 Anchitheriomys buceei (Rodentia, Castoridae) from the Miocene of Texas and a review of the Miocene beavers from the Texas Coastal Plain, USA
Anchitheriomys buceei (Rodentia, Castoridae) from the Miocene of Texas and a review of the Miocene beavers from the Texas Coastal Plain, USA
Article number: 26.1.a7
https://doi.org/10.26879/1236
Copyright Society of Vertebrate Paleontology, March 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 8 August 2022. Acceptance: 7 February 2023.
ABSTRACT
The record of fossil beavers from Miocene age strata in the Texas Coastal Plain is sparse. The first occurrence of fossil beavers is in Arikareean age faunas. One fossil beaver specimen was reported from a Hemingfordian fauna near Navasota, TX. Both Monosaulax and Anchitheriomys are described here from multiple early Barstovian faunas including a new taxon, Anchitheriomys buceei. The taxon, A. buceei, was a relatively large beaver, similar to A. fluminis in size, and shares characters with both A. fluminis and A. nanus. The holotype of Anchitheriomys buceei is a partial skull and endocast that preserves key features of the orbital foramina and palatine. Referred specimens include a partial dentary, isolated cheek teeth, and two post-cranial elements. The co-occurrence of this taxon and a small beaver identified as Monosaulax sp. in early Barstovian faunas is coeval with the first occurrence of proboscideans along the Texas Coastal Plain. Beavers are unknown in late Barstovian and Clarendonian faunas, although rodent fossils are uncommon from these faunas in general. Most of the fossil beaver specimens were obtained in the early twentieth century by collectors affiliated with Dr. Mark Francis at Texas A&M University, or with the State-Wide Paleontologic-Mineralogic Survey in Texas and are curated at the Texas Vertebrate Paleontology Collections. Archival materials housed at that facility have been instrumental in unraveling the history of collection, the distribution of localities and the evolving understanding of Miocene vertebrate biostratigraphy on the Texas Coastal Plain.
Steven R. May, Jackson School Museum of Earth History, The University of Texas at Austin, Austin, Texas 78758, USA. srmay@utexas.edu
Matthew A. Brown, Jackson School Museum of Earth History, The University of Texas at Austin, Austin, Texas 78758, USA. matthewbrown@utexas.edu
Keywords: new species; beaver; Miocene; Texas
Final citation: May, Steven R. and Brown, Matthew A. 2023. Anchitheriomys buceei (Rodentia, Castoridae) from the Miocene of Texas and a review of the Miocene beavers from the Texas Coastal Plain, USA. Palaeontologia Electronica, 26(1):a7.
https://doi.org/10.26879/1236
palaeo-electronica.org/content/2023/3775-texas-beavers
Copyright: March 2023 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
http://zoobank.org/09765039-68B6-45ED-8DC7-C79A997A2BAF
INTRODUCTION
The specimens described below are curated at the Texas Vertebrate Paleontology Collections at The University of Texas at Austin. Some of the material was initially housed in a museum at Texas A&M University. Most of the Texas A&M collection was assembled under the direction of Dr. Mark Francis, a veterinarian at the university and an early champion of Texas vertebrate paleontology. Dr. Francis and his colleagues collected fossil vertebrates from the Texas Coastal Plain from the late 1800s through the 1930s. Some of this material was being studied by C.J. Hesse, who was hired by Texas A&M in 1938, in part to act as curator of the Francis collections. Hesse’s (1942) paper describing the Miocene vertebrate localities from Southeast Texas remains a valuable reference, however, due to his death in 1945, much of the work Hesse started was left unfinished. The museum at Texas A&M was demolished in 1972 and many of the specimens were sent to Austin. In 1983, the collection was formally transferred by the President of Texas A&M to The University of Texas at Austin. Additional specimens described below were collected by the State-Wide Paleontologic-Mineralogic Survey conducted by the Works Progress Administration (WPA) under the direction of the University of Texas Bureau of Economic Geology (BEG) from 1939-1942. A few castorid specimens were later added in the 1950s by John A. Wilson and his students.
A number of papers have been published on Miocene vertebrates from the Texas Coastal Plain including Quinn (1952, 1955), Wilson (1956, 1960), Patton (1969), Forsten (1975), Webb and Hulbert (1986), Hulbert (1987a, 1987b, 1988a, 1988b), Prothero and Manning (1987), and May (2019). With a few exceptions, fossil rodents are poorly represented in the Miocene faunas. Wood and Wood (1937) described two castorid teeth from the Arikareean Cedar Run Local Fauna and Albright (1998) described a series of isolated teeth from the Toledo Bend Local Fauna of similar age in far east Texas. A review of all material in the TMM collection has resulted in the recognition of multiple specimens described here representing two different castorid taxa from a number of early Barstovian faunas. A large beaver is identified as a new species of Anchitheriomys, and a small beaver is identified as Monosaulax sp. Many of these specimens had been recognized as castorids on specimen cards, but not described in publication. Following these early Barstovian (Ba1) occurrences, there is a gap in the record of fossil beavers from the Texas Coastal Plain from the late Barstovian through the Pliocene. Relatively rare specimens of Castor and Castoroidies are known from the Quaternary although most specimens are from cave faunas of the Edwards Plateau region. Castor canadensis is a common element of modern faunas in the Texas Coastal Plain.
Materials and Methods
Abbreviations. AMNH, American Museum of Natural History; F:AM, Frick Collection, American Museum of Natural History; TAMU, Texas A & M University; TMM, Texas Vertebrate Paleontology Collections, The University of Texas at Austin; UCMP, Museum of Paleontology University of California, Berkeley; USNM, United States National Museum (Smithsonian Institution); NALMA, North American Land Mammal Age; AP, anteroposterior; HI, hypsodonty index; I incisor; M, upper molar; m, lower molar; P, upper premolar; p, lower premolar; TRL, tooth row length; L, left; R, right; mm, millimeters. Specimens and archival material at The University of Texas at Austin are curated in the Texas Vertebrate Paleontology Collections. Locality numbers are formatted TMM ##### and specimen numbers include the locality number, followed by a dash and the catalog number, i.e., TMM #####-####. Records of Mark Francis, Curtis Hesse, John Wilson and the Statewide Paleontologic-Mineralogic Survey are housed in the Texas Vertebrate Paleontology Archives.
Anatomical Terminology. Dental terminology used here is based on Stirton (1935) and the cranial terminology used for Anchitheriomys is based on Korth and Emry (1997).
Faunal Terminology. The terms local fauna and fauna are used following the definitions in Woodburne (2004). Locality, local fauna, and fauna names have been complicated historically by varied use in publications, archival records and specimen cards. We have largely followed the use of local fauna names as described in Tedford et al. (2004) for the Texas Coastal Plain.
Preparation. Fossils were originally prepared prior to this study by preparators at both The University of Texas and Texas A&M University using undocumented methods and materials. However, techniques likely followed the Handbook of Recommended Procedures (McAnulty, 1939) as outlined by Brown (2013). During this study, additional preparation was performed on TMM 40197-2665 by Deborah Wagner and Matthew Brown, and on TMM 31244-6 by Brown. Matrix was removed from the left and right orbital walls and palate to expose foramina, around the posterior margins of the palate, and other diagnostic areas of TMM 40197-2665. A rotated and displaced M3 was also removed from the matrix. Care was taken to preserve the endocast while uncovering additional bone surfaces. All preparation was done with a carbide needle and pin vise or an HW-1 airscribe under 10-50x magnification using a Wild M8 binocular microscope. Paraloid B-72 (Rohm & Haas) was used to consolidate friable bone. An unknown yellowed resin (possibly Duco Cement) was removed from TMM 31244-6, as well as a paper label attached with string and adhesive. A small amount of preparation using a pin vise was performed by Brown on TMM 1465-1.1 (Anchitheriomys nanus) to better expose foramina in the palatine region.
Photography. Specimens were photographed using an iPhone 11 or a Canon Power Shot D20 and processed using Adobe Photoshop.
Computed Tomography (CT). All CT imaging was performed at The University of Texas High-Resolution X-ray CT Facility at The University of Texas at Austin using a North Star Imaging micro-computed tomography scanner with a Fein Focus High Power source and a Perkin Elmer detector. TMM 40197-2665 was scanned at kV = 150; μA = 130 with a voxel size of 0.0799 mm x 0.0799 mm x 0.0799 mm. TMM 31244-6 was scanned at kV = 120; μA = 100 with a voxel size of 0.0914 mm x 0.0914 mm x.0914 mm. TMM 1465-1.1 was scanned at kV = 185; μA = 185 with a voxel size of 0.0417 mm x 0.0417 mm x 0.0417 mm. Scans are archived at https://www.morphosource.org. ImageVis3D, ImageJ, and Avizo software were used to visualize and analyze the CT data.
MIOCENE STRATIGRAPHY OF THE TEXAS COASTAL PLAIN
 Wilson (1956) reviewed the history of stratigraphic terminology and vertebrate biostratigraphy from Miocene strata of the Texas Coastal Plain. In that paper, he used the name Oakville Formation containing the Garvin Gully fauna and the Fleming Formation containing the younger Burkeville and Cold Spring faunas. However, Plummer (1932) and Renick (1936) had previously elevated the Fleming to group status composed of the underlying Oakville Formation and the overlying Lagarto Formation. Recent authors (Galloway et al., 1986; Young et al., 2010) consider the Oakville and Lagarto Formations to be part of the Fleming Group, and that is the stratigraphic nomenclature used here (Figure 1).
Wilson (1956) reviewed the history of stratigraphic terminology and vertebrate biostratigraphy from Miocene strata of the Texas Coastal Plain. In that paper, he used the name Oakville Formation containing the Garvin Gully fauna and the Fleming Formation containing the younger Burkeville and Cold Spring faunas. However, Plummer (1932) and Renick (1936) had previously elevated the Fleming to group status composed of the underlying Oakville Formation and the overlying Lagarto Formation. Recent authors (Galloway et al., 1986; Young et al., 2010) consider the Oakville and Lagarto Formations to be part of the Fleming Group, and that is the stratigraphic nomenclature used here (Figure 1).
The Fleming Group includes a number of large fluvial systems in up-dip areas that transition to deltaic and marginal marine systems down-dip and to fully marine offshore (Galloway et al., 1986; Morton et al., 1988). Measured thicknesses from outcrop include 215-430 meters in the Lagarto Formation and 90-215 meters in the underlying Oakville Formation (Galloway et al., 1986). Recognition of the up-dip, non-marine subdivisions of the Fleming Group is difficult. While the Oakville Formation is mapped in some areas, the Fleming Group is recognized as undifferentiated in others. Furthermore, the boundary between the Catahoula Formation and the Fleming Group is not well defined (Albright, 1998). In the offshore Gulf of Mexico, the formations of the Fleming Group thicken significantly. The Oakville Formation is a sandy progradational sequence above a maximum transgression associated with the Anahuac Shale. The Lagarto Formation is muddy with an aggradational to retrogradational architecture, including a peak transgression near the top of the formation associated with the Amphistega B Shale (Galloway et al., 1986). Projection of the maximum flooding surface up-dip into the outcrop is uncertain, but a possible candidate is the marginal marine facies described within the Fleming Group near Burkeville, Texas (Stenzel et al., 1944). Marine fossils from this area, as identified in Stenzel et al. (1944), include ray teeth, an oyster, and a foraminifer. As will be discussed later, the age of the Burkeville Local Fauna is early Barstovian (Ba1) consistent with the Langhian age for the Amphistega B transgression (Figure 1).
