 Erugomicula, a new genus of Arcellinida (testate lobose amoebae)
Erugomicula, a new genus of Arcellinida (testate lobose amoebae)
Article number: 24.1.a16
https://doi.org/10.26879/807
Copyright Paleontological Society, April 2021
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 24 July 2017. Acceptance: 27 April 2021.
ABSTRACT
Testate lobose amoebae of the order Arcellinida are a diverse, cosmopolitan group of shelled protists found in many environments, including freshwater habitats, peatlands, and soils. Their decay-resistant tests make them an important fossil group for reconstructing Quaternary environments. Within the family Difflugidae Stein, 1859 more than 300 species and 200 sub-species have been attributed to the genus Difflugia Leclerc, 1815. Although carried out on only a few taxa, molecular evidence has demonstrated that test morphology is more important than test composition in categorizing distinct taxa within the Arcellinida. The type species of Difflugia, D. proteiformis Lamarck, 1816, is characterized by a terminal aperture and an elongate acuminate test. The morphology of D. proteiformis is vastly different from most species assigned to Difflugia, explaining its polyphyletic status. We reclassify Difflugia bidens Penard, 1902 as type species of Erugomicula, a new genus within the Difflugidae, which is distinguished from other taxa within Difflugia by its broad, ovoid test, and distinct compression. Based on the compressed morphology of the test, which is not a characteristic of the Difflugiidae, we tentatively assign Erugomicula to the family Hyalospheniidae.
Nawaf A. Nasser. Ottawa-Carleton Geoscience Centre and Department of Earth Sciences, Carleton University, Ottawa K1S 5B6, Canada. nawaf.nasser@carleton.ca
Braden R.B. Gregory. Ottawa-Carleton Geoscience Centre and Department of Earth Sciences, Carleton University, Ottawa K1S 5B6, Canada. GregorBRB@gmail.com
David Singer. Department of Zoology, Institute of Biosciences, University of São Paulo, São Paulo, Brazil and Ottawa-Carleton Geoscience Centre and Department of Earth Sciences, Carleton University, Ottawa K1S 5B6, Canada. david.singer.bio@outlook.com
R. Timothy Patterson. Ottawa-Carleton Geoscience Centre and Department of Earth Sciences, Carleton University, Ottawa K1S 5B6, Canada. tim.patterson@carleton.ca
Helen M. Roe. School of Natural and Built Environments, Queen’s University Belfast, Belfast, United Kingdom, BT7 1NN. h.roe@qub.ac.uk
Keywords: Arcellinida; testate lobose amoebae; Quaternary; new genus
Final citation: Nasser, Nawaf A., Gregory, Braden R.B., Singer, David, Patterson, R. Timothy, and Roe, Helen M. 2021. Erugomicula, a new genus of Arcellinida (testate lobose amoebae). Palaeontologia Electronica, 24(1):a16. https://doi.org/10.26879/807
palaeo-electronica.org/content/2021/3356-erugomicula-a-new-genus-of-arcellinida
Copyright: April 2021 Paleontological Society.
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
https://zoobank.org/4BFD1CBC-4668-4431-A4FB-F0D2468108C9
INTRODUCTION
Arcellinida (testate lobose amoebae) are a diverse cosmopolitan group of shelled amoebae (Medioli et al., 1990). They are abundant in many environments including freshwater habitats (Patterson and Kumar, 2002), peatlands, and soils (Mitchell et al., 2008). Their ability to encase their cellular bodies in a robust and decay resistant test (Swindles and Roe, 2007) make Arcellinida an important fossil group, particularly in Quaternary paleoenvironments (McCarthy et al., 1995; Patterson and Kumar, 2002) and through the Phanerozoic into the Neoproterozoic (Porter and Knoll, 2000). They are important environmental proxies due to their short life spans and high sensitivity to environmental changes; e.g., water quality (Roe et al., 2010), water table fluctuation (Charman et al., 1998), pH variability (Patterson et al., 2013), lake acidity (Kumar and Patterson, 2000), land-use change (Patterson et al., 2002), nutrient loading (Patterson et al., 2012), seasonal environmental changes (Neville et al., 2011; Farooqui et al., 2012), ecosystem health (Neville et al., 2011), road salt contamination (Cockburn et al., 2020), and the impact of metal contaminants (Nasser et al., 2016, 2020).
Taxonomic discrimination of arcellinidan taxa at the genus and species level has been variously based on several criteria, including differences in test shape, size (e.g., Bonnet, 1975; Medioli et al., 1987; Beyens and Meisterfeld, 2001) and, to a lesser extent, test composition, and shape and number of nuclei (Penard, 1902; Awerinzew, 1907; Štepánek, 1952; Ogden and Meisterfeld, 1989; Chardez, 1991). This varied approach, coupled with the considerable morphologic variability observed within some taxa has resulted in the description of many new species with little regard to previous literature, the value of characters used, or even the rules of nomenclature (see discussions in Ogden and Hedley, 1980; Medioli and Scott, 1983; Tolonen, 1986; Medioli et al., 1987; Bobrov et al., 1999; Charman et al., 2000; Mazei and Warren, 2012). Meisterfeld (2000) carried out the last comprehensive taxonomic revision of the Arcellinida, grouping taxa primarily by test wall composition within the arcellinidan family Difflugidae Stein, 1859. Difflugia Leclerc, 1815 is by far the largest genus, comprised of more than 300 species and 200 subspecies (Leclerc, 1815; Meisterfeld, 2000; Meisterfeld and Mitchell, 2008; Gomaa et al., 2012). Although many of these taxa are legitimate species, the great taxonomic diversity has been partially attributed to inadequate descriptions, type material not being designated or preserved, and/or lack of diagnostic features (Lahr et al., 2008; Mazei and Warren, 2012). In efforts to rationalize the disparate species attributed to Difflugia several researchers have attempted to subdivide Difflugia into smaller, more manageable groups. For example, Medioli and Scott (1983) proposed a possible solution by suggesting that species be considered as widely variable groups that collectively accommodate 75% or more of the morphological variability within the entire population. This approach was refined with the establishment of an informal infrasubspecific nomenclature, which became known as ‘strains’ (Medioli et al., 1987; Asioli et al., 1996; Reinhardt et al., 1998). The ‘strain’ approach came out of the recognition that certain specimen morphologies, not necessarily linked to specific taxa, could be associated with specific environmental stressors, which during multivariate statistical analysis permitted the recognition of subtler environmental subdivision than would be otherwise possible (Patterson et al., 1996; Patterson and Kumar, 2002). In parallel, Gauthier-Lièvre and Thomas (1958), and more recently Mazei and Warren (2012, 2014, 2015), conducted surveys of the genus Difflugia that resulted in the unofficial subdivision of the genus into small subgroups based on test morphology. There have also been more formal efforts to describe new genera based on taxa formerly attributed to Difflugia (e.g., Patterson, 2014; Nasser and Patterson, 2015).
