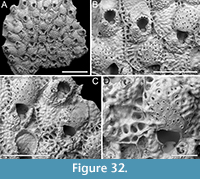 Etymology. Latin, meaning equipped with a ligula (i.e., the tongue-like skeletal projection on the pivotal bar), referring to the stout ligula of its avicularium.
Etymology. Latin, meaning equipped with a ligula (i.e., the tongue-like skeletal projection on the pivotal bar), referring to the stout ligula of its avicularium.
Diagnosis. Parasmittina with autozooids separated by raised sutures; tubercular, flat to slightly sunken frontal shield with single row of conspicuous marginal areolae located between ridges; orifice partly concealed by an asymmetrical peristome, having a denticle projecting inwardly on one side and an avicularium on the other, forming a broad asymmetrical pseudosinus proximally; anvil-shaped lyrula with sharp tips curved proximally; five oral spine bases, none visible in ovicellate zooids; avicularium small, teardrop-shaped, proximally directed, located on a swollen cystid lateral to the orifice; crossbar with stout ligula; ooecium nodular with pseudopores, formed by the maternal zooid and one or two adjacent distal zooids.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by raised sutures, longer than wide (mean ZL/ZW 1.43), rectangular to irregularly polygonal, quincuncially arranged. Frontal shield flat to slightly sunken, tubercular, imperforate except for a single row of 19-26, transversely elliptical to ovoid marginal areolar pores, 22-64 µm in maximum diameter, located between ridges, creating a costate appearance; additional smaller pores, about 10-15 µm in diameter, outlining proximally the avicularium cystid. Primary orifice partially concealed by an asymmetrical peristome, having a rounded triangular denticle projecting into the primary orifice on one side, an adventitious avicularium on the other, and a broad asymmetrical pseudosinus proximally; secondary orifice foot-shaped; lyrula anvil-shaped, 60-68 µm wide, with straight proximal margin sloping laterally into two pointed tips curved downward, often visible through the pseudosinus; acutely triangular condyles pointing medially, sometimes visible below the peristome; consistently five distal oral spine bases, 16-21 µm in diameter, none visible in ovicellate zooids. Avicularium adventitious, single or absent, located lateral to orifice on a swollen cystid, juxtaposed with the rim of the secondary orifice; rostrum rounded triangular, directed proximally; crossbar complete and equipped with a stout ligula, triangular to rectangular in shape, measuring 23-27 µm long by 18-27 µm wide, outlining a small semicircular opening distally, and a transversely C-shaped opening proximally. Ovicell hyperstomial, globular, formed by the maternal zooid and one or two adjacent distal zooids; ooecium striated, smooth (or tubercular when covered by variably developed secondary calcification), and pseudoporous; pseudopores circular, elliptical or drop-shaped roughly arranged in radial rows.
Measurements (µm). ZL 584±42, 483-639 (1, 15); ZW 410±47, 327-524 (1, 15); OL (secondary) 158±13, 140-183 (1, 7); OW 136±6, 127-143 (1, 7); AvL 83±12, 59-109 (1, 16); AvW 66±10, 46-80 (1, 16); OvL 292±22, 259-324 (1, 16); OvW 324±24, 296-369 (1, 16).
Remarks. We compared Parasmittina ligulata sp. nov. to all known North Pacific species of this genus and found that none had the same combination of characteristics. Among North Pacific species: P. acuta (Canu and Bassler, 1929) has avicularia placed adjacent to the proximal margin of the peristome; P. avicularissima Gontar, 1982 has a narrower lyrula, a large conical, nodular umbo, large frontal avicularia, and a single pore in the ooecium (see also Grischenko et al., 2007), P. contraria Seo, 1993 and P. pyriformis Seo, 2002 have only 1-2 spines and large, frontal, triangular avicularia distally directed; P. ragioidea Liu, 2001 in Liu et al. (2001) has 1-2 spines, two types of avicularia, triangular and spatulate (see also Taylor and Tan, 2015); P. spiculata Gluhak, Lewis and Popijak, 2007 lacks oral spines, and the avicularia are parallel-sided, long and slender; P. trispinosa granosa (Canu and Bassler, 1929), P. trispinosa macroavicularia (Androsova, 1958) and P. trispinosa nanshaensis Lu, 1991, all have 1-3 spines; P. winstonae Liu, 2001 in Liu et al. (2001) has 0-2 spines, serrated condyles, variably shaped avicularia, notably including giant avicularia directed distally, often extending over the frontal shield of the distal zooid (see also Taylor and Tan, 2015).
Among species from the Pliocene of Japan: P. chikagawaensis Hayami, 1975 is characterized by having 2-3 spines, and latero-oral and frontal avicularia distally directed; P. masudai Hayami, 1975 lacks spines and has prominent triangular avicularia directed distally, very large, occupying the entire frontal shield, sometimes covering one-quarter of the orifice and concealing its lyrula; P. shibikawaensis Hayami, 1975 seems to lack a lyrula and has a V-shaped peristomial sinus, with associated avicularia, two laterally, sometimes missing, and one proximally consistently present; P. okadai Hayami, 1975 has a prominent peristome forming a tube-like process with one to three associated avicularia, all directed distally.
Among species from the Pleistocene of Japan: P. aviculoumbonata Kataoka, 1961 has paired, umbonal avicularia lateral to the orifice and oriented in various directions, in addition to several rounded avicularia on the frontal shield, and two distal spines; P. peristoaviculata Kataoka, 1961 shows a well-developed peristome, thick and salient especially distally, and a long spatulate avicularium inside peristome; the peristome in P. plana Kataoka, 1961 forms a triangular pseudosinus and the frontal avicularia is placed nearly at the zooid axis pointing proximally.
Among Recent species described from or subsequently recorded in Japan, P. hanzawae Kataoka, 1960 has only two spines, a triangular pseudosinus, and median frontal avicularium, pointing obliquely distally; P. soulesi Scholz and Cusi, 1991 has 0-2 spines, elongate narrow avicularia, lyrula varying from minute to very large and conspicuous, and denticulate condyles (see Dick and Grischenko, 2017; note also that this species corresponds to P. lanceolata (Ortmann, 1890) in Hirose (2010)); P. triangularis (Mawatari, 1952) lacks spines and has four types of avicularia; P. trispinosa aomoriensis Kataoka, 1957 has two spines and slender obliquely directed avicularia.
Among Parasmittina species described and illustrated by Hirose (2010) from Sagami Bay, P. alanbanneri (Soule and Soule, 1973) has latero-oral avicularia with a narrow, elongate and serrated rostrum; P. areolata (Canu and Bassler, 1927) has rounded, denticulate condyles and slender avicularia with denticulate rostrum; P. japonica (Ortmann, 1890) has a strongly denticulate distal orifice margin, curved condyles that are also denticulate, and large frontal triangular avicularia pointing distally; the species reported as Parasmittina lateralis (Ortmann, 1890) n. comb. has a single spine and lateral, triangular avicularia almost as long as the entire length of the frontal shield; P. leviavicularia Soule and Soule, 1973 has two spines and avicularia with raised, strongly serrated edges, directed distally; P. parsevaliformis Soule and Soule, 1973 has 2-3 spines, a peristome that does not obstruct the orifice, narrow lyrula, and spatulate avicularia; P. projecta (Okada and Mawatari, 1937) is distinguished by its forward-projecting peristome, forming a narrow U-shaped sinus, three spines, large triangular avicularia with serrated edges that extend distally over the edge of the peristome, and ovicells opening on the peristome; P. serrula Soule and Soule, 1973 is distinguished by its larger rounded triangular avicularia and denticulate distal margin of the orifice; Parasmittina sp. nov. 1 has an erect colony form, in addition to a denticulate orificial margin, and large triangular avicularia distally directed; Parasmittina sp. nov. 2 has large, spatulate, triangular avicularia with serrated margins; Parasmittina sp. nov. 3 and Parasmittina sp. nov. 4 also have a denticulate orifice margin, and avicularia that are parallel-sided and slender in the former species, but spatulate in the latter species.
Family BITECTIPORIDAE MacGillivray, 1895
Genus METROPERIELLA Canu and Bassler, 1917
Metroperiella montferrandi (Audouin, 1826)
Figure 33
v. 1826 Flustra montferrandi Audouin, p. 240.
v. 1890 Lepralia acuta Ortmann, p. 41, pl. 3, fig. 12.
v. 1957 Codonellina montferrandii (Audouin); Harmer, p. 1049, pl. 69, figs. 25-26, 30.
v. 1975 Codonellina montferrandii (Audouin); Hayami, pl. 17, fig. 8.
v. 1984 Codonellina montferrandii (Audouin); Gordon, p. 77, pl. 26A.
v. 2001 Metroperiella montferrandii (Audouin); Tilbrook, Hayward and Gordon, p. 74, fig. 15F.
v. 2006 Metroperiella montferrandii (Audouin); Tilbrook, p. 182, pl. 39D.
Figured material. PMC EDM-Collection J.H.B.134a, sample 19227; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
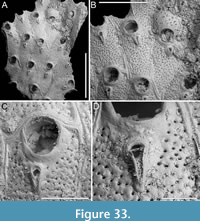 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by thick (13-23 µm) rims of smooth calcification outlining each perimeter and a thin furrow in-between, elongate, almost twice as long as wide (mean ZL/ZW 1.83), arranged in longitudinal rows. Frontal shield flat, nodular, evenly and densely pierced except for a narrow (c. 20-40 µm) band extending between margin of orifice and avicularium; pseudopores circular, 5-10 µm in diameter. Orifice terminal, raised relative to the frontal shield, ovoidal with horseshoe-shaped anter and a slightly narrower, shallow, bowl-shaped poster; condyles robust, rounded triangular, 12-24 µm long by 9-17 µm wide. Avicularia adventitious, typically one per zooid or occasionally absent, placed proximally to orifice along zooidal midline or slightly to either side, raised compared to the frontal shield; rostrum acutely triangular directed proximally; crossbar complete; distal opening semicircular, proximal opening rounded triangular. Ovicell hyperstomial, globular; ooecium smooth, wider than long, 185 µm long by 320 µm wide, perforated by circular pseudopores, slightly larger than those on the frontal shield (10-20 µm in diameter), except for a narrow imperforate proximal band; the periphery of the single ooecium observed with incipit of secondary calcification produced by distal zooid.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by thick (13-23 µm) rims of smooth calcification outlining each perimeter and a thin furrow in-between, elongate, almost twice as long as wide (mean ZL/ZW 1.83), arranged in longitudinal rows. Frontal shield flat, nodular, evenly and densely pierced except for a narrow (c. 20-40 µm) band extending between margin of orifice and avicularium; pseudopores circular, 5-10 µm in diameter. Orifice terminal, raised relative to the frontal shield, ovoidal with horseshoe-shaped anter and a slightly narrower, shallow, bowl-shaped poster; condyles robust, rounded triangular, 12-24 µm long by 9-17 µm wide. Avicularia adventitious, typically one per zooid or occasionally absent, placed proximally to orifice along zooidal midline or slightly to either side, raised compared to the frontal shield; rostrum acutely triangular directed proximally; crossbar complete; distal opening semicircular, proximal opening rounded triangular. Ovicell hyperstomial, globular; ooecium smooth, wider than long, 185 µm long by 320 µm wide, perforated by circular pseudopores, slightly larger than those on the frontal shield (10-20 µm in diameter), except for a narrow imperforate proximal band; the periphery of the single ooecium observed with incipit of secondary calcification produced by distal zooid.
Measurements (µm). ZL 653±61, 579-776 (1, 9); ZW 357±39, 280-414 (1, 9); OL 171±6, 165-183 (1, 7); OW 150±6, 141-161 (1, 7); AvL 129±14, 105-159 (1, 13); AvW 65±5, 57-73 (1, 13).
Remarks. Based on the available records, Metroperiella montferrandi appears to have a circumtropical distribution. Instances of its presence have been already documented in Japan, including both Recent (Ortmann, 1890; Harmer, 1957; Hirose, 2010) and Pleistocene (Hayami, 1975) occurrences. Within the limited size of the fragments examined here, the avicularia consistently are acutely triangular, with no observation of the spatulate form. It remains uncertain whether the absence of spatulate avicularia is genuine or due to the small size of the specimens. Specimens identified as M. montferrandi from Vanuatu (Tilbrook et al., 2001) and the Kermadec Ridge (Gordon, 1984) also lack spatulate frontal avicularia. Among Metroperiella species with monomorphic avicularia of acute triangular shape, M. cotoensis Dick, Ngai and Doan, 2020 differs in having a deeper orifice poster, while M. agassizi Winston and Woollacott, 2009 has a narrower poster and longer avicularia, which are more asymmetrically placed.
Genus PARKERMAVELLA Gordon and d’Hondt, 1997
Parkermavella daidokutsuensis sp. nov. Di Martino, Rosso and Taylor
Figure 34
zoobank.org/6C7829DD-A749-4C38-81E3-2E7EE147D179
Type material. Holotype PMC. B60. 29.7.2024a, sample 19113 (Figure 34A-C); paratype PMC. B60. 29.7.2024b1, sample 19135 (Figure 34D-G); paratype PMC. B60. 29.7.2024b2, sample 19160 (Figure 34H-J); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
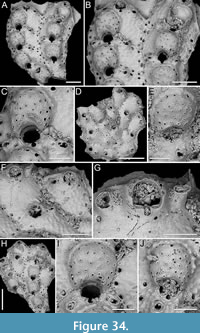 Etymology. Referring to the type locality, the Daidokutsu cave in Okinawa, Japan.
Etymology. Referring to the type locality, the Daidokutsu cave in Okinawa, Japan.
Diagnosis. Parkermavella with irregularly polygonal autozooids; nodular frontal shield with single row of marginal areolae; semicircular orifice with rectangular condyles and narrow, shallow U-shaped sinus; 8-9 oral spine bases, two visible in ovicellate zooids; 1-2 adventitious avicularia, elliptical parallel-sided or slightly spatulate, either placed laterally to orifice at different levels, aligned with the sinus or situated more distally, or suborally, located on raised cystid, directed proximally, proximolaterally or laterally; crossbar with ligula; ooecium smooth, striated, pseudoporous with a narrow distal band of secondary calcification.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by thin sutures of smooth calcification or shallow furrows, irregularly polygonal, squat, almost as long as wide (mean ZL/ZW 1.06), arranged in parallel rows. Frontal shield flat or slightly convex, nodular, imperforate apart from a continuous row of marginal areolar pores, circular to elliptical, 15-30 µm in maximum dimension. Orifice terminal with semicircular anter and narrow, shallow U-shaped sinus distally constricted; condyles rectangular occupying the proximal margin entirely; 8-9 oral spine bases, 12-16 µm in diameter, closely spaced or adjacent, forming an arch around the distal half of the anter; two spines visible in ovicellate zooids. Avicularia adventitious, elliptical, single or paired, placed laterally to orifice at various levels, aligned with the sinus, or more distally, aligned with the proximalmost pair of spines, or sometimes suborally, particularly when single, generally placed more centrally but still offset from the zooidal axis, elevated on a raised cystid; rostrum raised, parallel-sided or slightly spatulate, directed proximally, proximolaterally or laterally; crossbar complete with small, triangular ligula. Ovicell hyperstomial, globular, resting on the frontal shield of distal zooid, occupying a narrow portion thereof; ooecium smooth, striated, evenly perforated by circular, elliptical, rarely bean-shaped pseudopores, 7-26 µm in maximum diameter, typically larger distally, bordered by a raised margin, arranged radially; tubercular secondary calcification forming a narrow (20-30 µm wide) peripheral band, more pronounced distally.
Measurements (µm). ZL 361±54, 278-451 (3, 20); ZW 341±60, 246-446 (3, 20); OL (including sinus) 95±6, 83-107 (3, 15); OW 92±5, 82-102 (3, 15); AvL 49±5, 34-55 (3, 20); AvW 32±6, 19-49 (3, 20); OvL 190±10, 174-203 (3, 13); OvW 214±11, 191-232 (3, 13).
Remarks. Gordon and d’Hondt (1997) introduced the new genus Parkermavella for species resembling Schizomavella but with frontal shields imperforate except for marginal areolar pores. The genus is moderately diverse, encompassing 19 known Recent species (Bock, 2024), with only one species having a confirmed Pleistocene fossil record (Di Martino et al., 2017). The majority of Parkermavella species have been documented in the South Pacific, particularly in New Zealand, with Parkermavella orientalis (Androsova and Gontar, 1982 in Gontar, 1982) being the sole species reported from the North Pacific. Parkermavella orientalis differs from P. daidokutsuensis sp. nov. in the absence of oral spines, in having a wider oral sinus, and avicularia adjacent to the orifice margins.
?Bitectiporidae sp. indet.
Figure 35
Figured material. PMC EDM-Collection J.H.B.135a, sample 19229 (Figure 35A-B) and sample 19231 (Figure 35C); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
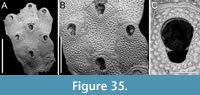 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves and a narrow band of smooth calcification outlining the zooidal perimeter, wider proximally (20-30 µm) than laterally (10-20 µm), rounded rectangular in shape with concave proximal and convex distal edge, longer than wide (mean ZL/ZW 1.53). Frontal shield flat to slightly convex, pustulose, with pustules 15-30 µm in diameter and sparse, minute, circular pseudopores, 3-13 µm in diameter, between pustules. Orifice keyhole-shaped, elongate (mean OL/OW 1.33), with horseshoe-shaped anter separated from a narrower, shallow bowl-shaped sinus by two stout triangular condyles, 19-27 µm long by 18-30 µm wide at the base, medially directed or curved proximally; oral spines absent. Avicularia and ovicells not observed.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves and a narrow band of smooth calcification outlining the zooidal perimeter, wider proximally (20-30 µm) than laterally (10-20 µm), rounded rectangular in shape with concave proximal and convex distal edge, longer than wide (mean ZL/ZW 1.53). Frontal shield flat to slightly convex, pustulose, with pustules 15-30 µm in diameter and sparse, minute, circular pseudopores, 3-13 µm in diameter, between pustules. Orifice keyhole-shaped, elongate (mean OL/OW 1.33), with horseshoe-shaped anter separated from a narrower, shallow bowl-shaped sinus by two stout triangular condyles, 19-27 µm long by 18-30 µm wide at the base, medially directed or curved proximally; oral spines absent. Avicularia and ovicells not observed.
Measurements (µm). ZL 710±81, 598-847 (2, 10); ZW 464±56, 367-556 (2, 10); OL 191±9, 179-203 (2, 9); OW 144±8, 133-157 (2, 9).
Remarks. Given the scarcity of material as well as the paucity of morphological characters (e.g., the lack of avicularia and ovicells, in both cases probably reflecting our very small sample size rather than a true absence in the species), we can only tentatively assign this taxon to a family. Assignment to Bitectiporidae is based on the presence of the following characters attributed to this family: lepralioid frontal shield that can have pseudopores, orifice with subcircular to oval anter and broad to narrow sinus, condyles that can be present, and spines that can be absent (see Martha et al., 2020). Other options we considered are Echinovadomidae Tilbrook, Hayward and Gordon, 2001; Gigantoporidae Bassler, 1935; Hippopodinidae Levinsen, 1909; and Schizoporellidae Jullien, 1882. Although these families have an orifice with condyles, the general shape is more subcircular or subquadrangular than elongate and keyhole-shaped. Keyhole-shaped orifices are also typical of families such as Hippaliosinidae Winston, 2005, in which the lepralioid frontal shield is imperforate, and Cleidochasmatidae Cheetham and Sandberg, 1964, which again includes genera with imperforate frontal shields except for a row of marginal areolae. Some genera in Phidoloporidae Gabb and Horn, 1862 may also have keyhole-shaped orifice but they are characterized by a denticulate anter.
Superfamily SCHIZOPORELLOIDEA Jullien, 1883
Family TETRAPLARIIDAE Harmer, 1957
Genus TETRAPLARIA Tenison Woods, 1879
Tetraplaria stellifera sp. nov. Di Martino, Rosso and Taylor
Figure 36
zoobank.org/70693D67-F6D1-4347-85DA-99AAD3FA285C
Type material. Holotype PMC. B61. 29.7.2024a, sample 19044 (Figure 36A-C); paratype PMC. B61. 29.7.2024b, sample 19028 (Figure 36D-G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
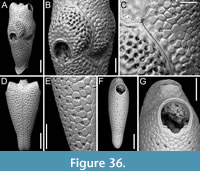 Etymology. Latin, meaning starry, referring to the stellate pattern of the frontal shield pseudopores.
Etymology. Latin, meaning starry, referring to the stellate pattern of the frontal shield pseudopores.
Diagnosis. Tetraplaria with internodes composed by at least 1-2 pairs of zooids, arranged back to back; frontal shield with a tessellated pattern formed by grooves in a rounded polygonal pattern interspersed with stellate pseudopores; orifice sinuate with rectangular condyles; ovicell kenozooidal, notably large, with proximal half flat and imperforate, and distal half sloping and pseudoporous.
Description. Colony erect, jointed; internodes consisting of at least 1-2 pairs of zooids arranged back to back; joints short (50-55 µm) and tubular, c. 100 µm in diameter. Autozooids distinct, separated by thin furrows, club-shaped, tapering proximally, elongate (mean ZL/ZW 2.69). Frontal shield convex, tessellated, featuring a pattern of rounded polygonal grooves, each polygon roughly 20-25 × 30-35 µm, interspersed with circular, stellate pseudopores, 10-17 µm in diameter, pseudopores missing immediately suborally. Orifice surrounded by a flat rim of smooth calcification, broader proximally (c. 18 µm) and tapering laterally and distally (6-9 µm), bell-shaped; anter horseshoe-shaped, sinus shallowly U-shaped occupying less than half the orifice width, condyles rectangular, 10 × 20 µm. Ovicell kenozooidal, globular, large, seemingly closed by zooidal operculum; ooecium proximal portion flat, tessellated and imperforate, distal portion sloping and densely and evenly pseudoporous (4-5 rows of pseudopores); pseudopores similar in size to those of the frontal shield but deeper, stellate pattern uncertain; a distal fissure interpreted as the remnants of the associated kenozooid participating in the development of the ooecium. Ovicellate zooid size (including the ooecium) same as non-ovicellate autozooids, 888 µm in length (including the ooecium) by 285 µm in width; ovicellate zooid orifice size also similar to that of autozooid, 158 × 132 µm. Kenozooid positioned distally to ooecium, in connection with the tubular joint, trapezoidal, 210 × 130 µm in size, functioning as a wedge separating two autozooids.
Measurements (µm). ZL 731±142, 595-889 (2, 4); ZW 272±38, 216-298 (2, 4); OL 156 (1, 1); OW 143 (1, 1); OvL 237 (1, 1); OvW 302 (1, 1).
Remarks. The genus Tetraplaria includes species with small hyperstomial, pseudoporous ooecia immersed in the distal zooid, such as T. ventricosa (Haswell, 1880), as well as species with internal ovisacs and enlarged fertile zooids, such as T. immersa (Haswell, 1880). Despite its general resemblance to T. ventricosa (e.g., Gordon and d’Hondt, 1997; Tilbrook, 2006), the new species stands out within Tetraplaria due to its distinctive large kenozooidal ovicell, which also displays a unique perforation pattern, with its proximal portion imperforate and distal portion densely and uniformly pseudoporous. Based on the observation of a single internode with ovicellate zooids, ooecium formation could potentially involve either a distal kenozooid or the distal autozooid, akin to certain species of cribrilinids (e.g., Rosso et al., 2021b) or Microporella (e.g., Di Martino and Rosso, 2021). Furthermore, to our knowledge, the stellate pattern of the frontal pseudopores is unique within the genus.
Family MARGARETTIDAE Harmer, 1957
Genus MARGARETTA Gray, 1843
Margaretta gracilior (Ortmann, 1892)
Figure 37
v. 1892 Tubucellaria gracilior Ortmann, p. 699.
v. 1957 Margaretta gracilior (Ortmann); Harmer, p. 835, pl. 55, figs. 23-28.
v. 2006 Margaretta gracilior (Ortmann); Tilbrook, p. 234, pl. 51D.
Figured material. PMC EDM-Collection J.H.B.136a, sample 19200 (Figure 37A-C) and sample 19195 (Figure 37D-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect, articulated with cylindrical, quadriserial internodes, either straight or slightly curved, narrow, measuring c. 100 µm in width at the undivided base of the rami, expanding to 335-600 µm; internode attachment base circular, 65-95 µm in diameter, undivided, located laterally on the autozooid frontal midway between the secondary orifice/peristome and ascopore. Autozooids arranged in whorls of back to back pairs, with each pair rotated 90º relative to the next, flask-shaped, elongate (mean ZL/ZW 2.56), with indistinct lateral boundaries. Frontal shield convex, smooth, regularly and evenly perforated by small, sunken, circular pseudopores (5-13 µm in diameter), centred in an elliptical to polygonal mesh (35-50 µm in maximum dimension), overall appearance reminiscent of a peanut shell; ascopore circular to transversely elliptical, measuring 28-56 µm × 41-51 µm, surrounded by a raised rim slightly crenulated distally, placed distomedially, 250-370 µm from the proximal margin of the secondary orifice. Secondary orifice transversely elliptical; peristome relatively short, projecting from the frontal plane of the branch by about 100-130 µm, at an angle of approximately 45°, not bordered by a basal ridge; peristome of brooding autozooids swollen at the base, longer and curved upward; brooding autozooids grouped in whorls.
Measurements (µm). ZL 909±120, 747-1205 (3, 13); ZW 354±39, 286-435 (3, 13); OL (secondary) 94±7, 85-108 (3, 7); OW (secondary) 141±11, 130-157 (3, 7).
Remarks. Key characters for distinguishing species of Margaretta include the number of autozooids in each whorl, the number of partitions at the base of the rami, the shape and development of the peristome, the position of the ascopore relative to the orifice, the patterning of the frontal shield, and the shape and arrangement of pseudopores. Our specimens align closely with the description of M. gracilior as provided by Harmer (1957) and Tilbrook (2006) in all these aspects. Margaretta gracilior was first described from the Red Sea and has since been widely reported across the Indo-Pacific region (Tilbrook, 2006).
Margaretta cf. tenuis Harmer, 1957
Figure 38
cf. 1957 Margaretta tenuis Harmer, p. 840, pl. 55, figs. 13-18.