Because formations of the Fleming Group are difficult to differentiate in up-dip outcrop areas of the Texas Coastal Plain, detailed stratigraphic correlation between localities is not possible. The relative stratigraphic positions of local faunas from the Fleming Group are based on general location with respect to the lower and upper lithostratigraphic boundaries. While the upper boundary with the Goliad Formation is reasonably well constrained, the lower boundary with the Catahoula Formation is often problematic.
Based on correlation of outcrop and subsurface, both planktonic and benthonic foraminifera constrain the age of the Oakville Formation to be Aquitanian through middle Burdigalian with the Lagarto Formation being middle Burdigalian through Langhian (Galloway et al., 1986). According to the time scale of Hilgren et al. (2012); Raffi, Wade and Pälike (2020), this correlation suggests that the Oakville Formation would be largely Arikareean (Ar3-Ar4) through early Hemingfordian (He1) in age and the Lagarto Formation would be late Hemingfordian and Barstovian (Figure 1). Although generally consistent with ages interpreted from the fossil vertebrates, late Barstovian (Ba2) faunas from the Lagarto Formation suggest that the upper part of the Fleming Group extends into the early Serrivalian.
Considering the vertebrate faunas, the age of the lower part of the Fleming Group may become younger from the Hemingfordian Garvin Gully locality on the west to the early Barstovian Moscow and Belts Creek localities to the east. However, as discussed previously, the Catahoula Formation - Fleming Group boundary is not well defined and this apparent diachroneity may be an artifact of geologic mapping (Albright, 1998).
MIOCENE VERTEBRATE BIOSTRATIGRAPHY
Two Arikareean local faunas (Cedar Run, Toledo Bend) include the three equids Archaeohippus, Anchippus, and Miohippus along with Diceratherium, Prosynthetoceras, Floridatragulus, Daphoenodon, and Dinohyus (Albright, 1998). The Hemingfordian fauna associated with the Garvin Gully localities includes the horses Archaeohippus, Anchitherium, and Parahippus, a diverse suite of camelids and Prosynthetoceras as well as the rhino Menoceras barbouri (Prothero and Manning, 1987) and the carnivore Amphicyon.
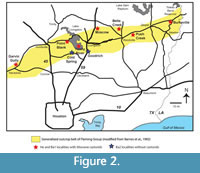 Vertebrate faunas from the early Barstovian (Ba1) localities (Point Blank, Belts Creek, Trinity River, Moscow, Push Creek, Burkeville, Figure 2; see Table 1 for locality names, numbers, and associated faunal names) are characterized by the continued presence of Prosynthetoceras and Floridatragulus, but also by the appearance of horses with hypsodont cheek teeth commonly assigned to Merychippus, Protohippus, and Cormohipparion (MacFadden, 1984). Instead of the single genus Menoceras, rhinos included Aphelops, Peraceras, and Teleoceras, all of which continued through the Ba2 Cold Spring Fauna (Prothero and Manning, 1987). The carnivores Paratomarctus (Wang et al., 1999) and possibly Tomarctus (T. brivirostris from Noble Farm according to Wang et al., 1999) and the procyonid Edaphocyon (Wilson, 1960) appeared in the Ba1 faunas as well. Based on studies of various vertebrate groups, Tedford et al. (2004) considered the Point Blank, Trinity River, Moscow, and Burkeville Local Faunas to be Ba1 in age. Proboscideans are known from the Ba1 faunas at Noble Farm, Belts Creek, and possibly Burkeville. Stenzel et al. (1944) provided a vertebrate faunal list for the Burkeville area that included ? Desmostylus. Reinhart (1976) reviewed these specimens and concluded they were fragments of a proboscidean tooth or perhaps were better considered indeterminate. Further examination leads us to the same conclusion. These are the fragments listed as proboscidean from the Burkeville Local Fauna by Tedford et al. (1987) and Tedford et al. (2004).
Vertebrate faunas from the early Barstovian (Ba1) localities (Point Blank, Belts Creek, Trinity River, Moscow, Push Creek, Burkeville, Figure 2; see Table 1 for locality names, numbers, and associated faunal names) are characterized by the continued presence of Prosynthetoceras and Floridatragulus, but also by the appearance of horses with hypsodont cheek teeth commonly assigned to Merychippus, Protohippus, and Cormohipparion (MacFadden, 1984). Instead of the single genus Menoceras, rhinos included Aphelops, Peraceras, and Teleoceras, all of which continued through the Ba2 Cold Spring Fauna (Prothero and Manning, 1987). The carnivores Paratomarctus (Wang et al., 1999) and possibly Tomarctus (T. brivirostris from Noble Farm according to Wang et al., 1999) and the procyonid Edaphocyon (Wilson, 1960) appeared in the Ba1 faunas as well. Based on studies of various vertebrate groups, Tedford et al. (2004) considered the Point Blank, Trinity River, Moscow, and Burkeville Local Faunas to be Ba1 in age. Proboscideans are known from the Ba1 faunas at Noble Farm, Belts Creek, and possibly Burkeville. Stenzel et al. (1944) provided a vertebrate faunal list for the Burkeville area that included ? Desmostylus. Reinhart (1976) reviewed these specimens and concluded they were fragments of a proboscidean tooth or perhaps were better considered indeterminate. Further examination leads us to the same conclusion. These are the fragments listed as proboscidean from the Burkeville Local Fauna by Tedford et al. (1987) and Tedford et al. (2004).
The Ba2 local faunas from the upper part of the Lagarto Formation (Cold Spring, Sam Houston, Goodrich) are clearly differentiated by the appearance of additional horse taxa including Calippus, Hipparion, Protohippus, and Neohipparion. The carnivore Aelurodon ferox appears in the Goodrich and Cold Spring Local Faunas (Wang et al., 1999). The Ba2 faunas also include Aepycamelus, Longirostomeryx, and Cranioceras (Tedford et al., 2004). These local faunas were referred to as the Cold Spring fauna by Quinn (1955), Wilson (1956, 1960), and Patton (1969).
DISTRIBUTION OF MIOCENE FOSSIL BEAVERS
The oldest fossil beavers from the Texas Coastal Plain are known from two Arikareean local faunas: Cedar Run (also referred to as Cedar Creek, Derrick Farm) and Toledo Bend. Wood and Wood (1937) described a small fauna of mostly isolated bones and teeth from “stream-channel” sandstones in what they interpreted as the basal part of the Oakville Formation. Geologic mapping compiled by Barnes et al. (1992) would place the Cedar Run locality well within the bounds of the Catahoula Formation. However, as discussed by Albright (1998), the boundary between the Catahoula Formation and Fleming Group has long been problematic and was never clearly defined. Jordan et al. (2019) report an Ar40/Ar39 radiometric date of 30.65 +/- 0.06 Ma for a volcanic ash from the Catahoula Formation near Sam Rayburn reservoir in east Texas. This is consistent with other dates from the Catahoula ranging from 29.98-32.72 Ma all of which suggest a Whitneyan age (early Oligocene) for at least part of the Catahoula (Jordan et al., 2019). Albright (1998) and Tedford et al. (2004) concluded that both the Cedar Run and Toledo Bend Local Faunas are most likely Ar3 in age or approximately 22-23 Ma. The age of the Cedar Run Local Fauna is consistent with the stratigraphic interpretation of Wood and Wood (1937) (Oakville Formation).
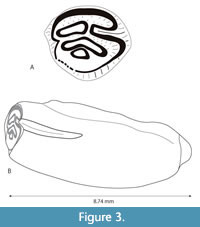 Wood and Wood (1937) included photos of two beaver teeth from the Cedar Run Local Fauna. AMNH No. 30088 was identified as a left p4 of Palaeocastor cf. simplicidens and AMNH No. 30090 was identified as a right M1? of Palaeocastor cf. barbouri. Albright (1998) included Palaeocastor cf. P. simplicidens and P. cf. P. barbouri in a faunal list for Cedar Run, but considered the possibility that only a single castorid taxon was present. Martin (1987) had reassigned P. barbouri (Stirton, 1935) to a new genus Pseudopalaeocastor, and Tedford et al. (2004) list both Palaeocastor and Pseudopalaeocastor in their faunal list from Cedar Run. Figure 3 illustrates a previously unreported castorid tooth from TMM 40068, which is the Cedar Run (Cedar Creek) locality of Wood and Wood (1937) that was collected by J. A. Wilson in 1950 (Wilson, 1960). TMM 40068-24 is a slightly worn left m1 with a simple hypoflexid and three simple fossettids (para, meso, and meta). It is small (3.38 mm x 4.06 mm AP x W) and has a hypsodonty index of approximately 2. The base of the enamel is preserved, but the roots are not. The hypoflexid would be lost after only modest wear leaving four simple fossettids. Short striae are characteristic of the palaeocastoines according to Korth (2001b). The size, occlusal pattern, and degree of hypsodonty are all similar to Palaeocastor fossor (Peterson, 1905). Martin (1987) described P. fossor as having “high-crowned cheek teeth”, a character that is also illustrated in Peterson (1905). It is often difficult to identify Miocene castorids on the basis of isolated cheek teeth. Although TMM 40068-24 is closely comparable to P. fossor, it is here identified as cf. Palaeocastor.
Wood and Wood (1937) included photos of two beaver teeth from the Cedar Run Local Fauna. AMNH No. 30088 was identified as a left p4 of Palaeocastor cf. simplicidens and AMNH No. 30090 was identified as a right M1? of Palaeocastor cf. barbouri. Albright (1998) included Palaeocastor cf. P. simplicidens and P. cf. P. barbouri in a faunal list for Cedar Run, but considered the possibility that only a single castorid taxon was present. Martin (1987) had reassigned P. barbouri (Stirton, 1935) to a new genus Pseudopalaeocastor, and Tedford et al. (2004) list both Palaeocastor and Pseudopalaeocastor in their faunal list from Cedar Run. Figure 3 illustrates a previously unreported castorid tooth from TMM 40068, which is the Cedar Run (Cedar Creek) locality of Wood and Wood (1937) that was collected by J. A. Wilson in 1950 (Wilson, 1960). TMM 40068-24 is a slightly worn left m1 with a simple hypoflexid and three simple fossettids (para, meso, and meta). It is small (3.38 mm x 4.06 mm AP x W) and has a hypsodonty index of approximately 2. The base of the enamel is preserved, but the roots are not. The hypoflexid would be lost after only modest wear leaving four simple fossettids. Short striae are characteristic of the palaeocastoines according to Korth (2001b). The size, occlusal pattern, and degree of hypsodonty are all similar to Palaeocastor fossor (Peterson, 1905). Martin (1987) described P. fossor as having “high-crowned cheek teeth”, a character that is also illustrated in Peterson (1905). It is often difficult to identify Miocene castorids on the basis of isolated cheek teeth. Although TMM 40068-24 is closely comparable to P. fossor, it is here identified as cf. Palaeocastor.
The Toledo Bend Local Fauna from near the Louisiana-Texas border was collected from strata mapped as Catahoula Formation on the Texas side of the Red River but as Fleming Group on the Louisiana side (Albright, 1998). Albright (1998) described the stratigraphic relationships and provided a complete review of the fauna with comparisons to the Great Plains and Florida. He concluded that the Toledo Bend Local Fauna was Ar3 in age and correlative with Cedar Run. Albright (1996) identified a castorid based on isolated teeth as “Monosaulax” hesperus but noted a number of issues with this generic assignment. Korth (1996) proposed the species hesperus, (previously assigned to Steneofiber or Monosaulax) as the type species for the new genus Neatocastor. He described the cheek teeth as having complex occlusal patterns, sub-hypsodont and rooted. Albright (1998) listed the beaver from the Toledo Bend Local Fauna as cf. Neatocastor hesperus.
