 Evaluating the impact of a barnacle parasite (Loxothylacus texanus) on the preservation of blue crabs (Callinectes sapidus) with experimental taphonomy
Evaluating the impact of a barnacle parasite (Loxothylacus texanus) on the preservation of blue crabs (Callinectes sapidus) with experimental taphonomy
Article number: 28.3.a47
https://doi.org/10.26879/1533
Copyright Paleontological Society, October 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Appendices
Submission: 15 January 2025. Acceptance: 3 October 2025.
ABSTRACT
The dynamics of parasitism in ancient ecosystems represent a considerable knowledge gap within paleontology, due to poorly preserved fossil evidence. Among many diverse extant parasite clades, fossil evidence is hindered by the parasites’ small bodies, relative lack of hard parts, and/or tendency for endoparasitism. However, these obstacles do not fully explain patterns in the fossil evidence of parasitism, particularly among parasites which produce distinct traces on host fossils. Characterizing the preservation of parasitism in the fossil record at the scale of individual species interactions is crucial for modeling and contextualizing efforts, to understand how parasitic interactions will respond to global change. Here, we use experimental tumbling to compare the post-mortem preservation of the blue crab, Callinectes sapidus, with and without infestation by the rhizocephalan barnacle parasite Loxothylacus texanus. We find minimal differences in the rate and pattern of degradation between specimens, suggesting no impact on the preservation potential of host Callinectes due to the presence of the parasite. This suggests that pre-burial preservation is not a primary control on the fidelity of the fossil record of rhizocephalan parasitism. Patterns in the fossil evidence of rhizocephalans may therefore reflect true ecological or evolutionary signals, other taphonomic and sampling influences, or a combination of these factors, which should be explored further.
Nathan L. Wright. Department of Geosciences, Baylor University, Waco, Texas, USA and Milwaukee Public Museum, Milwaukee, Wisconsin, USA. Wrightn@mpm.edu
Elizabeth Petsios. Department of Geosciences, Baylor University, Waco, Texas, USA. Elizabeth_Petsios@baylor.edu
Keywords: arthropod; marine; paleoecology; taphonomy; parasitism
Final citation: Wright, Nathan L., and Petsios, Elizabeth. 2025. Evaluating the impact of a barnacle parasite (Loxothylacus texanus) on the preservation of blue crabs (Callinectes sapidus) with experimental taphonomy. Palaeontologia Electronica, 28(3):a47.
https://doi.org/10.26879/1533
palaeo-electronica.org/content/2025/5692-blue-crab-preservation-with-parasites
Copyright: October 2025 Paleontological Society
This is an open access article distributed under the terms of Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0), which permits users to copy and redistribute the material in any medium or format, provided it is not used for commercial purposes and the original author and source are credited, with indications if any changes are made.
creativecommons.org/licenses/by-nc-sa/4.0/
INTRODUCTION
Parasitism in the fossil record, as in modern ecosystems, is a critical topic in ecology, as parasitism is the most species-rich mode of life on earth (Dobson et al., 2008; Larsen et al., 2017; Okamura et al., 2018; Carlson et al., 2020), parasites are essential to the structure and function of ecosystems (Dobson et al., 2008; Dougherty et al., 2016; Morton et al., 2021), and understanding the response of parasites to climate change is a pressing concern (Carlson et al., 2017; Cohen et al., 2020). Beyond the considerable data limitations encountered in modern parasitology, however, quantitatively rigorous evidence of parasitic relationships in the fossil record is often exceedingly rare (Poulin, 2007, 2014; Leung, 2017; Carlson et al., 2020). Current data on parasitism in the fossil record is subject to limitations of timescale, strong taxonomic unevenness, sampling influences, and is often over-represented by exceptional preservation. While cases of parasitism in the fossil record have an established history of publication (Cressey and Patterson, 1973; Baumiller, 1990; Feldmann, 1998; De Baets et al., 2015), and recent advances in data availability and analytical tools have allowed for some broader synthesis of fossil parasite data (De Baets et al., 2021), using fossil parasite information to model and contextualize ongoing and future ecological change requires intensive investigation of fossil parasitism at the scale of individual species interactions.
Preservation bias is considered a primary factor in the quantitative disparity between fossil parasitic evidence and extant parasitism, because many highly diverse and cosmopolitan extant parasites are small, lack mineralized skeletons, and do not produce diagnostic traces on the skeletal elements of their hosts (Littlewood and Donovan, 2003; De Baets and Littlewood, 2015). For these parasites, the only direct fossil evidence likely to be observed are relatively rare instances in which parasite body fossils are preserved in association with host body fossils (i.e., Baumiller, 2003; Nagler et al., 2016). In contrast, many extant marine parasites of invertebrates have been noted to produce distinct, diagnostic traces on their hosts’ exoskeletons (Klompmaker and Boxshall, 2015)(i.e., Klompmaker et al., 2014; Yamamori and Kato, 2020), but fossils with identified traces of these parasites are also uncommon. Some traces of biotic interactions, notably predatory drill holes, are associated with complex impacts on preservation (Roy et al., 1994; Kelley, 2008; Klompmaker, 2009; Dyer et al., 2018). The influence of preservation on the fidelity of the fossil record of parasitism is a largely unexplored topic, although recent study suggests the parasitic trace Kanthyloma crusta (Klompmaker et al., 2014), a swelling resulting from isopod parasitism on decapod crustacean hosts, may decrease preservation potential (Wright et al., 2024).
