 Introduction to Diplodocoidea
Introduction to Diplodocoidea
Article number: 28.2.a27
https://doi.org/10.26879/1518
Copyright Palaeontological Association, June 2025
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Diplodocoidea (Dinosauria, Sauropoda): Systematics, Phylogeny, Biogeography
Submission: 9 January 2025. Acceptance: 28 April 2025.
ABSTRACT
Diplodocoidea is one of the most iconic clades of the giant sauropod dinosaurs, known for their elongated necks and tails, and distinctive skull morphology. This group, existing from the Middle Jurassic to the early Late Cretaceous, encompasses three main families: Rebbachisauridae, Dicraeosauridae, and Diplodocidae. These sauropods were globally distributed, demonstrating significant diversity in body plans, feeding strategies, and ecological niches. Diplodocoid paleontology has been marked by extensive studies focusing on skeletal morphology, biomechanics, histology, and evolutionary relationships. Significant research has also explored their ontogeny and niche partitioning, suggesting that diplodocoids had specialized adaptations for low- to mid-level browsing. This contribution is the introduction to a special volume that aims to synthesize current research on Diplodocoidea, offering insights into their evolutionary success, with subsequent contributions addressing their phylogenetic relationships, ontogeny, and morphological variation.
Tom T.P. van der Linden. Oertijdmuseum, Bosscheweg 80, 5283 WB Boxtel, the Netherlands (corresponding author). tppaleo@gmail.com
Michael P. Taylor. University of Bristol, Wills Memorial Building, Queens Road, Bristol BS8 1QU, United Kingdom. dino@miketaylor.org.uk
Amy Campbell. Museum für Naturkunde, Leibniz-Institut für Evolutions-und Biodiversitätsforschung, Invalidenstraße 43, 10115 Berlin, Germany. Amy.Campbell@mfn.berlin
Brian D. Curtice. Arizona Museum of Natural History, 53 N Macdonald Mesa, Arizona 85201, USA. brian@fossilcrates.com
René Dederichs. University of Zurich, Department of Paleontology, Karl Schmid-Strasse 4, CH-8006 Zurich, Switzerland and University of Bonn, Regina-Pacis-Weg 3, 53113 Bonn, Germany. rededpaleo@gmail.com
Lucas N. Lerzo. (CONICET). Departamento de Paleontología, Centro de Ciencias Naturales, Ambientales y Antropológicas (Fundación Azara - Universidad Maimónides), Hidalgo 775, C1405BCK, Buenos Aires, Argentina. lerzo.lucas@maimonides.edu
John A. Whitlock. Mount Aloysius College, 7373 Admiral Peary Hwy, Cresson, Pennsylvania 16630, USA and Carnegie Museum of Natural History, 4400 Forbes Ave, Pittsburgh, Pennsylvania 15213, USA. jwhitlock@mtaloy.edu
D. Cary Woodruff. Phillip and Patricia Frost Museum of Science, 1101 Biscayne Boulevard, Miami 33132, Florida, USA and Museum of the Rockies, 600 West Kagy Boulevard, Bozeman 59717, Montana, USA. sauropod4@gmail.com
Emanuel Tschopp.Freie Universität Berlin, Institute of Geological Sciences, Malteserstr. 74-100, 12249 Berlin, Germany; University of Hamburg, Mittelweg 177, 20148 Hamburg, Germany; and American Museum of Natural History, Division of Paleontology, Central Park West @ 79th, New York, NY 10024, USA. e.tschopp@fu-berlin.de
Keywords: dinosaurs; Sauropoda; Diplodocoidea; evolution; phylogeny; biogeography
INSTITUTIONAL ABBREVIATIONS
AMNH FARB – American Museum of Natural History, New York, NY, USA. Fossil Amphibian, Reptile, and Bird Collections
ANS – Academy of Natural Sciences, Philadelphia, PA, USA
BYU – Brigham Young University Museum of Paleontology, Provo, UT, USA
CM – Carnegie Museum of Natural History, Pittsburgh, PA, USA
CMC – Cincinnati Museum Center, Cincinnati, OH, USA
CMNH – Cleveland Museum of Natural History, Cleveland, OH, USA
DINO – Dinosaur National Monument, Jensen, UT, USA
HMNS – Houston Museum of Natural Science, Houston, TX, USA
LM – Lingwu Museum, Lingwu, Ningxia, China
MACN – Museo Nacional de Ciencias Naturales “Bernardino Rivadavia,” Buenos Aires, Argentina
MB.R. – Museum für Naturkunde, Berlin, Germany
MDS – Museo de Dinosaurios de Salas de los Infantes, Salas de los Infantes, Burgos, Spain
ML – Museu da Lourinhã, Lourinhã, Portugal
MLL – Museo Municipal de Las Lajas, Las Lajas, Neuquén, Argentina
MMCh-PV – Museo Municipal ‘Ernesto Bachmann,’ Villa El Chocón, Neuquén, Argentina
MHNM – Muséum d’Histoire Naturelle de Marrakech, Marrakech, Morocco
MNN – Museé National du Niger, Niamey, Niger
MOZ – Museo Provincial de Ciencias Naturales “Dr. Prof. Juan A.Olsacher,” Zapala, Argentina
MUC – Museo de Geologia y Paleontologia, Universidad Nacional del Comahue, Neuquén, Argentina
NMMNH – New Mexico Museum of Natural History and Science, Albuquerque, NM, USA
NHMUK – Natural History Museum, London, England, UK
NMZ – Natural History Museum, University of Zurich, Zurich, Switzerland
ONM – Musée de l’Office National des Mines, Minestère de L’Industrie et de la Technologie, La Charguia, Tunis, Tunisia
RWR – Palaeontology Division, Geological Survey of India, Western Region, Jaipur, Rajasthan, India
SMA – Sauriermuseum Aathal, Aathal, Switzerland
SMNS – Staatliches Museum für Naturkunde Stuttgart, Stuttgart, Baden-Württemberg, Germany
TATE - Tate Geological Museum, Casper College, Casper, WY, USA
UFMA – Fossil collection of the Universidade Federal do Maranhão, São Luís, Brazil
UFRJ-DG – Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, Department of Geology
UFRJ-MN – Universidade Federal do Rio de Janeiro, Museu Nacional, Departamento de Geologia e Paleontologia, Paleovertebrate collection, Rio de janeiro, Brazil
UNPSJB-Pv – Universidad Nacional de la Patagonia “San Juan Bosco,” Comodoro Rivadavia, Chubut, Argentina, paleovertebrate collection
USNM – United States National Museum, Smithsonian Institution, Washington, D.C., USA
WN – ‘without number,’ an informal designation for specimens awaiting accession/Museum of Bale, Croatia
YPM – Yale Peabody Museum, New Haven, CT, USA
Final citation: van der Linden, Tom T.P., Taylor, Michael P., Campbell, Amy, Curtice, Brian D., Dederichs, René, Lerzo, Lucas N., Whitlock, John A., Woodruff, D. Cary, and Tschopp, Emanuel. 2025. Introduction to Diplodocoidea. Palaeontologia Electronica, 28(2):a27.
https://doi.org/10.26879/1506
palaeo-electronica.org/content/2025/5554-introduction-to-diplodocoidea
Copyright: June 2025 Palaeontological Association.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
creativecommons.org/licenses/by/4.0
INTRODUCTION
Sauropod dinosaurs were the largest terrestrial vertebrates to have ever lived (e.g., Jensen, 1985; Bonaparte and Coria, 1993; Novas et al., 2005; Calvo et al., 2007; Carballido et al., 2017; Carpenter, 2018; Pal and Ayyasami, 2022), and have been intensively studied ever since their initial discovery over 180 years ago (e.g., Owen, 1841b; Gomez et al., 2024a). Well known for their massive body mass and lengths (e.g., Calvo, 2023), graviportal stance (e.g., McPhee et al., 2018), and hyperelongate necks (e.g., Vidal et al., 2020b; Moore et al., 2023) and tails (e.g., Holland, 1915a; Conti et al., 2022), these megaherbivores were among the most successful dinosaur groups during the Mesozoic Era. The earliest members of Sauropoda evolved in the Late Triassic (e.g., Lallensack et al., 2017; Pol et al., 2021; Barrett et al., 2024) or Early Jurassic (e.g., Rauhut et al., 2020; Wang et al., 2024), and were globally one of the dominant clades of terrestrial herbivores until the end of the Maastrichtian in the latest Cretaceous (e.g., Gilmore, 1922; Wilson and Upchurch, 2003).
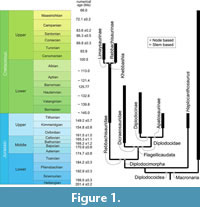 Within Sauropoda, neosauropods are divided into two clades (Figure 1): 1) Macronaria, containing the largest sauropods, the titanosaurs; and 2) Diplodocoidea (e.g., Salgado et al., 1997; Wilson and Sereno, 1998; Wilson, 2002; Whitlock, 2011a; Tschopp and Mateus, 2013; Tschopp et al., 2015; Bajpai et al., 2023). Diplodocoid sauropods are amongst the most iconic of all sauropods. With a more horizontal body plan compared to their macronarian counterparts (Taylor et al., 2009; Stevens, 2013), elongated tails (e.g., Conti et al., 2022), and specialized skulls with pencil-like teeth (e.g., Sereno and Wilson, 2005; Whitlock et al., 2010; Whitlock, 2011b; Tschopp and Mateus, 2013; Schwarz et al., 2015; Tschopp et al., 2015; Tschopp and Mateus, 2017; Peterson et al., 2022), this clade was globally successful from the Middle Jurassic until the early Late Cretaceous (Bajpai et al., 2023; Lerzo et al., 2024b).
Within Sauropoda, neosauropods are divided into two clades (Figure 1): 1) Macronaria, containing the largest sauropods, the titanosaurs; and 2) Diplodocoidea (e.g., Salgado et al., 1997; Wilson and Sereno, 1998; Wilson, 2002; Whitlock, 2011a; Tschopp and Mateus, 2013; Tschopp et al., 2015; Bajpai et al., 2023). Diplodocoid sauropods are amongst the most iconic of all sauropods. With a more horizontal body plan compared to their macronarian counterparts (Taylor et al., 2009; Stevens, 2013), elongated tails (e.g., Conti et al., 2022), and specialized skulls with pencil-like teeth (e.g., Sereno and Wilson, 2005; Whitlock et al., 2010; Whitlock, 2011b; Tschopp and Mateus, 2013; Schwarz et al., 2015; Tschopp et al., 2015; Tschopp and Mateus, 2017; Peterson et al., 2022), this clade was globally successful from the Middle Jurassic until the early Late Cretaceous (Bajpai et al., 2023; Lerzo et al., 2024b).
Diplodocoidea (stem-based definition: neosauropod taxa closer to Diplodocus than to Saltasaurus; Marsh, 1884; Upchurch, 1995; Taylor and Naish, 2005) consists of three main lineages: Rebbachisauridae, Dicraeosauridae, and Diplodocidae, which are united in the clade Diplodocimorpha (node-based definition: Rebbachisaurus tessonei + Diplodocidae, and all descendants of their common ancestor; Calvo and Salgado, 1995; Taylor and Naish, 2005) (Figure 1). Rebbachisaurids are primarily, and possibly exclusively, known from the Cretaceous (Lerzo et al., 2024a, 2024b, but see Carpenter, 2018), whereas Dicraeosauridae and Diplodocidae are primarily known from the Jurassic, though Cretaceous forms are known (Gallina et al., 2014; Tschopp et al., 2015; McPhee et al., 2016; Xu et al., 2018; Gallina et al., 2019; Whitlock and Wilson Mantilla, 2020; Bajpai et al., 2023). Non-diplodocimorph diplodocoid genera usually only include Haplocanthosaurus (Hatcher, 1903), though Amphicoelias (Cope, 1877b), has also been recovered as such (e.g., Mannion et al., 2021).
Previous studies on diplodocoid sauropods have focused on osteological descriptions (e.g., Hatcher, 1901, 1903; Lull, 1919; Janensch, 1929b; Gilmore, 1936; McIntosh and Williams, 1981; Gillette, 1991; Harris and Dodson, 2004; Harris, 2006a, 2006b, 2007; Tschopp and Mateus, 2013; Wilson and Allain, 2015; Tschopp and Mateus, 2017; Xu et al., 2018; Whitlock and Wilson Mantilla, 2020; Mannion et al., 2021; Bajpai et al., 2023; Lerzo et al., 2024a, 2024b), diplodocoid evolution (e.g., Whitlock, 2011a; Mannion et al., 2012; Tschopp et al., 2015; Bates et al., 2016; Bajpai et al., 2023; Lerzo et al., 2024a, 2024b), histology (e.g., Hedrick et al., 2014; Lambertz et al., 2018; Woodruff et al., 2018; Waskow, 2019; Price and Whitlock, 2022; Woodruff et al., 2024), pneumaticity (e.g., Wedel, 2003; Schwarz et al., 2007; Taylor and Wedel, 2021), ontogeny (e.g., Klein and Sander, 2008; Whitlock et al., 2010; Woodruff and Fowler, 2012; Tschopp and Mateus, 2013; Hanik et al., 2017; Woodruff et al., 2018), diseases (e.g., Woodruff et al., 2022), biomechanics (e.g., Wilhite, 2003; Taylor et al., 2009; Stevens, 2013; Taylor and Wedel, 2013a; Klinkhamer et al., 2018; Conti et al., 2022; Jannel et al., 2022), feeding mechanisms (e.g., Whitlock et al., 2010; Whitlock, 2011b; Young et al., 2012; D’Emic et al., 2013; Price and Whitlock, 2022), niche partitioning (e.g., Fiorillo, 1998; Whitlock et al., 2010; D’Emic et al., 2013; McHugh, 2018; Woodruff et al., 2018), and soft tissue reconstructions (e.g., Schwarz et al., 2007; Gallagher et al., 2021; Cerda et al., 2022). As such, diplodocoid sauropods represent an invaluable resource of information for sauropod paleobiological research.
In this contribution, we aim to summarize the status of research on diplodocoid sauropods. This introduction to diplodocoid sauropods will lead to a collection of studies describing new specimens, redescribing old specimens, deciphering ontogeny, revealing inter- and intraspecific variation, and describing skull morphology. These studies will inform a novel, extensive study on the phylogenetic relationships of these animals based on several matrices used in previous studies (e.g., Whitlock, 2011a; Tschopp et al., 2015, Xu et al., 2018; Whitlock and Wilson Mantilla, 2020; Mannion et al., 2021; Lerzo et al., 2024a, 2024b).
HISTORY OF DIPLODOCOID PALEONTOLOGY
The first sauropods to be described were Cardiodon (Owen, 1841a) and Cetiosaurus (Owen, 1841b), but the former was based only on a tooth, and the latter, also represented by only very fragmentary material, was initially interpreted as a gigantic marine predator (see summary in Taylor, 2010). It was only with the description of Cetiosaurus oxoniensis (Phillips, 1871) that Cetiosaurus was recognized to be a terrestrial, or at most amphibious, animal. Seven years later, the name Sauropoda was coined by Marsh (1878), for a group containing Atlantosaurus (Marsh, 1877b), Apatosaurus (Marsh, 1877b), Morosaurus (Marsh, 1878), and Diplodocus (Marsh, 1878), as well as “others... from this country and Europe described by various authors” (p. 412), including Cetiosaurus. Of these examples, three – Apatosaurus, Diplodocus, and the dubious Atlantosaurus – were diplodocids (though that name, and its corresponding superfamily name Diplodocoidea, had not yet been coined), so this group has always loomed large in the perception of sauropods.
Confusingly, the first diplodocoid to be described was “Titanosaurus” montanus (Marsh, 1877a), which was named in ignorance of Lydekker’s (1877) slightly earlier use of the same genus name for a very different Indian sauropod. Marsh swiftly replaced this homonym with the new name Atlantosaurus montanus (Marsh, 1877b), and in the same paper named the second diplodocoid, Apatosaurus ajax. These were followed later that same year by Amphicoelias altus (Cope, 1877b), then the next year by Diplodocus longus (Marsh, 1878), a year later by Brontosaurus excelsus (Marsh, 1879), then after 11 further years by Barosaurus lentus (Marsh, 1890). All these taxa were diplodocids; the first named non-diplodocid diplodocoid was Dicraeosaurus hansemanni (Janensch, 1914), followed by Rebbachisaurus garasbae (Lavocat, 1954).
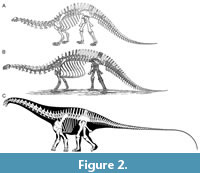 Most of these early descriptions were hopelessly inadequate by modern standards, in many cases illustrated by a single drawing (e.g., “Amphicoelias” fragillimus; Cope, 1878) or none (e.g., Amphicoelias altus), providing only the most cursory descriptive text, and giving no sense of the overall body-plan of these animals. This changed with Marsh’s (1883, plate I) skeletal reconstruction of Brontosaurus (Figure 2A). Marsh’s reconstruction showed a form readily recognizable today, but was erroneous in several respects: the back is too arched so that the anterior part of the trunk descends at a sharp angle; there are only 11 cervical vertebrae (rather than 15); the skull (then unknown) is based on that of a camarasaurid (YPM VP.001911); the forelimbs are strongly flexed rather than columnar, the forefeet are in the then-ubiquitous near-plantigrade posture, and the tail is much too short. Eight years later, Marsh (1891, plate XVI) published a revised reconstruction (Figure 2B). Although this correctly increased the number of cervical vertebrae – though only to 13, not 15 – it also increased the dorsal vertebral count from the correct 10 to 14 and provided a new but equally incorrect skull – this one based on YPM VP.001986, which is now considered brachiosaurid (Carpenter and Tidwell, 1998; D’Emic and Carrano, 2019). In modern, anatomically rigorous skeletal reconstructions such as that of Scott Hartman (Figure 2C), these errors are corrected, showing a very different animal with a compact torso supporting a longer neck and a much longer tail, with a characteristically diplodocid skull, and with erect forelimb and unguligrade forefeet.
Most of these early descriptions were hopelessly inadequate by modern standards, in many cases illustrated by a single drawing (e.g., “Amphicoelias” fragillimus; Cope, 1878) or none (e.g., Amphicoelias altus), providing only the most cursory descriptive text, and giving no sense of the overall body-plan of these animals. This changed with Marsh’s (1883, plate I) skeletal reconstruction of Brontosaurus (Figure 2A). Marsh’s reconstruction showed a form readily recognizable today, but was erroneous in several respects: the back is too arched so that the anterior part of the trunk descends at a sharp angle; there are only 11 cervical vertebrae (rather than 15); the skull (then unknown) is based on that of a camarasaurid (YPM VP.001911); the forelimbs are strongly flexed rather than columnar, the forefeet are in the then-ubiquitous near-plantigrade posture, and the tail is much too short. Eight years later, Marsh (1891, plate XVI) published a revised reconstruction (Figure 2B). Although this correctly increased the number of cervical vertebrae – though only to 13, not 15 – it also increased the dorsal vertebral count from the correct 10 to 14 and provided a new but equally incorrect skull – this one based on YPM VP.001986, which is now considered brachiosaurid (Carpenter and Tidwell, 1998; D’Emic and Carrano, 2019). In modern, anatomically rigorous skeletal reconstructions such as that of Scott Hartman (Figure 2C), these errors are corrected, showing a very different animal with a compact torso supporting a longer neck and a much longer tail, with a characteristically diplodocid skull, and with erect forelimb and unguligrade forefeet.
Several years after Marsh’s skeletal reconstructions, artists began creating life restorations of diplodocoids. The earliest known artistic life reconstruction of any sauropod is an 1897 drawing by Charles R. Knight, executed under Cope’s instruction, of several Amphicoelias individuals underwater (at the time, it was believed that sauropods were aquatic due to their great mass [see below]; reproduced by Osborn and Mook, 1921, figure 127). This was followed later that same year by Knight’s much better-known painting of a swamp-bound Brontosaurus (reproduced by Taylor, 2010, figure 6), which set the template that would dominate the perception of diplodocoid (and, more broadly, sauropod) ecology for 70 years.
The idea that sauropods were amphibious animals goes back to Phillips’s (1871) description of Cetiosaurus oxoniensis. Phillips noted that “all the articulations [of the limb bones] are such as to be suited for walking” (p. 294), but nevertheless concluded that “we have, therefore, a marsh-loving or river-side animal” (p. 294). Although Marsh (1877a) referred to Atlantosaurus montanus as “this largest of land animals,” a few years later he wrote of Brontosaurus that “the very small head and brain, and slender neural cord, indicate a stupid, slow-moving reptile. [...] In habits, Brontosaurus was more or less amphibious, and its food was probably aquatic plants or other succulent vegetation.” As noted above, Knight’s 1897 painting of an amphibious Brontosaurus helped to fix this notion in the minds of scientists and the public alike.
The first diplodocoid to receive a comprehensive description meeting today’s scientific standards was Diplodocus carnegii (Hatcher, 1901). Hatcher’s monograph included 13 plates culminating in a beautifully executed skeletal reconstruction. Hatcher (1901, p. 59-61) reaffirmed and expanded on the then-orthodox notion of sauropods in general, and diplodocoids in particular, as sluggish, swamp-bound creatures: “Though living for the most part in the more important rivers and freshwater lakes, it may not infrequently have left the water and taken temporarily to the land, either in quest of food or in migration from one to another of adjacent bodies of water.”
When King Edward VII of England visited Andrew Carnegie’s Scottish home at Skibo Castle in 1902, he was so taken with a framed print of the skeletal reconstruction from Hatcher’s monograph that he asked Carnegie to provide a Diplodocus skeleton for the British Museum. This led to the very first casting of a sauropod skeleton, which was donated by Carnegie to the Natural History Museum in London, where it stood until recently as the entry centerpiece of the museum. From these original molds, Carnegie had nine additional replica skeletons made which were donated and mounted in natural history museums in Berlin, Paris, Vienna, Bologna, St. Petersburg, La Plata, Madrid, and Mexico City, with a tenth cast traded to Munich but never mounted (Rea, 2001; Nieuwland, 2019; Taylor et al., 2025).
After the Carnegie Museum had finished with the molds used to make these cast skeletons, they were acquired by the Utah Field House of Natural History State Park Museum in Vernal, Utah, and used one last time to make a concrete Diplodocus that was exhibited outdoors from 1957 to 1989 (Taylor et al., 2023). When this cast was taken down, it was used to make second-generation molds which in turn have been used to make yet more copies of the Carnegie Diplodocus, which are now mounted in multiple locations in the USA, Canada, and Japan. As a result of this rich history, the Carnegie Diplodocus is probably the best-known individual dinosaur in the world, and certainly the sauropod that most formed public impressions of the sauropod form.
Around the same time that the Carnegie Museum was mounting Diplodocus, the American Museum of Natural History was creating a mounted skeleton of “Brontosaurus” (mainly based on the specimen AMNH FARB 460) – now considered an indeterminate apatosaurine (Tschopp et al., 2015). The question of which was the first mounted sauropod skeleton is a complex one. The Carnegie Diplodocus was the first to be erected, on 29 June 1904, but only as a trial mount before the cast was sent to England. This was not available to the public, nor did it include any real bone. The AMNH “Brontosaurus,” which largely consisted of real bone (albeit from several individuals), was unveiled to the public on 16 February 1905, three months before the public debut of the Carnegie cast in London on 12 May 1905.
The first diplodocoids recognized from outside North America were those discovered by the German expeditions in the Tendaguru Formation of Tanzania (then Deutsch Ostafrika). Janensch (1914) identified two sauropods that are relevant here. “Gigantosaurus” africanus was recognized as related to Diplodocus. The taxonomic history of “Gigantosaurus” is exceedingly complex, but the outcome is that the species described by Janensch is currently known as Tornieria africana (Remes, 2006), identified as a diplodocine. Perhaps more significant, it was in this paper that Janensch (1914) named Dicraeosaurus hansemanni, the first representative of the diplodocoid family Dicraeosauridae.
The last major group of diplodocoids to be recognized was Rebbachisauridae, first known from the remains of Rebbachisaurus garasbae (Lavocat, 1954), found in Morocco, and subsequently from numerous taxa from South America and elsewhere.
The “Dinosaur Renaissance” of the late 1960s and 1970s is often considered to have been catalyzed by Ostrom’s (1969) description of the bird-like, agile, active, and intelligent dromaeosaurid theropod Deinonychus. However, this was preceded by Bakker’s (1968) article “The Superiority of Dinosaurs,” in which he forcefully refuted the long-standing orthodoxy of swamp-bound sauropods. Critical to the impact of his argument was a pencil drawing of two Barosaurus individuals striding briskly across a dry Mesozoic landscape with their heads held high. Bakker’s arguments for terrestriality were placed on a firmer theoretical footing by Coombs (1975), and this perception of sauropod lifestyles has held sway ever since.
The vision of sauropods in general, and diplodocines such as Barosaurus in particular, as active terrestrial animals was sealed in the public imagination by the AMNH’s unveiling of a mounted cast skeleton of Barosaurus in a rearing posture in 1991, positioned as though to defend a juvenile from an attacking Allosaurus (Taylor et al., in prep.). Although this exhibit has provoked some controversy (e.g., Hicks and Badeer, 1992; Choy and Altman, 1992), it remains the iconic image of Barosaurus and was likely the inspiration for the briefly rearing Brachiosaurus in the 1993 film Jurassic Park.
