The macrofossil Lydonia jiggamintia gen. et sp. nov. from the Ediacaran of Newfoundland (Canada): From pseudofossil to metazoan-grade organism
The macrofossil Lydonia jiggamintia gen. et sp. nov. from the Ediacaran of Newfoundland (Canada): From pseudofossil to metazoan-grade organism
Article number: 28.3.a39
https://doi.org/10.26879/1386
Copyright Palaeontological Association, September 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendix
Submission: 5 March 2024. Acceptance: 30 August 2025.
ABSTRACT
The Ediacaran rocks from Discovery UNESCO Global Geopark on the Bonavista Peninsula, Newfoundland (CA), contain a rich fossil record, with remarkable preservation and an unusually great abundance of diverse Ediacaran organisms. This study focuses on the large (<40 cm), enigmatic taxon, previously described as “Blackbrookia”, that is particularly common on the Johnson Discovery Surface along with the super-abundant rangeomorph Fractofusus andersoni (<30.000 specimens). Unlike the pseudofossil Blackbrookia, the form under consideration is interpreted as a body fossil and reclassified herein (Lydonia jiggamintia gen. et sp. nov.). Lydonia is characterized by infilled pores on its upper surface, which we consider to be related to the presence of an aquiferous system. We compare this morphology to various extant and extinct macro-organisms and note that Lydonia might be analogous to modern encrusting Porifera in its body plan. Statistical analyses of Lydonia populations allow us to reconstruct the morphospace occupied by the species, as well as to consider aspects of ontogeny and population structure. We recognize a metazoan-grade morphology and mode of life, even though the phylogenetical affinity remains ambiguous. If accepted as a metazoan, Lydonia would constitute further evidence for animal life in the Ediacaran, with important implications for our understanding of the record of early animals and evidence for lengthening the Ediacaran food chain.
Giovanni Pasinetti. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada giovanni.pasinetti95@gmail.com
Latha Menon. University of Oxford, Department of Earth Science, S Parks Rd, Oxford OX1 3AN, Regno Unito, United Kingdom. menon891@btinternet.com
Nagi Chida. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada. nchida@mun.ca
Pascal Olschewski. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada. polschewski@mun.ca
Christopher McKean. School of Life Sciences, University of Essex, Wivenhoe Park, Colchester, Essex, CO4 3SQ, United Kingdom. cmckean@mun.ca
Daniel Pérez-Pinedo. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada. dperezpinedo@mun.ca
Rod S. Taylor. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada and Johnson GeoCenter, 175 Signal Hill Rd, St. John's, NL A1A 1B2, Canada. rodt@mun.ca
Duncan McIlroy. Memorial University of Newfoundland, Department of Earth Sciences, 1 Arctic Av., St. John's, NL A1C 5S7, Canada. dmcilroy@mun.ca
Keywords: Ediacaran; early animals’ evolution; ivesheadiomorph; porifera; evolution; palaeoecology; new genus; new species
Final citation: Pasinetti, Giovanni, Menon, Latha, Chida, Nagi, Olschewski, Pascal, McKean, Christopher, Pérez-Pinedo, Daniel, Taylor, Rod S., and McIlroy, Duncan. 2025. The macrofossil Lydonia jiggamintia gen. et sp. nov. from the Ediacaran of Newfoundland (Canada): From pseudofossil to metazoan-grade organism. Palaeontologia Electronica, 28(3):a39.
https://doi.org/10.26879/1386
palaeo-electronica.org/content/2025/5594-lydonia-jiggamintia
Copyright: September 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
https://zoobank.org/6AE0EF7B-26C0-44DC-A420-8295418C1F5D
INTRODUCTION
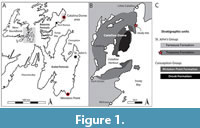 The Ediacaran strata of the Catalina Dome, Bonavista Peninsula (Figure 1A-B) have yielded a diverse biota of rangeomorphs, arboreomorphs and incertae sedis (Hofmann et al., 2008), belonging to the Avalon Assemblage, the oldest of the Ediacaran macrofossil assemblages (Waggoner, 2003). The biota is similar to the classic Mistaken Point biota (Liu et al., 2016), but with taxa having notably different stratigraphic ranges (Hofmann et al., 2008; Matthews et al., 2020; Pérez-Pinedo et al., 2022). The biota of the Catalina Dome was probably preserved in a shallower--perhaps offshore shelf--depositional environment than the classic Mistaken Point biota of the Avalon Peninsula (Hofmann et al., 2008). The most notable difference between the two biotas is the superabundance of Fractofusus andersoni in the Catalina Dome, whereas Fractofusus misrai is the numerically dominant taxon at Mistaken Point localities. One of the largest organisms in the Catalina Dome assemblage is the form referred to as “Blackbrookia” by Hofmann et al. (2008), a large (up to <40 cm) obovate to ovate fossil, which commonly has a folded/wrinkled axial region. The original material of Blackbrookia from Charnwood Forest, UK (Boynton and Ford, 1995) has been considered to be an ivesheadiomorph pseudofossil (Liu et al., 2011). When referring to the type material of Blackbrookia, and other related pseudofossils, the genus name is not italicized according to modern convention (cf. Arumberia, McIlroy and Walter, 1997; Kinneyia, Porada et al., 2008).
The Ediacaran strata of the Catalina Dome, Bonavista Peninsula (Figure 1A-B) have yielded a diverse biota of rangeomorphs, arboreomorphs and incertae sedis (Hofmann et al., 2008), belonging to the Avalon Assemblage, the oldest of the Ediacaran macrofossil assemblages (Waggoner, 2003). The biota is similar to the classic Mistaken Point biota (Liu et al., 2016), but with taxa having notably different stratigraphic ranges (Hofmann et al., 2008; Matthews et al., 2020; Pérez-Pinedo et al., 2022). The biota of the Catalina Dome was probably preserved in a shallower--perhaps offshore shelf--depositional environment than the classic Mistaken Point biota of the Avalon Peninsula (Hofmann et al., 2008). The most notable difference between the two biotas is the superabundance of Fractofusus andersoni in the Catalina Dome, whereas Fractofusus misrai is the numerically dominant taxon at Mistaken Point localities. One of the largest organisms in the Catalina Dome assemblage is the form referred to as “Blackbrookia” by Hofmann et al. (2008), a large (up to <40 cm) obovate to ovate fossil, which commonly has a folded/wrinkled axial region. The original material of Blackbrookia from Charnwood Forest, UK (Boynton and Ford, 1995) has been considered to be an ivesheadiomorph pseudofossil (Liu et al., 2011). When referring to the type material of Blackbrookia, and other related pseudofossils, the genus name is not italicized according to modern convention (cf. Arumberia, McIlroy and Walter, 1997; Kinneyia, Porada et al., 2008).
The collective term “ivesheadiomorphs” for these effaced forms is a taphonomic-based termionology intended to aid communication of a range of taphomorphs rather than define a taxonomically coherent grouping (Liu et al., 2011). The group is an important component of marine Ediacaran ecosystems of Avalonia and have been variously called by the informal names “pizza discs,” “lobate discs,” and “bubble discs” (Narbonne et al., 2009; Laflamme et al., 2012; Kenchington and Wilby, 2014; Mitchell and Butterfield, 2018), as well as by the Latin binomials “Ivesheadia lobata”, “Blackbrookia oaksi” and “Shepshedia palmata” (cf. Boynton and Ford, 1995: respectively, fig. 12, 17, 16). Ivesheadia, Blackbrookia and Shepshedia in the strict sense (not the “Blackbrookia” of Hofmann et al., 2008) are considered pseudofossils formed by post-mortem processes involving microbial degradation, matground overgrowth and sedimentation, which resulted in effacement of the original fossils (Liu et al., 2011).
While the ivesheadiomorphs are not body fossils sensu stricto -- rather they represent microbially mediated sedimentation associated with the carcasses of macroscopic organisms such as the Rangeomorpha and Arboreomorpha -- their importance stems from their being a record of necromass on the unbioturbated, ungrazed deep marine seafloors of Avalonia (Liu et al., 2011). It has been suggested that they can be taphomorphs in morphological continuum with well-known taxa (Liu et al., 2011; Antcliffe et al., 2015; Mitchell and Butterfield, 2018). Moreover, this evidence for microbial nutrient recycling demonstrates a significant change in the carbon cycle in which buried organisms are used by microbes and subsequently possible chemosymbiotic organisms such as Fractofusus (Dufour and McIlroy, 2017; McIlroy et al., 2021, 2022).
The large fossils described as “Blackbrookia” by Hofmann et al. (2008) from the Johnson Discovery Surface (JDS hereafter) of the Ediacaran of the Catalina Dome (also previously known as “Locality 14” in Hofmann et al., 2008; the “Discovery Surface”; and H14; (Figure 1B-C) are the focus of this study. The forms described by Hofmann et al. (2008) do not closely conform to the UK type material and present sharp boundaries and distinct morphologies, more fossil-like than any ivesheadiomorph. While they do have a broadly ivesheadiomorph morphology (i.e., ovate and wrinkled), they often differ in having a porose surface morphology (Hofmann et al., 2008), which has led to comparisons with the Cambrian sponge genus Crumillospongia (McIlroy et al., 2021). “Blackbrookia” does not show evidence for a single exhalating opening (“osculum”, in Porifera anatomy), but rather their porose body surface suggests the presence of an aquiferous system that is analogous to that of modern encrusting demosponges. This is in contrast to early Cambrian sponges, which are thought to have evolved from a primitive “ascon” body plan, with a single osculum (Botting and Muir, 2018). Some large “Blackbrookia” -shaped fossils from the JDS have a gross morphology strongly resembling the porose “Blackbrookia” , except they have an effaced surface texture and are thus more similar to the ivesheadiomorph pseudofossils. None of the ivesheadiomorph pseudofossil from the Charnian of the UK (described as Ivesheadia, Shepshedia and “Blackbrookia” ; Boynton and Ford, 1995) have porose surface textures. Since the Catalina Dome forms are more complex than the UK ivesheadiomorphs (including “Blackbrookia” )--and since the name “Blackbrookia” was applied to a pseudofossil (Liu et al., 2011)--the more fossil-like porose forms from the Catalina Dome warrant further attention and require renaming, especially in the context of the new discoveries presented herein.
