 A new baenid, “Trinitichelys” maini sp. nov., and other fossil turtles from the Upper Cretaceous Arlington Archosaur Site (Woodbine Formation, Cenomanian), Texas, USA
A new baenid, “Trinitichelys” maini sp. nov., and other fossil turtles from the Upper Cretaceous Arlington Archosaur Site (Woodbine Formation, Cenomanian), Texas, USA
Article number: 22.3.81
https://doi.org/10.26879/1001
Copyright Paleontological Society, December 2019
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 18 May 2019. Acceptance: 6 December 2019.
ABSTRACT
The Arlington Archosaur Site (AAS) is a Cenomanian (93-99 Mya) fossil locality in the Woodbine Formation of Texas. Woodbine deposits form primarily terrigenous near shore and shallow marine depositional systems, including shelf, deltaic, and fluvial environments. The AAS is the oldest Upper Cretaceous unit in the Gulf Coastal Plain, and the most complete fossil ecosystem discovered in southwestern Appalachia. It preserves a freshwater/brackish wetland situated in a low-lying coastal plain. The site contains diverse fossil vertebrate and invertebrate faunas and abundant carbonized plant material. The taphonomy of the site is complex, frequently resulting in fragmentary specimens due to a mixture of environmental transport, biological accumulation, pervasive crocodyliform predation, massive storms, wildfires, and widespread flooding events. Numerous new reptilian taxa (particularly crocodyliform) have been recently described, but turtles from the site have not been taxonomically identified. This study documents fossil shell material of AAS turtles, most of which were previously unknown from the site. Results include a new baenid species, “Trinitichelys” maini sp. nov., numerous elements of the helochelydrid Naomichelys, a small trionychid, and a bothremydid. Insights from the turtle faunas at the AAS contribute to our growing understanding of the coastal ecosystems of southwestern Appalachia at the beginning of the Upper Cretaceous.
Brent Adrian. Department of Anatomy, 19555 N. 59th Avenue, Midwestern University, Glendale, Arizona 85308 USA. badria@midwestern.edu
Heather F. Smith. Department of Anatomy, 19555 N. 59th Avenue, Midwestern University, Glendale, Arizona 85308 USA. hsmith@midwestern.edu
Christopher R. Noto. Department of Biological Sciences, University of Wisconsin-Parkside, Kenosha, 53141, Wisconsin, USA. noto@uwp.edu
Aryeh Grossman. Department of Anatomy, 19555 N. 59th Avenue, Midwestern University, Glendale, Arizona 85308 USA. agross@midwestern.edu
Keywords: New species; Baenidae; Woodbine Formation; Upper Cretaceous; Arlington Archosaur Site
Adrian, Brent, Smith, Heather F., Noto, Christopher R., and Grossman, Aryeh. 2019. A new baenid, "Trinitichelys" maini sp. nov., and other fossil turtles from the Upper Cretaceous Arlington Archosaur Site (Woodbine Formation, Cenomanian), Texas, USA. Palaeontologia Electronica 22.3.81 1–29. https://doi.org/10.26879/1001
palaeo-electronica.org/content/2019/2876-arlington-archosaur-turtles
Copyright: December 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/DAAC6949-9D98-4C41-9CDA-19D59EBBA48C
INTRODUCTION
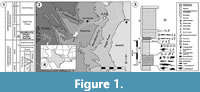 The Woodbine Formation [Fm] (lower middle Cenomanian) (Figure 1) is the oldest known Lower Cretaceous unit in the Gulf Coastal Plain, with exposures from Temple in central Texas to Lake Texoma in southern Oklahoma (Figure 1.2) (Hedlund, 1966; Dodge, 1969; Oliver, 1971; Johnson, 1974; Trudel, 1994; Noto, 2015; Adams et al., 2017). The formation sits unconformably between the older Grayson Marl of the Washita Group and the younger Eagle Ford Group [Gp] (Figure 1.1-2, Figure 2) (Oliver, 1971; Johnson, 1974; Noto, 2015). A period of marine deposition of at least 10 million years separates the Woodbine Fm from earlier terrestrial depositional systems characterizing the Lower Cretaceous Trinity Gp (Figure 1.1-2) (Winkler et al., 1995). General estimates indicate that the total Cretaceous section in north and central Texas includes a mostly complete interval from the Aptian (119 Ma) through the Cretaceous-Paleogene (K-Pg) boundary (Winkler et al., 1995; Denne et al., 2014). Ammonite biostratigraphy has established a minimum age of 95-96 Ma for the Upper Woodbine and Tarrant Fms of the lower Eagle Ford Gp, based on the presence of Conlinoceras tarrantense, a zonal marker for the base of the middle Cenomanian (Figure 1.1-2) (Kennedy and Cobban, 1990; Lee, 1997; Jacobs and Winkler, 1998; Noto, 2015; Adams et al., 2017). Except for the terrestrial facies of the Woodbine Fm, the Cretaceous rocks in central Texas are primarily marine after the mid-Albian (105 Ma) (Winkler et al., 1995). The Woodbine Fm. has produced plants (MacNeal, 1958; Dilcher and Crane, 1984), mammals (Krause and Baird, 1979), dinosaur teeth and tracks, crocodyliforms (Adams et al., 2011, 2017; Noto et al., 2019), turtles (Main, 2005; Noto et al., 2012; Main et al., 2013), invertebrates (Stephenson and Stenzel, 1952), and abundant fishes (McNulty and Slaughter, 1962). Here we present the first comprehensive description of the fossil turtles from the Arlington Archosaur Site (AAS) of the Woodbine Fm (Figure 1).
The Woodbine Formation [Fm] (lower middle Cenomanian) (Figure 1) is the oldest known Lower Cretaceous unit in the Gulf Coastal Plain, with exposures from Temple in central Texas to Lake Texoma in southern Oklahoma (Figure 1.2) (Hedlund, 1966; Dodge, 1969; Oliver, 1971; Johnson, 1974; Trudel, 1994; Noto, 2015; Adams et al., 2017). The formation sits unconformably between the older Grayson Marl of the Washita Group and the younger Eagle Ford Group [Gp] (Figure 1.1-2, Figure 2) (Oliver, 1971; Johnson, 1974; Noto, 2015). A period of marine deposition of at least 10 million years separates the Woodbine Fm from earlier terrestrial depositional systems characterizing the Lower Cretaceous Trinity Gp (Figure 1.1-2) (Winkler et al., 1995). General estimates indicate that the total Cretaceous section in north and central Texas includes a mostly complete interval from the Aptian (119 Ma) through the Cretaceous-Paleogene (K-Pg) boundary (Winkler et al., 1995; Denne et al., 2014). Ammonite biostratigraphy has established a minimum age of 95-96 Ma for the Upper Woodbine and Tarrant Fms of the lower Eagle Ford Gp, based on the presence of Conlinoceras tarrantense, a zonal marker for the base of the middle Cenomanian (Figure 1.1-2) (Kennedy and Cobban, 1990; Lee, 1997; Jacobs and Winkler, 1998; Noto, 2015; Adams et al., 2017). Except for the terrestrial facies of the Woodbine Fm, the Cretaceous rocks in central Texas are primarily marine after the mid-Albian (105 Ma) (Winkler et al., 1995). The Woodbine Fm. has produced plants (MacNeal, 1958; Dilcher and Crane, 1984), mammals (Krause and Baird, 1979), dinosaur teeth and tracks, crocodyliforms (Adams et al., 2011, 2017; Noto et al., 2019), turtles (Main, 2005; Noto et al., 2012; Main et al., 2013), invertebrates (Stephenson and Stenzel, 1952), and abundant fishes (McNulty and Slaughter, 1962). Here we present the first comprehensive description of the fossil turtles from the Arlington Archosaur Site (AAS) of the Woodbine Fm (Figure 1).
MATERIALS AND METHODS
Fossil specimens were collected and prepared by researchers, staff, and volunteers of the Perot Museum of Nature and Science. Specimens were measured with 6” Mitutoyo Absolute Digimatic calipers to the nearest 0.01 mm and rounded to the nearest 0.1 mm. Some distances and angles were measured from high quality digital photographs using ImageJ (Rasband, 1997-2018). A phylogenetic analysis was conducted by coding the AAS baenid for the characters present in the matrix from Lyson et al. (2016). Using TNT 1.5 (Goboloff et al., 2016), we used the Traditional Search algorithm using 1000 Wagner tree replicates and a TBR cycle. A 50% majority rule consensus tree was used to visualize the consensus topology among the minimum length trees. We apply the taxonomic scheme of turtles presented by Joyce (2007, 2017) unless otherwise specified. Also, family ranks are not used in accordance with PhyloCode guidelines (e.g., Laurin et al., 2005).
Abbreviations. DMNH = Perot Museum of Nature and Science (formerly the Dallas Museum of Paleontology), Dallas, Texas, USA; Ab = abdominal scute; An = anal scute; c = costal; Ce = cervical scute; ent = entoplastron; epi = epiplastron; Ex = extragular scute; Fe = femoral scute; Gu = gular scute; Hu = humeral scute; hyo = hyoplastron; hypo = hypoplastron; Im = inframarginal scute; n = neural; nu = nuchal; p = peripheral; Pe =pectoral scute; Pl = pleural scute; mes = mesoplatron; Ma = marginal scute; Ve = Vertebral scute; xi = xiphiplastron.
GEOLOGICAL SETTING
The four lithologic units designated by Dodge (1969), from oldest to youngest in the Woodbine are the Rush Creek, Dexter, Lewisville, and Arlington Members [Mbrs] (Main, 2005; Adams et al., 2017) (Figure 1.1). The Arlington Archosaur Site (AAS) is deposited in the lower to middle strata of the Lewisville Mbr. The AAS represents a diverse coastal community that inhabited a lower delta plain created during a regression of the southeastern margin of the Western Interior Seaway (WIS) (Figure 1.4) (Stephenson and Stenzel, 1952; Oliver, 1971; Main, 2005; Adams and Carr, 2010). The fossil-bearing exposures are organically rich and contain abundant arenaceous foraminifers, mollusks, and ammonites (Figure 1.3) (Kennedy and Cobban, 1990; Trudel, 1994; Noto, 2015). Strata are dominated by blue-gray to black, finely laminated to massive-bedded mud and shale interspersed with glauconitic sandstone lenses (Figure 1.3) (Oliver, 1971; Trudel, 1994; Main, 2005; Noto, 2015). The basal sandstone and lignite deposits recognized by Berquist (1949) are also now included in the Lewisville Mbr (Dodge, 1969; Trudel, 1994; Main, 2005).
Facies A comprises the primary and most productive fossil quarry at the AAS, and has yielded the specimens presented here and elsewhere (Figure 1.3; Adams et al., 2011, 2017; Noto et al., 2012, 2019). The geology and stratigraphy of the Woodbine Fm have been described recently for a diverse crocodyliform fauna (see Adams et al., 2017 and Noto et al., 2019). Sedimentological, paleobotanical, and microvertebrate evidence indicates the AAS was an intermittently inundated delta plain environment (Bennett et al., 2011). Collection efforts involved quarrying crocodilians and the ornithopod dinosaur ?Protohadros. The AAS preserves, in ascending order: a peat bed representing a low growth understory; a vertebrate-rich mudstone; a paleosol (histic gleysol) with a heavily rooted zone containing calcareous concretions associated with burned logs and tree stumps, indicating seasonal dryness and wildfires; and a fossil rich siderite sand transgressive lag (Bennett et al., 2011). Additional turtle material is currently being prepared at the Perot Museum and will be described in future work. Numerous crocodilian teeth (Woodbinesuchus sp.) are present throughout the section and are common in the basal peat layer. Ornithopod teeth attributed to ?Protohadros are present in the bone producing mudstone-paleosol horizon, but are rare in the basal peat bed and overlying lag deposits. Intensive surface collecting and screen washing began in 2009, revealing a diverse microfauna, including chondrichthyans (an indeterminant hybodont, Cretodus, Pseudohypolophus, and Onchopristis), the semionotiform Lepidotes, an indeterminate pycnodont, the tetraodontiform Stephanodus, and the lungfish Ceratodus carteri (Bennett et al., 2011; Main et al., 2011).
SYSTEMATIC PALAEONTOLOGY
TRINITICHELYS Gaffney, 1972
Type species. Trinitichelys hiatti Gaffney, 1972.
Remarks. For a recent review of baenid phylogeny, please refer to Joyce and Lyson (2015) and Lyson et al. (2016).
“Trinitichelys” maini sp. nov.
zoobank.org/2EC76641-C480-40F5-9CE0-EE8A5A0AAC2F
v. 2012 Noto, Main, and Drumheller, figs. 2, 4A.
 Holotype. DMNH 2013-07-0712, an anterior lobe of a plastron (Figure 3.1-4). This specimen was selected as the type because it shows diagnostic characters distinct from the known morphology of the basal baenids T. hiatti and Neurankylus. All additional baenid material from the AAS is referred to “T.” maini sp. nov. based on similarity in shell sculpturing and associated co-ossification.
Holotype. DMNH 2013-07-0712, an anterior lobe of a plastron (Figure 3.1-4). This specimen was selected as the type because it shows diagnostic characters distinct from the known morphology of the basal baenids T. hiatti and Neurankylus. All additional baenid material from the AAS is referred to “T.” maini sp. nov. based on similarity in shell sculpturing and associated co-ossification.
Etymology. In honor of the late Dr. Derek Main, for his vision and dedicated study and management of the AAS fossil collections.
Type strata and locality. Upper Cretaceous (Cenomanian) Woodbine Formation (Figure 1). The Arlington Archosaur Site, city of Arlington, Tarrant County, Texas. Exact locality data are on file at the Perot Museum of Nature and Science, Dallas, Texas.
Referred Material. DMNH 2013-07-0784, co-ossified partial anterior carapace and associated co-ossified posterior carapace (Figure 2.1-2, 2.4-6); DMNH 2013-07-0499, isolated p1 (Figure 2.3A-H); DMNH 2013-07-1711, an isolated nu (Figure 2.3E-H); DMNH 2013-07-0696 and -0704, left co-ossified bridge series; DMNH 2013-07-0873, right co-ossified bridge series (Figure 3.9-12); DMNH 2013-07-1703, isolated mes (Figure 3.13-17); DMNH 2013-07-1717, isolated xi (cf. juvenile) (Figure 3.18-3.21).
