 Stable isotope (ẟ13C, ẟ18O) paleoecology of the late Early Miocene mammalian fauna from Buluk, Kenya
Stable isotope (ẟ13C, ẟ18O) paleoecology of the late Early Miocene mammalian fauna from Buluk, Kenya
Article number: 27.1.a19
https://doi.org/10.26879/1335
Copyright Society of Vertebrate Paleontology, March 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 31 August 2023. Acceptance: 10 January 2024.
ABSTRACT
Early Miocene deposits at Buluk in northern Kenya have produced an abundant and diverse community of mammalian fossils, including catarrhine primates, and the site is an important resource for characterizing habitat heterogeneity across East Africa during the early-middle Miocene transition. Here we present the results of stable carbon and oxygen isotope analyses of fossil tooth enamel from Buluk’s ruminant artiodactyls, suoids, anthracotheres, rhinocerotids, proboscideans, and hyraxes, to address the nature of the C3 vegetation (i.e., open-canopy versus closed-canopy and/or degree of water stress) and to determine whether C4 plants were a dietary component of Buluk herbivores. δ13Cenamel and δ18Oenamel values indicate that most Buluk herbivores foraged in a C3-dominated mosaic of open canopy woodland habitats with no evidence of closed-canopy habitats, consistent with soil and biomarker analyses. Despite paleosol evidence for the presence of C4 vegetation at Buluk, δ13Cenamel results show no evidence for the consumption of C4 biomass. This discrepancy may be caused by the presence of C4 vegetation on the landscape that was not consumed by local herbivores in quantities that can be captured by bulk δ13Cenamel, lack of sampling of taxa that did consume C4 vegetation, or it may reflect differences in scale between these two data sources. Overall, the range of δ13Cenamel values from Buluk are like the early Miocene site of Moroto and are significantly more enriched than the middle Miocene localities Maboko Island and Fort Ternan, suggesting more water-stressed environments predominated at these earlier Miocene sites in East Africa.
Irisa D. Arney. Western University of Health Sciences, College of Osteopathic Medicine of the Pacific Northwest, Lebanon, Oregon 97355, USA. (corresponding author) iarney@westernu.edu
Ellis M. Locke. Idaho College of Osteopathic Medicine, Meridian, Idaho 83642, USA. elocke@icom.edu
Ellen R. Miller. Wake Forest University, Department of Anthropology, Winston Salem, North Carolina 27109, USA. millerer@wfu.edu
Isaiah O. Nengo, deceased. Formerly of Turkana Basin Institute, Social and Behavioral Sciences, Stony Brook University, Stony Brook, New York 11794, USA.
Key Words: carbon isotopes; East Africa; Miocene; paleoenvironment; herbivores; C3 diets
Final citation: Arney, Irisa D., Locke, Ellis M., Miller, Ellen R., and Nengo, Isaiah O. 2024. Stable isotope (ẟ13C, ẟ18O) paleoecology of the late Early Miocene mammalian fauna from Buluk, Kenya. Palaeontologia Electronica, 27(1):a19.
https://doi.org/10.26879/1335
palaeo-electronica.org/content/2024/5121-buluk-kenya-stable-isotopes
Copyright: March 2024 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Buluk is the easternmost early Miocene primate locality in Africa, and as such the site is a meaningful point of comparison for documenting both spatial and temporal heterogeneity of East African habitats across the early-middle Miocene transition. A growing body of paleoenvironmental evidence suggests that the early Miocene of East Africa was characterized by substantial spatial and temporal variation in climatic conditions and vegetation (Retallack et al., 2002; Bonnefille, 2010; Michel et al., 2014, 2020; Lukens et al., 2017a, 2021; Butts, 2019; Liutkus-Pierce et al., 2019; Baumgartner and Peppe, 2021; Peppe et al., 2023). While forests are documented at some early Miocene sites in western Kenya (e.g., Michel et al., 2014, 2020; Oginga, 2018; Baumgartner and Peppe, 2021), open woodland and wooded grassland environments are also well documented both in western Kenya (Driese et al., 2016; Lukens et al., 2017a) and northern Kenya (Lukens et al., 2017b, 2021; Butts, 2019; Liutkus-Pierce et al., 2019).
Soil and plant wax geochemical and palaeobotanical evidence for some contribution of C4 vegetation to open woodland habitats has been recovered at several early Miocene localities in East Africa, including at Buluk (Lukens et al., 2017a, 2021; Peppe et al., 2023). Whether early Miocene herbivorous mammals had begun to incorporate these C4 resources into their diets is less clear. Dental morphological data suggest that grazing adaptations were not present among ruminants until the late Miocene (Hall and Cote, 2021), and published information about herbivore enamel isotopic evidence prior to 10 Ma is largely lacking (Cerling et al., 1997; Arney et al., 2022; MacLatchy et al., 2023). Buluk’s mammalian assemblage provides an opportunity to add to the regional record of habitat variation in the early Miocene of East Africa and to retrodict the dietary behavior of herbivores that inhabited these environments. To this end, we report the results of enamel stable isotope analyses (δ13Cenamel, δ18Oenamel) from a range of fossil mammalian herbivores at Buluk with two objectives: 1) to establish dietary isotopic ranges for fossil herbivore guilds; and 2) to examine inter-site variation in δ13Cenamel values to assess niche partitioning among Early and Middle Miocene herbivores.
Toward the first objective, we establish carbon isotopic profiles for the major mammalian herbivore groups from Buluk to examine dietary niche partitioning at the site and to determine whether C4 vegetation was a dietary component for any of the sampled groups. Several past δ13 Cenamel studies exploring the vegetation history of East Africa during the Neogene found that landscapes in the Early and Middle Miocene were C3-dominated with little to no C4 biomass (Cerling et al., 1991, 1997; Uno et al., 2011, 2016; Feakins et al., 2013; Polissar et al., 2019). This paradigm was recently challenged by the recovery of geochemical evidence from plant wax and soil carbonates, which indicated heterogeneous environments including C4 grasses were present at multiple early Miocene localities (Peppe et al., 2023). δ13Cenamel values from herbivorous mammals at Buluk will allow us to assess whether C4 grasses or water-stressed C3 vegetation were being ingested by these animals. In addition, δ18O values are used to characterize the drinking habits utilized by mammalian herbivores, and to assess the implications for local environmental aridity at Buluk.
The second objective is to compare Buluk δ13Cenamel values with those from published records of two Middle Miocene localities in Western Kenya: Maboko Island (~15-14 Ma; Feibel and Brown, 1991) and Fort Ternan (13.7 Ma; Pickford et al., 2006), and the Ugandan Early Miocene locality Moroto (21 Ma; MacLatchy et al., 2023) to examine inter-site variation among common taxa. Inferred open habitats at Maboko (Evans et al., 1981; Retallack et al., 2002; Arney et al., 2022) and Fort Ternan (Evans et al., 1981; Shipman et al., 1981; Shipman, 1986; Retallack et al., 1990; Kappelman, 1991; Retallack, 1992; Cerling et al., 1991, 1997; Dugas and Retallack, 1993) have played a key role in framing the early-middle Miocene ecological transition. Additionally, Moroto, Maboko, and Fort Ternan are the only East African localities with δ13Cenamel values that provide comparative values for interpreting niche partitioning among presumed C3-dominated feeders before the spread of C4 grasslands at 10 Ma. Together with information from Buluk, these four enamel carbon isotope datasets will provide a snapshot of the temporal and spatial habitat variation across three distinct regions in the developing East African Rift Valley System during the early to middle Miocene.
BACKGROUND
Geology and Fauna
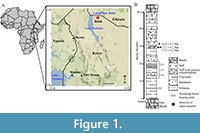 Buluk is a late Early Miocene fossil site in northern Kenya, situated between Lake Turkana and Lake Chew Bahir (Figure 1A). Fossiliferous sediments at Buluk are part of the Buluk Member of the Bakate Formation (Figure 1B), which is exposed along the uplift of the Suregai-Asille plateau (Harris and Watkins, 1974; Watkins, 1983; McDougall and Watkins, 1985). The upper section of the Buluk Member is a series of sterile, reworked pantelleritic tuffs, while the lower section includes fossiliferous channel lag deposits underlying a rhyolitic layer capped by a basalt flow (McDougall and Watkins, 1985; Hurford and Watkins, 1987). The published date of this capping basalt is 17.2 ± 0.2 Ma (McDougall and Watkins, 1985). At the time of fossil deposition, the landscape at Buluk was dominated by a mature river system associated with an extensive floodplain (Watkins, 1989).
Buluk is a late Early Miocene fossil site in northern Kenya, situated between Lake Turkana and Lake Chew Bahir (Figure 1A). Fossiliferous sediments at Buluk are part of the Buluk Member of the Bakate Formation (Figure 1B), which is exposed along the uplift of the Suregai-Asille plateau (Harris and Watkins, 1974; Watkins, 1983; McDougall and Watkins, 1985). The upper section of the Buluk Member is a series of sterile, reworked pantelleritic tuffs, while the lower section includes fossiliferous channel lag deposits underlying a rhyolitic layer capped by a basalt flow (McDougall and Watkins, 1985; Hurford and Watkins, 1987). The published date of this capping basalt is 17.2 ± 0.2 Ma (McDougall and Watkins, 1985). At the time of fossil deposition, the landscape at Buluk was dominated by a mature river system associated with an extensive floodplain (Watkins, 1989).
Buluk preserves a rich mammalian fauna (Table 1) including proboscideans, hyracoids, primates, carnivorans, hyaenodonts, rhinocerotids, suoids (i.e., sanitheriids and suids), tragulids, and pecorans (e.g., giraffoids, bovids). Since the initial publication of Early Miocene fossils from Buluk (Harris and Watkins, 1974), ongoing fieldwork has led to the collection of >1500 mammalian fossils, prompting several lineage-specific revisions of the fauna (Geraads et al., 2013; Locke et al., 2020; Morlo et al., 2021; Nishimura et al., 2022). The taxonomic composition of the mammalian fauna from Buluk largely overlaps with many other Early Miocene East African localities, with a few taxa hinting at affinities with Middle Miocene faunas. This is most apparent in the artiodactyls - a group that shows marked taxonomic and gradistic changes during the Miocene. The ruminant artiodactyl community at Buluk is like many other Early Miocene sites, being comprised primarily of tragulids in the genus Dorcatherium, the giraffid Canthumeryx, and multiple species of small-bodied pecorans of unknown familial status (Cote, 2010; Cote et al., 2018). Buluk lacks the more derived artiodactyl families found at Middle Miocene localities like Fort Ternan and Maboko (e.g., climacoceratid giraffoids, bovids, and hippopotamids), but shares with Maboko two closely related species of suids in the genus Lopholistriodon and Megalochoerus (Retallack et al., 2002; Pickford, 2006).
The diverse community of fossil primates from Buluk includes the large-bodied hominoid Afropithecus turkanensis (Leakey and Walker, 1985; Leakey and Walker, 1997), a small-bodied non-cercopithecoid catarrhine comparable in size to Simiolus enjiessi (Rose et al., 1992; Leakey et al., 2011; Nishimura et al., 2022) and at least two other as-yet-undescribed non-cercopithecoid catarrhine taxa (E.M.L. and E.R.M, personal observations). Buluk has also yielded numerous fossils of the stem cercopithecoid Noropithecus bulukensis (Leakey, 1985; Miller et al., 2009; Locke et al., 2020). In contrast to many Early Miocene sites, where cercopithecoids are scarce or absent, N. bulukensis comprises roughly half of all primate fossils recovered from Buluk (Locke et al., 2020).
Stable Carbon Isotopes
The ratio of stable carbon isotopes 13C/12C (often described as δ13C, see ‘Materials and Methods’ for equations) in plants consumed by herbivores is recorded in organismal tissues during development (Lee-Thorp et al., 1989, 1991; Cerling and Harris, 1999; Sponheimer et al., 2003). The high hydroxyapatite content of tooth enamel makes it resistant to diagenetic alteration during the fossilization process, making stable carbon isotope values from fossil herbivore enamel (δ13Cenamel) a valuable tool for dietary inference of extinct taxa and paleoenvironmental reconstruction (e.g., Bocherens et al., 1996; Cerling et al., 1997; 2003a, b; Schoeninger et al., 2003; Kingston and Harrison, 2007; Levin et al., 2008; Uno et al., 2011, 2018; Cerling et al., 2015; Du et al., 2019; Paquette and Drapeau, 2021; Robinson, 2021).
The utility of δ13Cenamel values derives from the fact that the δ13C composition of vegetation consumed by herbivores reliably discriminates between the C3 and C4 photosynthetic pathways used by plant groups for producing chemical energy. In modern eastern African ecosystems, woody vegetation and high-elevation grasses utilize the C3 photosynthetic pathway, which discriminates against the heavier 13C and results in lower δ13C values (-36‰ to -23‰) (Tieszen et al., 1979; Young and Young, 1983; Cerling and Harris, 1999). C4 vegetation includes low-elevation grasses, sedges, and some shrubs, which have relatively higher δ13C values (-14‰ to -10‰) (Tieszen et al., 1979; Young and Young, 1983; Cerling and Harris, 1999). The C4 pathway also discriminates against 13C but at a lower degree than the C3 photosynthetic pathway.