Figured material. PMC EDM-Collection J.H.B.137a, sample 19047 (Figure 38A-F) and sample 19185 (Figure 38G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
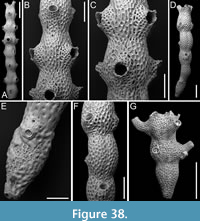 Description. Colony erect, articulated with straight or slightly curved internodes, cylindrical, measuring c. 180 µm in width at the bipartite base of the rami, expanding to 330-660 µm; internode attachment base not observed. Autozooids arranged back to back in alternating whorls of two, but mainly in whorls of three, flask-shaped, elongate (mean ZL/ZW 3.15), distinct, separated by shallow grooves. Frontal shield convex, sculptured, regularly perforated by small, sunken, circular pseudopores (7-20 µm in diameter), centred in an oval to polygonal mesh (40-80 µm in maximum dimension), with larger mesh size proximally on the frontal shield and finer on the peristome); ascopore circular, measuring 30-45 µm, positioned at the same level as the frontal shield, though not very noticeable, placed distomedially, 340-480 µm from the proximal margin of the secondary orifice, adjacent to or very close (28-46 µm below) to the basal ridge of the peristome. Secondary orifice transversely elliptical with the oral rim appearing slightly denticulate due to the frontal projection of the peristomial ridges. Peristome 250-520 µm long, with marked, broadly V-shaped, basal ridge; in standard autozooid, almost parallel to the frontal plane, causing the secondary orifice to face upwards with no distance between the distal margin of the secondary orifice and the distal margin of the autozooid; in brooding zooids, projecting from the frontal plane at an angle of almost 90°, swollen at the base, expanding the distance between the distal margin of the peristome and the distal margin of the autozooid, curved upwards, with marked longitudinal ridges and pseudopores, much larger than those on the frontal, measuring 20-40 µm in diameter, in valleys between ridges. Brooding autozooids grouped in whorls.
Description. Colony erect, articulated with straight or slightly curved internodes, cylindrical, measuring c. 180 µm in width at the bipartite base of the rami, expanding to 330-660 µm; internode attachment base not observed. Autozooids arranged back to back in alternating whorls of two, but mainly in whorls of three, flask-shaped, elongate (mean ZL/ZW 3.15), distinct, separated by shallow grooves. Frontal shield convex, sculptured, regularly perforated by small, sunken, circular pseudopores (7-20 µm in diameter), centred in an oval to polygonal mesh (40-80 µm in maximum dimension), with larger mesh size proximally on the frontal shield and finer on the peristome); ascopore circular, measuring 30-45 µm, positioned at the same level as the frontal shield, though not very noticeable, placed distomedially, 340-480 µm from the proximal margin of the secondary orifice, adjacent to or very close (28-46 µm below) to the basal ridge of the peristome. Secondary orifice transversely elliptical with the oral rim appearing slightly denticulate due to the frontal projection of the peristomial ridges. Peristome 250-520 µm long, with marked, broadly V-shaped, basal ridge; in standard autozooid, almost parallel to the frontal plane, causing the secondary orifice to face upwards with no distance between the distal margin of the secondary orifice and the distal margin of the autozooid; in brooding zooids, projecting from the frontal plane at an angle of almost 90°, swollen at the base, expanding the distance between the distal margin of the peristome and the distal margin of the autozooid, curved upwards, with marked longitudinal ridges and pseudopores, much larger than those on the frontal, measuring 20-40 µm in diameter, in valleys between ridges. Brooding autozooids grouped in whorls.
Measurements (µm). ZL 1408±211, 1083-1671 (3, 10); ZW 447±73, 330-569 (3, 10); OL (secondary) 131±22, 98-147 (3, 4); OW (secondary) 182±18, 163-201 (3, 4).
Remarks. The Recent Indo-Pacific M. tenuis closely resembles the Holocene Japanese species, sharing features such as the number of autozooids in a whorl, distinct boundaries of autozooids, a long tubular peristome with a prominent basal ridge projecting at right angle or obliquely, and an ascopore adjacent to the basal ridge. However, it differs in having a tripartite rather than bipartite base of the rami, a circular rather than elliptical secondary orifice, and a peristome basal ridge much closer to the proximal margin of the orifice (Harmer, 1957).
Family HIPPOPODINIDAE Levinsen, 1909
Genus HIPPOPODINA Levinsen, 1909
Hippopodina feegeensis (Busk, 1884)
Figure 39
v. 1884 Lepralia feegeensis Busk, p. 144, pl. 22, figs. 9, 9a, 9b.
v. 1957 Hippopodina feegeensis (Busk); Harmer, p. 974, pl. 67, figs. 8-9.
v. 1992 Hippopodina feegeensis (Busk); Ryland and Hayward, p. 256, pl. 67, fig. 17a.
v. 1999 Hippopodina feegeensis (Busk); Tilbrook, p. 451, fig. 1a-h.
v. 2015 Hippopodina feegeensis (Busk); Di Martino and Taylor, p. 11, pl. 27.
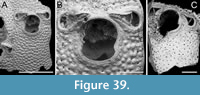 Figured material. PMC EDM-Collection J.H.B.138a, sample 19185 (Figure 39A-B) and sample 19160 (Figure 39C); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.138a, sample 19185 (Figure 39A-B) and sample 19160 (Figure 39C); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by thin furrows, rectangular, slightly longer than wide (mean ZL/ZW 1.39). Frontal shield slightly convex, tuberculate (tubercle diameter 25-52 μm), evenly punctuated by numerous circular pseudopores, 5-10 μm in diameter, arranged between tubercles. Orifice hoof-shaped, slightly longer than wide, with a pair of small, rounded, lateral condyles directed medially, separating a rounded anter from a smaller, bowl-shaped poster. Adventitious avicularia paired, located distolaterally of orifice facing medially; rostrum acutely triangular, raised, directed distolaterally and inwardly; crossbar complete. Ovicells not observed.
Measurements (µm). ZL 895±32, 873-918 (2, 2); ZW 643±124, 555-731 (2, 2); OL 286±11, 279-294 (2, 2); OW 265±33, 242-288 (2, 2); AvL 219±20, 202-252 (2, 5); AvW 133±14, 113-148 (2, 5).
Remarks. This species is identified as the Pacific H. feegeensis essentially based on the shape and positioning of avicularia and the shape of the orifice, where the distal portion is wider than the proximal portion (see Tilbrook, 1999).
Family ESCHARINIDAE Tilbrook, 2006
Genus BRYOPESANSER Tilbrook, 2006
Bryopesanser inflexus sp. nov. Di Martino, Rosso and Taylor
Figure 40
zoobank.org/36CD63CE-E101-4590-A347-9B3002932DF5
Type material. Holotype PMC. B62. 29.7.2024a, sample 19229; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
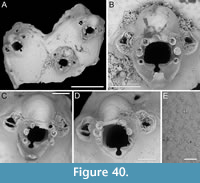 Etymology. Latin, meaning curved, referring to the peristomial proximal mucro, which is upwardly curved, and the ooecial process, which is downwardly curved.
Etymology. Latin, meaning curved, referring to the peristomial proximal mucro, which is upwardly curved, and the ooecial process, which is downwardly curved.
Diagnosis. Bryopesanser with faintly nodular frontal shield and multiporous pseudopores; orifice with straight proximal margin, longer-than-wide drop-shaped sinus, and minutely denticulate condyles; six oral spines in ovicellate zooids, the proximalmost pair slightly above the proximal margin of orifice; proximal peristome developing a narrow, median mucro curved upward; paired avicularia distolateral to orifice, aligned with the second proximalmost pair of spines, pear-shaped; rostrum open, distomedially directed; ooecium nodular, imperforate, with proximal projection curved downward.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by raised sutures and shallow grooves, pentagonal, slightly longer than wide (mean ZL/ZW 1.09), widest proximally, with thick lateral walls (175-225 µm); small, subcircular pore-chamber windows visible distally and distolaterally, approximately 30 µm in diameter. Frontal shield flat to slightly convex, convexity more obvious around the orifice, faintly nodular, densely pierced by multiporous pseudopores, 17-19 µm in diameter; a few marginal areolae also present at proximal and proximolateral zooidal corners, often obliterated by sediment. Orifice with semicircular anter, straight proximal margin occupied by rectangular, minutely denticulated condyles, 10 µm high, and longer-than-wide drop-shaped sinus, 45-50 µm long by 30-35 µm wide; six spine bases in ovicellate zooids, 25-40 µm in diameter, with the proximalmost pair slightly above the proximal margin of the orifice; proximal peristome 200-230 µm wide, developing into a narrow, squared, median mucro curved upward. Avicularia adventitious, paired, located distolaterally to orifice, aligned with the second proximalmost pair of spines, pear-shaped; rostrum open, distomedially directed; crossbar complete. Ovicell hyperstomial; ooecium cap-like, smooth, nodular, imperforate, with thick proximal margin developing into a narrow, squared, median projection curved downward.
Measurements (µm). ZL 764±16, 753-776 (1, 2); ZW 699±92, 634-764 (1, 2); OL (including sinus) 168±18, 156-181 (1, 2); OW 151±15, 141-162 (1, 2); AvL 128±9, 111-137 (1, 6); AvW 101±4, 95-105 (1, 6); OvL 176±17, 157-189 (1, 3); OvW 217±30, 183-236 (1, 3).
Remarks. Bryopesanser inflexus sp. nov. is distinguishable from all known species of Bryopesanser developing a suboral mucro. In Bryopesanser pesanseris (Smitt, 1873) from the Atlanto-Caribbean area, the frontal shield is densely granular with closely adjacent granules, whereas in the new species it is nodular yet smooth overall. The peristome of the new species produces a narrower mucro and the ooecium is smoother compared to B. pesanseris. Also, the sinus of the new species is deeper and more rounded, differing from the more elliptical sinus of B. pesanseris. Alignment and orientation of avicularia also differ: in the new species, they align with the second proximalmost pair of spines and are distomedially directed, while in B. pesanseris they align with the proximalmost pair of spines and are distally directed. The Indo-Pacific species B. capitaneus Tilbrook, 2006 has the proximalmost pair of spines placed more distally compared to the new species in which this pair is only slightly above the proximal margin of the orifice. Moreover, avicularia alignment in B. capitaneus is as in B. pesanseris. Bryopesanser latesco Tilbrook, 2006, widespread in the Pacific, differs in having condyles dipping medially and a wider-than-long sinus and a flared peristome only slightly raised medially; B. tonsillorum Tilbrook, 2012, widespread in the Indo-Pacific, differs in having distally directed avicularia placed below the proximalmost pair of spines, convex proximal border of orifice, and coarsely denticulate condyles; B. gardineri Tilbrook, 2012, from the western Pacific, has convex proximal margin of the orifice and serrated avicularia edges; B. hebelomaia Tilbrook, 2012, found in the South China Sea, has a smaller and shallower sinus, and distally directed avicularia; B. lobiones Tilbrook, 2012 has the proximal margin of the orifice sloping proximally from the midline; B. protrusus Winston and Jackson, 2021, from Jamaica, exhibits a conical proximal mucro and smooth condyles. The Miocene B. bragai Di Martino and Taylor, 2015 from East Kalimantan (Indonesia) develops a median mucro but differs in having large and simple circular pseudopores. The only species known from Japan, B. ascendolaris Tilbrook, 2012, lacks a proximal, median mucro.
Bryopesanser minutiporosus sp. nov. Di Martino, Rosso and Taylor
Figure 41
zoobank.org/74004D1D-D7C5-4467-9A03-5A91FD36CAD9
Type material. Holotype PMC. B63. 29.7.2024a, sample 19217 (Figure 41A-E); paratype PMC. B63. 29.7.2024b, sample 19210 (Figure 41F-H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
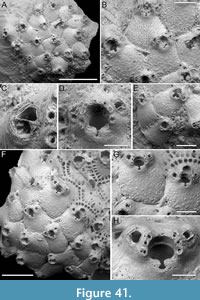 Etymology. Latin, meaning pierced by small pores, referring to the minute size of the lens-shaped pseudopores on its frontal shield.
Etymology. Latin, meaning pierced by small pores, referring to the minute size of the lens-shaped pseudopores on its frontal shield.
Diagnosis. Bryopesanser with proximally tubercular frontal shield densely pierced by minute lens-shaped pseudopores; orifice with longer-than-wide drop-shaped sinus, and minutely denticulate condyles; seven oral spines in autozooids, six visible in ovicellate zooids, the proximalmost pair slightly above the proximal margin of orifice; peristome lacking; paired avicularia distolateral to orifice, aligned with the second proximalmost pair of spines, oval; rostrum open, curved towards the orifice, distomedially directed; ooecium smooth, imperforate, with a transverse proximal crest but lacking proximal projection.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by raised sutures and shallow grooves, pentagonal, slightly longer than wide (mean ZL/ZW 1.18), widest proximally, with thick lateral walls (175 µm); small, subcircular pore-chamber windows visible laterally, approximately 20 µm in diameter. Frontal shield slightly convex, convexity more obvious around the orifice, tubercular proximally, tubercles rounded 20-30 µm in diameter, densely pierced by lens-shaped pseudopores, 3-9 µm long; an area of imperforate calcification distinguishable proximally and laterally to orifice; marginal areolae present at proximolateral zooidal corners, 20-25 µm long. Orifice with elliptical anter, slightly convex proximal margin occupied by rectangular, minutely denticulated condyles, 12-14 µm high, and longer-than-wide drop-shaped sinus, 38 µm long by 20-25 µm wide; seven oral spine bases in autozooids, six in ovicellate zooids, 18-28 µm in diameter, with the proximalmost pair slightly above the proximal margin of the orifice; peristome absent. Avicularia adventitious, paired, located distolaterally to orifice, aligned with the second proximalmost pair of spines, oval; rostrum open, curved towards the orifice, distomedially directed; crossbar complete and thick. Ovicell hyperstomial; ooecium cap-like, smooth, imperforate, with thick proximal margin and a transverse crest proximally, lacking a median projection. Intramural budding observed for autozooids with evident concentric rims around the orifice.
Measurements (µm). ZL 534±48, 457-618 (2, 12); ZW 453±70, 331-596 (2, 12); OL (including sinus) 135±9, 122-149 (2, 6); OW 110±11, 98-125 (2, 6); AvL 76±9, 67-91 (2, 14); AvW 59±6, 46-68 (2, 14); OvL 111±9, 104-120 (2, 4); OvW 178±13, 161-191 (2, 4).
Remarks. Bryopesanser minutiporosus sp. nov. differs from its congener from Daidokutsu cave mainly in the absence of a proximal peristome and ooecium projection, as well as in the appearance of the frontal shield, which has minute lens-shaped pseudopores and proximal tubercles in B. minutiporosus sp. nov. but is faintly nodular with multiporous pseudopores in B. inflexus sp. nov. Among Recent species of Bryopesanser lacking a proximal peristome: B. baderae Tilbrook, 2012 has nine oral spines and circular pseudopores; B. hebelomaia Tilbrook, 2012 develops proximal peristomes in ovicellate zooids; B. tilbrooki Winston and Vieira, 2013 from Brazil has a distinctive subquadrate sinus and multiporous pseudopores. The Miocene B. sanfilippoae Di Martino and Taylor, 2015 from East Kalimantan (Indonesia) also lacks a peristome but differs in having circular pseudopores.
Family GIGANTOPORIDAE Bassler, 1935
Genus GIGANTOPORA Ridley, 1881
Gigantopora sp. 1
Figure 42
Figured material. PMC EDM-Collection J.H.B.139a, sample 19140; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
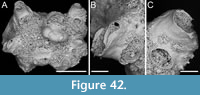 Description. Colony encrusting, multiserial, unilaminar. Autozooids oval to rhomboidal, longer than wide (ZL/ZW 1.40). Frontal shield bearing predominantly rounded tubercles and a few more prominent, presumably pointed processes, with some circular pores difficult to distinguish as either areolae or frontal pseudopores. Peristome tubular forming a broad, nearly smooth bridge, 110-190 µm wide, and a large elliptical to eight-shaped spiramen, with flared margin, 80-105 µm long by 150-250 µm wide. Secondary orifice rounded, 170 µm long by 180 µm wide. Avicularia present or absent, adventitious on the side of peristome, directed laterally inwards; rostrum raised, acutely triangular, with complete crossbar.
Description. Colony encrusting, multiserial, unilaminar. Autozooids oval to rhomboidal, longer than wide (ZL/ZW 1.40). Frontal shield bearing predominantly rounded tubercles and a few more prominent, presumably pointed processes, with some circular pores difficult to distinguish as either areolae or frontal pseudopores. Peristome tubular forming a broad, nearly smooth bridge, 110-190 µm wide, and a large elliptical to eight-shaped spiramen, with flared margin, 80-105 µm long by 150-250 µm wide. Secondary orifice rounded, 170 µm long by 180 µm wide. Avicularia present or absent, adventitious on the side of peristome, directed laterally inwards; rostrum raised, acutely triangular, with complete crossbar.
Measurements (µm). ZL 724 (1, 1); ZW 517 (1, 1); AvL 174 (1, 1); AvW 77 (1, 1).
Remarks. The specimen bears a striking resemblance to species of Gigantopora. However, its poor preservation prevents the observation of certain morphological features, such as the potential perforation of the frontal shield, and precise morphological measurements of most traits. Additionally, the small size of the specimen and the absence of ovicells impede thorough characterization and examination of intracolonial variability. The shape of the avicularia, with an acutely triangular rostrum, is typical of several Gigantopora species, including the widely distributed Indo-Pacific G. pupa (Jullien, 1903). Nonetheless, the smoothly tuberculate surface of the frontal shield, adorned with rounded rather than the more typical pointed tubercles, and the completely smooth texture of the peristomial bridge are less common.
Genus PARAGIGANTOPORA gen. nov. Di Martino, Rosso and Taylor
zoobank.org/A4286261-AE35-4FA4-BC49-48C5B7B52A6F
Type species. Paragigantopora echinata gen. et sp. nov. Di Martino, Rosso and Taylor.
Etymology. Greek prefix ‘para-’, meaning next to, referring to its resemblance to Gigantopora Ridley, 1881.
Diagnosis. Colony erect with rod-like branches and autozooids arranged in alternating longitudinal rows. Frontal shield tubercular, centrally imperforate with only marginal areolar pores, with some extending onto the proximal portion of the shield. Primary orifice surrounded on three sides by a raised, tubular peristome facing upwards and outwards, with peristomial bridge and large spiramen proximally. Spines absent. Avicularia, single or paired, on the sides of the peristome. Ooecium imperforate, opening into the peristome above the primary orifice.
Remarks. A new genus is introduced here to accommodate Paragigantopora echinata sp. nov., a Gigantopora -like species lacking pseudopores and having an imperforate ooecium. Additionally, the new combination Paragigantopora grandis (Gordon and Taylor, 2015) is suggested for an Eocene species, described from the Chatham Islands of New Zealand, sharing these features. Ridley (1881, p. 47) defined Gigantopora as having encrusting colonies, prominent autozooids with nodular frontal shields pierced by pseudopores, upwardly and outwardly directed tubular peristomes, single or paired avicularia flanking the peristome, a distinctive roundish, transversely broad pore visible frontally, and a small, globular, pseudoporous ooecium. While the new species and the fossil from the Chatham Islands are consistent with the diagnosis of Gigantopora in relation to the peristomial characteristics, including placement of the avicularia and the presence of a large spiramen, they differ in colony morphology, being erect rather than encrusting, and in frontal shield features. We agree with Gordon and Taylor (2015) in considering these ‘almost imperforate’ species as a more plesiomorphic form of Gigantopora, yet we propose that these distinctive traits necessitate the creation of a new genus within the Gigantoporidae. Similar actions have been taken previously in analogous situations, such as the introduction of Parkermavella Gordon and d’Hondt, 1997 for Schizomavella -like species with imperforate frontal shields, albeit with an umbonuloid distinction in that case. This pattern is observed in other families as well, like the Smittinidae, where genera such as Smittina are characterized by fully pseudoporous frontal shields, while Smittoidea and Hemismittoidea possess only marginal pores. Several species currently assigned to Gigantopora share certain traits with the new genus but not in its unique combination, simultaneously deviating from the typical diagnosis of Gigantopora. For instance, G. oropiscis Gordon and d’Hondt, 1997 is erect, yet has a proximally pseudoporous frontal shield and numerous marginal pores in the ooecium. Gigantopora foraminosa Hayward and Cook, 1983, and G. spathula Hayward and Winston, 2011 also have erect colonies but otherwise conform to the standard appearance of Gigantopora. An unusual Gigantopora is G. profunda Harmer, 1957, which is erect and cylindrical, with an imperforate frontal shield and an ooecium with only marginal pores, differing from species assigned to Paragigantopora gen. nov. in the position of avicularia, which are generally unilaterally placed at the base of the peristome on the marginal furrows outlining the zooidal perimeters. Gigantopora kirkpatricki Hayward, 1988 has an imperforate ooecium but its frontal shield is fully pseudoporous. Two other species, G. proximalis hispida d’Hondt, 1986 and G. verrucosissima Moyano, 2002, have prominently tubercular frontal shields that are also fully pseudoporous. Among the remaining genera in the family, Cosciniopsis Canu and Bassler, 1927, and Hemicosciniopsis Vigneaux, 1949, have pseudoporous frontal shields and lack a peristomial bridge and spiramen, while Barbadiopsis Winston and Woollacott, 2009, is distinguished by avicularia raised on swollen, pseudoporous cystids, with the rostra surrounding the orifice and meeting over it, small pores in the ooecium that can be obscured by proceeding calcification, and a rounded (not tubular) peristome.
Paragigantopora echinata sp. nov. Di Martino, Rosso and Taylor
Figure 43
zoobank.org/C048BFDE-F5A3-4B73-B5F0-6839B544B42A
Type material. Holotype PMC. B64. 29.7.2024a, sample 19140; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Etymology. Latin, meaning thorny, referring to the frontal shield tubercles giving a spinose appearance to the zooids.
Diagnosis. As for Paragigantopora gen. nov.
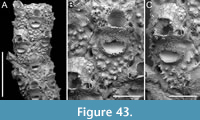 Description. Colony erect with cylindrical branches. Autozooids distinct, separated by narrow furrows, arranged in alternating longitudinal series, rhomboidal, elongate (mean ZL/ZW 1.84). Frontal shield flat, densely covered with evenly distributed tubercles, centrally imperforate; tubercles pointed, occasionally bifurcated, protruding from frontal shield surface by 25-35 µm, lending it a prickly appearance; narrowly elliptical marginal areolae, 35-55 µm long by 15-26 µm wide, distinguishable laterally, one on each side; a few (≤10), circular, smaller areolae, 7-12 µm in diameter, extending onto the proximal portion of the shield. Primary orifice concealed beneath a raised, tubular peristome extending upwards and outwards for approximately 170-250 µm from the branch axis, at an angle of c. 35º; peristomial bridge measuring 90-170 µm in width, lacking tubercles, forming a large, semicircular to reniform spiramen proximally, 100-110 µm long by 165-200 µm wide; secondary orifice elliptical, 100-190 µm long by 130-200 µm wide. Avicularia adventitious, single or paired, placed transversely to the elongation of the zooid and laterally on the peristome at the level of the proximal margin of secondary orifice (or distal margin of peristomial bridge), oval, with rounded rostrum directed laterally and inwards with complete crossbar. Ooecium partially immersed within the frontal shield of the distal zooid, opening into the peristome over the primary orifice; surface nodular and imperforate with a few (approximately five), small, circular marginal areolae visible on the periphery, 5-7 µm in diameter.
Description. Colony erect with cylindrical branches. Autozooids distinct, separated by narrow furrows, arranged in alternating longitudinal series, rhomboidal, elongate (mean ZL/ZW 1.84). Frontal shield flat, densely covered with evenly distributed tubercles, centrally imperforate; tubercles pointed, occasionally bifurcated, protruding from frontal shield surface by 25-35 µm, lending it a prickly appearance; narrowly elliptical marginal areolae, 35-55 µm long by 15-26 µm wide, distinguishable laterally, one on each side; a few (≤10), circular, smaller areolae, 7-12 µm in diameter, extending onto the proximal portion of the shield. Primary orifice concealed beneath a raised, tubular peristome extending upwards and outwards for approximately 170-250 µm from the branch axis, at an angle of c. 35º; peristomial bridge measuring 90-170 µm in width, lacking tubercles, forming a large, semicircular to reniform spiramen proximally, 100-110 µm long by 165-200 µm wide; secondary orifice elliptical, 100-190 µm long by 130-200 µm wide. Avicularia adventitious, single or paired, placed transversely to the elongation of the zooid and laterally on the peristome at the level of the proximal margin of secondary orifice (or distal margin of peristomial bridge), oval, with rounded rostrum directed laterally and inwards with complete crossbar. Ooecium partially immersed within the frontal shield of the distal zooid, opening into the peristome over the primary orifice; surface nodular and imperforate with a few (approximately five), small, circular marginal areolae visible on the periphery, 5-7 µm in diameter.
Measurements (µm). ZL 830±51, 770-875 (1, 4); ZW 450±43, 396-487 (1, 4); AvL 89±8, 75-100 (1, 7); AvW 60±9, 50-72 (1, 7); OvL 158 (1, 1); OvW 260 (1, 1).
Remarks. Paragigantopora echinata gen. et sp. nov. differs from the sole other species we propose including in this newly established genus, P. grandis comb. nov., primarily in the morphology and orientation of its peristomial avicularia. In P. grandis comb. nov., these avicularia have an acute rostrum curving obliquely proximally onto the peristomial bridge. Additionally, P. grandis comb. nov. has a more prominent ooecium. Although some partial similarities have been noted with other species currently assigned to Gigantopora, the differences remain more pronounced, as detailed above in the Remarks section of the new genus.
Family PHOCEANIDAE Vigneaux, 1949
Genus PHOCEANA Jullien in Jullien and Calvet, 1903
Phoceana gabrielei sp. nov. Di Martino, Rosso and Taylor
Figure 44
zoobank.org/60FC897E-2158-4FA2-BE0F-65F1D621E499
Type material. Holotype PMC. B65. 29.7.2024a, sample 19140 (Figure 44A-B); paratypes PMC. B65. 29.7.2024b1, sample 19185 (Figure 44C-E); paratype PMC. B65. 29.7.2024b2, sample 19125 (Figure 44F-H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
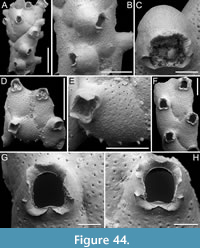 Etymology. Named after a close friend of the first author, Dr Gabriele Lanzafame, volcanologist at the University of Catania.
Etymology. Named after a close friend of the first author, Dr Gabriele Lanzafame, volcanologist at the University of Catania.
Diagnosis. Phoceana with encrusting colonies; autozooids lacking basal walls; frontal shield evenly pseudoporous with subconical tubercles around margins; hoof-shaped orifice with median pseudolyrula but lacking condyles, surrounded by a flared, undulose peristome; avicularia absent; ovicell peristomial with faintly nodular and imperforate ooecium.
Description. Holotype colony tubular, cylindrical, hollow, likely encrusting an ephemeral organic substrate; paratype colonies irregularly shaped, encrusting a small substrate (i.e., sediment grain or bioclast), continuing to develop independently afterward. Autozooids with entirely uncalcified basal walls, with almost indistinct boundaries marked by very shallow grooves or faint wrinkles, irregularly arranged, sometimes back to back, with opposite polarities or tilted relative to each other, typically hexagonal, occasionally rhomboidal, large and elongate (mean ZL/ZW 1.49). Frontal shield convex, faintly nodular, evenly and densely perforated by small, circular pseudopores, 5-13 µm in diameter, except around the peristome; marginal areolae not distinguishable; subconical tubercles developing around the zooidal margin, with a base characterized by two concentric rings: an outer ring 42-52 µm in diameter and an inner ring 17-32 µm in diameter; when preserved, tubercles ranging from 40 to 55 µm in length. Orifice hoof-shaped with a rounded anter, parallel sides, and a proximal margin bearing a pseudolyrula resembling the curve of a flattened Gaussian distribution, measuring 18-35 µm in length and 100-130 µm in width, occupying the centre of the proximal margin and about half of its total length, giving it a convex appearance, without condyles. Peristome relatively short, measuring 195-400 µm in lateral view, terminating in a flared, undulose collar with expanded wings 55-100 µm wide, which can be folded. Spines and avicularia absent. Ovicell peristomial with an imperforate and faintly nodular ooecium that has a more granular texture compared to the frontal shield; the proximal margin coinciding with the flared distal margin of the peristome; secondary orifice of ovicellate zooids more bell-shaped.
