 Reassessing floral diversity at Río Pichileufú, earliest middle Eocene of Río Negro, Argentina
Reassessing floral diversity at Río Pichileufú, earliest middle Eocene of Río Negro, Argentina
Article number: 27.3.a49
https://doi.org/10.26879/1383
Copyright Paleontological Society, September 2024
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendices
Submission: 1 March 2024. Acceptance: 12 September 2024.
ABSTRACT
The Río Pichileufú (RP) fossil locality contains one of Patagonia’s only well-dated middle Eocene floras, deposited ca. 47.7 Ma during the onset of global temperature decline and South America's tectonic isolation. In 1938, Edward W. Berry described 135 species from RP based on compressed angiosperm leaves and rare reproductive structures. The flora was considered highly diverse and to have predominantly Neotropical affinities; however, many of Berry’s identifications were botanically incorrect, confusing interpretations of composition, diversity, and biogeography. Only a fraction of the flora has been studied since, and substantial new collections have remained unevaluated. Here, we reassess the fossil leaves from RP, creating a stable platform for systematic and ecological analyses. We use a morphotype approach to bypass the numerous prior taxonomic errors, while preserving nomenclatural links to specimens. We jointly consider the type and cohort (n = 696) and recent (n = 1286) collections. We validate 82 leaf morphotypes in the type collections, much lower than Berry’s estimate of 131, and consider 43 species as indeterminate. We find that 44 historical species were improperly split, lumped, or misaligned to existing names. At least 12 plant families and 30 plant genera initially reported from the site are unreliable, including Poaceae, Cannabaceae, Ericaceae, Hydrangeaceae, and Rosaceae. However, considering all the collections, we recognize 158 total leaf morphotypes. Reliable taxa include ginkgophytes, Norfolk Island pines (Araucaria), legumes (Fabaceae), soapberries (Sapindaceae), and laurels (Lauraceae). Although Berry's initial assessment of diversity at RP was significantly overestimated, including new material re-establishes the flora as exceptionally diverse.
Gabriella Rossetto-Harris. Department of Geosciences and Earth and Environmental Systems Institute, Pennsylvania State University, University Park, Pennsylvania 16802, USA; Department of Earth Sciences, Denver Museum of Nature & Science, Denver, Colorado, 80205, USA; and Earth Sciences, Negaunee Integrative Research Center, Field Museum of Natural History, Chicago, Illinois 60605, USA. grossettoharris@gmail.com (corresponding author)
Peter Wilf. Department of Geosciences and Earth and Environmental Systems Institute, Pennsylvania State University, University Park, Pennsylvania 16802, USA. pwilf@psu.edu
Keywords: angiosperms; Argentina; Eocene; plant diversity; leaf architecture; Patagonia
Final citation: Rossetto-Harris, Gabriella and Wilf, Peter. 2024. Reassessing floral diversity at Río Pichileufú, earliest middle Eocene of Río Negro, Argentina. Palaeontologia Electronica, 27(3):a49.
https://doi.org/10.26879/1383
palaeo-electronica.org/content/2024/5337-diversity-at-rio-pichileufu
Copyright: September 2024 Paleontological Society
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
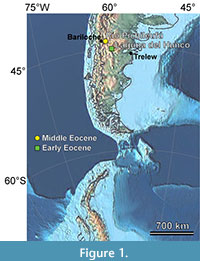 How the Paleogene floras of southern South America responded to climatic and tectonic changes and their relationships to the extant regional floras are topics of intense recent study (e.g., Barreda et al., 2020; Barreda and Palazzesi, 2021; Fernández et al., 2021; Vento et al., 2021). The Eocene Río Pichileufú fossil site, located east of Bariloche, Río Negro, Argentina (RP; Figure 1), produced what is still considered the most diverse published Cenozoic South American flora (Berry, 1935a, 1935b, 1935c, 1938). Dated at 47.74 ± 0.05 Ma from 40Ar/39Ar analyses of primary tuffs directly associated with the fossils (Wilf et al., 2005; Wilf, 2012), RP is the only known South American macroflora representing the time interval following the early Eocene climatic optimum and the initial isolation of South America (Hollis et al., 2012; Bijl et al., 2013; Korasidis et al., 2018; Westerhold et al., 2020). The flora contains many significant taxa (Table 1), including the first fossil Asteraceae flower, the last ginkgophyte of South America, and several conifers from groups that are mostly tropical today, as well as fossil helmeted frogs (Gómez et al., 2011) and the first fossil records of several insect families (Petrulevičius and Popov, 2014; Ramírez et al., 2016; Petrulevičius, 2018).
How the Paleogene floras of southern South America responded to climatic and tectonic changes and their relationships to the extant regional floras are topics of intense recent study (e.g., Barreda et al., 2020; Barreda and Palazzesi, 2021; Fernández et al., 2021; Vento et al., 2021). The Eocene Río Pichileufú fossil site, located east of Bariloche, Río Negro, Argentina (RP; Figure 1), produced what is still considered the most diverse published Cenozoic South American flora (Berry, 1935a, 1935b, 1935c, 1938). Dated at 47.74 ± 0.05 Ma from 40Ar/39Ar analyses of primary tuffs directly associated with the fossils (Wilf et al., 2005; Wilf, 2012), RP is the only known South American macroflora representing the time interval following the early Eocene climatic optimum and the initial isolation of South America (Hollis et al., 2012; Bijl et al., 2013; Korasidis et al., 2018; Westerhold et al., 2020). The flora contains many significant taxa (Table 1), including the first fossil Asteraceae flower, the last ginkgophyte of South America, and several conifers from groups that are mostly tropical today, as well as fossil helmeted frogs (Gómez et al., 2011) and the first fossil records of several insect families (Petrulevičius and Popov, 2014; Ramírez et al., 2016; Petrulevičius, 2018).
Despite several recent papers on the locality (Table 1), the Río Pichileufú flora is poorly understood and needs revision. Most current knowledge stems from the early twentieth century works of geologist Edward Wilber Berry, who described 135 fossil species and incorrectly considered the site to be Miocene (Berry, 1934, 1935a, 1935b, 1935c, 1938). However, Berry’s work contained several taxonomic inaccuracies that obscure understanding of the diversity and biogeography of the flora (i.e., Wilf et al., 2009, 2014; Wilf, 2012; Knight and Wilf, 2013; Table 1). The convention of the time was to place almost every fossil into a living genus, comparing fossils first with the extant floras near the fossil locality (Dilcher, 1971), and fragmentary or poorly preserved fossils were often assigned to new species without diagnostic characters. For example, many of the genera that Berry identified at RP correspond to living taxa with a range of Neotropical, West Pacific, and Antarctic affinities, contributing to the well-known “mixed paleoflora” interpretation (Romero, 1978, 1986) for Eocene Patagonia (see Floral Composition, below), and Berry (1934) especially emphasized relationships with South American living floras. Berry also incorrectly referred many fossils to existing botanical names from other fossil floras, further confusing the interpretation of floral diversity, composition, and turnover through time.
Over the past 20 years, several systematic studies of fossil plants initiated the reassessment of Berry’s taxonomic identifications at Río Pichileufú. These efforts have significantly enhanced understanding of the flora’s extensive biogeographic connections to Australasia and Southeast Asia, challenging previous assumptions about its connections to South America and the Neotropics (Table 1). Significant taxa now recognized include the conifers Agathis, Papuacedrus, Dacrycarpus, and Retrophyllum (Wilf et al., 2009, 2014, 2017), all native today to the West Pacific region, although Retrophyllum is also extant in the Neotropics. Notably, the discovery of the first known Asteraceae fossil flower at the site showed biogeographic connections to South America and Africa, potentially representing a dry-tolerant subtropical lineage (Barreda et al., 2010, 2012). In addition, several vertebrates (Gómez et al., 2011), insects (Dlussky and Perfilieva, 2003; Petrulevičius and Popov, 2014; Ramírez et al., 2016; Petrulevičius, 2018), and ichnotaxa for insect folivory (Sarzetti et al., 2008, 2009; Donovan et al., 2020) have been described from the Río Pichileufú locality in recent years.
Some discrepancies in Berry’s work on the RP flora have been detailed in earlier papers, illustrating the complex legacy of nomenclatural problems and the biogeographic and paleoclimatic implications of incorrect identifications. For example, two species that Berry reported from the site, “Fitzroya tertiaria” Berry (Berry, 1938) and Acmopyle (Podocarpus) engelhardti (Berry) Florin (Florin, 1940), putatively representing two conifer families (Cupressaceae and Podocarpaceae, respectively), actually represent a single species (Table 2, category 3; over-splitting) now attributable to the podocarp Dacrycarpus engelhardti (Berry) P. Wilf et A. Andruchow-Colombo (Andruchow-Colombo et al., 2023). Fitzroya is endemic to the Valdivian temperate rainforests of the southern Andes, but Wilf (2012) revised the “Fitzroya tertiaria” specimen to the bifacial foliage of Dacrycarpus puertae Wilf, showing Australasian rather than South American affinity. In addition, although the bilaterally flattened leaves of Dacrycarpus appeared to be rare in the RP collections (Wilf, 2012), Andruchow-Colombo et al. (2023) realized that Berry (1938) and Florin (1940) had incorrectly placed them into Acmopyle (Podocarpus) engelhardti and revised both the bifacial and bilateral foliage to Dacrycarpus engelhardti.