During the last decade, a revolution in the field of molecular research has resulted in this approach being used as a valuable taxonomic tool that has challenged many classical approaches to taxonomy (DeSalle and Goldstein, 2019). For example, molecular studies have been used to discriminate closely related species (Singer et al., 2015, 2019), as well as to improve upon, and correct the taxonomic placement of many iconic testate amoeba species (Duckert et al., 2018). For example, molecular research, albeit on a limited number of taxa, has provided evidence that test morphology closely aligns with natural phylogenetic relationships within the group (Lahr et al., 2012, 2013, 2019; Kosakyan et al., 2016). Phylogenetic reconstruction using single cell transcriptomic data has also provided evidence that the shape of the test is a key element to determine and characterize the relation between higher taxonomic ranks (Lahr et al., 2019). In an analysis of the SSU rRNA gene in species attributed to the genus Difflugia, Gomaa et al. (2012, 2017) showed that the group is polyphyletic. Other phylogenetic reconstructions based on the NAD9/NAD7 gene have also shown that Difflugia species can be definitively separated in different clades that correspond closely to morphologic features (Macumber et al., 2020). Based on molecular research, several morophologic characters including apertural shape, presence or absence of a neck, test shape (e.g., elongate, compressed, size, girth), test composition, and mixotrophy have been determined to be useful for discriminating separate arcellinidan species and genera (Macumber et al., 2020; Marcisz et al., 2020). These results indicate that a convergence has developed between the morphometric characters used in conventional systematics, and the similar characters recognized to be of systematic importance based on molecular-based approaches (e.g., Marcisz et al., 2020; Steele et al., 2020). This is fortunate considering the relatively few taxa that have been to date analyzed using molecular methodologies.
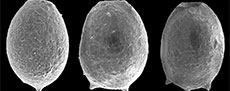
 An important starting point before initiating any taxonomic revision is to consider the underlying taxonomic basis for Difflugia. The type species of the genus is D. proteiformis Lamarck, 1816 (lectotype designated by Loeblich Jr. and Tappan, 1964, p. C35, as the specimen illustrated in Lamarck, 1816, pl. 17, figure 5), which is characterized by an acuminate test with a terminal aperture and a fundus tapering to a point (see typical specimens in Figure 1). Morphologically D. proteiformis differs significantly from many taxa presently attributed to Difflugia, and thus based on the molecular research described above many species presently attributed to the genus should be split into multiple morphological shape groups at the genus level (Gomaa et al., 2012; Kosakyan et al., 2012, 2016). Unfortunately, molecular sequencing of arcellinidan taxa has proven challenging due to the presence of an obscuring test, resulting in very few species having been sequenced. Therefore, characterizing new genera and species using the conventional methodology remains a fundamental taxonomic tool within the Arcellinida. Until such time as the large number of required barcode and transcriptome analyses have been carried to confirm relationships of the different species of Difflugia in the tree of life, it seems that traditional approaches to determining phylogenetic relations will continue to play an important role.
An important starting point before initiating any taxonomic revision is to consider the underlying taxonomic basis for Difflugia. The type species of the genus is D. proteiformis Lamarck, 1816 (lectotype designated by Loeblich Jr. and Tappan, 1964, p. C35, as the specimen illustrated in Lamarck, 1816, pl. 17, figure 5), which is characterized by an acuminate test with a terminal aperture and a fundus tapering to a point (see typical specimens in Figure 1). Morphologically D. proteiformis differs significantly from many taxa presently attributed to Difflugia, and thus based on the molecular research described above many species presently attributed to the genus should be split into multiple morphological shape groups at the genus level (Gomaa et al., 2012; Kosakyan et al., 2012, 2016). Unfortunately, molecular sequencing of arcellinidan taxa has proven challenging due to the presence of an obscuring test, resulting in very few species having been sequenced. Therefore, characterizing new genera and species using the conventional methodology remains a fundamental taxonomic tool within the Arcellinida. Until such time as the large number of required barcode and transcriptome analyses have been carried to confirm relationships of the different species of Difflugia in the tree of life, it seems that traditional approaches to determining phylogenetic relations will continue to play an important role.
As part of ongoing research to determine the positioning of arcellinidans within lake ecosystems (e.g., Patterson et al., 2013, 2015; Macumber et al., 2014; Nasser et al., 2016, 2020; Roe et al., 2017), we aim to integrate apparent taxonomic relationships into the work, which has resulted in the determination that many taxa, particularly many attributed to Difflugia, require to have their systematic taxonomic placements re-evaluated (e.g., Nasser and Patterson, 2015; Patterson et al., 2015).
Difflugia bidens Penard 1902 is a distinctive arcellinidan taxon characterized by a laterally-compressed, ovoid test, a circular aperture, and it may have a varying number of laterally aligned spines on the fundus (see typical specimens in Figure 2, Figure 3). Aside from both sharing the general arcellinidan characteristics of being unilocular and having an agglutinated test, D. bidens bears little similarity to the type species D. proteiformis. We therefore propose that D. bidens be recognized as the type species of the new genus Erugomicula n. gen.
