 Late Messinian mollusks and vertebrates from Moncucco Torinese, north-western Italy. Paleoecological and paleoclimatological implications
Late Messinian mollusks and vertebrates from Moncucco Torinese, north-western Italy. Paleoecological and paleoclimatological implications
Article number: 20.1.10A
https://doi.org/10.26879/658
Copyright Palaeontological Association, March 2017
Author biographies
Plain-language and multi-lingual abstracts
PDF version
Submission: 10 March 2017. Acceptance: 9 February 2017
{flike id=1749}
ABSTRACT
The systematic analysis of more than 20,000 fossils (Vertebrata and Mollusca), recovered from the post-evaporitic Messinian (5.41-5.33 Ma) succession of Moncucco Torinese (NW Italy), resulted in the identification of 90 vertebrate and 65 mollusk taxa that provide additional information about the paleoecological context and the paleoenvironmental settings of NW Italy slightly before the Mio-Pliocene boundary. Our analyses indicate a landscape dominated by open woodlands within a mosaic environment also including closed canopy forests, grasslands, rocky outcrops and limited water edges. The wide spectrum of habitats may have had a prominent role in determining the high paleobiodiversity observed in the paleocommunity of Moncucco Torinese. Slight variations in the abundances of the most common rodent species over the investigated succession are probably related to local changes in the paleolandscape. From a paleoclimatic point of view, the overall information provided by the fauna indicates mesic conditions in a subtropical climate, which is also consistent with the interpretation derived from paleobotanical and sedimentological analyses for the latest Messinian of Northern Italy.
Simone Colombero. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy. simone.colombero@unito.it
David M. Alba. Institut Català de Paleontologia Miquel Crusafont, Universitat Autònoma de Barcelona, Edifici ICTA-ICP, Carrer de les Columnes s/n, Campus de la UAB, E-08193 Cerdanyola del Vallès, Barcelona, Spain. david.alba@icp.cat
Carmine D’Amico. Dipartimento di Bioscienze e Territorio, Università degli Studi del Molise, Via C. da Fonte Lappone, I-86090 Pesche (Isernia), Italy. crmn.damico@gmail.com
Massimo Delfino. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy
and Institut Català de Paleontologia Miquel Crusafont, Universitat Autònoma de Barcelona, Edifici ICTA-ICP, Carrer de les Columnes s/n, Campus de la UAB, E-08193 Cerdanyola del Vallès, Barcelona, Spain. massimo.delfino@unito.it
Daniela Esu. Dipartimento di Scienze della Terra, Sapienza Università di Roma, Piazzale A. Moro 5, I-00185 Roma, Italy. daniela.esu@uniroma1.it
Piero Giuntelli. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy. pierogiuntelli@virgilio.it
Mathias Harzhauser. Geological-Paleontological Department, Natural History Museum Vienna, Burgring 7, A-1010 Vienna, Austria. mathias.harzhauser@nhm-wien.ac.at
Paul P.A. Mazza. Dipartimento di Scienze della Terra, Università degli Studi di Firenze, Via La Pira 4, 50121 Firenze, Italy. paul.mazza@unifi.it
Michele Mosca. Dipartimento di Economia e Statistica "Cognetti de Martiis", Università degli Studi di Torino, Lungo Dora Siena 100, 10153 Torino, Italy. michele.mosca@unito.it
Thomas A. Neubauer. Department of Animal Ecology & Systematics, Justus Liebig Universit, Heinrich-Buff-Ring 26-32 IFZ - 35392 Giessen, Germany. Thomas.a.neubauer@allzool.bio.uni-giessen.de
Giulio Pavia. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy giulio.pavia@unito.it
Marco Pavia. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy. marco.pavia@unito.it
Andrea Villa. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy. a.villa@unito.it
Giorgio Carnevale. Dipartimento di Scienze della Terra, Università degli Studi di Torino, Via Valperga Caluso 35, 10125 Torino, Italy. giorgio.carnevale@unito.it
Keywords: Paleoecology; Miocene; Messinian; Turolian; Italy; Vertebrata; Mollusca
Final citation: Colombero, Simone, Alba, David M., D’Amico, Carmine, Delfino, Massimo, Esu, Daniela, Giuntelli, Piero, Harzhauser, Mathias, Mazza, Paul P.A., Mosca, Michele, Neubauer, Thomas A., Pavia, Giulio, Pavia, Marco, Villa, Andrea, and Carnevale, Giorgio. 2017. Late Messinian mollusks and vertebrates from Moncucco Torinese, north-western Italy. Paleoecological and paleoclimatological implications. Palaeontologia Electronica 20.1.10A: 1-66. https://doi.org/10.26879/658
palaeo-electronica.org/content/2017/1749-moncucco-torinese-paleoecology
INTRODUCTION
Continental fossiliferous localities of late Messinian age are extremely rare in central Europe (sensu de Bruijn et al., 1992) and the record is thus very incomplete. This has serious impacts on the biochronological and paleobiogeographical correlations between different European faunal contexts. In recent years, newly discovered Italian localities provided information that partially bridges the chasm of knowledge. Until recently, the vertebrate fossil record ffrom the late Miocene of Italy have consisted solely of endemic faunal complexes mainly from two isolated areas: the Apulo-Abruzzi paleobioprovince (e.g., Freudenthal, 1971; Mazza and Rustioni, 2011; Delfino and Rossi, 2013; Masini et al., 2013; Meijer, 2013; Patacca et al., 2013; Pavia, 2013; Villier and Carnevale, 2013; Villier et al., 2013; Savorelli et al., 2016; Pavia et al., 2017) and the Tusco-Sardinian paleobioprovince (e.g., Engesser, 1989; Abbazzi et al., 2008a; Rook et al., 2011). However, some of the newly discovered Italian fossil localities yield non-endemic faunal assemblages. A few of them are from central Italian Messinian basins on the western side of Northern Apennines, such as the Tuscan basins of Baccinello-Cinigiano (Baccinello V3) (Rook et al., 2011), Valdelsa (Borro Strolla) (Abbazzi et al., 2008b), Casino (Abbazzi et al., 2008b) and Velona (Ghetti et al., 2002). Other rich faunal assemblages come from the upper Messinian karstic complex of the Monticino gypsum quarry near Brisighella, in Romagna. In Piedmont (NW Italy), two new sites, Verduno (Colombero et al., 2013) and Moncucco Torinese (Angelone et al., 2011), have recently been added to that of Ciabòt Cagna (Cavallo et al., 1993).
The fossiliferous deposits of Piedmont are referred to the late Messinian (Cavallo et al., 1993; Angelone et al., 2011; Colombero et al., 2013). The recently discovered sites from Piedmont are particularly important first, because Piedmont occupies a geographical position that was a crucial crossroads for faunas dispersing towards the emerging Apennine chain and peninsular territory of Italy, and second, because of the exceptional richness and variety of the Verduno and Moncucco Torinese fossil assemblages. The Verduno fossil record has been the subject of a recent extensive analysis (Colombero et al., 2014). The assemblage has tentatively been correlated to stage 3.1 (5.55-5.41 Ma) of the Messinian Salinity Crisis (MSC) (sensu Roveri et al., 2014). The fossil vertebrates from Moncucco Torinese have been cursorily described by Angelone et al. (2011). Later papers on the cercopithecid (Alba et al., 2014) and rodent (Colombero et al., 2014b, 2015; Colombero and Carnevale, 2016) remains as well as on non-marine gastropods (Harzhauser et al., 2015) of this site further confirmed the high biochronological and paleobiogeographical relevance of the Moncucco Torinese fauna. New excavations, conducted between 2012 and 2014, unearthed a huge amount of fossil vertebrate remains. The aim of this paper is to reconstruct the paleobiodiversity of the northwestern Italian mainland at the end of the Messinian based on a comprehensive analysis of the mollusk and vertebrate remains from Moncucco Torinese. This paper represents the outcomes of an in-depth case study of the paleoecological and paleoclimatic indications given by this outstanding Miocene terrestrial faunal community.
LOCALITY AND STRATIGRAPHY
Geology and Stratigraphy
 The uppermost Messinian locality of Moncucco Torinese (MCC) is located in the northern part of the Tertiary Piedmont Basin (TPB), along the southern slope of the Torino Hill (Figure 1). The TPB is an episutural basin masking the Alp-Apennine junction, which is filled with Eocene-to-Messinian deposits (Mosca et al., 2010; Dela Pierre et al., 2011). The Messinian succession is particularly well-exposed on the Torino Hill-side area as well as in the Langhe domain, which are situated in the northern and southern parts of the Tertiary Piedmont Basin, respectively. It has been thoroughly studied over the last few years and turned out to be particularly helpful in the interpretation of the events related to the MSC in the marginal basins of the peri-Mediterranean area (Dela Pierre et al., 2007, 2011, 2012; Natalicchio et al., 2014). The Messinian succession of the Tertiary Piedmont Basin (TPB) has been correlated in detail with the main events of the Messinian Salinity Crisis (Dela Pierre et al., 2007, 2011). In the TPB, the Messinian sequence starts with the pre-evaporitic deep-water marine marls of the Marne di Sant’Agata Fossili Formation. The latter are followed by alternated gypsum and clay beds of the Primary Lower Gypsum, which deposited in the evaporitic stage between 5.96 and 5.60 Ma (CIESM, 2008; Roveri et al., 2014). The Valle Versa Chaotic Complex (5.60-5.55 Ma) unconformably overlies the Primary Lower Gypsum. The Messinian succession is capped by the fluvial and brackish sediments of the Conglomerati di Cassano Spinola Formation.
The uppermost Messinian locality of Moncucco Torinese (MCC) is located in the northern part of the Tertiary Piedmont Basin (TPB), along the southern slope of the Torino Hill (Figure 1). The TPB is an episutural basin masking the Alp-Apennine junction, which is filled with Eocene-to-Messinian deposits (Mosca et al., 2010; Dela Pierre et al., 2011). The Messinian succession is particularly well-exposed on the Torino Hill-side area as well as in the Langhe domain, which are situated in the northern and southern parts of the Tertiary Piedmont Basin, respectively. It has been thoroughly studied over the last few years and turned out to be particularly helpful in the interpretation of the events related to the MSC in the marginal basins of the peri-Mediterranean area (Dela Pierre et al., 2007, 2011, 2012; Natalicchio et al., 2014). The Messinian succession of the Tertiary Piedmont Basin (TPB) has been correlated in detail with the main events of the Messinian Salinity Crisis (Dela Pierre et al., 2007, 2011). In the TPB, the Messinian sequence starts with the pre-evaporitic deep-water marine marls of the Marne di Sant’Agata Fossili Formation. The latter are followed by alternated gypsum and clay beds of the Primary Lower Gypsum, which deposited in the evaporitic stage between 5.96 and 5.60 Ma (CIESM, 2008; Roveri et al., 2014). The Valle Versa Chaotic Complex (5.60-5.55 Ma) unconformably overlies the Primary Lower Gypsum. The Messinian succession is capped by the fluvial and brackish sediments of the Conglomerati di Cassano Spinola Formation.  This lithostratigraphic unit is primarily characterized by clays, silts, sands and gravels that deposited between 5.55 and 5.33 Ma. This time period corresponds to the post-evaporitic phase of the Messinian Salinity Crisis, which slightly precedes the Mio-Pliocene boundary (Figure 2). It is partially associated with the so-called Lago-Mare event, when the Mediterranean experienced cyclic water salinity variations and is believed to have progressively established a stable connection with the Atlantic Ocean (CIESM, 2008; Cita et al., 1978; McCulloch and De Deckker, 1989; Carnevale et al., 2006a, 2006b; Do Couto et al., 2014). The fossiliferous layers of MCC are located at the top of a gypsum quarry. The investigated succession consists of nine layers (MCC1-MCC9, Figure 2), some of which contain a very abundant and extremely diverse fossil assemblage, with ostracods, mollusks and small and large vertebrates (Angelone et al., 2011; Colombero and Pavia, 2013; Alba et al., 2014; Colombero et al., 2014a, 2014b; Harzhauser et al., 2015; Grunert et al. 2016). Layers 4, 5 and 7 are constituted by several conglomerate lenses. During the sampling process, the lowermost parts of layers 4 and 5 were labeled MCC3/4 and MCC4/5, respectively. Vertebrate fossils are found only in the assemblages MCC3, MCC3/4, MCC4, MCC/4, MCC5 and MCC7. Nonetheless, layer MCC1, though barren of vertebrate remains, contains a moderately rich ostracod assemblage, which correlates with the Loxocorniculina djafarovi assemblage (Amnicythere propinqua, Cyprideis agrigentina, Loxoconcha kochi; Angelone et al., 2011), thereby implying that the overlying investigated succession is not older than 5.41 Ma (see Grossi et al., 2011). The Messinian succession is capped by the Zanclean marine clays of the Argille Azzurre Formation (Violanti et al., 2011). Therefore, the age of the fossiliferous layers is constrained between 5.41 and 5.33 Ma, within stage 3.2 of the MSC (sensu Roveri et al., 2014). However, the whole fossiliferous succession was likely deposited in a much shorter time lapse, based on the taphonomic features of the fauna as well as on the consistently stable taxonomical content of the fossiliferous layers. This implies that only minor paleoenvironmental changes occurred during the deposition of the fossiliferous succession.
This lithostratigraphic unit is primarily characterized by clays, silts, sands and gravels that deposited between 5.55 and 5.33 Ma. This time period corresponds to the post-evaporitic phase of the Messinian Salinity Crisis, which slightly precedes the Mio-Pliocene boundary (Figure 2). It is partially associated with the so-called Lago-Mare event, when the Mediterranean experienced cyclic water salinity variations and is believed to have progressively established a stable connection with the Atlantic Ocean (CIESM, 2008; Cita et al., 1978; McCulloch and De Deckker, 1989; Carnevale et al., 2006a, 2006b; Do Couto et al., 2014). The fossiliferous layers of MCC are located at the top of a gypsum quarry. The investigated succession consists of nine layers (MCC1-MCC9, Figure 2), some of which contain a very abundant and extremely diverse fossil assemblage, with ostracods, mollusks and small and large vertebrates (Angelone et al., 2011; Colombero and Pavia, 2013; Alba et al., 2014; Colombero et al., 2014a, 2014b; Harzhauser et al., 2015; Grunert et al. 2016). Layers 4, 5 and 7 are constituted by several conglomerate lenses. During the sampling process, the lowermost parts of layers 4 and 5 were labeled MCC3/4 and MCC4/5, respectively. Vertebrate fossils are found only in the assemblages MCC3, MCC3/4, MCC4, MCC/4, MCC5 and MCC7. Nonetheless, layer MCC1, though barren of vertebrate remains, contains a moderately rich ostracod assemblage, which correlates with the Loxocorniculina djafarovi assemblage (Amnicythere propinqua, Cyprideis agrigentina, Loxoconcha kochi; Angelone et al., 2011), thereby implying that the overlying investigated succession is not older than 5.41 Ma (see Grossi et al., 2011). The Messinian succession is capped by the Zanclean marine clays of the Argille Azzurre Formation (Violanti et al., 2011). Therefore, the age of the fossiliferous layers is constrained between 5.41 and 5.33 Ma, within stage 3.2 of the MSC (sensu Roveri et al., 2014). However, the whole fossiliferous succession was likely deposited in a much shorter time lapse, based on the taphonomic features of the fauna as well as on the consistently stable taxonomical content of the fossiliferous layers. This implies that only minor paleoenvironmental changes occurred during the deposition of the fossiliferous succession.
Taphonomic Notes
Angelone et al. (2011) performed a taphonomical analysis of the Messinian post-evaporitic assemblages of MCC. Just summary conclusions of that work are reported herein. All the bones of large and small vertebrates are well preserved and exhibit the same coloration (dark orange to dark brown). The hard parts of both small and large mammals are largely disarticulated and unabraded. Moreover, the absent or minimal degree of weathering, associated with the presence of fresh bone fractures, indicate relatively rapid burial after limited transportation. Angelone et al. (2011) therefore assumed a syn-sedimentary accumulation of the fossil vertebrate remains of MCC with the embedding sediment.
MATERIAL AND METHODS
The fossils described herein were unearthed during extensive excavations conducted between 2007 and 2014 by the paleontologists of the Dipartimento di Scienze della Terra of the Università degli Studi di Torino. Large vertebrate remains were consolidated with Paraloid B72, recovered and later on restored. Fossils of small vertebrates and mollusks were obtained by screen-washing the embedding sediments. The vertebrate and mollusk fossil remains studied in this work derive from six different assemblages (MCC3, MCC3/4, MCC4, MCC4/5, MCC5, MCC7) (Figure 2). A few fossils could not be assigned to a particular level and are labeled as “unassigned”. More than 18 tons of sediments were screen-washed. The identified fossil specimens include fish (180 otoliths), amphibian (886), reptile (11,383), bird (377), large (164) and small mammal (3,685) remains, as well as gastropod (3,300) and bivalve (1,600) shells. All the specimens are stored in the collections of the Museo di Geologia e Paleontologia of the Università degli Studi di Torino (Italy). The specimens are labeled with the abbreviation MGPT-PU. Measurements of the large mammal and avian remains were taken with a digital caliper Borletti CDEP15 to the nearest 0.02 mm. Small mammal teeth were measured with the digital measurement tools of the Leica Application Suite V 3.3 of a stereomicroscope Leica M205C. Anatomical nomenclature follows Sanchiz (1998) for anurans, Szyndlar (1984) for snakes, Baumel and Witmer (1993) for birds and von den Driesch (1976) for mammals. Nomenclature and measurements of small mammals follow Van de Weerd (1976), Daams (1981), Reumer (1984), Cuenca Bescós (2003) and Marivaux et al. (2004). Images of small specimens (smaller than 3 cm) were taken with a scanning electron microscope Cambridge S-360 of the Dipartimento di Scienze della Terra, Torino, or with a digital camera applied to a Leica M205C stereomicroscope. Images of the gastropods were taken with the SEM JEOL JSM 6610-LV (for specimens with a diameter < c. 1 cm) or the Nikon D700 at the Natural History Museum, Vienna.
Paleoecological Reconstruction
Paleoecological requirements of the terrestrial vertebrate taxa (Appendix 1) have been founded on the definitions given by several authors including Evans et al. (1981), Cuenca Bescós et al. (2005) and Andrews (2006). We have identified six habitat systems (HS) based on vegetation cover, presence of freshwater and type of substrate:
- closed canopy forests: forests with well-interconnected canopy and shaded herbaceous understory;
- woodlands/bushlands: areas with well-developed understory and gaps and clearings in the canopy. This category roughly corresponds to the woodland-bushland habitat used by Evans et al. (1981) to define the habitat spectra of the Miocene faunas of Western Kenya; it contains the woodland and bushland habitat systems described by Andrews (2006). In this paper it also includes degraded woodland habitats, such as forest edges but also semi-open successional stages, which comprise open forests and bushlands. The wide range of habitats was dictated by our dealing with extinct taxa whose ecological requirements can be only inferred or partially defined. Andrews (2006) advised that (degraded) woodland-adapted mammals are also frequently found in bushlands. Hence, separating bushland and open-woodland faunas is often quite problematic (Reed and Rector, 2007);
- grasslands: following Andrews (2006), grasslands are areas with dominant grasses with less than 20% of tree cover and shrubs, or no such cover at all. Humid and dry meadows are not distinguished from one another, primarily because some of the extinct taxa that frequented grasslands cannot be discriminated for such paleoecological requirements;
- rocky outcrops: barren, rocky landscapes;
- (semi)aquatic/water edges: freshwater basins and their surroundings; and
- sandy/soft soil: areas with a substrate suitable for burrowing.
Each of these six categories may include several habitat types, based on the wide ecological preferences of some taxa (Andrews, 2006). Small mammals are used to infer in detail the spectrum of habitat types that existed in the proximity of MCC. The very abundant and varied remains of these animals have been the subject of past detailed analyses (Colombero and Pavia, 2013; Colombero et al., 2014b, 2015). We follow Martín-Suárez et al. (2001), López Antoñanzas and Cuenca Bescos (2002) and García Alix et al. (2008), in order to define the criteria of assignment of the paleocological requirements of small mammals. The Habitat Weighting Method, which is related to the Taxonomic Habitat Index and Weighting Average methods (Evans et al., 1981; Andrews, 2006; Blain et al., 2008) has been used to avoid excessively restricted habitat assignments to the extinct small mammal taxa. Analogous methodologies had been previously applied to Miocene (Evans et al., 1981) and Pliocene or Pleistocene mammalian faunas (Andrews, 2006; Piñero et al., 2015). In particular, the Habitat Weighting Method has been previously used also with several extinct genera of small mammals (Piñero et al., 2015). The method of the score assignment follows Andrews et al. (1981) and Piñero et al. (2015). Summarizing, we assign a maximum possible score of 1 to each small mammal taxon. Then, we split this value according to the habitat preference of that taxon based on the inferred paleoecological requirements. For example, since Apodemus gudrunae occurred in both woodlands/bushlands and in rocky outcrops it was scored 0.5 in each of these HS. In order to detect possible variations in the habitat spectrum among the fossil assemblages, we followed Blain et al. (2008) and Piñero et al. (2015) in evaluating the incidence of a single HS in each fossil assemblage. The frequency of each HS was assessed adding up the values obtained by multiplying the relative abundances of each taxon by the score assigned to that taxon in that HS. We measured the relative abundances of the small mammals on the base of the nNISP (normalized Number of Identified Specimens) (see García-Alix et al., 2008). This methodology is devised to limit the overrepresentation of the taxa: the number of specimens of each small mammal taxon is divided by the number of the potential diagnostic elements of each taxon as follows: Leporidae, 28 derived from 26 dental elements (I2 excluded) plus two astragali; Ochotonidae, 24 dental elements (I2 excluded); Parasorex aff. ibericus, 42 dental elements; Soricidae, 20 dental elements; Talpidae, 46 derived from 44 dental elements plus two humeri; Muridae and Cricetidae, 12 molars; Gliridae and Sciuridae, 16 cheek teeth; Castoridae, 20 dental elements. Hystricidae have been excluded from this procedure, because they are represented only by a single fragmentary postcranial bone, which was recovered during manual excavation. In regards to the paleoecological requirements of the small mammals, they will be thoroughly discussed in the chapter “Paleoecology of small mammals.”
