 Fossil snakes of the Penny Creek Local Fauna from Webster County, Nebraska, USA, and the first record of snakes from the Early Clarendonian (12.5-12 Ma) of North America
Fossil snakes of the Penny Creek Local Fauna from Webster County, Nebraska, USA, and the first record of snakes from the Early Clarendonian (12.5-12 Ma) of North America
Article number: 27.1.a2
https://doi.org/10.26879/1220
Copyright Society of Vertebrate Paleontology, January 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 17 February 2022. Acceptance: 19 November 2023
ABSTRACT
The Penny Creek Local Fauna in southern Webster County, Nebraska, is an early Clarendonian fossil locality within the Ash Hollow Formation. Undescribed fossils from previously collected Penny Creek material represent the first record of snakes from this time interval and confirm the presence of multiple taxa immediately following the Mid-Miocene Climatic Optimum. We identified eight taxa from the locality, including one booid (Charina), three colubrines (Pantherophis, Lampropeltis, and Salvadora), a dipsadid (Heterodon/Paleoheterodon), and several natricids (Neonatrix elongata, Neonatrix magna, and Nerodia). Of these snakes, only Neonatrix is an extinct genus, Charina and Salvadora are presently extirpated from the area, and all other genera are represented in the Central Great Plains today. Habitats occupied by extant members of genera represented in the Penny Creek snake assemblage suggest a relatively open environment with loose substrates and plentiful ground cover near a permanent water source. This further corroborates previous geological and mammalian paleoecological assessments of the Penny Creek area as a somewhat open, woodland-prairie ecotone environment near a permanent, high-energy fluvial water source. Finally, the snakes of Penny Creek help contribute to our understanding of the modernization of North American snake assemblages in the Central Great Plains by providing data for a poorly understood time within the evolution of North American snakes
John J. Jacisin III. Department of Integrative Biology, The University of Texas. Austin, Texas, USA. john.jacisin@austin.utexas.edu
A. Michelle Lawing. Department of Ecology and Conservation Biology, Texas A&M University. College Station, Texas, USA. alawing@tamu.edu
Keywords: Neogene; Miocene; Clarendonian; snakes; Central Great Plains; Nebraska; climate envelope models
Final citation: Jacisin, John J., III, and Lawing, A. Michelle. 2024. Fossil snakes of the Penny Creek Local Fauna from Webster County, Nebraska, USA, and the first record of snakes from the Early Clarendonian (12.5-12 Ma) of North America. Palaeontologia Electronica, 27(1):a2.
https://doi.org/10.26879/1220
palaeo-electronica.org/content/2024/5074-fossil-snakes-of-penny-creek
Copyright: January 2024 Society of Vertebrate Paleontology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Snakes have a wide geographic distribution in the Cenozoic fossil record, with many modern North American groups first appearing in the Miocene of North America (Rage, 1984; Parmley and Holman, 1995; Holman 2000). Unfortunately, many Cenozoic North American snake faunas are chronically understudied, and specimens remain unidentified or misidentified/overidentified through covert biases such as gestalt or non-apomorphy-based identifications, thereby hindering opportunities to apply a deep time perspective to investigate the effects of environmental change on snake communities (Bell et al., 2010; Szyndlar, 2012; Head et al. 2016). Further complicating matters is the difficulty of identifying unique taxa based on isolated cranial and vertebral elements that may be weathered or otherwise damaged during or after the death of an organism (Rage, 1984). However, it is important to describe and evaluate these taxa because they provide an important key to understanding the evolution of snakes, as well as the deep time effects of environmental change on snake diversification and biogeography. Fossil Cenozoic snakes are potential tools for environmental reconstruction and investigating the long-term biotic effects of climate change, as the physiologies of many reptiles are strongly associated with their habitat (Webb and Shine, 1998; Head et al., 2009; Sinervo et al., 2010; Muthoni, 2010; Huey et al., 2012; Dupoué et al., 2017).
While a relatively small number of mostly diminutive fossil snakes from the Paleogene of the Central Great Plains (South Dakota, Nebraska, Kansas, and Oklahoma) have been described, the Neogene beds of the Central Great Plains - specifically Nebraska - include a comparatively long, well-preserved record of fossil snakes that is unparalleled in illustrating the history of richness, turnover, and modernization of snake assemblages in North America (Holman, 2000; Parmley and Holman, 2007; Parmley and Hunter, 2010). Within the changing environments leading up to, then following the Mid-Miocene Climatic Optimum (MMCO), the “archaic” booid-dominated faunas represented by Ogmophis, Calamagras, and Geringophis gradually gave way to Caenophidian-dominated faunas (Parmley and Holman, 1995; Parmley and Hunter, 2010). More specifically, North American faunas become primarily composed of Colubroidea (sensu Burbrink et al., 2020). The first recognized appearances of extant genera occur in the early Neogene of the Central Great Plains; these genera include Lampropeltis, Pantherophis (Elaphe), Salvadora, Heterodon, Nerodia, Thamnophis, Crotalus, Sistrurus, and Charina (Parmley and Holman, 1995; Parmley and Holman, 2007; Parmley and Hunter, 2010).
Despite the excellent state of the Central Great Plain’s fossil snake record and apparent peaks of genus-level richness in the late Barstovian and late Clarendonian (14.75-12.5 Ma and 10.0 Ma- 9.0 Ma; Holman, 2000; Parmley and Hunter, 2010; Jacisin et al., 2015), a paucity of fossil material has resulted in a knowledge gap on snake assemblages from the earliest Clarendonian (12.5-12 Ma). This temporal gap in the snake fossil record is present throughout North America and highlights a gap in our understanding about the temporal turnover and modernization of snake assemblages immediately following the late Barstovian (including the MMCO) of the Central Great Plains.
 Here, we report the first known early Clarendonian snake assemblage in North America Penny Creek Local Fauna in southern Webster County, Nebraska (Figure 1). This fossil material allows us to confirm the presence of a number of snake taxa in Nebraska and the Central Great Plains during the early Clarendonian, providing evidence to evaluate previous geological and biological interpretations of the local paleoenvironment and allowing for a more complete understanding of the modernization of North American snake faunas. Fossil reptiles were reported from this locality, but were unidentified, and the focus of previous studies was primarily given to the geological setting and composition of mammalian assemblages (Turner, 1972; Voorhies et al., 1987; Voorhies, 1990; Corner, 2014). Ours is the first study to explore the differences between Nebraska’s latest Barstovian and earliest Clarendonian snakes and the initial transition into a post-Middle Miocene Climatic Optimum (Zachos et al., 2001; Song et al. 2018) snake assemblage.
Here, we report the first known early Clarendonian snake assemblage in North America Penny Creek Local Fauna in southern Webster County, Nebraska (Figure 1). This fossil material allows us to confirm the presence of a number of snake taxa in Nebraska and the Central Great Plains during the early Clarendonian, providing evidence to evaluate previous geological and biological interpretations of the local paleoenvironment and allowing for a more complete understanding of the modernization of North American snake faunas. Fossil reptiles were reported from this locality, but were unidentified, and the focus of previous studies was primarily given to the geological setting and composition of mammalian assemblages (Turner, 1972; Voorhies et al., 1987; Voorhies, 1990; Corner, 2014). Ours is the first study to explore the differences between Nebraska’s latest Barstovian and earliest Clarendonian snakes and the initial transition into a post-Middle Miocene Climatic Optimum (Zachos et al., 2001; Song et al. 2018) snake assemblage.
Institutional abbreviations. UNSM, University of Nebraska State Museum, Nebraska, USA; TCWC, Texas A&M University Biodiversity and Research Teaching Collections, Texas, USA; and TMM, University of Texas at Austin Vertebrate Paleontology Collections, Texas, USA.
GEOLOGIC SETTING
 In general, the Ash Hollow Formation is composed of poorly sorted, heterogenous sediments (Diffendal et al., 1996). The basal Ash Hollow Fm. of the Ogallala Group is a cross-bedded conglomerate composed of partially rounded concretions pulled from the underlying Whitney Member of the Brule Formation and is mostly composed of stream-transported and stream-deposited sediments (Diffendal et al. 1996). Boellstorff (1978) first assigned a Miocene age for the formation based on the dates returned during the study of fission tracks in volcanic ash shards. This conglomerate is variable in thickness, and sequences upward into unstratified, massive pebbly sands and calcite-cemented pebbly sandstones (Diffendal et al., 1996; also known as pudding sands via Lueninghoener, 1934). These massive sandstones tend to form massive ledges with the underlying conglomeratic layer. At the type locality of the Ash Hollow Fm., the conglomeratic unit lies upon an erosional surface developed on top of a Whitney Member (Brule Fm.) concretion zone to form an unconformity. Overlying the Ash Hollow Fm. are coarse, stream-deposited sands and gravels that represent the Pliocene Broadwater Formation (Figure 2). While much of the Ash Hollow Fm. is epiclastic, it does contain several diatomite and ash beds (Thomasson, 1980), the latter of which includes the infamous Ashfall Fossil Beds located in Antelope County, Nebraska, although these beds are younger than the locality examined within this study (Voorhies, 1992; Diffendal et al., 1996).
In general, the Ash Hollow Formation is composed of poorly sorted, heterogenous sediments (Diffendal et al., 1996). The basal Ash Hollow Fm. of the Ogallala Group is a cross-bedded conglomerate composed of partially rounded concretions pulled from the underlying Whitney Member of the Brule Formation and is mostly composed of stream-transported and stream-deposited sediments (Diffendal et al. 1996). Boellstorff (1978) first assigned a Miocene age for the formation based on the dates returned during the study of fission tracks in volcanic ash shards. This conglomerate is variable in thickness, and sequences upward into unstratified, massive pebbly sands and calcite-cemented pebbly sandstones (Diffendal et al., 1996; also known as pudding sands via Lueninghoener, 1934). These massive sandstones tend to form massive ledges with the underlying conglomeratic layer. At the type locality of the Ash Hollow Fm., the conglomeratic unit lies upon an erosional surface developed on top of a Whitney Member (Brule Fm.) concretion zone to form an unconformity. Overlying the Ash Hollow Fm. are coarse, stream-deposited sands and gravels that represent the Pliocene Broadwater Formation (Figure 2). While much of the Ash Hollow Fm. is epiclastic, it does contain several diatomite and ash beds (Thomasson, 1980), the latter of which includes the infamous Ashfall Fossil Beds located in Antelope County, Nebraska, although these beds are younger than the locality examined within this study (Voorhies, 1992; Diffendal et al., 1996).
In Webster County, Nebraska, Turner (1972) first reported granitic sand and gravel with cross-bedding indicative of a braided stream fluvial environment for several Nebraska localities collectively named the Penny Creek Local Fauna; this included locality Wt 13B, from which the fossils of this study were collected (Figure 1). Turner (1972) assigned an early Clarendonian age to those localities based on the mammalian fauna. In addition to the dates reported in Boellstorff (1978), work by Swisher (1992) dating the sediments of Nebraska also indicated an early Clarendonian age based on Ar-Ar and K-Ar dates of glass and ash for the lower parts of the Ash Hollow Formation. Reviews of the biostratigraphy and geochronology of the North American Cenozoic further support these dates (Tedford et al., 2004). Research by Voorhies et al. (1987), Voorhies (1990) and Corner (2014) confirmed the assessment for the locality, and provided additional knowledge on the geology, vertebrate biostratigraphy, and mammalian fossils of Wt 13B as part of larger studies. They concluded that the sediments belonged to the Ogallala Group’s Ash Hollow Fm. and were earliest Clarendonian (middle Miocene) in age, or slightly older than the Ashfall Fossil Beds of northeastern Nebraska (Figure 2). They also noted that the sediments of the Penny Creek local fauna, and the Ogallala sediments as a whole, were predominantly epiclastic, as opposed to the volcaniclastic sediments of the underlying Arikaree and White River Group rocks in Nebraska (Voorhies, 1990).
MATERIALS AND METHODS
The fossils assessed in this study were collected from a single locality (Wt 13B), accessioned to the University of Nebraska State Museum’s collections, and are part of a diverse assemblage dubbed the “Penny Creek Local Fauna”, consisting of several other Webster County localities (Wt 11, Wt 12, Wt 13A, and Wt 15B; Turner, 1972; Voorhies et al., 1987; Corner, 2014). These localities were collected extensively, especially in the 1960’s and early 1970’s, under the supervision of the UNSM. Wt 13B was quarried annually for several years by students from the Red Cloud Community Schools under the supervision of J. L. Fitzgibbon (Voorhies et al., 1987). Collection methods included surface prospecting, quarrying, and screenwashing during these years (Corner, 2014).
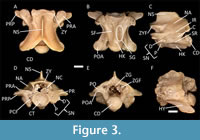 We used a binocular dissecting microscope to examine the snake fossils from Wt 13B, which were predominantly pre-cloacal trunk vertebrae and a small number of caudal vertebrae. Apomorphic characters (such as the presence of a hypapophysis on mid-trunk vertebrae; Holman, 2000) capable of uniquely identifying different snake groups (e.g., natricid vs. colubrine snakes) were used for fossil identification following the recommendations of Bell and colleagues (Bell et al., 2004, 2010). Character tables suggested by Holman (2000) and modified by Ikeda (2007) assisted in the process through generalized descriptions of the range of shape variation for the neural arch, prezygapophyseal articular facets in dorsal view, postzygapophyseal articular facets in ventral view, anterior edge of the zygosphene in anterior and dorsal views, zygosphenal articular facets in lateral view, anterior and posterior edges of the neural spine in lateral view, hypapophysis, hemal keel, cotyle, condyle, prezygopophyseal accessory processes in ventral view, developmental degree of the parapophyses, prezygapophyseal accessory processes, interzygapophyseal ridge in lateral view, and subcentral ridge in lateral view (see Figure 3 for morphological position of major vertebral structures). In addition, we made personal observations using comparative methods with available modern and fossil specimens at the UNSM, TCWC, and the TMM collections, as well as previously published literature and figures (including Gilmore, 1938; Auffenberg, 1963; Rage, 1984 and references therein; LaDuke, 1991; Holman, 2000; Parmley and Walker, 2003; Ikeda, 2007; Parmley and Hunter, 2010; Head et al., 2016; and Zaher et al., 2019). Extinct taxa are denoted by a dagger symbol (†).
We used a binocular dissecting microscope to examine the snake fossils from Wt 13B, which were predominantly pre-cloacal trunk vertebrae and a small number of caudal vertebrae. Apomorphic characters (such as the presence of a hypapophysis on mid-trunk vertebrae; Holman, 2000) capable of uniquely identifying different snake groups (e.g., natricid vs. colubrine snakes) were used for fossil identification following the recommendations of Bell and colleagues (Bell et al., 2004, 2010). Character tables suggested by Holman (2000) and modified by Ikeda (2007) assisted in the process through generalized descriptions of the range of shape variation for the neural arch, prezygapophyseal articular facets in dorsal view, postzygapophyseal articular facets in ventral view, anterior edge of the zygosphene in anterior and dorsal views, zygosphenal articular facets in lateral view, anterior and posterior edges of the neural spine in lateral view, hypapophysis, hemal keel, cotyle, condyle, prezygopophyseal accessory processes in ventral view, developmental degree of the parapophyses, prezygapophyseal accessory processes, interzygapophyseal ridge in lateral view, and subcentral ridge in lateral view (see Figure 3 for morphological position of major vertebral structures). In addition, we made personal observations using comparative methods with available modern and fossil specimens at the UNSM, TCWC, and the TMM collections, as well as previously published literature and figures (including Gilmore, 1938; Auffenberg, 1963; Rage, 1984 and references therein; LaDuke, 1991; Holman, 2000; Parmley and Walker, 2003; Ikeda, 2007; Parmley and Hunter, 2010; Head et al., 2016; and Zaher et al., 2019). Extinct taxa are denoted by a dagger symbol (†).