Institutional abbreviation. CANA: Canadian Museum of Nature
SYSTEMATIC PALAEONTOLOGY
Phylum AMOEBOZOA (Lühe, 1913) Corliss, 1984
Class TUBULINEA Smirnov et al., 2005 emend. [Lobosea (Carpenter, 1861) Cavalier-Smith, 1993]
Subclass NEOLOBOSIA Cavalier-Smith et al., 2016
Superorder EULOBOSIA Cavalier-Smith et al., 2016
Order ARCELLINIDA Kent, 1880
Family HYALOSPHENIIDAE Schulze 1877
Genus Erugomicula n. gen.
zoobank.org/A42A7EB9-B8CC-45E6-867E-2CDBCA038B23
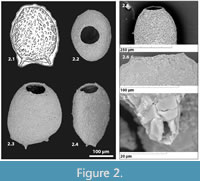 Type species. Difflugia bidens Penard, 1902, p. 264, figures 1-8 (figure 1 is re-illustrated here as Figure 2.1)
Type species. Difflugia bidens Penard, 1902, p. 264, figures 1-8 (figure 1 is re-illustrated here as Figure 2.1)
Diagnosis. A genus tentatively assigned to the Hyalospheniidae characterized by laterally-compressed, ovoid test, typically comprised of agglutinated particles, but is relatively smooth, generally with short hollow spines (Figure 2.7) oriented along the line of compression on the fundus (Figure 3); aperture round, simple (never slit-like) (Figure 2.2), occasionally with slight raised collar about the aperture (Figure 2.6).
Description. Test free, unilocular, wide, and ovoid in face view with a smooth outline (Figure 2.2); compressed in section (Figure 2.3-4); test wall comprised of finely agglutinated xenogenous particles, attached with an organic cement; anterior positioned circular aperture occasionally surrounded by slight raised collar; delicate hollow spines ranging in number from zero to three (two most common), aligned along the line of compression, generally characterize the fundus (Figure 3.1-3).
 Types and occurrence. In his description of Difflugia bidens, based on specimens collected from Lake Geneva, Switzerland, Pernard (1902) illustrated four distinct syntypes on page 265 (figures 1-4), but did not designate any as the holotype of the new species. Penard’s figure 1 (re-illustrated here as Figure 2.1) is the most typical morphotype of the species, being characterized by two basal processes from which the species name ‘bidens’ is derived. The specimen in Penard’s figure 2 is characterized by three basal processes, and figures 3 and 4 have no basal processes. As per Article 74.1 of the International Code of Zoological Nomenclature (1999) we designate Penard’s figure 1 as the lectotype for the species. The additional hypotypes illustrated here are included to provide higher quality scanning electron microscope (SEM) images of the morphologic variability within the species. They were obtained recently from Bell's Lake, Schomberg, Ontario, Canada (43°56.597’ N, 79°39.762’ W), Figure 2.2-4 (CANA 129300), Figure 3.1-6 (CANA129301). The SEM images of the specimens illustrated here were taking using a Tescan Vegall XMU SEM in the Nano Imaging Facility, Carleton University.
Types and occurrence. In his description of Difflugia bidens, based on specimens collected from Lake Geneva, Switzerland, Pernard (1902) illustrated four distinct syntypes on page 265 (figures 1-4), but did not designate any as the holotype of the new species. Penard’s figure 1 (re-illustrated here as Figure 2.1) is the most typical morphotype of the species, being characterized by two basal processes from which the species name ‘bidens’ is derived. The specimen in Penard’s figure 2 is characterized by three basal processes, and figures 3 and 4 have no basal processes. As per Article 74.1 of the International Code of Zoological Nomenclature (1999) we designate Penard’s figure 1 as the lectotype for the species. The additional hypotypes illustrated here are included to provide higher quality scanning electron microscope (SEM) images of the morphologic variability within the species. They were obtained recently from Bell's Lake, Schomberg, Ontario, Canada (43°56.597’ N, 79°39.762’ W), Figure 2.2-4 (CANA 129300), Figure 3.1-6 (CANA129301). The SEM images of the specimens illustrated here were taking using a Tescan Vegall XMU SEM in the Nano Imaging Facility, Carleton University.
 Dimensions. Hypotype specimens measured for this study (n = 24) were from Bell’s Lake, Ontario, Canada (length: 219 - 341 µm [mean = 274 µm]; width: 127 - 250 µm [mean = 207 µm]), with an average length:width ratio of 1.33 (Table 1, Figure 4). Specimens from Bell’s Lake showed little morphological variability, with only minor deviation about the mean length:width ratio, despite large size variation between specimens (Figure 4). Specimens of E. bidens measured by Penard (1902) from Lake Geneva, Switzerland, ranged from 250-270 µm in length, within the observed range of specimens from Bell’s Lake. Specimens retrieved from ditches in Naardermeer, Netherlands (Siemensma, 2017) were slightly larger than E. bidens found in Canada and Switzerland, ranging from 307-366 µm in length. The average apertural width of the Bell’s Lake hypotypes was 83 µm (50 - 103 µm), or approximately 40% of specimen width. Most specimens observed had two spines on the fundus, which was why the type species as named ‘bidens’, although specimens with zero, one, and three spines were also observed. It is likely that the presence or absence of spines is due to phenotypic plasticity and is not a suitable classification criterion (Jennings, 1916; Lahr et al., 2008; Gomaa et al., 2017).
Dimensions. Hypotype specimens measured for this study (n = 24) were from Bell’s Lake, Ontario, Canada (length: 219 - 341 µm [mean = 274 µm]; width: 127 - 250 µm [mean = 207 µm]), with an average length:width ratio of 1.33 (Table 1, Figure 4). Specimens from Bell’s Lake showed little morphological variability, with only minor deviation about the mean length:width ratio, despite large size variation between specimens (Figure 4). Specimens of E. bidens measured by Penard (1902) from Lake Geneva, Switzerland, ranged from 250-270 µm in length, within the observed range of specimens from Bell’s Lake. Specimens retrieved from ditches in Naardermeer, Netherlands (Siemensma, 2017) were slightly larger than E. bidens found in Canada and Switzerland, ranging from 307-366 µm in length. The average apertural width of the Bell’s Lake hypotypes was 83 µm (50 - 103 µm), or approximately 40% of specimen width. Most specimens observed had two spines on the fundus, which was why the type species as named ‘bidens’, although specimens with zero, one, and three spines were also observed. It is likely that the presence or absence of spines is due to phenotypic plasticity and is not a suitable classification criterion (Jennings, 1916; Lahr et al., 2008; Gomaa et al., 2017).