Measurements (µm). ZL 952±159, 756-1302 (4, 11); ZW 640±72, 539-734 (4, 11); OL 223±16, 189-238 (4, 8); OW 196±13, 170-208 (4, 8); OvL 334±110, 208-411 (2, 3); OvW 442±42, 393-467 (2, 3).
Remarks. We attributed this species to the genus Phoceana based on several distinct characters: the presence of a pseudolyrula (a median more or less semicircular denticle formed by the elevation of the peristomial wall), a peristomial ovicell, a flared peristome, and the absence of avicularia and of condyles in the orifice.
The genus Phoceana currently includes five species: three Recent species — P. columnaris Jullien, 1903, the type species of the genus from the Northeast Atlantic, P. acadiana Lagaaij, 1963, from Gulf of Mexico, and P. tubulifera (Heller, 1867) from the Mediterranean — and two fossils, P. pliocenica Pouyet, 1978, from the Pliocene of Spain, and the Eocene P. ? simulator (Canu and Bassler, 1920) from North Carolina. All previously known species are characterized by their erect and branched colonies, and long, tubular peristomes. Additionally, P. columnaris has pseudopores limited to the proximal and lateral portions of the frontal shield (Pouyet, 1978, pl. 1, figure 3), while P. ? simulator occasionally features a small avicularium on the peristome. The current distribution of the genus Phoceana is limited to the North Atlantic and the Mediterranean. Phoceana gabrielei sp. nov. represents the first record of the genus in the Pacific.
Family MICROPORELLIDAE Hincks, 1879
Genus CALLOPORINA Neviani, 1895
Calloporina biavicularia Kataoka, 1961
Figure 45
v. 1961 Calloporina biavicularia Kataoka, p. 255, pl. 33, fig. 2.
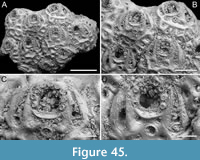 Figured material. PMC EDM-Collection J.H.B.140a., sample 19093; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.140a., sample 19093; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep furrows, oval to rounded hexagonal, longer than wide (mean ZL/ZW 1.22); circular pore chamber-windows, 30-40 µm in diameter, visible along distolateral margins. Frontal shield flat to slightly convex coarsely ornamented with sculptured ridges outlining polygonal depressions housing circular pores mainly along zooidal perimeter (i.e., marginal areolae, 30-55 µm in diameter), with a few centrally located (20-30 µm in diameter). Ascopore a short distance (60-90 µm) from proximal margin of orifice, circular (44-66 µm in diameter), encircled by a thin, raised rim. Orifice longitudinally elongate, semicircular and high-arched, with straight and crenulated proximal margin; nine oral spine bases (20-30 µm in diameter); a ridge of the frontal shield forming a small bowl-shaped platform below the proximal margin. Avicularia long, slender, single or paired, falciform, originating laterally to ascopore and extending the entire length of orifice; rostrum elongate, triangular, with a slight curvature towards the distal edge of orifice, directed distally; crossbar complete. Ovicells not observed.
Measurements (µm). ZL 612±12, 593-622 (1, 5); ZW 500±45, 438-563 (1, 5); OL 126±6, 120-134 (1, 5); OW 118±2, 116-120 (1, 5); AvL 248±26, 202-274 (1, 8); AvW 63±7, 55-71 (1, 8).
Remarks. This species was first described from the Pleistocene Ryukyu Limestone of Kagoshima, Japan (Kataoka, 1961). Despite the small size of our fragment, we observed some intracolonial variability, which was not mentioned by Kataoka (1961). This author noted the presence of generally eight spines. However, in the fragment available to us all zooids have nine spines. A similar discrepancy applies to avicularia; while Kataoka (1961) described them as paired, we observed both paired and single avicularia. Another species of Calloporina is known from the Pleistocene of Japan, C. hayamiae Arakawa, 1995. This species differs in having a crescentic ascopore (transversely C-shaped) and smaller avicularia with needle-like rostra positioned approximately at zooidal mid-length, aligned with the ascopore.
Family PETRALIIDAE Levinsen, 1909
Genus MUCROPETRALIELLA Stach, 1936
Mucropetraliella cf. robusta (Canu and Bassler, 1929)
Figure 46
cf. 1929 Petraliella robusta Canu and Bassler, p. 260, pl. 26, fig. 3.
cf. 1957 Mucropetraliella robusta (Canu and Bassler); Harmer, p. 717, pl. 46, fig. 13, text-fig. 65.
Figured material. PMC EDM-Collection J.H.B.141a, sample 19083 (Figure 46A-E), sample 19135 (Figure 46F), sample 19193 (Figure 46G), sample 19229 (Figure 46H), and sample 19178 (Figure 46I-J); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
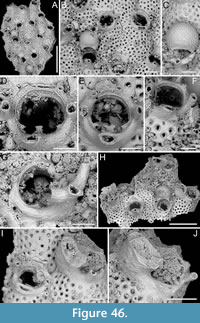 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, irregularly polygonal, elongate (mean ZL/ZW 1.77). Frontal shield slightly convex, smooth, striated, uniformly pseudoporous, with circular pseudopores 16-35 µm in diameter, indistinct from marginal areolae. Primary orifice subcircular (mean OL/OW 0.92), with a narrow anvil-shaped median denticle on the proximal margin (30-52 µm long by 30-70 µm wide at the tips), occupying one-tenth to one-third of the total width of the orifice, and two acutely triangular condyles placed proximally at about one-third from the orifice proximal margin, curved downward, measuring 26-33 µm in length by 20-43 µm at the base; the median denticle and condyles delimiting two transversely elliptical, slightly asymmetrical sinuses. Two distolateral spine bases, 15-35 µm in diameter. Suboral complex prominent, including a median bi-, tri- to quadrifurcate mucro connected to two lateral ones in a steering wheel shape, imperforate, striated and nodular; central mucro 150-305 µm long by 65-140 µm wide, lateral ones about 175 µm long. Avicularia not observed suborally but likely present and placed atop the mucro branches; two small elliptical avicularia positioned atop the lateral branches of the complex, directed proximally, with a complete crossbar; one of the small avicularia occasionally enlarged with a truncate cup-like rostrum curved upwards, directed proximally or proximolaterally inwards; in one instance, an avicularium observed laterally on the frontal shield on a raised column-like cystid, small and elliptical, directed laterally (Figure 46F). Ovicells hyperstomial, prominent, placed on the frontal shield of the distal zooid, occupying approximately one-third of its total length (mean OvL/ZL 0.32), smooth, minutely perforate with pseudopores 4-10 µm in diameter; an area near the orifice appearing imperforate or, at times, covered by incipient secondary calcification (Figure 46C). A subelliptical radicular pore chamber situated medially adjacent to the proximal margin of dorsal side of autozooid. Small conical pillars present in the distomedial area of certain autozooids.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, irregularly polygonal, elongate (mean ZL/ZW 1.77). Frontal shield slightly convex, smooth, striated, uniformly pseudoporous, with circular pseudopores 16-35 µm in diameter, indistinct from marginal areolae. Primary orifice subcircular (mean OL/OW 0.92), with a narrow anvil-shaped median denticle on the proximal margin (30-52 µm long by 30-70 µm wide at the tips), occupying one-tenth to one-third of the total width of the orifice, and two acutely triangular condyles placed proximally at about one-third from the orifice proximal margin, curved downward, measuring 26-33 µm in length by 20-43 µm at the base; the median denticle and condyles delimiting two transversely elliptical, slightly asymmetrical sinuses. Two distolateral spine bases, 15-35 µm in diameter. Suboral complex prominent, including a median bi-, tri- to quadrifurcate mucro connected to two lateral ones in a steering wheel shape, imperforate, striated and nodular; central mucro 150-305 µm long by 65-140 µm wide, lateral ones about 175 µm long. Avicularia not observed suborally but likely present and placed atop the mucro branches; two small elliptical avicularia positioned atop the lateral branches of the complex, directed proximally, with a complete crossbar; one of the small avicularia occasionally enlarged with a truncate cup-like rostrum curved upwards, directed proximally or proximolaterally inwards; in one instance, an avicularium observed laterally on the frontal shield on a raised column-like cystid, small and elliptical, directed laterally (Figure 46F). Ovicells hyperstomial, prominent, placed on the frontal shield of the distal zooid, occupying approximately one-third of its total length (mean OvL/ZL 0.32), smooth, minutely perforate with pseudopores 4-10 µm in diameter; an area near the orifice appearing imperforate or, at times, covered by incipient secondary calcification (Figure 46C). A subelliptical radicular pore chamber situated medially adjacent to the proximal margin of dorsal side of autozooid. Small conical pillars present in the distomedial area of certain autozooids.
Measurements (µm). ZL 818±61, 670-913 (5, 25); ZW 463±43, 368-534 (5, 25); OL 212±13, 189-236 (5, 19); OW 231±12, 209-253 (5, 19); AvL (small-sized) 52±8, 30-64 (5, 25); AvW (small-sized) 39±4, 32-48 (5, 25); AvL (large-sized) 221±41, 165-304 (5, 13); AvW (large-sized) 125±11, 109-152 (5, 13); OvL 265±13, 252-283 (3, 4); OvW 315±19, 298-342 (3, 4).
Remarks. The extant M. robusta from the Philippines is the most similar species, having a similarly developed suboral complex partially concealing the orifice, a narrow bidentate median denticle, rounded paired latero-oral avicularia, and a small frontal avicularium rarely present near the zooidal boundaries. Our “cf.” attribution is for two reasons. First, the suboral avicularium in our specimens is not preserved, while in M. robusta it is very large and slightly expanded at the distal end. Second, the number of spines differs; they were reported as absent in the original description of M. robusta by Canu and Bassler (1929). However, Harmer (1957) reported 4-6 spines and justified the discrepancy by noting that the previous authors examined dead specimens, which likely had detached spines, with the scars difficult to notice. Additionally, neither reference mentions the enlarged lateral avicularium we observed, which has a very similar shape to the suboral avicularium described by Harmer (1957). Mucropetraliella robusta was reported from water depths of 421 m in the Philippines (Canu and Bassler, 1929) and from 0-45 m in Indonesia (Harmer, 1957).
Two oral spines, as seen in our specimens, were described for M. bifidata Tilbrook, 2006 and M. bispinata Liu, 2001, from the Solomon Islands and China Sea, respectively, but they lack avicularia; M. asymmetrica Hayward and Cook, 1983, may have 2-3 spines, suboral avicularia at the base of a simpler mucro, and large lateral avicularia of different shape. The south-west Atlantic M. reticulata Figuerola, Gordon and Cristobo, 2018 has two oral spines, but also a peculiar suboral mucro forming a variable reticulate net of tubular kenozooids.
Compared to other Mucropetraliella species reported from Japan, the Recent Mucropetraliella japonica (Ortmann, 1890), and the Pliocene M. shibikawaensis Hayami, 1975 and M. tenuis Hayami, 1975 all lack spines. Spines are absent also in some Australian species: M. elleri (MacGillivray, 1869) has a simple short mucro and numerous avicularia sparse on the frontal surface; M. halei (Livingstone, 1928) has a simple mucro, numerous avicularia aligned on the lateral margins of the frontal shield, and avicularia instead of spines at the distolateral corners of the orifice; M. nodulosa Stach, 1936 is full of rounded tubercles around the orifice and on the frontal surface; and M. ligulata Stach, 1936 is characterized by its ligulate avicularia (Gordon, 1989b).
Spines are also absent in the following species from the Philippines: M. philippinensis (Canu and Bassler, 1929) which has large frontolateral spatulate avicularia; M. verrucosa (Canu and Bassler, 1929) has the aperture surrounded by small avicularia; and M. trita (Canu and Bassler, 1929) which lacks a mucro. Mucropetraliella malwanensis Sonar, Wayal and Badve, 2021 from the Holocene of India, has no spines, a simple mucro, and differently shaped lyrula and condyles. Two species from the Indian Ocean, M. multiaviculariata (Thornerly, 1912) and M. laccadivensis (Robertson, 1921), also lack spines; the former species is also characterized by supernumerary avicularia on the ovicell, around the orifice and along zooidal margins, with a large frontal avicularium directed proximally. Mucropetraliella armata (Waters, 1913) from East Africa also lacks spines (Hayward and Cook, 1983).
A group of species has up to four spines: M. capricornensis Tilbrook, Hayward and Gordon, 2001, from Vanuatu, has a wide, shallow median denticle, a short and simple mucro bearing an oval avicularium, and lateral avicularia, laterally directed, on cystids that are not much elevated; M. loculifera Harmer, 1957, from Indonesian stations of the Siboga Expedition, has 2-4 spines and a great number of small oval avicularia all aligned along the lateral margins of the zooid and surrounding the orifice, plus a large pointed avicularium budded more centrally on the frontal, obliquely oriented and directed proximolaterally, occupying one proximolateral corner of the zooid, and a broad lyrula with very pointed lateral corners; M. albirostris (Canu and Bassler, 1927) from Hawaii, has a straight and smooth mucro, and lateral avicularia directed laterally; and M. magnifica (Busk, 1884) also from Hawaii, lacks a median denticle and has hexagonal pores in the ovicell; M. neozelanica (Livingstone, 1929), from New Zealand, lacks an avicularian complex (Gordon, 1989b); M. tuberosa (Busk, 1884), from east Australia, has only a large suboral avicularium, and no lateral avicularia.
Several species have a greater number of oral spines. The Australian M. bennetti (Livingstone, 1926) has 7-9 (most commonly eight) thin oral spines, a small suboral mucro and associated avicularium, a large median denticle occupying one-third of orifice width (Tilbrook and Cook, 2004). The Philippine M. echinata (Canu and Bassler, 1929) has 12 oral spines. The western Pacific M. gaudialis d’Hondt, 1986 has eight spines and a large suboral avicularium with a spatuliform and curved rostrum.
A species of Mucropetraliella most reported from the Recent of Japan (Hirose, 2010; Arakawa, 2024) is M. thenardii (Audouin, 1826). The specimen from Sri-Lanka illustrated by Tilbrook and Cook (2005, figure 11A, B) has a similar general aspect to M. cf. robusta but differs in the orientation of the avicularia and the shape of the median denticle and condyles. In the original illustration by Savigny (Audouin, 1826; d’Hondt, 2006), the mucro is slender and trifurcate (similar to Figure 46F, G), but no avicularia or additional conical processes are shown. Harmer (1957) described numerous varieties under the name M. thenardii, making it difficult to understand what the real M. thenardii is, as with all species described by Audouin (1826), until a neotype is chosen. However, the choice of a neotype is problematic considering that the original localities are not well defined and sometimes it is not even clear whether it is a site in the Mediterranean or the Red Sea.
Mucropetraliella cf. tubulifera (Canu and Bassler, 1929)
Figure 47
cf. 1929 Petraliella tubulifera Canu and Bassler, p. 264, pl. 27, figs. 7-12.
Figured material. PMC EDM-Collection J.H.B.142a, sample 19035 (Figure 47A-E) and sample 19095 (Figure 47F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
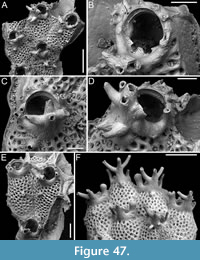 Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, irregularly polygonal, elongate (mean ZL/ZW 1.62). Frontal shield slightly convex, sculptured, uniformly pseudoporous, with circular pseudopores 15-40 µm in diameter, indistinct from marginal areolae, funnel-shaped, or centred in a polygonal mesh. Primary orifice subcircular (mean OL/OW 1.06), with a narrow trapezoidal median denticle on the proximal margin (c. 30 µm long by 40 µm wide at the base), occupying one-fifth of the total width of the orifice, and two acutely triangular condyles positioned proximally, very close (c. 40 µm) to the proximal margin of the orifice, pointing medially, measuring 20-26 µm in length by 17-23 µm at the base; the median denticle and condyles defining two transversely subcircular, slightly asymmetrical sinuses. Four to six distolateral spine bases, 19-39 µm in diameter. Suboral complex prominent, including a median tri- to quadri-furcate mucro connected to two lateral ones in a steering wheel shape, imperforate, nodular, striated, creating a polygonal pattern; central mucro 190-360 µm long, partially obscuring the orifice in frontal view, with branches reaching 150-240 µm in length; lateral mucrones about 80-160 µm long; additional subconical mucrones 75-150 µm long, developing either below the main suboral mucro complex or sparse on the frontal shield, being either straight or slightly curved. Avicularia and ovicells not observed. A circular radicular pore chamber situated in one corner of the proximal margin of the dorsal side of the autozooid. Small conical pillars present in the distomedial area of certain autozooids.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by shallow grooves, irregularly polygonal, elongate (mean ZL/ZW 1.62). Frontal shield slightly convex, sculptured, uniformly pseudoporous, with circular pseudopores 15-40 µm in diameter, indistinct from marginal areolae, funnel-shaped, or centred in a polygonal mesh. Primary orifice subcircular (mean OL/OW 1.06), with a narrow trapezoidal median denticle on the proximal margin (c. 30 µm long by 40 µm wide at the base), occupying one-fifth of the total width of the orifice, and two acutely triangular condyles positioned proximally, very close (c. 40 µm) to the proximal margin of the orifice, pointing medially, measuring 20-26 µm in length by 17-23 µm at the base; the median denticle and condyles defining two transversely subcircular, slightly asymmetrical sinuses. Four to six distolateral spine bases, 19-39 µm in diameter. Suboral complex prominent, including a median tri- to quadri-furcate mucro connected to two lateral ones in a steering wheel shape, imperforate, nodular, striated, creating a polygonal pattern; central mucro 190-360 µm long, partially obscuring the orifice in frontal view, with branches reaching 150-240 µm in length; lateral mucrones about 80-160 µm long; additional subconical mucrones 75-150 µm long, developing either below the main suboral mucro complex or sparse on the frontal shield, being either straight or slightly curved. Avicularia and ovicells not observed. A circular radicular pore chamber situated in one corner of the proximal margin of the dorsal side of the autozooid. Small conical pillars present in the distomedial area of certain autozooids.
Measurements (µm). ZL 717±69, 614-822 (3, 12); ZW 443±37, 376-489 (3, 12); OL 215±18, 202-235 (2, 3); OW 203±13, 194-218 (2, 3).
Remarks. Comparisons between these specimens and known species of Mucropetraliella featuring up to six oral spines will always be partial because crucial distinguishing characters — the shape, position, and size of avicularia — are not yet developed or preserved in our samples. Therefore, we use “cf.” for the identification of this species. Based on other Mucropetraliella species, including the congener found in Daidokutsu cave, we would expect avicularia to have developed atop the mucrones in this species as well. Hence, the Recent M. tubulifera (Canu and Bassler, 1929) from the Philippines is the most similar, having a variable number of spines (3-6 in the nominal species compared with 4-6 observed in the present specimens), and a similarly developed suboral complex. The avicularian complex in M. tubulifera consists of “two laterally salient avicularia, a suboral enormous avicularian mucro bifurcated or branching adorned with very salient tubules” (Canu and Bassler, 1929, p. 264). The “tubules” likely correspond to the frontal subconical mucrones observed in our specimens, either connected with the mucro complex or sparse on the frontal shield. Similar structures are also visible, sparsely distributed on the frontal shield in the original illustration (Canu and Bassler, 1929, pl. 27, figures 10, 12). In the Philippines, Mucropetraliella tubulifera was reported at five stations of increasing depth, from 37 m down to 622 m. Both the ornamentation of the shield and the complexity of the suboral mucro were observed to increase with the depth of the water (Canu and Bassler, 1929).
Other Mucropetraliella species with up to six spines are less similar. Mucropetraliella unimucronata Hayami, 1975, from the Pliocene of Japan, has 5-6 stout oral spines but only a simple suboral mucro. Among Recent Australian species, M. biaviculata (Waters, 1887) has six spines but a diminutive suboral avicularian complex (Gordon, 1989b); M. serrata (Livingstone, 1926) has six oral spines, large frontal avicularia and a suboral complex consisting either of only the avicularium or a short, narrow mucro; M. vultur (Hincks, 1882) has six spines and a bifid mucro with a pair of avicularia on the sides, in addition to latero-oral avicularia directed laterally or slightly proximolaterally. Among the other Recent species from the Philippines, M. robusta (Canu and Bassler, 1929) was originally described as lacking spines, although Harmer (1957) reported 4-6 spines and doubted their genuine absence because Canu and Bassler (1929) examined dead specimens that may have only preserved the spine bases, which could be easily overlooked. This species has a very massive suboral complex partially concealing the orifice, similar to M. cf. tubulifera, but there is no mention of additional “tubules” in its description. Conversely, M. robusta is characterized by paired latero-oral avicularia and frontal avicularia near the zooidal margins, like those in the other Mucropetraliella species found in Daidokutsu cave that we identified as M. cf. robusta (see description above).
Family LACERNIDAE Jullien, 1888
Genus ARTHROPOMA Levinsen, 1909
Arthropoma iuncta sp. nov. Di Martino, Rosso, and Taylor
Figure 48
zoobank.org/EE675AC2-CE56-47F8-9FEF-A1B32042AC8E
Type material. Holotype PMC. B67. 29.7.2024a, sample 19041 (Figure 48A-C); paratype PMC. B67. 29.7.2024b1, sample 19178 (Figure 48D); paratype PMC. B67. 29.7.2024b2, sample 19085 (Figure 48E); paratype PMC. B67. 29.7.2024b3, sample 19092 (Figure 48F); paratype PMC. B67. 29.7.2024b4, 19104 (Figure 48G-H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
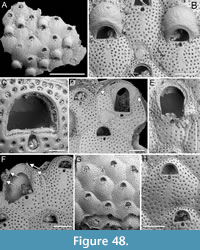 Etymology. Latin, meaning joined, referring to the orificial condyles joining over the sinus, thereby materially separating it from the orifice.
Etymology. Latin, meaning joined, referring to the orificial condyles joining over the sinus, thereby materially separating it from the orifice.
Diagnosis. Arthropoma with frontal shield evenly and densely pierced by circular pseudopores arranged in a regular pattern, except for a slender, imperforate, U-shaped strip below the orifice; orifice with robust condyles joined over the drop-shaped ‘sinus’, lacking spines; ooecium with mostly exposed, imperforate, minutely pitted endooecium marked by radial wrinkles, and smooth, calcified ectooecium reduced to a narrow band proximally; vicarious avicularia similar to autozooids but with an enlarged opesia and distal spines.
Description. Colony encrusting, multiserial, uni- to multilaminar. Autozooids distinct, delineated by fine furrows, quincuncially arranged, rounded hexagonal, slightly longer than wide (mean ZL/ZW 1.17). Frontal shield flat or slightly convex, densely pierced by pseudopores throughout except for a narrow, central, U-shaped strip below the orifice, 35-45 µm wide by 180-200 µm long; pseudopores circular, c. 14-20 µm in diameter, regularly arranged, with 1-2 rows distal to the orifice. Orifice semicircular, wider than long; the robust, rectangular, crenulated condyles (15 µm thick) bridging over the drop-shaped ‘sinus’, materially occupying the entire length of the proximal margin of the orifice and separating the two openings; peristomial rim and distal oral spines absent. Vicarious avicularia closely resembling autozooids in size, shape and frontal shield features, but with an enlarged, slightly spatulate opening/rostrum similarly characterized by joined condyles and drop-shaped ‘sinus’, and at least four distal spines; palatal shelf well developed distally, rapidly tapering laterally, absent proximally. Ovicell hyperstomial, globular; endooecium mostly exposed, imperforate, faintly marked by radial wrinkles, and minutely pitted; ectooecium partially calcified, covering the proximal periphery of the ooecium, smooth.
Measurements (µm). ZL 565±49, 457-671 (4, 20); ZW 484±46, 392-561 (4, 20); OL 122±5, 112-133 (4, 20); OW 170±8, 156-184 (20, 4); SinL 45±4, 39-53 (4, 20); SinW 32±3, 24-38 (4, 20); AvL 640±60, 572-713 (4, 4); AvW 481±67, 422-577 (4, 4); AvOpL 377±49, 326-426 (4, 4); AvOpW 303±27, 279-332 (3, 3); OvL 260±34, 206-300 (5, 13); OvW 322±16, 299-346 (5, 13).
Remarks. All species of Arthropoma described to date lack avicularia. Levinsen’s (1909) definition of the genus suggests that avicularia might be present, likely because Levinsen concurrently examined specimens belonging to both A. cecilii (Audouin, 1826) and Phonicosia circinata (MacGillivray, 1869). However, an additional species of Arthropoma, identified as A. cecilii in Hirose (2010, pl. 189D), has vicarious avicularia with a few distal oral spines. It differs from A. iuncta in having an orifice with thinner condyles that do not connect over the sinus, thus leaving it distally open, and in the shape of the avicularian rostrum, which is horseshoe-shaped while in the new species it is slightly spatulate. Another unusual feature of this new species among Arthropoma is the presence of the condyles that separate the orifice from a functional ascopore in both autozooids and avicularia. This feature is exceptionally rare in avicularia, with the only known example being in the Late Cretaceous genus Dysnoetopora Canu and Bassler, 1926 (Voigt, 1971).
Arthropoma semipunctata sp. nov. Di Martino, Rosso and Taylor
Figure 49
zoobank.org/71241B43-77B4-4CE2-9567-7B68F4F0F230
Type material. Holotype PMC. B66. 29.7.2024a, sample 19048 (Figure 49A-D); paratype PMC. B66. 29.7.2024b1, sample 19025 (Figure 49E-F); paratype PMC. B66. 29.7.2024b2, sample 19041 (Figure 49G-H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
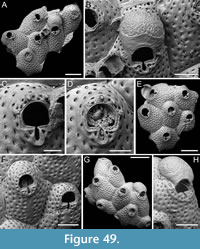 Etymology. Latin, meaning half-dotted, referring to the partially visible pseudoporous endooecium.
Etymology. Latin, meaning half-dotted, referring to the partially visible pseudoporous endooecium.
