 Quantifying intra- and interspecific variability in trilobite moulting behaviour across the Palaeozoic
Quantifying intra- and interspecific variability in trilobite moulting behaviour across the Palaeozoic
Article number: 22.2.34
https://doi.org/10.26879/940
Copyright Paleontological Society, June 2019
Author biography
Plain-language and multi-lingual abstracts
PDF version
Submission: 7 November 2018. Acceptance: 18 March 2019
{flike id=2489}
ABSTRACT
Moulting of a protective exoskeleton is a defining characteristic of Euarthropoda, and its evolution can be explored through analysing moults preserved in the fossil record. Our most complete record comes from the Trilobita, which were uniquely flexible in moulting compared to other arthropod groups. This study presents the first broad-scale quantitative analysis of trilobite moulting. Trends in moulting variability with taxonomy and through the Palaeozoic are explored by looking at the occurrences of six moulting characteristics: opening of the facial and ventral sutures; and disarticulation of the cephalon, cranidium, thorax, and pygidium. Significant differences in moulting across taxonomic and temporal groups were identified using chi-squared analyses, and biases with sampling and diversity identified through correlation analyses. Occurrences of facial and ventral suture opening, and cephalic disarticulation, significantly varied between orders and Epochs. These likely result from the prevalence of ventral suture moulting in Redlichiida and cephalic disarticulation in Phacopida, and their relevant diversity patterns. The data show high levels of intraspecific variability in moulting; ~40% of species showed multiple moulting characteristics. Redlichiida and the early Cambrian are the most intraspecifically variable, with greater variability likely an adaptation to the initial radiation and establishment of trilobites into new niches. The longest-lived group, Proetida, showed the lowest levels of intraspecific variability, which may suggest greater specialism later in the Palaeozoic. Ultimately, datasets such as this advocate the need to study behaviours in the fossil record on a broad-scale, because they help us build a comprehensive picture of extinct groups as living animals.
Harriet B. Drage. Department of Zoology, University of Oxford, South Parks Road, Oxford, United Kingdom, OX1 3PS. harriet.drage@zoo.ox.ac.uk
Keywords: behaviour; morphology; Cambrian; exoskeleton; exuviation; moulting
Drage, Harriet B. 2019. Quantifying intra- and interspecific variability in trilobite moulting behaviour across the Palaeozoic. Palaeontologia Electronica 22.2.34A 1-39. https://doi.org/10.26879/940
palaeo-electronica.org/content/2019/2489-trilobite-moulting-variability
Copyright: June 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
BACKGROUND
Arthropod Moulting
Moulting is the process of shedding the old exoskeleton and replacing it with a new one. This characteristic unites all Ecdysozoa (Aguinaldo et al., 1997), while arthropods in particular often have exoskeletons reinforced by biomineralisation. This provides a tough covering (for example chitin or calcite), which protects the individual from predation and parasitism, prevents desiccation in terrestrial groups, and acts as a muscle attachment point (Ewer, 2005). However, arthropods must go through numerous successive moult cycles in their lifetime as the exoskeleton restricts growth. Moulting consequently allows the individual to inflate, develop their morphology, and repair damage. Arthropods are extremely vulnerable during and immediately after moulting (Henningsmoen, 1975), and moulting events, either due to a failure to separate from the old exoskeleton, or predation, are responsible for up to 80-90% of individual arthropod deaths (Clarkson, 1979; Brandt, 2002), making it one of the most crucial recurring events in arthropod life history. Moulting is intrinsically linked to the behaviour, biochemistry, reproduction, morphology, and ecology of arthropods, and consequently of primary importance in their evolutionary history (Vevea and Hall, 1984; Brandt, 2002; Ewer, 2005).
There has been a great deal of research on extant arthropod moulting behaviours, particularly in insects, and the biochemical pathways (involving ecdysones) that stimulate the moulting process (e.g., Nijhout, 1994; Ewer, 2005; Song et al., 2017). However, there has been very little work using the fossil record to explore the evolution of this key characteristic. We can investigate moulting in the arthropod fossil record because moulted exoskeletons are preserved in much the same way as carcasses, and moult fossils are thought to be much more common than carcasses because each individual moults many times during its lifetime but produces only one carcass (Daley and Drage, 2016). These moults are recognisable because the moulting process in extinct arthropods is thought to comprise the same stages as for modern groups, which is consistent despite differences in exoskeleton composition (Ewer, 2005). This comprises a pre-moult stage, where the animal prepares for moulting (secreting moulting fluids, detaching the old exoskeleton cuticle); exuviation (or ecdysis, the actual exiting of the old exoskeleton); and a post-moult stage, where the new exoskeleton decompresses and hardens; followed by a return to the intermoult stage (Krishnakumaran and Schneiderman, 1970; Henningsmoen, 1975). The most complete and abundant early fossil record of moulting belongs to Trilobita. This is in part because of their thickly biomineralised calcitic exoskeletons with a correspondingly high preservation potential, but also their incomparable diversity, abundance, and global distribution (Tarver et al., 2007). For the most part the fossil record of moulting is based on isolated or grouped empty moults, and these remain uncommon outside Trilobita. Arthropods caught ‘in-the-act’ of exuviation, such as an individual of Marrella splendens Walcott 1912 from the Burgess Shale (García-Bellido and Collins, 2004), are extremely rare, and no convincing example has been described for trilobites (Drage and Daley, 2016). Consequently, trilobites provide us with the only large dataset with which to explore the origins of moulting for total group Arthropoda, which may have been central to the development of their dominance throughout the Phanerozoic into the present (Vevea and Hall, 1984; Ewer, 2005). A complete review of trilobite and other arthropod moulting evidence from the fossil record published to date is presented in Daley and Drage (2016).
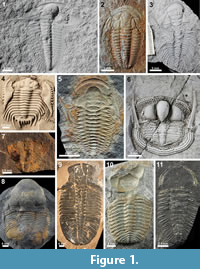 Trilobite moulting behaviour, i.e., the lines the old exoskeleton splits along and the behaviours that an individual uses to accomplish exuviation, has been shown to be both inter- and intraspecifically variable despite the reasonably consistent body plan in this group (Figure 1; Henningsmoen, 1975; Daley and Drage, 2016; Drage et al., 2018a). This is in contrast to the moulting behaviours of other arthropod groups, both extant and in the fossil record, which have morphologies and behaviours more specialised to one or two moulting methods (Daley and Drage, 2016). Trilobites, which appear earliest in the fossil record amongst crown-group arthropods (Daley et al., 2018), are therefore unique in their moulting. Previous studies of trilobite moulting, despite their clear importance, have been limited in scope or entirely qualitative observations (e.g., Henningsmoen, 1975; Whittington, 1980, 1990; McNamara and Rudkin, 1984; Busch and Swartz, 1985; Speyer, 1985; Speyer and Brett, 1985; McNamara, 1986; Brandt, 2002; Bruthansová, 2003; Clarkson et al., 2003; Budil and Bruthansová, 2005; Hunda et al., 2006; Paterson et al., 2007; Cederström et al., 2010; Rustán et al., 2011; Drage and Daley, 2016; Drage et al., 2018a), leaving many unanswered questions about the evolution of this behaviour. How flexible were trilobites in their moulting behaviour compared to other arthropods? What effect did this have on the evolution of their morphology, ecology, development, and geological longevity? Quantitative analysis of the history of trilobite moulting means that we can trace how this life history strategy affected the broad-scale evolution of a group.
Trilobite moulting behaviour, i.e., the lines the old exoskeleton splits along and the behaviours that an individual uses to accomplish exuviation, has been shown to be both inter- and intraspecifically variable despite the reasonably consistent body plan in this group (Figure 1; Henningsmoen, 1975; Daley and Drage, 2016; Drage et al., 2018a). This is in contrast to the moulting behaviours of other arthropod groups, both extant and in the fossil record, which have morphologies and behaviours more specialised to one or two moulting methods (Daley and Drage, 2016). Trilobites, which appear earliest in the fossil record amongst crown-group arthropods (Daley et al., 2018), are therefore unique in their moulting. Previous studies of trilobite moulting, despite their clear importance, have been limited in scope or entirely qualitative observations (e.g., Henningsmoen, 1975; Whittington, 1980, 1990; McNamara and Rudkin, 1984; Busch and Swartz, 1985; Speyer, 1985; Speyer and Brett, 1985; McNamara, 1986; Brandt, 2002; Bruthansová, 2003; Clarkson et al., 2003; Budil and Bruthansová, 2005; Hunda et al., 2006; Paterson et al., 2007; Cederström et al., 2010; Rustán et al., 2011; Drage and Daley, 2016; Drage et al., 2018a), leaving many unanswered questions about the evolution of this behaviour. How flexible were trilobites in their moulting behaviour compared to other arthropods? What effect did this have on the evolution of their morphology, ecology, development, and geological longevity? Quantitative analysis of the history of trilobite moulting means that we can trace how this life history strategy affected the broad-scale evolution of a group.
This study presents the first broad-scale quantification of the variability of trilobite moulting behaviour. Using a large global dataset this will present the extent of the intra- and interspecific variability in moulting, and how this differed taxonomically and temporally. Results will first look at interspecific variability in trilobite moulting in the total group, within each order, and within families or superfamilies. The intraspecific moulting variability of the sampled species will then be presented, followed by the same analyses looking at variability across Palaeozoic time bins rather than taxonomic groups. This work significantly advances our ability to answer the key questions about the evolutionary history of this ubiquitous characteristic central to the life histories of all arthropods.
Trilobite Moulting
Investigating the fossil record of moulting relies on being able to accurately distinguish preserved moults and carcasses. Henningsmoen (1975) and Daley and Drage (2016) outlined a number of criteria with which to do this based on recurrent patterns of exoskeleton separation (i.e., the presence of specific moulting characteristics; Table 1), and contextual information (e.g., lack of abiotic/biotic disturbance etc.). However, this can be difficult for specimens for which separated moulted sclerites are not found in close association with the remainder of the exoskeleton, and so detailed explorations of moulting are limited to trilobite-bearing assemblages in which we can be certain of a moult identification (i.e., often Konservat-Lagerstätten; Drage et al., 2018a). However, well-preserved specimens with all moulted exoskeleton sclerites in close association are found in many locations worldwide, not just Konservat-Lagerstätten. For trilobite specimens that do fulfil these criteria, and are therefore confidently interpreted as moults, we can identify six key exoskeleton disarticulations, and which describe the moult; these are moulting characteristics (Table 1). These moulting characteristics are: open facial sutures (separating the librigenae from the cranidium); open ventral sutures (separating the rostral plate and/or hypostome from the cephalon); disarticulation of the cranidium; disarticulation of the cephalon; separation between thoracic segments; and disarticulation of the pygidium (Table 1). These represent the anatomical features of trilobite moults, and in combination are what most moult identifications are based upon (see Daley and Drage, 2016, and references therein). These six moulting characteristics are used throughout this study to explore moulting trends and can be identified from all trilobites if most of their exoskeletal sclerites are preserved in association.
These moulting characteristics identified from the fossil record of trilobites can be summarised into three general styles of moulting, indicating extensive flexibility in this behaviour (Figure 1). Firstly, usage of the cephalic sutures during moulting, involving the opening of the facial sutures connecting the librigenae to the cranidium (most commonly), the rostral suture connecting the ventral rostral plate to the cephalon, the hypostomal suture connecting the hypostome to the cephalon, and/or a median suture running axially on the ventral side, depending on morphology. Some trilobites use the cephalothoracic joint to moult, meaning that the articulation connecting the cephalon to the thoracopygon is opened. Occasionally this may occur in association with use of the cephalic sutures in moulting. Finally, certain derived trilobite groups open the marginal suture during moulting, which is a suture usually found running around the anterior of the cephalon (similar to moulting in xiphosurans; Sekiguchi et al., 1988). In all cases, at these openings an exuvial gape is produced through which the animal can anteriorly egress the old exoskeleton. The trilobite individual would then be free to expand and harden the new exoskeleton.