Our taxonomic groups follow the terminology used in recent publications on snake systematics, including Burbrink et al. (2020), Georgalis and Smith (2020), and Zaher et al. (2019). These changes in taxonomic nomenclature resulted in a lack of diagnostic characters for some groups. This, combined with the overall rarity of vertebral morphological characters described for higher taxonomic levels in at least North American literature (pers. obs., but see also Holman, 2000 and Ikeda, 2007), hinders comparative morphological studies (see Bell et al., 2010 and Head et al., 2016 for further discussion of identification biases in fossil herpetofauna). As such, we have attempted to resolve more recent nomenclature for groups such as Colubroides (sensu Zaher et al., 2009) and Charinaidae (sensu Pyron et al., 2014) with vertebral characters for those groups, rather than vertebral diagnoses of the highest resolution identifications alone (e.g., genus or species).
 We assembled occurrences of snakes from 19 sites, Barstovian through Blancan, in the state of Nebraska in order to assess first and last occurrences and the transition to a more modern snake assemblage (Figure 4, Appendix Table 1). We examined the similarity of snake assemblages through time. It is important to note that absences may be representative of true absence, a lack of data or errors in identification. As such, true absences are exceptionally difficult or impossible to be distinguished from false absences, especially when considering communities with numerous rare or difficult to distinguish species (Boulinier et al., 1998).
We assembled occurrences of snakes from 19 sites, Barstovian through Blancan, in the state of Nebraska in order to assess first and last occurrences and the transition to a more modern snake assemblage (Figure 4, Appendix Table 1). We examined the similarity of snake assemblages through time. It is important to note that absences may be representative of true absence, a lack of data or errors in identification. As such, true absences are exceptionally difficult or impossible to be distinguished from false absences, especially when considering communities with numerous rare or difficult to distinguish species (Boulinier et al., 1998).
Beyond identifying taxa at each location, we visualized the similarity between assemblages at these sites by constructing a Euclidean distance matrix and using a principal coordinates analysis (PCoA) for the Nebraska localities. We also used this distance matrix to perform a hierarchical cluster analysis (HCA) with the complete-linkage method to look for groupings of localities that may suggest major shifts in the snake assemblages of Nebraska. Agglomerative HCA is a “bottom-up” analysis where each observation starts in its own cluster, and pairs of clusters are merged as the hierarchy is built up (Hammer et al., 2001). The complete-linkage or farthest neighbor clustering method, which forms compact clusters of near-equal diameters and avoids forcing clusters together based on single elements being close. Complete-link clustering builds clusters where the similarity of two clusters is the similarity of their most dissimilar members (thus, “farthest neighbor”).
Snake ectothermic physiology is highly dependent on ambient temperature and is therefore a practical choice for examining terrestrial climates of the past. To examine the potential climate of the Penny Creek local fauna, we identified area of overlap in climate envelope models for the snake assemblage of the Penny Creek local fauna. Climate envelope models characterize a set of suitable climates for a species or group of species derived from their location in climate space. While these models are constructed from the associations between the geographic location of a species and its climate without consideration of biotic interactions, dispersal, or evolutionary change, past review has suggested that climate envelope models are capable of producing useful initial approximations of the dynamics of species to climate interactions at appropriate scales (Lawing, 2021). Climate envelope models have been used to evaluate impacts of climate associated changes in the projected geographic distributions of snakes through time to model both the past and future dispersal of a clade (Lawing and Polly, 2011; Lawing et al., 2016).
To create climate envelope models for the Penny Creek snake assemblage, we selected representative congenerics of presumed closely related living species for each of the fossil taxa (see Appendix Table 2 for list of congenerics used for each fossil taxon). We obtained geographic range maps of those congenerics from the IUCN Redlist (IUCN, 2021). Climate data for Mean Annual Temperature (MAT) and Annual Precipitation (AP) from the Worldclim database Version 2.1 (Fick and Hijmans, 2017) at 10-minute resolution were extracted from the polygon overlap of the congeneric species. The two variables described the mean of temperature and sum of precipitation on an annual basis (Nix, 1986). All congeneric species were combined into a genus-level dataset to decrease the influence of specific differences. Finally, we produced density plot histograms to identify the area of overlap for all generic models across taxa of the Penny Creek local fauna. We used the area of overlap to infer the potential climate envelope of the depositional setting of Penny Creek.
SYSTEMATIC PALEONTOLOGY
Class REPTILIA Laurenti, 1768
Order SQUAMATA Oppel, 1811
Suborder SERPENTES Linnaeus, 1758
Infraorder ALETHINOPHIDIA Nopsca, 1923
Diagnosis. Vertebrae of Infraorder Alethinophidia are variable, but can typically be identified by the presence of a neural spine, a somewhat high neural arch, and the presence of a median notch on the posterior border of the neural arch has a median notch (Rage, 1984; Holman, 2000).
Remarks. While the above characters are true for most of Alethinophidia, both Rage (1984) and Holman (2000) noted exceptions in the Uropeltidae, which lack neural spines, and the genus Coniophis, where the vertebrae lack both a neural spine and a median notch in the posterior neural arch. The “higher neural arch” of Rage (1984) is relative to the lower neural arch of Scolecophidia. Ikeda (2007) examined these characters in additional taxa, and stated that despite the exceptions above, the character states of Rage (1984) and Holman (2000) can be considered diagnostic.
Suprafamily Constrictores Oppel, 1811
(Georgalis and Smith, 2020)
Diagnosis. The vertebrae of the Constrictores clade are massively built and anteroposteriorly short, with a low centrum length / neural arch width ratio (< 1.1), and generally have tall neural spines, and thick zygosphenes (Ivanov, 2000; Szyndlar and Rage, 2003; Georgalis and Scheyer, 2019; Georgalis and Smith, 2020).
Remarks. Georgalis and Smith (2020) applied the term Constrictores to the group uniting Booidea and Pythonoidea following the taxonomic revisions of Pyron et al. (2014). There are a few broad vertebral characters that unite Booidea and Pythonoidea, and exceptions within and outside of this group exist. Georgalis and Smith (2020) note that the centrum length / neural arch ratio of Constrictores is lower in all ingroup taxa except Ungaliophiinae and Xenopeltis, and perhaps some undescribed vertebral morphologies from taxa such as Xenophidion (Georgalis and Smith, 2020). Georgalis and Smith (2020) also note that this character is not unique to Constrictores, additionally occurring in Tropidophiidae (Bogert and Rowley, 1968), Madtsoiidae, and specific taxa such as Acrochordus (Hoffstetter and Gayrard, 1965; Zaher et al., 2019).
Superfamily BOOIDEA Gray, 1825
Diagnosis. There are not many vertebral characters defining the diverse group of booid snakes; the most commonly cited characters include the presence of lateral foramina and higher neural arches than those found in Anilioidea (Holman, 2000; modified from Rage, 1984; supported by Ikeda, 2007). Furthermore, in pythonids, the shape of the hemal keel is defined by grooves or depressions beginning at the cotylar rim, but projecting below the centrum only in the posterior part of each vertebra (Scanlon and Mackness, 2001; Szyndlar and Rage, 2003).
Remarks. Skeletal characters used to describe Booidea are primarily based on cranial elements (see Georgalis and Smith, 2020). In comparison to colubroids, booid vertebrae are generally less slender and elongate, and tend to have shorter and broader neural spines in at least North American species (Holman, 2000; Smith, 2013). Booidea can often be separated from Pythonoidea based on greater intracolumnar heterogeneity in the former (Szyndlar and Rage, 2003), and thicker zygosphenes in the latter when compared to similarly sized booids, although there is some amount of variability in this character (Georgalis and Smith, 2020).
Family CHARINAIDAE Gray, 1849 (sensu Pyron et al., 2014)
Diagnosis. The following vertebral osteological characters are modified from Brattstrom (1958), Kluge (1993), Bell and Mead (1996), Holman (2000), and Head (2015) to reflect the current nomenclature for Constrictores and Booidea (Reynolds et al., 2014; Zaher et al., 2019; Burbrink et al., 2020; Georgalis and Smith, 2020). The vertebrae possess a flattened neural arch. The neural spines are low, and in the caudal vertebrae are expanded, exhibiting a somewhat distended or distally lobate appearance relative to pre-caudal neural spines (Head, 2015). Prezygapophyseal accessory processes are reduced (Holman, 2000). Paracotylar foramina are absent (Kluge, 1993). Caudal vertebrae are very short, with a variety of processes giving them a complex appearance (with the exception of the genus Lichanura; Holman, 2000).
Remarks. Snakes of the Charinaidae are typically small to medium in size, robust in body form, with short tails and small eyes, all of which assist them in a semifossorial lifestyle (Holman, 2000). Many North American fossil booids, including extant genera Charina and Lichanura, were previously assigned to the Erycinae, which are generally similar in body form, vertebral morphology, and lifestyle (Holman, 2000; see Pyron et al., 2014 and ICZN, 2020 for additional details regarding taxonomic nomenclature). Under the most recent taxonomy, North American subfamilies Charinainae Gray, 1849 (Charina and Lichanura) and Ungaliophiinae McDowell, 1987 (Exiliboa and Ungaliophis) are now grouped within the Charinaidae (Pyron et al. 2014; Head, 2015; ICZN, 2020). This further complicates the fossil record of older North American snakes not found in Penny Creek, in that genera such as Calamagras, Ogmophis, Geringophis, Pterygoboa, and others are left with a somewhat uncertain taxonomic status, although some research has suggested that the fossil species Ogmophis compactus and Calamagras weigeli may represent loxocemid and ungaliophiine snakes, respectively (Smith, 2013). An extensive apomorphy-based redescription and reorganization of the older fossil taxa may be necessary to determine if it is possible to morphologically differentiate them at the species or genus level given the newer taxonomy of extant booids (Bell et al. 2010; Pyron et al., 2014; Head, 2015). The absence of paracotylar foramina in Charinaidae vertebrae differentiates the group from Boidae (sensu Pyron et al., 2014). This variable character is shared with pythonids and most non-boid booids, and as such does not differentiate Charinaidae from those groups (Kluge, 1993; Rage, 2001; Szyndlar and Rage, 2003; Georgalis, 2019; Georgalis and Smith, 2020).
Genus CHARINA Gray, 1849
Diagnosis. As diagnosed in Head (2015), Charina possesses a lobate neural spine that is laterally expanded similar to other Charinainae, and the pterapophyses are anteriorly directed in caudal vertebrae (Kluge, 1993; Szyndlar, 1994). Charina also exhibits a non-U-shaped zygosphene in dorsal view, a strongly concave zygosphene in anterior view, a relatively depressed neural arch, an incised posterior edge of the neural arch, and no paracotylar foramina (Holman, 2000).
Remarks. The fossils described here are similar in size and morphology to known species of Charina. The longer neural spine, the V-shaped (dorsal), strongly concave (anterior) zygosphene, and the depressed neural arch with a relatively deeply incised posterior edge suggest that these vertebrae do not belong to the genus Lichanura; however, it should be noted that Bell and Mead (1996) have observed some intraspecific variation in these characters. As in Parmley and Walker (2003), we instead attribute this fossil to the genus Charina based on the relative length of the neural spine, which is greater than that of Lichanura, and the lack of juvenile characteristics despite being relatively small in size. Parmley and Walker (2003) have observed that Lichanura of a similar size show juvenile characteristics such as a short, high overall morphology, thin neural arch, thin and highly arched zygosphene, exceptionally short neural spine, enlarged neural canal, and a condyle that appears too large for the centrum, none of which are visible in this specimen.
Charina cf. Charina prebottae Brattstrom, 1958 †
Figure 5
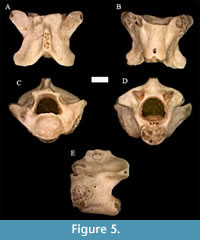 Material. UNSM 139981 (posterior middle trunk vertebra).
Material. UNSM 139981 (posterior middle trunk vertebra).
Description. The vertebra appears near square in shape from dorsal view, but is slightly wider at the prezygapophyses than the postzygapophyses. The postzygapophyses are rounded, while the prezygapophyses are moderately pointed with reduced accessory processes, and are raised antero-laterally. The neural spine is low, broad, and is longer than it is tall or wide. The top of the neural spine is weathered, but this does not appear to affect the overall shape of the neural spine in dorsal view. The neural spine tapers in width anteriorly and is incised posteriorly. The zygosphene is dorsally flat and v-shaped overall, with somewhat rounded lateral edges that extend slightly forward on the anterior face. In anterior view, the zygosphene is concave. The neural arch is somewhat flattened and deeply incised posteriorly. The cotyle is round and mildly angled ventrally, with the dorsal edge extending more anteriorly and the ventral edge extending more posteriorly. Paracotylar foramina are absent. The hemal keel is wide, flat, and smooth ending just before reaching the condylar head. It is bordered laterally by a flat indentation, primarily on the anterior end.
Remarks. The fossil described here is similar in size and morphology to known species of Charina (see above for description and comparisons with Lichanura). It is most similar to Charina prebottae specimens from other Nebraska localities, as it possesses a more strongly developed hemal keel when compared to the same vertebral region in extant species of Charina (Holman, 1987) and no break in the slope of the anterior neural spine in lateral view as it descends to the zygosphene (Bell and Mead, 1996). Other characters described by Brattstrom (1958) for C. prebottae may represent individual and intracolumnar variation as well as differences between his fossils from California and the fossils from Nebraska. Holman (2000) noted that Charina prebottae also exhibits notable variation across a wide geographic area throughout the Miocene. Previous literature suggests that C. prebottae may be a catch-all taxon for multiple species, as Brattstrom’s (1958) un-illustrated account and subsequent illustrated accounts do not completely match, warranting further in-depth study (Bell and Mead, 1996; Holman, 2000). Although this vertebra is weathered and missing the extreme parts of several of its structures, it exhibits features of the neural spine (Brattstrom, 1958; Bell and Mead, 1996) and hemal keel (Holman, 2000) matching those of C. prebottae. As such, we confer this vertebra to C. prebottae until the taxon is more comprehensively assessed and re-diagnosed.
Parvorder CAENOPHIDIA Hoffstetter, 1939
Diagnosis. The vertebral synapomorphies of crown Caenophidia include well-developed prezygapophyseal accessory processes, synapophyses that are well-differentiated into para- and diapophyseal articular facets, the presence of pleurocentral hypapophyses throughout the precloacal vertebral column, the presence of one or more paracotylar foramina, a condyle and cotyle that are relatively small (compared to Constrictores) and circular to ovoid and elongate in cross section, and well-developed paralymphatic channels that define the lateral margins of a distinct hemel keel (Holman, 2000; Head et al., 2016).
Remarks. The vertebral morphologies of Caenophidians is markedly different from that of Booidea, including many of the most notable components of a snake vertebrae (e.g., neural spine, centrum, synapophyses, etc.). The works of Head (2015) and Head et al., (2016) to provide fossil calibration dates for snakes also summarize the morphological synapomorphies that differentiate these groups after the taxonomic restructuring of Caenophidia by Zaher et al. (2009), and Constrictores by Pyron et al. (2014), and Georgalis and Smith (2020).