Diagnosis. Arthropoma with four distal spines in non-ovicellate autozooids but lacking in ovicellate autozooids, frontal shield pierced by slit-like pseudopores arranged in concentric ellipses leaving a slender imperforate strip beneath the orifice; orifice with deep, narrow sinus bordered by a flared, slim, proximal rim; ovicell kenozooidal; ooecium with exposed pseudoporous endooecium in its distal half, and smooth calcified ectooecium covering its proximal half.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, delineated by deep furrows, quincuncially or irregularly arranged, rounded hexagonal to irregularly polygonal, longer than wide (mean ZL/ZW 1.34). Frontal shield convex, pierced by pseudopores except for a narrow, central, rectangular strip below the orifice, 45-65 µm wide by 200-230 µm long, indistinct in some zooids; pseudopores slit-like, c. 15-25 µm long, arranged in concentric ellipses encircling the zooidal perimeter, with 4-5 rows distal to the orifice. Orifice placed at about 100-150 µm from distal zooidal margin, semicircular, wider than long, with a narrow, deep U-shaped sinus, flanked by two rectangular (10-15 µm wide by 50-65 µm long), crenulated condyles, slightly surpassing the length of the proximal margin on each side of the sinus and forming a distal constriction; a narrow (10-25 µm wide), flared rim surrounding the orifice (and sinus) proximally and laterally; four non-articulated distal oral spines present, short (20-35 µm long), tapering to a point with a small apical pore, the proximalmost pair positioned slightly above the distal half of the orifice, regularly and largely spaced, with pseudopores between pairs of spines, spine diameter at the base 18-26 µm; no spines visible in ovicellate zooids. Ovicell hyperstomial, globular, kenozooidal; endooecium partially exposed distally, with orange-peel texture, densely pierced by minute (2-3 µm in diameter), circular pseudopores; ectooecium partially calcified, covering the proximal half of the ooecium, the outline undulately rounded and W-shaped, smooth surface. Kenozooids associated with ovicells, irregularly polygonal, the portion exposed pseudoporous as zooidal frontal without further openings.
Measurements (µm). ZL 718±64, 647-834 (4, 12); ZW 537±54, 482-655 (4, 12); OL 135±5, 127-141 (4, 11); OW 170±6, 162-179 (4, 11); SinL 63±5, 52-71 (4, 12); OvL 313±32, 290-335 (2, 2); OvW 358±6, 353-362 (2, 2).
Remarks. Among Arthropoma species, only two others have autozooids with oral spines: A. subarensis Jain, Gordon, Huang, Kuklinski and Liow, 2022 and A. occidua Winston and Jackson, 2021. These species differ from A. semipunctata sp. nov. in features of the ooecium. Arthropoma subarensis is characterized by a reticulate dimpled patterning of the endooecium with minute perforations at the bottom of each dimple (Jain et al., 2022), while A. occidua features a helmet-shaped ooecium, longer than wide, with slightly granular calcification and a few small pseudopores (Winston and Jackson, 2021).
Genus CRIBELLOPORA Gautier, 1957
Cribellopora? fissurata sp. nov. Di Martino, Rosso, and Taylor
Figure 50
zoobank.org/5BF225A9-4B51-4CA3-988D-476030A73AB0
Type material. Holotype PMC. B68. 29.7.2024a, sample 19088; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
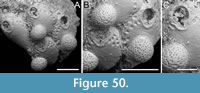 Etymology. Latin, meaning fissured, referring to the deep slit-like sinus of the orifice.
Etymology. Latin, meaning fissured, referring to the deep slit-like sinus of the orifice.
Diagnosis. Lacernid with exposed lateral gymnocyst forming a well defined boundary with the frontal wall, and a slightly raised rim below the orifice; frontal shield mostly imperforate except for 1-2 rows of peripheral pores, seemingly stellate; orifice semicircular with deep slit-like sinus, 6-7 distolateral spines in autozooids, four in ovicellate zooids; ovicell small and globular, uniformly pseudoporous with ooecial pseudopores circular.
Description. Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep grooves, rounded hexagonal, longer than wide (mean ZL/ZW 1.39). Gymnocyst smooth, visibly exposed laterally and around the orifice, forming a well-defined, slightly elevated boundary with the frontal wall; gymnocystal rim extending laterally to the orifice, aligning with the proximalmost pair of oral spine bases and lying at a short distance below the oral sinus. Frontal shield flat to slightly convex, smooth, mostly imperforate except for one or two rows of pseudopores along the zooidal lateral margins, the outermost row adjacent to the gymnocystal rim; pseudopores with a seemingly stellate pattern, 12-24 µm in diameter, situated within funnel-shaped depressions. Orifice semicircular, almost as wide as long with rectangular condyles spanning the entire length of the proximal margin of the orifice; sinus deep and slit-like; 6-7 oral spine bases in autozooids, 13-17 µm in diameter, the largest being the proximalmost pair; four spines retained in ovicellate zooids. Ovicell hyperstomial, globular; ooecium uniformly pseudoporous; pseudopores circular, 4-9 µm in diameter. Pore-chamber windows visible distally and distolaterally at colony edge, elliptical, 27-49 µm long by 9-21 µm wide.
Measurements (µm). ZL 415±87, 330-536 (1, 4); ZW 299±24, 267-324 (1, 4); OL 94±3, 92-98 (1, 4); OW 98±4, 94-101 (1, 4); SinL 27±2, 25-29 (1, 4); OvL 196±23, 171-217 (1, 3); OvW 201±12, 188-212 (1, 3).
Remarks. This species is tentatively assigned to Cribellopora because of its similarity with C. souleorum Dick, Tilbrook, and Mawatari, 2006 from Hawaii. These two species share characteristics such as autozooids with a lateral gymnocyst forming a well-defined boundary with the frontal wall, and a frontal shield mostly imperforate except for peripheral rows of seemingly stellate pores. They also share the semicircular orifice surrounded by smooth gymnocystal calcification with a narrow, deep sinus and long condyles flanking it, along with robust spine bases, and a globose ovicell with minute simple pores (see Dick et al., 2006, figure 13A-C and this paper Figure 50). The primary difference between the two species is in the shape of the sinus. In C. ? fissurata, the sinus is deep and slit-like with parallel sides, whereas in C. soleorum it is shallower and drop-shaped. Souto et al. (2010) questioned the generic assignment of C. soleorum after examining the type specimen of C. simplex Gautier, 1957, the type species of Cribellopora. Cribellopora simplex has a frontal shield uniformly perforated by compound (i.e., cribrate) pseudopores that are also present distally to the orifice, no visible lateral gymnocystal walls, and evanescent spines. Ovicells in C. simplex are flattened frontally with a central umbo, and imperforate except for a row of pseudopores arranged in an arc on its distal slope. Among the species currently assigned to the genus Cribellopora, there is significant morphological variability. For instance, C. connata (Ortmann, 1890) from Japan has uniformly porous ooecia with the same cribrate pseudopores as those on the frontal (Arakawa, 2024), while species like C. divisopora (Waters, 1887), C. napi Gordon, 1989b and C. siri Gordon, 1989b from New Zealand have imperforate ooecia except for a row of stellate or simple marginal pores (Gordon, 1989b). The Miocene European species C. latigastra (David, 1949) also displays this pattern (David and Pouyet, 1974). The Atlanto-Mediterranean C. trichotoma (Waters, 1918) appears more closely related to C. simplex. Although initially synonymized by Gautier (1962), they were later proven to be distinct (Souto et al., 2010). Cribellopora trichotoma has a wider sinus, stouter spine bases, and a more densely perforated ooecium. Cribellopora constellata Winston, 2005 features a frontal shield with numerous stellate pores, also found distally to the orifice and scarcely below it. This species also lacks oral spines and has an imperforate ooecium. The Miocene species C. hluchovensis Zágoršek, 2010 and C. trasoni Zágoršek, 2010 share an absence of oral spines and of marginal ooecial pores, although preservational issues, especially in the former species, might have obscured this characteristic (Zágoršek, 2010).
Within Lacernidae, Arthropoma, Lacerna Jullien, 1888 and Phonicosia Jullien, 1888 are other genera bearing a general resemblance to C. ? fissurata sp. nov., which however does not fit well into any of them. The shape of the sinus resembles that of some species of Arthropoma and Phonicosia, which differ in the arrangement of frontal pseudopores (present also distally to the orifice and more uniformly on the frontal except for limited central imperforate areas) and the development of the lateral gymnocyst. Additionally, variations in ovicell structure distinguish these genera. Arthropoma species may have a smooth, imperforate endooecium and a partially calcified ectooecium proximally, while ovicells in Phonicosia are smooth and imperforate except for marginal pores. Moreover, Phonicosia can have frontal adventitious avicularia. Lacerna has a frontal shield similar to C. ? fissurata sp. nov., with the lateral gymnocyst forming a sharp boundary and pseudopores predominantly limited to zooidal margins. However, the orifice typically displays a wider sinus, and the ovicell bears only marginal pores. This frontal structure, characterized by lateral gymnocystal walls forming a sharp boundary, as well as the arrangement of pseudopores, are reminiscent of species of Fenestrulina, a closeness supported by molecular sequencing data (see Orr et al., 2022).
While the similarities between our species and C. souleorum, along with their distinctive features, may suggest the need for a new genus within Lacernidae, we refrain from such action due to the scarcity of available specimens and preservational issues which impede the comprehensive observation of species variability and obscure pore shape, despite our certainty that they are not compound. Consequently, we choose provisionally to assign this species to Cribellopora based on its resemblance to C. souleorum.
Superfamily MAMILLOPOROIDEA Canu and Bassler, 1927
Family CLEIDOCHASMATIDAE Cheetham and Sandberg, 1964
Genus CHARACODOMA Maplestone, 1900
Characodoma erinaceum sp. nov. Di Martino, Rosso, and Taylor
Figure 51
zoobank.org/8E740FD0-F384-42D0-8B23-B461004FC248
Type material. Holotype PMC. B69. 29.7.2024a, sample 19183 (Figure 51E, G); paratype PMC. B69. 29.7.2024b1, sample 19025 (Figure 51A-B); paratype PMC. B69. 29.7.2024b2, sample 19041 (Figure 51C-D); paratype PMC. B69. 29.7.2024b3, sample 19068 (Figure 51F); paratype PMC. B69. 29.7.2024b4, sample 19228 (Figure 51H); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
 Etymology. Latin, meaning hedgehog, referring to the spiny appearance of its frontal shield.
Etymology. Latin, meaning hedgehog, referring to the spiny appearance of its frontal shield.
Diagnosis. Characodoma with both encrusting and erect colony growth phases, hexagonal autozooids with rather smooth frontal shield becoming spinous during ontogeny, imperforate except for two subcircular to slit-like marginal areolae at zooidal distolateral corners; orifice keyhole-shaped with small triangular condyles pointing proximally and shallow bowl-shaped sinus; avicularia rare, single, budded from one of the distolateral areolae, drop-shaped; rostrum rounded triangular, directed distolaterally; crossbar complete; ovicell resting on the frontal shield of the distal zooid at a certain distance from distal margin of orifice of maternal zooid, with large rounded triangular opening; ooecium smooth with spiny tubercles as in the frontal shield.
Description. Colony with both encrusting and erect growth phases; erect portion tubular or flat and bilamellar. Autozooids distinct, separated by shallow grooves and sometimes raised sutures, arranged quincuncially, hexagonal, longer than wide (mean ZL/ZW 1.27). Frontal shield smooth, developing spiny tubercles, up to 25 µm long, during ontogeny; tubercles aligned to form a collar proximally to orifice and additional tubercles developing concentrically to the collar or radially, usually occupying the central part of the frontal shield, leaving the proximal third bare; frontal shield appearing radially striated when tubercles are not yet developed; imperforate, with only 1-2 subcircular, slit-like or reniform marginal areolae, 20-30 µm long, placed at the distolateral corners of zooids, budding adventitious avicularia; occasionally a single areola present proximally. Orifice keyhole-shaped, with a horseshoe-shaped anter separated from a narrower, bowl-shaped sinus by two small, acutely triangular condyles pointing proximally, oral spines absent. Avicularium rarely present, placed on a raised cystid at zooidal lateral corner on either side, drop-shaped; rostrum rounded triangular, directed distolaterally; crossbar complete. Ovicell resting on the frontal shield of the distal zooid, not covering the distal orificial margin of the maternal zooid but at a certain distance from it (15-20 µm), globular, with large rounded triangular opening (60 µm long by 90 µm wide); ooecium smooth, developing spiny tubercles like the frontal shield. Avicularia occasionally showing evidence of intramural budding.
Measurements (µm). ZL 300±33, 236-361 (3, 20); ZW 235±34, 186-305 (3, 20); OL 111±8, 99-127 (3, 15); OW 103±7, 91-117 (3, 15); AvL 101±13, 83-120 (2, 12); AvW 64±5, 57-73 (2, 12); OvL 151±9, 140-164 (2, 6); OvW 190±18, 161-205 (2, 6).
Remarks. Most species of Characodoma have a granular, imperforate frontal shield with a row of conspicuous marginal pores. However, a few species have a rather smooth frontal shield with sparse tubercles and only one or two marginal areolae from which the avicularia are budded, as in this new species. Among these, the Indo-Pacific Characodoma longitudinale (Harmer, 1957) differs by having rounded tubercles (spiny in the Japanese species), and in the shape of the sinus, which is arrow-shaped in C. longitudinale but bowl-shaped in the new species (see also Di Martino et al., 2019, figure 9D-G). Additionally, the avicularia in C. longitudinale are smaller and rounded, whereas they are larger and triangular in C. erinaceum sp. nov. The Miocene species C. excubans (Waters, 1881) and C. rotundum (Waters, 1881) are the most similar, having a frontal shield that becomes spinose during ontogeny and both encrusting and erect colony growth phases (Cook and Bock, 1996). They differ in the position, direction and shape of avicularia: the former species has paired latero-oral avicularia directed proximolaterally outwards in addition to frontal spatulate avicularia, while the latter species has oval avicularia pointing to the orifice. ‘Characodoma’ latisinuatum Harmer, 1957 has a similar spiny appearance but is doubtfully placed in Characodoma because of its pseudoporous frontal shield. Based on the original drawing, the Japanese species Cleidochasma inermis Ortmann (1890) and C. japonica Ortmann (1890) seem to fit better in Plesiocleidochasma than in Characodoma.
Superfamily CELLEPOROIDEA Johnston, 1838
Family CELLEPORIDAE Johnston, 1838
Genus BUFFONELLARIA Canu and Bassler, 1927
Buffonellaria conformis sp. nov. Di Martino, Rosso, and Taylor
Figure 52
zoobank.org/E04D2C6A-0013-4D23-8861-13B536A1839E
Type material. Holotype PMC. B70. 29.7.2024a, sample 19174 (Figure 52A-D); paratype PMC. B70. 29.7.2024b, sample 19115 (Figure 52E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
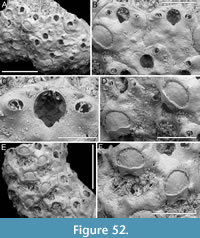 Etymology. Latin, meaning in compliance with, referring to the features of this species, such as the distolateral avicularia and the ooecium with ectooecial fenestra, which are typical and consistent with the generic diagnosis of the genus Buffonellaria.
Etymology. Latin, meaning in compliance with, referring to the features of this species, such as the distolateral avicularia and the ooecium with ectooecial fenestra, which are typical and consistent with the generic diagnosis of the genus Buffonellaria.
Diagnosis. Buffonellaria with flat, nodular frontal shield; single or paired oval avicularia, distolateral to orifice and placed above the level of condyles, rostrum smooth directed laterally outwards or slightly proximolaterally, with curved crossbar; orifice with widely U-shaped sinus; ooecium with large, semicircular, ectooecial fenestra, the proximal calcified portion with undulate distal margin and discontinuous from the lateral secondary calcification, endooecium only faintly ribbed.
Description. Colony encrusting, multiserial, uni- to multilaminar. Autozooids distinct, separated by thin furrows, irregularly arranged, irregularly polygonal, longer than wide (mean ZL/ZW 1.24). Frontal shield flat, nodular, imperforate except for one or two small, elliptical marginal pores placed laterally, approximately 20-35 µm from zooidal margins, occurring around zooidal mid-length to distal third, 5-9 µm long by 4-5 µm wide. Orifice slightly longer than wide; anter two-thirds of a full circle, proximal margin short and gently sloping, transitioning into a widely U-shaped sinus occupying roughly half of orifice width; condyles conspicuous, rhomboidal. Avicularia adventitious, single or paired, oval, placed distolaterally to orifice, slightly above the level of condyles; rostrum rounded, smooth, raised, directed laterally outwards or slightly proximolaterally; crossbar complete and curved with convexity opposite to rostrum direction. Ooecium recumbent upon frontal shield of distal zooid, surrounded distally and laterally by thick, secondary calcification; the proximal portion of the partially calcified ectooecium measuring 30-50 µm in length, appearing discontinuous from the secondary calcification surrounding the distal and lateral margins, its distal rim undulose; ectooeocial fenestra semicircular, 88-98 µm long by 125-150 µm wide; exposed endooecium faintly ribbed.
Measurements (µm). ZL 316±32, 271-370 (2, 10); ZW 255±43, 205-333 (2, 10); OL 91±6, 78-98 (2, 9); OW 82±4, 74-89 (2, 9); AvL 46±5, 37-56 (2, 14); AvW 33±4, 27-38 (2, 14); OvL 164±10, 151-175 (2, 6); OvW 180±8, 168-189 (2, 6).
Remarks. The new species differs from species of Buffonellaria previously reported from Japan. Buffonellaria divergens (Smitt, 1873), first described from Florida and later recorded by Hirose (2010) in the Sagami Sea and Sagami Bay, differs in having smaller distolateral avicularia, more distally placed on a well raised cystid, and more proximally directed (see also images of the lectotype in Berning and Kuklinski, 2008, p. 542, figure 3); an additional larger avicularium with acute rostrum proximolaterally to the orifice on one side although inconstant; and in the more terminally positioned orifice with steeper sides to the sinus. Buffonellaria sp. nov. of Hirose (2010) differs in having a coarsely tuberculate frontal shield, and a single avicularium on one side proximally to the orifice; it also lacks the non-calcified central ectooecial fenestra with the endooecium completely covered by secondary calcification, casting doubt that this species genuinely belongs to Buffonellaria. Berning and Kuklinski (2008) also mentioned a record of ‘ Schizoporella biaperta ’ from Japan by Androsova (1958), suggesting the need for re-examination of this material to ascertain its potential conspecificity with their newly described species B. arctica Berning and Kuklinski, 2008. Buffonellaria arctica is characterized by a distinct elliptical area of exposed endooecium, a more pronounced granulation of the frontal shield, and the presence of numerous, larger marginal areolae. Expanding the comparison to all northern Pacific Buffonellaria species, B. acutirostris Seo and Gong, 2006 is distinguished by its partially pseudoporous frontal shield (see also Seo and Min, 2009); B. bolini (Osburn, 1952) has an additional pair of avicularia proximal to orifice, sometimes supplemented by adventitious avicularia at the distal corners, along with a minutely pseudoporous frontal shield; B. ? indistincta (Canu and Bassler, 1929) also has distolateral avicularia directed proximally, a larger avicularium with an acutely triangular rostrum proximal to the orifice, and an erect bilaminar colony with zooids arranged back to back. Southern Pacific species also differ: B. depressa (Phillips, 1900) has paired distolateral avicularia directed proximally, frequent large spatulate avicularia with serrated rostra, and an extremely reduced distal ectooecial fenestra (see Gordon, 1984, pl. 47D); B. biavicularis (Powell, 1967) has distally directed, paired avicularia proximolateral to the orifice, a broader sinus, and coarsely tuberculate frontal shield (see Gordon, 1984, pl. 47A-B); B. christinelloides Gordon, 1984 has a vertical ooecial fenestra facing distally, frequent large spatulate frontal avicularia, paired distolateral avicularia on an elevated cystid, and additional protuberances on the frontal (Gordon, 1984, pl. 47C); B. regenerata (Powell, 1967) is characterized by paired distolateral avicularia with a denticulate rostrum, a distinctive hanging position of the ooecium, exposing the distal margin of the orifice and the distal zooidal margin, and a semilunar ridge proximal to the orifice (Gordon, 1984, pl. 47E). Lastly, B. turbula Gordon, 1989b shows jumbled autozooids due to intensive frontal budding, large marginal areolae, and frequent spatulate avicularia (Gordon, 1989b, pl. 35F).
Genus CELLEPORARIA Lamouroux, 1821
Celleporaria cf. calva Tilbrook, 2006
Figure 53, Figure 54
cf. 2006 Celleporaria calva Tilbrook, p. 140, pl. 25D-F.
cf. 2017 Celleporaria calva Tilbrook; Dick and Grischenko, p. 181, fig. 12.
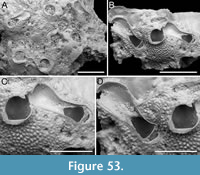 Figured material. PMC EDM-Collection J.H.B.144a. Colonies without spines: samples 19125 (Figure 53A) and 19114 (Figure 53B-D). Colony with spines: sample 19127 (Figure 54). Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.144a. Colonies without spines: samples 19125 (Figure 53A) and 19114 (Figure 53B-D). Colony with spines: sample 19127 (Figure 54). Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct or indistinct; when distinct, separated by shallow furrows between very thin, raised rims, irregularly polygonal, slightly longer than wide (ZL/ZW 1.13 with spines, 1.17 without spines), chaotically arranged. Frontal shield convex, coarsely, densely and evenly tuberculate with rounded tubercles measuring 20-30 µm in diameter, imperforate except for a peripheral row of circular, marginal areolar pores, each 22-28 µm in diameter. Orifice hoof-shaped with curved distal and straight proximal margin, nearly equidimensional (mean OL/OW 0.97 with spines, 0.83 without spines), with rounded condyles placed proximally, at reduced distance from the proximal orifice margin (c. 20-25 µm); a shelf-like peristome developing proximolaterally; oral spines present or absent, when present 2-4 spine bases (more commonly three) distally and distolaterally, 20-50 µm in diameter, the proximalmost pair with more robust bases. Avicularia vicarious, with rostrum parallel sided or slightly spatulate, randomly directed, obliquely placed on large cystids with the same frontal texture and sparse marginal areolae as the autozooids; avicularian opening rounded triangular, with a complete crossbar. In the smallest fragment found, consisting of two avicularia and one autozooid, the avicularia flank the autozooid and are distolaterally directed, one rostrum touching and indenting the lateral margin of the autozooid orifice. Ovicells not observed.
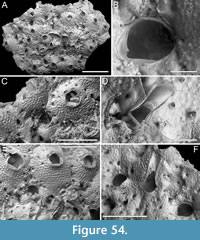 Measurements (µm). Colonies without spines: ZL 538 (1, 1); ZW 476 (1, 1); OL 174±4, 171-177 (2, 2); OW 179±1, 179-180 (2, 2); AvL 508±42, 478-571 (2, 4); AvW 198±25, 174-232 (2, 4). Colony with spines: ZL 497±49, 462-532 (1, 2); ZW 426±7, 421-431 (1, 2); OL 152±16, 132-175 (1, 5); OW 185±18, 167-207 (1, 5); AvL 300±93, 212-456 (1, 5); AvW 99±46, 74-181 (1, 5).
Measurements (µm). Colonies without spines: ZL 538 (1, 1); ZW 476 (1, 1); OL 174±4, 171-177 (2, 2); OW 179±1, 179-180 (2, 2); AvL 508±42, 478-571 (2, 4); AvW 198±25, 174-232 (2, 4). Colony with spines: ZL 497±49, 462-532 (1, 2); ZW 426±7, 421-431 (1, 2); OL 152±16, 132-175 (1, 5); OW 185±18, 167-207 (1, 5); AvL 300±93, 212-456 (1, 5); AvW 99±46, 74-181 (1, 5).
Remarks. Celleporaria calva was first described from the Solomon Islands (Tilbrook, 2006) and has since been reported from Okinawa at two sites: Sesoko Island and Minna Island (Dick and Grischenko, 2017). Colonies found at Sesoko Island lacked spines, like the nominal species, while those from Minna Island had two oral spines. Despite this difference, all other features matched, leading the authors to classify the two morphotypes as the same taxon. We encountered a similar situation and decided to interpret colony fragments lacking oral spines as well as the single colony having oral spines as belonging to the same species. The absence of spines appears to be genuine. In some zooids spines were obscured by spreading calcification from the distal zooid (e.g., Figure 54B with spines), but traces of their presence were always evident. In the two specimens lacking spines, they could be concealed behind the peristome, yet there is no sign of them in any of the zooids, not even in the larger fragment (Figure 53A without spines). Our specimen with spines differs from the Minna Island specimen in having 2-4 spines instead of consistently having two. All colonies from Daidokutsu (i.e., with and without spines) share the granular texture of the frontal shield and the hoof-shaped orifice, although the condyles are not obvious in the colony without spines. They also share the development of a shelf-like proximal peristome. The shape of the interzooidal/vicarious avicularia differs between the colonies. The colony with spines has a rostrum with parallel sides, while the one without spines has a more spatulate shape. However, Celleporaria calva from Okinawa, as described by Dick and Grischenko (2017), possesses both types of avicularia within the same colony. This suggests that the presence of only one type in our fragments may simply be due to the small and poorly preserved nature of our specimens. Given the fragmentary state and lack of ovicells in our specimens, we have decided against splitting the species or introducing a new species for the colonies with spines. Instead, we adopt a conservative approach in line with Dick and Grischenko (2017).
Celleporaria cf. repens (Canu and Bassler, 1929)
Figure 55
cf. 1929 Holoporella repens Canu and Bassler, p. 427, pl. 62, figs. 2-6.
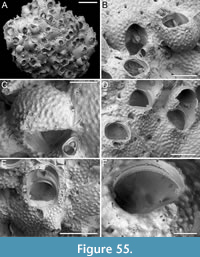 Figured material. PMC EDM-Collection J.H.B.145a, sample 19127; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.145a, sample 19127; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct or indistinct; when distinct, separated by shallow furrows, irregularly polygonal, longer than wide (mean ZL/ZW 1.21), chaotically arranged. Frontal shield convex, coarsely, densely, and evenly tuberculate with rounded tubercles measuring 15-25 µm in diameter, imperforate except for a few (4-5), sparse, circular, marginal areolar pores, each 10-50 µm in diameter. Orifice transversely D-shaped with a straight to slightly concave proximal margin, wider than long (mean OL/OW 0.78), with robust rounded triangular to trapezoidal condyles, 10-16 µm long, placed at proximal corners obliquely and projecting inwards; a short peristome surrounding the orifice; oral spines absent. Avicularium adventitious, suboral, oval, with a raised rostrum partially aligned on the proximal margin of the orifice and partially on the side of a variably developed subconical mucro, 95-125 µm long, directed obliquely and outwardly; avicularian openings semicircular; crossbar complete. Ovicells hyperstomial, globular; ooecium nearly encircling and completely obscuring the orifice, coarsely and densely granular like the frontal shield, sometimes developing a short mucro centrally or proximomedially.
Measurements (µm). ZL 413±32, 375-457 (1, 7); ZW 342±30, 290-384 (1, 7); OL 110±5, 105-116 (1, 4); OW 141±10, 129-152 (1, 4); AvL 74±8, 62-88 (1, 11); AvW 54±7, 42-64 (1, 11); OvL 171±17, 156-202 (1, 6); OvW 236±13, 213-249 (1, 6).
