 Investigating colour in marine Miocene molluscs: UV fluorescence patterns and pigment EDX spectroscopy in shells from the Murbko Marl, Murray Basin (South Australia)
Investigating colour in marine Miocene molluscs: UV fluorescence patterns and pigment EDX spectroscopy in shells from the Murbko Marl, Murray Basin (South Australia)
Article number: 28.1.a19
https://doi.org/10.26879/1394
Copyright Palaeontological Association, April 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 2 April 2024. Acceptance: 26 March 2025.
ABSTRACT
Molluscan shells are well known for their intricate and complex patterns of pigmentation, which provide valuable taxonomic information. Excluding cases of exceptional preservation, this feature is nearly always lost in fossil specimens, as pigment degrades quickly after death. However, traces of pigmentation may remain and can be revealed when viewed under ultraviolet (UV) light as these areas fluoresce. UV-induced fluorescence is becoming an increasingly common technique used to unlock this important diagnostic character, but there has been a heavy bias towards Northern Hemisphere material, with a notable lack of study on material from the Southern Hemisphere. Here, material from the mid-Miocene Murbko Marl Member of the Murray Basin, South Australia is imaged under UV light to reveal lost pigmentation patterns and reevaluate their taxonomic assignments. Eighteen species from three classes (Gastropoda, Bivalvia, and Scaphopoda) responded with fluorescence under UV light. Several shells of Maoricolpus murrayanus (Tate, 1885) are also reported on with partial preserved original pigmentation. Energy-dispersive X-ray spectroscopy (EDX) was undertaken on Maoricolpus murrayanus and modern relative Maoricolpus roseus (Quoy and Gaimard, 1834) to identify elements which may be associated with either the pigment or fluorescence and help understand their origin. No clear elemental association could be made with either. The pigmentation pattern observed in extinct Maoricolpus murrayanus is found to be comparable to modern Maoricolpus roseus from New Zealand, bringing into question their validity as separate species.
Mahala A. Fergusen. School of Biological Sciences, Faculty of Sciences, Engineering and Technology, University of Adelaide, North Terrace, Adelaide, South Australia 5005, Australia. mahala.fergusen@adelaide.edu.au
https://orcid.org/0000-0002-9190-8289
Elizabeth H. Reed. School of Biological Sciences, Faculty of Sciences, Engineering and Technology, University of Adelaide, North Terrace, Adelaide, South Australia 5005, Australia. liz.reed@adelaide.edu.au
https://orcid.org/0000-0001-6103-1861
Diego C. García-Bellido. School of Biological Sciences, Faculty of Sciences, Engineering and Technology, University of Adelaide, North Terrace, Adelaide, South Australia 5005, Australia and Earth Sciences Section, South Australian Museum, North Terrace, Adelaide, South Australia 5000, Australia. diego.garcia-bellido@adelaide.edu.au
https://orcid.org/0000-0003-1922-9836
Keywords: molluscs; UV fluorescence; colour pattern; pigment; Miocene; southern Australia
Final citation: Fergusen, Mahala A., Reed, Elizabeth H., and García-Bellido, Diego C. 2025. Investigating colour in marine Miocene molluscs: UV fluorescence patterns and pigment EDX spectroscopy in shells from the Murbko Marl, Murray Basin (South Australia). Palaeontologia Electronica, 28(1):a19.
https://doi.org/10.26879/1394
palaeo-electronica.org/content/2025/5498-uv-fossil-mollusc-fluorescence
Copyright: April 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Molluscs have long been admired for their eye-catching shells, many of which possess intricate colour patterns. In modern molluscan systematics, patterning is commonly a character included when describing species and can sometimes be the distinguishing feature between two taxa (Kelley and Swann, 1988; Dommergues et al., 2006; Caze et al., 2011a now). There is often a high correlation between DNA- and colour pattern-phylogenies, making the study of pigmentation patterns an informative phylogenetic feature in the absence of DNA, as is the case with fossil specimens (Gong et al., 2012). Finding fossil mollusc specimens with colour preserved is a rare occurrence as the chemical components of colour pigments break down rapidly after death (Caze et al., 2011a now, 2012). Abrasion from post-mortem transportation, prolonged exposure to sunlight, and degradation by bacteria and endolithic algae can all contribute to this loss (Hollingworth and Barker, 1991). Thus, pigmentation pattern is not typically a feature found in fossil taxa descriptions.
However, this patterning is not always completely lost. When viewed under ultraviolet light (UV light), some mollusc shells respond with fluorescence at the locations where pigment was once discernible under visible light. UV fluorescence has been observed in molluscan shells since the 1920s but the application of this technique has only become more commonplace in the literature over the past two decades (Miethe and Born, 1928; Wagner, 1928; Rolfe, 1965; Vokes and Vokes, 1968; Krueger, 1974; Hoerle, 1976; Swann and Kelley, 1985; Merle, 2003; Pacaud, 2003; Górka, 2008; Merle et al., 2008; Kase et al., 2008; Caze et al., 2010a, 2010b, 2011a, 2011b, 2012, 2015; Koskeridou and Thivaiou, 2012; Landau et al., 2013; Hendricks, 2015, 2018; Harzhauser and Landau, 2016; Williams et al., 2016; Crippa and Masini, 2022; Psarras et al., 2022; Pereira et al., 2022; Wolkenstein, 2022). Several papers have been able to develop systematics based upon the patterns revealed under UV light (Caze et al., 2011a now, 2015; Hendricks, 2015, 2018; Psarras et al., 2021, 2022, 2023). Most published works are on Cenozoic-age material (Vokes and Vokes, 1968; Swann and Kelley, 1985; Merle, 2003; Pacaud, 2003; Górka, 2008; Merle et al., 2008; Kase et al., 2008; Caze et al., 2010a, 2010b, 2011a, 2011b, 2012; Koskeridou and Thivaiou, 2012; Hendricks, 2015, 2018; Psarras et al., 2022), but this technique has successfully revealed pigmentation patterns on molluscs in several studies from material dating as far back as the Jurassic, Triassic, and Permian (Percival et al., 2012; Caze et al., 2015; Wolkenstein, 2022; Pereira et al., 2022).
The mechanisms behind the nature of original pigment and its fluorescence remain poorly understood. Early studies on molluscan pigment were primarily led by Alex Comfort, investigating pigment using chemical and biochemical methods (Comfort, 1949a, b, c, 1950a, b, c, d, 1951a, b; Nicholas and Comfort, 1949). More recent studies on molluscan pigment have largely employed Raman spectroscopy (Barnard and de Waal, 2006; Hedegaard et al., 2006; Bergamonti et al., 2013; de Oliveira et al., 2013; Ferrer et al., 2013; Stemmer and Nehrke, 2014; Ishikawa et al., 2019; Wade et al., 2019), whilst Williams et al. (2016) in addition to Raman spectroscopy investigated a wide variety of techniques, including several that focused on identification of trace elements associated with pigment. There are several different types of pigments currently identified to occur in molluscs: melanins, porphyrins, bile pigments, and polyenes (as carotenoids), but the detection of these pigments is not well developed (Williams, 2017). Melanin does not fluoresce at all under UV light, and though believed to be present in shells, thus far it has only been identified in the soft tissue of molluscs (Derby 2014; Speiser, DeMartini and Oakley, 2014; Williams, 2017). Porphyrins and bile pigments fluoresce red under UV light (Williams, 2017). As with melanin, carotenoids have been definitively identified in molluscan soft tissue, but not yet from the shell (Kennedy, 1979; Matsuno et al., 1984; Tsushima, Katsuyama and Matsuno, 1997; Borodina, Maoka and Soldatov, 2013; Williams 2017). Several studies do, however, strongly suggest carotenoid shell presence (Barnard and De Waal, 2006; Hedegaard et al., 2006; Zheng et al., 2010; de Oliveira et al., 2013; Williams, 2017). Raman spectroscopy found signals suggesting the presence of non-carotenoid polyenes of unknown type in Miocene gastropod shells from the Superfamily Cerithioidea (Wolkenstein et al., 2024). These shells did not display fluorescence and, conversely, all other gastropods from this study which did fluoresce lacked any signals of polyene presence. UV fluorescence has not been used as a presence indicator in any other polyene/carotenoid studies, but the work of Wolkenstein et al. (2024) would suggest the two are negatively linked.
In Australia, the application of UV within palaeontology has been used in a wide range of subjects to help from finding fossils in the field to seeing features with greater clarity. However, UV light as a tool for revealing lost pigmentation of Australian marine invertebrate fossils has very rarely been published and never as the focus of a study (Percival et al., 2012).
Here, we investigate material from the Murbko Marl Member of the Murray Basin, South Australia, under UV light to reveal lost pigmentation patterns, which we aim to use towards clarifying taxonomy. We also conduct Energy-dispersive X-ray spectroscopy on Maoricolpus murrayanus (Tate, 1885) and its modern relative Maoricolpus roseus (Quoy and Gaimard, 1834), aiming to identify elements which may be associated with either the pigment or fluorescence and help understand their origin.
