 The Lapara Creek Fauna: Early Clarendonian of south Texas, USA
The Lapara Creek Fauna: Early Clarendonian of south Texas, USA
Article number: 22.1.15
https://doi.org/10.26879/929
Copyright Society for Vertebrate Paleontology, April 2019
Author biography
Plain-language and multi-lingual abstracts
PDF version
Erratum for this article
Submission: 5 October 2018. Acceptance: 8 February 2019
{flike id=2445}
ABSTRACT
The Lapara Creek Fauna includes a large collection of fossil vertebrates obtained by the State-Wide Paleontologic-Mineralogic Survey in Texas (1939-1941) under the direction of the Bureau of Economic Geology and the Texas Memorial Museum. Of the 50 species of fossil vertebrates, five species are fish, seven are reptiles, two are birds and 36 are mammals. The 36 species of mammals represent 31 genera of which four are rodents, five are carnivores, two are proboscideans, 10 are artiodactyls and 10 are perissodactyls. These taxa are known from four separate local faunas distributed within the lower to middle parts of the Goliad Formation and are Clarendonian in age. The Ten Mile Waterhole Creek and Bridge Ranch local faunas compare well with Cl1 faunas in North America, while the Farish Ranch and Buckner Ranch local faunas compare well with early Cl2 faunas. The fauna includes the first occurrence, or at least very early occurrences, of cf. Trachemys sp., Apalone sp., Alligator cf. mississippiensis and cf. Eucyon sp. Identification of Ceratogaulus cf. rhinoceros extends the known geographic range of this taxon and represents the oldest occurrence of a mylagaulid from the Texas coastal plain. Blancotherium buckneri is a new generic name assigned to a longirostrine gomphothere that is represented by numerous specimens from the Buckner Ranch Local Fauna. The diverse horse fauna includes 12 species representing nine genera, all but one of which are hypsodont. The composition of the fauna is consistent with the widespread Clarendonian Chronofauna and with a mixed woodland-grassland environment on a broad floodplain associated with low-gradient rivers.
Steven R. May. Texas Vertebrate Paleontology Collections, The University of Texas at Austin, Austin, Texas, 78758, USA. srmay@utexas.edu
Keywords: vertebrate paleontology; Clarendonian; Miocene; Texas; stratigraphy; new genus; paleoenvironment; Blancotherium
May, Steven R. 2019. The Lapara Creek Fauna: Early Clarendonian of south Texas, USA. Palaeontologia Electronica 22.1.15A 1-129. https://doi.org/10.26879/929
palaeo-electronica.org/content/2019/2445-lapara-creek
Copyright: April 2019 Society of Vertebrate Paleontology
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0/creativecommons.org/licenses/by-nc-sa/4.0
http://zoobank.org/8732DBBC-6E45-4D0C-92E9-DAD2A20F8455
INTRODUCTION
Vertebrate fossils have been known from the Beeville area of the Texas coastal plain since at least the early 1930s. Weeks (1933) stated that vertebrate fossils had been collected from the “Upper Lagarto” beds along Medio Creek northeast of Beeville, Texas, including a “jaw bone” and tooth of a rhino “pertaining to the genus Teleoceras” based on identification by C.W. Gilmore at the U.S. National Museum. Weeks (1933) also noted the collection of vertebrate fossils about two miles southeast of Normanna along a tributary of Medio Creek that were sent to C.L. Camp at the University of California Museum of Paleontology (UCMP). Camp identified Hipparion, Pliohippus, Aphelops and a camel. Weeks (1933) suggested that similar horses were known from the Clarendon and Goodnight horizons of north Texas. At that time, these were considered Pliocene in age.
 The majority of vertebrate fossils known from Bee and Live Oak counties were collected by the State-Wide Paleontologic-Mineralogic Survey (hereafter referred to as the Survey), conducted by the Works Progress Administration (WPA) under the direction of the University of Texas Bureau of Economic Geology (BEG). The project ran from March 1939 through September 1941. By the end of June 1939, there were 97 workers and seven supervisors employed in four field units and two laboratory units (Figure 1). Over its history, the project included 23 field units engaged in both paleontological and mineralogical investigations. Fossil preparation was first conducted at a laboratory in San Antonio and later moved to Austin, Texas. With the coming of World War II, the focus of the project shifted from paleontology and mineralogy to just mineralogy in 1941. The project was executed under the leadership of Elias H. Sellards, Director of the BEG from 1932-1945 and Director of the Texas Memorial Museum from 1938-1945. Supervisors for the paleontologic field units in Bee and Live Oak counties included Glen L. Evans, Adolph H. Witte and Nolan McWhirter. Evans started as the Supervisor for the Bee County unit and was instrumental in initiating the major quarry at Buckner Ranch. It is clear from archived correspondence that Sellards relied heavily on Evans and by late April of 1939 offered him the role of “Geologist-in-Charge”. Supervisors for the paleontologic laboratory units included William H. McAnulty and Joseph T. Gregory. Correspondence, quarterly reports and the final report of the Survey (1941) provide a valuable record of both field and laboratory activities (archived at the Vertebrate Paleontology Collections and at the Briscoe Center for American History, The University of Texas at Austin). The majority of the Lapara Creek fossils are housed at the Vertebrate Paleontology Collections, The University of Texas at Austin. Small collections are also located at the American Museum of Natural History, New York and the University of California Museum of Paleontology. All photos from the Survey are reproduced courtesy of the Texas Archeological Research Laboratory, the University of Texas at Austin, unless otherwise noted.
The majority of vertebrate fossils known from Bee and Live Oak counties were collected by the State-Wide Paleontologic-Mineralogic Survey (hereafter referred to as the Survey), conducted by the Works Progress Administration (WPA) under the direction of the University of Texas Bureau of Economic Geology (BEG). The project ran from March 1939 through September 1941. By the end of June 1939, there were 97 workers and seven supervisors employed in four field units and two laboratory units (Figure 1). Over its history, the project included 23 field units engaged in both paleontological and mineralogical investigations. Fossil preparation was first conducted at a laboratory in San Antonio and later moved to Austin, Texas. With the coming of World War II, the focus of the project shifted from paleontology and mineralogy to just mineralogy in 1941. The project was executed under the leadership of Elias H. Sellards, Director of the BEG from 1932-1945 and Director of the Texas Memorial Museum from 1938-1945. Supervisors for the paleontologic field units in Bee and Live Oak counties included Glen L. Evans, Adolph H. Witte and Nolan McWhirter. Evans started as the Supervisor for the Bee County unit and was instrumental in initiating the major quarry at Buckner Ranch. It is clear from archived correspondence that Sellards relied heavily on Evans and by late April of 1939 offered him the role of “Geologist-in-Charge”. Supervisors for the paleontologic laboratory units included William H. McAnulty and Joseph T. Gregory. Correspondence, quarterly reports and the final report of the Survey (1941) provide a valuable record of both field and laboratory activities (archived at the Vertebrate Paleontology Collections and at the Briscoe Center for American History, The University of Texas at Austin). The majority of the Lapara Creek fossils are housed at the Vertebrate Paleontology Collections, The University of Texas at Austin. Small collections are also located at the American Museum of Natural History, New York and the University of California Museum of Paleontology. All photos from the Survey are reproduced courtesy of the Texas Archeological Research Laboratory, the University of Texas at Austin, unless otherwise noted.
Initially, fossils from Bee and Live Oak counties were transported to the Laboratory Unit in San Antonio, Texas. The prepared specimens were transferred to the University of Texas in Austin where they were accessioned into the collections of the Texas Memorial Museum. The Austin Laboratory opened in June,1939 within the Bureau of Economic Geology building at 18th and Red River Streets. In December of 1940, there were 50 workers at the Austin Laboratory. The majority of workers on the Survey were simply men in need of work. They neither were professional preparators nor paleontologists. A “Manual of suggested procedures to be employed by the State-Wide Paleontologic-Mineralogic Survey in Texas” was prepared by Glen Evans and Elias Sellards in 1939 (Evans, 1939). This document included best practices for field and laboratory techniques including procedures for fossil preparation. It stated: “When the specimen has been cleaned, hardened, reinforced, and all pieces cemented together, the next step is to restore the missing parts.” “Restored parts are grained and colored to imitate bone, with the coloring being a shade lighter than the natural bone so that the restoration can easily be detected should the specimen be used for study purposes.” This concept, that the goal of preparation was to produce “complete” specimens, is very different from practices employed today and explains why there is so much plaster restoration on many of the specimens.
 The Survey began work on Site 1 (TMM 30896) at the Buckner Ranch along Blanco Creek near Berclair, Texas, in the spring of 1939. Fossils were collected from both Pleistocene terrace deposits and the underlying Goliad Formation. At that time, the Goliad Formation was interpreted to be Pliocene in age. Approximately 20-25 feet of overburden were removed using a tractor and Fresno scraper (Figure 2). A quarry, approximately 400 feet long, 60 feet wide and 24 feet deep on the south bank of Blanco Creek, was excavated (Figure 3). 738 plaster jackets
The Survey began work on Site 1 (TMM 30896) at the Buckner Ranch along Blanco Creek near Berclair, Texas, in the spring of 1939. Fossils were collected from both Pleistocene terrace deposits and the underlying Goliad Formation. At that time, the Goliad Formation was interpreted to be Pliocene in age. Approximately 20-25 feet of overburden were removed using a tractor and Fresno scraper (Figure 2). A quarry, approximately 400 feet long, 60 feet wide and 24 feet deep on the south bank of Blanco Creek, was excavated (Figure 3). 738 plaster jackets  were reportedly collected over nearly a year. Most of the initial work was directed by Glen Evans, but when he was promoted to the Chief Geologist position, Adolph Witte took over as the Supervisor for the excavation. Archived correspondence shows Witte was taking direction from and communicating results to both Evans and Sellards. In July of 1939, Evans wrote to Witte stating that C.J. Hesse had identified some of the material from the Goliad Formation and considered it to be middle Pliocene in age. By mid-August of 1939, Witte wrote to Sellards and Evans that the eighth “mastodon skull” from Site 1 had been excavated. In October, Witte was transferred to Aransas Pass and Nolan McWhirter took over as Field Crew Supervisor for the Bee and Live Oak county work.
were reportedly collected over nearly a year. Most of the initial work was directed by Glen Evans, but when he was promoted to the Chief Geologist position, Adolph Witte took over as the Supervisor for the excavation. Archived correspondence shows Witte was taking direction from and communicating results to both Evans and Sellards. In July of 1939, Evans wrote to Witte stating that C.J. Hesse had identified some of the material from the Goliad Formation and considered it to be middle Pliocene in age. By mid-August of 1939, Witte wrote to Sellards and Evans that the eighth “mastodon skull” from Site 1 had been excavated. In October, Witte was transferred to Aransas Pass and Nolan McWhirter took over as Field Crew Supervisor for the Bee and Live Oak county work.
 The first quarterly report of the Survey noted that fossil bones were concentrated in the Goliad Formation along an erosional boundary between coarse-grained, partly-consolidated, calcareous-sandstone and an overlying bed of bentonitic-clay. Concentrations of bones and “fresh water mollusks” were located in shallow depressions along this erosional surface that were interpreted to represent “shallow ponds” (Survey First Quarterly Report, 1939). The same report stated that the most abundant fossils were those of “a long-jawed variety of mastodon” and the early collections included “at least two skulls with articulated tusks and lower jaws, one skull with tusks but lower jaw disconnected, five additional nearly complete lower jaws” and numerous post-cranial bones (Figure 4).
The first quarterly report of the Survey noted that fossil bones were concentrated in the Goliad Formation along an erosional boundary between coarse-grained, partly-consolidated, calcareous-sandstone and an overlying bed of bentonitic-clay. Concentrations of bones and “fresh water mollusks” were located in shallow depressions along this erosional surface that were interpreted to represent “shallow ponds” (Survey First Quarterly Report, 1939). The same report stated that the most abundant fossils were those of “a long-jawed variety of mastodon” and the early collections included “at least two skulls with articulated tusks and lower jaws, one skull with tusks but lower jaw disconnected, five additional nearly complete lower jaws” and numerous post-cranial bones (Figure 4).
Second in abundance, after the mastodon remains, were those of horses, primarily isolated teeth, followed by camels. The bones were described as “badly decomposed” and many were not collected. The fossils were very difficult to handle because of “chalky preservation” and many of the bones were broken and deformed by compaction. Nevertheless, fieldwork continued at the Bucker Ranch Site 1 until the first quarter of 1940. An average of 15 workers and one supervisor excavated a large number of specimens, including “15 skulls and 19 lower jaws of the shovel-jawed mastodon”. During the excavation, Sellards (1940b) published a description of the Buckner Ranch proboscidean naming it Gnathabelodon buckneri.
 Excavations at the second locality, Site 8 in Live Oak County was accomplished during the third quarter of 1939 (Survey Second Quarterly Report, 1939). The Survey referred to this locality as “Ten Mile Creek” and “Ten Mile Waterhole Creek” (TMM 30936) (Figure 5). The name was not based on a geographic feature, but instead denoted its location 10 miles east of George West, Texas, which was the center of operations for that field unit. At Site 8, vertebrate fossils were distributed through an 18-20-foot-thick interval of cross-bedded sand and gravel in what the Survey interpreted as the Lapara sandstone member of the Goliad Formation. Fossil wood was reported as abundant, “sometimes occurring as logs 10 or more feet long”, but none of this material is preserved in the collections at The University of Texas at Austin. Final preparation and cataloguing of material from Site 8 was completed in the third quarter of 1940. The Final Report of the Survey concluded that the Ten Mile Waterhole Creek fauna was “probably slightly older than the Clarendon fauna of the Texas Panhandle”, apparently based on the abundance of two horses also “known” from the Fleming Formation (Barstovian) (Survey Final Report, 1941). A note on related research in the sixth quarterly report of the Survey states that Dr. Joseph T. Gregory, Assistant Project Technician, was working on a description of fossils from Site 8. (Survey Sixth Quarterly Report, 1940). Apparently, this work was never published and no record of it has been found.
Excavations at the second locality, Site 8 in Live Oak County was accomplished during the third quarter of 1939 (Survey Second Quarterly Report, 1939). The Survey referred to this locality as “Ten Mile Creek” and “Ten Mile Waterhole Creek” (TMM 30936) (Figure 5). The name was not based on a geographic feature, but instead denoted its location 10 miles east of George West, Texas, which was the center of operations for that field unit. At Site 8, vertebrate fossils were distributed through an 18-20-foot-thick interval of cross-bedded sand and gravel in what the Survey interpreted as the Lapara sandstone member of the Goliad Formation. Fossil wood was reported as abundant, “sometimes occurring as logs 10 or more feet long”, but none of this material is preserved in the collections at The University of Texas at Austin. Final preparation and cataloguing of material from Site 8 was completed in the third quarter of 1940. The Final Report of the Survey concluded that the Ten Mile Waterhole Creek fauna was “probably slightly older than the Clarendon fauna of the Texas Panhandle”, apparently based on the abundance of two horses also “known” from the Fleming Formation (Barstovian) (Survey Final Report, 1941). A note on related research in the sixth quarterly report of the Survey states that Dr. Joseph T. Gregory, Assistant Project Technician, was working on a description of fossils from Site 8. (Survey Sixth Quarterly Report, 1940). Apparently, this work was never published and no record of it has been found.
 During the fourth quarter of 1939, the Survey began work at the third major locality known as Site 15 on Medio Creek (Figure 6). This was also known as the Farish Ranch locality (TMM 31081). This site was interpreted by the Survey to be from the middle member of the Goliad Formation, where fossils occurred in “a gravel and sandstone and below this is a bentonitic clay about five feet in thickness with sandstone bedrock” (Survey Third Quarterly Report, 1939). A 50 x 40 foot quarry was excavated after removal of about 18 feet of overburden along the bank of Medio Creek. During 1940, work at Site 15 was abandoned in favor of a site 100 m upstream and on the opposite bank of Medio Creek where there was less overburden (Site 15A). Fifteen workers spent about four months collecting specimens from the Farish Ranch sites, yielding a reported 23 rhinoceros skulls and a total of 489 jacketed specimens (Survey Final Report, 1941). In their sixth quarterly report, the Survey concluded that the Farish Ranch sites were “apparently somewhat lower in the section” than Site No.1 at Buckner Ranch and that fossils from the Farish Ranch sites were much better preserved than those from Buckner Ranch (Survey Sixth Quarterly Report, 1940). After preparation and cataloguing of the material, no significant differences were observed between the horses at Farish Ranch, Ten Mile Waterhole Creek and Buckner Ranch Site 1, although at the time, identifications were only attempted at a generic level.
During the fourth quarter of 1939, the Survey began work at the third major locality known as Site 15 on Medio Creek (Figure 6). This was also known as the Farish Ranch locality (TMM 31081). This site was interpreted by the Survey to be from the middle member of the Goliad Formation, where fossils occurred in “a gravel and sandstone and below this is a bentonitic clay about five feet in thickness with sandstone bedrock” (Survey Third Quarterly Report, 1939). A 50 x 40 foot quarry was excavated after removal of about 18 feet of overburden along the bank of Medio Creek. During 1940, work at Site 15 was abandoned in favor of a site 100 m upstream and on the opposite bank of Medio Creek where there was less overburden (Site 15A). Fifteen workers spent about four months collecting specimens from the Farish Ranch sites, yielding a reported 23 rhinoceros skulls and a total of 489 jacketed specimens (Survey Final Report, 1941). In their sixth quarterly report, the Survey concluded that the Farish Ranch sites were “apparently somewhat lower in the section” than Site No.1 at Buckner Ranch and that fossils from the Farish Ranch sites were much better preserved than those from Buckner Ranch (Survey Sixth Quarterly Report, 1940). After preparation and cataloguing of the material, no significant differences were observed between the horses at Farish Ranch, Ten Mile Waterhole Creek and Buckner Ranch Site 1, although at the time, identifications were only attempted at a generic level.
 The work at Farish Ranch was eventually abandoned in order to concentrate fieldwork on the Bridge Ranch localities near Normanna, Texas, in an attempt to obtain vertebrate fossils from what was believed to be a stratigraphically lower interval within the Goliad Formation. Three sites were collected from a small area along Medio Creek and an associated tributary (Sites 17, 18, 19: TMM 31132, TMM 31170, TMM 31184) (Figure 7). These localities were variably referred to as Bridge Estate and/or the Mrs. S.M. Richards Farm, although all are now referred to as Bridge Ranch. The eighth quarterly report of the Survey stated: “The fossils were in sand lenses interbedded with clay ball conglomerates, in a channel fill which cuts deeply into a resistant reddish brown sandstone” that was considered to likely represent the base of the Goliad Formation (Survey Eighth Quarterly Report, 1941). Although the Survey suspected that the Bridge Ranch localities were somewhat older than the Buckner and Farish Ranch sites, the generic level similarity of the horses was again interpreted to imply that all of the localities were approximately the same age.
The work at Farish Ranch was eventually abandoned in order to concentrate fieldwork on the Bridge Ranch localities near Normanna, Texas, in an attempt to obtain vertebrate fossils from what was believed to be a stratigraphically lower interval within the Goliad Formation. Three sites were collected from a small area along Medio Creek and an associated tributary (Sites 17, 18, 19: TMM 31132, TMM 31170, TMM 31184) (Figure 7). These localities were variably referred to as Bridge Estate and/or the Mrs. S.M. Richards Farm, although all are now referred to as Bridge Ranch. The eighth quarterly report of the Survey stated: “The fossils were in sand lenses interbedded with clay ball conglomerates, in a channel fill which cuts deeply into a resistant reddish brown sandstone” that was considered to likely represent the base of the Goliad Formation (Survey Eighth Quarterly Report, 1941). Although the Survey suspected that the Bridge Ranch localities were somewhat older than the Buckner and Farish Ranch sites, the generic level similarity of the horses was again interpreted to imply that all of the localities were approximately the same age.
The Bridge Ranch localities were excavated over a two-month period with a 15-man crew resulting in a reported “50 plastered specimens and about 1000 small bones and isolated teeth” (Survey Final Report, 1941). As the Survey neared its end, the ninth quarterly report discussed an increased focus on geologic mapping and the collection of a series of “hand auger test holes” connecting sites from Live Oak County to Goliad County to better assess correlations within the Goliad Formation (Survey Ninth Quarterly Report, 1941). None of this work was ever documented or published and, apparently, all of the original data has been lost.
In retrospect, the Survey amassed an impressive collection of fossil vertebrates from the Goliad Formation using mostly novice labor in the field and in the laboratory. This certainly reflects the skill of the supervisors Glen Evans, Adolph Witte, Nolan McWhirter, William McAnulty and Joseph Gregory. I believe it also reflects the strong desire of the workers to retain employment during the Great Depression. Unfortunately, no quarry maps showing fossil distributions have been found in the archives, but some photos from the Buckner Ranch quarry are available. Techniques utilized in the Survey preparation lab produce challenges for today’s paleontologists such as the use of abundant plaster to stabilize and “reconstruct” specimens and the confusion between matrix and cement on certain specimens, especially horse teeth. In 1941, the Survey rapidly transitioned to a focus on minerals and then was discontinued as the prospects of a world war loomed. Only a single paper was published at the time describing fossil vertebrates from the Lapara Creek Fauna; that being Sellards’ 1940 paper on the gomphothere. It was not until the mid 1950s that students and faculty at the University of Texas began studying and describing additional material from these collections.
A number of papers have been published about elements of the Lapara Creek Fauna including Sellards (1940a, b), Quinn (1952, 1955), Wilson (1956, 1960), Patton (1969), Patton and Taylor (1971, 1973), Forsten (1975), Webb and Hulbert (1986), Hulbert (1987a, 1987b, 1988a, 1988b) and Prothero and Manning (1987). Each of these papers dealt with a limited number of taxonomic groups and no complete synthesis of the entire fauna has been previously published. In addition, a number of field jackets dating from the Survey (1939-1941) have recently been prepared and additional specimens have been obtained though wet screening matrix from one of the original localities (TMM 30898). This paper is the outcome of work begun in 2014 to analyze fossil vertebrates from all four local faunas in light of current taxonomy, describe additional material and revisit the stratigraphic context of the main localities within the Goliad Formation. The Lapara Creek material continues to be one of the most important Clarendonian faunas from the Texas coastal plain and provides important insight into faunal composition, ecology and paleoenvironments during the Miocene.
Abbreviations. AMNH, American Museum of Natural History; F:AM, Frick Collection, American Museum of Natural History; MWSU, Midwestern State University; TMM, Texas Memorial Museum, The University of Texas at Austin; UCMP, Museum of Paleontology University of California, Berkeley; UO, University of Oregon, Museum of Natural History; USNM, United States National Museum (Smithsonian Institution); WPA, Works Progress Administration; WTSU, West Texas State University; NALMA, North American Land Mammal Age; Cl1 Clarendonian 1; Cl2 Clarendonian 2; AP, anteroposterior; C, canine; dp, lower deciduous premolar; DP, upper deciduous premolar; HI, hypsodonty index; I incisor; M, upper molar; m, lower molar; MCH, mesostyle crown height; P, upper premolar; p, lower premolar; PL, protocone length; PW, protocone width; TRL, tooth row length; L left; R right; mm millimeters. Specimens and archival material at The University of Texas at Austin are curated in the Vertebrate Paleontology Collections. Locality numbers and specimen numbers include the prefix TMM.
STRATIGRAPHY OF THE GOLIAD FORMATION
History and Terminology
The Goliad Formation is recognized regionally across the Texas coastal plain as an interval of dominantly fluvial siliciclastic strata that overlies the Miocene Fleming Formation and underlies Pleistocene terrace deposits (Hoel, 1982). The formation is now interpreted as a basinward-thickening progradational wedge of middle and late Miocene age. Stratigraphic thicknesses in outcrop range from 60-120 m, but offshore the interval thickens to as much as 365-425 m (Hoel, 1982). Structural dip in outcrop along Blanco and Medio Creeks is nearly flat lying and regionally is less than 1⁰ basinward (southerly). The coastline at Goliad time is interpreted to have been located near the modern coastline (Young et al., 2010).
Stratigraphic terminology associated with the Goliad Formation has a complicated history beginning with Dumble (1894), who first used the names Lapara and Lagarto for the lower and upper division of what he considered to be Pliocene strata. In the early 1930s, the BEG and USGS adopted the name Goliad Formation to include both the Lapara and Lagarto units of Dumble (Weeks, 1945).
Plummer (1932) described the Goliad Formation as consisting of three members including the Lapara sand, overlain by the Lagarto Creek beds, overlain by the Labahia beds with outcrop thicknesses ranging from 35 to 200 feet. The type section for the Lapara sand was recognized along La Para Creek in Live Oak County as defined by Dumble (1894). The base of the Lapara sand was recognized by Plummer (1932) as an erosional unconformity on the Lagarto Formation of the Fleming Group (note confusing use of Lagarto Formation in the Fleming Group and Lagarto Creek beds in the Goliad Formation). Plummer’s description of the Lapara sand included conglomerate, cross-bedded sandstone and limy claystone. According to him, the conglomerate included reworked material, including bone fragments and fossilized wood. The overlying Lagarto Creek beds were interpreted as a subset of the original unit described as “Lagarto” by Dumble (1894). Plummer defined the middle member of the Goliad Formation to include only the interval from the top of the Lapara sands (which he described as an unconformity) to the base of the Labahia beds, the uppermost sandy interval in the Goliad Formation. The Lagarto Creek beds were described as approximately 50 feet of predominantly claystone. The Labahia beds, sometimes referred to as the “upper Goliad sand”, were recognized for exposures of sandstones, conglomerates and “calcareous clay” along the San Antonio River near Goliad, Texas, where a measured thickness was cited as 10 feet (Plummer, 1932).
The Survey used the name Goliad Formation and a three-member internal stratigraphic terminology. According to various quarterly reports of the Survey, the Farish and Buckner Ranch localities were interpreted to be from the “medial member” of the Goliad Formation and both the Ten Mile Waterhole Creek and Bridge Ranch localities were located in the basal Lapara sandstone member of the Goliad Formation. This model for the stratigraphic distribution of the four main locality areas is clearly expressed in a letter dated December 9, 1940 from Glen Evans to Nolan McWhirter giving McWhirter guidance for a presentation he was expected to give at the annual Geological Society of America meeting in Austin, Txas December 26-28, 1940. “I believe it will be a good idea to mention in the address that the several sites which we have worked in the south Texas Pliocene are all within the Goliad formation, but which are distributed through a considerable vertical section. You will remember that the Live Oak County locality is in the Lapara member which as you know makes up the basal beds of the Goliad as it is now defined. We do not have the Blanco and Medio Creek exposures subdivided but are quite sure that they are somewhat higher in the section in (than) the Live Oak County site.” “We know then that vertebrates are at or near the base of the Goliad in Ten Mile Creek and east of Normanna, we know that in the medial beds of Blanco and Medio Creek there is another abundant fauna and lastly we know that some fossils at least are present in the upper-most Goliad of the La Bahia.”
Weeks (1945) reinterpreted stratigraphic relationships along the Texas coastal plain and included the Oakville and Cuero formations in the Fleming group. He introduced the name “Cuero formation” for red clays and sandstones stratigraphically below the “Goliad formation” and noted that “Fleming group” strata commonly exhibit steeper dips than those of the overlying “Goliad formation”.
Wilson (1956) reviewed the stratigraphy and vertebrate faunas from the Miocene of the Texas coastal plain and used the terms Fleming Formation and Goliad Formation. He included the upper part of the Oakville Formation and the Cuero Formation (Weeks, 1945) in the Fleming with age constraints based on the Burkeville and Cold Spring Faunas (Hemingfordian, Barstovian). Wilson (1956) also used the tri-member nomenclature of Lapara member, Lagarto Creek member and Labahia member for the Goliad Formation showing the Lapara Creek Fauna (Clarendonian) from the lower member and the Labahia Mission Local Fauna from the upper member. Although the Labahia Mission Local Fauna has been referred to as Hemphillian (Tedford et al., 2004), the material is very limited and not diagnostic (Baskin, 1991). Baskin and Hulbert (2012) reported a late Clarendonian fauna (Dinero Local Fauna) from near the top of the Goliad Formation.
Subsequent workers (Solis, 1981; Hoel, 1982; Morton et al., 1988; Young et al., 2010) incorporated abundant subsurface data in their stratigraphic analysis and abandoned the three-part stratigraphy, recognizing only a lower and upper Goliad Formation. Solis (1981) published a series of well log cross-sections and associated maps for the Late Tertiary and Quaternary of the central Texas coastal plain. Using a lithostratigraphic approach for correlation, he recognized lower and upper Goliad operational units. Derivative maps of depositional environments resulted in a complex pattern of roughly N-S oriented fluvial meander belts and interfluve floodplain in the Bee-Live Oak County area. Similarly, Hoel (1982) concluded that the tripartite subdivision of the Goliad Formation was not regionally useful, and the three members could not be mapped in the subsurface with well logs. Based largely on lithostratigraphic correlation, Hoel (1982) recognized four main depositional systems, each of which included axial fluvial deposits separated by overbank and floodplain.
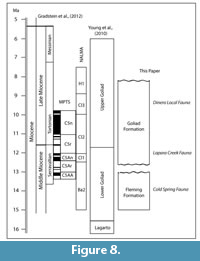 Morton et al. (1988) published a general sequence stratigraphic model for the middle and upper Miocene of the Texas coastal plain that was tied to marine biostratigraphy. In their paper, they chose not to subdivide the Goliad Formation, but correlated it with early Serravallian, Tortonian and Messinian stages. Their interpretation implied that the Goliad Formation spanned from approximately 14 Ma to 5 Ma. A sequence stratigraphic model was further refined by Young et al. (2010) in which they recognized lower and upper Goliad Formation units interpreted as separate progradational systems. They placed the boundary between the two informal members at the “Middle-Upper Miocene” boundary (Serravallian-Tortonian). Based on the most recent time scale of Gradstein et al. (2012), the Serravallian-Tortonian boundary is recognized at 11.63 Ma within the early part of Cl2 (Cl1-Cl2 boundary at 12.0 Ma). Baskin and Hulbert (2012) interpreted a late Clarendonian age for the Dinero Local Fauna from the upper part of the Goliad Formation. The boundary between the Goliad and Willis Formations probably occurs in the upper Miocene (late Hemphillian?) (Young et al., 2010) (Figure 8).
Morton et al. (1988) published a general sequence stratigraphic model for the middle and upper Miocene of the Texas coastal plain that was tied to marine biostratigraphy. In their paper, they chose not to subdivide the Goliad Formation, but correlated it with early Serravallian, Tortonian and Messinian stages. Their interpretation implied that the Goliad Formation spanned from approximately 14 Ma to 5 Ma. A sequence stratigraphic model was further refined by Young et al. (2010) in which they recognized lower and upper Goliad Formation units interpreted as separate progradational systems. They placed the boundary between the two informal members at the “Middle-Upper Miocene” boundary (Serravallian-Tortonian). Based on the most recent time scale of Gradstein et al. (2012), the Serravallian-Tortonian boundary is recognized at 11.63 Ma within the early part of Cl2 (Cl1-Cl2 boundary at 12.0 Ma). Baskin and Hulbert (2012) interpreted a late Clarendonian age for the Dinero Local Fauna from the upper part of the Goliad Formation. The boundary between the Goliad and Willis Formations probably occurs in the upper Miocene (late Hemphillian?) (Young et al., 2010) (Figure 8).
Depositional Environments
The Goliad Formation includes fluvial deposits in up-dip to offshore marine facies in the subsurface of the Gulf of Mexico. Hoel (1982) described fluvial depositional systems in the Goliad Formation comprised of channel-fill and inter-channel facies. The former includes medium- to coarse-grained sand and gravel with occasional large-scale cross-bedding, while the latter include sandy crevasse splays, muddy floodplain and playa lake facies. Mottled red and gray clays are common in the floodplain successions with soil profiles characterized by carbonate nodules. Root casts are locally abundant in the floodplain soils. Based on vertical stacking of sand fairways observed on well logs, fluvial channels apparently occupied consistent locations on the coastal plain (Morton et al., 1988). Hoel (1982) suggested that poorly developed channel levees likely resulted from the lack of bank-stabilizing vegetation. This is inconsistent with the composition of the Lapara Creek Fauna and the reported presence of abundant fossil wood, both of which indicate a vegetated woodland-savannah mosaic.
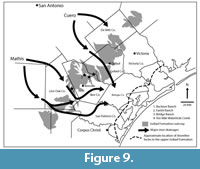 Young et al. (2010) provide separate maps of the lower and upper Goliad Formation that suggest the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas are from the lower Goliad Formation while the Farish and Bucker Ranch Local Faunas are from the upper Goliad. In their model, the lower Goliad Formation in Live Oak, Bee and Goliad counties includes up-dip fluvial meander belt facies adjacent to fluvial/coastal plain facies. Young et al. (2010) also recognized bay/lagoonal facies in the coastal counties extending north into southernmost Bee County. In the upper Goliad Formation, similar fluvial facies were recognized though central Bee and Goliad counties adjacent to fluvial/coastal plain facies in the southern parts of these counties. Shoreline facies were interpreted in San Patricio and Refugio counties suggesting that the shoreline at upper Goliad time was approximately 65-80 km south of the Buckner and Farish Ranch localities (Figure 9).
Young et al. (2010) provide separate maps of the lower and upper Goliad Formation that suggest the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas are from the lower Goliad Formation while the Farish and Bucker Ranch Local Faunas are from the upper Goliad. In their model, the lower Goliad Formation in Live Oak, Bee and Goliad counties includes up-dip fluvial meander belt facies adjacent to fluvial/coastal plain facies. Young et al. (2010) also recognized bay/lagoonal facies in the coastal counties extending north into southernmost Bee County. In the upper Goliad Formation, similar fluvial facies were recognized though central Bee and Goliad counties adjacent to fluvial/coastal plain facies in the southern parts of these counties. Shoreline facies were interpreted in San Patricio and Refugio counties suggesting that the shoreline at upper Goliad time was approximately 65-80 km south of the Buckner and Farish Ranch localities (Figure 9).
Outcrop Observations
The Goliad Formation was observed at multiple localities during the present study, although outcrops are generally limited to modern creek beds and are discontinuous due to abundant vegetation. Local lithology is variable, although gray to white mudstone, muddy sandstone and carbonate nodule conglomerates are common. At TMM 30898, the Goliad Formation is exposed in Blanco Creek and includes light-gray mudstone with well-developed soil profiles, muddy sandstone and carbonate nodule conglomerate.  Fragmentary vertebrate fossils and molds of invertebrates are common in a 15-20 cm thick bed of carbonate nodule conglomerate. This bed is very poorly sorted with a muddy sandstone matrix and pebble-size carbonate nodules. It is laterally discontinuous and pinches out into gray mudstones interpreted to represent floodplain deposits.
Fragmentary vertebrate fossils and molds of invertebrates are common in a 15-20 cm thick bed of carbonate nodule conglomerate. This bed is very poorly sorted with a muddy sandstone matrix and pebble-size carbonate nodules. It is laterally discontinuous and pinches out into gray mudstones interpreted to represent floodplain deposits.
Floodplain mudstones are commonly 1 to 2 m thick and display well-developed pedogenic profiles with distributed carbonate nodules that tend to increase in size and abundance upward towards exposure surfaces (Figure 10). The carbonate pebble conglomerate is interpreted to be sediment reworked from floodplain soils.
Along Medio Creek, just west of the Bridge Ranch localities, the Goliad Formation includes light gray, quartzose sandstone and carbonate-nodule conglomerate (Figure 11). The conglomerate also includes clasts of reddish mudstone, as well as bone fragments. The red mudstone clasts are pebble-cobble size, rounded and probably represent material reworked from the underlying Fleming Formation. These lithologies are very similar to those described by the Survey at the  Bridge Ranch localities. A sample of the Goliad Formation sandstone was analyzed for detrital zircon geochronology at the University of Arizona, Laserchron Center. Another sample was wet-screened because of its close association with bone-bearing conglomerate. The washed concentrate yielded rare bone fragments and bears further analysis.
Bridge Ranch localities. A sample of the Goliad Formation sandstone was analyzed for detrital zircon geochronology at the University of Arizona, Laserchron Center. Another sample was wet-screened because of its close association with bone-bearing conglomerate. The washed concentrate yielded rare bone fragments and bears further analysis.
Slightly farther west, outcrops along Medio Creek, include red fine-grained sandstone and dark-red mudstone overlain unconformably by gray carbonate-nodule conglomerate. The mudstone is lithologically indistinguishable from the clasts seen just to the east in the Goliad Formation. The red beds have a slightly greater south-southeast dip than do the overlying Goliad Formation strata. Weeks (1945) recognized that red clays and sandstones stratigraphically below the Goliad Formation commonly exhibited steeper dips. I interpret these relationships to represent the basal contact of the Goliad Formation along Medio Creek approximately 2.5 km west of the Bridge Ranch fossil localities. This is consistent with comments in Survey quarterly reports that the base of the Goliad Formation was interpreted east of Normanna where red sandstones of the Fleming Formation were overlain by gray caliche-pebble conglomerate containing fossil vertebrates.
Fossil Vertebrate Localities and Local Faunas
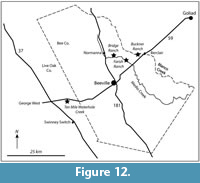 Fossil vertebrates are known from seven primary localities within the Goliad Formation in Bee and Live Oak Counties that comprise the Lapara Creek Fauna. All are located on private land. These seven localities can be grouped into four main locality areas that are treated here as four separate local faunas. Although the geographic separation between some of these localities is not great, uncertainty in their stratigraphic relationships requires independent analysis. From east to west, the four main locality areas/local faunas include Buckner Ranch (TMM 30896, TMM 30898), Farish Ranch (TMM 31081), Bridge Ranch (TMM 31132, TMM 31170, TMM 31184) and Ten Mile Waterhole Creek (TMM 30936) (Figure 12). Baskin and Hulbert (2012) described a small fauna (Dinero Local Fauna) from the upper part of the Goliad Formation near Swinney Switch that they interpreted as late Clarendonian.
Fossil vertebrates are known from seven primary localities within the Goliad Formation in Bee and Live Oak Counties that comprise the Lapara Creek Fauna. All are located on private land. These seven localities can be grouped into four main locality areas that are treated here as four separate local faunas. Although the geographic separation between some of these localities is not great, uncertainty in their stratigraphic relationships requires independent analysis. From east to west, the four main locality areas/local faunas include Buckner Ranch (TMM 30896, TMM 30898), Farish Ranch (TMM 31081), Bridge Ranch (TMM 31132, TMM 31170, TMM 31184) and Ten Mile Waterhole Creek (TMM 30936) (Figure 12). Baskin and Hulbert (2012) described a small fauna (Dinero Local Fauna) from the upper part of the Goliad Formation near Swinney Switch that they interpreted as late Clarendonian.
Three of the four locality areas occur within about 10 km of each other while the Ten Mile Waterhole Creek locality (TMM 30936) is located approximately 28 km west-southwest of Bridge Ranch (Figure 12). As discussed above, regional stratigraphic relationships suggest that the Ten Mile Waterhole Creek and Bridge Ranch localities are located in the lower part of the Goliad Formation while the Farish and Buckner Ranch localities are located higher in the formation. The very limited nature of the Goliad Formation outcrop, with abundant intervening vegetative cover, makes it impossible to unambiguously demonstrate the detailed stratigraphic relationships between the four main locality areas. However, the consistent nature of the very gentle south-southeast dip suggests a stratigraphic order from bottom to top of Ten Mile Waterhole Creek, Bridge Ranch, Farish Ranch and Buckner Ranch. Regional stratigraphic trends could be complicated by unobserved faults. One such fault was recognized by the Survey along Indian Creek to the east of Buckner Ranch.
I  have attempted to locate the original Survey quarry sites and was generally successful. However, 75 years of erosion and vegetation change have made it difficult to unambiguously identify the exact localities of some of the original excavations. In addition, Survey quarterly reports discuss work at the close of each excavation to fill in quarries. TMM 30898 was rediscovered along Blanco Creek with the help of current ranch owner J. Blackburn and became the focus for screen washing matrix (Figure 13.1). The general locations of the Farish Ranch localities along Medio Creek were relocated with the help of the current land owners, but the banks of Medio Creek are generally covered by vegetation (Figure 13.2). At Ten Mile Waterhole Creek, the author was welcomed by J.D. Grant, who was a young boy when the Survey was excavating on his father’s ranch in 1939. He was able to confirm the site shown on the Vertebrate Paleontology Collection locality map as the location of the Survey excavation. The only remaining evidence of the quarry is a shallow depression and some scattered bone fragments (Figure 13.3). R. Bridge kindly led the author onto private land along Medio Creek that included the original Bridge Ranch localities. Float from the Goliad Formation was found at the surface, but these localities are now heavily vegetated (Figure 13.4). Additional outcrops of the Goliad Formation were examined along Medio Creek east of Normanna and contained relatively abundant fossil bone fragments.
have attempted to locate the original Survey quarry sites and was generally successful. However, 75 years of erosion and vegetation change have made it difficult to unambiguously identify the exact localities of some of the original excavations. In addition, Survey quarterly reports discuss work at the close of each excavation to fill in quarries. TMM 30898 was rediscovered along Blanco Creek with the help of current ranch owner J. Blackburn and became the focus for screen washing matrix (Figure 13.1). The general locations of the Farish Ranch localities along Medio Creek were relocated with the help of the current land owners, but the banks of Medio Creek are generally covered by vegetation (Figure 13.2). At Ten Mile Waterhole Creek, the author was welcomed by J.D. Grant, who was a young boy when the Survey was excavating on his father’s ranch in 1939. He was able to confirm the site shown on the Vertebrate Paleontology Collection locality map as the location of the Survey excavation. The only remaining evidence of the quarry is a shallow depression and some scattered bone fragments (Figure 13.3). R. Bridge kindly led the author onto private land along Medio Creek that included the original Bridge Ranch localities. Float from the Goliad Formation was found at the surface, but these localities are now heavily vegetated (Figure 13.4). Additional outcrops of the Goliad Formation were examined along Medio Creek east of Normanna and contained relatively abundant fossil bone fragments.
 Buckner Ranch Site 1 (TMM 30896) has been unambiguously demonstrated by recent re-excavation. This locality was relocated through the work of David Calame and his colleagues with the encouragement of the Blackburn’s in 2016. Based on the original Survey notes and locality maps, brush was cleared and excavations were made at the presumed Site 1 locality on the south side of Blanco Creek (Figure 14).
Buckner Ranch Site 1 (TMM 30896) has been unambiguously demonstrated by recent re-excavation. This locality was relocated through the work of David Calame and his colleagues with the encouragement of the Blackburn’s in 2016. Based on the original Survey notes and locality maps, brush was cleared and excavations were made at the presumed Site 1 locality on the south side of Blanco Creek (Figure 14).
Excavations revealed a stratigraphic section in the Berclair Terrace that included about 4 m of fluvial sandstone, siltstone and mudstone exposed in an overall fining upward succession. The Berclair Terrace strata overlie light-gray mudstones of the Goliad Formation and are capped by a modern soil horizon. The backwall of the original Survey Site 1 was clearly delineated against chaotic backfilled material in which a glass bottle dated 1932 was found. Based on these excavations, the stratigraphy at TMM 30896 (Site 1) is clearly evident as depicted in Sellards (1940) (Figure 15). The light-gray mudstone and muddy sandstone of the Goliad Formation are consistent with matrix associated with fossils collected by the Survey.
 The Lapara Creek Fauna was originally referred to by Quinn (1952) as the Goliad Fauna, but in 1955 he renamed the fauna to be consistent with his interpretation that all of the fossils were from the Lapara Creek member of the Goliad Formation. Wilson (1956) followed this naming convention, as did Patton (1969) and most subsequent authors.
The Lapara Creek Fauna was originally referred to by Quinn (1952) as the Goliad Fauna, but in 1955 he renamed the fauna to be consistent with his interpretation that all of the fossils were from the Lapara Creek member of the Goliad Formation. Wilson (1956) followed this naming convention, as did Patton (1969) and most subsequent authors.
Wilson’s (1962) guidebook introduced some confusion in the list of key localities. His locality #22 is listed as 31132 from Bee County “Near Birnabba”. “Birnabba” is simply an unusual typo of Normanna (if your right hand is moved to the left by one set of keys). TMM 31132 is one of the Bridge Ranch localities. Also, Wilson’s locality #23 is listed as 31080 from near Berclair, Texas. TMM 31080 is actually a Rancholabrean site from the Berclair terrace deposits, but TMM 31081 is the Farish Ranch locality in the Goliad Formation, west of Berclair.
Prothero and Manning (1987) used different names for the Lapara Creek local faunas including the Berclair Local Fauna for TMM 30896 and equated this to the Farish Ranch Local Fauna. TMM 30896 is actually the primary locality for the Buckner Ranch Local Fauna. Prothero and Manning (1987) also used Berclair Local Fauna for TMM 31081, which is the Farish Ranch locality. The term Normanna Local Fauna was shown by these authors as equivalent to the Bridge Ranch Local Fauna, but only one of the Bridge Ranch localities was listed (TMM 31132). Prothero (2005) continued using the same local fauna names. Although the presumed intent was to tie local fauna names to places on a current map, rather than to ranch names that are no longer in use, they serve no purpose in clarifying localities or faunal relationships and are not followed in this paper.
Tedford et al. (1987) used the name Lapara Creek Fauna and suggested that the Bridge Ranch Local Fauna (Cl1) was older than the Farish/Buckner Ranch Local Fauna (Cl2). They made no mention of the Ten Mile Waterhole Creek Local Fauna in their summary. The same terminology and age interpretations were shown in Tedford et al. (2004). Janis et al. (1998) listed GC6 as Goliad Formation (E. Clarendonian-latest Hemphillian) with GC6A as “Lapara Member: Bridge Ranch and Buckner Ranch Local Faunas (E. Clarendonian)” and GC6B as “Lapara Member: Lapara Creek Fauna (inc. Farish Ranch Fauna and Navasota) (E. Clarendonian)”. It is unclear why this nomenclature for the local faunas/faunas was adopted, and it is not followed here.
SYSTEMATIC PALEONTOLOGY
All taxa from the Lapara Creek Fauna are described in the following section. Some taxa are common to multiple local faunas while others are apparently more restricted at least with respect to the current sample. Faunal lists from each local fauna are provided in the later discussion of age and correlation.
