 Oldest record of Alligator in southeastern North America
Oldest record of Alligator in southeastern North America
Article number: 26.1.a6
https://doi.org/10.26879/1223
Copyright Paleontological Society, March 2023
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 14 April 2022. Acceptance: 4 January 2023.
ABSTRACT
The genus Alligator has been represented by large-bodied, predatory species in southeastern North America for at least 18 million years (early Miocene), in what is now the southeastern United States. However, the first occurrences of the genus were from a smaller-bodied species, A. prenasalis, known from South Dakota and Nebraska that are about 34 million years old (latest Eocene to earliest Oligocene). Ancestors of A. prenasalis were likewise small-bodied and are from the Great Plains. This 16 million-year-gap has left open questions regarding the arrival and body size shift of Alligator from what is now the Great Plains to southeastern North America. Recently studied fossil material from Florida exhibits the oldest occurrence of Alligator in the region (about 28-26 million years ago). A well-preserved premaxilla (UF 422816) bears the diagnostic premaxillary 'notch' of Alligator. Additional material from this and two other Oligocene sites in Florida are indicative of Alligator as well. These include well-developed osteoderms, which suggest possible maturity at small body size. As of now, no records of larger Alligator from this time (or older) have been recovered from the region, possibly indicating body size may not have increased in Alligator until the Miocene.
Alexander K. Hastings. Science Museum of Minnesota, Saint Paul, Minnesota 55102, USA. ahastings@smm.org
Blaine W. Schubert. Center of Excellence in Paleontology and Department of Geosciences, East Tennessee State University, Johnson City, Tennessee 37614, USA. schubert@etsu.edu
Jason R. Bourque, Division of Vertebrate Paleontology, Florida Museum of Natural History, Gainesville, Florida 32611, USA. jbourque@flmnh.ufl.edu
Richard C. Hulbert, Jr. Division of Vertebrate Paleontology, Florida Museum of Natural History, Gainesville, Florida 32611, USA. rhulbert@flmnh.ufl.edu
Keywords: Oligocene; Miocene; Crocodylia; Alligatoridae; Florida; body size
Final citation: Hastings, Alexander K., Schubert, Blaine W., Bourque, Jason R., and Hulbert, Richard C., Jr. 2023. Oldest record of Alligator in southeastern North America. Palaeontologia Electronica, 26(1):a6.
https://doi.org/10.26879/1223
palaeo-electronica.org/content/2023/3765-oldest-alligator-in-se-usa
Copyright: March 2023 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
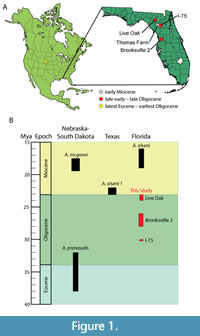 The earliest records of Alligator Cuvier, 1807, are small-bodied (1.1-2.6 m estimated total body length) and come from the latest Eocene of South Dakota (Higgins, 1971) and Nebraska (Figure 1; Whiting and Hastings, 2015). These belong to the basal species for the genus, Alligator prenasalis (Loomis, 1904), which are also found in earliest Oligocene strata from South Dakota (Higgins, 1971, with dates revised by Walker and Geissman, 2009). Alligatorine phylogeny is often poorly resolved at the node basal to Alligator, but typically the small-bodied Wannaganosuchus brachymanus Erickson, 1982 (Paleocene; North Dakota) is recovered as the next most basal form (e.g., Brochu, 2004; Piras and Buscalioni, 2006; Snyder, 2007; Brochu, 2011; Hastings et al., 2016). Moreover, other species commonly recovered as very closely related to Alligator are also small-bodied and from high latitude regions of western North America, including: Allognathosuchus wartheni Case, 1925 (early Eocene, Wyoming), Allognathosuchus polyodon (Cope, 1873) (early Eocene, Wyoming; Mook, 1961), Procaimanoidea kayi Mook, 1941 (early Eocene, Wyoming), and Procaimanoidea utahensis Gilmore, 1946 (middle Eocene, Utah).
The earliest records of Alligator Cuvier, 1807, are small-bodied (1.1-2.6 m estimated total body length) and come from the latest Eocene of South Dakota (Higgins, 1971) and Nebraska (Figure 1; Whiting and Hastings, 2015). These belong to the basal species for the genus, Alligator prenasalis (Loomis, 1904), which are also found in earliest Oligocene strata from South Dakota (Higgins, 1971, with dates revised by Walker and Geissman, 2009). Alligatorine phylogeny is often poorly resolved at the node basal to Alligator, but typically the small-bodied Wannaganosuchus brachymanus Erickson, 1982 (Paleocene; North Dakota) is recovered as the next most basal form (e.g., Brochu, 2004; Piras and Buscalioni, 2006; Snyder, 2007; Brochu, 2011; Hastings et al., 2016). Moreover, other species commonly recovered as very closely related to Alligator are also small-bodied and from high latitude regions of western North America, including: Allognathosuchus wartheni Case, 1925 (early Eocene, Wyoming), Allognathosuchus polyodon (Cope, 1873) (early Eocene, Wyoming; Mook, 1961), Procaimanoidea kayi Mook, 1941 (early Eocene, Wyoming), and Procaimanoidea utahensis Gilmore, 1946 (middle Eocene, Utah).
Fossil Alligator in Nebraska are not recovered again until the early Miocene, with Alligator mcgrewi Schmidt, 1941, in what was then referred to as the Marsland Formation, but is now split into the Runningwater Formation and Anderson Branch Formation (Tedford et al., 2004). Alligator mcgrewi possesses more derived features than A. prenasalis, including a dorsally facing external naris, also present in extant Alligator (Brochu, 1999; Brochu, 2011). Definitively identifiable Alligator fossils from southeastern North America first appeared in Florida from the early Miocene, belonging to the notably larger species Alligator olseni White, 1942 (Snyder, 2007), best known from the Thomas Farm site. Further reports of A. olseni have been assigned to material from the early Miocene of the Texas Gulf Coastal Plain (Albright, 1994). This has left a sizable gap between early Oligocene and early Miocene Alligator records (at least 9 million years; Figure 1).
