 New Neogene anourosoricin shrews from northern Asia
New Neogene anourosoricin shrews from northern Asia
Article number: 25.3.a29
https://doi.org/10.26879/1209
Copyright Paleontological Society, October 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 15 January 2022. Acceptance: 26 September 2022.
ABSTRACT
The shrews of the Anourosoricini tribe (Soricomorpha: Soricidae) were a broadly represented group of the subfamily Soricinae in the Neogene of Palaearctica and show high taxonomic diversity, up to now mostly in Europe. In the current study, the generic diversity of northern Asian anourosoricin is expanded to four: Crusafontina, Ishimosorex gen. nov., Paranourosorex and Anourosorex. Our investigation of original material from 22 Russian and Kazakh localities allowed us to describe fossil material for two endemic northern Asian genera, Ishimosorex gen. nov and Paranourosorex. Based on the dental features and stratigraphic position, we consider early Ishimosorex gen. nov. and later Paranourosorex to represent a single evolutionary lineage. The Ishimosorex-Paranourosorex lineage existed from the Late Miocene (late Vallesian, MN 10) to early Pliocene (Ruscinian, MN 15) over a broad geographic range in northern Asia from southwestern Siberia to the Inner Mongolia region and consists of five species: Ishimosorex ishimiensis gen. et sp. nov., P. seletiensis, P. inexspectatus, Paranourosorex intermedius sp. nov. and P. gigas.
Vladimir S. Zazhigin. Geological Institute (GIN), Russian Academy of Sciences, Pyzhevskii per. 7, Moscow, 109017, Russia. zazhvol@gmail.com
Leonid L. Voyta. Zoological Institute (ZIN), Russian Academy of Sciences, Universitetskaya nab. 1, Saint Petersburg, 199034, Russia. leonid.voyta@zin.ru
Keywords: Soricidae; Anourosoricini; Paranourosorex; Crusafontina; Ishimosorex; northern Asia; Neogene; new genus; new species
Final citation: Zazhigin, Vladimir S. and Voyta, Leonid L. 2022. New Neogene anourosoricin shrews from northern Asia. Palaeontologia Electronica, 25(3):a29. https://doi.org/10.26879/1209
palaeo-electronica.org/content/2022/3702-neogene-northern-asian-anourosoricini
Copyright: October 2022 Paleontological Society
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/1726FDAE-2EE5-4145-A124-6D24287C0514
INTRODUCTION
The shrews of Anourosoricini Anderson, 1879 (Soricomorpha: Soricidae), a tribe of the subfamily Soricinae broadly represented in the Neogene of Palaearctica, show high taxonomic diversity mostly in Europe (Rzebik-Kowalska, 1998; van Dam, 2004, 2010; Mészáros, 1997, 1999a, b, 2014; Furió and Agustí, 2017). Presently known Neogene Asian anourosoricins comprise two genera: Anourosorex Milne-Edwards, 1870 (Storch and Qiu, 1991; Storch et al., 1998; Qiu and Storch, 2005) and Paranourosorex Rzebik-Kowalska, 1975 (Storch, 1995; Storch and Zazhigin, 1996; Storch et al., 1998; Qiu and Storch, 2000, 2005). Members of Anourosorex survive to the present as four separate modern species: Anourosorex squamipes Milne-Edwards, 1870, A. yamashinai Kuroda, 1935, A. assamensis Anderson, 1875 and A. schmidi Petter, 1963 (Hutterer, 2005; Burgrin and He, 2018). Extinct members of this genus are represented by four Chinese Pleistocene species, A. kui Young and Liu, 1951, A. edwardsi Zheng, 1985, A. qianensis Zheng, 1985 and A. triangulatidens Zheng and Zhang, 1991 (Reumer, 1997; Li et al., 2014), and the Japanese Pleistocene A. japonicus Shikama and Hasegwa, 1958, which Storch and Qiu (1991: 611) considered to be closely related to recent A. squamipes. Older Anourosorex oblongus Storch and Qiu, 1991, is currently known from two late Miocene sites in China, from the type locality Lufeng (Storch and Qiu, 1991; Shihuiba by Qiu and Storch, 2005; MN 11-12) and Leilao (MN 11). New findings of Anourosorex (referred to “ Anourosorex, sp. nov.”) are known from the late Miocene Shuitangba site in China (Jablonski et al., 2014). The Paranourosorex genus was first described from the early Pliocene Podlesice site in Poland as Paranourosorex gigas by Rzebik-Kowalska (1975); however, this genus had a primarily Asian distribution (Storch, 1995; Storch and Zazhigin, 1996; Storch et al., 1998). Its representatives are known from western late Miocene and early Pliocene localities of China (Inner Mongolia), Kazakhstan (northern territories) and Russia (southwestern Siberia) against known Anourosorex from eastern China (Storch 1995; Storch and Zazhigin, 1996; Storch et al., 1998; Qiu and Storch, 2000, 2005), with expansion to Europe, where they rarely occurred in Ruscinian faunas (MN 14-15) in Eastern European Plain sites (Agadjanian and Kowalski, 1978; Topachevsky et al., 1988; Agadjanian, 2009) and elsewhere in Eastern Europe (Rzebik-Kowalska, 1975, 1998; Rzebik-Kowalska and Lungu, 2009; Rzebik-Kowalska and Recovets, 2016). At present, three species of Paranourosorex have been described: P. inexspectatus (Schlosser, 1924), P. gigas Rzebik-Kowalska, 1975 and P. seletiensis Storch and Zazhigin, 1996. In addition, in the paper by Storch and Zazhigin (1996), two undetermined forms were “ Paranourosorex sp. 1” from the Kazakh late Miocene Pavlodar 1A site (Turolian, MN 12) and “ Paranourosorex sp. 2” from two Russian early Pliocene sites, Borki 1A (Ruscinian, MN 14) and Novaya Stanitsa 1A (ibid.). The main purpose of the current paper is a reevaluation of the Storch and Zazhigin (1996) material from 22 late Miocene to early Pliocene localities in Russia (southwestern Siberia) and Kazakhstan with a special evaluation of the undetermined forms Paranourosorex sp. 1 and Paranourosorex sp. 2.
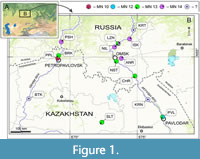 Geological setting. The fossils of Anourosoricini come from 22 late Miocene to early Pliocene sites in southwestern Siberia and northern and northeastern Kazakhstan (Figure 1; Appendix 1): Andreevka (ANR/1A, 2A), Borki 1 (BRK/1A, 1B, 1C), Biteke (BTK), Cherlak 1 (CHR/1A), Isakovka (ISK/1A, 2A), Krasnokutsk (KRN), Kartashovo (KRT), Lezhanka (LZN/1A, 2B), Nizhneil'inka (NIL), Novaya Stanitsa 1 (NST/1A, 1B), Petropavlovsk 1 (PPL/1A), Peshnevo 1 (PSH/1A, 1B), Pavlodar (PVL/1A, 2 ‘Quarry’) and Selety 1 (SLT/1A). These sites were mentioned by Storch and Zazhigin (1996), Storch et al. (1998), Vasilyan et al. (2017), Zazhigin and Voyta (2019). Lithological and biostratigraphic descriptions of the localities have been published in the following articles: Zazhigin and Zykin (1984), Zazhigin (2006), Zykin et al. (1987, 2007) and Zykin (2012).
Geological setting. The fossils of Anourosoricini come from 22 late Miocene to early Pliocene sites in southwestern Siberia and northern and northeastern Kazakhstan (Figure 1; Appendix 1): Andreevka (ANR/1A, 2A), Borki 1 (BRK/1A, 1B, 1C), Biteke (BTK), Cherlak 1 (CHR/1A), Isakovka (ISK/1A, 2A), Krasnokutsk (KRN), Kartashovo (KRT), Lezhanka (LZN/1A, 2B), Nizhneil'inka (NIL), Novaya Stanitsa 1 (NST/1A, 1B), Petropavlovsk 1 (PPL/1A), Peshnevo 1 (PSH/1A, 1B), Pavlodar (PVL/1A, 2 ‘Quarry’) and Selety 1 (SLT/1A). These sites were mentioned by Storch and Zazhigin (1996), Storch et al. (1998), Vasilyan et al. (2017), Zazhigin and Voyta (2019). Lithological and biostratigraphic descriptions of the localities have been published in the following articles: Zazhigin and Zykin (1984), Zazhigin (2006), Zykin et al. (1987, 2007) and Zykin (2012).
The 22 localities and their fossiliferous sediments with mammalian remains are attributed to seven regional stratotypic units: the late Miocene Ishim Formation (Zykin, 2012: 188, figure 4.6), late Miocene Pavlodar Formation (Zazhigin and Lopatin, 2001; Zazhigin et al., 2002; Zykin, 2012: 192, figure 4.10), late Miocene Novaya Stanitsa Formation (Zykin, 2012: 209, figure 4.12), late Miocene Rytov Formation (Zykin, 2012: 222), late Miocene Kedey Formation (Zazhigin, 2006), early Pliocene Isakov Formation (Zykin and Zazhigin, 2004; Zykin, 2012: 232, figure 4.13) and early Pliocene Peshnev Formation (Zykin and Zazhigin, 1984; Zykin, 2012: 238). The fossiliferous sediments of Borki/1A are attributed to the Novaya Stanitsa Formation, but the material contained in it probably came (partly at least) from the older underlying layers of the Ishim Formation. Topographically, the BRK/1A sediments lay on Ishim Formation sediments. The sediments of Nizhneil'inka are attributed to the Krutogor Formation (Zykin, 2012: 250), but its material is also probably redeposited from the older underlying layers of the Isakov Formation. In three localities, Biteke, Krasnokutsk and Kartashovo, the fossils were redeposited in Middle-late Pleistocene sediments. For these localities, the regional stratigraphic context is uncertain.
Institutional abbreviations. GIN, Geological Institute of the Russian Academy of Sciences, Moscow; ZIN, Zoological Institute of the Russian Academy of Sciences, St. Petersburg.
MATERIAL AND METHODS
The material of Anourosoricini investigated in this study comprises 184 fossils from 22 late Miocene to early Pliocene localities (Appendix 1). The findings primarily consist of isolated teeth, dentary fragments and rare skull fragments (Appendix 2).
Dental nomenclature follows Reumer (1984), Dannelid (1998) for the general terms and Lopatin (2006: S212) for the special terms of dental cristae and cristids. We do not use the term “W-shaped ectoloph” (Storch, 1995; Storch and Zazhigin, 1996; van Dam, 2004; and others), because we follow to the Lopatin nomenclature, in which the ectoloph compounds only a ‘middle part’ of the W-shaped line of shrew upper molars. To describe the W-shaped buccal crests of upper molars and its states we must discuss the combined features preparacrista + (postparacrista + premetacrista = centrocrista, ectoloph) + postmetacrista (see Lopatin, 2006: S213). Accordinly, in our description we use ‘W-shaped line of upper molar buccal crests.’ In a dentition description, we also use two narrowly used terms, ‘exaenodonty’ and ‘dimily.’ Exaenodonty is differences between lingual (shorter) and buccal (longer) sides of a tooth base, sense Rich et al. (2001:1). Dimily is tendency to reduce posterior molars, which inherent Dimylidae Schlosser, 1887 (Soricomorpha) and some Soricidae (Heterosoricinae: Ingentisorex Hutchison, 1966; Soricinae: Amblycoptus Kormos 1926 and Kordosia Mészáros, 1997). The term was introduced by Lopatin (2005: 139).