GEOLOGIC SETTING
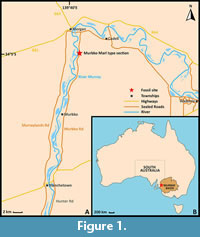 The continental rifting of the southern-Australian margin from Antarctica, commencing by the late Jurassic, led to the development of several basins across southeastern Australia (Norvick and Smith, 2001). The completion of the separation between the two initiated marine transgressions into these southern basins and subsequent deposition of marine sediment (Benbow et al., 1995). A prominent basin with large-scale marine successions which emerged from this prolonged period of rifting is the epicratonic Murray Basin (Figure 1B). The Murray Basin extends inland over 300 000 km2 across South Australia, Victoria, and New South Wales, with extensive Cenozoic successions up to 600 m in thickness, although generally less than 200 m thick (Brown and Stephenson, 1989; Rogers et al. 1995; Kingham, 1998).
The continental rifting of the southern-Australian margin from Antarctica, commencing by the late Jurassic, led to the development of several basins across southeastern Australia (Norvick and Smith, 2001). The completion of the separation between the two initiated marine transgressions into these southern basins and subsequent deposition of marine sediment (Benbow et al., 1995). A prominent basin with large-scale marine successions which emerged from this prolonged period of rifting is the epicratonic Murray Basin (Figure 1B). The Murray Basin extends inland over 300 000 km2 across South Australia, Victoria, and New South Wales, with extensive Cenozoic successions up to 600 m in thickness, although generally less than 200 m thick (Brown and Stephenson, 1989; Rogers et al. 1995; Kingham, 1998).
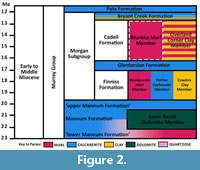 The Murray Basin experienced three major depositional periods in the Tertiary (Paleocene–Eocene to Lower Oligocene, Oligocene–Middle Miocene, and Upper Miocene–Pliocene), each correlating with episodes of sea level transgressions (Brown and Stephenson, 1989). The second of these Tertiary sequences saw an epicontinental sea develop due to major marine transgressions.
The Murray Basin experienced three major depositional periods in the Tertiary (Paleocene–Eocene to Lower Oligocene, Oligocene–Middle Miocene, and Upper Miocene–Pliocene), each correlating with episodes of sea level transgressions (Brown and Stephenson, 1989). The second of these Tertiary sequences saw an epicontinental sea develop due to major marine transgressions.  This facilitated the deposition of the Murray Group, a succession of units comprised largely of marls, limestones, and clays (Brown and Stephenson, 1989), as seen in Figure 2. All material presented in this study is from the Murbko Marl Member, a unit within the Cadell Formation of the Murray Group (Figure 2 and Figure 3). It is found in South Australia, outcropping between Blanchetown and Morgan. The Murbko Marl is described as a brownish-grey weathering marl, thinly bedded with laminated green-grey clay (Lukasik and James, 1998). Based on the presence of fossil foraminifera Astrononion obesum (Carter, 1964) and Praeorbulina curva (Blow, 1956) it is dated as Balcombian in age (15.5–15 Ma), equivalent to the Langhian Stage in the international scale, and this is supported by its stratigraphic position (Lindsay and Bonnett, 1973; Lukasik and James, 1998). It is rich in marine fossils, particularly molluscs (Figure 3C, D), being the formation from which Ralph Tate described dozens of species of the southern Australian Cenozoic molluscan fauna (Tate, 1886, 1888, 1889, 1890, 1893a, 1893b). The fauna shows similarities to those in tropical zones today, suggesting a warm-water depositional environment (Lindsay and Bonnett, 1973). It is dominated by the turritellid gastropod Maoricolpus murrayanus (in situ: Figure 3B, C and ex situ: Figure 3D.)
This facilitated the deposition of the Murray Group, a succession of units comprised largely of marls, limestones, and clays (Brown and Stephenson, 1989), as seen in Figure 2. All material presented in this study is from the Murbko Marl Member, a unit within the Cadell Formation of the Murray Group (Figure 2 and Figure 3). It is found in South Australia, outcropping between Blanchetown and Morgan. The Murbko Marl is described as a brownish-grey weathering marl, thinly bedded with laminated green-grey clay (Lukasik and James, 1998). Based on the presence of fossil foraminifera Astrononion obesum (Carter, 1964) and Praeorbulina curva (Blow, 1956) it is dated as Balcombian in age (15.5–15 Ma), equivalent to the Langhian Stage in the international scale, and this is supported by its stratigraphic position (Lindsay and Bonnett, 1973; Lukasik and James, 1998). It is rich in marine fossils, particularly molluscs (Figure 3C, D), being the formation from which Ralph Tate described dozens of species of the southern Australian Cenozoic molluscan fauna (Tate, 1886, 1888, 1889, 1890, 1893a, 1893b). The fauna shows similarities to those in tropical zones today, suggesting a warm-water depositional environment (Lindsay and Bonnett, 1973). It is dominated by the turritellid gastropod Maoricolpus murrayanus (in situ: Figure 3B, C and ex situ: Figure 3D.)
METHODOLOGY
Collection Localities
Fossil specimens were collected from the type section of the Murbko Marl Member (Figure 1A) in October 2021, June 2022, and August 2023 by the authors, in addition to a select amount from the private collection of Mr Peter Hunt (Adelaide). All figured fossil specimens are reposited in the South Australian Museum Adelaide Palaeontology collection (SAMA P). Modern specimens examined for comparison are housed in the Marine Invertebrate collection of the South Australian Museum, and originated from Christchurch, New Zealand (SAMA D51431); Auckland, New Zealand (SAMA D74439); Paihia, New Zealand (SAMA D74596) and an unspecified location in New Zealand (SAMA D74438).
Bleaching
Treating shells with common household bleach (sodium hypochlorite) not only enhances their fluorescence under UV light but can also induce it (Vokes and Vokes, 1968). A review of different preparation methods for UV photography of molluscs was compiled by Crippa and Masini (2022), recommending submergence of specimens for up to 72 hours in bleach prior to photographing. Bleach does not typically cause damage to the shell, aside from whitening, so it may be submerged for essentially as long as required for fluorescence to occur (Crippa and Masini, 2022). Greater care must be taken only if there is intention for the specimens to also be used for geochemical analysis as this can alter the trace chemical elements or introduce isotope biases (Wierzbowski, 2007; Schöne et al., 2017). Soaking in pure or diluted concentrations of bleach has no apparent difference on the strength of the effect, only perhaps in the time it takes for such effects to occur (Krueger, 1974; Crippa and Masini, 2022).
In this study, shells were first cleaned in water, then soaked in household bleach (sodium hypochlorite 42 g/L) for 72 hours. Many shells showed a weak fluorescence prior to the bath. Fluorescence was induced in the previously non-fluorescing shells after 24 hours, and most shells showed an increased intensity of fluorescence after 48 hours. Fluorescence intensity peaked for the majority after 48 hours, but a handful benefitted from 72 hours submergence. Once removed from the bleach, shells were again rinsed with water to remove any remaining traces of bleach and left to dry completely.
Shells were not prepared with bleach if they still possessed original pigment, if they already displayed an intense fluorescence (of which improvement by the treatment would be negligible), or if they were designated for energy-dispersive X-ray spectroscopy analysis.
Photography and Image Processing
Specimens were selected for photography based on the quality of fluorescence displayed. This did not always align with specimen quality otherwise, as some examples are fractured. Specimens were photographed with a Canon EOS 50D camera secured to a copy stand and operated remotely on a laptop using Canon EOS Utility 2. Specimens were photographed against a black velvet background for adequate aperture and exposure. Any small dust or lint particles upon this background were lifted with tape prior to photography as they fluoresce bright blue under UV light. Two Eagtac Compact MX30L3 UV torches were used for UV photography, positioned adjacent each side of the camera on the copy stand with a clamp arm. The wavelength emitted from these torches is 365 nm, in the longwave/UV-A range. Photographs of specimens in visible light and UV light were taken sequentially to ensure specimens were in the exact same position and camera focus for best comparison. During UV photography, all other light sources were eliminated from the room. Dark clothing and appropriate personal protection equipment was worn to prevent damage by exposure to UV light.
Images were focus-stacked in Helicon Focus 8.2.2 and figures prepared using Adobe Photoshop 2023 version 24.5.0. The white balance was corrected, and brightness and exposure adjusted where needed to improve lighting. Finally, colours were reversed using an inverse adjustment layer. This step is not necessary but helps to show the differences in colour contrast and visualise the pigmentation pattern more precisely (Hendricks, 2015). This is because in life the fluorescent areas are the ones which would have been darker in colour (Hendricks, 2015). It is important to note that nowhere in this photography and image editing process can the original colours of the pigments be seen or determined, it is for observation of patterns only.