Crustaceans are a useful study group for investigating fossil parasitism across scales, as they have a diverse, cosmopolitan fossil record spanning nearly the entire Phanerozoic (Hegna et al., 2020). Crustaceans exhibit a tremendous diversity both as parasitic taxa and as hosts for parasites, and novel parasitic ecologies have evolved independently many times within Pancrustacea (Klompmaker and Boxshall, 2015). Among these parasitic interactions are a number which can produce diagnostic traces on host skeletal elements (Klompmaker and Boxshall, 2015). Although these features benefit crustaceans as a study group for the fossil record of parasitism, there are substantial obstacles to studying the crustacean fossil record. The fossil record of crustaceans is limited by relatively light and heterogeneous skeletal calcification compared to some other ubiquitous marine invertebrate clades (i.e., molluscs, echinoderms), and disintegrate relatively rapidly in the absence of soft tissue (Allison, 1986; Bishop, 1986; Krause et al., 2011; Klompmaker et al., 2017). High fidelity crustacean fossils are often associated with calcite or siderite concretions, which represent exceptional physicochemical preservation conditions (Waugh et al., 2004; Wilson and Brett, 2013; McCoy et al., 2015).
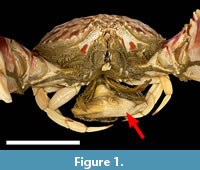 The subject of parasitism among extant crustaceans has received considerable attention, as crustacean parasites are nearly ubiquitous in marine ecosystems, and crustaceans are globally important ecologically and economically. Parasitism in the fossil record of crustaceans has been the subject of relatively limited research, owing in part to the significant obstacles to fossil evidence of parasitism (Klompmaker and Boxshall, 2015). Among the rarer examples of parasitism in the crustacean fossil record are rhizocephalan barnacles, a derived clade of barnacles which are obligate parasites of decapod crustaceans (Figure 1) (Glenner and Hebsgaard, 2006). Rhizocephalan barnacles are parasitic castrators of their hosts, and are diverse and widely distributed in modern ecosystems (O’brien and Van Wyk, 1985; Høeg and Lutzen, 1995; Høeg, 1995; Corral et al., 2021). As rhizocephalan barnacles lack any mineralized structure, and mature rhizocephalans live almost exclusively inside the body of their host, they do not fossilize readily, however rhizocephalans with juvenile male hosts are known to induce distinct sexually aberrant development of their host. The unique development of these hosts has been observed in fossils, such as in Miocene Tumidocarcinus from New Zealand (Feldmann, 1998). The profound impacts of rhizocephalan barnacles on their decapod hosts, including sterilization, aberrant development, and behavioral alteration, may result in considerable consequences for marine ecosystems and aquaculture if the changing ocean environment alters rhizocephalan prevalence, as there is no currently known treatment or cure for rhizocephalan parasite infestation (Waiho et al., 2021). Although rhizocephalan barnacles are soft-bodied endoparasites, they have a considerable whole-body impact on their hosts, including features known to influence fossil preservation potential, including decreased size, aberrant morphological development, behavioral changes, inhibited molting, and increased mortality (Bishop, 1986; Behrensmeyer et al., 2000; Waiho et al., 2021).
The subject of parasitism among extant crustaceans has received considerable attention, as crustacean parasites are nearly ubiquitous in marine ecosystems, and crustaceans are globally important ecologically and economically. Parasitism in the fossil record of crustaceans has been the subject of relatively limited research, owing in part to the significant obstacles to fossil evidence of parasitism (Klompmaker and Boxshall, 2015). Among the rarer examples of parasitism in the crustacean fossil record are rhizocephalan barnacles, a derived clade of barnacles which are obligate parasites of decapod crustaceans (Figure 1) (Glenner and Hebsgaard, 2006). Rhizocephalan barnacles are parasitic castrators of their hosts, and are diverse and widely distributed in modern ecosystems (O’brien and Van Wyk, 1985; Høeg and Lutzen, 1995; Høeg, 1995; Corral et al., 2021). As rhizocephalan barnacles lack any mineralized structure, and mature rhizocephalans live almost exclusively inside the body of their host, they do not fossilize readily, however rhizocephalans with juvenile male hosts are known to induce distinct sexually aberrant development of their host. The unique development of these hosts has been observed in fossils, such as in Miocene Tumidocarcinus from New Zealand (Feldmann, 1998). The profound impacts of rhizocephalan barnacles on their decapod hosts, including sterilization, aberrant development, and behavioral alteration, may result in considerable consequences for marine ecosystems and aquaculture if the changing ocean environment alters rhizocephalan prevalence, as there is no currently known treatment or cure for rhizocephalan parasite infestation (Waiho et al., 2021). Although rhizocephalan barnacles are soft-bodied endoparasites, they have a considerable whole-body impact on their hosts, including features known to influence fossil preservation potential, including decreased size, aberrant morphological development, behavioral changes, inhibited molting, and increased mortality (Bishop, 1986; Behrensmeyer et al., 2000; Waiho et al., 2021).