Variation in environmental and physiological factors that influence δ13C values in plants results in a greater range of δ13 C values for C3 plants than for C4 plants, reflecting a greater influence of environmental conditions on carbon isotope values using the C3 photosynthetic pathway. For example, the lowest δ13C values among C3 plants occur in closed canopy forests due to the recycling of 13C-depleted CO2 from soil respiration in the forest understory (Vogel, 1978; Ehleringer et al., 1986; van der Merwe and Medina, 1989, 1991). C3 plant parts found higher in the canopy and in more open canopy environments, where there is more exposure to solar radiation or more xeric conditions, have more positive δ13C values (e.g., Ehleringer et al., 1986; Heaton, 1999; Kohn, 2010). The δ13C ratios of C3 plants are also negatively correlated with mean annual precipitation (MAP), meaning plants from lower precipitation environments exhibit higher δ13C values. (Kohn, 2010).
Stable Oxygen Isotopes
In conjunction with δ13Cenamel values, the stable oxygen isotope values of herbivore tooth enamel (δ18Oenamel) can provide additional insights into aspects of an animal’s diet and interactions with the environment. Environmental factors such as variation in temperature, humidity, and precipitation influence δ18O values in drinking water and water within plant material, resulting in a downstream effect on δ18O enamel values of mammalian herbivores (Dansgaard, 1964; Rozanski et al., 1993; Higgins and McFadden, 2004). In addition to environmental variation and the δ18O composition of atmospheric oxygen, the drinking habits, physiology, and metabolic mechanisms of an animal also influence its δ18Oenamel values (Bryant and Froelich, 1995; Kohn et al., 1996, 1998; Kohn and Law, 2006; Sponheimer and Lee-Thorp, 1999; Levin et al., 2006). This confluence of biotic and abiotic factors complicates the interpretation of δ18Oenamel values.
Despite the intricacies involved in the oxygen flux within herbivore tissues, some general patterns have emerged from the literature. Plant tissue δ18O values are especially sensitive to changes in local temperature, humidity, and evapotranspiration (Sternberg et al., 1989). The δ18Oenamel values of herbivores that rely on plant resources to obtain their water (i.e., non-obligate drinkers) track local variation in humidity and evapotranspiration due to canopy height (Kohn et al., 1996; Levin et al., 2006; Krigbaum et al., 2013; Nelson, 2013; Carter and Bradbury, 2016; Blumenthal et al., 2017; Green et al., 2022). Even the extent of folivory might influence δ18Oenamelvalues (Carter and Bradbury, 2016; see also Fannin and McGraw, 2020). Herbivores that rely on drinking from local water sources for their water intake (i.e., obligate drinkers) track the δ18O of meteoric water more closely, and their δ18Oenamel values are depleted in 18O relative to those of non-obligate drinkers (Levin et al., 2006). Occupying an aquatic or semi-aquatic niche also affects δ18Oenamel values, with aquatic and semi-aquatic taxa having lower δ18Oenamel values relative to terrestrial herbivores (Cerling et al., 2003b; Levin et al., 2006; Kingston and Harrison, 2007; Clementz et al., 2008).
MATERIALS AND METHODS
Enamel Sampling
Samples of fossil tooth enamel (n=67) were collected from the postcanine teeth of 12 genera of non-primate mammalian herbivores from Buluk (Table 1). These samples represent a phylogenetically and ecologically diverse set of mammals including Hyracoidea, Proboscidea (Deinotheriidae, Gomphotheriidae, Mammutidae), Perissodactyla (Rhinocerotidae), and Artiodactyla (Anthracotheriidae, Giraffidae, Suidae, Sanitheriidae). Proboscidean specimens were identified by William Sanders, while all other previously unpublished specimens were identified by E.M.L and E.R.M. All specimens sampled for this study are housed in the collections of the National Museums of Kenya (NMK) in Nairobi, Kenya, and the Turkana Basin Institute (TBI) in Ileret, Kenya. All sampling was conducted with prior authorization from the NMK and by the Kenyan National Commission for Science, Technology, and Innovation (NACOSTI).
Sampling protocols were restricted to fragmentary or damaged teeth, which limits the identification of some specimens to higher taxonomic levels. Non-deinotheriid proboscidean teeth that could not be identified as gomphotheriid or mammutid were classified as “Elephantimorph indet.”. Rhinocerotid teeth that could not confidently be assigned to Brachypotherium minor were designated as “Rhinocerotidae indet.”, given evidence for the presence of multiple rhinocerotid taxa at Buluk (Geraads and Miller, 2013). These higher taxonomic level groups potentially include specimens representing multiple genera. For the entire sample, 75% were identified to the species level, 9% were identified to genus, and 16% were identified to superfamily (Elephantoidea) and family (Rhinocerotidae). When possible, enamel was collected from molars, specifically second and third molars, to ensure adult diet was captured.
Prior to sampling, fossil teeth were inspected for a suitable sampling surface along a broken face that would not interfere with occlusal surface morphology. The enamel surface was cleaned using a tungsten-carbide bur bit to remove matrix, cementum, and/or dentin before samples of pure enamel were removed. The mass of enamel collected reflects a compromise between obtaining a sufficient volume of enamel powder for analysis while preserving informative tooth morphology. Approximately 5 or more milligrams of enamel were collected for most taxa, except for those with small or thinly enameled dentitions (i.e., Tragulidae, Hyracoidea, and Sanitheriidae), for which samples were limited to a maximum of 3 mg.
Pretreatment and Isotopic Analysis
Following Uno and colleagues (2011), a subset of enamel samples (n=15) was analyzed with and without pretreatment to determine the effect of treatment on δ13Cenamel values. Enamel powder samples with a mass >6 mg were selected for pretreatment/treatment comparisons. Pretreatment was performed at the Paleoecology Lab at the University of Michigan. Samples undergoing pretreatment were soaked in 3% sodium hypochlorite (NaOCl) for 12 hours. Samples were centrifuged and NaOCl was decanted using a pipette without disturbing the enamel pellet. Enamel powder was rinsed with deionized water to neutrality. Samples were then treated with 0.1M solution of acetic acid (CH3 COOH) for 12 hours and rinsed with deionized water to neutrality. The enamel powder pellet was transferred to a glass vial and excess moisture was removed by freeze drying overnight. Results of treated versus untreated Buluk δ13Cenamel and δ18Oenamel values are shown in Appendix 1. The difference in δ13Cenamel and δ18Oenamel values between treated and untreated samples was less than ±0.6‰ for all 15 samples, indicating a minimal effect of treatment (Appendix 2).
Since a considerable amount of sample powder can be lost during pretreatment, standard protocols are to generally leave samples with ~1-4 mg of enamel powder untreated and to convert their values to match pretreated samples using regression equations (e.g., Levin et al., 2008; Cerling et al., 2015). Here, based on the results shown in Appendix 1, all other untreated samples were converted to match pretreated samples using the respective carbon and oxygen regression equations:
δ13C final = 0.96 * δ13C untreated - 0.67, R2 = 0.97 (1)
δ18O final = 0.99 * δ18O untreated - 0.26, R2 = 0.96 (2)
Treated and untreated samples were sent to the University of Florida, Department of Geosciences Light Stable Isotope Mass Spec Lab for analysis and determination of 13C/12C ratios. In glass vials, 3-5 mg of enamel powder sample and NBS-19 standards were reacted with 99% phosphoric acid (H3 PO4) at 70°C. The CO2 produced from this reaction was analyzed using mass spectrometry with a Kiel III carbonate preparation device coupled with a Finnigan-Mat 252 isotope ratio mass spectrometer. The standard deviation for NBS-19 standard analyses was ~0.03‰ for δ13C and ~0.07‰ for δ18O. Results are reported in standard parts per million notation (‰):
δ13C = (18O) = (Rsample/Rstandard - 1) * 1,000 (3)
R = 13C/12C or 18O/16O (4)
Interpretation of Enamel Stable Isotope Ratios
The δ13C value of atmospheric CO2 (δ13Catm) that is incorporated into plant tissues fluctuates through time and thus impacts the δ13C values of plant tissues. This flux in δ13Catm values must be standardized before comparing δ13Cenamel values from different time periods. Since the Industrial Revolution (estimated to be approximately A.D. 1750), the generation of 13C-depleted CO2 from anthropogenic processes has decreased global δ13Catm values (Francey et al., 1999). During the Early and Middle Miocene, estimated average δ13Catm values from North Atlantic benthic foraminifera varied between -4.8‰ to -6.6‰ (Tipple et al., 2010). A pre-Industrial Revolution δ13Catm value of −6.3‰ (δ13C1750) was used to standardize fossil enamel carbon isotope values and any modern enamel carbon isotope values mentioned in the text.
For the Early Miocene age (17.2±0.2 Ma, McDougall and Watkins, 1985), the δ13Catm values for 17.0 - 17.4 Ma from the high-resolution benthic dataset from Tipple et al. (2010) were averaged (mean = -5.66‰). Therefore, -0.64‰ was added to δ13Cenamel values for standardization to δ13C1750 values. Recently published paleoecological work has placed the age of Buluk closer to 16 Ma (Peppe et al., 2023). However, this relies on radiometric K-Ar (alkali feldspar) and fission-track (zircon) ages of 16.1± 0.2 Ma from the volcaniclastic tuffs overlying the Bakate Formation (Figure 1B; Watkins, 1989; Hurford and Watkins, 1987). We conservatively retain the use of the 17.2 Ma date to standardize enamel samples from Buluk with modern and other fossil carbon isotope data. For completeness, a comparison of the differences in δ13Cenamel value correction using a date of 16 Ma versus 17.2 Ma is provided in Appendix 3. All fossil and modern δ13C enamel results are reported as δ13C1750 values to accommodate fluctuations in atmospheric ẟ13C so modern and fossil enamel isotopic values can be compared. Published δ13Cenamel values from Moroto, Maboko, and Fort Ternan were also corrected using the high-resolution benthic δ13Catm estimates from Tipple et al. (2010) as follows: raw Maboko δ13Cenamel values were corrected following Arney et al., (2022); for the interval of 13.40-14.0 Ma (mean δ13Catm = -5.48‰), -0.82‰ was added to published Fort Ternan δ13Cenamel values; for the interval of 20.71-21.38 Ma (mean δ13Catm = -6.04‰), -0.26‰ was added to published Moroto δ13Cenamel values.
Isotopic dietary ranges that correspond with general biomes were used to assess habitat structure among presumed C3-dominated ecosystems (closed canopy vs. open canopy; mesic vs. xeric conditions). The following dietary categories are modified from the diet classifications described in Cerling and colleagues (2015): C3 closed canopy diets are defined as those with δ13Cenamel values < -14‰; C3 open canopy diets are defined as those with δ13Cenamel values between -14‰ and -8‰; and C3/C4 mixed diets are characterized as those with δ13Cenamel values > -8‰.
All statistical analyses and plots were made using R (version 4.2.1, R Core Team, 2021). δ13Cenamel data from Buluk was compared with published enamel stable isotope values from fossil mammals from Moroto (MacLatchy et al., 2023), Maboko (Arney et al., 2002) and Fort Ternan (Cerling et al., 1997) (Table 2). Shapiro-Wilk tests indicate a normal distribution for δ13Cenamel data from Buluk, Maboko, and Fort Ternan, but not from δ13Cenamel data Moroto. Intersite δ13Cenamel comparisons were conducted using pairwise nonparametric Mann-Whitney U tests. Bulk δ18Oenamel values between localities were not statistically compared, since each locality has a unique meteoric water source(s) with unknown δ18O values, which can further complicate interpretations. Results of the intersite carbon analysis and oxygen comparisons are reported in the main text of the Discussion.
RESULTS
General Patterns
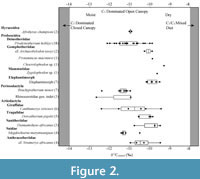 Buluk herbivore δ13Cenamel values range from -12.7‰ to -9.3‰ with a mean value of -11.2‰ (Figure 2; Table 2). These values are consistent with herbivores foraging in a C3 -dominated open
Buluk herbivore δ13Cenamel values range from -12.7‰ to -9.3‰ with a mean value of -11.2‰ (Figure 2; Table 2). These values are consistent with herbivores foraging in a C3 -dominated open canopy woodland ecosystem. Carbon isotopic profiles from Buluk herbivores do not fall within the range of values for closed canopy understory foraging, suggesting closed canopy habitats were either absent from the landscape or were not utilized consistently by herbivores sampled here. There is no evidence for the consumption of C4 resources, even in low proportions.
canopy woodland ecosystem. Carbon isotopic profiles from Buluk herbivores do not fall within the range of values for closed canopy understory foraging, suggesting closed canopy habitats were either absent from the landscape or were not utilized consistently by herbivores sampled here. There is no evidence for the consumption of C4 resources, even in low proportions.
The δ18Oenamel values from Buluk range from -6.4‰ to +3.5‰ (Figure 3). Differences in δ18Oenamel values among taxa potentially reflect variation in water intake strategies, physiological water flux, changes in meteoric water δ18O values due to environmental factors, and/or contributions from multiple water sources. While it is difficult to make specific inferences about drinking habits or environmental conditions given the lack of extant analogs for many of the fossil taxa sampled here, documenting intra and inter-specific variation in δ18Oenamel values is valuable for future comparative work. Taxon-specific summary statistics for δ13Cenamel and δ18Oenamel are reported in Table 3, and these values are plotted together for completeness in Appendix 4.