Plants
No plant fossils from the Goliad Formation exist in the current collections at The University of Texas at Austin, however, fossil wood was noted in Survey quarterly reports. Fossil wood, including relatively large logs, was reported as abundant at the Ten Mile Waterhole Creek locality (Survey Second Quarterly Report, 1939). Gray claystone was sampled at TMM 30898 and analyzed for fossil pollen, although none was recovered.
 Invertebrates
Invertebrates
Mudstone casts of pelecypods are relatively common. Original shell material is almost never preserved except for two anomalous specimens of Ostrea (TMM 30936-47, TMM 30936-237). Because of their common marine affinity and style of preservation, these two specimens are considered suspicious. It is likely that these specimens were reworked from older marine strata. Pelecypod casts were common in the material collected for screen washing from TMM 30898 but, again, no primary shell material was observed, and all specimens represent forms of freshwater bivalves (Figure 16).
Vertebrates
Vertebrata Indeterminate
(Figure 17)
 One of the most common biologic elements obtained from the matrix washed at TMM 30898 has not yet been identified. Specimens range in size from 1 to 3 mm and include two general end member morphologies with a range of intermediate shapes. Many of the objects are “T-shaped”. One side is relatively smooth but has a pair of ridges that run sub-parallel to the long axis. The other side is slightly bulbous and textured with tiny bumps. Another subset of the objects is teardrop-shaped, again having a side with longitudinal ridges and a textured side. The objects are hollow and broken ones are filled with matrix from the Goliad Formation.
One of the most common biologic elements obtained from the matrix washed at TMM 30898 has not yet been identified. Specimens range in size from 1 to 3 mm and include two general end member morphologies with a range of intermediate shapes. Many of the objects are “T-shaped”. One side is relatively smooth but has a pair of ridges that run sub-parallel to the long axis. The other side is slightly bulbous and textured with tiny bumps. Another subset of the objects is teardrop-shaped, again having a side with longitudinal ridges and a textured side. The objects are hollow and broken ones are filled with matrix from the Goliad Formation.
The association of these objects with isolated fish teeth as the most common elements recovered in the screen washing suggests some sort of aquatic vertebrate. Their abundance suggests a body element equivalent to scales or dermal denticles. They are hollow, show no evidence of wear patterns as seen in teeth and contain no enamel coating. They are composed of a material that macroscopically resembles some of the fossil fish bone from the Goliad Formation. A number of colleagues have suggested that these fossils represent dermal denticles, and they do compare reasonably well with some published figures of fossil shark denticles, but no convincing match has been found. The aquatic fauna (reptiles, fish and invertebrates) indicates a fresh water environment, and the distance from the Goliad Formation outcrops to the Clarendonian shoreline was 65-80 km. There are no fossil shark or ray teeth observed in the Goliad Formation in the study area. Other suggestions have included catastomid breeding tubercles or freshwater ray elements.
Class ACTINOPTERYGII Cope, 1887
Order LEPISOSTEIFORMES Hay, 1929
Family LEPISOSTEIDAE Cuvier, 1825
Genus LEPISOSTEUS Lacepè de, 1803
Lepisosteus sp.
Localities. Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30898).
Referred specimens. TMM 31132-457, TMM 31081-121, -122, -325, -615.
Description. Isolated, diamond shaped scales (5-25 mm).
Discussion. Isolated gar scales are present at multiple localities, but not common. None of the scales show annual growth rings as described by Voorhies (1969). Lepisosteus is a common lepisosteid fish in North America since the Cretaceous. Modern forms are found in streams, rivers, lakes, bayous, and estuaries and generally inhabit slow-water environments.
Order SILURIFORMES Cuvier, 1817
Family ICTALURIDAE Gill, 1861
Genus ICTALURUS Rafinesque, 1820
Ictalurus cf. lambda Hubbs and Hibbard, 1951 (Figure 18)
 Localities. Ten Mile Waterhole Creek (TMM 30936), Farish Ranch (TMM 31081).
Localities. Ten Mile Waterhole Creek (TMM 30936), Farish Ranch (TMM 31081).
Referred specimens. TMM 30936-312 pectoral spine, TMM 31081-1059 articular, -1127 pectoral spines (5), -1284 pectoral spines (2).
Description. Pectoral spines range from 68‒83 mm in length with barbs along the posterior edge set in a deep groove. The spines are dorsoventrally compressed and the sides are textured with curvilinear grooves/ridges. The anterior edge is a sharp ridge with marginal grooves on either side and has small dentations. The anterior process is well developed and L-shaped in ventral view as in the holotype of Ictalurus lambda (Hubbs and Hibbard, 1951). The width of the anterior fossa ranges from 2.5 to 3 mm.
Discussion. Multiple pectoral spines and one articular bone from an ictalurid catfish are known from Farish Ranch and a single spine from Ten Mile Waterhole Creek. Lundberg (1975) included the Lapara Creek specimens in Ictalurus lambda based on the size and morphology of the pectoral spines. The pectoral spines are similar in morphology and size to the type material of I. lambda (Hubbs and Hibbard, 1951), and to the spine figured by Lundberg (1975, plate VIII, G) of I. lambda from the Valentine Formation in Nebraska (Barstovian-Clarendonian, Voorhies, 1969). I. lambda was named by Hubbs and Hibbard (1951) on the basis of partial pectoral spines, however, as noted by Lundberg (1975), I. lambda is very similar to the modern catfish I. furcatus. The only consistent difference between the two taxa appears to be the presence of tubercles on the cleithral process in I. lambda. No catfish cleithra are known from the Lapara Creek Fauna.
Ictalurus has been reported from the Oligocene though Recent of North America and I. lambda has been recognized in Miocene faunas from Kansas, Nebraska and Florida (Lundberg, 1975). I. furcatus is commonly found in rivers and occasionally in estuarine environments.
Genus PYLODICTIS Rafinesque, 1819
Pylodictis olivaris Rafinesque, 1818
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081).
Referred specimens. TMM 30936-111 centrum; TMM 31132-767 centrum; TMM 31081-99 pectoral spine, --367 pectoral spine, -624 centrum (2), -850 centrum (2), -872 dentary.
Description. A number of specimens are represented by disc-shaped centra. The pectoral spines are significantly smaller than those referred to Ictalurus lambda and have barbs on both the anterior and posterior edges.
Discussion. Lundberg (1975) included a number of specimens from the Farish Ranch Local Fauna in Pylodictis olivaris. P. olivaris is the extant Flathead Catfish common to rivers and lakes throughout much of the central and eastern U.S. P. olivaris is the only known species of Pylodictis and is recognized from Miocene through Recent faunas (Lundberg, 1975).
Class ACTINOPTERYGII Cope, 1887
Indeterminate (Figure 19)
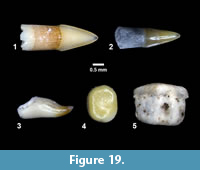 Localities. Buckner Ranch (TMM 30898).
Localities. Buckner Ranch (TMM 30898).
Referred Specimens. TMM 30898-29, -30, -31, -32, -33 isolated teeth. Numerous additional teeth in collection not assigned specimen numbers.
Description. Isolated teeth, blade-like to peg-like, 1-3 mm.
Discussion. Multiple types of isolated fish teeth were recovered from screen wash concentrate at TMM 30898 (Figure 19). Three primary morphologies include blade-like teeth with striated “roots”, hook-like teeth and peg-like teeth. It is likely that at least some of the blade-like teeth represent an ictalurid catfish based on comparison with modern specimens of Ictalurus and Pylodictis. The hook-like and peg-like teeth are similar to pharyngeal teeth of some modern catostomids or sciaenids (Eastman, 1977). The presence of these small and delicate teeth indicates that the screen washing process was not responsible for destroying small specimens, and that enamel is well preserved.
Class REPTILIA Laurenti, 1768
Order TESTUDINES Linnaeus, 1758
Family EMYDIDAE Bell, 1828
Genus TRACHEMYS Agassiz, 1857
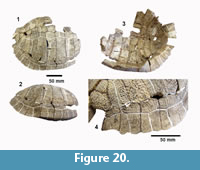
cf. Trachemys sp. Agassiz, 1857 (Figure 20, Figure 21)
 Localities. Farish Ranch (TMM 31081).
Localities. Farish Ranch (TMM 31081).
Referred specimens. TMM 31081-280.
Description. A relatively complete carapace and partial plastron represents a turtle that was approximately 250-300 mm long by 210-220 mm wide with a carapace height of approximately 100-120 mm (preserved dimensions are 220 mm x 185 mm x 95 mm). The carapace is strongly sculpted while the plastron is relatively smooth with only faint dimpling (Figure 20). The neurals are smooth and slightly keeled, while the costals and dorsal surface of the peripherals are strongly textured into ridges and dimples.
The nuchal bone (TMM 31081-280) is preserved, however, the adjacent peripherals are missing. The nuchal measures 26.9 mm at the anterior edge and is deeply notched with texturing on the dorsal surface that becomes smooth toward the anterior edge (Figure 21). The first vertebral scute is somewhat restricted anteriorly, and the anterolateral border is wholly contained within the nuchal. The first pleural scutes overlap the lateral edges of the nuchal bone. The four posterior peripherals have notches at the seams between marginal scutes and at the sutures. The pygal is missing although two suprapygals are present, the posterior being broad. There are eight costals and presumably eight neurals although only five are preserved in this specimen. A complete xiphiplastron (right and left sides) is 115.2 mm wide, by 57.4 mm in length. No vertebrae, limb bones or cranial material are known.
 Discussion. Lacking a skull, it is difficult to confidently differentiate genera within the emydid subfamily Deirochelyinae. Seidel and Smith (1986) concluded that Chrysemys, Pseudemys and Trachemys were distinct extant genera, and they included features of the carapace in their diagnoses. According to Seidel and Smith (1986) Pseudemys and Trachemys overlap in size, both having a rugose carapace (unlike Chrysemys) with serrated posterior peripherals. Pseudemys is described with a weakly keeled carapace while Trachemys is described as “usually” keeled. Seidel and Jackson (1990) recognized a series of characters to differentiate Trachemys from Pseudemys, Chrysemys, and Graptemys and proposed a phylogenetic model for these taxa. Based on these characters, TMM 30181-280 is most closely comparable to Trachemys. Seidel and Jackson (1990) proposed a Miocene or pre-Miocene origin for Trachemys, but only recognized T. hilli and T. inflata as valid late Miocene examples.
Discussion. Lacking a skull, it is difficult to confidently differentiate genera within the emydid subfamily Deirochelyinae. Seidel and Smith (1986) concluded that Chrysemys, Pseudemys and Trachemys were distinct extant genera, and they included features of the carapace in their diagnoses. According to Seidel and Smith (1986) Pseudemys and Trachemys overlap in size, both having a rugose carapace (unlike Chrysemys) with serrated posterior peripherals. Pseudemys is described with a weakly keeled carapace while Trachemys is described as “usually” keeled. Seidel and Jackson (1990) recognized a series of characters to differentiate Trachemys from Pseudemys, Chrysemys, and Graptemys and proposed a phylogenetic model for these taxa. Based on these characters, TMM 30181-280 is most closely comparable to Trachemys. Seidel and Jackson (1990) proposed a Miocene or pre-Miocene origin for Trachemys, but only recognized T. hilli and T. inflata as valid late Miocene examples.
In their review of amphibians and turtles from the Miocene Calvert Formation, Weems and George (2013) assigned a single posterior peripheral found in float to an indeterminate species of Trachemys. Zug (2001) also assigned a few isolated specimens from the Miocene-Pliocene Lee Creek Mine to “Chrysemys complex” and suggested that both Pseudemys and Trachemys were probably represented. Trachemys has been recognized from multiple Hemphillian faunas in North America including occurrences in Florida, Nebraska, and Tennessee (Hulbert (2001), Holman and Parmley (2005) and Parmalee et al. (2002)). Jasinski (2018) provided the most complete description of fossil Trachemys to date when he named Trachemys haugrudi from the late Hemphillian Gray Fossil Site in Tennessee (previously identified as Trachemys cf. inflata by Parmalee et al., 2002). T. haugrudi exhibits a number of very derived characters and has strongly serrated peripherals on the posterior carapace.
Fritz et al. (2012) analyzed the phylogeny, systematics, and biogeography of Central and South American slider turtles using sequence data from mitochondrial DNA. They concluded that Trachemys was a monophyletic group that diverged from its sister group at approximately 13 to 15 Ma. The early Clarendonian Farish Ranch occurrence of cf. Trachemys appears to be the earliest record of this genus in North America, consistent with the interpretation of Fritz et al. (2012). Jasinski (2018) provided a detailed discussion of the phylogenetic relationships within Trachemys and also concluded that the genus is monophyletic with multiple clades, both extinct and extant that are separated biogeographically. Although modern Trachemys is known to be saltwater tolerant for extended periods of time, it is commonly found in fresh water environments (Dunson and Seidel, 1986).
Genus TERRAPENE Merrem, 1820
cf. Terrapene sp.
Localities. Bridge Ranch (TMM 31132).
Referred specimens. TMM 31132-811 carapace fragment.
Description. TMM 31132-811 is a fragment of a carapace from a small turtle with an irregular or lumpy surface texture and sulci reflecting the presence of scutes. The fragment is thin (~4.5-5 mm) and is highly curved, reflecting a domed carapace.
Discussion. A single carapace fragment represents a relatively small, high-domed turtle most closely resembling the modern box turtle (Terrapene). The Miocene record of Terrapene in the USA was reviewed by Holman and Fritz (2005) including a number of occurrences from the Barstovian and Clarendonian of the Great Plains. In their description of the late Hemphillian T. parornata, Joyce, et al. (2012) concluded that the record of the Terrapene lineage extends back to the Barstovian of Nebraska, but the early occurrences are fragmentary and some are of uncertain provenance. The single specimen referred to cf. Terrapene from the Lapara Creek Fauna is too fragmentary to contribute to these discussions.
Family EMYDIDAE Bell, 1828
Emydidae indet. (Figure 22)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081).
 Referred specimens. TMM 30936-38 carapace/plastron fragments, -42 carapace fragment; TMM 31132-774 plastron fragments; TMM 31081-14 plastron fragment, -140 plastron fragments, -163 plastron fragment, -277 carapace fragment, -544 plastron, -888 posterior peripherals.
Referred specimens. TMM 30936-38 carapace/plastron fragments, -42 carapace fragment; TMM 31132-774 plastron fragments; TMM 31081-14 plastron fragment, -140 plastron fragments, -163 plastron fragment, -277 carapace fragment, -544 plastron, -888 posterior peripherals.
Description. A number of specimens represent medium to large size turtles with smooth carapaces and plastrons. TMM 31081-163 is a partial plastron approximately 34.4 cm long and 20.4 cm wide with a relatively thin shell of 5-15 mm (Figure 22.1). The ventral surface is smooth and the posterior edge of the xiphiplastron is relatively straight. TMM 31081-14 is the right half of a plastron approximately 35.4 cm long and 11.4 cm wide (full width would have been 22.8 cm) (Figure 22.2-22.3). The shell is smooth with a thickness of 5-15 mm. The anterior edge of the epiplastron is gently curved.
Discussion. Carapace and plastron fragments are identified as Emydidae indeterminate. They are all smoothly textured and relatively thin shelled. Estimated lengths of 35-40 cm for the original turtles compare well with the relatively large Pseudemys common today in ponds, lakes and streams from the Rio Grande to Florida.
Family TRIONYCHIDAE Gray, 1825
Genus APALONE Rafinesque, 1832
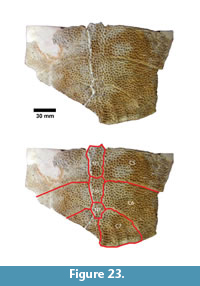
Apalone sp. (Figure 23, Figure 24)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31184), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
 Referred specimens. TMM 30936-58, -130, -168, -200, -230, -246, -266, -318, -340, -416 carapace fragments; TMM 31184-6 costal fragments; TMM 31132-8, -769, -812 carapace and plastron fragments, -519 costal, -521 plastron fragments; TMM 31081-139 xiphiplastron, -145 carapace fragment, -308 plastron fragment, -432 carapace fragment, -555, -601 plastron fragments, -725, -731, -737 costals, -864 neural, -1130 costal, -1488 hypoplastron, -1562 carapace fragments; TMM 30896-144 hyoplastron-hypoplastron, -377 costals, -635, -636 plastron fragments.
Referred specimens. TMM 30936-58, -130, -168, -200, -230, -246, -266, -318, -340, -416 carapace fragments; TMM 31184-6 costal fragments; TMM 31132-8, -769, -812 carapace and plastron fragments, -519 costal, -521 plastron fragments; TMM 31081-139 xiphiplastron, -145 carapace fragment, -308 plastron fragment, -432 carapace fragment, -555, -601 plastron fragments, -725, -731, -737 costals, -864 neural, -1130 costal, -1488 hypoplastron, -1562 carapace fragments; TMM 30896-144 hyoplastron-hypoplastron, -377 costals, -635, -636 plastron fragments.
Description. The most common turtle in the Lapara Creek Fauna is a trionychid. External surfaces of carapace and plastron fragments exhibit the sculptured texture characteristic of pan-trionychids, and both peripherals and scutes are absent. Costal margins are smooth and terminate distally in a convex to sub-pointed rib (following Gardner and Russell, 1994). The more complete specimens suggest an oval to sub-circular carapace shape similar to Apalone spinifera. Carapace and plastron thicknesses range from 8-12 mm. Assuming that a complete carapace would include a nuchal and seven costals, the larger specimens represent carapaces with a midline length of approximately 350-400 mm and a width of approximately 300 mm. The external texture of the carapace and plastron elements varies spatially from honeycomb to linear ridge patterns.
TMM 31081-145 is a partial carapace with neurals five-seven and costals five-seven (Figure 23). The posterior margins of the neurals are either convex or flat, and neural seven is very reduced. The suture between costals five and six intersects neural six and the suture between costals six and seven intersects neural seven. The seventh costals are the only preserved left and right pair that intersect along the midline.
 The hypoplastron (TMM 31081-1488, TMM 30896-144) has a strongly indented posterior margin as in A. spinifera and A. mutica (Figure 24). A partial xiphiplastron (TMM 31081-139) is very similar to mature A. spinifera.
The hypoplastron (TMM 31081-1488, TMM 30896-144) has a strongly indented posterior margin as in A. spinifera and A. mutica (Figure 24). A partial xiphiplastron (TMM 31081-139) is very similar to mature A. spinifera.
Discussion. Although comprised exclusively of carapace and plastron elements, specimens from the Lapara Creek Fauna represent one of the more complete records of early Clarendonian trionychids in North America. The Lapara Creek material can be diagnosed as a member of Pan-Trionychidae by the absence of scutes and peripherals and the presence of sculpturing that covers both carapace and plastron bones (following Vitek and Joyce, 2015). Matthew (1924) named Platypeltis mioceanus from the Snake Creek beds on the basis of a partial carapace. Vitek and Joyce (2015) referred this taxon to “Trionyx” mioceanus and considered it to be the only valid trionychid taxon from the Miocene of North America. Jasinski (2013) identified a trionychid carapace from a Hemphillian fauna in Alabama as Apalone cf. spinifera. Weems and George (2013) identified carapace and plastron fragments from the Miocene Calvert Formation in Delaware and Virginia as Apalone lima, but that material is very fragmentary and the diagnosis is largely based on the pattern of sculpting. A. lima is also probably a nomen dubium. Hulbert and Whitmore (2006) mention the presence of abundant Apalone specimens from the early Hemphillian Mauvilla Local Fauna in Alabama, but provided neither descriptions nor figures. Holman and Corner (1985) reported Trionyx sp. indet. from the Barstovian Myers Farm Local Fauna in Nebraska. Most workers have used the name Apalone for Neogene trionychids from North America following Meylan (1987), although a high degree of morphologic homoplasy in Trionychids adds to taxonomic uncertainty when faced with limited material (Vitek and Joyce, 2015). Valdes et al. (2017) named a new species Apalone amorense from the late Clarendonian Love Bone Bed in Florida that had previously been referred to Apalone (Trionyx) cf. ferox by Webb et al. (1981).
The Lapara Creek trionychid differs from Apalone amorense by its significantly larger size, shape of the hypoplastron, and the relationship of costal sutures and neurals (five-seven) in the carapace. It differs from the holotype of A. mioceanus (AMNH 6298) (Matthew, 1924) by having a more sub-rounded carapace. Relative to extant species of Apalone, the Lapara Creek specimens are similar to A. spinifera and A. mutica in the shape of the hypoplastron, and similar to A. spinifera in the geometry of the carapace. The geometry of costals and neurals in TMM 31081-145 is very similar to A. spinifera and different from A. ferox as figured in Gardner and Russell (1994, figure 1). Carapace dimensions are within the range of A. spinifera and A. ferox, but the latter has a more elongated, ovoid carapace. The record of Apalone from the Lapara Creek Fauna is one of the earliest occurrences of this taxon in North America. Although the available material is very similar to A. spinifera, without a skull, specific identification is impossible. The paleontological record differs from the DNA-based divergence estimates of Li et al. (2017) who suggested separation of Apalone in the Late Cretaceous and separation of A. ferox and A. spinifera in the Late Oligocene. This differs from prior results of a similar analysis by Le et al. (2014) who recognized divergence of Apalone in the Eocene and of the extant species of Apalone in the Miocene.
Apalone is common in creeks, rivers, ponds and lakes throughout the greater Mississippi River system and Gulf Coast.
Suborder CRYPTODIRA Cope, 1868
Family TESTUDINIDAE Gray, 1825
Genus Gopherus Rafinesque, 1832
cf. Gopherus sp. (Figure 25, Figure 26)
Localities. Farish Ranch (TMM 31081).
 Referred specimens. TMM 31081-1314 carapace and plastron.
Referred specimens. TMM 31081-1314 carapace and plastron.
Description. TMM 31081-1314 is a medium-size tortoise with a carapace size of 46.5 x 30.5 cm. The plastron is strongly concave (Figure 25). Although badly fractured, the shell was relatively broad and of moderate height, neural one and three are octagonal, and the hyoplastron and hypoplastron are subequal in length measured along the midline of the plastron. The nuchal is broader than long, and the peripherals define a relatively smooth edge (Figure 26).
 Discussion. A relatively complete shell of a fossil tortoise is assigned to cf. Gopherus sp. on the basis of size and shell morphology. Auffenberg (1976) described diagnostic characters of the shell of Gopherus as including octagonal alternate anterior neurals, hypoplastron and hyoplastron of sub-equal length, with shell broad and of moderate height. The nuchal compares favorably with Gopherus cf. polyphemus figured by Franz and Quitmyer (2005). TMM 31081-1314 is smaller than Hesperotestudo and within the size range of Gopherus (Auffenberg, 1976).
Discussion. A relatively complete shell of a fossil tortoise is assigned to cf. Gopherus sp. on the basis of size and shell morphology. Auffenberg (1976) described diagnostic characters of the shell of Gopherus as including octagonal alternate anterior neurals, hypoplastron and hyoplastron of sub-equal length, with shell broad and of moderate height. The nuchal compares favorably with Gopherus cf. polyphemus figured by Franz and Quitmyer (2005). TMM 31081-1314 is smaller than Hesperotestudo and within the size range of Gopherus (Auffenberg, 1976).
Although the early history of Gopherus is somewhat uncertain, Reynoso and Montellano-Ballesteros (2004) suggested an Oligocene origin for the genus in North America. There have been a number of reported occurrences of Gopherus from Hemingfordian and Barstovian faunas of the central plains and Gopherus sp. was reported from the Clarendon Fauna of Donley County, Texas (Schultz, 1990, McCord, 2002).
Genus HESPEROTESTUDO Williams, 1950
Hesperotestudo sp. (Figure 27)
Localities. Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
 Referred specimens. TMM 31132-82 dermal ossicle, -460 humerus, -770 carapace and plastron fragments; TMM 31081-82 caudal vertebrae, -364 dermal ossicle, -600 peripheral, -883 peripheral, -921 dermal ossicle, -1419 dermal ossicle; TMM 30896-328 carapace.
Referred specimens. TMM 31132-82 dermal ossicle, -460 humerus, -770 carapace and plastron fragments; TMM 31081-82 caudal vertebrae, -364 dermal ossicle, -600 peripheral, -883 peripheral, -921 dermal ossicle, -1419 dermal ossicle; TMM 30896-328 carapace.
Description. TMM 30896-328 is a carapace from a very large tortoise with a length of 85 cm and a width of 56 cm. Due to the degree of crushing and state of preservation, individual bones are difficult to identify but the shell is smoothly textured. Isolated peripherals like TMM 31081-600 represent even larger individuals, are smoothly textured, possess sulci reflecting the presence of scutes, and can be very thick (38 mm) (Figure 27.1-27.2). The caudal vertebra (TMM 31081-82) has very elongated transverse processes with a maximum width of 68.3 mm (Figure 27.3). A number of large dermal ossicles were recovered from multiple localities. TMM 31132-460 is a humerus assigned to Hesperotestudo sp. based on size with a mid-length shaft diameter of 31 mm, and a minimum length of 145 mm although both ends are broken.
Discussion. The large tortoise from the Lapara Creek Fauna is referred to the genus Hesperotestudo rather than Geochelone following Meylan and Sterrer (2000). Lacking a skull, specific assignment is not possible. The very large size, smooth carapace, elongated transverse processes on the caudal vertebra and the presence of dermal ossicles are consistent with Hesperotestudo.
Hesperotestudo has a reported range of Oligocene to Pleistocene in North America (Meylan and Sterrer, 2000) with occurrences in the type Clarendon faunas of North Texas (Schultz, 1990), as well as Clarendonian through Hemphillian faunas from the Great Plains (Czaplewski, 2008). The presence of this large, presumably non-burrowing tortoise suggests a relatively warm, equable climate (Hibbard, 1960).
Family ALLIGATORIDAE Gray, 1844
Genus ALLIGATOR Cuvier, 1807
Alligator cf. mississippiensis Daudin, 1801
(Figure 28, Figure 29, Figure 30, Figure 31)
 Localities. Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 30181), Buckner Ranch (TMM 30896).
Localities. Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 30181), Buckner Ranch (TMM 30896).
Referred specimens. TMM 31132-87, -520 osteoderms, -530, -766 teeth; TMM 31170-31, -42, -226 teeth; TMM 31081-33 teeth, -40 mandible fragment with symphysis, -48, -78, -106 teeth, -138 osteoderms, -215 left squamosal, -237, -276 osteoderms, -385 skull fragment, -400 femur, -579 humerus, -584 partial skull (left maxillary, partial right maxillary, partial nasals), -758 proximal rib fragment, -810 right premaxillary, -1393 mandible fragment, -1417 parietal, -1473 parietal; TMM 30896-280 partial skull, -335, -372 osteoderms, -357, -336 teeth.
Description. Cranial and post-cranial crocodilian material is present in three of the four local faunas. The partial skulls and mandibles exhibit a relatively broad, flat snout with upturned edges of the orbits, dorsally oriented nares and large external mandibular fenestra (Figure 28).
 The preserved splenial on TMM 31081-40 terminates approximately 10.0 mm prior to the dentary symphysis, but the dorsal portion of rostral tip is missing and, just anterior to this break, the mandible exhibits rugosity similar to the splenial suture (Figure 29). The Meckelian groove and the foramen intermandibularis oralis are exposed on the mandible. It is not possible to determine if an anterior foramen was present on the rostral tip of the splenial. The splenial suture is evident on the mandible posterior to the fragment of splenial preserved. The ventral portion of the splenial is very thin and, although it terminates near a break in the mandible, it is not clearly broken itself. Anterior to the ventral portion of the splenial, the mandible is smooth as in Alligator mississippiensis. The posterior termination of the symphysis is between the third and fourth tooth (Figure 30).
The preserved splenial on TMM 31081-40 terminates approximately 10.0 mm prior to the dentary symphysis, but the dorsal portion of rostral tip is missing and, just anterior to this break, the mandible exhibits rugosity similar to the splenial suture (Figure 29). The Meckelian groove and the foramen intermandibularis oralis are exposed on the mandible. It is not possible to determine if an anterior foramen was present on the rostral tip of the splenial. The splenial suture is evident on the mandible posterior to the fragment of splenial preserved. The ventral portion of the splenial is very thin and, although it terminates near a break in the mandible, it is not clearly broken itself. Anterior to the ventral portion of the splenial, the mandible is smooth as in Alligator mississippiensis. The posterior termination of the symphysis is between the third and fourth tooth (Figure 30).
A partial skull (TMM 30896-280, Figure 31) includes a quadrate that is ~149 mm wide, an interorbital width of 38.6 mm and orbital dimensions of 96 x 57 mm. The maximum dimensions of the cranial table are length = 87.5 mm and width = 155 mm. The cranial table narrows from approximately 155 mm at the posterior edge to 114 mm at the anterior corner. TMM 31081-215 is a squamosal from an alligator that was significantly larger than TMM 30896-280 (equivalent lengths of 82 mm for TMM 31081-215 vs. 48 mm for TMM 31081-280 and maximum thicknesses of 28 mm vs. 16.6 mm). Numerous preserved teeth are round to slightly oval in cross-section. and osteoderms have a strong central ridge.
 Discussion. The most diagnostic material, including partial skulls and mandibles from the Farish and Buckner Ranch Local Faunas, can be assigned to the genus Alligator based on the relatively broad, flat snout, upturned edges of the orbits, dorsally oriented nares and large external mandibular fenestra.
Discussion. The most diagnostic material, including partial skulls and mandibles from the Farish and Buckner Ranch Local Faunas, can be assigned to the genus Alligator based on the relatively broad, flat snout, upturned edges of the orbits, dorsally oriented nares and large external mandibular fenestra.
There are three species of Alligator generally regarded as valid from the late Miocene including A. olseni (White, 1942), A. thomsoni (Mook, 1923) and A. mefferdi (Mook, 1946), although Malone (1979) in an unpublished dissertation synonymized the latter two with A. mississippiensis. Relationships between these taxa are discussed in Brochu (1999), Snyder (2007) and Whiting et al., (2016). The Lapara Creek alligator differs to varying degrees from each of these taxa and appears most similar to the extant A. mississippiensis. Distinctions between the Miocene forms of Alligator are related to morphology of the skull and mandible and specifically the nature of the splenial. The lack of a splenial suture between the preserved splenial and the symphysis on the Lapara Creek alligator differs from A. olseni. Brochu (1999) suggested that while very similar, A. mefferdi could be distinguished from A. mississippiensis by the presence of the anterior foramen intermandibularis oralis in the splenial, a character never observed in the latter taxon. The splenial on the Lapara Creek alligator appears to terminate posterior of the foramen. Mook (1923, pg. 11) described the splenial on the paratype specimen (AMNH 1737) of A. thomsoni as extending to “within about one millimeter of the symphysis”. However, Malone (1979) interpreted this specimen to represent A. mcgrewi while stating that the other mandibles referred to A. thomsoni lacked splenials. Brochu (1999, pg. 100) stated that none of the mandibles of A. thomsoni from the holotype locality possess splenials so this character was unknown. A. mcgrewi differs from the Lapara Creek alligator by exhibiting a very blunt snout.
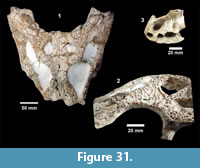 On TMM 30896-280, the pre-orbital step is much gentler than in A. olseni, a distinctive feature noted by White (1942) and described as similar to A. thomsoni. The Lapara Creek alligator has less prominent ridges along the interior margin of the orbitals and around the supratemporal fenestrae than in A. mefferdi. The mandible is relatively longer and not as robust as in A. thomsoni and the orbitals are more elongated. Whiting et al. (2016) reviewed the Miocene occurrences of Alligator in North America and tentatively assigned material from the Hemphillian Moss Acres Racetrack site in Florida to A. mississippiensis. Preserved morphologies and relative dimensions for the alligator from the Lapara Creek Fauna are most similar to A. mississippiensis.
On TMM 30896-280, the pre-orbital step is much gentler than in A. olseni, a distinctive feature noted by White (1942) and described as similar to A. thomsoni. The Lapara Creek alligator has less prominent ridges along the interior margin of the orbitals and around the supratemporal fenestrae than in A. mefferdi. The mandible is relatively longer and not as robust as in A. thomsoni and the orbitals are more elongated. Whiting et al. (2016) reviewed the Miocene occurrences of Alligator in North America and tentatively assigned material from the Hemphillian Moss Acres Racetrack site in Florida to A. mississippiensis. Preserved morphologies and relative dimensions for the alligator from the Lapara Creek Fauna are most similar to A. mississippiensis.
Some of the material from the Lapara Creek Fauna represents large individuals approximately 10-15 feet in length based on comparison with modern skeletal material of A. mississippiensis. This presence of Alligator is consistent with a meandering fluvial environment with local ponds and lakes. It also suggests that the climate was relatively warm and equable with few subfreezing temperatures during winter months. Alligators are relatively common today in Bee and Live Oak Counties and have been reported from as far north as San Antonio, Texas, within the San Antonio and Guadalupe River drainage systems.
Class AVES Linnaeus, 1758
Order Anseriformes Wagler, 1831
Family Anatidae Leach, 1820
cf. Anserinae Vigors, 1825 (Figure 32.1)
Localities. Farish Ranch (TMM 31081).
Referred specimens. TMM 31081-1263 ulna.
Description. TMM 31081-1263 is a partial ulna with the proximal end preserved. The maximum width of the proximal articular structure is 12.9 mm and the preserved length is ~102 mm.
Discussion. TMM 31081-1263 is similar in size and morphology to modern examples of geese. It compares well with material assigned to Branta by Bickart (1990) from the Hemphillian Big Sandy Formation, Arizona.
Order Ciconiiformes Bonaparte, 1854
Family Ciconiidae Gray, 1840
cf. Mycteria sp. Linnaeus, 1758 (Figure 32.2)
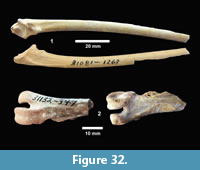 Localities. Bridge Ranch (TMM 31132).
Localities. Bridge Ranch (TMM 31132).
Referred specimens. TMM 31132-347 tarsometatarsus.
Description. TMM 31132-347 is a partial distal end of a tarsometatarsus that includes the trochlea metatarsi II and III. The maximum width across these preserved trochlea is 15.3 mm. A dorsoplantar foramen is adjacent to the trochlea metatarsi III. Although fragmentary, this specimen preserves enough morphology to recognize a close similarity to the modern wood stork including the geometry of the trochlea and the position of the dorsoplantar foramen.
Discussion. Birds are not well represented in the Lapara Creek Fauna probably due to the collecting techniques employed by the Survey and also to poor preservation of small bones. The two identifiable birds are consistent with a freshwater wetland or swampy environment. Modern wood storks are waders and feed primarily on small fish in shallow water. Geese are found in a wide variety of habitats including, but not restricted to, lakes, rivers and marshes.
Class MAMMALIA Linnaeus, 1758
Order LIPOTYPHLA Haeckel, 1866
Family TALPIDAE Fischer de Waldheim, 1817
Genus DOMNINOIDES Green, 1956
cf. Domninoides sp.
Localities. Bridge Ranch (TMM 31132).
Referred specimens. TMM 31132-531 partial left humerus; TMM 30898-22 partial M.
Description. A partial humerus of a mole is present in the Bridge Ranch Local Fauna. The maximum preserved distal width is 7.9 mm and the maximum length is 9.5 mm. The proximal end of the humerus is broken and the specimen appears slightly abraded. A partial upper molar was recovered in screen washing.
Discussion. TMM 31132-531 represents a small mole. This specimen compares very favorably in size and morphology with both the Barstovian Domninoides sp. from Quartz Basin, Oregon (Hutchison, 1968) and with a humerus (MWSU 12897) from the early Clarendonian Wisenhunt Quarry, Oklahoma. The latter specimen is smaller than the humerus (WTSU-V-7005) of D. hessei figured by Dalquest et al. (1996, figure 5) and probably represents D. knoxjonesi. The partial molar is comparable in size and morphology to D. knoxjonesi, also described by Dalquest et al. (1996), but is too incomplete for confident identification.
Order LAGOMORPHA Brandt, 1855
Family LEPORIDAE Fischer de Waldheim, 1817
Subfamily ARCHEOLAGNIAE Dice, 1917
Genus PRONOTOLAGUS White, 1991
cf. Pronotolagus apachensis Gazin, 1930
(Figure 33)
Localities. Buckner Ranch (TMM 30898).
Referred specimens. TMM 30898-24 P2, -25, -26, -27, -28 p/m.
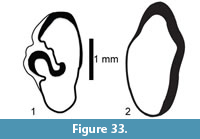 Description. TMM 30898-24 is a P2 that was recovered during screen washing (Figure 33.1). It represents a small species of rabbit with a length of 1.4 mm and a width of 2.45 mm. The depth of the main anterior reentrant (MAR) is 0.8 mm and that of the external anterior reentrant (EAR) is 0.3 mm. Simple crenulations in the enamel are present on the lingual wall of the MAR. Cementum is present filling both anterior reentrants. The enamel thickens significantly along the lingual side of the tooth and the labial side of the MAR. A number of partial cheek teeth were also recovered.
Description. TMM 30898-24 is a P2 that was recovered during screen washing (Figure 33.1). It represents a small species of rabbit with a length of 1.4 mm and a width of 2.45 mm. The depth of the main anterior reentrant (MAR) is 0.8 mm and that of the external anterior reentrant (EAR) is 0.3 mm. Simple crenulations in the enamel are present on the lingual wall of the MAR. Cementum is present filling both anterior reentrants. The enamel thickens significantly along the lingual side of the tooth and the labial side of the MAR. A number of partial cheek teeth were also recovered.
Discussion. The Lapara Creek rabbit is smaller than Hypolagus vetus but comparable to both H. fontinalis and to forms from the Clarendonian Cuyama Fauna in California. H. fontinalis was described by Dawson (1958), but the hypodigm included only lower teeth and one upper incisor. White (1987) published an emended diagnosis for this taxon and stated that P2 included a shallow EAR and deep MAR. Voorhies and Timperley (1997) figured numerous P2s that they assigned to H. fontinalis with very simple reentrants having no crenulations.
Gazin (1930) named H. apachensis from the Cuyama Fauna and noted that P2 had two anterior “infolds” and the internal reentrant fold extended beyond the middle of the tooth and possessed crenulations. Wood (1937) continued the usage of H. apachensis in his paper on the Cuyama Fauna and provided measurements for 3 P2 (APL: 1.1-1.25 mm, W: 2.04-2.23 mm). Based primarily on size, some of the Cuyama material was later separated into a new taxon that White (1987) named H. tedfordi based on lower dentitions. Later, White (1991) proposed a new genus Pronotolagus, for the apachensis morphotype with a diagnosis based solely on the morphology of p3 and with no upper teeth included in the hypodigm. Dalquest et al. (1996) followed White’s proposed taxonomy and assigned isolated teeth from the Clarendonian Wisenhunt Quarry Local Fauna, Oklahoma to P. apachensis. These isolated teeth included upper molariform teeth, but no P2. P. albus (Voorhies and Timperley, 1997), P. whitei (Korth, 1998) and P. nevadensis (Kelly, 2000) have since been proposed, but none of these species descriptions included P2.
Although P2 of Pronotolagus has not been described explicitly, the small P2s from Cuyama described by Gazin (1930) as Hypolagus apachensis, and presumably reflecting P. apachensis, provide the closest comparison with the Lapara Creek rabbit.
Prothero et al. (2008) correlated the magnetostratigraphic pattern from the Apache Canyon section of the Caliente Formation with early Clarendonian Chrons C5An through C5R. Tedford et al. (2004) correlated the Matthews Ranch Fauna from the Caliente Formation with Cl1 and early Cl2.
Order RODENTIA Bowdich, 1821
Superfamily Geomyoidea Bonaparte, 1845
Family HETEROMYIDAE Gray, 1868
Genus PEROGNATHUS Maximillian, 1839
Perognathus cf. minutus James, 1963
(Figure 34)
Localities. Buckner Ranch (TMM 30898).
Referred specimens. TMM 30898-21 left M2.
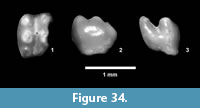 Description. A partial M2 obtained in screen washing is referable to the rodent family Heteromyidae. The tooth exhibits only slight wear so cusps are still prominent. It is strongly bilophodont, brachydont, with a slightly bulbous base (Figure 34). Although the posterior portion of the tooth is missing, the paracone and protocone are well developed with a smaller protostyle. A weak cingulum is present along the anterior base of the tooth. The metacone and hypocone are also well developed although partially missing. The maximum width of the preserved tooth is 0.88 mm.
Description. A partial M2 obtained in screen washing is referable to the rodent family Heteromyidae. The tooth exhibits only slight wear so cusps are still prominent. It is strongly bilophodont, brachydont, with a slightly bulbous base (Figure 34). Although the posterior portion of the tooth is missing, the paracone and protocone are well developed with a smaller protostyle. A weak cingulum is present along the anterior base of the tooth. The metacone and hypocone are also well developed although partially missing. The maximum width of the preserved tooth is 0.88 mm.
Discussion. A very small perognathine heteromyid is represented by a single, partial M2. It is morphologically similar to Perognathus furlongi, but significantly smaller. P. furlongi is well represented from the Barstow Formation where Lindsay (1972) documented M2 widths of 1.0-1.12 mm. James (1963) named P. minutus from the early Clarendonian Big Cat Quarry in the Cuyama badlands based on a partial right dentary. No upper teeth were available for description, but James (1963) stated that m1 is similar to other species of Perognathus except for its smaller size. Perognathus cf. minutus has been recognized at numerous additional localities from California to Florida and the Great Plains in both Barstovian and Clarendonian faunas. Bryant (1991) recognized P. cf. minutus from a Barstovian fauna in Florida. That sample included upper molars with measurements of 0.95 to 1.25 mm for the widths of M1s or M2s. Lindsay (1972) also recognized P. minutus from the Barstow Formation, California and documented a range in M2 widths of 0.97-1.06 mm. Whistler and Burbank (1992) report P. minutus from the Late Barstovian through the Early Hemphillian Dove Spring Formation also in California. Perognathus from the Lapara Creek Fauna is smaller than most previously described species, but as there is only a single tooth and it is incomplete, the form is referred to P. cf. minutus.
Family MYLAGAULIDAE Cope, 1881
Genus CERATOGAULUS Matthew, 1902
Ceratogaulus cf. rhinoceros Matthew, 1902
(Figure 35, Table 1)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170).
 Referred specimens. TMM 30936-92 upper incisor, -296 lower incisor; TMM 31132-143 left P4, TMM 31132-120 right p4; TMM 31170-67 left P4.
Referred specimens. TMM 30936-92 upper incisor, -296 lower incisor; TMM 31132-143 left P4, TMM 31132-120 right p4; TMM 31170-67 left P4.
Description. Three mylagaulid premolars from the Bridge Ranch Local Fauna are small and relatively elongated (Table 1). The p4 is simple with five fossettids and the P4s include six or seven fossettes. The branching pattern of the parafossette in TMM 31132-143 is like that in the Ceratogaulus, Mylagaulus and Alphagaulus douglassi group described by Korth (2000). The branching of the parafossette in TMM 31170-67 resembles the primitive “Hesperogaulus” pattern (Korth, 2000), but with additional wear would likely separate into a Ceratogaulus type pattern. P4 lacks the C-shaped posterolabial fossette that is diagnostic of Mylagaulus (Czaplewski, 2012).
Discussion. Two incisors from the Ten Mile Waterhole Creek Local Fauna represent a mylagaulid, but further identification is uncertain. P4s from the Bridge Ranch mylagaulid are morphologically similar to Ceratogaulus cf. rhinoceros from the Burge Fauna (Cl1, Nebraska), however, both upper and lower premolars are smaller than the material described by Korth (2000). The p4 from Bridge Ranch has five fossettids while Korth (2000) cited a range of five to nine for C. cf. rhinoceros from Burge. Isolated teeth of C. cf. rhinoceros from Burge are morphologically indistinguishable from C. rhinoceros according to Korth (2000), but they tend to be somewhat smaller. The width/length ratios shown in Table 1 compare closely with values for C. cf. rhinoceros from the Burge Fauna (P4 ~0.68, p4 ~0.53). Korth’s reasons for not assigning the Burge material to C. rhinoceros included minor differences in the cranial morphology. Only isolated teeth are known from the Bridge Ranch Local Fauna and they compare most closely in size with the Burge material. The P4s of the Bridge Ranch mylagaulid are significantly smaller than the type specimens of C. anecdotus from the Ash Hollow Formation, Nebraska, and p4 lacks the characteristic V-shaped anterior fossettid of that taxon (Korth, 2000).
Recognition of C. cf. rhinoceros from the Lapara Creek Fauna extends the known geographic range of this taxon and represents the oldest occurrence of a mylagaulid from the Texas coastal plain. Wilson (1962) included Mylagaulus in faunal lists from the Barstovian Cold Springs and Burkeville Faunas, however, Baskin and Hulbert (2012) noted that the Burkeville specimen is actually the beaver Amblycastor. Re-examination of the rodent material from the Burkeville and Cold Spring Faunas indicates that beavers tentatively assigned to Amblycastor, Anchitheriomys, and Monosaulux are present, but there are no mylagaulids. It appears that the Barstovian faunas from the Texas coastal plain include beavers but no mylagaulids, while the Clarendonian Lapara Creek and Dinero Faunas (Baskin and Hulbert, 2012) include mylagaulids but no beavers.
Order CARNIVORA Bowdich, 1821
cf. Mustelidae indeterminate
Localities. Farish Ranch (TMM 31081).
Referred specimens. TMM 31081-157, -1338 edentulous dentary.
Description. Two edentulous partial dentaries from the Farish Ranch Local Fauna represent two different potential mustelids based on size. Both rami are relatively shallow and curved with preserved lengths of 29.5 mm and 38.1 mm. The larger of the two specimens (TMM 31081-157) preserves the posterior portion of an alveolus for c and for p1 that appears to have been single rooted, double rooted alveoli for p2-m1 and a single alveolus for m2, although the posterior portion of the ramus is missing. Based on the alveoli, m1 was approximately 13 mm in length. TMM 31081-1338 displays two mental foramina and alveoli for c, a single rooted p1, double-rooted p2-m1 and a main alveolus for m2 with an additional very small lingual alveolus.
