 A new Cainotherioidea (Mammalia, Artiodactyla) from Palembert (Quercy, SW France): Phylogenetic relationships and evolutionary history of the dental pattern of Cainotheriidae
A new Cainotherioidea (Mammalia, Artiodactyla) from Palembert (Quercy, SW France): Phylogenetic relationships and evolutionary history of the dental pattern of Cainotheriidae
Article number: 23(3):a54
https://doi.org/10.26879/1081
Copyright Paleontological Society, November 2020
Author biographies
Plain-language and multi-lingual abstracts
PDF version
PE NOTE: Errata
Submission: 13 March 2020. Acceptance: 11 November 2020.
ABSTRACT
Cainotheriidae are small artiodactyls restricted to Western Europe deposits from the late Eocene to the middle Miocene. From their first occurrence in the fossil record, cainotheriids show a highly derived molar morphology compared to other endemic European artiodactyls, called the “Cainotherium plan”, and the modalities of the emergence of this family are still poorly understood. Cainotherioid dental material from the Quercy area (Palembert, France; MP18-MP19) is described in this work and referred to Oxacron courtoisii and to a new “cainotherioid” species. The latter shows an intermediate morphology between the “robiacinid” and the “derived cainotheriid” types. This allows for a better understanding of the evolution of the dental pattern of cainotheriids, and identifies the enlargement and lingual migration of the paraconule of the upper molars as a key driver. A phylogenetic analysis, based on dental characters, retrieves the new taxon as the sister group to the clade including Cainotheriinae and Oxacroninae. The new taxon represents the earliest offshoot of Cainotheriidae.
Romain Weppe. Institut des Sciences de l’Évolution de Montpellier, Université de Montpellier, CNRS, IRD, EPHE, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France. romain.weppe@umontpellier.fr
Cécile Blondel. Laboratoire Paléontologie Évolution Paléoécosystèmes Paléoprimatologie: UMR 7262, Bât. B35 TSA 51106, 6 rue M. Brunet, 86073 Poitiers Cedex 9, France. cecile.blondel@univ-poitiers.fr
Monique Vianey-Liaud. Institut des Sciences de l’Évolution de Montpellier, Université de Montpellier, CNRS, IRD, EPHE, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France. monique.vianey-liaud@umontpellier.fr
Thierry Pélissié. Réserve naturelle nationale géologique du Lot, Parc régional et Géoparc mondial UNESCO Causses du Quercy, 46240 Labastide-Murat, France. tpelissie@parc-causses-du-quercy.org
Maëva Judith Orliac. Institut des Sciences de l’Évolution de Montpellier, Université de Montpellier, CNRS, IRD, EPHE, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France. maeva.orliac@umontpellier.fr
Keywords: new genus; new species; “Cainotherium plan”; Eocene; karstic infillings; occlusion
Final citation: Weppe, Romain, Blondel, Cécile, Vianey-Liaud, Monique, Pélissié, Thierry, and Orliac, Maëva Judith. 2020. A new Cainotherioidea (Mammalia, Artiodactyla) from Palembert (Quercy, SW France): Phylogenetic relationships and evolutionary history of the dental pattern of Cainotheriidae. Palaeontologia Electronica, 23(3):a54. https://doi.org/10.26879/1081
palaeo-electronica.org/content/2020/3216-new-species-of-cainotherioidea
Copyright: November 2020 Paleontological Society. This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/04B4D9ED-3FCB-49DA-B7B9-2ED04F8C30F3
INTRODUCTION
Cainotheriidae are small artiodactyls documented from Western European deposits. They appeared abruptly in the fossil record at the end of the Eocene (MP18-MP19) and, unlike many endemic European ungulates that disappeared around the Eocene-Oligocene transition, they successfully crossed the Grande Coupure (Stehlin, 1910) and diversified during the Early Oligocene and at the beginning of the Miocene. They became extinct during the Middle Miocene. The morphology of their brachydont selenodont molars is, moreover, unique among mammals: named by Stehlin (1906) the “Cainotherium plan”, the cainotheriids pattern is characterized by the presence of a distolingual protocone on the upper molars, as well as by a large mediolingual cuspid on the lower molars (Weppe et al., 2020).
The family Cainotheriidae is composed of five genera: Oxacron Filhol, 1884, Paroxacron Hürzeler, 1936, Plesiomeryx Gervais, 1873, Caenomeryx Hürzeler, 1936, and Cainotherium Bravard, 1828 (Blondel, 2005). Hürzeler (1936) proposed to gather Oxacron and Paroxacron, mainly known from Late Eocene deposits, in the subfamily Oxacroninae Hürzeler, 1936, while including the other three genera, mainly Oligocene, in the Cainotheriinae Camp and VanDerHoof, 1940; these two subfamilies were each recovered as monophyletic by Weppe et al. (2020). Despite the great abundance of cainotheriid remains in collections, works on cainotheriid remain scarce, particularly for Eocene localities (Legendre, 1980; Hooker and Weidmann, 2000; Weppe et al., 2020). The last 50 years of excavations in Quercy area yielded abundant remains of cainotheriids in a rather precise chronological framework and allowed to clarify certain episodes in the evolutionary history of the group (Legendre, 1980; Blondel, 2005; Weppe et al., 2020). However, the modalities of the emergence of this family are still poorly understood. The first occurrence of cainotheriids in the fossil record is the species Oxacron courtoisii, documented and described by Gervais (1859) in the locality of La Débruge (Vaucluse, France) referred to the MP18 level. The dental morphology of this earliest species is derived compared to other endemic European artiodactyls and already shows the “Cainotherium plan”.
Because of their unique dental morphology, the phylogenetic position of Cainotheriidae within both endemic European artiodactyls and artiodactyls is still debated, and this family has been related to various endemic European families (Romer, 1966; Webb and Taylor, 1980; Gentry and Hooker, 1988) without reaching a consensus. Similarly, others have linked them to modern groups of artiodactyls such as ruminants (Geisler and Uhen, 2005; O’Leary and Gatesy, 2008; Lihoreau et al., 2015) or tylopods (Geisler and Uhen, 2003; Geisler et al., 2007; Thewissen et al., 2007). However, the recent phylogenetic study of Weppe et al. (2020) retrieved Cainotheriidae closely related to the endemic European families Mixtotheriidae, Anoplotheriidae, and Robiacinidae. According to their results, Robiacinidae is sister taxon to Cainotheriidae and forms with the latter the super-family Cainotherioidea. This relationship, already proposed by Sudre (1969, 1977, 1978), is supported in part by a distal migration of the protocone on the upper molars. The family Robiacinidae is monogeneric (Robiacina Sudre, 1969) and includes three species: Robiacina minuta Sudre, 1969, Robiacina quercyi Sudre, 1977, and Robiacina lavergnensis Sudre, 1977. The family is documented in the fossil record in MP16-MP17 levels. It presents a close dental morphology to Cainotheriidae, notably at the level of premolars and on the presence of a protocone subcentral and lingual (M3/) on the upper molars (Weppe et al., 2020). However, Cainotheriidae are dentally way more derived than Robiacinidae, and 15 synapomorphies define the cainotheriid node (Weppe et al., 2020). The earliest stages of the dental history of cainotheriids is therefore not yet documented by existing species.