GEOLOGICAL SETTING
Fossil specimens originally considered to be “Blackbrookia” have been reported in Newfoundland only from two localities in the Catalina Dome of the Bonavista Peninsula (the JDS and Locality 13 in Hofmann et al., 2008) (Figure 1A). The two surfaces have very similar fossil communities, dominated by super-abundant Fractofusus andersoni, but otherwise have low species diversity: Hofmann et al. (2008) report only three taxa from the JDS and six from Hoffman Locality 13. Both surfaces lie stratigraphically close to each other within the Catalina Member (Hofmann et al., 2008) (Figure 1B), which has been lithostratigraphically correlated with the Trepassey Formation of the St. John’s Group on the eastern Avalon Peninsula (O’Brien and King, 2005; Mason et al., 2013) (Figure 1C). The “Blackbrookia” -bearing surfaces lie within a mudstone-rich succession with some thickly bedded turbidites (1-2 m) characterized by normal grading, interbedded with thin cross-bedded sandstones (2-3 cm thick). Fossils are preserved on the JDS both as negative epireliefs (rangeomorphs) and positive epireliefs (the “Blackbrookia”) on a red-brown silty surface and overlain by a 0.5 mm thick layer of tuffite.
The surface is a classic example of conception-style preservation (Narbonne, 2005), which involves the casting of the specimens in-situ by a layer of tuff that preserves either the upper or lower surfaces of the organisms, depending on the rigidity of the original tissues. Other, previously undescribed, specimens of “Blackbrookia” come from the D and E surfaces of the Mistaken Point Formation of the Conception Group in the Mistaken Point Ecological Reserve (MPER) in the southern Avalon Peninsula, Newfoundland (Figure 1A).
MATERIALS AND METHODS
For the morphometric analyses that form part of this study, 39 specimens previously assigned to “Blackbrookia” (assigned to Lydonia gen. nov. below) from the Johnson Discovery Surface have been photographed and measured (2022 field season). To morphometrically characterize the new genus, a dataset was collected with the software imageJ and analysed in R studio (R Core Team, 2022). After identifying the major axis of the specimens, the longest measurable length perpendicular to the major axis was considered as the minor axis. Length of the major (V1) and the minor (V2) axes and their major/minor axial ratio (V3) were measured for each specimen and treated as continuous variables (Table 1), along with the qualitative variables “presence/absence of papillate texture” (V4; Table 1) and regularity of the profile of the organism (V5: sub-elliptical/irregular; Table 1). Specimens are recorded in our dataset by an identification code composed of the letters “r” and “i” (representing regular and irregular specimens, respectively), followed by an identification number.
Normality of the continuous variables (V1, V2, V3) was assessed by Shapiro-Wilk tests and only V1 was found to be normally distributed (Shapiro-Wilk test: α = 0.05; p-value[V1] = 0.057, p-value[V2] = 0.004, p-value[V3] = 0.020). Logarithmic (natural logarithm) transformation was therefore applied to the data, producing the transformed variables tV1, tV2 and tV3, which are all revealed to be normally distributed by Shapiro-Wilk tests (Shapiro-Wilk test: α=0.05; p-value[tV1] = 0.341, p-value[tV2] = 0.463, p-value[tV3] = 0.419). Even though approaches using non-transformed data have been attempted (e.g., Mitchell et al., 2015), transformed data are typically preferred as they usually produce a more precise representation of population structure (Bak and Meesters, 1998; Meesters et al., 2001; Darroch et al., 2013; Pérez-Pinedo et al., 2023).
Assumptions of linearity, homoscedasticity, and normality of the residuals and independence of residual error terms were satisfied for the transformed variables and therefore relationships between continuous variables were initially explored using linear models.
A Welch Two Sample t-test was applied to test whether the means of the two subsets of the population “r” and “i” are statistically different.
To investigate the morphospace occupied by the specimens of the Catalina Dome population, backtransform morphospace analyses were used. The method, developed by Olsen and Westneat (2015) and Olsen (2017) -- used previously in taxonomic studies of the Ediacaran biota (Laflamme et al., 2007; Pasinetti and McIlroy, 2023) -- employs equidistant landmarks and semi-landmarks, computed from digitized pictures of the 33 most complete specimens using the R package Stereomorph, version 1.6.4 (Olsen and Westneat, 2015; Olsen, 2017), from which generalized coordinates for each specimen were generated using Procrustes analyses. Due to the lack of evident homologous points on the outer organism profile, a landmark was chosen as the starting point at the conjunction of the major axis with the South-Western portion of the outer profile of the specimens. 100 equidistant semi-landmarks were then drawn around the outer profile and reconnected with the original landmark to capture the exterior two-dimensional shape of the organisms. The generalized coordinates were analysed with a Principal Component Analysis (PCA) and were replotted in a backtransform morphospace to visually represent the variability in shape of the population (see Olsen, 2017 for methodology). The resulting ordination was then compared with the qualitative assessment of the organism profile.
Size-frequency distribution histograms can be used to infer age classes and population structure within a monospecific population (Darroch et al., 2013; Pérez-Pinedo et al., 2023). The Gaussian finite mixture model-based clustering algorithms of the package MCLUST allows the identification of the most likely number of size modes (and therefore age/size classes) within a single population (Scrucca et al., 2016). A likelihood-based model selection criterion, BIC (Bayesian Information Criterion), was used to select the best model. Both univariate and bivariate size-frequency distribution analyses were performed to produce a model for the population structure of the assemblage and to infer the history of colonization and development on the JDS (see Darroch et al., 2013 and Pérez-Pinedo et al., 2023 for methodology).
RESULTS
Morphology of the Catalina Dome “Blackbrookia”
 The gross morphology of “Blackbrookia” in the Catalina Dome is highly variable, typically broadly sub-oval with one pointed end and one rounded end, sometimes with longitudinal Ivesheadia-like ridges (Figure 2A-B). The outlines of the JDS specimens show continuous variation from those with a smooth sub-oval profile (Figure 2A), to more irregular ones (Figure 2B). We qualitatively recognize two main morphogroups in the field (recorded in our dataset as the qualitative variable V5, regulars/irregulars) based on the aspect of their outline. Regular specimens have a neat elliptical profile characterized by a marginal ridge separating the body of the organism from the fossiliferous surface and elevating the upper surface of the fossil above the preservational plane (Figure 2A). Irregular specimens may include additional lobes or can deviate from the sub-elliptical shape towards being more sub-triangular (Figure 2B). We note here that there is, however, an undescribed rangeomorph with a central ridge (Figure 2C) from the JDS that is comparable to the common obovate form of “Blackbrookia” from the same locality.
The gross morphology of “Blackbrookia” in the Catalina Dome is highly variable, typically broadly sub-oval with one pointed end and one rounded end, sometimes with longitudinal Ivesheadia-like ridges (Figure 2A-B). The outlines of the JDS specimens show continuous variation from those with a smooth sub-oval profile (Figure 2A), to more irregular ones (Figure 2B). We qualitatively recognize two main morphogroups in the field (recorded in our dataset as the qualitative variable V5, regulars/irregulars) based on the aspect of their outline. Regular specimens have a neat elliptical profile characterized by a marginal ridge separating the body of the organism from the fossiliferous surface and elevating the upper surface of the fossil above the preservational plane (Figure 2A). Irregular specimens may include additional lobes or can deviate from the sub-elliptical shape towards being more sub-triangular (Figure 2B). We note here that there is, however, an undescribed rangeomorph with a central ridge (Figure 2C) from the JDS that is comparable to the common obovate form of “Blackbrookia” from the same locality.
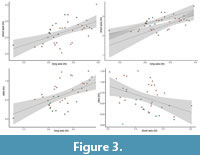 Statistical results: linear regressions. A linear regression (p -value = 0.00065) between the logarithms of the lengths of the major axes (tV1) and those of the minor axes (tV2) of the specimens in the JDS population shows a strong positive relationship between the two variables (y = 0.4914x+1.0306), albeit it has a low R2 value (0.2855) (Figure 3A). A linear regression between tV3 (log of ratio) and tV1 (log of long axis) shows a strong positive correlation (y=0.4988x-1.0088, R2 =0.2955, p -value=0.00034) (Figure 3C). A similar trend is found when computing the same linear regression in the two subsets of the JDS population: regulars and irregulars (Figure 3B). However, when comparing the ratio (tV3) with the short axis (tV2), a negative correlation is found (y=-0.4064x+1.7223, R2 =0.1656, p -value=0.01013) (Figure 3D). This negative correlation also stands true for the “regulars” subset of the JDS population, while for the “irregulars” subset the correlation is not statistically significant (α-value>>0.05).
Statistical results: linear regressions. A linear regression (p -value = 0.00065) between the logarithms of the lengths of the major axes (tV1) and those of the minor axes (tV2) of the specimens in the JDS population shows a strong positive relationship between the two variables (y = 0.4914x+1.0306), albeit it has a low R2 value (0.2855) (Figure 3A). A linear regression between tV3 (log of ratio) and tV1 (log of long axis) shows a strong positive correlation (y=0.4988x-1.0088, R2 =0.2955, p -value=0.00034) (Figure 3C). A similar trend is found when computing the same linear regression in the two subsets of the JDS population: regulars and irregulars (Figure 3B). However, when comparing the ratio (tV3) with the short axis (tV2), a negative correlation is found (y=-0.4064x+1.7223, R2 =0.1656, p -value=0.01013) (Figure 3D). This negative correlation also stands true for the “regulars” subset of the JDS population, while for the “irregulars” subset the correlation is not statistically significant (α-value>>0.05).
Statistical results: morphospace occupation and geographical orientations. The population of “Blackbrookia” from the JDS have highly variable outlines, from circular to elliptical and irregular, and maximum dimensions ranging from 9.8 cm to 52.7 cm in length. Regular specimens have long/short axial ratios (V3) from 1.35 to 3.52, with an average of 1.99 (Table 1), while irregular specimens have a lower eccentricity and V3 values that range from 1.02 and 2.51, with an average of 1.57.
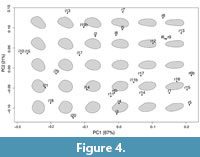 A backtransform morphospace analysis shows the range of variability within the population of the JDS. This methodology allows us to visually represent the morphospace occupied by a dataset by ordinating the specimens on two principal components (PC1 and PC2; Figure 4) computed on generalized coordinates to capture the shape variability of the specimens. PC1 (which explains 67% of variance) captures the eccentricity of the shape of the organisms, which range from sub-circular to sub-elliptical, and PC2 (explaining 21% of variance) appears to capture other shape irregularities, especially along the minor axis, such as the presence of a third lobe in the profile.