Paleoclimatology
Paleoclimatic parameters have been estimated using different methodologies based on small mammals as proxies. We follow Van Dam’s (2006) approach, which is based on several of the small mammal taxa, excluding bats, neomyine soricids and Castoridae (see Van Dam, 2006), to infer paleoprecipitation values (MAP=Mean Annual Precipitation; MINP= Precipitation in the driest month). We follow Montuire et al.’s (2006) method to estimate paleotemperatures (MAT=Mean Annual Temperature) using data from murids. Finally, identified rodent species permitted to estimate both MAP and MAT, as well as several other climatic parameters (MTW=Mean Temperature of the Warmest month; MTC=Mean Temperature of the Coldest month), according to the methodology developed by Hernández Fernández (2001). The definitions of the climate zones used in this paper follow Hernández Fernández et al. (2007). Hernández Fernández (2001), Hernández Fernández and Peláez-Campomanes (2003) and Hernández Fernández et al. (2007) were followed for the bioclimatic characterization of the extinct rodents, with few additions and modifications:
- Paraethomys meini is assigned to climate zones II, II/III, III and IV (see “Paleoecology of small mammals” for further details);
- Centralomys benericettii is assigned to climate zones II, II/III and IV (see “Paleoecology of small mammals” for further details);
- similarly to Allocricetus and Apocricetus, the genus Neocricetodon is assigned to climate zones IV, VI and VII; and
- Glis minor and Muscardinus vireti are assigned not only to climate zone VI but also to the climate IV, because the present Mediterranean distribution of Glis and Muscardinus includes the Italian peninsula as well as the Balkans (Panchetti et al., 2004; Capizzi and Filippucci, 2008).
Current climatic and weather data are copyright of Weatherbase (www.weatherbase.com, Frischling, 2016). Diversity indices of small mammals (Shannon Weaver Index) have been calculated with the software PAST v. 3.08 (Hammer et al., 2001).
Statistical Analyses
Murids are the most abundant mammals at MCC. They are represented by 2406 specimens belonging to seven taxa. They had been previously studied in great detail by Colombero and Pavia, (2013) and Colombero et al. (2014a). This large database has been used to verify possible variations in the structure of the original murid communities across the studied succession. The significance of the differences between the samples of fossil murids was assessed using Chi-square tests in PAST. The major purpose was also to verify if there are significantly different incidences across all the assemblages and to evaluate how and when the probability of finding a particular murid taxon at MCC varied across the succession. For this, we used Binary Logistic Regression (see Hosmer et al., 2013) to estimate the probability of finding one specific taxon at MCC. Seven logistic models were created, one for each murid taxon. The dataset used for our analyses derives from the murid collection of MCC. Binary Logistic Regression was performed using SAS/STAT ®9.3 (SAS Institute, Carey, NC, USA, SAS Institute Inc., 2011). The predictor variables of the models were the fossil assemblages (MCC3, MCC3/4, MCC4, MCC4/5, MCC5, MCC7). In this view, the coefficients of our models indicate the weight of each fossil assemblage in influencing the probability of finding a specific taxon in the whole succession of MCC. Since the assemblages are progressively younger from MCC3 to MCC7, the analyses of the coefficients reveal how the structure of the murid community varied across the studied succession. For each model (i.e., for each taxon), we assigned a value of 1 to each specimen of a given taxon found in a specific assemblage, whereas 0 was assigned to all the other murid specimens. The Beta coefficients of each predictor variable (= the assemblage in which murid specimens were found) were estimated to fit a logistic function given the data. MCC3 was chosen as the reference category because it is the lowest assemblage, stratigraphically, in which fossil murids are found. Since the aim was evaluating the (possible) differences accumulated along the succession, we focused our attention on the coefficients of the predictor variables (=the fossil assemblages). We first computed the 95% confidence interval (CI) of each coefficient with Wald Chi-square tests. Successively, the differences between the assemblages were assessed by comparing the beta coefficients. In each model (i.e., each taxon), any predictor variable (i.e., each assemblage) with coefficient lower (or higher) than zero indicates lower (or higher) weight than MCC3 in determining the probability (in terms of log odds) of finding that species at MCC. In other words, for each murid, the coefficient of a specific assemblage approaching zero indicates that, in the given assemblage, the probability of finding that given species is very similar to the one observed in MCC3. On the contrary, coefficients that are significantly different from zero indicate significant different probabilities of finding that species relative to MCC3. This method has the advantage of precisely estimating when the observed differences between the assemblages become statistically significant.
THE FAUNAL ASSEMBLAGE
The following sections report the fossil mollusks and vertebrates identified in the faunal assemblages of MCC. An exhaustive list of the identified taxa of mollusks and vertebrates can be found in Appendix 2.
Mollusks
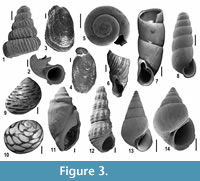 Gastropods. The 3,300 gastropod specimens found at MCC are assigned to 53 species (Harzhauser et al., 2015; Appendix 2). Most of these species are terrestrial and only 13 are aquatic species. Some of the latter, such as Bulinus meneghinii and Gyraulus sp., are considered to be parautochthonous. Preservation is generally good, but many shells were fragmented during screen-washing. All these species might have dwelled along and in ephemeral puddles and ponds. On the contrary, robust and solid shells of other aquatic gastropod species belong in an autochthonous Lago-Mare assemblage, which is dominated by Melanopsis narzolina, Melanoides curvicosta, Theodoxus doderleini, Theodoxus mutinensis and various species of Saccoia (Figure 3.9-14). Interestingly, the Lago-Mare gastropod assemblage is not related to coeval Pannonian-Pontian faunas of Lake Pannon, or of the Dacian Basin. Genera such as Saccoia and Melanoides did not settle in the Paratethyan basins and none of the typical Pannonian-Pontian endemic genera appears in the Lago-Mare faunas (Neubauer et al., 2015a, b).
Gastropods. The 3,300 gastropod specimens found at MCC are assigned to 53 species (Harzhauser et al., 2015; Appendix 2). Most of these species are terrestrial and only 13 are aquatic species. Some of the latter, such as Bulinus meneghinii and Gyraulus sp., are considered to be parautochthonous. Preservation is generally good, but many shells were fragmented during screen-washing. All these species might have dwelled along and in ephemeral puddles and ponds. On the contrary, robust and solid shells of other aquatic gastropod species belong in an autochthonous Lago-Mare assemblage, which is dominated by Melanopsis narzolina, Melanoides curvicosta, Theodoxus doderleini, Theodoxus mutinensis and various species of Saccoia (Figure 3.9-14). Interestingly, the Lago-Mare gastropod assemblage is not related to coeval Pannonian-Pontian faunas of Lake Pannon, or of the Dacian Basin. Genera such as Saccoia and Melanoides did not settle in the Paratethyan basins and none of the typical Pannonian-Pontian endemic genera appears in the Lago-Mare faunas (Neubauer et al., 2015a, b).
The assemblage is outstanding in evolutionary aspects as it represents an example of the earliest post-evaporitic gastropod fauna, which has passed the MSC. The composition of the terrestrial MCC malacofauna suggests a biochronologic transition from late Miocene to Pliocene communities, which is consistent with the late Turolian (MN13) age indicated by the mammalian assemblage. About 15% of the species have been known so far only from the European Miocene, 40% were described from the Pliocene and were unknown from older strata and about 42% are only known from the upper Messinian of MCC.
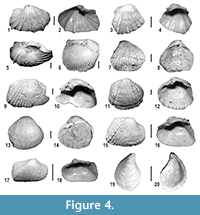 Bivalves. Brackish and freshwater bivalves also characterize the Messinian post-evaporitic deposits of MCC (Angelone et al., 2011; Harzhauser et al., 2015). Overall, the recorded Lago-Mare bivalves are poorly preserved, i.e., valves are always disarticulated, frequently abraded and often damaged (Figure 4). Twelve bivalve taxa of the cardiid subfamily Lymnocardiinae and a single species of the family Dreissenidae, Dreissena ex gr. rostriformis, were recognized, representing the typical Lago-Mare biofacies. Moreover, rare valves of the freshwater bivalve Pisidium sp. were also recorded. Among the Lymnocardiinae, five species of five different genera were identified; the other seven scarcely represented and poorly preserved taxa are left in open nomenclature. Taxa with either Paratethyan affinities or endemic of the late Messinian Mediterranean were recognized. The complete list of taxa and the abundance matrix are reported in Appendix 3.
Bivalves. Brackish and freshwater bivalves also characterize the Messinian post-evaporitic deposits of MCC (Angelone et al., 2011; Harzhauser et al., 2015). Overall, the recorded Lago-Mare bivalves are poorly preserved, i.e., valves are always disarticulated, frequently abraded and often damaged (Figure 4). Twelve bivalve taxa of the cardiid subfamily Lymnocardiinae and a single species of the family Dreissenidae, Dreissena ex gr. rostriformis, were recognized, representing the typical Lago-Mare biofacies. Moreover, rare valves of the freshwater bivalve Pisidium sp. were also recorded. Among the Lymnocardiinae, five species of five different genera were identified; the other seven scarcely represented and poorly preserved taxa are left in open nomenclature. Taxa with either Paratethyan affinities or endemic of the late Messinian Mediterranean were recognized. The complete list of taxa and the abundance matrix are reported in Appendix 3.
The bivalve assemblages are mainly composed by Lymnocardiinae with taxa of Pontian Paratethyan-type (Nevesskaya et al., 2001), such as the genera Pontalmyra, Euxinicardium, Prosodacnomya and Pseudocatillus. These genera possibly invaded the Mediterranean area from the Paratethys realm during the latest Messinian (Esu, 2007; Guerra-Merchán et al., 2010). Euxinicardium subodessae and Prosodacnomya sabbae are common in the Pontian deposits of the Paratethys, whilst Pontalmyra bollenenesis, Pontalmyra partschi and Pseudocatillus nevesskayae are endemic Mediterranean species (Esu, 2007; Angelone et al., 2011; Esu and Popov, 2012). The dreissenid D. ex gr. rostriformis, which is often found in the Lago-Mare deposits of the Mediterranean, is rather common in the Lymnocardiinae dominated assemblage of Moncucco Torinese (Figure 4).
Vertebrates
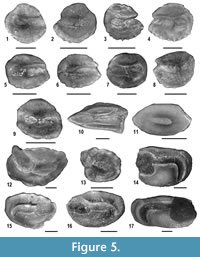 Fishes. Fish remains consist of well-preserved saccular otoliths (Figure 5, Appendix 2). The excellent preservation of the otoliths indicates autochthonous embedding, excluding any reworking from older rocks, or contamination from younger deposits. About 180 fish otoliths have been collected. They belong to a relatively diversified fish assemblage, with 17 species-level taxa of seven families. Each taxon has been assigned to an ecological guild, using a slightly modified version of the classifications proposed by Elliott and Dewailly (1995) and Mathieson et al. (2000). The use of ecological guilds is very useful to the understanding of the paleoenvironmental significance of the fish assemblage (e.g., see Carnevale et al., 2008). Overall, three guilds have been recognized, namely estuarine residents, marine adventitious species and marine migrants. The guild of estuarine residents includes the taxa that spend their life-cycles in brackish biotopes (primarily estuaries and lagoons) with thalassogenic waters; marine adventitious fishes are marine stenohaline taxa that might appear only occasionally in brackish biotopes; finally, marine migrants are taxa that regularly (seasonal) visit brackish biotopes during their adult or juvenile phases.
Fishes. Fish remains consist of well-preserved saccular otoliths (Figure 5, Appendix 2). The excellent preservation of the otoliths indicates autochthonous embedding, excluding any reworking from older rocks, or contamination from younger deposits. About 180 fish otoliths have been collected. They belong to a relatively diversified fish assemblage, with 17 species-level taxa of seven families. Each taxon has been assigned to an ecological guild, using a slightly modified version of the classifications proposed by Elliott and Dewailly (1995) and Mathieson et al. (2000). The use of ecological guilds is very useful to the understanding of the paleoenvironmental significance of the fish assemblage (e.g., see Carnevale et al., 2008). Overall, three guilds have been recognized, namely estuarine residents, marine adventitious species and marine migrants. The guild of estuarine residents includes the taxa that spend their life-cycles in brackish biotopes (primarily estuaries and lagoons) with thalassogenic waters; marine adventitious fishes are marine stenohaline taxa that might appear only occasionally in brackish biotopes; finally, marine migrants are taxa that regularly (seasonal) visit brackish biotopes during their adult or juvenile phases.
Considering the huge amount of sediment that has been processed, otoliths are relatively rare in the fossil assemblage. They reach the highest abundance in MCC 4, where more than 65% of the examined specimens have been found. About 60% of the specimens pertain to three taxa of the family Sciaenidae, most of which belong to a still undescribed taxon (Sciaenidarum sp. nov.) previously reported from the Messinian post-evaporitic Colombacci Formation (Carnevale et al., 2006a). Lanternfishes of the family Myctophidae represent about 30% of the specimens, whereas gobies (family Gobiidae) form slightly less than 7% of the specimens. Taxa belonging to other families (Bythitidae, Gadidae, Moridae, Trachichthyidae) are very rare, with a single specimen each. In this context, two sciaenid taxa are included in the guild of estuarine residents, marine migrants include a goby and a single sciaenid whereas the rest are placed here in the guild of marine adventitious taxa. Overall, estuarine residents dominate the assemblage, with slightly less than 60% of the specimens, followed by marine adventitious species which sum up to about one third of the specimens. Marine migrants are notably subordinate, with about 9% of the specimens. The relative proportions, however, vary in the different horizons. Estuarine residents are much more abundant in MCC4 and MCC7, whereas marine adventitious taxa dominate in MCC3 and MCC5.
Amphibians. Amphibians are represented by 886 remains, which belong to eight taxa (see Appendix 2). Due to the high fragmentation of most of the material, only few remains have been identified to genus or species level.
 Allocaudates are represented by about 40 tooth-bearing bones (premaxillae, maxillae and dentaries; Figure 6.1-2) that are here preliminarily referred to Albanerpeton sp., even if the morphology of the premaxilla is apparently congruent with that of Albanerpeton pannonicus, the geologically youngest species of the extinct clade of allocaudates (Venczel and Gardner, 2005). Albanerpeton was previously identified in Italy only in the lower Pleistocene of Rivoli Veronese, where it represents the geologically youngest evidence of the whole clade of Allocaudata (Delfino and Sala, 2007).
Allocaudates are represented by about 40 tooth-bearing bones (premaxillae, maxillae and dentaries; Figure 6.1-2) that are here preliminarily referred to Albanerpeton sp., even if the morphology of the premaxilla is apparently congruent with that of Albanerpeton pannonicus, the geologically youngest species of the extinct clade of allocaudates (Venczel and Gardner, 2005). Albanerpeton was previously identified in Italy only in the lower Pleistocene of Rivoli Veronese, where it represents the geologically youngest evidence of the whole clade of Allocaudata (Delfino and Sala, 2007).
A single trunk vertebra (MGPT-PU 132302; Figure 6.3-6.5) is referred to Chelotriton on the basis of the relatively large size (the centrum is 3.5 mm long) and the tall neural spine apically expanded to form a broad, triangular area, with numerous relatively large pits delimited by thin ridges. This extinct genus was reported in several Eocene to upper Pliocene European localities (Martín and Sanchiz, 2015), but never before in Italy.
A few caudate vertebrae can be preliminary referred to Lissotriton sp. on the basis of their small size (the centrum of MGPT-PU 132306 is 1.4 mm long) but also of the moderately high neural spine that develops posteriorly a small triangular area showing, at least in the case of MGPT-PU 132306 (Figure 6.6-7), a large pit (see Haller-Probst and Schleich, 1994).
A representative of the green toads (Bufo gr. B. viridis; Figure 6.8) is testified by five ilia characterized by the typical rod-like anterior branch, the multilobed tuber superior, as well as the preacetabular pit. Because the comparative osteology of the several species that had formerly been included in the taxon B. viridis is not known in detail, the remains from MCC are referred to it only at group level. For the generic identity of the green toads we followed Speybroeck et al. (2010), who suggested to group all the green toads in the genus Bufo.
At least one ilium (MGPT-PU 132316; Figure 6.9) has morphological traits suggestive of a green frog, Pelophylax sp., which include very broad dorsal wing on the anterior branch; distinct, laterally flattened tuber superior, forming an angle of about 90° with the anterior sloping surface of the pars ascendens ilii; relatively massive surface of contact with the ischium and with no deep groove (Bailon, 1999). Other ranid ilia, badly preserved, or very small in size and therefore possibly belonging to juvenile specimens that did not fully develop typical diagnostic characters, were identified as Rana s.l., including therefore both Pelophylax and Rana (the possible presence of brown frogs at Moncucco Torinese is unclear).
Ten isolated ilia (Figure 6.10) are referable to Hyla gr. H. arborea on the basis of their rod-like anterior branch, the often globular and prominent tuber superior and the very broad pars descendens ilii (Bailon, 1999). Seven Y-shaped scapulae and six small, lightly built vertebrae with very broad neural canal are referred to the same taxon. The European and Mediterranean species of tree frogs are not reliably diagnosable from their skeleton (despite Holman's, 1992, attempt). Their fossils are therefore here referred to a species group, named after the species that formerly included most of the currently recognized species.
Latonia sp. is represented by two angulars (Figure 6.11-12) that are characterized by the presence of distinct coronoid and paracoronoid processes and by a Meckelian canal that is deeply recessed at the level of the coronoid processes (Roček, 1994). A frontoparietal (MGPT-PU 132304), six ilia (Figure 6.13-14) and a trunk vertebra (MGPT-PU 132103) are referred to Pelobates sp. The most diagnostic characters are the dorsal ornamentation of the frontoparietal and the general morphology of the ilium (rod-like anterior branch, absence of the tuber superior and of the groove on the posteromedial surface and the contact surface with the ischium).
Reptiles. The herpetological sample includes 11,383 remains of reptiles. A single tooth (MGPT-PU  132316) is referred to Crocodylia indet. on the basis of its conical shape, reabsorbed root with conical basal depression, presence of mediodistal carinae delimiting a convex labial surface and a slightly concave lingual surface and presence of modest ridges on the labial and lingual surfaces (Figure 7.1-2). Despite the European endemic Diplocynodon likely was already extinct in the late Miocene (Martin et al., 2014), this tooth is not morphologically referable to Crocodylus, which was reported (in some cases tentatively) from other Italian late Miocene Italian localities (Delfino et al., 2007; Delfino and Rook, 2008; Delfino and Rossi, 2013). Undetermined crocodylians, based on isolated teeth, were also reported from the late Miocene localities of Fiume Santo (Abbazzi et al., 2008a) and Brisighella/Monticino (Rook et al., 2015).
132316) is referred to Crocodylia indet. on the basis of its conical shape, reabsorbed root with conical basal depression, presence of mediodistal carinae delimiting a convex labial surface and a slightly concave lingual surface and presence of modest ridges on the labial and lingual surfaces (Figure 7.1-2). Despite the European endemic Diplocynodon likely was already extinct in the late Miocene (Martin et al., 2014), this tooth is not morphologically referable to Crocodylus, which was reported (in some cases tentatively) from other Italian late Miocene Italian localities (Delfino et al., 2007; Delfino and Rook, 2008; Delfino and Rossi, 2013). Undetermined crocodylians, based on isolated teeth, were also reported from the late Miocene localities of Fiume Santo (Abbazzi et al., 2008a) and Brisighella/Monticino (Rook et al., 2015).
The presence of a tortoise of the genus Testudo (Testudo sp.; Figure 7.3-4) is testified by about 20 relatively thick and robust shell elements (plus several shell fragments). Particularly informative are the trapezoidal costals, the peripherals without pleuro-marginal sulcus and the trapezoidal pygal without sulcus. A partially preserved xiphiplastron (MGPT-PU 132005) does not permit to verify if this taxon had a hypo-xiphiplastral hinge. Testudo sp. was also reported at Verduno (Colombero et al., 2014a).
More abundant, and therefore more informative, are the shell elements of Mauremys (Figure 7.5-8). The peripherals are crossed not only by the intermarginal sulcus, but also by the pleuro-marginal sulcus. The left third peripheral MGPT-PU 132058 shows the musk pore. The small, rectangular pygal bears only one sagittal sulcus and a marked posterior notch. The notch on the right side corresponds to a small accessory ossicle that partly developed also on the right 11th peripheral (MGPT-PU 132339). The epiplastron is crossed by the gulo-humeral sulcus and has a very low epiplastral pad that does not develop the gular pocket. The entoplastra are vaguely rhomboidal in shape and (in addition to the sulci among the gulars and humerals) are transversally crossed nearly at mid height by the humero-pectoral sulci. Mauremys was likely present also at Verduno, where the scanty material referable to Geoemydidae indet. cannot be reliably identified (Colombero et al., 2014a). Mauremys was relatively common in Italy from at least the late Miocene to the end of the Pleistocene (Delfino and Bailon, 2000; Chesi et al., 2007, 2009; Chesi, 2009) when it became extinct along with other thermophilous taxa. Based on the available evidence the Mauremys material from MCC cannot be reliably related to Mauremys portisi from the Pliocene of Valleandona (Sacco, 1889).
MGPT-PU 132432 is a very small fragment of maxilla (about 1.3 mm in length; Figure 7.9) that has been attributed to a gekkotan because of the presence of a single pleurodont, cylindrical, slender and bicuspid tooth, with mesiolingually-aligned lingual and labial cusps (Sumida and Murphy, 1987). Based on its overall size and on the presence of a concavity on the anterior margin of the facial process, MGPT-PU 132432 is comparable morphologically to the maxillae of Euleptes europaea, but its very bad preservation prevents a dependable identification. The maxilla is therefore identified only as cf. Euleptes sp. A very small dorsal vertebra (MGPT-PU 132589; centrum length = 1.2 mm) and a very small fragmentary caudal vertebra (MGPT-PU 132590) can be tentatively attributed to the same taxon.
