 Anatomical sciuromorphy in "protrogomorph" rodents
Anatomical sciuromorphy in "protrogomorph" rodents
Article number: 23(2):a25
https://doi.org/10.26879/1049
Copyright Paleontological Society, May 2020
Author biographies
Plain-language and multi-lingual abstracts
PDF version
PE NOTE: Errata
Submission: 4 December 2019. Acceptance: 11 May 2020.
ABSTRACT
Historically, high-level rodent taxonomy has been intertwined with the anatomical condition of the masseter muscle, leading to the widespread use of grades based on four anatomical conditions: protrogomorphy, sciuromorphy, hystricomorphy, and myomorphy. Although many previous studies have since shown these grades to be paraphyletic, the idea of a “protrogomorph” rodent has remained popular for extinct species. We examined the oldest and most complete articulated skeleton yet known of Ischyromys (USNM 617532) from the late Duchesnean of West Canyon Creek, Wyoming. Ischyromys species are usually treated as anatomically protrogomorphous, but USNM 617532 shows attachment of the deep masseter anterior and dorsal to the infraorbital foramen on the rostrum, creating a zygomatic plate, and therefore exhibits anatomical sciuromorphy. A geometric morphometric analysis of cranial landmarks suggests that I. typus resembles extant, anatomically protrogomorphous rodents, whereas USNM 617532 falls within the range of non-protrogomorphous rodents. USNM 617532 differs from Ischyromys typus in terms of incisor procumbency, infraorbital foramen size and zygomatic arch shape. In addition to its distinctive rostrum and zygomatic plate, USNM 617532 possesses a sagittal crest, accessory cusps on the lower molars and large metaconules on the upper molars, features that help to diagnose this specimen as a member of Ischyromys douglassi. Masseteric patterns mapped onto a recent phylogenetic estimate suggest that none of the three main rodent clades, or even crown Rodentia itself, was characterized by an anatomically protrogomorphous common ancestor, although protrogomorphy did characterize many simplicidentate taxa on the stem leading to Rodentia.
Aime H. Rankin. Department of Zoology, University of Cambridge UK, ar835@cam.ac.uk
Robert J. Emry. Department of Paleobiology, Smithsonian Institution, Washington DC USA, emryr@si.edu
Robert J. Asher. Department of Zoology, University of Cambridge UK, r.asher-at-zoo.cam.ac.uk
Keywords: glires; Ischyromys; morphology; grade; clade; taxonomy; rostrum
Rankin, Aime H., Emry, Robert J., and Asher, Robert J. 2020. Anatomical sciuromorphy in "protrogomorph" rodents. Palaeontologia Electronica, 23(2):a25. https://doi.org/10.26879/1049
palaeo-electronica.org/content/2020/3043-protrogomorph-rodents
Copyright: May 2020 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
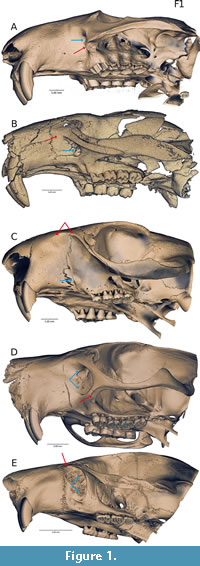 Several adaptations enable gnawing in rodents. They possess a single pair of ever-growing incisors in the upper and lower jaws, a reduced number of cheek teeth, a long diastema separating the incisors from the cheek teeth, and differentiation of the masseter muscle into three separate components to control jaw movement: the superficial masseter, deep masseter, and zygomaticomandibularis (terminology following Cox and Jeffery, 2011). The variation seen in the arrangement of the masseter tissues varies across rodents and falls into four morphologies: sciuromorphy, hystricomorphy, myomorphy, and protrogomorphy. These differences were first discussed by Waterhouse (1839) and then formally described by Brandt (1855). Protrogomorphy describes a condition where the origin of the masseter is limited to the zygomatic arch, a condition typically observed in non-rodents. Anatomical protrogomorphy is evident in many fossil rodents, but among living rodents only occurs in Aplodontia (Figure 1A) and some bathyergoids (Landry 1957; Wood 1965; Maier and Schrenk 1987; Cox and Faulkes, 2014). The other three arrangements are characterised by divisions of the masseter extending anteriorly to varying degrees. In sciuromorphy (Figure 1B-C), the origin of the deep masseter extends anteriorly from the zygomatic arch, past the infraorbital foramen, onto the rostrum. The rostrum features a correspondingly broadened and angled margin of the zygoma and lateral rostrum, called the zygomatic plate, which in some taxa (e.g., geomyids, sciurids; Figure 1C), approaches the dorsal and anterior margins of the skull. In hystricomorphy (Figure 1D), the deep masseter remains restricted to the ventrum of the zygomatic arch and the zygomaticomandibularis extends onto the rostrum, passing through an enlarged infraorbital foramen along its course. Myomorphy (Figure 1E) is a combination of both sciuromorphy and hystricomorphy; both the deep masseter and zygomaticomandibularis spread anteriorly, the latter through the infraorbital foramen and the former onto the zygomatic plate.
Several adaptations enable gnawing in rodents. They possess a single pair of ever-growing incisors in the upper and lower jaws, a reduced number of cheek teeth, a long diastema separating the incisors from the cheek teeth, and differentiation of the masseter muscle into three separate components to control jaw movement: the superficial masseter, deep masseter, and zygomaticomandibularis (terminology following Cox and Jeffery, 2011). The variation seen in the arrangement of the masseter tissues varies across rodents and falls into four morphologies: sciuromorphy, hystricomorphy, myomorphy, and protrogomorphy. These differences were first discussed by Waterhouse (1839) and then formally described by Brandt (1855). Protrogomorphy describes a condition where the origin of the masseter is limited to the zygomatic arch, a condition typically observed in non-rodents. Anatomical protrogomorphy is evident in many fossil rodents, but among living rodents only occurs in Aplodontia (Figure 1A) and some bathyergoids (Landry 1957; Wood 1965; Maier and Schrenk 1987; Cox and Faulkes, 2014). The other three arrangements are characterised by divisions of the masseter extending anteriorly to varying degrees. In sciuromorphy (Figure 1B-C), the origin of the deep masseter extends anteriorly from the zygomatic arch, past the infraorbital foramen, onto the rostrum. The rostrum features a correspondingly broadened and angled margin of the zygoma and lateral rostrum, called the zygomatic plate, which in some taxa (e.g., geomyids, sciurids; Figure 1C), approaches the dorsal and anterior margins of the skull. In hystricomorphy (Figure 1D), the deep masseter remains restricted to the ventrum of the zygomatic arch and the zygomaticomandibularis extends onto the rostrum, passing through an enlarged infraorbital foramen along its course. Myomorphy (Figure 1E) is a combination of both sciuromorphy and hystricomorphy; both the deep masseter and zygomaticomandibularis spread anteriorly, the latter through the infraorbital foramen and the former onto the zygomatic plate.
Brandt (1855) used these morphologies to split the rodents into three groups: Sciuromorpha, Hystricomorpha, and Myomorpha. Zittel (1893) proposed another group called “Protrogomorpha”, into which he placed Aplodontia and many fossil forms. Rodent specialists have long recognized that these groupings, as described by anatomical terms, do not represent monophyletic clades (e.g., Wood, 1965; Hartenberger, 1985; Fabre et al., 2012). Unlike the morphological patterns of the upper jaw, the lateral (or anatomically hystricognathous; Tullberg, 1899; Wood, 1965) position of the angular process of the mandible in relation to the plane of the lower incisors has proven to define the clade of caviomorph and hystricid rodents (i.e., Hystricognathi). This is opposed to a sciurognathous jaw, which indicates the position of the angular process in line with the lower incisor alveolus, as seen in all other rodents. Landry (1999) noted similarities of Hystricognathi with ctenodactylids and named this group Entodacrya, a term that has temporal priority over Ctenohystrica (Huchon et al., 2000) but is less widely known. Recent phylogenies (e.g., Marivaux et al., 2004; Churakov et al., 2010; Fabre et al., 2012; Asher et al., 2019; Swanson et al., 2019) support Ctenohystrica (i.e., guinea pig-related), squirrel-related, and mouse-related clades, with the masseter morphologies spread out across all three of these monophyletic groups.
The taxonomy of Brandt (1855) has priority over subsequent rodent classification schemes, and, arguably, should be at least partly retained to reflect broad consensus on the monophyly of each of these high-level clades. We, therefore, use Sciuromorpha and Myomorpha to indicate the squirrel- and mouse-related clades, and Ctenohystrica (Huchon, 2000) for the relatively novel clade including Hystricomorpha plus Ctenodactylidae. We also acknowledge the less well-known but prior suggestion of Entodacrya (Landry, 1999) for the clade of ctenodactylids-hystricomorphs; this term could serve as a total-clade cognate, encompassing crown Ctenohystrica and their stem relatives. When referring to muscular types, we use the nouns sciuromorphy, myomorphy, hystricomorphy, and protrogomorphy, and the adjectives sciuromorphous, myomorphous, hystricomorphous, and protrogomorphous. Our use of these terms with suffixes “-morphy” and “-morphous” is purely anatomical and does not imply membership in any particular clade.
Authors such as Wood recognized homoplasy in masticatory types and argued that they represented “adaptive levels” within Rodentia, involving changes in both cranial myology and dentition. His “Grade One - protrogomorph radiation” included Aplodontia along with many extinct taxa such as Paleocene-Eocene Paramys, Eocene-Oligocene Ischyromys, and groups such as Cylindrodontidae and Mylagualidae. Furthermore, Wood disassociated Phiomyidae from these animals, believing it to be closer to thryonomyids. While he wrote that his “Protrogomorpha may be considered to be a clade” (1965: 118), he believed that all other rodent groups existed as grades. For example, his “Grade Two” radiation was based on muscle and skull changes and contained anatomically sciuromorphous, hystricomorphous, and protrogomorphous species. His “Grade Three” focused on hypsodonty and included species from the first two grades, which had developed ever-growing cheek teeth. This included species from groups such as ground squirrels and geomyoids. Wood considered the adaptations seen in “Grade Two” to have arisen multiple times independently and, therefore, believed that the three classical suborders should make way for a correspondingly larger number of clades to represent such independent origins (Wood, 1965: 128).