Pictures of the craniomandibular remains and isolated teeth were taken with a digital camera (Canon EOS 60D) combined with a macro lens (Canon MP-E 65 mm). Features on the images were twice measured with tpsDig ver. 2.31 (Rohlf and Slice, 1990) to minimize ‘metering error.’ The statistical analysis was performed on the mean values of these replicates. The measurements were mainly taken according to Reumer (1984; for lower teeth) and van Dam (2004; for upper teeth) with some additions (added measurements: HI1c, LBcA1, LLgA1, MRWc, PLi1, RHI1); the measurements are illustrated in Appendix 3. All measurements are given in mm. Calculations were performed in PAST ver. 2.04 and ver. 3.15 (Hammer et al., 2001). The studied taxa measurements are provided in Appendix 4. High-resolution images were acquired using an electronic scanning microscope (Zeiss ESEM Quanta 250), with the surfaces covered by platinum sputter coating; some images were taken by using X-ray computed micro-tomography (NeoScan N80); the images were performed using equipment of the Core Facilities Centre “Taxon” of the Zoological Institute of the Russian Academy of Sciences (Saint Petersburg, Russia).
Association of fragmentary findings or ‘size recovery’ approach. The main problem of the current analysis is the presence of many poor sites with fragmentary remains, which represent a supposed taxon by upper teeth in one site and lower teeth in another site. Because the material should be analysed in view of the synthesis of disparate data on a finite number of known or unknown taxa, we attempted to associate fragmentary remains from different sites. To complete this task, we implemented the Larramendi (2016:539) approach for calculation of a proboscidean shoulder height using dimensions of the known mounted skeletons as references for a determination of ratio between bone lengths within the anatomical position in percents. The author has taken the ‘manus height’ as 100% and determined the percentages of each bone contribution for different proboscidean taxa. This approach allowed Larramendi (2016) and many others (e.g., Haynes, 2017; Haynes et al., 2018) to use fragmentary skeletal elements (i.e., not associated elements) for the large mammals body size calculation based on a scapula length (scapula-related shoulder height), humerus length (humerus-related shoulder height) or another bone.
We applied the Larramendi strategy to test the association of disparate elements to the same set/taxon as follows:
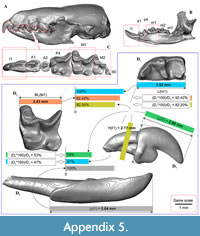 (a) determining the relationship between particular pairs of the dental and skull elements based on a 'sister' taxon (as reference); where, a ‘pair of particular elements’ is dictated by the fossils in question (e.g., first upper incisors from Petropavlovsk 1A site vs first lower incisors from Borki 1A site; details see in the main text); the relationship is determined as ratio between longer (100%) and shorter (percents of the longer element) elements. This phase is illustrated in the appendix (Appendix 5: figure 5.1).
(a) determining the relationship between particular pairs of the dental and skull elements based on a 'sister' taxon (as reference); where, a ‘pair of particular elements’ is dictated by the fossils in question (e.g., first upper incisors from Petropavlovsk 1A site vs first lower incisors from Borki 1A site; details see in the main text); the relationship is determined as ratio between longer (100%) and shorter (percents of the longer element) elements. This phase is illustrated in the appendix (Appendix 5: figure 5.1).
(b) ‘Size recovering’ approach implementation. We divide the matching phase to two steps: (b1) the element size recovery via the accepted ratio of the reference and using a simple formula [(A, mm*REF,%)/100) = B, mm], where, ‘A, mm’ is an observed measurement of the first anatomic element from one fossiliferous site (Appendix 5: figure 5.2: ‘Site AA’); ‘REF,%’ is reference value for the analysed pair of elements; ‘B, mm’ is a ‘supposed’ measurement of the second element from another site (Appendix 5: figure 5.2: 'Site BB'); (b2) matching the ‘supposed’ and ‘observed’ (Appendix 5: figure 5.2: ‘C, mm’) measurements of the second element in mm. We support the association two elements (i.e., they belong to a same set/taxon) when the supposed (recovered) and observed values lay within an allowable interval, which is determined by comparisons to reference set (RST). In this case, RST is any sample with five and more specimens (modern groups) or certainly associated fossils. In the current analysis RST is represented by the samples of A. squamipes (n = 17; completed skulls and jaws; ZIN Collection), several pairs of elements of P. gigas and P. seletiensis (GIN Collection). This phase is illustrated in the appendix (Appendix 5: figure 5.2). Museum numbers of RSTs are provided in the appendix (Appendix 5: table 5.1). Second table (Appendix 5: table 5.2) consists of supposed and observed values for a new species Ishimosorex ishimiensis gen. et sp. nov. after the ‘size recovering’ procedure; observed values in green mark association of the compared anatomic elements, and pink marks dissociation.
SYSTEMATIC PALAEONTOLOGY
Class MAMMALIA Linnaeus, 1758
Order EULIPOTYPHLA Waddell, Okada, and Hasegawa, 1999
Suborder SORICOMORPHA Gregory, 1910
Family SORICIDAE Fischer, 1817
Subfamily SORICINAE Fischer, 1817
Tribe ANOUROSORICINI Anderson, 1879
Genus ISHIMOSOREX gen. nov.
zoobank.org/67243F45-1EBF-46CC-A2C7-E6B73680B820
Type species. Ishimosorex ishimiensis sp. nov., by monotypy, see below.
Diagnosis. Small-sized anourosoricin shrew. I1 with an elongated and relatively low crown, and short slightly curved root; the hatchet-like (blade-like) talon with expressed narrow, deep notch (like a carnassial notch of lower molars). M1 has a subquadrate occlusal shape with a long and narrow hypoconal flange; the mesostyle is well-developed and protruded buccally; the metaloph is short and separated from the metacone base. i1 with the moderately upturned tip and two slender denticles (bicuspulate) on the cutting edge. p4 has a bulbous-like shape with the round (spot-shaped) wear facet and the very weak expressed central crest; the ecto- and entocingulids are wide and well developed. The hypoconid of m1 and m2 is buccally protruded and overhangs the base of the crown; the entostylid is presented as a small bulge and does not reach the entoconid level by height; entocristid is short. The interarticular area of the condylar process is moderately wide.
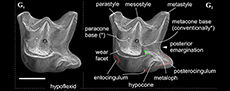
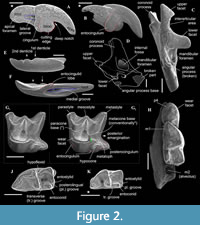 Differential diagnosis. Ishimosorex gen. nov. differs from Paranourosorex in presence of the deep notch and the long crown of I1 and its relatively short root (Figure 2A); in the equal lengths of the lingual and buccal sides of M1; in the deeper posterior emargination of M1 and more posterior protrusion of the hypoconal flange relative the hypocone position (Figure 2G); in presence of two denticles on the cutting edge of i1 and its long medial groove (Figure 3); in the wider interarticular area of the condylar process and the relatively large fossa of the temporal muscle of the mandibular ramus.
Differential diagnosis. Ishimosorex gen. nov. differs from Paranourosorex in presence of the deep notch and the long crown of I1 and its relatively short root (Figure 2A); in the equal lengths of the lingual and buccal sides of M1; in the deeper posterior emargination of M1 and more posterior protrusion of the hypoconal flange relative the hypocone position (Figure 2G); in presence of two denticles on the cutting edge of i1 and its long medial groove (Figure 3); in the wider interarticular area of the condylar process and the relatively large fossa of the temporal muscle of the mandibular ramus.
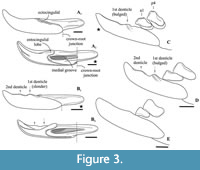 Ishimosorex gen. nov. differs from Crusafontina in the general proportion of M1 (subsquare vs. trapeziform outline shape), more expressed W-shaped line of the M1 buccal crests, more backwardly protruded the hypoconal flange of M1 (Figure 2G cf. M1 of C. endemica from Puente Minero 2, Teruel Basin by van Dam, 2004: figure 2-3); in the slender basal (first) denticle of i1 (Figure 3); in the bulbous-like crown of p4 with the extremely weak expression of the central crest (Figure 4); in a prominent buccal shifting of the protoconid of m1-m2 from the longitudinal axis of the crown and more central position of the entoconid of m1-m2 (Crusafontina has a more central position of the protoconid of m1 and a lingually shifted the entoconids of m1-m2 relatively to a basal crown outline; see van Dam, 2004: figure 2).
Ishimosorex gen. nov. differs from Crusafontina in the general proportion of M1 (subsquare vs. trapeziform outline shape), more expressed W-shaped line of the M1 buccal crests, more backwardly protruded the hypoconal flange of M1 (Figure 2G cf. M1 of C. endemica from Puente Minero 2, Teruel Basin by van Dam, 2004: figure 2-3); in the slender basal (first) denticle of i1 (Figure 3); in the bulbous-like crown of p4 with the extremely weak expression of the central crest (Figure 4); in a prominent buccal shifting of the protoconid of m1-m2 from the longitudinal axis of the crown and more central position of the entoconid of m1-m2 (Crusafontina has a more central position of the protoconid of m1 and a lingually shifted the entoconids of m1-m2 relatively to a basal crown outline; see van Dam, 2004: figure 2).
 Ishimosorex gen. nov. differs from Amblycoptus and Kordosia in the general proportion of I1 with the thin talon (Amblycoptus and Kordosia both show lateromedial inflation of the talon and expressed bulge of the buccal cingulum); in the proportion and outline shape of M1 (similar to Crusafontina buccal crest condition, see above); in the presence of a denticulation of the cutting edge of i1 (Figure 3); in more inflated the anterior part of p4 crown (‘subrectangle’ shape vs ‘subtriangle’ shape in Amblycoptus and Kordosia; Figure 4); in lesser exaenodonty of p4 crown; in a more buccal shifting of the protoconid and hypoconid of m1 from the longitudinal axis of the crown (Figure 2; also see van Dam, 2004: figure 5). The extreme dimily of Ishimosorex gen. nov. is doubtful but cannot be test due to lack of data.
Ishimosorex gen. nov. differs from Amblycoptus and Kordosia in the general proportion of I1 with the thin talon (Amblycoptus and Kordosia both show lateromedial inflation of the talon and expressed bulge of the buccal cingulum); in the proportion and outline shape of M1 (similar to Crusafontina buccal crest condition, see above); in the presence of a denticulation of the cutting edge of i1 (Figure 3); in more inflated the anterior part of p4 crown (‘subrectangle’ shape vs ‘subtriangle’ shape in Amblycoptus and Kordosia; Figure 4); in lesser exaenodonty of p4 crown; in a more buccal shifting of the protoconid and hypoconid of m1 from the longitudinal axis of the crown (Figure 2; also see van Dam, 2004: figure 5). The extreme dimily of Ishimosorex gen. nov. is doubtful but cannot be test due to lack of data.