The record of Arikareean castorids from the Texas Coastal Plain is sparse although it appears that both Palaeocastorines and Angotocastorines may be represented. The record of Hemingfordian castorids is even more limited. The only known Hemingfordian beaver from the Texas Coastal Plain is a single tooth (UCMP 32614) from the Garvin Gully Local Fauna collected by R.A. Stirton in 1935 and is curated at the University of California Museum of Paleontology (UCMP). Unfortunately, the status of this specimen is uncertain after it was borrowed in 1990 and never returned. The Garvin Gully Local Fauna is known from a few localities northeast of Navasota, TX (Figure 2). Stirton’s field notes from 1935 (UCMP archives) indicate that the UCMP material was collected at the Floyd Quarry (V-3507) and referred to as the Navasota fauna including a field identification of “Palaeocastor”. A sketch map with his notes suggests that the Floyd Quarry was located very close to the main Texas A&M locality, TAMU 20. Stirton’s field notes refer to discussions with “Dr. Francis” about collecting sites in the area. Mark Francis and his staff collected fossils in the Garvin Gully area from 1919-1922 (TAMU 20) resulting in a collection of numerous specimens now in The University of Texas Vertebrate Paleontology Collections. Based on correspondence in the TMM archives, Frick collectors also visited this site and there is a small collection at AMNH. Additional material was collected from Garvin Gully by The University of Texas in 1950 (TMM 31084). Wilson (1956) recognized that a number localities from the Oakville Formation (lower Fleming of others) were approximately correlative, and he referred to all of these occurrences as the Garvin Gully fauna. None of these localities other than the Floyd Quarry (V-3507) from the Garvin Gully area contain fossil castorids. Tedford et al. (1987) suggested comparison of the Garvin Gully taxa with those from the Runningwater Formation in Nebraska and Tedford et al. (2004) interpreted the Garvin Gully Local Fauna as Hemingfordian in age.
The castorids Monosaulax and Anchitheriomys are described in this paper from Ba1 faunas of the Texas Coastal Plain and make their appearance along with proboscideans, hypsodont equids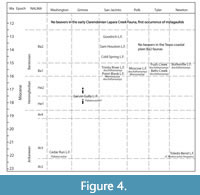 (Merychippus, Cormohipparion) the rhinos Aphelops, Peraceras, and Teleoceras, and the carnivores Paratomarctus, Tomarctus, and Edaphocyon. The Moscow (= Barrington Farm) Local Fauna and Belts Creek locality are from near the lower part of the Fleming Group as mapped by Barnes et al. (1992), while the Point Blank, Trinity River, Push Creek, and Burkeville Local Faunas are from localities stratigraphically higher near the middle part of the Fleming Group. All of these faunas are interpreted as Ba1 in age based on faunal composition and stratigraphic position.
(Merychippus, Cormohipparion) the rhinos Aphelops, Peraceras, and Teleoceras, and the carnivores Paratomarctus, Tomarctus, and Edaphocyon. The Moscow (= Barrington Farm) Local Fauna and Belts Creek locality are from near the lower part of the Fleming Group as mapped by Barnes et al. (1992), while the Point Blank, Trinity River, Push Creek, and Burkeville Local Faunas are from localities stratigraphically higher near the middle part of the Fleming Group. All of these faunas are interpreted as Ba1 in age based on faunal composition and stratigraphic position.
No fossil castorids are currently known from Ba2 faunas located in the upper part of the Fleming Group, nor from the Cl1-Cl2 Lapara Creek Fauna in the Goliad Formation (May, 2019) (Figure 4). Fossil castorids are not recognized again until the Quaternary of the Texas Coastal Plain where both Castor and one specimen assigned to Castoroides are present in the TMM collection.
SYSTEMATIC PALEONTOLOGY
Order Rodentia Bowdich, 1821
Family Castoridae Hemprich, 1820
Subfamily Anchitheriomyinae Korth, 2001a
Anchitheriomys Roger, 1898
Anchitheriomys buceei, new species
Amblycastor Matthew, 1918
Type Species. Anchitheriomys suevicus (Schlosser, 1884)
Referred Species. Anchitheriomys fluminis (Matthew, 1918), Anchitheriomys tungurensis (Stirton, 1934), Anchitheriomys stouti (Korth, 2001a), Anchitheriomys nanus (Korth, 2004), and Anchitheriomys buceei new species.
Range. Early to middle Miocene (Hemingfordian to Barstovian) of North America and Eurasia.
Anchitheriomys buceei new species
(Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11,
Figure 12, Figure 13, Figure 14, Figure 15, Table 2)
zoobank.org/517DFDD0-FE18-4A1F-99D0-7342CEF189C7
Type Specimen. TMM 40197-2665, partial skull with M3 and roots for P4-M3.
Referred Specimens. TMM 31057-7 LM2; TMM 31057-59 Rm2; TMM 31057-141 RM3; TMM 31057-163 Lm1; TMM 31057-185 Lm1; TMM 40622-13 LP4; TMM 40623-12 left dentary; TMM 71-2666 distal humerus, proximal ulna, TMM 71-2667 Rp4; TMM 71-2668 - molar; TMM 71-2669 - RM2. F:AM 64023A - partial left maxilla with P4; F:AM 64023B - P4; F:AM64024A edentulous partial left ramus; F:AM 64024B left lower cheek tooth. (Note: TMM 71-2770, an isolated tooth was borrowed in the 1940s and never returned. The specimen number at that time would have been TAMU 2770.)
Horizon and Locality. Lagarto Formation, Fleming Group, Burkeville, Newton Co. (TMM 40197); Push Creek, Belts Creek, Tyler Co. (TMM 40623, TMM 40622); Moscow (Barrington Farm), Polk Co. (TMM 31057); Trinity River (Pit 1 AMNH specimens), Point Blank, San Jacinto Co. (TMM 71); Texas.
Age. Early Barstovian, Ba1, (middle Miocene).
Etymology. Species named in recognition of Buc-ee’s roadside attraction travel centers that have re-popularized beavers in Texas.
 Diagnosis. Anchitheriomys buceei is larger than both A. stouti from the early Hemingfordian of Nebraska (Korth, 2001a) and A. nanus from the early Hemingfordian of Nebraska (Korth and Martin, 2007). The cheek teeth have much simpler enamel patterns than A. tungurensis (Stirton, 1934). The dentary has a well-developed symphyseal flange differing from both A. suevicus and A fluminis. A relatively large mental foramen is located on the labial side of the dentary anterior to p4 and well above the midline of the horizontal ramus with much smaller foramen just anterior to the primary foramen. The ridges on i1 are much more prominent than in A. nanus and possess secondary corrugations along their length. Anchitheriomys buceei is similar to A. fluminis in size and elongated rostrum, but differs in having grooves on the palatine, the more posterior position of the posterior palatine foramina, a single nasolacrimal foramen, the ethmoidal foramen is located more dorsal relative to the optic foramen, and the posterior divergence of the upper cheek teeth is less pronounced. The posterior terminus of the palatine in A. buceei forms a broadly curving arc as in A. fluminis and unlike the tight u-shaped terminus in A. nanus. The cheek teeth are large with a very large P4/p4 relative to the molars.
Diagnosis. Anchitheriomys buceei is larger than both A. stouti from the early Hemingfordian of Nebraska (Korth, 2001a) and A. nanus from the early Hemingfordian of Nebraska (Korth and Martin, 2007). The cheek teeth have much simpler enamel patterns than A. tungurensis (Stirton, 1934). The dentary has a well-developed symphyseal flange differing from both A. suevicus and A fluminis. A relatively large mental foramen is located on the labial side of the dentary anterior to p4 and well above the midline of the horizontal ramus with much smaller foramen just anterior to the primary foramen. The ridges on i1 are much more prominent than in A. nanus and possess secondary corrugations along their length. Anchitheriomys buceei is similar to A. fluminis in size and elongated rostrum, but differs in having grooves on the palatine, the more posterior position of the posterior palatine foramina, a single nasolacrimal foramen, the ethmoidal foramen is located more dorsal relative to the optic foramen, and the posterior divergence of the upper cheek teeth is less pronounced. The posterior terminus of the palatine in A. buceei forms a broadly curving arc as in A. fluminis and unlike the tight u-shaped terminus in A. nanus. The cheek teeth are large with a very large P4/p4 relative to the molars.
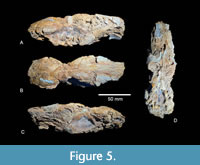
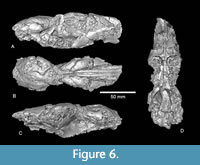 Description. TMM 40197-2665 is a partial skull of a relatively large castorid that preserves portions of the cranium and dentition, with much of the cranial anatomy preserved as an endocast (Figure 5, Figure 6). The length of the preserved skull is 152.6 mm, but with both anterior and posterior elements missing. The greatest width of the preserved rostrum is 47.3 mm, again representing a minimum value. Although the anterior portion of the rostrum is missing, the I1-P4 diastema is at least 55 mm. The depth of the skull from palate to the frontals is approximately 51 mm. This compares well with the value of 50.8 mm for the skull of Anchitheriomys fluminis (USNM 299914, Korth and Emry, 1997). The size of the skull of A. buceei is significantly larger than the Hemingfordian taxa A. nanus and A. stouti and similar to the mid Barstovian A. fluminis (USNM 299914, Korth and Emry, 1997).
Description. TMM 40197-2665 is a partial skull of a relatively large castorid that preserves portions of the cranium and dentition, with much of the cranial anatomy preserved as an endocast (Figure 5, Figure 6). The length of the preserved skull is 152.6 mm, but with both anterior and posterior elements missing. The greatest width of the preserved rostrum is 47.3 mm, again representing a minimum value. Although the anterior portion of the rostrum is missing, the I1-P4 diastema is at least 55 mm. The depth of the skull from palate to the frontals is approximately 51 mm. This compares well with the value of 50.8 mm for the skull of Anchitheriomys fluminis (USNM 299914, Korth and Emry, 1997). The size of the skull of A. buceei is significantly larger than the Hemingfordian taxa A. nanus and A. stouti and similar to the mid Barstovian A. fluminis (USNM 299914, Korth and Emry, 1997).