Hyracoidea
Two Afrohyrax championi teeth were sampled in this study, with δ13Cenamel values of -11.0‰. These values overlap with the δ13Cenamel values of several Buluk taxa, including deinotheres, the giraffid Canthumeryx sirtensis, the upper range of rhinocerotid values, and the low end of values for the anthracotheriid cf. Sivameryx africanus (Figure 2). δ18Oenamel values of the two A. championi specimens are -0.1‰ and +1.6‰. These oxygen isotopic values are relatively high, overlapping with cf. S. africanus, C. sirtensis, and the large-bodied suid Megalochoerus marymuunguae (Figure 3).
Proboscidea
The δ13C values for all sampled Buluk proboscideans range from -12.1‰ to -9.3‰ (Figure 2; Table 3). The range of δ13C values for Prodeinotherium hobleyi is -12.1‰ to -9.9‰, consistent with prior isotopic analyses from Buluk showing a pure C3 browsing signal for this taxon (Cerling et al., 1999). The range for the elephantimorph taxa (i.e., gomphtheriids and mammutids) is -10.7‰ to -9.5‰, suggesting that they may have fed on more water-stressed browse than the relatively δ13C depleted deinotheriid. These differences could also be the result of body size variation (Tejada-Lara et al., 2018), plant parts consumed (Carlson and Kingston, 2014; Blumenthal et al., 2016), and interspecific physiological and metabolic variation (Cerling et al., 2021).
The δ18Oenamel values of Buluk proboscideans range from -6.4‰ to -0.2‰, with a mean value of -3.1‰ (Figure 3). Oxygen signatures for the five proboscidean taxa overlap extensively, with the range of δ18Oenamel values of P. hobleyi encompassing all other proboscidean values. Overall, the lowest δ18Oenamel values of all taxa are from elephantimorph proboscideans and P. hobleyi specimens, suggesting proboscideans were dependent on drinking water from a variety of sources (e.g., rivers, springs, ponds, or lakes) for water intake. Proboscideans are the most δ18O depleted group of mammals sampled from Buluk.
Rhinocerotidae
Ten rhinocerotid teeth were sampled: seven belonging to Brachypotherium minor and three assigned to Rhinocerotidae gen. indet., which may represent a distinct species (Geraads and Miller, 2013). δ13Cenamel values for the total Rhinocerotidae sample range from -12.7‰ to -11.0‰. The range in B. minor δ13Cenamel values is from -12.4‰ to -11.0‰, which is bracketed by the Rhinocerotidae gen. indet. values (Figure 2). The rhinocerotid carbon isotope values overlap with those from the suid M. marymuunguae, the giraffid C. sirtensis, and the deinothere P. hobleyi, and are lower than δ13C values of the sanitheriid Diamantohyus africanus, the tragulid Dorcatherium pigotti, the anthracothere cf. S. africanus, and all elephantimorph proboscideans.
Rhinocerotid δ18Oenamel values range from -3.6‰ to +0.2‰ and have a mean value of -1.3‰ (Figure 3). These oxygen isotope values are higher than most δ18Oenamel samples from elephantimorph proboscideans and lower than most samples from C. sirtensis.
Giraffidae
Six Canthumeryx sirtensis teeth were sampled from Buluk. δ13Cenamel values for C. sirtensis range from -12.4‰ to -9.4‰ and have a mean value of -10.9‰ (Figure 2). C. sirtensis has the highest range of δ13Cenamel values among all sampled mammals from Buluk, which suggests that this taxon has a relatively broad dietary range.
The δ18Oenamel values for C. sirtensis range from -0.6‰ to +3.5‰, which is relatively enriched in 18O compared to all other taxa (Figure 3). Extant giraffes in many modern assemblages are similarly enriched relative to other herbivores on the landscape (Kingston and Harrison, 2007; Kohn et al., 1996; Sponheimer and Lee-Thorp, 1999). The 18O enriched C. sirtensis values are consistent with this taxon preferentially relying on diet for water intake, like extant Giraffa (Levin et al., 2006). These results also indicate C. sirtensis browsed on dietary items enriched in 18O, such as leaves and/or fruit from high in the woody canopy (Krigbaum et al., 2013; Carter and Bradbury, 2016; Roberts et al., 2017).
Tragulidae
The δ13Cenamel values for the five Dorcatherium pigotti teeth sampled range from -10.6‰ to -9.9‰ and have a mean value of -10.2‰ (Figure 2). This range of δ13Cenamel values overlaps with many other artiodactyls from Buluk, including Di. africanus, cf. S. africanus, and C. sirtensis. The D. pigotti carbon isotopic results are 13C enriched relative to the limited δ13Cenamel data available for the forest-dwelling, extant African tragulid, Hyemoschus aquaticus (-13.9‰; Cerling et al., 2004), indicating D. pigotti browsed in more open wooded habitats. There is a second, larger-bodied tragulid species present at Buluk, Dorcatherium chappuisi (Table 1), which could not be sampled. At the Middle Miocene site of Maboko, where these two taxa also co-occur, D. chappuisi is more enriched in 13 C than D. pigotti, but whether these taxa exhibit similar niche partitioning at Buluk cannot be determined at this time.
D. pigotti δ18Oenamel values range from -4.5‰ to -0.3‰ and have a mean value of -2.8‰. The distribution of D. pigotti overlaps with the distribution of δ18Oenamel values for deinotheres, the higher values of elephantimorphs, the lower values of rhinocerotids, and all other artiodactyls from Buluk (Figure 3).
Suoidea
Suoid species sampled here include three specimens of the sanithere Diamantohyus africanus and five specimens of the large-bodied suid Megalochoerus marymuunguae. Two smaller-bodied suids, Lopholistriodon pickfordi and cf. Kenyasus rusingensis, are also present at Buluk (Table 1) but could not be sampled. D. africanus δ13Cenamel values range from -10.8‰ to -9.5‰ and have a mean value of -10.0‰ (Table 3). These carbon isotopic values are 13C enriched compared to δ13Cenamel values from M. marymuunguae (Figure 2), which range from -11.4‰ to -10.5‰ and have a mean value of -11.2‰. The lower δ13Cenamel values of M. marymuunguae indicate it may have had a different foraging strategy than D. africanus, selecting plants or plant parts enriched in 13C or foraging in more open canopy.
The δ18Oenamel values for D. africanus range from -2.8‰ to -2.2‰ and have a mean value of -2.6‰. The δ18Oenamel values for M. marymuunguae range from -1.0‰ to +1.4‰ and have a mean value of +0.2‰, which are higher than the δ18Oenamel values of D. africanus (Figure 3). The 18O depleted δ18Oenamel values in D. africanus compared to M. marymuunguae oxygen profiles is consistent with a dependence on a more 18O depleted diet and water sources. Differences in body size and physiological metabolic processes could also have contributed to the divergent carbon and oxygen profiles between these two suoids (Bryant and Froelich, 1995; Kohn et al., 1996; Passey et al., 2005; Tejada-Lara et al., 2018; Cerling et al., 2021), as D. africanus is markedly smaller than M. marymuunguae (van der Made, 1996; Grossman, 2008). The higher δ18Oenamel values for M. marymuunguae, however, do not follow the expectations outlined in Bryant and Froelich (1995) for a much larger species, where larger body sizes take in more liquid water and rely less on food metabolism for oxygen intake.
Anthracotheriidae
Four anthracothere teeth were sampled, which are identified as cf. Sivameryx africanus. Anthracothere δ13Cenamel values range from -11.0‰ to -9.5‰ with a mean value of -10.4‰. The distribution of anthracothere δ13Cenamel values overlaps with those of tragulids, sanitheriids, and elephantimorph proboscideans (Figure 2).
The δ18Oenamel values for anthracotheres range from -5.2‰ to +1.9‰ and have a mean value of -2.7‰. Most anthracothere δ18Oenamel values are depleted in 18O and overlap with δ18Oenamel values of all proboscideans and D. pigotti. One δ18Oenamel value of cf. S. africanus is relatively 18O enriched, overlapping with δ18Oenamel values of C. sirtensis (Figure 3). While this sample came from a maxillary P4 of cf. S. africanus, the relatively enriched δ18O enamel value likely reflects a shift in either surface water and/or dietary δ18O values rather than a weaning signature, as the P4s of most extant ruminant and non-ruminant herbivores form and erupt after the permanent upper molars (Spinage, 1976; Davis, 1980; Levine, 1982; Hillman-Smith et al., 1986; Fricke and O’Niel, 1996; Kohn, 1996; Kohn et al., 1998; Fricke et al., 1998; Green et al., 2018, 2022).
DISCUSSION
Herbivore Enamel Isotopes, Diets, and Paleoecology
The range of Buluk herbivore δ13Cenamel values (-13.1‰ to -9.8‰) indicates these mammals had C3-dominated diets. Elephantimorph proboscideans exhibit the highest δ13Cenamel values in the sample and rhinocerotids exhibit the lowest values (Figure 2). Stable carbon isotope data from pedogenic carbonates at Buluk indicates the presence of a C4 component on the landscape (Peppe et al., 2023), but evidence from δ13Cenamel samples suggests the herbivorous mammals at Buluk were likely not consuming this resource. Using methods published by MacLatchy et al. (2023), estimated fractions of C4 biomass at Buluk from pedogenic carbonate δ13C values indicate a wide range of possible C4 biomass, with the lowest reconstructed C4 percentage of 19% (95% confidence interval = 0% to 46%; δ13Ccarbonate = -8.7‰) and the largest reconstructed C4 percentage of 72% (95% confidence interval = 46% to 97%; δ13Ccarbonate = -0.8‰).
Due to the opportunistic nature of the sampling strategy, it remains possible that some Buluk herbivores that were unable to be sampled for this analysis did indeed utilized C4 resources. It is also possible that the abundance of C4 vegetation was too low to be consistently incorporated into herbivore diets, or that C4 plants were not preferred dietary items. The tightly constrained range of δ13Cenamel values from Buluk suggests similar foraging patterns among mammalian herbivores, or niche partitioning along axes that are not reflected in isotopic analyses (Arney et al., 2022). The overall range of herbivore δ13Cenamel values reflects feeding in open environments without closed canopy components, water-stressed C3 plants, or abundantly consumed C4 plants.
These enamel carbon isotope results are consistent with many, but not all previous paleoecological findings from Buluk. Preliminary analyses of paleosol geochemistry and morphology suggest a relatively open woodland environment within a seasonal, subhumid precipitation regime (Lukens et al., 2017b, 2021). In this way, paleoenvironmental reconstructions at Buluk are consistent with the interpretation that heterogeneous open habitats were present across East Africa during the Early Miocene (Peppe et al., 2021). The fact that the Buluk herbivores exhibit C3-dominated diets within this open canopy habitat is also consistent with previous local and regional stable isotopic work showing that East African herbivore diets were C3-dominated prior to 10 Ma (Cerling et al., 1991, 1997; Uno et al., 2011; Feakins et al., 2013; Polissar et al., 2019).
Enamel oxygen profiles from Buluk mammals indicate variation in water intake strategies between herbivore guilds (Figure 3). The giraffid C. sirtensis displays the highest δ18Oenamel values, consistent with an interpretation of this taxon as a non-obligate drinker receiving most of its water intake from dietary vegetation, as seen in most extant giraffes (e.g., Sponheimer and Lee-Thorpe, 1999; Cerling et al., 2003a, b; Schoeninger et al., 2003; Levin et al., 2008) and δ18Oenamel results from Maboko and Fort Ternan (Appendix 4). The lowest δ18Oenamel values among the Buluk fauna are found in the elephantimorph proboscideans, which suggests that these taxa had a higher reliance on standing water (Levin et al., 2008). Although Buluk’s depositional environment reflects a mature river system (Watkins, 1989), intra- and inter-specific variation in δ18Oenamel values may reflect multiple sources of drinking water with varying δ18O values across the landscape. It is important to note that, while δ18Oenamel values have been used to draw inferences about obligate vs. non-obligate drinking herbivores from Plio-Pleistocene fossil localities (e.g., Blumenthal et al., 2017), the lack of modern analogs for some Early and Middle Miocene taxa makes inferences about their drinking behaviors (i.e., obligate vs. non-obligate) more tentative.
Precipitation seasonality has been previously proposed to be a driver in catarrhine evolution, specifically a shift from low to high precipitation seasonality in the Late Miocene (e.g., Temerin and Cant, 1983; Andrews and Martin, 1991; Pickford, 1995). Evidence for Miocene climatic seasonality, however, is limited both for the Turkana Basin and for eastern Africa more broadly (Green et al., 2022). The range of herbivore δ18Oenamel values from Buluk (9.9‰, n=67) is relatively high when compared to the bulk δ18Oenamel values of herbivores from Kalodirr (range = 7.8‰, n=66), an Early Miocene (~17 Ma) site in West Turkana where primate molar serial oxygen profiles have yielded clear evidence of seasonal precipitation variation (Green et al., 2022). While differences in δ18Oenamel values between Kalodirr and Buluk may reflect differences in local hydrological dynamics (i.e., different sources of rainwater with different δ18Oenamel values) and/or taxonomic sampling, the greater range of oxygen values from Buluk this provisionally could indicate that Buluk, like Kalodirr, was characterized by strong wet/dry seasonality. The frequency and intensity of wet/dry seasons at Buluk is currently unknown but may be better understood by future serial sampling of enamel from primates and/or associated fauna.