In this work we describe unpublished cainotherioid dental material collected in the 1980’s in the locality of Palembert (Quercy area, Tarn-et-Garonne, France; Crochet et al., 1981) close to the village of Caylus. The fossils were found in piles of excavated rocks extracted from a former phosphate infillings exploitation. The mammalian assemblage dated this locality from MP18-MP19 levels. The new material is here referred to Oxacron courtoisii (Gervais, 1852) and to a new cainotherioid taxon Palembertina deplasi gen. nov. sp. nov. showing an intermediate morphology between the “robiacinid” and the “derived cainotheriid” type. A phylogenetic analysis based on the taxon/characters matrix of Weppe et al. (2020), allows for discussing the phylogenetic affinities of this species and supports the proposed systematic framework. Palembertina deplasi gen. nov. sp. nov. provides a better understanding of the early evolutionary history of Cainotherioidea.
MATERIAL AND METHODS
Institutional and Anatomical Abbreviations
BMNH: British Museum of Natural History; PAL: specimen prefix for the Palembert locality in Montpellier University; MX/: upper molars; M/X: lower molars; PX/: upper premolars; P/X: lower molars.
Material
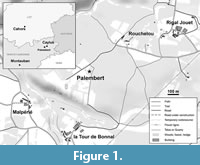 The material studied here comes from the locality of Palembert in the Quercy phosphorites area (South West of France) close to the village of Caylus (Tarn-et-Garonne, France), near to Malpérié and Rouchelou localities (Figure 1). The locality of Palembert is an area comprising various karstic excavations exploited at the end of the eighteenth century. The material was collected from excavated sediment piles mostly composed of clay, pisoliths, and calcareous fragments, disposed on one of the pits’ edge. Fossils were sorted out after washing and sieving the sediment with a 0,7 mm sieve. The Palembert material consists of six cainotherioid specimens: a right dentary fragment with P/3-M/3 (PAL 59) and a right dentary fragment with M/2-M/3 (PAL 61), referred to Oxacron courtoisii; a left M2/ (PAL 21), a right dentary fragment with M/3 (PAL 60), a right dentary fragment with P/4 and M/2 (PAL 62), and a right isolated M/3 (PAL 64), referred to Palembertina deplasi nov. gen. nov. sp. All these specimens are housed in the collection of Montpellier University. The age of the material described here was determined using biostratigraphic association of rodent species. The rodent assemblage documented in Palembert consists of the co-occurrence of Patriotheridomys altus? Vianey-Liaud, 1974, Pseudoltinomys cuvieri (Pomel, 1853), Suevosciurus indet. and Blainvillimys civracensis Vianey-Liaud and Ringeade, 1993, or Blainvillimys rotundidens Schlosser, 1884, pointing to a late Priabonian age. The scarcity and the partial preservation of the rodent material hindered the ability to precisely refer this locality to a Mammalian Paleogene (MP) reference level and the latter is here correlated with the MP18-MP19 levels.
The material studied here comes from the locality of Palembert in the Quercy phosphorites area (South West of France) close to the village of Caylus (Tarn-et-Garonne, France), near to Malpérié and Rouchelou localities (Figure 1). The locality of Palembert is an area comprising various karstic excavations exploited at the end of the eighteenth century. The material was collected from excavated sediment piles mostly composed of clay, pisoliths, and calcareous fragments, disposed on one of the pits’ edge. Fossils were sorted out after washing and sieving the sediment with a 0,7 mm sieve. The Palembert material consists of six cainotherioid specimens: a right dentary fragment with P/3-M/3 (PAL 59) and a right dentary fragment with M/2-M/3 (PAL 61), referred to Oxacron courtoisii; a left M2/ (PAL 21), a right dentary fragment with M/3 (PAL 60), a right dentary fragment with P/4 and M/2 (PAL 62), and a right isolated M/3 (PAL 64), referred to Palembertina deplasi nov. gen. nov. sp. All these specimens are housed in the collection of Montpellier University. The age of the material described here was determined using biostratigraphic association of rodent species. The rodent assemblage documented in Palembert consists of the co-occurrence of Patriotheridomys altus? Vianey-Liaud, 1974, Pseudoltinomys cuvieri (Pomel, 1853), Suevosciurus indet. and Blainvillimys civracensis Vianey-Liaud and Ringeade, 1993, or Blainvillimys rotundidens Schlosser, 1884, pointing to a late Priabonian age. The scarcity and the partial preservation of the rodent material hindered the ability to precisely refer this locality to a Mammalian Paleogene (MP) reference level and the latter is here correlated with the MP18-MP19 levels.
Measurements
Measurements (in millimetres) were taken using a mesuroscope Nikon 10 (binocular with measuring device). The general occlusal surface of the tooth is described by: L, the maximal mesiodistal length; and l, the maximal buccolingual width. The buccolingual aspect of the tooth is also expressed through two measurements: la, maximal width of mesial lobe; lp, maximal width of distal lobe. The height (h) corresponds to the maximal height of the crown (labial border for lower teeth and lingual border for upper teeth). L3 and l3, respectively, correspond to a maximal length and width of the third lobe (distal) of M/3. The variability of dental measures is estimated using the variation coefficient (V).
Phylogenetic Analysis
In order to test the phylogenetic position of Palembertina gen. nov. within Cainotherioidea, we performed a phylogenetic analysis based on the data matrix of Weppe et al. (2020). We modified five characters and included seven new ones. The character list and character processing (Appendix I), data matrix (Appendix II), and list of apomorphies (Appendix III) are provided in the online. Character coding is based on direct observations of specimens and on illustrations and descriptions from the following references: Gervais (1852); Gervais (1859); Stehlin (1906); Stehlin (1908); Matthew and Reed (1910); Hürzeler (1936); Berger (1959); Emry (1978); Sudre (1978); Gentry and Hooker (1988); Berthet (2003); Hooker and Weidmann (2000); Erfurt and Métais (2007); Hooker (2007); and Rincon et al. (2015). The matrix of characters was established with the software NDE 1.0 (Page, 2001). The phylogenetic analysis was performed with PAUP* version 4.0a166 (Swofford, 2020), using a heuristic search (>12 taxa) with TBR algorithm, and 1000 repetitions (10 trees retained by repetition; by stepwise addition).
Dental Nomenclature
The nomenclature of Boisserie et al. (2010; figure 2) and Weppe et al. (2020; figure 2) was used in this work to name crests/cristids and cuspids, respectively. It is detailed and figured in Appendix IV.
SYSTEMATIC PALAEONTOLOGY
Class MAMMALIA Linnaeus, 1758
Order ARTIODACTYLA Owen, 1848
Super-family CAINOTHERIOIDEA Camp and VanDerHoof, 1940
Family CAINOTHERIIDAE Camp and VanDerHoof, 1940
Emended diagnosis. Cainotherioidea with a size ranging from small rabbit-sized species as Romerolagus diazi (e.g., Palembertina, Oxacron, Paroxacron) to slightly smaller than the extant tragulid ruminant Tragulus javanicus (e.g., Cainotherium). Complete postorbital bar; enlarged bullae and orbits. Morphology of the mandible variable within the family but tends to become massive and deepen distally in several genera. Presence of diastemata within the premolar row in the Eocene species but tend to be reduced or lost (Cainotherium) in the Oligocene and Miocene forms. Lower incisors with two endocristulids; lower canine incisiform. Mesial premolars narrow to large; P/4 tends to be molarized; P1/ and P2/ without mesial lobe; P3/ triangular and labially concave, with a lingual cusp; P4/ triangular to sub-rectangular. Crowns of cheek teeth moderately high. Lower molars with a large and individualized centrolingual metaconulid; short to long mediocristid; centroconulid and postendocentrocristulid (neotrigonid of Sudre, 1977) present; absence of hypoconulid on M/1 and M/2. Upper molars sub-triangular to quadrangular with a “W”-shaped ectoloph and crescent-shaped lingual cusps; parastyle large like other styles; mediostyle and distostyle small to large; protocone subcentral to distal and paraconule mesiolingual; postparacristula present; lack of preprotocrista and paraconule junction. Limbs tetradactyl with a reduction of lateral digits (II and V); forelimb shorter than hind limb; tail long.