Suprafamily COLUBROIDES Zaher et al., 2009
Diagnosis. Characters that unite Colubroides and exclude Acrochordidae include a highly elongate centrum, the presence of distinct lateral foramina, and large, paired ventrolateral cotylar processes below the ventral margin of the cotyle that may form a paralymphatic channel (Head, 2005; Head et al., 2016). Additional vertebral synapomorphies of Acrochordus that differentiate Acrochordidae from Colubroides include smaller paired ventrolateral cotylar processes, a greater minimum number of paracotylar foramina (one or more in Colubroides, two or more in Acrochordidae), a shallow fossa or minute lateral foramina as opposed to an obvious foramen, synapophyseal articular surfaces with diapophyseal and synapophyseal facets strongly angled relative to each other, ventrally elongate and pendant synapophyses, and a hemispherical, vertically oriented, and blade-like prezygapophyseal accessory process (Head, 2005).
Remarks. Vertebral synapomorphies of this group have not been described under the current nomenclature of Colubroides, perhaps because of confusion between the use of Colubroides and the previous use of Colubroidea prior to the reorganization of Caenophidian taxonomy (see Zaher et al., 2009 for the history of these taxonomic terms). Here, we attempted to assemble some vertebral characters by which to define this group; however, some of these characters merely differentiate this group from Acrochordidae, and may not fully match some earlier taxa sometimes assigned to Colubroides (see Head et al., 2016’s discussion of Procerophis and Thaumastophis under Pan-Colubroidea).
Clade COLUBRIFORMES Günther, 1864
(sensu Zaher et al., 2019).
Diagnosis. Vertebrae can be assigned to Colubriformes based on the combination of the following features: vertebrae lightly built and elongate (longer than wide), paracotylar foramina and elongated, tapering prezygapophyseal accessory processes present, blade-like and uniformly thin neural spine reaches the roof of the zygosphene, and synapophyses differentiated into diapophyses and parapophyses (Rage, 1984; Zaher et al., 2019).
Remarks. Assigning vertebrae to Colubriformes is based on a combination of the characters listed above. Other groups may show some similarities, for example, Georgalis and Scheyer (2022) point out that elongate centra can be present in non-colubriforms, such as in some scolecophidians and ungaliophiids, but are quite different in other aspects of their morphology. The combination of elongate, lightly built and precloacal vertebrae, the presence of tapering prezygapophyseal processes, and ablade-like uniformly thin neural spine that reaches the roof of the zygosphene excludes Colubriformes from an association with the families Acrochordidae, Russellophiidae, Anomalophiidae, and Xenodermidae (Zaher et al., 2019) The differentiated para- and diapophysial articular facets further distinguishes Colubriformes from russellophiids and anomalophiids (Zaher et al., 2019). Finally, Xenodermidae differs from Colubriformes in possessing vertebrae with accessory processes on both zygapophyses and neural spines with broad lateral expansions (Bogert, 1964; Zaher et al., 2019).
Superfamily COLUBROIDEA Oppel, 1811
Diagnosis. Vertebral characters used to identify to Colubroidea include: vertebrae longer than wide (length at least 1.2-1.3 times as wide at the neural arch) and relatively lightly built (Holman, 2000; Smith, 2013); neural spines thin and long when compared to other groups (Holman, 2000); zygosphenal and zygantral areas less massive than in booids (Holman, 2000); synapophyses distinctly divided into parapophyseal and diapophyseal processes (Holman, 2000); mid- and posterior trunk vertebrae with sharp, relatively thin hemal keels, with hypapophyses often absent in these regions (Rage, 1984; Holman, 2000; Ikeda, 2007; Smith, 2013; Head et al., 2016). When present, trunk vertebral hypapophyses are relatively long and often pointed (Holman, 2000; Ikeda, 2007); both paracotylar and lateral foramina present (Rage, 1984; Ikeda, 2007).
Remarks. There is some disparity in what constitutes the defining characters of colubroid vertebrae because of the vast diversity of the group. As such, we saw it fitting to summarize known characters in this study. Zaher et al. (2009, 2019) point out that no known vertebral synapomorphies currently define Colubroidea, and vertebrae are typically assigned through the combination of the characters listed above. However, Rage (1984) and Ikeda (2007) identified the presence of both paracotylar and lateral foramina together on the vertebrae as consistent throughout the group. Holman (2000) provided a number of additional characters, but some of the proposed characters describe only some groups of colubroids, and as such, are not included in the diagnosis of the group at this time. These disputed characters include the lack of hypapophyses beyond the cervical region in several groups, and possibly the presence of well-developed prezygapophyseal accessory processes, which Ikeda (2007) was unable to find in several Asian viperids. It should be noted that Holman (2000) focused only on North America colubroids, and therefore may have defined the group primarily for North American taxa.
Family COLUBRIDAE Oppel, 1811
Diagnosis. Vertebral characters of Colubridae include: trunk vertebrae lightly built and longer than wide (Holman, 2000); neural spines long, thin, uniformly wide, and as high as or higher than they are long (Holman, 2000; Head et al., 2016); subcentral ridges of the centrum are deep (Holman, 2000; Jurestovsky, 2021); prezygapophyseal accessory processes are prominent (Holman, 2000); epizygophyseal spines extending posteriorly from the postzygapophyses present in some species (Holman, 2000); synapophyses distinctly divided into diapophyses and parapophyses (Holman, 2000); cotyle circular-to-oval in shape (Jurestovsky, 2021). The hemal keel is typically thin and may appear similar to one of three types: hemal keels present without parapophyseal process development, both hypapophysis and parapophyseal processes present, and hemal keel thin with somewhat round prezygpophyseal articular processes (Rage, 1984; Holman, 2000; Ikeda, 2007).
Remarks. The defining characters for colubrids are complicated by the diversity and degree of variation within the group, and are partially dependent on whether groups such as the natricids and dipsadids are included within the group. Post-cervical hypapophyseal characters would seem to be inappropriate for defining colubrids as a whole if natricids are included because, as noted in Ikeda (2007), several studies have pointed out that some natricids lack hypapophyses on their trunk vertebrae (McDowell, 1961; Malnate, 1972), while a small number of homalopsine snakes exhibit prominent hypapophyses on their trunk vertebrae (Gyi, 1970; McDowell, 1987). Furthermore, Ikeda (2007) noted the highly variable shape of the prezygapophyseal facets and hemal keel within Colubridae, and pointed out that the neural spine is often more long than high in small-bodied species, but more high than long in large-bodied species.
Subfamily COLUBRINAE Oppel, 1811
Diagnosis. Holman (2000) outlined some general osteological characters for colubrine snakes, which were later examined and modified by Ikeda (2007). The trunk vertebrae are typically longer than wide, are often lightly built, and lack hypapophyses (Holman, 2000; Ikeda, 2007; Ikeda et al., 2016). The neural spines are somewhat thin, tall, mostly uniform in width, and often project posteriorly over adjacent vertebrae (Holman, 2000). The prezygapophyseal accessory processes are well-projected and prominent (Holman, 2000; Ikeda, 2007; Ikeda et al., 2016). The hemal keels are well-projected from the centrum and may or may not be relatively thin (Holman, 2000; Ikeda, 2007; Ikeda et al., 2016), and the subcentral ridges and grooves are distinct (Holman, 2000, Ikeda, 2007; Ikeda et al., 2016). Epizygapophyseal spines may or may not be present (Holman, 2000).
Remarks. While Holman’s (2000) diagnosis of colubrines appears to fit North American taxa, Ikeda (2007) noted that the lightly build vertebrae and relatively thin hemal keels are not features consistent with some non-North American taxa, as these features show various states amongst extant species. These characters may only be consistently useful for North American taxa, and should not preclude taxa from being assigned to the subfamily Colubrinae if those characters are not present as described in Holman (2000). Furthermore, Head et al. (2016) point out that while a precloacal vertebral column without hypapophyses have been traditionally used to differentiate colubrines from natricids and elapids (Bell et al., 2004; Szyndlar, 2012), this absence also occurs in a number of dipsadid and elapid taxa as well (Dowling and Duellman, 1978 after Pyron et al., 2013). This character therefore cannot fully diagnose colubrines to the exclusion of other clades on its own (Head et al., 2016).
Genus LAMPROPELTIS Fitzinger, 1843
Diagnosis. Lampropeltis vertebrae are somewhat robust, relatively short, and wide for a colubrine (centrum about as wide as long; Parmley, 1987), with long neural spines that are moderate to low in height, overhang posteriorly, and are either straight or overhanging anteriorly (Auffenberg, 1963; Meylan, 1982; Parmley, 1988; Parmley, 1990). The neural spines may also be thickened dorsally (LaDuke, 1991). The neural arches are depressed and wide (Auffenberg, 1963; Meylan, 1982; LaDuke, 1991; Holman, 2000). The hemal keel is well-developed and usually widened posteriorly, with well-developed subcentral ridges that curve inward near the cotyle (Auffenberg, 1963; Meylan, 1982; Parmley, 1988), and may be bordered laterally by fossae (LaDuke, 1991). The cotyle is round (Auffenberg, 1963), the condyle is round and sometimes obliquely tilted upwards (Parmley, 1990), the zygosphenes are flat anteriorly (LaDuke, 1991) and epizygapophyseal spines are absent (Auffenberg, 1963; Holman, 2000).
Remarks. Lampropeltis vertebrae differ from those of Pantherophis and Pituophis in exhibiting more pronounced subcentral ridges, less vaulted neural arches, and relatively lower neural spines (Parmley, 1990). They may additionally differ from Pantherophis and Arizona in being relatively longer and more robust (LaDuke, 1991). Lampropeltis vertebrae differ from those of Rhinocheilus in being relatively longer, with relatively thinner and taller neural spines that do not project anteriorly beyond the zygosphene, narrower hemal keels, relatively wider cotyles, larger zygapophyses, and more developed subcentral ridges (Van Devender and Mead, 1978; LaDuke, 1991).
Holman (2000) suggested that Lampropeltis vertebrae are more easily diagnosed on a species-by-species basis, as there are greater differences among some species of Lampropeltis than there are among some other colubrine genera (such as Coluber and Masticophis). This idea was reexamined and discussed in Parmley and Hunter (2010), who found that Lampropeltis alterna and the Lampropeltis pyromelana-zonata grouping have diagnostic vertebral characters distinct from each other and from the rest of the genus, while Lampropeltis getula, Lampropeltis calligaster, and Lampropeltis triangulum form a discernable L. getula complex. Furthermore, Auffenberg (1963) noted that smaller species appeared to have neural spines that are relatively shorter in height compared to larger species and that the hemal keel showed variation in development between species and age groups. Where exactly the known fossil species of Lampropeltis fall within the genus could therefore be dependent on the ability to discern between these three main morphospaces on a case-by-case basis. A better understanding of these morphospaces and the morphology of fossil species could also help determine when the genus started exploring the various morphologies associated with these groupings.
Lampropeltis similis Holman, 1964 †
Figure 6
Material. UNSM 139982 (14 pre-cloacal trunk vertebrae).
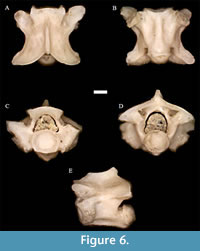 Description. In anterior view, the neural arch is moderately vaulted. The cotyle is a depressed oval bordered by deeply excavated pits, and is slightly larger than the ventrally restricted, inverted U-shaped neural canal. The zygosphene curves dorsally, and the prezygapophyseal articular facets tilt slightly upward. The diapophyses and parapophyses are distinct elements of the parapophyses, with the latter portion more distally pointed than the former; however, they are not as clearly separated as in most other colubrids.
Description. In anterior view, the neural arch is moderately vaulted. The cotyle is a depressed oval bordered by deeply excavated pits, and is slightly larger than the ventrally restricted, inverted U-shaped neural canal. The zygosphene curves dorsally, and the prezygapophyseal articular facets tilt slightly upward. The diapophyses and parapophyses are distinct elements of the parapophyses, with the latter portion more distally pointed than the former; however, they are not as clearly separated as in most other colubrids.
In dorsal view, the vertebrae are approximately as long as they are wide at the prezygapophyses, and the width at the well-developed, rounded prezygapophyseal accessory processes is greater than it is long through the zygapophyses. The neural spine tilts slightly ventrally in its anterior portion. The prezygapophyseal articular facets are oval to ovoid and slightly tilted upward. The epizygapophyseal spines are absent. The anterior edge of the zygosphene is slightly convex to slightly sinuate; the posterior notch of the zygosphene is V-shaped.
In lateral view, the neural spine is significantly longer than it is tall, and dips slightly downward cranially. The hemal keel is visible and quite strong.
In posterior view, the neural arch is moderately vaulted, and the condyle is a dorso-ventrally depressed oval.
In ventral view, the strong hemal keel is spatulate or oblong in shape, but not wide throughout most of its length. Subcentral ridges are present and concave from below, but not exceptionally developed. The postzygapophyseal articular facets are ovoid.
Remarks. These vertebrae in particular appear to be more similar to the general morphology of the milksnakes of the Lampropeltis triangulum complex or the kingsnake Lampropeltis calligaster as opposed to the L. alterna or L. pyromelana-zonata groupings. They possess long neural spines, moderately depressed neural arches, distinct hemal keels with deep subcentral grooves, and robust, distinct subcentral ridges (Parmley, 1990; Parmley and Hunter, 2010). More specifically, L. similis has previously been described as similar in appearance to L. triangulum; however, L. similis possesses a less depressed neural arch in the trunk region (more similar to that of L. calligaster) with an inverse U-shaped rather than depressed ovoid-shaped neural canal, a thinner hemal keel. Holman (2000) also stated a centrum that “is not as triangular from below” as an additional apomorphy for L. similis, but we are unable to confirm this as a consistent character throughout the vertebral column of the species.
Genus PANTHEROPHIS Fitzinger, 1843
Diagnosis. The vertebrae of North American Pantherophis are relatively short, but robustly built compared to most of their contemporary colubrids. The vertebrae have wide, high neural spines and broad hemal keels, but lack epizygapophyseal spines (Holman, 2000; Parmley and Hunter, 2010). The parapophyseal process is poorly developed on either side, and the interzygapophyseal ridge is straight in lateral view (Ikeda, 2007).
Remarks. The vertebrae of North American Pantherophis are similar to some species of other large-bodied colubrine genera. In North America, the vertebrae of Pantherophis differ from those of New World Masticophis and Coluber in that they are relatively shorter and more robustly built, have higher and wider neural spines, have wider hemal keels, and lack epizygapophyseal spines (Holman, 2000). Pantherophis generally differs from Lampropeltis in possessing a higher, more vaulted neural arch, a higher neural spine (compared to at least some species of Lampropeltis), straight and less defined subcentral ridges, and less robust vertebrae (Holman, 2000; Parmley and Hunter, 2010). Pantherophis differs from Pituophis in having a lower neural spine and a zygosphene that is rarely or never concave (Auffenberg, 1963). Pantherophis differs from Drymarchon in possessing a zygosphene that is rarely or never concave, greater posterolateral curvature of the neural arch, less posterior curvature of the neural spine, and a deeper concavity on the posteromedial portion of the neural arch (Jasinski and Moscato, 2017). There has been no work to definitively separate the vertebral morphology of Pantherophis and Elaphe, in part because their reclassification as separate genera has not been morphologically defined since their separation in Utiger et al. (2002).
Pantherophis kansensis Gilmore, 1938 †
Figure 7
Material. UNSM 139983 (21 pre-cloacal trunk vertebrae).