METHODS
Data Collection
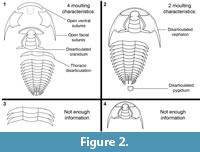 This study provides the first broad-scale, quantitative exploration of trilobite moulting behaviour variability, both taxonomically and temporally throughout the Palaeozoic. Trilobite moults from museum collections were identified using the criteria discussed in the Background Section (Henningsmoen, 1975; Daley and Drage, 2016). Some localities and geological ages produce more disarticulated material than others, for example the early Cambrian and late in the Palaeozoic, however, only reasonably complete specimens were used for data collection. Heavily disarticulated material (e.g., isolated sclerites; see Figure 2) cannot be confidently determined to represent a moult, and therefore less material from localities with poor preservation was included. From the identified moults, the six generalised moulting characteristics (locations of exoskeletal disarticulations associated with moulting, see Table 1) were recorded for 840 specimens from 355 species. These were separation/disarticulation of the: rostral plate and/or hypostome (= opening of the ventral sutures); librigenae (= opening of the facial sutures); entire cephalon (= opening of the cephalothoracic joint); cranidium (the cephalon minus the librigenae); thorax (between any two thoracic segments); and pygidium (= opening of the pygidiothoracic joint). The species were also categorised into the two main styles of moulting, use of cephalic sutures (species showing separation of the librigenae, ventral structures, and/or cranidium), and use of the cephalothoracic joint (species showing disarticulation of the cephalon or cranidium; cranidium disarticulation therefore falls under both general styles), which produce the combinations of these six moulting characteristics (see Figure 2). The ‘normal’ moulting characteristic was also recorded, which counted the number of species which in the majority of their sampled specimens displayed each of the six moulting characteristics.
This study provides the first broad-scale, quantitative exploration of trilobite moulting behaviour variability, both taxonomically and temporally throughout the Palaeozoic. Trilobite moults from museum collections were identified using the criteria discussed in the Background Section (Henningsmoen, 1975; Daley and Drage, 2016). Some localities and geological ages produce more disarticulated material than others, for example the early Cambrian and late in the Palaeozoic, however, only reasonably complete specimens were used for data collection. Heavily disarticulated material (e.g., isolated sclerites; see Figure 2) cannot be confidently determined to represent a moult, and therefore less material from localities with poor preservation was included. From the identified moults, the six generalised moulting characteristics (locations of exoskeletal disarticulations associated with moulting, see Table 1) were recorded for 840 specimens from 355 species. These were separation/disarticulation of the: rostral plate and/or hypostome (= opening of the ventral sutures); librigenae (= opening of the facial sutures); entire cephalon (= opening of the cephalothoracic joint); cranidium (the cephalon minus the librigenae); thorax (between any two thoracic segments); and pygidium (= opening of the pygidiothoracic joint). The species were also categorised into the two main styles of moulting, use of cephalic sutures (species showing separation of the librigenae, ventral structures, and/or cranidium), and use of the cephalothoracic joint (species showing disarticulation of the cephalon or cranidium; cranidium disarticulation therefore falls under both general styles), which produce the combinations of these six moulting characteristics (see Figure 2). The ‘normal’ moulting characteristic was also recorded, which counted the number of species which in the majority of their sampled specimens displayed each of the six moulting characteristics.
The complete trilobite collections of the following museums were surveyed, and the identified moults sampled and incorporated in the dataset: Lapworth Museum of Geology, Birmingham (BIRUG); Natural History Museum, London (NHMUK); Oxford University Museum of Natural History (OUMNH); the Uppsala University Museum of Evolution (PMU). A number of specimens were also sampled from the South Australian Museum (SAM) and National Museum Prague (NMP). Finally, species were sampled from moult specimens figured or described in the trilobite literature, although specimen counts were often not reported for these. Species of Trinucleidae and Harpetida were excluded from the final dataset due to their highly specialised morphology that constrained their unique moulting behaviours. Agnostida and uncategorised species were removed due to uncertain taxonomic assignment. Ordinal-level species assignments within the dataset therefore included the Asaphida, Corynexochida, Lichida (comprised of lichid and odontopleurid species), Phacopida, Proetida, Ptychopariida, and Redlichiida (Whittington et al., 1997; Jell and Adrain, 2002).
All species’ metadata were checked using comprehensive searches of the descriptive literature and the Paleobiology Database (Fossilworks, 2018; Kiessling et al., 2018). Species’ age assignments were time-binned using the ICS International Chronostratigraphic Chart 2017 (Cohen et al., 2013) into Palaeozoic Periods and Epochs. Taxa that occurred in two sequential time bins (i.e., across a boundary between two Periods or Epochs) were counted twice, once in each occupied time bin. Species taxonomic data (order, family) was obtained from Whittington et al. (1997) and Jell and Adrain (2002). Total-group trilobite diversity data was obtained from a FossilWorks and Palaeobiology Database search for all species entered and time-binned as Trilobita (Kiessling et al., 2018). These data were used to create raw and Shareholder Quorum Subsampled diversity curves using FossilWorks (from 26947 eligible occurrences, representing 8418 species throughout the Palaeozoic; generated 25/01/18; Fossilworks, 2018), which were then compared to sampling within the moulting dataset.
 Country and GPS coordinates were obtained for all species for which collection metadata was available. For species recorded from the literature, either locality information from the descriptive article or the type specimen locality was used, and the latitude and longitude coordinates obtained using Google Maps. Resulting coordinates were plotted for each sampled species on palaeogeographic maps representing the continental configurations at the mid-point age of each geological Period (Figure 3). These sampling maps were produced using GPlates and PaleoMap (Scotese, 2016). The Permian was excluded, as only one species was sampled.
Country and GPS coordinates were obtained for all species for which collection metadata was available. For species recorded from the literature, either locality information from the descriptive article or the type specimen locality was used, and the latitude and longitude coordinates obtained using Google Maps. Resulting coordinates were plotted for each sampled species on palaeogeographic maps representing the continental configurations at the mid-point age of each geological Period (Figure 3). These sampling maps were produced using GPlates and PaleoMap (Scotese, 2016). The Permian was excluded, as only one species was sampled.
Specimens were photographed using a Canon EOS 5D or 500D with a Macro Lens, under incident lighting, and using the Canon EOS Utility 2 remote camera access software. Graphs were produced in RStudio (RStudio Team, 2015) using custom code and the ggplot2 (Wickham et al., 2016) and Geoscale (Bell, 2015) packages. Figures were produced and/or modified using CS6 Adobe Illustrator and Photoshop.
Sampling
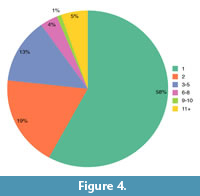 Overall, 29 species and 54 specimens were sampled from the BIRUG, 144 species and 336 specimens from the NHMUK, 70 species and 197 specimens from the OUMNH, three species and 31 specimens from the SAM, and 14 species and 142 from the PMU, although many more were observed. Finally, 148 species were sampled from the descriptive literature. A number of these provided data for the same species and not every species from the literature provided specimen measurement data, leaving a total of 355 species and 840 specimens. The number of species sampled for each order, suborder, superfamily, and family are displayed in Appendix 1, Table A1. The number of species sampled for each geological Epoch during the Palaeozoic are displayed in Appendix 1, Table A2, with a slightly higher total due to the presence of boundary taxa. Within the dataset, 58% of all trilobite species (196 species) were represented by moulting data from only one specimen, leaving 42% of species represented by two or more specimens (159 species; Figure 4). Nineteen percent of species (64) were represented by two specimens, 13% (45) by 3-5 and 10% (12) by 6+ (Figure 4). All data used to produce the results presented in this study are available in Appendix 2 and Appendix 3.
Overall, 29 species and 54 specimens were sampled from the BIRUG, 144 species and 336 specimens from the NHMUK, 70 species and 197 specimens from the OUMNH, three species and 31 specimens from the SAM, and 14 species and 142 from the PMU, although many more were observed. Finally, 148 species were sampled from the descriptive literature. A number of these provided data for the same species and not every species from the literature provided specimen measurement data, leaving a total of 355 species and 840 specimens. The number of species sampled for each order, suborder, superfamily, and family are displayed in Appendix 1, Table A1. The number of species sampled for each geological Epoch during the Palaeozoic are displayed in Appendix 1, Table A2, with a slightly higher total due to the presence of boundary taxa. Within the dataset, 58% of all trilobite species (196 species) were represented by moulting data from only one specimen, leaving 42% of species represented by two or more specimens (159 species; Figure 4). Nineteen percent of species (64) were represented by two specimens, 13% (45) by 3-5 and 10% (12) by 6+ (Figure 4). All data used to produce the results presented in this study are available in Appendix 2 and Appendix 3.
Analysis of Interspecific Moulting Variability with Taxonomy
Differences in the recorded moulting behaviours of trilobite taxonomic groups were explored over a number of levels. Firstly, at the superordinal-level, by comparing the proportion of all recorded trilobite species showing the two general styles of moulting (cephalic sutures, and cephalothoracic joint), and showing each of the six moulting characteristics. At the ordinal-level, comparisons were made of the proportions of species in each order showing both general styles of moulting (cephalic sutures, and cephalothoracic joint), and the moulting characteristics. For the latter, the ‘normal’ moulting characteristics were also plotted, i.e., the single moulting characteristic, which each species most commonly displayed. Finally, the proportion of species for each family or superfamily (dependent on sampling) displaying the six moulting characteristics were compared within each order (sample sizes too low for Lichida and Proetida). Taxonomic sampling quality within the dataset was explored by plotting the ordinal sample diversity against that obtained from a full search of Trilobita occurrences recorded in Fossilworks and the Paleobiology Database (Kiessling et al., 2018; n=9753 for all trilobite orders; Appendix 4, Table A2).
Chi-squared analyses were performed based on the null hypothesis (H0) that the trilobite clades (orders, superfamilies, or families) did not show significant differences in their moulting characteristics. These tested the alternate hypothesis (H1) that the clades showed significant differences in the number of species displaying each moulting characteristic. All analyses compared observed versus expected values. Expected numbers were calculated as the number of species that should display each moulting characteristic if all taxonomic groups moulted in the same way, when corrected for sampling. Analyses were performed in PAST3 (Hammer et al., 2001) with the “sample versus expected” setting. The total numbers (and proportions) of species summed to a greater total than that recorded in the dataset (or greater than 100%) because species could display more than one moulting characteristic. Full chi-squared tables for all analyses are available in Appendix 5. Correlation analyses (r2, Spearman’s Rank, and Kendall’s tau) were calculated in PAST3 (Hammer et al., 2001) to test for significant differences in sample ordinal species diversity and total group diversity (the latter from Fossilworks and the Paleobiology Database; Fossilworks, 2018; Kiessling et al., 2018).
The chi-squared test was chosen for the moulting variability analyses because the results can directly inform on the question of interest; ‘did trilobite moulting behaviour vary between taxonomic groupings?’ The expected numbers used in analyses were corrected for uneven group sampling, to give an accurate comparison between observed and expected data (Hammer et al., 2001). Chi-squared is also particularly appropriate as it is a non-parametric test, and much of the data analysed here, because it is nominal, may violate assumptions of normality (McHugh, 2013). The test can have reduced accuracy when including expected values totalling <5, however, this was inevitable given the behavioural nature of the variables tested (as some moulting characteristics occur rarely). Other statistical tests might be used to answer this question, for example ANOVAs or Mann-Whitney U tests to compare mean numbers of species displaying each moulting characteristic, however, these are parametric and would also require subsequent post-hoc tests to determine which groupings were causing the significant results. Tests such as Kolmogorov-Smirnoff are not appropriate to apply to this dataset, because this compares distributions of two samples, not a number of variables with summed counts for each grouping. Bonferroni corrections were applied to all chi-squared test p-values, in order to confirm that significant results were not due to chance through multiple testing, thereby correcting for family-wise error.
Analysis of Intraspecific Moulting Variability with Taxonomy
Intraspecific moulting variability between orders was explored by plotting the number of species in each order that displayed 1, 2, 3, 4, 5, or all 6 moulting characteristics. The impacts of dataset specimen sampling on this was determined by plotting the number of moulting characteristics shown by each species against the number of specimens sampled for it. This information was available for 326 species, the remainder of which were sampled from the literature where a usable specimen-count was not provided.
Analysis of Interspecific Moulting Variability through Time
Changes in the moulting behaviours of trilobites through time were explored by plotting the proportion of species in each geological Epoch displaying each of the six moulting characteristics. This was also plotted using the ‘normal’ moulting characteristics, i.e., the moulting characteristic most commonly observed for each species. Temporal sampling quality within the dataset was explored by plotting species diversity within each Epoch against total group diversity obtained from a full search of Trilobita occurrences recorded in Fossilworks and the Paleobiology Database (Fossilworks, 2018; Kiessling et al., 2018; n=8309; Appendix 4, Table A2), and against total diversity corrected for sampling using Shareholder Quorum Subsampling (SQS; carried out in Fossilworks, 2018). Geological time bins in the dataset were based on the International Chronostratigraphic Chart 2017 (Cohen et al., 2013). The time bins used by Fossilworks vary slightly, with the Cambrian remaining delineated as the lower Cambrian-middle Cambrian-Furongian, whereas the sample dataset uses the global designations of Series 2-Miaolingian-Furongian.