Remarks. The justification for the erection of the new genus and species Palembertina deplasi, and its integration in the Cainotheriidae family, is provided in the following section and follows the results of phylogenetic analysis.
Palembertina, new genus
zoobank.org/3C5849FC-3895-4100-8494-0ADFC2F617F7
Palembertina deplasi, new species
zoobank.org/AA39B90C-5786-457F-97B8-10D64E21CA10
Figure 2E-K, Figure 3C-D; Appendix V
Oxacron? courtoisii; Hooker and Weidmann 2000: 89 p., fig. 55j, (Left M1/ BMNH.30674, Bravard cat. no. 344, from La Débruge).
 Etymology. Palembertina refers to the Palembert locality. The species is named for Claude Deplas, who helped to discover many localities and fossils in the Quercy phosphorites.
Etymology. Palembertina refers to the Palembert locality. The species is named for Claude Deplas, who helped to discover many localities and fossils in the Quercy phosphorites.
Holotype. Left M2/ (PAL 21), from Palembert (Tarn-et-Garonne, France).
Paratype. Left dentary fragment with M/3 (PAL 60); left dentary fragment with P/4 and M/2 (PAL 62); right isolated M/3 (PAL 64), from Palembert (Tarn-et-Garonne, France).
Diagnosis. Genus of Cainotherioidea, similar in size to Oxacron (400-600g). Upper molar with a centrolingual protocone without crest; parastyle similar to other styles; looped and distally inclined mesostyle; distostyle and mediostyle isolated and large; short and continuous premetacristula; long postparacristula; mesial cingulum pronounced. P/4 with a talonid basin buccolingually compressed and a paraconid weakly lingually inclined. Lower molars with a small paraconid; strong lingual position of the protoconid; short mediocristid; talonid basin large with entoconid slightly more distal than hypoconid; large individualized metaconulid close to the metaconid; neotrigonid present; hypoconid absent on M/1 and M/2; large basin-shaped hypoconulid with a long and weakly marked posthypocristulid, and distal to the entoconid on M/3. Mandible with short diastema between P/2 and P/3; horizontal ramus gracile, relatively shallow and height is rather constant between the premolar area and the last molar; strong incisura vasorum.
Differential diagnosis. The upper molars of Palembertina gen. nov. differs from other Cainotheriidae by the presence of a centrolingual protocone without crests, by isolated and larger distostyle and mediostyle, by a larger mesial and distal cingulum, and by the absence of a distally inclined mesostyle. It differs also by the presence of a smaller talonid basin on the P/4. The lower molars of Palembertina gen. nov. are distinguished from those of other Caintotheriidae by a more lingual protoconid, a metaconulid closer to the metaconid with a shorter mediocristid, a larger talonid basin, a smaller centroconulid and shorter postendocentrocristulid, a less distal entoconid (close to the buccolingual position of hypoconid), and a basin-shaped hypoconulid more distal with a long posthypocristulid.
Palembertina gen. nov. differs from Robiacinidae on its upper molars by a stronger dilambdodonty, styles less crested, a looped mesostyle (postparacrista and premetacrista convex buccally), the presence of a postparacristula, the absence of preprotocrista, a shorter and continuous premetacristula, a longer preparacrista, the presence of a mediostyle and distostyle, and a larger mesial and distal cingulum. It differs also on its lower molars by an individual, larger and more distal metaconulid, the presence of a mediocristid and a neotrigonid, a slightly more distal entoconid (compared to buccolingual hypoconid position), and the absence of a hypoconulid on M/1 and M/2.
Material from Palembert. One left M2/ (PAL 21); one left mandibular fragment with M/3 (PAL 60); one left mandibular fragment with M/2 and P/4 (PAL 62); one right isolated M/3 (PAL 64). Palembert is located in the Quercy phosphorites area (South West of France) close to the village of Caylus (Tarn-et-Garonne, France).
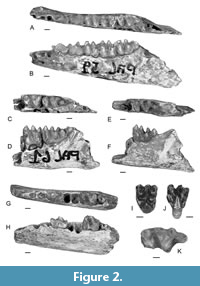
 Description. The upper dentition of Palembertina deplasi nov. gen. nov. sp. is only documented by the M2/ (PAL 21; Figure 2I-J, Figure 3C). It bears five main cusps. The paraconule is mesiolingual and bears a preparacristula connecting the preparacristyla; the metaconule is large and presents a short and continuous premetacristula, as well as a postmetacristula joining the postmetacristyla; the protocone lacks a crest and is centrolingually positioned. The styles are also well-pronounced: the buccal styles are weakly crested with a loop-like and distally inclined mesostyle; the lingual styles, distostyle and mediostyle, are isolated and large. Finally, the M2/ has pronounced mesial and distal cingula.
Description. The upper dentition of Palembertina deplasi nov. gen. nov. sp. is only documented by the M2/ (PAL 21; Figure 2I-J, Figure 3C). It bears five main cusps. The paraconule is mesiolingual and bears a preparacristula connecting the preparacristyla; the metaconule is large and presents a short and continuous premetacristula, as well as a postmetacristula joining the postmetacristyla; the protocone lacks a crest and is centrolingually positioned. The styles are also well-pronounced: the buccal styles are weakly crested with a loop-like and distally inclined mesostyle; the lingual styles, distostyle and mediostyle, are isolated and large. Finally, the M2/ has pronounced mesial and distal cingula.
The lower dentition of Palembertina deplasi nov. gen. nov. sp. is partly documented by a damaged P/4 and M/2, a complete, unworn M/3, and a damaged M/3. The crown of P/4 (PAL 62; Figure 2G-H) is short and narrow and presents three main cuspids: a large protoconid, metaconid, and paraconid. The protoconid and the metaconid are close to each other, the protoconid being more mesial than the metaconid. On the mesial part of the tooth, the paraconid is slightly inclined lingually. On the distal part, the talonid basin is buccolingually compressed and bears a small entoconid and a worn hypoconid. A postprotoconulid is visible between the protoconid and the hypoconid. The morphology of the lower molars is mainly based on M/3 (PAL 60, PAL 64; Figure 2E-F, 2K, Figure 3D), because of the poor preservation of the M/2 (PAL 62; Figure 2G-H). The lower molars present three lingual cuspids: a mesial metaconid, a sub-central metaconulid, and a distal entoconid. On the trigonid, the paraconid is small, and the protoconid is very lingual. The talonid is large with a small centroconulid close to the metaconulid, and a postendocentroconulid weakly expanded distolingually. The metaconulid is large, close to the metaconid, and the mediocristid is short. A large valley is present between the metaconulid and the entoconid. The entoconid has a loop-like shape and is slightly more distal than the hypoconid. M/3 (PAL 60, PAL 64; Figure 2E-F, 2K, Figure 3D) present a large hypoconulid, basin-shaped and distal to the entoconid. It connects to the rest of the talonid by the prehypocristulid, which joins the posthypocristulid and postentocristulid. Moreover, the posthypocristulid is weakly pronounced and extended lingually. Finally, the mesial cingulid is large.
The mandibular morphology of Palembertina deplasi nov. gen. nov. sp. is documented from the level of the second premolar (PAL 62; Figure 2G-H) mesially, to the base of the mandibular angle (PAL 60; Figure 2E-F) distally. The mandible (PAL 62) presents a short diastema between P/2 and P/3 and two mental foramina on the horizontal ramus, one below the mesial part of P/2 and one below the mesial part of P/4 (Figure 2H). The horizontal ramus is gracile, relatively shallow, and its height is rather constant between the premolar area and the last molar. A wide gap is present between the base of the ascending ramus and the distal margin of M/3 (Figure 2F). The angular apophysis is not preserved but seems to be medially inclined; it is mesially delimited by a shallow incisura vasorum (PAL 60).