Energy-dispersive X-ray Spectroscopy (EDX)
EDX was used to test for elements which may correlate to areas of pigmentation or fluorescence in fossil specimens of Maoricolpus murrayanus and its modern relative Maoricolpus roseus. M. murrayanus was chosen over other fluorescing species due to its larger size, larger area coverage of fluorescence, and interest as the most dominant species in the Murbko Marl. M. roseus was selected for the modern species as it is the more common of the two extant members of the genus Maoricolpus. The most promising specimens of M. murrayanus and M. roseus were chosen based on the intensity of their colour patterns and the fluorescence of the fossil specimen (M. roseus does not fluoresce). Analyses were carried out at Adelaide Microscopy using a FEI Quanta 450 FEG Environmental Scanning Electron Microscope equipped with an Oxford Ultim Max Large Area SDD EDS detector. The following microscope settings were used: low vacuum (chamber pressure of 130Pa), voltage of 15kV, spotsize 5, and working distance of 10 mm. Before analysing the shell, a small area known to fluoresce intensely was marked on the shell with pencil. This section was examined in the EDX machine as precisely as possible.
RESULTS
Pigmentation Pattern Descriptions
Fluorescence was observed in 18 molluscan taxa across three different classes: Gastropoda (14 taxa), Bivalvia (three taxa), and Scaphopoda (one taxon). The fluorescence observed in all of the specimens was yellow-orange in colour. Typically, the specimens with a greater intensity of fluorescence fluoresced orange. Fluorescence could be found on both internal surfaces (gastropods and bivalves) and external surfaces (all classes). Patterns ranged from solid colouration and simple patterns such as stripes found throughout all classes, to more complex shapes such as tent patterns only present on gastropods. Closely related taxa had similar patterning, e.g., turrids Maoricolpus murrayanus and Gazameda acricula (Tate, 1893) displayed axial waves, and the two Nannamoria species Nannamoria cadella Hawke, 2021 and Nannamoria sp. each displayed similar triangular motifs. This is not always the case, as demonstrated by a non-figured Nannamoria sp. (excluded due to the availability of only a single, fractured specimen), which possessed vertical lines with sharp dextral peaks.
Class GASTROPODA Cuvier, 1797
Order unassigned
Family TURRITELLIDAE Lovén, 1847
Maoricolpus murrayanus (Tate, 1885)
Figure 4A–F
 Pattern description. Yellow-orange fluorescence. Entirety of shell covered by banded axial waves. Waves are irregular in shape, sometimes connecting to the adjacent wave and other times remaining separate. The waves on each whorl do not align with adjacent whorls, i.e., they are not continuous. Frequently the posterior of the whorl will show a dashed checkerboard-like pattern (arrowheads in Figure 4A, D), rather than the continuation of the thicker main waves (Figure 4E, F). There is also occasionally partial pigmentation in the spiral striae, separate from the main patterning. The inner lip fluoresces solidly.
Pattern description. Yellow-orange fluorescence. Entirety of shell covered by banded axial waves. Waves are irregular in shape, sometimes connecting to the adjacent wave and other times remaining separate. The waves on each whorl do not align with adjacent whorls, i.e., they are not continuous. Frequently the posterior of the whorl will show a dashed checkerboard-like pattern (arrowheads in Figure 4A, D), rather than the continuation of the thicker main waves (Figure 4E, F). There is also occasionally partial pigmentation in the spiral striae, separate from the main patterning. The inner lip fluoresces solidly.
Comment. The extinct M. murrayanus is morphologically very similar to extant Maoricolpus roseus. The original description of M. murrayanus by Tate (1893a) differentiated it from M. roseus by only “the spiral ornament of the posterior whorls develops into ribs on the anterior whorls”. Garrard (1972) proposed there are strong grounds for considering M. murrayanus and M. roseus as synonymous due to the high morphological similarities. Whilst the protoconch was not preserved in the specimens in this study, the protoconch of both species has previously been described as multispiral, with three to four microscopic flatly globose whorls, apparently sinusigeroid (Garrard 1972). The main argument presented for not synonymising the species is the differing age and geographical ranges (Beu 2009).
M. roseus is one of two extant species in the genus Maoricolpus, together with Maoricolpus finlayi. Both are natively endemic to New Zealand, but M. roseus is much more common and has a wider distribution (Powell 1940; Donald and Spencer, 2014). M. roseus is now a dominant species in New Zealand waters, often the most dominant benthic species present (McKnight, 1969). This is like what is observed in M. murrayanus in the Murbko Marl—it is the most common species present (Figure 3D for abundance). M. roseus has been anthropogenically introduced to the east-coast of Australia, arriving in Tasmania in the 1920s and now found as far north as Botany Bay (Gunasakera et al., 2005). It is now considered a pest in Australian waters (Bax et al., 2003). M. roseus was previously separated into two subspecies, M. roseus roseus and M. roseus manukauensis, based on slight morphological variances and differences in distribution. Donald and Spencer (2014) used molecular data to conclude these subspecies were not valid, and comprised a single, morphologically variable species. M. roseus has also been merged with a fossil species, the now invalid Maoricolpus proroseus, described from Tertiary rocks of the Gisborne District in the northeast of New Zealand (MolluscaBase eds., 2023). Thus, there is a precedent for a fossil species to be combined with M. roseus. The colour patterning revealed here strengthens the argument that M. murrayanus and M. roseus are a single species with slight morphological variations through time which are insufficiently different to justify separation. The patterns on each species show banded axial waves. Both species show a variability in the presence of a checkerboard-like pattern towards the outer end of the whorl, as described above. This seems to be more commonly present on M. murrayanus than M. roseus but is present on specimens such as the ones in Figure 4H, I.
Essentially all 387 specimens of Murraycolpus murrayanus studied fluoresced very strongly. Unlike most of the other taxa, no bleach treatment was applied to the photographed M. murrayanus specimens as trials found no benefit due to the already highly fluorescent condition.
 In situ observation of M. murrayanus underneath UV light also displayed clear fluorescence when viewed in weak shade (see Supplementary Figure 1).
In situ observation of M. murrayanus underneath UV light also displayed clear fluorescence when viewed in weak shade (see Supplementary Figure 1).
Several specimens were found to partially retain the original pigmentation. The most intact of these specimens (Figure 4F) shows clearly defined pigmentation on the body whorl and first two spire whorls. The pigment is only present on the photographed side, while the side of the shell with the aperture lacks pigment, likely due to sun exposure. The other shells show a much fainter and smaller area of partial pigmentation. The fluorescence is not as strong in the partially pigmented specimens as it is in the non-pigmented specimens. The shells of modern relative M. roseus do not fluoresce under UV light.
Gazameda acricula (Tate, 1893)
Figure 5A
 Pattern description. Yellow-orange fluorescence. Thin, wavy axial lines. Lines vary on specimens from being relatively straight to more curved. Lines do not frequently overlap with other lines; they are independent.
Pattern description. Yellow-orange fluorescence. Thin, wavy axial lines. Lines vary on specimens from being relatively straight to more curved. Lines do not frequently overlap with other lines; they are independent.
Order LITTORINIMORPHA Golikov and Starobogatov, 1975
Family CYMATIIDAE Iredale, 1913
Gyrineum maccoyi (Pritchard, 1898)
Figure 5B
Pattern description. Yellow or orange fluorescence. Most notable area of fluorescence is the aperture, which fluoresces solidly. The fluorescence is strongest on the lips but is present across the entire aperture and interior siphonal canal surface. This is also apparent at the varices where the old outer lips were located during shell growth. There is also faint fluorescence on the body of the shell, but this is not clear enough to discern any pattern.
Family CYPRAEIDAE Rafinesque, 1815
Notoluponia murraviana (Tate, 1890)
Figure 6F
 Pattern description. Solid fluorescence on aperture, inner lip, and a band along the external side of the outer lip.
Pattern description. Solid fluorescence on aperture, inner lip, and a band along the external side of the outer lip.
Comment. In the photographed specimen the fluorescence is much more orange and intense on the outer lip than it is on the spire and inner lip. The fluorescence is also restricted to the section which is smooth and shiny.
Family NATICIDAE Guilding, 1834
Eunaticina subinfundibulum (Tate, 1893)
Figure 6C
Pattern description. Fluorescent spiral band positioned right after suture to middle whorl height. The spiral band consists of very closely related axial lines of variable thickness and brightness of the fluorescing areas. The axial lines are consistently thick near the edges of the spiral band, regardless of their thickness at the middle of the band.
Order NEOGASTROPODA Wenz, 1938
Family AUSTROSIPHONIDAE Cotton and Godfrey, 1938
Penion roblini simulans (Tate, 1888)
Figure 5C
Pattern description. Thin, yellow, fluorescent line running spirally through the centre of the whorls, concurrent with the apex of the sculptured peaks.
Family FASCIOLARIIDAE Gray, 1853
Dennantia murrayana (Tate, 1888)
Figure 5D
Pattern description. Rounded patches located on the sculptured nodules of the shell fluoresce a pale orange. This continues throughout the whorls of the shell but is less pronounced closer to the apex.