We describe here the first published record of identifiable Alligator material from the late Oligocene Brooksville 2 site in central Florida (Figure 1). Brooksville 2 has a diverse fauna, including the burrowing toad Rhinophrynus Duméril and Bibron, 1841 (Blackburn et al., 2019), kinosternid turtle Xenochelys Hay, 1906 (Bourque, 2013), lizard Anolis Daudin, 1802 (Chovanec, 2014), mormoopid bat Koopmanycteris Morgan, Czaplewski, and Simmons, 2019 (Morgan et al., 2019), and the early horse Miohippus Marsh, 1874 (Hayes, 2000). We report additional fossil material from two other Oligocene sites in Florida, the I-75 site (Patton, 1969; Morgan et al., 2019) and Live Oak site (Frailey, 1978), that also appear to come from a small species of Alligator. While the Alligator fossils described here are not diagnostic beyond genus, they significantly extend its temporal range in the region by 10-8 million years (see Locality and Horizon).
Institutional Abbreviation
UF, University of Florida, Florida Museum of Natural History, Division of Vertebrate Paleontology, Gainesville, Florida, USA.
SYSTEMATIC PALEONTOLOGY
CROCODYLIA Gmelin, 1789, sensu Benton and Clark, 1988
ALLIGATORIDAE Gray, 1844, sensu Norell et al., 1994
ALLIGATORINAE Kälin, 1939
Alligator Cuvier, 1807
Crocodilia Hayes, 2000, table 1
dwarf alligatorid Bourque, 2013, p. 461
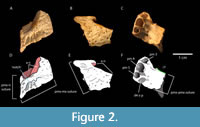 Referred specimens. Right premaxilla (UF 422816; Figure 2); complete parietal (UF 333984; Figure 3), fragment of a dorsal cranial bone (position uncertain; UF 425424; Figure 3), right articular (UF 422817; Figure 3), fragment of dentary (UF 425402; Figure 3), 12 isolated teeth (for catalog numbers, see Appendix 1; select teeth in Figure 3), neural arch of a dorsal vertebra (UF 424653; Figure 3), caudal vertebra (UF 425435; Figure 3), two unguals (UF 424655, UF 425405; Figure 3), and 83 partial to complete osteoderms (for catalog numbers, see Appendix 1; select osteoderms in Figure 3).
Referred specimens. Right premaxilla (UF 422816; Figure 2); complete parietal (UF 333984; Figure 3), fragment of a dorsal cranial bone (position uncertain; UF 425424; Figure 3), right articular (UF 422817; Figure 3), fragment of dentary (UF 425402; Figure 3), 12 isolated teeth (for catalog numbers, see Appendix 1; select teeth in Figure 3), neural arch of a dorsal vertebra (UF 424653; Figure 3), caudal vertebra (UF 425435; Figure 3), two unguals (UF 424655, UF 425405; Figure 3), and 83 partial to complete osteoderms (for catalog numbers, see Appendix 1; select osteoderms in Figure 3).
 Locality and horizon. Brooksville 2 is a series of at least five fissure-fill deposits of laminated clay and sand, formed within the older Suwannee Limestone, 35.5-33.3 Ma (Hayes, 2000). The site is near the town of Brooksville, in Hernando County, Florida (Figure 1). The age of the deposit was considered to be 26-25 Ma by Tedford et al. (2004) based on mammalian biochronology. Later analysis by Czaplewski and Morgan (2012) and Morgan et al. (2019) suggested a slightly older age range of 28-26 Ma for Brooksville 2. The premaxilla is from Quarry 1E, although some specimens are from Quarries 1A, 1B, 1D, and some of uncertain provenience within the site (further details in Appendix 1). Much of the material at this quarry is thought to have arrived via subaqueous transport through karst solution pipes (Hayes, 2000). There were no fissures or sinkholes with younger fossils in this part of the quarry, and the unusual orange color of the Alligator tooth enamel (Figure 3E-J) is also observed on Oligocene mammals from the same fissure-fills (e.g., Czaplewski and Morgan, 2012).
Locality and horizon. Brooksville 2 is a series of at least five fissure-fill deposits of laminated clay and sand, formed within the older Suwannee Limestone, 35.5-33.3 Ma (Hayes, 2000). The site is near the town of Brooksville, in Hernando County, Florida (Figure 1). The age of the deposit was considered to be 26-25 Ma by Tedford et al. (2004) based on mammalian biochronology. Later analysis by Czaplewski and Morgan (2012) and Morgan et al. (2019) suggested a slightly older age range of 28-26 Ma for Brooksville 2. The premaxilla is from Quarry 1E, although some specimens are from Quarries 1A, 1B, 1D, and some of uncertain provenience within the site (further details in Appendix 1). Much of the material at this quarry is thought to have arrived via subaqueous transport through karst solution pipes (Hayes, 2000). There were no fissures or sinkholes with younger fossils in this part of the quarry, and the unusual orange color of the Alligator tooth enamel (Figure 3E-J) is also observed on Oligocene mammals from the same fissure-fills (e.g., Czaplewski and Morgan, 2012).