Sub-family OXACRONINAE Hürzeler, 1936
Genus Oxacron Filhol, 1884
Oxacron courtoisii (Gervais, 1852)
Figure 2A-D; Appendix VI
Cainotherium courtoisii; Gervais, 1852: 80 p., plate XXXIV fig. 6 and plate XXXV fig. 4.
Cainotherium courtoisii; Gervais, 1859: 162 p., plate XXXIV fig. 6, plate XXXV fig. 4.
Plesiomeryx quinquedentatus; Filhol, 1877: 430 p., fig. 314-316.
Oxacron minimus; Filhol, 1884: 64 p.
Oxacron courtoisii (Gervais, 1852); Stehlin, 1906: 677 p., fig. XCIV-XCVI.
Lectotype. Left mandibular fragment with P/3-M/3 (Gervais, 1852), from the lignites of La Débruge (MP18; Vaucluse, France).
Paralectotype. Left maxillary fragment with P4/-M3/ (Gervais, 1852), from the lignites of La Débruge (MP18; Vaucluse, France).
Remarks. Gervais (1852) erected the species Cainotherium courtoisii, and Stehlin (1906) subsequently placed it in the genus Oxacron. Gervais (1852) described and figured the species based on the small sized cainotheriids specimens from La Debruge but did not define a holotype. According to the International Code of Zoological Nomenclature, the name Oxacron courtoisii is available but not valid; therefore, we define here a lectotype and a paralectotype for Oxacron courtoisii, corresponding to the material from La Debruge originally figured by Gervais (1852): left mandibular fragment with P/3-M/3 (plate XXXV, fig. 4) for the lectotype; left maxillary fragment with P4/-M3/ (plate XXXIV, fig. 6) for the paralectotype.
Emended Diagnosis. Sole species of Oxacron, with diastemata short to long between P1/-P2/ and P/2-P/3, and very short to absent between P/1-P/2 and C-P/1. Premolars short; P1/, P/1 and P/2 caniniforms; P3/ with short mesial lobe and small protocone; P4/ triangular and narrow buccolingually; upper molars subtriangular, slightly distally inclined, with small paraconule. Mandible with a gracile and relatively shallow horizontal ramus of constant height between the premolar area and the last molar; coronoid process of the mandible high above the occlusal surface; mandibular condyle relatively long mediolaterally; angular apophysis slightly extended ventrally and elongated distally; strong incisura vasorum. Cranium without ethmoidal fissures; rostrum elongated and mediolaterally constricted; contact maxillofrontal large; sagital crest lowly pronounced; choane large; basisphenoid apically rounded.
Differential diagnosis. Oxacron differs from Caintheriinae by the presence of subtriangular upper molars without crested styles, a shorter postparacristula, narrow premolars, a weaker postprotoconulid, a biradiculate P/1, diastemata between P/2-P/3 and P1/-P2/, and a protocone more mesial than the paracone on P4/. It differs also from Plesiomeryx and Caenomeryx species by smaller size, a smaller protocone on P3/, as well as the absence of a protocone on P2/. It differs from Cainotherium by smaller size, the absence of cingula/ids on premolars, a triangular P3/, and the presence of a valley between the entoconid and the mesoconid on lower molars. Oxacron is distinguished from the other Oxacroninae Paroxacron by shorter premolars, a P1/, P/1, and P/2 caniniforms, a shorter mesial lobe on the P3/, a triangular P4/, as well as a smaller paraconule on the upper molars. Finally, Oxacron differs from Palembertina gen. nov. by many characters (see above).
Material from Palembert. One right dentary fragment with P/3-M/3 (PAL 59); one right dentary fragment with M/2-M/3 (PAL 61). Palembert is located in the Quercy phosphorites area (South West of France) close to the village of Caylus (Tarn-et-Garonne, France).
Description. Oxacron courtoisii from Palembert is only documented by its lower dentition with two fragmentary dentaries. According to the alveola pattern of PAL 59 (Figure 2A-B), the P/1 is biradiculate. Very short diastemata are present between P/1-P/2 and P/2-P/3. P/3 and P/4 are short. The P/3 presents a strong protoconid and a short distal cristid lingually curved. The P/4 shows a protoconid more mesial than the metaconid. Its talonid basin is large with a small postprotoconulid and a small hypoconid. The entoconid is very weak.
The lower molars (PAL 59, PAL 61; Figure 2A-D) present the “Caintotherium plan” with three large lingual cuspids: a mesial metaconid, a medial metaconulid, and a distal entoconid. The paraconid is small. The metaconulid is large, far from the metaconid and separated by a long mediocristid. The talonid is bucco-lingually constricted with a large centroconulid and a postendocentroconulid expanded distolingually. The entoconid is looped and more distal than the hypoconid. The M/3 presents a large hypoconulid without a posthypocristulid. The mesial cingulid is rather large.
The mandible (PAL 59) shows two mental foramina on the horizontal ramus, one below the mesial part of P/2 and one below the mesial part of P/4 (Figure 2B). The horizontal ramus is gracile and shallow, and its height is rather constant between the premolar area and the last molar. The angular apophysis, not preserved, is mesially delimited by a strong incisura vasorum (PAL 59, PAL 60; Figure 2B, 2D).
RESULTS
Phylogenetic Analysis
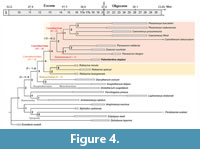 The cladistic analysis retained a single parsimonious tree (length = 183, Consistency Index [CI] = 0.48, Retention Index [RI] = 0.73, CI with only parsimony-informative characters = 0.45; Figure 4), reflecting a well-structured signal and relatively weak homoplasy. The topology is congruent with that of Weppe et al. (2020), the new taxon Palembertina deplasi nov. gen. nov. sp. finding its place within the monophyletic Cainotheriidae, as the sister taxon to the clade C (Oxacroninae, Cainotheriinae). The latter gathers two subfamilies: Oxacroninae, with Oxacron and Paroxacron; and Cainotheriinae, with Plesiomeryx, Caenomeryx, and Cainotherium.
The cladistic analysis retained a single parsimonious tree (length = 183, Consistency Index [CI] = 0.48, Retention Index [RI] = 0.73, CI with only parsimony-informative characters = 0.45; Figure 4), reflecting a well-structured signal and relatively weak homoplasy. The topology is congruent with that of Weppe et al. (2020), the new taxon Palembertina deplasi nov. gen. nov. sp. finding its place within the monophyletic Cainotheriidae, as the sister taxon to the clade C (Oxacroninae, Cainotheriinae). The latter gathers two subfamilies: Oxacroninae, with Oxacron and Paroxacron; and Cainotheriinae, with Plesiomeryx, Caenomeryx, and Cainotherium.