THE MAIN DIPLODOCOID GROUPS
Overview of Diplodocoid Phylogeny
Diplodocoidea had already been recognized as a major clade of neosauropod sauropods in the early years of cladistic analyses of Sauropoda (Calvo and Salgado, 1995; Upchurch 1995, 1998, 1999; Salgado et al., 1997, 1999; Wilson and Sereno, 1998). Its exact position within Sauropoda remained debated, however, with Upchurch (1995) recovering it as a sister clade to Titanosauria, whereas many other analyses (e.g., Calvo and Salgado, 1995; Salgado et al., 1997; Wilson and Sereno, 1998), including a subsequent iteration of Upchurch’s (1995) own analysis (Upchurch, 1998), found Diplodocoidea as a sister clade to a clade consisting of Camarasaurus, Brachiosaurus, and titanosaurs – a view that has since been confirmed by many other analyses. This sister clade to Diplodocoidea was named Macronaria by Wilson and Sereno (1998). These two major clades form Neosauropoda. As with the phylogenetic position of Diplodocoidea among sauropods, the composition of Diplodocoidea has also been debated.
Upchurch (1995, 1998, 1999) and Upchurch et al. (2004a) proposed that Nemegtosauridae was part of Diplodocoidea. Salgado and Calvo (1997), Curry Rogers and Forster (2001), and Wilson (2002) alternatively recovered Nemegtosauridae within Macronaria as derived titanosaurs. The genus Haplocanthosaurus has also regularly meandered between Diplodocoidea (e.g., Wilson, 2002), Macronaria (e.g., Upchurch, 1995; Wilson and Sereno, 1998), and even non-neosauropod Eusauropoda (e.g., Harris, 2006). The general structure and taxonomic contents of Diplodocoidea have converged since then, most importantly thanks to the in-depth analyses of diplodocoid relationships by Rauhut et al. (2005), Salgado et al. (2006), Sereno et al. (2007), Whitlock (2011a), Mannion et al. (2012), and Tschopp et al. (2015). These have mostly confirmed Wilson (2002) in that Diplodocoidea consisted of Haplocanthosaurus as its most basal member, and the three main lineages: Rebbachisauridae, Dicraeosauridae, and Diplodocidae (which are united in the clade Diplodocimorpha; originally proposed by Calvo and Salgado, 1995). Several additional clades within Diplodocoidea have been defined phylogenetically (Sereno, 1998; Harris and Dodson, 2004; Taylor and Naish, 2005); all of which were reviewed and summarized by Taylor and Naish (2005, table 1).
Based on their review, Taylor and Naish (2005) proposed a series of recommendations regarding the use and phylogenetic definition of these clades, which have since largely been followed. According to their scheme, Diplodocoidea is the stem-based taxon including all taxa more closely related to Diplodocus than to Saltasaurus. Diplodocimorpha is a node-based taxon including Diplodocus and Rebbachisaurus, their most recent common ancestor, and all its descendants (Figure 1). As such, it excludes Haplocanthosaurus in most phylogenetic analyses (see below). Included in Diplodocimorpha are the stem-based Rebbachisauridae and node-based Flagellicaudata (Figure 1). Rebbachisauridae is defined as all taxa more closely related to Rebbachisaurus than to Diplodocus. Flagellicaudata includes Dicraeosaurus, Diplodocus, their most recent common ancestor, and all its descendants (Figure 1). Dicraeosaurus and Diplodocus are also used to define the two stem-based taxa Dicraeosauridae and Diplodocidae that make up Flagellicaudata (Harris and Dodson, 2004). Finally, Diplodocidae includes the stem-based clades Apatosaurinae (all taxa more closely related to Apatosaurus than to Diplodocus) and Diplodocinae (all taxa more closely related to Diplodocus than to Apatosaurus) (Figure 1). These definitions have turned out to be applicable in a fairly stable way in a number of follow-up studies focusing on Diplodocoidea as a whole (e.g., Whitlock, 2011a; Mannion et al., 2012), or on distinct subgroups (Carballido et al., 2012; Tschopp et al., 2015; Canudo et al., 2018; Xu et al., 2018; Lindoso et al., 2019; Whitlock and Wilson, 2020; Windholz et al., 2022; Lerzo et al., 2024a, 2024b). These subgroups will be discussed below.
Rebbachisauridae (Table 1)
The first rebbachisaurid to be published, Rebbachisaurus garasbae (Lavocat, 1954), was based on a scapula, a posterior dorsal vertebra, and some undescribed elements. However, it was not until the nineties when Bonaparte (1997) recognized a new clade formed by Rebbachisaurus garasbae, “Rebbachisaurus” tessonei (Calvo and Salgado, 1995), and Rayososaurus agrioensis (Bonaparte, 1996), which he called Rebbachisauridae. The first phylogenetic definition of the family was published by Salgado et al. (2004) as all diplodocoids more closely related to Rebbachisaurus garasbae than to Diplodocus (Salgado et al., 2022). Since Bonaparte (1997), several articles have been published increasing the knowledge of the group (Dalla Vecchia, 1998; Pereda Suberbiola et al., 2003; Salgado et al., 2006, 2022; Carballido et al., 2010, 2012; Torcida Fernandéz-Baldor et al., 2011; Fanti et al., 2013, 2014, 2015; Ibiricu et al., 2013, 2015; Canudo et al., 2018; Lindoso et al., 2019; Bellardini et al., 2022a, 2022b, 2023; Lerzo et al., 2024a, 2024b; Simón and Salgado, 2025).
Whitlock (2011a) was the first to recognize two subclades within Rebbachisauridae: The South American Limaysaurinae (Limaysaurus not Nigersaurus) and the Euro-African Nigersaurinae (Nigersaurus not Limaysaurus). Limaysaurinae was an unstable clade supported solely by a character from the scapula (Carballido et al., 2012; Canudo et al., 2018). Recent analyses did not recover Limaysaurinae due to the multiple positions taken by Cathartesaura (Bellardini et al., 2022b, Lerzo et al., 2024a) or the different positions taken by Rebbachisaurus (Lerzo et al., 2024b). Wilson and Allain (2015) provided a complete description of Rebbachisaurus garasbae, recovering it within the Euro-African subclade. Consequently, following the ICZN rules, the clade name Rebbachisaurinae has priority over Nigersaurinae (Wilson and Allain, 2015; Canudo et al., 2018; Salgado et al., 2022). In the same year, Fanti et al. (2015) defined the clade Khebbashia as the least inclusive clade containing Limaysaurus tessonei, Nigersaurus taqueti, and Rebbachisaurus garasbae (Salgado et al., 2022).
Limaysaurus tessonei is based on an articulated, well-preserved skeleton, including cranial remains. The species was initially described as “Rebbachisaurus” tessonei and included in Diplodocimorpha (Calvo and Salgado, 1995). Later, Salgado et al. (2004) renamed this species from the Cenomanian Candeleros Formation of Argentina as Limaysaurus tessonei and recognized it as part of Rebbachisauridae. In recent years, L. tessonei has been a derived member within Rebbachisauridae (e.g., Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b).
Before the referral of “Rebbachisaurus” tessonei to its own genus Limaysaurus, several new rebbachisaurids were described. Rayososaurus agrioensis (Bonaparte, 1996; Carballido et al., 2010) is also from the Cenomanian Candeleros Formation, with the holotype consisting of appendicular elements. Upon description, R. agrioensis was not assigned to any family, but later works included this species in Rebbachisauridae (e.g., Salgado et al., 2004; Gallina and Apesteguía, 2005; Carballido et al., 2010). The species is tentatively considered to be a derived member closely related to Rebbachisaurus (Lerzo et al., 2024b), although several recent analyses omit this taxon due to its fragmentary nature (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a).
The geologically oldest unambiguous rebbachisaurid taxon is Histriasaurus boscarollii (Dalla Vecchia, 1998) from Istria (Croatia). It is solely represented by a partial middle/posterior dorsal vertebrae. H. boscarollii is one of the earliest-branching species in Rebbachisauridae (Bellardini et al., 2022; Lerzo et al., 2024a), which – combined with its Hauterivian-Barremian age – highlights its importance for untangling the biogeographic origin of Rebbachisauridae.
Agustinia ligabuei was originally described as a member of “Agustinidae” (Bonaparte, 1999) and later assigned to Titanosauria (Upchurch et al., 2004a; Curry Rogers, 2005). A recent redescription of the holotypic and newly referred materials, consisting of axial and appendicular elements, of Agustinia by Bellardini et al. (2022b) recovered rebbachisaurid affinities, and placed Agustinia as a basal member of the group. Other recent phylogenetic analyses find Agustinia as a problematic taxon, recovering the taxon in different positions within Rebbachisauridae (Lerzo et al., 2024a; Lerzo et al., 2024b).
The discovery and description of Nigersaurus taqueti (Sereno et al., 1999) informed the scientific community of the anatomy and ecology of rebbachisaurids. Represented by multiple individuals of different ontogenetic stages, including near complete skulls, N. taqueti from the Lower Cretaceous Elrhaz Formation shows feeding adaptations not previously documented in other sauropod skulls (Sereno et al., 2007). N. taqueti is nested well within Khebbashia and depending on the analysis included in Rebbachisaurinae (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a) or Nigersaurinae (Lerzo et al., 2024b).
Amazonsaurus maranhensis (Carvalho et al., 2003) was the first named rebbachisaurid from the Aptian-Albian of Brazil. Represented by axial and appendicular material, this species is often recognized as the most basal member of Rebbachisauridae (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b).
Following the redescription of Limaysaurus in 2004, several additional rebbachisaurid taxa have been established. Two new Argentinian rebbachisaurids were described shortly after the erection of Limaysaurus: Cathartesaura anaerobica (Gallina and Apesteguía, 2005) from the Cenomanian-Turonian Huincul Formation and Zapalasaurus bonapartei (Salgado et al., 2006) from the Barremian-Aptian La Amarga Formation. The holotype of C. anaerobica consists of several axial and appendicular elements. It represents a more derived rebbachisaurid (Bellardini et al., 2022a; Lerzo et al., 2024b), although some recent analyses (Lerzo et al., 2024a) suggest that this species may lie outside of Khebbashia. Z. bonapartei is represented also by axial and appendicular elements and has rather consistently been recovered as one of the earliest-branching members of the family (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b).
Demandasaurus darwini (Torcida Fernández-Baldor et al., 2011) is the most complete European rebbachisaurid, represented by cranial, axial, and appendicular elements. Although recovered as derived member (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b), it is also one the oldest rebbachisaurids, known from the Late Barremian to Early Aptian Castrillo de la Reina Formation (Torcida Fernández-Baldor et al., 2011). Its age and relative completeness aids investigations into the origins of the derived forms in Rebbachisauridae.
In 2013, a novel African and yet another Argentinian rebbachisaurid were described: Tataouinea hannibalis (Fanti et al., 2013) and Katepensaurus goicoecheai (Ibiricu et al., 2013). Originating from the Albian Ain el Guettar Formation of Tunesia, T. hannibalis was initially described based on fragmentary sacral and caudal remains, but in 2015, Fanti et al. described additional remains from the holotype, including a well-preserved articulated caudal vertebral series. K. goicoecheai comes from the Cenomanian-Turonian Bajo Barreal Formation, and its holotype consists of cranial, axial, and appendicular elements (Ibiricu et al., 2013, 2015). Both Tataouinea and Katepensaurus are considered to be derived members of Rebbachisauridae (Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b).
In subsequent years, two new South American taxa were named: Lavocatisaurus agrioensis (Canudo et al., 2018) and Itapeuasaurus cajapioensis (Lindoso et al., 2019). L. agrioensis is represented by significant skull and axial material, as well as appendicular elements, from the Aptian to lower Albian part of the Rayoso Formation in Argentina. I. cajapioensis was described from the Cenomanian Alcântara Formation and is represented by two dorsal and three caudal vertebrae. Lavocatisaurus was originally, and still is, thought to be a non-khebbashian rebbachisaurid (Canudo et al., 2018; Bellardini et al., 2022a, 2022b; Lerzo et al., 2024a, 2024b). Itapeuasaurus, however, was originally recovered as a derived member in Rebbachisauridae (Lindoso et al., 2019), but recent analyses have found it to be a non-khebbashian rebbachisaurid as well (Lerzo et al., 2024a, 2024b).
Most recently, three new rebbachisaurids were described: Sidersaura marae (Lerzo et al., 2024a), Campananeyen fragilissimus (Lerzo et al., 2024b), and Cienciargentina sanchezi (Simón and Salgado, 2025). All three species are represented by axial and appendicular materials, but Sidersaura and Campananeyen also preserve cranial elements. Sidersaura and Cienciargentina are both from the Cenomanian-Turonian Huincul Formation (Lerzo et al., 2024a; Simón and Salgado, 2025), whereas Campananeyen is from the Cenomanian Candeleros Formation (Lerzo et al., 2024b). Sidersaura and Campananeyen are recovered in a basal clade closely related to Zapalasaurus (Lerzo et al., 2024a; Lerzo et al., 2024b; Simón and Salgado, 2025). Cienciargentina possibly represents the earliest diverging member of Rebbachisauridae (Simón and Salgado, 2025). Sidersaura represents one of the largest known rebbachisaurid sauropods, with an estimated length of approximately 20 m and a weight of 15 tonnes. In contrast to Sidersaura, Campananeyen represents a small rebbachisaurid characterized by a novel sacral structure, resulting from the fusion of the dorsal ends of the sacral transverses processes (Lerzo et al., 2024b). Cienciargentina is characterized by a suite of ‘basal’ and ‘derived’ features and is important for our understanding of faunal turnovers in the Cretaceous (Simón and Salgado, 2025).
Over the years, several taxa have been part of Rebbachisauridae, but their taxonomic assignment requires further studies. This is the case for Nopcsaspondylus alarconensis, Maraapunisaurus fragillimus, Xenoposeidon proneneukos, and Dzharatitanis kingi (Apesteguía, 2007; Carpenter, 2018; Taylor, 2018; Averianov and Sues, 2021). In the case of Nopcsaspondylus alarconensis and Maraapunisaurus fragillimu s, all original material is lost (Carpenter, 2018; Salgado et al., 2022), and their referral to Rebbachisauridae was solely based on published figures (Apesteguía, 2007; Carpenter, 2018). Nopcsaspondylus alarconensis (Apesteguía, 2007) was represented by a missing middle or posterior dorsal vertebra and it represents one of the first dinosaur remains collected in Argentina (Canale et al., 2023). Nopcsa (1902) referred that vertebra to Bothriospondylus (Owen, 1985) but later, Hatcher (1903) referred to it as Haplocanthosaurus (Salgado et al., 2022; Canale et al., 2023). McIntosh (1990b) was the first to relate this vertebra with Rebbachisaurus garasbae. More findings are needed to corroborate the position of this species within Diplodocoidea.
Maraapunisaurus fragillimus was originally ‘described’ within two paragraphs and a single illustration in a popular science periodical in 1878 by Cope. Initially identified as the holotype for a second species of Amphicoelias (Cope, 1878), it was officially noted as ‘missing’ from the AMNH’s acquisition of Cope’s collection by Osborn and Mook (1921). With the original material being lost, this taxon has often been a synonym of Amphicoelias altus (Osborn and Mook, 1921; McIntosh, 1990b; Upchurch et al., 2004a; Woodruff and Foster, 2014) but was recently hypothesized to represent large rebbachisaurid (Carpenter, 2018).
Xenoposeidon proneneukos was originally placed within Neosauropoda (Taylor and Naish, 2007) and later reassigned to Rebbachisauridae based on similarities with Rebbachisaurus garasbae (Taylor, 2018). Xenoposeidon is represented only by a partial posterior dorsal vertebra. Both Xenoposeidon and Maraapunisaurus could be the first step in investigating the origin of diplodocoid sauropods because they could represent among the oldest rebbachisaurids. However, both assignments require new material, because one is based solely on a drawing and the other is a fragmentary specimen (Whitlock and Wilson Mantilla, 2020; Salgado et al., 2022).
Dzharatitanis kingi was originally described as a titanosaurian sauropod (Sues et al., 2015) and is only known from a single anterior caudal vertebra. Recently, Averianov and Sues (2021) redescribed this caudal vertebra as a rebbachisaurid sauropod. However, a subsequent phylogenetic analysis recovered Dzharatitanis within Titanosauria (Lerzo et al., 2021).
Dicraeosauridae (Table 2)
The clade Dicraeosauridae was first coined by Huene (1927) and is defined as all taxa more closely related to Dicraeosaurus than Diplodocus (Sereno, 1998). The first described genus was Dicraeosaurus (Janensch, 1914) of which there are two recognized species, D. hansemanni and D. sattleri, both from the Upper Jurassic Tendaguru Formation of Tanzania. Both species were distinguished based on differences in the robustness of their appendicular bones and geological age, with the more robust material from the Middle Dinosaur Member attributed to D. hansemanni and the more gracile elements from the Upper Dinosaur Member attributed to D. sattleri (Janensch, 1914). D. hansemanni is represented by a largely complete specimen, with all areas of the skeleton preserved, excluding the lower forelimbs and parts of the skull. Known D. sattleri material consists of more isolated remains, predominantly long bones and caudal vertebrae. Dicraeosaurus was the only genus included within Dicraeosauridae for 90 years, until the 1991 description of Amargasaurus cazaui (Salgado and Bonaparte, 1991) from the Early Cretaceous of Argentina. Since then, a further 8 species have been recovered as dicraeosaurids.
The holotype of Dicraeosaurus hansemanni (Janensch, 1914) is made up largely of a single articulated individual from quarry m within the Tendaguru area. Isolated remains of D. hansemanni have been found in numerous quarries across the Middle Dinosaur Member of the Tendaguru Formation, with all skull material having been found in a single quarry (dd). The holotype of D. sattleri consists of material from quarry M (note that this is not the same quarry as quarry m) within the Tendaguru area. Isolated elements of Dicraeosaurus sattleri (Janensch, 1914) from all regions of the postcranium are represented and have been discovered across multiple quarries within the Upper Dinosaur Member of the Tendaguru Formation.
Amargasaurus cazaui (Salgado and Bonaparte, 1991) is from the Lower Cretaceous La Amarga Formation of Argentina. It is represented by the basicranial and temporal skull region, and most elements of the postcranium. Brachytrachelopan mesai (Rauhut et al., 2005) from the Upper Jurassic Cañadón Calcáreo Formation of Argentina is known from an articulated partial postcranial skeleton consisting of cervical, dorsal, and sacral vertebrae, cervical and dorsal ribs, ilia, and distal elements of the left femur and left tibia (Rauhut et al., 2005). Dicraeosaurus, Brachytrachelopan, and Amargasaurus have been consistently recovered as forming a derived subclade within Dicraeosauridae across phylogenetic analyses (Whitlock, 2011a; Gallina, 2016; Tschopp and Mateus, 2017; Xu et al., 2018; Gallina et al., 2019; Whitlock and Wilson Mantilla, 2020; Bajpai et al., 2023).
Suuwassea emilieae (Harris and Dodson, 2004) from the Upper Jurassic Morrison Formation of Montana, USA, is represented by the basicranial, temporal, and partial rostral regions of the skull, cervical, dorsal, and caudal vertebrae, scapulocoracoid, humerus, and lower hind limb elements (Harris and Dodson, 2004). The phylogenetic analysis of Harris and Dodson (2004) recovered Suuwassea as a Flagellicaudatan, though not recovered in either Dicraeosauridae or Diplodocidae. Later, Lovelace et al. (2007) recovered Suuwassea as nested within Apatosaurinae, whereas all recent phylogenies recover it as a dicraeosaurid. The position of Suuwassea emilieae in Dicraeosauridae, however, is unstable, with analyses recovering the taxon as one of the most basal (Salgado et al., 2006; Whitlock, 2011a; Mannion et al., 2012; Tschopp and Mateus, 2017; Xu et al., 2018; Gallina et al., 2019; Bajpai et al., 2023) or belonging to a more derived clade as sister taxon to Amargatitanis (Whitlock and Wilson Mantilla, 2020).
Amargatitanis macni (Apesteguía, 2007) from the early Cretaceous La Amarga Formation of Argentina is known from a partial hind limb, ischium, and two partial caudal vertebrae. It was initially referred to Titanosauria (Apesteguía, 2007), subsequently considered a nomen dubium by D’Emic (2012) and Mannion et al. (2013); and then, following a redescription by Gallina (2016), was recovered nested within Dicraeosauridae as a sister taxon to Suuwassea. Subsequent analyses have recovered it within Dicraeosauridae (Coria et al., 2019; Whitlock and Wilson Mantilla, 2020; Mannion et al., 2021; Windholz et al., 2022; Bajpai et al., 2023; Windholz et al., 2023).
Lingwulong shenqi (Xu et al., 2018) from the Middle Jurassic of Lingwu, China is known from material of multiple individuals and referred material, and includes the basicrania, dentary teeth, and all postcranial regions. It has consistently been recovered as a basal member of Dicraeosauridae (Xu et al., 2018; Gallina et al., 2019; Whitlock and Wilson Mantilla, 2020; Windholz et al., 2022; Bajpai et al., 2023).
Pilmatueia faundezi (Coria et al., 2019) from the Lower Cretaceous Mulichino Formation of Argentina was first described based on a posterior dorsal vertebra and dorsal neural arch, a partial cervical vertebra, and two mid-caudal vertebrae. It was recovered in a derived position within Dicraeosauridae as the sister taxon to Amargasaurus (Coria et al., 2019). Subsequent analyses (partially) corroborated this finding, recovering Pilmatueia as belonging to a sister group of the clade Dicraeosaurus, Amargasaurus, and Brachytrachelopan (e.g., Gallina, 2019; Whitlock and Wilson Mantilla, 2020; Bajpai et al., 2023). Windholz et al. (2022) described three articulated cervical vertebrae, seven dorsal vertebrae, a caudal vertebra, and scapula belonging to Pilmatueia and scored the taxon in matrices from both Tschopp and Mateus (2017) and Whitlock and Wilson Mantilla (2020). From the Tschopp and Mateus (2017) matrix Pilmatueia formed a polytomy with Amargatitanis and Bajadasaurus with Amargasaurus, Brachytrachelopan, and Dicraeosaurus as successive steps down the tree. The Whitlock and Wilson Mantilla (2020) matrix resolved Pilmatueia in a group with Suuwassea and Amargatitanis as the sister group to the clade containing Amargasaurus, Brachytrachelopan, and Dicraeosaurus.
Bajadasaurus pronuspinax (Gallina et al., 2019; Garderes et al., 2023), from the Lower Cretaceous Bajada Colorada Formation of Argentina consists of a nearly complete skull, proatlases, atlantal neurapophyses, axis, and a mid-cervical vertebra from a single individual. Gallina et al. (2019) recovered the species as sister taxon to a group containing Pilmatueia and an unresolved grouping of Dicraeosaurus, Amargasaurus, and Brachytrachelopan. Later analyses have recovered Bajadasaurus in various positions basal to the Amargasaurus, Brachytrachelopan, and Dicraeosaurus group (Whitlock and Wilson Mantilla, 2020; Windholz et al., 2022; Bajpai et al., 2023).
Smitanosaurus agilis (Marsh, 1889; Whitlock and Wilson Mantilla, 2020) from the Morrison Formation of Colorado, USA was originally described as a species of ‘Morosaurus’ (Marsh, 1889) and consists of the braincase and parts of the skull roof, proatlases, and cervical vertebrae 1-3. In 2020 the material was redescribed and recovered within Dicraeosauridae as a basal dicraeosaurid (Whitlock and Wilson Mantilla, 2020).
The most recently described dicraeosaurid, Tharosaurus indicus (Bajpai et al., 2023) from the Middle Jurassic Fort Member of the Jaisalmer Formation of India is known from partial mid/posterior cervical vertebrae, a partial dorsal neural arch, dorsal neural spines, dorsal rib, and partial caudal vertebrae. It was recovered as a sister taxon of a clade containing Pilmatueia, Amargatitanis, Brachytrachelopan, Dicraeosaurus, and Amargasaurus (Bajpai et al., 2023). Its inclusion thus far in only a single analysis and the fragmentary nature of the specimen means that more evidence is needed in order to corroborate its position within Dicraeosauridae.
Several taxa have been proposed to represent putative dicraeosaurid sauropods. Kaatedocus siberi (Tschopp and Mateus, 2013) from the Upper Jurassic Morrison Formation of Wyoming, USA has traditionally been recovered within Diplodocinae (Tschopp et al., 2015; Tschopp et al., 2017) until Whitlock and Wilson Mantilla (2020) recovered it as the basal-most dicraeosaurid taxon. A supplementary analysis using a matrix derived from Mannion et al. (2019) corroborated the results of the main analysis (Whitlock and Wilson Mantilla, 2020).
Dyslocosaurus polyonychius (McIntosh et al., 1992) is known for fragmentary appendicular elements and was initially described as a diplodocid (McIntosh et al., 1992). The specimen is of particular interest, as its locality data suggests that Dyslocosaurus may be from the Maastrichtian Lance Formation. However, knowing the current temporal extent of diplodocoid sauropods, it is most likely that this specimen was excavated from nearby outcrops of the Morrison Formation (McIntosh et al., 1992). It was recovered as the most basal dicraeosaurid by Tschopp et al. (2015) and Coria et al. (2019). Mannion et al. (2021) recovered Dyslocosaurus nested within Dicraeosauridae as a polytomous group with Amargasaurus, Brachytrachelopan, and Dicraeosaurus on its inclusion in the matrix of Xu et al. (2018). Other isolated dicraeosaurid remains have been suggested to be present in the Wadi Milk Formation in Sudan (Rauhut, 1999), Kirkwood Formation in South Africa (McPhee et al., 2016), and Podosinki Formation in European Russia (Averianov and Zverkov, 2020).