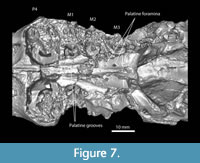 The upper tooth rows of Anchitheriomys buceei diverge posteriorly as in the skull of A. fluminis (USNM 299914) described by Korth and Emry (1997), but the degree of divergence is less (Figure 7). The width of the palatine on TMM 40197-2665 varies from 10.2 mm at P4 to 13.3 mm at M3. The ratio of palatal width measurements at P4 and M3 is 0.76 for A. buceei, but 0.56 for A fluminis. The two skulls of A. nanus described by Korth and Martin (2007) illustrate variability in this character with the larger skull at 0.67 and the smaller skull at 0.56. The anterior portion of the palatine is grooved, as in A. nanus, while the posterior portion is slightly concave with no distinct grooves. There is a prominent ridge along the midline of the palatine. The palatine grooves terminate anterior to the palatine foramina, similar to A. nanus (Korth and Martin, 2007)
The upper tooth rows of Anchitheriomys buceei diverge posteriorly as in the skull of A. fluminis (USNM 299914) described by Korth and Emry (1997), but the degree of divergence is less (Figure 7). The width of the palatine on TMM 40197-2665 varies from 10.2 mm at P4 to 13.3 mm at M3. The ratio of palatal width measurements at P4 and M3 is 0.76 for A. buceei, but 0.56 for A fluminis. The two skulls of A. nanus described by Korth and Martin (2007) illustrate variability in this character with the larger skull at 0.67 and the smaller skull at 0.56. The anterior portion of the palatine is grooved, as in A. nanus, while the posterior portion is slightly concave with no distinct grooves. There is a prominent ridge along the midline of the palatine. The palatine grooves terminate anterior to the palatine foramina, similar to A. nanus (Korth and Martin, 2007) 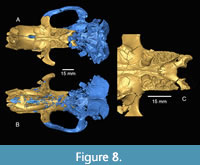 and unlike A. fluminis (Korth and Emry, 1997) (Figure 7). Recent preparation of A. nanus skull (TMM 1465-1.1) revealed that the palatine grooves terminate posteriorly in foramina. This appears to be the same in A. buceei, although the palatine bone is poorly preserved at that location. The palatine foramina are located entirely within the palatine bone as in other species of Anchitheriomys. Whereas A. nanus has a single palatine foramen on each side of the palatine bone, A. buceei has two, as does A. fluminis (Korth and Emry, 1997). The left side of the palatine includes a larger posterior foramen aligned with the middle of M3 and a smaller anterior foramen. The locations of the posterior palatine foramina in A. buceei are more posterior than in A. fluminis. The palatine/maxilla suture is not preserved, but the palatine bone extends at least to the posterior edge of M1. The posterior margin of the palatine structure in A. buceei forms a broadly curving arc as in A. fluminis (Figure 7). In contrast, the posterior edge of the palatine in A. nanus, forms a narrow, u-shaped margin along with the pterygoid processes (Figure 8C).
and unlike A. fluminis (Korth and Emry, 1997) (Figure 7). Recent preparation of A. nanus skull (TMM 1465-1.1) revealed that the palatine grooves terminate posteriorly in foramina. This appears to be the same in A. buceei, although the palatine bone is poorly preserved at that location. The palatine foramina are located entirely within the palatine bone as in other species of Anchitheriomys. Whereas A. nanus has a single palatine foramen on each side of the palatine bone, A. buceei has two, as does A. fluminis (Korth and Emry, 1997). The left side of the palatine includes a larger posterior foramen aligned with the middle of M3 and a smaller anterior foramen. The locations of the posterior palatine foramina in A. buceei are more posterior than in A. fluminis. The palatine/maxilla suture is not preserved, but the palatine bone extends at least to the posterior edge of M1. The posterior margin of the palatine structure in A. buceei forms a broadly curving arc as in A. fluminis (Figure 7). In contrast, the posterior edge of the palatine in A. nanus, forms a narrow, u-shaped margin along with the pterygoid processes (Figure 8C).
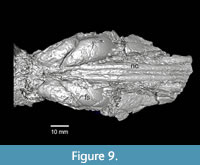 Although the nasal bones are mostly missing on TMM 40197-2665, the endocast of the nasal cavities is long and narrow with a preserved length of 78.0 mm and a combined width of approximately 10.6 mm (Figure 9, Figure 10). Fragments of both premaxilla are preserved. These fragments terminate at approximately the same position posteriorly probably reflecting the original suture with the frontal bones. A small bone fragment, presumably part of the right nasal, also terminates at approximately this position and the nasal cavity endocast exhibits a slight change in orientation at this position as well. The nasals and the premaxilla bones joined the frontals near the anterior terminus of the zygomatic arch. This geometry is similar to the skull of Anchitheriomys fluminis shown in Korth and Emry (1997).
Although the nasal bones are mostly missing on TMM 40197-2665, the endocast of the nasal cavities is long and narrow with a preserved length of 78.0 mm and a combined width of approximately 10.6 mm (Figure 9, Figure 10). Fragments of both premaxilla are preserved. These fragments terminate at approximately the same position posteriorly probably reflecting the original suture with the frontal bones. A small bone fragment, presumably part of the right nasal, also terminates at approximately this position and the nasal cavity endocast exhibits a slight change in orientation at this position as well. The nasals and the premaxilla bones joined the frontals near the anterior terminus of the zygomatic arch. This geometry is similar to the skull of Anchitheriomys fluminis shown in Korth and Emry (1997).
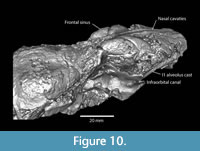 The frontal sinus is large and bilobed with dimensions of 44.1 mm x 14.6 mm (L x maximum width) (Figure 9, Figure 10). The infraorbital canal on the right side of the skull is relatively large with four small foramina present in two paired groups (Figure 9). A post-glenoid foramen is preserved on the right side of the skull and appears to be more rounded as in Anchitheriomys fluminis compared to the elongated geometry of A. nanus (Korth and Martin, 2007).
The frontal sinus is large and bilobed with dimensions of 44.1 mm x 14.6 mm (L x maximum width) (Figure 9, Figure 10). The infraorbital canal on the right side of the skull is relatively large with four small foramina present in two paired groups (Figure 9). A post-glenoid foramen is preserved on the right side of the skull and appears to be more rounded as in Anchitheriomys fluminis compared to the elongated geometry of A. nanus (Korth and Martin, 2007).
Approximate dimensions of the alveolus endocast for the right I1 are 12.5 mm x 11.5 mm and similar to Anchitheriomys fluminis (10.6 mm x 9.4 mm, 10.9 mm x 9.5 mm, I1 L x W in Korth and Emry, 1997). P4 is the largest of the cheek teeth and all cheek teeth have three roots. The lingual root is large and bladelike while the two labial roots are more peg-like (Figure 7). There is no evidence of cheek teeth anterior to P4.
Except for the right M3, only the roots of the cheek teeth are present in TMM 40197-2665. The left side tooth row is more complete with an alveolar length of ~41.6 mm. This is similar to the tooth row length of Anchitheriomys fluminis (41.4 mm, 39.2 mm in Korth and Emry, 1997). Measured at the roots, the left P4 is 13.1 mm x 15.3 mm. The right P4 at the roots is 12.0 mm x 14.1 mm.
The single isolated right M3 associated with TMM 40197-2665 is moderately worn. It had been rotated out of position and was prepared from the matrix of the original specimen. It is 7.8 mm x 8.3 mm (L x W) at the occlusal surface and 9.7 mm x 8.7 mm at the base of the enamel. The preserved crown height is 9.3 mm. The alveolar dimensions of the left M3 are 6.8 mm x 8.1 mm (L x W). The roots of the right M2 are also rotated so that they point laterally rather than ventrally.
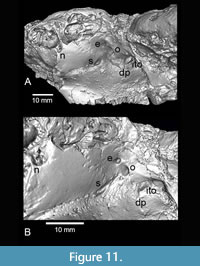 The orbital foramina are well preserved on TMM 40197-2665 and imaged in the CT data (Figure 11). The size and locations of these foramina are different in detail than either Anchitheriomys fluminis or A. nanus, but are more similar to A. nanus. According to Korth and Martin (2007), A. nanus differs from A. fluminis in the more posterior position of the optic and ethmoidal foramina which Korth and Martin (2007) interpreted as a primitive condition. The relative locations of the ethmoidal and optic foramina in A. buceei are similar to A. nanus, but the sphenopalatine foramen is elongate and dorso-ventrally oriented, and the optic foramen is sub-circular as in A. fluminis. Both the optic and interorbital foramina are larger than in A. fluminis. The ethmoidal foramen is located more dorsal relative to the optic foramen as in A. nanus. The large interorbital foramen in A. buceei is located just dorsal to a small dorsal palatine foramen. This positioning is similar to A. nanus and unlike A. fluminis. A pair of nasolacrimal foramina are present in A. buceei, as in A. fluminis, and unlike the single foramen described in A. nanus (Korth and Emry, 1997; Korth and Martin, 2007) (Figure 11).
The orbital foramina are well preserved on TMM 40197-2665 and imaged in the CT data (Figure 11). The size and locations of these foramina are different in detail than either Anchitheriomys fluminis or A. nanus, but are more similar to A. nanus. According to Korth and Martin (2007), A. nanus differs from A. fluminis in the more posterior position of the optic and ethmoidal foramina which Korth and Martin (2007) interpreted as a primitive condition. The relative locations of the ethmoidal and optic foramina in A. buceei are similar to A. nanus, but the sphenopalatine foramen is elongate and dorso-ventrally oriented, and the optic foramen is sub-circular as in A. fluminis. Both the optic and interorbital foramina are larger than in A. fluminis. The ethmoidal foramen is located more dorsal relative to the optic foramen as in A. nanus. The large interorbital foramen in A. buceei is located just dorsal to a small dorsal palatine foramen. This positioning is similar to A. nanus and unlike A. fluminis. A pair of nasolacrimal foramina are present in A. buceei, as in A. fluminis, and unlike the single foramen described in A. nanus (Korth and Emry, 1997; Korth and Martin, 2007) (Figure 11).
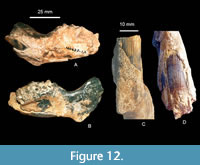 TMM 40623-12 is a partial left dentary assigned to Anchitheriomys buceei from the Push Creek Local Fauna (Figure 12). The dentary is robust. The medio-lateral width at the symphysis is 15.5 mm, at the diastema is 16.02 mm, at p4 is 21.7 mm, at m1 is 23.7 mm, and at m2 is 23.1 mm. The minimum dorso-ventral depth at the diastema is 22.9 mm while the depth below m1 is 30.6 mm (minimum measurement as the ventral portion of the dentary is missing). The diastema of A. buceei is relatively shallow compared to A. fluminis (FAM 64020 in Korth 2017b). The symphyseal flange (digastric process) is much better developed than in A. suevicus (Stefen and Mörs, 2008) and A. fluminis (Korth, 2017b). Anchitheriomys stouti (Korth, 2001a) also has a prominent symphyseal flange as does the large castorid (UF 223914) from Suwannee River, Florida (Mörs and Hulbert, 2010). Mörs and Hulbert (2010) assigned UF 223914 to Amblycastor fluminis, but Korth (2017b) argued that the Florida specimen is of uncertain taxonomic identity. The symphysis is oval in shape and narrows ventrally. It is 38.7 mm in dorso-ventral height with a maximum width of 23.7 mm.
TMM 40623-12 is a partial left dentary assigned to Anchitheriomys buceei from the Push Creek Local Fauna (Figure 12). The dentary is robust. The medio-lateral width at the symphysis is 15.5 mm, at the diastema is 16.02 mm, at p4 is 21.7 mm, at m1 is 23.7 mm, and at m2 is 23.1 mm. The minimum dorso-ventral depth at the diastema is 22.9 mm while the depth below m1 is 30.6 mm (minimum measurement as the ventral portion of the dentary is missing). The diastema of A. buceei is relatively shallow compared to A. fluminis (FAM 64020 in Korth 2017b). The symphyseal flange (digastric process) is much better developed than in A. suevicus (Stefen and Mörs, 2008) and A. fluminis (Korth, 2017b). Anchitheriomys stouti (Korth, 2001a) also has a prominent symphyseal flange as does the large castorid (UF 223914) from Suwannee River, Florida (Mörs and Hulbert, 2010). Mörs and Hulbert (2010) assigned UF 223914 to Amblycastor fluminis, but Korth (2017b) argued that the Florida specimen is of uncertain taxonomic identity. The symphysis is oval in shape and narrows ventrally. It is 38.7 mm in dorso-ventral height with a maximum width of 23.7 mm.