The monophyly of Cainotheriidae, including Palembertina gen. nov., is supported by nine non-ambiguous synapomorphies (Bremer index [BI] = 4). They are characterized by the presence on their upper molars, of: a large parastyle similar to other styles (81; RI = 0.67), a distostyle (111; RI = 0.89), a very strong dilambdodonty (221; RI = 0.88), a postparacristula (311; RI = 0.86), and a lack of preprotocrista and paraconule junction (170; RI = 0.67); on their lower molars, they present: a large (361; RI = 1.000) and individualized metaconulid (371; RI = 1.00), a neotrigonid (511; RI = 1.00), and the absence of hypoconulid on M/1 and M/2 (410; RI = 0.75). Within Cainotheriidae, Palembertina gen. nov. is the first offshoot and appears as the sister taxon to the clade C (Oxacroninae, Cainotheriinae). It shows three autapomorphies: a short diastema between P/2-P/3 (61; RI = 0.33), as well as a looped mesostyle (100; RI = 0.50) and a large distostyle (121) on upper molars. The clade C (BI = 2) is supported by six non-ambiguous synapomorphies, on upper molars: a distal protocone (132; RI = 0.94), a small cingulum surrounding the protocone (201; RI = 0.58), and a long postprotocrista (281; RI = 0.50); on lower molars: a very distal entoconid (432; RI = 1.00), and the absence of a basin-shaped hypoconulid (400; RI = 0.75) and of posthypocristulid (550; RI = 0.67) on M/3. Within clade C, Oxacroninae (BI=1) are supported by a protocone more mesial than the paracone on P4/ (621; RI = 0.50) and a biradiculate P/1 (771; RI = 1.00), and Cainotheriinae (BI = 1) are supported by a postparacristula buccally long joining the preprotocrista on upper molars (321; RI = 1.00) and a large postprotoconulid on lower premolars (761; RI = 1.00).
Robiacinidae are the sister taxon to Cainotheriidae and forms with them the superfamily Cainotherioidea. This position is supported by six non-ambiguous synapomorphies (BI = 4): a more distal (131-2; RI = 0.94) and lingual protocone (191; RI = 0.50) on upper molars and M3/, respectively, a sub-equal paracone and metacone on P3/ (661; RI = 1.00), the absence of protocone on P2/ (700; RI = 0.57), a small hypoconid on P/2-P/3 (740; RI = 0.75), and the presence of a postprotoconulid on the lower premolars (751; RI = 1.00). Robiacinidae (BI = 2) are characterized by the presence of a small paraconule (160; RI = 0.43) and a short preparacrista (271; RI = 1.00) on upper molars, as well as the presence of a metaconid on the P/3 (72 1; RI = 0.50).
Relationships outside Cainotherioidea are similar to that retrieved by Weppe et al. (2020). The Bremer index of some nodes is, however, higher in this new phylogenetic analysis.
DISCUSSION
Taxonomic Attribution of Palembert Cainotheriid Material
Two species of cainotherioid are described from the locality of Palembert: Palembertina deplasi gen. nov. sp. nov. (PAL 21, PAL 60, PAL 62, and PAL 64; Figure 2E-K, Figure 3C-D) and Oxacron courtoisii (PAL 59 and PAL 61; Figure 2A-D). These species are very similar in size (Appendix V, Appendix VI), but our decision to distinguish two species is based on numerous morphological differences: i) on the premolar proportion: the P/4 (PAL 62; Figure 2G) referred to P. deplasi gen. nov. sp. nov. presents a talonid basin strongly compressed buccolingually and a paraconid slightly inclined lingually, while the P/4 of O. courtoisii (PAL 59; Figure 2A) shows a large talonid basin and a paraconid strongly inclined lingually; ii) on the pattern structure of the upper molars: the material attributed to P. deplasi gen. nov. sp. nov. (PAL 21; Figure 2I-J, Figure 3C) presents a paraconule large and lingual, a protocone without crest in a centrolingual position, as well as large and individualized lingual styles. In contrast, the upper molars of O. courtoisii (“Cainotherium plan”; Figure 3E), not documented in the material of Palembert but present in other Quercy localities, bear a small paraconule, a crested distal protocone, and small lingual styles; iii) on the pattern of the lower molars structure: P. deplasi gen. nov. sp. nov. (PAL 60, PAL 62 and PAL 64; Figure 2E-H, 2K, Figure 3D) present molars with a trigonid and a talonid buccolingually large, a metaconulid in a mediolingual position, a neotrigonid weakly pronounced, and a crested basin-shaped hypoconulid. By opposition, PAL 59 and PAL 61 (Figure 2A-D) referred to O. courtoisii show molars with a compressed buccolingually trigonid and talonid, a more distal metaconulid, a neotrigonid with a large centroconulid and a long postendocentroconulid, as well as a hypoconulid without cristid (“Cainotherium plan”: Figure 3F); iv) on the mandiblar corpus: P. deplasi gen. nov. sp. nov. (PAL 60; Figure 2F) shows an angular apophysis with an incisura vasorum slightly marked and a wide gap between the base of the ascending ramus and the distal margin of M/3, while the mandibles of O. courtoisii (PAL 59 and PAL 61; Figure 2B, 2D) present a strongly marked incisura vasorum and a faintly visible gap. The number of specimens of P. deplasi gen. nov. sp. nov. is, however, small, and it is unfortunately not possible to discuss the intraspecific variability of this species. Intraspecific variation in the different cainotheriid species is generally manifested at the level of the length of the diastemata, the height of the mandibular corpus, and the degree of incisura vasorum marking.
Referral of the mandibles PAL 59 and PAL 61 (Figure 2A-D) to the species Oxacron courtoisii relies on the following characters: a biradiculate P/1, diastemata between P/2-P/3 and P/1-P/2, short and shallow premolars, a “Cainotherium plan” on the lower molars, and a strong incisura vasorum on the mandibular corpus. This association of characters is diagnostic of Oxacron courtoisii (Erfurt and Métais, 2007; Weppe et al., 2020). The specimens of Palembert are morphologically and biometrically similar to those described by Gervais (1852; plate XXXV, fig. 4; lectotype), Hürzeler (1936; fig. 67), and Legendre (1980) (Escamps (MP19; Quercy)), and referred to as Oxacron courtoisii. They are also similar to those from other Quercy localities: Rosière 1-4 (MP 19), Sindou D (MP 19), Tabarly (MP 20) and Pecarel (MP 20) referred to this species (Remy et al., 1987).
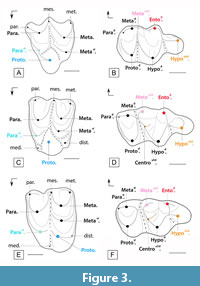
This study describes a new cainotherioid taxon: Palembertina deplasi nov. gen. nov. sp. The association between the isolated upper molar PAL 21 (Figure 2I-J, Figure 3C) and the lower dentition (mandibles PAL 60, PAL 62, and the isolated M/3 PAL 64; Figure 2E-H, 2K, Figure 3D) and the attribution to the same species has been validated by testing their occlusion (see Figure 5B, as paraconule and metaconulid). Palembertina gen. nov. presents a unique combination of characters, showing an intermediate morphology between the “robiacinid” (Figure 3A-B) and the “derived cainotheriid” pattern ("Cainotherium plan"; Figure 3E-F). It shows both: i) plesiomorphic characters retrieved the Robiacinidae: the protocone is centrolingual on the upper molars; on the lower molars, the talonid is large, the hypoconulid is in basin-shaped with a long posthypocristulid, and the entoconid is lowly distal; and on the P/4, the talonid basin is buccolingually compressed; ii) derived characters of the Cainotheriidae: on the upper molars, the dilambdodonty is strong, the parastyle size is similar to other styles, lingual styles (mediostyle and distostyle), and a postparacristula are present, and a preprotocrista and paraconule junction is absent; on the lower molars, the metaconulid is large, individualized and more distal, a neotrigonid is present, and a hypoconulid on M/1-M/2 is absent (Weppe et al., 2020; this study). We propose here to include Palembertina gen. nov. within the family Cainotheriidae. This contrasts with the definition of Stehlin (1906) who defined the Cainotheriidae by the presence of a distal protocone on the upper molars (“Cainotherium plan”; Figure 3E), which is not the case in Palembertina gen. nov. Indeed, according to our cladistic analysis, the character state “distal protocone” (132; RI = 0.94) is acquired at the node C, which could have been a good candidate as the Cainotheriidae node. However, we decided to include Palembertina gen. nov. within Cainotheriidae based on the sharing of nine non-ambiguous synapomorphies (see above; BI = 4), among which some characteristics are unique to this family: on upper molars, the presence of a mediostyle and distostyle; on lower molars, the presence of a large and individualized metaconulid, of a mediocristid, and of a neotrigonid. Palembertina gen. nov. therefore, represents the first offshoot of the Cainotheriidae family and differs from the other members of the family by i) retaining the plesiomorphic state for the six non-ambiguous synapomorphies defining the clade C (see above and phylogenetic analysis), gathering Oxacroninae and Cainotheriinae and ii) showing unique features (three autapomorphies), such as the presence large lingual styles (mediostyle and distostyle) on the upper molars, unique within artiodactyls. These characters altogether (see also Systemactic Palaeontology) led us to erect Palembertina deplasi as a new genus and a new species.