Agamid lizards are represented by 11 very fragmentary tooth-bearing bones (Figure 7.10) with acrodont teeth. The attribution of these remains to the family Chamaeleonidae can be ruled out by the marked teeth proximity (Delfino, 2002 and references therein). The past distribution of agamid lizards in Italy, which are now locally extinct, was summarized by Delfino et al. (2008), but new remains were recently reported by Colombero et al. (2014a) for the late Miocene locality of Verduno.
About 8,400 remains, mostly isolated osteoderms or osteoderm fragments, and a few vertebrae are referred to anguids (Figure 7.11-12). The size of the remains, together with the convergent (or vaguely parallel posteriorly) margins of the centrum of the trunk vertebrae, and the thickness, the subrectangular shape and the keeled external surface of most of the osteoderms, suggest the presence of a large-sized, non-Anguis anguine taxon. One of the vertebrae (MGPT-PU 132612) and one of the osteoderms (MGPT-PU 132367), in particular, are very large: the former has a centrum length of 8 mm, whereas the latter is 8 x 9 mm, with a thickness of 2 mm. A fragmentary maxilla, MGPT-PU 132443 and a fragmentary dentary, MGPT-PU 132482, bear slender and conical teeth, very slightly posteriorly curved by their tip. This tooth morphology is comparable to that of Ophisaurus according to Klembara et al. (2014), but the remains from Moncucco Torinese do not show striae on the lingual side. Another fragmentary maxilla, MGPT-PU 132543, has similar, but more robust teeth. The generic identification of these remains is difficult; they are here attributed only to indeterminate non- Anguis Anguinae.
More than 500 skeletal elements (Figure 7.13-16) are referable to an indeterminate species of a lacertid lizard whose size is consistent with that of extant Lacerta. Tooth-bearing bones are characterized by bicuspid teeth (in some cases the posterior ones can have also a third, small posterior cusp), whereas the vertebrae are procoelous, with nearly rounded cotyles and condyles and --if they are trunk vertebrae--with a convex ventral surface of the centrum that can express a modest keel. A couple of fused frontals, MGPT-PU 132532, show almost parallel lateral margins in the medial region, a feature typical of adult Lacerta bilineata and Lacerta schreiberi according to the analysis of Barahona and Barbadillo (1997). The quadrate MGPT-PU 132542, however, has a concave anterior platform, indicative of Timon lepidus (see Barahona and Barbadillo, 1997).
The presence of Amphisbaenia indet. (Figure 7.17-19) is testified by seven isolated trunk vertebrae sharing the following characters: small size (centrum length of about 2 mm), presence of very small prezygapophyses, absence of neural crest, neural spine replaced by a flattened area, bulbous and massive synapophyses, dorsoventrally flattened cotyle and condyle, ventral surface of the centrum flat and delimited by straight lateral margins (Estes, 1983). Isolated vertebrae of amphisbaenians are rather common in Italy, but they cannot be identified with precision (Delfino, 2003).
Scolecophidian snakes are represented by a single partial trunk vertebra (MGPT-PU 132027; Figure 7.20) that was preliminarily described by Delfino et al. (2013). The very scarce material from Moncucco Torinese and Verduno (Colombero et al., 2014a) represent the first evidence for the presence of these diminutive snakes in the Italian Peninsula.
Two partial caudal vertebrae (MGPT-PU 132625, 132626; Figure 7.21-22) characterized by additional processes on the neural arch (i.e., the pterapophyses on the neural arch) and a modestly expanded neural spine, are referred to erycine boids, Eryx sp. Erycine snakes are the only boids reported so far for the Italian peninsula and its islands (Delfino et al., 2014) and are also present in the nearly coeval assemblages of Ciabòt Cagna (Cavallo et al., 1993) and Brisighella/Monticino (Rook et al., 2015) as well as in the younger Sardinian site of Mandriola (Capo Mannu D1 Local Fauna; Delfino et al., 2011). There are no extant erycine snakes in continental Italy; the absence of Quaternary erycine fossils from Sicily and the recent discovery of a living population of Eryx jaculus on the island supports the hypothesis that the species was introduced by humans (Insacco et al., 2015).
Colubrid snake vertebrae with a centrum longer than wide and without hypapophysis have been referred to the working taxon “colubrines” (sensu Szyndlar, 1991a). At Moncucco Torinese several taxa are likely present, but the specimens are very badly preserved. Only two of them can be confidently identified: one with short and apically rounded prezygaphyseal processes (colubrines A; MGPT-PU 132662; Figure 7.23-25) and the other with long and apically pointed prezygaphyseal processes (colubrines B; MGPT-PU 132663; Figure 7.26-28).
Two hollow fangs (MGPT-PU 132045), a single basioccipital (MGPT-PU 132026) and three vertebrae (MGPT-PU 132025 and 132661; Figure 7.29-30) have been referred to large vipers of the Oriental group (sensu Szyndlar, 1991b). The best preserved vertebra MGPT-PU 132661 is rather large (length of the centrum about 6 mm), with the posterior edge of the neural arch distinctly flattened dorsoventrally, straight dorsal edges (in posterior view), proportionally large and dorsoventrally flattened cotyle and condyle and long, anteroventrally inclined parapophyseal processes.
Birds. Bird remains are quite abundant, but badly preserved. In particular, all the long bones, except a single carpometacarpus and some pedal phalanges, are fragmentary and represented only by the epiphyses, which in some cases are only partially complete.
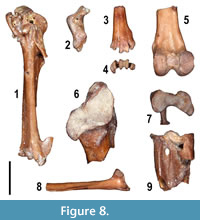 The available material reveals the presence of at least 12 taxa. Worth mentioning is the presence of Palaeortyx cf. gallica, which was originally described from the early Miocene of France and also reported from the Paleogene deposits of Quercy (Göhlich and Mourer-Chauviré, 2005). A complete carpometacarpus (Figure 8.1) as well as other specimens tentatively referred to this taxon, are comparable in size and morphology to the topotypical material from Saint-Gérand-le-Puy (Göhlich and Mourer-Chauviré, 2005) and differ from the other Phasianidae species of similar size, as for example the Neogene genus Palaeocryptonyx (see Pavia et al., 2012; Bedetti and Pavia, 2013). Few remains, including a proximal left coracoid (Figure 8.2), indicate the presence of a small Phasianidae referred to Coturnix sp., on the basis of its small size and the morphology of the coracoid. A left scapula (Figure 8.8) is the only remain indicating the presence of Apodidae, which is rarely reported in the Miocene fossil record (Mlíkovský, 2002), whereas more numerous are the remains of a small Columbidae (Figure 8.6), similar in size to the recent Streptopelia turtur, which is smaller than any other Neogene fossil Columbidae (Mlíkovský, 2002) (Figure 8). A number of remains, including fragments of tarsometatarsi (Figure 8.7 and 8.9), long bones and pedal phalanges, indicate the presence of a small Strigidae, similar in size to the extant Athene noctua. The only Miocene taxon comparable in size is Alasio collongensis, described from the middle Miocene of France (Mlíkovský, 1998), but none of the bones found at MCC correspond to the skeletal elements used to describe the species. At least two very fragmentary tarsometatarsi indicate the presence of Coliidae (Figure 8.3-4), a group with an extensive Paleogene and Neogene record in Europe, with at least two taxa known from the Miocene, but which has never been reported before in the latest Miocene (Mayr, 2010).
The available material reveals the presence of at least 12 taxa. Worth mentioning is the presence of Palaeortyx cf. gallica, which was originally described from the early Miocene of France and also reported from the Paleogene deposits of Quercy (Göhlich and Mourer-Chauviré, 2005). A complete carpometacarpus (Figure 8.1) as well as other specimens tentatively referred to this taxon, are comparable in size and morphology to the topotypical material from Saint-Gérand-le-Puy (Göhlich and Mourer-Chauviré, 2005) and differ from the other Phasianidae species of similar size, as for example the Neogene genus Palaeocryptonyx (see Pavia et al., 2012; Bedetti and Pavia, 2013). Few remains, including a proximal left coracoid (Figure 8.2), indicate the presence of a small Phasianidae referred to Coturnix sp., on the basis of its small size and the morphology of the coracoid. A left scapula (Figure 8.8) is the only remain indicating the presence of Apodidae, which is rarely reported in the Miocene fossil record (Mlíkovský, 2002), whereas more numerous are the remains of a small Columbidae (Figure 8.6), similar in size to the recent Streptopelia turtur, which is smaller than any other Neogene fossil Columbidae (Mlíkovský, 2002) (Figure 8). A number of remains, including fragments of tarsometatarsi (Figure 8.7 and 8.9), long bones and pedal phalanges, indicate the presence of a small Strigidae, similar in size to the extant Athene noctua. The only Miocene taxon comparable in size is Alasio collongensis, described from the middle Miocene of France (Mlíkovský, 1998), but none of the bones found at MCC correspond to the skeletal elements used to describe the species. At least two very fragmentary tarsometatarsi indicate the presence of Coliidae (Figure 8.3-4), a group with an extensive Paleogene and Neogene record in Europe, with at least two taxa known from the Miocene, but which has never been reported before in the latest Miocene (Mayr, 2010).
Most of the recognizable bird remains from MCC can be assigned to the order Passeriformes, including a single scapula of Corvidae and at least three size-group species. The osteology and the relationships of Miocene passeriforms to the extant taxa are almost unknown. It is therefore impossible to assign the badly preserved material from MCC to any extinct or extant taxon. The small sized Passeriformes recovered at MCC may include more than one species.
The MCC fossil bird assemblage is characterized by taxa that have already been reported from other early and middle Miocene localities. Modern bird taxa are absent from them. In particular, Palaeortix and Coliidae are still unknown in post-Miocene fossil localities (Göhlich and Pavia, 2008; Mlíkovský, 2002). Although very fragmentary, the bird remains of MCC indicate that the latest Miocene Italian bird communities were exclusively characterized by taxa of Miocene origin. Modern taxa only occurred from the Pliocene onwards. It is also worth noting that none of the taxa found at MCC has been reported from the almost coeval site of Verduno, in Piedmont (Colombero et al., 2014a).
Mammals.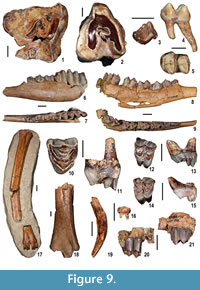 The large mammal sample includes 164 identified remains. Biometrics are available in Appendix 4. Two perissodactyl families, Rhinocerotidae and Tapiridae, are represented in the MCC sample. Three cheek teeth are morphologically and dimensionally indicative of Dihoplus schleiermacheri (Figure 9.1-2), which is present also in the nearby and slightly younger fossiliferous site of Verduno (Colombero et al., 2014a). Tapirus arvernensis (Figure 9.4-5) is represented by four lower molars and an incomplete left third metatarsal.
The large mammal sample includes 164 identified remains. Biometrics are available in Appendix 4. Two perissodactyl families, Rhinocerotidae and Tapiridae, are represented in the MCC sample. Three cheek teeth are morphologically and dimensionally indicative of Dihoplus schleiermacheri (Figure 9.1-2), which is present also in the nearby and slightly younger fossiliferous site of Verduno (Colombero et al., 2014a). Tapirus arvernensis (Figure 9.4-5) is represented by four lower molars and an incomplete left third metatarsal.
Artiodactyls occur with five taxa. A fragmentary lower cheek tooth of suid is compressed labiolingually and has the cuspids and talonid aligned in a trenchant, crest-like fashion. The metaconid is placed behind and close to the distal remnant of the protoconid. The size of the specimen falls in the ranges of the tribe Dicoryphochoerini (Figure 9.3).
Cervidae are the best represented artiodactyl family. Two saber-like, moschid type upper canines and several cheek teeth and postcranial elements show characters indicative of the muntiacine Euprox sp. (Figure 9.14-15, 9.16, 9.19), which is also present at Verduno (Colombero et al., 2014a). Several cranial, dental and postcranial remains attest to the presence of a cervid approximately the size of a modern fallow deer. The dental features and the size of the cranial and postcranial specimens are indicative of Pliocervus (Figure 9.8-9, 9.12-13, 9.17). The cheek teeth are quite similar to those of Cervavitus and Procapreolus, but they are more derived in the lack of the “ Palaeomeryx fold” and in the tendency to lose the cingulids in the lower molars (Czyzewska, 1968). The most prominent fossil ascribed to Pliocervus sp. is a fragmentary left frontal bone with pedicle and a morphotype A antler, based on Azanza et al.’s (2013) categorization (Figure 10). 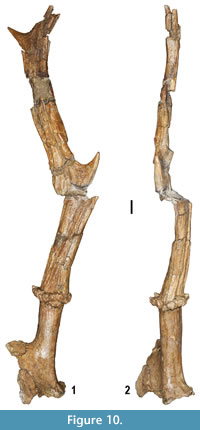 The antler is faulted at the level of the first fork and badly crushed mediolaterally in its upper part (Figure 10). It has an oval section at the base. In side view, from the base upwards, the beam curves at first backwards and then forwards. A short, upward pointed tine issues from each bend. The first fork is placed very high above the burr (about 70 mm measured medially, and around 110 mm from the medial base of the pedicle), whereas the second one is placed about 95 mm from the latter and directed posteriorly. The burr is located about 36 mm from the medial base of the pedicle (Figure 10). The pedicle shows a combination of characters of both Azanza et al.’s (2013) large morphotype 1 and morphotype 2 pedicles. It is moderately inclined backwards, somewhat compressed anteroposteriorly at the base and associated with a thin orbital rim. The fossa for the foramen supraorbitale is located somewhat laterally with respect to the pedicle. A long and slim tine fragment marked with shallow longitudinal grooves instead of tubercles closely resembles the tines of the antlers of Cervavitus and Pliocervus and differs from the cranial appendages of Capreolinae.
The antler is faulted at the level of the first fork and badly crushed mediolaterally in its upper part (Figure 10). It has an oval section at the base. In side view, from the base upwards, the beam curves at first backwards and then forwards. A short, upward pointed tine issues from each bend. The first fork is placed very high above the burr (about 70 mm measured medially, and around 110 mm from the medial base of the pedicle), whereas the second one is placed about 95 mm from the latter and directed posteriorly. The burr is located about 36 mm from the medial base of the pedicle (Figure 10). The pedicle shows a combination of characters of both Azanza et al.’s (2013) large morphotype 1 and morphotype 2 pedicles. It is moderately inclined backwards, somewhat compressed anteroposteriorly at the base and associated with a thin orbital rim. The fossa for the foramen supraorbitale is located somewhat laterally with respect to the pedicle. A long and slim tine fragment marked with shallow longitudinal grooves instead of tubercles closely resembles the tines of the antlers of Cervavitus and Pliocervus and differs from the cranial appendages of Capreolinae.
A few dental and postcranial remains are ascribed to aff. Palaeomeryx (Palaeomerycidae) with reservations (Figure 9.10-11, 9.18). These include 1) a large-sized left upper fourth premolar, with strong labial structures, a rounded, but fairly narrow lingual cone, a very weak mesial cingulum and rugose enamel and 2) the distal end of a large ruminant left tibia, with moderately-developed malleolus medialis, very deep and broad groove for the tendon of the tibialis posterior and flexor digitorum longus and two concave facets, a larger plantar one and a smaller dorsal one, for the fibula. Another tooth fragment, most similar to the left upper fourth premolar just described, was found in the nearby fossiliferous site of Verduno (Colombero et al., 2014a). These specimens closely resemble the premolars of the giraffid Palaeotragusrouenii. However, a mandibular fragment of this ruminant found at Verduno rules out any reference to the latter species because of its short p2-c diastema. Giraffid-like teeth are combined with a short mandibular diastema in Palaeomeryx. The latter genus, however, is hitherto known to have disappeared from Europe around 9 Ma. For this reason the remains from Moncucco Torinese are tentatively attributed to aff. Palaeomeryx.
An incomplete left hemimandible still preserving p3-m3 (Figure 9.6-7) and a left maxillary fragment of a bovid with M1 (Figure 9.20-21) have the same dental traits and size of an antelope classified as Gazella aff. pilgrimi found both in the nearby site of Verduno (Colombero et al., 2014a) and in Kohfidisch (Vislobokova 2007).
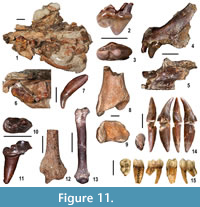 Moncucco Torinese yielded a rich and diverse carnivoran sample. A left maxillary bone with P3 and P4, five isolated teeth, four postcranial remains and, even more significantly, a crushed skull with moderately worn right P4 and M1, show the typical traits of viverrids (Figure 11.1, 11.6-7, 11.10-13). The skull bears a long, slender, labially-concave carnassial tooth, with a prominent parastyle, a small deuterocone (protocone), separated by a fairly evident constriction from the paracone, and a distinctive triangular M1 with a narrow and lingually-elongated protocone. The characters of the dental remains are suggestive of members of the subfamily Viverrinae. The distal epiphyses of a humerus and radius recall those of the extant terrestrial viverrine Civettictis civetta. We assign this material to Viverridae indet.
Moncucco Torinese yielded a rich and diverse carnivoran sample. A left maxillary bone with P3 and P4, five isolated teeth, four postcranial remains and, even more significantly, a crushed skull with moderately worn right P4 and M1, show the typical traits of viverrids (Figure 11.1, 11.6-7, 11.10-13). The skull bears a long, slender, labially-concave carnassial tooth, with a prominent parastyle, a small deuterocone (protocone), separated by a fairly evident constriction from the paracone, and a distinctive triangular M1 with a narrow and lingually-elongated protocone. The characters of the dental remains are suggestive of members of the subfamily Viverrinae. The distal epiphyses of a humerus and radius recall those of the extant terrestrial viverrine Civettictis civetta. We assign this material to Viverridae indet.
Of particular interest are the remains of two felids and an ursid. A rib, caudal vertebra and complete right fourth metacarpal attest to the presence of the wildcat Pristifelis attica, also reported from Verduno (Colombero et al., 2014a). The second felid is a pantherine cat (Pantherinae indet.) (Figure 11. 4-5, 11.8-9). A left premaxilla with I1, I3 and the alveoli of the second incisor and of the canine, the rostral portion of a right mandibular corpus with the alveoli of the canine, of a premolar and the diastema between them, a proximal half of a right fifth metacarpal and two fragments of tibia, have features and sizes most similar to those observed in the equivalent anatomical parts of present-day Panthera onca, Panthera concolor and Panthera pardus. Late Miocene finds of pantherine cats are particularly significant because the early history of these felids is still imperfectly known (Werdelin et al., 2010).
The ursid is represented by an unworn left upper carnassial with large paracone, quite smaller metacone, very small protocone located at the lingual side of the valley between the two former cones and cingula on both sides of the tooth (Figure 11.2-3). Morphologically and dimensionally, this specimen recalls the carnassials of the ursine bears from the Polish locality of Węże 1 (Qiu et al., 2009). The latter are ascribed to Euarctos pyrenaicus and dated to the late Ruscinian (MN 15), and are therefore slightly younger than the MCC specimen.
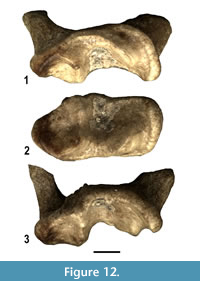 A moderately worn left M1belonging to a small ictonychin mustelid is referred to as Baranogale cf. helbingi (Figure 12). It displays subtrapezoidal outline with almost parallel mesial and distal borders, rather large and distinct paracone and metacone and a well-developed protocone bordered by a high anterolingual cingulum. Hypocone is absent. These morphological features are suggestive of Baranogale helbingi, a species previously reported in the Ruscinian and Villafranchian of Central Europe (Kormos, 1934; Viret, 1954; Kowalski, 1959; Morlo and Kundrát, 2001).
A moderately worn left M1belonging to a small ictonychin mustelid is referred to as Baranogale cf. helbingi (Figure 12). It displays subtrapezoidal outline with almost parallel mesial and distal borders, rather large and distinct paracone and metacone and a well-developed protocone bordered by a high anterolingual cingulum. Hypocone is absent. These morphological features are suggestive of Baranogale helbingi, a species previously reported in the Ruscinian and Villafranchian of Central Europe (Kormos, 1934; Viret, 1954; Kowalski, 1959; Morlo and Kundrát, 2001).
Primates are recorded at MCC by two cercopithecoids, based on dental and postcranial remains described in detail by Alba et al. (2014). These include a talus attributed to the extinct colobine monkey Mesopithecus pentelicus pentelicus, a male lower canine (Figure 11.14) and a proximal fragment of ulna tentatively referred to the same taxon, a lateral upper incisor and a third lower molar (Figure 11.15) assigned to the papionin cercopithecine cf. Macaca, and two phalanges unassigned to subfamily. The presence of a fossil macaque at MCC is further attested by an unpublished partial cranium (currently under study) that displays a typically papionin dental morphology (D.M.A., unpublished data). MCC thus uniquely records the co-occurrence of macaques and the Miocene colobine M. pentelicus (instead of the Pliocene Mesopithecus monspessulanus; Alba et al., 2014). It further represents, together with Almenara-Casablanca M in Spain (Köhler et al., 2000), one of two oldest records of Eurasian macaques. This suggests, given the lack of macaques in slightly older late Miocene sites such as Venta del Moro in Spain, that macaques did not disperse from Africa into Eurasia until the latest Miocene, coinciding with the sea level drop associated with the MSC (Alba et al., 2014, 2015). However, it is uncertain whether macaques dispersed through the Gibraltar area (Gibert et al., 2013) or otherwise using the Middle East route that was already available from pre-Messinian times and was most likely followed in the case of Mesopithecus (Gilbert et al., 2014; Alba et al., 2015).
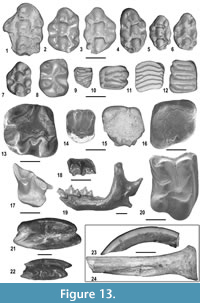 The small mammal assemblage of MCC consists of 3,685 specimens with 26 species (Figure 13) (see also Appendix 5, Appendix 6, and Appendix 7). They represent a very diverse fauna (two chiropterans, four insectivorans, two lagomorphs and 18 rodents). In the following paragraphs we will discuss the paleoecological requirements used to assign the habitat preferences to the small mammal taxa of MCC (Appendix 7).