Family ANCILLARIIDAE Swainson, 1840
Genus Alocospira Cossmann, 1899
Figure 5F
Pattern description. The tip of the apex strongly fluoresces yellow-orange, gradually fading in fluorescence away from the tip. The aperture fluoresces solidly. The upper half of the fasciolar band fluoresces strongly. The body whorl does not fluoresce but appears a darker, grey shade under UV light, though this colour difference did not translate well in photography.
Family VOLUTIDAE Rafinesque, 1815
Nannamoria cadella Hawke, 2021
Figure 6A
Pattern description. “False” triangle pattern (defined from the lack of pigment in a pigmented background) present on body whorl and first two whorls of spire. Pattern appearance the same as Nannamoria sp., aside from two medium-weight lines running spirally through the body whorl. These lines are interrupted by triangles.
Comment. Nannamoria cadella Hawke, 2021 in its original description was said to be comparable to Nannamoria trionyma Darragh, 1988 but with a smaller size, fewer shoulder nodules, which are also less developed, and with occurrence only in the Murbko Marl. No colour pattern for N. trionyma is known. Darragh (2024) synonymised the two species. We have continued to use N. cadella here for the following reasons: N. trionyma has not previously been recorded from the Murbko Marl, and each of the specimens studied follow the features given for N. cadella by Hawke (2021), with smaller shell sizes and fewer shoulder nodules (between 5 and 7).
As the colour pattern for N. trionyma is currently unknown and we do not have access to previously identified N. trionyma fossils, it is our hope that the colour pattern revealed here can be used in the future for comparison with analysis of Victorian material of N. trionyma to help address this issue, and either justify of refute the validity of N. cadella.
Nannamoria sp.
Figure 6B
Pattern description. Bright yellow-orange fluorescence. Body and first spire whorl show a “tent” pattern of solid, “false” triangles. Generally, one edge of the triangle is aligned to axis and the third point facing sinistrally. Location of triangles apparently random. Triangles occasionally overlap slightly, varying in size, but all are relatively small. Remaining whorls/protoconch show a solid yellow fluorescence.
Comment. Nannamoria strophodon (McCoy, 1876) is the closest match of the Australian tertiary species known in this genus and has been recorded from the Murbko Marl. However, this species is described as very morphologically variable, and it is the opinion of the authors that it likely comprises several species. For example, N. malonei Hawke, 2021 and N. gnotuki Hawke, 2021 were synonymised with N. strophodon by Daragh (2024), despite having distinct body shapes and differences in the number, size and shape of shoulder nodules. The specimens studied in this paper are distinctly different from N. gnotuki as described by Hawke (2021) and cannot be reasonably identified as the same species, i.e., N. strophodon. The patterns found through fluorescence here do not match those described from residual patterns of N. strophodon, which Darragh (1989) says to have thin, close set wavy lines. Thus, future comparison with previously identified specimens of N. strophodon is recommended to consider separation of the species.
Ternivoluta antiscalaris (McCoy, 1866)
Figure 5E
Pattern description. Orange fluorescence. Pattern of short, thick dashed lines. Start and end points of lines typically align with those above and below. Pattern covers entirety of shell but is greater defined on the body whorl than the more densely sculptured spire.
Superfamily CONOIDEA
Genus indet.
Figure 5G
Pattern description. Irregular, wavy pattern aligned to axis. Waves are not consistent in size or shape. Waves often overlap and connect with other waves. Pattern continuous throughout entire shell (excluding protoconch) but is greater defined on the body whorl compared to the spire, where the whorls are small and comparatively more sculptured.
Family MARGINELLIDAE Fleming, 1828
Serrata propinqua (Tate, 1878)
Figure 6D
Pattern description. Bright yellow fluorescence. The edge of the body whorl has a thick, solid band of fluorescence. The inner lip also fluoresces strongly. Scattered fluorescence along the whorl sutures.
Family TURRIDAE Adams and Adams, 1853 (1838)
Lophiotoma murrayana (Pritchard, 1904)
Figure 6E
Pattern description. Yellow-orange fluorescence. The ridged surface of the whorls shows fluorescence of lined chevrons, the apex of which point dextrally. Occasional fainter fluorescence in the depressed areas of the whorls showing vertical wavy lines continuing from the chevrons.
Comment. The patterns revealed here support the recent reassignment of L. murrayana from Lucerapex to Lophiotoma, where it had previously been placed. The move to Lucerapex was made by Powell (1963) who felt the original description of the species better matched Lucerapex, but did not study any specimens directly. Darragh (2024) returned L. murrayana back to Lophiotoma. Modern Lophiotoma frequently display patterns like what is observed here, whereas modern Lucerapex are described to typically be devoid of pattern and colour, with most species being pure white (Powell, 1966). Thus, we support the assignment to Lophiotoma proposed by Darragh (2024).
Class BIVALVIA Linnaeus, 1758
All the bivalve shells reported on here show evidence of pigmentation at the muscle scars. Hao et al. (2015) identified the pigment in the muscle scar of the Pacific oyster Crassostrea gigas as eumelanin and proposed that as melanin is elsewhere related to mechanical strength, its presence is related to increasing the strength and ability of the muscles to hold the shell closed when attacked by predators. However, melanin cannot be responsible for the pigmentation seen in the muscle scars here as melanin is not known to fluoresce (Williams, 2017).
Order MYIDA Stoliczka, 1870
Family CORBULIDAE Lamarck, 1818
Corbula ephamilla Tate, 1885
Figure 7A, B
 Pattern description. Solid, bright orange fluorescence on the entire external shell surface. The internal surface does not fluoresce aside from the muscle scars and pallial line, which appear yellow.
Pattern description. Solid, bright orange fluorescence on the entire external shell surface. The internal surface does not fluoresce aside from the muscle scars and pallial line, which appear yellow.
Comment. Fluorescence intensity varies among specimens, with some shells showing only the faintest external fluorescence even after bleach treatment.
Order ARCIDA Stoliczka, 1871
Family CUCULLAEIDAE Stewart, 1930
Cucullaea corioensis McCoy, 1876
Figure 7D
Pattern description. The external surface shows weak, yellow fluorescence as concentric banding. The internal surface is non-fluorescent, excluding the muscle scars and pallial line.
Family GLYCYMERIDIDAE Dall, 1908 (1847)
Tucetona subtrigonalis (Tate, 1886)
Figure 7E
Pattern description. External surface fluoresces yellow-orange in an irregular, mottled pattern. Fluorescent bands generally restricted to ribs–pattern does not usually end in the middle of a rib, rather it spans the width of the rib. Internal surface is non-fluorescent, excluding the muscle scars and pallial line.
Class SCAPHOPODA Bronn, 1862
Order DENTALIIDA Starobogatov, 1974
Family DENTALIIDAE Children, 1834
Fissidentalium mawsoni (Ludbrook, 1956)
Figure 7C
Pattern description. Yellow fluorescence. 1–2 thin transverse bands, located near the centre and just above the anterior aperture of the shell.
Energy-dispersive X-ray Spectroscopy
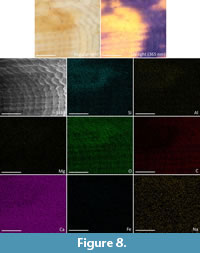 The EDX results on Maoricolpus murrayanus (Table 1) include data from both fluorescing and non-fluorescing areas of the shell. Whilst in the EDX machine it was not possible to see the fluorescing area due to a lack of UV light source, and SEM images being rendered without colour. To minimise this issue, a fluorescent section marked prior to analysis was examined as closely as possible. Thus, the elemental spectrum results are a combination of both the fluorescing area and non-pigmented area and are not able to be separated outside of visual interpretations made from the elemental distribution maps (Figure 8).
The EDX results on Maoricolpus murrayanus (Table 1) include data from both fluorescing and non-fluorescing areas of the shell. Whilst in the EDX machine it was not possible to see the fluorescing area due to a lack of UV light source, and SEM images being rendered without colour. To minimise this issue, a fluorescent section marked prior to analysis was examined as closely as possible. Thus, the elemental spectrum results are a combination of both the fluorescing area and non-pigmented area and are not able to be separated outside of visual interpretations made from the elemental distribution maps (Figure 8).
EDX of the fossil shell M. murrayanus identified O, C, and Ca, which constitute the calcium carbonate of the shell, with Si (apparent concentration 4.80, 2.54 Wt% –i.e., weight percentage–, 1.6 At%) and Al (apparent concentration 1.70, 100 Wt%, 0.6 At%) as the only other elements of notable amounts. Na, Mg, S, Cl, K, Fe, and W are all also present in miniscule amounts (see Table 1). Visual examination of the distribution map (Figure 8) shows a weak correlation between the density of Si, and to a lesser extent Al, and the area of remaining pigmentation/fluorescence. There also appears to be less carbon at this location than there is in the surrounding area. Other recorded elements show no apparent difference in density between the fluorescent and non-fluorescent areas.