The taxonomic studies required to revise the flora fully will take decades to complete because of the complex issues arising from Berry’s (1938) initial monograph and the large number of species making a comprehensive single-paper revision infeasible. There is also an urgent need to identify and stabilize potential species concepts in the flora, to provide a foundation for modern era paleoecological analyses and comparisons with other sites. We have found it challenging to perform even basic analyses, other than single-taxon revisions, of the RP flora using Berry’s framework, especially because he often incorrectly gave the same name to multiple entities within the RP assemblage and across fossil sites (Table 2). Instead, in this contribution, we thoroughly examined the entire floral sample (including historical and recent collections) as if Berry’s names did not exist, taking a morphotyping approach (Johnson and Hickey, 1990; Ellis et al., 2009). While maintaining all individual specimens’ links to their original nomenclature (Iglesias et al., 2021), we established 158 leaf morphotypes and described their distinguishing features. From this first comprehensive analysis of the RP flora in 85 years, we confirm high floral diversity during the middle Eocene in Patagonia. To provide context for our findings, we begin with a review of the RP fossil site, including an overview of its complex and little-known history and the status of investigations.
HISTORY OF THE RíO PICHILEUFÚ FOSSIL SITE
Discovery
The Río Pichileufú (Mapuche: “Little River”) flora was discovered in the austral summer of 1929-30, near the eponymous local stream, by Argentine geologist José Román Guiñazú (1897-1991), who worked for the Dirección de Minas, Geología e Hidrogeología, Argentina. Guiñazú found the locality during a field expedition to study the geology and geochronology of Patagonia that was commissioned by Dr. Carl Caldenius of the University of Uppsala and the Geochronological Institute of Stockholm, Sweden (Guiñazú, 1940; Pérez Gutiérrez de Sánchez Vacca, 2013). Guiñazú was known for his contributions to studying the hydrology and soils of Argentina (e.g., Guiñazú, 1934; Guiñazú and Arena, 1940), but he was also a naturalist. For example, he discovered dinosaurs in San Luis Province, leading to the founding of Parque Nacional Sierra de las Quijadas, and described medicinal plants (Pérez Gutiérrez de Sánchez Vacca, 2013; Codorniú et al., 2022).
 The RP locality is located about 40 km east of Bariloche, western Río Negro, Argentina (Figure 1, Figure 2; see Methods for coordinates). The original collection of about 1,000 fossil plants, one of the largest from any site in Argentina by that time, was made during a month and a half of continuous work (Guiñazú, 1940). Guiñazú’s photographs of the original localities are reproduced here and compared with photographs of more recently quarried layers to confirm that all collections originate from the same exposures (Figure 2). According to Guiñazú’s niece, Nilda del Cármen Guiñazú, he hypothesized that the area had a tropical climate based on the fossil species he collected that appeared, to him, like those currently found in the southern United States and Mexico (Pérez Gutiérrez de Sánchez Vacca, 2013).
The RP locality is located about 40 km east of Bariloche, western Río Negro, Argentina (Figure 1, Figure 2; see Methods for coordinates). The original collection of about 1,000 fossil plants, one of the largest from any site in Argentina by that time, was made during a month and a half of continuous work (Guiñazú, 1940). Guiñazú’s photographs of the original localities are reproduced here and compared with photographs of more recently quarried layers to confirm that all collections originate from the same exposures (Figure 2). According to Guiñazú’s niece, Nilda del Cármen Guiñazú, he hypothesized that the area had a tropical climate based on the fossil species he collected that appeared, to him, like those currently found in the southern United States and Mexico (Pérez Gutiérrez de Sánchez Vacca, 2013).
In 1932, Edward Wilber Berry received the collection from Guiñazú at Johns Hopkins University in Baltimore, Maryland, United States (Guiñazú, 1940). Despite never attending college, Berry was a self-taught academic who rose to become a professor, dean, and provost of Johns Hopkins University. He also served as the President of the Paleontological Society and Geological Society of America, and he made significant contributions to the field with over 500 publications (Cloos, 1974). Prior to his work on the RP flora, Berry described other fossil floras from Argentina, Peru, Bolivia, and Chile and became known as an expert in South American paleobotany (Berry, 1922, 1924, 1925, 1928, 1937a, 1937b). Berry’s reputation presumably influenced his selection to describe the RP flora. Although Guiñazú played a crucial role in leading the collection of fossils and studying the site’s geology (Guiñazú, 1940), there was limited ongoing discussion with Berry and a lack of international collaboration after the specimens were sent to the United States.
Geological Context
Guiñazú and Berry had differing points of view regarding the age and depositional setting of the Río Pichileufú flora. In 1935, Guiñazú wrote to Berry to share his interpretation of the flora, which he thought (correctly) to be “Eogene” (Paleogene; Guiñazú, 1940: 17). Guiñazú interpreted the strata as freshwater lake deposits of a coastal swamp (Guiñazú, 1940: 54), adjacent to an epicontinental sea that was part of an inferred Pacific marine transgression from the early Eocene through the late Oligocene. He also thought that RP was contemporaneous with the Arauco and Concepción floras from coal-bearing deposits of southern Chile (now known as Eocene). However, middle-late Eocene marine transgressions in Patagonia only occurred much further south, in the Austral Basin (Malumián and Náñez, 2011; Griffin et al., 2021), and the freshwater lake was of volcanic origin (Aragón and Romero, 1984). Still, Guiñazú did achieve a much closer approximation of the age of the flora than Berry.
Berry agreed with Guiñazú that the fossil deposit was lacustrine, based on his communications with Clarence S. Ross of the US Geological Survey, who found freshwater sponge spicules and diatoms from RP (Berry, 1938). However, Berry was adamant that the RP site was early Miocene based on perceived megafloral correlations. He cited 17 shared species with the Laguna del Hunco (called by him “Mirhoja”) flora from Chubut, Argentina (Berry, 1925) and 23 species in common with the Chilean Concepción-Arauco fossil flora, both of which he had earlier considered to be Miocene (Berry, 1922, 1925). In fact, all three of these localities are now known to be Eocene and not precisely contemporaneous (Collao et al., 1987; Wilf et al., 2005). In addition, as found here and elsewhere (Wilf et al., 2005, 2014), very few of the putative shared species are truly equivalent among the three sites.
For decades, the volcanic rocks associated with the RP flora were considered part of the Paleogene “Andesitic Series,” later defined as the Ventana Formation (González Bonorino and González Bonorino, 1978; Arguijo and Romero, 1981). Early radiometric dating of what was then considered the Ventana Formation in the Lago Nahuel Huapi area utilized whole-rock K-Ar isotope analyses, yielding an age range of 55 ± 3 Ma to 42 ± 1 Ma (early to late Eocene), which was consistent with palynology and biostratigraphy (González Díaz, 1979). However, the Andesitic Series was later recognized as comprising two distinct magmatic arcs, one being the extra-Andean Pilcaniyeu Belt, now associated with the Eocene Huitrera Formation, and the Oligocene - early Miocene Andean Maitén Belt, related to the Ventana Formation (Rapela et al., 1988; Cazau et al., 1989, 2005; Melendi et al., 2003; Iannelli et al., 2017). After being incorrectly referred to the Ventana Formation for many years, the RP locality is considered to belong to the Eocene Huitrera Formation (Cazau et al., 2005; Wilf et al., 2013).
In 2005, tuffs from the RP fossiliferous lake beds were dated for the first time, demonstrating that the RP flora is indeed Eocene in age and about 4.5 million years younger than Laguna del Hunco (52.2 Ma; Wilf et al., 2005, 2017). Radioisotopic analyses by M. Smith (in Wilf et al., 2005) of sanidines in three primary ashfall tuffs located immediately above the fossiliferous strata at RP produced concordant 40Ar-39Ar ages, resulting in a combined analytical age of 47.74 ± 0.05 Ma, adjusted for updated constants (Wilf et al., 2005; Wilf, 2012). The age of the RP flora is thus most likely earliest middle Eocene (Lutetian; Ypresian/Lutetian boundary is 47.8 Ma per Gradstein et al., 2012, as updated at www.stratigraphy.org).