Ishimosorex gen. nov. differs from Darocasorex in the general proportion of M1 outline shape (Darocasorex shows an anteroposterior compression of M1); in absence of the ectocingulid of i1; in absence of expressed crests of p4 (Darocasorex has the developed buccal and short lingual crests); in a more longitudinal direction of the oblique crestid of m1 and m2 (see van Dam, 2010).
Ishimosorex gen. nov. differs from Anourosorex in the general proportion of I1 and the buccal cingulum condition (Anourosorex has a short inflated ectocingulum); in more expressed W-shaped buccal crests and significantly lesser developed of the parastyle of M1 (Anourosorex has an extremely developed inflated and buccally protruded parastyle; see Storch and Qiu, 1991); in the bicuspulate i1; in the absence of the expressed and sharp central crest of p4 (Anourosorex has very developed, sharp and high central crest); in presence of the ecto- and entocingulids of p4; in the crown proportion of m1-m2 (similar to Amblycoptus and Kordosia see above).
Etymology. After the Ishim river, located in North Kazakhstan, the provenance of the fossil remains, and from Latin sorex, shrew.
Stratigraphic and geographic range. As for the only included species.
Ishimosorex ishimiensis sp. nov.
Figure 2
zoobank.org/DFBFD952-8B5C-4392-8522-867F4694C1E1
Type locality. Petropavlovsk 1A, right slope of the Ishim River Valley, within Petropavlovsk town (ca. N54°54' E69°07'), North Kazakhstan Region, Kazakhstan (Figure 1: PPL).
Type horizon. Stratotypic locality of Ishim Formation (ish), late Miocene (MN 10, ⁓9.7-8.7 Ma). Material was collected by VZ during fieldworks of 1964, 1976, 1980, 1982 and 1987.
Type material. Holotype: GIN 952/1153–right dentary fragment with p4-m1, the anterior alveolus of m2. Paratypes: (n = 7): GIN 952/1152–left fragment of mandibular ramus with whole coronoid and condylar processes; GIN 952/1154–isolated left m2; GIN 952/1055–isolated left M1; GIN 952/1158–isolated right m1; GIN 952/1159–isolated damaged crown part of the left i1; GIN 952/1160–isolated right I1 with damaged root; GIN 952/1161–isolated left I1.
Location of types. Holotype and paratypes in the collection of GIN, Moscow (Russia).
Etymology. As for genus name, after the Ishim river.
Material. Type material and four remains from type locality (GIN 952/1157–damaged right first lower molar; GIN 952/1400–damaged left P4; GIN 952/1401–damaged left P4; GIN 952/1402–damaged left P4); and three rediposited remains (GIN 1115/1148–worn left I1, crown part; GIN 1115/1149–right i1; GIN 1115/1150–left i1) from Borki 1A locality (Appendix 2: 1).
Measurements. See Appendix 4.
Diagnosis. As for genus.
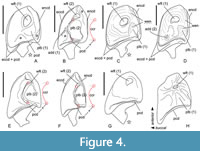
Description. I1 has an elongated massive crown; the large hatchet-like talon bears the sharp cutting edge and expressed narrow, deep notch; the apex of the incisor is not fissident. The buccal cingulum is wide and well expressed without a bulge and reaches half of the crown height. The root is notably shorter and more slender than the crown (Figure 3A, B). Only three damaged fragments of the left P4 without lingual part are known. The buccal part of these teeth has a relatively short postparacrista; the small parastyle is weakly separated from the paracone base. M1 has a subquadrate occlusal shape with an approximately equal length of the lingual and buccal sides (BL/LL ratio; see Appendix 4); the mesostyle is well-developed and buccally protruded outward of the M1 base level; the buccal crests (preparacrista + ectoloph + postmetacrista) are represented as an expressed W-shaped line; the metaloph is short and separated from the metacone base; the hypocone is rounded distinct cusp and shifted to the lingual margin of the tooth; the weak ridge lays across the hypocone tip obliquely. The posterior emargination of M1 is relatively deep; the hypoconal flange is narrow and elongated backward (Figure 2G). The relative distance between the hypocone and the posterior margin of the flange is distinctly larger than the other taxa have (see comparisons below). The crown of i1 is two times longer than the root (along the lateral side). The medial groove of the root is long and overreaches the crown-root junction. The cutting edge of i1 is bicuspidate; the basal (first) cuspule is slender without any bulge-like expression (opposite state to Crusafontina); the distal cuspule is also weak expressed; an ectocingulid is absent (Figure 2E, F). p4 has a massive and rounded crown; the central (single) wear facet is a rounded or ‘spot-shaped’ (opposite a ‘comma-shaped’ in Crisafontina; see van Dam, 2004: 744). The ecto- and entocingulids are well developed; the ectocindulid continuously passed to postcingulid (Figure 4A). p4 bears a shallow posterolingual basin and extremely weak the central crest, which doesn’t reach the postcingulid. The lower molars are graded in size: the first molar is the largest, the second is moderately smaller than the first (Figure 2H-K); the third molar is unknown. The hypoconid of m1 and m2 is protruded buccally and overhangs the base of the crown; the entostylid is presented as the small distinct bulge and does not reach the entoconid level. The entocristid is short and steeply descends to the metaconid base. The talonid basin opens posterolingually between the entoconid and entostylid through the posterolateral groove and lingually between the end of the entocristid and the metaconid base through the transverse groove. The narrow and well-distinguished ectocingulid does not reach the anterior side of the tooth (Figure 2J, K). The buccal side of m1 has a slightly wrinkled enamel surface. The ectocingulid of m2 is well developed along the tooth base with the plate-like extension in the first third (paraconid level).
The horizontal ramus of the lower jaw is narrow; the small mental foramen is situated slightly backwards of the m1 protoconid level without groove. The mandibular ramus is relatively low; its anterior border is notably tilted backwards. The internal fossa of the temporal muscle ('internal fossa') is moderately developed. The condylar process bears the widely divided upper and lower articular facets and a moderately broad interarticular area (Figure 2C, D).
Association of fragments. The studied remains from PPL/1A were each matched using the size recovery approach and several RSTs, A. squamipes and two Paranourosorex samples (Appendix 5: tables 5.1 and 5.2) for the following pairs of dental and mandibular elements and measurements: m1/M1: measurements L(m1)/BL(M1); m1/I1: measurements L(m1)/H(I1); I1/i1: measurements L(I1)/L(i1); m1/mandibular ramus: measuremets L(m1)/COR and L(m1)/MRWc. Most of the matched pairs displayed compliance; e.g., the matched m1 and M1 corresponded to each other in size based on the RST ratio. Noncompliance (pink blocks in Table 5.2 of Appendix 5) was revealed for matched upper and lower incisors from PPL/1A (and BRK/1A), i.e., the observed dimension of the lower incisor was less than the supposed dimension calculated based on the Anourosorex ratio (Appendix 5: Table 5.1). This noncompliance between calculated and observed incisor sizes could be a specific feature of the particular species, which can be explained in terms allometric heterochrony (sense Mitteroecker et al., 2005: 250). This feature (the ratio between upper and lower incisors) in Ishimosorex ishimiensis gen. et sp. nov. differs from Anourosorex. In addition, the short lower incisor of Ishimosorex ishimiensis gen. et sp. nov. can probably be compensated through a relatively long mandible protraction during a chewing cycle (for details, see below remark on a phenomenon of ‘cutting edges straightening’). Proof of this is the best value for the ratio L(m1)/L(i1); i.e., the Anourosorex ratio for L(m1)/L(i1) corresponds more to the observed ratio in Ishimosorex ishimiensis gen. et sp. nov. than to the ratio L(I1)/L(i1). Thus, the studied remains from PPL/1A can be treated here as belonging to Ishimosorex ishimiensis gen. et sp. nov. based on metric matching (Appendix 5: Table 5.2).
Comparisons. Ishimosorex ishimiensis gen. et sp. nov. (L(p4) = 1.75 mm; L(m1) = 2.47-2.73 mm, see Appendix 4) differs in larger size from C. endemica (L(m1) = 1.90-2.12 mm, see van Dam, 2004: table 4), Crusafontina exculta (Mayr and Fahlbusch, 1975) (L(m1) = 1.81-2.21 mm, see Prieto and van Dam, 2012), Crusafontina fastigata van Dam, 2004 (L(p4) = 1.43-1.44 mm, ibid.) and Crusafontina minima (Hutchinson and Bown, 1980) (L(m1) = 1.77-1.93 mm, see Bown, 1980: table VI). Ishimosorex ishimiensis gen. et sp. nov. differs from Crusafontina vandeweerdi van Dam, 2004 (L(p4) = 2.24 mm, van Dam, 2004: table 4) in its smaller size. Ishimosorex ishimiensis gen. et sp. nov. shows the similar teeth size to Crusafontina kormosi (Bachmayer and Wilson, 1970) (L(m1) = 2.38-2.84 mm, see Mészáros, 1998: table 3) and Crusafontina magna (Hutchinson and Bown, 1980) (L(m1) = 2.30-2.52 mm, Hutchinson and Bown, 1980: table V) but differs from both species in the well-developed mesostyle of M1, which contrary to C. kormosi and C. magna shows buccal protrusion of parastyle and metastyle tips (this is also a general difference from Anourosorex spp.); in the extremely weak central crest and the spot-shaped wear facet of the p4 opposite well-developed central crest and comma-shaped wear facet of the p4 of C. kormosi (Figure 4); p4 of C. magna is unknown.
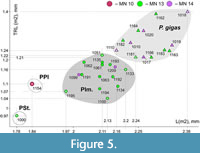 Ishimosorex ishimiensis gen. et sp. nov. differs from P. seletiensis in a slightly larger size; from Paranourosorex intermedius sp. nov. and P. gigas in a distinctly smaller size (Figure 5). Ishimosorex ishimiensis gen. et sp. nov. differs from P. inexspectatus in a slightly smaller size (Appendix 4). In addition, Ishimosorex ishimiensis gen. et sp. nov. morphometrically differs from the known Paranourosorex species in longer trigonid of m1 regarding the tooth length (TRL = 62.5% of m1 length) than show P. seletiensis (TRL = 57% of m1 length), Paranourosorex intermedius sp. nov. (TRL = 56% of m1 length) or P. gigas (TRL = 54% of m1 length).
Ishimosorex ishimiensis gen. et sp. nov. differs from P. seletiensis in a slightly larger size; from Paranourosorex intermedius sp. nov. and P. gigas in a distinctly smaller size (Figure 5). Ishimosorex ishimiensis gen. et sp. nov. differs from P. inexspectatus in a slightly smaller size (Appendix 4). In addition, Ishimosorex ishimiensis gen. et sp. nov. morphometrically differs from the known Paranourosorex species in longer trigonid of m1 regarding the tooth length (TRL = 62.5% of m1 length) than show P. seletiensis (TRL = 57% of m1 length), Paranourosorex intermedius sp. nov. (TRL = 56% of m1 length) or P. gigas (TRL = 54% of m1 length).
