 Diptera of the middle Eocene Kishenehn Formation.
Diptera of the middle Eocene Kishenehn Formation.
I. Documentation of diversity at the family level
Article number: 22.2.50
https://doi.org/10.26879/891
Copyright Paleontological Society, August 2019
Author biographies
Plain-language and multi-lingual abstracts
PDF version
See also Diptera of the Middle Eocene Kishenehn Formation II
Submission: 16 May 2018. Acceptance: 24 May 2019.
{flike id=2622}
ABSTRACT
The Coal Creek Member of the Kishenehn Formation in northwestern Montana, USA, is an emerging middle Eocene Lagerstätte. While fish, plant, mammal and molluscan fossils are present, the most numerous and well-preserved fossils are those of insects. In this study, we initiate an effort to enumerate, at the family level, the diversity of flies (Insecta: Diptera) at this locality. Seventeen specimens from 17 different families (15 families with Limoniinae and Cylindrotominae within Tipulidae s.l.), 15 new species and three new genera are described. These include Tipula fji sp. nov. (Tipulidae), Ellipteroides kishenehn sp. nov. (Limoniidae), Cyttaromyia lynnae sp. nov. (Cylindrotomidae), Sylvicola silibrarius sp. nov. (Anisopodidae), Efcookella nigra sp. nov. (first fossil known in the genus) (Scatopsidae), Bibiodes kishenehnensis sp. nov. (Bibionidae), Eosciarites hermes gen. et sp. nov. (Sciaridae), Rymosia hypnolithica sp. nov. (Mycetophilidae), Litoleptis araeostylus sp. nov. (Rhagionidae), Kishenehnoasilus bhl gen. et sp. nov. (Asilidae), Drapetis adelomedos sp. nov. (Hybotidae), Salishomyia eocenica gen. et sp. nov. (Dolichopodidae), Agathomyia eocenica sp. nov. (first known fossil in genus) (Platypezidae), Lonchoptera eocenica sp. nov. (Lonchopteridae) and Aenigmatias kishenehnensis sp. nov. (Phoridae). Two specimens in the families Psychodidae and Pipunculidae are described but not assigned to a genus. In addition, we revise several related fossil species housed at the NMNH. Asilopsis fusculus Cockerell, 1921, formerly described in Asilidae, is transferred to Cyttaromyia (Cylindrotomidae) as C. fuscula, Sciara florissantensis Cockerell, 1917 is assigned to Sciaroidea incertae sedis, and Sciara gurnetensis Cockerell, 1916, Sciara lacoei Cockerell, 1915 and Sciara protoberidis Cockerell, 1915, are assigned to Sciaridae incertae sedis. Given their diversity and high degree of preservation, continued characterization of the Coal Creek Member fossils may help elucidate the Eocene radiation of Diptera in North America.
Dale E. Greenwalt. Department of Paleobiology, National Museum of Natural History MRC 121, Smithsonian Institution, 10th & Constitution Ave. NW, Washington, D.C. 20013-7012, USA. GreenwaltD@si.edu
Daniel J. Bickel. Entomology, Australian Museum, 1 William Street, Sydney NSW 2010, Australia. dan.bickel@austmus.gov.au
Peter H. Kerr. Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, California 95832-1448, USA. pkerr@cdfa.ca.gov
Gregory R. Curler. Mississippi Entomological Museum, Mississippi State University, 100 Old Highway 12, Box 9775, Mississippi 39762-9775, USA. gcurler@gmail.com
Brian V. Brown. Entomology Section, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, USA. bbrown@nhm.org
Herman de Jong. Naturalis Biodiversity Center, Darwinweg 2, 2333 CR Leiden, The Netherlands. Herman.deJong@naturalis.nl
Scott J. Fitzgerald. Pacific Northwest Diptera Research Lab, 1460 SW Allen St., Corvallis, Oregon, 97333, USA. woodyfitz@gmail.com
Torsten Dikow, Smithsonian Institution, National Museum of Natural History, 10th & Constitution Ave. NW, Washington, DC 20560-0169, USA. dikowt@si.edu
Michal Tkoč. Department of Entomology, National Museum, Cirkusová 1740, CZ-193 00 Praha 9 - Horní Počernice, Czech Republic. michaltkoc@gmail.com
Christian Kehlmaier. Senckenberg Natural History Collections Dresden, Museum of Zoology, Königsbrücker Landstrasse 159, 01109 Dresden, Germany. kehlmaier@web.de
Dalton De Souza Amorim. Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes, 3900, 14040-901, Ribeirão Preto, SP, Brazil. dsamorim@usp.br
Keywords: fossil insects; Diptera; new species; Kishenehn Formation; middle Eocene
Final citation: Greenwalt, Dale E., Bickel, Daniel J., Kerr, Peter H., Curler, Gregory R., Brown, Brian V., de Jong, Herman, Fitzgerald, Scott J., Dikow, Torsten, Tkoč, Michal, Kehlmaier, Christian, and Amorim, Dalton De Souza . 2019. Diptera of the middle Eocene Kishenehn Formation. I. Documentation of diversity at the family level. Palaeontologia Electronica 22.2.50A 1-56. https://doi.org/10.26879/891
palaeo-electronica.org/content/2019/2622-kishenehn-formation-diptera
Copyright: August 2019 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/A6C79E56-3CCC-484E-B6AF-EAEEE1695FF6
INTRODUCTION
Diptera constitute one of the largest and most diverse groups of organisms on Earth. The order appears to have originated in the Permian, based both on molecular divergence studies (Wiegmann et al., 2011; Misof et al., 2014) and the existence of eight dipteran families in the 223-235 Ma Cow Branch Formation in Virginia (Blagoderov et al., 2007). The clade Schizophora, which includes the majority of dipteran families, is relatively recent, having undergone extensive and rapid radiations between the early Paleocene and the middle Eocene (Wiegmann et al., 2011). Despite their modern diversity, including about one-third of all extant dipteran species, Schizophora have a relatively poor fossil record. Evenhuis (2017) indicated that approximately 64% of all fossil species (45.6% of genera) were "Nematocera" or lower Diptera, and only 17% of all described fossil species (about 28.5% of genera) were schizophorans. Less than 1% of fossil species (2.25% of genera) belonged to the younger Calyptratae. Given these numbers, the discovery and proper description of new fossil flies is always relevant, but fossil discoveries documenting higher dipterans would be of critical importance for understanding the recent history of the order.
The middle Eocene Coal Creek Member of the Kishenehn Formation in northwestern Montana, USA, is an emerging Conservat Lagerstätte. Fossil insects from this site display a high degree of preservation of both morphological detail and original biomolecular components (Greenwalt et al., 2013, 2016). Taphonomic processes have produced a unique entomofauna, with a strong size bias against large specimens; fossil insects 1 cm or longer in length are rare and, with the exception of isolated wings, invariably poorly preserved. On the other hand, very small insects (e.g., ptiliid beetles and mymarid wasps) that are rarely found as compression fossils in other Lagerstätten, are frequently preserved in the Kishenehn Formation’s oil shales (Huber and Greenwalt, 2011; Shockley and Greenwalt, 2013; Greenwalt et al., 2015a). As a result, the Coal Creek Member entomofauna provides insights into insect diversity that are not available from other major North American Lagerstätten.
Although detailed studies of several individual families of Coal Creek Member insects have been published, documentation of the overall diversity of the entomofauna is more important and the goal of the current study. While there are at least 17 insect orders represented in the Coal Creek Member (Greenwalt et al., 2015a), enumeration of the total number of families has not been attempted. The current study of the families of the order Diptera is an initial effort towards this goal. Since documentation of diversity at the family level, vs. descriptions of new species per se, is the purpose of this study, new species descriptions are purposefully limited to one for each family. In some instances, families are represented by specimens not identified to genus level. A total of 17 dipteran families are identified herein; combined with those already published (Dixidae, Culicidae, Bolitophilidae and Bombyliidae), the total diversity of the Coal Creek Diptera, at the family level, currently numbers 21. This number will increase as the Kishenehn Formation specimens continue to be characterized. In addition to the description of these new specimens, we revise several fossil species in relevant families from similar geological ages in North America that are housed at the NMNH.
MATERIALS AND METHODS
Specimens described herein were collected from the Kishenehn Formation, exposed along the Middle Fork of the Flathead River in northwestern Montana, USA, between 2009 and 2016 in accordance with USFS Authorization HUN281. Exposures there are from the middle sequence of the Coal Creek Member, which have been estimated to be 46.2±0.4 Ma by 40Ar/39Ar analysis and 43.5±4.9 Ma by fission-track analysis (Constenius, 1996). Specimens were photographed with an Olympus SZX12 microscope equipped with a Q-Color5 Olympus camera. Image-Pro Plus 7.0 software (Media Cybernetics, Inc., Bethesda, MD) was used to capture and record the images. Kishenehn Formation fossils were immersed in 95% ethanol for examination and photography. Measurements were made with the Image-Pro Plus 7.0 software. A thin plate spline analysis was performed to show the direction of the changes from fossil to modern wing veination (for Lonchoptera eocenica). Comparison to a modern species was made by landmarking photographs using the program TPS-Dig. Landmarks were transformed using Procrustes analysis as implemented in PAST 3.22 (Hammer et al., 2001), and a comparative thin plate spline generated in the same program.
Venational terminology is from Cumming and Wood (2017). Although recent evidence (e.g., Ribeiro, 2008; Petersen et al., 2010; Zhang et al., 2016) suggests that Limoniidae is paraphyletic, this group is treated as a family herein. The holotypes of Asilopsis fuscula Cockerell, 1921 (USNM 66572), Rhagio fossitus Melander, 1949 (USNM 112626), Sciara florissantensis Cockerell, 1917 (Cockerell 1917a) (USNM 61995), S. gurnetensis Cockerell, 1915 (USNM 61435), S. locoei Cockerell, 1915 (USNM 61436) and S. protoberidis Cockerell, 1915 (USNM 61437) are housed at the NMNH, in Washington, D.C. Numbers of extant genera and species/family were taken from Pape et al. (2011). The number of fossil species for each individual family was obtained from the Paleobiology database. Institution acronyms and abreviations used herein are PBDB (Paleobiology Database), FDB (Florissant National Monument Fossil Database), EDNA (EDNA Fossil Insect Database), EOL (Encyclopedia of Life), NMNH (National Museum of Natural History), USNM (United States National Museum = NMNH depository), and LACM (Los Angeles County Museum).
Although specimens of modern, extant insect species have been reported in some older papers on Mexican and Baltic amber (Doutt, 1973; Masner, 1969; Mockford, 1972; Rozen, 1971), the assumed timespan of insect species has been estimated to be 3-10 My (Grimaldi and Engel, 2005). We have therefore limited comparisons of new species described herein to fossils of the Eocene Epoch.
Database Searches
The number of dipteran families from major Eocene localities was obtained from online digital databases and, for the Okanagan/Republic locality, a review of the literature. Given their universal value and huge potential, especially with the advent of database-based research, it is very disappointing that all on-line digital databases, including the PBDB (PBDB, 2018), FDB (Meyer, 2002), EDNA (EDNA, 2017), Bishop Museum Fossil Diptera Catalog (Evenhuis, 2017) and Systema Dipterorum (Pape and Thompson, 2013), are woefully underfunded. As a result, some databases are more up-to-date than others and different databases occasionally provide different results. The material that follows is not meant to be a criticism of the digital databases but rather straightforward documentation of the status of specific records.
The EDNA database was searched for Green River Formation dipteran families, with the parameters "Order equals Diptera", "Country equals U.S.A." and "Era equals Cenozoic" ("Period equals Paleogene" yields a null set) and yielded 442 records. Searches of "Formation contains Green", "Site: [any field] contains Green" and "Reference: Title contains Green" in place of "Country equals U.S.A." yielded 4, 28 and 10 records respectively. Obvious undesired locations (e.g., the Barstow, Kishenehn and Florissant Formations) as well as all junior synonyms were deleted from the 442 records to provide 155 records overall. Numerous records were assigned to local sites (e.g., Twin Creek, East Alkali Gulch, Little Duck Creek, Little Tommies Draw) that belonged to the Green River but were not originally recorded as such. Evidently, this is due to data entry based solely on the original publication; this also results in the geological age of Green River Formation specimens being listed as both Eocene and Oligocene. Most records of senior synonyms were entered without the Green River locality designation that accompanied the original junior synonym. The majority of the 155 records were listed solely as originating in the U.S.A. or, in some cases, a particular state (Colorado, Utah and Wyoming). Queries of the literature for more than 90 of these latter records eliminated many entries, nearly all of which were from the Florissant but not designated as such in the database. Corrections included transfer of Anthomyia winchesteri Cockerell, 1921 (Cockerell 1921a), from Anthomyiidae Robineau-Desvoidy, 1830, to unplaced Brachycera (Michelsen, 1996), Culex proavitus Scudder, 1877 from Culicidae Meigen, 1818, to Psychodidae Newman, 1834 (Edwards, 1923), and Heteromyza detecta Scudder, 1877, from Palaeopleciidae Rohdendorf, 1962 to Heleomyzidae Bezzi, 1911 (Evenhuis, 2017). Rhingia zephyrea Hull, 1945, is listed as a junior synonym of Geron oligocaenica Timon-David, 1944; however, Evenhuis (1994) transferred Phthiria oligocenica Timon-David, 1944, to the genus Geron Meigen, 1820 as G. oligocaenica within Bombyliidae. Nel (2006) considered the specimen to be Bombyliidae Latreille, 1802, subfamily and genus undetermined. In addition, Rhingia zephyrea was collected by Hull (1945) from the Florissant.
Corrections to the EDNA database relative to Baltic amber Diptera are as follows: Sciadoceridae, as the subfamily Sciadocerinae Schmitz, 1929, is currently placed in Phoridae Curtis, 1833 (Brown, 2007). Although often used during most of the twentieth century, Leptidae is an invalid synonym for Rhagionidae (Kertész, 1908; Malloch, 1931). Species of Palaeopleciidae are now included in the extinct family Protopleciidae Rohdendorf, 1946 (Blagoderov, 1996). Rachiceridae, as the subfamily Rachicerinae Curran, 1934, is placed in Xylophagidae (Clapham, 2016).
In the Florissant Fossil Database, the single species of Xylophagidae Fallén, 1810, Dialysis revelata Cockerell, 1908, was originally described in the family Leptidae (invalid family name; = Rhagionidae Latreille, 1802) and is listed in Systema Dipterorum in Xylophagidae. However, Melander (1949) placed D. revelata in Rhagionidae. The genus Dialysis Walker, 1850, was revised by Webb (1978). Melieria atavina Cockerell, 1917 (Cockerell 1917b), and M. calligrapha Melander, 1949, were originally placed in the family Otitidae Aldrich, 1932 and these two are the sole species ascribed to Otitidae in the FDB. However, Gentilini et al. (2006) have transferred both species to Ulidiidae Macquart 1835 (see Kameneva and Korneyev, 2006 for Otitidae as a junior synonym of Ulidiidae). The FDB lists five species in the family Anthomyiidae: Mecistoneuron perpetuum Melander, 1949, and Ophyra vetusta Melander, 1949, Anthomyia atavella Cockerell, 1913, A. persepulta Cockerell, 1917 (Cockerell 1917b) and A. laminarum Cockerell, 1917 (Cockerell 1917c). Evenhuis (1994) reassigned O. vetusta to Muscidae Latreille, 1802 and Michelsen (1996) reassigned M. perpetuum to Platypezidae Latreille, 1802. Michelsen (1996) designated all eleven compression fossils of the genus Anthomyia available at that time as nomina dubia.
A Note on Taxonomic Paleoentomology
The uncertain and often invalid status of many generic assignments of fossil insects is, if not fully appreciated, well known. Many such assignments were made in the nineteenth and early twentieth centuries, when attitudes and protocols were much less rigorous than they are today. Unfortunately, descriptions of new species with similarly suspect generic assignments continue to be published for both extinct and extant specimens (e.g., Hong, 2002; Park and Carlton, 2014). Poor preservation and/or the absence of the morphological detail required for rigorous generic identification forms the basis of most invalid assignments. In the absence of required morphological data, it is not uncommon for paleoentomologists to indicate uncertainty in their generic assignment. This has taken several forms. "Open nomenclature" wherein, for example, a question mark is added to the generic epithet (Richter, 1943; Bengtson, 1988) is commonly utilized. Unfortunately, subsequent literature rarely includes the punctuation mark and, with time, the generic assignment becomes more definitive than was originally intended. This is particularly problematic as it relates to modern digital databases, none of which record the question mark as an indicator of uncertainty.
Another common convention that can convey uncertainty and confusion in subsequent studies is the addition of prefixes such as litho-, archeo-, palaeo-, etc. to the stem of the name of an extant genus to create often unwarranted genera. For example, Lithobibio Beier, 1952, was based on an inaccurate interpretation of wing venation and was corrected by Nel (1994) to Bibio Geoffroy, 1762. Miopsiloptera savchenkoi Gentilini, 1984, from the Miocene of Italy, was placed in Symplecta Meigen, 1830, by Evenhuis (1994). The dipteran Mesotanyderus Riek, 1955 is now recognized as a mecopteran and Protocyrtus Rohdendorf, 1938, originally described as a fly, is now assigned to Hymenoptera (Evenhuis, 1994). Use of such prefixes, when applied to a genus that can be shown, based on preserved morphological details, to be related to but definitively distinct from an extant genus, can be informative and legitimate; when applied to specimens that lack enough detail to allow differentiation from another taxon, it simply confounds the taxonomy of that taxon.
Designation of specimens as incertae sedis, undetermined or simply "sp." may often be more appropriate and scientifically accurate. The problem is that most modern digital databases (e.g., the EDNA fossil insect database) are entirely species-based and do not record specimens that are designated as indeterminate, "sp." or incertae sedis. This results in the loss of valuable information since unassigned specimens can be of highly significant scientific value (e.g., Talamas and Buffington, 2015; Lak and Nel, 2009). Different approaches are currently taken regarding this conundrum. Talamas and Buffington (2015) figured specimens in Dominican amber from 25 extant genera, but described and assigned species names to only two. They argued that "quality morphology-based taxonomy requires examination of primary types and specimens from a broad geographical range to provide a context for interpreting morphology and intraspecific variation. Without synthetic work that provides a sound basis for accurate identification, the description of new species is of little use to taxonomy and can result in the proliferation of unstable species names, which are ultimately detrimental to understanding biodiversity and evolutionary history." A less extreme but still stringent approach was taken by Palmer et al. (1957) who stated that fossil "species are named only if critical morphological features of species rank are preserved on the specimens". An approach, perhaps at the opposite end of the spectrum, was taken by Pierce (1966) who stated simply "we need to have names to associate our findings."
SYSTEMATIC PALEONTOLOGY
Order Diptera Linnaeus, 1758
Family TIPULIDAE Latreille, 1802
Genus TIPULA Linnaeus, 1758
Type species. Tipula oleracea Linnaeus, 1758
Subgenus Trichotipula Alexander, 1915
Type species. Tipula oropezoides Johnson, 1909
Tipula (Trichotipula) fji De Jong, sp. nov.
Figure 1, Figure 2, Figure 3
zoobank.org/0CFD881A-D645-4490-99C0-95878A8C7E66
Etymology. The specific epithet (to be pronounced as efyaï) is the Latin genitive case for FJ, which stands for Floris-Jan Muys, a young Dutch researcher.
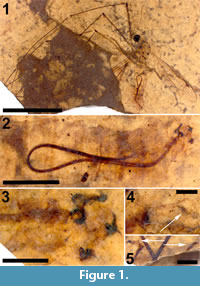 Holotype. USNM 625687, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Holotype. USNM 625687, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Tipula is distinguished by the short vein Rs, the parallel-sided and pentagonal discal cell, the petiolate cell m1, the length and position of crossvein m-cu, and the shape of the male terminalia.
Description
Adult male (Figure 1.1), body length about 13.5 mm, wing length about 11.5 mm. Specimen preserved in lateral view.
Head. Eyes well-developed, large, almost covering entire head, dorsally with narrow separation. Rostrum shorter than remainder of head, nasus invisible (Figure 2.1). Antenna about 4.5 mm long, longer than head and thorax combined, with elongate scape, pedicel not identifiable, flagellum consisting of 11 cylindrical flagellomeres with enlarged base that carries a set of verticils; flagellomeres becoming shorter towards apex of antenna, apical flagellomere abruptly much shorter than preceding one; most flagellomeres have become separated in fossil. Palp not clearly segmented, apparently densely set with setae (Figure 2.2).
Thorax. Scutum with dorsum moderately curved. Contours of halter indicated, halter about 1.2 mm long.
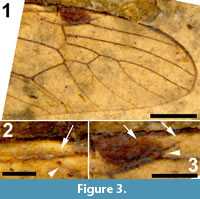
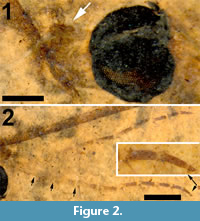 Wings. Right wing visible (Figure 3.1), although partly broken, apical part of left wing missing. Pterostigma distinct, dark-brown. Microtrichia on membrane visible. Subcosta long, terminating in R1 just apical of origin of Rs (Figure 3.2). R1 long, almost straight, terminating in costa near midlength of pterostigma. Rs very short and curved, forking near proximad side of pterostigma. R2+3+4 short, forking into R2+3 and the long R4 at distad end of pterostigma (Figure 3.3). R4 almost straight towards wing margin. Base of R5 aligned with crossvein r-m; long apical section of R5 slightly curved. M forking into short basal sections of M1+2 and M3+4. Discal cell with anterior and posterior margins almost parallel-sided, discal cell pentagonal. M1+2 forming petiole apicad of discal cell, then forks into a gradually widening cell m1 towards wing margin. M3 curves with anteriorly concave bow towards wing margin. M4 fuses with crossvein m-cu for a short distance near proximal part of discal cell and from there curves with an anteriorly concave arch towards wing margin. Crossvein m-cu strong and distinctly longer than Rs. CuA upturned and slightly angled at point of contact with crossvein m-cu; apical section of crossvein CuA rather abruptly curved just before wing margin. False vein immediately posterior to CuA present. CuP gradually deviating from CuA from wing base towards wing margin. A1 long, slightly sinuous. Anal area of wing well-developed.
Wings. Right wing visible (Figure 3.1), although partly broken, apical part of left wing missing. Pterostigma distinct, dark-brown. Microtrichia on membrane visible. Subcosta long, terminating in R1 just apical of origin of Rs (Figure 3.2). R1 long, almost straight, terminating in costa near midlength of pterostigma. Rs very short and curved, forking near proximad side of pterostigma. R2+3+4 short, forking into R2+3 and the long R4 at distad end of pterostigma (Figure 3.3). R4 almost straight towards wing margin. Base of R5 aligned with crossvein r-m; long apical section of R5 slightly curved. M forking into short basal sections of M1+2 and M3+4. Discal cell with anterior and posterior margins almost parallel-sided, discal cell pentagonal. M1+2 forming petiole apicad of discal cell, then forks into a gradually widening cell m1 towards wing margin. M3 curves with anteriorly concave bow towards wing margin. M4 fuses with crossvein m-cu for a short distance near proximal part of discal cell and from there curves with an anteriorly concave arch towards wing margin. Crossvein m-cu strong and distinctly longer than Rs. CuA upturned and slightly angled at point of contact with crossvein m-cu; apical section of crossvein CuA rather abruptly curved just before wing margin. False vein immediately posterior to CuA present. CuP gradually deviating from CuA from wing base towards wing margin. A1 long, slightly sinuous. Anal area of wing well-developed.
Legs. Left legs partly preserved, left foreleg almost complete. Femora and tibiae darkened at extreme tips. No tibial spurs identifiable. Apical tarsomere of foreleg with claw carrying a basal tooth (Figure 1.5).
Abdomen and genitalia. Abdomen made up of rather short segments. Male outer and inner genitalia partly visible (Figure 1.3). Posterior margin of tergite nine with a pair of lateral bulbous extensions that are ventrally set with dark spines; area between the lateral extensions U-shaped emarginate and (ventro-?) medially blackish, sclerotized; black spines along (ventral side of) posterior margin. A pair of blackish-brown gonostyles visible with broad base and slender, somewhat sinuous anterior part that ends in a narrow point; a bundle of thick black, curved setae on dorsal margin. Broad and dark-brown aedeagus clearly visible through integument, runs anterior from ill-defined sperm pump in segment seven to segment three and from there loops back to aedeagal guide in terminal segment (Figure 1.2).
Allotype. Female unknown.
Syncompressions. Coprolite (1).
 Remarks
Remarks
Tipulidae s.str. currently include 38 recent genera and 4,294 species and subspecies; the genus Tipula comprises 40 recent subgenera with 2,634 species and subspecies (Oosterbroek, 2018). The higher-level classification of the Tipulidae does not necessarily reflect phylogenetic relationships within the family and is in need of revision. Over 100 fossil species are described in Tipulidae, most of them are classified in Tipula sensu lato. The few fossil species that have been assigned to a subgenus of Tipula include T. (Electrotipula) pinetorum Alexander, 1931 (Alexander, 1931a), T. (Platytipula) anatolica Kania and Nel, 2013, T. (Tipula) oligocenica Kania and Nel 2013, and T. (Trichotipula) paicheleri Kania and Nel, 2013. Electrotipula Alexander, 1931 (Alexander, 1931a) is the only described extinct subgenus of Tipula.
The present fossil is placed in the genus Tipula because of its relatively small size, the presence of simple flagellomeres with a whorl of verticils at their enlarged bases (Figure 2.2), the long Sc ending apical of the origin of Rs, the petiolate cell m1 and the fusion of M4 with m-cu near the proximal part of the discal cell. It is provisionally placed in the subgenus Trichotipula because of the short vein Rs, the shape of the discal cell (Figure 3.1), the shape and armament of the posterior margin of tergite nine and the shape and sclerotization of the inner gonostylus. The inner gonostylus and the posterior margin of tergite nine are reminiscent of that of the type species of Trichotipula, T. oropezoides (cf. Alexander, 1965, figure 31). The exceptionaly well-preserved aedeagus may suggest that this particular cranefly was teneral and the post-eclosion period too short to have allowed cuticular sclerotization.