Remarks. Among Celleporaria species, the Daidokutsu specimen appears most similar to C. repens from an unknown locality in the Philippines, sharing with it several characters: a granular frontal shield with marginal pores, slightly erect zooids, a semielliptical transverse orifice without spines, suboral avicularia with salient rostrum, and an ovicell that nearly encircles the entire aperture. It also lacks interzooidal avicularia. Our uncertainty stems from the limited details in the original species description and illustration, making it impossible to confirm certain features, such as the presence of robust condyles at the corners of the orifice proximal margin. Additionally, our specimen shows sparse marginal areolae, whereas the original images depict a continuous series. However, it is well known that the images in the publications of F. Canu and R.S. Bassler were commonly retouched and might not accurately represent reality. Furthermore, we lack a precise description of the suboral avicularium shape.
Celleporaria species previously described from Japan are distinct: C. bicirrhata (Ortmann, 1890) has a denticulate orifice, two spines, and interzooidal avicularia; C. kataokai Hayami, 1975 has 6-8 spines and lacks suboral avicularia; C. transversa (Ortmann, 1890) has a denticulate proximal margin of the orifice; C. triacantha (Ortmann, 1890) has consistently three oral spines; C. trituberculata (Ortmann, 1890) has non-ovicellate zooids with three prominences surrounding the orifice, each with an avicularium atop.
Other Northwest Pacific species also differ: C. convexa (Canu and Bassler, 1929) has a smooth frontal and frequent interzooidal avicularia; C. discoidea (Busk, 1884) has spatulate avicularia; C. erectorostris (Canu and Bassler, 1929) has numerous interzooidal spatulate avicularia, condyles on the lower third of the orifice, and 3-4 oral spines.; C. inflata (Canu and Bassler, 1929) has very large zooids, frequent spatulate interzooidal avicularia, and frontal pores; C. melanodermorpha Liu in Liu, Yin, and Ma, 2001 has a tall columnar suboral columnar process and lacks orificial condyles (Harmelin, 2014); C. pygmaea (Canu and Bassler, 1929) has a smooth frontal, a very elevated cylindrical process bearing a suboral avicularium, with those of the deeper layer reaching the same height as the avicularium of the second layer; C. subflava (Canu and Bassler, 1929) has very finely granular frontal and frequent interzooidal avicularia.
Celleporaria cf. triangula Seo, 1994
Figure 56
cf. 1994 Celleporaria triangula Seo, p. 189, pls. 1, 2.
cf. 2005 Celleporaria triangula Seo; Seo, p. 397, pls. 120B, 121.
cf. 2017 Celleporaria triangula Seo; Dick and Grischenko, p. 187, fig. 14f-h.
Figured material. PMC EDM-Collection J.H.B.146a, sample 19057; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
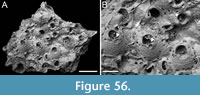 Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct in the flat portion of the colony, separated by a thin raised rim, irregularly polygonal, irregularly arranged, longer than wide (mean ZL/ZW 1.36). Frontal shield convex, coarsely tubercular with rounded tubercles 12-18 µm in diameter, imperforate except for 2-4 rounded to elliptical marginal pores per side, approximately 10-30 µm in maximum diameter. Orifice wider than long (mean OL/OW 0.78), transversely D-shaped with concave proximal margin and three median denticles; the two lateralmost denticles triangular and delicate, the central one stout and rectangular, 8-12 µm long, delineating two small, rounded sinuses, one slightly wider than the other or the same size (16-18 µm and 16-16 µm in the only two preserved orifices measured); oral spines generally absent, a single spine observed in one of the distolateral orifice corners of three autozooids, spine base 14-20 µm in diameter. Peristome usually absent in young autozooids, with older autozooids developing a short, thick collar-like peristome not concealing the orifice. Adventitious avicularium single, suboral, placed on a cystid becoming more raised when the peristome develops, parallel to the proximal margin of the orifice, directed laterally, elliptical with complete crossbar, rostrum tip seemingly denticulate. Interzooidal avicularia randomly distributed and randomly oriented, placed on polygonal cystids coarsely granular with marginal areolar pores like the autozooids; rostrum seemingly rounded triangular or parallel-sided with complete crossbar. Ovicells not observed.
Description. Colony encrusting, multiserial, multilaminar. Autozooids distinct in the flat portion of the colony, separated by a thin raised rim, irregularly polygonal, irregularly arranged, longer than wide (mean ZL/ZW 1.36). Frontal shield convex, coarsely tubercular with rounded tubercles 12-18 µm in diameter, imperforate except for 2-4 rounded to elliptical marginal pores per side, approximately 10-30 µm in maximum diameter. Orifice wider than long (mean OL/OW 0.78), transversely D-shaped with concave proximal margin and three median denticles; the two lateralmost denticles triangular and delicate, the central one stout and rectangular, 8-12 µm long, delineating two small, rounded sinuses, one slightly wider than the other or the same size (16-18 µm and 16-16 µm in the only two preserved orifices measured); oral spines generally absent, a single spine observed in one of the distolateral orifice corners of three autozooids, spine base 14-20 µm in diameter. Peristome usually absent in young autozooids, with older autozooids developing a short, thick collar-like peristome not concealing the orifice. Adventitious avicularium single, suboral, placed on a cystid becoming more raised when the peristome develops, parallel to the proximal margin of the orifice, directed laterally, elliptical with complete crossbar, rostrum tip seemingly denticulate. Interzooidal avicularia randomly distributed and randomly oriented, placed on polygonal cystids coarsely granular with marginal areolar pores like the autozooids; rostrum seemingly rounded triangular or parallel-sided with complete crossbar. Ovicells not observed.
Measurements (µm). ZL 399±40, 358-449 (1, 7); ZW 293±41, 245-363 (1, 7); OL 114±2, 110-116 (1, 5); OW 147±9, 137-159 (1, 5); AvL (adventitious) 59±23, 43-75 (1, 2); AvW (adventitious) 45±1, 44-46 (1, 2); AvL (interzooidal) 162±9, 154-171 (1, 3); AvW (interzooidal) 78±11, 69-90 (1, 3).
Remarks. The specimen of Celleporaria cf. triangula from Daidokutsu cave is poorly preserved and small, preventing the observation of all diagnostic characters of the species. Dick and Grischenko (2017), who recorded this species from a rocky-intertidal habitat on the west side of Okinawa (Japan), listed several diagnostic features to distinguish C. triangula from other Celleporaria with similar orificial denticles: 1) one or two ephemeral spines, which we observed in two zooids of the colony fragment; 2) oral denticles delineating one micro- and one macro- sinus: in our specimen the denticles are preserved only in two zooids, and the sinuses appear to be more or less the same size, with one only slightly smaller; 3) interzooidal avicularia with blunt triangular mandibles: although the mandibles are not preserved in our material, the rostrum in two out of three observed avicularia appears rounded triangular, suggesting a similarly shaped mandible. One avicularium, partially obscured by cemented sediment, appears more parallel-sided; 4) the bluntly denticulate rostral margin of both adventitious and interzooidal avicularia is not clearly discernible. While it seems serrated, it is difficult to determine if this denticulation is genuine or due to breakage, given the preservational state of the specimen. Another difference is in the development of the peristome, which is only visible in three autozooids of our specimen.
Family PHIDOLOPORIDAE Gabb and Horn, 1862
Genus RETEPORELLA Busk, 1884
Reteporella phimostoma sp. nov. Di Martino, Rosso, and Taylor
Figure 57
zoobank.org/D12C6C7C-9853-4B74-A74F-F720155C3EC0
Type material. Holotype PMC. B71. 29.7.2024a, sample 19061; Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
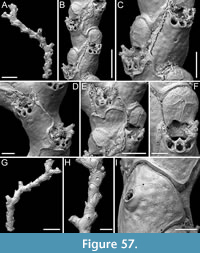 Etymology. Greek, from the combination of ‘phimos’, meaning muzzle, and ‘stoma’, meaning mouth, referring to its complex peristomial avicularian structure, which resembles a muzzle.
Etymology. Greek, from the combination of ‘phimos’, meaning muzzle, and ‘stoma’, meaning mouth, referring to its complex peristomial avicularian structure, which resembles a muzzle.
Diagnosis. Reteporella with dichotomously branching, non-fenestrate colony form, with biserial branches, becoming triserial at bifurcations; autozooids with nodular frontal shield; primary orifice obscured by the peristome and the complex peristomial avicularium; peristomial avicularium centrally positioned, parallel to autozooid axis, directed proximally, connected to a skeletal structure continuous with peristomial spines (5-6), defining a circular opening on each side of avicularium; spines coalescent in groups of 2-3; ovicell kenozooidal with median slit-like fenestra in the ectooecium revealing a fully calcified endooecium, sometimes covered by secondary calcification; dorsal side with raised vibices and rounded avicularia.
Description. Colony erect, rigid, non-fenestrate, dichotomously branching with bifurcations occurring at angles of 60-70º; branches generally narrow, biserial, becoming triserial and wider at bifurcations (216-425 µm wide). Autozooids distinct, separated by raised margins, arranged in alternating longitudinal rows, irregularly pentagonal to hexagonal, elongate (mean ZL/ZW 1.89). Frontal shield flat, slightly convex below the orifice, nodular, imperforate except for a single circular pore, placed medially at 25-40 µm from the orifice, with a diameter of 8-12 µm. Primary orifice deep, concealed by a short peristome and the complex structure of the peristomial avicularium; secondary orifice bean-shaped; 5-6 robust oral spine bases in a variable configuration, with those lateral coalescent in groups of two or three, appearing bi- to trifurcated, with the distalmost spine sometimes separated/not coalescent; spine base 12-18 µm in diameter; depending on their original position, 3-4 spines still visible in ovicellate zooids. Peristomial avicularium constantly present, placed medially in respect to the orifice and facing frontally, parallel to autozooid axis, flat or slightly sloping towards the orifice, elliptical, directed proximally, with a complete crossbar and a median trapezoidal denticle on the outer distal margin, connected with a complex skeletal structure to the distolateral spines, forming a sort of muzzle-like structure obscuring the orifice; between this structure and the avicularium are two small, circular openings, one on each side, c. 20 µm in diameter. Frontal avicularia seemingly absent. Ovicell pyriform, elongate (mean OvL/OvW 1.15), seemingly kenozooidal, modifying the proximal margin of the distal autozooid which is raised and covers the distal periphery of the ooecium; ooecium wrinkled, with a slit-like median fenestra 50-75 µm long, extending from the proximal margin of the ectooecium up to slightly more than half of its total length, narrow, slightly enlarging distally; endooecium fully calcified; labellum absent; secondary calcification gradually covering ooecia making the suture not visible and the surface characterized by raised margins corresponding to the outline of autozooids. Dorsal side nodular, with smooth, raised vibices outlining irregularly kenozooidal polygonal sectors of varying sizes; each sector with one or two sparse circular pores and sometimes a rounded raised avicularium, usually adjacent to the margin of a sector, randomly directed, with a complete crossbar. Secondary calcification patches, likely due to kenozooidal repair, observed on the frontal shield of some zooids.
Measurements (µm). ZL 260±32, 214-295 (1, 9); ZW 138±16, 118-167 (1, 9); AvL (suboral) 41±4, 34-47 (1, 13); AvW (suboral) 28±3, 24-34 (1, 13); AvL (dorsal) 33±3, 31-37 (1, 4); AvW (dorsal) 31±5, 26-36 (1, 4); OvL 113±9, 100-131 (1, 8); OvW 98±8, 88-107 (1, 8).
Remarks. We assigned this species to Reteporella based on several key characters: the presence of peristomial spines, the erect and rigid colony form that can be non-fenestrate, the appearance of the dorsal side of branches with vibices and avicularia, and the shape of the ooecium, which can be completely calcified. However, this species is unique among all identified Reteporella species due to its complex peristomial avicularian structure that forms a continuum with the peristomial spines. In addition to the absence of this feature, Japanese Reteporella species also differ in other characters. Reteporella anatina (Ortmann, 1890) and R. anatina limitata (Ortmann, 1890) have peristomes with two denticles and frequent duckbill-shaped frontal avicularia; R. crenulata (Okada, 1920) has an aperture with serrated proximal margin and three types of frontal avicularia; R. dendroides Ortmann, 1890 has a peristome with a pseudosinus and usually numerous frontal avicularia of two types; R. kinoshitai (Okada, 1920) has a peristomial pseudosinus and three types of frontal avicularia; R. minor Ortmann, 1890 has a peristomial slit-like pseudosinus; R. misakiensis (Okada, 1920) has a fenestrate colony, a slit-like pseudosinus, and bifid suboral avicularia; R. peripherica Ortmann, 1890 has branches with a greater number of autozooids, a pitted frontal shield, a pseudosinus, and frequent frontal avicularia; R. obtecta (Buchner, 1924) has a peristomial avicularium leaning on one side, and a circular pseudosinus on the other; R. terebrata (Buchner, 1924) has a peristomial round pseudosinus and large triangular frontal avicularia; R. watanabei (Okada, 1920) has a fenestrate colony and centrally placed frontal avicularia. The potential new species of Hirose (2010), Reteporella n. sp. 1 and Reteporella n. sp. 2, have several spines but peristomial avicularia defining only a single circular pseudosinus on the opposite side. They also differ in the presence of frontal avicularia.
Reteporella sp. 1
Figure 58
Figured material. PMC EDM-Collection J.H.B.147a, sample 19015 (Figure 58A-E) and sample 19025 (Figure 58F-K); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
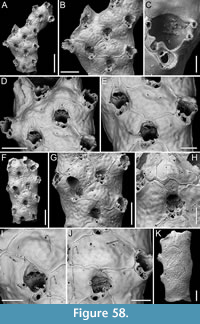 Description. Colony erect, rigid, non-fenestrate with dichotomous branching occurring at an angle of c. 60º; branches 410-750 µm wide, tri- to quadriserial, flat, and elliptical in cross-section. Autozooids arranged in alternating longitudinal rows, irregularly shaped, ranging from polygonal to flask-shaped to rounded/elliptical (mean ZL/ZW 1.35), covered by secondary calcification but remaining distinct, separated by raised margins, although it is uncertain if these boundaries correspond to the original margins of the autozooids. Frontal shield flat to slightly convex, faintly nodular, imperforate, except for 1-2 circular pores placed medially approximately at zooidal half length, 90-135 µm from the orifice, with a diameter of 7-13 µm. Primary orifice deep, concealed by a short peristome proximally lodging the suboral avicularium on one side (the avicularium occupying about two-thirds of the proximal margin length), and forming a narrow (18-20 µm), U-shaped to drop-shaped pseudosinus, 30-35 µm deep, on the other side; secondary orifice semicircular; up to 6 robust oral spines, with 2-4 persisting in ovicellate zooids and/or when secondary calcification occurs; spine base 12-22 µm in diameter. Suboral avicularium constantly present, placed transversely in respect to the orifice, and directed laterally, or positioned slightly obliquely and directed proximolaterally, oval, with a slightly spatulate rostrum and a complete crossbar. Frontal avicularia seemingly absent. Ovicell globular immersed in secondary calcification but always leaving out a median longitudinal fenestra; a short labellum sometimes visible. Dorsal side nodular and pitted, with smooth, raised vibices outlining irregularly kenozooidal polygonal sectors of varying sizes, each sector with a few sparse circular pores, without avicularia.
Description. Colony erect, rigid, non-fenestrate with dichotomous branching occurring at an angle of c. 60º; branches 410-750 µm wide, tri- to quadriserial, flat, and elliptical in cross-section. Autozooids arranged in alternating longitudinal rows, irregularly shaped, ranging from polygonal to flask-shaped to rounded/elliptical (mean ZL/ZW 1.35), covered by secondary calcification but remaining distinct, separated by raised margins, although it is uncertain if these boundaries correspond to the original margins of the autozooids. Frontal shield flat to slightly convex, faintly nodular, imperforate, except for 1-2 circular pores placed medially approximately at zooidal half length, 90-135 µm from the orifice, with a diameter of 7-13 µm. Primary orifice deep, concealed by a short peristome proximally lodging the suboral avicularium on one side (the avicularium occupying about two-thirds of the proximal margin length), and forming a narrow (18-20 µm), U-shaped to drop-shaped pseudosinus, 30-35 µm deep, on the other side; secondary orifice semicircular; up to 6 robust oral spines, with 2-4 persisting in ovicellate zooids and/or when secondary calcification occurs; spine base 12-22 µm in diameter. Suboral avicularium constantly present, placed transversely in respect to the orifice, and directed laterally, or positioned slightly obliquely and directed proximolaterally, oval, with a slightly spatulate rostrum and a complete crossbar. Frontal avicularia seemingly absent. Ovicell globular immersed in secondary calcification but always leaving out a median longitudinal fenestra; a short labellum sometimes visible. Dorsal side nodular and pitted, with smooth, raised vibices outlining irregularly kenozooidal polygonal sectors of varying sizes, each sector with a few sparse circular pores, without avicularia.
Measurements (µm). ZL 366±37, 314-464 (3, 18); ZW 271±46, 191-332 (3, 18); AvL 60±4, 54-71 (3, 18); AvW 44±5, 37-54 (3, 18).
Remarks. Among Reteporella species previously reported from Japan, R. obtecta (Buchner, 1924) is the only one with a similar suboral avicularium. However, it differs by having the rostrum projecting into the orifice (Harmer, 1934). Other Reteporella species from different regions of the world with similar suboral avicularium, such as the Mediterranean R. mediterranea Hass, 1948 and R. pelecanus Lopez de la Cuadra and Garcia-Gomez, 2001, or R. tenuitelifera Canu and Bassler, 1929 and R. millespinae Canu and Bassler, 1929 from the Philippines, all differ by having fenestrate colonies or trabeculae with a greater number of zooids, numerous and variable frontal avicularia, or avicularia on the dorsal side (Canu and Bassler, 1929; Ramalho et al., 2018). We leave this species in open nomenclature due to the heavy secondary calcification, which prevents observation, description, and measurements of ovicells.
Genus RETEPORELLINA Harmer, 1933
Reteporellina denticulata (Busk, 1884)
Figure 59
v. 1884 Retepora denticulata Busk, p. 109, text-fig. 18, pl. 26, fig. 13.
v. 1934 Reteporellina denticulata (Busk); Harmer, p. 581, figs. 25D, 33, pl. 35, figs. 21-23, pl. 38, figs. 27-32.
v. 2010 Reteporellina denticulata (Busk); Hirose, p. 73, pl. 127A-H.
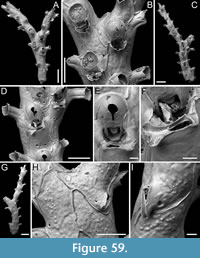 Figured material. PMC EDM-Collection J.H.B.148a, sample 19044 (Figure 59A-B) and sample 19021 (Figure 59C-I); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Figured material. PMC EDM-Collection J.H.B.148a, sample 19044 (Figure 59A-B) and sample 19021 (Figure 59C-I); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony erect, non-fenestrate, dichotomously branching with bifurcations occurring at angles of 60-80º; branches generally narrow, widest at bifurcations (145-700 µm wide), bi- to quadriserial. Autozooids distinct, separated by thin raised margins, arranged in alternating longitudinal rows, rectangular to rhomboidal, elongate (mean ZL/ZW 1.99). Frontal shield flat, faintly nodular, imperforate except for two marginal pores, slit-like and adjacent to the lateral sides, or rounded and located a certain distance from the margins, in both cases positioned in the proximal third of zooid, 8-30 µm in maximum dimension. Primary orifice concealed by a deep, curved peristome extending away from the branch axis at an angle of approximately 90º to the frontal surface, having a flared rim with rounded crenulations and a narrow U-shaped medioproximal notch. Avicularium present or absent, adventitious, suboral, transversely placed, occupying the entire length of the proximal margin of the peristome; rostrum duckbill-shaped with crenulated tip directed laterally on either side; crossbar complete. Ovicell pyriform, elongate (mean OvL/OvW 1.22), occupying half to two-thirds of the frontal shield of the distal zooid, gradually covered by secondary calcification except for a median slit-like or upside-down drop-shaped fissure, 65-100 µm long by 20-40 µm wide; aperture rectangular with a narrow semicircular to semielliptical labellum. Dorsal side nodular to coarsely granular, with smooth, raised vibices outlining irregularly polygonal sectors of varying size; each sector with one or two sparse circular pores; avicularia uncommon, obliquely and randomly placed within the dorsal surface, usually adjacent to the proximal corner of a sector, triangular, elevated and hump-shaped, with a needle-like rostrum directed proximally to proximolaterally, and complete crossbar.
Measurements (µm). ZL 434±35, 366-504 (5, 27); ZW 218±56, 147-306 (5, 27); PeL 168±34, 120-241 (5, 16); AvL (suboral) 131±16, 107-150 (3, 9); AvW (suboral) 46±7, 36-60 (3, 9); AvW (suboral; rostral tip) 65±10, 46-75 (3, 9); AvL (dorsal) 142±31, 124-178 (2, 3); AvW (dorsal) 46±5, 43-52 (2, 3); OvL 194±17, 162-222 (5, 17); OvW 159±14, 134-182 (5, 17).
Remarks. Reteporellina denticulata was first described from the Sandwich Islands at depths ranging from 36 to 73 m. Since then, it has been reported across a wide geographical range, from Australia (e.g., Hayward and Ryland, 1995) to the Indo-Pacific (e.g., Harmer, 1934), and into the North Pacific, with multiple records from Japan (Okada, 1920; Buchner, 1924; given in both publications as R. misakiensis (Okada, 1920) synonymized by Harmer, 1934; Hirose, 2010). Despite its seemingly implausible widespread distribution, the only notable morphological variation seems to be in the number of rows of autozooids in the branch, and the presence or absence of trabeculae lacking autozooids. Trabeculae are reported to be present in the type specimens, but we did not observe them in our samples. While their presence cannot be entirely ruled out due to the fragmented nature of our samples, the sheer number of fragments available (thousands) makes their presence highly unlikely. However, we prefer not to use these observed differences as the basis for introducing a new species currently, as a more thorough study, beyond the scope of this work, is needed.
Genus RHYNCHOZOON Hincks, 1895
Rhynchozoon lunifrons Dick and Grischenko, 2017
Figure 60
v. 2017 Rhynchozoon lunifrons Dick and Grischenko, p. 245, figs. 37-38.
Figured material. PMC EDM-Collection J.H.B.149a, sample 19033 (Figure 60A-C), sample 19015 (Figure 60D), sample 19031 (Figure 60E), sample 19041 (Figure 60F), and sample 19036 (Figure 60G-I); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
Description. Colony encrusting, subcircular, multiserial, uni- to multilaminar; second layer developing through frontal budding, originating from the centre of the colony. Two types of pseudoancestrular areas observed: first type, consisting of two small zooids budded in opposite directions, with a third, slightly larger zooid budded distally between them, surrounded by seven peripseudoancestrular autozooids (Figure 60B); second type, consisting of two small zooids budded in opposite directions, each budding another zooid laterally, creating a rounded cavity in the centre, also surrounded by seven autozooids (Figure 60F); pseudoancestrulae 215-275 µm in length by 150-230 µm in width, with an orifice 105 µm long by 115 µm wide; first zooids budded from pseudoancestrulae 250-315 µm long by 190-210 µm wide. Autozooids distinct only at the growing edge or in new, frontally budded laminae, separated by shallow furrows or a thin rim, hexagonal or irregularly polygonal, longer than wide (mean ZL/ZW 1.32), arranged radially or irregularly. Frontal shield convex, smooth to nodular and pitted, imperforate except for a row of 6-9 irregularly spaced, circular to elliptical, sometimes funnel-shaped or sloping outwards, conspicuous marginal pores, 10-70 µm in maximum diameter. Orifice deep and subcircular but slightly wider than long (mean OL/OW 0.85), with a finely denticulate anter (10-15 denticles), a broadly concave proximal margin, and rounded condyles. Four distolateral spine bases occasionally visible in some zooids, most commonly two, 16-25 µm in diameter, quickly becoming embedded in the frontal shield calcification of the distal autozooid. Peristome often obscuring the orifice, consisting of three mucrones: one suboral in the midline or slightly off-centre, developing first, and two paired laterally, developing later. Lateral mucrones often increasing to two on each side, forming a more complex structure of coalescent mucrones, sometimes bifurcating. Suboral mucro either slender and pointed or swollen accommodating an avicularium atop. Suboral avicularium sporadic, with triangular rostrum raised at a high angle to the frontal plane, directed frontolaterally; frontal avicularia rare, with the same shape and inclination as the suboral avicularia, the few examples observed placed proximally on the frontal shield, directed proximolaterally on either side; both types of avicularia with complete crossbar. Ovicell subimmersed, with extensive exposure of the smooth, faintly striated endooecium, the transversely elliptical to eight-shaped fenestra measuring 80-155 µm in length by 156-212 µm in width.
Measurements (µm). ZL 459±53, 390-605 (3, 20); ZW 348±67, 249-481 (3, 20); OL 107±8, 97-123 (4, 9); OW 125±7, 116-137 (4, 9); AvL (suboral) 89±21, 66-121 (4, 9); AvW (suboral) 61±5, 52-68 (4, 9); AvL (frontal) 85±30, 66-120 (2, 3); AvW (frontal) 65±8, 58-74 (2, 3); OvL 169±18, 134-214 (6, 18); OvW 236±17, 204-268 (6, 18).
Remarks. There are two main differences between the type specimens from Sesoko Island (Okinawa, Japan) and our specimens from Daidokutsu cave. First, the rostrum of the avicularia in the type specimens can become slightly hooked distally during late colony astogeny, a feature not observed in the specimens from Daidokutsu cave. This difference may be due to preservational issues or the fact that avicularia in our specimens (but also in general in this species) are rare and possibly from colonies that have not reached the appropriate age for this characteristic to develop. In the type specimens, oral spines are generally absent, except for one or two ephemeral distal oral spines observed in a so-called precocious form as defined by Dick and Grischenko (2017, figure 38b). In contrast, our specimens have up to four distal spines with robust bases. However, these spines quickly become embedded in the calcification spreading from the distal zooid. Despite these differences, other diagnostic characters and size measurements match, supporting the identification of the Daidokutsu colonies as R. lunifrons.
Genus STEPHANOLLONA Duvergier, 1921
Stephanollona pachycondylata sp. nov. Di Martino, Rosso and Taylor
Figure 61
zoobank.org/C59E2AC6-F1D6-491E-B3CE-6D26AE2F2AB7
Type material. Holotype PMC. B72. 29.7.2024a, sample 19185 (Figure 61A-E); paratype PMC. B72. 29.7.2024b, sample 19140 (Figure 61F-G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
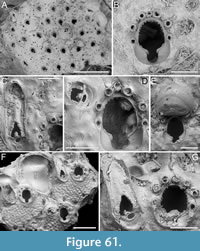 Etymology. Greek ‘pachys’, meaning thick, and Latin ‘condylus’, meaning articulation, referring to the robust orifice condyles of this species.
Etymology. Greek ‘pachys’, meaning thick, and Latin ‘condylus’, meaning articulation, referring to the robust orifice condyles of this species.
Diagnosis. Stephanollona with 6-7 oral spine bases (four in ovicellate zooids); keyhole-shaped orifice with distinctly elongate anter, distally denticulate, narrow and shallow sinus, and conspicuous condyles; paired or single dimorphic latero-oral avicularia, small and oval, directed laterally or slightly distolaterally outwards, and large and elliptical, directed distally or distolaterally outwards, all with ligulate crossbar; ooecium globular, opening with lateral indentations and short labellum.