Early Evolutionary History of Cainotheriidae
Cainotheriidae appeared abruptly in the fossil record in the middle levels of the Priabonian with Oxacron courtoisii from La Débruge (MP18; Gervais (1852)) and knew then an “amazing evolutionary success” (translated from Sudre (1977): 220). As soon as their first occurrence in the fossil record, the dental morphology of Cainotheriidae is highly derived and unique within artiodactyls: i) their upper molars present a five-cusps pattern, with two mesial and three distal cusps (Figure 3E), instead of the general artiodactyl pattern with three mesial and two distal cusps; ii) their lower molars present three well-developed and individualized lingual cuspids instead of the two generally observed in Artiodactyla (metaconid and entoconid). The “Cainotherium plan” therefore implies a rearrangement of upper molar cusps and an increase of the number of cuspids on lower molars.
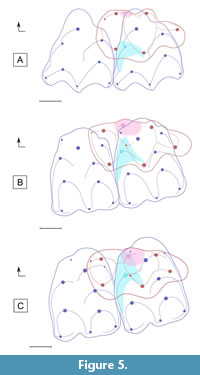 Different hypotheses of cusps/cuspids homology have been proposed to explain the “Cainotherium plan” (for a general review see Weppe et al., 2020). According to Stehlin (1910) and Hürzeler (1936), the cainotheriid morphology of the upper molars results from a distal migration of the protocone and a lingual migration of the paraconule. The peculiar pattern of the lower molars, according to Sudre (1977) and Weppe et al. (2020), results from the development and distal migration of a supernumerary cuspid (metaconulid; Weppe et al., 2020) originating from the metaconid. The dental morphology of the molars of the Robiacinidae (Figure 3A-B), sister group to Cainotheriidae, allows for a polarization of the dental characters of the molars and provides the start of an answer to understand the modalities of the cainotheriid pattern evolution (Figure 5, Figure 6). Compared to other Palaeogene artiodactyls, the protocone is displaced distally on the upper molars (centrolingual), and the lower molars bear a small metaconulid in contact with the metaconid (this character is also present in Anoplotheriidae; Weppe et al., 2020 and this study). However, a great morphological gap (Figure 3) remains between Robiacinidae and derived Cainotheriidae (Cainotheriinae and Oxacroninae; “Cainotherium plan”). The new genus Palembertina described in this study documents an early stage of the evolutionary history of the cainotheriid dental pattern. Indeed, the upper molars of Palembertina deplasi nov. gen. nov. sp. (Figure 3C-D) present a plesiomorphic pattern with a centrolingual location of the protocone, similar to Robiacinidae, while the lower molars present a more derived morphology similar to derived Cainotheriidae with, notably, a large metaconulid (see taxonomic attribution of palembert cainotheriid material for other characters). The “Cainotherium plan” of derived Cainotheriidae (Figure 3E-F) is then formed by a more distal migration of the protocone and metaconulid on the upper and lower molars.
Different hypotheses of cusps/cuspids homology have been proposed to explain the “Cainotherium plan” (for a general review see Weppe et al., 2020). According to Stehlin (1910) and Hürzeler (1936), the cainotheriid morphology of the upper molars results from a distal migration of the protocone and a lingual migration of the paraconule. The peculiar pattern of the lower molars, according to Sudre (1977) and Weppe et al. (2020), results from the development and distal migration of a supernumerary cuspid (metaconulid; Weppe et al., 2020) originating from the metaconid. The dental morphology of the molars of the Robiacinidae (Figure 3A-B), sister group to Cainotheriidae, allows for a polarization of the dental characters of the molars and provides the start of an answer to understand the modalities of the cainotheriid pattern evolution (Figure 5, Figure 6). Compared to other Palaeogene artiodactyls, the protocone is displaced distally on the upper molars (centrolingual), and the lower molars bear a small metaconulid in contact with the metaconid (this character is also present in Anoplotheriidae; Weppe et al., 2020 and this study). However, a great morphological gap (Figure 3) remains between Robiacinidae and derived Cainotheriidae (Cainotheriinae and Oxacroninae; “Cainotherium plan”). The new genus Palembertina described in this study documents an early stage of the evolutionary history of the cainotheriid dental pattern. Indeed, the upper molars of Palembertina deplasi nov. gen. nov. sp. (Figure 3C-D) present a plesiomorphic pattern with a centrolingual location of the protocone, similar to Robiacinidae, while the lower molars present a more derived morphology similar to derived Cainotheriidae with, notably, a large metaconulid (see taxonomic attribution of palembert cainotheriid material for other characters). The “Cainotherium plan” of derived Cainotheriidae (Figure 3E-F) is then formed by a more distal migration of the protocone and metaconulid on the upper and lower molars.
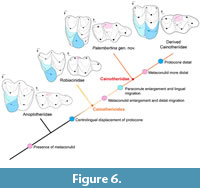 Concurrently to the “Cainotherium plan”, the Cainotheriidae also present a peculiar pattern of occlusion, directly linked to cusp/cuspids size and location (Figure 5). Robiacina shows rather triangular outlines of the upper molars related to the small size of the paraconule (Figure 3A, Figure 5A) while the Palembertina gen. nov. and more derived cainotheriid show more quadrangular upper molars with a bigger, more lingual, paraconule (Figure 3C, 3E, Figure 5B-C). In occlusion (Figure 5), the paraconule is interposed between the two mesiolingual cuspids of the lower molars (metaconid and metaconulid), and changes in size and position of these structures are related. The lingual development of the paraconule in Palembertina gen. nov. corresponds with the enlargement and distal displacement of the metaconulid on lower molars (Figure 5B). This tendency goes on to an extreme degree in more derived cainotheriids, accompanied by the fully distal migration of the protocone and metaconulid on the upper and lower molars (Figure 5C). The famous “Cainotherium plan” therefore results from a succession of dental changes (Figure 6); we agree with Sudre (1977) that the paraconule can be regarded as a key feature in the achievement of this pattern (Figure 5).
Concurrently to the “Cainotherium plan”, the Cainotheriidae also present a peculiar pattern of occlusion, directly linked to cusp/cuspids size and location (Figure 5). Robiacina shows rather triangular outlines of the upper molars related to the small size of the paraconule (Figure 3A, Figure 5A) while the Palembertina gen. nov. and more derived cainotheriid show more quadrangular upper molars with a bigger, more lingual, paraconule (Figure 3C, 3E, Figure 5B-C). In occlusion (Figure 5), the paraconule is interposed between the two mesiolingual cuspids of the lower molars (metaconid and metaconulid), and changes in size and position of these structures are related. The lingual development of the paraconule in Palembertina gen. nov. corresponds with the enlargement and distal displacement of the metaconulid on lower molars (Figure 5B). This tendency goes on to an extreme degree in more derived cainotheriids, accompanied by the fully distal migration of the protocone and metaconulid on the upper and lower molars (Figure 5C). The famous “Cainotherium plan” therefore results from a succession of dental changes (Figure 6); we agree with Sudre (1977) that the paraconule can be regarded as a key feature in the achievement of this pattern (Figure 5).