Discussion. Taxonomic assignment of these small carnivore jaws is uncertain. The specimens differ from Bassariscus, small canids and a number of modern skunks. They are most closely comparable to examples of Martes and Mustela. Based on size and morphology, TMM 31081-157 compares well with the modern Martes pennanti (fisher), although the ramus is slightly deeper in the modern form. TMM 31081-1338 represents a significantly smaller potential mustelid.
Family CANIDAE Fischer de Waldheim, 1817
Subfamily BOROPHAGINAE Simpson, 1945
Tribe BOROPHAGINI Simpson, 1945
Subtribe AELURODONTINA Wang, Tedford, and Taylor, 1999
Genus AELURODON Leidy, 1858
Aelurodon taxoides (Hatcher, 1893)
(Figure 36, Figure 37)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081).
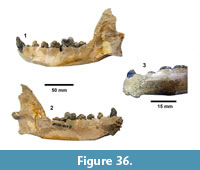 Referred specimens. TMM 30936-17 partial humerus, -40 partial right dentary with p2, -88 partial m1, -106 m1; TMM 31132-12 canine, -165 canine, -458 left dentary with c, p2-4, m1-2, -478 incisor, -787 canine; TMM 31170-64 partial left dentary with m1, -65 right M2; TMM 31081-197 canine, -271 i3, -1142 canine, -1143 m2.
Referred specimens. TMM 30936-17 partial humerus, -40 partial right dentary with p2, -88 partial m1, -106 m1; TMM 31132-12 canine, -165 canine, -458 left dentary with c, p2-4, m1-2, -478 incisor, -787 canine; TMM 31170-64 partial left dentary with m1, -65 right M2; TMM 31081-197 canine, -271 i3, -1142 canine, -1143 m2.
Description. Numerous specimens from multiple local faunas represent a medium-sized borophagine canid. TMM 31132-458 is a left dentary with c, p2-4 and m1-2 (Figure 36). This was the only specimen from the Lapara Creek Fauna recognized as Aelurodon by Wilson (1960, figure 5) and was partially figured by him. He assigned this specimen to A. taxoides on the basis of size and the relative lengths of premolar and molar series. The dentary is relatively slender with a total length of ~187 mm and a depth below p2 of ~33 mm. This compares well with measurements for A. taxoides from the Big Spring Canyon Fauna (Cl1, South Dakota) of 183 and 28 mm, respectively (Gregory, 1942). A weak symphyseal flange is apparent as illustrated in Figure 36.3. Although p1 is missing, a single alveolus is present clearly indicating a full complement of p1-p4. The first lower molar (m1) measures 30.3 mm x 12.0 mm while m2 is 12.0 x 8.6 mm. The p1-m3 length is approximately 105 mm. The m2 is reduced.
Another partial dentary from the Bridge Ranch Local Fauna (TMM 31170-64) is also identified as A. taxoides. As in TMM 31132-458, the masseteric fossa is well developed and extends to below m3. Only m1 is present and this tooth is broken, so the AP length of 29.7 mm is a minimum value while the width is 12.4 mm. Including the double-rooted alveolus for m2 and the single alveolus for m3, the length of the molar series would have been approximately 50 mm, very similar to TMM 31132-458. The depth of the ramus beneath m1 is 33 mm.
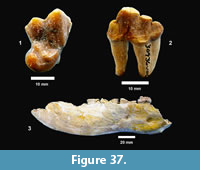 Three specimens assignable to Aelurodon are known from the Ten Mile Waterhole Creek Local Fauna. A partial m1 preserves the lingual half of the tooth with a minimum length of 31.5 mm. The p4 is moderately worn and 19.3 mm x 9.4 mm. The m1 and p4 are indistinguishable from the material assigned to A. taxoides from the Bridge Ranch Local Fauna. The partial right dentary includes a single root for p1, a broken p2 (14.9 x 9.4 mm) and then roots only for p3, p4 and m1 (Figure 37). The dentary is broken at the alveolus for m2. This dentary represents a somewhat larger and more robust individual than the specimens from the Bridge Ranch Local Fauna. The total estimated length of the tooth row from p1-m2 is ~114 mm while p1-p4 is ~65 mm. The depth of the dentary below m1 is 38 mm and there are two, 5-6 mm ovoid foramen, one below the anterior edge of p2 and the other below the center of p3.
Three specimens assignable to Aelurodon are known from the Ten Mile Waterhole Creek Local Fauna. A partial m1 preserves the lingual half of the tooth with a minimum length of 31.5 mm. The p4 is moderately worn and 19.3 mm x 9.4 mm. The m1 and p4 are indistinguishable from the material assigned to A. taxoides from the Bridge Ranch Local Fauna. The partial right dentary includes a single root for p1, a broken p2 (14.9 x 9.4 mm) and then roots only for p3, p4 and m1 (Figure 37). The dentary is broken at the alveolus for m2. This dentary represents a somewhat larger and more robust individual than the specimens from the Bridge Ranch Local Fauna. The total estimated length of the tooth row from p1-m2 is ~114 mm while p1-p4 is ~65 mm. The depth of the dentary below m1 is 38 mm and there are two, 5-6 mm ovoid foramen, one below the anterior edge of p2 and the other below the center of p3.
TMM 31170-65 is a nearly unworn right M2 assignable to A. taxoides. It is 15.9 mm in length by 18.6 mm in width. The hypocone is well developed as is a lingual cingulum.
Discussion. Wang et al. (1999) reviewed the borophagine canids and emended the diagnosis of Aelurodon to include: reduced M1-M2 and m2, m1 talonid narrowed, shortened m2, and m2 metaconid reduced. The material from the Bridge Ranch Local Fauna is consistent with these characters, and the size and morphologic details are consistent with both A. taxoides and A ferox. The left dentary (TMM 31132-458) has a weak symphyseal flange that distinguishes A. taxoides from A. ferox (Wang et al., 1999). Wilson (1960) identified TMM 31132-458 as A. taxoides, and Wang et al. (1999) included it in their list of referred specimens.
The more robust dentary from the Ten Mile Waterhole Creek Local Fauna (TMM 30936-40) may represent a different species of Aelurodon; however, it may also reflect variation due to sexual dimorphism and/or age. The width of the dentary below the anterior edge of m1 is 19.2 mm while this same dimension on TMM 31132-458 is 14.2 mm. Because most of the teeth are missing, the specimen is here referred to A. taxoides.
Family CANIDAE Fischer de Waldheim, 1817
Subfamily Caninae Fischer de Waldheim, 1817
Genus EUCYON Tedford and Qiu, 1996
cf. Eucyon sp. (Figure 38, Table 2)
Localities. Farish Ranch (TMM 31081).
Referred specimens. TMM 31081-1255 partial right maxilla with P4-M2.
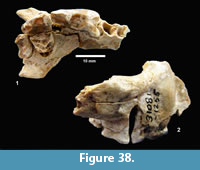 Description. TMM 31081-1255 is a partial right maxilla with well-worn P4-M2 and alveoli for P3. This specimen represents a mature, but relatively small canid, much smaller than Aelurodon or Ischyrocyon.
Description. TMM 31081-1255 is a partial right maxilla with well-worn P4-M2 and alveoli for P3. This specimen represents a mature, but relatively small canid, much smaller than Aelurodon or Ischyrocyon.
Two alveoli are preserved in the position of P3. M1 is sub-triangular while M2 is sub-rectangular. The paracone is slightly larger than the metacone on both M1 and M2 and the cusps are flattened with wear. A postprotocrista is present on M1 with a reduced metaconule. The paracone on P4 is large and aligned linearly with the metastyle. The protocone is relatively small and placed slightly anterior to the paracone.
Discussion. TMM 31081-1255 was figured and identified as an indeterminate canid by Wilson (1960). He recognized the significance of this specimen as potentially representing a very early example of Canis and compared the specimen in size and morphology to the modern coyote (Canis latrans). However, Wilson (1960) concluded that additional material was needed before this taxon could be appropriately characterized. More than 55 years after that opinion was published there are no new specimens of this taxon, however, additional material from other faunas is now available for comparison.
The sizes of P4, M1 and M2 are all larger than published ranges for Leptocyon, Vulpes, Metalopex, and Urocyon and smaller than Canis lepophagus, C. edwardii and C. latrans. These teeth compare closely in size to Eucyon davisi and Canis ferox. TMM 31081-1255 compares very closely in size and morphology with a specimen of Eucyon davisi figured by Tedford et al. (2009) (UO 26742, figure 33G) from the early Hemphillian in Oregon. The oldest published record of Eucyon in North America is E. skinneri from the late Clarendonian Ash Hollow Formation, Nebraska (Tedford et al., 2009). Although E. skinneri was described on the basis of lower dentition only, Tedford et al. (2009) stated that it compared closely with E. davisi. TMM 31081-1255 is identified here as cf. Eucyon sp. potentially extending the range of this taxon into the early Clarendonian as hypothesized in the phyletic model of Tedford et al. (2009, figure 66).
Genus LEPTOCYON Matthew, 1918
Leptocyon vafer (Leidy, 1858) (Figure 39, Table 3)
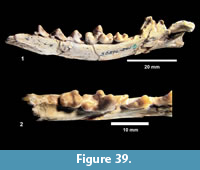 Localities. Buckner Ranch (TMM 30896).
Localities. Buckner Ranch (TMM 30896).
Referred specimens. TMM 30896-241 partial dentary with p2-m2.
Description. A small, foxlike canid is represented by a partial right dentary with p2-m2 and alveoli for p1 and m3. Represented by a single small alveolus, m3 would have been very reduced. The depth of the dentary below m1 is 9.7 mm and the total preserved length is 75.7 mm.
Discussion. Wilson (1960) identified TMM 30896-241 as Leptocyon vafer. The size of m1 is larger than in L. tejonensis, L. gregorii, and L. leidyi, but within the range of L. vafer and L. matthewi reported in Tedford et al. (2009). The morphology of the hypoconid on m1 is consistent with L. vafer. Jasinski (2015) recognized a shift from L. leidyi in the early Barstovian to L. vafer in the late Barstovian and Clarendonian in New Mexico. Munthe (1998) included the Lapara Creek Fauna as a valid occurrence of L. vafer. Tedford et al. (2009) recognized L. vafer as a common taxon with occurrences in the Great Plains and southwest to California from late Barstovian through late Clarendonian faunas.
Family AMPHICYONIDAE Trouessart, 1884
Genus ISCHYROCYON Matthew and Gidley, 1904
Ischyrocyon gidleyi (Matthew and Gidley, 1904) (Figure 40, Figure 41, Figure 42)
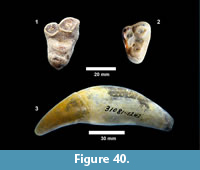 Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31184), Farish Ranch (TMM 31081).
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31184), Farish Ranch (TMM 31081).
Referred specimens. TMM 30936-236 m2, TMM 31132-29 M2, -259 M1, -328 M2, -412 P4; TMM 31184-2 M1; TMM 31081-465 humerus, -1243 C, -1166 C.
Description. A large amphicyonid is represented by isolated teeth and limited post-cranial material. The large molars exhibit the characteristic pattern of major cusps worn nearly flat. The size and morphology of M1, M2 compare well with the partial skull of Ischyrocyon gidleyi figured by Webb (1969) from the Minnechaduza Fauna (Cl2, Nebraska).
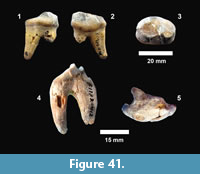 Measurements of isolated teeth from the Lapara Creek amphicyonid compare closely with those of I. gidleyi from the Burge and Minnechaduza Faunas (Webb, 1969). Reconstruction of pre-wear geometry suggests a very tall paracone and metacone on the sub-triangular M1 (TMM 31184-2) and a more reduced paracone-metacone height on the slightly more rectangular and smaller M2 (TMM 31132-29) (Figure 41). The well-worn m2 (TMM 30936-236) has a very reduced metaconid and suggests that the talonid and trigonid would eventually wear to a planar surface. Both m1 and m2 of I. gidleyi from the Burge and Minnechaduza Faunas have very reduced to absent metaconids (Webb, 1969). The canine (TMM 31081-1243) lacks a sharp posterior ridge. A P4 (TMM 31132-412) is well worn with only a limited rim of enamel remaining as is characteristic for amphicyonids and commonly invoked as evidence for a hyaenoid feeding style (Figure 41).
Measurements of isolated teeth from the Lapara Creek amphicyonid compare closely with those of I. gidleyi from the Burge and Minnechaduza Faunas (Webb, 1969). Reconstruction of pre-wear geometry suggests a very tall paracone and metacone on the sub-triangular M1 (TMM 31184-2) and a more reduced paracone-metacone height on the slightly more rectangular and smaller M2 (TMM 31132-29) (Figure 41). The well-worn m2 (TMM 30936-236) has a very reduced metaconid and suggests that the talonid and trigonid would eventually wear to a planar surface. Both m1 and m2 of I. gidleyi from the Burge and Minnechaduza Faunas have very reduced to absent metaconids (Webb, 1969). The canine (TMM 31081-1243) lacks a sharp posterior ridge. A P4 (TMM 31132-412) is well worn with only a limited rim of enamel remaining as is characteristic for amphicyonids and commonly invoked as evidence for a hyaenoid feeding style (Figure 41).
A very large humerus most likely represents Ischyrocyon based on size and morphology. This bone has a maximum length of 303 mm, a maximum proximal width of 72 mm, a distal transverse width of 90 mm, and the capitulum-trochlear surface is 61.5 mm wide (Figure 42). The deltopectoral crest is ~200 mm long. It compares closely with the partial humerus of I. gidleyi figured by Webb (1969) from the Minnechaduza Fauna.
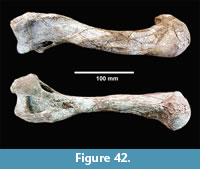 Discussion. Wilson (1960) recognized two teeth from the Bridge Ranch Local Fauna that he labeled amphicyonid in his figure 5, but then referred to them as Amphicyon sp. in the caption of figure 7. An additional 7 specimens are here referred to I. gidleyi making it and Aelurodon taxoides the two most common carnivores in the Lapara Creek Fauna.
Discussion. Wilson (1960) recognized two teeth from the Bridge Ranch Local Fauna that he labeled amphicyonid in his figure 5, but then referred to them as Amphicyon sp. in the caption of figure 7. An additional 7 specimens are here referred to I. gidleyi making it and Aelurodon taxoides the two most common carnivores in the Lapara Creek Fauna.
Ischyrocyon was described by Webb (1969) from the Burge and Minnechaduza Faunas and by Gregory (1942) from the Big Spring Fauna. In both cases, this taxon represents the largest carnivore in the fauna.
As discussed by Hunt (1998) and Sorkin (2006) the deltopectoral crest on amphicyonids is relatively long compared to the length of the humerus and is similar to large modern felids (Panthera) that use their front limbs to capture and hold prey. Measurements from the Lapara Creek amphicyonid humerus plot closely to Amphicyon and Ischyrocyon examples from Barstovian faunas documented in Sorkin (2006). According to Hunt (1998), Ischyrocyon and Pseudocyon can be distinguished from Amphicyon by having sub-rectangular M2s that are always smaller than the more triangular M1s. Ischyrocyo n can be distinguished from Pseudocyon by having m2 with a lower trigonid that is not crowded to the anterior and by having a canine that is somewhat more rounded in cross-section and lacks a sharp posterior ridge. Although the amount of material of this taxon is limited, the characters of M1, M2, m2 and C are most consistent with Ischyrocyon. I. gidleyi is the only recognized species of this genus in North America.
Order PROBOSCIDEA Illiger, 1811
Family GOMPHOTHERIIDAE Hay, 1922
Genus BLANCOTHERIUM n. gen.
zoobank.org/EEF70587-652F-4856-888A-E0F55835CD8F
Type species. Blancotherium buckneri (Sellards, 1940b)
Range. Early Clarendonian of Texas
Diagnosis. Long, gently upward curving upper tusks with no enamel band and no spiral; small, oval-shaped lower tusks with enamel band; downward deflected symphysis that becomes very elongated in old individuals; molars with pretrite trefoils, four main loph/lophids on M3/m3 with a smaller fifth loph/lophid and a posterior heel.
Etymology. Generic name reflects Blanco Creek locality where most of the known material of this taxon has been collected.
Remarks. Blancotherium n. gen. is a gomphotheriid proboscidean previously referred to Gnathabelodon buckneri by Sellards (1940b). Sellards (1940b) did not designate a type specimen.
Blancotherium buckneri (Sellards, 1940b)
(Figure 43, Figure 44, Figure 45, Figure 46, Figure 47, Figure 48, Figure 49, Figure 50, Figure 51, Figure 52, Figure 53, Figure 54, Figure 55, Figure 56, Figure 57, Figure 58, Figure 59, Figure 60, Figure 61, Figure 62, Figure 63, Figure 64, Figure 65, Figure 66, Table 4, Table 5)
Gnathabelodon buckneri (Sellards, 1940b)
Type specimen. TMM 30896-390 partial skull and mandible.
Localities. TMM 30936? (Ten Mile Waterhole Creek), TMM 31081 (Farish Ranch), TMM 30896 (Buckner Ranch).
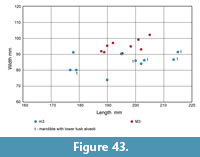 Referred specimens. TMM 30936-28 M3; TMM 31081-422 thoracic vertebra, -1158 partial palate with right and left M1-M3, -1287 right scapula; TMM 30896-51 right scapula, -64 mandible with left and right m2 and m3 (erupting), -85 femur, -90 humerus, -108 mandible with left and right m3, -141 M1, 197 tibia, -210 tibia, -218 humerus, -254 humerus, -232 humerus, -242 femur, -250 tibia, -257 stylohyoid, -260 femur, -269 partial palate with left M2-M3 and right M3, -278 partial right dentary with m2-m3, -285 femur, -295 right dentary with m3, -298 stylohyoid, -299 ulna, -309 mandible with left and right m1-m3 (m3 unerupted), -311 mandible with left and right m3, 318 mandible with left and right m3, -325 partial palate with right and left M2 and M3, -381 right M3, -386 M3, -389 skull with M2, M3, “M4”, -390 partial skull and mandible with M2-M3 and m3, -518 M3, -570 right dentary with m2-m3, -520 lower tusk, -526 lower tusk, -532 tibia, -655 left femur, -656 skull with right M2, M3, left M3 and left upper tusk, -657 mandible with left and right m3. Numerous cranial and post-cranial elements are still in field jackets.
Referred specimens. TMM 30936-28 M3; TMM 31081-422 thoracic vertebra, -1158 partial palate with right and left M1-M3, -1287 right scapula; TMM 30896-51 right scapula, -64 mandible with left and right m2 and m3 (erupting), -85 femur, -90 humerus, -108 mandible with left and right m3, -141 M1, 197 tibia, -210 tibia, -218 humerus, -254 humerus, -232 humerus, -242 femur, -250 tibia, -257 stylohyoid, -260 femur, -269 partial palate with left M2-M3 and right M3, -278 partial right dentary with m2-m3, -285 femur, -295 right dentary with m3, -298 stylohyoid, -299 ulna, -309 mandible with left and right m1-m3 (m3 unerupted), -311 mandible with left and right m3, 318 mandible with left and right m3, -325 partial palate with right and left M2 and M3, -381 right M3, -386 M3, -389 skull with M2, M3, “M4”, -390 partial skull and mandible with M2-M3 and m3, -518 M3, -570 right dentary with m2-m3, -520 lower tusk, -526 lower tusk, -532 tibia, -655 left femur, -656 skull with right M2, M3, left M3 and left upper tusk, -657 mandible with left and right m3. Numerous cranial and post-cranial elements are still in field jackets.
Diagnosis. Only species of the genus.
Age. Early Clarendonian (Cl1-Cl2) based on faunal composition of the Lapara Creek Fauna from the Goliad Formation, TX.
 Description. Blancotherium buckneri is a longirostrine gomphothere with long, upward curving upper tusks that lack enamel bands. These tusks show no evidence of helical spiraling. At least five mandibles preserve alveoli for lower tusks that are roughly oval in cross-section (~35-45 mm x 25-35 mm). Two isolated lower tusks (TMM 30896-520, -526) are compressed laterally, slightly curved with enamel bands on one side. Mandibles from the most mature individuals have very elongated symphyses and show only equivocal evidence for lower tusks. Mandibles are variable but generally have long narrow symphyses that progress from U-shaped posteriorly to flattened anteriorly. Some specimens suggest that the anterior end of the symphysis was bilobed. The symphysis is deflected downward relative to the tooth row with measured angles ranging from 24 to 38 degrees and a mean of 33 degrees. The length of the symphysis varies from 47 to 97 cm, but the anterior portion is often broken. The intermediate molars are trilophodont while the third molars commonly exhibit four well-developed lophs (ids) with pretrite trefoil patterns, a smaller fifth loph(id) and a posterior heel existing of 2-3 conules (conulids). The molars are relatively hypsodont and include accessory conules (ids) in the valleys between the primary lophs (ids). Average dimension for molars are given in Table 4, and third molar dimensions are illustrated in Figure 43.
Description. Blancotherium buckneri is a longirostrine gomphothere with long, upward curving upper tusks that lack enamel bands. These tusks show no evidence of helical spiraling. At least five mandibles preserve alveoli for lower tusks that are roughly oval in cross-section (~35-45 mm x 25-35 mm). Two isolated lower tusks (TMM 30896-520, -526) are compressed laterally, slightly curved with enamel bands on one side. Mandibles from the most mature individuals have very elongated symphyses and show only equivocal evidence for lower tusks. Mandibles are variable but generally have long narrow symphyses that progress from U-shaped posteriorly to flattened anteriorly. Some specimens suggest that the anterior end of the symphysis was bilobed. The symphysis is deflected downward relative to the tooth row with measured angles ranging from 24 to 38 degrees and a mean of 33 degrees. The length of the symphysis varies from 47 to 97 cm, but the anterior portion is often broken. The intermediate molars are trilophodont while the third molars commonly exhibit four well-developed lophs (ids) with pretrite trefoil patterns, a smaller fifth loph(id) and a posterior heel existing of 2-3 conules (conulids). The molars are relatively hypsodont and include accessory conules (ids) in the valleys between the primary lophs (ids). Average dimension for molars are given in Table 4, and third molar dimensions are illustrated in Figure 43.
 TMM 30896-390 is a partial skull with right and left upper tusks and mandible. Sellards (1940b) illustrated the right side of this specimen, but it was then mounted in a display case for the Texas Memorial Museum with the right side embedded in plaster. Figure 44 shows the left side of this specimen with most of the plaster reconstruction digitally removed. As described by Sellards (1940b), the upper tusks are long, upwardly curved and do not spiral. The left upper tusk is approximately 164 cm in length although the tip has been somewhat restored with plaster. The mandible is very long (152 cm) with the symphysis accounting for 94 cm of this length. The symphysis is down curved, U-shaped posteriorly, becoming more flattened anteriorly. The left side of the anterior tip of the symphysis is missing, but the right side suggests a broad, bilobed termination. The ventral labial side of the left dentary is crushed suggesting possible collapse of lower tusk alveolus, although this interpretation is unclear given the state of preservation and significant plaster repair. The mounted specimen includes both upper and lower, right and left third molars. An isolated M2 with the same specimen number was found in the collection. The third molars are very worn with pretrite trefoils, 5 lophs/lophids and a posterior heel.
TMM 30896-390 is a partial skull with right and left upper tusks and mandible. Sellards (1940b) illustrated the right side of this specimen, but it was then mounted in a display case for the Texas Memorial Museum with the right side embedded in plaster. Figure 44 shows the left side of this specimen with most of the plaster reconstruction digitally removed. As described by Sellards (1940b), the upper tusks are long, upwardly curved and do not spiral. The left upper tusk is approximately 164 cm in length although the tip has been somewhat restored with plaster. The mandible is very long (152 cm) with the symphysis accounting for 94 cm of this length. The symphysis is down curved, U-shaped posteriorly, becoming more flattened anteriorly. The left side of the anterior tip of the symphysis is missing, but the right side suggests a broad, bilobed termination. The ventral labial side of the left dentary is crushed suggesting possible collapse of lower tusk alveolus, although this interpretation is unclear given the state of preservation and significant plaster repair. The mounted specimen includes both upper and lower, right and left third molars. An isolated M2 with the same specimen number was found in the collection. The third molars are very worn with pretrite trefoils, 5 lophs/lophids and a posterior heel.
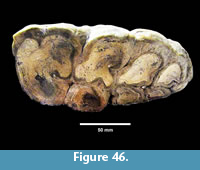 TMM 30896-570 is a right dentary with a very worn m2 and m3 and a partial left dentary. The symphysis is extremely elongated, and there is no evidence of lower tusks, but the symphysis is crushed flat by compaction and the distal tip is missing. As preserved, the symphysis curves downward in a continuous arc, but the approximate angle with the plane of the tooth row is 32 degrees (Figure 45). Measured from the anterior edge of m2, the length of the symphysis is 97 cm. The very worn lower molars exhibit a typical pretrite trefoil pattern (Figure 46). The ascending ramus is nearly complete except for the reconstructed condylar process and the tip of the coronoid process. Although no lower tusk is preserved, there is an elongated depression along the labial side of the crushed symphysis that may represent a lower tusk alveolus. There also appears to be an elongated “mental foramen”.
TMM 30896-570 is a right dentary with a very worn m2 and m3 and a partial left dentary. The symphysis is extremely elongated, and there is no evidence of lower tusks, but the symphysis is crushed flat by compaction and the distal tip is missing. As preserved, the symphysis curves downward in a continuous arc, but the approximate angle with the plane of the tooth row is 32 degrees (Figure 45). Measured from the anterior edge of m2, the length of the symphysis is 97 cm. The very worn lower molars exhibit a typical pretrite trefoil pattern (Figure 46). The ascending ramus is nearly complete except for the reconstructed condylar process and the tip of the coronoid process. Although no lower tusk is preserved, there is an elongated depression along the labial side of the crushed symphysis that may represent a lower tusk alveolus. There also appears to be an elongated “mental foramen”.
The partial left dentary was separated during recent re-preparation. It appears that the two molars (m1, m2) are completely surrounded by plaster and not in their proper location in the jaw. The third molar is missing on the left side.
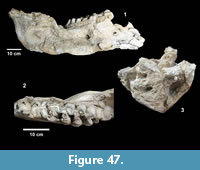 TMM 30896-278 is a partial right dentary with a very worn, trilophodont m2 and a moderately worn m3 with four main lophids, a small fifth lophid and a posterior conulid (Figure 47). Pretrite trefoils are well- developed (especially on the first and second lophids), and there is a well-developed labial cingulum. Although the anterior portion of the symphysis is broken, the maximum length of the mandible from the posterior edge of the ascending ramus to the preserved tip of the symphysis is ~80 cm. The depth of the ramus, below the anterior edge of m2, is 165 mm, and the approximate width of the preserved symphysis is 160 mm. Although broken, both right and left sides of the symphysis region are present, and both contain an alveolus for lower tusks. Both alveoli are oval in cross-section (left: 47 x 19 mm, right: 42 x 20 mm) and compare very well in size and shape with two isolated lower tusks from the same locality (TMM 30896-520, TMM 30896-526). The orientation of the lower tusks appears to be with the long-axis oriented dorsoventrally and positioned towards the labial margins of the symphysis. The symphysis turns down just anterior to m2 and forms an approximate angle with the tooth row of 37 degrees. Both coronoid process and condyle are missing.
TMM 30896-278 is a partial right dentary with a very worn, trilophodont m2 and a moderately worn m3 with four main lophids, a small fifth lophid and a posterior conulid (Figure 47). Pretrite trefoils are well- developed (especially on the first and second lophids), and there is a well-developed labial cingulum. Although the anterior portion of the symphysis is broken, the maximum length of the mandible from the posterior edge of the ascending ramus to the preserved tip of the symphysis is ~80 cm. The depth of the ramus, below the anterior edge of m2, is 165 mm, and the approximate width of the preserved symphysis is 160 mm. Although broken, both right and left sides of the symphysis region are present, and both contain an alveolus for lower tusks. Both alveoli are oval in cross-section (left: 47 x 19 mm, right: 42 x 20 mm) and compare very well in size and shape with two isolated lower tusks from the same locality (TMM 30896-520, TMM 30896-526). The orientation of the lower tusks appears to be with the long-axis oriented dorsoventrally and positioned towards the labial margins of the symphysis. The symphysis turns down just anterior to m2 and forms an approximate angle with the tooth row of 37 degrees. Both coronoid process and condyle are missing.
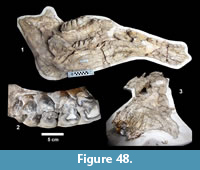 TMM 30896-311 is a mandible crushed flat with the labial side of the right dentary facing up and the labial side of the left dentary embedded in a plaster cradle (Figure 48). Both right and left m3s are present and are moderately worn with four main lophids, a smaller fifth and a posterior conulid. The coronoid process is preserved on the left dentary although both condyles are missing. The maximum apparent length from the posterior edge of the ascending ramus to the broken anterior tip of the symphysis is 120 cm. The right dentary appears to possess an alveolus for a lower tusk. The symphysis changes geometry from U-shaped at the posterior end to a flattened, spatulate shape at the anterior end. The approximate width at the broken anterior end of the symphysis is 175 mm and the length of the preserved symphysis is 56 cm. The depth of the ramus below the anterior edge of m3 is 144 mm and the height of the ascending ramus is 357 mm. Pretrite trefoils are well-developed.
TMM 30896-311 is a mandible crushed flat with the labial side of the right dentary facing up and the labial side of the left dentary embedded in a plaster cradle (Figure 48). Both right and left m3s are present and are moderately worn with four main lophids, a smaller fifth and a posterior conulid. The coronoid process is preserved on the left dentary although both condyles are missing. The maximum apparent length from the posterior edge of the ascending ramus to the broken anterior tip of the symphysis is 120 cm. The right dentary appears to possess an alveolus for a lower tusk. The symphysis changes geometry from U-shaped at the posterior end to a flattened, spatulate shape at the anterior end. The approximate width at the broken anterior end of the symphysis is 175 mm and the length of the preserved symphysis is 56 cm. The depth of the ramus below the anterior edge of m3 is 144 mm and the height of the ascending ramus is 357 mm. Pretrite trefoils are well-developed.
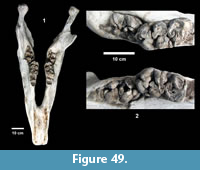 TMM 30896-64 includes the mandible of a juvenile gomphothere with alveoli for m1, a trilophodont m2 and m3 just beginning to erupt (Figure 49). This specimen experienced dorsoventral crushing during compaction and suffers from significant plaster reconstruction in the Survey laboratory. Examination under UV light reveals variable amounts of original bone and all geometries described for the mandible should be treated as approximate. Pretrite trefoil patterns are well developed on the m2s.
TMM 30896-64 includes the mandible of a juvenile gomphothere with alveoli for m1, a trilophodont m2 and m3 just beginning to erupt (Figure 49). This specimen experienced dorsoventral crushing during compaction and suffers from significant plaster reconstruction in the Survey laboratory. Examination under UV light reveals variable amounts of original bone and all geometries described for the mandible should be treated as approximate. Pretrite trefoil patterns are well developed on the m2s.
In cross-section, the symphysis progresses from a flattened U-shape at the posterior end to a flat, spatulate shape at the anterior tip. However, at least some of this geometry appears to have been sculpted by the Survey preparators. The amount of plaster makes it impossible to evaluate the presence of alveoli for lower tusks.
 TMM 30896-271 is a relatively small mandible that includes a very worn left m1, moderately worn left and right m2s and unerupted m3s. The preserved length of the mandible is 80 cm including a shallow, U-shaped symphysis that is 13 cm in length. Although the anterior tip of the symphysis is missing and the broken edge had been restored with plaster, skillful removal of the plaster during recent re-preparation reveals what appears to be an alveolus for a right lower tusk. The anterior portion of the left dentary is fractured as if a lower tusk alveolus in this location had collapsed (Figure 50). Based on the state of wear of the m2s and the unerupted m3s, this specimen represents a young individual with a short symphysis and apparent alveoli for lower tusks.
TMM 30896-271 is a relatively small mandible that includes a very worn left m1, moderately worn left and right m2s and unerupted m3s. The preserved length of the mandible is 80 cm including a shallow, U-shaped symphysis that is 13 cm in length. Although the anterior tip of the symphysis is missing and the broken edge had been restored with plaster, skillful removal of the plaster during recent re-preparation reveals what appears to be an alveolus for a right lower tusk. The anterior portion of the left dentary is fractured as if a lower tusk alveolus in this location had collapsed (Figure 50). Based on the state of wear of the m2s and the unerupted m3s, this specimen represents a young individual with a short symphysis and apparent alveoli for lower tusks.
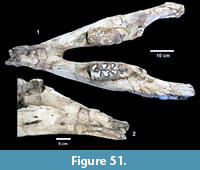 TMM 30896-657 is a relatively small mandible containing moderately worn m3s (Figure 51). These molars have four main lophids, a small fifth lophid and a posterior heel. The state of wear suggests a mature adult (Right: 179 x 80 mm, left: 177 x 80 mm). The length of the mandible is 84 cm. The preserved symphysis is U-shaped, ~26 cm long and tapers from 177 mm to 95 mm at the broken distal end. The presence of alveoli for lower tusks is equivocal due to the poor preservation of the anterior end of the symphysis. However, the ventrallabial area of the right dentary appears to be collapsed into a possible lower tusk alveolus. This specimen is somewhat enigmatic because of the small size, narrow symphysis and yet moderate maturity given the wear on m3s. This may reflect the early mature nature of this individual, sexual dimorphism or both. The dimensions of the m3s occur at the small end of the range observed in this taxon.
TMM 30896-657 is a relatively small mandible containing moderately worn m3s (Figure 51). These molars have four main lophids, a small fifth lophid and a posterior heel. The state of wear suggests a mature adult (Right: 179 x 80 mm, left: 177 x 80 mm). The length of the mandible is 84 cm. The preserved symphysis is U-shaped, ~26 cm long and tapers from 177 mm to 95 mm at the broken distal end. The presence of alveoli for lower tusks is equivocal due to the poor preservation of the anterior end of the symphysis. However, the ventrallabial area of the right dentary appears to be collapsed into a possible lower tusk alveolus. This specimen is somewhat enigmatic because of the small size, narrow symphysis and yet moderate maturity given the wear on m3s. This may reflect the early mature nature of this individual, sexual dimorphism or both. The dimensions of the m3s occur at the small end of the range observed in this taxon.
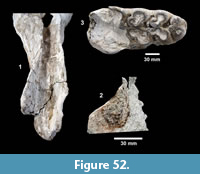 TMM 30896-318 includes a mandible with a relatively well-preserved symphysis although the anterior tip is broken. The maximum preserved length of the right dentary is 148.8 cm. The symphysis is moderately elongated and U-shaped with a maximum width of 175 mm and a preserved length of 733 mm (Figure 52). A break in the anterior portion of the symphysis reveals a possible alveolus for a lower tusk that is filled with matrix. The alveolus is ellipsoidal in cross-section approximately 41 x 29 mm. It narrows to 32 x 19 mm at the anterior edge of the symphysis. The right dentary includes an elongated structure along the ventral labial margin that may represent a collapsed alveolus for a lower tusk. Both right and left m3s are very worn and the right m3 is broken with significant plaster repair. The left m3 is 214 mm x 87 mm.
TMM 30896-318 includes a mandible with a relatively well-preserved symphysis although the anterior tip is broken. The maximum preserved length of the right dentary is 148.8 cm. The symphysis is moderately elongated and U-shaped with a maximum width of 175 mm and a preserved length of 733 mm (Figure 52). A break in the anterior portion of the symphysis reveals a possible alveolus for a lower tusk that is filled with matrix. The alveolus is ellipsoidal in cross-section approximately 41 x 29 mm. It narrows to 32 x 19 mm at the anterior edge of the symphysis. The right dentary includes an elongated structure along the ventral labial margin that may represent a collapsed alveolus for a lower tusk. Both right and left m3s are very worn and the right m3 is broken with significant plaster repair. The left m3 is 214 mm x 87 mm.
 TMM 30896-108 includes a mandible with extremely worn m3s and significant plaster reconstruction. The specimen represents a very mature individual with a relatively short symphysis (616 mm) and possible alveoli for lower tusks. When this specimen was first examined in 2018, a suspicious looking lower tusk was present in the left alveolus (Figure 53). Upon further examination, this tusk was found to be entirely composed of plaster and had been sculpted and painted by the Survey preparators. Removal of the plaster tusk revealed a possible alveolus for a lower tusk, but much of the anterior portion of the symphysis appears “hollow” and filled with plaster.
TMM 30896-108 includes a mandible with extremely worn m3s and significant plaster reconstruction. The specimen represents a very mature individual with a relatively short symphysis (616 mm) and possible alveoli for lower tusks. When this specimen was first examined in 2018, a suspicious looking lower tusk was present in the left alveolus (Figure 53). Upon further examination, this tusk was found to be entirely composed of plaster and had been sculpted and painted by the Survey preparators. Removal of the plaster tusk revealed a possible alveolus for a lower tusk, but much of the anterior portion of the symphysis appears “hollow” and filled with plaster.
TMM 30896-295 includes a right dentary with a moderately worn and broken m3, a very elongated symphysis and no evidence of lower tusks. The length of the preserved symphysis is 807 mm and of the total dentary is 1560 mm. The depth of the dentary below the posterior termination of the symphysis is 163 mm, and the broken m3 is approximately 190 x 74 mm. Although somewhat smaller, the geometry of this mandible is similar to TMM 30896-570.
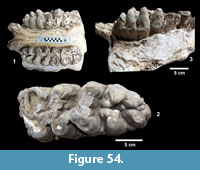 TMM 30896-325 is a partial palate with left and right M2 and M3 (Figure 54). The M2s are fractured and very worn while the M3s are moderately worn, especially the first and second lophs. The M3s have four main lophs, a smaller fifth loph and a posterior complex of two or three conules. Pretrite trefoils are well developed and the teeth are relatively hypsodont with a crown height of 74 mm on the labial side of the unworn fourth loph (Figure 54.3). A well-developed anterolingual cingulum is present. Although portions of both left and right maxillae are preserved, at least some of the palate appears to be a plaster reconstruction.
TMM 30896-325 is a partial palate with left and right M2 and M3 (Figure 54). The M2s are fractured and very worn while the M3s are moderately worn, especially the first and second lophs. The M3s have four main lophs, a smaller fifth loph and a posterior complex of two or three conules. Pretrite trefoils are well developed and the teeth are relatively hypsodont with a crown height of 74 mm on the labial side of the unworn fourth loph (Figure 54.3). A well-developed anterolingual cingulum is present. Although portions of both left and right maxillae are preserved, at least some of the palate appears to be a plaster reconstruction.
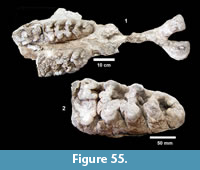 A partial palate is represented by TMM 30896-269 (Figure 55). This specimen includes a very worn and broken left M2 and moderately worn left and right M3 with well-developed pretrite trefoils. The fifth loph and posterior heel are expressed as a semi-circular collection of posterior conules. Although embedded in plaster, continuous bone appears to associate the palate with the occipital condyles.
A partial palate is represented by TMM 30896-269 (Figure 55). This specimen includes a very worn and broken left M2 and moderately worn left and right M3 with well-developed pretrite trefoils. The fifth loph and posterior heel are expressed as a semi-circular collection of posterior conules. Although embedded in plaster, continuous bone appears to associate the palate with the occipital condyles.
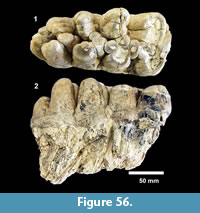 Isolated upper molars include TMM 30896-381, a slightly worn right M3 (Figure 56) and TMM 30936-28, a left M3. The latter is the only specimen assignable to B. buckneri from the Ten Mile Waterhole Creek Local Fauna. The moderately worn tooth is broad (195 x 90 mm) and the valleys are filled with accessory conules. The specimen is anomalously encrusted with carbonate cement.
Isolated upper molars include TMM 30896-381, a slightly worn right M3 (Figure 56) and TMM 30936-28, a left M3. The latter is the only specimen assignable to B. buckneri from the Ten Mile Waterhole Creek Local Fauna. The moderately worn tooth is broad (195 x 90 mm) and the valleys are filled with accessory conules. The specimen is anomalously encrusted with carbonate cement.
 TMM 30896-389 is a skull that was partially figured by Sellards (1940) to support his comments about anomalous dentition in some specimens (Figure 57). Archival records include Survey photos of this skull after preparation, but the location of the specimen is now unknown. The photos, and Sellards (1940) figure, clearly show a small molar posterior to M3 possibly representing a rare case of proboscidean hyperdontia. The M3 also appears anomalously long and relatively narrow with five lophs and a posterior heel composed of multiple conules.
TMM 30896-389 is a skull that was partially figured by Sellards (1940) to support his comments about anomalous dentition in some specimens (Figure 57). Archival records include Survey photos of this skull after preparation, but the location of the specimen is now unknown. The photos, and Sellards (1940) figure, clearly show a small molar posterior to M3 possibly representing a rare case of proboscidean hyperdontia. The M3 also appears anomalously long and relatively narrow with five lophs and a posterior heel composed of multiple conules.
 One of the most complete skulls known of B. buckneri (TMM 30896-656, Figure 58) was removed from the original field jacket and prepared at the Vertebrate Paleontology Collections during 2017-2018. Although crushed laterally, the approximate dimensions of the cranium are: height of cranium from cranial vertex to alveolar plane of the molars 69 cm, length from pre-orbitals to occipital condyles >76 cm (occipital condyles are missing), angle between tusk alveolus and molar alveolar plane 38 degrees.
One of the most complete skulls known of B. buckneri (TMM 30896-656, Figure 58) was removed from the original field jacket and prepared at the Vertebrate Paleontology Collections during 2017-2018. Although crushed laterally, the approximate dimensions of the cranium are: height of cranium from cranial vertex to alveolar plane of the molars 69 cm, length from pre-orbitals to occipital condyles >76 cm (occipital condyles are missing), angle between tusk alveolus and molar alveolar plane 38 degrees.  The skull exhibits a gently sloping frontal profile as is common in gomphotheriids. The jugal is preserved and is ~36 cm long. The first rib was displaced post-mortem and is wedged under the jugal, suggesting that the skull and post-cranial material were separate bones, probably lacking flesh and skin at the time of burial. The skull includes a very worn right M2 and very worn right and left M3. TMM 30896-656 was a mature adult based on the significant wear on M3 (Figure 59). The first three lophs are well worn, the fourth has moderate wear and the conules of the heel are unworn.
The skull exhibits a gently sloping frontal profile as is common in gomphotheriids. The jugal is preserved and is ~36 cm long. The first rib was displaced post-mortem and is wedged under the jugal, suggesting that the skull and post-cranial material were separate bones, probably lacking flesh and skin at the time of burial. The skull includes a very worn right M2 and very worn right and left M3. TMM 30896-656 was a mature adult based on the significant wear on M3 (Figure 59). The first three lophs are well worn, the fourth has moderate wear and the conules of the heel are unworn.
 TMM 30896-656 also includes an upper left tusk that was sawed off in the field and jacketed separately (Figure 60). The tusk is roughly oval in cross-section with a maximum dimension at the proximal end of 175 mm and a total length of ~160 cm (Figure 61). The tusk has no enamel band and is slightly curved with no expression of a helical spiral. There are approximately 12 macroscopic concentric growth rings at the position where the tusk was sawed off by the Survey workers near the skull. These growth rings are composed of a thicker lighter colored interval and a thin darker interval. There are numerous upper tusks from the Buckner Ranch Site 1 locality, many of which are still in plaster field jackets. Field photos from the Survey illustrate the likely upward and outward curvature of the upper tusks (Figure 62).
TMM 30896-656 also includes an upper left tusk that was sawed off in the field and jacketed separately (Figure 60). The tusk is roughly oval in cross-section with a maximum dimension at the proximal end of 175 mm and a total length of ~160 cm (Figure 61). The tusk has no enamel band and is slightly curved with no expression of a helical spiral. There are approximately 12 macroscopic concentric growth rings at the position where the tusk was sawed off by the Survey workers near the skull. These growth rings are composed of a thicker lighter colored interval and a thin darker interval. There are numerous upper tusks from the Buckner Ranch Site 1 locality, many of which are still in plaster field jackets. Field photos from the Survey illustrate the likely upward and outward curvature of the upper tusks (Figure 62).
Two lower tusks are small, slightly curved and oval in cross-section although both are fractured and were restored with plaster (Figure 63). Both tusks exhibit fragments of an enamel band that was apparently present on only one side of the tusk (presumably the labial side). These tusks match very closely the size and geometry of the alveoli in mandible TMM 30896-278 and others.
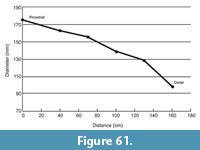 Post-cranial Material
Post-cranial Material
Two stylohyoid bones from two different individuals were recovered from the Buckner Ranch site TMM 30896 (Figure 64). The stylohyoids are interpreted to represent B. buckneri because they were recovered in association with B. buckneri skulls and post-cranial material. Following the characters described by Shoshani et al. (2007), these bones show clear affinity with the Gomphotheriidae. Both specimens are flattened somewhat due to burial, so some characters cannot be assessed. A posterior ramus is well developed and the ratio of the superior ramus length to the posterior ramus length is 0.98-1.0. The ratio of the inferior ramus-superior ramus length to the superior ramus length to the “point” (as defined in Shoshani et al., 2007) is 2.56-2.32. The angle between the inferior ramus and the posterior ramus is 62-63 degrees. The shelf at the intersection of all three rami is present, and there is no angulus on the posterior ramus. The qualitative metrics are identical to the average gomphothere values in Shoshani et al. (2007). The angle between the inferior ramus and the posterior ramus is greater than other proboscidean groups documented by Shoshani et al. (2007) except for Mammuthus. A number of factors can result in measurement variability including preserved length of inferior ramus and compaction deformation.