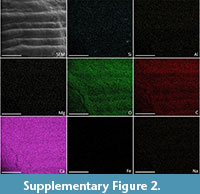 In comparison, the EDX of the modern shell, M. roseus, indicated that there were no levels of anything over 1At% (atomic percentage) except for the expected O (51.4 At%), C (36.9 At%), and Ca (11.2 At%). Na, S, Si, Cl, Fe, Mg, and Sr were present but in miniscule amounts. There was no apparent visual correlation in the elemental maps between the pigmented area and any of the identified elements (Supplementary Figure 2).
In comparison, the EDX of the modern shell, M. roseus, indicated that there were no levels of anything over 1At% (atomic percentage) except for the expected O (51.4 At%), C (36.9 At%), and Ca (11.2 At%). Na, S, Si, Cl, Fe, Mg, and Sr were present but in miniscule amounts. There was no apparent visual correlation in the elemental maps between the pigmented area and any of the identified elements (Supplementary Figure 2).
ENERGY-DISPERSIVE X-RAY SPECTROSCOPY ANALYSIS OF SHELL SURFACE ELEMENTS
All specimens presented here fluoresced either yellow or orange. Yellow-orange fluorescence has been identified in a diverse range of molluscs, including caenogastropods, patellogastropod limpets, and bivalves from Arcoida, Protobranchia, Mytiloidea, Pectinoida, and Heterodonta (Caze et al., 2011a now, 2012, 2015; Hendricks 2015, 2018). Despite yellow-orange fluorescence being the most observed type within the molluscs, the pigment/s responsible have not yet been identified, nor have any associated elements (Williams 2017).
The Energy-dispersive X-ray spectroscopy performed in this study found no elements were present in significant amounts at the pigmented locations of either the modern or fossil shell – excluding those which comprise the calcium carbonate of the shell (Ca, C, and O). The most significant element present was silicon, which does appear in higher density at the location of fluorescence in the fossil specimen (Figure 8). As there is no difference in the density of silicon visible in the modern specimen, it can be interpreted that the denser silicon in the fossil specimen is either a product of pigment breakdown or has been obtained from the environment during diagenesis.
Pure silicon has a high absorption coefficient, peaking at around 200–400 nm (Green and Keevers, 1995). A high absorption coefficient means a material more readily absorbs photons, in turn exciting electrons. Fluorescence occurs when excited electrons return to their ground state. Thus, the higher the absorption coefficient, the greater the potential for fluorescence. McDougall (1952) reported that practically no pure elements or simple compounds exhibit fluorescence. Therefore, the silicon must be bound to something complex. Similarly, Bayrakceken and Yegin (2012) state that fused silica (SiO2) exhibits coherent visible fluorescence in the UV-C range (also known as shortwave fluorescence, from 200–400 nm). Thus, we would expect a much more complicated molecule if silicon is responsible for the fluorescence observed. This cannot be identified with EDX alone and will need further investigation in the future.
An alternative explanation can be presented by considering how fluorescent minerals behave. In minerals, fluorescence is most often triggered by the presence of an “activator”, usually an element which is “foreign” (Robbins, 1983). The difference between a mineral fluorescing or not is the presence of its activator. Sometimes the activator can be effective with only miniscule amounts present, creating issues in identifying said activator. Minerals in which the activator is not foreign are called “self-activated”. In minerals it is the activator which typically controls the colour of the fluorescence: e.g., europium gives a blue fluorescence when present in several minerals (Robbins, 1983). The combination of two activators can also change the fluorescence colour emitted.
Robbins (1983) listed elements known to be activators in minerals. From that list, only tungsten (W) was present in our EDX analysis. W was the element detected with the smallest amount present, with only 0.03 apparent concentration, k ratio 0.00026 and Wt% 0.02. Although activators can be present in miniscule amounts, W typically induces a blue fluorescence in minerals, not a yellow-orange fluorescence as witnessed here. Thus, tungsten is unlikely to be an activator in this instance.
Williams et al. (2016) investigated trace elements associated with pigmentation using EDX (alongside a range of other techniques) in modern Clanculus shells, which fluoresce red. From Robbins’s activator list Williams et al. (2016) found manganese and copper present. Manganese is an activator which commonly causes red fluorescence in minerals (Robbins, 1983). Copper causes a range of coloured fluorescence, but not typically red. Williams et al. (2016) disregarded the presence of these elements as there was no clear elemental mapping to areas of pigmentation. However, if these two elements are behaving as activators the combination of their presence with the unknown components of the unidentified pigment could together be enabling fluorescence.
CONCLUSIONS
Our study has used UV fluorescence to investigate pigmentation patterns found in Miocene fossil molluscan shells from the 15 m.y.o. Murbko Marl of the Cadell Formation, north of Blanchetown, South Australia. These patterns were found represented in 18 taxa across three separate classes of molluscs—predominantly gastropods, but also bivalves and scaphopods.
The taxonomic placement of fossil species can be reconsidered with the new characters presented by UV fluorescence. This has been discussed predominantly in the case of Maoricolpus murrayanus, Lophiotoma murrayana, and the genus Nannamoria. M. murrayanus shows very similar patterning to extant Maoricolpus roseus, a species from which it is already distinguished by a negligible margin. Both species have variability in the patterning but show similarities in these variations which continues to call into question the long-debated validity of their separation. Clarification of the origin of either pigmentation or fluorescence in fossil Maoricolpus murrayanus or modern Maoricolpus roseus has not been ascertained despite employing Energy-dispersive X-ray spectroscopy on both.
Lophiotoma murrayana, on the other hand, shows patterning which supports its recent return to the genus from Lucerapex from which extant species are typically colourless. Patterns revealed in Nannamoria species can be used in future works to help justify species validity.
ACKNOWLEDGEMENTS
We acknowledge that the Murbko Marl lies in the traditional lands of the Meru people and pay our respect to their Elders past, present and emerging. Funding was received from the University of Adelaide Student Support Fund and Australian Research Council Discovery Project DP220102772 (to D.C.G.B). We thank Dr. M-A Binnie for access to the South Australian Museum Palaeontological Collection; Dr. A. Crowther and S. Sorokin for access to the Marine Invertebrate collection of the South Australian Museum; P. Hunt for the loan of modern mollusc specimens for comparison; Adelaide Microscopy for the use of the FEI Quanta 450 FEG Environmental Scanning Electron Microscope; and M-A Binnie, T. Botha, P. Hunt, M. Burrell, and N. Spooner for help, advice, and scientific discussions.
REFERENCES
Adams, H. and Adams, A. 1853. The genera of recent Mollusca: arranged according to their organization. Van Voorst, London.
https://doi.org/10.5962/bhl.title.4772
Barnard, W. and de Waal, D. 2006. Raman investigation of pigmentary molecules in the molluscan biogenic matrix. Journal of Raman Spectroscopy, 37:342–352.
https://doi.org/10.1002/jrs.1461
Bax, N.J., McEnnulty, F.R., and Gowlett-Holmes, K.L. 2003. Distribution and biology of the introduced gastropod, Maoricolpus roseus (Quoy and Gaimard, 1834) (Caenogastropoda: Turritellidae) in Australia. Centre for Research on Introduced Marine Pests. Technical Report No. 25. CSIRO Marine Research, Hobart. p. 40.
https://doi.org/10.4225/08/585d6674c8ae9
Bayrakceken, F. and Yegin, K. 2012. Resonance Fluorescence of Fused Silica by the Depopulation of the Ground State. International Journal of Photoenergy, 2012:1–3.
https://doi.org/10.1155/2012/359384
Benbow, M.C., Alley, N.F., and Lindsay, J.M. 1995. Tertiary Introduction, p. 151. In Drexel, J.F. and Preiss, W.V. (ed.), The geology of South Australia. Geological Survey of South Australia, Adelaide.
Bergamonti, L., Bersani, D., Mantovan, S., and Lottici, P.P. 2013. Micro-Raman investigation of pigments and carbonate phases in corals and molluscan shells. European Journal of Mineralogy, 25:845–853.
https://doi.org/10.1127/0935-1221/2013/0025-2318
Beu, A. 2010. Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 3. Gastropoda (Vetigastropoda - Littorinimorpha). Journal of the Royal Society of New Zealand, 40(3-4), 59–180.
https://doi.org/10.1080/03036758.2010.500717
Borodina, A.V., Maoka, T., and Soldatov, A.A. 2013. Composition and content of carotenoids in body of the Black Sea gastropod Rapana venosa (Valenviennes, 1846). Journal of Evolutionary Biochemistry and Physiology, 49 283–290.
https://doi.org/10.1134/s002209301303002x
Bronn, H.G. 1862. Die Klassen und Ordnungen der Weichthiere (Malacozoa). Kopflose Weichthiere (Malacozoa Acephala) Weichthiere. Leipzig.
Brown, C.M. and Stephenson, A.E. 1991. Geology of the Murray Basin (1:1 000 000 scale map). Bureau of Mineral Resources, Canberra.