Diagnosis. Basal baenid, as indicated by similarly-sized Gu and Ex, a mostly co-ossified shell, and robust bridge peripherals. Tentatively included in “Trinitichelys” based on shared paired intergular scutes, absent fenestra, and a rectangular cervical scute. Medial contact of Ex differentiates this taxon from T. hiatti and Neurankylus. This character alone differentiates the AAS baenid from the limited material known for T. hiatti. However, additional shell, cranial, and postcranial material is currently being prepared and new diagnostic characters will warrant either the erection of a new genus or more definitive inclusion in Trinitichelys. “Trinitichelys” maini sp. nov. is thus placed in “Trinitichelys” as a derived turtle relative to T. hiatti, but lacks the set of derived characters that diagnose Baenodda. No midline contact of the Ex is the derived condition found in Plesiobaena, Palatobaena, Stygiochelys, Baena, and Chisternon. Ex-Gu sulci curved, unlike Dinochelys, T. hiatti, N. baueri, and N. lithographicus. It is also diagnosed by its lack of epiplastral processes and a single undivided Ce that is taller than wide, as in T. hiatti, N. eximius, and N. notos. Fenestrae absent, as in T. hiatti, N. eximius, Boremys, and P. brinkman, but unlike Plesiobaena antiqua and Palatobaena cohen (Joyce and Lyson, 2015). It has a femoro-anal sulcus that is relatively straight, rather than omega-shaped, which is found in more derived baenodds (Joyce and Lyson, 2015; Lyson et al., 2016).
Description
Carapace (Figure 2). DMNH 2013-07-0784 is a co-ossified partial anterior carapace (Figure 2.1-2, 4-7). The dorsal side of the specimen is badly contorted and crushed, and the left anterior carapace is collapsed ventrally. The entire surface of the carapace is covered in a dimpled pattern of shallow depressions (~2 mm diameter) with rounded, irregular polygons between them. Sulci are thin (<1 mm) and slightly deeper than the surface indentations (Figure 2.1, 4). The Ce is wider than long, and its lateral edges converge anteriorly. Additional isolated specimens are described below to clarify some scute and bone arrangements that are obscured by co-ossification in DMNH 2013-07-0784 (Figure 2.1-2, 4-6).
DMNH 2013-07-0499 is a left p1 with a generally thin, flat cross-section typical of the posterior shell (Figure 2.3A-D). The dorsal surface is ornamented with irregular, vermicular grooves between low, parallel ridges dorsally, and a fine network of short interwoven grooves ventrally. The dorsal grooves are mostly parallel with the intercostal sutures within a single scute, and transition to become parallel with the free peripheral edges (Figure 2.1-2, Figure 3E-F). Inter- and pleuro-marginal sulci divide the dorsal surface unequally between Ma5-6 and Pl1-2 (Figure 2.3B). The sulci are thin (≤1 mm), deeper than the sinuous netting of dorsal grooves, and do not have raised edges. From a ventral view, the bone is thickest in a wedge-shaped portion of the peripheral, which narrows to a point anteriorly (Figure 2.3C-D). The surface texture and intermarginal sulcus wrap around the free edge onto the bone’s ventral aspect (Figure 2.3C-D).
DMNH 2013-07-1711 is a tectangular nu whose anterior and posterior edges have a similar width, and a rounded free margin (Figure 2.3E-H). The bone’s posterior margin is comprised of sutural crenulations, but lacks the distinctive costiform processes of kinosternoids or chelydrids. Its anterior edge is narrower than the posterior, and a vertebro-marginal sulcus travels posterolaterally from the rear corners of the Ce. Nuchals from several individuals of “T.” maini sp. nov. demonstrate slight variation, but all have Ce that are rectangular, wider than long, and wider posteriorly than anteriorly (Figure 2.3E-F). Sulci are thinner than 1 mm, flat on the bottom, and the edges are not raised (Figure 2.3E-F). The sculpture of the dorsal surface is composed of parallel longitudinal striations in its posterior half. The anterior half is covered by an irregular arrangement of small indentations between rounded crenulations (Figure 2.3E-F). The ventral surface is smooth and a thin hemispherical shelf of superficial bone forms the posterior border of the concavity at the midline (Figure 2.3G-H). The bone is thickest along a rounded, slightly upturned rim that is ~10 mm wide along the free margin. Cervical articulation with the ventral nu is lacking. The vertebral scutes are generally rectangular and wider than long; however, a complete Ve3 is not present. Pleural scutes are roughly equal in length and width.
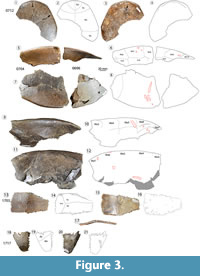 Plastron (Figure 3). DMNH 2013-07-0712 constitutes the type specimen for “Trintichelys” maini sp. nov., an anterior plastral lobe that includes the bilateral Gu and Ex, partial bilateral Hu, and partial Pe (Figure 3.1-4). It preserves the midline contact of the Ex, known from more derived baenodds, but not in T. hiatti. The plastral lobe has a similarly subtriangular shape in T. hiatti and “T.” maini sp. nov., unlike the squared profile of neurankylids (Gaffney, 1972; Joyce and Lyson, 2015; Lyson et al., 2016). The anterior border of the plastron is convex with a narrow gutter along the dorsal aspect of its anterior edge. All the bones of the anterior plastral lobe (epi, ento, and hyo) are co-ossified with no visible sutures, and the entire lobe is thin medially and posteriorly (Figure 3.1-4). The plate reaches a minimum thickness of ~3 mm at the midline. Ventral surface texture consists of short, curved, irregular grooves (Figure 3.1). Sulci are thin (~0.5 mm) and incised slightly deeper than the surrounding vascular grooves. The Hu-Ex sulci curve anterolaterally at 50º, and the curved Gu-Ex sulci project 10º anterolaterally from horizontal (Figure 3.1-2). The Pe-Hu sulcus is partially intact on the left, coursing laterally and slightly anteriorly from the midline toward the free edge of the plastron (Figure 3.1-2).
Plastron (Figure 3). DMNH 2013-07-0712 constitutes the type specimen for “Trintichelys” maini sp. nov., an anterior plastral lobe that includes the bilateral Gu and Ex, partial bilateral Hu, and partial Pe (Figure 3.1-4). It preserves the midline contact of the Ex, known from more derived baenodds, but not in T. hiatti. The plastral lobe has a similarly subtriangular shape in T. hiatti and “T.” maini sp. nov., unlike the squared profile of neurankylids (Gaffney, 1972; Joyce and Lyson, 2015; Lyson et al., 2016). The anterior border of the plastron is convex with a narrow gutter along the dorsal aspect of its anterior edge. All the bones of the anterior plastral lobe (epi, ento, and hyo) are co-ossified with no visible sutures, and the entire lobe is thin medially and posteriorly (Figure 3.1-4). The plate reaches a minimum thickness of ~3 mm at the midline. Ventral surface texture consists of short, curved, irregular grooves (Figure 3.1). Sulci are thin (~0.5 mm) and incised slightly deeper than the surrounding vascular grooves. The Hu-Ex sulci curve anterolaterally at 50º, and the curved Gu-Ex sulci project 10º anterolaterally from horizontal (Figure 3.1-2). The Pe-Hu sulcus is partially intact on the left, coursing laterally and slightly anteriorly from the midline toward the free edge of the plastron (Figure 3.1-2).
DMNH 2013-07-0704 and DMNH 2013-07-0696 are two continuous elements from a partial left bridge, including portions of Ma3-8 and all four Im (Figure 3.5-3.6). The two elements have a gap between them, and the bone is significantly collapsed medially, especially away from the heavy, thick shell periphery. The shell margin is rounded and not uniformly curved, and becomes more angular near the posterior edges of Ma4 and 7 (Figure 3.5-6). Dorsal surface sculpture is a dense pattern of thin overlapping vermiform grooves. The marginal-intermarginal and interpleural sulci are thin and have no raised edges. The lateral edge of the bridge is rounded, transitioning from thick and obtuse anteriorly (~70º from horizontal) to thin and acute posteriorly (Figure 3.5-6). The bone surface has clear evidence of bite marks and tooth scores (Figure 3.5-6) (Noto et al., 2012). Another specimen, DMNH 2013-07-0783 (Figure 3.9-12), retains an additional portion of the bridge which preserves Ma3-7.
DMNH 2013-07-1703 is a right Mes that is trapezoidal in shape and reaches a maximum length of 67.3 mm and width of 118.3 mm (Figure 3.13-17). An intersection of dorsal sulci is located just medial to a longitudinal break where the plate bends ventrally (Figure 3.17). Sulci separate the dorsal surface between the Pe, Ab, and Im2-3 (Figure 3.13-14). The sulcus between Pe and Ab is slightly curved along the medial third of the proximal third of the dorsal surface (Figure 3.13-14). Sulci are approximately 1 mm wide and flat at the edges, where they are incised through orthogonal ridges along the anterior and posterior Ic sutures. Small channels divide the center of the dorsal surface into an increasingly short and compact mesh of raised, rounded polygons (Figure 3.13). The dorsal surface is quite smooth in comparison, and the only non-taphonomic marks are associated with scattered patterns of foramina directly opposite the most compact portion of ventral sculpture (Figure 3.13). Very fine striations converge toward the same nexus at the plane break described above (Figure 3.17). The flat, medial suture suggests two mesoplastra that share a median contact.
DMNH 2013-07-1708 is a complete right xi that is fractured posterior to the pubic scar (Figure 3.18-21). Coarsely serrated edges contribute to sutures with the hyo anteriorly and the left xi medially (Figure 3.18-21). The prominence of the sutures, lack of co-ossification, and small size suggest that the xiphiplastron (DMNH 2013-07-1708) belonged to a juvenile. The Fe-An sulcus is thin (~1 mm wide) and crosses the anterior quarter of the bone’s ventral surface obliquely (Figure 3.18). The suture is relatively straight, and does not cross the hypo-xi suture, unlike the transgressive, omega-shaped sulcus in derived Baenodda (Joyce and Lyson, 2015; Lyson et al., 2016). Ventral surface ornamentation forms a reticulated pattern of subparallel, overlapping grooves, each approximately 10 mm long. Additional striations run perpendicular to the bone’s medial and anterior edges in bands and overlap to form a network of short, gently sinuous grooves. These surround irregular, rhomboidal tubercles that become smaller and increasingly compact near the plate’s lateral boundary. Nearly all the ventral surface striations converge just posterior to the Fe-An scute (Figure 3.18). This area corresponds to a ventral process on the dorsal xiphiplastron that is associated with a pelvic connection, where another convergence of faint striations occurs across the otherwise smooth surface (Figure 3.20). The ventral prominence forms a raised, posteriorly-directed triangle 22.4 mm long, 5.2 mm wide. This process is associated with a pelvic articulation, and is much reduced compared to the homologous structure in Pleurodira. This indicates a less rigid adherence of the axial and appendicular bones in Baenidae than Pleurodira certainly, but also a reduction compared to DMNH 2013-07-1717, Naomichelys sp. Otherwise, the xiphiplastron has a subtle V-shaped anal notch.
Remarks. Baenidae is endemic to North America and became distinct from other Jurassic North American turtles (i.e., pleurosternids) in the Lower Cretaceous. Baenids remained restricted to North America, where they reached peak diversity after the Cenomanian, primarily along the eastern coast of Laramidia (Hutchison and Archibald, 1986; Hirayama et al., 2000; Hutchison, 2000; Joyce and Lyson, 2015; Joyce et al., 2016b; Lyson et al., 2016; Smith et al., 2017; Avrahami et al., 2018). Baenids are closely related to other primitive freshwater paracryptodires (i.e., Pleurosternidae, Compsemydidae) that ranged across Laurasia beginning in the Jurassic (Pérez-García et al., 2015; Joyce et al., 2016b). Heavy shells with frequent co-ossification and a preference for riverine habitats all contribute to a robust baenid fossil record (Hutchison, 1984; Lyson and Joyce, 2009; Joyce and Lyson, 2015; Lyson et al., 2016). Specifically, fine- to coarse-grained channel sandstone accounts for nearly all deposits from which relatively complete (≥50% of the shell) baenid shells have been recovered (Hutchison, 1980; Hutchison, 1984; Hutchison and Archibald, 1986). Baenids typically have moderately-domed hydrodynamic shells that form a teardrop shape in lateral view and a relatively large plastron which protects much of the shell from below (Joyce and Lyson, 2015). This study considers the new baenid in the context of known riverine taxa (Hutchison and Archibald, 1986; Lyson et al., 2016).
Baenids are conspicuously absent from Arctic regions (West and Dawson, 1977; Brinkman and Tarduno, 2005), suggesting that distribution may have been constrained by lower temperatures (Joyce and Lyson, 2015). Thus, baenids did not utilize Arctic routes for intercontinental dispersal during the Cretaceous and Paleogene, unlike several other turtle families (Joyce and Lyson, 2015; Joyce et al., 2016b). The Western Interior Seaway [WIS] also limited baenid distribution by dividing North America into eastern Appalachian and western Laramidian components (Joyce and Lyson, 2015). A previously described hiatus of recovered fossil baenids from Cenomanian to Santonian deposits (Joyce and Lyson, 2015; Lyson et al., 2016) may be explained by recurring cyclothems (alternating transgressive/regressive phases) of the WIS. Pre-Woodbine deposits have been substantially eroded east of the Comanche Shelf (see previous work by Scott, 2003, 2016). Vast river drainages in southern Appalachia after the Cenomanian have also significantly obscured many older terrestrial strata via extensive erosion (Schwimmer et al., 1993; Cumbaa et al., 2010). In any case, the dinosaur fossil record indicates that Appalachia was dominated by relict forms isolated by the WIS, and the evolution of Appalachian forms is poorly understood (Brownstein, 2018). Evolutionary relationships of Laramidian and Appalachian baenids are mostly unresolved, but baenids only became diverse in Laramidia. The Woodbine Fm represents the earliest known Upper Cretaceous faunas from Appalachia (Adams et al., 2017; Noto et al., 2019). “T”. maini is currently the only known Late Cretaceous baenid from Appalachia. Additional shell, cranial, and postcranial material is currently being prepared and will likely provide important additional information on baenid turtles in the middle of the Cretaceous.
TESTUDINATA Klein, 1760
HELOCHELYDRIDAE Nopska, 1928
NAOMICHELYS Hay, 1908
Naomichelys sp.
v. 2012 Noto, Main, and Drumheller, figs.4B-E, 6.
Type species. Naomichelys speciosa Hay, 1908. Definite or tentatively helochelydrids have been widely referred from abundant fragmentary material, largely identified solely based on texture. Please see a recent review of the clade by Joyce (2017).