There has been no modern sedimentological analysis in the area, other than the stratigraphic section and lithologic descriptions of Aragón and Romero (1984). However, the current paleoenvironmental interpretation is that the RP fossil deposit preserves a volcanic caldera-lake bed, based on the interbedded, laminated mudstones and tuffs and the presence of fossil frogs (Aragón and Romero, 1984; Báez, 1986, 2000; Gómez et al., 2011). The depositional environment was probably similar to the well-studied early Eocene Laguna del Hunco flora, which is also part of the Huitrera Formation (Aragón and Romero, 1984; Wilf et al., 2005; Gosses et al., 2021). However, in contrast to the Laguna del Hunco flora, which is preserved throughout the ~30 km diameter Piedra Parada caldera and is accessible through 170 m of a single fossiliferous stratigraphic section at Laguna del Hunco (Wilf et al., 2003), the RP flora comes from strata with limited exposure, consisting of a single fossiliferous horizon that crops out sporadically around a single drainage over a few hundred meters laterally (Figure 2; Aragón and Romero, 1984; Wilf et al., 2005).
The Need to Re-Assess Floral Composition and Diversity
Berry described 131 leaf and four reproductive species that he assigned to 99 genera from Río Pichileufú (Berry, 1935a, 1935b, 1935c, 1938). Families such as Sapindaceae, Lauraceae, Myrtaceae, Rubiaceae, Anacardiaceae, and others were included among a total of 57 families (Berry, 1938). Berry (1938) published the extant range maps of the various genera he described from RP, some of which have disjunct distributions between the Americas, the West Pacific, and SE Asia. He grappled with the idea of Antarctica as a center of dispersal to explain the paleobotanical record because he disagreed with the then-controversial theory of continental drift (now plate tectonics). The presence of supposed Fitzroya, Libocedrus, Podocarpus, Myrcia, Lomatia, and Laurelia fit the description of a subantarctic flora composed of elements present in the southern Andean region today, although several of those genera are also found in Australasia. Overall, Berry (1934: 282) considered the floristic composition to be “typically American.” Fossils that he assigned to several genera, including Cochlospermum, Annona, Nectandra, and Cupania, are still considered to be related to living American subtropical or tropical species (Markgraf et al., 1996; Hinojosa and Villagrán, 1997), although several also have ranges elsewhere.
Much later, Romero (1978, 1986) incorporated the RP assemblage into his concept of a “mixed paleoflora” (Paleoflora Mixta), characterized by the coexistence of tropical-subtropical and cold-temperate species under unique ecological conditions. The mixed paleoflora was based on the idea that a Cretaceous South American Neotropical flora extended southward into Patagonia, where it mixed with Antarctic elements that either evolved in situ or migrated northward during the late Paleocene to middle Eocene. Then, according to this model, by the late Eocene, the cold-temperate flora became the dominant vegetation in Patagonia, while the Neotropical component retreated northward (Romero, 1978, 1986). Thus, southern South American floral evolution was explained primarily by a tropical, American origin and progressive climatic changes.
The mixed paleoflora concept remained the predominant interpretation of the evolution of Paleogene South American floras for some time, but there has been a recent shift in perspective. The Gondwanan connection is now understood to have influenced the origins of Patagonian floras significantly, whereas few of the putative Neotropical genera have withstood modern taxonomic revisions. Some of the many examples of Gondwanic taxa now known at RP (Table 1) were known to Berry, including Lomatia (Berry, 1938; González et al., 2007) and Araucaria Section Eutacta (Berry, 1938; Rossetto-Harris et al., 2020). The ongoing discovery of diverse tropical to subtropical Asian and Australian elements in the Patagonian paleofloras increasingly supports the idea of a trans-Antarctic rainforest that persisted through the early and early-middle Eocene (Wilf et al., 2013, 2023). Tectonic isolation from Antarctica and cooling eventually resulted in the survival of the Antarctic cold-temperate remnants of the Gondwanan paleoflora in Patagonia, supporting one component of the “mixed floras” concept (Wilf et al., 2009; Barreda et al., 2020). However, there is little or possibly no evidence remaining for Neotropical influence in Paleogene Patagonian floras (Jaramillo and Cárdenas, 2013).
Renewed investigations of LH and RP by Argentine-US collaborative teams began during the late 1990s, leading to extensive new field-census collections and a small but growing number of taxonomic revisions of the RP flora (see Methods). Nine taxa from RP have been recently studied, some of which were not known to Berry (Table 1). Most of the taxonomic work has focused on the gymnosperms, which are crucial for paleoclimate interpretations based on the functional ecology of several nearest living relatives. For example, several podocarp conifers, like Retrophyllum, have single-veined, broad leaves with lateral transfusion tissues that are susceptible to embolism from water stress during drought (e.g., Brodribb and Hill, 2004; Brodribb, 2011; Wilf, 2012; Wilf et al., 2017). In addition, the last known ginkgophyte from South America is from RP (Berry, 1935c; Villar de Seoane et al., 2015).
Fewer angiosperm taxa have been recently studied, but the discoveries are significant. Knight and Wilf (2013) revised Laurelia guinazui (Berry 1935) to a fossil genus of Atherospermataceae similar to Daphnandra, an Australian rainforest understory component. Notably, Barreda et al. (2010, 2012) described an extinct taxon of Asteraceae flower with characters of extant South American and African subfamilies, using a collection separate from those studied here, suggesting an earlier divergence of the family than previously thought and providing the earliest evidence for possible bird pollination by 47.7 Ma (Barreda et al., 2010, 2012).
Wilf et al. (2005) completed an initial evaluation of species diversity at RP using the field-censused collections mentioned above. From a preliminary sample (n = 341 specimens), they identified 39 dicot species using a morphotype approach. Wilf et al. (2005) listed several species apparently shared between RP and LH, but they noted the need to address incorrect identifications and nomenclatural issues in the original (Berry, 1938) taxonomy.
Although most of the RP flora has yet to be revised, Berry’s species concepts continue to be used to assess compositions of other Paleogene floras in Patagonia (Gayó et al., 2005; Vento and Prámparo, 2018) and the origins of the extant South American flora (Hinojosa and Villagrán, 1997, 2005; Pérez-Escobar et al., 2022). Additionally, fossils misaligned to botanical names can lead to erroneous divergence times if used to calibrate molecular phylogenies (Sauquet et al., 2012). Thus, there is a significant need to further clarify the species composition of the RP flora.
The goal of the species reassessment is to establish a stable framework for further taxonomic, diversity, abundance, and insect-damage studies on the RP flora. Our research is necessary to improve understanding of the flora’s ecological, biogeographic, and evolutionary significance. Here, the type and cohort collections, as well as additional collections made at RP in 2002 (amounting to ~500 specimens) and 2005 (~650 specimens) as part of a larger collaboration between institutions in Argentina and the USA (see Materials and Methods), are studied together in a whole-flora context for the first time to reassess floral diversity at RP.
MATERIALS AND METHODS
Specimens and Repositories
The specimens analyzed in this study include the original gathering from RP collected by Guiñazú (Berry, 1938) and newer (mostly 2002 and 2005) collections, each made with standard bench quarrying methods and either selective (biased) or field-census (unbiased) collection strategies (Table 3 and discussion below). Among the historical Río Pichileufú collections that supported Berry’s work (n = 700 specimens), there is a combination of type and figured specimens (243 specimens; Berry, 1935a, 1935b, 1935c, 1938) and unillustrated specimens from the original gathering, referred to here as the cohort collection (457 specimens). Fossils in the cohort collection are housed with notes in Berry’s handwriting and were presumably used in his formation of species concepts (Rossetto-Harris et al., 2020). The type and cohort collections are housed in the Paleobotanical Division of the National Museum of Natural History, Smithsonian Institution (repository acronym USNM; Washington, District of Columbia, USA).
The recent collections from RP examined here amount to 1,272 specimens (Table 3). Excavations included four quarries (RP1: S41.15737° W70.83218°; RP2: S41.15735° W70.83245°; RP3: S41.15576° W70.83451°; and RP4: S41.15536° W70.83423°) within the single fossiliferous horizon, the most productive being RP3. The quarried strata are, beyond a reasonable doubt, the same as where Guiñazú collected. Correlating his localities as shown in the original photographs (Figure 2), location 1 roughly corresponds to our quarries RP1 and RP2, location 2 corresponds to our RP3, and location 3 was not collected by us. Guiñazú did not collect at the location of RP4, which is nevertheless very close to RP3.