Trichotipula includes 46 recent species, of which 34 are known from the Nearctic region; eight species are recorded from the Neotropical region, four from the East Palaearctic and one from the Oriental region; one species occurs in both the Nearctic and Neotropical regions. Most, but not all, Trichotipula species have at least some macrotrichia on the membrane of the wingtip; in the present fossil, microtrichia can be observed quite clearly, but macrotrichia are absent.
The fossil species from the late Oligocene of Turkey classified by Kania and Nel (2013) in Trichotipula does not belong to this subgenus, or even to the genus Tipula. The very short vein Rs, the sessile cell m1 and the position of crossvein m-cu proximal of the discal cell indicate that it belongs to the genus Nephrotoma Meigen, 1803. For these reasons, the species paicheleri is formally transferred to the genus Nephrotoma as Nephrotoma paicheleri (new combination).
Family LIMONIIDAE Rondani, 1856
Genus ELLIPTEROIDES Becker, 1907
Type species. Ellipteroides piceus Becker, 1907
Subgenus Ellipteroides Becker, 1907
Type species. Ellipteroides piceus Becker, 1907
Ellipteroides (Ellipteroides) kishenehn De Jong, sp. nov.
Figure 4
zoobank.org/8BD7F94B-69ED-43DE-8792-F2AA9196DFC3
Etymology. The specific epithet is regarded here to be a noun in apposition to the genus name Ellipteroides, which is masculine.
Holotype. USNM 621123, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of the genus Ellipteroides is distinguished by the length of vein Sc, absence of vein R2, wide cell r3, absence of the discal cell and the shape of cell m1.
 Description
Description
Adult male (Figure 4.1), body length about 5.0 mm, wing length about 5.1 mm. Specimen preserved in dorsal view.
Head. Head round. Eyes large covering most of sides of head, widely separated medially. Rostrum hardly visible but by inference very short. Antenna about 0.7 mm long, number of segments not distinguishable, basal segments short and somewhat bulbous, becoming more elongate and slender apically towards antennal tip; intermediate and apical flagellomeres with long verticils that exceed length of segments (Figure 4.4). Palp only vaguely indicated. Occiput dark brown.
Thorax. Hardly any distinguishing characters; metatergite distinct from remainder of thoracic dorsum. Thorax dark brown.
Wings. Both wings entirely preserved, right wing somewhat folded along M. Pterostigma not visible (Figure 4.2-3). Sc terminating in costa at level of first fork of Rs. The position of crossvein sc-r is uncertain, but it is possibly present at some distance proximad of apex of Sc. R1 long, straight, terminating in costa near level of fork of R(2+)3 and R4. Rs long, originating at level of apex of A1, gradually curved. R(2+)3+4 with short petiole, free section of R2 absent, R3 slightly sinuous and subparallel to apex of R1, R4 long, slightly sinuous. R5 long, evenly curved towards wing tip. Crossvein r-m rather long, a bit curved and oblique. Discal cell absent. M branches into M1+2 and M4 (M3 absent). M1+2 with short petiole before branching into M1 and M2. M4 aligned with M. Crossvein m-cu touches M at its branching point, appearing somewhat curved possibly due to deformation of fossil. CuA almost straight, not upcurved at contact with crossvein m-cu, apical section of CuA aligned with preceding part of vein. False vein immediately posterior to CuA distinct from base of wing to level of crossvein m-cu. CuP gradually diverging from CuA from wing base to margin. A1 long, gradually bowed to posterior wing margin. Anal area well developed, anal corner evenly rounded.
Legs. Missing.
Abdomen and genitalia. Abdomen entirely present, but covered by wings, dark-brown. Genitalia preserved in dorsolateral view, but no details discernible.
Allotype. Female unknown.
Syncompressions. None.
Remarks
The family Limoniidae currently includes 147 recent genera and 10,578 described species (Oosterbroek, 2018). The genus Ellipteroides is divided into six subgenera, Ellipteroides sensu stricto (with 15 species), Progonomyia Alexander, 1920 (55), Protogonomyia Alexander, 1934 (38), Ptilostenodes Alexander, 1931b (9), Ramagonomyia Alexander, 1968 (2) and Sivagonomyia Alexander, 1968 (1). Ellipteroides is a taxonomic derivative of a huge clade that is dominated by the large genus Gonomyia Meigen, 1818. The systematics of this group is based on venational characters as the length of Sc, presence or absence of R2, depth of cell r3, presence or absence of the discal cell, shape of cell m1 and the structure of the male and female terminalia. The classification is in need of revision and given the present situation, the fossil is best filed under the subgenus Ellipteroides (Ellipteroides). Placement in the genus Ellipteroides is based on the long Rs, the apically wide cell r3, the very long R4 (much longer than R(2+)3+4), the position of m-cu near the fork of M, branching of M into M1+2 and M4. Placement in the subgenus Ellipteroides s.s is based on the absence of a free section of R2, the absence of a discal cell, and the petiole of M1+2 being shorter than its fork. In the Nearctic region, the genus Ellipteroides is represented by only four recent species that are classified in the subgenus Progonomyia. No fossils of Ellipteroides s.l. have previously been described.
Family CYLINDROTOMIDAE Schiner, 1863
Genus CYTTAROMYIA Scudder, 1877
Type species. Cyttaromyia fenestrata Scudder, 1877
Cyttaromyia lynnae De Jong, sp. nov.
Figure 5, Figure 6
zoobank.org/D2C5FED7-813C-418A-B1B4-A35C286BD427
Etymology. The specific epithet is the Latin genitive case of the first name of the wife of the author (HDJ).
Holotype. USNM 621109, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Cyttaromyia is distinguished by the presence of an additional crossvein r'-m', a wide and long discal cell, four complete but relatively short medial veins, and a female terminalia with a long and curved extension of tergite 10, slender and curved cerci and broad hypogynial valves.
Description
Adult female (Figure 5.1), body length about 10 mm, wing length about 10 mm. Specimen preserved in dorsal view.
Head. Eyes well-developed, large, dorsally widely separated though distance not measurable due to crushed state of head (Figure 6.3). Occiput dark brown colored. Antenna 2.4 mm long, about as long as head and thorax combined, consisting of short scape and pedicel, and slender flagellum including 14 cylindrical flagellomeres. Some verticils at base of flagellomeres preserved, distinctly shorter than length of flagellomeres. Rostrum short. Palp with third to fifth segments visible, this part measuring about 0.9 mm, third and fourth segments robust, fifth irregularly shaped and about as long as third and fourth combined (Figure 6.1).
Thorax. Pronotum with well-developed antepronotum and broad postpronotum, scutum with visible transverse suture in posterior part. Thorax brownish in groundcolor, dark-brown on postpronotum and with broad dark-brown medial stripe and lateral sides on scutum.
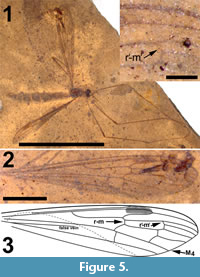 Wings. Elongate and slender, anal areas folded under in both wings (Figure 5.2-3). Pterostigma distinct, located between C and first section of R2+3+4, dark-brown. Sc long, terminating in C well beyond level of first fork of Rs and anterior to pterostigma. The positions of crossvein sc-r and R1, if they exist, are uncertain. Rs long, almost 2.0 mm, about 0.75 X as long as entire R2+3+4. R5 evenly curved towards wing margin. Crossvein r-m connecting Rs with discal cell slightly distad of first fork of Rs. Vein M long, forking at proximad end of discal cell in a long first section of M1+3 and a short first section of M3+4. Discal cell elongate, gradually widening towards wing margin, cell remarkably longer than apical sections of M1 -M4. M1+2 shortly petiolate distad of discal cell where it forks into M1 and M2. Base of apical part of M1 almost perpendicular to M2, before turning towards apex of wing; M1 here connected to R5 by additional crossvein (r'-m'). Apical section of M2 almost continuous with petiole of fork of M1+2. M3+4 forks at distal part of discal cell, apical section of M4 almost continuous with M3+4 and base of M3 making angle of about 70o with M3+4 and M4. Apical section of M3 subsinuous from discal cell towards wing margin. Crossvein m-cu located at proximad corner of discal cell. CuA only very slightly bent at point of contact with crosvein m-cu; visible part of apical section of CuA straight. Remainder of venation invisible.
Wings. Elongate and slender, anal areas folded under in both wings (Figure 5.2-3). Pterostigma distinct, located between C and first section of R2+3+4, dark-brown. Sc long, terminating in C well beyond level of first fork of Rs and anterior to pterostigma. The positions of crossvein sc-r and R1, if they exist, are uncertain. Rs long, almost 2.0 mm, about 0.75 X as long as entire R2+3+4. R5 evenly curved towards wing margin. Crossvein r-m connecting Rs with discal cell slightly distad of first fork of Rs. Vein M long, forking at proximad end of discal cell in a long first section of M1+3 and a short first section of M3+4. Discal cell elongate, gradually widening towards wing margin, cell remarkably longer than apical sections of M1 -M4. M1+2 shortly petiolate distad of discal cell where it forks into M1 and M2. Base of apical part of M1 almost perpendicular to M2, before turning towards apex of wing; M1 here connected to R5 by additional crossvein (r'-m'). Apical section of M2 almost continuous with petiole of fork of M1+2. M3+4 forks at distal part of discal cell, apical section of M4 almost continuous with M3+4 and base of M3 making angle of about 70o with M3+4 and M4. Apical section of M3 subsinuous from discal cell towards wing margin. Crossvein m-cu located at proximad corner of discal cell. CuA only very slightly bent at point of contact with crosvein m-cu; visible part of apical section of CuA straight. Remainder of venation invisible.
Legs. Front legs and left midleg almost completely preserved; what appears as right midleg with femur and part of tibia preserved. Femora somewhat broader at tip, apex darkened. Tibiae without visible apical spurs. No claws detected.
Abdomen and genitalia. Abdomen, 7.6 mm long, broadening from segment one to five and from there narrowing towards ovipositor. Ovipositor preserved in lateral view, showing long curved extension of tergite ten dorsal of cerci and hypogynial valves. Cerci long, curved and rather slender, hypogynial valves shorter than cerci and broad (Figure 6.2).
Allotype. Male unknown.
Syncompressions. Gastropods (8).
Remarks
Cylindrotomidae is a small family of Tipuloidea with only nine recent genera, including 70 species (Oosterbroek, 2018). The recent genera occurring in North America are Cylindrotoma Macquart, 1834 (with two recent North American species), Liogma Osten Sacken, 1869 (1), Phalacrocera Schiner, 1863 (4), and Triogma Schiner, 1863 (1). The genera can be easily separated using wing venational characters (Alexander and Byers, 1981). In a phylogenetic analysis of both molecular and morphological data, Petersen et al. (2010) recovered this group as monophyletic and sister to Tipulinae but were unable to confidently resolve the combined group within Tipuloidea; they treat the group as a subfamily within Tipulidae.
Fossil Cylindrotomidae have been described in the extant genera Cylindrotoma and Diogma Edwards, 1938 and the extinct genus Cyttaromyia Scudder, 1877 (Table 1) (Cockerell 1921a, 1925, 1926; Freiwald, 1991; Freiwald and Krzemiński, 1991; Krzemiński, 1998; Podenas, 2000; Scudder, 1877, 1894; Séguy, 1934). All fossil Cylindrotomidae are characterized by the presence of four medial veins reaching the wing margin (recent Diogma always show only three medial veins). The genus Cyttaromyia was created by Scudder (1877) based on the apical half of an isolated wing. Scudder redefined the genus in 1894 based on several more intact specimens from the Florissant Formation. Members of Cyttaromyia differ from most Cylindrotoma and Diogma (and the other recent Cylindrotomidae) by the presence of additional crossvein r'-m'. This additional crossvein connecting the base of M1 with R5 is sometimes also found in aberrant specimens of both the North American and Palaearctic subspecies of the recent Cylindrotoma distinctissima Meigen, 1818 (cf. Brodo, 1967, fig. 46; Peus, 1952, fig. 14) and in the Eastern Palaearctic and Oriental C. taiwanica Alexander, 1929 (Alexander, 1929, fig. 4).
Scudder (1894) described Cyttaromyia as lacking tibial spurs. This character state, in addition to the presence of r'-m', could more definitively define Cyttaromyia relative to Cylindrotoma. However, Cyttaromyia frelloi Krzemiński, 1998, the only specimen of the genus in Baltic amber, was described as with "distinct tibial spurs present; single on the forelegs and midlegs, paired on hind legs. The spur of the foreleg is especially large, of a size not met till now in the Tipulidae and Limoniidae." Tibial spurs have not been reported in the more poorly preserved compression fossils of this genus.
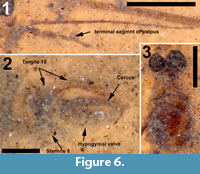 The structure of the ovipositor of the fossil described here (Figure 6.2) shows close similarity with that known from recent Cylindrotoma, where tergite ten is posteriorly extended into a long, curved and apically forked extension that is positioned dorsal of the cerci (cf Brodo, 1967, figs. 21, 22; Peus, 1952, figure 7b, c, 30). Krzemiński (1991a) previously suggested the similarity of the ovipositor of Cyttaromyia to that of Cylindrotoma. The species is placed in the genus Cyttaromyia because of the presence of additional crossvein r'-m', which it shares with all other species of Cyttaromyia. The crossvein can be present in aberrant specimens of some species of the extant genus Cylindrotoma.
The structure of the ovipositor of the fossil described here (Figure 6.2) shows close similarity with that known from recent Cylindrotoma, where tergite ten is posteriorly extended into a long, curved and apically forked extension that is positioned dorsal of the cerci (cf Brodo, 1967, figs. 21, 22; Peus, 1952, figure 7b, c, 30). Krzemiński (1991a) previously suggested the similarity of the ovipositor of Cyttaromyia to that of Cylindrotoma. The species is placed in the genus Cyttaromyia because of the presence of additional crossvein r'-m', which it shares with all other species of Cyttaromyia. The crossvein can be present in aberrant specimens of some species of the extant genus Cylindrotoma.
Cyttaromyia lynnae differs from C. vahldieki Freiwald, 1991, and C. rayona Freiwald and Krzemiński, 1991, in not having a distinct patterning of the wings and from C. frelloi, C. quievreuxi Séguy, 1934, and C. reclusa Cockerell, 1925, in being female. There are numerous differences in the venation of C. lynnae compared to both C. princetoniana Scudder, 1894, and C. fenestrate Scudder, 1877. In the former, Rs is relatively short (Rs/R2+3 = 1.4; 1.8 in C. lynnae), R1 is distinct and the distance between the 1st fork of M and m-cu is subequal to the length of r-m whereas that value is < 0.25 in C. lynnae. The ratio of the discal cell’s L/W = 2.4 in C. fenestrata and 3.3 in C. lynnae. In addition, Sc terminates in C in-line with the 2nd half of the supplemental discal cell in C. fenestrata but just beyond the r-m in C. lynnae. Cells m1 and m2 are equal in length in C. fenestrata whereas m2 is longer in C. lynnae. The terminus of r-r is in line with r'-m' in C. fenestrata but greatly basad of r'-m' in C. lynnae. Given the poor preservation of C. scudderi, it is difficult to identify differences with respect to C. lynnae except perhaps the shape of the pterostigma. The venation of C. obdurescens Cockerell, 1925 is also very similar to C. lynnae, although Cockerell (1926) stated that it was "similar" to C. oligocena Scudder, 1894 and "may prove a synonym of C. reclusa". The reliance on slight differences in venational morphology potentially diminishes the probable status of many of the fossil Cyttaromyia as separate species. Brodo (1967) has figured a large degree of intraspecific variability in the venation of multiple different specimens of the extant species Cylindrotoma distinctissima and C. tarsalis Johnson, 1912, as well as in specimens from three additional related genera. Given the existence of numerous specimens of some of the North American species (for example, there are 12 specimens of Cyttaromyia reclusa [Brown, 1988]), it would be of interest to study the intraspecific variability in their venation patterns.
Cyttaromyia fuscula Cockerell, 1921 (Cockerell, 1921a, Brodo, 1967)
Figure 7
Asilopsis fusculus Cockerell, 1921 (Cockerell, 1921a)
Asilopsis fuscula Cockerell, 1921 (Evenhuis, 1994)
Material examined. Holotype, wing only. USNM 66572 (NMNH; examined).
Type horizon. Middle Eocene, Green River Formation.
Type locality. White River, Colorado, USA
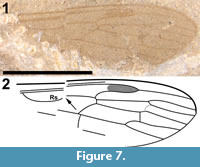 Redescription
Redescription
This specimen consists of a single wing (Figure 7.1-2). The shape of what can be interpreted as R2+3+4 and R1, the shape and size of the discal cell, the position of what appears to be M1, and the shape of cell m2, which narrows toward the wing margin, indicate that this is a representative of Cylindrotomidae. Scudder (1877) described Cyttaromyia fenestrata from White River, but C. fenestrata has a shorter and apically much wider discal cell, and a very wide cell m3 compared to C. fuscula. The short section between the first forking of vein M and the position where crossvein m-cu touches the discal cell in C. fusculus differs from the other known Cyttaromyia species; Cyttaromyia fuscula appears to be a distinct species.
Remarks
Originally assigned to Asilidae by Cockerell (1921a), Asilopsis fusculus was discussed as possibly a member of Asilinae or Laphriinae or its own new subfamily Asilopsinae. Hull (1962) discussed the fossil and stated "... the ultimate interpretation of Asilopsis Cockerell must rest upon the presence or absence of a proboscis and the character of the pretarsus. Without further material and for the reasons given above, I reject a subfamily based upon this fly." The specimen was subsequently assigned to Tipulidae by Brodo (1967). Twenty years later, Brown (1988), in a review of fossil Cyttaromyia, relied on the input of Curtis Sabrosky and Aubrey Scarbrough who stated "We believe it is a primitive asilid... and not at all tipuloid." However, neither Sabrosky nor Scarbrough, who were Brachycera specialists, appear to have been well-acquainted with the diversity of crane flies. Brodo (1967) followed the North American concept of "Tipulidae" in which Tipulidae s.l. includes Tipulidae s.str. (as Tipulinae), Cylindrotominae, Limoniinae and Pediciinae; the rest of the world treats these four taxa as families. We believe that this specimen represents neither Tipulidae s.str. nor Limoniidae; the only other crane fly possibility other than Cylindrotomidae would be Pediciidae (based on what in that case would be R4+5), but then the shape of R1, R2 and R3 would be very unusual, the discal cell much too large and its shape atypical, and the number of M veins would be 'incorrect' for Pediciidae. With the caveat that the specimen is poorly preserved, we propose that wing venation is similar to that of Cylindrotomidae: Cyttaromyia.
Family PSYCHODIDAE Newman, 1834
BRUCHOMYIINAE Alexander, 1921
Bruchomyiinae incertae sedis
Figure 8
Holotype. USNM 619952, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Disbrow Creek site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. Based only on the wing, Bruchomyiinae is distinguished from all other Psychodidae by the following combination of character states: measured at their greatest value, distance from base to apex at least three times that of anterior to posterior wing margin; five radial veins present; base of R1 prominent, thickened, setose; apex of CuA reaching wing margin.
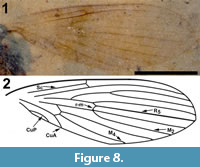 Description
Description
Two wings (Figure 8.1-2), the left wing missing a portion of it postero-apical end, attached to remnants of the mesosoma.
Wings. Wing length, 3.08 mm, 1.02 mm wide; macrotrichia not present/preserved. No costal breaks beyond base. Distances from wing base - Rs fork - Sc terminus - r-m - R2+3 fork - wing apex are 1.08, 0.22, 0.12, 0.25 and 1.35 mm; ratio of the length of the stem of R2+3 to the length of the fork is 0.5. Apex of wing between R4 and R5. M2 originating at the end of second basal cell (i.e., at r-m).
Allotype. Sex unknown.
Syncompressions. None
Remarks
Approximately 150 genera and 3,020 species of Psychodidae have been described (Pape et al., 2011); although estimates suggest the actual diversity is much greater (Wagner and Ibañez-Bernal, 2009). In addition to Bruchomyiinae, there are an additional six subfamilies including Datziinae Stebner et al., 2015, which are known only from fossil species. Despite their occurrence in a variety of habitats and frequent numerical abundance (Brown, 2005; Wagner and Ibañez-Bernal, 2009), most psychodids are poorly known. In contrast, many phlebotomine species are well known due to their role as vectors of Leishmania Ross, 1903 spp. and other disease agents.
There is a rich fossil record for psychodids dating to the early Jurassic (Ansorge, 1994) and possibly the late Triassic (Fraser et al., 1996; Blagoderov et al., 2007); however, Blagoderov et al. (2007) note that Triassopsychoda olseni Blagoderov and Grimaldi in Blagoderov, Grimaldi and Fraser, 2007 has a unique wing veination with several plesiomorphies and its relationship to other psychodomorphs is unclear. As summarized by Stebner et al. (2015), approximately 30 genera and more than 100 fossil psychodid species have been described, yet, like the extant fauna, many species remain undescribed.
Bruchomyiinae, with six genera, 53 extant species and eight fossil species (Curler and Jacobson, 2012; Wagner and Stuckenberg, 2012; Wagner and Stuckenberg, 2016; Stebner et al., 2015), is among the less-taxonomically diverse subfamilies of Psychodidae. Adults of this group are readily distinguished from other psychodids by their relatively large size as well as the diagnostic wing characters provided previously. Male and female genitalia characters are important for distinguishing genera and species (Curler and Jacobson, 2012; Wagner and Stuckenberg, 2016).
Fossil Bruchomyiinae are described from Baltic, Dominican and Burmese amber with the oldest specimen preserved in middle Cretaceous amber from Myanmar (Schluter, 1978; Stebner et al., 2015; Wagner, 2006; Wagner and Stuckenberg, 2012; Wagner, 2017). All fossil species of this subfamily, previously placed in Nemopalpus Macquart, 1838, were recently transferred to other genera (Wagner, 2017). Baltic amber species are placed in Palaeosycorax Meunier, 1905 or Hoffeinsodes Wagner, 2017 while Burmese amber species are grouped in Palaeoglaesum Wagner, 2017 and Dominican amber species are included in Boreofairchildia Wagner and Stuckenberg, 2016.
The wing venation of the Kishenehn specimen is similar to, for example, extant Bruchomyia Alexander, 1921, species and fossil Hoffeinsodes species in that M2 originates at the level of r-m. Regardless, this character state occurs in other extant and fossil genera (Wagner and Stuckenberg, 2012; Wagner personal commun.); therefore, it may be a plesiomorphy within the subfamily. Considering the ambiguity of wing venation and the lack of other preserved characters, it is impossible to identify the Kishenehn specimen beyond subfamily.
This is the first compression fossil of a bruchomyiine species to be discovered and it is the first fossil of this subfamily from the Nearctic Region to be reported. With the exception of Notofairchildia zelandiae Alexander, 1921 (New Zealand), and N. stuckenbergi Wagner, 2012 (Chile, Valdivia) (Wagner and Stuckenberg, 2012), extant Bruchomyiinae are apparently absent from the temperate zone. Nonetheless, this new record and the growing number of species described from various ambers (Wagner, 2006, 2017) indicate that the group was once more widespread and morphologically diverse. In addition to the bruchomyiine specimen, psychodids taken from the Kishenehn Formation include over 20 specimens of Psychodidinae with potential for further study.
Family ANISOPODIDAE Knab, 1912
Genus SYLVICOLA Harris, 1776
Type species. Sylvicola brevis Coquillett, 1910 (SD)
Sylvicola silibrarius Greenwalt, sp. nov.
Figure 9, Figure 10, FIgure 11
zoobank.org/A1401C87-3CD1-40F7-8995-A63FBD5C4034
Etymology. The specific epithet is a combination of the abbreviation SI (for the Smithsonian Institution) and the Latin term librarius (pertaining to books) and is in appreciation for the essential services that the Smithsonian libraries perform.
Holotype. USNM 626077, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Deep Ford site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Sylvicola is distinguished by the basal separation of M1 -M2 1/6 of that between M2 and M3; pterostigma extended along cell r1.
Description
 Female (Figure 9.1), head and thorax dark brown/black, abdomen brown. Body length 5.3 mm, wing length 4.4 mm.
Female (Figure 9.1), head and thorax dark brown/black, abdomen brown. Body length 5.3 mm, wing length 4.4 mm.
Head. Antenna setose; with, probably, 14 flagellomeres, basally wider than long, becoming thinner, longer than wide apically; apical flagellomere 2.5 x long as wide. Scape with ring of fine setulae in a single row. Terminal segment of palpus visible, protruding well beyond oral margin. Three occipital bristles present (Figure 10.1).
Thorax. 1.25 mm long. Scutum setose. Femur distinctly shorter than tibia; tarsomere 1 twice the length of tarsomere 2 which, in turn, is about twice the length of tarsomere 3, tarsomeres 4 and 5 short; legs setose.
Wing. Slightly dusky, membrane covered with macrotrichia. Wing shorter than body, 1.82 mm wide. C ending at R4+5; pterostigma extended along cell r1; radial veins much thicker than posterior veins, with fine setulae. M1, M2 and M3 connected directly to discal cell, no forks. Distance between M1 and M2 at base about 1/6 of that between M2 and M3. CuP faint, anal lobe present (Figure 9.2-3).
Abdomen and genitalia. Abdomen, 3.4 mm long, 1.2 mm wide; uniformly brown, evenly covered with black setulae. A single sclerotized spermatheca present, 82 μm in diameter. Cerci indistinct, poorly preserved (Figure 10.2).
Allotype. Male unknown.
Syncompressions. None.
Paratype. A second specimen of Sylvicola silibrarius (USNM621508) is designated as a paratype (Figure 11). The specimen more clearly shows the small spherical shape of the head, the setose flagellomeres, a short arched scutum and additional legs. The specimen was collected at the Dakin site which is 0.6 km from the Deep Ford site, a possible indication of the of prevalence of the species.