Our aim was to investigate the preservation of blue crabs Callinectes sapidus (Rathbun, 1896) hosting rhizocephalan parasites relative to the preservation of individuals without parasites, to assess the impact of the parasite on the early fossilization potential of hosts. Experimental taphonomy has, for decades (Kidwell and Baumiller, 1990; Briggs, 1995; Gorzelak and Salamon, 2013; Klompmaker et al., 2017), proven a useful tool for characterizing pre-burial fossil preservation dynamics. Experimental tumbling is a type of taphonomy experiment in which specimens are sealed in rotary tumblers, to simulate rapid, controlled post-mortem transport and decay processes. Tumbling experiments have limitations; they do not simulate the full spectrum of taphonomic processes, such as burial and in situ decay. However, tumbling experiments provide advantages in logistical accessibility and experimental control, as well as offering unique insight into long-distance transport and high-energy preservation conditions (Kidwell and Baumiller, 1990; Gorzelak and Salamon, 2013). Here, we apply experimental taphonomy to the study of fossil parasitism, investigating the impact of the rhizocephalan barnacle Loxothylacus texanus (Boschma, 1933) on its host, the blue crab Callinectes sapidus (Hochberg et al., 1992; Lázaro-Chávez et al., 1996), as a key step toward disentangling ecological and evolutionary signals from extraneous fossil data influences.
MATERIAL AND METHODS
Specimens Studied
Recently collected blue crabs were used for tumbling experiments to simulate postmortem taphonomic processes that impact preservation potential, such as transport and decay. Crabs were collected from the Gulf of Mexico, and were either supplied by the Gulf Specimen Marine Lab (Gulf Specimen Marine Laboratories, Inc.) or collected by hand (under Florida FWC Special Activity License SAL-22-2460-SR, locality information in Appendix 1). Specimens were dispatched by freezing, as in previous studies (Plotnick, 1986; Krause et al., 2011; Klompmaker et al., 2017). In total, 16 specimens, seven with rhizocephalan parasites and nine without, were included in the experiments across one preliminary trial and seven experimental trials (Table 1). Of the parasitized Callinectes specimens collected, all were female. As a result, sex-specific preservation impacts of rhizocephalan parasites are not tested herein. Of the specimens without parasites, six were male and three were female. All specimens with parasites were relatively small adults, with a maximum carapace width of 7.4 cm, and an average width of 6.2 cm. The specimens without parasites included specimens at relatively full adult size (n=5), with a maximum carapace width of 16.2 cm and an average width of 13.8 cm, as well as relatively small younger adults (n=4), with a maximum carapace width of 7 cm, and an average width of 6.7 cm.
Experimental Tumbling
Tumbling experiments were conducted using two Thumler’s Tumbler Model B rotary tumblers (Tru-Square Metal Products), at room temperature, rotating at a rate of 20 revolutions per minute. At the beginning of each trial, each tumbler barrel was loaded with four liters of water, 140 g of Instant Ocean Reef Salt (Spectrum Brands), and 250 ml of washed and graded coarse quartz sand. The tumblers were run for five minutes before adding specimens to thoroughly mix the salt, sand, and water. Following mixing, one frozen specimen was added to each barrel, and the tumblers were reactivated. For the preliminary trial, two full-sized adult male specimens were tumbled. For each subsequent experimental trial, one specimen with a rhizocephalan parasite and one specimen without were tumbled concurrently. During trials, specimens were tumbled at a rate approximately equivalent to 720 meters per hour. At the beginning of each trial and after each 24 hour interval of tumbling, the tumblers were halted and opened for up to 20 minutes, during which the specimen material was removed from the barrels, the taphonomic character states of each specimen (Table 2) were assessed semi-quantitatively, and the specimens were photographed. This research was conducted in accordance with local ethical guidelines. Recorded taphonomic scores for each specimen are accessible in Appendix 2.
Analytical Methods
At each 24-hour interval, each specimen was given scores for its state of preservation in a number of categories: completeness of the carapace, the presence of soft tissue, separation of the pleon and carapace, completeness of the pleon, completeness of the telson, completeness of carapace spines, and discoloration. The scoring scheme (Table 2) was adapted from previous experimental taphonomy studies of crustaceans, (Krause et al., 2011; Klompmaker et al. 2017) using observations from the preliminary tumbling trial. After the trials concluded and the scores were compiled, the scores were normalized to give each category equal weight by dividing each score in each category by the maximum value of the category. The cumulative taphonomic scores for each specimen were then converted to an index value and inverted, by dividing each cumulative score by the maximum value, then subtracting one and taking the absolute value of the result. For the resulting index values, a value of one indicates a pristine specimen, and a value of zero indicates the maximum extent of disintegration. For each trial, and for the combined averaged data from each trial including and excluding larger parasite-free specimens, a paired two-tailed Mann-Whitney test was conducted to determine the statistical significance of taphonomic score differences between specimens with and without a rhizocephalan parasite. All data analysis was performed in R version 4.4.1.