Apatosaurinae (Table 3)
Apatosaurines were among the first diplodocoids to be described, with Apatosaurus (Marsh, 1877b) named in the same year as the first diplodocoid, the dubious Atlantosaurus (Marsh, 1877a), and with Brontosaurus (Marsh, 1879) following only two years later. The subfamily name Apatosaurinae was first used by Janensch (1929a, p. 31), without comment, alongside Diplodocinae, Dicraeosaurinae, and “Titanosaurinae” as subfamilies within his proposed family Homalosauropodidae in a ranked taxon list. Bakker (1998) used the term as “an informal category for all the massive limbed, wide necked-wide tailed diplodocids” (p. 74). It was not given a phylogenetic definition until that of Taylor and Naish (2005) as the clade of all individuals more closely related to Apatosaurus than to Diplodocus.
For many years, Apatosaurus and Brontosaurus were the only known apatosaurines, and Riggs’s (1903) synonymization of the latter with the former left only a single apatosaurine genus, though one containing several species. Although the name Brontosaurus continued to be used in informal contexts, scientific writing mostly followed Riggs’s scheme until the phylogenetic analysis of Tschopp et al. (2015) argued based on morphological differences for the generic separation of the species Brontosaurus excelsus from Apatosaurus ajax. The separation has generally been followed (e.g., Vidal et al., 2020a; King et al., 2023; Taylor and Wedel, 2023; Wedel and Taylor, 2023).
Peterson and Gilmore (1902) named Elosaurus parvus for a small specimen, CM 566, which they considered to belong to “Morosauridae” (i.e., Camarasauridae). This specimen has been generally considered a juvenile individual of Apatosaurus (=Brontosaurus) excelsus (e.g., McIntosh, 1995), or a separate species Apatosaurus parvus (e.g., Upchurch et al., 2004b). Tschopp et al. (2015) recovered it as more closely related to Brontosaurus excelsus, yielding the new combination Brontosaurus parvus. The immature status of the holotype influences character scoring and makes it impossible to be confident about its low-level taxonomic affinities, but all authors since Peterson and Gilmore seem to have agreed that it is an apatosaurine.
Filla and Redman (1994) described the new species Apatosaurus yahnahpin, which they considered to be a primitive species of Apatosaurus (i.e., a basal apatosaurine, as they were working within Riggs’s schema where all apatosaurines were considered species of Apatosaurus). Bakker (1998) moved this species to its own new genus as Eobrontosaurus yahnahpin, considering it ancestral to Brontosaurus, but giving no specific rationale for generic separation. Tschopp et al. (2015) found the species to be closely related to Brontosaurus excelsus and referred it to the new combination Brontosaurus yahnahpin. As with B. parvus, the exact phylogenetic position of B. yahnahpin is not certain, but it seems to be unambiguously apatosaurine.
Some other taxa were recovered as apatosaurines in the past, too. In Lovelace et al.’s (2007) description of a second specimen of Supersaurus, they found that this taxon, previously considered diplodocine (e.g., Curtice, 1996), was recovered as an apatosaurine in a phylogenetic analysis. However, the result has not been replicated, and Whitlock (2011), Mannion et al. (2012), and Tschopp et al. (2015) found Supersaurus to be diplodocine.
In some trees of Tschopp et al. (2015), including those obtained by equal weighting (figure 115), Amphicoelias altus was also found to be apatosaurine. This is in contrast to earlier analyses such as that of Whitlock (2011a), in which Amphicoelias was found as a very basal diplodocoid, outside Diplodocimorpha. A reassessment of Amphicoelias altus (Mannion et al., 2021) also concluded that this taxon is unlikely to be an apatosaurine.
Diplodocinae (Table 4)
For a long time, the term Diplodocidae (Marsh, 1884) – erected based on the type genus Diplodocus (Marsh, 1878) – was used for the clade that is now called Diplodocoidea (e.g., McIntosh 1990a, 1990b). Given that, the name Diplodocinae first appeared in Janensch (1929a, p. 31), who proposed it as a name for the sister clade to his “Dicraeosaurinae.” In its current use, with Diplodocidae forming the sister clade to Dicraeosauridae, Diplodocinae includes all taxa more closely related to Diplodocus than Apatosaurus (Taylor and Naish, 2005). Until the 1990s, what we now call Diplodocinae (following Taylor and Naish, 2005) mostly included the genera Diplodocus and Barosaurus. However, additional genera have been recognized to belong to this clade in more recent years. At least 10 species and seven genera are currently considered valid by most researchers (Table 4).
The first diplodocine genus to be named was Diplodocus (Marsh, 1878). Its type species, Diplodocus longus (Marsh, 1878) consists of an incomplete tail (YPM VP.001920; McIntosh and Carpenter, 1998; Tschopp and Mateus, 2016; Tschopp et al., 2018), which is often considered undiagnostic, with the result that the species should be treated as a nomen dubium (Tschopp et al., 2015, 2018; Tschopp and Mateus, 2016; Lucas, 2017; Taylor, 2017). Three more species were described later (Marsh, 1884; Hatcher, 1901; Holland, 1924). Of these, Diplodocus lacustris (Marsh, 1884) comprises a single articulated tooth row (YPM VP.001922), which also does not bear any diagnostic features (Tschopp et al., 2015; Tschopp and Mateus, 2016). Diplodocus carnegii (Hatcher, 1901) is known from at least two partial postcranial skeletons that were complete enough to allow Hatcher (1901) to publish the first full-skeleton reconstruction (CM 84, CM 94). Diplodocus hayi (Holland, 1924) consists of a single, nearly complete specimen (HMNS 175), and was later referred to its own diplodocine genus Galeamopus (Tschopp et al., 2015) as the type species. Another species, initially described as Seismosaurus halli (Gillette, 1991) was later referred to Diplodocus with its corrected species epithet as Diplodocus hallorum (Lucas et al., 2006). Several specimens initially referred to Diplodocus longus were later assigned to Diplodocus hallorum (Tschopp et al., 2015), so this species is now known from all parts of the skeleton except for the skull.
Given that the undiagnosability of the type species of Diplodocus may in the future lead to an invalidation of all connected higher-level taxa, Tschopp and Mateus (2016) proposed an ICZN case to replace the type species with the third described species, Diplodocus carnegii (see also Tschopp et al., 2018). That case, however, was declined two years later (ICZN 2018), after having received three comments in support (Lucas, 2017; Taylor, 2017; Woodruff, 2017), and three in opposition (Carpenter, 2017; Demirjian, 2017; Mortimer, 2017). With the status of Diplodocus longus being debated, there are currently two Diplodocus species that are universally considered valid: D. carnegii (Hatcher, 1901) and D. hallorum (Gillette, 1991).
The second described diplodocine genus was Barosaurus. The only currently accepted species of Barosaurus is its type species Barosaurus lentus (Marsh, 1890). It is represented by at least two fairly complete specimens (Lull, 1919; McIntosh, 2005; Tschopp et al., 2015). Two other proposed species, B. affinis (nomen dubium) and B. gracilis (nomen nudum) are no longer considered valid (Tschopp et al., 2015). Specimens that were referred to B. africanus (Janensch, 1914), a diplodocine from the Upper Jurassic Tendaguru Formation of Tanzania, are now placed in Tornieria africana or are indeterminate diplodocines (Remes, 2006, 2009; Tschopp et al., 2015).
Tornieria africana is the only known species of Tornieria and was initially described as Gigantosaurus africanus (Fraas, 1908). The genus Gigantosaurus, however, was preoccupied, so alternatively, Sternfeld (1911) proposed Tornieria as a replacement name. It was the first taxon in this clade from outside North America. Janensch (1922) later referred the species Tornieria africana to Barosaurus. In 2006, Remes demonstrated generic distinction from Barosaurus and reinstated Tornieria africana as a valid taxon. This has since been confirmed by several phylogenetic analyses that recovered Tornieria distinct from Barosaurus (Remes, 2006; Whitlock, 2011a; Mannion et al., 2012; Tschopp et al., 2015). Two specimens can be confidently referred to the species, which preserve cranial and postcranial material (Remes, 2006, 2009; Tschopp et al., 2015).
Diplodocinae also includes the more recently described North American species Supersaurus vivianae (Jensen, 1985). In the first phylogenetic analysis including this species, it was recovered as an apatosaurine (Lovelace et al., 2007), but it has since been consistently recovered as a diplodocine (Whitlock, 2011a; Mannion et al., 2012; Tschopp et al., 2015; Mannion et al., 2021). It is represented by at least two partial skeletons, one of which includes the holotypic dorsal vertebra of Ultrasauros macintoshi (BYU 725-9044; Curtice et al., 1996) and Dystylosaurus edwini (BYU 725-4503; Curtice and Stadtman, 2001). More recently, Tschopp et al. (2015) suggested that the only named European diplodocine, Dinheirosaurus lourinhanensis (Bonaparte and Mateus, 1999), is alternatively a second species of Supersaurus. If corroborated, Supersaurus would be the only currently known diplodocine genus present on two continents.
An important taxon belonging to Diplodocinae is Leinkupal laticauda (Gallina et al., 2014) from the Lower Cretaceous Bajada Colorado Formation of Argentina. It is so far the only named diplodocid from South America and from the Cretaceous. A single caudal vertebra from the Cretaceous of South Africa has also been referred to Diplodocinae, but the authors refrained from erecting a new species (McPhee et al., 2016). The description of Leinkupal contradicted the general understanding at the time that diplodocids went extinct at the Jurassic-Cretaceous boundary (Gallina et al., 2014). The taxon is represented by a proportionally small anterior caudal vertebra and possibly other cranial and postcranial material from the same locality. However, the disarticulated nature of the material and the fact that multiple taxa were recovered from this locality disallows unambiguous attribution of all diplodocid bones to a single species (Gallina et al., 2014, 2022; Garderes et al., 2022).
The new genus Galeamopus was proposed by Tschopp et al. (2015) for the species “Diplodocus” hayi, for which they confirmed earlier proposals of generic distinction based on their phylogenetic analysis (Holland, 1924). A second species, Galeamopus pabsti (Tschopp and Mateus, 2017) was erected shortly thereafter, based on a specimen with a nearly complete skull and a fairly complete postcranial skeleton (NMZ 1000011 – formerly SMA 0011; lacking its tail). Several other individual skeletons have since been tentatively referred to that species, including both cranial and postcranial elements (Tschopp et al., 2019).
The most recent addition to Diplodocinae is Ardetosaurus viator (van der Linden et al., 2024). Consisting of cervical, dorsal, sacral, and caudal vertebrae, as well as appendicular elements mainly from the hindlimbs, this taxon likely represents a more basal member of diplodocine sauropod, similar to Galeamopus (Tschopp and Mateus, 2017) and possibly Kaatedocus (Tschopp and Mateus, 2013).
Other Diplodocoids (Table 5)
Several sauropod species have been, or are currently, included in Diplodocoidea, which have fluctuated in and outside the clade. We focus here on taxa that remain taxonomically valid. Former diplodocoid taxa that are no longer considered to be taxonomically valid (e.g., Diplodocus lacustris or Apatosaurus laticollis) and their current phylogenetic positions can be found in Tschopp et al. (2015) and references therein.
In 1877 and 1878, several diplodocoid taxa were erected. Two species of Atlantosaurus were named: Atlantosaurus montanus (Marsh, 1877b) and Atlantosaurus immanis (Marsh, 1878). Atlantosaurus montanus, from Lakes Quarry 1 in the Morrison Formation, consists solely of an incomplete sacrum (Marsh, 1877b; Ostrom and McIntosh, 1966). This dubious taxon has never been included in any phylogenetic analysis but likely represents an apatosaurine diplodocoid (Foster, 2020). Atlantosaurus immanis was included in Tschopp et al. (2015) and possibly constitutes a new genus and species within Apatosaurinae. Dystrophaeus viaemalae (Cope, 1877a) has been regarded as a diplodocoid (McIntosh, 1990b), but is most likely a non-neosauropod eusauropod, though its affinities remain dubious due to its fragmentary nature (Tschopp et al., 2015; Foster et al., 2016). The affinities of Amphicoelias altus (Cope, 1877b), although generally found inside Diplodocoidea, also remain uncertain, with different analyses grouping this taxon in different clades within Diplodocoidea (e.g., Whitlock, 2011a; Tschopp et al., 2015; Mannion et al., 2021).
One of the more complete non-diplodocimorph diplodocoid taxa is Haplocanthosaurus. Haplocanthosaurus currently comprises two species, Haplocanthosaurus priscus (Hatcher, 1903) and Haplocanthosaurus delfsi (McIntosh and Williams, 1981) and has proven to be a problematic taxon throughout most sauropod phylogenetic analyses. Various analyses have recovered Haplocanthosaurus as a macronarian (Upchurch, 1995; Wilson and Sereno, 1998; Upchurch et al., 2004a), a non-neosauropod eusauropod (Upchurch, 1998, 1999; Rauhut et al., 2005; Harris, 2006c), and a basal neosauropod (Harris and Dodson, 2004), although most analyses favor a non-diplodocimorph diplodocoid placement (Wilson, 2002; Salgado et al., 2004; Barco et al., 2005, 2006; Salgado et al., 2006; Lovelace et al., 2007; Whitlock, 2011a; Tschopp and Mateus, 2013, 2017; Tschopp et al., 2015; Whitlock and Wilson Mantilla, 2020; Mannion et al., 2021). However, recently, some analyses have again favored non-diplodocoid positions for Haplocanthosaurus (Bajpai et al., 2023; Gomez et al., 2024b).
Several other diplodocoid taxa were erected during the 1900s. Formerly considered to be an apatosaurine, “Apatosaurus” minimus (Mook, 1917) consists solely of a sacrum and a pelvic girdle. Currently, this species is thought to represent some form of macronarian sauropod (Upchurch et al., 2004a; Tschopp et al., 2015), though its affinities remain uncertain (Taylor and Wedel, 2012; Mannion et al., 2021). Although diplodocoid affinities have been suggested for the European taxon Cetiosauriscus stewarti (e.g., Upchurch et al., 2004a), more recent analyses place it as a non-neosauropod eusauropod (e.g., Tschopp et al., 2015; Schwarz et al., 2020). In earlier analyses investigating (diplodocoid) sauropod interrelationships, the Mongolian taxa Nemegtosaurus and Quaesitosaurus were placed in Diplodocoidea (Upchurch, 1995, 1998, 1999; Upchurch et al., 2004a); these are currently considered to be lithostrotian titanosaurs (e.g., Wilson, 2002, 2005; Filippi et al., 2024). The Portuguese sauropod Apatosaurus alenquerensis was erected by Lapparent and Zbyszewski (1957) and later referred to Camarasaurus by McIntosh (1990b). Dantas et al. (1998) erected a new genus for the species, Lourinhasaurus, and subsequent analyses have shown basal macronarian affinities for Lourinhasaurus alenquerensis (e.g., Mocho et al., 2014; Tschopp et al., 2015). Dyslocosaurus polyonychius was originally described as a diplodocid diplodocoid (McIntosh et al., 1992), but reanalysis has shown that it is more likely to be a dicraeosaurid diplodocoid (Tschopp et al., 2015).
In the last 25 years, interrelationships of diplodocoid sauropods have been more thoroughly studied, such that few new taxa have been erected with dubious affinities to the clade. Losillasaurus giganteus was a basal diplodocoid (Casanovas et al., 2001), but is currently placed in Turiasauria (e.g., Royo-Torres et al., 2021). Galvesaurus herreroi from Spain was placed in Diplodocoidea in its initial description (Barco et al., 2005) but is currently thought to be a brachiosaurid macronarian (Barco et al., 2006; Pérez-Pueyo et al., 2019). Most recently, the Tendaguru sauropod Australodocus bohetii (Remes, 2007) was described as a diplodocine diplodocoid but is currently considered to represent a titanosauriform sauropod (Whitlock, 2011a, 2011c; Mannion et al., 2013; Mannion et al., 2019).
OVERVIEW OF DIPLODOCOID MORPHOLOGY
Diplodocoid sauropods can be distinguished from other sauropods based on a series of postcranial features, as well as potentially some cranial traits. The latter, however, hinges on the unknown cranial morphology and still debated phylogenetic position of Haplocanthosaurus. Consequently, the following cranial synapomorphies could be either valid for Diplodocoidea or Diplodocimorpha.
The premaxilla of diplodocoid (or diplodocimorph) sauropods is an elongate unit with a straight ascending process in lateral view, which is not separated from the tooth-bearing portion by a step. This ascending process of the premaxilla connects to the dorsal process of the maxilla, which reaches more posteriorly than the posterior process of the same bone. The nasal opening is retracted dorsally, and relatively small, whereas the antorbital fenestra is large relative to the orbit. The entire rear part of the skull is tilted and the snout elongated and rather squared. This results from an oblique orientation of the quadrate and the basipterygoid processes on the parabasisphenoid in relation to the skull roof, as well as an elongation of the basipterygoid processes, the ectopterygoid flanges on the pterygoid, and anterior rami of the quadratojugals. Moreover, the jugal contributes to the antorbital fenestra, and there are multiple generations of pencil-shaped replacement teeth present in the jaws. The teeth do not occlude (possible exception of Nigersaurus, see Sereno and Wilson, 2005; Canudo et al., 2018), and their wear produced one or two planar facets on the tooth apex (Berman and McIntosh, 1978; McIntosh, 1990b; Calvo and Salgado, 1995; Upchurch, 1995; Wilson, 2002; Upchurch et al., 2004a; Rauhut et al., 2005; Tschopp et al., 2015). Postcranial synapomorphies include short cervical ribs and an obliquely oriented fibular facet on the astragalus (Berman and McIntosh, 1978; Tschopp et al., 2015).
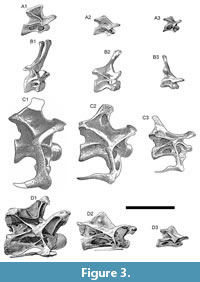 Gross morphology of cervical vertebrae is among the most distinctive difference among diplodocoids (Figure 3). More basal forms, such as Haplocanthosaurus (Figure 3A) and rebbachisaurids show an unsplit neural spine. In Dicraeosauridae, Apatosaurinae, and Diplodocinae, the neural spines are split into two metapophyses. Dicraeosaurid cervical vertebrae are smaller in absolute terms, lack deep invasive pneumatic structures, and show dorsoventral elongation of the hemispinous processes (Figure 3B), with extremes seen in Amargasaurus and Bajadasaurus. The cervical vertebrae of apatosaurines (Figure 3C) are far more robustly constructed than those of diplodocines and are notable especially for their tall neural arches and very deep and robust cervical ribs. In diplodocines (Figure 3D), the cervical vertebrae are more slender and anteroposteriorly elongated, showing extreme lengths in Barosaurus and Supersaurus. Both rebbachisaurids, apatosaurines and diplodocines independently evolved more complex laminar structures, whereas the ‘simpler’ morphology was retained in Haplocanthosaurus and the dicraeosaurids.
Gross morphology of cervical vertebrae is among the most distinctive difference among diplodocoids (Figure 3). More basal forms, such as Haplocanthosaurus (Figure 3A) and rebbachisaurids show an unsplit neural spine. In Dicraeosauridae, Apatosaurinae, and Diplodocinae, the neural spines are split into two metapophyses. Dicraeosaurid cervical vertebrae are smaller in absolute terms, lack deep invasive pneumatic structures, and show dorsoventral elongation of the hemispinous processes (Figure 3B), with extremes seen in Amargasaurus and Bajadasaurus. The cervical vertebrae of apatosaurines (Figure 3C) are far more robustly constructed than those of diplodocines and are notable especially for their tall neural arches and very deep and robust cervical ribs. In diplodocines (Figure 3D), the cervical vertebrae are more slender and anteroposteriorly elongated, showing extreme lengths in Barosaurus and Supersaurus. Both rebbachisaurids, apatosaurines and diplodocines independently evolved more complex laminar structures, whereas the ‘simpler’ morphology was retained in Haplocanthosaurus and the dicraeosaurids.
Rebbachisaurid skulls (mostly known from Limaysaurus, Nigersaurus, and Lavocatisaurus; Calvo and Salgado, 1995; Sereno et al., 2007; Canudo et al., 2018) are characterized by an elongate jugal, which articulates with the squamosal, which is ventrally expanded (Canudo et al., 2018; Salgado et al., 2022). In this way, the postorbital is not delimiting the infratemporal fenestra as it does in Flagellicaudata (Tschopp and Mateus, 2013, 2017; Canudo et al., 2018; Garderes et al., 2023). The skull roof is closed – lacking a frontoparietal foramen – except for Sidersaura (Lerzo et al., 2024a). The postcranial skeleton is characterized by racquet-shaped scapular blades and a hook-like acromion process, and tetralaminated and petal-shaped middle to posterior dorsal neural spines (Haluza et al., 2012; Salgado et al., 2022; Lerzo et al., 2024a). Rebbachisaurids present extreme postcranial pneumatization. Within Diplodocoidea, Rebbachisauridae is the only clade that presents a laterodiapophyseal fossa/fenestra and an intradiapophyseal chamber in the dorsal vertebrae (Ibiricu et al., 2013, 2015, 2017; Lerzo et al., 2024b). The pneumatization is extended to anterior to middle caudal vertebrae and invades the pelvic girdle (Fanti et al., 2015; Ibiricu et al., 2017). Indeed, the most recent phylogenetic analyses of the group recovered the camerate pneumatization of the ilium as one of the synapomorphies of Rebbachisauridae (Lerzo et al., 2024a, 2024b).
Skull features that unite all dicraeosaurids as recovered by the analysis of Whitlock and Wilson Mantilla (2020) include the presence of a large foramen posterior to the anterior maxillary foramen, dorsal to preantorbital fossa on the maxilla; a reduced or absent preantorbital fenestra; a prominent, ventrally directed ‘prong’ on the posteroventral margin of the squamosal; distance of the supratemporal fenestrae of the parietal are greater than 1.5 the length of the supratemporal fenestrae; the presence of a postparietal foramen; a nearly flat distal margin of the paroccipital processes; maximum diameter of the supratemporal fenestra is equal to the greatest length of the foramen magnum; the sagittal nuchal crest of the supraoccipital is narrow and distinct; the crista prootica is expanded laterally into a distinct sheet-like process; the basipterygoid processes diverge narrowly; and the basioccipital depression between the foramen magnum and basal tubera is absent. Postcranial characteristics recovered by the same analysis include the presence of an epipophyseal-prezygapophyseal lamina in the anterior cervical vertebrae; a dorsally divided centroprezygapophyseal lamina in the middle and posterior cervical vertebrae, whereby the medial branch connects to the intraprezygapophyseal lamina, and not to the prezygapophysis; and the bifid neural spines in the posterior cervical and anterior dorsal vertebrae are narrow, parallel to converging.
Apatosaurines are immediately distinguishable from other diplodocoids because of their much heavier build (Wilhite, 2005). Their humeri, for example, are far more robust than those of most diplodocines (although Galeamopus pabsti is an exception: see Tschopp and Mateus, 2017, figures 60-61). The most distinctive apatosaurine feature is the neck, the vertebrae of which are topologically similar to those of diplodocines – bifid neural spines after the first few cervicals, similar lamination – but constructed more robustly and are far taller dorsoventrally, partly because the cervical ribs are suspended so far below the centra. For example, the cervical vertebrae of Apatosaurus louisae CM 3018 are about 85% as long as those of Diplodocus carnegii CM 84, but nearly twice as tall from the tip of the high neural spine to the lowest point of the attachment of the cervical ribs to the capitulum (Figure 3C, D). Similarly, the cervical vertebrae of apatosaurines are much wider than in diplodocines: compare Gilmore (1936, plate XXIV) with Hatcher (1901, plate V).
In the analysis of Tschopp et al. (2015), the clade Apatosaurinae was supported by six synapomorphies, four of them in cervical vertebrae: paired pneumatic fossae absent from ventral surface of anterior cervical vertebrae; postzygapophyseal centrodiapophyseal fossa extends onto posterior face of transverse process in cervical vertebrae; cervical ribs projecting well beneath centrum; anterior process of posterior cervical ribs reduced to a short bump-like process or absent; postspinal lamina or rugosity terminates at or beneath dorsal margin of neural spine in anterior caudals; rectangular anteroventral margin of coracoid. More derived apatosaurines were separated from the most basal, “Atlantosaurus” immanis, by 14 additional synapomorphies, so that the group of well-recognized apatosaurs is separated from the sister group Diplodocinae by 20 characters.
Diplodocines can be morphologically distinguished from most other flagellicaudatans by a generally more strongly developed postcranial pneumatization of the vertebral column and often elongate mid-cervical and mid-caudal vertebral centra. Additionally, most diplodocines have rather gracile limbs, except for Galeamopus (McIntosh, 1990a, 1990b, 2005; Tschopp et al., 2015, 2019; Tschopp and Mateus, 2017). Synapomorphic features found to represent Diplodocinae by Tschopp et al. (2015) include one cranial and several postcranial traits. The only cranial synapomorphy found by Tschopp et al. (2015) were box-like basal tubera. The proposed postcranial synapomorphies were dorsally elongate coels on posterior cervical neural spines; convex prezygapophyseal facets in mid- and posterior cervical vertebrae (not flat, as erroneously stated in Tschopp et al., 2015, p. 249); relatively weakly developed triangular aliform processes on mid- and posterior dorsal neural spines, which do not project as far laterally as their respective postzygapophyses; parapophyseal centrodiapophyseal fossae that are deeply excavated and triangular in posterior dorsal neural arches; ‘fan’-shaped caudal ribs that transition to ‘normal’ caudal ribs between caudal vertebrae 6 and 7, or more posteriorly; triangular lateral processes on caudal neural spines; a posteriorly displaced scapular acromial process, lying nearly at midpoint of the scapular body; a pubis that contributes equally or more to the acetabular opening compared to the ischium; an elongate muscle scar on the ischial shaft; a subtriangular proximal articular surface of the tibia; and several foramina marking the dorsal/anterior surface of the metatarsal I. The validity of some of these synapomorphies was questioned by Whitlock and Wilson Mantilla (2020), however, in part because their analysis recovered Kaatedocus siberi as a dicraeosaurid, whereas Tschopp et al. (2015) recovered this species as a diplodocine.