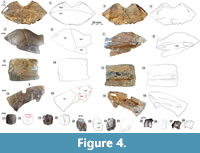 Material. DMNH 2013-07-1715, nu (Figure 4.1-8); DMNH 2013-07-0530, left c1 (Figure 4.5-4.8); DMNH 2013-07-0543, partial left c3 (Figure 4.9-4.12); DMNH 2013-07-0559, left c7 (Figure 4.13-16); DMNH 2013-07-0706, cf. n4 (Figure 4.17-20); DMNH 2013-07-0770, right partial n7/8 (Figure 4.21-24); DMNH 2013-07-0570, pygal (Figure 4.25-28); DMNH 2013-07-0711, right hyoplastron and Im 2 (Figure 5.1-4); DMNH 2013-07-0511, left hyoplastron (Figure 5.5-8); DMNH 2013-07-1717, right xiphiplastron (Figure 5.9-12); DMNH 2013-07-0603, partial mesoplastron (Figure 5.13-16).
Material. DMNH 2013-07-1715, nu (Figure 4.1-8); DMNH 2013-07-0530, left c1 (Figure 4.5-4.8); DMNH 2013-07-0543, partial left c3 (Figure 4.9-4.12); DMNH 2013-07-0559, left c7 (Figure 4.13-16); DMNH 2013-07-0706, cf. n4 (Figure 4.17-20); DMNH 2013-07-0770, right partial n7/8 (Figure 4.21-24); DMNH 2013-07-0570, pygal (Figure 4.25-28); DMNH 2013-07-0711, right hyoplastron and Im 2 (Figure 5.1-4); DMNH 2013-07-0511, left hyoplastron (Figure 5.5-8); DMNH 2013-07-1717, right xiphiplastron (Figure 5.9-12); DMNH 2013-07-0603, partial mesoplastron (Figure 5.13-16).
Description
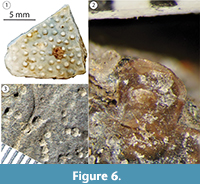
Carapace (Figure 4). DMNH 2013-07-1715 is a complete nu that has complex fractures near the midline (Figure 4.1-4). Its thickness decreases sharply from 30.0 mm at the free anterior edge to 6.8 mm at the posterior midline. The width of the nu is approximately twice the length, and there is no clear definition of the Ce (Figure 4.1). The visceral surface near the midline has no indication of cervical articulation. The external carapace surface is covered with the remnants of a repeating pattern of pustules. When broken, these leave shallow, sometimes coalesced circular pits with variable spacing (Figure 6) (Joyce et al., 2014). The free edge of the nu is rounded cranially and deeply concave (~20 mm), with a raised, 11.1 mm wide gutter along its dorsal free edge (Figure 4.1-4). The remaining edges have large, distinct peg-like articulations with p1 laterally, c1 posterolaterally, and n1 posteriorly. Differs from Helochelys danubina by having a deeper nu emargination and a shallow Ce. There is a dorsal sulcus between Ma1 and Ve1, which bifurcates at each lateral end to meet the bone on either side of the lateralmost points. The lateral articular surface shared with p1 is folded posteriorly onto itself and angled medially to form a wedge-shaped ledge set between the dorsal and ventral portions of the suture. There is a shallow (~10 mm wide) channel that travels toward the midline along the rounded anterior free edge. This is associated with a fused nu/cleithra complex that is apparent in some stem turtles (Figure 4.3-4) (Lyson et al., 2013; Joyce et al., 2014), rather than the more robust costiform processes known in some taxa.
DMNH 2013-07-0530 is a left c1 that includes sulci between Ve1-2, and between the vertebral scutes and p11 laterally (Figure 4.5-8). The sulci are thin and deeply impressed with raised edges. There is no evidence of the supernumerary scute described in N. speciosa (Joyce et al., 2014). However, the dorsal surface is covered with small, regularly-spaced pits that correspond to the surface of N. speciosa with mostly dislocated tubercles (Joyce et al., 2014). Near its anterior and posterior sutures, there is a series of fine parallel striations perpendicular to the sutures. There are stacked terraces of bone, which are raised along growth margins, as in N. speciosa (Joyce et al., 2014). C1 articulates medially with n1, the nu anteromedially, c2 posteriorly, and p1-3 anteriorly and laterally (Figure 4.5). The raised and flattened second thoracic rib (tr 2) protrudes from the visceral surface and reaches a length 22.0 mm. The rib head is broken posteriorly, and its medial end curves ventrally. The rib widens into a flat articular surface as it extends past a set of parallel ventral ripples (Figure 4.7).
DMNH 2013-07-0543 is the lateral half of the left c3 (Figure 4.9-12). It is broken lateral to the intersection of the costo-vertebral and intervertebral sulci. A rib end protrudes slightly beyond the thinner lateral edge of the bone, and the dorsal surface is terraced approaching the edge. The dorsal surface pits are irregularly spaced and are more numerous and distinct laterally (Figure 4.9). A raised, flattened rib travels along the middle of the ventral surface of the bone, reaching 29.5 mm wide, with a missing distal tip (Figure 4.11).
DMNH 2013-07-0559 is a left c7 that is missing a quadrangular portion of its anterior edge (Figure 4.13-16). It is nearly uniform in width (~50 mm) but widens laterally and has a rounded posteromedial projection (Figure 4.13-16). The flattened rib occupies much of the ventral surface, reaching 25.0 mm wide, and protrudes ~5 mm past the posterior margin.
The rib head is small and broken flush with the bone surface (Figure 4.15-16). There is a rough, semielliptical concavity just posterior to it. In general, dorsal sulci are less than 1 mm thick, and the pitted ornamentation is smaller and more closely arranged along the medial and lateral edges. There are bands of short, parallel striations near the midline, and at least three shallow elliptical troughs are located on the dorsal surface (Figure 4.13-14). Similar marks were interpreted as scores from crocodyliform teeth, as described above (Noto et al., 2012). Most AAS turtle specimens show multiple tooth marks, which may occur singularly or serially (Noto et al., 2012). Of the numerous fossils collected at the AAS, only a relatively small proportion of turtle shell elements surveyed had definitive crocodyliform tooth marks, and further study may elucidate detailed faunal relationships.
DMNH 2013-07-0706 is a rounded, octagonal neural (Figure 4.17-20). It has a single scar that articulated with the vertebral arch of a corresponding vertebra (Figure 4.19-20). It is most likely a fourth neural based on the absence of a transverse intervertebral sulcus and a generally rounded rectangular shape and relatively small size (Joyce et al., 2014). Naomichelys speciosa has a low keel on the dorsal surface of n2, and from n5 through the n7/8 (Joyce et al., 2014). However, a keel is absent in the AAS Naomichelys material, and only fine short ridges along and between parallel rows of tiny pits. A cluster of shallow concavities approximately 20 mm wide and 10 mm long is along the bone’s anterior edge (Figure 4.18). These crushed excavations are interpreted as crocodyliform tooth marks, whose round and smooth edges are distinct from other predators (Njau and Blumenschine, 2006; Noto et al., 2012).
DMNH 2013-07-0770 is the medial portion of n7/8 (Figure 4.21-24). It forms an irregularly shaped polyhedron that is longer than wide, becoming thicker medially to a maximum of 28.5 mm. The edges are broken except for a moderately serrated posterior edge, and a pair of 8-10 mm diameter elliptical sockets is located along the posterior face (Figure 4.23-24). These may have been sockets that received corresponding articular pegs from c8. The broken edge of the fused neurals reveals a dense exterior cortical layer approximately 3-4 mm thick and a generally subequal internal cortical surface (Figure 4.21-22). The sulcus between Ve4-5 is uniform, has no raised edges, and is approximately 1 mm thick. The sulcus is constrained to c7, differing from N. speciosa, in which the sulcus traverses and returns across the suture articulating c7-8. It curves gently across the dorsal surface, traveling between its posterolateral and anteromedial corners (Figure 4.21-22). The dorsal ornamentation forms a “chainlink” arrangement of densely arranged elliptical pits 1-2 mm in diameter, which are separated by thin, rounded ridges (Figure 4.21). Pits are most distinct along a band parallel to the sulcus, but a similar, less distinct texture is present near the posterolateral corner of the ventral bone surface. The ventral surface is convex and the bone becomes thicker in a broad, longitudinal elevation that gives way to a medial channel that is 7.5 mm wide (Figure 4.23-24). In this region, longitudinal midline struts provide structural support to a typically robust, differentiated posterior carapace in N. speciosa and are also seen here (Joyce et al., 2014).
DMNH 2013-07-0570 is a pygal whose broken edges are shared with c8 anteriorly and p1 laterally (Figure 4.25-28). Its dorsal surface bulges on each side of flat-bottomed midline sulcus (Figure 4.25-26). As described above, the dorsal shell surface is covered in small, circular uniformly spaced pits. Two bony protuberances extend from a shared origin on the anterior ventral surface. Each protrudes posteriorly and is approximately 5 mm wide (Figure 4.25-26).
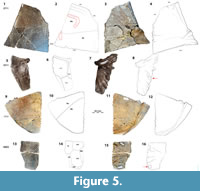 Plastron (Figure 5). DMNH 2013-07-0711 is a partial right hyo that is divided into Hu and Pe, with a short sulcus that separates Im2 at the posterolateral corner of the plate (Figure 5.1-4). A central plastral fontanelle is absent, as in H. nopscai, but unlike N. speciosa and Solemys spp. It shares serrated edges with the entoplastron medially, the epiplastron anteriorly, and the mes posteriorly (Figure 5.1-2). The Hu-Pe sulcus crosses the bone transversely on its dorsal surface, and its edges are flat. The small dorsal surface pits are generally arranged into rows that are parallel to the midline. Pits become smaller and shallower laterally, and the dorsal surface of the carapace is elevated in concentric terraces near the lateral extent of the Hu-Pe sulcus, anterior to the axillary buttress (Figure 5.1-2). Rod-like projections extend medially and anteriorly from the ventral surface, and a thickened gutter runs along the lateral free edge, reaching 25.7 mm wide. The free margin is acute and rounded, and most sutural edges are coarsely serrated (Figure 5.1-4). The dorsal surface of the hyo of this specimen is scored by crocodyliform teeth, both in bisected and hooked forms on the ventral surface lateral and also anterior to the axillary buttress (Figure 5.1-2) (see Noto et al., 2012). Significantly hooked scores may be diagnostic of inertial feeding behavior, and bisected scores are often associated with crushed or distorted underlying bone (Njau and Blumenschine, 2006; D’Amore and Blumenschine, 2009; Noto et al., 2012).
Plastron (Figure 5). DMNH 2013-07-0711 is a partial right hyo that is divided into Hu and Pe, with a short sulcus that separates Im2 at the posterolateral corner of the plate (Figure 5.1-4). A central plastral fontanelle is absent, as in H. nopscai, but unlike N. speciosa and Solemys spp. It shares serrated edges with the entoplastron medially, the epiplastron anteriorly, and the mes posteriorly (Figure 5.1-2). The Hu-Pe sulcus crosses the bone transversely on its dorsal surface, and its edges are flat. The small dorsal surface pits are generally arranged into rows that are parallel to the midline. Pits become smaller and shallower laterally, and the dorsal surface of the carapace is elevated in concentric terraces near the lateral extent of the Hu-Pe sulcus, anterior to the axillary buttress (Figure 5.1-2). Rod-like projections extend medially and anteriorly from the ventral surface, and a thickened gutter runs along the lateral free edge, reaching 25.7 mm wide. The free margin is acute and rounded, and most sutural edges are coarsely serrated (Figure 5.1-4). The dorsal surface of the hyo of this specimen is scored by crocodyliform teeth, both in bisected and hooked forms on the ventral surface lateral and also anterior to the axillary buttress (Figure 5.1-2) (see Noto et al., 2012). Significantly hooked scores may be diagnostic of inertial feeding behavior, and bisected scores are often associated with crushed or distorted underlying bone (Njau and Blumenschine, 2006; D’Amore and Blumenschine, 2009; Noto et al., 2012).
DMNH 2013-07-0511 is an anterior portion of the left hyo with Im series immediately posterior to the axillary buttress (Figure 5.5-8). The plate is widest proximally and gradually tapers laterally and ventrally from the midline (Figure 5.5-8). Sulci and ornamentation cover the shell surface as a dense web of curved, interwoven channels, which are interspersed with variable arrangements of small pits. The sulcus edges are beveled, elevated, and distinct from the sculpture pattern. Sulci define the border between Ma10-11, and between each of these and Ve4 (Figure 5.5-8). This bone is generally thick and flat but becomes robust and cylindrical at the midline. It decreases from approximately 15 mm thick medially to 8 mm laterally. Eight rod-like projections radiate anterolaterally from a thick, common area along the posteromedial aspect of the visceral surface (Figure 5.5-6). Each is ~8 mm wide, and the remainder are each approximately a third as wide. There is a smooth knob-like process (~15 mm diameter) near the middle of the element, among a set of finger-like projections that diverge from a common point on the internal surface of the bone (Figure 5.5-6). The process occurs along a significant decrease in plate thickness that is only apparent on the visceral surface. There is a similar process on the visceral surface of a partial mesoplastron (DMNH 2013-07-0603), and both are located near buttresses (see Joyce et al., 2014: figure 8.2). Typically, this area is dominated by attachment of the lateral portion of the pectoralis muscle, which varies considerably in extent and organization among turtles (Zhu, 2011).
DMNH 2013-07-1717 is a subtriangular right xi with coarsely serrated anterior and medial edges (Figure 5.9-12). Its thickness increases from 6.3 mm at the midline to 20.9 mm posteriorly and 20.5 mm laterally. Ventral surface pit size decreases and becomes shallower, forming compact rows approaching the posterolateral free margin (Figure 5.9). There is a flattened cylindrical gutter that wraps dorsally around the lateral and posterior free edges. It becomes thinner in its middle, and its cross-section is generally compressed and cylindrical. The maximum width of the gutter is 34.7 mm at the hyp-xi suture, and it is ornamented with a sculpture similar to that described above (Figure 5.11-12). Otherwise, the remainder of the plate’s ventral surface is relatively smooth (Figure 5.11). The Fe-An sulcus crosses the ventral surface of the xi posterolaterally at approximately 55º from the midline (Figure 5.9-10). No anal notch or central plastral fontanelle is present in the AAS Naomichelys, unlike N. speciosa (Figure 5.9-12) (Joyce et al., 2014). The AAS xi has a straighter lateral edge and is more curved than in N. speciosa (Joyce et al., 2014). The width of the posterior lobe of the plastron (DMNH 2013-07-1717) (Figure 5.9-12) is estimated to be approximately the same size (304 mm) as N. speciosa (FMNH PR273) (310 mm) (Joyce et al., 2014).