Among the Buluk herbivores, the most positive δ13Cenamel values are from elephantimorph proboscideans (gomphotheres and mammutids). Elephantimorph δ13Cenamel values overlap with some deinothere samples, but deinotheres exhibit a significantly larger variance in δ13Cenamel values than elephantimorphs (F[18,12]=4.733, p=0.009), which extends into a more δ13C-depleted range than any other proboscidean sampled from Buluk. This greater range of variation is likely due to the larger sample size of deinothere teeth compared to other fauna (Figure 3), which captures more isotopic variation compared with the limited samples of available gomphothere and mammutid teeth. Despite these sample size limitations, it is also possible that elephantimorph proboscideans consumed vegetation or plant parts that were enriched in δ13C compared to deinotheres (Carlson and Kingston, 2014; Blumenthal et al., 2016), or that isotopic enrichment differs between these groups as a result of differences in digestive physiology, metabolism, and body size (Passey et al., 2005; Tejada-Lara et al., 2018; Cerling et al., 2021). Enamel microwear features indicate that Buluk P. hobleyi, Archaeobelodon sp. nov., and Zygolophodon sp. nov. taxa likely fed on tough, fibrous browse (Sanders et al., 2020). Mesowear angles for Archaeobelodon sp. nov. molars suggest browsing with some mixed feeding for this species (Saarinen and Lister, 2023). Mixed feeding among elephantimorphs that incorporated C3 browse or grasses enriched in 13C could be driving the higher δ13Cenamel values relative to the other proboscidean taxa sampled.
There is no clear pattern of oxygen isotopic partitioning among the Buluk proboscideans based on δ18Oenamel values. Deinothere and elephantimorph δ18Oenamel values overlap extensively between deinotheres, gomphotheres, and mammutids (Figure 3). Deinothere δ18O values are widely variable and overlap with rhinocerotids, tragulids, sanitheres, suids, and anthracotheres. This broad range of proboscidean δ18O values may be related to methodology (e.g., sampling different tooth positions and areas of the crown; Kohn et al., 1996; Malone et al., 2021) or dietary ecology (e.g., drinking water from across multiple sources or consuming food sources with widely varying δ18O values; Kohn et al., 1996; Carter and Bradbury, 2016).
Relatively depleted δ18Oenamel values from early Miocene anthracotheres at Kalodirr appear to corroborate previous inferences of an aquatic or semi-aquatic niche for this clade (Green et al., 2022), which was previously suggested based on the association of anthracothere skeletal remains with lacustrine sediments (Pickford, 1981, 1983). Extant semi-aquatic herbivores like the hippopotamus (Hippopotamus amphibius) have δ18Oenamel values lower than associated terrestrial fauna (Bocherens et al., 1996; Clementz et al., 2008). This technique has been applied to fossil anthracotheres from the Eocene and Oligocene of Egypt as well as the Oligocene of Germany to infer both semi-aquatic and terrestrial niches for members of this clade (Clementz et al., 2008; Liu et al., 2008; Tütken and Absolon, 2015). In contrast with Kalodirr specimens of Sivameryx africanus, the Buluk specimens of cf. Sivameryx africanus have relatively enriched δ18Oenamel values (Figure 3) that overlap with oxygen profiles of terrestrial herbivores from the same site (e.g., elephantimorphs, deinotheres, tragulids). One possible interpretation of these results is that cf. Sivameryx from Buluk was occupying a more terrestrial niche rather than the semi-aquatic one proposed for Sivameryx from Kalodirr.
Buluk preserves a diverse suoid community that includes the small-bodied selenodont sanithere Diamantohyus africanus, the small-bodied lophodont suid Lopholistriodon pickfordi and two bunodont kubanochoerine suids, cf. Kenyasus rusingensis and the much larger Megalochoerus marymuunguae (Pickford, 1983a; van der Made, 1996; Bishop, 2010). Only M. marymuunguae and D. africanus could be sampled for this analysis (Table 1), but variation in their carbon and oxygen isotopic values suggests possible niche partitioning among the Buluk suoids. Megalochoerus marymuunguae has lower δ13 Cenamel values than D. africanus, suggesting it fed on more 13C depleted vegetation (Carlson and Kingston, 2014; Blumenthal et al., 2016). Given the size difference between D. africanus and M. marymuunguae, variation in body mass-related digestive physiologies and metabolic processes could have contributed to these δ13Cenamel results (Passey et al., 2005; Tejada-Lara et al., 2018; Cerling et al., 2021).
The δ18Oenamel values of M. marymuunguae are enriched relative to most other herbivores at Buluk, including D. africanus, suggesting that this species was less water-dependent and received a larger portion of its water from dietary vegetation. These higher δ18Oenamel values could also be attributed to consuming foods enriched in δ18O, such as fruits from higher in the woody canopy or a large proportion of leafy browse (Carter and Bradbury, 2016; Roberts et al., 2017). Other species of large-bodied kubanochoerine suids from Early Miocene deposits on Rusinga Island (Garrett, 2016) and the Middle Miocene deposits on Maboko Island (Arney et al., 2022) exhibit a much lower δ18Oenamel signal relative to other fauna than M. marymuunguae from Buluk. At both Rusinga and Maboko Islands, kubanochoerine suids exhibit δ18Oenamel values that are more depleted than most other herbivore taxa (Garrett, 2016; Arney et al., 2022), which is ecologically similar to the depleted δ18Oenamel values of the extant, water-dependent giant forest hog (Hylochoerus meinertzhageni) and the red river hog (Potamochoerus porcus) (Harris and Cerling, 2002; Nelson, 2013; Cerling et al., 2015; Martin et al., 2015; Lazagabaster et al., 2021). The contrasting enriched δ18Oenamel values of M. marymuunguae suggest feeding and/or drinking behavior that is unlike these extant taxa and other fossil kubanochoerines from localities with closed forest elements in the local habitat.
Only one of the two tragulid species at Buluk, Dorcatherium pigotti, could be sampled in this study. While no specimens of the larger congeneric D. chappuisi from Buluk were available for sampling, these two species exhibit overlapping δ13Cenamel and δ18Oenamel values at the Early Miocene locality of Kalodirr (Butts, 2019; Green et al., 2022), but disparate carbon and oxygen values at the Middle Miocene site of Maboko (Arney et al., 2022). The δ13Cenamel values of D. pigotti from Buluk are enriched (mean δ13Cenamel value = -10.7‰) relative to the water chevrotain (Hyemoschus aquaticus), the only extant African tragulid species (mean δ13Cenamel value = -13.9‰, Cerling et al., 2004). Extant H. aquaticus is a nocturnal, forest-dwelling herbivore that primarily consumes fallen fruits, but also occasionally consumes insects and carrion (Dubost, 1987; Gautier-Hion et al., 1980; Hart, 1986). Dorcatherium pigotti from Buluk, along with the majority of Miocene tragulids from East Africa and Eurasia, appears to have had a wider range of browsing diets outside of forested habitats than the modern H. aquaticus (Nelson, 2007; Aiglstorfer et al., 2014).
Early and Middle Miocene Intersite Comparisons
Stable carbon isotope data from herbivore enamel is available from relatively few East African Miocene sites older than 9 Ma (Cerling et al., 1997; Arney et al., 2022; MacLatchy et a., 2023), yet the few published δ13Cenamel datasets have contributed substantially to our understanding of herbivore dietary isotope ecology within C3 -dominated habitats. The new δ13Cenamel data from Buluk can be compared with data from Moroto (21 Ma), Maboko (~16 Ma), and Fort Ternan (13.7 Ma) to help fill a gap in what is known about East African dietary paleoecology across the early to Middle Miocene transition (16-15 Ma) (Table 2).
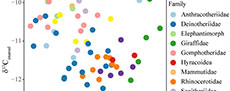
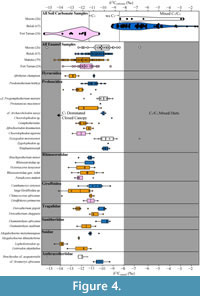 The bulk δ13Cenamel values from Buluk are not significantly different from the Early Miocene site Moroto (Figure 4; Table 4). Moroto’s paleoenvironment has been reconstructed as a woodland setting in which herbivores consumed primarily water-stressed C3 vegetation and potentially some C4 vegetation (MacLatchy et al., 2023). The lack of statistically significant differences between bulk δ13Cenamel values from Buluk and Moroto suggests that the landscape at Buluk likely also included open woodland elements with water-stressed C3 vegetation. Herbivores from Buluk, however, exhibit a narrower range of δ13Cenamel values than those from Moroto, and there is no isotopic evidence for any closed canopy forest elements nor of minor C4 (or CAM) dietary components, as indicated by the end members of the Moroto δ13Cenamel distribution (MacLatchy et al., 2023). While bulk δ13Cenamel distributions are influenced by the taxonomic sampling and overall sample size, the narrower range of the larger Buluk sample (n=67) compared to the broader range of the smaller Moroto samples (n=25) implies that the differences in δ13Cenamel range are likely not an artifact of sampling strategy.
The bulk δ13Cenamel values from Buluk are not significantly different from the Early Miocene site Moroto (Figure 4; Table 4). Moroto’s paleoenvironment has been reconstructed as a woodland setting in which herbivores consumed primarily water-stressed C3 vegetation and potentially some C4 vegetation (MacLatchy et al., 2023). The lack of statistically significant differences between bulk δ13Cenamel values from Buluk and Moroto suggests that the landscape at Buluk likely also included open woodland elements with water-stressed C3 vegetation. Herbivores from Buluk, however, exhibit a narrower range of δ13Cenamel values than those from Moroto, and there is no isotopic evidence for any closed canopy forest elements nor of minor C4 (or CAM) dietary components, as indicated by the end members of the Moroto δ13Cenamel distribution (MacLatchy et al., 2023). While bulk δ13Cenamel distributions are influenced by the taxonomic sampling and overall sample size, the narrower range of the larger Buluk sample (n=67) compared to the broader range of the smaller Moroto samples (n=25) implies that the differences in δ13Cenamel range are likely not an artifact of sampling strategy.
The δ13Cenamel values from Buluk and from Moroto are significantly more enriched than the two Middle Miocene sites, Maboko and Fort Ternan (Figure 4; Table 4). Middle Miocene deposits at Maboko include multiple time-successive beds, which have been reconstructed as primarily open forest/woodland mosaics without the presence of C4 biomass or water-stressed C3 vegetation (Arney et al., 2022). Fort Ternan, despite a long history of paleoenvironmental debate (Shipman et al., 1981; Pickford, 1983a, 1983b; Shipman, 1986; Retallack et al., 1990; Cerling et al., 1991; Kappleman, 1991; Retallak, 1992; Dugas and Retallack, 1993; Cerling et al., 1997), has yielded stable carbon isotope data from soil carbonates and herbivore enamel that indicates a relatively open canopy C3-dominated ecosystem without the presence of C4 biomass (Cerling et al., 1991; Cerling et al., 1997). Comparisons of the bulk δ13Cenamel distributions between these Middle Miocene sites and Buluk suggest that herbivores at Buluk foraged within more open-canopy, relatively drier habitats with greater proportions of water-stressed C3 vegetation than at Maboko or Fort Ternan. While multiple paleoecological proxies have demonstrated temporal and spatial habitat heterogeneity across East African Early Miocene localities (Peppe et al., 2023), landscape variation across East Africa during the Middle Miocene is less well known, due in large part to the small number of known Middle Miocene fossil localities, and it is difficult to say whether the differences in δ13Cenamel values reported here reflect any spatial and/or temporal trends across the Early-Middle Miocene transition.
A comparison of representative taxonomic groups (hyracoids, proboscideans, rhinocerotids, giraffoids, tragulids, suoids, and anthracotheres) reveals substantial differences in herbivore foraging behaviors between Early and Middle Miocene localities in Kenya (Figure 4). The δ13Cenamel values of Buluk taxa are higher than their Middle Miocene counterparts.
Specifically, comparisons of species that occur at both Buluk and Maboko (the deinothere P. hobleyi, the tragulid D. pigotti, and the hyracoid A. championi) demonstrate dietary differences that likely reflect environmental differences between these two sites. While the gomphothere P. macinnesi also occurs at both Buluk and Maboko, the single sample from Buluk limits comparisons that can be made for this taxon, although this specimen is more enriched than any P. macinnesi specimen from Maboko. All D. pigotti and A. championi δ13Cenamel values from Buluk are relatively enriched and do not overlap with the values of their conspecifics from Maboko (mean D. pigotti value is 2.1‰ higher; mean A. championi value is 2.0‰ higher). There is overlap among the δ13Cenamel distributions of P. hobleyi specimens from Buluk and Maboko, but the mean δ13Cenamel value of the Buluk specimens is relatively enriched. These results suggest that species co-occurring between Buluk and Maboko likely had flexible diets and consumed various proportions of dietary items (e.g., leaves, fruits, C3 grasses) depending on the local environment.
Additional comparisons at higher taxonomic levels broadly demonstrate greater δ13Cenamel enrichment at Buluk and Moroto compared to Maboko and Fort Ternan (Figure 4). Among elephantimorphs, both Eozygodon morotoensis from Moroto (which has the highest δ13Cenamel values of any elephantimorph in this sample) and the two taxa from Buluk demonstrate more enriched values than the Maboko and Fort Ternan specimens. Among suoids, M. marymuunguae and D. africanus from Buluk have higher δ13Cenamel values than their congeners (Megalochoerus khinzikebirus and Diamantohyus nadirum) from Maboko, indicating different dietary preferences and/or vegetation availability between these sites. The sole giraffoid from Buluk, C. sirtensis, exhibits slightly enriched δ13Cenamel values relative to Climacoceras africanus from Maboko but overlaps with the large Giraffoidea sp. from Maboko and Giraffokeryx primaevus from Fort Ternan. Rhinocerotidae is the only taxonomic group that demonstrates considerable overlap in δ13Cenamel values across Early and Middle Miocene sites. The extensive overlap between the rhinocerotid samples from Buluk, Maboko, and Fort Ternan suggests limited dietary variation among these species and possibly less flexible feeding ecology than other mammalian groups.
