Diptera of the Middle Eocene Kishenehn Formation II
Diptera of the Middle Eocene Kishenehn Formation II
Article number: 25.2.a22
https://doi.org/10.26879/1215
Copyright Paleontological Society, July 2022
Author biographies
Plain-language and multi-lingual abstracts
PDF version
See also Diptera of the middle Eocene Kishenehn Formation I
Submission: 31 January 2022. Acceptance: 27 May 2022.
ABSTRACT
Eleven new species of Diptera are reported from the middle Eocene Kishenehn Formation in northwestern Montana, USA. Belonging to 10 families, four of which (Apsilocephalidae, Keroplatidae, Opetiidae and Scenopinidae) are new to the formation, they expand the record of Kishenehn Formation Diptera to 25 families and 38 fossil species. Taxa are as follows with significance noted: Palaeoapsilocephala kishenehnensis Hauser and Greenwalt, gen. et sp. n. (Apsilocephalidae); Dilophus idanos Fitzgerald and Greenwalt, sp. n. (Bibionidae), the first fossil of the genus in the Nearctic and the oldest known fossil imago of the genus; Tmemophlebia carolinae Evenhuis and Greenwalt, sp. n. (Bombyliidae), the first fossil of the genus and the oldest fossil of the subfamily Poecilognathini; Microphorella fragilis Cumming and Greenwalt, sp. n. (Dolichopodidae), the first fossil of the genus and the first fossil parathalassiine from the Nearctic; Hoplocyrtoma eocenica Sinclair and Greenwalt, sp. n. (Hybotidae), the first fossil of the genus; Macrocera apithanos Kerr and Greenwalt, sp. n. (Keroplatidae), the first fossil species of the genus from the Nearctic; Azana akarenos Kerr and Greenwalt, sp. n. (Mycetophilidae), the first fossil of the genus in the Nearctic and the oldest known fossil of the genus; Opetia americana Amorim and Greenwalt, sp. n. (Opetiidae), the first definitive fossil of the genus and the first of its family from the Nearctic; (Psectrosciara makrochaites Amorim and Greenwalt, sp. n. and P. crassieton Amorim and Greenwalt, sp. n. (Scatopsidae); Brevitrichia messogenes Greenwalt and Winterton, sp. n. (Scenopinidae), the first fossil of the genus and, if Proratites simplex Grimaldi and Cumming, 1999 proves not to be a scenopinid, the oldest representative of the family. The bibionid species Dilophus magnus Dürrenfeldt, 1968 was re-examined and designated as Bibionidae incertae sedis. In addition, two species previously assigned to Apsilocephala, Psilocephala pusilla Hennig, 1967, and Rueppellia vagabunda Cockerell, 1927, are reassigned to the new genus as Palaeoapsilocephala.
Dale E. Greenwalt. Department of Paleobiology, National Museum of Natural History MRC 121, Smithsonian Institution, 10th & Constitution Ave. NW, Washington, D.C., 20013-7012, USA. GreenwaltD@si.edu
Dalton De Souza Amorim. Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes, 3900, 14040-901, Ribeirão Preto, SP, Brazil. dsamorim@usp.br
Martin Hauser. Department of Food and Agriculture, Plant Pest Diagnostics Branch, 3294 Meadowview Road, Sacramento, California 95832, USA. Phycus@gmail.com
Peter H. Kerr. Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, California 95832-1448, USA. pkerr@cdfa.ca.gov00
Scott J. Fitzgerald. Pacific Northwest Diptera Research Lab, 1460 SW Allen St., Corvallis, Oregon, 97333 USA. woodyfitz@gmail.com
Shaun L. Winterton. California State Collection of Arthropods, Sacramento, California, USA. wintertonshaun@gmail.com
Jeffrey M. Cumming. Invertebrate Biodiversity, Agriculture and Agri-Food Canada, K.W. Neatby Bldg., C.E.F., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6 jeff.cumming@agr.gc.ca
Neal L. Evenhuis. J. Linsley Gressitt Center for Research in Entomology, Bernice Pauahi Bishop Museum, 1525 Bernice Street, Honolulu, Hawai‘i 96817-2704, USA. NealE@bishopmuseum.org
Bradley J. Sinclair. Canadian National Collection of Insects and Canadian Food Inspection Agency, OPL-Entomology, K.W. Neatby Bldg., C.E.F., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6. bradley.sinclair@inspection.gc.ca
Keywords: fossil Diptera; new species; Kishenehn Formation; middle Eocene
Final citation: Greenwalt, Dale E., De Souza Amorim, Dalton, Hauser, Martin, Kerr, Peter H., Fitzgerald, Scott J., Winterton, Shaun L., Cumming, Jeffrey M., Evenhuis, Neal L., and Sinclair, Bradley J. 2022. Diptera of the Middle Eocene Kishenehn Formation II. Palaeontologia Electronica, 25(2):a22. https://doi.org/10.26879/1215
palaeo-electronica.org/content/2022/3630-kishenehn-formation-diptera
Copyright: July 2022 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
http://zoobank.org/5CC7CF97-AE37-4717-9340-6310AC3ACB84
INTRODUCTION
The order Diptera is one of the four hyperdiverse orders of the class Insecta, members of which thrive in nearly every environment on Earth. The order includes predators, hematophages, parasites and parasitoids, fungivores, herbivores, plant gallers, scavengers and pollinators that greatly affect–and sometimes dominate–the ecosystems within which they live. There are over 170,000 described taxonomically valid species of Diptera, and the order is unquestionably much more diverse with estimates of over a million extant species (Herbert et al., 2016; Evenhuis and Pape, 2021). The Diptera were well established in the Middle Triassic and, over the ensuing 160 million years, they have radiated into a total of 220 families, approximately 161 of which are extant (Krzemiński and Krzemińska, 2003; Grimaldi and Engel, 2005; Evenhuis, 2017). The more advanced flies, the Brachycera, fossils of which date from the Early Jurassic, are largely terrestrial and, with 115 families, constitute the vast majority of dipteran diversity (Michelsen, 1996; Grimaldi and Engel, 2005; Wiegmann et al., 2011). Their radiation, to a large extent, paralleled both that of angiosperm plants approximately 130 million years ago, and a concomitant prolonged period of global warming. However, the more recent radiation of the Schizophora in the Cenozoic, gave rise to what is, at the family level, the most diverse clade of the order, with about 81 families and approximately 50,000 extant species (Grimaldi and Engel, 2005; Wiegmann et al., 2011).
The fossil record of Diptera of the North American Eocene is provided by several Lagerstätte, all of which are rich sources of compression fossils. Unfortunately, there are no productive Paleogene amber deposits in North America. To date, the Florissant (33.9-37.2 Mya), Green River (46.2-50.3 Mya), Kishenehn (45.8-46.6 Mya) and Okanagan (47.8-56 Mya) sites have provided described specimens of flies from 35, 25, 25 (including those in the present study) and 12 families, respectively, the total number of different dipteran families numbering 56. Of these, only one, Eophlebomyiidae Cockerell, 1925, from the Green River, is extinct (Cockerell, 1925). Of the 55 extant families, 20, 21 and 14 are nematoceran flies, flies of the clade Brachycera-Schizophora, and Schizophora, respectively. These numbers translate to over 60% and less than 20% of the known non-schizophoran and schizophoran families, respectively, being represented in these four Eocene localities.
The numbers and identities of the various families found in the four different Lagerstätte show significant differences. For example, the Kishenehn Formation has produced 17 nematoceran families vs. six from the Green River and 15 from the Florissant. Most striking, however, is the paucity of Kishenehn Schizophora. To date, the Kishenehn Formation has not produced any described species of the Schizophora while the Florissant, Green River and Okanagan have produced described species in 11, seven and one families, respectively. The reasons for these differences are a topic of great interest and ongoing research. The large numbers of families from the Green River and Florissant localities can be ascribed to the simple fact that they have been worked for well over a hundred years, whereas concerted studies of the Okanagan insect fauna were only initiated by M.V.H. Wilson in the late 1970s and S.B. Archibald since 2000 (Archibald et al., 2018); the Kishenehn Formation has only been actively worked for the last decade. Another factor is the area of fossiliferous shale available at the various sites. For example, the area covered by the Green River localities is more than an order of magnitude larger than that of the Kishenehn, with the fossil lake beds covering portions of three states and approximately 30,000 square miles. However, much of the observed site-specific diversity is undoubtedly a function of taphonomic windows.
The four North American Eocene localities are all lacustrine in nature although aspects of their environments differ. Wilson (1977, 1978, 1982) argued for a deep-water environment in the Okanagan while that at the Coal Creek Member of the Kishenehn Formation has been described as shallow water (Greenwalt et al., 2015). The fact that immature stages of the nematoceran flies, including the culicids, chaoborids, chironomids and ceratopogonids that are common to the Kishenehn Formation, are usually found in shallow aquatic environments, supports this conclusion (Harbach and Greenwalt, 2012; Baranov et al., 2022). Diatomaceous mats have been implicated in the preservational processes at the Florissant, while there is evidence that cyanobacterial mats were essential to the preservation of insects at the Kishenehn Formation (Harding and Chant, 2000; O’Brien et al., 2002, 2008). One of the more quantifiable differences is the distinct size bias that is obvious in Kishenehn insect fossils. This locality rarely produces well-preserved insect specimens that are more than 1 cm long while preserving tiny insects such as parasitic wasps of the family Mymaridae and a beetle, 650 μm in length, in the family Ptiliidae (Huber and Greenwalt, 2011; Shockley and Greenwalt, 2013; Greenwalt et al., 2015). Moreover, quantitative studies have shown statistically significant differences in the lengths of both curculionid (Coleoptera) and hymenopteran insects from the Kishenehn, Florissant and Green River localities (Munro, 2021, personal commun.; Greenwalt et al., 2011).
This is the second paper in a series devoted to the diversity of the Diptera of the middle Eocene (Lutetian) Coal Creek Member of the Kishenehn Formation of Northwestern Montana (Greenwalt et al., 2019). We report new species in four families new to the Kishenehn locality: Keroplatidae (Macrocera apithanos Kerr and Greenwalt), Opetiidae (Opetia americana Amorim and Greenwalt), Scenopinidae (Brevitrichia messogenes Greenwalt and Winterton) and Apsilocephalidae (Palaeoapsilocephala kishenehnensis Hauser and Greenwalt). Two specimens previously assigned to Apsilocephala, A. pusilla and A. vagabunda, are reassigned to the new genus as Palaeoapsilocephala pusilla (Hennig, 1967) comb. n. and Palaeoapsilocephala vagabunda (Cockerell, 1927) comb. n. In addition, new species in the genera Dilophus (Bibionidae), Tmemophlebia (Bombyliidae), Microphorella (Dolichopodidae), Hoplocyrtoma (Hybotidae), Azana (Mycetophilidae) and Psectrosciara (Scatopsidae) are described.
MATERIALS AND METHODS
Specimens described in this study were collected from the Kishenehn Formation in northwestern Montana, USA, in accordance with United States Forest Service Authorization HUN281. Exposures there are from the middle sequence of the Coal Creek Member, which has been estimated to be 46.2 ± 0.4 Ma by 40Ar/39 Ar analysis and 43.5 ± 4.9 Ma by fission-track analysis (Constenius et al., 1989; Constenius, 1996). Ages of other formations mentioned herein were obtained from the Paleobiology Database (2022). Kishenehn Formation specimens were photographed with either an Olympus SZX12 microscope equipped with a Q-Color5 Olympus camera and Image-Pro Plus 7.0 software (Media Cybernetics, Inc., Bethesda, MD) or, in some cases, focus stacking images were obtained with an Olympus DSX 100 microscope. Specimens were immersed in 95% ethanol for examination and photography. Measurements were made with the Image-Pro Plus 7.0 software. Dilophus magnus (GZG.W.14836) was photographed at the Geowissenschaftliches Museum in Göttingen, Germany, with a Sony SLT-A99V camera.
Heyden (1870) reported the length of Dilophus krantzii as “61/* Linie” - the original text was rife with typographical and/or typesetting errors; the unit of measurement, the Linie, at that time was equal to 2.188 mm (in some usage, 2.0 mm) (Tanner, 1894). The value of 2.0 mm is used herein. Venational terminology is from Cumming and Wood (2017) though details of wing venation homology for opetiids follows Amorim et al. (2018). Numbers of genera and species/family were taken from Evenhuis and Pape (2021). The number of fossil species for each individual family were obtained from the Paleobiology Database and other pertinent literature. Institutional acronyms and abbreviations used herein are GZGM (Geowissenschaftliches Museum, Göttingen), GBIF (Global Biodiversity Information Facility), NMNH (National Museum of Natural History) and USNM (United States National Museum = NMNH depository).
SYSTEMATICS
Family Scatopsidae Newman, 1834
Subfamily Psectrosciarinae Cook, 1963
Genus Psectrosciara Kieffer, 1911
Psectrosciara makrochaites Amorim and Greenwalt sp. n.
Figure 1D-F
zoobank.org/96E79AEC-90A4-471F-AF16-2E825AB247A0
Type species. Psectrosciara mahensis Kieffer, 1911 [= Psectrosciara brunnescens (Brunetti, 1911)], by original description.
Holotype. Female,USNM 625934, deposited in the Paleobiology collections of the National Museum of Natural History in Washington, D.C.
Locality and horizon. Deep Ford site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The species epithet makrochaites is derived from the Greek words makro (long) and chaite (long hair, mane) in reference to the long hair on the abdomen of this species.
Diagnosis. Psectrosciara makrochaites i s differentiated from Psectrosciara fossilis by M1 incomplete, interrupted at its base and from Psectrosciara crassieton by its relatively slim habitus.
Description. Female. Body length, 3.0 mm. Head dark brown, 0.24 mm long, 0.29 mm high; antenna uniformly dark brown, 0.25 mm long, flagellum with seven flagellomeres, terminal flagellomere elongated, 68 μm long, 60 μm wide; palpus light brown, elongated, 70 μm long, 24 μm wide, setose; labellum light brown. Thorax dark brown, longer than wide, 0.6 mm long; scutum apparently bare–some recent species have fine scattered setae, which is probably the case in the fossils, with these setae not preserved (Figure 1A-B). Fore leg dark brown, mid and hind legs lighter in color, coxae light brown; length of femora, tibiae and tarsomeres 1-5: 0.33 mm, 0.30 mm, 0.12 mm, 0.05 mm, 0.05 mm, 0.05 mm and 0.06 mm long (fore leg), 0.24 mm, 0.21 mm, 0.13 and 0.07 mm long (mid leg, last three tarsomeres not visible); and 0.36 mm, 0.30 mm, 0.17 mm, 0.08 mm, 0.06 mm, 0.04 mm 0.05 mm long (hind leg). Anteroapical quarter of hind femur not pigmented; claws pointed, apparently no tooth on tarsal claw. First tarsomere of fore and hind legs not particularly modified, with differentiated spines or stout setae (Figure 1E). Wing, 1.5 mm long, 0.5 mm wide, macrotrichia present. Wing length (WL)/C section 1 (C1) ratio, 3.43; WL/C2, 2.43; WL/C3, 3.21; C1+C2/WL, 0.70. Sc apparently absent, basal portion of M1 absent, basal portions of M2 and M4 not visible, CuA sigmoid (Figure 1C-D). Abdomen, 2.2 mm long, 0.4 mm high; tergites brown, with light intersegmental areas, tergites covered laterally with long, thin hairs, 0.1 mm long, perhaps twice that long; spermatheca sclerotized, 85 mm in diameter. Tergite and sternite 7 dark brown, sternite 8 thinly sclerotized, yellowish, tergite 8+9 yellowish, slightly projecting beyond tip of T7 (Figure 1F).
Male. Unknown.
Synimpressions. Diptera (1).
Psectrosciara crassieton Amorim and Greenwalt, sp. n.
Figure 1G
zoobank.org/379D327C-CA5D-4EDF-867A-DCBE30BB84E6
Holotype. Female, USNM 625035, deposited in the Paleobiology collections of the National Museum of Natural History in Washington, D.C.
Locality and horizon. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The species epithet crassieton is derived from the Greek words crassus (fat, stout) and eton (abdomen, belly) in reference to the more stout habitus of this specimen relative to P. makrochaites.
Diagnosis. Psectrosciara crassieton is differentiated from extant or the two other extinct species of the genus, P. makrochaites and P. fossilis, by its stout size and M1 incomplete, interrupted at its base, respectively.
Description. Female. Body length, 2.65 mm (Figure 1G). Head dark brown, 0.28 mm long, 0.27 mm high; antenna uniformly dark brown, 0.28 mm long, terminal flagellomere elongated, bulbous, 87 μm long, 72 μm wide, number of flagellomeres not discernible; palpus brown, narrow, elongated and setose; labellum brown. Thorax dark brown, longer than wide, 0.65 mm long; scutum bare. Legs dark reddish brown, fore coxa large. Wing 1.06 mm long, 0.5 mm wide, Sc not visible, ratio wing length/section costal 1, 3.32; WL/C2, 2.58; WL/C3, 3.19; C1+C2/WL = 0.69. Abdomen 1.85 mm long, 0.54 mm high, tergites reddish brown with light intersegmental areas, lateral aspects of tergites covered with long, thin hair, 0.1 mm long, perhaps twice that long; spermatheca sclerotized, 0.09 mm in diameter, tergite 8+9 dark, sclerotized.
Synimpressions. Impression of a coprolite
Remarks. The superfamily Scatopsoidea is comprised of the small families Scatopsidae, Canthyloscelidae and Valeseguyidae, the latter with a single extant species. There is a controversy about the position of the superfamily, with authors assigning it either to the Psychodomorpha (Wood and Borkent, 1989; Amorim, 1994) or to the Bibionomorpha (e.g., Wiegmann et al., 2011). The largest of these families, Scatopsidae, contains nearly 400 extant species in 36 genera, the vast majority of which (31) are in Scatopsinae (Haenni and Amorim, 2017). The subfamily Psectrosciarinae is composed of the genera Psectrosciara and Anapausis Enderlein, 1912, with 25 and 43 described extant species, respectively (Haenni and Amorim, 2017). Psectrosciarinae has a single described fossil, Psectrosciara fossilis, from 16 Ma Chiapas amber (Nel and Coty, 2016).
Psectrosciara makrochaites and P. crassieton both clearly fit in the genus Psectrosciara. The scutum without an elevated U-shaped anterior ridge, scutellum without long setae, the fore tibia not produced beyond the base of the tarsi, the apex of hind tibia not club-shaped and strongly swollen apically, C not swollen at junction with R4+5, r-m present, M not fused with R4+5, base of M1 absent and wing with macrosetae (not observed in P. crassieton) are Psectrosciarinae features observed in both species described here. The specimens can be recognized as belonging to the genus (i.e., not to Anapausis) based on R4+5 long, gradually approaching C and body elongate (Cook, 1958, 1981).
Both P. makrochaites and P. crassieton belong to the brunnescens group of Psectrosciara, as indicated by the sinuose CuA, the absence of modification of the fore and hind tarsomeres and the modified setae at the tip of the tibiae; as such, both species fit well in the brunnescens group. Females of the group- scatopsiformis have an elongate spermatheca; the males have stout, short spine-like setae on the tibiae and the tarsi (Amorim, 1982). Ten species of Psectrosciara are known from North America, six of which belong to the brunnescens group (Amorim and Brown, 2020). In Cook’s (1958) study of the genus, the females of P. forcipata and P. stonei belong to the brunnescens group. Psectrosciara makrochaites is clearly more similar to P. stonei based on the shape of the female terminalia sclerites.
The holotype of P. makrochaites and P. crassieton, both females, differ from P. fossilis (known from the male holotype), also a species of the brunnescens group (Nel and Coty, 2016) in that in this latter species M1 is complete, not interrupted at the base. Psectrosciara makrochaites and P. crassieton differ from one another in that the latter is more stout. The ratios of the length of the scutum to the length of the abdomen is 4.4 in P. makrochaites vs. 2.9 in P. crassieton; the ratio of the length of the abdomen to the height of the abdomen is 5.3 in P. makrochaites vs. 3.2 in P. crassieton.