A relatively large mental foramen is located on the labial side of the dentary anterior to p4 and well above the midline of the horizontal ramus. A much smaller foramen is located just anterior to the primary foramen. Anchitheriomys suevicus exhibits a single mental foramen located near the middle of the horizontal ramus (Stefen and Mörs, 2008), while A. stouti has a double mental foramen that is located below the center of the diastema at about the dorso-ventral midline of the horizontal ramus (Korth, 2001a). TMM 40623-12 clearly differs from both of these morphologies. Anchitheriomys fluminis (FAM 64020) shown in Korth (2017b) appears to have a single small foramen located near the midline of the horizontal ramus and more posterior than in A. buceei. However, the holotype of A. fluminis figured in Matthew (1918) shows a large sub-circular mental foramen slightly posterior to the midline of the dentary.
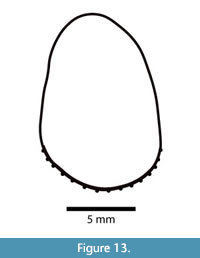 The lower incisor of Anchitheriomys buceei is ovoid in cross-section with very prominent longitudinal ridges on the anterior face (Figure 13). Approximately 15-16 ridges are generally sub-parallel, but occasionally anastomose. The ridges are individually corrugated with small culminations along their length as shown in Figure 14. Because the ventral portion of the dentary is broken, the ridges can be seen to run the entire length of the incisor (Figure 12). Measured at the natural break, i1 is 12.8 mm x 11.3 mm in cross section with a preserved length of 73.5 mm. The ridges on i1 are much more prominent than in A. nanus.
The lower incisor of Anchitheriomys buceei is ovoid in cross-section with very prominent longitudinal ridges on the anterior face (Figure 13). Approximately 15-16 ridges are generally sub-parallel, but occasionally anastomose. The ridges are individually corrugated with small culminations along their length as shown in Figure 14. Because the ventral portion of the dentary is broken, the ridges can be seen to run the entire length of the incisor (Figure 12). Measured at the natural break, i1 is 12.8 mm x 11.3 mm in cross section with a preserved length of 73.5 mm. The ridges on i1 are much more prominent than in A. nanus.
 The dimensions of p4 on TMM 40623-12 measured at the roots (alveolar) are 14.0 mm x 13.6 mm (L x W). This is much larger than A. stouti (Korth 2001a), A. nanus (Korth 2004) and near the largest reported dimensions for A. fluminis and A. suevicus (Stefen and Mörs, 2008), but smaller than the large castorid described by Mörs and Hulbert (2010). The alveolar tooth row length is difficult to measure, but is at least 39 mm.
The dimensions of p4 on TMM 40623-12 measured at the roots (alveolar) are 14.0 mm x 13.6 mm (L x W). This is much larger than A. stouti (Korth 2001a), A. nanus (Korth 2004) and near the largest reported dimensions for A. fluminis and A. suevicus (Stefen and Mörs, 2008), but smaller than the large castorid described by Mörs and Hulbert (2010). The alveolar tooth row length is difficult to measure, but is at least 39 mm.
I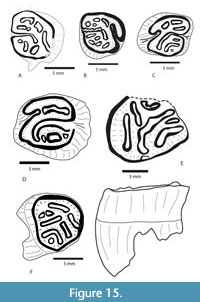 solated Cheek Teeth: Six isolated upper cheek teeth are illustrated in Figure 15 including two P4s, two M2s and two M3s at various stages of wear. These teeth are from the Burkeville, Moscow, Belts Creek, and Point Blank Local Faunas. Dimensions are summarized in Table 2. TMM 40197-2665 (Figure 15) is a moderately worn R M3 that was associated with the skull of the same specimen number. This tooth was embedded in the matrix surrounding the skull, but was rotated out of the alveolus. It has a well-developed mesoflexus that curves posteriorly and an antero-lingually oriented hypoflexus. There are four anterior fossettes and three posterior fossettes. The fossette pattern is somewhat simpler than A. fluminis (Korth and Emry, 1997), but more complex than A. nanus (Korth and Martin, 2007). The size is slightly smaller than A. fluminis, but significantly larger than A. nanus.
solated Cheek Teeth: Six isolated upper cheek teeth are illustrated in Figure 15 including two P4s, two M2s and two M3s at various stages of wear. These teeth are from the Burkeville, Moscow, Belts Creek, and Point Blank Local Faunas. Dimensions are summarized in Table 2. TMM 40197-2665 (Figure 15) is a moderately worn R M3 that was associated with the skull of the same specimen number. This tooth was embedded in the matrix surrounding the skull, but was rotated out of the alveolus. It has a well-developed mesoflexus that curves posteriorly and an antero-lingually oriented hypoflexus. There are four anterior fossettes and three posterior fossettes. The fossette pattern is somewhat simpler than A. fluminis (Korth and Emry, 1997), but more complex than A. nanus (Korth and Martin, 2007). The size is slightly smaller than A. fluminis, but significantly larger than A. nanus.
TMM 40622-13 (Figure 15) is a moderately worn left P4 with a well-developed mesoflexus that curves posteriorly and an antero-lingually oriented hypoflexus. The parafossette is broad and the metafossette is relatively narrow with accessory fossettes on either end. The lack of accessory anterior fossettes seems to differ from A. fluminis (Emry and Korth, 1997). This specimen is 9.6 mm x 10.7 mm at the occlusal surface (L x W) and broadens to 10.2 mm x 11.6 mm at the base of the enamel.
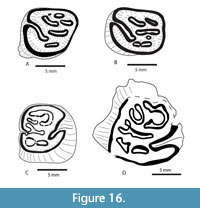 Four isolated lower cheek teeth are illustrated in Figure 16 including one p4, two m1’s, and one m2. Three of the four specimens are from the Moscow Local Fauna (TMM 31057), and one is from the Point Blank Local fauna (TMM 71). TMM 71-2667 is the only p4 in the collection (Figure 16). It is very worn and broken, but illustrates some of the occlusal pattern. It is a large tooth measuring 12.3 mm x 10.8 mm near the occlusal surface, but broadens to 13.3 mm x 12.5 mm at the base of the enamel. This is consistent with the alveolar measurements of p4 on TMM 40623-12 of 14.0 mm x 13.6 mm (L x W). The dimensions of p4 for A. buceei are similar to those reported for A. suevicus and for A. fluminis and much larger than A. nanus and A. stouti (Stefen and Mörs, 2008). The occlusal pattern is similar to A. suevicus with crescentic and branching fossettids.
Four isolated lower cheek teeth are illustrated in Figure 16 including one p4, two m1’s, and one m2. Three of the four specimens are from the Moscow Local Fauna (TMM 31057), and one is from the Point Blank Local fauna (TMM 71). TMM 71-2667 is the only p4 in the collection (Figure 16). It is very worn and broken, but illustrates some of the occlusal pattern. It is a large tooth measuring 12.3 mm x 10.8 mm near the occlusal surface, but broadens to 13.3 mm x 12.5 mm at the base of the enamel. This is consistent with the alveolar measurements of p4 on TMM 40623-12 of 14.0 mm x 13.6 mm (L x W). The dimensions of p4 for A. buceei are similar to those reported for A. suevicus and for A. fluminis and much larger than A. nanus and A. stouti (Stefen and Mörs, 2008). The occlusal pattern is similar to A. suevicus with crescentic and branching fossettids.
TMM 31057-185 and TMM 31057-163 are both very worn left m1s. The posteriorly directed hypoflexid is distinct. The anterior parafossetid complex includes four separate fossettids on TMM 31057-185 and only two on TMM 31057-163, while the mesofossettid is a single loop at this stage of wear. The metafossettid is a single loop on TMM 31057-185 but includes a pair of fossettids on TMM 31057-163 in addition to a small accessory fossettid. This variability is at least partly due to slight variation in the amount of wear on these two teeth.
TMM 31057-59 is a moderately well-worn right m2 with a distinct posteriorly directed hypoflexid, and a short mesoflexid. The parafossettid is crescent shaped as seen in specimens of A. suevicus (Stefen and Mörs, 2008). The metafossettid appears to be two separate fossettids.
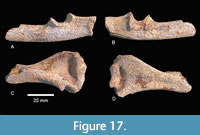 Post Cranial: Two post-cranial elements were recovered from the Point Blank Local Fauna (TMM 71) (Figure 17). A distal right humerus (TMM 71-2666) and a proximal right ulna (TMM 71-2666) were associated with the box of isolated teeth labeled “topotype material” discussed above. Both specimens are broken and appear to have been tumbled. The epicondylar width of the humerus is 32.0 mm. The trochlear surface is shallow as is the olecranon fossa. There is no trochlear foramen. The lateral supracondylar ridge is not strongly developed as it is in modern Castor canadensis. The olecranon process of the partial right ulna is relatively short. The trochlear notch is relatively shallow and the coronoid process is not strongly developed. Based on multiple measurements of the ulna from A. buceei and multiple specimens of C. canadensis in the TMM comparative collection, this element of A. buceei was approximately 25-30% larger than adult specimens of the modern American beaver.
Post Cranial: Two post-cranial elements were recovered from the Point Blank Local Fauna (TMM 71) (Figure 17). A distal right humerus (TMM 71-2666) and a proximal right ulna (TMM 71-2666) were associated with the box of isolated teeth labeled “topotype material” discussed above. Both specimens are broken and appear to have been tumbled. The epicondylar width of the humerus is 32.0 mm. The trochlear surface is shallow as is the olecranon fossa. There is no trochlear foramen. The lateral supracondylar ridge is not strongly developed as it is in modern Castor canadensis. The olecranon process of the partial right ulna is relatively short. The trochlear notch is relatively shallow and the coronoid process is not strongly developed. Based on multiple measurements of the ulna from A. buceei and multiple specimens of C. canadensis in the TMM comparative collection, this element of A. buceei was approximately 25-30% larger than adult specimens of the modern American beaver.
Discussion. Stenzel et al. (1944) discussed the stratigraphy and paleontology of Miocene sedimentary rocks exposed near Burkeville, TX. The area includes rocks of both brackish marine and non-marine facies including both marine invertebrate and non-marine vertebrate fossils. The significance of this stratigraphic relationship had been recognized for years and discussed in publications by Veatch (1902, 1906), Dall (1913), Ellisor (1936), Stenzel and Turner (1944), and others. In his 1942 paper, Hesse lists Amblycastor n. sp. (skull) from the A. & M. Museum Locality No. 42 (TAMU 42) (p. 175). This is undoubtedly a reference to TMM 40197-2665. Stenzel et al. (1944) list Amblycastor n. sp. Hesse, without a date, but cite a paper by C.J. Hesse listed as “Fossil Vertebrates of the Burkeville Area, Newton County, Texas”, American Journal of Science, in press (1944). Stenzel and Turner (1944) improperly list the name Amblycastor stirtoni citing the same Hesse paper as “in preparation”. Hesse never described this material nor officially proposed the name in a publication, as he died in 1945. An untitled, unpublished manuscript housed in the archives at the Texas Vertebrate Paleontology Archives (Mark Francis Papers, Series I: Correspondence/Manuscripts), includes a note from Hesse: “For a description of this material see Amblycastor stirtoni, in the discussion of the Burkeville fauna.” There is no record of that part of the manuscript, and discussion of the Burkeville fauna only includes the name Amblycastor n. sp. Although it seems clear that Hesse intended to name a new species of castorid, in fact, the specimens were never described in the literature and no holotype was ever specified. Furthermore, much of the cranial anatomy has only become observable since 2019 through preparation of TMM 40197-2665. The use of Amblycastor stirtoni, therefore, does not conform to the International Code of Zoological Nomenclature Article 13.1, and the name used in Stenzel and Turner (1944) is a nomen nudum.