Most of the recent collections were made in December 2002 and March 2005, where 1,208 specimens were collected on expeditions based out of the Museo Paleontológico Egidio Feruglio (MEF, Trelew, Chubut, Argentina). Those collections were made using a census system, where all material deemed potentially identifiable was collected, following quantitative bench-quarrying methods described in many other papers (Wilf, 2000; Johnson, 2002). The 2002 census collection from the RP3 quarry was the basis for the preliminary analyses in Wilf et al. (2005). We also include some small additional collections (18 specimens, collected in unknown years) from the Museo Paleontológico de Bariloche. In 2017, we made additional, taxonomically selective collections (see Table 3) with the Universidad Nacional del Comahue (San Carlos de Bariloche, Río Negro, Argentina) that were largely used to revise the conifer Araucaria pichileufensis (Rossetto-Harris et al., 2020). Thus, the 2002 and 2005 census collections are considered unbiased compared with the historical and other collections in Table 3, where the collection method is either unknown or known to be selective. Fossils collected on these field trips are housed at the Museo Paleontológico de Bariloche (repository acronym BAR; San Carlos de Bariloche, Río Negro, Argentina) and were studied on loan at the Museo Paleontológico Egidio Feruglio (MEF; Trelew, Argentina). Several fossils from the new collections have also been examined in previous systematic studies (Table 1).
When possible, specimens are cited through the text (i.e., in Figures, Appendices) using the repository acronym and collection number–for example, “USNM 40483” or “BAR 4487.” In most of the 2005 collections, official museum specimen numbers have yet to be assigned. Those fossils are cited here using the repository acronym plus the field number as the unique identifier. The field-number format is: (BAR) RP quarry_ year collected_field tally number. For example, “(BAR) RP3_2005_610” is a BAR specimen collected from RP3 in 2005 with the unique field number “610.”
The fossils from RP are primarily preserved as compressions, and approximately 94% of all collections consist of angiosperm and gymnosperm leaves. For convenience, we refer to both leaves and leaflets of compound leaves as “leaves.” Although in situ cuticle has been studied in some gymnosperms (Villar de Seoane et al., 2015; Rossetto-Harris et al., 2020), it has not yet been found in angiosperms from the site. Rare reproductive structures (~5% of the census collection) are generally poorly preserved, but this fraction includes described gymnosperm cone scales and pollen cones (Wilf et al., 2014; Rossetto-Harris et al., 2020), and an exquisite Asteraceae capitulum was described from a different collection (Barreda et al., 2010, 2012). Due to their poor preservation and limited sample sizes, we do not include the reproductive structures in the morphotype schema or describe them, but we illustrate them here for completeness.
Imaging and Digital Image Library
The USNM collections and most BAR collections (on loan at MEF) were imaged on-site in several sessions using a Nikon D90 camera with a 60 mm macro lens (Nikon, Melville, New York, USA), a polarizing filter, and low-angle, unidirectional light. The small additional collection at BAR (Table 3, “unknown”) was photographed by P. Wilf and Ari Iglesias in 2010 using a D90 with a tripod and remote flashes.
An image library containing all specimens was consolidated digitally and keyworded for leaf architecture characters using Adobe Bridge (version 11.1.1; Adobe Inc., San Jose, California, USA), a visual file browser that can be used to manage metadata and aid in the digital morphotyping process, as described by Rossetto-Harris et al. (2022). To help sort specimens into species-like entities (morphotypes; see Morphotype Approach; Appendix 1), we utilized keywording in Bridge following the hierarchical characters in the Manual of Leaf Architecture (Ellis et al., 2009) for leaf shape, venation, and tooth features (Rossetto‐Harris et al., 2022; Wilf et al., 2022). By embedding detailed, searchable character metadata into the images, our method enabled simultaneous virtual processing of collections housed in the U.S. and Argentina, complemented by several in-person comparisons. The resulting leaf-architecture character data for each dicot morphotype were exported and formatted as a matrix (Appendix 2) suitable for analyses. In addition to the illustrations provided here, a full-resolution image library of all collections examined is provided in an accompanying Figshare item (Table 4). Appendix 3 provides identifications of every specimen and serves as a look up table for the Figshare archive, while Appendix 4 reports abundances. Additional specimen information and images for USNM material are available at https://collections.nmnh.si.edu/search/paleo.
Morphotype Approach
To assess the extensive collection and bypass the numerous issues in Berry’s (1938) taxonomy of the RP flora (summarized in Table 2), we adopted a conservative morphotype approach. Leaf morphotypes are species-like entities based on leaf architectural characters (see Rossetto-Harris et al., 2022: appendix 2). We assigned morphotypes to the material from all collections as if prior names did not exist, noting all references to original nomenclature associated with the morphotype (Appendix 1). Therefore, instead of full taxonomic descriptions, we list the distinguishing features of each morphotype (Appendix 1) and provide a full leaf-architecture character matrix for all dicots (Appendix 2). This approach allows us to estimate the number of species present to more accurately represent the floral diversity and leaf traits at Río Pichileufú.
A new morphotype was only distinguished, regardless of any prior nomenclature, if sufficient characters were present to reliably distinguish it from others. For example, to recognize a morphotype, a leaf should be mostly complete and include a base and/or the margin, and higher-order venation should be preserved. Otherwise, the specimen was marked indeterminate (these included many type specimens). When choosing the most representative leaf to illustrate for each morphotype, we selected a fossil that demonstrates many of the distinguishing characters, to facilitate comparison with morphotypes that may appear similar at first glance. However, in the space available here, not all characters may be easily visible in the illustrated exemplars at the sizes shown, and we refer readers to the supplemental Figshare image libraries to view full-resolution images and many additional specimens (Table 4). In Appendix 1, we also describe the differences between similar morphotypes. The morphotype prefix used here is “GZ,” after the discoverer and initial collector of the Río Pichileufú locality, José Román Guiñazú. Morphotypes are listed numerically (Appendix 1), independent of systematic inferences but grouped according to general morphologic similarity to facilitate comparisons.
Because most of Berry’s unrevised botanical identifications are doubtful, we use quotes on all generic names except those confirmed by subsequent studies (i.e., Table 1). In Appendix 5, we list for convenience the updated family placement of the extant genera that Berry listed (using World Flora Online Plant List, accessed 18 September 2023). In the cases where Berry incorrectly referred RP material to species established earlier in other fossil floras, our comparisons of type specimens and illustrations usually showed that the specimens with the same names are clearly dissimilar among floras (see Discussion; also Wilf, 2012; Wilf et al., 2014, 2019). In this case, the entire taxonomic name is placed in quotes to designate that the name from a previous fossil flora is not congruent with the RP fossil sensu Berry (1938). Finally, Berry oversplit many taxa that we more conservatively lump, particularly for groups notorious for having variable leaf morphology such as the Sapindales, Fabaceae, and Myrtaceae. Also, certain families, such as Lauraceae, require cuticular characters to differentiate leaves into species under modern standards (Carpenter et al., 2010). Our approach is to more conservatively lump rather than split lauraceous leaves without fossilized cuticles preserved, as seems to be the case for all RP specimens.
Statistical Analysis
We used RStudio (version 2021.9.2.382; RStudio Team, 2022) to run a Kolmogorov-Smirnov non-parametric test comparing abundance distributions on the historic and census datasets.
RESULTS
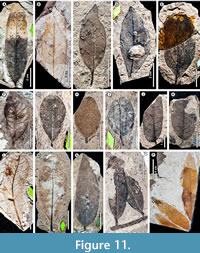
We recognize a total of 158 angiosperm, conifer, and fern leaf morphotypes from 1,092 specimens of the 1,982 total examined (Table 3); the remaining 892 specimens (44%) are considered indeterminate. A representative of each leaf morphotype is illustrated in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, and distinguishing features of the morphotypes are detailed in Appendix 1. Appendix 2 contains a leaf-architecture character matrix for all dicot leaf morphotypes, and Appendix 3 includes the morphotype identification of every specimen with repository and field numbers (if any) and serves as a lookup table for the Figshare image archives, which contain full-resolution images (see Materials and Methods). Finally, Appendix 4 summarizes the abundance of each morphotype in non-census, census, and combined collections. Examples of distinctive reproductive structures, including fertile fern foliage, gymnosperm pollen cones and cone scales, and dicot seeds, flowers, and fruits, are not included in the totals or further described (see Materials and Methods) but are illustrated for reference in Figure 14.
 |
 |
 |
 |
 |
 |
Among the 158 leaf morphotypes are four ferns, eight gymnosperms, four monocots, and 142 dicots (non-monocot angiosperms). From the type and cohort collection (Berry, 1938), we recognize 82 leaf morphotypes compared with the 131 total leaf species described in that work. We find that 43 species listed in Berry (1938) are based on specimens that are too poorly preserved to diagnose, which we consider indeterminate (313 of 696 specimens from the type and cohort collections). In addition, at least 19 of the historical species exhibited multiple issues identified in Table 2 (see Discussion). For example, we consider fifteen species listed by Berry (1938) to be misaligned to previously existing fossil names, 21 as overly split, and 15 as overly lumped. Appendix 5 assesses each species of Berry (1938), including any updates to nomenclature, corresponding GZ morphotype number(s), and, if applicable, the issue(s) identified from Table 2. Out of the 82 morphotypes based on the original gathering, 35 (~43%) are also present in the new collections. This is expected when sampling at the same locality, even seven or more decades later, and emphasizes the impact of additional collections on the observed richness of highly diverse fossil floras.