Of the taxonomic groups that overlap between Buluk and Moroto, only the anthracotheres show differences in δ13Cenamel values. The Moroto anthracothere, Brachyodus cf. aequatorialis, has depleted δ13Cenamel values relative to cf. Sivameryx africanus from Buluk. One interpretation of these results is that B. cf. aequatorialis at Moroto inhabited wetter, more densely vegetated habitats relative to cf. S. africanus at Buluk. This may be more indicative of differences in the behavior and biology of these taxa, which differ in size and dental morphology, rather than environmental differences between these sites. The low δ18Oenamel values of B. cf. aequatorialis relative to other Moroto herbivore δ18Oenamel values (Appendix 5; see also fig. S12 of MacLatchy et al., 2023) support the idea that this taxon might have been more water dependent than cf. S. africanus at Buluk. Brachyodus and Sivameryx have been suggested to be a hydrophilic taxon based on depositional and taphonomic settings at North African and some East African Miocene localities (Pickford, 1983; Holroyd et al., 2010; Miller et al., 2014), although cranial remains of Sivameryx africanus from the Early Miocene locality of Kalodirr have cast doubt on the proposal that this species was semi-aquatic (Rowan et al., 2015).
Prior to the publication of stable carbon isotope data from the Early Miocene in East Africa, variation in 13C among plants in C3-dominated ecosystems was presumed to be too limited for dietary inference or palaeoecological reconstruction (MacLatchy et al., 2023). Here, however, the collective ranges of dietary carbon signals from herbivore guilds at Moroto, Buluk, Maboko, and Fort Ternan document shifting foraging strategies across the Early and Middle Miocene. While caution should be used when using herbivore δ13Cenamel values for paleoenvironmental comparisons, especially when inferring differences in woody cover (Robinson et al., 2021), the considerable C3 -dominated dietary heterogeneity documented in these intersite results is consistent with independent lines of palaeoecological evidence for variable habitat cover and vegetation at these Early and Middle Miocene sites (e.g., Lukens et al., 2021; MacLatchy et al., 2023; Peppe et al., 2023).
East African Miocene Environments and Catarrhine Evolution
 Early models of the environmental context for early catarrhine evolution emphasized pervasive complex canopy cover across eastern Africa during the Early Miocene, eventually giving way to more fragmented woodlands in the late Early Miocene and the Middle Miocene (e.g., Andrews and Van Couvering, 1975; Andrews and Kelley, 2007; Couvreur et al., 2008). A more recent body of soil carbonate and plant wax isotopic datasets from multiple Early Miocene sites has indicated the presence of more eclectic habitats across this region, ranging from closed canopy forests to wooded grasslands with arid conditions that could support substantial C4 plant components (Lukens et al., 2017a; Liutkus-Pierce et al., 2018; MacLatchy et al., 2023; Peppe et al., 2023). Buluk, along with the Karungu, Moroto, and Napak have been identified as some of the sites with C4 isotopic biomass signatures from pedogenic carbonates, and the presence of PACMAD (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristioideae, Danthonoideae) phytoliths at six Early Miocene sites suggests the presence of potentially C4 grasses on the landscape (Peppe et al., 2023) (Figure 5A, B).
Early models of the environmental context for early catarrhine evolution emphasized pervasive complex canopy cover across eastern Africa during the Early Miocene, eventually giving way to more fragmented woodlands in the late Early Miocene and the Middle Miocene (e.g., Andrews and Van Couvering, 1975; Andrews and Kelley, 2007; Couvreur et al., 2008). A more recent body of soil carbonate and plant wax isotopic datasets from multiple Early Miocene sites has indicated the presence of more eclectic habitats across this region, ranging from closed canopy forests to wooded grasslands with arid conditions that could support substantial C4 plant components (Lukens et al., 2017a; Liutkus-Pierce et al., 2018; MacLatchy et al., 2023; Peppe et al., 2023). Buluk, along with the Karungu, Moroto, and Napak have been identified as some of the sites with C4 isotopic biomass signatures from pedogenic carbonates, and the presence of PACMAD (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristioideae, Danthonoideae) phytoliths at six Early Miocene sites suggests the presence of potentially C4 grasses on the landscape (Peppe et al., 2023) (Figure 5A, B).
In contrast, evidence from herbivore δ13Cenamel values reveals the persistence of pure C3 or C3 -dominated diets during this time period (Figure 5C). The C3-dominated diets of herbivores at Buluk, as well as Moroto, contrast with the considerable C4 signal derived from paleosol carbonates, suggesting that these C4 plants were not consumed in sufficient quantities to be reflected in the δ13Cenamel values of the taxa sampled here. The nature of the opportunistic sampling strategy allows for the possibility that one or more of the taxa that were not sampled may have consumed some amount of C4 vegetation. That is, sampling biases from the availability of dental remains among both the Buluk and Moroto collections could be underestimating the total range of herbivore δ13Cenamel values, particularly at the higher end of the range.
Differences in temporal scale between isotopic proxies may also help explain the discrepancy between paleosol and herbivore enamel isotope results. Enamel isotopes reflect vegetation consumed over a shorter duration of time (months to years), while soil isotopes reflect a local vegetation signal that is averaged over thousands of years (Koch, 1998). The mobility of herbivores also suggests that δ13Cenamel values reflect vegetation from a wider geographic area than paleosol carbonates, and the discrepancy between the two proxies is possible if herbivores foraged on C3 resources in more distant areas from where carbonate nodules were collected (Du et al., 2019). As a result, enamel isotopes likely capture a short-term phase of vegetation and a wider area of foraging where C4 plants were not abundant or utilized, while carbonates capture long-term, seasonally drier intervals in a more localized area that would have favored spikes in C4 plants and the formation of soil carbonates (Peppe et al., 2023). In a mature river system, like the one interpreted for Buluk, it is also possible that the pedogenic carbonates might not have recorded a C3 endmember signal since the time of carbonate formation exceeds that of the channel position with its C3 vegetation (Levin et al., 2004). In other words, pedogenic carbonate forming beneath a riverbank and its riparian woodland would have migrated further distally or eroded by a change in the river channel position. Additionally, it is also possible that the presence of diagenesis in enamel and/or pedogenic carbonate samples could be influencing this incongruity.
Beginning in the late Oligocene and Early Miocene, tectonic activity in eastern Africa (e.g., rift valley formation, volcanism, uplift of the East African Plateau) is believed to have been a force shaping regional climatic variation and local scale habitat heterogeneity across eastern Africa (Sepulchre et al., 2006; Wichura et al., 2015; Linder, 2017; Peppe et al., 2023; Munday et al., 2023). The accumulation of multi-proxy paleoenvironmental data documenting extensive habitat heterogeneity across East Africa during the Early and Middle Miocene (Michel et al., 2014, 2020; Driese et al., 2016; Lukens et al., 2017; Liutkus-Pierce et al., 2019; Baumgartner and Peppe, 2021; Maclatchy et al., 2023; Peppe et al., 2023) has complicated previous adaptive models of catarrhine evolution that posited the emergence of hominoid diet and locomotion within widespread forest environments (e.g., Napier, 1967; Temerin and Cant, 1983; Hunt, 2016). Concomitant with this emerging picture of eastern African habitat heterogeneity is a growing body of evidence for a wide array of locomotor and dietary diversity among Early and Middle Miocene catarrhines (e.g., Wuthrich et al. 2019; Nishimura et al., 2022; MacLatchy et al., 2023). The Buluk herbivore enamel isotope results presented here provide additional evidence documenting the presence of open canopy woodland habitats inhabited by Early Miocene catarrhines. Additionally, the extent of niche separation among Buluk herbivores, as well as the separation between conspecific and congeneric taxa at Buluk and Maboko demonstrates that Early and Middle Miocene herbivores utilized complex and variable dietary behaviors on this heterogenous East African landscape,and the primates may have done the same.
CONCLUSIONS
Buluk is a terminal Early Miocene locality that preserves a rich mammalian fauna including a diversity of fossil catarrhines. Herbivore δ13Cenamel values indicate that Buluk mammals lived and foraged in an arid, C3 -dominated, open canopy woodland environment, as indicated by the tightly constrained range of δ13Cenamel values (-12.7‰ to -9.4‰). Herbivores at Buluk had C3 -dominated diets with no evidence for consumption of C4 resources, despite their presence on the landscape (Peppe et al., 2023). Taxa with the lowest δ13Cenamel values include rhinocerotids and deinotheres, while the highest values are among elephantimorph proboscideans and sanitheriid suoids. The relatively high δ18Oenamel range among Buluk herbivores may reflect extensive fluctuation in δ18O values, which may be a consequence of precipitation seasonality, multiple local water sources, or sampling several water sources during seasonal migration. Results of serial oxygen isotopic analyses of herbivore teeth would test for seasonality (e.g., Green et al., 2022), while comparisons of Buluk herbivore strontium isotopic ratios with bioavailable 87Sr/86Sr could potentially test for herbivore mobility (Janzen et al., 2020).
Comparisons with δ13Cenamel data from fauna at the Early Miocene site Moroto and the Middle Miocene sites Maboko and Fort Ternan indicate a shift from more enriched signals in the Early Miocene to more depleted ones in the Middle Miocene. The similarity between the Moroto and Buluk δ13Cenamel value distributions suggests the dominance of water-stressed C3 vegetation at these sites, although the broader range of values from Moroto implies greater habitat variability, including the presence of some closed-canopy elements and the consumption of limited C4 grasses, which are not documented at Buluk. Stable carbon isotope data have previously affirmed the similarity in paleoenvironments between the Middle Miocene sites of Maboko and Fort Ternan (Arney et al., 2022). Compared to the herbivores from these Middle Miocene sites, herbivores from Buluk foraged in a more open canopy, water-stressed ecosystem. Shared taxa between Buluk and Maboko (P. hobleyi, A. championi, and D. pigotti) have δ13Cenamel values that reflect consistently 13C enriched diets at Buluk. These findings are consistent with recent reconstructions of a subhumid woodland environment at Buluk based on geochemical data (Lukens et al., 2017b, 2021) and contribute to the growing body of evidence for environmental and dietary heterogeneity across space and time in the East African Miocene (Peppe et al., 2023).
Despite the growing body of geochemical and palaeobotanical evidence for C4 biomass in eastern Africa during the Early and Middle Miocene (Kingston et al., 1994; Morgan et al., 1994; Lukens et al., 2017a; MacLatchy et al., 2023; Peppe et al., 2023), δ13Cenamel data from Buluk provides no evidence of a C4 dietary signal among mammalian herbivores. The absence of a C4 component in herbivore diets could reflect the differences in temporal scale captured by stable isotopes from tooth enamel and pedogenic carbonates. It may be that the C4 biomass present at Buluk during the lifespan of herbivores sampled was in low enough proportion that it was not a preferred dietary item for herbivorous mammals, or they consumed this material in quantities too low to impact bulk enamel δ13C values.
Future research could expand the understanding of individual dietary variability and dietary variation across herbivore guilds in the C3 -dominated ecosystem at Buluk by applying additional dietary proxies (e.g., mesowear, microwear) and serial isotopic sampling of herbivore molars. To further assess shifting diets and environments across the Early-Middle Miocene transition, more targeted sampling of additional Buluk taxa that persisted into the Middle Miocene (e.g., Dorcatherium chappuisi, Lopholistriodon pickfordi, cf. Kenyasus rusingensis, and cf. Propaleoryx nyanzae; see Table 1) should be prioritized if additional dental specimens are recovered.
ACKNOWLEDGEMENTS
Funding for this work was provided by the Leakey Foundation, the Turkana Basin Institute, National Geographic, and the Foothill De-Anza Foundation. We would like to thank the Kenyan National Commission for Science, Technology, and Innovation (NACOSTI) for permission to conduct this research. We would also like to thank the Department of Earth Sciences at the National Museums of Kenya and the Turkana Basin Institute for access to fossil specimens and permission for sampling. Thanks to B. Sanders for identifying the proboscideans fossils from Buluk and to J. Kingston, N. Levin, and L. MacLatchy for guidance and feedback during the project development phase. We are grateful for comments from K. Uno and one anonymous reviewer. Finally, many thanks to the Buluk field crew without whom this work would not have been possible.
REFERENCES
Aiglstorfer, M., Bocherens, H., and Böhme, M. 2014. Large mammal ecology in the late middle Miocene gratkorn locality (Austria). Palaeobiodiversity and Palaeoenvironments, 94:189–213.
https://doi.org/10.1007/s12549-013-0145-5
Andrews, P. and Kelley, J. 2007. Middle Miocene dispersals of apes. Folia primatologica, 78:328–343. https://doi.org/10.1159/000105148
Andrews, P. and Martin, L. 1991. Hominoid dietary evolution. Philosophical Transactions of the Royal Society, 334:199–209.
https://doi.org/10.1098/rstb.1991.0109
Andrews, P. and VanCouvering, J.A. 1975. Palaeoenvironments in the East African Miocene, p. 62–103. In Szalay, F.S. (ed.), Approaches to Primate Paleobiology, Contributions to Primateology, Volume 5. Karger.