A backtransform morphospace analysis shows the range of variability within the population of the JDS. This methodology allows us to visually represent the morphospace occupied by a dataset by ordinating the specimens on two principal components (PC1 and PC2; Figure 4) computed on generalized coordinates to capture the shape variability of the specimens. PC1 (which explains 67% of variance) captures the eccentricity of the shape of the organisms, which range from sub-circular to sub-elliptical, and PC2 (explaining 21% of variance) appears to capture other shape irregularities, especially along the minor axis, such as the presence of a third lobe in the profile.
The specimens distribute in the ordination without any evident pattern of aggregation. Regular specimens appear to be restricted to the right side of the ordination (as expected, as they were selected partly because of their higher eccentricity), but irregular specimens appear to occupy the entire morphospace (Figure 4).
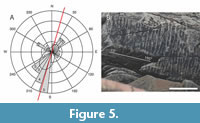 Most specimens (23 of the 30 measured, 76%) on the JDS are oriented with their long axis parallel to the regional S-SW directed paleocurrent (15°-195°; Figure 5A) as determined from current ripple foresets (Figure 5B) and consistent with regional basin reconstructions of Mason et al. (2013) while some specimens (seven out of 31, two regulars and five irregulars) are oriented broadly orthogonal to the paleocurrent. The observed wavy ripples may be indicative of internal waves in the basin, as the beds were deposited below the lowest wave base, suggesting that the organisms experienced periodic reversal of the current direction. The specimens oriented with the long axis in the direction of the inferred palaeocurrent show either the pointed or the blunt end upstream, with a V3 ranging from 1.02 to 3.52, with an average of 2.10. The remaining specimens are oriented broadly perpendicular to the palaeocurrent, with the pointy end on either side of it: such specimens have a low long/short axial ratio (1.12 to 1.66, with one outlier at 2.23 and an average of 1.50), which a Welch Two Sample t-test confirms to be significantly lower than the average V3 of oriented specimens.
Most specimens (23 of the 30 measured, 76%) on the JDS are oriented with their long axis parallel to the regional S-SW directed paleocurrent (15°-195°; Figure 5A) as determined from current ripple foresets (Figure 5B) and consistent with regional basin reconstructions of Mason et al. (2013) while some specimens (seven out of 31, two regulars and five irregulars) are oriented broadly orthogonal to the paleocurrent. The observed wavy ripples may be indicative of internal waves in the basin, as the beds were deposited below the lowest wave base, suggesting that the organisms experienced periodic reversal of the current direction. The specimens oriented with the long axis in the direction of the inferred palaeocurrent show either the pointed or the blunt end upstream, with a V3 ranging from 1.02 to 3.52, with an average of 2.10. The remaining specimens are oriented broadly perpendicular to the palaeocurrent, with the pointy end on either side of it: such specimens have a low long/short axial ratio (1.12 to 1.66, with one outlier at 2.23 and an average of 1.50), which a Welch Two Sample t-test confirms to be significantly lower than the average V3 of oriented specimens.
Observations in the field suggest that some strings of regular specimens appear to be arranged in a line at a low angle with respect to the paleocurrent, with a few meters between each specimen (cf. Delahook et al., 2024).
 Upper surface morphology. It has been noted that the upper surfaces of many of the Catalina Dome “Blackbrookia” have millimetric circular structures, which have been described as pores (Hofmann et al., 2008; Dufour and McIlroy, 2017; McIlroy et al., 2021). An extensive ferruginous mesh-like layer covers both the upper surface of the fossils, as well as the rest of the fossiliferous surface (Figure 6A), with the pores representing an interruption of this layer as features of the upper surface of the “Blackbrookia” organism. The pores are filled with siltstone. Pyritic replacement of soft tissues is not otherwise known in the Ediacaran of Avalonia but is the common mode of preservation of some lightly biomineralized Ediacaran taxa (e.g., Cloudina; Smith et al., 2016). The ferruginous layer extends uninterruptedly above the “Blackbrookia” specimens and beyond their margins, covering the entire surface and corresponding to the layer upon which Fractofusus specimens leave their impression. Such layers are typically interpreted as the oxidative pyritic replacement of an extensive microbial matground (Gehling, 1999; Liu, 2016; Pasinetti and McIlroy, 2023), suggesting that “Blackbrookia” was procumbent on the seafloor, possibly covered by the microbial matground, piercing through it, and accessing the water column with tubular structures whose positions correspond to the pores on the upper surface of the fossil. Other Ediacaran taxa are considered to have been preserved due to the presence of a pyritic death mask associated with a microbial matground (Gehling, 1999; Mapstone and McIlroy, 2006; Liu, 2016; Pasinetti and McIlroy, 2023) or even a pyritic envelope, in the case of more three dimensionally preserved fossils (McKean et al., 2023).
Upper surface morphology. It has been noted that the upper surfaces of many of the Catalina Dome “Blackbrookia” have millimetric circular structures, which have been described as pores (Hofmann et al., 2008; Dufour and McIlroy, 2017; McIlroy et al., 2021). An extensive ferruginous mesh-like layer covers both the upper surface of the fossils, as well as the rest of the fossiliferous surface (Figure 6A), with the pores representing an interruption of this layer as features of the upper surface of the “Blackbrookia” organism. The pores are filled with siltstone. Pyritic replacement of soft tissues is not otherwise known in the Ediacaran of Avalonia but is the common mode of preservation of some lightly biomineralized Ediacaran taxa (e.g., Cloudina; Smith et al., 2016). The ferruginous layer extends uninterruptedly above the “Blackbrookia” specimens and beyond their margins, covering the entire surface and corresponding to the layer upon which Fractofusus specimens leave their impression. Such layers are typically interpreted as the oxidative pyritic replacement of an extensive microbial matground (Gehling, 1999; Liu, 2016; Pasinetti and McIlroy, 2023), suggesting that “Blackbrookia” was procumbent on the seafloor, possibly covered by the microbial matground, piercing through it, and accessing the water column with tubular structures whose positions correspond to the pores on the upper surface of the fossil. Other Ediacaran taxa are considered to have been preserved due to the presence of a pyritic death mask associated with a microbial matground (Gehling, 1999; Mapstone and McIlroy, 2006; Liu, 2016; Pasinetti and McIlroy, 2023) or even a pyritic envelope, in the case of more three dimensionally preserved fossils (McKean et al., 2023).
The specimens considered herein commonly have a folded or torn upper surface (Figure 6B), consistent with the post-mortem collapse of an organism that was somewhat convex in life.
Our studies have revealed, for the first time, short subconical projections in association with a single specimen of “Blackbrookia” (Figure 6C) from the JDS. These suggest the presence of papillae on the upper surface of the large ovate morph. The projections are filled with the same siltstone that underlies the ferruginous layer and can be interpreted to be internal moulds of tubular papillae (Figure 6D). The mouldic preservation has previously been suggested for the common porose morphology (McIlroy et al., 2021).
 The size and spacing of pores on the upper surface of “Blackbrookia” from the Catalina Dome is uneven both within, and between, specimens (Figure 7A-B). There is no clear relationship between “pore” diameter and specimen length, which is perhaps to be expected if the pores are preserved internal moulds of the bases of tubular papillae, although some of the smaller specimens do have larger pores (Figure 7B).
The size and spacing of pores on the upper surface of “Blackbrookia” from the Catalina Dome is uneven both within, and between, specimens (Figure 7A-B). There is no clear relationship between “pore” diameter and specimen length, which is perhaps to be expected if the pores are preserved internal moulds of the bases of tubular papillae, although some of the smaller specimens do have larger pores (Figure 7B).
Irregular specimens are also often associated with less prominent pores on the upper surface (Figure 2B), which may be a taphonomic artefact, perhaps resulting from decay and microbial overgrowth of “Blackbrookia” or related taxon (47% of irregular specimens do not have pores vs 6% of regular specimens).
The upper surface of reclining rangeomorph fossils is generally not preserved in the Ediacaran biotas of Avalonia. Several rangeomorphs from the Upper Island Cove biota do have well preserved upper surfaces (Narbonne, 2004; Brasier et al., 2012; Mckean et al., 2023), none of which resemble the porose upper surface of the Catalina Dome “Blackbrookia”. However, the upper surface of a specimen of Charnia sp. has been documented from the Ediacaran of the White Sea (Butterfield, 2020) showing rangeomorph-type architecture and pores on the upper surface, which are regularly spaced and cannot be compared with “Blackbrookia”.
 Lower surface morphology. The most surprising component of our revisiting of the Catalina Dome “Blackbrookia” has been the discovery of two specimens with rangeomorph-type branching structures preserved on the lower surface of the organism. Most specimens from the JDS do not preserve the lower surface. Both specimens with preserved rangeomorph branching are the obovate morph (Figure 8A, C-D), which is comparable to the gross morphology of an undescribed rangeomorph (Figure 2C).
Lower surface morphology. The most surprising component of our revisiting of the Catalina Dome “Blackbrookia” has been the discovery of two specimens with rangeomorph-type branching structures preserved on the lower surface of the organism. Most specimens from the JDS do not preserve the lower surface. Both specimens with preserved rangeomorph branching are the obovate morph (Figure 8A, C-D), which is comparable to the gross morphology of an undescribed rangeomorph (Figure 2C).
The clearest example of a “Blackbrookia” overlying a rangeomorph has large cm-wide displayed rangeomorph units with second order branching (Figure 8C) morphologically comparable to that of Fractofusus andersoni (Figure 8B), which is super-abundant on the same surface (Hofmann et al., 2008: figure 25.3). The branches are however anomalously long and wide for F. andersoni and the gross morphology of the obovate “Blackbrookia” is unlike the typical ovate morphology of F. andersoni (Gehling and Narbonne, 2007). Some broken/truncated specimens of F. andersoni are known from the surface, which would create a more obovate shape, but all of those are an order of magnitude smaller than the figured “Blackbrookia” (Figure 8C).
The second obovate “Blackbrookia”- topped rangeomorph is better preserved in that finer details of the rangeomorph branching are evident, though these are longer and narrower than in the other specimen described above and the branching is poorly ordered, with some resemblance to Bradgatia -like branching (cf. Brasier et al., 2012; fig. 8A, D). The “Blackbrookia” expression on this specimen is the perforated-pinacoderm-type preservation, which is best preserved towards the pointed end of the fossil.