Description. The premaxilla recovered from Brooksville 2 (UF 422816) bears the characteristic premaxillary 'notch' alongside the naris that defines the genus Alligator (Figure 2; Brochu, 1999; Brochu, 2011). Moreover, it has a forward-facing external naris, consistent with A. prenasalis (Brochu, 1999). This is counter to the more dorsal-facing naris of A. olseni and the extant Alligator mississippiensis (Daudin, 1802) (Brochu, 1999). Because the anterior end of the premaxilla is missing, and no part of the nasal is preserved, it is unclear whether or not the naris would have been fully bisected, as in other Alligator (Brochu, 1999). However, as the premaxilla curves back anteriorly along the nasal suture, we can say the nasal almost certainly penetrated well into the naris. UF 422816 bears three preserved alveoli, although more were likely present in the anterior broken portion. Based on the presence of the suture with the maxillary, these would be the third through fifth positions. Of these three preserved alveoli, the fourth is the largest, followed by the third, then the fifth (see measurements in Table 1). Sutural surfaces are preserved with the right maxilla (posteriorly), left premaxilla (ventrally), and right nasal (medially). Part of an occlusal pit is preserved at the sutural contact with the right maxilla, for reception of the fourth dentary tooth. This character is consistent across all Alligator species (Brochu, 1999; Brochu, 2011), and is distinct from Crocodylidae as well as Oligocene Thecachampsa antiquus Leidy, 1852 (synonymized with Gavialosuchus; Myrick, 2001; Brochu, 2011). The dorsal premaxillary process extends posteriorly only slightly between the nasal and maxillary bones, indicating a rather short snout. This process is also short in the fairly brevirostrine Alligator species, A. mcgrewi (Schmidt, 1941), and makes a similar angle from the acute posterior end between the nasal and maxillary sutures. This angle of the dorsal premaxillary process is wider in other Alligator species, including extant A. mississippiensis. The dorsal ornamentation is highly developed and rugose for such a small bone. The degree of pitting is typical of extant Alligator that are well beyond sexual maturity, yet the size is similar to an immature individual.
The parietal recovered from Brooksville 2 (UF 333984) is isolated, but complete, including sutures with the frontal, postorbital, squamosal, and supraoccipital (Figure 3). The frontoparietal suture is convex and remains on the skull table. The dermal part of the parietal overhangs the supratemporal fenestra weakly. There is a shallow fossa at the anteromedial corner of the supratemporal fenestra, and the medial parietal wall is imperforate. The parietal and squamosal meet along the posterior wall of the supratemporal fenestra. The skull table is planar at maturity and does not slope ventrally. There is no supraoccipital exposure on the dorsal surface of the parietal (Figure 2), which is consistent with A. mcgrewi, A. olseni and A. mississippiensis but not with A. prenasalis (Brochu, 1999). The supratemporal fenestra is longer than wide, with a thick posterior bar. There is a recess that connects with the pneumatic system (sensu Brochu, 2011).
The 12 preserved teeth from Brooksville 2 exhibit morphology that is consistent with Alligator (Figure 3). These include the globidontan morphology of posterior dentition (Figure 3E), as well as spade-shaped morphology from the middle of the jaw (Figure 3F-G). Several specimens include full roots, indicating they were likely not shed teeth. These teeth are also consistent in size with the premaxilla (UF 422816) and are inconsistent with that of contemporary Thecachampsa, which are longer and recurved in the middle of the jaw and more triangular in the rear (Myrick 2001).
A caudal vertebra was recovered at Brooksville 2 (Figure 3U-V). Although only partial, it appears to represent roughly the mid-section of the tail. This vertebra is of small size (14.78 mm centrum length) and exhibits a fully closed neurocentral suture.
A total of 83 partial to complete osteoderms were recovered from Brooksville 2 (Figure 3). These have the characteristic patterns of a square or anteroposteriorly rectangular shape, median keel, anterior imbricating shelf, and dorsal pitting found in Alligator. The more complete osteoderms can be recognized as belonging to the dorsal shield, rather than the nuchal shield, due to the development of the highly rectangular shape. Several osteoderms are complete enough to exhibit fully-developed edges and lateral sutures (Figure 3), which are consistent with individuals well beyond yearling age (see Discussion). The presence of a keel is inconsistent with Thecachampsa, which also has mediolaterally rectangular osteoderms, rather than anteroposteriorly rectangular ones (Myrick, 2001).
CROCODYLIA Gmelin, 1789, sensu Benton and Clark, 1988
ALLIGATORIDAE Gray, 1844, sensu Norell et al., 1994
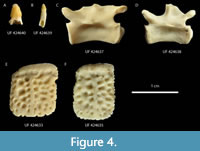 Referred specimens. Material from Live Oak (Figure 4) includes: three isolated teeth (UF 424639-41), two caudal vertebrae (UF 424637-8), and four osteoderms (UF 424633-36). A partial osteoderm (UF 16729; Figure 5) from the I-75 site is referred as well.
Referred specimens. Material from Live Oak (Figure 4) includes: three isolated teeth (UF 424639-41), two caudal vertebrae (UF 424637-8), and four osteoderms (UF 424633-36). A partial osteoderm (UF 16729; Figure 5) from the I-75 site is referred as well.
Localities and horizons. The Live Oak fossil site (a.k.a. SB-1A; Figure 1) represents an unstratified sequence of conglomerate deposited in a single fissure in the Suwanee Limestone (Frailey, 1978). Mammalian biochronology suggests this site is slightly younger than Brooksville 2, at about 25-24 Ma (Figure 1; Tedford et al., 2004). Fossils from the I-75 site (Figure 1) were recovered from a small karst solution fill, exposed during road construction. The geologic age is around 30 Ma, either late Whitneyan or early Arikareean (Patton, 1969; Hayes, 2000; Morgan et al., 2019). Other than the fossils of shark teeth and other marine fishes from the limestone bedrock, all the age-diagnostic fossils from these two localities provide a consistent date with no evidence of contamination of younger material.