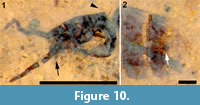 Remarks
Remarks
The family Anisopodidae consists of 15 extant genera and more than 200 species and is widely distributed (Kania et al., 2019). The fossil record, with 49 described species (EOL, 2017; PBDB, 2018), is rich, ancient and controversial. Michelsen (1999), in reference to the unsettled Mesozoic record of anisopod stem groups, stated "all fossil family-group names... may by necessity be referred incertae sedis to the lineage Anisopodidae." New Mesozoic species continue to be described, however, and the genera Mesorhyphus Handlirsch, 1920, and Megarhyphus Kovalev, 1990, date back to the Lower Jurassic (Ewa et al., 2010). The family has been proposed as sister to the Bibionomorpha (Wiegmann et al, 2011). Twelve of the fossil species date from the Eocene epoch. The genus Sylvicola contains 78 extant species and nine fossil species, the oldest of which, Sylvicola prisca Brodie, 1845, is from the Early Cretaceous of the Middle Purbeck; seven described species are from the Eocene (Wojtoń et al., 2018; PBDB, 2018). Pratt and Pratt (1980) proposed division of the genus Sylvicola into two subspecies, Anisopus Meigen, 1803, and Sylvicola Harris, 1776 s. str., based, in part, on the distance between M1 and M2 at base of cell m1 ≤ 1/4 (Anisopus) or ≥ 2/3 (Sylvicola) the length of the vein separating M2 and M3 (Krivosheina and Menzel, 1998). This proposal was rejected by Amorim and Tonzoni (1994) and Michelsen (1999).
 Sylvicola silibrarius differs from the four Eocene species of Sylvicola as follows: S. cadaver Scudder, 1890, from the Green River Formation, is slightly smaller (wing length, 3.5 mm vs. 4.4 for S. silibrarius), its bm is "about half as long as the wing", and its br terminates apically "scarcely beyond the tip of" Sc. In addition, M1 -M2 is 2/3 (left wing) or nearly equal to (right wing) the length of M2 -M3, as determined from the Scudder’s figure 17. Sylvicola hooleyi Cockerell, 1921 (Cockerell 1921b), from the Isle of Wight, is an isolated wing. The distance between the bases of M1 and M2, as determined from Cockerell’s original figure, is 62% of that between M2 and M3 (vs. 17% for S. silibrarius). In addition, in S. silibrarius, the wing length is slightly shorter (4.4 mm vs 5.2 mm), lacks apical pigmentation, has the r-m crossvein contacting the discal cell at its apical half rather than its basal half and has a much wider cell m3 relative to the discal cell (1.5 x vs. 0.8 x) measured at or as a continuation of the vein separating M2 and M3. The description of S. splendida Meunier, 1907, mentions only the large pulvilli and claws of this species. S. silibrarius, which is slightly smaller (5.3 mm vs. 5.75 mm in length) differs from S. splendida, a male from Baltic amber, in having smaller tarsal claws (< the width of the base of the tarsomere). Sylvicola silibrarius differs from the male and female specimens of S. thiriona Meunier, 1904, also from Baltic amber, in that S. thiriona has a filiform antenna, pedicel < twice the width of F12 (pedicel more stout, > twice the width of the terminal flagellomere in S. silibrarius), R1 reaching C closer to Sc than to the end of R2+3, M1 -M2 ≥ 2/3 of M2 -M3 and terminal tarsomeres with small claws (plate 17, fig. 14 in Meunier, 1904a).
Sylvicola silibrarius differs from the four Eocene species of Sylvicola as follows: S. cadaver Scudder, 1890, from the Green River Formation, is slightly smaller (wing length, 3.5 mm vs. 4.4 for S. silibrarius), its bm is "about half as long as the wing", and its br terminates apically "scarcely beyond the tip of" Sc. In addition, M1 -M2 is 2/3 (left wing) or nearly equal to (right wing) the length of M2 -M3, as determined from the Scudder’s figure 17. Sylvicola hooleyi Cockerell, 1921 (Cockerell 1921b), from the Isle of Wight, is an isolated wing. The distance between the bases of M1 and M2, as determined from Cockerell’s original figure, is 62% of that between M2 and M3 (vs. 17% for S. silibrarius). In addition, in S. silibrarius, the wing length is slightly shorter (4.4 mm vs 5.2 mm), lacks apical pigmentation, has the r-m crossvein contacting the discal cell at its apical half rather than its basal half and has a much wider cell m3 relative to the discal cell (1.5 x vs. 0.8 x) measured at or as a continuation of the vein separating M2 and M3. The description of S. splendida Meunier, 1907, mentions only the large pulvilli and claws of this species. S. silibrarius, which is slightly smaller (5.3 mm vs. 5.75 mm in length) differs from S. splendida, a male from Baltic amber, in having smaller tarsal claws (< the width of the base of the tarsomere). Sylvicola silibrarius differs from the male and female specimens of S. thiriona Meunier, 1904, also from Baltic amber, in that S. thiriona has a filiform antenna, pedicel < twice the width of F12 (pedicel more stout, > twice the width of the terminal flagellomere in S. silibrarius), R1 reaching C closer to Sc than to the end of R2+3, M1 -M2 ≥ 2/3 of M2 -M3 and terminal tarsomeres with small claws (plate 17, fig. 14 in Meunier, 1904a).
Sylvicola baltica, S. hoffeinsorum and S.punctata were recently described by Wojtoń et al. (2018); S. baltica (male) and S. punctata (female) are both based on single specimens from Baltic amber whereas eight specimens of S. hoffeinsorum (both male and female) were studied, seven from Baltic amber and one from Bitterfield amber. Sylvicola silibrarius differs from all of these specimens in having m’-m’ much shorter than m-m. In addition, S. baltica has a terminal flagellomere six times as long as wide and all flagellomeres longer than wide; the terminal flagellomere of S. librarius is three times as long as wide. Sylvicola punctata has an undulating M4 and the ratio of the distances between Sc and R1 and R1 and R2+3 is 3 whereas it is about 2 in S. librarius.; this ratio is 1.5 in S. hoffeinsorum. In all three of the species described by Wojtoń et al. (2018), r-m terminates in the middle of dm whereas the cross vein intersects with dm at the distal third of the cell in S. librarius.
Notes on some other fossil Sylvicola: Lewis (1987) figured the wing of an anisopodid from the Oligocene Ruby River Basin in southwestern Montana. The entire insect was said to have been preserved. The specimen was identified as resembling the type species of Sylvicola, S. brevis Harris, 1780 [Tipula fenestralis Scopoli, 1763]. The specimen was not described, and a repository was not designated. Although the specimen was collected by H. Becker, of the New York Botanical Gardens, sometime between 1950 and 1970, an inquiry made to the New York Botanical Gardens established that the specimen was not housed at the Gardens. Inquiries to St. Cloud State University, where Lewis’ collection is reported to be housed, have gone unanswered.
Evenhuis (1994) treated the name Rhyphus lugubris Heer, 1849, under Sylvicola. However, Heer (1849) neither described nor mentioned Rhyphus lugubris. Evidently, Evenhuis had made a typographical error for Plecia lugubris Heer, 1849, which is in the text immediately after the description of Sylvicola maculata Heer, 1849 (N.L. Evenhuis., personal commun., 2017).
Family SCATOPSIDAE Newman, 1834
Genus EFCOOKELLA Haenni, 1998
Type species. Efcookella albitarsis Zetterstedt, 1850
Efcookella nigra Greenwalt, sp. nov.
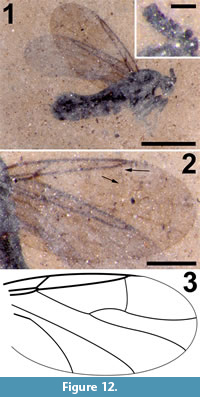
Figure 12
zoobank.org/B16260A9-30BA-4765-9CEA-98FFDF806172
Etymology. The specific epithet is derived from the Latin term nigra for black, the colour of the insect.
Holotype. USNM 618088, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Efcookella is distinguished by R1 very short, C terminating just beyond R4+5 significantly basal of the wing apex, the presence of a complete R4+5 -M1 cross vein, M1 and M2 diverging abruptly close to the wing margin and CuA not sigmoidal in shape. Flagellomeres compact, much wider than long.
 Description
Description
Body length 2.3 mm long, sex undetermined; entire body black (Figure 12.1).
Head. Head 0.24 mm long, 0.30 mm high; antennal flagellum, 0.28 mm long, eight flagellomeres, transverse, approximately 25 μm long and 65 μm wide.
Thorax. Scutum 0.64 mm long, tibia about 2/3 length of tarsus.
Wing. Membrane uniformly covered with microtrichia; wing length 1.99 (left), 1.81 mm (right), right wing width, 0.96 mm (Figure 12.2-3). Anterior veins C, R1 and R4+5 darkly pigmented; R1 short, extending only about 1/4 the length of the wing, costa ending just distal of apex of R4+5, well before wing apex; cross vein r-m minimal. Relatively long R4+5 extends to 0.62 (left) and 0.65 (right) of wing length. M very lightly sclerotized, forked, M1 and M2 diverging abruptly before wing margin, faint R4+5 -M1 cross vein present, CuA strongly curved towards wing margin but not sigmoidal, CuP not visible.
Abdomen and genitalia. Abdomen 1.50 mm long, 0.4 mm in height, bulbous apically, genitalia not preserved sufficiently for characterization.
Allotype. Sex unknown.
Syncompressions. None.
Remarks
Scatopsidae is a family of very small flies, with 407 species in four subfamilies and 34 genera (Pape et al., 2011). The genus Efcookella contains 21 extant species (EOL, 2017). The fossil record of Scatopsidae was reviewed by Amorim (1998), subsequent to which new species have been added by Nel and Prokop (2004), Fate et al., (2013) and Nel and Coty (2016); there currently are 18 species of fossil Scatopsidae. Of the fossil species, 13 belong to extant genera although Scatopse grassaris Meunier, 1907 and S. crassicornis Meunier, 1907 are considered Scatopsidae incertae sedis. Additionally, Amorim (1998) has suggested that Procolobostema incisum Cook, 1971, and P. obscurum Cook, 1971 may be synonyms of P. hurdi Cook, 1971. According to Amorim (1998), most males of this genus lack the crossvein R4+5 -M1 while females have an incomplete R4+5 -M1, cross vein. Several specimens from Cretaceous amber from Myanmar, Canada, New Jersey and Lebanon have been reported but not described (Pike, 1994; Grimaldi et al., 2000; Rasnitsyn and Ross, 2000; Poinar and Milki, 2001).
Sinoscatopse eocenica Hong, 2002, is a potentially interesting specimen but it does not appear to be a scatopsid. As described by Hong (2002), S. eocenica has a four segmented palpus (vs. a one segmented palpus in Scatopsidae), antennae "like the antennae of a female mosquito" with its flagellomeres longer than wide and "plumose" with a pair of setae/segment ≥ in length than the flagellomeres themselves, whereas the flagellomeres of scatopsids are compact and wider than long. In addition, S. eocenica has prominent tibial spurs on all legs (absent in Scatopsidae). Most interesting is the wing venation: Rs originates at the mid-point of the wing with the first abscissa of M > 1/2 wing length. In all scatopsids, the cubital fork is at the very base of the wing vs. the presence of a significant stem in S. eocenica. The specimens described in Hong (2002) are stated to be housed in the Chinese Geology Museum, the Beijing Natural History Museum or personal collections; however, no single specimen has its location indicated. Until the specimen can be located and re-examined, it must be considered as Diptera incertae sedis.
 Of the 18 described fossils of Scatopsidae, 17 are in amber. The exception, Reichertella fasciata Melander, 1949, was described from the 34 myo Florissant Formation. This specimen is unique amongst the Scatopsidae in having a long R4+5 that closely parallels the costa for most of its length. Amorim (1998) elected to preserve the original placement. Meyer (2002) lists and figures another specimen (PU-6943) of R. fasciata that was collected by the Princeton scientific expedition of 1877. This specimen, housed at the NMNH (USNM 112563), is not a type specimen. Inspection of this specimen (Figure 13) does not support its assignment to the family Scatopsidae. The body is 4.5 mm in length, large for a scatopsid, with numerous bristles on the head, scutum and scutellum. This specimen is either lacking its antennae or has antennae unlike that in Scatopsidae. Most importantly, the venation is very unlike that of a scatopsid. The anterior veins are not particularly more strongly pigmented than the posterior veins. Both medial and cubital forks are present, and CuA is subparallel to M4 and does not curve markedly towards the posterior margin, as is the case in all Scatopsidae. This specimen is assigned to Diptera indeterminate.
Of the 18 described fossils of Scatopsidae, 17 are in amber. The exception, Reichertella fasciata Melander, 1949, was described from the 34 myo Florissant Formation. This specimen is unique amongst the Scatopsidae in having a long R4+5 that closely parallels the costa for most of its length. Amorim (1998) elected to preserve the original placement. Meyer (2002) lists and figures another specimen (PU-6943) of R. fasciata that was collected by the Princeton scientific expedition of 1877. This specimen, housed at the NMNH (USNM 112563), is not a type specimen. Inspection of this specimen (Figure 13) does not support its assignment to the family Scatopsidae. The body is 4.5 mm in length, large for a scatopsid, with numerous bristles on the head, scutum and scutellum. This specimen is either lacking its antennae or has antennae unlike that in Scatopsidae. Most importantly, the venation is very unlike that of a scatopsid. The anterior veins are not particularly more strongly pigmented than the posterior veins. Both medial and cubital forks are present, and CuA is subparallel to M4 and does not curve markedly towards the posterior margin, as is the case in all Scatopsidae. This specimen is assigned to Diptera indeterminate.
Efcookella nigra keys to Scatopsinae Newman, 1834, as Aspistinae Rondani, 1840 (C swollen at junction of R4+5), Ectaetiinae Enderlein, 1936 (stem of M1+2 arising distal to base of R4+5) and Psectrosciarinae Cook, 1963 (base of M2 arising at base of R4+5) are characterized by states not found in the fossil. Critical character states that define the tribes of Scatopsinae, Rhegmoclematini Cook, 1955 (CuA sigmoid in shape), Scatopsini Newman, 1834 (basal third of CuA gently curved towards wing margin and medial fork without a "constriction" midway to apex/R4+5 -M1 cross vein absent), Swammerdamellini Cook, 1972 (R4+5 not extending beyond middle of wing—this is not the case for the genus Pararhexosa Freeman, 1990; this genus however has ten flagellomeres) and Colobostematini Amorim, 1994 (basal third of CuA strongly curved towards wing margin) suggest that this specimen can be assigned to Colobostematini (Amorim, 2009). Of the six genera in Colobostematini, the specimen keys to Efcookella (formerly Cookella—see Haenni, 1998) based on CuA not sigmoidal, R4+5 -M1 cross vein present and scutum longer than wide. The single fossil species of Efcookella, E. eocenica Nel and Prokop, 2004, was described from 53 Mya amber from Le Quesnoy, France. Efcookella nigra is larger than E. eocenica (2.3 mm body length vs. 1.86 mm, 124%), its wings are approximately 50% longer, its scutum is 71% longer (0.64 mm vs. 0.375 mm) and the shape of the cell formed by the cross vein R4+5 -M1 and the terminal abscissa of M1 is markedly different in shape: the terminal abscissa of M1 in E. eocenica angles downwards from R4+5 -M1 at an angle of 33° while that of E. nigra is inline with the more basal abscissa of M1.
Family BIBIONIDAE Fleming, 1821
Genus BIBIODES Coquillett, 1904
Type species. Bibiodes halteralis Coquillett, 1904
Bibiodes kishenehnensis Fitzgerald, sp. nov.
Figure 14, Figure 15
zoobank.org/E6A8DA89-FB03-4061-B16B-25FD8DD11FDF
Etymology. The specific epithet is named after the Kishenehn Formation in which the holotype was preserved.
Holotype. USNM 625738 deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. Bibiodes kishenehnensis is a typical representative of the bibionid genus Bibiodes that is distinguished from other genera by the elongated coalescence of the stem of M and Rs veins. B. kishenehnensis is distinguished from fossil congeners by the following combination of characters: coalescence of stem of M and Rs longer (coalescence 0.43 mm and slightly longer than base of Rs), wings brown fumose (especially at anterior apical portion of wing), stigma strongly pigmented, legs black and hind basitarsus about four times as long as wide (width measured at mid-point).
 Description
Description
Female (Figure 14.1), body length (excluding antennae) 6.8 mm.
Head. Black, antennae and palps black, number of flagellomeres not discernible, as base of antennae are hidden (Figure 15.1).
Thorax. Ventral and visible lateral portions black, dorsum hidden from view.
Wings. 5.2 mm long (base of wing estimated for measurement) by 1.8 mm wide (measured at level of apical end of coalescence of stem of M and Rs (Figure 14.2-3). Anterior veins except Sc (C, radial veins, base of M including junction with Rs) bold, strongly pigmented dark brown. Sc and apical tips of medial veins faint, light brown, remainder of veins unpigmented. Sc long, fading out before stigma (presumably not reaching C as in extant species). Pterostigma strongly pigmented, dark brownish black, elongate. Wing membrane distinctly brown fumose along anterior margin from stigma to just beyond apical end of C and slightly light brown fumose elsewhere, but especially along wing edge to about M4. Costa continued only slightly as tiny stump beyond junction with R4+5.
Legs. Black. Length of spur of anterior tibia not discernible. Hind femur about 1.4 mm long (base of femur estimated), swollen, 0.44 mm wide (width measured at widest area on apical third). Hind tibia not swollen, straight-sided, but gradually thickened distally, 1.5-1.6 mm long by 0.28-0.30 mm wide (width measured at apex; Figure 15.3). Hind basitarsus slender, gradually slightly more robust distally, about four times as long as wide, 0.48-0.60 mm long by 0.16 wide (width measured at mid-point of basitarsus).
Abdomen and genitalia. Abdomen brown, broad, as is typical for females. Cerci light brown, ovate, with fine setae, projecting posteriorly (Figure 15.2).
Allotype. Male unknown.
Syncompressions. None.
 Remarks
Remarks
The family Bibionidae sensu stricto (excluding Hesperinus Walker, 1848, but including both fossil and extant forms) consists of nine genera (Fitzgerald, 2004; Skartveit, 2008) and 1,102 species (Pape et al., 2011), and is distributed worldwide. Roughly 328 of these are fossil species (PBDB, 2018), an unusually large number given the relatively small number of extant species, which is perhaps an indication of the clade being more diverse or at least more abundant in the past. However, Skartveit and Nel (2017) recently synonymized the fossil Bibio conformans Théobald, 1937, with B. celasensis Theobald, 1937, and suggested that B. obtusus Théobald, 1937, and B. tenuiapacalis Théobald, 1937, may also be synonyms. The validity of many other fossils is uncertain. Most (70%) of the fossil species date from the Miocene and Oligocene, with 79 species from the Eocene epoch.
The genus Bibiodes contains four extant species. Five fossil species, B. balticus Skartveit 2008, B. intermedia James, 1937, B. confluens Cockerell 1915, B. provincialis Skartveit and Nel, 2017, and B. nanus Skartveit, 2008, have been described from the Eocene and Oligocene (Cockerell, 1915; James, 1937; Skartveit, 2008; Skartveit and Nel, 2017). Bibiodes kishenehnensis differs from most fossil congeners in part by the longer coalescence of the stem of M with the base of Rs (Figure 14; see additional characters in diagnosis) and in this regard is more similar to extant western Nearctic species of Bibiodes.
The Kishenehn Formation fossil insect collection contains 162 specimens of Bibionidae, including additional specimens of Bibiodes and several putative new species. The holotypes of Plecia akerionana Fitzgerald, 1999, and Bibiodes (= Bibiodites) confluens are housed at the NMNH.
Family SCIARIDAE Billberg, 1820
Genus EOSCIARITES Greenwalt, gen. nov.
zoobank.org/B5BA26D4-17C9-47B6-BFF3-7CA229FB6999
Type species. Eosciarites hermes Greenwalt, gen. et sp. nov., by monotypy.
Eosciarites hermes Greenwalt, sp. nov.
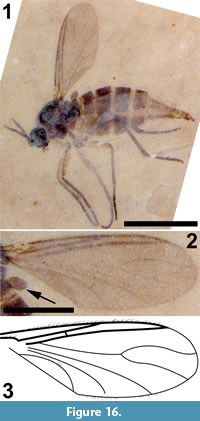
Figure 16, Figure 17
zoobank.org/C0541528-F65E-4593-9B09-9C1CB318C6C2
Etymology. The generic epithet is a combination of the greek word Eos (early, dawn), the genus name Sciara and the suffix "-ites" (Latin for "having the nature of"). Eosciarites is a collective parataxon as defined by Rasnitsyn (1986; 1996). The specific epithet is the Greek word Hermes (mythical messenger of the gods).
Holotype. USNM 624633, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. The absence of macrotrichia on veins M and Cu and flagellomeres with cylindrical nodes or necks differentiates this specimen from Sciara. A three-segmented palpus, R1 joining C prior to medial fork and significantly longer than half the length of R, M+CuA significantly greater than bm-m, middle and hind tibia with a single apical tibial spur are all diagnostic of this specimen.
 Description
Description
Female (Figure 16.1). Total length 2.2 mm, light brown in colour.
Head. Black, spherical, 0.27 mm diameter; eye about 0.12 mm in diameter. Maxillary palpus three-segmented, about 0.19 mm in length, terminal two segments slightly longer than wide. Antennal base situated in a distinct depression, flagellomeres brown, quadrate basally. Apical flagellomeres not preserved (Figure 17.1).
Thorax. Black to dark red, legs light brown. Scutum covered with short scattered setae. Mediotergite with several bristles on its posterior margin, katepisternum not attentuated. Haltere light brown, knob about 0.09 mm wide and 0.16mm long.
Legs. Setose, light brown basally, tibia and tarsi darker. Forecoxa with long setae along ventral margin, hind tibia with line of spines posteriorly; middle and hind tibia with one and possibly two apical spurs respectively, spurs longer than tibia diameter; foretibia with a spur longer than foretibial diameter on either side of a dark triangular area of setae (Figure 17.2).
Wings. Length: 1.45 mm, width: 0.52 mm (Figure 16.2-3). Membrane with microtrichia, macrotrichia restricted to C, R1 and R4+5. C extending beyond apex of R4+5, about half way to M1. Sc short, free, R1 significantly longer than half of R, R1 with a short, brief posterior turn basally, ending well-short of medial fork. R4+5 reaching C well before wing tip. Rs oblique to R4+5. Base of M and stem of M4 both shorter than r-m cross vein, ratio of r-m to M-petiole 1:2.6. M1+2 inconspicuous, slightly longer than medial fork, M1 and M2 slightly divergent; cubital fork very long, with origin basal to origin of M1+2. CuP inconspicuous or not preserved.
Abdomen and genitalia. Female, length 1.58 mm, brown; basal tergites wider than long. Tergites 3-5 with short setae at posterior margin; cercus two-segmented (Figure 17.3).
Allotype. Male unknown.
Syncompressions. Thysanoptera (1), Diptera (1).
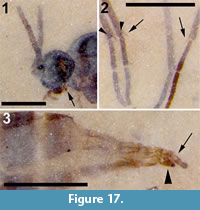 Remarks
Remarks
The family has 92 genera and 2,455 species, with a very complex taxonomy. Sciara Meigen, 1803, with approximately 700 extant species, is one of the most species-rich genera in the class Insecta—although many of these species may be misplaced. Sciara is the largest genus in the family Sciaridae and constitutes nearly 30% of the family’s extant species. Sciara has obviously served as a default assignment for poorly preserved fossils; 89 of all 168 fossil Sciaridae are assigned to the genus (PBDB, 2018). Species epithets such as rottensis, defectuosa, deperdita, diabolica, difficilis, ignorata, etc. may reflect authors’ frustrations in the identification of their fossil specimens (Heyden, 1870; Scudder, 1878; Meunier, 1904b). The presence or absence of macrotrichia on veins M and/or Cu is an essential morphological character in the identification of Sciara. A common question with fossil specimens is whether the macrothrichia were ever present or whether they were just not preserved. The near universal presence of these structures on the costal and radial veins however, should serve as an internal control. Neither macrotrichia nor their sockets are preserved in veins C, R1 and R4+5 in the three species re-examined below.
While this new specimen does not appear to belong to the genus Sciara, due to the absence of macrotrichia on veins M, the fact that it is a female and the inability to determine morphological characters such as the absence or presence of a neck on the flagellomeres (Figure 17.1), make it difficult to assign the specimen to an extant genus. For an impression fossil, preservation of this specimen is exceptional, but although the claws do not appear to have teeth and the labial palps do not appear to have setae, their absence can not be definitively established. Dolichociara and Angustosciara can be eliminated due to the absence of macrotrichia on the M veins; Sciarotrichia can be eliminated due to the length of the terminal segments of its labial palps; Edidapus and Pnyxia can be eliminated as they lack a patch of anteroapical setae on their foretibia and the presence of an attenuated katepisternum; Scatopsciara can be eliminated due to R1 less than half the length of R, Euricrium can be eliminated as it has an M fork bell-shaped, wider basally than distally; Bradysia is eliminated as its foretibia have two apical spurs; Rhynchomegalosphys and Scythropochroa can be eliminated as M+CuA is much longer than bm-m; Ceratiosciara is eliminated as it has strongly shortened convex flagellomeres; Eugnoriste is eliminated due to its greatly elongated mouthparts; Cratyna and Archicratyna are eliminated due to the presence of two midtibial spurs; Hyperlasion and Cosmosciara are eliminated due to the presence of an attenuated katepisternum (Menzel and Smith, 2017). This does not mean that the specimen does not belong to an extant genus. However, until additional specimens are collected that may assist in such an assignment, the generic epithet Eosciarites is provided.
Sciara florissantensis Cockerell, 1917, reassigned.
Figure 18
Material examined. Holotype, USNM 61995, housed in the National Museum of Natural History, Washington, D.C., USA.
Type horizon. Florissant Formation, latest Eocene.
Type locality. Florissant, Colorado.