The small mammal assemblage of MCC consists of 3,685 specimens with 26 species (Figure 13) (see also Appendix 5, Appendix 6, and Appendix 7). They represent a very diverse fauna (two chiropterans, four insectivorans, two lagomorphs and 18 rodents). In the following paragraphs we will discuss the paleoecological requirements used to assign the habitat preferences to the small mammal taxa of MCC (Appendix 7).
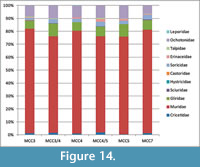
Among rodents, the Muridae are the most abundant family, representing more than 75% of the small mammal (based on nNISP) in all the studied layers (Figure 14). Gliridae are much rarer, ranging from 6% to 9%. Cricetids are always present but uncommon, ranging from 0.3% to 1.5%. All the other rodent families (Sciuridae, Castoridae and Hystricidae) are extremely rare (less than 1% when present), or even absent in some assemblages (Figure 14). Lagomorphs are represented by Ochotonidae and Leporidae. Ochotonids are regularly found in all layers, ranging from 6% (MCC7) to 10% (MCC3), whereas leporids have been found only in MCC4 and MCC5, where they sum up to less than 0.5% of the small mammals. Insectivorans include the families Soricidae, Talpidae and Erinaceidae. The latter are represented only by Galericinae. Soricids range from 1.6% to 3%, galericines are usually below 2% and moles represent less than 1% of the small mammals. In summary, several taxa are commonly found in each layer. Others are sporadic and extremely rare, particularly among families Sciuridae, Castoridae, Hystricidae and Leporidae, which are sometimes represented only by very few specimens (see Appendix 5, Appendix 6, and Appendix 7). The absence of the latter taxa in some layers is probably related to their rarity in origin and mainly due to the very low probability of being recorded in the fossil record. Therefore, it cannot be considered of biochronologic or paleoecological relevance.
 Bats from MCC are currently under study. There are at least two taxa of different size, the smaller Chiroptera indet. sp. 1, which is reported in all layers, and the larger and much rarer, Chiroptera indet. sp. 2.
Bats from MCC are currently under study. There are at least two taxa of different size, the smaller Chiroptera indet. sp. 1, which is reported in all layers, and the larger and much rarer, Chiroptera indet. sp. 2.
The Eulipotyphla (Figure 13.17-13.20) are the most diverse group of small mammals after rodents and are represented by several isolated dental remains and very few fragmentary mandibles. Four taxa are recorded, belonging to three families, Erinacaeidae, Talpidae and Soricidae. Galericine erinaceids are currently under study. They are moderately frequent in the MCC assemblage. They are recorded by 129 remains including isolated teeth and fragmentary mandibular corpora. Their size is only slightly smaller than that of Parasorex depereti (see Crochet, 1986). However, the proportions of the teeth and several morphological features, including the nearly rectangular M2 and the more developed labial cingula of the lower molars, are reminiscent of Parasorex ibericus, a small-sized species reported in the late Miocene of Europe (Mein and Martín-Suárez, 1993; Furió, 2007). For this reason, the galericine material from MCC is assigned here to Parasorex aff. ibericus.
A few isolated dental remains, a single mandibular fragment, as well as some partial humeri, are assigned to an indeterminate species of the genus Talpa. The m1 displays a U-shaped trigonid with a moderately curved paralophid and an oblique cristid connected with, or very close to, the metaconid; the single available mandibular corpus displays three mental foramina; the upper molars are subtriangular in shape and bear a well-developed protocone. The humeri are robust and fully comparable to those of extant species of the genus Talpa. The size is smaller than that of Talpa gilothi and Talpavallesensis and similar to that of Talpa minor from some Pliocene localities of Poland (Sulimski, 1959). However, in the coeval locality of Maramena, material of Talpa similar in size to that from MCC was referred to Talpa fossilis (see Doukas et al., 1995).
Soricids are the most abundant insectivorans, being represented by isolated dental remains and, more rarely, by partially preserved mandibles. The assemblage is dominated by far by remains of neomyin shrews, identified by a mandibular morphology exhibiting a slightly concave anterior margin of the coronoid process, well-developed coronoid spicule placed halfway the coronoid, shallow external temporal fossa, narrow interarticular facet and mental foramen below the reentrant valley, or the hypoconid of the m1. Additional features include fissident upper incisors, monocuspulate, or bicuspulate lower incisors, vaguely pigmented teeth, stout lower teeth with well-developed cingula, moderately high entoconid crests and poorly reduced talonid in m3. Within the tribe Neomyini, the genera Neomysorex and Asoriculus share most of the morphological features described above. They differ from each other in the presence of a fifth upper antemolar in Neomyosrex. The co-occurrence of two neomyins, the smaller Neomysorex alpinoides and the larger Asoriculus gibberodon, is documented at Podlesice (MN14, Poland, Rzebik-Kowalska, 1994). Pending thorough analyses and comparisons with neomyin material from the Neogene of Europe, we assign the material from MCC to Neomyini indet.
The soricin material consists of two upper incisors, a single lower incisor and a single m2, which are assigned to Petenyia cf. hungarica. The molar displays a dark-red to dark-brown coloration almost covering half of the crown and a subrectangular “bat-like” aspect with well-developed cingula. The upper incisors are not fissident, being characterized by a marked talon. The lower incisor is bicuspulate, with a tendency to develop a third distal tiny cuspule. These morphological features, but also the size of the examined dental elements, fit well with those reported for P. hungarica (Reumer, 1984; Rzebik-Kowalska, 1989, Marchetti et al., 2000; Popov, 2003; Siori et al., 2014). The size of the teeth is larger than that of Petenyia dubia, a species reported in several Miocene and Pliocene localities of Europe (Bachmayer and Wilson, 1970; Furió et al., 2014), whereas Petenyiakatrinae is only documented in the early Yushean (early Pliocene) of Eastern Asia (Qiu and Storch, 2000). If confirmed, this would be the oldest Italian record of this soricid. This taxon is also documented at Rivoli Veronese (late Villanyian), as well as at Monte la Mesa, Monte Argentario and Pirro Nord (early Biharian; Kotsakis et al., 2003). Moreover, MCC may represent one of the oldest records of this species in Europe. Its broad biochronological range spans from the late Turolian of Greece (Doukas et al., 1995) to the Ruscinian, Villanyian and Biharian of a vast area of Europe extending from the Iberian Peninsula (Minwer-Barakat et al., 2010) to Central and Eastern Europe (Reumer, 1984; Popov, 2003).
Rodents (Figure 13.1-13.16, 13.23-13.24) are the most diverse and abundant mammals of the MCC assemblage, consisting of 18 taxa included in six different families: Hystricidae, Castoridae, Sciuridae, Gliridae, Cricetidae and Muridae.
Hystricidae are represented by a single fragmentary radius. In a recent paper, Colombero et al. (2015) identified this bone as Hystrix (Hystrix) depereti. This taxon was widespread in Southwest Europe during the Turolian and early Ruscinian (Sen and Purabrishemi, 2010).
A single fragment of a left upper incisor is referred to as Castorinae indet. The cross section is subtriangular with an almost flattened enamel surface. The wear facet is short with a steep part at the tip of the tooth and a posterior angled part. The cutting edge is slightly more worn on the distal (lateral) side. The dimensions (L: 7.5 mm; W; 8.5 mm) and morphological features are indicative of a medium-to-large sized castorid, which rules out smaller representatives of the genera Dipoides and Euroxenomys. During the late Turolian (MN13), large-sized castorines are rather rare in Europe (Kotsakis, 1989; Stefen, 2011). The size of the studied specimen is consistent with, or only slightly larger than those of Chalicomys jaegeri, which has been previously reported in the MN13 of Polgardi and is rather common in the Vallesian and early Turolian of Europe (Kotsakis, 1989; Stefen, 2009), and Castor praefiber (see Dahlmann, 2001). In Italy, Castor cf. praefiber has been reported in the slightly older MN13 locality of Baccinello V3 (6.7-6.4 Ma; Kotsakis, 1989; Hugueney, 1999).
Squirrels are only sporadically found at MCC. They are only represented by nine teeth (which represent less than 0.005% of the whole rodent assemblage). Nonetheless, sciurids are rather diverse and provide significant paleoecological data. Four taxa have been identified, all belonging to the subfamily Sciurinae (Colombero et al., 2014b; Colombero and Carnevale, 2016). Among flying squirrels (tribe Pteromyini), a single M1 is attributed to Hylopetes hungaricus for the small size, the presence of short accessory ridges and constricted protoloph and metaloph . This taxon is known based on rare material from the late Miocene and Pliocene of Central Europe (Kretzoi, 1959; Sulimski, 1964; Black and Kowalski, 1974; Daxner-Höck, 2004; Bosma et al., 2013). Two rather large teeth, with a labyrinthic pattern produced by crenulated enamel and accessory ridges, have been assigned to Pliopetaurista pliocaenica, a species widespread in the late Turolian and Ruscinian of Europe (Colombero and Carnevale, 2016). A small number of teeth, including a few medium- to large-sized lower and upper ones and square lower molars with well-developed entoconids and without anterosinusids, represent the oldest occurrence of the genus Sciurus in Europe (Colombero and Carnevale, 2016). They are assigned here to Sciurus warthae, a species previously reported only in the Pliocene and Pleistocene of Italy and Central Europe (Sulimski, 1964; Black and Kowalski, 1974). Finally, a single fragmentary m1-2 is referred to Sciurinae indet.
Four species of glirids are documented by numerous isolated cheek teeth. The most frequent is Muscardinus vireti, a medium-sized hazel dormouse that was previously reported in the late Turolian of Italy, France and Spain (Colombero et al., 2014b). Dental remains of Glis minor, with an occlusal pattern very similar to that of the extant Glis glis, but slightly smaller in size, are reported in all the stratigraphic levels. Glis minor is common in the Miocene and early Pliocene of Central and Southeastern Europe (Daxner-Höck and Höck, 2009). The garden dormouse Eliomys yevesi, previously identified as Eliomys aff. intermedius by Colombero et al. (2014b), is rarely documented at MCC. This taxon is intermediate in size between Eliomys intermedius and Eliomys truci and displays a dental pattern rather similar to that of the former species, with a posterior centroloph in the upper molars and a somewhat more rounded outline of the cheek teeth. This glirid is rarely found around the Mio-Pliocene boundary of the northern Mediterranean area (Mansino et al., 2015a). The rarest glirid at MCC is Glirulus lissiensis, whose occurrence is revealed only by four teeth with a complex pattern of five main and five (lower molars) or four (upper molars) accessory ridges. This species has a long presence in Central and Western Europe throughout the late Miocene (Daxner-Höck and Höck, 2009).
Cricetids are rare at MCC. They occur with a single species, the large Neocricetodon magnus, previously reported in the early Ruscinian of Central Europe (Fahlbusch, 1969). This hamster is characterized by the presence of fully developed mesoloph(id)s in the upper and lower molars, poorly incised and continous anteroconid in m1 and strongly reduced M3.
Murids are the most abundant and diverse rodents of the MCC assemblage. Seven taxa have been recognized. Occitanomys brailloni, a medium-sized, weakly stephanodont murid, is the most abundant species in all the fossiliferous horizons. This species is rather common in the Pliocene of Southwestern Europe (Michaux, 1969). It has also been reported in Southeastern Europe around the Mio-Pliocene boundary (Koufos and Vasileiadou, 2015). The relatively large-sized field mouse Apodemus gudrunae is rather abundant at MCC. It is common in the MN13 of Southern Europe and Western Asia (Colombero et al., 2014b and references therein). Slightly less common are Paraethomys meini, a large murid widely distributed in the Mediterranean basin in the late Miocene and early Pliocene (Agustí et al., 2006), and Centralomys benericettii, a small stephanodont murid endemic of the Italian peninsula (De Giuli, 1989). Less common, but anyhow present in all the horizons, are Micromys bendai, a large Micromys species documented in the Balkans around the Mio-Pliocene boundary and in the Pliocene of Southwestern Europe (Hordijk and de Bruijn, 2009), and Apodemus atavus, a species widespread in the European Mediterranean area (Martín Suárez and Mein, 1998).
All the species mentioned so far had already been described in detail by Colombero et al. (2014). The newly added material includes very few remains of a large-sized murid, with a notable stephanodonty consisting of a long longitudinal spur on a single m1 and of an evident ridge connecting t3-t5 in M1 and M2. These specimens are only slightly smaller than Stephanomys ramblensis and much smaller than Stephanomys degiulii from Brisighella (De Giuli, 1989), or Stephanomys donezzani from Borro Strolla (Abbazzi et al., 2008b). This material is therefore assigned here to Stephanomys sp.
Lagomorphs (Figure 13.21-13.22) are currently under study. They are represented by two taxa. The first species is the ochotonid Prolagus sorbinii, which is commonly reported in the Messinian of Italy and Greece (Angelone, 2007) and is also rather common in the various levels of MCC (Angelone et al., 2011). The second lagomorph is represented by a few dental remains and a right astragalus belonging to a leporid. The Messinian record of European leporids includes Alilepus, Trischizolagus and Hypolagus (Flynn et al., 2014). In Italy, Alilepus meini has been reported in the locality of Baccinello V3, which is slightly older than MCC. Trischizolagus sp. has been described from Borro Strolla, a site that is nearly coeval to MCC (Angelone and Rook, 2012). Due to its scantiness, we prefer to assign the material from MCC to Leporidae indet.
PALEOECOLOGY
Paleoecology of Mollusks
In absolute numbers, Cochlostoma esuanum, Parmacella sp., Lucilla miocaenica and Limacidae/Agriolimacidae dominate among the terrestrial taxa of Gastropoda (Figure 3.1-5). Modern species of Cochlostoma prefer rocky limestone habitats in open or forested environments. They often live on rock rubble (Welter-Schultes, 2012). Stony open habitats are also reported for extant Parmacella species (Kerney et al., 1979). Similar ecological requirements are indicated for the very frequent Aciculidae, e.g., Acicula cf. lineata (Figure 3.8). Aciculids are typically found in deciduous forests and deep under rock rubble, where they find shelter from desiccation (Boeters et al., 1989). Among the clausiliids, the closing apparatuses of Nordsieckia pontica and Truciella ballesioi indicate adaptations to at least periodical arid conditions (Figure 3.6-7). Some moisture, within leaf litter, deadwood and soil, is indicated by the genera Craspedopoma, Hydrocena, Helicodiscus and Argna (Welter-Schultes, 2012). The deeper parts of the soil cover was inhabited by the numerous subterranean Lucilla and Cecilioides. The freshwater species are represented only by few specimens, indicating that freshwater environments were rather rare. Similarly, species adapted to moist lakeside habitats, such as Carychium cf. rufolabiatum, Carychiella puisseguri and an unidentified Succineidae, were very rare. All these species might have dwelt along and in ephemeral puddles and ponds. Among bivalves, the very rare Pisidium may indicate at least the presence of ephemeral freshwater bodies in the surroundings. Dreissenids and limnocardiids along with melanopsids and hydrobiids, among gastropods, point to a Lago-Mare biofacies (Esu, 2007; Guerra-Merchán et al., 2010). Overall, the Lago-Mare gastropod and bivalve assemblage from the Upper Messinian deposits of Moncucco is indicative of a shallow water environment with low (oligo- to mesohaline) salinity.
Paleoecology of Fishes
The aquatic macrofauna of MCC is indicative of a brackish environment. As demonstrated by Mariani (2001), fish assemblages represent a very useful complementary tool in defining the influence of sea level in paralic biotopes. The fish assemblages reveal a sharp dominance of estuarine residents associated with marine migrants, thereby suggesting a brackish depositional environment characterized by thalassogenic waters and a reduced degree of confinement (see Guelorget and Perthuisot, 1992).
The diversity of lanternfishes and other oceanic taxa is not fully consistent with any kind of brackish biotope. However, the occurrence of otoliths of this kind of fishes in brackish deposits can be explained on taphonomic grounds. Nolf (1985, 2013) showed that the predatory activity of fishes and marine mammals largely contributes to the formation of otolith taphocoenoses. Large piscivorous vertebrates that forage in open-ocean environments frequently accumulate otoliths in their excreta (Schäfer, 1966). They often transport them in shallow water biotopes, including those with brackish thalassogenic waters. Many predatory fishes extensively consume lanternfishes, as well as small gadiforms, and accumulate great amounts of their otoliths in the stomachs. As a consequence, otoliths of these oceanic fishes are commonly transported into shallow marine or brackish biotopes by the predatory activity of large fishes, like scombrids or carangids, which periodically visit paralic environments attracted by habitat availability and great seasonal abundance of food (Elliott and Dewailly, 1995; Whitfield, 1999; Nordlie, 2003). The presumed taphonomic processes connected with the migratory activity of predatory fishes have substantial implications for interpreting the physiography of the original depositional environment, suggesting the existence of a permanent, persistent connection between the paralic biotope and the open sea. In summary, the structure of the fish assemblage and the relative abundance of the recognized taxa and ecological guilds concur to suggest that the Messinian post-evaporitic fossiliferous deposits of Moncucco Torinese accumulated in a coastal lagoon permanently connected with a marine environment with normal marine waters. Some environmental features of this coastal lagoon can be defined based on the ecological characteristics of the taxa of the guild of estuarine residents, in this particular case, the sciaenids. Sciaenids occur worldwide in tropical to temperate coastal waters and estuarine settings (e.g., Sasaki, 1989). These fishes are usually demersal carnivores (piscivores, infaunal feeders, epipsammivores) with a relevant ecological role in estuarine ecosystems. The very reduced taxonomic diversity of estuarine residents and their relative abundances are probably indicative of a paralic context characterized by fluctuating salinity and highly turbid waters. Such a context is usually characterized by a simplified food web. Here Sciaenidarum sp. nov. was the most successful taxon and occupied the broadest niche.
Paleoecology of Amphibia and Reptilia
The identified amphibian and reptiles indicate, altogether, a varied ecological spectrum. Most of the amphibians require the presence of at least temporary fresh waters, but Pelophylax is tied to more permanent waters. Newts in general prefer temporary, standing waters devoid of fishes. Otherwise, they select water bodies with abundant vegetation providing shelter from fishes (Lanza et al., 2007). The ecology of Albanerpeton, a taxon belonging to an extinct clade, is not known in detail. Even if it had been considered as a fossorial, ‘dry adapted’ taxon because the first fossils came from karstic areas (that could be superficially dry), its recent finding in different environments (from floodplain, to coastal deltaic and lacustrine deposits) lead to the conclusion that stable, moist and shaded conditions were preferred, and that in karstic areas it could have exploited existing crevices, similarly to the cave salamanders that were found co-occurring in the geologically youngest site with allocaudates of Rivoli Veronese (see Delfino and Sala, 2007, and references therein).
Reptiles also include burrowing taxa, such as worm lizards, worm snakes and sand boas. According to the ecological needs of the extant European representatives of these taxa, worm lizards often occur “in rather moist places, both in soils with a lot of humus and in ones that are predominantly sandy [...] and avoid densely packed soils and clay” (Arnold and Ovenden, 2002: 194). Worm snakes and sand boas are more dry-adapted. The latter, in particular, are found “principally in dry habitats, usually with a good covering of light soil or sand” (Arnold and Ovenden, 2002: 202). The only extant European agamid inhabits a variety of dry, often rocky habitats (Arnold and Ovenden, 2002), ecological requirements that it shares with all the other Mediterranean agamid species. In contrast, crocodylians and water terrapins confirm the local presence of water bodies that only temporarily can dry up. The extant Mediterranean species of the water terrapin Mauremys can even survive in brackish waters (Arnold and Ovenden, 2002), including coastal lagoons. None of the identified amphibians and reptiles indicates the presence of dense forests, but most of them could have lived in ecotonal areas at the edge of forests.
Paleoecology of Birds
Most of the identified birds were fairly ubiquitous ecologically or had unknown ecological needs. Noteworthy is the occurrence of Picidae and Coliidae, which are indicative of woodlands and bushlands, whereas Phasianidae might also imply the presence of open areas. It is also worth mentioning that no remains of species connected with aquatic ecosystems have been found.
Paleoecology of Large Mammals
The large mammal community from MCC includes thermophilic taxa indicative of a mixture of habitats, from forested to open landscapes. Tapirus arvernensis occurred mainly in humid to wet jungles and rainforests (Lacombat et al., 2008), seeking out rivers and marshes. Dihoplus schleiermacheri and Pliocervus preferentially inhabited humid woodlands. They may have occasionally ventured into more open settings, but anyhow in moist contexts close to wooded areas (van der Made et al., 2006; Spassov et al., 2006).
Dicoryphochoerini, Euprox and the gazelle are more typically associated with open, moist environments. Dicoryphochoerini lived in grasslands with mixtures of shrubs and trees (Liu et al., 2004). Euprox habitats included areas of dense vegetation, close to a water source (Merceron et al., 2012). The gazelle of Moncucco Torinese is quite similar to Gazella pilgrimi, which is associated with open grasslands (Koufos et al., 2009).
The carnivorans of MCC are ubiquitous species. Euarctos pyrenaicus can be considered ecologically analogous to the extant American black bear and is therefore indicative of woodlands, scrub forests and riparian areas (Larivière, 2001). The viverrine remains resemble those of the extant Civettictis civetta, which inhabits different kinds of forests (rainforests and scrub forests), but also open savannas and grasslands, generally with access to permanent water bodies (Ray, 1995; Salesa et al., 2006). Also Pristifelis attica lived either in forested areas, or in open landscapes (Salesa et al., 2012). Exant representatives of Ictonychini, such as Vormela and Ictonyx libycus, mainly frequent subdesertic areas dominated by steppes (Gorsuch and Larivière, 2005; Hoffman et al., 2015), whereas Poecilogale and Ictonyx striatus have a wide habitat tolerance that extends from open/sub-desertic areas to open woodlands (Larivière, 2002; Stuart et al., 2008). Baranogale cf . helbingi from MCC may be indicative of both open areas and woodlands.
The cercopithecoid primates recorded at MCC do not have very strict environmental requirements. Cf. Macaca would be generally indicative of moderately warm and humid environments with forested areas and water bodies, but not incompatible with the presence of more arid and open biotopes nearby (Alba et al., 2014). In turn, Mesopithecus pentelicus would be suggestive of a mosaic habitat, including patches of forest alternating with grasslands, bushy areas and gallery forests (Alba et al., 2014, 2015, and references therein), although the arboreal adaptations of the Mesopithecus talus from Moncucco further attests to the presence of some densely forested areas (Alba et al., 2014).