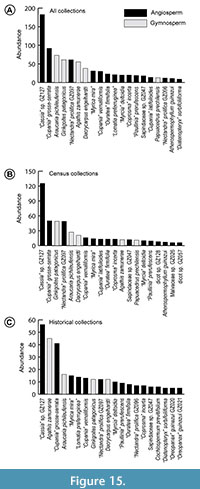
Figure 15 shows the top 20 species at RP in abundance rank order, using all (Figure 15A), census (Figure 15B), and type and cohort (Figure 15C) collections. A Kolmogorov-Smirnov test found that the cumulative distributions for census (Figure 15B) and historical (Figure 15C) relative abundances are not significantly different (P = 0.38), providing justification for combining the datasets in future paleoecological analyses.
 |
 |
 |
 |
 |
 |
Questionable Assignment to Living Taxa
Berry (1938) assigned many specimens to extant genera without sufficient characters preserved either for taxonomic placement or to justify a morphotype under modern standards (issue category 1 of Table 2). Our categorization of these specimens from the type and cohort collections as indeterminate eliminates reliable evidence of several families at the site, including Poaceae, Annonaceae, Berberidaceae, Burseraceae, Hydrangeaceae, Primulaceae, Nyctaginaceae, and Rosaceae (Appendix 5).
Incorrect Referral to Prior Fossil Species
We found 15 species from Berry (1938) to be incorrectly referred to a botanical name established in a prior fossil flora (issue category 2 of Table 2), adding Cannabaceae, Ericaceae, Myricaceae, and Myristicaceae to the list of families not reliably present at the site. There are several examples of taxonomic names that originated in Berry’s earlier work on the early Eocene Laguna del Hunco flora (Berry, 1925) that he applied to specimens from the RP collections, implying shared species; however, from examining the Laguna del Hunco types at USNM and many other collections from that site, we found that few of the RP specimens match the Laguna del Hunco material available to Berry or any subsequent Laguna del Hunco collections. This confounds interpretations based on the similarity of the two floras, which traditionally were considered coeval and to represent the same type of forest (Berry, 1938; Petersen, 1946; Romero, 1978; Arguijo and Romero, 1981; Aragón and Romero, 1984; Markgraf et al., 1996; Hinojosa and Villagrán, 2005; Wilf et al., 2005) but later recognized as temporally distinct (Wilf et al. 2005a). This finding, to be detailed in a companion paper, supports at a whole-flora level the emerging trend in systematics work (Table 1) for fewer shared taxa (e.g., Wilf et al., 2019, 2024; Andruchow-Colombo, 2023).
An additional example (see also Introduction) further demonstrates the significance of recognizing botanical errors and the complexities involved in formal taxonomic revisions. “Banara” prehernandiensis (putative Salicaceae, formerly Flacourtiaceae) was initially named from Laguna del Hunco (Berry, 1925) based on a leaf specimen with an acute, cuneate base. In 1938, Berry assigned material from RP to the same taxon; however, the RP “Banara prehernandiensis” specimen (Figure 8P) has an obtuse base, naked basal acute secondaries, and agrophic veins, so it clearly does not belong under the same name. Abundant material from RP with this morphology (Figure 8P) is ascribed here to morphotype GZ078, which we consider having affinities to ziziphoid Rhamnaceae (under separate study; interestingly, GZ078 appears to match a different, unnamed Laguna del Hunco species). In this case, the misidentification of these fossils to a genus of living South American Salicaceae has clear ecological and biogeographic implications because some of the fossils may instead have affinities to extant Asian rhamnaceous genera.
Over-splitting
We determined 21 Berry (1938) species to be over-split (issue category 3 of Table 2). For example, Berry reported four “Cassia” (Fabaceae) species in the flora. Here, we lump the “Cassia” spp. along with three other species from Berry (1938) into a single legume morphotype (GZ127, Figure 11O; see Appendix 1, Appendix 5) due to the expected variation in leaflet shape and venation seen within legume species and among leaflets on a compound leaf. Similarly, for Sapindaceae, Berry reported 13 species, although we more conservatively recognize five morphotypes of probable Sapindalean affinity (GZ045‒GZ048, Figure 6J-L, Figure 7A; GZ128, Figure 11P). Another example of over-splitting is seen with “Myrica mira” and “Allophylus” graciliformis, where we determined that the latter represents small or immature leaves of some specimens assigned to the former, and we united these under a single morphotype (GZ080, Figure 9B).
Over-lumping
We found 15 of Berry’s species to be over-lumped (issue category 4 of Table 2), such that specimens assigned to a single species represented two or more distinct morphotypes. For example, in “Casearia” patagonica, the two originally figured specimens are both toothed, but the similarities end there; one is elliptic with a convex base and regular, high-angled secondaries (USNM 40454b; Figure 8C), but the other is ovate with a cuneate base and more numerous, irregular, and closer-spaced secondaries (USNM 40454a; Figure 8D). Thus, we separated the two fossils into respective morphotypes, GZ65 and GZ66.
DISCUSSION
Our conservative reassessment of species across collections supports the idea that diversity during the earliest middle Eocene at Río Pichileufú was very high. Upon vetting the 131 historical leaf species, we identified 82 as valid morphotypes and, thus, likely species entities, lower than Berry’s original count. However, our assessment of the recent collections increased the overall diversity to 158 species. This underscores the importance of new collections for capturing the full diversity of fossil assemblages (Table 3), even when legacy collections are substantial. Solidifying the morphological categories that may lead to species concepts for the RP flora establishes a foundation for future taxonomic and paleoecological research.
 Berry (1938) identified 87 genera and 41 families in the RP flora. Our revision eliminates at least 30 genera and 12 families previously reported (see Questionable assignment to living taxa and Incorrect referral to prior fossil species; Appendix 5). Further taxonomic work beyond the scope of this study will probably invalidate many more genera and families from Berry’s original identifications (see Introduction).
Berry (1938) identified 87 genera and 41 families in the RP flora. Our revision eliminates at least 30 genera and 12 families previously reported (see Questionable assignment to living taxa and Incorrect referral to prior fossil species; Appendix 5). Further taxonomic work beyond the scope of this study will probably invalidate many more genera and families from Berry’s original identifications (see Introduction).
Although the RP flora was angiosperm-dominated in richness (~90% of species), a few gymnosperm species account for some of the most abundant taxa in the flora (Figure 15). A legume morphotype (GZ127) has the top abundance rank, although these are small leaflets of compound leaves counted individually and, thus, potentially came from a less abundant source species than indicated by raw count data. Another compound-leaved species, “Cupania” grosse-serrata (Sapindaceae; GZ046) ranks among the top three species in all abundance analyses (Figure 15). Ginkgoites patagonicus (GZ006), Araucaria pichileufensis (GZ007), Dacrycarpus engelhardti (GZ011), “Nectandra” prolifica (GZ097), “Cupania” vernaliformis, and “Myrica mira” are all among the top 10 most abundant species. There is an increased presence of Lomatia preferruginea in the historical collections, ranked sixth among type and cohort but not within the top 20 of the census collections. In addition, there is a higher frequency of lobed species among the historical collections, with three species (“Cochlospermum” previtifolium, “Oreopanax” guinazui GZ20, and “Oreopanax” guinazui GZ21) not otherwise found to be abundant in the census collections. Eighty-seven of our revised leaf morphotypes are singletons. Thus, based on leaf counts, we find that the most likely dominant taxa in the ancient vegetation included Fabaceae, Sapindaceae, Lauraceae, Araucaria, and Dacrycarpus. A more detailed analysis of the RP floral composition, using the Laguna del Hunco flora as a primary comparison, will appear in a companion paper.
CONCLUSIONS
Although Berry (1938) made many botanical and nomenclatural errors while assigning 131 leaf species from the original Guiñazú collections, we confirm that the Río Pichileufú flora was highly diverse. Upon reconsideration of the Berry (1938) type and cohort collections taken alone, we found 82 leaf morphotypes (likely species equivalents). Our analysis calls into question Berry's reported occurrences of many plant families, including Poaceae, Annonaceae, Berberidaceae, Burseraceae, Cannabaceae, Ericaceae, Hydrangeaceae, Primulaceae, Myricaceae, Myristicaceae, Nyctaginaceae, and Rosaceae. When we combined all collections, we validated 158 leaf morphotypes. Updating the morphotype categorizations across all collections stabilizes potential species concepts in the flora and provides a platform for future taxonomic and paleoecological work. Additionally, this reassessment produces a dataset that will be used in a companion article for quantitative comparison with the early Eocene climatic optimum flora from Laguna del Hunco to assess species turnover and changes in paleoecology and climate during the global cooling and tectonic isolation that began during the middle Eocene.