Description. The additional three teeth preserved from Live Oak likewise display morphology entirely consistent with Alligator (Figure 4). More specifically, these teeth display the typical spade-shaped morphology from the middle of the jaw. One specimen also includes a full root, suggesting it was likely not a shed tooth (UF 424639; Figure 4B). Again, these teeth are consistent in size with the premaxilla from Brooksville 2 (UF 422816) and are inconsistent with that of contemporary Thecachampsa (Myrick, 2001).
 Two caudal vertebrae were recovered at Live Oak (Figure 4). Similar to Brooksville 2, these seem to represent the mid-section of the tail. These vertebrae are of even smaller size (10.34-12.00 mm centrum length) and also exhibit fully closed neurocentral sutures.
Two caudal vertebrae were recovered at Live Oak (Figure 4). Similar to Brooksville 2, these seem to represent the mid-section of the tail. These vertebrae are of even smaller size (10.34-12.00 mm centrum length) and also exhibit fully closed neurocentral sutures.
Four osteoderms have been recovered at Live Oak (Figure 4) and a partial osteoderm has been recovered from the I-75 fossil site (Figure 5). Much like the remains at Brooksville 2, these are all of small size, are anteroposteriorly rectangular, and possess a median keel, an anterior imbricating shelf, and dorsal pitting like that found in Alligator. The more complete osteoderms can be recognized as belonging to the dorsal shield. Again, these appear to represent individuals that were at least beyond yearling age (see Discussion). Not only are they much smaller, but they are inconsistent with the contemporary Thecachampsa (Myrick, 2001).
DISCUSSION
The premaxilla, UF 422816, exhibits the diagnostic character of a premaxillary ‘notch’ adjacent to the external naris, placing it within the genus Alligator. The morphology of the Alligator premaxilla indicates the ancestral condition of a forward-facing external naris, much like the older A. prenasalis of Nebraska and South Dakota (Whiting and Hastings, 2015). Likewise, the small size of the premaxilla, paired with its notable dorsal ornamentation indicates small body size despite relative maturity. Alligator prenasalis has been estimated at 1.30-1.92 m in body length at adult size (Whiting and Hastings, 2015). In addition to similar morphology, UF 422816 has comparable size to the premaxillae of more completely preserved A. prenasalis. Conversely, this element is much smaller than the premaxillae of adult A. olseni, which typically has a larger body size of 1.71-2.41 m (calculated from data in Snyder, 2007, equations in Farlow et al., 2005). However, the lack of supraoccipital exposure of the parietal (UF 333984) is not characteristic of A. prenasalis, but would be consistent with A. olseni (Brochu, 1999). Thus, the Alligator from Brooksville 2 may indicate a transitional form between these two species; however, having material that can reliably connect both features as belonging to a single specimen would be needed.
The features of the material included here likely indicate a new species of Alligator, but due to the lack of material from other parts of the skull typically used to diagnose Alligator species, we refrain from assigning a new taxon here. We included the postcranial remains from Brooksville 2 as referred specimens due to the total lack of other crocodylian material from the site and their close proximity in size to the individual that would have yielded the premaxilla. Due to the nature of fossil preservation, it is not possible to confidently assign any two bones as having belonged to the same individual. Although all seem to indicate small body size, they do seem to have some size variation, indicating that this was almost certainly more than one individual.
Given the presence of early caimanines in North America during the Paleogene, it is worth some comparison to this group. For example, Tsoabichi is known from multiple individuals in southwest Wyoming from the early Eocene, ranging from 51.98 to 49.25 million years old (Walter et al., 2021). This taxon is similar to the Brooksville 2 Alligator in having an anterodorsally facing naris, but is missing the diagnostic thin crest encircling it. In addition, Tsoabichi has a broad exposure of the supraoccipital bone on the skull table (Walter et al., 2021), which the Brooksville 2 specimen does not. Similarly, the parietal of Protocaiman (Paleocene of Argentina) also possesses dorsal exposure of the supraoccipital bone on the skull table (Bona et al., 2018). Unfortunately, the premaxilla is unknown for this taxon. A newly described taxon from the Eocene of Texas, Chinatichampsus wilsonorum includes a skull with a partial premaxilla (Stocker et al., 2021). This bone preserves an occlusal pit lying medial to the tooth row and nearly entirely anterior to the premaxillary-maxillary suture. This is unlike the occlusal pit of UF 422816, which is much more in-line with the tooth row and split across the suture. Premaxillary tooth positions were not assigned for Chinatichampsus, but images indicate the relative proportions of the alveoli anterior to the premaxillary-maxillary suture are in quite different proportions from UF 422816 (Stocker et al., 2021). Moreover, Chinatichampsus also has dorsal exposure of the supraoccipital bone (Stocker et al., 2021).
Another alligatoroid that could potentially have occurred in Florida at one time, Bottosaurus harlani, a Cretaceous-Paleocene taxon known from New Jersey, lacks the premaxillary notch and has four alveoli rather than five; the incisive foramen is also much larger (Cossette and Brochu, 2018). In addition, Bottosaurus has broad exposure of the supraoccipital on the skull table (Cossett and Brochu, 2018). There is very little overlapping material of Bottosaurus fustidens from the Paleocene of Texas, only a small portion of one premaxilla and the anterior portion of the parietal (Cossette, 2021). These are not known well enough for a meaningful comparison with the Brooksville 2 fossil Alligator.