Overall, the Moncucco Torinese large mammals seem to form an ecologically coherent community. They suggest extensive arboreal cover, scattered with open grassy steppe areas and pools, under warm temperate and mesic climatic conditions.
Paleoecology of Small Mammals
The water shrews of the Neomyini tribe have semiaquatic habits and live near water edges in wet and warm regions (García-Alix et al., 2008). The ecological requirements of the galericine Parasorex aff. ibericus are virtually unknown. Morphological analyses, however, suggest a diet based on herbaceous plants (van den Hoek Ostende, 2001). Petenya is considered an opportunistic taxon (Popov, 2003). The presence of Talpa is indicative of humid conditions in a variety of different habitats (García-Alix et al., 2008).
Hystrix (Hystrix) depereti is considered an eurytopic species, based on the present occurrence of Hystrix in many different habitats (Santini, 1980). Subgenus Hystrix is currently spread in areas with an average annual temperature above 10° C (Tong, 2008).
Like the extant Castor, extinct beavers of the subfamily Castorinae had (semi)aquatic habits (Rybczynski, 2007).
Even if extremely rare, the sciurids from MCC include at least four different taxa. Pteromyini includes Hylopetes hungaricus and Pliopetaurista pliocaenica, two flying squirrels indicative of forested environments (García-Alix et al., 2008; Thorington et al., 2012). Sciurus warthae, the earliest Old World squirrel of Europe, and Sciurinae indet. were also related to the presence of forests (Thorington et al., 2012).
Neocricetodon is rather rare, but regularly present at MCC. It is related to wet conditions (Daams et al., 1988); van Dam and Weltje (1999) suggested that this hamster was eurytopic.
Gliridae and Muridae include more than 80% of the small mammals from MCC. The great abundance of these taxa with highly selective environmental requirements calls for special attention to the ecological information they have provided.
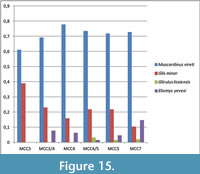 Glirids are mainly arboreal taxa with affinity for different forested biotopes. Although only moderately frequent, all the glirids of MCC are related to extant taxa with specific ecological needs. They are therefore extremely useful in defining the nature and the variation of the forested habitats of MCC. The most common dormouse at MCC is Muscardinus vireti (4%-7% of the small mammals), followed by Glis minor (0.8%-2%). Eliomys yevesi is rather infrequent (0%-1%) and Glirulus lissiensis is extremely rare, with only four specimens found in the layers MCC4/5, MCC5 and MCC7. The analysis of these four species reveals that Muscardinus vireti maintains a stable abundance throughout the whole studied succession, with only slightly lower relative abundance in MCC3 (Figure 15). On the contrary, Glis minor decreases somewhat in abundance moving upwards along the succession, reaching its lowest frequency in layer MCC7. Today, the species of the genus Glis prefer wooded areas with well interconnected canopy, whereas Muscardinus tends to avoid closed forests, preferring those with clearings and gaps that favour growth of understory (Capizzi and Filippucci, 2008; Juškaitis and Šiožinytė, 2008). Extant Eliomys quercinus and Eliomys melanuru s, which are the less arboreal glirids of Europe, can frequent woodlands and bushlands, but they can also be found in rocky areas (Capizzi and Filippucci, 2008). Therefore, Eliomys yevesi is interpreted here as indicative of “woodland/bushland” and “rocky outcrop” habitats. Glirulus japonicus is a small, strictly arboreal glirid, currently widespread in the primary and secondary temperate forests of Japan (Ishii and Kaneko, 2008). We postulate similar habitat requirements for G. lissiensis. These data suggest that the wooded areas of MCC consisted mainly of thinned out forests with well-developed shrubby understory and limited closed canopy areas. The slightly lower abundance of Glis and the parallel, moderately increased frequency of Eliomys in the upper layers, especially in the samples MCC3/4 -MCC4 and MCC7, may indicate a further slight contraction of the closed canopy in the forested habitats, which further favored the development of shrubberies or of other shade-intolerant plants in the upper portion of the succession.
Glirids are mainly arboreal taxa with affinity for different forested biotopes. Although only moderately frequent, all the glirids of MCC are related to extant taxa with specific ecological needs. They are therefore extremely useful in defining the nature and the variation of the forested habitats of MCC. The most common dormouse at MCC is Muscardinus vireti (4%-7% of the small mammals), followed by Glis minor (0.8%-2%). Eliomys yevesi is rather infrequent (0%-1%) and Glirulus lissiensis is extremely rare, with only four specimens found in the layers MCC4/5, MCC5 and MCC7. The analysis of these four species reveals that Muscardinus vireti maintains a stable abundance throughout the whole studied succession, with only slightly lower relative abundance in MCC3 (Figure 15). On the contrary, Glis minor decreases somewhat in abundance moving upwards along the succession, reaching its lowest frequency in layer MCC7. Today, the species of the genus Glis prefer wooded areas with well interconnected canopy, whereas Muscardinus tends to avoid closed forests, preferring those with clearings and gaps that favour growth of understory (Capizzi and Filippucci, 2008; Juškaitis and Šiožinytė, 2008). Extant Eliomys quercinus and Eliomys melanuru s, which are the less arboreal glirids of Europe, can frequent woodlands and bushlands, but they can also be found in rocky areas (Capizzi and Filippucci, 2008). Therefore, Eliomys yevesi is interpreted here as indicative of “woodland/bushland” and “rocky outcrop” habitats. Glirulus japonicus is a small, strictly arboreal glirid, currently widespread in the primary and secondary temperate forests of Japan (Ishii and Kaneko, 2008). We postulate similar habitat requirements for G. lissiensis. These data suggest that the wooded areas of MCC consisted mainly of thinned out forests with well-developed shrubby understory and limited closed canopy areas. The slightly lower abundance of Glis and the parallel, moderately increased frequency of Eliomys in the upper layers, especially in the samples MCC3/4 -MCC4 and MCC7, may indicate a further slight contraction of the closed canopy in the forested habitats, which further favored the development of shrubberies or of other shade-intolerant plants in the upper portion of the succession.
Murids are the most diverse and abundant small mammals of Moncucco Torinese. The sample includes 2406 teeth, which have been assigned to seven different species. Moreover, except for Stephanomys sp., which is represented by only five teeth, the other species are fairly uniformly distributed in all the examined layers. Given this large amount of data, the samples of murids from the different layers have been analyzed systematically in order to detect any possible significant variation through the succession. Figure 16 shows that Occitanomys brailloni is always the most abundant taxon (25%-35% of the murids). Apodemus gudrunae is usually very well represented (20%-30%) and the second most common taxon except in MCC7. Centralomysbenericettii and Paraethomys meini usually occur in a range between 10% and 20 % of the murids; they are slightly more frequent in the upper layers, particularly in MCC7. Apodemusatavus and Micromysbendai are rather uncommon, each representing less than 10% of the murid remains.
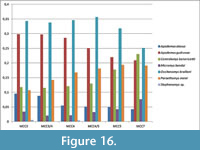 From the base to the top of the studied succession, the most evident datum is, on the one hand, the reduction of Apodemus atavu s, Apodemus gudrunae and Occitanomys brailloni and, on the other, the increased abundance of Centralomys benericettii, Paraethomys meini and Micromys bendai (Figure 16). Chi-square tests reveal that there are no significant differences between murid samples of the assemblages MCC3, MCC3/4 and MCC4 (Table 1). Moreover, contiguous assemblages do not show significant differences (except MCC5 and MCC7). By contrast, the most evident differences are observed between the basal (MCC3-MCC3/4-MCC4) and the uppermost (MCC5 and MCC7; Table 1) part of the succession. These data suggest that the structure of the murid community changes significantly from the older (MCC3) to the youngest (MCC7) assemblages. This is confirmed by the models obtained through Binary Logistic Regression, where most murid species (Stephanomys sp. is excluded due to its extreme rarity) exhibit significantly different incidences in layer MCC7 with respect to MCC3 (Figure 17 and Appendix 8). However, this important discrepancy results from successive gradual changes recorded across the whole succession, which increasingly affect the murid assemblage. In more detail, Apodemus atavus and Paraethomys meini apparently display the most evident changes in the basal part of the succession. Between MCC3 and MCC4, there is a rapid decline of Apodemus atavus and an increase of Paraethomys meini (Figure 17). Micromys bendai, Centralomys benericettii, Apodemus gudrunae and Occitanomys brailloni show significant changes only in the upper part of the succession, particularly in layers MCC5 and MCC7. A. gudrunae and Occitanomys brailloni decline, whereas Centralomys benericetti and Micromys bendai proliferate (Figure 17). These variations were probably induced by environmental changes. Unfortunately, the paleoecological requirements of some of these murid taxa is imperfectly known, because the genera Paraethomys, Stephanomys, Occitanomys and Centralomys have no living counterparts. However, the large amount of information disclosed by the many proxies used in this study allows inferences on the paleoecological preferences of these extinct taxa.
From the base to the top of the studied succession, the most evident datum is, on the one hand, the reduction of Apodemus atavu s, Apodemus gudrunae and Occitanomys brailloni and, on the other, the increased abundance of Centralomys benericettii, Paraethomys meini and Micromys bendai (Figure 16). Chi-square tests reveal that there are no significant differences between murid samples of the assemblages MCC3, MCC3/4 and MCC4 (Table 1). Moreover, contiguous assemblages do not show significant differences (except MCC5 and MCC7). By contrast, the most evident differences are observed between the basal (MCC3-MCC3/4-MCC4) and the uppermost (MCC5 and MCC7; Table 1) part of the succession. These data suggest that the structure of the murid community changes significantly from the older (MCC3) to the youngest (MCC7) assemblages. This is confirmed by the models obtained through Binary Logistic Regression, where most murid species (Stephanomys sp. is excluded due to its extreme rarity) exhibit significantly different incidences in layer MCC7 with respect to MCC3 (Figure 17 and Appendix 8). However, this important discrepancy results from successive gradual changes recorded across the whole succession, which increasingly affect the murid assemblage. In more detail, Apodemus atavus and Paraethomys meini apparently display the most evident changes in the basal part of the succession. Between MCC3 and MCC4, there is a rapid decline of Apodemus atavus and an increase of Paraethomys meini (Figure 17). Micromys bendai, Centralomys benericettii, Apodemus gudrunae and Occitanomys brailloni show significant changes only in the upper part of the succession, particularly in layers MCC5 and MCC7. A. gudrunae and Occitanomys brailloni decline, whereas Centralomys benericetti and Micromys bendai proliferate (Figure 17). These variations were probably induced by environmental changes. Unfortunately, the paleoecological requirements of some of these murid taxa is imperfectly known, because the genera Paraethomys, Stephanomys, Occitanomys and Centralomys have no living counterparts. However, the large amount of information disclosed by the many proxies used in this study allows inferences on the paleoecological preferences of these extinct taxa.
 Apodemus atavus is closely related to Apodemus sylvaticus and Apodemus flavicollis. It lived in densely woody areas or at the edges of forests (García-Alix et al., 2008). Apodemusgudrunae is considered an ancestor of the lineage that led to the extant subgenus Karstomys (see Hernández Fernández, 2001), which is currently widespread in the Eastern Mediterranean open and degraded forests with dense understory and sparse cover of grasses and shrubs, as well as on rocky areas (Amori et al., 2008; Bego et al., 2009). Today, the abundance of Apodemus (Karstomys) mystacinus is strictly related to the amount of foliage present at 30-200 cm from the ground’s surface. This species therefore has a preference for shrubs and bushlands (Abramsky, 1981). Apodemus gudrunae is here supposed to have had similar ecological preferences. The genus Micromys is related to grasslands in humid contexts. This conclusion is mainly based on the ecological requirements of Micromys minutus, the extant harvest mouse (García-Alix et al., 2008; Koufos and Vasileiadou, 2015). Stephanomys sp. is considered here as an eurytopic species (see García-Alix et al., 2008).
Apodemus atavus is closely related to Apodemus sylvaticus and Apodemus flavicollis. It lived in densely woody areas or at the edges of forests (García-Alix et al., 2008). Apodemusgudrunae is considered an ancestor of the lineage that led to the extant subgenus Karstomys (see Hernández Fernández, 2001), which is currently widespread in the Eastern Mediterranean open and degraded forests with dense understory and sparse cover of grasses and shrubs, as well as on rocky areas (Amori et al., 2008; Bego et al., 2009). Today, the abundance of Apodemus (Karstomys) mystacinus is strictly related to the amount of foliage present at 30-200 cm from the ground’s surface. This species therefore has a preference for shrubs and bushlands (Abramsky, 1981). Apodemus gudrunae is here supposed to have had similar ecological preferences. The genus Micromys is related to grasslands in humid contexts. This conclusion is mainly based on the ecological requirements of Micromys minutus, the extant harvest mouse (García-Alix et al., 2008; Koufos and Vasileiadou, 2015). Stephanomys sp. is considered here as an eurytopic species (see García-Alix et al., 2008).
The habitat needs of Occitanomys brailloni, which is the most abundant mammal of MCC, are difficult to define. Some authors consider it typical of open areas, mainly on the basis of the stephanodonty that characterizes the molar pattern of this species (Vasileiadou et al., 2003; Koufos and Vasileiadou, 2015). Stephanodont molars display well-developed longitudinal connections. These dental patterns, associated with hypsodonty and broad crowns, are usually interpreted as indicative of adaptation to an abrasive, herbaceous diet (Van Dam, 1996; Renaud et al., 1999). However, grasses are also an important component of the ground vegetation in open forests (Andrews, 2006). Other authors (Hernández Fernández and Peláez-Campomanes, 2003) believe that Occitanomys preferred forested biotopes. Finally, according to García-Alix et al. (2008) and Mansino et al. (2015b), some species of Occitanomys would have been eurytopic. It is worth noting that the molars of Occitanomys brailloni do not display a marked stephanodonty and are particularly brachyodont. Moreover, a similarly moderate or even more pronounced stephanodonty is currently observed in some African murids that live in a variety of open forested habitats (see Monadjem et al., 2015), including Acacia bushlands and thicket vegetation in savannah biomes (Thallomys, Grammomys), bushlands and thickets in coastal, riverine or montane forests (Grammomys, Thamnomys, Desmomys) or tangled vegetation in clearings or at the edge of rain forests (Oenomys). This indicates that stephanodonty is not exclusively related to dry open habitats. Moreover, recent paleofloristic analyses suggest that during stage 3.2 of the MSC, the mesic lowland territories of Piedmont were largely dominated by subhumid forests mainly constituted by Mediterranean taxa (Bertini and Martinetto, 2011). Since Occitanomys brailloni is by far the most abundant mammal taxon at MCC, a strict preference for extensive open areas devoid of woodlands does not seem to be consistent with the paleovegetational reconstruction of the latest Messinian Piedmont. Moreover, the significant decrease of Occitanomys brailloni from MCC3 to MCC7, in conjunction with that of Apodemus and of arboreal species such as Glis, further suggests that Occitanomys brailloni preferred wooded areas. In this view, a slight expansion of open habitats, connected with the reduction of woodlands, would have negatively affected the population of Occitanomys brailloni. Therefore, similarly to most of the African stephanodont taxa, and in agreement with Hernández Fernández and Peláez-Campomanes (2003), Occitanomysbrailloni is considered here typical of woodlands/bushlands habitats.
During the latest Miocene Paraethomys meini dispersed into Europe from Northern Africa (Agustí et al., 2006), where it co-occurred with rodent taxa typical of arid/desertic open environments, including Ctenodactylidae, Gerbillidae and ground squirrels. In line with Martín-Suárez et al. (2001) and García-Alix et al. (2008), this taxon is here believed as an indicator of dry and warm climates, with a preference for open/herbaceous biotopes. The habitat requirements of Centralomys benericettii, a latest Miocene endemic of the Italian Peninsula, are only partially defined. The moderate stephanodonty and hypsodonty may suggest an herbaceous diet (Colombero and Pavia, 2013). Its maximum abundance (MCC7) actually coincides with the greatest abundance of taxa that inhabited open habitats (Paraethomys and Micromys), but also with the reduction of taxa related to woodlands (Apodemus, Glis). In the cases of Centralomys benericettii and Paraethomys meini, these hypotheses are corroborated by the analysis of rodents from the Piedmont locality of Verduno (Colombero et al., 2013). In that slightly older locality, the vertebrate fauna includes camels, gazelles and canids, as well as other taxa indicative of a savannah-like biome (Colombero et al., 2014a, 2016). Centralomys benericettii dominates the small mammal assemblages of Verduno. Together with Paraethomys meini it represents more than 95% of the recovered rodents (Colombero et al., 2013). This oligotypic rodent assemblage, in which arboreal taxa are nearly absent, can be correlated with a quite uniform habitat, mainly constituted of rather extended open areas, devoid of (or with extremely reduced) wooded districts, in which Paraethomys and Centralomys were dominant rodents (Colombero et al., 2013). In any case, it is not possible to conclusively exclude that these murid taxa also frequented bushlands and open forests, even considering that, in these habitats, grasses are primary elements of the ground vegetation. Hence, these two species are associated here with both woodlands/bushlands and grasslands habitats. Bioclimatically, Centralomys benericettii is referred to the climate types II, II/III and IV. In contrast to Hernández Fernández and Peláez-Campomanes (2003), who considered it an equatorial species, and following the indications of García-Alix et al. (2008), Paraethomys meini is believed to have been adapted to drier climates and is associated with climate types II, II/III, III and IV.
Leporidae indet. from MCC had unknown habitat preferences. Following Vasileiadou et al. (2003), we attribute a eurytopic affinity to Prolagus sorbinii. However, by analogy with the extant cottontail rabbit Sylvilagus, Prolagus sorbinii is considered a forest element by some authors (López-Martínez, 2001). The slight decrease of Prolagus sorbinii in layer MCC7 may indicate that, like other woodland taxa, this ochotonid possibly preferred woodlands/bushlands rather than wide open habitats. However, further analyses are necessary to confirm such a hypothesis.
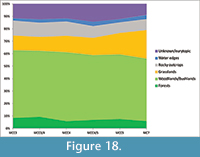 Paleoenvironmental indications of small mammals. In all the studied vertebrate fossil assemblages from MCC, the small mammals exhibit a similar and rather high diversity index (Table 2). As evidenced above, the small mammal taxa from Moncucco Torinese are indicative of a variety of habitats. The presence of forested areas is suggested by the occurrence of Sciurini, Pteromyini, Gliridae and also by some murids (Apodemus and Occitanomys brailloni). However, small mammals as a whole indicate that woodlands were primarily constituted by open, thinned out forests with well-developed understory, whereas closed canopy forests could grow only on moist or wet soils, such as near ponds or rivers. In contrast, open areas are suggested by some rodents, including Micromys bendai, Paraethomys meini and Centralomys benericettii. Rocky outcrops and rare humid areas were important elements of the inferred paleolandscape, enhancing the spectrum of available habitats (Figure 18 and Appendix 9).
Paleoenvironmental indications of small mammals. In all the studied vertebrate fossil assemblages from MCC, the small mammals exhibit a similar and rather high diversity index (Table 2). As evidenced above, the small mammal taxa from Moncucco Torinese are indicative of a variety of habitats. The presence of forested areas is suggested by the occurrence of Sciurini, Pteromyini, Gliridae and also by some murids (Apodemus and Occitanomys brailloni). However, small mammals as a whole indicate that woodlands were primarily constituted by open, thinned out forests with well-developed understory, whereas closed canopy forests could grow only on moist or wet soils, such as near ponds or rivers. In contrast, open areas are suggested by some rodents, including Micromys bendai, Paraethomys meini and Centralomys benericettii. Rocky outcrops and rare humid areas were important elements of the inferred paleolandscape, enhancing the spectrum of available habitats (Figure 18 and Appendix 9).
The moderate decrease in the abundance of Apodemus and Occitanomys brailloni, and the associated increase of Paraethomys, Micromys and Centralomys from MCC3 to MCC7, may indicate a slight reduction of woodlands and a modest expansion of open/herbaceous areas in the upper part of the sequence. A moderate reduction of closed canopy forests and woodlands may have occurred between layers MCC3 and MCC4, as testified by the conspicuous decrease in abundance of A. atavus and the concomitant increase of P. meini (Figure 18, Appendix 8 and Appendix 9). Subsequently, between MCC4/5 and MCC7, the significant reduction of frequency of O. brailloni and A. gudrunae, associated with the concurrent increase of C. benericettii and M. bendai, may suggest a further, slight expansion of the open areas. The reduction of the forested areas seems to be corroborated by the rarefaction of Glis minor between MCC3 and MCC3/4, followed by a further decrease of its incidence in layer MCC7. Conversely, Eliomys became the second most common glirid. It should be remarked that layer 6 of the succession of MCC, which is positioned between MCC5 and MCC7, is barren of fossils. It may correspond to a phase of no water influx and development of an incipient calcareous soil (Angelone et al., 2011), which is also indicative of a slight modification of the local paleoenvironmental conditions. In any case, these data document moderate variations within the fairly uniform small mammal community and not important modifications in the fauna as a whole (which, in contrast, would be characterized by abrupt turnovers of predominant taxa). Based on this, it can be speculated that paleoenvironmental variations were somewhat limited and consisted merely of local modification of the paleolandscape. The heterogeneous paleoenvironmental conditions postulated here may have led to an increase of richness of the small mammal community of MCC, especially considering that open forest habitats display a highly diverse vegetation that can expand the amount of small mammal species (Williams et al., 2002).