“Ischyromyoidea”, as defined by Wood (1965), comprises a paraphyletic assemblage of North American taxa. Historically, temporally diverse groups such as Paleocene and early Eocene Paramys, Franimys, Reithroparamys, and much younger, late Eocene-Oligocene Ischyromys have been associated with “Ischyromyoidea” or “Ischyromyidae” (Wood, 1965; Korth, 1994: fig. 5.5). Following Korth (1994:37), these genera have been regarded as anatomically protrogomorph: “the attachment for the masseter on the skull is limited to the ventral base of the zygoma (protrogomorphous) and the infraorbital foramen is small, opens anteriorly, and is not enlarged by muscle invasion or compressed by any modification of a zygomatic plate” (1994:37).
Wood spent many years reviewing these rodents (e.g., Wood, 1937, 1962, 1965, and 1976) and was the advisor of another eminent vertebrate paleontologist, Craig Call Black. Both were experts in rodent anatomy and examined the same specimens. However, they did not agree on the supposed protrogomorphous nature of many fossil rodents. Black (1968) believed that all members of “Protrogomorpha”, including Ischyromys, were anatomically protrogomorphous. This meant that the term “protrogomorph” was sometimes used taxonomically to denote members of what Black or Wood would have recognized as a natural group, as well as to describe the anatomical condition in which the masseter does not extend anteriorly or dorsally onto the rostrum from its attachment sites on the zygomatic arch.
In contrast to Black, Wood (1976) argued that some "protrogomorph" ischyromyids were, in fact, anatomically sciuromorphous. Wood observed that some Chadronian specimens of Ischyromys had a more derived masticatory musculature than those from the Orellan (Wood, 1937, 1980) and referred these derived forms to a different genus, Titanotheriomys. Wood and Black’s debate revolved around these two genera. Black (1968) synonymized Titanotheriomys with Ischyromys; Wood (1937, 1976) regarded them as closely related, but distinct. This was based heavily on Woodʼs myological interpretation of the crania. As summarized below, he (e.g., Wood, 1976) argued that at least some ischyromyines were anatomically sciuromorph, whereas Black (1968) regarded them all as protrogomorph.
Their disagreement, both anatomically and taxonomically, has yet to be resolved. Korthʼs (1994) comprehensive treatment of North American fossil rodents concluded “it is most economical to include all ischyromyines in a single genus Ischyromys” pending a systematic revision of relevant material including juveniles. Heaton (1996: fig. 14) undertook a cluster analysis of lower jaws, based on “mean population values for 83 characters: 25 on each molar and eight on the jaw.” His analysis yielded two clusters, corresponding to geologically older (largely Chadronian) and younger (Orellan and Whitneyan) groups of Ischyromys. He placed the Duchesnean West Canyon Creek population among the taxa in the older cluster, along with one other Duchesnean population (Porvenir, Texas) and nine from the Chadronian, including I. douglassi from McCartyʼs Mountain (Montana). He thus concluded that the Duchesnean-Chadronian specimens of Ischyromys were morphometrically distinct from the younger populations, and used the subgenus Ischryomys (Titanotheriomys) for the former. We do not dispute this usage, but until a phylogenetic analysis is undertaken of all relevant species of ischyromyines (including many specimens unavailable to us), we follow Korth (1994) in using the generic name Ischyromys for these late Eocene and early Oligocene populations.
Wood (1976) focused on specimens from Montana, Wyoming, and Texas. From Montana, he examined material from Pipestone Springs and McCarty’s Mountain (from the middle and early Chadronian, respectively; see Tabrum et al., 1996, Prothero and Emry, 2004). In Wyoming, the major sites of interest were Beaver Divide (White River Formation, Chadronian; Emry, 1975) and Bates Hole (early to middle Chadronian). Most Texan specimens he studied came from Ash Spring (middle Chadronian). He observed that some specimens of Ischyromys had a tilted zygomatic plate that sloped from the maxillary root of the zygoma to the infraorbital foramen (Wood, 1976). Furthermore, he observed a crest that curved medially across the plate, separating the fossa of the deep masseter from the incline below the infraorbital foramen (Wood, 1976: his fig. 2). To Wood, this suggested that there was some migration of what he called the “masseter lateralis” (e.g., Wood, 1976: figs. 3b, 3c, referred to as the deep masseter here following Cox and Jeffery, 2011) off the ventral surface of the zygoma, signaling important changes had occurred in the gnawing mechanism. Black agreed to some anterior migration of the deep masseter and slight compression of the infraorbital foramen in some specimens of Ischyromys. However, he still considered specimens in this genus to be anatomically protrogomorph (Black, 1968).
Wood described many Ischyromys specimens (his Titanotheriomys) as anatomically sciuromorph, without crests cutting across the zygomatic plate denoting the anterior end of the deep masseter, but with the dorsal limit of this muscle extended forwards, anterodorsal to the infraorbital foramen (Wood, 1976: fig. 3). In contrast, Black (1968: 279-282) argued that specimens from Beaver Divide were distorted and that Wood’s observations resulted from this distortion and a failure to recognise morphological variation within the population. Wood, however, was not convinced by the latter and claimed that no Chadronian collection from a restricted spatio-temporal location showed a transitional series of forms, as expected if there were variation within a population. As recounted by Wood (1976), both men were civil, meeting in person to look at the specimens together and yet, ultimately, their debate was never resolved. Wood’s idea of anatomical sciuromorphy within “Ischyromyidae” did not play a major role in subsequent literature, and Ischyromys (including Titanotheriomys) has since been described as “protrogomorph”, at least in a taxonomic sense (e.g., Dawson, 1977; Vianey-Liaud, 1985; Korth, 1994).
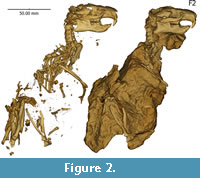 In this study, we describe one of the oldest and best preserved skeletons of Ischyromys (USNM 617532, Figure 2) and consider its evolution in the context of a recent phylogenetic study (Asher et al., 2019). This fossil was collected by one of us (Emry) in 1971 and is from the White River Fm of West Canyon Creek, Wyoming. It is Duchesnean in age (i.e., 40-37 Ma, Prothero and Emry, 1996) and among the geologically oldest localities from which ischyromyines (including Woodʼs Titanotheriomys) are known. We consider the USNM 617532 skeleton to belong to Ischyromys douglassi, a taxon originally described from McCarty’s Mountain, Montana (Black, 1968). Based on the presence of Ardynomys occidentalis in both the Porvenir fauna of west Texas and the McCarty’s Mountain fauna (and nowhere else), and on the similarity of Ischyromys blacki from the Porvenir and I. douglassi from McCarty’s Mountain, Wood (1974) concluded that the two faunas were close in age, with the latter perhaps being slightly younger. The Porvenir fauna is widely regarded as late Duchesnean in age (Prothero, 1996). Several other taxa of the West Canyon Creek fauna suggest that it is a close correlate of the Porvenir and McCarty’s Mountain faunas, perhaps closer to the former than the latter. Thus, we regard the specimens of I. douglassi discussed herein as being of late Duchesnean age.
In this study, we describe one of the oldest and best preserved skeletons of Ischyromys (USNM 617532, Figure 2) and consider its evolution in the context of a recent phylogenetic study (Asher et al., 2019). This fossil was collected by one of us (Emry) in 1971 and is from the White River Fm of West Canyon Creek, Wyoming. It is Duchesnean in age (i.e., 40-37 Ma, Prothero and Emry, 1996) and among the geologically oldest localities from which ischyromyines (including Woodʼs Titanotheriomys) are known. We consider the USNM 617532 skeleton to belong to Ischyromys douglassi, a taxon originally described from McCarty’s Mountain, Montana (Black, 1968). Based on the presence of Ardynomys occidentalis in both the Porvenir fauna of west Texas and the McCarty’s Mountain fauna (and nowhere else), and on the similarity of Ischyromys blacki from the Porvenir and I. douglassi from McCarty’s Mountain, Wood (1974) concluded that the two faunas were close in age, with the latter perhaps being slightly younger. The Porvenir fauna is widely regarded as late Duchesnean in age (Prothero, 1996). Several other taxa of the West Canyon Creek fauna suggest that it is a close correlate of the Porvenir and McCarty’s Mountain faunas, perhaps closer to the former than the latter. Thus, we regard the specimens of I. douglassi discussed herein as being of late Duchesnean age.
Heatonʼs (1996) analysis of dental evolution in Ischyromys regarded this West Canyon Creek species as new but did not name it. His analysis focused on the lower dentitions of ischyromyines, including jaws and teeth from West Canyon Creek. The dental crown morphology of USNM 617532 remains occluded by matrix, but is nonetheless visible with computerized tomography (CT) and thus amenable to comparison to other West Canyon Creek specimens. We also compare USNM 617532 and other West Canyon Creek specimens to younger species of Ischyromys, including I. typus (USNM 16828, AMNH 144628, ROM V1007 and BMNH PV M 7855) and note previously missed details of the Ischyromys ear region. Finally, we provide a geometric morphometric assessment of the masticatory configuration of these specimens in light of Wood and Black’s debate.
MATERIALS AND METHODS
Institutional Acronyms
AMNH, American Museum of Natural History (New York); BMNH, The Natural History Museum (London); ROM, Royal Ontario Museum (Toronto); USNM, National Museum of Natural History (Washington, D.C.).