Population Structure of the Johnson Discovery Surface
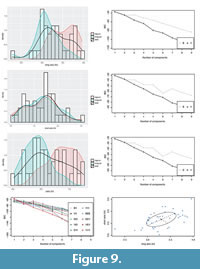 Untransformed length and width frequency distributions were right-skewed and moderately depart from normality. Log-transformed quantitative variables (tV1, tV2, tV3 in Figure 9A, C, E; following Darroch et al., 2013) and ratio of major axis/minor axis (V3) were normally distributed.
Untransformed length and width frequency distributions were right-skewed and moderately depart from normality. Log-transformed quantitative variables (tV1, tV2, tV3 in Figure 9A, C, E; following Darroch et al., 2013) and ratio of major axis/minor axis (V3) were normally distributed.
When normality of the two subsets (regular and irregular) is tested separately, Shapiro-Wilk tests always find a normal distribution of all the variables, non-transformed and transformed.
Size-frequency distributions graphs (Figure 9A, C, E) can be helpful in reconstructing population structure and can be used to suggest reproductive and growth models within a population (Meesters et al., 2001; Darroch et al., 2013; Pérez-Pinedo et al., 2023). Irregular specimens show distribution peaks at lower values of tV1 (Figure 9A), at similar values of tV2 (Figure 9C) and at higher values of tV3 (Figure 9E) compared to regular specimens (Figure 9A, C, E). A Welch Two Sample t-test indicates that the means of all the variables, untransformed and transformed, are statistically different for the two subsets of “regular” and “irregular” specimens, with regular specimens showing greater mean values for the variables V1, tV1, V3 and tV3 and smaller values for V2 and tV2 (Table 1). The lower V3 and tV3 values measured in the irregular specimens are consistent with the lower eccentricity shown by backtransform morphospace analyses (Figure 4).
To find the most likely number of modes, and therefore possible age classes within the population, a likelihood-based model selection criterion (BIC) was used to choose the most probable clustering solutions produced by Gaussian finite mixture model-based clustering algorithms (Scrucca et al., 2016). Univariate analyses on the transformed variables tV1 (Figure 9B), tV2 (Figure 9D) and tV3 (Figure 9F) resulted in a single mode as the best fitting model, assuming both equal and unequal variance. When conducting multivariate analyses, Mclust generates best-fitting models that assume ellipsoidal, diagonal, and spherical distributions. Models that assume ellipsoidal distributions are the most biologically realistic, as they allow unequal variances on two axes. The single mode found by the univariate analyses is confirmed from bivariate [tV1+tV2] clustering solutions (Figure 9G-H), which also finds a single mode ellipsoidal distribution as the most likely grouping solution for the analysed specimens.
Other Porose-Textured Taxa from the Mistaken Point Biota
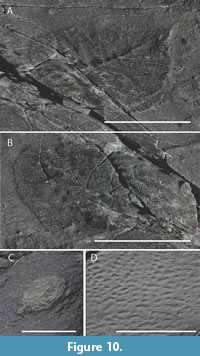 Mistaken Point on the southeast coast of Newfoundland’s Avalon Peninsula is well known for its abundant deep marine Ediacaran biotas (Narbonne, 2005), with most work having focused on fossils from the taxonomically diverse E Surface (Seilacher, 1992; Narbonne, 2005; Darroch et al., 2013; Taylor et al., 2021, 2023; Vixseboxse et al., 2021; McIlroy et al., 2022). The underlying D Surface is however under-studied and includes examples of recently described arboreomorphs (Pérez-Pinedo et al., 2022) and the abundant rangeomorphs Fractofusus (Taylor et al., 2023), Bradgatia (Flude and Narbonne, 2008) and Pectinifrons (Bamforth et al., 2008), as well as rare Hapsidophyllas flexibilis (Taylor et al., 2021). Previously overlooked on the D and parts of the E surface are surface textures comparable to those of “Blackbrookia”, except preserved as internal moulds of the pores (Figure 10A-C). In some cases, the low relief porose texture pseudomorphs another--possibly arboreomorph--taxon (Figure 10A), but it also exists as small circular positive epirelief features with no associated branching structures preserved (Figure 10C) or as a less pronounced low-relief obovate shape (Figure 10B). When wet, these fossils are distinguished by their slightly lighter colour, suggesting that there may be some biomineral present or there is a differential fill of a leuconoid-like aquiferous cavity (Figure 10C).
Mistaken Point on the southeast coast of Newfoundland’s Avalon Peninsula is well known for its abundant deep marine Ediacaran biotas (Narbonne, 2005), with most work having focused on fossils from the taxonomically diverse E Surface (Seilacher, 1992; Narbonne, 2005; Darroch et al., 2013; Taylor et al., 2021, 2023; Vixseboxse et al., 2021; McIlroy et al., 2022). The underlying D Surface is however under-studied and includes examples of recently described arboreomorphs (Pérez-Pinedo et al., 2022) and the abundant rangeomorphs Fractofusus (Taylor et al., 2023), Bradgatia (Flude and Narbonne, 2008) and Pectinifrons (Bamforth et al., 2008), as well as rare Hapsidophyllas flexibilis (Taylor et al., 2021). Previously overlooked on the D and parts of the E surface are surface textures comparable to those of “Blackbrookia”, except preserved as internal moulds of the pores (Figure 10A-C). In some cases, the low relief porose texture pseudomorphs another--possibly arboreomorph--taxon (Figure 10A), but it also exists as small circular positive epirelief features with no associated branching structures preserved (Figure 10C) or as a less pronounced low-relief obovate shape (Figure 10B). When wet, these fossils are distinguished by their slightly lighter colour, suggesting that there may be some biomineral present or there is a differential fill of a leuconoid-like aquiferous cavity (Figure 10C).
Competing Models for “Blackbrookia” Morphology
Competing models for the “Blackbrookia”- rangeomorph association are explored, as well as possible systematic interpretations of the taxon. Any viable model should account for all the observations and such models compared to determine whether there is a likely best fit.
Association with underlying frondose organisms. The association of rangeomorph branching at low topographic levels in some “Blackbrookia”, makes it tempting to suggest that the Rangeomorpha might have had a leuconoid-like aquiferous system with ostia and oscula. In such a model, rangeomorphs might be reclining organisms with a high surface area lower surface, possibly for harbouring chemosymbionts (cf. Dufour and McIlroy, 2017; McIlroy et al., 2021; Pasinetti and McIlroy, 2023; McIlroy, 2025), in which case the upper sponge-like surface might be used for feeding and respiration, with an aquiferous system in the intervening cavity.
Since the upper surface of some rangeomorph taxa are known from rare examples of three-dimensional preservation, including: the Upper Island Cove biota in Newfoundland (Narbonne, 2004; Narbonne et al., 2009; Brasier et al., 2012; McKean et al., 2023); rangeomorphs from the Flinders Ranges, Australia (Gehling, 1999); and two specimens of Charnia from the White Sea (Butterfield, 2020). Of these, only the White Sea Charnia have pores (compared to the Cnidaria by Butterfield, 2022) and those specimens have rangeomorph-type architecture on the upper surface. None of these examples are similar to the morphology of the Catalina Dome “Blackbrookia”, which is considered highly unlikely to be a rangeomorph and alternate hypotheses must be sought. The preservational style seen might reflect the lower surface of reclining rangeomorphs being rather firm, even postmortem (Dufour and McIlroy, 2017), and potentially being further cast by the basal tissues of the “Blackbrookia”.
Associations between necromass (ivesheadiomorphs) and subsequent rangeomorphs (especially Fractofusus) resulting from community succession is common in the Ediacaran of Newfoundland (Liu et al., 2011; Dufour and McIlroy, 2017), giving cause for caution in interpreting the Catalina Dome “Blackbrookia” as the top of a rangeomorph. The association of pustulate surfaces with an arboreomorph on the D surface at Mistaken Point (Figure 10A) and other taxa such as a strap-like form on the E surface suggest that the morphology is not restricted to the Rangeomorpha, which are a clade distinct from the Arboreomorpha (Dececchi et al., 2017; Pérez-Pinedo et al., 2022). As such, the hypothesis that the “Blackbrookia” of the JDS are the tops of rangeomorphs is rejected.
If not rangeomorph then what? Many possibilities have been proposed for the affinities of different elements of the classic frondose members of the Ediacaran biota, with authors suggesting that some taxa might be ancestors of modern taxonomic groups, including fungi, metazoans, protists and algae (e.g., Retallack, 1994; Boynton and Ford, 1995; Dunn et al., 2021) extinct “stem” groups of modern clades (Xiao and Laflamme, 2009) or, in some cases, even extinct kingdoms (Seilacher, 1992). It is clear however that the Ediacaran biota includes a large diversity of taxa, often superficially similar to each other but with substantially different Bauplans (for example, frondose rangeomorphs and arboreomorphs; Dececchi et al., 2017), suggesting that the diversity of Ediacaran ecosystem has been largely underestimated and the taxonomic position of each fossil should be considered separately.
The appearance of the proposed perforate upper surface of the “Blackbrookia” component does resemble some end members of Kinneyia-type microbial matground surfaces (Noffke et al., 2021; Figure 10D), though the expression of matground pustules is often trigonous rather than circular and the porose structures in “Blackbrookia” are limited to the top of the fossil and are not observed in the surrounding matground. The porose and particularly the papillate textures of the Catalina Dome “Blackbrookia” are difficult to reconcile with a microbial matground model and are better explained as infilled structures that represented raised openings in the body of the organisms in life. While it seems evident that “Blackbrookia” is the body fossil of a collapsed macro-organism, determining its systematic nature remains, as for many other Ediacaran taxa, problematic.
It is unlikely that “Blackbrookia” is an algal taxon or any other photosynthetic group, as the Ediacaran successions of the Catalina Dome have been inferred to have been deposited at substantial water depths, including at the bottom of the continental slope (O’Brien and King, 2004, 2005; Mason et al., 2013). Similarly, we can reject the hypothesis that “Blackbrookia” was fungal, as most marine fungi are microscopic, and no marine mushrooms are known (Cunliffe, 2023). Instead, the size of the organisms, as well as their complex morphology, suggest that they could be investigated as candidate metazoans. The discovery of the crown group staurozoan cnidarians Haootia quadriformis and Mamsetia manunis (Liu et al., 2014; McIlroy et al., 2024) in nearby strata of similar age, confirms that eumetazoans had already evolved and differentiated during the Ediacaran. However, there is sparse evidence for bilaterians in the Avalon Assemblage (Liu and McIlroy, 2015) and “Blackbrookia” does not have any characteristic eumetazoan traits.