There are fossil crocodyliforms from the Oligocene and Miocene of the Caribbean that do not (as of yet) have a record from the mainland, but hypothetically could have appeared in Florida. Aktiogavialis is a gryposuchine gavialoid known from a braincase in the Oligocene of Puerto Rico (Velez-Juarbe et al., 2007). The parietal from Brooksville 2 differs significantly from Aktiogavialis, in that the suproccipital has no dorsal exposure, and the mid-point of the posterior edge does not form a point with indentations directed anteriorly on either side (Figure 3; Velez-Juarbe et al., 2007). Moreover, other gryposuchines have premaxillae with a very different shape, including: (1) four alveoli rather than five, (2) very elongate dorsal and ventral posterior processes, (3) no ‘notch’ present on the dorsal surface, and (4) do not have the deep pit for occlusion with the lower jaw seen at the premaxillary-maxillary suture, and instead have a tight constriction allowing for the lower dentition to sit outside of the premaxilla entirely (e.g., De Souza et al., 2018). Additional unidentified crocodyliform remains from the Oligocene and Miocene of Puerto Rico, as well as the Miocene of Cuba are rather fragmentary, and there is very little overlap from which to compare the material (Brochu and Jimenez-Vazquez, 2014). The two osteoderms from the late Oligocene of Puerto Rico bear more resemblance to the rectangular and low, small keels of gavialoids than the ones presented here (Brochu et al., 2007). Of the two crocodyliform osteoderms described from the Miocene of Cuba, one is square and has a modest keel that somewhat resembles the Florida material described here. The other is very round with a prominent keel, which does not match the ones described here. Both are significantly larger as well.
 Given the limited material available, a new method was developed to estimate the body size of UF 422816. A study set of 22 extant Alligator skulls were measured for six dimensions that could be compared to UF 422816: length of the premaxillary-maxillary suture, length of the premaxillary-premaxillary suture, length and width of the fourth premaxillary alveolus, and length and width of the fifth premaxillary alveolus. These were then compared to dorsal skull length. Of these six, by far the greatest correlation between premaxillary measurement (Table 2) and dorsal skull length (Table 3) was the length of the premaxillary-maxillary suture (R2 = 0.9917; Table 4). Using a best-fit linear regression, determined from this study set (Figure 6), the dorsal skull length of UF 422816 is estimated as 15.9 cm. Established correlations have been found between dorsal skull length and total body length in Alligator (Woodward et al., 1995; Young et al., 2011). Applying a known regression for extant Alligator yields an estimated total body length for UF 422816 as 1.18 m.
Given the limited material available, a new method was developed to estimate the body size of UF 422816. A study set of 22 extant Alligator skulls were measured for six dimensions that could be compared to UF 422816: length of the premaxillary-maxillary suture, length of the premaxillary-premaxillary suture, length and width of the fourth premaxillary alveolus, and length and width of the fifth premaxillary alveolus. These were then compared to dorsal skull length. Of these six, by far the greatest correlation between premaxillary measurement (Table 2) and dorsal skull length (Table 3) was the length of the premaxillary-maxillary suture (R2 = 0.9917; Table 4). Using a best-fit linear regression, determined from this study set (Figure 6), the dorsal skull length of UF 422816 is estimated as 15.9 cm. Established correlations have been found between dorsal skull length and total body length in Alligator (Woodward et al., 1995; Young et al., 2011). Applying a known regression for extant Alligator yields an estimated total body length for UF 422816 as 1.18 m.
The osteoderms, which come from the dorsal shield over the trunk, are at least somewhat informative in terms of relative maturity. A study of development in Alligator has shown that osteoderm calcification occurs relatively late in ontogeny: “In A. mississippiensis the earliest sign of calcification occurs approximately 1 year after hatching, in the area of the presumptive nuchal shield” (Vickaryous and Hall, 2008). Even at this stage, osteoderms begin development along the center, where the median keel eventually forms, then grow outward to the lateral edges (Vickaryous and Hall, 2008). Development of additional features such as lateral sutures and imbricating shelves occurs even later in ontogeny, further indicating growth well past the yearling stage for the Oligocene individuals. However, without morphological studies that track osteoderm features with maturity, there is not currently a way to be more definitive about how old these individuals were outside osteohistological analyses, which are beyond the scope of this paper.
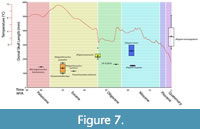 Shortly after Alligator appears in the fossil record of the Great Plains, the region experienced a massive drop in mean annual temperature (8.2 ± 3.1 °C), at the beginning of the Oligocene (Zanazzi et al., 2007). Following this, temperature generally warmed through the Oligocene, particularly at the time of the Brooksville 2 fauna (Zachos et al., 2001), which has been further supported by pollen analysis on the eastern coast of North America (Kotthoff et al., 2015; Figure 7). Body size in ectothermic vertebrates often relates to mean annual temperature (Makarieva et al., 2005 a,b; Head et al., 2009). Paleotemperature data are currently lacking for the Oligocene of Florida, and thus temperature differences between the Great Plains and Florida at this time remain unknown. Given our current knowledge, Alligator may have originated in the Great Plains during the middle Paleocene-late Eocene and dispersed to the southeastern region of North America sometime prior to the late Oligocene. Large temperature drops in the Great Plains could have been a factor in this hypothetical dispersal. However, despite potentially being a warmer climate in Florida, small body size may have been retained, as no large-bodied individuals have yet been recovered at any Oligocene Florida site. This may indicate a lag between the relationships of body size and latitudinal migration. Figure 7 shows dorsal skull length (as a proxy for body size) across the ancestral Alligator taxa and Oligocene-Miocene species against global temperatures. The occurrence of UF 422816 is actually smaller than the known Alligator prenasalis adults recorded from higher latitudes, but again, sub-adult status cannot be fully ruled out. The position of UF 422816 in time; however, is prior to a significant increase in temperature that precedes the occurrence of the larger Alligator olseni, which may have been able to grow larger in these generally warmer temperatures. Meanwhile, Alligator mcgrewi retained small size in the presumably cooler latitude of Nebraska at the time. The morphological similarity and potential body size similarity between the Oligocene Alligator of Florida and those of the Great Plains indicates a possible faunal affinity between the two regions. Florida has been suggested as a post-Eocene refugium for the turtle Xenochelys, which has its latest fossil occurrence at Brooksville 2 and was previously known from the Eocene of the midwestern United States (Bourque, 2012).