Taxon Sample
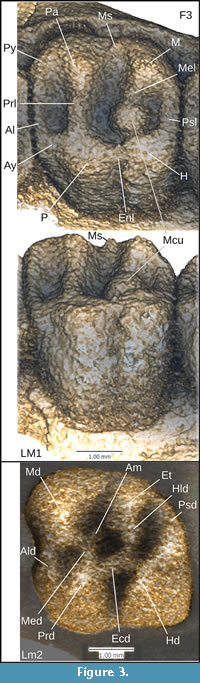 We obtained microCT scans of 39 rodent and two lagomorph skulls (Table 1), chosen to include representatives of each of the four anatomical conditions of the masseter muscle from the three major extant rodent groups and a number of specimens representing four fossil taxa. These were Gomphos elkema (MAE BU14467, a mimotonid from the early Eocene Bumbanian Asian Land Mammal Age), Paradelomys crusafonti (UM-ACQ-6618, a theridomyid from the middle Eocene following Vianey-Liaud and Marivaux, 2016), Ischyromys from the Orellan Brule formation (USNM 16828 from Wyoming, ROM V1007 from Nebraska, AMNH 144628 from Stark County North Dakota, CMNH 588 from Badland Creek Nebraska, and BMNH PV M 7855 from the White River Fm of South Dakota), and USNM 617532 from the Duchesnean of West Canyon Creek, Wyoming. Computerized tomography scans of two of our USNM skulls (617532 and 16828) are available via morphosource.org project 945 (https://www.morphosource.org/Detail/ProjectDetail/Show/project_id/945). We considered the anatomy of several additional Ischyromys specimens, also from West Canyon Creek, from a collection housed at the USNM. Nomenclature used for dental morphology is depicted in Figure 3 and follows Wood and Wilson (1936) and Marivaux et al. (2004).
We obtained microCT scans of 39 rodent and two lagomorph skulls (Table 1), chosen to include representatives of each of the four anatomical conditions of the masseter muscle from the three major extant rodent groups and a number of specimens representing four fossil taxa. These were Gomphos elkema (MAE BU14467, a mimotonid from the early Eocene Bumbanian Asian Land Mammal Age), Paradelomys crusafonti (UM-ACQ-6618, a theridomyid from the middle Eocene following Vianey-Liaud and Marivaux, 2016), Ischyromys from the Orellan Brule formation (USNM 16828 from Wyoming, ROM V1007 from Nebraska, AMNH 144628 from Stark County North Dakota, CMNH 588 from Badland Creek Nebraska, and BMNH PV M 7855 from the White River Fm of South Dakota), and USNM 617532 from the Duchesnean of West Canyon Creek, Wyoming. Computerized tomography scans of two of our USNM skulls (617532 and 16828) are available via morphosource.org project 945 (https://www.morphosource.org/Detail/ProjectDetail/Show/project_id/945). We considered the anatomy of several additional Ischyromys specimens, also from West Canyon Creek, from a collection housed at the USNM. Nomenclature used for dental morphology is depicted in Figure 3 and follows Wood and Wilson (1936) and Marivaux et al. (2004).
Geometric Morphometrics and Character State Reconstructions
We made virtual reconstructions with Drishti v.2.6.4 (Limaye, 2012; Figure 4A) and identified 21 digital landmarks in 3D based on Morris et al. (2018) from the right side of each cranium (Table 2; Figure 4B). 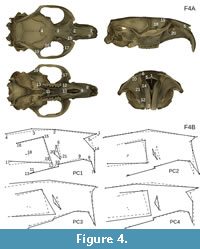 Of the five Ischyromys typus specimens, only USNM 16828 preserved the structures required for the landmarking. The effects of specimen size, position, and orientation were eliminated by performing a Procrustes fit on the superimposed sets of landmarks using MorphoJ (Klingenberg, 2011). The locations of the specimens across morphospace were then visualised by performing a principal component analysis (PCA). The anatomical condition of the masseter for each specimen of an extant species, based on Maier and Schrenk (1987), Hautier et al. (2008), and Cox and Faulkes (2014), was then superimposed onto the PCA results to be able to observe overlap among fossils and non-rodents in our sample. In addition, we used the phylogeny of Asher et al. (2019: fig. 4) to reconstruct the evolution of rodent masticatory types using parsimony ancestral state reconstruction in PAUP 4.0a build 167 (Swofford, 2003) and Mesquite v3.51 (Maddison and Maddison, 2019), also taking into account character optimizations in fossil taxa as described by Wang (1997), Meng et al. (2003), Marivaux et al. (2004), Wible et al. (2005), and this paper.
Of the five Ischyromys typus specimens, only USNM 16828 preserved the structures required for the landmarking. The effects of specimen size, position, and orientation were eliminated by performing a Procrustes fit on the superimposed sets of landmarks using MorphoJ (Klingenberg, 2011). The locations of the specimens across morphospace were then visualised by performing a principal component analysis (PCA). The anatomical condition of the masseter for each specimen of an extant species, based on Maier and Schrenk (1987), Hautier et al. (2008), and Cox and Faulkes (2014), was then superimposed onto the PCA results to be able to observe overlap among fossils and non-rodents in our sample. In addition, we used the phylogeny of Asher et al. (2019: fig. 4) to reconstruct the evolution of rodent masticatory types using parsimony ancestral state reconstruction in PAUP 4.0a build 167 (Swofford, 2003) and Mesquite v3.51 (Maddison and Maddison, 2019), also taking into account character optimizations in fossil taxa as described by Wang (1997), Meng et al. (2003), Marivaux et al. (2004), Wible et al. (2005), and this paper.
RESULTS
Description
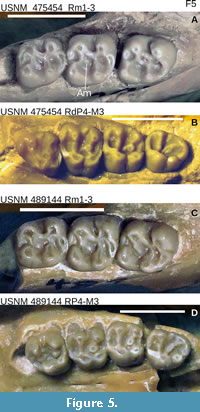 Ischyromys douglassi USNM 617532 (Figure 2) is embedded in matrix and consists of a complete skull with a dorsally fragmented braincase and upper and lower dentitions, plus a nearly complete skeleton. The dentition consists of the right and left dP4, M1-3 and dp4, m1-3, with alveoli for upper right and left deciduous and permanent P3 loci. The skeleton consists of cervical, anterior thoracic, and posterior lumbar vertebrae, partially articulated but displaced ribs, sacrum, fragmentary os coxae with acetabulum present only on left side, partial left scapula with articulated left humerus, radius, ulna and partial manus, glenoid fossa of right scapula and damaged right humerus and proximal ulna, articulated left femur, tibia, fibula, and broken but articulated left astragalus and calcaneum (missing their posterior aspect), distal right femur and proximal tibia, and disarticulated phalanges and some caudal vertebrae. Several additional specimens from West Canyon Creek further illustrate the anatomy of I. douglassi, including skulls with at least intact rostra and some associated upper and lower dentitions (USNM 475454 and 489144 illustrated in Figure 5, plus USNM 475451, 475453, 475457, 475458, and 489148).
Ischyromys douglassi USNM 617532 (Figure 2) is embedded in matrix and consists of a complete skull with a dorsally fragmented braincase and upper and lower dentitions, plus a nearly complete skeleton. The dentition consists of the right and left dP4, M1-3 and dp4, m1-3, with alveoli for upper right and left deciduous and permanent P3 loci. The skeleton consists of cervical, anterior thoracic, and posterior lumbar vertebrae, partially articulated but displaced ribs, sacrum, fragmentary os coxae with acetabulum present only on left side, partial left scapula with articulated left humerus, radius, ulna and partial manus, glenoid fossa of right scapula and damaged right humerus and proximal ulna, articulated left femur, tibia, fibula, and broken but articulated left astragalus and calcaneum (missing their posterior aspect), distal right femur and proximal tibia, and disarticulated phalanges and some caudal vertebrae. Several additional specimens from West Canyon Creek further illustrate the anatomy of I. douglassi, including skulls with at least intact rostra and some associated upper and lower dentitions (USNM 475454 and 489144 illustrated in Figure 5, plus USNM 475451, 475453, 475457, 475458, and 489148).
Upper dentition. USNM 617532 has one deciduous premolar (dP4) and three molars on each side of the upper jaw (Figure 6A-F). Although P3s and permanent P4s are missing in the maxilla of this specimen, dorsal and anterior to each dP4 are empty alveoli with sufficient space on each side to house both a P3 root and P4 crown. We therefore infer for this specimen the typical Ischyromys dental formula of 1.0.2.3/1.0.1.3. The dP4 is heavily worn and lacks detail of its occlusal anatomy, such as transverse crests or metaconules that may have been present. The dP4 differs from the molars in having no hypostria (vertical grooves) on the lingual surface of the tooth. The paracone and metacone are still visible; the protocone and hypocone are almost entirely worn down.
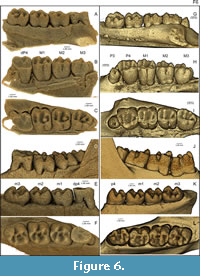 Molars show a large metaconule on the lingual end of the metaloph in M1-3, which dams the posterolingual valley (Figure 3, Figure 6). M1 and M2 are quadritubercular with pronounced hypocones. There is a distinct metacone, metaconule, and hypocone, with very weak development of the metaloph connecting them. The anterior arm of the paracone is extended to form a small parastyle. The anterior arm of the protocone is also extended, forming a small anterostyle which is connected to the parastyle by an anteroloph. The posterior end of the protocone and anterior end of the hypocone are similarly extended, forming the endoloph that joins them. The posterior arm of the hypocone extends lingually, producing a prominent posteroloph. A very small mesostyle is present on M1 between the paracone and metacone, joined by the posterior and anterior arms of these cusps respectively. The M3 is similar and also shows a prominent mesostyle, but differs from the other molars in having an obliquely oriented metaloph and consequently short transverse dimension of the posterior side of the tooth. The molars feature a metaconule on the lingual end of the metaloph, well developed on M1-3, which almost dams the entire posterior lingual valley. Due to the oblique metaloph on M3, the metaconule shows a very slight metacone-protocone connection. Large upper molar metaconules are reported in Ischyromys blacki, I. douglassi (Figure 3, Figure 6C, and I. junctus (Wood, 1980) but not species of Ischyromys such as I. typus (Figure 6I).