RESULTS
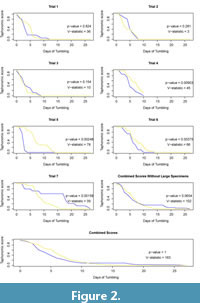 Taphonomic scores through time for each trial, as well as the normalized cumulative taphonomic scores through all trials including and excluding larger parasite-free specimens are shown in Figure 2. The p-value and V statistic from the Mann-Whitney tests are also shown for each trial and data treatment in Figure 2. Experimental trials one through three and the combined values (Figure 2A-C;I), which include larger parasite-free specimens, do not have statistically significant differences between the taphonomic scores of parasitized and non-parasitized specimens (p-values = 0.824, 0.281, 0.154, 1, a = 0.05). Trials four through seven (Figure 2D-G), which included parasite-free specimens similar in size to the rhizocephalan host specimens, do exhibit statistically significant differences (p-values = 0.009, 0.002, 0.004, 0.002, a = 0.05)., although the combined data excluding larger specimens narrowly fails statistical significance (p-value = 0.07, a = 0.05). Photographs of individuals after 24 hours, 120 hours, and 240 hours of tumbling (one, five, and ten days of tumbling, respectively) are shown in Figure 3 and Figure 4.
Taphonomic scores through time for each trial, as well as the normalized cumulative taphonomic scores through all trials including and excluding larger parasite-free specimens are shown in Figure 2. The p-value and V statistic from the Mann-Whitney tests are also shown for each trial and data treatment in Figure 2. Experimental trials one through three and the combined values (Figure 2A-C;I), which include larger parasite-free specimens, do not have statistically significant differences between the taphonomic scores of parasitized and non-parasitized specimens (p-values = 0.824, 0.281, 0.154, 1, a = 0.05). Trials four through seven (Figure 2D-G), which included parasite-free specimens similar in size to the rhizocephalan host specimens, do exhibit statistically significant differences (p-values = 0.009, 0.002, 0.004, 0.002, a = 0.05)., although the combined data excluding larger specimens narrowly fails statistical significance (p-value = 0.07, a = 0.05). Photographs of individuals after 24 hours, 120 hours, and 240 hours of tumbling (one, five, and ten days of tumbling, respectively) are shown in Figure 3 and Figure 4.
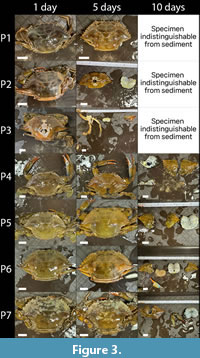 In each trial, the specimens exhibited little damage for the first two to five days of tumbling, often sustaining only minor perforation, abrasion, and discoloration of the carapace. Following this phase, specimens entered into rapid phase of disarticulation and damage, typically initiated by the separation of the carapace and the pleon, and followed by fragmentation of the carapace.
In each trial, the specimens exhibited little damage for the first two to five days of tumbling, often sustaining only minor perforation, abrasion, and discoloration of the carapace. Following this phase, specimens entered into rapid phase of disarticulation and damage, typically initiated by the separation of the carapace and the pleon, and followed by fragmentation of the carapace. Specimens which remained after this rapid phase of damage entered into a slower final phase of decay, in which the fragments of the carapace, pleon, and telson were often stable across multiple days of tumbling, before concluding with the complete or near-complete disintegration of the carapace. The parasite-free specimen in trial seven (specimen H7), and to a lesser extent the parasitized specimen in trial five (specimen P5), exhibited a different pattern, in which a two-phase decline is caused by the lack of major carapace fragmentation after the initial separation of the carapace and pleon. One specimen, the parasitized specimen tumbled in trial seven (specimen P7), was unusual in that there were two clear rhizocephalan externae present (Figure 5). Notably, in trial seven, the taphonomic scores of the parasite host and the parasite-free individual were significantly different, and it is the only trial with significant results in which the parasitized specimen experienced an earlier phase of rapid decay than the parasite-free specimen.
Specimens which remained after this rapid phase of damage entered into a slower final phase of decay, in which the fragments of the carapace, pleon, and telson were often stable across multiple days of tumbling, before concluding with the complete or near-complete disintegration of the carapace. The parasite-free specimen in trial seven (specimen H7), and to a lesser extent the parasitized specimen in trial five (specimen P5), exhibited a different pattern, in which a two-phase decline is caused by the lack of major carapace fragmentation after the initial separation of the carapace and pleon. One specimen, the parasitized specimen tumbled in trial seven (specimen P7), was unusual in that there were two clear rhizocephalan externae present (Figure 5). Notably, in trial seven, the taphonomic scores of the parasite host and the parasite-free individual were significantly different, and it is the only trial with significant results in which the parasitized specimen experienced an earlier phase of rapid decay than the parasite-free specimen.