The differences of Ishimosorex ishimiensis gen. et sp. nov. from species of Amblycoptus, Kordosia and Darocasorex were described in the new genus comparisons (see above the Differential Diagnosis section).
Remarks. Ishimosorex ishimiensis gen. et sp. nov. shows an intermediate combination of 'omnivorous' features of Paranourosorex and ‘carnivorous’ features of Crusafontina in the dentition. M1 bears expressed W-shaped buccal crests and a buccally protruding well-developed mesostyle similar to Paranourosorex conditions. In addition, both taxa have a moderately developed parastyle of M1, the bulbous and anteriorly inflated p4 without sharp crests and wide lower molars (buccal shifting of the protoconid and hypoconid). These features are intended for tearing and crushing, i.e., they can be determined as ‘omnivore-like features.’ In contrast, Crusafontina (American and European species, except C. vandeweerdi), Amblycoptus, Kordosia and Anourosorex show different degrees of straightening of the buccal crests and increasing (from Crusafontina to Kordosia) parastyle size of M1. The change in the parastyle together with the trapeziform M1 outline of these taxa probably are related to carnivorous adaptations such as the lengthening of cutting edges; e.g., to the sharp and well-developed central crest of p4 of American and European Crusafontina; the central position of the protoconid of m1 and the corresponding lengthening of the preprotocristid of Crusafontina, Amblycoptus, Kordosia and Anourosorex to different degrees. Thus, some characteristics of Ishimosorex ishimiensis sp. nov. together with C. vandeweerdi can be determined as ‘carnivore-like’ compared with pronounced carnivorous anourosoricins. The deep notch in the talon of I1 and two sharp, slender denticles of the cutting edge of i1 are similar to the American and European species of the more carnivorous Crusafontina.
Stratigraphic and geographic range. Known from the type locality of the species (Petropavlovsk 1A, MN 10, Ishim Formation; North Kazakhstan Region) and Borki 1A locality (MN 13, Novaya Stanitsa Formation; North Kazakhstan Region) to which the material was redeposited from Ishim Formation.
Genus PARANOUROSOREX Rzebik-Kowalska, 1975
Type species. Paranourosorex gigas Rzebik-Kowalska, 1975
Previous diagnosis. Storch and Zazhigin (1996: 259) stated, “Large-sized shrews. Mandibular articulation highly specialized, lower articular facet shifted anteriorly and interarticular area formed by very narrow ridge. P4/4 and M1/1 accentuated, M2/2 and M3/3 reduced. M1/ with W-shaped ectoloph and labially protruding mesostyle, with slight posterior emargination and usually a distinct metaconule. Lower molars with well-developed hypoflexid. P/4 inflated and with strong tendency to reduce the posterolingual basin. Parastyle P4/ protruded anteriorly and protocone shifted lingually. Trigonid on M/1 not particularly elongated. M/1-2 with moderately to weakly developed entocristids. Upper incisor not fissident. Lower incisor with smooth cutting edge. Three upper antemolars and one lower antemolar in front 4th premolar. The teeth are rather bulbous; cingula are well developed. Coronoid process strong and spatulate, with prominent coronoid spicule; internal temporal fossa located anteriorly.”
Emended diagnosis. From small-sized to large-sized shrews. The generalized Anourosoricini dental formula with three upper (A1-A3) and one lower (a1) antemolars and retained third molars (M3 and m3); the dental formula is 1.3.1.3/1.1.1.3. The teeth bear generalized omnivorous characters such as the massive and bulbous-like lower teeth, the hatchet-like talon of I1, the wrinkled enamel on different parts of the teeth, the expressed W-shaped buccal crests line of M1 with well-developed mesostyle, a presence of the short metaloph; the extremely short metastyle (i.e., short postmetacrista) of M2; the wide trigone basin of M1 and trigonid and talonid basins of m1-m2, the well-developed hypoflexid of m1-m2, p4 subrectangle in shape with the spot-shaped wear facet and extremely weak developed the crown structures (central crest and posterolingual basin).
Differential diagnosis. Paranourosorex differs from Crusafontina, Amblycoptus, Kordosia and Anourosorex in its best developed parastyle of P4 together with a long postcrista; in the mostly expressed W-shaped line of the M1 buccal crests and distinctly buccal protruding of the mesostyle together with the weak developed posterior emargination of P4 and M1 (i.e., a short hypoconal flange); in the subrectangular inflated p4 without developed crown elements and developed ecto- and entocingulids (the compared genera have p4 subtriangle in shape with developed central crista and posterolingual basin in different degree); in the shape of I1 talon (the compared genera have hatchet-like talon with deep notch); in relatively short and wide m1 and m2 (the compared genera have the elongated m1 and m2). In addition, Paranourosorex differs from Crusafontina in the smooth cutting edge of i1 (Figure 3) and the strongly shorter postmetacrista of M2 together with expressed W-shaped buccal crests of this tooth in general. In addition, Paranourosorex differs from Amblycoptus, Kordosia and Anourosorex also in the dental formula composition (Anourosorex : 1.2.1.3/1.1.1.3; expressed dimily in Amblycoptus : 1.3.1.2/1.1.1.2; and Kordosia : 1.2.1.2/1.1.1.2). Differences between Paranourosorex and Ishimosorex gen. nov. see above. Paranourosorex differs from Darocasorex in the general proportion of M1 outline shape (Darocasorex shows an anteroposterior compression of M1).
Remarks. Paranourosorex is probably the Asian origin group that supposedly arose long before the first appearance of P. seletiensis in the regional paleontological record. The main evolutionary trend of the genus connects with body size increase of species from early to latter members while maintaining omnivorous, which is marked by basic dental characters adapted for tearing and crushing and consumption of various types of food resources. After the study of Storch and Zazhigin (1996) we revised the available north Asian material and expanded the regional species list for Russia and Kazakhstan to three taxa: P. seletiensis Storch and Zazhigin, 1996, Paranourosorex intermedius sp. nov. and P. gigas.
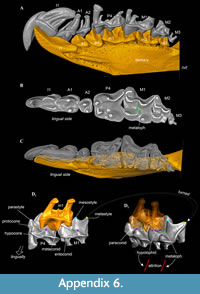 The Storch and Zazhigin (1996: 259) diagnosis includes several morphological traits that need clarification. A highly specialized craniomandibular articulation is a common character of the tribe. A statement “usually a distinct metaconule” is not entirely accurate; in our interpretation, Anourosoricini has not a true metaconule of M1. Actually, we can see a short metaloph on an unworn tooth. Due to the fact that a metaloph does not reach a metacone base, we observe a metaconule-like bulge on a worn tooth. In addition, the metaloph area of M1 and posterior border of the m1 talonid (hypolophid/entostylid area) shows variations in presence/absence of important characters precisely because these areas are in occlusal contact (Appendix 6) and susceptible to wear.
The Storch and Zazhigin (1996: 259) diagnosis includes several morphological traits that need clarification. A highly specialized craniomandibular articulation is a common character of the tribe. A statement “usually a distinct metaconule” is not entirely accurate; in our interpretation, Anourosoricini has not a true metaconule of M1. Actually, we can see a short metaloph on an unworn tooth. Due to the fact that a metaloph does not reach a metacone base, we observe a metaconule-like bulge on a worn tooth. In addition, the metaloph area of M1 and posterior border of the m1 talonid (hypolophid/entostylid area) shows variations in presence/absence of important characters precisely because these areas are in occlusal contact (Appendix 6) and susceptible to wear.
Stratigraphic and geographic range. At present, the distribution includes 22 localities from Kazakhstan and Russia (Appendix 2) and two European localities (Rzebik-Kowalska, 1975, 1998; Rzebik-Kowalska and Lungu, 2009). The stratigraphic range covers an interval between the late Miocene Kedey Formation (Turolian, MN 12/13; Kazakhstan) to early Pliocene (Ruscinian, MN 14, Russia (Peshnev Formation); Ruscinian, MN 15, Slovakia).
Paranourosorex seletiensis Storch and Zazhigin, 1996
Figure 6A, B
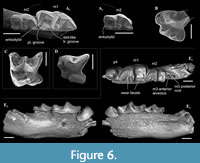 Material. Left hemimandible with m1-m2, alveoli of m3 and slightly damaged mandibular ramus without angular process (GIN 951/1000, holotype); isolated left P4 without end of the postparacrista (GIN 951/1001, paratype) from Selety 1A locality. One paratype specimen, isolated right i1 (GIN 951/1002), is lost (Appendix 2: 2).
Material. Left hemimandible with m1-m2, alveoli of m3 and slightly damaged mandibular ramus without angular process (GIN 951/1000, holotype); isolated left P4 without end of the postparacrista (GIN 951/1001, paratype) from Selety 1A locality. One paratype specimen, isolated right i1 (GIN 951/1002), is lost (Appendix 2: 2).
Description. Smallest Paranourosorex species. The general proportion of P4 similar to other Paranourosorex species: the moderately developed parastyle is separated from the paracone base by the shallow groove; the well-developed anterior notch between the parastyle and protocone; the well-developed massive cone-like hypocone and the shallow posterior emargination; the parastyle shifts lingually from the paracone longitudinal axis; the postcingulum begins with well-distinguished cingular cuspule (Figure 6B). The hypolophid of m1 ends by the entostylid, which visibly turns backwards; the talonid basin of m1 opens posterolingually by the long posterolingual groove; the entocristid separated from metaconid base by the narrow slot-like transverse groove (Figure 6A). The hypolophid and entoconid of m2 are heavily worn. The lower molars have narrow well-distinguished ectocingulids.
Measurements. See Appendix 4.
Remarks. P. seletiensis clearly differs from other Paranourosorex species in smallest size (Appendix 4). P. seletiensis differs from P. inexspectatus (according to the description of Storch, 1995, and Storch and Zazhigin, 1996) and P. gigas in the more expressed and turned entostylid of m1; in longer posterolingual groove between the entostylid and entoconid of m1; in presence of the slot-like groove between the entocristid and the metaconid base (other compared species have a wider groove).
Stratigraphic and geographic range. At present, the distribution includes only the type locality (Appendix 2) the late Miocene Kedey Formation (Turolian, MN 12/13).
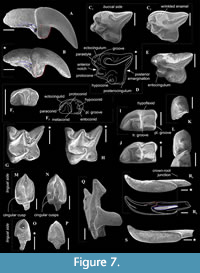
Paranourosorex intermedius sp. nov.