PALEOCLIMATOLOGY
The paleoclimatic parameters obtained with different methodologies are consistent with each other (Table 3). This supports that the large amount of specimens collected during extensive excavations are useful proxies for the actual faunal composition of the small mammal communities and of their changes through time. The MAP estimated for MCC is very similar to that obtained from the roughly coeval MN13/MN14 locality of Maramena and higher than those estimated based on the faunas of other MN13 localities of Europe (van Dam, 2006). Moreover they are very close and only slightly lower than those estimated by Fauquette et al. (2006) for the post-evaporitic (5.40-5.33 Ma) locality of Torre Sterpi (Piedmont) based on the analysis of pollens (MAP=1100-1400 mm). These data are in line with the increased humidity recorded in the Mediterranean during phase 3.2 of the MSC, which slightly precedes the Miocene-Pliocene boundary (Roveri et al., 2014). The estimated temperature values for MCC partially overlap those hypothesized for Piedmont in phase 2 of the MSC (5.96-5.6 Ma) based on the analysis of the physiognomy of the leaves (MAT=13-16 °C; Martinetto et al., 2007) and are comparable to those reported by Fauquette et al. (2006) for Torre Sterpi (5.40-5.33 Ma) in Piedmont after pollen analyses (MAT=15.6-20 °C; MTW=20.0-27.7°C; MTC=4.9-16.2°C).
GENERAL PALEOECOLOGICAL AND PALEOCLIMATIC DISCUSSION
Paleoenvironmental Insights
The taxonomic identification of 16,675 vertebrate specimens and 4,900 terrestrial, freshwater and brackish mollusk shells from MCC depicted highly diversified upper Messinian fossil assemblages. Vertebrates occur with 90 taxa, including 17 fishes, eight amphibians, 13 reptiles, 12 birds and 40 mammals. Mollusks include at least 15 bivalves and 53 gastropods. These taxa indicate a varied and heterogeneous paleolandscape, characterized by a wide spectrum of habitats and many different ecological niches. The ecological affinities of the fish taxa indicate that faunal remains from the mainland accumulated in coastal lagoons after relatively short water transport (Angelone et al., 2011; this paper). Therefore, the hypothesis of a wide drainage basin that extended through different habitats across a wide area seems to be unlikely, because the fossils do not exhibit evidence of prolonged transportation from distant areas. Alternatively, we can hypothesize the presence of a mosaic landscape in which different localized habitat patches, possibly maintained by patch dynamics and mild disturbances (Pickett and White, 1985) similar to those characteristic of open forests and shrublands in subtropical climates (e.g., see Trabaud, 1994; Kirkman et al., 2004; Safford and Harrison, 2004), provided differentiated resources and shelters, further amplifying the diversity of the faunal communities (Torre and Díaz, 2004; Swanson et al., 2010; Zozaya et al., 2011). The terrestrial fossil fauna of MCC indicates that dry, thinned out forests with well-developed understory, including bushlands, forest edges and early successional stages of forests, were the dominant elements of the inferred paleoenvironment. This conclusion is implied in particular by mollusks and mammals, but reptiles and birds are also consistent with such a reconstruction. A landscape dominated by open forests with clearings in the canopy is also consistent with the presence of a mosaic landscape that favors an increase in productivity and resource availability (Young, 1995). However, more closed forests, probably confined to humid areas such as riparian gallery forests, were also present, as indicated by sporadic flying squirrels and rare taxa including tapirs and some other large mammals. Vast open areas dominated by herbs and grasses were probably less common, though grasslands slightly expanded when the upper part of the succession accumulated, as suggested by the change in the composition of the small mammal assemblages (Figure 18). Rocky outcrops, basical for some of the most common gastropods, as well as for various reptiles and several rodents, were also certainly present. The thick gypsum layers, which currently represent the most important rocky substrate of the territory of Moncucco Torinese, were most likely exposed during the post-evaporitic Messinian (Dela Pierre et al., 2007). It is worth noting that gypsum can be easily affected by karstic processes that create crevices and fissures, which are ideal shelters for many terrestrial animals. Freshwater reservoirs were rare and probably represented by ephemeral ponds, given the rarity of freshwater mollusks and of amphibians that prefer fish-free (therefore temporary) water bodies. However, the occurrence of tapirs, beavers and water shrews indicate that a few, maybe limited, permanent internal basins were present in the sorroundings. The paleoenvironmental conditions inferred here are consistent with the floristic evidence (Kovar-Eder et al., 2006; Bertini and Martinetto, 2011), which indicates, during phase 3.2 of the MSC in northwestern Italian territory, the presence of subtropical and sub-humid open forests associated with the more closed forests of the riparian belts or near humid zones. These paleocarpological and palynological analyses indicate for the Piedmont area high abundance of Cupressus cf. sempervirens, but also of other taxa adapted to dry-mesic conditions, such as evergreen oaks (Quercusilex-type), chastetrees (Vitex) and medicks (Medicago). In this context, moderately high humidity would have enhanced the spread of thinned out forests with clearings that supported the development of the understory. Currently, C. sempervirens forms open woodlands with well-developed understory in many poor and dry soils, or even rocky areas of the Mediterranean region (Brofas et al., 2006; Papanastasis et al., 2009). Taxa requiring more humid conditions, such as the riparian Populus or Salix and also Magnolia and Zanthoxylum, were present in Piedmont area, indicating the presence of gallery forests with more closed canopy along freshwater reservoirs and humid areas.
A slight expansion of open areas with the concomitant reduction of woodlands can be hypothesized towards the upper part of the section, based on the presence of some rodents, particularly glirids and murids (Figure 18). However, the diversity of the rodent paleocommunity was not affected by these environmental changes, as shown by the lack of any marked faunal turnover. Instead, from the base to the upper portion of the succession, some species grew more abundant, while others declined. These changes possibly reflect slight modifications of the paleolandscape that are unlikely related to large scale climatic variations. For example, it can be conjectured that the mammal paleocommunity was subjected to humidity-driven changes, connected with tectonically-induced interruption or reduction of water afflux, or with variation in the endhoreic or exhorheic nature of internal basins as previously supposed for other similar slight variations of the fossil faunal communities (García-Alix, 2015). Local droughts can also occur after moderately severe disturbances such as wildfires, which favor the development of grasslands and the reduction of woodlands, thereby increasing the abundance of species that require grasses and herbs (Recher et al., 2009). Moreover, different patterns of variation in the abundances of large and small mammals are typically observed in many Mediterranean-type ecosystems due to recurrent disturbances, such as wildfires (Quinn, 1994). A community dominated by tree life forms can shift to shrublands or to a dominance of herbs after high disturbance rates (Runkle, 1985) and the early successional stages after severe disturbances are usually dominated by annual and perennial herbs (Swanson et al., 2010). For example, in the extant Greek forests, the mixed stands of coniferous plants, including Pinus alepensis, Pinus brutia and Cupressus sempervirens, can be gradually replaced by earlier successional stages after continuous wildfires (Zagas et al., 2001). In conclusion, it cannot be excluded that local factors, such as severe or constant mild disturbances or variations of the structure of the drainage basins, may have played a major role in the modification of the paleolandscape in the territory surrounding the paleobiotopes recorded at MCC during the studied time interval.
Paleoclimatic Insights
The paleoclimatic information disclosed by the small mammals of MCC indicates that the MAT and MAP at the end of the Messinian were roughly similar to those currently observed for coastal areas of Italy and the Balkans, which are characterized by Mediterranean and subtropical temperate climates (Frischling, 2016). The bioclimatic spectra resulting from the analyses (Appendix 10) reveal a slight prevalence of the Mediterranean climate (26.9%) over a typical temperate climate (19.4%). Tropical climate with summer rains (13.5%) has much lower incidence. These results agree with those reached by paleobotanical analyses, which indicate sub-humid conditions in a subtropical climate (Bertini and Martinetto, 2011). The moderately high MAP estimated by the analyses performed here is consistent with the increase in humidity hypothesized for the post-evaporitic phase 3.2 of the MSC (Roveri et al., 2014). An increase in precipitation in the latest Miocene is also postulated based on the higher sedimentation rates recorded in the Po Basin during the post-evaporitic Messinian (Willet et al., 2006). The increase in sediment yield was probably driven by global climatic changes correlated with the end of the late Miocene glacial period at 5.5 Ma, slightly before the Lago-Mare event (Willet et al., 2006). In summary, the results of the present study concur with previous analyses showing that moderately humid, mesic conditions occurred in NW Italy at least during the stage 3.2 of the MSC. This period was probably wetter than the preceding stage 3.1 (5.55-5.42 Ma), when the Italian Peninsula experienced moderate xeric conditions, as testified by the spread of open vegetation in central and northern Italy, including typical steppe elements of northern Africa origin, such as Lygeum (see Bertini, 2006).
CONCLUSIONS
The thorough analyses on the highly diverse fossil fauna of the latest Miocene locality of MCC lead to the following conclusions:
- The fish assemblage includes 17 species. These indicate that deposition occurred in a paralic environment in which sediments of continental origin, rich in terrestrial faunal remains, accumulated together with otoliths of marine fish taxa. Additional evidence from sea fish remains shows that normal marine conditions were already established in the Mediterranean during the post-evaporitic Lago-Mare phase of the MSC (see also Carnevale et al., 2006a, 2006b, 2008; Grunert et al., 2016).
- Gastropods (53 taxa) and bivalves (15 taxa) are dominated by terrestrial and brackish taxa. Freshwater elements are extremely rare, thus suggesting that permanent freshwater bodies had limited extent or were absent from the nearest surroundings. The terrestrial assemblage indicates the presence of rocky outcrops and open woodlands developed in relatively dry/mesic conditions, with limited moist environments. Brackish taxa indicate a Lago-Mare biofacies with oligo-meso-haline salinity.
- Some of the amphibians (eight taxa) and reptiles (13 taxa) found in the MCC samples, such as Chelotriton sp. and Scolecophidia indet., are reported from Italy for the first time. Overall, the herpetofaunal assemblage is indicative of a broad range of habitats, including ephemeral and permanent freshwater basins and rocky outcrops.
- Bird remains include at least 12 taxa. Piciidae and Coliidae are distinctly indicative of bushlands/woodlands.
- Large mammals consist of 12 species. Overall, these indicate a mosaic landscape dominated by woodlands alternated with scattered and limited grasslands.
- Small mammals are represented by 26 species. They indicate the presence of woodlands and rare open areas, rocky outcrops and water edges. Small changes in the abundances of murids and glirids are recorded throughout the succession, probably in relation to local variations of the paleolandscape. Small mammals indicate mesic climatic conditions, strongly supporting previous paleoclimatic interpretations that indicate a subtropical sub-humid climate, with moderately high precipitation rates and relatively warm temperatures in northern Italy slightly before the Pliocene.
- Overall, the high diversity of the terrestrial fossil fauna of Moncucco Torinese indicates a mosaic landscape characterized by thinned out, open woodlands associated with grasslands, rocky outcrops and rather rare humid areas with more closed forests.
CONTRIBUTIONS OF AUTHORS
SC drafted the manuscript and homogenized the contributions of the different authors. CD’A, DE, GP, MH, TAN and PG studied the mollusks (Gastropoda and Bivalvia); GC studied fish otoliths; MD and AV studied Amphibia and Reptilia; MP studied Aves; PPAM studied large mammals (Perissodactyla, Artiodactyla and Carnivora) with the exclusion of Primates that were studied by DMA; SC studied Eulipotyphla, Rodentia and Lagomorpha. Statistical analyses were performed by MM.
ACKNOWLEDGEMENTS
Field research was facilitated by collaboration with the Fassa Bortolo factory (owner of the gypsum quarry of Moncucco Torinese) with particular attention to Ing. Igor Muller and by synergy with the Soprintendenza ai Beni Archeologici del Piemonte with particular participation of Dr. P. Aurino. We are grateful to O. Mandic (Natural History Museum Vienna) for the useful discussion on the systematics of Lymnocardiinae and to E. Tschopp (Dipartimento Scienze della Terra di Torino) for his comments and help during the preparation of the MS. L. Ferro, B. Orrù and O. Giuliano took some of the photos used for the figures of the herpetofauna. This manuscript also benefited from the comments of one anonymous reviewer and from the comments of the handling editor B. Beatty and the style editor K. Travouillon. This study contributes to the FWF (Austrian Science Fund) project P25365-B25 and has been supported by Rome University Sapienza grant (2012) to DE and the Generalitat de Catalunya (2014 SGR 416 GRC and CGL2016-76431-P [AEI/FEDER, EU]) to DMA and MD, MIUR PRIN 2009MSSS9L_002 and University of Torino ex-60% 2015, 2016 grants to GC. AV acknowledges support of Synthesys grant FR-TAF-5007.
REFERENCES
Abbazzi, L., Delfino, M., Gallai, G., Trebini, L., and Rook, L. 2008a. New data on the vertebrate assemblage of Fiume Santo (North-western Sardinia, Italy), and overview on the Late Miocene Tusco-Sardinian paleobioprovince. Palaeontology, 51:425-451.
Abbazzi, L., Benvenuti, M., Ceci, M.E., Esu, D., Faranda, C., Rook, L., and Tangocci, F. 2008b. The end of the Lago-Mare time in the SE Valdelsa Basin (Central Italy): interference between local tectonism and regional sea-level rise. Geodiversitas, 30:611-639.
Abramsky, Z. 1981. Habitat relationships and competition in two Mediterranean Apodemus spp. Oikos, 36:219-225.
Agustí, J., Garcés, M., and Krijgsman, W. 2006. Evidence for African-Iberian exchanges during the Messinian in the Spanish mammalian record. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:5-14.
Alba, D.M., Delson, E., Carnevale, G., Colombero, S., Delfino, M., Giuntelli, P., Pavia, M., and Pavia, G. 2014. First joint record of Mesopithecus and cf. Macaca in the Miocene of Europe. Journal of Human Evolution, 67:1-18.
Alba, D.M., Montoya, P., Pina, M., Rook, L., Abella, J., Morales, J., and Delson, E. 2015. First record of Mesopithecus (Cercopithecidae, Colobinae) from the Miocene of the Iberian Peninsula. Journal of Human Evolution, 88:1-14.
Amori, G., Hutterer, R., Kryštufek, B., Yigit, N., Mitsain, G., and Muñoz, L.J.P. 2008. Apodemus mystacinus. The IUCN Red List of Threatened Species 2008: e.T1898A8770676. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T1898A8770676.en.
Andrews, P. 2006. Taphonomic effects of faunal impoverishment and faunal mixing. Palaeogeography, Palaeoclimatology, Palaeoecology, 241:572-589.
Angelone, C. 2007. Messinian Prolagus (Ochotonidae, Lagomorpha) of Italy. Geobios, 40:407-421.
Angelone, C., Colombero, S., Esu, D., Giuntelli, P., Marcolini, F., Pavia, M., Trenkwalder, S., Van den Hoek Ostende, L.W., Zunino, M., and Pavia, G. 2011. Moncucco Torinese, a new post-evaporitic Messinian fossiliferous site from Piedmont (NW Italy). Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 259:89-104.
Angelone, C. and Rook, L. 2012. Late Neogene and Quaternary lagomorphs from Tuscany: a revision based on specimens in Basel Naturhistorisches Museum and Florence University collections. Swiss Journal of Palaeontology, 131:127-145.
Antoñanzas, R.L. and Bescós, G.C. 2002. The Gran Dolina site (Lower to Middle Pleistocene, Atapuerca, Burgos, Spain): new palaeoenvironmental data based on the distribution of small mammals. Palaeogeography, Palaeoclimatology, Palaeoecology, 186:311-334.
Arnold, N. and Ovenden, D. 2002. A Field Guide to the Reptiles and Amphibians of Britain and Europe. HarperCollins Publisher, London.
Azanza Baumel, J.J. and Witmer, L.M. 1993. Osteologia, p. 45-132. In Baumel, J.J. (ed.), Handbook of avian anatomy: Nomina Anatomica Avium. Second Edition. Publications of the Nuttal Ornithological Club 23. Nuttall Ornithological Club, Cambridge, Massachusets.
Azanza, B., Rössner, G.E., and Ortiz-Jaureguizar, E. 2013. The early Turolian (late Miocene) Cervidae (Artiodactyla, Mammalia) from the fossil site of Dorn-Dürkheim 1 (Germany) and implications on the origin of crown cervids. Palaeobiodiversity and Palaeoenvironments, 93:217-258.
Bachmayer, F. and Wilson, R.W. 1970. Die Fauna der altpliozänen Höhlen-und Spaltenfüllungen bei Kohfidisch, Burgenland (Österreich). Small Mammals (Insectivora, Chiroptera, Lagomorpha, Rodentia) from the Kohfidisch Fissures of Burgenland, Austria. Annalen des Naturhistorischen Museums in Wien, 74:533-587.
Bailon, S. 1999. Différenciation ostéologique des anoures (Amphibia, Anura) de France, p. 1-38. In Desse, J. and Desse-Berset, N. (eds.), Fiches d'Ostéologie Animale pour l'Archéologie, Série C: Varia. Centre de Recherches Archéologiques-CNRS, Valbonne.
Barahona, F. and Barbadillo, L.J. 1997. Identification of some Iberian lacertids using skull characters. Revista Española de Herpetología, 11:47-62.
Bedetti, C. and Pavia, M. 2013. Early Pleistocene birds from Pirro Nord (Puglia, southern Italy). Palaeontographica Abteilung A-Palaozoologie-Stratigraphie, 298:31-53.
Bego, F., Kryštufek, B., Paspali, G., and Rogozi, E. 2009. Small terrestrial mammals of Albania: annotated list and distribution. Hystrix, the Italian Journal of Mammalogy, 19:83-101.
Bertini, A. 2006. The Northern Apennines palynological record as a contribute for the reconstruction of the Messinian palaeoenvironments. Sedimentary Geology, 188:235-258.
Bertini, A. and Martinetto, E. 2011. Reconstruction of vegetation transects for the Messinian-Piacenzian of Italy by means of comparative analysis of pollen, leaf and carpological records. Palaeogeography, Palaeoclimatology, Palaeoecology, 304:230-246.
Black, C.C. and Kowalski, K. 1974. The Pliocene and Pleistocene Sciuridae (Mammalia, Rodentia) from Poland. Acta Zoologica Cracoviensia, 19:461-484.
Blain, H.A., Bailon, S., and Cuenca-Bescós, G. 2008. The Early-Middle Pleistocene palaeoenvironmental change based on the squamate reptile and amphibian proxies at the Gran Dolina site, Atapuerca, Spain. Palaeogeography, Palaeoclimatology, Palaeoecology, 261:177-192.
Boeters, H.D., Gittenberger, K., and Subai, P. 1989. Die Aciculidae (Mollusca Gastropoda, Prosobranchia). Zoologische Verhandelungen, 252:1-234.
Bosma, A.A., De Bruijn, H., and Wessels, W. 2013. Late Miocene Sciuridae (Mammalia, Rodentia) from Anatolia, Turkey. Journal of Vertebrate Paleontology, 33:924-942.
Brofas, G., Karetsos, G., Dimopoulos, P., and Tsagari, C. 2006. The natural environment of Cupressus sempervirens in Greece as a basis for its use in the Mediterranean region. Land Degradation & Development, 17:645-659.
Capizzi, D. and Filippucci, M.G. 2008. Famiglia Gliridae Muirhead, 1819, p. 378-441. In Amori, G., Contoli, L., and Nappi, A. (eds.), Fauna d’Italia - Mammalia II - Erinaceomorpha, Soricomorpha, Lagomorpha, Rodentia. Calderini, Milano.
Carnevale, G., Caputo, D., and Landini, W. 2006a. Late Miocene fish otoliths from the Colombacci Formation (Northern Apennines, Italy): Implications for the Messinian ‘Lago-mare’ event. Geological Journal, 41:537-555.
Carnevale, G., Landini, W., and Sarti, G. 2006b. Mare versus Lago-mare. Marine fishes and the Mediterranean environment at the end of the Messinian Salinity Crisis. Journal of the Geological Society, London, 163:75-80.
Carnevale, G., Longinelli, A., Caputo, D., Barbieri, M., and Landini, W. 2008. Did the Mediterranean marine reflooding precede the Mio-Pliocene boundary? Paleontological and geochemical evidence from upper Messinian sequences of Tuscany, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology, 257:81-105.
Cavallo, O., Sen, S., Rage, J.C., and Gaudant, J. 1993. Vertébrés messiniens du faciès à congéries de Ciabòt Cagna, Corneliano d’Alba (Piémont, Italie). Rivista Piemontese di Storia Naturale, 14:3-22.
Chesi, F. 2009. Il registro fossile italiano dei cheloni. Unpublished PhD Thesis, University of Florence, Italy.
Chesi, F., Delfino, M., Abbazzi, L., Carboni, S., Lecca, L., and Rook, L. 2007. New fossil vertebrate remains from San Giovanni di Sinis (Late Pleistocene, Sardinia): the last Mauremys (Reptilia, Testudines) in the Central Mediterranean. Rivista Italiana di Paleontologia e Stratigrafia, 113:287-297.
Chesi, F., Delfino, M., and Rook, L. 2009. Late Miocene Mauremys (Testudines, Geoemydidae) from Tuscany (Italy): evidence of terrapin persistence after a mammal turnover. Journal of Paleontology, 83:379-388.
CIESM 2008. The Messinian Salinity Crisis from mega-deposits to microbiology - a consensus report. F. Briand (ed.), CIESM Workshop Monographs, 33:1-168.
Cita, M.B., Colalongo, M.L., d'Onofrio, S., Iaccarino, S., and Salvatorini, G. (1978). Biostratigraphy of Miocene deep-sea sediments (Sites 372 and 375), with special reference to the Messinian/pre-Messinian interval. Initial Reports DSDP, 42:671-685.
Colombero, S., Angelone, C., Bonelli, E., Carnevale, G., Cavallo, O., Delfino, M., Giuntelli, P., Mazza, P., Pavia, G., Pavia, M., and Repetto, G. 2014a. The upper Messinian assemblages of fossil vertebrate remains of Verduno (NW Italy): Another brick for a latest Miocene bridge across the Mediterranean. Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen, 272:287-324.
Colombero, S., Bonelli, E., Kotsakis, T., Pavia, G., Pavia, M., and Carnevale, G. 2013. Late Messinian rodents from Verduno (Piedmont, NW Italy): Biochronological, paleoecological and paleobiogeographic implications. Geobios, 46:111-125.
Colombero, S., Bonelli, E., Pavia, M., Repetto, G., and Carnevale, G. 2016. Paracamelus (Mammalia, Camelidae) remains from the late Messinian of Italy: insights into the last camels of western Europe. Historical Biology, 29: 509-518.