ACKNOWLEDGEMENTS
We thank A. Iglesias and A.P. Carabajal, Museo Paleontológico Bariloche, and Secretaría de Estado de Cultura de Río Negro for assistance with specimen loans to the MEF and Instituto de Investigaciones Aplicadas for land access. At MEF, we thank I. Escapa, E. Dudu Ruigomez, L. Reiner, M. Caffa, and L. Canessa for their assistance in curation and preparation of the RP fossils. Sincere thanks to S. Wing and J. Wingerath, Smithsonian Institution, for assistance with collections visits and specimen inventories. We appreciate the efforts of many individuals who assisted with fieldwork in recent decades, including P. Puerta, M. Caffa, L. Canessa, K. Johnson, C. González, B. Cariglino, and A. Iglesias. We gratefully acknowledge the efforts of C. Cleveland, A. Whitaker, and E. Spagnuolo in imaging hundreds of fossil specimens. Thank you to our editor, M. Pole, and two anonymous reviewers for their helpful feedback and to S. Ivory, M. Patzkowsky, and J. Lasky of Penn State University for discussions. We are thankful for funding that supported this project, including (to G.R.H.) a Charles A. & June R.P. Ross Research Grant from the Geological Society of America, a Leo J. Hickey Award from the Paleontological Society, and the P.D. Krynine Memorial Fund of the Penn State Geosciences department; and to P.W. and others, National Science Foundation grants DEB-1556666 and EAR-1925755. This work partially fulfills the requirements for a Ph.D. in Geosciences at Penn State University for G.R.H.
REFERENCES
Andruchow-Colombo, A., Rossetto-Harris, G., Brodribb, T.J., Gandolfo, M.A., and Wilf, P. 2023. A new fossil Acmopyle with accessory transfusion tissue and potential reproductive buds: direct evidence for ever-wet rainforests in Eocene Patagonia. American Journal of Botany, 110:e16221.
https://doi.org/10.1002/ajb2.16221
Aragón, E. and Romero, E.J. 1984. Geología, paleoambientes y paleobotánica de yacimientos Terciarios del occidente de Río Negro, Neuquén y Chubut, Actas del IX Congreso Geológico Argentino, San Carlos de Bariloche, Argentina, pp. 475–507.
Arguijo, M.H. and Romero, E.J. 1981. Análisis bioestratigráfico de formaciones portadores de tafofloras Terciarias, Actas del VIII Congreso Geológico Argentino, pp. 691–717.
Báez, A. 1986. El registro Terciario de los anuros en el territorio Argentino: una revaluación, Congresso Argentino de Paleontología y Bioestatigrafía, 4. Mendoza, Argentina, pp. 107–118.
Báez, A. 2000. Tertiary anurans from South America. Amphibian Biology, 4:1388–1401.
Barreda, V.D., Palazzesi, L., Tellería, M.C., Katinas, L., Crisci, J.V., Bremer, K., Passalia, M.G., Corsolini, R., Rodríguez Brizuela, R., and Bechis, F. 2010. Eocene Patagonia fossils of the daisy family. Science, 329:1621.
https://doi.org/10.1126/science.1193108
Barreda, V.D., Palazzesi, L., Katinas, L., Crisci, J.V., Tellería, M.C., Bremer, K., Passalia, M.G., Bechis, F., and Corsolini, R. 2012. An extinct Eocene taxon of the daisy family (Asteraceae): evolutionary, ecological, and biogeographical implications. Annals of Botany, 109:127–134.
https://doi.org/10.1093/aob/mcr240
Barreda, V.D., Palazzesi, L., Pujana, R., Panti, C., Tapia, M., Fernández, D., and Noetinger, S. 2020. The Gondwanan heritage of the Eocene-Miocene Patagonian floras. Journal of South American Earth Sciences:103022.
https://doi.org/10.1016/j.jsames.2020.103022
Barreda, V.D. and Palazzesi, L. 2021. Role of climate and tectonism on the modernization of Patagonian floras: evidence from the fossil record. Global and Planetary Change, 204:103556.
https://doi.org/10.1016/j.gloplacha.2021.103556
Berry, E.W. 1922. The flora of the Concepción-Arauco coal measures of Chile. Johns Hopkins University Studies in Geology, 4:73–143.
Berry, E.W. 1924. Mesozoic plants from Patagonia. American Journal of Science, 7:473–482.
Berry, E.W. 1925. A Miocene flora from Patagonia. Johns Hopkins University Studies in Geology, 6:183–251.
Berry, E.W. 1928. Tertiary fossil plants from the Argentine Republic. Proceedings of the United States National Museum, 73:1–27.
https://doi.org/10.5479/si.00963801.73-2743.1
Berry, E.W. 1934. Miocene Patagonia. Proceedings of the National Academy of Sciences USA, 20:280–282.
Berry, E.W. 1935a. A fossil Cochlospermum from northern Patagonia. Bulletin of the Torrey Botanical Club, 62:65–67.
Berry, E.W. 1935b. The Monimiaceae and a new Laurelia. Botanical Gazette, 96:751–754.
https://doi.org/10.1086/334520
Berry, E.W. 1935c. A Tertiary Ginkgo from Patagonia. Torreya, 35:11–13.
Berry, E.W. 1937a. A Paleocene flora from Patagonia. Johns Hopkins University Studies in Geology, 12:33–50.
Berry, E.W. 1937b. An Upper Cretaceous flora from Patagonia. Johns Hopkins University Studies in Geology, 12:11–31.
Berry, E.W. 1938. Tertiary flora from the Río Pichileufú, Argentina. Geological Society of America Special Papers, 12:1–149.
Bijl, P.K., Bendle, J.A.P., Bohaty, S.M., Pross, J., Schouten, S., Tauxe, L., Stickley, C.E., McKay, R.M., Röhl, U., Olney, M., Sluijs, A., Escutia, C., Brinkhuis, H., and Expedition 318 Scientists. 2013. Eocene cooling linked to early flow across the Tasmanian Gateway. Proceedings of the National Academy of Sciences USA, 110:9645-9650.
https://doi.org/10.1073/pnas.1220872110
Brodribb, T.J. 2011. A functional analysis of podocarp ecology. Smithsonian Contributions to Botany, 95:165–173.
https://doi.org/10.5479/si.0081024x.95.165
Brodribb, T. and Hill, R.S. 2004. The rise and fall of the Podocarpaceae in Australia ‒ a physiological explanation, p. 381–399. In Hemsley, A.R. and Poole, I. (eds.), The evolution of plant physiology: from whole plants to ecosystems. Academic Press, London, UK,
https://doi.org/10.1016/b978-012339552-8/50020-2
Carpenter, R.J., Truswell, E.M., and Harris, W.K. 2010. Lauraceae fossils from a volcanic Palaeocene oceanic island, Ninetyeast Ridge, Indian Ocean: ancient long-distance dispersal? Journal of Biogeography, 37:1202–1213.
https://doi.org/10.1111/j.1365-2699.2010.02279.x
Cazau, L., Mancini, D., Cangini, J., and Spalletti, L.A. 1989. Cuenca de Ñirihuau, p. 299–318. In Chebli, G.A. and Spalletti, L.A. (eds.), Cuencas Sedimentarias Argentinas, Serie Correlación Geológica, vol. 6. Universidad Nacional de Tucumán, San Miguel de Tucuman, Argentina.
Cazau, L., Cortiñas, J., Reinante, S., Asensio, M., Bechis, F., and Apreda, D. 2005. Cuenca de Ñirihuau, p. 251–273. In Chebli, G., Cortiñas, J.S., Spalletti, L., Legarreta, L., and Vallejo, E.L. (eds.), Frontera Exploratoria de la Argentina, VI Congreso de Exploración y Desarrollo de Hidrocarburos, Mar del Plata, Argentina.
Cloos, E. 1974. Edward Wilber Berry: February 10, 1875-September 20, 1945. Biographical Memoirs, National Academy of Sciences 45: 60–99.
https://doi.org/10.17226/568
Codorniú, L.S., Rivarola, D.L., Castillo-Elías, G., Gianechini, F.A., and Rivarola, M. 2022. Los excepcionales reptiles voladores y otros hallazgos de San Luis. Publicación Electrónica de la Asociación Paleontológica Argentina, 22: 175–187.
https://doi.org/10.5710/PEAPA.21.04.2021.346
Collao, S., Oyarzun, R., Palma, S., and Pineda, V. 1987. Stratigraphy, palynology and geochemistry of the lower Eocene coals of Arauco, Chile. International Journal of Coal Geology, 7:195–208.
https://doi.org/10.1016/0166-5162(87)90049-8
Dilcher, D. 1971. Revision of the Eocene flora of southeastern north America. The Palaeobotanist, 20:7–18. https://doi.org/10.54991/jop.1971.882
Dlussky, G.M. and Perfilieva, K.S. 2003. Paleogene ants of the genus Archimyrmex Cockerell, 1923. Paleontological Journal, 37:39–47.