Remarks. Erugomicula, n. genus, differs from Difflugia Leclerc, 1815 in the distinct compression of a wide ovoid test (Figure 2-Figure 3). Erugomicula is distinguished by having a simple round aperture in contrast to Awerintzewia Schouteden, 1906, which has a compressed to oval aperature and inhabits forest soils, and sphagnum and peat bogs. The new genus is also distinct from Heleopera Leidy, 1879, which is characterized by a slit like aperture and roughly agglutinated aboral region. Erugomicula differs from Nebela (Leidy, 1874; sensu Kosakyan et al., 2016) by being composed of finely agglutinated xenogenous mineral particles, while Nebela is composed primarily of oval and circular siliceous plates or organic cement. Nebela species are also characterize by distinctive apertures which can be either linear, slightly or strongly curved.
The type species of the new genus, E. bidens, is usually present in small numbers in most lacustrine assemblages. Erugomicula bidens has been observed in lakes across Canada, including eastern Canada (Medioli and Scott, 1983; McCarthy et al., 2012; Patterson et al., 2012; Macumber et al., 2014), western Canada (Torigai et al., 2000; Neville et al., 2010), and the Northwest Territories (Nasser et al., 2016, 2020). It has also been found in Europe in Bulgaria (Serafimov et al., 1995; Golemansky et al., 2003), Estonia (Lokko et al., 2014), Finland (Kauppila et al., 2006; Kihlman and Kauppila, 2012), France (Thomas, 1954), Lithuania (Šatkauskienė, 2014), Portugal (Camacho et al., 2015), and of course Switzerland (Penard, 1902; Golemansky et al., 2003), where the species was first described. Based on the similarly compressed test we also tentatively place Difflugia biconcava Ertl, 1965 and the possible junior synonym of that species Difflugia balcanica Ogden and Zivovich, 1983 in Erugomicula. Difflugia lucida Penard, 1890 is not placed in Erugomicula as that very coarsely agglutinated taxa has a very compressed aperture and due to the presence of a neck. Similarly, the compressed and coarsely agglutinated Difflugia nodosa Leidy, 1879 is also excluded from the new genus due to the presence of a pronounced neck.
Erugomicula is here tentatively placed in the family Hyalospheniidae based on recent molecular studies that suggest that test shape is an inherited fundamental trait of deep time ancestral phylogenetic importance (Lahr et al., 2019). Although genera of the family Hyalospheniidae are compressed, tests of current genera within that family are variously identified as chitinoid, clear, completely organic, or if agglutinated, comprised of the shell plates of small euglyphids. As E. bidens is comprised of mineral agglutinated particles further research will be required to determine whether Erugomicula is actually attributable to the Hyalospheniidae or Difflugiidae Stein 1859 where many agglutinated test genera are placed, or neither. If proven to be the case, then the test composition description of the Hyalospheniidae will require amendment. The work of Macumber et al. (2020) may provide additional support for placing the new genus in the Hyalospheniidae, based on recognition in that study of two distinct morphologically-based clades (lanceolate and pyriform) within Difflugia. Interestingly the “pyriform” clade of Macumber et al. (2020) was determined to be a sister clade of Hyalosphenia papilio Leidy, 1874 (Family Hyalospheniidae). Nevertheless, these recent studies provide only a preliminary indication of the relationship between the species of Difflugia, with much additional research required (Gomaa et al., 2012). Erugomicula be a key genus that requires particular attention in future molecular investigations of the group.
Erugomicula bidens is considered to be an indicator of increased terrigenous erosion of minerals and organic matter associated with land-use change (Patterson et al., 1985; Kihlman and Kauppila, 2012; Macumber et al., 2014). However, Patterson et al. (2002) did not observe an increase in D. bidens abundance in Swan Lake, Ontario in a stratigraphic section where an increase in sediment runoff into the lake was observed. The sediment runoff was predominantly a nutrient poor glacial clay; the lack of nutrients is most likely an additional important limiting factor on the distribution for D. bidens. An association of D. bidens with nutrient status was further supported by Patterson et al. (2012) as it was observed to have one of the higher optima and tolerances for Olsen's phosphorus (150-400 ppm). It has also been observed in mesotrophic and hypereutrophic lakes (Neville et al., 2010) and lakes impacted by industrial contaminants (Kauppila et al., 2006; Neville et al., 2011; Nasser et al., 2016).
Etymology. From the Latin Erugo, clear of wrinkles, smooth; and mico, shine, sparkle, f. dim., with reference to the relatively smooth surface of the type species Difflugia bidens Penard, 1902.
Stratigraphic range. Although some arcellinidan species have been found in sediments dating as far back as the Permian (Singh et al., 2015), the type species of the new genus Erugomicula bidens has, to date, only been observed in Holocene lacustrine sediments (Medioli and Scott, 1983).
ACKNOWLEDGEMENTS
The authors acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant to RTP, (#RGPIN05329) and the Swiss National Science Foundation project (P2NEP3_178543 to DS).
REFERENCES
Asioli, A., Medioli, F.S., and Patterson, R.T. 1996. Thecamoebians as the tool for reconstruction of paleoenvironments in some southern Alpine Lakes (Orta, Varese and Candia). Journal of Foraminiferal Research, 26:248-263. https://doi.org/10.2113/gsjfr.26.3.248
Awerinzew, S. 1907. Über einige Arten gehäusetragender Rhizopoden des Süβwassers. Archiv für Protistenkunde, 8:86-94.
Beyens, L. and Meisterfeld, R. 2001. Protozoa: testate amoebae, p. 121-153. In Smol, J.P., Birks, H.J.B., and Last, W.M. (eds.), Tracking Environmental Change Using Lake Sediments. Volume 3: Terrestrial, Algal, and Siliceous Indicators. Kluwer Academic Press, Dordrecht, the Netherlands. https://doi.org/10.1007/0-306-47668-1_7
Bobrov, A., Charman, D.J., and Warner, B.G. 1999. Ecology of testate amoebae (Protozoa: Rhizopoda) on peatlands in western Russia with special attention to niche separation in closely related taxa. Protist, 150:125-136. https://doi.org/10.1016/s1434-4610(99)70016-7
Bonnet, L. 1975. Types morphologiques, écologie et évolution de la thèque chez les thécamoebiens. Protistologica, 11:363-378.