The porose morphology of the best-preserved example leads us to suggest that “Blackbrookia”, from the Catalina Dome in particular, are candidates for being considered sponges. The pores in “Blackbrookia” resemble “oscula” (i.e., the exhalent openings) of modern encrusting demosponges. However, encrusting demosponges are typically amorphous in outline (in contrast with the regular outlines of “Blackbrookia”). Additionally, early sponges are believed to have evolved from thin-bodied taxa with only one exhalant opening (Botting and Muir, 2018). Botting and Muir (2018) refute the presence of Ediacaran sponges, including specimens with articulated spicules (Li et al., 1998; Wörheide et al., 2012; Dohrmann and Wörheide, 2017) in favour of an Early Cambrian evolution and diversification, though the newly described Helicolocellus cantori would seem to meet all criteria for being a crown group sponge (Wang et al. 2024). This hypothesis is supported by some molecular evidence, which determine Ctenophora as sister group of all other animals rather than the Porifera, which have traditionally been considered the most basal of all metazoans (Jákely et al., 2015; Ryan and Chiodin, 2015; Schultz et al., 2023).
Even if molecular analyses were to positively identify the Ctenophora as sister group of all other Metazoa, this would make the Porifera a sister group of the Parahoxozoa (or “Eumetazoa”, sensu Erives and Fritzsch, 2020), the clade including the Placozoa, Cnidaria, and Bilateria, of which the Cnidaria have been confirmed to be present in the Avalon Assemblage (Menon et al. 2013; Liu et al., 2014; 2015; McIlroy et al., 2024). Therefore, even though there is not enough evidence to positively assess “Blackbrookia” as a poriferan, it is also not possible to reject this hypothesis yet on stratigraphic grounds.
Lastly, the possibility remains that “Blackbrookia” is an extinct Porifera-like clade, with no Phanerozoic record, as has been proposed for almost all Ediacaran groups at some stage (e.g., Seilacher, 1992). This possibility should be taken into consideration, even though it is not possible to falsify it.
DISCUSSION
The macrofossils of the Ediacaran biota are difficult to treat systematically, partly because of the difficulties in preserving soft-bodied Ediacaran organisms (Gehling, 1999; McIlroy et al., 2009); their complex morphology (Brasier and Antcliffe, 2004, 2009; Narbonne, 2004); and the fact that we know little about their life cycles (Brasier and Antcliffe, 2004; Mitchell et al., 2015; Liu and Dunn, 2020) and especially affinities (Ford, 1958; Jenkins, 1985; Seilacher, 1992, 1999; Retallack, 1994; Sperling et al., 2007; Sperling and Vinther, 2010; Butterfield, 2020; Dunn et al., 2021). There is also a lack of clarity over whether many Ediacaran taxa should be covered by the International Code of Zoological Nomenclature (ICZN) or the International Code of Nomenclature for algae, fungi and plants (ICN; formerly the International Code of Botanical Nomenclature). Many Ediacaran macrofossils are known primarily from their morphologically simple basal disks that have few diagnostic characters (e.g., Aspidella s.l.; Hiemalora). Some discoidal taxa have even been found in association with more than one frondose taxon. For example, both Primocandelabrum hiemaloranum (Hofmann et al., 2008) and Arborea sp. (Wang et al., 2020) have both been observed with a Hiemalora base, despite apparently belonging to two unrelated clades of frondose taxa (the Rangeomorpha and Arboreomorpha respectively; see Dececchi et al., 2017; Pérez-Pinedo et al., 2022). Additionally, many occurrences of Hiemalora do not have any associated frond (Fedonkin, 1982). For this reason, organ taxa--which are accepted in the ICN but not the ICZN--have been invoked (Hofmann et al., 2008), despite recent suggestions that the Rangeomorpha and Arboreomorpha belong in the Eumetazoa (Dunn et al., 2019, 2021).
The discussion above suggests that the taxa referred to by the obsolete name “Blackbrookia” from the Catalina Dome (Figure 1) are distinct from the pseudofossil Blackbrookia from the UK (Liu et al., 2011). As such the material requires formal taxonomic treatment.
SYSTEMATIC PALAEONTOLOGY
LYDONIA gen. nov.
zoobank.org/BE15838D-C027-419B-AFCA-0DCB16578C5C
V* 2008 Blackbrookia sp., Hofmann et al., figs. 25, 1-5.
V* 2015 ‘Blackbrookia’, Liu et al., fig. 5g.
V* 2017 Blackbrookia, Dufour and McIlroy, fig. 2a-c.
V* 2017 ‘Blackbrookia’, Liu et al., fig. 14f.
V* 2021 cf. Crumillospongia, McIlroy et al., fig. 5a.
Etymology. Named for the punk rock legend John Lydon, with whom this taxon is considered to have shared a spiky “hairstyle”.
Diagnosis. Ovate to obovate fossils commonly preserved in positive relief and covered in small pores (<0.5 cm), or a mesh-like reticulate structure. Upper surface is typically longitudinally folded, sometimes with a positive relief rim. Typical size range is from 15-40 cm in the longest dimension.
Type species. Lydonia jiggamintia by monotypy.
Lydonia jiggamintia sp. nov.
zoobank.org/57A63DFC-41BF-4F47-AC91-290E459110BF
Etymology. Jiggamintia is latinized from jiggamint, the Beothuk word for gooseberry (a spiky current). We use the Beothuk word to honour the original inhabitants of the island of Newfoundland who were made culturally extinct by European colonizers. Isolated words were recorded from captured Beothuk in the early 1800’s (especially from Oubee, Desmasduwit and Shanawdithit).
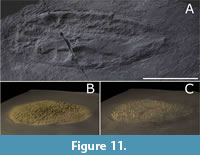
Holotype. Specimen remains in situ on the Johnson Discovery Surface (Figure 2A, Figure 11), from the Trepassey Formation of the Catalina Dome, Bonavista Peninsula, Newfoundland, Canada (Figure 1B-C).
Plastotype. A cast of the holotype and paratype A has previously been accessioned to the Rooms Corporation of Newfoundland and Labrador (St. John’s, NL) with the accession number NFM F-534 (Hofmann et al., 2008). Since the type material remains in the field as required by provincial law, the accessioned material is to act for the type material.
Paratype. Paratype A: regular specimen, in situ on the JDS (Figure 2A, bottom); paratype B: irregular specimen, in situ on the JDS (Figure 2B).
Description. The holotype is an obovate specimen with a smooth profile and a clear polarity along the longest axis from a rounded end to a pointed end and is preserved as a positive epirelief on the fossiliferous surface, from which it is separated by an abrupt positive rim. The long axis is 32.3 cm long and the short axis is 10.4 cm long, with a ratio of 3.11. The holotype is symmetrical with respect to the long axis. The holotype has a longitudinal fold in its central portion that runs all the way from the rounded pole to the pointed one. Both sides of the ridge have an extensive mesh-like pattern of small pores of regular size, with a diameter of about 0.5 cm. Two main morphologies are present: regular specimens, such as paratype A, which strongly resemble the obovate shape of the holotype, and irregular specimens, such as paratype B, which has a lobate profile but a similar porose surface and raised rim.
Discussion. Lydonia are variable in shape, with ovate, circular, sock-shaped, semi-circular and almost square forms known. The upper surface may be covered in small pores, which might have been openings in life, possibly with raised rims or papillae. The size distribution of pores or mesh size is not systematic but spacing in-between is typically less than 0.5 cm. Common gross outlines of Lydonia are obovate and ovate (cf. Holotype, paratype A), but many specimens have an irregular profile that diverge from a regular ovoid with lobes and convex curves in their profile (cf. Paratype B). Both ovoid and irregular forms may have one or more folded ridges and a positive relief rim.
Morphological Reconstruction
The mode of life of Lydonia is that of a sessile epibenthic (Figure 11B) or semi-infaunal organism (Figure 11C), which potentially colonized other macro-organisms. To date, Lydonia is only documented from surfaces with abundant Fractofusus, though the two are not thought to be taxonomically related. The wrinkled upper surface of Lydonia resembles that of a collapsed upper surface that was originally slightly inflated in life (Figure 6B). The distribution of pores and the presence of papillae is based on direct morphological evidence from the holotype in the form of short silty internal moulds of papillae, and by comparison with modern papillate sponges such as the demosponge Polymastia.
Other pustulate taxa from Mistaken Point in south-eastern Newfoundland (Figure 10A-C) are likely to have had similar morphologies; they are found to be overgrowing other organisms (arboreomorphs) or as discrete colonies. The Mistaken Point fossils extend laterally beyond the underlying organism to grow in contact with the seafloor and its matground. The lack of wrinkling in those Mistaken Point Lydonia suggests that they had a thinner body than the Lydonia from the Catalina Dome, potentially analogous to that of modern encrusting demosponges (Goodwin et al., 2021). A single specimen has bulging 3D textures near the presumed centre of the body, suggesting that the middle of the organism was thicker than nearer the margins (Figure 10C).
Palaeobiology and Development of the Catalina Dome Specimens
Linear regressions between tV2 (short axis) and tV3 (ratio) show a negative correlation (Figure 3D), indicating that the species was likely subject to allometric growth. In an isometric growth model, we would expect tV3 to remain constant through the life of the specimen, but it appears that the long axis was subject to faster growth than the short, possibly to increase the surface exposed to the current without too much increase in drag. Under the assumption of allometric growth, V3 and tV3 can be interpreted to be loosely correlated to the age of the organism. This would create a fast flow of current on top of the specimen along its long axis, increasing its access to particulate and dissolved organic matter and enabling improved gas exchange with the water column. This is also consistent with the lower long/short axis ratio characteristic of specimens oriented perpendicular to the current: an increase of the short axis relative to the long axis would have elongated the specimen in the direction parallel to the current. Similar allometric growth was described by Darroch et al. (2013) for several taxa from the Mistaken Point E and D surfaces, including Thectardis avalonensis and Fractofusus misrai.