Remarks
Sciara florissantensis was described by Cockerell (1917a) as a male. The body length is 4.5 mm, wing 3.6 mm; wings hyaline, veins not setose; Sc colorless, R1 long, its length 60% of R4+5; r-m approximately 0.38 mm long, fork of media not preserved; first two antennal joints short and broad, following ones cylindrical, longer than broad; apical part of gonostyle slender (Figure 18.1-3). This specimen was not figured by Cockerell. Figure 18.3 herein shows that nothing of the medial or cubital veins is preserved. Although Cockerell provided a measurement for the stem of M, this vein is not visible in the fossil. There is some pigmentation at the base of the costal cell, but the preservation of Sc is debatable—a tibia covers part of this portion of the wing. There is nothing to indicate that the specimen belongs to Sciaridae let alone Sciara. We therefore assign this specimen to Sciaroidea incertae sedis.
Sciara gurnetensis Cockerell, 1915, reassigned
Figure 19.1
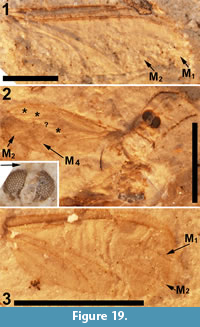 Material examined. Holotype, USNM 61435, housed in the National Museum of Natural History, Washington, D.C., USA.
Material examined. Holotype, USNM 61435, housed in the National Museum of Natural History, Washington, D.C., USA.
Type horizon. Bembridge Marls, UK, latest Eocene
Type locality. Gurnard Bay, Isle of Wight, UK
Remarks
Sciara gurnetensis was described, but not figured, by Cockerell (1916) as an isolated wing, 3.4 mm in length. However, the apical portion of the wing is deformed; its width is 1.27 mm. None of the veins have macrotrichia or remnants thereof. R1 and R4+5 are 0.6 and 0.9 of the wing length. Both the base of M and the cubital stem are shorter than cross vein r-m; the stem and fork of M are 1.2 mm and 1.5 mm long, respectively (4:5). The very long "cubital fork" (actually M4 originating very basally in the wing) in this species and in S. lacoei is seen in a few Cecidomyiidae, the rangomaramid genera Ohakunea, Colonomyia etc. and some mycetophilids. It is impossible to establish Sciara gurnetensis as a member of Sciaridae let alone Sciara. We, therefore, assign S. gurnetensis to Sciaroidea incertae sedis.
Sciara lacoei Cockerell, 1915, reassigned
Figure 19.2-3
Material examined. Holotype, USNM 61436, and specimen 7583, both housed in the National Museum of Natural History, Washington, D.C., USA.
Type horizon. Bembridge Marls, UK, latest Eocene
Type locality. Gurnard Bay, Isle of Wight, UK
Remarks
This fossil is of the dorsal aspect of the insect (Figure 19.2). The specimen is an impression fossil with the head, with well-preserved and clearly separated eyes, having formed a deep hemispherical depression in fine-grained mud; the face of the head contacted the bottom of the depression. Poorly preserved remnants of the basal antennomeres lie on the surface of the fossil above the hemispherical depression. The posterior portion of the head is not preserved and it is therefore impossible to determine whether or not an eye bridge is present.
C, R1 and R4+5 are clearly present; Sc is absent (there is a gouge in the fossil at that point). R1 and R4+5 extend to 0.48 and 0.89 of the wing's total length. The cubital veins are well preserved, but the medial veins are not. The fossil contains several creases that confound identification of these veins. In Cockerell’s figure, there is a short apical portion of M2 preserved, and this vein does indeed appear to be present in the fossil. M1, labeled with a "?" in figure 18.3, if not a crease, takes an uncharacteristically straight path to the margin; the base of the medial fork appears to be too basal for Sciaridae. As discussed above, M4 originating very basally in the wing in this species is seen in some Cecidomyiidae, Rangomaramidae and Mycetophilidae. The very long R5 also suggests that S. lacoei is not a sciarid. We, therefore, assign this specimen to Sciaroidea incertae sedis.
A second specimen (#7583, also labelled USNM 61436) is not a type but is labelled Sciara lacoei. This specimen (Figure 19.3) is 1.7 mm long, although the end of the abdomen is not visible; it contains no structures of value other than the wing. The preserved portion of the wing (the apical portion is missing) is approximately 1.4 mm in length, the same length as that of the holotype. R1 is preserved, but the end of R4+5 is not. Apical portions of M1 and M2 are preserved, but the base of the fork is not. This specimen appears to be a sciarid, although it is impossible to assign this specimen to Sciara; we assign it to Sciaridae incertae sedis.
Sciara protoberidis Cockerell, 1915, reassigned
Figure 20
Material examined. Holotype, USNM 61437, housed in the National Museum of Natural History, Washington, D.C., USA.
Type horizon. Bembridge Marls, UK, Latest Eocene
Type locality. Gurnard Bay, Isle of Wight, UK
 Remarks
Remarks
Sciara protoberidis is an insect body of 1.83 mm in length through tergite 5, 2.23 mm to the end of what may be the terminalia. Head not elongate. Wing length, 1.87 mm (left) and 1.71 mm (right), width 0.63 mm. No macrotrichia visible on any of the veins. Right wing R1 and R4+5 1.16 mm and 1.5 mm in length, respectively (7:9). Fork of M 0.73 mm in length, maximum width twice that at the wing’s margin, as figured by Cockerell; stem of M not preserved, at most as long as fork, probably shorter. There are no morphological characters present that distinguish this specimen from the many genera of the family or the families within Sciaroidea. We assign S. protoberidis to Sciaroidea incertae sedis.
Family MYCETOPHILIDAE Newman, 1834
Genus RYMOSIA Winnertz, 1863
Type species. Mycetophila discoidea Meigen, 1818, p. 268 = Rymosia fasciata (Meigen, 1804, p. 131); by designation of Johannsen, 1909, p. 102.
Rymosia hypnolithica Kerr, sp. nov.
Figure 21, Figure 22
zoobank.org/8C1B7B7A-C82A-400D-AC71-CA596FF9DC50
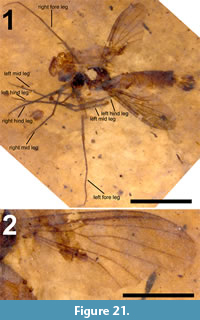 Etymology. The specific epithet is a combination of the Greek terms hypnos (sleep) and litho (stone), meaning asleep in stone.
Etymology. The specific epithet is a combination of the Greek terms hypnos (sleep) and litho (stone), meaning asleep in stone.
Holotype male. USNM 624134, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA.).
Differential diagnosis. This species of Rymosia is differentiated from all other fossils of the genus by the combination of its small size; cubital fork basal of r-m terminus; wings hyaline, without color; and heavily sclerotized gonostylus with dorsoapical process.
Description
Body length 3.50 mm (4.05 mm including genitalia), male, head and scutum black, abdomen light brown to brown, darkening distally (Figure 21.1).
Head. Ovoid, 0.5 mm high, 0.40 mm wide, vertex and face setose. Antenna with 14 flagellomeres, flagellomere two poorly defined, terminal flagellomere longer than wide, 0.40 mm wide, 0.10 mm long. Pedicel poorly defined, with two longer setae dorsally, approx. 0.60 mm and 0.10 mm in length, respectively (Figure 21.1).
Thorax. Length 0.90 mm. Scutal setae on dorsal surface, proepisternal bristles present, but other pleura not distinguishable, a single pair of very long (0.44 mm) bristles at posterior end of scutellum.
Wings. Left wing, 2.75 mm long, 1.0 mm wide; right wing 2.65 mm long, 1.15 mm wide (Figure 21.2); wings hyaline, covered with microtrichia arranged in rows. Stem of M much shorter than fork (0.225 mm to 1.5 mm, left; 0.225 mm to 1.55 mm, right). Branches of anterior fork do not reach wing margin. M and CuA veins without setulae. CuP strong, extending beyond CuA + M4 furcation.
Legs. Fore coxa, 0.48 mm long; fore and mid femur 0.92 mm and 0.96 mm, respectively [hind femur not observable]; tibia of fore, mid and hind legs, 0.86, 1.18 mm and 1.46 mm in length, respectively; tarsomeres (T1-T5) of fore leg, 1.02-1.08 mm, 0.52-0.54 mm, 0.36-0.38 mm, 0.28 mm and 0.22-0.24 mm in length, respectively; mid leg tarsomeres (T1-T5), 0.96 mm, 0.48 mm, 0.34-0.36 mm, 0.22-0.24 mm, and 0.16-0.20 mm in length, respectively; hind leg tarsomeres (T1-T5), 1.04 mm, 0.40 mm, 0.28 mm, 0.20 mm and 0.18 mm in length, respectively (Figure 22.2). All legs with setae in parallel rows. Tibial spurs 1: 2: 2; fore tibial spurs, 0.22-0.23 mm, tibial spurs at midleg 0.27 mm and 0.37 mm long, and tibial spurs at hind leg, 0.38-0.47 mm and 0.59 mm long.
Abdomen and genitalia. Length, 2.35 mm (not counting genitalia), width, 0.7 mm (at tergite 4), uniformly setose. Genitalia, 0.52 mm long, 0.52 mm wide; tergite nine with pair of elongate setae, approximately 0.4 mm long. Dorsal branch of gonostylus subovate with dorsoapical process measuring 0.09 mm, ventral branch of gonostylus elongate, heavily setose (Figure 22.3).
Allotype. Female unknown.
Syncompressions. None.
Remarks
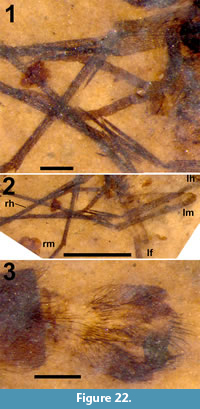 The large family Mycetophilidae consists of 180 extant genera with 4,150 species and is distributed worldwide. There are 415 known fossil species, the oldest of which date from the earliest Cretaceous. Most (220) of the fossil mycetophilids are Eocene in age. The genus Rymosia s.l. (Kjærandsen, 2006; Søli et al., 2000) contains 87 extant species and eight fossil species, six of which are from the Eocene; R. miocenica Lewis, 1969 and R. foersteri Theobald, 1937 date to the Miocene and Oligocene respectively (PBDB, 2018).
The large family Mycetophilidae consists of 180 extant genera with 4,150 species and is distributed worldwide. There are 415 known fossil species, the oldest of which date from the earliest Cretaceous. Most (220) of the fossil mycetophilids are Eocene in age. The genus Rymosia s.l. (Kjærandsen, 2006; Søli et al., 2000) contains 87 extant species and eight fossil species, six of which are from the Eocene; R. miocenica Lewis, 1969 and R. foersteri Theobald, 1937 date to the Miocene and Oligocene respectively (PBDB, 2018).
Unfortunately, the original descriptions of R. edwardsi Cockerell, 1921 (Cockerell, 1921b), R. ferruginea Cockerell, 1921 (Cockerell, 1921b), R. grisea Cockerell, 1921 (Cockerell, 1921b) and R. rufescens Cockerell, 1921 (Cockerell, 1921b), all from the Isle of Wight, were exceedingly terse. None of the specimens was identified as either male or female. All four descriptions, however, were accompanied by a figure of a wing. Rymosia hypnolithica differs from R. edwardsi in that the latter’s wing is 5.1 mm long, two and three times that of the male and a possible female of the new species, respectively. In addition, the wing of R. edwardsi is markedly patterned. The cubital forks of R. ferruginea and R. grisea are quite basal of the medial terminus of r-m whereas these two points are nearly aligned in R. hypnolithica. While the stem of M1+2 in R. grisea is half the length of r-m, in R. hypnolithica, the two are nearly the same length. The wing of R. rufescens is about as long as that of the male of R. hypnolithica, but the wing of R. rufescens (and R. ferruginea) was reported to be reddish in color. Krzemiński et al. (in press) recently suggested that Rymosia edwardsi Cockerell, 1921, R. ferruginea Cockerell, 1921 and R. rufescens Cockerell, 1921, most probably belong to the tribe Mycetophilini. The body of R. longicalcar Meunier 1904 (Meunier 1904b) is 7 mm long vs. 3.5 mm for R. hypnolithica, its flagellomeres are twice as long as wide, and its cubital fork is distal to its medial fork. Rymosia strangulata Scudder 1890 is a nearly complete specimen, although the apical quarter of the wing and the genitalia are still buried under matrix material. Scudder (1890) stated that "the drawing is incorrect" and, in his figure, Rs and r-m are both missing. He also stated that "the stalk of the upper discoidal vein" as drawn was too long. The specimen is in the collections of Harvard University Museum of Comparative Zoology and a photograph clearly shows a short r-m. The ratio of its length to the distance from the beginning of the fork in M to the anterior edge of the wing is 2.3; this ratio is 1.5 in R. hypnolithica.
Paratype specimens of Rymosia hypnolithica provide supportive morphological details. USNM 622565 is a paratype male with a dorsal view of the genitalia; USNM 626133 is a paratype male with black gonostyli with apical process and 9th (epandrial) sclerite with pair of long bristles. Male specimens USNM 626148 and USNM 621302 may constitute a distinct species, with gonostyli black, of a different form (more compact/elongate), without visible apical process. In addition, leg setation appears relatively longer and antennal segments more compact (not as long relative to width). Unfortunately, these fossils are not preserved with enough morphological detail to merit description of another new species at this time. USNM 623928 (male) represents an additional related, undescribed mycetophilid. Since the mycetophilids found here belong to more than one closely related species, and species are primarily distinguished by male features, the species identity of female USNM 624494, also an exechiine mycetophilid, remains uncertain.
Family RHAGIONIDAE Latreille, 1802
Genus LITOLEPTIS Chillcott, 1963
Type species. Litoleptis alaskensis Chillcott, 1963
Litoleptis araeostylus Greenwalt, sp. nov.
Figure 23
zoobank.org/A3D6DD5B-109E-4C06-84AD-110E46CF4C54
Etymology. The specific epithet is a combination of the Latin term araios (thin, narrow) and stylus and refers to the thin seta at the end of the flagellum.
Holotype. USNM 624657, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Litoleptis araeostylus is distinguished by its small size, cerci widely separated, tergum 9 short, partially retracted within T8, tergum 10 absent, majority or entire length of the length of flagellum long, tapered, ending in a relatively long seta.
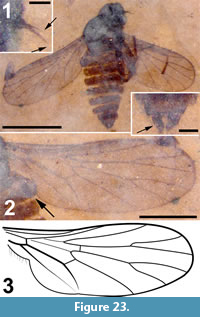 Description
Description
Female (Figure 23.1) 2.0 mm in length, not including cerci; head and notum black, abdomen reddish brown.
Head. Unfortunately, it is difficult to determine if the visible basal-most portion of the antenna is the pedicel or the basal bulbous portion of F1. Observable antennal length 0.257 mm; if pedicel not visible (this portion of the antenna lacks setae), basal portion of F1 bulbous, 48 μm long x 60 μm long, with thin tapering style 157 μm long and 18 μm wide at its base; needle-like seta approximately 40 μm in length at its terminus.
Thorax. Wing,1.72 mm long and 0.65 mm maximum width (Figure 23.2-3). Halter, 0.3 mm in length, knob, 134 μm wide and 180 μm long, stem 65 μm wide. Legs without tibial spurs.
Abdomen and genitalia. Abdomen,1.18 mm in length and 0.65 mm wide at maximum width; lengths and widths of T7, T8 and T9, 0.46 x 0.13, 0.34 x 0.16 and 0.22 x 0.36 mm, respectively. Basal half of T9 appears to be withdrawn into T8 (Figure 23.1 inset). Spermatheca not preserved/sclerotized. Cercus 2-segmented, lengths and widths of C1 and C2 60 x 46 μm and 50 x 40 μm, respectively.
Allotype. Male unknown.
Syncompressions. Thysanoptera (3), Chaoboridae (8), dipteran pupae (3), Diptera (1), Corixidae (1), Bibionidae (1), Hymenoptera (1), Aphididae (1), Hemiptera (1).
Remarks
The family Rhagionidae consists of 47 genera and 756 described species. There are 89 species of fossil Rhagionidae mostly in extinct Mesozoic genera. Of the fossil species, 57 date to the Mesozoic and 27 to the Eocene, with 21 of the latter in Baltic amber. The oldest known Rhagionidae s.l., Gallia alsatica Krzemiński and Krzemińska, 2003, is from the early Triassic. Solórzano Kraemer and Nel (2009) reviewed the fossil record of Rhagionidae. Litoleptis Chillcott, 1963 is a small genus with nine described extant members (Imada and Kato, 2016). The EDNA (EDNA, 2017) and Bishop Museum fossil insect databases (Evenhuis, 2017) list Litoleptis in the family Spaniidae Stuckenberg, 2001, a taxon proposed by Stuckenberg, but rejected by Kerr (2010). The subfamily Spaniinae Nagatomi, 1982, is unique within the Rhagionidae in the absence of a discal cell, tergite 9 short and withdrawn into T8, tibia, without spurs amongst other characters.
The only described fossil of Litoleptis, L. fossilis Arillo et al., 2009, from Lower Albian San Just amber (Spain) (Arillo et al., 2009), is not closely related to either L. araeostylus or the extant members of the genus, in that the costa does not extend around the wing margin but terminates at R5, the tibia of the middle leg (the only preserved leg) has a spur as well as marked differences in the female genitalia (e.g., cerci not widely separated). It also differs from all other species of the genus in that the stem of R and M are both longer than their forks, with R4 curving anteriorly, parallel to the terminal portion of R2+3.
 The six extant species of Litoleptis from Japan have been divided into two groups, one of which contains only L. japonica Imada and Kato, 2016. This species is differentiated from the others by a number of characters, including the presence of a long stout seta at the tip of the flagellomere, a character shared by L. araeostylus. Litoleptis japonica is differentiated from L. araeostylus by its larger size, 3.4 mm wing length vs. 1.7) and wing longer than body. Litoleptis araeostylus differs from L. chilensis Hennig, 1972, in that the latter has the fork of R4+5 distal of the fork of M1+2, CuA reaching the wing margin and wing 3.0 mm long (Hennig, 1972). Litoleptis orientalis is larger than L. araeostylus (2.7 mm wing length vs. 1.7 mm), and the fork of R4+5 is distal to the fork of M1+2. Litoleptis alaskensis Chillcott, 1963, also has the fork of R4+5 significantly distal to the fork of M1+2; it also lacks the needle-like seta at the terminus of the flagellum (Chillcott, 1963).
The six extant species of Litoleptis from Japan have been divided into two groups, one of which contains only L. japonica Imada and Kato, 2016. This species is differentiated from the others by a number of characters, including the presence of a long stout seta at the tip of the flagellomere, a character shared by L. araeostylus. Litoleptis japonica is differentiated from L. araeostylus by its larger size, 3.4 mm wing length vs. 1.7) and wing longer than body. Litoleptis araeostylus differs from L. chilensis Hennig, 1972, in that the latter has the fork of R4+5 distal of the fork of M1+2, CuA reaching the wing margin and wing 3.0 mm long (Hennig, 1972). Litoleptis orientalis is larger than L. araeostylus (2.7 mm wing length vs. 1.7 mm), and the fork of R4+5 is distal to the fork of M1+2. Litoleptis alaskensis Chillcott, 1963, also has the fork of R4+5 significantly distal to the fork of M1+2; it also lacks the needle-like seta at the terminus of the flagellum (Chillcott, 1963).
Litoleptis is distributed worldwide, albeit sparsely, with extant specimens described from Alaska, Japan and the Philippines. It is of particular interest to note that, while a number of rhagionids are hematophagous, the larvae of Litoleptis are obligate miners of liverwort thalli (Imada and Kato, 2016), with species restricted to a single genus of host. Of those species whose host is known, only L. japonica feeds on a species in the liverwort family Conocephalaceae Grolle, 1972. There are 13 fossil species of the genus Rhagio Fabricus, 1775 (Evenhuis, 2017); the holotype of one of these, Rhagio fossitus (USNM 112626), is housed at the NMNH and is refigured here (Figure 24). Although the posterior margin of the wing is not preserved, the separation between the distal termini of CuP and CuA suggests the presence of an open anal cell, a characteristic that distinguishes Rhagio from all other genera in the family Rhagionidae. The specimen is left as is without additional comment.
Family ASILIDAE Latreille, 1802
Genus KISHENEHNOASILUS Dikow, gen. nov.
zoobank.org/9C563B33-C11B-4B0D-9D8B-080B10DDB05F
Type species. Kishenehnoasilus bhl Dikow, gen. et sp. nov., by monotypy.
 Etymology. Kishenehn refers to the Kishenehn formation from which this species is described, asilus is a common part of generic names in Asilidae: Asilinae. Refers to the placement of this genus in the Asilinae and is to be treated as masculine.
Etymology. Kishenehn refers to the Kishenehn formation from which this species is described, asilus is a common part of generic names in Asilidae: Asilinae. Refers to the placement of this genus in the Asilinae and is to be treated as masculine.
Holotype. USNM 624491, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. Small asilid flies, with antennal postpedicel tapered distally and a simple cylindrical stylus; compound eye posterior margin straight or slightly curved throughout; cells r1, m3, and cua closed and petiolate; all femora expanded; femora and tibiae with numerous macrosetae in rows; presutural and postsutural dorsocentral macrosetae present; female with ovipositor comprised of 8th and following segments.
Kishenehnoasilus bhl Dikow, sp. nov.
Figure 25, Figure 26
zoobank.org/E2AEF6A9-90EB-4882-AC79-AA7E2257FA99
Etymology. The specific epithet refers to the Biodiversity Heritage Library (www.biodiversitylibrary.org), abbreviated BHL and pronounced "bee aitch el", and is to be treated as a noun in apposition. Kishenehnoasilus bhl sp. nov. is providing us a window in to the past and so is the digitization effort of the BHL by making available digitally natural history literature with emphasis on publications published prior to 1923.
Description
 Female (Figure 25.1), 12 mm in length, including genitalia; head, thorax and abdomen black.
Female (Figure 25.1), 12 mm in length, including genitalia; head, thorax and abdomen black.
Head. Head shape in anterior view not observable, black; vertex shape not observable; facial swelling not observable, pubescence not observable; mystax not observable, mystax extent not observable; ommatidia size not observable; postgena posterior margin simple, smooth; frons (at level of antennal insertion) not observable, pubescence not observable, setation not observable; ocellar tubercle not observable, setation not observable; vertex pubescence not observable, setation not observable; median occipital sclerite setation not observable; postocular (pocl) setae not observable; occiput setation not observable, pubescence not observable; compound eye posterior margin straight or slightly curved throughout (in lateral view). Proboscis and maxillary palpus: proboscis straight, black; postmentum plate-like, straight, setation not observable; prementum cross section not observable, dorso-median development not observable, setation not observable; labella rounded; maxillary palpus not observable; stipites not observable (Figure 26.2).
Antenna. Black, pubescence not observable; scape not observable; pedicel setation not observable; postpedicel tapering distally, asetose; stylus comprised of one element, asetose; apical seta-like sensory element situated apically on stylus.
Thorax. Dark brown to black; prosternum not observable; proepisternum not observable; cervical sclerite not observable; antepronotum not observable; postpronotum not observable; postpronotal lope not observable; pleuron pubescence not observable; proepimeron not observable; anepisternum setation not observable; anterior basalare not observable, posterior basalare not observable; setation of anepimeron, katepisternum, katepimeron, katatergite, meron + metanepisternum, metakatepisternum, metepimeron, and anatergite not observable; scutum pubescence not observable, scutum setation only partially observable: one npl seta, spa and pal setae absent, two presutural and two postsutural dc macrosetae, acrostichal setae not observable, median posterior scutum setation not observable; scutellum pubescence not observable, ds sctl setae not observable, ap sctl setae present, two long macrosetae; postmetacoxal area not observable.
Leg. Light brown to brown, pubescence not observable, all setae circular in cross section (Figure 26.3); pro coxa black, pubescence not observable, asetose; pro femur black, no macrosetae, setation not observable; pro tibia light brown to brown, short black setose, black macrosetose: three long in one ventro-posterior row, four short in 1 posterior row, distal tip with four thick, black, medium length macrosetae; mes coxa black, pubescence not observable, asetose; mes femur black, distally brown, short black setose, black macrosetose: 2 long in one anterior row distally, two long in one ventral row; mes tibia light brown to brown, short black setose, black macrosetose: three long in one anterior row, two to three short in distal ½ anteriorly and dorsally; met coxa black, pubescence not observable, asetose, anteriorly without any protuberance; met trochanter setation and median shape not observable; met femur black, brown proximally and distally, short black setose, black macrosetose: two long in one anterior row distally, five long in one antero-ventral row; met tibia light brown to brown, straight, short black setose, long black macrosetose: four in one antero-dorsal row, one to two in one antero-ventral row distally, two to three antero-distally; proximal pro and mes tarsomeres slightly longer than tarsomere two, proximal met tarsomere as long as following two tarsomeres combined, pro tarsomeres short black setose dorsally, longer black macrosetose ventrally; mes tarsomeres short black setose dorsally, longer black macrosetose ventrally, met tarsomeres short black setose dorsally, longer black macrosetose ventrally; pulvilli well-developed (as long as claw); claw abruptly angled distally, pointed; empodium setiform, minute or entirely absent.
Wing. Length, 6.3 mm, hyaline, evenly microtrichose (Figure 25.2-3); C circumambient, R2+3 distally relatively straight, r1 closed, R1 and R2+3 meet apically and form a stalk vein (petiolate); R4 terminating anterior to wing apex, relatively straight, stump vein (R3) absent; r4 open, R4 and R5 more or less parallel; R5 terminating posterior to wing apex; r5 open; M1 terminating posterior to wing apex; cell d closed by base of M2 and m-m, M2 and m-m not aligned, r-m situated in distal half; m3 closed and petiolate; cua closed and petiolate; alula not observable; microtrichia on posterior wing margin arranged in a single plane.
Abdomen. Black, tergites smooth, setae with small sockets only; T1 setation and pubescence not observable, dorsal surface smooth, without protuberances; T2-8 entirely sclerotized, predominantly black, pubescence not observable, setation in general not observable (some distal setae on T6-7 discernible), marginal macrosetae absent from T2-8, medial macrosetae absent from T2-8; S1-8 color, setation and pubescence not observable, asetose.
Female abdomen. T7 and S7 without modifications, ovipositor comprised of 8th and following segments, T6-8 pubescence and setation not observable; T8 internal apodeme not observable, S8 keel-like throughout; T9 and T10 not fused, T10 undivided (single sclerite), acanthophorite spines absent (Figure 26.1).