DISCUSSION
 Most specimens’ taphonomic scores loosely follow a logarithmic decline, in which an initial phase of minimal damage is followed by a phase of rapid decline, which is followed by a final phase of slowed decline. In trials one through three, which included full-sized adult parasite-free individuals, the two specimens’ taphonomic score patterns are statistically indistinct. There are significant differences between the scores of parasitized and parasite-free specimens in the trials with parasite-free specimens similar in size to the parasitized specimens (trials four through seven), although these differences are not uniform. In each of these trials, the parasite-free individual entered an initial phase of decline earlier than the parasitized individual. In trials four and six, the taphonomic score pattern of the specimens were similar in shape and rate, but were offset in time. In trial five, the parasite-free specimen declined the most rapidly in taphonomic score of all specimens, reaching complete disintegration of taphonomic characters within five days, while the parasite host underwent a two-phase decline across 14 days.
Most specimens’ taphonomic scores loosely follow a logarithmic decline, in which an initial phase of minimal damage is followed by a phase of rapid decline, which is followed by a final phase of slowed decline. In trials one through three, which included full-sized adult parasite-free individuals, the two specimens’ taphonomic score patterns are statistically indistinct. There are significant differences between the scores of parasitized and parasite-free specimens in the trials with parasite-free specimens similar in size to the parasitized specimens (trials four through seven), although these differences are not uniform. In each of these trials, the parasite-free individual entered an initial phase of decline earlier than the parasitized individual. In trials four and six, the taphonomic score pattern of the specimens were similar in shape and rate, but were offset in time. In trial five, the parasite-free specimen declined the most rapidly in taphonomic score of all specimens, reaching complete disintegration of taphonomic characters within five days, while the parasite host underwent a two-phase decline across 14 days.
These results do not indicate a decisive impact of the rhizocephalan barnacle on blue crab preservation, but instead indicate nuances and patterns which should be investigated further. Primarily, the impact of size on the preservation of these specimens is complex, as smaller and larger parasite-free individuals declined in taphonomic score differently in terms of timing, relative to the parasitized individuals. The lack of significant differences between full-sized adults and parasite hosts suggests that individuals with parasites may preserve at a similar rate to normal adult specimens. The lack of clear directionality among the trials with significant differences between individuals suggests that despite being similar in size, smaller specimens without parasites exhibit greater variability in preservation than those with rhizocephalan parasites. The overall trend, as seen in the taphonomic score patterns combined across trials, both with and without larger specimens (Figure 2H, I), suggests that patterns of post-mortem, pre-burial decay are similar among blue crabs with and without rhizocephalan parasite.
As the specimens were dispatched by freezing and subsequently stored frozen, freezing may have had an impact on the preservation patterns observed in this study. Freezing can impact the stability of chitin and proteins (Bhatnagar et al., 2007; Liu et al., 2010; Figueroa-Pizano et al., 2018), the primary components of crustacean exoskeletons, but is considerably less impactful on animal soft tissue and skeletal elements than storage in ethanol (Chen et al., 2008; Vesper et al., 2017; Leonard et al., 2022). As all specimens in this study, both with and without a parasite, were frozen in identical conditions, freezing had no impact on taphonomic observation comparisons between individuals. In addition, freezing was the only logistically accessible form of dispatch which would not have a direct impact on initial taphonomic character states, such as spiking or instantaneous maceration.
CONCLUSIONS
Although the preservation patterns of blue crabs with and without rhizocephalan parasites are similar, there is a tremendous discrepancy between the prevalence of rhizocephalan parasites in modern ecosystems and the dearth of fossil evidence for them. Much of this discrepancy may be explained by the difficulty of recognizing rhizocephalan hosts without soft tissue; Without the externae, the only clear, direct morphological impact on their hosts is seen in the sexually aberrant development of juvenile males with rhizocephalan parasites. Although this has been recognized in the fossil record, such as by Feldmann (1998), distinguishable fossils of rhizocephalan developmental abnormalities must provide ventral views of the specimen, as well as sufficient intact morphology of the pleon and secondary sexual characteristics (such as major claw size for many decapods) to determine sexual abnormalities. Decapod fossils that meet these conditions are generally restricted to exceptional preservation scenarios, such as concretion formation. In addition, a large sample of fossil decapods is necessary to distinguish rhizocephalan evidence from the morphological spectra of sexual dimorphism and ontogeny, and such samples of fossil decapods are rare. The results of this study suggest that under tumbling conditions, and therefore in idealized instances of post-mortem transport prior to burial, the preservation of blue crabs is not strongly controlled by the presence of a rhizocephalan parasite. Although there is much further study to be done in understanding the nuances of the rhizocephalan barnacle fossil record, these results may indicate greater than predicted fidelity in the prevalence of these parasites in the fossil record.
ACKNOWLEDGMENTS
We thank the Fish and Wildlife Research Institute, in particular P. Larson and C. Fuchs, and the Gulf Specimen Marine Laboratories, Inc., for providing or facilitating the collection of Callinectes specimens for this project. This project was partially supported by a Graduate Student Research Grant awarded by the Geological Society of America.
REFERENCES
Allison, P.A. 1986. December 1. Soft-bodied animals in the fossil record: The role of decay in fragmentation during transport. Geology 14:979-981.
https://doi.org/10.1130/0091-7613(1986)14<979:SAITFR>2.0.CO;2
Baumiller, T.K. 1990. Non-predatory drilling of Mississippian crinoids by platyceratid gastropods. Palaeontology 33:743-748.