 Post-cranial elements are shown in Figure 65-Figure 66 and dimensions are provided in Table 5. There are numerous additional post-cranial specimens, many still in field jackets at the Vertebrate Paleontology Collections.
Post-cranial elements are shown in Figure 65-Figure 66 and dimensions are provided in Table 5. There are numerous additional post-cranial specimens, many still in field jackets at the Vertebrate Paleontology Collections.
Discussion. A large collection of both cranial and post-cranial material from a longirostrine gomphothere was obtained by the Survey from TMM 30896 (Buckner Ranch Site 1). The abundance of proboscidean material at this locality from a relatively restricted horizon is anomalous relative to the other Lapara Creek localities, and suggests unique paleoenvironmental and/or taphonomic conditions. Photos from the Survey document the association of some of the gomphothere material with multiple skulls and post-cranial material found in a relatively restricted quarry area, although very little of this material is articulated (Figure 62). Other than a few photos, no records were made of the detailed distribution of specimens in the quarry.
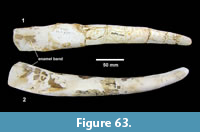 Sellards (1940b) described Gnathabelodon buckneri based on a small subset of the proboscidean material from the Buckner Ranch Site 1 quarry. Although he did not designate a holotype for G. buckneri, TMM 30896-390 (Figure 44) is the most complete specimen figured in his 1940 paper, where the name was proposed and all of the diagnostic morphologies he noted are displayed in this specimen. Sellards (1940b) stated that G. buckneri was characterized by long lower jaws that were downcurved with slightly expanded “tips” being flat on top and bifid. He further stated that lower tusks were lacking, based exclusively on TMM 30896-390, which is a very mature individual. Additional mandibles from less mature individuals are now known to include alveoli for lower tusks and a pair of isolated lower tusks with enamel bands from the Buckner Ranch Site 1 quarry, was apparently recovered after Sellards submitted his publication.
Sellards (1940b) described Gnathabelodon buckneri based on a small subset of the proboscidean material from the Buckner Ranch Site 1 quarry. Although he did not designate a holotype for G. buckneri, TMM 30896-390 (Figure 44) is the most complete specimen figured in his 1940 paper, where the name was proposed and all of the diagnostic morphologies he noted are displayed in this specimen. Sellards (1940b) stated that G. buckneri was characterized by long lower jaws that were downcurved with slightly expanded “tips” being flat on top and bifid. He further stated that lower tusks were lacking, based exclusively on TMM 30896-390, which is a very mature individual. Additional mandibles from less mature individuals are now known to include alveoli for lower tusks and a pair of isolated lower tusks with enamel bands from the Buckner Ranch Site 1 quarry, was apparently recovered after Sellards submitted his publication.
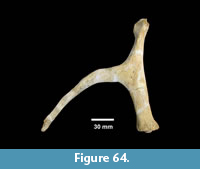 In his diagnosis, Sellards (1940b) included “intermediate molars trilophodont” with M/m3s commonly having five lophs (ids), but with some variability. Upper tusks were characterized as slender, 5 to 6 feet long, lacking enamel bands and upcurved. Sellards (1940b) argued that the Buckner Ranch gomphothere was excluded from Megabelodon because of the long, upcurved upper tusks that lacked enamel bands. He also argued that it differed from Gnathabelodon because of the morphology of the symphysis and the curvature of the upper tusks, but he still used that name. We now know it differs from Gnathabelodon in both upper tusk morphology, in the presence of lower tusks and in molar morphology.
In his diagnosis, Sellards (1940b) included “intermediate molars trilophodont” with M/m3s commonly having five lophs (ids), but with some variability. Upper tusks were characterized as slender, 5 to 6 feet long, lacking enamel bands and upcurved. Sellards (1940b) argued that the Buckner Ranch gomphothere was excluded from Megabelodon because of the long, upcurved upper tusks that lacked enamel bands. He also argued that it differed from Gnathabelodon because of the morphology of the symphysis and the curvature of the upper tusks, but he still used that name. We now know it differs from Gnathabelodon in both upper tusk morphology, in the presence of lower tusks and in molar morphology.  The skull of the only specimen of G. thorpei, collapsed during excavation, and much of it was lost including the anterior portion of the mandibular symphysis. Nevertheless, Barbour and Sternberg (1935) chose to erect Gnathabelodon as a new genus and placed this taxon in its own subfamily. Because of its unique attributes, Gnathabelodon has been difficult to place in phylogenetic models (Shoshani and Tassy, 2005). In their diagnosis, Barbour and Sternberg (1935) described the upper tusks as long, thick, slightly helical and lacking an enamel band. The upper tusks were considered to be different from the generally short and downturned geometry in all known longirostrine gomphotheres. In this sense, the Buckner Ranch gomphothere is similar to G. thorpei. The mandible of G. thorpei was described as long, “strong”, massive and tuskless. The shape of the symphysis was reconstructed and described as having a shoehorn-like geometry. Although they argued that lower tusks were “obviously precluded” based on the shape of the symphysis, Barbour and Sternberg (1935) confused the issue somewhat by describing alveoli for lower tusks that were “ossified throughout”. Most recent analyses do not place Gnathabelodon in the Amebelodontinae (Shoshani and Tassy, 2005). The upper and lower third molars of G. thorpei have well-developed trefoils but differ from Blancotherium in having only four main lophs (ids) and a small posterior heel. The figured upper molar in Barbour and Sternberg (1935) illustrates three main lophs, a smaller fourth loph and a much smaller posterior heel. (Note this tooth is a right M3 rather than a left as labeled in their figure 189.) The M3 of G. thorpei (209 mm x 108 mm) is longer and relatively wider than the average M3 of B. buckneri (196 x 95 mm) while m3 (165 mm x 88.9 mm in G. thorpei) is significantly shorter and relatively wider (B. buckneri: 190 x 86 mm average). Based on the amount of wear, the holotype of G. thorpei was a mature adult.
The skull of the only specimen of G. thorpei, collapsed during excavation, and much of it was lost including the anterior portion of the mandibular symphysis. Nevertheless, Barbour and Sternberg (1935) chose to erect Gnathabelodon as a new genus and placed this taxon in its own subfamily. Because of its unique attributes, Gnathabelodon has been difficult to place in phylogenetic models (Shoshani and Tassy, 2005). In their diagnosis, Barbour and Sternberg (1935) described the upper tusks as long, thick, slightly helical and lacking an enamel band. The upper tusks were considered to be different from the generally short and downturned geometry in all known longirostrine gomphotheres. In this sense, the Buckner Ranch gomphothere is similar to G. thorpei. The mandible of G. thorpei was described as long, “strong”, massive and tuskless. The shape of the symphysis was reconstructed and described as having a shoehorn-like geometry. Although they argued that lower tusks were “obviously precluded” based on the shape of the symphysis, Barbour and Sternberg (1935) confused the issue somewhat by describing alveoli for lower tusks that were “ossified throughout”. Most recent analyses do not place Gnathabelodon in the Amebelodontinae (Shoshani and Tassy, 2005). The upper and lower third molars of G. thorpei have well-developed trefoils but differ from Blancotherium in having only four main lophs (ids) and a small posterior heel. The figured upper molar in Barbour and Sternberg (1935) illustrates three main lophs, a smaller fourth loph and a much smaller posterior heel. (Note this tooth is a right M3 rather than a left as labeled in their figure 189.) The M3 of G. thorpei (209 mm x 108 mm) is longer and relatively wider than the average M3 of B. buckneri (196 x 95 mm) while m3 (165 mm x 88.9 mm in G. thorpei) is significantly shorter and relatively wider (B. buckneri: 190 x 86 mm average). Based on the amount of wear, the holotype of G. thorpei was a mature adult.
 Additional material has been prepared since Sellards’ publication and provides the basis for a revised description and a new name for this proboscidean. The sample from the Buckner Ranch Site 1 quarry is interpreted to represent a single taxon based on molar morphology and variable mandible morphology appears to be related to maturity and possibly gender. The most mature specimens, based on tooth wear, have very elongated symphyses and, although they lack clear evidence of lower tusks, dentaries exhibit breakage possibly associated with collapse of alveoli. Immature to moderately mature specimens have relatively shorter symphyses and possess alveoli for lower tusks. The lower tusks are not flattened, as in classic “shovel-tuskers”, but are small and oval in cross section. Evidence of lower tusks is now recognized in mandibles from immature through mature individuals. The two mandibles from the most mature individuals (TMM 30896-390, TMM 30897-570) have very elongated symphyses, and the evidence for lower tusk alveoli is equivocal. It may be that lower tusks were lost in late stages of life as the symphysis became very elongated. Loss of lower tusks with maturity has been described in the gomphothere Cuvieronius (Mothe et al., 2016).
Additional material has been prepared since Sellards’ publication and provides the basis for a revised description and a new name for this proboscidean. The sample from the Buckner Ranch Site 1 quarry is interpreted to represent a single taxon based on molar morphology and variable mandible morphology appears to be related to maturity and possibly gender. The most mature specimens, based on tooth wear, have very elongated symphyses and, although they lack clear evidence of lower tusks, dentaries exhibit breakage possibly associated with collapse of alveoli. Immature to moderately mature specimens have relatively shorter symphyses and possess alveoli for lower tusks. The lower tusks are not flattened, as in classic “shovel-tuskers”, but are small and oval in cross section. Evidence of lower tusks is now recognized in mandibles from immature through mature individuals. The two mandibles from the most mature individuals (TMM 30896-390, TMM 30897-570) have very elongated symphyses, and the evidence for lower tusk alveoli is equivocal. It may be that lower tusks were lost in late stages of life as the symphysis became very elongated. Loss of lower tusks with maturity has been described in the gomphothere Cuvieronius (Mothe et al., 2016).
The Buckner Ranch gomphothere differs from all known longirostrine gomphotheres based on the following suite of characters:
1. Long, slender, upcurved upper tusks that lack enamel bands and do not spiral.
2. Small lower tusks, oval in cross-section, with an enamel band.
3. Downward deflected (24-38 degrees), U-shaped mandibular symphysis that becomes very elongated in mature individuals
4. Third molars with four main lophs, a smaller fifth loph and a posterior heel.
The stylohyoid of the Buckner Ranch proboscidean shows clear affinity with the Gomphotheriidae as described in Shoshani and Marchant (2001). Lower tusks are not flattened as in Amebelodon, Serbelodon, Platybelodon, Torynobelodon, or Eurybelodon. Small, oval lower tusks are present at least until late stages of life. The Buckner Ranch proboscidean represents a longirostrine gomphotheriid with pretrite trefoils on the lower and upper molars and trilophodont intermediate cheek teeth with tetralophodont third molars, often with a small fifth loph and a posterior heel. It differs from Gomphotherium in having no enamel bands on the upper tusks. It differs from the amebelodontine gomphotheriids in having long, relatively slender upper tusks that diverge up and out and do not spiral. It differs from Eubelodon and Megabelodon in having mandibular tusks, although it is similar to these taxa in having a flattened mandibular symphysis that becomes elongated with maturity. It also differs from Eubelodon and Megabelodon in having long, slender upturned upper tusks, and from Eubelodon in having a downward deflected symphysis. It is similar to Rynchotherium in having lower tusks with enamel bands and a downward deflected symphysis, however, Rynchotherium maintains spiral upper tusks with enamel bands. Quinn (1955) recognized that the Buckner Ranch gomphothere was not Gnathabelodon and used the name Gomphotherium buckneri in his Lapara Creek Faunal list. For reasons stated above, the Buckner Ranch gomphothere is not Gomphotherium.
Sellards (1940) described the anomalous condition of a fourth molar in some of the Buckner Ranch specimens and speculated that the small posterior tooth was a vestigial M3, thus making the dominant molar M2. Although TMM 30896-389 figured by Sellards (1940) could not be relocated, the Survey photo of this specimen (Figure 57) clearly illustrates the small molar posterior to M3, perhaps representing a rare case of hyperdontia.
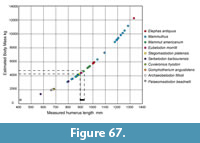 The measured lengths of three humeri from B. buckneri show a range of 910-935 mm (mean: 921). Based on the work of Christiansen (2004), this reflects a large gomphotheriid with an estimated body mass of ~4300-4700 kg (Figure 67). This is significantly larger than the measured lengths for Serbelodon, Gomphotherium and Cuvieronius, but similar in size to Stegomastodon, Eubelodon and Mammut. Tibia lengths (590-630 mm, mean: 615 mm) suggest a body mass of 3300 to 3900 kg according to Christiansen’s data. The discrepancy between estimates suggests that the relative proportions of humerus and tibia length to body mass in Blancotherium were different than in extant proboscideans from which Christiansen (2004) derived his relationships. Nevertheless, B. buckneri was a relatively large gomphothere with body mass probably in the 3500-4500 kg range (~4-5 tons).
The measured lengths of three humeri from B. buckneri show a range of 910-935 mm (mean: 921). Based on the work of Christiansen (2004), this reflects a large gomphotheriid with an estimated body mass of ~4300-4700 kg (Figure 67). This is significantly larger than the measured lengths for Serbelodon, Gomphotherium and Cuvieronius, but similar in size to Stegomastodon, Eubelodon and Mammut. Tibia lengths (590-630 mm, mean: 615 mm) suggest a body mass of 3300 to 3900 kg according to Christiansen’s data. The discrepancy between estimates suggests that the relative proportions of humerus and tibia length to body mass in Blancotherium were different than in extant proboscideans from which Christiansen (2004) derived his relationships. Nevertheless, B. buckneri was a relatively large gomphothere with body mass probably in the 3500-4500 kg range (~4-5 tons).
B. buckneri is the most abundant taxon from the Buckner Ranch Site 1 locality (TMM 30896) and represents one of the larger samples of a Clarendonian gomphothere from North America. Although a second gomphothere is known from the Lapara Creek Fauna, no material from that taxon has been recognized at either the Buckner Ranch or Farish Ranch localities. Furthermore, B. buckneri is not present in the Bridge Ranch Local Fauna and only a single tooth assignable to this taxon is known from the Ten Mile Waterhole Creek locality. However, this latter specimen is of suspect provenance due to an unusual coating of carbonate cement. Based on the unique morphological attributes described above and the large number of specimens, the Buckner Ranch gomphothere is removed from the genus Gnathabelodon and is designated as the type species of a new genus, Blancotherium, with the genotypic and only know species being Blancotherium buckneri (Sellards, 1940b).
Genus GOMPHOTHERIUM Frick, 1933
cf. Gomphotherium sp. (Figure 68, Figure 69, Figure 70, Figure 71)
Localities. Bridge Ranch, Ten Mile Waterhole Creek
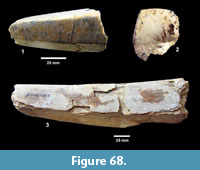 Referred specimens. TMM 30936-90 upper tusk fragment, -97 right M3, -289 left m3, -331 M/m2; TMM 31170-47 upper tusk fragment; TMM 31132-274 m3, -365 lower tusk fragment; TMM 31081-1 juvenile right dentary with dp2 and dp4.
Referred specimens. TMM 30936-90 upper tusk fragment, -97 right M3, -289 left m3, -331 M/m2; TMM 31170-47 upper tusk fragment; TMM 31132-274 m3, -365 lower tusk fragment; TMM 31081-1 juvenile right dentary with dp2 and dp4.
Description. A second proboscidean is represented in the Lapara Creek Fauna by isolated molars, molar fragments, upper tusk fragments and a single fragment of a lower tusk. The upper tusk fragments (TMM 30936-90, TMM 30936-47) preserve an enamel band that forms an approximate right angle with a flattened dorsal wear surface. Cross-hatching (engine-turning pattern) is apparent in the dentinal core of TMM 30936-90 (Figure 68). It is uncertain how long the upper tusks may have originally been, as both fragments are relatively distal. There is a slight curvature apparent in TMM 30936-47.
 The lower tusk fragment is pear-shaped in cross-section and shows concentric rings with well-developed cross-hatching (engine-turning pattern) and no evidence of dentinal rods (Figure 69). The specimen is approximately 55 mm wide and has a maximum thickness of 35 mm.
The lower tusk fragment is pear-shaped in cross-section and shows concentric rings with well-developed cross-hatching (engine-turning pattern) and no evidence of dentinal rods (Figure 69). The specimen is approximately 55 mm wide and has a maximum thickness of 35 mm.
Isolated molars are relatively narrow with open valleys lacking accessory cusps and are relatively low crowned (Figure 70). Third molars exhibit 4 main lophs/lophids and a posterior heel with a weak anterior cingulum. Pretrite trefoils are weakly developed relative to Blancotherium buckneri. Second molars are trilophodont with a posterior heel. Three third molars range in size from 150-170 x 66-75 mm. TMM 30936-331, an M2, is 115 x 68 mm. These teeth are significantly smaller than in B. buckneri.
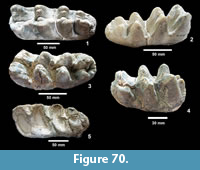 Discussion. The gomphothere from the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas is characterized by relatively narrow molars and clean valleys between lophs, although still exhibiting a weakly developed pretrite trefoil pattern. These molars are associated with upper tusk fragments that possess broad enamel bands and with a fragment of a flattened lower tusk. Nothing is known about the morphology of the mandible or skull, although this proboscidean is clearly distinct from Blancotherium buckneri. The lower tusk fragment has a cross-sectional geometry similar to examples of Serbelodon barbourensis (Frick, 1933). However, the molars are unlike Serbelodon. Quinn (1955) listed “Gomphotherium cf. productus” as a taxon from the Lapara Creek Fauna with no discussion. Wang et al. (2017) reviewed Gomphotherium and documented the paraphyletic nature of this taxon. In their phylogenetic analysis, they recognized three main groups. The “derived Gomphotherium” group includes the complex of North American species they refer to G. productum. Some third molars figured by Wang et al. (2017) of G. tassyi, that also belongs to the “derived Gomphotherium” group, are very similar to cf. Gomphotherium from the Lapara Creek Fauna.
Discussion. The gomphothere from the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas is characterized by relatively narrow molars and clean valleys between lophs, although still exhibiting a weakly developed pretrite trefoil pattern. These molars are associated with upper tusk fragments that possess broad enamel bands and with a fragment of a flattened lower tusk. Nothing is known about the morphology of the mandible or skull, although this proboscidean is clearly distinct from Blancotherium buckneri. The lower tusk fragment has a cross-sectional geometry similar to examples of Serbelodon barbourensis (Frick, 1933). However, the molars are unlike Serbelodon. Quinn (1955) listed “Gomphotherium cf. productus” as a taxon from the Lapara Creek Fauna with no discussion. Wang et al. (2017) reviewed Gomphotherium and documented the paraphyletic nature of this taxon. In their phylogenetic analysis, they recognized three main groups. The “derived Gomphotherium” group includes the complex of North American species they refer to G. productum. Some third molars figured by Wang et al. (2017) of G. tassyi, that also belongs to the “derived Gomphotherium” group, are very similar to cf. Gomphotherium from the Lapara Creek Fauna. 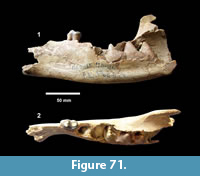 Due to the limited amount of material, and the uncertain taxonomic status of similar proboscideans, the gomphothere from the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas is referred to cf. Gomphotherium sp.
Due to the limited amount of material, and the uncertain taxonomic status of similar proboscideans, the gomphothere from the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas is referred to cf. Gomphotherium sp.
TMM 31081-1 is a right dentary of a juvenile gomphothere with a moderately worn, double rooted dp2, double rooted alveoli for dp3 and an unerupted dp4 (Figure 71). It is not possible to tell which gomphothere taxon this specimen represents. However, the dp4 is a zygodont tooth with clean valleys most similar to mature cf. Gomphotherium. The occurrence of this specimen in the Farish Ranch Local Fauna may suggest it is a juvenile Blancotherium buckneri. The symphyseal region is incomplete, but there is no evidence of lower tusks.
Order ARTIODACTYLA Owen, 1848
Family MERYCOIDODONTIDAE Thorpe, 1923
Subfamily USTATOCHOERINAE Stevens and Stevens, 2007
Genus USTATOCHOERUS Schultz and Falkenbach, 1941
Ustatochoerus cf. medius (Leidy, 1858)
(Figure 72, Table 6)
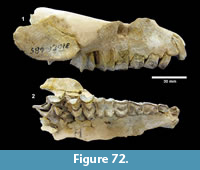 Localities. Bridge Ranch (TMM 31170), Farish Ranch (TMM 31081).
Localities. Bridge Ranch (TMM 31170), Farish Ranch (TMM 31081).
Referred specimens. TMM 31170-25 partial right dentary with p4-m2, -114 left M2; TMM 31081-685 partial right maxilla with C, P1-M3, -1157 partial right maxilla with M2-M3 and alveoli for P1-M1.
Description. TMM 31081-685 is a partial right maxilla with C, P1-M3 (Figure 72). The cheek teeth through M1 are very worn, although M3 has only minor wear. Premolars exhibit both labial and lingual cingula, and the molars have well developed labial styles and ribs. M1-M2 possess anterolingual cingula. The diastema between C and P1 is approximately 5 mm.
The P1-P4 length for TMM 31081-685 is ~47 mm and for M1-M3 is ~69.6 mm. The infraorbital foramen above P4 is sub-triangular in cross-section with a maximum width of ~7mm. The angle of the premaxillary bone on both TMM 31081-685 and -1157 steepens significantly above P2.
Discussion. Oreodonts are rare in the Lapara Creek Fauna. The few specimens that are known compare well with Ustatochoerus medius based on dentition, the geometry of the maxilla and general size. The upper premolars exhibit well-developed labial cingula, as is characteristic of the genus (Stevens and Stevens, 2007). Schultz and Falkenbach (1968) report a length range (P1-M3) of 106-122.5 mm for U. medius (including subspecies) from various localities in California, New Mexico, Colorado, Nebraska and Kansas. The equivalent dimensions from the Lapara Creek specimens are approximately 112-116 mm. Although not complete, the relatively steep angle of the maxilla (Figure 72) suggests that the nasals were not significantly retracted.
Stevens and Stevens (2007) recognized Ustatochoerus as a valid taxon that could be separated from Ticholeptis and Merychyus on the basis of dentition. Following their usage, U. medius is known from late Barstovian through early Clarendonian faunas in the Great Plains and U.S. Southwest. Patton (1969) referred the Lapara Creek oreodont to U. profectus but noted difficulty in separating large U. medius from small U. profectus. However, as discussed by Stevens and Stevens (2007), U. profectus may be considered a junior synonym of U. major until more cranial material is recovered. The Lapara Creek oreodont is significantly smaller than U. major and appears to have a steeper maxillary profile. Because of the limited material available from the Lapara Creek Fauna, the oreodont is assigned to U. cf. medius.
In a number of papers, Lander (1998, 2005, 2008) has argued for an alternative interpretation of the taxonomy of U. medius and placed ‘medius’ in the genus Merychyus, and Merychyus medius in Ticholeptinae and Oreodontidae (rather than in Ustatochoerinae and Merycoidodontidae). The P1-M3 TRL of the Lapara Creek oreodont is consistent with M. medius medius from the type Clarendon Fauna in Texas as used in Lander (2008). It is beyond the scope of this paper to evaluate the complex taxonomy of oreodonts, but it may be shown that Merychyus cf. medius is the correct name for the Lapara Creek oreodont.
Family GELOCIDAE Schlosser, 1886
Subfamily PSEUDOCERATINAE Frick, 1937
Genus PSEUDOCERAS Frick, 1937
Pseudoceras skinneri (Frick, 1937) (Figure 73)
Localities. Bridge Ranch (TMM 31132, TMM 31170), Buckner Ranch (TMM 30896).
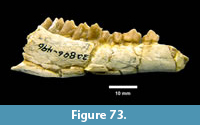 Referred specimens. TMM 31132-4 partial dentary with dp4, -331 partial left dentary with m2-m3; TMM 31170-109 partial left dentary with p4-m1; TMM 30896-326 partial right dentary with p3-m3, -496 partial dentary with p3-m3.
Referred specimens. TMM 31132-4 partial dentary with dp4, -331 partial left dentary with m2-m3; TMM 31170-109 partial left dentary with p4-m1; TMM 30896-326 partial right dentary with p3-m3, -496 partial dentary with p3-m3.
Description. TMM 30896-496 is a partial dentary with p3-m3 present and alveoli for p2. The diastema is not preserved. Using an estimate for p2 based on the preserved alveoli, the p2-m3 TRL is ~49.2 mm. The premolars have weakly developed lingual cingulids and the premolar to molar length ratio is approximately 70%. The metaconid on p3 and p4 rises centrally above each tooth. An enclosed talonid basin on p4 is oriented posterolingually. The molars are mesodont with prominent cingula and stylids. The metaconid and entoconid of m2 and m3 are elongated and flattened as is typical of the pseudoceratines (Webb, 2008). A groove separates the metaconid and entoconid on m2 and m3 (Figure 73). The posterior lophid of the hypoconid fails to join the entoconid leaving a lingual opening, a feature that Webb (2008) described as characteristic of pseudoceratine lower molars. Dimensions of the teeth (p3: 6.2 x 2.6 mm, p4: 7.7 x 3.3 mm, m1: 8.4 x broken, m2: 9.0 x 5.9 mm, m3: 12.4 x 4.9 mm) are consistent with the holotype of P. skinneri (F:AM 33723) figured in Frick (1937, figure 66).
Discussion. Although originally identified as Blastomeryx elegans by Patton (1969), Webb (2008) recognized TMM 30896-496 as Pseudoceras skinneri in his review of the Pseudoceratinae, based on dental morphology and dimensions. Prothero (2008) considered Blastomeryx elegans (Matthew and Cook, 1909) and all previously named species to be junior synonyms of B. gemmifer (Cope, 1874) and chose not to include the Lapara Creek material in his hypodigm for the taxon. Data provided in Prothero (2008) suggest that the premolar length of the Lapara Creek taxon is slightly longer than is typical for Blastomeryx. Four additional partial dentaries represent small artiodactyls similar in size and morphology to TMM 30896-496. TMM 30896-326, a partial right dentary also from the Buckner Ranch quarry, almost certainly represents the same taxon, but is very worn. The assignment of three partial dentaries from the Bridge Ranch Local Fauna is less certain. TMM 31170-109, a partial left dentary, includes two teeth that are so worn it is difficult to be sure of their homology, but the general dimensions of the ramus and teeth are consistent with P. skinneri. TMM 31132-331 is a partial left dentary with m2-m3 that compares well with P. skinneri, and TMM 31132-4 is a partial dentary with dp4.
The occurrence of Pseudoceras skinneri from the Bridge Ranch Local Fauna is likely synchronous with the earliest record of this taxon in North America from the Cl1 Clayton Quarry (Ash Hollow Formation), Nebraska (Webb, 2008). These two faunas share the conspecific taxa of Calippus martini, Calippus placidus, Protohippus supremus, Neohipparion affine and Aelurodon taxoides (Hulbert, 1989; Wang et al., 1999). Webb (2008) reported the youngest occurrence of P. skinneri from Hh2 sites in northern Nebraska, with additional occurrences from the Cl3 Higgins Locality in northern Texas and the Cl3 Love Bone Bed, Florida. Their habitat has been interpreted as one of stream-border forests, consistent with depositional environments of the Goliad Formation and other elements of the Lapara Creek Fauna.
Family ANTILOCAPRIDAE Owen, 1841
Subfamily MERYCODONTINAE Matthew, 1904
Genus RAMOCEROS Frick, 1937
Ramoceros ramosus (Cope, 1874) (Figure 74)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081).
 Referred specimens. TMM 30936-56 m3 (2), -117 m3 (2), -294 partial horn (tine); TMM 31132-73 partial horn; TMM 31170-28 m3; TMM 31081- various post-cranial elements.
Referred specimens. TMM 30936-56 m3 (2), -117 m3 (2), -294 partial horn (tine); TMM 31132-73 partial horn; TMM 31170-28 m3; TMM 31081- various post-cranial elements.
Description. TMM 31132-73 is a partial horn with breaks at the base of each of four tines (Figure 74). The total length of the specimen is 101 mm and the maximum dimension of the shaft just below tine 1 is 12.2 mm. Tine 1 is interpreted to be the characteristic brow tine described by Frick (1937). Sub-circular near the base, the horn flattens distally where tines 2-4 project. No burr is preserved and the horn is hollow at the broken proximal end. In addition to the partial horn, a number of isolated teeth are indistinguishable from Ramoceros, although they possess no characters unique to R. ramosus.
Discussion. Patton (1969) recognized Ramoceros ramosus from the Lapara Creek Fauna claiming that the Lapara Creek specimen was “inseparable from horn material assigned by Frick to Ramoceros ramosus from New Mexico, Colorado and Nebraska.” Although Patton didn’t list any referred specimens, he did include a line drawing of specimen TMM 31132-73 that he mislabeled as 311-73 (figure 32). This partial horn is similar in morphology to examples of Ramoceros ramosus figured in Frick (1937), however, it is smaller and thinner. Without further material, it is possible that this simply reflects a young individual. A number of isolated third molars are also consistent with Ramoceros. These teeth, as well as isolated post-cranial elements of a very small antilocaprid, document the presence of merycodontine antilocaprids in the Lapara Creek Fauna.
Family PROTOCERATIDAE Marsh, 1891
Subfamily SYNTHETOCERATINAE Frick, 1937
Genus SYNTHETOCERAS Stirton, 1932
Synthetoceras tricornatus (Stirton, 1932)
(Figure 75, Table 7)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081).
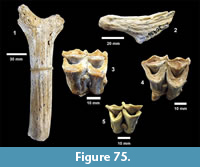 Referred specimens. TMM 30936-20 M1, -23 rostral horn fragment, -89 molars, -115 m, -116 m, -214 M3, -280 M2, -413 m, -506 frontal horn fragment; TMM 31132-563 M2; TMM 31170-84 m3; TMM 31081-525 metapodial, -612 metapodial, -1416 M2, -1471 M2.
Referred specimens. TMM 30936-20 M1, -23 rostral horn fragment, -89 molars, -115 m, -116 m, -214 M3, -280 M2, -413 m, -506 frontal horn fragment; TMM 31132-563 M2; TMM 31170-84 m3; TMM 31081-525 metapodial, -612 metapodial, -1416 M2, -1471 M2.
Description. TMM 30936-23 represents a diagnostic fragment of a bifurcate rostral horn. The maximum preserved diameter is 33 mm while the length of the fragment is 175 mm. The cross-sectional shape of the main shaft changes from sub-triangular towards the proximal end to oval just prior to the bifurcation. A canal with a central septum is exposed along the outer edge of the left tine. A number of isolated teeth can also be referred to S. tricornatus. (Note: dental terminology follows Patton and Taylor, 1971.) Molars are brachydont with well-developed anterolingual cingula, lingual styles between the protocone and metaconule, a strong mesostyle and ribs on the metacone and paracone. Some specimens also exhibit accessory styles (Figure 75). Dimensions of the isolated upper molars compare well with data reported in Patton and Taylor (1971) for S. tricornatus (Table 7).
Discussion. Patton (1969) figured two of the isolated molars of Synthetoceras tricornatus although there are a number of mistakes in that reference. He referred to “figure 11B and 11C” although the figure is actually 29A and 29B. In his description of figure 29, he only mentioned TMM 31132-563 (called UTBEG 31132-563 at that time) as a right m2, which is the specimen shown in 29B. Figure 29A is TMM 30936-214, which Patton (1969) did include in his list of referred specimens. Furthermore, 31132-563 is misidentified as 31153-563 in the referred specimens list. In the discussion, 31132-563 is identified as an M2 rather than m2 as stated in the figure caption. It is unclear why Patton (1969) didn’t mention the other specimens of S. tricornatus, especially the diagnostic nasal horn. Following Patton (1969), Patton and Taylor (1971) included only the same two specimens in their list of referred material for S. tricornatus from the Lapara Creek Fauna.
TMM 30936-23 represents a diagnostic fragment of a bifurcate rostral horn (Figure 75.1). This specimen compares closely in size and morphology with S. tricornatus described by Stirton (1932) from the type Clarendon beds in Donley County, Texas. A series of isolated molars also compare well in both size and morphology with S. tricornatus.
Synthetoceras has relatively straight, unfused metapodials with distal articular heads adjacent to each other (Patton and Taylor, 1971). Patton (1969) assigned multiple unfused metapodials to the camel Protolabis, but all, except TMM 31132-319, are relatively straight, robust and probably belong to Synthetoceras.
Synthetoceras tricornatus is the most common artiodactyl in the Ten Mile Waterhole Creek Local Fauna. Based on size of limited post-cranial material of various artiodactyls, there were probably two camels (similar in size to Procamelus occidentalis and P. grandis) at Ten Mile Waterhole Creek. The dominance of protoceratids in the artiodactyl fauna is consistent with the composition of the Barstovian Cold Spring Fauna from the Fleming Formation that immediately underlies the Goliad Formation. In that fauna, Prosynthetoceras is relatively abundant, and the only camelids that have been described are Aepycamelus and Floridatragulus (Patton, 1969). Aepycamelus sp. was recognized by Patton (1969) on the basis of very limited post-cranial material, and Floridatragulus hesperus was named by Patton (1969) on the basis of a single specimen, with no referred specimens. Whether or not F. hesperus from the Cold Spring Fauna is a valid name is questionable as the only specimen is a partial mandible with well-worn m1-m3. Regardless, the composition of the Barstovian and early Clarendonian artiodactyl faunas in the Texas Gulf Coast is similar. It is not until the time of the Bridge Ranch Local Fauna, and especially the Farish and Buckner Ranch Local Faunas, that the camelids become significantly more diverse and dominate the artiodactyl population. S. tricornatus persisted through the Farish Ranch Local Fauna even as camelid diversity expanded. No specimens of S. tricornatus have been found in the Buckner Ranch Local Fauna although a number of other artiodactyls are known. Synthetoceras. tricornatus is best known from the Clarendon beds of Donley Co., Texas (Patton and Taylor, 1971). Synthetoceras cf. tricornatus has been reported from the Hemphillian of Florida and Alabama (Hirschfeld and Webb, 1968; Isphording and Lamb, 1971).
Family PALAEOMERYCIDAE Lydekker, 1883
Subfamily DROMOMERYCINAE Frick, 1937
Tribe CRANIOCERATINI Frick, 1937
Genus CRANIOCERAS Matthew, 1918
Cranioceras teres (Cope, 1874) (Figure 76)
Localities. Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081).
Referred specimens. TMM 31132-455 partial skull with proximal horn; TMM 31081-300 M2, -628 M3, -1434 m, -1446 M2, -1480 partial right dentary with dp2-4, m1.
 Description. A relatively small palaeomerycid is represented in the Bridge and Farish Ranch Local Faunas by a partial skull with proximal horn, a partial dentary of an immature animal, and isolated molars. The horn is oval in cross-section near the base with maximum and minimum dimensions of 23.9 and 15.5 mm and lacks any evidence of a burr (Figure 76). The oval cross-section of the post-orbital horn distinguishes Cranioceras from Procranioceras (Frick, 1937). The isolated molars are brachydont with well-developed labial ribs, anterior and posterior cingula and a lingual style (Figure 76.3). Dimensions include: TMM 31132-300 M2 14.4 x 16.6 mm, TMM 31081-1480 m1 17.9 x 11.1 mm, TMM 31081-1446 M2 15.5 x 17.2 mm, TMM 31081-628 M3 17.0 x 19.0 mm. Upper molar dimensions are consistent with the smaller form of Cranioceras (C. teres).
Description. A relatively small palaeomerycid is represented in the Bridge and Farish Ranch Local Faunas by a partial skull with proximal horn, a partial dentary of an immature animal, and isolated molars. The horn is oval in cross-section near the base with maximum and minimum dimensions of 23.9 and 15.5 mm and lacks any evidence of a burr (Figure 76). The oval cross-section of the post-orbital horn distinguishes Cranioceras from Procranioceras (Frick, 1937). The isolated molars are brachydont with well-developed labial ribs, anterior and posterior cingula and a lingual style (Figure 76.3). Dimensions include: TMM 31132-300 M2 14.4 x 16.6 mm, TMM 31081-1480 m1 17.9 x 11.1 mm, TMM 31081-1446 M2 15.5 x 17.2 mm, TMM 31081-628 M3 17.0 x 19.0 mm. Upper molar dimensions are consistent with the smaller form of Cranioceras (C. teres).
Discussion. Patton (1969) identified the partial horn (TMM 31132-455) and an “immature dentition” as Cranioceras clarendonensis. Prothero and Liter (2008) concluded that there were only two valid species of Cranioceras: C. unicornis (larger form) and C. teres (smaller form). By their reasoning, C. clarendonensis is a junior synonym of C. teres and included the specimens reported by Patton (1969) from the Lapara Creek Fauna in this taxon. TMM 31132-455 is indistinguishable from the type specimen of Cranioceras teres, both of which include a small skull fragment attached to the basal part of the post-orbital horn. The type specimen of C. teres (USNM 2044) is from the early Clarendonian Chamita Formation, New Mexico. C. teres has been reported from Barstovian through early Clarendonian faunas in New Mexico, Texas, South Dakota and California (Prothero and Liter, 2008). Although generally considered to be browsers based on tooth and skull morphology, wear patterns on the teeth of Cranioceras studied by Semprebon et al. (2004) suggest at least seasonal or regional grazing was part of their ecology, and that C. teres may have inhabited relatively closed, forest-edge systems.
Family CAMELIDAE Gray, 1821
Subfamily MIOLABINAE Hay, 1902
Genus NOTHOTYLOPUS Patton, 1969
Nothotylopus camptognathus (Patton, 1969) (Figure 77)
Localities. Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
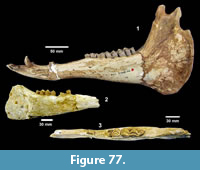 Referred specimens. TMM 31081-26 (holotype) left dentary with i1-3, c, p1, p2-4, m1-3; TMM 30896-198 partial right dentary.
Referred specimens. TMM 31081-26 (holotype) left dentary with i1-3, c, p1, p2-4, m1-3; TMM 30896-198 partial right dentary.
Description. Patton (1969) provided a complete description of the holotype, but listed no referred material. A second specimen of N. camptognathus is now recognized from the Buckner Ranch Local Fauna (Figure 77). TMM 30896-198 is a partial right dentary with p1 (roots only), p3 (roots only), p4, m1 (roots only), m2 (posterior ½), and m3. Dimensions for this specimen include: m3 37 mm x 14.6 mm, m2 25.5 mm x 14.8 mm and p4 12.6 mm x 6.1 mm. There is no lower second premolar, and the root/alveoli for p1 suggests a caniniform geometry. The p1-p3 diastema is approximately 38 mm. The anterior edge of m3 exhibits the buttress described by Patton (1969). Although broken, the posteroventral edge of the ramus exhibits the beginning of a labial flare as in the holotype.
Discussion. Nothotylopus camptognathus was named by Patton (1969) from the Farish Ranch Local Fauna on the basis of a single specimen (TMM 31081-26). A second specimen (TMM 30896-198) is now recognized from the Buckner Ranch Local Fauna. Although slightly smaller, dental morphology and incipient labial flare of the ramus compare well with the holotype. However, the p1-p3 diastema is significantly shorter (38 mm vs. 61.5). The depth of the dentary beneath the p1-p3 diastema is similar to the holotype (38 mm vs. 35.8). N. camptognathus and Protolabis cf. yavapaiensis are the only camelids from the Lapara Creek Fauna that lack p2.
Patton (1969) suggested that Nothotylopus was probably related to Protolabis. Honey et al. (1998) include Nothotylopus in the Miolabinae and recognized N. camptognathus as the only known species. According to those authors, Nothotylopus has been recognized in Barstovian faunas in New Mexico (Tesuque Formation), the Clarendon Local Fauna (Texas), the Barstovian-Clarendonian Valentine Formation and the Clarendonian Ash Hollow Formation (Nebraska). The oldest occurrence of Nothotylopus appears to be from the late Hemingfordian Nambé Fauna in New Mexico (Morgan, 2015). Based on the older occurrences in New Mexico and Nebraska, and the lack of Nothotylopus in the Barstovian faunas of the Texas coastal plain, it appears that this taxon was an immigrant into the Texas coastal plain during the early Clarendonian. Nothotylopus has not been recognized at either the Ten Mile Waterhole Creek or the Bridge Ranch Local Faunas.
Subfamily CAMELINAE Gray, 1821
Tribe LAMINI Webb, 1965
Genus AEPYCAMELUS MacDonald, 1956
Aepycamelus sp. (Figure 78, Figure 79, Figure 80, Figure 81)
Localities. Farish Ranch (TMM 31081).
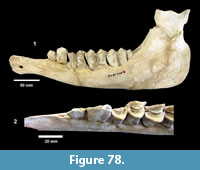 Referred specimens. TMM 31081-18A partial right dentary with p3-m3, -460 left dentary with alveolus for p2, p3-m3, -532 cervical vertebra, -561 metapodial (on display at TMM), -578 cervical vertebra, -636 astragalus, -682 cervical vertebrae.
Referred specimens. TMM 31081-18A partial right dentary with p3-m3, -460 left dentary with alveolus for p2, p3-m3, -532 cervical vertebra, -561 metapodial (on display at TMM), -578 cervical vertebra, -636 astragalus, -682 cervical vertebrae.
Description. TMM 31081-460 was described by Patton (1969). On the basis of this specimen, he proposed the new species Megatylopus primaevus and referred an additional partial right dentary and a metapodial to this taxon. Key features of the holotype TMM 31081-460 include large size, relatively hypsodont premolars and molars, presence of a reduced p2 based on double rooted alveoli, presence of p1 (just erupting), double rooted p3 and p4, slender ramus and symphysis (Figure 78). The integrity of TMM 31081-460 was questioned by this author as p3 and p4 were clearly broken off at some point and glued in place without clear evidence of a convincing fit. The illustration of the holotype in Patton (1969, figure 19) is misleading. To test this concern, imaging was performed at The University of Texas at Austin High Resolution X-ray Computed Tomography (CT) Facility. The resulting CT scan (Figure 79.3) clearly reveals that, although they are broken with no clear fit, p3 and p4 clearly match the roots in the dentary and their mutual association is confirmed. The CT imaging further confirms the value of this non-invasive exploration of the interior structure of certain fossil specimens.
 TMM 31081-18A is from an older individual with more advanced wear, but displays most of the characteristic features (Figure 80). Patton (1969) commented on the smaller size of this specimen and the less well-developed stylids, but the significance of these differences is still unclear. Dimensions for TMM 31081-18A include: p3-15 x 6.4 mm, p4-19.8 x 9.5 mm, m1-31 x 17.5 mm, m2-36.6 x 17.4 mm and m3-47.5 x 18 mm. These dimensions are slightly smaller than in TMM 31081-460 and the depth of the dentary beneath the anterior edge of m1 is 39.4 mm vs. 39.7 mm in the holotype.
TMM 31081-18A is from an older individual with more advanced wear, but displays most of the characteristic features (Figure 80). Patton (1969) commented on the smaller size of this specimen and the less well-developed stylids, but the significance of these differences is still unclear. Dimensions for TMM 31081-18A include: p3-15 x 6.4 mm, p4-19.8 x 9.5 mm, m1-31 x 17.5 mm, m2-36.6 x 17.4 mm and m3-47.5 x 18 mm. These dimensions are slightly smaller than in TMM 31081-460 and the depth of the dentary beneath the anterior edge of m1 is 39.4 mm vs. 39.7 mm in the holotype.
In addition to the two dentaries, more specimens representing a very large, long-necked camel are now recognized including a series of elongated cervical vertebrae ranging from 142-160 mm in length (at centrum) (Figure 81). TMM 31081-636 is a very large astragalus measuring 110.9 x 74.2 mm. TMM 31081-561 is a very elongated metapodial (720 mm) that was identified by Patton (1969) as Aepycamelus.
 Discussion. Patton (1969) named and diagnosed M. primaevus on the basis of limited material with TMM 31081-460 designated as the holotype. He cited Matthew and Cook (1909) for characters of the generic diagnosis including the absence of upper and lower second premolars. Although he acknowledged that the absence of p2 was diagnostic for Megatylopus, he interpreted the reduced p2 in TMM 31081-460 as evidence for it being the most primitive species of Megatylopus and hence the name primaevus. He further noted a strong similarity with M. major. Patton (1969) made no comparison with Aepycamelus even though he identified the latter taxon on the basis of a metapodial from the same quarry.
Discussion. Patton (1969) named and diagnosed M. primaevus on the basis of limited material with TMM 31081-460 designated as the holotype. He cited Matthew and Cook (1909) for characters of the generic diagnosis including the absence of upper and lower second premolars. Although he acknowledged that the absence of p2 was diagnostic for Megatylopus, he interpreted the reduced p2 in TMM 31081-460 as evidence for it being the most primitive species of Megatylopus and hence the name primaevus. He further noted a strong similarity with M. major. Patton (1969) made no comparison with Aepycamelus even though he identified the latter taxon on the basis of a metapodial from the same quarry.
Harrison (1985) did not include M. primaevus in the genus Megatylopus. According to her analysis, the only large early Clarendonian camel was Aepycamelus. Furthermore, she included Megatylopus major in Aepycamelus citing personal communication from B. Taylor’s analysis of specimens in the Frick Collection at the AMNH. Honey et al. (1998) agreed with the inclusion of M. major in Aepycamelus. Although Harrison (1985) stated that p2 was always present in Aepycamelus, Honey et al. (1998) claimed that P2/p2 is sometimes lost in derived species.
 The only other occurrence of Megatylopus primaevus was reported by Leite (1990) as M. cf. primaevus from the Ash Hollow Formation, Nebraska. However, that identification was apparently based on a single juvenile specimen (skull and mandible) with a broken dp2 “indicated by a single small alveolus”. The comparison seems to be based largely on the size of m1, m2 and the depth of the jaw at the diastema. Leite (1990) described the camel from the Shoreline Local Fauna as “a very large camel with large, relatively hypsodont teeth and reduction in number and size of premolars”. Identification of this material as M. cf. primaevus is considered suspect.