 DMNH 2013-07-0603 is a partial mes (Figure 5.13-16). The two pieces form a flat, bony plate that includes the posterior portion of Im3, and posterior and anterior parts of Im2 and 4, respectively (Figure 5.14). Narrow wedges of marginal scutes are located at the broken lateral margin, and the Pe-Ab sulcus extends medially from Im3. Sulci are deep, less than 1 mm wide, and have flat bottoms. The ventral surface has a pattern of small, irregular elliptical pits which frequently coalesce to form short serpentine channels (~3 mm long). Faint, parallel, longitudinal striations form a band along the anterior margin of the hyo (Figure 5.13). As described above in the hyoplastron (DMNH 2013-07-0711), four rod-shaped forms radiate from a common point on the medial aspect of the dorsal surface. The plate is fractured transversely through their origin, approximately between the second and third projections. The bone immediately around the origin is 5.7 mm thicker than at the ends (Figure 5.15). The posteriormost projection is approximately a third the width of the others, and there is an elliptical, raised knob (~12.5 mm diameter) in the posterolateral corner of the plate (Figure 5.15-16). Additionally, Im3 is generally asymmetrical and hexagonal. Its maximum length is 76.8 mm, maximum width is 67.3 mm, and it is consistently ~9.2 mm thick.
DMNH 2013-07-0603 is a partial mes (Figure 5.13-16). The two pieces form a flat, bony plate that includes the posterior portion of Im3, and posterior and anterior parts of Im2 and 4, respectively (Figure 5.14). Narrow wedges of marginal scutes are located at the broken lateral margin, and the Pe-Ab sulcus extends medially from Im3. Sulci are deep, less than 1 mm wide, and have flat bottoms. The ventral surface has a pattern of small, irregular elliptical pits which frequently coalesce to form short serpentine channels (~3 mm long). Faint, parallel, longitudinal striations form a band along the anterior margin of the hyo (Figure 5.13). As described above in the hyoplastron (DMNH 2013-07-0711), four rod-shaped forms radiate from a common point on the medial aspect of the dorsal surface. The plate is fractured transversely through their origin, approximately between the second and third projections. The bone immediately around the origin is 5.7 mm thicker than at the ends (Figure 5.15). The posteriormost projection is approximately a third the width of the others, and there is an elliptical, raised knob (~12.5 mm diameter) in the posterolateral corner of the plate (Figure 5.15-16). Additionally, Im3 is generally asymmetrical and hexagonal. Its maximum length is 76.8 mm, maximum width is 67.3 mm, and it is consistently ~9.2 mm thick.
Remarks. Naomichelys is the only North American genus of Helochelydridae, a family of large-bodied stem cryptodires from the Upper Jurassic (Tithonian) to Upper Cretaceous (Maastrichtian) of Euramerica (Hirayama et al., 2000; Anquetin, 2012; Joyce et al., 2014). Other large stem turtles (including O. cunicularius, M. efremovi, and meiolaniids) evolved alongside crown-group turtles until the Pleistocene (Hirayama et al., 2000; Joyce, 2007; Anquetin, 2012; Scheyer et al., 2014). These turtles achieved global distribution during the Mesozoic and early Cenozoic, with representatives in North and South America, Asia, Australia, and likely Europe (Lapparent de Broin and Murelaga, 1996, 1999; Milner, 2004; Anquetin, 2012; Joyce et al., 2016b). The extensive range of stem turtles in North America makes this clade a vital part of turtle assemblages across Cretaceous North America (Anquetin, 2012). During the Jurassic and part of the Lower Cretaceous, North America was part of the faunal realm that includes Europe, and the geographic distribution of Helochelydridae overlaps with that of Paracryptodira (including Baenoidea) over their entire known history (Gaffney, 1975; Hutchison, 2000; Joyce, 2007; Joyce et al., 2016b). This pattern is confirmed at the AAS, where we report both a baenid (“T.” maini) and a helochelydrid (Naomichelys sp.). The helochelydrid material from the AAS differs most significantly in the shape and proportions of the xi in the type material (Joyce et al., 2014). However, a more detailed description is needed to describe possible ontogenetic and sexually dimorphic variation observed between the xi described here and the type material described in Joyce et al. (2014). If sexual dimorphism or ontogenetic variation is demonstrably ruled out, the material referred to Naomichelys sp. from the AAS could be considered for assignment to a new species. However, the fragmentary nature of the current material and lack of available comparative material precludes a new helochelydrid taxon at this time.
Pantrionychidae indet.
Figure 7.1-4
Material. DMNH 2013-07-1304, isolated left nu (Figure 7.1-4).
 Description. Small pantrionychid, indicated by absent sulci on the carapace, indicating carapace scutes are absent. DMNH 2013-07-1304 is an ensiform left partial nu broken near the midline (Figure 7.1-4). The dorsal ornamentation consists of a pattern of shallow circular and quadrangular pits that are typically 0.5-0.7 mm in diameter. Thin ridges surround each pit, and some adjacent pits have coalesced. The pits are smaller medially and anteriorly, and have a narrow gutter along the free edge of the bone. The posterior edge of the nu is slightly concave near the midline and may form the articulation with a preneural as in some trionychids (Joyce and Lyson, 2017). The ventral surface has metaplastic ossification obscuring fused cleithral portions of the nu (Figure 7.1-4; Lyson et al., 2013). The nu has only a slight bend near the midline, indicating a generally flat carapace typical of softshell turtles. Nuchal embayment is absent as in Aspideretoides foveatus, which also shares similar nu proportions of juveniles or subadults (Gardner et al., 1995; Li et al., 2015; Joyce et al., 2018). Referral beyond this level requires additional diagnostic, verifiably adult material, and may help clarify the evolutionary stem of the clade in North America.
Description. Small pantrionychid, indicated by absent sulci on the carapace, indicating carapace scutes are absent. DMNH 2013-07-1304 is an ensiform left partial nu broken near the midline (Figure 7.1-4). The dorsal ornamentation consists of a pattern of shallow circular and quadrangular pits that are typically 0.5-0.7 mm in diameter. Thin ridges surround each pit, and some adjacent pits have coalesced. The pits are smaller medially and anteriorly, and have a narrow gutter along the free edge of the bone. The posterior edge of the nu is slightly concave near the midline and may form the articulation with a preneural as in some trionychids (Joyce and Lyson, 2017). The ventral surface has metaplastic ossification obscuring fused cleithral portions of the nu (Figure 7.1-4; Lyson et al., 2013). The nu has only a slight bend near the midline, indicating a generally flat carapace typical of softshell turtles. Nuchal embayment is absent as in Aspideretoides foveatus, which also shares similar nu proportions of juveniles or subadults (Gardner et al., 1995; Li et al., 2015; Joyce et al., 2018). Referral beyond this level requires additional diagnostic, verifiably adult material, and may help clarify the evolutionary stem of the clade in North America.
Remarks. Soft-shelled turtles (Pan-Trionychidae) have a rich and diverse global fossil record from the Aptian to the Holocene (Joyce and Lyson, 2017). The systematics of North American fossil trionychids have been studied extensively, but remain largely unresolved (Webb, 1962; Dalrymple, 1977; Gardner and Russell, 1994; Hutchison and Holroyd, 2003; Joyce et al., 2004; Vitek and Joyce, 2015). Aspideretoides is the most commonly recognized North American trionychine genus of the Upper Cretaceous (Brinkman, 2003; Hutchison and Holroyd, 2003; Vitek and Joyce, 2015). Among fossil and extant trionychid forms, nu shapes vary extensively between taxa, ranging from relatively long, narrow, and subtriangular to short, wide, and lenticular (Gardner and Russell, 1994; Vitek and Joyce, 2015).
The limbs of North American fossil trionychids are nearly identical in morphology and proportion to modern species, so it is reasonable to presume that fossil trionychids were highly aquatic like their modern relatives (Hay, 1908; Vitek, 2011; Jasinski, 2013; Vitek and Joyce, 2015). All extant species are either carnivorous or omnivorous (Ernst and Barbour, 1989). Several fossil trionychid species have been recovered from near-shore marine strata, suggesting a limited ability to tolerate marine conditions (Weems, 1988, 2014; Kasparek, 2001; Vitek and Joyce, 2015). Their first remains in North America are isolated fragments from the Albian in Laramidia (Willow Tank and Cedar Mountain Fms) (Ostrom, 1970; Bonde et al., 2008). Another early North American trionychid was recovered from high latitude strata from the mid-Cenomanian Dunvegan Formation in Alberta (Bhattacharya and Walker, 1991; Brinkman et al., 2003). A small indeterminate trionychid at the AAS (Figure 7.1-4) indicates a rapid dispersal of (presumably stem) trionychids into southern waters of the WIS and proto-Gulf of Mexico (GOM) coastal environments. Aside from resolving taxonomic uncertainty, more trionychid material from the AAS may allow better understanding of relative paucity (as in pleurodiran material) compared with other cryptodires (Table 1). The AAS trionychid specimen is potentially a juvenile, and confirmed smaller body size or early ontogenetic stage would inform our assessment as to whether the AAS, as previously hypothesized, was a reptile nesting site or nursery in addition to clear indications that it was a crocodyliform feeding site (Noto et al., 2012).
Most known Cretaceous trionychid species have nuchals with a width at least four times wider than they are long (Hay, 1908; Gardner et al., 1995; Joyce et al., 2009; Vitek and Danilov, 2010; Vitek, 2012, 2011; Vitek and Joyce, 2015). The AAS trionychid nu is almost five times wider than long, and the dorsal texture is a netting of subcircular shallow pits with concave bottoms (Figure 7.1). A specific identification is not possible due to the paucity of material, but the AAS material most closely resembles Aspideretoides foveatus based on the general shape and proportions of the nu (Hay, 1908; Gardner et al., 1995). It is remarkable that only the single specimen described here is referable to Trionychidae out of hundreds of fossil turtle specimens collected from this site (Noto et al., 2012; Adams et al., 2017). This is perhaps due to significant competition from other aquatic carnivorous freshwater faunas, and/or taphonomic effects on small fragmentary elements (Ryan et al., 2001). Also, various small animals are known to have washed from coastal river deltas into marine or brackish basins during storms and floods (Joyce and Gauthier, 2004). Small trionychids are also known from near-shore marine strata where they were deposited as part of freshwater overflow onto brackish or marine waters (Joyce and Gauthier, 2004).
PLEURODIRA Cope, 1865
PELOMEDUSOIDES Broin, 1988
BOTHREMYDIDAE Baur, 1891
cf. Algorachelus sp.
Figure 7.5-8
Material. DMNH 2013-07-1405, isolated left c4 (Figure 7.5-8).
Description. DMNH 2013-07-1405 is a left c4 with finely serrated margins (Figure 7.5-8). The interpleural sulcus 2-3 follows the contour of the posterior Ic suture on the lateral two thirds of the dorsal surface. It splits medially to separate Pl2, Pl3, and V2. On approximately the lateral two-thirds of the dorsal surface, the sulcus between Pl2-3 follows the contour of the nearby posterior Ic suture. It splits to form sulci between Pl2, Pl3, and V2 (Figure 7.5-6). The lateral third of the ventral surface is significantly crumbled, and a gracile rib head emerges near the center of the medial quarter of the bone (Figure 7.7-8).
The isolated costal is referred to Pleurodira based on the texture of its dorsal surface, which has fine, winding channels, and no pits or protuberances, differing from the other taxa at the AAS (Table 1). Referred to Bothremydidae by inferred contact of the inguinal buttress with p5 (Joyce et al., 2016a). Damage described below on the ventral surface is similar to the pattern of disarticulation described in Algorachelus peregrinus (Pérez-García et al., 2017: figure 3D). The dorsal texture is similar to the finely incised, vermiculating striations described for A. tibert (Joyce et al., 2016a: figure 4) and the irregular grooves described in A. peregrinus (Pérez-García et al., 2017: figure 3C; Pérez-García, 2018). Further, bothremydids are the only pleurodires known to successfully disperse from Africa to North America in the Cenomanian (Joyce et al., 2016a; Pérez-García et al., 2017).
Remarks. Bothremydidae was a Gondwanan clade of side-necked turtles that inhabited a wide range of shallow waters associated with the Hispanic Corridor, during the fracturing of Pangaea in the Upper Jurassic (Joyce et al., 2016b; Pérez-García, 2016, 2018). The genus Algorachelus is now recognized in the early or middle Cenomanian in the Middle East (A. parvus), in the Iberian Peninsula in the middle to lowermost upper Cenomanian interval (A. peregrinus), and in North American Naturita Fm (uppermost Cenomanian) of Utah (A. tibert) (Joyce et al., 2016a; Pérez-García, 2016; Pérez-García et al., 2017; Pérez-García, 2018). Biogeographical and paleoecological implications for the AAS pleurodiran material are discussed below. However, the relative paucity of pleurodiran material is remarkable and unexplained for similar reasons as the trionychid material.
Panpleurodires were restricted to Gondwana until ~100─105 Ma when Africa and South America began to separate from the rest of Gondwana (e.g., Ferriera et al., 2018). Prior to this time, they inhabited primarily freshwater habitats. Pleurodires, both extinct and modern, are known to utilize the sandy coastal deposits of bars and channels, which have subtidal and intertidal events (Pérez-García et al., 2017; Pérez-García 2018a, b). The opening of the South Atlantic led to separation of populations, diversification including adaptations to marine environments, and dispersal into North America, Europe, and North Africa.
Most pleurodires were exclusively freshwater forms, but the adaptation of some bothremydids to brackish coastal environments facilitated the dispersal of several lineages (e.g., Algorachelus spp.; Lapparent de Broin and Werner, 1998, Rabi et al., 2012; Joyce et al., 2016a; Pérez-García, 2016; Pérez-García et al., 2017). Considering they already dominated many equatorial Gondwanan environments, shallow freshwater or brackish waters were available in coastal areas surrounding the Hispanic Corridor. For example, Algorachelus is by far the most abundant turtle known from fossil sites at Algora, constituting approximately 90% of fossil turtles (Pérez-García, 2018a, b). A large sample of complete shells even allowed for a detailed study of patterns of disarticulation, and shell scute variation (see Pérez-García, 2018b). Disarticulation begins in turtles with separation of the carapace and the plastron, and the disarticulation of individual costals (DMNH 2013-07-1405) occurs toward the end of the process (Pérez-García, 2018b). The bothremydid recovered from the AAS is part of the earliest dispersal event recognized for bothremydids of African origin. Prior to the current study, the genus Algorachelus was previously known from Laramidia, and the material presented here comprises the first definitive bothremydid from Appalachia. Cf. Algorachelus sp. was part of the earliest dispersal event recognized particularly for bothremydids from Africa (Pérez-García, 2018a, b). Bothremydid turtles reached the Atlantic shores of North America by at least the middle Cenomanian, the early Gulfian Woodbine Fm near the mouth of the WIS by the late middle Cenomanian, and the east coast of Laramidia, further into the WIS by the latest Cenomanian (Joyce et al., 2016a,b; Pérez-García et al., 2017). Algorachelus is the oldest panpleurodiran genus known from Laramidia, and the oldest North American panpleurodire known from North America (Joyce et al., 2016a). A. tibert is the only species known from Cenomanian deposits in North America and is a representative of Gondwanan crown pleurodires in Laurasian deposits (Pérez-García et al., 2017).