Colombero, S. and Carnevale, G. 2016. Late Miocene (Turolian, MN13) squirrels from Moncucco Torinese, NW Italy. Comptes Rendus Palevol, 15: 515-526.
Colombero, S. and Pavia, G. 2013. Centralomysbenericettii (De Giuli, 1989) (Mammalia, Rodentia): a latest Messinian murid from northern and central Italy. New data from Piedmont. Bollettino della Società Paleontologica Italiana, 52:27-34.
Colombero, S., Pavia, G., and Carnevale, G. 2014. Messinian rodents from Moncucco Torinese, NW Italy: palaeobiodiversity and biochronology. Geodiversitas, 36:421-475.
Colombero, S., Pavia, M., and Carnevale, G. 2015. Old World porcupine (Rodentia, Hystricidae) remains from the late Messinian of Piedmont, NW Italy. Rivista Italiana di Paleontologia e Stratigrafia, 121:243-253.
Crochet, J.Y. 1986. Insectivores pliocènes du sud de la France (Languedoc-Roussillon) et du nord-est de l’Espagne. Palaeovertebrata, 16:145-171.
Cuenca Bescós, G. 2003. Analisis filogenético de Allocricetus del Pleistoceno (Cricetidae, Rodentia, Mammalia). Coloquios de Paleontología, 1:95-113.
Cuenca-Bescós, G., Rofes, J., and García-Pimienta, J. 2005. Environmental change across the Early-Middle Pleistocene transition: small mammalian evidence from the Trinchera Dolina cave, Atapuerca, Spain. Geological Society, London, Special Publications, 247:277-286.
Czyzewska, T. 1968. Deers from Węże and their relationship with the Pliocene and Recent Eurasiatic Cervidae. Acta Palaeontologica Polonica, 13:537-593.
Daams, R. 1981. The dental pattern of the dormice Dryomys, Myomimus, Microdyromys and Peridyromys. Utrecht Micropaleontological Bulletins. Special Publication, 3:1-115.
Daams, R., Freudenthal, M., and Van der Meulen, A.J. 1988. Ecostratigraphy of micromammal faunas from the Neogene of Spain. Scripta Geologica, Special Issue, 1:287-302.
Dahlmann, T. 2001. Die Kleinsäuger der unter-pliozänen Fundstelle Wölfersheim in der Wetterau (Mammalia: Lipotyphla, Chiroptera, Rodentia). Courier Forschungsinstitut Senckenberg, 227:1-129
Daxner-Höck, G. 2004. Flying squirrels (Pteromyinae, Mammalia) from the upper Miocene of Austria. Annalen des Naturhistorischen Museums in Wien. Serie A, 106:387-423.
Daxner-Höck, G. and Höck, E. 2009. New data on Eomyidae and Gliridae (Rodentia, Mammalia) from the late Miocene of Austria. Annalen des Naturhistorischen Museums in Wien. Serie A, 111:375-444.
De Bruijn, H., Daams, R., Daxner-Höck, G., Fahlbusch, V., Ginsburg, L., Mein, P., Morales, J., Heizmann, E., Mayhew, D.F., Van der Meulen, A.J., Schidt-Kittler, N., and Telles Antunes, M. 1992. Report of the RCMNS working group on fossil mammals, Reisensburg 1990. Newsletters on Stratigraphy, 2/3:65-118.
De Giuli, C. 1989. The rodents of the Brisighella latest Miocene fauna. Bollettino della Società Paleontologica Italiana, 28:197-212.
Dela Pierre, F., Bernardi, E., Cavagna, S., Clari, P., Gennari, R., Irace, A., Lozar, F., Lugli, S., Manzi, V., Natalicchio, M., Roveri, M., and Violanti, D. 2011. The record of the Messinian salinity crisis in the Tertiary Piedmont Basin (NW Italy): The Alba section revisited. Palaeogeography, Palaeoclimatology, Palaeoecology, 310:238-255.
Dela Pierre, F., Clari, P., Bernardi, E., Natalicchio, M., Costa, E., Cavagna, S., Lozar, F., Lugli, S., Manzi, V., Roveri, M., and Violanti, D. 2012. Messinian carbonate-rich beds of the Tertiary Piedmont Basin (NW Italy): microbially-mediated products straddling the onset of the salinity crisis. Palaeogeography, Palaeoclimatology, Palaeoecology, 344:78-93.
Dela Pierre, F., Festa, A., and Irace, A. 2007. Interaction of tectonic, sedimentary, and diapiric processes in the origin of chaotic sediments: An example from the Messinian of Torino Hill (Tertiary Piedmont Basin, northwestern Italy). Geological Society of America Bulletin, 119:1107-1119.
Delfino, M., 2002. Erpetofaune italiane del Neogene e del Quaternario. Unpublished PhD Thesis. University of Modena and Reggio Emilia, Italy.
Delfino, M. 2003. A Pleistocene amphisbaenian from Sicily. Amphibia-Reptilia, 24:407-414.
Delfino, M. and Bailon, S. 2000. Early Pleistocene herpetofauna from Cava Dell’Erba and Cava Pirro (Apulia, Southern Italy). Herpetological Journal, 10:95-110.
Delfino, M. and Rook, L. 2008. African crocodylians in the late Neogene of Europe. A revision of Crocodylus bambolii Ristori, 1890. Journal of Paleontology, 82:336-343.
Delfino, M. and Rossi, M.A. 2013. Fossil crocodylid remains from Scontrone (Tortonian, Southern Italy) and the Late Neogene Mediterranean biogeography of crocodylians. Geobios, 46:25-31.
Delfino, M. and Sala, B. 2007. Late Pliocene albanerpetontid (Lissamphibia) from Italy. Journal of Vertebrate Paleontology, 27:716-719.
Delfino, M., Böhme, M., and Rook, L. 2007. First European evidence for transcontinental dispersal of Crocodylus (late Neogene of southern Italy). Zoological Journal of the Linnean Society, 149:293-307.
Delfino, M., Kotsakis, T., Arca, M., Tuveri, C., Pitruzzella, G., and Rook, L. 2008. Agamid lizards from the Plio-Pleistocene of Sardinia (Italy) and an overview of the European fossil record of the family. Geodiversitas, 30:641-656.
Delfino, M., Pitruzzella, G., and Bailon, S. 2011. The Late Pliocene amphibians and reptiles from “Capo Mannu D1 Local Fauna” (Mandriola, Sardinia, Italy). Geodiversitas, 33:357-382.
Delfino, M., Ferro, L., Giuntelli, P., and Pavia, M. 2013. First Italian record of scolecophidian snakes (late Miocene, Moncucco Torinese), p. 106-107. In Scillitani, G., Liuzzi C., Lorusso, L., Mastropasqua, F., and Ventrella, P. (eds.), Atti IX Congresso Nazionale della Societas Herpetologica Italica, Bari-Conversano.
Delfino, M., Zoboli, D., Carnevale, G., and Pillola, G.L. 2014. The rediscovered holotype of Palaeopython sardus Portis, 1901 from the Miocene of Sardinia belongs to a fish, not a snake. Bollettino della Società Paleontologica Italiana, 53: 89-92.
Do Couto, D., Popescu, S.-M., Suc, J.-P., Melinte-Dobrinescu, M.C., Barhoun, N., Gorini, C., Jolivet, L., Poort, J., Jouannic, G., and Auxietre, J.-L. 2014 . Lago Mare and the Messinian Salinity Crisis: Evidence from the Alboran Sea (S. Spain) . Marine and Petroleum Geology, 52:57-76.
Doukas, C.S., Van den Hoek Ostende, L.W., Theocharopoulos, C., and Reumer, J.W.F. 1995. Insectivora (Erinaceidae, Talpidae, Soricidae, Mammalia), p. 43-64. In Schmidt-Kittler, N. (ed.), The Vertebrate Locality Maramena (Macedonia, Greece) at the Turolian-Ruscinian Boundary. Münchner Geowissenschaftliche Abhandlandlungen A, 28.
Elliott, M. and Dewailly, F. 1995. The structure and components of European estuarine fish assemblages. Netherlands Journal of Aquatic Ecology, 29:397-417.
Engesser, B. 1989. The late tertiary small mammals of the Maremma region (Tuscany, Italy); 2nd part: Muridae and cricetidae (Rodentia, Mammalia). Bollettino della Società Paleontologica Italiana, 28:227-252.
Estes, R. 1983. Sauria terrestria, Amphisbaenia. Handbuch der Paläoherpetologie, part 10A. Gustav Fischer Verlag, Stuttgart/New York.
Esu, D. 2007. Latest Messinian “Lago-Mare” Lymnocardiinae from Italy: Close relations with the Pontian fauna from the Dacic basin. Geobios, 40:291-302.
Esu, D. and Popov, S.V. 2012. Revision of late Messinian Lymnocardiinae (Bivalvia) from Piedmont (NW Italy). Rivista Italiana di Paleontologia e Stratigrafia, 118:343-356.
Evans, E.N., Van Couvering, J.A., and Andrews, P. 1981. Palaeoecology of Miocene sites in western Kenya. Journal of Human Evolution, 10:99-116.
Fahlbusch, V. 1969. Pliozäne und Pleistozäne Cricetinae (Rodentia, Mammalia) aus Polen. Acta Zoologica Cracoviensia, 14:99-138.
Fauquette, S., Suc, J.P., Bertini, A., Popescu, S.M., Warny, S., Taoufiq, N.B., Perez Villa, M.-J., Chikhi, H., Feddi, N., Subally, D., Clauzon, G., and Ferrier, J. 2006. How much did climate force the Messinian salinity crisis? Quantified climatic conditions from pollen records in the Mediterranean region. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:281-301.
Flynn, L.J., Winkler, A.J., Erbaeva, M., Alexeeva, N., Anders, U., Angelone, C., Čermák, S., Fladerer, F.A., Kraatz, B., Ruedas, L.A., Ruf, I., Tomida, Y., Veitschegger, K., and Zhang, Z. 2014. The Leporid Datum: a late Miocene biotic marker. Mammal Review, 44:164-176.
Freudenthal, M. 1971. Neogene Vertebrates from the Gargano Peninsula, Italy . Scripta Geologica, 3:1-10.
Frischling, B. 2016. Weather records and averages. http://www.weatherbase.com.
Furió, M. 2007. Los Insectívoros (Soricomorpha, Erinaceomorpha, Mammalia) del Neógeno superior del Levante Ibérico. PhD dissertation Universitat Autònoma de Barcelona. 299 pp.
Furió, M., Van Dam, J., and Kaya, F. 2014. New insectivores (Lipotyphla, Mammalia) from the late Miocene of the Sivas Basin, Central Anatolia. Bulletin of Geosciences, 89:163-181.
García-Alix, A. 2015. A multiproxy approach for the reconstruction of ancient continental environments. The case of the Mio-Pliocene deposits of the Granada Basin (southern Iberian Peninsula). Global and Planetary Change, 131:1-10.
García-Alix, A., Minwer-Barakat, R., Suárez, E.M., Freudenthal, M., and Martín, J.M. 2008. Late Miocene-Early Pliocene climatic evolution of the Granada Basin (southern Spain) deduced from the paleoecology of the micromammal associations. Palaeogeography, Palaeoclimatology, Palaeoecology, 265:214-225.
Ghetti, P., Anadón, P., Bertini, A., Esu, D., Gliozzi, E., Rook, L., and Soulié-Märsche, I. 2002. The Early Messinian Velona basin (Siena, central Italy): paleoenvironmental and paleobiogeographical reconstructions. Palaeogeography, Palaeoclimatology, Palaeoecology, 187:1-33.
Gilbert, C.C., Bibi, F., Hill, A., and Beech, M.J. 2014. Early guenon from the late Miocene Baynunah Formation, Abu Dhabi, with implications for cercopithecoid biogeography and evolution. Proceedings of the National Academy of Sciences, 111: 10119-10124.
Gibert, L., Scott, G.R., Montoya, P., Ruiz-Sánchez, F.J., Morales, J., Luque, L., Abella, J., and Lería, M. 2013. Evidence for an African-Iberian mammal dispersal during the pre-evaporitic Messinian. Geology, 41:691-694.
Göhlich, U.B. and Mourer-Chauviré, C. 2005. Revision of the phasianids (Aves: Galliformes) from the lower Miocene of Saint-Gérand-le-Puy (Allier, France). Palaeontology, 48:1331-1350.
Göhlich, U.B. and Pavia, M., 2008. A new species of Palaeortyx (Aves: Galliformes: Phasianidae) from the Neogene of Gargano, Italy. Orycto s, 7:95-108.
Gorsuch, W.A. and Larivière, S. 2005. Vormela peregusna. Mammalian Species, 779:1-5.
Grossi, F., Gliozzi, E., and Cosentino, D. 2011. Paratethyan ostracod immigrants mark the biostratigraphy of the Messinian Salinity Crisis. Joannea, Geologie und Paläontologie, 11:66-68.
Grunert, P., Harzhauser, M., Rosenthal, Y., and Carnevale, G. 2016. Estuarine Lago Mare fauna from the Tertiary Piedmont Basin indicates episodic Atlantic/Mediterranean exchange during the final stage of the Mediterranean Salinity Crisis. Palaeogeography, Palaeoclimatology, Palaeoecology, 457:70-79.
Guelorget, O. and Perthuisot, J.P. 1992. Paralic ecosystems: Biological organization and functioning. Vie et Milieu, 42:215-251.
Guerra-Merchán, A., Serrano, F., Garcés, M., Gofas, S., Esu, D., Gliozzi, E., and Grossi, F. 2010. Messinian Lago-Mare deposits near the Strait of Gibraltar (Malaga Basin, S Spain). Palaeogeography, Palaeoclimatology, Palaeoecology, 285:264-276.
Haller-Probst, M. and Schleich, H.-H. 1994. Vergleichende osteologische Untersuchungen an einigen Urodelen Eurasiens (Amphibia: Urodela, Salamandridae, Proteidae). Courier Forschungsinstitut Senckenberg, 173:23-77.
Hammer, Ø., Harper, D.A.T., and Ryan, P.D. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4.1.4:1-9 palaeo-electronica.org/2001_1/past/past.pdf
Harzhauser, M., Neubauer, T.A., Georgopoulou, E., Esu, D., D’Amico, C., Pavia, G., Giuntelli, P., and Carnevale, G. 2015. Late Messinian continental and Lago-Mare gastropods from the Tertiary Piedmont Basin, NW Italy. Bollettino della Società Paleontologica Italiana, 54:1-53.
Hernández Fernández, M. 2001. Análisis paleoecológico y paleoclimático de las sucesiones de mamíferos del Plio-Pleistoceno ibérico. Unpublished PhD Thesis. Universidad Complutense de Madrid, Madrid, Spain.
Hernández Fernández, M. and Peláez-Campomanes de Labra, P. 2003. Ecomorphological characterization of Murinae and hypsodont “Cricetidae”(Rodentia) from the Iberian Plio-Pleistocene. Coloquios de Paleontología, 1:237-251.
Hernández Fernández, M., Sierra, M.Á.Á., and Peláez-Campomanes, P. 2007. Bioclimatic analysis of rodent palaeofaunas reveals severe climatic changes in Southwestern Europe during the Plio-Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 251:500-526.
Hoffmann, M., Cuzin, F., and De Smet, K. 2015. Ictonyx libycus. The IUCN Red List of Threatened Species 2015: e.T41645A82561597.
http://dx.doi.org/10.2305/IUCN.UK.2015.RLTS.T41645A82561597.en
Holman, J.A. 1992. Hyla meridionalis from the Late Pleistocene (last interglacial age: Ipswichian) of Britain. British Herpetological Society Bulletin, 41:12-14.
Hordijk, K. and de Bruijn, H. 2009. The succession of rodent faunas from the Mio/Pliocene lacustrine deposits of the Florina-Ptolemais-Servia Basin (Greece). Hellenic Journal of Geosciences, 44:21-103.
Hosmer, Jr., D.W., Lemeshow, S., and Sturdivant, R.X. 2013. Applied Logistic Regression. Third edition. John Wiley & Sons. Hoboken, New Jersey.
Hugueney, M. 1999. Family Castoridae, p. 281-300. In Rössner, G.E. and Heissig, K. (eds.), The Miocene Land Mammals of Europe, Dr. Friedrich Pfeil, München.
Insacco, G., Spadola, F., Russotto, S., and Scaravelli, D. 2015. Eryx jaculus (Linnaeus, 1758): a new species for the Italian herpetofauna (Squamata: Erycidae). Acta Herpetologica, 10:149-153.
Ishii, N. and Kaneko, Y. 2008. Glirulus japonicus. The IUCN Red List of Threatened Species 2008: e.T9246A12971678. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T9246A12971678.en.
Juškaitis, R. and Šiožinytė, V. 2008. Habitat requirements of the common dormouse (Muscardinus avellanarius) and the fat dormouse (Glis glis) in mature mixed forest in Lithuania. Ekológia, 27:143-151.
Kerney, M.P., Cameron, R.A.D., and Jungbluth, J.H. 1979. Die Landschnecken Nord- und Mitteleuropas. Ein Bestimmungsbuch für Biologen und Naturfreunde. 384 pp., Parey, Hamburg.
Kirkman, L.K., Goebel, P.C., Palik, B.J., and West, L.T. 2004. Predicting plant species diversity in a longleaf pine landscape. Ecoscience, 11:80-93.
Klembara, J., Hain, M., and Dobiašová, K. 2014. Comparative anatomy of the lower jaw and dentition of Pseudopus apodus and the interrelationships of species of subfamily Anguinae (Anguimorpha, Anguidae). Anatomical Record, 297:516-544.
Kotsakis, T. 1989. Quelques observations sur les Castoridae du Turolien et du Ruscinien. Bolletino della Società Paleontologica Italiana, 28:271-276.
Köhler, M., Moyà-Solà, S., and Alba, D. M. 2000. Macaca (Primates, Cercopithecidae) from the Late Miocene of Spain. Journal of human evolution, 38:447-452.
Kormos, T. 1934. Neue und wenig bekannte Musteliden aus dem ungarischen Oberpliozän. Folia Zoologica Hydrobiologica 5:129-158.
Kotsakis, T., Abbazzi, L., Angelone, C., Argenti, P., Barisone, G., Fanfani, F., Marcolini, F., and Masini, F. 2003. Plio-Pleistocene biogeography of Italian mainland micromammals. Deinsea, 10:313-342.
Koufos, G.D., Kostopoulos, D.S., and Merceron, G. 2009. The late Miocene mammal faunas of the Mytilinii Basin, Samos Island, Greece: New Collection: 17. Palaeoecology-Palaeobiogeography. Beitrage zur Paläontologie, 31:409-430.
Koufos, G. D. and Vasileiadou, K. 2015. Miocene/Pliocene mammal faunas of southern Balkans: implications for biostratigraphy and palaeoecology. Palaeobiodiversity and Palaeoenvironments, 95:285-303.
Kovar-Eder, J., Kvaček, Z., Martinetto, E., and Roiron, P. 2006. Late Miocene to early Pliocene vegetation of southern Europe (7-4 Ma) as reflected in the megafossil plant record. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:321-339.
Kowalski, K. 1959. Baranogale helbingi Kormos and other Mustelidae from the bone breccia in Podlesice near Kroczyce (Poland). Acta Palaeontologica Polonica, 4: 61-69.
Kretzoi, M. 1959. Insectivoren, nagetiere und lagomorphen der jüngstpliozänen fauna von Csarnóta im Villányer Gebirge (Südungarn). Vertebrata Hungarica, 1:237-246.
Lacombat, F., Abbazzi, L., Ferretti, M.P., Martínez-Navarro, B., Moullé, P.E., Palombo, M.R., Rook, L., Turner, A., and Valli, A.M.F. 2008. New data on the early Villafranchian fauna from Vialette (Haute-Loire, France) based on the collection of the Crozatier Museum (Le Puy-en-Velay, Haute-Loire, France). Quaternary International, 179:64-71.
Lanza, B., Andreone, F., Bologna, M.A., Corti, C., and Razzetti, E. (eds.), 2007. Fauna d’Italia, Vol. XLII, Amphibia. Calderini, Bologna.
Larivière, S. 2001. Ursus amercanus. Mammalian Species, 647:1-11.
Larivière, S. 2002. Ictonyx striatus. Mammalian Species, 698:1-5.
Liu, L.P., Kostopoulos, D.S., and Fortelius M. 2004. Late Miocene Microstonyx remains (Suidae, Mammalia) from northern China. Geobios, 37:49-64.
López Martínez, N. 2001. Palaeobiogeographical history of Prolagus, a European ochotonid (Lagomorpha). Lynx, 32:215-231.
Lozar, F., Clari, P., Dela Pierre, F., Natalicchio, M., Bernardi, E., Violanti, D., Costa, E., and Giardino, M. 2015. Virtual tour of past environmental and climate change: the Messinian succession of the Tertiary Piedmont Basin (Italy). Geoheritage, 7:47-56.
Mansino, S., García-Alix, A., Ruiz-Sánchez, F.J., and Montoya, P. 2015a. A new Eliomys from the late Miocene of Spain, and its implications for the phylogeny of the genus. Acta Palaeontologica Polonica, 60:577-588.
Mansino, S., Ruiz-Sánchez, F.J., Fierro, I., and Montoya, P. 2015b. Mio-Pliocene rodent assemblages from Alcoi Forn (Alcoy Basin, Eastern Spain). Biostratigraphical and palaeoclimatical inferences. Historical Biology,
http://dx.doi.org/10.1080/08912963.2015.1102238]
Marchetti, M., Parolin, K., and Sala, B. 2000. The Biharian fauna from Monte La Mesa (Verona, northeastern Italy). Acta Zoologica Cracoviensia, 43:79-105.
Mariani, S. 2001. Can spatial distribution of ichthyofauna describe marine influence on coastal lagoons? A Central Mediterranean case study. Estuarine Coastal and Shelf Science, 52:261-267.
Marivaux, L., Vianey-Liaud, M., and Jaeger, J.J. 2004. High-level phylogeny of early Tertiary rodents: dental evidence. Zoological Journal of the Linnean Society, 142:105-134.
Martin, J.E., Smith, T., de Lapparent de Broin, F., Escuillie, F., and Delfino, M. 2014. Late Paleocene eusuchian remains from Mont de Berru, France and the origin of the alligatoroid Diplocynodon. Zoological Journal of the Linnean Society, 172:867-891.