The only other occurrence of Megatylopus primaevus was reported by Leite (1990) as M. cf. primaevus from the Ash Hollow Formation, Nebraska. However, that identification was apparently based on a single juvenile specimen (skull and mandible) with a broken dp2 “indicated by a single small alveolus”. The comparison seems to be based largely on the size of m1, m2 and the depth of the jaw at the diastema. Leite (1990) described the camel from the Shoreline Local Fauna as “a very large camel with large, relatively hypsodont teeth and reduction in number and size of premolars”. Identification of this material as M. cf. primaevus is considered suspect.
In their review of the Camelidae, Honey et al. (1998) concluded that the taxonomic status of M. primaevus was uncertain. Citing the previous work of Patton (1969) and Harrison (1985), they suggested that M. primaevus might better be assigned to Aepycamelus. In their diagnosis of Aepycamelus, Honey et al. (1998) included “p3 with two roots, in contrast to most Megatylopus”. The presence of double rooted p2 and p3, the very elongated metapodial and the elongated cervical vertebrae all suggest that this large camel from the Farish Ranch Local Fauna should be referred to Aepycamelus. M. primaevus is therefore considered a nomen dubium and the tall, long-necked camel from the Farish Ranch Local Fauna is assigned to Aepycamelus. The elongated metapodial (~720 mm) is similar in size to A. bradyi and is significantly larger than A. giraffinus (Patton, 1969). The astragalus from the Farish Ranch Local Fauna is significantly larger than that of A. bradyi (MacDonald, 1956). Harrison (1985) stated that A. bradyi was the largest known species of the genus, however, a metapodial assigned to A. major from the Clarendonian Love Bone Bed, Florida, was reported as ~870 mm long by Webb et al. (1981). Based on size, Aepycamelus from the Lapara Creek Fauna is probably close to A. bradyi or A. major.
Tribe CAMELINI Gray, 1821
Genus PROCAMELUS Leidy, 1858
Procamelus occidentalis Leidy, 1858
(Figure 82.1, Table 8)
Localities. Ten Mile Waterhole Creek? (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
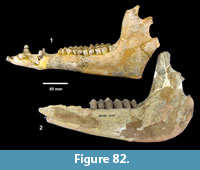 Referred Specimens. TMM 30936-52, -53 astragali, -125 metapodial, -181 astragalus; TMM 31132-374 partial left maxillary P3-M3; TMM 31170-6 partial right dentary p1-m3, TMM 31170-63 partial right dentary m2, m3 (broken); TMM 31081-241 partial left dentary with p2 (roots only), p3-m3, -665 nearly complete mandible with i1-3, c, p1-4, and m1-3; TMM 31081-49 partial left dentary with p2 (roots only), p3-m3 (m2 broken), -313 partial right dentary with m1-m3, -543 partial left maxillary P2-M3, -738 partial right dentary p1, p2 and p3 (roots only), p4, m1(broken), m2-m3; TMM 30896-475 partial mandible i2 (broken), i3-c, p1-p3 (alveoli), p4-m3; numerous post-cranial elements.
Referred Specimens. TMM 30936-52, -53 astragali, -125 metapodial, -181 astragalus; TMM 31132-374 partial left maxillary P3-M3; TMM 31170-6 partial right dentary p1-m3, TMM 31170-63 partial right dentary m2, m3 (broken); TMM 31081-241 partial left dentary with p2 (roots only), p3-m3, -665 nearly complete mandible with i1-3, c, p1-4, and m1-3; TMM 31081-49 partial left dentary with p2 (roots only), p3-m3 (m2 broken), -313 partial right dentary with m1-m3, -543 partial left maxillary P2-M3, -738 partial right dentary p1, p2 and p3 (roots only), p4, m1(broken), m2-m3; TMM 30896-475 partial mandible i2 (broken), i3-c, p1-p3 (alveoli), p4-m3; numerous post-cranial elements.
Description. Patton (1969) provided an adequate description of P. occidentalis from the Lapara Creek Fauna. This is the most common camel in the fauna and is found in at least three, and possibly all four, of the local faunas. Post-cranial material (astragali, metapodial) from the Ten Mile Waterhole Creek Local Fauna are consistent in size and morphology with P. occidentalis. Some of the key features include the dental formula of i3, c, p4, m3; a single-rooted caniniform p1, double rooted p2-p4; triangular-shaped p4 with a strong anterolingual flexid and posterior enamel lake; moderately hypsodont molars lacking strong ribs or styles and relatively slender ramus with no lateral deflections. The p1-p2 diastema ranges from 22-32 mm and the p2-m3 TRL varies between 113 and 122.5 mm. TMM 31081-543 is a partial left maxillary with P2-M3 and tooth dimensions of: P2 10.4 x 5.5 mm, P3 12.1 x 7.9 mm, P4 12.4 x 11.4 mm, M1 18.8 x 17.3 mm, M2 24.2 x 19.9 mm, M3 24.2 x 19.0 mm.
Discussion. The most common camel in the Lapara Creek Fauna is Procamelus occidentalis. This taxon is known from Barstovian and Clarendonian faunas in Nebraska, Montana and Texas (Honey et al., 1998). This taxon has not been recognized from similar age faunas in California, New Mexico or Florida.
Procamelus grandis Gregory, 1939
(Figure 82.2, Table 9)
Localities. Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
Referred specimens. TMM 31081-429 partial left dentary, -453 partial dentary with m2-m3, -469 partial maxillary with P2-M2, -689 partial left dentary with dp4, m1, m2; TMM 30896-531 partial right dentary with p4-m3.
Description. Four partial dentaries from two local faunas compare well with Procamelus, but are significantly larger than P. occidentalis. The molars are simple, relatively hypsodont and the premolar series includes at least p2-p4, all of which are double rooted. None of the specimens preserves the ramus anterior to p2. The size and shape of the molars compare well with the material described by Gregory (1939, 1942) from the Big Spring Canyon Fauna in South Dakota. The m1-m3 length is approximately 104 mm (TMM 31081-429) and the p2-m3 length is approximately 154 mm. These compare well to ranges provided by Gregory of 98.5-102.9 mm and 144-146 mm, respectively. The depth of the dentary below m1 is approximately 43 mm compared to a range of 37-49 in Gregory (1942).
Discussion. Patton (1969) recognized P. grandis from the Lapara Creek Fauna on the basis of two partial dentaries, one from Farish Ranch and one from Buckner Ranch. In addition, two partial dentaries and a partial maxilla are now identified from the Farish Ranch Local Fauna. P. grandis is significantly larger than P. occidentalis (m3 length 44-46 mm vs. 30-36 mm, respectively). Unlike P. occidentalis, which is known primarily from the Great Plains and Texas, P. grandis is known from Central America, Florida, Texas, the Great Plains and Oregon (Honey et al., 1998).
Subfamily PROTOLABINAE Zittel, 1893
Genus PROTOLABIS Cope, 1876
Protolabis coartatus (Stirton, 1929) (Figure 83)
Protolabis notiochorinus (Patton, 1969)
Localities. Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081).
Referred specimens. TMM 31132-319 metapodial, -332 partial mandible with p1-m3; TMM 31081-261, -293 metapodial.
 Description. TMM 31132-332 includes a partial mandible (Figure 83). The right dentary includes double-rooted alveolus for p1, double roots for p2-p3 and complete p4-m3. The left dentary includes roots for p2-p4, and m1-m3 (broken). The dentaries exhibit a strong angular flare and possess two mental foramina. The depth of the dentary decreases rapidly towards the symphysis. The depth below the anterior edge of m1 is 38.9 mm and below the posterior edge of m3 is 57.3 mm. The tooth measurements provided by Patton (1969) for this specimen are accurate. The right p2-m3 TRL is ~108 mm and the left is ~106 mm. The symphysis was broken and has been reconstructed so the number and morphology of incisors is unknown. The left canine is represented by a single root and the C-p2 diastema is ~25.6 mm. Patton (1969) stated that the anterolingual flexid of p4 was weakly expressed and that the posterior lobe of this tooth was narrower than the medial lobe, however, this tooth is very worn. Lower molars are very simple with no lamine buttresses and with weak ribs present only on the anterolingual corner of m3.
Description. TMM 31132-332 includes a partial mandible (Figure 83). The right dentary includes double-rooted alveolus for p1, double roots for p2-p3 and complete p4-m3. The left dentary includes roots for p2-p4, and m1-m3 (broken). The dentaries exhibit a strong angular flare and possess two mental foramina. The depth of the dentary decreases rapidly towards the symphysis. The depth below the anterior edge of m1 is 38.9 mm and below the posterior edge of m3 is 57.3 mm. The tooth measurements provided by Patton (1969) for this specimen are accurate. The right p2-m3 TRL is ~108 mm and the left is ~106 mm. The symphysis was broken and has been reconstructed so the number and morphology of incisors is unknown. The left canine is represented by a single root and the C-p2 diastema is ~25.6 mm. Patton (1969) stated that the anterolingual flexid of p4 was weakly expressed and that the posterior lobe of this tooth was narrower than the medial lobe, however, this tooth is very worn. Lower molars are very simple with no lamine buttresses and with weak ribs present only on the anterolingual corner of m3.
Discussion. On the basis of a single specimen (TMM 31132-332), Patton (1969) erected the species Protolabis notiochorinus primarily on the basis of size, reduction of the premolars (all but one of which was represented only by roots), the shape of the p4 (based on a very worn specimen) and the shape of the masseteric region of the mandible. Honey and Taylor (1978) included Protolabis notiochorinus as a junior synonym of P. coartatus, the type specimen (UCMP 19820) of which was described by Stirton (1929) from the early Clarendonian, Stewart Valley, Nevada and originally included in Procamelus. Honey et al. (1998) also recognized the synonymy.
Honey and Taylor (1978) amended the diagnosis of Protolabis to include: smaller size than Procamelus, p2-p4 shorter and more brachydont with p2 sometimes lost in most derived species; molars lower crowned, narrower and less anteroposteriorly expanded; weak to strong lateral flare and weak to strong mesial tuberosity or shelf on angle of mandible. In addition, they stated that the presence of a double-rooted p1 in Protolabis and a single-rooted p1 in Procamelus was useful in separating the two genera. Webb (1969) noted that the width of the posterior cusps of p3 and p4 were often greater than that of the middle cusps in Procamelus. In Protolabis, the medial cusp tends to be equal in width or wider than the posterior cusp. Although the molars of these two genera are similar, differences in the proportions of the molars have been noted. Frick and Taylor (1971) pointed out that the molars of Procamelus are more anteroposteriorly elongated and higher crowned than those of Protolabis. Specifically, at a similar wear stage m1 and m2 of Procamelus are longer relative to the length of m3 than those of Protolabis. Although both m3s are broken, the ratio of m1/m3 length is 0.41 and of m2/m3 length is 0.65. The same ratios for specimens of Procamelus occidentalis at Lapara Creek are 0.58 and 0.70, respectively, and for specimens of P. grandis are 0.62 and 0.70, respectively.
The angle of the type mandible of P. coartatus is broken. However, most workers consider outward flare of the angle to be a distinguishing character of this taxon (Honey et al, 1998). Specimens from the Clarendonian Milk Creek Formation in Arizona exhibit the most extreme development of the outward flare of the angle (Honey and Taylor, 1978). The Bridge Ranch specimen shows modest evidence of an angular flare and, although Patton (1969) considered this a distinctive feature of the Lapara Creek form, it is within the range of other examples of this taxon and less pronounced than in P. coartatus from Milk Creek.
Webb’s (1969) diagnosis of Protolabis included unfused metapodials, however, Honey and Taylor (1978) listed fused metapodials as a character of the genus. Patton (1969) referred five unfused metapodials to P. notiochorinus. Re-inspection of these metapodials indicates that TMM 31081-261, -293 and TMM 31132-319 are bowed and either represent a primitive character state for Protolabis from Lapara Creek or a different taxon. TMM 31081-525 and TMM 31081-612 are straight, unfused metapodials and belong to Synthetoceras.
Protolabis cf. yavapaiensis (Honey and Taylor, 1978) (Figure 84, Table 10)
Localities. TMM 31081 (Farish Ranch), TMM 30896 (Buckner Ranch).
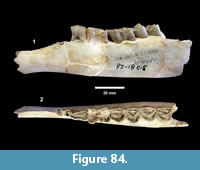 Referred Specimens. TMM 31081-28 partial left dentary with p3 (roots only), p4-m3 (broken); TMM 30896-296 partial left dentary with p3 (roots only), p4, m1, m2, m3 (early eruption).
Referred Specimens. TMM 31081-28 partial left dentary with p3 (roots only), p4-m3 (broken); TMM 30896-296 partial left dentary with p3 (roots only), p4, m1, m2, m3 (early eruption).
Description. TMM 31081-28 is a partial left dentary that clearly shows p2 is absent with a shallow ramus and a diastema that narrows to a depth of 18 mm and a width of 8.8 mm (Figure 84). The posterior margin of an alveolus for p1 is preserved at the broken anterior edge of the specimen. The p1-p3 diastema length is approximately 29-30 mm. The molars are simple and lack ribs and stylids with no lamine buttresses. TMM 30896-296 represents the same taxon, but from a younger individual with the premolars and last molar at an early stage of eruption. There is no evidence of a p2, although p3 and p4 are just erupting. The diastema is slender and shallow.
Discussion. TMM 31081-28 is a relatively small camel that lacks p2. Although the p4 is similar to Procamelus, the specimen compares most closely with Protolabis yavapaiensis (Honey and Taylor, 1978). It is smaller than Procamelus occidentalis with a slenderer diastema and lacks p2. Relative to Protolabis coartatus, the diastema is slenderer and p4 differs being widest at the posterior edge and narrowing anteriorly with a strong anterolingual flexid and a posterior enamel lake. Both p3 and p4 are double rooted. The diastema is also slenderer than in Nothotylopus camptognathus and m2-m3 lack anterolabial stylids. Measurements of the lower teeth (Table 10) compare well with those of P. yavapaiensis in Honey and Taylor (1978): p4 8.8-12.9 mm x 4.6-7.0 mm), m1 15.6-18.9 mm x 9.7-11.9 mm, m2 18.5-24.8 mm x 11.6-14.5 mm. The depth of the dentary at the diastema also compares well: 18 mm for TMM 31081-28 and 13.3-19.1 mm for P. yavapaiensis.
Honey and Taylor (1978) defined Michenia yavapaiensis from material collected in the Clarendonian Milk Creek Formation of Arizona. Material from the early Clarendonian Avawatz Formation in California (Henshaw, 1940) was considered by Honey and Taylor (1978) to compare well with M. yavapaiensis, but due to the limited number of specimens they refrained from conclusive identification. Lozinsky and Tedford (1991) reported M. sp. cf. yavapaiensis from the late Clarendonian-early Hemphillian Popotosa Formation in the Albuquerque Basin of New Mexico. In his review of protolabidines, Honey (2007) concluded that yavapaiensis belongs to Protolabis rather than Michenia on the basis of skull proportions and other differences relative to the genotypic species M. agatensis. P. yavapaiensis from the Lapara Creek Fauna is one of the earliest records of this taxon and extends the range eastward.
Family TAYASSUIDAE Palmer, 1897
Genus “PROSTHENNOPS” Gidley, 1904
“Prosthennops” xiphidonticus Barbour, 1925 (Figure 85, Figure 86, Figure 87, Figure 88, Table 11)
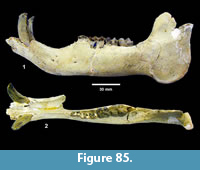 Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
Referred Specimens. TMM 30936-62 P4; TMM 31132-202 partial mandible with dp3-m2; TMM 31081-363 partial dentary with dp4-m1, -629 partial mandible with p4-m3, -673 partial mandible with right and left p2(alveoli), p3-m3, -1128 M1 or M2; TMM 30896-412 M1.
Description. Tayassuid specimens are known from each of the four local faunas in the Lapara Creek Fauna, and there is no reason, based on available material, to separate these into different taxa. TMM 31132-202 is a partial mandible with alveoli for three incisors on each side, a large canine that is sub-triangular in cross section, a long diastema with a concave dorsal surface profile and a trough-like symphysis (Figure 85, Figure 86). 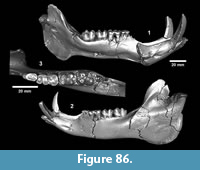 The jaw is from an immature animal with very worn dp3 and dp4 still present, a slightly worn m1, m2 unworn and m3 unerupted. Dimensions include dp3: 11.7 x 6.4 mm, dp4: 17.6 x 9.7 mm, m1: 14.5 x 10.2 mm, m2: 17.8 x 12.0 mm. Small roots, anterior to dp3, presumably represent dp2. The first and second molars are bunodont with a weak anterior cingulum. The m1 exhibits subequal protoconid and metaconid, a small central entoconulid, a relatively large hypoconid, a smaller entoconid and a small posterior hypoconulid. There are four small foramina on the labial side of the left ramus and three on the lingual side. The posterior angle is rounded with an elevated ridge around the outer edge. The right ramus is missing posterior to the diastema.
The jaw is from an immature animal with very worn dp3 and dp4 still present, a slightly worn m1, m2 unworn and m3 unerupted. Dimensions include dp3: 11.7 x 6.4 mm, dp4: 17.6 x 9.7 mm, m1: 14.5 x 10.2 mm, m2: 17.8 x 12.0 mm. Small roots, anterior to dp3, presumably represent dp2. The first and second molars are bunodont with a weak anterior cingulum. The m1 exhibits subequal protoconid and metaconid, a small central entoconulid, a relatively large hypoconid, a smaller entoconid and a small posterior hypoconulid. There are four small foramina on the labial side of the left ramus and three on the lingual side. The posterior angle is rounded with an elevated ridge around the outer edge. The right ramus is missing posterior to the diastema.
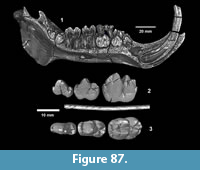 A CT scan of TMM 31132-202 clearly shows unerupted p2-p4 and m3 (Figure 87). The p3 has a single trigonid cusp (protoconid) and no distinct paraconid although two weak cingula extend anteriorly and posteriorly from the labial side of the cusp. The talonid is well developed although much lower than the trigonid and includes a hypoconid, a larger entoconid and a small hypoconulid. A well-developed anterior cingulum is also present. The unerupted p4 includes an anterior cingulum, subequal protoconid and metaconid, a clear paraconid (and equivalent cusp anterior to the metaconid), and a well-developed talonid with hypoconid, entoconid, hypoconulid and entoconulid.
A CT scan of TMM 31132-202 clearly shows unerupted p2-p4 and m3 (Figure 87). The p3 has a single trigonid cusp (protoconid) and no distinct paraconid although two weak cingula extend anteriorly and posteriorly from the labial side of the cusp. The talonid is well developed although much lower than the trigonid and includes a hypoconid, a larger entoconid and a small hypoconulid. A well-developed anterior cingulum is also present. The unerupted p4 includes an anterior cingulum, subequal protoconid and metaconid, a clear paraconid (and equivalent cusp anterior to the metaconid), and a well-developed talonid with hypoconid, entoconid, hypoconulid and entoconulid.
TMM 31081-673 is a partial mandible of a mature individual with very worn p3-m3 and double rooted alveoli for p2 on both sides (Figure 88). Although the symphysis region was repaired with plaster, this specimen illustrates the robust nature of the horizontal rami under the tooth row. The teeth are too worn to provide useful description. The p4 is molariform and the third molar exhibits a posterior hypoconulid complex.
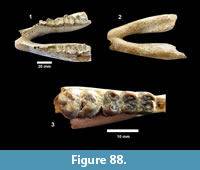 TMM 31081-363 is a partial right dentary of an immature individual with roots for dp2-dp3, a moderately worn dp4 and an unworn m1. While the dp4 has three sets of laterally opposed conids, the m1 exhibits subequal protoconid and metaconid, a well-developed central entoconulid with an additional accessory conulid, a hypoconid slightly larger than the entoconid and a well-developed posterior hypoconulid with a posterior cingulum (Figure 88).
TMM 31081-363 is a partial right dentary of an immature individual with roots for dp2-dp3, a moderately worn dp4 and an unworn m1. While the dp4 has three sets of laterally opposed conids, the m1 exhibits subequal protoconid and metaconid, a well-developed central entoconulid with an additional accessory conulid, a hypoconid slightly larger than the entoconid and a well-developed posterior hypoconulid with a posterior cingulum (Figure 88).
Discussion. Wright (1998) provided the most recent overview of the Miocene Tayassuidae and concluded that taxa previously included in Prosthennops were actually distributed across multiple monophyletic clades. He recognized a series of monophyletic groups belonging to Tayassuinae including “Prosthennops” xiphidonticus, the “Prosthennops” niobrarensis - Tayassu clade and the Macrogenis-Tayassu clade. Wright (1998) separated “Prosthennops” niobrarensis and “Prosthennops” xiphidonticus from Prosthennops serus which he retained as the only valid species of Prosthennops sensu stricto, however, no new generic names have yet been proposed. Wright (1991) concluded that none of the 16 species of Prosthennops named after 1904 were valid and that Prosthennops sensu stricto was a monotypic genus that included P. serus (Cope 1878) (Gidley, 1904), Dicotyles serus (Cope, 1878), P. serus (Gidley, 1904), P. serus (Hesse, 1935), P. serus (Colbert, 1938) and P. (Macrogenis) graffhami (Schultz and Martin, 1975). Diagnostic characters of the lower cheek teeth of P. serus include: p3 with two anteroposteriorly elongated trigonid cusps and no paraconid, a low talonid that includes both hypoconid and entoconid and a posterior cingulum (Wright, 1991). As used by Wright (1998), P. serus is restricted to Hemphillian faunas.
According to Wright (1991) the p3 of “Prosthennops” niobrarensis possess a metaconid differentiating this taxon from “P.” xiphidonticus. Macrogenis has a further developed trigonid on p3 with distinct cusps (protoconid and metaconid). Wright (1998) suggests that the Macrogenis-Tayassu clade can further be distinguished by transversely broad cheek teeth with p3 lacking a paraconid and having a large talonid. Following the taxonomy of Wright (1991), “P.” xiphidonticus included P. xiphidonticus (Barbour, 1925), Prosthennops (Stirton and McGrew, 1935), “Cynorca” proterva (Gazin and Collins, 1950), Prosthennops. sp. (Voorhies, 1969), “Cynorca” proterva (Woodburne, 1969), Dyseohyus stirtoni (Woodburne, 1969), P. xiphidonticus (Webb, 1969) and Dyseohyus xiphidonticus (Corner, 1975).
Macrogenis and “P.” niobrarensis are the only two taxa of Tayassuinae currently recognized from the early Clarendonian (Wright, 1998). The Lapara Creek peccary lacks a metaconid on p3 and therefore differs from these taxa. The lower molar morphology is most similar to “P.” xiphidonticus, a taxon previously reported from Barstovian faunas of the Texas Gulf Coast, the central plains, and Maryland. “P.” xiphidonticus, from the Fleming Formation in San Jacinto Co., Texas (Cold Spring Fauna) was included in the hypodigm of Dyseohyus stirtoni by Woodburne (1969). Compared to the average length of m2 reported in Wright (1998) (12.6-14.6 mm), and measurements in Woodburne (1998), “P.” xiphidonticus from the Lapara Creek Fauna is slightly larger than the Barstovian forms (Table 11).
Order PERISSODACTYLA Owen, 1848
Family RHINOCEROTIDAE Owen, 1845
Tribe TELEOCERATINI Hay, 1902
Genus TELEOCERAS Hatcher, 1894
Teleoceras major (Hatcher, 1894)
(Figure 89, Figure 90, Figure 91, Table 12, Table 13, Table 14)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081) and Buckner Ranch (TMM 30896).
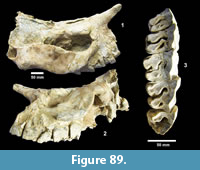 Referred specimens. TMM 30936-16 p4, m3, -27 p4, m3, -2 P2 (2), -55 P2, -85 tibia, -233 m2, -321 p3; TMM 31132-193 atlas, -305 metapodials (2); TMM 31081 skulls: (including partial skulls/maxilla): 29, -150, -294, -347, -534, -576, -639, -744, -748, -979, -980, -983, -993, -1151, -1152, -1312. TMM 31081 mandibles: 108, -146, -435, -462, -523, -571, -668, -834, -845, -1153, -1156, -1394. TMM 31081 isolated teeth: 4 M1, -5 p/m, -38 P4, M2?, -39 p/m, -104 P2, -198 P3, -244 P3, -254 M1, -263 P2, -285 P3, -294 dP3?, -369 i2, -370 m3, -393 M3, -395 p3, -454 i2 female, -523 i2 female, -528 i2 female, -546 P3?, -567 i2 male, -575 P4, -602 M3, -603 P4, -689 p/m, -720 i2, -728 M2, -811 p/m, -832 P3, -833 M3, -836 i2 male, -859 i2 female, -897 dP2, -910 p/m, -1092 M2, -1094 i2, -1164 M2, -1168 dP1, -1213 i2?, -1328 i2, -1389 i2 female, -1396 P4?, -1437 P3?, M3, -1460 M1?, -1475 P2, -1487 P3, P4, -1491 p3, m1, m2, m3, -1492 i2 female. TMM 31081 femurs: 312, -399, -402, -434, -568, -574, -978, -1079, -1528. TMM 31081 tibias: 8, -349, -529, -535, -538, -736. TMM 31081 humeri: 11, -410, -415, -531, -569, -573, -577, -774, -777. TMM 31081 radii: 25, -483, -504, -526, -667. TMM 31081 ulna: 401, -412, -489, -539, -686; TMM 30896-124 humerus, -152 right dentary with p3, p4, m1, and m2 unerupted, -337 p3, -654 atlas.
Referred specimens. TMM 30936-16 p4, m3, -27 p4, m3, -2 P2 (2), -55 P2, -85 tibia, -233 m2, -321 p3; TMM 31132-193 atlas, -305 metapodials (2); TMM 31081 skulls: (including partial skulls/maxilla): 29, -150, -294, -347, -534, -576, -639, -744, -748, -979, -980, -983, -993, -1151, -1152, -1312. TMM 31081 mandibles: 108, -146, -435, -462, -523, -571, -668, -834, -845, -1153, -1156, -1394. TMM 31081 isolated teeth: 4 M1, -5 p/m, -38 P4, M2?, -39 p/m, -104 P2, -198 P3, -244 P3, -254 M1, -263 P2, -285 P3, -294 dP3?, -369 i2, -370 m3, -393 M3, -395 p3, -454 i2 female, -523 i2 female, -528 i2 female, -546 P3?, -567 i2 male, -575 P4, -602 M3, -603 P4, -689 p/m, -720 i2, -728 M2, -811 p/m, -832 P3, -833 M3, -836 i2 male, -859 i2 female, -897 dP2, -910 p/m, -1092 M2, -1094 i2, -1164 M2, -1168 dP1, -1213 i2?, -1328 i2, -1389 i2 female, -1396 P4?, -1437 P3?, M3, -1460 M1?, -1475 P2, -1487 P3, P4, -1491 p3, m1, m2, m3, -1492 i2 female. TMM 31081 femurs: 312, -399, -402, -434, -568, -574, -978, -1079, -1528. TMM 31081 tibias: 8, -349, -529, -535, -538, -736. TMM 31081 humeri: 11, -410, -415, -531, -569, -573, -577, -774, -777. TMM 31081 radii: 25, -483, -504, -526, -667. TMM 31081 ulna: 401, -412, -489, -539, -686; TMM 30896-124 humerus, -152 right dentary with p3, p4, m1, and m2 unerupted, -337 p3, -654 atlas.
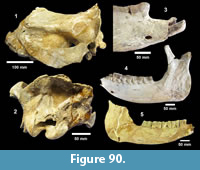 Description. A large sample of rhino specimens was collected at the Farish Ranch locality (TMM 31081) including 16 skulls or partial skulls, 12 mandibles or partial mandibles, numerous isolated teeth and abundant post-cranial material (Figure 89, Figure 90. Figure 91, Table 12, Table 13, Table 14). This collection represents a relatively small, short limbed rhino, with a brachycephalic skull. The molariform teeth are relatively hypsodont with strong antecrochets (M1-M2) (Figure 89.3), reduced premolars, narrow nasals, relatively broad zygomatic arches, relatively broad lambdoid crest and teardrop-shaped i2 morphology (cross-section).
Description. A large sample of rhino specimens was collected at the Farish Ranch locality (TMM 31081) including 16 skulls or partial skulls, 12 mandibles or partial mandibles, numerous isolated teeth and abundant post-cranial material (Figure 89, Figure 90. Figure 91, Table 12, Table 13, Table 14). This collection represents a relatively small, short limbed rhino, with a brachycephalic skull. The molariform teeth are relatively hypsodont with strong antecrochets (M1-M2) (Figure 89.3), reduced premolars, narrow nasals, relatively broad zygomatic arches, relatively broad lambdoid crest and teardrop-shaped i2 morphology (cross-section).
Specimens of this rhino are present, but very rare, at the other three Lapara Creek Fauna localities as well. The Ten Mile Waterhole Creek Local Fauna includes a tibia and five isolated cheek teeth that fall within the range of the Farish Ranch material. The Bridge Ranch Local Fauna includes an atlas vertebra, two metapodials and a series of isolated teeth. The two metapodials (TMM 31132-305) are shorter than any of the equivalent elements from Farish Ranch. The Buckner Ranch collection includes two post-cranial elements, an isolated tooth and a right dentary (TMM 30896-152) with p3, p4 and m1 (m2 unerupted). Most of these specimens fall within the range of measurements and morphology observed in the Farish Ranch collection.
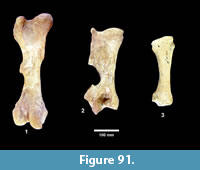 Discussion. The abundant rhino material from the Farish Ranch locality can be confidently assigned to Teleoceras based on size and body proportions, relatively hypsodont teeth, strong antecrochets, reduced premolars, narrow nasals, broad zygomatic arches, broad lambdoid crest and i2 morphology.
Discussion. The abundant rhino material from the Farish Ranch locality can be confidently assigned to Teleoceras based on size and body proportions, relatively hypsodont teeth, strong antecrochets, reduced premolars, narrow nasals, broad zygomatic arches, broad lambdoid crest and i2 morphology.
The following comparisons rely on measurements published in Mead (2000) and Prothero (2005). P2-M3 length of the Farish Ranch rhino is indistinguishable from T. major and T. brachyrhinum. The mean TRL is longer than T. americanum and T. medicornutum and shorter than T. fossiger and T. proterum. The M1- M3 length is indistinguishable from T. major, T. brachyrhinum, T. medicornutum and T. proterum. The mean length is larger than T. americanum and T. meridianum and smaller than T. fossiger. The mean length of p3-m3 falls between the female and male subsets of T. major, however, at one standard deviation, p3-m3 length is indistinguishable from T. major, T. americanum, T. proterum, and T. medicornutum, T. fossiger and T. brachyrhinum. The mean femur length is indistinguishable from T. major, T. proterum, T. meridianum, T. brachyrhinum. The femur is shorter than the male subset of T. major, T. medicornutum and T. fossiger. The femur is longer than that of T. americanum. The mean humerus length is indistinguishable from T. proterum, T. major, T. fossiger, T. brachyrhinum and T. medicornutum, but larger than T. meridianum. The mean ulna length of the Farish Ranch rhino is longer than all other taxa for which measurements were available, but at one standard deviation overlaps with T. medicornutum.
Although M1-M3 length is slightly larger than T. medicornutum, the rhino from the Lapara Creek Fauna is smaller than T. medicornutum for almost all other cranial and post-cranial measurements. In the Farish Ranch material, cristae are present on a few M1s (e.g., TMM 31081-1312, TMM 31081-980). The Farish Ranch rhino is generally smaller than T. fossiger, has a less-derived dentition than T. proterum and is generally larger than T. meridianum and T. americanum. The skull of the Farish Ranch rhino is less brachycephalic and the nasals are narrower than in T. brachyrhinum. The Farish Ranch rhino also lacks p2, which is usually present in T. brachyrhinum (Prothero, 2005).
The rhino from Farish Ranch compares closely with Teleoceras major, especially the subset of female T. major specimens described by Mead (2000). Prothero and Manning (1987) referred specimens from the Lapara Creek Fauna to Teleoceras cf. major and Prothero (2005) included specimens from this fauna in his hypodigm of T. major. Although Prothero (2005) noted “dozens of TMM specimens” in the Bridge Ranch Local Fauna (“Berclair l.f.”), he only listed TMM 31081-744. However, he referenced a skull from the Bridge Ranch Local Fauna (“Normana l.f.”) as 31132-29 (Prothero, 2005, pg. 109). The specimen with this number is a calcaneum, but TMM 31081-29 is a rhino skull from the Farish Ranch Local Fauna. The only rhino skulls known from the Lapara Creek Fauna are from the Farish Ranch locality.
Quinn (1955) included T. proterus (now T. proterum) in his faunal list from the Lapara Creek Fauna, but did not include any description of the material and only a brief discussion. Although similar in some attributes (e.g., femur length), the Farish Ranch rhino differs from T. proterum by having a much shorter P2-M3 length, shorter occipital height, much longer tibia, and broader crochets and antecrochets.
Based on the above comparisons, the Farish Ranch rhino is assigned to T. major and compares very well with the female subset of T. major from Nebraska described by Mead (2000). The fact that the range of variation in the Farish Ranch sample is much smaller than that of the combined (female + male) population of T. major from Nebraska (Mead, 2000) may suggest that sexual dimorphism was less well developed. Although there is a suggestion of slight bimodal distribution in some characters (M1-M3 length, depth of dentary below m1), the only strong evidence for dimorphic morphology is in the geometries of the tusks (i2).
The concentration of rhino material at the Farish Ranch quarry is anomalous. Over 90% of the rhino specimens from the Lapara Creek Fauna were collected in this quarry and, although no articulated skeletons were found, both cranial and postcranial material was abundant. Skulls or partial skulls represent a range of maturity evenly divided between early mature (slight wear on M3), mature (moderate wear on M3) and very mature (significant wear on M3). Unfortunately, there are neither photographs nor maps of the quarry with which to evaluate specimen associations.
Teleoceras ranges from Hemingfordian to latest Hemphillian in North America (Prothero, 2005) and possibly into the early Blancan (Gustafson, 2012). T. major is known from numerous Clarendonian localities from the Great Plains through Texas and Nevada.
Family EQUIDAE Gray, 1821
Horses from the Lapara Creek Fauna have been discussed at length beginning with Quinn (1955). They are the most common group in the fauna based on number of specimens and are very diverse in terms of number of taxa. Early mentions of the fossil equids from the Goliad Formation can be found in Sellards (1940b), Hesse (1942), and Quinn (1952). However, Quinn (1955) was the first to provide a systematic overview in the context of Arikareean through Clarendonian faunas from the Texas coastal plain. He included 17 taxa in his faunal list of equids from the Lapara Creek Fauna with two new genera and eight new species. Although many of the new taxa proposed by Quinn (1955) have been subsequently synonymized, his conceptual model for the evolution of horses in the Miocene was an important departure from previous schemes. Twenty years later, Forsten (1975) published an alternative assessment of the Gulf Coast Miocene equids with only eight taxa in part because of a stated opinion that Quinn (1955) had not appropriately considered normal variability within taxonomic units. Indeed, Quinn’s approach to taxonomy was very focused on type specimens and lists of referred specimens were far from exhaustive. Forsten (1975) stated that Quinn’s “extreme vertical phylogeny” was “hard to follow in taxonomic practice”, so her conceptual model for equid evolution more closely followed that of Stirton (1940).
A series of publications, beginning in the mid-1980s and continuing into the 1990s, significantly clarified the identification and relationships of Miocene equids from the Gulf Coast. MacFadden (1984), Webb and Hulbert (1986), Hulbert (1987a, 1987b, 1988a, 1988b, 1989), Hulbert and MacFadden (1991) and Hulbert (1993) provided revised interpretations of equid phylogeny including analysis of specimens from the Lapara Creek Fauna. The following discussion of fossil horses from the Lapara Creek Fauna is based largely on these works, although careful attention has been paid to taxonomic composition within each of the local faunas and to material that had not been previously included. In addition, lists of referred specimens have been updated to incorporate previous re-numbering of numerous specimens. Twelve species representing nine different genera are now recognized in the equid fauna of Lapara Creek.
Family EQUIDAE Gray, 1821
Subfamily EQUINAE Steinmann and Döderlein, 1890
Tribe HIPPARIONINI Quinn, 1955
Genus PSEUDIHIPPARION Ameghino, 1904
Pseudhipparion curtivallum (Quinn, 1955)
(Figure 92, Table 15)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
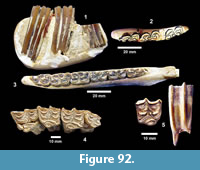 Referred specimens. TMM 30936-74 M, -134 partial dentary with p2-3, -141 pm, -146 M, -258 M, -363 M, -421 partial dentary with m1-2; 422 p2, -423-426 pm, -427-430 m, -431 m3. (Note: Webb and Hulbert (1986) included TMM 30896-80 and TMM 30896-351 as “upper premolars” of P. curtivallum, but these specimens are post-cranial elements of a camel. I suspect some confusion with TMM 30936-80 and TMM 30936-353 that were the upper premolars referred by Quinn (1955), but explicitly excluded from P. curtivallum by Webb and Hulbert (1986)). TMM 31132-20 m, -106 PM, -119 m, -126 PM, -133 PM, -168 m1-2, -236 LP4-M3, -250 p2-m3, -265 PM, -336 m, -368 PM, -375 PM, -377 m, -405 pm, -438 PM, -500 M3, -538 PM, -617-622 PM; -623-627 M, -628-631 M3, -632-634 m, -635-636 m3; TMM 31170-36 PM, -38 P2, -69 P2, -130 P2, -131 P2, -132-136 PM, -137-138 M; TMM 31081-371 PM, -479 partial dentary with p2-m3, -597 PM, -1138 m (mislabeled in Webb and Hulbert (1986) as locality 31018), -1017 RP2-3, RM1-3, LP3, LM1-2, -1139 M, -1161 PM, -1182 LP3-M1, -1438 M, -1501 M; TMM 30896-177 R P3-M3, -581 p4-m3.
Referred specimens. TMM 30936-74 M, -134 partial dentary with p2-3, -141 pm, -146 M, -258 M, -363 M, -421 partial dentary with m1-2; 422 p2, -423-426 pm, -427-430 m, -431 m3. (Note: Webb and Hulbert (1986) included TMM 30896-80 and TMM 30896-351 as “upper premolars” of P. curtivallum, but these specimens are post-cranial elements of a camel. I suspect some confusion with TMM 30936-80 and TMM 30936-353 that were the upper premolars referred by Quinn (1955), but explicitly excluded from P. curtivallum by Webb and Hulbert (1986)). TMM 31132-20 m, -106 PM, -119 m, -126 PM, -133 PM, -168 m1-2, -236 LP4-M3, -250 p2-m3, -265 PM, -336 m, -368 PM, -375 PM, -377 m, -405 pm, -438 PM, -500 M3, -538 PM, -617-622 PM; -623-627 M, -628-631 M3, -632-634 m, -635-636 m3; TMM 31170-36 PM, -38 P2, -69 P2, -130 P2, -131 P2, -132-136 PM, -137-138 M; TMM 31081-371 PM, -479 partial dentary with p2-m3, -597 PM, -1138 m (mislabeled in Webb and Hulbert (1986) as locality 31018), -1017 RP2-3, RM1-3, LP3, LM1-2, -1139 M, -1161 PM, -1182 LP3-M1, -1438 M, -1501 M; TMM 30896-177 R P3-M3, -581 p4-m3.
Description. Webb and Hulbert (1986) provided a description of Pseudhipparion curtivallum with a revised diagnosis based largely on the material from the Lapara Creek Fauna. Because no cranial material has been recognized for P. curtivallum, the diagnosis relies on dental morphology and size. The type specimen (TMM 30896-196) includes three teeth (p4-m2) embedded in plaster, with some encasing mandibular bone. Figure 92.1 illustrates the lingual side of the specimen with some of the plaster and mandibular bone removed. Much of the cementum was prepared off of the teeth above the original “plaster line” and the anterior-most tooth was previously sectioned with a saw. The anterior-most tooth has been previously interpreted as p4 although the restored relationship with m1 is somewhat questionable. The occlusal surface of p4 is unworn reflecting an immature animal. The type specimen illustrates the long, but rounded metaconid-metastylid, deep metaflexid, relatively shallow linguaflexid and a clear protostylid that Webb and Hulbert (1986) noted in their revised diagnosis. Webb and Hulbert (1986) also figured TMM 31132-250 from the Bridge Ranch Local Fauna as P. curtivallum. This specimen is much more complete than the holotype and represents a more mature individual (Figure 92.3). It displays morphological differences relative to the type that presumably reflect age differences and natural variability within this taxon. The most notable differences include the depth of the ectoflexid especially on m1 and m2, the roundness of the metaconid-metastylid, and the geometry of the entoconid-hypoconulid.
Webb and Hulbert (1986) provided extensive measurements for P. curtivallum from the Lapara Creek Fauna and showed that this taxon is a relatively small species of Pseudhipparion, with a mean TRL of 95 mm. TMM 31132-236 (Figure 92.4) illustrates a number of the characters of the upper dentition, including an isolated protocone, a hypoconal groove that closes to a fossette and moderately complex plications on the fossette borders. The upper cheek teeth are not strongly curved (Figure 92.5).
Discussion. As with most of the horses from the Lapara Creek Fauna, the taxonomic history of Pseudhipparion curtivallum is complicated. Quinn (1955) proposed the name Astrohippus curtivallis as a new species and designated TMM 30896-196 as the type specimen with two upper premolars figured as referred specimens. Webb (1969) considered A. curtivallis to be a junior synonym of Calippus anatinus. He recognized Pseudhipparion retrusum and P. gratum from the Burge and Minnechaduza Faunas and included Griphippus retrusus and G. gratus as junior synonyms. Forsten (1975) proposed that A. curtivallis belonged to Pseudhipparion. Webb and Hulbert (1986) reviewed the record of Pseudhipparion in North America and recognized P. curtivallum, in which they included Quinn’s (1955) type specimen but not the referred upper premolars. Furthermore, they concluded that Griphippus (Quinn, 1955) was a junior synonym of Pseudhipparion and included all the Griphippus material from the Lapara Creek Fauna as P. curtivallum. According to Webb and Hulbert (1986) the P. curtivallum specimens from the Lapara Creek Fauna are indistinguishable from material collected from the Agricola Fauna, Florida. MacFadden (1998) included P. curtivallum as a valid species of Pseudhipparion and listed the Lapara Creek and Agricola Faunas as the only known occurrences.
Genus NANNIPPUS Matthew, 1926
Nannippus sp. (Figure 93-Figure 94, Table 16)
Localities. Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
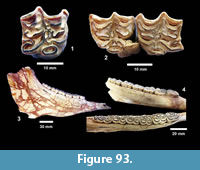 Referred specimens. Modified from Hulbert (1987a): TMM 31132-306 juvenile dentary, -307 M, -402 P3/P4, -742 M1/M2, -653 M3, -730 M, -731 M, -735 dentaries, -738 M1/M2, -742 M, -747 M1/M2, -748 M; TMM 31081-16 partial mandible, -27 dentary, -168 p, -176 dentary, -303 dentary, -319 P, -566 dentary, -630 associated R P2, M1-M2, -658 M, -659 M, -660 associated R P2-P3, -675 dentary, -735 dentary, -825 P3/P4 (2), -953 p, -956 p (3), -1159 associated L M1-M2, -1411 M, -1413 M, -1421A M (5), -1424 m(2), -1506 dp2, -1511 dentary; TMM 30896-400 M1/M2, -450A-B associated L P3, M1, -455 m.
Referred specimens. Modified from Hulbert (1987a): TMM 31132-306 juvenile dentary, -307 M, -402 P3/P4, -742 M1/M2, -653 M3, -730 M, -731 M, -735 dentaries, -738 M1/M2, -742 M, -747 M1/M2, -748 M; TMM 31081-16 partial mandible, -27 dentary, -168 p, -176 dentary, -303 dentary, -319 P, -566 dentary, -630 associated R P2, M1-M2, -658 M, -659 M, -660 associated R P2-P3, -675 dentary, -735 dentary, -825 P3/P4 (2), -953 p, -956 p (3), -1159 associated L M1-M2, -1411 M, -1413 M, -1421A M (5), -1424 m(2), -1506 dp2, -1511 dentary; TMM 30896-400 M1/M2, -450A-B associated L P3, M1, -455 m.
Description. A new species of Nannippus from the Lapara Creek Fauna was recognized by Hulbert (1987a) but has not been formally named and described. Hulbert (1993) referred to this taxon as Nannippus n. sp. and compared it to other species in the genus. MacFadden (1984) provided a revised diagnosis for Nannippus that included: small, hypsodont and gracile hipparion, mean TRL ranges from 82.25 to 115.90 mm, cheek teeth hypsodont to very hypsodont; upper cheek teeth with oval protocones and moderately complex enamel plications on fossette borders; lower cheek teeth with relatively deep ectoflexids, pli caballinids rudimentary or absent, widely separated metaconids and metastylids and protostylids poorly developed or absent.
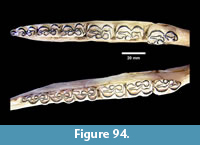 Characteristics of upper cheek teeth in the Lapara Creek sample include relatively small size, moderately hypsodont, double pli-caballins, oval to slightly flattened protocones, moderately complex plications on the fossette borders, well developed parastyle and deep hypoconal groove (Figure 93). Lower cheek teeth exhibit well developed, rounded metastylids and metaconids, shallow ectoflexid in the premolars, but deep in the molars (Figure 94). Pli caballinids are variably present, relatively small on premolars and generally absent on molars. Unlike MacFadden’s (1984) generic diagnosis, the Lapara Creek specimens have well developed protostylids on p3-m2. TRL for the cheek teeth measured in seven partial mandibles ranges from 106-114 mm. Additional measurements are provided in Table 16. No cranial material for this taxon is present in the Vertebrate Paleontology Collections.