Fossils of ectaetiine scatopsids are known from late Albian/early Cenomanian mid Cretaceous amber fossils of France (Fate et al., 2013). The Ectaetiinae are assumed to be sister to all remaining scatopsids except the Aspistinae (Amorim, 1982, 1994), meaning that the Psectrosciarinae should not be considered a young offshoot in the evolution of the family, but rather a group that may have originated at the mid or late Cretaceous. Nevertheless, Psectrosciara makrochaites and P. crassieton are the oldest known representatives of their subfamily, already corresponding to differentiated members of the brunnescens group.
Family Bibionidae Fleming, 1821
Genus Dilophus Meigen, 1803
Dilophus idanos Fitzgerald and Greenwalt sp. n.
Figure 2, Figure 3, Figure 4
zoobank.org/C3C58815-8BCE-43EB-A3D5-0547CC742A43
Type species. Tipula febrilis Linnaeus, 1758, by subsequent designation of Latreille, 1810.
Holotype. Male, USNM 624840; deposited in the Ppaleobiology collections at the National Museum of Natural History, in Washington, D.C.
 Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet is the Greek term idanos, meaning fair, comely.
Diagnosis. Dilophus idanos is distinguished from fossil congeners by a combination of its small size (wing 2.4 mm), fore tibial spines 2:2:6(8?) and patterns and numbers of the mesonotal spines (see comparison to previously described fossil Dilophus in Table 1).
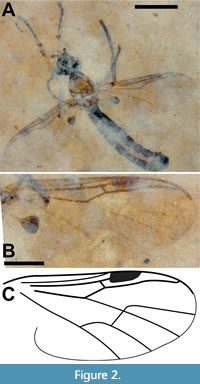
Description (male). Body length (excluding antennae) 3.7 mm; black except thorax, tibiae and femora. Head black, 0.52 mm long; antenna short (0.2 mm), base hidden; approximately six flagellomeres present with terminal flagellomeres bulbous with several apical setae. 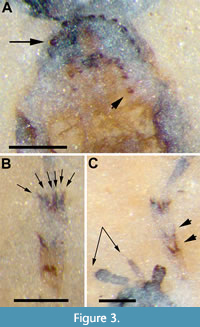 Terminal palpal segment 83 μm long with several apical setae (Figure 2A). Thorax light brown, 0.93 mm long, 0.60 mm wide; anterior margin of mesonotum with broad semicircular arrangement of 12 spines; just posterior to anterior semicircle of spines,mesonotum with narrower semicircular pattern of 10 spines with additional two spines below apex of semicircle forming diamond-shaped pattern (Figure 3A). Wings 2.4 mm long, 0.91 mm wide, hyaline with microtrichia on surface; pterostigma strongly pigmented, dark brown. Costa, with marginal anterior setae, continued beyond R4+5 to slightly less than halfway to wing apex; Sc long, reaching C just before pterostigma; r-m long, 3.2 times as long as bRs (left wing); anterior veins except Sc (C, radial veins, base of M prior to junction with r-m, r-m) thick, strongly pigmented dark brown. Sc and apical tips of M1, M2, M4 and CuA faint, light brown; CuP not visible (Figure 2B-C). Hind legs not preserved. Tarsi black, tibiae and femora light brown. Fore tibia with two pairs of spines below mid-point, one pair above other; apex of fore tibia with circlet of six visible spines with probable total of eight (2:2:6[8?]) (Figure 3B-C). Abdomen black, narrow (2.3 mm x 0.45 mm) as typical for males of family. Terminal segment bulbous, details of genitalia not preserved.
Terminal palpal segment 83 μm long with several apical setae (Figure 2A). Thorax light brown, 0.93 mm long, 0.60 mm wide; anterior margin of mesonotum with broad semicircular arrangement of 12 spines; just posterior to anterior semicircle of spines,mesonotum with narrower semicircular pattern of 10 spines with additional two spines below apex of semicircle forming diamond-shaped pattern (Figure 3A). Wings 2.4 mm long, 0.91 mm wide, hyaline with microtrichia on surface; pterostigma strongly pigmented, dark brown. Costa, with marginal anterior setae, continued beyond R4+5 to slightly less than halfway to wing apex; Sc long, reaching C just before pterostigma; r-m long, 3.2 times as long as bRs (left wing); anterior veins except Sc (C, radial veins, base of M prior to junction with r-m, r-m) thick, strongly pigmented dark brown. Sc and apical tips of M1, M2, M4 and CuA faint, light brown; CuP not visible (Figure 2B-C). Hind legs not preserved. Tarsi black, tibiae and femora light brown. Fore tibia with two pairs of spines below mid-point, one pair above other; apex of fore tibia with circlet of six visible spines with probable total of eight (2:2:6[8?]) (Figure 3B-C). Abdomen black, narrow (2.3 mm x 0.45 mm) as typical for males of family. Terminal segment bulbous, details of genitalia not preserved.
Description (female). Unknown.
Synimpressions. None
Paratype. Male,USNM 768228; deposited in the Paleobiology collections at the National Museum of Natural History, in Washington, D.C.
Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Remarks. The genus Dilophus contains about 232 described extant species and is most diverse in the tropics of the Southern Hemisphere; approximately 18 and 36 species are found in the Nearctic and Palaearctic regions, respectively (Hardy, 1965; Haenni and Bosák, 2007; GBIF, 2021; Skartveit, 2017). Including D. idanos, there are eleven valid fossil species assigned to the genus, four of which are in amber: D. crassicornis Skartveit, 2008, D. palaeofebrilis Skartveit, 2008, and D. succineus Skartveit, 2008 from Baltic amber and D. matilei Waller, Nel and Menier, 2000 from Dominican amber. Species described from compression fossils include: D. krantzii Heyden, 1870 and D. luteipennis Théobald, 1937 (transferred from Bibio Geoffroy, 1762 by Skartveit and Nel, 2017) from the Oligocene of Germany and France respectively (Heyden, 1870; Skartveit and Nel, 2017), D. pumilio Skartveit and Pika, 2014 from the Miocene of Germany, D. pinguis (Heer, 1849) from the Miocene of Croatia (transferred from Bibio by Skartveit and Krizmanic, 2020), D. andrewrossi Nel, Collomb and Waller, 2019 from Isle of Wight deposits (Krzemiński et al., 2019) and D. magnus Dürrenfeldt,1968 from Pliocene Germany (Dürrenfeldt, 1968; Skartveit and Pika, 2014; but see treatment of this species below). Dilophus campbelli, a compression fossil of a larval specimen, was reported from Eocene New Zealand by Harris (1983). Dilophus priscus Loew, 1850, a Baltic amber specimen which may be lost, was considered a nomen nudum (Skartveit, 2008). However, Loew (1850) stated that “the throax has thick horns,” which is sufficient to make the name available. Dilophus deletus Heyden, 1859 was treated as nomen dubium by Skartveit and Wedmann (2021). Numerous specimens not preserved sufficiently for assignment to species have been reported from Baltic amber (Skartveit, 2008), the Oligocene of France (Skartveit and Nel, 2017), the Miocene of Germany (Skartveit and Pika, 2014) and Miocene of Iceland (Skartveit et al., 2017).
Dilophus idanos is an exceptionally well-preserved specimen. It is the oldest adult representative of the genus, the first fossil of the genus to be reported from the Nearctic, and distinct from all known fossils of the genus (see comparison to previously described fossil Dilophus in Table 1, which roughly summarizes much of this Remarks section). With the exceptions of D. crassicornis, D. succineus and D. matilei, it is smaller than most fossils of the genus. However, size and color are not reliable characters for distinguishing different fossil species. For example, Collomb et al. (2008) reported that body lengths of the extant species Bibio hortulanus Linnaeus, 1758 ranged from 4.5 to 11 mm in males and from 6 to 11 mm in females. That said, Dilophus idanos is of typical size to most extant species of Dilophus and is unlikely to be mistaken with the unusually large fossil Dilophus species D. krantzii (male) and D.pinguis (male and female), which have body lengths greater than 1 cm. Dilophus idanos differs from D. luteipennis (female) in the ratio of the lengths of r-m relative to that of the basal portion of Rs (bRs); the ratio for D. idanos is 3.2 (left wing, Figure 2B-C). Skartveit and Nel (2017) indicated that this ratio for D. luteipennis was slightly greater than one, but the specimen was figured with r-m slightly shorter than bRs and data in their table 5 showed bRs significantly longer than r-m. Although their values (their table 5) for the r-m and Rs veins of D. luteipennis varied by approximately 50%, the ratio of the average of the measurements of r-m/bRs is about 0.6, which is the value reported here in Table 1. However, it should be noted that Skartveit and Nel (2017) stated that “As usual with fossil insects ... it is not possible to calculate ratios between different measurements from the data as here presented.” Heyden (1870) figured the wing of D. krantzii with crossvein r-m approximately half the length of bRs (plate 45, figure 24). Skartveit and Pika (2014) state that “crossvein r-m (in D. pumilio) is markedly longer than the basal part of Rs, which is a diagnostic character for Dilophus” (the r-m/bRs ratio for D. pumilio = 2.1). However, Collomb et al. (2008) documented extreme intraspecific variation in the length of the r-m cross vein in the extant Bibio hortulanus, including an r-m/bRs ratio that would be a “diagnostic character for Dilophus” (their figure 5c); the value of the ratio of the lengths of r-m to bRs in Dilophus would be of greater value if intraspecific variation data were available. Dilophus idanos differs from the male of D. palaeofebrilis and both the male and female of D. succenius in that the antennae of the latter two species has twice as many (12) flagellomeres and are 2.5 times as long. The costa of D. luteipennis, which did not extend beyond R4+5, distinguishes this species from D. idanos (Figure 2B-C).
However, one of the more informative characters for distinguishing between different species of Dilophus is the pattern and number of fore tibial and mesonotal spines. The fore tibial spines consist of those that form a circlet at the apex of the tibia as well as one or more short rows or clusters of spines mesally. Dilophus idanos has two mesal rows of spines, one above the other, of two spines each, and six visible spines at the apex of the fore tibia (a few additional apical spines may be obscured so the number could be about 8) (Figure 3B-C); a shorthand description of this pattern can be given as 2:2:6(8?) with the far right-hand value always indicating the number of spines in the apical circlet. The fore tibial patterns of D. palaeofebrilis (male), D. succineus (male, female), D. matilei (male) and D. crassicornis (male, female) are 1:2:8, 2?:3:6, 2:3:6, 2:4:8, 3:6 and 3:6, respectively. Dilophus luteipennis was described as “possibly with 3 mesal spines” (Skartveit and Nel, 2017) and D. pinguis as “possibly with two small spines mesally” (Skartveit and Krizmanić, 2020). Heyden (1870) stated “die Vorderschienen mit einem Stachelkranz endingen” (apical end of fore tibia with a ring of spines) in his description of D. krantzii, but provided no information about mesal spines.
The description of D. andrewrossi makes no mention of mesal spines (absence of mesal spines is unknown in the genus) and states that only two apical spines are present in the fore tibia instead of the typical crown of apical spines (Krzeminski et al., 2019).
Several of the fossil species also differ in the number, size and pattern of pro- and mesonotal spines. Dilophus idanos has a broad semicircular arrangement of 12 spines at the anterior margin of the mesonotum and, more posteriorly, a narrower semicircular pattern of 10 spines with an additional two spines below the apex of the semicircle forming a diamond-shaped pattern (Figure 3A). The D. succineus specimens are described as having transverse rows of spines (Skartveit, 2008); the posterior row of mesonotal spines in D. palaeofebrilis, as figured, is also transverse (Skartveit, 2008). The female of D. crassicornis has two rows of eight mesonotal spines with “one spine on each side laterally between the two spine rows” (Skartveit, 2008), while the male has approximately ten spines in a semicircular pattern somewhat similar to D. idanos. The two rows of mesonotal spines in D. matilei (male) are transverse (anterior) or nearly so (posterior) as originally figured (Waller et al., 2000). The mesonotum of a Dilophus sp. from the late Miocene of Iceland (Skartveit et al., 2017) is described as having “traces of two transverse spine rows.” In an examination of the female holotype and a female paratype of D. luteipennis, Skartveit and Nel (2017) stated that mesonotal spines were not visible. Dilophus pumilio, a female from the Miocene of Öhningen, Germany, was described with “Probable traces of protibial spines preserved, their exact pattern not possible to make out but may have consisted of one basal (two spines?) and one mesal (two or three spines?) group.” and a “vague trace of pronotal spine row” (Skartveit and Pika, 2014). The M4 vein of D. pumilio was figured nearly touching M1+2 just apical of r-m but this may have been an artifact of the preservation process. The venation was described as “veins brownish, no difference in pigmentation between anterior and posterior veins.” This latter character would distinguish this specimen from D. idanos.
The paratype of Dilophus idanos appears to differ from the holotype in several respects. The terminal palpal segment of the latter is much longer than wide while that of the paratype is ovoid in shape. There are also subtle differences in both the fore tibial and mesonotal spines. In the holotype of D. idanos, the rows of the latter are more strongly arched although the number of spines appears to be the same in both specimens. In addition, while the holotype appears to have four spines above the apex of the fore tibia, the paratype may have five (Figure 3B-C, Figure 4C). We do not consider these differences sufficient to suggest that specimen USNM 768228 is a different species. Fossilization-related changes to minute structures often give rise to spurious shapes. For example, the more distal row of fore tibial spines of the left leg of D. idanos have distinctly crenate termini (Figure 3B-C), while those of the right leg are sharply pointed.
Perhaps more importantly, species descriptions of extant Dilophus often report the number of spines as a range. For example, “...the posterior has twelve to fourteen minute teeth” (Hardy, 1953) or “Pronotum with a transverse row of 8-11 spines, anterior margin of mesonotum with a transverse row of 11-12 smaller spines” (Haenni and Baez, 2001). In a key to Nearctic Bibionidae, Hardy (1945) stated “comb comprised of seven to ten teeth” in female D. strigilatus McAtee, 1922 and “comb... usually twelve to sixteen teeth” in female D. oklahomensis Hardy, 1937 (currently treated as a junior synonym of D. occipitalis Coquillett [in Baker, 1904]) and D. breviceps Loew, 1869 (currently treated as a junior synonym as a subspecies of D. tibialis loew, 1869).
Bibionidae incertae sedis stat. rev. (Dilophus magnus Dürrenfeldt, 1968)
Figure 5
Holotype. GZG.W.14836 (originally 612-6 [Dürrenfeldt, 1968]), both part and counterpart, is housed at the Geowissenschaftliches Museum in Göttingen, Germany.
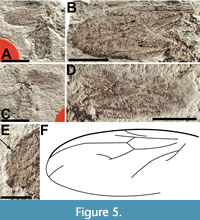 Redescription. Sex unknown. ventral view (abdomen and part of thorax) and dorsal view (part of thorax) body length approximately 2 cm. Details of head and antennae not preserved (Figure 5A-C). Dorsal portion of thorax, with left wing attached, partially preserved. Mesonotum, probably light brown and concolorous with ventral part of thorax, with distinct convergent mesonotal furrows; mesonotal spines absent. Wings, overlapped and flipped horizontally relative to one another, preserved on right side of body, at least 1.65 cm long, 6.7 mm wide; Costa apparently terminates at R4+5; Sc long, reaching C at level distal to the distal end of r-m; pterostigma slightly oval, not touching R4+5; bRs veins 0.92 and 0.81 mm in length, r-m veins 0.96 and 0.96 mm in length; M1+M2 and portions of M1 present, M2 and M4 not visible; CuA very prominent, CuP not preserved (Figure 5). Hind femur, tibia and tarsus 4.1, 5 and 4.6 mm long respectively; abdomen 1.2.cm long, 5 mm wide details of genitalia not preserved.
Redescription. Sex unknown. ventral view (abdomen and part of thorax) and dorsal view (part of thorax) body length approximately 2 cm. Details of head and antennae not preserved (Figure 5A-C). Dorsal portion of thorax, with left wing attached, partially preserved. Mesonotum, probably light brown and concolorous with ventral part of thorax, with distinct convergent mesonotal furrows; mesonotal spines absent. Wings, overlapped and flipped horizontally relative to one another, preserved on right side of body, at least 1.65 cm long, 6.7 mm wide; Costa apparently terminates at R4+5; Sc long, reaching C at level distal to the distal end of r-m; pterostigma slightly oval, not touching R4+5; bRs veins 0.92 and 0.81 mm in length, r-m veins 0.96 and 0.96 mm in length; M1+M2 and portions of M1 present, M2 and M4 not visible; CuA very prominent, CuP not preserved (Figure 5). Hind femur, tibia and tarsus 4.1, 5 and 4.6 mm long respectively; abdomen 1.2.cm long, 5 mm wide details of genitalia not preserved.
Remarks. Dilophus magnus was collected from the Willershausen clay pit, a Piacenzian (2.6 - 3.6 Ma) pond marl in Germany and described by Dürrenfeldt (1968). The description consisted mostly of body coloration with some limited data on wing venation. As originally described, the specimen is unusual relative to Dilophus in a number of respects (e.g., a relatively short r-m and a relatively long distance between the origin of Rs and the terminus of Sc). Dürrenfeldt stated that, despite the absence of the fore legs and their diagnostic tibial spines, the specimen could be identified to the genus Dilophus based on C terminating beyond R4+5. Based on study of the holotype we disagree with Dürrenfeldt’s interpretation and believe C terminates at R4+5. However, regardless of the interpretation of the termination point of C, we argue here that this character alone is insufficient for such an identification. The Neotropical genus Bibionellus Edwards, 1935 (e.g., B. barrettoi Lane and Forattini, 1948) and some African species of Bibio (e.g., B. turneri Edwards, 1925) have the costa extending beyond the last radial vein (Hardy, 1950; Pinto and Amorim, 1997). In addition, the length of r-m in Dilophus is often quite a bit longer (often 2-3 times as long) than the base of Rs, which is not the case in D. magnus where r-m and base of Rs are almost subequal (it should be noted that Skartveit and Nel (2017) reassigned D. luteipennis, from Bibio, although its wing has r-m/bRs nearly subequal [their figure 213]). However, this species has neither C extending beyond R4+5 nor evidence of fore tibial spines. Lastly, there is no indication of mesonotal spines on the piece of the mesonotum associated with the left wing. We treat D. magnus as Bibionidae incertae sedis.
Family Mycetophilidae Newman, 1834
Subfamily Sciophilinae Rondani, 1840
Genus Azana Walker, 1856
Azana akarenos Kerr and Greenwalt sp. n.
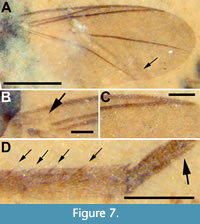
Figure 6-Figure 7
zoobank.org/465EEEB4-8583-4425-8878-FF79F8A4D252
Type species. Azana scatopsoides Walker, 1856, by monotypy (= Azana anomala Staeger, 1840).
Holotype. Female, USNM 625099, deposited in the Paleobiology collections of the National Museum of Natural History in Washington, D.C.
 Locality and horizon. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Spring site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The species epithet is derived from the Greek term akarenos, meaning headless.
Diagnosis. Head is either not present or barely visible on left side of thorax below anterior margin of wing; thorax arched; costa produced beyond tip of R4+5; subcosta short, ending free; r-m long, arising near base of wing; Rs essentially absent as R1 and r-m veins touch; M1 obsolete at its base, M4 faintly present at wing margin.
Description (female). Body length 2.05 mm (from terminus of genitalia to middle of scutum); scutum, scutellum, abdomen and terminalia dark brown, legs light brown (Figure 6). Wings 1.61 mm (right) and 1.66 mm (left) in length, 0.64 mm wide, lightly infuscate without markings, membrane densely microtrichose, veins thick and brown (except base of M1 obsolete); costal vein extends beyond R4+5, approximately 0.18 of distance between R4+5 and M1; Sc short; R1 reaching C at 0.47 times length of wing; Rs imperceptible, R1 touching longitudinal r-m/R4+5; ratio of R1 to R4+5 0.27 (given difficulty of locating Rs, this value could range from 0.24 to 0.27); r-m long and longitudinal; M1 obsolete at base, but well defined in apical 2/3, extending straight to wing margin far beyond apex of wing; M2 absent, M4 present at right wing margin, about 0.2 mm in length (about twice the distance between M4 and CuA), delaminated and very faint in left wing; CuA strong, more angled than smoothly curved (i.e., with defined inflection point) (Figure 7A-C); halter relatively small, 0.19 mm long, knob 0.07 mm wide, stem and knob light brown. Femur covered in short, light brown setae; all tibial setae shorter than width of tibia; mid and hind tibiae with row of several larger spines; abdomen1.3 mm long, 0.62 mm wide; tibial spurs 1:2:2 (Figure 7D). Gonostylus absent; abdomen suggestive of a female (Figure 6).