Backtransform morphospace analyses suggest that differences in the profile shape of the specimens on the JDS do not represent taxonomic differences. Regular and irregular comprise a continuum, with differences probably arising from developmental and palaeobiological factors or, in some cases, such as the Ivesheadia-like specimens, post-mortem processes. This would also be consistent with the large percentage of irregular specimens lacking pores, possibly due to necrosis and/or microbial overgrowth (cf. Liu et al., 2011). We observe that regular specimens are typically longer than irregular specimens (Figure 9A), suggesting that they might have either 1) colonized the surface earlier, 2) have a micro-environmental advantage or 3) have a rheotropic advantage.
As there is supporting evidence for the organisms being reclining on the seafloor or semi-infaunal, possibly covered in microbial matground (Figure 11C), it is possible that the obovate shape of the strongly parallel-oriented end members of the population represents an advantageous adaptation to the inferred directional paleocurrents, by increasing the exposed surface without increasing drag or lift.
If we interpret Lydonia as an epibenthic organism, which potentially settled by encrusting and overgrowing other organisms, it is possible that the differences in shape could also be attributed to the characteristics of the host organism. Lydonia encrusting small organisms or highly decomposed organisms might have soon run out of space (or nutrients if they performed saprotrophy in some way) and might also exhibit secondary growth (Figure 2B).
Population Models
Our analyses suggest that the specimens on the JDS all belong to the same species and that there are no distinctive size modes or age classes. Darroch et al. (2013) gives three possible explanations to the absence of size modes within Ediacaran benthic communities: 1) the all for one model, where all of the specimens belong to the same age class and are the result of a single colonization/recruitment event; 2) the slow and steady model, in which growth rates are slower relative to the seasonal reproductive rates and 3) the continuous reproduction model, in which the absence of age classes is the result of continuous aseasonal reproduction. All the three models have been documented in benthic marine invertebrate communities. The all for one model typically results in organisms of very similar sizes (e.g., holothurians Billett and Hansen, 1982), and it would require invoking local environmental differences to explain the high size variability observed in the JDS community. Ediacaran seafloors are typically interpreted as being rather homogeneous (Butterfield, 1997; Darroch et al., 2013), in terms of spatial distribution of nutrients and for this reason the all for one model was deemed unlikely to explain the single size modes in several Ediacaran communities in Newfoundland (Darroch et al., 2013). Moreover, we observe a large variability for the V3 and tV3 variables in the JDS population, which has been interpreted as a possible proxy for age. In a slow and steady model, age classes resulting from seasonal reproductive events are present, but slow growth rates of the organisms result in a continuous size-frequency distribution and a single size mode, while in a continuous reproduction model the absence of different size modes is the result of aseasonal reproduction.
Support for a slow and steady model in Ediacaran communities can be found in modern deep-marine benthic communities, which are characterized by slower growth rates than similar taxa in shallow waters (e.g., bivalves, Turekian et al., 1975; and octocorals Cordes et al., 2001). Modern sponges have been found to reproduce seasonally in both deep and shallow water settings (Witte, 1996; Shaffer et al., 2020); this is coupled with slow growth rates and great longevity (Leys and Lauzon, 1998; Baquiran et al., 2020). In some cases, growth rates might also be correlated with seasonality (Leys and Lauzon, 1998). When growth rates are fast, as in the case of the giant barrel sponge, seasonal reproduction could result in distinct size modes within a population (McMurray et al., 2010).
Since we could not recognize seasonal or aseasonal reproductive patterns in the Lydonia assemblage of the JDS, it is not possible to distinguish between the two hypotheses and a combination of the two (aseasonal reproduction and slow growth rates) is also possible.
Candidate Sponges of Ediacaran Age from Newfoundland
Candidate sponges among the Ediacaran biota are generally contentious but of significant importance for the calibration of molecular clocks (e.g., Cummings et al., 2017). The Ediacaran taxon that is most widely attributed to the Porifera or pre-sponges is the inferred infundibuliform Thectardis avalonensis (Clapham et al., 2004; Sperling et al., 2007; Dufour and McIlroy, 2017; Aragonés Suarez and Leys, 2022), which likely lived in a reclined position commonly with the open end of the cone either orientated directly into or away from a weak current, at least at Mistaken Point E Surface (McIlroy et al., 2022b), and is also present on the Johnson Discovery Surface. Specimens of Thectardis do not preserve spicules and as such do not meet the bar that has been set for confirming a fossil poriferan by Antcliffe et al. (2014). While the fossil record of the Porifera might be contentious, there is also scope for the preservation of Cavalier-Smith (2017) “pre-sponge” grade of organization (Dufour and McIlroy, 2017, 2018). It is perhaps possible that Thectardis meets the criteria for this pre-sponge grade of organization. Other porose taxa without spicules that could be considered as possible sponges include Kuckaraukia sp. and Gibbavasis kushkii from what may be the latest Ediacaran of Iran (Razumovskiy et al., 2015; Vaziri et al., 2018).
CONCLUSIONS
Determining the clade to which Ediacaran macrofossils belong is challenging. An unequivocal sponge fossil would need evidence for an aquiferous system including ostia/oscula, along with evidence for a spiculate skeleton. Spiculae and a clear osculum have not been recognized in Lydonia or the other sponge-like taxa in the offshore shelf to deep-marine Ediacaran strata of Avalonian Newfoundland and therefore does not meet the bar for being an unequivocal poriferan (Antcliffe et al., 2014). The presence of an aquiferous system is evinced by the inferred originally inflated morphology of Lydonia and the evidence for numerous openings (similar to demosponge osculae) of the upper surface, which were possibly raised into the water column on short papillae. The presence of pores on the upper surface of Lydonia is likely correlated with a filter-feeding mode of life, possibly similar to that of modern encrusting sponges.
The superimposition of Lydonia on rangeomorph and arboreomorph fossils is unlikely to be accidental. Having excluded Lydonia as being part of the underlying organism, we are left with the possibility that the dead bodies of other organisms would have offered a settling substrate for larval or juvenile Lydonia, and it could be possibly related to a saprotrophic secondary feeding strategy. The population structure of a Lydonia -rich assemblage in the Ediacaran of Avalonia reveals a single size mode, similar to that of other Ediacaran assemblages (Darroch et al., 2013) and consistent with modern poriferan population structure (Baquiran et al., 2020), but not unequivocally.
The poriferan-like morphology of Lydonia suggests that other evidence of sponges--such as spicules--might yet be found in the Ediacaran of Avalonia. Such evidence would then meet the bar set by Antcliffe et al. (2014) for conclusive demonstration of Ediacaran sponges that could then be used to help unravel the early history of animal life.
ACKNOWLEDGMENTS
Fossil surfaces in the Bonavista Peninsula are protected under Reg. 67/11, of the Historic Resources Act 2011 and their study is only allowed under permission from the Government of Newfoundland and Labrador. Fieldwork near Port Union was conducted under permit (P21.05) from the Government of Newfoundland and Labrador. Thanks are due to E. and N. Samson for their kind support;. M. Steele for help collecting field data; D. Johnson for his discovery of the Johnson Discovery Surface.
REFERENCES
Antcliffe, J.B., Callow, R.H.T., and Brasier, M.D. 2014. Giving the early fossil record of sponges a squeeze. Biological Reviews, 89:972-1004.
https://doi.org/10.1111/brv.12090
Antcliffe, J.B., Hancy, A.D., and Brasier, M.D. 2015. A new ecological model for the ∼565Ma Ediacaran biota of Mistaken Point, Newfoundland. Precambrian Research, 268:227-242.
https://doi.org/10.1016/j.precamres.2015.06.015
Aragonés Suarez, P. and Leys, S.P. 2022. The sponge pump as a morphological character in the fossil record. Paleobiology, 48:446-461.
Bak, R. and Meesters, E. 1998. Coral population structure: the hidden information of colony size-frequency distributions. Marine Ecology Progress Series, 162:301-306.
https://doi.org/10.3354/meps162301
Bamforth, E.L., Narbonne, G.M., and Anderson, M.M. 2008. Growth and ecology of a multi-branched Ediacaran rangeomorph from the Mistaken Point assemblage, Newfoundland. Journal of Paleontology, 82:763-777.
https://doi.org/10.1666/07-112.1
Baquiran, J.I.P., Nada, M.A.L., Posadas, N., Manogan, D.P., Cabaitan, P.C., and Conaco, C. 2020. Population structure and microbial community diversity of two common tetillid sponges in a tropical reef lagoon. PeerJ, 8:e9017.
https://doi.org/10.7717/peerj.9017
Billett, D.S.M. and Hansen, B. 1982. Abyssal aggregations of Kolga hyaline, Danielssen and Koren (Echinodermata: Holothurioidea) in the northeast Atlantic Ocean: a preliminary report. Deep-Sea Research, 29:799-818.
https://doi.org/0198-0149/82/070799-19 $03.00/0
Botting, J.P. and Muir, L.A. 2018. Early sponge evolution: A review and phylogenetic framework. Palaeoworld, 27:1-29.
https://doi.org/10.1016/j.palwor.2017.07.001
Boynton, H.E. and Ford, T.D. 1995. Ediacaran fossils from the Precambrian (Charnian Supergroup) of Charnwood Forest, Leicestershire, England. Mercian Geologist, 13:156-182.