ECOLOGY AND ONTOGENY OF DIPLODOCOIDS
Ecology
Diplodocoids are generally characterized by an anteroposteriorly elongated skull and teeth with peg-shaped crowns (Upchurch and Barrett, 2000), all of which differ from the non-titanosaurian macronarian cranial conditions (e.g., Wilson and Sereno, 1998; D’Emic et al., 2013; Button et al., 2014; Peterson et al., 2022). The necks of diplodocoids, as in other sauropods, supported energy-efficient feeding as the animal would need to move less to gather food (Stevens and Parrish, 1999; Sander, 2013; Woodruff, 2016).
Diplodocoidea, as herbivorous dinosaurs, co-occurred with other herbivores like macronarian sauropods and ornithischian dinosaurs (Paulina Carabajal et al., 2014; Foster, 2020; Melstrom et al., 2021). Contrary to the general belief that all herbivores found in e.g., the Morrison Formation and the Tendaguru beds are coeval or even coexisting, only a fraction of the found taxa would share the same environment, due to the extensive temporal and spatial distribution of these fossils within these formations (Aberhan et al., 2002; Maidment, 2024). For example, Aberhan et al. (2002) list Dicraeosaurus hansemanni, the macronarian Giraffatitan, and the stegosaur Kentrosaurus as co-occurring in the Middle Saurian bed, whereas Dicraeosaurus sattleri and Tornieria (referred as Barosaurus africanus, prior to the revision of Remes, 2006) appear only in the Upper Saurian Bed, suggesting two ecosystems of different ages and faunal compositions. More prominent, the faunal compositions in the Morrison Formation are not only separated by time but also by different types of segregation (Maidment, 2024). Whereas Apatosaurus, Diplodocus, and Camarasaurus are abundant and found in several different localities (Foster, 2020), examples like Kaatedocus, Suuwassea, and the stegosaur Hesperosaurus seem to be restricted to the northern regions and systems tracts of different age (Maidment, 2024). Regardless, the occurrence of several large herbivores might result in competition for food sources. Niche partitioning, which has been proposed several times for these ecosystems (e.g., Fiorillo, 1998; Upchurch and Barrett, 2000; Hummel and Claus, 2011; Whitlock, 2011b; McHugh, 2018) could explain the sustainability of an ecosystem with several large herbivores of different clades.
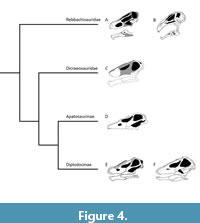 Although diplodocoids are generally considered to prefer low- to medium-level browsing (Stevens and Parrish, 1999; Whitlock, 2011b), there are noteworthy specializations on certain feeding strategies in the large groups of Diplodocimorpha. Examples of such adaptations can be seen in the diversity of skull morphologies (Figure 4), the microwear of teeth combined with a rapid tooth replacement rate (D´Emic et al., 2013; Hummel and Claus, 2011; Whitlock, 2011b; Melstrom et al., 2021), and the anatomy of the cervical region, which is connected to the osteological neutral position and the range of motion of the cervical series (Stevens and Parrish, 1999; Christian and Dzemnski, 2011; Taylor, 2014; Peterson et al., 2022).
Although diplodocoids are generally considered to prefer low- to medium-level browsing (Stevens and Parrish, 1999; Whitlock, 2011b), there are noteworthy specializations on certain feeding strategies in the large groups of Diplodocimorpha. Examples of such adaptations can be seen in the diversity of skull morphologies (Figure 4), the microwear of teeth combined with a rapid tooth replacement rate (D´Emic et al., 2013; Hummel and Claus, 2011; Whitlock, 2011b; Melstrom et al., 2021), and the anatomy of the cervical region, which is connected to the osteological neutral position and the range of motion of the cervical series (Stevens and Parrish, 1999; Christian and Dzemnski, 2011; Taylor, 2014; Peterson et al., 2022).
Rebbachisauridae
Rebbachisaurids are generally considered to be small- to medium-sized (Salgado et al., 2022) with total body length estimates for Nigersaurus between 9 m (Sereno et al., 2007) and possibly 15 m (Sereno et al., 1999; Mannion, 2009) and Rebbachisaurus similar in size to some dicraeosaurids (Wilson and Allain, 2015). Rebbachisaurid cranial remains are sparse, even compared to the other families in Diplodocoidea. However, based on the known skulls and their size, their diet is more likely connected to low-level browsing. The potentially most drastic adaptation for low-level browsing in any diplodocoid skull is found in Nigersaurus taqueti from the Lower Cretaceous Elrhaz Formation of Niger (Sereno et al., 1999). This species had tooth batteries housed in the anterior-most section of the snout. Although often described as a “dental battery” similar to those in ornithischian dinosaurs (Sereno and Wilson, 2005), the ornithischian dental battery is composed of extensive, overlapping tooth rows that work in unison to create a large slicing and grinding surface (Erickson et al., 2012). Conversely, the elongated single upper and lower tooth rows of N. taqueti are sensu stricto not a “battery.” The reconstructed neutral cranial posture (and subsequent cervical posture), derived from the orientation of the semicircular canals observed in the endocast of the holotype, results in a downward orientation of the muzzle (Sereno et al., 2007). Whitlock (2011b) supports the finding of N. taqueti as a low-level browsing animal, which could be compared today with grazing mammals (Sereno et al., 2007).
Another important taxon for inferring feeding strategies within Rebbachisauridae is Lavocatisaurus agrioensis (Canudo et al., 2018) from the Lower Cretaceous Rayoso Formation of Argentina. Canudo et al. (2018) recovered L. agrioensis in a more stemward phylogenetic position than N. taqueti. L. agrioensis has a different morphology of the snout and positioning of the teeth compared to N. taqueti. The microwear of the teeth differs in L. agrioensis from N. taqueti resulting in a different method of biting (Canudo et al., 2018), implying a different preferred level of browsing, which is less specialized than more derived rebbachisaurids.
Dicraeosauridae
Several dicraeosaurid species are known from both cranial and postcranial remains. The cranial remains of Dicraeosaurus hansemanni (Janensch, 1914), from the Tendaguru beds (Upper Jurassic) of Tanzania and those of Amargasaurus cazaui (Salgado and Bonaparte, 1991), from the Lower Cretaceous La Amarga Formation of Argentina, suggest that dicraeosaurids were adapted for low to mid-level browsing (Whitlock 2011b; Paulina Carabajal et al., 2014; Schwarz et al., 2015). This corresponds with their general small to medium body size, as exemplary body size estimates reach between 9 m in Amargasaurus (Mazzetta et al., 2004) and 12 m in Dicraeosaurus (Schwarz et al., 2015), as well as the comparatively small necks and more robust morphology in contrast to diplodocids (Gallina, 2022). Whitlock (2011b) proposed that dicraeosaurids favored more forested ecosystems, rather than savannah-type ecosystems. A thoroughly studied dicraeosaurid regarding feeding strategies is D. hansemanni (Whitlock, 2011b; Schwarz et al., 2015). D. hansemanni is considered to be a mid-level browser, which is supported by the results of carbon isotope analysis by Tütken (2011). Schwarz et al. (2015) suggested that D. hansemanni fed specifically on lower mid-level foliage and speculated on the possible use of the tongue for feeding.
A bizarre example of the diversity of ecological niches in dicraeosaurids is Brachytrachelopan mesai (Rauhut et al., 2005) from the Upper Jurassic Cañadón Calcáreo Formation of Argentina. B. mesai is estimated to be less than 10 m long and has, compared to other diplodocoids, extremely anteroposteriorly shortened cervical vertebrae (Rauhut et al., 2005). Rauhut et al. (2005) suggested that this morphology paired with a restricted dorsiflexion of the cervical series might be an adaptation to the lowermost level of browsing (Rauhut et al., 2005). Based on the phylogenetic position of B. mesai and other dicraeosaurids (such as Lingwulong shenqi, Xu et al., 2018), this clade in general evolved shortened cervical series.
Diplodocidae
Diplodocids show a large range of total body lengths. Smaller taxa like Kaatedocus (assuming diplodocid affinities) are estimated at 14 m in length (Tschopp and Mateus, 2013) whereas larger taxa like Diplodocus and Supersaurus are found to reach body lengths beyond 25 m (Woodruff et al., 2024). Several diplodocid species are represented by well-preserved cranial and axial remains (see Tschopp et al., 2015), which can be studied to reconstruct their feeding strategies. Diplodocus (Marsh, 1878), was studied extensively regarding its ecology and diet (e.g., Fiorillo, 1998; Tütken, 2011; Whitlock 2011b, 2017; Young et al., 2012; Price and Whitlock, 2022) which can be generally summarized as follows: 1) Diplodocus seems to have preferred a diet of low-level vegetation based on analysis of δ13C isotopes (Tütken, 2011), further supported by neck posture and dental microwear (Stevens and Parrish, 1999; Tütken, 2011; Whitlock, 2011b); 2) recent analyses suggest that the bite was orthal and used for cutting/cropping, rather than the historical portrayal of stripping leafy foliage (Price and Whitlock, 2022); and 3) additionally, the elongation of the skull is connected to accumulating more food rather than reducing stress while feeding (Young et al., 2012).
Conversely, Apatosaurus appears to differ from Diplodocus, which might support the niche partitioning within diplodocids (Peterson et al., 2022). A recently studied Apatosaurus skull (TATE-099) by Peterson et al. (2022) underlined that apatosaurines had a larger capacity of replacement teeth than Diplodocus. That capacity, noted especially for the premaxillae, is only eclipsed by Nigersaurus (Peterson et al., 2022). This variation in replacement teeth was suggested by McHugh (2018) and Peterson et al. (2022) to indicate that Apatosaurus fed on tougher vegetation than Diplodocus. Tütken (2011) noted a higher value of δ13C isotopes in Apatosaurus than in Diplodocus teeth, further supporting different dietary preferences. Tütken (2011) also noted that seasonal and ontogenetic shifts in the diet of sauropods need to be considered when analyzing the diet of the tested taxa. Such shifts seemed to be a crucial factor in the life cycle of diplodocoids (Whitlock et al., 2010; Woodruff et al., 2018).
Ontogeny
Griffin et al. (2021) suggested that when using the concept of ontogenetic stages, a combination of independent proxies is more beneficial in delimiting maturational ranges. At first glance, the seemingly low amount of fossil remains of immature diplodocoids contradicts estimates of their percentage in the total population of their ecosystem (Farlow et al., 2022). However, as exemplified by the ceratopsian Triceratops, specimens that were historically thought to represent the ‘adult morphological condition’ have since been osteohistologically demonstrated to have been ‘subadults’ (Scannella and Horner, 2010). Likely the same holds true for most iconic, museum-mounted sauropod taxa (although this needs osteohistological verification), but former maturational assumptions, a rapid growth rate during early ontogeny, and potentially a high predator-driven mortality rate during early ontogeny, are all factors that contribute to our paucity of exceptionally small-statured (i.e., ‘baby’) diplodocoids. Nevertheless, some studies have been able to trace important ontogenetic patterns and developmental pathways in Diplodocoidea, especially diplodocids (e.g., Curry, 1999; Klein and Sander, 2008; Whitlock et al., 2010; Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Woodruff et al., 2017, 2018, Waskow, 2019; Wiersma-Weyand et al., 2021).
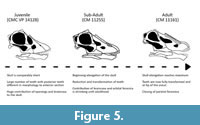 Although less common, smaller-statured, immature diplodocoids are known from the Morrison Formation and assigned to flagellicaudatans like Apatosaurus, Barosaurus, Diplodocus, Kaatedocus, and Suuwassea (e.g., Curtice and Wilhite, 1996; Harris and Dodson, 2004; Foster, 2005; Whitlock et al., 2010; Tschopp and Mateus, 2013; Melstrom et al., 2016; Hanik et al., 2017); although relative body size has primarily served as the means for generalizing a specimen’s maturation. Though osteohistological analyses have not yet been conducted, several diplodocoid holotypes (e.g., the apatosaurine “Elosaurus” parvus [Peterson and Gilmore, 1902], the diplodocine Kaatedocus siberi, and Smitanosaurus agilis [Whitlock and Wilson-Mantilla, 2020]) are likely not skeletally mature. However, taxonomic referral of immature specimens is challenging (Woodruff, 2019) and some studies questioned the validity of certain taxa (see Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Tschopp et al., 2015). The lack of skeletal maturity and full osteological development may lead to the potential absence of apomorphies which are needed for a clear taxonomic assignment, leading to a different phylogenetic position categorically (Tschopp et al., 2015). Similar to the increasing skeletal pneumaticity and complexity of vertebrae through ontogenetic stages (Wedel, 2007; Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Tschopp and Mateus, 2017; Woodruff et al., 2017), heterochrony seems to be a large factor in the growth of sauropods. For instance, the skull changes in variable dimensions throughout growth (Figure 5); a prime example of allometric, not isometric, development in sauropods (Salgado, 1999; Whitlock et al., 2010; Tschopp and Mateus, 2017; Woodruff et al., 2018; Fabbri et al., 2021). These changes might also be linked to shifts in the ecological niche as their size restricts the potential food sources (Woodruff et al., 2018). But as exemplified by the allometry of the skull, an organism that hatched out of an approximately cantaloupe-sized egg (13-15 cm in diameter; Chiappe et al., 1998) and grows to more than 25 m in body length in some cases (Woodruff et al., 2024), is expected to show numerous, variable degrees of ontogenetic changes throughout the skeleton towards adulthood.
Although less common, smaller-statured, immature diplodocoids are known from the Morrison Formation and assigned to flagellicaudatans like Apatosaurus, Barosaurus, Diplodocus, Kaatedocus, and Suuwassea (e.g., Curtice and Wilhite, 1996; Harris and Dodson, 2004; Foster, 2005; Whitlock et al., 2010; Tschopp and Mateus, 2013; Melstrom et al., 2016; Hanik et al., 2017); although relative body size has primarily served as the means for generalizing a specimen’s maturation. Though osteohistological analyses have not yet been conducted, several diplodocoid holotypes (e.g., the apatosaurine “Elosaurus” parvus [Peterson and Gilmore, 1902], the diplodocine Kaatedocus siberi, and Smitanosaurus agilis [Whitlock and Wilson-Mantilla, 2020]) are likely not skeletally mature. However, taxonomic referral of immature specimens is challenging (Woodruff, 2019) and some studies questioned the validity of certain taxa (see Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Tschopp et al., 2015). The lack of skeletal maturity and full osteological development may lead to the potential absence of apomorphies which are needed for a clear taxonomic assignment, leading to a different phylogenetic position categorically (Tschopp et al., 2015). Similar to the increasing skeletal pneumaticity and complexity of vertebrae through ontogenetic stages (Wedel, 2007; Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Tschopp and Mateus, 2017; Woodruff et al., 2017), heterochrony seems to be a large factor in the growth of sauropods. For instance, the skull changes in variable dimensions throughout growth (Figure 5); a prime example of allometric, not isometric, development in sauropods (Salgado, 1999; Whitlock et al., 2010; Tschopp and Mateus, 2017; Woodruff et al., 2018; Fabbri et al., 2021). These changes might also be linked to shifts in the ecological niche as their size restricts the potential food sources (Woodruff et al., 2018). But as exemplified by the allometry of the skull, an organism that hatched out of an approximately cantaloupe-sized egg (13-15 cm in diameter; Chiappe et al., 1998) and grows to more than 25 m in body length in some cases (Woodruff et al., 2024), is expected to show numerous, variable degrees of ontogenetic changes throughout the skeleton towards adulthood.
Taxonomic assignments are difficult when dealing with unique immature diplodocids that have no readily evident adult counterpart. In several documented cases, cranial remains in particular have been challenging to taxonomically identify. CM 11255, which consists of an isolated skull (Holland, 1924; Whitlock et al., 2010), is considered to be either a specimen of Diplodocus or Barosaurus (Whitlock et al., 2010; Woodruff et al., 2018). Another skull, CMC VP 14128 was found together with some cervical material and is referred to cf. Diplodocus (Woodruff et al., 2018). However, several diplodocine taxa are not yet known by cranial remains. For example, there is no unambiguous specimen of Diplodocus with a clear association of a skull and a postcranial skeleton (Tschopp et al., 2015; Woodruff et al., 2018); therefore, an unquestionable assignment to Diplodocus or Barosaurus based strictly on cranial material is not yet possible. However, other juvenile diplodocids, like CM 79038 and DINO 2921, are known from vertebrae and appendicular remains which could be compared and assigned to Barosaurus (Melstrom et al., 2016; Hanik et al., 2017).
Subadult diplodocoid specimens, as evident by morphological and osteohistological examinations, are known in Kaatedocus siberi (morphological), Nigersaurus taqueti, and Suuwassea emilieae (osteohistological) which have nevertheless developed enough morphologies to be differentiated from known taxa (Hedrick et al., 2014; Tschopp and Mateus, 2013; Wedel and Taylor, 2013; Tschopp et al., 2015). However, as the holotypes of Kaatedocus and Suuwassea are incomplete and show features known from both immature diplodocoids and dicraeosaurids, their phylogenetic position remains debated (Harris, 2006c; Whitlock, 2011a; Woodruff and Fowler, 2012; Wedel and Taylor, 2013; Tschopp et al., 2015; Whitlock and Wilson-Mantilla, 2020). Until a series of ontogenetic stages of a taxon is known, repositionings of non-adult specimens must be expected, unless ontogenetic morphological characters are outweighed by characters with a connection to diagnostic features and no connection to ontogeny (Tschopp et al., 2015).
BIOGEOGRAPHY OF DIPLODOCOIDS
The earliest unambiguous diplodocoid sauropod remains represent the dicraeosaurid Lingwulong, from the Middle Jurassic of China (Xu et al., 2018). Tharosaurus indicus from the Middle Jurassic of India (Bajpai et al., 2023) may represent an even earlier diplodocoid, but its fragmentary nature sheds doubt on its dicraeosaurid affinities and thus the origins of Diplodocoidea. Other remains from the Callovian (latest Middle Jurassic) of western Eurasia may also represent indeterminate diplodocoids (Holwerda et al., 2019; Averianov and Zverkov, 2020). Additionally, fragmentary pedal elements from the Bathonian-Callovian of Mexico were assigned to Flagellicaudata (Rivera-Sylva and Espinosa-Arrubarrena, 2020), although the assignation of that material to the group is based on characters that may have a wider distribution and are likely not diagnostic at this level (Mannion and Whitlock, in press). An isolated tooth from the Bathonian of Madagascar has also been suggested as a potential diplodocoid (Bindellini and Dal Sasso, 2021), but identification of isolated teeth can be troublesome.
Late Jurassic sediments, by contrast, are relatively rich with diplodocoids and, in particular, flagellicaudatans. The Upper Jurassic Morrison Formation (Kimmeridgian-Tithonian) of North America is especially dense taxonomically, including the diplodocids Amphicoelias (Cope, 1877b), Apatosaurus (Marsh, 1877), Barosaurus (Marsh, 1890), Brontosaurus (Marsh, 1879; Tschopp et al., 2015), Diplodocus (Marsh, 1878), Galeamopus (Tschopp et al., 2015; Tschopp and Mateus, 2017), and Supersaurus (Jensen, 1985), the dicraeosaurids Dyslocosaurus (McIntosh et al., 1992; Tschopp et al., 2015), Smitanosaurus (Marsh, 1889; Whitlock and Wilson Mantilla, 2020), and Suuwassea (Harris and Dodson, 2004; Whitlock, 2011a), the putatively basal diplodocoid Haplocanthosaurus (Hatcher, 1903; Wilson, 2002; Whitlock, 2011a), the putative rebbachisaurid Maraapunisaurus (Carpenter, 2018, but see Carpenter, 2006 and Woodruff and Foster, 2014), and the flagellicaudatan Kaatedocus (Tschopp and Mateus, 2013, 2017; Tschopp et al., 2015; Whitlock and Wilson Mantilla, 2020). The Late Jurassic record of Portugal includes the diplodocid Dinheirosaurus (Bonaparte and Mateus, 1999; Mannion et al., 2012, but see Tschopp et al., 2015, Mannion et al., 2019, and Whitlock and Wilson Mantilla, 2020). Additional Late Jurassic diplodocoid material is known from Georgia and Spain (see Mannion et al., 2012 and references therein).
The Gondwanan Late Jurassic is less diverse, probably owing to a less extensive rock record. The Tendaguru Formation of Tanzania includes both the diplodocid Tornieria (Janensch, 1922) and the dicraeosaurid Dicraeosaurus (Janensch, 1914). The Upper Jurassic outcrops of Argentina have thus far only yielded the dicraeosaurid Brachytrachelopan (Rauhut et al., 2005), though more unnamed diplodocoid material is known from Argentina and Chile (Rauhut et al., 2015; Salgado et al., 2015). This situation is reversed in the Cretaceous, however, with limited Laurasian exposures (and accompanying reduction in fossil specimens), and radiation in Gondwanan taxa. There are no known North American Cretaceous diplodocoids. The probable rebbachisaurid Xenoposeidon is known from the Early Cretaceous of England (Taylor, 2018). Histriasaurus from the Early Cretaceous of Istria (Croatia) is also commonly recovered as a basal rebbachisaurid (Dalla Vecchia, 1998). Finally, the Spanish rebbachisaurid Demandasaurus (Pereda Suberbiola et al., 2003; Torcida Fernández-Baldor et al., 2011) is known from the Barremian-Aptian, making it the latest-appearing Laurasian diplodocoid.
The Gondwanan record is dominated by Argentinian fossils, including the latest-appearing members of both Diplodocidae and Dicraeosauridae. Leinkupal, from the late Berriasian-Valanginian (Early Cretaceous), is the only named diplodocid to have survived past the Jurassic (Gallina et al., 2014). The dicraeosaurid record is much richer, however, boasting the Early Cretaceous taxa Amargasaurus (Salgado and Bonaparte, 1991), Amargatitanis (Gallina, 2016; Whitlock and Wilson Mantilla, 2020), Bajadasaurus (Gallina et al., 2019) and Pilmatueia (Coria et al., 2018), comprising roughly one-third of known dicraeosaurid diversity. Rebbachisaurids primarily flourished in South America during this time, including two taxa from Brazil, the Aptian-Albian aged Amazonsaurus (Carvalho et al., 2003) and the Cenomanian Itapeuasaurus (Lindoso et al., 2019). The remainder of South American taxa are Argentinean, including Agustinia (Bellardini et al., 2022b), Campananeyen (Lerzo et al., 2024b), Cathartesaura (Gallina and Apesteguía, 2005), Comahuesaurus (Carballido et al., 2012), Katepensaurus (Ibiricu et al., 2013), Lavocatisaurus (Canudo et al., 2018), Limaysaurus (Calvo and Salgado, 1995), Nopcsaspondylus (Apesteguía, 2007), Sidersaura (Lerzo et al., 2024a), and Zapalasaurus (Salgado et al., 2006). The African Cretaceous record is less speciose but still contains the rebbachisaurids Rebbachisaurus (Lavocat, 1954), Nigersaurus (Sereno et al., 1999), and Tataouinea (Fanti et al., 2013), which belong to the same radiation (Rebbachisaurinae) as Demandasaurus and a handful of South American taxa. Additional unnamed remains belonging to dicraeosaurids and diplodocids have been reported from the Lower Cretaceous Kirkwood Formation of South Africa (McPhee et al., 2016).
Historically, the presence of early-diverging members of both Dicraeosauridae and Diplodocidae (and putatively Rebbachisauridae), as well as putative basal members lying outside of Diplodocimorpha (e.g., Haplocanthosaurus, Amphicoelias) has led some researchers to indicate North America as a center of origin for either Flagellicaudata or Diplodocoidea as a whole (i.e., Whitlock, 2011a; but see Remes, 2006 for a contrary opinion). The recent discovery of the derived dicraeosaurid Lingwulong from the late Middle Jurassic of China, however, casts some doubt on this hypothesis (Xu et al., 2018; Mannion et al., 2019; Bajpai et al., 2023), although as noted by those authors, a Laurasian origin for Flagellicaudata remains plausible. Under this scenario, Gondwanan appearances of flagellicaudatan sauropods would be the result of multiple excursions out of Laurasia (Whitlock, 2011a; Mannion et al., 2019). Multiple studies have suggested the presence of a distinctly Gondwanan clade within dicraeosaurids (e.g., Rauhut et al., 2005; Coria et al., 2019; Gallina et al., 2019; Whitlock and Wilson Mantilla, 2020), which would suggest geographic isolation of a lineage independent from the putatively more plesiomorphic North American forms. Similarly, a sister relationship between the Argentinean diplodocid Leinkupal and the African Tornieria has been posited (Mannion et al., 2019), which would be further evidence of expansion out of North America. The fragmentary sauropod Tharosaurus, being possibly the oldest flagellicaudatan, presents some challenges to a North American origin, however. Bajpai et al. (2023) argue for an Indian (and thus Gondwanan) origin for the clade through a pan-Pangean dispersal event. However, given the highly fragmentary nature of Tharosaurus, the evidence is somewhat thin and requires accepting several caveats to make this the most probable scenario. Nonetheless, it appears that the center of origin for Flagellicaudata is far from a settled question.