Synimpressions. Chironomidae (32, one at mid-eclosion), Parasitica (Hymenoptera) (1), Corixidae (2), Hemiptera (1), Dipteran pupae (6), unidentified larvae (2), Diptera (2)
Remarks. Mycetophilidae is a diverse group of small nematocerous Diptera with approximately 4,900 described species including 403 described fossil species (Evenhuis and Pape, 2021). However, the Sciophiline genus Azana contains only 13 extant species, although it is widely distributed (Kerr, 2010). Species of the genus are easily distinguished by reduced wing venation; Sc short, ending free; M1 obsolete at its base; M4 vestigially present, near wing margin. The habitus of extant species of Azana provides a rationale for the missing head of A. akarenos. The middle of the scutum is the anterior-most aspect of the bodies of these flies, with the entire head tucked below the ventral aspect of the thorax. From a dorsal view, therefore, the head is typically not visible. In the fossil, the head is probably mostly buried in the shale matrix although a portion of it may be seen to the left, near the base of the wing (computed tomography using a GE Phoenix micro CT failed to image the specimen.). Azana akarenos is distinct from the single fossil known for the genus, A. rarissima Meunier, described from Baltic amber by Meunier (1904). Meunier (1904) described the antenna of A. rarissima, figured its wing and stated that the 2.0 mm-long insect had tibial spines and setose tarsi. The two Eocene species are distinguished from one another based on the ratio of R1 to R4+5, 0.22 in A. rarissima and 0.24-0.27 in A. akarenos. More obvious, however, is the difference in the length of Rs. In A. akarenos, this vein - in both the left and right wings - is short to the point of not existing; R1 and the junction of r-m and R4+5 touch one-another. In contrast, the length of Rs in A. rarissima is 80% of the distance between R1 and C, at that point.
Given that differentiation of the various species of the genus are based mostly on characteristics of the head and male terminalia, and color, it would be futile to attempt a detailed comparison of A. akarenos with extant members of the genus. However, the four extant species of Azana from North America differ from A. akarenos with respect to the same character states used to distinguish the two fossil species of the genus. Azana malinamoena Kerr, 2010, A. frizzelli Kerr, 2010 and A. sinusa Coher, 1995 (= A. pilosa Taber) have distinctly longer R1 veins relative to the length of R4+5 (ratios = 0.37, 0.34, 0.39 and 0.36, respectively). In addition, all three of these species have a distinct Rs vein, although in A. sinusa the length of Rs is only about the width of the vein itself (Coher, 1995; Taber, 2017). In A. atlantica Oliveira and Balbi, 2008 (in Amorim et al., 2008a), collected in Brazil, Rs and r-m are separated by slightly more than a vein width but the base of r-m is absent and “r-m indistinct from Rs” (Amorim et al., 2008b). Again, the ratio of R1 to R4+5 is large, in this case 0.37 (vs. 0.27 in A. akarenos).
Family Keroplatidae Meigen, 1803
Subfamily Macrocerinae Rondani, 1856
Genus Macrocera Meigen, 1803
Macrocera apithanos Kerr and Greenwalt sp. n.
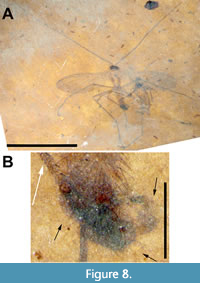
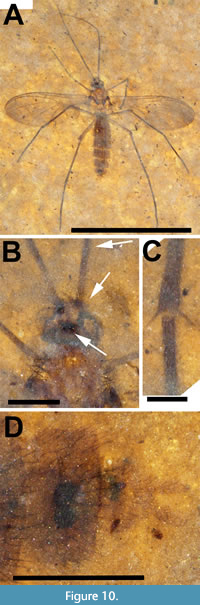
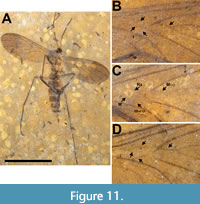
Figure 8, Figure 9, Figure 10, Figure 11
zoobank.org/608DC8E0-57E9-48E6-BC7F-F1B9B70A9BBB
Type species. Macrocera lutea Meigen, 1804, by subsequent designation (Guérin, 1826).
Holotype. Male, USNM 624407, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet is a Greek term that means unlikely, improbable, and refers to the rarity of fossils that preserve very long, thin antennae.
Diagnosis. This species of Macrocera is distinguished from other fossils of the genus by its very long antennae, wing length/body length ratio and short vein Sc.
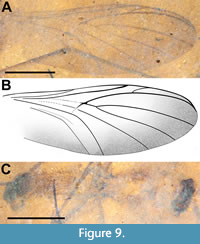 Description (male). (Figure 8-Figure 9, Figure 11B). Body length 3.3 mm. Head dark brown, much wider than long, 0.61 mm x 0.23 mm. Antenna (left) 8.8 mm long, narrow, 2.7 times body length; F1 66 μm wide at base, terminal flagellomere half that width at tip, basal flagellomeres ≥ 6 x pedicel length. Thorax yellowish, 0.54 mm wide, length difficult to determine due to poor preservation of relatively long, wide and less pigmented pronotum. Scutum pillose, uniformly covered by long (0.15 mm) dark brown setae; subcoxal pleural sclerites prominent, black (Figure 8A, Figure 9C). Wing 3.9 mm long, 1.4 mm wide, with microtrichia. Sc terminates in C at level slightly distal of m-cu origin; costa extending 0.2 distance from R5 to M1. R2+3 angled about 10° from horizontal, origin in line with terminus of R1; Rs+M fusion minimal, present as heavily sclerotized junction only; base of M4 with very slight curve; m-cu acutely angled (about 45°). Distal quarter of wing along anterior, apical and posterior margins of the wing infuscate (Figure 9A-B, Figure 11B). Legs light brown, setose; fore, mid and hind femora 0.86, 1.62 and 1.62 mm long, respectively; fore, mid and hind tibiae 1.37, 2.42 and 1.87 mm long, respectively; fore, mid and hind tarsi 1.71, 2.02 and 2.19 mm long, respectively; apical ¾ of fore tibia with single row of short stout anteroventral spines; abdomen 1.87 mm long (genitalia not included), uniformly wide at approximately 0.4 mm, entire length of abdomen pilose, covered with uniformly long (0.15 mm) setae. Genitalia dark brown/black, gonostylus curved, hook-like, about 0.32 mm long, terminal quarter black, highly sclerotized (Figure 8B).
Description (male). (Figure 8-Figure 9, Figure 11B). Body length 3.3 mm. Head dark brown, much wider than long, 0.61 mm x 0.23 mm. Antenna (left) 8.8 mm long, narrow, 2.7 times body length; F1 66 μm wide at base, terminal flagellomere half that width at tip, basal flagellomeres ≥ 6 x pedicel length. Thorax yellowish, 0.54 mm wide, length difficult to determine due to poor preservation of relatively long, wide and less pigmented pronotum. Scutum pillose, uniformly covered by long (0.15 mm) dark brown setae; subcoxal pleural sclerites prominent, black (Figure 8A, Figure 9C). Wing 3.9 mm long, 1.4 mm wide, with microtrichia. Sc terminates in C at level slightly distal of m-cu origin; costa extending 0.2 distance from R5 to M1. R2+3 angled about 10° from horizontal, origin in line with terminus of R1; Rs+M fusion minimal, present as heavily sclerotized junction only; base of M4 with very slight curve; m-cu acutely angled (about 45°). Distal quarter of wing along anterior, apical and posterior margins of the wing infuscate (Figure 9A-B, Figure 11B). Legs light brown, setose; fore, mid and hind femora 0.86, 1.62 and 1.62 mm long, respectively; fore, mid and hind tibiae 1.37, 2.42 and 1.87 mm long, respectively; fore, mid and hind tarsi 1.71, 2.02 and 2.19 mm long, respectively; apical ¾ of fore tibia with single row of short stout anteroventral spines; abdomen 1.87 mm long (genitalia not included), uniformly wide at approximately 0.4 mm, entire length of abdomen pilose, covered with uniformly long (0.15 mm) setae. Genitalia dark brown/black, gonostylus curved, hook-like, about 0.32 mm long, terminal quarter black, highly sclerotized (Figure 8B).
Synimpressions. Aphididae (5), Mymaridae (1), Chironomidae (1), Hemiptera (1), Thysanoptera (3)
Allotype. Female, USNM 729602. Allotype and paratype USNM 768010 deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Type locality and horizon. Park site (allotype), Dakin site (paratype), Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Description. Allotype female (Figure 10A) body length 4.2 mm. Head dark brown, wider than long, 0.55 mm x 0.36 mm; cerebral sclerite apparently present, roughly square in shape, approximately 0.18 mm/side. Antenna (left) setose, 3.9 mm long, narrow, terminal flagellomeres missing. Pedicel about 75 μm in diameter, uniformly setose; F1 60 μm wide at base, 0.52 mm long (Figure 10A-B). Thorax yellowish, 0.57 mm wide, 1.1 mm long, alar setae plentiful, dorsocentral setae less so. Subcoxal pleural sclerites present, black. Wing 3.6 mm long, 1.4 mm wide, with microtrichia. Sc terminates in C at level slightly distal of m-cu origin; costa extending 1/3 distance from R5 to M1. R2+3 origin in line with terminus of R1; Rs+M fusion not visible, base of M4 with distinct curve towards CuA (as in paratype, Figure 11D), more pronounced than that in holotype male; m-cu not acutely angled (parallel to costa in paratype). Entire distal half of wing infuscate (Figure 10A, Figure 11C). Legs light brown, uniformly setose, mid and hind femora 1.2 and 1.4 mm long, respectively; fore, mid and hind tibiae 1.1, 1.7 and 2.0 mm long, respectively; fore, mid and hind tarsi 1.25, 1.9 and 2.1 mm long, respectively; hind tibia with two prominent spurs (Figure 10C). Abdomen 2.5 mm long (genitalia not included), maximum width approximately 0.7 mm, entire length of abdomen pilose, individual segments well defined. Genitalia light brown, two black spermathecae present, cerci about 40 mm in diameter and extend about 0.14 beyond sternite 8 (Figure 10D).
Synimpressions. Aphididae (1)
 Remarks. The family Keroplatidae (superfamily Sciaroidea) consists of 130 genera and about 1,000 species, 67 of which are fossils distributed among 29 genera (Evenhuis and Pape, 2021). The family’s most speciose genus, Macrocera, has about 200 extant species and a worldwide distribution (Evenhuis, 2006; Sasakawa, 2zhe antennae are “protruded, filiform, long and ... cylindrical”. Although the antenna of the holotype for the genus, M. lutea (designated by Guérin [1826]), was described by Meigen (1804) as “long or longer than the insect”, the antennae of this genus may or may not be longer than the body and in some species, they are relatively short (reviewed in Vockeroth, 1981; Blagoderov and Ševčík, 2017).
Remarks. The family Keroplatidae (superfamily Sciaroidea) consists of 130 genera and about 1,000 species, 67 of which are fossils distributed among 29 genera (Evenhuis and Pape, 2021). The family’s most speciose genus, Macrocera, has about 200 extant species and a worldwide distribution (Evenhuis, 2006; Sasakawa, 2zhe antennae are “protruded, filiform, long and ... cylindrical”. Although the antenna of the holotype for the genus, M. lutea (designated by Guérin [1826]), was described by Meigen (1804) as “long or longer than the insect”, the antennae of this genus may or may not be longer than the body and in some species, they are relatively short (reviewed in Vockeroth, 1981; Blagoderov and Ševčík, 2017).
The holotype of Macrocera apithanos has an intact antennae 2.7 times as long as the body of the fly as well as vein M4 slightly curved towards CuA at its base (Figure 8A, Figure 9A-B, Figure 11B). Although the thorax and abdomen are poorly preserved, with individual segments of the abdomen indiscernible, specimen USNM 624407 is designated as the holotype as it is a male and its genitalia are preserved, albeit poorly. The allotype (Figure 10A-D, Figure 11C) and its paratype (Figure 11A, D) are better preserved although, in each case, the antennae appear to be incomplete. The wings of both the allotype and paratype females differ from the male in the greater extent of infuscation. The base of vein M4 in the females is better preserved and curved to a greater degree towards CuA than in the male (Figure 11B-D).
A large number of Cretaceous species of Sciaroidea have been described, including Burmacrocera petiolata Cockerell, 1917 (sex undetermined) from Myanmar (Blagoderov and Grimaldi, 2004). Burmacrocera petiolata is a small species with a wing length of 2 mm and an antennal length of 1.2 mm. The venation of B. petiolata is similar to M. apithanos but the Eocene species a shorter Sc, R1 and R2+3 less steeply angled to C, M1+2 shorter, M1 reaching margin below wing apex, M4 bent towards the posterior and CuA reaching the wing margin. The older literature contains a number of species designated as Macrocera that were subsequently reassigned: Meunier (1899) listed, but neither appropriately described nor figured, M. grandis Lundström, 1912 and M. minuta Meunier, 1899 from Baltic amber and both have been declared nomina nuda by Evenhuis (2006). Macrocera abundare Meunier, 1904, M. ciliata Meunier, 1904 and M. filiformis Meunier, 1904 were described and figured by Meunier (1904) but were subsequently assigned to the extinct genus Kelneria Matile, 1979 which differs from Macrocera in that R1 is shorter (less than half the length of the wing) and its basal cell is reduced. Six fossil species of the genus, all from the Cenozoic, are currently known. Macrocera electracornis Evenhuis, 2006 [M. longicornis Meunier, 1904], a male from Baltic amber, was figured with an antenna three times body length but with Sc long (0.64 x wing length vs. 0.38 x wing length for M. apithanos). Macrocera soccata Meunier, 1899 (male) and M. elegantissima Meunier, 1904 (female), both from Baltic amber, have antennae about half the body length, and antennae reaching the end of the abdomen, respectively. The genitalia of Macrocera soccata are figured as long and thin; M. elegantissima has flagellomeres < 4 x the length of the pedicel, vs. ≥ 6 x in M. apithanos. The antenna of Macrocera melanopoda Hong, 1974 (male) from 50 Ma amber of the Guchengzi Formation in the Liaoning Provence of China, is relatively short, about 1.3 mm in length (Hong et al., 1974). Additionally, the curvature of the gonostylus of Macrocera melanopoda is much less than that of M. apithanos (about 90° vs. 180°, respectively).
Two fossil species are preserved as compression fossils, M. archaica Armbruster, 1938 (Armbruster, 1938), sex unknown, from the Miocene Randeck Maar and M. umbonata Statz, 1944 (Statz, 1944), a female from the Oligocene Rott Formations of Germany. Neither described the antennae - the antennae are missing in M. umbonata. The former species, as originally figured, has the M fork - which Armbruster referred to as “Archaic” - at the level of the terminus of R1, while in M. apithanos, R1 ends far distad of the fork. In addition, R2+3 originates far distal to the terminus of R1 and is closer to vertical than horizontal (50°) in M. archaica while, in M. apithanos, this vein is closer to horizontal (10°) and originates below the terminus of R1. Both M. umbonata and M. archaica are larger than M. apithanos (4.7 mm and 5.5 mm, respectively, vs. 3.3 mm) but more importantly, the ratio of wing length to body length in these two species is less than one (0.91 and 0.76, respectively) vs. 1.18 in M. apithanos. Both M. archaica and M. umbonata have the fusion of Rs and M (Rs+M) equal to or longer than the basal sector of M1+2, whereas in M. apithanos, the junction of Rs and M1+2 is no more than a thickening of the veins at that point. Macrocera umbonata also differs from the North American fossil in that m-cu in the former is horizontal while it is acutely angled at about 45° in M. apithanos. The genitalia of Macrocera umbonata were neither described nor figured.
Family Bombyliidae Latreille, 1802
Subfamily Phthiriinae Becker, 1913
Genus Tmemophlebia Evenhuis, 1986
Tmemophlebia carolinae Evenhuis and Greenwalt sp. n.
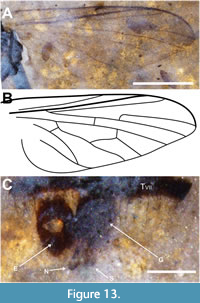
Figure 12-Figure 13
zoobank.org/7BB0C21B-2DCA-4831-B0E7-9BB85B110674
Type species. Cyclorhynchus testaceus Macquart, 1840, automatic (same type species as for Cyclorhynchus Macquart).
Holotype. Male, USNM 768156, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
 Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The new species is named for Dr. Carolina Yamaguchi (of São Paulo), for her current work on the phylogenetics and taxonomy of New World Phthiriinae.
Diagnosis. Tmemophlebia carolinae is the first described fossil species of the New World genus Tmemophlebia. It can be distinguished from the congeners by the costal vein ending just beyond the end of R4 (others in the genus have this ending at M1 or M2; other phthiriines have the costa entire); the presence of a short ventral prong at the tip of the postpedicel (congeners usually without it or very reduced), and the shallow cleft of the epandrium (congeners usually have this cleft much deeper or broader).
Description (male). Ventrolateral view. Black to dark brown, body length 4.3 mm (including genitalia, artefactual intersegmental portions of abdomen not included). Head dark brown/black, subspherical, 0.45 mm long, nearly all morphologcal aspects distorted (Figure 12A). Proboscis long, 1.44 mm in length, 0.2 mm wide; labellum 0.31 mm long, 0.1 mm wide; palpus 0.57 mm long, 50 mm wide; labrum 1.13 mm long, 60 mm wide. Postpedicel short, slightly diamond-shaped, 0.27 mm long, 0.1 mm wide, with apical sulcus, 15 μm deep, with short triangular ventral and larger bulbous dorsal prongs (Figure 12B-C). Thorax black, 1.65 mm long, maximum width 1.4 mm; wing (left) 3.2 mm long, 1.35 mm wide, L/W = 2.4, wing/body length = 0.74; C and R1 heavy, darkly pigmented, C ending at wing apex and R4, distance between termini of Sc and R1 equal to that between R1 and R4, R4 origin at basal quarter of cell r5, cell dm 0.95 mm long; M2 present, CuA distinctly recurved to meet CuA+CuP about 0.18 mm from wing margin (Figure 13A-B). Legs poorly preserved, distorted. Abdomen dark brown, setose, length 2.25 mm, maximum width 1.3 mm; tergite VII narrow, about half the width of tergite VI; epandrium 0.25 mm long, 0.16 mm wide, with distinct apical notch; gonocoxite and phallic complex 0.23 mm long, 0.16 mm wide; gonostylus 0.13 mm long, 30 mm wide (Figure 13C).
Synimpressions. None
Remarks. The genus was first created under the name Cyclorhynchus by Macquart in 1840. Subsequently determined to be preoccupied, Evenhuis proposed the replacement Tmemophlebia in 1986. The genus currently contains 16 extant species and is endemic to Nearctic and Neotropical regions (Evenhuis and Greathead, 2015). While the family Bombyliidae, with 59 described fossil species, has a reasonable fossil record, that of the subfamily Phthiriinae is poor with only two previously known. Elektrophthiria magnifica Nel, 2006 was described from the Eocene French Oise deposits and the Oligocene Geronites stigmalis Cockerell, 1914, was assigned to Poecilognathus Jaennicke, 1867 by Evenhuis (1994) after examination of the type specimen. The Oligocene Phthiria fossa Lewis, 1975 is incertae sedis within the subfamily (Nel, 2006) and Phthiria oligocaenica Timon-David, 1943 was assigned to Geron Meigen, 1820 (Evenhuis, 1994) although more recently suggested to be incertae sedis within Bombyliidae (Nel, 2006). Tmemophlebia carolinae is the third phthiriine fossil known as identified to the tribe Poecilognathini based on vein R4+5 forked, M2 present and postpedicel with apical sulcus. The cleft in the epandrium and the relatively small genitalia puts it in or close to Tmemophlebia and the short antennae are reminiscent of some species of Poecilognathus and Tmemophlebia. The incomplete costa is a feature distinguishing Tmemophlebia. Tmemophlebia are small, 2 to 6 mm in length (Evenhuis, 1990); Tmemophlebia carolinae fits well within that range.