Characteristics of upper cheek teeth in the Lapara Creek sample include relatively small size, moderately hypsodont, double pli-caballins, oval to slightly flattened protocones, moderately complex plications on the fossette borders, well developed parastyle and deep hypoconal groove (Figure 93). Lower cheek teeth exhibit well developed, rounded metastylids and metaconids, shallow ectoflexid in the premolars, but deep in the molars (Figure 94). Pli caballinids are variably present, relatively small on premolars and generally absent on molars. Unlike MacFadden’s (1984) generic diagnosis, the Lapara Creek specimens have well developed protostylids on p3-m2. TRL for the cheek teeth measured in seven partial mandibles ranges from 106-114 mm. Additional measurements are provided in Table 16. No cranial material for this taxon is present in the Vertebrate Paleontology Collections.
Discussion. R. Hulbert (pers. comm., 2014) plans to formally describe this taxon. It not only represents a new species of Nannippus but significantly extends the temporal range of this genus into the early Clarendonian. The taxon is not common in the Lapara Creek Fauna, although it is more common in Florida sites including Phosphoria Mine (Hulbert, 1987). Hulbert (1993) stated, by comparison, that “Nannippus n. sp.” was slightly larger than N. westoni and similar to N. lenticularis and N. beckensis, but much larger than N. aztecus and N. morgani. Furthermore, N. sp. has a more complex fossette pattern than N. westoni, with a larger, commonly double, pli caballin and a deeper hypoconal groove.
Although Forsten (1975) referred all of the medium-sized hipparionin horses from the Lapara Creek Fauna to Nannippus ingenuum, the work of Webb and Hulbert (1986) and Hulbert (1987a, 1988a) suggests at least four distinct taxa including Hipparion, Cormohipparion, Neohipparion and Nannippus. Nannippus sp. is most common in the Farish and Bridge Ranch Local Faunas, rare in the Buckner Ranch Local Fauna and apparently absent in the Ten Mile Waterhole Creek Local Fauna.
Genus HIPPARION de Cristol, 1832
Hipparion tehonense (Stirton, 1940)
(Figure 95, Table 17)
Localities. Bridge Ranch TMM 31170, TMM 31132, Farish Ranch TMM 31081, Buckner Ranch TMM 30896.
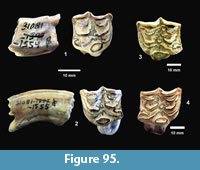 Referred specimens. Modified from Hulbert (1987a). TMM 31170-90 M1/M2, -188 M1/M2, -189 P3/P4, -190 P3/P4, -191 P3/P4, -192 m1/m2, -193 M1/M2, -194 M3, -195 M3, -196 M1/M2, 198 M1/M2, -288 P3/P4, -920 M1/M2; TMM 31132-22 M1, -731 M; TMM 31081-50 M, -162 R maxilla with DP3-M1, -397 p2, -627 M3, -705 P2, -822 M, -823 M, -952 M, -1033 M, -1072 p2, -1117 m, -1222 M3, -1240 M, -1440 P2, -1482 P2, -1463 M; TMM 30896-537 M3, -629 P4, -630 M1/M2, -631 M1/M2.
Referred specimens. Modified from Hulbert (1987a). TMM 31170-90 M1/M2, -188 M1/M2, -189 P3/P4, -190 P3/P4, -191 P3/P4, -192 m1/m2, -193 M1/M2, -194 M3, -195 M3, -196 M1/M2, 198 M1/M2, -288 P3/P4, -920 M1/M2; TMM 31132-22 M1, -731 M; TMM 31081-50 M, -162 R maxilla with DP3-M1, -397 p2, -627 M3, -705 P2, -822 M, -823 M, -952 M, -1033 M, -1072 p2, -1117 m, -1222 M3, -1240 M, -1440 P2, -1482 P2, -1463 M; TMM 30896-537 M3, -629 P4, -630 M1/M2, -631 M1/M2.
Description. A medium-size hipparionin horse (Figure 95, Table 17). Upper cheek teeth with oval protocones, sometimes with an anterior spur. Protocone connects to the protoloph in late wear stages, hypoconal groove is well developed and persistent with wear. Moderately complex plications on the fossette borders. Pli caballins are well developed but simple. Lower cheek teeth show deepening of ectoflexids toward the molars; metaconids and metastylids are widely separated. No cranial material is known from the Lapara Creek Fauna.
Discussion. MacFadden (1984) and Hulbert (1987a) provided the current diagnosis for this taxon. Quinn (1955) included Nannippus tehonensis in his faunal list from Lapara Creek, but provided no description. Forsten (1975) identified this medium-size hipparionin horse as Nannippus cf. ingenuum, but noted similarities with Nannippus tehonensis. MacFadden (1980, 1984) referred the population of medium-size hipparionin horses from the Clarendon Fauna to H. tehonense and stated that a portion of the Lapara Creek sample was also referable to that taxon. MacFadden (1998) included the Lapara Creek Fauna as a valid occurrence of H. tehonense. H. tehonense is best known from Clarendonian faunas in California (Whistler and Burbank, 1992; Kelly and Stewart, 2008) and Texas (MacFadden, 1980, 1984) but has also been reported from faunas in the Great Plains and Florida (Hulbert, 1988a).
Genus CORMOHIPPARION Skinner and MacFadden, 1977
Cormohipparion ingenuum (Leidy, 1885)
(Figure 96)
Localities. Farish Ranch (TMM 31081).
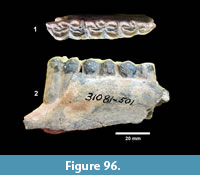 Referred specimens. TMM 31081-501 partial dentary with p2-p4.
Referred specimens. TMM 31081-501 partial dentary with p2-p4.
Description. Two specimens were recognized as Cormohipparion ingenuum by Hulbert (1988b). TMM 31081-501 is a partial mandible with p2-p4 from the Farish Ranch Local Fauna. As described by Hulbert (1988b), this specimen lacks the pli-caballinid seen in C. occidentale and exhibits simpler fossette patterns.
The other specimen, TMM 31204-1 includes isolated, but associated upper premolars and molars from a locality labeled John Dye Farm Site. This site is on Manahuilla Creek north of Goliad and was collected by the Survey in 1941. TMM 31204-1 is the only specimen known from this locality so the age is uncertain. The locality does plot within the mapped boundaries of the Goliad Formation.
Discussion. The taxonomic history, diagnosis and description of C. ingenuum was presented by Hulbert (1988b). In that paper, he reviewed the previous work of Quinn (1955) and Forsten (1975), regarding the suite of medium-size hipparionin horses from the Lapara Creek Fauna and concluded that a single specimen from the Farish Ranch Local Fauna was assignable to C. ingenuum. No new material has been added to the collection and my review of the Lapara Creek horses did not reveal any additional specimens that could be assigned with confidence. C. ingenuum is known from early Clarendonian through early Hemphillian faunas of central Florida and early Hemphillian of Honduras (Hulbert, 1988a).
Cormohipparion occidentale Leidy, 1856
(Figure 97, Figure 98, Figure 99, Table 18)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
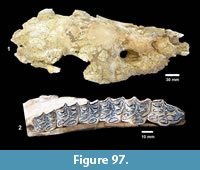 Referred specimens. Forsten (1975) referred numerous specimens from the Lapara Creek Fauna to Neohipparion occidentale. MacFadden (1984) recognized this horse as Cormohipparion occidentale and noted its occurrence from “numerous localities” associated with the Lapara Creek Fauna and published a figure of TMM 31081-619 (P2-M1). Hulbert (1987a, 1988a) agreed with this identification and cited the list of referred specimens from Forsten (1975) with a few exceptions that he recognized as Neohipparion affine. The following list differs from Forsten (1975) in that some specimens have been re-numbered since 1975, some specimens in her list appear to have been typographical errors (as they refer to non-equine material), and some of the specimens are either missing or indeterminate. I have not included any post-cranial elements from Forsten (1975).
Referred specimens. Forsten (1975) referred numerous specimens from the Lapara Creek Fauna to Neohipparion occidentale. MacFadden (1984) recognized this horse as Cormohipparion occidentale and noted its occurrence from “numerous localities” associated with the Lapara Creek Fauna and published a figure of TMM 31081-619 (P2-M1). Hulbert (1987a, 1988a) agreed with this identification and cited the list of referred specimens from Forsten (1975) with a few exceptions that he recognized as Neohipparion affine. The following list differs from Forsten (1975) in that some specimens have been re-numbered since 1975, some specimens in her list appear to have been typographical errors (as they refer to non-equine material), and some of the specimens are either missing or indeterminate. I have not included any post-cranial elements from Forsten (1975).
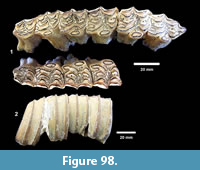 TMM 30936-66 P, -133 p/m (multiple teeth), -190 p, -210 M3, -269 P, -324 m, -326 M; TMM 31132-3 P/M, -70 L ramus with p2-p4, -82 M, -114 p, -131 M, -91 m (multiple teeth), -189 P, -223 M, -275 M (two teeth), -287 m (multiple teeth), -338 m (multiple teeth), -383 P, -441 p (two teeth), -467 m3, -468 M, -551 M, -670 P4, -673 M, -695 M3, -697 M1, -699 M, -759 m3; TMM 31170-17 p/m (three teeth), -41 L p2-m3, -49 juvenile ramus with dp2-dp4, -55 p/m (2p, 2m, one sectioned), -72 p/m (four teeth), -91 p/m (three teeth including m1-m2), -154 dentary with p2-m1, -202 M3; TMM 31081-7 juvenile dentary with dp2-m2, -217 ramus with p2-m3, -329 M1, -272 DP/P/M (5 teeth, DP4-M3), -390 M1, -456 juvenile dentary with dp2-m2, -516 ramus with p2-m3, -520 P4-M3 (in plaster), -527 dentary with p2-m3, -559 M, -560 dentary with p2-p3, -595 P, -619 P2-M1, -637 dentary with p2-m3, -638 juvenile dentary with dp2-m1, -780 dentary with p2-m3, -785 M2, -844 skull with R and L P2-M3, -858 P2, -865 P2, -1031 m3 (two teeth), -1071 p4, -1076 R P2-M3, LP4-M3 (likely one individual), -1089 p2-m3 (six teeth), -1114 P, -1115 M, -1116 M, -1223 dentary fragment with p3-p4, -1288 M (2 teeth), -1440 P2, -1441 M, -1465 P, -1464 m2-m3 (two teeth), -1472 P, -1559 M, -1500 M, -1544 p, -1546 M2, -1560 M1; TMM 30896-159 R P4-M3 (in plaster with some maxillary bone), -392 M3?, -393 P2?, -396 M, -457 M3, -495 juvenile dentary with dp2-dp4, -573 p (sectioned), -591 P/M, -595 M, -614 m3, -629 M, -630 M.
TMM 30936-66 P, -133 p/m (multiple teeth), -190 p, -210 M3, -269 P, -324 m, -326 M; TMM 31132-3 P/M, -70 L ramus with p2-p4, -82 M, -114 p, -131 M, -91 m (multiple teeth), -189 P, -223 M, -275 M (two teeth), -287 m (multiple teeth), -338 m (multiple teeth), -383 P, -441 p (two teeth), -467 m3, -468 M, -551 M, -670 P4, -673 M, -695 M3, -697 M1, -699 M, -759 m3; TMM 31170-17 p/m (three teeth), -41 L p2-m3, -49 juvenile ramus with dp2-dp4, -55 p/m (2p, 2m, one sectioned), -72 p/m (four teeth), -91 p/m (three teeth including m1-m2), -154 dentary with p2-m1, -202 M3; TMM 31081-7 juvenile dentary with dp2-m2, -217 ramus with p2-m3, -329 M1, -272 DP/P/M (5 teeth, DP4-M3), -390 M1, -456 juvenile dentary with dp2-m2, -516 ramus with p2-m3, -520 P4-M3 (in plaster), -527 dentary with p2-m3, -559 M, -560 dentary with p2-p3, -595 P, -619 P2-M1, -637 dentary with p2-m3, -638 juvenile dentary with dp2-m1, -780 dentary with p2-m3, -785 M2, -844 skull with R and L P2-M3, -858 P2, -865 P2, -1031 m3 (two teeth), -1071 p4, -1076 R P2-M3, LP4-M3 (likely one individual), -1089 p2-m3 (six teeth), -1114 P, -1115 M, -1116 M, -1223 dentary fragment with p3-p4, -1288 M (2 teeth), -1440 P2, -1441 M, -1465 P, -1464 m2-m3 (two teeth), -1472 P, -1559 M, -1500 M, -1544 p, -1546 M2, -1560 M1; TMM 30896-159 R P4-M3 (in plaster with some maxillary bone), -392 M3?, -393 P2?, -396 M, -457 M3, -495 juvenile dentary with dp2-dp4, -573 p (sectioned), -591 P/M, -595 M, -614 m3, -629 M, -630 M.
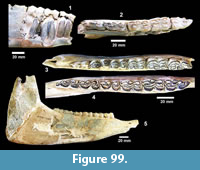 Description. Cormohipparion occidentale from the Lapara Creek Fauna has been well described by Forsten (1975), MacFadden (1984) and Hulbert (1987a, 1988a). Protocones are isolated and oval and the pli caballins are often bifurcate especially in P3-M1 (Figure 97, Figure 98). Fossette borders have relatively complex plications (commonly 4-8), and the hypostyle is well developed sometimes with plications. Lower molars commonly display deep ectoflexids, and the protoconid and hypoconid labial borders are sub-rounded (Figure 99). Mesostyle crown heights for slightly worn to unworn M1-2 range from 48-52 mm (50-54 mm reported by Hulbert, 1988a). Tooth row lengths range from 129-146 mm, falling within the range for C. occidentale reported by MacFadden (1984) as 125-151 mm. Protocones display a mean length/width ratio of 2.07 with a standard deviation of 0.33.
Description. Cormohipparion occidentale from the Lapara Creek Fauna has been well described by Forsten (1975), MacFadden (1984) and Hulbert (1987a, 1988a). Protocones are isolated and oval and the pli caballins are often bifurcate especially in P3-M1 (Figure 97, Figure 98). Fossette borders have relatively complex plications (commonly 4-8), and the hypostyle is well developed sometimes with plications. Lower molars commonly display deep ectoflexids, and the protoconid and hypoconid labial borders are sub-rounded (Figure 99). Mesostyle crown heights for slightly worn to unworn M1-2 range from 48-52 mm (50-54 mm reported by Hulbert, 1988a). Tooth row lengths range from 129-146 mm, falling within the range for C. occidentale reported by MacFadden (1984) as 125-151 mm. Protocones display a mean length/width ratio of 2.07 with a standard deviation of 0.33.
As described by Hulbert (1988b), TMM 31081-844 is an important specimen, as it includes much of the skull and upper tooth series, although it is crushed and partially restored with plaster (Figure 97.1). The right-side facial fossa is pocketed, ~60 mm x 30 mm, oval but narrowing towards the anterior. It extends from above the posterior edge of M1 to above P3. Measurements and morphology of the pre-orbital region, described in Hulbert (1988b) are consistent with C. occidentale. TMM 31081-7 is an interesting specimen with dp2-dp4 preserved above p2-p4 (Figure 99.1, 2). The deciduous premolars also exhibit an isolated protoconid that is especially evident on dp3.
Cormohipparion occidentale is relatively common in the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas. It is very common in the Farish Ranch Local Fauna, but rare in the Buckner Ranch Local Fauna.
Discussion. Cormohipparion was named by Skinner and MacFadden (1977). Prior to that, the species “occidentale” was commonly referred to the genus Neohipparion, as Forsten (1975) did for the Lapara Creek material. MacFadden (1984) and Hulbert (1988a) recognized Cormohipparion occidentale from the Lapara Creek Fauna but Woodburne (2007) did not comment on this occurrence and restricted usage of the name to Cl2 faunas in Nebraska and South Dakota. He introduced five new species of Cormohipparion, including two from the Clarendon Beds of north Texas that had previously been referred to C. occidentale. Although material included in C. occidentale may represent a more diverse suite of species, the material from Lapara Creek is consistent with the description of C. occidentale in Woodburne (2007). While clearly present in three of the four local faunas, C. occidentale is not well represented in the stratigraphically highest Buckner Ranch Local Fauna. Following the more common usage, C. occidentale is known from late Barstovian through early Hemphillian faunas in North America (MacFadden, 1998).
Genus NEOHIPPARION Gidley, 1903
Neohipparion affine (Leidy, 1869)
(Figure 100)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Buckner Ranch (TMM 30896).
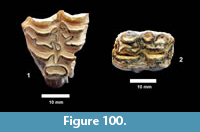 Referred specimens. TMM 30936-440, p3/p4, -466 M1/M2, -468 M; TMM 31132-432 P3/P4, -616 M1/M2; TMM 30896-392 M3, -395 M1/M2, -454 p3/p4 [figured by Quinn (1955) plate 14 as Equus laparensis ], -480 R dentary with p2-m3 [figured by Quinn (1955) as Equus or Neohipparion ], -571 M, -572 P3/P4, -573 p, -574 p, -576 P2, -577 P2; 578P2, -579 P2, -580 m1/m2.
Referred specimens. TMM 30936-440, p3/p4, -466 M1/M2, -468 M; TMM 31132-432 P3/P4, -616 M1/M2; TMM 30896-392 M3, -395 M1/M2, -454 p3/p4 [figured by Quinn (1955) plate 14 as Equus laparensis ], -480 R dentary with p2-m3 [figured by Quinn (1955) as Equus or Neohipparion ], -571 M, -572 P3/P4, -573 p, -574 p, -576 P2, -577 P2; 578P2, -579 P2, -580 m1/m2.
Description. Hulbert (1987b) presented measurements and a description of specimens representing N. affine in the Lapara Creek Fauna. He argued that this taxon could be separated from other hipparionin horses in the fauna on the basis of size, protocone shape, fossette shape, pli caballin development and flattened protoconid-hypoconid borders on the lower teeth. Woodburne (2007) noted that N. affine differed from Cormohipparion occidentale in having less complex fossette borders, a single pli-caballin, a more lingually angled axis of the hypoconal groove in the premolars and a less prominent pli protoconule. These characters are consistent with specimens from the Lapara Creek Fauna.
Discussion. Quinn (1955) did not describe Neohipparion from the Lapara Creek Fauna, although he included N. coloradense in his faunal list. Some of the specimens referred to N. affine by Hulbert (1987b) had been previously identified by Quinn (1955) as Equus. Forsten (1975) recognized Neohipparion, but only as the species N. occidentale, that is now included in Cormohipparion (MacFadden, 1984, Hulbert, 1988a). Hulbert (1987b) was the first to recognize N. affine in the Lapara Creek collection. A number of specimens, identified as Neohipparion coloradense by Forsten (1975) from the Ten Mile Waterhole Creek Local Fauna, are now identified as N. affine. It is a relatively rare taxon in the Lapara Creek Fauna.
N. affine is present, but often rare in Clarendonian faunas from the Great Plains. Outside of the Texas Gulf Coast, N. affine is known from late Barstovian through late Clarendonian faunas in New Mexico, Texas, South Dakota, Nebraska and Kansas (MacFadden, 1998).
Tribe Equini (Gray, 1821)
Genus CALIPPUS Matthew and Stirton, 1930
Calippus placidus (Leidy, 1869)
(Figure 101, Table 19)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
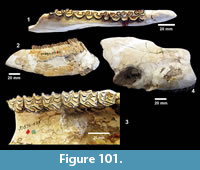 Referred specimens. Modified after Hulbert (1988a). TMM 30936-80 P3/P4, -160 P3/P4, -353 P3/P4; TMM 31132-105 associated P3-M2, -617 M1/M2; TMM 31081-75 P2, -1139 M3, -1375 M1/M2; TMM 30896-187 DP2-M1, -200 associated P4-M2, -419 associated R DP2-DP4, -479 R dentary with dp2-m1, -528 and 530 associated palate with R L P2-M3 and R dentary with p3-m3 [C. optimus syntypes of Quinn (1955)].
Referred specimens. Modified after Hulbert (1988a). TMM 30936-80 P3/P4, -160 P3/P4, -353 P3/P4; TMM 31132-105 associated P3-M2, -617 M1/M2; TMM 31081-75 P2, -1139 M3, -1375 M1/M2; TMM 30896-187 DP2-M1, -200 associated P4-M2, -419 associated R DP2-DP4, -479 R dentary with dp2-m1, -528 and 530 associated palate with R L P2-M3 and R dentary with p3-m3 [C. optimus syntypes of Quinn (1955)].
Description. Hulbert (1988a) provided a revised diagnosis and description for this taxon in part based on material from the Lapara Creek Fauna, although the type material is from northern Nebraska. C. placidus is a relatively small, hypsodont protohippine. The cheek teeth are more hypsodont than in C. proplacidus and are larger than in C. regulus. Protocones are connected and fossette borders are relatively simple. Although figured by Quinn (1955, plate 6) as the type specimens of C. optimus, Figure 101 further illustrates the associated partial palate and dentary from the Buckner Ranch Local Fauna (TMM 30896-528, -530). TMM 30896-528 includes part of the skull preserving the anterior rim of the orbit located above M2. It is difficult to evaluate the evidence of facial fossa as the skull is crushed and repaired with plaster.
Discussion. Calippus placidus is a relatively small horse with average TRL of ~100 mm. The taxonomy of C. placidus and the history of nomenclature applied to the small protohippine horses from the Lapara Creek Fauna is well documented in Hulbert (1988a). C. placidus is recognized in all four of the local faunas, but is not a common taxon. C. placidus has been documented in late Barstovian through late Clarendonian faunas of the Great Plains (MacFadden, 1998).
Calippus regulus (Johnston, 1937)
(Figure 102, Figure 103)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132, TMM 31170, TMM 31184), Farish Ranch (TMM 31081) and Buckner Ranch (TMM 30896).
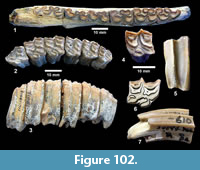 Referred Specimens. TMM 30936-31 L p4-m3, -71 m, -76 P/M, -78 p2, -79 M3, -82 p3, -99 R dentary p4-m3, -128 P/M, -153 R dentary p2-m3, -155 m, -157 m, -352 M, -402 p3, -446 P/M, -449 P/M, -450 P/M 451 P/M, -477 m1, -479 m2, -480 p3, -481 p3, -483 p4, -496 M3, -497 M3; TMM 31132-25 p, -105 P3-M2, -225 M, -229 p/m, -398 M, -461 L dentary p2-m2, -466 m, -647 p/m, -648 p/m, -654 P/M, -683 P/M, -685 p2, -687 m, -688 m3, -689 p3, -690 m3, -691 m, -717 m, -719 P/M, -720 M, -740 M, -761 m, -762 m, -765 m; TMM 31170-19 m1/m2, -39 p/m, -50 P/M, -76 P/M, -88 P/M, -89 p/m, -94 R dentary with p2-m3, -146 P/M, -147 P2, -149 P/M, -208 p/m, -209 p/m, -210 p/m, -211 p/m, -212 p/m, -213 p/m 214 p/m, -215 m3, -216 p/m, -217 p2, -245 p/m, -249 P2, -253 m3, -256 p/m, -258 p/m; TMM 31184-7 R dentary p2-m3; TMM 31081-97 M1/M2, -171 M1/M2, -374 M, -661 d, -711 R L P2-M3, -714 L dentary p4-m3, -863 p4, -1042 M, -1122 M, -1160 p2 p4 m2 m3, -1290 p2; TMM 30896-173 juvenile skull R and L D2-M1 [holotype of C. anatinus according to Quinn (1955)], -356 p, -360 p, -361 p2, -402 p, -462 m2 m3, -538 m, -599 p2, -600 p2, -601 p/m, -602 p/m, -608 M1/M2, -610 P4, -611 M2.
Referred Specimens. TMM 30936-31 L p4-m3, -71 m, -76 P/M, -78 p2, -79 M3, -82 p3, -99 R dentary p4-m3, -128 P/M, -153 R dentary p2-m3, -155 m, -157 m, -352 M, -402 p3, -446 P/M, -449 P/M, -450 P/M 451 P/M, -477 m1, -479 m2, -480 p3, -481 p3, -483 p4, -496 M3, -497 M3; TMM 31132-25 p, -105 P3-M2, -225 M, -229 p/m, -398 M, -461 L dentary p2-m2, -466 m, -647 p/m, -648 p/m, -654 P/M, -683 P/M, -685 p2, -687 m, -688 m3, -689 p3, -690 m3, -691 m, -717 m, -719 P/M, -720 M, -740 M, -761 m, -762 m, -765 m; TMM 31170-19 m1/m2, -39 p/m, -50 P/M, -76 P/M, -88 P/M, -89 p/m, -94 R dentary with p2-m3, -146 P/M, -147 P2, -149 P/M, -208 p/m, -209 p/m, -210 p/m, -211 p/m, -212 p/m, -213 p/m 214 p/m, -215 m3, -216 p/m, -217 p2, -245 p/m, -249 P2, -253 m3, -256 p/m, -258 p/m; TMM 31184-7 R dentary p2-m3; TMM 31081-97 M1/M2, -171 M1/M2, -374 M, -661 d, -711 R L P2-M3, -714 L dentary p4-m3, -863 p4, -1042 M, -1122 M, -1160 p2 p4 m2 m3, -1290 p2; TMM 30896-173 juvenile skull R and L D2-M1 [holotype of C. anatinus according to Quinn (1955)], -356 p, -360 p, -361 p2, -402 p, -462 m2 m3, -538 m, -599 p2, -600 p2, -601 p/m, -602 p/m, -608 M1/M2, -610 P4, -611 M2.
Description. As with C. placidus, Hulbert (1988a) provided a revised diagnosis and description of C. regulus. Numerous specimens representing this taxon were also described by Forsten (1975), and by Quinn (1955) as C. anatinus. C. regulus is a very small equid distinguished by its size, very simple fossette borders, connected protocone and high degree of hypsodonty. Measured TRLs ranged from 78-88.5 mm.
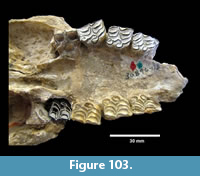 Discussion. Hulbert (1988a) agreed with Forsten (1975) in considering Quinn’s (1955) Calippus anatinus from the Lapara Creek Fauna a junior synonym of C. regulus. C. regulus is recognized in all four of the Lapara Creek Local Faunas. C. regulus was first described by Johnston (1937) from the middle Clarendonian Grant Lease Site in Donley County, Texas. Hulbert (1988a) considered its distribution to include late Barstovian faunas of the northern Great Plains, early and middle Clarendonian faunas of the southern Great Plains and early Clarendonian faunas of the Gulf Coast. MacFadden (1998) included occurrences in the late Barstovian Cold Spring Fauna from Texas, along with the Lapara Creek Fauna, Clarendonian faunas in the Southern Plains and late Barstovian to early Clarendonian faunas in the Great Plains.
Discussion. Hulbert (1988a) agreed with Forsten (1975) in considering Quinn’s (1955) Calippus anatinus from the Lapara Creek Fauna a junior synonym of C. regulus. C. regulus is recognized in all four of the Lapara Creek Local Faunas. C. regulus was first described by Johnston (1937) from the middle Clarendonian Grant Lease Site in Donley County, Texas. Hulbert (1988a) considered its distribution to include late Barstovian faunas of the northern Great Plains, early and middle Clarendonian faunas of the southern Great Plains and early Clarendonian faunas of the Gulf Coast. MacFadden (1998) included occurrences in the late Barstovian Cold Spring Fauna from Texas, along with the Lapara Creek Fauna, Clarendonian faunas in the Southern Plains and late Barstovian to early Clarendonian faunas in the Great Plains.
Calippus martini (Hesse, 1936)
(Figure 104, Figure 105, Table 20)
Localities. Bridge Ranch (TMM 31132, TMM 31170), Farish Ranch (TMM 30181), Buckner Ranch (TMM 30896).
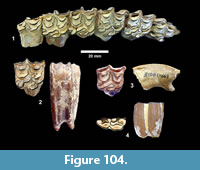 Referred specimens. Modified from Hulbert, 1988b. TMM 31132-169 M1/M2, -343 p4-m3, -357 dp3/dp4, -430 p3-p4, -498 p3/p4, -569 m1/m2, -675 P3/P4, -676 P3/P4, -815 dp3/dp4, -816 m1/m2, -820 P3/P4; TMM 31170-13 m1/m2, -73 P3/P4, -74 m1/m2, -280 dp3/dp4; TMM 31081-169 M1/M2, -1244 M1/M2, -1565 p3/p4; TMM 30896-127 P2-M3, -188 palate with R DP2-DP4 and L DP2- M1, -195 P2-P4 (holotype of Equus laparensis Quinn, 1955), -207 R dentary with dp2-m1, -418 P4-M1, -501 L dentary with dp2-dp4, -533 p2, -535 dp2, -545 R p2-p4 and L p2-m3, -569 skull with R P4-M3.
Referred specimens. Modified from Hulbert, 1988b. TMM 31132-169 M1/M2, -343 p4-m3, -357 dp3/dp4, -430 p3-p4, -498 p3/p4, -569 m1/m2, -675 P3/P4, -676 P3/P4, -815 dp3/dp4, -816 m1/m2, -820 P3/P4; TMM 31170-13 m1/m2, -73 P3/P4, -74 m1/m2, -280 dp3/dp4; TMM 31081-169 M1/M2, -1244 M1/M2, -1565 p3/p4; TMM 30896-127 P2-M3, -188 palate with R DP2-DP4 and L DP2- M1, -195 P2-P4 (holotype of Equus laparensis Quinn, 1955), -207 R dentary with dp2-m1, -418 P4-M1, -501 L dentary with dp2-dp4, -533 p2, -535 dp2, -545 R p2-p4 and L p2-m3, -569 skull with R P4-M3.
Description. Hulbert (1988a) provided a revised diagnosis and description of C. martini based in part on the sample from the Lapara Creek Fauna. C. martini is the largest of the three species of Calippus present in the Lapara Creek Fauna. 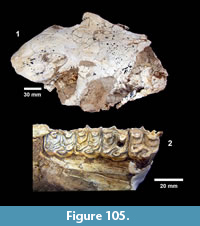 It is characterized by a connected protocone and moderately simple fossette borders on the upper cheek teeth that become very simple with wear. Hulbert (1988a) noted that C. martini is the largest species of Calippus and can be difficult to separate from the other medium- to large-size protohippines in the Gulf Coast faunas on the basis of isolated teeth. The approximate TRL based on TMM 30896-127 is 125 mm.
It is characterized by a connected protocone and moderately simple fossette borders on the upper cheek teeth that become very simple with wear. Hulbert (1988a) noted that C. martini is the largest species of Calippus and can be difficult to separate from the other medium- to large-size protohippines in the Gulf Coast faunas on the basis of isolated teeth. The approximate TRL based on TMM 30896-127 is 125 mm.
Discussion. Hulbert (1988a) described the complex taxonomic history of C. martini. It is relatively rare in the Bridge, Farish and Buckner Ranch Local Faunas and has not been recognized at Ten Mile Waterhole Creek. Hulbert (1988a) used a cross-plot of protocone length vs. basal crown length to demonstrate that C. martini from the Lapara Creek Fauna grouped closely with examples of this taxon from the type Clarendon Fauna and from the Minnechaduza Fauna. C. martini was described by Hulbert (1988a) from the early Clarendonian Agricola Fauna in the lower Bone Valley Formation of Florida, and he also noted an occurrence in the Hookers Prairie Mine Fauna. MacFadden (1998) included occurrences in Clarendonian faunas of the Ash Hollow Formation in South Dakota and the early Clarendonian WaKeeney Fauna in the Ogallala Formation of Kansas.
Genus PROTOHIPPUS Leidy, 1869
Protohippus supremus (Leidy, 1869)
(Figure 106)
Hippotigris sellardsi Quinn (1955)
Hippotigris clarendonensis Quinn (1955)
Hippotigris parastylus Quinn (1955)
Pliohippus martini (Hesse), Forsten (1975) (in part)
Pliohippus (Pliohippus) cf. supremus (Leidy), Forsten (1975) (in part)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), Farish Ranch (TMM 31081), Buckner Ranch (TMM 30896).
Referred specimens. TMM 30936-60 P3, -140 p4, -342 M2, -343 M1; TMM 31132-104 M1/M2, -224 M1/M2, -459 partial L dentary with p2-m3, -679 P/M; TMM 31081-168 p3, -664 partial R dentary with p2-m3, -1183 L p3/p4, -1210 p2, -1540 m1, -1541 m2, -1543 m; TMM 30896-206 partial R dentary with p3-m3, -502 partial R juvenile dentary with p2-m2, -503 associated R and L P2-M3, -541 partial L dentary with p2-m3.
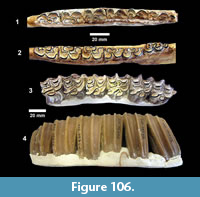 Description. A relatively small number of specimens represent a large protohippine horse with tooth row lengths of 137-142 mm (Figure 106). Hulbert (1987a) provided a description of these and a revised diagnosis of Protohippus supremus. No cranial material is known from Lapara Creek so comparisons have been made on isolated teeth and a few partial dentaries. Some of the more distinctive characters include: protocones are isolated from the protoselene in early stages of wear, protocones are ovoid with pointed ends and fossette borders show simple to moderately complex plications. Relative to Pliohippus pernix, the protostylid is well developed in P. supremus, the ectoflexid is deeper on p3/p4 and the metaconid-metastylid loops are more symmetrical with a larger metastylid.
Description. A relatively small number of specimens represent a large protohippine horse with tooth row lengths of 137-142 mm (Figure 106). Hulbert (1987a) provided a description of these and a revised diagnosis of Protohippus supremus. No cranial material is known from Lapara Creek so comparisons have been made on isolated teeth and a few partial dentaries. Some of the more distinctive characters include: protocones are isolated from the protoselene in early stages of wear, protocones are ovoid with pointed ends and fossette borders show simple to moderately complex plications. Relative to Pliohippus pernix, the protostylid is well developed in P. supremus, the ectoflexid is deeper on p3/p4 and the metaconid-metastylid loops are more symmetrical with a larger metastylid.
Discussion. Specimens of Protohippus supremus are recognized in all four local faunas and include specimens previously figured and described by Quinn (1955) as Hippotigris sellardsi, Hippotigris clarendonensis, and Hippotigris parastylus. Although Quinn (1955) recognized a large number of protohippine horses in the Lapara Creek Fauna, Webb (1969) synonymized many of them as did Forsten (1975), with a different regrouping. Hulbert (1988a) suggested that there were three large protohippine horses common in the Clarendonian faunas of the Gulf Coast, one of which was P. supremus. P. supremus has been reported from late Barstovian through Clarendonian faunas of the Great Plains (Hulbert, 1988b).
Genus PLIOHIPPUS Marsh, 1874
Pliohippus pernix (Marsh, 1874) (Figure 107)
Localities. Ten Mile Waterhole Creek (TMM 30936), Bridge Ranch (TMM 31132), and Farish Ranch (TMM 31081).
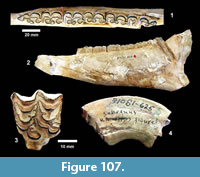 Referred specimens. TMM 30936-61 m3, -158 M1/M2; TMM 31132-24 P3/P4, -39 m, -41 P3/P4, -147 P/M, -190 P3/P4, -267 P3/P4, -333 right partial dentary with p2-m3 [(holotype of Dinohippus subvenus by Quinn (1955)], -435 P3/P4, -496 p4; TMM 31170-86 M3; TMM 31081-625 M1/M2.
Referred specimens. TMM 30936-61 m3, -158 M1/M2; TMM 31132-24 P3/P4, -39 m, -41 P3/P4, -147 P/M, -190 P3/P4, -267 P3/P4, -333 right partial dentary with p2-m3 [(holotype of Dinohippus subvenus by Quinn (1955)], -435 P3/P4, -496 p4; TMM 31170-86 M3; TMM 31081-625 M1/M2.
Description. The largest horse in the Lapara Creek Fauna is represented primarily by isolated teeth and a partial dentary (Figure 107). Many of these were figured and described by Quinn (1955) as Dinohippus subvenus.
Discussion. Dinohippus subvenus was proposed by Quinn (1955) with TMM 31132-333 designated as the type specimen. Hulbert (1988a) reviewed the subsequent taxonomic history, as revised by Webb (1969) and Forsten (1975), and considered D. subvenus a subjective synonym of Pliohippus pernix. MacFadden (1998) included D. subvenus within Dinohippus and listed Bridge Ranch as an occurrence, but also listed Pliohippus supremus and Protohippus supremus as valid taxa from Bridge Ranch. I have followed Hulbert (1988a) in recognizing Pliohippus pernix, Protohippus supremus, and Calippus martini as the three large protohippine horses in the Lapara Creek Fauna. However, as noted by previous authors, these taxa are difficult to differentiate without cranial material.
Subfamily ANCHITHERIINAE Leidy, 1869
Genus HYPOHIPPUS Leidy, 1869
Hypohippus cf. affinis (Leidy, 1858) (Figure 108)
Localities. Ten Mile Waterhole Creek (TMM 30936).
 Referred specimens. TMM 30936-348 P/M.
Referred specimens. TMM 30936-348 P/M.
Description. A single tooth from the Ten Mile Waterhole Creek Local Fauna represents a low-crowned browsing equid (Figure 108). Forsten (1975) also referred a single, unworn lower molar or premolar (TMM 31132-447) from the Bridge Ranch Local Fauna to this taxon, but that specimen is here considered Equidae indeterminate. TMM 30936-348 includes portions of the maxillary bone that are quite delicate, suggesting that this specimen is not reworked from older strata.
Discussion. While most of the horses from the Lapara Creek Fauna exhibit well developed hypsodonty, at least one specimen represents a low crowned anchitherine equid. Hypohippus cf. affinis represents a relatively primitive browsing equid found only at the Ten Mile Waterhole Creek Local Fauna. This is consistent with this local fauna being from the lower part of the Goliad Formation and slightly older than the Buckner and Farish Ranch Local Faunas. The lack of Hypohippus in any of the younger local faunas apparently records the extinction of anchitherine horses in the Texas Gulf Coast in the early Clarendonian.
BIOCHRONOLOGY
Comparison of the Four Local Faunas
 Faunal composition for all four local faunas is shown in Figure 109. Fifty species of fossil vertebrates include five species of fish, seven species of reptiles, two species of birds and 36 species of mammals. The 36 species of mammals represent 31 genera of which four are rodents, five are carnivores, two are proboscideans, 10 are artiodactyls and 10 are perissodactyls. Seven taxa, of which four are equids, are common to all four local faunas (Nine and five if questionable occurrences are included).
Faunal composition for all four local faunas is shown in Figure 109. Fifty species of fossil vertebrates include five species of fish, seven species of reptiles, two species of birds and 36 species of mammals. The 36 species of mammals represent 31 genera of which four are rodents, five are carnivores, two are proboscideans, 10 are artiodactyls and 10 are perissodactyls. Seven taxa, of which four are equids, are common to all four local faunas (Nine and five if questionable occurrences are included).
Both Simpson and Pickford indices were calculated from mammalian taxa for comparison of faunal composition following de Bonis et al. (1992) (Table 21). Rodents and rabbits known only from TMM 30898 (Buckner Ranch) were excluded as the same kind of wet screen sample is not available from all four local faunas.
The Ten Mile Waterhole Creek Local Fauna is most similar to the Bridge Ranch Local Fauna and least similar to the Buckner Ranch Local Fauna with an intermediate Pickford Index distance calculated with respect to the Farish Ranch Local Fauna. The calculated indices do not demonstrate significant differences between the Bridge, Farish and Buckner Ranch Local Faunas. Even though these metrics do not reveal an unambiguous ordering, relationships, based on faunal composition, are consistent with the relative stratigraphic distribution of the local faunas. The general similarity of the local faunas and the consistency of depositional environments throughout the Goliad Formation suggests that time is a significant component of this ordering. Although they did not consider the Ten Mile Waterhole Creek Local Fauna, the chronostratigraphic ordering recognized here is consistent with the previous interpretation of Tedford et al. (1987), who considered the Bridge Ranch Local Fauna slightly older than the Farish Ranch-Buckner Ranch Local Fauna.
Age and Correlation
 The known Hemingfordian through Hemphillian age ranges in North America for taxa from the Lapara Creek Fauna are shown in Figure 110.
The known Hemingfordian through Hemphillian age ranges in North America for taxa from the Lapara Creek Fauna are shown in Figure 110.
Lepisosteus is known from the Cretaceous through Recent.
Ictalurus has been reported from the Oligocene through recent of North America and I. lambda has been recognized in Miocene faunas from Kansas, Nebraska and Florida (Lundberg, 1975). Voorhies (1969) identified Ictalurus from the Barstovian Valentine Formation.
Pylodictis olivaris is known from Hemingfordian to recent faunas (Lundberg, 1975).
Trachemys has been reported from the Barstovian Calvert Formation where Weems and George (2013) identified Trachemys sp. based on a single posterior peripheral found in float. Zug (2001) also assigned a few isolated specimens from the Zanclean (latest Hemphillian-early Blancan) Yorktown Formation to the “Chrysemys complex” and suggested that both Pseudemys and Trachemys were probably represented. Trachemys has been recognized from multiple Hemphillian faunas in North America, including occurrences in Florida, Nebraska and Tennessee (Hulbert (2001), Holman and Parmley (2005), Parmalee et al. (2002)). The presence of cf. Trachemys sp. in the Farish Ranch Local Fauna potentially represents one of the earliest occurrences of this genus in North America.
Hulbert and Whitmore (2006) mentioned the presence of abundant Apalone specimens from the early Hemphillian Mauvilla Local Fauna, Alabama and Apalone cf. spinifera was identified from these deposits by Jasinski (2013). Weems and George (2013) identified carapace and plastron fragments from the Barstovian Calvert Formation in Delaware and Virginia as Apalone lima, but that material is very fragmentary and the diagnosis is largely based on the pattern of sculpting. Holman and Corner (1985) reported Trionyx sp. indet. from the late Barstovian Myers Farm Local Fauna, Nebraska, which most workers would now refer to Apalone, following the analysis of Meylan (1987). Valdes et al. (2017) named a new species Apalone amorense from the late Clarendonian Love Bone Bed, Florida. The Love Bone Bed material had previously been referred to Apalone (Trionyx) cf. ferox by Webb et al. (1981). Apalone sp. from the Lapara Creek Fauna, represents one of the earliest occurrences of this genus in North America.
Terrapene has been reported from Barstovian and younger faunas in North America (Holman and Fritz, 2005; Joyce et al., 2012).
Gopherus is known from Eocene to recent faunas in North America (Brattstrom, 1961; McCord, 2002; Ehret, 2007).
Hesperotestudo has a reported range of Oligocene to Pleistocene in North America (Meylan and Sterrer, 2000).
Alligator mississippiensis has been reported from the Hemphillian Moss Acres Racetrack site, Florida (Whiting et al., 2016). Alligator cf. mississippiensis from the Lapara Creek Fauna may represent the earliest occurrence of this taxon in North America.
Pronotolagus apachensis is known from the Cl1-Cl2 Apache Canyon section of the Caliente Formation, California (Prothero et al., 2008). Dalquest et al. (1996) also reported this taxon from the Clarendonian Wisenhunt Quarry Local Fauna, Oklahoma.
Domninoides is known from numerous Barstovian and Clarendonian faunas from California, Oregon and the Great Plains. The youngest documented occurrence is from the Hemphillian Bidahochi Formation, Arizona (Baskin, 1979).
Perognathus minutus has been recognized at numerous localities from California to Florida and the Great Plains in both Barstovian and Clarendonian faunas (Lindsay, 1972; Bryant, 1991). Whistler and Burbank (1992) report P. minutus from the Late Barstovian through the Early Hemphillian in the Dove Spring Formation, California.
Ceratogaulus rhinoceros is known from the Barstovian through early Clarendonian of Colorado and Nebraska (Korth, 2000).
Aelurodon taxoides is well known from numerous Clarendonian faunas in the Great Plains, New Mexico, California and Florida and from the Late Barstovian of New Mexico (Wang et al., 1999).
Tedford et al. (2009) recognized Eucyon from late Clarendonian through Hemphillian faunas in North America. The material assigned to cf. Eucyon sp. from the Farish Ranch Local Fauna potentially represents the oldest known occurrence of this taxon.
Leptocyon vafer is a common taxon with occurrences in the Great Plains through California from late Barstovian through late Clarendonian faunas (Tedford et al., 2009).
Ischyrocyon gidleyi is known from late Barstovian through late Clarendonian faunas in the northern Great Basin and Great Plains (Hunt, 1998).
Blancotherium buckneri is known only from the Lapara Creek Fauna.
Gomphotherium is generally considered an immigrant taxon that arrived from Eurasia in the Barstovian. It was a common element of faunas during the Clarendonian and then became scarce by the late Hemphillian. Tedford et al. (1987) stated the range as Ba2-Hh3, although Lambert and Shoshani (1998) noted both an early Barstovian record in Oregon and an early Blancan record in Florida.
Ustatochoerus medius is known from late Barstovian through early Clarendonian faunas in the Great Plains and southwestern U.S. (Stevens and Stevens, 2007). Lander (2008) shows various subspecies of Merychyus medius from late Barstovian through middle Clarendonian faunas.
The record of Pseudoceras skinneri in North America includes the Cl1 Clayton Quarry in Nebraska, the Cl3 Higgins Locality in northern Texas, the Cl3 Love Bone Bed of Florida and Hh2 sites in northern Nebraska (Webb, 2008).
Ramoceros ramosus is primarily known from various Barstovian through early Clarendonian localities in the Tesuque Formation of New Mexico (Janis and Manning, 1998).
Synthetoceras tricornatus is best known from the Clarendon beds of Donley Co., Texas (Patton and Taylor, 1971). Synthetoceras tricornatus probably evolved in the Gulf Coast from Prosynthetoceras, a common taxon in the late Barstovian Cold Spring Fauna. Synthetoceras tricornatus is present in the Ten Mile Waterhole Creek, Bridge Ranch and Farish Ranch Local Faunas, but absent in the Buckner Ranch Local Fauna. This distribution seems to record the local demise of the protoceratids and the diversification of the camelids. Synthetoceras cf. tricornatus has been reported from the Hemphillian of Florida and Alabama (Hirschfeld and Webb, 1968; Ispohording and Lamb, 1971).