Chi-squared analyses performed were based on the null hypothesis (H0) that the geological time bins (Epochs or Periods) did not show significant differences in the moulting characteristics of their constituent species. These tested the alternate hypothesis (H1) that the time bins showed significant differences in the number of species displaying each moulting characteristic. All analyses compared observed versus expected values. Expected numbers were calculated as the number of species that should display each moulting characteristic if all time bins showed the same moulting behaviour, when corrected for sampling. Analyses were performed in PAST3 (Hammer et al., 2001) with the “sample versus expected” setting. The total numbers (and proportions) of species summed to a greater total than that recorded in the dataset (or greater than 100%), because each could display more than one moulting characteristic. The Late Devonian to early Permian Epochs were removed due to having too small sample sizes. Full chi-squared tables are available in Appendix 5. Significant deviations in dataset and total/SQS diversity through time were tested for using correlation analyses (r2, Spearman’s Rank, and Kendall’s tau) calculated in PAST3 (Hammer et al., 2001).
Analysis of Intraspecific Moulting Variability through Time
Intraspecific moulting variability between geological Epochs was explored by plotting the number of species in each Epoch that displayed 1, 2, 3, 4, 5, or all 6 moulting characteristics. The Pennsylvanian and early Permian Epochs were removed due to having too low sample sizes. The mean average and 95% Confidence Intervals for the number of moulting characteristics shown in each Epoch was calculated. ANOVA and Kruskal-Wallis tests were carried out in PAST3 (Hammer et al., 2001) to compare the means, testing for a significant change in intraspecific moulting variability through time.
RESULTS
Moulting in Highly Derived Trilobites
 Trilobites of the family Trinucleidae (Figure 5.1) and order Harpetida (Figure 5.2) were excluded from the moulting behaviour dataset. This is because they show an unusual derived morphology, with this necessitating a different moulting behaviour in comparison to those observed for the rest of Trilobita. Trinucleid and harpetid trilobites convergently evolved a fused cephalic shield, with a broader shape divided into dorsal and ventral halves (Stubblefield, 1959; Sekiguchi et al., 1988). This appears to have been a feeding adaptation, with the cephalic fringe pits in trinucleids apparently adaptive for a filter-feeding lifestyle (Fortey and Owens, 1999). Consequently, moulting in these groups appears to have taken place through opening of the marginal suture (Figure 5.1, 5.2), with an anterior exuvial gape suture created between the dorsal and ventral parts of the cephalic shield. This would extend along the entire anterior margin on the cephalon, and the animal would then emerge forwards. Other trilobites also evolved similar cephalic structures, such as later brachymetopid species with a greatly expanded rostral plate and fused facial sutures (Fortey and Owens, 1975), and many olenid and phacopid species which secondarily lost the facial sutures and moulted through disarticulation of the entire cephalon (discussed in detail later; Crônier, 2013; Drage et al., 2018b). Modern xiphosurans moult in a comparable manner to harpetids and trinucleids, also having a fused cephalic shield (carapace) in which a marginal gape opens between the dorsal and ventral parts (Sekiguchi et al., 1988). For trinucleids, this could produce a moult assemblage with the dorsal cephalic fringe displaced away from the remainder of the exuvia (Figure 5.1). Moulted harpetids are difficult to recognise, like xiphosurans, as the anterior gape appeared to have closed post-moulting (Figure 5.2-5.4). These trilobites may also have moulted through disarticulating the entire cephalon, but complete configurations of these were rarely observed.
Trilobites of the family Trinucleidae (Figure 5.1) and order Harpetida (Figure 5.2) were excluded from the moulting behaviour dataset. This is because they show an unusual derived morphology, with this necessitating a different moulting behaviour in comparison to those observed for the rest of Trilobita. Trinucleid and harpetid trilobites convergently evolved a fused cephalic shield, with a broader shape divided into dorsal and ventral halves (Stubblefield, 1959; Sekiguchi et al., 1988). This appears to have been a feeding adaptation, with the cephalic fringe pits in trinucleids apparently adaptive for a filter-feeding lifestyle (Fortey and Owens, 1999). Consequently, moulting in these groups appears to have taken place through opening of the marginal suture (Figure 5.1, 5.2), with an anterior exuvial gape suture created between the dorsal and ventral parts of the cephalic shield. This would extend along the entire anterior margin on the cephalon, and the animal would then emerge forwards. Other trilobites also evolved similar cephalic structures, such as later brachymetopid species with a greatly expanded rostral plate and fused facial sutures (Fortey and Owens, 1975), and many olenid and phacopid species which secondarily lost the facial sutures and moulted through disarticulation of the entire cephalon (discussed in detail later; Crônier, 2013; Drage et al., 2018b). Modern xiphosurans moult in a comparable manner to harpetids and trinucleids, also having a fused cephalic shield (carapace) in which a marginal gape opens between the dorsal and ventral parts (Sekiguchi et al., 1988). For trinucleids, this could produce a moult assemblage with the dorsal cephalic fringe displaced away from the remainder of the exuvia (Figure 5.1). Moulted harpetids are difficult to recognise, like xiphosurans, as the anterior gape appeared to have closed post-moulting (Figure 5.2-5.4). These trilobites may also have moulted through disarticulating the entire cephalon, but complete configurations of these were rarely observed.
 Superordinal-level Interspecific Moulting Variability
Superordinal-level Interspecific Moulting Variability
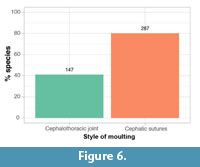
The majority of trilobite species sampled (except those that display marginal suture moulting; Figure 5) show use of the cephalic sutures for moulting (80% of species), where cephalic sutures (facial, rostral, and/or hypostomal) are opened to create an anterior exuvial gape (Figure 6). Approximately 40% of species show use of the cephalothoracic joint where this is disarticulated to create an exuvial gape between the cephalon and thoracopygon (Figure 6). This means that for 20% of species moult configurations were found displaying both styles of moulting, and these taxa had the flexibility to use both cephalic and cephalothoracic exuvial gapes.
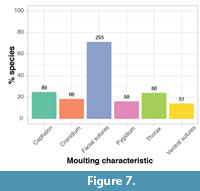 We can divide these general styles of moulting into the moulting characteristics they produced; opening of the ventral and/or facial sutures and cranidial disarticulation all showing use of cephalic sutures, and cephalic or cranidial disarticulation requiring opening of the cephalothoracic joint. Thoracic and pygidial disarticulations occur indiscriminately with the two styles, because they are not associated with the cephalon. Seventy-one percent of all trilobite species display moult configurations with open facial sutures and disarticulated librigenae (Figure 7); the additional 9% utilising cephalic sutures (Figure 6) but not the facial sutures must have moulted via opening of the ventral sutures. The other moulting characteristics are all observed in fewer than 30% of species, with 18% showing disarticulated cranidia, 16% disarticulated pygidia, and 14% opening of the ventral sutures (Figure 7). An approximately equal proportion of species show disarticulation of the cephalon (25%) and the thorax (24%) in moult configurations.
We can divide these general styles of moulting into the moulting characteristics they produced; opening of the ventral and/or facial sutures and cranidial disarticulation all showing use of cephalic sutures, and cephalic or cranidial disarticulation requiring opening of the cephalothoracic joint. Thoracic and pygidial disarticulations occur indiscriminately with the two styles, because they are not associated with the cephalon. Seventy-one percent of all trilobite species display moult configurations with open facial sutures and disarticulated librigenae (Figure 7); the additional 9% utilising cephalic sutures (Figure 6) but not the facial sutures must have moulted via opening of the ventral sutures. The other moulting characteristics are all observed in fewer than 30% of species, with 18% showing disarticulated cranidia, 16% disarticulated pygidia, and 14% opening of the ventral sutures (Figure 7). An approximately equal proportion of species show disarticulation of the cephalon (25%) and the thorax (24%) in moult configurations.
Ordinal-level Interspecific Moulting Variability
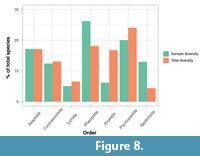 Total diversity (as sampled from Fossilworks; Kiessling et al., 2018) corresponds quite closely with database sampled diversity at an ordinal level (Figure 8; Appendix 4, Table A1). For the Asaphida, Corynexochida, Lichida, and Ptychopariida the sampled species diversity is reasonably similar to total diversity. However, the samples for Phacopida, Proetida, and Redlichiida are less representative of their total group diversities. Correlation analyses show that the sample diversity does not significantly correlate with total group diversity (r2: 0.57, p=0.18; Spearman’s Rank: 0.71, p=0.088; Kendall’s tau: 0.52, p=0.099). Although for the non-parametric tests this is close to the significance threshold of α=0.05.
Total diversity (as sampled from Fossilworks; Kiessling et al., 2018) corresponds quite closely with database sampled diversity at an ordinal level (Figure 8; Appendix 4, Table A1). For the Asaphida, Corynexochida, Lichida, and Ptychopariida the sampled species diversity is reasonably similar to total diversity. However, the samples for Phacopida, Proetida, and Redlichiida are less representative of their total group diversities. Correlation analyses show that the sample diversity does not significantly correlate with total group diversity (r2: 0.57, p=0.18; Spearman’s Rank: 0.71, p=0.088; Kendall’s tau: 0.52, p=0.099). Although for the non-parametric tests this is close to the significance threshold of α=0.05.
All seven trilobite orders display both styles of moulting in relatively high frequencies (Figure 9). Use of the cephalic sutures for moulting is the most common style for all orders except Phacopida, as expected by the almost ubiquitous presence of librigenae with facial sutures in trilobite morphology (Stubblefield, 1959; Whittington et al., 1997). For these six orders, the percentage of sampled species with at least one specimen displaying open cephalic sutures varies from 80% (Asaphida) to 100% (Redlichiida). However, 25% 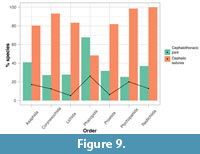 (Ptychopariida) to 41% (Asaphida) of species also show use of the cephalothoracic joint, previously considered to be very rare amongst non-phacopid trilobites (e.g., Henningsmoen, 1975). Phacopida is the only order to have a higher percentage of species displaying an open cephalothoracic joint (68%) than cephalic sutures (48%). Chi-squared analyses show significant p-values and therefore reject the null hypothesis (p=0.027 and 1.8x10-4, respectively, at α=0.025; Appendix 5, Table A1), suggesting that the number of species displaying the two styles significantly differ between the orders. For use of the cephalic sutures this mainly results from corynexochid and ptychopariid species using these more than expected (41 observed to 34 expected species, and 68 observed to 56 expected, respectively), and less often than expected in phacopids (43 observed to 69 expected). Unsurprisingly the result is the opposite for use of the cephalothoracic joint in moulting, with phacopids displaying this more often than expected (63 observed to 37 expected), and corynexochids and ptychopariids less often (12 observed to 19 expected, and 18 observed to 30 expected). All other trilobite orders show counts close to those expected (Figure 9).
(Ptychopariida) to 41% (Asaphida) of species also show use of the cephalothoracic joint, previously considered to be very rare amongst non-phacopid trilobites (e.g., Henningsmoen, 1975). Phacopida is the only order to have a higher percentage of species displaying an open cephalothoracic joint (68%) than cephalic sutures (48%). Chi-squared analyses show significant p-values and therefore reject the null hypothesis (p=0.027 and 1.8x10-4, respectively, at α=0.025; Appendix 5, Table A1), suggesting that the number of species displaying the two styles significantly differ between the orders. For use of the cephalic sutures this mainly results from corynexochid and ptychopariid species using these more than expected (41 observed to 34 expected species, and 68 observed to 56 expected, respectively), and less often than expected in phacopids (43 observed to 69 expected). Unsurprisingly the result is the opposite for use of the cephalothoracic joint in moulting, with phacopids displaying this more often than expected (63 observed to 37 expected), and corynexochids and ptychopariids less often (12 observed to 19 expected, and 18 observed to 30 expected). All other trilobite orders show counts close to those expected (Figure 9).