Four specimens of a relatively large castorid were recovered from the Trinity River Pit 1 locality by Frick collectors in 1964 and are now curated at the AMNH (F:AM 64023A, B, F:AM 64024A, B). 64023A is a partial left maxilla with P4 and 64023B is an isolated P4. F:AM64024A is an edentulous partial left ramus, while 64024B is an isolated left lower cheek tooth. Due to inaccessibility during the Covid pandemic, these specimens have only been studied in photographs, but are consistent in size and morphology with A. buceei.
Matthew (1918) described the type material (AMNH 17213) for Amblycastor fluminis from the Snake Creek fauna of Nebraska. The holotype is a waterworn partial mandible with p4 and a partial i1. Additional specimens of A. fluminis from the Ba1 Olcott Formation have been described by Stirton (1935) and Korth (2017b). These Ba1 occurrences of A. fluminis include dentaries, fragments of dentaries, and isolated teeth. The skull of A. fluminis is only known from the Ba2 Valentine Formation (Korth and Emry, 1997). No skulls of A. suevicus are known from Europe. Anchitheriomys tungurensis is known from dentaries, partial dentaries, and isolated teeth. Stefen and Mörs (2008) reviewed the age constraints for A. tungurensis and concluded that it is from latest Barstovian to earliest Clarendonian equivalent faunas and therefore younger than both A. fluminis (Ba) and A. suevicus (MN5-MN6). The only other species of Anchitheriomys known from skull material is A. nanus from the early Hemingfordian Runningwater Formation in Nebraska (Korth and Martin, 2007).
The skull, dentary, and isolated teeth of A. buceei from the Ba1 Lagarto Formation of the Texas Coastal Plain are similar in size to A. fluminis, but exhibit a number of characters that differ from that taxon. Some characters are similar to A. nanus such as the grooved palatine. A. buceei represents a permissible member of the A. nanus - A. fluminis lineage.
Family Castoridae Hemprich, 1820
Subfamily Castoroidinae Allen, 1877
Tribe Castoroidini Allen, 1877
Genus Monosaulax Stirton, 1935
Steneofiber Cope, 1874
Type Species. Steneofiber pansus Cope, 1874; Barstovian (Miocene), New Mexico, USA.
Referred Species. Monosaulax curtus Matthew and Cook (1909); Monosaulax typicus Shotwell (1968); Monosaulax skinneri (in Evander, 1999); Monosaulax tedi Korth (1999).
Range. Barstovian (middle Miocene).
Monosaulax sp.
(Figure 18, Figure 19, Figure 20)
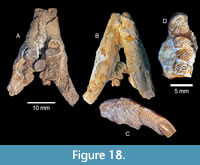 Description. A small castorid from two Ba1 local faunas of the Texas Coastal Plain is identified as Monosaulax sp. based primarily on dental and mandibular morphology and size. TMM 31057-300 (Moscow Local Fauna, Barringer Farm) is a partial maxilla with P4-M3 (Figure 18). TMM 31244-6 was collected by the Statewide Paleontologic-Mineralogic Survey at the Wanza Farm locality in San Jacinto County (Point Blank Local Fauna). The specimen includes a partial mandible with fragmentary right and left i1, p4-m3. P4-M2 of presumably the same individual is wedged between the right and left dentaries (Figure 18). This artifact of taphonomy results in a clear association of upper and lower cheek teeth. The cheek teeth are well worn in both specimens but appear to be mesodont. It is not possible to determine if the cheek teeth have two or three roots. The fourth premolar is the largest of the cheek teeth.
Description. A small castorid from two Ba1 local faunas of the Texas Coastal Plain is identified as Monosaulax sp. based primarily on dental and mandibular morphology and size. TMM 31057-300 (Moscow Local Fauna, Barringer Farm) is a partial maxilla with P4-M3 (Figure 18). TMM 31244-6 was collected by the Statewide Paleontologic-Mineralogic Survey at the Wanza Farm locality in San Jacinto County (Point Blank Local Fauna). The specimen includes a partial mandible with fragmentary right and left i1, p4-m3. P4-M2 of presumably the same individual is wedged between the right and left dentaries (Figure 18). This artifact of taphonomy results in a clear association of upper and lower cheek teeth. The cheek teeth are well worn in both specimens but appear to be mesodont. It is not possible to determine if the cheek teeth have two or three roots. The fourth premolar is the largest of the cheek teeth.
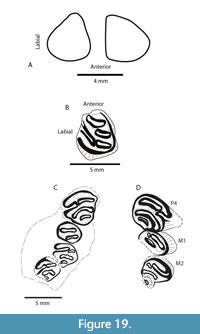 The incisors of TMM 31244-6 are subtriangular in cross section, rounded anteriorly, and have a slight rugosity, but lack ridges (Figure 19). The width of the left incisor where it is broken at the alveolus is 3.8 mm. The left p4 of TMM 31244-6 is 4.4 mm x 4.1 mm (L x W) at the occlusal surface but the length increases to 5.0 mm near the base of the enamel. The parafossetid is curved rather than straight, one of the characters separating Monosaulax from Eucastor (Korth, 1999). Accessory fossettes (fossettids) are absent in these two specimens and generally rare in the genus Monosaulax as compared to the similarly sized Temperocastor (Korth, 2008). At the moderate level of wear in both of the specimens, the hypoflexus (id) is the only striae (id) still open. All others have closed as fossettes (ids) indicating that the striae (ids) are short, a primitive character for the genus and similar to M. tedi (Korth, 1999) and M. typicus (Shotwell, 1968). Because the striae (ids) are short, the molars lack the S-pattern. At a similar degree of wear, the lingual striid is still open in M. pansus (RAM 7618) from the Barstow Formation (Lofgren et al., 2019). Korth (1999) stated that M. pansus has deeper lingual striids than M. tedi.
The incisors of TMM 31244-6 are subtriangular in cross section, rounded anteriorly, and have a slight rugosity, but lack ridges (Figure 19). The width of the left incisor where it is broken at the alveolus is 3.8 mm. The left p4 of TMM 31244-6 is 4.4 mm x 4.1 mm (L x W) at the occlusal surface but the length increases to 5.0 mm near the base of the enamel. The parafossetid is curved rather than straight, one of the characters separating Monosaulax from Eucastor (Korth, 1999). Accessory fossettes (fossettids) are absent in these two specimens and generally rare in the genus Monosaulax as compared to the similarly sized Temperocastor (Korth, 2008). At the moderate level of wear in both of the specimens, the hypoflexus (id) is the only striae (id) still open. All others have closed as fossettes (ids) indicating that the striae (ids) are short, a primitive character for the genus and similar to M. tedi (Korth, 1999) and M. typicus (Shotwell, 1968). Because the striae (ids) are short, the molars lack the S-pattern. At a similar degree of wear, the lingual striid is still open in M. pansus (RAM 7618) from the Barstow Formation (Lofgren et al., 2019). Korth (1999) stated that M. pansus has deeper lingual striids than M. tedi.
Monosaulax typicus (Shotwell, 1968) from the early Barstovian of southeastern Oregon is slightly smaller than Monosaulax sp. from the Texas Coastal Plain, but appears to be similar in terms of crown height and length of striae (ids). The occlusal patterns of M. typicus figured in Shotwell (1968) are similar to Monosaulax sp. from Texas except that the parafossettid on p4 is straighter. Shotwell (1968) stated that the cheek teeth of M. typicus were shorter crowned and possessed shorter striae (ids) than M. pansus. Monosaulax baylei from the early Hemingfordian Runningwater Formation of Nebraska (Korth, 2004) is slightly larger, but the p4 has a v-shaped parafossettid unlike the gently curved parafossettid in Monosaulax sp.
The p4 of TMM 31244-6 is significantly smaller than Monosaulax skinneri (Korth, 2008) (Figure 19). The p4-m3 tooth row length (13.1-13.4 mm) is shorter than M. skinneri (Korth, 2008), M. pansus (Korth, 2002) and M. baylei (Korth, 2004), and similar to M. tedi and M. curtus (Korth, 1999), but the latter has a straight parafossetid while that of TMM 31244-6 is curved. The tooth row length is just slightly longer than the measurements reported for M. typicus (Shotwell, 1968). The P4-M2 length of TMM 31244-6 is approximately 10.4 mm.
 The preserved length of the right dentary is 35.5 mm, and the minimum depth below the diastema is 9.5 mm. The diastema is relatively shallow. A single mental foramen is located anterior to p4 near the midline of the horizontal ramus and is 1.8 mm in its long dimension (Figure 20). There is no symphyseal flange. These characters are similar to the dentaries of M. typicus figured in Shotwell (1968). A virtual slice through the CT volume of TMM 31244-6 shows two roots on p4 (Figure 20). Korth (1999) described this as the common character state in all species of Monosaulax except for M. skinneri.
The preserved length of the right dentary is 35.5 mm, and the minimum depth below the diastema is 9.5 mm. The diastema is relatively shallow. A single mental foramen is located anterior to p4 near the midline of the horizontal ramus and is 1.8 mm in its long dimension (Figure 20). There is no symphyseal flange. These characters are similar to the dentaries of M. typicus figured in Shotwell (1968). A virtual slice through the CT volume of TMM 31244-6 shows two roots on p4 (Figure 20). Korth (1999) described this as the common character state in all species of Monosaulax except for M. skinneri.
The P4-M3 tooth row length of TMM 31057-300 is 12.9 mm. Individual tooth measurements are P4: 4.6 mm x 4.3 mm (L x W), M1: 2.8* mm x 3.6 mm (*broken), M2: 2.8 mm x 3.2 mm, M3: 2.7 mm x 2.7 mm. The occlusal patterns of the upper cheek teeth are simple with 2-3 fossettes and a hypoflexus. Even the hypoflexus on M1 has been lost with wear so the tooth only contains two fossettes (Figure 19).
Discussion. A small castorid from the early Barstovian (Ba1) Moscow and Point Blank Local Faunas is identified as Monosaulax sp. The small size, low crown height, lack of accessory fossettes(ids), short striae (ids), and lack of S-shaped molars are all interpreted to be primitive characters for the genus. The two specimens of Monosaulax from the Moscow and Point Blank Local Faunas are most similar to M. typicus, but minor differences in occlusal patterns (e.g., less curved parafossettid on M. typicus) and the limited number of specimens from Texas causes us to be cautious about that assignment.