Molars show a large metaconule on the lingual end of the metaloph in M1-3, which dams the posterolingual valley (Figure 3, Figure 6). M1 and M2 are quadritubercular with pronounced hypocones. There is a distinct metacone, metaconule, and hypocone, with very weak development of the metaloph connecting them. The anterior arm of the paracone is extended to form a small parastyle. The anterior arm of the protocone is also extended, forming a small anterostyle which is connected to the parastyle by an anteroloph. The posterior end of the protocone and anterior end of the hypocone are similarly extended, forming the endoloph that joins them. The posterior arm of the hypocone extends lingually, producing a prominent posteroloph. A very small mesostyle is present on M1 between the paracone and metacone, joined by the posterior and anterior arms of these cusps respectively. The M3 is similar and also shows a prominent mesostyle, but differs from the other molars in having an obliquely oriented metaloph and consequently short transverse dimension of the posterior side of the tooth. The molars feature a metaconule on the lingual end of the metaloph, well developed on M1-3, which almost dams the entire posterior lingual valley. Due to the oblique metaloph on M3, the metaconule shows a very slight metacone-protocone connection. Large upper molar metaconules are reported in Ischyromys blacki, I. douglassi (Figure 3, Figure 6C, and I. junctus (Wood, 1980) but not species of Ischyromys such as I. typus (Figure 6I).
Lower dentition. The lower dp4 of USNM 617532 is also heavily worn (Figure 6E-F), with only a trace of the metaconid and hypoconid remaining. This tooth is buccolingually narrower, particularly anteriorly, than the square-shaped molars. The lower molars possess a trigonid consisting of the metaconid and protoconid. The two cusps are connected by the metalophid, which curves posteriorly from the metaconid to the protoconid. The posterior arm of the metaconid is well developed, almost reaching the entoconid. The anterior arm of the protoconid is extended, forming an “anterolophulid” according to Marivaux et al. (2004: fig. 1). The talonid is wider than the trigonid and comprises a large hypoconid, smaller entoconid, and smaller still, a hypoconulid. The hypoconid is connected to the protoconid via a curved ectolophid but no mesoconid is observable on this crest. The hypolophid intersects this crest, connecting the entoconid to the hypoconid. The posterior arm of the hypoconid extends medially to form a pronounced posterolophid that reaches the lingual side of the tooth. A distinct anterior medial cusp originates on the posterior edge of the metalophid (labelled “Am” on Figure 3, Figure 5) and almost divides the lingual valley. This cusp is best observed on m2 and m3 where the cusp almost extends as far as the entoconid, splitting the anterior lingual valley in two. Heaton illustrated this cusp and referred to it in his text as the “anterior medial accessory cusp” (Heaton, 1996, his fig. 1 and p.375). The lower molars of USNM 617532 are larger than those of Ischyromys blacki and smaller than those of I. typus. Its lower m1 is slightly shorter, and its m2 and m3 lengths are within the range for I. douglassi given by Black (1968: table 2). The m3 of USNM 617532 is larger than that of I. veterior, but its m1-2 are slightly shorter in length and broader in width. Overall, its dental lengths are close to those recorded for I. douglassi as reported by Black (1968:table 2), although some are near or slightly below the lower end of his ranges.
All of the Ischyromys typus specimens we examined date to the Orellan and have the typical Ischyromys dental formula of 1.0.2.3/1.0.1.3, although only USNM 16828 (Figure 7E-H) and BNMH PV M 7855 are sufficiently complete to retain a full complement of teeth. The P3 is a small peg-like tooth with a cone and partially developed peripheral cingulum (Figure 6H-I). The P4 is molariform with a pronounced hypocone, protocone, paracone, and metacone. The paracone and protocone are connected by a straight transverse crest, the protoloph. The metacone and hypocone are likewise joined by the metaloph (Figure 3I). The P4 is distinguished from the molars by the absence of the hypostria between the protocone and hypocone, on the lingual surface of the tooth. Neither paraconule nor metaconule are present. Anterolophs and posterolophs are present, although the former is weak and terminates just lingual to the parastyle. As for P4, the molars are quadritubercular and have pronounced hypocones. From M1 to M2 to M3, the metaloph becomes progressively shorter and more oblique, such that the M3 is posteriorly narrower than the M2, which is posteriorly narrower than M1. The anteroloph is more developed in the molars than in P4 and extends to the lingual margin of the tooth.
The teeth of the lower jaw of Ischyromys typus (e.g., USNM 16828) are also lophate (Figure 6J-L). The p4 has a small trigonid, appearing reduced anteriorly in comparison to m1 and m2. The m3 is extended and rectangular in shape. The lower teeth are fairly simple with a narrow trigonid, featuring a metaconid and protoconid. These two cusps are connected by a metalophid, which curves posteriorly from the metaconid towards the protoconid. The anterior side of the protoconid extends slightly to form a short anterolophid. This is best seen in the slightly more worn p4 and m1. The talonid is wider than the trigonid (especially in p4) which has a large hypoconid and smaller entoconid. A hypolophid connects these two cusps. The hypoconid is joined to the protoconid via a curved ectolophid, which lacks a mesoconid. A well-developed posterolophid runs from the hypoconid to the lingual side of the tooth. There is no distinct hypoconulid.
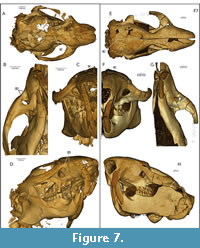 Skull. The skull of USNM 617532 is broad and the rostrum comes to a blunt end. This and other specimens (see below) show that the species is anatomically sciuromorphous, consistent with the interpretations of Wood (1976). USNM 617532 and other West Canyon Creek specimens exhibit a scar of the deep masseter extending anterodorsally from the ventral surface of the zygoma, passing above and anterior to the infraorbital foramen and terminating close to the maxillary-premaxillary suture, contributing to a zygomatic plate on the lateral surface of the rostrum (Figure 1B, Figure 7B, D).
Skull. The skull of USNM 617532 is broad and the rostrum comes to a blunt end. This and other specimens (see below) show that the species is anatomically sciuromorphous, consistent with the interpretations of Wood (1976). USNM 617532 and other West Canyon Creek specimens exhibit a scar of the deep masseter extending anterodorsally from the ventral surface of the zygoma, passing above and anterior to the infraorbital foramen and terminating close to the maxillary-premaxillary suture, contributing to a zygomatic plate on the lateral surface of the rostrum (Figure 1B, Figure 7B, D).
USNM 457454 from West Canyon Creek also shows a parenthesis-shaped scar on the bottom of the arch, which curves inward toward the P4. This distinct scar is also present in Ischryomys typus (Figure 7H); however, unlike I. typus, it shows a rounded but prominent ridge that continues forward and upward beyond the infraorbital foramen to merge with the dorso-lateral surface of the rostrum, with an anterior extent at or near the maxilla-premaxilla suture. Other West Canyon Creek specimens with a well-preserved rostrum (USNM 457453, 475757, 475458) show a similar rostral morphology as in USNM 617532 (Figure 7B-D), except for one particularly young specimen (USNM 489148), which retains dP3-dP4 and lacks any sign of a mineralized M3. Here, the scar is relatively weak with a termination slightly posterior to the premaxilla-maxilla suture.
Although USNM 617523 has jaw closing musculature resembling specimens referred to Titanotheriomys by Wood (1976), it shows several differences. Titanotheriomys was described by Wood (1976) as having an arched dorsal profile of the skull; this is relatively flat in USNM 617532. Furthermore, Wood characterized Titanotheriomys as lacking confluent supraorbital crests. In most Titanotheriomys specimens Wood examined, the supraorbital crests did not converge but showed a lyrate shaped space dividing them. In USNM 617532, however, the two supraorbital crests unite at the frontal suture and join to form a single sagittal crest (Figure 7A), as in other West Canyon Creek specimens (e.g., USNM 475458).
The zygoma and rostrum of USNM 617532 differ from those of Ischyromys typus in a number of ways. From a dorsal view, the skull appears proportionally wider than that of I. typus (e.g., USNM 16828, Figure 7). The rostrum is also proportionally shorter than in I. typus and rather than tapering to a point (Figure 7E), it is blunt (Figure 7A). These two features of USNM 617532 bear a superficial resemblance to Aplodontia rufa. The maxillary root of the zygoma is broader in USNM 617532 than in I. typus, and there are two notable scars which, based on comparisons with extant rodents, indicate attachment sites of the deep masseter. As in I. typus, there is a scar which curves medially at the anterior end of the zygoma. However, unlike I. typus, this scar is very short and weak. The area between the dorsal edge of this scar and the infraorbital foramen is very inset, giving the ventral edge of the foramen a pronounced lip (Figure 7C). Furthermore, a second scar of the deep masseter can be observed to extend from the ventral surface of the zygoma. This scar follows the dorsal border of the maxillary root, ascending at a 45 degree angle. It reaches past the dorsal border of the infraorbital foramen, continuing upwards until it curves antero-medially to reach the maxillary-premaxillary suture. These features define a zygomatic plate (Figure 1B; “zp” in Figure 7B, D), with its dorsal limit superior to the infraorbital foramen. The muscle scars indicate that the deep masseter originated on the rostrum, anterior to and above the infraorbital foramen, as well as covering the surface area below the infraorbital foramen. These scars demonstrate that I. douglassi is anatomically sciuromorphous, as described by Wood (1976) in Ischyromys veterior (referred to by Wood as “Titanotheriomys wyomingensis”). The infraorbital foramen, although much smaller than those seen in anatomically hystricomorphous rodents (Figure 1D), is proportionally larger than the foramina seen in protrogomorphous rodents, such as Aplodontia rufa. As in other specimens of Ischyromys (e.g., I. typus, Figure 7F) the foramen in I. douglassi narrows towards its ventromedial edge (Figure 7C). Other notable features on the rostrum of I. douglassi are two well-defined pits, anterior to each upper toothrow and ventral to the infraorbital foramen (Figure 7D). One of these pits is a small but deep depression antero-lateral to the alveolus of the P3. Following Wood (1976), this is likely to be a fossa for the attachment of the buccinator muscle. The other larger and more pronounced depression lies anteroventrally to the infraorbital foramen. This marks the attachment site of the tendon of the superficial masseter. The site of attachment for the tendon appears particularly large in USNM 617532 (Figure 7D) relative to USNM 16828 (Figure 7H).