Baumiller, T.K. 2003. Evaluating the interaction between platyceratid gastropods and crinoids: a cost-benefit approach. Palaeogeography, Palaeoclimatology, Palaeoecology: Drilling Predation in the Fossil Record 201:199-209.
https://doi.org/10.1016/S0031-0182(03)00625-4
Behrensmeyer, A.K., Kidwell, S.M. and Gastaldo, R.A. 2000. Taphonomy and paleobiology. Paleobiology 26:103-147.
https://doi.org/10.1017/S0094837300026907
Bhatnagar, B.S., Bogner, R.H. and Pikal, M.J. 2007. Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharmaceutical Development and Technology 12:505-523.
https://doi.org/10.1080/10837450701481157
Bishop, G.A. 1986. Taphonomy of the North American Decapods. Journal of Crustacean Biology 6:326-355.
https://doi.org/10.1163/193724086X00190
Boschma, H. 1933. New species of Sacculinidae in the collection of the United States National Museum. Tijdschrift der Nederlandsche Dierkundige Vereeniging 3:219-241.
Briggs, D.E.G. 1995. Experimental Taphonomy. PALAIOS 10:539-550.
https://doi.org/10.2307/3515093
Carlson, C.J., Burgio, K.R., Dougherty, E.R., Phillips, A.J., Bueno, V.M., Clements, C.F., Castaldo, G., Dallas, T.A., Cizauskas, C.A., Cumming, G.S., Doña, J., Harris, N.C., Jovani, R., Mironov, S., Muellerklein, O.C., Proctor, H.C. and Getz, W.M. 2017. Parasite biodiversity faces extinction and redistribution in a changing climate. Science Advances 3:e1602422.
https://doi.org/10.1126/sciadv.1602422
Carlson, C.J., Dallas, T.A., Alexander, L.W., Phelan, A.L. and Phillips, A.J. 2020. What would it take to describe the global diversity of parasites? Proceedings of the Royal Society B: Biological Sciences 287:20201841.
https://doi.org/10.1098/rspb.2020.1841
Chen, P.-Y., Lin, A.Y.-M., McKittrick, J. and Meyers, M.A. 2008. Structure and mechanical properties of crab exoskeletons. Acta Biomaterialia 4:587-596.
https://doi.org/10.1016/j.actbio.2007.12.010
Cohen, J.M., Sauer, E.L., Santiago, O., Spencer, S. and Rohr, J.R. 2020. Divergent impacts of warming weather on wildlife disease risk across climates. Science 370: eabb1702.
https://doi.org/10.1126/science.abb1702
Corral, J.M., Henmi, Y. and Itani, G. 2021. Differences in the parasitic effects of a bopyrid isopod and rhizocephalan barnacle on the portunid crab, Charybdis bimaculata. Parasitology International 81:102283.
https://doi.org/10.1016/j.parint.2021.102283
Cressey, R. and Patterson, C. 1973. Fossil Parasitic Copepods from a Lower Cretaceous Fish. Science 180:1283-1285. American Association for the Advancement of Science.
https://doi.org/10.1126/science.180.4092.1283
De Baets, K. and Littlewood, D.T.J. 2015. The Importance of Fossils in Understanding the Evolution of Parasites and Their Vectors, p. 90, 1-51. Advances in Parasitology. Elsevier.
https://doi.org/10.1016/bs.apar.2015.07.001
De Baets, K., Dentzien-Dias, P., Upeniece, I., Verneau, O. and Donoghue, P.C.J. 2015. Constraining the Deep Origin of Parasitic Flatworms and Host-Interactions with Fossil Evidence, p. 90, 93-135. Advances in Parasitology. Elsevier.
https://doi.org/10.1016/bs.apar.2015.06.002
De Baets, K., Huntley, J.W., Scarponi, D., Klompmaker, A.A. and Skawina, A. 2021. Phanerozoic parasitism and marine metazoan diversity: dilution versus amplification. Philosophical Transactions of the Royal Society B: Biological Sciences 376:20200366.
https://doi.org/10.1098/rstb.2020.0366
Dobson, A., Lafferty, K.D., Kuris, A.M., Hechinger, R.F. and Jetz, W. 2008. Homage to Linnaeus: How many parasites? How many hosts? Proceedings of the National Academy of Sciences of the United States of America 105:11482-11489.
https://doi.org/10.1073/pnas.0803232105
Dougherty, E.R., Carlson, C.J., Bueno, V.M., Burgio, K.R., Cizauskas, C.A., Clements, C.F., Seidel, D.P. and Harris, N.C. 2016. Paradigms for parasite conservation. Conservation Biology 30:724-733.
https://doi.org/10.1111/cobi.12634
Dyer, A.D., Ellis, E.R., Molinaro, D.J. and Leighton, L.R. 2018. Experimental fragmentation of gastropod shells by sediment compaction: Implications for interpreting drilling predation intensities in the fossil record. Palaeogeography, Palaeoclimatology, Palaeoecology 511:332-340.
https://doi.org/10.1016/j.palaeo.2018.08.018
Feldmann, R.M. 1998. Parasitic Castration of the Crab, Tumidocarcinus giganteus Glaessner, from the Miocene of New Zealand: Coevolution within the Crustacea. Journal of Paleontology 72:493-498.