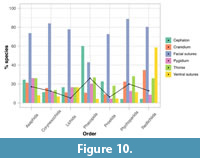 Variability in trilobite moulting characteristics (i.e., the specific disarticulations produced during moulting) is relatively consistent across all orders. All orders display each of the six moulting characteristics (Figure 10), even with the lowest sample size of 18 species (Lichida). Moulting behaviour, movements during exuviation, and moulting configurations are therefore extremely variable across all of Trilobita. Opening of the facial sutures is the most common moulting characteristic for all orders excepting Phacopida, meaning most of the species that used the cephalic sutures for moulting (Figure 6, Figure 9) did so by disarticulating the librigenae (congruent with Figure 7). Opening of the ventral sutures and disarticulation of the rostral plate is also very common for Redlichiida (59% of species), meaning these sutures were important in redlichiids for moulting. Disarticulation of the cephalon is the most common moulting characteristic in phacopids (60%, Figure 10) because they more commonly opened the cephalothoracic joint (Figure 9). Proportions of the remaining moulting characteristics are reasonably consistent, and relatively uncommon, within all orders. Disarticulation of the pygidium in moult configurations varies between 26% of species at its highest incidence in Asaphida, to 5% in Proetida. Thoracic disarticulation is slightly more common, varying between 14% (Corynexochida) and 28% (Ptychopariida) of species. Opening of the cephalothoracic joint in tandem with the facial sutures, causing disarticulation of the cranidium, is relatively common in Redlichiida (35% of species) (Figure 10), but less so in other orders. Chi-squared analyses (Appendix 5, Table A2) indicate that the orders have significantly different numbers of species displaying opening of the ventral sutures (p=1.3x10-9), facial sutures (p=0.0038), and cephalic disarticulation (p=6.8x10-14). These are significant at the 0.05 threshold (with Bonferroni correction α=0.0083). For opening of the ventral sutures in moulting this relates to its much greater prevalence in Redlichiida than expected (27 observed to 8 expected species), and low incidence in Phacopida (4 observed to 13 expected). For opening of the facial sutures this results from the same trends seen for the broader styles of moulting (Appendix 5, Table A1), namely a greater count than expected in Corynexochida and Ptychopariida and fewer than expected in Phacopida (Appendix 5, Table A2). The inverse is true for disarticulation of the cephalon, although Redlichiida also has fewer species showing this moulting characteristic than expected (two observed to 15 expected). The remaining three moulting characteristics do not show significant results, indicating that the number of species displaying them do not vary between the orders.
Variability in trilobite moulting characteristics (i.e., the specific disarticulations produced during moulting) is relatively consistent across all orders. All orders display each of the six moulting characteristics (Figure 10), even with the lowest sample size of 18 species (Lichida). Moulting behaviour, movements during exuviation, and moulting configurations are therefore extremely variable across all of Trilobita. Opening of the facial sutures is the most common moulting characteristic for all orders excepting Phacopida, meaning most of the species that used the cephalic sutures for moulting (Figure 6, Figure 9) did so by disarticulating the librigenae (congruent with Figure 7). Opening of the ventral sutures and disarticulation of the rostral plate is also very common for Redlichiida (59% of species), meaning these sutures were important in redlichiids for moulting. Disarticulation of the cephalon is the most common moulting characteristic in phacopids (60%, Figure 10) because they more commonly opened the cephalothoracic joint (Figure 9). Proportions of the remaining moulting characteristics are reasonably consistent, and relatively uncommon, within all orders. Disarticulation of the pygidium in moult configurations varies between 26% of species at its highest incidence in Asaphida, to 5% in Proetida. Thoracic disarticulation is slightly more common, varying between 14% (Corynexochida) and 28% (Ptychopariida) of species. Opening of the cephalothoracic joint in tandem with the facial sutures, causing disarticulation of the cranidium, is relatively common in Redlichiida (35% of species) (Figure 10), but less so in other orders. Chi-squared analyses (Appendix 5, Table A2) indicate that the orders have significantly different numbers of species displaying opening of the ventral sutures (p=1.3x10-9), facial sutures (p=0.0038), and cephalic disarticulation (p=6.8x10-14). These are significant at the 0.05 threshold (with Bonferroni correction α=0.0083). For opening of the ventral sutures in moulting this relates to its much greater prevalence in Redlichiida than expected (27 observed to 8 expected species), and low incidence in Phacopida (4 observed to 13 expected). For opening of the facial sutures this results from the same trends seen for the broader styles of moulting (Appendix 5, Table A1), namely a greater count than expected in Corynexochida and Ptychopariida and fewer than expected in Phacopida (Appendix 5, Table A2). The inverse is true for disarticulation of the cephalon, although Redlichiida also has fewer species showing this moulting characteristic than expected (two observed to 15 expected). The remaining three moulting characteristics do not show significant results, indicating that the number of species displaying them do not vary between the orders.
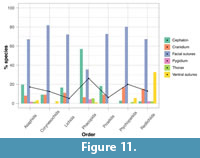 Differences in the moulting behaviours of the trilobite orders are more readily apparent when condensing the data to the ‘normal’ moulting characteristic of each species. This is the moulting characteristic most commonly observed for each species (Figure 11). Moulting through opening of the facial sutures is again the most common characteristic for all orders (67-82% of species) except Phacopida (35%), which is dominated by a prevalence of cephalic disarticulation (57%). However, the other moulting characteristics display lower prevalences than when plotting all the data (Figure 10). Only 33% of redlichiids usually open the ventral sutures for moulting, compared to the 59% of species that show this characteristic in any of their sampled specimens (Figure 10, Figure 11). Very few species show thoracic or pygidial disarticulation as their most commonly observed moulting characteristic, although these are slightly more common for Phacopida. Disarticulation of the cranidium for moulting, despite being reasonably prevalent in the sample as a whole (Figure 10), is not often a main moulting characteristic, with only Ptychopariida and Redlichiida containing 15% of species for which this is the case (Figure 11). The chi-squared analyses and corrected α-level show the same results (Appendix 5, Table A3) as for the analyses using all the data (Appendix 5, Table A2). The orders show significant differences in the number of species displaying rostral plate (p=8.8x10-9) and facial suture opening (p=0.0022), and cephalic disarticulation (p=9.9x10-14), with deviations in the same orders seemingly causing this result. Table 2 summarises the most commonly observed moulting characteristics and sampling for the seven trilobite orders.
Differences in the moulting behaviours of the trilobite orders are more readily apparent when condensing the data to the ‘normal’ moulting characteristic of each species. This is the moulting characteristic most commonly observed for each species (Figure 11). Moulting through opening of the facial sutures is again the most common characteristic for all orders (67-82% of species) except Phacopida (35%), which is dominated by a prevalence of cephalic disarticulation (57%). However, the other moulting characteristics display lower prevalences than when plotting all the data (Figure 10). Only 33% of redlichiids usually open the ventral sutures for moulting, compared to the 59% of species that show this characteristic in any of their sampled specimens (Figure 10, Figure 11). Very few species show thoracic or pygidial disarticulation as their most commonly observed moulting characteristic, although these are slightly more common for Phacopida. Disarticulation of the cranidium for moulting, despite being reasonably prevalent in the sample as a whole (Figure 10), is not often a main moulting characteristic, with only Ptychopariida and Redlichiida containing 15% of species for which this is the case (Figure 11). The chi-squared analyses and corrected α-level show the same results (Appendix 5, Table A3) as for the analyses using all the data (Appendix 5, Table A2). The orders show significant differences in the number of species displaying rostral plate (p=8.8x10-9) and facial suture opening (p=0.0022), and cephalic disarticulation (p=9.9x10-14), with deviations in the same orders seemingly causing this result. Table 2 summarises the most commonly observed moulting characteristics and sampling for the seven trilobite orders.
Superfamilial/Familial-level Moulting Variability
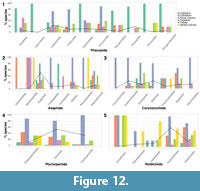 The well-sampled trilobite orders show some differences in the moulting characteristics observed within their constituent families or superfamilies (Figure 12). Familial/superfamilial moulting variability seems relatively unaffected by sampling, as many of the clades with smaller sample sizes show almost all of the characteristics. Better sampled clades do not necessarily show more characteristics (Figure 12), and all orders show high variability in moulting behaviour. Phacopid families are notably disparate in their moulting characteristics. Seven phacopid families show a bias to cephalic disarticulation over opening of the facial sutures for moulting (see the total-group phacopid data; Figure 9, Figure 10, Figure 11), with only the Calymenidae, Cheiruridae and Encrinuridae showing the reverse, although all families contain species with moult configurations with disarticulated cephala (Figure 12.1). Thoracic disarticulation is relatively common across all phacopid families (>15% of species). The Acastidae and Dalmanitidae show high levels of pygidial disarticulation (33-50%), but they, along with the Pliomeridae, Calmoniidae, and Phacopidae do not show cranidial disarticulation. Only two families (the Calymenidae and Pliomeridae) contain species that use the ventral sutures in moulting (Figure 12.1). The better sampled asaphid families show a tendency toward opening the facial sutures for moulting, except the Raphiophoridae which show high moulting variability, and the Cyclopygidae which only display disarticulation of the cephalon and pygidium (Figure 12.2). Within the Corynexochida (Figure 12.3) the Dorypygidae and Styginidae show greater variability in moulting characteristics, with a higher prevalence of thoracic disarticulation, as well as separation of the cranidium in the latter and the Oryctocephalidae. The Ptychopariida and Redlichiida (Figure 12.4, 12.5) displayed particularly high levels of variability, but this may be because their sample sizes meant testing at the superfamily-level. Although, it is probably a more true representation to consider the Ptychopariida and Redlichiida at the superfamily or family-level as these orders are widely considered to represent para- or polyphyletic groupings with unclear phylogenetic relationships (Fortey, 2001), and so order-level analyses may have lower accuracies for the Redlichiida and Ptychopariida. Ptychopariid superfamilies show relatively uniform variability in the six moulting characteristics with opening of the facial sutures being most common, although the Olenoidea and Ellipsocephaloidea contain higher proportions of species showing thoracic and cranidial disarticulation (Figure 12.4). All redlichiid superfamilies show disproportionately high incidences of ventral suture opening for moulting. This is observable in at least 50% of species belonging to all superfamilies and is as common or more so than opening of the facial sutures for three out of five superfamilies (Figure 12.5). The Emuelloidea, Paradoxidoidea, and Redlichioidea also show notably high proportions of cranidial and thoracic disarticulation.
The well-sampled trilobite orders show some differences in the moulting characteristics observed within their constituent families or superfamilies (Figure 12). Familial/superfamilial moulting variability seems relatively unaffected by sampling, as many of the clades with smaller sample sizes show almost all of the characteristics. Better sampled clades do not necessarily show more characteristics (Figure 12), and all orders show high variability in moulting behaviour. Phacopid families are notably disparate in their moulting characteristics. Seven phacopid families show a bias to cephalic disarticulation over opening of the facial sutures for moulting (see the total-group phacopid data; Figure 9, Figure 10, Figure 11), with only the Calymenidae, Cheiruridae and Encrinuridae showing the reverse, although all families contain species with moult configurations with disarticulated cephala (Figure 12.1). Thoracic disarticulation is relatively common across all phacopid families (>15% of species). The Acastidae and Dalmanitidae show high levels of pygidial disarticulation (33-50%), but they, along with the Pliomeridae, Calmoniidae, and Phacopidae do not show cranidial disarticulation. Only two families (the Calymenidae and Pliomeridae) contain species that use the ventral sutures in moulting (Figure 12.1). The better sampled asaphid families show a tendency toward opening the facial sutures for moulting, except the Raphiophoridae which show high moulting variability, and the Cyclopygidae which only display disarticulation of the cephalon and pygidium (Figure 12.2). Within the Corynexochida (Figure 12.3) the Dorypygidae and Styginidae show greater variability in moulting characteristics, with a higher prevalence of thoracic disarticulation, as well as separation of the cranidium in the latter and the Oryctocephalidae. The Ptychopariida and Redlichiida (Figure 12.4, 12.5) displayed particularly high levels of variability, but this may be because their sample sizes meant testing at the superfamily-level. Although, it is probably a more true representation to consider the Ptychopariida and Redlichiida at the superfamily or family-level as these orders are widely considered to represent para- or polyphyletic groupings with unclear phylogenetic relationships (Fortey, 2001), and so order-level analyses may have lower accuracies for the Redlichiida and Ptychopariida. Ptychopariid superfamilies show relatively uniform variability in the six moulting characteristics with opening of the facial sutures being most common, although the Olenoidea and Ellipsocephaloidea contain higher proportions of species showing thoracic and cranidial disarticulation (Figure 12.4). All redlichiid superfamilies show disproportionately high incidences of ventral suture opening for moulting. This is observable in at least 50% of species belonging to all superfamilies and is as common or more so than opening of the facial sutures for three out of five superfamilies (Figure 12.5). The Emuelloidea, Paradoxidoidea, and Redlichioidea also show notably high proportions of cranidial and thoracic disarticulation.