Camacho, S., Connor, S., Asioli, A., Boski, T., and Scott, D.B. 2015. Testate amoebae and tintinnids as spatial and seasonal indicators in the intertidal margins of Guadiana Estuary (southeastern Portugal). Ecological Indicators, 58:426-444. https://doi.org/10.1016/j.ecolind.2015.05.041
Carpenter, W.B. 1861. On the systematic arrangement of the Rhizopoda. Natural History Review, 1:456-472.
Cavalier-Smith, T. 1993. Kingdom protozoa and its 18 phyla. Microbiological Reviews, 57:953-994. https://doi.org/10.1128/mmbr.57.4.953-994.1993
Cavalier-Smith, T., Chao, E.E., and Lewis, R. 2016. 187-gene phylogeny of protozoan phylum Amoebozoa reveals a new class (Cutosea) of deep-branching, ultrastructurally unique, enveloped marine Lobosa and clarifies amoeba evolution. Molecular Phylogenetics and Evolution, 99:275-296. https://doi.org/10.1016/j.ympev.2016.03.023
Chardez, D. 1991. Note sur Difflugia humilis sp. n. (Protozoa: Rhizopoda: Testacea). Acta Protozoologica, 1:45-47.
Charman, D.J., Roe, H.M., and Gehrels, W.R. 1998. The use of testate amoebae in studies of sea-level change: a case study from the Taf Estuary, south Wales, UK. The Holocene, 8:209-218. https://doi.org/10.1191/095968398676389446
Charman, D.J., Hendon, D., and Woodland, W.A. 2000. The Identification of Peatland Testate Amoebae. Technical Guide. Quaternary Research Association, London.
Cockburn, C.F., Gregory, R.B., Nasser, N.A., Patterson, R.T. 2020. Intra-lake Arcellinida (testate lobose amoebae) response to winter de-icing contamination in an eastern Canada road-side ‘Salt Belt’ lake. Microbial Ecology, 80:366-383. https://doi.org/10.1007/s00248-020-01513-w
Corliss, J.O. 1984. The kingdom Protista and its 45 phyla. BioSystems, 17:87-126. https://doi.org/10.1016/0303-2647(84)90003-0
DeSalle, R. and Goldstein, P. 2019. Review and interpretation of trends in DNA barcoding. Frontiers in Ecology and Evolution, 7:302. https://doi.org/10.3389/fevo.2019.00302
Duckert, C., Blandenier, Q., Kupferschmid, F.A., Kosakyan, A., Mitchell, E.A., Lara, E., and Singer, D. 2018. En garde! Redefinition of Nebela militaris (Arcellinida, Hyalospheniidae) and erection of Alabasta gen. nov. European Journal of Protistology, 66:156-165. https://doi.org/10.1016/j.ejop.2018.08.005
Ertl, M. 1965. Zur Kenntnis der Testaceenfauna der slowakischen Reisfelder. Hydrobiologia, 26:13-20. https://doi.org/10.1007/bf00142249
Farooqui, A., Kumar, A., and Swindles, G.T. 2012. Thecamoebian communities as proxies of seasonality in Lake Sadatal in the Ganga-Yamuna Plains of North India. Palaeontologia Electronica, 15.1.3A:1-19. https://doi.org/10.26879/200
Gauthier-Lièvre, L. and Thomas, R. 1958. Les genres Difflugia, Pentagonia, Maghrebia et Hoogenraadia (Rhizopodes testacés) en Afrique. Archiv für Protistenkunde, 103:241-370.
Golemansky, V.G., Todorov, M.T., and Zhecheva, A. 2003. A check-list of the Rhizopods (Amoebida and Testacea) in the collection of Dr P Pateff deposited at the Institute of Zoology (Sofia). Acta Zoologica Bulgarica, 55:95-104.
Gomaa, F., Todorov, M., Heger, T.J., Mitchell, E.A.D., and Lara, E. 2012. SSU rRNA phylogeny of Arcellinida (Amoebozoa) reveals that the largest Arcellinid genus, Difflugia Leclerc 1815, is not monophyletic. Protist, 163:389-399. https://doi.org/10.1016/j.protis.2011.12.001
Gomaa, F., Lahr, D.J.G., Todorov, M.T., Lin, J.H., and Lara, E. 2017. A contribution to the phylogeny of agglutinating Arcellinida (Amoebozoa) based on SSU rRNA gene sequences. European Journal of Protistology, 59:99-107. https://doi.org/10.1016/j.ejop.2017.03.005
International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature. Fourth Edition. The Natural History Museum, London.
Jennings, H.S. 1916. Heredity, variation and the results of selection in the uniparental reproduction of Difflugia corona. Genetics, 1:407-534. https://doi.org/10.1093/genetics/1.5.407
Kauppila, T., Kihlman, S., and Mäkinen, J. 2006. Distribution of arcellaceans (testate amoebae) in the sediments of a mine water impacted bay of Lake Retunen, Finland. Water, Air, and Soil Pollution, 172:337-358. https://doi.org/10.1007/s11270-006-9099-9
Kent, W.S. 1880. A Manual of the Infusoria: including a Description of all known Flagellate, Ciliate, and Tentaculiferous Protozoa, British and Foreign, and an Account of the Organization and Affinities of Sponges, vol. 1. Bogue, London.