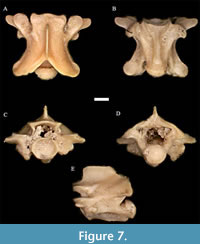 Description. In anterior view, the neural spine is relatively low for a colubrid, the neural arch is moderately vaulted, and the zygosphene is convex dorsally. The zygosphenal articular facets are strongly tilted dorso-ventrally. The neural canal is rounded and slightly smaller than the cotyle, which is also round. The prezygapophyseal articular facets tilt slightly upward, and the diapophyses and parapophyses are rounded, distinct sections of the synapophyses.
Description. In anterior view, the neural spine is relatively low for a colubrid, the neural arch is moderately vaulted, and the zygosphene is convex dorsally. The zygosphenal articular facets are strongly tilted dorso-ventrally. The neural canal is rounded and slightly smaller than the cotyle, which is also round. The prezygapophyseal articular facets tilt slightly upward, and the diapophyses and parapophyses are rounded, distinct sections of the synapophyses.
In dorsal view, the neural spine is long and located in the middle of the vertebra. The anterior edge of the zygosphene is concave. The prezygapophyseal accessory processes are moderately pointed, the prezygapophyseal articular facets are rounded to oval and tilted slightly upward, and the epizygapophyseal spines are absent. The posterior edge of the neural arch in dorsal view is slightly rounded.
In lateral view, the neural spine is approximately twice as long as it is high. The condyle is tilted slightly forward. The hemal keel is distinct from the centrum, and the synapophyses can clearly be divided into diapophyseal and parapophyseal sections. The interzygapophyseal ridge is bowed downward, and the subcentral ridge is bowed upward.
In posterior view, the neural arch is somewhat vaulted. The condyle is round and approximately the same size as the neural canal, which is an inverted U-shape. The zygosphenal articular facets and the postzygapophyseal articular facets are all tilted sharply upward. The diapophyses and parapophyses are distinct parts of the synapophyses.
In ventral view, the centrum is triangular and bordered by visible subcentral ridges. The hemal keel is well-developed, moderately wide, and constricted in the middle relative to the ends. The prezygapophyseal accessory processes are distinct, oblique, and directed anterolaterally. As in other views, the synapophyses are distinctly divided into the diapophyses and parapophyses.
Remarks. Pantherophis kansensis has vertebrae with a lower neural spine and more anterolaterally directed prezygapophyseal accessory processes than any other known species of Pantherophis or Bogertophis. This neural spine is relatively taller in some specimens, and is more proportionally more similar to Pantherophis obsoletus in that regard; these vertebrae appear to be from a more anterior portion of the vertebral column.
Genus SALVADORA Baird and Girard, 1853
Diagnosis. Generally, Salvadora can be identified by the combination of the following characters: (1) a thin, relatively low neural spine that gets shorter posteriorly through the trunk region; (2) obsolete to absent epizygapophyseal spines; (3) vertebrae that are approximately as wide as they are long; and (4) dorsally convex subcentral ridge in lateral view (Holman, 1973).
Remarks. The delicate neural spine and invariably thin hemal keel is similar to that found in other North American colubrids such as Coluber or Masticophis. However, Salvadora can be identified separately from those taxa on the basis of a proportionally shorter vertebra that appears to be nearly as wide as it is long, whereas the other two taxa appear elongate, more dorsally convex subcentral ridges, and obsolete to absent epizygapophyseal spines. Pantherophis, Pituophis, and Lampropeltis possess more robust and relatively wider vertebrae with less delicate neural spines and broader hemal keels than those of Salvadora. Jurestovsky (2021) also noted that extant Salvadora differ from Carphophis, Diadophis, and Gyalopion in possessing longer, more pointed accessory processes and a uniformly thinner hemal keel. Carphophis also possesses a narrower zygosphene, more elongate centrum, a shorter neural spine, less laterally directed postzygapophyses, and a less dorsoventrally tall cotyle (Jurestovsky, 2021). Salvadora has less robust postzygapophyses than Diadophis and possesses a more elongate centra, a taller neural spine, and a dorsoventrally taller cotyle than in Gyalopion (Jurestovsky, 2021).
Salvadora paleolineata Holman, 1973 †
Figure 8
Material. UNSM 139984 (8 pre-cloacal trunk vertebrae).
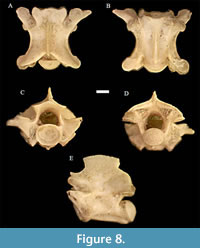 Description. The description is similar to Holman (2000) unless otherwise noted. In anterior view, the cotyle is a slightly depressed oval bookmarked by relatively large paracotylar foramen to each side. The neural canal is similar in size to the cotyle and is an inverted U-shape with medially convex sides to the neural arch. The zygosphene is dorsally convex in this view. The prezygapophyses are tilted slightly upward, and the prezygapophyseal accessory processes are well developed.
Description. The description is similar to Holman (2000) unless otherwise noted. In anterior view, the cotyle is a slightly depressed oval bookmarked by relatively large paracotylar foramen to each side. The neural canal is similar in size to the cotyle and is an inverted U-shape with medially convex sides to the neural arch. The zygosphene is dorsally convex in this view. The prezygapophyses are tilted slightly upward, and the prezygapophyseal accessory processes are well developed.
In dorsal view, the vertebra is approximately as wide as it is long. The neural spine is thin and slightly overhangs the neural arch posteriorly. The zygosphene is slightly convex anteriorly. The prezygapophyseal articular facets are subrounded, and the prezygapophyseal accessory processes are approximately two-thirds as long as the width of the prezygapophyseal articular facets and are well-developed and moderately pointed. The diapophyses are only somewhat directed laterally and are barely visible from this orientation.
In lateral view, the neural spine is delicate and is low, over three times as long as it is tall, with a slight anterior and posterior overhang. The subcentral groove is relatively deep, with dorsally convex subcentral ridges and a clear, uniform hemal keel. The condyle is tilted somewhat postero-dorsally, while the cotyle is oriented slightly antero-ventrally. The lateral foramen is positioned near the center of the vertebrae in this orientation, just below the interzygapophyseal ridge. Epizygapophyseal spines are absent.
In posterior view, the subcentral ridges are well-developed and narrow, and the hemal keel is strongly developed and uniformly thin. The postzygapophyseal articular facets are subrounded. The condyle is a slightly depressed oval. Epizygapophyseal spines are absent, as also seen in the lateral orientation.
In ventral view, the zygosphene is slightly convex anteriorly. The prezygapophyseal accessory processes are well developed. The hemal keel is uniformly thin. Both the cotyle and the condyle are oval in shape and the condyle extends to be approximately even with the postzygapophyseal articular facets.
Remarks. According to Holman (2000) and our own comparisons, the vertebrae from Penny Creek assigned to S. paleolineata are primarily from the middle trunk region, as noted by neural spines somewhat taller than in the type specimen and the larger condylar-cotylar articulation. As in Holman’s description, none of the specimens appear to have epizygapophyseal spines; as such, taxonomic assignment to S. paleolineata is possible. S. paleolineata is suggested as an ancestral species to modern Salvadora species, which differ only in possessing obsolete epizygapophyseal spines (see description of genus above; Holman, 1973). S. paleolineata also differs from Coluber and Masticophis in vertebral proportions, where the former has proportionally shorter, wider vertebrae and less dorsally convex subcentral ridges, as well as in the complete absence of epizygapophyseal spines (Holman, 2000). Dakotaophis greeni possesses smaller zygapophyseal articular facets than S. paleolineata, and the articular facets are more elongate or oval in shape and oriented less laterally (Holman, 2000). It is possible that, as in Heterodon/Paleoheterodon, there may be greater morphological differences in the elements of the skull, should they ever be found for this species.
Family DIPSADIDAE Bonaparte, 1838
Diagnosis. Vertebrae can be assigned to Dipsadidae through a combination of characters that individually are not necessarily exclusive to the group. Dipsadid snakes generally have vertebrae that are square to slightly longer than wide in dorsal view, an elongated centrum, and long, low, and narrow neural spines that overhang posteriorly (some taxa also overhang anteriorly) (Holman, 2000; Parmley and Hunter, 2010; Holman et al., 2011; Mead and Steadman, 2017; Jurestovsky, 2021; Syromyatnikova et al., 2021). The neural arch is depressed to moderately vaulted in different species, and the zygosphene is usually crenate, but can be concave in some species when viewed dorsally (Holman, 2000; Mead and Steadman, 2017). The prezygapophyses generally protrude only slightly beyond the ovoid prezygapophyseal facets to form small points (Mead and Steadman, 2017). The cotyle and condyle are round to slightly dorsoventrally compressed, where the cotyle is flattened on the ventral portion in anterior view (Mead and Steadman, 2017; Jurestovsky, 2021) Epizygapophyseal spines are absent (Holman, 2000; Mead and Steadman, 2017; Jurestovsky, 2021). The synapophyses are divided into distinct diapophyses and parapophyses (Holman, 2000; Syromyatnikova et al., 2021). Distinct hemal keels with adjacent subcentral ridges are present, and hypapophyses are absent from trunk vertebrae except potentially in a few species (Holman, 2000; Ikeda, 2007; Head et al., 2016; Mead and Steadman, 2017; Syromyatnikova et al., 2021)
Remarks. The vertebrae of many extant dipsadid snakes have not yet been described and is outside the scope of this study, making it difficult to confirm the above characters across the entire group. We have, however, assembled the common characters expressed across several publications (Holman, 2000; Parmley and Hunter, 2010; Holman et al., 2011; Mead and Steadman, 2017; Jurestovsky, 2021; Syromyatnikova et al., 2021) - and our personal observations of some North American and West Indian taxa - as a way to delimit dipsadids from colubrids and natricids through a combination of characters.
Genus HETERODON Latreille, 1801 (in Sonnini and Latreille, 1801)
or PALEOHETERODON Holman, 1964 †
Diagnosis. The trunk vertebrae of Heterodon and Paleoheterodon can exhibit a very depressed to slightly more vaulted neural arch (though relatively depressed overall compared to other dipsadids), a narrow neural spine that is longer than it is high, and a wide but flattened hemal keel (Holman, 2000). The vertebrae are slightly more long than wide, including the centrum, but still somewhat squarish appearance (Holman, 2000). The zygpophyseal articular facets are ovoid, and the prezygapophyseal accessory processes are moderately well-developed, end in somewhat obtuse points, and extend just beyond the prezygapophyseal articular facets (Holman, 2000; Mead and Steadman, 2017; Jurestovsky, 2021). The condyle and cotyle are mostly round but may be slightly dorsoventrally compressed and flattened on the ventral side; both are similar in size to the neural canal (Holman, 2000). The synapophyses are divided into distinct diapophyses and parapophyses. Subcentral ridges are present adjacent to the hemal keel but are not prominent, the epizygapophyseal spines are absent, and hypapophyses are absent from post-cervical vertebrae (Holman, 2000). The zygosphene is variably crenate/convex or concave (Holman, 2000).
Remarks. Though it is possible to identify trunk vertebrae to genus for Heterodon or possibly Paleoheterodon, individual vertebrae of this group are difficult to distinguish at the species level. They share most diagnostic features with the genus Farancia, but the vertebrae of Farancia generally exhibit greater anteroposterior compression (leading to a laterally wider appearance overall; Jurestovsky, 2021) a more vaulted neural arch, and a neural spine that is more deeply undercut both anteriorly and posteriorly than in Heterodon (Holman, 2000). Vertebrae associated with specimens previously assigned to Paleoheterodon may show the same differences from Farancia as Heterodon, except in the neural arch, which overlaps with both Farancia and Heterodon in how depressed/vaulted the shape of that region is (Holman, 2000; Parmley and Hunter, 2010; Head et al., 2016). Farancia and the Heterodon/Paleoheterodon group are both identifiable within Dipsadidae in possessing comparatively flattened and depressed neural arches (Head et al., 2016), longer than high neural spines (Jurestovsky, 2021) and wide, relatively flat hemal keels (Holman, 2000, Jurestovsky, 2021).
Heterodon Latreille in Sonnini and Latreille, 1801
or Paleoheterodon Holman, 1964 † sp. indet.
Figure 9
Material. UNSM 139985 (12 pre-cloacal trunk vertebrae).
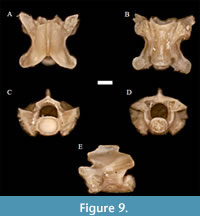 Description. In dorsal view, the vertebrae are nearly square to slightly longer than wide. The anterior edge of zygosphene is convex, and the prezygapophyseal facets, when preserved, are ovoid in shape. The prezygapophyseal accessory processes are well-developed and the tips are moderately pointed to obtuse. Epizygapophyseal spines are absent. The postzygapophyseal accessory processes are ovoid in shape.
Description. In dorsal view, the vertebrae are nearly square to slightly longer than wide. The anterior edge of zygosphene is convex, and the prezygapophyseal facets, when preserved, are ovoid in shape. The prezygapophyseal accessory processes are well-developed and the tips are moderately pointed to obtuse. Epizygapophyseal spines are absent. The postzygapophyseal accessory processes are ovoid in shape.
In anterior view, both articular facets are visible on the synapophyses. The cotyle is mostly round, but sometimes slightly taller than wide. The neural arch is depressed. The neural canal is similar in shape to a ventrally restricted semi-cylinder, even somewhat squarish, and similar in size to the cotyle. The cotyle is somewhat dorso-ventrally compressed, with well-excavated pits on either side.
In lateral view, the neural spine is longer than it is tall, somewhat depressed, and is typically more undercut posteriorly than anteriorly. In posterior view, the shape of the condyle is a dorso-ventrally compressed oval, and similar overall to the cotyle.
In ventral view, the hemal keel generally is weak, wide, and oblong, and very depressed to flat. In some specimens, the hemal keel is slightly more distinct, and is slightly constricted between the synapophyses, indicating a more anterior trunk position for these vertebrae. The subcentral ridges are indistinct. The centrum itself is longer than it is wide.
Remarks. The somewhat more vaulted nature of the neural arch in the specimens from Wt 13B when compared to extant Heterodon vertebrae (a morphological difference also shared with Farancia, as stated by Head et al., 2016) would previously have suggested that these fossils belong to or are comparable to Heterodon (or Paleoheterodon) tiheni, a species that is more easily determined based on skull morphology, rather than vertebral morphology (Holman, 1964; Holman, 2000; Parmley and Hunter, 2010). However, Parmley and Hunter (2010) determined that this neural arch character showed considerable and overlapping variation in specimens assigned to Paleoheterodon and Heterodon, and that vertebral characters are not sufficient to differentiate between the two taxa. Unfortunately, we lack reproducible or statistical data on both the variation and the degree of overlap between these taxa, as both Holman (1977; 2000) and Parmley and Hunter (2010) included only personal observations on the topic. Furthermore, these vertebrae lack the apomorphies of the fossil species Heterodon meadi, which exhibits a zygosphenal groove and anteroposteriorly directed sculpturing on the neural arch, but lacks skull elements to clarify its association with Heterodon/Paleoheterodon (Jurestovsky, 2021). Similarly, the unnamed but potentially different taxon from the Sappa Creek Fauna in Kansas also lacks cranial elements (Holman et al., 2011). Because we presently do not have enough information regarding both vertebral and skull characters to confidently differentiate these taxa, and because these taxa overlap temporally (albeit in younger deposits; Parmley and Hunter, 2010), we describe it here as Heterodon or Paleoheterodon of an indeterminate species. Future work using quantitative and morphometric methods with isolated skeletal elements may potentially help untangle this taxonomic issue.