Brasier, M. and Antcliffe, J.B. 2004. Decoding the Ediacaran Enigma. Science, 305:1115-1117.
https://doi.org/10.1126/science.1102673
Brasier, M.D., and Antcliffe, J.B. 2009. Evolutionary relationships within the Avalonian Ediacara biota: new insights from laser analysis. Journal of the Geological Society, 166:363-384.
https://doi.org/10.1144/0016-76492008-011
Brasier, M.D., Liu, A. G., Menon, L., Matthews, J.J., McIlroy, D., and Wacey, D. 2012. Explaining the exceptional preservation of Ediacaran rangeomorphs from Spaniard’s Bay, Newfoundland: A hydraulic model. Precambrian Research, 231:122-135.
https://doi.org/10.1016/j.precamres.2013.03.013
Butterfield, N J. 1997. Plankton ecology and the Proterozoic-Phanerozoic transition. Paleobiology, 23:247-262.
https://doi.org/10.1017/S009483730001681X
Butterfield, N.J. 2022. Constructional and functional anatomy of Ediacaran rangeomorphs. Geological Magazine, 159:1148-1159.
https://doi.org/10.1017/S0016756820000734
Cavalier-Smith, T. 2017. Origin of animal multicellularity: precursors, causes, consequences--the choanoflagellate/sponge transition, neurogenesis and the Cambrian explosion. Philosophical Transitions of the Royal Society of Biology, 372:20150476.
https://doi.org/10.1098/rstb.2015.0476
Clapham, M.E., Narbonne, G.M., Gehling, J.G., Greentree, C., and Anderson, M.M. 2004. Thectardis avalonensis: A new Ediacaran fossil from the Mistaken Point biota, Newfoundland. Journal of Paleontology, 78:1031-1036.
https://doi.org/10.1666/0022-3360(2004)078<1031:taanef>2.0.co;2
Cordes, E.E., Nybakken, J.W., and VanDykhuizen, G. 2001. Reproduction and growth of Anthomastus ritteri (Octocorallia: Alcyonacea) from Monterey Bay, California, USA. Marine Biology, 138:491-501.
https://doi.org/10.1007/s002270000470
Cunliffe, M. 2023. Who are the marine fungi? Environmental Microbiology, 25:131-134.
https://doi.org/10.1111/1462-2920.16240
Darroch, S.A.F., Laflamme, M., and Clapham, M.E. 2013. Population structure of the oldest known macroscopic communities from Mistaken Point, Newfoundland. Paleobiology, 39:591-608.
https://doi.org/10.1666/12051
Dececchi, T.A., Narbonne, G.M., Greentree, C., and Laflamme, M. 2017. Relating Ediacaran Fronds. Paleobiology, 43:171-180.
https://doi.org/10.1017/pab.2016.54
Dohrmann, M. and Wörheide, G. 2017. Dating early animal evolution using phylogenomic data. Scientific Reports, 7:3599. https://doi.org/10.1038/s41598-017-03791-w
Dufour, S.C. and McIlroy, D. 2017. Ediacaran pre-placozoan diploblasts in the Avalonian biota: the role of chemosynthesis in the evolution of early animal life. Geological Society, London, Special Publications, 448:211-219.
https://doi.org/10.1144/SP448.5
Dufour, S.C. and McIlroy, D. 2018. An Ediacaran pre-placozoan alternative to the pre-sponge route towards the Cambrian explosion of animal life: a comment on Cavalier-Smith 2017. Philosophical Transactions of the Royal Society of Biology, 373.
https://doi.org/10.1098/rstb.2017.0148
Dunn, F.S., Grazhdankin, D.V., Liu, A.G., and Wilby, P.R. 2021. The developmental biology of Charnia and the eumetazoan affinity of the Ediacaran rangeomorphs. Science Advances, 7:eabe0291.
https://doi.org/10.1126/sciadv.abe0291
Erives A. and Fritzsch B. 2020. A Screen for Gene Paralogies Delineating Evolutionary Branching Order of Early Metazoa. G3 (Bethesda). 10(2):811-826.
https://doi.org/10.1534/g3.119.400951
Fedonkin, M.A. 1982. A new generic name for some Precambrian Coelenterates. Paleontologicheskiy Zhurnal, 2:137-154.
Flude, L.I. and Narbonne, G.M. 2008. Taphonomy and ontogeny of a multibranched Ediacaran fossil: Bradgatia from the Avalon Peninsula of Newfoundland. Canadian Journal of Earth Science, 45:1095-1109.
https://doi.org/10.1139/E08-057
Ford, T.D. 1958. Pre-Cambrian fossils from Charnwood Forest. Proceedings of the Yorkshire Geological Society, 31:211-217.
https://doi.org/10.1144/pygs.31.3.211
Gehling, J.G. 1999. Microbial mats in terminal Proterozoic siliciclastics: Ediacaran death masks. PALAIOS, 14:40.
https://doi.org/10.2307/3515360
Gehling, J.G. and Narbonne, G.M. 2007. Spindle-shaped Ediacara fossils from the Mistaken Point assemblage, Avalon Zone, Newfoundland. Canadian Journal of Earth Science, 44:367-387.
https://doi.org/10.1139/e07-003
Goodwin, C., Dinn, C., Nefedova, E., Nijhof, F., Murillo, F.J., and Nozères, C. 2021. Two new species of encrusting sponge (Porifera, family Crellidae) from eastern Canada. Canadian Journal of Zoology, 99:760-772.
https://doi.org/10.1139/cjz-2021-0041
Hofmann, HJ., O’Brien, S. J., and King, A.F. 2008. Ediacaran biota on Bonavista Peninsula, Newfoundland, Canada. Journal of Paleontology, 82:1-36.
https://doi.org/10.1666/06-087.1
Jákely, G., Paps, J., and Nielsen, C. 2015. The phylogenetic position of ctenophores and the origins of nervous systems. EvoDevo, 6:1.
https://doi.org/10.1186/2041-9139-6-1
Jenkins, R.J.F. 1985. The enigmatic Ediacaran (late Precambrian) genus Rangea and related forms. Paleobiology, 11:336-355.
https://doi.org/10.1017/S0094837300011635
Kenchington, CG. and Wilby, P.R. 2014. Of time and taphonomy: preservation in the Ediacaran. The Paleontological Society Papers, 20:23.
https://doi.org/https://doi.org/10.1017/S1089332600002825
Laflamme, M., Narbonne, G.M., Greentree, C., and Anderson, M.M. 2007. Morphology and taphonomy of an Ediacaran frond: Charnia from the Avalon Peninsula of Newfoundland. Geological Society, London, Special Publications, 286:237-257.
https://doi.org/10.1144/SP286.17
Laflamme, M., Flude, L.I., and Narbonne, G.M. 2012. Ecological tiering and the evolution of a stem: the oldest stemmed frond from the Ediacaran of Newfoundland, Canadian Journal of Paleontology, 86:193-200.
https://doi.org/10.1666/11-044.1
Leys, S.P. and Lauzon, N.R.J. 1998. Hexactinellid sponge ecology: growth rates and seasonality in deep water sponges. Journal of Experimental Marine Biology and Ecology, 230:111-129.
https://doi.org/10.1016/S0022-0981(98)00088-4
Li, C.-W., Chen, J.-Y., and Hua, T.-E. 1998. Precambrian sponges with cellular structures. Science, 279:879-882.
https://doi.org/10.1126/science.279.5352.879
Liu, A.G. 2016. Framboidal pyrite shroud confirms the “Death Mask” model for moldic preservation of Ediacaran soft-bodied organism. PALAIOS, 31:259-274.
https://doi.org/10.2110/palo.2015.095
Liu, A.G., McIlroy, D., Antcliffe, J.B., and Brasier, M.D. 2011. Effaced preservation in the Ediacara biota and its implications for the early macrofossil record. Palaeontology, 54:607-630.
https://doi.org/10.1111/j.1475-4983.2010.01024.x
Liu, A.G., Matthews, J.J., Menon, L.R., McIlroy, D., and Brasier, M.D. 2014. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the Late Ediacaran period approx. 560 Ma. Proceedings of the Royal Society of Biology, 28:20141202.
https://doi.org/10.1098/rspb.2014.1202
Liu, A.G. and McIlroy, D. 2015. Horizontal surface traces from the Fermeuse Formation, Ferryland Newfoundland, Canada, and their place within the Late Ediacaran ichnological revolution. Geological Association of Canada, Miscellaneous Publication 9:41-156.
Liu, A.G., Matthews, J.J., and McIlroy, D. 2016. The Beothukis/Culmofrons problem and its bearing on Ediacaran macrofossil taxonomy: evidence from an exceptional new fossil locality. Palaeontology, 59:45-58.
https://doi.org/10.1111/pala.12206
Liu, A.G. and Dunn, F.S. 2020. Filamentous connections between Ediacaran fronds. Current Biology, 30:1322-1328.e3.
https://doi.org/10.1016/j.cub.2020.01.052
Mapstone, N.B. and McIlroy, D. 2006. Ediacaran fossil preservation: Taphonomy and diagenesis of a discoid biota from the Amadeus Basin, central Australia. Precambrian Research, 149:126-148.
https://doi.org/10.1016/j.precamres.2006.05.007
Mason, S.J., Narbonne, G.M., Dalrymple, R.W., and O’Brien, S.J. 2013. Paleoenvironmental analysis of Ediacaran strata in the Catalina Dome, Bonavista Peninsula, Newfoundland. Canadian Journal of Earth Science, 50:197-212.
https://doi.org/10.1139/cjes-2012-0099
Matthews, J.J., Liu, AG., Yang, C., McIlroy, D., Levell, B., and Condon, D. J. 2020. A chronostratigraphic framework for the rise of the Ediacaran macrobiota: New constraints from Mistaken Point Ecological Reserve, Newfoundland. Geological Society of America Bulletin, 133:612-524.
https://doi.org/10.1130/B35646.1
McIlroy D. 2016 Architectural modelling of the fractal-like Ediacaran rangeomorph Charnia masoni. Journal of the Geological Society, 182 (3):2024-242.
https://doi.org/10.1144/jgs2024-242
McIlroy, D. and Walter, M.R. 1997. A reconsideration of the biogenicity of Arumberia banksi Glaessner & Walter. Alcheringa: An Australasian Journal of Palaeontology, 21:79-80.
https://doi.org/10.1080/03115519708619187
McIlroy, D., Brasier, M.D., and Lang, AS. 2009. Smothering of microbial mats by macrobiota: implications for the Ediacara biota. Journal of the Geological Society, 166:1117-1121.
https://doi.org/10.1144/0016-76492009-073
McIlroy, D., Dufour, S. C., Taylor, R., and Nicholls, R. 2021. The role of symbiosis in the first colonization of the seafloor by macrobiota: Insights from the oldest Ediacaran biota (Newfoundland, Canada). Biosystems, 205:104413.
https://doi.org/10.1016/j.biosystems.2021.104413
McIlroy, D., Pérez-Pinedo, D., Pasinetti, G., McKean, C., Taylor, R.S., and Hiscott, R.N. 2022. Rheotropic epifaunal growth, not felling by density currents, is responsible for many Ediacaran fossil orientations at Mistaken Point. Frontiers in Earth Science, 10:849194.
https://doi.org/10.3389/feart.2022.849194
McIlroy, D. Pasinetti, G., Pérez-Pinedo, D., McKean, C., Dofour, S.C., Matthews, J.J., Menon, L.R., Nichols, B., Taylor, R.S. 2024. The Palaeobiology of Two Crown Group Cnidarians: Haootia quadriformis and Mamsetia manunis gen. et sp. nov. from the Ediacaran of Newfoundland, Canada. Life, 14(9): 1096.
https://doi.org/10.3390/life14091096
Mckean, C., Taylor, R.S., and McIlroy, D. 2023. New taphonomic and sedimentological insights into the preservation of high-relief Ediacaran fossils at Upper Island Cove, Newfoundland. Lethaia, 56:1-17.
https://doi.org/10.18261/let.56.4.2
McMurray, S.E., Henkel, TP., and Pawlik, J. R. 2010. Demographics of increasing populations of the giant barrel sponge Xestospongia muta in the Florida Keys. Ecology, 91:560-570.
https://doi.org/10.1890/08-2060.1
Meesters, E., Hilterman, M., Kardinaal, E., Keetman, M., De Vries, M., and Bak, R. 2001. Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation. Marine Ecology Progress Series, 209:43-54.
https://doi.org/10.3354/meps209043
Menon, L.R., McIlroy, D., and Brasier, M.D. 2013. Evidence for Cnidaria-like behaviour in ca. 560Ma Ediacaran Aspidella. Geology, 41, 895-898.