Allotype. Male unknown.
Syncompressions. None.
Remarks
Kishenehnoasilus gen. nov. clearly belongs to the Asilinae. Although it is in general well-preserved, few characters can be observed that would help to provide more information on it’s placement within this diverse taxon. Features that support the placement within the Asilinae are (1) antenna (postpedicel tapering distally, stylus comprised of one element, apical seta-like sensory element situated apically on stylus), (2) wing venation (r1 closed, R4 and R5 more or less parallel, cell d closed by base of M2 and m-m, m3 closed and petiolate, cua closed and petiolate, Figure 25), (3) prothoracic and mesothoracic coxae orientation (directed ventrally and not posteriorly), (4) femora development (all femora expanded), (5) development of dorsocentral (dc) macrosetae (prominent pre- and postsutural dc setae present), (6) abdomen shape (somewhat tapered abdomen), and (7) ovipositor development (elongated, simple for placing eggs on vegetation or dropping them to the ground; Figure 25.2, Figure 26.1 and 26.3).
Dikow (2009) published a comprehensive morphological phylogeny of Asilidae in which some 32 species from 29 Asilinae genera (of 183 currently known genera) were included. The relationships among these genera could not be resolved in much detail based on the characters employed. Despite the general good preservation of Kishenehnoasilus bhl sp. nov., it cannot be placed within this phylogeny because of the many features that cannot be observed. A more in-depth phylogeny of Asilinae is in preparation by Rodrigo Vieira (personal commun.), and it is hoped that the findings in that study will shed some light on the relationships within Asilinae and of Kishenehnoasilus bhl sp. nov.
Notes on Asilopsis fusculus Cockerell, 1921
While preparing the description of Kishenehnoasilus bhl sp. nov., it seemed important to re-examine the holotype of Asilopsis fusculus, which is deposited in the NMNH. This fossil (Figure 7.1-2) is based on a single, partially broken wing, which might make a placement difficult. It was immediately obvious that the wing does not have any features of an Asilidae, but that it clearly belongs to another family. Through the help of Jon Gelhaus and Herman de Jong, we established that the wing venation is similar to that of Cylindrotomidae (Tipuloidea) and Asilopsis fusculus is here transferred to that family (see under Cylindrotomidae for a redescription and further comments).
Family Dolichopodidae Latreille, 1809
Subfamily MEDETERINAE Fischer von Waldheim, 1819
Genus SALISHOMYIA Bickel, gen. nov.
zoobank.org/DD02F2B7-F02B-4083-92BF-B2D10F4B30B3
Type species. Salishomyia eocenica Bickel, gen. et sp. nov., by monotypy.
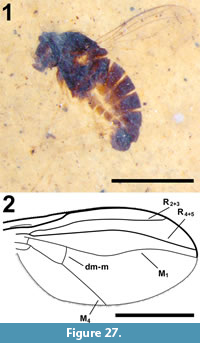 Etymology. Salishomyia is a combination of the name of the indigenous Salish people who inhabited the region in Montana where the Kishenehn formation occurs (and who were referred to by outsiders as "Flatheads"), and "myia" from Greek meaning "fly". The gender is feminine.
Etymology. Salishomyia is a combination of the name of the indigenous Salish people who inhabited the region in Montana where the Kishenehn formation occurs (and who were referred to by outsiders as "Flatheads"), and "myia" from Greek meaning "fly". The gender is feminine.
Holotype. USNM 622501, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana).
Differential diagnosis. Small (<1.5 mm body length) flies in the subfamily Medeterinae; antenna short; postpedicel subrectangular and rounded, with apical arista, dorsal postcranium concave; posterior mesonotum apparently flattened; legs elongate, without strong setation and without evidence of anterior preapical setae on femora II and III; hypopygium with epandrium spheroidal and fully exserted, not enclosed by anterior postabdominal segments; wing rather broad; R2+3 joining costa at 5/6 distance from base; R4+5 in gentle anterior arc and joining C just anterior to wing apex; vein M basally diverging from R4+5, and at midlength with gentle bend to arch forward towards R4+5 with the two veins becoming subparallel in distal sixth of wing, and M joining margin at apex; crossvein dm-m positioned basally, with ratio of length of dm-m crossvein/distal section M4 = 0.3.
Salishomyia eocenica Bickel, sp. nov.
Figure 27
zoobank.org/ACCE57D9-7CDD-49F8-ADAF-8E2674E9124A
Etymology. The specific epithet refers to this species’ occurrence in the Eocene period.
Description
Male, body length: 1.7 mm (Figure 27.1).
Head. Ovate in lateral view; dorsal postcranium concave and appearing to partially enclose anterior mesonotum; palp with short apical seta; proboscis short, subrectangular; antenna short; postpedicel subrectangular and rounded (possibly reniform) with apical arista; arista threadlike and shorter than head height.
Thorax. Dark brown to black; dorsal setae (probably dorsocentral setae) evident; posterior mesononotum distinctly flattened, scutellum with distinct lateral and median setae.
Leg. Coxa I apparently infuscated basally, with coxae II and III yellowish; remainder of legs apparently yellow, with little evident setation and no indication of anterior preapical setae on femora II and III; [relative lengths of podomeres are representative ratios, not measurements and given in the following formula and punctuation for each leg: femur; tibia; tarsomere 1/ 2/ 3/ 4/ 5]; leg I: 3.7; 3.6, distal podomeres obscured; leg II: all podomeres obscured; leg III: 4.0; 3.4; 1.0/ 1.2/ 0.6/ 0.4/ 0.4.
Wing. Length and width, 1.4 and 0.7 mm (Figure 27.2); hyaline, C reaches wing apex, joining vein M; R2+3 joining costa at 5/6 distance from base; R4+5 in gentle anterior arc and joining costa just anterior to wing apex; M basally diverging from R4+5, and at midlength with gentle bend to arch towards R4+5 with the two veins becoming subparallel in distal sixth of wing, and M joining margin at apex; crossvein dm-m positioned basally, with ratio of length of dm-m crossvein/distal section M4 = 0.3; lower calypter and halter not visible.
Abdomen. Tergites with only short setation, without long marginal setae; tergite one short and adjacent to metapostnotum; tergites two to five each well-developed, with corresponding sternite; tergite six prominent; hypopygium spheroidal with short digitiform surstylus; cercus subtriangular; hypandrium (or phallus?) curved and projecting beyond hypopygium near sternite 5.
Allotype. Female unknown.
Syncompressions. None.
Remarks
The family Dolichopodidae comprises some 7,300 species in 230 genera in the recent fauna. It is often rich and abundant in Tertiary amber deposits, a result of their use of tree trunks for both feeding and mating, thereby increasing their chance of becoming entrapped by resin flows. Dolichopodids are also numerous in marine and lacustrine littoral habitats and correspondingly are known as compression fossils from a number of fine-grained lacustrine deposits. The new genus Salishomyia from the Kishenehn Formation clearly belongs to the dolichopodid subfamily Medeterinae, based on the following characters: antenna short; postpedicel subrectangular and rounded (possibly reniform) with apical arista, dorsal postcranium concave; posterior mesonotum apparently flattened; legs without strong setation, femora II and III without preapical setae, hypopygium large and external, not enclosed by anterior postabdominal segments. Salishomyia appears to have a classical Medetera-like venation, with vein M basally diverging from R4+5, and at midlength bending gently to arch forward towards vein R4+5. However, in Medetera, the dm-m crossvein connects M4 with M at the bend in vein M, while in Salishomyia, the dm-cu crossvein is positioned basad of the vein M bend, and the wing is broader. This wing character is diagnostic for Salishomyia.
The Kishenehn Formation dolichopodid fauna, as of 2017, consists of 78 specimens. Other than Salishomyia eocenica, 30 additional specimens are of potential interest. Among these are two species in a genus near Hercostomus Loew (possibly Gymnopternus Loew), one with six males (USNM 621409, 622506, 622656, 625273, 625526 and 626174) and the other with one male (USNM 621182) and two females (USNM 622026 and 624021) of uncertain specific association. The two species can be separated by the shape of surstyli projecting from the hypopygium. Hercostomus acts as a cosmopolitan "holding genus" for many described species and remains poorly defined and is undoubtedly a polyphyletic assemblage (Brooks, 2005). Although additional work is needed to resolve the phylogenetic relationships of this complex cosmopolitan genus, it is important to note the presence of Hercostomus-like species in the Kishenehn formation to demonstrate regional historical-biogeographical relationships. The genus Gymnopternus is closely related to Hercostomus; Gymnopternus lacustris was described from the Miocene Florissant beds of Colorado (Bickel, 1995).
There are a number of additional specimens that are of interest but lack critical morphological detail. These include: 1) a species with some similarities to the recent genus Chrysotimus Loew but with a slighter build and an enlarged hypopygium (12 specimens, both males [USNM 620106, 620817, 621111, 622587 and 623229] and females [USNM 621246, 621939, 623788, 623828, 624434, 712964 and 712965]), 2) a species where veins R4+5 and M bowed with respect to each other beyond the dm-m crossvein (three females [USNM 609598, 624638 and 712963] and one male [USNM 620605]), 3) a species with similar venation to "Chrysotus molestus Meunier" (which is not in the genus Chrysotus) from Baltic amber, with a wide wing and vein M ending well behind the wing apex (two females [USNM 620633 and 620464] and a possible male [USNM 620994]), 4) a species possibly near the abundant Baltic amber genus Palaeomedeterus Meunier (one male [USNM 624586]), and 5) a species appearing similar to the recent genus Rhaphium Meigen (one male [USNM 620398]).
Family HYBOTIDAE Macquart, 1827
Genus DRAPETIS Meigen, 1822
Type species. Drapetis exilis Meigen, 1822
Drapetis adelomedos Greenwalt, sp. nov.
Figure 28, Figure 29
zoobank.org/A993F24D-BC6A-4035-B36A-02E3DFE65FE1
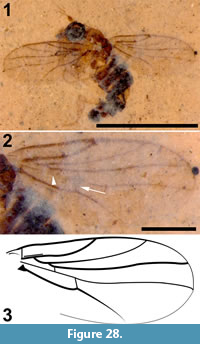 Etymology. The specific epithet is derived from the Greek words adelos (obscure) and medos (plan) and references the lack of preserved morphological detail of the genitalia.
Etymology. The specific epithet is derived from the Greek words adelos (obscure) and medos (plan) and references the lack of preserved morphological detail of the genitalia.
Holotype. USNM 621705, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Tunnel Creek site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. Antenna with long terminal arista-like stylus, pedicel with marginal setae but without visible ventral bristle; proboscis stronger at base. One pair of vertical bristles visible on head. Rs longer than bm-cu cross vein, cell br much shorter than cell bm, cell dm absent, and radial and medial veins not forked; cup absent/not visible, CuP very weak. Scutum about as long as broad.
Description
Male. (Figure 28.1). Length, including terminalia, 2.5 mm.
Head. (Figure 29.1-2). Black/dark brown, spherical, diameter 0.4 mm, dark. Proboscis subtriangular in shape, 0.22 mm in length, 0.12 mm wide at base, heavily setose at base. Antennae reddish brown, 0.52 mm in length with apically concave pedicel with marginal fringe of short setae; first flagellomere triangular, concave at base, 90 μm long, 67 μm wide, L/W = 1.35. Arista 0.35 mm long, covered with short setae, approximately 8 μm in length. Head with one pair of occipital bristles, about 0.1 mm in length.
Thorax. Redish, 0.73 mm long, scutum barely longer than wide, as long as head.
Wings. (Figure 28.2-3). Length, 1.75 mm (right), width 0.8 mm; hyaline; microtrichia arranged in parallel longitudinal lines; costal setulae well developed; Rs about one-third length of cell bm, longer than bm-cu cross vein, cell br length about half that of cell bm; R2+3 strongly arched upwards, extending to beyond midlength of wing, R4+5 slightly sinuous, barely divergent from M1+2; crossvein r-m nearly transverse, dm-m less so.
Legs. Forelegs and middle legs not preserved; hind femur with row of several short weakly sclerotized posteroventral bristles (Figure 29.3); hind tibia 0.52 mm long, 0.12 mm wide, with posteroventral row of stout bristles, about 0.06 mm in length. Hind tarsus 0.75 mm in length, 1st tarsal segment longest, 0.34 mm long.
Abdomen and genitalia. 1.37 mm in length, reddish/dark brown. Details of the male genitalia not preserved.
Allotype. Female unknown.
Syncompressions. None.
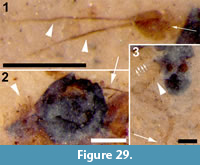 Remarks
Remarks
The family Hybotidae consists of 2,005 species in 75 genera. It contains 48 described fossils, most (27) of which are from the Eocene. The genus Drapetis was originally separated into two subgenera, Drapetis and Crossopalpus Bigot, 1857, by Melander (1918), but the two are now recognized as separate genera. Crossopalpus, which contains 34 species, is distinguished by the pedicel with a distinct ventral bristle. Drapetis, with 156 extant species, is represented in the fossil record by six species, five in Baltic amber (Meunier, 1908) and one, D. dissentis Solórzano Kraemer et al., 2005, from Miocene Chiapas amber. The Chiapis specimen and the Baltic amber species described by Meunier are all smaller than D. adelomedos, ranging from 0.75 to 1.75 mm in length. Other differences exist. Drapetis brevis Meunier, 1908 has a subapical arista and r-m and m-cu nearly touching. In our view, this specimen does not belong to Drapetis and is here designated as Hybotidae undetermined. Drapetis decolorata Meunier, 1908 has cell bm triangular in shape, with origin of m-cu cross vein at the level of the Rs fork. D. vitiosum Meunier, 1908 has m-cu origin much closer to the level of the Rs fork than the R1 terminus. However, CuP of Drapetis, when present, is weak, faint and concave (absent in D. adelomedos) whereas CuP in D. vitiosum is figured by Meunier as strong and distinctly convex. Drapetis vitiosum does not appear to belong to Drapetis and is here designated as Hybotidae undetermined. Drapetis decoratum Meunier, 1908, has a single apical tibial spine. Its wings were not figured by Meunier, who described them as "a little longer than the body and quite wide. Length = ¾ mm." Given the absence of a detailed description of the venation, these specimens (both the male and female were described) must be re-examined before they can be reliably assigned to the genus Drapetis. Drapetis mortuum Meunier, 1908, has F1 convex at its base and an L/Wmax ratio = 2.2 (vs. 1.3 for Drapetis sp.). Drapetis dissentis differs from D. adelomedos in that its R4+5 is strongly divergent from M1+2.
Family PLATYPEZIDAE Latreille, 1829
Genus AGATHOMYIA Verral, 1901
Type species. Callomyza antennata Zetterstedt, 1819
Agathomyia eocenica Tkoč, sp. nov.
Figure 30, Figure 31
zoobank.org/AD4A9FE2-B217-444B-84BA-770423E2F470
 Etymology. The specific epithet, a Latin adjective, refers to the geologic epoch (Eocene) in which this species lived.
Etymology. The specific epithet, a Latin adjective, refers to the geologic epoch (Eocene) in which this species lived.
Holotype. Agathomyia eocenica Tkoč, female; NMNH, USNM 553697.
Type horizon. Middle Eocene Coal Creek member, Kishenehn Formation.
Type locality. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana).
Differential diagnosis. This species has the typical wing shape and wing venation of Agathomyia, i.e., it has seven longitudinal veins (Sc, R1, R2+3, R4+5, M1+2, M4, CuA+CuP), two crossveins (r-m, dm-m) and three non-costal cells are present (rm, bm, cua). Body and legs are dark brown in color; head and abdomen are black to gray; wing with microtrichia, M1+2 not forked; antenna with first flagellomere conical and several basal aristomeres; tarsomeres of hind leg broad, tarsomere I the longest, both tarsomere II and III wider than long.
Description
Female (Figure 30.1), body dark brown, body length, 4.4 mm, wing length 3.6 mm.
Head. Dark brown,0.49 mm long, 0.87 mm high (Figure 31.4). Labellum 0.31 mm long, setose with setae approximately 50 μm long; first flagellomere 0.15 mm long, conical, with maximum width 0.10 mm, narrowed apically with three basal aristomeres approximately 50 μm x 30 μm (L x W). Arista not preserved. Proboscis and palpus light brown.
Thorax. Length, 1.51 mm; 4 notopleural setae (two short, two long) visible, one long prescutellar dorsocentral seta and one supraalar seta.
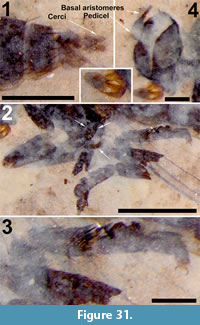 Wing. Length, 3.6 mm, at the middle, width 1.3 mm, with microtrichia (Figure 30.2). First longitudinal vein (R1) devoid of any spines. Costal cell (c) a little longer than portion of C on subcostal cell (sc), 1.26:1.10 mm. Length of discal cell (d) 1.55 mm, cell cup, 0.89 mm, bm, 0.57 mm, and rm, 0.77 mm. Plane of crossvein r-m intersects cell c at 59% of its 1.26 mm length. Posterior crossvein (dm-m) almost twice as long (0.34 mm) as distal part of M4, 0.20 mm. Cell cua elongated, its length about three times portion of vein (CuA+CuP) beyond it.
Wing. Length, 3.6 mm, at the middle, width 1.3 mm, with microtrichia (Figure 30.2). First longitudinal vein (R1) devoid of any spines. Costal cell (c) a little longer than portion of C on subcostal cell (sc), 1.26:1.10 mm. Length of discal cell (d) 1.55 mm, cell cup, 0.89 mm, bm, 0.57 mm, and rm, 0.77 mm. Plane of crossvein r-m intersects cell c at 59% of its 1.26 mm length. Posterior crossvein (dm-m) almost twice as long (0.34 mm) as distal part of M4, 0.20 mm. Cell cua elongated, its length about three times portion of vein (CuA+CuP) beyond it.
Legs. Legs dark brown (Figure 31.2-3). Front coxa with long setae. Apex of front femur and basal parts of tibia (= "knees") of lighter color. Setation of front and middle legs not visible. Visible hind tarsomeres slightly flattened, with dark bifurcated setae (an apomorphic character for family Platypezidae, see Tkoč et al. [2017]).
Abdomen and genitalia. Length, 2.63 mm long, maximum height 0.76 mm; abdominal segments narrowed gradually towards apex, T6-T7 with a row of erect blackish setae at posterior margin (Figure 31.1), approximately 0.13 mm in length (margins of the more anterior tergites not visible). Setae of two basal segments not visible. Cercus 0.16 mm in length, 43 μm in height.
Allotype. Male unknown.
Syncompressions. Diptera (1).
Remarks
The family Platypezidae, with the other flies in Platypezoidea, is thought to be sister to all other Cyclorrhapha (Wiegmann et al., 2011). Within the Platypezoidea, the family is sister to the clade (Opetiidae + Microsania) (Tkoč et al., 2017). The family is relatively small, with more than 250 extant species in 17 extant genera (Tkoč et al., 2017). The Paleobiology Database reports 17 fossils of Platypezoidea, most of which from the early Cretaceous (Mostovski, 1996). Evenhuis (1994) listed 11 species of Platypezidae (including Opetiidae) in nine genera. Amorim et al. (2018) have recently updated the positions of the Cretaceous biota. Four platypezid fossils are Eocene in age: Two species have been described from the Green River Formation (Callomyia hypolitha Cockerell, 1909 and C. torporata Scudder, 1890), one species from the Florissant (Eucallimyia fortis Cockerell, 1911) and Oppenheimiella baltica Meunier, 1893 from Baltic amber.
The specimen Agathoymia eocenica sp. nov. from the Kishenehn Formation is the first described extinct species of Agathomyia. Its inclusion into any Agathomyia species group is, however, problematic. Some of the important characters required to establish its position within the genus (exact colouration, color of setae on tergites 1-2, setation on middle tibia) are not preserved in the fossil. Figure 30.3 provides a comparison with the recent species Agathomyia antennata (Zetterstedt, 1819). The venation is very similar to this species, but there are observable differences: 1) cell cua is more elongated in A. eocenica; 2) the anal lobe has a different shape; 3) the cell between M4 and CuA+CuP is narrower and not lobate posteriorly in A. eocenica; 4) the costal cell (c) is slightly longer than the portion of costa on subcostal cell (sc) in A. eocenica.
Family LONCHOPTERIDAE Macquart, 1835
Genus LONCHOPTERA Meigen, 1803
Type species. Lonchoptera lutea Panzer, 1809, p. 20, by subsequent monotypy.
Lonchoptera eocenica Amorim and Brown, sp. nov.
Figure 32, Figure 33, Figure 34
zoobank.org/D0AB2A31-72B7-4B1B-A42F-D47E39F4E481
 Etymology. The specific epithet, a Latin adjective, refers to the geologic epoch in which this species lived.
Etymology. The specific epithet, a Latin adjective, refers to the geologic epoch in which this species lived.
Holotype. USNM 625379, compression fossil, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek member, Kishenehn Formation.
Type locality. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana).
Differential diagnosis. Similar to modern lonchopterids, but can be clearly separated from the recent species by the broader shape of the wing, slightly more rounded apically; R2+3 not as close to R4+5 as in modern species of the genus; R4+5 reaching C slightly before wing tip (while in recent species R4+5 characteristically ends at tip of wing); probably related to the general shape of the wing, the medial fork wider, with M1 and M2 relatively more separated at the wing margin; the distal end of M4, close to the margin, is almost straight, while in recent species it is slightly curved posteriorly.
Description
Female (Figure 32), body dark brown, body length 2.8 mm, wing length 2.7 mm.
Head. Relatively small, flattened; flagellomere 1 apically pointed.
Thorax and wings. Thorax stout. Sc weakly sclerotized (as in recent species), slightly separated from R1; R1 short, extending to about one-third of visible wing length; R2+3 convex, slightly converging towards wing apex; R4+5 extended to wing apex, reaching C just before wing tip; costal setae ending at R4+5; r-m originating at R4+5 just beyond origin of R2+3, strongly curved basally; CuA+CuP joining M4 before wing margin (as in modern females); fused vein CuA+CuP about as long as base of medial fork; wing cells br, bm, and cua (this latter hard to delimit) small, displaced to the base of the wing. Setulae visible on veins R1, M1+2, M2, M4, and base of CuA+CuP.
Legs. Legs apparently slightly shorter than in recent species.
Abdomen and genitalia. Abdomen shorter than recent species, tergites and sternites apparently well sclerotized. Terminalia short, only a short cercus visible.
Allotype. Male unknown.
Syncompressions. None.
Remarks
Lonchopteridae is a small family of extant flies that is found nearly worldwide. The modern fauna is represented by a single genus, Lonchoptera Meigen, for which about 50 species have been described. The Nearctic species and much of the information about the genus were reviewed by Klymko and Marshall (2008). The common name for this group, "spear-winged flies", is based on the narrowed wing with pointed apex. Other distinctive characters are the setulose wing veins, the shortened wing veins R1 and Sc, and the sexually dimorphic wing venation, with CuA+CuP joining M4 in females, but extended to the wing margin in males.
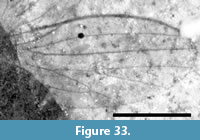 Fossils assigned to this family are few, basically two species of uncertain relationships to modern lonchopterids (Grimaldi and Cumming 1999). They both are, however, considerably different relative to the modern genus Lonchoptera and, if they belong at all to the clade, they would clearly belong to the stem group of the family. Indeed, Amorim et al. (2018) suggested that Lonchopterites Grimaldi and Cumming could be a stem-group Opetiidae, not a Lonchopteridae.
Fossils assigned to this family are few, basically two species of uncertain relationships to modern lonchopterids (Grimaldi and Cumming 1999). They both are, however, considerably different relative to the modern genus Lonchoptera and, if they belong at all to the clade, they would clearly belong to the stem group of the family. Indeed, Amorim et al. (2018) suggested that Lonchopterites Grimaldi and Cumming could be a stem-group Opetiidae, not a Lonchopteridae.
Few details of the head, thorax, legs, abdomen, and terminalia are visible in the specimen (Figure 32). Nevertheless, the wings are largely well preserved and visible, and there is scarcely any doubt that this species fits together with the recent species of Lonchoptera as a clade. Recent Lonchoptera species are considerably similar in the wing shape and the wing venation. Some modified wing venation features present in Lonchoptera are shared with Opetiidae and Phoridae within the Platypezoidea (Amorim et al., 2018), particularly the very basal origin of R1, the stronger R4+5, the reduction in size of cells bm, br, and cua, the loss of the dm-m crossvein and the shape of the long medial fork (some of these features secondarily modified in the phorids). A number of apomorphic conditions are seen in the wing of the recent species of Lonchoptera. These include the typical elongate shape of the wing, with a pointed tip, the displacement of R1 to a considerably basal position in the wing, the weakly sclerotized and short Sc, a convex R2+3, the tip of R2+3 displaced to close to the wing tip, R4+5 reaching precisely the acute wing tip, the strong displacement of the posterior end of r-m towards the base of the wing, CuA distally fused to M4 in females and a very short CuP actually coming out from the wing margin (actually, the circumambient C) to join CuA.
All of these features are present in the Eocene species described here, except the lack of the general elongate shape and pointed wing apex—while the CuP emerging from the wing margin cannot be verified (Figure 33). Some of the features in L. eocenica are modified, but not to the same degree as in recent species: this includes the displacement R1 to the base, of R2+3 to the apex and R4+5 ending at the very tip of the wing.
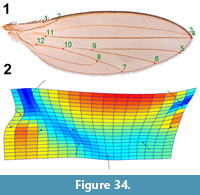 This set of features does not leave any doubt about the position of the species together with the recent Lonchoptera. A thin plate spline analysis was performed to show the direction of the changes from the fossil to modern wing veins (Figure 34.1-2; dots indicate modern venation, line bases the fossil equivalent). The analysis makes clear that the distance between R1 and R2+3 is expanded (orange/red) in the recent species, while R2+3 is compressed towards R4+5 (light/dark blue). Also, R1 is in an even more basal position in the recent species. The process of narrowing the wing in the modern species takes place in both the anterior and posterior portions of the wing.