The extant species of Tmemophlebia are primarily dune or light-colored sand dwellers and have a pale color that either acts as camouflage or helps reflect sunlight and regulates temperature in such arid regions. Pale colors are characteristic of many bee fly species that inhabit arid environments while dark coloration is a common adaptation in forested/non-arid areas. Bombylius albicapillus Loew, 1872, collected in the southern Sierra of California (Walker Pass), consisted of a pale variety in the Mojave Desert side of the pass and a dark variety in the adjacent pine woodland (NLE, pers. obs.). The lacustrine environment of the Coal Creek Member of the Kishenehn Formation is thought to have consisted of the shores and shallow waters of a large lake and associated semitropical forest. That Tmemophlebia carolinae is dark may reflect on its more lacustrine habitat. We speculate that Tmemophlebia evolved from a less arid dwelling taxon to one that transitioned successfully through the desertification process in the western United States since the Miocene.
Family Scenopinidae Burmeister, 1835
Subfamily Scenopininae Burmeister, 1835
Genus Brevitrichia Hardy, 1944
Tribe Metatrichini
Brevitrichia messogenes Greenwalt and Winterton sp. n.
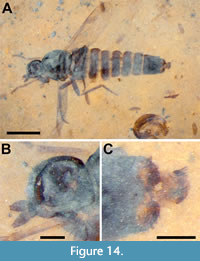
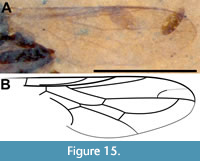
Figure 14-Figure 15
zoobank.org/2DC66356-B877-4D33-B68D-A2C3A9717D91
Type species. Pseudatrichia griseola Coquillett,1900, by original designation.
Holotype. Female, USNM 620163, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
 Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet is derived from the Greek, messogenes, middle aged, relating to the Paleogene age of the deposit bearing this fossil.
Diagnosis. Brevitrichia messogenes is the first described fossil species of the rather species-rich New World genus Brevitrichia. Most species of Brevitrichia are pale colored, frequently with a fine, grayish cuticular surface texture. Brevitrichia messogenes differs from these by the apparent uniformly dark body coloration. Additional discriminating features are lacking to enable further differentiation from other species in the genus.
Description (female). Black to brown, body length 4.0 mm (Figure 14A). Head dark brown/black, subspherical, 0.52 mm long, 0.48 mm high, flat ventrally; eye oval, 0.3 mm wide, 0.37 mm high; antenna short, 0.28 mm long, pedicel 66 μm high, 55 μm long, F1 0.18 mm long, slightly diamond-shaped, 92 μm maximum width, 33 μm at concave terminus Figure 14B). Thorax black, 0.89 mm long; wing short, not reaching tergite 7, 2.0 mm long, 0.65 mm wide, wing/body length = 0.5; C and R1 pigmented though pigmentation increasingly weak posteriorly, C setose with terminus at wing apex and R5 +M1, distance between Sc and R1 equal to that between R1 and R2+3, R4 origin in distal third of cell r5, cell dm 0.5 mm long, M1 fused with R5, CuA distinctly recurved to meet CuA+CuP, M4 meeting wing margin (Figure 15A-B). Fore, mid and hind femora, tibiae and tarsi 0.33, NA, 0.6, 0.43, 0.54, 0.72, 0.42, 0.50 and 0.52 mm, respectively; fore leg tarsomeres 1-5 0.16 mm, 78 μm, 63 μm, 49 μm, 67 μm respectively. Abdomen dark brown, 2.73 mm long, maximum basal width twice terminal width; posterior edge of tergite 8 heavily spinose, tergite 9+10 0.18 mm long, with acanthophorite spines about 80 μm long, cercus without setae, about 25 μm wide (Figure 14C).
Male. Unknown.
Synimpressions. Dipteran pupae
 Remarks. The family Scenopinidae contains 25 genera and approximately 420 species that have been separated into three distinct subfamilies (Winterton and Ware, 2015). The tribe Metatrichini, one of two in the subfamily Scenopininae, is distinguished from all other scenopinids by R5 merged with M1. The tribe contains 183 extant species in 14 genera, many endemic to the Southern Hemisphere (Winterton and Gaimari, 2017). Three fossil species have been assigned to the family - all differ markedly from B. messogenes. Metatrichia pria Yeates and Grimald, 1993 was described from two female specimens in amber from the Dominican Republic. They are large, 7.6 mm in length, and have M1 fused with R5. The species is characterized by a bulging face, wing extending beyond abdomen; wing/body ratio = 0.57 vs. 0.48 for B. messogenes, R4 fork origin in basal third of cell r5 and at the level of the termination of R2+3 in C (Yeates and Grimaldi, 1993). Eocenotrichia magnifica Garrouste, Azar and Nel, 2016 is from the lowermost Eocene amber of Le Quesnoy, France (Garrouste et al., 2016). Garrouste et al. (2016) keyed E. magnifica to the extant genus Propebrevitrichia Kelsey, 1969 but noted its larger size (>7 mm vs. <4 mm) and tergite 8 slightly longer than sternite 8 and, as a result, created the new genus Eoceotrichia. The specimen is 7.6 mm in length, with M1 fused with R5, wing/body ratio = 0.57, R4 fork origin in distal third of cell r5 and R4 distal to the termination of R2+3 in C. A third and much older species, Proratites simplex Grimaldi and Cumming, 1999, is from 90-94 MA New Jersey amber; unlike M. pria and E. magnifica, it does not have M1 fused with R5 and, as such, does not belong to Metatrichini (Grimaldi and Cumming, 1999). It differs from all extant Scenopinidae in having a long thin arista-like stylus four times as long as the postpedicel. Winterton and Ware (2015) have argued that P. simplex cannot be conclusively placed in Scenopinidae.
Remarks. The family Scenopinidae contains 25 genera and approximately 420 species that have been separated into three distinct subfamilies (Winterton and Ware, 2015). The tribe Metatrichini, one of two in the subfamily Scenopininae, is distinguished from all other scenopinids by R5 merged with M1. The tribe contains 183 extant species in 14 genera, many endemic to the Southern Hemisphere (Winterton and Gaimari, 2017). Three fossil species have been assigned to the family - all differ markedly from B. messogenes. Metatrichia pria Yeates and Grimald, 1993 was described from two female specimens in amber from the Dominican Republic. They are large, 7.6 mm in length, and have M1 fused with R5. The species is characterized by a bulging face, wing extending beyond abdomen; wing/body ratio = 0.57 vs. 0.48 for B. messogenes, R4 fork origin in basal third of cell r5 and at the level of the termination of R2+3 in C (Yeates and Grimaldi, 1993). Eocenotrichia magnifica Garrouste, Azar and Nel, 2016 is from the lowermost Eocene amber of Le Quesnoy, France (Garrouste et al., 2016). Garrouste et al. (2016) keyed E. magnifica to the extant genus Propebrevitrichia Kelsey, 1969 but noted its larger size (>7 mm vs. <4 mm) and tergite 8 slightly longer than sternite 8 and, as a result, created the new genus Eoceotrichia. The specimen is 7.6 mm in length, with M1 fused with R5, wing/body ratio = 0.57, R4 fork origin in distal third of cell r5 and R4 distal to the termination of R2+3 in C. A third and much older species, Proratites simplex Grimaldi and Cumming, 1999, is from 90-94 MA New Jersey amber; unlike M. pria and E. magnifica, it does not have M1 fused with R5 and, as such, does not belong to Metatrichini (Grimaldi and Cumming, 1999). It differs from all extant Scenopinidae in having a long thin arista-like stylus four times as long as the postpedicel. Winterton and Ware (2015) have argued that P. simplex cannot be conclusively placed in Scenopinidae.
The presence of acanthophorite spines, well-developed mouthparts, fused M1 +R5, relatively narrow abdomen, and relatively small size place this new fossil species in Metatrichini among the group of genera comprising Brevitrichia Hardy, 1944, Heteromphrale Kröber, 1937, Irwiniana Kelsey, 1971, Propebrevitrichia Kelsey, 1969, Paramonova Kelsey, 1970 and Riekiella Paramonov, 1955 (Winterton and Ware, 2015). Specifically, this species is placed in the New World genus Brevitrichia as it exhibits the following (female) characteristics: mouthparts well developed, M1 and R5 fused apically, acanthophorite spines well developed but not tufted apically, body size relatively small and abdomen not broadly flattened. The shape of sternite 8 helps differentiate B. messogenes from Irwiniana and Heteromphrale as it is slightly rounded apically, resembling that of other species of Brevitrichia. Sternite 8 in Irwiniana is trilobate apically, or elongated in Heteromphrale (Winterton and Gharali, 2011; Winterton and Gaimari, 2011). Based on the angle this specimen was preserved, the characteristic rounded lateral lobes typically found in Brevitrichia are not readily apparent. In addition, wing R4 diverges from R5 along the basal half of cell r3 in Heteromphrale and Brevitrichia compared to along the distal half of the cell in Propebrevitrichia, Riekella, Irwiniana, Paratrichia and Paramonova. Brevitrichia messogenes displays the diverging R4 from R5 in the distal half of the cell, suggesting that the utility of the character in isolation may be questionable. Brevitrichia messogenes is differentiated from extant Brevitrichia species by the uniformly dark coloration, as most species are pale coloured. Few other discriminating characters are evident in this fossil.
Family Apsilocephalidae Nagatomi, Saigusa, Nagatomi and Lyneborg, 1991a
Type genus and species Apsilocephala longistyla Kröber, 1914
Palaeoapsilocephala Hauser and Greenwalt gen. n.
Figure 16-Figure 17
zoobank.org/81EF97C3-8AB6-4C83-BD9B-D61C4D043F32
Type species. Palaeoapsilocephala kishenehnensis Hauser and Greenwalt by present designation.
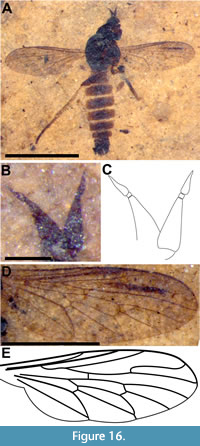 Included species. Palaeoapsilocephala pusilla (Hennig, 1967; Psilocephala) comb. n.; Palaeoapsilocephala vagabunda (Cockerell, 1927; Rueppellia) comb. n.
Included species. Palaeoapsilocephala pusilla (Hennig, 1967; Psilocephala) comb. n.; Palaeoapsilocephala vagabunda (Cockerell, 1927; Rueppellia) comb. n.
Diagnosis. Antenna with scape less than twice as long as thick and pedicel square. Postpedicel elongate triangular; first stylus segment short and square, second stylus segment elongate, four to five time as long as its greatest width. Hind femur thickened, more than twice the width as the associated tibia. Epandrium without articulated surstyli.
Differential diagnosis. Palaeoapsilocephala differs from the extant Apsilocephala by the shape of the antenna, which has the last stylus segment more than twice as long as the nearly rounded postpedicel, the hind femur being only slightly thicker than the tibia, as well as the presence of articulated surstyli on the epandrium. Kaurimyia Winterton and Irwin, 2008 can be separated from Palaeoapsilocephala by the apically enlarged hind tibia and the swollen hind tarsus. A number of morphological similarities are shared between Palaeoapsilocephala and Clesthentia White, 1914. While the postpedicel in Clesthentia is more egg-shaped in contrast to the more triangular shaped postpedicel in Palaeoapsilocephala, this is not a necessarily a significant character to separate two genera. Other differences are the elongated, ventrally projected surstyli of Clesthentia, which could be an autapomorphy for this genus. The epandrium of Kaurimyia is much more similar to P. pusilla and P. kishenehnensis, with the triangular edges laterally expanding over the tip of the oval cerci. The lack of an apical bristle on the apex of the antenna in Clesthentia could also be considered a character to distinguish the two genera. The last two characters could not be confirmed with all the fossil taxa, because, for P. vagabunda only one female is known, and the apical bristle on the antenna can only be discerned in the amber fossil P. pusilla. The extinct genera (See Remarks) are distinguished from Palaeoapsilocephala by long spines on the hind femur (Irwinimyia Zhang, Wang and Yeates, 2018), the row of posterior setae on the fore femur and the lack of a pterostigma (Kumaromyia Grimaldi and Hauser, 2011).
Palaeoapsilocephala kishenehnensis Hauser and Greenwalt sp. nov.
zoobank.org:act:CDF58467-53D2-47C7-9265-9C700DF9E1E2
Holotype. Male, USNM 717195, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The generic name is from the prefix “palaeo” (from the Greek palaios for ancient, old) an indication that this is an extinct genus, and “apsilocephala”, which refers to the type genus of this family. The species epithet is derived from the name of the Kishenehn Formation, where the specimen was found.
Differential diagnosis. This species is smaller (3.8 mm) than P. vagabunda (5.6 mm) and P. pusilla (5 mm). In addition, P. kishenehnensis has a much shorter Sc (ending at r-m) than does P. vagabunda (Hauser and Irwin, 2005) and the basal flagellomere of the stylus of P. kishenehnensis is quadrate whereas that of P. pusilla is three times as long as wide (Hennig, 1967).
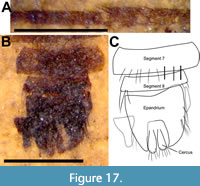
Description (male). Body 3.8 mm long (Figure 16A); head dark brown, distorted due to fossilization process; antenna 0.33 mm long, shorter than head; scape and pedicel short, lighter in color than postpedicel, pedicel 66 μm wide, wider than long with short, dark setae; postpedicel dark brown, length 0.18 mm, maximum width 80 mm, gradually tapered, not pyriform. Stylus 2-segmented, 0.55 times length of postpedicel, basal section very short, wider than long (13 μm long, 17 μm wide); terminal segment of stylus conical, 89 μm long (Figure 16B-C). Thorax black to brown, 1.1 mm long, without bristles. Wing 2.28 mm long, 0.8 mm wide with dense microtrichia, with long narrow pterostigma surrounding terminal portion of R1; R4 parallel for long distance with R5; R5 meeting margin exactly at apex of wing; vein M3 complete, cells m3 and cua closed (Figure 16D-E). Legs pale yellow, femora darker, twice as wide as tibiae; hind tibia 1.07 mm long, with longer setae at apex (part of circlet of apical setae?) (Figure 17); hind tarsus 0.94 mm long. Abdomen elongate, length 2.2 mm, maximum width 0.71 mm, dark brown with posterolateral margins of tergites cream-colored; sparsely setose (Figure 17A). Genitalia partially torn; elongate cerci surrounded laterally by triangular extensions of epandrium; without articulated surstyli; other parts not discernable (Figure 17B-C).
Female. Unknown.
Synimpressions. Chaoborid (Diptera) pupae (3), Notonectidae (Hemiptera) and chaoborid adults (2)
Remarks. The family Apsilocephalidae, as established in 1991, contained three species in three genera: Apsilocephala Kröber, 1914, Clesthentia, White, 1914 and Clesthentiella, Nagatomi, Saigusa, Nagatomi and Lyneborg, 1991. Apsilocephala and Clesthentia had previously been assigned to different families (Therevidae and Rhagionidae, respectively (Nagatomi et al., 1991a)). The family was diagnosed based in part, on vein R5 ending before or at wing apex, first flagellomere round-triangular or pyriform with pointed 1- or 2-segmented stylus, frons concave and narrow, scutum strongly arched, apex of tibiae with circlet of setae and no spurs, empodium bristle like and male with posterolateral epandrial surstylus (Nagatomi et al., 1991b; 1991c). Because only the genus Apsilocephala possesses articulated surstyli, they are not considered a synapomorphy for the family, and their homology with the surstyli of the Empidoidea has been questioned (Sinclair et al., 1994). Another character for this family would be the absence of acanthophorite spines in the females, which is clearly a secondary loss, because they are found in most related families. Although Nagatomi and Yang (1998) later synonymized Apsilocephalidae under the fossil family Rhagionempididae Rohdendorf, 1951, this change was never followed by other dipterists. This is partially due to the uncertain position of Rhagionempididae, for which the absence or presence of pulvilli or a pad like empodium as well as the male and female genitalia are unknown. The position of Apsilocephalidae in the therevoid clade of Asiloidea has been confirmed by molecular and morphological evidence (Yeates et al., 2003; Yeates and Wiegmann, 2005; Trautwein et al., 2010, Wiegmann et al., 2011; Winterton and Ware, 2015; Shin et al., 2017). In the molecular phylogenies of Winterton and Ware (2015) and Shin et al. (2017), Evocoidae is the sister to Apsilocephalidae, while Therevidae and Scenopinidae form a clade that is the sister to Apsilocephala and Evocoidae.
The extremely relictual fauna that constitutes Apsilocephalidae currently consists of four extant described species in three genera: The monotypic Apsilocephala with A. longistyla Kröber, 1914 in western North America (with several undescribed closely related species in the southwestern USA); Clesthentia, with the two species from Tasmania: C. aberrans White, 1914 and C. crassioccipitis (Nagatomi, 1991). The latter were originally described in the genus Clesthentiella Nagatomi, 1991 and synonymized with Clesthentia by Winterton and Irwin (2008); the monotypic Kaurimyia Winterton and Irwin, 2008 with K. thorpei Winterton and Irwin, 2008 from New Zealand. The authors have seen images of an undescribed Apsilocephalidae from southern Australia.
Extant Apsilocephalidae Genera and Their Characters
Because of the rarity of both specimens and species in this family, the characters used to distinguish genera and species have been contentious. The genus Apsilocephala is characterized by the epandrial surstylus as well as the very long last flagellomere and the long and thin legs (especially the hind femur). Vein M3 is present in Apsilocephala and Kaurimyia, but variable in Clesthentia. Nagatomi et al. (1991a) described Clesthentia with M3 but C. crassioccipitis without M3 while Winterton and Irwin (2008) reported specimens of Clesthentia with an incomplete M3. The absence or reduction of a wing vein can affect only part of a population and should not be used for classification purposes if only very few specimens are available. Vein R5 was originally reported to terminate before or at the wing apex of Apsilocephala but was figured ending just below the apex (figure 5 in Nagatomi et al., 1991a), while R5 in Clesthentia it ends before the apex (the partial wing of C. crassioccipitis did not contain the terminus of R5); R5 terminates at the wing apex in Kaurimyia (Winterton and Irwin, 2008). It is sometimes difficult to evaluate if R5 terminates at, or slightly below or above the wing apex, and it is, therefore, not a good character for generic limitation; unfortunately, this character is often used in the even more difficult task of describing fossil taxa. However, it is a good character to distinguish this family from the closely related Therevidae, which has R5 always distinctly ending below the wing apex (the wing apex is always between R4 and R5). In a limited study of intraspecies variation, examination of seven specimens of Apsilocephala longistylus housed at the NMNH from six different sites in four western North American states, R5 terminated either at or slightly below the wing apex (data not shown). These preliminary data support the statement by Winterton and Irwin (2008) that this character, by itself, should not be justification for generic separation.
While Apsilocephala was originally reported as not having a pterostigma, Apsilocephala and Evocoa Yeates, Irwin and Wiegmann, 2006–a replacement name for preoccupied Ocoa Yeates, Irwin and Wiegmann, 2003–in the family Evocoidea, both have a distinct darkening between the R1 and Sc veins. Nagatomi et al. (1991a) reported a female specimen of Clesthentia sp. with a pterostigma very much like that of Apsilocephala and Kaurimyia; White (1914) figured Clesthentia as having a long dark pterostigma that filled cell sc but the depicted intensity of the pigmentation may have been an artifact of printing. Clestenthia crassioccipitis was originally reported with an area that “may be brown” at the apex of sc (Nagatomi et al., 1991a). It seems that all Apsilocephalidae and Evocoidae have a similar pterostigma.