The general features of the rostrum in Ischryomys typus agree with the descriptions provided by Wood (1937) and Black (1968) of various I. typus skulls found in the White River Formation. There is a clear muscle scar for the deep masseter on the ventral surface of the zygoma (Figure 7F-H). The scar curves medially at the anterior end of the zygoma, and continues until the maxillary root of the zygoma, marking the anterior limit of the muscle. As described by Wood (1976), the anterior surface of the maxillary root of the zygoma in I. typus is tilted anteriorly, from the scar of the deep masseter to just below the infraorbital foramen (Figure 7H). Wood described this area as a “diagonal zygomatic plate” (Wood, 1976:252), although there is no evidence on the specimens examined in this study to suggest that the deep masseter attached here. Both I. douglassi (USNM 617532, Figure 7C) and I. typus (USNM 16828, Figure 7F) show large infraorbital foramina, mediolaterally wider than dorsoventrally tall. Wood (1976) described a depression for the tendon of the superficial masseter anterior and lateral to the P3 on I. typus. Although there are no rugose areas observable on USNM 16828 (Figure 7H), there is a distinct pit for the tendon of the superficial masseter in AMNH 144628. These specimens of I. typus are consistent with the generally held view that I. typus was anatomically protrogomorphous. Additionally, the angle between the coronoid process relative to the antero-posterior axis of the mandibular ramus is slightly smaller in USNM 617532 (about 114 degrees; Figure 7D) compared to I. typus (about 121 degrees in USNM 16828; see Figure 7H).
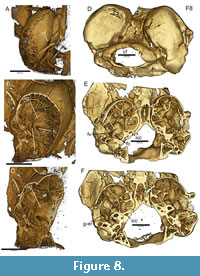 The inner ear region of USNM 617532 is well preserved. Wahlert 1974:393 writes that based on absence of a stapedial foramen, the stapedial artery is absent in Ischyromys. Bertrand and Silcox (2016:12) cite Wahlert and note further that “in ROM V1007 and AMNH 144628, the cast of the stapedial artery is absent....” However, the carotid foramen, evident along the medial aspect of the auditory bulla, shows a connection with a substantial groove on the ventrum of the promontorium of the periotic (or “promontory”) in all CT scanned specimens of Ischyromys examined so far, including USNM 617532 (Figure 8A-C; see also Asher et al. 2019: fig. 2), ROM V1007 (Figure 8D-F), USNM 16828 and AMNH 144628. This is further illustrated in media #M578238 of morphobank.org project 2769 (Asher et al., 2019). This groove traverses the fenestra vestibuli (Figure 8B, E), is continuous with the internal carotid foramen and canal proximally (Figure 8C, E, F), and leads to a gap in the roof of the epitympanic recess and adjacent foramina distally (Figure 8F), consistent with the presence of a patent stapedial artery. Given the size of the promontory groove (Figure 8B, C, E, F), comparable to those seen in Exmus and Cocomys (Li et al., 1989: figs. 3, 4; Wible et al., 2005: figs. 6, 7), we regard this as more likely than presence of a nerve or vein only. Black (1968: fig. 19) mistakenly labeled a “stapedial” foramen on the medial aspect of the auditory bulla in a specimens of Ischyromys douglassi (CMNH 1122), but the structure in question is actually a jugular (also known as posterior lacerate) foramen, anterolateral to the hypoglossal foramen and posterolateral to the carotid foramen. These three structures are correctly labeled for a specimen of Ischyromys typus (AMNH 694) in Wahlert (1974:fig. 9).
The inner ear region of USNM 617532 is well preserved. Wahlert 1974:393 writes that based on absence of a stapedial foramen, the stapedial artery is absent in Ischyromys. Bertrand and Silcox (2016:12) cite Wahlert and note further that “in ROM V1007 and AMNH 144628, the cast of the stapedial artery is absent....” However, the carotid foramen, evident along the medial aspect of the auditory bulla, shows a connection with a substantial groove on the ventrum of the promontorium of the periotic (or “promontory”) in all CT scanned specimens of Ischyromys examined so far, including USNM 617532 (Figure 8A-C; see also Asher et al. 2019: fig. 2), ROM V1007 (Figure 8D-F), USNM 16828 and AMNH 144628. This is further illustrated in media #M578238 of morphobank.org project 2769 (Asher et al., 2019). This groove traverses the fenestra vestibuli (Figure 8B, E), is continuous with the internal carotid foramen and canal proximally (Figure 8C, E, F), and leads to a gap in the roof of the epitympanic recess and adjacent foramina distally (Figure 8F), consistent with the presence of a patent stapedial artery. Given the size of the promontory groove (Figure 8B, C, E, F), comparable to those seen in Exmus and Cocomys (Li et al., 1989: figs. 3, 4; Wible et al., 2005: figs. 6, 7), we regard this as more likely than presence of a nerve or vein only. Black (1968: fig. 19) mistakenly labeled a “stapedial” foramen on the medial aspect of the auditory bulla in a specimens of Ischyromys douglassi (CMNH 1122), but the structure in question is actually a jugular (also known as posterior lacerate) foramen, anterolateral to the hypoglossal foramen and posterolateral to the carotid foramen. These three structures are correctly labeled for a specimen of Ischyromys typus (AMNH 694) in Wahlert (1974:fig. 9).
Geometric Morphometric Analysis and Character Evolution
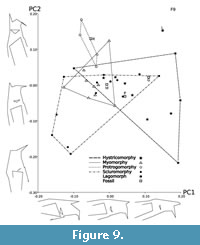 The PCA analysis of the 21 3D cranial landmarks (Figure 4) shows that 90% of the shape variation is explained by the first 14 principal components and just over 50% of variation is explained by the first four (see Table 3 for all eigenvalues). Interpretation of wireframe graphs indicate several cranial shapes changes associated with PC1 (17% of variation; Figure 9). Increasingly positive values along PC1 represent a shorter length of the naso-premaxillary suture in relation to the internasal suture, an increasingly anterior limit of the incisive foramina, and increased elevation of the posterior root of the zygoma in relation to the anterior root. PC1 also shows the infraorbital foramen increasing in diameter. The negative values along PC1 capture cranial shape changes associated with the presence of a large zygomatic plate and the positive values with extreme anatomical hystricomorphy. Increasingly positive values of PC2 (13.6% of variation) represent a trend towards increasing incisor procumbency, increasing length of the naso-premaxillary suture in relation to the internasal suture, and decreasing infraorbital foramen size. PC2 also describes the position of the anterior limit of the interior zygomatic arch edge. For positive values of PC2, the interior edge is in-line with the orbital margin; for negative values, the anterior limit extends anteriorly past the orbital margin. PC3 (11.6% of variation) separates the extant rodents and fossils from the lagomorph Lepus. Lepus represents the most negative value along the PC3 axis, associated with a smaller infraorbital foramen, shorter naso-premaxillary suture, decreasing incisor procumbency, and an increasingly anterior limit of the frontal suture as it crosses the orbital margin. Negative values of PC4 (9.7% of variation) also indicate a smaller infraorbital foramen. The remaining 48.2% of cranial shape variation is explained by PC5-35, each accounting for progressively less variation explained.
The PCA analysis of the 21 3D cranial landmarks (Figure 4) shows that 90% of the shape variation is explained by the first 14 principal components and just over 50% of variation is explained by the first four (see Table 3 for all eigenvalues). Interpretation of wireframe graphs indicate several cranial shapes changes associated with PC1 (17% of variation; Figure 9). Increasingly positive values along PC1 represent a shorter length of the naso-premaxillary suture in relation to the internasal suture, an increasingly anterior limit of the incisive foramina, and increased elevation of the posterior root of the zygoma in relation to the anterior root. PC1 also shows the infraorbital foramen increasing in diameter. The negative values along PC1 capture cranial shape changes associated with the presence of a large zygomatic plate and the positive values with extreme anatomical hystricomorphy. Increasingly positive values of PC2 (13.6% of variation) represent a trend towards increasing incisor procumbency, increasing length of the naso-premaxillary suture in relation to the internasal suture, and decreasing infraorbital foramen size. PC2 also describes the position of the anterior limit of the interior zygomatic arch edge. For positive values of PC2, the interior edge is in-line with the orbital margin; for negative values, the anterior limit extends anteriorly past the orbital margin. PC3 (11.6% of variation) separates the extant rodents and fossils from the lagomorph Lepus. Lepus represents the most negative value along the PC3 axis, associated with a smaller infraorbital foramen, shorter naso-premaxillary suture, decreasing incisor procumbency, and an increasingly anterior limit of the frontal suture as it crosses the orbital margin. Negative values of PC4 (9.7% of variation) also indicate a smaller infraorbital foramen. The remaining 48.2% of cranial shape variation is explained by PC5-35, each accounting for progressively less variation explained.
When comparing the locations of species in morphospace across the first two principal components (30.5% of variation), anatomically sciuromorphous species form a wide cluster (Figure 9). Hystricomorphous species form an equally large cluster, which overlaps with the sciuromorphous and the more compact myomorphous cluster at the centre of morphospace. Anatomically protrogomorphous species form a small cluster which overlaps slightly with the myomorphous cluster and even less with the hystricomorphous cluster. Ischyromys douglassi (USNM 617532) is centrally placed and nested within the overlap between the hystricomorphous, myomorphous, and sciuromorphous species. Ischyromys typus (USNM 16828) lies on the margin of the protrogomorphous cluster (Figure 9), closer to Aplodontia and bathyergoids than any other rodent based on the first two principal components. In contrast, and as noted above, I.douglassi is anatomically sciuromorph by virtue of its zygomatic plate defined in part by the masseter scar anterior and dorsal to the infraorbital foramen (Figure 1, Figure 7). In terms of shape space, it appears in a region in which all three extant, non-protrogomorph clusters overlap (Figure 9), and is separated from I. typus by a larger distance on PC2 than PC1. Ischyromys typus occupies a more positive position on the PC2 axis than I. douglassi indicating that in comparison, the latter has less profound incisor procumbency, a larger infraorbital foramen, a shorter naso-premaxillary suture, and that the interior edge of the zygomatic arch is anterior to the frontal suture as it crosses the orbital margin. Paradelomys crusafonti (Vianey-Liaud and Marivaux, 2016) is an anatomically hystricomorphous theridomyid and lies within the overlap of hystricomorphous and sciuromorphous clusters. Gomphos, a mimotonid stem-lagomorph, also lies within this region of overlap.