RESULTS
Four turtles are reported from the AAS, representing the clades Baenidae, Helochelydridae, Trionychidae, and Bothremydidae (Table 1). We conducted a phylogenetic analysis on the AAS material referred to “Trinitichelys” maini sp. nov. (Figure 2-Figure 3) using the matrix of Joyce and Lyson, 2015, as emended by Lyson et al., 2016. Our resulting coding was as follows:
“T.” maini. ???????????????????????????????????????0210200?10000?0000011?010?????
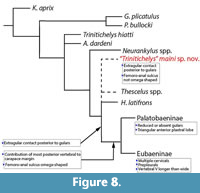 Our analysis produced 30 minimum trees of length 181 (Figure 8). In the 50% majority rule consensus tree, “T.” maini sp. nov. was retrieved in an unresolved polytomy with the genera Thescelus, Scabremys, and Hayemys, all of which fall as outgroups to the Eubaeninae and Palatobaeninae. This node was supported at 100% bootstrap values. In our analysis, S. ornata was retrieved as the sister group to eubaenids and palatobaenids (Figure 8). Our phylogenetic analysis recovers “T.” maini sp. nov. as more derived than T. hiatti, but without the full suite of known baenodd traits, and completely distinct from the genus Neurankylus. Though not a baenodd, “T.” maini sp. nov. shares several of the morphological traits of that clade. Like H. latifrons, Thescelus spp. (T. rapiens and T. insiliens), “T.” maini sp. nov. shares the synapomorphy of midline contact between Ex posterior to the Gu (Figure 3, Figure 8). Additionally, the new species shares primitive plastral traits with H. latifrons and Thescelus spp. including a Fe-An sulcus that is relatively straight, unlike the omega shape found in derived baenids (Figure 3, Figure 8; Joyce and Lyson, 2015; Lyson et al., 2016). Additionally, “T.” maini sp. nov. does not have the following synapomorphies characteristic of Baenodda: reduced or absent Gu, multiple Ce, prepleural scutes, or a Ve5 that is longer than wide (Figure 2). Significantly, “T.” maini sp. nov. also lacks the key defining feature of Baenodda, the contribution of the most posterior vertebral scute to the margin of the carapace (Figure 3, Figure 8) (Lyson and Joyce, 2015). Instead, as in Neurankylus spp. and T. hiatti, the posterior marginals are located between Ve5 and the carapace margin. The sum of these characters suggests that “T.” maini sp. nov. is a more derived version of the type species T. hiatti that displays some, but not all, of the diagnostic traits of the Baenodda (Gaffney, 1972; Joyce and Lyson, 2015; Lyson et al., 2016). It is thus interpreted as a transitional form between early baenids and the highly derived Eubaeninae and Palatobaeninae.
Our analysis produced 30 minimum trees of length 181 (Figure 8). In the 50% majority rule consensus tree, “T.” maini sp. nov. was retrieved in an unresolved polytomy with the genera Thescelus, Scabremys, and Hayemys, all of which fall as outgroups to the Eubaeninae and Palatobaeninae. This node was supported at 100% bootstrap values. In our analysis, S. ornata was retrieved as the sister group to eubaenids and palatobaenids (Figure 8). Our phylogenetic analysis recovers “T.” maini sp. nov. as more derived than T. hiatti, but without the full suite of known baenodd traits, and completely distinct from the genus Neurankylus. Though not a baenodd, “T.” maini sp. nov. shares several of the morphological traits of that clade. Like H. latifrons, Thescelus spp. (T. rapiens and T. insiliens), “T.” maini sp. nov. shares the synapomorphy of midline contact between Ex posterior to the Gu (Figure 3, Figure 8). Additionally, the new species shares primitive plastral traits with H. latifrons and Thescelus spp. including a Fe-An sulcus that is relatively straight, unlike the omega shape found in derived baenids (Figure 3, Figure 8; Joyce and Lyson, 2015; Lyson et al., 2016). Additionally, “T.” maini sp. nov. does not have the following synapomorphies characteristic of Baenodda: reduced or absent Gu, multiple Ce, prepleural scutes, or a Ve5 that is longer than wide (Figure 2). Significantly, “T.” maini sp. nov. also lacks the key defining feature of Baenodda, the contribution of the most posterior vertebral scute to the margin of the carapace (Figure 3, Figure 8) (Lyson and Joyce, 2015). Instead, as in Neurankylus spp. and T. hiatti, the posterior marginals are located between Ve5 and the carapace margin. The sum of these characters suggests that “T.” maini sp. nov. is a more derived version of the type species T. hiatti that displays some, but not all, of the diagnostic traits of the Baenodda (Gaffney, 1972; Joyce and Lyson, 2015; Lyson et al., 2016). It is thus interpreted as a transitional form between early baenids and the highly derived Eubaeninae and Palatobaeninae.
DISCUSSION
Evolutionary implications of AAS turtles. With a baseline of known taxa, especially in light of limited articulated material, isolated fragments fill gaps. Small or fragmentary fossil specimens are typically more easily transported hydrologically over significant distances, completely disarticulated, and even preserved in the coprolites of predators (Konuki, 2008; Noto et al., 2012; Schwimmer, 2015; Pérez-García, 2018b). Several juvenile turtles have been discovered in apparent association with a large crocodyliform (Noto et al., 2012; Main, 2013). Additional taxonomic certainty regarding AAS taxa may also allow for more nuanced analysis of variation in morphology or taphonomic processes, as reported for Algorachelus (Pérez-García, 2018b). Fossil material previously published from the AAS should be reviewed to confirm proper taxonomic assignment (e.g., Noto et al., 2012; Main, 2013).
Cenomanian-age deposits are globally uncommon and littoral/marine forms can obscure strong intracontinental biogeographic signatures (Joyce et al., 2016a, b; Pérez-García, 2018a, b). Deposits at the AAS are constrained within a distinct layer of dark brown sandy siltstone (>50 cm thick) overlain by a 30-40 cm thick dark gray layer of carbonaceous sandy siltstone, which contains slickensides in its upper portion (Figure 1.3) (Adams et al., 2017). The turtles of the AAS are distinct from known Laramidian units of similar age (e.g. Cedar Mtn., Cloverly, and Dakota Fms) (Table 2). Most rich terrestrial fossil localities in North America are concentrated during either the Aptian or Albian of the Lower Cretaceous, or the Campanian and Maastrichtian of the Upper Cretaceous, leaving an approximately 20 million year interval during which few fossils are known (Jacobs and Winkler, 1998; Weishampel et al., 2004; Zanno and Makovicky, 2013; Noto et al., 2019). The relative rarity of pleurodiran and trionychid material suggests that invading taxa (one specimen each for Trionychidae indet. and cf. Algorachelus sp.) may have had a minimal presence. The AAS turtle assemblage resembles those of other North American assemblages (e.g., Cloverly, Dakota, Cedar Mountain Fms), where freshwater aquatic cryptodiran forms are generally dominant among stem Testudines (Joyce et al., 2016b; Avrahami et al., 2018). We report no indication of chelonioid or deep water marine forms.
Fossil sites in Texas are currently unrivaled in turtle diversity among Lower and middle Cretaceous deposits in North America, especially if the Aptian/Albian record is considered. Recently described theropod material from the Mussentuchit Member of the Cedar Mountain Fm is associated with an adocid and Helochelydridae indet. and provided histological thin sections, but few other morphometric details regarding described turtle fossils (Avrahami et al., 2018).
Major shell and skull adaptations to aquatic habitats occurred primarily in the stem of Testudines (Foth et al., 2016). It is important to note that the relict forms Naomichelys sp. and "T". maini sp. nov. persisted since the Upper Jurassic, and fell outside Testudines (Table 1) (Joyce and Gauthier, 2004; Joyce, 2007, 2017). Apparent vacancies in paleoecological niches are suggested primarily in deep water marine and terrestrial herbivorous, neither of which are typically associated with good preservation of fossils (Table 1) (Gaffney, 1972; Joyce et al., 2014; Joyce and Lyson, 2015; Lyson et al., 2016; Pérez-García, 2018). Phylogenetic analyses have struggled to resolve inferred habitat preferences of the stem and crown Pantestudines (Joyce and Gauthier, 2004; Joyce et al., 2004). Additional fossil material, particularly with definitive shell-skull associations from key North American units, may clarify these relationships.
North American turtle distribution in the Early Cretaceous is similar to that known in dinosaurs, with a generally homogenous fauna existing and separating primarily in marine habitats or within proximity of a reliable source of fresh water, especially before more sophisticated marine adaptation in Cretaceous turtles (Weishampel et al., 2004; Gates et al., 2010; Zanno and Makovicky, 2013; Brownstein, 2018). “Trinitichelys” maini sp. nov. and Naomichelys sp. represent endemic Laurasian lineages that persisted in eastern North America from the Jurassic (Lyson et. al, 2016; Smith et al., 2017). Helochelydridae survived into younger Cretaceous deposits in western Appalachia and diversified in near-coastal ecosystems of Europe (Pérez-García et al., 2014; Joyce et al., 2016b; Joyce, 2017; Pérez-García, 2018). The co-occurrence of a representative of the Baenidae (“T.” maini sp. nov.) and Helochelydridae (Naomichelys sp.) is typical in Laurasian assemblages beginning in the Aptian (Table 2) (Brinkman et al., 2017; Joyce, 2017).
The unique combination of derived and symplesiomorphic characters in “T.” maini sp. nov. in comparison to other baenids complicates the interpretation of the evolutionary history of the Baenodda (Figure 7). It also suggests that while “T.” maini sp. nov. may be phylogenetically positioned along the baenodd lineage, it is unlikely to be ancestral to Baenodda. Tentative referral of AAS material to the genus Trinitichelys provides continuity of the baenid lineage into the Upper Cretaceous of Appalachia (Table 2). Its occurrence in the Cenomanian places this evolutionary grade earlier than previously understood, and the baenid genus Neurankylus is not known until approximately 8 million years later in the Santonian (Joyce and Lyson, 2015).
Similar continuity among older forms in Texas is also possible with the genus Naomichelys. Perhaps some of the morphological differences between the type and AAS materials can be attributed to evolutionary change during the approximately 20-million-year interval between known Lower and Upper Cretaceous helochelydrids (Noto et al., 2019). Also, a detailed description is needed to describe possible ontogenetic and sexually dimorphic variation observed between the xiphiplastron described here and the type material described in Joyce et al. (2014).
Shell surface anatomy in Naomichelys (Figure 6). The surface sculpture of the genus Naomichelys is distinctive and a primary diagnostic character differentiating helochelydrid taxa (Joyce et al., 2014). When intact, the ornamentation typically consists of distinct, non-coalesced tubercles on the surface of the cranium, shell, and osteoderms (Joyce et al., 2011, 2014). Its ornamentation is considered analogous to that of the European helochelydrids Helochelydra nopscai from the Aptian/Albian and Helochelys danubina from the Cenomanian (von Meyer, 1855; Scheyer, 2007; Joyce et al., 2014). Raised pustules have a 0.75-2.0 mm diameter and are easily dislodged by taphonomic process from the shell surface due to their height (Figure 6) (Joyce et al., 2014). Tubercle morphology and arrangement varies somewhat in different regions of the shell, as described for N. speciosa (Joyce et al., 2014). Tubercles may range from low, narrow, and closely-spaced to large and widely-spaced (Figure 4, dorsal views) and are sometimes fused toward the center of the carapace in sequences of up to four (Joyce et al., 2014). Well-defined concave scars remain where the tall, cylindrical bodies break at the bases, suggesting a higher density than the underlying bone (von Nopsca, 1928; Joyce et al., 2014). Tubercles are often at least partially obliterated by complex post-depositional diagenesis at the AAS, and intact tubercles are rarely preserved (Figure 6). Thick skin has been hypothesized to occupy the surface between the tubercles, and extensive osteoderms are known in N. speciosa (Joyce et al., 2014). It is also possible that the scutes replicated the bone surface to create a rough pattern that could be used as camouflage or perhaps to resist pushing on unstable substrate (Joyce et al., 2014). Additional research is ongoing regarding the morphology and histology of Naomichelys bone from the AAS. In general, additional studies are exploring the meso- and microanatomical structure of turtles at the AAS, particularly seeking to better understand the periosteal interfaces between fossilized bone and missing soft tissue.
Turtle paleoecology at the AAS. In light of additional discoveries of multiple new crocodyliform and turtle taxa, the association of particular depositional groupings, specimen clusters, or taphonomic traits (e.g., tooth size and spacing) may clarify relationships between taxa and expand our knowledge of crocodyliform feeding behavior compared with extant forms (Erickson et al., 2003; Njau and Blumenschine, 2006). For example, specimens of Algorachelus sp. have been discovered associated with helochelydrid and theropod dinosaurs (Pérez-García, 2018a, b). More associated fossil material may also clarify niche partitioning among AAS turtles, similar to the current understanding of Cenomanian European strata.
Baenids are known from freshwater fluvial-lacustrine and estuarine deposits in semi-arid to humid paleoenvironments (Table 1) (Hirayama et al., 2000; Joyce and Lyson, 2015). The habitat preferences of helochelydrid turtles have been debated, but terrestriality is clearly indicated by sedimentological and morphological data (Scheyer, 2007; Vullo et al., 2010; Scheyer et al., 2014; Joyce, 2017). Histological analyses of the highly derived autapomorphic shell bone microstructures also indicate terrestrial preference in Helochelydridae (Table 1) (Scheyer, 2007; Scheyer and Sander, 2007; Scheyer et al., 2014). Generally, turtles increasingly evolved an aquatic habitat preference in the stem of Testudines (Foth et al., 2016). It is important to note that the relict forms Naomichelys sp. and “T.” maini sp. nov. persisted since the Upper Jurassic, and were outside Testudines (Table 1) (Joyce and Gauthier, 2004; Joyce, 2007, 2017). Apparent vacancies in paleoecological niches are suggested primarily in deep water marine and terrestrial herbivorous habitats, neither of which are typically associated with good preservation of fossils (Table 1) (Gaffney, 1972; Joyce et al., 2014; Joyce and Lyson, 2015; Lyson et al., 2016; Pérez-García, 2018). Phylogenetic analyses have struggled to resolve inferred habitat preferences of the stem and crown Pantestudines (Joyce and Gauthier, 2004; Joyce et al., 2004). Additional fossil material, particularly with definitive shell-skull associations from key North American units, may clarify these relationships.