CONCLUSIONS
This study presents the first description of a new cainotherioid, Palembertina deplasi nov. gen. nov. sp. from the locality of Palembert (Quercy, France). It documents an early stage in the evolutionary history of the dental pattern of cainotheriids and shows an intermediate morphology between the “robiacinid” type and “derived cainotheriid” type (“Cainotherium plan”). The dental morphology of Palembertina gen. nov. allows us to understand better the evolution of the dental pattern of cainotheriids, defining the development and the lingual migration of the paraconule of the upper molars as a key feature of its formation.
Our phylogenetic analysis, based mainly on the matrix of Weppe et al. (2020), places Palembertina gen. nov. as the sister group to the clade including Cainotheriinae and Oxacroninae. Because of the number and non homoplasic nature of synapomorphies supporting their node, we consider Palembertina gen. nov. as an early offshoot of the Cainotheriidae family.
ACKNOWLEDGEMENTS
We are grateful to S. Legendre (LEHNA, Lyon) and J. Sudre (ISEM, Montpellier) for his preliminary work on the Palembert material and to all of the team of the Cloup d’Aural and the Quercy research team (M. Godinot, MNHN, Paris; ISEM, Montpellier; G. Escarguel, LEHNA, Lyon; C. Bousquet, Cloup d’Aural) for their work in the field. We thank S. Jiquel for her help in the Montpellier University’s collections. We are also grateful to S. Agret and L. Marivaux for kindly welcoming us in their office to take measurements and photos of the material. Finally, we acknowledge the anonymous reviewers for their constructive comments. This work was financially supported by the ANR program DEADENDER (ANR- 18-CE02-0003-01) - P.I. M. J. Orliac. This is ISEM publication 2020-168.
REFERENCES
Antunes, M.T. 1986. Anoplotherium (mammalia, artiodactyla) et geochelone (reptilia, testudines) à côja: les vertébrés fossiles et l’éocène supérieur au Portugal. Ciências da Terra, 99-110. http://hdl.handle.net/10362/3287
Berger, F.E. 1959. Untersuchungen an Schädel und Gebissresten von Cainotheriidae. Palaeontographica, Abteilung A, 112:1-58. https://www.schweizerbart.de/papers/pala/detail/A112/70560
Berthet, D. 2003. Le genre Cainotherium (Mammalia, Artiodactyla): étude morphométrique, révision systématique, implications évolutives et paléobiogéographiques, extinction. Unpublished PhD Thesis, University of Lyon 1, France. https://www.theses.fr/2003LYO10067
Blondel, C. 1997. Les ruminants de Pech Desse et de Pech du Fraysse (Quercy; MP28); évolution des ruminants de l’Oligocène d’Europe. Geobios, 30(4):573-591. https://doi.org/10.1016/s0016-6995(97)80123-4
Blondel, C. 2005. New data on the Cainotheriidae (Mammalia, Artiodactyla) from the early Oligocene of south-western France. Zoological Journal of the Linnean Society, 144(2):145-166. https://doi.org/10.1111/j.1096-3642.2005.00166.x
Boisserie, J.R., Lihoreau, F., Orliac, M.J., Fisher, R.E., Weston, E.M., and Ducrocq, S. 2010. Morphology and phylogenetic relationships of the earliest known hippopotamids (Cetartiodactyla, Hippopotamidae, Kenyapotaminae). Zoological Journal of the Linnean Society, 158(2):325-366. https://doi.org/10.1111/j.1096-3642.2009.00548.x
Bravard, A. 1828. Monographie de la Montagne de Perrier, près d’Issoire (Puy-de-Dome) et de deux espèces fossiles du genre Felis, découvertes dans l’une de ses couches d'alluvion: avec une carte et deux planches. Dufour et Docagne, Paris. https://reader.digitale-sammlungen.de/de/fs1/object/display/bsb10283074_00010.html
Camp, C.L. and VanDerHoof, V.L. 1940. Bibliography of fossil vertebrates, 1928-1933. Geological Society America Special Papers, 27:1-503. https://doi.org/10.1130/spe27-p1
Crochet, J.Y., Hartenberger, J.L., Rage, J.C., Rémy, J.A., Sigé, B., Sudre, J., and Vianey-Liaud, M. 1981. Les nouvelles faunes de vertébrés antérieures à la « Grande Coupure » découvertes dans les phosphorites du Quercy. Bulletin du Muséum national d’Histoire naturelle, 4(3):255-266.
Da Silva Pires, A. 2008. Géoportail. Le portail Internet des territoires et des citoyens, Vanves, Foucher. https://www.geoportail.gouv.fr
Emry, R.J. 1978. A new hypertragulid (Mammalia, Ruminantia) from the early Chadronian of Wyoming and Texas. Journal of Paleontology, 52(5):1004-1014. https://www.jstor.org/stable/1303846
Erfurt, J. and Métais, G. 2007. Endemic European Paleogene artiodactyls: Cebochoeridae, Choeropotamidae, Mixtotheriidae, Cainotheriidae, Anoplotheriidae, Xiphodontidae, and Amphimerycidae, p. 59-84. In Prothero, D.R. and Foss, S.E. (eds.), The Evolution of Artiodactyls. Johns Hopkins University Press, Baltimore.
https://jhupbooks.press.jhu.edu/title/evolution-artiodactyls
Filhol, H. 1877. Recherches sur les phosphorites du Quercy: étude des fossiles qu’on y rencontre et spécialement des mammifères. Annales de la société géologique de France, 8:1-340.
Filhol, H. 1884. Observations relatives à des mammifères fossiles nouveaux provenant des dépôts de phosphate de chaux du Quercy. Bulletin des Sciences physiques et naturelles de Toulouse, 5(2):159-203.
Geisler, J.H., Theodor, J.M., Uhen, M.D., and Foss, S.E. 2007. Phylogenetic relationships of cetaceans to terrestrial artiodactyls, p. 19-31. In Prothero, D.R. and Foss, S.E. (eds.), The Evolution of Artiodactyls. Johns Hopkins University Press, Baltimore. https://doi.org/10.1007/s10914-005-4963-8
Geisler, J.H. and Uhen, M.D. 2003. Morphological support for a close relationship between hippos and whales. Journal of Vertebrate Paleontology, 23(4):991-996. https://doi.org/10.1671/32
Geisler, J.H. and Uhen, M.D. 2005. Phylogenetic relationships of extinct cetartiodactyls: results of simultaneous analyses of molecular, morphological, and stratigraphic data. Journal of Mammalian Evolution, 12:145-160. https://doi.org/10.1007/s10914-005-4963-8
Gentry, A.W. and Hooker, J.J. 1988. The phylogeny of the Artiodactyla, p. 235-272. In Benton M.J. (ed.), The Phylogeny and Classification of the Tetrapods: Mammals. Clarendon Press, Oxford.