Figueroa-Pizano, M.D., Vélaz, I., Peñas, F.J., Zavala-Rivera, P., Rosas-Durazo, A.J., Maldonado-Arce, A.D. and Martínez-Barbosa, M.E. 2018. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydrate Polymers 195:476-485.
https://doi.org/10.1016/j.carbpol.2018.05.004
Glenner, H. and Hebsgaard, M.B. 2006. Phylogeny and evolution of life history strategies of the parasitic barnacles (Crustacea, Cirripedia, Rhizocephala). Molecular Phylogenetics and Evolution 41:528-538.
https://doi.org/10.1016/j.ympev.2006.06.004
Gorzelak, P. and Salamon, M.A. 2013. Experimental tumbling of echinoderms -- Taphonomic patterns and implications. Palaeogeography, Palaeoclimatology, Palaeoecology 386:569-574.
https://doi.org/10.1016/j.palaeo.2013.06.023
Hegna, T.A., Luque, J. and Wolfe, J.M. 2020. The Fossil Record of the Pancrustacea, p. 1st ed., 21-52. In Thiel, M. and Poore, G. (eds.), Evolution and Biogeography. Oxford University PressNew York.
https://doi.org/10.1093/oso/9780190637842.003.0002
Herbst, J.F.W. 1794. Versuch einer Naturgeschichte der Krabben und Krebse nebst einer systematischen Beschreibung ihrer verschiedenen Arten. Gottlieb August Lange, Berlin and Stralsund.
Hochberg, R.J., Bert, T.M., Steele, P. and Brown, S.D. 1992. Parasitization of Loxothylacus Texanus on Callinectes Sapidus: Aspects of Population Biology and Effects on Host Morphology. Bulletin of Marine Science 50:117-132.
Høeg, J. and Lutzen, J. 1995. Life cycle and reproduction in the Cirripedia, Rhizocephala. Oceanography and Marine Biology: an annual review. Vol. 33 33:427-485.
Høeg, J.T. 1995. The biology and life cycle of the Rhizocephala (Cirripedia). Journal of the Marine Biological Association of the United Kingdom 75:517-550.
https://doi.org/10.1017/S0025315400038996
Kelley, P.H. 2008. Role of bioerosion in taphonomy: effect of predatory drillholes on preservation of mollusc shells, p. 451-470. In Wisshak, M. and Tapanila, L. (eds.), Current Developments in Bioerosion. Springer, Berlin, Heidelberg.
https://doi.org/10.1007/978-3-540-77598-0_23
Kidwell, S.M. and Baumiller, T. 1990. Experimental Disintegration of Regular Echinoids: Roles of Temperature, Oxygen, and Decay Thresholds. Paleobiology 16:247-271. Paleontological Society.
Klompmaker, A.A. 2009. Taphonomic bias on drill-hole predation intensities and paleoecology of Pliocene mollusks from Langenboom (Mill), the Netherlands. Palaios 24:772-779.
https://doi.org/10.2110/palo.2009.p09-023r
Klompmaker, A.A., Artal, P., Bakel, B.W.M. van, Fraaije, R.H.B. and Jagt, J.W.M. 2014. Parasites in the Fossil Record: A Cretaceous Fauna with Isopod-Infested Decapod Crustaceans, Infestation Patterns through Time, and a New Ichnotaxon. PLOS ONE 9:e92551.
https://doi.org/10.1371/journal.pone.0092551
Klompmaker, A.A. and Boxshall, G.A. 2015. Chapter Six - Fossil Crustaceans as Parasites and Hosts, p. 90, 233-289. In De Baets, K. and Littlewood, D.T.J. (eds.), Advances in Parasitology. Fossil Parasites. Academic Press.
https://doi.org/10.1016/bs.apar.2015.06.001
Klompmaker, A.A., Portell, R.W. and Frick, M.G. 2017. Comparative experimental taphonomy of eight marine arthropods indicates distinct differences in preservation potential. Palaeontology 60:773-794.
https://doi.org/10.1111/pala.12314
Krause, R.A., Parsons-Hubbard, K. and Walker, S.E. 2011. Experimental taphonomy of a decapod crustacean: Long-term data and their implications. Palaeogeography, Palaeoclimatology, Palaeoecology: Special Issue: The Shelf and Slope Experimental Taphonomy Initiative (SSETI): Thirteen years of taphonomic observations on carbonate and wood in the Bahamas and Gulf of Mexico 312:350-362.
https://doi.org/10.1016/j.palaeo.2011.03.020
Larsen, B.B., Miller, E.C., Rhodes, M.K. and Wiens, J.J. 2017. Inordinate Fondness Multiplied and Redistributed: the Number of Species on Earth and the New Pie of Life. The Quarterly Review of Biology 92:229-265.
https://doi.org/10.1086/693564
Lázaro-Chávez, E., Alvarez, F. and Rosas, C. 1996. Records of Loxothylacus texanus (Cirripedia: Rhizocephala) Parasitizing the Blue Crab Callinectes sapidus in Tamiahua Lagoon, Mexico. Journal of Crustacean Biology 16:105-110.