Mitchell, E.G. and Butterfield, NJ. 2018. Spatial analyses of Ediacaran communities at Mistaken Point. Paleobiology, 44:40-57.
https://doi.org/10.1017/pab.2017.35
Mitchell, E. G., Kenchington, C.G., Liu, A.G., Matthews, J.J., and Butterfield, N.J. 2015. Reconstructing the reproductive mode of an Ediacaran macro-organism. Nature, 524:343-346.
https://doi.org/10.1038/nature14646
Narbonne, G.M. 2004. Modular construction of Early Ediacaran complex life forms. Science, 305:1141-1144.
https://doi.org/10.1126/science.1099727
Narbonne, G.M. 2005. The Ediacara biota: Neoproterozoic origin of animals and their ecosystems. Annual Review of Earth and Planetary Science, 33:421-442.
https://doi.org/10.1146/annurev.earth.33.092203.122519
Narbonne, G.M., Laflamme, M., Greentree, C., and Trusler, P. 2009. Reconstructing a lost world: Ediacaran rangeomorphs from Spaniard’s Bay, Newfoundland. Journal of Paleontology, 83:503-523.
https://doi.org/10.1666/08-072R1.1
Noffke, N., Beraldi-Campesi, H., Callefo, F., Carmona, NB., Cuadrado, D. G., Hickman-Lewis, K., Homann, H., Mitchell R.L., Sheldon, N., Westall, F., Xiao, S. 2021. “Microbially induced sedimentary structures (MISS),” in Encyclopaedia of Geology (Elsevier), 545-554.
https://doi.org/10.1016/B978-0-08-102908-4.00109-0
O’Brien, SJ. and King, A.F. 2005. Late Neoproterozoic (Ediacaran) stratigraphy of Avalon Zone sedimentary rocks, Bonavista Peninsula, Newfoundland. Geological Survey, 05:13.
Olsen, A.M. 2017. Feeding ecology is the primary driver of beak shape diversification in waterfowl. Functional Ecology, 31:1985-1995.
https://doi.org/10.1111/1365-2435.12890
Olsen, AM. and Westneat, M. W. 2015. StereoMorph: an R package for the collection of 3D landmarks and curves using a stereo camera set-up. Methods in Ecology and Evolution, 6:351-356.
https://doi.org/10.1111/2041-210X.12326
Pasinetti, G. and McIlroy, D. 2023. Palaeobiology and taphonomy of the rangeomorph Culmofrons plumosa. Palaeontology, 66:e12671.
https://doi.org/10.1111/pala.12671
Pérez-Pinedo, D., McKean, C., Taylor, R., Nicholls, R., and McIlroy, D. 2022. Charniodiscus and Arborea are separate genera within the Arboreomorpha: Using the holotype of C. concentricus to resolve a taphonomic/taxonomic tangle. Frontiers in Earth Science, 9:785929.
https://doi.org/10.3389/feart.2021.785929
Pérez-Pinedo, D., Neville, J.M., Pasinetti, G., McKean, C., Taylor, R., and McIlroy, D. 2023. Frond orientations with independent current indicators demonstrate the reclining rheotropic mode of life of several Ediacaran rangeomorph taxa. Paleobiology, 1-22.
https://doi.org/10.1017/pab.2023.2
Porada, H., Ghergut, J., and Bouougri, E.H. 2008. Kinneyia-type wrinkle structures--critical review and model of formation. PALAIOS, 23:65-77.
https://doi.org/10.2110/palo.2006.p06-095r
Razumovskiy, A.A., Ivantsov, A. Yu., Novikov, I.A., and Korochantsev, A.V. 2015. Kuckaraukia multituberculata: A new Vendian fossil from the basal formation of the Asha Group in the South Urals. Paleontology Journal, 49:449-456.
https://doi.org/10.1134/S0031030115050111
Retallack, GJ. 1994. Were the Ediacaran fossils lichens? Paleobiology, 20:523-544.
https://doi.org/10.1017/S0094837300012975
Ryan, J.F. and Chiodin, M. 2015. Where is my mind? How sponges and placozoans may have lost neural cell types. Philosophical Transactions of the Royal Society of Biology, 370:20150059.
https://doi.org/10.1098/rstb.2015.0059
Schultz, D.T., Haddock, S.H.D., Bredeson, J.V., Green, R.E., Simakov, O., and Rokhsar, D.S. 2023. Ancient gene linkages support ctenophores as sister to other animals. Nature, 618:110-117.
https://doi.org/10.1038/s41586-023-05936-6
Scrucca, L., Fop, M., Murphy, T., Brendan, and Raftery, A., E. 2016. mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. The R Journal, 8:289.
https://doi.org/10.32614/RJ-2016-021
Seilacher, A. 1992. Vendobionta and Psammocorallia: lost constructions of Precambrian evolution. Journal of the Geological Society, 149:607-613.
https://doi.org/10.1144/gsjgs.149.4.0607
Seilacher, A. 1999. Biomat-related lifestyles in the Precambrian. PALAIOS,14:86.
https://doi.org/10.2307/3515363
Shaffer, M.R., Davy, S.K., Maldonado, M., and Bell, J J. 2020. Seasonally driven sexual and asexual reproduction in temperate Tethya species. The Biological Bulletin, 238:89-105.
https://doi.org/10.1086/708624
Smith, E.F., Nelson, L.L., Strange, M.A., Eyster, A.E., Rowland, S.M., Schrag, D.P., Macdonald, F.A. 2016. The end of the Ediacaran: Two new exceptionally preserved body fossil assemblages from Mount Dunfee, Nevada, USA. Geology, 44:911-914.
https://doi.org/10.1130/G38157.1
Sperling, E.A., Pisani, D., and Peterson, K.J. 2007. Poriferan paraphyly and its implications for Precambrian palaeobiology. Geological Society, London, Special Publications, 286:355-368.
https://doi.org/10.1144/SP286.25
Sperling, EA. and Vinther, J. 2010. A placozoan affinity for Dickinsonia and the evolution of late Proterozoic metazoan feeding modes: Placozoan affinity for Dickinsonia. Evolution & Development, 12:201-209.
https://doi.org/10.1111/j.1525-142X.2010.00404.x
Taylor, R.S., Matthews, J.J., Nicholls, R., and McIlroy, D. 2021. A re-assessment of the taxonomy, palaeobiology and taphonomy of the rangeomorph organism Hapsidophyllas flexibilis from the Ediacaran of Newfoundland, Canada. Paleontologische Zeitschrift, 95:187-207.
https://doi.org/10.1007/s12542-020-00537-4
Taylor, R.S., Nicholls, R., Neville, J.M., and McIlroy, D. 2023. Morphological variation in the rangeomorph organism Fractofusus misrai from the Ediacaran of Newfoundland, Canadian Geological Magazine, 160:146-166. https://doi.org/10.1017/S0016756822000723
Turekian, K.K., Cochran, J.K., Kharkar, D.P., Cerrato, R.M., Vaisnys, J.R., Sanders, H.L., Grassle, J.F., Allen, J.A. 1975. Slow growth rate of a deep-sea clam determined by 228Ra chronology. Proceedings of the National Academy of Science of the U.S.A., 72:2829-2832.
https://doi.org/10.1073/pnas.72.7.2829
Vaziri, S.H., Majidifard, M.R., and Laflamme, M. 2018. Diverse assemblage of Ediacaran fossils from Central Iran. Scientific Reports, 8:50-60.
https://doi.org/10.1038/s41598-018-23442-y
Vixseboxse, PB., Kenchington, C. G., Dunn, F.S., and Mitchell, E.G. 2021. Orientations of Mistaken Point fronds indicate morphology impacted ability to survive turbulence. Frontiers in Earth Science, 9:762824.
https://doi.org/10.3389/feart.2021.762824
Waggoner, B. 2003. The Ediacaran biotas in space and time. Integrative and Comparative Biology, 43:104-113.
https://doi.org/10.1093/icb/43.1.104
Wang, X., Pang, K., Chen, Z., Wan, B., Xiao, S., Zhou, C., Yuan, X. 2020. The Ediacaran frondose fossil Arborea from the Shibantan limestone of South China. Journal of Paleontology, 94:1034-1050.
https://doi.org/10.1017/jpa.2020.43
Wang, X., Liu, A.G., Chen, Z., Wu, C., Liu, Y., Wan, B., Pang, K., Zhou, C., Yuan, X., and Xiao S. 2024. A late-Ediacaran crown-group sponge animal. Nature, 630, 905-911.
Witte, U. 1996. Seasonal reproduction in deep-sea sponges-triggered by vertical particle flux? Marine Biology, 124:571-581. https://doi.org/10.1007/BF00351038
Wörheide, G., Dohrmann, M., Erpenbeck, D., Larroux, C., Maldonado, M., Voigt, O., Borchiellini, C., Lavrov, D.V. 2012. “Deep phylogeny and evolution of sponges (Phylum Porifera),” in Advances in Marine Biology (Elsevier), 61:1-78.
https://doi.org/10.1016/B978-0-12-387787-1.00007-6
Xiao, S. and Laflamme, M. 2009. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends in Ecology & Evolution, 24:31-40.
https://doi.org/10.1016/j.tree.2008.07.015