Caze, B., Didier, M., Jean-Michel, P., and Jean-Paul Saint, M. 2010a. First Systematic Study using the Variability of the Residual Colour Patterns: The Case of the Paleogene Seraphsidae (Mollusca, Gastropoda, Stromboidea). Geodiversitas, 32:417–477.
https://doi.org/10.5252/g2010n3a4
Caze, B., Saint Martin, J.-P., Merle, D., and Martin, S. 2010b. Intérêt des motifs colorés résiduels des coquilles de mollusques pour la valorisation des sites paléontologiques et des collections: l’exemple du Badénien de Roumanie, p. 27–38. In Martin, J.-P.S., Martin, S.S., Oaie, G., and Sedgedi, Antoneta. (eds.), Le patrimoine paléontologique, Des trésors du fond des temps. GeoEcoMar.
Caze, B., Merle, D., Saint Martin, J.-P., and Pacaud, J.-M. 2011a. Contribution of residual colour patterns to the species characterization of Caenozoic molluscs (Gastropoda, Bivalvia). Comptes Rendus Palevol, 10:171–179.
https://doi.org/10.1016/j.crpv.2010.10.005
Caze, B., Merle, D., Le Meur, M., Pacaud, J.-M., Ledon, D., and Saint Martin, J.-P. 2011b. Taxonomic implications of the residual colour patterns of ampullinid gastropods and their contribution to the discrimination from naticids. Acta Palaeontologica Polonica, 56:329–347.
https://doi.org/10.4202/app.2009.0084
Caze, B., Merle, D., Saint Martin, J.-P., and Pacaud, J.-M. 2012. Les mollusques éocènes se dévoilent sous ultraviolet. Fossiles, special series 3:15–57.
Caze, B., Merle, D., and Schneider, S. 2015. UV Light Reveals the Diversity of Jurassic Shell Colour Patterns: Examples from the Cordebugle Lagerstätte (Calvados, France). PLOS One, 10:e0126745.
https://doi.org/10.1371/journal.pone.0126745
Colchester, D., Webb, G., and Emseis, P. 2006. Amber-like fossil resin from north Queensland, Australia. Australian Gemmologist, 22:378–386.
Comfort, A. 1949a. Acid-soluble pigments of shells. 1. The distribution of porphyrin fluorescence in molluscan shells. Biochemical Journal, 44:111–117.
https://doi.org/10.1042/bj0440111
Comfort, A.1949b. Acid-soluble pigments of molluscan shells. 2. Pigments other than porphyrins. Biochemical Journal, 45:199–204.
https://doi.org/10.1042/bj0450199
Comfort, A.1949c. Acid soluble pigments of molluscan shells. 3. The indigoid character of the blue pigment of Haliotis cracherodii Leach. Biochemical Journal, 45:204–208.
https://doi.org/10.1042/bj0450204
Comfort, A. 1950a. A Subsidiary Shell Pigment of Haliotis cracherodii Leach. Nature, 166:194–195.
https://doi.org/10.1038/166194b0
Comfort, A.1950b. Acid-soluble pigments of molluscan shells. 5. Identity of some subsidiary fractions derived from Pinctada vulgaris. Biochemical Journal, 47:254–255.
https://doi.org/10.1042/bj0470254
Comfort, A. 1950c. Biochemistry of molluscan shell pigments. Proceedings of the Malacological Society of London, 28:79–85.
https://doi.org/10.1093/oxfordjournals.mollus.a064570
Comfort, A.1950d. Molluscan shells as a practical source of uroporphyrin I. Science, 112:279–280.
https://doi.org/10.1126/science.112.2906.279
Comfort, A. 1951a. Observations on the shell pigments of land pulmonates. Proceedings of the Malacological Society of London, 29:35–44.
https://doi.org/10.1093/oxfordjournals.mollus.a064598
Comfort, A. 1951b. The pigmentation of molluscan shells. Biological Reviews, 26:285–301.
https://doi.org/10.1111/j.1469-185x.1951.tb01358.x
Cossmann, M. 1895-1924. Essais de paléoconchologie comparée. Cossmann, Paris, France.
https://doi.org/10.5962/bhl.title.52314
Cotton, B.C. and Godfrey, F.K. 1938. A systematic list of the gastropoda the marine, freshwater and land univalve mollusca of south and central Australia. Malacological Society of South Australia, Adelaide.
Cowley, W.M. and Barnett, S.R. 2007. Revision of Oligocene-Miocene Murray Group stratigraphy for geological and groundwater studies in South Australia. MESA Journal:17–20.
Crippa, G. and Masini, S. 2022. Photography in the ultraviolet and visible violet spectra: Unravelling methods and applications in palaeontology. Acta Palaeontologica Polonica, 67:685–702.
https://doi.org/10.4202/app.00948.2021
Cuvier, G. 1795. Second Mémoire sur l’organisation et les rapports des animaux à sang blanc, dans lequel on traite de la structure des Mollusques et de leur division en ordre. Magasin Encyclopédique: ou Journal des Sciences, des Lettres et des Arts, 2:433–449.
Dall, W.H. 1908. Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U.S. Fish Commission steamer “Albatross,” during 1891, Lieut.-Commander Z.L. Tanner, U.S.N., commanding. XXXVII. Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission steamer “Albatross”, from October, 1904 to March, 1905, Lieut.-Commander L.M. Garrett, U.S.N., commanding. XIV. The Mollusca and Brachiopoda. Bulletin of the Museum of Comparative Zoology, 43:205–487.
Darragh, T. 1988. A revision of the Tertiary Volutidae (Mollusca: Gastropoda) of southeastern Australia. Memoirs of the Museum of Victoria, 49:195–307.
https://doi.org/10.24199/j.mmv.1988.49.12
Darragh T. 2024. A checklist of Australian marine Cenozoic Mollusca. Memoirs of Museum Victoria, 83:37–206.
https://doi.org/10.24199/j.mmv.2024.83.02
de Oliveira, L.N., de Oliveira, V.E., D’ávila, S., Edwards, H.G., and de Oliveira, L.F. 2013. Raman spectroscopy as a tool for polyunsaturated compound characterization in gastropod and limnic terrestrial shell specimens. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 114:541–546.
https://doi.org/10.1016/j.saa.2013.05.095
Derby, C.D. 2014. Cephalopod ink: production, chemistry, functions and applications. Mar Drugs, 12:2700–30.
https://doi.org/10.3390/md12052700
Dommergues, E., Dommergues, J.-L., and Dommergues, C. 2006. Deux espèces sous un même masque. Le point de vue paléontologique piégé par les coquilles de deux espèces européennes de Trivia (Mollusca, Gastropoda). Revue de Paléobiologie, Geneva, 25:775–790.
Donald, K.M. and Spencer, H.G. 2015. New Zealand screw shells Maoricolpus roseus (Gastropoda: Turritellidae): two species, two subspecies or a single variable species? Molluscan Research, 35:123–127.
https://doi.org/10.1080/13235818.2014.977838
Ferrer, P., Ruiz-Moreno, S., De Torres, A.R., and López-Gil, A. 2013. Molecular stratigraphic analysis with Raman spectroscopy of the shell of a mussel. Conference Proceedings of the MARTECH 2013; 5th International Workshop on Marine Technology, Girona, SARTI.
Fleming, J. 1828. History of British animals. Bell & Bradfute, Edinburgh.
https://doi.org/10.5962/bhl.title.2156
Frese, M., Gloy, G., Oberprieler, R.G., and Gore, D.B. 2017. Imaging of Jurassic fossils from the Talbragar Fish Bed using fluorescence, photoluminescence, and elemental and mineralogical mapping. PLOS One, 12:e0179029.
https://doi.org/10.1371/journal.pone.0179029
Garrard, T.A. 1972. A Revision of Australian Recent and Tertiary Turritellidae (Gastropoda: Mollusca). Journal of the Malacological Society of Australia, 2:267–338.
https://doi.org/10.1080/00852988.1972.10673858
Golikov, A.N. and Starobogatov, Y.I. 1975. Systematics of prosobranch gastropods. Malacologia, 15:185–232.
Gong, Z., Matzke, N., Ermentrout, B., Song, D., Vendetti, J., Slatkin, M., and Oster, G. 2012. Evolution of patterns on Conus shells. Proceedings of the National Academy of Sciences of the United States of America, 109:E234–41.
https://doi.org/10.1073/pnas.1119859109
Górka, M. 2008. Shell colour pattern in two fossil helicid snails, Tropidomphalus incrassatus (KLEIN, 1853) and Cepaea sylvestrina gottschicki WENZ, 1919, from the Middle Miocene of Poland. Acta Geologica Polonica, 58:105–111.
Green, M.A. and Keevers, M.J. 1995. Optical properties of intrinsic silicon at 300 K. Progress in Photovoltaics: Research and Applications, 3:189–192.
https://doi.org/10.1002/pip.4670030303
Guérin-Méneville, F.É. and Société, C. 1849. Revue et magasin de zoologie pure et appliquée, sér.2:t.1 (1849). Bureau de la Revue et Magasin de Zoologie, Paris.