Family NATRICIDAE Bonaparte, 1840
Diagnosis. North American natricid vertebrae have well-developed, pointed hypapophyses directed posteriorly on the trunk vertebrae (Holman, 2000). These hypapophyses are usually sigmoid in shape (Szyndlar, 1991). The vertebrae overall are lightly built and elongate, with long centra, strong subcentral ridges, posteriorly vaulted neural arches, and somewhat short parapophyseal processes (Szyndlar, 1991).
Remarks. Szyndlar (1991) differentiated natricid snakes from other snake groups known to possess hypapophyses on their trunk vertebrae. Natricids differ from viperids in exhibiting hypapophyses that are somewhat sigmoidal in shape, and in possessing a relatively longer centra, posteriorly vaulted neural arches, and shorter parapophyseal processes. They differ from elapids in being more lightly build overall, with much longer centra and prominent subcentral ridges. Despite the hypapophyses being presented as a definitive character for natricids as a whole, McDowell (1961), Malnate (1972), and Ikeda (2007) showed that there are a few exceptions to this rule outside of North America, possibly representing a loss of this character later in the evolution of some species.
Genus NEONATRIX Holman, 1973 †
Diagnosis. Neonatrix trunk vertebrae are most easily characterized by very short hypapophyses that strongly project posteriorly, are ventrally beveled in most North American species (except Neonatrix elongata) but are weakly developed, terminally rounded, and do not extend beyond the end of the condyle. Furthermore, they are relatively small and distinctly longer than wide. The neural spines of Neonatrix are much longer (up to 4x) than wide (Holman, 1973, 2000; Parmley and Hunter, 2010), with reduced or absent hooked projections at each terminal end. The zygosphene is convex, but less so in caudal vertebrae (Jasinski and Moscato, 2017).
Remarks. The hypapophyses of North American species of Neonatrix are less well-developed than the reported species from Europe (Holman, 1973, 1982, 1996; Rage and Holman, 1984) and shorter than in any other natricid genus (Holman, 1973, 2000). Neonatrix also differs from Thamnophis and Nerodia in possessing an anteriorly taller neural spine that lacks hooked projections and a less prominant subcentral ridge (Jasinski and Moscato, 2017). The neural spine of Neonatrix is also taller than in Storeria, Tropidoclonion, Virginia, and Micronatrix, but shorter than in Seminatrix (Parmley and Hunter, 2010; Jasinski and Moscato, 2017)
Neonatrix elongata Holman, 1973 †
Figure 10
Material. UNSM 139986 (17 pre-cloacal trunk vertebrae).
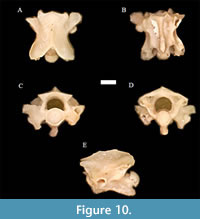 Description. The diagnosis follows that of Holman (2000) and is only modified where otherwise noted. Neonatrix elongata trunk vertebrae are longer than wide, with a neural spine that is drastically longer than tall or wide. The vertebrae possess poorly developed hypapophyses reaching only the posterior portion of the centrum, and unbeveled neural spines that end just short of the posterior end of the cotyle.
Description. The diagnosis follows that of Holman (2000) and is only modified where otherwise noted. Neonatrix elongata trunk vertebrae are longer than wide, with a neural spine that is drastically longer than tall or wide. The vertebrae possess poorly developed hypapophyses reaching only the posterior portion of the centrum, and unbeveled neural spines that end just short of the posterior end of the cotyle.
In anterior view, the neural canal is shaped like a ventrally restricted semi-cylinder, is medially convex at the sides, and is slightly narrower than the round cotyle. The zygosphene is convex dorsally. The synapophysis is developed. For the first time in this genus and species, we confirm the presence of paracotylar foramina in anterior view, adjacent to the cotyle on each side, approximately half-way down the cotyle. Previous descriptions exhibited excavated cavities on either side of the cotyle, but could not observe any foramina based on the specimens available.
In dorsal view, the centrum is longer than wide. The prezygapophyseal articular facets are ovoid in shape. This is a correction of Holman (2000), which appears to have mistakenly labeled the prezygapophyseal processes as ovoid, rather than the articular facets. The prezygapophyseal processes are long and somewhat pointed on the anterior end. The diapophyses only slightly extend out laterally. The epizygapophysel spines are absent or obsolete.
In lateral view, the vertebrae are elongate. The neural spine is over two times as long as it is high. The neural spine is rarely preserved at the ends in Penny Creek fossils and in Neonatrix throughout the fossil record, and so is difficult to observe, but a few mostly preserved neural spines from Penny Creek material allow us to determine the absence of an anterior projection, and an extremely reduced to absent - typically absent - posterior overhang. The subcentral ridge is convex dorsally. The prezygapophyses tilt somewhat dorsally. The hypapophysis is short and ends short of the posterior-most part of the condyle; we find that it is does not extend past even the anterior-most part of that articulation in these specimens.
In posterior view, we find that the condyle is near circular, but is slightly depressed and closer to an oval shape in some vertebrae. The neural arch is vaulted, steeply incised by the zygantral articular facets, and similar in size to the condyle. The hypapophysis is visible below the condyle around one-half of the condyle’s height when undamaged. The postzygapophyses tilt slightly upward, just as in the prezygapophyses. In ventral view, the centrum is long and narrow. The subcentral grooves are shallow, and the hemal keel narrows slightly anteriorly to the robust but truncated hypapophysis.
Remarks. Like other species of North American Neonatrix, N. elongata have less well-developed hypapophyses that do not extend as far posteriorly (relative to the condyle) than found in the European species (Rage and Holman, 1984). N. elongata occurs earlier than other North American species of Neonatrix and is known to occur more broadly in a temporal and geographic manner throughout the Miocene. In the Penny Creek specimens, the unbeveled neural spine and unbeveled hypapophysis that end just short of the posterior end of the cotyle indicate that these fossils belong to N. elongata, as opposed to other known species of Neonatrix. There is some variation on how rounded (or pointed) the posterior tips of the hypapophyses are, likely indicating a small amount of intracolumnar variation in the hypapophyseal morphology of the species (LaDuke, 1991; McCartney, 2015). Nonetheless, we have not observed more variation within an individual taxon than between species, and the morphology is generally consistent with what is described above. Neonatrix elongata is typically smaller than Neonatrix magna and Neonatrix infera, but the taxon’s relative length and neural spine height are intermediate between the other two species, which are either more elongate with a shorter neural spine (N. infera) or less elongate with a taller neural spine (N. magna; Holman, 2000). It is noteworthy that these specimens show some measure of variation in terms of the neural spine height and the shape of the hypapophyses, some of which appear similar to some morphologies known in the other North American species. Given that N. elongata was considered to have “intermediate” morphologies by Holman (2000), it may be necessary to further study the intracolumnar variation with Neonatrix for additional definitive characters and consider potential changes to the taxonomy of the species within.
Neonatrix magna Holman, 1982 †
Figure 11
Material. UNSM 139987 (8 pre-cloacal trunk vertebrae).
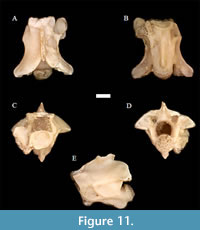 Description. The diagnosis is based on that of Holman (2000) but is modified where noted. The vertebrae are mainly distinguished by (1) a very poorly developed hypapophysis that typically terminates near the posterior part of the centrum; (2) ventral portion of the hypapophysis beveled and ending well anterior to the condyle; (3) vertebral size relatively large (at least relative to other North American Neonatrix at >5 mm); (4) vertebrae nearly the same width and length; and (5) neural spine near same height and length.
Description. The diagnosis is based on that of Holman (2000) but is modified where noted. The vertebrae are mainly distinguished by (1) a very poorly developed hypapophysis that typically terminates near the posterior part of the centrum; (2) ventral portion of the hypapophysis beveled and ending well anterior to the condyle; (3) vertebral size relatively large (at least relative to other North American Neonatrix at >5 mm); (4) vertebrae nearly the same width and length; and (5) neural spine near same height and length.
In anterior view, the cotyle is round and similar in size to the neural canal, which is an inverted U-shape with slightly convex lateral sides. The zygosphene is dorsally straight. The prezygapophyses are slightly tilted dorsally. Short excavations with single, centered paracotylar foramen border each side of the cotyle. We observe that the synapophyses that are missing from previous descriptions are moderately well developed and differentiated.
In dorsal view, the centrum’s length and width are similar. The prezygapophyses are ovoid. The neural spine is somewhat thick and slightly overhangs posteriorly when preserved. Epizygapophyseal spines are absent. The zygosphene is slightly convex anteriorly. The diapophyses are produced laterally enough to be seen from the dorsal orientation.
In lateral view, the vertebra is only slightly elongate. The neural spines are not completely preserved but appear as though they are similar in height and length. There is a slight overhang on the posterior border of the neural spine. The hypapophysis is beveled ventrally, about as tall as it is long, and ends anterior to the condyle, but appears to have rounded rather than the pointed tips observed by Holman (1982; 2000).
In posterior view, the neural arch is significantly vaulted. We observed that the condyle is mostly round and slightly compressed, rather than truly oval, and is similar in size to the neural canal. The hypapophyses strongly projects ventrally and is clearly visible below the condyle.
In ventral view, the hypapophysis is somewhat narrow, ends clearly anterior to the anterior end of the condyle, and is beveled on the ventral surface. The subcentral ridges are well developed.
Remarks. Neonatrix magna has the shortest relative length and widest appearance, the highest neural spine, and most anteriorly terminating hypapophyses of any species of Neonatrix in North America or Europe. It also has somewhat less strongly developed subcentral ridges and deep subcentral groups than N. elongata or N. infera.
Several of our observations contribute to or differ slightly from previous descriptions of the species. We observe that the synapophyses are moderately developed. The hypapophyses of our specimens have rounded rather than pointed tips, and the condyle, while slightly depressed, is better described as round rather than oval. Comparison with specimens of N. elongata and examination of intracolumnar differences in vertebral morphology in extant natricid snakes suggest that the hypapophysis does change shape along the column; however, the differences in the hypapophysis alone between the two species described from fossils seems to be greater than generally seen in other natricids, except between anterior or middle trunk vertebrae and precloacal vertebrae. Given other morphological differences discussed above and the observation that N. magna is not smaller but would appear to have a morphology more similar to the smaller posterior trunk and precloacal vertebrae, we do not believe that these vertebrae represent the same species. Nonetheless, little is known about the intracolumnar variation of Neonatrix at this time, as preserved elements are mainly trunk vertebrae; this would seem to warrant a more complete investigation, as also suggested by Jasinski and Moscato (2017).
Genus NERODIA Baird and Girard, 1853
Diagnosis. The trunk vertebrae of Nerodia are typically medium to large in size and relatively short and wide, with an elongate centrum (Holman, 2000). The neural spines and hypapophyses are prominent (Holman, 2000). The neural spine is tall and undercut on both the anterior and posterior sides. The robust hypapophyses on each precaudal vertebra are well-developed, laterally compressed, directed posteriorly, and usually end in a somewhat pointed tip extending beyond the condylar head (Holman, 2000). Epizygapophyseal spines are absent (Holman, 2000).
Remarks. The trunk vertebrae of Nerodia relative to other North American natricids are typically medium to large in size, relatively robust in appearance, and exhibit higher neural spines (Holman, 2000). The vertebrae are still elongate relative to large North American colubrines (Holman, 2000). Nerodia vertebrae are typically less elongate than Thamnophis vertebrae, with a more ventrally-oriented hypapophysis with a steeper angle relative to the centrum (LaDuke, 1991; Holman, 2000; Jasinski and Moscata, 2017). Nerodia also exhibits a more vaulted neural arch, broader and more robust hypapophyses, but more gracile prezygapophyses compared to Thamnophis (Jasinski and Moscato, 2017). They possess higher neural spines than Storeria, Tropidoclonion, and Virginia (but relatively shorter than Regina), and the hypapophyses are longer and less squared in shape when compared to Regina (Holman, 2000). It should be noted that the degree to which the hypapophysis extends beyond the condylar head appears to differ between some taxa, and perhaps between individuals, though this has not been extensively studied at this time. Other characters of Nerodia, such as neural spine height, hypapophysis shape, and the degree to which the neural spine is undercut all vary to some degree at least between species of the genus (Holman, 2000; pers. obs.).
Nerodia sp. indet.
Figure 12
Material. UNSM 139988 (24 pre-cloacal trunk vertebrae).
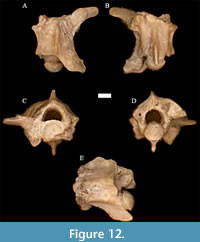 Description. These trunk vertebrae are large in size and relatively wide when including the prezygapophyseal accessory processes, but are still relatively elongate along the centrum. The neural spines are tall and straight dorsally and somewhat undercut both anteriorly and posteriorly, but are rarely preserved in their entirety, hindering full comparisons between neural spine height and length. The neural arch is vaulted. The zygosphene is convex when viewed dorsally. The condyle and cotyle are round, and larger than the neural canal. The prezygapophyseal and postzygapophyseal facets are ovaloid in shape (sensu Holman, 2000) and oblique to the long axis of the centrum. The epizygapophyseal spines are absent. The subcentral ridges are pronounced and form deep grooves along the hypapophysis. The hypapophyses on each precaudal vertebra are well-developed, laterally compressed, directed posteriorly, and end in a somewhat pointed tip that extends posteriorly beyond the condylar head when complete.
Description. These trunk vertebrae are large in size and relatively wide when including the prezygapophyseal accessory processes, but are still relatively elongate along the centrum. The neural spines are tall and straight dorsally and somewhat undercut both anteriorly and posteriorly, but are rarely preserved in their entirety, hindering full comparisons between neural spine height and length. The neural arch is vaulted. The zygosphene is convex when viewed dorsally. The condyle and cotyle are round, and larger than the neural canal. The prezygapophyseal and postzygapophyseal facets are ovaloid in shape (sensu Holman, 2000) and oblique to the long axis of the centrum. The epizygapophyseal spines are absent. The subcentral ridges are pronounced and form deep grooves along the hypapophysis. The hypapophyses on each precaudal vertebra are well-developed, laterally compressed, directed posteriorly, and end in a somewhat pointed tip that extends posteriorly beyond the condylar head when complete.
Remarks. The only other concurrent species during the Miocene is Nerodia hillmani and an indeterminate species from the Pratt Slide (Bw 123) locality, which is ~10.5-9.5 Ma (Clarendonian 3). Based on the larger size, longer hypapophysis, and longer prezygapophyseal articular facets, the Nerodia fossils described here are more similar to the unnamed Pratt Slide species than to N. hillmani. Comparison to more specimens, extant Nerodia sipedon and the somewhat younger Nerodia hibbardi from the Pliocene of Idaho and possibly Texas (Holman, 1968, 2000) may be necessary before assigning a species-level classification to these fossils.
RESULTS AND DISCUSSION
The Neogene fossil record of the Central Great Plains has produced the most significant and continuous record of the evolution and modernization of snakes in North America (Figure 2). While the number of taxa represented in Penny Creek locality Wt 13B is low when compared to other Nebraska localities, such as the older Late Barstovian Myers Farm Local Fauna or the younger Late Clarendonian Pratt Slide Local Fauna (Holman, 1977; Parmley and Hunter, 2010), the Penny Creek snake assemblage is a crucial piece of evidence to understand diversification and modernization of North American snake faunas. As the only described snake assemblage from the earliest Clarendonian (12.5-12.0 Ma), the presence of the snake species identified here can now be confirmed as having been present in the area, whereas the presence of these taxa could previously only be assumed based on bracketing snake assemblages from older and younger localities (Figure 2). Furthermore, the snakes of Wt 13B represent the first documentation of non-testudine reptiles documented in a period temporally bracketed by the earlier peak of reptilian diversity in the warmer, more humid, and likely more closed environments of the Late Barstovian of the Central Great Plains (Holman, 1977) and the later Yellowstone hotspot eruption that preserved the Ashfall Fossil Beds lagerstätten in an opening, cooler, and more arid northeastern Nebraska (Voorhies, 1992). Penny Creek, therefore, provides the first opportunity to look at not only what snakes were present during this period, but also allows for paleoenvironmental predictions based on their poikilothermic physiologies and modern proxies.