Cranioceras teres has been reported from the Barstovian through early Clarendonian of New Mexico, Texas, South Dakota and California (Prothero and Liter, 2008).
Nothotylopus is first known from the late Hemingfordian of New Mexico (Morgan, 2015). Nothotylopus camptognathus has been recognized in Barstovian through early Clarendonian faunas in New Mexico (Tesuque Formation), Texas (Clarendon Local Fauna) and Nebraska (Valentine and Ash Hollow Formations) (Honey et al., 1998). Based on the older occurrences in New Mexico and Nebraska, and the lack of Nothotylopus in the Barstovian faunas of the Texas coastal plain, it appears that this taxon was an immigrant into the Texas coastal plain during the early Clarendonian. Nothotylopus only appears in the younger of the two Lapara Creek local faunas (Farish Ranch, Buckner Ranch).
Aepycamelus is known from late Hemingfordian through mid Hemphillian faunas in North America (Honey et al., 1998).
Procamelus occidentalis is known from Barstovian and Clarendonian faunas in Nebraska, Montana and Texas (Honey et al., 1998). Procamelus grandis has a broader range including the late Barstovian-Clarendonian of the Great Plains (South Dakota, Nebraska, Texas), the late Clarendonian of Florida (Love Bone Bed), late Clarendonian of Oregon and the early Hemphillian of Honduras and El Salvador (Honey et al., 1998).
Protolabis coartatus has been reported from the Clarendonian Milk Creek Formation, Arizona and the early Clarendonian Esmeralda Formation, California (Honey et al., 1998).
Protolabis yavapaiensis was named by Honey and Taylor (1978) from material collected in the Cl2 Milk Creek Formation, Arizona. Lozinsky and Tedford (1991) reported material that probably represents P. yavapaiensis from the late Clarendonian-early Hemphillian Popotosa Formation in New Mexico.
“Prosthennops” xiphidonticus has previously been reported from Barstovian faunas in North America including the Barstovian, Cold Spring Fauna from the Texas Gulf Coast (Wright, 1991). “P.” xiphidonticus from the Lapara Creek Fauna is slightly larger than Barstovian examples and apparently represents the youngest occurrence of this taxon.
Teleoceras major is known from early to late Clarendonian faunas in Nebraska, Kansas, South Dakota, Texas, Nevada and Washington (Prothero, 2005).
Pseudhipparion curtivallum is known from the Lapara Creek Fauna and from the early Clarendonian Agricola Fauna in Florida (MacFadden, 1998).
Nannippus sp. is known from the Bridge, Farish and Buckner Ranch Local Faunas but not from Ten Mile Waterhole Creek. It is also recorded in the Cl2 Phosphoria Mine Fauna in Florida. Hulbert (1987a) suggested that the slightly lower unworn crown heights in the Florida specimens could indicate a slightly older age than at Lapara Creek.
Hipparion tehonense is known from Clarendonian faunas in California (Whistler and Burbank, 1992; Kelly and Stewart, 2008) and Texas (MacFadden, 1980, 1984) as well as the Great Plains and Florida (Hulbert 1988a).
Cormohipparion ingenuum is known from early Clarendonian through early Hemphillian faunas of central Florida and the early Hemphillian of Honduras (Hulbert, 1988a).
Cormohipparion occidentale has been described from late Barstovian through early Hemphillian faunas in North America (Hulbert, 1988a; MacFadden, 1998).
Neohipparion affine is known from late Barstovian through late Clarendonian faunas in New Mexico, Texas, South Dakota, Nebraska, and Kansas (MacFadden, 1998).
Calippus placidus has been documented in late Barstovian through late Clarendonian faunas of the Great Plains (MacFadden, 1998).
Calippus regulus is recorded in the late Barstovian Cold Spring Fauna from the Fleming Formation in the Texas coastal plain as well as late Barstovian through late Clarendonian faunas in the Texas panhandle, Colorado and Nebraska including the Cl1 Burge Fauna (MacFadden, 1998).
Calippus martini is restricted to Cl1 and Cl2 faunas of the Great Plains and Florida (Hulbert, 1988b).
Protohippus supremus has been reported from late Barstovian through Clarendonian faunas of the Great Plains (Hulbert, 1988b).
Pliohippus pernix has been reported from latest Barstovian through Cl2 faunas in South Dakota, Nebraska and north Texas (MacFadden, 1998).
Hypohippus affinis is known from late Barstovian through Cl2 faunas in the Great Plain (MacFadden, 1998).
The base of the Clarendonian has long been problematic, primarily due to interpretation of the assignment of the Burge Fauna, Nebraska either to the latest Barstovian or earliest Clarendonian. Tedford, et al. (2004) modified the position of Tedford et al. (1987) and concluded that the Burge Fauna was earliest Clarendonian. They recognized the first occurrence of Pseudoceras as the defining taxon for the base of the Clarendonian and considered the following taxa characteristic of Cl1: Eucastor planus, E. dividerus, Eubelodon and Megabelodon, Cynarctus voorhiesi, Aelurodon stirtoni, Paratomarctus euthos, Pliohippus pernix, Cormohipparion occidentale, Pseudhipparion retrusum, Protohippus supremus, Megahippus matthewi, Ustatochoerus major, and Cranioceras. Pseudoceras skinneri is known from both the Bridge Ranch and Buckner Ranch Local Faunas but, as noted previously, its full biochronologic range is Cl1 through at least Hh2. Based on this taxon, at least the Bridge Ranch Local Fauna is no older than Cl1. The occurrence of Teleoceras major at Ten Mile Waterhole Creek also suggests an age no older than Cl1 for this stratigraphically oldest local fauna. The mylagaulid Ceratogaulus rhinoceros, present at Bridge Ranch and probably Ten Mile Waterhole Creek, is not known in faunas younger than Cl1. Of the characterizing taxa listed in Tedford et al. (2004), the Lapara Creek Fauna includes only Pliohippus pernix, Cormohipparion occidentale, Protohippus supremus and Cranioceras. Pliohippus pernix is known from the stratigraphically lowest three local faunas, but not from the Buckner Ranch Local Fauna. Cormohipparion occidentale and Protohippus supremus are known from all four local faunas, while Cranioceras is known from the stratigraphically intermediate Bridge Ranch and Farish Ranch Local Faunas. We now recognize additional taxa restricted to Cl1 and early Cl2 faunas including Pseudhipparion curtivallum, Nannippus sp. and Protolabis coartatus. Pseudhipparion curtivallum is known from all four local faunas, Nannippus sp. is known from the stratigraphically younger three local faunas, and Protolabis coartatus is known from the intermediate Bridge Ranch and Farish Ranch Local Faunas.
Similarly, Tedford et al. (2004) defined the beginning of Cl2 by the first occurrence of Barbourofelis in North America, and characterized this interval with Epicyon, Carpocyon robustus, Aelurodon taxoides, Cynarctus crucidens, Pseudhipparion gratum, Hipparion tehonense, Neohipparion affine, Dinohippus and Synthetoceras. Of these, A. taxoides, H. tehonense, N. affine and Synthetoceras are known from the Lapara Creek Fauna. A. taxoides occurs in the stratigraphically oldest three local faunas, but is not known at Buckner Ranch. H. tehonense occurs in the stratigraphically youngest three local faunas. N. affine is known from the full stratigraphic range of local faunas (although no specimens have been recognized in the Farish Ranch Local Fauna). None of the taxa considered characteristic of Cl3 faunas by Tedford et al. (2004) are present in the Lapara Creek Fauna.
Considering the known biochronologic ranges of taxa from the Lapara Creek Fauna and their distribution within the four stratigraphically arranged local faunas, the most parsimonious assignment of age for the Ten Mile Waterhole Creek and Bridge Ranch Local Faunas is Cl1 and for the Farish Ranch and Buckner Ranch Local Faunas is early Cl2. The only taxa unique to the stratigraphically highest Buckner Ranch Local Fauna include Leptocyon vafer, Pronotolagus apachensis, and Perognathus sp. However, the occurrences of the lagomorph and rodent almost certainly reflect sampling bias, as these taxa are only known from the single site that has produced micro-fossils through screen washing (TMM 30898). Leptocyon vafer is a long-ranging taxon from the late Barstovian through the Clarendonian. The Farish Ranch Local Fauna is statistically similar to both Bridge Ranch and Buckner Ranch, but the temporal affinity of Farish and Buckner may be suggested by the shared occurrences of Blancotherium buckneri, Nothotylopus camptognathus, Procamelus grandis, and Protolabis cf. yavapaiensis. Of these, Protolabis yavapaiensis is known only from Cl2 through Hh1 faunas. B. buckneri is known only from these two localities (with the possible exception of TMM 30936-28 discussed above), while N. camptognathus ranges from the Barstovian through Clarendonian and Procamelus grandis from the late Barstovian through early Hemphillian. Both of these faunas also lack cf. Gomphotherium sp., Ceratogaulus cf. rhinoceros and Hypohippus affinis.
Tedford et al. (1987) were the first to propose that the Lapara Creek Fauna could be divided into the Cl1 Bridge Ranch Local Fauna and the Cl2 Farish Ranch Local Fauna. They suggested that the horses from TMM 31132 (Bridge Ranch) differed significantly from succeeding assemblages, but also acknowledged that the equid taxonomy of Quinn (1955) was difficult to apply. Based on the current understanding of equid taxonomy and occurrences in the four local faunas, there is limited evidence to indicate significant age variation. However, the last occurrence of Hypohippus affinis in the Ten Mile Waterhole Creek l.f. and the appearance of Nannippus sp., Hipparion tehonense, Cormohipparion ingenuum and Calippus martini in the local faunas stratigraphically younger than Ten Mile Waterhole Creek Local Fauna, suggest a general evolution of the equid fauna. Tedford et al. (1987) suggested that the presence of Aelurodon and a merycodont indicated correlation with the Burge Fauna, Nebraska, but Aelurodon taxoides is now known from late Barstovian through Clarendonian faunas and the merycodont Ramoceros ramosus is primarily known from Barstovian through early Clarendonian faunas in New Mexico. Tedford et al. (1987) assigned TMM 31081 (Farish Ranch) and TMM 30896 (Buckner Ranch) to the Farish Ranch Local Fauna and interpreted correlation with the lower part of the Clarendon beds of Texas and the Minnechaduza Fauna of Nebraska that they considered as early Cl2 faunas. They cite advanced species of Procamelus, a late miolabine camel (Nothotylopus), early Megatylopus, Cranioceras clarendonensis, Ustatochoerus profectus and Teleoceras major as evidence for this correlation. Neither species of Procamelus is restricted to Cl2 faunas. The “early Megatylopus” is now recognized as Aepycamelus, a taxon that ranges from early Barstovian into the Hemphillian. Cranioceras clarendonensis is now known to be C. teres known from Ba1 through Cl1 faunas, while the oreodont Ustatochoerus medius is known from Ba2 through Cl2 faunas. Teleoceras major is a valid identification and is most common in the Farish Ranch Local Fauna, however, it is present in all four local faunas. T. major is perhaps best known from the early Cl2 Cap Rock Member of the Ash Hollow Formation, but this species is recognized from early to late Clarendonian faunas in Nebraska, Kansas, South Dakota, Texas, Nevada and Washington (Prothero, 2005). Therefore, none of these taxa provide an unambiguous correlation with the Clarendon and Minnechaduza Faunas. Nevertheless, for reasons stated above, the current analysis suggests that the ages proposed by Tedford et al. (2004) were accurate.
Although there is general faunal stability within the suite of Lapara Creek local faunas, a few trends can be recognized. As is characteristic for Clarendonian faunas in North America, the equid fauna is very diverse with 12 different species distributed across nine different genera. The final transition locally from a mixed browser-grazer equid fauna to an exclusively grazing community may be reflected by the last occurrence of the low-crowned Hypohippus at Ten Mile Waterhole Creek. The protoceratid-dominated artiodactyl community seen in the Barstovian Cold Spring Fauna transitions to a camelid-dominated community within the Lapara Creek Fauna. Synthetoceras tricornatus is the most common artiodactyl in the Ten Mile Waterhole Creek Local Fauna while the last occurrence of Synthetoceras is seen in the Farish Ranch Local Fauna. A diverse camelid fauna including Nothotylopus, Aepycamelus, Procamelus grandis and Protolabis cf. yavapaiensis appears in the Farish Ranch and Buckner Ranch Local Faunas. The last occurrence of Synthetoceras is locally coincident with the last occurrence of the antilocaprid Ramoceros ramosus and the paleomerycid Cranioceras teres.
The gomphothere cf. Gomphotherium sp. seems to have been replaced by Blancotherium buckneri, with only a single common proboscidean in any given local fauna. While Barstovian faunas of the Texas coastal plain have beavers but no mylagaulids, the Clarendonian faunas, including the late Clarendonian Dinero Local Fauna (Baskin and Hulbert, 2012), have mylagaulids but no beavers. The carnivore fauna was generally conventional and included two possible mustelids, the borophagine canid Aelurodon and the large amphicyonid Ischyrocyon. However, cf. Eucyon sp. (from the Farish Ranch Local Fauna) is possibly the oldest known record of this taxon in North America. An early Clarendonian divergence between Canini and Vulpini was hypothesized by Tedford et al. (2009) who considered Eucyon the earliest member of the Tribe Canini.
Despite concerted screen washing efforts, the full composition of the rodent/lagomorph fauna is still poorly known. The earliest, or near earliest, occurrence in North America of the turtles cf. Trachemys and Apalone and the earliest occurrence of Alligator cf. mississippiensis suggests that the fluvial systems of the Clarendonian Texas coastal plain may have been a locus for reptilian diversification. This conclusion awaits further confirmation of taxonomic identifications.
Detrital Zircon Geochronology
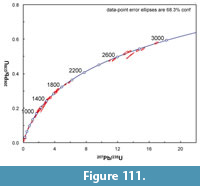 One hundred seven zircons were analyzed from a sandstone sample of the Goliad Formation along Medio Creek (Figure 11). Analysis was performed at the University of Arizona Laserchron Center using standard procedures as described in May et al. (2013). Results from 98 grains satisfied the applied discordance filters (Figure 111) and exhibit uranium concentrations less than 600 ppm.
One hundred seven zircons were analyzed from a sandstone sample of the Goliad Formation along Medio Creek (Figure 11). Analysis was performed at the University of Arizona Laserchron Center using standard procedures as described in May et al. (2013). Results from 98 grains satisfied the applied discordance filters (Figure 111) and exhibit uranium concentrations less than 600 ppm.
Seven percent of the grains yield Tertiary ages with the youngest grain dated at 28.7 +/- 1.9 Ma. Twenty-eight percent of the grains yield Mesozoic ages, five percent Paleozoic (Cambrian and Permian) and the remaining 60% having Precambrian ages. Although eight percent of the grains yield Archean ages, the oldest statistically significant age peak was calculated at 1674 Ma, with additional age peaks at 1435 Ma, 1176 Ma, 1086 Ma and 1010 Ma (Figure 112). These are relatively common grain ages reflecting original source areas in the Yavapai-Mazatzal, Midcontinent granitic and Grenville crustal domains. The abundance of Mesozoic and Cenozoic grains (35%) clearly reflects sediment contributions from the Cordilleran orogen to the west. These results are consistent with detrital zircon age spectra reported by Xu et al. (2017) from the early Miocene Oakville Formation, Texas. The zircon age distribution from the middle Miocene Goliad Formation reported here is similar to the Rio Grande embayment pattern recognized by Xu et al. (2017) suggesting that first order early Miocene drainage patterns continued into the middle Miocene, at least in south Texas.
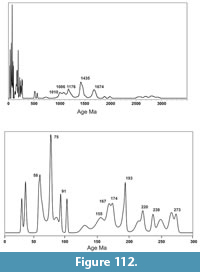 Although the detrital zircon age data from the Goliad Formation provide some evidence for regional fluvial patterns and paleogeography, they do not help constrain the age of the Goliad Formation.
Although the detrital zircon age data from the Goliad Formation provide some evidence for regional fluvial patterns and paleogeography, they do not help constrain the age of the Goliad Formation.
In summary, the age of the Goliad Formation is best constrained by correlation with equivalent marine strata to the south and by the vertebrate fossils themselves. Based on these constraints, the outcrop extent of the Goliad Formation ranges from Cl1 near its base or approximately 12-12.5 Ma to early Cl2 or 11.5-12 Ma at the Buckner Ranch locality near Berclair, Texas. Based on the Dinero Local Fauna described by Baskin and Hulbert (2012), the Goliad Formation gets as young as Cl3, or approximately 9-10 Ma in outcrop. A few specimens, from near the La Bahia Mission assigned to the La Bahia Local Fauna, were cited by some authors as evidence for a Hemphillian age for the uppermost part of the Goliad Formation (Wilson, 1956; Tedford et al., 1987). However, as noted by Baskin (1991), the few specimens from the La Bahia locality are not sufficient to interpret a confident age assignment. Although there is local outcrop evidence for a slight angular unconformity between the underlying Fleming Formation and the Goliad Formation, there is little evidence for a significant temporal gap on the basis of the vertebrate faunas.
TAPHONOMY AND DEPOSITIONAL ENVIRONMENT
 Although very little was recorded about the detailed distribution of fossils by the Survey, some observations can be made from Survey quarterly reports and field photos. Most of the materials collected were isolated teeth and bones, but two anomalies from this pattern stand out. The predominance of proboscidean remains at the Bucker Ranch locality is anomalous. The vast majority of proboscidean specimens in the Lapara Creek Fauna were collected from TMM 30896. As shown in Figure 113, this concentration included both cranial and post-cranial material, almost none of which appear to have been articulated. The type specimen of Blancotherium buckneri is the only specimen with articulated jaw and skull. Within the Buckner Ranch quarry, skulls with and without upper tusks, isolated upper tusks, mandibles and post-cranial bones were found in close association. The concentration apparently records animals that died on the floodplain environment, became disarticulated through decay and scavenging and were deposited in shallow ponds. There is no sedimentologic nor biologic evidence to suggest the concentration represents a catastrophic event, although seasonal flood events would not be unexpected.
Although very little was recorded about the detailed distribution of fossils by the Survey, some observations can be made from Survey quarterly reports and field photos. Most of the materials collected were isolated teeth and bones, but two anomalies from this pattern stand out. The predominance of proboscidean remains at the Bucker Ranch locality is anomalous. The vast majority of proboscidean specimens in the Lapara Creek Fauna were collected from TMM 30896. As shown in Figure 113, this concentration included both cranial and post-cranial material, almost none of which appear to have been articulated. The type specimen of Blancotherium buckneri is the only specimen with articulated jaw and skull. Within the Buckner Ranch quarry, skulls with and without upper tusks, isolated upper tusks, mandibles and post-cranial bones were found in close association. The concentration apparently records animals that died on the floodplain environment, became disarticulated through decay and scavenging and were deposited in shallow ponds. There is no sedimentologic nor biologic evidence to suggest the concentration represents a catastrophic event, although seasonal flood events would not be unexpected.
The second anomalous concentration is the predominance of rhino remains at the Farish Ranch locality. Although there are no quarry photos from the Survey showing the distribution of bones, the quarry produced numerous skulls and post-cranial material of Teleoceras major. Skulls, or partial skulls, represent a range of maturity evenly divided between early mature with slight wear on M3, mature with moderate wear on M3 and very mature with significant wear on M3. No juvenile skulls are present although isolated teeth indicate young individuals. The sense of “shear” with respect to the geometry of skull crushing from compaction is roughly equivalent between “top-to-the-left” and “top-to-the-right,” which would be expected from a random orientation of skulls at the time of burial. As with the Blancotherium material from TMM 30896, there are no articulated skeletons although within the scale of the quarry there is abundant post-cranial material associated with the skulls. The lack of both juvenile skulls and articulated skeletons suggests that the accumulation of rhino material at TMM 31081 was probably not the result of a catastrophic event. There is no obvious explanation for the abundance of gomphothere material at TMM 30896 and of rhino material at TMM 31081, however, the two anomalies are associated with both the largest mammals and those that probably displayed herding behavior. Although the Farish Ranch Local Fauna may be slightly older than the Buckner Ranch Local Fauna, both are quite diverse and share a number of common taxa suggesting that environmental conditions were not drastically different. Both local faunas contain Blancotherium and Teleoceras. It may be that the two localities represent slightly different sub-environments that provided a preservation bias within the large mammals. Most of the gomphothere material from TMM 30896 was recovered from gray mudstones and sandy mudstone deposited in floodplain environments and associated shallow ponds. The lithology at TMM 31081 was reported by the Survey as “gravels and sands” below a mudstone interval. The Farish Ranch locality and associated rhinos may represent a fluvial channel environment rather than a floodplain environment.
Material collected by screen washing from TMM 30898 was recovered from a thin (15 cm) bed of light-gray, poorly sorted, carbonate nodule conglomerate with light-gray, overbank mudstone above and below. Vertebrate bone fragments were common as were isolated large mammal teeth and dense, compact post-cranial bones such as equid phalanges. Small mammal fossils were very rare and commonly broken, although isolated fish teeth were relatively abundant and well preserved. Internal molds of complete bivalves are very common in the conglomerate, although actual shell is rarely preserved. The types and distribution of fossils in this depositional unit, the abundance of both carbonate nodules within a muddy matrix and of articulated pelecypods suggest that this unit represents a flood-event debris-flow that collected material from the adjacent floodplain and deposited it in a shallow channel or pond. The bed is very poorly sorted with a muddy matrix and no obvious sedimentary structures. The delicate fish teeth probably represent the in situ fauna within the original pond environment. Very small root casts are also common within this unit and probably represent grasses and small plants that were growing either on the floodplain or at the edge of the pond.
Archived notes from the Survey record mostly isolated specimens, commonly poor (chalky) preservation and evidence of surface weathering prior to burial. Within the limited exposures, strata are not disrupted as might be expected from a spring boil. Most of the specimens were probably disarticulated and weathered on interfluve flood plains and then “swept” up into fluvial channel deposits or floodplain ponds. Even the concentration of gomphotheres at TMM 30896 (Buckner Ranch) and rhinos at TMM 31081 (Farish Ranch) suggest transport of skeletal material by fluvial and perhaps scavenging processes. There is little evidence for predation or scavenger damage on the existing bones, but identification is hindered by poor preservation as well as the practices of the Survey preparators. Some bones show evidence of post-mortem weathering prior to burial and fossilization.
ENVIRONMENT OF THE TEXAS COASTAL PLAIN DURING THE CLARENDONIAN
Hoel (1982) interpreted the paleoclimate during deposition of the Goliad Formation as arid to semi-arid and lacking vegetation that would have stabilized channel levees. The presence of pedogenic profiles associated with exposure surfaces in floodplain mudstones is consistent with a semi-arid to semi-humid environment, but the vertebrate fauna suggests that vegetation, both grasses and trees/shrubs, was abundant.
Two samples of the overbank mudstones above and below the bone bed at TMM 30898 were analyzed for palynology, but proved to be barren. No other plant fossils are known in association with the Lapara Creek Fauna; however, small root casts are very common in the screen washing concentrate from TMM 30898. These probably represent grasses or small plants growing on the floodplain or in the pond margin environment. Fossil logs as long as 10 feet in length were noted in the Survey notes from the Ten Mile Waterhole Creek locality although none of that material was apparently collected. Although there is almost no direct paleofloral evidence for climatic conditions from the Goliad Formation, the sedimentary facies and the composition of the vertebrate fauna suggest a woodland-grassland mosaic associated with a low relief fluvial environment with extensive interfluve floodplains and shallow ponds. The ungulates include both browsing and grazing forms, and the abundance of large mammals, including Blancotherium and Teleoceras, suggest abundant vegetation.
The Lapara Creek Fauna is typical of the Clarendonian Chronofauna (Webb, 1983; Tedford et al., 1987) and includes few endemic forms. Many of the taxa are known from correlative faunas of the Great Plains and some reflect broader distributions into the southwest (Southern Rockies) and eastward to Florida. As noted in numerous publications (Behrensmeyer et al., 1992; Webb and Opdyke, 1995; Janis et al., 2002), the faunal composition of the Clarendonian Chronofauna was similar to the structure of the savanna faunas of Africa today with a diverse ungulate fauna, a proboscidean (cf. Gomphotherium, Blancotherium buckneri), a hippo-like rhino (Teleoceras), a giraffe-like camel (Aepycamelus), a limited suite of carnivore taxa (Eucyon, Aelurodon, Ischyrocyon), a crocodilian, wading birds, turtles and fish. There is little evidence that the paleoenvironments and faunas of the Texas coastal plain in the early Clarendonian were significantly different from much of the mid-continent. Global temperatures were generally warmer, and the thermal gradient was less pronounced during the Miocene than today (Pound et al., 2012).
The depositional facies of the Goliad Formation, including fluvial and floodplain strata with local ponds, is consistent with a mixed woodland-grassland savanna mosaic that was widespread during the Clarendonian in North America (Stromberg, 2005). This condition apparently persisted until the middle Hemphillian, when the Clarendonian Chronofauna was replaced (Webb and Opdyke, 1995) in association with expansion of C4 grasslands (Wang et al., 1994). During the Clarendonian and early Hemphillian, paleobotanical evidence from North America suggests that a drying climate was reflected by the warm temperate mixed evergreen and broadleaf biome becoming more restricted and transitioning to more open biomes (Pound, et. al., 2012). Fossil phytoliths have been interpreted to suggest that open-habitat grasses were common in the northern Great Plains by the early Miocene even if C4 grasses weren’t widespread until the late Miocene (Stromberg, 2005).
It is now generally accepted that C4 grasslands only became widespread during the Hemphillian (Cerling et al., 1993, 1997). However, the complexity of grassland ecosystems and the mechanisms that influenced this expansion are still not well understood (Edwards, et al., 2010). While evidence for C4 grasses is known from the early Clarendonian Dove Spring Formation, isotopic data from enamel of hypsodont horse teeth in those deposits indicate a diet dominated by C3 grasses (Cerling et al., 1998). In a recent study of isotopic data from fossil mammal teeth from late Barstovian faunas in Nebraska, Nguy (2017) found that only a single taxon, Neohipparion, exhibited clear evidence for a dominantly C4 diet. Nguy (2017) concluded that the Barstovian of Nebraska was characterized predominantly by open woodland, bushland or wooded savanna environments as the majority of fossils analyzed exhibited a dominantly C3 diet with small amounts (~5%) of C4 vegetation. Feranec and Pagnac (2013) studied enamel geochemistry from a variety of fossil herbivores from the Barstow Formation in the Mojave Desert. They found carbon isotopic values, especially in the equids, indicative of some C4 plants (<20%) in the diets of these mammals.
The Lapara Creek Fauna suggests that the very diverse equids had transitioned to a grazing community by Cl1 time based on the dominance of hypsodonty. A more diverse browsing and mixed feed community was still represented by a number of taxa including artiodactyls and proboscideans.
Artiodactyl teeth, appropriate for quantitative estimation of hypsodonty [following Janis et al. (2004) and using unworn or slightly worn second and third molars], are rare but yielded the following estimations: Aepycamelus: 2.1 (slightly worn m3), Procamelus: 2.1 (moderately worn m3), Teleoceras: 1.3-1.8 (slightly worn to unworn M3). These generally fall within the range of mesodont teeth (1.5-3.5 HI) commonly attributed to mixed feeders. “Prosthennops”, Hypohippus and the gomphotheres are the only truly brachydont browsers for which HI estimates could be made, although taxa such as Cranioceras, Pseudoceras and some oreodonts were noted as brachydont elements in other North American faunas of similar age by Janis et al. (2004). Therefore, the Lapara Creek Fauna seems to have been composed of a dominantly equine grazing community, a mixed feed artiodactyl community, and a more limited number of true browsers. Together with the depositional facies, the faunal composition is consistent with a mixed woodland-grassland savanna mosaic environment developed along low-relief fluvial systems and associated floodplains with local ponds.
Although the Lapara Creek Fauna is generally consistent with the notional Clarendonian Chronofauna, some apparent differences may reflect local paleoenvironmental conditions of the Texas coastal plain. For example, while the low-crowned browsing equids (Hypohippus, Megahippus) persist throughout much of the temporal range of the Clarendonian Chronofauna farther north, Hypohippus barely persists into early Cl1 time in south Texas.
CONCLUSIONS
The Lapara Creek Fauna is the best example of early Clarendonian life in the Texas coastal plain. The collection, that including more than 3900 specimens, was obtained largely through the efforts of the Statewide Paleontologic and Mineralogic Survey during 1939-1941. This project was a joint effort between the Bureau of Economic Geology and the Works Progress Administration and employed numerous workers in both the field and laboratory. Four main collecting localities are treated as four separate local faunas due to geographic and stratigraphic variability. Both stratigraphic and faunal evidence suggests that the local faunas can be arranged chronologically in the following sequence from oldest to youngest: Ten Mile Waterhole Creek Local Fauna, Bridge Ranch Local Fauna, Farish Ranch Local Fauna and Buckner Ranch Local Fauna.
Fifty species of fossil vertebrates include five species of fish, seven species of reptiles, two species of birds and 36 species of mammals. The 36 species of mammals represent 31 genera of which four are rodents, five are carnivores, two are proboscideans, 10 are artiodactyls and 10 are perissodactyls. The four separate local faunas are distributed from the lower to middle parts of the Goliad Formation and are Clarendonian in age. The Ten Mile Waterhole Creek and Bridge Ranch Local Faunas compare well with Cl1 faunas in North America while the Farish Ranch and Buckner Ranch Local Faunas compare well with early Cl2 faunas. This assemblage of fossil vertebrates includes first, or at least very early, occurrences of cf. Trachemys sp., Apalone sp., Alligator cf. mississippiensis and cf. Eucyon sp. Blancotherium buckneri is a new generic name assigned to a longirostrine gomphothere that is represented by numerous specimens from the Buckner Ranch Local Fauna. The equid fauna is diverse with 12 different species distributed across nine different genera. The final transition locally from brachydont to hypsodont equids may be reflected by the last occurrence of the low-crowned Hypohippus at Ten Mile Waterhole Creek. The protoceratid dominated artiodactyl community seen in the Barstovian Cold Spring Fauna transitions to a camelid dominated community with the last occurrence of Synthetoceras in the Farish Ranch Local Fauna and the appearance of Nothotylopus, Aepycamelus, Procamelus grandis and Protolabis cf. yavapaiensis in the Farish Ranch and Buckner Ranch Local Faunas. The last occurrence of Synthetoceras is locally coincident with the last occurrence of the antilocaprid Ramoceros ramosus and the paleomerycid Cranioceras teres. The composition of the fauna is consistent with the widespread Clarendonian Chronofauna and with a mixed woodland-grassland environment on a broad floodplain associated with low-gradient rivers.
No articulated skeletons were recovered from the Goliad Formation although anomalous concentrations of gomphothere remains at Buckner Ranch and of rhino remains at Farish Ranch included cranial and post-cranial material in close proximity. It is unclear if these two anomalous concentrations represent mass-mortality events or if they reflect variability in local environment and local faunal distribution. At TMM 30896 (Buckner Ranch), Survey quarterly reports and Sellards (1940a, figure 4) both noted the concentration of fossils in mudstones that seemed to infill swales in the underlying sandy interval within the Goliad Formation. These were interpreted as representing local pond deposits within the fluvial floodplain environment. However, the rhino concentration at Farish Ranch was collected from fluvial sandstones and gravels. Teleoceras has been previously interpreted to have exhibited a hippopotamus-type lifestyle and may have lived in close association with the Goliad rivers.
At the Ten Mile Waterhole Creek and Bridge Ranch localities, fossils were associated with fluvial sandstones and all specimens were isolated. In these localities, the fossils most probably reflect material that had accumulated on the floodplain, were disarticulated through decay and scavenging, and then were “collected” by fluvial processes into local accumulations.
CT scanning proved to be very useful for examining the internal structure of two mandibles, one from a camel and one from a peccary. CT data conclusively demonstrated the association of two premolars that had been broken off the camel jaw and re-attached by Survey preparators with no obvious “fit”. CT scanning was preferred as an initial, non-invasive approach, as this specimen had been designated a holotype by a previous author. Similarly, CT scanning allowed un-erupted cheek teeth in the peccary jaw to be imaged and described without physically dismantling the specimen. This permitted a more confident identification of the taxon.
Work will continue on selected elements of the Lapara Creek Fauna. Additional screen washing will hopefully add to our knowledge of the rodent fauna. There are still ~86 specimens including skulls, tusks, mandibles and post-cranial elements of the Buckner Ranch gomphothere (presumably Blancotherium buckneri) in plaster jackets. Preparation of this material is ongoing, but is labor intensive given the style of fossil preservation. Stable isotope analysis will be performed to further evaluate the diets and paleoenvironments associated with the Lapara Creek Fauna.
ACKNOWLEDGEMENTS
J. and L. Blackburn graciously provided access to their ranch as well as helped me locate original Survey localities and scouted additional outcrops of the Goliad Formation along Blanco Creek. D.W. Grant provided access to his ranch as did his father to the Survey in 1939. Although only a young boy at the time, he remembered where the Survey excavation had been located. Robert Bridge spent a day enthusiastically guiding me around Medio Creek localities where the Survey collected the Bridge Ranch Local Fauna. R. Mostyn provided access to his ranch on Medio Creek. D. Calame and his crew helped confirm the original Buckner Ranch Site 1 locality on Blanco Creek with skillful backhoe work and research into the Berclair Terrace stratigraphy and archeology. P. Hughes also assisted in the search for the Site 1 locality. D. Lambert provided useful discussions on the gomphotheres. R. Hulbert provided helpful input on the horses. M. Emery provided useful discussion on the oreodont. B. Jacobs helped with the attempted recovery of pollen from the Goliad Formation. A. Cohen helped in the quest to identify Vertebrata Indeterminate. L. Boucher helped with microphotography at The Non-Vertebrate Paleontology Laboratory. Numerous colleagues provided help at the Texas Vertebrate Paleontology Collections. E. Lundelius and M. Brown helped with numerous discussions about Texas paleontology and the Survey archives. C. Sagebiel assisted with his knowledge of the curation of the Lapara Creek specimens and with the database. K. Bader helped with the modern osteology collection and with preparation of specimens. D. Wagner helped with specimen preparation and supervised a number of students in the preparation laboratory. University of Texas students B. Chapman, M. Chiappone, D. Ledesma, C. Malone, G. Self, Y. Shinoda and L. Staskus assisted in the preparation laboratory with financial support from the Geology Foundation and the Discovering Texas Fossils HornRaiser campaign. D. Knapp-Smith volunteered much appreciated efforts in the preparation lab. J. Lively assisted with photography and discussions about turtles. J. Jarvis provided access to the Texas Archeological Research Laboratory archives. M. Collins provided access to notes and papers by G. Evans. D. Trombatore at the Walter Geology Library and C. Lyon at the University of Texas Libraries were instrumental in getting the Survey reports digitized and available online. J. Maisano and M. Colbert provided data obtained at The University of Texas at Austin High Resolution X-ray Computed Tomography (CT) Facility. Special thanks to M. May who helped with field work, scanned photos at the Texas Archeological Research Laboratory and significantly improved the manuscript by reading and editing it twice. The manuscript was also improved by technical reviews from S. Jasinski and one anonymous reviewer.
REFERENCES
Agassiz, L. 1857. North American Testudinata. Contributions to the Natural History of the United States of America, First Monograph. 1:233-452. Little, Brown and Co., Boston, Massachusetts. https://doi.org/10.5962/bhl.title.12644
Ameghino, F. 1904. Reserches de morphologie phylogenetique sur les molaires superiors des ongules. Anales del Museo Nacional de Historia Natural de Buenos Aires, 3:1-541. https://doi.org/10.5962/bhl.title.15704
Auffenberg, W. 1976. The genus Gopherus (Testudinae): Pt. 1. Osteology and relationships of extant species. Bulletin of the Florida State Museum, Biological Sciences, 20:47-110.
Barbour, E.H. 1925. Prosthennops xiphidonticus, sp. nov. A new fossil peccary from Nebraska. Bulletin of the University of Nebraska State Museum, 1:25-32.
Barbour, E.H. and Sternberg, G. 1935. Gnathabelodon thorpei, gen. et sp. nov. A new mud-grubbing mastodont. Bulletin of the Nebraska State Museum, 1:395-404.
Barnes, V.E. 1992. Geologic map of Texas. The University of Texas at Austin, Bureau of Economic Geology, State Map No. 3.
Baskin, J.A. 1979. Small mammals of the Hemphillian age White Cone Local Fauna, northeastern Arizona. Journal of Paleontology, 53:695-708.
Baskin, J.A. 1991. Early Pliocene horses from late Pleistocene fluvial deposits, Gulf Coastal Plain, south Texas. Journal of Paleontology, 65:995-1006. https://doi.org/10.1017/S0022336000033308
Baskin, J.A. and Hulbert, R.C. 2012. A late Clarendonian local fauna from the Goliad Formation of south Texas. Paludicola, 8:187-193.
Behrensmeyer, A.K., Damuth, J.D., DiMichele, W.A., Potts, R., Sues, H-D., and Wing, S. 1992. Terrestrial Ecosystems Through Time. Evolutionary Paleoecology of Terrestrial Plants and Animals. The University of Chicago Press, Chicago, Illinois. https://doi.org/10.1046/j.1420-9101.1993.6050767.x
Bell, T. 1828. Characters of the order, families, and genera of the Testudinata. Zoological Journal, London, 3:513-616.
Bickart, K.J. 1990. Recent advances in the study of Neogene fossil birds. 1. The birds of the late Miocene-early Pliocene Big Sandy Formation, Mohave County, Arizona. Ornithological Monographs, 44:1-72. https://doi.org/10.2307/40166673
Bonaparte, C.L. 1845. Catalogo Metodico dei Mammiferi Europei. Giacomo Pirola, Milan, Italy. 36 p. https://doi.org/10.5962/bhl.title.77311
Bonaparte C.L. 1854. Coup d’œil sur les pigeons. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences, Séance du 6 Novembre, 39:869-880.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia, for the use of Students and Travelers. J. Smith, Paris. 115 p.
Brandt, J.F. 1855. Beiträge zur nahern Kenntniss der Säugethiere Russlands. Mémoires de l'Académie Impériale des Sciences de St. Pétersbourg, 6(9):1-375.
Brattstrom, B.H. 1961. Some new fossil tortoises from western North America with remarks on the zoogeography and paleoecology of tortoises. Journal of Paleontology, 35:543-560.
Brochu, C.A. 1999. Phylogenetics, taxonomy, and historical biogeography of Alligatoroidea. Journal of Vertebrate Paleontology, 19(S2):9-100. https://doi.org/10.1080/02724634.1999.10011201
Bryant, J.D. 1991. New early Barstovian (middle Miocene) vertebrates from the upper Torreya Formation, eastern Florida Panhandle. Journal of Vertebrate Paleontology, 11:472-489. https://doi.org/10.1080/02724634.1991.10011416
Cerling, T.E., Ehleringer, J.R., and Harris, J.M. 1998. Carbon dioxide starvation, the development of C4 ecosystems, and mammalian evolution. Philosophical Transactions of the Royal Society of London, 353:159-171. https://doi.org/10.1098/rstb.1998.0198
Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J., Elsenmann, V., and Ehleringer, J.R. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature, 389:153-158. https://doi.org/10.1038/38229
Cerling, T.E., Wang, Y., and Quade, J. 1993. Expansion of C4 ecosystems as an indicator of global ecological change in the late Miocene. Nature, 361:344-345. https://doi.org/10.1038/361344a0
Christiansen, P. 2004. Body size in proboscideans, with notes on elephant metabolism. Zoological Journal of the Linnaean Society, 140:523-549. https://doi.org/10.1111/j.1096-3642.2004.00113.x
Colbert, E.H. 1938. Pliocene peccaries from the Pacific Coast region of North America. Publications of the Carnegie Institution of Washington, 487:241-269.
Cope, E.D. 1887. Zittel's Manual of Palaeontology. American Naturalist, 21:1014-1019. https://doi.org/10.1086/274597
Cope, E.D. 1868. On the origin of genera. Proceedings of the Academy of Natural Sciences of Philadelphia, 1868:242-300.
Cope, E.D. 1874. Notes on the Santa Fe marls and some of the contained vertebrate fossils. Proceedings of the Philadelphia Academy of Natural Sciences, 1984:147-152.
Cope, E.D. 1876. On a new genus of Camelidae. Proceedings of the Academy of Natural Sciences of Philadelphia, 28:144-147.
Cope, E.D. 1878. Descriptions of new Vertebrata from the upper Tertiary formations of the west. Proceedings of the American Philosophical Society, 17:219-231.
Cope, E.D. 1881. Review of the Rodentia of the Miocene period of North America. American Naturalist, 15:586-587. https://doi.org/10.5962/bhl.title.135451
Cope, E.D. 1889. A review of the North American species of Hippotherium. American Philosophical Society, Proceedings, 26:429-458.
Corner, R.G. 1975. The early Valentinian peccary, Dyseohyus xiphidonticus (Barbour), new combination. Proceedings of the Nebraska Academy of Sciences, 85:39.
Cristol, J. de. 1832. No title: description of Hipparion. Annales Science Industrie, Midi, France, 1:180-1.
Cuvier, G. 1807. Sur les différentes especes de crocodiles vivans et sur leurs caracteres distinctifs. Annales du Muséum d'Histoire Naturelle, Paris t, 10:8-66.
Cuvier, C. 1817. Le règne animal distribué d'après son organisation, pour servir de base à l'histoire naturelle des animaux et d'introduction à l'anatomie comparée. Avec figures, dessinées d'après nature. Tome II, contenant les reptiles, les poissons, les mollusques et les annélides. Paris, Chez Deterville, Libraire.
Cuvier, G. 1825. Recherches sur les Ossemens Fossils, our l’ on Rètablit les Charactèrs des Plusieurs Animax don't les Révolutions du Globe ont Détruit les Espèces 3. G. Dufour et E. d'Ocagne, Paris.
Czaplewski, N.J. 2008. Miocene vertebrates from Ogallala Formation sites in western Oklahoma, p. 1-14. In Lucas, S.G., Morgan, G.S., Spielmann, J.A., and Prothero, D.R. (eds.), Neogene Mammals. New Mexico Museum of Natural History and Science Bulletin 44.
Czaplewski, N.J. 2012. A Mylagaulus (Mammalia, Rodentia) with nasal horns from the Miocene (Clarendonian) of western Oklahoma. Journal of Vertebrate Paleontology, 32:139-150. https://doi.org/10.1080/02724634.2012.620677
Dalquest, W.W., Baskin, JA., and Schultz, G.E. 1996. Fossil mammals from a late Miocene (Clarendonian) site in Beaver County, Oklahoma, p. 107-137. In Genoways, H.H., and Baker, R.J. (eds.), Contributions in Mammalogy. A Memorial Volume Honoring Dr. J. Knox Jones, Jr. Lubbock, Texas, Museum of Texas Tech University.
Daudin, F.M. 1801. Histoire Naturelle, Générale et Particulière des Reptiles; Ouvrage Faisant Suite à l'Histoire Naturelle Générale et Particulière, Composée par Leclerc de Buffon; et Rédigée par C.S. Sonnini, Membre de Plusieurs Sociétés Savantes. Volume 2. Paris, De Limprimerie De F. Dufart. https://doi.org/10.5962/bhl.title.60678
Dawson, M.R. 1958. Later Tertiary Leporidae of North America. University of Kansas Paleontological Contributions, Vertebrata, 6:1-75.
de Bonis, L., Bouvrain, G., Geraads, D., and Koufos, G. 1992. Diversity and paleoecology of Greek late Miocene mammalian faunas. Palaeogeography, Palaeoclimatology, Palaeoecology, 91:99-121. https://doi.org/10.1016/0031-0182(92)90035-4
Dice, L.R. 1917. Systematic position of several American Tertiary lagomorphs. University of California Publication, Bulletin of the Department of Geological Sciences, 10:179-183. https://doi.org/10.2307/41168738
Dumble, E.T. 1894. The Cenozoic deposits of Texas. Journal of Geology, 2:549-567. https://doi.org/10.1086/607017
Dunson W.A. and Seidel M.E. 1986. Salinity tolerance of estuarine and insular emydid turtles (Pseudemys nelsoni and Trachemys decussata). Journal of Herpetology, 20:237-245. https://doi.org/10.2307/1563949
Eastman, J.T. 1977. The pharyngeal bones and teeth of catostomid fishes. American Midland Naturalist, 97:68-88. https://doi.org/10.2307/2424686
Edwards, E.J., Osborne, C.P., Strömberg, C.A.E., Smith, S.A., and the C4 Grasses Consortium. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science, 328:587-591. https://doi.org/10.1126/science.1177216
Ehret, D.J. 2007. Skeletochronology: a method for determining the individual age and growth of modern and fossil tortoises (Reptilia: Testudines). Bulletin of the Florida Museum of Natural History, 47:49-72.
Evans, G.L. 1939. Manual of Suggested Procedures to be Employed by the State-wide Paleontologic-Mineralogic Survey in Texas. Bureau of Economic Geology, Austin, Texas. http://hdl.handle.net/2152/63113
Feranec, R.S. and Pagnac, D. 2013. Stable carbon isotope evidence for the abundance of C4 plants in the middle Miocene of southern California. Palaeogeography, Palaeoclimatology, Palaeoecology, 388:42-47. https://doi.org/10.1016/j.palaeo.2013.07.022
Fischer de Waldheim, G. 1817. Adversaria zoological. Memoir Societe Naturelle (Moscow), 5:368-428.
Forsten, A. M. 1975. The fossil horses of the Texas Gulf Coastal Plain: a revision. Texas Memorial Museum Pierce-Sellards Series, 22:1-86.
Franz, R. and Quitmyer, I.R. 2005. A fossil and zooarchaeological history of the gopher tortoise (Gopherus polyphemus) in the southeastern United States. Bulletin of the Florida Museum of Natural History, 45:179-199.
Frick, C. 1933. New remains of trilophodont-tetrabeleodont mastodons. Bulletin of the American Museum of Natural History, 59:505-552.
Frick, C. 1937. Horned ruminants of North America. Bulletin of the American Museum of Natural History, 69:1-669.
Frick, C. and Taylor, B.E. 1971. Michenia, a new Protolabine (Mammalia, Camelidae) and a brief review of the early taxonomic history of the genus Protolabis. American Museum Novitates, 2444:1-24.