Martín, C. and Sanchiz, B. 2015. Lisanfos KMS. Version 1.2. Online reference accessible at http://www.lisanfos.mncn.csic.es/. Museo Nacional de Ciencias Naturales, MNCN-CSIC. Madrid, Spain. Accessed 14 August 2015.
Martín-Suárez, E. and Mein, P. 1998. Revision of the genera Parapodemus, Apodemus, Rhagamys and Rhagapodemus (Rodentia, Mammalia). Geobios, 31:87-97.
Martı́n-Suárez, E., Freudenthal, M., and Civis, J. 2001. Rodent palaeoecology of the continental upper Miocene of Crevillente (Alicante, SE Spain). Palaeogeography, Palaeoclimatology, Palaeoecology, 165:349-356.
Martinetto, E., Uhl, D., and Tarabra, E. 2007. Leaf physiognomic indications for a moist warm-temperate climate in NW Italy during the Messinian (Late Miocene). Palaeogeography, Palaeoclimatology, Palaeoecology, 253:41-55.
Masini, F., Rinaldi, P.M., Savorelli, A., and Pavia, M. 2013. A new small mammal assemblage from the M013 Terre Rosse fissure filling (Gargano, South-Eastern Italy). Geobios, 46:49-61.
Mathieson, S., Cattrijsse, A., Costa, M.J., Drake, P., Elliott, M., Gardner, J., and Marchand, J. 2000. Fish assemblages of European tidal marshes: a comparison based on species, families and functional guilds. Marine Ecology Progress Series, 204:225-242.
Mayr, G. 2010. Mousebirds (Coliiformes), parrots (Psittaciformes), and other small birds from the late Oligocene/early Miocene of the Mainz Basin, Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 258:129-144.
Mazza, P. and Rustioni, M. 2011. Five new species of Hoplitomeryx from the Neogene of Abruzzo and Apulia (central and southern Italy) with revision of the genus and of Hoplitomeryx matthei Leinders, 1983. Zoological Journal of the Linnean Society, 163:1304-1333.
McCulloch, M. T. and Deckker, P. D. 1989. Sr isotope constraints on the Mediterranean environment at the end of the Messinian salinity crisis. Nature, 342: 62-65.
Meijer, H.J.M. 2013. A peculiar anseriform (Aves: Anseriformes) from the Miocene of Gargano (Italy). Comptes Rendus Palevol, 13:19-26.
Mein, P. and Martín-Suárez, E.M. (1993). Galerix iberica sp. nov. (Erinaceidae, Insectivora, Mammalia) from the late Miocene and early Pliocene of the Iberian Peninsula. Geobios, 26:723-730.
Merceron, G., Costeur, L., Maidet, O., Ramdarshan, A., and Göhlich, U.B. 2012. Mult-proxy approach detects heterogeneous habitats for primates during the Miocene climatic optimum in Central Europe. Journal of Human Evolution, 63:150-161.
Michaux, J. 1969. Muridae (Rodentia) du Pliocène supérieur d'Espagne et du Midi de la France. Palæovertebrata, 3:1-25.
Minwer-Barakat, R., García-Alix, A., Suárez, E.M., and Freudenthal, M. 2010. Soricidae (Soricomorpha, Mammalia) from the Pliocene of Tollo de Chiclana (Guadix Basin, Southern Spain). Journal of Vertebrate Paleontology, 30:535-546.
Mlíkovský, J. 1998. Two new owls (Aves: Strigidae) from the early Miocene of the Czech Republic, with comments on the fossil history of the subfamily Striginae. Buteo, 10:5-22.
Mlíkovský, J. 2002. Cenozoic birds of the World. Part 1: Europe. Ninox Press, Praha.
Monadjem, A., Taylor, P.J., Denys, C., and Cotterill, F.P.D. 2015. Rodents of Sub-Saharan Africa. A biogeographic and taxonomic synthesis. Walter de Gruyter GmbH, Berlin/Munich/Boston.
Montuire, S., Maridet, O., and Legendre, S. 2006. Late Miocene-early Pliocene temperature estimates in Europe using rodents. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:247-262.
Morlo, M. and Kundrát, M. 2001. The first carnivoran fauna from the Ruscinium (Early Pliocene, MN 15) of Germany. Paläontologische Zeitschrift, 75:163-187.
Mosca, P., Polino, R., Rogledi, S., and Rossi, M. 2010. New data for the kinematic interpretation of the Alps-Apennines junction (Northwestern Italy). International Journal of Earth Sciences, 99:833-849.
Natalicchio, M., Dela Pierre, F., Lugli, S., Lowenstein, T.K., Feiner, S.J., Ferrando, S., Manzi, V., Roveri, M., and Clari, P. 2014. Did Late Miocene (Messinian) gypsum precipitate from evaporated marine brines? Insights from the Piedmont Basin (Italy). Geology, 42:179-182.
Neubauer, T.A., Harzhauser, M., Georgopoulou, E., Kroh, A., and Mandic, O. 2015b. Tectonics, climate, and the rise and demise of continental aquatic species richness hotspots. Proceedings of the National Academy of Sciences USA, 112:11478-11483.
Neubauer, T.A., Harzhauser, M., Kroh, A., Georgopoulou, E., and Mandic, O. 2015a. A gastropod-based biogeographic scheme for the European Neogene freshwater systems. Earth-Science Reviews, 143:98-116.
Nevesskaya L.A., Paramonova N.P., and Popov S.V. 2001. History of Lymnocardiinae (Bivalvia, Cardiidae). Paleontological Journal, 35 (Supplement 3):147-217.
Nolf, D. 1985. Otolithi Piscium, p. 1-145 In Schultze, H.-P. (ed.), Handbook of Paleoichthyology. Günther Fischer Verlag, Stuttgart, New York.
Nolf, D. 2013. The Diversity of Fish Otoliths Past and Present. Royal Belgian Institute of Natural Sciences, Bruxelles.
Nordlie, F.G. 2003. Fish communities of estuarine salt marshes of Eastern North America, and comparisons with temperate estuaries of other continents. Review of Fish Biology and Fisheries, 13:281-325.
Panchetti, F., Amori, G., Carpaneto, G.M., and Sorace, A. 2004. Activity patterns of the common dormouse (Muscardinus avellanarius) in different Mediterranean ecosystems. Journal of Zoology, 262:289-294.
Papanastasis, V.P., Mantzanas, K., Dini-Papanastasi, O., and Ispikoudis, I. 2009. Traditional agroforestry systems and their evolution in Greece, p. 89-109. In Rigueiro-Rodroguez, A., McAdam, J., and Mosquera-Losada, M.R. (eds.), Agroforestry in Europe: Current Status and Future Prospects. Springer, Dordrecht,
Patacca, E., Scandone, P., and Carnevale, G. 2013. The Miocene vertebrate-bearing deposits of Scontrone (Abruzzo, Central Italy): Stratigraphic and paleoenvironmental analysis. Geobios, 46:5-23.
Pavia, M. 2013. The Anatidae and Scolopacidae (Aves: Anseriformes, Charadriiformes) from the late Neogene of Gargano, Italy. Geobios, 46:43-48.
Pavia, M., Göhlich, U.B., and Mourer-Chauviré, C. 2012. Description of the type-series of Palaeocryptonyx donnezani Depéret, 1892 (Aves: Phasianidae) with the selection of a lectotype. Comptes Rendus Palevol, 11:257-263.
Pavia, M., Meijer, H.J.M., Rossi, M.A., and Göhlich, U.B. 2017. The extreme insular adaptation of Garganornis ballmanni Meijer, 2014: a giant Anseriformes of the Neogene of the Mediterranean Basin. Royal Society Open Science, 4:160722.
Pickett, S.T. and White, P.S. (eds.) 1985. The Ecology of Natural Disturbance and Patch Dynamics. Academic Press. Orlando - United Kingdom Edition, London.
Piñero, P., Agustí, J., Blain, H.A., Furió, M., and Laplana, C. 2015. Biochronological data for the Early Pleistocene site of Quibas (SE Spain) inferred from rodents assemblage. Geologica Acta, 13:229-241.
Popov, V.V. 2003. Late Pliocene Soricidae (Insectivora, Mammalia) from Varshets (North Bulgaria). Acta zoologica cracoviensia, 46:43-72
Qiu, Z. and Storch, G. 2000. The early Pliocene micromammalian fauna of Bilike, Inner Mongolia, China (Mammalia: Lipotyphla, Chiroptera, Rodentia, Lagomorpha). Senckenbergiana lethaea, 80:173-229.
Qiu, Z.X., Deng, T., and Wang, B.Y. 2009. First ursine bear material from Dongxiang, Gansu-Addition to the Longdan Mammalian Fauna (2). Vertebrata PalAsiatica, 4:245-264.
Quinn, R.D. 1994. Animals, fire, and vertebrate herbivory in Californian Chaparral and other Mediterranean-type ecosystems, p. 46-78. In Moreno, J.M., and Oechel, W.C. (eds.), The Role of Fire in Mediterranean-Type Ecosystems. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Ray, J. 1995. Civettictis civetta. Mammal species, 488:1-7.
Recher, H.F., Lunney, D., and Matthews, A. 2009. Small mammal populations in a eucalypt forest affected by fire and drought. I. Long-term patterns in an era of climate change. Wildlife Research, 36:143-158.
Reed, K.E. and Rector, A.L. 2007. African Pliocene paleoecology: Hominin habitats, resources, and diets, p. 262-288. In Ungar, P.S. (ed.), Evolution of the Human Diet: The Known, the Unknown, and the Unknowable. Oxford University Press, Oxford,
Renaud, S., Benammi, M., and Jaeger, J.J. 1999. Morphological evolution of the murine rodent Paraethomys in response to climatic variations (Mio-Pleistocene of North Africa). Paleobiology, 25:369-382.
Reumer, J.W.F. 1984. Ruscinian and early Pleistocene Soricidae (Insectivora, Mammalia) from Tegelen (The Netherlands) and Hungary. Scripta Geologica, 73:1-173.
Roček, Z. 1994. Taxonomy and distribution of Tertiary discoglossid (Anura) of the genus Latonia V. Meyer, 1843. Geobios, 27:717-751.
Rook, L., Delfino, M., and Sami, M. 2015. I vertebrati fossili della cava del Monticino di Brisighella: una finestra sui popolamenti continentali del Mediterraneo nel Miocene superiore. In Lucci, P. and Piastra, S. (eds.), I Gessi di Brisighella e Rontana. Studio multidisciplinare di un’area carsica nella Vena del Gesso Romagnola. Memorie dell’Istituto Italiano di Speleologia, 28:79-100.
Rook, L., Oms, O., Benvenuti, M.G., and Papini, M. 2011. Magnetostratigraphy of the Late Miocene Baccinello-Cinigiano basin (Tuscany, Italy) and the age of Oreopithecus bambolii faunal assemblages. Palaeogeography, Palaeoclimatology, Palaeoecology, 305:286-294.
Roveri, M., Lugli, S., Manzi, V., Gennari, R., and Schreiber, B.C. 2014. High-resolution strontium isotope stratigraphy of the Messinian deep Mediterranean basins: Implications for marginal to central basins correlation. Marine Geology, 349:113-125.
Runkle, J.R. 1985. Disturbances regimes in temperate Forest, p. 17-33. In Pickett, S.T.A. and White, P.S. (eds.), The Ecology of Natural Disturbances and Patch Dynamics. Academic Press. Orlando - United Kingdom Edition, London.
Rybczynski, N. 2007. Castorid phylogenetics: implications for the evolution of swimming and tree-exploitation in beavers. Journal of Mammalian Evolution, 14:1-35.
Rzebik-Kowalska, B. 1989. Pliocene and Pleistocene Insectivora (Mammalia) of Poland. V. Soricidae: Petenyia Kormos, 1934 and Blarinella Thomas, 1911. Acta Zoologica Cracoviensia, 32:521-546.
Rzebik-Kowalska, B. 1994. Pliocene and Quaternary Insectivora [Mammalia] of Poland. Acta Zoologica Cracoviensia, 1:77-136.
Sacco, F. 1889. I cheloni astiani del Piemonte. Memorie della Reale Accademia delle Scienze di Torino, 39:427-461
Safford, H.D. and Harrison, S. 2004. Fire effects on plant diversity in serpentine vs. sandstone chaparral. Ecology, 85:539-548.
Salesa, M.J., Antón, M., Turner, A., and Morales, J. 2006. Inferred behaviour and ecology of the primitive sabre-toothed cat Paramachairodus ogygia (Felidae, Machairodontinae) from the late Miocene of Spain. Journal of Zoology, 268:243-54.
Salesa, M.J., Pesquero,, M.D., Siliceo G., Antón, M., Alcalá, L., and Morales, J. 2012. A rich community of Felidae (Mammalia, Carnivora) from the Late Miocene (Turolian, MN 13) site of Las Casiones (Villalba Baja, Teruel, Spain). Journal of Vertebrate Paleontology, 32: 658-676.
Sanchiz, B. 1998. Salientia. Handbuch der Paläoherpetologie, part 4. Pfeil, München.
Santini, L. 1980. The habits and influence on the environment of the Old World Porcupine Hystrix cristata L. in the northernmost part of its range, p. 149-153. In Clark, J.P. and Marsh, R.E. (eds.), Proceedings of the 9th Vertebrate Pest Conference (1980). University of California, Davis, California.
SAS Institute Inc. 2011. SAS/STAT® 9.3 User’s Guide. Cary, NC:SAS Institute Inc.
Sasaki, K. 1989. Phylogeny of the family Sciaenidae, with notes on its zoogeography (Teleostei, Perciformes). Memoirs of the Faculty of Fisheries, Hokkaido University, 36:1-137.
Savorelli, A., Colombero, S., and Masini, F. 2016. Apatodemus degiulii n. gen et sp. (Rodentia, Muridae), a hitherto undescribed endemite from the terre Rosse of Gargano (Late Miocene, Southeastern Italy). Palaeontographica Abteilung A: Palaeozoology - Stratigraphy, 306:25-49.
Schäfer, W. 1966. Aktuopaläontologische Beobachtungen 6. Otolithen-Anreicherungen. Natur und Museum, 69:439-444.
Sen, S. and Purabrishemi, Z. 2010. First porcupine fossils (Mammalia, Rodentia) from the late Miocene of NW Iran, with notes on late Miocene-Pliocene dispersal of porcupines. Paläontologische Zeitschrift, 84:239-248.
Siori, M.S., Boero, A., Carnevale, G., Colombero, S., Delfino, M., Sardella, R., and Pavia, M. 2014. New data on Early Pleistocene vertebrates from Monte Argentario (Central Italy). Paleoecological and biochronological implications. Geobios, 47:403-418.
Spassov, N., Tzankov, T., and Geraads, D. 2006. Late Neogene stratigraphy, biochronology, faunal diversity and environments of South-West Bulgaria (Struma River Valley). Geodiversitas, 28:477-498.
Speybroeck, J., Beukema, W., and Crochet, P.A. 2010. A tentative species list of the European herpetofauna (Amphibia and Reptilia) - an update. Zootaxa, 2492:1-27.
Stefen, C. 2009. The European Tertiary beaver Chalicomys jaegeri (Rodentia: Castoridae) revisited. Kaupia, 16:161-175.
Stefen, C. 2011. A brief overview of the evolution of European tertiary beavers. Baltic Forestry, 17: 148-153.
Stuart, C., Stuart, T., and Hoffmann, M. 2008. Poecilogale albinucha. The IUCN Red List of Threatened Species 2008: e.T41662A10530450. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T41662A10530450.en
Sulimski, A. 1959. Pliocene insectivores from Weze. Acta Palaeontologica Polonica, 4:119-173.
Sulimski, A. 1964. Pliocene Lagomorpha and Rodentia from Weze 1 (Poland). Acta Palaeontologica Polonica, 9:149-240.
Sumida, S.S. and Murphy, R.W. 1987. Form and function of the tooth crown structure in gekkonid lizards (Reptilia, Squamata, Gekkonidae). Canadian Journal of Zoology, 65:2886-2892.
Swanson, M.E., Franklin, J.F., Beschta, R.L., Crisafulli, C.M., Della Sala, D.A., Hutto, R.L., Lindenmaver, D.B., and Swanson, F.J. 2010. The forgotten stage of forest succession: early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment, 9:117-125.
Szyndlar, Z. 1984. Fossil snakes from Poland. Acta Zoologica Cracoviensia, 28:1-156.
Szyndlar, Z. 1991a. A review of Neogene and Quaternary snakes of Central and Eastern Europe. Part I: Scolecophidia, Boidae, Colubrinae. Estudios Geológicos, 47:103-126.
Szyndlar, Z. 1991b. A review of Neogene and Quaternary snakes of Central and Eastern Europe. Part II: Natricinae, Elapidae,Viperidae. Estudios Geológicos, 47:237-266.
Thorington, Jr, R.W., Koprowski, J.L., Steele, M.A., and Whatton, J.F. 2012. Squirrels of the world. JHU Press, Baltimore.
Tong, H. 2008. Quaternary Hystrix (Rodentia, Mammalia) from North China: Taxonomy, stratigraphy and zoogeography, with discussions on the distribution of Hystrix in Palearctic Eurasia. Quaternary International, 179:126-134.
Torre, I. and Díaz, M. 2004. Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecologica, 25:137-142.
Trabaud, L. 1994. Postfire Plant community dynamics in the Mediterranean basin, p. 1-15. In Moreno, J.M., and Oechel, W.C. (eds.), The Role of Fire in Mediterranean-Type ecosystems. Springer-Verlag, New York.
Van Dam, J. 1996. Stephanodonty in fossil murids, p. 449-461. In Marcus, L.F., Corti, M., Loy, A., Naylor, G.J.P., and Slice, E. (eds.), Advances in morphometrics Springer US., New York.
Van Dam, J.A. 2006. Geographic and temporal patterns in the late Neogene (12-3 Ma) aridification of Europe: the use of small mammals as paleoprecipitation proxies. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:190-218.
Van Dam, J.A. and Weltje, G.J. 1999. Reconstruction of the late Miocene climate of Spain using rodent palaeocommunity successions: an application of end-member modelling. Palaeogeography, Palaeoclimatology, Palaeoecology, 151:267-305.
Van de Weerd, A. 1976. Rodent faunas of the Mio-Pliocene continental sediments of the Teruel-Alfambra region, Spain. Utrecht Micropaleontological Bulletins.Special Publication, 2:1-217.
Van den Hoek Ostende, L.W. 2001. A revised generic classification of the Galericini (Insectivora, Mammalia) with some remarks on their palaeobiogeography and phylogeny. Geobios, 34:681-695.
Van der Made, J., Morales J., and Montoya, P. 2006. Late Miocene turnover in the Spanish mammal record in relation to palaeoclimate and the Messinian Salinity Crisis. Palaeogeography, Palaeoclimatology, Palaeoecology, 238:228-246.
Vasileiadou, K.V., Koufos, G.D., and Syrides, G.E. 2003. Silata, a new locality with micromammals from the Miocene/Pliocene boundary of the Chalkidiki peninsula, Macedonia, Greece. Deinsea, 10:549-562.
Venczel, M. and Gardner, J.D. 2005. The geologically youngest albanerpetontid amphibian, from the lower Pliocene of Hungary. Palaeontology, 48:1273-1300.
Villier, B. and Carnevale, G. 2013. A new skeleton of the giant hedgehog Deinogalerix from the Miocene of Gargano, southern Italy. Journal of Vertebrate Paleontology, 33:902-923.
Villier, B., van den Hoek Ostende, L.W., de Vos, J., and Pavia, M. 2013. New discoveries on the giant hedgehog Deinogalerix from the Miocene of Gargano (Apulia, Italy). Geobios, 46:63-75.
Violanti, D., Dela Pierre, F., Trenkwalder, S., Lozar, F., Clari, P., Irace, A., and D’Atri, A. 2011. Biostratigraphic and palaeoenvironmental analyses of the Messinian/Zanclean boundary and Zanclean succession in the Moncucco quarry (Piedmont, northwestern Italy). Bulletin de la Société Géologique de France, 182:149-162.
Vislobokova, I. A. 2007. New data on late Miocene mammals of Kohfidisch, Austria. Paleontological Journal, 41:451-460.
Viret, J. 1954. Le Loess à bancs durcis de Saint-Vallier, Drôme, et sa faune de mammifères villafranchiens. Nouvelles archives du Muséum d'histoire naturelle de Lyon, 4:1-200.
Von Den Driesch, A. 1976. A guide to the measurement of animal bones from archaeological sites: as developed by the Institut für Palaeoanatomie, Domestikationsforschung und Geschichte der Tiermedizin of the University of Munich (Vol. 1). Peabody Museum Press, 1:1-137.
Young, T.P. 1995. Landscape mosaics created by canopy gaps, forest edges and bushland glades. Selbyana, 16:127-134.
Welter-Schultes, F.W. 2012. European non-marine mollusks, a guide for species identification. Planet Poster Editions, Göttingen.
Werdelin, L., O’Brien, S., Johnson, W., and Yamaguchi, N. 2010. Phylogeny and evolution of cats (Felidae), p. 60-82. In Macdonald, D.W and Loveridge, A.J. (eds.), Biology and Conservation of Wild Felids. Oxford University Press. Oxford, UK.
Whitfield, A.K. 1999. Ichthyofaunal assemblages in estuaries: A South African case study. Review of Fish Biology and Fisheries, 9:151-186.
Willett, S.D., Schlunegger, F., and Picotti, V. 2006. Messinian climate change and erosional destruction of the central European Alps. Geology, 34:613-616.
Williams, S.E., Marsh, H., and Winter, J. 2002. Spatial scale, species diversity, and habitat structure: small mammals in Australian tropical rain forest. Ecology, 83:1317-1329.
Zagas, T., Tsitsoni, T., and Hatzistathis, A. 2001. The mixed forests of Greece. Silva Gandavensis, 66:68-75.
Zozaya, E.L., Brotons, L., and Vallecillo, S. 2011. Bird community responses to vegetation heterogeneity following non-direct regeneration of Mediterranean forests after fire. Ardea, 99:73-84.