Figure 6E, Figure 7B, C-D, F-G, I, K, M, O, Q-R
zoobank.org/FA6D006C-2C2A-4860-BE5A-BBAD043A2D49
 1996 Paranourosorex sp. 2 Storch and Zazhigin, p. 264, figs. 3c, 4l-o.
1996 Paranourosorex sp. 2 Storch and Zazhigin, p. 264, figs. 3c, 4l-o.
Type locality. Novaya Stanitsa 1A, right slope of the Irtysh River Valley near Novaya Stanitsa village (ca. N54°50' E73°24'), Omskaya Oblast', Russia (Figure 1: NST).
Type horizon. Stratotypic locality of Novaya Stanitsa Formation (nst), late Miocene (Turolian, MN 13). Material was collected by VZ during fieldworks of 1980s, 2000 and 2001.
Type material. Holotype: GIN 948/1051–right dentary fragment with moderately worn p4 and heavily worn m1-m3. Paratypes: (n =12): GIN 948/1052–left dentary fragment with i1, m1 and alveoli of a1-p4 (i1 heavily and m1 moderately worn); GIN 948/1056–isolated right m1 (slightly worn); GIN 948/1063–isolated left m2 (slightly worn); GIN 948/1064–isolated right m3 (slightly worn);GIN 948/1065–isolated left P4 (slightly worn); GIN 948/1069–isolated right M1 (metacone damaged; moderately worn); GIN 948/1070–isolated right M1 (paracone and metacone damaged; moderately worn); GIN 948/1073–isolated left I1 (slightly worn); GIN 948/1075–isolated right i1 (tip of root is broken off; moderately worn); GIN 948/1078–isolated left p4 (without roots; unworn); GIN 948/1081–isolated left a1 (slightly damaged and worn); GIN 948/1089–isolated left A1 (slightly worn).
Location of types. Holotype and paratypes in the collection of GIN, Moscow (Russia).
Etymology. From the Latin intermedius, intermediate (between smallest P. seletiensis and largest P. gigas).
Material. Type material and 27 remains from type locality (NST/1A), 60 remains from other 8 localities: NST/1B (n = 1), BRK/1A (n = 26), BRK/1B (n = 18), LZN/2B (n = 8), PVL/2 (n = 1), ISK/1A (n = 1), ISK/2A (n = 3), KRT (n = 1) (see Appendix 2: 3).
Measurements. See Appendix 4.
Diagnosis. Large-sized Paranourosorex species. Lower teeth, mostly p4 and m1, are inflated. Upper antemolar row consists of three teeth with vestigial A3. P4 has trapeziform shape with a deep anterior notch and very shallow the posterior emargination. M1 has subquadrate shape with the expressed W-shaped buccal crests, moderately expressed short metaloph without the metacone base contact. p4 is moderately exaenodont, bulbous-like in shape with the spot-shaped wear facet; the posterolingual basin of p4 is very weak. The entocristid of m1 is separated from the metaconid base by a distinctly marked transverse groove; the hypolohid is low; the small entostylid is weakly separated from the entoconid base.
Description. I1 crown proportional in size to the root; the talon has an oblique tilted cutting edge without any notch in the base; the ectocingulum is moderately developed and rises up to the middle of the crown (Figure 7B). The upper antemolars are dramatically decreased in size from the largest A1 to middle-sized A2 (less than 1/2 of A1) and vestigial A3. The addition of cingular cusps (usually one cusp) on the lingual part of the crown is developed on A1; the entocingulum of A2 is smooth. A1 and A2 have developed both anterior and central crests; A1 displays a wrinkled enamel (Figure 7M). P4 has trapeziform crown, developed anterior notch and the shallow posterior emargination. The parastyle is separated from the paracone base by a shallow groove. P4 has a short ectocingulum, well-developed postcingulum and wrinkled enamel on the paracone buccal side (Figure 7C); the entocingulum is absent. M1 has subquadrate occlusal shape with the expressed W-shaped line of the buccal crests. The metaconule is absent; the metaloph is short and separated from the base of the metacone (Figure 7G). The posterior emargination of M1 is shallow. M2 has well-developed cones; the parastyle is longest, the mesostyle is buccally prominent, the metastyle undeveloped; the hypocone round in the base with a spot-shaped wear facet. M3 unknown. i1 has a smooth cutting edge and distinctly upturned tip. The ectocingulid of i1 is undeveloped. The medial groove of the root is short and did not overreach the crown-root junction. a1 has a bulbous-like crown and massive root; the crown with well-developed ecto- and entocingulids, spot-shaped wear facet and short anterior crest. The central crests of a1 is undeveloped. The inflated p4 bears the spot-shaped wear facet, extremely weak developed the crown structures and distinctly wrinkled enamel (Figure 4C, Figure 7K). m1 is relatively short and wide and so seems slightly inflated. The enamel surface of the trigonid buccal side is slightly wrinkled. The protoconid and metaconid of m1 are brought closer, the hypoconid and entoconid are widely separated. The hypoflexid of m1 is a deep almost vertical groove that does slightly not reach the ectocingulid. The hypolophid is worn in different degrees in all examined specimens, but despite this, we can see two morphotypes: the hypolophid is weakly separated from the entoconid, the entostylid is absent and the posterolingual groove very weak (Morphotype I, GIN 948/1056, NST/1A) (Figure 7I); the hypolophid is clearly separated from the entoconid, the small entostylid and the shallow and short posterolingual groove are present (Morphotype II, GIN 1115/1126, BRK/1B). The entoconid is high and relatively narrow; the entocristid is separated from the metaconid base by a narrow transverse groove. The ectocingulid is well-distinguished along the base of the tooth; entocingulid is absent. m2 notably less than m1; the crown bears the high trigonid and the low talonid. The entostylid is absent; the hypolophid contacts the entoconid without the groove. The entocristid is separated the base of the metaconid by a narrow slot. The ectocingulid m2 is very well-developed along the tooth base with the plate-like extension in the first third (paraconid level). The small m3 has the well-developed trigonid and the reduced talonid without a talonid basin; the talonid consists of the small hypoconid and entoconid that separated by the shallow posterolingual groove (Figure 7F). The ectocingulid of m3 bears the plate-like extension in the first third that is similar to m2.
A single left premaxilla-maxilla fragment with I1 and A2 from PSH/1B (GIN 1118/1027) was examined. I1 is moderately worn; double-rooted A2 is slightly worn. Two alveoli of A1 indicate the presence of the small separated anterior root of A1. Thus, A1 and A2 are both double-rooted.
The mandibular body is long and relatively low without a visible inward curve of the lower outline. The mental foramen is positioned under the protoconid of m1 without any grooves in a wide and shallow depression. The mandibular ramus is massive with a small internal fossa for the temporal muscle; its maximal length is lesser than the condylar height (HC). The coronoid process includes a well-developed long coronoid spicule. The single mandibular foramen is large. The lower articular facet of the condylar process shifts far anteriorly; an interarticular area is relatively narrow (Figure 7Q). An angular process is unknown.
Comparisons. Paranourosorex intermedius sp. nov. differs from P. seletiensis in a larger size (Figure 5; Appendix 4) and some dental characters (the first species has longer hypoconal flange of P4 and more inflated m1 and m2 than has the second species; Figure 6B cf. Figure 7D). Paranourosorex intermedius sp. nov. differs from P. inexspectatus in a slightly larger size; in the characters of P4 such as a more developed anterior notch and a more shallow posterior emargination, absent the entocingulum; in a more developed metastyle of M1 (the metastyle of P. inexspectatus is relatively shorter and narrower); in a more lingual position of the hypocone and a slightly deeper posterior emargination of M1 (for P. inexspectatus see Storch, 1995: plate 4). Paranourosorex intermedius sp. nov. differs from P. gigas in a slightly smaller size; in a more shallow anterior notch and posterior emargination of P4 (Figure 7C cf. E); in a more developed metastyle of M1 (the metastyle of P. gigas is relatively narrower); in a more lingual position of the hypocone, a slightly longer hypoconal flange and a slightly deeper posterior emargination of M1; in absence of the entocingulum of M1 (Figure 7G cf. H); in strongly exaenodont buccal half of a1 (Figure 7O cf. P); in presence of a weak posterolingual groove of m1 (P. gigas has not the groove, the hypolophid contacts the entoconid; Morphotype I).
Remarks. The fossils of Paranourosorex intermedius sp. nov. correspond to ‘ Paranourosorex sp. 2’ in Storch and Zazhigin (1996: 264). That description comprises several discordant features that require clarification. Authors stated: “its [p4] apex is weakly two-cusped.” We re-evaluated the apex of p4 as a distinctly single cusp with a spot-shaped wear facet (Figure 7K). Authors also stated: “the hypolophid on M/1 is separated from the entoconid by a groove (even in worn specimens).” We found two morphotypes of hypolophid/entoconid of m1 state when the hypolophid is weakly separated from the entoconid with a very weak groove, either the hypolophid is clearly separated from the entoconid with the small entostylid and the shallow and short posterolingual groove.
Much material of Paranourosorex intermedius sp. nov. originates from NST/1A (n = 39), BRK/1A (n = 26) and BRK/1B (n = 18). The species is more poorly represented in the other nine sites (Appendix 2). Paranourosorex intermedius sp. nov. probably coexisted with larger shrew P. gigas in CHR/1A (Turolian, MN 13, Rytov Formation), ISK/1A (Ruscinian, MN 14, Isakov Formation), PSH/1A and PSH/1B (Ruscinian, MN 14, Peshnev Formation) localities.
Stratigraphic and geographic range. At present, the distribution includes the type locality and 11 localities from Kazakhstan and Russia (Appendix 2) between the late Miocene Novaya Stanitsa Formation (Turolian, MN 13) and the early Pliocene Peshnev Formation (Ruscinian, MN 14).
Paranourosorex gigas Rzebik-Kowalska, 1975
Figure 6A, E, H, J, L, N, P, S
1978 Crocidura sp. Agadjanian and Kowalski, p. 33.
1988 Paranourosorex sp. Topachevsky et al., p. 20.
2009 Soricidae gen. Agadjanian, p. 102.
Material. The analysed material of P. gigas comprises remains mainly from CHR/1A (n = 20) and PSH/1B (n = 33) localities. Single remains originate from ISK/1A (n = 1), NIL (n = 2), PSH/1A (n = 1), ANR/1A (n = 2), ANR/2A (n = 1), LZN/1A (n = 3), BRK/1C (n = 1) and KRN (n = 2) localities (see Appendix 2: 4).
Description. Largest Paranourosorex species. In general, the dental characters are typical for the genus but some teeth display specific characters that distinguish this species such as the most undulated and sculptured P4 outline between the parastyle and hypocone with the well-developed anterior notch, the short portion of the entocingulum under the protocone, the moderately developed notch before the hypocone and the protocone lobe; the relatively deep posterior emargination of P4 (Figure 7E); the moderately developed metastyle of M1; the massive cone-like hypocone of M1, relatively short the hypoconal flange and the shallow posterior emargination of M1 (Figure 7H); M1 has a weak entocingulum under the protocone; the moderately exaenodont a1 has a wide postcingulid (Figure 7P); the hypolophid of m1 contacts the entoconid base without a groove; a tip of the entoconid of m1 is slightly shifted anteriorly; the entocristid of m1 is separated from the metaconid base through the moderately developed transverse groove. m3 is unknown.