Shortly after Alligator appears in the fossil record of the Great Plains, the region experienced a massive drop in mean annual temperature (8.2 ± 3.1 °C), at the beginning of the Oligocene (Zanazzi et al., 2007). Following this, temperature generally warmed through the Oligocene, particularly at the time of the Brooksville 2 fauna (Zachos et al., 2001), which has been further supported by pollen analysis on the eastern coast of North America (Kotthoff et al., 2015; Figure 7). Body size in ectothermic vertebrates often relates to mean annual temperature (Makarieva et al., 2005 a,b; Head et al., 2009). Paleotemperature data are currently lacking for the Oligocene of Florida, and thus temperature differences between the Great Plains and Florida at this time remain unknown. Given our current knowledge, Alligator may have originated in the Great Plains during the middle Paleocene-late Eocene and dispersed to the southeastern region of North America sometime prior to the late Oligocene. Large temperature drops in the Great Plains could have been a factor in this hypothetical dispersal. However, despite potentially being a warmer climate in Florida, small body size may have been retained, as no large-bodied individuals have yet been recovered at any Oligocene Florida site. This may indicate a lag between the relationships of body size and latitudinal migration. Figure 7 shows dorsal skull length (as a proxy for body size) across the ancestral Alligator taxa and Oligocene-Miocene species against global temperatures. The occurrence of UF 422816 is actually smaller than the known Alligator prenasalis adults recorded from higher latitudes, but again, sub-adult status cannot be fully ruled out. The position of UF 422816 in time; however, is prior to a significant increase in temperature that precedes the occurrence of the larger Alligator olseni, which may have been able to grow larger in these generally warmer temperatures. Meanwhile, Alligator mcgrewi retained small size in the presumably cooler latitude of Nebraska at the time. The morphological similarity and potential body size similarity between the Oligocene Alligator of Florida and those of the Great Plains indicates a possible faunal affinity between the two regions. Florida has been suggested as a post-Eocene refugium for the turtle Xenochelys, which has its latest fossil occurrence at Brooksville 2 and was previously known from the Eocene of the midwestern United States (Bourque, 2012).
Alternatively, the records of Alligator in the Great Plains prior to the new material, combined with the sparse record of Eocene-Oligocene terrestrial deposits in eastern North America, may give an illusion of migration towards the southeast. Thus, it is possible that Alligator had a distribution that expanded to coastal areas when they occurred in the Great Plains, and over time climate change resulted in the extirpation of more northern populations. Further fossil material from the southeastern United States may clarify the origin and paleogeography of Alligator.
Regardless, the presence of Alligator in Florida during the Oligocene increases the antiquity of the genus in southeastern North America by an additional 10-8 million years, so it now has a ca. 26 million-year history in the region. During much of this time, Alligator has been the largest predator in its aquatic environment, but this fossil occurrence suggests the possibility that this niche may not have been the ancestral condition for southeastern populations.
ACKNOWLEDGMENTS
Thanks to the East Tennessee State University Center of Excellence in Paleontology for travel funding that benefited this study. Permission for UF to collect the Brooksville 2 fossils was granted by Florida Rock Industries. Thanks to M.C. Vallejo-Pareja and L.W. Vinola-Lopez for assistance with relocating UF 333984.
REFERENCES
Albright, L.B. 1994. Lower vertebrates from an Arikareean (Earliest Miocene) fauna near the Toledo Bend Dam, Newton County, Texas. Journal of Paleontology, 68:1131–1145.
https://doi.org/10.1017/S002233600002672X
Benton, M.J. and Clark, J.M. 1988. Archosaur phylogeny and the relationships of the Crocodylia, p. 295–338. In Benton, M.J. (ed.), The Phylogeny and Classification of the Tetrapods. Clarendon Press, Oxford.
Blackburn, D.C., Roberts, L., Vallejo-Pareja, M.C., and Stanley, E.L. 2019. First record of the anuran Family Rhinophrynidae from the Oligocene of Eastern North America. Journal of Herpetology, 53:316–323. https://doi.org/10.1670/19-044
Bourque, J.R. 2013. Fossil Kinosternidae from the Oligocene and Miocene of Florida, USA, p. 459–475. In Brinkman, D.B., Holroyd, P.A., Gardner, J.D. (eds.), Morphology and Evolution of Turtles, Vertebrate Paleobiology and Paleoanthropology. Springer, Dordrecht.
https://doi.org/10.1007/978-94-007-4309-0_25
Brochu, C.A. 1999. Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. Journal of Vertebrate Paleontology, 19:sup002:9–100.
https://doi.org/10.1080/02724634.1999.10011201
Brochu, C.A. 2004. Alligatorine phylogeny and the status of Allognathosuchus Mook, 1921. Journal of Vertebrate Paleontology, 24:857–873.
https://doi.org/10.1671/0272-4634(2004)024[0857:APATSO]2.0.CO;2
Brochu, C.A., Nieves-Rivera, A.M., Vélez-Juarbe, J., Daza-Vaca, J.D., and Santos, H. 2007. Tertiary crocodylians from Puerto Rico: evidence for Late Tertiary endemic crocodylians in the West Indies? Geobios, 40:51–59. https://doi.org/10.1016/j.geobios.2005.10.008
Brochu, C.A. 2011. Phylogenetic relationships of Necrosuchus ionensis Simpson, 1937 and the early history of caimanines. Zoolological Journal of the Linnean Society, 163:S228–S256. https://doi.org/10.1111/j.1096-3642.2011.00716.x
Brochu, C.A. and Jiménez-Vázquez, O. 2014. Enigmatic crocodyliforms from the early Miocene of Cuba. Journal of Vertebrate Paleontology, 34:1094–1101.
https://doi.org/10.1080/02724634.2014.855225
Case, E.C. 1925. Note on a new species of the Eocene crocodilian Allognathosuchus, A. wartheni. Contributions from the Museum of Geology University of Michigan, 2:93–97.