 The plot of the third and fourth principal components (Figure 10) displays more overlap between the different masseter morphologies than PC1 plotted against PC2 (Figure 9) and largely separates rodents from Lepus. It also explains less cranial shape variation (21.3%). Anatomically hystricomorphous species occupy a larger cluster in this plot compared to sciuromorphous species. As in the plot of PC1 and PC2, myomorphous and protrogomorphous species occupy the tightest clusters. Ischyromys douglassi and Ischyromys typus are close to each other in morphospace on both PC3 and PC4 and occupy the hystricomorphous cluster. Gomphos and Paradelomys occupy the sciuromorphous cluster.
The plot of the third and fourth principal components (Figure 10) displays more overlap between the different masseter morphologies than PC1 plotted against PC2 (Figure 9) and largely separates rodents from Lepus. It also explains less cranial shape variation (21.3%). Anatomically hystricomorphous species occupy a larger cluster in this plot compared to sciuromorphous species. As in the plot of PC1 and PC2, myomorphous and protrogomorphous species occupy the tightest clusters. Ischyromys douglassi and Ischyromys typus are close to each other in morphospace on both PC3 and PC4 and occupy the hystricomorphous cluster. Gomphos and Paradelomys occupy the sciuromorphous cluster.
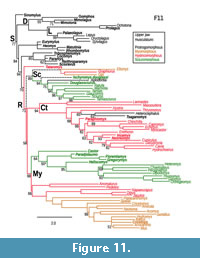 Based on the phylogenetic study of Asher et al. (2019), Ischryomys is well supported (posterior probability of 0.94) as a member of the squirrel-related clade (Sciuromorpha), sister to Aplodontia -Sciuridae (Figure 11). Anatomically, and in agreement with Wood (1976), the genus Ischryomys shows polymorphic masseter architecture, with some species (e.g., I. douglassi) exhibiting anatomical sciuromorphy and others (e.g., I. typus) anatomical protrogomorphy (Figure 7). Figure 11 provides a reconstruction of masseter types as a single, multistate character mapped onto the phylogeny of Asher et al. (2019: fig. 2). Several key taxa have not yet been incorporated into that phylogenetic analysis, such as anatomically hystricomorphous theridomyids (Vianey Liaud et al., 2016), protrogomorphous Eogliravus (Storch and Seiffert, 2007), and protrogomorphous, possible stem muroids such as Knightomys (Wood, 1965) and Pauromys (Dawson, 1968). Nonetheless, optimal topologies from Asher et al. (2019) support the novel interpretation that the anatomically hystricomorphous Tataromys comprises the sister taxon of crown Rodentia, and that anatomically polymorphic Ischyromys (with sciuromorphous I. douglassi and protrogomorphous I. typus) comprises the sister taxon of Aplodontia plus sciurids.
Based on the phylogenetic study of Asher et al. (2019), Ischryomys is well supported (posterior probability of 0.94) as a member of the squirrel-related clade (Sciuromorpha), sister to Aplodontia -Sciuridae (Figure 11). Anatomically, and in agreement with Wood (1976), the genus Ischryomys shows polymorphic masseter architecture, with some species (e.g., I. douglassi) exhibiting anatomical sciuromorphy and others (e.g., I. typus) anatomical protrogomorphy (Figure 7). Figure 11 provides a reconstruction of masseter types as a single, multistate character mapped onto the phylogeny of Asher et al. (2019: fig. 2). Several key taxa have not yet been incorporated into that phylogenetic analysis, such as anatomically hystricomorphous theridomyids (Vianey Liaud et al., 2016), protrogomorphous Eogliravus (Storch and Seiffert, 2007), and protrogomorphous, possible stem muroids such as Knightomys (Wood, 1965) and Pauromys (Dawson, 1968). Nonetheless, optimal topologies from Asher et al. (2019) support the novel interpretation that the anatomically hystricomorphous Tataromys comprises the sister taxon of crown Rodentia, and that anatomically polymorphic Ischyromys (with sciuromorphous I. douglassi and protrogomorphous I. typus) comprises the sister taxon of Aplodontia plus sciurids.
DISCUSSION
Among Ischyromys teeth from West Canyon Creek, Heaton (1996: 395) noted “a unique feature that separates it from all other species of Ischyromys... the exceptionally high incidence of medial and lingual accessory cusps.” However, such accessory cuspules are not unique to the West Canyon Creek specimens but are also evident in I. douglassi from McCartyʼs Mountain. In his description of I. douglassi, Black (1968, p. 286), describes this as “a small cusp on the posterior face of the protoconid which bulges into the basin behind the metalophid” and that “this structure is quite prominent on M1-M3” and it is shown in his figures 10-12. Blackʼs illustrations (1968: fig. 10-12) of the lower dentition (CMNH 1053, 1125, and 10963) show more wear than the lower molars of USNM 617532 and other specimens from West Canyon Creek, but they are consistent with the presence of lower accessory cuspules in I. douglassi, evident in USNM 475454, 489144 (Figure 5), 617532 (Figure 3) and other specimens from West Canyon Creek.
One of the most striking features of Ischyromys douglassi (and in particular USNM 617532) is the presence of a broad zygomatic plate and muscle scars of the deep masseter reaching onto the rostrum, anterodorsal to the infraorbital foramen (Figure 1B, Figure 7B, D). These are not as extensive or well defined as in extant sciuromorphous taxa such as Sciurus, Castor, or Ratufa (Figure 1C) but neither are they present in anatomically protrogomorphous rodents (Figure 1A, Figure 7G-H). In agreement with Wood (1976), species of Ischyromys such as I. douglassi and I. veterior (Wood, 1976) match Brandt’s (1855) definition of anatomical sciuromorphy. Previous generations of paleomammalogists have often mixed the anatomical conditions of the masseter with rodent taxonomy, leading to the widespread, historical classification of Ischyromys among “protrogomorph”-grade rodents (Wood, 1937; Chaline and Mein, 1979; Fahlbusch, 1985). Black (1968) was concerned that crushing had given Wood’s fossils an altered appearance, but that is not the case here. Although parts of the occiput, braincase, nasals, and premaxilla show damage (Figure 2, Figure 7A), the specimen (and particularly the infraorbital region) of USNM 617532 is otherwise well-preserved (Figure 7B-7D) and consistent with our interpretation that one of the oldest specimens of Ischyromys exhibits a zygomatic plate that extends anterior to the infraorbital foramen. It is, thus, anatomically sciuromorph.
Anatomical sciuromorphy has long been defined in the literature based on expansion of the deep masseter along the anterior zygomatic arch (Hautier et al., 2008; Cox et al., 2012). The first two principal components of the geometric morphometric analysis undertaken here places USNM 617532 within the shape space occupied by extant, anatomically sciuromorphous rodents and outside of that exhibited by extant protrogomorphous rodents (Figure 9). However, there is substantial overlap among the hystricomorph, sciuromorph, and myomorph clusters based on the first two principal components (Figure 9), which collectively account for about 31% of the variation, and even more overlap among all categories based on extant species using principal components 3 and 4 (Figure 10, accounting for ca. 21% of the variation). The cranial landmarks used here show that there is a great amount of shared shape variation among these rodents, despite their different masseter structures. Morris et al. (2018) investigated convergences in cranial variation across Euarchontoglires, and their results reflect this finding in rodents. They found that the cranial variation within the squirrel-related clade of rodents, most of which are anatomically sciuromorphous (except for Aplodontia and glirids; see Figure 11 and Maier et al., 2002), overlapped with that of both mouse-related and ctenohystrican clades. When considering the first two principal components in our study (Figure 9), Ischyromys douglassi overlaps with the sciuromorphous, hystricomorphous, and myomorphous clusters, but not the protrogomorphous cluster (as defined by extant rodents only). In contrast, I. typus appears adjacent to the protrogomorph cluster. Their distance in morphospace further demonstrates that although these two species are closely related, their rostral morphology (and by extension myology) is different. When the third and fourth principal components are considered (Figure 10), both species of Ischyromys appear within the hystricomorphous cluster and close to the margins of the sciuromorphous and myomorphous clusters.
The landmarks chosen for this analysis likely capture some shape variation not directly related to the masseter morphology, and each principal component captures a slightly different aspect of variation. Thus, Ischyromys typus may not resemble other protrogomorphous species closely due to other aspects of the skull shape that are not shared, or they might be represented by the other principal components. The species we regard as anatomically protrogomorphous are all visually quite different. For example, Heterocephalus glaber has relatively smaller infraorbital foramina than I. typus and A. rufa. Furthermore, although the masseter in each of these species never penetrates the infraorbital foramen, it comes close in some bathyergoids (Cox and Faulkes, 2014). The zygomaticomandibularis of H. glaber extends past the zygoma until it reaches the anterior orbit, but does not pass through the small infraorbital foramen (Cox and Faulkes, 2014). Although a member of Ctenohystrica (an otherwise exclusively hystricomorphous group), bathyergoids have a secondarily derived protrogomorphous condition, which is likely to be an adaptation for fossorial life (Cox and Faulkes, 2014).
Much like the other masseteric conditions, protrogomorphy displays homoplasy, having evolved multiple times among the different rodent clades. Following the phylogenetic study of Asher et al. (2019), the anatomically protrogomorphous Heterocephalus and Tsaganomys are both deeply nested within the clade of Ctenohystrica and share multiple common ancestors with anatomically hystricomorphous species such as Thryonomys, Hystrix, and ctenodactylids. Similarly, protrogomorphous Aplodontia is nested within the squirrel-related clade and shares common ancestors with sciuromorphous Ischyromys douglassi (and by extension the protrogomorphous Ischyromys typus), and myo- and hystricomorphous glirids (Figure 11).