Of the eight taxa identified at Penny Creek, all taxa identified to the species level are extinct; however, only Neonatrix is extinct at the genus level. Charina persists in western North America and Salvadora persists in the southwestern United States and Mexico, but are extirpated from Nebraska and the Central Great Plains. All other genera described from Penny Creek currently persist in the Central Great Plains. Thus, approximately 88.9% of this assemblage can be considered taxonomically “modern” (i.e., extant at the genus level). Furthermore, the low abundance of booid relative to colubroid taxa supports the idea of a taxonomically “modern” assemblage. This site bridges the previous temporal gap in the transitional modernization of North American snake assemblages.
The lack of identified material for other previously common booids, such as Calamagras, Geringophis, Ogmophis, Pterygoboa, and Tregophis, does not reject the possibility of these taxa persisting over a wide geographic range in the post-Barstovian Central Great Plains, but their absence (or relative rarity) at both this locality and nearly all other published younger Miocene localities in the Central Great Plains is notable. Only two documented younger Clarendonian localities in the Central Great Plains have identified booids not attributable to Charina or Lichanura and these localities are associated with environmental settings that differ from similarly-aged localities in the same region. The WaKeeney local fauna preserves a “small stream-filled basin” depositional environment with vegetation and climate apparently comparable to present-day southeastern Texas during the Middle Clarendonian (Holman, 1975). One vertebra each - both of which represent the type specimens for both taxa - of Tregophis brevirachis and Ogmophis pliocompactus were described out of 1230 total snake vertebrae, an exceptionally low occurrence rate for the total number of snake fossils (Holman, 1975). The Pratt Slide local fauna has been suggested as a wooded valley occupied by a riparian stream that is adjacent to a savanna landscape; this idea is supported by geological, paleontological, and isotopic data and differs from other localities of the Late Clarendonian in Nebraska (Rudnick, 1994; Parmley and Hunter, 2010; Parmley et al., 2015; Kita et al., 2014). This assemblage contains Calamagras sp. indet. or Ogmophis sp. indet., along with the second documented occurrence of Tregophis brevirachis. Both taxa are represented at Pratt Slide by three identifiable vertebrae each. As far as we are currently able to determine based on known occurrences and the above information, it seems possible that the state of the North American booid fossil record represents a true decline in extinct booid taxa in terms of both geographic range and relative abundance, rather than a lack of preservation during the Clarendonian and later. In broader terms, it is possible that the Clarendonian of North America represents a near-complete decline (but not complete loss) of now-extinct booid taxa as soon as the Central Great Plains began transitioning to a more open, arid, cooler, and seasonal environment from more closed, humid Barstovian environments. If that is the case, then the aforementioned examples above may represent a few persistent pockets containing relatively rare holdovers, where environmental conditions were more reminiscent of those in the Late Barstovian of the same region.
The transition away from extinct colubroids, for the most part, appears more prolonged. Starting with the Late Barstovian, Dakotaophis makes its last appearance in the Bijou Hills local fauna of South Dakota (Holman, 1978), and Micrurus is extirpated from the region (Holman, 1977), and then does not reappear until the Early Pleistocene (Irvingtonian I) Inglis IA site of Florida and the Middle to Late Pleistocene (Irvingtonian 2) Fyllan Cave Fauna of Texas (Meylan, 1982; Holman and Winkler, 1987). As in the booids above, Texasophis and Ameiseophis make their final appearances at the unique Wakeeney, Ashfall, and Pratt Slide localities in the Middle and Late Clarendonian of the Central Great Plains (Parmley and Hunter, 2010). Paracoluber and Nebraskophis last appear in the Hemphillian (Late Miocene) Lemoyne Quarry of Nebraska; and Salvadora is extirpated from the region following the Hemphillian. Finally, Neonatrix makes its last known appearance in the Central Great Plains at Lemoyne Quarry, but may potentially be present in the Hemphillian Mio-Pliocene Gray Fossil Site of Northeastern Tennessee as well (Parmley and Holman, 1995; Jasinski and Moscato, 2017).
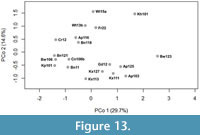 Our PCoA (Figure 13) and complete-linkage HCA (Figure 14) produced similar results regarding the dissimilarity of the 19 selected fossil localities from Nebraska. The PCoA axes 1 and 2, which represented 29.7% and 14.6% (44.3% total) of the variation in the dataset, indicate that the loss of “archaic” booids and colubroids (particularly natricids) and the introduction of “modern” colubroids - including a more diverse crotaline and colubrine presence - lead to the separation of the localities into multiple groups (Figure 13-Figure 14). Our HCA further clarifies these clusters based on a dissimilarity matrix of each locality (Figure 14
Our PCoA (Figure 13) and complete-linkage HCA (Figure 14) produced similar results regarding the dissimilarity of the 19 selected fossil localities from Nebraska. The PCoA axes 1 and 2, which represented 29.7% and 14.6% (44.3% total) of the variation in the dataset, indicate that the loss of “archaic” booids and colubroids (particularly natricids) and the introduction of “modern” colubroids - including a more diverse crotaline and colubrine presence - lead to the separation of the localities into multiple groups (Figure 13-Figure 14). Our HCA further clarifies these clusters based on a dissimilarity matrix of each locality (Figure 14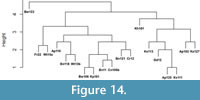 ). The Pratt Slide local fauna (Bw 123) was an outlier for the localities and forms the first main cluster. This is likely because of its high genus-level richness, which includes an uncommon combination of extinct, novel, and extant taxa. Our second main cluster contains the ten other localities from the Barstovian through the Clarendonian, with the addition of the Hemphillian Cn 106b, which only has Thamnophis and indeterminate colubrines identified. All other localities in this cluster seem to be defined by the presence of now extinct or extirpated taxa, including taxa such as Neonatrix and Pterygoboa. Our third and final cluster includes all seven remaining Hemphillian through Blancan snake assemblages, with appearances from extant taxa such as Regina, Storeria, and Rhinocheilus, and more consistent identifications of extant crotaline genera. These results imply that the major compositional shift of the Central Great Plains occurred through the Clarendonian and the snake fauna was functionally a group of near extant assemblages by the Hemphillian.
). The Pratt Slide local fauna (Bw 123) was an outlier for the localities and forms the first main cluster. This is likely because of its high genus-level richness, which includes an uncommon combination of extinct, novel, and extant taxa. Our second main cluster contains the ten other localities from the Barstovian through the Clarendonian, with the addition of the Hemphillian Cn 106b, which only has Thamnophis and indeterminate colubrines identified. All other localities in this cluster seem to be defined by the presence of now extinct or extirpated taxa, including taxa such as Neonatrix and Pterygoboa. Our third and final cluster includes all seven remaining Hemphillian through Blancan snake assemblages, with appearances from extant taxa such as Regina, Storeria, and Rhinocheilus, and more consistent identifications of extant crotaline genera. These results imply that the major compositional shift of the Central Great Plains occurred through the Clarendonian and the snake fauna was functionally a group of near extant assemblages by the Hemphillian.
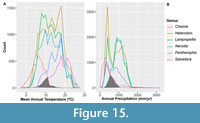 Our climate envelope models using congenerics produced clear ranges of temperature and precipitation overlap, with differences on which specific congenerics provided the upper and lower bounds (Figure 15). The MAT variable (Figure 15a) suggests that the lowest overlapping values are bounded by Salvadora, while the highest overlapping values are bounded by Charina, as these taxa respectively have higher minimum MAT and lower maximum MAT tolerances today. The overlapping MAT values range from just below 5°C to 16°C, with a maximum density between 11°C-12°C. The maximum density of the area of overlap may suggest that MAT in the earliest Clarendonian of Webster Co., NE was higher than the present (~10.55°C); however, the range of projections encompasses higher and lower temperatures. Additionally, this would suggest that MAT in the early Clarendonian of the Penny Creek local fauna was lower than proposed for the older Late Barstovian Myers Farm local fauna, which has been considered warm and temperate to sub-tropical (Holman, 1977; Holman, 2000; Corner, 2014), and supports an overall cooling climate in the early Clarendonian. The AP variable (Figure 15b) is bounded at the lower values by Nerodia and at the higher values by both Charina and Salvadora, although Nerodia and Charina respectively occupy the lower and upper limits at peak density. The overlapping AP values range from approximately 200-1100 mm, with maximum density at 550-600 mm. While these values range from well-below to well-above the present AP of Webster Co., the maximum density suggest a slightly drier environment in the earliest Clarendonian, again indicating a more open, temperate, and cooler environment after the MMCO. The combined MAT and AP values suggest that the most likely biome of the Penny Creek local fauna was a shrubland/woodland (Bailey, 1998; 2005), especially given the maximum density values for each. Slightly less likely biomes based on maximum and minimum values of MAT and AP also include temperate grassland/cold desert (lower AP) or temperate seasonal forest (higher AP); however, the majority of MAT and AP combinations suggest the shrubland/woodland biome.
Our climate envelope models using congenerics produced clear ranges of temperature and precipitation overlap, with differences on which specific congenerics provided the upper and lower bounds (Figure 15). The MAT variable (Figure 15a) suggests that the lowest overlapping values are bounded by Salvadora, while the highest overlapping values are bounded by Charina, as these taxa respectively have higher minimum MAT and lower maximum MAT tolerances today. The overlapping MAT values range from just below 5°C to 16°C, with a maximum density between 11°C-12°C. The maximum density of the area of overlap may suggest that MAT in the earliest Clarendonian of Webster Co., NE was higher than the present (~10.55°C); however, the range of projections encompasses higher and lower temperatures. Additionally, this would suggest that MAT in the early Clarendonian of the Penny Creek local fauna was lower than proposed for the older Late Barstovian Myers Farm local fauna, which has been considered warm and temperate to sub-tropical (Holman, 1977; Holman, 2000; Corner, 2014), and supports an overall cooling climate in the early Clarendonian. The AP variable (Figure 15b) is bounded at the lower values by Nerodia and at the higher values by both Charina and Salvadora, although Nerodia and Charina respectively occupy the lower and upper limits at peak density. The overlapping AP values range from approximately 200-1100 mm, with maximum density at 550-600 mm. While these values range from well-below to well-above the present AP of Webster Co., the maximum density suggest a slightly drier environment in the earliest Clarendonian, again indicating a more open, temperate, and cooler environment after the MMCO. The combined MAT and AP values suggest that the most likely biome of the Penny Creek local fauna was a shrubland/woodland (Bailey, 1998; 2005), especially given the maximum density values for each. Slightly less likely biomes based on maximum and minimum values of MAT and AP also include temperate grassland/cold desert (lower AP) or temperate seasonal forest (higher AP); however, the majority of MAT and AP combinations suggest the shrubland/woodland biome.
The results of the climate envelope models are supported by previous research using phytoliths (Strömberg 2004, 2006, 2011), vertebrate faunal composition (Turner, 1972; Corner, 2014), mammal hypsodonty (Janis et al., 2000, 2002, 2004; Strömberg, 2006), paleosols (Retallack, 2007), carbonate isotopes (Fox and Koch, 2004), and isotope values for mammalian teeth from the late Miocene (Wang et al., 1994; Kita et al., 2014), as well as the snakes in this study. Interestingly, Charina and Salvadora impose opposing limits for MAT, but share much of the upper limit for AP in our climate envelope models. While both snakes are presently located in western North America and overlap somewhat in the southwestern United States today, Charina is adapted to cooler temperatures and Salvadora to warmer temperatures compared to all other congenerics in this analysis, thus limiting the overlap area of MAT. The shared upper limit of AP composed of Charina and Salvadora likely reflects the high degree of drier biomes occupied by those taxa; the long tail of Charina’s data in AP when compared to Salvadora’s signifies the former’s presence in the Pacific Northwest of the United States and Canada. Conspicuously, both taxa were the only genera from this assemblage to be extirpated from Nebraska to the West and South during the Neogene, likely during the Hemphillian based on the last appearance datum for each (Jacisin et al., 2015). Nerodia, on the other hand, is typically found near permanent sources of water, which likely imposes a lower limit on AP in our plots. It should be noted that the results of our climate envelope models are slightly complicated by the lack of extant species identified from the Penny Creek material. By choosing closely related congeneric species from the present, we assume that our fossil species share similar ecologies and ecomorphologies, to their extant relatives, as suggested by niche conservatism. It is possible that directional trends of evolution related to climate are not detected when fossils are not included, as seen in other squamates such as Sceloporus (Lawing et al., 2016). Stronger predictions can be made by incorporating the entire known fossil record of a group in these models, but that is beyond the scope of this study.
Based on the ecologies of extant relatives for the taxa present at Penny Creek, the recovery of Charina, Lampropeltis, Pantherophis, Heterodon, and multiple natricid genera allows us to make a few assumptions on what the Penny Creek environment may have looked like. Extant Charina, Heterodon, Lampropeltis, and Salvadora all prefer loose substrates such as sandy soils, along with areas with plenty of ground-level cover (Holman, 1977; St. Clair, 1999; Plummer and Mills, 2000; Pyron and Burbrink, 2009, Davis and LaDuc, 2018). Nerodia, Thamnophis, and some species of Lampropeltis generally prefer to reside near permanent sources of water and the surrounding grassy areas (Holman, 1977; Holman and Schroeder, 1991, McVay et al., 2015). Finally, the presence of Charina may indicate a relatively mild, temperate-to-semi-arid environment, as extant members of that genus are relatively cold-adapted booids (St. Clair, 1999; Holman, 2000; Rodriguez‐Robles et al., 2001). Most of these snakes, including Pantherophis and crotaline viperids, are found across wide geographic swaths of North America today. The majority of the taxa would today be consistent with a transitional or mosaic woodland and prairie ecotone, making it somewhat similar to the Barstovian Egelhoff fauna (Holman, 1987); however, there is notably less amphibian and turtle material presently known from Wt 13b. This difference suggests a permanent stream or river as a local water source, instead of a pond or lake. Furthermore, the severely weathered state of the fossil vertebrates at Wt 13B additionally suggest a high energy fluvial source as a depositional environment.
The interpretation of a somewhat open, permanent fluvial environment surrounded by a transitional or mosaic prairie and woodland is consistent with previous studies focused on the geology and mammalian paleoecology of the Penny Creek Local Fauna (Turner, 1972; Voorhies et al., 1987; Voorhies, 1990). Furthermore, the Penny Creek fossil snakes represent the only presently described snake assemblage from 12.5-12.0 Ma in North America, providing us with an initial glimpse into the Clarendonian 1 North American Land Mammal Age for small herpetofauna. The description of the Penny Creek fossil snakes confirms the persistence of multiple taxa across the MMCO and contributes to our understanding of the interactions between post-MMCO environmental changes and the gradual modernization and evolution of North American snakes. Extensive future work is a requirement to better understand the radiation of the modern North American snake fauna, particularly in time intervals with sparse material and in regions outside of the Central Great Plains, but Penny Creek is a significant step towards filling the extensive gap of knowledge in our present understanding of ophidian history.