Guilding, L. 1834. Observations on Naticina and Dentalium, two Genera of Molluscous Animals. Transactions of the Linnean Society of London. 17:29–35.
https://doi.org/10.1111/j.1095-8339.1834.tb00016.x
Hao, S., Hou, X., Wei, L., Li, J., Li, Z., and Wang, X. 2015. Extraction and Identification of the Pigment in the Adductor Muscle Scar of Pacific Oyster Crassostrea gigas. PLOS One, 10:e0142439.
https://doi.org/10.1371/journal.pone.0142439
Harzhauser M. and Landau B. 2016. A revision of the Neogene Conidae and Conorbidae (Gastropoda) of the Paratethys Sea. Zootaxa, 4210 (1):1–178.
https://doi.org/10.11646/ZOOTAXA.4210.1.1
Hawke, A.L. 2021. New species of Nannamoria from the Pliocene and Miocene of Australia (Caenogastropoda: Volutidae). The Festivus, 53:245–260.
https://doi.org/10.54173/f534245
Hendricks, J.R. 2015. Glowing Seashells: Diversity of Fossilized Coloration Patterns on Coral Reef-Associated Cone Snail (Gastropoda: Conidae) Shells from the Neogene of the Dominican Republic. PLOS One, 10:e0120924.
https://doi.org/10.1371/journal.pone.0120924
Hendricks, J.R. 2018. Diversity and preserved shell coloration patterns of Miocene Conidae (Neogastropoda) from an exposure of the Gatun Formation, Colón Province, Panama. Journal of Paleontology, 92:804–837.
https://doi.org/10.1017/jpa.2017.153
Hoerle S.E. 1976. The genus Conus (Mollusca: Gastropoda) from the Alum Bluff Group of Northwestern Florida. Tulane Studies in Geology and Paleontology, 12 (1):1–32.
Hollingworth, N.T.J. and Barker, M.J. 1991. Colour pattern preservation in the fossil record: taphonomy and diagenetic significance, p. 105–119. In Donovan, S.K. (ed.), The processes of Fossilization. Belhaven Press, London.
Iredale, T. 1913. The generic name to be used for Murex tritonis Linne. The Nautilus, 27:55–56.
Iredale, T. 1929. Mollusca from the Continental Shelf of eastern Australia. No. 2. Records of the Australian Museum, 17:157–189.
https://doi.org/10.3853/j.0067-1975.17.1929.759
Ishikawa, M., Kagi, H., Sasaki, T., and Endo, K. 2019. Chemical basis of molluscan shell colors revealed with in situ micro-Raman spectroscopy. Journal of Raman Spectroscopy, 50:1700–1711.
https://doi.org/10.1002/jrs.5708
Kase, T., Kitao, F., Aguilar, Y., Kurihara, Y., and Pandita, H. 2008. Reconstruction of color markings in Vicarya, a Miocene potamidid gastropod (Mollusca) from SE Asia and Japan. Paleontological Research, 12:345–353.
https://doi.org/10.2517/prpsj.12.345
Kelley, P.H. and Swann, C.T. 1988. Functional significance of preserved color patterns of mollusks from the Gosport Sand (Eocene) of Alabama. Journal of Paleontology, 62:83–87.
https://doi.org/10.1017/s0022336000018035
Kennedy, G.Y. 1979. Pigments of marine invertebrates. Advances in Marine Biology, 16:309–381.
https://doi.org/10.1016/S0065-2881(08)60295-3
Kingham, R. 1998. Geology of the Murray-Darling Basin: simplified lithostratigraphic groupings. Australian Geological Survey Organisation, Dept. of Primary Industries & Energy, Canberra.
Koskeridou, E. and Thivaiou, D 2012. Problematic species: Color patterns revealed by UV light as a character for systematics in mollusc fossils– an example from the Hellenic region. Proceedings of the 10th Symposium on Oceanography & Fisheries, Athens, Greece.
Krueger, K.K. 1974. The Use of Ultraviolet Light in the Study of Fossil Shells. Curator: The Museum Journal, 17:36–49.
https://doi.org/10.1111/j.2151-6952.1974.tb01222.x
Krzemiński, W., Soszyńska-Maj, A., Bashkuev, A.S., and Kopeć, K. 2015. Revision of the unique Early Cretaceous Mecoptera from Koonwarra (Australia) with description of a new genus and family. Cretaceous Research, 52:501–506.
https://doi.org/10.1016/j.cretres.2014.04.004
Lamarck, J-B. 1818. Histoire naturelle des animaux sans vertèbres... précédée d'une introduction offrant la détermination des caractères essentiels de l'animal, sa distinction du végétal et des autres corps naturels, enfin, l'exposition des principes fondamentaux de la zoologie. Verdière, Paris.
https://doi.org/10.5962/bhl.title.12712
Landau, B., Harzhauser, M., Büyükmeriç, Y., and Silva C. 2013. Systematics and palaeobiogeography of the gastropods of the middle Miocene (Serravallian) Karaman Basin, Turkey. Cainozoic Research, 11-13:3–576.
Lindsay J. M. and Bonnett J.E. 1973. Tertiary stratigraphy of three deep bores in the Waikerie area of the Murray Basin. Geological Survey of South Australia Report of Investigation 38.
Linnaeus, C. 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata, 1:824. Laurentius Salvius, Holmiae.
https://doi.org/10.5962/bhl.title.542
Lovén, S. 1847. Malacozoologi. Öfversigt af Kongliga Vetenskaps Akademiens Förhandlingar, 4:175–199.
Ludbrook, N.H. 1956. The molluscan fauna of the Pliocene strata underlying the Adelaide Plains, part III – Scaphopoda, Polyplacophora, Gastropoda (Haliotidae to Tornidae). Transactions of the Royal Society of South Australia 79:1–36.
Lukasik, J.J. and James, N.P. 1998. Lithostratigraphic revision and correlation of the Oligo-Miocene Murray Supergroup, Western Murray Basin, South Australia. Australian Journal of Earth Sciences, 45:889–902.
https://doi.org/10.1080/08120099808728443
Matsuno, T., Katagiri, K., Maoka, T., and Komori, T. 1984. Carotenoids of the spindle shell Fusinus perplexus. Bulletin of the Japanese Society of Scientific Fisheries, 50:1583–1588.
https://doi.org/10.2331/suisan.50.1583
McCoy, F. 1866. On some new species of fossil volutes from the Tertiary beds near Melbourne. Annals and Magazine of Natural History, 18:375–381.
McCoy, F. 1876. Prodomus of the Palaeontology of Victoria; or figures and descriptions of Victorian organic remains, Vol. 4. Government Printer, Melbourne.
https://doi.org/10.5962/bhl.title.13870
McKnight, D.G. 1969. Infaunal benthic communities of the New Zealand continental shelf. New Zealand Journal of Marine and Freshwater Research, 3:409–444.
https://doi.org/10.1080/00288330.1969.9515308
Merle, D. 2003. First record of coloured patterns in Palaeogene Muricidae (Mollusca, Gastropoda). Annales de Paléontologie, 89:191–203.
https://doi.org/10.1016/S0753-3969(03)00024-7
Merle, D., Pacaud, J.-M., Kriloff, A., and Loubry, P. 2008. Les motifs colorés résiduels des coquilles lutétiennes du bassin de Paris, p. 182–227. In Didier, M. (ed.), Stratotype Lutétien. Publications scientifiques du Muséum, Paris, Biotope Mèze, BRGM Orléans.
Miethe, A. and Born, A. 1928. Die Fluorographie von Fossilien. Palaeontologische Zeitschrift, 9:343–356.
https://doi.org/10.1007/bf03041560
MolluscaBase eds. 2023. MolluscaBase. Maoricolpus proroseus Marwick, 1931 †. Records accessed 12 October 2023. World Register of Marine Species.
https://www.marinespecies.org/aphia.php?p=taxdetails&id=1055344
Nicholas, R.E.H. and Comfort, A. 1949. Acid-soluble pigments of molluscan shells. 4. Identification of shell porphyrins with particular reference to conchoporphyrin. Biochemical Journal, 45:208–210.
https://doi.org/10.1042/bj0450208
Norvick, M.S. and Smith, M.A. 2001. Mapping the plate tectonic reconstruction of southern and southeastern Australia and implications for petroleum systems. The APPEA Journal, 41:15–35.
https://doi.org/10.1071/AJ00001
Pacaud, J.-M. 2003. First fossil records of the Recent Ovulid genus Pseudocypraea Schilder, 1927 (Mollusca, Gastropoda) with description of a new species. Geodiversitas, 25:451–462.
Percival, I., Meakin, S., Sherwin, L., Vanderlaan, T., and Flitcroft, P. 2012. Permian fossils and palaeoenvironments of the northern Sydney Basin, New South Wales. Quarterly Notes Geological Survey of New South Wales, 138:1–23.