A complex signal of habitat preference is not surprising in a turtle known to inhabit brackish coastlines, alluvial plains, and marshes, which are generally within a short distance of freshwater, at least perennially (Stephenson, 1915; Joyce et al., 2014). Naomichelys is known from brackish depositss, as are all other AAS turtles except “T.” maini sp. nov. The AAS was deposited during an important interval in turtle evolution, when stem Testudine groups were developing early adaptations to marine environments, but prior to the known explosive diversity later in the Cretaceous (Hirayama et al., 2000; Hutchison, 2000; Joyce et al., 2016b)
However, a semi-aquatic or aquatic habitat has also been proposed (Lapparent de Broin and Murelaga, 1999; Marmi et al., 2009; Suarez et al., 2012). Helochelydridae have been identified in coastal, fluvial, estuarine, deltaic, and lagoonal deposits (Table 1) (Hirayama et al., 2000; Rylaarsdam et al., 2006; Vullo et al., 2010). Turtles living near shorelines or in swamps may provide a complex or unclear habitat preference because marshes are typically formed by a patchwork of dry and submerged land areas (Joyce, 2017). Slickensides, dessication cracks, and clastic dikes are indicative of a seasonally dry climate (Retallack, 2001; DiMichele et al., 2006; Adams et al., 2017). Stem Testudinata are represented at AAS by Naomichelys sp. and “T.” maini sp. nov. Crown testudinate turtles (Testudines) are represented by the crown pleurodiran cf. Algorachelus sp. and a small basal trionychid. Both of these taxa were likely to be brackish tolerant (Table 1), and if representative of adult individuals (thereby fully grown), perhaps divided littoral habitats and smaller channels.
The AAS contains facies that represent a freshwater or brackish wetland deposited in a low-energy, deltaic system, which allowed for organisms to mix across coastal environments, even postmortem (Noto et al., 2012, 2019). Aquatic turtles, crocodyliforms, and snails at the AAS suggest that surface water was perennially available, at least locally near alluvial fans (Table 1) (Stephenson and Stenzel, 1952; Dorr, 1985; Noto et al., 2019). The AAS crocodyliforms currently known are: Terminonaris cf. T. rubusta (Osborn and Brown, 1905; Adams et al., 2011); Woodbinesuchus byersmauricei (Lee, 1997), Deltasuchus motherali (Adams et al., 2017), and Scolomastax sahlsteini (Noto et al., 2019).
Of the taxa in the AAS turtle assemblage (Table 1), cf. Algorachelus sp. was likely the most adapted to intrusion of salt water, however, its small body size and lack of powerful swimming adaptation likely made it unsuited for open marine conditions (Joyce et al., 2016a, b; Pérez-García et al., 2017). The congeneric A. peregrinus is known from coastal deposits in Europe, and A. tibert inhabited brackish environments along the east coast of Laramidia (Joyce et al., 2016a; Pérez-García et al., 2017; Pérez-García, 2018). The Woodbine Fm (Figure 1) is composed primarily of sand, shale, and silty sediments (Johnson, 1974), which suggests an interface between active channels and channel margins (siltstone-mudstone) (Figure 1.3) (Hutchison and Archibald, 1986). In summary, the turtles at the AAS apparently divided primarily freshwater aquatic systems between riverine and fresh water channels that originated from springs and upland drainages from the Ouachita Mountains to the north (Stephenson, 1915; Noto et al., 2019). A high abundance of terrestrial palynomorphs (primarily ferns), rare dinoflagellates and the absence of foraminifera indicate a fluvial deposition with minor marine input; fragmentary remains of elasmobranchs and osteichythyans representing individuals a meter or more in length suggest the nearby presence of deeper water; invertebrate remains consist mainly of gastropods, and contain a mixture of freshwater and brackish groups.
Early angiosperm evolution in North America. Coastal beaches and estuaries form vital sanctuaries for reptile nests and nurseries, though it should be noted that most of our understanding of turtle taphonomy is based on only a few individuals, and their preservation is favored by larger body size, shell thickness, and common co-ossification and expansion of midline scutes that cross sutures (i.e., baenids) (Joyce and Lyson, 2015). Life-history strategies of the highly aquatic taxa represented at the AAS were influenced by local environmental conditions, natural streamflow patterns, rates of deposition, and disturbance events such as flood pulses (Tornabene, 2014). Juvenile marine turtles are also known to transition to life in the open sea among accumulations of land debris and floating crinoids (Everhart, 2017; Konuki, 2008).
The Early Cretaceous of North America, particularly in the proto-GOM, is known to have a diverse range of terrestrial and aquatic floras, and the majority of basal turtle taxa were primarily carnivorous (Foth et al., 2016). Freshwater and marine grasses were known from Early Cretaceous deposits, and it is known that modern turtles (as well as epiphytes, invertebrates, and fishes) utilize aquatic angiosperms and macroalgae as forage, protection, and nutrient recycling (Hearne et al., 2019). Aquatic angiosperms (e.g., Montsechia) are perhaps basal to all other angiosperms, and have been recently recognized as important components of early submerged and shoreline ecosystems (Barendse and Scheffer, 2009; Gomez et al., 2015; Jud et al., 2018; Wang and Dilcher, 2018). Organic accumulations in coastal environments may have also provided ample fuel for frequent wildfires (Main, 2005; Brown et al., 2012).
Many of the floral samples reported by Main (2013) were useful while conducting the current analysis: broad, lenticular mats of featureless carbonized plant remains as much as 4 cm thick, compressed but well-preserved carbonized tree sections 15-20 cm wide, and smaller fragments of permineralized wood. Earlier work in the Gulfian region has noted the presence of fossilized wood (Mudge, 1877; Williston, 1894; Everhart, 2017). The AAS preserves similar charcoal-bearing horizons, including clastic debris flows, in situ burned root systems, and abundant small charcoal fragments (Main et al., 2010; Adams et al., 2017; Noto et al., 2019). Carbonized plant matter is commonly associated with vertebrate fossils resulting from frequent, widespread fires that characterized terrestrial sedimentary systems globally from the Valanginian throughout the Upper Cretaceous (Main, 2005; Brown et al., 2012). A study of an assemblage of AAS leaves could also be compared with fossil flora known from the Dakota Fm, providing insight into the early diversification of angiosperms and its effect on the evolution of herbivory in reptiles (Wang and Dilcher, 2018).
Taphonomy and Integrative Paleobiology at the AAS. Paleoecological hypotheses combining the most recent contributions from different faunal groups are somewhat inseparable from taphonomic analyses (Noto et al., 2019). Revisiting of collections of Woodbine Fm fossils is necessary to improve taxonomic accuracy, sample size, and diagnoses. Large volumes of Woodbine Fm fossil material from various vertebrate, invertebrate, and floral groups have long sat in museum collections. We hope that this contribution spurs the future description, analysis, and integration of available fossil material.
Descriptions and biogeographical analyses allow a more complete understanding of the complex ecosystems of the ancient coasts of the proto-GOM. A biogeographic profile of southern Appalachia is taking shape, and it is distinct from contemporaneous deposits in Laramidia (Avrahami et al., 2018; Brownstein, 2018; Noto et al., 2019). Much of the difference regards geography of the North American interior, which differed drastically from the modern Great Plains (Figure 1.3). The proximity of the AAS to the mouth of the WIS is reflected in a grouping of largely brackish tolerant channel occupants who were simultaneously effective predators and frequently predated. The turtle component of the AAS is, broadly speaking: (i) more aquatic than terrestrial, (ii) minimally adapted to marine environments, (iii) small to medium in size (~30-60 cm), and (iv) more carnivorous than herbivorous (Table 1).
Future taphonomic studies are also now possible with more accurate turtle taxonomy. Additionally, geochemical analyses of sediments from the AAS could provide insight into particular layers and the water conditions under which they were deposited. Most of the AAS material is quite fragmentary, but the bone is usually not degraded and could be of value in a sedimentological study. In particular, pervasive black dust impregnates the matrix embedding the fossil including the interior cavities of cancellous bone. At the AAS, organic preservation was enhanced by a locally high water table, which promoted anoxic and reducing conditions (Noto et al., 2012). There may also be diagenetic degradation of the cancellous bone or a thin crust of fine particulate found on covered specimens, likely precluding simply dust (Figure 6.2). Additional work should be conducted on charcoal inclusions that are associated with fossils of Facies A (see Brown et al., 2012 and fire horizons in Main et al., 2013).
ACKNOWLEDGEMENTS
The authors would like to thank the Perot Museum of Nature and Science and the many researchers, students, staff, and volunteers who were vital in fossil collection and processing at the AAS since its discovery, especially A. Fiorillo, R. Tykoski, and K. Morton. Thanks to A. Williams, K. Ezell, and A. Lee for assistance with the specimens and general support to the project. We also thank Dr. W. Joyce and an anonymous referee for feedback that greatly enhanced the study. Research was supported by Midwestern University Intramural Funds (AG, HFS).
REFERENCES
Adams, R.L. and Carr, J.P. 2010. Regional depositional systems of the Woodbine, Eagle Ford, and Tuscaloosa of the U.S. Gulf Coast. Gulf Coast Association of Geological Societies Transactions, 60:3-27.
Adams, T.L., Noto, C.R., and Drumheller, S. 2017. A large neosuchian crocodyliform from the Upper Cretaceous (Cenomanian) Woodbine Formation of North Texas. Journal of Vertebrate Paleontology, 37:1349776. https://doi.org/10.31233/osf.io/2ck7v
Adams, T.L., Polcyn, M.J., Mateus, O., Winkler, D.A., and Jacobs LL. 2011. First occurrence of the long-snouted crocodyliform Terminonaris (Pholidosauridae) from the Woodbine Formation (Cenomanian) of Texas. Journal of Vertebrate Paleontology, 31:712-716. https://doi.org/10.1080/02724634.2011.572938
Anquetin, J. 2012. Reassessment of the phylogenetic interrelationships of basal turtles (Testudinata). Systematic Paleontology, 10:3-45. https://doi.org/10.1080/14772019.2011.558928
Avrahami, H.M., Gates, T.A., Heckert, A.B., Makovicky, P.J., and Zanno, L.E. 2018. A new microvertebrate assemblage from the Mussentuchit Member, Cedar Mountain Formation: Insights into the paleobiodiversity and paelobiogeography of early Late Cretaceous ecosystems in western North America. PeerJ, 6:e5883. https://doi.org/10.7717/peerj.5883
Baur, G. 1891. Notes on some little known American fossil tortoises. Proceedings of the Academy of Natural Sciences Philadelphia, 43:411-430.
Bennett, G.E., Main, D., Anderson K.B., and Peterson, R. 2011. Microvertebrate paleoecology of the Cretaceous Woodbine Formation (Cenomanian) at the Arlington Archosaur Site, North-central Texas. Geological Society of America Abstracts with Programs, Minneapolis, 43(2):89.
Berendse, F. and Scheffer, M. 2009. The angiosperm radiation revisited, an ecological explanation for Darwin's 'abominable mystery'. Ecology Letters, 12:865-872. https://doi.org/10.1111/j.1461-0248.2009.01342.x
Bergquist, H. R. 1949. Geology of the Woodbine formation of Cooke, Grayson, and Fannin Counties, Texas. United States Geological Survey Oil and Gas Investigations Map, OM-98. https://doi.org/10.3133/om98
Bhattacharya, J.P. and Walker, R.G. 1991. Allostratigraphic subdivision of the Upper Cretaceous Dunvegan, Shaftesbury, and Kaskapau formations in the northwestern Alberta subsurface. Bulletin of Canadian Petroleum Geology, 39:165-191.
Bonde, J., Varricchio, D., Jackson, F., Loope, D., and Shirk, A. 2008. Dinosaurs and dunes! Sedimentology and paleontology of the Mesozoic in the Valley of Fire State Park. Geological Society of America, Field Guide, 11:249-262. https://doi.org/10.1130/2008.fld011(11)
Bowker, K.A. 2007. Barnett Shale gas production, Fort Worth Basin: Issues and discussion. American Association of Petroleum Geologists Bulletin, 91(4):523-533. https://doi.org/10.1306/06190606018
Brinkman, D.B. 2003. A review of nonmarine turtles from the Late Cretaceous of Alberta. Canadian Journal of Earth Sciences, 40:557-571. https://doi.org/10.1139/e02-080
Brinkman, D.B. and Tarduno, J.A. 2005. A Late Cretaceous (Turonian-Coniacian) high-altitude turtle assemblage from the Canadian Arctic. Canadian Journal of Earth Sciences, 42:2073-2080. https://doi.org/10.1139/e05-074
Brinkman, D.B., Rabi, M., and Zhao, L. 2017. Lower Cretaceous fossils from China shed light on the ancestral body plan of crown softshell turtles (Trionychidae, Cryptodira). Scientific Reports, 7:1-11. https://doi.org/10.1038/s41598-017-04101-0
Brown, S.A.E., Scott, A.C., Glasspool, I.J., and Collinson, M.E. 2012. Cretaceous wildfires and their impact on the Earth system. Cretaceous Research, 36:162-190. https://doi.org/10.1016/j.cretres.2012.02.008
Brownstein, C.D. 2018. The biogeography and ecology of the Cretaceous non-avian dinosaurs of Appalachia. Palaeontologia Electronica, 21.1.5A:1-56. https://doi.org/10.26879/801
palaeo-electronica.org/content/2018/2123-appalachia-biogeography
Cope, E.D. 1865. Sketch of the primary groups of Batrachia Salienta. Natural History Review, New Series 5:97-120.
Cope, E.D. 1873. On the extinct Vertebrata of the Eocene of Wyoming, observed by the expedition of 1872, with notes on the geology. United States Geological Survey of the Territories, 6:545-649.
Cumbaa, S.L., Shimada, K., and Cook, T.D. 2010. Mid-Cenomanian vertebrate faunas of the Western Interior Seaway of North America and their evolutionary, paleobiogeographical, and paleoecological implications. Palaeogeography, Palaeoclimatology, Palaeoecology, 295:199-214. https://doi.org/10.1016/j.palaeo.2010.05.038
D’Amore, D.C. and Blumenschine, R.J. 2009. Komodo monitor (Varanus komodoensis) feeding behavior and dental function reflected through tooth marks on bone surfaces, and the application to ziphodont paleobiology. Paleobiology, 35:525-552. https://doi.org/10.1666/0094-8373-35.4.525
Denne, R.A., Hinote, R.E., Breyer, J.A., Kosanke, T.H., Lees, J.A., Engelhardt-Moore, N., Spaw, J.M., and Tur, N. 2014. The Cenomanian-Turonian Eagle Ford Group of South Texas: Insights on timing and paleoceanographic conditions from geochemistry and micropaleontologic analyses. Palaeogeography, Palaeoclimatology, Palaeoecology, 413:2-28. https://doi.org/10.1016/j.palaeo.2014.05.029
Dilcher, D. and Crane, P. 1984. Archaenthus: An early angiosperm from the Cenomanian of the Western Interior of North America. Annals of the Missouri Botanical Garden, 71:351-383. https://doi.org/10.2307/2399030
DiMichele, W., Tabor, T., Chaney, D., and Nelson, W. 2006. From wetlands to wet spots: environmental tracking and the fate of Carboniferous elements in Early Permian tropical floras. Geological Society of America Special Papers, 399:223-248. https://doi.org/10.1130/2006.2399(11)
Dodge, C.F. 1969. Stratigraphic nomenclature of the Woodbine Formation Tarrant County, Texas. Texas Journal of Science, 21:43-62.