The origins of Rebbachisauridae have often been recovered as South American (e.g., Whitlock, 2011a; Carballido et al., 2012; Fanti et al., 2015), although there are several caveats to this. The first and most obvious is that the earliest occurrences of the group are Laurasian (Histriasaurus, Maraapunisaurus, and Xenoposeidon), as are the putatively most basal members of the more inclusive clade Diplodocoidea (Amphicoelias, Haplocanthosaurus). Additionally, the internal relationships of Rebbachisauridae point to a more complicated origin as well. Although limaysaurines (if monophyletic) appear to have been endemic to Patagonia (Whitlock, 2011a; Mannion et al., 2019), the sister clade Rebbachisaurinae was more cosmopolitan, boasting members from South America (Katepensaurus, possibly Itapeuasaurus), Africa (Nigersaurus, Rebbachisaurus, and Tataouinea) and Europe (Demandasaurus). Lerzo et al. (2024b) recover Nigersaurinae, synonymizing Limaysaurinae and Rebbachisaurinae, thus showing a different distribution of taxa among the rebbachisaurid subclades. However, all taxa outside Khebbashia are South American in Lerzo et al. (2024b), supporting a South American origin regardless of the change in interrelationships within Rebbachisauridae. The distribution of Rebbachisauridae is often thought to be the result of an “Apulian Route” between North Africa and Europe (Pereda Suberbiola et al., 2003; Gheerbrant and Rage, 2006; Canudo et al., 2009; Pereira et al., 2020; Lerzo et al., 2024b), with the European taxa being descendants from North African taxa, themselves being the result of a pre-Aptian dispersal into Africa from South America (Canudo et al., 2018; Lerzo et al., 2024b). This fits with current analyses placing the South American taxa Amazonsaurus, Agustinia, Comahuesaurus, and Lavocatisaurus as more basal members in Rebbachisauridae. However, given the probable Laurasian origins of the sister clade Flagellicaudata, and the timing of the first occurrence of members of the group, it remains possible (if less well supported by phylogeny at this time) that the known distribution of Rebbachisauridae is the result of dispersal into Gondwana out of Laurasia, as for flagellicaudatans.
LIFE APPEARANCE OF DIPLODOCOIDS
As our scientific knowledge of diplodocoids has expanded, so has our understanding of their life appearance. From their earliest life restorations, diplodocids (and all sauropods) were depicted as Hippopotamus-like behemoths that were restricted to aquatic realms given their immense girth (e.g., Amphicoelias reconstruction, see Osborn and Mook, 1921, figure 127). Although Phillips (1871) correctly noted anatomy indicative of an active terrestrial quadruped, given the then hypothesized taxonomic relationship between dinosaurs and reptiles, in 1910, O.P. Hay depicted the life restoration of Diplodocus with greatly splayed lizard-like limbs and locomotion (echoed by G. Tornier in 1909). Using drawings, anatomical dissections, scaled models, and even life-sized sauropod casts, this sprawling hypothesis was quickly challenged and put to rest by W.J. Holland later that same year. Although capable of some form of aquatic movements, we know now from extensive evidence, such as numerous global occurrences of trackways (e.g., Day et al., 2002; Santos et al., 2009; Calvo et al., 2022), that all sauropods expressed a graviportal, not sprawling stance, and were fully terrestrial (morphologically and ichnologically championed by Coombs, 1975).
Interestingly, the reconstruction of the external nares of diplodocids (and all sauropods) also derives from the historic aquatic hypothesis. Depicted as early as E.D. Cope’s 1897 sketch, to C. Knight’s 1897 scientific reconstruction, Amphicoelias altus was depicted with its long neck vertically outstretched in the water column with the head just above water level as a snorkel. In his later works, such as his 1907 depiction of a tripodally rearing Diplodocus, Knight reconstructed the external nares as medial to the orbits, occupying the actual bony nares. Unlike many of his contemporaries, Knight variably reconstructed the external nares in his sauropod reconstructions. In his 1897 Amphicoelias and his 1897, 1906, and 1939 Diplodocus, the nares are anteriorly positioned on the skull near the snout, whereas his 1897, 1929, and 1946 Brontosaurus and 1907 Diplodocus have external nares dorsally situated within the bony nares. Whereas many of the sauropod life reconstructions for much of the 20th Century had such dorsally situated external nares, Z. Burian’s 1957 Brachiosaurus is likely the most iconic, and explicit, functional depiction of the position of the external nares as a snorkel.
In his same 1975 paper championing fully terrestrial sauropods, W.P. Coombs additionally noted the morphology of the bony nares in comparison to mammals. Though cautiously not advocating the idea, Coombs (1975) noted the morphology of the bony nares in sauropods was like that of elephants and tapirs; thus, a sauropod with a proboscis-like soft-tissue structure was not entirely implausible. Inspired by Coombs’s (1975) observation between the bony nares of sauropods and elephants, Bakker (1986, p. 141) reconstructed a Diplodocus with an elephant-style trunk. The factual implausibilities of Diplodocus (and all sauropods) with elephantine trunks were examined and refuted by Knoll et al. (2006).
In a review of the bony nares in Dinosauria with respect to the external nares, Witmer (2001) proposed that the fleshy nostrils were contrarily located anteriorly on the skull near the tip of the ‘snout’. Witmer (2001) cited evidence for this alternative positioning from the extant phylogenetic bracket, and importantly from osteological remnants of the nasal vestibular vascular plexus which all advocated for rostroventrally placed nares. Whereas the study of Witmer (2001) is of extreme importance to the life history reconstructions of all Dinosauria, pertinent to this volume is that the Witmer study was selected as the cover article for the Volume 293 Number 5531 issue of Science, and the cover art synthesizing these findings was of a rostroventrally fleshy nostriled Diplodocus.
Perhaps the most debated aspect of diplodocid reconstructions and function is the neck. Since the days of C. Knight’s submersible Brontosaurus, diplodocids (and all sauropods) were historically reconstructed with a vertical, swan-like cervical series. Once sauropods were determined to be terrestrial, not aquatic organisms (Riggs, 1904; Bakker, 1971; Coombs, 1975), their bauplan was likened to that of giraffes (a similar comparison was made by Cope, 1877b). From then on, the hyperelongated cervical series was inferred to have evolved for feeding high in trees. This depiction of a vertically necked, canopy-feeding sauropod has become a classic cultural image (e.g., Jurassic Park).
Whereas the high-browsing sauropod depiction is an established cultural reference, the legitimacy of such cervical posturing was called into question by the studies of Martin (1987) and Stevens and Parrish (1999). Examining the degrees of cervical pre- and postzygapophyseal overlap of Apatosaurus louisae and Diplodocus carnegii, Stevens and Parrish (1999) concluded that the cervical series of diplodocids was incapable of being oriented in such a position. Instead, Stevens and Parrish (1999) proposed that in these diplodocids, the neck was habitually held at a more horizontal angle and that the cervical range of movement was primarily in a lateral plane. From such, Stevens and Parrish (1999) suggested that diplodocids like Apatosaurus and Diplodocus were alternatively low, not high, browsers. The study of Stevens and Parrish (1999) has been received with mixed academic opinions. However, the work of Stevens and Parrish (1999) has generated several analyses exploring numerous avenues of the neck posture debate (Upchurch, 2000; Christian, 2002; Stevens and Parrish, 2005a, 2005b; Christian and Dzemski, 2007; Dzemski and Christian, 2007; Sereno et al., 2007; Taylor et al., 2009; Christian, 2010; Clauss, 2011; Woodruff and Fowler, 2012; Cobley et al., 2013; Preuschoft and Klein, 2013; Stevens, 2013; Taylor and Wedel, 2013a; Taylor, 2014; Woodruff, 2016; Vidal et al., 2020a, 2020b). Whereas the topic of diplodocid (and all sauropods) neck posture is still the subject of investigation, we have at least reached a general agreement that the necks were not habitually held vertically. What might be tentatively deemed as a ‘general consensus’ is that diplodocids had a more horizontal habitual neck posture than their macronarian counterparts. More recently, both Stevens and Parrish (2005a) and Vidal et al. (2020a) advocated that contra the century-old idea that a long neck allowed sauropods to feed at greater vertical heights, as exemplified by the macronarian Brachiosaurus, placement of the shoulder girdle, and most importantly, increasing the length of the forelimbs automatically raises the head height (i.e., it is more effective to stand on a step stool as opposed to straining on your tiptoes with an outstretched arm). But pertinent to diplodocids, the study of Vidal et al. (2020b) recognized a new synapomorphy for Eusauropoda: a 10° anterodorsally oriented sacrum. By anterodorsally inclining the hips, the dorsal series is likewise raised, which is further expressed in the cervical series. Therefore, diplodocids may have exhibited some anterodorsally oriented postures simply from a shallow inclination of the pelvis. However, the low forelimb/hindlimb ratio observed in diplodocoids and vertebral angles in the cervico-dorsal transition (e.g., Tschopp et al., 2015) shows that this newly proposed synapomorphy must be studied further, as these factors may nullify the effect of the wedge-shaped sacrum.
Moving on from the head and neck, perhaps the most unique physical trait first recognized in diplodocids, which has since seemingly been ubiquitously applied to all Sauropoda, are the dermal scales. In 1992, S. Czerkas reported on sauropod integument from excavations at the Howe Quarry in Wyoming, USA. In addition to “pebbly” skin impressions, Czerkas (1992) documented a minimum of 14 conical to spine-like ornaments (then attributed to either Diplodocus or Barosaurus). Unlike an osteoderm, which has an osseous core with a keratinous sheath, these dermal spines had no osseous core (Czerkas, 1992), and instead appear to have been preserved as a three-dimensional carbonaceous structure infilled with sediment (E. Tschopp, personal obs., 2020) – the very thin carbonaceous layer presumably representing a diagenetically altered remnant of the original keratinous structure (Tschopp et al., 2020).
Although few of the reported 14 dermal spines were complete, Czerkas (1992) estimated the largest ones were approximately 18 cm in dorsoventral height. Regarding their morphology, Czerkas (1992) described them as quite variable; some were more mediolaterally compressed, while others were more conical, and some with straight and pointed apices, others with recurved apices. Czerkas (1992) claimed that while some of these conical dermal scales were found isolated, others were found associated, and others in semi-articulation, with caudal vertebrae. For the few in association/articulation, Czerkas (1992) reported they formed a single, midline row along the distalmost region of the caudal series. Czerkas (1992) reported that these dermal spines were thus far only known from the caudal region but given the distribution of similar structures in hadrosaurs (and in many extant lizards like Iguana spp.), he reconstructed a Diplodocus skeleton with these midline dermal spines running from the back of the skull continuously to the tip of the tail. Since Czerkas (1992), these midline dermal spines have been near ubiquitously reconstructed on all sauropods (not just diplodocids), with some being more conservative (such as the Diplodocus in Walking with Dinosaurs), to those with more artistic licensing being depicted nearly buzzsaw or mohawk-like. To date, no other sauropods have been documented with similar dermal spines, nor have any geochemical analyses been conducted on the specimens reported in Czerkas (1992). Whereas such integumentary reconstructions are likely in diplodocids (and any sauropod), much like the documented osteoderms in titanosaurs, patchworks or singular occurrences have been reconstructed more broadly anatomically and phylogenetically.
Unlike the extreme rarity of the dermal spines, both skin and skin impressions from Morrison Formation sauropods are known from several localities. Whereas skin impressions have been reported from the Morrison Formation – from tracks (though likely macronarian; Platt and Hasiotis, 2006) to isolated occurrences (such as one is tentatively referred to as “Sauropoda?”; Foster and Hunt-Foster, 2011) – there are well-documented examples in context with diplodocid remains. Czerkas (1992, 1994) reported on skin impressions and likely diagenetically altered skin from the Howe Quarry in Wyoming, USA. B. Brown noted the abundance of skin remains from the Howe Quarry in popular articles, and in one 1935 article he wrote, “Patches of skin impression, in many cases overlaid by the actual substance of the epidermal covering, were found all over the quarry in such profusion that much of it had to be destroyed in preparing the bones for shipment.” Whereas only a few tiny fragments of this Brown-era skin survive, Czerkas (1994) reported on new material from the Howe Quarry collected by the Sauriermuseum Aathal during expeditions in 1990 and 1991. Czerkas (1994) states that the scales are polygonal in shape (pentagonal to heptagonal according to his drawing), do not overlap, and vary in size from less than 1 cm to over 3 cm.
Czerkas (1994) further mentions that some skin fragments from Dinosaur National Monument (DNM; Vernal, Utah, USA), that are presumed, given proximity, to belong to Barosaurus bear a resemblance to his reported Howe Quarry (HQ) material. Czerkas (1994) noted the similar large size of the polygonal, non-overlapping scales, and that the scales appeared to be arranged in an ornamental cluster. This ornamental cluster consisted of smaller scales surrounding progressively larger scales at the center (Czerkas, 1994). Additionally, on both the DNM and HQ specimens, Czerkas (1994; figures 2 and 4) shows that the large polygonal scales are each covered in a small, rounded, papilliform-like texture. Only one section of skin from the HQ reported by Czerkas (1994) appeared to be associated with skeletal remains, and Czerkas (1994) reported that it was from the belly region. In this sample, Czerkas (1994) reports that the scales are 2-3 cm in width, non-overlapping, and are not in an ornamental pattern as observed in the other examples.
Our best examples to date of how integument varies across the body of a diplodocid comes from the cf. Diplodocus reported by Gallagher et al. (2021). From an in-situ slab preserved potentially in association with dorsal ribs, Gallagher et al. (2021) documented up to six different scale morphologies. Gallagher et al. (2021) describe these scales as non-overlapping, and other than their size (likely an indication of the young maturation of the individuals from this quarry), some of the scale morphotypes are like those reported by Czerkas (1994). From comparisons to crocodilian skin, Gallagher et al. (2021) hypothesized the approximate location on the body. Gallagher et al. (2021) noted: 1) polygonal scales were the most common, with smaller scales (<5 mm) more ventral than the larger (>5 mm) dorsal scales; 2) pebble scales were the smallest observed (1-2 mm), and were located ventrally along the polygonal scales; 3) rectangular scales that varied from 2-10 mm in length, with these rectangular scales “abruptly” intersecting the ventral polygonal scales, creating a demarcated change in scale type; 4) irregular globular scales that had three-dimensional (3D) relief; 5) ovoid scales are the largest (~10 mm), have 3D relief as well, the pointed ends all face the same direction, and they also abruptly intersect the adjacent polygonal scales; and 6) domed scales which are also 3D, located near the ovoid scales, and come in two distinct sizes (5 mm and <5 mm). In a life reconstruction based on these samples, Gallagher et al. (2021) placed the large ovoid scales dorsally along the sacral-caudal transition, with the remaining scale types positioned laterally and ventral – all indicative of changing scale morphology across and along different regions of the body.
Although the research mainly focused on diplodocid diplodocoids, meaningful inferences can be made for Rebbachisauridae and Dicraeosauridae as well. Rebbachisaurids are generally characterized by small to large body sizes, see above, and show specialized skull forms (e.g., Nigersaurus). However, the lack of complete specimens has resulted in a poor understanding of their overall appearance. Possible skin impressions of rebbachisaurids have been discovered in trackways from the Candeleros Formation in Argentina, but the lack of associated skeletal material makes the referral ambiguous (Apesteguía et al., 2023). Dicraeosaurids are characterized by their relatively small body size (see ecology and ontogeny of diplodocoids) and their elongated cervical and dorsal neural spines, resulting in a ‘hump’ or ‘sail’ like structure (e.g., Janensch, 1914; Salgado and Bonaparte, 1991; Bailey, 1997; Rauhut et al., 2005; Xu et al., 2018). In at least two dicraeosaurid taxa, namely Amargasaurus and Bajadasaurus, the cervical region shows hyperelongated hemispinous processes, which have variably been reconstructed as being covered in keratinous sheaths (like a bovid horn), to support a sail of skin thinly stretched between the hemispinous processes, to variable combinations of ‘sail and spikes’. These – then unique – cervical spines of Amargasaurus made this taxon the first of what would popularly and generationally be referred to as “bizarre dinosaurs.” However, in 2022, Cerda et al. osteohistologically examined these hyperelongated hemispinous processes and demonstrated that in life, thin sheet muscles would have covered and anteroposteriorly spanned the entire hemispinous processes (though questions regarding the presence of connective tissues transversely between the bifurcated spines remain). Much like the historic reconstructions of external nares, examination and study of these bizarre vertebral structures upends decades-long popular notions and reconstructions; yet, despite the Cerda et al. (2022) study, the function of these thinly muscled covered hyperelongate cervical processes remains unknown. In summary, notable advances have been made in reconstructing diplodocoids to their life appearance (Figure 6), which in turn reflects, at least partially, their ecological role.
(e.g., Janensch, 1914; Salgado and Bonaparte, 1991; Bailey, 1997; Rauhut et al., 2005; Xu et al., 2018). In at least two dicraeosaurid taxa, namely Amargasaurus and Bajadasaurus, the cervical region shows hyperelongated hemispinous processes, which have variably been reconstructed as being covered in keratinous sheaths (like a bovid horn), to support a sail of skin thinly stretched between the hemispinous processes, to variable combinations of ‘sail and spikes’. These – then unique – cervical spines of Amargasaurus made this taxon the first of what would popularly and generationally be referred to as “bizarre dinosaurs.” However, in 2022, Cerda et al. osteohistologically examined these hyperelongated hemispinous processes and demonstrated that in life, thin sheet muscles would have covered and anteroposteriorly spanned the entire hemispinous processes (though questions regarding the presence of connective tissues transversely between the bifurcated spines remain). Much like the historic reconstructions of external nares, examination and study of these bizarre vertebral structures upends decades-long popular notions and reconstructions; yet, despite the Cerda et al. (2022) study, the function of these thinly muscled covered hyperelongate cervical processes remains unknown. In summary, notable advances have been made in reconstructing diplodocoids to their life appearance (Figure 6), which in turn reflects, at least partially, their ecological role.
CONCLUDING REMARKS
Diplodocoidea is a thoroughly studied group of highly successful sauropods, but there remain numerous questions that need answers. This summarizing contribution leads into a selection of studies which together form the volume titled: “Diplodocoidea (Dinosauria, Sauropoda): Systematics, Phylogeny, Biogeography.” Through studying inter- and intraspecific variation and ontogeny, and the description of several new taxa, we aim to provide more insight into the origin and relationships of diplodocoid sauropods, as well as their ecology and biogeography.
ACKNOWLEDGEMENTS
We thank S. Hartman, O. Zant, J. González, A. Atuchin, and M. Witton for kindly providing their art for this contribution. We thank Palaeontologia Electronica for hosting this special volume and thank the reviewers for their feedback on earlier versions of the manuscript.
REFERENCES
Aberhan, M., Bussert, R., Heinrich, W.D., Schrank, E., Schultka, S., Sames, B., Kriwet, J., and Kapilima, S. 2002. Palaeoecology and depositional environments of the Tendaguru beds (late Jurassic to early Cretaceous, Tanzania). Fossil Record, 5, 1:19–44.
https://doi.org/10.1002/mmng.20020050103
Apesteguía, S. 2007. The sauropod diversity of the La Amarga Formation (Barremian), Neuquén (Argentina). Gondwana Research, 12, 4:533–546.
https://doi.org/10.1016/j.gr.2007.04.007
Apesteguía, S., Díaz-Martínez, I., Mayoral, J.P., Riguetti, F., Veiga, G.D., de Valais, S., and Dumont, L.F. 2023. Dinosaur tracks with skin impressions in the La Buitrera Paleontological Area (Candeleros Formation, Cenomanian), Río Negro Province, Argentina. Cretaceous Research, 150:105584.
https://doi.org/10.1016/j.cretres.2023.105584
Averianov, A.O., and Zverkov, N.G. 2020. New diplodocoid sauropod dinosaur material from the Middle Jurassic of European Russia. Acta Palaeontologica Polonica, 65:499–509.
https://doi.org/10.4202/app.00724.2020
Averianov, A. and Sues, H.D. 2021. First rebbachisaurid sauropod dinosaur from Asia. PloS ONE, 16, 2:e0246620.
https://doi.org/10.1371/journal.pone.0246620
Ayer, J. 2000. The Howe Ranch Dinosaurs. Sauriermuseum Aathal, Aathal.
Bailey, J.B. 1997. Neural spine elongation in dinosaurs: sailbacks or buffalo-backs? Journal of Paleontology, 71, 6:1124–1146.
https://doi.org/10.1017/S0022336000036076
Bajpai, S., Datta, D., Pandey, P., Ghosh, T., Kumar, K., and Bhattacharya, D. 2023. Fossils of the oldest diplodocoid dinosaur suggest India was a major centre for neosauropod radiation. Scientific Reports, 13, 1:12680.
https://doi.org/10.1038/s41598-023-39759-2
Bakker, R.T. 1968. The superiority of dinosaurs. Discovery, 3, 2:11–22.
Bakker, R.T. 1971. Ecology of the brontosaurs. Nature, 229:172–174.
https://doi.org/10.1038/229172a0
Bakker, R.T. 1986. The dinosaur heresies: New theories unlocking the mystery of the dinosaurs and their extinction. William Morrow, New York.
Bakker, R.T. 1998. Dinosaur mid-life crisis: the Jurassic-Cretaceous transition in Wyoming and Colorado. In Lucas, S.G., Kirkland, J.I., and Estep, J.W. (eds.), Lower and Middle Cretaceous Terrestrial Ecosystems. Vol. 14. New Mexico Museum of Natural History and Science Bulletin. pp. 67–77.
Barco, J.L., Canudo, J.I., Cuenca-Bescós, G., and Ruiz-Omeñaca, J.I. 2005. Un nuevo dinosaurio saurópodo Galvesaurus herreroi gen. nov., sp. nov., del tránsito Jurásico-Cretácico en Galve (Teruel, NE de España). Naturaleza Aragonesa, 15, 4:e17.
Barco, J.L., Canudo, J.I., and Cuenca-Bescós, G. 2006. Descripción de las vértebras cervicales de Galvesaurus herreroi Barco, Canudo, Cuenca-Bescos & Ruiz-Omeñaca, 2005 (Dinosauria, Sauropoda) del tránsito Jurásico-Cretácico en Galve (Teruel, Aragón, España). Spanish Journal of Palaeontology, 21, 2:189–205.
Barrett, P.M., Chapelle, K.E., Sciscio, L., Broderick, T.J., Zondo, M., Munyikwa, D., and Choiniere, J.N. 2024. A new Late Triassic sauropodomorph dinosaur from the Mid-Zambezi Basin, Zimbabwe. Acta Palaeontologica Polonica, 69, 2:227–241.
https://doi.org/10.4202/app.01100.2023
Bates, K.T., Mannion, P.D., Falkingham, P.L., Brusatte, S.L., Hutchinson, J.R., Otero, A., Sellers, W.I., Sullivan, C., Stevens, K.A., and Allen, V. 2016. Temporal and phylogenetic evolution of the sauropod dinosaur body plan. Royal Society Open Science, 3, 3:150636.
https://doi.org/10.1098/rsos.150636
Bellardini, F., Filippi, L.S., Garrido, A.C., Carballido, J.L., and Baiano, M.A. 2022a. New rebbachisaurid remains from the Huincul Formation (Middle Cenomanian-Early Turonian) of the Central Neuquén Basin, Patagonia, Argentina. Publicación Electrónica de la Asociación Paleontológica Argentina, 22, 2:1–24.
https://doi.org/10.5710/PEAPA.22.04.2022.419
Bellardini, F., Coria, R.A., Windholz, G.J., Martinelli, A.G., and Baiano, M.A. 2022b. Revisiting the Early Cretaceous sauropod Agustinia ligabuei (Dinosauria: Diplodocoidea) from southern Neuquén Basin (Patagonia, Argentina), with implications on the early evolution of rebbachisaurids. Historical Biology, 1–27.
https://doi.org/10.1080/08912963.2022.2142911
Bellardini, F., Filippi, L.S., Carballido, J.L., Garrido, A.C., and Baiano, M.A. 2023. Exploring rebbachisaurid hind-limb anatomy on the basis of a new articulated specimen from the Huincul Formation (upper Cenomanian) of Neuquén Basin, Patagonia, Argentina. Historical Biology, 36, 12:2587–2603.
https://doi.org/10.1080/08912963.2023.2268638
Berman, D.S. and McIntosh, J.S. 1978. Skull and relationships of the Upper Jurassic sauropod Apatosaurus (Reptilia, Saurischia). Bulletin of Carnegie Museum of Natural History, 8:1–35.
Bindellini, G. and Dal Sasso, C. 2021. Sauropod teeth from the Middle Jurassic of Madagascar, and the oldest record of Titanosauriformes. Papers in Paleontology, 7:137–161.
https://doi.org/10.1002/spp2.1282
Boisvert, C., Curtice, B., Wedel, M., and Wilhite, R. 2024. Description of a new specimen of Haplocanthosaurus from the Dry Mesa Dinosaur Quarry. Anatomical Record, 2024, 1–19.
https://doi.org/10.1002/ar.25520
Bonaparte, J. 1997. Rayososaurus agrioensis Bonaparte 1995. Ameghiniana 34, 1:116.
Bonaparte, J. 1999. An armoured sauropod from the Aptian of northern Patagonia, Argentina. Proceedings of the Second Gondwanan Dinosaur Symposium, National Science Museum Monographs, 15:1–12.
Bonaparte, J. and Coria, R. 1993. Un nuevo y gigantesco saurópodo titanosaurio de la Formación Río Limay (Albiano-Cenomaniano) de la Provincia del Neuquén, Argentina. Ameghiniana 30, 3:271–282.