The antennal flagellum of Apsilocephala, Clesthentia and Kaurimyia were all reported to have three segments although C. crassioccipitis was originally described as having only two segments. The only specimen of C. crassioccipitis could not be located (it is not in the collection in Copenhagen, where it should be), and therefore whether or not the antenna has only two flagellomeres or if the third one is broken off, can not be verified. The second flagellomere in Apsilocephala and Clesthentia is much shorter than the terminal third flagellomere. In Kaurimyia, the terminal segment of the flagellum is figured as about 1/8 the length of the “deflexed” part of the antenna, which consisted of the last two flagellomeres. In the key, it states “apical segment [of flagellum] greatly elongate”, but there is no description of the proportions of the last two flagellomeres in the description (Winterton and Irwin, 2008). It is likely that the illustration is incorrect, and Kaurimyia has the same configuration as the other apsilocephalid genera, with the second flagellomere being the shortest. The diagnosis of the genus Kaurimyia lists two antennal characters: “Antennal style approximately equal length to base of flagellum, deflexed ventrally at base”. According to the key to genera in Winterton and Irwin (2008), the antennal style is “approximately equal length of basal segment of flagellum” in Kaurimyia, while “less than half the length of basal segment of flagellum” in Clesthentia, a character also adopted in the key by Zhang et al. (2018). Ratios of length of last two flagellomeres to the first flagellomere is 0.8 in Kaurimyia when measured from the figure in Winterton and Irwin (2008), 0.7 (female) to 0.4 (male) in Clesthentia sp., 0.15 in C. crassioccipitis, and 1.4 (female) to nearly 3 (male) in Apsilocephala (Nagatomi et al., 1991a; Winterton and Irwin, 2008). This indicates that use of the antennal length ratio to separate Kaurimyia and Clesthentia in the keys of Winterton and Irwin (2008) and Zhang et al. (2018) are not practical. Winterton and Irwin (2008) stated that “Considering also, the variability in the length and number of flagellar segments at genus and species level in related Therevidae, this character (and wing venation) by itself should not be justification for generic separation.” It is therefore surprising that Winterton and Irwin (2008) synonymized Clesthentiella with Clesthentia, even though Clesthentiella is the only apsilocephalid with a two segmented flagellum (and a significantly enlarged occiput) and then erected the new genus Kaurimyia by using even more subtle antennal characters to diagnose it. In addition, the deflexed last flagellomere is listed as an important character, but from photographs of the type of Kaurimyia, the angle is not 90° as in the drawing, and a ventral deflexion is also found in Clesthentia and Apsilocephala. This deflexion could be a postmortem artifact. The synonymy of Clesthentiella might have been a bit hasty, considering the strange antennal character of Clesthentiella and the enlarged occiput, but a final decision has to await additional material. Kaurimyia is clearly a separate genus from Clesthentia, by the characters of the male genitalia, the number of spermathecae and the unusual hindlegs. Its description mentions that the “hind legs are distinctly longer and thicker than mid and fore legs”, but the authors failed to mention that the hind tibia is club-shaped and twice as thick apically as basally. Even the tarsal segments, especially the basitarsus, are significantly thickened. Unfortunately, the size of the specimens and the configuration of the palps (one or two segmented) are not mentioned in the description.
The Fossil Record of Apsilocephalidae
Several fossil species of Apsilocephalidae have been reported (See Zhang et al., 2018 for overview and key). Fossils of Cretaceous age include Burmapsilocephala cockerelli Gaimari and Mostovski, 2000, Burmapsilocephala evocoa, Grimaldi, 2016, Kumaromyia burmitica Grimaldi et al., 2011, Myanmarpsilocephala grimaldii Zhang et al., 2018, Irwinimyia spinosa Zhang et al., 2018, and Cascomixticus tubuliferous Poinar and Vega, 2021 (Gaimari and Mostovski, 2000; Grimaldi et al., 2011; Grimaldi, 2016; Zhang et al., 2018; Poinar and Vega, 2021). Solórzano Kraemer and Cumming (2019) added the genus Kuhwahldyia as family incertae sedis, near Apsilocephlidae, and compared it with all known genera in Zhang et al., 2018. Noteworthy is the description of Cretothereva antiqua Carmo et al., 2021, the oldest Therevidae fossil. We will discuss these taxa and their taxonomic placements.
Cretothereva Carmo, Lamas and Ribeiro, 2021. C. antiqua is a beautifully preserved species from the Crato Formation (115 Ma), which was described as a Therevidae. The open cell m3 is a character state not found in the more basal Therevidae lineages Phycusinae and Xestomyzinae, but it is present in the Therevinae and Agapophytinae. The absence of a hypandrium, which is present and large in all Xestomyzinae and present in many Phycusinae, indicates that this genus is not part of these subfamilies. As Carmo et al. (2021) stated, it is not possible to place the genus in one of the recent four subfamilies of Therevinae, and it displays several characters which do not indicate a basal position in the Therevidae. We don’t know where this fossil should be placed, but it might not have a close relationship with Therevidae or even the therevoid clade.
Kuhwahldyia Solórzano Kraemer and Cumming, 2019. The head of Kuhwahldyia is much higher than broad in lateral view compared to the more spherical head shape of other Apsilocephalidae, a character shared only with Irwinimyia Zhang, Li, Wang and Yeates, 2017; the pterostigma is absent, as it is in Kumaromyia Grimaldi and Hauser, 2011; scape and pedicel are lacking setae as in Kumaromyia; the frons seem to be relatively broader than the rather narrow frons in most Apsilocephalidae; the last abdominal segment appears to be much smaller (more narrow in dorsal view and thinner in lateral view) than in other Apsilocephalidae; the one segmented female cercus in Kuhwahldyia is cylindrical and not flat and triangular as in the modern Apsilocephalidae genera. The illustration of the wing (figure 6 in Solórzano Kraemer and Cumming [2019]) has an additional crossvein in cell br, which is not visible in the photograph of the fossil, and the cell bm has an unusually pointed extension leading into vein M4, which is not present in the photograph of the wing. We agree that this fossil has a certain similarity with Apsilocephalidae, and that the best placement for the moment is in incertae sedis, near Apsilocephalidae.
Cascomixticus Poinar and Vega, 2021. The unique species C. tubuliferous differs from all known Apsilocephalidae by its long mouthparts. Although the wing venation resembles that of an apsilocephalid, it is also a rather plesiomorphic venation, which is similar even to families outside the Asiloidea clade. If the interpretation of the antennal structures is correct, the antenna has an unusually elongate pedicel. In the description of the antennae, the authors state that the flagellum is only two segmented, but this needs to be confirmed. The empodium is described as “spine-like”, which is different from the empodium of Apsilocephala, Evocoa and Therevidae, in which the unguitractor plate has only a modest anterior extension. We consider Cascomixticus to be insertae sedis within Asilomorpha, likely not close to the Asiloidea.
Burmapsilocephala Gaimari and Mostovski, 2000 and Myanmarpsilocephala Zhang, Li, Wang and Yeates, 2017. From the descriptions of Burmapsilocephala and Myanmarpsilocephala it is evident to us that these two genera belong in the family Evocoidae Yeates, Irwin and Wiegmann, 2006. Evocoidae is a monotypic family including Evocoa chilensis (Yeates, Irwin and Wiegmann, 2003), which is endemic to Chile (Yeates et al., 2003, 2006). This family is characterized by its long legs, a postpedicel with a bulbous base and a long threadlike terminal element, M3 lacking, tibial spurs absent, anterior surface of hind coxa with a strong, knoblike, bulbous projection; the epandrium divided into two sickle-like halves, gonostyli articulate in a horizontal plane, female with acanthophorite spines and hypoproct with ventrally projecting needlelike setae. Evocoa differs from Burmapsilocephala and Myanmarpsilocephala by having the cell cua open and M3 absent. These differences are discussed in Grimaldi (2016) under the genus Burmapsilocephala. Despite recognition of a close relationship between the three genera–as indicated by the species epithet evocoa–B. evocoa was placed in Apsilocephalidae and not Evocoidae. Burmapsilocephala evocoa was described after a male which has the typical sickle-like epandrium of Evocoa as well as the bulbous postpedicel with the long threadlike terminal. It differs by being holoptic, while Evocoa is dichoptic in both sexes. The genus itself was described by Gaimari and Mostovski (2000) based on a female specimen of Burmapsilocephala cockerelli and, although the structures at the end of the abdomen are not visible, the antenna and the wing venation is very similar to Burmapsilocephala evocoa. The two Burmese amber genera and Evocoa share vein M4 originating from the cell dm and not bm, as it is the case in all Apsilocephalidae. We consider this character more important than the open cua cell (and/or the reduction of M3). A convergent system of wing venation can be found in the Vermileonidae: M3 can be reduced (Vermileo Marquart, 1834) or present and cell m3 can be open (Isalomyia Stuckenberg, 2002a) or closed (Namaquamyia Stuckenberg, 2002b). The general habitus of Evocoa as a gracile, long-legged fly is strikingly similar to Burmapsilocephala and Myanmarpsilocephala. The configuration of the female abdominal tip with acanthophorite spines and long ventral setae in the holotype of Myanmarpsilocephala grimaldii Zhang et al., 2018 (figures 3C and D in Zhang et al., 2018) is nearly identical with Evocoa (figure 14 in Yeates et al., 2003). None of the other Apsilocephalidae have acanthophorite spines, except an undescribed fossil in Cretaceous amber from Wealden (UK) mentioned by Grimaldi et al. (2011) and depicted by Chandler (2010). Because of the great similarities between Evocoa, Burmapsilocephala and Myanmarpsilocephala we are transferring these two fossil genera from Apsilocephalidae to Evocoidae.
The undescribed Wealden-fossil has been associated with Apsilocephalidae (Grimaldi et al., 2011), and the wing venation is very indicative of this placement. However, it has short, thick acanthophorite spines and lacks a circlet of apical setae on the tibia, both characters that differ from modern Apsilocephalidae. While the antennal proportions are very similar to Kumaromyia, the tip of the abdomen of Kumaromyia was not discernible, and therefore it is not clear if there are acanthophorite spines present. We agree with Grimaldi et al. (2011) that “It is quite possible that Kumaromyia is a stem-group taxon for the therevid-family group, not necessarily belonging within Apsilocephalidae or Therevidae” a statement which might also be true for the undescribed Wealden amber fossil and even for Kuhwahldyia.
Irwinimyia Zhang, Li, Wang and Yeates, 2017. The genus Irwinimyia is a very interesting and unusual taxon, with its distinct spines on the hind femur and a two segmented palpus. The head shape and the crossvein r-m located in the middle of the discal cell are conditions also found in Kuhwahldyia. The scape is longer in Irwinimyia than in Kuhwahldyia, but the proportions of the three flagellomeres are similar, and both species seem to possess a very small bristle on the apex of the last flagellomere. This is not included in the description of Irwinimyia or in the drawing of the antenna which is wrongfully depicted with numerous short setae covering all flagellomeres (figure 6A in Zhang et al., 2018); the bristle is visible in the photograph of the head (figure 5B in Zhang et al., 2018). Cell m3 is closed and petiolate in Irwinimyia, while it is open in Kuhwahldyia. Solórzano Kraemer and Cumming (2019) gave an extensive comparison between the Kuhwahldyia and all other Apsilocephalidae genera.
Palaeoapsilocephala Hauser and Greenwalt, 2022. Two species, Psilocephala pusilla Hennig, 1967 described from Baltic amber, and Rueppellia vagabunda Cockerell, 1927 from the 34 Ma Florissant Formation in western North American, are Eocene in age (Cockerell, 1927; Hennig, 1967; Hauser and Irwin, 2005; Hauser, 2007). Both species have been placed in the genus Apsilocephala (Hauser and Irwin, 2005; Hauser, 2007). A number of morphological features characteristic of extant Apsilocephala are not shared in the fossil species assigned to this genus (e.g., antennal stylus very elongate). As a result, we conclude that the fossil species A. pusilla and A. vagabunda do not belong in Apsilocephala as the last flagellomere is much shorter, the femora are thicker and the epandrium is without articulated surstyli. Consequently, these two species, together with the new species described herein, are placed in the new genus Palaeoapsilocephala.
Family Dolichopodidae Agassiz, 1846
Subfamily Parathalassiinae Chvála, 1981
Genus Microphorella Becker, 1909
Microphorella fragilis Cumming and Greenwalt sp. n.
Figure 18-Figure 19
zoobank.org/4653A0BC-9E19-43B1-B7FE-D266140FC121
Type species. Microphorus praecox Loew, 1864a (original designation)
Holotype. Male, USNM 624117, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
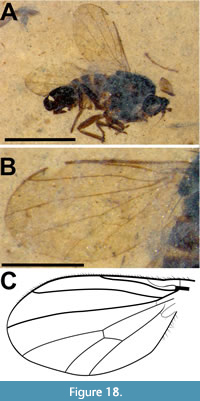 Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Dakin site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet is derived from the Latin fragilis (= fragile) referring to the delicate nature of the preservation of this fossil in thin oil shale.
Diagnosis. This species of Microphorella is the first described fossil in the genus. Within the Parathalassiinae, Microphorella is primarily characterized by a reduced anal area of the wing and complete wing venation with cell dm closed and vein M2 present, plus the lack of features (i.e., well-developed gena, greatly lengthened antennal stylus, enlarged palpus and double row of costal spine-like setae) that characterize other parathalassiine genera with this type of wing (Cumming and Brooks, 2019). Within this broad definition of the genus, Microphorella fragilis can be separated from other species of Microphorella by the combination of a broad wing with a short R1 vein and an abruptly tapered postpedicel apically.
Description (male). (Figure 18A). Head, thorax and genitalia black, abdomen and legs dark brown, body length 1.75 mm. Head 0.28 mm long, 0.37 mm high, subquadrate (possibly deformed) with large ocellar triangle; ocellar setae 67 μm in length; dichoptic, ommatrichia on eyes not discernible, frons and face broad; gena narrow. Antenna inserted just above middle of head, reddish brown (left antenna detached and inverted towards head), scape and pedicel not visible, flagellum 0.29 mm long, postpedicel 0.11 mm long, abruptly tapered apically, basal portion of postpedicel spherical, 55 μm in length, stylus with single article, 0.18 mm in length (1.6 times length of postpedicel). Palpus narrowly ovoid (Figure 19A, arrow), about 90 μm long, 30 μm wide, setose apically; proboscis short, projecting ventrally. Thorax 0.70 mm long, strong postpronotal seta apparently absent, various scutal setae and a single pair of long apical scutellar setae, 0.20 mm in length present (Figure 19B). Wing (Figure 18B-C) broad with anal lobe not developed, 1.24 mm long, 0.61 mm wide, covered with minute microtrichia, pterostigma absent. Costa circumambient, weaker posteriorly, with single row of short evenly spaced setae anteriorly; Sc faint apically; R1 short, reaching costa just before mid-length of wing (or before level of base of M2); base of Rs originating opposite humeral crossvein; R2+3 diverging from R4+5 apically; M1 diverging from R4+5 beyond cell dm; M1 and M2 strongly diverging beyond cell dm; M2 and M4 nearly parallel beyond cell dm; short crossvein r-m present; crossvein bm-m nearly complete basally; cell dm present and closed; cell cua closed, rounded apically with CuA recurved; CuA+CuP slightly extended beyond cell cua. Femur, tibia and tarsus of hind and mid-legs 0.40, 0.35, 0.33 mm and 0.30, 0.40 and 0.31 mm long, respectively. Hind trochanter without spine bearing tubercle; hind femur with several widely-spaced, long thin setae, 70 μm in length; hind tarsus with tarsomere 1 as long as combined length of tarsomeres 2-5. Abdomen slightly longer than thorax, stout, 1.0 mm long including terminalia. Terminalia large and globular, about half length of abdomen, asymmetrical, lateroflexed to right and inverted with posterior end directed anteriorly; in right lateral view with large hypandrium dorsally and probable right cercus posteroventrally (Figure 19C, arrow) with additional lobes anteroventrally.
present (Figure 19B). Wing (Figure 18B-C) broad with anal lobe not developed, 1.24 mm long, 0.61 mm wide, covered with minute microtrichia, pterostigma absent. Costa circumambient, weaker posteriorly, with single row of short evenly spaced setae anteriorly; Sc faint apically; R1 short, reaching costa just before mid-length of wing (or before level of base of M2); base of Rs originating opposite humeral crossvein; R2+3 diverging from R4+5 apically; M1 diverging from R4+5 beyond cell dm; M1 and M2 strongly diverging beyond cell dm; M2 and M4 nearly parallel beyond cell dm; short crossvein r-m present; crossvein bm-m nearly complete basally; cell dm present and closed; cell cua closed, rounded apically with CuA recurved; CuA+CuP slightly extended beyond cell cua. Femur, tibia and tarsus of hind and mid-legs 0.40, 0.35, 0.33 mm and 0.30, 0.40 and 0.31 mm long, respectively. Hind trochanter without spine bearing tubercle; hind femur with several widely-spaced, long thin setae, 70 μm in length; hind tarsus with tarsomere 1 as long as combined length of tarsomeres 2-5. Abdomen slightly longer than thorax, stout, 1.0 mm long including terminalia. Terminalia large and globular, about half length of abdomen, asymmetrical, lateroflexed to right and inverted with posterior end directed anteriorly; in right lateral view with large hypandrium dorsally and probable right cercus posteroventrally (Figure 19C, arrow) with additional lobes anteroventrally.
Female. Unknown.
Synimpressions. Dipteran pupae (5), Chironomidae (20), Hymenoptera (1), Chalcidoid (1), Diptera (1) and Notonectidae (1)
Remarks. The subfamily Parathalassiinae, along with the Microphorinae, is one of the basal subfamilies in the Dolichopodidae s.lat. (Sinclair and Cumming, 2006). Worldwide, the Parathalassiinae currently includes 51 described extant species in eight genera and approximately 45 additional undescribed species (Cumming and Brooks, 2019). The described genera are as follows: Amphithalassius Ulrich, 1991, Chimerothalassius Shamshev and Grootaert, 2002, Eothalassius Shamshev and Grootaert, 2005, Microphorella Becker, 1909, Neothalassius Brooks and Cumming, 2016, Parathalassius Mik, 1891, Plesiothalassius Ulrich, 1991 and Thalassophorus Saigusa, 1986. In addition, there are three fossil genera (Cumming and Brooks, 2019), namely Archichrysotus Negrobov, 1978, Cretomicrophorus Negrobov, 1978 and Retinitus Negrobov, 1978, from various Late Cretaceous ambers (Grimaldi and Cumming, 1999), and Electrophorella Cumming and Brooks, 2002 from Baltic amber.
As presently classified, Microphorella differs from the taxa listed above primarily by wing venation and wing shape. The genus possesses wing cell dm, which is closed by the base of M2 and crossvein dm-m and emits three veins, M1, M2 and M4. Cell cua is apically rounded with vein CuA recurved, and the shape of the wing is characterized by a reduced anal area (Cumming and Brooks, 2019). In addition, Microphorella has a narrow gena, antennal stylus that is not greatly lengthened, short palpus, single pair of apical scutellar setae and single row of costal spine-like setae, which are features that also separate it from other parathalassiine genera.
Microphorella fragilis is the first fossil of the genus Microphorella and the first Nearctic parathalassiine fossil from the Cenozoic. It can be distinguished from other species of the genus by the combination of a broad wing with short R1 vein and an abruptly tapered postpedicel apically. The affinities of M. fragilis within Microphorella are probably with the short R1 group as defined by Cumming and Brooks (2019), a species group currently known only from western North America and one that may eventually need elevation to generic level.
Family Hybotidae Macquart, 1827
Subfamily Bicellariinae Sinclair and Cumming, 2006
Genus Hoplocyrtoma Melander, 1928
Hoplocyrtoma eocenica Sinclair and Greenwalt sp. n.
Figure 20-Figure 21
zoobank.org/B9FBCA7E-96C1-4702-AD62-AFCFA2813778
Type species. Cyrtoma procera Loew, 1864b (original designation)
Holotype. Female, USNM 621518, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
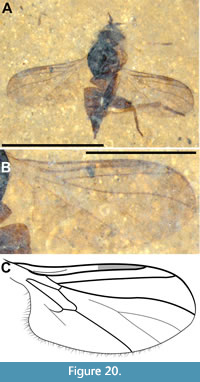 Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet refers to this species occurrence in the Eocene period.
Diagnosis. Hoplocyrtoma eocenica is identified as belonging to Hoplocyrtoma due to a combination of C ending at R4+5, M forked and evanescent - weakest at base, cell dm absent, CuA and cell cu a present, anal lobe well developed and hind femur enlarged, hind leg raptorial.