ACKNOWLEDGMENTS
We thank R. Short, L. Siciliano-Martina, J. West, J. Head, J. Mead, G. Corner, and C. Bell for input and beneficial discussion. We thank our anonymous reviewers for their constructive feedback. We thank the University of Nebraska State Museum and Vertebrate Paleontology Collections for access to fossils, as well as G. Corner and S. Tucker assistance and support. C. Sagebiel and M. Brown provided access to the University of Texas Vertebrate Paleontology Collections, and Toby Hibbitts and Heather Prestridge provided access to the Texas A&M University Biodiversity Collections for comparative specimens. J. Jacisin III was funded by a Texas A&M University College of Agricultural and Life Sciences Graduate Merit Fellowship and an Ecosystem Science and Management Research Mini-Grant from Texas A&M University.
REFERENCES
Auffenberg, W. 1963. The fossil snakes of Florida. Tulane Studies in Zoology, 10:131–216.
https://doi.org/10.5962/bhl.part.4641
Bailey, R.G. 1998. Ecoregions map of North America. U.S. Forest Service Miscellaneous Publications, 1548:1–10.
Bailey, R.G. 2005. Identifying Ecoregion Boundaries. Environmental Management, 34(Supplement 1):S14-S16. https://doi.org/10.1007/s00267-003-0163-6
Baird, S.F. and Girard, C. 1853. Catalogue of North American Reptiles in the Museum of the Smithsonian Institution. Part I. Serpentes. Smithsonian Miscellaneous Collections, 2:v–xvi + 172 p.
Bell, C.J. and Mead, J.I. 1996. Charina Gray, 1849 (Boidae: Erycinae) from the Pleistocene of California. Herpetological Natural History, 4:161–168.
Bell, C.J., Head, J.J., and Mead, J.I. 2004. Synopsis of the herpetofauna from Porcupine Cave, p. 117–126. In Barnosky, A.D. (ed.), Biodiversity Response to Climate Change in the Middle Pleistocene. The Porcupine Cave Fauna from Colorado. Berkeley: University of California Press.
https://doi.org/10.1525/california/9780520240827.003.0011
Bell, C.J., Gauthier, J.A., and Bever, G.S. 2010. Covert biases, circularity, and apomorphies: A critical look at the North American Quaternary Herpetofaunal Stability Hypothesis. Quaternary International, 217:30–36.
https://doi.org/10.1016/j.quaint.2009.08.009
Boellstorff, J. 1978. Chronology of some late Cenozoic deposits from the central United States and the Ice Ages. Nebraska Academy of Sciences Transactions, 6:35–49.
Bogert, C.M. 1964. Snakes of the genera Diaphorolepis and Synophis and the colubrid subfamily Xenoderminae. Senckenbergiana biologica, 45:509–531.
Bogert, C.M. and Rowley, J.S. 1968. A new genus and species of dwarf boa from southern Mexico. American Museum Novitates, 2354:1–38.
Bonaparte, C.L. 1838. Amphibiorum tabula analytica. Nuovi Annali delle Scienze Naturali, Bologna, 1:391–393.
Bonaparte, C.L. 1840. Amphibia europaea ad systema nostrum vertebratorum ordinate. Memorie della Reale Accademia delle Scienze di Torino, 2:385–456.
Boulinier, T., Nichols, J.D., Sauer, J.R., Hines, J.E., and Pollock, K.H. 1998. Estimating species richness: the importance of heterogeneity in species detectability. Ecology, 79:1018–1028.
https://doi.org/10.1890/0012-9658(1998)079[1018:esrtio]2.0.co;2
Brattstrom, B.H. 1958. New records of Cenozoic amphibians and reptiles from California. Bulletin of the Southern California Academy of Sciences, 57:5–12.
Burbrink, F.T., Grazziotin, F.G., Pyron, R.A., Cundall, D., Donnellan, S., Irish, F., Keogh, J.S., Kraus, F., Murphy, R.W., Noonan, B., Raxworthy, C.J., Ruane, S., Lemmon, A.R., Lemmon, E.M., and Zaher, H. 2020. Interrogating Genomic-Scale Data for Squamata (Lizards, Snakes, and Amphisbaenians) Shows no Support for Key Traditional Morphological Relationships. Systematic Biology, 69(3):502–520.
https://doi.org/10.1093/sysbio/syz062
Corner, G.R. 2014. Newly acquired vertebrates from Webster County, South-Central Nebraska, USA. Abstract of the 48th Annual Meeting of the Geological Society of America, North-Central Section.
Davis, D.R. and LaDuc, T.J. 2018. Amphibians and reptiles of C.E. Miller Ranch and the Sierra Vieja, Chihuahuan Desert, Texas, USA. Zookeys, 735:97–130.
https://doi.org/10.3897/zookeys.735.22200
Diffendal, R.F. Jr., Pabian, R.K., and Thomasson, J.R. 1996. Geologic history of Ash Hollow Park, Nebraska. Conservation and Survey Division, Institute of Agriculture and Natural Resources, University of Nebraska, Educational Circular, 5:1–33.
Dowling, H.G. and Duellman, W.E. 1978. Systematic herpetology: a synopsis of families and higher categories. HISS Publications, New York.
Dupoué, A., Rutschmann, A., Le Galliard, J.F., Clobert, J., Angelier, F., Marciau, C., Ruault, S., Miles, D., and Meylan, S. 2017. Shorter telomeres precede population extinction in wild lizards. Scientific Reports, 7:1–8.
https://doi.org/10.1038/s41598-017-17323-z
Fick, S.E. and Hijmans, R.J. 2017. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37:4302–4315.
https://doi.org/10.1002/joc.5086
Fitzinger, L. 1843. Systema reptilium. Fasciculus primus. Amblyglossae. Vindobonae. https://doi.org/10.5962/bhl.title.4694
Fox, D.L. and Koch, P.L. 2004. Carbon and oxygen isotopic variability in Neogene paleosol carbonates: constraints on the evolution of the C4-grasslands of the Great Plains, USA. Palaeogeography, Palaeoclimatology, Palaeoecology, 207:305–329.
https://doi.org/10.1016/j.palaeo.2003.09.030
Georgalis, G.L. 2019. Poor but classic: The squamate fauna from the late Miocene of Pikermi, near Athens, Greece. Comptes Rendus Palevol, 18:801–815.
https://doi.org/10.1016/j.crpv.2019.09.004
Georgalis, G.L. and Scheyer, T.M. 2019. A new species of Palaeopython (Serpentes) and other extinct squamates from the Eocene of Dielsdorf (Zurich, Switzerland). Swiss Journal of Geosciences, 112:383–417.
https://doi.org/10.1007/s00015-019-00341-6
Georgalis, G.L. and Scheyer, T.M. 2022. Crushed but not lost: a colubriform snake (Serpentes) from the Miocene Swiss Molasse, identified through the use of micro-CT scanning technology. Swiss Journal of Geosciences, 115:15.
https://doi.org/10.1186/s00015-022-00417-w
Georgalis, G. and Smith, K. 2020. Constrictores Oppel, 1811 - the available name for the taxonomic group uniting boas and pythons. Vertebrate Zoology, 70:291–304.
https://doi.org/10.26049/VZ70-3-2020-03
Gilmore, C.W. 1938. Fossil snakes of North America. Geological Society of North America Special Paper, 9:1–96.
https://doi.org/10.1130/spe9-p1
Gray, J.E. 1825. A synopsis of the genera of Reptilia and Amphibia. Annals of Philosophy, 10:193–217.
Gray, J.E. 1849. Catalogue of the Specimens of Snakes in the Collection of the British Museum. Printed by order of the Trustees.
Günther, A.C.L.G. 1864. The Reptiles of British India. R. Hardwicke, London.
Gyi, K. K. 1970. A revision of colubrid snakes of the subfamily Homalopsinae. University of Kansas Publications, Museum of Natural History, 20:47–223.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4:9.
https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Head, J.J. 2005. Snakes of the Siwalik Group (Miocene of Pakistan): systematics and relationship to environmental change. Palaeontologia Electronica, 8:1–33.
https://palaeo-electronica.org/2005_1/head18/issue1_05.htm
Head, J.J. 2015. Fossil calibration dates for molecular phylogenetic analysis of snakes 1: Serpentes, Alethinophidia, Boidae, Pythonidae. Palaeontologia Electronica, 18.1.6FC:1–17.
https://doi.org/10.26879/487
Head, J.J. and Polly, P.D. 2015. Evolution of the snake body form reveals homoplasy in amniote Hox gene function. Nature, 520:86–89.
https://doi.org/10.1038/nature14042
Head, J.J., Bloch, J.I., Hastings, A.K., Bourque, J.R., Cadena, E.A., Herrera, F.A., Polly, P.D., and Jaramillo, C.A. 2009. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature, 457:715–718.
https://doi.org/10.1038/nature07671
Head, J.J., Mahlow, K., and Müller, J. 2016. Fossil calibration dates for molecular phylogenetic analysis of snakes 2: Caenophidia, Colubroidea, Elapoidea, Colubridae. Palaeontologia Electronica, 19.2.2FC:1–21.
https://doi.org/10.26879/625
Hoffstetter, R. 1939. Contribution a l’étude des Elapidae actuels et fossiles et de l’ostéologie des ophidians. Archives du Muséum d'Histoire Naturelle de Lyon, 15:1–78.
Hoffstetter, R. and Gayrard, Y. 1965. Observations sur l’ostéologie et la classification des Acrochordidae (Serpentes). Bulletin du Muséum National d'Historie Naturelle, 36:677–696.
Holman, J.A. 1964. Fossil snakes from the Valentine Formation of Nebraska. Copeia, 1964:631–637.
https://doi.org/1441438
Holman, J.A. 1968. Upper Pliocene snakes from Idaho. Copeia, 1:152–158.
Holman, J.A. 1973. Reptiles of the Egelhoff local fauna (upper Miocene) of Nebraska. Contributions of the Museum of Paleontology, University of Michigan, 24:125–134.
Holman, J.A. 1975. Herpetofauna of the WaKeeney Local Fauna (Lower Pliocene: Clarendonian) of Trego County, Kansas. University of Michigan Papers on Paleontology, 12:49–66.
Holman, J.A. 1977. Upper Miocene snakes (Reptilia, Serpentes) from southeastern Nebraska. Journal of Herpetology, 11:323–335.
https://doi.org/10.2307/1563245
Holman, J.A. 1978. Herpetofauna of the Bijou Hills Local Fauna (Late Miocene: Barstovian) of South Dakota. Herpetologica, 34:253–257.
https://www.jstor.org/stable/3891548
Holman, J.A. 1982. New herpetological species and records from the Norden Bridge fauna (Miocene: Late Barstovian) of Nebraska. Transactions of the Nebraska Academy of Science, 10:31–36.
Holman, J.A. 1987. Herpetofauna of the Egelhoff Site (Miocene: Barstovian) of north-central Nebraska. Journal of Vertebrate Paleontology, 7:109–120.
https://doi.org/10.1080/02724634.1987.10011646
Holman, J.A. 1996. Herpetofauna of the Trinity River local fauna (Miocene: Early Barstovian, San Jacinto County, Texas, USA. Tertiary Research, 17:5–10.
Holman, J.A. 2000. Fossil snakes of North America: Origin, evolution, distribution, paleoecology. Indiana University Press, Bloomington.
Holman, J.A. and Schroeder, M.E. 1991. Fossil herpetofauna of the Lisco C quarries (Pliocene: Early Blancan) of Nebraska. Transactions of the Nebraska Academy of Sciences, XVIII:19–29.
Holman, J.A. and Winkler, A.J. 1987. A mid-Pleistocene (Irvingtonian) herpetofauna from a cave in southcentral Texas. Pearce-Sellards Series, Texas Memorial Museum, The University of Texas at Austin, 44:1–17.
Holman, J.A., Fay, L.P., and Korth, W.W. 2011. Herpetofauna of Late Miocene Sappa Creek Fauna, Northwestern Kansas. Paludicola, 8:91–99.
Huey, RB., Kearney, M. R., Krockenberger, A., Holtum, J.A., Jess, M., and Williams, S.E. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B: Biological Sciences, 367:1665–1679.
https://doi.org/10.1098/rstb.2012.0005
ICZN (International Code of Zoological Nomenclature). 2020. Opinion 2454 (Case 3688) - Charinidae Gray, 1849 (Reptilia, Squamata, Serpentes): emended to Charinaidae to remove homonymy with Charinidae Quintero, 1986 (Arachnida, Amblypygi); Ungaliopheinae McDowell, 1987 (Reptilia, Squamata, Serpentes): emended to Ungaliophiinae McDowell, 1987. Bulletin of Zoological Nomenclature, 77.
Ikeda, T. 2007. A comparative morphological study of the vertebrae of snakes occurring in Japan and adjacent regions. Current Herpetology, 26:13–34.
https://doi.org/10.3105/1345-5834(2007)26[13:ACMSOT]2.0.CO;2
Ikeda, T., Otsuka, H., and Ota, H. 2016. Early Pleistocene fossil snakes (Reptilia: Squamata) from Okinawajima Island in the Ryukyu Archipelago, Southwestern Japan. Herpetological Monographs, 30:143–156.
https://doi.org/10.1655/HERPMONOGRAPHS-D-15-00005.1
IUCN (International Union for Conservation of Nature). 2021. The IUCN Red List of Threatened Species. Version 2021-1. https://www.iucnredlist.org. Downloaded on 10 June 2021.
Ivanov, M. 2000. Snakes of the lower/middle Miocene transition at Vieux Collonges (Rhône, France), with comments on the colonisation of western Europe by colubroids. Geodiversitas, 22:559–588.
Jacisin III, J.J., Whiting, E.T., Ricker, A., Wallace, J., and Head, J.J. 2015. Neogene herpetofaunas from the Central Great Plains: diversity, modernization, and relationships to climate change. 75th Annual Meeting of the Society of Vertebrate Paleontology, Dallas, Texas, U.S.A.
Janis, C.M., Damuth, J., and Theodor, J.M. 2000. Miocene ungulates and terrestrial primary productivity: where have all the browsers gone? Proceedings of the National Academy of Sciences, 97:899.
https://doi.org/10.1073/pnas.97.14.7899
Janis, C.M., Damuth, J., and Theodor, J.M. 2002. The origins and evolution of the North American grassland biome: the story from the hoofed mammals. Palaeogeography, Palaeoclimatology, Palaeoecology, 177:183-0198.
https://doi.org/10.1016/S0031-0182(01)00359-5
Janis, C.M., Damuth, J., and Theodor, J.M. 2004. The species richness of Miocene browsers, and implications for habitat type and primary productivity in the North American grassland biome. Palaeogeography, Palaeoclimatology, Palaeoecology, 207:371–398.
https://doi.org/10.1016/j.palaeo.2003.09.032
Jasinski, S.E. and Moscato, D.A. 2017. Late Hemphillian Colubrid Snakes (Serpentes, Colubridae) from the Gray Fossil Site of Northeastern Tennessee. Journal of Herpetology, 51:245–257.
https://doi.org/10.1670/16-020
Joeckel, R.M., Tucker, S.T., and Howard, L.M. 2017. Geology and Paleontology Along Part of the Niobrara River in Northern Nebraska. University of Nebraska, Conservation and Survey Division, Lincoln, Nebraska, U.S.A.