Doody, J.S., Pauza, M., Stewart, B., and Comacho, C. 2009. Nesting behavior of the pig-nosed turtle, Carettochelys insculpta, in Australia. Chelonian Conservation and Biology, 8 (2):185-191. https://doi.org/10.2744/ccb-0764.1
Dorr, J.A. 1985. Newfound Early Cretaceous dinosaurs and other fossils in southeastern Idaho and westernmost Wyoming. Contributions from the Museum of Paleontology, The University of Michigan, 27(3):73-85.
Erickson, G.M., Lappin, A.K., and Vliet, K.A. 2003. The ontogeny of bite-force performance in American alligator (Alligator mississppiensis). Journal of Zoology, 260:317-327.
Ernst, C. and Barbour, R. 1989. Turtles of the World. Smithsonian Institution Press, Washington, D.C.
Everhart, M.J. 2017. Oceans of Kansas: A Natural History of the Western Interior Sea. Indiana University Press, Bloomington.
Foth, C., Rabi, M., and Joyce, W.G. 2016. Skull shape variation in extant and extinct Testudinata and its relation to habitat and feeding ecology. Acta Zoologica (Stockholm), 98:310-325. https://doi.org/10.1111/azo.12181
Gaffney, E.S. 1972. The systematics of the North American family Baenidae (Reptilia, Cryptodira). Bulletin of the American Museum of Natural History, 147(5):1-84.
Gaffney, E.S. 1975. A revision of the side-necked turtle Taphrosphys sulcatus (Leidy) from the Cretaceous of New Jersey. American Museum Novitiates, 2571:1-24.
Gaffney, E.S. and Meylan, P.A. 1988. A phylogeny of turtles, p. 157-219. In Benton, M.J. (ed.), The Phylogeny and Classification of the Tetrapods. Vol. 1: Amphibians, Reptiles, Birds. Clarendon Press, Oxford.
Gaffney, E.S., Tong, H., and Meylan, P.A. 2006. Evolution of the side-necked turtles: the families Bothremydidae, Euraxemydidae, and Araripemydidae. Bulletin of the American Museum of Natural History, 300:1-318. https://doi.org/10.1206/0003-0090(2006)300[1:eotstt]2.0.co;2
Gardner, J.D. and Russell, A.P. 1994. Carapacial variation among soft-shelled turtles (Testudines: Trionychidae), and its relevance to taxonomic and systematic studies of fossil taxa. Neues Jahrbuch fur Geologie und Palaontologie, 193:209-244.
Gardner, J.D., Russell, A.P., and Brinkman, D.B. 1995. Systematics and taxonomy of softshelled turtles (Family Trionychidae) from the Judith River Group (mid-Campanian) of North America. Canadian Journal of Earth Sciences, 32:631-643. https://doi.org/10.1139/e95-053
Gates, T., Sampson, S., Zanno, L., Roberts, E., Eaton, J., Nydam, R., Hutchison, J.H., Smith, J., Loewen, M., and Getty, M. 2010. Biogeography of terrestrial and freshwater vertebrates from the Late Cretaceous (Campanian) Western Interior of North America. Palaeogeography, Palaeoclimatology, Palaeoecology, 291(3-4):371-387. https://doi.org/10.1016/j.palaeo.2010.03.008
Georgialis, G.L. and Joyce, W.G. 2017. A review of the fossil record of Old World turtles of the clade Pan-Trionychidae. Bulletin of the Peabody Museum of Natural History, 58:115-208. https://doi.org/10.3374/014.058.0106
Goboloff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics, 32(3):221-238. https://doi.org/10.1111/cla.12160
Hay, O.P. 1908. The Fossil Turtles of North America. Carnegie Institute of Washington, Washington DC. https://doi.org/10.5962/bhl.title.12500
Hearne, E.L., Johnson, R.A., Gulick, A.A., Candelmo, A., Bolten, A.B., and Bjorndal, K.A. 2019. Effects of green turtle grazing on seagrass and macroalgae diversity vary spatially among seagrass meadows. Aquatic Botany, 152:10-15. https://doi.org/10.1016/j.aquabot.2018.09.005
Hedlund, R.W. 1966. Palynology of the Red Branch Member of the Woodbine Formation (Cenomanian), Bryan County, Oklahoma. Oklahoma Geological Survey, 112:1-69.
Hirayama, R., Brinkman, D.B., and Danilov, I.C. 2000. Distribution and biogeography of non-marine Cretaceous turtles. Russian Journal of Herpetology, 7:181-198.
Hummel, K. 1929. Die fossilen Weichschildkroten (Trionychia). Geolgische und Palaeontologische Abhandlungen, 16:360-487.
Hutchison, J.H. 1980. Turtle stratigraphy of the Willwood Formation, Wyoming: Preliminary results. Papers on Paleontology, 24:115-118.
Hutchison, J. 1984. Determinate growth in the Baenidae (Testudines): taxonomic, ecologic and stratigraphic significance. Journal of Vertebrate Paleontology, 3:148-151. https://doi.org/10.1080/02724634.1984.10011968
Hutchison, J.H. 2000. Diversity of Cretaceous turtle faunas of Eastern Asia and their contribution to the turtle faunas of North America. Paleontological Society of Korea Special Publication, 4:27-38.
Hutchison, J.H. and Archibald, D.J. 1986. Diversity of turtles across the Cretaceous/Tertiary boundary in Northeastern Montana. Palaeogeography, Palaeoclimatology, Palaeoecology, 55:1-32. https://doi.org/10.1016/0031-0182(86)90133-1
Hutchison, J.H. and Holroyd, P.A. 2003. Late Cretaceous and early Paleocene turtles of the Denver Basin. Colorado Rocky Mountain Geology, 38:121-142. https://doi.org/10.2113/gsrocky.38.1.121
Jacobs, L., Polcyn, M., Winkler, D., Myers, T., Kennedy, J., and Wagner, J. 2013. Late Cretaceous strata and vertebrate fossils of North Texas, p. 1-13. In Hunt, B. and Catlos, E. (eds.), Late Cretaceous to Quaternary Strata and Fossils of Texas: Field Excursions Celebrating 125 Years of GSA and Texas Geology. Geological Society of America South-Central Section Meeting, Austin, Texas. https://doi.org/10.1130/2013.0030(01)
Jacobs, L.L. and Winkler, D.A. 1998. Mammals, archosaurs, and the Early to Late Cretaceous transition in north-central Texas, p. 253-280. In Tomida, Y., Flynn, L.J., and Jacobs, L.L. (eds.), Advances in Vertebrate Paleontology and Geochronology. National Science Museum Monographs, Tokyo.
Jasinski, S.E. 2013. Review of the fossil Trionychidae (Testudines) from Alabama, including the oldest record of Trionychid turtles from Eastern North America. Bulletin of the Alabama Museum of Natural History, 31:46-59.
Johnson, R.O. 1974. Lithofacies and Depositional Environments of the Rush Creek Member of the Woodbine Formation (Gulfian) of North Central Texas. Unpublished Master’s Thesis, University of Texas Arlington, Arlington.
Joyce, W.G. 2007. Phylogenetic relationships of Mesozoic turtles. Bulletin of the Peabody Museum of Natural History, 48:1-102. https://doi.org/10.3374/0079-032x(2007)48[3:promt]2.0.co;2
Joyce, W.G. 2017. A review of the fossil record of basal Mesozoic turtles. Bulletin of the Peabody Museum of Natural History, 58:65-113. https://doi.org/10.3374/014.058.0105
Joyce, W.G., Chapman, S.D., Moody, R.T.J., and Walker, C.A. 2011. The skull of the solemydid turtle Helochelydra nopscai from the Early Cretaceous of the Isle of Wight (UK) and a review of Solemydidae. Special Papers in Palaeontology, 86:75-97.
Joyce, W.G. and Gauthier, J.A. 2004. Palaeoecology of Triassic stem turtles sheds new light on turtle origins. Proceedings of the Royal Society B, 271(1534):1-5. https://doi.org/10.1098/rspb.2003.2523
Joyce, W.G., Lucas, S.C., Scheyer, T.M., Heckert, A.B., and Hunt, A.P. 2009. A thin-shelled reptile from the Late Triassic of North America and the origin of the turtle shell. Proceedings of the Royal Society B, 276(1656):507-513. https://doi.org/10.1098/rspb.2008.1196
Joyce, W.G. and Lyson, T.R. 2015. A review of the fossil record of turtles of the clade Baenidae. Bulletin of the Peabody Museum of Natural History, 56 (2):147-183. https://doi.org/10.3374/014.056.0203
Joyce, W.G. and Lyson, T.R. 2017. The shell morphology of the latest Cretaceous (Maastrichtian) trionychid turtle Helopanoplia Distincta. PeerJ, 5:4169. https://doi.org/10.7717/peerj.4169
Joyce, W.G., Lyson, T.R. and Kirkland, J.I. 2016a. An early bothremydid (Testudines, Pleurodira) from the Late Cretaceous (Cenomanian) of Utah, North America. PeerJ, 4:e2502. https://doi.org/10.7717/peerj.2502
Joyce, W.G., Parham, J.F., and Gauthier, J.A. 2004. Developing a protocol for the conversion of rank-based taxon names to phylogenetically defined clade names, as exemplified by turtles. Journal of Paleontology, 78(5):989-1013. https://doi.org/10.1666/0022-3360(2004)078<0989:dapftc>2.0.co;2
Joyce, W.G., Rabi, M., Clark, J.M., and Xu, X. 2016b. A toothed turtle from the Late Jurassic of China and the global biogeographic history of turtles. BMC Evolutionary Biology, 16:1-29. https://doi.org/10.1186/s12862-016-0762-5
Joyce, W.G., Sterli, J., and Chapman, S. 2014. The skeletal morphology of the solemydid turtle Naomichelys speciosa from the Early Cretaceous of Texas. Journal of Paleontology, 88:1257-1287. https://doi.org/10.1666/14-002
Jud, N.A., D'Emic, M.D., Williams, S.A., Mathews, J.C., Tremaine, K.M., and Bhattacharya, J. 2018. A new fossil assemblage shows that large angiosperm trees grew in North America by the Turonian (Late Cretaceous). Science Advances, 4:eaar8568. https://doi.org/10.1126/sciadv.aar8568
Kasparek, M. 2001. Priorities for the conservation of the Nile soft-shelled turtle, Trionyx triunguis, in the Mediterranean. Testudo, 5(3):4945.
Kennedy, W.J. and Cobban, W.A. 1990. Cenomanian ammonite faunas from the Woodbine Formation and lower part of the Eagle Ford Group, Texas. Palaeontology, 33:75154.
Klein, I.T. 1760. Klassification und kurze Geschichte der Vierfübigen Thiere. Jonas Schmidt (transl. by F. D. Behn), Lübeck, Germany.
Konuki, R. 2008. Biostratigraphy of sea turtles and possible bite marks on a Toxochelys (Testudines, Chelonioidea) from the Niobara Formation (Late Santonian), Logan County, Kansas and paleoecological implications for predator-prey relationships among large marine vertebrates. Unpublished Master’s thesis, Fort Hays State University, Hays, Kansas, USA.
Krause, D.W. and Baird, D. 1979. Late Cretaceous mammals east of the North American Western Interior Seaway. Journal of Paleontology, 53:562-565.
Langston, W. 1965. Fossil Crocodilians from Colombia and the Cenozoic History of the Crocodilia in South America. University of California Press, Berkeley.
Lapparent de Broin, F. and Murelaga. X. 1999. Turtles from the Upper Cretaceous of Laño (Iberian Peninsula). Estudios del Museo de Ciencias Naturales de Álava, 14(Núm. Espec. 1):135-211.
Lapparent de Broin, F. and Murelaga. X. 1996. Une nouvelle faune de chéloniens dans le Crétacé supérieur européen. Comptes Rendus de l’Académie des Sciences Paris, 323(2):729-735.
Lapparent de Broin, F. and Werner, C. 1998. New Late Cretaceous turtles from the Western Desert, Egyptian Annales of Paléontologie, 84(2):131-214. https://doi.org/10.1016/S0753-3969(98)80005-0
Laurin, M., de Queiroz, K., Cantino, P., Cellinese, N., and Olmstead, R. 2005. The PhyloCode, types, ranks, and monophyly: a response to Pickett. Cladistics 21:605-607. https://doi.org/10.1111/j.1096-0031.2005.00090.x
Lee, Y.N. 1997. The Archosauria from the Woodbine Formation (Cenomanian) in Texas. Journal of Paleontology, 71:1147-1156. https://doi.org/10.1017/s0022336000036088
Li, L., Joyce, W., and Liu, J. 2015. The first soft-shelled turtle from the Jehel Biota of China. Journal of Vertebrate Paleontology, e909450. https://doi.org/10.1080/02724634.2014.909450
Lipka, T.R., Therrien, F., Weishampel, D.B., Jamniczky, H.A., Joyce, W.G., Colbert, M.W., and Brinkman, D.B. 2006. A new turtle from the Arundel Clay facies (Potomac Formation. Early Cretaceous) of Maryland, USA. Journal of Vertebrate Paleontology, 26:300-307. https://doi.org/10.1671/0272-4634(2006)26[300:antfta]2.0.co;2
Lyson, T.R., Bever, G.S., Scheyer, T.M., Hsiang, A.Y., and Gauthier, J.A. 2013. Evolutionary origin of the turtle shell. Current Biology, 23(12):1113-9. https://doi.org/10.1016/j.cub.2013.05.003
Lyson, T.R. Joyce, W.G., Lucas, S.G., and Sullivan, R.M. 2016. A new baenid turtle from the early Paleocene (Torrejonian) of New Mexico and a species-level phylogenetic analysis of Baenidae. Journal of Paleontology, 90:305-316. https://doi.org/10.1017/jpa.2016.47
MacNeal, D.L. 1958. The flora of the Upper Cretaceous Woodbine Sand in Denton County, Texas. Academy of Natural Sciences of Philadelphia Monograph 10:1-152.