The chi-squared analyses show little significant variation in these moulting characteristic occurrences within each order (Appendix 5, Table A4). Only the number of species opening the ventral sutures (p=0.035) and disarticulating the cephalon (p=0.022) during moulting vary significantly between asaphid families, and the facial sutures (p=0.0036) and cephalon (p=0.002) for Phacopida (Appendix 5, Table A4). For the cephalic disarticulation in Asaphida this is likely due to it occurring more often than expected in the Cyclopygidae and less than in the Nileidae, and in Phacopida this is more common than expected in the Pliomeridae, Acastidae, Dalmanitidae, Phacopidae, and Pterygometopidae, but less than in the Calymenidae, Cheiruridae, and Encrinuridae. Opening of the facial sutures shows the opposite pattern to this in Phacopida. For the Corynexochida, Ptychopariida, and Redlichiida the sampled families or superfamilies do not significantly vary in their displayed moulting characteristics.
Intraspecific Moulting Variability with Taxonomy
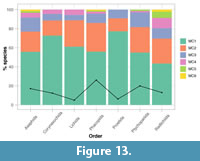 Intraspecific moulting variability, or the proportion of species displaying multiple moulting characteristics, varies between the orders. Three of the orders (Asaphida, Phacopida, and Redlichiida) contain species that display all six of the moulting characteristics (Figure 13). These orders therefore appear to show greater intraspecific moulting variation than the others. Asaphida and Phacopida have large sample sizes, however, Ptychopariida has the second-largest sample size and contains only species displaying up to five of the moulting characteristics, while Lichida has the lowest sample size and contains a number of species showing four of the moulting characteristics (Figure 13). Overall, the patterns of intraspecific moulting variation are reasonably consistent across the trilobite orders. Of the trilobite species, 43-77% display only a single moulting characteristic (see sampling discussion for Figure 4), and for all orders except Redlichiida (with 80%), >90% of species displayed three or fewer moulting characteristics (Figure 13). Redlichiida is, therefore, the most intraspecifically variable order for moulting behaviour. It is uncommon for trilobite species to show more than two of the moulting characteristics, so most species are either not at all or only moderately intraspecifically variable.
Intraspecific moulting variability, or the proportion of species displaying multiple moulting characteristics, varies between the orders. Three of the orders (Asaphida, Phacopida, and Redlichiida) contain species that display all six of the moulting characteristics (Figure 13). These orders therefore appear to show greater intraspecific moulting variation than the others. Asaphida and Phacopida have large sample sizes, however, Ptychopariida has the second-largest sample size and contains only species displaying up to five of the moulting characteristics, while Lichida has the lowest sample size and contains a number of species showing four of the moulting characteristics (Figure 13). Overall, the patterns of intraspecific moulting variation are reasonably consistent across the trilobite orders. Of the trilobite species, 43-77% display only a single moulting characteristic (see sampling discussion for Figure 4), and for all orders except Redlichiida (with 80%), >90% of species displayed three or fewer moulting characteristics (Figure 13). Redlichiida is, therefore, the most intraspecifically variable order for moulting behaviour. It is uncommon for trilobite species to show more than two of the moulting characteristics, so most species are either not at all or only moderately intraspecifically variable.
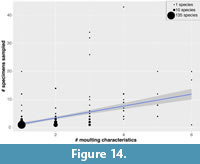 However, the number of moulting characteristics shown per species does increase with the number of specimens sampled (Figure 14). This is supported by significant results from correlation tests (r2: 0.48, p=1.03x10-19; Spearman’s Rank: 0.49, p=3.046x10-21; Kendall’s Tau: 0.44, p=1.519x10-32), however, these values indicate only a moderate positive correlation between specimen sampling and the number of moulting characteristics shown (i.e., below 0.5). This suggests that intraspecific moulting variability would increase with better specimen-level sampling, but likely only at the lower end of sampling (1-10 specimens), and not at higher levels of sampling, which do not fit the trend line as well (Figure 14).
However, the number of moulting characteristics shown per species does increase with the number of specimens sampled (Figure 14). This is supported by significant results from correlation tests (r2: 0.48, p=1.03x10-19; Spearman’s Rank: 0.49, p=3.046x10-21; Kendall’s Tau: 0.44, p=1.519x10-32), however, these values indicate only a moderate positive correlation between specimen sampling and the number of moulting characteristics shown (i.e., below 0.5). This suggests that intraspecific moulting variability would increase with better specimen-level sampling, but likely only at the lower end of sampling (1-10 specimens), and not at higher levels of sampling, which do not fit the trend line as well (Figure 14).
Interspecific Moulting Variability through Time
Raw total group trilobite species diversity across the Palaeozoic closely tracks the numbers of species sampled within the dataset (Figure 15; Appendix 4, Table A2). The lines are slightly offset from one 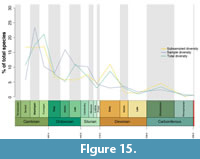 another (i.e., the total diversity peaks occur slightly after the sample diversity peaks), likely due to the difference in time bin dates between this study and Fossilworks (2018). An r2 test shows that these are significantly correlated (0.68, p=0.0076), as do non-parametric tests for correlation (Spearman’s rank: 0.77, p=0.0013; Kendall’s tau: 0.58, p=0.0042). The raw total group diversity curve suggests trilobite diversity declined overall from the end-Cambrian, with these data not showing an early Ordovician radiation (unlike that observed for other groups during the Great Ordovician Biodiversification Event; see Servais et al., 2010), although there were clear diversity peaks in the Late Ordovician and Early Devonian (potentially reflecting the origination, and then diversification, of Phacopida; Crônier, 2013; and Proetida during the latter Epoch; Stubblefield, 1959). Figure 15 also supports the notion of trilobites having dramatically radiated early in the Cambrian (Webster, 2007). When corrected for sampling (using Shareholder Quorum Subsampling), the total group diversity shows similar trends (Figure 15), also closely tracing the sample diversity (again, with a significant positive correlation; r2: 0.58, p=0.03; Spearman’s rank: 0.7, p=0.0051; Kendall’s tau: 0.55, p=0.0059), except for some slight divergences in the Ordovician.
another (i.e., the total diversity peaks occur slightly after the sample diversity peaks), likely due to the difference in time bin dates between this study and Fossilworks (2018). An r2 test shows that these are significantly correlated (0.68, p=0.0076), as do non-parametric tests for correlation (Spearman’s rank: 0.77, p=0.0013; Kendall’s tau: 0.58, p=0.0042). The raw total group diversity curve suggests trilobite diversity declined overall from the end-Cambrian, with these data not showing an early Ordovician radiation (unlike that observed for other groups during the Great Ordovician Biodiversification Event; see Servais et al., 2010), although there were clear diversity peaks in the Late Ordovician and Early Devonian (potentially reflecting the origination, and then diversification, of Phacopida; Crônier, 2013; and Proetida during the latter Epoch; Stubblefield, 1959). Figure 15 also supports the notion of trilobites having dramatically radiated early in the Cambrian (Webster, 2007). When corrected for sampling (using Shareholder Quorum Subsampling), the total group diversity shows similar trends (Figure 15), also closely tracing the sample diversity (again, with a significant positive correlation; r2: 0.58, p=0.03; Spearman’s rank: 0.7, p=0.0051; Kendall’s tau: 0.55, p=0.0059), except for some slight divergences in the Ordovician.
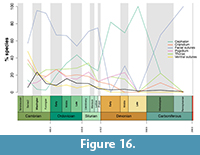 The proportion of all trilobite species displaying each of the six moulting characteristics varied through the Palaeozoic. Opening of the facial sutures and disarticulation of the cephalon during moulting displays almost opposing trends (Figure 16). The former is extremely common from the early Cambrian to end Silurian (>50% of species), although in the Cambrian Series 2 moulting seems to be more variable, with all characteristics except pygidial disarticulation exhibiting a small peak (which do not correspond with a peak in sampling). When disarticulation of the cephalon is extremely prevalent during the Devonian opening of the facial sutures is less common. Cephalic disarticulation also became conspicuously more common over the Ordovician Period. The chi-squared analyses over geological Periods (Permian excluded due to sample size) indicate that the number of species displaying both of these characteristics during moulting is different to that expected (Appendix 5, Table A5; p=0.0046 and 6.1x10-18, respectively). This is also true of opening of the ventral sutures (p=0.00053), which is very common in the early Cambrian (48% of species in Series 2, decreasing to 24% in Miaolingian), became less prevalent during the Ordovician and Silurian, and is absent by the start of the Devonian (Figure 16). The remaining three moulting characteristics follow similar patterns over a longer trajectory, becoming generally less prevalent from the early Silurian, but these do not have significant chi-squared results. None of the characteristics closely track the total number of species sampled (black lines, Figure 16, Figure 17). The same chi-squared results were also produced when these data were further divided into geological Epoch bins. Opening of the facial sutures, ventral sutures, and cephalic disarticulation vary significantly (Appendix 5, Table A6; p=0.018, 0.0011, and 7.03x10-13 respectively), resulting from much the same deviations from expected values as described above.
The proportion of all trilobite species displaying each of the six moulting characteristics varied through the Palaeozoic. Opening of the facial sutures and disarticulation of the cephalon during moulting displays almost opposing trends (Figure 16). The former is extremely common from the early Cambrian to end Silurian (>50% of species), although in the Cambrian Series 2 moulting seems to be more variable, with all characteristics except pygidial disarticulation exhibiting a small peak (which do not correspond with a peak in sampling). When disarticulation of the cephalon is extremely prevalent during the Devonian opening of the facial sutures is less common. Cephalic disarticulation also became conspicuously more common over the Ordovician Period. The chi-squared analyses over geological Periods (Permian excluded due to sample size) indicate that the number of species displaying both of these characteristics during moulting is different to that expected (Appendix 5, Table A5; p=0.0046 and 6.1x10-18, respectively). This is also true of opening of the ventral sutures (p=0.00053), which is very common in the early Cambrian (48% of species in Series 2, decreasing to 24% in Miaolingian), became less prevalent during the Ordovician and Silurian, and is absent by the start of the Devonian (Figure 16). The remaining three moulting characteristics follow similar patterns over a longer trajectory, becoming generally less prevalent from the early Silurian, but these do not have significant chi-squared results. None of the characteristics closely track the total number of species sampled (black lines, Figure 16, Figure 17). The same chi-squared results were also produced when these data were further divided into geological Epoch bins. Opening of the facial sutures, ventral sutures, and cephalic disarticulation vary significantly (Appendix 5, Table A6; p=0.018, 0.0011, and 7.03x10-13 respectively), resulting from much the same deviations from expected values as described above.
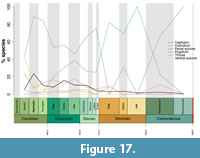 Plotting the ‘normal’ moulting characteristic for the species in each geological Epoch results in similar patterns (Figure 17 compared to Figure 16). Disarticulation of the cephalon again shows an opposing pattern to opening of the facial sutures, with the former becoming more prevalent during the Devonian (82-100% of species). The chi-squared results confirm that these two characteristics significantly vary through the Palaeozoic (Appendix 5, Table A7; p=0.00098 for the facial sutures and 1.03x10-15 for the cephalon). Presenting only the most common moulting characteristic for each species means that disarticulation of the thorax, pygidium, or cranidium decreases from around 10-40% species (Figure 16) to under 10% for the thorax and pygidium, and 18% of species at the most for the cranidium (Figure 17). The chi-squared results suggest that any variation in these three characteristics through time is again not significant (Appendix 5, Table A7). The proportion of species most commonly displaying open ventral sutures in moults is also lower throughout the Palaeozoic, with no species using this as their main mode of moulting after the Late Ordovician (despite there being moult specimens with open ventral sutures until the Early Devonian; Figure 16). However, opening the ventral sutures is notably common during the Cambrian Series 2 at 43% of species (Figure 17), producing a significant chi-squared result (Appendix 5, Table A7; p=0.00096).
Plotting the ‘normal’ moulting characteristic for the species in each geological Epoch results in similar patterns (Figure 17 compared to Figure 16). Disarticulation of the cephalon again shows an opposing pattern to opening of the facial sutures, with the former becoming more prevalent during the Devonian (82-100% of species). The chi-squared results confirm that these two characteristics significantly vary through the Palaeozoic (Appendix 5, Table A7; p=0.00098 for the facial sutures and 1.03x10-15 for the cephalon). Presenting only the most common moulting characteristic for each species means that disarticulation of the thorax, pygidium, or cranidium decreases from around 10-40% species (Figure 16) to under 10% for the thorax and pygidium, and 18% of species at the most for the cranidium (Figure 17). The chi-squared results suggest that any variation in these three characteristics through time is again not significant (Appendix 5, Table A7). The proportion of species most commonly displaying open ventral sutures in moults is also lower throughout the Palaeozoic, with no species using this as their main mode of moulting after the Late Ordovician (despite there being moult specimens with open ventral sutures until the Early Devonian; Figure 16). However, opening the ventral sutures is notably common during the Cambrian Series 2 at 43% of species (Figure 17), producing a significant chi-squared result (Appendix 5, Table A7; p=0.00096).