Jurestovsky, D.J. 2021. Small Colubroids from the Late Hemphillian Gray Fossil Site of Northeastern Tennessee. Journal of Herpetology, 55:422–431.
https://doi.org/10.1670/21-008
Kita, Z.A., Secord, R., and Boardman, G.S. 2014. A new stable isotope record of Neogene paleoenvironments and mammalian paleoecologies in the western Great Plains during the expansion of C4 grasslands. Palaeogeography, Palaeoclimatology, Palaeoecology, 399:160–172.
https://doi.org/10.1016/j.palaeo.2014.02.013
Kluge, A.G. 1993. Calabaria and the phylogeny of erycine snakes. Zoological Journal of the Linnaean Society, 107:293–351.
https://doi.org/10.1111/j.1096-3642.1993.tb00290.x
LaDuke, T.C. 1991. The fossil snakes of Pit 91, Racho La Brea, California. Natural History Museum of Los Angeles County Contributions in Science, 424:1–28.
Latreille, P.A. 1801. In Sonnini de Manoncourt, C.N.S. and Latreille, P.A. 1801. Histoire naturelle des reptiles, avec figures dessinees d'apres nature. Deterville, Paris, France.
Laurenti, J.N. 1768. Specimen medicum, exhibens synopsin Reptilium emendatam cum experimentis circa venena et antidota Reptilium austriacorum. Typis Joannis Thomae Trattner, Vindobonae.
Lawing, A.M. 2021. The geography of phylogenetic paleoecology: integrating data and methods to better understand biotic response to climate change. Paleobiology, 47:178–197.
https://doi.org/10.1017/pab.2021.14
Lawing A.M. and Polly, P.D. 2011. Pleistocene climate, phylogeny, and climate envelope models: an integrative approach to better understand species’ response to climate change. PLoS ONE, 16:e28554.
https://doi.org/10.1371/journal.pone.0028554
Lawing, A.M., Polly, P.D., Hews, D.K., and Martins, E.P. 2016. Including fossils in phylogenetic climate reconstructions: A deep time perspective on the climatic niche evolution and diversification of spiny lizards (Sceloporus). The American Naturalist, 188:133–148.
https://doi.org/10.5061/dryad.69fc0
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Laurenti Salvi, Holmiae [Stockholm].
Lueninghoener, G.C. 1934. A lithologic study of some typical exposures of the Ogallala Formation in western Nebraska. Unpublished MSc thesis. University of Nebraska, Lincoln, U.S.A.
Malnate, E.V. 1972. Observations on the vertebral hypapophyses and associated musculature in some snakes, with special reference to the Colubridae. Zoologische Mededelingen, 47(18):225–239.
McCartney, J. 2015. Morphology and function of the ophidian vertebral column: implications for the paleobiology of fossil snakes. Stony Brook University, New York, U.S.A.
McDowell, S.B. 1961. (Review of) Systematic division and evolution of the colubrid snake genus. Natrix, with comments on the subfamily Natricinae, by Edmond V. Malnate. Copeia, 1961:502–506.
McDowell, S.B. 1987. Systematics. p. 3–50. In: Collins, J.T., Novak, S.S., and Seigel, R.A. (eds.), Snakes: Ecology and Evolutionary Biology. McGraw-Hill Publishing Company, New York, U.S.A.
McVay, J.D., Flores-Villela, O., and Carstens, B. 2015. Diversification of North American natricine snakes. Biological Journal of the Linnean Society, 116(1):1–12.
https://doi.org/10.1111/bij.12558
Mead, J.I. and Steadman, D.W. 2017. Late Pleistocene snakes (Squamata: Serpentes) from Abaco, The Bahamas. Geobios, 50:431–440.
https://doi.org/10.1016/j.geobios.2017.09.001
Meylan, P.A. 1982. The squamate reptiles of the Inglis IA fauna (Irvingtonian: Citrus County, Florida). Bulletin of the Florida State Museum, Biological Sciences, 27:1–85.
Muthoni, F.K. 2010. Modelling the spatial distribution of snake species under changing climate scenario in Spain. Unpublished MSc thesis, University of Twente, The Netherlands.
Nix, H.A. 1986. A biogeographic analysis of Australian Elapid Snakes, p. 415. In Longmore, R. (ed.), Atlas of Elapid Snakes of Australia. Australian Flora and Fauna Series, 7.
Nopsca, F. 1923. Eidolosaurus und Pachyophis. Zwei neue Neocom-Reptilien. Palaeontographica, 65:97–154.
Oppel, M. 1811. Die Ordnungen, Familien und Gattungen der Reptilien als Prodom einer Naturgeschichte derselben. Joseph Lindauer Verlag, München.
Parmley, D. 1987. Lampropeltis similis from the Coffee Ranch local fauna (Hemphillian Land Mammal Age) of Texas. Texas Journal of Science, 39:123–128.
Parmley, D. 1988. Early Hemphillian (late Miocene) snakes from the Higgins local fauna of Lipscomb County, Texas. Journal of Vertebrate Paleontology, 8:322–327.
https://www.jstor.org/stable/4523209
Parmley, D. 1990. Late Pleistocene snakes from Fowlkes Cave, Culberson County, Texas. Journal of Herpetology, 24:266–274.
https://doi.org/10.2307/1564393
Parmley, D. and Holman, J.A. 1995. Hemphillian (Late Miocene) snakes from Nebraska, with comments on Arikareean through Blancan snakes of midcontinental North America. Journal of Vertebrate Paleontology, 15:79–95.
https://doi.org/10.1080/02724634.1995.10011208
Parmley, D. and Holman, J.A. 2007. Earliest fossil record of a pigmy rattlesnake (Viperidae: Sistrurus Garman). Journal of Herpetology, 41(1):141–144.
https://doi.org/10.1670/0022-1511(2007)41[141:EFROAP]2.0.CO;2
Parmley, D. and Hunter, K.B. 2010. Fossil Snakes of the Clarendonian (Late Miocene) Pratt Slide Local Fauna of Nebraska, with the description of a new natricine colubrid. Journal of Herpetology, 44:526–543.
https://doi.org/10.1670/09-248.1
Parmley, D. and Walker, D. 2003. Snakes of the Pliocene Taunton local fauna of Adams County, Washington with the description of a new colubrid. Journal of Herpetology, 37(2):235–244.
https://doi.org/10.1670/0022-1511(2003)037[0235:SOTPTL]2.0.CO;2
Parmley, D., Chandler, R., and Chandler, L. 2015. Fossil frogs of the late Clarendonian (late Miocene) Pratt slide local fauna of Nebraska, with the description of a new genus. Journal of Herpetology, 49:143–149.
https://doi.org/10.1670/13-171
Plummer, M.V. and Mills, N.E. 2000. Spatial ecology and survivorship of resident and translocated hognose snakes (Heterodon platirhinos). Journal of Herpetology, 34:565–575.
https://doi.org/10.2307/1565272
Pyron, R.A. and Burbrink, F.T. 2009. Lineage diversification in a widespread species: roles for niche divergence and conservatism in the common kingsnake, Lampropeltis getula. Molecular Ecology, 18:3443–3457.
https://doi.org/10.1111/j.1365-294X.2009.04292.x
Pyron, R.A., Burbrink, F.T., and Wiens, J.J. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology, 13:93.
https://doi.org/10.1186/1471-2148-13-93
Pyron, R.A., Reynolds, R.G., and Burbrink, F.T. 2014. A taxonomic revision of boas (Serpentes: Boidae). Zootaxa, 3846:249–260.
https://doi.org/10.11646/zootaxa.3846.2.5
Rage, J.-C. 1984. Serpentes. Part 11. Handbuch der Paläoherpetologie. Gustav Fisher Verlag, Stuttgart, Germany.
Rage, J.-C. 2001. Fossil snakes from the Paleocene of São José de Itaboraí, Brazil. Part II. Boidae. Palaeovertebrata, 30:111–150.
Rage, J.-C. and Holman, J.A. 1984. Des serpents (Reptilia, Squamata) de type nord-américain dans le Miocène Français. Évolution parallèle ou dispersion? Geobios, 17:89–104.
https://doi.org/10.1016/S0016-6995(84)80007-8
Retallack, G.J. 2007. Cenozoic paleoclimate on land in North America. The Journal of Geology, 115:271–294.
https://doi.org/10.1086/512753
Reynolds, R.G., Niemiller, M.L., and Revell, L.J. 2014. Towards a Tree-of-Life for the boas and pythons: multilocus species-level phylogeny with unprecedented taxon sampling. Molecular Phylogenetics and Evolution, 71:201–213.
https://doi.org/10.1016/j.ympev.2013.11.011
Rodriguez‐Robles, J.A., Stewart, G.R., and Papenfuss, T.J. 2001. Mitochondrial DNA‐based phylogeography of North American rubber boas, Charina bottae (Serpentes: Boidae). Molecular Phylogenetics and Evolution, 18:227–237.
https://doi.org/10.1006/mpev.2000.0886
Rudnick, C.E. 1994. Insectivores from the Late Clarendonian (Miocene) Pratt Slide, North Central Nebraska. Unpublished MSc thesis. University of Nebraska, Lincoln, U.S.A.
Scanlon, J.D. and Mackness, B.S. 2001. A new giant python from the Pliocene Bluff Downs Local Fauna of northeastern Queensland. Alcheringa, 25:425–437.
Sinervo, B., Mendez-De-La-Cruz, F., Miles, D.B., Heulin, B., Bastiaans, E., Villagrán-Santa Cruz, M., et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science, 328:894–899.
https://doi.org/10.1126/science.1184695
Smith, K.T. 2013. New constraints of the evolution of the snake clades Ungaliophiinae, Loxocemidae and Colubridae (Serpentes), with comments of the fossil history of erycine boids in North America. Zoologischer Anzeiger, 252:157–182.
https://doi.org/10.1016/j.jcz.2012.05.006
Song, Y., Wang, Q., An, Z., Qiang, X., Dong, J., Chang, H., Zhang, M., and Guo, X. 2018. Mid-Miocene climatic optimum: Clay mineral evidence from the red clay succession, Longzhong Basin, Northern China. Palaeogeography, Palaeoclimatology, Palaeoecology, 512:46–55.
https://doi.org/10.1016/j.palaeo.2017.10.001
Sonnini de Manoncourt, C.N.S. and Latreille, P.A. 1801. Histoire naturelle des reptiles, avec figures dessinees d'apres nature. Deterville, Paris, France.
St. Clair, R.C. 1999. Identifying critical habitats for a vulnerable snake species, the rubber boa. Report to Columbia Basin Fish and Wildlife Compensation Program, BC Hydro, Nelson, British Columbia.
Strömberg, C.A.E. 2004. Using phytolith assemblages to reconstruct the origin and spread of grass-dominated habitats in the Great Plains during the late Eocene to early Miocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 207:239–275.
https://doi.org/10.1016/j.palaeo.2003.09.028
Strömberg, C.A.E. 2006. Evolution of hypsodonty in equids: testing a hypothesis of adaptation. Paleobiology, 32:236–258.
https://doi.org/10.1666/0094-8373(2006)32[236:EOHIET]2.0.CO;2
Strömberg, C.A.E. 2011. Evolution of grasses and grassland ecosystems. Annual Review of Earth and Planetary Sciences, 39:517–544.
https://doi.org/10.1146/annurev-earth-040809-152402
Swisher, C.C. III. 1992. 40Ar/39 Ar dating and its application to the calibration of North American land-mammal ages. Ph.D. dissertation, University of California, Berkeley.
Syromyatnikova, E., Aranda, E., and González, S.F. 2021. First insight into the diversity of snakes in the Pleistocene of Cuba. Acta Palaeontologica Polonica, 66:395–407.
https://doi.org/10.4202/app.00766.2020
Szyndlar, Z. 1991. A review of Neogene and Quaternary snakes of central and Eastern Europe. Part II: Natricinae, Elapidae, Viperidae. Estudios Geológicos, 47:237–266.
Szyndlar, Z. 1994. Oligocene snakes of southern Germany. Journal of Vertebrate Paleontology, 14:24–37.
Szyndlar, Z. 2012. Early Oligocene to Pliocene Colubridae of Europe: a review. Bulletin de la Société Géologique de France, 183:661–681.
Szyndlar, Z. and Rage, J.-C. 2003. Non-erycine Booidea from the Oligocene and Miocene of Europe. Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Kraków.
Tedford, R.H., Albright III, B., Barnosky, A.D., Ferrusquia-Villafranca, I., Hunt, R.M., Jr., Storer J.E., Swisher III, C.C., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian Biochronology of the Arikareean Through Hemphillian Interval (Late Oligocene Through Early Pliocene Epochs), p. 169–231. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Columbia University Press, New York, U.S.A.
Thomasson, J.R. 1980. Archaeoleersia nebraskensis gen. et sp. nov. (Gramineae-Oryzeae), a new fossil grass from the Late Tertiary of Nebraska. American Journal of Botany, 67:876–882.
https://doi.org/10.1002/j.1537-2197.1980.tb07717.x
Turner, M.A. 1972. A faunal assemblage from the Lower Ash Hollow Formation (Neogene) of southern Nebraska. Unpublished MSc thesis. University of Nebraska, Lincoln, U.S.A.
Utiger, U., Helfenberger, N., Schätti, B., Schmidt, C., Ruf, M., and Ziswiler, V. 2002. Molecular systematics and phylogeny of Old and New World Rat Snakes, Elaphe auct., and related genera (Reptilia, Squamata, Colubridae). Russian Journal of Herpetology, 9:105–124.
Van Devender, T.R. and Mead, J.I. 1978. Early Holocene and late Pleistocene amphibians and reptiles in Sonoran Desert packrat middens. Copeia, 1978:464–475.
Voorhies, M.R. 1990. Vertebrate biostratigraphy of the Ogallala Group in Nebraska, pp. 115–151. In Gustavson, T.C. (ed.), Geologic Framework and Regional Hydrology Upper Cenozoic Blackwater Draw and Ogallala Formation, Great Plains. Bureau of Economic Geology, University of Texas, Austin, U.S.A.
Voorhies, M.R. 1992. Ashfall: life and death at a Nebraska waterhole ten million years ago. University of Nebraska State Museum, Museum Notes, 81:1–4.
Voorhies, M.R., Corner, R.G., and Fitzgibbon, J.L. 1987. Calippus regulus (Mammalia: Equidae) in the Penny Creek Local Fauna (Clarendonian), Webster County, Nebraska. Transactions of the Nebraska Academy of Sciences and Affiliated Societies, Paper 206.
Wang, Y., Cerling, T.E., and MacFadden, B.J. 1994. Fossil horses and carbon isotopes: new evidence for Cenozoic dietary, habitat, and ecosystem changes in North America. Palaeogeography, Palaeoclimatology, Palaeoecology, 107:269–279.
https://doi.org/10.1016/0031-0182(94)90099-X
Webb, J.K., and Shine, R. 1998. Using thermal ecology to predict retreat-site selection by an endangered snake species. Biological Conservation, 86:233–242.
https://doi.org/10.1016/S0006-3207(97)00180-8
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292:686–693.
https://doi.org/10.1126/science.1059412
Zaher, H., Grazziotin, F.G., Cadle, J.E., Murphy, R.W., Moura-Leite, J.C.D., and Bonatto, S.L. 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia (São Paulo), 49:115–153.
Zaher H., Murphy R.W., Arredondo J.C., Graboski R., Machado-Filho P.R., Mahlow K., Montingelli G.G., Quadros A.B., Orlov N., Wilkinson M., Zhang, Y.P., and Grazziotin, F.G. 2019. Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes (Squamata: Serpentes). PLoS ONE, 14:e0216148.
https://doi.org/10.1371/journal.pone.0216148