Measurements. See Appendix 4.
Remarks. P. gigas is the biggest species of the tribe, especially in comparison to P. seletiensis (Figure 5). To differentiate P. gigas from Paranourosorex intermedius sp. nov. see above. VZ has seen materials from Obukhovka 1 referred to as ‘ Paranourosorex sp.’ (Topachevsky et al., 1988); from Antipovka and Chugunovka referred to as ‘ Crocidura sp.’ by Agadjanian and Kowalski (1978) and ‘Soricidae gen.’ by Agadjanian (2009) and determined all findings as P. gigas.
Much material of P. gigas originates from CHR/1A (n = 20) and PSH/1B (n = 33).
Stratigraphic and geographic range. At present, the distribution includes the type locality Podlesice (early Pliocene, Ruscinian, MN 14, Poland), Slovakian locality (early Pliocene, Ruscinian, MN 15) (Rzebik-Kowalska, 1975, 1998), three Eastern European Plain localities such as Obukhovka 1 (Topachevsky et al., 1988), Antipovka and Chugunovka (Agadjanian and Kowalski, 1978; Agadjanian, 2009; early Pliocene, Ruscinian, MN 14) and also 10 localities from Kazakhstan and Russia (Appendix 2). The stratigraphic range in Asia covers an interval between the late Miocene Rytov Formation (Turolian, MN 13) and early Pliocene Peshnev Formation (Ruscinian, MN 14).
Genus CRUSAFONTINA Gibert, 1974
Type species. Crusafontina endemica Gibert, 1975
1966 Anourosoricodon Topachevsky, p. 91. (? nomen oblitum)
1980 Anouroneomys Hutchison and Bown in Bown, 1980, p. 105, figs. 2d, 3-6, 7d.
Emended diagnosis. See van Dam (2004).
Remarks. VZ has seen type material of Anourosoricodon pidoplitschkoi Topachevsky, 1966 (no. 42-1, a fragment of the right dentary with i1 and m1, and alveoli; see Topachevsky, 1966; Gureev, 1979: 461) from the early Pliocene locality on the left shore of the Kakhovskoie water storage basin near Kamenskoie Village (Ukraine), stored in the Zoological Institute of the Ukraine Academy of Sciences (Kiev, Ukraine) and revealed the conspecifity between Anourosoricodon Topachevsky, 1966 and Crusafontina Gibert, 1974. Thus, Anourosoricodon is the senior synonym. According to Article 23 of ICZN (1999) Anourosoricodon is a valid genus name. However, we propose to use point 23.9.3 of ICZN (1999) further refer the matter to the Commission for a ruling under the plenary power with the proposal to use the junior synonym to maintain the stability of the Anourosoricini tribe system because Crusafontina name used 47 years and clearly corresponds to particular European taxa and time span. Previously, van Dam (2004: 763) referred to Nesin and Nadachowski (2001) and used Crusafontina as a synonym of Anourosoricodon without taxonomic remarks.
Type specimen of Anourosoricodon pidoplitschkoi corresponds to C. kormosi in the i1 and m1 qualitative features and size of m1 (L(m1) = 2.6 mm, see Topachevsky, 1966; Gureev, 1979: 461).
Stratigraphic and geographic range. At present, the distribution includes numerous European localities (Rzebik-Kowalska, 1998; van Dam, 2004), two North American localities (Bown, 1980) and two Asian localities with two undetermined forms, Crusafontina sp. 1 (Pavlodar 1A, Kazakhstan) and Crusafontina sp. 2 (Selety 1A, Kazakhstan). The stratigraphic range covers an interval between middle Miocene (MN 7/8) to early Pliocene (MN 15) of Europe (see van Dam, 2004: appendix 1); the New World late Miocene late Clarendonian Stage (Juntura Formation) and the early Hemphillian Stage (Ash Hollow Formation; see Bown, 1980); and Asian late Miocene interval restricted by the Turolian Pavlodar (MN 12) and Kedey (MN 12/13) Formations (Kazakhstan).
Crusafontina sp.1
Figure 6C
1996 Paranourosorex sp. 1 Storch and Zazhigin, p. 261, fig. 4a.
2004 Crusafontina? vandeweerdi ? van Dam, p. 763.
Material. Isolated left M1 (GIN 640/1004) from Pavlodar 1A locality (Appendix 2: 5).
Description. The large and moderately worn first upper molar displays clearly defined crown features. The buccal crests are expressed W-shaped line like a condition of Paranourosorex (Figure 6D cf. Figure 7G). The metaconule is absent; the short metaloph is similar to the Paranourosorex condition and has a weak broadening of the base (‘poorly developed metaconule’ by van Dam 2004: 752) and contacts with the metacone base. The hypocone is elongated in the base and shifts to the lingual margin of the flange. The posterior emargination is well-expressed; the hypoconal flange is protruded posteriorly and narrow in shape.
Measurements. BL(M1) = 2.54 mm; AW(M1) = 2.68 mm; PW(M1) = 2.56 mm; LL(M1) = 2.42 mm; PE(M1) = 2.07 mm (abbr. see in Appendix 3).
Remarks. The M1 is slightly larger than the known M1 of C. vandeweerdi (BL = 2.48 mm, AW = 2.58 mm, see van Dam, 2004: 750) and distinctly larger than other species of Crusafontina. The crown features of M1 such as the buccal crests shape and the buccal outline are similar to Paranourosorex conditions. This fact did not lead Storch and Zazhigin (1996) to determine this molar as a Crusafontina. On the other hand, the authors could not associate this molar to Paranourosorex without a doubt because it has deep posterior emargination, contact between the metaloph and the base of the metacone and the narrow hypoconal flange. Later, van Dam (2004) supposed the conspecifity of the Pavlodar’s form and the new Spanish species C. vandeweerdi (Teruel Basin, MN 12), pointed its intermediate feature conditions to both Crusafontina and Paranourosorex. The species has Crusafontina -like p4 with a central crest and distinct posterolingual basin; the ecto- and entocingulids of p4 are absent. We determined the Pavlodar’s molar as Crusafontina sp. 1 due to the absence of any tooth material for more detailed comparisons with type materials of C. vandeweerdi.
Stratigraphic and geographic range. Crusafontina sp. 1 known only from the single Kazakh locality, Pavlodar 1A, late Miocene Pavlodar Formation (Turolian, MN 12).
Crusafontina sp. 2
Figure 6D
Material. Isolated left P4 (GIN 951/1003 (1040)) from the Selety 1A locality (Appendix 2: 6).
Description. The small P4 has a strongly undulated and sculptured crown outline between the parastyle and hypocone with a distinguished anterior notch, moderate protrusion of the protocone anteriorly and strong protrusion of the hypocone lingually; the parastyle forms a pointed anterior outline. The parastyle also modestly shifted anteriorly from the paracone base and is separated from it by a groove. The postcrista is relatively short, and tooth seems shortened in general. The hypocone is inflated and seems more massive than the protocone. The hypoconal flange is relatively long and moderately narrow with an angulated posterior outline; the posterior emargination is deep and shifted lingually. The short ectocingulum is present (Figure 6D).
Measurements. BL(P4) = 2.18; W(P4) = 2.06; LL(P4) = 1.76; PE(P4) = 1.41.
Remarks. The studied P4 from the Selety 1A locality is distinctly larger than that from C. exculta (BL = 1.97-2.08 mm, see van Dam, 2010: 750) and C. minima (BL = 1.84-1.96 mm, see Bown, 1980: 114) and smaller than that from C. kormosi (BL = 2.34-2.77 mm, see Mészáros, 1999a: 9) and especially C. vandeweerdi (BL mean = 2.72 mm, see van Dam, 2004: 750). This P4 is slightly larger than the P4 of C. endemica from European localities (BL = 1.97-2.16 mm, see van Dam, 2004: 750). Initially, we determined the tooth from Selety 1A as belonging to C. endemica because it quite precisely fits the crown features and outline of P4 of C. endemica from the Spanish Can Llobateres 1 locality (van Dam, 2004: figure 2:16, but see point 3: 749). However, C. endemica is not known in the fossil record later than the end of the Vallesian. Therefore, either C. endemica survived in Asia to the beginning of the late Turolian, or we have a tooth of an unknown species of Crusafontina. On the other hand, based on the tooth size, outline shape and stratigraphic range, P4 from Selety 1A best matches the New World species C. magna (BL = 2.12-2.34 mm; W = 2.15-2.37 mm, see Bown, 1980). Morphologically, P4 from Selety 1A is similar to the tooth of C. magna in the distinct anterior projection of the parastyle (with the slight lingual shift from the paracone axis); in the well-developed long notch between the parastyle and protocone and shorter notch between the protocone and huge cone-like hypocone; and in the narrow and posteriorly protruded hypoconal flange with clear cingulum along the border and relatively deep posterior emargination. The poor quality of the Hutchinson and Bown images (Bown, 1980: figure 3A) does not allow us to judge clear similarity in the small crown features. In summary, at present, we determined the tooth from Selety 1A as Crusafontina sp. 2 due to the absence of any tooth material for more detailed comparisons with type materials of C. magna. In addition, the similarity between P4 from Selety 1A and New World species does not contradict the data on the existence of the Beringian terrestrial bridge during the late Miocene (Wen et al., 2016; Jiang et al., 2019), when C. magna could enter Asia from the New World (or vice versa), as Reumer (1999: 393) proposed for Blarinini members.
Stratigraphic and geographic range. At present, the distribution of this form includes only the Kazakh locality, Selety 1A, late Miocene Kedey Formation (Turolian, MN 12/13).
DISCUSSION
Our investigation of original material taken from the Siberian and Kazakh localities revealed the presence of diverse, endemic northern Asian anourosoricin fauna comprising Ishimosorex gen. nov. and Paranourosorex genus. The new genus has specific features of M1 (expressed W-shaped buccal crests), p4 (an inflated crown, spot-shaped facet and and unexpressed crown elements), m1 (a buccal shift of the protoconid and hypoconid) and m2 (a plate-like ectocingulum), which later appeared in Paranourosorex. It is possible that both groups, Ishimosorex gen. nov. and Paranourosorex, derived from a common ancestor. Therefore, Ishimosorex-Paranourosorex lineage existed from the late Miocene (late Vallesian, MN 10) to early Pliocene (Ruscinian, MN 15) within a broad geographic range from southwestern Siberia to the Inner Mongolia region and consisted of five species: Ishimosorex ishimiensis gen. et sp. nov., P. seletiensis, P. inexspectatus, Paranourosorex intermedius sp. nov. and P. gigas. The latter species expanded to Eastern Europe (Rzebik-Kowalska 1975, 1998).
Northern Asian anourosoricin fauna also included allochthonous elements, which came from Europe and/or North America: two findings of undetermined Crusafontina species, namely, Crusafontina sp.1 from the Pavlodar 1A locality with similar crown features and dimensions to the late Miocene species C. vandeweerdi in Spain, and Crusafontina sp.2 from the Selety 1A locality with similar crown features and dimensions to the late Miocene C. endemica in Europe or late Miocene C. magna in the New World.