Intraspecific Moulting Variability through Time
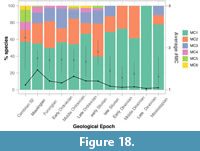 Intraspecific moulting variability also varies temporally within the dataset (Figure 18). In general, intraspecific moulting variability decreased over the Palaeozoic, going from 57% of species showing a single characteristic in the Cambrian Series 2 to 78% in the Mississippian (Figure 18). All Epochs from the early Cambrian to early Silurian contain species that displayed at least four moulting characteristics (between 29% in the Cambrian Series 2 and 3% in the Furongian), while the late Silurian and Mississippian both contain species showing three characteristics. The proportion of species showing two moulting characteristics varies stochastically through the Palaeozoic, but the overall proportion showing three to six moulting characteristics decreases through time (Figure 18). From the late Silurian this may be a product of smaller sample sizes, however, this is not consistent with sampling bias prior to the Silurian. For example, the Cambrian Series 2 shows a high level of intraspecific moulting variation, with by far the highest proportion of species showing three or more moulting characteristics (29%), but the lowest level of sampling (21 species) before the late Silurian. While the mean average number of moulting characteristics shown by the species is higher at this time (2.24) than in any other Epoch (1 to 1.89), the broad 95% confidence intervals overlap, thereby suggesting the means do not differ, and that intraspecific moulting variability does not change through the Palaeozoic. However, these appear more different when comparing the means in the first half of the Palaeozoic to later Epochs. This is supported by ANOVA and Kruskal-Wallis tests comparing the means of the geological Periods which are close to the significance threshold (p=0.064 for both). Decreasing moulting variability through the Palaeozoic (i.e., increasing proportion of one moulting characteristic, and decrease in three to six) also trends with decreasing diversity through time (both in the sample, but also total group and subsampled diversity; see Figure 15).
Intraspecific moulting variability also varies temporally within the dataset (Figure 18). In general, intraspecific moulting variability decreased over the Palaeozoic, going from 57% of species showing a single characteristic in the Cambrian Series 2 to 78% in the Mississippian (Figure 18). All Epochs from the early Cambrian to early Silurian contain species that displayed at least four moulting characteristics (between 29% in the Cambrian Series 2 and 3% in the Furongian), while the late Silurian and Mississippian both contain species showing three characteristics. The proportion of species showing two moulting characteristics varies stochastically through the Palaeozoic, but the overall proportion showing three to six moulting characteristics decreases through time (Figure 18). From the late Silurian this may be a product of smaller sample sizes, however, this is not consistent with sampling bias prior to the Silurian. For example, the Cambrian Series 2 shows a high level of intraspecific moulting variation, with by far the highest proportion of species showing three or more moulting characteristics (29%), but the lowest level of sampling (21 species) before the late Silurian. While the mean average number of moulting characteristics shown by the species is higher at this time (2.24) than in any other Epoch (1 to 1.89), the broad 95% confidence intervals overlap, thereby suggesting the means do not differ, and that intraspecific moulting variability does not change through the Palaeozoic. However, these appear more different when comparing the means in the first half of the Palaeozoic to later Epochs. This is supported by ANOVA and Kruskal-Wallis tests comparing the means of the geological Periods which are close to the significance threshold (p=0.064 for both). Decreasing moulting variability through the Palaeozoic (i.e., increasing proportion of one moulting characteristic, and decrease in three to six) also trends with decreasing diversity through time (both in the sample, but also total group and subsampled diversity; see Figure 15).
Results Summary
Trilobite moulting behaviour is interspecifically variable within all trilobite orders (Figure 6, Figure 7, Figure 9, Figure 10, Figure 11), and at lower taxonomic levels (Figure 12). Firstly, around 20% of all species show the use of both cephalic sutures and the cephalothoracic joint for moulting (Figure 6), despite these representing quite different methods of creating an anterior exuvial gape. Trilobite species commonly display moulting using the facial sutures (Figure 6, Figure 7, Figure 9). It is thought that the facial sutures originated as a tool for moulting (Henningsmoen, 1975; Whittington, 1990), and are present in most species and in some closely related, non-biomineralised artiopodans (Stubblefield, 1959; Hunda et al., 2006; Hou et al., 2017; Du et al., 2018), potentially even being the ancestral condition uniting these groups with trilobites (Du et al., 2018). All six moulting characteristics are seen in every order (Figure 10), and the family/superfamily-level data also shows high levels of interspecific variability with most groups showing at least three of the moulting characteristics (Figure 12). Moulting behaviour is similarly variable when plotting the data through geological time. All six moulting characteristics occur throughout the Palaeozoic, and usually during each Epoch, although ventral sutures were not used from the Devonian onwards, Figure 16). The sampled species are also generally intraspecifically variable in moulting behaviour, with moults of the same species often displaying more than one characteristic (Figure 18). This includes >30% of sampled species for most of the Palaeozoic, with the mean number of moulting characteristics per species in each Epoch ranging from 1.3-2.2. Trilobite orders are not consistent in their intraspecific moulting variability (Figure 13). All seven orders contain species showing at least four moulting characteristics, but differ in the proportion of species displaying 2 or more, with Redlichiida the most at 57% of species, to Proetida the least at 23% (Figure 13).
DISCUSSION
Trilobite Moulting Variability and Diversity
This study presents the first broad-scale quantitative exploration of trilobite exoskeleton moulting behaviours. Previous qualitative studies named moult configurations and reconstructed behaviours, while noting that despite the overall similarity of body forms within Trilobita, their moulting behaviour is seemingly highly variable (see Henningsmoen, 1975; Whittington, 1990; Brandt, 2002; Hughes, 2003; Daley and Drage, 2016; Drage and Daley, 2016; Drage et al., 2018a). This is in comparison to groups like chelicerates or decapod crustaceans, which have several body forms, and each has one specialised mode of moulting (Daley and Drage, 2016, and references therein). This study demonstrates for the first time the unusually high behavioural variability in trilobite moulting, confirming the preliminary work of Daley and Drage (2016) that identified this from a smaller dataset over the Cambrian and Ordovician.
A number of differences in moulting variability between taxonomic groups and throughout the Palaeozoic have been identified and are summarised at the end of the Results section. These may be affected by sampling given the moderate positive correlation between specimen sampling and the number of moulting characteristics displayed (Figure 14). However, Redlichiida and Proetida are at the extremes of intraspecific moulting variability, but did not have the highest or lowest sample sizes (Figure 13). Intraspecific moulting variability also slightly decreases through the Palaeozoic (Figure 18), with the proportion of species showing only one moulting characteristic increasing through time. The Cambrian Series 2 shows the highest levels of intraspecific moulting variability. This corresponds with their elevated diversity (total group and sampled) in the Cambrian and early Ordovician (Figure 15) and high levels of morphological variability (Fortey and Owens, 1999; Webster, 2007), presumably due to their ecological expansion after the Cambrian Explosion. Behavioural flexibility, particularly for a risky recurrent event like moulting, might be adaptive when expanding into new habitats, such as during the Cambrian. Both trilobite diversity and intraspecific moulting variability slowly decline after the Cambro-Ordovician, before the extinction of trilobites in the Permian (Song et al., 2013). This decrease in moulting variability is not simply a result of the reduced species diversity because groups with the highest variability in moulting behaviour generally went extinct earlier, such as the Redlichiida (Stubblefield, 1959). Only the Proetida survived until the Permian (Fortey and Owens, 1975; Mikulic, 1981; Owens, 2003), but show low intraspecific variability (Figure 13) and comparably low morphological variability, particularly in the few surviving groups after the early Carboniferous (Fortey and Owens, 1975; Owens, 2003; Webster, 2007; Lerosey-Aubril and Feist, 2012). This suggests a curtailment in moulting behaviour variability and potentially specialisation in moulting behaviour over the Permian, which may have been an evolutionary advantage of the less intraspecifically flexible proetids. Brandt (2002) suggested that moulting flexibility may have been detrimental to trilobite evolutionary success, because later-evolving arthropod groups were more specialised and efficient in their moulting behaviours. As such, the less behaviourally diverse proetids may therefore have survived for such a prolonged period of time because they were more specialised. However, Lerosey-Aubril and Feist (2012) found that proetids underwent a generic-level diversification following Devonian and Carboniferous extinctions accompanied by a burst of adaptations to more ecological niches; as such, further investigation of Permo-Carboniferous proetid moulting behaviour might reveal an increase in flexibility comparable to the early Cambrian. Proetids sampled here mainly correspond to the Proetidae, although several aulacopleurid species were included which have an uncertain phylogenetic affinity (Adrain, 2011, removed them from Proetida, but phylogenetic analyses argue for their proetid assignment; Lamsdell and Selden, 2015) and this may have affected the results. In any case, future increased sampling of the Proetida, if suitable material were available, would be informative on late Palaeozoic changes in moulting behaviour.
The initial flexibility in moulting behaviour may have been adaptive for trilobites owing to their generally thick, heavily-calcified exoskeleton, which would make moulting more problematic than for other arthropod groups (Miller and Clarkson, 1980; Fortey and Wilmot, 1991). This provided increased protection from predation and parasitism during the Cambrian Explosion and later in the Palaeozoic (Brandt, 2002), but was balanced by having exuvial gapes that were presumably harder to create. Trilobites may have therefore been prone to moulting failures, where the planes of weakness (facial and ventral sutures, and/or the cephalothoracic joint) did not open and the animal was trapped, necessitating the use of other ‘back-up’ moulting behaviours (McNamara, 1986; Brandt, 2002; Budil and Bruthansová, 2005; Paterson et al., 2007; Tortello and Clarkson, 2008). That this behavioural flexibility was adaptive and a response to dire circumstances is supported by looking at the ‘normal’ moulting behaviours of the sampled species. Most species known from multiple specimens (42% of the dataset, Figure 14) have one characteristic most commonly associated with their moult configurations, with other characteristics being generally rarer (Figure 11). Facial sutures (usually present in most species; Henningsmoen, 1975; Whittington, 1990; Brandt, 2002; Du et al., 2018) and the cephalothoracic joint are most commonly used for moulting, with a much smaller number of species usually showing cranidial disarticulation or opening of the ventral sutures (the latter being almost confined to Redlichiida; Figure 11). The lack of significant temporal or taxonomic variation (Appendix 5, Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7) in the percentage of species showing thoracic or pygidial disarticulation during moulting indicate that these characteristics were rarely involved in moulting and occurred incidentally, in association with another moulting characteristic. The comparable weakness at the articulation joints between thoracic segments, and the pygidiothoracic joint (see Whittington, 1980; McNamara and Rudkin, 1984; Drage et al., 2018a), meant these areas could disarticulate fairly readily when put under pressure, such as during moulting. Some authors have considered most post-cephalic disarticulation to be the result of decay and/or post-mortum disturbance of trilobite carcasses, and not always attributed to moulting (e.g., Hunda et al., 2006). Although, decay and disturbance could equally happen to moults pre-preservation, which would also explain the occurrence of thoracic and pygidial disarticulation usually being associated with another moulting characteristic.
Moulting and Morphology
Certain aspects of trilobite moulting variability can be explained by morphology and taxonomy. The significant chi-squared analyses for the temporal and taxonomic variation of ventral suture use in moulting (Figure 10, Figure 11, Figure 16, Figure 17; Appendix 5, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7) largely result from redlichiids opening the ventral sutures more than expected (27:8, Figure 10). This is because many taxa support a broad and easily recognisable rostral plate often with rostral sutures, a conterminant hypostome, and because the early Cambrian Olenelloidea do not have facial sutures like most trilobites (Stubblefield, 1959; Fortey, 1990). For many redlichiids usage of the functional ventral connective sutures (e.g., rostral, hypostomal) is therefore important for moulting (Fortey, 1990). Redlichiida are reasonably abundant in the early Cambrian (18/21 and 29/87 species in the Cambrian Series 2 and Miaolingian, respectively) and went extinct soon after (in particular the olenellids; Stubblefield, 1959) explaining the significant Cambrian peak in ventral suture usage and its subsequent decrease in later Epochs (Figure 16). Stubblefield (1959) argued that groups with functioning facial sutures outcompeted these early-evolving redlichiids, and many of these later groups repeatedly lost the rostral sutures (Fortey, 1990), making moulting by ventral sutures alone less frequent. Although it is likely that Redlichiida represents a para- or polyphyletic grouping (Fortey, 2001), and so further family-level study would be useful for further exploring this result.