Kihlman, S. and Kauppila, T. 2012. Effects of mining on testate amoebae in a Finnish lake. Journal of Paleolimnology, 47:1-15. https://doi.org/10.1007/s10933-011-9541-x
Kosakyan, A., Heger, T.J., Leander, B.S., Todorov, M., Mitchell, E.A.D., and Lara, E. 2012. COI barcoding of nebelid testate amoebae (Amoebozoa: Arcellinida): extensive cryptic diversity and redefinition of the Hyalospheniidae Schultze. Protist, 163:415-434. https://doi.org/10.1016/j.protis.2011.10.003
Kosakyan, A., Gomaa, F., Lara, E., and Lahr, D.J.G. 2016. Current and future perspectives on the systematics, taxonomy and nomenclature of testate amoebae. European Journal of Protistology, 55:105-117. https://doi.org/10.1016/j.ejop.2016.02.001
Kumar, A. and Patterson, R.T. 2000. Arcellaceans (Thecamoebians): new tools for monitoring long- and short-term changes in lake bottom acidity. Environmental Geology, 39:689-697. https://doi.org/10.1007/s002540050483
Lahr, D.J.G., Bergmann, P.J., and Lopes, S.G.B.C. 2008. Taxonomic identity in microbial eukaryotes: A practical approach using the testate amoeba Centropyxis to resolve conflicts between old and new taxonomic descriptions. Journal of Eukaryotic Microbiology, 55:409-416. https://doi.org/10.1111/j.1550-7408.2008.00339.x
Lahr, D.J.G., Lara, E., and Mitchell, E.A.D. 2012. Time to regulate microbial eukaryote nomenclature. Biological Journal of the Linnean Society, 107:469-476. https://doi.org/10.1111/j.1095-8312.2012.01962.x
Lahr, D.J.G., Grant, J.R., and Katz, L.A. 2013. Multigene phylogenetic reconstruction of the Tubulinea (Amoebozoa) corroborates four of the six major lineages, while additionally revealing that shell composition does not predict phylogeny in the Arcellinida. Protist, 164:323-339. https://doi.org/10.1016/j.protis.2013.02.003
Lahr, D.J., Kosakyan, A., Lara, E., Mitchell, E.A., Morais, L., Porfirio-Sousa, A.L., Ribeiro, G.M., Tice, A.K., Pánek, T., Kang, S., and Brown, M.W. 2019. Phylogenomics and morphological reconstruction of Arcellinida testate amoebae highlight diversity of microbial eukaryotes in the Neoproterozoic. Current Biology, 29:991-1001. https://doi.org/10.1016/j.cub.2019.01.078
Lamarck, J.B. 1816. Naturelle des Animaux sans Vèrtèbres Tome 2. Verdière, Paris.
Leclerc, M. 1815. Note sur la Difflugie, nouveau genre de polype amorphe. Memoires du (Paris) Museum D’Histoire Naturelle, 2:474-479.
Leidy, J. 1874. Notice of some new freshwater rhizopods. Journal of Natural History, 14:383-385.
Leidy, J. 1879. Fresh-Water Rhizopods of North America. United States Geological Survey of the Territories, Washington, D.C.
Loeblich Jr., A.R. and Tappan, H. 1964. Sarcodina, chiefly “Thecamoebians” and Foraminiferida, p. 1-900. In Moore, R.C. (ed.), Treatise on Invertebrate Paleontology, Part C, Protista 2. Geological Society of America and University of Kansas Press, Lawrence, Kansas.
Lokko, K., Virro, T., and Kotta, J. 2014. Taxonomic composition of zoopsammon in fresh and brackish waters of Estonia, a Baltic province ecoregion of Europe. Estonian Journal of Ecology, 63:242-261. https://doi.org/10.3176/eco.2014.4.04
Lühe, M. 1913. Faunistische Untersuchung der Moore Ostpreußens. Schriften der physikalisch-ökonomischen Gesellschaft Königsberg, 54:84-86.
Macumber, A.L., Patterson, R.T., Roe, H.M., Reinhardt, E.G., Neville, L.A., and Swindles, G.T. 2014. Autoecological approaches to resolve subjective taxonomic divisions within Arcellacea. Protist, 165:305-316. https://doi.org/10.1016/j.protis.2014.03.004
Macumber, A.L., Blandenier, Q., Todorov, M., Duckert, C., Lara, E., Lahr, D.J., Mitchell, E.A., and Roe, H.M. 2020. Phylogenetic divergence within the Arcellinida (Amoebozoa) is congruent with test size and metabolism type. European Journal of Protistology, 72:125645. https://doi.org/10.1016/j.ejop.2019.125645
Marcisz, K., Jassey, V.E.J., Kosakyan, A., Krashevska, V., Lahr, D.J.G., Lara, E., Lamentowicz, Ł., Lamentowicz, M., Macumber, A., Mazei, Y., Mitchell, E.A.D., Nasser, N.A., Patterson, R.T., Roe, H.M., Singer, D., Tsyganov, A.N., and Fournier, B. 2020. Testate amoeba functional traits and their use in paleoecology. Frontiers in Ecology and Evolution, 8:575966.6. https://doi.org/10.3389/fevo.2020.575966
Mazei, Y. and Warren, A. 2012. A survey of the testate amoeba genus Difflugia Leclerc, 1815 based on specimens in the E. Penard and C.G. Ogden collections of the Natural History Museum, London. Part 1: Species with shells that are pointed aborally and/or have aboral protubera. Protistology, 7:121-171.
Mazei, Y. and Warren, A. 2014. A survey of the testate amoeba genus Difflugia Leclerc, 1815 based on specimens in the E. Penard and C.G. Ogden collections of the Natural History Museum, London. Part 2: Species with shells that are pyriform or elongate. Protistology, 8:133-171.
Mazei, Y. and Warren, A. 2015. A survey of the testate amoeba genus Difflugia Leclerc, 1815 based on specimens in the E. Penard and C.G. Ogden collections of the Natural History Museum, London. Part 3: Species with shells that are spherical or ovoid. Protistology, 9:1-49.
McCarthy, F.M.G., Collins, E.S., McAndrews, J.H., Kerr, H.A., Scott, D.B., and Medioli, F.S. 1995. A comparison of postglacial arcellacean (“Thecamoebian”) and pollen succession in Atlantic Canada, illustrating the potential of arcellaceans for paleoclimatic reconstruction. Journal of Paleontology, 69:980-993. https://doi.org/10.1017/s0022336000035630
McCarthy, F.M.G., Tiffin, S., Sarvis, A., McAndrews, J.H., and Blasco, S. 2012. Early Holocene brackish closed basin conditions in Georgian Bay, Ontario, Canada: microfossil (thecamoebian and pollen) evidence. Journal of Paleolimnology, 47:429-445. https://doi.org/10.1007/s10933-010-9415-7
Medioli, F.S. and Scott, D.B. 1983. Holocene Arcellacea (Thecamoebians) from eastern Canada. Cushman Foundation for Foraminiferal Research Special Publication, 21:1-63.