Andrews, P., Lord, J.M., and Nesbit Evans, E.M. 1979. Patterns of ecological diversity in fossil and modern mammalian faunas. Biological Journal of the Linnean Society, 11:177–205.
https://doi.org/10.1111/j.1095-8312.1979.tb00034.x
Andrews, P., Meyer, G.E., Pilbeam, D.R., Van Couvering, J.A., and Van Couvering, J.A.H. 1981. The Miocene fossil beds of Maboko Island, Kenya: Geology, age, taphonomy and palaeontology. Journal of Human Evolution, 10:35–48.
https://doi.org/10.1016/S0047-2484(81)80024-3
Arney, I., Benefit, B.R., McCrossin, M.L., MacLatcy, L., and Kingston, J.D. 2022. Herbivore isotopic dietary ecology of the middle Miocene Maboko Formation, Kenya. Palaeogeography, Palaeoclimatology, Palaeoecology, 601:111061.
https://doi.org/10.1016/j.palaeo.2022.111061
Baumgartner, A. and Peppe, D.J. 2021. Paleoenvironmental changes in the Hiwegi Formation (lower Miocene) of Rusinga Island, Lake Victoria, Kenya. Palaeogeography, Palaeoclimatology, Palaeoecology, 574:110458.
https://doi.org/10.1016/j.palaeo.2021.110458
Benefit, B. 1999. Victoriapithecus: the key to Old World monkey and catarrhine origins. Evolutionary Anthropology, 7:155–174.
https://doi.org/10.1002/(SICI)1520-6505(1999)7:5<155::AID-EVAN2>3.0.CO;2-D
Blumenthal, S.A., Rothman, J.M., Chritz, K.L., and Cerling, T.E. 2016. Stable isotopic variation in tropical forest plants for applications in primatology. Journal of Primatology, 78:1041–1054.
https://doi.org/10.1002/ajp.22488
Blumenthal, S.A., Levin, N.E., Brown, F.H., Brugal, J.-P., Chritz, K.L., Harris, J.M., Jehle, G.E., and Cerling, T.E. 2017. Aridity and hominin environments. Proceedings from the National Academy of Science, 114:7331–7336.
https://doi.org/10.1073/pnas.1700597114
Bocherens, H., Koch, P.L., Mariotti, A., Geraads, D., and Jaeger, J.J. 1996. Isotopic Biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene Hominid Sites. Palaios, 11:306–318.
https://doi.org/10.2307/3515241
Bonnefille, R. 1984. Cenozoic vegetation and environments of early hominids in East Africa. The Evolution of the East Asian Environment, 2:579–612.
Bonnefille, R. 2010. Cenozoic vegetation, climate changes and hominid evolution in tropical Africa. Global and Planetary Change, 72:390–411.
https://doi.org/10.1016/J.GLOPLACHA.2010.01.015
Bryant, D.J. and Froelich, P.N. 1995. A model of oxygen isotope fractionation in body water of large mammals. Geochimica et Cosmochimica Acta, 59:4523–4537.
Butts, C.F.R. 2019. Paleoenvironmental Reconstruction of Kalodirr and Moruorot, Kenya using Stable Carbon Isotopes. Unpublished Masters Thesis, University of Calgary, Calgary, AB, Canada.
Carlson, B.A. and Kingston, J.D. 2014. Chimpanzee isotopic ecology: A closed canopy C3 template for hominin dietary reconstruction. Journal of Human Evolution, 76:107–115.
https://doi.org/10.1016/J.JHEVOL.2014.06.001
Carter, M.L. and Bradbury, M.W. 2016. Oxygen isotope ratios in primate bone carbonate reflect amount of leaves and vertical stratification in the diet. American Journal of Primatology, 78:1086–1097.
https://doi.org/10.1002/AJP.22432
Cerling, T.E. and Harris, J.M. 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia, 120:347–363.
https://doi.org/10.1007/s004420050868
Cerling, T.E., Quade, J., Ambrose, S.H., and Sikes, N.E. 1991. Fossil soils, grasses, and carbon isotopes from Fort Ternan, Kenya: grassland or woodland? Journal of Human Evolution, 21:295–306.
https://doi.org/10.1016/0047-2484(91)90110-H
Cerling, T.E., Harris, J.M., Ambrose, S.H., Leakey, M.G., and Solounias, N. 1997. Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. Journal of Human Evolution, 33:635–650.
https://doi.org/10.1006/jhev.1997.0151
Cerling, T.E., Harris, J.M., and Leakey, M.G. 1999. Browsing and grazing in elephants: the isotope record of modern and fossil proboscideans. Oecologia, 120:364–374.
https://doi.org/10.1007/s004420050869
Cerling, T.E., Harris, J.M., Leakey, M.G. and Mudida, N. 2003a. Stable isotope ecology of northern Kenya, with emphasis on the Turkana Basin, p. 583–603. In Leakey, M.G., Harris J.M., (eds.), Lothagam: the dawn of humanity in eastern Africa. New York: Columbia University Press.
Cerling, T.E., Harris, J.M. and Leakey, M.G. 2003b. Isotope Paleoecology of the Nawata and Nachukui Formations at Lothagam, Turkana Basin, Kenya, p. 605–624. In Leakey, M.G. and Harris J.M., (eds.), Lothagam: The Dawn of Humanity in Eastern Africa.
Cerling, T.E., Hart, J.A., and Hart, T.B. 2004. Stable isotope ecology in the Ituri Forest. Oecologia, 138:5–12.
https://doi.org/10.1007/s00442-003-1375-4
Cerling, T.E., Andanje, S.A., Blumenthal, S.A., Brown, F.H., Chritz, K.L., Harris, J.M., Hart, J.A., Kirera, F.M., Kaleme, P., Leakey, L.N., Leakey, M.G., Levin, N.E., Manthi, F.K., Passey, B.H., and Uno, K.T. 2015. Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 Ma. Proceedings from the National Academy of Science, 112:11467–11472.
https://doi.org/10.1073/pnas.1513075112
Cerling, T.E., Bernasconi, S.M., Hofstetter, L.S., Jaggi, M., Wyss, F., Rudolf von Rohr, C., and Clauss, M. 2021. CH4 /CO2 Ratios and carbon isotope enrichment between diet and breath in herbivorous mammals. Frontiers in Ecology and Evolution, 9:638568.
https://doi.org/10.3389/fevo.2021.638568
Clementz, M.T., Holroyd, P.A., and Koch, P.L. 2008. Identifying aquatic habits of herbivorous mammals through stable isotope analysis. Palaios, 23:574–585.
https://doi.org/10.2110/palo.2007.p07-054r
Cote, S., Kingston, J., Deino, A., Winkler, A., Kityo, R., and MacLatchy, L. 2018. Evidence for rapid faunal change in the early Miocene of East Africa based on revised biostratigraphic and radiometric dating of Bukwa, Uganda. Journal of Human Evolution, 116:95–107.
https://doi.org/10.1016/j.jhevol.2017.12.001
Couvreur, T.L.P., Chatrou, L.W., Sosef, M.S.M., and Richardson, J.E. 2008. Molecular phylogenetics reveal multiple tertiary vicariance origins of the African rain forest trees. BMC Biology, 6:54.
https://doi.org/10.1186/1741-7007-6-54
Dansgaard, W. 1964. Stable isotopes in precipitation. Tellus, 16:436–468.
https://doi.org/10.3402/tellusa.v16i4.8993
Davis, S.J.M. 1980. A note on the dental and skeletal ontogeny of Gazella. Israel Journal of Zoology, 29:129–134.
Driese, S.G., Peppe, D.J., Beverly, E.J., DiPietro, L.M., Arellano, L.N., and Lehmann, T. 2016. Paleosols and paleoenvironments of the early Miocene deposits near Karungu, Lake Victoria, Kenya. Palaeogeography, Palaeoclimatology, Palaeoecology, 443:167–182.
https://doi.org/10.1016/j.palaeo.2015.11.030
Du, A., Rowan, J., Lazagabaster, I.A., Behrensmeyer, A.K., and Robinson, J.R. 2019. Stable carbon isotopes from paleosol carbonate and herbivore enamel document differing paleovegetation signals in the eastern African Plio-Pleistocene. Review of Palaeobotany and Palynology, 261:41–52.
https://doi.org/10.1016/j.revpalbo.2018.11.003
Dugas, D.P. and Retallack, G.J. 1993. Middle Miocene fossil grasses from Fort Ternan. Journal of Paleontology, 67:113–128.
https://doi.org/10.1017/S0022336000021223
Ehleringer, J.R., Field, C.B., Lin, Z., and Kuo, C. 1986. Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia, 70:520–526.
https://doi.org/10.1007/BF00379898
Fannin, L.D. and McGraw, W.S. 2020. Does oxygen stable isotope composition in primates vary as a function of vertical stratification or folivorous behaviour? Folia Primatologica, 91:219–227.
https://doi.org/10.1159/000502417
Feakins, S.J., Levin, N.E., Liddy, H.M., Sieracki, A., Eglinton, T.I., and Bonnefille, R. 2013. Northeast African vegetation change over 12 m.y. Geology, 41:295–298.
https://doi.org/10.1130/G33845.1
Feibel, C.S. and Brown, F.H. 1991. Age of the primate-bearing deposits on Maboko Island, Kenya. Journal of Human Evolution, 21:221–225.
https://doi.org/10.1016/0047-2484(91)90063-2
Francey, R.J., Allison, C.E., Etheridge, D.M., Trudinger, C.M., Enting, I.G., Leuenberger, M., Langenfelds, R.L., Michel, E., and Steele, L.P. 1999. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus, 51:170–193.
https://doi.org/10.1034/j.1600-0889.1999.t01-1-00005.x
Fricke, H.C. and O’Neil, J.R. 1996. Inter-and intra-tooth variation in the oxygen isotope composition of mammalian tooth enamel phosphate: implications for palaeoclimatological and palaeobiological research. Palaeogeography, Palaeoclimatology, Palaeoecology, 126:91–99.
https://doi.org/10.1016/S0031-0182(96)00072-7
Fricke, J.R., Clyde, W.C., and O’Neil, J.R. 1998. Intra-tooth variations in δ18O (PO4) of 839 mammalian tooth enamel as a record of seasonal variations in continental climate 840 variables. Geochimica et Cosmochimica Acta, 62:1839–1850.
https://doi.org/10.1016/S0016-7037(98)00114-8
Garrett, N. 2016. Stable isotope paleoenvironmental reconstructions of the Late Pleistocene and Early Miocene sites on Rusinga and Mfangano Islands, Lake Victoria, Kenya. Unpublished Ph.D. Dissertation, University of Minnesota, Minneapolis, MN, USA.
Gautier-Hion, A., Emmons, L.H, and Dubost, G. 1980. A comparison of the diets of three major groups of primary consumers of Gabon (primates, squirrels and ruminants). Oecologia, 45:182–189.
https://doi.org/10.1007/BF00346458
Geraads, D. and Miller, E. 2013. Brachypotherium minor n. sp., and other Rhinocerotidae from the Early Miocene of Buluk, Northern Kenya. Geodiversitas, 35:359–375.
https://doi.org/10.5252/g2013n2a5
Green, D.R., Olack, G., and Colman, A.S. 2018. Determinants of blood water δ18O variation in a population of experimental sheep: Implications for paleoclimate reconstruction. Chemical Geology, 485:32–43.
https://doi.org/10.1016/J.CHEMGEO.2018.03.034
Green, D.R., Ávila, J.N., Cote, S., Dirks, W., Lee, D., Poulsen, C.J., Williams, I.S., and Smith, T.M. 2022. Fine-scaled climate variation in equatorial Africa revealed by modern and fossil primate teeth. Proceedings of the National Academy of Sciences, 119:e2123366119.
https://doi.org/10.1073/pnas.2123366119
Grossman, A. 2008. Ecological and morphological diversity in catarrhine primates from the Miocene of Africa. Unpublished Ph.D. Dissertation, Stony Brook University, Stony Brook, New York, USA.
Hall, A.S. and Cote, S. 2021. Ruminant mesowear reveals consistently browse-dominated diets throughout the early and middle Miocene of eastern Africa. Palaeogeography, Palaeoclimatology, Palaeoecology, 567:110253.
https://doi.org/10.1016/j.palaeo.2021.110253
Harris, J.M. and Cerling, T.E. 2002. Dietary adaptations of extant and Neogene African suids. Journal of Zoology, 256:45–54.
https://doi.org/10.1017/S0952836902000067
Harris, J.M. and Watkins, R. 1974. New early Miocene vertebrate locality near Lake Rudolf, Kenya. Nature, 252:576–577.
https://doi.org/10.1038/252576a0
Hart, J. 1986. Comparative dietary ecology of a community of frugivorous forest ungulates in Zaire (ruminants, feeding habits, rainforest). PhD Dissertation, Michigan State University, East Lansing, MI, USA.