Albeit weakly supported (posterior probability of 71 in Figure 11), the node separating crown Rodentia from the anatomically hystricomorphous taxon Tataromys (from the Oligocene-Miocene of central Asia; Wang, 1997) underscores the possibility that the ancestor of crown rodents was itself non-protrogomorphous. Tataromys has generally been regarded as an Asian relative of ctenodactylids (Wang, 1997; Marivaux et al., 2004; Wible et al., 2005; Oliver and Daxner-Höck, 2017), not a simplicidentate outside of crown Rodentia. However, Tataromys was reconstructed using both probabilistic optimality criteria and parsimony (Asher et al., 2019: figs. 4 and S2) as the sister taxon of crown rodents, not a member of Ctenohystrica (Figure 11). Further phylogenetic analyses with better samples of (for example) fossil Ctenohystrica would be necessary to further test this possibility.
Swanson et al. (2019) considered the evolution of rodent masseteric architecture in a phylogenetic study of a large UCE dataset. They discussed several key fossils, including anatomically protrogomorphous species that, according to the literature (Wood, 1959, 1965, 1985; Dawson, 1968; Marivaux et al., 2004; Storch and Seiffert, 2007), are likely stem glirids (Eogliravus), stem mouse-related (Pauromys, Prolapsus, Knightomys), or sister to crown Rodentia (Bumbanomys). With these fossils in their literature-inferred placements, Swanson et al. (2019: fig. 2) reconstructed the common ancestors of two of the three major rodent clades (excluding Ctenohystrica), and of Rodentia itself, as most likely anatomically protrogomorph, consistent with the long-held and legitimate inference that, at some point, the total clade Simplicidentata (encompassing Rodentia) evolved from an anatomically protrogomorph common ancestor.
The phylogenetic relationships of the 51 extant taxa sampled by Swanson et al. (2019) was based on a large molecular dataset, comprising an alignment over 2000 UCEs (ultraconserved elements, or genomic fragments of DNA) summing to nearly 900 kb. Asher et al. (2019) sampled a much smaller molecular dataset (ca. 17kb) for slightly more extant taxa (60), plus fossilizable morphological characters sampled for all living taxa and another 42 fossils. These two datasets yielded highly congruent topologies overall, but with differences in the position of the rodent root. Swanson et al. (2019) reconstructed the mouse related clade (Myomorpha) as sister to Ctenohystrica-Sciuromorpha; Asher et al. (2019; shown here in Figure 11) reconstructed Sciuromorpha as sister to Ctenohystrica-Myomorpha. The much larger sequence dataset of Swanson et al. (2019) comprises a stronger basis on which to hypothesize the affinities of extant rodents. However, their fossils were positioned in their tree based on the literature, not on a phylogenetic data matrix including those fossils along with extant species. In contrast, Asher et al. (2019) did undertake a phylogenetic analysis of DNA and morphology for living taxa along with morphological data for fossils. In order to definitively arbitrate between the competing hypotheses regarding masseteric architectures at key nodes in the early radiation of rodents, it will clearly be necessary to integrate the samples of living taxa and fossils discussed by both Swanson et al. (2019) and Asher et al. (2019), for example by including anatomically protrogomorphous (e.g., Eogliravus) and non-protrogomorphous (e.g., Tataromys) fossils long assumed to be within crown Rodentia, but seldom tested in a total evidence framework.
Heaton’s (1996) cluster analysis of 31 ischyromyid populations, based on dental and jaw morphometrics, shown in his figure 14 and without the data on rostrum morphology discussed here, provides evidence of further masseteric homoplasy within ischyromyids. Populations he describes as having the sciuromorphous condition, placed in Ischyromys (Titanotheriomys), form a cluster with two populations, West Canyon Creek and Porvenir (late and latest Duchesnean of Wyoming and Texas, respectively). These are geologically older than the other Ischyromys populations in his sample. Heaton (1996) considered the West Canyon Creek population to be a new species, but still categorized it as anatomically protrogomorph (Heaton, 1996: table 8), despite clustering with Woodʼs sciuromorphous Titanotheriomys. He suggests later on in a proposed evolutionary tree (Heaton, 1996: fig. 15), that the West Canyon Creek population is the basal-most species of Ischyromys and that (based on its “Ischyromys like skull“ and “lack of an elongate m3”) it “makes a likely ancestor for all later species.” There are few populations of Ischyromys that predate West Canyon Creek (Lac Pelletier, Badwater Creek, and possibly Porvenir), but their lack of cranial and associated material have so far made it impossible to identify species or determine the anatomical features of the masseter (Heaton, 1996). Thus, I. douglassi from West Canyon Creek (including USNM 617532), is the oldest, anatomically best-known ischyromyine, and to our knowledge it is also the oldest rodent known to be anatomically sciuromorph.
CONCLUSIONS
Rodent classification now reflects the structure of the increasingly well-corroborated phylogenetic tree (Marivaux et al., 2004; Churakov et al., 2010; Fabre et al., 2012; Asher et al., 2019; Swanson et al., 2019), not typological concepts of rodent masticatory patterns as articulated in the nineteeth century and used to varying extents well into the twentieth century. Authors such as Wood (1965), Luckett and Hartenberger (1985), Korth (1994) and many others were aware of the homoplasy of rodent masticatory types of the upper jaw. However, most authors prior to this decade did not have the advantage of the well-corroborated evolutionary tree upon which to anchor their taxonomies or reconstruct character evolution. We, therefore, use this phylogeny to recognize the paraphyly of “ischyromyoids”, some of which (e.g., Paramys, Reithroparamys) are outside of crown Rodentia and some of which (e.g., Ischryomys) are within the squirrel-related clade (or Sciuromorpha, Figure 11). Our results also underscore the long-recognized homoplasy in the masticatory musculature of the upper jaw, including within the genus Ischyromys.
Previous authors have often referred to ischyromyine rodents as “protrogomorphous”, in some cases implying the anatomical condition (Heaton, 1996), others a taxonomic grade (Dawson, 1977; Vianey-Liaud, 1985; Korth, 1994) and sometimes a mixture of both (Wood, 1980). Anatomically, our study confirms the views of Wood (1976) that at least some members of this group, including Ischyromys douglassi, possess diagnostic features of sciuromorphy. Geometric morphometrics demonstrate cranial similarities between this species and extant rodents, whilst highlighting the mosaic nature of jaw musculature evolution across rodents. The age of this specimen, and phylogenetic affinities postulated by Heaton (1996: fig. 15), suggest that rather than being the derived condition, an anatomically sciuromorphous rostrum characterizes the geologically oldest populations of Ischyromys yet known. Our phylogenetic placement of the fossil Tataromys as a non-rodent simplicidentate, and the possible, non-protrogomorphous character optimizations at the common ancestors of major rodent clades, and even for Rodentia itself (Figure 11), is tentative pending a phylogenetic analysis that includes more fossils. Nonetheless, our results underscore the possibility that one or more early-diverging rodent clades were not anatomically protrogomorph.
ACKNOWLEDGEMENTS
We are grateful for the support provided to A.H.R. by a Whitten Studentship from the Department of Zoology, University of Cambridge. We also thank Phil Cox (York), Phil Morris (Florida) and Madeleine Geiger (Zürich) for sharing their expertise on geometric morphometric methods. For access to specimens, we thank L. Hautier, P.H. Fabre and M. Vianey Liaud (Montpellier), B. Kraatz (Pomona), J. Meng (New York), O. Bertrand and M. Silcox (Toronto), Matthew Lowe, Natalie Jones and Ewan St. John Smith (Cambridge), Pip Brewer, Jerry Hooker, and Roberto Portela Miguez (London), and Jessica Nakano and Jennifer Strotman (Washington DC). For review and handling of our paper we thank Dan Hembree, Dariusz Nowakowski, and two anonymous referees. We are also grateful to the online resources at morphobank.org (New York), morphosource.org (Durham) and digimorph.org (Austin).
REFERENCES
Asher, R.J., Smith, M.R., Rankin, A.H., and Emry, R.J. 2019. Congruence, fossils and the evolutionary tree of rodents and lagomorphs. Royal Society Open Science, 6:190387. https://doi.org/10.1098/rsos.190387
Bertrand, O. and Silcox, M. 2016. First virtual endocast of a fossil rodent: Ischyromys typus (Ischyromyidae, Oligocene) and brain evolution in rodents. Journal of Vertebrate Paleontology, 36(3):e1095762. https://doi.org/10.1080/02724634.2016.1095762
Black, C.C. 1968. The Oligocene rodent Ischyromys and discussion of the family Ischyromyidae. Annals of the Carnegie Museum, 39:273-305. https://archive.org/details/biostor-238037/page/n1
Brandt, J.F. 1855. Beiträge zur nähern Kenntniss der Säugethiere Russlands. Buchdruckerei der Kaiserlichen Akad. der Wissenschaften, St. Petersburg. https://hdl.handle.net/2027/nyp.33433011578691
Chaline, J. and Mein, P. 1979. Les Rongeurs et L’evolution. Doin, Paris.
Churakov, G., Sadasivuni, M.K., Rosenbloom, K.R., Huchon, D., Brosius J., and Schmitz, J. 2010. Rodent evolution: back to the root. Molecular Biology and Evolution, 27:1315-1326. https://doi.org/10.1093/molbev/msq019
Cox, P.G. Rayfield, E.J., Fagan, M.J., Herrel, A., Pataky, T.C., and Jeffery, N. 2012. Functional evolution of the feeding system in rodents. PLoS ONE, 7(4):1-11. https://doi.org/10.1371/journal.pone.0036299
Cox, P.G. and Faulkes, C.G. 2014. Digital dissection of the masticatory muscles of the naked mole-rat, Heterocephalus glaber (Mammalia, Rodentia). PeerJ, e448. https://doi.org/10.7717/peerj.448
Cox, P.G. and Jeffery, N. 2011. Reviewing the morphology of the jaw-closing musculature in squirrels, rats, and guinea pigs with contrast-enhanced microCT. The Anatomical Record, 294:915-928. https://doi.org/10.1002/ar.21381
Dawson, M.R. 1968. Middle Eocene rodents (Mammalia) from northeastern Utah. Annals of Carnegie Museum, 39:327-370.