This set of features does not leave any doubt about the position of the species together with the recent Lonchoptera. A thin plate spline analysis was performed to show the direction of the changes from the fossil to modern wing veins (Figure 34.1-2; dots indicate modern venation, line bases the fossil equivalent). The analysis makes clear that the distance between R1 and R2+3 is expanded (orange/red) in the recent species, while R2+3 is compressed towards R4+5 (light/dark blue). Also, R1 is in an even more basal position in the recent species. The process of narrowing the wing in the modern species takes place in both the anterior and posterior portions of the wing.
It is quite unfortunate that the some details of the fossil cannot be fully described, particularly the head, thorax, and legs. The very characteristic shape of the head of recent Lonchoptera and the quite elongate thorax cannot be properly checked in L. eocenica, but the fossil seems to be slightly stouter. The legs of L. eocenica seem to be also shorter. Based on the discussion above, L. eocenica appears to be the sister species to the recent species of Lonchoptera. In other words, the Eocene fossil described here is a stem Lonchoptera. Despite some differences in the wing shape and in the wing venation, we do not see any particular reason to have a separate monotypic taxon of generic rank to hold the fossil species. It is worth noting that Bouchenak-Khelladi et al. (2010) date the crown node of Poaceae (i.e., BEP+PACCMAD) at the early Eocene, 57 Ma. The larvae of recent species have quite varied habitats, but adults are associated with grassy habitats (Klymko and Marshall, 2008), and the genus may have largely expanded its distribution with the diversification of grassy, open environments at the mid of the first half of the Cenozoic. This fossil will certainly bring important benefits to calibration in studies on age divergence in the Cyclorrhapha.
Family PHORIDAE Curtis, 1833
Genus AENIGMATIAS Meinert, 1890
Type species. Aenigmatias blattoides Meinert, 1890
Aenigmatias kishenehnensis Brown, sp. nov.
Figure 35
zoobank.org/28A4C2F4-40E2-45AF-83A4-FADAD0EBD616
Etymology. The specific epithet denotes the geological Formation in which the specimen was preserved.
Holotype. USNM 625132, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. The Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This species of Aenigmatias is distinguished by the presence of wings, very short C, R1 and Rs veins, the absence of R2+3 and an apparently nonlimuloid body shape.
 Description
Description
Body length (head to the end of tergite 6) 1.5 mm. Female (Figure 35.1).
Head. Four large setae present at vertex and at least six large setae on frons (all frontal setae absent in modern species, often reduced in number and size in fossils). Postpedicel rounded. Palpus with well-developed setae.
Thorax. Most details of thorax not visible, but not appearing to be highly limuloid. One pair of short setae visible (possibly posterior dorsocentral setae) and one longer pair (scutellars?) near base of wing.
Wings. C short, but costal setae long. R2+3 absent. Base of radial veins possibly with row of setulae.
Legs. Foretibia not preserved. Midtibia with one large seta near base. Hind legs preserved, but details not visible.
Abdomen and genitalia. Abdomen unmodified, unlike flattened modern species. Abdominal segment 7 with dense striation and blunt thick setae. (Figure 35.2, bts; as in modern species, Figure 35.3).
Allotype. Male unknown.
Syncompressions. None.
Remarks
The family Phoridae consists of 302 genera and about 4,300 species. The fossil record of the family is quite good, with 103 fossil species (47 genera), 95% of which are in amber (about half of these in Baltic amber). Many extant phorids exhibit a parasitoid lifestyle and several genera are myrmecophilous (Brown, 2018). The 14 extant species of the genus Aenigmatias are presumed to be larval parasitoids of ant pupae. Members of the genus are markedly sexually dimorphic, with the female wingless and limuloid in shape, the latter an adaptation that provides defense against attack by ants (Brown, 2017; Brown et al., 2017). Brown (1999), in an examination of fossil phorids in Baltic and Fushun amber, concluded that many of the specimens assigned to extant genera actually belong to more primitive stem-groups. Protophorites fimbriatus Brues, 1939, was synonymized with Protoplatyphora tertiaria Brues, 1939 (holotype lost), which was thought to be a stem group of a clade with the modern Aenigmatias species, based on a less limuloid body shape, the presence of wings (Protophorites fimbriatus is a female) and the presence of large setae on the vertex and scutellum. Brown (2017), in a phylogenetic analysis of the fossil Aenigmatias and related genera, transferred Protoplatyphora tertiaria and Chaetopleurophora multisetosa Brown, 2007 to Aenigmatias and described three new species from Baltic amber. With Aenigmatias kishenehnensis, there are currently a total of six fossil species in the genus: A. tertiarius Brues, 1939, A. bisetosa Brown, 2007, A. longicornis Brown, 2017, A. primitivus Brown, 2017 and A. nigeroticus Brown, 2017. Only A. tertiarius, Brues 1939, A. kishenehnensis and an additional specimen (LACM 159804) are females.
Aenigmatias kishenehnensis can be differentiated from A. tertiaria in having a much shorter costa (0.24 mm vs. 0.5 mm), R2+3 vein absent, and the frons with large setae; from A. bisetosa in having a much shorter body length (1.5 mm vs. 4.3-4.4 mm), a much shorter costa and R2+3 absent; from A. longicornis in having a rounded first flagellomere (vs. elongate) and a much shorter costa (0.24 mm vs. 0.43 mm); from A. primitivus in having a much shorter costa (0.24 mm vs. 0.48 mm); and from A. nigeroticus in having a much shorter costa (0.24 mm vs. 0.48 mm), R2+3 absent and the frons with large setae. Aenigmatias kishenehnensis is also differentiated from Aenigmatias sp. indet. (LACM ENT 159804; Brown, 2017), a female, in having a much shorter costa (0.24 mm vs. 0.71 mm). Apparently, the set of extinct species of Aenigmatias do not constitute a clade sister to the extant species of the genus, but rather a grade.
The 21 fossil phorid flies of the Kishenehn formation are intriguing. With the exception of A. kishenehnensis, they are difficult to place to any modern group. None of the specimens exhibit proclinate supra-antennal setae, a condition found in most phorids of the subfamily Metopininae, which is the numerically dominant group today. The earliest undoubted metopinines are known from Baltic amber, and even there they are a smaller portion of the fauna than today, suggesting that the metopinine radiation is indeed an evolutionarily recent (i.e., post-Eocene) event. Other character states visible in the Kishenehn phorids are not considered synapomorphic of any modern groups, with a single exception of A. kishenehnensis.
Modern Aenigmatias, and most fossil species of the genus (Brown, 2017), have a series of setulae along the radial vein, more than four scutellar setae and longitudinal, irregular rows of tightly-packed setulae (setal palisades) on the hind tibia in addition to the limuloid body form. None of these character states was convincingly observed in A. kishenehnensis, but the much more distinctive structure of the female terminal segments is clearly visible: the ovipositor has heavily striate membrane and thick, peglike setae. This structure is known from at least one fossil species, A. tertiarius, and all examined modern species, but no other phorids. The life history of two of the 14 modern species has been studied and both found to be parasitoids of Formica Linnaeus 1758 ant pupae (Donisthorpe, 1927). No studies have been done on the function of this peculiar ovipositor, which is unlike those of other parasitoid phorids that attack adult ants, but it is likely related to the parasitic lifestyle.
Family PIPUNCULIDAE Walker, 1834
PIPUNCULINAE Walker, 1834
Type species. Pipunculus campestris Latreille, 1802
Pipunculinae incertae sedis
Figure 36
Type horizon. Middle Eocene Coal Creek Member, Kishenehn Formation.
Type locality. Deep Ford site, Middle Fork of the Flathead River (Pinnacle, Montana, USA).
Differential diagnosis. This specimen is distinguished by antennal pedicel with prominent bristle and shorter setae, and a long, thin arista; body without long setae/bristles; wing with costa extending beyond apex to M1, R4+5 ending at the apex of the wing, M2 present, CuA2 merges with A1 just before margin.
Description. Ventral aspect, female. Total length 3.64 mm (actual), 4.0 mm (estimated); color black. NMNH, USNM 625934 (Figure 36.1).
Head. Large, 1.27 mm wide, 0.89 mm long. Ocellar bristles absent. Occiput, if present, obscured/destroyed by crack. Antenna well-preserved. Scape not clearly distinguishable. Pedicel 0.13 mm wide, length 74 μm, with one prominent ventral bristle 0.12 mm long plus some smaller bristles and at least eight smaller but distinct (30-80 μm) setae on the dorsal surface. Visible portion of F1 spherical, 73 μm wide, with several thin 33 μm setae. Arista long (0.46 mm), thin, basal portion 86 μm long, 26 μm wide (Figure 36.1).
Thorax. Quadrate, both length and width 1.43 mm. Scutum and scutellum not visible as the fossil is of the ventral aspect of the insect.
Wings. Length,4.0 mm, width, 1.33 mm (left), 1.48 mm (right) (Figure 36.2). Pterostigma present, as long as 3rd costal section. Ratio of length of 2nd, 3rd and 4th costal sections 4.5:2.2:1. R4+5 meets margin at wing apex, C extends beyond apex to reach M1; r-m at 0.27 of length of discal cell. M1+2 distal of r-m curved, convex. M2 present, 0.61 of length of last abscissa of M1+2; M1 curved, convex, dm-cu slightly curved, CuA merges with CuP just before margin. Spurious veins in both wings in the basal radial cell and cells r1+2 and r4+5. Anal lobe present.
Legs. Five legs visible including yellow fore and hind tibiae; tibiae with short black setae. Front femur wide, black.
Abdomen. Sternites 1-5 length, 0.2 mm, 0.23 mm, 0.27 mm, 0.26 mm and 0.41 mm, respectively, wider than long, sternite 3, 1.3 mm wide. Lateral abdominal setae, if present, short. Terminalia not preserved. Length of sternite 5 strongly suggests that the specimen is a female, since males have sternite 5 considerably reduced compared to sternites 2-4 due to the genitalia complex that follows S5.
Allotype. Male unknown.
Syncompressions. None.
 Remarks
Remarks
Pipunculidae contains approximately 1,428 species in 22 extant genera placed in four subfamilies: Chalarinae Aczél, 1939a, Nephrocerinae Aczél, 1939b, Pipunculinae Walker, 1834, and Protonephrocerinae Aczél, 1948. According to molecular dating, the diversification of this family began approximately 85 Mya (Wiegmann et al., 2011). The oldest, albeit undescribed, fossil is from the Fur Formation (about 55 Mya; Bonde et al., 2008). Members of the crown group of the family (with M2 absent), however, existed as early as 51 Mya (Archibald et al., 2014a). To date, 16 fossil specimens have been described (Archibald et al. 2014a; Kehlmaier et al., 2014; this study).
The crack in this fossil has destroyed any remnant of an occiput. The first flagellomere is very small (smaller than the pedicel, which is not the case in any extant species), which raises the possibility that only the uppermost portion of this structure is visible. Except for the pedicel, no other long setae or bristles are visible on the head, thorax, legs or abdomen. This feature, in combination with the wing venation, eliminates the possibility that this species belongs to the Chalarinae, Protonephrocerinae (eliminated based on R4+5 ending at the apex of the wing), part of Nephrocerinae (Nephrocerus Zetterstedt, 1838) and part of Pipunculinae. Priabona Archibald et al., 2014 (Nephrocerinae) is not well enough known. In extant Pipunculinae, long and numerous (>5) bristles on pedicel occur especially in Claraeola Aczél, 1940 (Eudorylini Rafael and De Meyer, 1992) of which many extant species also have M2. Skevington and Yeates (2001) regard long bristles on the pedicel as a plesiomorphy while a larger number of bristles as an apomorphic condition. Despite the destroyed occiput, the fossil can be safely placed in the Pipunculinae. The characters required for a confident assignment to a tribe, however, let alone a genus, cannot be observed. The specimen is here assigned to Pipunculinae incertae sedis.
This Kishenehn Formation pipunculine is small relative to most fossils of the family. Pipunculidae species A (Archibald et al., 2014a), Metanephrocerus belgardeae Archibald, Kehlmaier and Mathewes, 2014, Protoverallia succinea Meunier, 1903, Nephrocerus oligocenicus, Carpenter and Hull, 1939, Metanephrocerus collini Carpenter and Hull, 1939, M. hoffeinsorum Kehlmaier and Skevington in Kehlmaier, Dierick and Skevington, 2014, and M. groehni Kehlmaier and Skevington in Kehlmaier, Dierick and Skevington, 2014, have wing lengths of 5.9, 9.2, 6, 8, 6, 6.7 and 6 mm, respectively. The body length of Eudorylas Aczél, 1940 species A is only 2.2 mm, while Eudorylas species A and B (De Meyer, 1995) and Pipunculinae species A (Archibald et al., 2014a) lack vein M2. Unlike this incertae sedis Pipunculinae, both Protoverallia succinea and Metanephrocerus collini have long abdominal bristles; Metanephrocerus collini also has M2 at the wing margin; Cephalosphaera baltica, according to Carpenter and Hull, 1939, has "smoky wings", but this feature was not mentioned by Aczél (1948). Cephalosphaera baltica is larger than the Kishenehn Formation specimen as its wings are 5.5 mm in length (Aczél, 1948). Pipunculidae species A, Metanephrocerus hoffeinsorium and M. groehni differ markedly from this Pipunculinae incertae sedis in the ratios of the 2nd, 3rd and 4th costal sections length. Carpenter and Hull (1939) briefly reviewed Meunier’s (1903) fossils and indicated that Verrallia exstincta Meunier, 1903, was conspecific with what Meunier termed a "variety" of V. exstincta which he referred to as V. kerteszia Meunier, 1903. Aczél (1948) however, stated that "according to the figure, the 3rd and 4th costal sections are shorter than the 5th (in V. kerteszia), while the 3rd and 4th costal sections of V. exstincta are much longer than the 5th." In any case, both of these fossil specimens of Verrallia are twice the length of the Kishenehn specimen.
DISCUSSION
There are currently 163 extant families in the order Diptera (Pape et al., 2011; Papp, 2011), including the families Mythicomyiidae (Melander, 1902), Pseudopomyzidae (McAlpine, 1966) and Uluromyiidae (Michelsen and Pape, 2017). Most (116 or 71%) of these are represented in the fossil record. There are 54 additional taxa with family rank that are extinct (Nagatomi and Yang, 1998; Poinar, 2010; Pape et al., 2011; Zhang et al., 2011; Zhang, 2012). In North America, the Eocene Florissant, Green River and Okanagan/Republic Lagerstätten have, to date, produced described species in 36, 26 and 15 families, respectively (Table 2) (Rice, 1959; Wilson, 1977, 1978; Douglas and Stockey, 1996; Wehr and Barksdale, 1996, 1998; Meyer, 2003; Archibald, 2007; Archibald et al., 2010, 2013, 2014a, 2014b). For reference, Baltic amber has produced described species in 81 dipteran families, only one of which, Hoffeinsmyiidae, is extinct (Michelsen, 2009; EDNA, 2017). The insect fauna of the Coal Creek Member of the Kishenehn Formation in northwestern Montana is dominated by flies, and studies to date, including the material herein addressed, have described 23 species of Diptera in 21 families (including Limoniidae and Cylindrotomidae; Table 2).
Of the 21 familes described from the Kishenehn Formation, including the 17 described herein, only three, Platypezidae, Phoridae and Pipunculidae belong to Cyclorrhapha. Pipunculidae has recently been proposed to be sister to the Schizophora (Pauli et al., 2018). No schizophorans have been described to date from the Kishenehn Formation although Calyptratae near Muscidae and acalyptrates near Chloropidae have been identified (Bickel, personal commun., 2017). In contrast, 18 families of Cyclorrhapha have been described from the three other North American Eocene compression fossil Lagerstätten, including 10 Schizophora and five Calyptratae (Glossinidae Theobald, 1903, Muscidae Latreille, 1802, Oestridae Leach, 1815, Scathophagidae Robineau-Desvoidy, 1830 and Tachnidae Robineau-Desvoidy, 1830 [Table 3]).
Classification of Kishenehn Formation specimens to extant genera reflects, in part, their high degree of preservation of morpholpgical detail. It also reflects the relative longevity of dipteran genera. Analysis of all described fossil Diptera (Evenhuis, 2017) reveals that 55% of all genera represented by species from the Eocene epoch are extant. The percentage increases to 79% for genera in which the oldest representative is from the Oligocene. The former percentage is actually much higher as the value is negatively affected by the 79 new and mostly unnecessary genera created by Hong (2002). In the current studies, different approaches have been taken by different authors. Two of the specimens have been identified to subfamily incertae sedis. Although the bruchomyiine (Psychodidae) specimen can be keyed to the genus Nemopalpus (Quate and Alexander, 2000), the observations that all existing bruchomyiine fossils have been assigned to this genus and many, if not the majority of these assignments are thought to be inaccurate (Wagner and Stuckenberg, 2016) have elicited a conservative assignment of this specimen. In the case of the asiline (Asilidae) specimen, the new genus Kishenehnoasilus is, in essence, a placeholder assignment, useful until additional and better preserved specimens can be obtained and/or the extant North American fauna is better understood.
Several factors affect the preservation of dipteran diversity in the Kishenehn Formation. Very small insects are often well-preserved (Huber and Greenwalt, 2011; Shockley and Greenwalt, 2013). Culicidae, of which over 70 specimens have been collected from the Coal Creek Member, have not been recorded as described species from either the Florissant or the Okanagan. Specimens of the families Dixidae Schiner, 1868, and Bolitophilidae Winnertz, 1863, both described from the Kishenehn Formation (Greenwalt and Moulton, 2016; Greenwalt and Blagoderov, 2019), have not been reported from any of the three North American Lagerstätten. There appears to be a taphonomic size bias such that specimens greater than 1 cm in length are rarely preserved in the Kishenehn Formation. For example, large 3-dimensional flies such as horse flies, house flies, etc. are, if present, invariably very poorly preserved. This of course has a negative impact on the documentation of existing diversity at that time. The Diptera of the Coal Creek Member of the Kishenehn Formation are dominated by families that have aquatic immatures. As with the size bias, the shallow lacustrine environment in which the insects were preserved both limits and promotes the preservation of diversity. Kishenehn Formation specimens from numerous additional families including Ceratopogonidae Newman, 1834, Chaoboridae Newman, 1834, Chironomidae Newman, 1834, Chloropidae Rondani, 1856, Empididae Latreille, 1804, Microphoridae Collin 1960, Muscidae Latreille, 1802, Syrphidae Latreille, 1802, Xylomyidae Verrall, 1901 and others, have been identified and await description.
ACKNOWLEDGMENTS
The authors wish to thank C.F. Thompson, R. Wagner and J. Gelhaus for their valuable input. B. Archibald compiled the list of dipteran families of the Okanagan. We also thank two reviewers (D. Amorim and V. Blagoderov), whose careful reviews and constructive criticisms significantly improved the manuscript. DEG wishes to thank C. Labandeira for the opportunity to continue his research activities, as a Research Associate, at the National Museum of Natural History in Washington, D.C. MT was supported by the Ministry of Culture of the Czech Republic (DKRVO 2019-2023/5.I.a, National Museum, Prague, 00023272) and by the Institutional Research Support grant of the Charles University, Prague (SVV No. 260 434 / 2019). We also wish to thank Alicia Reuter, Jinghong Cai and Rosanne D. Johnson of the Smithsonian Translation Service for translation of Freiwald (1991) and Hong (2002). We also wish to thank those individuals who produce and support the EDNA, PBDB, Bishop Museum, Florissant National Monument and Systema Diptorum digital.
REFERENCES
Aczél, M.L. 1939a. Die Untergattung Dorylomorpha m. von Tomosvaryella m. (Doryl. Stud. 2). Zoologischer Anzeiger, 125(3,4):49-69.
Aczél, M.L. 1939b. Das System der Familie Dorylaidae. Dorylaiden-Studien I. Zoologischer Anzeiger, 125:15-23.
Aczél, M.L. 1940. Vorarbeiten zu einer Monographie der Dorylaiden (Diptera). Dorylaiden-Studien V. Zoologische Anzeiger, 132:149-169.
Aczél, M. 1948. Grundlagen einer Monographie der Dorilaiden (Diptera). Dorilaiden-Studien VI. Acta Zoologica Lilloan, 6:5-168.
Aldrich, J.M. 1932. New Diptera, or two-winged flies, from America, Asia, and Java, with additional notes. Proceedings of the United States National Museum, 80:1-10.
Alexander, C.P. 1915. New or little-known crane-flies from the United States and Canada: Tipulidae, Diptera. Part 2. Proceedings of the Academy Natural Sciences of Philadelphia, 67:458-514.
Alexander, C.P. 1920. The crane-flies of New York. Part II. Biology and Phylogeny. Memoirs of the Cornell University Agricultural Experiment Station, 38:699-1133.
Alexander, C.P. 1921. A new subfamily of tanyderid flies (Diptera). Annals of the Entomological Society of America, 13:402-406.
Alexander, C.P. 1929. New or little-known Tipulidae from eastern Asia (Diptera). V. Philippine Journal of Science, 40:519-547.
Alexander, C.P. 1931a. Crane-flies of the Baltic amber (Diptera). Bernstein Forschungen, 2:1-135.
Alexander, C.P. 1931b. Deutsche Limnologische Sunda-Expedition. The crane-flies (Tipulidae, Diptera). Archiv fur Hydrobiologie, 9:135-191.
Alexander, C.P. 1934. New or little-known Tipulidae from eastern Asia (Diptera). XXI. Philippine Journal of Science, 55:19-60.
Alexander, C.P. 1965. New subgenera and species of crane-flies from California (Diptera: Tipulidae). Pacific Insects, 7:333-386.
Alexander, C.P. 1968. New or little known Tipulidae from eastern Asia (Diptera) LXII. Philippine Journal of Science, 96:29-72.
Alexander, C.P. and Byers, G.W. 1981. Tipulidae. p. 153-190. In McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., and Wood, D.M. (eds.), Manual of Nearctic Diptera, Volume 1. Research Branch, Agriculture Canada, Quebec.
Amorim, D.S. 1994. A new suprageneric classification of the Scatopsidae (Diptera: Psychodomorpha). Iheringia, Série Zoologia, 77:107-112.
Amorim, D.S. 1998. Amber fossil Scatopsidae (Diptera: Psychodomorpha). I. considerations on described taxa, Procolobostema roseni, new species, from Dominican amber, and the position of Procolobostema in the family. American Museum Novitates Amorim, D.S. 2009. Scatopsidae. p. 347-355. In Brown, B., Borkent, A., Cumming, J.M., Wood, D.M., and Zumbado, M.A. (eds.), Diptera of Central América, Vol. 1. NRC-CNRC, Ottawa.
Amorim, D.S. and Tozoni, S.H.S. 1994. Phylogenetic and biogeographic analysis of the Anisopodoidea (Diptera, Bibionomorpha), with an area cladogram for intercontinental relationships. Revista Brasiliera de Entomologia, 38:517-543.
Amorim, D.S., Silva, V.C., and Brown, B.V. 2018. Puyehuemyia chandleri, gen. n., sp. n. (Diptera: Opetiidae): remnant of a Cretaceous biota in Chile. American Museum Novitates, 3892:1-27. https://doi.org/10.1206/3892.1
Ansorge, J. 1994. Tanyderidae and Psychodidae (Insecta: Diptera) from the Lower Jurassic of northeastern Germany. Palaontologische Zeitschrift, 68:199-210. https://doi.org/10.1007/bf02989440
Archibald, S.B. 2007. Climate and Species Diversity: The Eocene Okanagan Highlands Insect View, Vols. 1-2. Unpublished Ph.D. Thesis, Harvard University, Cambridge, Massachusetts, USA.
Archibald, S.B., Bossert, W.H., Greenwood, D.R., and Farrell, B.D. 2010. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology, 36(3):374-398. https://doi.org/10.1666/09021.1
Archibald, S.B., Greenwood, D.R., and Mathewes, R.W. 2013. Seasonality, montane beta diversity, and Eocene insects: Testing Janzen's dispersal hypothesis in an equable world. Palaeogeography, Palaeoclimatology, Palaeoecology, 371:1-8. https://doi.org/10.1016/j.palaeo.2012.10.043
Archibald, S.B., Kehlmaier, C., and Mathewes, R.W. 2014a. Early Eocene big headed flies (Diptera: Pipunculidae) from the Okanagan Highlands, western North America. The Canadian Entomologist, 146 (4):429-443. https://doi.org/10.4039/tce.2013.79
Archibald, S.B., Morse, G E., Greenwood, D.R., and Mathewes, R. W. 2014b. Fossil palm beetles refine upland winter temperatures in the Early Eocene Climatic Optimum. Proceedings of the National Academy of Sciences, 111(22):8095-8100. https://doi.org/10.1073/pnas.1323269111
Arillo, A., Penalver, E., and Garcia-Gimeno, V. 2009. First fossil Litoleptis (Diptera: Spaniidae) from the Lower Cretaceous amber of San Just (Teruel Province, Spain). Zootaxa, 2026:33-39.
Becker, T. 1907. Die Ergebnisse meiner dipterologischen Fruhjahrsreise nach Algier und Tunis, 1906. Zeitschrift für Systematische Hymenopterologie und Dipterologie, 7:33-61.
Beier, M. 1952. Miozäne und oligozäne Insekten aus Österreich und den unmittelbar angrenzenden Gebieten. Sitzungsberichte, Österreichische Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse, Abteilung I. Biologie, Mineralogie, Erdkunde und verwandte Wissenschaften, 161:129-134.
Bengtson, P. 1988. Open nomenclature. Palaeontology, 31 (1):223-227.
Bezzi, M. 1911. Biospeologica. XX. Dipteres (premiere serie) suivi d'un appendice sur les dipteres cavernicoles recueillis par le Dr Absolon dans les Balcans. Archives de Zoologie Expérimentale et Générale, 5(8):1-87.
Bickel, D.J. 1995. A fossil Gymnopternus Loew (Diptera: Dolichopodidae) from the Florissant beds, Colorado. Psyche, 102:169-172. https://doi.org/10.1155/1995/18952
Bigot, J.M.F. 1857. Essai d'une classification generale et synoptique de l'ordre des insectes dipteres. 5e memoire. Tribu des Asilidi (mihi). Annales de la Société Entomologique de France, 3eme Series, 5:517-564.