Similarly, the significant for variation in the proportion of species disarticulating the cephalon are likely linked to the diversity of the Phacopida. This is the only trilobite order with a higher percentage of species displaying disarticulation of the cephalothoracic joint for moulting (68%, Figure 9) than opening of the cephalic sutures (46%; Figure 10, Figure 11). The suborder Phacopina has secondarily fused facial sutures during adulthood (Crônier, 2013; Drage et al., 2018b), preventing the librigenae from being disarticulated for moulting. Phacopines diversified later in the Palaeozoic (25/30 for the Devonian; Crônier, 2013), and repeated convergence of blindness and loss of facial sutures was common in Devonian trilobites (Feist, 1995), likely causing the peak in cephalic moulting at this point (Figure 16, Figure 17). Those phacopid families that retained operable facial sutures, like other trilobite groups, separate the librigenae more often than disarticulating the cephalon in moulting (Figure 12.1). Use of the facial sutures and cephalic disarticulation are mutually exclusive moulting characteristics (together, they equate to cranidial disarticulation), and so the facial suture trends mirror the cephalic data. For the majority of orders and geological Epochs, separation of the librigenae is the most common moulting characteristic, owing to the almost ubiquitous existence of the facial sutures (potentially even as an ancestral condition in Artiopoda; Du et al., 2018), which probably originated as a tool for moulting (Stubblefield, 1959; Henningsmoen, 1975).
Disarticulation of the cranidium mostly follows the same trend as thoracic and pygidial disarticulation, and does not significantly vary or show much prevalence amongst the ‘normal’ moulting characteristics for the sampled species. This is in contrast to Drage et al. (2018a), who found that disarticulation of the cranidium was extremely common for moult configurations of Estaingia bilobata Pocock 1964, and did not follow the stochastic occurrence of thoracic and pygidial disarticulations (again, presumably due to incidental occurrence during moulting, or because they would have readily occurred due to taphonomic effects pre-preservation). This may be because this characteristic is simply not morphologically or taxonomically linked (at least above species-level), and therefore does not show broader-scale trends.
Sampling a Rarely Preserved Behaviour
Quantitative studies will always be enriched by sampling greater numbers of specimens. For example, this work shows a moderate positive correlation between specimen sampling and intraspecific moulting variability, probably owing to the 58% of species for which only one specimen was recorded (Figure 14). However, it would be almost impossible to sample moulting behaviour for the over 20,000 described species of trilobite (Lerosey-Aubril and Peel, 2018), especially as many are not known from fully-articulated specimens. Further, greater specimen sampling would likely reinforce the major findings of this work as more specimens would generally even higher levels of intraspecific variability. Greater specimen sampling may even strengthen the trend of decreasing variability through time, since the longest surviving group, the proetids, appeared to be comparably specialised in moulting behaviour (Figure 13). In depth sampling of families from certain groups (in particular the ptychopariids and redlichiids) would also allow for analyses to be carried out predominantly at the family-level, which might circumnavigate issues of uncertain high-level trilobite phylogeny (Fortey, 2001).
Increased species-level sampling would likely have little effect on the broad-scale results. There is a significant correlation between sampled species diversity and total group (raw and subsampled) diversity (Figure 15), and these diversity curves are similar to those presented by other researchers (Brandt, 2002; Webster, 2007; Adrain, 2008). Further, with the exception of a couple of orders, taxonomic species sampling is also reasonably similar to total diversity (Figure 8; although data from Fossilworks and the Paleobiology Database are also subject to missing records; Fossilworks, 2018). These factors suggest that the dataset sampling is representative of the ‘true’ diversity of trilobites through the Palaeozoic, and that the interspecific moulting results are robust. Specimen sampling is also less of an issue than might be supposed. Firstly, specimen sampling is overall underestimated in the results reported here (Figure 14) because accurate counts were impossible to obtain from specimens described in the literature. Secondly, the total number of trilobite specimens actually surveyed and interpreted to produce the dataset was several times greater than the 840 specimens included (in the order of 5-10,000 accessioned specimens). Excepting for Konservat-Lagerstätten, the preservation of an unambiguous moult configuration is reasonably rare, and therefore these data are not available for the majority of Palaeozoic localities, or even many described species. This is because specimens must be mostly articulated (with most sclerites found in association) to be confidently identified as moults, helping to ensure carcasses that have simply been disarticulated prior to preservation (e.g., by water currents) are not falsely included within the dataset. For the majority of species in the dataset multiple specimens were therefore examined, but could not be included because the material was too disarticulated. These singleton species could be excluded from the dataset to improve overall specimen sampling, but, owing to their rarity, all of these are informative and important for broad-scale studies of behaviour. Given the requirement for reasonably articulated specimens, if exploring trilobite behaviours at a finer-scale than that presented here, it would be logical to look at Konservat-Lagerstätten with their greater abundance of samples (e.g., Drage et al., 2018a).
Finally, the higher-level taxonomy used here is subject to change (Fortey, 1990) because of the problematic phylogenetic relationships of Trilobita. This is owing to widespread cryptogenesis within the group (Stubblefield, 1959) and a paucity of broad-scale phylogenetic studies, although the latter is greatly improving (see Lieberman and Karim, 2010). Hence, a more in-depth study on a family or superfamily-level (such as presented in Figure 12) would likely prove worthwhile for investigating phylogenetic differences in moulting. Further studies on aspects such as moulting and ontogeny (e.g., Drage et al., 2018b; Wolfe and Hegna, 2014) may even help to elucidate these problematic relationships.
CONCLUSIONS
This study presents the first quantification and detailed exploration of total-group trilobite moulting behaviour and the largest dataset on trilobite behaviour ever assembled. It describes variation in trilobite moulting and tracks broad-scale trends in the occurrence of their characteristic moulting disarticulations. This has been explored both intra- and interspecifically on taxonomic and temporal scales, and compared to diversity estimates through the Palaeozoic. The sample presented here is representative of the total-group (Figure 15), and is currently the largest dataset feasible given the huge number of specimens examined to produce it and the reasonably high preservational requirements of valid specimens. The data capture a broad scale of morphological and taxonomic diversity, as well as rarer examples of moulting.
Despite their reasonably consistent body plan, trilobite moulting behaviour is extremely variable across all orders, superfamilies, families (Figure 6, Figure 7, Figure 9, Figure 10, Figure 11, Figure 12), and throughout the Palaeozoic (Figure 16, Figure 17, Figure 18). This represents an inherent moulting flexibility within the group, and within a large proportion of trilobite species. Opening of the facial sutures and separation of the librigenae for moulting is generally ubiquitous across Trilobita, because this represents a specialism for moulting found in most of the group (Du et al., 2018). Some groups and time bins show significant differences in the prevalence of moulting characteristics. In particular, Redlichiida from lower Cambrian Epochs often moulted by opening the ventral sutures (Figure 11, Figure 17). For many of the early trilobite species (e.g., olenellids) this is due to a morphological constraint, but this characteristic is also seen throughout most of the Palaeozoic and in all orders (Figure 10). Similarly, the Phacopida are biased toward disarticulating the entire cephalon for moulting (Figure 11), and are responsible for peaks in this behaviour during the Ordovician and Devonian when they were particularly diverse (Figure 17). Again, a subgroup of phacopids (the phacopines) were constrained to this behaviour (Drage et al., 2018b), but it is also frequently observed in all of the orders (Figure 7, Figure 9) and throughout the Palaeozoic (Figure 16).
Moulting behaviour flexibility generally appeared to decrease through time in concert with decreasing trilobite diversity (Figure 15, Figure 18). This may reflect high levels of behavioural flexibility (along with morphological variability) during their early radiation and establishment after the Cambrian Explosion, and subsequent specialism in the more conservative later-Palaeozoic groups (such as the long-lived Proetida; Figure 13).
This work conclusively demonstrates that quantitative behavioural hypothesis testing using the fossil record is not only feasible but can reveal broad-scale evolutionary trends. Moulting is of singular importance in the life histories of arthropods, and has been central to their establishment as the most abundant and diverse animal group from the early Cambrian to the present. It has thereby dramatically shaped the evolution of groups like trilobites, and in doing so affected all of Earth’s ecosystems, past and present. Quantitative analyses based on large datasets of moulting behaviour, beginning with that presented here, allow us to start to fully understand the evolution and importance of this behaviour in deep time.
ACKNOWLEDGEMENTS
The author wishes to gratefully thank A.C. Daley, S. Pates, and G. Edgecombe for their continued discussion and support with this work. C. Mellish (NHMUK), J.O. Ebbestad (PMU), M.A. Binnie (SAM), K. Riddington (BIRUG), and E. Howlett (OUMNH) provided access to and support with museum collections, and data collection would have been impossible without them. A Natural Environment Research Council Doctoral Training Partnership studentship (NE/L002612/1) to H.B. Drage, and support from the Insitut des Sciences de la Terre (Université de Lausanne), funded this research. Two anonymous reviewers and the editors are thanked for providing helpful feedback on this manuscript. All the data generated for this study and used to produce the results herein are included in this published article and its supplementary information files. The Fossilworks/Paleobiology Database datasets generated and analysed during the current study are publically available in the Fossilworks repository, at http://fossilworks.org.
REFERENCES
Adrain, J.M. 2008. A global species database of Trilobita: progress, results, and revision of the Treatise, p. 27-28. In Rábano, I., Gozalo, R., and García-Bellido, D.C. (eds.), Advances in Trilobite Research. Cuadernos del Museo Geominero 9, Publicaciones del Instituto Geológico y Minero de España, Madrid.
Adrain, J.M. 2011. Class Trilobita Walch, 1771. In Zhang, Z.-Q. (ed.), Animal Biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148:104-109.
Aguinaldo, A.M.A., Turbeville, J.M., Linford, L.S., Rivera, M.C., Garey, J.R., Raff, R.A., and Lake, J.A. 1997. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature, 387:489-493. https://doi.org/10.1038/387489a0
Bell, M.A. 2015. Package ‘geoscale’. https://cran.r-project.org/web/packages/geoscale
Brandt, D.S. 2002. Ecdysial efficiency and evolutionary efficacy among marine arthropods: implications for trilobite survivorship. Alcheringa, 26:399-421. https://doi.org/10.1080/03115510208619264
Bruthansová, J. 2003. Exuviation of selected Bohemian Ordovician trilobites. Special Papers in Palaeontology, 70:293-308.
Budil, P. and Bruthansová, J. 2005. Moulting in Ordovician dalmanitoid and acastoid trilobites of the Prague Basin. Preliminary observation. Geologica Acta, 3:373-383.
Busch R.M. and Schwartz, F.M. 1985. Molting and description of a new homolonotid trilobite from Pennsylvania. Journal of Paleontology, 59:1062-1074.
Cederström, P., Ahlberg, P., Nilsson, C.H., Ahlgren, J., and Eriksson, M.E. 2010. Moulting, ontogeny and sexual dimorphism in the Cambrian ptychopariid trilobite Strenuaeva inflata from the northern Swedish Caledonides. Palaeontology, 54:685-703. https://doi.org/10.1111/j.1475-4983.2010.01021.x
Clarkson, E.N.K. 1979. Invertebrate Palaeontology and Evolution. George Allen & Unwin, London.
Clarkson, E.N.K., Ahlgren, J., and Taylor, C.M. 2003. Structure, ontogeny, and moulting of the olenid trilobite Ctenopyge (Eoctenopyge) angusta Westergård, 1922 from the upper Cambrian of Västergötland, Sweden. Palaeontology, 46:1-27. https://doi.org/10.1111/1475-4983.00284
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J-X. 2013 updated. The ICS International Chronostratigraphic Chart. Episodes, 36:199-204.