Donovan, M.P., Wilf, P., Iglesias, A., Cúneo, N.R., and Labandeira, C.C. 2020. Persistent biotic interactions of a Gondwanan conifer from Cretaceous Patagonia to modern Malesia. Communications Biology, 3:708.
https://doi.org/10.1038/s42003-020-01428-9
Ellis, B., Daly, D., Hickey, L.J., Johnson, K.R., Mitchell, J., Wilf, P., and Wing, S.L. 2009. Manual of Leaf Architecture. Cornell University Press, Ithaca, New York.
Fernández, D.A., Palazzesi, L., Estebenet, M.S.G., Tellería, M.C., and Barreda, V.D. 2021. Impact of mid Eocene greenhouse warming on America’s southernmost floras. Communications Biology, 4:176.
https://doi.org/10.1038/s42003-021-01701-5
Florin, R. 1940. Die heutige und frühere Verbreitung der Koniferengattung Acmopyle Pilger. Svensk Botanisk Tidskrift, 34:117–140.
Gayó, E., Hinojosa, L.F., and Villagrán, C. 2005. On the persistence of tropical paleofloras in central Chile during the early Eocene. Review of Palaeobotany and Palynology, 137:41–50.
https://doi.org/10.1016/j.revpalbo.2005.09.001
Gómez, R.O., Báez, A.M., and Muzzopappa, P. 2011. A new helmeted frog (Anura: Calyptocephalellidae) from an Eocene subtropical lake in northwestern Patagonia, Argentina. Journal of Vertebrate Paleontology, 31:50–59.
https://doi.org/10.1080/02724634.2011.539654
González Bonorino, F.G. and González Bonorino, G.G. 1978. Geología de la region de San Carlos de Bariloche: un estudio de las formaciones Terciarias del Grupo Nahuel Huapi. Revista de la Asociación Geológica Argentina, 33:175–210.
González, C.C., Gandolfo, M.A., Zamaloa, M.C., Cúneo, N.R., Wilf, P., and Johnson, K.R. 2007. Revision of the Proteaceae macrofossil record from Patagonia, Argentina. Botanical Review, 73:235–266.
https://doi.org/10.1663/0006-8101(2007)73[235:rotpmr]2.0.co;2
González Díaz, E.F. 1979. La edad de la Formación Ventana, en el área al norte y al este del Lago Nahuel Huapi. Revista de la Asociación Geológica Argentina, 34:113–124.
Gosses, J., Carroll, A.R., Bruck, B.T., Singer, B.S., Jicha, B.R., Aragón, E., Walters, A.P., and Wilf, P. 2021. Facies interpretation and geochronology of diverse Eocene floras and faunas, northwest Chubut Province, Patagonia, Argentina. Geological Society of America Bulletin, 133:740–752.
https://doi.org/10.1130/b35611.1
Gradstein, F.M., Ogg, J.G., Schmitz, M., and Ogg, G. 2012. The Geologic Time Scale 2012. Elsevier, Amsterdam.
Griffin, M., Pagani, M.A., and Damborenea, S. 2021. Past sea incursions into Patagonia and the resulting record of marine invertebrates, p. 91–113, In Helbling, E.W., Narvarte, M.A., González, R.A., and Villafañe, V.E. (eds.), Global Change in Atlantic Coastal Patagonian Ecosystems. Natural and Social Sciences of Patagonia. Springer, Cham, Switzerland.
https://doi.org/10.1007/978-3-030-86676-1_5
Guiñazú, J.R. 1934. Los depósitos de turba de Tierra del Fuego. Su extensión y posibles usos, Ministerio de Agricultura, Dirección de Minas y Geología, Buenos Aires, Argentina.
Guiñazú, J.R. 1940. El Terciario Carbonifero del sur Argentino y Chileno. Su posición estratigráfica, Ministerio de Agricultura, Buenos Aires, Argentina.
Guiñazú, J.R. and Arena, A. 1940. La erosión eólica de los suelos centro-oeste de la Argentina: reconocimiento preliminar del efecto del viento sobre los suelos del territorio de La Pampa y zonas limítrofes, Ministerio de Agricultura, Dirección de Suelos, Buenos Aires, Argentina.
Hinojosa, L.F. and Villagrán, C. 1997. Historia de los bosques del sur de Sudamérica, I: antecedentes paleobotánicos, geológicos y climáticos del Terciario del Cono Sur de América. Revista Chilena de Historia Natural, 70:225–239.
Hinojosa, L.F. and Villagrán, C. 2005. Did South American Mixed Paleofloras evolve under thermal equability or in the absence of an effective Andean barrier during the Cenozoic? Palaeogeography, Palaeoclimatology, Palaeoecology, 217:1–23.
https://doi.org/10.1016/j.palaeo.2004.11.013
Hollis, C.J., Taylor, K.W.R., Handley, L., Pancost, R.D., Huber, M., Creech, J.B., Hines, B.R., Crouch, E.M., Morgans, H.E.G., Crampton, J.S., Gibbs, S., Pearson, P.N., and Zachos, J.C. 2012. Early Paleogene temperature history of the Southwest Pacific Ocean: reconciling proxies and models. Earth and Planetary Science Letters, 349-350:53–66.
https://doi.org/10.1016/j.epsl.2012.06.024
Iannelli, S.B., Litvak, V.D., Fernández Paz, L., Folguera, A., Ramos, M.E., and Ramos, V.A. 2017. Evolution of Eocene to Oligocene arc-related volcanism in the North Patagonian Andes (39-41°S), prior to the break-up of the Farallon plate. Tectonophysics, 696-697:70–87.
https://doi.org/10.1016/j.tecto.2016.12.024
Iglesias, A., Wilf, P., Stiles, E., and Wilf, R. 2021. Patagonia’s diverse but homogeneous early Paleocene forests: Angiosperm leaves from the Danian Salamanca and Peñas Coloradas formations, San Jorge Basin, Chubut, Argentina. Palaeontologia Electronica, 24:a2.
https://doi.org/10.26879/1124
Jaramillo, C. and Cárdenas, A. 2013. Global warming and Neotropical rainforests: a historical perspective. Annual Review of Earth and Planetary Sciences, 41:741–766.
https://doi.org/10.1146/annurev-earth-042711-105403
Johnson, K.R. 2002. The megaflora of the Hell Creek and lower Fort Union formations in the western Dakotas: vegetational response to climate change, the Cretaceous-Tertiary boundary event, and rapid marine transgression. Geological Society of America Special Papers, 361:329–391.
https://doi.org/10.1130/0-8137-2361-2.329
Johnson, K.R. and Hickey, L.J. 1990. Megafloral change across the Cretaceous/Tertiary boundary in the northern Great Plains and Rocky Mountains, U.S.A. Geological Society of America Special Papers 247: 433–444.
https://doi.org/10.1130/SPE247-p433
Knight, C.L. and Wilf, P. 2013. Rare leaf fossils of Monimiaceae and Atherospermataceae (Laurales) from Eocene Patagonian rainforests and their biogeographic significance. Palaeontologia Electronica, 16: 3.26A.
https://doi.org/10.26879/386
Korasidis, V.A., Wallace, M.W., Wagstaff, B.E., and Hill, R.S. 2018. Terrestrial cooling record through the Eocene-Oligocene transition of Australia. Global and Planetary Change, 173:61–72.
https://doi.org/10.1016/j.gloplacha.2018.12.007
Malumián, N. and Náñez, C. 2011. The Late Cretaceous−Cenozoic transgressions in Patagonia and the Fuegian Andes: foraminifera, palaeoecology, and palaeogeography. Biological Journal of the Linnean Society, 103:269–288.
https://doi.org/10.1111/j.1095-8312.2011.01649.x
Markgraf, V., Romero, E.J., and Villagrán, C. 1996. History and paleoecology of South American Nothofagus forest, p. 354–386. In Veblen, T.T., Hill, R.S., and Read, J. (eds.), The Ecology and Biogeography of Nothofagus Forests. Yale University Press, New Haven, Connecticut.