Monosaulax is known from a number of Barstovian localities in North America. The older and more primitive species (M. typicus, M. tedi) are most similar to Monosaulax sp. from the Texas Coastal Plain. The suite of primitive characters and similarity with M. typicus are consistent with a Ba1 age assignment for the Moscow and Point Blank local faunas. Korth (1999) suggested that Monosaulax was close to the basal castoroidine. Prisaulax was described as a new genus by Korth and Bailey (2006) from the late Arikareean and early Hemingfordian of Nebraska, and they proposed a basal castoroidine phylogenetic position for that taxon. Monosaulax and Prisaulax appear to be closely related, and Korth (2017a) removed the species serundi from the genus Monosaulax and placed it in the genus Prisaulax.
DISCUSSION
Fossil castorids are rare in the Miocene vertebrate faunas from the Texas Coastal Plain. The first record appears to be that of Dumble (1915), who listed Hystricops sp. based on a report from W.D. Matthew concerning an “upper jaw with M1 and a lower molar” from “one and one-fourth miles north of Cold Springs”, TX. Stirton (1935) referred this material to Amblycastor, but did not examine it. The descriptions of Anchitheriomys buceei and Monosaulax sp. presented in this paper are based on specimens that were collected in the first half of the twentieth century. The holotype of the new species Anchitheriomys buceei was collected from outcrops near Burkeville, Texas, by H. Turner, F. Stenzel, and C. Hesse in 1941. A number of the referred specimens were collected between 1932 and 1940 from near Point Blank, TX (TMM 71) by C. Riley, a collector for Dr. Mark Francis. Dr. Francis and his collectors made important contributions to the knowledge of fossil vertebrates in Texas from the late 1800s through the 1920s. His contributions have been recognized by a number of taxa with patronyms reflecting his influence (e.g., Aleurodon francisi, Equus francisi, Rhynchotherium francisi). Many of the fossils collected by Francis were described by W.D. Matthew of the American Museum and by O.P. Hay of the Smithsonian Institution and, after his death, became part of the first museum at Texas A & M University, then known as the Museum of the Agricultural and Mechanical College of Texas. C.J. Hesse was hired in 1938 and promoted to curator at the museum in 1943. He recognized the significance of the castorid material from Miocene localities on the Texas Coastal Plain. Although it is clear from publications by Stenzel et al. (1944), Stenzel and Turner (1944), as well as archival material at the University of Texas that Hesse intended to name a new species, in fact, the specimens were never described in the literature, were never illustrated, and no holotype was ever specified. Hesse died in 1945 at the age of 40 before he could complete his study.
In addition to the TAMU material, specimens of Miocene castorids were collected by the Statewide Paleontologic-Mineralogic Survey including material from the Barringer Farm near Moscow (TMM 31057) collected by J.T. Gregory in 1940, and material from the Wanza Farm locality in San Jacinto Co. (TMM 31244) collected by G.H. Schafer in 1941. The material from Push Creek (TMM 40623) and Belts Creek (TMM 40622) was collected by J.A. Wilson in 1963.
Previous work on Miocene castorids from the Texas Coastal Plain included the recognition of rare occurrences from Arikareean and Hemingfordian faunas. Palaeocastor and perhaps Pseudopalaeocastor are reported from the Cedar Run Local Fauna and cf. Neatocastor from the Toledo Bend Local Fauna (Wood and Wood, 1937; Tedford et al., 2004; Albright, 1998). There is also an unpublished report of Palaeocastor from Garvin Gully (R.A. Stirton field notes, 1935). These occurrences are limited to isolated teeth and identifications remain uncertain. The palaeocastorines are best known from fossil burrows in the Great Plains and were interpreted by Rybczynski (2007) to be part of a North American clade of castorids associated with an open-habitat fossorial environments.
A number of Miocene castorid fossils in The University of Texas Vertebrate Paleontology Collection represent a large form now recognized as a new species of Anchitheriomys. Anchitheriomys buceei is distinct from other species of this genus based on a suite of cranial and dental characters. We interpret the skull, mandible, isolated cheek teeth, and limited post-cranial elements as all representing this new taxon. Reconstruction of the skull and measurements of the post-cranial material suggests that this castorid was about 20-30% larger than adult individuals of the modern Castor canadensis. Rybczynski (2007) proposed that Anchitheriomys was part of a “swimming clade” of Holarctic castorids. According to her analysis, semi-aquatic/woodcutting behaviors interpreted for this clade also appear to be associated with immigration between Europe and North America. The oldest occurrence of Anchitheriomys is from the early Hemingfordian Runningwater Formation of Nebraska (Korth, 2001a; Korth and Martin, 2007). Anchitheriomys suevicus and A. tungurensis appear in European and Asian faunas correlative with the Barstovian (Stefen and Mörs, 2008). Interpretation of migration between North America and Eurasia is based in part on the synonymy of Amblycastor with Anchitheriomys as discussed in Mörs and Hulbert (2010) and Korth (2017a).
A small castorid is identified as Monosaulax sp. The specimens from the Texas Coastal Plain represent a primitive form of the genus most similar to the early Barstovian M. typicus and M. tedi. Monosaulax is known from Barstovian faunas in North America and is thought to be a primitive member of the Eucastor-Dipoides-Castoroides lineage. Although not addressed in the analysis of Rybczynski (2007), the phylogenetic association of Monosaulax with castoridines such as Eucastor, Dipoides, and Castoroides suggests that Monosaulax was also a semi-aquatic form.
Both Anchitheriomys buceei and Monosaulax. sp. are currently known from Ba1 local faunas in the Texas Coastal Plain. These faunas include Prosynthetoceras, Floridatragulus, Merychippus, Protohippus, Cormohipparion, Aphelops, Peraceras, Teleoceras, Paratomarctus, Tomarctus? and Edaphocyon. The later Barstovian faunas from the upper part of the Lagarto Formation contain no known castorids, but are differentiated by the appearance of Calippus, Hipparion, Protohippus, Neohipparion, Aelurodon ferox, Aepycamelus, Longirostomeryx and Cranioceras. At least two of the Ba1 local faunas also contain the earliest records of fossil proboscideans in the Texas Coastal Plain.
ACKNOWLEDGMENTS
At the Texas Vertebrate Paleontology Collections, C. Sagebiel assisted with his knowledge of the curation of castorid specimens and with the database and K. Bader helped with the modern osteology collection. D. Wagner began preparation of the holotype specimen of Anchitheriomys buceei. M. Colbert and J. Maisano scanned and helped visualize data at The University of Texas at Austin High Resolution X-ray Computed Tomography (CT) Facility. P. Holroyd at the University of California Museum of Paleontology and A. Mellone at the American Museum of Natural History provided useful information concerning specimens in those collections. S. May benefited from correspondence with B. Welton, B. Albright, and W. Korth. This manuscript was improved by critical reviews by M. May and two anonymous reviewers. E. Cundieff helped us contact A. Aplin who is an important supporter of Texas wildlife including beavers. Project funding was provided in part by the Jackson School of Geosciences Geology Foundation and the Texas Historical Foundation.
REFERENCES
Albright, L.B. 1996. Insectivores, rodents, and carnivores of the Toledo Bend Local Fauna: an Arikareean (Earliest Miocene) assemblage from the Texas Coastal Plain. Journal of Vertebrate Paleontology, 16:458-473. https://doi.org/10.1080/02724634.1996.10011334
Albright, L.B. 1998. The Arikareean Land Mammal Age in Texas and Florida: southern extension of Great Plains faunas and Gulf Coastal Plain endemism. Geological Society of America Special Paper, 325:167-183. https://doi.org/10.1130/0-8137-2325-6.167
Allen, J.A. 1877. Castoroididae, Castoridae. In Coues, E. and Allen, J.A. (eds.), Monographs of North American Rodentia. Report of the United States Geological Survey of the Territories, 11:415-454, Government Printing Office, Washington, D.C.
Barnes, V.E., Hartmann, B.M., and Scranton, D.F. 1992. Geologic Map of Texas. The University of Texas at Austin, Bureau of Economic Geology.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia, for the use of Students and Travelers. J. Smith, Paris. 115 p.
Brown, M.A. 2013. The development of “modern” laboratory methods: a century of progress. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 103: 205-216. https://doi.org/10.1017/S1755691013000352
Cope, E.D. 1874. On a new mastodon and rodent. Proceedings of the Academy of Natural Sciences of Philadelphia, 26:221-223.
Dall, W.H. 1913. On a brackish water Pliocene fauna of the southern coastal plain. Proceedings of the United States National Museum, 46:225-237. https://doi.org/10.5479/si.00963801.46-2023.225
Dumble, E.T. 1915. Problem of the Texas Tertiary sands. Bulletin of the Geological Society of America, 26:447-476. https://doi.org/10.1130/gsab-26-447
Ellisor, A.C. 1936. Potamides mastoni Zone of Texas (Burkeville Beds). Bulletin of the American Association of Petroleum Geologists, 20: 494-495. https://doi.org/10.1306/3d932dd4-16b1-11d7-8645000102c1865d
Evander, R.L. 1999. Rodents and lagomorphs (Mammalia) of the Railway Quarries Local Fauna (Miocene, Barstovian) of Nebraska. Paludicola, 2:240-257.
Forsten, A.M. 1975. The fossil horses of the Texas Gulf Coastal Plain: a revision. Texas Memorial Museum Pierce-Sellards Series, 22:1-86.
Galloway, W.E., Jirik, L.A., Morton, R.A., and DuBar, J.R. 1986. Lower Miocene (Fleming) depositional episode of the Texas Coastal Plain and continental shelf: structural framework, facies, and hydrocarbon resources. Bureau of Economic Geology, Report of Investigations, 150, 50 pp., 20 figs., 4 tables, 1 appendix, 7 plates. https://doi.org/10.23867/ri0150d
Hemprich, W. 1820. Gundriss der Naturgeschichte fur hohere Lehranstalten, Entworfen von Dr. W. Hemprich. 432 pp. August Rucker, Berlin.
Hesse, C.J. 1942. A preliminary report on the Miocene vertebrate faunas of Southeast Texas. Proceedings and Transactions of The Texas Academy of Science, 1941, 25:157-179.
Hilgren, F.J., Lourens, L.J., and Van Dam, J.A., 2012, The Neogene Period. In Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G., (eds.) The Geologic Time Scale 2012, Elsevier, Oxford. https://doi.org/10.1016/B978-0-444-59425-9.00029-9
Hulbert, R.C. 1987a. Phylogenetic systematics, biochronology, and paleobiology of Late Neogene horses (Family Equidae) of the Gulf Coastal Plain and the Great Plains. Unpublished Ph.D. dissertation. University of Florida, Gainesville, Florida. https://doi.org/10.5962/bhl.title.42484
Hulbert, R.C. 1987b. Late Neogene Neohipparion (Mammalia: Equidae) from the Gulf Coastal Plain of Florida and Texas. Journal of Paleontology, 61:809-830. https://doi.org/10.1017/S0022336000029152
Hulbert, R.C. 1988a. Calippus and Protohippus (Mammalia, Perissodactyla, Equidae) from the Miocene (Barstovian-early Hemphillian) of the Gulf Coastal Plain. Bulletin of the Florida State Museum, Biological Sciences, 32:221-340.
Hulbert, R.C. 1988b. Cormohipparion and Hipparion (Mammalia, Perissodactyla, Equidae) from the Late Neogene of Florida. Bulletin of the Florida State Museum, Biological Sciences, 33:229-338.