Crônier, C. 2013. Morphological disparity and developmental patterning: contribution of phacopid trilobites. Palaeontology, 56:1263-1271. https://doi.org/10.1111/pala.12024
Daley, A.C., Antcliffe, J.B., Drage, H.B., and Pates, S. 2018. Early fossil record of Euarthropoda and the Cambrian Explosion. Proceedings of the National Academy of Sciences of the United States of America, 115:5323-5331. https://doi.org/10.1073/pnas.1719962115
Daley, A.C. and Drage, H.B. 2016. The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Arthropod Structure and Development, 45:71-96. https://doi.org/10.1016/j.asd.2015.09.004
Drage, H.B. and Daley, A.C. 2016. Recognising moulting behaviour in trilobites by examining morphology, development and preservation: Comment on Błażejowski et al. 2015. BioEssays, 38:981-990. https://doi.org/10.1002/bies.201600027
Drage, H.B., Holmes, J.D., García-Bellido, D.C., and Daley, A.C. 2018a. An exceptional record of Cambrian trilobite moulting behaviour preserved in the Emu Bay Shale, South Australia. Lethaia, 51:473-492. https://doi:10.1111/let.12266
Drage, H.B., Laibl, L., and Budil, P. 2018b. Post-embryonic development of Dalmanitina, and the evolution of facial suture fusion in Phacopina. Paleobiology, 44:638-659. https://doi.org/10.1017/pab.2018.31
Du, K.-S., Ortega-Hernández, J., Yang, J., and Zhang, X.-G. 2019. A soft-bodied euarthropod from the early Cambrian Xiaoshiba Lagerstätte of China supports a new clade of basal artiopodans with dorsal ecdysial sutures. Cladistics. 35:269–281. https://doi:10.1111/cla.12344
Ewer, J. 2005. How the ecdysozoan changed its coat. PLoS Biology, 3:e349. https://doi.org/10.1371/journal.pbio.0030349
Feist, R. 1995. Effect of paedomorphosis in eye reduction on patterns of evolution and extinction in trilobites, p. 225-244. In: McNamara, K.J. (ed.), Evolutionary Change and Heterochrony. John Wiley & Sons Ltd, Chichester.
Fortey, R.A. 1990. Ontogeny, hypostome attachment and trilobite classification. Palaeontology, 33:529-576.
Fortey, R.A. and Owens, R.M. 1975. Proetida - a new order of trilobites. Fossils and Strata, 4:227-239.
Fortey, R.A. and Owens, R.M. 1999. Feeding habits in trilobites. Palaeontology, 42:429-465. http://doi.org/10.1111/1475-4983.00080
Fortey, R.A. and Wilmot, N.V. 1991. Trilobite cuticle thickness in relation to palaeoenvironment. Paläontologische Zeitschrift, 65:141-151. https://doi.org/10.1007/BF02985779
Fortey, R.A. 2001. Trilobite systematics: the last 75 years. Journal of Paleontology, 75:1141-1151. https://doi.org/10.1017/S0022336000017194
Fossilworks and the Paleobiology Database. 2018. Macquarie University, Sydney. http://fossilworks.org
García-Bellido, D.C. and Collins, D.H. 2004. Moulting arthropod caught in the act. Nature, 429:40. https://doi.org/10.1038/429040a
Green, J. 1832. A Monograph of the Trilobites of North America: with Coloured Models of the Species. Clark and Raser, Philadelphia.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4:1-9.
https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Henningsmoen, G. 1975. Moulting in trilobites. Fossils and Strata, 4:179-200.
Hou, X.-G., Williams, M., Gabbott, S., Siveter, D.J., Siveter, D.J., Cong, P.-Y., Ma, X.-Y., and Sansom, R. 2017. A new species of the artiopodan arthropod Acanthomeridion from the lower Cambrian Chengjiang Lagerstätte, China, and the phylogenetic significance of the genus. Journal of Systematic Palaeontology, 15:733-740. https://doi.org/10.1080/14772019.2016.1229695
Hughes, N.C. 2003. Point/Counterpoint: Trilobite molting and survivorship. The Trilobite Papers, 15:8-11.
Hunda, B.R., Hughes, N.C., and Flessa, K.W. 2006. Trilobite taphonomy and temporal resolution in the Mt. Orab Shale Bed (Upper Ordovician, Ohio, U.S.A.). Palaios, 21:26-45. https://doi.org/10.2110/palo.2005.p05-01
Jell, P.A. and Adrain, J.M. 2002. Available generic names for trilobites. Memoirs of the Queensland Museum, 48:331-553.
Kiessling, W., Miller, A.I., Foote, M., Wagner, P.J., Kröger, B., Patzkowsky, M.E., Hopkins, M.J., Holland, S.M., Clapham, M.E., Alroy, J., and Novack-Gottshall, P.M. 2018. Taxonomic occurrences of Trilobita, 8418 records downloaded 25 January 2018. Fossilworks and the Paleobiology Database. http://fossilworks.org
Krishnakumaran, A. and Schneiderman, H.A. 1970. Control of molting in mandibulate and chelicerate arthropods by ecdysones. The Biological Bulletin, 139:520-538. https://doi.org/10.2307/1540371
Lamsdell, J.C. and Selden, P.A. 2014. Phylogenetic support for the monophyly of proetide trilobites. Lethaia, 48:375-386. https://doi.org/10.1111/let.12113
Lerosey-Aubril, R. and Feist, R. 2012. Quantitative approach to diversity and decline in Late Palaeozoic trilobites, p. 535-555. In Talent, J.A. (ed.), Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations Through Time. Springer Science and Media, Dordrecht. https://doi.org/10.1007/978-90-481-3428-1_16
Lerosey-Aubril, R. and Peel, J.S. 2018. Gut evolution in early Cambrian trilobites and the origin of predation on infaunal macroinvertebrates: evidence from muscle scars in Mesolenellus. Palaeontology, 61:747-760. https://doi.org/10.1111/pala.12365
Lieberman, B.S. and Karim, T.S. 2010. Tracing the trilobite tree from the root to the tips: A model marriage of fossils and phylogeny. Arthropod Structure & Development, 39:111-123. https://doi.org/10.1016/j.asd.2009.10.004
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Impensis Direct. Laurentii Salvii, Holmiae.
Lu, Y. 1950. On the genus Redlichia with description of its new species. Geological Review, 15:157-170.
McHugh, M.L. 2013. The chi-square test of independence. Biochemia Medica, 23:143-149. https://doi.org/10.11613/BM.2013.018
McNamara, K.J. 1986. Techniques of exuviation in Australian species of the Cambrian trilobite Redlichia. Alcheringa, 10:403-412. https://doi.org/10.1080/03115518608619148
McNamara, K.J. and Rudkin, D.M. 1984. Techniques of trilobite exuviation. Lethaia, 17:153-173. https://doi.org/10.1111/j.1502-3931.1984.tb01722.x
Mikulic, D.G. 1981. Trilobites in Paleozoic carbonate buildups. Lethaia,14:45-56. https://doi.org/10.1111/j.1502-3931.1981.tb01073.x
Miller, J. and Clarkson, E.N.K. 1980. The post-ecdysial development of the cuticle and the eye of the Devonian trilobite Phacops rana milleri Stewart 1927. Philosophical Transactions of the Royal Society of London Series B, 288:461-480. https://doi.org/10.1098/rstb.1980.0023
Nijhout, H.F. 1994. Insect Hormones. Princeton University Press, New Jersey.
Novák, O. and Perner, J. 1918. Die trilobiten der zone D-d 1y von Prag und Umgebung. Palaeontographica Bohemiae, 9:1-51.
Owens, R.M. 2003. The stratigraphical distribution and extinctions of Permian trilobites. Special Papers in Palaeontology, 70:377-397.
Paterson, J.R., Jago, J.B., Brock, G.A., and Gehling, J.G. 2007. Taphonomy and palaeocology of the emuellid trilobite Balcoracania dailyi (early Cambrian, South Australia). Palaeogeography, Palaeoclimatology, Palaeoecology, 249:302-321. https://doi.org/10.1016/j.palaeo.2007.02.004
Pocock, K.J. 1964. Estaingia, a new trilobite genus from the Lower Cambrian of South Australia. Palaeontology, 7:458-471.
Richter, R. 1856. Beitrag zur Paläontologie des Thüringer Waldes. Erster Theil. Denkschriften der kaiserlichen Akademie der Wissenschaften, mathematisch−naturwissenschaftlichen, 11:87-138.
Rominger, C. 1887. Description of primordial fossils from Mount Stephens, N.W. Territory of Canada. Proceedings of the Academy of Natural Sciences of Philadelphia, 39:12-19.
RStudio Team. 2015. RStudio: Integrated Development for R. RStudio, Inc., Boston. http://www.rstudio.com.
Rustán, J.J., Balseiro, D., Waisfeld, B., Foglia, R.D., and Vaccari, N.E. 2011. Infaunal molting in Trilobita and escalatory responses against predation. Geology, 39:495-498. https://doi.org/10.1130/G31879.1
Salter, J.W. 1853. British organic remains. Memoirs of the Geological Survey UK, 1-78.
Scotese, C.R. 2016. Atlas of Earth History, Volume 1, Paleogeography. PALEOMAP Project, Arlington. http://www.scotese.com.
Sekiguchi, K., Seshimo, H., and Sugita, H. 1988. Chapter VI-3: Post-embryonic development, p. 181-195. In Sekiguchi, K. (ed.), Biology of Horseshoe Crabs. Science House Co., Ltd., Tokyo.
Servais, T., Owen, A.W., Harper, D.A.T., Kröger, B., and Munnecke, A. 2010. The Great Ordovician Biodiversification Event (GOBE): the palaeoecological dimension. Palaeogeography, Palaeoclimatology, Palaeoecology, 294:99-119. https://doi.org/10.1016/j.palaeo.2010.05.031
Šnajdr, M. 1957. O nových trilobitech z českého kambria. Věstnik Ústředniho ústavu geologickéh o, 32:235-44
Song, Y., Villeneuve, D.L., Toyota, K., Iguchi, T., and Tollefsen, K.E. 2017. Ecdysone receptor agonism leading to lethal molting disruption in arthropods: review and adverse outcome pathway development. Environmental Science & Technology, 51:4142-4157. https://doi.org/10.1021/acs.est.7b00480
Song, H., Wignall, P.B., Tong, J., and Yin, H. 2013. Two pulses of extinction during the Permian-Triassic crisis. Nature Geoscience, 6:52-56. https://doi.org/10.1038/ngeo1649
Speyer, S.E. 1985. Moulting in phacopid trilobites. Transactions of the Royal Society of Edinburgh, 76:239-253. https://doi.org/10.1017/S0263593300010476
Speyer, S.E. and Brett, C.E. 1985. Clustered trilobite assemblages in the Middle Devonian Hamilton Group. Lethaia, 18:85-103. https://doi.org/10.1111/j.1502-3931.1985.tb00688.x
Sternberg, K. 1833. Rede von Kaspar Grafen von Sternberg, Beilage. Verhandlung Gesellschaft Vaterland Museum Bohmisch, 3:45-56.
Stubblefield, C.J. 1959. Evolution in trilobites. Quarterly Journal of the Geological Society of London, 115:145-162. https://doi.org/10.1144/GSL.JGS.1959.115.01.08
Tarver, J.E., Braddy, S.J., and Benton, M.J. 2007. The effects of sampling bias on Palaeozoic faunas and implications for macroevolutionary studies. Palaeontology, 50:177-184. https://doi.org/10.1111/j.1475-4983.2006.00618.x
Tortello, M.F. and Clarkson, E.N.K. 2008. Ontogeny, structure, and moulting of Parabolina frequens argentina (Kayser) (Trilobita, Olenidae) from the Furongian of Northwestern Argentina. Ameghiniana, 45:13-31.
Vevea, D. and Hall, K.D. 1984. The effects of water temperature on molting and egg production following eyestalk ablation in two species of crayfish, Orconectes rusticus and Orconectes propinquus. BIOS, 55:135-143.
Walcott, C.D. 1912. Middle Cambrian Branchiopoda, Malacostraca, Trilobita and Merostomata. Cambrian Geology and Paleontology II. Smithsonian Miscellaneous Collections, 57:145-228.
Webster, M. 2007. A Cambrian peak in the morphological variation within trilobite species. Science, 317:499-502. https://doi.org/10.1126/science.1142964
Whittington, H.B. 1980. Exoskeleton, moult stage, appendage morphology and habits of the Middle Cambrian trilobite Olenoides serratus. Palaeontology, 23:171-204.
Whittington, H.B. 1990. Articulation and exuviation in Cambrian trilobites. Philosophical Transactions of the Royal Society of London Series B, 329:27-46. https://doi.org/10.1098/rstb.1990.0147
Whittington, H.B., Chatterton, B.D.E., Speyer, S.E., Fortey, R.A., Owens, R.M., Chang, W.T., Dean, W.T., Jell, P.A., Laurie, J.R., Palmer, A.R., Repina, L.N., Rushton, A.W.A., Shergold, J.H., Clarkson, E.N.K., Wilmot, N.V., and Kelly, S.R.A. 1997. Treatise on Invertebrate Paleontology, Part O: Trilobita, revised. The Geological Society of America, Inc. & University of Kansas, Boulder, Colorado.
Wickham, H., Chang, W., and RStudio. 2016. Package ‘ggplot2’. https://cran.r-project.org/web/packages/ggplot2
Wolfe, J.M. and Hegna, T.A. 2014. Testing the phylogenetic position of Cambrian pancrustacean larval fossils by coding ontogenetic stages. Cladistics, 30:366-390. https://doi.org/10.1111/cla.12051