Chovanec, K. 2014. Non-anguimorph Lizards of the Late Oligocene and Early Miocene of Florida and Implications for the Reorganization of the North American Herpetofauna. Master’s Thesis, East Tennessee State University, Johnson City, Tennessee, USA.
Cope, E.D. 1873. On the extinct vertebrata of the Eocene of Wyoming, observed by the expedition of 1872, with notes on the geology. Annual Report of the U. S. Geological and Geographic Survey of the Territories, 6:545–649.
Cossette, A.P. and Brochu, C.A. 2018. A new specimen of the alligatoroid Bottosaurus harlani and the early history of character evolution in alligatorids. Journal of Vertebrate Paleontology, 38:1–22. https://doi.org/10.1080/02724634.2018.1486321
Cossette, A.P. 2021. A new species of Bottosaurus (Alligatoroidea: Caimaninae) from the Black Peaks Formation (Palaeocene) of Texas indicates an early radiation of North American caimanines. Zoological Journal of the Linnean Society, 191:276–301. https://doi.org/10.1093/zoolinnean/zlz178
Cuvier, G. 1807. Sur les différentes espèces de crocodiles vivans et sur leurs caractères distinctifs. Annales du Muséum d’Histoire Naturelle de Paris, 10:8–66.
Czaplewski, N.J. and Morgan G.S. 2012. New basal noctilionoid bats (Mammalia: Chiroptera) from the Oligocene of subtropical North America, p. 162–209. In Gunnell, G.F. and Simons, N.B. (eds.), Evolutionary History of Bats: Fossils, Molecules, and Morphology. Cambridge University Press, New York, USA.
Daudin, F.M. 1802. Histoire Naturelle, Générale et Particulière des Reptiles. L’Imprimerie de F. Dufart, Paris, France. https://doi.org/10.5962/bhl.title.60678
De Souza, R.G., Riff, D., De Souza-Filho, J.P., and Kellner, A.W. 2018. Revisiting Gryposuchus jessei Gürich, 1912 (Crocodylia: Gavialoidea): specimen description and comments on the genus. Zootaxa, 4457:167–178. https://doi.org/10.11646/zootaxa.4457.1.9
Duméril, A.M.C. and Bibron, G. 1841. Erpétologie générale ou histoire naturelle complète des reptiles, vol. 8. Paris, France.
Erickson, B.R. 1982. Wannaganosuchus, a new alligator from the Paleocene of North America. Journal of Paleontology, 56:492–506. https://www.jstor.org/stable/1304478
Farlow, J.O., Hurlburt, G.R., Elsey, R.M., Britton, A.R., and Langston, Jr. W. 2005. Femoral dimensions and body size of Alligator mississippiensis: estimating the size of extinct mesoeucrocodylians. Journal of Vertebrate Paleontology, 25:354–369.
https://doi.org/10.1671/0272-4634(2005)025[0354:FDABSO]2.0.CO;2
Frailey, D. 1978. An early Miocene (Arikareean) fauna from northcentral Florida (the SB-1A local fauna). Occasional papers of the Museum of Natural History, the University of Kansas, 75:1–20.
Gilmore, C.W. 1946. A new crocodilian from the Eocene of Utah. Journal of Paleontology, 20:62–67.
Gmelin, J. 1789. Linnei Systema Naturae. GE Beer, Germany.
Gray, J.E. 1844. Catalogue of Tortoises, Crocodilians, and Amphisbaenians in the Collection of the British Museum. British Museum (Natural History), United Kingdom.
Hastings, A.K., Reisser, M., and Scheyer, T.M. 2016. Character evolution and the origin of Caimaninae (Crocodylia) in the New World Tropics: new evidence from the Miocene of Panama and Venezuela. Journal of Paleontology, 90:317–332.
https://doi.org/10.1017/jpa.2016.37
Hay, O.P. 1906. Descriptions of two new genera (Echmatemys and Xenochelys) and two new species (Xenochelys formosa and Terrapene putnami) of fossil turtles. Bulletin of the American Museum of Natural History, 22:27–31.
Hayes, F.G. 2000. The Brooksville 2 local fauna (Arikareean, latest Oligocene): Hernando County, Florida. Bulletin of the Florida Museum of Natural History, 43:1–47.
Head, J.J., Bloch, J.I., Hastings, A.K., Bourque, J.R., Cadena, E.A., Herrera, F.A., Polly, P.D., and Jaramillo, C.A. 2009. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature, 457:715–718. https://doi.org/10.1038/nature07671
Higgins, D.W. 1971. A Review of Oligocene Alligators from the Big Badlands of South Dakota. Master’s Thesis, South Dakota School of Mines and Technology, Rapid City, South Dakota, USA.
Kälin, J.A. 1939. Ein extrem kurzschnauziger Crocodilide aus den Phosphoriten des Quercy, Arambourgia (nov. gen.) gaudryi de Stefano. Abhandlungen der Schweizerischen Palaeontologischen Gesellschaft, 62:1–18.
Kotthoff, U., Greenwood, D.R., McCarthy, F.M.G., Müller-Navarra, K., Prader, S., and Hesselbo, S.P. 2014. Late Eocene to middle Miocene (33 to 13 million years ago) vegetation and climate development on the North American Atlantic Coastal Plain (IODP Expedition 313, Site M0027). Climate of the Past, 10:1523–1536.
Leidy, J. 1852. Description of a new species of crocodile from the Miocene of Virginia. Journal of the Academy of Natural Sciences of Philadelphia, 2:135–138.
Loomis, L.B. 1904. Two new river reptiles from the Titanothere beds. American Journal of Science, Series 4, 18:427–432.