Description (female). Dorsolateral aspect (Figure 20A). Body length 2.15 mm, (left) wing shorter than body, 1.81 mm x 0.79 mm; ratio of wing length to body length = 0.84; head dark brown/black, thorax black, abdomen brown. Head oval, approximately 0.39 mm in diameter; palpus 3 times as long as broad, 0.12 mm long; pedicel suboval, approximately 50 μm long, with row of short setae at apical border; postpedicel conical, gradually tapered, 165 μm long, 60 μm maximum width; stylus 105 μm in length (Figure 21A). Thorax approximately 0.75 mm long, at least one pair of long bristles at apex of scutellum. Wing (right) 2.3 times long as wide, 1.81 mm long, 0.79 mm wide; covered with microtrichia; costa with short setae, terminates at R4+5, setae longer along the posterior margin of wing and along well-developed anal lobe; Sc light, close to and parallel to R1, fading away at proximal end of pterostigma; R1 ending in C ¾ of the length of wing, pterostigma anterior to and along the distal half of R1; C, R1, R2+3 and R4+5 more heavily sclerotized, than other longitudinal veins, though all weaker at base of wing; R2+3 straight, R4+5 gently sinuous, with both veins broadly divergent at wing margin. M nearly absent at mid-wing, forked into evanescent M1 and M2, both of which reach wing margin. Cross vein r-m origin just distal to R1 fork, cell br shorter than cell bm, cell bm longer than cell cua, cell dm absent; M4 reaching wing margin; CuA recurrent, stronger at base, reaching CuA+CuP; CuA+CuP very weak, possibly not reaching wing margin (Figure 20B-C). Fore tarsus and hind legs preserved. Hind leg raptorial, femur enlarged, length and width 0.79 and 0.20 mm, respectively; width to length ratio = 0.25. Hind femur darkly pigmented on dorsal surface, light brown elsewhere, with two interspersed rows of short stout spines and thinner, longer setae on ventral surface. Hind tibia geniculate at base, much shorter than corresponding femur, 0.59 mm long, 80 μm wide just below bend, 117 μm at apex; tarsus 0.59 mm long (Figure 21B). Abdomen excluding genitalia, 1.15 mm long, setae present along apical surface of at least tergite 2. Abdomen terminating in pair of elongate cerci; cercus thin, approximately 0.14 mm long (Figure 21C).
Male. Unknown.
Synimpressions. One Hymenoptera (Parasitica)
 Remarks. Given the age of the Empidoidea - fossils from the Jurassic are known and evidence suggests that extant families and subfamilies were well established by the Early Cretaceous (Grimaldi and Cumming, 1999; Grimaldi and Engel, 2005) - and the unique and readily recognizable morphology of members of Bicellariinae, the absence of this family from the fossil record up to this point is surprising. Hoplocyrtoma eocenica is the first known fossil of the subfamily, a monophyletic clade suggested by Sinclair and Cumming (2006) as Bicellariini, to be sister to Hybotinae. Wahlberg and Johanson (2018) elevated this lineage to subfamily rank and assigned it as sister to all other Hybotidae based on molecular phylogenetic analysis. The subfamily contains three genera, Bicellaria Maquart, 1823, Hoplocyrtoma and Leptocyrtoma Saigusa, 1986, all of which are characterized by an unique wing venation that includes cell dm absent and the branches of M evanescent, especially at their base. Both Bicellaria and Leptocyrtoma are defined, in part, by the absence of enlarged raptorial hind legs, a distinguishing feature of Hoplocyrtoma (Melander, 1928; Saigusa, 1986). Melander (1928) stated: “The hind femora are swollen and abundantly armed beneath with a mixture of spines and thorns. The hind tibiae are much shorter than the femora, not clavate but nearly straight and cylindrical, geniculate at the knee”.
Remarks. Given the age of the Empidoidea - fossils from the Jurassic are known and evidence suggests that extant families and subfamilies were well established by the Early Cretaceous (Grimaldi and Cumming, 1999; Grimaldi and Engel, 2005) - and the unique and readily recognizable morphology of members of Bicellariinae, the absence of this family from the fossil record up to this point is surprising. Hoplocyrtoma eocenica is the first known fossil of the subfamily, a monophyletic clade suggested by Sinclair and Cumming (2006) as Bicellariini, to be sister to Hybotinae. Wahlberg and Johanson (2018) elevated this lineage to subfamily rank and assigned it as sister to all other Hybotidae based on molecular phylogenetic analysis. The subfamily contains three genera, Bicellaria Maquart, 1823, Hoplocyrtoma and Leptocyrtoma Saigusa, 1986, all of which are characterized by an unique wing venation that includes cell dm absent and the branches of M evanescent, especially at their base. Both Bicellaria and Leptocyrtoma are defined, in part, by the absence of enlarged raptorial hind legs, a distinguishing feature of Hoplocyrtoma (Melander, 1928; Saigusa, 1986). Melander (1928) stated: “The hind femora are swollen and abundantly armed beneath with a mixture of spines and thorns. The hind tibiae are much shorter than the femora, not clavate but nearly straight and cylindrical, geniculate at the knee”.
Hoplocyrtoma eocenica is differentiated from the four extant species in the genus, H. japonica Saigusa and Kato, 2002, H. watanabei, Saigusa and Kato, 2002, H. procera Loew, 1864b and H. femorata Loew, 1864b, as follows: the former three species are characterized by having the postpedicel sharply attenuated apically, whereas the postpedicel in H. femorata and H. eocenica is gradually tapered. Hoplocyrtoma eocenica is much smaller than H. femorata, with a wing length of more than 4.0 mm in the latter species.
Family Opetiidae Rondani, 1856
Genus Opetia Meigen, 1830
Opetia americana Amorim and Greenwalt sp. n.
Figure 22, Figure 23, Figure 24, Figure 25
zoobank.org/4287685D-213B-4002-8F14-3EE44B6B8B06
Type species. Opetia nigra Meigen, 1830: 357 (= ? Opetia aberrans Shatalkin, 1985 = Opetia lonchopteroides Curtis, 1834), by subsequent monotypy.
Holotype. Female, USNM 621520, compression fossil, deposited in the Department of Paleobiology, National Museum of Natural History (NMNH), Smithsonian Institution, Washington, District of Columbia, USA.
Locality and horizon. Park site, Middle Fork of the Flathead River (Pinnacle, Montana, USA). Middle Eocene Coal Creek Member, Kishenehn Formation.
Etymology. The specific epithet refers to the species being the first record of the genus for North America.
Differential diagnosis. First flagellomere slender, not elongate oval. Sc reaching C at basal third of wing, tip of R1 reaching C well beyond base of medial fork, at distal third of wing. R1 curved towards anterior margin along basal third, distal end gradually approaching C. M1+2 beyond origin of r-m three fourths the length of medial fork. Female abdominal tergite 5 slightly more slender than tergite 4, tergites 6, 7 and terminalia slender.
Description. Female. Head blackish, dichoptic. Ocellar triangle not visible. Frons apparently with four well-developed fronto-orbital setae evenly spaced on each side; ocellars and postocellars not visible. Eyes apparently strongly holoptic, anterior end closely approximated, frons triangular from dorsal view (Figure 22B). Antenna inserted at tip of frons, proportion between scape and pedicel not clear, both much shorter than flagellomere 1; first flagellomere slender, not widening midway to apex, with scattered small setae on basal half, arista with two slender articles of about same length, texture similar to first flagellomere, covered with microtrichia, lacking setation (Figure 22B, Figure 23A). Thorax  with scutum dark brown, dorsocentrals not visible; setae along lateral margin of scutum present, but not distinguishable. Scutellum concolorous with scutum, apparently marginal scutellars present. Legs delicate, fore tibia and tarsi, mid femur and tibia, and hind femur, tibia and tarsi visible. Apparently tibial spurs absent on all legs, tarsomeres gradually shorter (Figure 23A). Wing length, 2.5 mm. Membrane light fumose brown, slightly darker along anterior margin, no maculae. Hu present, transverse, Sc complete, reaching C at basal third of wing; R1 long, gradually approaching C, reaching margin at distal third of wing; R2+3 long, reaching C close to wing tip, apparently devoid of dorsal macrotrichia, R5 ending at wing tip. Circumambient costal vein not verifiable. Transverse vein r-m discrete, at basal fourth of wing; M1+2 (including sector basal to r-m) about as long as medial fork; M4 thicker than other posterior veins. Cells bm and br small, closed, m-cu present, cell cua present, closed, CuA+CuP reaching wing margin (Figure 23B, Figure 24A). Abdominal tergites and sternites brown, segments 1-3 well developed, segment 4 slightly more slender at distal end, segment 5 slender. Segments 6-8 strongly modified to constitute well sclerotized, elongated ovipositor, single sclerotized line dorsally on terminalia (Figure 24B).
with scutum dark brown, dorsocentrals not visible; setae along lateral margin of scutum present, but not distinguishable. Scutellum concolorous with scutum, apparently marginal scutellars present. Legs delicate, fore tibia and tarsi, mid femur and tibia, and hind femur, tibia and tarsi visible. Apparently tibial spurs absent on all legs, tarsomeres gradually shorter (Figure 23A). Wing length, 2.5 mm. Membrane light fumose brown, slightly darker along anterior margin, no maculae. Hu present, transverse, Sc complete, reaching C at basal third of wing; R1 long, gradually approaching C, reaching margin at distal third of wing; R2+3 long, reaching C close to wing tip, apparently devoid of dorsal macrotrichia, R5 ending at wing tip. Circumambient costal vein not verifiable. Transverse vein r-m discrete, at basal fourth of wing; M1+2 (including sector basal to r-m) about as long as medial fork; M4 thicker than other posterior veins. Cells bm and br small, closed, m-cu present, cell cua present, closed, CuA+CuP reaching wing margin (Figure 23B, Figure 24A). Abdominal tergites and sternites brown, segments 1-3 well developed, segment 4 slightly more slender at distal end, segment 5 slender. Segments 6-8 strongly modified to constitute well sclerotized, elongated ovipositor, single sclerotized line dorsally on terminalia (Figure 24B).
Male. Unknown.
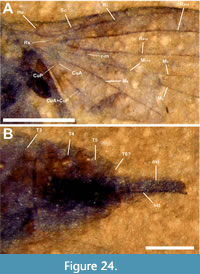 Synimpressions. One Hymenoptera (Ichneumonoidea).
Synimpressions. One Hymenoptera (Ichneumonoidea).
Remarks. The Opetiidae are a small family of Platypezoidea that includes Opetia Meigen, 1830–known from the northern hemisphere, with one species from Europe and two species from east Asia (see Chandler, 2001)–and Puyehuemyia Amorim, Silva and Brown, 2018, known in the southern hemisphere only from southern Chile (Amorim, Silva and Brown, 2018).
Most of the Mesozoic fossils originally included in the Opetiidae–Palaeopetia Zhang, 2018, Pseudopetia Zhang et al., 2018, Mesopetia Zhang et al., 2018, Lithopetia Zhang et al., 2018 and Sinolesta Hong and Wang, 1974–do not fit into the family (Chandler, 2001). The Cretaceous New Jersey amber fossil Electrosania Grimaldi and Cumming, 1999 was assigned by Grimaldi and Cumming (1999) to the Platypezidae, a position with which Chandler (2001) agreed, mentioning that it “has a thoracic chaetotaxy and aristal structure as in Opetiidae”. Coram et al. (2000) described Opetiala from Early Cretaceous Purbeck Limestone, but the fossil does not belong either to the crown Opetiidae or to its stem (Amorim et al., 2018). Grimaldi and Cumming (1999) discussed the similarities between the Neocomian early Cretaceous Lebanon amber Lonchopterites prisca Grimaldi and Cumming, 1999 and Opetia. Some features of the fossil suggest, according to Amorim et al. (2018), that it could be a stem Opetiidae. Grimaldi (2018) has provided an extensive review of platypezoid fossils, with clear stem and crown Platypezidae, stem Ironomyiidae, stem Lonchopteridae, and stem and crown Phoridae.
Information on Cenozoic fossil crown opetiids is extremely limited. Meunier (1893) described Oppenhemiella baltica Meunier, 1893 and carefully compared his Baltic amber fossil with Calomyia Röser, 1840 and Opetia, but illustrated only the tip of the hind tibia and hind tarsus. Hennig (1964) mentioned that the type is lost and questioned Meunier’s assumption of proximity of Oppenhemiella Meunier, 1893 to the platypezids. Meunier’s comments on the antennal structure of Oppenhemiella apply either to a platypezid or to an opetiid. With the type lost, it will not be possible to solve this riddle.
Opetia atra Statz, 1940 from the Oligocene Rott locality, was largely overlooked in the literature although it was listed by Evenhuis (1994) in Platypezidae. We hardly have any question that his illustrated specimen is a male Opetia, but the holotype should be examined for a careful understanding of the morphology. The fossil is not particularly well preserved and the antennae cannot be observed. The hind tarsi do not suggest a platypezid. There is a rather long medial fork and no dm-cu crossvein connecting M1+2 to M4. R2+3 is shorter and curved towards the anterior margin, differing from both extant genera of Opetiidae, but the wing cell cua is shorter than in most platypezids (although slightly longer than in O. nigra and Puyehuemyia) and the crossveins have position and size similar to that of opetiids (Statz,1940, figure 26). There is an additional reference to an Oligocene fossil of Opetia in the Rott Statz Collection from Germany (http://paleobiodb.org).
In this paper, we describe a fly from the middle Eocene Kishenehn Formation of Montana, that belongs to the extant genus Opetia, the first record of the genus for North America and the first definite Cenozoic fossil record of the genus. We here formally describe and illustrate this species and discuss its implications for the understanding of the evolution of the family. The fossil is very well preserved, with the exception of the unexpectedly triangular shape of the head, the antennae placed at its tip. The most reasonable explanation for this unique head shape seems to be that the eyes collapsed and were not preserved, only the frons being seen, giving a general triangular shape to the head.
 There are quite clear differences between Opetia americana and O. nigra, in such a way that a diagnosis can be made, especially in the wing (Figure 25A-C). In the Eocene Kishenehn species, Sc and R1 are longer than in O. nigra, with the tip of these veins reaching C more distally. The ratio M1+2 /medial fork, on the other hand, is smaller in O. americana than in O. nigra. Interestingly, the conditions of these features in O. americana is more similar to the condition seen in Puyehuemyia. Apparently R2+3 does not bear a row of dorsal setae. The basal third of the sclerotized ovipositor in O. nigra has a constriction, but this feature cannot be verified in the holotype of O. americana, since the anterior half of the ovipositor is inside the distal segments of the abdomen.
There are quite clear differences between Opetia americana and O. nigra, in such a way that a diagnosis can be made, especially in the wing (Figure 25A-C). In the Eocene Kishenehn species, Sc and R1 are longer than in O. nigra, with the tip of these veins reaching C more distally. The ratio M1+2 /medial fork, on the other hand, is smaller in O. americana than in O. nigra. Interestingly, the conditions of these features in O. americana is more similar to the condition seen in Puyehuemyia. Apparently R2+3 does not bear a row of dorsal setae. The basal third of the sclerotized ovipositor in O. nigra has a constriction, but this feature cannot be verified in the holotype of O. americana, since the anterior half of the ovipositor is inside the distal segments of the abdomen.
The extensive study of Chandler (2001) on Opetia and Amorim et al.’s (2018) discussion on the southern Neotropical representative member of the family helps in the analysis of the fossil specimen of Opetia americana. The specimen is a female and shares the absence of a well-developed anal lobe and the shape of the ovipositor. One striking difference between Opetia and Puyehuemyia is the number of articles in the arista. Opetia has typically two segments, while Puyehuemyia has three, a plesiomorphic condition in the Cyclorrhapha. The fossil has two segments and is assigned to the extant species of Opetia.
Opetia has only three known extant species, including Opetia nigra from Europe, and two species from Japan and Taiwan. Opetia americana obviously differs from O. nigra in details of the wing venation. Indeed, there seems to be a gradual displacement of the tip of veins Sc, R1 and R2+3 to a more basal position along the anterior margin, from the condition seen in Puyehuemyia to the condition seen in Opetia americana and O. nigra.
Amorim et al. (2018) discussed the question of the age of the Opetiidae. The estimated divergence age for the Cyclorrhapha (Wiegmann et al., 2011), about 150 mya, is compatible with the Neocomian age of the Lebanon amber fossil Lonchopterites (Grimaldi and Cumming, 1999) and a disjunction with the southern temperate Chilean opetiid genus Puyehuemyia. This suggests a possible Jurassic origin for the stem Opetia, and the Opetia americana Kishenehn fossil would be a much later divergence in the genus. The presence of an extinct Eocene Nearctic species of an extant genus also known from the Palaearctic Region is not new. A fossil species of the canthyloscelid genus Synneuron Lundström, 1910 that also has Palaearctic representatives, was recently described, also from the Eocene Kishenehn (Amorim and Greenwalt, 2020). Another example is the genus Reissa, extant species of which were originally described from the Canary Islands by Greathead and Evenhuis (2001). The Eocene Reissa kohlsi was described from the Green River Formation in western North America (Evenhuis, 2019). The second half of the Cenozoic may have witnessed the loss of an important number of Holarctic elements in North America.
ACKNOWLEDGEMENTS
The authors wish to thank G. Hundertmark and A. Gehler from the Geoscience Museum of the University of Göttingen for photography of D. magnas. We thank S. Gaimari and an anonymous reviewer, whose careful reviews and constructive criticisms significantly improved the manuscript. DSA acknowledges financial support from CNPq (grant #440549/2015-9) and FAPESP (grants #2016/50369-9 and #2021/08713-2). DEG wishes to thank C. Labandeira for his continued support. We particularly wish to thank those individuals and organizations that produce and support the Biodiversity Heritage Library as well as the online databases of the PBDB, Bishop Museum, Florissant National Monument and Systema Dipterorum.
REFERENCES
Agassiz, L. 1846. Nomenclatoris zoologici index universalis, continens nomina systematica classium, ordinum, familiarum et generum animalium omnium, tam viventium quam fossilium, secundum ordinem alphabeticum unicum disposita, adjectis homonymiis plantarum, nec non variis adnotationibus et emendationibus. Fasc. XII. Jent and Gassmann, Solothurn, Switzerland.
Amorim, D.S. 1982. Sistemática filogenética dos Scatopsidae (Diptera: Oligoneura: Bibionomorpha). Unpublished PhD Thesis, Universidade de Sao Paulo, Sao Paulo.
Amorim, D.S. 1994. A new suprageneric classification of the Scatopsidae (Diptera: Psychodomorpha). Iheringia, Zoologia, 77:107-112.
Amorim, D.S. and Brown, B.V. 2020. Urban Scatopsidae (Diptera) of Los Angeles, California, USA. Insects Systematics and Diversity, 4(1):1-41. https://doi.org/10.1093/isd/ixaa001
Amorim, D.S. and Greenwalt, D.E. 2020. Cretaceous and Eocene fossils of the rare extant genus Synneuron Lundstrom (Diptera: Canthyloscledidae): evidence of a true Pangean clade. Cladistics, 36(4):413-423. https://doi.org/10.1111/cla.12413
Amorim, D.S, Oliveira, S.S., and Balbi, M.I.P.A. 2008a. First Neotropical species of genus Azana (Diptera: Mycetophilidae: Sciophilinae). Zootaxa, 1937:67-68. https://doi.org/10.11646/zootaxa.1937.1.5
Amorim, D.S., Oliveira, S.S., and Balbi, M.I.P.A. 2008b. Azana atlantica, n. sp., with reduced mouthparts and two ocelli: first record of Azana for the Neotropical region (Diptera: Mycetophilidae: Sciophilinae). Zootaxa, 1789(1):57-65. https://doi.org/10.3958/059.042.0113
Amorim, D.S., Silva, V.C., and Brown, B.V. 2018. Puyehuemyia chandleri, gen. n., sp. n. (Diptera: Opetiidae): remnant of a Cretaceous biota in Chile. American Museum Novitates, 3892:1-27. https://doi.org/10.1206/3892.1
Archibald, S.B., Rasnitsyn, A.P., Brothers, D.J., and Mathewes, R.W. 2018. Modernisation of the Hymenoptera: ants, bees, wasps, and sawflies of the early Eocene Okanagan Highlands of western North America. The Canadian Entomologist, 150(2):205-257. https://doi.org/10.4039/tce.2017.59
Armbruster, L. 1938. Versteinerte Honigbienen aus dem obermiocänen Randecker Maar. Archiv für Bienenkunde, 19:97-133.
Baker, C.F. 1904. Diptera. Reports on Californian and Nevadan Diptera, I. Invertebrata Pacifica, 1:17-39.