Bonaparte, J. and Mateus, O. 1999. A new diplodocid, Dinheirosaurus lourinhanensis gen. et sp. nov., from the Late Jurassic beds of Portugal. Revista del Museo Argentino de Ciencias Naturales, 5, 2:13–29.
Button, D.J., Rayfield, E.J., and Barrett, P.M. 2014. Cranial biomechanics underpins high sauropod diversity in resource-poor environments. Proceedings of the Royal Society B: Biological Sciences, 281:20142114.
https://doi.org/10.1098/rspb.2014.2114
Calvo, J.O. 2023. What is the most giant sauropod from Argentina? Diversity of large titanosaurs from Patagonia. Metode Science Studies Journal, 14:65–75.
https://doi.org/10.7203/metode.14.24556
Calvo, J.O. and Salgado, L. 1995. Rebbachisaurus tessonei sp. nov. a new Sauropoda from the Albian-Cenomanian of Argentina; new evidence on the origin of the Diplodocidae. Gaia, 11:13–33.
Calvo, J.O., Porfiri, J.D., González-Riga, B.J., and Kellner, A.W. 2007. A new Cretaceous terrestrial ecosystem from Gondwana with the description of a new sauropod dinosaur. Anais da Academia Brasileira de Ciências, 79:529–541.
https://doi.org/10.1590/S0001-37652007000300013
Calvo, J.O., González Riga, B.J., Apesteguía, S., and Tomaselli, M.B. 2022. Sauropod Ichnology: Overview and New Research Lines from a South American Perspective, p. 503–540. In Otero, A., Carballido, J.L., and Pol, D. (eds.), South American Sauropodomorph Dinosaurs: Record, Diversity and Evolution. Springer Earth System Sciences, Springer International Publishing.
https://doi.org/10.1007/978-3-030-95959-3_14
Canale, J.I., Garrido, A., and Lerzo, L.N. 2023. Sobre la procedencia geográfica y estratigráfica de Nopcsaspondylus alarconensis (Sauropoda, Diplodocoidea, Rebbachisauridae). Reunión de comunicaciones de la Asociación Paleontológica Argentina (RCAPA). Libro de Resúmenes, 29–30.
Canudo, J.I., Barco, J.L., Pereda Suberbiola, X., Ruiz-Omeñaca, J.I., Salgado, L. Torcida Fernández-Baldor, F., and Gasulla, J.M. 2009. What Iberian dinosaurs reveal about the bridge said to exist between Gondwana and Laurasia in the Early Cretaceous. Bulletin de la Société Géologique de France, 180:5–11.
https://doi.org/10.2113/gssgfbull.180.1.5
Canudo, J., Carballido, J., Salgado, L., and Garrido, A. 2018. A new rebbachisaurid sauropod from the Aptian-Albian, Lower Cretaceous Rayoso Formation, Neuquén, Argentina. Acta Palaeontologica Polonica, 63:679–691.
https://doi.org/10.4202/app.00524.2018
Carballido, J.L., Salgado, L., Pol, D., Canudo, J.I., and Garrido, A. 2012. A new basal rebbachisaurid (Sauropoda, Diplodocoidea) from the Early Cretaceous of the Neuquén Basin; evolution and biogeography of the group. Historical Biology, 24:631–654.
https://doi.org/10.1080/08912963.2012.672416
Carballido, J.L., Pol, D., Otero, A., Cerda, I.A., Salgado, L., Garrido, A.C., Ramezani, J., Cúneo, N.R., and Krause, J.M. 2017. A new giant titanosaur sheds light on body mass evolution among sauropod dinosaurs. Proceedings of the Royal Society B: Biological Sciences, 284, 1860:20171219.
https://doi.org/10.1098/rspb.2017.1219
Carpenter, K. 2006. Biggest of the big – a critical re-evaluation of the megasauropod Amphicoelias fragillimus, p. 131–137. In Foster, J. and Lucas, S.G. (eds.), Paleontology and geology of the Upper Jurassic Morrison Formation: New Mexico Museum of Natural History and Science Bulletin, 36.
Carpenter, K. 2010. Species concept in North American stegosaurs. Swiss Journal of Geosciences, 103:155–162.
https://doi.org/10.1007/s00015-010-0020-6
Carpenter, K. 2017. Comment (Case 3700) – opposition against the proposed designation of Diplodocus carnegii Hatcher, 1901 as the type species of Diplodocus Marsh, 1878 (Dinosauria, Sauropoda): The Bulletin of Zoological Nomenclature, 74:47–49.
https://doi.org/10.21805/bzn.v74.a014
Carpenter, K. 2018. Maraapunisaurus fragillimus, n.g. (formerly Amphicoelias fragillimus), a basal rebbachisaurid from the Morrison Formation (Upper Jurassic) of Colorado. Geology of the Intermountain West, 5:227–244.
https://doi.org/10.31711/giw.v5.pp227-244
Carpenter, K. and Tidwell, V. 1998. Preliminary description of a Brachiosaurus skull from Felch Quarry 1, Garden Park, Colorado. Modern Geology, 23:69–84.
Carvalho, I. de S., Avilla, L. dos S., and Salgado, L. 2003. Amazonsaurus maranhensis gen. et sp. nov. (Sauropoda, Diplodocoidea) from the Lower Cretaceous (Aptian-Albian) of Brazil. Cretaceous Research, 24:697–713.
https://doi.org/10.1016/j.cretres.2003.07.005
Casanovas, M.L., Santafé, J.V., and Sanz, J.L. 2001. Losillasaurus giganteus, a new sauropod from the transitional Jurassic-Cretaceous of the Los Serranos basin (Valencia, Spain). Paleontologia y Evolució, 32:99–122.
Cerda, I.A., Novas, F.E., Carballido, J.L., and Salgado, L. 2022. Osteohistology of the hyperelongate hemispinous processes of Amargasaurus cazaui (Dinosauria: Sauropoda): Implications for soft tissue reconstruction and functional significance. Journal of Anatomy, 240, 6:1005–1019.
https://doi.org/10.1111/joa.13659
Christian, A. 2002. Neck posture and overall body design in sauropods. Fossil Record, 5, 1:271–281.
https://doi.org/10.1002/mmng.20020050116
Christian, A. 2010. Some sauropods raised their necks - evidence for high browsing in Euhelopus zdanskyi. Biology Letters, 6, 6:823–825.
https://doi.org/10.1098/rsbl.2010.0359
Christian, A., and Dzemski, G. 2007. Reconstruction of the cervical skeleton posture of Brachiosaurus brancai Janensch, 1914 by an analysis of the intervertebral stress along the neck and a comparison with the results of different approaches. Fossil Record, 10, 1:38–49.
https://doi.org/10.1002/mmng.200600017
Christian, A. and Dzemski, G. 2011. Neck Posture in Sauropods, p. 251–260. In Klein, N., Remes, K., Gee, C.T., and Sander, P.M. (eds.), Biology of the sauropod dinosaurs. Understanding the life of giants. Indiana University Press, Bloomington, Indiana.
Choy, D.S.J. and Altmann, P. 1992. The cardiovascular system of Barosaurus: an educated guess. Lancet 340, 8818:534–536.
Clauss, M. 2011. Sauropod biology and the evolution of gigantism: what do we know?, p. 3–7. In Klein, N., Remes, K., Gee, C.T., and Sander, P.M (eds.), Biology of the sauropod dinosaurs. Understanding the life of giants. Bloomington and Indianapolis: Indiana University Press.
Cobley, M.J., Rayfield, E.J., and Barrett, P.M. 2013. Inter-vertebral flexibility of the ostrich neck: implications for estimating sauropod neck flexibility. PLoS One, 8, 8:e72187.
https://doi.org/10.1371/journal.pone.0072187
Conti, S., Tschopp, E., Mateus, O., Zanoni, A., Masarati, P., and Sala, G. 2022. Multibody analysis and soft tissue strength refute supersonic dinosaur tail. Scientific Reports, 12, 1:19245.
https://doi.org/10.1038/s41598-022-21633-2
Coombs, W. 1975. Sauropod Habits and Habitats. Palaeogeography, Palaeoclimatology, Palaeoecology, 17:1–33.
Cope, E.D. 1877a. On a dinosaurian from the Trias of Utah. Proceedings of the American Philosophical Society, 16:579–584.
Cope, E.D. 1877b. On Amphicoelias, a genus of saurians from the Dakota Epoch of Colorado. Paleontology Bulletin, 27:1–5.
Cope, E.D. 1878. A new species of Amphicoelias. American Naturalist, 12:563–565.
https://doi.org/10.1086/272176
Coria, R.A., Windholz, F.J., Ortega, F., and Currie, P.J. 2019. A new dicraeosaurid sauropod from the Lower Cretaceous (Mulichinco Formation, Valanginian, Neuquén Basin) of Argentina. Cretaceous Research, 93:33–48.
https://doi.org/10.1016/j.cretres.2018.08.019
Curry, K.A. 1999. Ontogenetic histology of Apatosaurus (Dinosauria: Sauropoda): new insights on growth rates and longevity. Journal of Vertebrate Paleontology, 19:654–665.
https://doi.org/10.1080/02724634.1999.10011179
Curtice, B.D. 1996. Codex of diplodocid caudal vertebrae from the Dry Mesa dinosaur quarry. Masters Thesis, Brigham Young University, Department of Geology.
Curtice, B.D. and Wilhite, D.R. 1996. A Re-Evaluation of the Dry Mesa Dinosaur Quarry Sauropod Fauna with a Description of Juvenile Sauropod Elements. Geology and Resources of the Paradox Basin, 325–338.
Curtice, B.D. and Stadtman, K.L. 2001. The demise of Dystylosaurus edwini and a revision of Supersaurus vivianae. Mesa Southwest Museum Bulletin, 8:33–39.
Czerkas, S.A. 1992. Discovery of dermal spines reveals a new look for sauropod dinosaurs. Geology, 20, 12:1068–1070.
https://doi.org/10.1130/0091-7613(1992)020<1068:DODSRA>2.3.CO;2
Czerkas, S.A. 1994. The history and interpretation of sauropod skin impressions. Gaia, 10:173–182.
Dalla Vecchia, F. 1998. Remains of Sauropoda (Reptilia, Saurischia) in the Lower Cretaceous (Upper Hauterivian/Lower Barremian) limestones of SW Istria (Croatia). Geologia Croatica, 51, 2:105–134.
Dantas, P., Sanz, J., da Marques Silva, C., Ortega, F., Santos, V., and Cachão, M. 1998. Lourinhasaurus n gen. novo dinossáurio saurópode do Jurássico superior (Kimeridgiano superior-Titoniano inferior) de Portugal. Actas Do V Congresso Nacional de Geologia, 84:91–94.
Day, J.J., Upchurch, P., Norman, D.B., Gale, A.S., and Powell, H.P. 2002. Sauropod trackways, evolution, and behavior. Science, 296, 5573:1659–1659.
https://doi.org/10.1126/science.1070167
de Lapparent, A.F. and Zbyszewski, G. 1957. Les dinosauriens du Portugal. Memoires Des Services Géologiques Du Portugal, 2:1–63.
D’Emic, M.D. and Carrano, M.T. 2019. Redescription of brachiosaurid sauropod dinosaur material from the Upper Jurassic Morrison Colorado, Formation, USA. The Anatomical Record 303:732–758.
https://doi.org/10.1002/ar.24198
D’Emic, M.D., Whitlock, J.A., Smith, K.M., Fisher, D.C., and Wilson, J.A. 2013. Evolution of high tooth replacement rates in sauropod dinosaurs. PLoS ONE, 8, 7:e69235.
https://doi.org/10.1371/journal.pone.0069235
Demirjian, V.D. 2017. Comment (Case 3700) – on the proposed designation of Diplodocus carnegii Hatcher, 1901 as the type species of Diplodocus Marsh, 1878 (Dinosauria, Sauropoda) – application should be rejected based on new data. The Bulletin of Zoological Nomenclature, 73, 2-4:132–133.
https://doi.org/10.21805/bzn.v73i2.a15
Dzemski, G. and Christian, A. 2007. Flexibility along the neck of the ostrich (Struthio camelus) and consequences for the reconstruction of dinosaurs with extreme neck length. Journal of Morphology, 268, 8:701–714.
https://doi.org/10.1002/jmor.10542
Erickson, G.M., Krick, B.A., Hamilton, M., Bourne, G.R., Norell, M.A., Lilleodden, E., and Sawyer, W.G. 2012. Complex dental structure and wear biomechanics in hadrosaurid dinosaurs. Science, 338, 6103:98–101.
https://doi.org/10.1126/science.1224495
Fabbri, M., Navalón, G., Mongiardino Koch, N., Hanson, M., Petermann, H., and Bhullar, B. 2021. A shift in ontogenetic timing produced the unique sauropod skull. Evolution 75:819–831.
https://doi.org/10.1111/evo.14190
Fanti, F., Cau, A., Hassine, M., and Contessi, M. 2013. A new sauropod dinosaur from the Early Cretaceous of Tunisia with extreme avian-like pneumatization. Nature Communications 4:2080.
https://doi.org/10.1038/ncomms3080
Fanti, F., Cau, A., and Hassine, M. 2014. Evidence of titanosauriforms and rebbachisaurids (Dinosauria: Sauropoda) from the Early Cretaceous of Tunisia. Journal of African Earth Sciences, 90:1–8.
https://doi.org/10.1016/j.jafrearsci.2013.10.010
Fanti, F., Cau, A., Cantelli, L., Hassine, M., and Auditore, M. 2015. New information on Tataouinea hannibalis from the Early Cretaceous of Tunisia and implications for the tempo and mode of rebbachisaurid sauropod evolution. PloS ONE, 10, 4:e0123475.
https://doi.org/10.1371/journal.pone.0123475
Farlow, J.O., Coroian, D., Currie, P.J., Foster, J.R., Mallon, J.C., and Therrien, F. 2022. “Dragons” on the landscape: Modeling the abundance of large carnivorous dinosaurs of the Upper Jurassic Morrison Formation (USA) and the Upper Cretaceous Dinosaur Park Formation (Canada). The Anatomical Record, 306:1669–1696.
https://doi.org/10.1002/ar.25024
Fernández-Baldor, F.T., Canudo, J.I., Huerta, P., Montero, D., Suberbiola, X.P., and Salgado, L. 2011. Demandasaurus darwini, a New Rebbachisaurid Sauropod from the Early Cretaceous of the Iberian Peninsula. Acta Palaeontologica Polonica, 56:535–552.
https://doi.org/10.4202/app.2010.0003
Filippi, L.S., Valieri, R.D.J., Gallina, P.A., Méndez, A.H., Gianechini, F.A., and Garrido, A.C. 2024. A rebbachisaurid-mimicking titanosaur and evidence of a Late Cretaceous faunal disturbance event in South-West Gondwana. Cretaceous Research, 154:105754.
https://doi.org/10.1016/j.cretres.2023.105754
Filla, B.J. and Redman, P.D. 1994. Apatosaurus yahnahpin: a preliminary description of a new species of diplodocid dinosaur from the Late Jurassic Morrison Formation of Southern Wyoming, the first sauropod dinosaur found with a complete set of “belly ribs”, p. 159–178. In Nelson, G.E. (ed.), The dinosaurs of Wyoming. Wyoming Geological Association 44th annual field conference guidebook. Wyoming Geological Association, Casper.
Fiorillo, A.R. 1998. Dental micro wear patterns of the sauropod dinosaurs Camarasaurus and Diplodocus: evidence for resource partitioning in the Late Jurassic of North America. Historical Biology, 13, 1:1–16.
https://doi.org/10.1080/08912969809386568
Foster, J.R. 2005. New Juvenile Sauropod Material from Western Colorado, and the Record of Juvenile Sauropods from the Upper Jurassic Morrison Formation, p. 141–153. In Tidwell, V. and Carpenter, K. (eds.), Thunderlizards: the sauropodomorph dinosaurs. Indiana University Press, Bloomington, Indiana.
Foster, J.R. 2020. Jurassic west: the dinosaurs of the Morrison Formation and their world, second edition. Indiana University Press, Bloomington.
https://doi.org/10.2307/j.ctv18sqxpx
Foster, J.R. and Hunt-Foster, R.K. 2011. New occurrences of dinosaur skin of two types (Sauropoda? and Dinosauria indet.) from the Late Jurassic of North America (Mygatt-Moore Quarry, Morrison Formation). Journal of Vertebrate Paleontology, 31, 3:717–721.
https://doi.org/10.1080/02724634.2011.557419
Foster, J.R., Irmis, R.B., Trujillo, K.C., McMullen, S.K., and Gillette, D.D. 2016. Dystrophaeus viaemalae Cope from the basal Morrison Formation of Utah: Implications for the origin of eusauropods in North America. Journal of Vertebrate Paleontology, Program and Abstracts, 138.
Fraas, E. 1908. Ostafrikanische Dinosaurier. Palaeontographica, 15:105–144.
Gallagher, T., Poole, J., and Schein, J.P. 2021. Evidence of integumentary scale diversity in the late Jurassic sauropod Diplodocus sp. from the Mother’s Day Quarry, Montana. PeerJ, 9:e11202.
https://doi.org/10.7717/peerj.11202
Gallina, P.A. 2016. Reappraisal of the Early Cretaceous sauropod dinosaur Amargatitanis macni (Apesteguía, 2007), from northwestern Patagonia, Argentina. Cretaceous Research, 64:79–87.
https://doi.org/10.1016/j.cretres.2016.04.002
Gallina, P.A. and Apesteguía, S. 2005. Cathartesaura anaerobica gen. et sp. nov., a new rebbachisaurid (Dinosauria, Sauropoda) from the Huincul Formation (Upper Cretaceous), Río Negro, Argentina. Revista del Museo Argentino de Ciencias Naturales, 7:153–166
Gallina, P.A., Apesteguía, S., Haluza, A., and Canale, J.I. 2014. A Diplodocid Sauropod Survivor from the Early Cretaceous of South America. PLoS ONE, 9:e97128.
https://doi.org/10.1371/journal.pone.0097128
Gallina, P.A., Apesteguía, S., Canale, J.I., and Haluza, A. 2019. A new long-spined dinosaur from Patagonia sheds light on sauropod defense system. Scientific Reports, 9:1392.
https://doi.org/10.1038/s41598-018-37943-3
Gallina, P.A., Apesteguía, S., Carballido, J.L., and Garderes, J.P. 2022. Southernmost Spiny Backs and Whiplash Tails: Flagellicaudatans from South America, p. 209–236. In Otero, A., Carballido, J.L., and Pol, D. (eds.), South American Sauropodomorph Dinosaurs. Springer Earth System Sciences. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-030-95959-3_6
Garderes, J.P., Gallina, P.A., Whitlock, J.A., and Toledo, N. 2022. Neuroanatomy of a diplodocid sauropod dinosaur from the Lower Cretaceous of Patagonia, Argentina. Cretaceous Research, 129:105024.
https://doi.org/10.1016/j.cretres.2021.105024
Garderes, J.P., Gallina, P.A., Whitlock, J.A., and Toledo, N. 2023. Cranial osteology of Bajadasaurus pronuspinax (Sauropoda, Dicraeosauridae). Historical Biology, 36:1343–1367.
https://doi.org/10.1080/08912963.2023.2212389
Gheerbrant, E. and Rage, J.-C. 2006. Paleobiogeography of Africa: How distinct from Gondwana and Laurasia?. Palaeogeography, Palaeoclimatology, Palaeoecology, 241:224–246.
https://doi.org/10.1016/j.palaeo.2006.03.016
Gilmore, C.W. 1922. A new sauropod dinosaur from the Ojo Alamo Formation of New Mexico. Smithsonian Miscellaneous Collections, 72:1–9.
Gilmore, C.W. 1936. Osteology of Apatosaurus: with special reference to specimens in the Carnegie Museum. Memoirs of the Carnegie Museum, 11:175–300.
Gillette, D.D. 1991. Seismosaurus halli, gen. et sp. nov., a new sauropod dinosaur from the Morrison Formation (Upper Jurassic/Lower Cretaceous) of New Mexico, USA. Journal of Vertebrate Paleontology, 11, 4:417–433.
https://doi.org/10.1080/02724634.1991.10011413
Gomez, K.L., Pérez-Moreno, A., Meso, J.G., Bellardini, F., Baiano, M.A., Pol, D., Garrido, A., Kaluza, J., Muci, L., and Pittman, M. 2024a. Unraveling sauropod diversity in the Portezuelo Formation of Patagonia through a comprehensive analysis of new and existing material. BMC Ecology and Evolution, 24, 96:1–16.
https://doi.org/10.1186/s12862-024-02280-9
Gomez, K.L., Carballido, J.L., and Pol, D. 2024b. Cranial anatomy of Bagualia alba (Dinosauria, Eusauropoda) from the Early Jurassic of Patagonia and the implications for sauropod cranial evolution. Journal of Systematic Palaeontology, 22:1.
https://doi.org/10.1080/14772019.2024.2400471
Griffin, C.T., Stocker, M.R., Colleary, C., Stefanic, C.M., Lessner, E.J., Riegler, M., Formoso, K., Koeller, K., and Nesbitt, S.J. 2021. Assessing ontogenetic maturity in extinct saurian reptiles. Biological Reviews, 96:470–525.
https://doi.org/10.1111/brv.12666
Haluza, A., Canale, J.I., Otero, A., Pérez, L.M., and Scanferla, C.A. 2012. Changes in vertebral laminae across the cervicodorsal transition of a well-preserved rebbachisaurid (Dinosauria, Sauropoda) from the Cenomanian of Patagonia, Argentina. Journal of Vertebrate Paleontology, 32, 1:219–224.
https://doi.org/10.1080/02724634.2012.620674
Hanik, G.M., Lamanna, M.C., and Whitlock, J.A. 2017. A juvenile specimen of Barosaurus Marsh, 1890 (Sauropoda: Diplodocidae) from the upper jurassic Morrison formation of dinosaur national monument, Utah, USA. Annals of Carnegie Museum, 84, 3:253–263.
https://doi.org/10.2992/007.084.0301
Harris, J.D. and Dodson, P. 2004. A new diplodocoid sauropod dinosaur from the Upper Jurassic Morrison Formation of Montana, USA. Acta Palaeontologica Polonica, 49, 2:197.
Harris, J.D. 2006a. Cranial osteology of Suuwassea emilieae (Sauropoda: Diplodocoidea: Flagellicaudata) from the upper jurassic morrison formation of montana, USA. Journal of Vertebrate Paleontology, 26, 1:88–102.
https://doi.org/10.1671/0272-4634(2006)26[88:COOSES]2.0.CO;2
Harris, J.D. 2006b. The axial skeleton of the dinosaur Suuwassea emilieae (Sauropoda: Flagellicaudata) from the Upper Jurassic Morrison Formation of Montana, USA. Palaeontology, 49:1091–1121.
https://doi.org/10.1111/j.1475-4983.2006.00577.x
Harris, J.D. 2006c. The significance of Suuwassea emilieae (Dinosauria: Sauropoda) for flagellicaudatan intrarelationships and evolution. Journal of Systematic Palaeontology, 4, 2:185–198.
https://doi.org/10.1017/S1477201906001805
Harris, J.D. 2007. The appendicular skeleton of Suuwassea emilieae (Sauropoda: Flagellicaudata) from the upper Jurassic Morrison Formation of Montana (USA). Geobios, 40, 4:501–522.
https://doi.org/10.1016/j.geobios.2006.02.002
Hatcher, J.B. 1901. Diplodocus (Marsh): its osteology, taxonomy, and probable habits, with a restoration of the skeleton. Memoirs of the Carnegie Museum, 1:1–63.
Hatcher, J.B. 1903. Osteology of Haplocanthosaurus, with description of a new species and remarks on the probable habits of the Sauropoda and the age and origin of the Atlantosaurus beds: additional remarks on Diplodocus. Memoirs of the Carnegie Museum, 2:1–72.
Hay, O.P. 1908. On the habits and the pose of the sauropodous dinosaurs, especially of Diplodocus. The American Naturalist, 42, 502:672–681.
https://doi.org/10.1086/278992
Hedrick, B.P., Tumarkin-Deratzian, A.R., and Dodson, P. 2014. Bone microstructure and relative age of the holotype specimen of the diplodocoid sauropod dinosaur Suuwassea emilieae. Acta Palaeontologica Polonica, 59, 2:295–304.
https://doi.org/10.4202/app.2012.0049
Hicks, J.W. and Badeer, H.S. 1992. Barosaurus and its circulation. The Lancet, 340:1229.
Holland, W.J. 1915a. Heads and tails: a few notes relating to the structure of the sauropod dinosaurs. Annals of the Carnegie Museum, 9:272–278.
Holland, W.J. 1915b. A new species of Apatosaurus. Annals of the Carnegie Museum, 10:143–145.
Holland, W.J. 1924. The skull of Diplodocus. Memoirs of the Carnegie Museum, 9:378–403.
Holwerda, F.M., Evans, M., and Liston, J.J. 2019. Additional sauropod material from the Callovian Oxford Clay Formation, Peterborough, UK: evidence for higher sauropod diversity. PeerJ, 7:e6404.
https://doi.org/10.7717/peerj.6404
Huene, F.V. 1927. Short review of the present knowledge of the Sauropoda. Memoirs of the Queensland Museum, 9, 1:121–126.
Hummel, J. and Clauss, M. 2011. Sauropod feeding and digestive physiology, p. 11–33. In Klein, N., Remes, K., Gee, C.T., and Sander, P.M. (eds.), Biology of the sauropod dinosaurs. Understanding the life of giants. Indiana University Press, Bloomington, Indiana.