Gervais, P. 1852. Zoologie et paléontologie françaises (animaux vertébrés): ou nouvelles recherches sur les animaux vivants et fossiles de la France. Arthus Bertrand, Paris.
https://doi.org/10.5962/bhl.title.39473
Gervais, P. 1859. Zoologie et paléontologie françaises: nouvelles recherches sur les animaux vertébrés dont on trouve les ossements enfouis dans le sol de France et sur leur comparaison avec les espèces propres aux autres régions du globe. Arthus Bertrand, Paris. https://numelyo.bm-lyon.fr/f_view/BML:BML_00GOO0100137001100032726#
Gervais, P. 1873. Mammifères dont les ossements accompagnent les dépôts de chaux phosphatée des départements du Tarn-et-Garonne et du Lot. Journal de Zoologie (Paris), 2:356-380. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=GEODEBRGMFR2030192
Hooker, J.J. 2007. Bipedal browsing adaptations of the unusual Late Eocene-earliest Oligocene tylopod Anoplotherium (Artiodactyla, Mammalia). Zoological Journal of the Linnean Society, 151(3):609-659. https://doi.org/10.1111/j.1096-3642.2007.00352.x
Hooker, J.J. and Weidmann, M. 2000. The Eocene mammal faunas of Mormont, Switzerland: systematic revision and resolutions of dating problems. Schweizerische Paläontologische Abhandlungen, 120:1-143.
Hürzeler, J. 1936. Osteologie und odontologie der Caenotheriden. Abhandlungen der schweizerischen paläeontologischen, Gesellschaft, 58-59:1-111.
Legendre, S. 1980. Etude du gisement de Port-la-Nouvelle; étude des Cainotheriidae d’Escamps. Unpublished DEA of Paleontology, University of Montpellier, France.
Lihoreau, F., Boisserie, J.R., Manthi, F.K., and Ducrocq, S. 2015. Hippos stem from the longest sequence of terrestrial cetartiodactyl evolution in Africa. Nature Communications, 6(6264):1-8. https://doi.org/10.1038/ncomms7264
Linnæus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima tertia. Laurentius Salvius, Holmia. https://www.biodiversitylibrary.org/item/10277#page/3/mode/1up
Matthew, W.D. and Reed, W.H. 1910. On the skull of Apternodus and the skeleton of a new artiodactyl. Bulletin of the American Museum of Natural History, 28(5):33-42.
https://www.biodiversitylibrary.org/bibliography/89572#/summary
Ménouret, B. 2014. Gisements paléontologiques à mammifères ou empreintes de pas de mammifères du Parc naturel régional du Luberon. Courrier scientifique du Parc naturel régional du Luberon et de la Réserve de biosphère Luberon-Lure, 12:56-74. http://documents.irevues.inist.fr/handle/2042/58152
O’Leary, M.A. and Gatesy, J. 2008. Impact of increased character sampling on the phylogeny of Cetartiodactyla (Mammalia): combined analysis including fossils. Cladistics, 24(4):397-442. https://doi.org/10.1111/j.1096-0031.2007.00187.x
Owen, R. 1848. Description of teeth and portions of jaw of two extinct anthracotherioid quadrupeds (Hyopotamus vectianus and Hyop. bovines) discovered by the Marchioness of Hastings in the Eocene deposits on the NW coast of the Isle of Wight: with an attempt to develop Cuvier's idea of the classification of pachyderms by the number of their toes. Quarterly Journal of the Geological Society of London, 4:103-141.
https://doi.org/10.1144/gsl.jgs.1848.004.01-02.21
Page, R.D. 2001. Nexus Data Editor. Version 0.5.0. Glasgow.
Pomel, A. 1853. Catalogue méthodique et descriptif des vertébrés fossiles découverts dans le bassin hydrographique supérieur de la Loire et surtout dans la vallée de son affluent principal de l’Allier. Baillière, Paris. https://gallica.bnf.fr/ark:/12148/bpt6k97953010.texteImage
Prothero, D.R. 1998. Oromerycidae, p. 426. In Janis, C.M., Scott, K.M., Jacobs, L.L., Gunnell, G.F., and Uhen, M.D. (eds.), Evolution of Tertiary Mammals of North America: Volume 1, Terrestrial Carnivores, Ungulates, and Ungulate Like Mammals. Cambridge University Press, Cambridge.
Remy, J.A, Crochet, J.Y., Sigé, B., Sudre, J., De Bonis, L., Vianey-Liaud, M., Godinot, M., Hartenberger, J.L., Lange-Badré, B., and Comte, B. 1987. Biochronologie des phosphorites du Quercy: mise à jour des listes fauniques et nouveaux gisements de mammifères fossiles. Münchner Geowissenschaftliche Abhandlungen, 10(A):169-188.
Rincon, A.F., Bloch, J.I., MacFadden, B.J., and Jaramillo, C.A. 2015. New early Miocene protoceratids (Mammalia, Artiodactyla) from Panama. Journal of Vertebrate Paleontology, 35(5):1-22. https://doi.org/10.1080/02724634.2015.970688
Romer, A.S. 1966. Vertebrate Paleontology. Third Edition. University of Chicago Press, Chicago.
Schlosser, M. 1884. Die Nager des Europäischen Tertiärs: nebst Betrachtungen über die Organisation und die geschichtliche. Palaeontographica, 21:1-143.
https://www.schweizerbart.de/papers/palae/detail/31/60902/Die_Nager_des_europaischen_Tertiars_nebst_Betrachtungen_uber_die_Organisation_und_die_geschichtliche_Entwicklung_der_Nager_uberhaupt
Stehlin, H.G. 1906. Die Säugetiere des schweizerischen Eocaens. Abhandlungen der schweizerischen paläontologischen Gesellschaft, 33: 596-690.
Stehlin, H.G. 1908. Die Säugetiere des Schweizerischen Eocaens, Cristischer Catalog der Materialen, Tome 5. Abhandlungen der schweizerischen paläontologischen Gesellschaft, 34:691-837.
Stehlin, H.G. 1910. Remarques sur les faunules de mammifère des couches éocènes et oligocènes du Bassin de Paris. Bulletin de la Société géologique de France, 19:488-520.
Sudre, J. 1969. Les gisements de Robiac (Eocène supérieur) et leurs faunes de Mammifères. Palaeovertebrata, 2:95-156. https://doi.org/10.18563/pv.2.3.95-156
Sudre, J. 1977. L’évolution du genre Robiacina Sudre 1969, et l’origine des Cainotheriidae; implications systématiques. Geobios, 10:213-231. https://doi.org/10.1016/s0016-6995(77)80020-x
Sudre, J. 1978. Les artiodactyles de l’Eocène moyen et supérieur d’Europe occidentale: (systématique et évolution). Mémoires et Travaux de l’E.P.H.E., Montpellier, France. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=9133649
Sudre, J. and Erfurt, J. 1996. Les artiodactyles du gisement yprésien terminal de Prémontré (Aisne, France). Palaeovertebrata, 25(2-4):391-414. https://palaeovertebrata.com/Articles/view/215
Swofford, D.L. 2020. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0a161. Sinauer Associates, Sunderland, Massachusetts. http://paup.csit.fsu.edu.
Thewissen, J.G., Cooper, L.N., Clementz, M.T., Bajpai, S., and Tiwari, B.N. 2007. Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature, 450:1190-1195. https://doi.org/10.1038/nature06343
Vianey-Liaud, M. 1974. Les rongeurs de l’Oligocène inférieur d’Escamps. Palaeovertebrata, 6:197-241. https://palaeovertebrata.com/articles/view/172
Weppe, R., Blondel, C., Vianey-Liaud, M., Escarguel, G., Pélissié, T., Antoine, P.O., and Orliac, M.J. 2020. Cainotheriidae (Mammalia, Artiodactyla) from Dams (Quercy, SW France): phylogenetic relationships and evolution around the Eocene-Oligocene transition (MP19-MP21). Journal of Systematic Palaeontology, 18(7):541-572. https://doi.org/10.1080/14772019.2019.1645754