Pereira, P.A., Prado, L.A.C.d., Araripe, R.V.C., Oliveira, D.H.d., Lemos, F.A.P., Lobo, L.R.d.S., Tomé, M.E.T.R., and Barreto, A.M.F. 2022. Gastropods Colour Patterns in Cassiopids and Naticids from Romualdo Formation, Araripe Basin, Northeast Brazil. Anuário do Instituto de Geociências, 45:51358.
https://doi.org/10.11137/1982-3908_2022_45_51358
Powell, A.W B. 1940. The marine Mollusca of the Aupourian Province, New Zealand. Transactions of the Royal Society of New Zealand, 70:205–248.
Powell, A.W.B. 1966. The molluscan families Speightiidae and Turridae: an evaluation of the valid taxa, both recent and fossil, with lists of characteristic species. Bulletin of the Auckland Institute and Museum, 5:1–184.
Pritchard, G.B. 1898. Contributions to the palaeontology of the Older Tertiary of Victoria. Gastropoda–Part I. Proceedings of the Royal Society of Victoria, 11:96–111.
Pritchard, G.B. 1904. Contributions to the Palaeontology of the Older Tertiary of Victoria. Gastropoda–Part II. Proceedings of the Royal Society of Victoria, 17:320–337.
Psarras, C., Koskeridou, E., and Merle, D. 2021. Late Miocene Conidae (Mollusca: Gastropoda) of Crete (Greece). Part 1: genera Conilithes Swainson, 1840 and Conus (Kalloconus) da Motta, 1991. Geodiversitas, 43:1309–1339.
https://doi.org/10.5252/geodiversitas2021v43a24
Psarras, C., Merle, D., and Koskeridou, E. 2022. Late Miocene Conidae (Mollusca: Gastropoda) of Crete (Greece). Part 2. European Journal of Taxonomy, 816:1–70.
https://doi.org/10.5852/ejt.2022.816.1747
Psarras C., Merle D., Antonarakou A., and Koskeridou E. 2023. Late Miocene Conidae (Mollusca: Gastropoda) of Crete, (Greece). Part 3: subgenus Conus (Monteiroconus) da Motta, 1991. Bollettino della Società Paleontologica Italiana, 62 (1):27–52.
https://doi.org/10.4435/BSPI.2023.01
Rafinesque, C.S. 1815. Analyse de la nature ou tableau de l’univers et des corps organisés. Rafinesque, Palerme.
https://doi.org/10.5962/bhl.title.106607
Robbins, M. 1983. The Collector’s Book of Fluorescent Minerals. Springer Science + Business Media, New York, New York.
https://doi.org/10.1007/978-1-4757-4792-8
Rogers, P.A., Lindsay, J.M., Alley, N.F, Barnett, S.R., Lablack, K.L., and Kwitko, G. 1995. Murray Basin, p. 157–163. In Drexel, J.F. and Preiss, W.V. (eds.), The geology of South Australia. Geological Survey of South Australia, Adelaide.
Rolfe, W.D.I. and Brett, D.W. 1969. Fossilization Processes, p. 213–244. In Eglinton, G. and Murphy, M.T.J. (eds.), Organic Geochemistry: Methods and Results. Springer Berlin Heidelberg, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-642-87734-6_9
Schöne, B.R., Schmitt, K., and Maus, M. 2017. Effects of sample pretreatment and external contamination on bivalve shell and Carrara marble δ18O and δ13C signatures. Palaeogeography, Palaeoclimatology, Palaeoecology, 484:22–32.
https://doi.org/10.1016/j.palaeo.2016.10.026
Simpson, G.G. 1926. Are Dromatherium and Microconodon Mammals? Science, 63:548–549.
https://doi.org/10.1126/science.63.1639.548
Speiser, D.I., DeMartini, D.G., and Oakley, T.H. 2014. The shell-eyes of the chiton Acanthopleura granulata (Mollusca, Polyplacophora) use pheomelanin as a screening pigment. Journal of Natural History, 48:2899–2911.
https://doi.org/10.1080/00222933.2014.959572
Swainson, W. and Berry, S.S. 1840. Treatise on Malacology; or, the natural classification of shells and shell fish. By William Swainson. Longman, Orme, Brown, Green and Longmans, London.
https://doi.org/10.5962/bhl.title.8027
Swann, C. and Kelley, P. 1985. Residual color patterns of molluscs from the Gosport Sand (Eocene), Alabama. Mississippi Geology, 5:1–8.
Starobogatov, Y.I. 1974. Xenoconchias and their bearing on the phylogeny and systematics of some molluscan classes. Paleontologicheskii Zhurnal 1:3–18.
Stemmer, K. and Nehrke, G. 2014. The distribution of polyenes in the shell of Arctica islandica from North Atlantic localities: a confocal Raman microscopy study. Journal of Molluscan Studies, 80:365–370.
https://doi.org/10.1093/mollus/eyu033
Tate, R. 1878. The Fossil Marginellidae of Australasia. Transactions and Proceedings and Report of the Philosophical Society of Adelaide, South Australia, 1:90–98.
Tate, R. 1885. Description of new species of Mollusca of the upper Eocene beds at Table Cape. Papers and Proceedings of the Royal Society of Tasmania for 1884, 226–231.
Tate, R. 1886. The Lamellibranchs of the older Tertiary of Australia. (Part I.). Transactions and Proceedings and Report of the Royal Society of South Australia, 8:96–158.
Tate, R. 1888. The Gastropods of the Older Tertiary of Australia. (Part I.). Transactions and Proceedings and Report of the Royal Society of South Australia, 10:91–176.
Tate, R. 1889. The Gastropods of the Older Tertiary of Australia. (Part II.). Transactions and Proceedings and Report of the Royal Society of South Australia, 11:116–174.
Tate, R. 1890. The Gastropods of the Older Tertiary of Australia (Part III.). Transactions and Proceedings and Report of the Royal Society of South Australia, 13:185–235.
Tate, R. 1893a. The gastropods of the Older Tertiary of Australia. Part IV. (including Supplement to Part III.) Transactions and Proceedings and Report of the Royal Society of South Australia, 17:316–345.
Tate, R. 1893b. Unrecorded genera of the Older Tertiary fauna of Australia, including diagnoses of some new genera and species. Journal and Proceedings of the Royal Society of New South Wales, 27:167–197.
https://doi.org/10.5962/p.359147
Tsushima, M., Katsuyama, M., and Matsuno, T. 1997. Metabolism of carotenoids in the Apple Snail, Pomacea canaliculata. Comparative Biochemistry and Physiology Part B, 118:431–436.
https://doi.org/10.1016/S0305-0491(97)00215-0
Vokes, H.E. and Vokes, E.H. 1968. Variation in the genus Orthaulax (Mollusca: Gastropoda). Tulane Study in Geology and Paleontology, 6:71–79.
Wade, J., Pugh, H., Nightingale, J., Kim, J.-S., and Williams, S.T. 2019. Colour in bivalve shells: Using resonance Raman spectroscopy to compare pigments at different phylogenetic levels. Journal of Raman Spectroscopy, 50:1527–1536.
https://doi.org/10.1002/jrs.5639
Wagner, E. and Born, A. 1928. Zur Priorität der UV-Untersuchung von Fossilien. Palaeontologische Zeitschrift, 10:215–216.
https://doi.org/10.1007/BF03041573
Wierzbowski, H. 2007. Effects of pre-treatments and organic matter on oxygen and carbon isotope analyses of skeletal and inorganic calcium carbonate. International Journal of Mass Spectrometry, 268:16–29.
https://doi.org/10.1016/j.ijms.2007.08.002
Williams, S.T. 2017. Molluscan shell colour. Biological Reviews, 92:1039–1058.
https://doi.org/10.1111/brv.12268
Williams, S.T., Ito, S., Wakamatsu, K., Goral, T., Edwards, N.P., Wogelius, R.A., Henkel, T., de Oliveira, L.F.C., Maia, L.F., Strekopytov, S., Jeffries, T., Speiser, D.I., and Marsden, J.T. 2016. Identification of Shell Colour Pigments in Marine Snails Clanculus pharaonius and C. margaritarius (Trochoidea; Gastropoda). PLOS One, 11:e0156664. https://doi.org/10.1371/journal.pone.0156664
Wolkenstein, K. 2022. Fluorescent colour patterns in the basal pectinid Pleuronectites from the Middle Triassic of Central Europe: origin, fate and taxonomic implications of fluorescence. Palaeontology, 65:e12625.
https://doi.org/10.1111/pala.12625
Xiao, L., Fucun, W., Hongen, Z., Guofan, Z., and Ximing, G. 2009. A Novel Shell Color Variant of the Pacific Abalone Haliotis Discus Hannai Ino Subject to Genetic Control and Dietary Influence. Journal of Shellfish Research, 28:419–424.
https://doi.org/10.2983/035.028.0226
Zheng, H., Liu, H., Zhang, T., Wang, S., Sun, Z., Liu, W., and Li, Y. 2010. Total carotenoid differences in scallop tissues of Chlamys nobilis (Bivalve: Pectinidae) with regard to gender and shell colour. Food Chemistry, 122:1164–1167.
https://doi.org/10.1016/j.foodchem.2010.03.109