Ibiricu, L.M., Casal, G.A., Martínez, R.D., Lamanna, M.C., Luna, M., and Salgado, L. 2013. Katepensaurus goicoecheai, gen. et sp. nov., a Late Cretaceous rebbachisaurid (Sauropoda, Diplodocoidea) from central Patagonia, Argentina. Journal of Vertebrate Paleontology, 33, 6:1351–1366.
https://doi.org/10.1080/02724634.2013.776562
Ibiricu, L.M., Casal, G.A., Martínez, R.D., Lamanna, M.C., Luna, M., and Salgado, L. 2015. New material of Katepensaurus goicoecheai (Sauropoda: Diplodocoidea) and its significance for the morphology and evolution of Rebbachisauridae. Ameghiniana, 52, 4:430–446.
https://doi.org/10.5710/AMGH.24.04.2015.2830
Ibiricu, L.M., Lamanna, M.C., Martínez, R.D., Casal, G.A., Cerda, I.A., Martínez, G., and Salgado, L. 2017. A novel form of postcranial skeletal pneumaticity in a sauropod dinosaur: Implications for the paleobiology of Rebbachisauridae. Acta Palaeontologica Polonica, 62, 2:221–236.
https://doi.org/10.4202/app.00316.2016
Janensch, W. 1914. Übersicht über die Wirbeltierfauna der Tendaguru-Schichten nebst einer kurzen Charakterisierung der neu aufgeführten Arten von Sauropoden. Archiv für Biontologie, 3:81–110.
Janensch, W. 1922. Das Handskelett von Gigantosaurus robustus und Brachiosaurus brancai aus den Tendaguru-Schichten Deutsch-Ostafrikas. Centralblatt Für Mineralogie, Geologie Und Paläontologie, 15:464–480.
Janensch, W. 1929a. Material und Formengehalt der Sauropoden in der Ausbeute der Tendaguru-Expedition. Palaeontographica Supplement, 7:1-34.
Janensch, W. 1929b. Die Wirbelsäule der Gattung Dicraeosaurus. Palaeontographica Supplement, 7:38–133.
Jannel, A., Salisbury, S.W., and Panagiotopoulou, O. 2022. Softening the steps to gigantism in sauropod dinosaurs through the evolution of a pedal pad. Science Advances, 8, 31:eabm8280.
https://doi.org/10.1126/sciadv.abm8280
Jensen, J.A. 1985. Three new sauropod dinosaurs from the Upper Jurassic of Colorado. Western North American Naturalist, 45:697–709.
King, J.L., McHugh, J.B., Wedel, M.J., and Curtice, B. 2023. A previously unreported form of dorsal rib pneumaticity in Apatosaurus (Dinosauria: Sauropoda) and implications for pneumatic variation among diplodocid dorsal ribs. Journal of Vertebrate Paleontology, 43, 5:e2316665.
https://doi.org/10.1080/02724634.2024.2316665
Klein, N. and Sander, M. 2008. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology, 34, 2:247–263.
https://doi.org/10.1666/0094-8373(2008)034[0247:OSITLB]2.0.CO;2
Klinkhamer, A.J., Mallison, H., Poropat, S.F., Sinapius, G.H., and Wroe, S. 2018. Three‐dimensional musculoskeletal modeling of the sauropodomorph hind limb: the effect of postural change on muscle leverage. The Anatomical Record, 301, 12:2145–2163.
https://doi.org/10.1002/ar.23950
Knoll, F., Galton, P.M., and López-Antoñanzas, R. 2006. Paleoneurological evidence against a proboscis in the sauropod dinosaur Diplodocus. Geobios, 39, 2:215–221.
https://doi.org/10.1016/j.geobios.2004.11.005
Lallensack, J.N., Klein, H., Milàn, J., Wings, O., Mateus, O., and Clemmensen, L.B. 2017. Sauropodomorph dinosaur trackways from the Fleming Fjord Formation of East Greenland: evidence for Late Triassic sauropods. Acta Palaeontologica Polonica, 62, 4:833–843.
https://doi.org/10.4202/app.00374.2017
Lambertz, M., Bertozzo, F., and Sander, P.M. 2018. Bone histological correlates for air sacs and their implications for understanding the origin of the dinosaurian respiratory system. Biology letters, 14, 1:20170514.
https://doi.org/10.1098/rsbl.2017.0514
Lavocat, R. 1954. Sur les dinosauriens du continental intercalaire des Kem Kem de la Daoura. Comptes Rendus de la Dix-Neuvième Session, Congrès Géologique International 21, París, 3:65–68.
Lerzo, L.N., Carballido, J.L., and Gallina, P.A. 2021. Rebbachisaurid sauropods in Asia? A re-evaluation of the phylogenetic position of Dzharatitanis kingi from the late Cretaceous of Uzbekistan. Publicación Electrónica de la Asociación Paleontológica Argentina, 21, 1:18–27.
https://doi.org/10.5710//PEAPA.24.03.2021.389
Lerzo, L.N., Gallina, P.A., Canale, J.I., Otero, A., Carballido, J.L., Apesteguía, S., and Makovicky, P.J. 2024a. The last of the oldies: a basal rebbachisaurid (Sauropoda, Diplodocoidea) from the early Late Cretaceous (Cenomanian-Turonian) of Patagonia, Argentina. Historical Biology, 37:208–233.
https://doi.org/10.1080/08912963.2023.2297914
Lerzo, L.N., Torcida Fernández-Baldor, F., Canale, J.I., Whitlock, J.A., Otero, A., and Gallina, P.A. 2024b. They all floated in the cretaceous: new rebbachisaurid (Sauropoda, Diplodocoidea) with a highly pneumatized skeleton from the Upper Cretaceous (lower Cenomanian) of Patagonia, Argentina. Historical Biology, 1–14.
https://doi.org/10.1080/08912963.2024.2383708
Lindoso, R.M., Medeiros, M.A.A., de Souza Carvalho, I., Pereira, A.A., Mendes, I.D., Iori, F.V., and Silva, T.C.M. 2019. A new rebbachisaurid (Sauropoda: Diplodocoidea) from the middle Cretaceous of northern Brazil. Cretaceous Research, 104, 104191.
https://doi.org/10.1016/j.cretres.2019.104191
Lovelace, D.M., Hartman, S.A., and Wahl, W.R. 2007. Morphology of a specimen of Supersaurus (Dinosauria, Sauropoda) from the Morrison Formation of Wyoming, and a re-evaluation of diplodocid phylogeny. Arquivos do Museu Nacional, Rio de Janeiro, 65, 4:527–544.
Lucas, S.G. 2017. Comment (Case 3700) – support for designating Diplodocus carnegii Hatcher, 1901 as the type species of Diplodocus Marsh, 1878 (Dinosauria, Sauropoda). The Bulletin of Zoological Nomenclature, 73, 2-4:128.
https://doi.org/10.21805/bzn.v73i2.a13
Lucas, S.G., Spielmann, J.A., Rinehart, L.F., Heckert, A.B., Herne, M.C., Hunt, A.P., Foster, J.R., and Sullivan, R.M. 2006. Taxonomic status of Seismosaurus hallorum, a Late Jurassic sauropod dinosaur from New Mexico. New Mexico Museum of Natural History and Science Bulletin, 36:149–162.
Lull, R.S. 1919. The sauropodous dinosaur Barosaurus Marsh. Memoirs of the Connecticut Academy of Arts and Sciences, 6:1–42.
Lydekker, R. 1877. Notices of new and other Vertebrata from Indian Tertiary and Secondary rocks. Records of the Geological Survey of India, 10:30–43.
Maidment, S.C. 2024. Diversity through time and space in the Upper Jurassic Morrison Formation, Western USA. Journal of Vertebrate Paleontology, 43, 5:e2326027.
https://doi.org/10.1080/02724634.2024.2326027
Mannion, P.D. 2009. A rebbachisaurid sauropod from the Lower Cretaceous of the Isle of Wight, England. Cretaceous Research, 30:521–526.
https://doi.org/10.1016/j.cretres.2008.09.005
Mannion, P.D. and Whitlock, J.A. in press. Diplodocoidea. In Weishampel, D.B., Barrett, P.M., Carrano, M.T., and Makovicky, P.J. (eds.), The Dinosauria (3rd Edition). Cambridge University Press.
Mannion, P.D., Upchurch, P., Mateus, O., Barnes, R.N., and Jones, M.E. 2012. New information on the anatomy and systematic position of Dinheirosaurus lourinhanensis (Sauropoda: Diplodocoidea) from the Late Jurassic of Portugal, with a review of European diplodocoids. Journal of Systematic Palaeontology, 10, 3:521–551.
https://doi.org/10.1080/14772019.2011.595432
Mannion, P.D., Upchurch, P., Barnes, R.N., and Mateus, O. 2013. Osteology of the Late Jurassic Portuguese sauropod dinosaur Lusotitan atalaiensis (Macronaria) and the evolutionary history of basal titanosauriforms. Zoological Journal of the Linnean Society, 168, 1:98–206.
https://doi.org/10.1111/zoj.12029
Mannion, P.D., Upchurch, P., Schwarz, D., and Wings, O. 2019. Taxonomic affinities of the putative titanosaurs from the Late Jurassic Tendaguru Formation of Tanzania: phylogenetic and biogeographic implications for eusauropod dinosaur evolution. Zoological Journal of the Linnean Society, 185, 3:784–909.
https://doi.org/10.1093/zoolinnean/zly068
Mannion, P.D., Tschopp, E., and Whitlock, J.A. 2021. Anatomy and systematics of the diplodocoid Amphicoelias altus supports high sauropod dinosaur diversity in the Upper Jurassic Morrison Formation of the USA. Royal Society Open Science, 8:210377.
https://doi.org/10.1098/rsos.210377
Marsh, O.C. 1877a. Notice of a new and gigantic dinosaur. American Journal of Science, 3-14, 79:87–88.
https://doi.org/10.2475/ajs.s3-14.79.87
Marsh, O.C. 1877b. Notice of new dinosaurian reptiles from the Jurassic Formation. American Journal of Science, 3-14, 84:514–516.
https://doi.org/10.2475/ajs.s3-14.84.514
Marsh, O.C. 1878. Principal characters of American Jurassic dinosaurs, Part I. American Journal of Science, 3, 16:411–416.
https://doi.org/10.2475/ajs.s3-16.95.411
Marsh, O.C. 1879. Notice of new Jurassic reptiles. American Journal of Science 3, 8:501–505. https://doi.org/10.2475/ajs.s3-18.108.501
Marsh, O.C. 1883. Principal characters of American Jurassic dinosaurs. Pt. VI. Restoration of Brontosaurus. American Journal of Science, Series 3, 26, 81–85 and plate 1.
https://doi.org/10.2475/ajs.s3-26.152.81
Marsh, O.C. 1884. Principal characters of American Jurassic dinosaurs, Part VII, Diplodocidae, a new family of Sauropoda. American Journal of Science, 27:161–167.
https://doi.org/10.2475/ajs.s3-27.158.161
Marsh, O.C. 1889. Notice of new American Dinosauria. American Journal of Science, 3:331–336.
https://doi.org/10.2475/ajs.s3-37.220.331
Marsh, O.C. 1890. Description of new dinosaurian reptiles. American Journal of Science, 3, 39:81–86.
https://doi.org/10.2475/ajs.s3-39.229.81
Marsh, O.C. 1891. Restoration of Triceratops. American Journal of Science, 3, 41: 339–342 and plates XV-XVI.
https://doi.org/10.2475/ajs.s3-41.244.339
Martin, J. 1987. Mobility and feeding of Cetiosaurus (Saurischia: Sauropoda) - why the long neck? p. 154–159. In Currie, P.J. and Koster, E.H. (eds.), Fourth Symposium on Mesozoic Terrestrial Ecosystems, Short Papers. Boxtree Books, Drumheller, Alberta.
Mazzetta, G.V., Christiansen, P., and Fariña, R.A. 2004. Giants and bizarres: Body size of some southern South American cretaceous dinosaurs. Historical Biology, 16:71–83.
https://doi.org/10.1080/08912960410001715132
McHugh, J. 2018. Evidence for niche partitioning among ground-height browsing sauropods from the Upper Jurassic Morrison Formation of North America. Geology of the Intermountain West, 5:95–103.
https://doi.org/10.31711/giw.v5.pp95-103
McIntosh, J.S. 1990a. Species determination in sauropod dinosaurs with tentative suggestions for their classification, p. 53–69. In Carpenter, K. and Currie, P.J. (eds.), Dinosaur systematics: perspectives and approaches. Cambridge University Press, New York.
McIntosh, J.S. 1990b. Sauropoda, p. 345–401. In Weishampel, D.B., Dodson, P., and Osmólska, H. (eds.). The Dinosauria. University of California Press, Berkeley.
McIntosh, J.S. 1995. Remarks on the North American sauropod Apatosaurus Marsh. 6th Symposium on Mesozoic Terrestrial Ecosystems, Short Papers, 119–123.
McIntosh, J.S. 2005. The Genus Barosaurus Marsh (Sauropoda, Diplodocidae), p. 38–77. In Tidwell, V. and Carpenter, K. (eds.), Thunderlizards: the sauropodomorph dinosaurs. Indiana University Press, Bloomington, Indiana.
McIntosh, J.S. and Williams, M.E. 1988. A new species of sauropod dinosaur, Haplocanthosaurus delfsi sp. nov., from the Upper Jurassic Morrison Fm. of Colorado. Kirtlandia, 43:3–26.
McIntosh, J.S., and Carpenter, K. 1998. The holotype of Diplodocus longus, with comments on other specimens of the genus. Modern Geology, 23:85–110.
McIntosh, J.S., Coombs Jr., W.P., and Russell, D.A. 1992. A new diplodocid sauropod (Dinosauria) from Wyoming, USA. Journal of Vertebrate Paleontology, 12, 2:158–167.
https://doi.org/10.1080/02724634.1992.10011446
McPhee, B.W., Mannion, P.D., de Klerk, W.J., and Choiniere, J.N. 2016. High diversity in the sauropod dinosaur fauna of the Lower Cretaceous Kirkwood Formation of South Africa: implications for the Jurassic-Cretaceous transition. Cretaceous Research, 59:228–248.
https://doi.org/10.1016/j.cretres.2015.11.006
McPhee, B.W., Benson, R.B., Botha-Brink, J., Bordy, E.M., and Choiniere, J.N. 2018. A giant dinosaur from the earliest Jurassic of South Africa and the transition to quadrupedality in early sauropodomorphs. Current Biology, 28, 19:3143–3151.
https://doi.org/10.1016/j.cub.2018.07.063
Melstrom, K.M., D’emic, M.D., Chure, D., and Wilson, J.A. 2016. A juvenile sauropod dinosaur from the Late Jurassic of Utah, U.S.A., presents further evidence of an avian style air-sac system. Journal of Vertebrate Paleontology, 36:e1111898.
https://doi.org/10.1080/02724634.2016.1111898
Melstrom, K.M., Chiappe, L.M., and Smith, N.D. 2021. Exceptionally simple, rapidly replaced teeth in sauropod dinosaurs demonstrate a novel evolutionary strategy for herbivory in Late Jurassic ecosystems. BMC Ecology and Evolution, 21:202.
https://doi.org/10.1186/s12862-021-01932-4
Michelis, I. 2004. Taphonomie des Howe Quarry’s (Morrison-Formation, Oberer Jura), Bighorn County, Wyoming, USA. Unpublished PhD thesis, Institute of Palaeontology, University of Bonn.
Mocho, P., Royo-Torres, R., and Ortega, F. 2014. Phylogenetic reassessment of Lourinhasaurus alenquerensis, a basal Macronaria (Sauropoda) from the Upper Jurassic of Portugal. Zoological Journal of the Linnean Society, 170, 4, 875–916.
https://doi.org/10.1111/zoj.12113
Mook, C.C. 1917. Criteria for the determination of species in the Sauropoda, with description of a new species of Apatosaurus. Bulletin of the AMNH, 37:16.
Moore, A.J., Barrett, P.M., Upchurch, P., Liao, C.C., Ye, Y., Hao, B., and Xu, X. 2023. Re-assessment of the Late Jurassic eusauropod Mamenchisaurus sinocanadorum Russell and Zheng, 1993, and the evolution of exceptionally long necks in mamenchisaurids. Journal of Systematic Paleontology, 21,1:2171818.
https://doi.org/10.1080/14772019.2023.2171818
Mortimer, M. 2017. Comment (Case 3700) – a statement against the proposed designation of Diplodocus carnegii Hatcher, 1901 as the type species of Diplodocus Marsh, 1878 (Dinosauria, Sauropoda): The Bulletin of Zoological Nomenclature, 73, 2-4:129–131.
https://doi.org/10.21805/bzn.v73i2.a14
Nieuwland, I. 2019. American dinosaur abroad: a cultural history of Carnegie's plaster Diplodocus. University of Pittsburgh Press.
https://doi.org/10.2307/j.ctvh4zh5n
Nopcsa, F. 1902. Notizen über die Cretacischen Dinosaurier. Pt. 3. Wirbel eines südamerikanischen Sauropoden. Sitz Ber Aka Wissens, 3:108–114
Novas, F., Salgado, L., Calvo, J., and Agnolin, F. 2005. Giant titanosaur (Dinosauria, Sauropoda) from the Late Cretaceous of Patagonia. Revista del Museo Argentino de Ciencias Naturales Nueva Serie, 7, 1:31–36.
Osborn, H.F. and Mook, C.C. 1921. Camarasaurus, Amphicoelias, and other sauropods of Cope. Memoirs of the American Museum of Natural History, 3:247–387.
Ostrom, J.H. 1969. Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Peabody Museum of Natural History Bulletin 30:1–165.
Ostrom, J.O. and McIntosh, J.S. 1966. Marsh’s dinosaurs: the collection from Como Bluff, Volume 1. Yale University Press, New Haven.
Owen, R. 1841a. Odontography, Part II. Hippolyte Bailliere, London.
Owen, R. 1841b. A description of a portion of the skeleton of the Cetiosaurus, a gigantic extinct saurian reptile occurring in the oolitic formations of different portions of England. Proceedings of the Geological Society of London, 3, 80:457–462.
Owen, R. 1875. A monograph on the Fossil Reptilia of the Mesozoic Formations. Monograph on the Genus Bothriospondylus. Palaeontographical Society Monograph, 29:15–26.
https://doi.org/10.5962/bhl.title.100403
Pal, S. and Ayyasami, K. 2022. The lost titan of Cauvery. Geology Today, 38, 3:112–116.
https://doi.org/10.1111/gto.12390
Paulina Carabajal, A., Carballido, J.L., and Currie, P.J. 2014. Braincase, neuroanatomy, and neck posture of Amargasaurus cazaui (Sauropoda, Dicraeosauridae) and its implications for understanding head posture in sauropods. Journal of Vertebrate Paleontology, 34:870–882.
https://doi.org/10.1080/02724634.2014.838174
Pereda Suberbiola, X., Torcida, F., Izquierdo, L.A., Huerta, P., Montero, D., and Pérez, G. 2003. First rebbachisaurid dinosaur (Sauropoda, Diplodocoidea) from the Early Cretaceous of Spain: palaeobiogeographical implications. Bulletin de la Société Géologique de France, 174, 5:471–479.
https://doi.org/10.2113/174.5.471
Pereira, P.V.L.G.C., Veiga, I.M.M.G., Ribeiro, T.B., Cardozo, R.H.B., Candeiro C.R.A., and Bergqvist, L.P. 2020. The path of giants: a new occurrence of Rebbachisauridae (Dinosauria, Diplodocoidea) in the Açu Formation, NE Brazil, and its paleobiogeographic implications. Journal of South American Earth Sciences, 100:102515.
https://doi.org/10.1016/j.jsames.2020.102515
Pérez-Pueyo, M., Moreno-Azanza, M., Barco, J.L., and Canudo, J.I. 2019. New contributions to the phylogenetic position of the sauropod Galvesaurus herreroi from the Tithonian (Jurassic) of Spain (Teruel). Boletín Geológico y Minero, 130, 3:375–392.
https://doi.org/10.21701/bolgeomin.130.3.001
Peterson, J.E., Lovelace, D., Connely, M., and McHugh, J.B. 2022. A novel feeding mechanism of diplodocid sauropods revealed in an Apatosaurine skull from the Upper Jurassic Nail Quarry (Morrison Formation) at Como Bluff, Wyoming, USA. Palaeontologia Electronica, 25.2.a21.
https://doi.org/10.26879/1216
Peterson, O.A. and Gilmore, C.W. 1902. Elosaurus parvus: a new genus and species of the Sauropoda. Annals of the Carnegie Museum, 1:490–499.
https://doi.org/10.5962/p.78087
Phillips, J. 1871. Geology of Oxford and the Valley of the Thames. Clarendon Press, Oxford.
Platt, B.F. and Hasiotis, S.T. 2006. Newly discovered sauropod dinosaur tracks with skin and foot-pad impressions from the Upper Jurassic Morrison Formation, Bighorn Basin, Wyoming, USA. Palaios, 21, 3:249–261.
https://doi.org/10.2110/palo.2004.p04-69
Pol, D., Otero, A., Apaldetti, C., and Martínez, R.N. 2021. Triassic sauropodomorph dinosaurs from South America: The origin and diversification of dinosaur dominated herbivorous faunas. Journal of South American Earth Sciences, 107:103145.
https://doi.org/10.1016/j.jsames.2020.103145
Preuschoft, H. and Klein, N. 2013. Torsion and bending in the neck and tail of sauropod dinosaurs and the function of cervical ribs: insights from functional morphology and biomechanics. PloS one, 8, 10:e78574.
https://doi.org/10.1371/journal.pone.0078574
Price, J.R. and Whitlock, J.A. 2022. Dental Histology of Diplodocus (Sauropoda, Diplodocoidea). Journal of Vertebrate Paleontology, 42, 1:e2099745.
https://doi.org/10.1080/02724634.2022.2099745
Rauhut, O.W. 1999. A dinosaur fauna from the Late Cretaceous (Cenomanian) of northern Sudan. Palaeontologia Africana 35, 61–84.
Rauhut, O.W., Remes, K., Fechner, R., Cladera, G., and Puerta, P. 2005. Discovery of a short-necked sauropod dinosaur from the Late Jurassic period of Patagonia. Nature, 435, 7042:670–672.
https://doi.org/10.1038/nature03623
Rauhut, O.W., Carballido, J.L., and Pol, D. 2015. A diplodocid sauropod dinosaur from the late Jurassic Canadon Calcareo formation of Chubut, Argentina. Journal of Vertebrate Paleontology, 35, 5:e982798.
https://doi.org/10.1080/02724634.2015.982798
Rauhut, O.W., Holwerda, F.M., and Furrer, H. 2020. A derived sauropodiform dinosaur and other sauropodomorph material from the Late Triassic of Canton Schaffhausen, Switzerland. Swiss Journal of Geosciences, 113:1–54.
https://doi.org/10.1186/s00015-020-00360-8
Rea, T. 2001. Bone Wars: The Excavation and Celebrity of Andrew Carnegie’s Dinosaur. University of Pittsburgh Press, Pittsburgh, PA.
Remes K. 2006. Revision of the Tendaguru sauropod dinosaur Tornieria africana (Fraas) and its relevance for sauropod paleobiogeography. Journal of Vertebrate Paleontology, 26:651–669.
https://doi.org/10.1671/0272-4634(2006)26[651:ROTTSD]2.0.CO;2
Remes, K. 2007. A second Gondwanan diplodocid dinosaur from the Upper Jurassic Tendaguru beds of Tanzania, East Africa. Palaeontology, 50, 3:653–667.
https://doi.org/10.1111/j.1475-4983.2007.00652.x
Remes, K. 2009. Taxonomy of Late Jurassic diplodocid sauropods from Tendaguru (Tanzania). Fossil Record, 12, 1:23–46.
https://doi.org/10.1002/mmng.200800008
Riggs, E.S. 1903. Structure and relationships of opisthocoelian dinosaurs. Part I: Apatosaurus Marsh. Field Columbian Museum, Geological Series 2, 4:165–196.
Riggs, E.S. 1904. Structure and relationships of opisthocoelian dinosaurs. Part II, the Brachiosauridae. Field Columbian Museum, Geological Series 2, 6:229–247.
Rivera-Sylva, H.E. and Espinosa-Arrubarrena, L. 2020. Remains of a diplodocoid (Sauropoda: Flagellicaudata) from the Otlaltepec Formation Middle Jurassic (Bathonian-Callovian) from Puebla, Mexico. Paleontología Mexicana, 9:145–150.
https://doi.org/10.22201/igl.05437652e.2020.9.2.173
Royo-Torres, R., Cobos, A., Mocho, P., and Alcalá, L. 2021. Origin and evolution of turiasaur dinosaurs set by means of a new ‘rosetta’ specimen from Spain. Zoological Journal of the Linnean Society, 191, 1:201–227.
https://doi.org/10.1093/zoolinnean/zlaa091
Salgado, L. 1999. The macroevolution of the Diplodocimorpha (Dinosauria; Sauropoda): a developmental model. Ameghiniana, 36:203–216.
Salgado, L. and Bonaparte, J.F. 1991. Un nuevo saurópodo Dicraeosauridae, Amargasaurus cazaui gen et sp. nov., de la Formación La Amarga, Neocomiano de la provincia del Neuquén, Argentina. Ameghiniana, 28:333–346.
Salgado, L., Coria, R.A., and Calvo, J.O. 1997. Evolution of titanosaurid sauropods. I: phylogenetic analysis based on the postcranial evidence. Ameghiniana 34:3–32.