Jordan, B.J., Yancey, T.E., Heinrich, P.V., Miller, B.V., McGuire, K. 2019. The age and geochemistry of volcanic ash in the Catahoula Formation of Louisiana, Mississippi, and Texas, USA. The Journal of Geology, 127: 207-222. https://doi.org/10.1086/701677
Korth, W.W. 1996. A new genus of beaver (Mammalia: Castoridae: Rodentia) from the Arikareean (Oligocene) of Montana and its bearing on castorid phylogeny. Annals of Carnegie Museum, 65:167-179. https://doi.org/10.5962/p.215134
Korth, W.W. 1999. A new species of beaver (Rodentia, Castoridae) from the earliest Barstovian (Miocene) of Nebraska and the phylogeny of Monosaulax Stirton. Paludicola, 2:258-264.
Korth, W.W. 2001a. A new species of Anchitheriomys (Rodentia, Castoridae) and a review of the Anchitheriomyine beavers from North America. Paludicola, 3: 51-55.
Korth, W.W. 2001b. Comments on the systematics and classification of the beavers (Rodentia, Castoridae). Journal of Mammalian Evolution, 8:279-296. https://doi.org/10.1023/A:1014468732231
Korth, W.W. 2004. Beavers (Rodentia, Castoridae) from the Runningwater Formation (Early Miocene, early Hemingfordian) of western Nebraska. Annals of Carnegie Museum, 73:1-11. https://doi.org/10.5962/p.316082
Korth, W.W. 2008. Cranial morphology, systematics and succession of beavers from the Middle Miocene Valentine Formation of Nebraska, USA. Acta Palaeontologica Polonica, 53:169-182. https://doi.org/10.4202/app.2008.0201
Korth, W.W. 2017a. A new tribe of castoridine beavers from the late Arikareean to Hemphillian (Oligocene-Miocene) of western North America. Acta Palaeontologica Polonica, 62:249-258. https://doi.org/10.4202/app.00339.2017
Korth, W.W. 2017b. Comments on the distinction between the Miocene beavers Anchitheriomys Roger, 1898, and Amblycastor Matthew, 1918 (Rodentia, Castoridae). Annals of Carnegie Museum, 84:179-181. https://doi.org/10.2992/007.084.0201
Korth, W.W. and Emry, R.J. 1997. The skull of Anchitheriomys and a new subfamily of beavers (Castoridae, Rodentia). Journal of Paleontology, 71:343-347. https://doi.org/10.1017/s0022336000039251
Korth, W.W. and Bailey, B.E. 2006. Earliest castoridine beaver (Rodentia, Castoridae) from the late Arikareean (Early Miocene) of Nebraska. Annals of Carnegie Museum, 75:237-245. https://doi.org/10.2992/0097-4463(2006)75[237:ecbrcf]2.0.co;2
Korth, W.W. and Martin, J.E. 2007. Additional cranial material of the Miocene beaver Anchitheriomys (Rodentia, Castoridae) from Nebraska. Bulletin of Carnegie Museum of Natural History, 39:165-171. https://doi.org/10.2992/0145-9058(2007)39[165:acmotm]2.0.co;2
Lofgren, D., Bi, A., Gardner, D., Henry, K., Raus, P., Stoddard, M., and Nalbandi, K. 2019. Paleoecology of the Slug Bed and other mollusk-bearing sites from the Barstow Formation. In Miller, D.D. (ed.), Exploring Ends of Eras in the Eastern Mojave Desert, 2019 Desert Symposium.
MacFadden, B.J. 1984. Systematics and phylogeny of Hipparion, Neohipparion, Nannippus, and Cormohipparion (Mammalia, Equidae) from the Miocene and Pliocene of the New World. Bulletin of the American Museum of Natural History, 179:1-196.
Martin, L.D. 1987. Beavers from the Harrison Formation (Early Miocene) with a revision of Euhapsis. In Martin, J.E. and Ostrander, G.E. (eds.). Papers in Vertebrate Paleontology in Honor of Morton Green. Dakoterra, 3: 73-91.
Matthew, W.D. 1918. Contributions to the Snake Creek Fauna. Bulletin of the American Museum of Natural History, 38:183-229.
Matthew, W.D. and Cook, H.J. 1909. A Pliocene fauna from western Nebraska. Bulletin of the American Museum of Natural History, 26:361-414.
May, S.R. 2019. The Lapara Creek Fauna: Early Clarendonian of south Texas, USA. Palaeontologia Electronica 22.1.15A:1-129. https://doi.org/10.26879/929
McAnulty, W.N. 1939. The Laboratory Unit. In Evans, G. (ed.), Manual of suggested procedures to be employed by the state-wide paleontologic-mineralogic survey in Texas, 73-91. Austin, TX: Bureau of Economic Geology. http://hdl.handle.net/2152/63113
Mörs, T. and Hulbert, R.C. 2010. Anchitheriomys Roger, 1898 or Amblycastor Matthew, 1918 (Rodentia, Castoridae)? Taxonomic implications of a mandible from the Miocene of Florida. Journal of Vertebrate Paleontology, 30:1899-1902. https://doi.org/10.1080/02724634.2010.521533
Morton, R.A., Jirik, L.A., and Galloway, W.E. 1988. Middle-Upper Miocene depositional sequences of the Texas Coastal Plain and continental shelf: geologic framework, sedimentary facies, and hydrocarbon plays. The University of Texas at Austin, Bureau of Economic Geology, Report of Investigations, 174, 40pp. https://doi.org/10.23867/ri0174d
Patton, T.H. 1969. Miocene and Pliocene artiodactyls, Texas Gulf Coastal Plain. Bulletin Florida State Museum Biological Sciences, 14:115-226.
Peterson, O.A. 1905. Description of new rodents and discussion of the origin of Daemonelix. Memoirs of the Carnegie Museum, 2:139-200.
Plummer, F.B. 1932. Cenozoic Systems in Texas. In Sellards, E.H., Adkins, W.S., and Plummer, F.B. (eds.), The Geology of Texas, Volume 1, Stratigraphy. The University of Texas at Austin, Bureau of Economic Geology Bulletin, 3232:519-818.
Prothero, D.R. and Manning, E.M. 1987. Miocene rhinoceroses from the Texas Gulf Coastal Plain. Journal of Paleontology, 61:388-423. https://doi.org/10.1017/S0022336000028559
Quinn, J.H. 1952. Recognition of hipparions and other horses in the middle Miocene mammalian faunas of the Texas Gulf region. University of Texas Bureau of Economic Geology Report of Investigations, 14:5-6.
Quinn, J.H. 1955. Miocene Equidae of the Texas Gulf Coastal Plain. Bureau of Economic Geology, The University of Texas at Austin, 5516:1-102.
Raffi, I., Wade, B.S., and Pälike, H., 2020, The Neogene Period. In Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G., (eds.) The Geologic Time Scale 2020, Elsevier, Oxford. https://doi.org/10.1016/B978-0-12-824360-2.00029-2
Reinhart, R.H. 1976. Fossil sirenians and desmostylids from Florida and elsewhere. Bulletin of the Florida State Museum Biological Sciences, 20:187-300.
Renick, B.C. 1936. The Jackson Group and the Catahoula and Oakville Formations in a part of the Texas Gulf coastal plain. The University of Texas Bulletin, 3619:1-104.
Roger, O. 1898. Wirbeltierreste aus dem Dinotheriensande der bayerischschwabischen Hochebene. Bericht des Naturwissenschaftlichen Vereins fur Schwaben und Neuberg, 33:1-46.
Rybczynski, N. 2007. Castorid phylogenetics: Implications for the evolution of swimming and tree-exploitation in beavers. Journal of Mammalian Evolution, 14:1-35.
https://doi.org/10.1007/s10914-006-9017-3
Schlosser, M. 1884. Die Nager des europaischen Tertiars nebst Betrachtungen uber die Organisation und die geschichtliche Entwicklung der Nager uberhaupt. Palaeontographica, 31:19-162.
Shotwell, J.A. 1968. Miocene mammals of southeast Oregon. University of Oregon Museum of Natural History Bulletin, 14:67 pp.
Stefen, C. and Mörs, T. 2008. The beaver Anchitheriomys from the Miocene of central Europe. Journal of Paleontology, 82: 1009-1020. https://doi.org/10.1666/06-049.1
Stenzel, H.B. and Turner, F.E. 1944. A Miocene invertebrate fauna from Burkeville, Newton County, Texas. American Journal of Science, 242: 289-308. https://doi.org/10.2475/ajs.242.6.289
Stenzel, H.B., Turner, F.E., and Hesse, C.J. 1944. Brackish and non-marine Miocene in southeastern Texas. Bulletin of the American Association of Petroleum Geologists, 28:977-1011. https://doi.org/10.1306/3d933696-16b1-11d7-8645000102c1865d
Stirton, R.A. 1934. A new species of Amblycastor from the Platybelodon beds, Tung Gur Formation, of Mongolia. American Museum Novitates, 694:1-4.
Stirton, R.A. 1935. A review of the Tertiary beavers. University of California Publications in Geological Sciences, 23:391-458.
Tedford, R.H., Galusha, T., Skinner, M.F., Taylor, B.E., Fields, R.W., Macdonald, J.R., Rensberger, J.M., Webb, S.D., and Whistler, D.P. 1987. Faunal succession and biochronology of the Arikareean through Hemphillian interval (late Oligocene through earliest Pliocene epochs) in North America, p. 153-210. In Woodburne, M.O. (ed.), Cenozoic Mammals of North America, Geochronology and Biostratigraphy. University of California Press, Berkeley and Los Angeles, California.
Tedford, R.H., Albright, L.B., Barnosky, A.D., Ferrusquia-Villafranca, I., Hunt, R.M., Storer, J.E., Swisher, C.C., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (late Oligocene through early Pliocene epochs). p. 169-231. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York. https://doi.org/10.7312/wood13040-008
VanderHoof, V.L. and Gregory, J.T. 1940. A review of the genus Aelurodon. University of California Publications in Geological Sciences, 25:143-164.
Veatch, A.C. 1902. The geography and geology of the Sabine River. Louisiana Geological Survey, Part 6, Report for 1902, Special Report 3, 136 pp.
Wang, X., Tedford, R.H., and Taylor, B.E. 1999. Phylogenetic systematics of the Borophaginae (Carnivora: Canidae). Bulletin of the American Museum of Natural History, 243:1-391.
Webb, S.D. and Hulbert, R.C. 1986. Systematics and evolution of Pseudhipparion (Mammalia, Equidae) from the late Neogene of the Gulf Coastal Plain and the Great Plains. Rocky Mountain Geology, 24 (special paper 3):237-272. https://doi.org/10.2113/gsrocky.24.special_paper_3.237
Wilson, J.A. 1956. Miocene formations and vertebrate biostratigraphic units, Texas coastal plain. Bulletin of the American Association of Petroleum Geologists, 40:2233-2246. https://doi.org/10.1306/5ceae571-16bb-11d7-8645000102c1865d
Wilson, J.A. 1960. Miocene carnivores, Texas coastal plain. Journal of Paleontology, 34:983-1000.
Wilson, R.W. 1960. Early Miocene rodents and insectivores from northeastern Colorado. The University of Kansas Paleontological Contributions, 7:1-92.
Wood, H.E. and Wood, A.E. 1937. Mid-Tertiary vertebrates from the Texas coastal plain: fact and fable. American Midland Naturalist, 18:129-146.
Young, S.C., Budge, T., Knox, P.R., Kalbouss, R., Baker, E., Hamlin, S., Galloway, W.E., and Deeds, N. 2010. Hydrostratigraphy of the Gulf Coast Aquifer from the Brazos River to the Rio Grande. Austin, Texas Water Development Board Report.