Description. Colony encrusting, multiserial, uni- to multilaminar. Autozooids becoming indistinct due to secondary calcification and frontal budding, appearing polygonal, nearly square-shaped, almost as long as wide (mean ZL/ZW 1.11). Frontal nodular, imperforate except for elliptical marginal areolae along lateral and proximal zooidal margins, 20-40 µm in maximum dimension. Orifice terminal, immersed, keyhole-shaped, with short, smooth proximal peristome; anter elongate, denticulated, denticles measuring 6-7 µm in length, placed no further proximally than the proximalmost pair of oral spines; sinus U-shaped, accounting for one-third of the total orifice width; condyles smooth, rounded, conspicuous, outlining the proximal sloping margin of the orifice with distal prominences; 6-7 distolateral oral spine bases with the proximalmost pair situated above the level of condyles, 21-33 µm in diameter, four remaining in ovicellate zooids; peristome beginning from the proximalmost pair of spines, usually smaller than more distal pairs. Avicularia adventitious, latero-oral, single or rarely paired, dimorphic; avicularia type 1: large, elliptical, almost parallel sided with rounded rostrum, directed distally or slightly distolaterally outwards, placed at the level of the proximal margin of the peristome, with triangular opesia; avicularia type 2: small, oval, directed laterally or slightly distolaterally outwards, placed more distally than type 1, at the level of the second proximalmost pair of spines, with semicircular opesia; all avicularia with complete, ligulate crossbar and somewhat beaded opesia. Ovicell hyperstomial, globular; ooecium smooth, striated by concentric lines; opening with short labellum and lateral indentations.
Measurements (µm). ZL 510±47, 438-587 (2, 14); ZW 460±37, 397-530 (2, 14); OL (including sinus) 150±8, 140-163 (2, 9); OW 103±4, 97-111 (2, 9); AvL (type 1) 290±32, 253-332 (2, 6); AvW (type 1) 93±13, 77-112 (2, 6); AvL (type 2) 87±7, 77-97 (2, 12); AvW (type 2) 68±9, 56-85 (2, 12); OvL 197±12, 189-206 (1, 2); OvW 244±19, 231-258 (1, 2).
Remarks. This new species differs from the two species of Stephanollona previously identified from the North Pacific: S. eopacifica Soule, Soule and Chaney, 1991 from the northeastern Pacific is distinguished by a wider orificial sinus and avicularia with a toothed rostral tip directed more proximally; and S. boreopacifica Yang, Seo, and Gordon, 2018, found in South Korea, is characterized by 5-6 spines, avicularia with a toothed rostral tip, and small, oval avicularia also associated with the ooecium. Among species with 6-7 spines, S. ignota (Hayward and Cook, 1983) from eastern South Africa, differs in having avicularia directed distomedially; S. longispinata (Busk, 1884) from the South Atlantic has small, oval avicularia directed laterally or proximolaterally, and large, spatulate avicularia directed distally or distolaterally and medially; S. cardiophora Winston and Jackson, 2021 from Jamaica, has a broader sinus; S. angusta Vieira, Gordon, Souza, and Haddad, 2010 from Brazil, differs in the shape of the orifice which has a less elongate, almost semicircular anter, and the shape of avicularia with narrow, rounded triangular rostra and crossbars without a ligula; S. arborescens Vieira, Gordon, Souza, and Haddad, 2010, also from Brazil, is characterized by its erect and bilaminar colonies, a shallower orificial sinus, less conspicuous condyles, and avicularian rostra that are narrow and rounded triangular; S. asper (Canu and Bassler, 1923) from the Gulf of Mexico has an orifice with a less elongate anter, shallower and broader sinus, and less developed condyles (Winston, 2005).
Genus TRIPHYLLOZOON Canu and Bassler, 1917
Triphyllozoon angustum sp. nov. Di Martino, Rosso, and Taylor
Figure 62
zoobank/F8FBE39D-5A4E-4966-9652-2F33E524E64F
Type material. Holotype PMC. B73. 29.7.2024a, sample 19113 (Figure 62A-D, G-I); paratype PMC. B73. 29.7.2024b1, sample 19055 (Figure 62E-F); paratype PMC. B73. 29.7.2024b2, sample 19105 (not figured); paratype PMC. B73. 29.7.2024b3, sample 19200 (not figured); paratype PMC. B73. 29.7.2024b4, sample 19094 (not figured); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
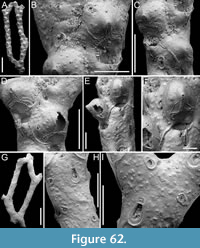 Etymology. Latin, meaning narrow, referring to its narrow, bi- to triserial trabeculae.
Etymology. Latin, meaning narrow, referring to its narrow, bi- to triserial trabeculae.
Diagnosis. Triphyllozoon with elongate fenestrae and bi- to triserial trabeculae; autozooids with sparsely tubercular frontal shield and frontal avicularia of three types: small and subcircular, medium-sized subelliptical/spatulate, and large spatulate; oval suboral avicularium embedded in the proximal peristome, the two lobes fusing and forming a deep teardrop-shaped pseudosinus; two lateral spines present or absent; ovicell projecting frontally from the branch, with trifoliate dentate suture with longer median arm and lateral arms diverging at 160-180°, curving downward to form a semicircle, central mucro fairly developed; dorsal side sparsely granular with numerous avicularia of the same three types seen on the frontal side, with the medium-sized spatulate being the most common.
Description. Colony erect, rigid, reticulate; fenestrae elongate, c. 3.6 mm long by 0.6 mm wide; trabeculae 340-600 µm wide, consisting of 2-3 alternating autozooidal series. Autozooids flask-shaped, longer than wide (mean ZL/ZW 1.80), with indistinct boundaries; frontal shield slightly convex, sparsely tubercular, with tubercles measuring 12-30 µm in diameter, imperforate except for a few, small, round to elliptical pores along the lateral margins, 7-20 µm in maximum diameter. Peristome well developed proximally with the two lobes fusing to form a deep (100-130 µm), teardrop-shaped pseudosinus, 20-30 µm in maximum width, slightly off-centre; frequently a small, oval avicularium enclosed on the rim of one lobe, either side, placed transversely and directed laterally (type 1). Secondary orifice eye-shaped with two oral spine bases sometimes present at its lateral corners, also visible in ovicellate zooids, c. 15 µm in diameter at the base. Numerous frontal avicularia present in three types: small, subcircular avicularia usually placed on the proximal portion of the zooid frontal and directed proximolaterally (type 2); medium-sized avicularia, variable in shape from subelliptical with parallel sides to slightly spatulate, usually placed at mid-length or more distally on the zooid, perpendicular or oblique to the zooid axis and directed proximally to proximolaterally (type 3); large spatulate avicularia, usually placed and directed as the medium-sized ones (type 4); each zooid with one or more avicularia in various combinations. On the dorsal side of the colony, all three types (i.e., types 2 to 4) present, with the medium-sized slightly spatulate avicularia being the most frequent. All avicularia with complete crossbar. Ovicell hyperstomial, prominent, projecting frontally from the branch surface with a fairly developed distomedial mucro; ooecium slightly longer than wide (mean OvL/OvW 1.15), initially smooth but becoming sparsely granular as the frontal when covered by secondary calcification, with trifoliate dentate suture, with a longer median arm (85-160 µm) compared to the lateral arms (70-110 µm), diverging at 160-180° and curving downward to form semicircles; width of the suture similar in median and lateral arms (18-39 µm vs 17-33 µm); labellum short and square. Dorsal side sparsely granular like the frontal, with spaced oblique vibices (15-30 µm wide) outlining subrectangular or irregularly polygonal kenozooidal sectors, including a few sparse elliptical and circular marginal pores (19-25 µm in diameter) and numerous avicularia, usually placed around the sector perimeter and randomly directed, of the types described above.
Measurements (µm). ZL 541±30, 505-577 (2, 5); ZW 301±39, 263-352 (2, 5); AvL (type 1, suboral) 41±6, 35-51 (1, 5); AvW (type 1, suboral) 38±5, 30-44 (1, 5); AvL (type 2, frontal) 45±4, 35-50 (4, 16); AvW (type 2, frontal) 46±7, 31-53 (4, 16); AvL (type 3, frontal) 82±11, 66-108 (4, 15); AvW (type 3, frontal) 52±5, 42-62 (4, 15); AvL (type 4, frontal) 177±31, 155-213 (2, 3); AvW (type 4, frontal) 57±23, 40-83 (2, 3); AvL (type 2, dorsal) 43±7, 34-55 (2, 7); AvW (type 2, dorsal) 39±5, 31-46 (2, 7); AvL (type 3, dorsal) 98±9, 76-113 (3, 20); AvW (type 3, dorsal) 66±8, 51-80 (3, 20); AvL (type 4, dorsal) 220±23, 163-244 (3, 12); AvW (type 4, dorsal) 107±13, 76-123 (3, 12); OvL 301±16, 263-323 (3, 17); OvW 262±18, 236-298 (3, 17).
Remarks. This species has been closely compared to other bi- to triserial species of Triphyllozoon known to date and is found to be distinct. Triphyllozoon bimunitum (Ortmann, 1890), T. bimunitum trimunitum (Buchner, 1924), and T. bimunitum sparsimunitum (Buchner, 1924) all have bicuspid avicularia (Hayward, 2000; Hirose, 2010). Triphyllozoon contortuplicata (Busk, 1884) lacks dorsal avicularia, and its frontal avicularia are nearly suboral and triangular. Triphyllozoon formosoides Hayward, 2004 has avicularia with cusps and a peristome formed by two lateral flaps without a deep pseudosinus. In Triphyllozoon gracile Gordon and d’Hondt, 1997, avicularia are oval rather than spatulate. Triphyllozoon inornatum Harmer, 1934 has large tricuspid avicularia on the dorsal side and at the fenestrae, and its ovicell is non-mucronate. Triphyllozoon patulum Harmer, 1934 shares spatulate avicularia with the new species but differs in having a non-mucronate ovicell with no strongly curved suture arms and a peristome with a short pseudosinus. Spatulate avicularia and non-mucronate ovicells are also typical of T. punctiligerum (Ortmann, 1890). Triphyllozoon separatum Harmer, 1934 and T. tuberculiferum Harmer, 1934 have elongate triangular avicularia. Triphyllozoon trifoliatum Harmer, 1934 has a short pseudosinus, and an avicularium associated with the ovicell.
Family CONESCHARELLINIDAE Levinsen, 1909
Genus CONESCHARELLINA d’Orbigny, 1852
Conescharellina brevirostris Silén, 1947
Figure 63
v. 1947 Conescharellina brevirostris Silén, p. 39, text-fig. 24, pl. 1, figs. 4-6.
Figured material. PMC EDM-Collection J.H.B.150a, sample 19053 (Figure 63A-B), sample 19095 (Figure 63C-D), and sample 19081 (Figure 63E-F); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
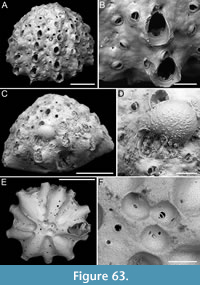 Description. Colony small, conical, height (H) 0.765-1.200 mm and basal diameter (BD) 1.125-1.535 mm in our specimens (mean H/BD 0.75), with 12-18 slightly prominent, narrow, radial costules corresponding with the raised peristomes of the autozooids, alternating with intercostular valleys occupied by rows of interzooidal avicularia. Autozooids arranged in radial rows corresponding to the prominent costules, with 3-4 autozooids per row; orifices of autozooids in the same costule vertically aligned but alternating with orifices of autozooids in neighbouring costules; autozooids observed on the antapical surface distinct, separated by sloping vertical walls, outlined by a lateral row of circular, widely spaced marginal pores (10-15 µm in diameter) on each side, elliptical to oval, elongate (mean ZL/ZW 1.73); interzooidal communication through small, circular, uniporous pore-chamber windows, 3-5 along each lateral margin of the zooids, 8-18 µm in diameter. Frontal shield convex around the orifice, flat centrally, smooth, nodular, imperforate except for one or two elliptical marginal areolar pores (10-15 µm in maximum diameter) placed laterally. Primary orifice with semielliptical anter and rounded triangular condyles, 8-10 µm long, pointing mediodistally and defining a shallow U-shaped sinus, longer than wide (mean OL/OW 1.22); apical pore generally absent unless there is an adjacent avicularium; peristome collar-like, well developed laterally and proximally, forming a proximal concave shelf about 25-30 µm wide, absent or little developed distally. Interzooidal avicularia circular, arranged in radial, sinuous or linear rows along the furrows between costules, with 6-8 avicularia per row, rostrum slightly raised, randomly directed, either distolaterally or proximolaterally, crossbar complete. An additional adventitious avicularium only in some autozooids, placed distolaterally to the orifice, lying on the peristome, elliptical, with blunt triangular rostrum directed proximolaterally, and with complete crossbar. Apical surface coarsely tubercular, occupied by kenozooids and avicularia; antapical surface with kenozooidal pits and avicularia in radial rows, continuous with those of the lateral surface of the colony, and centrally. Ovicell hyperstomial, globular, placed obliquely on the frontal of distal zooid to avoid obstructing its orifice, extending to the adjacent avicularian valley; ectooecium mostly uncalcified, forming a smooth, slightly raised peripheral rim, endooecium rugose and finely granular, with granules roughly arranged in radial rows, nearly disappearing in the medial distal area.
Description. Colony small, conical, height (H) 0.765-1.200 mm and basal diameter (BD) 1.125-1.535 mm in our specimens (mean H/BD 0.75), with 12-18 slightly prominent, narrow, radial costules corresponding with the raised peristomes of the autozooids, alternating with intercostular valleys occupied by rows of interzooidal avicularia. Autozooids arranged in radial rows corresponding to the prominent costules, with 3-4 autozooids per row; orifices of autozooids in the same costule vertically aligned but alternating with orifices of autozooids in neighbouring costules; autozooids observed on the antapical surface distinct, separated by sloping vertical walls, outlined by a lateral row of circular, widely spaced marginal pores (10-15 µm in diameter) on each side, elliptical to oval, elongate (mean ZL/ZW 1.73); interzooidal communication through small, circular, uniporous pore-chamber windows, 3-5 along each lateral margin of the zooids, 8-18 µm in diameter. Frontal shield convex around the orifice, flat centrally, smooth, nodular, imperforate except for one or two elliptical marginal areolar pores (10-15 µm in maximum diameter) placed laterally. Primary orifice with semielliptical anter and rounded triangular condyles, 8-10 µm long, pointing mediodistally and defining a shallow U-shaped sinus, longer than wide (mean OL/OW 1.22); apical pore generally absent unless there is an adjacent avicularium; peristome collar-like, well developed laterally and proximally, forming a proximal concave shelf about 25-30 µm wide, absent or little developed distally. Interzooidal avicularia circular, arranged in radial, sinuous or linear rows along the furrows between costules, with 6-8 avicularia per row, rostrum slightly raised, randomly directed, either distolaterally or proximolaterally, crossbar complete. An additional adventitious avicularium only in some autozooids, placed distolaterally to the orifice, lying on the peristome, elliptical, with blunt triangular rostrum directed proximolaterally, and with complete crossbar. Apical surface coarsely tubercular, occupied by kenozooids and avicularia; antapical surface with kenozooidal pits and avicularia in radial rows, continuous with those of the lateral surface of the colony, and centrally. Ovicell hyperstomial, globular, placed obliquely on the frontal of distal zooid to avoid obstructing its orifice, extending to the adjacent avicularian valley; ectooecium mostly uncalcified, forming a smooth, slightly raised peripheral rim, endooecium rugose and finely granular, with granules roughly arranged in radial rows, nearly disappearing in the medial distal area.
Measurements (µm). ZL (measured on the antapical surface) 549±84, 438-587 (2, 11); ZW (measured on the antapical surface) 317±49, 246-371 (2, 11); OL 112±9, 101-122 (2, 5); OW 92±4, 86-97 (2, 5); AvL (frontal) 50±5, 39-62 (3, 20); AvW (frontal) 47±5, 38-56 (3, 20); AvL (base) 29±2, 28-33 (2, 4); AvW (base) 32±2, 30-35 (2, 4); OvL 206 (1, 1); OvW 267 (1, 1).
Remarks. We have observed and described for the first time a complete ovicell in C. brevirostris. Silén (1947) noted a wide and shallow excavation emanating from the right distolateral margin of an autozooid peristome and extending into the neighbouring valley, which he interpreted as a broken ooecium (see Silén, 1947, text-figure 24). However, this structure was not observed in the syntype colonies re-examined by Di Martino (2023) using SEM. The discrepancy may be because Silén had access to more colonies than are currently available in the type collection at the Swedish Museum of Natural History in Stockholm. Silén (1947) also suggested that the ectooecium was delicate and prone to breaking. This assumption is supported by our observations, as the ectooecium is largely uncalcified. Additionally, Silén (1947) anticipated that the ooecium would develop obliquely to the right, as seen in another species of the genus, C. striata Silén, 1947, and this inference has also been confirmed.
Although slightly larger than the subsequently described Conescharellina sp. 1, this colony is still regarded as relatively young based on its size and the number of autozooids per row.
Conescharellina sp. 1
Figure 64
Figured material. PMC EDM-Collection J.H.B.151a, sample 19063 (Figure 64A-F) and sample 19206 (Figure 64G); Core 19, Daidokutsu cave, Okinawa, Japan, Holocene.
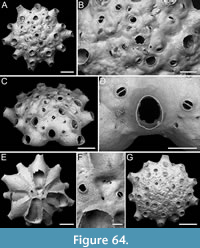 Description. Colony small, flat, discoidal, height 0.512-0.674 mm and basal diameter 0.946-1.072 mm in our specimens (mean H/BD 0.58), with 10-12 slightly prominent, radial rows corresponding with the projecting peristomes of the autozooids, alternating with valleys occupied by irregular rows of interzooidal avicularia. Autozooids 2-3 per row; orifices of autozooids in the same row vertically aligned but alternating with orifices of autozooids in neighbouring rows; autozooids observed on the antapical surface distinct, separated by sloping vertical walls, with 2-3 circular to slit-like, regularly spaced marginal pores (9-20 µm in diameter) on each side, elliptical to oval, elongate (mean ZL/ZW 1.63); interzooidal communication through small, circular, uniporous pore-chamber windows, 3-4 along each lateral margin of the zooids, 8-18 µm in diameter. Frontal shield convex around the orifice, flat centrally, smooth, nodular, imperforate except for one or two elliptical marginal areolar pores (15-16 µm in maximum diameter) placed laterally. Primary orifice with semielliptical anter and rounded triangular condyles, 6-8 µm long, pointing mediodistally and defining a shallow U-shaped sinus, longer than wide (mean OL/OW 1.30), often concealed by the peristome; apical pore absent; peristome tubular, well developed all around the orifice, 25-50 µm long. Interzooidal avicularia circular arranged in radial, sinuous or linear rows between autozooids, with up to four avicularia per row, rostrum slightly raised, randomly directed, either distolaterally or proximolaterally, crossbar complete. Apical surface coarsely tubercular, occupied by kenozooids with circular openings (42-80 µm in diameter) and sparse avicularia; antapical surface with kenozooidal pits and avicularia, like those on the frontal, centrally.
Description. Colony small, flat, discoidal, height 0.512-0.674 mm and basal diameter 0.946-1.072 mm in our specimens (mean H/BD 0.58), with 10-12 slightly prominent, radial rows corresponding with the projecting peristomes of the autozooids, alternating with valleys occupied by irregular rows of interzooidal avicularia. Autozooids 2-3 per row; orifices of autozooids in the same row vertically aligned but alternating with orifices of autozooids in neighbouring rows; autozooids observed on the antapical surface distinct, separated by sloping vertical walls, with 2-3 circular to slit-like, regularly spaced marginal pores (9-20 µm in diameter) on each side, elliptical to oval, elongate (mean ZL/ZW 1.63); interzooidal communication through small, circular, uniporous pore-chamber windows, 3-4 along each lateral margin of the zooids, 8-18 µm in diameter. Frontal shield convex around the orifice, flat centrally, smooth, nodular, imperforate except for one or two elliptical marginal areolar pores (15-16 µm in maximum diameter) placed laterally. Primary orifice with semielliptical anter and rounded triangular condyles, 6-8 µm long, pointing mediodistally and defining a shallow U-shaped sinus, longer than wide (mean OL/OW 1.30), often concealed by the peristome; apical pore absent; peristome tubular, well developed all around the orifice, 25-50 µm long. Interzooidal avicularia circular arranged in radial, sinuous or linear rows between autozooids, with up to four avicularia per row, rostrum slightly raised, randomly directed, either distolaterally or proximolaterally, crossbar complete. Apical surface coarsely tubercular, occupied by kenozooids with circular openings (42-80 µm in diameter) and sparse avicularia; antapical surface with kenozooidal pits and avicularia, like those on the frontal, centrally.
Measurements (µm). ZL (measured on the antapical surface) 425±28, 398-475 (1, 6); ZW (measured on the antapical surface) 261±36, 216-303 (1, 6); OL 100 (1, 1); OW 77 (1, 1); AvL (frontal) 41±8, 19-52 (2, 18); AvW (frontal) 41±6, 33-52 (2, 18); AvL (base) 39±4, 36-42 (1, 2); AvW (base) 39±1, 38-40 (1, 2).
Remarks. Although Conescharellina brevirostris found in the same samples appears very similar to this species, there are some differences. Conescharellina sp. 1 has flatter more discoidal colonies than the subconical colonies of C. brevirostris. It also has fewer autozooids per row, more irregularly placed interzooidal avicularia, no avicularia distolateral to the orifice, and no associated apical pore. Additionally, the autozooids are slightly smaller, while the avicularia are similar in size. The orifice shape is very similar, but the peristome is less developed distally in C. brevirostris. We have left this species in open nomenclature due to the absence of ovicells. We also believe that all the observed differences could be due to the age of the colony. It is possible that this flatter form with smaller and fewer autozooids, the lack of avicularia associated with the orifice, and the absence of ovicells (although these are rare also in C. brevirostris) could indicate a young stage of the same species. We have kept it separated in anticipation of discovering additional specimens in the future that may provide further information.
DISCUSSION
The cheilostome bryozoan fauna from sediment Core 19 in the Daidokutsu cave comprises 63 species, 45 genera, and 29 families. Of these, 17 species (27%) are anascan-grade, and the remaining 46 (73%) are ascophoran-grade cheilostomes. The most diverse families are Phidoloporidae with five genera and six species, Candidae with four genera and seven species, and Smittinidae with three genera and five species. Seven families are represented by two genera each: Celleporidae with four species; Bitectiporidae, Cribrilinidae, and Lacernidae with three species each; and Calloporidae, Gigantoporidae, and Romancheinidae with two species each. Six families are represented by a single genus but multiple species, including Exechonellidae with three species, and Conescharellinidae, Escharinidae, Margarettidae, Petraliidae, and Steginoporellidae with two species each. The remaining 13 families are represented by one genus and one species each.
Approximately 48% (30 out of 63 species) are new to science, with 23% (seven species) being anascan-grade and 77% (23 species) being ascophorans. Additionally, three out of the 45 genera (7%) are new: one belonging to each of the families Calloporidae, Catenicellidae, and Gigantoporidae. These percentages align with Winston’s (1988) statement, subsequently reported by Scholtz et al. (2008), noting that only about 50% of bryozoan species dwelling in the Indo-Pacific are known. Other studies of Japanese bryozoan faunas show similar richness in new species. For instance, Dick and Grischenko (2017) found that 21% (11 out of 52) of the species they collected from rocky intertidal sites near the Sesoko Biological Station in west-central Okinawa were new species, while 86.5% of species found were new records for Japan. In their review of Japanese Pleistocene bryozoan faunas, Dick et al. (2008) reported that in the Boso Peninsula, on the Pacific side of central Honshu, 30 out of 145 species (21%) were new; in SW Hokkaido and N Honshu near the Tsugaru Strait, 32 out of 129 species (25%) were new; in the Noto Peninsula, three out of 26 species (12%) were new; and in Niigata, central Honshu, Sea of Japan, and Kikai-jima Island in the Nansei Archipelago south of Kyushu, 32 out of 133 species (24%) were new. Given that this habitat and time interval have not yet been studied, it is normal to find a higher percentage of new species.
Of the remaining taxa, 26 were previously known from the Pleistocene of Japan or were Recent records from the NW Pacific or the Indo-Pacific, originating from areas such as South Korea, the Philippines, Indonesia, Solomon Islands, and Hawaii, with about half being subsequently reported from Japan, and the other half being new records.
The diversity is relatively high for a single cave comparad to thoroughly studied Recent cave habitats, like those in the Mediterranean, which host a total of 220 species, including cyclostomes (Rosso and Di Martino, 2016). Cyclostomes are missing from the current study, but a preliminary estimate suggests about 17-18 species. For comparison with single caves or groups of caves, the number of species including cyclostomes, in the Plemmirio caves (SE Sicily) ranges from 36 to 51 (Rosso et al., 2013a), totalling 72 species, and from 32 to 63 species in Lesvos Island (Aegean Sea), totalling 67 species (Rosso et al., 2019), while French Mediterranean caves host 110 species in total (Harmelin, 2000). However, it should be noted that those caves represent Recent habitats, a snapshot of the present-day situation, while the fauna from Daidokutsu cave spans 7,000 years, implying an evolution of the cave community and possibly species turnover.
This work provides the taxonomic baseline needed for a significant ecological investigation of the cave fauna evolution in response to environmental and climate changes throughout the Holocene.
ACKNOWLEDGEMENTS
We thank Sophie Crump and Tom Perkins for helping sorting bryozoans from the core samples during their year as volunteers at the Department of Earth Sciences of the Natural History Museum, London, and Masato Hirose for providing essential literature. EDM received support from the Leverhulme Trust Research Project ‘Origin of high tropical diversity: a test using bryozoans’ (2016-2018) (award no. RGP‐2015‐036 to PDT), the Young Research Talent Grant of the Research Council of Norway (2019-2025) (award no. 314499 to EDM), and the University of Catania through PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 - Linea di Intervento 3 “Starting Grant” (2024). AR was funded by the University of Catania through PIAno di inCEntivi per la RIcerca di Ateneo 2020/2022 - Linea di Intervento 2. Additional support to EDM and AR was provided by PiaCeRi 2024/2026. MY was funded by Research Grants Council of the Hong Kong Special Administrative Region, China (project codes: RFS2223-7S02, C7013-19G to MY); the Seed Funding Programme for Basic Research of the University of Hong Kong (project codes: 202111159167, 2302101483 to MY); and SKLMP Seed Collaborative Research Fund (project codes: SKLMP/SCRF/0073 to MY). Two anonymous reviewers and the Executive Editor Oleksandr Kovalchuk provided comments and suggestions that greatly improved the original submitted manuscript. This is Catania Paleontological Research Group contribution no. 522.
REFERENCES
Achilleos, K., Gordon, D.P., and Smith, A. 2020. Cellaria (Bryozoa, Cheilostomata) from the deep: new species from the southern Zealandian region. Zootaxa, 4801:201-236.
https://doi.org/10.11646/zootaxa.4801.2.1
Allmann, G.J. 1856. A monograph of the Freshwater Polyzoa, including all the known species, both British and Foreign. The Ray Society, London.
https://doi.org/10.5962/bhl.title.9143
Androsova, E.I. 1958. Bryozoa of the Order Cheilostomata from the northern part of the Sea of Japan [in Russian]. Issledovaniya Dal'nevostochnykh Morei SSSR, 5:90-204.