Salgado, L., Garrido, A., Cocca, S.E., and Cocca, J.R. 2004. Lower Cretaceous rebbachisaurid sauropods from Cerro Aguada del León (Lohan Cura Formation), Neuquén Province, northwestern Patagonia, Argentina. Journal of Vertebrate Paleontology, 24, 4:903–912.
https://doi.org/10.1671/0272-4634(2004)024[0903:LCRSFC]2.0.CO;2
Salgado, L., Carvalho, I.D.S., and Garrido, A.C. 2006. Zapalasaurus bonapartei, a new sauropod dinosaur from La Amarga Formation (Lower Cretaceous), northwestern Patagonia, Neuquén Province, Argentina. Geobios, 39:695–707.
https://doi.org/10.1016/j.geobios.2005.06.001
Salgado, L., Novas, F.E., Suarez, M., De La Cruz, R., Isasi, M., Rubilar-Rogers, D., and Vargas, A. 2015. Late Jurassic sauropods in Chilean Patagonia. Ameghiniana, 52,4:418–429.
https://doi.org/10.5710/AMGH.07.05.2015.2883
Salgado, L., Gallina, P.A., Lerzo, L.N., and Canudo, J.I. 2022. Highly Specialized Diplodocoids: The Rebbachisauridae, p. 165–208. In Otero, A., Carballido, J.L., and Pol, D. (eds.), South American Sauropodomorph Dinosaurs. Springer Earth System Sciences. Springer International Publishing, Cham.
https://doi.org/10.1007/978-3-030-95959-3_5
Sander, P.M. 2013. An Evolutionary Cascade Model for Sauropod Dinosaur Gigantism - Overview, Update and Tests. PLoS ONE, 8:e78573.
https://doi.org/10.1371/journal.pone.0078573
Santos, V.F., Moratalla, J.J., and Royo-Torres, R. 2009. New sauropod trackways from the Middle Jurassic of Portugal. Acta Palaeontologica Polonica, 54, 3:409–422.
https://doi.org/10.4202/app.2008.0049
Scannella, J.B. and Horner, J.R. 2010. Torosaurus Marsh, 1891, is Triceratops Marsh, 1889 (Ceratopsidae: Chasmosaurinae): synonymy through ontogeny. Journal of Vertebrate Paleontology, 30, 4:1157–1168.
https://doi.org/10.1080/02724634.2010.483632
Schwarz-Wings, D. and Böhm, N. 2014. A morphometric approach to the specific separation of the humeri and femora of Dicraeosaurus from the Late Jurassic of Tendaguru, Tanzania. Acta Palaeontologica Polonica, 59, 1:81–98.
https://doi.org/10.4202/app.2011.0095
Schwarz, D., Frey, E., and Meyer, C.A. 2007. Pneumaticity and soft-tissue reconstructions in the neck of diplodocid and dicraeosaurid sauropods. Acta Palaeontologica Polonica, 52:167–188.
Schwarz, D., Kosch, J.C.D., Fritsch, G., and Hildebrandt, T. 2015. Dentition and tooth replacement of Dicraeosaurus hansemanni (Dinosauria, Sauropoda, Diplodocoidea) from the Tendaguru Formation of Tanzania. Journal of Vertebrate Paleontology, 35:e1008134.
https://doi.org/10.1080/02724634.2015.1008134
Schwarz, D., Mannion, P.D., Wings, O., and Meyer, C.A. 2020. Re-description of the sauropod dinosaur Amanzia (“Ornithopsis/Cetiosauriscus”) greppini n. gen. and other vertebrate remains from the Kimmeridgian (Late Jurassic) Reuchenette Formation of Moutier, Switzerland. Swiss Journal of Geosciences, 113, 1:1–48.
https://doi.org/10.1186/s00015-020-00355-5
Sereno, P.C. 1998. A rationale for phylogenetic definitions, with application to the higher-level taxonomy of Dinosauria. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 210, 1:41–83.
Sereno, P. and Wilson, J.A. 2005. Structure and evolution of a sauropod tooth battery, p. 157–177. In Curry Rogers, K. and Wilson, J.A. (eds.), The Sauropods: Evolution and Paleobiology. University of California Press.
Sereno, P.C., Beck, A.L., Dutheil, D.B., Larsson, H.C.E., Lyon, G.H., Moussa, B., Sadleir, R.W., Sidor, C.A., Varricchio, D.J., Wilson, G.P., and Wilson, J.A. 1999. Cretaceous Sauropods from the Sahara and the Uneven Rate of Skeletal Evolution Among Dinosaurs. Science, 286:1342–1347.
https://doi.org/10.1126/science.286.5443.1342
Sereno, P.C., Wilson, J.A., Witmer, L.M., Whitlock, J.A., Maga, A., Ide, O., and Rowe, T.A. 2007. Structural Extremes in a Cretaceous Dinosaur. PLoS ONE, 2:e1230.
https://doi.org/10.1371/journal.pone.0001230
Simón, M.E. and Salgado, L. 2025. New rebbachisaurid (Dinosauria, Sauropoda) from the Huincul Formation (upper Cenomanian-Turonian) of Villa El Chocón (Neuquén Province, Argentina). Cretaceous Research, 106137.
https://doi.org/10.1016/j.cretres.2025.106137
Sternfeld, R. 1911. Zur Nomenklatur der Gattung Gigantosaurus Fraas. Sitzungsberichte Der Gesellschaft Naturforschender Freunde Zu Berlin, 8:398.
Stevens, K.A. 2013. The articulation of sauropod necks: methodology and mythology. PLoS One, 8, 10:e78572.
https://doi.org/10.1371/journal.pone.0078572
Stevens, K.A. and Parrish, J.M. 1999. Neck posture and feeding habits of two Jurassic sauropod dinosaurs. Science, 284, 5415:798–800.
https://doi.org/10.1126/science.284.5415.798
Stevens, K.A. and Parrish, J.M. 2005a. Neck posture, dentition, and feeding strategies in Jurassic sauropod dinosaurs, p. 212–232. In Tidwell, V. and Carpenter, K. (eds), Thunder-Lizards: The Sauropodomorph Dinosaurs. Bloomington: Indiana University Press.
Stevens, K.A. and Parrish, J.M. 2005b. Digital reconstructions of sauropod dinosaurs and implications for feeding, p. 178–200. In Curry Rogers, K.A. and Wilson, J.A. (eds.), The Sauropods: Evolution and Paleobiology. University of California Press, Berkeley, California.
https://doi.org/10.1525/9780520932333-009
Sues, H.D., Averianov, A., Ridgely, R.C., and Witmer, L.M. 2015. Titanosauria (Dinosauria, Sauropoda) from the Upper Cretaceous (Turonian) Bissekty Formation of Uzbekistan. Journal of Vertebrate Paleontology, 35, 1:e889145.
https://doi.org/10.1080/02724634.2014.889145
Taylor, M.P. 2010. Sauropod dinosaur research: a historical review. p. 361–386. In Moody, R.T.J., Buffetaut, E., Naish, D. and Martill, D.M. (eds.), Dinosaurs and Other Extinct Saurians: a Historical Perspective. Geological Society of London, Special Publication, 343.
https://doi.org/10.1144/SP343.22
Taylor, M.P. 2014. Quantifying the effect of intervertebral cartilage on neutral posture in the necks of sauropod dinosaurs. PeerJ, 2:e712.
https://doi.org/10.7717/peerj.712
Taylor, M.P. 2017. Comment (Case 3700) – support for Diplodocus carnegii Hatcher, 1901 being designated as the type species of Diplodocus Marsh, 1878. The Bulletin of Zoological Nomenclature, 73, 2-4:134–135.
https://doi.org/10.21805/bzn.v73i2.a16
Taylor, M.P. 2018. Xenoposeidon is the earliest known rebbachisaurid sauropod dinosaur. PeerJ, 6, e5212.
https://doi.org/10.7717/peerj.5212
Taylor, M.P. and Naish, D. 2005. The phylogenetic taxonomy of Diplodocoidea (Dinosauria: Sauropoda). PaleoBios, 25:1–7.
Taylor, M.P. and Naish, D. 2007. An unusual new neosauropod dinosaur from the lower cretaceous hastings beds group of East Sussex, England. Palaeontology, 50, 6:1547–1564.
https://doi.org/10.1111/j.1475-4983.2007.00728.x
Taylor, M.P., Wedel, M.J., and Naish, D. 2009. Head and neck posture in sauropod dinosaurs inferred from extant animals. Acta Palaeontologica Polonica, 54, 2:213–220.
https://doi.org/10.4202/app.2009.0007
Taylor, M.P. and Wedel, M.J. 2012. Re-evaluating “Apatosaurus” minimus, a bizarre Morrison Formation sauropod with diplodocoid and macronarian features. p. 23. In Friedman. M. and Lloyd, G. (eds), Programme and Abstracts, 60th Annual Symposium of Vertebrate Palaeontology and Comparative Anatomy. University of Oxford, UK.
Taylor, M.P. and Wedel, M.J. 2013a. The effect of intervertebral cartilage on neutral posture and range of motion in the necks of sauropod dinosaurs. PLoS One, 8, 10:e78214.
https://doi.org/10.1371/journal.pone.0078214
Taylor, M.P. and Wedel, M.J. 2013b. Why sauropods had long necks; and why giraffes have short necks. PeerJ 1:e36.
https://doi.org/10.7717/peerj.36
Taylor, M.P. and Wedel, M.J. 2021. Why is vertebral pneumaticity in sauropod dinosaurs so variable. Qeios, 4:1–13.
https://doi.org/10.32388/1G6J3Q.5
Taylor, M.P., Sroka, S.D., and Carpenter, K. 2023. The Concrete Diplodocus of Vernal – a Cultural Icon of Utah. Geology of the Intermountain West, 10:65–91.
https://doi.org/10.31711/giw.v10.pp65-91
Taylor, M.P. and Wedel, M.J. 2023. Novel pneumatic features in the ribs of the sauropod dinosaur Brachiosaurus altithorax. Acta Palaeontologica Polonica 68, 4:709–718.
https://doi.org/10.4202/app.01105.2023
Taylor, M.P., Henrici, A.C., Church, L.J., Nieuwland, I., and Lamanna, M.C. 2025. The history and composition of the Carnegie Diplodocus. Annals of the Carnegie Museum, 91, 1:55–91.
Torcida Fernández-Baldor, F., Canudo, J.I., Huerta, P., Montero, D., Suberbiola, X.P., and Salgado, L. 2011. Demandasaurus darwini, a new rebbachisaurid sauropod from the Early Cretaceous of the Iberian Peninsula. Acta Palaeontologica Polonica, 56, 3:535–552.
https://doi.org/10.4202/app.2010.0003
Tornier, G. 1909. Wie war der Diplodocus carnegii wirklich gebaut. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin, 4, 193–209.
Tschopp, E. and Mateus, O. 2013. The skull and neck of a new flagellicaudatan sauropod from the Morrison Formation and its implication for the evolution and ontogeny of diplodocid dinosaurs. Journal of Systematic Palaeontology, 11, 7:853–888.
https://doi.org/10.1080/14772019.2012.746589
Tschopp, E., Mateus, O., and Benson, R.B.J. 2015. A specimen-level phylogenetic analysis and taxonomic revision of Diplodocidae (Dinosauria, Sauropoda). PeerJ 3:e857.
https://doi.org/10.7717/peerj.857
Tschopp, E. and Mateus, O. 2016. Case 3700 Diplodocus Marsh, 1878 (Dinosauria, Sauropoda): proposed designation of D. carnegii Hatcher, 1901 as the type species. The Bulletin of Zoological Nomenclature 73.1:17–24.
https://doi.org/10.21805/bzn.v73i1.a22
Tschopp, E. and Mateus, O. 2017. Osteology of Galeamopus pabsti sp. nov. (Sauropoda: Diplodocidae), with implications for neurocentral closure timing, and the cervico-dorsal transition in diplodocids. PeerJ, 5:e3179.
https://doi.org/10.7717/peerj.3179
Tschopp, E., Brinkman, D., Henderson, J., Turner, M.A., and Mateus, O. 2018. Considerations on the replacement of a type species in the case of the sauropod dinosaur Diplodocus Marsh, 1878. Geology of the Intermountain West, 5:245–262.
https://doi.org/10.31711/giw.v5.pp245-262
Tschopp, E., Maidment, S.C.R., Lamanna, M.C., and Norell, M.A. 2019. Reassessment of a Historical Collection of Sauropod Dinosaurs from the Northern Morrison Formation of Wyoming, with Implications for Sauropod Biogeography. Bulletin of the American Museum of Natural History, 437:1–79.
https://doi.org/10.1206/0003-0090.437.1.1
Tschopp, E., Mehling, C., and Norell, M.A. 2020. Reconstructing the specimens and history of Howe Quarry (Upper Jurassic Morrison Formation; Wyoming). American Museum Novitates, 2020, 3956:1–56.
https://doi.org/10.1206/3956.1
Tütken, T. 2011. The Diet of Sauropod Dinosaurs: Implications of Carbon Isotope Analysis on Teeth, Bones, and Plants, p. 57–79. In Klein, N., Remes, K., Gee, C.T., and Sander, P.M. (eds.), Biology of the sauropod dinosaurs. Understanding the life of giants. Indiana University Press, Bloomington, Indiana.
Upchurch, P. 1995. The evolutionary history of sauropod dinosaurs. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 349, 1330:365–390.
https://doi.org/10.1098/rstb.1995.0125
Upchurch, P. 1998. The phylogenetic relationships of sauropod dinosaurs. Zoological journal of the Linnean Society, 124, 1:43–103.
https://doi.org/10.1111/j.1096-3642.1998.tb00569.x
Upchurch, P. 1999. The phylogenetic relationships of the Nemegtosauridae (Saurischia, Sauropoda). Journal of Vertebrate Paleontology, 19, 1:106–125.
https://doi.org/10.1080/02724634.1999.10011127
Upchurch, P. 2000. Neck posture of sauropod dinosaurs. Science, 287, 5453:547–547.
https://doi.org/10.1126/science.287.5453.547b
Upchurch, P. and Barrett, P.M. 2000. The evolution of sauropod feeding mechanism. Evolution of Herbivory in Terrestrial Vertebrates. Cambridge University Press.
Upchurch, P., Barrett, P.M., and Dodson, P. 2004a. Sauropoda, p. 259–324. In Weishampel, D.B., Dodson, P., and Osmolska, H. (eds.), The Dinosauria, 2nd Edition. University of California Press, Berkeley.
Upchurch, P., Tomida Y., and Barrett, P.M. 2004b. A new specimen of Apatosaurus ajax (Sauropoda: Diplodocidae) from the Morrison Formation (Upper Jurassic) of Wyoming, USA. National Science Museum Monographs No. 26, Tokyo.
van der Linden, T.T.P., Tschopp, E., Sookias, R.B., Wallaard, J.J., Holwerda, F.M., and Schulp, A.S. 2024. A new diplodocine sauropod from the Morrison Formation, Wyoming, USA. Palaeontologia Electronica, 27(3):a50.
https://doi.org/10.26879/1380
Vidal, D., Ortega, F., and Sanz, J.L. 2020a. Sauropodomorph skeletal mounts as scientific devices for testing hypotheses. Journal of Iberian Geology, 46, 2:177–193.
https://doi.org/10.1007/s41513-020-00122-3
Vidal, D., Mocho, P., Aberasturi, A., Sanz, J.L., and Ortega, F. 2020b. High browsing skeletal adaptations in Spinophorosaurus reveal an evolutionary innovation in sauropod dinosaurs. Scientific Reports, 10, 1:6638.
https://doi.org/10.1038/s41598-020-63439-0
Wang, Y.M., Zhao, Q., and You, H.L. 2024. Reassessment of ‘Gyposaurus’ sinensis Young, 1941 (Dinosauria: Sauropodomorpha) from the Early Jurassic Lufeng Basin, Yunnan Province, China. Zoological Journal of the Linnean Society, zlae032.
https://doi.org/10.1093/zoolinnean/zlae032
Waskow, K. 2019. Patterns of life history recorded in the dorsal rib histology of amniotes. Unpublished PhD Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn.
Wedel, M.J. 2003. The evolution of vertebral pneumaticity in sauropod dinosaurs. Journal of Vertebrate Paleontology, 23, 2:344–357.
https://doi.org/10.1671/0272-4634(2003)023[0344:TEOVPI]2.0.CO;2
Wedel, M.J. and Taylor, M.P. 2013. Neural spine bifurcation in sauropod dinosaurs of the Morrison formation: Ontogenetic and phylogenetic implications. PalArch’s Journal of Vertebrate Palaeontology, 10:1–34.
Wedel, M.J. and Taylor, M.P. 2023. The biomechanical significance of bifurcated cervical ribs in apatosaurine sauropods. Vertebrate Anatomy Morphology Palaeontology, 11:91–100.
https://doi.org/10.18435/vamp29394
Whitlock, J.A. 2011a. A phylogenetic analysis of Diplodocoidea (Saurischia: Sauropoda). Zoological Journal of the Linnean Society 161, 4:872–915.
https://doi.org/10.1111/j.1096-3642.2010.00665.x
Whitlock, J.A. 2011b. Inferences of diplodocoid (Sauropoda: Dinosauria) feeding behavior from snout shape and microwear analyses. PLoS One, 6, 4:e18304.
https://doi.org/10.1371/journal.pone.0018304
Whitlock, J.A. 2011c. Re-evaluation of Australodocus bohetii, a putative diplodocoid sauropod from the Tendaguru Formation of Tanzania, with comment on Late Jurassic sauropod faunal diversity and palaeoecology. Palaeogeography, Palaeoclimatology, Palaeoecology, 309, 3-4:333–341.
https://doi.org/10.1016/j.palaeo.2011.07.001
Whitlock, J.A. 2017. Was Diplodocus (Diplodocoidea, Sauropoda) capable of propalinal jaw motion? Journal of Vertebrate Paleontology, 37:e1296457.
https://doi.org/10.1080/02724634.2017.1296457
Whitlock, J.A. and Wilson Mantilla, J.A. 2020. The Late Jurassic sauropod dinosaur ‘Morosaurus’ agilis Marsh, 1889 reexamined and reinterpreted as a dicraeosaurid. Journal of Vertebrate Paleontology 40:e1780600.
https://doi.org/10.1080/02724634.2020.1780600
Whitlock, J.A., Wilson, J.A., and Lamanna, M.C. 2010. Description of a nearly complete juvenile skull of Diplodocus (Sauropoda: Diplodocoidea) from the Late Jurassic of North America. Journal of Vertebrate Paleontology, 30, 2:442–457.
https://doi.org/10.1080/02724631003617647
Wiersma-Weyand, K., Canoville, A., Siber, H.-J., and Sander, P.M. 2021. Testing hypothesis of skeletal unity using bone histology: the case of the sauropod remains from the Howe-Stephens and Howe Scott quarries, Morrison Formation, Wyoming, USA. Palaeontologia Electronica, 24, 1:a10.
https://doi.org/10.26879/766
Wilhite, D.R. 2003. Biomechanical reconstruction of the appendicular skeleton in three North American Jurassic sauropods. PhD dissertation, Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College.
Wilhite D.R. 2005. Variation in the appendicular skeleton of North American sauropod dinosaurs: taxonomic implications, p. 268–301. In Tidwell, V. and Carpenter, K. (eds.), Thunder-lizards: the sauropodomorph dinosaurs. Bloomington: Indiana University Press.
Wilson, J.A. 2002. Sauropod dinosaur phylogeny: critique and cladistic analysis. Zoological Journal of the Linnean Society, 136, 2:215–275.
https://doi.org/10.1046/j.1096-3642.2002.00029.x
Wilson, J.A. 2005. Redescription of the Mongolian sauropod Nemegtosaurus mongoliensis Nowinski (Dinosauria: Saurischia) and comments on Late Cretaceous sauropod diversity. Journal of Systematic Palaeontology, 3, 3:283–318.
https://doi.org/10.1017/S1477201905001628
Wilson, J.A. and Sereno, P.C. 1998. Early evolution and higher-level phylogeny of sauropod dinosaurs. Journal of Vertebrate paleontology, 18, S2:1–79.
https://doi.org/10.1080/02724634.1998.10011115
Wilson, J.A. and Upchurch, P. 2003. A revision of Titanosaurus Lydekker (Dinosauria‐Sauropoda), the first dinosaur genus with a ‘Gondwanan’ distribution. Journal of Systematic Palaeontology, 1, 3:125–160.
https://doi.org/10.1017/S1477201903001044
Wilson, J.A. and Allain, R. 2015. Osteology of Rebbachisaurus garasbae Lavocat, 1954, a diplodocoid (Dinosauria, Sauropoda) from the early Late Cretaceous-aged Kem Kem beds of southeastern Morocco. Journal of Vertebrate Paleontology, 35, 4:e1000701.
https://doi.org/10.1080/02724634.2014.1000701
Windholz, G.J., Baiano, M.A., Bellardini, F., and Garrido, A. 2021. New Dicraeosauridae (Sauropoda, Diplodocoidea) remains from the la amarga formation (Barremian-aptian, lower cretaceous), Neuquén Basin, Patagonia, Argentina. Cretaceous Research, 117:104629.
https://doi.org/10.1016/j.cretres.2020.104629
Windholz, G.J., Coria, R.A., Bellardini, F., Baiano, M.A., Pino, D.A., Ortega, F., and Currie, P. 2022. On a dicraeosaurid specimen from the Mulichinco Formation (Valanginian, Neuquén Basin) of Argentina and phylogenetic relationships of the South American dicraeosaurids (Sauropoda, Diplodocoidea). Comptes Rendus Palevol 21:991–1019.
https://doi.org/10.5852/cr-palevol2022v21a45
Windholz, G.J., Carballido, J.L., Coria, R.A., Zurriaguz, V.L., and Rauhut, O.W. 2023. How pneumatic were the presacral vertebrae of dicraeosaurid (Sauropoda: Diplodocoidea) dinosaurs? Biological Journal of the Linnean Society, 138, 1:103–120.
https://doi.org/10.1093/biolinnean/blac131
Witmer, L.M. 2001. Nostril position in dinosaurs and other vertebrates and its significance for nasal function. Science, 293, 553:850–853.
https://doi.org/10.1126/science.1062681
Woodruff, D.C. 2016. Nuchal ligament reconstructions in diplodocid sauropods support horizontal neck feeding postures. Historical Biology, 29, 3:1–12.
https://doi.org/10.1080/08912963.2016.1158257
Woodruff, D.C. 2017. Comment (Case 3700) – support for the proposed designation of Diplodocus carnegii Hatcher, 1901, as type species of Diplodocus Marsh, 1878: The Bulletin of Zoological Nomenclature, 73, 2-4:12–127.
https://doi.org/10.21805/bzn.v73i2.a12
Woodruff, D.C. 2019. What factors influence our reconstructions of Morrison Formation sauropod diversity? Geology of the Intermountain West, 6:93–112.
https://doi.org/10.31711/giw.v6.pp93-112
Woodruff, D.C. and Fowler, D.W. 2012. Ontogenetic influence on neural spine bifurcation in Diplodocoidea (Dinosauria: Sauropoda): a critical phylogenetic character. Journal of Morphology, 273, 7:754–764.
https://doi.org/10.1002/jmor.20021
Woodruff, D.C. and Foster, J.R. 2014. The fragile legacy of Amphicoelias fragillimus (Dinosauria: Sauropoda; Morrison Formation – Latest Jurassic). Volumina Jurassica, 12, 2:211–220.
Woodruff, D.C., Fowler, D.W., and Horner, J. 2017. A new multi-faceted framework for deciphering diplodocid ontogeny. Palaeontologia Electronica, 20.3.43A:1–53.
https://doi.org/10.26879/674
Woodruff, D.C., Carr, T.D., Storrs, G.W., Waskow, K., Scannella, J.B., Nordén, K.K., and Wilson, J.P. 2018. The smallest diplodocid skull reveals cranial ontogeny and growth-related dietary changes in the largest dinosaurs. Scientific Reports, 8, 1:14341.
https://doi.org/10.1038/s41598-018-32620-x
Woodruff, D.C., Wolff, E.D., Wedel, M.J., Dennison, S., and Witmer, L.M. 2022. The first occurrence of an avian-style respiratory infection in a non-avian dinosaur. Scientific Reports, 12, 1:1954.
https://doi.org/10.1038/s41598-022-05761-3
Woodruff, D.C., Curtice, B.D., and Foster, J.R. 2024. Seis‐ing up the Super‐Morrison formation sauropods. Journal of Anatomy, 00:1–17.
https://doi.org/10.1111/joa.14108
Xu, X., Upchurch, P., Mannion, P.D., Barrett, P.M., Regalado-Fernandez, O.R., Mo, J., Ma, J., and Liu, H. 2018. A new Middle Jurassic diplodocoid suggests an earlier dispersal and diversification of sauropod dinosaurs. Nature Communications, 9:2700.
https://doi.org/10.1038/s41467-018-05128-1
Young, M.T., Rayfield, E.J., Holliday, C.M., Witmer, L.M., Button, D.J., Upchurch, P., and Barrett, P.M. 2012. Cranial biomechanics of Diplodocus (Dinosauria, Sauropoda): testing hypotheses of feeding behaviour in an extinct megaherbivore. Naturwissenschaften, 99:637–643.
https://doi.org/10.1007/s00114-012-0944-y
Yu, C. 1993. Reconstruction of the skull of Diplodocus and the phylogeny of the Diplodocidae (Dinosauria: Sauropoda). PhD dissertation, University of Chicago, Chicago, USA.