In the current study, we find four tendencies in north Asian anourosoricin faunal evolution: (1) the northern Asian endemic Ishimosorex-Paranourosorex lineage developed simultaneously or slightly later than the true Crisafontina lineage in Europe within the Vallesian Stage (MN 9, 10; see Rzebik-Kowalska, 1998; van Dam, 2004); (2) the first appearance of Crusafontina representatives in northern Asian fossil records was associated with the end of the early Turolian Stage (MN 12, MN 12/13). At present, we revealed just two findings of Crusafontina from Kazakh localities not later than Kedey Formation deposits (MN 12/13); (3) the evolutionary trajectory of the Ishimosorex-Paranourosorex lineage assumed the appearance and disappearance of the earlier Ishimosorex ishimiensis gen. et sp. nov. with the further appearance of an earlier Paranourosorex species, P. seletiensis. The evolutionary lineage of proper Paranourosorex species exhibited an increase in overall size from the earlier small-sized form, P. seletiensis, to the large-sized form, P. gigas. This tendency was already noted by Storch and Zazhigin (1996). We can add a new tendency for this evolutionary lineage, namely the shortening of the related trigonid length of m1 from 62% of the tooth length in Ishimosorex ishimiensis gen. et sp. nov. to 54% in P. gigas; (4) the Paranourosorex species seems to have disappeared in the fossil records with an appearance in Asia of Beremendia fissidens (late Ruscinian, MN 15, Biteke Formation; see Zazhigin and Voyta, 2019). This was also mentioned by European authors (Mészáros, 2014: 109, figure 4).
Variability of dental features. The teeth of all studied species of Paranourosorex and Ishimosorex ishimiensis gen. et sp. nov. display wrinkled enamel. This characteristic of the enamel surface is developed to different degrees and varies within and between species. Wrinkled enamel is most expressed in Paranourosorex intermedius sp. nov. (Figure 7C). The most significant variable characteristics are the presence of a metaconule in M1 and the variation in the hypolophid/entostylid states of m1 (I and II morphotypes). We state that the metaconule of M1 was absent in the species of Ishimosorex-Paranourosorex lineage. Most likely, this is true for other anourosoricin groups. The review of the metaloph area in Ishimosorex gen. nov., Paranourosorex and several species of Crusafontina, Amblycoptus and Kordosia revealed different degrees of metaloph development: a short metaloph without contact with the metacone base (Asian taxa) and a long metaloph with weak or expressed contact with the metacone (C. vandeweerdi, A. oligodon, and K. topali). However, some teeth show a metaconule; e.g., van Dam (2004: 752) described C. vandeweerdi as “The metaloph shows a 90-degree angle, buccally of which a poorly developed metaconule is present.” This is not a true metaconule but rather a partly worn metaloph crest. The selective wear of the metaloph forms by its attrition at the posterior structures of the m1 talonid–the hypoconid-hypolophid-(entostylid)-entoconid line (Appendix 6). We suggest that selective wear is subject to individual variability in relation to different kinds of foods due to various habitats similar to those revealed in other mammalian taxa (e.g., see Smuts et al., 1978 for carnivores; Anders et al., 2011 for artiodactyls) and age variability. Therefore, in well-represented material (e.g., Paranourosorex intermedius sp. nov. type locality), we can see the same worn stage of several M1 but different states of the metaloph. The posterior structures of the m1 talonid display similar conditions to the stages of wear of the metaloph. These structures are also subject to variability (individual and age variability). Partly, this is evidenced by the presence of two morphotypes of the entostylid absent/present in m1 of Paranourosorex intermedius sp. nov. (see species description). The slightly and moderately worn stages of m1 nevertheless allow us to consider the presence of talonid grooves (posterolingual and transverse) and the presence/absence of entostylid (and its size); consequently, these characteristics are included in species comparisons.
CONCLUSIONS
The shrew tribe Anourosoricini consists of seven genera: Crusafontina, Darocasorex, Paranourosorex, Amblycoptus, Kordosia, Anourosorex and Ishimosorex gen. nov. In the current study, the generic diversity of northern Asian anourosoricin is expanded from two (Storch et al., 1998) to four genera, Crusafontina, Ishimosorex gen. nov., Paranourosorex and Anourosorex.
We consider Ishimosorex gen. nov. and Paranourosorex genera as a single evolutionary lineage. The dental analysis of this endemic northern Asian lineage in comparison with other anourosoricin genera revealed a unique combination of ancestral (plesiomorphic) and derived (apomorphic) dental features that were developed within northern Asian genera: ancestral features of M1 (an expressed W-shaped line of the buccal crests), p4 (an inflated crown, spot-shaped facet and poor crown elements), m1 (a buccal shift of the protoconid and hypoconid), and m2 (a plate-like ectocingulum) of Ishimosorex gen. nov., which later appeared in Paranourosorex species; and derived features of I1 (a talon with an anteriorly tilted cutting edge) and i1 (a smooth cutting edge) of Paranourosorex.
In the current paper, we were able to resolve one taxonomic issue of Storch and Zazhigin (1996) with the undetermined form Paranourosorex sp. 2, which here was determined to be a new species, Paranourosorex intermedius sp. nov. The undetermined Paranourosorex sp. 1 of Storch and Zazhigin (1996) is reevaluated to be Crusafontina sp. 1. In addition, we revealed a new undetermined form, Crusafontina sp. 2 from the Selety 1A locality. Neither species of Crusafontina can be resolved without new fossil material.
ACKNOWLEDGEMENTS
Authors are grateful to Dr. Lukács Mészáros for provided access to anourosoricin species from late Miocene Hungarian Polgárdi 4 locality (materials on Crusafontina kormosi and Amblycoptus oligodon used for composition of Figure 3 and Figure 4 under LM permission) and Dr. Alexey V. Abramov for provided access to materials of the extant Anourosorex squamipes (ZIN). Authors also are grateful to three anonymous reviewers who refereed our manuscript and contributing to its improvement. Authors thank Dr. Lars W. Van den Hoek Ostende for help with rare references in Chinese. Authors would like to thank Dr. Marc Furió and Dr. Lu Li for help with translating abstract to Spanish and Chinese, correspondingly. This study was completed within the framework of the Federal themes of GIN "Paleontological grounds for the stratigraphic scale of the upper Cenozoic of Northern Eurasia" (by Vladimir Zazhigin), and partly funded by Project no. 22-24-00510 of the Russian Scientific Foundation (in parts: data analysis, descriptions, comparisons, text and images creating by Leonid Voyta). The study used the collection materials of the Geological Institute of the Russian Academy of Sciences (Moscow), and Zoological Institute of the Russian Academy of Sciences (St. Petersburg).
REFERENCES
Agadjanian, A.K. 2009. Pliocene-Pleistocene small mammals of the Russian Plain. Nauka, Moscow. (In Russian)
Agadjanian, A.K. and Kowalski, K. 1978. Prosomys insuliferus (Kowalski 1958) (Rodentia, Mammalia) from the Oliocene of Poland and of the European Part of the U.S.S.R. Acta Zoologica Cracoviensia, 23:29-53.
Anders, U., von Koenigswald, W., Ruf, I., and Smith, B.H. 2011. Generalized individual dental age stages for fossil and extant placental mammals. Paläontologische Zeitschrift, 85:321-339. https://doi.org/10.1007/s12542-011-0098-9
Bown, T.M. 1980. The fossil Insectivora of Lemoyne Quarry (Ash Hollow Formation, Hemphillian), Keith County, Nebraska. Transactions of the Nebraska Academy of Sciences and Affiliated Scoieties, 8:99-122.
Burgin, C.J. and He, K. 2018. Family Soricidae, p. 332-551. In Wilson, D.E. and Russell, A.M. (eds.), Handbook of the mammals of the world. Vol. 8. Insectivores, sloths and colugos. Lynx Edicions, Barcelona.
Dannelid, E. 1998. Dental adaptations in shrew, p. 157-174. In Wójcik, J.M. and Wolsan, M. (eds.), Evolution of Shrews. Mammal Research Institute Polish Academy of Sciences, Białowieża.
Furió, M. and Augustí, J. 2017. Latest Miocene insectivores from Eastern Spain: Evidence for enhanced latitudinal differences during the Messinian. Geobios, 50:123-140. https://doi.org/10.1016/j.geobios.2017.02.001
Gureev, A.A. 1979. The Fauna of the USSR. Mammals, v. IV, Is. 2. Insectivorous: Hedgehogs, moles, and shrews (Erinaceidae, Talpidae, Soricidae). Izdatel'stvo Nauka, Leningrad. (In Russian)
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological Statistics software package for and data analysis. Palaeontologia Electronica, 4, 4A:1-9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Haynes, G. 2017. Finding meaning in mammoth age profiles. Quaternary International, 443:65-78. https://doi.org/10.1016/j.quaint.2016.04.012
Haynes, G., Klimowicz, J., and Wojtal, P. 2018. A comparative study of woolly mammoths from the Gravettian site Kraków Spadzista (Poland) based on estimated shoulder heights, demography, and life conditions. Quaternary Research, 90:483-502. https://doi.org/10.1017/qua.2018.60
Hutterer, R. 2005. Order Soricomorpha, p. 220-311. In Wilson, D.E. and Reeder, D.A (eds.), Mammal species of the world: a taxonomical reference. 3rd edition. Vol. 1. Johns Hopkins University Press, Baltimore.
ICZN. 1999. International Code of Zoological Nomenclature. The international Trust for Zoological Nomenclature, London.
Jablonski, N.G., Su, D.F., Flynn, L.J., Ji, X., Deng, C., Kelly, Y., Zhang, Y., Yin, J., You, Y., and Yang, X. 2014. The site of Shuitangba (Yunnan, China) preserves a unique, terminal Miocene fauna. Journal of Vertebrate Paleontology, 34:1251-1257. https://doi.org/10.1080/02724634.2014.843540
Jiang, D., Klaus, S., Zhang, Y.-P., Hillis, D.M., and Li, J.-T. 2019. Asymmetric biotic interchange across the Bering land bridge between Eurasia and North America. National Science Review, 6:739-745. https://doi.org/10.1093/nsr/nwz035
Larramendi, A. 2016. Shoulder height, body mass, and shape of proboscideans. Acta Palaeontologica Polonica, 61:537-574. https://doi.org/10.4202/app.00136.2014
Li, C., Qiu, Z., Tong, Y., Zheng S., and In, X. 2014. Palaeovertebrata Sinica Volume III Basal Synapsids and Mammals Fascicles 3 (Series no.16) Eulipotyphlans, Proteutheres, Chiropterans, Euarchontans, and Anagalids. Science Press, Beijing.