Heaton, T.H.E. 1999. Spatial, species, and temporal variations in the 13C/12C ratios of C3 plants: implications for paleodiet studies. Journal of Archaeological Sciences, 26:637–649. https://doi.org/10.1006/jasc.1998.0381
Higgins, P. and MacFadden, B.J. 2004. “‘Amount Effect’” recorded in oxygen isotopes of Late Glacial horse (Equus) and bison (Bison) teeth from the Sonoran and Chihuahuan deserts, southwestern United States. Palaeogeography, Palaeoclimatology, Palaeoecology, 206:337–353. https://doi.org/10.1016/j.palaeo.2004.01.011
Hillman-Smith, A.K.K., Anderson, J.L., Hall-Martin, A.J., and Selaladi, D.J.P. 1986. Age estimation of the White rhinoceros (Ceratotherium simum). Journal of Zoology, 210:355–379. https://doi.org/10.1111/j.1469-7998.1986.tb03639.x
Holroyd, P.A., Lihoreau, F., Gunnell, G.F., and Miller, E. 2010. Anthracotheriidae, p. 851–859. In Werdelin, L. and Sanders, W. (eds.), Cenozoic Mammals of Africa. University of California Press, Berkeley, California.
Hunt, K.D. 2016. Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. Journal of Anatomy, 228:630–685.
https://doi.org/10.1111/joa.12454
Hurford, A.J. and Watkins, R.T. 1987. Fission-track age of the tuffs of the Buluk member, Bakate formation, Northern Kenya: A suitable fission-track age standard. Chemical Geology: Isotope Geoscience section, 66:209–216.
https://doi.org/10.1016/0168-9622(87)90042-X
Janzen, A., Bataille, C., Copeland, S.R., Quinn, R.L., Ambrose, S.H., Reed, D., Hamilton, M., Grimes, V., Richards, M.P., le Roux, P., and Roberts, P. 2020. Spatial variation in bioavailable strontium isotope ratios (87Sr/86Sr) in Kenya and northern Tanzania: Implications for ecology, paleoanthropology, and archaeology. Palaeogeography, Palaeoclimatology, Palaeoecology, 560: 109957.
https://doi.org/10.1016/j.palaeo.2020.109957
Kappleman, J. 1991. The paleoenvironment of Kenyapithecus at Fort Ternan. Journal of Human Evolution, 20:95–129.
https://doi.org/10.1016/0047-2484(91)90053-X
Kingston, J.D. and Harrison, T. 2007. Isotopic dietary reconstructions of Pliocene herbivores at Laetoli: Implications for early hominin paleoecology. Palaeogeography, Palaeoclimatology, Palaeoecology, 243:272–306.
https://doi.org/10.1016/j.palaeo.2006.08.002
Kingston, J.D., Hill, A., and Marino, B.D. 1994. Isotopic evidence for Neogene hominid paleoenvironments in the Kenyan Rift Valley. Science, 264:955–959.
https://doi.org/10.1126/science.264.5161.955
Koch, P.L. 1998. Isotopic reconstruction of past continental environments. Annual Review of Earth and Planetary Sciences, 26:573–613.
https://doi.org/10.1146/annurev.earth.26.1.573
Kohn, M.J. 1996. Predicting animal δ18O: Accounting for diet and physiological adaptation. Geochimica et Cosmochimica Acta, 60:4811–4829.
https://doi.org/10.1016/S0016-7037(96)00240-2
Kohn, M.J. 2010. Carbon isotope compositions of terrestrial C3 plants as indicators of paleoecology and paleoclimate. Proceedings of the National Academy of Sciences, 46:19691–19695.
https://doi.org/10.1073/pnas.1004933107
Kohn M.J. and Law, J.M. 2006. Stable isotope chemistry of fossil bone as a new paleoclimate indicator. Geochimica et Cosmochimica Acta, 70:931–946.
https://doi.org/10.1016/j.gca.2005.10.023
Kohn, M.J., Schoeninger, M.J., and Valley, J.W. 1996. Herbivore tooth oxygen isotope compositions: Effects of diet and physiology. Geochimica et Cosmochimica Acta, 60:3889–3896.
https://doi.org/10.1016/0016-7037(96)00248-7
Kohn, M.J., Schoeninger, M.J., and Valley, J.W. 1998. Variability in oxygen isotope compositions of herbivore teeth: reflections of seasonality or developmental physiology. Chemical Geology, 152:97–112.
https://doi.org/10.1016/S0009-2541(98)00099-0
Krigbaum, J., Berger, M.H., Daegling, D.J., and McGraw, W.S. 2013. Stable isotope canopy effects for sympatric monkeys at Taï Forest, Côte d’Ivoire. Biology Letters, 9:20130466.
https://doi.org/10.1098/rsbl.2013.0466
Lazagabaster, I.A., Cerling, T.E., and Faith, J.T. 2021. A Late Pleistocene third molar of Hylochoerus (Suidae, Mammalia) from Rusinga Island, Kenya: paleoenvironmental implications and a note on the hypsodonty of African forest hogs. Historical Biology, 33:3673–3685.
https://doi.org/10.1080/08912963.2021.1887861
Leakey, M. 1985. Early Miocene cercopithecids from Buluk, northern Kenya. Folia Primatologica, 44:1–14.
https://doi.org/10.1159/000156194
Leakey, M. and Walker, A. 1997. Afropithecus, p. 225–239. In Begun, D.R., Ward, C.V., and Rose, M.D. (eds.), Function, Phylogeny, and Fossils. Advances in Primatology. Springer, Boston, MA.
https://doi.org/10.1007/978-1-4899-0075-3_11
Leakey, M., Grossman, A., Gutierrez, M., and Fleagle, J.G. 2011. Faunal change in the Turkana Basin during the Late Oligocene and Miocene. Evolutionary Anthropology, 20:238–253.
Leakey, R.E.F. and Walker, A. 1985. New higher primates from the early Miocene of Buluk, Kenya. Nature, 318:173–175.
https://doi.org/10.1038/318173a0
Lee-Thorp, J.A. and van der Merwe, N.J. 1991. Aspects of the chemistry of modern and fossil biological apatites. Journal of Archaeological Science, 18:343–354.
https://doi.org/10.1016/0305-4403(91)90070-6
Lee-Thorp, J.A., Sealy, J.C., and van der Merwe, N.J. 1989. Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. Journal of Archaeological Science, 16:585–599.
https://doi.org/10.1016/0305-4403(89)90024-1
Levin, N.E., Quade, J., Simpson, S.W., Semaw, S., and Rogers, M. 2004. Isotopic evidence for Plio-Pleistocene environmental change at Gona, Ethiopia. Earth and Planetary Science Letters, 219:93–110.
https://doi.org/10.1016/S0012-821X(03)00707-6
Levin, N.E., Cerling, T.E., Passey, B.H., Harris, J.M., and Ehleringer, J.R. 2006. A stable isotope aridity index for terrestrial environments. Proceedings of the National Academy of Sciences of the United States of America, 103:11201–11205.
https://doi.org/10.1073/pnas.0604719103
Levin, N.E., Simpson, S.W., Quade, J., Cerling, T.E., and Frost, S.R. 2008. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. In Quade, J. and Wynn, J.G. (eds.), The Geology of Early Humans in the Horn of Africa (Geological Society of America), 446:215–234.
https://doi.org/10.1130/2008.2446(10)
Linder, H.P. 2017. East African Cenozoic vegetation history. Evolutionary Anthropology: Issues, News, and Reviews, 26:300–312.
https://doi.org/10.1002/evan.21570
Liu, A.G.S.C., Seiffert, E.R., and Simons, E.L. 2008. Stable isotope evidence for an amphibious phase in early proboscidean evolution. Proceedings of the National Academy of Sciences, 105:5786–5791.
https://doi.org/10.1073/pnas.0800884105
Liutkus-Pierce, C.M., Takashita-Bynum, K.K., Beane, L.A., Edwards, C.T., Burns, O.E., Mana, S., Hemming, S., Grossman, A., Wright, J.D., and Kirera, F.M. 2019. Reconstruction of the Early Miocene Critical Zone at Loperot, Southwestern Turkana, Kenya. Frontiers in Ecology and Evolution, 7:44.
https://doi.org/10.3389/fevo.2019.00044
Locke, E.M., Benefit, B.R., Kimock, C.M., Miller, E.R., and Nengo, I. 2020. New dentognathic fossils of Noropithecus bulukensis (Primates, Victoriapithecidae) from the late Early Miocene of Buluk, Kenya. Journal of Human Evolution, 148:102886.
https://doi.org/10.1016/j.jhevol.2020.102886
Lukens, W.E., Lehmann, T., Peppe, D.J., Fox, D.L., Driese, S.G., and McNulty, K.P. 2017a. The Early Miocene Critical Zone at Karungu, Western Kenya: an equatorial, open habitat with few primate remains. Frontiers in Earth Science, 5:87.
https://doi.org/10.3389/feart.2017.00087
Lukens, W.E., Peppe, D.J., Locke, E., Miller, E., Deino, A.L., and Oginga, K.O. 2017b. Paleoenvironments and mammalian fauna of the early Miocene fossil site at Buluk, Kenya. American Journal of Physical Anthropology, 162(S64):268–269.
Lukens, W., Peppe, D., Deino, A.L., Fox, D.L., Beverly, E., and Miller, E. 2021. A woodland-savanna mosaic in the early middle Miocene at Buluk, Turkana Basin, Kenya. Geological Society of America Abstracts with Programs, 53:6.
https://doi.org/10.1130/abs/2021AM-363879
Luyt, J., Hare, V.J., and Sealy, J. 2019. The relationship of ungulate δ13C and environment in the temperate biome of southern Africa, and its palaeoclimatic application. Palaeogeography, Palaeoclimatology, Palaeoecology, 514:282–291.
https://doi.org/10.1016/j.palaeo.2018.10.016
MacLatchy, L.M., Cote, S.M., Deino, A.L., Kityo, R.M., Mugume, A.A., Rossie, J.B., Sanders, W.J., Cosman, M.N., Driese, S.G., Fox, D.L., Freeman, A.J., Jansma, R.J.W., Jenkins, K.E.H., Kinyanjui, R.N., Lukens, W.E., McNulty, K.P., Novello, A., Peppe, D.J., Strömberg, C.A.E., Uno, K.T., Winkler, A.J., and Kingston, J.D. 2023. The evolution of hominoid locomotor versatility: evidence from Moroto, a 21 Ma site in Uganda. Science, 380:eabq2835.
https://doi.org/10.1126/science.abq2835
Malone, M.A., MacLatchy, L.M., Mitani, J.C., Kityo, R., and Kingston, J.D. 2021. A chimpanzee enamel-diet δ13C enrichment factor and a refined enamel sampling strategy: Implications for dietary reconstructions. Journal of Human Evolution, 159:103062.
https://doi.org/10.1016/J.JHEVOL.2021.103062
Martin, J.E., Vance, D., and Balter, V. 2015. Magnesium stable isotope ecology using mammal tooth enamel. Proceedings of the National Academy of Sciences, 112:430–435.
https://doi.org/10.1073/pnas.1417792112
Mayr, A. and Mayr, G. 2014. A hoatzin fossil from the middle Miocene of Kenya documents the past occurrence of modern-type Opisthocomiformes in Africa. The Auk: Ornithological Advances,131:55–60.
https://doi.org/10.1642/AUK-13-134.1
Mayr, G. 2014. On the Middle Miocene avifauna of Maboko Island, Kenya. Geobios, 47:133–146.
https://doi.org/10.1016/j.geobios.2014.03.001
McDougall, I. and Watkins, R.T. 1985. Age of hominoid-bearing sequence at Buluk, northern Kenya. Nature, 318:175–178.
https://doi.org/10.1038/318175a0
Michel, L.A., Peppe, D.J., Lutz, J.A., Driese, S.G., Dunsworth, H.M., Harcourt-Smith, W.E.H., Horner, W.H., Lehmann, T., Nightingale, S., and McNulty, K.P. 2014. Remnants of an ancient forest provide ecological context for Early Miocene fossil apes. Nature Communications, 5:611–614.
https://doi.org/10.1038/ncomms4236
Michel, L.A., Lehmann, T., Mcnulty, K.P., Driese, S.G., Dunsworth, H., Fox, D.L., Harcourt-Smith, W.E.H., Jenkins, K., and Peppe, D.J. 2020. Sedimentological and palaeoenvironmental study from Waregi Hill in the Hiwegi Formation (early Miocene) on Rusinga Island, Lake Victoria, Kenya. Sedimentology, 67:3567–3594.
https://doi.org/10.1111/sed.12762
Miller, E.R., Benefit, B.R., McCrossin, M.L., Plavcan, J.M., Leakey, M.G., El-Barkooky, A.N., Hamdan, M.A., Abdel Gawad, M.K., Hassan, S.M., and Simons, E.L. 2009. Systematics of early and middle Miocene Old World monkeys. Journal of Human Evolution, 57:195–211.
https://doi.org/10.1016/j.jhevol.2009.06.006
Miller, E.R., Gunnell, G.F., Gawad, M.A., Hamdan, M., El-Barkooky, A.N., Clementz, M.T., and Hassan, S.M. 2014. Anthracotheres from Wadi Moghra, Early Miocene, Egypt. Journal of Paleontology, 88: 967–981.
https://doi.org/10.1666/13-122
Morgan, M.E., Kingston, J.D., and Marino, B.D. 1994. Carbon isotopic evidence for the emergence of C4 plants in the Neogene from Pakistan and Kenya. Nature, 367:162–165.
https://doi.org/10.1038/367162a0
Morlo, M., Friscia, A., Miller, E., Locke, E., and Nengo, I. 2021. Systematics and paleobiology of Carnivora and Hyaenodonta from Buluk, Early Miocene, Kenya. Acta Palaeontologica Polonica, 66: 465–484.
https://doi.org/10.4202/app.00794.2020
Munday, C., Savage, N., Jones, R.G., and Washington, R. 2023. Valley formation aridifies East Africa and elevates Congo Basin rainfall. Nature, 615: 276–279.
https://doi.org/10.1038/s41586-022-05662-5
Napier, J.R. 1967. Evolutionary aspects of primate locomotion. American Journal of Physical Anthropology, 27:333–341.
https://doi.org/10.1002/ajpa.1330270306
Nelson, S.V. 2007. Isotopic reconstructions of habitat change surrounding the extinction of Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology, 243:204–222.
https://doi.org/10.1016/j.palaeo.2006.07.017
Nelson, S.V. 2013. Chimpanzee fauna isotopes provide new interpretations of fossil ape and hominin ecologies. Proceedings of the Royal Society B: Biological Sciences, 280:20132324.
https://doi.org/10.1098/rspb.2013.2324
Nesbit Evans, E.M., Wildlife, V., Van Couvering, J.A.H., and Andrews, P. 1981. Palaeoecology of Miocene Sites in Western Kenya. Journal of Human Evolution, 10:99–116.