Dawson, M.R. 1977. Late Eocene rodent radiations: North America, Europe and Asia. Géobios, 10:195-209. https://doi.org/10.1016/S0016-6995(77)80018-1
Emry, R.J. 1975. Revised Tertiary stratigraphy and paleontology of the western Beaver Divide, Fremont County, Wyoming. Smithsonian Contributions to Paleobiology, 25:1-20. https://doi.org/10.5479/si.00810266.25.1
Fabre, P.H., Hautier, L., Dimitrov, D., and Douzery, E.J. 2012. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evolutionary Biology, 12(88):1-9. https://doi.org/10.1186/1471-2148-12-88
Fahlbusch, V. 1985. Origin and evolutionary relationships among geomyoids, p. 617-629. In Luckett, W.P. and Hartenberger, J. (eds.), Evolutionary Relationships Among Rodents: A Multidisciplinary Analysis. Springer, Boston. https://doi.org/10.1007/978-1-4899-0539-0_23
Hartenberger, J.-L. 1985. The Order Rodentia: major questions on their evolutionary origin, relationships and suprafamilial systematics, p. 1-33. In Luckett, W.P. and Hartenberger, J.-L. (eds.), Evolutionary Relationships Among Rodents. Springer, Boston. https://doi.org/10.1007/978-1-4899-0539-0_1
Hautier, L., Michaux, J., Marivaux, L., and Vianey-Liaud, M. 2008. Evolution of the zygomasseteric construction in Rodentia, as revealed by a geometric morphometric analysis of the mandible of Graphiurus (Rodentia, Gliridae). Zoological Journal of the Linnean Society, 154(4):807-821. https://doi.org/10.1111/j.1096-3642.2008.00453.x
Heaton, T.H. 1996. Ischyromyidae, p. 373-398. In Prothero, D.R. and Emry, R.J. (eds.), The Terrestrial Eocene-Oligocene Transition in North America. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9780511665431.019
Huchon, D., Catzeflis, F., and Douzery, E.J.P. 2000. Variance of molecular datings, evolution of rodents and the phylogenetic affinities between Ctenodactylidae and Hystricognathi. Proceedings of the Royal Society B, 267(1441):393-402. https://doi.org/10.1098/rspb.2000.1014
Klingenberg, C.P. 2011. MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11:353-357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Korth, W.W. 1994. The Tertiary Record of Rodents in North America. Plenum Press, New York. https://doi.org/10.1007/978-1-4899-1444-6
Landry, S.O., Jr. 1957. The interrelationships of the New and Old World hystricomorph rodents. University of California Publications in Zoology, 56:1-118.
Landry, S.O., Jr. 1999. A proposal for a new classification and nomenclature for the Glires (Lagomorpha and Rodentia). Zoosystematics and Evolution, 75:283-316. https://doi.org/10.1002/mmnz.19990750209
Li, C.K., Zheng, J.J., and Ting, S.Y. 1989. The skull of Cocomys lingchaensis, an early Eocene ctenodactyloid rodent of Asia. Natural History Museum of Los Angeles County Science Series, 33:179-192.
Limaye, A. 2012. Drishti - volume exploration and presentation tool. Proceedings SPIE Developments in X-Ray Tomography VIII, 85060X. https://doi.org/10.1117/12.935640
Luckett, W.P. and Hartenberger, J.-L. (eds.). 1985. Evolutionary Relationships among Rodents: A Multidisciplinary Analysis. Springer, New York. https://doi.org/10.1007/978-1-4899-0539-0
Maddison, W.P. and Maddison, D.R. 2019. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61. http://www.mesquiteproject.org
Maier, W., Klingler, P., and Ruf, I. 2002. Ontogeny of the medial masseter muscle, pseudo-myomorphy, and the systematic position of the Gliridae (Rodentia, Mammalia). Journal of Mammalian Evolution, 9:253-269. https://doi.org/10.1023/A:1023979212759
Maier, W. and Schrenk, F. 1987. The hystricomorphy of the Bathyergidae, as determined from ontogenetic evidence. Zeitschrift für Säugetierkunde, 52:156-164.
Marivaux, L., Vianey-Liaud, M., and Jaeger, J. 2004. High-level phylogeny of early Tertiary rodents: dental evidence. Zoological Journal of the Linnean Society, 142:105-134. https://doi.org/10.1111/j.1096-3642.2004.00131.x
Meng, J., Hu, Y., and Li, C. 2003. The osteology of Rhombomylus (Mammalia, Glires): implications for phylogeny and evolution of Glires. Bulletin of the American Museum of Natural History, 275:1-247. https://doi.org/10.1206/0003-0090(2003)275%3C0001:toormg%3E2.0.co;2
Morris, P.J.R., Cobb, S.N.F., and Cox, P.G. 2018. Convergent evolution in the Euarchontoglires. Biology Letters, 14:1-4. https://doi.org/10.1098/rsbl.2018.0366
Oliver, O. and Daxner-Höck, G. 2017. Large-sized species of Ctenodactylidae from the Valley of Lakes (Mongolia): an update on dental morphology, biostratigraphy, and paleobiogeography. Palaeontologia Electronica, 20.1.1A:1-22. https://doi.org/10.26879/649
Prothero, D.R. 1996. Magnetic stratigraphy of the White River Group in the High Plains, p. 262. In Prothero, D.R. and Emry, R.J. (eds.), The Terrestrial Eocene-Oligocene Transition in North America. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9780511665431.014
Prothero, D.R. and Emry, R.J. (eds.). 1996. The Terrestrial Eocene-Oligocene Transition in North America. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9780511665431
Prothero, D.R. and Emry, R.J. 2004. The Chadronian, Orellan, and Whitneyan North American Land Mammal Ages, p. 156-168. In Woodburne, M.O. (ed.), Late Cretaceous and Cenozoic Mammals of North America. Columbia University Press, New York. https://doi.org/10.7312/wood13040-007
Storch, G., and Seiffert, C. 2007. Extraordinarily preserved specimen of the oldest known glirid from the middle Eocene of Messel (Rodentia). Journal of Vertebrate Paleontology, 27:189-194. https://doi.org/10.1671/0272-4634(2007)27[189:epsoto]2.0.co;2
Swanson, M.T., Oliveros, C.H., and Esselstyn, J.A. 2019. A phylogenomic rodent tree reveals the repeated evolution of masseter architectures. Proceedings of the Royal Society B, 286:20190672. https://doi.org/10.1098/rspb.2019.0672
Swofford, D.L. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods).
Version 4. Sinauer Associates, Sunderland, Massachusetts.
Tabrum, A.R., Prothero, D.R., Garcia, D., and Emry, R.J. 1996. Magnetostratigraphy and biostratigraphy of the Eocene-Oligocene transition, southwestern Montana, p. 278-311. In Prothero, D.R. and Emry, R.J. (eds.), The Terrestrial Eocene-Oligocene Transition in North America. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9780511665431.015
Tullberg, T. 1899. Ueber das System der Nagetiere: eine Phylogenetische Studie. Akademische Buchdruckeri, Uppsala. https://doi.org/10.5962/bhl.title.1733
Vianey-Liaud, M. 1985. Possible evolutionary relationships among Eocene and Lower Oligocene rodents of Asia, Europe and North America, p. 277-309. In Luckett, W.P. and Hartenberger, J. (eds.), Evolutionary Relationships Among Rodents: A Multidisciplinary Analysis. Springer, Boston. https://doi.org/10.1007/978-1-4899-0539-0_10
Vianey-Liaud, M. and Marivaux, L. 2016. Autopsie d’une radiation adaptive: phylogénie des Theridomorpha rongeurs endémiques du Paléogène d’Europe - histoire, dynamique évolutive et intérêt biochronologique. Palaeovertebrata, 40(3):1-68. https://doi.org/10.18563/pv.40.3.e1
von Zittel, K.A. 1893. Handbuch der Palaeontologie, Sect. I. Palaeozoologie, Vol. IV, Vertebrata (Mammalia). R. Oldenbourg, Munich.
Wahlert, J.H. 1974. The cranial foramina of protrogomorphous rodents; an anatomical and phylogenetic study. Bulletin of the Museum of Comparative Zoology, 146(8):363-410.
Wang, B. 1997. The mid-Tertiary Ctenodactylidae (Rodentia, Mammalia) of eastern and central Asia. Bulletin of the American Museum of Natural History, 234:1-88. http://hdl.handle.net/2246/1627
Waterhouse, G.R. 1839. Observations on the Rodentia with a view to point out groups as indicated by the structure of the crania in this order of mammals. Magazine of Natural History, 3:90-96.
Wible, J.R., Wang, Y., Li, C., and Dawson, M.R. 2005. Cranial anatomy and relationships of a new ctenodactyloid (Mammalia, Rodentia) from the early Eocene of Hubei Province, China. Annals of Carnegie Museum, 74:91-150. https://doi.org/10.2992/0097-4463(2005)74[91:caaroa]2.0.co;2
Wood, A.E. 1937. The mammalian fauna of the White River Oligocene Part II - Rodentia. Transactions of the American Philosophical Society, 28(2):155-269. https://doi.org/10.2307/1005501
Wood, A.E. 1962. The early Tertiary rodents of the family Paramyidae. Transactions of the American Philosophical Society, 52:1-261. https://doi.org/10.2307/1005914
Wood, A.E. 1965. Grades and clades among rodents. Evolution, 19:115-130. https://doi.org/10.2307/2406300
Wood, A.E. 1974. Early Tertiary vertebrate faunas, Vieja Group, Trans-Pecos Texas: Rodentia. Texas Memorial Museum Bulletin, 21:1-112. http://hdl.handle.net/2152/29944
Wood, A.E. 1976. The Oligocene rodents Ischyromys and Titanotheriomys and the content of the family Ischyromyidae, p. 244-277. In Churcher, C.S. (ed.), Essays on Palaeontology in Honour of Loris Shano Russell. University of Toronto Press, Toronto. https://doi.org/10.5962/bhl.title.60760
Wood, A.E. 1980. The Oligocene rodents of North America. Transactions of the American Philosophical Society, 70(5):1-68. https://doi.org/10.2307/1006314
Wood, A.E. and Wilson, R.W., 1936. A suggested nomenclature for the cusps of the cheek teeth of rodents. Journal of Paleontology, 10(5):388-391.