https://doi.org/10.1163/193724096X00324
Leonard, K.C., Worden, N., Boettcher, M.L., Dickinson, E. and Hartstone-Rose, A. 2022. Effects of freezing and short-term fixation on muscle mass, volume, and density. Anatomical Record (Hoboken, N.J.: 2007) 305:199-208.
https://doi.org/10.1002/ar.24639
Leung, T.L.F. 2017. Fossils of parasites: what can the fossil record tell us about the evolution of parasitism? Biological Reviews 92:410-430.
https://doi.org/10.1111/brv.12238
Littlewood, D.T.J. and Donovan, S.K. 2003. Fossil Parasites: A Case of Identity. Geology Today 19:136-142.
https://doi.org/10.1046/j.1365-2451.2003.00406.x
Liu, T., Li, B., Zheng, X., Liang, S., Song, X., Zhu, B., Kennedy, J. and Xia, J. 2010. Effects of freezing on the condensed state structure of chitin in alkaline solution. Carbohydrate Polymers 82:753-760.
https://doi.org/10.1016/j.carbpol.2010.05.047
McCoy, V.E., Young, R.T. and Briggs, D.E.G. 2015. Factors controlling exceptional preservation in concretions. Palaios 30:272-280.
https://doi.org/10.2110/palo.2014.081
Morton, D.N., Antonino, C.Y., Broughton, F.J., Dykman, L.N., Kuris, A.M. and Lafferty, K.D. 2021. A food web including parasites for kelp forests of the Santa Barbara Channel, California. Scientific Data 8:99. Nature Publishing Group.
https://doi.org/10.1038/s41597-021-00880-4
Nagler, C., Haug, C., Resch, U., Kriwet, J. and Haug, J.T. 2016. 150 million years old isopods on fishes; a possible case of palaeo-parasitism. Bulletin of Geosciences 91:1-12.
https://doi.org/10.3140/bull.geosci.1586
O’Brien, J. and Wyk, P.V. 1985. Effects of Crustacean Parasitic Castrators (Epicaridean Isopods and Rhizocephalan Barnacles) on Growth of Crustacean Hosts, p. Crustacean Issues 3. Routledge.
Okamura, B., Hartigan, A. and Naldoni, J. 2018. Extensive Uncharted Biodiversity: The Parasite Dimension. Integrative and Comparative Biology 58:1132-1145.
https://doi.org/10.1093/icb/icy039
Plotnick, R.E. 1986. Taphonomy of a Modern Shrimp: Implications for the Arthropod Fossil Record. PALAIOS 1:286-293.
https://doi.org/10.2307/3514691
Poulin, R. 2007. Are there general laws in parasite ecology? Parasitology 134:763-776.
https://doi.org/10.1017/S0031182006002150
Poulin, R. 2014. Parasite biodiversity revisited: frontiers and constraints. International Journal for Parasitology 44:581-589.
https://doi.org/10.1016/j.ijpara.2014.02.003
Rathbun, M.J. 1896. The genus Callinectes. Proceedings of the United States National Museum 18:349-375.
https://doi.org/10.5479/si.00963801.18-1070.349
Roy, K., Miller, D.J. and Labarbera, M. 1994. Taphonomic Bias in Analyses of Drilling Predation: Effects of Gastropod Drill Holes on Bivalve Shell Strength. PALAIOS 9:413-421.
https://doi.org/10.2307/3515059
Vesper, E.O., Hammond, M.A., Allen, M.R. and Wallace, J.M. 2017. Even With Rehydration, Preservation in Ethanol Influences the Mechanical Properties of Bone and How Bone Responds to Experimental Manipulation. Bone 97:49-53.
https://doi.org/10.1016/j.bone.2017.01.001
Waiho, K., Glenner, H., Miroliubov, A., Noever, C., Hassan, M., Ikhwanuddin, M. and Fazhan, H. 2021. Rhizocephalans and their potential impact on crustacean aquaculture. Aquaculture 531:735876.
https://doi.org/10.1016/j.aquaculture.2020.735876
Waugh, D.A., Feldmann, R.M., Crawford, R.S., Jakobsen, S.L. and Thomas, K.B. 2004. Epibiont preservational and observational bias in fossil marine decapods. Journal of Paleontology 78:961-972.
https://doi.org/10.1666/0022-3360(2004)078<0961:EPAOBI>2.0.CO;2
Wilson, D.D. and Brett, C.E. 2013. Concretions as sources of exceptional preservation, and decay as a source of concretions: examples from the Middle Devonian of New York. Palaios 28:305-316.
https://doi.org/10.2110/palo.2012.p12-086r
Wright, N.L., Klompmaker, A.A. and Petsios, E. 2024. Exploring the preservation of a parasitic trace in decapod crustaceans using finite elements analysis. PLOS ONE 19:e0296146.
https://doi.org/10.1371/journal.pone.0296146
Yamamori, L. and Kato, M. 2020. Shift of Feeding Mode in an Epizoic Stalked Barnacle Inducing Gall Formation of Host Sea Urchin. iScience 23:100885.
https://doi.org/10.1016/j.isci.2020.100885