Nishimura, A.C., Russo, G.A., Nengo, I.O., and Miller, E.R. 2022. Morphological affinities of a fossil ulna (KNM-WS 65401) from Buluk, Kenya. Journal of Human Evolution, 166:103177.
https://doi.org/10.1016/j.jhevol.2022.103177
Paquette, J. and Drapeau, M.S.M. 2021. Environmental comparisons of the Awash Valley, Turkana Basin and lower Omo Valley from upper Miocene to Holocene as assessed from stable carbon and oxygen isotopes of mammalian enamel. Palaeogeography, Palaeoclimatology, Palaeoecology, 562:110099.
https://doi.org/10.1016/j.palaeo.2020.110099
Passey B.H., Robinson, T.F., Ayliffe, L.K., Cerling, T.E., Sponheimer, M., Dearing, M.D., Roeder, B.L., and Ehleringer, J.R. 2005. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. Journal of Archaeological Science, 32:1459–1470.
https://doi.org/10.1016/j.jas.2005.03.015
Peppe, D., Cote, S.M., Deino, A.L., Fox, D.L., Kingston, J.D., Kinyanjui, R.N., Lukens, W.E., MacLatchy, L.M., Novello, A., Strömberg, C.A.E., Driese, S.G., Garrett, N.D., Hillis, K.R., Jacobs, B.F., Jenkins, K.E.H., Kityo, R.M., Lehmann, T., Manthi, F.K., Mbua, E.N., Michel, L.A., Miller, E.R., Mugume, A.A.T., Muteti, S.N., Nengo, I.O., Oginga, K.O., Phelps, S.R., Polissar, P., Rossie, J.B., Stevens, N.J., Uno, K.T., and McNulty, K.P. 2023. Oldest evidence of abundant C4 grasses and habitat heterogeneity in eastern Africa. Science, 380:173–177.
https://doi.org/10.1126/science.abq2834
Pickford, M. 1981. Preliminary Miocene mammalian biostratigraphy for Western Kenya. Journal of Human Evolution, 10:73–97.
https://doi.org/10.1016/S0047-2484(81)80026-7
Pickford, M. 1983a. Sequence and environments of the Lower and Middle Miocene hominoids of Western Kenya, p. 421–439. In Ciochon, R.L. and Corruccini, R.D. (eds.), New Interpretations of Ape and Human Ancestry. Plenum Press, New York.
https://doi.org/10.1007/978-1-4684-8854-8_16
Pickford, M. 1983b. On the origins of Hippopotamidae together with descriptions of two new species, a new genus and a new subfamily from the Miocene of Kenya. Geobios, 16:193–217.
https://doi.org/10.1016/S0016-6995(83)80019-9
Pickford, M. 1995. Fossil land snails of East Africa and their palaeoecological significance. Journal of African Earth Sciences, 20:167–226.
https://doi.org/10.1016/0899-5362(95)94397-R
Pickford, M., Sawada, Y., Tayama, R., Matsuda, Y., Itaya, T., Hyodo, H., and Senut, B. 2006. Refinement of the age of the Middle Miocene Fort Ternan Beds, Western Kenya, and its implications for Old World biochronology. Comptes Rendus Geoscience, 338:545–555.
https://doi.org/10.1016/J.CRTE.2006.02.010
Polissar, P.J., Rose, C., Uno, K.T., Phelps, S.R., and deMenocal, P. 2019. Synchronous rise of African C4 ecosystems 10 million years ago in the absence of aridification. Nature Geoscience, 12:657–660.
https://doi.org/10.1038/s41561-019-0399-2
R Core Team 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Retallack, G.J. 1992. Middle Miocene fossil plants from Fort Ternan (Kenya) and evolution of African grasslands. Paleobiology, 18:383–400.
https://doi.org/10.1017/S0094837300010964
Retallack, G.J., Dugas, D.P., and Bestland, E.A. 1990. Fossil soils and grasses of a middle Miocene East African Grassland. Science, 247:1325–1328.
https://doi.org/10.1126/science.247.4948.1325
Retallack, G.J., Wynn, J.G., Benefit, B.R., and Mccrossin, M.L. 2002. Paleosols and paleoenvironments of the middle Miocene, Maboko Formation, Kenya. Journal of Human Evolution, 42:659–703.
https://doi.org/10.1006/JHEV.2002.0553
Roberts, P., Blumenthal, S.A., Dittus, W., Wedage, O., and Lee-Thorp, J.A. 2017. Stable carbon, oxygen, and nitrogen, isotope analysis of plants from a South Asian tropical forest: Implications for primatology. American Journal of Primatology, 79:e22656.
https://doi.org/10.1002/ajp.22656
Robinson, J.R., Rowan, J., Barr, W.A., and Sponheimer, M. 2021. Intrataxonomic trends in herbivore enamel δ13C are decoupled from ecosystem woody cover. Nature Ecology & Evolution, 5:995–1002.
https://doi.org/10.1038/s41559-021-01455-7
Rose, M.D., Leakey, M.G., Leakey, R.E.F., and Walker, A.C. 1992. Postcranial specimens of Simiolus enjiessi and other primitive catarrhines from the early Miocene of Lake Turkana, Kenya. Journal of Human Evolution, 22:171–237.
https://doi.org/10.1016/S0047-2484(05)80006-5
Rowan, J., Adrian, B., and Grossman, A. 2015. The first skull of Sivameryx africanus (Anthracotheriidae, Bothriodontinae) from the early Miocene of East Africa. Journal of Vertebrate Paleontology, 35:e928305.
https://doi.org/10.1080/02724634.2014.928305
Rozanski, K., Araguas-Araguas, L., and Gonfiantini, R. 1993. Isotopic patterns in modern global precipitation, p. 1–36. In Swart, P.K., Lohmann, K.C., McKenzie, J., and Savin, S. (eds.), Climate Change in Continental Isotopic Records, Volume 78. The American Geophysical Union, Washington, DC.
https://doi.org/10.1029/GM078p0001
Saarinen, J. and Lister, A.M. 2023. Fluctuating climate and dietary innovation drove ratcheted evolution of proboscidean dental traits. Nature Ecology & Evolution, 7:1490–1502.
https://doi.org/10.1038/s41559-023-02151-4
Schoeninger, M.J., Reeser, H., and Hallin, K. 2003. Paleoenvironment of Australopithecus anamensis at Allia Bay, East Turkana, Kenya: evidence from mammalian herbivore enamel stable isotopes. Journal of Anthropological Archaeology, 22:200–207.
https://doi.org/10.1016/S0278-4165(03)00034-5
Sepulchre, P., Ramstein, G., Fluteau, F., Schuster, M., Tiercelin, J.J., and Brunet, M. 2006. Tectonic uplift and Eastern Africa aridification. Science, 313:1419–1423.
https://doi.org/10.1126/science.1129158
Shipman, P. 1986. Paleoecology of Fort Ternan reconsidered. Journal of Human Evolution, 15:193–204.
https://doi.org/10.1016/S0047-2484(86)80045-8
Shipman, P., Walker, A., Van Couvering, J.A., Hooker, P.J., and Miller, J.A. 1981. The Fort Ternan hominoid site, Kenya: Geology, age, taphonomy and paleoecology. Journal of Human Evolution, 10:49–72. https://doi.org/10.1016/S0047-2484(81)80025-5
Spinage, C.A. 1976. Age determination of the female Grant's gazelle. African Journal of Ecology, 14:121–134.
https://doi.org/10.1111/j.1365-2028.1976.tb00157.x
Sponheimer, M. and Lee-Thorp, J.A. 1999. Oxygen isotopes in enamel carbonate and their ecological significance. Journal of Archaeological Science, 26:723–728.
https://doi.org/10.1006/JASC.1998.0388
Sponheimer, M., Lee-Thorp, J.A., DeRuiter, D.J., Smith, J.M., van der Merwe, N.J., Reed, K., Grant, C.C., Ayliffe, L.K., Robinson, T.F., Heidelberger, C, and Marcus, W. 2003. Diets of Southern African Bovidae: stable isotope evidence. Journal of Mammalogy, 84:471–479.
https://doi.org/10.1644/1545-1542(2003)084<0471:DOSABS>2.0.CO;2
Sternberg, L.S.L., Mulkey, S.S., and Wright, S.J. 1989. Oxygen isotope ratio stratification in a tropical moist forest. Oecologia, 81:51–56.
https://doi.org/10.1007/BF00377009
Tejada-Lara, J.V., MacFadden, B.J., Bermudez, L., Rojas, G., Salas-Gismondi, R., and Flynn, J.J. 2018. Body mass predicts isotope enrichment in herbivorous mammals. Proceedings of the Royal Society B, 285:20181020.
https://doi.org/10.1098/RSPB.2018.1020
Temerin, L. and Cant, J. 1983. The evolutionary divergence of Old World monkeys and apes. American Naturalist, 122:335–351.
https://doi.org/10.1086/284139
Tieszen, L.L., Senyimba, M.M., Imbamba, S.K., and Troughton, J.H. 1979. The distribution of C3 and C4 grasses and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia, 37:337–350.
https://doi.org/10.1007/BF00347910
Tipple, B.J., Meyers, S.R., and Pagani, M. 2010. Carbon isotope ratio of Cenozoic CO2: A comparative evaluation of available geochemical proxies. Paleoceanography, 25:1–11.
https://doi.org/10.1029/2009PA001851
Tütken, T. and Absolon, J. 2015. Late Oligocene ambient temperatures reconstructed by stable isotope analysis of terrestrial and aquatic vertebrate fossils of Enspel, Germany. Palaeobiodiversity and Palaeoenvironments, 95:17–31.
https://doi.org/10.1007/s12549-014-0183-7
Uno, K.T., Cerling, T.E., Harris, J.M., Kunimatsu, Y., Leakey, M.G., Nakatsukasa, M., and Nakaya, H. 2011. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proceedings of the National Academy of Sciences, 108:6509–6514.
https://doi.org/10.1073/pnas.1018435108
Uno, K.T., Polissar, P.J., Jackson, K.E., and deMenocal, P.B. 2016. Neogene biomarker record of vegetation change in eastern Africa. Proceedings of the National Academy of Sciences, 113:6355-6363.
https://doi.org/10.1073/pnas.1521267113
Uno, K.T., Rivals, F., Bibi, F., Pante, M., and Njau, J. 2018. Large mammal diets and paleoecology across the Oldowan-Acheulean transition at Olduvai Gorge, Tanzania from stable isotope and tooth wear analyses. Journal of Human Evolution, 120:76–91.
https://doi.org/10.1016/J.JHEVOL.2018.01.002
van der Made, J. 1996. Listriodontinae (Suidae, Mammalia), their evolution, systematics and distribution in time and space. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie, 33:3–254.
van der Merwe, N.J. and Medina, E. 1989. Photosynthesis and 13C12C ratios in Amazonian rain forests. Geochimica et Cosmochimica Acta, 53:1091–1094.
https://doi.org/10.1016/0016-7037(89)90213-5
van der Merwe, N.J. and Medina, E. 1991. The canopy effect, carbon isotope ratios and foodwebs in Amazonia. Journal of Archaeological Science, 18:249–259.
https://doi.org/10.1016/0305-4403(91)90064-V
Vogel, J.C. 1978. Recycling of carbon in a forest environment. Oecologia Plantarum, 13:89–94.
Watkins, R.T. 1989. The Buluk Member, a fossil hominoid-bearing sedimentary sequence of Miocene age from northern Kenya. Journal of African Earth Sciences (and the Middle East), 8:107–112.
https://doi.org/10.1016/S0899-5362(89)80015-6
Wheeler, E.A., Wiemann, M.C., and Fleagle, J.G. 2007. Woods from the Miocene Bakate Formation, Ethiopia Anatomical characteristics, estimates of original specific gravity and ecological inferences. Review of Palaeobotany and Palynology, 146:193–207.
https://doi.org/10.1016/j.revpalbo.2007.04.002
Wichura, H., Jacobs, L.L, Lin, A., and Clemens, M. 2015. A 17-My-old whale constrains onset of uplift and climate change in East Africa. Proceedings of the National Academy of Sciences, 112:3910–3915.
https://doi.org/10.1073/pnas.1421502112
Young, H.J. and Young, T.P. 1983. Local distribution of C3 and C4 grasses in sites of overlap on Mount Kenya. Oecologia, 58:373–377.
https://doi.org/10.1007/BF00385238