Arakawa, S. 1995. Bryozoan fauna in the Jizodo Formation (Pleistocene), Boso Peninsula, Honshu, Japan. Natural History Research, 3:75-110.
Arakawa, S. 2024. Descriptions of three cheilostomatid bryozoan species from the continental shelf off Boso Peninsula, Japan. Species Diversity, 29:99-110.
https://doi.org/10.12782/specdiv.29.99
Audouin, J.V. 1826. Explication sommaire des planches de polypes de l'Egypte et de la Syrie, publiées par Jules-Cesar Savigny. In: Audouin, J.V. (ed.), Description de l'Egypte, ou recueil des observations et des recherches qui ont été faites en Egypte pendant l'expédition de l'armée française. Histoire naturelle, Imprimerie Impériale, Paris.
Bassler, R.S. 1935. Bryozoa. Generum et Genotyporum. Index et Bibliographica. Fossilium Catalogus I. Animalia. Junk, W. s'Gravenhage.
Berning, B. 2013. New and little-known Cheilostomata (Bryozoa, Gymnolaemata) from the NE Atlantic. European Journal of Taxonomy, 44:1-25.
https://doi.org/10.5852/ejt.2013.44
Berning, B. and Kukliński, P. 2008. North-east Atlantic and Mediterranean species of the genus Buffonellaria (Bryozoa, Cheilostomata): implications for biodiversity and biogeography. Zoological Journal of the Linnean Society, 152:537-566.
https://doi.org/10.1111/j.1096-3642.2007.00379.x
Bishop, J.D.D. and Househam, B.C. 1987. Puellina (Bryozoa; Cheilostomatida; Cribrilinidae) from British and adjacent waters. Bulletin of the British Museum (Natural History), Zoology, 53:1−63.
Bock, P.E. 2024. Bryozoa Home Page.
https://bryozoa.net/
Boonzaaier-Davids, M.K., Ma, K.C.K., and McQuaid, C.D. 2023. Epibiotic association of encrusting cheilostome bryozoans on shells of an invasive mussel from rocky shores of South Africa, with the description of a new aviculiferous species of Chaperia. Zootaxa, 5258: 197-210.
https://doi.org/10.11646/zootaxa.5258.2.2
Buchner, P. 1924. Studien über den Polymorphismus der Bryozoen. 1. Anatomische und systematische Untersuchungen an japanischen Reteporiden. Zoologischer Jahrbücher, Abtheilung für Systematik, Geographie und Biologie der Thiere, 48:155-216.
Busk, G. 1852. Catalogue of marine Polyzoa in the collection of the British Museum. I. Cheilostomata. Trustees of the British Museum, London:1-54.
https://doi.org/10.1242/jcs.s1-1.2.136b
Busk, G. 1854. Catalogue of marine Polyzoa in the collection of the British Museum. II. Cheilostomata. Trustees of the British Museum, London:1-120.
https://doi.org/10.1242/jcs.s1-2.8.277a
Busk, G. 1860. Zoophytology. Catalogue of the Polyzoa collected by J.Y. Johnson, Esq., at Madeira, in the years 1859 and 1860, with descriptions of new species. Quarterly Journal of Microscopical Science, 8:213-214, 280-285.
https://doi.org/10.1242/jcs.s1-8.32.280
Busk, G. 1884. Report on the Polyzoa collected by H.M.S. Challenger during the years 1873-1876. Part 1. The Cheilostomata. Report on the Scientific Results of the Voyage of the H.M.S. “Challenger”, Zoology, 10:1-216.
Cáceres-Chamizo, J.P., Sanner, J., Tilbrook, K.J., and Ostrovsky, A.N. 2017. Revision of the Recent species of Exechonella Canu and Bassler in Duvergier, 1924 and Actisecos Canu and Bassler, 1927 (Bryozoa, Cheilostomata): systematics, biogeography and evolutionary trends in skeletal morphology. Zootaxa, 4305:1-79.
https://doi.org/10.11646/zootaxa.4305.1
Canu, F. 1908. Les Bryozoaires des terrains tertiaires des environs de Paris, II. Annales de Paléontologie, Paris, 3:61-104.
Canu, F. and Bassler, R.S. 1917. A synopsis of American early Tertiary cheilostome Bryozoa. United States National Museum Bulletin, 96:1-87.
https://doi.org/10.5479/si.03629236.96.1
Canu, F. and Bassler, R.S. 1920. North American early Tertiary Bryozoa. United States National Museum Bullettin, 106:1−879.
https://doi.org/10.5479/si.03629236.106.i
Canu, F. and Bassler, R.S. 1923. North American later Tertiary and Quaternary Bryozoa. United States National Museum Bulletin, 125:1-302.
https://doi.org/10.5479/si.03629236.125.i
Canu, F. and Bassler, R.S. 1925. Les Bryozoaires du Maroc et de la Mauritanie. Mémoires de la Société des Sciences Naturelles du Maroc, 10:1-79.
Canu, F. and Bassler, R.S. 1926. Studies on the cyclostomatous Bryozoa. Proceedings of the U.S. National Museum, 67:1-124.
https://doi.org/10.5479/si.00963801.67-2593.1
Canu, F. and Bassler, R.S. 1927. Classification of the cheilostomatous Bryozoa. Proceedings of the United States National Museum, 69:1-42.
https://doi.org/10.5479/si.00963801.69-2640.1
Canu, F. and Bassler, R.S. 1928. Fossil and Recent Bryozoa of the Gulf of Mexico region. Proceedings of the United States National Museum, 72:1-199.
https://doi.org/10.5479/si.00963801.72-2710.1
Canu, F. and Bassler, R.S. 1929. Bryozoa of the Philippine region. United States National Museum Bulletin, 100:1-685.
Cheetham, A.H. and Sandberg, P.A. 1964. Quaternary Bryozoa from Louisiana mudlumps.
Journal of Paleontology, 38:1013-1046.
Chiu, W.-T.R., Yasuhara, M., Iwatani, H., Kitamura, A., and Fujita, K. 2016. An enigmatic Holocene podocopid ostracod from a submarine cave, Okinawa, Japan: “living fossil” or adaptive morphotype. Journal of Systematic Palaeontology, 14:643-652.
https://doi.org/10.1080/14772019.2015.1094754
Chiu, W-T.R., Yasuhara, M., Iwatani, H., Kitamura, A., and Fujita, K. 2017. Response of subtropical submarine-cave ecosystem to Holocene cave development and Asian monsoon variability. Paleobiology, 43(3):425-434.
http://doi/org/10.1017/pab.2016.53
Cook, P.L. and Bock, P.E. 1996. Characodoma Maplestone, a senior synonym of Cleidochasma Harmer Cleidochasmatidae, p. 81–88. In Gordon, D.P., Smith, A.M., and Grant-Mackie, J.A., (eds.), Bryozoans in Space and Time. NIWA, Wellington.
Cook, P.L. and Bock, P.E. 2004. Exechonella (Exechonellidae: Bryozoa, Cheilostomata) from the Recent and Miocene of southern Australia. Proceedings of the Royal Society of Victoria, 116:265-282.
Cook, P.L., Bock, P.E., Hayward, P.J., and Gordon, D.P. 2018. Class Gymnolaemata, order Cheilostomata, p. 61-280. In Cook, P.L., Bock, P.E., Gordon, D.P., and Weaver, H. (eds.), Australian Bryozoa Volume 2: Taxonomy of Australian families. CSIRO Publishing, Melbourne.
https://doi.org/10.1071/9781486306831
Dick, M.H. and Grischenko, A.V. 2017. Rocky-intertidal cheilostome bryozoans from the vicinity of the Sesoko Biological Station, west-central Okinawa, Japan. Journal of Natural History, 51:141-266.
https://doi.org/10.1080/00222933.2016.1253797
Dick, M.H., Tilbrook, K.J., and Mawatari, S.F. 2006. Diversity and taxonomy of rocky-intertidal Bryozoa on the Island of Hawaii, USA. Journal of Natural History, 40:2197-2258.
https://doi.org/10.1080/00222930601062771
Dick, M.H., Takashima, R., Kaneko, N., and Mawatari, S.F. 2008. Overview of Pleistocene bryozoans in Japan, p. 83-91. In Okada, H., Mawatari, S.F., Suzuki, N., and Gautam, P. (eds.), Proceedings of the International Symposium, The Origin and Evolution of Natural Diversity, 1-5 October 2007. Hokkaido University, Sapporo.
Dick, M.H., Ngai, N.D., and Doan, H.D. 2020. Taxonomy and diversity of coelobite bryozoans from drift coral cobbles on Co To Island, northern Vietnam. Zootaxa, 4747:201-252.
https://doi.org/10.11646/zootaxa.4747.2.1
Di Martino, E. 2023. Scanning electron microscopy study of Lars Silén’s cheilostome bryozoan type specimens in the historical collections of natural history museums in Sweden. Zootaxa, 5379:1-106.
https://doi.org/10.11646/zootaxa.5379.1.1
Di Martino, E. and Rosso, A. 2021. Seek and ye shall find: new species and new records of Microporella (Bryozoa, Cheilostomatida) in the Mediterranean. Zookeys, 1053:1-42.
https://doi.org/10.3897/zookeys.1053.65324
Di Martino, E. and Taylor, P.D. 2014. Miocene Bryozoa from East Kalimantan, Indonesia. Part I: Cyclostomata and Cheilostomata ‘Anasca’. Scripta Geologica, 146:17-126.
Di Martino, E. and Taylor, P.D. 2015. Miocene Bryozoa from East Kalimantan, Indonesia. Part II: ‘Ascophoran’ Cheilostomata. Scripta Geologica, 148:1-142.
Di Martino, E., Taylor, P.D., and Portell, R.W. 2017 Bryozoans from the lower Miocene Chipola Formation, Calhoun County, Florida, USA. Bulletin of the Florida Museum of Natural History, 53:97-200.
https://doi.org/10.58782/flmnh.pgmm1110
Di Martino, E., Taylor, P.D., Gordon, D.P., and Liow, L.H. 2017. New bryozoan species from the Pleistocene of the Wanganui Basin, North Island, New Zealand. European Journal of Taxonomy, 345:1-15.
https://doi.org/10.5852/ejt.2017.345
Di Martino, E., Taylor, P.D., Fernando, A.G.S., Kase, T., and Yasuhara, M. 2019. First bryozoan fauna from the middle Miocene of Central Java, Indonesia. Alcheringa, 43:461-478.
https://doi.org/10.1080/03115518.2019.1590639
Duvergier, J. 1921. Note sur les Bryozoaires du Néogène de l'Aquitaine. Actes de la Société Linnéenne, Bordeaux, 72:145-182.
Duvergier, J. 1924. Deuxième note sur les Bryozoaires du Néogène de l'Aquitaine. Actes de la Société Linnéenne, Bordeaux, 75:145-190.
Ehrenberg, C.G. 1831. Symbolae Physicae, seu Icones et descriptiones Corporum Naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegyptum, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam. Studio Annis 1820-1825. Berolini, Berlin.
Ellis, J. and Solander, D.C. 1786. The natural history of many curious and uncommon zoophytes, collected from various parts of the globe. White and Elmsly, London.
https://doi.org/10.5962/bhl.title.64985
Figuerola, B., Gordon, D.P., and Cristobo, J. 2018. New deep Cheilostomata (Bryozoa) species from the Southwestern Atlantic: shedding light in the dark. Zootaxa, 4375:211-249.
https://doi.org/10.11646/zootaxa.4375.2.3
Fleming, J. 1828. A history of British animals, exhibiting their descriptive characters and systematic arrangement of the genera and species of quadrupeds, birds, reptiles, fishes, Mollusca, and Radiata of the United Kingdom. Bell and Bradfute, Edinburgh.
https://doi.org/10.5962/bhl.title.12859
Gabb, W.M. and Horn, G.H. 1862. The fossil Polyzoa of the Secondary and Tertiary Formations of North America. Journal of the Academy of Natural Sciences of Philadelphia, 5:111-179.
Gautier, Y.V. 1957. Bryozoaires des Iles Baléares. Vie et Milieu, 2:205-222.
Gluhak, T., Lewis, J.E., and Popijac, A. 2007. Bryozoan fauna of Green Island, Taiwan: first indications of biodiversity. Zoological Studies, 46:397-426.
Gontar, V.I. 1982. New species of the order Cheilostomata (Bryozoa) from the region of the Kurile Islands. Zoologicheskii Zhurnal, 61:543-553. (in Russian)
Gontar, V.I. 1993. New deepwater species of Cheilostomatida from the Kuril Islands and the Pacific Ocean (Bryozoa). Zoosystematica Rossica, 2:41-45.
Gordon, D.P. 1984. The marine fauna of New Zealand. Bryozoa Gymnolaemata from the Kermadec Ridge. New Zealand Oceanographic Institute Memoir, 91:1-198.
Gordon, D.P. 1989a. Intertidal bryozoans from coral reef-flat rubble in Sa'aga, Western Samoa. New Zealand Journal of Zoology, 16:447-463.
https://doi.org/10.1080/03014223.1989.10422912
Gordon, D.P. 1989b. The marine fauna of New Zealand. Bryozoa Gymnolaemata (Cheilostomida Ascophorina) from the Western South Island continental shelf and slope. New Zealand Oceanographic Institute Memoir, 97:1-158.
Gordon, D.P. 1993. Bryozoan frontal shields: studies on umbonulomorphs and impacts on classification. Zoologica Scripta, 22:203-221.
https://doi.org/10.1111/j.1463-6409.1993.tb00352.x
Gordon, D.P. 2016. Bryozoa of the South China Sea-an overview. Raffles Bulletin of Zoology, 34 (Supplement):604-618.
Gordon, D.P. and Braga, G. 1994. Bryozoa: living and fossil species of the catenicellid subfamilies Ditaxiporinae Stach and Vasignyellidae nov. Mémoires du Muséum national d'Histoire naturelle, 161:55-85.
Gordon, D.P. and d'Hondt, J.-L. 1985. Talivittaticella, a new genus of Catenicellidae (Bryozoa) from the deep sea. Records of the New Zealand Oceanographic Institute, 5:13-19.
Gordon, D.P. and d'Hondt, J.-L. 1997. Bryozoa: Lepraliomorpha and other Ascophorina from New Caledonian waters. Mémoires du Muséum national d'Histoire naturelle, 176:9-124.
Gordon, D.P. and Taylor, P.D. 2015. Bryozoa of the early Eocene Tumaio Limestone, Chatham Island, New Zealand. Journal of Systematic Palaeontology, 13:983-1070.
https://doi.org.org/10.1080/14772019.2014.991905
Gray, J.E. 1843. Additional radiated animals and Annelides. Travels in New Zealand: with Contributions to the Geography, Geology, Botany, and Natural History of That Country, 2:292-295.
Gray, J.E. 1848. List of the specimens of British animals in the collections of the British Museum. Part 1. Centrionae or radiated animals. Trustees of the British Museum, London:91−151.
Grischenko, A.V., Dick, M.H., and Mawatari, S.F. 2007. Diversity and taxonomy of intertidal Bryozoa (Cheilostomata) at Akkeshi Bay, Hokkaido, Japan. Journal of Natural History, 41:1047-1161.
https://doi.org/10.1080/00222930701391773
Harmelin, J.-G. 1986. Patterns in the distribution of bryozoans in the Mediterranean marine caves. Stygologia, 2:10-25.
Harmelin, J.-G. 1997. Diversity of bryozoans in a Mediterranean sublittoral cave with bathyal-like conditions: role of dispersal processes and local factors. Marine Ecology Progress Series, 153:139-152.
https://doi.org/10.3354/meps153139
Harmelin, J.-G. 2000. Ecology of cave and cavity dwelling bryozoans, p. 38-55. In Herrera Cubilla, A. and Jackson, J.B.C. (eds.), Proceedings of the 11th IBA Conference, 1998, Republic of Panama. Smithsonian Tropical Research Institute, Balboa.
Harmelin, J.-G. 2003. Biodiversité des habitats cryptiques marins du parc national de Port-Cros (Méditerranée, France). Assemblages de bryozoaires d’une grotte sous-marine et des faces inférieures de pierres. Scientific Reports Port-Cros National Park, France, 19:101-1015.
Harmelin, J.-G. 2014. Alien bryozoans in the eastern Mediterranean Sea - new records from the coast of Lebanon. Zootaxa, 3893:301-308.
https://doi.org/10.11646/zootaxa.3893.3.1
Harmer, S.F. 1900. A revision of the genus Steganoporella. Quarterly Journal of Microscopical Science, 43:225-297.
https://doi.org/10.1242/jcs.s2-43.170.225
Harmer, S.F. 1926. Polyzoa of the Siboga Expedition. Part 2. Cheilostomata Anasca. Siboga Expedition Reports, 28b:183-501.
Harmer, S.F. 1934. The Polyzoa of the Siboga Expedition. Part 3. Cheilostomata Ascophora, I. Family Reteporidae. Siboga Expedition Reports, 28c:502-640.
Harmer, S.F. 1957. The Polyzoa of the Siboga Expedition, Part 4. Cheilostomata Ascophora II. Siboga Expedition Reports, 28d:641-1147.
Hass, H. 1948. Beitrag zur kenntnis der Reteporiden. Zoologica, 101:1-138.
Haswell, W.A. 1880. On some Polyzoa from the Queensland Coast. Proceedings of the Linnean Society of New South Wales, 5:33-44.
https://doi.org/10.5962/bhl.part.15866
Hayami, T. 1971. Some Neogene Cheilostomata (Bryozoa) from Okinawa-jima. Transactions and Proceedings of the Palaeontological Society of Japan, 82:73-92.
Hayami, T. 1973. The recent Cheilostomata (Bryozoa) from Kuroko-Shima, Northern Honshu, Japan. Saito Ho-on Kai Museum Research Bulletin, 42:47-56.
Hayami, T. 1975. Neogene Bryozoa from northern Japan. Science Reports Tôhoku University, 45:83-126.
Hayami, I. and Kase, T. 1993. Submarine cave Bivalvia from the Ryukyu Islands: systematics and evolutionary significance. Bulletin of The University Museum, University of Tokyo, 35:1-133.
Hayami, I. and Kase, T. 1996. Characteristics of submarine cave bivalves in the northwestern Pacific. American Malacological Bulletin, 12:59-65.
Hayward, P.J. 1988. Mauritian cheilostome Bryozoa. Journal of Zoology, London, 215:269-356.
https://doi.org/10.1111/j.1469-7998.1988.tb04900.x
Hayward, P.J. 2000. Lace corals (Bryozoa: Phidoloporidae) from Australia and the tropical south-west Pacific. Journal of Zoology, London, 252:109-136.
https://doi.org/10.1017/S0952836900009122
Hayward, P.J. 2004. Taxonomic studies on some Indo-West Pacific Phidoloporidae (Bryozoa: Cheilostomata). Systematics and Biodiversity, 1:305-326.
https://doi.org/10.1017/S1477200003001191
Hayward, P.J. and Cook, P.L. 1983. The South African Museum's Meiring Naude Cruises. Part 13, Bryozoa II. Annals of the South African Museum, 91:1-161.
Hayward, P.J. and Parker, S.A. 1994. Notes on some species of Parasmittina Osburn, 1952 (Bryozoa: Cheilostomatida). Zoological Journal of the Linnean Society, 110:53-75.
https://doi.org/10.1111/j.1096-3642.1994.tb01471.x
Hayward, P.J. and Ryland, J.S. 1998. Cheilostomatous Bryozoa. Part 1. Aeteoidea-Cribrilinoidea. Synopses of the British Fauna (n.s.), 10:1-366.
Hayward, P.J. and Winston, J.E. 2011. Bryozoa collected by the United States Antarctic Research Program: new taxa and new records. Journal of Natural History, 46:2259-2338.
https://doi.org/10.1080/00222933.2011.574922
Heller, C. 1867. Die Bryozoen des adriatischen Meeres. Verhandlungen der Zoologisch-botanischen Gesellschaft in Wien, 17:77-136.
Hincks, T. 1879. On the classification of the British Polyzoa. Annals and Magazine of Natural History, 3:153-164.
https://doi.org/10.1080/00222937908682494
Hincks, T. 1882. Polyzoa of the Queen Charlotte Islands: preliminary notice of new species. Annals and Magazine of Natural History (Series 5), 10:248-256.
https://doi.org/10.1080/00222938209459702
Hincks, T. 1884. Contributions towards a general history of the marine Polyzoa. Part XII. Polyzoa from India (coast of Burmah). Part XIII. Polyzoa from Victoria and western Australia. Annals and Magazine of Natural History (Series 5), 13:356-369.
https://doi.org/10.1080/00222938409459254
Hincks, T. 1887. On the Polyzoa and Hydroida of the Mergui Archipelago collected for the Trustees of the Indian Museum, Calcutta, by Dr J. Anderson, F.R.S., Superintendent of the Museum. Journal of the Linnean Society (Zoology), London, 21:121-135.
https://doi.org/10.1111/j.1096-3642.1887.tb00970.x
Hincks, T. 1895. Contributions towards a general history of the marine Polyzoa, 1880-1891. Appendix (no. 5):1-6.
https://doi.org/10.1080/00222938009458895
Hirose, M. 2010. Cheilostomatous Bryozoa (Gymnolaemata) from Sagami Bay, with notes on Bryozoan diversity and faunal changes over the past 130 years. Unpublished PhD Thesis, Graduate School of Science, Hokkaido University, Japan.
Hirose, M. 2017. Diversity of freshwater and marine Bryozoans in Japan, p. 629-649. In Motokawa, M. and Kajihara, H. (eds.), Species Diversity of Animals in Japan. Springer, Tokyo.
https://doi.org/10.1007/978-4-431-56432-4_24
Hirose, M., Mawatari, S.F., and Scholz, J. 2013. Distribution and diversity of erect bryozoan assemblages along the Pacific coast of Japan, p. 121-136. In Ernst, A., Schäfer, P., and Scholz, J. (eds.), Bryozoan Studies 2010. Springer, Heidelberg.
https://doi.org/10.1007/978-3-642-16411-8_9
Hondt, d’ J.-L. 1986. Bryozoaires de Nouvelle-Caledonie et du plateau des Chesterfield. Bulletin du Muséum national d'Histoire naturelle. Section A, Zoologie, Biologie et Ecologie animales, 8:697-756.
https://doi.org/10.5962/p.287554
Hondt, d’ J-L. 2006. Bryozoaires. Nouvelle explications des planches de polypes de la description de l'Egypte (dessinées sous la direction de Jules-César Savigny, et commentées sommairement à l'origine par Victor Audouin). Collection Nouvelle Description de l'Egypte, 2:1-86.
ICZN. 1999. International Code of Zoological Nomenclature. Fourth edition. The International Trust for Zoological Nomenclature, London, UK.
https://www.iczn.org/
Johnston, G. 1838. A history of British zoophytes. Lizars, Edinburgh, London and Dublin.
https://doi.org/10.5962/bhl.title.110844
Jullien, J. 1881. Remarques sur quelques espèces des Bryozoaires cheilostomiens. Bulletin de la Société zoologique de France, 6:163-168.
Jullien, J. 1882. Note sur une nouvelle division des Bryozoaires cheilostomiens. Bulletin de la Société zoologique de France, 6:271-285.
Jullien, J. 1883. Dragages du ‘Travailleur’. Bryozoaires, espèces draguées dans l'Océan Atlantique en 1881. Bulletin de la Société zoologique de France, 7:497-529.
https://doi.org/10.5962/bhl.part.27178
Jullien, J. 1888. Bryozoaires. Mission Scientifique du Cap Horne 1882-1883, 6:1-92.
Jullien, J. and Calvet, L. 1903. Bryozoaires provenant des campagnes de l'Hirondelle (1886-1888). Resultats des Campagnes Scientifiques accomplies sur son yacht par Albert Ier, Prince Souverain de Monaco, 23:1-188.
Kase, T. and Hayami, I. 1992. Unique submarine cave mollusc fauna: composition, origin and adaptation. Journal of Molluscan Studies, 58:446-449.
https://doi.org/10.1093/mollus/58.4.446
Kataoka, J. 1957. Bryozoa form the Daishaka Formation (Pliocene), Minami-tsugaru-gun, Aomori Prefecture. Transactions and Proceedings of the Palaeontological Society of Japan, 28:143-153.
Kataoka, J. 1960. Bryozoa from Mogami-Tai, Japan Sea. Science Reports Tôhoku University, 4:393-399.
Kataoka, J. 1961. Bryozoan fauna from the “Ryukyu Limestone” of Kikai-jima, Kagoshima Prefecture, Japan. The Science Reports of Tohoku University, Geology, 32:213-272.
Kirkpatrick, R. 1890. Reports on the zoological collections made in Torres Straits by Professor A.C. Haddon, 1888-1889. Hydroida and Polyzoa. Scientific Proceedings of the Royal Dublin Society, New Series, 6:603-626.
Kitamura, A., Hiramoto, M., Kase, T., Yamamoto, N., Amemiya, M., and Ohashi, S. 2007a. Changes in cavernicolous bivalve assemblages and environments within a submarine cave in the Okinawa Islands during the last 5,000 years. Paleontological Research, 11:163-182.
https://doi.org/10.2517/1342-8144(2007)11[163:CICBAA]2.0.CO;2
Kitamura, A., Yamamoto, N., Kase, T., Ohashi, S., Hiramoto, M., Fukusawa, H., Watanabe, T., Irino, T., Kojitani, H., Shimamura, M., and Kawakami, I. 2007b. Potential of submarine-cave sediments and oxygen isotope composition of cavernicolous micro-bivalve as a late Holocene paleoenvironmental record. Global and Planetary Change, 55:301-316.
https://doi.org/10.1016/j.gloplacha.2006.09.002
Kitamura, A., Kobayashi, K., Tamaki, C., Yamamoto, N., Irino, T., Miyairi, Y., and Yokoyama, Y. 2013. Evidence of recent warming in the Okinawa region, subtropical northwestern Pacific, from an oxygen isotope record of a cave-dwelling marine micro-bivalve. Paleontological Research, 17:58-68.
https://doi.org/10.2517/1342-8144-17.1.58
Lagaaij, R. 1963. New additions to the bryozoan fauna of the Gulf of Mexico. Institute of Marine Science Texas Publication, 9:181-236.
Lamouroux, J.V.F. 1816. Histoire des Polypiers Coralligènes Flexibles, vulgairement nommés Zoophytes. Caen.
https://doi.org/10.5962/bhl.title.11172
Lamouroux, J.V.F. 1821. Exposition méthodique des genres de l’ordre des polypiers. Agasse, Paris.
Lamouroux, J.V.F. 1824-1826. Description des Polypiers flexibles, p. 603-642. In Quoy, J.R.C. and Gaimard, J.P. (eds.), Voyage autour du Monde executée sur l'Uranie et la Physicienne pendant les années 1817-1820. Vol. 3, Zoologie IV. Pillet Ane, Paris.