Fritz, U., Stuckas, H., Vargas-Ramirez, M., Hunsdorfer, A.K., Maran, J., and Packert, M. 2012. Molecular phylogeny of Central and South American slider turtles: implications for biogeography and systematics (Testudines: Emydidae: Trachemys). Journal of Zoological Systematics and Evolutionary Research, 50:125-136. https://doi.org/10.1111/j.1439-0469.2011.00647.x
Gardner, J.D. and Russell, A.P. 1994. Carapacial variation among soft-shelled turtles (Testudines: Trionychidae), and its relevance to taxonomic and systematic studies of fossil taxa. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 193:209-244.
Gazin, C. L. 1930. A Tertiary vertebrate fauna from the upper Cuyama drainage basin, California. Carnegie Institution of Washington Publication, 404:55-76.
Gazin, C.L. and Collins, R.L. 1950. Remains of land mammals from the Miocene of the Chesapeake Bay region. Smithsonian Miscellaneous Collections, 116:1-21.
Gidley, J.W. 1903. A new three-toed horse. Bulletin of the American Museum of Natural History, 19:465-476.
Gidley, J.W. 1904. New or little known mammals from the Miocene of South Dakota; American Museum Expedition of 1903. Bulletin of the American Museum of Natural History, 20:241-268.
Gill, T.N. 1861. Synopsis of the subfamily of Percinae. Proceedings of the Academy of Natural Sciences of Philadelphia, 13:44-52.
Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G. 2012. The Geologic Time Scale 2012 (first edition). Elsevier, Oxford. https://doi.org/10.1016/B978-0-444-59425-9.00029-9
Gray, G. R. 1840. A List of the Genera of Birds, with an Indication of the Typical Species of each Genus. Richard and John E. Taylor, London. https://doi.org/10.5962/bhl.title.13777
Gray, J.E. 1821. On the natural arrangements of vertebrose animals. London Medical Repository Review, 15:296-310.
Gray, J.E. 1825. A synopsis of the genera of reptiles and amphibia, with a description of some new species. Annals of Philosophy, 10:193-217.
Gray, J.E. 1844. Catalogue of Tortoises, Crocodilians, and Amphisbaenians in the collection of the British Museum. British Museum of Natural History, London. https://doi.org/10.5962/bhl.title.21639
Gray, J.E. 1868. Synopsis of the species of Saccomyinae, or pouched mice, in the collection of the British Museum. Proceedings of the Scientific Meetings of the Zoological Society of London, 199-206.
Green, M. 1956. The lower Pliocene Ogallala-Wolf Creek vertebrate fauna, South Dakota. Journal of Paleontology, 30:146-169.
Gregory, J.T. 1939. Two new camels from the late lower Pliocene of South Dakota. Journal of Mammalogy, 20:366-368. https://doi.org/10.2307/1374267
Gregory, J.T. 1942. Pliocene vertebrates from Big Spring Canyon, South Dakota. University of California Publications, Bulletin of the Department of Geological Sciences, 26:307-446.
Gustafson, E.P. 2012. New records of rhinoceroses from the Ringold Formation of central Washington and the Hemphillian-Blancan boundary. Journal of Vertebrate Paleontology, 32:727-731. https://doi.org/10.1080/02724634.2012.658481
Haeckel, E. 1866. Generelle Morphologie der Organismen: Allgemeine Grundzuge der Organischen Formen-Wissenschaft, Mechanisch Begrundet durch die von Charles Darwin Reformierte Descendenz-Theorie 1 & 2. Georg Reimer, Berlin.
Harrison, J.A. 1985. Giant camels from the Cenozoic of North America. Smithsonian Contributions to Paleobiology, 57:1-29. https://doi.org/10.5479/si.00810266.57.1
Hatcher, B. 1894. A median horned rhinoceros from the Loup Fork beds of Nebraska. American Geologist, 13:149-150.
Hatcher, J. B. 1893. On a small collection of vertebrate fossils from the Loup Fork beds of northwestern Nebraska, with note on the geology of the region. The American Naturalist, 28:236-248. https://doi.org/10.1086/275895
Hay, O.P. 1902. Bibliography and catalogue of the fossil Vertebrata of North America. United States Geological Survey Bulletin, 179:868. https://doi.org/10.5962/bhl.title.20094
Hay, O.P. 1908. The fossil turtles of North America. Carnegie Institution of Washington Publication, 75:1-568. https://doi.org/10.5962/bhl.title.12500
Hay, O.P. 1922. Further observations on some extinct elephants. Proceedings of the Biological Society of Washington, 35:97-101.
Hay, O.P. 1929. Second bibliography and catalogue of the fossil Vertebrata of North America. Publications of the Carnegie Institute of Washington, 390:1-2003.
Henshaw, P.C. 1940. A Tertiary mammalian fauna from the Avawatz Mountains, San Bernardino County, California. In Studies of Cenozoic vertebrates and stratigraphy of western North America. Carnegie Institution of Washington Publication, 514:1-30.
Hesse, C.J. 1935. A vertebrate fauna from the type locality of the Ogallala Formation. University of Kansas Science Bulletin, 22:79-117.
Hesse, C.J. 1936. Lower Pliocene vertebrate fossils from the Ogallala Formation (Lavern Zone) of Beaver County, Oklahoma. Carnegie Institution of Washington Publication, 476:47-72.
Hesse, C.J. 1942. Vertebrate paleontology in Texas. Bulletin of the Texas Archeological and Paleontological Society, 14:97-119.
Hibbard, C. W. 1960. An interpretation of Pliocene and Pleistocene climates in North America. Michigan Academy of Science, Annual Report, 62:5-30.
Hirschfeld, S.E. and Webb, S.D. 1968. Plio-Pleistocene megalonychid sloths of North America. Bulletin of the Florida State Museum, Biological Sciences, 12:213-296.
Hoel, H.D. 1982. Goliad Formation of the South Texas Gulf Coastal Plain: Regional Genetic Stratigraphy and Uranium Mineralization. Unpublished Master’s Thesis. The University of Texas at Austin, Austin, Texas.
Holman, J.A. 1975. Herpetofauna of the WaKeeney local fauna (lower Pliocene: Clarendonian) of Trego County, Kansas. University of Michigan Papers on Paleontology, 12:49-66.
Holman, J.A. and Corner, R.G. 1985. A Miocene Terrapene (Testudines: Emydidae) and other Barstovian turtles from South-Central Nebraska. Herpetologica, 41:88-93.
Holman, J. A. and Fritz, U. 2005. The box turtle genus Terrapene (Testudines: Emydidae) in the Miocene of the U.S.A. The Herpetological Journal, 15:81-90.
Holman, J.A. and Parmley, D. 2005. Noteworthy turtle remains from the late Miocene (late Hemphillian) of northeastern Nebraska. Texas Journal of Science, 57:307-316.
Honey, J.G. 2007. Family Camelidae, p. 177-188. In Prothero, D.R. and Foss, S.E. (eds.), The Evolution of Artiodactyls. The Johns Hopkins University Press. Baltimore, Maryland.
Honey, J.G., Harrison, J.A., Prothero, D.R., and Stevens, M.S. 1998. Camelidae, p. 439-462. In Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Honey, J.G. and Taylor, B.E. 1978. A generic revision of the Protolabidini (Mammalia, Camelidae), with a description of two new protolabidines. Bulletin of the American Museum of Natural History, 161:371-425.
Hubbs, C.L. and Hibbard, C.W. 1951. Ictalurus lambda, a new catfish, based on a pectoral spine from the lower Pliocene of Kansas. Copeia, 1951:8-14. https://doi.org/10.2307/1438044
Hulbert, R.C. 1987a. Phylogenetic systematics, biochronology, and paleobiology of Late Neogene horses (Family Equidae) of the Gulf Coastal Plain and the Great Plains. Unpublished Ph.D. dissertation. University of Florida, Gainesville, Florida. https://doi.org/10.5962/bhl.title.42484
Hulbert, R.C. 1987b. Late Neogene Neohipparion (Mammalia: Equidae) from the Gulf Coastal Plain of Florida and Texas. Journal of Paleontology, 61:809-830. https://doi.org/10.1017/S0022336000029152
Hulbert, R.C. 1988a. Calippus and Protohippus (Mammalia, Perissodactyla, Equidae) from the Miocene (Barstovian-early Hemphillian) of the Gulf Coastal Plain. Bulletin of the Florida State Museum, Biological Sciences, 32:221-340.
Hulbert, R.C. 1988b. Cormohipparion and Hipparion (Mammalia, Perissodactyla, Equidae) from the Late Neogene of Florida. Bulletin of the Florida State Museum, Biological Sciences, 33:229-338.
Hulbert, R.C. 1989. Phylogenetic interrelationships and evolution of North American Late Neogene Equinae, p. 176-196. In Prothero, D.R. and Schoch, R.M. (eds.), The Evolution of Perissodactyls. Oxford University Press, New York.
Hulbert, R.C. 1993. Late Miocene Nannippus (Mammalia: Perissodactyla) from Florida, with a description of the smallest hipparionine horse. Journal of Vertebrate Paleontology, 13:350-366. https://doi.org/10.1080/02724634.1993.10011515
Hulbert, R.C. 2001. The Fossil Vertebrates of Florida. University of Florida Press, Gainesville, Florida. 368 p.
Hulbert, R.C. and MacFadden, B.J. 1991. Morphological transformation and cladogenesis at the base of the adaptive radiation of Miocene hypsodont horses. American Museum Novitates, 3000:1-61.
Hulbert, R.C. and Whitmore F.C. 2006. Late Miocene mammals from the Mauvilla local fauna, Alabama. Bulletin of the Florida Museum of Natural History, 46:1-28.
Hunt, R.M., Jr. 1998. Amphicyonidae, p. 196-227. In Janis, C., Scott, K., and Jacobs, L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Hutchison, J.H. 1968. Fossil Talpidae (Insectivora, Mammalia) from the late Tertiary of Oregon. Bulletin of the Museum of Natural History, University of Oregon, 11:1-117.
Illiger, J. 1811. Prodromus Systematis Mammalium et Avium. C. Salfeld, Berlin. https://doi.org/10.5962/bhl.title.106965
Isphording, W.C. and Lamb, G.M. 1971. Age and origin of the Citronelle Formation in Alabama. Geological Society of America Bulletin, 82:775-780. https://doi.org/10.1130/0016-7606(1971)82[775:AAOOTC]2.0.CO;2
James, G.T. 1963. Paleontology and nonmarine stratigraphy of the Cuyama Valley Badlands, California, Part I, geology, faunal interpretations, and systematic descriptions of Chiroptera, Insectivora, and Rodentia. University of California Publications in Geological Sciences, 451:1-154.
Janis, C.M., J. Damuth, J.M. Theodor. 2002. The origins and evolution of the North American grassland biome: the story from the hoofed mammals. Palaeogeography, Palaeoclimatology, Palaeoecology, 177:183-198. https://doi.org/10.1016/S0031-0182(01)00359-5
Janis, C.M. and Manning, E. 1998. Antilocapridae, p. 491-507. In Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.) 1998. Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Jasinski, S.E. 2013. Review of the fossil Trionychidae (Testudines) from Alabama, including the oldest record of trionychid turtles from eastern North America. Bulletin of the Alabama Museum of Natural History, 31:46-59.
Jasinski, S.E. 2015. Middle Miocene Carnivora of New Mexico (Tesuque Formation): species patterns, richness, and faunal turnover. New Mexico Museum of Natural History and Science Bulletin, 67:89-105.
Jasinski, S.E. 2018. A new slider turtle (Testudines: Emydidae: Deirochelyinae: Trachemys) from the late Hemphillian (late Miocene/early Pliocene) of eastern Tennessee and the evolution of the deirochelyines. PeerJ, 6:e4338. https://doi.org/10.7717/peerj.4338
Johnston, C.S. 1937. Description of a new horse, Calippus regulus from the Clarendon Beds of Donley County, Texas. American Midland Naturalist, 18:905-907. https://doi.org/10.2307/2420424
Joyce, W.A., Petricevic, A., Lyson, T.R., and Czaplewski, N.J. 2012. A new box turtle from the Miocene/Pliocene boundary (Latest Hemphillian) of Oklahoma and a redefined chronology of box turtle diversification. Journal of Paleontology, 86:177-190. https://doi.org/10.1666/11-073.1
Kelly, T.S. 2000. A new Hemphillian (late Miocene) mammalian fauna from Hoye Canyon, west central Nevada. Contributions in Science, Natural History Museum of Los Angeles County, 481:1-21.
Kelly, T.S. and Stewart, J.D. 2008. New records of middle and late Miocene Perissodactyla and Artiodactyla from the western border of the San Joaquin Valley, Diablo Range, Fresno County, California. Contributions in Science, Natural History Museum of Los Angeles County, 516:1-29.
Korth, W. 1998. Rodents and lagomorphs (Mammalia) from the late Clarendonian (Miocene) Ash Hollow Formation, Brown County, Nebraska. Annals of Carnegie Museum, 67:299-348.
Korth, W.W. 2000. Review of Miocene (Hemingfordian to Clarendonian) mylagaulid rodents (Mammalia) from Nebraska. Annals of Carnegie Museum, 69:227-280.
Lacépède, B.GE. 1803. Histoire Naturelle des Poissons, Vol. 5, P. Plassan, Paris.
Lambert, W.D. and Shoshani, J. 1998. Proboscidea, p. 606-621. In Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Lander, B.E. 1998. Oreodontoidea. P. 402-425. In Janis, C.M., Scott, K.M., Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume I: Terrestrial Carnivores, Ungulates, and Ungulate-like Mammals, Cambridge University Press, Cambridge, UK.
Lander, E. B. 2005. Revised correlation and age assignments of fossil land mammal assemblages of late Hemingfordian to earliest Hemphillian (early middle to early late Miocene) age in California, Nebraska, and Texas, based on occurrences of ticholeptine oreodonts (Mammalia, Artiodactyla, Oreodontidae, Ticholeptinae) and other land mammal taxa. Bulletin of the Southern California Academy of Sciences, 104:29-30.
Lander, B.E. 2008. Early Clarendonian (late middle Miocene) fossil land mammal assemblages from the Lake Mathews Formation, Riverside County, southern California and a preliminary review of Merychyus (Mammalia, Artiodactyla, Oreodontidae). In Wang, X. and Barnes, L. G., (eds.), Geology and vertebrate paleontology of western and southern North America, contributions in honor of David P. Whistler. Natural History Museum of Los Angeles County, Science Series, 41:181-212.
Laurenti, J.N. 1768. Specimen Medicum, Exhibens Synopsin Reptilium Emendatem cum Experimentis Circa Venena et Antidota Reptilium Austrazcorm. A. Asher, Amsterdam, 214 p. https://doi.org/10.5962/bhl.title.5108
Le, M., Duong, H.T., Dinh, L.D., Nguyen, T.Q., Pritchard, P.C.H., McCormack, T. 2014. A phylogeny of soft-shell turtles (Testudines: Trionychidae) with reference to the taxonomic status of the critically endangered, giant softshell turtle, Rafetus swinhoei. Organisms Diversity & Evolution, 14:279-293. https://doi.org/10.1007/s13127-014-0169-3
Leach, W.E. 1820. Synopsis of the Contents of the British Museum: Eleventh Room. British Museum, London. https://doi.org/10.5962/bhl.title.144385
Leidy, J. 1856. Notice of some remains of extinct Mammalia, recently discovered by Dr. F. V. Hayden in the bad lands of Nebraska. Proceedings of the Academy of Natural Sciences of Philadelphia, 8:59-60.
Leidy, J. 1858. Notice of remains of extinct Vertebrata, from the valley of the Niobrara River, collected during the exploring expedition of 1857, in Nebraska, under the command of Lieut. G.K. Warren, U. S. Topographical Engineer, by Dr. F.V. Hayden, Geologist to the expedition. Proceedings of Academy of Natural Sciences of Philadelphia, 10:20-29.
Leidy, J. 1869. The extinct mammalian fauna of Dakota and Nebraska, including an account of some allied forms from other localities, together with a synopsis of the mammalian remains of North America. Journal of the Academy of Natural Sciences of Philadelphia, 7:1-472. https://doi.org/10.5962/bhl.title.20910
Leidy, J. 1885. Rhinoceros and Hippotherium from Florida. Proceedings of the Academy of Natural Sciences of Philadelphia, 37:32-33.
Leite, M.B. 1990. Stratigraphy and mammalian paleontology of the Ash Hollow Formation (upper Miocene) on the north shore of Lake McConaughy, Keith County, Nebraska. Contributions to Geology, University of Wyoming, 28:1-29.
Li, H., Liu, J., Xiong, L., Zhang, H., Zhou, H., Yin, H., Jing, W., Li, J., Shi, Q., Wang, Y., and Nie, L. 2017. Phylogenetic relationships and divergence dates of soft-shell turtles (Testudines: Trionychidae) inferred from complete mitochondrial genomes. Journal of Evolutionary Biology, 30:1011-1023. https://doi.org/10.1111/jeb.13070
Lindsay, E. H. 1972. Small mammal fossils from the Barstow Formation, California. University of California Publications in Geological Sciences, 93:1-104.
Linnaeus, C. 1758. Systema Naturae per Regna tria Naturae, Secundum Classes, Ordines, Genera, Species cum Characeribus, Differentiis, Synonymis, Locis. Editio decima, reformata. Laurentii Salvii, Stockholm, 1. https://doi.org/10.5962/bhl.title.542
Lozinsky, R.P. and Tedford, R.H. 1991. Geology and paleontology of the Santa Fe Group, southwestern Albuquerque Basin, Valencia County, New Mexico. New Mexico Bureau of Mines and Mineral Resources, Bulletin, 132:1-39.
Lundberg, J.G. 1975. The fossil catfishes of North America. Claude W. Hibbard Memorial Volume 2. University of Michigan Museum of Paleontology, Papers on Paleontology No. 11.
Lydekker, R. 1883. Synopsis of the fossil Vertebrata of India. Records of the Geological Survey of India, 16:61-69.
MacDonald, J.R. 1956. A new Clarendonian mammalian fauna from the Truckee Formation of western Nevada. Journal of Paleontology, 30:186-202.
MacFadden, B.J. 1980. The Miocene horse Hipparion from North America and from the type locality in southern France. Palaeontology, 23:617-635.
MacFadden, B.J. 1984. Systematics and phylogeny of Hipparion, Neohipparion, Nannippus, and Cormohipparion (Mammalia, Equidae) from the Miocene and Pliocene of the New World. Bulletin of the American Museum of Natural History, 179:1-196.
MacFadden, B.J. 1998. Equidae, p. 537-559. In Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial carnivores, ungulates and ungulatelike mammals. Cambridge University Press, Cambridge, UK.
Malone, B. 1979. The Systematics, Phylogeny and Paleobiology of the Genus Alligator. unpublished Ph.D. dissertation, City University of New York, New York City, NY.
Marsh, O.C. 1874. Notice of new equine mammals from the Tertiary formation. American Journal of Science, 7:247-258. https://doi.org/10.2475/ajs.s3-7.39.247
Marsh, O.C. 1891. A horned artiodactyle (Protoceras celer) from the Miocene. American Journal of Science, 41:81-82. https://doi.org/10.2475/ajs.s3-41.241.81
Matthew, W.D. 1902. A horned rodent from the Colorado Miocene with a revision of the Mylagauli, beavers and hares of the American Tertiary. Bulletin of the American Museum of Natural History, 16:291-310.
Matthew, W.D. 1904. A complete skeleton of Merycodus. Bulletin of the American Museum of Natural History, 19:197-226.
Matthew, W.D. 1918. Contributions to the Snake Creek Fauna. Bulletin of the American Museum of Natural History, 38:183-229.
Matthew, W.D. 1924. Third contribution to the Snake Creek Fauna. Bulletin of the American Museum of Natural History, 50:59-210.
Matthew, W.D. 1926. The evolution of the horse: a record and its interpretation. The Quarterly Review of Biology, 1:139-185. https://doi.org/10.1086/394242
Matthew, W.D. and Cook, H.J. 1909. A Pliocene fauna from western Nebraska. Bulletin of the American Museum of Natural History, 26:361-414.
Matthew, W.D. and Gidley G.W. 1904. New or little known mammals from the Miocene of South Dakota, American Museum Expedition of 1903. Bulletin American Museum of Natural History, 20:241-268.
Matthew, W.D. and Stirton, R.A. 1930. Equidae from the Pliocene of Texas. Bulletin of the Department of Geological Sciences, University of California, 19:349-396.
Maximilian, Prinze zu Wied. 1839. Uber einige Nager mit Ausseren Backentaschen aus dem westlichen Nord-America. Nova acta physico-medica Academiae Caesareae Leopoldino-Carolinae Naturae Curiosum, 368-369.
May, S.R., Gray, G.G., Summa, L.L., Stewart N.R., Gehrels, G.E., and Pecha, M.E. 2013. Detrital zircon geochronology from the Bighorn Basin, Wyoming, USA: implications for tectonostratigraphic evolution and paleogeography. The Geological Society of America Bulletin, 125:1403-1422. https://doi.org/10.1130/B30824.1
McCord, R.D. 2002. Fossil history and evolution of the gopher tortoises (genus Gopherus). p. 52-66. In Van Devender, T.R. (ed.), The Sonoran Desert Tortoise, Natural History, Biology and Conservation. Arizona-Sonora Desert Museum Studies in Natural History. University of Arizona Press, Tucson, Arizona.
Mead, A.J. 2000. Sexual dimorphism and paleoecology in Teleoceras, a North American Miocene rhinoceros. Paleobiology, 26:689-706. https://doi.org/10.1666/0094-8373(2000)026<0689:SDAPIT>2.0.CO;2
Merrem, B. 1820. Versuch eines Systems der Amphibien. J.C. Krieger, Marburg, Germany. https://doi.org/10.5962/bhl.title.5037
Meylan, P.A. 1987. The phylogenetic relationships of soft-shelled turtles (Family Trionychidae). Bulletin of the American Museum of Natural History, 186:1-101.
Meylan, P.A. and Sterrer, W. 2000. Hesperotestudo (Testudines: Testudinidae) from the Pleistocene of Bermuda, with comments on the phylogenetic position of the genus. Zoological Journal of the Linnaean Society, 128:51-76. https://doi.org/10.1111/j.1096-3642.2000.tb00649.x
Mook, C.C. 1923. A new species of Alligator from the Snake Creek beds. American Museum Novitates, 73:1-13.
Mook, C.C. 1946. A new Pliocene Alligator from Nebraska. American Museum Novitates, 1311:1-12.
Morgan, G.S. 2015. Oligocene and Miocene vertebrates of New Mexico. p. 159-232. In Lucas, S.G. and Sullivan, R.M. (eds.), Vertebrate Paleontology in New Mexico. New Mexico Museum of Natural History and Science Bulletin 68.
Morton, R.A., Jirik, L.A., and Galloway, W.E. 1988. Middle-Upper Miocene Depositional Sequences of the Texas Coastal Plain and Continental Shelf. The University of Texas at Austin, Bureau of Economic Geology Report of Investigations 174. https://doi.org/10.23867/RI0174D
Mothe, D., Ferretti, M.P., and Avilla, L.S. 2016. The dance of tusks: rediscovery of lower incisors in the Pan-American proboscidean Cuvieronius hyodon revises incisor evolution in Elephantimorpha. PLoS ONE, 11:1-18. e0147009. https://doi.org/10.1371/journal.pone.0147009
Munthe, K. 1998. Canidae, p. 124-143. In Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Nguy, W.H. 2017. Middle Miocene paleoenvironmental reconstruction of the central Great Plains from stable carbon isotopes in large mammals. Unpublished Master of Science Thesis, University of Nebraska-Lincoln, Lincoln, Nebraska, USA.
Owen, R. 1841. Description of some fossil remains of Chaeropotamus, Palaeotherium, Anoplotherium and Dichobune from the Eocene formation, Isle of Wight. Transactions of the Geological Society of London, 6:41-45. https://doi.org/10.1144/transgslb.6.1.41
Owen, R. 1845. A History of British Fossil Mammals and Birds. J. Van Voorst, London. https://doi.org/10.1017/CBO9781139104029.006
Owen, R. 1848. Description of teeth and portions of jaws of the extinct anthracotheroid quadrupeds (Hyopotomus vectianus and H. bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the northwest coast of the Isle of Wight, with an attempt to develop Cuvier's idea of the classification of pachyderms by the number of their toes. The Quarterly Journal of the Geological Society of London, 4:104-141. https://doi.org/10.1144/GSL.JGS.1848.004.01-02.21
Palmer, T.S. 1897. Notes on the nomenclature of four genera of Tropical American mammals. Proceedings of the Biological Society, Washington, 11:173-174.
Parmalee, P.W., Klippel, W.E., Meylan, P.A., and Holman, J.A. 2002. A late Miocene-early Pliocene population of Trachemys (Testudines: Emydidae) from east Tennessee. Annals of Carnegie Museum, 71:233-239.
Patton, T.H. 1969. Miocene and Pliocene artiodactyls, Texas Gulf Coastal Plain. Bulletin Florida State Museum Biological Sciences, 14:115-226.
Patton, T.H. and Taylor, B.E. 1971. The Synthetoceratinae (Mammalia, Tylopoda, Protocertaidae). Bulletin of the American Museum of Natural History, 145:119-218.
Patton, T.H. and Taylor, B.E. 1973. The Protoceratinae (Mammalia, Tylopoda, Protoceratidae) and the systematics of the Protoceratidae. Bulletin of the American Museum of Natural History, 150:347-414.
Plummer, F.B. 1932. Cenozoic Systems in Texas. In Sellards, E.H., Adkins, W.S., and Plummer, F.B. (eds.), The Geology of Texas, Volume 1, Stratigraphy. The University of Texas at Austin, Bureau of Economic Geology Bulletin, 3232:519-818.
Pound, M.J., Haywood, A.M., Salzmann, U., and Riding, J.B. 2012. Global vegetation dynamics and latitudinal temperature gradients during the mid to late Miocene (15.97-5.33 Ma). Earth Science Reviews, 112:1-22. https://doi.org/10.1016/j.earscirev.2012.02.005
Prothero, D.R. 2005. The Evolution of North American Rhinoceroses. Cambridge University Press, Cambridge, UK.
Prothero, D.R. 2008. Systematics of the musk deer (Artiodactyla: Moschidae: Blastomerycinae) from the Miocene of North America, p. 207-223. In Lucas. S.G., Morgan, G.S., Spielmann, J.A., and Prothero, D.R. (eds.), Neogene Mammals. New Mexico Museum of Natural History and Science Bulletin 44.
Prothero, D.R. and Liter, M.R. 2008. Family Paleomerycidae, p. 241-248. In Prothero, D.R. and Foss, S.E. (eds.), The Evolution of Artiodactyls. The Johns Hopkins University Press. Baltimore, Maryland.
Prothero, D.R., Kelly, T.S., McCardel, K.J., and Wilson, E.L. 2008. Magnetostratigraphy, biostratigraphy, and tectonic rotation of the Miocene Caliente Formation, Ventura County, California, p. 255-272. In Lucas, S.G., Morgan, G.S., Spielmann, J.A., and Prothero, D.R. (eds.), Neogene Mammals. New Mexico Museum of Natural History and Science Bulletin 44.
Prothero, D.R. and Manning, E.M. 1987. Miocene rhinoceroses from the Texas Gulf Coastal Plain. Journal of Paleontology, 61:388-423. https://doi.org/10.1017/S0022336000028559
Quinn, J.H. 1952. Recognition of hipparions and other horses in the middle Miocene mammalian faunas of the Texas Gulf region. University of Texas Bureau of Economic Geology Report of Investigations, 14:5-6.
Quinn, J.H. 1955. Miocene Equidae of the Texas Gulf Coastal Plain. Bureau of Economic Geology, The University of Texas at Austin, 5516:1-102.
Rafinesque, C.S. 1818. Discoveries in natural history, made during a journey through the western region of the United States. American Monthly Magazine and Critical Revue, 3:354-356.
Rafinesque, C.S. 1819. Prodrome de 70 nouveaux genres d'animaux découverts dans l'intérieur des États-Unis d'Amérique, durant l'année 1818. Journal de Physique, de Chimie, d'Histoire Naturelle et des Arts, Paris, 88:417-429.
Rafinesque, C.S. 1820. Ichthyologia Ohiensis [Part 7]. Western Review and Miscellaneous Magazine, 2:355-363. https://doi.org/10.5962/bhl.title.6892
Rafinesque, C.S. 1832. Description of two new genera of soft shell turtles of North America. Atlantic Journal and Friend of Knowledge, 1:64-65.
Reynoso, V. and Montellano-Ballesteros, M. 2004. A new giant turtle of the genus Gopherus (Chelonia: Testudinidae) from the Pleistocene of Tamaulipas, México, and a review of the phylogeny and biogeography of gopher tortoises. Journal of Vertebrate Paleontology, 24:822-837. https://doi.org/10.1671/0272-4634(2004)024[0822:ANGTOT]2.0.CO;2
Schlosser, M. 1886. Beitrage zur kenntniss der Stammesgeschichte der Huftiere und versuch einer Systematik der Paar-und Unpaarhufer. Morphologisches Jahrbuch, 12:1-136.
Schultz, C.B. and Falkenbach, C.H. 1941. Ticholeptinae: a new subfamily of oreodonts. Bulletin of the American Museum of Natural History, 79:1-105.
Schultz, C.B. and Falkenbach, C.H. 1968. The phylogeny of the oreodonts, parts 1 and 2. Bulletin of the American Museum of Natural History, 139:1-498.
Schultz, C.B. and Martin, L.D. 1975. A new Kimballian peccary from Nebraska. Bulletin of the University of Nebraska State Museum, 10:35-46.
Schultz, G.E. 1990. Clarendonian and Hemphillian vertebrate faunas from the Ogallala Formation (late Miocene-early Pliocene) of the Texas Panhandle and adjacent Oklahoma, p. 56-97. In Gustavson, T.C. (ed.), Geologic Framework and Regional Hydrology: Upper Cenozoic Blackwater Draw and Ogallala Formations, Great Plains. Bureau of Economic Geology, The University of Texas at Austin, Austin, TX.
Seidel, M.E. and Jackson, D.R. 1990. Evolution and fossil relationships of slider turtles, p. 68-73. In Whitfield, G.J. (ed.), Life History and Ecology of the Slider Turtle. Smithsonian Institution Press, Washington, D.C.
Seidel, M.E. and Smith, H.M. 1986. Chrysemys, Pseudemys, Trachemys (Testudines: Emydidae): did Agassiz have it right? Herpetologica, 42:242-248.
Sellards, E.H. 1940a. Pleistocene artifacts and associated fossils from Bee County, Texas. Bulletin of the Geological Society of America, 51:1627-1658. https://doi.org/10.1130/GSAB-51-1627
Sellards, E.H. 1940b. New Pliocene mastodon. Bulletin of the Geological Society of America, 51:1659-1664. https://doi.org/10.1130/GSAB-51-1659
Semprebon, G., C. Janis, N. Solounias. 2004. The diets of the Dromomerycidae (Mammalia: Artiodactyla) and their response to Miocene vegetational change. Journal of Vertebrate Paleontology, 24:427-444. https://doi.org/10.1671/2431
Shoshani, J., Ferretti, M.P., Lister, A.M., Agenbrod, L.D., Saegusa, H., Mol, D., and Takahashi, K. 2007. Relationships within the Elephantine using hyoid characters. Quaternary International, 169:174-185. https://doi.org/10.1016/j.quaint.2007.02.003
Shoshani, J. and Marchant, G.H. 2001. Hyoid apparatus: a little-known complex of bones and its contribution to proboscidean evolution, p. 668-675. In Cavarretta, G., Gioia, P., Mussi, M., and Palombo, M.R. (eds.), Proceedings of the First International Congress of La Terra degli Elefanti, The World of Elephants. Consiglio Nazionale delle Ricerche, Rome.
Shoshani, J. and Tassy, P. 2005. Advances in proboscidean taxonomy and classification, anatomy and physiology, and ecology and behavior. Quaternary International, 126:5-20. https://doi.org/10.1016/j.quaint.2004.04.011
Simpson, G.G. 1945. The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History, 85:1-350.
Skinner, M.F. and MacFadden, B.J. 1977. Cormohipparion n. gen. (Mammalia, Equidae) from the North American Miocene (Barstovian-Clarendonian). Journal of Paleontology, 51:912-926.
Snyder, D. 2007. Morphology and systematics of two Miocene alligators from Florida, with a discussion of Alligator biogeography. Journal of Paleontology, 81:917-928. https://doi.org/10.1666/pleo05-104.1
Solis, R.F. 1981. Upper Tertiary and Quaternary Depositional Systems, Central Coastal Plain, Texas: Regional Geology of the Coastal Aquifer and Potential Liquid-waste Repositories. The University of Texas at Austin, Bureau of Economic Geology, Report of Investigations 108.
Sorkin, B. 2006. Ecomorphology of the giant bear-dogs Amphicyon and Ischyrocyon. Historical Biology, 18:375-388. https://doi.org/10.1080/08912960600618073
State-Wide Paleontologic-Mineralogic Survey. 1939. The First Quarterly Report Covering the Quarter Ending June 30, 1939. http://hdl.handle.net/2152/65941
State-Wide Paleontologic-Mineralogic Survey in Texas. 1939. The Second Quarterly Report Covering the Quarter Ending September 30, 1939. http://hdl.handle.net/2152/65942
State-Wide Paleontologic-Mineralogic Survey in Texas. 1939. The Third Quarterly Report Covering the Quarter Ending December 31, 1939. http://hdl.handle.net/2152/72400
State-Wide Paleontologic-Mineralogic Survey in Texas. 1940. The Sixth Quarterly Report Covering the Quarter Ending September 30, 1940. http://hdl.handle.net/2152/72403
State-Wide Paleontologic-Mineralogic Survey in Texas. 1941. The Eighth Quarterly Report Covering the Quarter Ending March 31, 1941. http://hdl.handle.net/2152/72405
State-Wide Paleontologic-Mineralogic Survey in Texas. 1941. The Ninth Quarterly Report Covering the Quarter Ending June 30, 1941. http://hdl.handle.net/2152/72406
State-Wide Paleontologic-Mineralogic Survey in Texas: A Federal Works Agency, Work Projects Administration Project. 1941. Final report covering the period from March 4, 1939 to September 30, 1941. http://hdl.handle.net/2152/70316
Steinmann, G. and Doderlein, L. 1890. Elemente der Palaontologie. Wilhelm Engelmann, Leipzig, Germany. 848 p. https://doi.org/10.5962/bhl.title.15050
Steven, M.S. and Stevens, J.B. 2007. Family Merycoidodontidae, p. 157-168. In Prothero, D.R. and Foss, S.E. (eds.), The Evolution of Artiodactyls. The Johns Hopkins University Press. Baltimore, Maryland.
Stirton, R.A. 1929. Artiodactyla from the fossil beds of Fish Lake Valley, Nevada. University of California Publications in Geological Sciences, 18:291-302.
Stirton, R.A. 1932. A new genus of Artiodactyla from the Clarendon lower Pliocene of Texas. University of California Publications in Geological Sciences, 21:147-168.
Stirton, R.A. 1940. Phylogeny of North American Equidae. University of California Publications, Bulletin of the Department of Geological Sciences, 25:165-198.
Stirton, R.A. and McGrew, P.O. 1935. A preliminary notice on the Miocene and Pliocene mammalian faunas near Valentine, Nebraska. American Journal of Science, 29:125-132. https://doi.org/10.2475/ajs.s5-29.170.125
Stromberg, C.A. 2005. Decoupled taxonomic radiation and ecological expansion of open-habitat grasses in the Cenozoic of North America. Proceedings of the National Academy of Sciences of the United States of America, 102:11980-11984. https://doi.org/10.1073/pnas.0505700102
Tedford, R.H., Albright, L.B., Barnosky, A.D., Ferrusquia-Villafranca, I., Hunt, R.M., Storer, J.E., Swisher, C.C., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (late Oligocene through early Pliocene epochs). p. 169-231. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York. https://doi.org/10.7312/wood13040-008
Tedford, R.H., Galusha, T., Skinner, M.F., Taylor, B.E., Fields, R.W., Macdonald, J.R., Rensberger, J.M., Webb, S.D., and Whistler, D.P. 1987. Faunal succession and biochronology of the Arikareean through Hemphillian interval (late Oligocene through earliest Pliocene epochs) in North America, p. 153-210. In Woodburne, M.O. (ed.), Cenozoic Mammals of North America, Geochronology and Biostratigraphy. University of California Press, Berkeley and Los Angeles, California.
Tedford, R.H. and Qiu Z.-x. 1996. A new canid genus from the Pliocene of Yushe, Shanxi Province. Vertebrata PalAsiatica, 34:27-40.
Tedford, R.H., Wang, X., and Taylor, B.E. 2009. Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae). Bulletin of the American Museum of Natural History, 325:1-218. https://doi.org/10.1206/574.1
Thorpe, M.R. 1923. The primitive and carnivore-like characters of the Merycoidodontidae. American Journal of Science, 6:239-246. https://doi.org/10.2475/ajs.s5-6.33.239
Trouessart, E. 1884. Catalogue des mammifères vivants et fossiles (Carnivores). Bulletin de la Société d'Études Scientifiques d'Angers, 14:1-108. https://doi.org/10.5962/bhl.title.63808
Valdes, N., Bourque, J., and Vitek, N.S. 2017. A new soft-shelled turtle (Trionychidae, Apalone) from the late Miocene of north-central Florida. Bulletin of the Florida Museum of Natural History, 55:117-138.
Vigors, N.A. 1825. A description of a new species of Scolopax lately discovered in the British Islands: with observations on the Anas glocitans of Pallas, and a description of the female of that species. Transactions of the Linnaean Society, 14:556-562. https://doi.org/10.1111/j.1095-8339.1823.tb00102.x
Vitek, N.S. and Joyce, W.G. 2015. A review of the fossil record of New World turtles of the clade Pan-Trionychidae. Bulletin of the Peabody Museum of Natural History, 56:185-244. https://doi.org/10.3374/014.056.0204
Voorhies, M.R. 1969. Taphonomy and population dynamics of an early Pliocene vertebrate fauna, Knox County, Nebraska. Contributions to Geology, University of Wyoming, Special Paper, 1:1-69. https://doi.org/10.2113/gsrocky.8.special_paper_1.1
Voorhies, M.R. and Timperley, C.L. 1997. A new Pronotolagus (Lagomorpha: Leporidae) and other leporids from the Valentine Railway Quarries (Barstovian, Nebraska), and the Archaeolagine-Leporine transition. Journal of Vertebrate Paleontology, 17:725-737. https://doi.org/10.1080/02724634.1997.10011020
Wagler, J.G. 1831. Einige Mittheilungen über Thiere Mexicos. Isis von Oken, 24:510-535.
Wang, S., Li, Y., Duangkrayom, J., Yang, X., He, W., and Chen, S. 2017. A new species of Gomphotherium (Proboscidea, Mammalia) from China and the evolution of Gomphotherium in Eurasia. Journal of Vertebrate Paleontology, 37, e1318284, 15 p. https://doi.org/10.1080/02724634.2017.1318284
Wang, X., Tedford, R. H., and Taylor, B. E. 1999. Phylogenetic systematics of the Borophaginae (Carnivora: Canidae). Bulletin of the American Museum of Natural History, 243:1-391.
Wang, Y., Cerling, T.E., and MacFadden, B.J. 1994. Fossil horses and carbon isotopes: new evidence for Cenozoic dietary, habitat, and ecosystem changes in North America. Palaeogeography, Palaeoclimatology, Palaeoecology, 107:269-279. https://doi.org/10.1016/0031-0182(94)90099-X
Webb, S.D. 1965. The osteology of Camelops. Bulletin of the Los Angeles County Museum, Science, 1:1-54.
Webb, S.D. 1969. The Burge and Minnechaduza Clarendonian mammalian faunas of north-central Nebraska. University of California Publications in Geological Sciences, 78:1-191. https://doi.org/10.1016/0031-0182(71)90046-0
Webb, S.D. 2008. Revision of the extinct Pseudoceratinae (Artiodactyla: Ruminantia: Gelocidae). Bulletin of the Florida Museum of Natural History, 48:17-58.
Webb, S.D. 1983. The rise and fall of the late Miocene ungulate fauna in North America, p. 267-306. In Nitecki, M.H. (ed.), Coevolution. University of Chicago Press, Chicago, Illinois.
Webb, S.D. and Hulbert, R.C. 1986. Systematics and evolution of Pseudhipparion (Mammalia, Equidae) from the Late Neogene of the Gulf Coastal Plain and the Great Plains, p. 237-272. In Flanagan, K.M. and Lillegraven, J.A. (eds.), Vertebrates, Phylogeny, and Philosophy: A Tribute to George Gaylord Simpson. University of Wyoming Contributions to Geology, Special Paper 3. https://doi.org/10.2113/gsrocky.24.special_paper_3.237
Webb, S.D., MacFadden, B.J., Baskin, J.A. 1981. Geology and paleontology of the Love Bone Bed from the late Miocene of Florida. American Journal of Science, 281:513-544. https://doi.org/10.2475/ajs.281.5.513
Webb, S.D. and Opdyke, N.D. 1995. 11. Global Climatic Influence on Cenozoic land Mammal Faunas. National Research Council. Effects of Past Global Change on Life. Washington, DC, The National Academies Press. https://doi.org/10.17226/4762
Weeks, A.W. 1933. Lissie, Reynosa, and upland terrace deposits of coastal plain of Texas between Brazos River and Rio Grande. Bulletin of the American Association of Petroleum Geologists, 17:453-487.
Weeks, A.W. 1945. Oakville, Cuero, and Goliad formations of Texas coastal plain between Brazos River and Rio Grande. Bulletin of the American Association of Petroleum Geologists, 29:1721-1732.
Weems, R.E. and George, R.A. 2013. Amphibians and nonmarine turtles from the Miocene Calvert Formation of Delaware, Maryland, and Virginia (USA). Journal of Paleontology, 87:570-588. https://doi.org/10.1666/12-071
Whistler, D.P. and Burbank, D.W. 1992. Miocene biostratigraphy and biochronology of the Dove Spring Formation, Mojave Desert, California, and characterization of the Clarendonian mammal age (late Miocene) in California. Geological Society of America Bulletin, 104:644-658. https://doi.org/10.1130/0016-7606(1992)104<0644:MBABOT>2.3.CO;2
White, J.A. 1987. The Archaeolaginae (Mammalia, Lagomorpha) of North America, excluding Archaeolagus and Panolax. Journal of Vertebrate Paleontology, 7:425-450. https://doi.org/10.1080/02724634.1988.10011674
White, J.A. 1991. North American Leporinae (Mammalia: Lagomorpha) from late Miocene (Clarendonian) to latest Pliocene (Blancan). Journal of Vertebrate Paleontology, 11:67-89. https://doi.org/10.1080/02724634.1991.10011376
White, T.E. 1942. A new alligator from the Miocene of Florida. Copeia, 1:3-7. https://doi.org/10.2307/1437933
Whiting, E.T., Steadman, D.W., and Kent, A.V. 2016. Cranial polymorphism and systematics of Miocene and living Alligator in North America. Journal of Herpetology, 50:306-315. https://doi.org/10.1670/15-023
Williams, E. 1950. Testudo cubensis and the evolution of western hemisphere tortoises. Bulletin of the American Museum of Natural History, 95:1-36.
Wilson, J.A. 1956. Miocene formations and vertebrate biostratigraphic units, Texas coastal plain. Bulletin of the American Association of Petroleum Geologists, 40:2233-2246.
Wilson, J.A. 1960. Miocene carnivores, Texas coastal plain. Journal of Paleontology, 34:983-1000.
Wilson, J.A. 1962. Tertiary formations between Austin and Houston with special emphasis on the Miocene and Pliocene. Geology of the Gulf Coast and Central Texas, and Guidebook of Excursions. Field Excursion No. 10:342-353.
Wood, A.E. 1937. Additional material from the Tertiary of the Cuyama Basin of California. American Journal of Science, 33:29-43. https://doi.org/10.2475/ajs.s5-33.193.29
Woodburne, M.O. 1969. Systematics, biogeography, and evolution of Cynorca and Dyseohyus (Tayassuidae). Bulletin of the American Museum of Natural History, 141:273-356.
Woodburne, M.O. 2007. Phyletic diversification of the Cormohipparion occidentale complex (Mammalia; Perissodactyla, Equidae), late Miocene, North America, and the origin of the Old World Hippotherium Datum. Bulletin of the American Museum of Natural History, 306:138. https://doi.org/10.1206/0003-0090(2007)306[1:PDOTCO]2.0.CO;2
Wright, D.B. 1991. Cranial Morphology, Systematics, and Evolution of Neogene Tayassuuidae (Mammalia). Unpublished Ph.D. dissertation. University of Massachusetts.
Wright, D.B. 1998. Tayassuidae, p. 389-401. In Janis, C.M., Scott, K.M., and Jacobs, L.L., (eds.), Evolution of Tertiary Mammals of North America. Volume 1: Terrestrial Carnivores, Ungulates and Ungulate-like Mammals. Cambridge University Press, Cambridge, UK.
Xu, J., Snedden, J.W., Stockli, D.F., Fulthorpe, C.S., and Galloway, W.E. 2017. Early Miocene continental-scale sediment supply to the Gulf of Mexico Basin based on detrital zircon analysis. The Geological Society of America Bulletin, 129:3-22. https://doi.org/10.1130/B31465.1
Young, S.C., Budge, T., Knox, P.R., Kalbouss, R., Baker, E., Hamlin, S., Galloway, W.E., and Deeds, N. 2010. Hydrostratigraphy of the Gulf Coast Aquifer from the Brazos River to the Rio Grande. Austin, Texas Water Development Board Report.
Zittel, K.A. 1893. Handbuch der Palaeontologie. Abtheilung I. Palaeozologie. Band IV, Vertebrata (Mammalia). R. Oldenbourg, Munich.
Zug, G.R. 2001. Turtles of the Lee Cree Mine (Pliocene: North Carolina). In Ray, C.E. and Bohaska, D.J. (eds.), Geology and Paleontology of the Lee Creek Mine, North Carolina. III. Smithsonian Contributions to Paleobiology, 9:203-218. https://doi.org/10.5479/si.00810266.90.1