Main, D.J. 2005. Paleoenvironments and Paleoecology of the Cenomanian Woodbine Formation of Texas: Paleobiogeography of the Hadrosaurs (Dinosauria: Ornithischia). Unpublished Master’s Thesis, The University of Texas Arlington, Arlington, Texas.
Main, D.J. 2013. Appalachian Delta Plain Paleoecology of the Cretaceous Woodbine Formation at the Arlington Archosaur Site, North Texas. Unpublished PhD Thesis, The University of Texas Arlington, Arlington, Texas.
Main, D.J., Parris, D., Grandstaff, B., and Carter, B. 2011. A new lungfish (Dipnoi:Ceratodontidae) from the Cretaceous Woodbine Formation, Arlington Archosaur Site, North Texas. Texas Journal of Science, 56:112-126.
Magwene, P.M. and Socha, J.J. 2013. Biomechanics of turtle shells: how whole shells fail in compression. Journal of Experimental Zoology A: Ecology, Genetics, and Physiology, 319:86-89. https://doi.org/10.1002/jez.1773
Marmi, J., Vila, B., and Galobart, A. 2009. Solemys (Chelonii, Solemydidae) remains from the Maastrichtian of Pyrenees: evidence for a semi-aquatic lifestyle. Cretaceous Research 30:1307-1312. https://doi.org/10.1016/j.cretres.2009.07.008
McNulty, M. and Slaughter, B. 1962. A new sawfish from the Woodbine Formation (Cretaceous) of Texas. Copeia, 1962:775-776. https://doi.org/10.2307/1440678
Meylan, P.A. 1987. The phylogenetic relationships of soft-shelled turtles (Family Trionychidae). Bulletin of the American Museum of Natural History, 186(1):1-103.
Milner, A.R. 2004. The turtles of the Purback Limestone Group of Dorset, Southern England. Palaeontology, 47:1441-1467. https://doi.org/10.1111/j.0031-0239.2004.00418.x
Mudge, B.F. 1877. Annual Report of the Committee on Geology, for the year ending November 1, 1876. Kansas Academy of Science, Transactions, 4-5.
Njau, J.K. and Blumenschine, R.J. 2006. A diagnosis of crocodile feeding traces on larger mammal bone, with fossil examples from the Plio-Pleistocene Olduvai Basin, Tanzania. Journal of Human Evolution, 50:142-162. https://doi.org/10.1016/j.jhevol.2005.08.008
Nopska, B.F. 1928. Palaeontological notes on reptiles. V. On the skull of the Upper Cretaceous dinosaur Euoplocephalus. Geologica Hungarica, Series Palaeontologica, 1(1):1-54.
Noto, C.R. 2015. Archosaur fossil localities in the Woodbine Formation (Cenomanian) of north-central Texas, p. 38-51. In Noto, C.R. (ed.), Early- and Mid-Cretaceous Archosaur Localities of north-central Texas. Fieldtrip Guidebook. Society of Vertebrate Paleontology, Dallas, TX.
Noto, C.R., Drumheller, S., Adams, T.L., and Turner, A.H. 2019. An enigmatic small neosuchian crocodyliform from the Woodbine Formation of Texas. The Anatomical Record. https://doi.org/10.1002/ar.24174
Noto, C.R., Main, D.J., and Drumheller, S.K. 2012. Feeding traces and paleobiology of a Cretaceous (Cenomanian) crocodyliform: example from the Woodbine Formation of Texas. Palaios, 27:105-115. https://doi.org/10.31233/osf.io/hjf63
Oliver, W.B. 1971. Depositional systems in the Woodbine Formation (Upper Cretaceous), north-east Texas: Bureau of Economic Geology Report of Investigations, 73:28. https://doi.org/10.1306/5d25ce07-16c1-11d7-8645000102c1865d
Osborn, H.F., and Brown B. 1905. Teleorhinus browni-a teleosaur in the Fort Benton. The Journal of Geology, 13(2):239-240. https://doi.org/10.1086/621218
Ostrum, J. 1970. Stratigraphy and Paleontology of the Cloverly Formation (Lower Cretaceous) of the Bighorn Basin Area, Wyoming and Montana. Bulletin of the Peabody Museum of Natural History, 35:1-205.
Parrish, J.M., Parrish, J.T., Hutchison, H.J., and Spicer, R.A. 1987. Late Cretaceous vertebrate fossils from the North Slope of Alaska and implications for dinosaur ecology. Palaios, 2:377-389. https://doi.org/10.2307/3514763
Pérez-García, A. 2016. A new turtle confirms the presence of Bothremydidae (Pleurodira) in the Cenozoic of Europe and expands the biogeographic range of Foxemydina. Naturwissenschaften, 103(708):1-50. https://doi.org/10.1007/s00114-016-1375-y
Pérez-García, A. 2018a. New insights on the only bothremydid turtle (Pleurodira) identified in the British record: Palemys bowerbankii new combination. Palaeontologia Electronica, 21.2.28A:1-12. https://doi.org/10.26879/849
palaeo-electronica.org/content/2018/2268-british-bothremydid-turtle
Pérez-García, A. 2018b. New information on the Cenomanian bothremydid turtle Algorachelus based on new, well-preserved material from Spain. Fossil Record, 21:119-135. https://doi.org/10.5194/fr-21-119-2018
Pérez-García, A., Antunes, M.T., Barroso-Barcenilla, F., Callapez, P.M., Segura, M., Soares, A.F., and Torices, A. 2017. A bothremydid from the middle Cenomanian of Portugal identified as one of the oldest pleurodiran turtles in Laurasia. Cretaceous Research, 78:61-70. https://doi.org/10.1016/j.cretres.2017.05.031
Pérez-Garciá, A., Espílez, E., Mampel, L., and Alcalá, L. 2015. A new European Albian turtle that extends the known stratigraphic range of the Pleurosternidae (Paracryptodira). Cretaceous Research, 55:74-83. https://doi.org/10.1016/j.cretres.2015.02.007
Pérez-García, A., Royo-Torres, R., and Cobos, A. 2014. A new European Late Jurassic pleurosternid (Testudines, Paracryptodira) and a new hypothesis of paracrypotodiran phylogeny. Journal of Systematic Paleontology, 13:351-369. https://doi.org/10.1080/14772019.2014.911212
Rabi, M., Tong, H., and Botfalvai, G. 2012. A new species of the side-necked turtle Foxemys (Pelomedusoides: Bothremydidae) from the Late Cretaceous of Hungary and the historical biogeography of the Bothremydini. Geology Magazine, 149(4):662-674. https://doi.org/10.1017/S0016756811000756
Rasband, W.S. 1997-2018. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/
Retallack, G.J. 2001. Soils of the Past. Blackwell Science, Malden. https://doi.org/10.1002/9780470698716
Ryan, M.J., Russell, A.P., Eberth, D.A., and Currie, P.J. 2001. The taphonomy of a Centrosaurus (Ornithischia: Certopsidae) bone bed from the Dinosaur Park Formation (Upper Campanian), Alberta, Canada, with Comments on Cranial Ontogeny. Palaios, 16(5):482-506. https://doi.org/10.2307/3515564
Rylaarsdam, J., Varban, B., Plint, A., Buckley, L., and McCrea, R. 2006. Middle Turonian dinosaur paleoenvironments in the Upper Cretaceous Kaskapau Formation, northeast British Columbia. Canadian Journal of Earth Sciences, 43:631-652. https://doi.org/10.1139/e06-014
Schwimmer, D.R., Weems, R.E., and Sanders, A.E. 2015. A Late Cretaceous shark coprolite with baby freshwater turtle vertebrae inclusions. Palaois, 30:707-713. https://doi.org/10.2110/palo.2015.019
Schwimmer, D.R., Williams, G.D., Dobie, J.L., and Siesser, W.G. 1993. Late Cretaceous dinosaurs from the Blufftown Formation in western Georgia and eastern Alabama. Journal of Vertebrate Paleontology, 67(2):288-296. https://doi.org/10.1017/s0022336000032212
Scheyer, T.M. 2007. Comparative Bone Histology of the Turtle sSell (Carapace and Plastron): Implications for Turtle Systematics, Functional Morphology and Turtle Origins. Unpublished PhD Thesis, University of Bonn, Sweden.
Scheyer, T.M., Pérez-García, A., and Murelaga, X. 2014. Shell bone histology of solemydid turtles (stem Testudines): palaeoecological implications. Organisms, Diversity & Evolution, 15:199212. https://doi.org/10.1007/s13127-014-0188-0
Scheyer, T.M. and Sander, P.M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society B, 274:1885-1893. https://doi.org/10.1098/rspb.2007.0499
Scott, R.W., Benson, D.G., Morin, D.G., Shaffer, B.L., and Oboh-Ikuenobe, F.E. 2003. Integrated Albian-Lower Cenomanian chronostratigraphic standard, Trinity River section Texas. Gulf Coast Section SEPM Foundation: U.S. Gulf Coast Cretaceous Stratigraphy and Paleoecology Perkins Memorial Volume: 277-334.
Scott, R.W., Campbell, W., Hojnacki, R., Wang, Y., and Lai, X. 2016. Albian rudist biostratigraphy (Bivalvia), Comanche Shelf to shelf margin. Carnets Geology, 16(21):513-541.
Smith, H.F., Hutchison, H.J., Townsend, K.E.B., Adrian, B., and Jager, D. 2017. Morphological variation, phylogenetic relationships, and geographic distribution of the Baenidae (Testudines), based on new specimens from the Uinta Formation (Uinta Basin), Utah (USA). PlosOne, 12(e0180574):1-40. https://doi.org/10.1371/journal.pone.0180574
Stephenson, L.W. 1915. The Cretaceous-Eocene contact in the Atlantic and Gulf Coastal Plain. United States Geological Survey Professional Paper, 90J. https://doi.org/10.3133/pp90J
Stephenson, L.W. and Stenzel, H.B. 1952. Larger invertebrate fossils of the Woodbine Formation (Cenomanian) of Texas, with decapod crustaceans from the Woodbine Formation of Texas. USGS Professional Paper, 242:1-225. https://doi.org/10.3133/pp242
Suarez, C.A., González, L.A., Ludvigson, G.A., Cifelli, R.L., and Tremain, E. 2012. Water utilization of the Cretaceous Mussentuchit Member local vertebrate fauna, Cedar Mountain Formation. Utah, USA: Using oxygen isotopic composition of phosphate. Palaeogeography, Palaeoclimatology, Palaeoecology, 313:78-92. https://doi.org/10.1016/j.palaeo.2011.10.011
Thurmond, J.T. and Jones, D.E. 1981. Fossil Vertebrates of Alabama. University Alabama Press, Tuscaloosa, Alabama.
Tornabene, B.J. 2014. Movements, Habitats, and Nesting Ecology of Spiny Softshells in the Missouri River: the Influence of Natural and Anthropogenic Factors. Unpublished Master’s thesis, Montana State University, Bozeman, Montana, USA.
Trudel, P. 1994. Stratigraphic Sequences and Facies Architecture of the Woodbine-Eagle Ford Interval, Upper Cretaceous. Unpublished Master’s Thesis, Tarleton State University, Stephenville, Texas.
Vitek, N.S. 2011. Insights into the taxonomy and systematics of North American Eocene soft-shelled turtles from a well-preserved specimen. Bulletin of the Peabody Museum of Natural History, 52:189-208. https://doi.org/10.3374/014.052.0201
Vitek, N.S. 2012. Giant fossil soft-shelled turtles of North America. Palaeontologia Electronica, 15.1.13A:1-43. https://doi.org/10.26879/299
palaeo-electronica.org/content/2012-issue-1-articles/210-giant-soft-shelled-turtles
Vitek, N.S. and Danilov, I.C. 2010. New material and a reassessment of soft-shelled turtles (Trionychidae) from the Late Cretaceous of Middle Asia and Kazakhstan. Journal of Vertebrate Paleontology, 30:383-393. https://doi.org/10.1080/02724631003617548
Vitek, N.S. and Joyce, W.G. 2015. A review of the fossil record of New World turtles of the clade Pan-Trionychidae. Bulletin of the Peabody Museum of Natural History, 56:185-244. https://doi.org/10.3374/014.056.0204
Vlachos, E. 2018. A review of the fossil record of North American turtles of the clade Pan-Testudinoidea. Bulletin of the Peabody Museum of Natural History, 59(1):3-94.
von Nopsca, B. 1928. Palaeontological notes on reptiles IV: Helochelydra and Hylaeochelys, two little known tortoises from the Wealden and Purbeck Formations. Geologica Hungarica Series Palaeontologica, 1:44-50.
Vullo, R., Lapparent de Broin, F., Neraudeau, D., and Durieu, N. 2010. Turtles from the early Cenomanian paralic deposits (Late Cretaceous) of Charentes, France. Oryctos, 9:37-48.
Wang, H. and Dilcher, D.L. 2018. Early Cretaceous angiosperm leaves from the Dakota Formation, Hoisington III locality, Kansas, USA. Paleontologia Electronica, 21.3.34A(3):1-49. https://doi.org/10.26879/841
palaeo-electronica.org/content/2018/2270-early-cretaceous-leaves
Webb, R.G. 1962. North American recent soft-shelled turtles (Family Trionychidae). University of Kansas Publications, 13:429-611.
Weems, R.E. 1988. Paleocene turtles from the Aquia and Brightseat Formations, with a discussion of their bearing on sea turtle evolution and phylogeny. Proceedings of the Biological Society of Washington, 101:109-145.
Weems, R.E. 2014. Paleogene chelonians from Maryland and Virginia. PaleoBios, 31:1-32.
Weishampel, D.B., Barrett, P.M., Coria, R.A., Le Loeuff, J., Xing, X., Xijin, Z., Sahni, A., Gomani, E.M.P., and Noto, C.R. 2004. Dinosaur distribution, p. 517-606. In Weishampel, D.B., Dodson, P., and Osmolska, H. (eds.), The Dinosauria, University of California Press, Berkeley.
Williston, S.W. 1894. A new turtle from the Benton Cretaceous. University of Kansas Quarterly, 3:5-19.
Winkler, D., Jacobs, L., Lee, Y., and Murry, P. 1995. Sea level fluctuation and terrestrial faunal change in North-Central Texas, p. 175-177. In Sun, A. and Wang, Y. (eds.), Sixth Symposium on Mesozoic Terrestrial Ecosystems and Biota. Ocean Press, China.
Zanno, L.E. and Makovicky, P.J. 2013. Neovenatrid theropods are apex predators in the Late Cretaceous of North America. Nature Communications, 4:2827. https://doi.org/10.1038/ncomms3827
Zhu, H. 2011. Plastron Reduction and Associated Myology in Turtles, and Its Implications for Functional Morphology and Natural History. Unpublished Master’s Thesis, Marshall University, Ann Arbor.