Levinsen, G.M.R. 1902. Studies on Bryozoa. Videnskabelige Meddelelser frå den naturhistoriske Forening i Kjøbenhavn, 54:1-31.
Levinsen, G.M.R. 1909. Morphological and systematic studies on the cheilostomatous Bryozoa. Nationale Forfatterers Forlag, Copenhagen.
https://doi.org/10.5962/bhl.title.54983
Liu, X. 1991. A study on the anascan-cribrimorphan bryozoans from Nansha Islands of China. Bulletin of Marine Biology Nansha Island and Neighbouring Seas, 5:56-81. (in Chinese)
Liu, X., Yin, X., and Ma, J. 2001. Biology of marine-fouling bryozoans in the coastal waters of China. Science Press, Beijing.
Livingstone, A.A. 1926. Studies on Australian Bryozoa. No. 3. Records of the Australian Museum, 15:79-99.
https://doi.org/10.3853/j.0067-1975.15.1926.801
Livingstone, A.A. 1928. Bryozoa from South Australia. Records of the South Australian Museum, 4:111-124.
Livingstone, A.A. 1929. Papers from Dr Th. Mortensen’s Pacific Expedition 1914-16. XLIX. Bryozoa Cheilostomata from New Zealand. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kjøbenhavn, 87:45-104.
López de la Cuadra, C.-M. and Garcia-Gomez, J.C. 2001. New and little-known ascophoran bryozoans from the Western Mediterranean, collected by the ‘Fauna Iberica’ expeditions. Journal of Natural History, 35:1717-1732.
https://doi.org/10.1080/002229301317092414
Lu, L. 1991. Holocene bryozoans from the Nansha Sea area. Quaternary Biological Groups of the Nansha Islands and the Neighbouring Waters. Zhongshan University Publishing House, Guangzhou: 11-81, 473-486.
MacGillivray, P.H. 1869. Descriptions of some new genera and species of Australian Polyzoa to which is added a list of species found in Victoria. Transactions and Proceedings of the Royal Society of Victoria, 9:126-148.
MacGillivray, P.H. 1887. Descriptions of new or little-known Polyzoa, Part 11. Transactions and Proceedings of the Royal Society of Victoria, 23:64-72.
MacGillivray, P.H. 1891. Descriptions of new, or little-known, Polyzoa, Part 14. Proceedings of the Royal Society of Victoria (new series), 3:77-83.
MacGillivray, P.H. 1895. A monograph of the Tertiary Polyzoa of Victoria. Transactions of the Royal Society of Victoria, 4:1-166.
https://doi.org/10.5962/bhl.title.6076
Maplestone, C.M. 1899. Further descriptions of the Tertiary Polyzoa of Victoria. 2. Proceedings of the Royal Society of Victoria (new series), 12:1-13.
Maplestone, C.M. 1900. Further descriptions of the Tertiary Polyzoa of Victoria. 4. Proceedings of the Royal Society of Victoria (new series), 13:1-9.
Maplestone, C.M. 1909. Polyzoa from the Gilbert Islands. Proceedings of the Royal Society of Victoria (new series), 21:410-419.
Martha, S.O., Vieira, L.M., Souto-Derungs, J., Grischenko, A.V., Gordon, D.P., and Ostrovsky, A.N., 2020. 11. Gymnolaemata, Cheilostomata, p. 317-423. In Schwaha, T. (ed.), Phylum Bryozoa. de Gruyter, Berlin.
https://doi.org/10.1515/9783110586312-011
Mawatari, S. 1952. Bryozoa of Kii Peninsula. Publications of the Seto Marine Biological Laboratory, 2:261-288.
https://doi.org/10.5134/174675
Mawatari, S. 1956. Cheilostomatous Bryozoa from the Kurile Islands and the neighbouring districts. Pacific Science, 10:113-135.
Mawatari, S. 1962. Bryozoa of the eastern shore of Noto Peninsula. Annual Report of Noto Marine Laboratory, Kanazawa University, 3:5-10.
Mawatari, S. 1987. A preliminary list of cheilostomatous bryozoans from Sesoko, Okinawa. Galaxea, 6:99-107.
Mawatari, S.F. and Mawatari, S. 1981. A preliminary list of cheilostomatous bryozoans collected along the coasts of Hokkaido. Proceedings of the Japanese Society of Systematic Zoology, 21:41-58.
Milne-Edwards, H. 1836. Les Polypes. In Lamarck, J.B.P.A. 1816. Histoire naturelle des Animaux sans Vertèbres. Tome II. Paris.
Moyano, G.H.I. 2002. Bryozoa from oceanic south eastern Pacific islands: diversity and zoogeography, p. 229-238. In Wyse Jackson, P.N., Buttler, C.J., and Spencer-Jones, M. (eds.), Bryozoan Studies 2001. A.A. Balkema Publishers, Lisse, Abingdon, Exton, Tokyo.
Neviani, A. 1895. Briozoi neozoici di alcune località d’Italia. Parte 1. Bollettino della Società Romana per gli Studi Zoologici, 4:109-123.
Norman, A.M. 1903. Notes on the natural history of East Finmark, Polyzoa. Annals and Magazine of Natural History, 11:567−598.
https://doi.org/10.1080/00222930308678818
Okada, Y. 1920. Notes on some species of Retepora and Adeonella occurring in Japan, with descriptions of one new variety and five new species. Annotationes Zoologicae Japonenses, 9:613-634.
Okada, Y. 1923. On a collection of Bryozoa from the Straits of Corea. Annotationes Zoologicae Japonenses, 10:215-234.
Okada, Y. 1928. Report of the biological survey of Mutsu Bay. 8. Cyclostomatous Bryozoa of Mutsu Bay. Science Report of Tôhoku Imperial University, Ser. 4, Biology, 3:481-496.
Okada, Y. 1929. Report of the biological survey of Mutsu Bay. 12. Cheilostomatous Bryozoa of Mutsu Bay. Science Report of Tôhoku Imperial University, Ser. 4, Biology, 4:11-35.
Okada, Y. 1934. Bryozoa fauna in the vicinity of the Shimoda Marine Biological Station. Science Reports of the Tokio University of Literature and Science, Section B, 2:1-20.
Okada, Y. and Mawatari, S. 1935. Bryozoa fauna collected by the ‘Misago’ during the Zoological Survey around Izu Peninsula (I). Science Reports of the Tokyo Bunrika Daigaku, Section B, 2:127-147.
Okada, Y. and Mawatari, S. 1936. Bryozoa fauna collected by the ‘Misago’ during the Zoological Survey around Izu Peninsula (II). Science Reports of the Tokyo Bunrika Daigaku, Section B, 3:53-73.
Okada, Y. and Mawatari, S. 1937. On the collection of Bryozoa along the coast of Onagawa Bay and its vicinity, the northern part of Honshu, Japan. Science Reports Tôhoku University, 11:433-445.
Okada, Y. and Mawatari, S. 1938. On the collection of Bryozoa along the coast of Wakayam-ken, the middle part of Honshu, Japan. Annotationes Zoologicae Japonenses, 17:445-462.
Omori, A., Kitamura, A., Fujita, K., Honda, K., and Yamamoto, N. 2010. Reconstruction of light conditions within a submarine cave during the past 7,000 years based on the temporal and spatial distribution of algal symbiont-bearing large benthic foraminifers. Palaeogeography, Palaeoclimatology, Palaeoecology, 292:443-452.
https://doi.org/10.1016/j.palaeo.2010.04.004
Orbigny, d’ A. 1851-1854. Paléontologie française. Description des Mollusques et Rayonées fossils. Terrains crétacés. Tome 5, Bryozoaires. Victor Masson, Paris.
Ortmann, A. 1890. Die Japanische Bryozoenfauna. Bericht über die von Herrn Dr.L.Döderlein in Jahre 1880-81, gemachten Sammlungen. Archiv für Naturgeschichte, 56:1-74.
Ortmann, A. 1892. Die Koralriffe van Dar-es-Salaam und umgegend. Zoologische Jahrbücher. Abteilung für Systematik, Geographie und Biologie der Tiere, 6:631-670.
Osburn, R.C. 1950. Bryozoa of the Pacific coast of America, part 1, Cheilostomata-Anasca. Report of the Allan Hancock Pacific Expeditions, 14:1-269.
https://doi.org/10.5962/bhl.title.6542
Osburn, R.C. 1952. Bryozoa of the Pacific coast of America, part 2, Cheilostomata-Ascophora. Report of the Allan Hancock Pacific Expeditions, 14:271-611.
Ostrovsky, A.N. 2013. Evolution of sexual reproduction in marine invertebrates. Example of Gymnolaemate bryozoans. Springer, Dordrecht.
https://doi.org/10.1007/978-94-007-7146-8
Philipps, E.G. 1900. Report on the Polyzoa collected by Dr. Willey from the Loyalty Isles; New Guinea and New Britain, p. 439-450. In Willey, A. (ed.), Zoological Results based on material from New Britain, New Guinea, Loyalty Islands and elsewhere, collected during the years 1895, 1896 and 1897. University Press, Cambridge.
Pourtalès, L.F. 1867. Contributions to the fauna of the Gulf Stream at great depths. Bulletin of the Museum of Comparative Zoology at Harvard College in Cambridge, 1:103-120.
Pouyet, S. 1978. Phoceana pliocenica espèce fossile du genre Phoceana (Bryozoa Cheilostomata). Geobios, 11:119-123.
https://doi.org/10.1016/S0016-6995(78)80026-6
Pouyet, S. and David, L. 1979. Revision of the genus Steginoporella (Bryozoa Cheilostomata), p. 565-584. In Larwood, G.P. and Abbott, M.B. (eds.), Advances in Bryozoology. Academic Press, London.
Powell, N.A. 1967. Polyzoa (Bryozoa) - Ascophora - from north New Zealand. Discovery Reports, 34:199-393.
Ramalho, L.V., López-Fé, C.M., and Rueda, J.L. 2018. Three species of Reteporella (Bryozoa: Cheilostomata) in a diapiric and mud volcano field of the Gulf of Cádiz, with the description of Reteporella victori n. sp. Zootaxa, 4375:90-104.
https://doi.org/10.11646/zootaxa.4375.1.4
Ramalho, L.V., Moraes, F.C., Salgado, L.T., Bastos, A.C., and Moura, R.M. 2021. Bryozoa from the reefs off the Amazon River mouth: checklist, thirteen new species, and notes on their ecology and distribution. Zootaxa, 4950:1-45.
https://doi.org/10.11646/zootaxa.4950.1.1
Ridley, S.O. 1881. Account of the zoological collections made during the survey of H.M.S. Alert in the straits of Magellan and on the coast of Patagonia. Proceedings of the Zoological Society of London, 1881:44-61.
Ristedt, H. 1985. Cribrilaria -Arten (Bryozoa) des Indopazifiks (Rotes Meer, Seychellen, Philippinen). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg, 59:15-38.
Robertson, A. 1921. Report on a collection of Bryozoa from the Bay of Bengal and other eastern seas. Records of the Indian Museum, 22:33-65.
https://doi.org/10.5962/bhl.part.1465
Rosso, A. and Di Martino, E. 2016. Bryozoan diversity in the Mediterranean Sea: an update. Mediterranean Marine Science, 17:567-607.
https://doi.org/10.12681/mms.1706
Rosso, A., Di Martino, E., Sanfilippo, R., and Di Martino, V. 2013a. Bryozoan communities and thanatocoenoses from submarine caves in the Plemmirio Marine Protected Area (SE Sicily), p. 251-269. In Ernst, A., Schäfer, P., and Scholz, J. (eds.), Bryozoan Studies 2010. Proceedings of the 15th International Bryozoology Association Conference, 2010 Kiel, Germany. Lecture Notes in Earth System Sciences 143. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-642-16411-8_17
Rosso, A., Sanfilippo, R., Taddei Ruggiero, E., and Di Martino, E. 2013b. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Bollettino della Società Paleontologica Italiana, 52:167-176.
Rosso, A., Gerovasileiou, V., Sanfilippo, R., and Guido, A., 2018a. Bryozoan assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean). Marine Biodiversity, 49:707-726.
https://doi.org/10.1007/s12526-018-0846-0
Rosso, A., Beuck, L., Vertino, A., Sanfilippo, R., and Freiwald, A. 2018b. Cribrilinids (Bryozoa, Cheilostomata) associated with deep-water coral habitats at the Great Bahama Bank slope (NW Atlantic), with description of new taxa. Zootaxa, 4524:401-439.
https://doi.org/10.11646/zootaxa.4524.4.1
Rosso, A., Gerovasileiou, V., Sanfilippo, R., and Guido, A. 2019. Undisclosed bryodiversity of submarine caves of the Aegean Sea (Eastern Mediterranean). 2nd Mediterranean Symposium on the conservation of Dark Habitats. Antalya, Turkey, 16 January 2019:1-25.
Rosso, A., Sanfilippo, R., Guido, A., Gerovasileiou, V., Taddei Ruggiero, E., and Belmonte, G., 2021a. Colonisers of the dark: biostalactite-associated metazoans from “lu Lampiùne” submarine cave (Apulia, Mediterranean Sea). Marine Ecology, 42:e12634.
https://doi.org/10.1111/maec.12634
Rosso, A., Di Martino, E., and Ostrovsky, A.N. 2021b. Cribrilinid bryozoans from Pleistocene Mediterranean deep-waters, with the description of new species. Journal of Paleontology, 95:268-290.
https://doi.org/10.1017/jpa.2020.93
Rucker, J.B. 1967. Paleoecological analysis of cheilostome Bryozoa from Venezuela-British Guiana shelf sediments. Bulletin of Marine Science, 17:787-839.
Ryland, J.S. 1984. Phylum Bryozoa - Lace corals and their relatives, p. 69-80. In Mather, P. and Bennett, I. (eds.), A Coral Reef Handbook. Handbook No.1. Australian Coral Reef Society, Brisbane.
Ryland, J.S. and Hayward, P.J. 1992. Bryozoa from Heron Island, Great Barrier Reef. Memoirs of the Queensland Museum, 32:223-301.
Sakakura, K. 1935. Pliocene and Pleistocene Bryozoa from the Boso peninsula. I. Bryozoa of the Dizôdô Beds. Journal of the Faculty of Science, Imperial University of Tokyo, 4:253-344.
Sakakura, K. 1938. Bryozoaires pleistocenes aux environs de Tako-mati, Prefecture de Tiba. Journal of the Geological Society of Japan, 45:717-722.
https://doi.org/10.5575/geosoc.45.717
Scholz, J. and Cusi, M.A.V. 1991. Paleoecologic implications of modern coral and bryozoan communities from southern Leyte, Philippines. Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg, 71:405-431.
Scholz, J., Mawatari, S.F., and Hirose, M. 2008. The bryozoan diversity mystery: Why do we have about 1000 species in Japanese waters?, p. 129-135. In Okada, H., Mawatari, S.F., Suzuki, N., and Gautam, P. (eds.), Proceedings of the International Symposium “The Origin and Evolution of Natural Diversity”, 1-5 October 2007, Sapporo.
Seo, J.E. 1993. Systematic study on bryozoans from the South Sea in Korea 2. Smittinidae. Korean Journal of Systematic Zoology, 9:35-50.
Seo, J.E. 1994. Two species of Celleporaria (Cheilostomata: Bryozoa) from Korea. Korean Journal of Systematic Zoology, 10:189-197.
Seo, J.E. 2002. A new record of smittinid Bryozoa (Gymnolaemata, Cheilostomata) from Manjae Island, Korea. Korean Journal of Systematic Zoology, 18:135-141.
Seo, J.E. 2005. Illustrated encyclopedia of fauna and flora of Korea. Vol. 40. Bryozoa. Seoul: Daehon Printing and Publishing. (in Korean)
Seo, J.E. and Gong, Y.H. 2006. A new species and two new records of Cheilostomata (Bryozoa) from Korea. Korean Journal of Systematic Zoology, 22:13-16.
Seo, J.E. and Bum, S.M. 2009. A faunistic study on cheilostomatous bryozoans from the shoreline of South Korea, with two new species. Korean Journal of Systematic Zoology, 25:19-40.
https://doi.org/10.5635/KJSZ.2009.25.1.019
Silén, L. 1938. Zur Kenntnis des Polymorphismus der Bryozoen. Die Avicularien der Cheilostomata Anasca. Zoologiska Bidrag från Uppsala, 17:149-366.
Silén, L. 1941. Cheilostomata Anasca (Bryozoa) collected by Prof. Dr. Sixten Bock’s expedition to Japan and the Bonin Islands 1914. Arkiv för Zoologi, 33A:1-130.
Silén, L. 1947. Conescharellinidae (Bryozoa Gymnolaemata) collected by Prof. Dr. Sixten Bock’s expedition to Japan and the Bonin Islands 1914. Arkiv för Zoology, 39A:1-59.
Smitt, F.A. 1873. Floridan Bryozoa, collected by Count L.F. de Pourtales. II. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 11:1-83.
Sonar, M.A., Wayal, D.V., and Badve, R.M. 2021. Lepraliomorph bryozoans from Holocene deposits along the Konkan Coast of Western India. Alcheringa, 45:274-287.
https://doi.org/10.1080/03115518.2021.1916836
Soule, D.F. and Soule, J.D. 1973. Morphology and speciation of Hawaiian and eastern Pacific Smittinidae (Bryozoa, Ectoprocta). Bulletin of the American Museum of Natural History, 152:365-440.
Soule, J.D., Soule, D.F., and Chaney, H.W. 1991. New tropical Pacific and Indian Ocean Cleidochasmatidae (Cheilostomata: Ascophora), p. 465-486. In Bigey, F.P. and Hondt, d’ J.-L. (eds.), Bryozoaires Actuels et Fossiles. Bulletin de la Société des Sciences Naturelles de l'Ouest de la France Mémoire HS 1.
Soule, D.F., Soule, J.D., and Chaney, H.W. 1999. New species of Thalamoporella (Bryozoa) with acute or subacute avicularium mandibles and review of known species worldwide. Irene McCulloch Foundation Monograph Series, 4:1-57.
Stach, L.W. 1935. The genera of Catenicellidae. Proceedings of the Royal Society of Victoria, 47:389-396.
Stach, L.W. 1936. Studies on Recent Petraliidae (Bryozoa). Records of the Australian Museum, 19:355-379.
https://doi.org/10.3853/j.0067-1975.19.1936.706
Tabuki, R. and Hanai, T. 1994. A 'living fossil ostracod' in submarine caves a new saipanettid genus. Fossils, 57:16-20.
Tabuki, R. and Hanai, T. 1999. A new sigillid ostracod from submarine caves of the Ryukyu Islands, Japan. Palaeontology, 42:569-593.
https://doi.org/10.1111/1475-4983.00086
Taylor, P.D. and Tan, S.-H.A. 2015. Cheilostome Bryozoa from Penang and Langkawi, Malaysia. European Journal of Taxonomy, 149:1-34.
https://doi.org.org/10.5852/ejt.2015.149
Tenison Woods, J.E. 1879. On some Australian Tertiary fossil corals and Polyzoa. Journal and Proceedings of the Royal Society of New South Wales, 12:57-61.
https://doi.org/10.5962/p.358822
Thomson, C.W. 1858. On new Genera and Species of Polyzoa in the collection of Professor W.H. Harvey. Natural History Review and Quarterly Journal of Science, 5:134-147.
Thornely, L.R. 1905. Report on the Polyzoa collected by Professor Herdmann, at Ceylon, in 1902. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manar, 4:107-130.
Thornely, L.R. 1907. Report on the marine Polyzoa in the collection of the Indian Museum. Records of the Indian Museum, 1:179-196.
https://doi.org/10.26515/rzsi/v1/i3/1907/163390
Thornely, L.R. 1912. Marine Polyzoa of the Indian Ocean. Transactions of the Linnean Society of London, 15:137-157.
https://doi.org/10.1111/j.1096-3642.1912.tb00095.x
Tilbrook, K.J. 1998. The species of Antropora Norman, 1903 (Bryozoa: Cheilostomatida), with the description of a new genus in the Calloporoidea. Records of the South Australian Museum, 31:25-49.
Tilbrook, K.J. 1999. Description of Hippopodina feegeensis and three other species of Hippopodina Levinsen, 1909 (Bryozoa: Cheilostomatida). Journal of Zoology, London, 247:449-456.
https://doi.org/10.1017/S0952836999004045
Tilbrook, K.J. 2006. Cheilostomatous Bryozoa from the Solomon Islands. Santa Barbara Museum of Natural History Monographs, 4:1-386.
Tilbrook, K.J. 2012. Review of the bryozoan genus Bryopesanser Tilbrook, 2006 (Escharinidae: Cheilostomata) with the description of 11 new species. Zootaxa, 3165:39-63.
https://doi.org/10.11646/zootaxa.3165.1.3
Tilbrook, K.J. and Cook, P.L. 2005. Petraliellidae Harmer, 1957 (Bryozoa: Cheilostomata) from Queensland, Australia. Systematics and Biodiversity, 2:319-339.
https://doi.org/10.1017/S1477200004001525
Tilbrook, K.J. and Vieira, L.M. 2012. Scrupocellaria (Bryozoa: Cheilostomata) from the Queensland coast, with the description of three new species. Zootaxa, 3528:29-48.
https://doi.org/10.11646/zootaxa.3528.1.2
Tilbrook, K.J., Hayward, P.J., and Gordon, D.P. 2001. Cheilostomatous Bryozoa from Vanuatu. Zoological Journal of the Linnean Society, London, 131:35-109.
https://doi.org/10.1111/j.1096-3642.2001.tb01309.x
Vieira, L.M., Gordon, D.P., and Correia, M.D. 2007. First record of a living ditaxiporine catenicellid in the Atlantic, with a description of Vasignyella ovicellata n. sp. (Bryozoa). Zootaxa, 152:49-58.
https://doi.org/10.11646/zootaxa.1582.1.5
Vieira, L.M., Gordon, D.P., Souza, F.B.C., and Haddad, M.A. 2010. New and little-known cheilostomatous Bryozoa from the south and southeastern Brazilian continental shelf and slope. Zootaxa, 2722:1-53.
https://doi.org/10.11646/zootaxa.2722.1.1
Vieira, L.M., Spencer Jones, M.E., Winston, J.E., Migotto, A.E., and Marques, A.C. 2014. Evidence for polyphyly of the genus Scrupocellaria (Bryozoa: Candidae) based on a phylogenetic analysis of morphological characters. PLOS ONE, 9:e95296.
https://doi.org.org/10.1371/journal.pone.0095296
Vigneaux, M. 1949. Révision des Bryozoaires néogènes du Bassin d'Aquitaine et essai de classification. Mémoires de la Société Géologique de France (Nouvelle Série), 28:1-153.
Voigt, E. 1971. The cheilostomate nature of the alleged cyclostomatous bryozoan genus Dysnoetopora. Lethaia, 4:79-100.
https://doi.org/10.1111/j.1502-3931.1971.tb01281.x
Wass, R.E. and Yoo, J.J. 1975. Bryozoa from Site 282 west of Tasmania. Initial Reports of the Deep-sea Drilling Project, 29:809-831.
https://doi.org/10.2973/dsdp.proc.29.121.1975
Wass, R.E. and Yoo, J.J. 1976. Distribution and taxonomy of some recent catenicelliform Bryozoa from Australia, p. 281-297. In Pouyet, S. (ed.), Bryozoa 1974, Documents de Laboratoires de Géologie Faculté de Sciences de Lyon HS 3. Université Claude Bernard, Lyon.
Waters, A.W. 1881. On fossil chilostomatous Bryozoa from south-west Victoria, Australia. Quarterly Journal of the Geological Society (London), 37:309-347.
https://doi.org/10.1144/GSL.JGS.1881.037.01-04.29
Waters, A.W. 1887. Bryozoa from New South Wales, North Australia, etc. Part II. Annals and Magazine of Natural History, 20:181-203.
https://doi.org/10.1080/00222938709460036
Waters, A.W. 1909. Reports on the Marine Biology of the Sudanese Red Sea. 12. The Bryozoa, Part 1, Cheilostomata. Journal of the Linnean Society (Zoology) London, 31:123-181.
https://doi.org/10.1111/j.1096-3642.1909.tb00458.x
Waters, A.W. 1913. The marine fauna of British East Africa and Zanzibar, from collections made by Cyril Crossland M.A., B.Sc., F.Z.S., in the years 1901-1902. Bryozoa - Cheilostomata. Proceedings of the Zoological Society of London, 1913:458-537.
https://doi.org/10.1111/j.1469-7998.1913.tb06141.x
Winston, J.E. 1986. An annotated check-list of coral-associated bryozoans. American Museum Novitates, 2859:1-39.
Winston, J.E. 1988. The systematist’s perspective, p. 1-6. In Fautin, D.G. (ed.), Biomedical importance of marine organisms. California Academy of Sciences, San Francisco.
Winston, J.E. 2005. Re-description and revision of Smitt’s “Floridan Bryozoa” in the collection of the Museum of Comparative Zoology, Harvard University. Virginia Museum of Natural History Memoir, 7:1-147.
Winston, J.E. and Woollacott, R.M. 2009. Scientific Results of the Hassler Expedition. Bryozoa. No.1. Barbados. Bulletin of the Museum of Comparative Zoology, 159:239-300.
https://doi.org/10.3099/0027-4100-159.5.239
Winston, J.E. and Vieira, L.M. 2013. Systematics of interstitial encrusting bryozoans from southeastern Brazil. Zootaxa, 3710:101-146.
https://doi.org/10.11646/zootaxa.3710.2.1
Winston, J.E. and Jackson, J.B.C. 2021. Coral reef-associated bryozoans of Jamaica. Zootaxa, 4988:1-281.
https://doi.org/10.11646/zootaxa.4988.1.1
Yamamoto, N., Kitamura, A., Ohmori, A., Morishima, Y., Toyofuku, T., and Ohashi, S. 2009. Long-term changes in sediment type and cavernicolous bivalve assemblages in Daidokutsu submarine cave, Okinawa Islands: evidence from a new core extending over the past 7,000 years. Coral Reefs, 28:967-976.
https://doi.org/10.1007/s00338-009-0536-2
Yamamoto, N., Kitamura, A., Irino, T., Kase, T., and Ohashi, S. 2010. Climatic and hydrologic variability in the East China Sea during the last 7,000 years based on oxygen isotope records of the submarine cavernicolous micro-bivalve Carditella iejimensis. Global and Planetary Change, 72:131-140.
https://doi.org/10.1016/j.gloplacha.2010.01.025
Yanagi, N. and Okada, Y. 1918. On a collection of Japanese Cheilostomatous Bryozoa. I. Annotationes Zoologicae Japonenses, 9:407-429.
Yang, H.J., Seo, J.E., and Gordon, D.P. 2018. Sixteen new generic records of Korean Bryozoa from southern coastal waters and Jeju Island, East China Sea: evidence of tropical affinities. Zootaxa, 4422:493-518.
https://doi.org/10.11646/zootaxa.4422.4.3
Zágoršek, K. 2003. Upper Eocene Bryozoa from Waschberg Zone (Austria). Beitrage zur Palaontologie und Geologie osterreich Ungarns und des Orients, Wien, 28:101-263.
https://doi.org/10.1127/njgpm/2003/2003/439