Billberg, G.J. 1820. Enumeratio Insectorum in Museo Gustaf Johan Billberg. Typis Gadelianis, Stockholm.
Blagoderov, V.A. 1996. Revision of the nematoceran family Protopleciidae (Insecta: Diptera) from the Early Jurassic Sogyuty locality, Kyrgyzstan. Paleontologicheskii Zhurnal, 30:210-216.
Blagoderov, V.A., Grimaldi, D.A., and Fraser, N.C. 2007. How time flies for flies: diverse Diptera from the Triassic of Virginia and early radiation of the order. American Museum Novitates, 3572:1-39. https://doi.org/10.1206/0003-0082(2007)509[1:htfffd]2.0.co;2
Bonde, N., Andersen, S., Hald, N., and Jakobsen, S.L. 2008. Danekræ - Danmarks Bedste Fossiler. Gyldendal, Copenhagen, Denmark.
Borkent, A. and Grimaldi, D.A. 2011. The Cretaceous fossil Burmaculex antiquus confirmed as the earliest known lineage of mosquitoes (Diptera: Culicidae). Zootaxa, 4079(4):457-466. https://doi.org/10.11646/zootaxa.4079.4.5
Bouchenak-Khelladi, Y., Verboom, G.A., Savolainen, V., and Hodkinson, T.R. 2010. Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal evolutionary history in geographical space and geological time. Botanical Journal of the Linnean Society, 162(4):543-557. https://doi.org/10.1111/j.1095-8339.2010.01041.x
Brodie, P.B. 1845. A History of the Fossil Insects in the Secondary Rocks of England Accompanied by a Particular Account of the Strata in which they Occur, and of the Circumstances Connected with their Preservation. John Van Voorst, London.
Brodo, F. 1967. A review of the subfamily Cylindrotominae in North America (Diptera, Tipulidae). The University of Kansas Science Bulletin, 47:71-115.
Brooks, S.E. 2005. Systematics and phylogeny of Dolichopodinae (Diptera: Dolichopodidae). Zootaxa, 857:1-158.
Brown, B.V. 1999. Re-evaluation of the fossil Phoridae (Diptera). Journal of Natural History, 33(10):1561-1573. https://doi.org/10.1080/002229399299897
Brown, B.V. 2005. Malaise trap catches and the crisis in Neotropical Dipterology. American Entomologist, 51:180-183. https://doi.org/10.1093/ae/51.3.180
Brown, B.V. 2007. Novel character states in fossil species of modern phorid genera (Diptera: Phoridae). Studia Dipterologica, 14:107-116.
Brown, B.V. 2017. Fossil evidence of social insect commensalism in the Phoridae (Insecta: Diptera). Journal of Systematic Palaeontology, 15(4):275-285. https://doi.org/10.1080/14772019.2016.1172676
Brown, B.V. 2018. A second contender for "world’s smallest fly" (Diptera: Phoridae). Biodiversity Data Journal, 6:e22396. https://doi.org/10.3897/BDJ.6.e22396
Brown, B.V., Hash, J.M., Hartop, E.A., Porras, W., and Amorim, D. de S. 2017. Baby killers: Documentation and evolution of Scuttle fly (Diptera: Phoridae) parasitism of ant (Hymenoptera: Formicidae) brood. Biodiversity Data Journal, 5:e11277. https://doi.org/10.3897/bdj.5.e11277
Brown, F.M. 1988. The extinct genus Cyttaromyia Scudder in Colorado (Diptera: Tipulidae). Journal of the Kansas Entomological Society, 61(2):232-234.
Brues, C.T. 1939. Fossil Phoridae in Baltic amber. Bulletin of the Museum of Comparative Zoology, Harvard University, 85:413-436.
Carpenter, F.M. and Hull, F.M. 1939. The fossil Pipunculidae. Bernstein-Forschungen, 4:8-17.
Chillcott, J.G. 1963. A new genus of Rhagionidae (Diptera) with notes and descriptions of Bolbomyia Loew. The Canadian Entomologist, 95:1185-1190.
Clapham, M.E. 2016. Insect taxonomy information from the internet, with fixing of species orphaned by genus synonymy. Paleobiology Database. http://PaleoDB.org/
Cockerell, T.D.A. 1908. Two fossil Diptera. Canadian Entomologist, 40:173-175.
Cockerell, T.D.A. 1909. Descriptions of Tertiary insects, 5. Some new Diptera. American Journal of Science (Series 4), 27:53-58.
Cockerell, T.D.A. 1911. Fossil insects from Florissant, Colorado. Bulletin of the American Museum of Natural History, 30:71-82.
Cockerell, T.D.A. 1913. The first fossil anthomyiid fly from Florissant (Dipt.). Entomological News, 24:295-296.
Cockerell, T.D.A. 1915. British fossil insects. Proceedings of the United States National Museum, 49:469-499.
Cockerell, T.D.A. 1917a. Some American fossil insects. Proceedings of the United States National Museum, 51(2146):89-106.
Cockerell, T.D.A. 1917b. New Tertiary insects. Proceedings of the United States National Museum, 52:373-384.
Cockerell, T.D.A. 1917c. Fossil insects. Annals of the Entomological Society of America, 10:1-22.
Cockerell, T.D.A. 1921a. Eocene insects from the Rocky Mountains. Proceedings of the United States National Museum, 57:233-260.
Cockerell, T.D.A. 1921b. Fossil arthropods in the British Museum - VI. Oligocene insects from Gurnet Bay, Isle of Wight. The Annals and Magazine of Natural History, Ninth Series, 7:453-480.
Cockerell, T.D.A. 1925. Fossil insects in the United States National Museum. Proceedings of the United States National Museum, 64(2503):1-15.
Cockerell, T.D.A. 1926. Plant and insect fossils from the Green River Eocene of Colorado. Proceedings of the United States National Museum, 66(2556):1-13.
Constenius, K.N. 1996. Late Paleogene extensional collapse of the Cordilleran foreland fold and thrust belt. Geological Society of America Bulletin, 108:20-39. https://doi.org/10.1130/0016-7606(1996)108%3C0020:lpecot%3E2.3.co;2
Cook, E.F. 1955. A contribution toward a monograph of the family Scatopsidae (Diptera). Annals of the Entomological Society of America, 48(4):240-251.
Cook, E.F. 1963. Guide to the insects of Connecticut, pt. 6. The Diptera or true flies, fasc. 8. Family Scatopsidae. Bulletin of the Geological Survey of the Connecticut, 93:1-37.
Cook, E.F. 1971. Fossil Scatopsidae in Mexican amber (Diptera: Insecta). University of California Publications in Entomology, 63:57-61.
Cook, E.F. 1972. A synopsis of the Scatopsidae of the Palaearctic. Part II. Swammerdamellini. Journal of Natural History, 6:625-634.
Coquillett, D.W. 1904. New North American Diptera. Proceedings of the Entomology Society of Washington, 6:166-192.
Coquillett, D.W. 1910. The type-species of the North American genera of Diptera. Proceedings of the United States National Museum, 37:499-647.
Cumming, J.M. and Wood, D.M. 2017. Adult morphology and terminology, pp. 89-133. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.), Manual of Afrotropical Diptera, Vol. 1. Introductory Chapters and Keys to Diptera Families, Suricata 4. SANBI, Pretoria.
Curler, G.R. and Jacobson, A.J. 2012. New species of Psychodidae (Diptera) from Australasia, with a checklist of the world species of Bruchomyiinae and Sycoracinae. Zootaxa, 3552:43-65. https://doi.org/10.11646/zootaxa.3552.1.3
Curran, C.H. 1934. The Families and Genera of North American Diptera. Ballou Press, New York.
Curtis, J. 1833. British Entomology: Being Illustrations and Descriptions of the Genera of Insects Found in Great Britain and Ireland, Volume 10. Privately published, London.
De Meyer, M. 1995. Short note on fossil Pipunculidae (Diptera) from Dominican amber. Journal of the New York Entomological Society, 103:208-214.
Dikow, T. 2009. Phylogeny of Asilidae inferred from morphological characters of imagines (Insecta: Diptera: Brachycera: Asiloidea). Bulletin of the American Museum of Natural History 319:1-175. https://doi.org/10.1206/603.1
Donisthorpe, H.S.J.K. 1927. The Guests of British Ants, Their Habits and Life Histories. George Routledge and Sons, London.
Douglas, S.D. and Stockey, R.A. 1996. Insect fossils in middle Eocene deposits from British Columbia and Washington State: Faunal diversity and geological range extensions. Canadian Journal of Zoology, 74:1140-1157. https://doi.org/10.1139/z96-126
Doutt, R.L. 1973. The fossil Mymaridae (Hymenoptera: Chalcidoidea). Pan-Pacific Entomologist, 49:221-228.
EDNA. 2017. EDNA Fossil Insect Database. http://edna.palass-hosting.org/search.php (accessed April 27, 2017 and additional dates).
Edwards, F.W. 1923. Oligocene mosquitoes in the British Museum, with a summary of our present knowledge concerning fossil Culicidae. Quarterly Journal of the Geological Society, 79:139-155.
Edwards, F.W. 1938. British short-palped craneflies. Taxonomy of adults. Transactions of the Society for British Entomology, 5:1-168.
EOL. 2017. Encyclopedia of Life. http://eol.org/pages/80583/overview (accessed February 16, 2017 and additional dates).
Enderlein, G. 1936. Diptera (Zweiflügler). Tierwelt Mitteleuropas 6, Insekten, 3:1-259.
Evenhuis, N.L. 1994. Catalogue of the Fossil Flies of the World (Insecta: Diptera). Backhuys Publishers, Leiden.
Evenhuis, N.L. 2017. Catalogue of the Fossil Flies of the World (Insecta: Diptera): On-line Digital Database. http://hbs.bishopmuseum.org/fossilcat/ (accessed May 1, 2017 and additional dates).
Ewa, K., Robert, C.A., and Wiesław, K. 2010. A new species of Megarhyphus, an interesting discovery from the Lower Jurassic of England (Diptera, Anisopodidae). Acta Geologica Sinica (English Edition), 84(4):693-695.
Fallén, C.F. 1810. Specim. entomolog. novam Diptera disponendi methodum exhibens. Berlingianis, Lund.
Fate, C., Perrichot, V., and Nel, A. 2013. A mid Cretaceous representative of the modern scatopsid genus Ectaetia (Diptera: Scatopsidae: Ectaetiinae). Zootaxa, 3686:396-400. https://doi.org/10.11646/zootaxa.3686.3.9
Fitzgerald, S.J. 1999. A new species of Plecia from the Green River Formation and new combinations of fossil Bibionidae (Diptera). Great Basin Naturalist, 59:182-187.
Fitzgerald, S.J. 2004. Evolution and Classification of Bibionidae (Diptera: Bibionomorpha). Unpublished Ph.D. Thesis. Oregon State University, Corvallis, Oregon. Available from http://ir.library.oregonstate.edu/xmlui/handle/1957/10998 (accessed January 29, 2018).
Fleming, J. 1821. Insecta, p. 41-56. In Stewart, D., Playfair, J., and Brande, W.T. (eds.), Supplement to the Fourth, Fifth and Sixth Editions of the Encyclopedia Britannica, Vol. 5, Pt. 1. A. Constable and Co., Edinburgh.
Fraser, N.C., Grimaldi, D.A., Olsen, P.E., and Axsmith, B. 1996. A Triassic lagerstatte from eastern North America. Nature, 380(6575):615-619. https://doi.org/10.1038/380615a0
Freeman, P. 1990. Redescription of seven Oriental species of Scatopsidae (Diptera) described by F.W. Edwards in the genus Scatopse. Entomologists’ Monthly Magazine, 126:9-19.
Freiwald, A. 1991. Insekten aus der Fur-Formation von Dänemark (Moler, ob. Paleozän/unt. Eozän?). 5. Cylindrotomidae (Diptera: Tipulomorpha). Meyniana, 43:97-123.
Freiwald, A. and Krzemiński, W. 1991. Cylindrotomidae (Diptera, Tipulomorpha) from the Paleogene of Bolshaya Svetlovodnaya (Eastern Asiatic USSR). Paläontologische Zeitschrift, 65:339-344.
Gentilini, G. 1984. Limoniidae and Trichoceridae (Diptera Nematocera) from the upper Miocene of Monte Castellaro (Pesaro, Central Italy). Bollettino del Museo Civico di Storia Naturale di Verona, 11:171-190.
Gentilini, G., Korneyev, V.A., and Kameneva, E.P. 2006. Fossil tephritoid flies (Diptera: Pallopteridae, Ulidiidae, Tephritidae) from the Upper Miocene of Monte Castellaro, Italy, and a review of fossil European tephritoids. Instrumenta Biodiversitatis, 7:1-20.
Geoffroy, E.L. 1762. Histoire Abregée des Insectes qui se Trouvent aux Environs de Paris, Volume 2. Durand, Paris.
Greenwalt, D., Goreva, Y., Siljeström, S., Rose, T., and Harbach, R.E. 2013. Hemoglobin-derived porphyrins preserved in a Middle Eocene blood-engorged mosquito. Proceedings of the National Academy of Sciences, 110(46):18496-18500. https://doi.org/10.1073/pnas.1310885110
Greenwalt, D.E. and Blagoderov, V.A. 2019. Review of the fossil record of Bolitophilidae, with description of new taxa and discussion of position of Mangas Kovalev (Diptera: Sciaroidea). Zootaxa, 4567(3):546-560. https://doi.org/10.11646/zootaxa.4567.3.6
Greenwalt, D.E. and Moulton, J.K. 2016. The first fossil New World Dixidae with a critical discussion of generic definitions. Palaeontologia Electronica, 19.3.55A:1-32. https://doi.org/10.26879/656
palaeo-electronica.org/content/2016/1698-new-world-fossil-dixidae
Greenwalt, D.E., Rose, T.R., and Chatzimanolis, S. 2016. Preservation of mandibular zinc in a beetle from the Eocene Kishenehn Formation of Montana. Canadian Journal of Earth Science, 53:614-621. https://doi.org/10.1139/cjes-2015-0157
Greenwalt, D.E., Rose, T.R., Siljeström, S.M., Goreva, Y.S., Constenius, K.N., and Wingerath, J.G. 2015a. Taphonomy of the fossil insects of the middle Eocene Kishenehn Formation. Acta Palaeontologica Polonica, 60(4):931-947. https://doi.org/10.4202/app.00071.2014
Greenwalt, D.E., Wingerath, J.G., and Evenhuis, N.L. 2015b. Two new and disparate fossil bee flies (Bombyliidae: Anthracinae) from the Americas and reassessment of Anthrax dentoni Lewis, 1969. Palaeontologia Electronica, 18.3.51A:1-10. https://doi.org/10.26879/582
palaeo-electronica.org/content/2015/1350-two-new-fossil-bee-flies
Grimaldi, D. 1990. Insects from the Santana Formation, Lower Cretaceous, of Brazil. Bulletin of the American Museum of Natural History, 195:164-183.
Grimaldi, D. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, New York.
Grimaldi, D.A. and Cumming, J.M. 1999. Brachyceran Diptera in Cretaceous ambers and Mesozoic diversification of the Eremoneura. Bulletin of the American Museum of Natural History, 239:1-124.
Grimaldi, D.A., Shedrinsky, A., and Wappler, T.P. 2000. A remarkable deposit of fossiliferous amber from the Upper Cretaceous (Turonian) of New Jersey, p. 1-76. In Grimaldi, D.A. (ed.), Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Backhuys Publishers, Leiden.
Grolle, R. 1972. Die Namen der Familien und Unterfamilien der Lebermoose (Hepaticopsida). Journal of Bryology, 7:201-236.
Haenni, J.-P. 1998. Scatopsidae. p. 141-144. In Merz, B., Bächli, G., Haenni, J.-P., and Gonseth, Y. (eds.), Diptera - Checklist. Fauna Helvetica. Vol. 1. CSCF/SEG, Neuchâtel.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4.1.4A:1-9.
https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Handlirsch, A. 1920. Palaeontologie. Handbuch der Entomologie, 3:117-208.
Harbach, R.E. and Greenwalt, D. 2012. Two Eocene species of Culiseta (Diptera: Culicidae) from the Kishenehn Formation in Montana. Zootaxa, 3530:25-34. https://doi.org/10.11646/zootaxa.3530.1.2
Harris, M. 1776-1780. An Exposition of English Insects. Robson Co., London.
Heer, O. 1849. Die Insektenfauna der Tertiärgebilde von Oeningen und von Radoboj in Croatien. Zweite Theil: Heuschrecken, Florfliegen, Aderflügler, Schmetterlinge und Fliegen. W. Engelmann, Leipzig.
Hennig, W. 1970. Insektenfossilien aus der unteren Kreide. II. Empididae (Diptera, Brachycera). Stuttgarter Beiträge zur Naturkunde, 214:1-12.
Hennig. W. 1972. Eine neue Art der Rhagionidengattung Litoleptis aus Chile, mit Bemehungen uber Fuhlerbildung und Verwandtschaftsbeziehungen einiger Bachycerenfamilien (Diptera: Brachycera). Stuttgarter Beitraege zur Naturkunde, Serie A, Biologie, 174:1-51.
Heyden, C.H.G., von. 1870. Fossile Dipteren aus der Braunkohle von Rott im Siebengebirge. Palaeontographica, 17:237-266.
Hong, Y.-C. 2002. Amber Insects of China. Beijing Science and Technology Press, Beijing.
Huber, J.T. and Greenwalt, D. 2011. Compression fossil Mymaridae (Hymenoptera) from Kishenehn oil shales, with description of two new genera and review of Tertiary amber genera. ZooKeys, 130:473-494.
https://doi.org/10.3897/zookeys.130.1717
Hull, F.M. 1945. A revisional study of the fossil Syrphidae. Bulletin of the Museum of Comparative Zoology, 95:251-355.
Hull, F.M. 1962. Robber flies of the world - The genera of the family Asilidae. Bulletin of the United States National Museum, 224(1):1-430.
Imada, Y. and Kato, M. 2016. Bryophyte-feeding of Litoleptis (Diptera: Rhagionidae) with descriptions of new species from Japan. Zootaxa, 4097(1):41-58. https://doi.org/10.11646/zootaxa.4097.1.2
James, M.T. 1937. A preliminary review of certain families of Diptera from the Florissant Miocene Beds. Journal of Paleontology, 11(3):241-247.
Johannsen, O.A. 1909. Diptera. Family Mycetophilidae, p. 1-141. In Wytsman, P. (ed.), Genera Insectorum, Fasicle 93. V. Verteneuil and L. Desmet, Brussels.
Johnson, C.W. 1909. New and little known Tipulidae. Proceedings of the Boston Society of Natural History, 34:115-133.
Johnson, C.W. 1912. New North American Diptera. Psyche, 19:1-5.
Kaddumi, H.F. 2007. Amber of Jordan. The Oldest Prehistoric Insects in Fossilized Resin. Eternal River Museum of Natural History, Amman, Jordan.
Kameneva, E.P. and Korneyev, V.A. 2006. Biotaxonomy of Tephritoidea. Myennidini, a new tribe of the subfamily Otitinae (Diptera: Ulidiidae), with discussion of the suprageneric classification of the family. Israel Journal of Entomology, 35-36:497-586.
Kania, I. and Nel, A. 2013. New fossil Tipulidae from the volcano-sedimentary Latest Oligocene of Bes-Konak (Turkey). Polish Journal of Entomology, 82:327-338. https://doi.org/10.2478/v10200-012-0046-3
Kania, I., Wojtoń, M., Lukashevich, E., Stanek-Tarkowska, J., Wang, B., and Krzemiński, W. 2019. Anisopodidae (Insecta: Diptera) from Upper Cretaceous amber of northern Myanmar. Cretaceous Research, 94:190-206. https://doi.org/10.1016/j.cretres.2018.10.013
Kehlmaier, C., Dierick, M., and Skevington, J.H. 2014. Micro-CT studies of amber inclusions reveal internal genitalic features of big-headed flies, enabling a systematic placement of Metanephrocerus Aczél, 1948 (Insecta: Diptera: Pipunculidae). Arthropod Systematics and Phylogeny, 72:23-36.
Kerr, P.H. 2010. Phylogeny and classification of Rhagionidae, with implications for Tabanomorpha (Diptera: Brachycera). Zootaxa, 2592:1-133.
Kertész, K. 1908. Catalogus Dipterorum Hucusque Descriptorum, Volume 3. G. Engelmann, Leipzig.
Kjærandsen, J. 2006. Review of fungus gnats of the genus Tarnania Tuomikoski, with a phylogeny of the Rymosia s.l. genus group (Diptera: Mycetophilidae). Insect Systematics and Evolution, 37:121-148. https://doi.org/10.1163/187631206788831146
Klymko, J. and Marshall, S. 2008. Review of the Nearctic Lonchopteridae (Diptera), including descriptions of three new species. The Canadian Entomologist, 140:649-673. https://doi.org/10.4039/n08-034
Knab, F. 1912. New species of Anisopidae (Rhyphidae) from tropical America. (Diptera: Nemocera.). Proceedings of the Biological Society of Washington, 25:111-113.
Kovalev, V.G. 1990. Diptera, Muscida, in Pozdne-Mezozoyskie Nasekomye Vostochnogo Zabaykal'ya. Akademiya Nauk SSSR, Trudy Paleontologicheskogo Instituta, 239:123-177.
Krivosheina, N.P. and Menzel, F. 1998. The Palaearctic species of the genus Sylvicola Harris, 1776. Beiträge zur Entomologie, 48:201-217.
Krzemińska, E., Coram, R.A., and Krzemiński, W. 2010. A new species of Megarhyphus, an interesting discovery from the Lower Jurassic of England (Diptera, Anisopodidae). Acta Geologica Sinica, 84(4):693-695. https://doi.org/10.1111/j.1755-6724.2010.00231.x
Krzemiński, W. 1991a. Revision of the fossil Cylindrotomidae (Diptera, Nematocera) from Florissant and White River, USA. Paläontologische Zeitschrift, 65:333-338.
Krzemiński, W. 1991b. A first fossil Helius (Diptera, Limoniidae) from North America. Acta Zoologica Cracoviensia, 34(1):311-313.
Krzemiński, W. 1992. Triassic and Lower Jurassic stage of Diptera evolution. Bulletin de la Société Entomologique Suisse, 65:39-59.
Krzemiński, W. 1998. Cyttaromyia frelloi sp. n., the first representative of the family Cylindrotomidae in Baltic amber (Diptera: Tipulomorpha). Polskie Pismo Entomologiczne, 67:303-308.
Krzemiński, W., Blagoderov, V., Azar, D., Lukashevich, E., Szadziewski, R., Wedmann, S., Nel, A., Collomb, F-M., Waller, A., and Nicholson, D.B. In press. True flies (Insecta: Diptera) from the late Eocene Insect Limestone (Bembridge Marls) of the Isle of Wight, England, UK. Earth and Environmental Science, Transactions of the Royal Society of Edinburgh.
Krzemiński, W. and Krzemińska, E. 2003. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta Zoologica Cracoviensia, 46(Suppl.):153-184.
Lak, M. and Nel, A. 2009. An enigmatic diapriid wasp (Insecta, Hymenoptera) from French Cretaceous amber. Geodiversitas, 31(1):137-144. https://doi.org/10.5252/g2009n1a12
Latreille, P.A. 1802. Histoire Naturelle Générale et Particulière des Crustacés et des Insects. Dufart, Paris.
Latreille, P.A. 1804. Tableau méthodique des insectes, pp. 129-200. In (editor unknown), Nouveau Dictionnaire d’Histoire Naturelle, Appliqué aux Arts, Principalement à l’Agriculture et à l’Économie Rurale et Domestique. Tome XXIV. [Section 3:] tableaux méthodiques d’histoire naturelle. Déterville, Paris.
Latreille, P.A. 1809. Genera Crustaceorum et Insectorum Secundum Ordinem Naturalem in Familias Disposita, Iconibus Exemplisque Plurimis Explicata. Tomus Quartus et Ultimas. A. Koenig, Paris and Strasbourg.
Latreille, P.A. 1829. Suite et fin des insectes, p. 321-583. In Cuvier, G.L.C. F.D. (ed.), Le Regne Animal, Distribué d'Après son Organisation, pour Servir de Base à l'Histoire Naturelle des Animaux et d'Introduction à l'Anatomie Comparée. Tome V. Deterville et Crochard, Paris.
Leach, W.E. 1815. Entomology, p. 57-172. In Brewster, D. (ed.), The Edinburgh Encyclopedia. Vol. 9, Part 1. ENG-FLU. Baldwin, Edinburg.
Lewis, S.E. 1969. Fossil insects of the Latah Formation (Miocene) of eastern Washington and northern Idaho. Northwest Science, 43(3):99-115.
Lewis, S.E. 1987. Fossil flies (Diptera) from the Ruby River Basin (Oligocene) of southwestern Montana. Occasional Papers in Paleobiology, 1(4):1-22.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Editio Decima L. Salvii, Stockholm.
Loew, W. 1850. Uber den Bernstein und die Bernsteinfauna. Programme der Realschule Meseritz. E.S. Mittler, Berlin.
Lukashevich, E.D. 1996. Mesozoic Dixidae (Insects: Diptera) and systematic position of Dixamima Rohdendorf, 1964 and Rhaetomyia Rohdendorf, 1962. Paleontological Journal, 30:46-51
Macquart, J. 1827. Insectes Dipteres du Nord de la France. Platypezines, Dolichopodes, Empides, Hybotides. Leleux, Lille.
Macquart, J. 1834. Histoire Naturelle des Insectes. Dipteres. Tome Premiere. Roret, Paris.
Macquart, J. 1835 Histoire Naturelle des Insectes. Dipteres. Tome Deuxieme. Roret, Paris.
Macquart, J. 1838. Insectes Dipteres Nouveaux ou peu Connus. Tome Premier. 1re Partie. Roret, Paris.
Malloch, J.R. 1931. Notes on Australian Diptera. XXVIII. Proceedings of the Linnean Society of New South Wales, 51:273-276.
Masner, L. 1969. A scelionid wasp surviving unchanged since Tertiary (Hymenoptera: Proctotrupoidea). Proceedings of the Entomological Society of Washington, 71:397-400.