Medioli, F.S., Scott, D.B., and Abbott, B.H. 1987. A case study of protozoan intraclonal variability: taxonomic implications. Journal of Foraminiferal Research, 17:28-47. https://doi.org/10.2113/gsjfr.17.1.28
Medioli, F.S., Scott, D.B., Collins, E.S., and McCarthy, F.M.G. 1990. Fossil thecamoebians: present status and prospects for the future, p. 813-840. In Hemleben, C., Kaminksi, M.A., Kuhnt, W., and Scott, D.B. (eds.), Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera. NATO Advanced Study Institute Series, Series C, Mathematical and Physical Sciences. Springer, The Netherlands.
Meisterfeld, R. 2000. Arcellinida, 2nd ed., p. 690-1432. In Lee, J.J., Leedale, G.F., and Bradbury, P. (eds.), An Illustrated Guide to the Protozoa Volume II. Society of Protozoologists, Lawrence, Kansas.
Meisterfeld, R. and Mitchell, E.A.D. 2008. Difflugia Leclerc, 1815. The Tree of Life Web Project. Retrieved from http://tolweb.org/Difflugia/124487/2008.09.02
Mitchell, E.A.D., Charman, D.J., and Warner, B. 2008. Testate amoebae analysis in ecological and paleoecological studies of wetlands: past, present and future. Biodiversity and Conservation, 17:2115-2137. https://doi.org/10.1007/s10531-007-9221-3
Nasser, N.A. and Patterson, R.T. 2015. Conicocassis, a new genus of Arcellinina (testate lobose amoebae). Palaeontologia Electronica, 18.3.46A:1-11. https://doi.org/10.26879/538
Nasser, N.A., Patterson, R.T., Roe, H.M., Galloway, J.M., Falck, H., Palmer, M.J., Spence, C., Sanei, H., Macumber, A.L., and Neville, L.A. 2016. Lacustrine Arcellinina (testate amoebae) as bioindicators of arsenic contamination. Microbial Ecology, 72:130-149. https://doi.org/10.1007/s00248-016-0752-6
Nasser, N.A., Patterson, R.T., Roe, H.M., Galloway, J.M., Falck, H., and Sanei, H. 2020. Use of Arcellinida (testate lobose amoebae) arsenic tolerance limits as a novel tool for biomonitoring arsenic contamination in lakes. Ecological Indicators, 113:106177. https://doi.org/10.1016/j.ecolind.2020.106177
Neville, L.A., Christie, D.G., McCarthy, F., and MacKinnon, M.D. 2010. Biogeographic variation in Thecamoebian (Testate amoeba) assemblages in lakes within various vegetation zones of Alberta, Canada. International Journal of Biodiversity and Conservation, 2:215-224.
Neville, L.A., McCarthy, F.M.G., MacKinnon, M.D., Swindles, G.T., and Marlowe, P. 2011. Thecamoebians (testate amoebae) as proxies of ecosystem health and reclamation success in constructed wetlands in the Oil Sands of Alberta, Canada. The Journal of Foraminiferal Research, 41:230-247. https://doi.org/10.2113/gsjfr.41.3.230
Ogden, C.G. and Hedley, R.H. 1980. An Atlas of Freshwater Testate Amoebae. British Museum (Natural History), London and Oxford University Press, Oxford.
Ogden, C.G. and Meisterfeld, R. 1989. The taxonomy and systematics of some species of Cucurbitella, Difflugia and Netzelia (Protozoa: Rhizopoda); with an evaluation of diagnostic characters. European Journal of Protistology, 25:109-128. https://doi.org/10.1016/s0932-4739(89)80022-7
Ogden C.G. and Živković A. 1983. Morphological studies on some Difflugiidae from Yugoslavia (Rhizopoda, Protozoa). Bulletin British Museum Natural History (Zoology), 44:341-375.
Patterson, R.T. 2014. Mediolus, a new genus of Arcellacea (testate lobose amoebae). Palaeontologia Electronica, 17.1.28A:1-8. https://doi.org/10.26879/440
Patterson, R.T., MacKinnon, M.D., Scott, D.B., and Medioli, F.S. 1985. Arcellaceans (“thecamoebians”) in small lakes of New Brunswick and Nova Scotia: Modern distribution and Holocene stratigraphic changes. Journal of Foraminiferal Research, 15:114-137. https://doi.org/10.2113/gsjfr.15.2.114
Patterson, R.T., Baker, T., and Burbidge, S.M. 1996. Arcellaceans (thecamoebians) as proxies of arsenic and mercury contamination in northeastern Ontario lakes. Journal of Foraminiferal Research, 26:172-183. https://doi.org/10.2113/gsjfr.26.2.172
Patterson, R.T., Dalby, A.P., Kumar, A., Henderson, L.A., and Boudreau, R.E.A. 2002. Arcellaceans (thecamoebians) as indicators of land-use change: settlement history of the Swan Lake area, Ontario as a case study. Journal of Paleolimnology, 28:297-316.
Patterson, R.T. and Kumar, A. 2002. A review of current testate rhizopod (thecamoebian) research in Canada. Palaeogeography, Palaeoclimatology, Palaeoecology, 180:225-251. https://doi.org/10.1016/s0031-0182(01)00430-8
Patterson, R.T., Roe, H.M., and Swindles, G.T. 2012. Development of a thecamoebian (testate amoebae) based transfer function for sedimentary phosphorous in lakes. Palaeogeography, Palaeoclimatology, Palaeoecology, 348-349:32-44. https://doi.org/10.1016/j.palaeo.2012.05.028
Patterson, R.T., Lamoureux, E.D.R., Neville, L.A., and Macumber, A.L. 2013. Arcellacea (testate lobose amoebae) as pH indicators in a pyrite mine-acidified lake, Northeastern Ontario, Canada. Microbial Ecology, 65:541-554. https://doi.org/10.1007/s00248-012-0108-9
Patterson, R.T., Huckerby, G., Kelly, T.J., Swindles, G.T., and Nasser, N.A. 2015. Hydroecology of Amazonian lacustrine Arcellinida (testate amoebae): A case study from Lake Quistococha, Peru. European Journal of Protistology, 51:460-469. https://doi.org/10.1016/j.ejop.2015.06.009
Penard, M.E. 1902. Faune rhizopodique du bassin du Leman. (H. Kundig, Ed.). Librairie de L’Institut, Geneva.