Melendi, D.L., Scafati, L.H., and Volkheimer, W. 2003. Palynostratigraphy of the Paleogene Huitrera Formation in N-W Patagonia, Argentina. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 228:205–273.
https://doi.org/10.1127/njgpa/228/2003/205
Pérez-Escobar, O.A., Zizka, A., Bermúdez, M.A., Meseguer, A.S., Condamine, F.L., Hoorn, C., Hooghiemstra, H., Pu, Y., Bogarín, D., and Boschman, L.M. 2022. The Andes through time: evolution and distribution of Andean floras. Trends in Plant Science, 27:364–378.
https://doi.org/10.1016/j.tplants.2021.09.010
Pérez Gutiérrez de Sánchez Vacca, S. 2013. Personalidades de San Luis que dejaron huella, Colección Bicentenario. San Luis Libro, San Luis, Argentina.
Petersen, C.S. 1946. Estudios geológicos en la región del Río Chubut medio, Secretaría de Industria y Comercio, Dirección de Minas, Geología e Hidrogeología Argentina, Buenos Aires, Argentina.
Petrulevičius, J.F. 2018. A new malachite damselfly (Synlestidae: Odonata) from the Eocene of Patagonia, Argentina. Life: The Excitement of Biology, 6:39–43.
https://doi.org/10.9784/LEB6(2)Petrulevicius.01
Petrulevičius, J.F. and Popov, Y.A. 2014. First fossil record of Discocephalinae (Insecta, Pentatomidae): a new genus from the middle Eocene of Río Pichileufú, Patagonia, Argentina. ZooKeys, 422:23–33.
https://doi.org/10.3897/zookeys.422.6750
Ramírez, L.C., Corsolini, J., and Di Iorio, O. 2016. First fossil record of parasitic flat-bark beetle (Coleoptera: Passandridae) from the Eocene of Patagonia, Argentina. Ameghiniana, 53:160–169.
https://doi.org/10.5710/amgh.29.11.2015.2920
Rapela, C.W., Spalletti, L.A., Merodio, J.C., and Aragón, E. 1988. Temporal evolution and spatial variation of early Tertiary volcanism in the Patagonian Andes (40° S-42°30' S). Journal of South American Earth Sciences, 1:75–88.
https://doi.org/10.1016/0895-9811(88)90017-x
Romero, E.J. 1978. Paleoecología y paleofitografía de las tafofloras del Cenofitico de Argentina y areas vecinas. Ameghiniana, 15:209–227.
Romero, E.J. 1986. Paleogene phytogeography and climatology of South America. Annals of the Missouri Botanical Garden, 73:449–461.
https://doi.org/10.2307/2399123
Romero, E.J. and Hickey, L.J. 1976. A fossil leaf of Akaniaceae from Paleocene beds in Argentina. Bulletin of the Torrey Botanical Club, 103:126–131.
https://doi.org/10.2307/2484888
Rossetto-Harris, G., Wilf, P., Escapa, I.H., and Andruchow-Colombo, A. 2020. Eocene Araucaria Sect. Eutacta from Patagonia and floristic turnover during the initial isolation of South America. American Journal of Botany, 107:806–832.
https://doi.org/10.1002/ajb2.1467
Rossetto‐Harris, G., Stiles, E., Wilf, P., Donovan, M.P., and Zou, X. 2022. Rapid character scoring and tabulation of large leaf-image libraries using Adobe Bridge. Applications in Plant Sciences, 10:e11500.
https://doi.org/10.1002/aps3.11500
RStudio Team. 2022. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Sarzetti, L.C., Labandeira, C.C., and Genise, J.F. 2008. A leafcutter bee trace fossil from the middle Eocene of Patagonia, Argentina, and a review of megachilid (Hymenoptera) ichnology. Palaeontology, 51:933–941.
https://doi.org/10.1111/j.1475-4983.2008.00787.x
Sarzetti, L.C., Labandeira, C.C., Muzón, J., Wilf, P., Cúneo, N.R., Johnson, K.R., and Genise, J.F. 2009. Odonatan endophytic oviposition from the Eocene of Patagonia: the ichnogenus Paleoovoidus and implications for behavioral stasis. Journal of Paleontology, 83:431–447.
https://doi.org/10.1666/08-121.1
Sauquet, H., Ho, S.Y.W., Gandolfo, M.A., Jordan, G.J., Wilf, P., Cantrill, D.J., Bayly, M., Bromham, L., Brown, G.K., Carpenter, R.J., Lee, D.M., Murphy, J.M., Sniderman, J.M.K., and Udovicic, F. 2012. Testing the impact of calibration on molecular divergence times using a fossil-rich group: the case of Nothofagus (Fagales). Systematic Biology, 61:289–313.
https://doi.org/10.1093/sysbio/syr116
Vento, B. and Prámparo, M.B. 2018. Angiosperm association from the Río Turbio Formation (Eocene-?Oligocene) Santa Cruz, Argentina: revision of Hünicken’s (1955) fossil leaves collection. Alcheringa, 42:125–153.
https://doi.org/10.1080/03115518.2017.1408854
Vento, B., Puebla, G.G., Pinzón, D., and Prámparo, M. 2021. Paleoclimate estimates for the Paleogene-Neogene in southern South America using fossil leaves as proxies. Comptes Rendus Palevol, 20:29–48.
https://doi.org/10.5852/cr-palevol2021v20a3
Villar de Seoane, L., Cúneo, N.R., Escapa, I.H., Wilf, P., and Gandolfo, M.A. 2015. Ginkgoites patagonica (Berry) comb. nov. from the Eocene of Patagonia, last ginkgoalean record in South America. International Journal of Plant Sciences, 176:346–363, plus Erratum p. 364.
https://doi.org/10.1086/680221
Westerhold, T., Marwan, N., Drury, A.J., Liebrand, D., Agnini, C., Anagnostou, E., Barnet, J.S., Bohaty, S.M., De Vleeschouwer, D., and Florindo, F. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science, 369:1383–1387.
https://doi.org/10.1126/science.aba6853
Wilf, P. 2012. Rainforest conifers of Eocene Patagonia: attached cones and foliage of the extant Southeast Asian and Australasian genus Dacrycarpus (Podocarpaceae). American Journal of Botany, 99:562–584.
https://doi.org/10.3732/ajb.1100367
Wilf, P., Cúneo, N.R., Johnson, K.R., Hicks, J.F., Wing, S.L., and Obradovich, J.D. 2003. High plant diversity in Eocene South America: evidence from Patagonia. Science, 300:122–125.
https://doi.org/10.1126/science.1080475
Wilf, P., Johnson, K.R., Cúneo, N.R., Smith, M.E., Singer, B.S., and Gandolfo, M.A. 2005. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. American Naturalist, 165:634–650.
https://doi.org/10.1086/430055
Wilf, P., Little, S.A., Iglesias, A., Zamaloa, M.C., Gandolfo, M.A., Cúneo, N.R., and Johnson, K.R. 2009. Papuacedrus (Cupressaceae) in Eocene Patagonia: a new fossil link to Australasian rainforests. American Journal of Botany, 96:2031–2047.
https://doi.org/10.3732/ajb.0900085
Wilf, P., Cúneo, N.R., Escapa, I.H., Pol, D., and Woodburne, M.O. 2013. Splendid and seldom isolated: the paleobiogeography of Patagonia. Annual Review of Earth and Planetary Sciences, 41:561–603.
https://doi.org/10.1146/annurev-earth-050212-124217
Wilf, P., Escapa, I.H., Cúneo, N.R., Kooyman, R.M., Johnson, K.R., and Iglesias, A. 2014. First South American Agathis (Araucariaceae), Eocene of Patagonia. American Journal of Botany, 101:156–179.
https://doi.org/10.3732/ajb.1300327
Wilf, P., Donovan, M.P., Cúneo, N.R., and Gandolfo, M.A. 2017. The fossil flip-leaves (Retrophyllum, Podocarpaceae) of southern South America. American Journal of Botany, 104:1344–1369.
https://doi.org/10.3732/ajb.1700158
Wilf, P., Nixon, K.C., Gandolfo, M.A., and Cúneo, N.R. 2019. Eocene Fagaceae from Patagonia and Gondwanan legacy in Asian rainforests. Science, 364:eaaw5139.
https://doi.org/10.1126/science.aaw5139
Wilf, P., Zou, X., Donovan, M.P., Kocsis, L., Briguglio, A., Shaw, D., Slik, J.F., and Lambiase, J.J. 2022. First fossil-leaf floras from Brunei Darussalam show dipterocarp dominance in Borneo by the Pliocene. PeerJ, 10:e12949.
https://doi.org/10.7717/peerj.12949
Wilf, P., González, C.C., Gandolfo, M.A., and Zamaloa, M.C. 2024. Putative Celtis leaves from Eocene Patagonia are allied with Asian Anacardiaceae. Ameghiniana, 61: 73–92.
https://doi.org/10.5710/AMGH.21.02.2024.3586