Makarieva, A.M., Gorshkov V.G., and Li, B.-L. 2005. Gigantism, temperature and metabolic rate in terrestrial poikilotherms. Proceedings of the Royal Society, London B, 272:2325–2328. https://doi.org/10.1098/rspb.2005.3223
Makarieva, A.M., Gorshkov, V.G., and Li, B.-L. 2005. Temperature-associated upper limits to body size in terrestrial poikilotherms. Oikos, 111:425–436.
https://doi.org/10.1111/j.1600-0706.2005.14095.x
Marsh, O.C. 1874. Fossil horses in America. The American Naturalist, 8:288–294.
https://www.jstor.org/stable/2447952
Miller-Camp, J. 2016. Patterns in alligatorine evolution. PhD Dissertation, University of Iowa, Iowa City, Iowa, USA. https://doi.org/10.17077/etd.2l4bqxdg
Mook, C.C. 1941. A new crocodilian, Hassiacosuchus kayi, from the Bridger Eocene beds of Wyoming. Annals of the Carnegie Museum, 28:207–220.
Mook, C.C. 1961. Notes on the skull characters of Allognathosuchus polyodon. American Museum Novitates, 2072:1–5.
Morgan, G.S., Czaplewski, N.J., and Simmons, N.B. 2019. A new mormoopid bat from the Oligocene (Whitneyan and Early Arikareean) of Florida, and phylogenetic relationships of the major clades of Mormoopidae (Mammalia: Chiroptera). Bulletin of the American Museum of Natural History, 2019:1–146. https://doi.org/10.1206/0003-0090.434.1.1
Myrick, Jr. A.C. Thecachampsa antiqua (Leidy, 1852) (Crocodylidae: Thoracosaurinae) from fossil marine deposits at Lee Creek Mine, Aurora, North Carolina, USA. Smithsonian Contributions to Paleobiology, 90:219–225.
Norell, M.A., Clark, J.M., and Hutchison, J.H. 1994. The Late Cretaceous alligatoroid Brachychampsa montana (Crocodylia): new material and putative relationships. American Museum Novitates, 3116:1–26.
Patton, T.H. 1969. An Oligocene land vertebrate fauna from Florida. Journal of Paleontology, 43:543–546.
Piras, P. and Buscalioni, A.D. 2006. Diplocynodon muelleri comb. nov., an Oligocene diplocynodontine alligatoroid from Catalonia (Ebro Basin, Lleida province, Spain). Journal of Vertebrate Paleontology, 26:608–620.
https://doi.org/10.1671/0272-4634(2006)26[608:DMCNAO]2.0.CO;2
Schmidt, K.P. 1941. A new fossil alligator from Nebraska. Geological Series of Field Museum of Natural History, 8:27–32.
Snyder, D. 2007. Morphology and systematics of two Miocene alligators from Florida, with a discussion of Alligator biogeography. Journal of Paleontology, 81:917–928.
https://doi.org/10.1666/pleo05-104.1
Stocker, M.R., Brochu, C.A., and Kirk, E.C. 2021. A new caimanine alligatorid from the Middle Eocene of Southwest Texas and implications for spatial and temporal shifts in Paleogene crocodyliform diversity. PeerJ, 9:e10665. https://doi.org/10.7717/peerj.10665
Tedford, R.H., Albright, III, L.B., Barnosky, A.D., Ferrusquia-Villafranca, I., Hunt, Jr. R.M., Storer, J.E., Swisher, III, C.C., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (late Oligocene through early Pliocene epochs), p. 169-231.
Vélez-Juarbe, J., Brochu, C.A., and Santos, H. 2007. A gharial from the Oligocene of Puerto Rico: transoceanic dispersal in the history of a non-marine reptile. Proceedings of the Royal Society B: Biological Sciences, 274:1245–1254. https://doi.org/10.1098/rspb.2006.0455
Vickaryous, M.K. and Hall, B.K. 2008. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. Journal of Morphology, 269: 398–422. https://doi.org/10.1002/jmor.10575
Walker, J.D. and Geissman, J.W. 2009. GSA geologic time scale. GSA Today, 19:60.
https://doi.org/10.1130/2009.CTS004R2C
Walter, J., Darlim, G., Massonne, T., Aase, A., Frey, E., and Rabi, M. 2022. On the origin of Caimaninae: insights from new fossils of Tsoabichi greenriverensis and a review of the evidence. Historical Biology, 34:580–595. https://doi.org/10.1080/08912963.2021.1938563
White, T.E. 1942. A new alligator from the Miocene of Florida. Copeia, 1942:3–7.
https//doi.org/10.2307/1437933
Whiting, E.T. and Hastings, A.K. 2015. First Fossil Alligator from the Late Eocene of Nebraska and the Late Paleogene Record of Alligators in the Great Plains. Journal of Herpetology, 49:560–569. https://doi.org/10.1670/14-069
Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York, USA.
Woodward, A.R., White, J.H., and Linda, S.B. 1995. Maximum size of the alligator (Alligator mississippiensis). Journal of Herpetology, 29:507–513. https://doi.org/10.2307/1564733
Young, M.T., Bell, M.A., De Andrade, M.B., and Brusatte, S.L. 2011. Body size estimation and evolution in metriorhynchid crocodylomorphs: implications for species diversification and niche partitioning. Zoological Journal of the Linnean Society, 163:1199–1216.
https://doi.org/10.1111/j.1096-3642.2011.00734.x
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292:686–693.
https://doi.org/10.1126/science.1059412
Zanazzi, A., Kohn, M.J., MacFadden, B.J., and Terry, D.O. 2007. Large temperature drop across the Eocene-Oligocene transition in central North America. Nature 445:639–642.
https://doi.org/10.1038/nature05551