Penard, E. 1890. Etudes sur les Rhizopodes d'eau douce. Memoires de la Societe de Physique et d'Histoire Naturelle de Geneve, 2:1-230.
Porter, S.A. and Knoll, A.H. 2000. Testate amoeba in the Neoproterozoic Era: evidence from vase-shaped microfossils in the Chuar Group, Grand Canyon. Paleobiology, 26:360-385. https://doi.org/10.1666/0094-8373(2000)026<0360:TAITNE>2.0.CO;2
Reinhardt, E.G., Dalby, A.P., Kumar, A., and Patterson, R.T. 1998. Arcellaceans as pollution indicators in mine tailing contaminated lakes near Cobalt, Ontario, Canada. Micropaleontology, 44:1-18.
Roe, H.M., Patterson, R.T., and Swindles, G.T. 2010. Controls on the contemporary distribution of lake thecamoebians (testate amoebae) within the Greater Toronto Area and their potential as water quality indicators. Journal of Paleolimnology, 43:955-975. https://doi.org/10.1007/s10933-009-9380-1
Roe, H.M., Elliot, S.M. and Patterson, R.T. 2017. Re-assessing the vertical distribution of testate amoeba communities in surface peats: implications for palaeohydrological studies. European Journal of Protistology, 60:13-27. https://doi.org/10.1016/j.ejop.2017.03.006
Šatkauskienė, I. 2014. Testate amoebae (Testaceae) of Lithuania. Biologija, 60, 59-69. https://doi.org/10.6001/biologija.v60i2.2906
Schouteden, H. 1906. Les Rhizopodes testaces d'eau douce, d'apres la Monographie du Prof. A. (Sic) Awerintzew. Annales de Biologie Lacustre, 1(3):327-382.
Schultze, F.E. 1877. Rhizopodenstudien VI. Archiv für mikroskopische Anatomie, 13:9-30.
Serafimov, B.L., Golemansky, V.G., and Todorov, M.T. 1995. Testacean taxocenoses (Rhizopoda, Testacea) in two quarry lakes of Sofia district. Acta Zoologica Bulgarica, 48:23-33.
Siemensma, F.J. 2017. Microworld, world of amoeboid organisms. Retrieved from http://www.arcella.nl
Singer, D., Kosakyan, A., Pillonel, A., Mitchell, E.A.,, and Lara, E. 2015. Eight species in the Nebela collaris complex: Nebela gimlii (Arcellinida, Hyalospheniidae), a new species described from a Swiss raised bog. European Journal of Protistology, 51:79-85. https://doi.org/10.1016/j.ejop.2014.11.004
Singer, D., Mitchell, E. A., Payne, R. J., Blandenier, Q., Duckert, C., Fernández, L. D., Fournier, B., Hernández, C.E., Granath, G., Rydin, H., Bragazza, L., Koronatova, N.G., Goia, I., Harris, L.I., Kajukało, K., Kosakyan, A., Lamentowicz, M., Kosykh, N.P., Vellak, K., and Lara, E. 2019. Dispersal limitations and historical factors determine the biogeography of specialized terrestrial protists. Molecular Ecology, 28(12):3089-3100. https://doi.org/10.1111/mec.15117
Singh, V., Pandita, S.K., Tewari, R., van Hengstum, P.J., Pillai, S.S., Agnihotri, D., Kumar, K., and Bhat, G.D. 2015. Thecamoebians (testate amoebae) straddling the Permian-Triassic boundary in the Guryul Ravine section, India: evolutionary and palaeoecological implications. PLoS One, 10:e0135593. https://doi.org/10.1371/journal.pone.0135593
Smirnov, A., Nassonova, E., Cedric, B., Fahrni, J., Bolivar, I., and Pawlowski, J. 2005. Molecular phylogeny and classification of the lobose amoebae. Protist, 156:129-142. https://doi.org/10.1016/j.protis.2005.06.002
Steele, R.E., Patterson, R.T., Hamilton, P.B., Nasser, N.A., and Roe, H.M. 2020. Assessment of FlowCam technology as a potential tool for rapid semi-automatic analysis of lacustrine Arcellinida (testate lobose amoebae). Environmental Technology & Innovation, 17:100580. https://doi.org/10.1016/j.eti.2019.100580
Stein, S.F.N. 1859. Über die ihm aus eigener Untersuchung bekannt gewordenen Süßwasser-Rhizopoden. Königliche Böhmische Gesellschaft der Wissenschaften Abhandlungen, Ser. 5, 10:41-43.
Štepánek, M. 1952. Testacea of the pond of Hrádek at Kunratice (Prague). Acta Musei Nationalis Pragae, Series B - Historia Naturalis Praha: Národní Muzeum, 8:1-55.
Swindles, G.T. and Roe, H.M. 2007. Examining the dissolution characteristics of testate amoebae (Protozoa: Rhizopoda) in low pH conditions: Implications for peatland palaeoclimate studies. Palaeogeography, Palaeoclimatology, Palaeoecology, 252:486-496. https://doi.org/10.1016/j.palaeo.2007.05.004
Thomas, R. 1954. Thécamoebiens de la région bordelaise. Bulletin de la Sociéte d’Histoire Naturelle de Toulouse, 89:245-264.
Tolonen, K. 1986. Rhizopod analysis, p. 645-666. In Berglund, B.E. (ed.), Handbook of Holocene Palaeoecology and Palaeohydrology. John Wiley and Sons, New York.
Torigai, K., Schröder-Adams, C.J., and Burbridge, S.M. 2000. A variable lacustrine environment in Lake Winnipeg, Manitoba: evidence from modern thecamoebian distribution. Journal of Paleolimnology, 23:305-318. https://doi.org/10.1023/A:1008148027142