Baranov, V., Haug, J., Harbach, R., and Greenwalt, D. 2022. Diversity of culicomorphan dipterans in the Eocene Kishenehn Konservat-Lagerstätte (Montana, USA) and its palaeoecological implications. Palaeontologia Electronica, 25(1):a04. https://doi.org/10.26879/1165
Becker, T. 1909. Microphorus Macq. und seine nächsten Verwandten (Diptera). Wiener Entomologische Zeitung, 28:25-28.
Becker, T. 1913. Genera Bombyliidarum. Ezhegodnik Zoologicheskago Museya Imperatorskoi Akademiia Nauk, St. Petersburg 17:421-502.
Blagoderov, V. and Grimaldi, D. 2004. Fossil Sciaroidea (Diptera) in Cretaceous ambers, exclusive of Cecidomyiidae, Sciaridae, and Keroplatidae. American Museum Novitates, 3433:1-76. http://hdl.handle.net/2246/2798
Blagoderov, V. and Ševčík, J. 2017. 18. Keroplatidae (Predaceous Fungus Gnats), p. 505-525. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.), Manual of Afrotropical Diptera. Volume 2. Nematocerous Diptera and lower Brachycera. Suricata 5. South African National Biodiversity Institute, Pretoria.
Brooks, S.E. and Cumming, J.M. 2016. Neothalassius, a new genus of Parathalassiinae (Diptera: Dolichopodidae s.lat.) from the Pacific coast of South America. Zootaxa, 4066 (3):311-322. https://doi.org/10.11646/zootaxa.4066.3.7
Brunetti, E. 1911. New Oriental Nemocera. Records of the Indian Museum, 4:259-316.
Burmeister, H.C.C. 1835. Bericht über die Fortschritte der Entomologie 1834-35. Archiv für Naturgeschichte, 1(2):7-74.
Carmo, D.D., Lamas, C.J.E., and Ribeiro, G.C. 2021. The oldest fossil Stiletto fly: a new genus and species from the Lower Cretaceous Crato Formation of Brazil (Diptera: Therevidae). Cretaceous Research, 130:105039. https://doi.org/10.1016/j.cretres.2021.105039
Chandler, P.J. 2001. The flat-footed flies (Diptera: Opetiidae and Platypezidae) of Europe. Fauna Entomologica Scandinavica, volume 36. Brill, Leiden.
Chandler, P.J. 2010. A Dipterist’s Handbook, 2nd Edition. The Amateur Entomologist, volume 15. The Amateur Entomologist’s Society, Kent, UK.
Chvála, M. 1981. Classification and phylogeny of Empididae, with a presumed origin of Dolichopodidae (Diptera). Entomologica Scandinavica, 15 (supplement): 225-236.
Cockerell, T.D.A. 1914. The fossil and recent Bombyliidae compared. Bulletin of the American Museum of Natural History, 33:229-236.
Cockerell, T.D.A. 1917. Insects in Burmese amber. Annals of the Entomological Society of America, 10:323-329.
Cockerell, T.D.A. 1925. The Eocene fossil fly Eophlebomyia. Psyche, 32:229-230.
Cockerell, T.D.A. 1927. Fossil insects from the Miocene of Colorado. The Annals and Magazine of Natural History, 9:161-166.
Coher, E.I. 1995. A contribution to a revision of the genus Azana Walker, 1856 (Insecta: Diptera: Mycetophilidae: Sciophilinae). Reichenbachia, 31:83-91.
Collomb, F.M., Nel, A., Fleck, G., and Waller, A. 2008. March flies and European Cenozoic palaeoclimates (Diptera: Bibionidae). Annales de la Société Entomologique de France, 44(2):161-179. https://doi.org/10.1080/00379271.2008.10697553
Constenius, K.N. 1996. Late Paleogene extensional collapse of the Cordilleran foreland fold and thrust belt. Geological Society of America Bulletin, 108:20-39. https://doi.org/10.1130/0016-7606(1996)108<0020:lpecot>2.3.CO;2
Constenius, K.N., Dawson, M.R., Pierce, H.G., Walter, R.C., and Wilson, M.V.H. 1989. Reconnaissance paleontologic study of the Kishenehn Formation, northwestern Montana and southeastern British Columbia. Montana Geological Society 1989 Field Conference, Montana Centennial. Geological Resources of Montana, 1:189-203.
Cook, E.F. 1958. A contribution toward a monograph of family Scatopsidae (Diptera). Part VII. The genus Psectrosciara Kieffer. Annals of the Entomological Society of America, 51:587-595.
Cook, E.F. 1963. Scatopsidae and Hyperoscelidae. p. 1-37. In Guide to the Insects of Connecticut. Part VI. The Diptera or true flies of Connecticut. 8 fascicle. Bulletin of the Conneticut State Geological and Natural History Survey, 93.
Cook, E.F. 1981. Scatopsidae, p. 313-319. In McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., and Wood, D.M. (eds.), Manual of Nearctic Diptera, 1, Biosystematic Research Institute, Ottawa, Ontario.
Coquillett, D.W. 1900. New Scenopinidae from the United States. Entomological News, 11:500-501.
Coram, R., Jarzembowski, E.A., and Mostovski, M.B. 2000. Two rare eremoneuran flies (Diptera: Empididae and Opetiidae) from the Purbeck Limestone group. Paleontological Journal (supplement), 34:370-373.
Cumming, J.M. and Brooks, S.E. 2002. Electrophorella, a new genus of parathalassiine flies from Baltic amber, with a cladistic analysis of the Microphorinae + Dolichopodidae lineage (Diptera: Empidoidea). Studia dipterologica, 9:41-54.
Cumming, J.M. and Wood, D.M. 2017. 3. Adult morphology and terminology, p. 89-133. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.), Manual of Afrotropical Diptera, vol. 1. Introductory chapters and keys to Diptera families, Suricata 4, SANBI, Pretoria.
Cumming, J.M. and Brooks, S.E. 2019. Phylogenetic analysis and preliminary classification of the Parathalassiinae (Diptera: Empidoidea: Dolichopodidae sensu lato). Zootaxa, 4648(1):111-129. https://doi.org/10.11646/zootaxa.4648.1.5
Curtis, J. 1834. British entomology; being illustrations and descriptions of the genera of insects found in Great Britain and Ireland: containing coloured figures from nature of the most rare and beautiful species, and in many instances of the plants upon which they are found. 8: Diptera. Omaloptera. London.
Dürrenfeldt, A. 1968. Dipteren aus dem Oberpliozän von Willershausen. Beihefte zu den Berichten der Naturhistorischen Gesellschaft zu Hannover, 6:43-81.
Edwards, F.W. 1925. Mycetophilidae and Bibionidae (Diptera) in the Collections of the South African Museum. Annals of the South African Museum, 19:601-616.
Edwards, F.W. 1935. New Neotropical Bibionnae (Diptera). Stylops, 4:19-24.
Enderlein, G. 1912. Zur Kenntnis der Zygophthalmen. Über die Gruppierung der Sciariden und Scatopsiden. Zoologischer Anzeiger, 40:261-282.
Evenhuis, N.L. 1986. The Genera of the Phthiriinae of Australia and the New World:(Diptera: Bombyliidae). Privately published, Honolulu.
Evenhuis, N.L. 1990. Systematics and Evolution of the Genera in the Subfamilies Usiinae and Phthiriinae-Diptera-Bombyliidae-of the World (Vol. 3). Brill Archive.
Evenhuis N.L. 1994. Catalogue of the Fossil Flies of the World (Insecta: Diptera). Backhuys Publishers, Leiden.
Evenhuis, N.L. 2006. Catalog of the Keroplatidae of the world (Insecta: Diptera). Bishop Museum Bulletin in Entomology, 13:1-178.
Evenhuis, N.L. 2017. Catalog of the fossil flies of the world (Insecta: Diptera) website. http://hbs.bishopmuseum.org/fossilcat
Evenhuis, N.L. 2019. First fossil record of the genus Reissa Evenhuis & Báez (Diptera: Mythicomyiidae: Mythicomyiinae) from the Eocene Green River Formation of North America, and discussion of biogeographical implications. Palaeoentomology, 2(3):223-228. https://doi.org/10.11646/palaeoentomology.2.3.5
Evenhuis, N.L. and Greathead, D.J. 2015. World catalog of bee flies (Diptera: Bombyliidae) web site. http://hbs.bishopmuseum.org/bombcat
Evenhuis, N.L. and Pape, T. 2021. Systema Dipterorum. Version 3.5, 1 December 2021 (Last accessed 15 January 2022). http://diptera.org
Fate, C., Perrichot, V., and Nel, A. 2013. A Mid Cretaceous representative of the modern scatopsid genus Ectaetia (Diptera: Scatopsidae: Ectaetiinae). Zootaxa, 3686(3):396-400. https://doi.org/10.11646/zootaxa.3686.3.9
Fleming, J. 1821. Insecta. vol. 5, Pt. 1, p. 41-56. In Stewart, D., Playfair, J., and Brande, W.T. (eds.), Supplement to the fourth, fifth and sixth editions of the Encyclopedia Britannica. A. Constable and Co., Edinburgh.
Gaimari, S.D. and Mostovski, M.B. 2000. Burmapsilocephila cockerelli, a new genus and species of Asiloidea (Diptera) from Burmese amber. Bulletin of the Natural History Museum (Geology), 56:43-46.
Garrouste, R., Azar, D., and Nel, A. 2016. The oldest accurate record of Scenopinidae in the Lowermost Eocene amber of France (Diptera: Brachycera). Zootaxa, 4093(3):444-450. https://doi.org/10.11646/zootaxa.4093.3.10
GBIF.org (14 November 2021) GBIF Occurrence Download https://www.gbif.org/species/1590478
Geoffroy, E.L. 1762. Histoire abrégée des insectes qui se trouvent aux environs de Paris; dans laquelle ces animaux sont rangés suivant un ordre méthodique. Tome second. Durand, Paris. 690 pp.
Greathead, D.J. and Evenhuis, N.L., 2001. Annotated keys to the genera of African Bombylioidea (Diptera: Bombyliidae; Mythicomyiidae). African Invertebrates, 42:105-224. https://doi.org/10.11646/zootaxa.3745.2.3
Greenwalt, D., Marsh, F., and Labandeira, C. 2011. Preliminary Characterization of the Entomofauna of the Middle Eocene Kishenehn Basin. GSA Joint Rocky Mountain Cordilleran Sections meeting.
Greenwalt, D.E., Rose, T.R., Siljeström, S.M., Goreva, Y.S., Constenius, K.N., and Wingerath, J.G. 2015. Taphonomy of the fossil insects of the middle Eocene Kishenehn Formation. Acta Palaeontologica Polonica, 60(4):931-947.
https://doi.org/10.4202/app.00071.2014
Greenwalt, D.E., Bickel, D.J., Kerr, P.H., Curler, G.R., Brown, B., De Jong, H., Fitzgerald, S.J., Dikow, T., Tkoč, M., Kehlmaier, C., and Amorim, D.S. 2019. Diptera of the middle Eocene Kishenehn formation. I. Documentation of diversity at the family level. Palaeontologia Electronica, 22.2.50:1-56. https://doi.org/10.26879/891
Grimaldi, D.A. 2016. Diverse orthorrhaphan flies (Insecta: Diptera: Brachycera) in amber from the Cretaceous of Myanmar: Brachycera in Cretaceous amber, part VII. Bulletin of the American Museum of Natural History, 408:1-131. https://doi.org/10.1206/0003-0090-408.1.1
Grimaldi, D.A. 2018. Basal Cyclorrhapha in amber from the Cretaceous and Tertiary (Insecta, Diptera), and their relationships. Bulletin of the American Museum of Natural History, 423:1-97. https://doi.org/10.1206/0003-0090-423.1.1
Grimaldi, D.A. and Cumming, J.M. 1999. Brachyceran Diptera in Cretaceous ambers and Mesozoic diversification of the Eremoneura. Bulletin of the American Museum of Natural History, 239:1-124. http://hdl.handle.net/2246/1583
Grimaldi, D.A. and Engel, M.S. 2005. Evolution of the Insects. Cambridge University Press, Cambridge.
Grimaldi, D.A., Arillo, A., Cumming, J.M., and Hauser, M. 2011. Brachyceran Diptera (Insecta) in Cretaceous ambers. Part IV, Significant new Orthorrhaphous taxa. ZooKeys, 148:293-332. https://doi.org/10.3897/zookeys.148.1809
Guérin, F.E. 1826. Macrocère. In Dictionnaire classique d’histoire naturelle. Volume 10, In Bory de Saint-Vincent, J.B.B.M. (ed.), Rey and Gravier and Baudouin Frères, Paris.
Haenni, J.-P. and Amorim, D.S. 2017. Chapter 26. Scatopsidae. Minute black scavenger flies or dung midges, p. 641-652. In Kirk-Spriggs, A.H., Sinclair, B.J., and Muller, B.S. (eds.), Manual of Afrotropical Diptera. Vol. 2. Nematocerous Diptera and Lower Brachycera. Suricata 5. South African National Biodiversity Institute, Pretoria.
Haenni, J.-P. and Baez, M. 2001. The Madeiran species of Dilophus Meigen (Diptera, Bibionidae. Bulletin de la Societe Entomologique Suisse, 74:85-90.
Haenni, J.-P. and Bosák, J. 2007. An unusual new species of Dilophus (Diptera, Bibionidae) from Afghanistan. Mitteilungen-Schweizerische Entomologische Gesellschaft, 80(3/4):185.
Harbach, R.E. and Greenwalt, D. 2012. Two Eocene species of Culiseta (Diptera: Culicidae) from the Kishenehn Formation in Montana. Zootaxa, 3530(1):25-34. https://doi.org/10.11646/zootaxa.3530.1.2
Harding, I.C. and Chant, L.S. 2000. Self-sedimented diatom mats as agents of exceptional fossil preservation in the Oligocene Florissant lake beds, Colorado, United States. Geology, 28:195-198. https://doi.org/10.1130/0091-7613(2000)28<195:SDMAAO>2.0.CO;2
Hardy, D.E. 1937. New Bibionidae (Diptera) from nearctic America. Utah Academy of Sciences, Arts and Letters, Proceedings, 14:199-213.
Hardy, D.E. 1944. A revision of North American Omphralidae (Scenopinidae). Journal of the Kansas Entomological Society, 17:31-40.
Hardy, D.E. 1945. Revision of Nearctic Bibionidae including Neotropical Plecia and Penthetria (Diptera). University of Kansas, Science Bulletin, 30:367-547.
Hardy, D.E. 1950. A monographic study of the African Bibionidae I. Introduction and genus Bibio Geoffroy. Journal of the Kansas Entomological Society, 23(4):137-153.
Hardy, D.E. 1953. The Argentine Bibionidae. Acta Zoologica Lilloana, 12:343-376.
Hardy, D.E. 1965. Family Bibionidae, p. 191-196. In Stone, A., Sabrosky, C.W., Wirth, W.W., Foote, R.H., and Coulson, J.R. (eds.), A catalog of the Diptera of America North of Mexico. Smithsonian Institution Press, Washington, D.C.
Harris, A.C. 1983. An Eocene larval insect fossil (Diptera: Bibionidae) from North Otago, New Zealand. Journal of the Royal Society of New Zealand, 13:93-105.
Hauser, M. 2007. Baltic amber Therevidae and Apsilocephalidae (Diptera). Studia Dipterologica, 14(2):37-59.
Hauser, M. and M.E. Irwin. 2005. Fossil Therevidae (Insecta: Diptera) from Florissant, Colorado (Upper Eocene). Journal of Systematic Palaeontology, 3(4):393-401. https://doi.org/10.1017/S1477201905001690
Hebert, P.D., Ratnasingham, S., Zakharov, E.V., Telfer, A.C., Levesque-Beaudin, V., Milton, M.A., Pedersen, S., Jannetta, P., and DeWaard, J.R. 2016. Counting animal species with DNA barcodes: Canadian insects. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1702):20150333. https://doi.org/10.1098/rstb.2015.0333
Heer, O. 1849. Die Insektenfauna der Tertiärgebilde von Oeningen und von Radoboj in Croatien. Zweiter Theil: Heuschrecken, Florfliegen, Aderflügler, Schmetterlinge und Fliegen. W. Engelmann, Leipzig.
Hennig, W. 1964. Die Dipteren-Familie Sciadoceridae im Baltischen Bernstein (Diptera: Cyclorrhapha Aschiza). Stuttgarter Beiträge zur Naturkunde, 127:1-10.
Hennig, W. 1967. Therevidae aus dem baltischen Bernstein mit einigen Bemerkungen über Asilidae und Bombyliidae (Diptera, Brachycera). Stuttgarter Beiträge zur Naturkunde, 176:1-14.
Heyden, L.F.J.D. 1870. Fossile Dipteren aus der Braunkohle von Rott dem Tertiar-Thon von Nieder-Florscheim in Rhein-Hessen. Palaeontographica, 17:237-266.
Hong, Y-C., Yang, Z-q., Wang, S-t., Wang, S-e., Li, Y., Sun, M-r., Sun, X-j., and Du, N-q. 1974. Stratigraphy and palaeontology of Fushun coal-field, Liaoning Province. Acta Geologica Sinica, 48:13-150.
Huber, J.T. and Greenwalt, D. 2011. Compression fossil Mymaridae (Hymenoptera) from Kishenehn oil shales, with description of two new genera and review of Tertiary amber genera. ZooKeys, 130:473-494. https://doi.org/10.3897/zookeys.130.1717
Jaennicke, F. 1867. Neue exotische Dipteren. Abhandlungen der Senckenbergische Naturforschenden Gesellschaft, 6:311-407.
Kelsey, L.P. 1969. A revision of the Scenopinidae (Diptera) of the World. Bulletin of the United States National Museum, 277:1-336.
Kelsey, L.P. 1970. The Scenopinidae (Diptera) of Australia: including the description of one new genus and six new species. Journal of the Australian Entomological Society, 9:103-148.
Kelsey, L.P. 1971. A new scenopinid genus with three new species from Chile. The Pan-Pacific Entomologist, 47:279-284.
Kerr, P.H., 2010. New Azana species from Western North America (Diptera: Mycetophilidae). Zootaxa, 2397(1):1-14. https://doi.org/10.11646/zootaxa.2397.1.1
Kieffer, J.J. 1911. In Enderlein, G. (ed.), Die phyletischen Beziehungen der Lycoriiden (Sciariden) zu den Fungivoriden (Mycetophiliden) und Itonididen (Cecidomyiiden) und ihre systematische Gliederung. Archiv für Naturgeschichte, 77:116-201.
Kröber, O. 1914. Beitrage zur Kenntnis der Thereviden und Omphraliden. Mitteilungen aus dem Naturhistorischen Museum in Hamburg, 31(2):29-74.
Kröber, O. 1937. Ein Beitrag zur Kenntnis der Omphraliden (Scenopiniden), Diptera. Stettiner Entomologische Zeitung, 98:211-231.
Krzemiński, W. and Krzemińska, E. 2003. Triassic Diptera: descriptions, revisions and phylogenetic relations. Acta zoologica cracoviensia, 46 (Supplement-Fossil Insects):153-184.
Krzemiński, W., Blagoderov, V., Dany, A.Z.A.R., Lukashevich, E., Szadziewski, R., Wedmann, S., André, N.E.L., Collomb, F.M., Waller, A., and Nicholson, D.B. 2019. True flies (Insecta: Diptera) from the late Eocene insect limestone (Bembridge Marls) of the Isle of Wight, England, UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 110(3-4):495-554. https://doi.org/10.1017/S1755691018000464
Lane, J. and Forattini, O.P. 1948. Duas espécies novas de Bibionellus Edwards, 1935 (Diptera, Bibionidae). Revista brasileira de Entomologia, Sāo Paulo, 19(3):569-574.
Latreille, P.A. 1802. Histoire naturelle, generale et particuliere, des crustaces et des insectes. Vol. 3. Dufart, Paris.
Latreille, P.A. 1810. Considérations générales sur l'ordre naturel des animaux composant les classes des crustacés, des arachnides, et des insectes; avec un tableau méthodique de leurs genres, disposés en familles. F. Schoell, Paris.
Lewis, S.E. 1975. A new species of fossil bombyliid (Diptera: Bombyliidae) from the Ruby River basin (Oligocene) of Southwestern Montana. Journal of Paleontology, 49:422-429.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum caracteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. L. Salvii, Holmiae.