McAlpine, D.K. 1996. Relationships and classification of the Pseudopomyzidae (Diptera: Nerioidea). Proceedings of the Linnean Society of New South Wales, 116:223-232.
Meigen, J.W. 1803. Versuch einer neuen Gattungs-Eintheilung der europaischen zweiflugligen Insekten. Magazin für Insektenkunde, 2:259-281.
Meigen, J.W. 1804. Klassifikazion und Beschreibung der europaischen zweiflugeligen Insekten (Diptera Linn.). Erster Band. Reichard, Brunswick.
Meigen, J.W. 1818. Systematische Beschreibung der Bekannten Europäischen Zweiflügeligen Insekten. Schulz-Wundermann, Hamm.
Meigen, J.W. 1820. Systematische Beschreibung der Bekannten Europäischen Zweiflügeligen Insekten. Zweiter Theil. F.W. Forstmann, Aachen.
Meigen J.W. 1822. Systematische Beschreibung der Bekannten Europäischen Zweiflügeligen Insekten. Dritter Theil. Schulz-Wundermann, Hamm.
Meigen J.W. 1830. Systematische Beschreibung der Bekannten Europäischen Zweiflügeligen Insekten. Sechster Theil. Schulz, Hamm.
Meinert, F. 1890. Aenigmatias blattoides, dipteron novum apterum. Entomologiske Meddelelser, 2:212-226.
Melander, A.L. 1902. A monograph of the North American Empididæ part I. Transactions of the American Entomological Society, 28(3/4):195-367.
Melander, A.L. 1918. The dipterous genus Drapetis Meigen (Family Empididae). Annals of the Entomological Society of America, 11:183-221.
Melander, A.L. 1949. A report on some Miocene diptera from Florissant, Colorado. American Museum Novitates, 1407:1-63.
Meunier, F. 1893. Note sur les Platypezidae de l'ambre tertiaire. Bulletin de la Societe Zoologique de France, 18:230-234.
Meunier, F. 1903. Les Pipunculidae de l’ambre baltique. Revue Scientifique du Bourbonnais, 16:148-151.
Meunier, F. 1904a. Contribution à la faune des Helomyzinae de l’ambre de la Baltique. Feuille des Jeunes Naturalistes, 35:21-27.
Meunier, F. 1904b. Monographie des Cecidomyidae, des Sciaridae, des Mycetophilidae et des Chironomidae de l'ambre de la Baltique. Annales de la Société Scientifique de Bruxelles, 28:12-92.
Meunier, F. 1905. Monographie des Psychodidae de l'Ambre de la Baltique. Annales Musei Nationalis Hungarici, 3:235-255.
Meunier, F. 1907. Beitrag zur Fauna der Bibioniden, Simuliden und Rhyphiden des Bernsteins. Jahrbuch der Königlich Preussischen Geologischen Landesanstalt und Bergakadamie zu Berlin, 24:391-404.
Meunier, F. 1908. Monographie des Empidae de l'ambre de la Baltique et catalogue bibliographique complet sur les diptères fossiles de cette résine. Annales des Sciences Naturelles Zoologie, 7:81-135.
Meyer, H.W. 2002. Florissant Fossil Beds National Monument Database. http://planning.nps.gov/flfo/ (accessed April 24, 2017 and other dates).
Meyer, H.W. 2003. The Fossils of Florissant. Smithsonian Books, Washington, D.C.
Michelsen, V. 1996. First reliable record of a fossil species of Anthomyiidae (Diptera), with comments on the definition of recent and fossil clades in phylogenetic classification. Biological Journal of the Linnean Society, 58:441-451. https://doi.org/10.1111/j.1095-8312.1996.tb01445.x
Michelsen, V. 1999. Wood gnats of the genus Sylvicola (Diptera, Anisopodidae): taxonomic status, family assignment, and review of nominal species described by JC Fabricius. Tijdschrift voor Entomologie, 142(1-2):69-75.
Michelsen, V. 2009. Hoffeinsmyiidae, a new extinct family of Schizophora (Diptera) in Baltic amber. Studia Dipterologica, 15:211-222.
Michelsen, V. and Pape, T. 2017. Ulurumyiidae-a new family of calyptrate flies (Diptera). Systematic Entomology. 42(4):826-36. https://doi.org/10.1111/syen.12252
Misof, B., Liu, S., Meusemann, K., Peters, R.S., Donath, A., Mayer, C., Frandsen, P.B., Ware, J., Flouri, T., Beutel, R.G., Niehuis, O., Petersen, M., Izquierdo-Carrasco, F., Wappler, T., Rust, J., Aberer, A.J., Aspöck, U., Aspöck, H., Bartel, D., Blanke, A., Berger, S., Böhm, A., Calcott, B., Chen, J., Friedrich, F., Fukui, M., Fujita, M., Greve, C., Grobe, P., Gu, S., Huang, Y., Jermiin, L.S., Kawahara, A.Y., Krogmann, L., Kubiak, M., Lanfear, R., Letsch, H., Li, Y., Li, Z., Li, J., Lu, H., Machida, R., Mashimo, Y., Kapli, P., McKenna, D.D., Meng, G., Nakagaki, Y., Navarrete-Heredia, J.L., Ott, M., Ou, Y., Pass, G., Podsiadlowski, L., Pohl, H., von Reumont, B.M., Schütte, K., Sekiya, K., Shimizu, S., Slipinski, A., Stamatakis, A., Song, W., Su, X., Szucsich, N.U., Tan, M., Tan, X., Tang, M., Tang, J., Timelthaler, G., Tomizuka, S., Trautwein, M., Tong, X., Uchifune, T., Walzl, M.G., Wiegmann, B.M., Wilbrandt, J., Wipfler, B., Wong, T.K.F., Wu, Q., Wu, G., Xie, Y., Yang, S., Yang, Q., Yeates, D.K., Yoshizawa, K., Zhang, Q., Zhang, R., Zhang, W., Zhang, Y., Zhao. J., Zhou, C., Zhou, L., Ziesmann, T., Zou, S., Li, Y., Xu, X., Zhang, Y., Yang, H., Wang, J., Wang, J., Kjer, K.M., and Zhou, X. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science, 346:763-767. https://doi.org/10.1126/science.1257570
Mockford, E.L. 1972. New species, records, and synonymy of Florida Belaphotroctes (Psocoptera: Liposcelidae). Florida Entomologist, 55:153-163.
Mostovski, M.B. 1995. New Mesozoic Platypezidae (Diptera) and the main directions of evolution of the family. Paleontological Journal, 29(2):130-146.
Mostovski, M.B. 1996. New taxa of Ironomyiidae (Diptera, Phoromorpha) from the Cretaceous of Siberia and Mongolia. Paleontological Journal (translation of Paleontologicheskii Zhurnal), 30:318-331.
Nagatomi, A. 1982. The genera of Rhagionidae (Diptera). Journal of Natural History, 16:31-70. https://doi.org/10.1080/00222938200770041
Nagatomi, A. and Yang, D. 1998. A review of extinct Mesozoic genera and families of Brachycera (Insecta, Diptera, Orthorrhapha). Entomologist's Monthly Magazine, 134(8-9):95-196.
Nel, A. 1994. Revision of the Miocene genus Lithobibio (Diptera: Bibionidae). European Journal of Entomology, 91:451-454.
Nel, A. 2006. Oldest records of Bombyliidae: Phthiriinae and Mythicomyiidae: Glabellulinae from the Lowermost Eocene amber of France (Diptera: Bombylioidea). European Journal of Entomology, 103(1):109-114. https://doi.org/10.14411/eje.2006.016
Nel, A. 2008. The oldest bee fly in the French Paleocene (Diptera: Bombyliidae). Comptes Rendus Palevol, 7:401-405. https://doi.org/10.1016/j.crpv.2008.08.001
Nel, A. and Coty, D. 2016. A fossil dung midge in Mexican amber (Diptera: Scatopsidae). Palaeontologia Electronica, 19.2.22A:1-6. https://doi.org/10.26879/633
palaeo-electronica.org/content/2016/1527-fossil-scatopsidae-in-amber
Nel, A. and Prokop, J. 2004. New Paleogene Scatopsidae (Diptera: Nematocera) in amber from the Czech Republic and France. Acta Societatis Zoologicae Bohemicae, 68:91-98.
Newman, E. 1834. Attempted division of the British Insects into natural Orders. Entomologist´s Magazine, 2:379-441.
Oosterbroek, P. 2018. Catalogue of the Craneflies of the World. http://ccw.naturalis.nl (accessed February 5, 2018).
Osten Sacken, C.R. 1869. Monographs of the Diptera of North America. Part IV. Smithsonian Miscellaneous Collection, 8:1-345.
PBDB, 2018. Paleobiology Database. https://paleobiodb.org/#/ (accessed April 27, 2018 and other dates).
Palmer, A.R., Carvalho, J.C.M., and Cook, D.R. 1957. Miocene arthropods from the Mojave Desert, California. Geological Survey Professional Paper, 294:237-277.
Panzer, G.W.F. 1809. Faunae insectorum germanicae initiae oder Deutschlands Insecten. Heft 108. Felsecker, Nürnberg.
Pape, T. and Thompson, F.C. (eds.). 2013. Systema Dipterorum, Version 1.5. http://www.diptera.org/ (accessed on April 27, 2017).
Pape, T., Blagoderov, V., and Mostovski, M.B. 2011. Order Diptera Linnaeus, 1758. Zootaxa, 3148:222-229.
Papp, L. 2011. Description of a new genus and a new family Circumphallidae Fam. Nov., of the acalyptrate flies (Diptera). Acta Zoologica Academiae Scientiarum Hungaricae, 57(4):315-341.
Park, J.-S. and Carlton, C.E. 2014. A revision of the New Zealand species of the genus Sagola Sharp (Coleoptera: Staphylinidae: Pselaphinae: Faronitae). The Coleopterists Bulletin, 68(4):1-156. https://doi.org/10.1649/072.068.0mo4.1
Pauli, T., Burt, T.O., Meusemann, K., Bayless, K., Donath, A., Podsiadlowski, L., Mayer, C., Kozlov, A., Vasilikopoulos, A., Liu, S., and Zhou, X. 2018. New data, same story: Phylogenomics does not support Syrphoidea (Diptera: Syrphidae, Pipunculidae). Systematic Entomology, 43(3):447-459. https://doi.org/10.1111/syen.12283
Petersen, M.J., Bertone, M.A., Wiegmann, B.M., and Courtney, G.W. 2010. Phylogenetic synthesis of morphological and molecular data reveals new insights into the higher-level classification of Tipuloidea (Diptera). Systematic Entomology, 35(3):526-545. https://doi.org/10.1111/j.1365-3113.2010.00524.x
Peus, F. 1952. Cylindrotomidae, p. 1-80. In Lindner, E. (ed.), Die Fliegen der Palaearktischen Region, Vol. 17. E. Schweizerbart, Stuttgart.
Pierce, W.D. 1966. Fossil arthropods of California. 29. Silicified Miocene pupae of ceratopogonid flies. Bulletin of the Southern California Academy Sciences, 65(2):81-98.
Pike, E.M. 1994. Historical changes in insect community structure as indicated by hexapods of Upper Cretaceous Alberta (Grassy Lake) amber. The Canadian Entomologist, 126:695-702. https://doi.org/10.4039/Ent126695-3
Podenas, S. 2000. A new species of Diogma Edwards, 1938 (Diptera, Cylindrotomidae) from Baltic amber (Eocene). Transactions of the American Entomological Society, 126:103-107.
Poinar, G. 2010. Cascoplecia insolitis (Diptera: Cascopleciidae), a new family, genus, and species of flower-visiting, unicorn fly (Bibionomorpha) in Early Cretaceous Burmese amber. Cretaceous Research, 31(1):71-76. https://doi.org/10.1016/j.cretres.2009.09.007
Poinar, G.O., jr. and Milki, R. 2001. Lebanese Amber. The Oldest Ecosystem in Fossilized Resin. Oregon State University Press, Corvallis.
Pratt, G.K. and Pratt, H.D. 1980. Notes on Nearctic Sylvicola (Diptera: Anisopodidae). Proceedings of the Entomological Society of Washington, 82:86-98.
Quate, L.W. and Alexander, J B. 2000. Synopsis of the New World Nemapalpus (Diptera, Psychodidae, Bruchomyiinae) with description of four new species. Annals of the Entomological Society of America, 93(2):185-193. https://doi.org/10.1603/0013-8746(2000)093[0185:sotnwn]2.0.co;2
Rafael, J.A. and De Meyer, M. 1992. Generic classification of the family Pipunculidae (Diptera): A cladistic analysis. Journal of Natural History, 26:637-658. https://doi.org/10.1080/00222939200770391
Rasnitsyn, A.P. 1986. Parataxon and paranomenclature. Paleontologicheskii Zhurnal, 3:11-21. (Paleontological Journal, 20[3]:25-33)
Rasnitsyn, A.P. 1996. Conceptual issues in phylogeny, taxonomy, and nomenclature. Contributions to Zoology, 66:3-41.
Rasnitsyn, A.P. and Ross, A.J. 2000. A preliminary list of arthropod families present in the Burmese amber collection at the Natural History Museum, London. Bulletin of the Natural History Museum, London, Geology, 56(1):21-24.
Ren, D., Lu, L.W., Guo, Z.G., and Ji, S.A. 1995. Faunae and Stratigraphy of Jurassic-Cretaceous in Beijing and the Adjacent Areas. Vol. 8. Geological Publishing House, Beijing.
Ribeiro, G.C. 2008. Phylogeny of the Limnophilinae (Limoniidae) and early evolution of the Tipulomorpha (Diptera). Invertebrate Systematics, 22:627-694. https://doi.org/10.1071/IS08017
Rice, H.M.A. 1959. Fossil Bibionidae (Diptera) from British Columbia. Bulletin of the Geological Survey of Canada, 55:137.
Richter, R. 1943. Einführung in die Zoologische Nomenklatur. Senckenbergische Naturforschende Gesellschaft, Frankfurt.
Riek, E.F. 1955. Fossil insects from the Triassic beds at Mt. Crosby, Queensland. Australian Journal of Zoology, 3(4):654-691.
Robineau-Desvoidy, J.B. 1830. Essai sur les myodaires. Mémoires présentés par divers Savans à l'Académie Royale des Sciences de l'Institut de France, 2:1-813.
Rohdendorf, B.B. 1938. Dvukrylye basekomye Mezozoya Kara-Tau. I. Brachycera i chast' Nematocera. Akademiya Nauk SSSR, Trudy Paleontologicheskogo Instituta, 7:29-67. (In Russian)
Rohdendorf, B.B. 1946. Эволюция крыла и филогения Oligoneura (Diptera, Nematocera). [Evolution of the wing and the phylogeny of Oligoneura (Diptera, Nematocera).] Trudy Paleontologicheskogo Instituta, 13(2):5-108. (In Russian)
Rohdendorf, B.B. 1962. Order Diptera, p. 444-576. In Rohdendorf, B.B. (ed.), Fundamentals of Palaeontology. Vol. 9. Arthropoda, Tracheata, Chelicerata. Izdatel’stvo Akademii Nauk SSSR, Moscow (In Russian; English translation: Amerind Publishing, New Delhi).
Rondani, C. 1840. Sopra Alcuni Nuovi Generi di Insetti Ditteri per Servire alia Dipterologia Italiana. Donati, Parma.
Rondani, C. 1856. Dipterologiae Italicae Prodromus. Vol. I. Genera Italica Ordinis Dipterorum Ordinatim Disposita et Distincta et in Familias et Stirpes Aggregata. A. Stocchi, Parma.
Ross, R. 1903. Further notes on Leishman's bodies. British Medical Journal, 2:1401.
Rozen, J.G. 1971. Micromalthus debilis Leconte from amber of Chiapas, Mexico (Coleoptera: Micromalthidae). University of California Publications in Entomology, 63:75-76.
Schiner, I.R. 1863. Vorlaufiger Commentar zum dipterologischen Theile der "Fauna Austriaca", mit einer naheren Begrundung der in derselben aufgenommenen neuen Dipteren-Gattungen. V. [concl]. Wiener Entomologische Monatschrift (Vienna), 7:217-226.
Schiner, I.R. 1868. Diptera, p. 1-388. In Von Wullerstorf-Urbair, B. (ed.), Reise der österreichischen Fregatte Novara. Zoologie, 2(1). B. K. Gerold’s Sohn, Wien.
Schlüter, T. 1978. Die Schmetterlingsmücken-Gattung Nemopalpus (Diptera: Psychodidae) aus dem oligozänen Harz der Dominikanischen Republik. Entomologia Germanica, 4:242-249.
Schmitz, H. 1929. Diptera of Patagonia and South Chile, Based Mainly on Material in the British Museum (Nat. Hist.). Part VI. Fasc. 1. Sciadoceridae and Phoridae. British Museum (Natural History), London.
Scopoli, J.A. 1763. Entomologia Carniolica Exhibens Insecta Carnioliae, Indigena et Distributa in Ordines, Genera, Species, Varietates, Methodo Linnaeana. I.T. Trattner, Vienna.
Scudder, S.H. 1877. The first discovered traces of fossil insects in the American Tertiaries. Bulletin of the U.S. Geological and Geographical Survey of the Terranes, 3:741-762.
Scudder, S.H. 1878. Additions to the Insect Fauna of the Tertiary Beds at Quesnel (British Colombia). Report of Progress of the Geological Survey of Canada 1876-1877. Geological Survey of Canada, Montreal.
Scudder, S.H. 1890. The Tertiary Insects of North America. Report of the United States Geological Survey of the Territories, 13:1-734.
Scudder, S.H. 1894. Tertiary Tipulidae, with special reference to those of Florissant, Colorado. Proceedings of the American Philosophical Society, 32:163-245.
Séguy, E. 1934. Un nouveau Cylindrotomine fossile (Tipulidae). Encyclopédie Entomologie (B) 11, 7:47-48.
Shockley, F.W. and Greenwalt, D. 2013. Ptenidium kishenehnsis, a new fossil described from the Kishenehn oil shales (Coleoptera: Ptiliidae), with a checklist of previously known fossil ptiliids. Proceedings of the Entomological Society of Washington, 115(2):173-181. https://doi.org/10.4289/0013-8797.115.2.173
Skartveit, J. 2008. Fossil Hesperinidae and Bibionidae (Diptera: Bibionoidea) from Baltic amber. Studia Dipterologica, 15:3-42.
Skartveit, J. and Nel, A. 2017. Revision of fossil Bibionidae (Insecta: Diptera) from French Oligocene deposits. Zootaxa, 4225:1-83. https://doi.org/10.11646/zootaxa.4225.1.1
Skevington, J.H. and Yeates, D.K. 2001. Phylogenetic classification of Eudorylini (Diptera: Pipunculidae). Systematic Entomology, 26(4):421-452. https://doi.org/10.1046/j.0307-6970.2001.00160.x
Søli, G.E.E., Vockeroth, J.R., and Matile, L. 2000. Families of Sciaroidea, p. 49-92. In Papp, L. and Darvas, B. (eds.), Contributions to a Manual of Palaearctic Diptera. Science Herald, Budapest.
Solórzano Kraemer, M.M. and Nel, A. 2009. First recorded evidence in the fossil record of snipe flies (Diptera: Rhagionidae) in Cretaceous amber, France. Cretaceous Research, 30(6):1367-1375. https://doi.org/10.1016/j.cretres.2009.08.001
Solórzano Kraemer, M.M., Sinclair, B.J., and Cumming, J.M. 2005. Five new species of Tachydromiinae (Diptera: Empididae s.l.) from New World Tertiary ambers. Zootaxa, 1010:37-52. https://doi.org/10.11646/zootaxa.1010.1.4
Stebner, F., Solórzano Kraemer, M.M., Ibáñez-Bernal, S., and Wagner, R. 2015. Datziinae as a new subfamily name for the unavailable name Protopsychodinae Stebner et al., 2015, (Diptera: Psychodidae). PeerJ, 3:e1423. https://doi.org/10.7717/peerj.1423
Stuckenberg, B.R. 2001. Pruning the tree: a critical review of classifications of the Homeodactyla (Diptera, Brachycera), with new perspectives and an alternative classification. Studia Dipterologica, 8:3-41.
Talamas, E. and Buffington, M. 2015. Fossil Platygastroidea in the National Museum of Natural History, Smithsonian Institution. Journal of Hymenoptera Research, 47:1-52. https://doi.org/10.3897/JHR.47.5730
Theobald, F.V. 1903. Report on a collection of mosquitoes or Culicidae, etc., from Gambia, and descriptions of new species, p. i-xi, plates 6-7. In (editor unknown), Memoirs of the Liverpool School of Tropical Medicine, 10. Liverpool School of Tropical Medicine, Liverpool.
Theobald, N. 1937. Les insectes fossiles des terrains oligocènes de France. Bulletin Mensuel (Mémoires) de la Société des Sciences de Nancy, 1:1-473.
Timon-David, J. 1944. Insectes fossiles de l’Oligocène inférieur des Camoins 1. Diptères Brachycères. Bulletin de la Société entomologique de France, 48:128-134.
Tkoč, M., Tóthová, A., Ståhls, G., Chandler, P.J., and Vaňhara, J. 2017. Molecular phylogeny of flat-footed flies (Diptera: Platypezidae): Main clades supported by new morphological evidence. Zoologica Scripta, 46:429-444. https://doi.org/10.1111/zsc.12222
Verrall, G.H. 1901. A List of British Diptera. University Press, Cambridge.
Wagner, R. 2006. Amber Bruchomyiinae, descriptions of already known and new species, and the position of the ‘subfamily’ within Psychodidae (s.l.) (Diptera). Studia Dipterologica, 13:83-95.
Wagner, R. 2017. Synopsis of extinct Bruchomyiinae (Diptera, Psychodidae) from Burmese, Baltic and Dominican amber, with descriptions of new genera and species. Zootaxa, 4320:100-120. https://doi.org/10.11646/zootaxa.4320.1.6
Wagner, R. and Ibáñez-Bernal, S. 2009. Psychodidae (sand flies, and moth flies or owl flies). p. 293-300. In Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., and Zumbado, M.A. (eds.), Manual of Central American Diptera: Volume I. NRC Research Press, Ottawa.
Wagner, R. and Stuckenberg, B.R. 2012. New fossil and extant species of Nemopalpus Macquart (Diptera: Psychodidae: Bruchomyiinae). African Invertebrates, 53(1):355-367. https://doi.org/10.5733/afin.053.0119
Wagner, R. and Stuckenberg, B. 2016. Cladistic analysis of subfamily Bruchomyiinae (Diptera: Psychodidae). Zootaxa, 4092(2):151-174. https://doi.org/10.11646/zootaxa.4092.2.1
Walker, F. 1834. Observations on the British species of Pipunculidae. Entomological Magazine, 2:262-270.
Walker, F. 1848. List of the Specimens of Dipterous Insects in the Collection of the British Museum. Part 1. British Museum, London.
Walker, F. 1850. Diptera. Part I, p. 1-76. In Saunders, W.W. (ed.), Insecta Saundersiana: or Characters of Undescribed Insects in the Collection of William Wilson Saunders, Esq., F.R.S., F.L.S. Vol. 1. Van Voorst, London.
Webb, D. 1978. A revision of the Nearctic genus Dialysis (Diptera: Rhagionidae). Journal of the Kansas Entomological Society, 51(3):405-431.
Wehr, W.C. 1998. Middle Eocene insects and plants of the Okanogan Highlands, p. 99-109. In Martin, J.E. (ed.), Contributions to the Paleontology and Geology of the West Coast: In Honor of V. Standish Mallory. Burke Museum, Research Report No. 6. University of Washington Press, Seattle.
Wehr, W.C. and Barksdale, L.L. 1996. A checklist of fossil insects from Republic, Washington. Washington Geology, 24:29.
Wiegmann, B.M., Trautwein, M.D., Winkler, I.S., Barr, N.B., Kim, J.W., Lambkin, C., Bertone, M.A., Cassel, B.K., Bayless, K.M., Heimberg, A.M., and Wheeler, B.M. 2011. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences, 108(14):5690-5695. https://doi.org/10.1073/pnas.1012675108
Wilson, M.V.H. 1977. New records of insect families from the freshwater Middle Eocene of British Columbia. Canadian Journal of Earth Science, 14:1139-1155. https://doi.org/10.1139/e77-104
Wilson, M.V.H. 1978. Evolutionary significance of North American Paleogene insect faunas. Quaestiones Entomologicae, 14:35-42.
Winnertz, J. 1863. Beitrag zu einer Monographie der Pilzmucken. Abhandlungen der Zoologisch-Botanischen Gesellschaft Wien, 13:637-964.
Wojtoń, M., Kania, I., and Kopeć, K. 2018. Sylvicola Harris, 1780 (Diptera: Anisopodidae) in the Eocene resins. Annales Zoologici, 68(4): 849-867. https://doi.org/10.3161/00034541ANZ2018.68.4.009
Zetterstedt, J.W. 1819. Nagra nya Svenska insekt-arter fundne och beskrifne. Kungliga Svenska Vetenskaps-akademiens Handlingar, 1819:69-86.
Zetterstedt, J.W. 1838. Sectio tertia. Diptera, p. 477-868. In (editor unknown), Insecta Lapponica. L. Voss, Leipzig.
Zetterstedt, J.W. 1842-1860. Diptera Scandinaviae. Officina Lundbergiana, Lund.
Zhang, J. 2012. Orientisargidae fam. n., a new Jurassic family of Archisargoidea (Diptera, Brachycera), with review of Archisargidae from China. ZooKeys, 238:57-76. https://doi.org/10.3897/zookeys.238.3624
Zhang, K., Yang, D., and Ren, D. 2011. A new brachyceran family Origoasilidae fam. nov. from the Late Mesozoic of China (Insecta: Diptera). Acta Geologica Sinica (English Edition), 85(5):994-997. https://doi.org/10.1111/j.1755-6724.2011.00533.x
Zhang, X., Kang, Z., Mao, M., Li, X., Cameron, S.L., de Jong, H., Wang, M., and Yang, D. 2016. Comparative Mt genomics of the Tipuloidea (Diptera: Nematocera: Tipulomorpha) and its implications for the phylogeny of the Tipulomorpha. PloS ONE, 11(6):e0158167. https://doi.org/10.1371/journal.pone.0158167