Lopatin, A.V. 2005. Early Paleogene insectivores and modern taxonomic system of Lipotyphla, p. 133-154. In Rozanov, A.Yu., Lopatin, A.V., and Parkhaev, P.Yu. (eds.), A modern palaeontology: Classical and modern methods. Paleontological Institute of the Russian Academy of Sciences, Moscow. (In Russian, with English summary)
Lopatin, A.V. 2006. The origin of shrew family (Soricidae, Mammalia): paleontological data, p. 233-245. In Rozhnov, S.V. (ed.), Evolution of biosphere and biodiversity. KMK Scientific Press Ltd., Moscow. (In Russian)
Mayr, H. and Fahlbusch, V. 1975. Eine unterpliozäne Kleinsäugerfauna aus der Oberen Süßwasser-Molasse Bayerns. Mitteilungen der Bayerischen Staatssamlung für Paläontologie und Historische Geologie, 15:91-111.
Mészáros, L. 1997. Kordosia, a new genus for some Late Miocene Amblycoptini shrews (Mammalia, Insectivora). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte, 2:65-78.
Mészáros, L. 1998. Late Miocene Soricidae (Mammalia) fauna from Tardosbánya (Western Hungary). Hantkeniana, 2:103-125.
Mészáros, L. 1999a. An exceptionally rich Soricidae (Mammalia) fauna from the upper Miocene localities of Polgárdi (Hungary). Annales Universitatis Scientiarum Budapestinensis, Sectio Geologica, 32:5-34.
Mészáros, L. 1999b. Some insectivore (Mammalia) remains from the Late Miocene locality of Alsótelekes (Hungary). Annales Universitatis Scientiarum Budapestinensis, Sectio Geologica, 32:35-47.
Mészáros, L. 2014. A possible taphonomical evidence for the palaeoecological role of the giant shrews (Mammalia, Soricidae) in the Carpathian Basin. Hantkeniana, 9:107-116.
Mitteroecker, P., Gunz, P., and Bookstein, F.L. 2005. Heterochrony and geometric morphometrics: a comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evolution & Development, 7:244-258. https://doi.org/10.1111/j.1525-142x.2005.05027.x
Nesin, V.A. and Nadachowski, A. 2001. Late Miocene and Pliocene small mammal faunas (Insectivora, Lagomorpha, Rodentia) of South-eastern Europe. Acta Zoologica Cracoviensa, 44:107-135.
Prieto, J. and van Dam, J.A. 2012. Primitive Anourosoricini and Allosoricinae from the Miocene of Germany. Geobios, 45:581-589. https://doi.org/10.1016/j.geobios.2012.03.001
Qiu, Z. and Storch, G. 2000. The early Pliocene micromammalian fauna of Bilike, Inner Mongolia, China (Mammalia: Lipotyphla, Chiroptera, Rodentia, Lagomorpha). Senckenbergiana Lethaea, 80:173-229.
Qiu, Z. and Storch, G. 2005. China, p. 37-50. In Hoek Ostende, L.W. van den, Doukas, C.S., and Reumer, J.W.F. (eds.), The fossil record of the Eurasian Neogene insectivores (Erinaceomorpha, Soricomorpha, Mammalia), Part I. Scripta Geologica Special Issue. Leiden.
Reumer, J.W.F. 1984. Ruscinian and Early Pleistocene Soricidae (Insectivora, Mammalia) from Tegelen (The Netherlands) and Hungary. Scripta Geologica, 73:1-173.
Reumer, J.W.F. 1997. De evolutiebiologie van de spitsmuizen (Mammalia, Insectivora, Soricidae). III. De subfamilie Soricinae en het literatuuroverzicht. Cranium, 14:13-36.
Reumer, J.W.F. 1999. Shrews (Mammalia, Insectivora, Soricidae) as paleoclimatic indicators in the European Neogene, p. 390-396. In Agusti, J, Rook, L., and Andrews, P. (eds.), The evolution of Neogene terrestrial ecosystems in Europe, Volume 1: Hominoid evolution and climatic change in Europe. Cambridge University Press, New York.
Rich, T.R., Flannery, T.F., Trusler, P., Kool, L., van Klaveren, N.A., and Vickers-Rich, P. 2001. A second tribosphenic mammal from the Mesozoic of Australia. Records of the Queen Victoria Museum Launceston, 110:1-9.
Rohlf, F.G. and Slice, D.E. 1990. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39:40-59. https://doi.org/10.2307/2992207
Rzebik-Kowalska, B. 1975. The Pliocene and Pleistocene insectivores (Mammalia) of Poland. II. Soricidae: Paranourosorex and Amblycoptus. Acta Zoologica Cracoviensia, 20:167-182.
Rzebik-Kowalska, B. 1998. Fossil history of shrews in Europe, p. 23-92. In Wójcik, J.M. and Wolsan, M. (eds.), Evolution of Shrews. Mammal Research Institute Polish Academy of Sciences, Białowieża.
Rzebik-Kowalska, B. and Lungu, A. 2009. Insectivore mammals from the Late Miocene of the Republic of Moldova. Acta Zoologica Cracoviensia, 52A:11-60.
Rzebik-Kowalska, B. and Rekovets, L.I. 2016. New data on Eulipotyphla (Insectivora, Mammalia) from the Late Miocene to the Middle Pleistocene of Ukraine. Palaeontologia Electronica, 19.1.9A:1-31. https://doi.org/10.26879/573
Smuts, G.L., Anderson, J.L., and Austin, J.C. 1978. Age determination of the African lion (Panthera leo). Journal of Zoology, 185:115-146. https://doi.org/10.1111/j.1469-7998.1978.tb03317.x
Storch, G. 1995. The Neogene mammalian faunas of Ertemte and Harr Obo in Inner Mongolia (Nei Mongol), China.–11. Soricidae (Insectivora). Senckenbergiana Lethaea, 75:221-251.
Storch, G. and Qiu, Z. 1991. Insectivores (Mammalia: Erinaceidae, Soricidae, Talpidae) from the Lufeng hominoid locality, late Miocene, China. Geobios, 24:601-621. https://doi.org/10.1016/0016-6995(91)80025-U
Storch, G. and Zazhigin, V.S. 1996. Taxonomy and phylogeny of the Paranourosorex lineage, Neogene of Eurasia (Mammalia: Soricidae: Anourooricini). Paläontologische Zeitschrift, 70:257-268. https://doi.org/10.1007/BF02988282
Storch, G., Qui, Z., and Zazhigin, V.S. 1998. Fossil history of shrews in Asia, p. 93-120. In Wójcik, J.M. and Wolsan, M. (eds.), Evolution of Shrews. Mammal Research Institute Polish Academy of Sciences, Białowieża.
Topachevsky, V.A. 1966. New genus of a large white-toothed shrew (Insectivora, Soricidae) from Pliocene deposits of southern Ukraine, p. 90-96. In Voinctvenski, M.A., Kistjakivski, O.B., Abelentsev, V.I., Sokur, I.T., Gizenko, O.I. and Girenko, L.L. (eds.). Ecology and history of vertebrates of Ukrainian fauna. Naukova Dumka, Kyiv. (In Ukrainian)
Topachevsky, V.A., Nesin, Y.A., Rekovets, L.I., Topachevsky, I.V., and Pashkov, A.V. 1988. A new location of small mammalian remains in Pliocene of the northern Azov Sea area. Dopovidi Akademii Nauk Ukrainskoi RSR, Seria B, 11:18-21. (In Ukrainian)
Van Dam, J.A. 2004. Anourosoricini (Mammalia: Soricidae) from the Mediterranean region: A pre-Quaternary example of recurrent climate-controlled north-south range shifting. Journal of Paleontology, 78:741-764. https://www.jstor.org/stable/4094903
Van Dam, J.A. 2010. The systematic position of Anourosoricini (Soricidae, Mammalia): paleontological and molecular evidence. Journal of Vertebrate Paleontology, 30:1221-1228. https://doi.org/10.1080/02724634.2010.483553
Vasilyan, D., Zazhigin, V.S., and Böhme, M. 2017. Neogene amphibians and reptiles (Caudata, Anura, Gekkota, Lacertilia, and Testudines) from the south of Western Siberia, Russia, and Northeastern Kazakhstan. PeerJ, 5:e3025:1-65. https://doi.org/10.7717/peerj.3025
Wen, J., Nie, Z.-L., and Ickert-Bond, S.M. 2016. Intercontinental disjunctions between eastern Asia and western North America in vascular plants highlight the biogeographic importance of the Bering land bridge from late Cretaceous to Neogene. Journal of Systematics and Evolution, 54:469-490. https://doi.org/10.1111/jse.12222
Zazhigin, V.S. 2006. On fauna of small mammals of the arid zone of south of West Siberia in late Miocene, p. 198-202. In Matishov, G.G. (ed.), Late Cenozoic geological history of the north of the arid zone. YuNC RAN, Rostov-on-Don. (In Russian)
Zazhigin, V.S. and Zykin, V.S. 1984. The new data on the stratigraphy of the Pliocene of the south of West-Siberian Valley, p. 29-53. In Arkhipov, S.A. (ed.), Stratigrafia pogranichnikh otlozhenii neogena i antropogena Sibiri. Institut geologii i geofiziki SO AN SSSR, Novosibirsk. (In Russian)
Zazhigin, V.S. and Lopatin, A.V. 2001. The history of the Dipodoidea (Rodentia, Mammalia) in the Miocene of Asia: 4. Dipodinae at the Miocene-Pliocene Transition. Paleontological Journal, 35:60-74.
Zazhigin, V.S., Lopatin, A.V. and Pokatilov, A.G. 2002. The history of the Dipodidae (Rodentia, Mammalia) in the Miocene of Asia: 5. Lophocricetus (Lophocricetinae). Paleontological Journal, 36:180-194.
Zazhigin, V.S. and Voyta, L.L. 2019. Northern Asian Pliocene-Pleistocene beremendiin shrews (Mammalia, Lipotyphla, Soricidae): a description of material from Russia (Siberia), Kazakhstan, and Mongolia and the paleobiology of Beremendia. Journal of Paleontology, 93:1234-1257. https://doi.org/10.1017/jpa.2019.51
Zykin, V.S. 2012. Stratigraphy and evolution of environments and climate during Late Cenozoic in the Southern West Siberia. Academic Publishing House “Geo”, Novosibirsk. (In Russian)
Zykin, V.S. and Zazhigin, V.S. 1984. On determination of the Peshnev Formation in Pliocene of south of West-Siberian Plain. Geology and Geophysics, 2:46-50. (In Russian)
Zykin, V.S., Zazhigin, V.S., and Presyazhnjuk, V.A. 1987. Stratigraphy of Pliocene and Eopleistocene deposits from the Biteke river valley (Northern Kazakhstan). Geologia i Stratigrafia, 3:12-19. (In Russian)
Zykin, V.S. and Zazhigin, V.S. 2004. A new biostratigraphic level of Pliocene of West Siberia and age of the lower-middle Miocene Besheul horyzon stratotipic age. Doklady Akademii Nauk, 398:214-217. (In Russian)
Zykin, V.S., Zykina, V.S., and Zazhigin, V.S. 2007. Issue in separating and correlating Pliocene and Quaternary sediments of Southwestern Siberia. Archeology, Ethnology & Anthropology of Eurasia, 2(30):24-40.