Loew, H. 1850. Ueber den Bernstein und die Bernsteinfauna. Programm der Königlichen Realschule zu Meseritz, 1850:1-44.
Loew, H. 1864a. Ueber die schlesischen Arten der Gattungen Tachypeza Meig. (Tachypeza, Tachista, Dysaletria) und Microphorus Macq. (Trichina und Microphorus). Zeitschrift für Entomologie, 14(1860):1-50.
Loew, H. 1864b. Diptera Americae septentrionalis indigena. Centuria quinta. Berliner Entomologische Zeitschrift, 8:49-104.
Loew, H. 1869. Diptera Americae septentrionalis indigena. Centuria nona. Berliner Entomologische Zeitschrift, 13:129-186.
Loew, H. 1872. Diptera Americae septentrionalis indigena. Centuria decima. Berliner Entomologische Zeitschrift, 16:49-124.
Lundström, C. 1910. Beiträge zur Kenntnis der Dipteren Finlands. V. Bibionidae. Acta Societatis pro Fauna et Flora Fennica, 33(1):4-15.
Lundström, C. 1912. Beiträge zur Kenntnis der Dipteren Finlands. VIII. Supplement 2. Mycetophilidae, Tipulidae, Cylindrotomidae und Limnobiidae. Acta Societatis pro Fauna et Flora Fennica, 36(1):1-70.
Macquart, P.J.M. 1823. Monographie des insectes dipteres de la famille des empides, observes dans le nord-ouest de la France. Recueil des travaux de la Société (d'Amateurs) des sciences, de l'agriculture et des arts à Lille, 1823:137-165.
Macquart, P.J.M. 1827. Insectes dipteres du nord de la France. Platypezines, dolichopodes, empides, hybotides. Lille.
Macquart, P.J.M. 1834. Histoire naturelle des insectes. Diptères. Tome premiere. Roret, Paris.
Macquart, P.J.M. 1840, Dipteres exotiques nouveaux oupeu connus. Tome deuxieme.–I re partie, N. E. Roret, Paris.
Matile, L. 1979. Un nouveau genre de Keroplatidae de l’ambre oligocène de la Baltique [Diptera: Mycetophilidae]. Revue Française d’Entomologie, 1(1):36-41.
McAtee, W.L. 1922. Notes on Nearctic bibionid flies. United States National Museum Proceedings, 60(11):1-27.
Meigen, J.W. 1803. Versuch einer neuen Gattungseintheilung der europäischen zweiflügeligen Insekten. Magazin für Insektenkunde, 2:259-281.
Meigen, J.W. 1804. Klassifikazion und Beschreibung der europäischen zweiflügeligen Insekten (Diptera Linn.). Reichard, Braunschweig.
Meigen, J.W. 1820. Systematische Beschreibung der bekannten europäische n zweiflügeligen Insekten. Zweiter Theil. Forstmann, Aachen.
Meigen, J.W. 1830. Systematische Beschreibung der bekannten europäischen zweiflügeligen Insekten (Diptera Linn.). Sechster Theil. Verlag der Schultz- Wundermann'schen Buchhandlung, Hamm.
Melander, A.L. 1928. Diptera. Family Empididae. Fasc. 185, p 1-434. In Wytsman, P. (ed.), Genera insectorum. V. Verteneuil and L. Desmet, Bruxelles.
Meunier, F. 1893. Note sur les Platypezidae de l’ambre tertiaire. Bulletin de la Société Zoologique de France, 18:230-234.
Meunier, F. 1899. Révision des diptères fossiles types de Loew conservés au Musée Provincial de Koenigsberg. Miscellanea Entomologica, 7:169-182.
Meunier, F. 1904. Monographie des Cecidomyidae, des Sciaridae, des Mycetophilidae et des Chironomidae de l'ambre de la Baltique. Annales de la Société scientifique de Bruxelles, 28:12-92.
Michelsen, V. 1996. Neodiptera: new insights into the adult morphology and higher level phylogeny of Diptera (Insecta). Zoological Journal of the Linnean Society, 117(1):71-102.
Mik, J. 1891. Vorläufige Notiz über Parathalassius Blasigii [sic], ein neues Dipteron aus Venedig. Wiener Entomologische Zeitung, 10:216-217.
Nagatomi, A., Saigusa, T., Nagatomi, H., and Lyneborg, L. 1991a. Apsilocephalidae, a new family of the orthorrhaphous Brachycera (Insecta, Diptera). Zoological Science, 8:579-591.
Nagatomi, A., Saigusa, T., Nagatomi, H., and Lyneborg, L. 1991b. The genitalia of the Apsilocephalidae (Diptera). Japanese Journal of Entomology, 59(2):409-423.
Nagatomi, A., Saigusa, T., Nagatomi, H., and Lyneborg, L. 1991c. The systematic position of the Apsilocephalidae, Rhagionempididae, Protempididae, Hilarimorphidae, Vermileonidae and some genera of Bombylidae (Insecta, Diptera). Zoological Science, 8:593-607.
Nagatomi, A. and Yang, D. 1998. A review of extinct Mesozoic genera and families of Brachycera (Insecta, Diptera, Orthorrhapha). Entomologists Monthly Magazine, 134:95-192.
Negrobov, O.P. 1978. Flies of the superfamily Empidoidea (Diptera) from Cretaceous retinite in northern Siberia. Paleontologicheskii Zhurnal,1978(2):81-90.
Nel, A. 2006. Oldest records of Bombyliidae: Phthiriinae and Mythicomyiidae: Glabellulinae from the Lowermost Eocene amber of France (Diptera: Bombylioidea). European Journal of Entomology, 103(1):109-114.
Nel, A. and Coty, D. 2016. A fossil dung midge in Mexican amber (Diptera: Scatopsidae). Palaeontologia Electronica, 19.2.22A. https://doi.org/10.26879/633
Newman, E. 1834. Attempted division of British insects into natural orders. Entomological Magazine, 2:379-431.
O’Brien, N.R., Meyer, H.W., Reilly, K., Ross, A.M., and Maguire, S. 2002. Microbial taphonomic processes in the fossilization of insects and plants in the late Eocene Florissant Formation. Colorado. Rocky Mountain Geology, 37:1-11.
O’Brien, N.R., Meyer, H.W., and Harding, I.C. 2008. The role of biofilms in fossil preservation, Florissant Formation, Colorado, p. 19-31, In Meyer, H.W. and Smith, D.M. (eds.), Paleontology of the Upper Eocene Florissant Formation, Colorado. Geological Society of America Special Paper 435.
Palaeobiology Database, accessed May 10, 2022. Data were downloaded using the taxon “Diptera” and the following parameters: time interval = Eocene, location = North America and stratigraphy. https://training.paleobiodb.org/classic/displayDownloadGenerator
Paramonov, S.J. 1955. A review of Australian Scenopinidae (Diptera). Australian Journal of Zoology, 3:634-653.
Pinto, L.G. and Amorim, D.S. 1997. Taxonomy and phylogeny of the Neotropical genus Bibionellus (Diptera: Bibionidae). Iheringia Série Zoologia, 83:65-84.
Poinar Jr., G. and Vega, F.E. 2021. A new genus of Apsilocephalidae (Diptera) in Mid-Cretaceous Burmese Amber. Biosis: Biological Systems, 2(1):183-190. https://doi.org/10.37819/biosis.002.01.0091
Rohdendorf, B.B. 1951. The organs of locomotion of the Diptera and their origin. Transactions of the Trudy Paleontologicheskogo Instituta, 35:1-180.
Rondani, C. 1840. Sopra alcuni nuovi generi di insetti ditteri. Memoria seconda per servir alla ditterologia italiana. Donati, Parma.
Rondani, C. 1856. Dipterologiae italicae prodromus. Vol: I. Genera Italica ordinis dipterorum ordinatim disposita et distincta et in familias et stirpes aggregata. A. Stocchi, Parmae.
Saigusa, T. 1986. New genera of Empididae (Diptera) from eastern Asia. Sieboldia, 5:97-118.
Saigusa, T. and Kato, A. 2002. Discovery of the Nearctic empidid genus Hoplocyrtoma Melander (Diptera, Brachycera) from Japan. Special Bulletin of the Japanese Society of Coleopterology, 5:485-493.
Sasakawa, M. 2009. Oriental and Australasian Macrocera (Insecta: Diptera: Keroplatidae), with Descriptions of Eleven New Species. Species Diversity, 14(3):173-196. https://doi.org/10.12782/specdiv.14.173
Shamshev, I.V. and Grootaert, P. 2002. A new genus of Microphorinae (Diptera: Empidoidea) from New Zealand. Belgian Journal of Entomology, 4:129-144.
Shamshev, I.V. and Grootaert, P. 2005. Eothalassius, a new genus of parathalassiine flies (Diptera: Empidoidea: Dolichopodidae) from Southeast Asia and Papua New Guina. European Journal of Entomology, 102:107-118.
Shatalkin, A.I. 1985. A survey of Platypezidae (Diptera) of the USSR fauna. Sbornik Trudov Zoologicheskogo Muzeya Mgu (Moskovskogo Gosudarstvennogo Universiteta), 23:69-136.
Shin, S., Bayless, K.M., Winterton, S.L., Dikow, T., Lessard, B.D., Yeates, D.K., Wiegmann, B.M., and Trautwein, M.D., 2017. Taxon sampling to address an ancient rapid radiation: a supermatrix phylogeny of early brachyceran flies (Diptera). Systematic Entomology, 43:277-289. https://doi.org/10.1111/syen.12275
Shockley, F.W. and Greenwalt, D.E. 2013. Ptenidium kishenehnsis, a new fossil described from the Kishenehn oil shales (Coleoptera: Ptiliidae), with a checklist of previously known fossil ptiliids. Proceedings of the Entomological Society of Washington, 115 (2):173-181. https://doi.org/10.4289/0013-8797.115.2.173
Sinclair, B.J. and Cumming, J.M. 2006. The morphology, higher level phylogeny and classification of the Empidoidea (Diptera). Zootaxa, 1180:1-172. https://doi.org/10.11646/zootaxa.1180.1.1
Sinclair, B.J., Cumming, J.M., and Wood, D.M. 1994. Homology and phylogenetic implications of male genitalia in Diptera - Lower Brachycera. Entomologica Scandinavica, 24:407-432.
Skartveit, J. 2008. Fossil Hesperinidae and Bibionidae from Baltic amber (Diptera: Bibionoidea). Studia dipterologica, 15:3-42.
Skartveit, J. 2017. Bibionidae, p. 497-504. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.), Manual of Afrotropical Diptera. Volume 2. Nematocerous Diptera and lower Brachycera. Suricata 5. South African National Biodiversity Institute, Pretoria. http://hdl.handle.net/20.500.12143/6089
Skartveit, J. and Krizmanić, K. 2020. Revision of fossil Bibionidae (Insecta: Diptera) from the Miocene of Radoboj, Croatia. Zootaxa, 4759(3):351-378. https://doi.org/10.11646/zootaxa.4759.3.3
Skartveit, J. and Nel, A. 2017. Revision of fossil Bibionidae (Insecta: Diptera) from French Oligocene deposits. Zootaxa, 4225(1):1-83. https://doi.org/10.11646/zootaxa.4225.1.1
Skartveit, J. and Pika, M. 2014. Revision of Bibionide (Diptera) named by Oswald Heer from the Miocene of Öhningen, Southern Germany. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 87(1-2):103. http://hdl.handle.net/20.500.11850/96648
Skartveit, J. and Wedmann, S. 2021. A Revision of fossil Bibionidae (Insecta: Diptera) from the Oligocene of Germany. Zootaxa, 4909(1):1-77. https://doi.org/10.11646/zootaxa.4909.1.1
Skartveit, J., Grímsson, F., and Wappler, T. 2017. Bibionidae (Diptera) from the late Miocene of Hrútagil (Mókollsdalur), Iceland. Paläontologische Zeitschrift, 91(2):195-205. https://doi.org/10.1007/s12542-017-0341-0
Solórzano-Kraemer, M.M. and Cumming, J.M. 2019. New genera of brachyceran flies (Diptera: Xylomyidae and Apsilocephalidae sensu auctorum) from mid-Cretaceous Hukawng Valley Burmese amber. Palaeoentomology, 2(3):251-261. https://doi.org/10.11646/palaeoentomology.2.3.10
Staeger, R.C. 1840. Systematisk fortegnelse over de i Danmark hidtil fundne Diptera. Naturhistorisk Tidsskrift, 1:228-288.
Statz, G. 1940. Neue Dipteren (Brachycera et Cyclorhapha) aus dem Oberoligozän von Rott. Palaeontographica Abteilung A, 91:120-174
Statz, G. 1944. Neue Dipteren (Nematocera) aus dem Oberoligocän von Rott. II. Familie Fungivoridae (Pilzmücken). Palaeontographica Abteilung A, 95:67-92.
Stuckenberg, B.R. 2002a. A new genus and species Vermileonidae (Diptera: Brachycera) from Madagascar. Tijdschrift voor Entomologie, 145:1-8.
Stuckenberg, B.R. 2002b. Namaquamyia, a replacement name for a South African genus of Vermileonidae (Diptera). African Invertebrates, 43:123.
Taber, S.W. 2017. A new Nearctic species of Azana Walker fungus gnat. Southwestern Entomologist, 42(1):137-143. https://doi.org/10.3958/059.042.0113
Tanner, Z.L. 1894. Report upon the investigations of the U. S. fish commission steamer Albatross for the year ending June, 30, 1892. In Report of the commissioner - United States Commission of fish and fisheries. Part 18. Washington.
Théobald, N. 1937. Les insectes fossiles des terrains oligocènes de France. Bulletin Mensuel (Mémoires) de la Sociéte des Sciences de Nancy, 1:1-473.
Timon-David, J. 1943. Insectes fossiles de l’Oligocène inférieur des Camoins 1. Diptères Brachycères. Bulletin de la Société entomologique de France, 48:128-134.
Trautwein, M.D., Wiegmann, B.M., and Yeates, D.K. 2010. A multigene phylogeny of the fly superfamily Asiloidea (Insecta): taxon sampling and additional genes reveal the sister-group to all higher flies (Cyclorrhapha). Molecular Phylogenetics and Evolution, 56:918-930. https://doi.org/10.1016/j.ympev.2010.04.017
Ulrich, H. 1991. Two new genera of parathalassiine-like flies from South Africa (Diptera, Empidoidea). Bonner Zoologische Beiträge, 42:187-216.
Vockeroth, JR. 1981. 14. Mycetophilidae, p. 223-246. In McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., and Wood, D.M. (eds.), Manual of Nearctic Diptera. Monograph No. 27. Research Branch, Agriculture Canada.
von Heyden, C.H.G. 1859. Fossile Insekten aus der rheinischen Braunkohle. Palaeontographica, 8:1-15.
von Röser, K.L.F. 1840. Erster Nachtrag zu dem im Jahre 1834 bekannt gemachten Verzeichnisse in Württemberg vorkommender zweiflügliger Insekten. Correspondenzblatt des Königlich Württembergischen Landwirtschaftlichen Vereins. Stuttgart, 37(1):49-64.
Wahlberg, E. and Johanson, K.A. 2018. Molecular phylogenetics reveals novel relationships within Empidoidea (Diptera). Systematic Entomology, 43:619-636. https://doi.org/10.1111/syen.12297
Walker, F. 1856. Catalogue of the dipterous insects collected in Singapore and Malacca by Mr. A.R. Wallace, with descriptions of new species. Journal of the Proceedings of the Linnean Society of London, Zoology, 1:4-39.
Waller, A., Nel, A., and Menier, J.-J. 2000. Le premier Dilophus fossile de l’ambre dominicain (Diptera, Bibionidae). Revue Francaise d’ Entomologie (Nouvelle Serie), 22:149-153.
White, A. 1914. The Diptera-Brachycera of Tasmania. Part I. Families Leptidae, Stratiomyidae, Nemestrinidae, and Cyrtidae. Papers and Proceedings of the Royal Society of Tasmania, 1914:35-74.
Wiegmann, B.M., Trautwein, M.D., Winkler, I.S., Barr, N.B., Kim, J., Lambkin, C., Bertone, M.A., Cassel, B.K., Heimberg, A.M., Wheeler, B.M., Peterson, K.J., Pape, T., Sinclair, B.J., Skevington, J.H., Blagoderov, F.C., Grimaldi, D.A., Beckenbach, A.T., Courtney, G.W., Friedrich, M., Meier, R., and Yeates, D.K., 2011. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences. U.S.A. 108:5690-5695. https://doi.org/10.1073/pnas.1012675108
Wilson, M.V.H. 1977. Paleoecology of Eocene lacustrine varves at Horsefly, British Columbia. Canadian Journal of Earth Sciences, 14:953-962.
Wilson, M.V.H. 1978. Paleogene insect faunas of western North America. Quaestiones Entomologicae, 14:13-34.
Wilson, M.V.H. 1982. Early Cenozoic insects: paleoenvironmental biases and evolution of the North American insect fauna. Proceedings of the Third North American Paleontological Convention, 2:585-588.
Winterton, S.L. and Irwin, M.E. 2008. Kaurimyia gen. nov.: discovery of Apsilocephalidae (Diptera: Therevoid clade) in New Zealand. Zootaxa, 1779:38-44. https://doi.org/10.11646/zootaxa.1779.1.3
Winterton, S.L. and Gaimari, S.D. 2011. Revision of the South American window fly genus Heteromphrale Kröber, 1937 (Diptera, Scenopinidae). ZooKeys, 84:39-57. https://doi.org/10.3897/zookeys.84.774
Winterton, S.L. and Gharali, B. 2011. Iranotrichia gen. n., a new genus of Scenopinidae (Diptera) from Iran, with a key to window fly genera of the world. ZooKeys, 138:75-92. https://doi.org/10.3897/zookeys.138.1821
Winterton, S.L. and Ware, J.L. 2015. Phylogeny, divergence times and biogeography of window flies (Scenopinidae) and the therevoid clade (Diptera: Asiloidea). Systematic Entomology, 40(3):491-519. https://doi.org/10.1111/syen.12117
Winterton, S.L. and Gaimari, S.D. 2017. 14. Scenopinidae (Window Flies), p. 1-51. In Kirk-Spriggs, A.H. and Sinclair, B.J. (eds.), Manual of Afrotropical Diptera. Volume 2. Nematocerous Diptera and lower Brachycera. Suricata 5. South African National Biodiversity Institute, Pretoria.
Wood, D.M. and Borkent, A. 1989. Phylogeny and classification of the Nematocera. In McAlpine JF and Wood DM (Coords), Manual of Nearctic Diptera. Vol. 3. Agriculture Canada Monograph, 32:1333-1370.
Yeates, D.K. and Grimaldi, D. A. 1993. A new Metatrichia window fly (Diptera: Scenopinidae) in Dominican amber, with a review of the systematics and biogeography of the genus. American Museum Novitates, 3078:1-8.
Yeates, D.K. and Wiegmann, B.M., 2005. Phylogeny and evolution of Diptera: recent insights and new perspectives. p. 14-44. In Yeates, D.K. and Wiegmann, B.M. (eds.), The Evolutionary Biology of Flies, Chapter 2. Columbia University Press, New York, New York.
Yeates, D.K., Irwin, M.E., and Wiegmann, B.M. 2003. Ocoidae, a new family of asiloid flies (Diptera: Brachycera: Asiloidea), based on Ocoa chilensis gen. and sp. n. from Chile, South America. Systematic Entomology, 28:417-431. https://doi.org/10.1046/j.1365-3113.2003.00224.x
Yeates, D.K., Irwin, M.E., and Wiegmann, B.M. 2006. Evocoidae (Diptera: Asiloidea), a new family name for Ocoidae, based on Evocoa, a replacement name for the Chilean genus Ocoa Yeates, Irwin, and Wiegmann 2003. Systematic Entomology, 31(2):373. https://doi.org/10.1111/j.1365-3113.2006.00332.x
Zhang, Q.Q., Li, X.K., Xu, B.Q., Zhu, Y.M., Lu, R.Q., Wang, B., and Yeates, D.K. 2018. Two new genera of Apsilocephalidae from mid-Cretaceous Burmese amber. Cretaceous Research, 84:525-532. https://doi.org/10.1016/j.cretres.2017.11.026